Abstract
Background
Fixed prosthodontic treatment (crowns, fixed dental prostheses (FDPs), complete arch prostheses) involves the use of several different materials to replace missing tooth structure. Traditionally full metal or metal frameworks veneered with ceramic (metal‐ceramic (MC)) have been used. In recent years several different metal‐free systems have become available to clinicians and patients. In general, metal‐free restorations should allow practitioners to better reproduce natural tooth colour, avoiding shortcomings of MC restorations. The comparative in service clinical performance of fixed prosthodontic treatments of different materials is unclear.
Objectives
To assess the effects of metal‐free materials for prosthodontic restorations compared to metal‐ceramic or other conventional all‐metal materials.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (searched 3 May 2017), Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4) in the Cochrane Library (searched 3 May 2017), MEDLINE Ovid (1946 to 3 May 2017), and Embase Ovid (1980 to 3 May 2017). The US National Institutes of Health Trials Registry (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials (searched 3 May 2017). No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
Randomised controlled trials (RCTs) in which the clinical performance of metal‐free fixed prosthodontic restorations was compared with metal‐ceramic (MC) or other conventional restorations in adult patients requiring prosthodontic treatment. RCTs in which the clinical performance of different kinds of metal‐free systems were compared among themselves were also considered.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Screening of eligible studies, assessment of the methodological quality of the trials and data extraction were conducted independently and in duplicate. Trial authors were contacted for missing information. Available results for the outcomes of interest of the systematic review of the studies included were tabulated as they could not be included in a formal meta‐analysis.
Main results
Nine trials involving a total of 448 participants were included. We judged two trials to be at unclear risk of bias and seven to be at high risk of bias. The majority of items of risk of bias were evaluated to be at unclear or high risk level in more than 50% of the included trials. Each trial except two was addressing a different type of intervention. All evidence was rated as being of very low quality due to problems with risk of bias and imprecision of results, the latter being due to very small sample sizes, low event rates, 95% confidence intervals including the possibility of benefit for both the test and control groups, or combinations of these problems. This means that we are very uncertain about all of the results presented in this review.
One trial compared metal‐free single crowns (full contour zirconia) to cast gold single crowns in 224 participants and found insufficient evidence of a difference in failure rate after one year, but after five years there was some evidence of a benefit for the gold crowns. There was insufficient evidence of a difference for crown complications at either time of assessment.
One trial compared three‐unit metal‐free FDPs (lithium disilicate) to three‐unit metal‐ceramic FDPs in 37 participants. There was insufficient evidence of a difference in bridge failure at one and six years, but some evidence of a benefit for the lithium disilicate group in terms of bridge complications at six years. One trial compared zirconia‐ceramic FDPs to metal‐ceramic FDPs in 34 participants but found insufficient evidence of a difference in bridge failures (i.e. no failures in either treatment group), bridge complications or patients' aesthetic evaluation at any time of assessment up to three years.
One trial compared metal‐free cantilevered FDPs to metal‐ceramic cantilevered FDPs in 21 participants. There was insufficient evidence of a difference for any primary outcome: bridge failures (i.e. no failures in either treatment group), bridge complications, or patients' aesthetic evaluation at any time of assessment up to three years.
One trial compared metal‐free implant‐supported screw retained single crowns (zirconia veneered with feldspathic ceramic) to metal‐ceramic implant‐supported screw‐retained single crowns in 20 participants. There was insufficient evidence of a difference for any primary outcome: crown failures (i.e. no failures in either treatment group), crown complications, or satisfaction/aesthetic evaluation at any time of assessment up to two years.
Two trials compared metal‐free implant abutments (zirconia) to metal implant abutments both supporting single crowns in 50 participants. There was insufficient evidence of a difference in abutment failure at one year.
One trial compared metal‐free implant‐supported FDPs made of two different types of zirconia ceramic in 18 participants. There was insufficient evidence of a difference in failures at any time of assessment up to 10 years (i.e. no failures in either treatment group). There was some evidence of a benefit for the zirconia‐toughened alumina group in terms of complications (chipping).
One trial compared metal‐free tooth‐supported FDPs made with two different veneering techniques (pressed versus layered) in 40 participants. There was insufficient evidence of a difference for failures (i.e. no failures in either treatment group) or complications at any time of assessment up to three years.
Authors' conclusions
There is insufficient evidence to support or refute the effectiveness of metal‐free materials for fixed prosthodontic treatment over metal‐ceramic or other type of standard restorations. The overall quality of existing evidence was very low, therefore great caution should be exercised when generalising the results of the included trials. Until more evidence becomes available clinicians should continue to base decisions on which material to use for fixed prosthodontic treatment on their own clinical experience, whilst taking into consideration the individual circumstances and preferences of their patients. There is urgent need of properly designed RCTs.
Plain language summary
Metal‐free materials for making crowns and bridges
Review question
To compare the effects of metal‐free materials to metal‐ceramic or other conventional all‐metal materials for prosthodontic treatments aimed to restore severely damaged teeth or to replace missing teeth.
Background
Fixed prosthodontic treatment is a routine dental procedure in which one or more missing or severely damaged teeth are replaced by artificial substitutes. The material used to make the prosthesis may be made of a metal framework with a veneering of an aesthetic material (ceramic) or entirely in metal or it can be made with different non‐metal structures (metal‐free materials). There is still uncertainty regarding metal‐free long‐term performance compared to metal‐based crowns and bridges.
Study characteristics
This review of existing studies was carried out by Cochrane Oral Health authors and the evidence is current up to 3 May 2017. We searched scientific databases for randomised controlled trials (studies where people are randomly put into one of two or more treatment groups) comparing different types of materials for prosthodontic treatment in people who were followed up for at least one year.
Of the nine included trials three were conducted in Germany, one in Sweden, one in Spain, one in Switzerland and the USA, one in Denmark, one in Italy, and one in Switzerland. All the included trials were single‐centre conducted at university dental clinics and had a parallel‐group study design. All the included trials received support from industry.
Key results
The review included nine studies with 448 participants in which a total of 224 crowns and 132 bridges on natural teeth, and a total of 74 crowns and 25 bridges on implants were used. Each trial was addressing a different type of intervention. The studies had durations up to 10 years but included very small numbers of participants and were assessed as at unclear or high risk of bias. Based on these studies, there is currently insufficient reliable evidence to support which of these materials are more effective.
Quality of the evidence
Two trials were at unclear risk of bias and seven were at high risk of bias. The overall quality of evidence was very low, therefore caution should be exercised when generalising the results of the included trials. Future research should aim to provide more reliable information which can help clinicians to decide on appropriate materials for fixed prosthodontic treatment whilst taking into consideration the individual circumstances and preferences of their patients.
Summary of findings
Summary of findings for the main comparison. Summary of findings: all comparisons.
| Metal‐free materials compared with metal‐ceramic or other conventional all‐metal materials for prosthodontic restorations |
|
Patient or population: adults (18 years of age or older) with prosthodontic restorations Settings: primary or secondary care Intervention: metal‐free materials Comparison: metal‐ceramic or other conventional all‐metal materials |
| This review is made up almost entirely of single‐study comparisons of very small studies. For each comparison, the evidence for the primary outcomes 'failure of the prosthesis', 'complications' and 'aesthetic evaluation' at all times of assessment was rated as being very low quality. All bodies of evidence were downgraded by 1 level for risk of bias and by 2 levels for imprecision (due to single‐study comparisons with either very small sample sizes, low event rates, 95% CIs including the possibility of benefit for both the test and control groups, or combinations of these problems) This review has included studies assessing the following comparisons 1) Metal‐free single crowns compared to conventional crowns 2) Metal‐free FDPs compared to metal‐ceramic FDPs 3) Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs 4) Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns 5) Metal‐free abutments compared to metal abutments supporting single crowns 6) Metal‐free implant‐supported FDPs made of different materials 7) Metal‐free tooth‐supported FDPs made of different materials |
| CI: confidence interval; FDPs: fixed dental prostheses. |
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate |
Background
Description of the condition
Missing or severely damaged teeth may result in a functional and aesthetic deficit and have traditionally been replaced with fixed prosthodontic treatment (crowns or bridges).
Description of the intervention
When fabricating conventional metal–ceramic prosthodontic restorations (crowns, fixed dental prostheses (FDPs), complete arch prostheses), the presence of a metal framework makes it more difficult to imitate natural aesthetics, especially in anterior areas, where space is limited and challenging aesthetic demands need to be satisfied (high translucency). Metal‐ceramic (MC) restorations have been widely used in fixed prosthodontics for more than 50 years. The aesthetic quality and functional longevity of MC restorations may vary, but the clinical performance of these restorations is rather predictable. Indeed, the long‐term survival rate of MC restorations has been estimated to be approximately 92% after 10 years (Scurria 1998) and 75% after 15 years (Creugers 1994; Scurria 1998). In the search for more aesthetic restorations, alumina‐reinforced porcelain jacket crowns were introduced in the mid1960s (McLean 1967), but they had a high failure rate. In the last 15 years many metal‐free systems have been proposed (Conrad 2007; Manicone 2007). Many ceramics, such as spinel, alumina, ceramic reinforced with lithium disilicate, yttrium‐stabilized zirconia have been proposed for the construction of metal‐free restorations (Conrad 2007; Harder 2009). Polymeric materials have also been used both in tooth‐supported crowns, FDPs and in implant‐supported prostheses (Behr 2003; Bergendal 1995), due to their lower cost. Currently, several different metal‐free systems are available to clinicians and patients. In general, metal‐free restorations not only allow practitioners to better reproduce natural tooth colour, but also to avoid discolouration of the gingival tissues (greying or lower value), as often occurs with MC restorations.
Despite growing interest, however, concern exists about possible adverse outcomes of metal‐free materials, such as an increased risk of failure of the restoration. Of course to be suitable for reliable clinical applications, long‐term results similar to those of metal–ceramic reconstructions should be achieved with metal‐free systems. Several systematic reviews attempted to calculate long‐term survival of metal‐free restorations in comparison to conventional therapies (Pjetursson 2007; Sailer 2007; Sailer 2009a, Schley 2010).
Why it is important to do this review
The choice of restorative material for fixed prosthodontic treatment is critical for long‐term effectiveness. However, there is still uncertainty about the comparative clinical performance of crowns, FDPs, and complete arch prostheses made with different materials with or without metal used to restore severely damaged or missing teeth. The results of this review may better inform clinical decision making in the choice of either of these materials for different clinical situations. Moreover some patients are 'metalphobic', feeling that metals in their restorations could cause systemic health problems, although the possibility of such influence is not demonstrated.
Objectives
To assess the effects of metal‐free materials for prosthodontic restorations compared to metal‐ceramic or other conventional all‐metal materials.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) were considered in which the clinical performance of metal‐free fixed prosthodontic restorations was compared with metal‐ceramic (MC) or other conventional restorations with a minimum follow‐up of 12 months. RCTs in which the clinical performance of different kinds of metal‐free systems were compared among themselves were also included. Trials performed in a primary or secondary care setting were included.
Types of participants
Adult patients (18 years of age or older) who received fixed prosthodontic restorative treatment.
Types of interventions
All types of metal‐free materials for fixed prosthodontic treatment. Studies comparing metal‐free systems (single crowns, fixed dental prostheses (FDPs), complete arch prostheses) among themselves and to MC systems or other conventional prosthodontic restorations were considered. Studies comparing metal‐free prosthodontic components connected to implants (abutments, crowns, FDPs, complete arch prostheses) to all metal (gold, titanium, semiprecious, etc.) components were included.
Studies comparing implant materials were excluded.
Types of outcome measures
Primary outcomes
Failure of the prosthesis
The main primary outcome was longevity of the restoration. The nature of the outcome data is dichotomous. The following events were defined as failures.
Non‐repairable fracture of the prosthesis.
Fracture or loss of supporting tooth.
Fracture or loss of implant abutment.
Non‐restorable secondary caries on natural tooth.
Loosening of an implant abutment screw leading to replacement of the prosthesis.
Complications
The following adverse events were defined as complications.
Repairable fractures of the prosthesis (cracks, chipping, delamination).
Restorable secondary caries on natural tooth.
Severe unfavourable periodontal or peri‐implant response or severe reaction of the adjacent mucosa.
Loosening of an implant abutment screw not leading to replacement of the prosthesis.
The nature of the outcome data is dichotomous.
Aesthetic evaluation
The following aesthetic parameters were considered.
Aesthetics evaluated by dentist by any validated aesthetic index (continuous or ordinal outcome).
Aesthetics evaluated by the patient (continuous or ordinal outcome).
Aesthetic preference evaluated by the patient in split‐mouth design studies (ordinal outcome: better, no difference or worse).
Primary outcome data were recorded at 12 months (all studies), 36 months and 60 months (when available) time intervals.
Secondary outcomes
-
Periodontal or peri‐implant status evaluated through:
plaque index (PI, dichotomous outcome);
bleeding on probing (BOP, dichotomous outcome);
probing attachment level (PAL, continuous outcome);
gingival recession (REC, continuous outcome);
marginal bone level (MBL) around implants measured on intraoral radiographs taken with a parallel technique (continuous outcome).
Occlusal wear evaluated through any validated system (continuous outcome).
Secondary outcome data were recorded at 12 months (all studies), 36 months and 60 months (when available) time intervals.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 3 May 2017) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4) in the Cochrane Library (searched 3 May 2017) (Appendix 2);
MEDLINE Ovid (1946 to 3 May 2017) (Appendix 3);
Embase Ovid (1980 to 3 May 2017) (Appendix 4).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Searching other resources
The following trial registries were searched for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 3 May 2017) (Appendix 5);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 3 May 2017) (Appendix 6).
The reference lists of any articles about clinical trials identified and the review authors' personal lists of previously found articles were cross‐checked for additional trials published outside the searched databases.
Authors of RCTs identified and personal contacts were written to in an attempt to identify unpublished or ongoing trials.
Metal‐free system manufacturers were contacted to request information about possible ongoing trials.
Data collection and analysis
Selection of studies
The titles and abstracts (when available) of all reports identified through the electronic searches were examined independently by two review authors (Carlo E Poggio (CEP), Carlo Ercoli (CE)). Reports not matching the inclusion criteria were excluded. For studies appearing to meet the inclusion criteria, or for those for which there was insufficient data in the title and abstract to make a clear decision, the full report was obtained. The full reports obtained from all the electronic and other methods of searching were assessed independently by two review authors to establish whether the studies meet the inclusion criteria or not. Duplicate records of the same report were removed. When necessary, authors were contacted for clarification. Disagreements were resolved by discussion. Where resolution would not be possible, a third review author (Marco Esposito (ME)) was consulted. All irrelevant records were excluded and the details and the reasons for their exclusion were noted in the 'Characteristics of excluded studies' section of this review.
Data extraction and management
Study details were entered into the 'Characteristics of included studies' table in Review Manager 5 (Review Manager 2014).
Two review authors (CEP and Lorena Rispoli (LR)) extracted data independently and in duplicate using specially designed data collection forms. The data collection forms were piloted on several papers and modified as required before use. The review authors included data only if there was an independently reached consensus; any disagreements were resolved by consulting with a third review author (ME).
Where necessary, authors were asked for clarification or missing information.
We extracted the following details for each trial.
Year of publication, country of origin and source of study funding.
Details of the participants including demographic characteristics and criteria for inclusion.
Details of the type of intervention.
Details of the outcomes reported, including method of assessment and time intervals.
Risk of bias assessment
Assessment of risk of bias in included studies
The recommended approach for assessing risk of bias in studies included in Cochrane Reviews was followed (Higgins 2011). A two‐part tool was used, addressing the domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other bias. Each domain included one or more specific entries in a 'Risk of bias' table. Within each entry, the first part of the tool involved describing what was reported to have happened in the study. The second part of the tool involved assigning a judgement relating to the risk of bias for that entry.
Two review authors independently carried out the risk of bias assessment as part of the data extraction process (CEP, CE).
After taking into account the additional information provided by the authors of the trials, the studies were grouped into the following categories.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
A 'Risk of bias' table was completed for each included study in the 'Characteristics of included studies' table and the results were presented graphically.
Measures of treatment effect
For dichotomous data, the estimates of effect of an intervention were expressed as odds ratios together with 95% confidence intervals.
For continuous outcomes, mean differences and 95% confidence intervals were used to summarise the data for each group where the mean difference and standard deviations were calculable from the data presented.
Unit of analysis issues
The statistical unit was the patient and not the prosthesis. No split‐mouth studies were found.
Dealing with missing data
Whenever possible, we contacted the original investigators to request missing data. If no additional information was available no imputation was performed and data for only those participants whose results were known were included. The potential impact of the missing data was addressed in the assessment of risk of bias.
Assessment of heterogeneity
The paucity of studies included in this review did not permit any assessment of heterogeneity but in future updates and if further studies are included, the following methods will apply.
The significance of any discrepancies in the estimates of the treatment effects from the different trials will be assessed by means of Cochran's test for heterogeneity and the I2 statistic, which describes the percentage total variation across studies that is due to heterogeneity rather than chance. Heterogeneity will be considered statistically significant if the P value is < 0.1. A rough guide to the interpretation of the I2 statistic given in the Cochrane Handbook for Systematic Reviews of Interventions is: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, 75% to 100% considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
If a sufficient number of studies assessing similar interventions had been identified for inclusion in this review we planned to assess publication bias according to the recommendations on testing for funnel plot asymmetry as described in Section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If asymmetry was identified we would attempt to assess other possible causes and these would be explored in the discussion if appropriate.
Data synthesis
Available results for the outcomes of interest of the systematic review of the studies included were tabulated as they could not be included in a formal meta‐analysis.
If future updates include a sufficient number studies (> 2) investigating similar interventions, the data analysis will be conducted in Review Manager 5 (Review Manager 2014) and the following methods will apply: odds ratios will be combined for dichotomous data, and mean differences for continuous data, using random‐effects models if there are more than three trials, otherwise fixed‐effect models will be used.
If future updates include data from split‐mouth studies they will be combined with data from parallel‐group trials with the method outlined by Elbourne (Elbourne 2002), using the generic inverse variance method in Review Manager 5.
Subgroup analysis and investigation of heterogeneity
If sufficient data are available in future updates, a subgroup analysis for anterior (canine, lateral and central incisors) and posterior (premolars and molars) restorations (single crowns and fixed dental prostheses (FDPs)) will be conducted.
Sensitivity analysis
If there are sufficient included trials in future updates, sensitivity analyses will be undertaken to assess the robustness of the review results, excluding trials at high risk of bias on the assessment of the overall estimates of effect.
Presentation of main results
We intended to produce 'Summary of findings' tables for the main comparisons and primary outcomes of this review using GRADEpro GDT software (GRADEpro GDT 2015). However, there were too many comparisons with a very small amount of evidence and we decided to summarise everything in one table. To assess the quality of the evidence we considered the following factors: risk of bias, imprecision, inconsistency, indirectness and potential for publication bias.
Results
Description of studies
Results of the search
The electronic searches retrieved 3143 references to studies after de‐duplication, out of which 3087 did not match our inclusion criteria, were clearly ineligible and were eliminated. We obtained full‐text copies of the remaining potentially eligible 56 studies. After evaluation 22 studies (30 reports) were excluded for reasons described in the Characteristics of excluded studies section of this review. We included nine studies (14 reports, five being follow‐up articles of three trials (Encke 2009; Larsson 2006; Ohlmann 2012)). No additional study over and above those that had already been identified in the electronic search was found.
Our searches of the trial registries did identify 12 ongoing trials potentially relevant to this review (DRKS00005452; DRKS00010423; DRKS00011173; NCT01229995; NCT01729858; NCT01835821; NCT02175329; NCT02188212; NCT02272491; NCT02758457; NCT02937220; NCT03039985).
See Figure 1.
1.
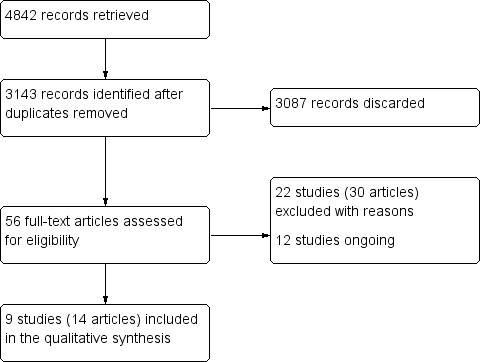
Study flow diagram.
Included studies
See Characteristics of included studies table.
We identified nine trials to be included in this review (Albornoz 2014; Baldini 2016; Encke 2009; Gallucci 2011; Larsson 2006; Makarouna 2011; Naenni 2015; Nicolaisen 2016; Ohlmann 2012).
Characteristics of trial setting and investigators
Of the nine included trials three were conducted in Germany (Encke 2009; Makarouna 2011; Ohlmann 2012), one in Spain (Albornoz 2014), one in Sweden (Larsson 2006), one in Switzerland and USA (Gallucci 2011), one in Switzerland (Naenni 2015), one in Italy (Baldini 2016), and one in Denmark (Nicolaisen 2016).
All the included trials were single centre.
All the included trials had a parallel‐group study design.
All the included trials were conducted at university dental clinics or hospitals.
All the included trials received support from industry.
Characteristics of the participants
A total of 448 participants were included in the nine trials. All patients were adults.
Inclusion criteria
Generic inclusion/exclusion criteria were common to all studies (patients above 18 to 21 years of age, no relevant medical conditions).
Specific inclusion criteria were different according to the different types of interventions planned: need of a single crown in the molar or premolar region (Encke 2009), one missing tooth in the aesthetic zone for single implant supported crowns (Albornoz 2014; Baldini 2016; Gallucci 2011), need of an implant‐supported bridge in the posterior region (Larsson 2006), need to replace one premolar or incisor (Ohlmann 2012), need of a three‐unit bridge (Makarouna 2011; Naenni 2015; Nicolaisen 2016).
Inclusion criteria for each study are described in the Characteristics of included studies tables.
Exclusion criteria
Exclusion criteria for each study are described in the Characteristics of included studies tables.
Four trials indicated bruxism as an exclusion criteria (Albornoz 2014; Larsson 2006; Ohlmann 2012; Nicolaisen 2016), while one study generically indicated "pronounced parafunctions" as an exclusion criteria (Makarouna 2011).
Characteristics of the interventions
(1) Metal‐free single crowns compared to conventional crowns.
One trial (Encke 2009) compared metal‐free single crowns (full contour zirconia, Everest HPC, KaVo Dental GmbH, Biberach/Riss, Germany) to cast gold single crowns (Degulor M, Degudent). Treatments were carried out in the University Dental Hospital Freiburg. Different standardized procedures for tooth preparation were used for the two groups: for the metal‐free crowns a deep (1.2 mm) chamfer margin and an occlusal reduction of 1.5 mm while for the conventional metal crowns a 0.8 mm chamfer and an occlusal reduction of 1.2 mm. Zirconia crowns were milled out of KaVo Everest HPC blanks, and subsequently sintered before try‐in and cementation.
(2) Metal‐free fixed dental prostheses (FDPs) compared to metal‐ceramic FDPs.
One trial (Makarouna 2011) compared metal‐free three‐unit FDPs (lithium disilicate, Ivoclar Vivadent) to metal‐ceramic three‐unit FDPs. 37 patients treated in 2001 to 2003 were split into two groups. The metal‐free group received 18 lithium disilicate FPDs, while the metal‐ceramic group received 19 conventional FPDs. The clinical protocol was standardized for the two groups and comprised chamfer preparation with rounded smooth contours, a monophase impression with a custom tray, try‐in and luting with Vivaglass CEM glass ionomer cement (Ivoclar Vivadent).
One trial (Nicolaisen 2016) compared metal‐free three‐unit FDPs (zirconia framework, BEGO veneered with ceramic VITA Zahnfabrik) to metal‐ceramic three‐unit FDPs (gold platinum alloy framework, Bio Ponto Star BEGO, veneered with ceramic VITA Zahnfabrik). 34 patients split in two groups of 17 each received either an all ceramic or a metal‐ceramic FDP. A chamfer preparation was used for the metal‐free group while a mixed preparation with a shoulder and a chamfer was used for the metal‐ceramic group. Frameworks were tried in and subsequently veneered and cemented with a resin enhanced glass ionomer cement (Ketac Cem Plus, 3M ESPE).
(3) Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs.
One trial (Ohlmann 2012) compared metal‐free cantilevered FDPs (Lava, 3M ESPE) to metal‐ceramic cantilevered FDPs. Tooth preparation had for both groups same standards (minimal occlusal reduction 1.5 mm, axial reduction (chamfer design) 1.2 mm, convergence preparation angle 6 degrees). For the metal‐free group frameworks were milled from prefabricated zirconia blanks and then sintered. Frameworks were veneered with feldspathic ceramic, tried‐in, adjusted, repolished and cemented with a resin cement (Rely X Unicem, 3M ESPE). For the metal‐ceramic group cantilevered FDPs were made according to standardized manufacturer's instructions.
(4) Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns.
One trial (Gallucci 2011) compared metal‐free implant‐supported single crowns (zirconia) to metal‐ceramic implant‐supported single crowns. For the metal‐free group, a screwed retained all ceramic crown was fabricated using a blank composed of 90% alumina with glass infiltration (synOcta, InCeram blank, and synOcta abutment, Straumann Co.) and veneered with alumina ceramic. For the metal‐ceramic group, a screwed retained metal‐ceramic crown was fabricated and veneered with feldspathic ceramic.
(5) Metal‐free implant abutments compared to metal implant abutments.
One trial (Albornoz 2014) compared metal‐free implant abutments to metal implant abutments. For the metal‐free group a zirconia abutment (SPIART; Thommen Medical AG, Grenchen, Switzerland) was used to support a metal‐free single crown (feldspathic veneered zirconia). For the metal group a commercially pure titanium grade 4 abutment (CPTi Gr 4; SPIEASY; Thommen Medical AG, Grenchen, Switzerland) was used to support a metal‐free single crown (feldspathic veneered zirconia).
One trial (Baldini 2016) compared metal‐free implant abutments to metal implant abutments. For the metal‐free group a zirconia abutment (SPIART; Thommen Medical AG, Grenchen, Switzerland) was used to support a metal‐free single crown (feldspathic veneered zirconia). For the metal group a commercially pure titanium grade 4 abutment (CPTi Gr 4; SPIEASY; Thommen Medical AG, Grenchen, Switzerland) was used to support a metal‐ceramic single crown.
(6) Metal‐free implant‐supported FDPs made of different materials.
One trial (Larsson 2006) compared metal‐free implant‐supported FDPs made of two different types of zirconia ceramic. Titanium implant abutments were prepared with a cervical shoulder depth of 1.2 mm and slightly rounded inner angles, a minimum occlusal thickness of 1.7 mm and minimum buccal, approximal, and lingual/palatal thicknesses of 1.5 mm. Frameworks of zirconia‐toughened alumina (In‐Ceram Zirconia, Vita Zahnfabrik) and of yttria‐stabilized tetragonal zirconia polycrystal material (Denzir, Decim) were fabricated and veneered with porcelain. FDPs were cemented permanently with zinc phosphate cement (De Trey zinc crown and bridge Fixodont Plus, Dentsply) in one sitting.
(7) Metal‐free tooth‐supported FDPs made of different materials.
One trial (Naenni 2015) compared metal‐free tooth‐supported FDPs made with two different techniques of veneering. 20 patients were allocated to two groups. Test group (20 patients) received a zirconia–ceramic FDP (IPS e.max ZirCAD, Ivoclar Vivadent) with pressed veneering ceramic, control group (20 patients) received a zirconia–ceramic FDP (IPS e.max ZirCAD, Ivoclar Vivadent) with conventionally layered veneering ceramic.
Characteristics of the outcome measures
The primary outcome failure of the prosthesis was reported in all the included trials.
The primary outcome complications not leading to replacement of the prosthesis was reported in all the included trials.
The primary outcome aesthetics evaluated by the dentist was reported in three trials (Albornoz 2014; Baldini 2016; Gallucci 2011). The Implant Crown Aesthetic Index (ICAI) was calculated in Albornoz 2014 and in Baldini 2016. The ICAI was assessed at follow‐ups by a blinded observer on standardized pictures. It included the following parameters: mesiodistal dimension of the crown, position of the incisal edge of the crown, labial convexity of the crown, colour and translucency of the crown, surface of the crown, position of the labial margin of the peri‐implant mucosa, position of the interdental papilla, contour of the labial surface of the mucosa, colour and surface of the labial mucosa. When compared to the adjacent teeth, penalty points were assigned (0, excellent; 1 or 2, satisfactory; 3 or 4, moderate; 5 or more, poor). Pink aesthetic score (PES) and white aesthetic score (WES) were calculated in Gallucci 2011 for both groups by three independent observers at the end of the study. The PES included the following parameters: mesial and distal papilla, curvature of the facial mucosa, level of the facial mucosa, root convexity, soft‐tissue colour, texture. The WES included: tooth form, tooth volume/outline, colour, translucency, and characterization. Both scores were recorded for each group and subsequently compared between groups.
The primary outcome aesthetics evaluated by the patient was reported in three trials (Gallucci 2011; Naenni 2015; Ohlmann 2012). In Gallucci 2011 the patient answered on a visual analogue scale (VAS) to a question regarding the aesthetic outcome. In a 100 mm straight line where the left end read 'not satisfied at all' and the right end 'fully satisfied', participants were asked to mark a cross line representing their level of satisfaction. Answers were measured form left to right to obtain a numeric value for the patients' blinded answer. In Ohlmann 2012 the aesthetic performance of the FDPs was subjectively evaluated by the patient using a visual rating scale in which 0 = perfect and 5= completely inadequate. In Naenni 2015 aesthetics evaluated by the patient was reported dichotomous as yes or no. Two trials (Albornoz 2014; Baldini 2016) included a questionnaire and a VAS to rate the patient's aesthetics satisfaction but did not report data.
The secondary outcome periodontal/peri‐implant evaluation was reported in five trials (Albornoz 2014; Baldini 2016; Gallucci 2011; Naenni 2015; Ohlmann 2012). In Albornoz 2014 and in Baldini 2016 clinical and radiographic outcomes were reported: implant probing pocket depths, gingival/mucosal recession and probing attachment levels, radiographic vertical distance from the contact point to the bone crest at mesial and distal sides, radiographic vertical distance from the implant shoulder (1 mm supracrestally) to the most coronal bone in contact with the implant at mesial and distal sites, radiographic horizontal distance from the implant shoulder to the adjacent teeth at mesial and distal sides. In Gallucci 2011 the following secondary outcomes were available: (1) recession (expressed as changes in clinical crown length (CLi) at the implant site); (2) marginal bone level (expressed as first bone to implant contact (FBIC)). In Ohlmann 2012 plaque index and gingival index were analyzed and reported. In Naenni 2015 plaque index, bleeding on probing and pocket probing depth were analyzed and reported. Two trials (Larsson 2006 and Nicolaisen 2016) included in follow‐up visit registration of pocket depth, bleeding on probing, and mobility but did not report the data. No information was reported in two other trials (Encke 2009; Makarouna 2011).
The secondary outcome occlusal wear was not reported in any of the included trials.
Excluded studies
See Characteristics of excluded studies table.
Twenty‐two studies were analyzed and excluded for the following main reasons:
inadequate randomisation (Bindl 2005; Cehreli 2011; Christensen 2010; Henriksson 2004; Li 2007; Sagirkaya 2012; Vanoorbeek 2010);
study design combining both parallel and split‐mouth characteristics (Andersson 1999; Andersson 2001; Borg 2014; Cehreli 2009; Chen 2008; Esquivel‐Upshaw 2012; Esquivel‐Upshaw 2014: Esquivel‐Upshaw 2014b; Etman 2008; Ohlmann 2006; Pelaez 2012; Sailer 2009; Sailer 2009b; Vanoorbeek 2010);
follow‐up shorter than one year (Batson 2014; Jung 2008).
Risk of bias in included studies
The risk of bias of the included trials is summarised in Figure 2 and in Figure 3. Two studies were assessed as at unclear risk of bias (Naenni 2015; Ohlmann 2012), the remaining seven as at high risk of bias.
2.
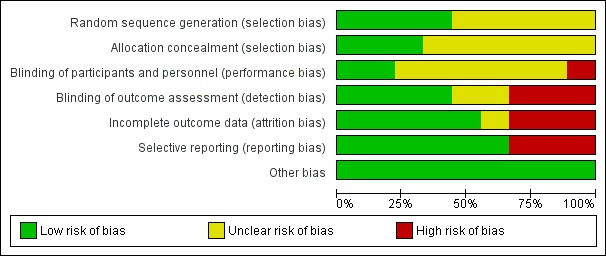
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
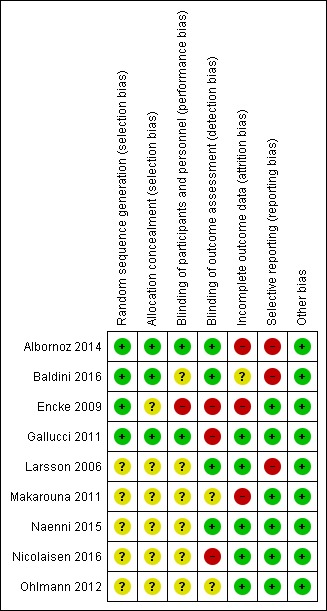
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Sequence generation
Four trials (Albornoz 2014; Baldini 2016; Encke 2009; Gallucci 2011) described an adequate method of sequence generation and were assessed as being at low risk of bias for this domain. The other five trials (Larsson 2006; Makarouna 2011; Naenni 2015; Nicolaisen 2016; Ohlmann 2012) did not provide an adequate description of sequence generation, therefore were judged to be at unclear risk of bias.
Allocation
Three trials (Albornoz 2014; Baldini 2016; Gallucci 2011) described an adequate method of allocation concealment and were assessed as being at low risk of bias for this domain. The other six trials (Encke 2009; Larsson 2006; Makarouna 2011; Naenni 2015; Nicolaisen 2016; Ohlmann 2012) did not provide an adequate description of allocation concealment, therefore were judged to be at unclear risk of bias.
Blinding
For one trial, Encke 2009, the comparison involved interventions that did not allow blinding (zirconia crowns compared to cast gold crowns), therefore both performance and detection bias were assessed to be at high risk of bias. In one trial (Gallucci 2011) performance bias was assessed to be at low risk of bias while detection bias was assessed to be at high risk of bias (zirconia crowns compared to metal abutments and metal‐ceramic crowns). In one trial (Albornoz 2014) both performance and detection bias were assessed to be at low risk. In the remaining trials the description of blinding was assessed as unclear due to limited description (Baldini 2016; Larsson 2006; Makarouna 2011; Naenni 2015; Nicolaisen 2016; .Ohlmann 2012).
Incomplete outcome data
There were low numbers of dropouts in all but three trials (Albornoz 2014; Encke 2009; Makarouna 2011), which were assessed as at high risk of attrition bias.
Selective reporting
In one trial (Larsson 2006) follow‐up visits included pocket depth, bleeding on probing and mobility assessment but data were not reported, therefore study was assessed as at high risk of bias. In two trials (Albornoz 2014; Baldini 2016) follow‐up visits included questionnaires and a visual analogue scale to rate the patient's aesthetic satisfaction but data were not reported, therefore studies were assessed as at high risk of reporting bias.
Other potential sources of bias
No other potential sources of bias were identified.
Effects of interventions
See: Table 1
Comparison 1 Metal‐free single crowns compared to conventional crowns
One trial (Encke 2009) compared full contour zirconia to cast gold single crowns. The study was assessed to be at high risk of bias.
Primary outcomes: failures and complications of the prosthesis
Data for primary outcome failure could be calculated at 12 and 60 months intervals.
At 12 months 194 participants were available for analysis. With regard to crown failure or crown complications there was insufficient evidence of a difference between either treatment approach (odds ratio (OR) 0.83 (95% confidence interval (CI) 0.05 to 13.44) and OR 4.31 (95% CI 0.49 to 37.57)) respectively (Analysis 1.1; Analysis 1.3).
1.1. Analysis.
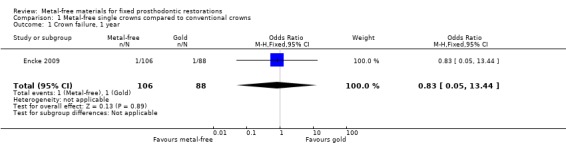
Comparison 1 Metal‐free single crowns compared to conventional crowns, Outcome 1 Crown failure, 1 year.
1.3. Analysis.
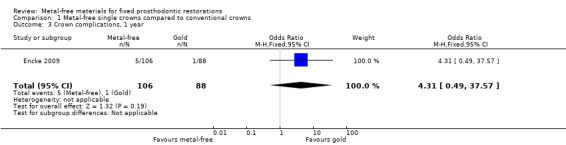
Comparison 1 Metal‐free single crowns compared to conventional crowns, Outcome 3 Crown complications, 1 year.
At 60 months 158 participants were available for analysis. A difference was shown in terms of crown failure (OR 17.52 (95% CI 5.07 to 60.54) (Analysis 1.2) in favour of conventional metal crowns. There was insufficient evidence of a difference when evaluating crown complications (OR 1.44 (95% CI 0.59 to 3.52) (Analysis 1.4).
1.2. Analysis.
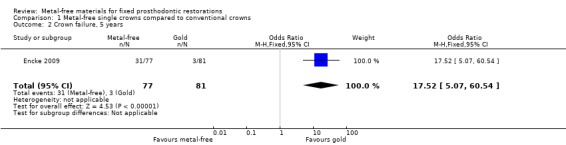
Comparison 1 Metal‐free single crowns compared to conventional crowns, Outcome 2 Crown failure, 5 years.
1.4. Analysis.
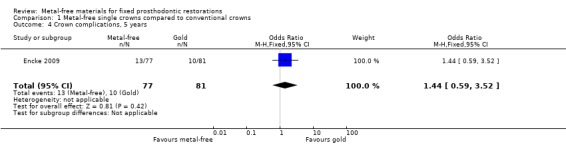
Comparison 1 Metal‐free single crowns compared to conventional crowns, Outcome 4 Crown complications, 5 years.
Primary outcomes: aesthetic evaluation
No data were presented with regard to aesthetics.
Secondary outcomes
No data were presented for any of the pre‐specified secondary outcomes.
Comparison 2 Metal‐free fixed dental prostheses (FDPs) compared to metal‐ceramic FDPs
One trial (Makarouna 2011) compared the clinical performance of lithium disilicate FDPs to metal‐ceramic FDPs. The study was assessed to be at high risk of bias.
Primary outcomes: failures and complications of the prosthesis
Data for primary outcome failure could be calculated at 12 and 72 months intervals.
At 12 months 37 participants were available for analysis. With regard to FDP failure there was insufficient evidence of a difference between either treatment approach (OR 9.00 (95% CI 0.96 to 84.50) (Analysis 2.1). Data for complications were not available at 12 months.
2.1. Analysis.
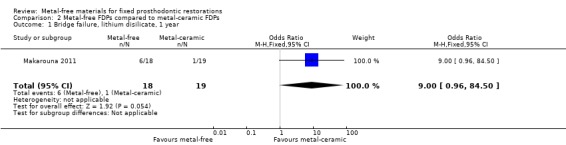
Comparison 2 Metal‐free FDPs compared to metal‐ceramic FDPs, Outcome 1 Bridge failure, lithium disilicate, 1 year.
At 72 months 22 participants were available for analysis. There was insufficient evidence of a difference in terms of FDP failure (OR 6.86 (95% CI 0.66 to 71.72)) (Analysis 2.2) while a difference for complications was found in favour of metal‐free FDPs (OR 0.07 (95% CI 0.01 to 0.75)) (Analysis 2.3).
2.2. Analysis.
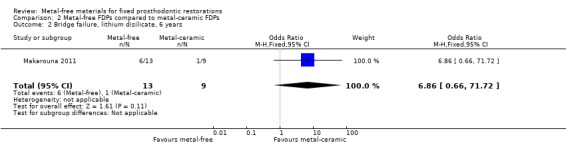
Comparison 2 Metal‐free FDPs compared to metal‐ceramic FDPs, Outcome 2 Bridge failure, lithium disilicate, 6 years.
2.3. Analysis.
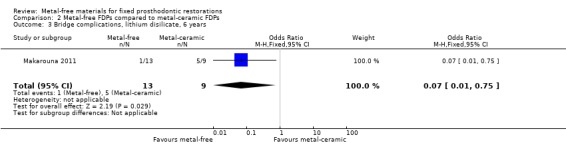
Comparison 2 Metal‐free FDPs compared to metal‐ceramic FDPs, Outcome 3 Bridge complications, lithium disilicate, 6 years.
Primary outcomes: aesthetic evaluation
No data were presented with regard to aesthetics.
Secondary outcomes
No data were presented for any of the pre‐specified secondary outcomes.
One trial (Nicolaisen 2016) compared the clinical performance of zirconia ceramic FDPs to metal‐ceramic FDPs. The study was assessed to be at high risk of bias.
Primary outcomes: failures and complications of the prosthesis
Data for primary outcome failure could be calculated at 12 and 36 months intervals.
At 12 months 34 participants were available for analysis. No FDP failure was reported in either treatment approach. Data for complications were not available at 12 months.
At 36 months 34 participants were available for analysis. No FDP failure was reported in either treatment approach. There was insufficient evidence of a difference for complications (OR 1.94 (95% CI 0.38 to 9.88) (Analysis 2.6).
2.6. Analysis.
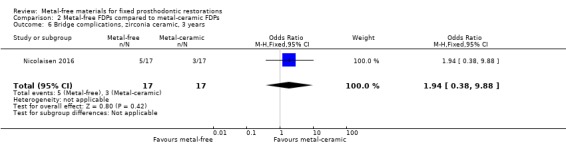
Comparison 2 Metal‐free FDPs compared to metal‐ceramic FDPs, Outcome 6 Bridge complications, zirconia ceramic, 3 years.
Primary outcomes: aesthetic evaluation
At 36 months, an analysis of 34 participants showed insufficient evidence of a difference in patient aesthetic satisfaction changes (OR 15.40 (95% CI 0.78 to 304.61) (Analysis 2.7).
2.7. Analysis.
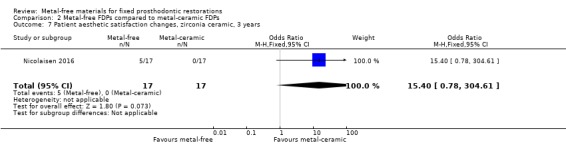
Comparison 2 Metal‐free FDPs compared to metal‐ceramic FDPs, Outcome 7 Patient aesthetic satisfaction changes, zirconia ceramic, 3 years.
Secondary outcomes
No data were presented for any of the pre‐specified secondary outcomes.
Comparison 3 Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs
One trial (Ohlmann 2012) compared metal‐free (feldspathic veneered zirconia) FDPs to metal‐ceramic FDPs. The study was assessed to be at unclear risk of bias.
Primary outcomes: failures and complications of the prosthesis
At 12 months 19 participants were available for analysis. No failure and no complication were reported in either test and control group.
At 24 months 19 participants were available. While no failure was reported there was insufficient evidence of a difference in terms of occurrence of complications (OR 5.59 (95% CI 0.23 to 133.61)) (Analysis 3.4).
3.4. Analysis.
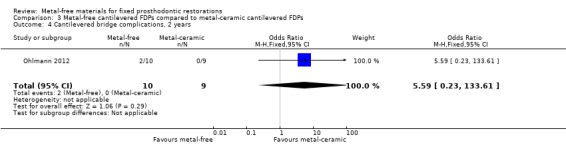
Comparison 3 Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs, Outcome 4 Cantilevered bridge complications, 2 years.
At 36 months 19 participants were still available. No failure was reported and there was insufficient evidence of a difference for complications (OR 2.00 (95% CI 0.15 to 26.73)) (Analysis 3.6).
3.6. Analysis.
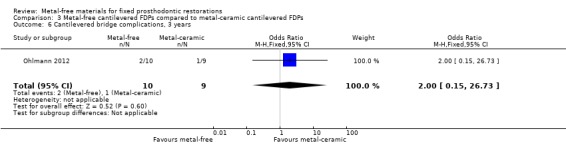
Comparison 3 Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs, Outcome 6 Cantilevered bridge complications, 3 years.
Primary outcomes: aesthetic evaluation
Patients' subjective ratings for aesthetic performance through visual analogue scale (VAS) was provided at the 24 months time interval. No statistically significant difference was shown (mean difference (MD) ‐0.34 (95% CI ‐0.85 to 0.17)) (Analysis 3.9).
3.9. Analysis.
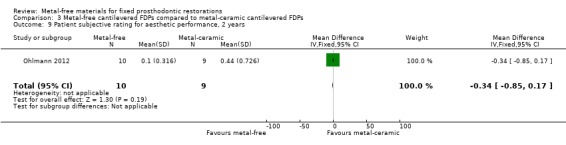
Comparison 3 Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs, Outcome 9 Patient subjective rating for aesthetic performance, 2 years.
Secondary outcomes
Secondary outcomes Plaque Index (PI) and Gingival Index (GI) were available at two years. There was insufficient evidence of a difference for either outcome (PI 2 years MD ‐0.10 (95% CI ‐1.04 to 0.84); GI 2 years MD 0.03 (95% CI ‐0.75 to 0.81)) (Analysis 3.7; Analysis 3.8).
3.7. Analysis.
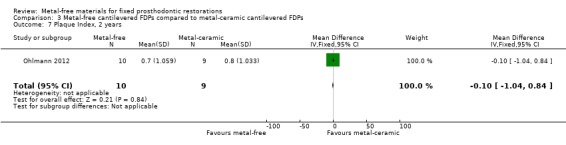
Comparison 3 Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs, Outcome 7 Plaque Index, 2 years.
3.8. Analysis.
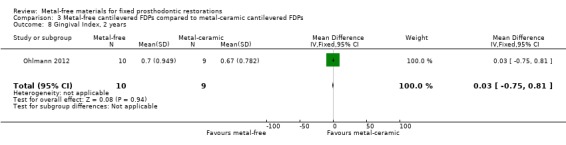
Comparison 3 Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs, Outcome 8 Gingival Index, 2 years.
Comparison 4 Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns
One trial (Gallucci 2011) compared metal‐free implant‐supported single crowns (zirconia) to metal‐ceramic implant‐supported single crowns. The study was assessed to be at high risk of bias.
Primary outcomes: failures and complications of the prosthesis
At 12 and 24 months 18 participants were available. No failure was reported for the test or control groups at either 12 or 24 months. At 24 months, there was insufficient evidence of a difference for complications (OR 6.33 (95% CI 0.26 to 152.86)) (Analysis 4.3).
4.3. Analysis.
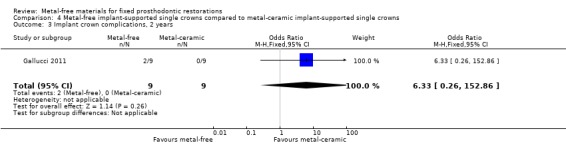
Comparison 4 Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns, Outcome 3 Implant crown complications, 2 years.
Primary outcomes: aesthetic evaluation
VAS for patient satisfaction (mm) was provided at one‐ and two‐year time intervals. There was insufficient evidence of any differences (VAS 1 year MD ‐4.65 (95% CI ‐18.75 to 9.45); VAS 2 years MD ‐0.03 (95% CI ‐7.65 to 7.59)) (Analysis 4.8; Analysis 4.9).
4.8. Analysis.
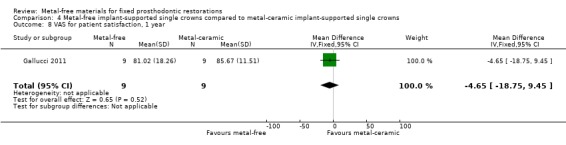
Comparison 4 Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns, Outcome 8 VAS for patient satisfaction, 1 year.
4.9. Analysis.
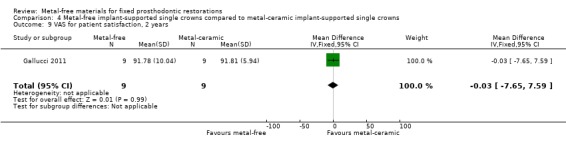
Comparison 4 Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns, Outcome 9 VAS for patient satisfaction, 2 years.
Aesthetic evaluation through pink aesthetic score (PES) and white aesthetic score WES total was provided at two years with insufficient evidence of a difference (MD ‐0.77 (95% CI ‐3.00 to 1.46)) (Analysis 4.10).
4.10. Analysis.
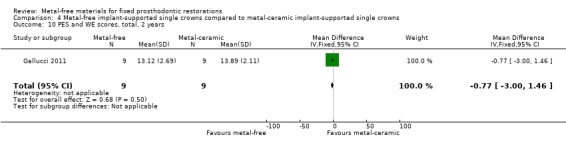
Comparison 4 Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns, Outcome 10 PES and WE scores, total, 2 years.
Secondary outcomes
First bone to implant contact (FBIC) mesial and distal to implant were available at one and two years. There was insufficient evidence of any differences (FBIC mesial 1 year MD ‐0.03 (95% CI ‐0.42 to 0.36); FBIC distal 1 year MD 0.09 (95% CI ‐0.28 to 0.46); FBIC mesial 2 years MD ‐0.01 (95% CI ‐0.93 to 0.91); FBIC distal 2 years MD ‐0.18 (95% CI ‐0.89 to 0.53)) (Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7).
4.4. Analysis.
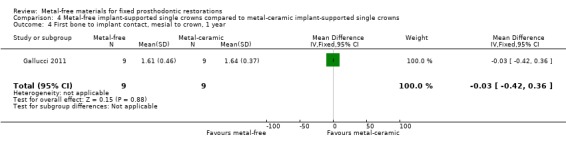
Comparison 4 Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns, Outcome 4 First bone to implant contact, mesial to crown, 1 year.
4.5. Analysis.
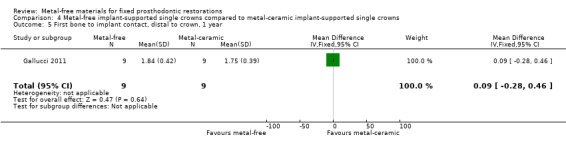
Comparison 4 Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns, Outcome 5 First bone to implant contact, distal to crown, 1 year.
4.6. Analysis.
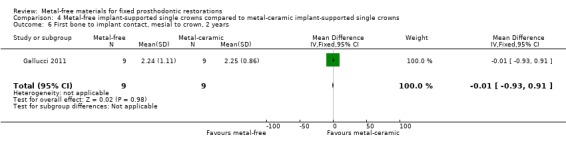
Comparison 4 Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns, Outcome 6 First bone to implant contact, mesial to crown, 2 years.
4.7. Analysis.
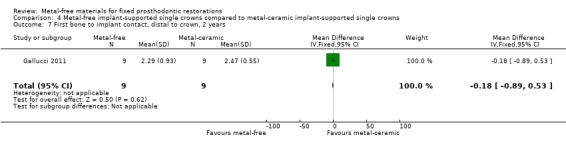
Comparison 4 Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns, Outcome 7 First bone to implant contact, distal to crown, 2 years.
Comparison 5 Metal‐free implant abutments compared to metal implant abutments
Two trials (Albornoz 2014; Baldini 2016) compared metal‐free implant abutments (zirconia) to metal implant abutments supporting single crowns. The studies were assessed to be at high risk of bias.
Primary outcomes: failures and complications of the prosthesis
At 12 months 47 participants were available. With regard to abutment failure there was insufficient evidence of a difference between either treatment approach (OR 7.63 (95% CI 0.33 to 177.14) (Analysis 5.1), whilst no complications were reported.
5.1. Analysis.
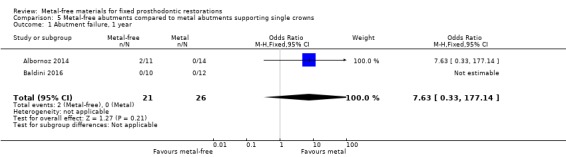
Comparison 5 Metal‐free abutments compared to metal abutments supporting single crowns, Outcome 1 Abutment failure, 1 year.
Primary outcomes: aesthetic evaluation
Primary outcome aesthetic evaluation data was reported as frequency of distribution of the Implant Crown Aesthetic Index (ICAI).
Secondary outcomes
Implant probing pocket depths (PPD), gingival/mucosal recession (REC), marginal bone level changes (MBLC) mesial and distal to implant were available at 12 months and there was insufficient evidence of any differences (PPD MD ‐0.22 (95% CI ‐0.60 to 0.16); REC MD 0.00 (95% CI ‐0.22 to 0.22); MBLC mesial MD ‐0.39 (95% CI ‐0.43 to ‐0.35); MBLC distal MD ‐0.05 (95% CI ‐0.15 to 0.04)) (Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5).
5.2. Analysis.
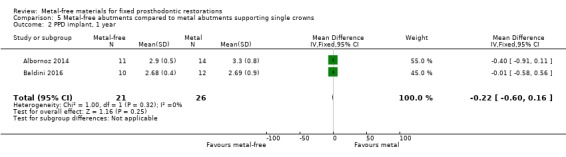
Comparison 5 Metal‐free abutments compared to metal abutments supporting single crowns, Outcome 2 PPD implant, 1 year.
5.3. Analysis.
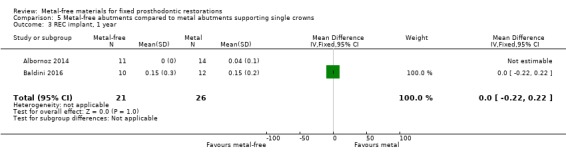
Comparison 5 Metal‐free abutments compared to metal abutments supporting single crowns, Outcome 3 REC implant, 1 year.
5.4. Analysis.
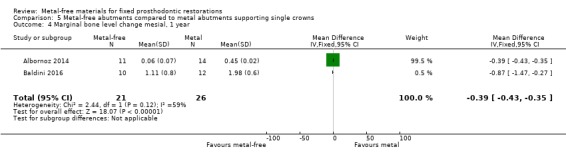
Comparison 5 Metal‐free abutments compared to metal abutments supporting single crowns, Outcome 4 Marginal bone level change mesial, 1 year.
5.5. Analysis.
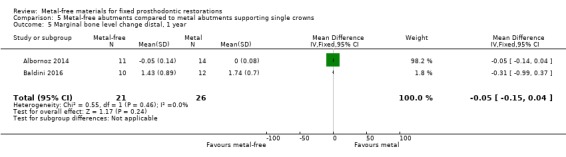
Comparison 5 Metal‐free abutments compared to metal abutments supporting single crowns, Outcome 5 Marginal bone level change distal, 1 year.
Comparison 6 Metal‐free implant‐supported FDPs made of different materials
One trial (Larsson 2006) compared metal‐free implant‐supported FDPs made of two different materials veneered with porcelain (zirconia‐toughened alumina (In‐Ceram Zirconia, Vita Zahnfabrik) and of yttria‐stabilized tetragonal zirconia polycrystal material (Denzir, Decim). The study was assessed to be at high risk of bias
Primary outcomes: failures and complications of the prosthesis
At 12 months 18 participants were available. No FDPs failures were reported and with regard to complications there was a difference in favour of zirconia‐toughened alumina (OR 0.04 (95% CI 0.00 to 0.48)) (Analysis 6.2).
6.2. Analysis.
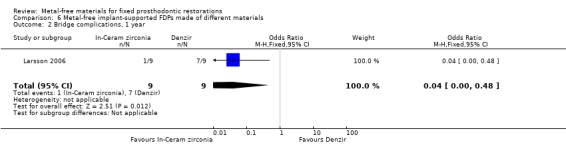
Comparison 6 Metal‐free implant‐supported FDPs made of different materials, Outcome 2 Bridge complications, 1 year.
At 36 months 18 participants were available. No FDPs failures were reported and with regard to complications there was a difference in favour of zirconia‐toughened alumina (OR 0.02 (95% CI 0.00 to 0.30)) (Analysis 6.4).
6.4. Analysis.
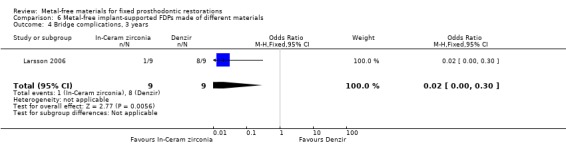
Comparison 6 Metal‐free implant‐supported FDPs made of different materials, Outcome 4 Bridge complications, 3 years.
At 60 months 18 participants were available. No FDPs failures were reported and with regard to complications there was a difference in favour of zirconia‐toughened alumina (OR 0.02 (95% CI 0.00 to 0.42)) (Analysis 6.6).
6.6. Analysis.
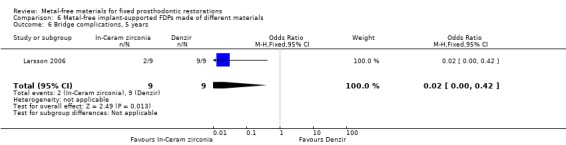
Comparison 6 Metal‐free implant‐supported FDPs made of different materials, Outcome 6 Bridge complications, 5 years.
At 120 months 17 participants were available. No FDPs failures were reported and with regard to complications there was a statistically significant difference in favour of zirconia‐toughened alumina (OR 0.02 (95% CI 0.00 to 0.48)) (Analysis 6.8).
6.8. Analysis.
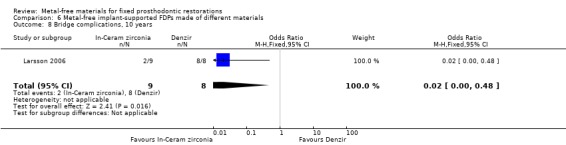
Comparison 6 Metal‐free implant‐supported FDPs made of different materials, Outcome 8 Bridge complications, 10 years.
Primary outcomes: aesthetic evaluation
No data were presented with regard to aesthetics.
Secondary outcomes
No data were presented for any of the pre‐specified secondary outcomes.
Comparison 7 Metal‐free tooth‐supported FDPs made of different materials
One trial (Naenni 2015) compared metal‐free tooth‐supported FDPs made with two different veneering techniques (pressed versus layered). The study was assessed to be at unclear risk of bias.
Primary outcomes: failures and complications of the prosthesis
At 12 months 40 participants were available. No FDPs failures were reported, no complications were reported.
At 36 months 36 participants were available. No FDPs failures were reported and with regard to complications there was insufficient evidence of a difference (OR 2.80 (95% CI 0.66 to 11.92) (Analysis 7.3).
7.3. Analysis.
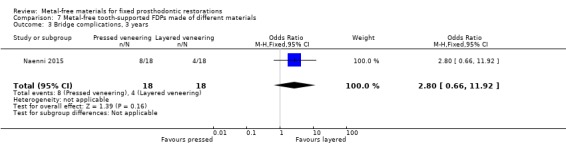
Comparison 7 Metal‐free tooth‐supported FDPs made of different materials, Outcome 3 Bridge complications, 3 years.
Primary outcomes: aesthetic evaluation
No data were presented with regard to aesthetics.
Secondary outcomes
No data were presented for any of the pre‐specified secondary outcomes.
Discussion
Summary of main results
Nine trials involving a total of 448 participants were included. Each trial except two (Albornoz 2014; Baldini 2016) was addressing a different type of intervention: metal‐free single crowns (full‐contour zirconia) compared to cast gold single crowns (Encke 2009); metal‐free implant‐supported fixed dental prostheses (FDPs) made of two different types of zirconia ceramic (Larsson 2006); metal‐free implant‐supported single crowns (zirconia veneered with feldspathic ceramic) compared to metal‐ceramic implant‐supported single crowns (Gallucci 2011); metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs (Ohlmann 2012); metal‐free FDPs (lithium disilicate) compared to metal‐ceramic FDPs (Makarouna 2011); metal‐free FDPs (zirconia ceramic) compared to metal‐ceramic FDPs (Nicolaisen 2016); metal‐free tooth‐supported FDPs made of two different types of zirconia ceramic (Naenni 2015); two trials (Albornoz 2014; Baldini 2016) investigated metal‐free (zirconia) versus metal (titanium) abutments for single tooth implant‐supported cemented crowns.
Except for one trial (Encke 2009) comparing metal‐free with gold crowns which had a large number of participants (224) all the other studies had limited size ranging from 18 to 40 participants.
Overall completeness and applicability of evidence
There was insufficient evidence to draw any useful conclusions. In general, treatments were administered in university clinics and hospitals under strict follow‐up regimens. The generalisation of the results to other clinical conditions should be considered with caution. It is unlikely to know if in different settings the results could be similar.
Quality of the evidence
The overall quality of evidence was very low. All bodies of evidence (i.e. for each comparison and primary outcome) were downgraded by one level for risk of bias and by two levels for imprecision (due to single‐study comparisons with either very small sample sizes, low event rates, 95% confidence intervals including the possibility of benefit for both the test and control groups, or combinations of these problems). Therefore caution should be exercised when generalising the results of the included trials.
The most striking aspect is that only 9 out of 44 potentially eligible randomised controlled trials could be included in the present review. The most common problems were not being randomised and study designs combining the features of two different study designs (parallel and split‐mouth) in the same study.
Investigators should design studies carefully deciding on either a parallel‐group or a split‐mouth design on outset, not combining the two different study designs in the same study as this leads to strong statistical limitations when willing to perform meta‐analysis of data. 14 of the 22 excluded studies were potentially interesting trials which could not be included for this limitation (Andersson 1999; Andersson 2001; Borg 2014; Cehreli 2009; Chen 2008; Esquivel‐Upshaw 2012; Esquivel‐Upshaw 2014: Esquivel‐Upshaw 2014b; Etman 2008; Ohlmann 2006; Pelaez 2012; Sailer 2009; Sailer 2009b; Vanoorbeek 2010).
Potential biases in the review process
No known potential bias was identified.
Agreements and disagreements with other studies or reviews
We identified no other systematic reviews with similar objectives and methodology.
Authors' conclusions
Implications for practice.
Based on the results of the included randomised controlled trials (RCTs), there is insufficient evidence to support or refute the effectiveness of metal‐free materials for fixed prosthodontic treatment over metal‐ceramic or other type of standard restorations. The overall quality of existing evidence was very low, therefore great caution should be exercised when generalising the results of the included trials. Until more evidence becomes available clinicians should continue to base decisions on which material to use for fixed prosthodontic treatment on their own clinical experience, whilst taking into consideration the individual circumstances and preferences of their patients. There is urgent need of properly designed RCTs.
Implications for research.
Future research should aim to provide more reliable information which can help clinicians to decide on appropriate material for fixed prosthodontic treatment whilst taking into consideration the individual circumstances and preferences of their patients. More well‐designed, long‐term RCTs are required to understand if metal‐free materials have the same in‐service clinical performance of conventional metal‐based fixed prosthodontic treatments.
It is recommended that such trials include:
test and control treatments performed in the same way when possible;
a sufficient number of participants to disclose a true difference, if any;
a proper group allocation concealment;
independent outcome assessors when blinding is not possible to minimise detection bias.
Such trials should be reported according to CONSORT guidelines (www.consort‐statement.org).
Investigators should design studies carefully deciding on either a parallel‐group or a split‐mouth design on outset, not combining the two different study designs in the same study.
What's new
| Date | Event | Description |
|---|---|---|
| 21 November 2019 | Review declared as stable | This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future |
Notes
This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future.
Acknowledgements
The review authors would like to express gratitude to Dr Carlo Monaco for help in the initial development of the protocol; Professor Chengge Hua for translation support; Dr Haralampos Petridis for providing comments on the review draft; and Cochrane Oral Health for all their support.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
From March 2015, searches of the Cochrane Oral Health's Trials Register for this review were undertaken using the Cochrane Register of Studies and the search strategy below:
1 ((crown* or "full cast*" or full‐cast* or "indirect restor*" or abutment* or prosthes* or denture* or bridge* or pontic*):ti,ab) AND (INREGISTER) 2 ((prosthodontic* AND fix* AND restor*):ti,ab) AND (INREGISTER) 3 (#1 or #2) AND (INREGISTER) 4 ((ceramic* or porcelain* or alumina or "aluminium oxide" or zirconium or zirconia or "lithium disilicate" or leucite or polymer* or compomer* or "composite resin*"):ti,ab) AND (INREGISTER) 5 ((fibre‐reinforced or "fibre reinforced" or fiber‐reinforced or "fiber reinforced" or spinell or spinel or metal‐free or "metal free" or "non metal" or non‐metal):ti,ab) AND (INREGISTER) 6 (#4 or #5) AND (INREGISTER) 7 (#3 and #6) AND (INREGISTER)
Previous searches for this review were undertaken using the Procite software and the search strategy below:
((crown* or "full cast*" or full‐cast* or "indirect restor*" or abutment* or prosthes* or denture* or bridge* or pontic* or (prosthodontic* AND fix* AND restor*)) AND (ceramic* or porcelain* or alumina or "aluminium oxide" or zirconium or zirconia or "lithium disilicate" or leucite or polymer* or compomer* or "composite resin*" or fibre‐reinforced or "fibre reinforced" or fiber‐reinforced or "fiber reinforced" or spinell or spinel or metal‐free or "metal free" or "non metal" or non‐metal))
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 MeSH descriptor Crowns explode all trees #2 MeSH descriptor Denture, Partial, Fixed explode all trees #3 MeSH descriptor Dental Prosthesis, Implant‐Supported this term only #4 MeSH descriptor Dental Abutments this term only #5 ((dental in All Text near/5 crown* in All Text) or (oral in All Text near/5 crown* in All Text) or (implant* in All Text near/5 crown* in All Text)) #6 ((dental in All Text or oral in All Text or implant* in All Text) and (full‐cast in All Text or "full cast*" in All Text)) #7 "indirect restor*" in All Text #8 ((dental in All Text near/5 abutment* in All Text) or (implant* in All Text near/5 abutment* in All Text)) #9 (dental* in All Text and (arch* in All Text near/5 prosthes* in All Text)) #10 (denture* in All Text near/5 partial in All Text) #11 ("fixed partial denture*" in All Text or "fixed dental prosthes*" in All Text) #12 ((dental in All Text or dentist* in All Text or implant* in All Text or teeth in All Text or tooth in All Text) and (bridge* in All Text or pontic* in All Text))˜ #13 (prosthodontic* in All Text near/3 fix* in All Text near/3 restor* in All Text) #14 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13) #15 MeSH descriptor Ceramics explode all trees #16 ((dental* in All Text or implant* in All Text or oral* in All Text) and (ceramic* in All Text or porcelain* in All Text)) #17 MeSH descriptor Aluminum Oxide this term only #18 (alumina in All Text or "aluminium oxide" in All Text) #19 MeSH descriptor Zirconium this term only #20 (zirconium in All Text or zirconia in All Text) #21 ("lithium disilicate" in All Text or leucite in All Text) #22 MeSH descriptor Polymers this term only #23 MeSH descriptor Composite resins explode all trees #24 ((dental in All Text near/6 polymer* in All Text) or (oral in All Text near/6 polymer* in All Text) or (implant* in All Text near/6 polymer in All Text) or (crown* in All Text near/6 polymer* in All Text)) #25 ((dental in All Text near/6 compomer* in All Text) or (oral in All Text near/6 compomer* in All Text) or (implant* in All Text near/6 compomer in All Text) or (crown* in All Text near/6 compomer* in All Text)) #26 ((dental in All Text near/6 "composite resin*" in All Text) or (oral in All Text near/6 "composite resin*" in All Text) or (implant* in All Text near/6 "composite resin*" in All Text) or (crown* in All Text near/6 "composite resin*" in All Text)) #27 (fibre‐reinforced in All Text or "fibre reinforced" in All Text or fiber‐reinforced in All Text or "fiber reinforced" in All Text) #28 (spinell in All Text or spinel in All Text) #29 (metal‐free in All Text or "metal free" in All Text or non‐metal in All Text or "non metal" in All Text #30 (#15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29) #31 (#14 and #30)
Appendix 3. MEDLINE Ovid search strategy
1. exp Crowns/ 2. exp Fixed Partial Denture/ 3. Dental Prosthesis, Implant‐Supported/ 4. Dental Abutments/ 5. ((dental$ or oral$ or implant) adj5 crown$).ti,ab. 6. ((dental$ or oral or implant$) adj5 ("full cast$" or full‐cast$)).ti,ab. 7. "indirect restor$".ti,ab. 8. ((dental$ or implant$) adj5 abutment$).ti,ab. 9. (dental$ and (arch adj5 prosthesis)).ti,ab. 10. (dental$ and (arch adj5 prostheses)).ti,ab. 11. ("complete arch prosthesis" or "complete arch prostheses").ti,ab. 12. (denture$ adj partial).ti,ab. 13. "fixed dental prosthes$".ti,ab. 14. ((dental or dentist$ or implant$ or teeth or tooth) adj5 (bridge$ or pontic$)).ti,ab. 15. (prosthodontic adj3 fix$ adj3 restor$).ti,ab. 16. or/1‐15 17. exp Ceramic/ 18. ((dental$ or implant$ or oral$) and (ceramic$ or porcelain$)).ti,ab. 19. Alumina/ 20. (alumina or "aluminium oxide").ti,ab. 21. Zirconium/ 22. (zirconium or zirconia).ti,ab. 23. ("lithium disilicate" or leucite).ti,ab. 24. Polymers/ 25. exp Composite Resins/ 26. ((dental$ or oral$ or implant$ or crown$) adj6 (polymer$ or compomer$ or "composite resin$")).ti,ab. 27. (fibre‐reinforced or "fibre reinforced" or fiber‐reinforced or "fiber reinforced").ti,ab. 28. (spinell or spinel).ti,ab. 29. (metal‐free or "metal free" or "non metal" or non‐metal).ti,ab. 30. or/17‐29 31. 16 and 30
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Higgins 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. exp Tooth crown/ 2. Tooth prosthesis/ 3. Denture/ 4. ((dental$ or oral$ or implant) adj5 crown$).ti,ab. 5. ((dental$ or oral or implant$) adj5 ("full cast$" or full‐cast$)).ti,ab. 6. "indirect restor$".ti,ab. 7. ((dental$ or implant$) adj5 abutment$).ti,ab. 8. (dental$ and (arch adj5 prosthesis)).ti,ab. 9. (dental$ and (arch adj5 prostheses)).ti,ab. 10. ("complete arch prosthesis" or "complete arch prostheses").ti,ab. 11. (denture$ adj partial).ti,ab. 12. "fixed dental prosthes$".ti,ab. 13. ((dental or dentist$ or implant$ or teeth or tooth) adj5 (bridge$ or pontic$)).ti,ab. 14. (prosthodontic adj3 fix$ adj3 restor$).ti,ab. 15. or/1‐14 16. exp Ceramics/ 17. ((dental$ or implant$ or oral$) and (ceramic$ or porcelain$)).ti,ab. 18. Aluminium oxide/ 19. (alumina or "aluminium oxide").ti,ab. 20. Zirconium/ 21. (zirconium or zirconia).ti,ab. 22. ("lithium disilicate" or leucite).ti,ab. 23. Polymers/ 24. exp Resin/ 25. ((dental$ or oral$ or implant$ or crown$) adj6 (polymer$ or compomer$ or "composite resin$")).ti,ab. 26. (fibre‐reinforced or "fibre reinforced" or fiber‐reinforced or "fiber reinforced").ti,ab. 27. (spinell or spinel).ti,ab. 28. (metal‐free or "metal free" or "non metal" or non‐metal).ti,ab. 29. or/16‐28 30. 15 and 29
The above subject search was linked to the Cochrane Oral Health filter for identifying RCTs in Embase via Ovid:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Appendix 5. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
(dental and ceramic and (crown or prosthesis)) (tooth and ceramic and (crown or prosthesis)) (dental and porcelain and (crown or prosthesis)) (tooth and porcelain and (crown or prosthesis)) (dental and alumina and (crown or prosthesis)) (tooth and alumina and (crown or prosthesis)) (dental and “aluminium oxide” and (crown or prosthesis)) (tooth and “aluminium oxide” and (crown or prosthesis)) (dental and zirconium and (crown or prosthesis)) (tooth and zirconium and (crown or prosthesis)) (dental and zircona and (crown or prosthesis)) (tooth and zircona and (crown or prosthesis)) (dental and leucite and (crown or prosthesis)) (tooth and leucite and (crown or prosthesis)) (dental and “lithium disilicate” and (crown or prosthesis)) (tooth and “lithium disilicate” and (crown or prosthesis)) (dental and polymer and (crown or prosthesis)) (tooth and polymer and (crown or prosthesis)) (dental and compomer and (crown or prosthesis)) (tooth and compomer and (crown or prosthesis)) (dental and composite and (crown or prosthesis)) (tooth and composite and (crown or prosthesis)) (dental and fibre and (crown or prosthesis)) (tooth and fibre and (crown or prosthesis)) (dental and fiber and (crown or prosthesis)) (tooth and fiber and (crown or prosthesis)) (dental and spinel* and (crown or prosthesis)) (tooth and spinel* and (crown or prosthesis))
Appendix 6. WHO International Clinical Trials Registry Platform search strategy
dental and crown and ceramic or tooth and crown and ceramic or dental and prosthesis and ceramic or tooth and prosthesis and ceramic dental and crown and porcelain or tooth and crown and porcelain or dental and prosthesis and porcelain or tooth and prosthesis and porcelain dental and crown and alumina or tooth and crown and alumina or dental and prosthesis and alumina or tooth and prosthesis and alumina dental and crown and aluminium oxide or tooth and crown and aluminium oxide or dental and prosthesis and aluminium oxide or tooth and prosthesis and aluminium oxide dental and crown and zirconium or tooth and crown and zirconium or dental and prosthesis and zirconium or tooth and prosthesis and zirconium dental and crown and zirconia or tooth and crown and zirconia or dental and prosthesis and zirconia or tooth and prosthesis and zirconia dental and crown and leucite or tooth and crown and leucite or dental and prosthesis and leucite or tooth and prosthesis and leucite dental and crown and lithium disilicate or tooth and crown and lithium disilicate or dental and prosthesis and lithium disilicate or tooth and prosthesis and lithium disilicate dental and crown and polymer or tooth and crown and polymer or dental and prosthesis and polymer or tooth and prosthesis and polymer dental and crown and compomer or tooth and crown and compomer or dental and prosthesis and compomer or tooth and prosthesis and compomer dental and crown and composite or tooth and crown and composite or dental and prosthesis and composite or tooth and prosthesis and composite dental and crown and fibre or tooth and crown and fibre or dental and prosthesis and fibre or tooth and prosthesis and fibre dental and crown and fiber or tooth and crown and fiber or dental and prosthesis and fiber or tooth and prosthesis and fiber dental and crown and spinel or tooth and crown and spinel or dental and prosthesis and spinel or tooth and prosthesis and spinel
Data and analyses
Comparison 1. Metal‐free single crowns compared to conventional crowns.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Crown failure, 1 year | 1 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.05, 13.44] |
| 2 Crown failure, 5 years | 1 | 158 | Odds Ratio (M‐H, Fixed, 95% CI) | 17.52 [5.07, 60.54] |
| 3 Crown complications, 1 year | 1 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.31 [0.49, 37.57] |
| 4 Crown complications, 5 years | 1 | 158 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.59, 3.52] |
Comparison 2. Metal‐free FDPs compared to metal‐ceramic FDPs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bridge failure, lithium disilicate, 1 year | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.96, 84.50] |
| 2 Bridge failure, lithium disilicate, 6 years | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.86 [0.66, 71.72] |
| 3 Bridge complications, lithium disilicate, 6 years | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.75] |
| 4 Bridge failure, zirconia ceramic, 1 year | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Bridge failure, zirconia ceramic, 3 years | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Bridge complications, zirconia ceramic, 3 years | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.38, 9.88] |
| 7 Patient aesthetic satisfaction changes, zirconia ceramic, 3 years | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 15.4 [0.78, 304.61] |
2.4. Analysis.
Comparison 2 Metal‐free FDPs compared to metal‐ceramic FDPs, Outcome 4 Bridge failure, zirconia ceramic, 1 year.
2.5. Analysis.
Comparison 2 Metal‐free FDPs compared to metal‐ceramic FDPs, Outcome 5 Bridge failure, zirconia ceramic, 3 years.
Comparison 3. Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cantilevered bridge failure, 1 year | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Cantilevered bridge complications, 1 year | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cantilevered bridge failure, 2 years | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cantilevered bridge complications, 2 years | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.59 [0.23, 133.61] |
| 5 Cantilevered bridge failure, 3 years | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cantilevered bridge complications, 3 years | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.15, 26.73] |
| 7 Plaque Index, 2 years | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.04, 0.84] |
| 8 Gingival Index, 2 years | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.75, 0.81] |
| 9 Patient subjective rating for aesthetic performance, 2 years | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.85, 0.17] |
3.1. Analysis.
Comparison 3 Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs, Outcome 1 Cantilevered bridge failure, 1 year.
3.2. Analysis.
Comparison 3 Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs, Outcome 2 Cantilevered bridge complications, 1 year.
3.3. Analysis.
Comparison 3 Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs, Outcome 3 Cantilevered bridge failure, 2 years.
3.5. Analysis.
Comparison 3 Metal‐free cantilevered FDPs compared to metal‐ceramic cantilevered FDPs, Outcome 5 Cantilevered bridge failure, 3 years.
Comparison 4. Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Implant crown failure, 1 year | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Implant crown failure, 2 years | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Implant crown complications, 2 years | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.33 [0.26, 152.86] |
| 4 First bone to implant contact, mesial to crown, 1 year | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.42, 0.36] |
| 5 First bone to implant contact, distal to crown, 1 year | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.28, 0.46] |
| 6 First bone to implant contact, mesial to crown, 2 years | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.93, 0.91] |
| 7 First bone to implant contact, distal to crown, 2 years | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.89, 0.53] |
| 8 VAS for patient satisfaction, 1 year | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐4.65 [‐18.75, 9.45] |
| 9 VAS for patient satisfaction, 2 years | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐7.65, 7.59] |
| 10 PES and WE scores, total, 2 years | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐3.00, 1.46] |
4.1. Analysis.
Comparison 4 Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns, Outcome 1 Implant crown failure, 1 year.
4.2. Analysis.
Comparison 4 Metal‐free implant‐supported single crowns compared to metal‐ceramic implant‐supported single crowns, Outcome 2 Implant crown failure, 2 years.
Comparison 5. Metal‐free abutments compared to metal abutments supporting single crowns.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abutment failure, 1 year | 2 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.63 [0.33, 177.14] |
| 2 PPD implant, 1 year | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.60, 0.16] |
| 3 REC implant, 1 year | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.22, 0.22] |
| 4 Marginal bone level change mesial, 1 year | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.43, ‐0.35] |
| 5 Marginal bone level change distal, 1 year | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.15, 0.04] |
Comparison 6. Metal‐free implant‐supported FDPs made of different materials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bridge failure, 1 year | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Bridge complications, 1 year | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.48] |
| 3 Bridge failure, 3 years | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Bridge complications, 3 years | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.30] |
| 5 Bridge failure, 5 years | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Bridge complications, 5 years | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.42] |
| 7 Bridge failure, 10 years | 1 | 17 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Bridge complications, 10 years | 1 | 17 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.48] |
6.1. Analysis.
Comparison 6 Metal‐free implant‐supported FDPs made of different materials, Outcome 1 Bridge failure, 1 year.
6.3. Analysis.
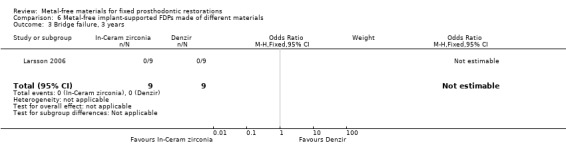
Comparison 6 Metal‐free implant‐supported FDPs made of different materials, Outcome 3 Bridge failure, 3 years.
6.5. Analysis.
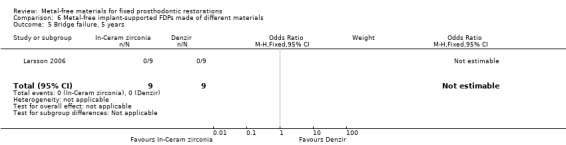
Comparison 6 Metal‐free implant‐supported FDPs made of different materials, Outcome 5 Bridge failure, 5 years.
6.7. Analysis.
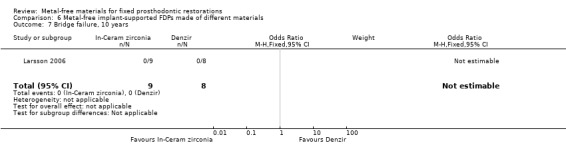
Comparison 6 Metal‐free implant‐supported FDPs made of different materials, Outcome 7 Bridge failure, 10 years.
Comparison 7. Metal‐free tooth‐supported FDPs made of different materials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bridge failure, 1 year | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Bridge failure, 3 years | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Bridge complications, 3 years | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.8 [0.66, 11.92] |
7.1. Analysis.
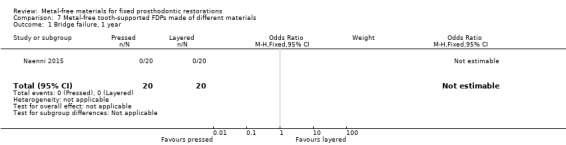
Comparison 7 Metal‐free tooth‐supported FDPs made of different materials, Outcome 1 Bridge failure, 1 year.
7.2. Analysis.
Comparison 7 Metal‐free tooth‐supported FDPs made of different materials, Outcome 2 Bridge failure, 3 years.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albornoz 2014.
| Methods | 1‐year follow‐up, single centre, parallel, double‐blind clinical trial | |
| Participants | 38 subjects ≥18 years of age screened at the Periodontology Clinic of the Faculty of Odontology, Complutense University, Madrid, Spain, 7 patients excluded as they did not satisfy the inclusion/exclusion criteria, 1 patient withdrew before baseline data collection
Inclusion criteria:
Exclusion criteria:
30 subjects were allocated after 3 months of healing to either test or control group, 26 received the allocated treatment 25 subjects were available at the 1‐year examination: 11 (test group) and 14 (control group) |
|
| Interventions | Implant abutments made of 2 different materials supporting single crowns in the anterior maxilla Group 1: 12 zirconia abutment (SPIART; Thommen Medical AG, Grenchen, Switzerland) Group 2: 14 titanium abutment (SPIEASY; Thommen Medical AG, Grenchen, Switzerland) Implant insertion:
Impressions and lab work:
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All subjects were randomized using computer‐generated permuted block randomization with an allocation ratio of 1:1" |
| Allocation concealment (selection bias) | Low risk | Quote: "The treatment allocation was assigned by means of opaque sealed envelopes containing a code derived from the randomized list and handled directly to the restorative dentist responsible for placing the crown" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the study examiner and the patient were blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the study examiner and the patient were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 dropouts were recorded. The reasons for the loss of patient follow‐up were economic problems for crown placement in 3 subjects, the start of orthodontic therapy in 1 subject, and the move to another city in another 1 subject. 4 subjects were lost after allocation (3 in the test group and 1 in the control group) and 1 subject was lost at follow‐up at 12 months. At 1 year, 25 of the 30 randomised patients were analysed: 11 ( zirconia abutments) and 14 ( titanium abutments). 2 patients in the test group were reported with fracture of the metal‐free abutment at the time of insertion, but the study does not report them as failure |
| Selective reporting (reporting bias) | High risk | Follow‐up visits included a written questionnaire for evaluating patient satisfaction with regard to aesthetic appearance, phonetic ability, and their overall satisfaction with treatment, graded using a 6‐grade ordinal scale ranked from extremely negative to extremely positive and a VAS but data were not reported |
| Other bias | Low risk | |
Baldini 2016.
| Methods | 1‐year follow‐up, single centre, parallel, double‐blind clinical trial | |
| Participants | 24 patients with mono‐edentulism in the aesthetic zone of either the maxillary or mandibular region (from 1.5 to 2.5 or 3.5 to 4.5) screened at the Department of Periodontology of the University of Siena, Italy Inclusion criteria:
Exclusion criteria:
24 subjects were screened, 24 subjects were allocated to the 2 treatment groups, all received the allocated treatment, 22 were available at the 1‐year examination: 10 (test group) and 12 (control group) |
|
| Interventions | Implant abutments made of 2 different materials supporting single crowns in the anterior maxilla and mandible Group 1: 12 zirconia abutment (SPIART; Thommen Medical AG, Grenchen, Switzerland) Group 2: 12 titanium abutment (SPIEASY; Thommen Medical AG, Grenchen, Switzerland) Implant insertion:
Impressions and lab work:
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All patients were randomly assigned to the test or control group using a computer permuted block randomization system with an allocation ratio of 1:1" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was performed by the dentist responsible for the prosthetic restoration, by means of sealed envelopes containing a code: both patients and analysing statisticians were blinded" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "...both patients and analysing statisticians were blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two clinicians not involved in patient treatment were trained in calibration prior to the beginning of the study to record all outcome measurements. They were blinded about treatment group assignment; only one of the two examiners performed the aesthetic analysis (AC), the second examiner recorded all secondary outcome parameters (CD)" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 dropout patients in test group, dropout reasons not explained |
| Selective reporting (reporting bias) | High risk | Follow‐up visits included a written questionnaire for evaluating patient satisfaction concerning items such as the aesthetically related variables (harmonization of gingival margin, overall aesthetic satisfaction) and lifestyle related variables (confidence when smiling, phonic ability, comfort when chewing or biting) assessed using VAS but data were not reported |
| Other bias | Low risk | |
Encke 2009.
| Methods | 5‐year follow‐up, randomised, single‐blind, parallel‐group study | |
| Participants | 536 patients aged above 18 years recruited through newspaper advertisements Inclusion criteria:
Exclusion criteria:
308 allocated to 2 groups (152 zirconia, 156 gold) with at least 1 tooth in the premolar or molar region in need of crowning. 224 patients received allocated intervention |
|
| Interventions | Single crowns on mandibular and maxillary premolars and molars Group 1: 123 patients with 123 all ceramic ZrSiO4 crowns (Everest HPC, KaVo Dental GmbH, Biberach/Riss, Germany) Group 2: 101 patients with 101 gold crowns (Degulor M, Degudent) All treatments were carried out in the University Dental Hospital Freiburg, Germany, by staff dentists Tooth preparation:
Provisionalization:
Impressions and lab work:
Try‐in and cementation:
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were assigned a unique identification number, which was forwarded to a statistician (Institute of Medical Biometry and Medical Informatics, University of Freiburg). Randomization was carried out using the Bernoulli distribution for each patient identification number. Accordingly, the patients received either the ceramic (test) or gold crown (control). No balancing was carried out" Comment: of the 308 patients allocated to 2 groups (152 zirconia, 156 gold) only 224 patients received allocated intervention, resulting in 123 zirconia versus 101 gold crowns |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the obvious differences between the 2 crown designs (gold versus ceramic), blinding was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to the obvious differences between the 2 crown designs (gold versus ceramic), blinding was not possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the ceramic crown group, 15 patients were not followed up at the 12‐month examination, 24 patients at the 24‐month follow‐up, 50 patients at the 36‐month recall and 46 patients at the 48‐ and 60‐month follow‐ups In the gold group, 7 patients were lost to follow‐up at the 12‐month recall, 16 patients at the 24‐month follow‐up, 27 patients at the 36‐month recall examination, 18 patients after 48 months and 19 patients at the 60‐month follow‐up 2 patients allocated to gold group were not included in the analysis, but reasons were provided |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | |
Gallucci 2011.
| Methods | 2‐year follow‐up, randomised, parallel‐group study | |
| Participants | 20 patients with 1 missing tooth in the anterior maxilla (first bicuspid to first bicuspid) and presence of 2 intact adjacent teeth Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 2 different types of screw‐retained single implant crowns Group 1: 10 all ceramic single crowns Group 2: 10 PFM single crowns Implant insertion:
Provisionalization:
Impressions and lab work:
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All participants were randomly assigned to one the two treatment options. A random permuted block system was generated by a collaborator not involved in the study. Six permuted blocks containing three test and three control subjects were generated and included in 24 sealed envelopes. A copy of the randomization sequence was preserved for accuracy assessment at the end of the study. The permuted block randomization system ensured the uniformity of the patient allocation during the clinical trial by randomly distributing three participants to the test and three participants to the control group every six treated patients" |
| Allocation concealment (selection bias) | Low risk | Quote: "Upon patient's enrolment, a sealed envelope was assigned by order of inclusion in the study. In order to avoid bias during the prosthodontic treatment, the individually assigned sealed envelopes were only opened after final impression taking and were subsequently sent to the dental laboratory for fabrication of the implant crowns" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "For the objective assessment at baseline, the study design was double blinded because neither the investigators nor the patients were aware of the assigned group" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "For the objective assessment at baseline, the study design was double blinded because neither the investigators nor the patients were aware of the assigned group. For the objective measurements at CI, 1Y, and 2Y, the study design was single‐blinded being only the participants unaware of the group they were allocated. For the subjective evaluation, the trial design was double blinded. Neither the patients nor external expert clinicians were informed about the results of the randomization" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three dropouts were recorded. Two patients moved abroad before receiving the final crowns and one patient was unreachable after completing the 1Y" |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Larsson 2006.
| Methods | 10‐year follow‐up, randomised, parallel‐group study | |
| Participants | 320 individuals responded to an advertisement in a local newspaper. After preliminary interview, and panoramic X‐ray, 18 partially dentate patients (12 women, 6 men; age range: 37 to 70 years) in need of 1 or more 2‐ to 5‐unit implant‐supported FDPs with satisfactory oral hygiene were randomised to 2 groups Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 2‐ to 5‐unit implant‐supported FDPs Group 1: 9 patients with 12 FDPs of a zirconia‐toughened alumina, 35 units (InZ) Group 2: 9 patients with 13 FDPs of a yttria‐stabilized tetragonal zirconia polycrystal material, 31 units (DZ) Implant insertion:
Impressions and lab work:
Try‐in and cementation:
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized to the two groups by drawing lots" |
| Allocation concealment (selection bias) | Unclear risk | Patients were divided into 2 groups after healing of implants |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examinations were performed blindly and the examiners were not aware of which material system each patient had received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Follow‐up visits included PD, BOP and mobility assessment but data were not reported |
| Other bias | Low risk | None identified |
Makarouna 2011.
| Methods | 6‐year follow‐up, randomised, parallel‐group study | |
| Participants | 37 patients in need of 3‐unit FPDs treatment (23 women, 14 men; mean age: 47 years) received 1 FPD each Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Group 1: test group (n = 18) received lithium disilicate FPDs Group 2: control group (n = 19) received PFM FPDs Tooth preparation:
Impressions and lab work:
Try‐in and cementation:
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description available |
| Allocation concealment (selection bias) | Unclear risk | No description available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 15 patients were lost to follow‐up (study group: n = 5, control: n = 10) for reasons unrelated to the dental treatment |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
Naenni 2015.
| Methods | 3‐year follow‐up, multicentre, parallel, double‐blind randomised controlled trial | |
| Participants | 40 patients in need of 1 maxillary or mandibular 3‐unit FDP in second premolar or molar area recruited and treated at 2 separate centres at the University of Zurich, Switzerland Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Group 1 (test group): 20 patients received a zirconia–ceramic FDP with pressed veneering ceramic Group 2 (control group): 20 patients received a FDP with conventionally layered veneering ceramic Tooth preparation:
Impressions and lab work:
Try‐in and cementation:
All FDPs were evaluated at baseline, at 6 months and at 1 and 3 years of clinical service |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to the treatment modality and the respective clinic by means of a random list with even and uneven numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available concerning participants blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In order to avoid bias the FDPs were examined by two clinicians who were not involved in the reconstructive treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Four patients (2 test and 2 control) were not available for the 3‐year examination. They did show up for the 1‐year recall and later moved away without giving notice" |
| Selective reporting (reporting bias) | Low risk | None known |
| Other bias | Low risk | |
Nicolaisen 2016.
| Methods | 3‐year follow‐up, single centre, parallel randomised trial | |
| Participants | 34 patients in need of replacement of a second premolar or first molar A convenience sample was obtained from the clinic at the Department of Dentistry, Aarhus University, Denmark and by referral from private practitioners in the Aarhus area Inclusion criteria:
Exclusion criteria
|
|
| Interventions | Group 1: test group (n = 17) all‐ceramic (AC) FDPs with a zirconia framework Group 2: control group (n = 17) metal‐ceramic (MC) FDPs with a high‐noble metal framework Tooth preparation:
Impressions and lab work:
Try‐in and cementation:
FDPs were examined at baseline, 6 months, 1, 2, and 3 years |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not known. Quote: "The patients were randomly allocated..." |
| Allocation concealment (selection bias) | Unclear risk | Not known |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not known |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The evaluations were performed by the operator and another clinician who had not been involved in the treatments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | None known |
| Other bias | Low risk | None known |
Ohlmann 2012.
| Methods | 3‐year follow‐up, randomised, parallel‐group study | |
| Participants | 21 patients (12 women and 9 men) between 26 and 74 years of age, with a mean age of 56 years (SD 12.6 years) received a total of 21 cantilever FDPs Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Group 1: 11 patients with 1 zirconia cantilever FDPs Group 2: 10 patients with 1 metal‐ceramic cantilever FDPs Tooth preparation:
Impressions and lab work:
Try‐in and cementation:
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | No description available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Clinical evaluations were performed by a clinician not involved in the treatment of the individual patient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients left the study without providing a reason before the 2‐year recall. Thus, a total of 19 FDPs (10 all‐ceramic FDPs and 9 metal‐ceramic FDPs) were included for further analysis |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | None identified |
CAD/CAM = computer‐aided design and computer‐aided manufacturing; FDPs = fixed dental prostheses; FMBS = full‐mouth bleeding score; FMPS = full‐mouth plaque score; MC = metal‐ceramic; PAL = probing attachment levels; PD = pocket depth; PFM = porcelain‐fused‐to‐metal; PI = plaque index; PII = prosthetic implant infection; PPD = probing pocket depth; REC = gingival/mucosal recession; SD = standard deviation; VAS = visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersson 1999 | Study design is neither parallel‐group nor split‐mouth. 69 prosthetic abutments have been randomised in 60 patients to 2 interventions. Some patients had 1 intervention, some others had both interventions |
| Andersson 2001 | Study design is neither parallel‐group nor split‐mouth. 103 prosthetic abutments have been randomised in 32 patients to 2 interventions. Some patients had 1 intervention, some others had both interventions |
| Batson 2014 | Follow‐up shorter than 1 year |
| Bindl 2005 | Not randomised |
| Borg 2014 | Study design is neither parallel‐group nor split‐mouth. 18 implant‐supported FDPs have been randomised in 16 patients to 2 interventions. Some patients had 1 intervention, some others had both interventions |
| Cehreli 2009 | Study design is neither parallel‐group nor split‐mouth. 30 prosthetic crowns have been randomised in 20 patients to 2 interventions. Some patients had 1 intervention, some others had both interventions |
| Cehreli 2011 | It is unclear whether randomisation was done according to patients or restorations. 33 consecutive patients included in the study were randomised to 2 interventions, feldspathic porcelain crowns (12 patients, 50 crowns) and glass‐infiltrated alumina all‐ceramic crowns (21 patients, 51 crowns). Authors were contacted via mail but did not answer |
| Chen 2008 | Study design is neither parallel‐group nor split‐mouth. 35 implants in 23 patients have been randomised to 2 interventions. Some patients had 1 intervention, some others had both interventions |
| Christensen 2010 | Unclear randomisation. Study is described as randomised with 10 different treatment modalities but 2 "were not available initially." 2 other groups were limited in size compared to the others because of investigators "concern about framework fractures shortly after placement" |
| Esquivel‐Upshaw 2012 | Study design is neither parallel‐group nor split‐mouth. 36 posterior crowns in 31 patients have been randomised to 3 interventions. Some patients had 1 intervention, some others had more than 1 intervention |
| Esquivel‐Upshaw 2014 | Study design is neither parallel‐group nor split‐mouth. 72 FDPs in 55 patients have been randomised to 2 interventions. Some patients had 1 intervention, some others had more than 1 intervention |
| Esquivel‐Upshaw 2014b | Study design is neither parallel‐group nor split‐mouth. 89 FDPs in 68 patients have been randomised to 2 interventions. Some patients had 1 intervention, some others had more than 1 intervention |
| Etman 2008 | Study design is neither parallel‐group nor split‐mouth. 90 posterior crowns in 48 patients have been randomised to 3 interventions. Some patients had 1 intervention, some others had more than 1 intervention |
| Henriksson 2004 | Not randomised |
| Jung 2008 | Follow‐up shorter than 1 year |
| Li 2007 | Not randomised |
| Ohlmann 2006 | Study design is neither parallel‐group nor split‐mouth. 120 prosthetic crowns have been randomised in 66 patients to 3 interventions. Some patients had 1 intervention, some others had more than 1 intervention |
| Pelaez 2012 | Study design is neither parallel‐group nor split‐mouth. 40 3 units FPDs have been randomised in 37 patients to 2 interventions. Some patients had 1 intervention, some others had both interventions |
| Sagirkaya 2012 | It is unclear how randomisation was done. 59 subjects with excessive loss of tooth structure requiring full veneer crowns, crowns/FPDs needing replacement, and missing tooth or teeth requiring tooth‐ or implant‐supported crowns and/or 3 to 6 units FPDs were randomised to 4 interventions. According to materials and methods section patients were randomised, but the number of patient randomised to each group is not reported neither in materials and methods nor in results sections. Results report data according to units, and the 4 groups have large differences in size of reported units (Cercon, 24 units; ZirkonZahn, 118 units; Lava, 40 units; Katana, 85 units). Authors were contacted via mail but did not answer |
| Sailer 2009 | Study design is neither parallel‐group nor split‐mouth. 40 implant sites have been randomised in 22 patients to 2 interventions. Some patients had 1 intervention, some others had both interventions |
| Sailer 2009b | Study design is neither parallel‐group nor split‐mouth. 76 FPDs have been randomised in 59 patients to 2 interventions. Some patients had 1 intervention, some others had both interventions |
| Vanoorbeek 2010 | Study design is neither parallel‐group nor split‐mouth. 200 prosthetic crowns have been randomised in 130 patients to 2 interventions. Some patients had 1 intervention, some others had both interventions. Moreover randomisation was terminated after the first 120 restorations due to early complications in 1 group |
FDPs = fixed dental prostheses.
Characteristics of ongoing studies [ordered by study ID]
DRKS00005452.
| Trial name or title | 'Prospective randomized controlled interventional study of chair‐side generated monolithic single implant supra‐structures made of lithium disilicate ceramic' |
| Methods | Randomised, parallel‐group study |
| Participants | 30 adult patients with indication for single implant(s) in the posterior region |
| Interventions | Group 1: monolithic single implant suprastructures made of lithium disilicate Group 2: titanium abutment and crown made of lithium disilicate |
| Outcomes | Survival rate Complication rate over a time period of at least 3 years |
| Starting date | November 2013 |
| Contact information | sreich@ukaachen.de |
| Notes |
DRKS00010423.
| Trial name or title | 'Monolithic zirconia fixed dental prosthesis in the posterior region: a randomized controlled clinical trial' |
| Methods | Randomised, parallel‐group study |
| Participants | 60 patients in need of a FDP in the posterior region |
| Interventions | Group 1: monolithic zirconia FDP (posterior region) Group 2: monolithic zirconia FDP (posterior region), buccaly and occlusaly veneered Group 3: monolithic zirconia FDP (posterior region), circumferentially veneered |
| Outcomes | Survival Technical complication rate after 3 years of clinical service by means of standardized USPHS criteria; secondary caries (assessed on a radiograph and by clinical examination), bleeding on probing, pocket depth, plaque score Wear rate of the FDP and the antagonists (assessed by means of a silicone impression) |
| Starting date | June 2016 |
| Contact information | 4c57314a547939677566784631424653635a75784f62586535535a65783445596f5338452f5947614546453d@drks.de |
| Notes |
DRKS00011173.
| Trial name or title | 'Different materials for posterior all‐ceramic CAD/CAM generated single crowns: a randomized prospective controlled clinical trial' |
| Methods | Randomised, parallel‐group study |
| Participants | 45 patients in need of a single crown in the posterior region of the maxilla or mandible |
| Interventions | Group 1: monolithic CAD/CAM single crown: e.max CAD Group 2: monolithic CAD/CAM single crown: VITA Enamic Group 3: monolithic CAD/CAM single crown: VITABLOCS Mark II |
| Outcomes | Survival after 1, 3 and 5 years (the crown remained in situ) Technical and biological complication rates after 1, 3 and 5 years |
| Starting date | March 2017 |
| Contact information | 4c57314a547939677566784631424653635a75784f62586535535a65783445596f5338452f5947614546453d@drks.de |
| Notes |
NCT01229995.
| Trial name or title | 'A comparison of zirconia CAD/CAM and conventionally fabricated single implant abutments and restorations in the aesthetic zone: a randomized controlled clinical trial' |
| Methods | Randomised, parallel‐group study |
| Participants | 30 adult patients in need of replacing 1 missing tooth in the aesthetic area |
| Interventions | Group 1: implant‐supported single crowns made with CAD/CAM 'Etkon' technology (zirconia abutment and ceramic crown) Group 2: implant‐supported single crowns made with conventional technique (crossfit titanium abutment and a ceramometal crown) |
| Outcomes | Failure, chipping, periodontal/peri‐implant measurements, aesthetic evaluation through pink aesthetic score (PES) and white aesthetic score (WES), VAS questionnaire for aesthetics assessed by patient |
| Starting date | May 2009 |
| Contact information | |
| Notes |
NCT01729858.
| Trial name or title | 'Factors influencing the survival of implant‐supported all‐ceramic prostheses' |
| Methods | Randomised, parallel‐group study |
| Participants | 150 adult patients with partial edentulism |
| Interventions | Group 1: metal‐ceramic prosthesis with press on veneer with different thicknesses, different diameters of curvature of gingival embrasure and connector heights Group 2: zirconia computer‐aided design and computer‐milled cores with press on veneers with different thicknesses, gingival embrasure diameters and connector heights |
| Outcomes | Primary: any fracture or chipping of the prostheses reported by the participant or noted at recall periods Secondary: wear of prosthesis and enamel antagonist |
| Starting date | December 2008 |
| Contact information | |
| Notes | Preliminary data published 2012 |
NCT01835821.
| Trial name or title | 'A clinical evaluation of hand‐veneered, porcelain‐fused NobelProcera crown shaded zirconia and NobelProcera(TM) full contour crown IPS e.Max CAD on molars' |
| Methods | Randomised, split‐mouth study |
| Participants | 11 adult patients in need of at least 2 paired contralateral single‐tooth full coverage molar restorations in the maxilla or mandible or both |
| Interventions | Group 1: single cemented ceramic crowns made with shaded zirconia (NobelProcera Shaded Zirconia) Group 2: single cemented full contour NobelProcera crowns made with IPS e.max CAD lithium disilicate |
| Outcomes | Success defined according to CDA (Canadian Dental Association) index (Romeo or Sierra; excellent or acceptable) |
| Starting date | March 2010 |
| Contact information | |
| Notes |
NCT02175329.
| Trial name or title | 'A randomized clinical trial of 3‐unit posterior zirconia‐ceramic fixed dental prosthesis (FDPs) veneered with layered and milled (CAD‐on) veneering ceramics |
| Methods | Randomised, parallel‐group study |
| Participants | 60 adult patients |
| Interventions | Group 1: 3‐unit posterior CAD/CAM fabricated zirconia bridge framework veneered with CAD/CAM fabricated lithium disilicate ceramic veneer Group 2: 3‐unit posterior CAD/CAM fabricated zirconia bridge framework veneered manually layered ceramic |
| Outcomes | Clinical success |
| Starting date | January 2010 |
| Contact information | |
| Notes |
NCT02188212.
| Trial name or title | 'A clinical evaluation of hand‐veneered, porcelain‐fused NobelProceraTM crown shaded zirconia and NobelProceraTM full contour crown IPS e.Max CAD on molars' |
| Methods | Randomised, split‐mouth study |
| Participants | 143 adult patients in need of 2 single tooth restorations on contralateral teeth in the same arch (molars) |
| Interventions | Group 1: single cemented ceramic crowns made with shaded zirconia (NobelProcera Shaded Zirconia) Group 2: single cemented full contour NobelProcera crowns made with IPS e.max CAD lithium disilicate |
| Outcomes | Longevity |
| Starting date | March 2010 |
| Contact information | |
| Notes |
NCT02272491.
| Trial name or title | 'Monolithic zirconia crowns for single implants in the molar region: a multicenter randomized controlled clinical trial' |
| Methods | Randomised, parallel‐group study |
| Participants | 80 adult patients in need of molar implant‐supported single crowns |
| Interventions | Group 1: ZrO2 crown (Straumann CARES)
The monolithic zirconia crown (Straumann CARES Full Contour Zerion HT) will be bonded to the titanium base (Straumann CARES Variobase Abutment RN) Group 2: PFM crown (Straumann Gold) Porcelain‐fused‐to‐metal crown consisting of a gold abutment, a gold core, and veneering ceramic |
| Outcomes | "The primary outcome of the study is the technical complication rate. This outcome represents an indicator for the prosthetic success of the implant‐supported crown. The main biological secondary outcomes are marginal bone level, histological signs of inflammation, and presence of pathogenic bacteria. Further outcomes are crown survival, wear of the crown and of the antagonist" |
| Starting date | October 2014 |
| Contact information | research.kbtm@zzm.uzh.ch |
| Notes |
NCT02758457.
| Trial name or title | 'Zirconia and metal‐based single crown posterior restorations' |
| Methods | Randomised, parallel‐group study |
| Participants | 72 adult patients in need of a single posterior ceramic crown |
| Interventions | Group 1: a metal‐based restorations (single crown with a metal framework and pressed ceramic) Group 2: a zirconia‐based restorations (single crown with a zirconia framework and pressed ceramic) |
| Outcomes | Survival Technical complication rate (time frame: 5 years) assessed by USPHS criteria Bleeding on probing, pocket probing depth |
| Starting date | January 2008 |
| Contact information | carlo.monaco2@unibo.it |
| Notes |
NCT02937220.
| Trial name or title | 'Influence of superstructure material on crestal bone resorption and aesthetic outcome of dental implants' |
| Methods | Randomised, parallel‐group study |
| Participants | 34 patients in need of a single implant‐supported crown in the aesthetic region |
| Interventions | Group 1: implant‐supported monolithic zirconia crown Group 2: implant‐supported metal ceramic crown |
| Outcomes | Survival PES (time frame: 1 year, pink aesthetic scoring system for soft tissue aesthetics) Crestal bone resorption |
| Starting date | December 2016 |
| Contact information | Ahmed Roshdy Radwan, Cairo University, Egypt |
| Notes |
NCT03039985.
| Trial name or title | 'All‐ceramic crowns in patients with sleep bruxism ‐ a randomized clinical trial' |
| Methods | Randomised, parallel‐group study |
| Participants | 100 patients in need of a single molar crown |
| Interventions | Group 1: monolithic zirconia molar crowns (Prettau) in patients with bruxism Group 2: monolithic zirconia molar crowns (Prettau) in patients without bruxism Group 3: monolithic lithium disilicate molar crowns (IPSe.max) in patients with bruxism Group 4: monolithic lithium disilicate molar crowns (IPSe.max) in patients without bruxism |
| Outcomes | Success of all ceramic crowns as determined by complication rate (time frame up to 5 years) |
| Starting date | February 2015 |
| Contact information | Brigitte_Ohlmann@40med.uni‐heidelberg.de |
| Notes |
CAD/CAM = computer‐aided design and computer‐aided manufacturing; FDPs = fixed dental prostheses; USPHS = United States Public Health Service; VAS = visual analogue scale.
Differences between protocol and review
The search of the International Association for Dental Research (IADR) abstracts was planned in the protocol but not completed in the final review due to poor yield in preliminary phase.
Contributions of authors
Development of the protocol: Carlo E Poggio (CEP), Carlo Ercoli (CE), Marco Esposito (ME). Examination of titles and abstracts: CEP, CE, Lorena Rispoli (LR). Retrieval of full‐text reports: CEP, CE, LR, Carlo Maiorana (CM). Linking multiple reports of the same study: CEP, LR. Examination of full‐text reports and final decisions on study inclusion: CEP, CE, LR, ME. Data extraction and management: CEP, CE, LR, CM, ME. Risk of bias assessment: CEP, LR, ME. Drafting the final review: CEP.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland
Declarations of interest
‐ Carlo E Poggio: none known. ‐ Carlo Ercoli: Carlo has received consulting fees from Ivoclar NA in the past. ‐ Lorena Rispoli: none known. ‐ Carlo Maiorana: none known. ‐ Marco Esposito: from April 2011 Marco Esposito became a full‐time freelance consultant in dentistry specialising in implantology. Therefore he receives funding for conducting clinical trials and presenting the results of trials/Cochrane Reviews at international dental meetings. This funding comes from universities, companies (in alphabetical order: Apollonia e Fama Implant, Biomax, Biomet 3i, Bioteck, Bone System, Branemark Integration, CMS Dental, Dentsply‐Friadent, Geistlich Pharma, Geass, Keystone Dental, MegaGen Implant, Mozo‐Grau, Nano Bridging molecules, Nobel Biocare, Ricerfarma, Saint Jude Medical, Southern Implants, Supercharched production, Techoss Dental Thommen Medical, Tutogen Medical, Zimmer Dental, Z‐Systems), scientific societies, publishing companies, and private dentists. This list of companies was provided by Marco on Friday 4 November 2011 and the funders will change all the time.
This is to certify that: a) Marco does not own stock in companies that produce products included in the reviews of which he is an author; b) he does not have patents on any of the products included in the reviews; c) his salary will not be affected by company sales of any of the products included in the reviews.
Marco's authorship has been authorised by The Cochrane Collaboration Funding Arbiter (reference 071111/057: Cochrane Oral Health).
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Albornoz 2014 {published data only}
- Carrillo de Albornoz A, Vignoletti F, Ferrantino L, Cardenas E, Sanctis M, Sanz M. A randomized trial on the aesthetic outcomes of implant supported restorations with zirconia or titanium abutments. Journal of Clinical Periodontology 2014;41:1161–9. [DOI] [PubMed] [Google Scholar]
Baldini 2016 {published data only}
- Baldini N, D'Elia C, Clementini M, Carrillo de Albornoz A, Sanz M, Sanctis M. Esthetic outcomes of single‐tooth implant‐supported restorations using metal‐ceramic restorations with zirconia or titanium abutments: a randomized controlled clinical study. International Journal of Periodontics and Restorative Dentistry 2016;36(4):e59‐66. [DOI] [PubMed] [Google Scholar]
Encke 2009 {published data only}
- Encke BS, Heydecke G, Wolkewitz M, Strub JR. Results of a prospective randomized controlled trial of posterior ZrSiO(4)‐ceramic crowns. Journal of Oral Rehabilitation 2009;36(3):226‐35. [DOI] [PubMed] [Google Scholar]
- Passia N, Stampf S, Strub JR. Five‐year results of a prospective randomised controlled clinical trial of posterior computer‐aided design‐computer‐aided manufacturing ZrSiO4 ‐ceramic crowns. Journal of Oral Rehabilitation 2013;40(8):609‐17. [DOI] [PubMed] [Google Scholar]
Gallucci 2011 {published data only}
- Gallucci GO, Grutter L, Nedir R, Bischof M, Belser UC. Esthetic outcomes with porcelain‐fused‐to‐ceramic and all‐ceramic single‐implant crowns: a randomized clinical trial. Clinical Oral Implants Research 2011;22(1):62‐9. [DOI] [PubMed] [Google Scholar]
Larsson 2006 {published data only}
- Larsson C, Vult von Steyern P. Five‐year follow‐up of implant‐supported Y‐TZP and ZTA fixed dental prostheses. A randomized, prospective clinical trial comparing two different material systems. International Journal of Prosthodontics 2010;23(6):555‐61. [PubMed] [Google Scholar]
- Larsson C, Vult von Steyern P. Ten‐year follow‐up of implant‐supported all‐ceramic fixed dental prostheses: a randomized prospective clinical trial. International Journal of Prosthodontics 2016;29(1):31‐4. [DOI] [PubMed] [Google Scholar]
- Larsson C, Vult von Steyern P, Sunzel B, Nilner K. All‐ceramic two‐ to five‐unit implant‐supported reconstructions. A randomized, prospective clinical trial. Swedish Dental Journal 2006;30(2):45‐53. [PubMed] [Google Scholar]
Makarouna 2011 {published data only}
- Makarouna M, Ullmann K, Lazarek K, Boening KW. Six‐year clinical performance of lithium disilicate fixed partial dentures. International Journal of Prosthodontics 2011;24(3):204‐6. [PubMed] [Google Scholar]
Naenni 2015 {published data only}
- Naenni N, Bindl A, Sax C, Hämmerle C, Sailer I. A randomized controlled clinical trial of 3‐unit posterior zirconia‐ceramic fixed dental prostheses (FDP) with layered or pressed veneering ceramics: 3‐year results. Journal of Dentistry 2015;43(11):1365‐70. [DOI] [PubMed] [Google Scholar]
Nicolaisen 2016 {published data only}
- Nicolaisen MH, Bahrami G, Schropp L, Isidor F. Comparison of metal‐ceramic and all‐ceramic three‐unit posterior fixed dental prostheses: a 3‐year randomized clinical trial. International Journal of Prosthodontics 2016;29(3):259‐64. [DOI] [PubMed] [Google Scholar]
- Nicolaisen MH, Bahrami G, Schropp L, Isidor F. Functional and esthetic comparison of metal‐ceramic and all‐ceramic posterior three‐unit fixed dental prostheses. International Journal of Prosthodontics 2016;29(5):473‐81. [DOI] [PubMed] [Google Scholar]
Ohlmann 2012 {published data only}
- Ohlmann B, Eiffler C, Rammelsberg P. Clinical performance of all‐ceramic cantilever fixed dental prostheses: results of a 2‐year randomized pilot study. Quintessence International 2012;43(8):643‐8. [PubMed] [Google Scholar]
- Zenthöfer A, Ohlmann B, Rammelsberg P, Bömicke W. Performance of zirconia ceramic cantilever fixed dental prostheses: 3‐year results from a prospective, randomized controlled pilot study. Journal of Prosthetic Dentistry 2015;114:34‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andersson 1999 {published data only}
- Andersson B, Glauser R, Maglione M, Taylor A. Ceramic implant abutments for short‐span FPDs: a prospective 5‐year multicenter study. International Journal of Prosthodontics 2003;16(6):640‐6. [PubMed] [Google Scholar]
- Andersson B, Scharer P, Simion M, Bergstrom C. Ceramic implant abutments used for short‐span fixed partial dentures: a prospective 2‐year multicenter study. International Journal of Prosthodontics 1999;12(4):318‐24. [PubMed] [Google Scholar]
Andersson 2001 {published data only}
- Andersson B, Taylor A, Lang BR, Scheller H, Scharer P, Sorensen JA, et al. Alumina ceramic implant abutments used for single‐tooth replacement: a prospective 1‐ to 3‐year multicenter study. International Journal of Prosthodontics 2001;14(5):432‐8. [PubMed] [Google Scholar]
Batson 2014 {published data only}
- Batson RB, Cooper LF, Duqum I, Mendonça G. Clinical outcomes of three different crown systems with CAD/CAM technology. Journal of Prosthetic Dentistry 2014;112:770‐7. [DOI] [PubMed] [Google Scholar]
Bindl 2005 {published data only}
- Bindl A, Richter B, Mormann WH. Survival of ceramic computer‐aided design/manufacturing crowns bonded to preparations with reduced macroretention geometry. International Journal of Prosthodontics 2005;18(3):219‐24. [PubMed] [Google Scholar]
Borg 2014 {published data only}
- Borg M, Vult von Steyern P, Larsson C. Titanium‐ and zirconia‐based implant‐supported fixed dental prostheses. A randomized, prospective clinical pilot study. Swedish Dental Journal 2014;38(1):23‐30. [PubMed] [Google Scholar]
Cehreli 2009 {published data only}
- Cehreli MC, Kokat AM, Akca K. CAD/CAM zirconia vs slip‐cast glass‐infiltrated alumina/zirconia all‐ceramic crowns: 2‐year results of a randomized controlled clinical trial. Journal of Applied Oral Science 2009;17(1):49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cehreli 2011 {published data only}
- Cehreli MC, Kokat AM, Ozpay C, Karasoy D, Akca K. A randomized controlled clinical trial of feldspathic versus glass‐infiltrated alumina all‐ceramic crowns: a 3‐year follow‐up. International Journal of Prosthodontics 2011;24(1):77‐84. [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen ZF, Nang PH, Wang Y, Luo ZB. Clinical evaluation of ceramic implant abutments in anterior restorations. Annals of the Royal Australasian College of Dental Surgeons 2008;19:67‐70. [PubMed] [Google Scholar]
Christensen 2010 {published data only}
- Christensen RP, Ploeger BJ. A clinical comparison of zirconia, metal and alumina fixed‐prosthesis frameworks veneered with layered or pressed ceramic: a three‐year report. Journal of the American Dental Association 2010;141(11):1317‐29. [DOI] [PubMed] [Google Scholar]
Esquivel‐Upshaw 2012 {published data only}
- Esquivel‐Upshaw J, Rose W, Oliveira E, Yang M, Clark AE, Anusavice K. Randomized, controlled clinical trial of bilayer ceramic and metal‐ceramic crown performance. Journal of Prosthodontics 2013;22(3):166‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquivel‐Upshaw JF, Rose WF Jr, Barrett AA, Oliveira ER, Yang MC, Clark AE, et al. Three years in vivo wear: core‐ceramic, veneers, and enamel antagonists. Dental Materials 2012;28(6):615‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esquivel‐Upshaw 2014 {published data only}
- Esquivel‐Upshaw JF, Clark AE, Shuster JJ, Anusavice KJ. Randomized clinical trial of implant‐supported ceramic‐ceramic and metal‐ceramic fixed dental prostheses: preliminary results. Journal of Prosthodontics 2014;23(2):73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esquivel‐Upshaw 2014b {published data only}
- Esquivel‐Upshaw JF, Mehler A, Clark AE, Neal D, Anusavice KJ. Fracture analysis of randomized implant‐supported fixed dental prostheses. Journal of Dentistry 2014;42(10):1335‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Etman 2008 {published data only}
- Etman MK, Woolford M, Dunne S. Quantitative measurement of tooth and ceramic wear: in vivo study. [Erratum appears in International Journal of Prosthodontics 2008;21(6):508]. International Journal of Prosthodontics 2008;21(3):245‐52. [PubMed] [Google Scholar]
- Etman MK, Woolford MJ. Three‐year clinical evaluation of two ceramic crown systems: a preliminary study. Journal of Prosthetic Dentistry 2010;103(2):80‐90. [DOI] [PubMed] [Google Scholar]
Henriksson 2004 {published data only}
- Henriksson K, Jemt T. Measurements of soft tissue volume in association with single‐implant restorations: a 1‐year comparative study after abutment connection surgery. Clinical Implant Dentistry and Related Research 2004;6(4):181‐9. [DOI] [PubMed] [Google Scholar]
Jung 2008 {published data only}
- Jung RE, Holderegger C, Sailer I, Khraisat A, Suter A, Hammerle CH. The effect of all‐ceramic and porcelain‐fused‐to‐metal restorations on marginal peri‐implant soft tissue color: a randomized controlled clinical trial. International Journal of Periodontics and Restorative Dentistry 2008;28(4):357‐65. [PubMed] [Google Scholar]
Li 2007 {published data only}
- Li ZY, Niu W, Fang W, Hu J, Wang YN. Clinical evaluation of ceramic and titanium abutment for implant‐supported all‐ceramic single crown. Chinese Journal of Stomatology 2007;42(7):391‐4. [PubMed] [Google Scholar]
Ohlmann 2006 {published data only}
- Ohlmann B, Bermejo JL, Rammelsberg P, Schmitter M, Zenthöfer A, Stober T. Comparison of incidence of complications and aesthetic performance for posterior metal‐free polymer crowns and metal‐ceramic crowns: results from a randomized clinical trial. Journal of Dentistry 2014;42(6):671‐6. [DOI] [PubMed] [Google Scholar]
- Ohlmann B, Dreyhaupt J, Schmitter M, Gabbert O, Hassel A, Rammelsberg P. Clinical performance of posterior metal‐free polymer crowns with and without fiber reinforcement: one‐year results of a randomised clinical trial. Journal of Dentistry 2006;34(10):757‐62. [DOI] [PubMed] [Google Scholar]
- Ohlmann B, Trame JP, Dreyhaupt J, Gabbert O, Koob A, Rammelsberg P. Wear of posterior metal‐free polymer crowns after 2 years. Journal of Oral Rehabilitation 2008;35(10):782‐8. [DOI] [PubMed] [Google Scholar]
- Ohlmann B, Uekermann J, Dreyhaupt J, Schmitter M, Mussotter K, Rammelsberg P. Clinical wear of posterior metal‐free polymer crowns. one‐year results from a randomized clinical trial. Journal of Dentistry 2007;35(3):246‐52. [DOI] [PubMed] [Google Scholar]
Pelaez 2012 {published data only}
- Pelaez J, Cogolludo PG, Serrano B, Serrano JF, Suarez MJ. A four‐year prospective clinical evaluation of zirconia and metal‐ceramic posterior fixed dental prostheses. International Journal of Prosthodontics 2012;25(5):451‐8. [PubMed] [Google Scholar]
Sagirkaya 2012 {published data only}
- Sagirkaya E, Arikan S, Sadik B, Kara C, Karasoy D, Cehreli M. A randomized, prospective, open‐ended clinical trial of zirconia fixed partial dentures on teeth and implants: interim results. International Journal of Prosthodontics 2012;25(3):221‐31. [PubMed] [Google Scholar]
Sailer 2009 {published data only}
- Sailer I, Zembic A, Jung RE, Siegenthaler D, Holderegger C, Hammerle CH. Randomized controlled clinical trial of customized zirconia and titanium implant abutments for canine and posterior single‐tooth implant reconstructions: preliminary results at 1 year of function. Clinical Oral Implants Research 2009;20(3):219‐25. [DOI] [PubMed] [Google Scholar]
- Zembic A, Bosch A, Jung RE, Hammerle CH, Sailer I. Five‐year results of a randomized controlled clinical trial comparing zirconia and titanium abutments supporting single‐implant crowns in canine and posterior regions. Clinical Oral Implants Research 2013;24(4):384‐90. [DOI] [PubMed] [Google Scholar]
- Zembic A, Sailer I, Jung RE, Hammerle CH. Randomized‐controlled clinical trial of customized zirconia and titanium implant abutments for single‐tooth implants in canine and posterior regions: 3‐year results. Clinical Oral Implants Research 2009;20(8):802‐8. [DOI] [PubMed] [Google Scholar]
Sailer 2009b {published data only}
- Sailer I, Gottnerb J, Kanelb S, Hammerle CH. Randomized controlled clinical trial of zirconia‐ceramic and metal‐ceramic posterior fixed dental prostheses: a 3‐year follow‐up. International Journal of Prosthodontics 2009;22(6):553‐60. [PubMed] [Google Scholar]
Vanoorbeek 2010 {published data only}
- Vanoorbeek S, Vandamme K, Lijnen I, Naert I. Computer‐aided designed/computer‐assisted manufactured composite resin versus ceramic single‐tooth restorations: a 3‐year clinical study. International Journal of Prosthodontics 2010;23(3):223‐30. [PubMed] [Google Scholar]
References to ongoing studies
DRKS00005452 {published data only}
- DRKS00005452. Prospective randomized controlled interventional study of chair‐side generated monolithic single implant supra‐structures made of lithium disilicate ceramic. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00005452 (first received 19 November 2013).
DRKS00010423 {published data only}
- DRKS00010423. Monolithic zirconia fixed dental prosthesis in the posterior region: a randomized controlled clinical trial. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00010423 (first received 26 April 2016).
DRKS00011173 {published data only}
- DRKS00011173. Different materials for posterior all‐ceramic CAD/CAM generated single crowns: a randomized prospective controlled clinical trial. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00011173 (first received 17 January 2017).
NCT01229995 {published data only}
- NCT01229995. Randomized controlled clinical trial of CAD/CAM and conventionally fabricated singl implant abutments [A comparison of zirconia CAD/CAM and conventionally fabricated single implant abutments and restorations in the esthetic zone: a randomized controlled clinical trial]. clinicaltrials.gov/show/NCT01229995 (first received 27 October 2010).
NCT01729858 {published data only}
- NCT01729858. Survival of implant‐supported all‐ceramic prostheses [Factors influencing the survival of implant‐supported all‐ceramic prostheses]. clinicaltrials.gov/show/NCT01729858 (first received 14 November 2012).
NCT01835821 {published data only}
- NCT01835821. Clinical study of porcelain‐fused and IPS e.Max CAD crowns [A clinical evaluation of hand‐veneered, porcelain‐fused NobelProcera(TM) crown shaded zirconia and NobelProcera(TM) full contour crown IPS e.Max CAD on molars]. clinicaltrials.gov/show/NCT01835821 (first received 5 April 2013).
NCT02175329 {published data only}
- NCT02175329. Clinical investigation on zirconia‐ceramic three‐unit bridges with CAD‐on veneering [A randomized clinical trial of 3‐unit posterior zirconia‐ceramic fixed dental prosthesis (FDPs) veneered with layered and milled (CAD‐on) veneering ceramics]. clinicaltrials.gov/show/NCT02175329 (first received 23 June 2014).
NCT02188212 {published data only}
- NCT02188212. NobelProcera crown shaded zirconia and NobelProceraTM full contour crown IPS e.Max CAD [A clinical evaluation of hand‐veneered, porcelain‐fused NobelProceraTM crown shaded zirconia and NobelProceraTM full contour crown IPS e.Max CAD on molars]. clinicaltrials.gov/show/NCT02188212 (first received 3 July 2014).
NCT02272491 {published data only}
- NCT02272491. Monolithic zirconia crowns for single implants in the molar region: a multicenter clinical trial (MonoZrO2crown) [Monolithic zirconia crowns for single implants in the molar region: a multicenter randomized controlled clinical trial]. clinicaltrials.gov/show/NCT02272491 (first received 10 October 2014).
NCT02758457 {published data only}
- NCT02758457. Zirconia and metal‐based single crown posterior restorations [Zirconia‐based and metal‐based single crown posterior restorations: 5‐year results of a randomized controlled clinical study]. clinicaltrials.gov/show/NCT02758457 (first received 23 April 2016).
NCT02937220 {published data only}
- NCT02937220. Influence of superstructure material on crestal bone resorption and esthetic outcome of dental implants [Influence of superstructure material on crestal bone resorption and esthetic outcome of dental implants]. clinicaltrials.gov/show/NCT02937220 (first received 16 October 2016).
NCT03039985 {published data only}
- NCT03039985. All‐ceramic crowns in patients with sleep bruxism [All‐ceramic crowns in patients with sleep bruxism ‐ a randomized clinical trial]. clinicaltrials.gov/show/NCT03039985 (first received 13 January 2017).
Additional references
Behr 2003
- Behr M, Rosentritt M, Handel G. Fiber‐reinforced composite crowns and FPDs: a clinical report. International Journal of Prosthodontics 2003;16(3):239–43. [PubMed] [Google Scholar]
Bergendal 1995
- Bergendal T, Ekstrand K, Karlsson U. Evaluation of implant‐supportedcarbon/graphite fiber‐reinforced poly (methyl methacrylate) prostheses. A longitudinal multicenter study. Clinical Oral Implants Research 1995;6(4):246‐53. [DOI] [PubMed] [Google Scholar]
Conrad 2007
- Conrad HJ, Seong WJ, Pesun IJ. Current ceramic materials and systems with clinical recommendations: a systematic review. Journal of Prosthetic Dentistry 2007;98(5):389‐404. [DOI] [PubMed] [Google Scholar]
Creugers 1994
- Creugers NH, Käyser AF, van't Hof MA. A meta‐analysis of durability data on conventional fixed bridges. Community Dentistry and Oral Epidemiology 1994;22(6):448‐52. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 1 April 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Harder 2009
- Harder S, Kern M. Survival and complications of computer‐aided designing and computer‐aided manufacturing vs conventionally fabricated implant supported reconstructions: a systematic review. Clinical Oral Implants Research 2009;20(Suppl 4):48‐54. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Manicone 2007
- Manicone PF, Rossi Iommetti P, Raffaelli L. An overview of zirconia ceramics: basic properties and clinical applications. Journal of Dentistry 2007;35(11):819‐26. [DOI] [PubMed] [Google Scholar]
McLean 1967
- McLean JW. The alumina reinforced porcelain jacket crown. Journal of the American Dental Association 1967;75(3):621‐8. [DOI] [PubMed] [Google Scholar]
Pjetursson 2007
- Pjetursson BE, Sailer I, Zwahlen M, Hämmerle CH. A systematic review of the survival and complication rates of all‐ceramic and metal‐ceramic reconstructions after an observation period of at least 3 years. Part I: single crowns. Clinical Oral Implants Research 2007;18(Suppl 3):73‐85. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sailer 2007
- Sailer I, Pjetursson BE, Zwahlen M, Hämmerle CH. A systematic review of the survival and complication rates of all‐ceramic and metal‐ceramic reconstructions after an observation period of at least 3 years. Part II: fixed dental prostheses. Clinical Oral Implants Research 2007;18(Suppl 3):86–96. [DOI] [PubMed] [Google Scholar]
Sailer 2009a
- Sailer I, Philipp A, Zembic A, Pjetursson BE, Hämmerle CHF, Zwahlen M. A systematic review of the performance of ceramic and metal implant abutments supporting fixed implant reconstructions. Clinical Oral Implants Review 2009;20(Suppl 4):4‐31. [DOI] [PubMed] [Google Scholar]
Schley 2010
- Schley JS, Heussen N, Reich S, Fischer J, Haselhuhn K, Wolfart S. Survival probability of zirconia‐based fixed dental prostheses up to 5 yr: a systematic review of the literature. European Journal of Oral Sciences 2010;118(5):443‐50. [DOI] [PubMed] [Google Scholar]
Scurria 1998
- Scurria MS, Bader JD, Shugars DA. Meta‐analysis of fixed partial denture survival: prostheses and abutments. Journal of Prosthetic Dentistry 1998;79(4):459‐64. [DOI] [PubMed] [Google Scholar]


