Abstract
Background
Oral mucositis is a side effect of chemotherapy, head and neck radiotherapy, and targeted therapy, affecting over 75% of high‐risk patients. Ulceration can lead to severe pain and difficulty with eating and drinking, which may necessitate opioid analgesics, hospitalisation and supplemental nutrition. These complications may disrupt cancer therapy, which may reduce survival. There is also a risk of death from sepsis if pathogens enter the ulcers of immunocompromised patients. Ulcerative oral mucositis can be costly to healthcare systems, yet there are few preventive interventions proven to be beneficial. Cytokines and growth factors may help the regeneration of cells lining of the mouth, thus preventing or reducing oral mucositis and its negative effects.
Objectives
To assess the effects of cytokines and growth factors for preventing oral mucositis in patients with cancer who are receiving treatment.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (searched 10 May 2017); the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4) in the Cochrane Library (searched 10 May 2017); MEDLINE Ovid (1946 to 10 May 2017); Embase Ovid (7 December 2015 to 10 May 2017); CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 10 May 2017); and CANCERLIT PubMed (1950 to 10 May 2017). The US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials.
Selection criteria
We included parallel‐design randomised controlled trials (RCTs) assessing the effects of cytokines and growth factors in patients with cancer receiving treatment.
Data collection and analysis
Two review authors independently screened the results of electronic searches, extracted data and assessed risk of bias. For dichotomous outcomes, we reported risk ratios (RR) and 95% confidence intervals (CI). For continuous outcomes, we reported mean differences (MD) and 95% CIs. We pooled similar studies in random‐effects meta‐analyses. We reported adverse effects in a narrative format.
Main results
We included 35 RCTs analysing 3102 participants. Thirteen studies were at low risk of bias, 12 studies were at unclear risk of bias, and 10 studies were at high risk of bias.
Our main findings were regarding keratinocyte growth factor (KGF) and are summarised as follows.
There might be a reduction in the risk of moderate to severe oral mucositis in adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers (RR 0.89, 95% CI 0.80 to 0.99; 6 studies; 852 participants; low‐quality evidence). We would need to treat 11 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 6 to 112). There might be a reduction in the risk of severe oral mucositis in this population, but there is also some possibility of an increase in risk (RR 0.85, 95% CI 0.65 to 1.11; 6 studies; 852 participants; low‐quality evidence). We would need to treat 10 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 5 to prevent the outcome to 14 to cause the outcome).
There is probably a reduction in the risk of moderate to severe oral mucositis in adults receiving radiotherapy to the head and neck with cisplatin or fluorouracil (RR 0.91, 95% CI 0.83 to 1.00; 3 studies; 471 participants; moderate‐quality evidence). We would need to treat 12 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 7 to infinity). It is very likely that there is a reduction in the risk of severe oral mucositis in this population (RR 0.79, 95% CI 0.69 to 0.90; 3 studies; 471 participants; high‐quality evidence). We would need to treat 7 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 5 to 15).
It is likely that there is a reduction in the risk of moderate to severe oral mucositis in adults receiving chemotherapy alone for mixed solid and haematological cancers (RR 0.56, 95% CI 0.45 to 0.70; 4 studies; 344 participants; moderate‐quality evidence). We would need to treat 4 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 3 to 6). There might be a reduction in the risk of severe oral mucositis in this population (RR 0.30, 95% CI 0.14 to 0.65; 3 studies; 263 participants; low ‐quality evidence). We would need to treat 10 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 8 to 19).
Due to the low volume of evidence, single‐study comparisons and insufficient sample sizes, we found no compelling evidence of a benefit for any other cytokines or growth factors and there was no evidence on children. There did not appear to be any serious adverse effects of any of the interventions assessed in this review.
Authors' conclusions
We are confident that KGF is beneficial in the prevention of oral mucositis in adults who are receiving: a) radiotherapy to the head and neck with cisplatin or fluorouracil; or b) chemotherapy alone for mixed solid and haematological cancers. We are less confident about a benefit for KGF in adults receiving bone marrow/stem cell transplant after conditioning therapy for haematological cancers because of multiple factors involved in that population, such as whether or not they received total body irradiation (TBI) and whether the transplant was autologous (the patients' own cells) or allogeneic (cells from a donor). KGF appears to be a relatively safe intervention.
Due to limited research, we are not confident that there are any beneficial effects of other cytokines and growth factors. There is currently insufficient evidence to draw any conclusions about the use of cytokines and growth factors in children.
Plain language summary
Can cytokines and growth factors help prevent mouth soreness and ulcers (oral mucositis) in patients being treated for cancer?
Review question
This review has been produced to assess whether or not the use of cytokines and growth factors during cancer treatment, can help prevent mouth soreness and ulcers.
Background
Sore mouth and ulcers (oral mucositis) is a side effect of treatment for cancer including chemotherapy, head and neck radiotherapy, and targeted therapy, affecting over 75% of high‐risk patients. Ulcers can lead to severe pain and difficulty with eating and drinking. Sufferers may need strong painkillers, possibly have to go into hospital and even be fed through a tube into their stomach or their veins.
These complications may disrupt their cancer therapy, meaning they are not receiving the best treatment, which may reduce survival. Cancer patients have weakened immune systems due to their treatment and are less able to fight infections. An ulcer is an open wound and there is a risk that bacteria can enter the body leading to infection or sepsis (a dangerous inflammatory reaction of the body to infection).
Mouth soreness and ulcers can be costly to healthcare systems, yet there are few preventive interventions or treatments proven to be beneficial. Cytokines and growth factors may help the regeneration of cells lining the mouth, thus preventing or reducing oral mucositis and its negative effects.
Study characteristics
Authors from Cochrane Oral Health carried out this review of existing studies and the evidence is current up to 10 May 2017. It includes 35 studies (published between 1993 and 2017) with 3102 participants, all patients being treated for cancer, aged from 1 to 87 years old. Review authors included studies comparing cytokines and growth factors for the prevention of oral mucositis. The studies were carried out all over the world and often featured multiple sites, although most took place in high‐income countries.
Main results
The main findings were regarding keratinocyte growth factor (KGF). KGF is likely to reduce the risk of oral mucositis in adults who are receiving either radiotherapy to the head and neck with chemotherapy (cisplatin or fluorouracil), or chemotherapy alone for mixed solid and blood cancers. KGF may also reduce the risk of oral mucositis in adults receiving bone marrow/stem cell transplant after conditioning therapy for blood cancers, but these results are less clear because of multiple complicating factors. KGF appears to be a relatively safe intervention. There did not appear to be any serious adverse effects of any of the interventions assessed in this review.
Due to limited research, review authors are uncertain of any beneficial effects of other cytokines and growth factors. There is currently insufficient evidence to draw any conclusions about the use of cytokines and growth factors in children.
Quality of the evidence
For reducing oral mucositis in adults receiving radiotherapy to the head and neck with chemotherapy, review authors rated the evidence for KGF as moderate to high quality. For reducing oral mucositis in adults receiving chemotherapy alone for mixed solid and blood cancers, they rated the evidence for KGF as low to moderate quality. This evidence was downgraded due to there not being enough data and because some results have not yet been published. For reducing oral mucositis in adults receiving bone marrow/stem cell transplant after conditioning therapy for blood cancers, they rated the evidence for KGF as low quality because results were not similar across the studies and some results have not yet been published. Evidence on side effects of KGF was poorly reported and inconsistent.
Summary of findings
Summary of findings for the main comparison. Keratinocyte growth factor (KGF) compared to placebo for preventing oral mucositis in adults with cancer receiving treatment.
| KGF compared to placebo for preventing oral mucositis in adults with cancer receiving treatment | ||||||
| Patient or population: adults** receiving treatment for cancer (see subgroup for treatment type) Setting: hospital Intervention: KGF Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with KGF | |||||
| Oral mucositis (moderate + severe) | BMT/SCT after conditioning for haematological cancers | RR 0.89 (0.80 to 0.99) | 852 (6 studies) | ⊕⊕⊝⊝ LOW1 | There might be a benefit for KGF in this population NNTB = 11 (95% CI 6 to 112) |
|
| 848 per 1000 | 755 per 1000 (678 to 839) | |||||
| RT to head and neck with cisplatin/5FU | RR 0.91 (0.83 to 1.00) | 471 (3 studies) | ⊕⊕⊕⊝ MODERATE2 | There is probably a benefit for KGF in this population NNTB = 12 (95% CI 7 to ∞) |
||
| 932 per 1000 | 848 per 1000 (773 to 932) | |||||
| CT alone for mixed cancers | RR 0.56 (0.45 to 0.70) | 344 (4 studies) | ⊕⊕⊕⊝ MODERATE3 | It is likely that there is a benefit for KGF in this population NNTB = 4 (95% CI 3 to 6) |
||
| 631 per 1000 | 353 per 1000 (284 to 441) | |||||
| Oral mucositis (severe) | BMT/SCT after conditioning for haematological cancers | RR 0.85 (0.65 to 1.11) | 852 (6 studies) | ⊕⊕⊝⊝ LOW4 | There might be a benefit for KGF in this population, but there is also some possibility of an increase in risk NNTB = 10 (95% CI 5 NNTB to 14 NNTH) |
|
| 677 per 1000 | 575 per 1000 (440 to 751) | |||||
| RT to head and neck with cisplatin/5FU | RR 0.79 (0.69 to 0.90) | 471 (3 studies) | ⊕⊕⊕⊕ HIGH | It is very likely that there is a benefit for KGF in this population NNTB = 7 (95% CI 5 to 15) |
||
| 700 per 1000 | 553 per 1000 (483 to 630) | |||||
| CT alone for mixed cancers | RR 0.30 (0.14 to 0.65) | 263 (3 studies) | ⊕⊕⊝⊝ LOW5 | There might be a benefit for KGF in this population NNTB = 10 (95% CI 8 to 19) |
||
| 154 per 1000 | 46 per 1000 (22 to 100) | |||||
| Adverse events | Adverse events that were attributed to the study drugs rather than the cancer therapy were typically oral‐related or skin‐related. Events were mostly mild to moderate with very few incidences of serious events. However, reporting was poor and inconsistent, meaning that it was not appropriate to meta‐analyse data | |||||
|
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**Only 1 study in the subgroup BMT/SCT after conditioning for haematological cancers' included some children (but the median age of participants was 46 years). ***The number of people that would need to receive KGF in order to prevent 1 additional person from developing the outcome. Calculated as 1 divided by the absolute risk reduction (which is the control arm event rate minus the experimental arm event rate). NNTH means the number of people that would need to receive KGF to cause 1 additional person to develop the outcome. All decimal places have been rounded up to the nearest whole number (i.e. 6.1 = 7). ∞: infinity; 5FU: fluorouracil; BMT: bone marrow transplantation; CI: confidence interval; CT: chemotherapy; KGF: keratinocyte growth factor; NNTB: number needed to treat to benefit***; NNTH: number needed to treat to harm; RR: risk ratio; RT: radiotherapy; SCT: stem cell transplantation. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by 1 level for inconsistency (substantial heterogeneity: I2 = 50% to 90%, P < 0.1); downgraded 1 further level for publication bias as there are 2 references in Studies awaiting classification that would be included in the conditioning/transplant subgroup, but the data are not available (NCT02313792; Spielberger 2001). 2Downgraded by 1 level for inconsistency (substantial heterogeneity: I2 = 50% to 90%, P < 0.1). 3Downgraded by 1 level for publication bias as there is 1 reference in Studies awaiting classification that would be included in the chemotherapy alone subgroup, but the data are not available (NCT00393822). 4Downgraded by 1 level for inconsistency (substantial heterogeneity: I2 = 50% to 90%, P < 0.1); downgraded 1 further level for publication bias as there are 2 references in Studies awaiting classification that would be included in the conditioning/transplant subgroup, but the data are not available (NCT02313792; Spielberger 2001); we did not downgrade for imprecision because, despite the confidence interval including a small chance of an increase in risk, it is a fairly narrow interval and a rating of 'very low quality' would seem an overly harsh rating for this body of evidence. 5Downgraded by 1 level for imprecision (wide confidence interval, small sample size and low event rate); downgraded 1 further level for publication bias as there is 1 reference in Studies awaiting classification that would be included in the chemotherapy alone subgroup, but the data are not available (NCT00393822).
Summary of findings 2. Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) compared to placebo/no treatment for preventing oral mucositis in adults with cancer receiving treatment.
| GM‐CSF compared to placebo/no treatment for preventing oral mucositis in adults with cancer receiving treatment | ||||||
| Patient or population: adults** receiving treatment for cancer (see subgroup for treatment type) Setting: hospital Intervention: GM‐CSF Comparison: placebo/no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo/no treatment | Risk with GM‐CSF | |||||
| Oral mucositis (moderate + severe) | BMT/SCT after conditioning for haematological cancers | RR 0.94 (0.79 to 1.13) | 109 (1 study) | ⊕⊝⊝⊝ VERY LOW1 | There is insufficient evidence to determine a benefit for GM‐CSF in this population NNTB = 20 (95% CI 6 NNTB to 10 NNTH) |
|
| 839 per 1000 | 789 per 1000 (663 to 948) | |||||
| RT to head and neck | RR 0.72 (0.49 to 1.06) | 29 (1 study) | ⊕⊝⊝⊝ VERY LOW2 | There is insufficient evidence to determine a benefit for GM‐CSF in this population NNTB = 4 (95% CI 3 NNTB to 14 NNTH) |
||
| 929 per 1000 | 669 per 1000 (455 to 984) | |||||
| Oral mucositis (severe) | BMT/SCT after conditioning for mixed cancers | RR 0.74 (0.33 to 1.67) | 235 (3 studies) | ⊕⊕⊝⊝ LOW3 | There is insufficient evidence to determine a benefit for GM‐CSF in this population NNTB = 12 (95% CI 5 NNTB to 5 NNTH) |
|
| 347 per 1000 | 257 per 1000 (115 to 580) | |||||
| RT to head and neck | RR 0.31 (0.01 to 7.09) | 29 (1 study) | ⊕⊝⊝⊝ VERY LOW4 | There is insufficient evidence to determine a benefit for GM‐CSF in this population NNTB = 21 (95% CI 15 NNTB to 3 NNTH) |
||
| 71 per 1000 | 22 per 1000 (1 to 506) | |||||
| CT alone for mixed cancers | RR 0.59 (0.05 to 7.11) | 65 (2 studies) | ⊕⊝⊝⊝ VERY LOW5 | There is insufficient evidence to determine a benefit for GM‐CSF in this population NNTB = 5 (95% CI 3 NNTB to 2 NNTH) |
||
| 500 per 1000 | 295 per 1000 (25 to 1000) | |||||
| Adverse events | Adverse events that were attributed to the study drugs rather than the cancer therapy were typically bone pain, nausea, fever and headache. Events were not reported as being serious. Some studies did not report adverse events and 1 even reported that there were none. However, reporting was poor and inconsistent, meaning that it was not appropriate to meta‐analyse data | |||||
|
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**There were no studies conducted on children. ***The number of people that would need to receive GM‐CSF in order to prevent 1 additional person from developing the outcome. Calculated as 1 divided by the absolute risk reduction (which is the control arm event rate minus the experimental arm event rate). NNTH means the number of people that would need to receive GM‐CSF to cause 1 additional person to develop the outcome. All decimal places have been rounded up to the nearest whole number (i.e. 6.1 = 7). BMT: bone marrow transplantation; CI: confidence interval; CT: chemotherapy; GM‐CSF: granulocyte‐macrophage colony‐stimulating factor; NNTB: number needed to treat to benefit***; NNTH: number needed to treat to harm; RR: risk ratio; RT: radiotherapy; SCT: stem cell transplantation. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by 2 levels for imprecision (single study with a small sample size and the confidence interval includes a possible increase in risk that is of a similar magnitude to the possible reduction in risk); downgraded 1 further level for indirectness (single study so not widely generalisable). 2Downgraded by 2 levels for imprecision (wide confidence interval and very small sample size); downgraded by 1 further level for high risk of performance bias; downgraded by 1 further level for indirectness (single study so not widely generalisable); downgraded by 1 further level for publication bias as there are 2 references in Studies awaiting classification that would be included in the RT to head and neck subgroup, but the data are not currently available (Antonadou 1998; NCT00293462). 3Downgraded by 2 levels for imprecision (small sample size and the confidence interval includes a possible increase in risk that is of a similar magnitude to the possible reduction in risk); downgraded by 1 further level for inconsistency (substantial heterogeneity: I2 = 50% to 90%, P < 0.1). 4Downgraded by 2 levels for imprecision (extremely wide confidence interval incorporating both very large increase and reduction in risk, very small sample size and very low event rate); downgraded by 1 further level for high risk of performance bias; downgraded by 1 further level for indirectness (single study so not widely generalisable); downgraded by 1 further level for publication bias as there are 2 references in Studies awaiting classification that would be included in the RT to head and neck subgroup, but the data are not currently available (Antonadou 1998; NCT00293462). 5Downgraded by 2 levels for imprecision (extremely wide confidence interval incorporating both very large increase and reduction in risk and very small sample size); downgraded by 1 further level for high risk of performance bias; downgraded by 1 further level for inconsistency (substantial heterogeneity: I2 = 50% to 90%, P < 0.1).
Summary of findings 3. Granulocyte‐colony stimulating factor (G‐CSF) compared to placebo/no treatment for preventing oral mucositis in adults with cancer receiving treatment.
| G‐CSF compared to placebo/no treatment for preventing oral mucositis in patients with cancer receiving treatment | ||||||
| Patient or population: adults** receiving treatment for cancer (see subgroup for treatment type) Setting: hospital Intervention: G‐CSF Comparison: placebo/no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo/no treatment | Risk with G‐CSF | |||||
| Oral mucositis (moderate + severe) | CT alone for breast cancer | RR 0.33 (0.12 to 0.95) | 14 (1 study) | ⊕⊝⊝⊝ VERY LOW1 | There is very weak evidence that there might be a benefit for G‐CSF in this population NNTB = 2 (95% CI 2 to 20) |
|
| 1000 per 1000 | 330 per 1000 (120 to 950) | |||||
| Oral mucositis (severe) | RT to head and neck | RR 0.37 (0.15 to 0.87) | 54 (2 studies) | ⊕⊕⊝⊝ LOW2 | There is weak evidence that there might be a benefit for G‐CSF in this population NNTB = 3 (95% CI 3 to 15) |
|
| 519 per 1000 | 192 per 1000 (78 to 451) | |||||
| Adverse events | There was limited evidence of adverse events for G‐CSF. 2 of the 6 studies did not report adverse events. There were low rates of mild to moderate events, the most common of which appeared to be bone pain. However, reporting was poor and inconsistent, meaning that it was not appropriate to meta‐analyse data | |||||
|
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**There were no studies conducted on children. ***The number of people that would need to receive G‐CSF in order to prevent 1 additional person from developing the outcome. Calculated as 1 divided by the absolute risk reduction (which is the control arm event rate minus the experimental arm event rate). NNTH means the number of people that would need to receive G‐CSF to cause 1 additional person to develop the outcome. All decimal places have been rounded up to the nearest whole number (i.e. 6.1 = 7). CI: confidence interval; CT: chemotherapy; G‐CSF: granulocyte‐colony stimulating factor; NNTB: number needed to treat to benefit***; NNTH: number needed to treat to harm; RR: risk ratio; RT: radiotherapy. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by 2 levels for imprecision (wide confidence interval and very small sample size); downgraded by 1 further level for high risk of performance bias; downgraded by 1 further level for indirectness (single study so not widely generalisable). 2Downgraded by 2 levels for imprecision (wide confidence interval and very small sample size).
Background
Description of the condition
Treating cancer with chemotherapy, radiotherapy of the head and neck, or targeted therapy can cause toxic oral side effects (Al‐Dasooqi 2013; Scully 2006; Sonis 2004). Perhaps the most widely researched of these side effects is oral mucositis (Al‐Dasooqi 2013), which affects at least 75% of high risk patients (those receiving head and neck radiotherapy or high‐dose chemotherapy) (Scully 2006). Oral mucositis may be under‐reported in lower risk groups for various reasons: their tendency to be outpatients with less observation; less reporting of moderate mucositis; or patients and clinicians wishing to avoid any disruption to optimal cancer treatment (Scully 2006).
Simply put, oral mucositis affects the oral mucosa (the mucous membrane of moist tissue lining the oral cavity) and can lead to the development of lesions (ulcers). However, the process that leads to oral mucositis is complex and multifactorial, with Sonis' five‐phase model being a widely accepted description of the sequence of events underlying the condition (Sonis 2004; Sonis 2009).
Initiation: DNA damage caused by chemotherapy or radiotherapy results in the loss of ability to proliferate in the basal cells of the epithelium (the external layers of cells lining the oral mucosa). This produces reactive oxygen species (ROS).
Primary damage response: radiotherapy, chemotherapy, ROS, and DNA strand breaks all contribute to the activation of transcription factors such as nuclear factor kappa beta (NF‐Kβ), and sphingomyelinases. All this leads to the upregulation of pro‐inflammatory cytokines (e.g. tumour necrosis factor alpha ‐ TNF‐α), nitric oxide, ceramide, and matrix metalloproteinases, resulting in the thinning of the epithelium through tissue injury and cell death, culminating with the destruction of the oral mucosa.
Signal amplification: some of the molecules in the previous phase can lead to the exacerbation and prolonging of tissue injury through positive or negative feedback (e.g. TNF‐α can positively feedback on NF‐Kβ thus inducing more pro‐inflammatory cytokine production).
Ulceration: bacteria colonise ulcers and their cell wall products infiltrate the submucosa (the connective tissues beneath the oral mucosa), activating tissue macrophages (white blood cells that respond to infection or damaged/dead cells), which results in further production of pro‐inflammatory cytokines, inflammation, and pain.
Healing: signalling from the extracellular matrix of the submucosa results in epithelial proliferation and differentiation, and thus a thickening of the epithelium. The local oral flora are reinstated.
However, there remains a lack of clarity around mechanisms and risk factors for oral mucositis, particularly areas such as genetic predisposition and microbial effects. Understanding of the pathobiology leading to mucosal toxicity as a result of targeted therapies (e.g. mammalian target of rapamycin (mTOR) inhibitor‐associated stomatitis ‐ mIAS) is also currently limited, but it is thought to differ from chemotherapy‐ and radiotherapy‐induced mucositis, and the clinical presentation of the ulcers is more similar to aphthous stomatitis (Al‐Dasooqi 2013; Boers‐Doets 2013; Peterson 2015).
Oral mucositis is an acute condition and, when caused by chemotherapy, ulceration normally occurs one week after treatment and resolves within three weeks of treatment (Sonis 2009). Radiotherapy‐induced oral mucositis takes longer both to develop and to heal, with ulceration normally occurring around two weeks into a seven‐week treatment cycle, and resolving three to four weeks after treatment has ended (Sonis 2009).
Ulceration is the most significant phase as it leads to pain of varying severity, and difficulties with eating, swallowing, and talking (Scully 2006). This in turn leads to the consumption of pain relief medication, nutritional support (i.e. nasogastric or intravenous feeding), treatment of the oral mucositis, specialist oral hygiene care, increased medical appointments and use of staff and resources, and, in some instances, hospitalisation (Jensen 2014; Miller 2001; Trotti 2003). Thus the negative impact on the quality of life of cancer patients, when they are already suffering, is severe (Elting 2008; Epstein 1999). Further problems can occur in immunosuppressed patients if whole bacteria on the ulcer surface cross into the underlying submucosa, potentially leading to bacteraemia and sepsis, which require antibiotics and hospitalisation, and can cause death (Jensen 2014; Peterson 2015; Scully 2006).
Therefore, oral mucositis can be a dose‐limiting condition, disrupting a patient's optimal cancer treatment plan (Jensen 2014; Peterson 2015; Sonis 2004). The additional costs associated with oral mucositis are significant, with one study reporting a median incremental cost of USD 18,515 per patient (Nonzee 2008). These costs have been reported to be as much as USD 42,749 more per patient when ulcerative oral mucositis is present (Sonis 2001).
Description of the intervention
As described above, oral mucositis occurs partly as result of the loss of regenerative ability of the oral epithelial cells. Growth factors and anti‐inflammatory cytokines are used to counteract the biological processes leading to this loss of proliferative ability. Growth factors and anti‐inflammatory cytokines include (Raber‐Durlacher 2013):
keratinocyte growth factor;
colony‐stimulating factors;
epidermal growth factor;
transforming growth factor‐beta;
whey‐derived growth factor;
interleukin‐11;
ATL‐104;
trefoil factor.
How the intervention might work
The growth factors described here are proteins that bind to receptors of target cells and either increase the proliferation of the epithelial cells that form the mucous membrane lining of the oral cavity, or promote the recovery of the white blood cells that contribute to the maintenance of oral health following conventional or high dose chemotherapy (with or without radiotherapy) (Raber‐Durlacher 2013). Anti‐inflammatory cytokines are also proteins or glycoproteins that bind to receptors of target cells, and are thought to alter the complex balance of pro‐ and anti‐inflammatory cytokines involved in the pathogenesis of oral mucositis (Raber‐Durlacher 2013).
Currently, evidence‐based guidelines recommend growth factors for the prevention of oral mucositis in patients with haematological cancers undergoing high‐dose chemotherapy and total body irradiation prior to haematopoietic stem cell transplantation (Lalla 2008). It has been postulated that tumour cells may also have receptors accommodating cytokines and growth factors, thus encouraging the proliferation of cancer cells in solid tumours (Lalla 2008; von Bültzingslöwen 2006). A 2010 systematic review suggested that the risk of acute myeloid leukaemia (AML) or myelodysplastic syndrome (MDS) is increased in people with various cancers receiving chemotherapy with granulocyte colony‐stimulating factor (G‐CSF) when compared to those receiving chemotherapy without G‐CSF (Lyman 2010). The authors concluded that it was not clear whether the increased AML/MDS risk was due to G‐CSF or due to the increased chemotherapy dose‐intensity in those patients. However, the review also reported a reduction in overall mortality for those receiving G‐CSF.
Why it is important to do this review
This Cochrane Review is part of a series that will replace the previously published Cochrane Review covering all interventions for the prevention of oral mucositis in patients with cancer receiving treatment (Worthington 2011). The Mucositis Study Group (MSG) of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) is a group that was set up in 1998 for the purpose of producing international evidence‐based clinical practice guidelines for managing mucositis (both oral and gastrointestinal), which they first published in 2004, with the latest update published in 2014 (Lalla 2014). In order to facilitate the future updating of Cochrane Reviews on this topic, and also to make them more usable to clinicians, guideline developers, and consumers, we have decided to divide the original Cochrane Review into the same intervention categories as those used by MASCC/ISOO, which are as follows:
basic oral care/good clinical practice;
growth factors and cytokines;
anti‐inflammatory agents;
antimicrobials, mucosal coating agents, anaesthetics, and analgesics;
laser and other light therapy;
cryotherapy;
natural and miscellaneous agents;
amifostine.
We believe that following the MASCC/ISOO structure will better enable the Cochrane Reviews to feed into such guidelines. We can also be more thorough and rigorous in our assessment and summarising of the evidence in each of the categories, which was not feasible in a single Cochrane Review approaching 150 included studies.
It is also important to do this review as it is consistently shown to be the most used review produced by Cochrane Oral Health (in terms of full‐text downloads). It was also ranked by an expert panel of oral medicine specialists as being the most important topic in the field of oral medicine in an international prioritisation exercise carried out by Cochrane Oral Health in 2014 (Worthington 2015).
Objectives
To assess the effects of cytokines and growth factors for preventing oral mucositis in patients with cancer who are receiving treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) of parallel design. It is possible to conduct cross‐over studies in this area as patients may receive several treatment sessions/cycles, with any mucositis completely healing in the periods between the sessions. However, we did not include cross‐over data as we could not discount any period effects, with mucositis risk increasing as patients receive further cycles of treatment (Scully 2006; Sonis 2009). Instead, we used the first‐period data only and treated such studies as parallel group studies.
Types of participants
We included all patients with cancer who were receiving treatment.
Types of interventions
We included studies comparing growth factors and cytokines for the prevention of oral mucositis (we would also have included targeted therapy‐induced stomatitis had such studies been identified) against usual care, no treatment, or any other treatment to prevent oral mucositis. We also included studies comparing different growth factors and cytokines or different regimens of growth factors and cytokines against each other (head‐to‐head studies).
We excluded studies with 'complex' interventions for the prevention of mucositis, such as lasers plus growth factors and cytokines versus lasers. This is because it is difficult to attribute any effect shown to any particular component of the intervention. We excluded studies assessing different cancer treatments where the primary outcome was survival/cure, with mucositis as a toxicity.
Types of outcome measures
We are in agreement with Williamson 2012 that, if clinical trials and systematic reviews are to be utilised, the outcomes assessed should be those considered important to patients, healthcare professionals, and other key stakeholders. If outcomes and outcome measures are inconsistent across studies, it will not be possible to compare and summarise research, and there is potential for outcome reporting bias, with the selective reporting of results based on statistical significance and favourability (Clarke 2007; Dwan 2008; Williamson 2005). This can lead to exaggerated estimates of effect in systematic reviews of interventions, leading to an incorrect belief that an intervention is more beneficial that it truly is (Clarke 2007). It is thought that the way to address this problem is to develop disease‐ or condition‐specific core outcome sets to be used as a minimum when conducting and reporting clinical trials (Clarke 2007; Williamson 2012).
Therefore we used the core outcome set produced by Bellm 2002, which is registered on the COMET (Core Outcome Measures in Effectiveness Trials) Initiative's website (www.comet‐initiative.org), and is the only core outcome set for oral mucositis known to us. We added the outcomes 'interruptions to cancer treatment' and 'adverse events'.
Primary outcomes
Mucositis incidence of any severity. We used mucositis measured on a 0 to 4 point scale (none to severe) and dichotomised it as any mucositis (0 versus 1+), moderate to severe mucositis (0 to 1 versus 2+), and severe mucositis (0 to 2 versus 3+).
Some studies measure the effects of mucositis using a composite scale. If it was possible to extract the 'mucositis only' data from the total score, we would have included the data in the analyses. If it was not possible, we would have recorded the composite data in an additional table.
Secondary outcomes
Interruptions to cancer treatment.
Oral pain.
Quality of life.
Normalcy of diet (including use of percutaneous endoscopic gastrostomy (PEG) feeding tubes or total parenteral nutrition (TPN)).
Adverse events.
Number of days in hospital.
Number of days of treatment with opioid analgesics.
Number of days unable to take medicine orally.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials without language or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 10 May 2017) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4) in the Cochrane Library (searched 10 May 2017) (Appendix 2);
MEDLINE Ovid (1946 to 10 May 2017) (Appendix 3);
Embase Ovid (7 December 2015 to 10 May 2017) (Appendix 4);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 10 May 2017) (Appendix 5);
CANCERLIT (Cancer subset within PubMed; 1950 to 10 May 2017) (Appendix 6).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Due to the Cochrane Embase Project to identify all clinical trials in the database and add them to CENTRAL, only most recent months of the Embase database were searched. See the searching page on the Cochrane Oral Health website for more information. No other restrictions were placed on the date of publication when searching the electronic databases.
Searching other resources
We searched the following trial registries for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 10 May 2017) (Appendix 7);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 10 May 2017) (Appendix 8).
We included only handsearching done as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL.
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts retrieved from the electronic searches. We obtained full‐text copies of all studies that appeared to meet the inclusion criteria of the review, or where there was insufficient information in the title or abstract to make a clear judgement. Two review authors independently assessed the full‐text copies for eligibility and attempted to resolve any disagreements through discussion. We consulted a third review author when we were unable to resolve disagreements.
On assessing the full‐text article, we discarded any studies that clearly did not meet the inclusion criteria. We recorded all other studies that did not meet the inclusion criteria, along with reasons for exclusion, in the Characteristics of excluded studies table.
Data extraction and management
Two review authors independently extracted the data from each included study using a specially designed data extraction form, which we first piloted on a small sample of studies. We contacted study authors for clarification or missing data where necessary and feasible. We resolved any disagreements through discussion, consulting a third review author to achieve consensus when necessary.
We recorded the following data for each included study in the Characteristics of included studies table.
Trial design, location, number of centres, recruitment period.
Inclusion/exclusion criteria, age and gender of participants, number randomised/analysed, any other potentially important prognostic factors (e.g. cancer type, cancer treatment, etc.).
Detailed description of the intervention and comparator, including timing and duration. Information on compliance with the intervention.
Details of the outcomes reported, including method of assessment and time(s) assessed.
Details of sample size calculations, adverse effects, funding sources, declarations/conflicts of interest.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study using the Cochrane domain‐based, two‐part tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We contacted study authors for clarification or missing information where necessary and feasible. We resolved any disagreements through discussion, consulting a third review author to achieve consensus when necessary.
We completed a 'Risk of bias' table for each included study. For each domain of risk of bias, we first described what was reported to have happened in the study. This provided the rationale for our judgement of whether that domain was at low, high, or unclear risk of bias.
We assessed the following domains:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective outcome reporting (reporting bias);
other bias.
We categorised the overall risk of bias of individual studies. Studies were categorised as being at low, high, or unclear risk of bias according to the following criteria:
low risk of bias (plausible bias unlikely to seriously alter the results) if all domains were at low risk of bias;
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more domains were at high risk of bias; or
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more domains were at unclear risk of bias.
We also presented the 'Risk of bias' summary graphically.
Measures of treatment effect
For continuous outcomes (e.g. oral pain on a visual analogue scale) where studies used the same scale, we used the mean values and standard deviations (SDs) reported in the studies in order to express the estimate of effect as mean difference (MD) with 95% confidence interval (CI). Where different scales were used, we expressed the treatment effect as standardised mean difference (SMD) with 95% CI.
For dichotomous outcomes (e.g. mucositis of any severity/no mucositis), we expressed the estimate of effect as a risk ratio (RR) with 95% CI.
We did not use area under the curve (AUC) data due to variation in length of follow‐up for outcome assessment, variation in the length of the scale used to measure the outcome and also variation or lack of clarity whether the results were reported in terms of total area under the curve or average over the time period.
Unit of analysis issues
The participant was the unit of analysis.
Dealing with missing data
We attempted to contact the author(s) of all included studies, where feasible, for clarification, and missing data. We would have used the methods described in Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions to estimate missing SDs (Higgins 2011). We did not use any other statistical methods or perform any further imputation to account for missing data.
Assessment of heterogeneity
When a sufficient number of studies were included in any meta‐analyses, we assessed clinical heterogeneity by examining the characteristics of the studies, the similarity between the types of participants, the interventions, and the outcomes. We also assessed heterogeneity statistically using a Chi2 test, where a P value < 0.1 indicates statistically significant heterogeneity. We quantified heterogeneity using the I2 statistic. A guide to interpretation of the I2 statistic given in Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions is as follows (Higgins 2011):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
If at least 10 studies were included in a meta‐analysis, we planned to assess publication bias according to the recommendations on testing for funnel plot asymmetry (Egger 1997), as described in Section 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If asymmetry were identified, we would have examined possible causes. We were not able to assess publication bias in this way because, although we had a sufficient number of studies in our meta‐analyses for the primary outcome in one comparison, they were split into subgroups containing less than 10 studies, with no pooling of the subgroup totals.
Data synthesis
We only carried out meta‐analyses where there were studies of similar comparisons reporting the same outcomes. We combined MDs for continuous data, and RRs for dichotomous data. Our general approach was to use a random‐effects model. With this approach, the CIs for the average intervention effect were wider than those that would have been obtained using a fixed‐effect approach, leading to a more conservative interpretation.
We used an additional table to report the results from studies not suitable for inclusion in a meta‐analysis, but only for the primary outcome.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analyses according to type of cancer treatment. We also would have considered age group (children versus adults) as a category for subgroup analyses, if there had been sufficient numbers of studies with these differing populations.
Sensitivity analysis
If there had been sufficient numbers of studies in the meta‐analyses, we would have tested the robustness of our results by performing sensitivity analyses based on excluding the studies at unclear or high risk of bias from the analyses.
If any meta‐analyses had included several small studies and a single very large study, we would have carried out a sensitivity analysis comparing the effect estimates from both random‐effects and fixed‐effect models. If these were different we would have reported on both analyses as part of the results section, and considered possible interpretation.
Presentation of main results
We produced a 'Summary of findings' table for each comparison in which there was more than one study in at least one of the subgroups based on cancer treatment. We included the incidence of moderate to severe oral mucositis, the incidence of severe oral mucositis and adverse events. We used GRADE methods (GRADE 2004), and the GRADEpro GDT online tool for developing 'Summary of findings' tables (www.guidelinedevelopment.org). We assessed the quality of the body of evidence for each comparison and outcome by considering the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, and the risk of publication bias. We categorised the quality of each body of evidence as high, moderate, low, or very low.
Results
Description of studies
Results of the search
Our electronic searches identified 5125 records. After removing duplicates, this number was reduced to 3145. We examined the titles and abstracts of these records and discarded 3042, leaving 103 records to examine in more detail. Where possible, we obtained full‐text copies of these potentially relevant records and linked any references pertaining to the same study under a single study ID. These 103 records represented 73 studies. We excluded 24 studies at this stage. The remaining 49 studies met our inclusion criteria and we included 35 of these studies in the review. The remaining 14 studies are awaiting assessment because we do not have enough information to be able to include them in the review. We present this process as a study flow chart in Figure 1.
1.
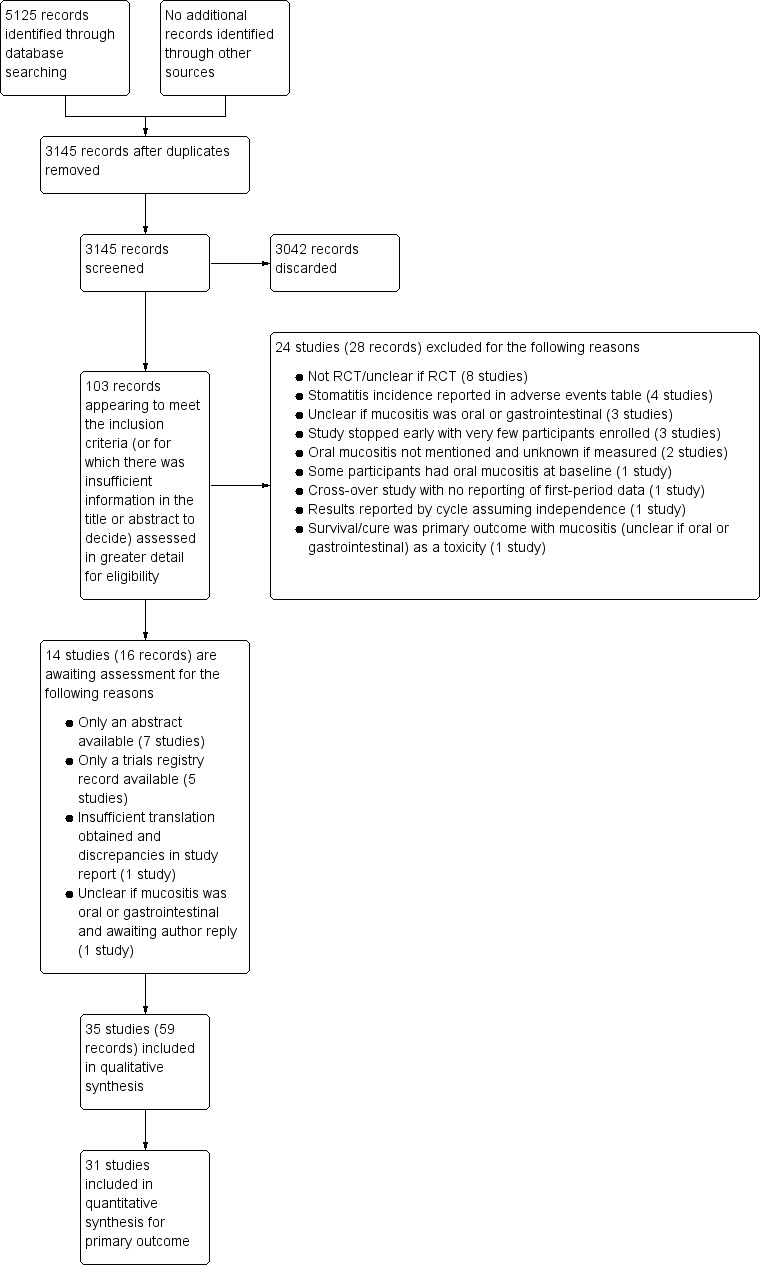
Study flow diagram. RCT = randomised controlled trial.
Included studies
We included 35 studies in this review. For further information see the Characteristics of included studies tables.
Characteristics of the trials
Study design
One study was a cross‐over design that reported the first‐period data separately (Chi 1995), whilst the remaining studies all used a parallel design.
Number of arms
Twenty‐seven studies had two arms, three studies had three arms (Blijlevens 2013; Freytes 2004; Peterson 2009), one study had four arms (Wu 2009), two studies had five arms (Cartee 1995; Linch 1993), and two studies had seven arms (Blazar 2006; Meropol 2003). Where studies had more than two arms, this was because they tested a range of doses of the cytokine/growth factor. In such instances we combined the arms testing different doses to make pairwise comparisons against the control group. Where possible, we also made head‐to‐head comparisons of doses (Blijlevens 2013; Cartee 1995; Freytes 2004; Meropol 2003; Peterson 2009).
Country
Nine studies were conducted in the USA (Blazar 2006; Cartee 1995; Crawford 1999; Freytes 2004; Meropol 2003; Schneider 1999; Spielberger 2004; Su 2006; Vadhan‐Raj 2010), four in Italy (Cesaro 2013; Dazzi 2003; Lucchese 2016a; Lucchese 2016b), two in each of South Korea (Kim 2017; Wu 2009), the UK (Linch 1993; McAleese 2006), Iran (Gholizadeh 2016; Hosseinjani 2017), Finland (Makkonen 2000; Saarilahti 2002), and one in each of the Netherlands (van der Lelie 2001), Russia (Peterson 2009), Japan (Katano 1995), Germany (Fink 2011), China (Chi 1995), Australia (Bradstock 2014), and France (Antoun 2009). The remaining seven studies were conducted across more than one country: USA and Australia (Jagasia 2012; Rosen 2006); USA and Canada (Nemunaitis 1995); Australia, Canada and the USA (Brizel 2008); Australia, Canada and Europe (Henke 2011); Canada, USA and Europe (Le 2011); and 14 European countries (Blijlevens 2013).
Number of centres
Fifteen studies were conducted at a single‐centre (Antoun 2009; Cartee 1995; Chi 1995; Dazzi 2003; Fink 2011; Hosseinjani 2017; Katano 1995; Kim 2017; Lucchese 2016a; Lucchese 2016b; McAleese 2006; Saarilahti 2002; Su 2006; Vadhan‐Raj 2010; van der Lelie 2001). Eighteen studies were multicentric, ranging from two sites (Blazar 2006; Makkonen 2000) to 46 sites (Le 2011). It was unclear how many centres were involved in the remaining two studies (Gholizadeh 2016; Schneider 1999).
Trials registries
We were able to find a trials registry number for 13 studies (Blijlevens 2013; Bradstock 2014; Cesaro 2013; Fink 2011; Gholizadeh 2016; Henke 2011; Hosseinjani 2017; Jagasia 2012; Kim 2017; Le 2011; McAleese 2006; Spielberger 2004; Vadhan‐Raj 2010), although only six studies mentioned it in the study report (Bradstock 2014; Cesaro 2013; Gholizadeh 2016; Hosseinjani 2017; Kim 2017; Vadhan‐Raj 2010), whilst a further study mentioned an obsolete number (Jagasia 2012).
Sample size calculation
Twenty‐one studies reported details of sample size calculations, but four of these were not based on oral mucositis (Cesaro 2013; Crawford 1999; Jagasia 2012; Su 2006). One further study stated that 36 participants "should be enough to demonstrate a clinically significant difference", with no details reported (van der Lelie 2001).
Funding and conflicts of interest
This information is difficult to summarise as it was not always adequately reported.
Nineteen studies appeared to be funded by industry alone i.e. it was explicitly stated that they received industry funding or that industry supplied the interventions or both. Five studies appeared to be funded by government/public sector alone and did not state whether or not the interventions were supplied by industry (Cartee 1995; Lucchese 2016a; Lucchese 2016b; Su 2006; Wu 2009). Four studies reported both government and industry funding, three of which stated that industry provided the interventions (Bradstock 2014; Chi 1995; Kim 2017), and one of which was not clear (Blazar 2006). Two studies stated that there was no funding for the study (Cesaro 2013; Hosseinjani 2017). The remaining five studies did not mention funding (Dazzi 2003; Freytes 2004; Gholizadeh 2016; McAleese 2006; Saarilahti 2002).
Ten studies, all industry funded, declared conflicts of interest for reasons such as board membership of the funder, employment or leadership roles with the funder, receipt of lecture fees or consultancy fees or research funding or honoraria from the funder (Antoun 2009; Blijlevens 2013; Brizel 2008; Henke 2011; Jagasia 2012; Le 2011; Peterson 2009; Rosen 2006; Spielberger 2004; Vadhan‐Raj 2010). Six of those studies also declared that some authors owned equity/stock with the funder (Brizel 2008; Henke 2011; Jagasia 2012; Le 2011; Rosen 2006; Spielberger 2004). Three studies did not explicitly declare conflicts of interest, but some authors were employed by the funder (Crawford 1999; Linch 1993; Nemunaitis 1995). Eight studies stated that there were no conflicts of interest (Bradstock 2014; Cesaro 2013; Gholizadeh 2016; Hosseinjani 2017; Kim 2017; Lucchese 2016a; Lucchese 2016b; Su 2006). The remaining 14 studies did not mention conflicts of interest.
Characteristics of the participants
Number randomised/analysed
The studies randomised 3218 participants, of whom 3102 were included in the studies' analyses (the latter number does not include any participants from Makkonen 2000, as this study did not report how many of the 40 randomised participants were analysed).
Age and sex
The age of the participants ranged from 1 to 87 years, with four studies only including children and young adults (i.e. up to 18 years) (Cesaro 2013; Gholizadeh 2016; Lucchese 2016a; Lucchese 2016b). Of the 31 studies including adult participants, one had a median age of 29 (Dazzi 2003), two had mean or median ages in their 30s (Linch 1993; Nemunaitis 1995), nine in their 40s (Blazar 2006; Bradstock 2014; Cartee 1995; Chi 1995; Hosseinjani 2017; Jagasia 2012; Spielberger 2004; Vadhan‐Raj 2010; van der Lelie 2001), 11 in their 50s (Blijlevens 2013; Brizel 2008; Fink 2011; Freytes 2004; Henke 2011; Katano 1995; Kim 2017; Le 2011; Peterson 2009; Saarilahti 2002; Wu 2009), seven in their 60s (Antoun 2009; Crawford 1999; Makkonen 2000; McAleese 2006; Meropol 2003; Rosen 2006; Su 2006), and one study did not report the age, although the inclusion criteria stated that they must be at least 18 years old (Schneider 1999). In 24 studies, there was a clear majority of male participants, whilst the male to female ratio was roughly equal in seven studies. In three studies there were more female participants, although two of these exclusively included breast cancer patients (Cartee 1995; Katano 1995), whilst the third included colorectal cancer patients (Peterson 2009).
Cancer type
Fourteen studies enrolled participants with haematological cancers (Blazar 2006; Blijlevens 2013; Bradstock 2014; Fink 2011; Freytes 2004; Gholizadeh 2016; Hosseinjani 2017; Jagasia 2012; Kim 2017; Lucchese 2016a; Lucchese 2016b; Nemunaitis 1995; Spielberger 2004; van der Lelie 2001). Eighteen studies enrolled participants with solid cancers: head and neck (Brizel 2008; Chi 1995; Henke 2011; Le 2011; Makkonen 2000; McAleese 2006; Saarilahti 2002; Schneider 1999; Su 2006; Wu 2009); colorectal (Antoun 2009; Meropol 2003; Peterson 2009; Rosen 2006); breast (Cartee 1995; Katano 1995); lung (Crawford 1999); and sarcoma (Vadhan‐Raj 2010). The remaining three studies enrolled a mixture of participants with solid cancers and participants with haematological cancers, two of which were 80% to 90% solid (Cesaro 2013; Dazzi 2003), and the other study only 3% solid (Linch 1993).
Cancer treatment
In 11 studies, the participants received chemotherapy only (Antoun 2009; Bradstock 2014; Cartee 1995; Chi 1995; Crawford 1999; Gholizadeh 2016; Katano 1995; Meropol 2003; Peterson 2009; Rosen 2006; Vadhan‐Raj 2010). Of the 15 studies in which the participants received conditioning therapy prior to stem cell or bone marrow transplantation, five of these involved chemotherapy only (Blijlevens 2013; Dazzi 2003; Fink 2011; Hosseinjani 2017; Kim 2017), and one involved total body irradiation (TBI) only (Lucchese 2016b). In the remaining nine transplant studies, all the participants had chemotherapy, but the proportion of participants also receiving TBI differed: 100% (Lucchese 2016a; Nemunaitis 1995; Spielberger 2004); around 50% (Blazar 2006; Jagasia 2012; van der Lelie 2001); 29% (Linch 1993); 10% or less (Cesaro 2013; Freytes 2004). The remaining nine studies were all on head and neck cancer patients where the participants either had radiotherapy to the head and neck alone (Makkonen 2000; McAleese 2006; Saarilahti 2002; Schneider 1999; Su 2006), or radiotherapy to the head and neck plus chemotherapy (Brizel 2008; Henke 2011; Le 2011; Wu 2009), although in one of those studies only 50% of participants had the chemotherapy (Wu 2009).
Of the 15 transplant studies, four involved allogeneic transplants (Blazar 2006; Jagasia 2012; Lucchese 2016b; Nemunaitis 1995), nine involved autologous transplants (Blijlevens 2013; Cesaro 2013; Dazzi 2003; Fink 2011; Freytes 2004; Hosseinjani 2017; Kim 2017; Lucchese 2016a; Spielberger 2004), with the remaining two involving a mixture (Linch 1993; van der Lelie 2001).
In six studies, all participants received granulocyte‐colony stimulating factor (a growth factor) as part of the cancer treatment to prevent neutropenia. Four of these studies were investigating keratinocyte growth factor (Blazar 2006; Bradstock 2014; Spielberger 2004; Vadhan‐Raj 2010), and two were investigating granulocyte‐macrophage colony‐stimulating factor (Cartee 1995; Dazzi 2003). Giving all participants this growth factor would have the potential to lessen the impact of the study intervention.
Characteristics of the interventions and comparisons
Keratinocyte growth factor (KGF)
Of the 16 studies investigating KGF, one study assessed KGF‐2 (repifermin) (Freytes 2004), whilst the remaining studies assessed KGF‐1 (palifermin).
Fourteen studies used a placebo comparator (Blazar 2006; Blijlevens 2013; Bradstock 2014; Brizel 2008; Freytes 2004; Henke 2011; Jagasia 2012; Le 2011; Lucchese 2016a; Lucchese 2016b; Meropol 2003; Rosen 2006; Spielberger 2004; Vadhan‐Raj 2010), one was KGF plus standard care versus standard care alone (Fink 2011), and the remaining study used a chlorhexidine mouthwash comparator (Gholizadeh 2016).
In all studies, KGF was given intravenously. The most common total dosage received was 360 µg/kg in seven studies (Bradstock 2014; Fink 2011; Gholizadeh 2016; Jagasia 2012; Lucchese 2016a; Lucchese 2016b; Spielberger 2004). The dosage varied greatly in the other studies: 120 µg/kg (Rosen 2006); 180 µg/kg (Vadhan‐Raj 2010); 600 µg/kg (Brizel 2008); 840 μg/kg to 960 µg/kg depending on resection type (Henke 2011); 1440 µg/kg (Le 2011). The dosages varied within the remaining studies due to multiple arms receiving different doses: 3 μg/kg to 240 µg/kg (Meropol 2003); 180 μg/kg to 360 µg/kg (Blijlevens 2013); 240 μg/kg to 720 µg/kg (Blazar 2006); 325 μg/kg to 650 µg/kg (Freytes 2004).
The number of doses received ranged from one (Vadhan‐Raj 2010) to 13 (Freytes 2004), but the most common was six (Blijlevens 2013; Bradstock 2014; Fink 2011; Gholizadeh 2016; Lucchese 2016a; Lucchese 2016b; Spielberger 2004).
Reporting of compliance varied too greatly to summarise succinctly but compliance was generally high (see Characteristics of included studies).
Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF)
Of the eight studies investigating GM‐CSF, four used a placebo comparator (Cartee 1995; Dazzi 2003; Nemunaitis 1995; van der Lelie 2001), two used a no‐treatment comparator (Chi 1995; McAleese 2006), one was GM‐CSF plus sucralfate versus sucralfate alone (Makkonen 2000), and the remaining study used a sucralfate comparator (Saarilahti 2002).
In three studies, GM‐CSF was given by subcutaneous injection (Chi 1995; Makkonen 2000; McAleese 2006). In Makkonen 2000, both arms received sucralfate mouthwash that was swallowed after rinsing. In three studies, GM‐CSF was taken as a mouthwash (Cartee 1995; Dazzi 2003; Saarilahti 2002). In Saarilahti 2002, both the GM‐CSF and sucralfate comparator mouthwashes were swallowed after rinsing. In one study, GM‐CSF was given as an oral gel and swallowed after holding in the mouth (van der Lelie 2001). In the remaining study, GM‐CSF was given intravenously (Nemunaitis 1995).
Total dosage varied greatly: 40 µg (Chi 1995); 2100 µg (McAleese 2006); 5250 µg/m2 (Nemunaitis 1995). The dosages ranged from 12.6 µg to 12,600 µg within one study due to multiple arms receiving different doses (Cartee 1995). Another study reported a mean total dosage of 3398 µg, but this total ranged from 300 µg to 7200 µg depending on the participant's weight and the length of radiotherapy course (Makkonen 2000). In two studies, the dose was 150 µg per day but the total received varied depending on neutrophil recovery (Dazzi 2003), and the length of radiotherapy course (Saarilahti 2002). In the remaining study, the dose was 300 µg per day but the total received varied depending on neutrophil recovery (van der Lelie 2001).
As is obvious from the variation in total dosage, the number of doses received varied greatly both between studies and within studies. Compliance was also reported inconsistently but was generally high (see Characteristics of included studies).
Granulocyte‐colony stimulating factor (G‐CSF)
Of the six studies investigating G‐CSF, four used a placebo comparator (Crawford 1999; Linch 1993; Schneider 1999; Su 2006), one used a no‐treatment comparator (Katano 1995), and the remaining study compared a type of G‐CSF that is given as a single dose (pegfilgrastim) with the standard G‐CSF that is given in multiple doses (filgrastim) (Cesaro 2013).
Four studies reported that G‐CSF was given by subcutaneous injection (Crawford 1999; Katano 1995; Schneider 1999; Su 2006), whilst one did not specify, but was probably subcutaneous (Cesaro 2013), and the remaining study was intravenous delivery (Linch 1993).
Total dosage varied: 3220 µg/m2 (Crawford 1999); 3 µg/kg per day with the total dependent on neutrophil counts and the length of radiotherapy course (Schneider 1999; Su 2006); 2 µg/kg to 15 µg/kg per day due to multiple arms receiving different dosages with the total was depending on neutrophil recovery (Linch 1993); 125 µg per day with total depending on neutrophil recovery (Katano 1995); 100 µg/kg in the pegfilgrastim arm and at least 45 µg/kg in the filgrastim arm (Cesaro 2013).
The number of doses received varied both between studies and within studies. Compliance was reported as being 100% in one study (Cesaro 2013), whilst one study only reported that the interventions were well tolerated (Schneider 1999), and the remaining four studies did not report on compliance.
Epidermal growth factor (EGF)
Two studies investigated an oral spray of EGF, both using a placebo comparator (Kim 2017; Wu 2009). Total dosage was unclear in both studies but the daily dose was 50 µg/mL (six sprays twice daily) in one study (Kim 2017), and 10 µg to 100 µg per day (due to multiple arms receiving different dosages) in the other study (Wu 2009). The number of doses varied depending on neutrophil recovery and resolution of oral mucositis in Kim 2017, whilst participants in Wu 2009 received the interventions daily for five weeks but it was not clear if that meant only on the radiotherapy days (five days per week) or seven days per week. Compliance was reported as a median of 93% and 92% in the EGF and placebo groups respectively in Kim 2017, but compliance was not reported in Wu 2009.
Intestinal trefoil factor (ITF)
One study investigated an oral spray of ITF using a placebo comparator (Peterson 2009). The ITF was not expectorated. The study included two ITF arms with total dosages of 336 mg and 2688 mg. The mode of administration was three sprays to the oral mucosa eight times daily for 14 days. Patient‐reported compliance was 97%.
Erythropoietin
One study investigated a mouthwash of erythropoietin using a placebo comparator (Hosseinjani 2017). Neither swallowing nor expectoration was reported. The mouthwash was taken as 15 mL (50 IU/mL) four times daily (daily dosage of 3000 IU) for 14 days or until neutrophil recovery, whichever occurred first. Compliance was reported narratively as being low but no reason was stated.
Transforming growth factor (TGF)
One study investigated TGF‐beta(2) using a placebo comparator (Antoun 2009). The dosage was 2 ng of TGF per mg protein mixed with cool boiled water at 0.23 g/mL (100 kcl/100 mL). During each cycle participants received 750 mL to 1000 mL per day plus any other food desired. The formula was administered for two days before, two days during, and three days after chemotherapy (seven days/cycle), for one to eight cycles. Compliance was poor i.e. nine participants did not eat the formula and were excluded.
Characteristics of the outcomes
Primary outcome
For the primary outcome of oral mucositis, we were interested in both the presence/absence of oral mucositis, and also different levels of severity. All 35 studies assessed and reported the incidence of oral mucositis. Twenty‐two studies primarily used the WHO (World Health Organization) 0 to 4 scale, whilst four used the NCI‐CTC (National Cancer Institute common toxicity criteria) 0 to 4 scale (Brizel 2008; Dazzi 2003; Freytes 2004; Kim 2017), four used the RTOG (Radiation Therapy Oncology Group) 0 to 4 scale (Chi 1995; McAleese 2006; Saarilahti 2002; Wu 2009), one used the CALGB (Cancer and Leukemia Group B) 0 to 4 scale (Cartee 1995), one used an unnamed 0 to 2 scale (Makkonen 2000), one used an unnamed 0 to 3 scale (Su 2006), one used an unnamed 0 to 4 scale (Nemunaitis 1995), and the remaining study did not mention a scale and only reported the incidence of stomatitis (Linch 1993). The different oral mucositis assessment scales are described in Appendix 9.
Twelve studies reported the data in our preferred format which was the maximum oral mucositis score experienced by each participant over the length of the study, allowing us to dichotomise the data into various levels of severity as described in the section Primary outcomes. Eighteen studies reported a particular level of severity (e.g. grade 3 or above). One study reported the incidence of each oral mucositis grade on multiple assessment days. We were unable to use the data from the remaining four studies for analysis due to unclear or lack of reporting (Linch 1993; Lucchese 2016a; Lucchese 2016b; Makkonen 2000).
The frequency of oral mucositis assessment and the duration for which it was assessed varied greatly across the studies, often depending on whether the participants received radiotherapy, and often depending on the speed of neutrophil recovery, resolution of oral mucositis, or duration of hospitalisation. Four studies did not report the frequency of assessment (Antoun 2009; Cesaro 2013; Linch 1993; Nemunaitis 1995), whilst a further study was unclearly reported (Lucchese 2016b). Twelve studies reported daily assessments, eight reported weekly assessments, with the remainder falling somewhere in between these two frequencies. Where participants had multiple cycles of treatment, we only reported the results for the first cycle if these data were available separately.
Secondary outcomes
Interruptions to cancer treatment
Six studies reported data that we were able to use in analyses (Brizel 2008; Henke 2011; Le 2011; Saarilahti 2002; Su 2006; Wu 2009), whilst a further two studies assessed this outcome but either did not report the interruption by treatment arm (Makkonen 2000), or narratively reported that there were no differences, with no numerical data (Schneider 1999).
Two studies reported this outcome as the incidence of unscheduled radiotherapy breaks of five or more days (Brizel 2008; Henke 2011; Le 2011). Two of those studies also reported on chemotherapy delays/discontinuations (Henke 2011; Le 2011). The remaining studies all reported on the incidence of interruptions to radiotherapy treatment, one of which stated that interruptions were specifically due to oral mucositis (Saarilahti 2002), and another reporting the incidence of three or more consecutive days of interruption (Wu 2009).
Oral pain
Four studies reported data that we were able to use in analyses (Dazzi 2003; Freytes 2004; Henke 2011; Le 2011). Two of these studies used a 0 to 4 scale and reported the mean (Henke 2011; Le 2011), whilst the other two studies used a 0 to 10 scale and reported the mean worst score experienced (Dazzi 2003; Freytes 2004).
Of the 11 other studies that reported that oral pain was an outcome of the study, five reported the results as area under the curve (AUC) but, for reasons stated in the section Measures of treatment effect, we did not meta‐analyse these data (Blijlevens 2013; Kim 2017; Lucchese 2016a; Rosen 2006; Spielberger 2004). Two studies reported medians, which are not suitable for meta‐analysis (Vadhan‐Raj 2010; van der Lelie 2001). One study reported the data graphically as a mean over time with no standard deviation (Saarilahti 2002). One study narratively reported that there were no differences, with no numerical data (Wu 2009). The remaining two studies used two different scales: one reported as "no difference" and another reported on a graph with no standard deviation (Makkonen 2000); both reported on a graph over time, with one also reported as AUC (Meropol 2003).
Quality of life
Four studies assessed quality of life using various assessment scales: European Quality Of Life Utility Scale ‐ EQ‐5D (Blijlevens 2013); modified Oral Mucositis Daily Questionnaire ‐ OMDQ (Kim 2017); Functional Assessment of Cancer Therapy ‐ FACT (Spielberger 2004); an unnamed 1 to 7 scale (Vadhan‐Raj 2010). We did not use the data in our analyses as they were either reported as AUC (Kim 2017; Spielberger 2004), as a median (Vadhan‐Raj 2010), or the mean was reported at one very early time point with no standard deviation (Blijlevens 2013).
Normalcy of diet ‐ including use of percutaneous endoscopic gastrostomy (PEG) feeding tubes or total parenteral nutrition (TPN)
Fourteen studies reported data that we were able to use in analyses in the form of: incidence of TPN (Blijlevens 2013; Cesaro 2013; Fink 2011; Jagasia 2012; Kim 2017; Spielberger 2004; van der Lelie 2001); incidence of PEG (Brizel 2008; Saarilahti 2002; Su 2006); incidence of TPN, PEG, nasogastric tube or intravenous (IV) hydration (Henke 2011; Le 2011); incidence of "tube feeding" (McAleese 2006); ability to eat using a 1 to 4 scale (Freytes 2004). Only one of these studies explicitly stated that supplemental feeding was due to oral mucositis (Henke 2011).
Two further studies only reported the duration of TPN (Lucchese 2016a; Lucchese 2016b), and another study used 0 to 4 scales to assess difficulty in eating and drinking, but reported median scores (Vadhan‐Raj 2010).
We combined studies reporting incidence of TPN, PEG, etc., in meta‐analyses of 'supplemental feeding'.
Adverse events
This outcome was very poorly reported with some studies reporting numerical data and some reporting narratively. Some studies only reported adverse events if there was a minimum incidence (which varied between studies) or if there was a specified difference in incidence between treatment arms. It was also difficult to determine whether or not many adverse effects were due to the study interventions, or due to the underlying cancer treatment. We presented adverse event data/information only in an additional table.
Number of days in hospital
Two studies reported data that we were able to use in analyses i.e. mean and standard deviations (Blijlevens 2013; Hosseinjani 2017).
Five further studies reported medians (Cesaro 2013; Fink 2011; Linch 1993; Nemunaitis 1995; van der Lelie 2001). One study reported data graphically with no standard deviation or P value (Crawford 1999). One study listed this as an outcome of the study but did not actually report it (Kim 2017). One study reported the incidence of hospitalisation (Saarilahti 2002).
Number of days of treatment with opioid analgesics
Two studies reported data that we were able to use in analyses i.e. mean and standard deviations (Blijlevens 2013; Dazzi 2003; Freytes 2004). Only one study specified that the opioid use was due to oral mucositis (Freytes 2004).
Four further studies reported medians (Fink 2011; Kim 2017; Lucchese 2016a; Spielberger 2004), whilst another study did not state whether the data were means or medians, and there were no standard deviations or P value (Lucchese 2016b). Three studies reported total dose of opioid analgesic (Henke 2011; Le 2011; Vadhan‐Raj 2010), whilst four studies reported the incidence of its use (Hosseinjani 2017; Jagasia 2012; Saarilahti 2002; van der Lelie 2001). One study stated that it assessed the use of opioid analgesics, but did not specify whether this was in terms of duration, quantity or incidence, and did not actually report any data (Wu 2009).
Number of days unable to take medicine orally
No studies reported this outcome.
Excluded studies
We excluded 24 studies from this review for the following reasons.
Not a randomised controlled trial or unclear (Foncuberta 2001; Gordon 1993; Horsley 2007; Hunter 2009; Iwase 1997; Limaye 2013; Throuvalas 1995; Vitale 2014).
Stomatitis incidence reported in adverse events table (Kubo 2016; Lee 2016; Nabholtz 2002; Tsurusawa 2016).
Unclear if mucositis was oral or gastrointestinal (Jones 1996; Legros 1997; Pettengell 1992).
Study stopped early with very few participants enrolled (Antin 2002; NCT00360971; NCT00626639).
Oral mucositis not mentioned and unknown if measured (Gebbia 1994; Gladkov 2013).
Some participants had oral mucositis at baseline (Ryu 2007).
Cross‐over study with no reporting of first‐period data (de Koning 2007).
Results reported by cycle assuming independence (Karthaus 1998).
Survival/cure was primary outcome with mucositis (unclear if oral or gastrointestinal) as a toxicity (Ifrah 1999).
Risk of bias in included studies
Allocation
Random sequence generation
Nineteen studies described an adequate method of generating a random sequence, so we assessed these as at low risk of bias. The remaining 16 studies stated that they were randomised without providing a description of how the random sequence was generated, so we assessed these as at unclear risk of bias.
Allocation concealment
Seventeen studies described a process that would have concealed the random sequence from those involved in the study, thus allowing it to be applied as it was generated. We assessed these 17 studies as at low risk of bias. The remaining 18 studies did not describe any methods used to conceal the random sequence, so we assessed them as at unclear risk of bias.
In total, 16 studies are at low risk of selection bias, meaning that we assessed both of the above domains as low risk of bias. The remaining 19 studies are at unclear risk of selection bias because one or both of the above domains were rated as unclear.
Most studies were carried out in middle‐income and high‐income countries with strict controls and regulations and we feel that many of them probably had adequate randomisation, and that the unclear ratings for these two domains were probably due to reporting issues rather than actual bias. Therefore, when incorporating risk of bias into our GRADE assessments, we did not downgrade any evidence based on selection bias.
Blinding
Blinding of participants and personnel (performance bias)
We assessed 28 studies as at low risk of bias. Twenty‐seven of these studies used a placebo comparator and this ensured that blinding was performed successfully. One further study compared GM‐CSF with sucralfate, but the interventions were supplied as identical‐looking mouthwashes, the study was described as double‐blind, and there was no reason to suspect that participants or personnel were not blinded (Saarilahti 2002).
We assessed seven studies as at high risk of bias. Three of these studies used a no‐treatment comparator, so blinding was not possible (Chi 1995; Katano 1995; McAleese 2006). Two other studies were similar in that they compared KGF plus best supportive care with best supportive care alone (Fink 2011), and GM‐CSF plus sucralfate with sucralfate alone (Makkonen 2000). One study compared intravenous KGF with a chlorhexidine mouthwash (Gholizadeh 2016). The remaining study compared two types of G‐CSF, but the dosing schedule was very different, ensuring that blinding was not possible (Cesaro 2013).
Blinding of outcome assessment (detection bias)
We assessed 29 studies as at low risk of bias. We assessed four studies as at unclear risk of bias because blinding of outcome assessment was not mentioned, but we judged that it would have been possible to achieve (Cesaro 2013; Chi 1995; Katano 1995; Makkonen 2000). We assessed the remaining two studies as at high risk of bias because they either stated that there was no blinding of outcome assessors (Fink 2011), or it was implied by the description "single‐blind" (Linch 1993).
Incomplete outcome data
Attrition was generally very low and we assessed 31 studies as at low risk of bias. We assessed two studies as at unclear risk of bias because one did not report how many of the randomised participants were included in the analyses (Makkonen 2000), and the other did not report the attrition by treatment arm but there was potential for bias if the dropouts were mostly from one arm and had developed the outcome of severe oral mucositis (Cartee 1995). We assessed two studies as at high risk of bias because one had very high attrition (Antoun 2009), and the other had 19% attrition in one arm compared to none in the other arm (Fink 2011).
Selective reporting
It is important to note that we have perhaps been quite lenient when rating bias under this domain. We have tended to focus on the primary outcome because the vast majority of the data are for this outcome. Many studies have only reported a particular level of oral mucositis severity, for example grade 2 to 4 (ulcerative/moderate to severe), when they could have reported more usable data by reporting the maximum grade experienced per patient, allowing us to dichotomise this into all severities. Some readers may consider this to be bias but we have reported all this information transparently in the Characteristics of included studies tables, thus allowing the reader to decide if they would judge the risk of bias differently. Furthermore, many secondary outcomes were reported poorly or in a way that was not amenable to meta‐analysis, which in most cases is a reporting issue rather than a bias issue. This highlighted the problem with the current Cochrane risk of bias tool in that meta‐analyses are being biased due to missing information, but this is not being accounted for in the meta‐analysis. It does not seem appropriate to rate a study at high risk of bias due to a secondary outcome when it is contributing data to the meta‐analysis for the primary outcome, and it is the meta‐analysis for the secondary outcome that is affected by bias. Again, all this information is clearly reported in the Characteristics of included studies tables.
We assessed 32 studies as at low risk of bias. We assessed the remaining three studies as at high risk of bias, two because there were no usable data for the primary outcome (Linch 1993; Makkonen 2000), and one because several outcomes were assessed but not reported (Wu 2009).
Other potential sources of bias
We did not consider there to be any issues arising from other potential sources of bias in any of the studies and we therefore assessed them all as at low risk of other bias.
Overall risk of bias
Thirteen studies (37%) were at low overall risk of bias (Blijlevens 2013; Dazzi 2003; Freytes 2004; Henke 2011; Hosseinjani 2017; Kim 2017; Le 2011; Lucchese 2016a; Lucchese 2016b; Saarilahti 2002; Schneider 1999; Su 2006; Vadhan‐Raj 2010).
Twelve studies (34%) were at unclear overall risk of bias (Blazar 2006; Bradstock 2014; Brizel 2008; Cartee 1995; Crawford 1999; Jagasia 2012; Meropol 2003; Nemunaitis 1995; Peterson 2009; Rosen 2006; Spielberger 2004; van der Lelie 2001).
Ten studies (29%) were at high overall risk of bias (Antoun 2009; Cesaro 2013; Chi 1995; Fink 2011; Gholizadeh 2016; Katano 1995; Linch 1993; Makkonen 2000; McAleese 2006; Wu 2009).
Risk of bias can be viewed graphically in Figure 2.
2.
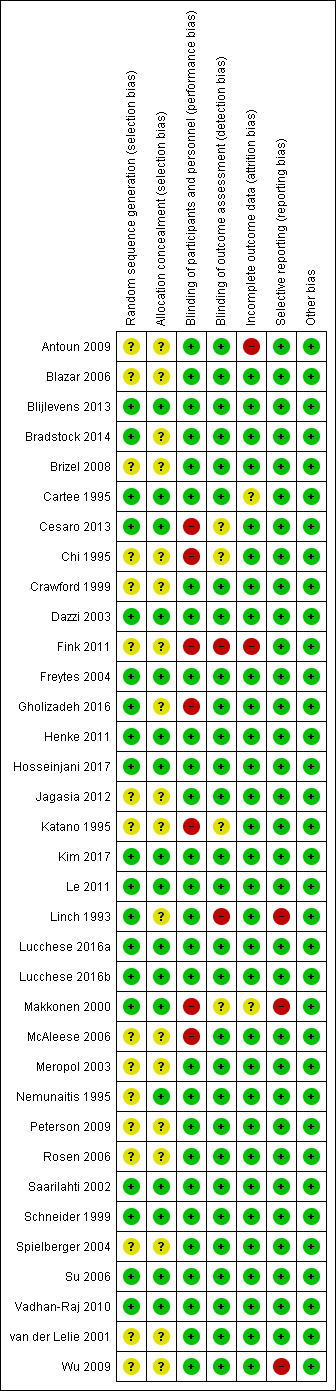
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3
We used GRADE methods to assess the quality of the body of evidence for each comparison in which there was more than one study in at least one of the subgroups based on cancer treatment. We included the incidence of moderate to severe oral mucositis, the incidence of severe oral mucositis and adverse events. These assessments are presented in Table 1; Table 2; Table 3.
Keratinocyte growth factor (KGF) versus placebo
Oral mucositis
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers
There was insufficient evidence from four studies, one at low (Blijlevens 2013), two at unclear (Blazar 2006; Spielberger 2004), and one at high risk of bias (Fink 2011), to determine whether or not KGF reduces the risk of any level of oral mucositis: risk ratio (RR) 0.96, 95% confidence interval (CI) 0.88 to 1.05; 655 participants (Analysis 1.1).
1.1. Analysis.
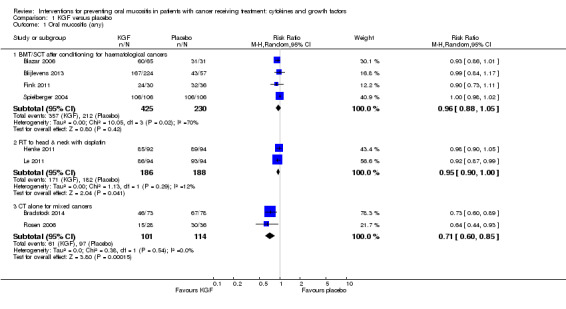
Comparison 1 KGF versus placebo, Outcome 1 Oral mucositis (any).
Six studies, two at low (Blijlevens 2013; Freytes 2004), three at unclear (Blazar 2006; Jagasia 2012; Spielberger 2004), and one at high risk of bias (Fink 2011), showed a reduction in the risk of moderate to severe oral mucositis in favour of KGF: RR 0.89, 95% CI 0.80 to 0.99; 852 participants (Analysis 1.2).
1.2. Analysis.
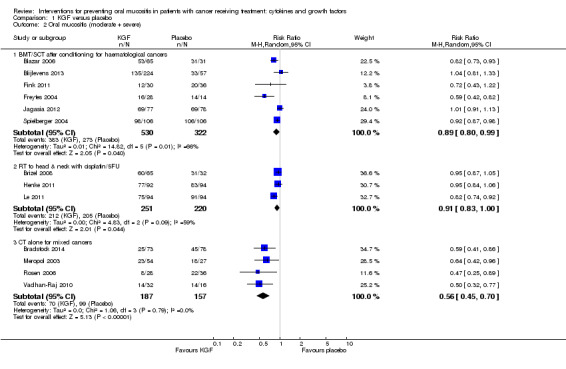
Comparison 1 KGF versus placebo, Outcome 2 Oral mucositis (moderate + severe).
The same six studies showed a possible reduction in the risk of severe oral mucositis in favour of KGF, but there is also some possibility of an increase in risk: RR 0.85, 95% CI 0.65 to 1.11; 852 participants (Analysis 1.3).
1.3. Analysis.

Comparison 1 KGF versus placebo, Outcome 3 Oral mucositis (severe).
Heterogeneity present in these meta‐analyses may partly be due to differences between studies where transplants were autologous or allogeneic.
Adults receiving radiotherapy to the head and neck with cisplatin/fluorouracil (5FU)
Two studies, both at low risk of bias (Henke 2011; Le 2011), showed a reduction in the risk of any level of oral mucositis in favour of KGF: RR 0.95, 95% CI 0.90 to 1.00; P = 0.04; 374 participants (Analysis 1.1).
Three studies, two at low (Henke 2011; Le 2011), and one at unclear risk of bias (Brizel 2008), showed a reduction in the risk of moderate to severe oral mucositis in favour of KGF: RR 0.91, 95% CI 0.83 to 1.00; P = 0.04; 471 participants (Analysis 1.2).
The same three studies showed a reduction in the risk of severe oral mucositis in favour of KGF: RR 0.79, 95% CI 0.69 to 0.90; 471 participants (Analysis 1.3).
Adults receiving chemotherapy alone for mixed cancers
Two studies, both at unclear risk of bias (Bradstock 2014; Rosen 2006), showed a reduction in the risk of any level of oral mucositis in favour of KGF: RR 0.71, 95% CI 0.60 to 0.85; 215 participants (Analysis 1.1).
Four studies, one at low (Vadhan‐Raj 2010), and three at unclear risk of bias (Bradstock 2014; Meropol 2003; Rosen 2006), showed a reduction in the risk of moderate to severe oral mucositis in favour of KGF: RR 0.56, 95% CI 0.45 to 0.70; 344 participants (Analysis 1.2).
Three studies, one at low (Vadhan‐Raj 2010), and two at unclear risk of bias (Bradstock 2014; Rosen 2006), showed a reduction in the risk of severe oral mucositis in favour of KGF: RR 0.30, 95% CI 0.14 to 0.65; 263 participants (Analysis 1.3).
Interruptions to cancer treatment
Adults receiving radiotherapy to the head and neck with cisplatin/fluorouracil (5FU)
There was insufficient evidence from three studies, two at low (Henke 2011; Le 2011), and one at unclear risk of bias (Brizel 2008), to determine whether or not KGF reduces the risk of having unscheduled radiotherapy breaks of five or more days: RR 1.01, 95% CI 0.65 to 1.59; 473 participants (Analysis 1.4).
1.4. Analysis.
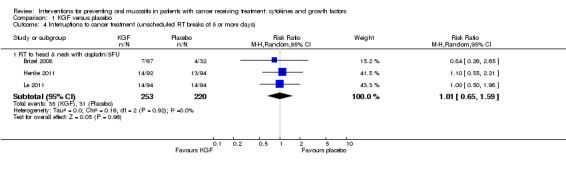
Comparison 1 KGF versus placebo, Outcome 4 Interruptions to cancer treatment (unscheduled RT breaks of 5 or more days).
There was insufficient evidence, from the same two studies at low risk of bias, to determine whether or not KGF reduces the risk of having chemotherapy delays/discontinuations: RR 0.96, 95% CI 0.62 to 1.47; 374 participants (Analysis 1.5).
1.5. Analysis.
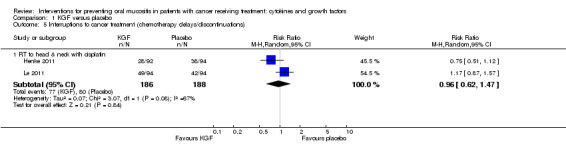
Comparison 1 KGF versus placebo, Outcome 5 Interruptions to cancer treatment (chemotherapy delays/discontinuations).
Oral pain
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers
There was insufficient evidence, from one study at low risk of bias (Freytes 2004), to determine whether or not KGF reduces the mean worst pain experienced on a 0 (no pain) to 10 (worst pain) scale: mean difference (MD) ‐0.85, 95% CI ‐3.00 to 1.30; 42 participants (Analysis 1.6).
1.6. Analysis.
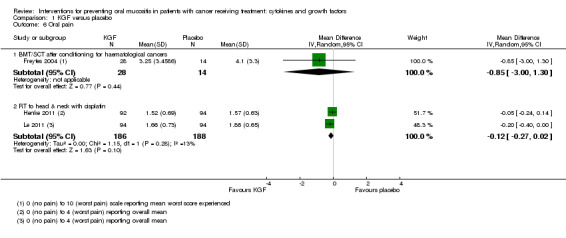
Comparison 1 KGF versus placebo, Outcome 6 Oral pain.
Adults receiving radiotherapy to the head and neck with cisplatin
There was some evidence, from two studies at low risk of bias (Henke 2011; Le 2011), that KGF might lead to a reduction in the mean pain score on a 0 (no pain) to 4 (worst pain) scale: MD ‐0.12, 95% CI ‐0.27 to 0.02; 374 participants (Analysis 1.6).
Normalcy of diet
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers
There was insufficient evidence from four studies, one at low (Blijlevens 2013), two at unclear (Jagasia 2012; Spielberger 2004), and one at high risk of bias (Fink 2011), to determine whether or not KGF reduces the risk of total parenteral nutrition: RR 0.89, 95% CI 0.58 to 1.34; 714 participants (Analysis 1.7).
1.7. Analysis.
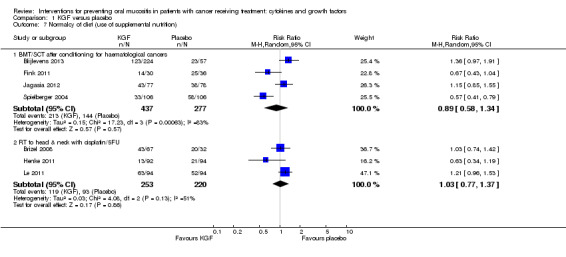
Comparison 1 KGF versus placebo, Outcome 7 Normalcy of diet (use of supplemental nutrition).
There was further insufficient evidence, from one study at low risk of bias (Freytes 2004), to determine whether or not KGF reduces the mean worst ability to eat score on a 1 (normal) to 4 (no solids or liquids) scale: MD ‐0.50, 95% CI ‐1.21 to 0.21; 42 participants (Analysis 1.8).
1.8. Analysis.

Comparison 1 KGF versus placebo, Outcome 8 Normalcy of diet (worst ability to eat score ‐ 1 to 4 scale).
Adults receiving radiotherapy to the head and neck with cisplatin/fluorouracil (5FU)
There was insufficient evidence from three studies, two at low (Henke 2011; Le 2011), and one at unclear risk of bias (Brizel 2008), to determine whether or not KGF reduces the risk of receiving supplemental nutrition (total parenteral nutrition, percutaneous endoscopic gastrostomy, nasogastric tube or intravenous (IV) hydration): RR 1.03, 95% CI 0.77 to 1.37; 473 participants (Analysis 1.7).
Adverse events
This outcome was difficult to summarise due to poor and inconsistent reporting, and we did not meta‐analyse any data. However, there do not appear to be any serious concerns regarding adverse effects of KGF. We have tabulated relevant information in Additional Table 4.
1. Adverse events: KGF.
| Study ID | Adverse events results |
| Blazar 2006 |
|
| Blijlevens 2013 |
|
| Bradstock 2014 | Insufficient evidence of a difference in infection or Grade 3 to 4 (NCI‐CTC 0 to 4 toxicity scale) skin rash/desquamation |
| Brizel 2008 | The study authors state that most adverse events were considered to be caused by the cancer treatment or the underlying cancer itself and not related to study treatment. 2 participants in the palifermin group had serious adverse events considered to be related to the intervention: 1 had increased sputum production; the other had dehydration, dysphagia, pain (including abdominal), pancreatitis, and subsequently had schistosomiasis |
| Fink 2011 | Total of 28 side effects in palifermin group occurring in 11 of 22 patients (50%) who received at least 4 of the 6 doses. Most frequent (90.9%) were cases of erythema or exanthema, often associated with itching (54.5%). Often (54.5%) a swelling of the oral mucosa including the tongue occurred. In 4 out of 6 patients, this was accompanied by taste disturbance. The severity of side effects were classified as mild to moderate. The CTC Grade 3 occurred only once in the form of a strong heat sensation In 1 of the 11 cases, there was premature discontinuation of palifermin due to severe facial swelling with eyelid and laryngeal pain as well as painful swelling of the hands following the second injection |
| Freytes 2004 | 25 different adverse events were reported and were mostly not KGF‐related. There was insufficient evidence of a difference for diarrhoea, abdominal pain, infection or rash |
| Gholizadeh 2016 | "..two patients reported knee joint pain, skin rash was observed in one patient, two patients had abnormal taste, and one showed lingual mucosal thickening" (control group was chlorhexidine mouthrinse. The authors do not report the events by treatment group) |
| Henke 2011 |
|
| Jagasia 2012 | KGF‐related AEs with incidence ≥ 5% in KGF group: higher rate of gastrointestinal disorders in KGF group (18/78 versus 2/73; RR 8.42, 95% CI 2.02 to 35.04; P = 0.003). Insufficient evidence of a difference in any AE, tongue coating, tongue disorder, skin and subcutaneous tissue disorders, rash, pruritus, or erythema |
| Le 2011 | "Study drug–related AEs were reported for 35% of palifermin and 11% of placebo patients. The most frequent study drug–related AEs (palifermin, placebo) were rash (9%, 2%), flushing (5%, 0%), dysgeusia (5%, 1%), nausea (4%, 1%),and vomiting (3%, 1%). None of these events led to study withdrawal. Serious AEs considered related to study treatment were reported for five palifermin patients (5%; one each with necrotic pancreatitis, hypersensitivity, tracheostomy malfunction, peritoneal carcinoma, and convulsion) and two placebo patients (2%; one each with hepatitis/hepatic enzyme increase and cryptogenic organizing pneumonia)" |
| Lucchese 2016a | "The administration of palifermin was generally safe and without considerable complications. The only adverse reactions were rashes (lasting for 48–72 hours) localized to the face, upper neck and shoulders, erythema, and altered taste (consistent with the pharmacologic action of palifermin of oral epithelium and skin), most of which were of NCI grade 1 or 2 severity" |
| Lucchese 2016b | "The administration of palifermin was mostly safe and without substantial complications. The mean duration of the OM and the number of adverse event was significant less in the palifermin group (Tables II, III, Figure 1). The main adverse episodes were erythema, cutaneous rashes and altered taste and three of the patients in the palifermin group showed a light thickness of the tongue, mouth and palate" |
| Meropol 2003 | "Although the predefined frequency of DLTs attributable to KGF was not reached with KGF doses between 1 and 80 µg/kg/d, there were three adverse reactions involving the skin that required discontinuation of KGF in the 18 patients treated with 60 or 80 µg/kg (Table 5). Overall, skin and oral adverse events (rash, flushing, pruritis, edema, hypoesthesia, paresthesia, tongue disorder [thickening], and alteration in taste sensation) attributed to KGF occurred in 13 of 18 patients treated with 60 and 80 µg/kg of KGF (eight patients, grade 1; four patients, grade 2; and one patient, grade 3) and in three of 11 patients treated with 40 µg/kg (all grade 1). These events were reported in 16 of 39 patients (41%) dosed with KGF at > 20 µg/kg/d, whereas these symptoms were reported in only two of 21 subjects (10%) treated with placebo. The skin and oral toxicities associated with KGF were generally mild to moderate in severity, with onset approximately 36 hours after the first dose of KGF and resolution 7 to 10 days thereafter" |
| Rosen 2006 |
|
| Spielberger 2004 | "The incidence, frequency, and severity of adverse events were similar in the two groups, and most were attributable to the underlying cancer, cytotoxic chemotherapy, or total‐body irradiation. Those that occurred with an incidence that was at least 5 percentage points higher in the palifermin group than in the placebo group are listed in Table 3. Most of these adverse events were consistent with the pharmacologic action of palifermin on skin and oral epithelium (e.g., rash, pruritus, erythema, paresthesia, mouth and tongue disorders, and taste alteration). All these events were mild to moderate in severity, transient (occurring approximately three days after the third dose of palifermin and lasting approximately three days), and not a cause for the discontinuation of study drug. Serious adverse events considered to be related to treatment occurred in one palifermin recipient (rash) and one placebo recipient (hypotension)" |
| Vadhan‐Raj 2010 |
|
AE = adverse event; CI = confidence interval; KGF = keratinocyte growth factor; NCI‐CTC = National Cancer Institute common toxicity criteria; RR = risk ratio; WHO = World Health Organization.
Number of days in hospital
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers
There was insufficient evidence, from one study at low risk of bias (Blijlevens 2013), to determine whether or not KGF reduces the mean number of days in hospital: MD 0.00, 95% CI ‐1.64 to 1.64; 281 participants (Analysis 1.9).
1.9. Analysis.
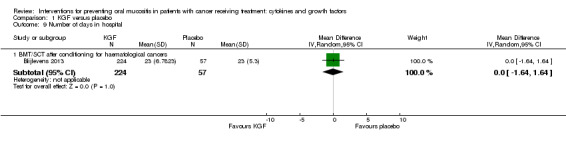
Comparison 1 KGF versus placebo, Outcome 9 Number of days in hospital.
Number of days of treatment with opioid analgesics
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers
There was some imprecise evidence, from two studies at low risk of bias (Blijlevens 2013; Freytes 2004), that KGF might lead to a reduction in the mean number of days of treatment with opioid analgesics: MD ‐1.41, 95% CI ‐3.33 to 0.51; 323 participants (Analysis 1.10). The average effect is around 1.5 days reduction, but the confidence interval is compatible with both a reduction of almost 3.5 days and an increase of half a day.
1.10. Analysis.
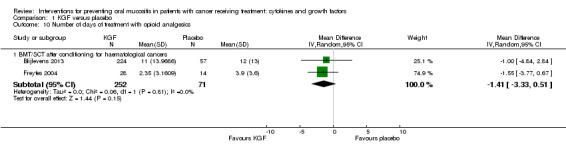
Comparison 1 KGF versus placebo, Outcome 10 Number of days of treatment with opioid analgesics.
No studies assessed the outcomes 'quality of life' and 'number of days unable to take medicine orally'.
Keratinocyte growth factor (KGF) dose comparisons
There was some inconsistent evidence from which no conclusions can be drawn regarding different dosages of KGF (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8).
2.1. Analysis.
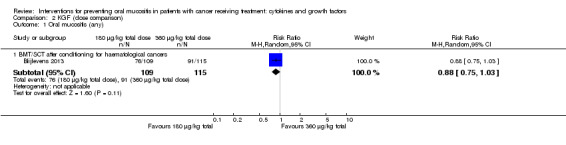
Comparison 2 KGF (dose comparison), Outcome 1 Oral mucositis (any).
2.2. Analysis.
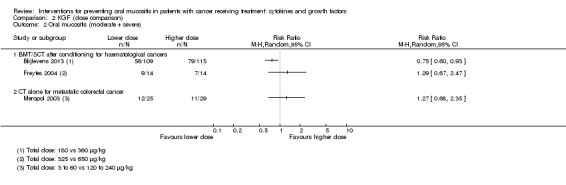
Comparison 2 KGF (dose comparison), Outcome 2 Oral mucositis (moderate + severe).
2.3. Analysis.
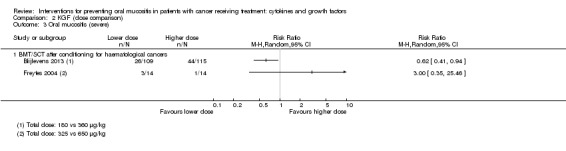
Comparison 2 KGF (dose comparison), Outcome 3 Oral mucositis (severe).
2.4. Analysis.
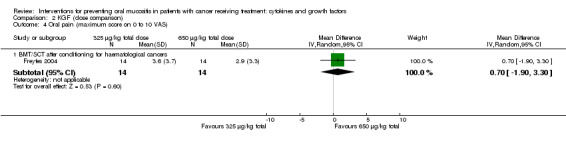
Comparison 2 KGF (dose comparison), Outcome 4 Oral pain (maximum score on 0 to 10 VAS).
2.5. Analysis.
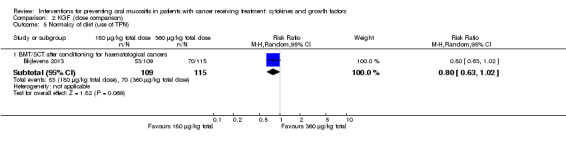
Comparison 2 KGF (dose comparison), Outcome 5 Normalcy of diet (use of TPN).
2.6. Analysis.
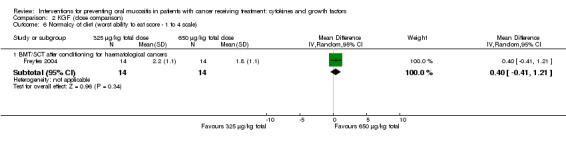
Comparison 2 KGF (dose comparison), Outcome 6 Normalcy of diet (worst ability to eat score ‐ 1 to 4 scale).
2.7. Analysis.
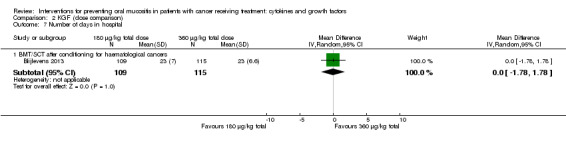
Comparison 2 KGF (dose comparison), Outcome 7 Number of days in hospital.
2.8. Analysis.
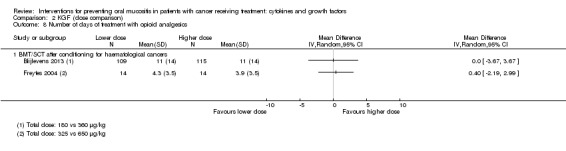
Comparison 2 KGF (dose comparison), Outcome 8 Number of days of treatment with opioid analgesics.
Keratinocyte growth factor (KGF) versus chlorhexidine
One study, at high risk of bias and analysing 90 children receiving mixed chemotherapy alone for acute lymphoblastic leukaemia (Gholizadeh 2016), compared KGF by IV infusion with chlorhexidine mouthwash. There was weak evidence (due to risk of bias and low sample size) that KGF performs better than chlorhexidine in reducing the risk of any level of oral mucositis (RR 0.67, 95% CI 0.54 to 0.85; Analysis 3.1), moderate to severe oral mucositis (RR 0.12, 95% CI 0.05 to 0.28; Analysis 3.2), and severe oral mucositis (RR 0.01, 95% CI 0.00 to 0.19; Analysis 3.3).
3.1. Analysis.
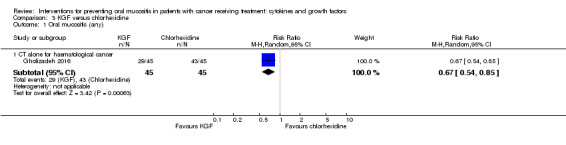
Comparison 3 KGF versus chlorhexidine, Outcome 1 Oral mucositis (any).
3.2. Analysis.
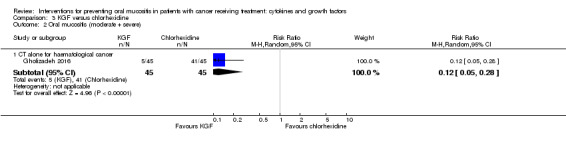
Comparison 3 KGF versus chlorhexidine, Outcome 2 Oral mucositis (moderate + severe).
3.3. Analysis.
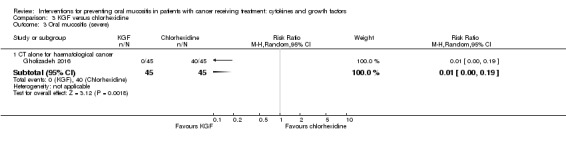
Comparison 3 KGF versus chlorhexidine, Outcome 3 Oral mucositis (severe).
Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) versus placebo/no treatment
Oral mucositis
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for mixed cancers
There was some evidence, from one study at low risk of bias (Dazzi 2003), that GM‐CSF might lead to a reduction in the risk of any level of oral mucositis: RR 0.91, 95% CI 0.80 to 1.04; 90 participants (Analysis 4.1).
4.1. Analysis.
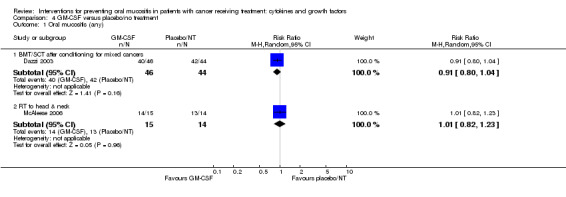
Comparison 4 GM‐CSF versus placebo/no treatment, Outcome 1 Oral mucositis (any).
There was insufficient evidence, from one study at unclear risk of bias (Nemunaitis 1995), to determine whether or not GM‐CSF reduces the risk of moderate to severe oral mucositis: RR 0.94, 95% CI 0.79 to 1.13; 109 participants (Analysis 4.2).
4.2. Analysis.
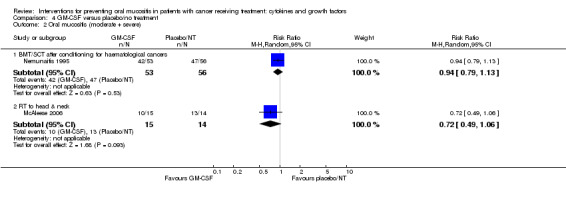
Comparison 4 GM‐CSF versus placebo/no treatment, Outcome 2 Oral mucositis (moderate + severe).
There was insufficient evidence from three studies, one at low (Dazzi 2003), and two at unclear risk of bias (Nemunaitis 1995; van der Lelie 2001), to determine whether or not GM‐CSF reduces the risk of severe oral mucositis: RR 0.74, 95% CI 0.33 to 1.67; 235 participants (Analysis 4.3).
4.3. Analysis.
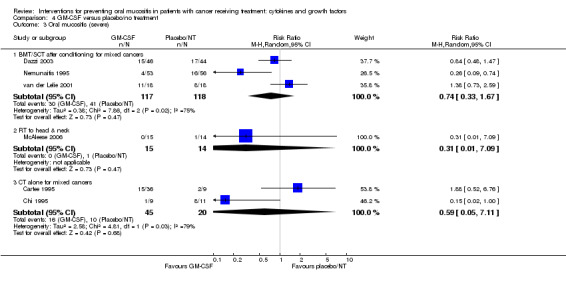
Comparison 4 GM‐CSF versus placebo/no treatment, Outcome 3 Oral mucositis (severe).
Adults receiving radiotherapy to the head and neck
There was insufficient evidence, from one study at high risk of bias (McAleese 2006), to determine whether or not GM‐CSF reduces the risk of any level of oral mucositis (RR 1.01, 95% CI 0.82 to 1.23; 29 participants; Analysis 4.1), moderate to severe oral mucositis (RR 0.72, 95% CI 0.49 to 1.06; 29 participants; Analysis 4.2), or severe oral mucositis (RR 0.31, 95% CI 0.01 to 7.09; 29 participants; Analysis 4.3).
Adults receiving chemotherapy alone for mixed cancers
There was insufficient evidence from two studies, one at unclear (Cartee 1995), and one at high risk of bias (Chi 1995), to determine whether or not GM‐CSF reduces the risk of severe oral mucositis: RR 0.59, 95% CI 0.05 to 7.11; 65 participants (Analysis 4.3).
Oral pain
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for mixed cancers
There was insufficient evidence, from one study at low risk of bias (Dazzi 2003), to determine whether or not GM‐CSF reduces the mean pain score on a 0 (no pain) to 10 (worst pain) scale: MD 0.60, 95% CI ‐0.85 to 2.05; 90 participants (Analysis 4.4).
4.4. Analysis.
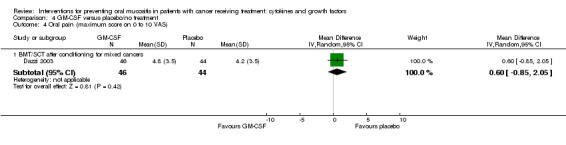
Comparison 4 GM‐CSF versus placebo/no treatment, Outcome 4 Oral pain (maximum score on 0 to 10 VAS).
Normalcy of diet
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers
There was insufficient evidence, from one study at unclear risk of bias (van der Lelie 2001), to determine whether or not GM‐CSF reduces the risk of total parenteral nutrition: RR 1.10, 95% CI 0.63 to 1.91; 36 participants (Analysis 4.5).
4.5. Analysis.
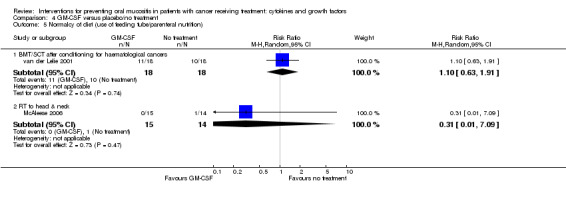
Comparison 4 GM‐CSF versus placebo/no treatment, Outcome 5 Normalcy of diet (use of feeding tube/parenteral nutrition).
Adults receiving radiotherapy to the head and neck
There was insufficient evidence, from one study at high risk of bias (McAleese 2006), to determine whether or not GM‐CSF reduces the risk of tube feeding: RR 0.31, 95% CI 0.01 to 7.09; 29 participants (Analysis 4.5).
Adverse events
This outcome was difficult to summarise due to poor and inconsistent reporting, and we did not meta‐analyse any data. However, there do not appear to be any serious concerns regarding adverse effects of GM‐CSF. We have tabulated relevant information in Additional Table 5.
2. Adverse events: GM‐CSF.
| Study ID | Adverse events results |
| Cartee 1995 | 2 participants (group allocation not reported) withdrew by day 3 due to intolerance to their mouthwash (dry mouth); 1 participant receiving GM‐CSF (1 µg/mL) had mouthwash withdrawn by day 3 due to possible allergic reaction (sensation of fullness in the posterior pharyngeal area) but resolved within 4 hours (the participant was withdrawn but appears to have been included in the analysis) |
| Chi 1995 | "One patient had fever and chills, and two patients had general malaise and headache during GM‐CSF treatment. No patient had evidence of fluid retention after GM‐CSF" |
| Dazzi 2003 | Not reported |
| Makkonen 2000 | (Sucralfate given to both groups) "Only 2 of the 20 patients treated with GM‐CSF and sucralfate did not experience any side effects related to the drugs, but most side effects were mild (WHO Grade 1 or 2). The most common side effects were local skin reactions, fever, bone pain, and mild nausea...In the control group only 1 patient complained of nausea possibly related to the use of sucralfate, and another patient interrupted sucralfate treatment because of the same reason" |
| McAleese 2006 | "12 patients who received GM‐CSF had elevated white cell counts (WCC). The range of maximal WCC was 7.2–30.5 (median 19.7). All WCC had returned to normal within 3 weeks of completing injections (median 2 weeks). Three patients developed influenza‐like symptoms with the GM‐CSF and in one patient the injections were stopped because of this symptom. One patient developed an erythematous rash at his injection sites after completing his course of 14 injections (Figure 3). He had a past history of allergy to radiographic contrast medium" |
| Nemunaitis 1995 |
|
| Saarilahti 2002 | (Comparator was sucralfate) "Both mouthwashes were well tolerated, and none of the patients reported any adverse effects related to the mouthwashes. Adverse effects commonly associated with subcutaneous GM‐CSF administration, such as nausea, vomiting, bone pain, headaches, and fever, were not observed" |
| van der Lelie 2001 | Not reported |
GM‐CSF = granulocyte‐macrophage colony‐stimulating factor.
Number of days of treatment with opioid analgesics
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for mixed cancers
There was some evidence, from one study at low risk of bias (Dazzi 2003), that GM‐CSF might lead to a reduction in the mean number of days of treatment with opioid analgesics: MD ‐1.10, 95% CI ‐1.91 to ‐0.29; 90 participants (Analysis 4.6).
4.6. Analysis.
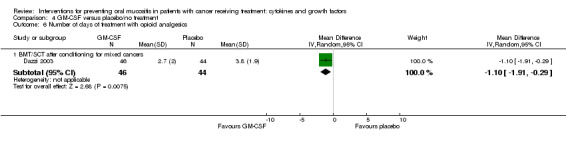
Comparison 4 GM‐CSF versus placebo/no treatment, Outcome 6 Number of days of treatment with opioid analgesics.
No studies assessed the outcomes 'interruptions to cancer treatment', 'quality of life', 'number of days in hospital' and 'number of days unable to take medicine orally'.
Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) dose comparison
There is some very weak evidence, from one study at unclear risk of bias and analysing 36 adults receiving mixed chemotherapy alone for breast cancer (Cartee 1995), that a higher dose of GM‐CSF (range 1260 µg to 12,600 µg) reduces the risk of severe oral mucositis when compared to a lower dose (range 12.6 µg to 126 µg): RR 2.75, 95% CI 1.07 to 7.04; 36 participants (Analysis 5.1).
5.1. Analysis.
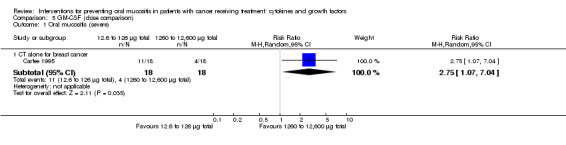
Comparison 5 GM‐CSF (dose comparison), Outcome 1 Oral mucositis (severe).
Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) versus sucralfate
One study, at low risk of bias and analysing 40 adults receiving radiotherapy to the head and neck (Saarilahti 2002), compared GM‐CSF with sucralfate, both as a mouthwash. There was insufficient evidence to determine whether GM‐CSF or sucralfate perform better in reducing the risk of moderate to severe oral mucositis (RR 0.96, 95% CI 0.80 to 1.14; Analysis 6.1), severe oral mucositis (RR 0.54, 95% CI 0.24 to 1.21; Analysis 6.2), interruptions to cancer treatment (RR 0.13, 95% CI 0.01 to 2.36; Analysis 6.3), or percutaneous endoscopic gastrostomy (RR 0.18, 95% CI 0.01 to 3.56; Analysis 6.4).
6.1. Analysis.
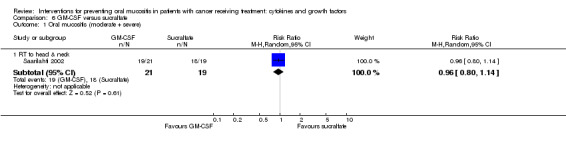
Comparison 6 GM‐CSF versus sucralfate, Outcome 1 Oral mucositis (moderate + severe).
6.2. Analysis.
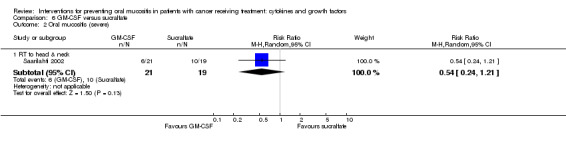
Comparison 6 GM‐CSF versus sucralfate, Outcome 2 Oral mucositis (severe).
6.3. Analysis.
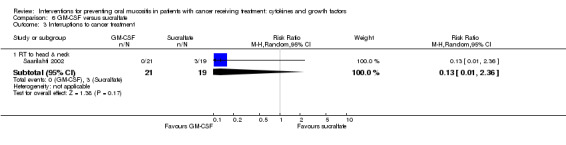
Comparison 6 GM‐CSF versus sucralfate, Outcome 3 Interruptions to cancer treatment.
6.4. Analysis.
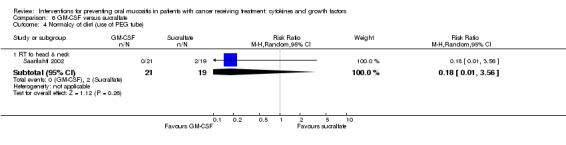
Comparison 6 GM‐CSF versus sucralfate, Outcome 4 Normalcy of diet (use of PEG tube).
Granulocyte‐colony stimulating factor (G‐CSF) versus placebo/no treatment
Oral mucositis
Adults receiving radiotherapy to the head and neck
There was insufficient evidence, from two studies at low risk of bias (Schneider 1999; Su 2006), to determine whether or not G‐CSF reduces the risk of any level of oral mucositis: RR 1.02, 95% CI 0.86 to 1.22; 54 participants (Analysis 7.1).
7.1. Analysis.
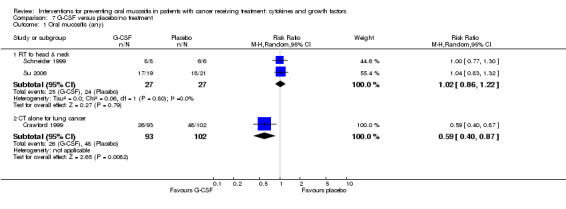
Comparison 7 G‐CSF versus placebo/no treatment, Outcome 1 Oral mucositis (any).
The same two studies showed weak evidence (due to a wide confidence interval and low sample size) of a reduction in the risk of severe oral mucositis in favour of G‐CSF: RR 0.37, 95% CI 0.15 to 0.87; 54 participants (Analysis 7.3).
7.3. Analysis.
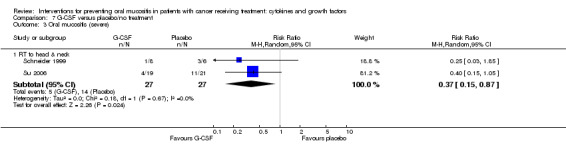
Comparison 7 G‐CSF versus placebo/no treatment, Outcome 3 Oral mucositis (severe).
Adults receiving chemotherapy alone for mixed cancers
One study on lung cancer, at unclear risk of bias (Crawford 1999), showed a reduction in the risk of any level of oral mucositis in favour of G‐CSF: RR 0.59, 95% CI 0.40 to 0.87; 195 participants (Analysis 7.1).
One study on breast cancer, at high risk of bias (Katano 1995), showed very weak evidence (due to risk of bias, very low sample size and a wide confidence interval) of a reduction in the risk of moderate to severe oral mucositis in favour of G‐CSF: RR 0.33, 95% CI 0.12 to 0.95; 14 participants (Analysis 7.2).
7.2. Analysis.
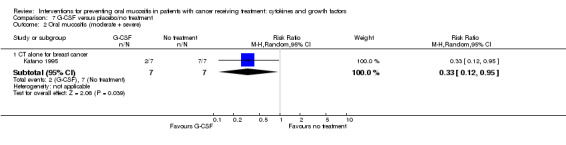
Comparison 7 G‐CSF versus placebo/no treatment, Outcome 2 Oral mucositis (moderate + severe).
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers
One study, at high risk of bias and analysing 121 participants (Linch 1993), did not provide any details of how oral mucositis was measured, so it is not clear what severity the information refers to. There were no numerical results reported, only the statement: "There was no difference in the frequency of stomatitis (defined as a sore, infected or ulcerated mouth, lips or pharynx), the incidence being between 29 and 33% in all groups" (Additional Table 6).
3. G‐CSF versus placebo.
| Study ID | Population | Outcome | GM‐CSF | Placebo | Result |
| Linch 1993 | BMT/SCT after conditioning for haematological cancers | Oral mucositis: no scale described | No data | No data | "There was no difference in the frequency of stomatitis (defined as a sore, infected or ulcerated mouth, lips or pharynx), the incidence being between 29 and 33% in all groups" |
BMT = bone marrow transplantation; G‐CSF: granulocyte‐colony stimulating factor; SCT = stem cell transplantation.
Interruptions to cancer treatment
Adults receiving radiotherapy to the head and neck
There was insufficient evidence, from one study at low risk of bias (Su 2006), to determine whether or not G‐CSF reduces the risk of radiotherapy interruptions: RR 0.22, 95% CI 0.01 to 4.31; 40 participants (Analysis 7.4).
7.4. Analysis.
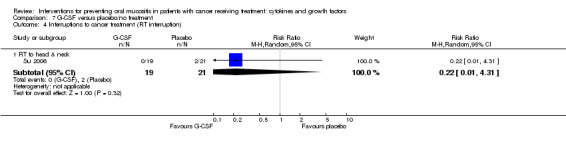
Comparison 7 G‐CSF versus placebo/no treatment, Outcome 4 Interruptions to cancer treatment (RT interruption).
Normalcy of diet
Adults receiving radiotherapy to the head and neck
There was insufficient evidence, from one study at low risk of bias (Su 2006), to determine whether or not G‐CSF reduces the risk of percutaneous endoscopic gastrostomy: RR 0.16, 95% CI 0.01 to 2.86; 40 participants (Analysis 7.5).
7.5. Analysis.
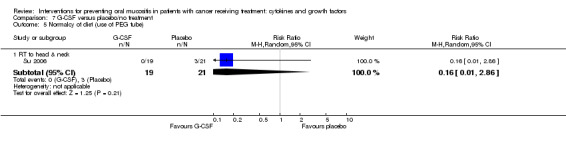
Comparison 7 G‐CSF versus placebo/no treatment, Outcome 5 Normalcy of diet (use of PEG tube).
Adverse events
This outcome was difficult to summarise due to poor and inconsistent reporting, and we did not meta‐analyse any data. However, there do not appear to be any serious concerns regarding adverse effects of G‐CSF. We have tabulated relevant information in Additional Table 7.
4. Adverse events: G‐CSF.
| Study ID | Adverse events results |
| Cesaro 2013 | "Both pegfilgrastim and filgrastim were well tolerated and no significant adverse effects were associated with their use" (G‐CSF versus G‐CSF) |
| Crawford 1999 | Approximately 20% of participants receiving G‐CSF experienced mild to moderate skeletal pain which was resolved by using oral analgesics; 6% of participants in both groups reported mild generalised rash/itching; 3 participants experienced an event thought to be G‐CSF‐related and which caused them to request withdrawal from the study: abdominal pain, diffuse aches and pains, and a flare‐up of pre‐existing eczema |
| Katano 1995 | Not reported |
| Linch 1993 | "There was no difference in the overall frequency of adverse clinical or laboratory events between the groups or in the frequency of adverse events thought by the clinicians to be possibly or probably due to study medication" |
| Schneider 1999 | Not reported |
| Su 2006 | "In general, toxicities typical of postoperative RT to the head and neck were observed. Additional toxicities attributable to G‐CSF and/or daily injections were as follows: elevated WBC requiring G‐CSF dose reduction by prospectively planned guidelines occurred in nine patients in the GCSF arm; grade 2–3 bone pain was observed in two patients in the G‐CSF arm; three patients refused injection (2 G‐CSF, 1 placebo)" |
G‐CSF = granulocyte‐colony stimulating factor.
No studies assessed the outcomes 'oral pain', 'quality of life', 'number of days in hospital', 'number of days of treatment with opioid analgesics' and 'number of days unable to take medicine orally'.
G‐CSF (pegfilgrastim) versus G‐CSF (filgrastim)
There was insufficient evidence, from one study at high risk of bias and analysing 61 children receiving bone marrow/stem cell transplantation after conditioning therapy for mixed cancers (Cesaro 2013), to determine whether pegfilgrastim or filgrastim perform better in reducing the risk of any level of oral mucositis (RR 1.02, 95% CI 0.82 to 1.27; Analysis 8.1), moderate to severe oral mucositis (RR 0.78, 95% CI 0.55 to 1.11; Analysis 8.2), or total parenteral nutrition (RR 1.00, 95% CI 0.94 to 1.06; Analysis 8.3).
8.1. Analysis.

Comparison 8 G‐CSF (pegfilgrastim) versus G‐CSF (filgrastim), Outcome 1 Oral mucositis (any).
8.2. Analysis.
Comparison 8 G‐CSF (pegfilgrastim) versus G‐CSF (filgrastim), Outcome 2 Oral mucositis (moderate + severe).
8.3. Analysis.
Comparison 8 G‐CSF (pegfilgrastim) versus G‐CSF (filgrastim), Outcome 3 Normalcy of diet (use of supplemental nutrition).
Epidermal growth factor (EGF) versus placebo
Oral mucositis
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers
There was insufficient evidence, from one study at low risk of bias and analysing 136 participants (Kim 2017), to determine whether or not EGF reduces the risk of moderate to severe oral mucositis (RR 1.06, 95% CI 0.78 to 1.43; Analysis 9.1), or severe oral mucositis (RR 1.03, 95% CI 0.59 to 1.80; Analysis 9.2).
9.1. Analysis.

Comparison 9 EGF versus placebo, Outcome 1 Oral mucositis (moderate + severe).
9.2. Analysis.
Comparison 9 EGF versus placebo, Outcome 2 Oral mucositis (severe).
Adults receiving radiotherapy to the head and neck with/without cisplatin
One study, at high risk of bias (Wu 2009), showed weak evidence (due to risk of bias and low sample size) of a reduction in the risk of moderate to severe oral mucositis in favour of EGF: RR 0.67, 95% CI 0.45 to 0.99; 103 participants (Analysis 9.1).
Interruptions to cancer treatment
Adults receiving radiotherapy to the head and neck with/without cisplatin
There was insufficient evidence, from one study at high risk of bias (Wu 2009), to determine whether or not EGF reduces the risk of having radiotherapy breaks longer than two days: RR 4.38, 95% CI 0.25 to 75.44; 113 participants (Analysis 9.3).
9.3. Analysis.
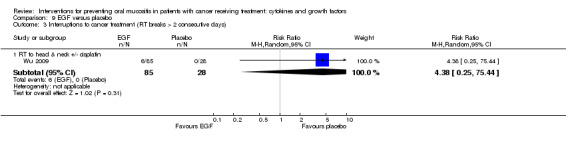
Comparison 9 EGF versus placebo, Outcome 3 Interruptions to cancer treatment (RT breaks > 2 consecutive days).
Normalcy of diet
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers
There was insufficient evidence, from one study at low risk of bias (Kim 2017), to determine whether or not EGF reduces the risk of total parenteral nutrition: RR 1.03, 95% CI 0.55 to 1.94; 136 participants (Analysis 9.4).
9.4. Analysis.
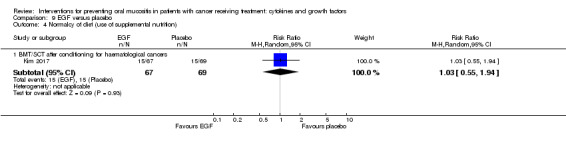
Comparison 9 EGF versus placebo, Outcome 4 Normalcy of diet (use of supplemental nutrition).
Adverse events
There do not appear to be any serious concerns regarding adverse effects of EGF. We have tabulated relevant information in Additional Table 8.
5. Adverse events: EGF.
| Study ID | Adverse events results |
| Kim 2017 | "Adverse events were similar in both groups (Table 3). The most common adverse event in the rhEGF group was nausea (n = 7, 10.4%). The incidence of other adverse events including oral pain, dry mouth, and taste alteration was low. All the adverse events were mild and transient. No grade 3 or 4 adverse events were noted during the study period" (there were no differences between groups in any adverse event) |
| Wu 2009 | "The frequency of minor and serious adverse events was similar in all groups. Most adverse events were related to primary disease status and treatment modalities" |
EGF = epidermal growth factor.
No studies assessed the outcomes 'oral pain', 'quality of life', 'number of days in hospital', 'number of days of treatment with opioid analgesics' and 'number of days unable to take medicine orally'.
Intestinal trefoil factor (ITF) versus placebo
Oral mucositis
Adults receiving chemotherapy alone for colorectal cancer
One study, at unclear risk of bias and analysing 99 participants (Peterson 2009), showed weak evidence (due to low sample size) of a reduction in the risk of any level of oral mucositis (RR 0.52, 95% CI 0.35 to 0.79; Analysis 10.1), and moderate to severe oral mucositis (RR 0.22, 95% CI 0.10 to 0.48; Analysis 10.2), both in favour of ITF.
10.1. Analysis.

Comparison 10 ITF versus placebo, Outcome 1 Oral mucositis (any).
10.2. Analysis.
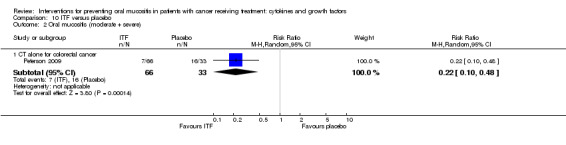
Comparison 10 ITF versus placebo, Outcome 2 Oral mucositis (moderate + severe).
There was insufficient evidence, from the same study, to determine whether or not EGF reduces the risk of severe oral mucositis: RR 1.52, 95% CI 0.06 to 36.39 (Analysis 10.3).
10.3. Analysis.
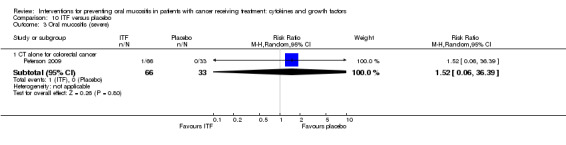
Comparison 10 ITF versus placebo, Outcome 3 Oral mucositis (severe).
Adverse events
There do not appear to be any serious concerns regarding adverse effects of ITF. We have tabulated relevant information in Additional Table 9.
6. Adverse events: ITF.
| Study ID | Adverse events results |
| Peterson 2009 | "Only a minority of patients (six [6.1%] of 99 patients) reported mild to moderate treatment‐emergent adverse events on the study. The symptoms included abdominal pain, diarrhea, oral pain, headache, and hypertension (Table 2). Of these, four were considered related to study drug: one (3%) was in the placebo group, two (6%) were in the low‐dose rhITF group, and one (3%) was in the high‐dose rhITF group. The events were isolated and resolved spontaneously without sequelae" |
ITF = intestinal trefoil factor.
No studies assessed the outcomes 'interruptions to cancer treatment', 'oral pain', 'quality of life', 'normalcy of diet', 'number of days in hospital', 'number of days of treatment with opioid analgesics' and 'number of days unable to take medicine orally'.
Intestinal trefoil factor (ITF) dose comparison
There was insufficient evidence, from one study at unclear risk of bias and analysing 66 adults receiving chemotherapy alone for colorectal cancer (Peterson 2009), to determine whether a lower dose (336 mg) or a higher dose (2688 mg) perform better in reducing the risk of oral mucositis of any severity (Analysis 11.1; Analysis 11.2; Analysis 11.3).
11.1. Analysis.
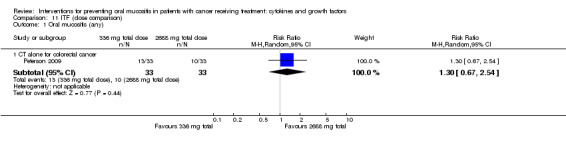
Comparison 11 ITF (dose comparison), Outcome 1 Oral mucositis (any).
11.2. Analysis.
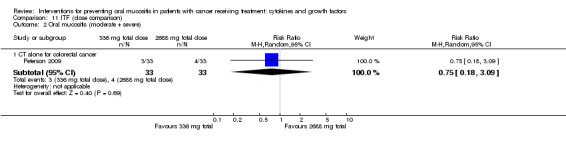
Comparison 11 ITF (dose comparison), Outcome 2 Oral mucositis (moderate + severe).
11.3. Analysis.
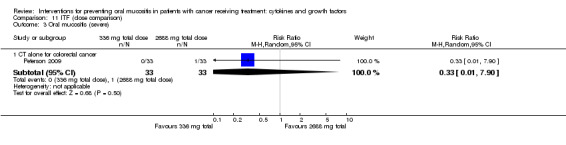
Comparison 11 ITF (dose comparison), Outcome 3 Oral mucositis (severe).
Erythropoietin versus placebo
Oral mucositis
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers
One study, at low risk of bias and analysing 80 participants (Hosseinjani 2017), showed weak evidence (due to low sample size) of a reduction in the risk of any level of oral mucositis (RR 0.35, 95% CI 0.21 to 0.60; Analysis 12.1), and moderate to severe oral mucositis (RR 0.43, 95% CI 0.24 to 0.79; Analysis 12.2), both in favour of erythropoietin.
12.1. Analysis.
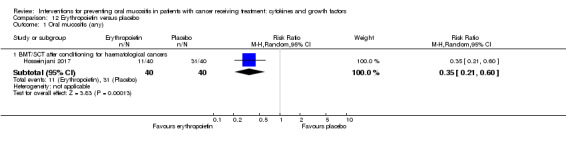
Comparison 12 Erythropoietin versus placebo, Outcome 1 Oral mucositis (any).
12.2. Analysis.
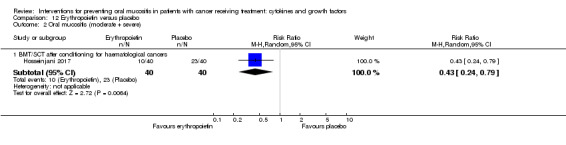
Comparison 12 Erythropoietin versus placebo, Outcome 2 Oral mucositis (moderate + severe).
The same study showed weak evidence (due to low sample size and a wide confidence interval) that erythropoietin might reduce the risk of severe oral mucositis, but there is also some possibility of an increase in risk: RR 0.40, 95% CI 0.14 to 1.17 (Analysis 12.3).
12.3. Analysis.
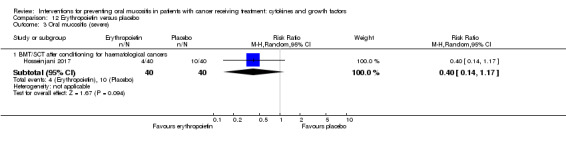
Comparison 12 Erythropoietin versus placebo, Outcome 3 Oral mucositis (severe).
Number of days in hospital
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers
There was insufficient evidence, from one study at low risk of bias (Hosseinjani 2017), to determine whether or not erythropoietin reduces the mean number of days in hospital: MD ‐2.95, 95% CI ‐7.73 to 1.83; 80 participants (Analysis 12.4).
12.4. Analysis.
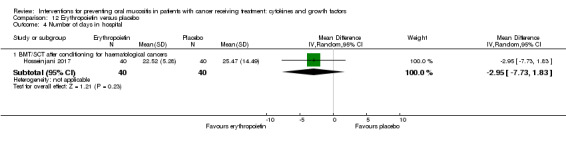
Comparison 12 Erythropoietin versus placebo, Outcome 4 Number of days in hospital.
No studies assessed the outcomes 'interruptions to cancer treatment', 'oral pain', 'quality of life', 'normalcy of diet', 'adverse events', 'number of days of treatment with opioid analgesics' and 'number of days unable to take medicine orally'.
Transforming growth factor (TGF) versus placebo
Oral mucositis
Adults receiving chemotherapy alone for colorectal cancer
There was insufficient evidence, from one study at high risk of bias and analysing 13 participants (Antoun 2009), to determine whether or not TGF reduces the risk of any level of oral mucositis: RR 0.10, 95% CI 0.01 to 1.71 (Additional Table 10).
7. TGF‐beta(2) versus placebo.
| Study ID | Population | Outcome | TGF‐beta(2) | Placebo | Result |
| Antoun 2009 | CT alone for colorectal cancer | Oral mucositis (WHO 0 to 4 scale): any oral mucositis | 0/9 | 2/4 | RR 0.10 (95% CI 0.01 to 1.71); P = 0.11 |
CI = confidence interval; CT = chemotherapy; RR = risk ratio; TGF = transforming growth factor; WHO = World Health Organization.
No studies assessed the outcomes 'interruptions to cancer treatment', 'oral pain', 'quality of life', 'normalcy of diet', 'adverse events', 'number of days in hospital', 'number of days of treatment with opioid analgesics' and 'number of days unable to take medicine orally'.
Discussion
Summary of main results
Thirty‐five studies met our eligibility criteria and were included in this review. We used GRADE methodology to assess the quality of the body of evidence for each of the main comparisons and for the primary outcome of incidence and severity of oral mucositis (GRADE 2004). Most of the evidence we found was for keratinocyte growth factor (KGF: Table 1), granulocyte‐macrophage colony‐stimulating factor (GM‐CSF: Table 2), and granulocyte‐colony stimulating factor (G‐CSF: Table 3).
Our main findings were as follows.
Keratinocyte growth factor (KGF)
Moderate to severe oral mucositis
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancer: might be a reduction in risk (11% and ranging from 20% to 1%).
Adults receiving radiotherapy to the head and neck with cisplatin/fluorouracil (5FU): probably a reduction in risk (9% and ranging from 17% to no reduction).
Adults receiving chemotherapy alone for mixed cancers: likely to be a reduction in risk (44% and ranging from 55% to 30%).
Severe oral mucositis
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancer: might be a reduction in risk, but some possibility of an increase in risk (15% reduction and ranging from 35% reduction to 11% increase).
Adults receiving radiotherapy to the head and neck with cisplatin/fluorouracil (5FU): very likely a reduction in risk (21% and ranging from 31% to 10%).
Adults receiving chemotherapy alone for mixed cancers: might be a reduction in risk (60% and ranging from 86% to 35%).
Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF)
Moderate to severe oral mucositis
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancer: insufficient evidence of a benefit.
Adults receiving radiotherapy to the head and neck: insufficient evidence of a benefit.
Severe oral mucositis
Adults receiving bone marrow/stem cell transplantation after conditioning therapy for mixed cancers: insufficient evidence of a benefit.
Adults receiving radiotherapy to the head and neck: insufficient evidence of a benefit.
Adults receiving chemotherapy alone for mixed cancers: insufficient evidence of a benefit.
Granulocyte‐colony stimulating factor (G‐CSF)
Moderate to severe oral mucositis
Adults receiving chemotherapy alone for breast cancer: very weak evidence of a possible reduction in risk (67% and ranging from 88% to 5%).
Severe oral mucositis
Adults receiving radiotherapy to the head and neck: weak evidence of a possible reduction in risk (63% and ranging from 85% to 13%).
The remaining evidence for the primary outcome was from single‐study comparisons.
Epidermal growth factor might reduce the risk of moderate to severe oral mucositis in adults receiving radiotherapy to the head and neck with or without cisplatin, but there was insufficient evidence of a reduction in the risk of either moderate to severe, or severe oral mucositis in adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancer.
Intestinal trefoil factor might reduce the risk of moderate to severe oral mucositis in adults receiving chemotherapy alone for colorectal cancer.
Erythropoietin might reduce the risk of moderate to severe oral mucositis in adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancer.
There was mostly insufficient evidence of a benefit regarding the secondary outcomes of this review. The interventions investigated all appear to be relatively safe, with only mild to moderate adverse effects reported.
Overall completeness and applicability of evidence
The evidence we have presented in this review allows for some conclusions to be made regarding the effects of KGF for preventing oral mucositis in adults receiving certain types of cancer treatment. However, the evidence is lacking for other cytokines and growth factors, and for children. It is unfortunate that the two studies we found on KGF versus placebo in children were unclear in their reporting and we were unable to present any data. All studies reported on our primary outcome, but the evidence for the secondary outcomes of this review is lacking.
The evidence for KGF should have reasonable external validity as most of the adult population were covered in terms of the types of treatment people have for different types of cancer. The studies were also carried out all over the world and often involved multiple sites. One limitation, however, may be the fact that most studies were done in middle‐income and high‐income countries, so may be less generalisable to people in low‐income countries.
Numerous studies reported on some of our secondary outcomes but did not report the data in a suitable format for inclusion in our meta‐analyses e.g. as median with or without range, area under the curve, or as mean (or a graph) but with no standard deviation/standard error/P value. In such cases, the meta‐analysis is biased by missing information. However, the Cochrane risk of bias tool and meta‐analyses do not currently address this issue adequately. The study may be assessed at high risk of selective outcome reporting, but if the study is not included in the meta‐analysis due to having no data, then this is not reflected or accounted for. This highlights the need for standardisation in both 'what to measure' and 'how to measure it' in clinical trials in this area of research. Otherwise there will continue to be research waste, with data that are not able to be pooled in data syntheses. There are initiatives such as COMET (Core Outcome Measures in Effectiveness Trials) and COSMIN (COnsensus‐based Standards for the selection of health Measurement INstruments) that can help with these issues, and future research in these areas would be beneficial.
During the systematic review process, we developed further concerns regarding the usefulness of the secondary outcomes because it was not clear whether or not they were due to oral mucositis. Hospitalisation, the use of supplemental nutrition or opioid analgesics, and interruptions to cancer treatment could all occur due to reasons other than oral mucositis. Furthermore, it was not always clear if adverse effects were due to the interventions given to prevent oral mucositis. These issues could be improved by clearer and more explicit reporting.
Cost is an issue that we did not consider in this review, but it is one that may affect whether or not the evidence can be applied in some settings. Taking KGF as an example, cost per dose is high but there is currently an absence of high quality health economic evaluations, rendering decision making difficult.
Quality of the evidence
We included 35 randomised controlled trials (RCTs) analysing 3102 participants. Despite this large volume of research, we were not able to make robust conclusions about the effects of most cytokines and growth factors. The strongest body of evidence, both in terms of volume and quality, was for the different populations receiving KGF. We assessed the evidence for KGF in preventing severe oral mucositis in adults receiving radiotherapy to the head and neck with or without cisplatin or fluorouracil as high quality. In the same population, we downgraded the evidence for prevention of moderate to severe oral mucositis by one level due to inconsistency in the individual study results (heterogeneity), resulting in moderate‐quality evidence. In adults receiving chemotherapy alone for mixed cancers, we downgraded the evidence for KGF in preventing moderate to severe oral mucositis once due to publication bias, as we found an unpublished study that would be included in the meta‐analysis, resulting in moderate‐quality evidence (NCT00393822). In the same population, we downgraded the evidence for prevention of severe oral mucositis by two levels: one for publication bias and one for imprecision due to a small sample size, low event rates and a wide confidence interval. This resulted in low‐quality evidence. In adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers, the evidence for KGF in preventing both moderate to severe and severe oral mucositis was assessed as low quality. We downgraded the evidence by two levels: one for heterogeneity and one for publication bias, as there were two studies for which we could not find published full reports (NCT02313792; Spielberger 2001). There were no concerns over risk of bias in the KGF studies as they are often large multicentre trials which are carried out well, mostly using placebos for blinding purposes, and with very low attrition.
The evidence for GM‐CSF and G‐CSF was weaker, and consequently was rated as being low or very low quality. The reasons for downgrading were mostly due to imprecision because the volume of evidence was much lower and the studies often recruited very few participants, leading to very wide confidence intervals that frequently included both the possibility of a decrease in risk and an increase. Further reasons were risk of performance bias due to lack of blinding in some of these studies, inconsistency, and also because some of the evidence was from single studies. When a body of evidence was from a single study, we automatically downgraded a level. The reasoning behind this was because often, when using GRADE methodology, bodies of evidence are downgraded for inconsistency due to different effect estimates in the individual studies. This inconsistency is not possible for a single‐study body of evidence and therefore not downgrading would falsely inflate the rating of quality, whilst at the same time the larger body of evidence is unfairly penalised, in comparison, due to having more studies. In such cases, we downgraded the single‐study evidence due to indirectness as it may only be generalisable to the particular population who took part in the study.
The remaining evidence for other interventions was from single‐study comparisons and therefore was all considered to be of low to very low quality, mainly for indirectness (as described above) and imprecision.
Potential biases in the review process
Although systematic review methodology is designed to minimise biases in the process, decisions are often made out of necessity or for practical reasons, and this can introduce some potential bias.
Once we began to assess the literature identified by the searches, we became concerned that we may have missed some relevant studies because we had not included search terms for other conditions for which cytokines and growth factors have been used to manage (e.g. diarrhoea, graft‐versus‐host disease, and neutropenia). In order to assess the extent of this potential problem, a rough scoping search was run including the search terms. The yield was very high and a single review author assessed a sample of 500 records, but no further studies were identified. Therefore, we decided not to amend the search by adding the new search terms. We acknowledge the possibility that we have missed some studies that have measured and reported on oral mucositis but not mentioned it in the abstract. This could introduce bias if there are relevant data missing from the review.
There were some studies that had multiple treatment arms with different doses of the cytokine or growth factor. In all instances, we combined the arms to make a pairwise comparison against the control arm thus losing some possible subtleties of the data and potentially biasing the results.
Agreements and disagreements with other studies or reviews
The Mucositis Study Group (MSG) of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) is the leading international group in this area of research. In 2013, they published a series of systematic reviews on the different interventions for managing oral mucositis, including one on cytokines and growth factors (Raber‐Durlacher 2013). These reviews feed into the MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy (Lalla 2014). The MASCC/ISOO systematic review is not limited to RCTs. The current guidance from this group is as follows.
Recommendations in favour of an intervention (i.e. strong evidence supporting effectiveness): the panel recommends that recombinant human keratinocyte growth factor‐1 (KGF‐1/palifermin) be used to prevent oral mucositis (at a dose of 60 µg/kg per day for three days prior to conditioning treatment and for three days after transplant) in patients receiving high‐dose chemotherapy and total body irradiation, followed by autologous stem cell transplantation, for a haematological malignancy (level II evidence).
Suggestions against an intervention (i.e. weaker evidence indicating lack of effectiveness): the panel suggests that granulocyte‐macrophage colony‐stimulating factor mouthwash not be used to prevent oral mucositis in patients receiving high‐dose chemotherapy, for autologous or allogeneic stem cell transplantation (level II evidence).
For our meta‐analyses for KGF in the above mentioned population, we combined studies of all types of KGF, both with autologous and allogeneic transplants, and with total body irradiation (TBI), without TBI or a mixture of TBI/no TBI. The MASCC/ISOO systematic review separated all of these factors. However, looking at the individual studies in our meta‐analyses, the first recommendation appears to be a valid one. Furthermore, the MASCC/ISOO systematic review states "Evidence on the efficacy of palifermin in autologous HSCT without TBI conditioning is conflicting...and these rather small studies did not allow a guideline. In addition, no guideline could be provided for the use of palifermin in the setting of allogeneic HSCT with or without TBI." Despite our meta‐analyses including some further RCTs not included in the other review, these statements also appear to be valid.
The suggestion against GM‐CSF mouthwash is also a valid one as, although we did not separate studies by mode of administration, it is clear that the two mouthwash studies in our analysis (Analysis 4.3) have conflicting results. However, based on one study on GM‐CSF given intravenously in this population (Nemunaitis 1995), there is promising evidence of a benefit, but the MASCC/ISOO systematic review considered this evidence alongside other studies that we did not include, and concluded that there was no guideline possible.
Our results are not in agreement with the following statements from the MASCC/ISOO systematic review regarding other populations receiving KGF.
"No guideline could be provided for the use of palifermin in the setting of CT for solid and hematological tumors...due to insufficient evidence."
"In addition, no guideline could be provided for the use of palifermin in H&N RT due to insufficient evidence."
We present some moderate‐ to high‐quality evidence of a benefit for KGF in these populations, possibly warranting new guideline statements in their next update. This evidence would equate to level I evidence in the grading system used in the guidelines ("evidence obtained from meta‐analysis of multiple, well‐designed, controlled studies"). In another Cochrane Review on preventing salivary gland dysfunction in patients receiving radiotherapy to the head and neck, with or without chemotherapy, KGF did not appear to have any detrimental effect on overall survival or progression‐free survival (Riley 2017).
Authors' conclusions
Implications for practice.
We are confident that keratinocyte growth factor (KGF) is beneficial in the prevention of oral mucositis in adults who are receiving: a) radiotherapy to the head and neck with cisplatin or fluorouracil; or b) chemotherapy alone for mixed solid and haematological cancers. We are less confident about a benefit for KGF in adults receiving bone marrow/stem cell transplant after conditioning therapy for haematological cancers because of multiple factors involved in that population, such as whether or not they received total body irradiation (TBI) and whether the transplant was autologous (the patients' own cells) or allogeneic (cells from a donor). KGF appears to be a relatively safe intervention.
Due to limited research, we are not confident that there are any beneficial effects of other cytokines and growth factors. There is currently insufficient evidence to draw any conclusions about the use of cytokines and growth factors in children.
Implications for research.
Despite a large volume of research, once studies are categorised by cancer treatment type/population, there is very little we can conclude regarding the effects of most cytokines and growth factors. It is clear that much more research is needed in this area, especially as many of the interventions have shown promise in some populations, yet we have not been able to make robust conclusions due to the limited volume/low sample sizes. Strong evidence from randomised controlled trials (RCTs) using placebos should be generated before head‐to‐head comparisons of different interventions are undertaken. More RCTs of KGF are needed in the population receiving bone marrow/stem cell transplant after conditioning therapy so that in future updates we may be able to include separate subgroups to account for differing factors such as TBI/no TBI and autologous/allogeneic transplant. Further large confirmatory RCTs of KGF would be beneficial in the other two populations: a) radiotherapy to the head and neck with chemotherapy (and possibly without chemotherapy); and b) chemotherapy alone for mixed cancers.
More research is needed on all other cytokines and growth factors in the various populations, including in children. Placebo controls should be used in the first instance to establish whether or not they are effective, and only then should head‐to‐head comparisons of active interventions be made.
Future RCTs should be adequately powered to detect a difference if one actually exists and they should be reported according to the CONSORT Statement (Consolidated Standards of Reporting Trials). They should measure and report in full all the outcomes listed in this review, most of which are recommended in the core outcome set produced by Bellm et al (Bellm 2002). For our primary outcome of oral mucositis incidence, we urge trialists to use a measurement tool such as the WHO (World Health Organization) or NCI‐NCT (National Cancer Institute common toxicity criteria) scale (Appendix 9), to allow us to combine the data with those already included in this review. Reporting the maximum grade of oral mucositis experienced per participant would allow us to assess the incidence of different severities, thus maximising the usefulness of the data. It would also be useful if oral pain was measured on a 0 to 10 scale and reported as an overall mean and mean maximum score experienced per participant. Numbers included in any analysis should always be reported and any continuous data should be reported as means and standard deviations. Furthermore, measurement of outcomes should be taken with appropriate frequency so as to avoid any problems with ascertainment bias.
History
Protocol first published: Issue 12, 2015 Review first published: Issue 11, 2017
| Date | Event | Description |
|---|---|---|
| 26 January 2016 | Amended | Minor edit (hyperlink) |
Acknowledgements
We would like to thank the Cochrane Oral Health editorial team (in particular, Tanya Walsh, Laura MacDonald and Janet Lear) for their help in preparing this Cochrane Review. We also thank Joanne Elliott for her comments on our search strategy. We thank external referees Andrei Barasch and Douglas E Peterson (Please note that referees provided comments on a personal basis and not as representatives of any organisation with which they are connected.)
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
1 ((neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or neutropeni* or neutropeni* or carcino* or adenocarcinoma* or lymphoma*):ti,ab) AND (INREGISTER) 2 ((radioth* or radiat* or irradiat*):ti,ab) AND (INREGISTER) 3 ((("bone marrow" and transplant*) or "hematopoietic stem cell transplant*" or "haematopoietic stem cell transplant*" or HSCT):ti,ab) AND (INREGISTER) 4 (chemo*:ti,ab) AND (INREGISTER) 5 (#1 or #2 or #3 or #4) AND (INREGISTER) 6 ((stomatitis or mucositis):ti,ab) AND (INREGISTER) 7 ((oral and mucos*):ti,ab) AND (INREGISTER) 8 ((mycosis or mycotic):ti,ab) AND (INREGISTER) 9 (mIAS:ti,ab) AND (INREGISTER) 10 (#6 or #7 or #8 or #9) AND (INREGISTER) 11 (("growth factor*" or "growth substance*" or "immunologic factor*"):ti,ab) AND (INREGISTER) 12 ("fibroblast growth factor*":ti,ab) AND (INREGISTER) 13 ((keratinocyte* or cytokine*):ti,ab) AND (INREGISTER) 14 ((palifermin* or KGF or FGF):ti,ab) AND (INREGISTER) 15 ((keprivance or velafermin or repifermin):ti,ab) AND (INREGISTER) 16 (glycoprotein*:ti,ab) AND (INREGISTER) 17 ((colony‐stimulat* or "macrophage‐granulocyte inducer*" or "myeloid cell‐growth inducer*" or "protein inducer MGI"):ti,ab) AND (INREGISTER) 18 ((GM‐CSF or G‐CSF):ti,ab) AND (INREGISTER) 19 ((molgramostim or Growgen‐GM or Leucomax or Molcass or Gramostim or Leucocitim or Mielogen or Meustim or Bagomol or Gramal):ti,ab) AND (INREGISTER) 20 ((rhEGF or "recombinant epithelial growth factor*" or "epidermal growth factor*" or EGF):ti,ab) AND (INREGISTER) 21 (("platelet‐derived growth factor*" or PDGF or "platelet lysate"):ti,ab) AND (INREGISTER) 22 (("transforming growth factor*" or "bone‐derived transforming growth factor*" or "milk growth factor*" or "platelet transforming growth factor*" or TGF‐beta or TGFBeta):ti,ab) AND (INREGISTER) 23 (("hepatocyte growth factor*" or hepatopoietin or "scatter factor"):ti,ab) AND (INREGISTER) 24 ((somatomedin* or "insulin‐like growth factor*" or "sulfation factor*" or Mecasermin or Increlex or Iplex or IGF‐1 or IGF1):ti,ab) AND (INREGISTER) 25 (erythropoietin*:ti,ab) AND (INREGISTER) 26 ((thrombopoietin* or "mpl Ligand" or "megakaryocyte colony stimulating factor*" or "megakaryocyte growth and development factor*" or "MGDF factor" or "myeloproliferative leukemia virus oncogene ligand" or "thrombocytopoiesis‐stimulating factor" or thrombocytopoietin*):ti,ab) AND (INREGISTER) 27 (("interleukin 11" or "adipogenesis inhibitory factor*" or IL‐11 or IL11):ti,ab) AND (INREGISTER) 28 ((ghrelin* or "GHRL protein" or obestatin):ti,ab) AND (INREGISTER) 29 ((ATL‐104 or ATL104):ti,ab) AND (INREGISTER) 30 ((whey or "milk derived protein" or "milk derived growth factor"):ti,ab) AND (INREGISTER) 31 (("glucagon‐like peptide 2" or "amino acid*" or proglucagon or GLP‐2 or teduglutide or Gattex or Revestive):ti,ab) AND (INREGISTER) 32 (("trefoil factor" or "carcinoembryonic antigen cell adhesion molecule 1" or glutathione or isethion):ti,ab) AND (INREGISTER) 33 (("vascular endothelial growth factor*" or VEGF*):ti,ab) AND (INREGISTER) 34 (("targeted therap*" or "targeted agent*"):ti,ab) AND (INREGISTER) 35 ("biologic* therap*":ti,ab) AND (INREGISTER) 36 ((#11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35)) AND (INREGISTER) 37 (#5 and #10 and #36) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 [mh neoplasms] #2 [mh radiotherapy] #3 [mh "antineoplastic agents"] #4 [mh "^bone marrow transplantation"] #5 [mh ^"hematopoietic stem cell transplantation"] #6 MeSH descriptor: [Antineoplastic Combined Chemotherapy Protocols] explode all trees #7 (neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or neutropeni* or neutropeni* or carcino* or adenocarcinoma* or lymphoma*) #8 (radioth* or radiat* or irradiat*) #9 (("bone marrow" and transplant*) or "hematopoietic stem cell transplant*" or "haematopoietic stem cell transplant*" or HSCT) #10 chemo* #11 {or #1‐#10} #12 [mh Stomatitis] #13 [mh ^"oral candidiasis"] #14 stomatitis #15 mucositis #16 (oral near/6 mucos*) #17 (mycosis or mycotic) #18 mIAS #19 {or #12‐#18} #20 ("growth factor*" or "growth substance*" or "immunologic factor*") #21 [mh ^"Fibroblast growth factor 7"] #22 "fibroblast growth factor*" #23 (keratinocyte* or cytokine*) #24 (palifermin* or KGF or FGF) #25 (keprivance or velafermin or repifermin) #26 glycoprotein* #27 [mh "colony‐stimulating factors"] #28 (colony‐stimulat* or "macrophage‐granulocyte inducer*" or "myeloid cell‐growth inducer*" or "protein inducer MGI") #29 (GM‐CSF or G‐CSF) #30 (molgramostim or Growgen‐GM or Leucomax or Molcass or Gramostim or Leucocitim or Mielogen or Meustim or Bagomol or Gramal) #31 [mh ^"Epidermal growth factor"] #32 (rhEGF or "recombinant epithelial growth factor*" or "epidermal growth factor*" or EGF) #33 [mh ^"Platelet‐derived growth factor"] #34 ("platelet‐derived growth factor*" or PDGF or "platelet lysate") #35 [mh ^"Transforming growth factor beta"] #36 ("transforming growth factor*" or "bone‐derived transforming growth factor*" or "milk growth factor*" or "platelet transforming growth factor*" or TGF‐beta or TGFBeta) #37 [mh ^"Hepatocyte growth factor"] #38 ("hepatocyte growth factor*" or hepatopoietin or "scatter factor") #39 [mh Somatomedin] #40 (somatomedin* or "insulin‐like growth factor*" or "sulfation factor*" or Mecasermin or Increlex or Iplex or IGF‐1 or IGF1) #41 erythropoietin* #42 [mh ^Thrombopoietin] #43 (thrombopoietin* or "mpl Ligand" or "megakaryocyte colony stimulating factor*" or "megakaryocyte growth and development factor*" or "MGDF factor" or "myeloproliferative leukemia virus oncogene ligand" or "thrombocytopoiesis‐stimulating factor" or thrombocytopoietin*) #44 [mh ^Ghrelin] #45 [mh "Interleukin 11"] #46 ("interleukin 11" or "adipogenesis inhibitory factor*" or IL‐11 or IL11) #47 (ghrelin* or "GHRL protein" or obestatin) #48 (ATL‐104 or ATL104) #49 (whey or ("milk derived" next (protein or "growth factor"))) #50 [mh ^"Glucagon‐like peptide 2"] #51 ("glucagon‐like peptide 2" or "amino acid*" or proglucagon or GLP‐2 or teduglutide or Gattex or Revestive) #52 [mh ^Glutathione] #53 ("trefoil factor" or "carcinoembryonic antigen cell adhesion molecule 1" or glutathione or isethion) #54 [mh ^"Vascular endothelial growth factors"] #55 ("vascular endothelial growth factor*" or VEGF*) #56 [mh ^"Molecular targeted therapy"] #57 (targeted near/3 (therap* or agent*)) #58 (biologic* next therap*) #59 {or #20‐#58} #60 #11 and #19 and #59
Appendix 3. MEDLINE Ovid search strategy
1. exp NEOPLASMS/ 2. exp RADIOTHERAPY/ 3. exp Antineoplastic agents/ 4. Anti‐neoplastic combined chemotherapy protocols/ 5. Bone Marrow Transplantation/ 6. Hematopoietic Stem Cell Transplantation/ 7. (neoplasm$ or cancer$ or leukaemi$ or leukemi$ or tumour$ or tumor$ or malignan$ or neutropeni$ or carcino$ or adenocarcinoma$ or lymphoma$).ti,ab. 8. (radioth$ or radiat$ or irradiat$).ti,ab. 9. ((bone adj marrow adj5 transplant$) or "hematopoietic stem cell transplant$" or "haematopoietic stem cell transplant$" or HSCT).ti,ab. 10. chemo$.ti,ab. 11. or/1‐10 12. exp STOMATITIS/ 13. Candidiasis, Oral/ 14. stomatitis.ti,ab. 15. mucositis.ti,ab. 16. (oral adj6 mucos$).ti,ab. 17. (mycosis or mycotic).ti,ab. 18. mIAS.ti,ab. 19. or/12‐18 20. ((growth adj factor$) or (growth adj substance$) or (immunologic adj factor$)).ti,ab. 21. Fibroblast growth factor 7/ 22. "fibroblast growth factor$".ti,ab. 23. (keratinocyte$ or cytokine$).ti,ab. 24. (palifermin$ or KGF or FGF).ti,ab. 25. (kepivance or velafermin or repifermin).ti,ab. 26. glycoprotein$.ti,ab. 27. exp Colony‐stimulating factors/ 28. ("colony‐stimulat$" or "macrophage‐granulocyte inducer$" or "myeloid cell‐growth inducer$" or "protein inducer MGI").ti,ab. 29. (GM‐CSF or G‐CSF).ti,ab. 30. (molgramostim or Growgen‐GM or Leucomax or Molcass or Gramostim or Leucocitim or Mielogen or Meustim or Bagomol or Gramal).ti,ab. 31. Epidermal growth factor/ 32. (rhEGF or "recombinant epithelial growth factor$" or "epidermal growth factor$" or EGF).ti,ab. 33. Platelet‐derived growth factor/ 34. ("platelet‐derived growth factor$" or PDGF or "platelet lysate").ti,ab. 35. Transforming Growth Factor beta/ 36. ("transforming growth factor$" or "bone‐derived transforming growth factor$" or "milk growth factor$" or "platelet transforming growth factor" or TGF‐beta or TGFbeta).ti,ab. 37. Hepatocyte growth factor/ 38. ("hepatocyte growth factor$" or hepatopoietin or "scatter factor").ti,ab. 39. exp Somatomedin/ 40. (somatomedin$ or "insulin‐like growth factor$" or "sulfation factor" or Mecasermin or Increlex or Iplex or IGF‐1 or IGF1).ti,ab. 41. erythropoietin$.ti,ab. 42. Thrombopoietin/ 43. (thrombopoietin$ or "mpl Ligand" or "megakaryocyte colony stimulating factor$" or "megakaryocyte growth and development factor$" or "MGDF factor" or "myeloproliferative leukemia virus oncogene ligand" or "thrombocytopoiesis‐stimulating factor" or thrombocytopoietin$).ti,ab. 44. Ghrelin/ 45. Interleukin 11/ 46. ("interleukin 11" or "Adipogenesis Inhibitory Factor$" or IL‐11 or IL11).ti,ab. 47. (ghrelin$ or "GHRL protein" or obestatin).ti,ab. 48. (ATL‐104 or ATL104).ti,ab. 49. (whey or ("milk derived" adj (protein or growth factor))).ti,ab. 50. Glucagon‐Like Peptide 2/ 51. ("glucagon‐like peptide 2" or (amino adj acid$) or proglucagon or GLP‐2 or teduglutide or Gattex or Revestive).ti,ab. 52. Glutathione/ 53. ("trefoil factor" or "carcinoembryonic antigen cell adhesion molecule 1" or glutathione or isethion).ti,ab. 54. Vascular endothelial growth factors/ 55. ("vascular endothelial growth factor$" or VEGF$).ti,ab. 56. Molecular targeted therapy/ 57. (targeted adj3 (therap$ or agent$)).ti,ab. 58. (biologic$ adj therap$).ti,ab. 59. or/20‐58 60. 11 and 19 and 59
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Higgins 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. exp NEOPLASM/ 2. exp RADIOTHERAPY/ 3. exp Antineoplastic agent/ 4. Bone Marrow Transplantation/ 5. Hematopoietic Stem Cell Transplantation/ 6. (neoplasm$ or cancer$ or leukaemi$ or leukemi$ or tumour$ or tumor$ or malignan$ or neutropeni$ or carcino$ or adenocarcinoma$ or lymphoma$).ti,ab. 7. (radioth$ or radiat$ or irradiat$).ti,ab. 8. ((bone adj marrow adj5 transplant$) or "hematopoietic stem cell transplant$" or "haematopoietic stem cell transplant$" or HSCT).ti,ab. 9. chemo$.ti,ab. 10. or/1‐9 11. exp STOMATITIS/ 12. Thrush/ 13. stomatitis.ti,ab. 14. mucositis.ti,ab. 15. (oral adj6 mucos$).ti,ab. 16. (mycosis or mycotic).ti,ab. 17. mIAS.ti,ab. 18. or/11‐17 19. ((growth adj factor$) or (growth adj substance$) or (immunologic adj factor$)).ti,ab. 20. "fibroblast growth factor$".ti,ab. 21. (keratinocyte$ or cytokine$).ti,ab. 22. (palifermin$ or KGF or FGF).ti,ab. 23. (kepivance or velafermin or repifermin).ti,ab. 24. glycoprotein$.ti,ab. 25. exp Colony‐stimulating factor/ 26. ("colony‐stimulat$" or "macrophage‐granulocyte inducer$" or "myeloid cell‐growth inducer$" or "protein inducer MGI").ti,ab. 27. (GM‐CSF or G‐CSF).ti,ab. 28. (molgramostim or Growgen‐GM or Leucomax or Molcass or Gramostim or Leucocitim or Mielogen or Meustim or Bagomol or Gramal).ti,ab. 29. Epidermal growth factor/ 30. (rhEGF or "recombinant epithelial growth factor$" or "epidermal growth factor$" or EGF).ti,ab. 31. Platelet derived growth factor/ 32. ("platelet‐derived growth factor$" or PDGF or "platelet lysate").ti,ab. 33. Transforming Growth Factor beta/ 34. ("transforming growth factor$" or "bone‐derived transforming growth factor$" or "milk growth factor$" or "platelet transforming growth factor" or TGF‐beta or TGFbeta).ti,ab. 35. Scatter factor/ 36. ("hepatocyte growth factor$" or hepatopoietin or "scatter factor").ti,ab. 37. exp Somatomedin/ 38. (somatomedin$ or "insulin‐like growth factor$" or "sulfation factor" or Mecasermin or Increlex or Iplex or IGF‐1 or IGF1).ti,ab. 39. erythropoietin$.ti,ab. 40. Thrombopoietin/ 41. (thrombopoietin$ or "mpl Ligand" or "megakaryocyte colony stimulating factor$" or "megakaryocyte growth and development factor$" or "MGDF factor" or "myeloproliferative leukemia virus oncogene ligand" or "thrombocytopoiesis‐stimulating factor" or thrombocytopoietin$).ti,ab. 42. Ghrelin/ 43. Interleukin 11/ 44. ("interleukin 11" or "Adipogenesis Inhibitory Factor$" or IL‐11 or IL11).ti,ab. 45. (ghrelin$ or "GHRL protein" or obestatin).ti,ab. 46. (ATL‐104 or ATL104).ti,ab. 47. (whey or ("milk derived" adj (protein or growth factor))).ti,ab. 48. ("glucagon‐like peptide 2" or (amino adj acid$) or proglucagon or GLP‐2 or teduglutide or Gattex or Revestive).ti,ab. 49. Glutathione/ 50. ("trefoil factor" or "carcinoembryonic antigen cell adhesion molecule 1" or glutathione or isethion).ti,ab. 51. Vasculotropin/ 52. ("vascular endothelial growth factor$" or VEGF$).ti,ab. 53. Molecularly targeted therapy/ 54. (targeted adj3 (therap$ or agent$)).ti,ab. 55. (biologic$ adj therap$).ti,ab. 56. or/19‐55 57. 10 and 18 and 56
The above subject search was linked to adapted version of the Cochrane Embase Project filter for identifying RCTs in EMBASE via OVID (see www.cochranelibrary.com/help/central‐creation‐details.html for information).
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 5. CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) search strategy
S53 S10 and S18 and S52 S52 S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 S51 (biologic* N1 therap*) S50 (targeted N3 (therap* or agent*)) S49 ("vascular endothelial growth factor*" or VEGF*) S48 (mh Vascular endothelial growth factors) S47 ("trefoil factor" or "carcinoembryonic antigen cell adhesion molecule 1" or glutathione or isethion) S46 (mh Glutathione) S45 ("glucagon‐like peptide 2" or "amino acid*" or proglucagon or GLP‐2 or teduglutide or Gattex or Revestive) S44 (mh Glucagon‐like peptide 2) S43 (whey or ("milk derived" N1 (protein or "growth factor"))) S42 (ATL‐104 or ATL104) S41 (ghrelin* or "GHRL protein" or obestatin) S40 ("interleukin 11" or "adipogenesis inhibitory factor*" or IL‐11 or IL11) S39 (mh Ghrelin) S38 (thrombopoietin* or "mpl Ligand" or "megakaryocyte colony stimulating factor*" or "megakaryocyte growth and development factor*" or "MGDF factor" or "myeloproliferative leukemia virus oncogene ligand" or "thrombocytopoiesis‐stimulating factor" or thrombocytopoietin*) S37 erythropoietin* S36 (somatomedin* or "insulin‐like growth factor*" or "sulfation factor*" or Mecasermin or Increlex or Iplex or IGF‐1 or IGF1) S35 ("hepatocyte growth factor*" or hepatopoietin or "scatter factor") S34 ("transforming growth factor*" or "bone‐derived transforming growth factor*" or "milk growth factor*" or "platelet transforming growth factor*" or TGF‐beta or TGFBeta) S33 (mh Transforming growth factor beta) S32 ("platelet‐derived growth factor*" or PDGF or "platelet lysate") S31 (mh Platelet‐derived growth factor) S30 (rhEGF or "recombinant epithelial growth factor*" or "epidermal growth factor*" or EGF) S29 (MH "Epidermal Growth Factors") S28 (molgramostim or Growgen‐GM or Leucomax or Molcass or Gramostim or Leucocitim or Mielogen or Meustim or Bagomol or Gramal) S27 (GM‐CSF or G‐CSF) S26 (colony‐stimulat* or "macrophage‐granulocyte inducer*" or "myeloid cell‐growth inducer*" or "protein inducer MGI") S25 (mh colony‐stimulating factors) S24 glycoprotein* S23 (keprivance or velafermin or repifermin) S22 (palifermin* or KGF or FGF) S21 (keratinocyte* or cytokine*) S20 "fibroblast growth factor*" S19 ("growth factor*" or "growth substance*" or "immunologic factor*") S18 S11 or S12 or S13 or S14 or S15 or S16 or S17 S17 mIAS S16 (mycosis or mycotic) S15 (oral N6 mucos*) S14 mucositis S13 stomatitis S12 (MH "Candidiasis, Oral") S11 (mh stomatitis+) S10 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 S9 chemo* S8 (("bone marrow" and transplant*) or "hematopoietic stem cell transplant*" or "haematopoietic stem cell transplant*" or HSCT) S7 (radioth* or radiat* or irradiat*) S6 (neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or neutropeni* or neutropeni* or carcino* or adenocarcinoma* or lymphoma*) S5 (mh hematopoietic stem cell transplantation) S4 (mh bone marrow transplantation) S3 (mh antineoplastic agents+) S2 (mh radiotherapy+) S1 (mh neoplasms+)
The above subject search was linked to Cochrane Oral Health's filter for CINAHL via EBSCO.
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi center study") or AB ("multicentre study" or "multicenter study" or "multi centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S3 TI random* or AB random* S4 AB "latin square" or TI "latin square" S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S6 MH Placebos S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*) S8 TI blind* or AB mask* or AB blind* or TI mask* S9 S7 and S8 S10 TI Placebo* or AB Placebo* or SU Placebo* S11 MH Clinical Trials S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 6. CANCERLIT PubMed search strategy
We searched CANCERLIT by limiting a PubMed search with the Cancer subject filter and the following search strategy.
#60 (#11 and #19 and #59) #59 (#20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58) #58 "biologic* therap*" #57 ("targeted therap*" or "targeted agent*") #56 Molecular targeted therapy [mh:noexp] #55 (("vascular endothelial growth factor*" or VEGF*)) #54 Vascular endothelial growth factors [mh:noexp] #53 (("trefoil factor" or "carcinoembryonic antigen cell adhesion molecule 1" or glutathione or isethion)) #52 Glutathione [mh:noexp] #51 (("glucagon‐like peptide 2" or "amino acid*" or proglucagon or GLP‐2 or teduglutide or Gattex or Revestive)) #50 Glucagon‐like peptide 2 [mh:noexp] #49 ((whey or "milk derived protein" or "milk derived growth factor")) #48 ((ATL‐104 or ATL104)) #47 ((ghrelin* or "GHRL protein" or obestatin)) #46 (("interleukin 11" or "adipogenesis inhibitory factor*" or IL‐11 or IL11)) #45 Interleukin 11 [mh:exp] #44 Ghrelin [mh:noexp] #43 ((thrombopoietin* or "mpl Ligand" or "megakaryocyte colony stimulating factor*" or "megakaryocyte growth and development factor*" or "MGDF factor" or "myeloproliferative leukemia virus oncogene ligand" or "thrombocytopoiesis‐stimulating factor" or thrombocytopoietin*)) #42 Thrombopoietin [mh:noexp] #41 erythropoietin* #40 ((somatomedin* or "insulin‐like growth factor*" or "sulfation factor*" or Mecasermin or Increlex or Iplex or IGF‐1 or IGF1)) #39 Somatomedin [mh:exp] #38 (("hepatocyte growth factor*" or hepatopoietin or "scatter factor")) #37 Hepatocyte growth factor [mh:noexp] #36 (("transforming growth factor*" or "bone‐derived transforming growth factor*" or "milk growth factor*" or "platelet transforming growth factor*" or TGF‐beta or TGFBeta)) #35 Transforming growth factor beta [mh:noexp] #34 (("platelet‐derived growth factor*" or PDGF or "platelet lysate")) #33 Platelet‐derived growth factor [mh:noexp] #32 ((rhEGF or "recombinant epithelial growth factor*" or "epidermal growth factor*" or EGF)) #31 Epidermal growth factor [mh:noexp] #30 ((molgramostim or Growgen‐GM or Leucomax or Molcass or Gramostim or Leucocitim or Mielogen or Meustim or Bagomol or Gramal)) #29 ((GM‐CSF or G‐CSF)) #28 ((colony‐stimulat* or "macrophage‐granulocyte inducer*" or "myeloid cell‐growth inducer*" or "protein inducer MGI")) #27 colony‐stimulating factors [mh:exp] #26 glycoprotein* #25 ((keprivance or velafermin or repifermin)) #24 ((palifermin* or KGF or FGF)) #23 ((keratinocyte* or cytokine*)) #22 "fibroblast growth factor*" #21 Fibroblast growth factor 7 [mh:noexp] #20 (("growth factor*" or "growth substance*" or "immunologic factor*")) #19 (#12 or #13 or #14 or #15 or #16 or #17 or #18) #18 mIAS #17 ((mycosis or mycotic)) #16 "oral mucos*" #15 mucositis #14 stomatitis #13 oral candidiasis [mh:noexp] #12 Stomatitis [mh:exp] #11 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10) #10 chemo* #9 ((("bone marrow" and transplant*) or "hematopoietic stem cell transplant*" or "haematopoietic stem cell transplant*" or HSCT)) #8 ((radioth* or radiat* or irradiat*)) #7 ((neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or neutropeni* or neutropeni* or carcino* or adenocarcinoma* or lymphoma*)) #6 Antineoplastic Combined Chemotherapy Protocols [mh:noexp] #5 "hematopoietic stem cell transplantation" [mh:noexp] #4 bone marrow transplantation [mh:noexp] #3 antineoplastic agents [mh:exp] #2 radiotherapy [mh:exp] #1 neoplasms [mh:exp]
This search was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.a of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Lefebvre 2011).
#1 randomized controlled trial [pt] #2 controlled clinical trial [pt] #3 randomized [tiab] #4 placebo [tiab] #5 drug therapy [sh] #6 randomly [tiab] #7 trial [tiab] #8 groups [tiab] #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 animals [mh] not humans [mh] #11 #9 NOT #10
Appendix 7. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
oral mucositis and cytokines oral mucositis and growth factor oral mucositis and palifermin oral mucositis and GM‐CSF
Appendix 8. World Health Organization International Clinical Trials Registry Platform search strategy
oral mucositis and cytokines oral mucositis and growth factor oral mucositis and palifermin oral mucositis and GM‐CSF
Appendix 9. Oral mucositis measurement scales
Oral mucositis assessment scale (OMAS)
(as reported in Freytes 2004)
| Oral cavity ulceration | Oral cavity erythema | ||
| Grade 0 | No lesion | Grade 0 | None |
| Grade 1 | Lesion < 1 cm2 | Grade 1 | Not severe |
| Grade 2 | Lesion 1 cm2 to 3 cm2 | Grade 2 | Severe |
| Grade 3 | Lesion > 3 cm2 | ‐ | ‐ |
Ulceration and erythema are measured at 9 different sites (upper and lower labial mucosa, right and left buccal mucosa, right and left lateral and ventral portions of the tongue, floor of the mouth, soft palate (fauces), and hard palate (gingival)) and summated to give a total mucositis score. Therefore score could range from 0 to 45.
Radiation Therapy Oncology Group (RTOG) scale
(physician‐rated objective gross score as reported in Chi 1995)
| Grade 0 | None |
| Grade 1 | Erythematous sores |
| Grade 2 | Patchy mucositis (< 1/2 mucosa) |
| Grade 3 | Confluent fibrinous mucositis (> 1/2 mucosa) |
| Grade 4 | Haemorrhage and necrosis |
Radiation Therapy Oncology Group (RTOG) scale
(as reported in Wu 2009)
| Grade 0 | None |
| Grade 1 | Injection; may experience mild pain not requiring analgesia |
| Grade 2 | Patchy mucositis that may produce an inflammatory serosanguineous discharge; may experience moderate pain requiring analgesia |
| Grade 3 | Confluent fibrinous mucositis; may include severe pain requiring narcosis |
| Grade 4 | Ulceration, haemorrhage, or necrosis |
Cancer and Leukemia Group B (CALGB) scale
(as reported in Turhal 2000)
| Grade 0 | No mucositis |
| Grade 1 | Painless ulcers, erythema or mild soreness |
| Grade 2 | Painful erythema, edema, or ulcers, but can eat |
| Grade 3 | Painful erythema, edema, or ulcers, and cannot eat |
| Grade 4 | Requires parenteral or enteral support |
National Cancer Institute common toxicity criteria (NCI‐CTC)
(as reported in Dazzi 2003)
| Grade 0 | No mucositis |
| Grade 1 | Painless ulcers, erythema or mild soreness in the absence of lesions |
| Grade 2 | Painful erythema, edema or ulcers, but can eat or swallow |
| Grade 3 | Painful erythema, edema or ulcers requiring intravenous hydration |
| Grade 4 | Severe ulceration or requires parenteral or enteral nutritional support |
World Health Organization (WHO) scale
(as reported in Vadhan‐Raj 2010)
| Grade 0 | No mucositis |
| Grade 1 | Soreness ± erythema |
| Grade 2 | Erythema, ulcers. Patient can swallow solid diet |
| Grade 3 | Ulcers, extensive erythema. Patient cannot swallow solid diet |
| Grade 4 | Mucositis to the extent that alimentation is not possible |
Data and analyses
Comparison 1. KGF versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (any) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 BMT/SCT after conditioning for haematological cancers | 4 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.05] |
| 1.2 RT to head & neck with cisplatin | 2 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.90, 1.00] |
| 1.3 CT alone for mixed cancers | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.60, 0.85] |
| 2 Oral mucositis (moderate + severe) | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 BMT/SCT after conditioning for haematological cancers | 6 | 852 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.80, 0.99] |
| 2.2 RT to head & neck with cisplatin/5FU | 3 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.83, 1.00] |
| 2.3 CT alone for mixed cancers | 4 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.45, 0.70] |
| 3 Oral mucositis (severe) | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 BMT/SCT after conditioning for haematological cancers | 6 | 852 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.11] |
| 3.2 RT to head & neck with cisplatin/5FU | 3 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.90] |
| 3.3 CT alone for mixed cancers | 3 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.14, 0.65] |
| 4 Interruptions to cancer treatment (unscheduled RT breaks of 5 or more days) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 RT to head & neck with cisplatin/5FU | 3 | 473 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.65, 1.59] |
| 5 Interruptions to cancer treatment (chemotherapy delays/discontinuations) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 RT to head & neck with cisplatin | 2 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.62, 1.47] |
| 6 Oral pain | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 BMT/SCT after conditioning for haematological cancers | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐3.00, 1.30] |
| 6.2 RT to head & neck with cisplatin | 2 | 374 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.27, 0.02] |
| 7 Normalcy of diet (use of supplemental nutrition) | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 BMT/SCT after conditioning for haematological cancers | 4 | 714 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.58, 1.34] |
| 7.2 RT to head & neck with cisplatin/5FU | 3 | 473 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.77, 1.37] |
| 8 Normalcy of diet (worst ability to eat score ‐ 1 to 4 scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 BMT/SCT after conditioning for haematological cancers | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.21, 0.21] |
| 9 Number of days in hospital | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 BMT/SCT after conditioning for haematological cancers | 1 | 281 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.64, 1.64] |
| 10 Number of days of treatment with opioid analgesics | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 BMT/SCT after conditioning for haematological cancers | 2 | 323 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐3.33, 0.51] |
Comparison 2. KGF (dose comparison).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (any) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 BMT/SCT after conditioning for haematological cancers | 1 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.75, 1.03] |
| 2 Oral mucositis (moderate + severe) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 BMT/SCT after conditioning for haematological cancers | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 CT alone for metastatic colorectal cancer | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Oral mucositis (severe) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 BMT/SCT after conditioning for haematological cancers | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Oral pain (maximum score on 0 to 10 VAS) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 BMT/SCT after conditioning for haematological cancers | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.90, 3.30] |
| 5 Normalcy of diet (use of TPN) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 BMT/SCT after conditioning for haematological cancers | 1 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.02] |
| 6 Normalcy of diet (worst ability to eat score ‐ 1 to 4 scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 BMT/SCT after conditioning for haematological cancers | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.41, 1.21] |
| 7 Number of days in hospital | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 BMT/SCT after conditioning for haematological cancers | 1 | 224 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.78, 1.78] |
| 8 Number of days of treatment with opioid analgesics | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 BMT/SCT after conditioning for haematological cancers | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. KGF versus chlorhexidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (any) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 CT alone for haematological cancer | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.54, 0.85] |
| 2 Oral mucositis (moderate + severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 CT alone for haematological cancer | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.05, 0.28] |
| 3 Oral mucositis (severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 CT alone for haematological cancer | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.19] |
Comparison 4. GM‐CSF versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (any) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 BMT/SCT after conditioning for mixed cancers | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.04] |
| 1.2 RT to head & neck | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.82, 1.23] |
| 2 Oral mucositis (moderate + severe) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 BMT/SCT after conditioning for haematological cancers | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.13] |
| 2.2 RT to head & neck | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.49, 1.06] |
| 3 Oral mucositis (severe) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 BMT/SCT after conditioning for mixed cancers | 3 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.33, 1.67] |
| 3.2 RT to head & neck | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.09] |
| 3.3 CT alone for mixed cancers | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.05, 7.11] |
| 4 Oral pain (maximum score on 0 to 10 VAS) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 BMT/SCT after conditioning for mixed cancers | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.85, 2.05] |
| 5 Normalcy of diet (use of feeding tube/parenteral nutrition) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 BMT/SCT after conditioning for haematological cancers | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.1 [0.63, 1.91] |
| 5.2 RT to head & neck | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.09] |
| 6 Number of days of treatment with opioid analgesics | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 BMT/SCT after conditioning for mixed cancers | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.91, ‐0.29] |
Comparison 5. GM‐CSF (dose comparison).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 CT alone for breast cancer | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 2.75 [1.07, 7.04] |
Comparison 6. GM‐CSF versus sucralfate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (moderate + severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 RT to head & neck | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.80, 1.14] |
| 2 Oral mucositis (severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 RT to head & neck | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.24, 1.21] |
| 3 Interruptions to cancer treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 RT to head & neck | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.36] |
| 4 Normalcy of diet (use of PEG tube) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 RT to head & neck | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.56] |
Comparison 7. G‐CSF versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (any) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 RT to head & neck | 2 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.22] |
| 1.2 CT alone for lung cancer | 1 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.40, 0.87] |
| 2 Oral mucositis (moderate + severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 CT alone for breast cancer | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.12, 0.95] |
| 3 Oral mucositis (severe) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 RT to head & neck | 2 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.15, 0.87] |
| 4 Interruptions to cancer treatment (RT interruption) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 RT to head & neck | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.01, 4.31] |
| 5 Normalcy of diet (use of PEG tube) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 RT to head & neck | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.01, 2.86] |
Comparison 8. G‐CSF (pegfilgrastim) versus G‐CSF (filgrastim).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (any) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 BMT/SCT after conditioning for mixed cancers | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.27] |
| 2 Oral mucositis (moderate + severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 BMT/SCT after conditioning for mixed cancers | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.55, 1.11] |
| 3 Normalcy of diet (use of supplemental nutrition) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 BMT/SCT after conditioning for mixed cancers | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.94, 1.06] |
Comparison 9. EGF versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (moderate + severe) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 BMT/SCT after conditioning for haematological cancers | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.78, 1.43] |
| 1.2 RT to head & neck +/‐ cisplatin | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 0.99] |
| 2 Oral mucositis (severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 BMT/SCT after conditioning for haematological cancers | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.59, 1.80] |
| 3 Interruptions to cancer treatment (RT breaks > 2 consecutive days) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 RT to head & neck +/‐ cisplatin | 1 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 4.38 [0.25, 75.44] |
| 4 Normalcy of diet (use of supplemental nutrition) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 BMT/SCT after conditioning for haematological cancers | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.55, 1.94] |
Comparison 10. ITF versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (any) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 CT alone for colorectal cancer | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.35, 0.79] |
| 2 Oral mucositis (moderate + severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 CT alone for colorectal cancer | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.10, 0.48] |
| 3 Oral mucositis (severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 CT alone for colorectal cancer | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.06, 36.39] |
Comparison 11. ITF (dose comparison).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (any) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 CT alone for colorectal cancer | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 1.3 [0.67, 2.54] |
| 2 Oral mucositis (moderate + severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 CT alone for colorectal cancer | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.18, 3.09] |
| 3 Oral mucositis (severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 CT alone for colorectal cancer | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.90] |
Comparison 12. Erythropoietin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral mucositis (any) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 BMT/SCT after conditioning for haematological cancers | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.21, 0.60] |
| 2 Oral mucositis (moderate + severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 BMT/SCT after conditioning for haematological cancers | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.24, 0.79] |
| 3 Oral mucositis (severe) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 BMT/SCT after conditioning for haematological cancers | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.4 [0.14, 1.17] |
| 4 Number of days in hospital | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 BMT/SCT after conditioning for haematological cancers | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐2.95 [‐7.73, 1.83] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antoun 2009.
| Methods |
Trial design: parallel (2 arms) Location: Institut Gustave Roussy, Villejuif, France Number of centres: 1 Study duration: February 2005 to September 2006 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: adults with metastatic colorectal adenocarcinoma (grade 3 to 4); life expectancy greater than 3 months; receiving 5FU‐based chemotherapy Exclusion criteria: HIV; pregnant or lactating; unlikely to comply with interventions; participation in another trial in the previous 12 months (unless regarding chemotherapeutic protocols); undergone a total colectomy; state of subocclusion; chronic inflammatory diseases of the digestive tract; radiation enteropathy Cancer type: metastatic colorectal adenocarcinoma (grade 3 to 4) Cancer treatment: 5FU‐based chemotherapy Age at baseline (years): median 60 (not reported by group) Gender: not reported Number randomised: 22 (not reported by group) Number evaluated: 13 (Group A: 9; Group B: 4) |
|
| Interventions |
Comparison: TGF‐beta(2) versus placebo Group A: nutritional supplement of proteins, carbohydrates, fats, vitamins and minerals, with TGF‐beta(2) (2 ng/mg protein); formulas were in powder form, mixed with cool previously boiled water at 0.23 g/mL (100 kcl/100 mL); during each cycle participants received 750 mL to 1000 mL per day plus any other food desired; formula administered for 2 days before, 2 days during, and 3 days after chemotherapy (7 days/cycle) Group B: same as above without the TGF‐beta(2) Compliance: "Nine randomised patients who never ate the formula were excluded from the study" (not reported by group) Duration of treatment: "3 months (test or control formula), for a minimum of one and a maximum of eight cycles of treatment" |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: "This study was funded by Nestec Ltd" ‐ Nestlé (manufacturer of the intervention) Declarations/conflicts of interest: 6 of the 9 authors were either consultants (1) or employees (5) of Nestlé Data handling by review authors: reported in additional table Other information of note: "Due to low accrual of patients (22 patients were enrolled and randomised in 18 months), the study was prematurely stopped" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly assigned" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The test formula differed only by containing an additional..." Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: it is not clear who was blinded. There are subjective elements to the assessment of oral mucositis using this scale, requiring the patient's assessment of pain/soreness and their ability to swallow but, as the participants were unaware of their group allocation, the assessment of oral mucositis can be considered to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall attrition was 41% although it was not reported by group |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Blazar 2006.
| Methods |
Trial design: parallel (7 arms) dose‐ranging study Location: Universities of Minnesota and Michigan, USA Number of centres: 2 Study duration: not reported Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: aged 3 to 65 years; diagnosed with haematological malignancy (including myelodysplastic syndromes); ECOG score of 0 to 2; eligible for allogeneic HSCT after conditioning treatment with chemotherapy with or without TBI Exclusion criteria: received previous allogeneic HSCT; due to receive a T‐cell‐depleted donor graft; active chronic skin disease; pre‐existent inflammatory bowel disease; uncontrolled (antibiotic‐resistant) bacterial infection; hepatitis; HIV Cancer type: haematologic: ALL (Group A: 12%; Group B: 3%); AML (Group A: 35%; Group B: 39%); CML (Group A: 10%; Group B: 26%); MDS (Group A: 9%; Group B: 19%); NHL (Group A: 19%; Group B: 3%); Hodgkin's (Group A: 1%; Group B: 0%); Other (Group A: 14%; Group B: 10%) Cancer treatment: both centres had allogeneic HSCT on day 0 but differed in conditioning regimen and GVHD prophylaxis as follows:
Both centres received G‐CSF (filgrastim) 5 µg/kg per day from 24 hours after HSCT until neutrophil recovery Age at baseline (years): Group A: median 46 (range 7 to 65); Group B: median 46 (range 7 to 63) Gender: both groups 58% male Number randomised: 100 (Group A: 69; Group B: 31) Number evaluated: 96 (Group A: 65; Group B: 31) |
|
| Interventions |
Comparison: KGF (palifermin) versus placebo (4 KGF arms and 3 placebo arms were each combined into a single arm) Group A: KGF
Group B: placebo with matching schedule to either the 6, 9 or 12 dose regimen Mode of administration not described but presumably IV as in other KGF studies Compliance: Group A: 20 did not receive all study doses (17 of these were replaced to allow a full assessment of safety); Group B: 2 did not receive all study doses (1 replaced) Duration of treatment: varied from 13 days (6 doses) to 27 days (12 doses) ‐ see above |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: government grants from NIH and FDA, and also supported by Amgen (pharmaceutical industry) Declarations/conflicts of interest: not reported Data handling by review authors: the data for incidence of mucositis were not reported separately for each dose and therefore it was not possible to include head‐to‐head comparisons of different dosages in this review; the data for incidence of mucositis were presented in subgroups of those that did or did not receive the final methotrexate infusion on day 11 but we used the overall data in our meta‐analyses (the study authors report that there was no difference between these subgroups) Other information of note: the study authors report a greater decrease in incidence of grade 3 to 4 (severe) oral mucositis due to palifermin in the Minnesota participants (who received a more mucotoxic conditioning regimen) than in the Michigan participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients...were randomly assigned..." Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients...were randomly assigned..." Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" Comment: it is not clear who was blinded. There are subjective elements to the assessment of oral mucositis using this scale, requiring the patient's assessment of pain/soreness and their ability to swallow but, as the participants were unaware of their group allocation, the assessment of oral mucositis can be considered to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Data for all patients randomly assigned and who received a transplant were used in all other analyses (intent‐to‐treat)" Comment: although 18 participants (Group A: 17; Group B: 1) were replaced to allow a full assessment of safety, it seems that the originally randomised participants were included in the analyses Overall attrition was 4% (Group A: 6%; Group B: 0%) for the oral mucositis incidence outcome. The reasons were unclear but this proportion of attrition is unlikely to have biased the results |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Blijlevens 2013.
| Methods |
Trial design: parallel (3 arms) Location: Europe (Italy, France, the Netherlands, Ireland, Germany, UK, Denmark, Austria, Switzerland, Czech Republic, Sweden, Hungary, Belgium, Finland) Number of centres: 39 Study duration: December 2006 to February 2009 Trials registry number: NCT00434161 |
|
| Participants |
Inclusion criteria: aged between 18 and 70 years; due to receive high‐dose melphalan; ECOG score of 0 to 2 (or 3, if reason was due to multiple myeloma); at least 2 x 10⁶ CD34+ cells per kg; corrected carbon monoxide diffusing capacity 50% or higher of predicted; absolute neutrophil count at least 1.5 x 10⁹/L and platelets at least 100 x 10⁹/L; total bilirubin 2 mg/dL or lower; aspartate aminotransferase and/or alanine aminotransferase 4 x institutional upper limit of normal or lower Exclusion criteria: not reported Cancer type: multiple myeloma Cancer treatment: 1‐day administration of high‐dose melphalan (200 mg/m²) on day ‐2, followed by auto‐SCT on day 0 Age at baseline (years): Group A: median 55 (range 32 to 69); Group B: median 58 (range 40 to 68); Group C: median 58 (range 41 to 68) Gender: Group A: 54% male; Group B: 55% male; Group C: 58% male Number randomised: 281 (Group A: 109; Group B: 115; Group C: 57) Number evaluated: 281 (Group A: 109; Group B: 115; Group C: 57) |
|
| Interventions |
Comparison: KGF versus placebo Group A: KGF (60 µg/kg) daily IV on days ‐6, ‐5, and ‐4, then placebo on days 0 (the day of auto‐SCT), 1, and 2 (total dose = 180 µg/kg) Group B: KGF (60 µg/kg) daily IV on days ‐6, ‐5, ‐4, 0, 1, and 2 (total dose = 360 µg/kg) Group C: placebo daily IV on days ‐6, ‐5, ‐4, 0, 1, and 2 Compliance: Group A: 8% discontinued; Group B: 12% discontinued; Group C: 4% discontinued; (point of discontinuation or number of treatments not stated for any group) Duration of treatment: 6 treatment days (over 9 days) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: 275 participants required at 95% power and 5% significance to detect an odds ratio of at least 3.5 between placebo and KGF in grade 2 to 4 oral mucositis Funding: sponsored by Swedish Orphan Biovitrum (pharmaceutical industry); KGF and placebo manufactured and packaged by Amgen (pharmaceutical industry) Declarations/conflicts of interest: 2 authors were employees of the sponsors; the remaining authors declared no competing financial interests Data handling by review authors: we combined the 2 KGF groups to make a single pairwise comparison against placebo; we also made a separate comparison of the 2 KGF regimens Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by using an interactive‐voice‐response‐system before planned admission" Comment: large multicentre trial using high‐tech randomisation method ‐ likely to be done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by using an interactive‐voice‐response‐system before planned admission" Comment: large multicentre trial using high‐tech randomisation method ‐ likely to be done properly |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" and "Study drug...packaged...in identical vials" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" Comment: it is not clear who was blinded. There are subjective elements to the assessment of lower grades of oral mucositis using the WHO scale, requiring the patient's assessment of pain/soreness and their ability to swallow. Higher grades have more objective elements so may not be affected by potential lack of blinding of the assessor. This would be the same for other subjective and objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately (although quality of life reported with no SD or P values, this does not affect the risk of bias judgement for other outcomes) |
| Other bias | Low risk | No other sources of bias are apparent |
Bradstock 2014.
| Methods |
Trial design: parallel (2 arms) Location: Australia Number of centres: 23 Study duration: recruitment from September 2006 to April 2010 Trials registry number: ACTRN012605000095662 (mentioned in trial report) |
|
| Participants |
Inclusion criteria: aged 15 to 60 years with newly diagnosed and previously untreated (except for hydroxycarbamide for high presenting white blood cell count) acute myeloid leukaemia ‐ all subtypes except t(15;17) or variants, or core‐binding factor AML (t(8;21) or inv(16) or variants); ECOG score of 0 to 3; no history of cancer (other than basal cell skin cancer or carcinoma of the cervix in situ, or other localised cancer treated by surgical excision only more than 5 years earlier without evidence of recurrence in the intervening period) Exclusion criteria: not reported Cancer type: acute myeloid leukaemia Cancer treatment: induction chemotherapy consisting of: idarubicin 9 mg/m² daily IV infusion on days 1 to 3; etoposide 75 mg/m² daily IV infusion on days 1 to 7; cytarabine 3 g/m² 12‐hourly IV infusion on days 1, 3, 5, and 7 All participants received G‐CSF (pegfilgrastim) 6 mg subcutaneously on day 8 Age at baseline (years): Group A: mean 46 (SD 12; range 17 to 60); Group B: mean 44 (SD 12; range 16 to 60) Gender: Group A: 61% male; Group B: 67% male Number randomised: 160 (Group A: 79; Group B: 81) Number evaluated: 151 (Group A: 73; Group B: 78) |
|
| Interventions |
Comparison: KGF (palifermin) versus placebo Group A: KGF (60 µg/kg) daily IV on days ‐3, ‐2, ‐1 prior to chemotherapy and for 3 days after completion of chemotherapy (total dose = 360 µg/kg) Group B: same schedule with placebo Compliance: received all 3 pre‐chemotherapy doses: Group A: 97%; Group B: 100%; received all 3 post‐chemotherapy doses: Group A: 95%; Group B: 96% Duration of treatment: 6 treatment days (over 14 days) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: 128 per group required to detect a reduction in grade 3 to 4 mucositis from 22% to 10% at 70% power and 5% significance Funding: "This study was funded in part from Project Grant 302133 from the National Health and Medical Research Council of Australia" (government); KGF and placebo provided by Amgen (pharmaceutical industry) Declarations/conflicts of interest: "The authors have no conflicts of interest to declare" Data handling by review authors: N/A Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomized 1:1 using a block randomization technique and stratification by participating centre to receive placebo or palifermin" Comment: large multicentre trial using block randomisation and stratification ‐ likely to be done properly |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eligible patients were randomized 1:1 using a block randomization technique and stratification by participating centre to receive placebo or palifermin" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo‐controlled" and "Both investigators and patients were blinded to the randomization outcome" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "placebo‐controlled" and "Both investigators and patients were blinded to the randomization outcome" Comment: investigators assessed oral mucositis, which would have partly relied on patient's assessment of pain/soreness and their ability to swallow; both investigators and patients were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was 6% (Group A: 8%; Group B: 4%) for the oral mucositis incidence outcome. The reasons were similar between groups and this proportion of attrition is unlikely to have biased the results |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Brizel 2008.
| Methods |
Trial design: parallel (2 arms) Location: Australia, Canada and USA Number of centres: 22 Study duration: September 1999 to May 2001 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: adults with newly diagnosed stage III/IVa or IVb squamous carcinoma of the oral cavity, oropharynx, nasopharynx, hypopharynx, and larynx (or with unknown primary and extensive neck disease) undergoing CRT intended to be curative; Karnofsky performance score of 60 or higher; haemoglobin 10 g/dL or higher; white blood cell count 3.5 x 10⁹/L or higher or absolute neutrophil count 1.5 x 10⁹/L or higher; platelet count 100 x 10⁹/L or higher; serum bilirubin 1.5 mg/dL or lower; serum creatinine lower than 2.0 mg/dL (plus a 24‐hour urinary creatinine clearance 50 mL/min in those aged 60 years or older) Exclusion criteria: previous RT to the head and neck; previous surgery for the primary tumour (not including biopsy); previous CT; allergy to Escherichia coli‐derived products; participation in any other investigational study within the 30 days prior to this study; refusal to use adequate contraception during the study; pregnant or breastfeeding Cancer type: head and neck: oral (Group A: 12%; Group B: 6%); oropharynx/nasopharynx (Group A: 61%; Group B: 66%); hypopharynx/larynx (Group A: 27%; Group B: 28%) Cancer treatment:
Age at baseline (years): Group A: mean 54 (SD 10; range 25 to 80); Group B: mean 56 (SD 10; range 42 to 75) Gender: Group A: 82% male; Group B: 84% male Number randomised: 101 (Group A: 69; Group B: 32) Number evaluated: 97 (Group A: 65; Group B: 32) |
|
| Interventions |
Comparison: KGF (palifermin) versus placebo Group A: KGF 60 µg/kg once weekly by IV bolus injection starting on the Friday before CRT began (on the following Monday), then each Friday after completion of RT for 7 weeks, and 2 more doses after completion of CRT i.e. 10 doses in total (total dose = 600 µg/kg) Group B: same schedule with matching placebo Compliance: 99 participants (Group A: 67; Group B: 32) received at least 1 dose of their allocated intervention; 69 participants completed the full course (Group A: 47; Group B: 22); mean number of doses (Group A: 8.4; Group B: 9.1) Duration of treatment: 9 weeks (10 doses) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: based on a previous study, 99 participants required to detect a 30% difference in the duration of grade 2 or higher oral mucositis with 80% power provided that the mean duration in the placebo arm was 56 days Funding: "Supported by Amgen Inc" (pharmaceutical industry) Declarations/conflicts of interest: multiple and involving: employment or leadership positions with the funders (Amgen); consultant or advisory roles with the funders and other pharmaceutical companies; stock ownership with the funders; honoraria from the funders and other pharmaceutical companies; research funding from the funders and other pharmaceutical companies Data handling by review authors: the data for incidence of mucositis were presented in subgroups of those that received standard or hyperfractionated RT but we used the overall data in our meta‐analyses; for the interruptions to radiotherapy outcome, we used the data for breaks longer than 4 days as these could be pooled with other studies in this comparison Other information of note: the study authors report a greater decrease in incidence due to palifermin in the hyperfractionated subgroup than in the standard subgroup (see figure 3A in the study report) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned..." Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned..." Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blinded" and "Palifermin...or matching placebo was administered by intravenous bolus" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blinded" and "Palifermin...or matching placebo was administered by intravenous bolus" Comment: it is not clear who was blinded. There are subjective elements to the assessment of oral mucositis using this scale, requiring the patient's assessment of pain/soreness and their ability to swallow but, as the participants were unaware of their group allocation, the assessment of oral mucositis can be considered to be blinded. The other outcomes are objective and therefore unlikely to be affected by any potential lack of blinding of the outcome assessor(s) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was 4% (Group A: 6%; Group B: 0%) for the oral mucositis incidence outcome. The reasons were unclear but this proportion of attrition is unlikely to have biased the results |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Cartee 1995.
| Methods |
Trial design: parallel (5 arms) dose‐ranging study Location: Duke University Medical Centre, Durham, North Carolina, USA Number of centres: 1 Study duration: not reported Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: premenopausal or perimenopausal patients with histologically confirmed metastatic breast cancer who had chemotherapy for inoperable or metastatic disease; performance status of 0 or 1 (CALGB criteria) Exclusion criteria: metastatic disease involving the central nervous system; pregnant Cancer type: stage IV breast Cancer treatment: AFM regimen (21‐day cycle): 5FU (500 mg/m²/day) continuous infusion on days 1 to 5; adriamycin (25 mg/m²) IV bolus on days 3 to 5; methotrexate (250 mg/m²) IV on day 15 (if oral mucositis less than grade 3) All participants received G‐CSF (filgrastim) subcutaneously (5 µg/kg/day) on days 7 to 13 and from day 16 until resolution of neutropenia Age at baseline (years): mean 44 (not reported by group) Gender: 49 female; 1 male (not reported by group) Number randomised: 50 (not reported by group) Number evaluated: 45 (Group A: 36; Group B: 9) |
|
| Interventions |
Comparison: GM‐CSF (molgramostim) versus placebo Group A: GM‐CSF
Group B: (n = 9 analysed) same schedule with matching placebo mouthwash Compliance: (not reported by group) mouthwash therapy was discontinued if the participant experienced oral mucositis of grade 3 or above; 30 participants took at least 80% of their prescribed doses; 11 participants discontinued mouthwash therapy within 3 days prior to day 15; 4 participants discontinued mouthwash therapy between day 15 and day 21 Duration of treatment: 21 days (first treatment cycle of AFM) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: this was done but the numbers required are not reported Funding: "...supported in part by National Cancer Institute (Bethesda, MD) grant number PO1‐47741‐A4" Declarations/conflicts of interest: not reported Data handling by review authors: we combined the 4 GM‐CSF groups to make a single pairwise comparison against placebo and, in order to make a head‐to‐head comparison of doses, we grouped the 2 lower doses (0.01 µg/mL and 0.1 µg/mL) together and grouped the 2 higher doses (1 µg/mL and 10 µg/mL) together to make pairwise groups for comparison Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized by the Duke Cancer Center Protocol Office according to a block randomization scheme and assigned a unique identifier number which designated the GM‐CSF dose level to be received" Comment: method of sequence generation not fully described but was done by a dedicated specialist centre so was probably done adequately |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized by the Duke Cancer Center Protocol Office" and "The patient supply of mouthwash was labelled to correspond with the assigned identifier number and dispensed by the Pharmacy. The patient assignment information was maintained by the Pharmacy" Comment: the entire randomisation process was performed by third party so the random sequence is unlikely to have been manipulated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" and "The patient assignment information was maintained by the Pharmacy" Comment: it is not clear who was blinded. There are subjective elements to the assessment of oral mucositis using this scale, requiring the patient's assessment of pain/soreness and their ability to swallow but, as the participants were unaware of their group allocation, the assessment of oral mucositis can be considered to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall attrition was 10%. Reasons for attrition fully reported. If all participants would have developed severe oral mucositis or dropped out due to severe oral mucositis and were all from 1 particular group, this would have biased the results. However, attrition was not reported by group, so it is unclear |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Cesaro 2013.
| Methods |
Trial design: parallel (2 arms) Location: Italy Number of centres: 4 Study duration: May 2007 to June 2011 Trials registry number: EudraCT 2007‐001430‐14 (mentioned in trial report) |
|
| Participants |
Inclusion criteria: aged 0 to 17 years with leukaemia, lymphoma or solid tumour; due to receive a first autologous PBSCT Exclusion criteria: not reported Cancer type: leukaemia/lymphoma (Group A: 22%; Group B: 17%); solid (Group A: 78%; Group B: 83%) (neuroblastoma, Ewing sarcoma/peripheral neuroectodermal tumour, medulloblastoma, Wilms tumour, central nervous system tumour) Cancer treatment: all participants had autologous PBSCT on day 0 but differed in conditioning regimen as follows:
Age at baseline (years): Group A: median 11.1 (range 1.7 to 17.4); Group B: median 11.9 (range 1.6 to 17.2) Gender: Group A: 66% male; Group B: 59% male Number randomised: 61 (Group A: 32; Group B: 29) Number evaluated: 61 (Group A: 32; Group B: 29) |
|
| Interventions |
Comparison: G‐CSF (pegfilgrastim) versus G‐CSF (filgrastim) Group A: pegfilgrastim single dose (100 µg/kg; maximum 6 mg) injected on day 3 Group B: filgrastim (5 µg/kg per day; maximum 300 µg per day) injected by 9 or more doses starting on day 3 (total dose = at least 45 µg/kg) Mode of administration not described but presumably subcutaneously as in other G‐CSF studies Compliance: all participants received their allocated intervention with no discontinuations Duration of treatment: Group A: 1 day; Group B: 9 or more days |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: based on the noninferiority of pegfilgrastim versus filgrastim in speeding the recovery of polymorphonuclear cells Funding: "The authors have no support or funding to report" Declarations/conflicts of interest: "The authors have declared that no competing interests exist" Data handling by review authors: N/A Other information of note: G‐CSF administration only began after the chemotherapy and PBSCT were completed, by which point oral mucositis may have already begun to develop |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomisation list was drawn up at Data Office Centre of AIEOP in Bologna, Italy, by a statistician not involved in patient management" Comment: adequate method used |
| Allocation concealment (selection bias) | Low risk | Quote: "The list was stored by sequentially numbered sealed envelopes that was concealed to investigators until the completion of recruitment. The local investigator ...assigned each eligible patient to randomization list by phoning to AIEOP Data Office Centre" Comment: ideal method of concealment used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment regimens were different so blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It would be possible to blind the outcome assessor for oral mucositis, but it was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Chi 1995.
| Methods |
Trial design: cross‐over (2 arms) Location: Cancer Centre and Department of Otolaryngology, Veterans General Hospital, Taiwan, Republic of China Number of centres: 1 Study duration: not reported Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: diagnosed stage IV SCC of head and neck, previously untreated or locally recurrent after previous surgery or radiotherapy or both; ECOG score of 2 or above; adequate bone marrow, liver and renal function Exclusion criteria: concurrent medical illness; local radiotherapy to oropharynx region in the previous 3 months Cancer type: head and neck: nasopharyngeal (Group A: 44%; Group B: 27%); tongue (Group A: 22%; Group B: 36%); hypopharynx (Group A: 11%; Group B: 18%); buccal (Group A: 11%; Group B: 9%); tonsillar (Group A: 11%; Group B: 9%) Cancer treatment: PFL regimen (21‐day cycle): cisplatin (20 mg/m²/day), 5FU (800 mg/m²/day) and leucovorin (90 mg/m²/day) IV for days 1 to 4; cycle repeated every 3 weeks (study consisted of 2 cycles) Age at baseline (years): Group A: median 44 (range 36 to 62); Group B: median 49 (range 40 to 66) Gender: Group A: 89% male; Group B: 91% male Number randomised: 20 (Group A: 9; Group B: 11) ‐ figures for first cycle Number evaluated: 20 (Group A: 9; Group B: 11) |
|
| Interventions |
Comparison: GM‐CSF versus no treatment Group A: GM‐CSF (4 µg/kg) subcutaneously from day 5 to 14 (total dose = 40 µg) Group B: no treatment Compliance: not reported Duration of treatment: 10 days (days 5 to 14 of 21‐day cycle) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: "..supported in part by Department of Health, Taiwan, Republic of China, research grant no. DOH 83‐HR‐202" and "GM‐CSF (supplied by Schering Plough Corp, Kenilworth, NJ)" (pharmaceutical industry) Declarations/conflicts of interest: not reported Data handling by review authors: as stated in the methods section, we would only include first‐period data from cross‐over studies due to potential for period effects (which were reported in this study). The only usable data in this study were reported in the text as incidence of severe gross mucositis for the first cycle Other information of note: the authors report a significant period effect of GM‐CSF (P < 0.01), whereby the benefits continued into the second cycle GM‐CSF administration only began after the 4‐day chemotherapy was completed, by which point oral mucositis may have already begun to develop |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized to receive GM‐CSF or no therapy" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomized to receive GM‐CSF or no therapy" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comparison with no treatment so blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It would be possible to blind the outcome assessor, as the data we used were assessed by a physician using an objective scale. However, it was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants appear to be included in the analyses |
| Selective reporting (reporting bias) | Low risk | Although most of the data were not usable in this review, this does not seem to be due to selective reporting |
| Other bias | Low risk | No other sources of bias are apparent |
Crawford 1999.
| Methods |
Trial design: parallel (2 arms) Location: USA Number of centres: 14 Study duration: recruitment from May 1988 to November 1989 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: newly diagnosed small‐cell lung cancer meeting standard criteria for end‐organ function; ECOG score of 0 to 2 Exclusion criteria: previous radiotherapy; other serious medical illnesses precluding participation Cancer type: small‐cell lung cancer Cancer treatment: CAE regimen (21‐day cycle): cyclophosphamide (1000 mg/m²) and doxorubicin (50 mg/m²) on day 1; etoposide (120 mg/m²) on days 1 to 3; all by IV; repeated for up to 6 cycles Age at baseline (years): Group A: mean 61 (SD 10; range 31 to 78); Group B: mean 62 (SD 8; range 31 to 80) Gender: Group A: 65% male; Group B: 63% male Number randomised: 211 (Group A: 101; Group B: 110) Number evaluated: 195 (Group A: 93; Group B: 102) ‐ figures for first cycle |
|
| Interventions |
Comparison: G‐CSF (r‐metHuG‐CSF) (filgrastim) versus placebo Group A: G‐CSF (230 µg/m²) self‐administered subcutaneously on days 4 to 17 (total dose = 3220 µg/m²) Group B: as above but with placebo G‐CSF stopped if postnadir neutrophil count exceeded 10 x 10⁹/L after day 12; participants kept receiving their allocated intervention until they experienced fever with neutropenia, then they received unblinded G‐CSF (230 µg/m²) in subsequent cycles; participants in the G‐CSF group who experienced fever with neutropenia were allowed 25% reduction in chemotherapy dosages in subsequent cycles Compliance: not reported Duration of treatment: 14 days during a 21‐day cycle |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: based on a difference of 20% in the incidence of fever with neutropenia over the 6 cycles Funding: "The study was designed, coordinated, and analyzed in conjunction with Amgen, the supplier of the G‐CSF" Declarations/conflicts of interest: not reported but some authors were employed by Amgen (pharmaceutical industry) Data handling by review authors: for oral mucositis, we only used the data from the first cycle due to the reasons listed above (under 'Interventions') Other information of note: G‐CSF administration only began after the 3‐day chemotherapy was completed, by which point oral mucositis may have already begun to develop. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to chemotherapy followed by study drug (either placebo or G‐CSF)" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to chemotherapy followed by study drug (either placebo or G‐CSF)" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo was supplied in matching vials for double blinding" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Placebo was supplied in matching vials for double blinding" Comment: it is not clear who was blinded. There are subjective elements to the assessment of oral mucositis using this scale, requiring the patient's assessment of pain/soreness and their ability to swallow but, as the participants were unaware of their group allocation, the assessment of oral mucositis can be considered to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was 8% (Group A: 8%; Group B: 7%) for the oral mucositis incidence outcome. The reasons were reported and similar between groups, and this proportion of attrition is unlikely to have biased the results |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Dazzi 2003.
| Methods |
Trial design: parallel (2 arms) Location: Ravenna, Italy Number of centres: 1 Study duration: recruitment from July 1997 to February 2002 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: 14 years of age or older; hospitalised for high‐dose chemotherapy with autologous PBSCT Exclusion criteria: not reported Cancer type: breast (Group A: 17.5%; Group B: 27.5%); Ewing's sarcoma (Group A: 41.5%; Group B: 28.5%); osteosarcoma (Group A: 13%; Group B: 14%); NHL (Group A: 15%; Group B: 11.5%); germ cell tumours (Group A: 11%; Group B: 16%); small‐cell lung (Group A: 2%; Group B: 0%); soft tissue sarcoma (Group A: 0%; Group B: 2.5%) Cancer treatment: high‐dose chemotherapy with autologous PBSCT; chemotherapy regimens were categorised into high risk and low risk and this was used as a stratification factor for randomisation, therefore high‐ and low‐risk participants were equally distributed across groups All participants received subcutaneous G‐CSF (300 µg/day) until haematopoietic reconstitution Age at baseline (years): Group A: median 29 (range 15 to 57); Group B: median 29 (range 17 to 61) Gender: Group A: 59% male; Group B: 55% male Number randomised: 90 (Group A: 46; Group B: 44) Number evaluated: 90 (Group A: 46; Group B: 44) |
|
| Interventions |
Comparison: GM‐CSF versus placebo Group A: GM‐CSF mouthwash 150 µg/day in 100 cm³ of sterile water taken in 4 doses per day; mouthrinsing performed for 1 minute each time; treatment started on the day after the completion of chemotherapy and continued until bone marrow recovery (absolute neutrophil count > 500/mm³) or resolution of mucositis if still persistent after bone marrow recovery (total dose = variable) Group B: as above but with placebo (sterile water) All participants received 0.2% oral chlorhexidine and amphotericin B Compliance: all but 7 participants regularly completed mouthwashes: 1 participant in the placebo group had none due to persistent vomiting; 6 (4 in placebo group and 2 in GM‐CSF group) started treatment but interrupted it early due to nausea and vomiting Duration of treatment: variable and dependent on bone marrow recovery/resolution of mucositis |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: 90 participants required to detect 25% minimal difference in the rate of severe mucositis at 90% power and 5% significance Funding: no external funding (from correspondence with authors) Declarations/conflicts of interest: not reported Data handling by review authors: the data for incidence of mucositis were presented in subgroups of those at low or high risk of mucositis but we used the overall data in our meta‐analyses Other information of note: there does not appear to be any difference in risk of any or severe mucositis between the low‐ and high‐risk subgroups GM‐CSF administration only began after the chemotherapy was completed, by which point oral mucositis may have already begun to develop |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from correspondence with authors: "The randomization list was centralized" Comment: centralised randomisation method ‐ likely to be done properly |
| Allocation concealment (selection bias) | Low risk | Quote from correspondence with authors: "The randomization list was centralized" Comment: centralised randomisation method ‐ likely to be done properly |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, randomized, placebo‐controlled study" and "The color, odor, texture and taste of both solutions were virtually identical" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, randomized, placebo‐controlled study" Comment: it is not clear who was blinded. There are subjective elements to the assessment of oral mucositis using this scale, requiring the patient's assessment of pain/soreness and their ability to swallow but, as the participants were unaware of their group allocation, the assessment of oral mucositis can be considered to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Fink 2011.
| Methods |
Trial design: parallel (2 arms) Location: Department of Hematology and Oncology, University Hospital Freiburg, Germany Number of centres: 1 Study duration: March 2006 to December 2010 Trials registry number: EudraCT 2008‐001833‐87; DRKS00000043 |
|
| Participants |
Inclusion criteria: adults aged 18 to 75 years with either: high‐grade non‐Hodgkin's lymphoma with high‐risk syndrome (> 2 risk factors according to age‐adapted IPI = international prognostic index) in the first complete remission; Hodgkin's lymphoma in the first recurrence; recurrence of follicular lymphoma; primary therapy of a coat‐cell lymphoma (MCL) in stage II‐IV; due to receive BEAM chemotherapy followed by autologous PBSCT; Karnofsky performance score more than 60%; life expectancy more than 3 months Exclusion criteria: previous therapy using palifermin; severe concomitant diseases with organ failure; pregnancy, lactation, positive pregnancy test; hypersensitivity to 1 of the trial drugs; severe psychiatric illness; HIV disease or immunologic deficiency; known central nervous system involvement Cancer type: haematologic: diffuse large‐cell lymphoma (Group A: 33%; Group B: 42%); B‐cell type acute lymphocytic leukaemia (Group A: 10%; Group B: 6%); T‐cell non‐Hodgkin's lymphoma (Group A: 13%; Group B: 11%); follicular/mantle cell lymphoma (Group A: 27%; Group B: 28%); Hodgkin's lymphoma (Group A: 17%; Group B: 14%) Cancer treatment: prior to receiving autologous PBSCT on day 0, participants received BEAM conditioning regimen from day ‐8 to ‐2: carmustine (BCNU) 300 mg/m²; etoposide 800 mg/m²; cytosine arabinoside 1600 mg/m²; melphalan 140 mg/m² Age at baseline (years): (ITT population) Group A: median 50 (range 22 to 71); Group B: median 55 (range 22 to 73) Gender: (ITT population) Group A: 57% male; Group B: 61% male Number randomised: 73 (Group A: 37; Group B: 36) Number evaluated: ITT: 66 (Group A: 30; Group B: 36); PP: 54 (Group A: 22; Group B: 32) |
|
| Interventions |
Comparison: KGF (palifermin) plus best supportive care versus best supportive care alone Group A: KGF (60 µg/kg) by IV daily for 3 days (days ‐10, ‐9, ‐8) prior to conditioning regimen and autologous PBSCT and then for 3 days after (days 0, 1, 2) (total dose = 360 µg/kg) Group B: best supportive care ("effective oral hygiene like teeth brushing, oral rinsing") beginning on day ‐8 (the day of hospital admission for BEAM conditioning) Compliance: Group A: 7/37 withdrew before therapy started, 3/37 had a different conditioning regimen to that specified in the study protocol (unclear if they still received KGF), 5/37 either had no KGF or did not receive all doses; Group B: 4/36 had a different conditioning regimen to that specified in the study protocol (unclear if they still received control intervention) Duration of treatment: 6 treatment days (over 13 days) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: 76 participants required to detect 30% difference in the rate of severe mucositis at 80% power and 5% significance Funding: Amgen (pharmaceutical industry) Declarations/conflicts of interest: not reported Data handling by review authors: data for ITT population used Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly assigned" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The randomization result was known to the patient as well as to the practitioners before the start of therapy" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The randomization result was known to the patient as well as to the practitioners before the start of therapy" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall attrition was 10% (Group A: 19%; Group B: 0%) for the ITT population. All 7 participants died before therapy started. Although this reason is not related to the outcomes, the balance created by randomisation may have been lost |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Freytes 2004.
| Methods |
Trial design: parallel (3 arms) Location: USA Number of centres: 8 Study duration: not reported Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: aged 18 years or older; due to receive autologous HSCT with a conditioning regimen with a high propensity for producing mucositis (typically 50% incidence of NCI‐CTC grade 3 or 4 when receiving standard mucositis management); Karnofsky performance score of 70%; free of acute or significant chronic dental or periodontal disease at baseline examination Exclusion criteria: previous HSCT; visible oral ulcerations at screening; pregnant or breastfeeding; childbearing potential or not using adequate contraception; history of allergy to Escherichia coli‐derived products; posterior subcapsular cataract identified at screening; history of thyroid disease prior to receiving chemotherapy (except for hypothyroidism adequately controlled with replacement therapy); history or clinical evidence of active significant acute or chronic diseases that may affect evaluation or interpretation of the effects of the study medication on mucositis; following medications: interleukin 11, topical steroids, sucralfate, hydrogen peroxide, pilocarpine, misoprostol, oral chlorhexidine rinses, or any agent that would affect the assessment of changes in the appearance of mucositis during the study Cancer type: lymphoma (Group A: 64%; Group B: 71%; Group C: 57%); other haematologic malignancy (Group A: 36%; Group B: 29%; Group C: 43%) Cancer treatment: prior to receiving autologous HSCT, participants received the following conditioning regimens:
Age at baseline (years): Group A: mean 54 (SD 10); Group B: mean 47 (SD 10); Group C: mean 51 (SD 15) Gender: Group A: 79% male; Group B: 64% male; Group C: 79% male Number randomised: 42 (Group A: 14; Group B: 14; Group C: 14) Number evaluated: 42 (Group A: 14; Group B: 14; Group C: 14) |
|
| Interventions |
Comparison: KGF‐2 (repifermin) versus placebo Group A: KGF‐2 (25 µg/kg) by IV daily for 3 days prior to conditioning regimen and autologous HSCT and then for 10 days after (total dose = 325 µg/kg) Group B: KGF‐2 (50 µg/kg) as above (total dose = 650 µg/kg) Group C: placebo as above Compliance: not reported Duration of treatment: 13 treatment days (over a longer period dependent on conditioning regimen) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported Data handling by review authors: we combined the 2 KGF‐2 groups to make a single pairwise comparison against placebo and we also made a comparison of the 2 different KGF‐2 dosages against each other Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from correspondence with authors: "The method to implement the random allocation was by central telephone" Comment: centralised randomisation method ‐ likely to be done properly |
| Allocation concealment (selection bias) | Low risk | Quote from correspondence with authors: "The method to implement the random allocation was by central telephone" Comment: centralised randomisation method ‐ likely to be done properly |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blinded, placebo‐controlled" Comment: the use of a placebo and identical schedule of treatment for all 3 arms should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blinded, placebo‐controlled" Comment: it is not clear who was blinded. There are subjective elements to the assessment of oral mucositis using the NCI‐CTC scale, requiring the patient's assessment of pain/soreness and their ability to swallow but, as the participants were unaware of their group allocation, the assessment of oral mucositis can be considered to be blinded. This would be the same for other subjective outcomes. The objective outcomes are unlikely to be affected by any potential lack of blinding of the outcome assessor(s) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Gholizadeh 2016.
| Methods |
Trial design: parallel (2 arms) Location: Iran Number of centres: not reported Study duration: not reported Trials registry number: IRCT2013021812510N1 (mentioned in trial report) |
|
| Participants |
Inclusion criteria: previously untreated acute lymphoblastic leukaemia patients; aged between 5 and 18 years Exclusion criteria: any other systemic disease; presence of oral mucositis or other oral lesions prior to chemotherapy; history of dermatology or respiratory hypersensitivity; acute lymphoblastic leukaemia recurrence Cancer type: acute lymphoblastic leukaemia (ALL) Cancer treatment: induction chemotherapy protocol consisted of standard risk B‐precursor ALL (COG)/dexamethasone, vincristine, L‐asparaginase, intrathecal (methotrexate + ara‐C + hydrocortisone). The intensification protocol was dexamethasone, vincristine, L‐asparaginase/ dexamethasone, cyclophosphamide/6‐thioguanine + cytarabine + intrathecal methotrexate Age at baseline (years): Group A: mean 8.8 (SD 2.5); Group B: mean 8.4 (SD 2.2); overall range: 5 to 18 Gender: Group A: 49% male; Group B: 49% male Number randomised: 90 (Group A: 45; Group B: 45) Number evaluated: 90 (Group A: 45; Group B: 45) |
|
| Interventions |
Comparison: KGF (palifermin) versus chlorhexidine Group A: KGF (60 µg/kg) by IV bolus daily for 3 days prior to chemotherapy regimen and then for 3 days after (total dose = 360 µg/kg) Group B: chlorhexidine (concentration not reported) mouthwash used for 1 minute once daily for 3 days prior to chemotherapy regimen and then for 3 days after Compliance: not reported Duration of treatment: 6 treatment days (over an unspecified longer period) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: "There is no conflict of interest in relation to this study" Data handling by review authors: we report the data at 2 weeks as they represent the maximum oral mucositis score experienced better than those at 1 week Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly assigned to the palifermin or control group by using the table of random numbers" Comment: adequate method used |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to the palifermin or control group by using the table of random numbers" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comparison with chlorhexidine so blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each patient was evaluated for oral lesions one and two weeks after the chemotherapy completion by the same specialist who was blind to the type of treatment" and "This limited use of chlorhexidine was to prevent the adverse effects like tooth discoloration and temporally taste changes" Comment: grade 1 on this scale would require the unblinded participant's assessment of soreness but other aspects of the scale are more objective and were assessed by a blinded assessor. Also, blinding may not have been broken by staining/discolouration due to limited use of chlorhexidine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Henke 2011.
| Methods |
Trial design: parallel (2 arms) Location: Australia, Canada, and Europe (Austria, France, Germany, Italy, Spain, UK) Number of centres: 38 Study duration: recruitment from January 2005 to August 2007 Trials registry number: NCT00131638; 2004‐002016‐28 (EudraCT number) |
|
| Participants |
Inclusion criteria: more than 18 years old; resected for pathohistologically documented high‐risk stage 2 to 4B SCC of the oral cavity, oropharynx, hypopharynx, or larynx; ECOG score of 0 to 2; at least 2 of 9 areas of the oral or oropharyngeal mucosa due to receive at least 50 Gy RT Exclusion criteria: tumours of the lips, paranasal sinuses, salivary glands, or unknown primary site; metastatic disease; history of chronic pancreatitis or acute pancreatitis within the last year; prior RT to the head and neck region or prior chemotherapy; previous treatment on this study or with other KGFs Cancer type: head and neck: oropharynx (Group A: 47%; Group B: 48%); oral cavity (Group A: 32%; Group B: 27%); larynx (Group A: 11%; Group B: 15%); hypopharynx (Group A: 10%; Group B: 10%); other (Group A: 1%; Group B: 1%) Cancer treatment: after R0 or R1 resection:
Age at baseline (years): Group A: mean 56 (SD 8); Group B: mean 57 (SD 9) Gender: Group A: 85% male; Group B: 80% male Number randomised: 186 (Group A: 92; Group B: 94) Number evaluated: 186 (Group A: 92; Group B: 94) |
|
| Interventions |
Comparison: KGF (palifermin) versus placebo Group A: KGF (120 µg/kg) 3 days prior to start of, and then once per week during radiochemotherapy, i.e. 7 doses for those with R0 resection, 8 doses for those with R1 resection (total dose = 840 µg/kg or 960 µg/kg respectively) Group B: same schedule with placebo Mode of administration not described but presumably IV as in other KGF studies Compliance: 78% of participants in KGF group completed all planned doses compared to 86% in placebo group Duration of treatment: 7 or 8 treatment days (over 7 or 8 weeks), depending on R0 or R1 resection respectively |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: assuming 60% of placebo group would develop grade 3 to 4 mucositis, 90 per group required to detect a reduction of at least 25% at 90% power and 5% significance Funding: "This study was supported by Amgen" (Amgen also named as sponsor on trials registry ‐ pharmaceutical industry) Declarations/conflicts of interest: some authors had both employment or leadership positions and stock ownership within Amgen Data handling by review authors: N/A Other information of note: study originally randomised participants to 3 arms (180 µg/kg once per week for 7 weeks, 180 µg/kg once per week for 4 weeks followed by placebo for the next 3 doses, or placebo throughout) but, after 1 serious adverse event of respiratory insufficiency reported in 1 of the first 10 participants, the data monitoring committee decided to restart the study using 120 µg/kg doses, excluding the 17 randomised participants from the efficacy assessments. The arm with KGF for 4 weeks followed by placebo was stopped due to slow recruitment, after enrolment of 38 participants, and the results analysed in a separate appendix |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment was made by a centralized interactive voice response system" Comment: large multicentre trial using high‐tech randomisation method ‐ likely to be done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "Random assignment was made by a centralized interactive voice response system" Comment: large multicentre trial using high‐tech randomisation method ‐ likely to be done properly |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, randomized, placebo‐controlled" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, randomized, placebo‐controlled" Comment: it is not clear who was blinded. There are subjective elements to the assessment of lower grades of oral mucositis using the WHO scale, requiring the patient's assessment of pain/soreness and their ability to swallow. Higher grades have more objective elements so may not be affected by potential lack of blinding of the assessor. This would be the same for other subjective and objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Hosseinjani 2017.
| Methods |
Trial design: parallel (2 arms) Location: Tehran University of Medical Sciences, Tehran, Iran Number of centres: 1 Study duration: February 2014 to March 2015 Trials registry number: IRCT2015042518842N8 (mentioned in trial report) |
|
| Participants |
Inclusion criteria: aged 18 years or older with non‐Hodgkin's lymphoma, Hodgkin's disease or multiple myeloma; due to receive autologous HSCT; adequate cardiac, pulmonary, renal and hepatic function Exclusion criteria: Karnofsky performance score less than 70%; participation in another study using an unlicensed product Cancer type: haematologic: non‐Hodgkin's lymphoma (Group A: 25%; Group B: 25%); Hodgkin's disease (Group A: 23%; Group B: 23%); multiple myeloma (Group A: 53%; Group B: 53%) Cancer treatment: prior to receiving autologous HSCT, participants received the following conditioning regimens:
Age at baseline (years): Group A: mean 43 (SD 14); Group B: mean 45 (SD 16) Gender: Group A: 55% male; Group B: 48% male Number randomised: 80 (Group A: 40; Group B: 40) Number evaluated: 80 (Group A: 40; Group B: 40) |
|
| Interventions |
Comparison: Erythropoietin (recombinant human) versus placebo Group A: 50 IU/mL erythropoietin mouthwash in aqueous vehicle (sodium benzoate, sodium citrate, citric acid, sodium hydroxide, sugar and distilled water) supplied in glass bottle stored at 4°C, 15 mL 4 times daily, starting from the first day of conditioning chemotherapy until 14 days after HSCT or until discharge from hospital (i.e. neutrophil recovery), whichever occurred first, oral intake not permitted for 1 hour following mouthwashing Group B: same schedule with placebo (aqueous vehicle‐only) All participants received oral hygiene care in addition to 20 drops of nystatin every 3 hours, mouthwashes containing 10 mL chlorhexidine 0.02% plus 10 mL diluted povidone iodine every 3 hours Compliance: "However, it was a limitation of our study that EPO mouthwash administration might be affected by patients' low compliance" (no data reported) Duration of treatment: variable and dependent on neutrophil recovery |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: 40 per group required assuming a 30% decrease in incidence of grade 2 to 4 mucositis at 5% significance and 80% power Funding: "There was no applicable funding source for the clinical trial" Declarations/conflicts of interest: "The authors have no conflict of interests to report" Data handling by review authors: there is a discrepancy in the incidence of grade 2 to 4 mucositis between Figure 2 and Table 2 (the latter has 1 extra event per group). However, this does not change the effect estimate. We have used the data in Table 2 as it reports numbers of participants along with percentages Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated...in a blocked randomization schedule" and "Both patient randomization and drug preparation were performed in the pharmaceutical laboratory of Pharmacy Department" Comment: method of random sequence generation not described but done by university hospital pharmacy and therefore probably done adequately |
| Allocation concealment (selection bias) | Low risk | Quote: "Both patient randomization and drug preparation were performed in the pharmaceutical laboratory of Pharmacy Department" Comment: not explicitly described but pharmacy‐controlled randomisation should have ensured concealment of the random sequence from those recruiting participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, randomized, placebo‐controlled" and "The study participants, the attending physician and the outcome assessor were all blind to the treatment assignment" and "There were no differences in colour, flavour, taste or container of the study drug and the placebo" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, randomized, placebo‐controlled" and "The study participants, the attending physician and the outcome assessor were all blind to the treatment assignment" Comment: all parties were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Jagasia 2012.
| Methods |
Trial design: parallel (2 arms) Location: USA (16 sites) and Australia (4 sites) Number of centres: 20 Study duration: December 2005 to November 2008 Trials registry number: NCT00189488 (mentions obsolete number in trial report: NCT00964899) |
|
| Participants |
Inclusion criteria: aged 18 years or older with haematologic malignancy (including myelodysplastic syndromes) and due to receive allogeneic SCT (marrow or PBPC) after a conditioning regimen; Karnofsky performance score of 70% or more; related donor or HLA‐matched unrelated donor identical at 6/6 HLA‐A, ‐B and ‐DRB1 loci (molecular typing of class I and class II for unrelated donors) Exclusion criteria: other malignancies; prior SCT; previous use of KGF; active infection or oral mucositis; congestive heart failure (NYHA class III or IV); use of a T‐cell depleted graft for GVHD prophylaxis; inadequate renal, liver or pulmonary function; pregnant or breastfeeding; refusal to use adequate contraception during study; participation in another investigational device or drug trial in previous 30 days Cancer type: haematologic: leukaemia (Group A: 71%; Group B: 79%); myelodysplastic syndrome (Group A: 16%; Group B: 12%); non‐Hodgkin's lymphoma (Group A: 12%; Group B: 8%); multiple myeloma (Group A: 0%; Group B: 1%); Hodgkin's disease (Group A: 1%; Group B: 0%) Cancer treatment: prior to receiving allogeneic SCT on day 0, participants received 1 of the following conditioning regimens from day ‐11 to ‐2:
Participants received methotrexate (with a calcineurin inhibitor ‐ either cyclosporine or tacrolimus) for GVHD prophylaxis on days 1, 3 and 6 (planned), and on day 11 (if toxicity allowed) at doses of 15 mg/m², 10 mg/m², 10 mg/m² and 10 mg/m² respectively Age at baseline (years): Group A: median 42 (range 18 to 62); Group B: median 44 (range 18 to 64) Gender: Group A: 52% male; Group B: 63% male Number randomised: 155 (Group A: 77; Group B: 78) Number evaluated: 155 (Group A: 77; Group B: 78) |
|
| Interventions |
Comparison: KGF (palifermin) versus placebo Group A: KGF (60 µg/kg) by IV bolus daily for 3 days prior to start of conditioning therapy, then a single 180 µg/kg dose after conditioning, but often 1 or 2 days before SCT (total dose = 360 µg/kg) Group B: same schedule with placebo Compliance: received at least 1 dose: Group A: 99%; Group B: 96%; received all doses: Group A: 92%; Group B: 88% Duration of treatment: 4 treatment days (over roughly 14 days) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: based on GVHD (not met due to early stopping) Funding: "This study was supported by research funding from Amgen Inc. Jonathan Latham of PharmaScribe, LLC received funding from Amgen Inc. to provide assistance with the preparation of the manuscript. Xuesong Guan of Amgen Inc. provided assistance with statistical analyses" (pharmaceutical industry) Declarations/conflicts of interest: some authors were employees and stockholders of Amgen and some received compensation from Amgen for consultation Data handling by review authors: N/A Other information of note: planned sample size was 200 participants but the study was stopped due to slow recruitment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned..." Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned..." Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" Comment: it is not clear who was blinded but grades 2 to 4 on the WHO scale are sufficiently objective and unlikely to be affected by any lack of blinding of the assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Katano 1995.
| Methods |
Trial design: parallel (2 arms) Location: Saga Medical School, Saga, Japan Number of centres: 1 Study duration: not reported Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: breast cancer patients Exclusion criteria: not reported Cancer type: breast: primary advanced (Group A: 2; Group B: 5); inflammatory (Group A: 4; Group B: 1); recurrent (Group A: 1; Group B: 1) Cancer treatment: preoperative IA high‐dose adriamycin (10 mg to 40 mg every 2 to 3 days to a total dose of 70 mg to 170 mg) Age at baseline (years): Group A: mean 53 (SD 11; range 38 to 69); Group B: mean 52 (SD 10; range 45 to 69) Gender: all female Number randomised: 14 (Group A: 7; Group B: 7) Number evaluated: 14 (Group A: 7; Group B: 7) |
|
| Interventions |
Comparison: G‐CSF versus no treatment Group A: G‐CSF (125 µg) by daily subcutaneous injection until leukocyte counts > 8000/mm³; timing in relation to chemotherapy not specifically reported, but the group was further divided into 2 subgroups where one (n = 4) received G‐CSF during/as an adjunct to the chemotherapy, and the other (n = 3) received G‐CSF afterwards (after the leukocyte counts were likely to drop below 2000/mm³) Group B: no treatment Compliance: not reported Duration of treatment: variable and dependent on leukocyte recovery (to > 8000/mm³) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: "G‐CSF (Neutrogin) was provided by Chugai Pharmaceutical" Declarations/conflicts of interest: not reported Data handling by review authors: the data for incidence of mucositis were presented in subgroups of those receiving G‐CSF during or after chemotherapy but we used the overall data in our meta‐analyses Other information of note: both cases of mucositis were in the subgroup who received G‐CSF after chemotherapy. Oral mucositis may have already begun to develop in this subgroup |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized into two groups" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomized into two groups" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comparison with no treatment so blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It would be possible to blind the outcome assessor, as the data we used were assessed by an examiner looking for erythema and ulcers. However, it was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Kim 2017.
| Methods |
Trial design: parallel (2 arms) Location: Seoul National University Hospital, Seoul, South Korea Number of centres: 1 Study duration: recruitment from March 2009 to August 2014 Trials registry number: NCT00845819 (mentioned in trial report) |
|
| Participants |
Inclusion criteria: aged 18 years or older with haematologic malignancy; due to receive intensive chemotherapy followed by autologous or allogeneic HSCT; normal oral cavity (grade 0 mucositis); ECOG score of 0 to 2 Exclusion criteria: received chemotherapy, radiotherapy or surgery within previous 3 weeks; history of allergy to the intervention or similar drugs; participation in other clinical trials within the previous 4 weeks with the potential to affect study results Cancer type: haematologic: multiple myeloma (Group A: 57%; Group B: 55%); lymphoma (Group A: 36%; Group B: 38%); other (Group A: 7%; Group B: 7%) Cancer treatment: prior to receiving autologous (Group A: 96%; Group B: 96%) or allogeneic HSCT, participants received the following conditioning regimens:
Age at baseline (years): Group A: median 53 (range 18 to 65); Group B: median 51 (range 19 to 65) Gender: Group A: 49% male; Group B: 54% male Number randomised: 138 (Group A: 69; Group B: 69) Number evaluated: 136 (Group A: 67; Group B: 69) |
|
| Interventions |
Comparison: EGF (recombinant human) versus placebo Group A: EGF (50 µg/mL) daily by oral spray, applied twice daily, sprayed (6 sprays per application) over the entire oral mucosa and then swallowed, no oral intake for 30 minutes afterwards; starting on first day of conditioning therapy and continuing until absolute neutrophil count recovered more than 1000 µL for 3 days and mucositis had resolved Group B: placebo as above Compliance: median patient compliance rate: Group A: 93% (range 35% to 100%); Group B: 92% (range 18% to 100%) Duration of treatment: variable and dependent on neutrophil recovery/resolution of mucositis |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: 62 participants per group required to detect 27% difference in incidence of grade 2 to 4 mucositis with 80% power and 5% significance Funding: multiple government grants; Daewoong Pharmaceutical Company (Seoul, Korea) only supplied interventions but provided no further funding and had no involvement with data collection, analysis or manuscript writing Declarations/conflicts of interest: none apparent Data handling by review authors: N/A Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned...using a computer‐generated randomization protocol, by the Medical Research Collaborating Center, Seoul National University Hospital" Comment: adequate method used |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly assigned...using a computer‐generated randomization protocol, by the Medical Research Collaborating Center, Seoul National University Hospital" Comment: although concealment not explicitly mentioned, use of centralised/third party randomisation ‐ likely to be done properly |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo‐controlled, double‐blind" and "clinicians, patients, and investigators responsible for assessing outcomes and analyzing data were masked to treatment assignments" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "placebo‐controlled, double‐blind" and "clinicians, patients, and investigators responsible for assessing outcomes and analyzing data were masked to treatment assignments" Comment: all parties were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 of 138 randomised participants were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | Although most of the data were not usable in this review, this does not seem to be due to selective reporting |
| Other bias | Low risk | No other sources of bias are apparent |
Le 2011.
| Methods |
Trial design: parallel (2 arms) Location: Canada, USA and Europe (Hungary, Poland, Austria, Germany, Italy, Czech Republic) Number of centres: 46 Study duration: recruitment started August 2005, 4‐month follow‐up finished September 2007 Trials registry number: NCT00101582; 2005‐000213‐35 (EudraCT number) |
|
| Participants |
Inclusion criteria: newly diagnosed, unresected stage 3 to 4B SCC of the oral cavity, oropharynx, nasopharynx, hypopharynx, or larynx; no evidence of secondary malignancy; at least 2 of 9 areas of the oral or oropharyngeal mucosa due to receive more than 50 Gy RT Exclusion criteria: not reported Cancer type: head and neck: oropharynx (Group A: 59%; Group B: 54%); oral cavity (Group A: 5%; Group B: 10%); larynx (Group A: 17%; Group B: 10%); hypopharynx (Group A: 15%; Group B: 23%); nasopharynx (Group A: 4%; Group B: 3%) Cancer treatment:
Age at baseline (years): Group A: mean 56 (SD 9); Group B: mean 55 (SD 8) Gender: Group A: 84% male; Group B: 85% male Number randomised: 188 (Group A: 94; Group B: 94) Number evaluated: 188 (Group A: 94; Group B: 94) |
|
| Interventions |
Comparison: KGF (palifermin) versus placebo Group A: KGF (180 µg/kg) by IV bolus over 30 to 60 seconds, 3 days prior to start of, and then once per week during radiochemotherapy, i.e. 8 doses (total dose = 1440 µg/kg) Group B: same schedule with placebo Compliance: 93% (SD 19%) of planned KGF doses were administered compared to 96% (SD 14%) in placebo group Duration of treatment: 8 treatment days (over 8 weeks) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: assuming 60% of placebo group would develop grade 3 to 4 mucositis, 90 per group required to detect a reduction of at least 25% at 90% power and 5% significance Funding: "Supported by Amgen" (Swedish Orphan Biovitrum named as sponsor on trials registry, Amgen named as collaborator ‐ both pharmaceutical industry) Declarations/conflicts of interest: some authors had both employment or leadership positions and stock ownership within Amgen; some authors had received research funding from Amgen Data handling by review authors: N/A Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A centralized randomization system assigned patients to either palifermin or placebo in a 1:1 ratio" Comment: large multicentre trial using centralised randomisation method ‐ likely to be done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "A centralized randomization system assigned patients to either palifermin or placebo in a 1:1 ratio" Comment: large multicentre trial using centralised randomisation method ‐ likely to be done properly |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo‐controlled, double‐blind" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "placebo‐controlled, double‐blind" Comment: it is not clear who was blinded. There are subjective elements to the assessment of lower grades of oral mucositis using the WHO scale, requiring the patient's assessment of pain/soreness and their ability to swallow. Higher grades have more objective elements so may not be affected by potential lack of blinding of the assessor. This would be the same for other subjective and objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Linch 1993.
| Methods |
Trial design: parallel (5 arms) Location: UK Number of centres: 12 Study duration: recruitment from August 1989 to July 1990 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: adults due to receive BMT after conditioning Exclusion criteria: myeloid malignancies Cancer type: mixed haematologic and solid (not reported by group): Hodgkin's disease (29%); non‐Hodgkin's lymphoma (33%); multiple myeloma (Group A: 20%); ALL (15%); solid (3%) Cancer treatment: prior to receiving BMT (autologous 84%; allogeneic 16%), participants received a conditioning regimen which consisted of chemotherapy only (71%) or with TBI (29%) Age at baseline (years): median 36 (range 17 to 64) (not reported by group) Gender: 69% male (not reported by group) Number randomised: 121 (Group A: 96; Group B: 25); Group A represents 4 arms with different dosages Number evaluated: 121 (Group A: 96; Group B: 25) |
|
| Interventions |
Comparison: G‐CSF versus placebo Group A: G‐CSF (2 µg/kg, 5 µg/kg, 10 µg/kg or 15 µg/kg) by 30‐minute IV daily starting from the day after BMT transplant and continuing until neutrophil count was > 1.0 x 10⁹/L for 3 consecutive days or until day 28, whichever occurred first Group B: as above but with placebo Compliance: not reported Duration of treatment: variable and dependent on neutrophil recovery |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: "Financial support for this trial was provided by Chugai Rhone Poulene" (pharmaceutical industry) Declarations/conflicts of interest: 1 author was employed by the funders Data handling by review authors: oral mucositis reported narratively in additional table Other information of note: G‐CSF administration only began after the conditioning and BMT transplant were completed, by which point oral mucositis may have already begun to develop (although time scale of conditioning/BMT transplant not reported) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised...in blocks of five by a computer‐generated randomisation schedule" Comment: adequate method used |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomised...in blocks of five by a computer‐generated randomisation schedule" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "single blind...vehicle‐controlled" Comment: the use of a placebo (the 'vehicle') should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "single blind...vehicle‐controlled" Comment: the quote implies that outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | High risk | Oral mucositis not mentioned in the methods section as 1 of the study end points. It is only mentioned in the results narratively as there being no difference between any group, but with no data or P value |
| Other bias | Low risk | No other sources of bias are apparent |
Lucchese 2016a.
| Methods |
Trial design: parallel (2 arms) Location: Vita‐Salute San Raffaele University Hospital, Milan, Italy Number of centres: 1 Study duration: conducted from April 2009 to January 2015 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: children aged 7 years or older with B‐cell lineage acute lymphoblastic leukaemia; scheduled to receive autologous HSCT after a conditioning regimen; Karnofsky performance score of 70 or more; were to have at least 1.5 x 10⁶ CD34+ cells reinfused per kilogram available for transplant; adequate cardiac, pulmonary, renal and hepatic function Exclusion criteria: not reported Cancer type: B‐cell lineage acute lymphoblastic leukaemia Cancer treatment: prior to receiving autologous HSCT (on day 0), participants received a conditioning regimen which consisted of TBI delivered in 8 fractions over 3 days (‐3 to ‐1) with at least 6 hours between fractions, followed by chemotherapy on day ‐1 (type and dose of radiotherapy and chemotherapy not reported) Age at baseline (years): Group A: median 11 (range 7 to 16); Group B: median 11 (range 7 to 16) Gender: Group A: 52% male; Group B: 44% male Number randomised: 60 (Group A: 30; Group B: 30) Number evaluated: 54 (Group A: 27; Group B: 27) |
|
| Interventions |
Comparison: KGF (palifermin) versus placebo Group A: KGF (60 µg/kg) by IV, on days ‐6 (3 days prior to start of conditioning regimen), ‐5 and ‐4, and on days 0 (the day of HSCT), 1, and 2 after transplant (total dose = 360 µg/kg) Group B: same schedule with placebo Compliance: not reported Duration of treatment: 6 treatment days (over 9 days) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: numbers required not reported but was estimated at 80% power and 5% significance Funding: "This work was performed with Departmental funding only" Declarations/conflicts of interest: the authors report that they have no conflict of interest (supplemental material on journal website) Data handling by review authors: we emailed the lead author June 2017 for clarification of the oral mucositis data but, until we receive a response, we are unable to use those data Other information of note: unclear definitions of ulcerative (should be grade 2 to 4) and severe (should be grade 3 to 4): "...ulcerative OM (WHO grades 3 and 4), incidence and duration of severe OM (WHO grades 3 and 4)..." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A centralized randomization system assigned patients either to palifermin or conventional treatment in a 1:1 ratio......The statistician gave randomization list to the pharmacy, so the patient and the clinical research team (who assessed outcomes) were blinded to the study treatment" Comment: randomisation 'system' used and done by a statistician, likely to be done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "A centralized randomization system assigned patients either to palifermin or conventional treatment in a 1:1 ratio......The statistician gave randomization list to the pharmacy, so the patient and the clinical research team (who assessed outcomes) were blinded to the study treatment" Comment: centralised randomisation with pharmacy assigning participants to groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" and "The statistician gave randomization list to the pharmacy, so the patient and the clinical research team (who assessed outcomes) were blinded to the study treatment. The pharmacy provided the research team with the blinded study medication" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The statistician gave randomization list to the pharmacy, so the patient and the clinical research team (who assessed outcomes) were blinded to the study treatment. The pharmacy provided the research team with the blinded study medication" Comment: outcome assessors were clearly blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was same in both groups (10%) with the same reason given |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Lucchese 2016b.
| Methods |
Trial design: parallel (2 arms) Location: Vita‐Salute San Raffaele University Hospital, Milan, Italy Number of centres: 1 Study duration: conducted from April 2010 to January 2014 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: children aged 9 to 15 years with B‐cell lineage acute lymphoblastic leukaemia; scheduled to receive allogeneic HSCT after a conditioning regimen Exclusion criteria: not reported Cancer type: B‐cell lineage acute lymphoblastic leukaemia Cancer treatment: prior to receiving allogeneic HSCT, participants received a conditioning regimen of a total of 12 Gy TBI delivered in 8 fractions of 1.5 Gy twice daily over 4 days Age at baseline (years): Group A: median 12 (range 8 to 15); Group B: median 12 (range 8 to 15) Gender: Group A: 50% male; Group B: 55% male Number randomised: 46 (Group A: 24; Group B: 22) Number evaluated: 46 (Group A: 24; Group B: 22) |
|
| Interventions |
Comparison: KGF (palifermin) versus placebo Group A: KGF (60 µg/kg) by IV, for 3 days prior to start of conditioning regimen and for 3 days after completion (total dose = 360 µg/kg) Group B: same schedule with placebo Compliance: not reported Duration of treatment: 6 treatment days (over unclear period of time ‐ not fully described) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: "This work has been performed with departmental funding only" Declarations/conflicts of interest: "The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript" Data handling by review authors: we emailed the lead author June 2017 for clarification of the oral mucositis data but, until we receive a response, we are unable to use those data Other information of note: "Patients who had severe (grade 3 or 4) OM during blinded cycles received open‐label palifermin at the same dosages as in the other group. The research team assessed patients for OM at baseline before the cycle; at days 1, 2, 3 and at the end of the transplant cycle." ‐ nowhere else in the report suggests that there were multiple cycles of treatment; unclear definitions of ulcerative mucositis i.e. described correctly in one section as grades 2 to 4, but described in another section as grades 1 to 4 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "No stratification was performed and two distinct computer‐generated a randomization lists. The statistician gave both randomization lists to the pharmacy, so the patient and the clinical research team (who assessed outcomes) were blinded to the study treatment" Comment: adequate method done by a statistician (although it is unclear why there were 2 lists) |
| Allocation concealment (selection bias) | Low risk | Quote: "No stratification was performed and two distinct computer‐generated a randomization lists. The statistician gave both randomization lists to the pharmacy, so the patient and the clinical research team (who assessed outcomes) were blinded to the study treatment" Comment: randomisation 'system' used and done by a statistician, likely to be done properly |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" and "The statistician gave both randomization lists to the pharmacy, so the patient and the clinical research team (who assessed outcomes) were blinded to the study treatment. The pharmacy provided the research team with the blinded study medication" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The statistician gave both randomization lists to the pharmacy, so the patient and the clinical research team (who assessed outcomes) were blinded to the study treatment. The pharmacy provided the research team with the blinded study medication" Comment: outcome assessors were clearly blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Makkonen 2000.
| Methods |
Trial design: parallel (2 arms) Location: Turku University Central Hospital and Helsinki University Central Hospital, Finland Number of centres: 2 Study duration: recruitment from November 1994 to August 1996 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: scheduled to receive total target dose of at least 56 Gy to the oropharyngeal mucosa Exclusion criteria: prior chemotherapy or radiotherapy; concurrent use of anticholinergic drugs; autoimmune thrombocytopenic purpura; WHO performance status higher than 2 Cancer type: head and neck: mobile tongue (Group A: 30%; Group B: 20%); oral cavity, other (Group A: 40%; Group B: 25%); oropharynx (Group A: 20%; Group B: 15%); nasopharynx (Group A: 5%; Group B: 10%); supraglottic larynx (Group A: 5%; Group B: 15%); hypopharynx (Group A: 0%; Group B: 15%) Cancer treatment:
Total target dose: Group A: median 65 Gy (range 56 to 68); Group B: median 66 Gy (range 58 to 73); oral surgery prior to RT: Group A: 20%; Group B: 30%; oral surgery after radiotherapy: 23 (not reported by group) Age at baseline (years): Group A: mean 63 (SD 10; range 47 to 87); Group B: mean 59 (SD 13; range 33 to 79) Gender: Group A: 60% male; Group B: 55% male Number randomised: 40 (Group A: 20; Group B: 20) Number evaluated: unclear; results presented as percentages |
|
| Interventions |
Comparison: GM‐CSF (molgramostim) plus sucralfate versus sucralfate alone
Both groups rinsed mouth with sucralfate (1 g) suspension for 1 minute before swallowing, 6 times per day, starting after 1 week of RT and continued until the end of RT (including weekends and planned/unplanned treatment breaks). Both groups advised to rinse their mouths with saline solution in between the sucralfate rinses if required Group A: after cumulative radiation dose of 10 Gy, GM‐CSF (150 µg to 300 µg ‐ dependent on body weight) by daily subcutaneous injection until the last day of RT; not given over weekends or during planned/unplanned treatment breaks; mean total dose = 3398 µg (range 300 µg to 7200 µg) Group B: no other treatment Oral pain was treated with anti‐inflammatory analgesics and with local anaesthetic mouthwashes (lidocaine hydrochloride, Xylocain 0.5%) Compliance: not reported Duration of treatment: variable and dependent on duration of RT |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: GM‐CSF and sucralfate were both provided by pharmaceutical industry (Schering‐Plough Corporation and Orion‐Farmos Pharmaceuticals respectively) Declarations/conflicts of interest: not reported Data handling by review authors: no usable data Other information of note: GM‐CSF administration began after cumulative radiation dose of 10 Gy, by which point oral mucositis may have already begun to develop |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "assignment to the treatment groups was carried out via a phone call to the randomization center located at the Finnish Cancer Registry, Helsinki" Comment: use of a specialist randomisation centre |
| Allocation concealment (selection bias) | Low risk | Quote: "assignment to the treatment groups was carried out via a phone call to the randomization center located at the Finnish Cancer Registry, Helsinki" Comment: third party/remote randomisation would have ensured concealment of the random sequence from those recruiting participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible as no placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It would be possible to blind the outcome assessor for oral mucositis as the scale was fairly objective, but it was not mentioned. It would not be possible to blind oral pain assessment as this was done by the participant who was not blinded (however, we could not use the pain data so there was no bias for this outcome) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not clear how many participants were included in the analyses |
| Selective reporting (reporting bias) | High risk | Poor reporting of oral mucositis and oral pain |
| Other bias | Low risk | No other sources of bias are apparent |
McAleese 2006.
| Methods |
Trial design: parallel (2 arms) Location: Royal Marsden Hospital, London, UK Number of centres: 1 Study duration: recruitment from September 1997 to October 2000 Trials registry number: NCT00004256 |
|
| Participants |
Inclusion criteria: histologically proven T1 N0 or T2 N0 glottic carcinoma; due to receive RT with a 16‐fraction 3‐week regimen; WHO performance status 0 or 1 Exclusion criteria: renal or hepatic impairment; serious infections requiring antibiotics; taking corticosteroids or likely to require them; known allergy to GM‐CSF Cancer type: laryngeal Cancer treatment: accelerated radiotherapy: once‐daily fractions of 3.125 Gy, to a total dose of 50 Gy in 16 fractions over 21 days Age at baseline (years): Group A: median 60 (range 48 to 79); Group B: median 65 (range 32 to 70) Gender: Group A: 93% male; Group B: 86% male Number randomised: 29 (Group A: 15; Group B: 14) Number evaluated: 29 (Group A: 15; Group B: 14) |
|
| Interventions |
Comparison: GM‐CSF (molgramostim) versus no treatment Group A: GM‐CSF (150 µg) by daily subcutaneous injection, starting on day 14 of RT and continuing for 14 days i.e. for the last week of RT and for 1 week after completion of RT (total dose = 2100 µg) Group B: no treatment Compliance: 2 participants did not complete their course of GM‐CSF Duration of treatment: 14 days |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: 17 per group needed at 90% power and 5% significance to detect reduction from an anticipated 60% incidence of severe mucositis to 10% Funding: not reported Declarations/conflicts of interest: not reported Data handling by review authors: N/A Other information of note: imbalance in stage distribution, with more T2 patients in the GM‐CSF group. Consequently more of this group were treated with larger fields GM‐CSF administration only began after 14 days of radiotherapy, by which point oral mucositis may have already begun to develop |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomly assigned to the active or control arms" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "were randomly assigned to the active or control arms" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible as no placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "At each visit one of two independent observers, blinded to group allocation, scored mucositis..." Comment: a physician's objective version of the RTOG scale was used. Use of feeding tube is also an objective outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Analgesic use reported as "No differences" with no data, however mucositis fully reported |
| Other bias | Low risk | No other sources of bias are apparent |
Meropol 2003.
| Methods |
Trial design: parallel (7 arms) Location: USA Number of centres: 10 Study duration: not reported Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: 18 years or older; metastatic colon or rectal adenocarcinoma; scheduled to receive palliative 5FU and leucovorin chemotherapy; ECOG performance status 0 to 2 (ambulatory at least 50% of waking hours); life expectancy of at least 4 months; free of lesions and no recent history (within 30 days before baseline examination) of oral ulceration, herpes simplex, oral candidiasis, severe gingivitis, or the presence of active or chronic mucositis, xerostomia, or diarrhoea; absolute neutrophil count ≥ 1.5 x 109/L; platelet count ≥ 100 x 109/L; serum creatinine ≤ 2.0 mg/dL; serum bilirubin ≤ 2.0 mg/dL; serum aspartate amino transferase less than 5 times the upper limit of normal; absence of other serious concurrent medical illness Exclusion criteria: received chemotherapy, radiotherapy, or other investigational drugs within 4 weeks of enrolment (6 weeks for chemotherapy with mitomycin or nitrosoureas); any unresolved adverse event from previous therapy; major surgery within 2 weeks before study entry; history of insulin‐dependent diabetes mellitus; pregnant or breastfeeding; of child‐bearing potential and not using adequate contraceptive precautions; previous hypersensitivity reaction to leucovorin calcium or Escherichia coli‐derived material Cancer type: metastatic colorectal Cancer treatment: (palliative) leucovorin 20 mg/m² by rapid IV injection, followed immediately by 5FU 425 mg/m² by rapid IV injection for 5 consecutive days on days 4 to 8 of each 28‐day cycle Age at baseline (years): Group A: mean 62 (SD 10; range 44 to 84); Group B: mean 66 (SD 13; range 41 to 86) Gender: Group A: 57% male; Group B: 59% male Number randomised: 81 (Group A: 54; Group B: 27) Number evaluated: 81 (Group A: 54; Group B: 27) |
|
| Interventions |
Comparison: KGF (recombinant human) versus placebo Group A: KGF
Group B: placebo as above Compliance: 3 participants stopped KGF treatment due to adverse reactions involving the skin (1 in 60 and 2 in 80 µg/kg group) Duration of treatment: 3 days |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: "Supported by a grant from Amgen, Inc" (pharmaceutical industry) Declarations/conflicts of interest: not reported Data handling by review authors: we combined the 6 KGF groups to make a single pairwise comparison against placebo; in order to make a head‐to‐head comparison of doses we grouped the 3 lower doses (1 µg/kg, 10 µg/kg and 20 µg/kg) together and grouped the 3 higher doses (40 µg/kg, 60 µg/kg and 80 µg/kg) together to make pairwise groups for comparison Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This study was a multicenter, randomized, double‐blinded, placebo‐controlled, phase I study" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "This study was a multicenter, randomized, double‐blinded, placebo‐controlled, phase I study" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blinded, placebo‐controlled" Comment: the use of a placebo should have ensured that blinding was successful; incidence of adverse events does not appear to differ enough between groups to cause unblinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blinded, placebo‐controlled" Comment: it is not clear who was blinded but the outcome is grade 2 to 4 WHO‐scale mucositis and it is unlikely that this would be affected by any lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Nemunaitis 1995.
| Methods |
Trial design: parallel (2 arms) Location: USA and Canada Number of centres: 7 Study duration: 1 November 1990 to 1 July 1993 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: any age; undergoing allogeneic BMT (from HLA‐identical sibling) for haematological malignancy Exclusion criteria: due to receive methotrexate as part of GVHD prophylaxis; chronic lymphocytic leukaemia; chronic myelogenous leukaemia in blast crisis; acute lymphoblastic leukaemia or acute myelogenous leukaemia with failure at first induction or progressed beyond a second relapse; previously received cytokines; HIV; life expectancy less than 7 days Cancer type: haematologic malignancy: lymphoid malignancy (Group A: 21%; Group B: 16%); acute myeloid malignancy (Group A: 34%; Group B: 27%); chronic myeloid malignancy (Group A: 32%; Group B: 30%); multiple myeloma (Group A: 6%; Group B: 5%); myelodysplastic syndrome (Group A: 6%; Group B: 11%); aplastic anaemia (Group A: 2%; Group B: 11%) Cancer treatment: prior to receiving allogeneic BMT (from HLA‐identical sibling), participants received the following conditioning regimens: busulfan and cyclophosphamide with TBI (Group A: 9%; Group B: 16%) or without TBI (Group A: 32%; Group B: 34%); busulfan, cyclophosphamide, cytosine arabinoside, and methotrexate with TBI (Group A: 23%; Group B: 13%) or without TBI (Group A: 8%; Group B: 11%); cyclophosphamide and steroid with TBI (Group A: 0%; Group B: 2%); cyclophosphamide, cytosine arabinoside, and steroid with TBI (Group A: 23%; Group B: 18%); etoposide with TBI (Group A: 0%; Group B: 2%); etoposide, cytosine arabinoside, and cyclophosphamide with TBI (Group A: 2%; Group B: 0%); etoposide, cytosine arabinoside, cyclophosphamide, and asparaginase with TBI (Group A: 0%; Group B: 2%); etoposide, methotrexate, cytosine arabinoside, and steroid with TBI (Group A: 4%; Group B: 4%) All participants received cyclosporine and methylprednisolone for GVHD prophylaxis Age at baseline (years): Group A: mean 32; Group B: mean 34 Gender: Group A: 60% male; Group B: 54% male Number randomised: 109 (Group A: 53; Group B: 56) Number evaluated: 109 (Group A: 53; Group B: 56) |
|
| Interventions |
Comparison: GM‐CSF (yeast‐derived recombinant human) versus placebo Group A: GM‐CSF (250 µg/m²) by 4‐hour IV infusion starting on day 0 (the day of BMT) to day 20 (total dose = 5250 µg/m²) Group B: as above with placebo Compliance: Group A: 13 participants (25%) stopped their intervention early: 11 due to adverse events (1 rash, 7 bone pain, 2 acute dyspnoea, 1 seizure); 2 (with no apparent toxicity) due to participant or physician request; Group B: 9 participants (16%) stopped their intervention early: 6 due to adverse events (2 rash, 2 bone pain, 1 elevated liver enzymes, 1 infections); 2 (on day 19) due to miscalculation of the number of days to receive the intervention; 1 (on day 9) due to severe mucositis and veno‐occlusive disease of the liver Duration of treatment: 21 days |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: "..product provided by Immunex" (pharmaceutical). Also mention of "sponsoring company" which is likely to be Immunex Declarations/conflicts of interest: not reported but 4 authors employed by Immunex Data handling by review authors: N/A Other information of note: GM‐CSF administration only began after the conditioning regimen, by which point oral mucositis may have already begun to develop |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Assignment to treatment was made via a randomization schema prepared by Almedica Corporation" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "Blinded numbered vials containing placebo or rhGM‐CSF were provided to each participating centre. The pharmacists, principal investigators, patients, support care personnel and sponsoring company were blinded to the study medication for the entire course of the study" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled" and "The pharmacists, principal investigators, patients, support care personnel and sponsoring company were blinded to the study medication for the entire course of the study" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled" and "The pharmacists, principal investigators, patients, support care personnel and sponsoring company were blinded to the study medication for the entire course of the study" Comment: everyone involved in the study was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | 5 participants in the placebo group received cytokines off study during the first 42 days after BMT but unlikely to bias the results in a meaningful way |
Peterson 2009.
| Methods |
Trial design: parallel (3 arms) Location: Russia Number of centres: 9 Study duration: recruitment from August 2006 to May 2007 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: 18 years or older with colorectal cancer (stage I to IV); undergoing chemotherapy as primary cancer therapy; experienced WHO grade 2 or higher oral mucositis during first cycle of chemotherapy, but recovered prior to enrolment (i.e. grade 0); ECOG score of 0 to 2 Exclusion criteria: pregnant (or risk of pregnancy) or lactating; scheduled to receive RT to the head and neck; received other investigational drugs within 14 days of the start of the study; evidence of alcohol and drug abuse; pre‐existing conditions such as active fungal or herpetic infection Cancer type: colorectal (stage I to IV) Cancer treatment: all participants received 5FU (97% with leucovorin) but 1 received capecitabine; 6 participants (3 in low‐dose ITF, 3 in placebo) also received oxaliplatin; doses or regimens comparable between groups Age at baseline (years): Group A: median 62 ; Group B: median 56 ; Group C: median 59 Gender: Group A: 42% male; Group B: 33% male; Group C: 48% male Number randomised: 99 (Group A: 33; Group B: 33; Group C: 33) Number evaluated: 99 (Group A: 33; Group B: 33; Group C: 33) |
|
| Interventions |
Comparison: ITF (recombinant human) versus placebo Group A: ITF (10 mg/mL) oral spray, 300 µL (3 sprays) to the oral mucosa 8 times daily, starting on the first day (day 1) of the second chemotherapy cycle for total 14 days (total dose = 336 mg) Group B: ITF (80 mg/mL) oral spray as above (total dose = 2688 mg) Group C: placebo oral spray as above Compliance: patient‐reported compliance was 97% Duration of treatment: 14 days |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: based on previous study, 80% power to detect 40% difference in incidence of WHO grade 2 or above Funding: The GI Company (pharmaceutical) Declarations/conflicts of interest: some authors had roles with the funding pharmaceutical company (and other companies) both compensated and uncompensated Data handling by review authors: we combined the 2 ITF groups to make a single pairwise comparison against placebo; we also made a separate comparison of the 2 ITF dosages Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomly assigned" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" Comment: it is not clear who was blinded. There are subjective elements to the assessment of oral mucositis using this scale, requiring the patient's assessment of pain/soreness and their ability to swallow but, as the participants were unaware of their group allocation, the assessment of oral mucositis can be considered to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Rosen 2006.
| Methods |
Trial design: parallel (2 arms) Location: USA and Australia Number of centres: 15 Study duration: not reported Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: 18 years or older with advanced (Duke's D) colon or rectal adenocarcinoma; scheduled to receive bolus 5FU and low‐dose leucovorin (Mayo regimen) as first‐line or subsequent therapy; normal oral cavity examination (no ulceration, herpes simplex, candidiasis, or severe gingivitis); ECOG score of 0 to 2; life expectancy of 4 months or more; absolute neutrophil count of 1.5 x 10⁹/L or higher; platelet count of 100 x 10⁹/L or higher; normal renal and hepatic function Exclusion criteria: previous radiotherapy or chemotherapy within 4 weeks or major surgery within 2 weeks of study day 1; insulin‐dependant diabetes; known allergy to leucovorin; known hypersensitivity to Escherichia coli‐derived material Cancer type: advanced colorectal (Duke's D) Cancer treatment: low‐dose leucovorin (20 mg/m²) by IV immediately followed by 5FU (425 mg/m²) by rapid IV once daily for 5 consecutive days on days 4 to 8 of each 28‐day cycle; 2 cycles (5FU dose could be decreased by 20% during cycle 2 if moderately severe toxicities occurred in cycle 1) Age at baseline (years): Group A: mean 65 (SD 11.2); Group B: mean 65 (SD 11.1) Gender: Group A: 57% male; Group B: 72% male Number randomised: 65 (not reported by group) Number evaluated: 64 (Group A: 28; Group B: 36) |
|
| Interventions |
Comparison: KGF (palifermin) versus placebo Group A: KGF (40 µg/kg) by IV for 3 consecutive days (days 1 to 3 of each 28‐day cycle) before the start of chemotherapy (total dose = 120 µg/kg) Group B: as above with placebo Compliance: not reported (only reports "palifermin was generally well tolerated") Duration of treatment: 3 days |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: "Supported by Amgen Inc" (pharmaceutical industry) Declarations/conflicts of interest: all authors were linked to the funding pharmaceutical company in terms of employment, consultancy, stock ownership, honoraria, and receipt of research funding Data handling by review authors: data were reported separately for the chemotherapy cycles 1 and 2 ‐ we used the data for cycle 1 Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned (by center and prior chemotherapy)" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned (by center and prior chemotherapy)" Comment: insufficient information to determine method of random sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" Comment: it is not clear who was blinded. There are subjective elements to the assessment of oral mucositis using this scale, requiring the patient's assessment of pain/soreness and their ability to swallow but, as the participants were unaware of their group allocation, the assessment of oral mucositis can be considered to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 randomised participant was not included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Saarilahti 2002.
| Methods |
Trial design: parallel (2 arms) Location: Helsinki University Central Hospital, Finland Number of centres: 1 Study duration: recruitment from October 1999 to April 2001 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: undergone radical surgery for head and neck cancer and scheduled to receive postoperative RT to a total dose of 50 Gy or more to the oral and oropharyngeal mucosa Exclusion criteria: prior chemotherapy or RT; chronic autoimmune or inflammatory disease; WHO performance score > 2 Cancer type: head and neck: mobile tongue (Group A: 33%; Group B: 37%); floor of mouth (Group A: 19%; Group B: 16%); tonsil (Group A: 29%; Group B: 32%); oral cavity other (Group A: 19%; Group B: 16%) Cancer treatment: mean time from radical surgery to start of RT: Group A: 39 days (range 20 to 73); Group B: 38 days (range 20 to 56); conventionally fractionated RT to a total dose of 50 Gy to 60 Gy in 2‐Gy daily fractions, 5 times weekly (on weekdays) for 5 to 6 weeks, to the primary site and locoregional lymph nodes Age at baseline (years): Group A: median 56 (range 43 to 87); Group B: median 60 (range 24 to 72) Gender: Group A: 57% male; Group B: 79% male Number randomised: 40 (Group A: 21; Group B: 19) Number evaluated: 40 (Group A: 21; Group B: 19) |
|
| Interventions |
Comparison: GM‐CSF versus sucralfate Group A: starting after a cumulative RT dose of 10 Gy (after 1 week of RT); mouth was cleaned with water, then GM‐CSF (37.5 µg per 25 mL rinse) mouthwash rinsed around the mouth for 3 minutes then swallowed, 4 times daily after meals, on RT‐days (weekdays), until the end of RT (total dose dependent on duration of RT) Group B: as above but with sucralfate (1 g per 25 mL rinse) (total dose dependent on duration of RT) Compliance: not reported (only reports "both mouthwashes were well tolerated, and none of the patients reported any adverse effects related to the mouthwashes") Duration of treatment: 4 to 5 weeks (dependent on duration of RT) |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported Data handling by review authors: we used oral mucositis incidence (maximum score experienced per patient) data provided by the authors; all participants experienced mucositis (grade 1 or above) as they had already received a week of RT before the intervention started, therefore we felt an analysis of incidence of any grade of mucositis should not be included Other information of note: GM‐CSF/sucralfate administration only began after 1 week of RT, by which point oral mucositis may have already begun to develop |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using computer‐generated random digits" Comment: adequate method used |
| Allocation concealment (selection bias) | Low risk | Quote: "they were assigned to a treatment group by way of a telephone call to the randomization office" Comment: third party/remote randomisation would have ensured concealment of the random sequence from those recruiting participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "Both solutions looked alike, and neither the investigators nor the patients were aware of the contents of the solutions given. The drug vials were marked with a study code that prevented identification of the allocation group" Comment: adequate methods to ensure blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blinded, placebo‐controlled" Comment: nobody involved in the study was aware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Schneider 1999.
| Methods |
Trial design: parallel (2 arms) Location: USA Number of centres: not reported Study duration: recruitment from January 1995 to April 1996 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: 18 years or older with histologically proven head and neck malignancy; mucosa of the oropharynx and/or oral cavity to be included in RT portal; Karnofsky performance score of 60 or more; no known hypersensitivity to E coli‐derived proteins; haemoglobin and platelet counts 100 x 10⁹/L or higher; absolute neutrophil count higher than 1.5 x 10⁹/L; normal blood pressure; agreement to bring dentition to acceptable level prior to RT (teeth with periapical abscesses or severe periodontal disease extracted, followed by a 10‐day healing period allowance) Exclusion criteria: lactating/pregnant/females not taking effective form of contraception; scheduled to receive chemotherapy or radiosensitising drugs during the planned RT; history of myeloid malignancy; underlying collagen vascular disease; active rheumatoid arthritis Cancer type: head and neck: nasopharynx (Group A: 0; Group B: 1); oropharynx (Group A: 2; Group B: 1); tongue (Group A: 2; Group B: 1); larynx (Group A: 2; Group B: 1); other/unknown (Group A: 2; Group B: 2) Cancer treatment: radiotherapy in 1.8 Gy to 2 Gy standard daily fractions from Monday to Friday, to total dose 50 Gy or more Age at baseline (years): not reported Gender: Group A: 100% male; Group B: 83% male Number randomised: 14 (Group A: 8; Group B: 6) Number evaluated: 14 (Group A: 8; Group B: 6) |
|
| Interventions |
Comparison: G‐CSF (r‐metHuG‐CSF) (filgrastim) versus placebo Group A: G‐CSF (starting at 3 µg/kg and titrated to keep neutrophil count between 10 x 10⁹/L and 30 x 10⁹/L) by daily subcutaneous injection, starting on the first day of RT (weekdays), until the end of RT (total dose dependent on duration of RT and neutrophil counts) Group B: placebo as above Compliance: not reported (only reports "Filgrastim was well tolerated") Duration of treatment: variable and dependent on duration of RT |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: 54 required to detect 30% decrease in incidence of grade 2 or 3 mucositis at 80% power at the 5% significance level Funding: "Financial support was provided through a grant from Amgen Inc" (pharmaceutical industry) Declarations/conflicts of interest: not reported Data handling by review authors: interruptions to cancer treatment outcome only reported narratively Other information of note: study was stopped after an interim analysis. Authors state that "owing to administrative obstacles, completion of the trial is not possible", rather than due to previously stated early stopping rules |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomized equally between the two treatment groups" and "The study material and randomization list were held by the UCLA Pharmacy for the duration of the study" Comment: method of random sequence generation not described but done by UCLA Pharmacy and therefore probably done adequately |
| Allocation concealment (selection bias) | Low risk | Quote: "Amgen Inc...prepared and packaged all drug and placebo in identical containers, with the only designator being the randomization number. The study material and randomization list were held by the UCLA Pharmacy for the duration of the study" Comment: pharmacy‐controlled randomisation would have ensured concealment of the random sequence from those recruiting participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" and "Amgen Inc...prepared and packaged all drug and placebo in identical containers, with the only designator being the randomization number" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" and "A single examiner...who was blinded to the patients' randomization status...scored mucositis on a weekly basis" Comment: both participants and outcome assessor were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | Although the study reports an interim analysis only, the study was stopped due to administrative reasons and is unlikely to introduce bias |
Spielberger 2004.
| Methods |
Trial design: parallel (2 arms) Location: USA Number of centres: 13 Study duration: recruitment from March 2001 to October 2002 Trials registry number: NCT00041665 |
|
| Participants |
Inclusion criteria: 18 years or older; Karnofsky performance score of 70 or more; scheduled to undergo autologous HSCT after conditioning regimen of fractionated TBI plus etoposide and cyclophosphamide for haematological cancers; adequate cardiac, pulmonary, renal, and hepatic function Exclusion criteria: not reported Cancer type: haematologic: NHL (Group A: 68%; Group B: 65%); Hodgkins (Group A: 20%; Group B: 22%); multiple myeloma (Group A: 10%; Group B: 8%); leukaemia (Group A: 2%; Group B: 5%) Cancer treatment: prior to autologous HSCT, participants received the following conditioning regimen:
All participants received G‐CSF (filgrastim) 5 µg/kg per day from HSCT until neutrophil recovery Age at baseline (years): Group A: median 48 (range 18 to 69); Group B: median 49 (range 19 to 68) Gender: Group A: 56% male; Group B: 68% male Number randomised: 214 (Group A: 107; Group B: 107) Number evaluated: 212 (Group A: 106; Group B: 106) |
|
| Interventions |
Comparison: KGF (palifermin) versus placebo Group A: KGF (60 µg/kg) by IV for 3 consecutive days starting 3 days before RT, followed by 3 more doses after HSCT starting on the day of HSCT (total dose = 360 µg/kg) Group B: placebo as above Compliance: 212 participants received at least 1 dose of their allocated intervention and 205 participants (Group A: 103; Group B: 102) "completed the study" (it is not clear whether or not this means that they received all doses) Duration of treatment: 6 treatment days over 13 to 14 days |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: 210 participants required to detect a mean difference in duration of severe oral mucositis of at least 3 days (SD 6.6) at 90% power and 5% significance Funding: "Funded by Amgen" (pharmaceutical industry) Declarations/conflicts of interest: the majority of authors were linked to the funding pharmaceutical company in terms of employment, consulting fees, lecture fees, receipt of research funding, and ownership of equity Data handling by review authors: N/A Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned in a 1:1 ratio" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly assigned in a 1:1 ratio" Comment: insufficient information to determine method of random sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo‐controlled, double‐blind" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "placebo‐controlled, double‐blind" Comment: it is not clear who was blinded. There are subjective elements to the assessment of lower grades of oral mucositis using the WHO scale, requiring the patient's assessment of pain/soreness and their ability to swallow. Higher grades have more objective elements so may not be affected by potential lack of blinding of the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 randomised participants (1 per group) were not included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Su 2006.
| Methods |
Trial design: parallel (2 arms) Location: USA Number of centres: 1 Study duration: recruitment from January 1992 to December 1996 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: 18 years or older with histologically proven AJCC stage II to IV squamous cell carcinoma of the head and neck; undergone gross complete resection with negative pathological surgical margins and medically fit to begin RT within 8 weeks of surgery; Karnofsky performance score of 80% or more; adequate haematologic and serum metabolic laboratory indices Exclusion criteria: nasopharyngeal cancer; concurrent active malignancy other than localised; nonmelanoma skin cancer; previous RT to head and neck; previous chemotherapy; positive serum β‐human chorionic gonadotropin in women of procreative potential; need for tube feeding at the start of RT Cancer type: stage II to IV squamous cell carcinoma of the head and neck: hypopharynx (Group A: 11%; Group B: 5%); larynx (Group A: 16%; Group B: 23%); oral cavity (Group A: 26%; Group B: 27%); oropharynx (Group A: 21%; Group B: 32%); unknown primary (Group A: 26%; Group B: 14%) Cancer treatment: postoperative external beam RT in once‐daily fractions of 1.8 Gy, 5 days per week, to total dose of 63 Gy at primary site and involved neck (54 Gy to regional lymphatics at risk for subclinical metastasis; spinal cord dose limited to 45 Gy) Age at baseline (years): Group A: median 67 (interquartile range 59 to 75); Group B: median 61 (interquartile range 54 to 67) Gender: Group A: 79% male; Group B: 73% male Number randomised: 41 (Group A: 19; Group B: 22) Number evaluated: 40 (Group A: 19; Group B: 21) |
|
| Interventions |
Comparison: G‐CSF versus placebo Group A: G‐CSF (3 µg/kg) by daily subcutaneous injection, 7 days per week, starting 3 days before the start of RT and continued to end of RT (total dose = dependent on duration of RT) Group B: placebo as above Compliance: not reported Duration of treatment: variable and dependent on duration of RT |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: planned sample sample size was 66 per group to detect absolute difference of 20% in PEG tube use (from 10% in one group to 30% in the other) at 80% power and 5% significance Funding: "This study was supported by NIH grant #CA69913" (government) Declarations/conflicts of interest: "No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this article" Data handling by review authors: although the scale for assessing oral mucositis does not completely match the 0 to 4 scales (such as WHO, etc), it was possible to dichotomise it for use in the 'any mucositis' meta‐analysis, and we also used grade 3 (confluent mucositis) as incidence of severe mucositis Other information of note: planned to enrol 132 participants but stopped due to slow recruitment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized...by randomly permuted blocks...A randomization list was prepared by the Memorial Sloan‐Kettering Cancer Center Biostatistics Service and held by the pharmacy" Comment: specialist centre used |
| Allocation concealment (selection bias) | Low risk | Quote: "A randomization list was prepared by the Memorial Sloan‐Kettering Cancer Center Biostatistics Service and held by the pharmacy. Investigators did not have access to this list, ensuring that allocation could not be predicted before registration or changed afterwards" Comment: central/remote randomisation would have ensured concealment of the random sequence from those recruiting participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" and "All patients and treating physicians were blinded to treatment group assignment" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" and "All patients and treating physicians were blinded to treatment group assignment" Comment: the treating physician was the outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 randomised participant was not included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Vadhan‐Raj 2010.
| Methods |
Trial design: parallel (2 arms) Location: University of Texas MD Anderson Cancer Center, USA Number of centres: 1 Study duration: recruitment from December 2005 to February 2008; last follow‐up in May 2008 Trials registry number: NCT00267046 (mentioned in trial report) |
|
| Participants |
Inclusion criteria: aged 15 to 65 years with sarcoma; due to start chemotherapy at the centre; Karnofsky performance score of 80% or more; adequate bone marrow, renal, and hepatic function Exclusion criteria: history of pelvic RT; clinically significant cardiac disease; undergone surgery within the previous 2 weeks Cancer type: sarcoma Cancer treatment: doxorubicin (total dosage 90 mg/m²) administered by continuous IV infusion over 72 hours, and ifosfamide (total dosage 10 g/m²) administered by 3‐hour IV infusion for 4 days; participants with osteosarcoma (Group A: 2; Group B: 1) received the same dosage of doxorubicin but with intra‐arterial cisplatin (120 mg/m²); all participants received G‐CSF (pegfilgrastim) the day after chemotherapy; cycle repeated every 21 days; planned 6 cycles Age at baseline (years): Group A: median 42 (range 17 to 63); Group B: median 39 (range 15 to 64) Gender: Group A: 53% male; Group B: 50% male Number randomised: 48 (Group A: 32; Group B: 16) Number evaluated: 48 (Group A: 32; Group B: 16) |
|
| Interventions |
Comparison: KGF (palifermin) versus placebo Group A: KGF (180 µg/kg) by IV as single dose 3 days before the start of each chemotherapy cycle Group B: placebo as above Compliance: the proportion of participants that completed all 6 blinded cycles (i.e. they took their allocated intervention) was higher in Group A (63%) than Group B (31%) Duration of treatment: 1 day per 3‐week cycle; planned total 6 days of intervention over 18 weeks |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: 48 participants required to detect absolute difference of 50% (from 26% in Group A to 76% in Group B) in grade 2 to 4 mucositis at 88% power and 5% significance Funding: "Amgen provided the palifermin and partial funding for the study" (pharmaceutical industry) Declarations/conflicts of interest: principal investigator is a member of the Amgen board Data handling by review authors: we only report data from the first 2 cycles (blinded phase) as very few participants received placebo in the remaining cycles Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two distinct computer‐generated randomization lists were prepared by the University of Texas M.D. Anderson Cancer Center, Department of Biostatistics, one for the 20 patients who consented to pharmacokinetic sampling and the other for the 28 patients who did not. For the pharmacokinetics cohort, the treatment allocation ratio was 4 patients receiving palifermin to 1 receiving placebo, in blocks of 5; for the other cohort, the ratio was 4 patients receiving palifermin to 3 receiving placebo, in blocks of 14" Comment: adequate method used |
| Allocation concealment (selection bias) | Low risk | Quote: "The statistician provided both randomization lists to the pharmacy, so the patient and the clinical research team (who assessed outcomes) were blinded to the study treatment. At patient enrolment, the research team notified the pharmacy, which assigned the patient the next sequential slot and treatment from the appropriate randomization list on the basis of whether he or she had consented to pharmacokinetic sampling. The pharmacy provided the research team with the blinded study medication. Upon completion of the study, pharmacy provided the statistician with the 2 randomization lists, including individual patient treatment assignments, for analysis" Comment: pharmacy‐controlled randomisation would have ensured concealment of the random sequence from those recruiting participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" and "the patient and the clinical research team (who assessed outcomes) were blinded to the study treatment" and "Blinding might not have been maintained because of adverse effects of palifermin" Comment: some personnel may not have been blinded due to increased adverse effects, although adverse effects from palifermin may have been difficult to isolate from adverse effects of cancer treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" and "the patient and the clinical research team (who assessed outcomes) were blinded to the study treatment" and "Blinding might not have been maintained because of adverse effects of palifermin" and "patients were assessed at each cycle by both research and clinical teams, including those without direct knowledge of the protocol" Comment: it seems unlikely that lack of blinding would affect outcomes as the higher grades of oral mucositis assessed in this study are more objective; also, some were assessed by personnel without knowledge of the protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
van der Lelie 2001.
| Methods |
Trial design: parallel (2 arms) Location: the Netherlands Number of centres: 1 Study duration: recruitment from May 1997 to August 1999 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: malignant disease and due to have myeloablative treatment followed by autologous or allogeneic BMT or PBSCT Exclusion criteria: not reported Cancer type: haematologic: lymphoma (Group A: 28%; Group B: 50%); acute leukaemia (Group A: 28%; Group B: 28%); CML (Group A: 22%; Group B: 6%); multiple myeloma (Group A: 22%; Group B: 17%) Cancer treatment: prior to autologous or allogeneic BMT or PBSCT, participants received the following conditioning regimens:
Lymphoma patients received BEAM; other patients received CYTBI or BUCY; nearly all participants received autologous or allogeneic PBSCT Age at baseline (years): Group A: median 47 (range 25 to 63); Group B: median 48 (range 18 to 62) Gender: Group A: 61% male; Group B: 39% male Number randomised: 36 (Group A: 18; Group B: 18) Number evaluated: 36 (Group A: 18; Group B: 18) |
|
| Interventions |
Comparison: GM‐CSF versus placebo Group A: GM‐CSF (300 µg daily dose) gel, 5 mL twice daily (early in the morning and before going to sleep) kept in the mouth for as long as possible and then swallowed; no oral intake for 1 hour afterwards; starting on day 1 (the day after the day of transplant) and continued until neutrophil recovery (typically up to 14 days after transplant) Group B: placebo as above All participants followed hospital's standard protocol for mouth care: toothbrushing after meals, rinsing with 0.9% saline, and if there was inflammation, 0.12% chlorhexidine rinse 6 times daily Compliance: 8 participants (Group A: 4; Group B: 4) did not complete the study; they all cited the main reason being nausea and the taste of the gel Duration of treatment: variable and dependent on neutrophil recovery |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: not reported ("GM‐CSF was supplied by the sponsor for 18 patients so that 36 patients could be included in the study. This should be enough to demonstrate a clinically significant difference") Funding: "We thank Novartis and Schering‐Plough for supplying the GM‐CSF" (pharmaceutical industry) Declarations/conflicts of interest: not reported Data handling by review authors: N/A Other information of note: GM‐CSF administration only began after the conditioning treatment had been completed, by which point oral mucositis may have already begun to develop |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the patients were randomized to receive GM‐CSF or placebo" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the patients were randomized to receive GM‐CSF or placebo" Comment: insufficient information to determine method of random sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled" and "There was no difference in taste or appearance between the placebo and the GM‐CSF" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled" and "There was no difference in taste or appearance between the placebo and the GM‐CSF" Comment: it is not clear who was blinded. There are subjective elements to the assessment of lower grades of oral mucositis using the WHO scale, requiring the patient's assessment of pain/soreness and their ability to swallow. Higher grades have more objective elements so may not be affected by potential lack of blinding of the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
Wu 2009.
| Methods |
Trial design: parallel (4 arms) Location: South Korea Number of centres: 6 Study duration: recruitment from January to August 2007 Trials registry number: none/unknown |
|
| Participants |
Inclusion criteria: 18 years or older with histological evidence of head and neck cancer; due to receive at least 5 weeks of primary RT, primary chemoradiotherapy, or postoperative RT; agreement to have complete medical history and physical examination; ECOG score of 0 to 2; white blood counts 3 x 10³/L or higher; platelet counts 100 x 10³/L or higher Exclusion criteria: oral ulcers; herpes simplex virus; severe periodontal disease; serum creatine levels greater than 2 mg/dL; ALT/AST values greater than 200 IU/L; cytotoxic chemotherapy or RT within 3 weeks of the start of the study; systemic or topical corticosteroids within 30 days of the start of the study; participated in another clinical study within 30 days of the start of the study Cancer type: head and neck: nasopharynx (Group A: 36%; Group B: 32%); oropharynx (Group A: 24%; Group B: 25%); oral cavity (Group A: 19%; Group B: 25%); hypopharynx (Group A: 2%; Group B: 4%); other (Group A: 19%; Group B: 14%) Cancer treatment:
Age at baseline (years): Group A: median 56 (range 18 to 75); Group B: median 51 (range 18 to 77) Gender: Group A: 69% male; Group B: 57% male Number randomised: 113 (Group A: 85; Group B: 28) Number evaluated: 103 (Group A: 76; Group B: 27) for incidence of moderate to severe mucositis; 94 (Group A: 70; Group B: 24) for incidence of severe mucositis |
|
| Interventions |
Comparison: EGF (recombinant human) versus placebo Group A: EGF
Group B: placebo as above All participants gargled with chlorhexidine to maintain oral hygiene Compliance: not reported Duration of treatment: 5 weeks |
|
| Outcomes |
|
|
| Notes |
Sample size calculation: allowing for 10% attrition, 26 participants per group required Funding: "This study was supported by a grant from the National R&D Program for Cancer Central, Ministry of Health, Welfare and Family Affairs, Republic of Korea (0620270)" (government) and "The EGF and placebo treatments were supplied free of charge" (presumably pharmaceutical industry) Declarations/conflicts of interest: not reported Data handling by review authors: we combined the 3 EGF groups to make a single pairwise comparison against placebo; no formal statistical analysis was undertaken for the different EGF dosages Other information of note: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We assigned patients randomly to 4 arms" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "We assigned patients randomly to 4 arms" Comment: insufficient information to determine method of random sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" and "Three concentrations of EGF and a placebo containing the drug delivery vehicle were manufactured, packaged, and supplied in a double‐blind fashion" Comment: the use of a placebo should have ensured that blinding was successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled" Comment: it is not clear who was blinded. There are subjective elements to the assessment of most grades of oral mucositis using the RTOG scale, requiring the patient's assessment of pain. Grade 4 is more objective so may not be affected by potential lack of blinding of the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was 9% (Group A: 11%; Group B: 4%). Slight differential between groups but the reasons were reported and mostly unrelated to interventions/outcomes. Unlikely to have biased the results |
| Selective reporting (reporting bias) | High risk | Several outcomes were assessed but not properly reported ("No secondary endpoint showed any difference between the placebo and study groups") |
| Other bias | Low risk | No other sources of bias are apparent |
5FU = fluorouracil; AJCC = American Joint Committee on Cancer; ALL = acute lymphoblastic leukaemia; allogeneic = cells from donor; AML = acute myelogenous leukaemia; AUC = area under the curve; autologous = patients' own cells; BMT = bone marrow transplantation; CALGB = Cancer and Leukaemia Group B; cGy = centigray; CML = chronic myelogenous leukaemia; CRT = chemoradiotherapy; CT = chemotherapy; ECOG = Eastern Co‐operative Oncology Group; EGF = epidermal growth factor; EQ‐5D = European Quality Of Life Utility Scale; FACT = Functional Assessment of Cancer Therapy; FDA = US Food and Drug Administration; G‐CSF = granulocyte‐colony stimulating factor; GM‐CSF = granulocyte‐macrophage colony‐stimulating factor; GVHD = graft‐versus‐host disease; Gy = gray; HLA = human leukocyte antigen; HSCT = haematopoietic stem cell transplantation; IA = intra‐arterial; ITF = intestinal trefoil factor; ITT = intention‐to‐treat; IV = intravenous; KGF = keratinocyte growth factor; MDS = myelodysplastic syndrome; N/A = not applicable; NCI‐CTC = National Cancer Institute common toxicity criteria; NHL = non‐Hodgkin's lymphoma; NIH = National Institutes of Health; OMAS = oral mucositis assessment scale; OMDQ = oral mucositis daily questionnaire; OMWQ‐HN = Oral Mucositis Weely Questionnaire ‐ Head and Neck cancer; PBPC = peripheral blood progenitor cell; PBSCT = peripheral blood stem cell transplantation; PEG = percutaneous endoscopic gastrostomy; PP = per protocol; RT = radiotherapy; RTOG = Radiation Therapy Oncology Group; SCC = squamous cell carcinoma; SCT = stem cell transplantation; SD = standard deviation; SMD = standardised mean difference; TBI = total‐body irradiation; TGF = transforming growth factor; TPN = total parenteral nutrition; VAS = visual analogue scale; WBC = white blood cell; WHO = World Health Organization; WCCNR = Western Consortium for Cancer Nursing Research.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Antin 2002 | Recombinant human interleukin‐11 versus placebo. Study stopped early due to adverse event triggering preset stopping rule ‐ data only for 10 patients in rhIL group and 3 placebo |
| de Koning 2007 | TGF‐beta(2) versus placebo. Cross‐over study with no first period data reported (see Types of studies) |
| Foncuberta 2001 | TGF‐beta(3) versus placebo. Participants assigned sequentially, not randomised |
| Gebbia 1994 | G‐CSF versus thymopentin versus G‐CSF plus thymopentin versus placebo. Oral mucositis not mentioned (unknown if measured) |
| Gladkov 2013 | G‐CSF (lipegfilgrastim) versus G‐CSF (pegfilgrastim). Oral mucositis not mentioned (unknown if measured). Insufficient information (abstract) |
| Gordon 1993 | GM‐CSF versus no treatment. Unclear if randomised or not. Only 13 participants. Insufficient information (abstract) |
| Horsley 2007 | KGF (palifermin) versus standard care. No random allocation |
| Hunter 2009 | ATL‐104 versus placebo. Not RCT ‐ this study combines patients who were in cohorts with increasing doses of mouthrinse to assess safety, with an RCT |
| Ifrah 1999 | GM‐CSF versus placebo. Primary outcome was survival/cure, with mucositis as a toxicity. Unclear if mucositis was oral or gastrointestinal |
| Iwase 1997 | G‐CSF versus no treatment. No mention of randomisation and no description of when intervention given in relation to cancer treatment |
| Jones 1996 | GM‐CSF versus placebo. Unclear if mucositis was oral or gastrointestinal |
| Karthaus 1998 | G‐CSF versus placebo. Only 8 patients, 32 chemotherapy cycles and results presented assuming independent |
| Kubo 2016 | G‐CSF (pegfilgrastim) versus G‐CSF (filgrastim). Incidence of stomatitis reported in adverse events table Trials registry number: JapicCTI‐111394 |
| Lee 2016 | G‐CSF (pegteograstim) versus G‐CSF (pegfilgrastim). Incidence of stomatitis reported in adverse events table Trials registry number: NCT01328938 |
| Legros 1997 | GM‐CSF versus placebo. Unclear if mucositis was oral or gastrointestinal |
| Limaye 2013 | AG013 versus placebo. AG013 is composed of recombinant Lactococcus lactis engineered to secrete human Trefoil Factor 1. Randomised at first but participants not developing oral mucositis in chemotherapy cycle 1 were excluded from the next phase where oral mucositis was measured, so randomisation was lost Trials registry number: NCT00938080 |
| Nabholtz 2002 | G‐CSF (leridistim) versus G‐CSF (filgrastim). Incidence of stomatitis reported in adverse events table |
| NCT00360971 | KGF (palifermin) versus placebo. Study terminated at 21 participants (298 planned) due to positive preliminary results from other palifermin studies |
| NCT00626639 | KGF (palifermin) versus placebo. Study terminated at 5 participants due to departure of principal investigator and slow enrolment |
| Pettengell 1992 | G‐CSF versus no treatment. Unclear if mucositis was oral or gastrointestinal |
| Ryu 2007 | GM‐CSF versus placebo. Some participants (6%) had oral mucositis at baseline Trials registry number: NCT00008398 |
| Throuvalas 1995 | GM‐CSF versus no treatment. Probably not RCT ‐ described as comparative study. Only 10 participants. Insufficient information (abstract) |
| Tsurusawa 2016 | G‐CSF versus no treatment. Incidence of stomatitis reported in adverse events table Trials registry number: UMIN ID: 000000675 |
| Vitale 2014 | KGF (palifermin) versus no treatment. From full text it is not RCT ‐ retrospective and allocation of KGF/no KGF based on doctor's decision |
G‐CSF = granulocyte‐colony stimulating factor; GM‐CSF = granulocyte‐macrophage colony‐stimulating factor; KGF = keratinocyte growth factor; RCT = randomised controlled trial; rhIL = recombinant human interleukin; TGF = transforming growth factor.
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12606000083594.
| Methods | Multicentre, double‐blind, randomised placebo‐controlled trial |
| Participants | 18 years or older with lymphoma and due to undergo high‐dose BEAM chemotherapy and autologous stem cell transplantation as inpatient |
| Interventions | Whey growth factor extract at 13.5 mg/mL in sterile saline versus placebo (sterile saline) |
| Outcomes |
|
| Notes | Funding: commercial sector/industry (TGR Biosciences, Australia) Contact: correspondence with pharmaceutical company suggests no benefit |
Antonadou 1998.
| Methods | Multicentre, randomised controlled trial (no placebo) |
| Participants | Head and neck cancer; receiving continuous course of radiotherapy for 6 to 7 weeks |
| Interventions | GM‐CSF (subcutaneous) versus no treatment |
| Outcomes | Signs and symptoms of oral mucositis: erythema, pain and dysphagia each measured on 0 to 3 scale |
| Notes | Abstract only (we were unable to link this abstract to a full‐text report) Some P values suggest benefit for GM‐CSF at some time points Quote: "GM‐CSF reduces the incidence and severity of radiation mucositis and allows the completion of the XRT treatment without protraction" |
Elsaid 2001.
| Methods | Randomised controlled trial (no placebo) |
| Participants | Anaemic participants with head and neck cancer receiving radiotherapy once daily (1.8 Gy or 2 Gy) to doses of 66 Gy to 70 Gy |
| Interventions | Recombinant human erythropoietin versus no treatment |
| Outcomes | Incidence of grade 3 mucositis and dermatitis |
| Notes | Abstract only (we were unable to link this abstract to a full‐text report) Higher rate of mucositis in the no‐treatment group (5.9% versus 0%) |
Grzegorczyk 2006.
| Methods | Randomised placebo‐controlled trial |
| Participants | Adults (aged 19 to 68 years) undergoing haematopoietic stem cell transplantation |
| Interventions | G‐CSF versus placebo |
| Outcomes |
|
| Notes | Translation provided insufficient information. Discrepancy between graph legends and descriptions Unable to contact author |
Koga 2015.
| Methods | Randomised controlled trial (no placebo) |
| Participants | Children with B‐cell non‐Hodgkin's lymphoma (B‐NHL) receiving chemotherapy |
| Interventions | G‐CSF versus no treatment |
| Outcomes |
|
| Notes | Abstract only (we were unable to link this abstract to a full‐text report) Quote: "G‐CSF+ patients showed a positive impact on the meantime to neutrophil recovery and hospital stay, but they had no impact in incidences of febrile neutropenia, infections, stomatitis, and total cost" |
NCT00293462.
| Methods | Double‐blind, randomised placebo‐controlled trial (1 of the 3 arms is a cross‐over) |
| Participants | 18 years or older with head and neck cancer due to receive conventional or hyperfractionated radiotherapy or intensity‐modulated radiotherapy (IMRT) with or without concurrent chemotherapy |
| Interventions | GM‐CSF mouthwash versus salt and soda mouthwash versus both (cross‐over arm) |
| Outcomes |
|
| Notes | Trials registry number: NCT00293462 Funding: University of California, San Francisco; National Cancer Institute (NCI) |
NCT00393822.
| Methods | Double‐blind, randomised placebo‐controlled trial |
| Participants | 18 years or older with resected colon cancer (American Joint Committee on Cancer Stage 2B or 3) and due to receive 5‐FU and leucovorin |
| Interventions | KGF versus placebo |
| Outcomes |
|
| Notes | Trials registry number: NCT00393822 Funding: Amgen (pharmaceutical industry) |
NCT02303197.
| Methods | Randomised controlled trial (no placebo) |
| Participants | Adults (aged 18 to 75 years) with head and neck cancer due to receive radiotherapy |
| Interventions | Recombinant human EGF versus Chining |
| Outcomes |
|
| Notes | Trials registry number: NCT02303197 Funding: Tianjin Medical University Cancer Institute and Hospital |
NCT02313792.
| Methods | Double‐blind, randomised placebo‐controlled trial |
| Participants | 16 years or older due to receive preparative cancer treatment regimen followed by autologous or allogeneic HSCT (cancer type not reported) |
| Interventions | KGF versus placebo |
| Outcomes |
|
| Notes | Trials registry number: NCT02313792 Funding: The Catholic University of Korea; Collaborator: "BLNH" (probably pharmaceutical industry) |
Patte 2002.
| Methods | Randomised controlled trial (no placebo) |
| Participants | Children with non‐Hodgkin's lymphoma due to receive 2 courses of COPAD induction chemotherapy |
| Interventions | G‐CSF versus no treatment |
| Outcomes |
|
| Notes | Quote: "The incidence of grade 3 and 4 mucositis was similar in both arms" Contact: emailed corresponding author July 2017 to clarify if mucositis is oral or gastrointestinal |
Schuster 2007a.
| Methods | Multicentre, double‐blind, randomised placebo‐controlled trial |
| Participants | 18 years or older with multiple myeloma or lymphoma due to receive high‐dose chemotherapy (with or without TBI) followed by autologous HSCT |
| Interventions | Recombinant human FGF‐20 (velafermin) (3 arms with different dosages) versus placebo |
| Outcomes |
|
| Notes | Abstract only (we were unable to link this abstract to a full‐text report) Trials registry number: NCT00104065 |
Schuster 2007b.
| Methods | Multicentre, double‐blind, randomised placebo‐controlled trial |
| Participants | 18 years or older with multiple myeloma or lymphoma undergoing high‐dose chemotherapy (with or without TBI) followed by autologous HSCT |
| Interventions | Recombinant human FGF‐20 (velafermin) (3 arms with different dosages) versus placebo |
| Outcomes |
|
| Notes | Abstract only (we were unable to link this abstract to a full‐text report) Trials registry number: NCT00323518 |
Shea 2007.
| Methods | Randomised controlled trial (no placebo) |
| Participants | Aged 18 to 74 years with lymphoma, leukaemia or multiple myeloma; TBI plus high‐dose chemotherapy followed by autologous peripheral blood stem cell transplantation |
| Interventions | KGF (4 arms with different schedules) |
| Outcomes |
|
| Notes | Abstract only (we were unable to link this abstract to a full‐text report) Trials registry number: NCT00109031 |
Spielberger 2001.
| Methods | Multicentre, double‐blind, randomised placebo‐controlled trial |
| Participants | Haematologic malignancies; eligible for TBI plus high‐dose chemotherapy followed by autologous peripheral blood stem cell transplantation; aged 12 to 65 years |
| Interventions | KGF (7 doses) versus KGF (4 doses + 3 doses of placebo) versus placebo (7 doses) |
| Outcomes |
|
| Notes | Abstract only (we were unable to link this abstract to a full‐text report) Trials registry number: NCT00004132 |
5FU = fluorouracil; EGF = epidermal growth factor; EORTC QLQ‐H&N35 = European Organisation for Research and Treatment of Cancer, Quality of Life Questionnaire, Head and Neck Module; G‐CSF = granulocyte‐colony stimulating factor; GM‐CSF = granulocyte‐macrophage colony‐stimulating factor; HSCT = haematopoietic stem cell transplantation; IV = intravenous; KGF = keratinocyte growth factor; NCI‐CTC = National Cancer Institute common toxicity criteria; OMDQ = oral mucositis daily questionnaire; FGF‐20 = fibroblast growth factor‐20; TBI = total‐body irradiation; VAS = visual analogue scale; WHO = World Health Organization.
Differences between protocol and review
For the 'Summary of findings' tables, we decided to limit the outcomes to the incidence of moderate to severe, and the incidence of severe oral mucositis, and also adverse events. This was partly because the scales used to assess oral mucositis often incorporate aspects of some of the secondary outcomes, and it is the most clinically relevant outcome, and partly because the tables would become unwieldy and difficult to read once the secondary outcomes had been divided into their subgroups based on cancer treatment type. We also eliminated the incidence of any oral mucositis from the tables because ulcerative and severe grades are more important.
We decided not to report data from secondary outcomes that were not suitable for meta‐analysis in additional tables as we felt that this would not be helpful and could result in 'vote‐counting' i.e. xx studies reported a difference and yy studies reported no difference.
Contributions of authors
Philip Riley: writing the Background and Methods sections, screening searches, data extraction, risk of bias assessment, interpreting the results, writing the review. Anne‐Marie Glenny: writing the Methods section, screening searches, data extraction, risk of bias assessment, interpreting the results. Helen V Worthington: writing the Methods section, screening searches, data extraction, risk of bias assessment, interpreting the results. Anne Littlewood: writing the Methods section, designing the search strategy and carrying out the searches, screening searches, data extraction, risk of bias assessment, interpreting the results. Luisa M Fernandez Mauleffinch: writing the Methods section, screening searches, data extraction, risk of bias assessment, interpreting the results, copy editing the review. Jan E Clarkson: interpreting the results and providing a clinical perspective. Martin G McCabe: interpreting the results and providing a clinical perspective.
Sources of support
Internal sources
School of Dentistry, The University of Manchester, UK.
Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
Declarations of interest
There was no industry funding to support the undertaking of this Cochrane Review.
Philip Riley: I am a salaried member of the Cochrane Oral Health editorial team. Anne‐Marie Glenny: none known. I am Deputy Co‐ordinating Editor of Cochrane Oral Health. Helen V Worthington: none know. I am Co‐ordinating Editor of Cochrane Oral Health. Anne Littlewood: I am a salaried member of the Cochrane Oral Health editorial team. Luisa M Fernandez Mauleffinch: I am a salaried member of the Cochrane Oral Health editorial team. Jan E Clarkson: none known. I am Co‐ordinating Editor of Cochrane Oral Health. Martin G McCabe: none known. I am an Editor with Cochrane Oral Health.
New
References
References to studies included in this review
Antoun 2009 {published data only}
- Antoun S, Boige V, Ducreux M, Paintain M, van't Hof M, Enslen M, et al. Protective effect of an enteral formula containing TGF‐beta(2) in the prevention of chemotherapy‐induced diarrhoea: a pilot study. e‐SPEN, European e‐Journal of Clinical Nutrition and Metabolism 2009;4(6):e348‐50. [Google Scholar]
Blazar 2006 {published data only}
- Blazar BR, Weisdorf DJ, Defor T, Goldman A, Braun T, Silver S, et al. Phase 1/2 randomized, placebo‐control trial of palifermin to prevent graft‐versus‐host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT). Blood 2006;108(9):3216‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blijlevens 2013 {published data only}
- Blijlevens N, Château M, Krivan G, Rabitsch W, Szomor A, Pytik R, et al. In a high‐dose melphalan setting, palifermin compared to placebo had no effect on oral mucositis or related patient's burden. Supportive Care in Cancer 2011;19(Suppl 2):S227‐8. [DOI] [PubMed] [Google Scholar]
- Blijlevens N, Château M, Krivan G, Rabitsch W, Szomor A, Pytik R, et al. Medical resource use of two different dosing regimens of palifermin to prevent mucositis in multiple myeloma patients receiving one‐day administration of high‐dose melphalan. Bone Marrow Transplantation 2011;46(Suppl 1s):S163‐4. [Google Scholar]
- Blijlevens N, Château M, Krivan G, Rabitsch W, Szomor A, Pytik R, et al. Randomized controlled trial of two different dosing regimens of palifermin to prevent mucositis in multiple myeloma patients receiving one‐day administration of high‐dose melphalan. Blood 2010;116(21):904. [Google Scholar]
- Blijlevens N, Château M, Krivan G, Rabitsch W, Szomor A, Pytlik R, et al. In a high‐dose melphalan setting, palifermin compared with placebo had no effect on oral mucositis or related patient's burden. Bone Marrow Transplantation 2013;48(7):966‐71. [DOI] [PubMed] [Google Scholar]
Bradstock 2014 {published data only}
- Bradstock KF, Link E, Collins M, Iulio J, Lewis ID, Schwarer A, et al. A randomized trial of prophylactic palifermin on gastrointestinal toxicity after intensive induction therapy for acute myeloid leukaemia. British Journal of Haematology 2014;167(5):618‐25. [DOI] [PubMed] [Google Scholar]
Brizel 2008 {published data only}
- Brizel DM, Murphy BA, Rosenthal DI, Pandya KJ, Glück S, Brizel HE, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. Journal of Clinical Oncology 2008;26(15):2489‐96. [DOI] [PubMed] [Google Scholar]
Cartee 1995 {published data only}
- Cartee L, Petros WP, Rosner G, Gilbert C, Moore S, Affronti M, et al. Evaluation of GM‐CSF mouthwash for prevention of chemotherapy‐induced mucositis: a randomized double‐blind dose escalating study. Blood 1993;82(10 Suppl 1):496a. [Abs No 1968] [DOI] [PubMed] [Google Scholar]
- Cartee L, Petros WP, Rosner GL, Gilbert C, Moore S, Affronti ML, et al. Evaluation of GM‐CSF mouthwash for prevention of chemotherapy‐induced mucositis: a randomized, double‐blind, dose‐ranging study. Cytokine 1995;7(5):471‐7. [DOI] [PubMed] [Google Scholar]
Cesaro 2013 {published data only}
- Cesaro S, Nesi F, Tridello G, Abate M, Panizzolo IS, Balter R, et al. A randomized, non‐inferiority study comparing efficacy and safety of a single dose of pegfilgrastim versus daily filgrastim in pediatric patients after autologous peripheral blood stem cell transplant. PLOS One 2013;8(1):e53252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaro S, Nesi F, Tridello G, Fagioli F, Abate M, Panizzolo I, et al. A non‐inferiority study comparing efficacy and safety of a single dose of pegfilgrastim versus daily filgrastim in paediatric patients after autologous peripheral blood stem cell transplant. Bone Marrow Transplantation 2012;47(Suppl 1s):S372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chi 1995 {published data only}
- Chi KH, Chen CH, Chan WK, Chow KC, Chen SY, Yen SH, et al. Effect of granulocyte‐macrophage colony‐stimulating factor on oral mucositis in head and neck cancer patients after cisplatin, fluorouracil and leucovorin chemotherapy. Journal of Clinical Oncology 1995;13(10):2620‐8. [DOI] [PubMed] [Google Scholar]
- Chi KH, Chen CH, Chan WK, Yen SH, Liang MJ, Chou KC, et al. Effect of granulocyte‐macrophage colony‐stimulating factor (GH‐CFS) on oral mucositis in head and neck cancer patients after cisplatin, 5‐FU and leucovorin chemotherapy. Proceedings of the American Society of Clinical Oncology 1994;13:428. [Abs No 1469] [DOI] [PubMed] [Google Scholar]
Crawford 1999 {published data only}
- Crawford J, Glaspy J, Vincent M, Tomita D, Mazanet R. Effect of filgrastim (r‐metHuG‐CSF) on oral mucositis in patients with small cell lung cancer (SCLC) receiving chemotherapy (cyclophosphamide, doxorubicin and etoposide, CAE). Proceedings of the American Society of Clinical Oncology 1994;13:442. [Abs No A1523] [Google Scholar]
- Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, et al. Reduction by granulocyte colony‐stimulating factor of fever and neutropenia induced by chemotherapy in patients with small‐cell lung cancer. New England Journal of Medicine 1991;325(3):164‐70. [DOI] [PubMed] [Google Scholar]
- Crawford J, Tomita DK, Mazanet R, Glaspy J, Ozer H. Reduction of oral mucositis by filgrastim (r‐metHuG‐CSF) in patients receiving chemotherapy. Cytokines, Cellular and Molecular Therapy 1999;5(4):187‐93. [PubMed] [Google Scholar]
Dazzi 2003 {published data only}
- Dazzi C, Cariello A, Giovanis P, Monti M, Vertogen B, Leoni M, et al. Prophylaxis with GM‐CSF mouthwashes does not reduce frequency and duration of severe oral mucositis in patients with solid tumors undergoing high‐dose chemotherapy with autologous peripheral blood stem cell transplantation rescue: a double‐blind, randomized, placebo‐controlled study. Annals of Oncology 2003;14(4):559‐63. [DOI] [PubMed] [Google Scholar]
- Dazzi C, Cariello A, Monti M, Giovanis P, Vertogen B, Nanni O, et al. Prophylaxis with GM‐CSF mouthwash does not reduce frequency and duration of severe oral mucositis in patients with solid tumors undergoing high dose chemotherapy with autologous PBPC rescue: a double‐blind randomized placebo‐controlled study. Annals of Oncology 2002;13 Suppl 5:167. [Abs No 617O] [DOI] [PubMed] [Google Scholar]
Fink 2011 {published and unpublished data}
- Fink G, Ihorst G, Burbeck M, Kleber M, Kohlweyer U, Engelhardt M. Prospective randomized trial of palifermin (keratinocyte growth factor) versus best supportive care measures (BSC) for the decrease of oral mucositis (OM) in lymphoma patients receiving high‐dose BEAM‐(BCNU, Etoposide, Ara‐C, Melphalan) conditioning and autologous stem‐cell transplantation (ASCT). Onkologie. Jahrestagung der Deutschen, Österreichischen und Schweizerischen Gesellschaften für Hämatologie und Onkologie; 2011 Sept‐Oct 30‐04; Basel (Switzerland). Basel (Switzerland): S Karger AG, 2011:302.
Freytes 2004 {published data only}
- Freytes CO, Ratanatharathorn V, Taylor C, Abboud C, Chesser N, Restrepo A, et al. Phase I/II randomized trial evaluating the safety and clinical effects of repifermin administered to reduce mucositis in patients undergoing autologous hematopoietic stem cell transplantation. Clinical Cancer Research 2004;10(24):8318‐24. [DOI] [PubMed] [Google Scholar]
Gholizadeh 2016 {published data only}
- Gholizadeh N, Mehdipoor M, Sajadi H, Moosavi MS. Palifermin and chlorhexidine mouthwashes in prevention of chemotherapy‐induced mucositis in children with acute lymphocytic leukemia: a randomized controlled trial. Journal of Dentistry (Shiraz, Iran) 2016;17(4):343‐7. [PMC free article] [PubMed] [Google Scholar]
Henke 2011 {published data only}
- Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo‐controlled trial. Journal of Clinical Oncology 2011;29(20):2815‐20. [DOI] [PubMed] [Google Scholar]
- Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, et al. Palifermin significantly reduces severe oral mucositis in subjects with resected locally advanced head and neck cancer undergoing post‐operative concurrent radio‐chemotherapy. Radiotherapy and Oncology 2008;88 Suppl 2:S152. [Google Scholar]
Hosseinjani 2017 {published data only}
- Hosseinjani H, Hadjibabaie M, Gholami K, Javadi M, Radfar M, Jahangard‐Rafsanjani Z, et al. The efficacy of erythropoietin mouthwash in prevention of oral mucositis in patients undergoing autologous hematopoietic SCT: a double‐blind, randomized, placebo‐controlled trial. Hematological Oncology 2017;35(1):106‐12. [DOI] [PubMed] [Google Scholar]
Jagasia 2012 {published data only}
- Jagasia MH, Abonour R, Long GD, Bolwell BJ, Laport GG, Shore TB, et al. Palifermin for the reduction of acute GVHD: a randomized, double‐blind, placebo‐controlled trial. Bone Marrow Transplantation 2012;47(10):1350‐5. [DOI] [PubMed] [Google Scholar]
Katano 1995 {published data only}
- Katano M, Nakamura M, Matsuo T, Iyama A, Hisatsugu T. Effect of granulocyte colony‐stimulating factor (G‐CSF) on chemotherapy‐induced oral mucositis. Surgery Today 1995;25(3):202‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2017 {published data only}
- Kim J‐W, Kim KI, Lee HJ, Kim B‐S, Bang S‐M, Kim I, et al. Interim analysis of randomized phase II study of recombinant human epidermal growth factor on oral mucositis induced by intensive chemotherapy with stem cell transplantation for hematologic malignancies. Blood 2010;116(21):905. [Google Scholar]
- Kim JW, Kim MG, Lee HJ, Koh Y, Kwon JH, Kim I, et al. Topical recombinant human epidermal growth factor for oral mucositis induced by intensive chemotherapy with hematopoietic stem cell transplantation: final analysis of a randomized, double‐blind, placebo‐controlled, phase 2 trial. PLOS One 2017;12(1):e0168854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KI, Kim JW, Lee HJ, Kim BS, Bang SM, Kim I, et al. Recombinant human epidermal growth factor on oral mucositis induced by intensive chemotherapy with stem cell transplantation. American Journal of Hematology 2013;88(2):107‐12. [DOI] [PubMed] [Google Scholar]
Le 2011 {published data only}
- Le Q, Kim H, Schneider C, Muraközy G, Skladowski K, Reinisch S, et al. Palifermin reduces severe oral mucositis in subjects with locally advanced head and neck cancer undergoing chemoradiotherapy. International Journal of Radiation Oncology, Biology, Physics 2008;72 Suppl 1:S32. [Google Scholar]
- Le QT, Kim HE, Schneider CJ, Muraközy G, Skladowski K, Reinisch S, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo‐controlled study. Journal of Clinical Oncology 2011;29(20):2808‐14. [DOI] [PubMed] [Google Scholar]
Linch 1993 {published data only}
- Linch DC, Scarffe H, Proctor S, Chopra R, Taylor PR, Morgenstern G, et al. Randomised vehicle‐controlled dose‐finding study of glycosylated recombinant human granulocyte colony‐stimulating factor after bone marrow transplantation. Bone Marrow Transplantation 1993;11(4):307‐11. [PubMed] [Google Scholar]
Lucchese 2016a {published data only}
- Lucchese A, Matarese G, Ghislanzoni LH, Gastaldi G, Manuelli M, Gherlone E. Efficacy and effects of palifermin for the treatment of oral mucositis in patients affected by acute lymphoblastic leukemia. Leukemia and Lymphoma 2016;57(4):820‐7. [DOI] [PubMed] [Google Scholar]
Lucchese 2016b {published data only}
- Lucchese A, Matarese G, Manuelli M, Ciuffreda C, Bassani L, Isola G, et al. Reliability and efficacy of palifermin in prevention and management of oral mucositis in patients with acute lymphoblastic leukemia: a randomized, double‐blind controlled clinical trial. Minerva Stomatologica 2016;65(1):43‐53. [PubMed] [Google Scholar]
Makkonen 2000 {published data only}
- Makkonen TA, Minn H, Jekunen A, Vilja P, Tuominen J, Joensuu H. Granulocyte macrophage‐colony stimulating factor (GM‐CSF) and sucralfate in prevention of radiation‐induced mucositis: a prospective randomized study. International Journal of Radiation Oncology, Biology, Physics 2000;46(3):525‐34. [DOI] [PubMed] [Google Scholar]
- Minn HR, Makkonen TA, Jekunen A, Vilja P, Tuominen J, Joensuu H. Granulocyte macrophage‐colony stimulating factor (GM‐CSF) and sucralfate in prevention of radiation‐induced mucositis: a prospective randomized study. International Journal of Radiation Oncology, Biology, Physics 1999;45 Suppl 3:239. [DOI] [PubMed] [Google Scholar]
McAleese 2006 {published data only}
- McAleese JJ, Bishop KM, A'Hern R, Henk JM. Randomized phase II study of GM‐CSF to reduce mucositis caused by accelerated radiotherapy of laryngeal cancer. British Journal of Radiology 2006;79(943):608‐13. [DOI] [PubMed] [Google Scholar]
Meropol 2003 {published data only}
- Meropol NJ, Somer RA, Gutheil J, Pelley RJ, Modiano MR, Rowinsky EK, et al. Randomized phase I trial of recombinant human keratinocyte growth factor plus chemotherapy: potential role as mucosal protectant. Journal of Clinical Oncology 2003;21(8):1452‐8. [DOI] [PubMed] [Google Scholar]
Nemunaitis 1995 {published data only}
- Nemunaitis J, Rosenfeld CS, Ash R, Freedman MH, Deeg HJ, Appelbaum F, et al. Phase III randomized, double‐blind placebo‐controlled trial of rhGM‐CSF following allogeneic bone marrow transplantation. Bone Marrow Transplantation 1995;15(6):949‐54. [PubMed] [Google Scholar]
Peterson 2009 {published data only}
- Barker NP, Peterson DE, Akhmadullina LI, Rodionova I, Sherman NZ, Gertner JM, et al. Prophylaxis of recurrent chemotherapy‐induced oral mucositis: a phase II multicenter, randomized, placebo‐controlled trial of recombinant human intestinal trefoil factor (rhITF). Journal of Clinical Oncology 2008;26:505. [Abs No 9514] [Google Scholar]
- Peterson DE, Barker NP, Akhmadullina LI, Rodionova I, Sherman NZ, Davidenko IS, et al. Phase II, randomized, double‐blind, placebo‐controlled study of recombinant human intestinal trefoil factor oral spray for prevention of oral mucositis in patients with colorectal cancer who are receiving fluorouracil‐based chemotherapy. Journal of Clinical Oncology 2009;27(26):4333‐8. [DOI] [PubMed] [Google Scholar]
Rosen 2006 {published data only}
- Clarke SJ, Abdi E, Davis ID, Schnell FM, Zalcberg JR, Gutheil J, et al. Recombinant human keratinocyte growth factor (rHuKGF) prevents chemotherapy‐induced mucositis in patients with advanced colorectal cancer: a randomized phase II trial. Proceedings of the American Society of Clinical Oncology 2001;20 (Pt 1):383a. [Abs No 1529] [Google Scholar]
- Rosen LS, Abdi E, Davis ID, Gutheil J, Schnell FM, Zalcberg J, et al. Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil‐based chemotherapy. Journal of Clinical Oncology 2006;24(33):5194‐200. [DOI] [PubMed] [Google Scholar]
Saarilahti 2002 {published data only}
- Saarilahti K, Kajanti M, Joensuu T, Kouri M, Joensuu H. Comparison of granulocyte‐macrophage colony‐stimulating factor and sucralfate mouthwashes in the prevention of radiation‐induced mucositis: a double‐blind prospective randomized phase III study. International Journal of Radiation Oncology, Biology, Physics 2002;54(2):479‐85. [DOI] [PubMed] [Google Scholar]
Schneider 1999 {published data only}
- Schneider SB, Nishimura RD, Zimmerman RP, Tran L, Shiplacoff J, Tormey M, et al. Filgrastim (r‐metHuG‐CSF) and its potential use in the reduction of radiation‐induced oropharyngeal mucositis: an interim look at a randomized, double‐blind, placebo‐controlled trial. Cytokines, Cellular and Molecular Therapy 1999;5(3):175‐80. [PubMed] [Google Scholar]
Spielberger 2004 {published data only}
- Emmanouilides C, Spielberger R, Stiff P, Rong A, Isitt J, Bensinger W, et al. Palifermin treatment of mucositis in transplant patients reduces health resource use: phase III results. Journal of Supportive Oncology 2004;2(2):77‐8. [Google Scholar]
- Spielberger R, Emmanouilides C, Stiff P, Bensinger W, Gentile T, Weisdorf D, et al. Use of recombinant human keratinocyte growth factor (palifermin) can reduce severe oral mucositis in patients with hematologic malignancies undergoing autologous peripheral blood progenitor cell transplantation after radiation‐based conditioning. Journal of Supportive Oncology 2004;2(2):73‐4. [Google Scholar]
- Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. New England Journal of Medicine 2004;351(25):2590‐8. [DOI] [PubMed] [Google Scholar]
- Stiff P, Bensinger W, Emmanouilides C, Gentile T, Weisdorf D, Shea T, et al. Treatment of mucositis with palifermin improves patient function and results in a clinically meaningful reduction in mouth and throat soreness: phase III results. Journal of Supportive Oncology 2004;2(2):75‐6. [Google Scholar]
- Stiff PJ, Emmanouilides C, Bensinger WI, Gentile T, Blazar B, Shea TC, et al. Palifermin reduces patient‐reported mouth and throat soreness and improves patient functioning in the hematopoietic stem‐cell transplantation setting. Journal of Clinical Oncology 2006;24(33):5186‐93. [DOI] [PubMed] [Google Scholar]
Su 2006 {published data only}
- Su YB. Double‐blind, randomized trial of granulocyte‐colony stimulating factor (GCSF) versus (v) placebo during postoperative radiation (RT) for advanced resectable squamous cell head and neck cancer (SCCHN): impact on mucositis. Journal of Clinical Oncology 2004;22 Suppl:14S. [Google Scholar]
- Su YB, Vickers AJ, Zelefsky MJ, Kraus DH, Shaha AR, Shah JP, et al. Double‐blind, placebo‐controlled, randomized trial of granulocyte‐colony stimulating factor during postoperative radiotherapy for squamous head and neck cancer. Cancer Journal 2006;12(3):182‐8. [DOI] [PubMed] [Google Scholar]
Vadhan‐Raj 2010 {published data only}
- Vadhan‐Raj S, Trent J, Patel S, Zhou X, Johnson MM, Araujo D, et al. Single‐dose palifermin prevents severe oral mucositis during multicycle chemotherapy in patients with cancer: a randomised trial. Annals of Internal Medicine 2010;153(6):358‐67. [DOI] [PubMed] [Google Scholar]
- Vadhan‐Raj S, Trent JC, Patel SR, Araujo DM, Ludwig LA, Bailey D, et al. Randomized, double‐blind, placebo‐controlled study of palifermin for the prevention of mucositis in patients receiving doxorubicin‐based chemotherapy. Journal of Clinical Oncology 2008;26(15 Suppl):9547. [Google Scholar]
van der Lelie 2001 {published data only}
- Lelie H, Thomas BL, Oers RH, Ek‐Post M, Sjamsoedin SA, Dijk‐Overtoom ML, et al. Effect of locally applied GM‐CSF on oral mucositis after stem cell transplantation: a prospective placebo‐controlled double‐blind study. Annals of Hematology 2001;80(3):150‐4. [DOI] [PubMed] [Google Scholar]
Wu 2009 {published data only}
- Ahn Y, Wu H, Song S, Kim Y, Oh Y, Lee C, et al. The therapeutic effect of recombinant human epidermal growth factor (rhEGF) on mucositis in patients with head and neck cancer undergoing radiotherapy with or without chemotherapy: a double‐blind placebo‐controlled prospective phase II multi‐institutional study. Journal of Clinical Oncology 2008;26(15 Suppl):6021. [Google Scholar]
- Lee S, Song S, Kim Y, Oh Y, Lee C, Keum K, et al. The therapeutic effect of recombinant human epidermal growth factor (rhEGF) on mucositis in patients with head and neck cancer undergoing radiotherapy with or without chemotherapy: a double‐blind placebo‐controlled prospective phase II multi‐institutional clinical trial. International Journal of Radiation Oncology, Biology, Physics 2008;72(1):S32. [Google Scholar]
- Wu HG, Song SY, Kim YS, Oh YT, Lee CG, Keum KC, et al. Therapeutic effect of recombinant human epidermal growth factor (RhEGF) on mucositis in patients undergoing radiotherapy, with or without chemotherapy, for head and neck cancer: a double‐blind placebo‐controlled prospective phase 2 multi‐institutional clinical trial. Cancer 2009;115(16):3699‐708. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Antin 2002 {published data only}
- Antin JH, Lee SJ, Neuberg D, Alyea E, Soiffer RJ, Sonis S, et al. A phase I/II double‐blind, placebo‐controlled study of recombinant human interleukin‐11 for mucositis and acute GVHD prevention in allogeneic stem cell transplantation. Bone Marrow Transplantation 2002;29(5):373‐7. [DOI] [PubMed] [Google Scholar]
de Koning 2007 {published data only}
- Koning BA, Philipsen‐Geerling B, Hoijer M, Hahlen K, Buller HA, Pieters R. Protection against chemotherapy induced mucositis by TGF‐beta(2) in childhood cancer patients: results from a randomized cross‐over study. Pediatric Blood & Cancer 2007;48(5):532‐9. [DOI] [PubMed] [Google Scholar]
Foncuberta 2001 {published data only}
- Foncuberta MC, Cagnoni PJ, Brandts CH, Mandanas R, Fields K, Derigs HG, et al. Topical transforming growth factor‐beta3 in the prevention or alleviation of chemotherapy‐induced oral mucositis in patients with lymphomas or solid tumors. Journal of Immunotherapy 2001;24(4):384‐8. [DOI] [PubMed] [Google Scholar]
Gebbia 1994 {published data only}
- Gebbia V, Valenza R, Testa A, Cannata G, Borsellino N, Gebbia N. A prospective randomized trial of thymopentin versus granulocyte‐colony stimulating factor with or without thymopentin in the prevention of febrile episodes in cancer patients undergoing highly cytotoxic chemotherapy. Anticancer Research 1994;14(2B):731‐4. [PubMed] [Google Scholar]
Gladkov 2013 {published data only}
- Gladkov O, Buchner A, Bias P, Mueller U, Elsaesser R. Chemotherapy‐associated treatment burden in breast cancer patients receiving lipegfilgrastim or pegfilgrastim: secondary efficacy data from a phase III study. Haematologica 2013;98(Suppl 1):426. [DOI] [PubMed] [Google Scholar]
Gordon 1993 {published data only}
- Gordon B, Spadinger A, Hodges E, Coccia P. Effect of granulocyte macrophage colony stimulating factor (GMCSF) on oral mucositis after autologous bone marrow transplantation. Proceedings of the American Society of Clinical Oncology 1993;12:432. [Abs No 1489] [DOI] [PubMed] [Google Scholar]
Horsley 2007 {published data only}
- Horsley P, Bauer JD, Mazkowiack R, Gardner R, Bashford J. Palifermin improves severe mucositis, swallowing problems, nutrition impact symptoms, and length of stay in patients undergoing hematopoietic stem cell transplantation. Supportive Care in Cancer 2007;15(1):105‐9. [DOI] [PubMed] [Google Scholar]
Hunter 2009 {published data only}
- Hunter A, Mahendra P, Wilson K, Fields P, Cook G, Peniker A, et al. A randomized, double‐blind, placebo‐controlled, multicenter trial of ATL‐104, a swallowable mouthwash, in patients with oral mucositis following peripheral blood stem cell transplantation. Journal of Supportive Oncology 2007;5(4 Suppl 2):52‐3. [Google Scholar]
- Hunter A, Mahendra P, Wilson K, Fields P, Cook G, Peniket A, et al. Treatment of oral mucositis after peripheral blood SCT with ATL‐104 mouthwash: results from a randomized, double‐blind, placebo‐controlled trial. Bone Marrow Transplantation 2009;43(7):563‐9. [DOI] [PubMed] [Google Scholar]
Ifrah 1999 {published data only}
- Ifrah N, Witz F, Jouet JP, Francois S, Lamy T, Linassier C, et al. Intensive short term therapy with granulocyte‐macrophage‐colony stimulating factor support, similar to therapy for acute myeloblastic leukemia, does not improve overall results for adults with acute lymphoblastic leukemia. American Cancer Society 1999;86(8):1496‐505. [DOI] [PubMed] [Google Scholar]
Iwase 1997 {published data only}
- Iwase M, Yoshiya M, Kakuta S, Nagumo M. Clinical trial of recombinant granulocyte colony‐stimulating factor for chemotherapy‐induced neutropenia patients with oral cancer. Journal of Oral and Maxillofacial Surgery 1997;55(8):836‐40. [DOI] [PubMed] [Google Scholar]
Jones 1996 {published data only}
- Jones SE, Schottstaedt MW, Duncan LA, Kirby RL, Good RH, Mennel RG, et al. Randomized double‐blind prospective trial to evaluate the effects of sargramostim versus placebo in a moderate‐dose fluorouracil, doxorubicin, and cyclophosphamide adjuvant chemotherapy program for stage II and III breast cancer. Journal of Clinical Oncology 1996;14(11):2976‐83. [DOI] [PubMed] [Google Scholar]
Karthaus 1998 {published data only}
- Karthaus M, Rosenthal C, Huebner G, Paul H, Elser C, Hertenstein B, et al. Effect of topical oral G‐CSF on oral mucositis: a randomised placebo‐controlled trial. Bone Marrow Transplantation 1998;22(8):781‐5. [DOI] [PubMed] [Google Scholar]
- Karthaus M, Rosenthal C, Paul H, Novotny J, Hóbner G, Fenchel K, et al. Effect of topical oral G‐CSF application on oral mucositis in high‐grade lymphoma patients treated with hd‐methotrexate (hd‐mtx) ‐ results of a randomized placebo‐controlled trial. Proceedings of the American Society of Clinical Oncology 1998;17:235. [Google Scholar]
Kubo 2016 {published data only}
- Kubo K, Miyazaki Y, Murayama T, Shimazaki R, Usui N, Urabe A, et al. A randomized, double‐blind trial of pegfilgrastim versus filgrastim for the management of neutropenia during CHASE(R) chemotherapy for malignant lymphoma. British Journal of Haematology 2016;174(4):563‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee KH, Kim JY, Lee MH, Han HS, Lim JH, Park KU, et al. A randomized, multicenter, phase II/III study to determine the optimal dose and to evaluate the efficacy and safety of pegteograstim (GCPGC) on chemotherapy‐induced neutropenia compared to pegfilgrastim in breast cancer patients: KCSG PC10‐09. Supportive Care in Cancer 2016;24(4):1709‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legros 1997 {published data only}
- Legros M, Fleury J, Bay JO, Choufi B, Basile M, Condat P, et al. rhGM‐CSF vs placebo following rhGM‐CSF‐mobilized PBPC transplantation: a phase III double‐blind randomized trial. Bone Marrow Transplantation 1997;19(3):209‐13. [DOI] [PubMed] [Google Scholar]
Limaye 2013 {published data only}
- Limaye SA, Haddad RI, Cilli F, Sonis ST, Colevas AD, Brennan MT, et al. Phase 1b, multicenter, single blinded, placebo‐controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Cancer 2013;119(24):4268‐76. [DOI] [PubMed] [Google Scholar]
- Murphy B, Limaye SA, Sonis ST, Cilli F, Brennan MT, Hu KS, et al. Phase 1B study assessing safety, tolerability and efficacy of AG013 in subjects with head and neck cancer receiving induction chemotherapy. Supportive Care in Cancer 2012;20(1 Suppl):S259. [Abs No 1078] [Google Scholar]
Nabholtz 2002 {published data only}
- Nabholtz JM, Cantin J, Chang J, Guevin R, Patel R, Tkaczuk K, et al. Phase III trial comparing granulocyte colony‐stimulating factor to leridistim in the prevention of neutropenic complications in breast cancer patients treated with docetaxel/doxorubicin/cyclophosphamide: results of the BCIRG 004 trial. Clinical Breast Cancer 2002;3(4):268‐75. [DOI] [PubMed] [Google Scholar]
NCT00360971 {published data only}
- NCT00360971. Palifermin in lessening oral mucositis in patients undergoing radiation therapy and chemotherapy for locally advanced head and neck cancer [A randomized, phase III, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of palifermin (NSC# 740548; IND # 6370) for the reduction of oral mucositis in patients with locally advanced head and neck cancer receiving radiation therapy with concurrent chemotherapy (followed by surgery for selected patients)]. clinicaltrials.gov/show/NCT00360971 (first received 3 August 2006).
NCT00626639 {published data only}
- NCT00626639. A study of palifermin for the reduction of oral mucositis in patients with locally advanced head and neck cancer receiving postoperative radiotherapy and concurrent chemotherapy [A phase 1/2 study to evaluate safety, pharmacokinetics, pharmacodynamics and preliminary efficacy of weekly doses of palifermin (rHuKGF) for the reduction of oral mucositis in subjects with locally advanced head and neck cancer (HNC) receiving postoperative radiotherapy with concurrent chemotherapy]. clinicaltrials.gov/show/NCT00626639 (first received 21 February 2008).
Pettengell 1992 {published data only}
- Pettengell R, Gurney H, Radford JA, Deakin DP, James R, Wilkinson PM, et al. Granulocyte colony‐stimulating factor to prevent dose‐limiting neutropenia in non‐Hodgkin's lymphoma: a randomized controlled trial. Blood 1992;80(6):1430‐6. [PubMed] [Google Scholar]
Ryu 2007 {published data only}
- Hoffman KE, Pugh SL, James JL, Scarantino C, Movsas B, Valicenti RK, et al. The impact of concurrent granulocyte‐macrophage colony‐stimulating factor on quality of life in head and neck cancer patients: results of the randomized, placebo‐controlled Radiation Therapy Oncology Group 9901 trial. Quality of Life Research 2014;23(6):1841‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, Swann S, LeVeque F, Scarantino CW, Johnson D, Chen A, et al. The impact of concurrent granulocyte macrophage‐colony stimulating factor on radiation‐induced mucositis in head and neck cancer patients: a double‐blind placebo‐controlled prospective phase III study by Radiation Therapy Oncology Group 9901. International Journal of Radiation Oncology, Biology, Physics 2007;67(3):643‐50. [DOI] [PubMed] [Google Scholar]
Throuvalas 1995 {published data only}
- Throuvalas N, Antonadou D, Pulizzi M, Sarris G. Evaluation of the efficacy and safety of GM‐CSF in the prophylaxis of mucositis in patients with head and neck cancer treated with RT. European Journal of Cancer 1995;31 Suppl 5:S93. [Abs No 431] [Google Scholar]
Tsurusawa 2016 {published data only}
- Tsurusawa M, Watanabe T, Gosho M, Mori T, Mitsui T, Sunami S, et al. Randomized study of granulocyte colony stimulating factor for childhood B‐cell non‐Hodgkin lymphoma: a report from the Japanese pediatric leukemia/lymphoma study group B‐NHL03 study. Leukemia & Lymphoma 2016;57(7):1657‐64. [DOI] [PubMed] [Google Scholar]
Vitale 2014 {published data only}
- Vitale KM, Violago L, Cofnas P, Bishop J, Jin Z, Bhatia M, et al. Impact of palifermin on incidence of oral mucositis and healthcare utilization in children undergoing autologous hematopoietic stem cell transplantation for malignant diseases. Pediatric Transplantation 2014;18(2):211‐6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12606000083594 {published data only}
- ACTRN12606000083594. Safety and efficacy trial of whey growth factor extract for oral mucositis [A multicentre, double‐blind, placebo‐controlled, safety and efficacy trial of whey growth factor extract (WGFEA) for oral mucositis in lymphoma patients undergoing carmustine, etoposide, cytosine arabinoside and melphalan (BEAM) chemotherapy and autologous stem cell transplantation]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=1142 (first received 27 February 2006).
Antonadou 1998 {published data only}
- Antonadou D, Athanassiou E, Synodinou M, Koliarakis N, Panoussaki K, Karageorgis P, et al. Evaluation of the efficacy of granulocyte macrophage colony stimulating factor (GM‐CSF) in the prevention of radiation induced mucositis. Radiotherapy and Oncology 1998;48 Suppl 1:S39. [Google Scholar]
Elsaid 2001 {published data only}
- Elsaid A, Farouk M. Significance of anemia and role of erythropoietin in radiation induced mucositis in head and neck cancer patients. International Journal of Radiation Oncology, Biology, Physics 2001;51(3 Suppl 1):368. [Google Scholar]
Grzegorczyk 2006 {published data only}
- Grzegorczyk‐Jazwinska A, Dwilewicz‐Trojaczek J, Kozak I, Karakulska‐Prystupiuk E, Gorska R. Effect of locally applied G‐CSF on oral mucositis after autogeneic and allogeneic stem cell transplantation. Acta Haematologica Polonica 2006;37(2):225‐40. [Google Scholar]
- Grzegorczyk‐Jazwinska A, Dwilewicz‐Trojaczek J, Kozak I, Karakulska‐Prystupiuk E, Gorska R. Effect of locally applied G‐CSF on oral mucositis after autologic stem cell transplantation. Dental and Medical Problems 2004;41(4):695‐701. [Google Scholar]
Koga 2015 {published data only}
- Koga Y, Tsurusawa M, Watanabe T, Gosho M, Mori T, Mitsui T, et al. Randomized study of granulocyte colony stimulating factor for childhood B‐cell non‐Hodgkin lymphoma: a report from the Japanese pediatric leukemia/lymphoma study Group B‐NHL03 study. British Journal of Haematology 2015;171(Suppl S1):63‐4. [DOI] [PubMed] [Google Scholar]
NCT00293462 {published data only}
- NCT00293462. GM‐CSF mouthwash for preventing and treating mucositis in patients who are undergoing radiation therapy for head and neck cancer [Management of mucositis with GM‐CSF (sargramostim) mouthwash study protocol]. clinicaltrials.gov/show/NCT00293462 (first received 16 February 2006).
NCT00393822 {published data only}
- NCT00393822. A study of palifermin for the reduction of oral mucositis in subjects with stage 2B or 3 locally advanced, colon cancer [A randomized, double‐blind, placebo‐controlled study evaluating the efficacy and safety of palifermin (recombinant human keratinocyte growth factor) for reduction of oral mucositis in subjects with stage 2B or 3 locally advanced, colon cancer receiving 5‐FU and leucovorin as adjuvant therapy]. clinicaltrials.gov/show/NCT00393822 (first received 26 October 2006).
NCT02303197 {published data only}
- NCT02303197. Clinical trial on the efficacy and safety of Chining decoction in the treatment of radiation stomatitis [A randomized, controlled clinical trial on the efficacy and safety of Chining decoction in the treatment of radiation stomatitis]. clinicaltrials.gov/show/NCT02303197 (first received 25 November 2014).
NCT02313792 {published data only}
- NCT02313792. Palifermin for patients receiving hematopoietic stem cell transplantation [Efficacy, safety and quality of life of palifermin on reducing oral mucositis in patients with hematopoietic stem cell transplantation, prospective double‐blind randomized phase III trial]. clinicaltrials.gov/show/NCT02313792 (first received 5 December 2014).
Patte 2002 {published data only}
- Patte C, Laplanche A, Bertozzi AI, Baruchel A, Frappaz D, Schmitt C, et al. Granulocyte colony‐stimulating factor in induction treatment of children with non‐Hodgkin's lymphoma: a randomized study of the French Society of Pediatric Oncology. Journal of Clinical Oncology 2002;20(2):441‐8. [DOI] [PubMed] [Google Scholar]
Schuster 2007a {published data only}
- Schuster MW, Anaissie E, Hurd D, Bensinger W, Mason J, McCarty J, et al. Final analysis of the phase II, randomized, double‐blind, placebo‐controlled trial of single‐dose velafermin (CG53135‐05) for the prevention of oral mucositis. Journal of Supportive Oncology 2007;5(4 Suppl 2):58‐9. [Google Scholar]
Schuster 2007b {published data only}
- Schuster MW, Mehta J, Waller EK, Rifkin RM, Micallef I, Hurd D, et al. A randomized placebo controlled phase II trial of prevention of severe oral mucositis using single dose velafermin in patients receiving myeloablative therapy and autologous hematopoietic stem cell transplant (AHSCT). Blood 2007;110(11):615. [Google Scholar]
Shea 2007 {published data only}
- Shea TC, Kewalramani T, Mun Y, Jayne G, Dreiling LK. Evaluation of single‐dose palifermin to reduce oral mucositis (OM) in fractionated total body irradiation (fTBI) and high dose (HD) chemotherapy with autologous peripheral blood progenitor cell (PBPC) transplantation. Blood 2006;108(11 Part 1):875‐6. [Google Scholar]
- Shea TC, Kewalramani T, Mun Y, Jayne G, Dreiling LK. Evaluation of single‐dose palifermin to reduce oral mucositis in fractionated total‐body irradiation and high‐dose chemotherapy with autologous peripheral blood progenitor cell transplantation. Journal of Supportive Oncology 2007;5(4 Suppl 2):60‐2. [Google Scholar]
Spielberger 2001 {published data only}
- Spielberger RT, Stiff P, Emmanouilides C, Yanovich S, Bensinger W, Hedrick E, et al. Efficacy of recombinant human keratinocyte growth factor (rHuKGF) in reducing mucositis in patients with hematologic malignancies undergoing autologous peripheral blood progenitor cell transplantation (auto‐PBPCT) after radiation‐based conditioning ‐ results of a phase 2 trial. Proceedings of the American Society of Clinical Oncology 2001;20(Pt 1):7a. [Abs No 25] [Google Scholar]
Additional references
Al‐Dasooqi 2013
- Al‐Dasooqi N, Sonis ST, Bowen JM, Bateman E, Blijlevens N, Gibson RJ, et al. Emerging evidence on the pathobiology of mucositis. Supportive Care in Cancer 2013;21(11):3233‐41. [DOI] [PubMed] [Google Scholar]
Bellm 2002
- Bellm LA, Cunningham G, Durnell L, Eilers J, Epstein JB, Fleming T, et al. Defining clinically meaningful outcomes in the evaluation of new treatments for oral mucositis: oral mucositis patient provider advisory board. Cancer Investigation 2002;20(5‐6):793‐800. [DOI] [PubMed] [Google Scholar]
Boers‐Doets 2013
- Boers‐Doets CB, Raber‐Durlacher JE, Treister NS, Epstein JB, Arends AB, Wiersma DR, et al. Mammalian target of rapamycin inhibitor‐associated stomatitis. Future Oncology 2013;9(12):1883‐92. [DOI] [PubMed] [Google Scholar]
Clarke 2007
- Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials 2007;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwan 2008
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLOS One 2008;3(8):e3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elting 2008
- Elting LS, Keefe DM, Sonis ST, Garden AS, Spijkervet FK, Barasch A, et al. Patient‐reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 2008;113(10):2704‐13. [DOI] [PubMed] [Google Scholar]
Epstein 1999
- Epstein JB, Emerton S, Kolbinson DA, Le ND, Phillips N, Stevenson‐Moore P, et al. Quality of life and oral function following radiotherapy for head and neck cancer. Head & Neck 1999;21(1):1‐11. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jensen 2014
- Jensen SB, Peterson DE. Oral mucosal injury caused by cancer therapies: current management and new frontiers in research. Journal of Oral Pathology and Medicine 2014;43(2):81‐90. [DOI] [PubMed] [Google Scholar]
Lalla 2008
- Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dental Clinics of North America 2008;52(1):61‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lalla 2014
- Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014;120(10):1453‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lyman 2010
- Lyman GH, Dale DC, Wolff DA, Culakova E, Poniewierski MS, Kuderer NM, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony‐stimulating factor: a systematic review. Journal of Clinical Oncology 2010;28(17):2914‐24. [DOI] [PubMed] [Google Scholar]
Miller 2001
- Miller M, Kearney N. Oral care for patients with cancer: a review of the literature. Cancer Nursing 2001;24(4):241‐54. [DOI] [PubMed] [Google Scholar]
Nonzee 2008
- Nonzee NJ, Dandade NA, Patel U, Markossian T, Agulnik M, Argiris A, et al. Evaluating the supportive care costs of severe radiochemotherapy‐induced mucositis and pharyngitis. Cancer 2008;113(6):1446‐52. [DOI] [PubMed] [Google Scholar]
Peterson 2015
Raber‐Durlacher 2013
- Raber‐Durlacher JE, Bültzingslöwen I, Logan RM, Bowen J, Al‐Azri AR, Everaus H, et al. Systematic review of cytokines and growth factors for the management of oral mucositis in cancer patients. Supportive Care in Cancer 2013;21(1):343‐55. [DOI] [PubMed] [Google Scholar]
Riley 2017
- Riley P, Glenny AM, Hua F, Worthington HV. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD012744] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scully 2006
- Scully C, Sonis S, Diz PD. Oral mucositis. Oral Diseases 2006;12(3):229‐41. [DOI] [PubMed] [Google Scholar]
Sonis 2001
- Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem‐cell transplantation. Journal of Clinical Oncology 2001;19(8):2201‐5. [DOI] [PubMed] [Google Scholar]
Sonis 2004
- Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer‐Jensen M, et al. Perspectives on cancer therapy‐induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100(9 Suppl):1995‐2025. [DOI] [PubMed] [Google Scholar]
Sonis 2009
- Sonis ST. Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncology 2009;45(12):1015‐20. [DOI] [PubMed] [Google Scholar]
Trotti 2003
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiotherapy and Oncology 2003;66(3):253‐62. [DOI] [PubMed] [Google Scholar]
Turhal 2000
- Turhal NS, Erdal S, Karacay S. Efficacy of treatment to relieve mucositis‐induced discomfort. Supportive Care in Cancer 2000;8(1):55‐8. [DOI] [PubMed] [Google Scholar]
von Bültzingslöwen 2006
- Bültzingslöwen I, Brennan MT, Spijkervet FK, Logan R, Stringer A, Raber‐Durlacher JE, et al. Growth factors and cytokines in the prevention and treatment of oral and gastrointestinal mucositis. Supportive Care in Cancer 2006;14(6):519‐27. [DOI] [PubMed] [Google Scholar]
Williamson 2005
- Williamson PR, Gamble C, Altman DG, Hutton JL. Outcome selection bias in meta‐analysis. Statistical Methods in Medical Research 2005;14(5):515‐24. [DOI] [PubMed] [Google Scholar]
Williamson 2012
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision‐making. Evidence‐based Dentistry 2015;16(3):69‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Riley 2015
- Riley P, Glenny AM, Worthington HV, Littlewood A, Clarkson JE, McCabe MG. Interventions for preventing oral mucositis in patients with cancer receiving treatment: cytokines and growth factors. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011990] [DOI] [PMC free article] [PubMed] [Google Scholar]
Worthington 2011
- Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny AM, Littlewood A, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD000978.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]


