Abstract
Background
Lung transplantation has become a valuable and well‐accepted treatment option for most end‐stage lung diseases. Lung transplant recipients are at risk of transplanted organ rejection, and life‐long immunosuppression is necessary. Clear evidence is essential to identify an optimal, safe and effective immunosuppressive treatment strategy for lung transplant recipients. Consensus has not yet been achieved concerning use of immunosuppressive antibodies against T‐cells for induction following lung transplantation.
Objectives
We aimed to assess the benefits and harms of immunosuppressive T‐cell antibody induction with ATG, ALG, IL‐2RA, alemtuzumab, or muromonab‐CD3 for lung transplant recipients.
Search methods
We searched the Cochrane Renal Group's Specialised Register to 4 March 2013 through contact with the Trials Search Co‐ordinator using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE and EMBASE.
Selection criteria
We included all randomised controlled trials (RCTs) that compared immunosuppressive monoclonal and polyclonal T‐cell antibody induction for lung transplant recipients. An inclusion criterion was that all participants must have received the same maintenance immunosuppressive therapy within each study.
Data collection and analysis
Three authors extracted data. We derived risk ratios (RR) for dichotomous data and mean differences (MD) for continuous data with 95% confidence intervals (CI). Methodological risk of bias was assessed using the Cochrane risk of bias tool and trial sequential analyses were undertaken to assess the risk of random errors (play of chance).
Main results
Our review included six RCTs (representing a total of 278 adult lung transplant recipients) that assessed the use of T‐cell antibody induction. Evaluation of the included studies found all to be at high risk of bias.
We conducted comparisons of polyclonal or monoclonal T‐cell antibody induction versus no induction (3 studies, 140 participants); polyclonal T‐cell antibody versus no induction (3 studies, 125 participants); interleukin‐2 receptor antagonists (IL‐2RA) versus no induction (1 study, 25 participants); polyclonal T‐cell antibody versus muromonab‐CD3 (1 study, 64 participants); and polyclonal T‐cell antibody versus IL‐2RA (3 studies, 100 participants). Overall we found no significant differences among interventions in terms of mortality, acute rejection, adverse effects, infection, pneumonia, cytomegalovirus infection, bronchiolitis obliterans syndrome, post‐transplantation lymphoproliferative disease, or cancer.
We found a significant outcome difference in one study that compared antithymocyte globulin versus muromonab‐CD3 relating to adverse events (25/34 (74%) versus 12/30 (40%); RR 1.84, 95% CI 1.13 to 2.98). This suggested that antithymocyte globulin increased occurrence of adverse events. However, trial sequential analysis found that the required information size had not been reached, and the cumulative Z‐curve did not cross the trial sequential alpha‐spending monitoring boundaries.
None of the studies reported quality of life or kidney injury. Trial sequential analyses indicated that none of the meta‐analyses achieved required information sizes and the cumulative Z‐curves did not cross the trial sequential alpha‐spending monitoring boundaries, nor reached the area of futility.
Authors' conclusions
No clear benefits or harms associated with the use of T‐cell antibody induction compared with no induction, or when different types of T‐cell antibodies were compared were identified in this review. Few studies were identified that investigated use of antibodies against T‐cells for induction after lung transplantation, and numbers of participants and outcomes were also limited. Assessment of the included studies found that all were at high risk of methodological bias.
Further RCTs are needed to perform robust assessment of the benefits and harms of T‐cell antibody induction for lung transplant recipients. Future studies should be designed and conducted according to methodologies to reduce risks of systematic error (bias) and random error (play of chance).
Keywords: Adult; Humans; Lung Transplantation; Alemtuzumab; Antibodies, Monoclonal; Antibodies, Monoclonal/therapeutic use; Antibodies, Monoclonal, Humanized; Antibodies, Monoclonal, Humanized/therapeutic use; Antilymphocyte Serum; Antilymphocyte Serum/therapeutic use; Basiliximab; Daclizumab; Graft Rejection; Graft Rejection/immunology; Graft Rejection/prevention & control; Immunoglobulin G; Immunoglobulin G/therapeutic use; Immunosuppression Therapy; Immunosuppression Therapy/adverse effects; Immunosuppression Therapy/methods; Immunosuppressive Agents; Immunosuppressive Agents/adverse effects; Immunosuppressive Agents/therapeutic use; Muromonab‐CD3; Muromonab‐CD3/therapeutic use; Randomized Controlled Trials as Topic; Receptors, Interleukin‐2; Receptors, Interleukin‐2/antagonists & inhibitors; Recombinant Fusion Proteins; Recombinant Fusion Proteins/therapeutic use; T‐Lymphocytes; T‐Lymphocytes/immunology
Plain language summary
Can antibody induction therapy help to reduce organ rejection for lung transplant recipients?
People who receive transplanted lungs are at significant risk of organ rejection. To help reduce the risk of organ rejection, antibodies against T‐cells (a type of white blood cell that plays a central role in immunity) are given to patients within the first two weeks after transplantation. Several types of antibodies have been used, but their benefits and harms are unclear.
We evaluated the use of antibodies against T‐cells following lung transplantation to find out whether this therapy was safe, beneficial or harmful, and which type of antibodies work best with fewest adverse effects.
We analysed six studies that investigated the use of several different types of antibody therapies in 278 adult patients following lung transplantation. Flaws in study designs were found that indicated the studies were at risk of overestimating benefits and underestimating harms.
Our analysis compared several types of antibodies, but with one exception ‐ that antithymocyte globulin seemed to increase some adverse events ‐ we found no significant differences in lung survival or rejection for any of the treatments. There was some uncertainty about this effect because the study was too small to be sure that observed benefits would apply to a larger population. We found no significant differences among therapies in terms of infection, bronchiolitis obliterans syndrome, post‐transplantation lymphoproliferative disease, or cancer.
Few investigated the use of T‐cell antibodies after lung transplantation, and these included small numbers of participants. These limitations meant that our findings did not necessarily indicate no differences existed among comparisons in our analysis. To overcome this problem, larger and more robust randomised studies that assess the benefits and harms of antibodies against T‐cells for people following lung transplantation are needed.
Summary of findings
Summary of findings for the main comparison. T‐cell antibody induction compared with no antibody induction for lung transplant recipients.
| T‐cell antibody induction compared with no antibody induction for lung transplant recipients | ||||||
| Patient or population: lung transplant recipients Settings: patients with end‐stage lung failure who underwent lung transplantation Intervention: antibody induction Comparison: no antibody induction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No antibody induction | Antibody induction | |||||
| Mortality Follow‐up: 2 to 8 years | Study population | RR 0.99 (0.69 to 1.41) | 140 (3) | ⊕⊕⊕⊝ moderate¹ | ||
| 448 per 1000 | 444 per 1000 (309 to 632) | |||||
| Moderate | ||||||
| 400 per 1000 | 396 per 1000 (276 to 564) | |||||
| Acute rejection grade II or higher Follow‐up: 2 to 8 years | Study population | RR 0.66 (0.43 to 1.02) | 140 (3) | ⊕⊕⊕⊝ moderate¹ | ||
| 483 per 1000 | 319 per 1000 (208 to 492) | |||||
| Moderate | ||||||
| 500 per 1000 | 330 per 1000 (215 to 510) | |||||
| Infection Follow‐up: 2 to 8 years | Study population | RR 1.4 (0.97 to 2.01) | 104 (2) | ⊕⊕⊕⊝ moderate¹ | ||
| 458 per 1000 | 642 per 1000 (445 to 921) | |||||
| Moderate | ||||||
| 458 per 1000 | 641 per 1000 (444 to 921) | |||||
| Bronchiolitis obliterans syndrome Follow‐up: 2 to 8 years | Study population | RR 0.77 (0.55 to 1.09) | 140 (3) | ⊕⊕⊕⊝ moderate¹ | ||
| 534 per 1000 | 412 per 1000 (294 to 583) | |||||
| Moderate | ||||||
| 400 per 1000 | 308 per 1000 (220 to 436) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
¹ All studies were assessed to be at high risk of bias using the Cochrane risk of bias tool
Summary of findings 2. Polyclonal T‐cell antibody compared with no antibody induction for lung transplant recipients.
| Polyclonal T‐cell antibody compared with no antibody induction for lung transplant recipients | ||||||
| Patient or population: lung transplant recipients Settings: patients with end‐stage lung disease who underwent lung transplantation Intervention: polyclonal antibody Comparison: no antibody induction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No antibody induction | Polyclonal antibody | |||||
| Mortality Follow‐up: 2 to 8 years | Study population | RR 1.02 (0.71 to 1.47) | 125 (3) | ⊕⊕⊕⊝ moderate¹ | ||
| 448 per 1000 | 457 per 1000 (318 to 659) | |||||
| Moderate | ||||||
| 400 per 1000 | 408 per 1000 (284 to 588) | |||||
| Acute rejection grade II or higher Follow‐up: 2 to 8 years | Study population | RR 0.68 (0.44 to 1.04) | 125 (3) | ⊕⊕⊕⊝ moderate¹ | ||
| 483 per 1000 | 328 per 1000 (212 to 502) | |||||
| Moderate | ||||||
| 500 per 1000 | 340 per 1000 (220 to 520) | |||||
| Infection Follow‐up: 2 to 8 years | Study population | RR 1.4 (0.97 to 2.01) | 104 (2) | ⊕⊕⊕⊝ moderate¹ | ||
| 458 per 1000 | 642 per 1000 (445 to 921) | |||||
| Moderate | ||||||
| 458 per 1000 | 641 per 1000 (444 to 921) | |||||
| Bronchiolitis obliterans syndrome Follow‐up: 2 to 8 years | Study population | RR 0.81 (0.57 to 1.16) | 125 (3) | ⊕⊕⊕⊝ moderate¹ | ||
| 534 per 1000 | 433 per 1000 (305 to 620) | |||||
| Moderate | ||||||
| 400 per 1000 | 324 per 1000 (228 to 464) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ All studies were assessed to be at high risk of bias using the Cochrane risk of bias tool
Summary of findings 3. IL‐2 receptor antagonist induction compared with no antibody induction for lung transplant recipients.
| IL‐2 receptor antagonist induction compared with no antibody induction for lung transplant recipients | ||||||
| Patient or population: lung transplant recipients Settings: patients with end‐stage lung disease who underwent lung transplantation Intervention: interleukin‐2 receptor antagonist (IL‐2RA) induction Comparison: no antibody induction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No antibody induction | IL‐2RA induction | |||||
| Mortality Follow‐up: mean 2 years | Study population | RR 0.67 (0.22 to 2.07) | 25 (1) | ⊕⊕⊕⊝ moderate¹ | ||
| 400 per 1000 | 268 per 1000 (88 to 828) | |||||
| Moderate | ||||||
| 400 per 1000 | 268 per 1000 (88 to 828) | |||||
| Acute rejection Follow‐up: mean 2 years | Study population | RR 1.07 (0.49 to 2.33) | 25 (1) | ⊕⊕⊕⊝ moderate¹ | ||
| 500 per 1000 | 535 per 1000 (245 to 1000) | |||||
| Moderate | ||||||
| 500 per 1000 | 535 per 1000 (245 to 1000) | |||||
| Bronchiolitis obliterans syndrome Follow‐up: mean 2 years | Study population | RR 0.33 (0.07 to 1.49) | 25 (1) | ⊕⊕⊕⊝ moderate¹ | ||
| 400 per 1000 | 132 per 1000 (28 to 596) | |||||
| Moderate | ||||||
| 400 per 1000 | 132 per 1000 (28 to 596) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
¹ All studies were assessed to be at high risk of bias using the Cochrane risk of bias tool
Summary of findings 4. Polyclonal T‐cell antibody induction compared with interleukin‐2 receptor antagonist induction for lung transplant recipients.
| Polyclonal T‐cell antibody induction compared with IL‐2RA induction for lung transplant recipients | ||||||
| Patient or population: lung transplant recipients Settings: patients with end‐stage lung disease who underwent lung transplantation Intervention: polyclonal antibody induction Comparison: interleukin‐2 receptor antagonist (IL‐2RA) induction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IL‐2RA induction | Polyclonal antibody induction | |||||
| Mortality Follow‐up: 0.5 to 2 years | Study population | RR 1.41 (0.55 to 3.64) | 100 (3) | ⊕⊕⊕⊝ moderate¹ | ||
| 113 per 1000 | 160 per 1000 (62 to 412) | |||||
| Moderate | ||||||
| 77 per 1000 | 109 per 1000 (42 to 280) | |||||
| Acute rejection Follow‐up: 1 to 2 years | Study population | RR 1.33 (0.93 to 1.92) | 76 (2) | ⊕⊕⊕⊝ moderate¹ | ||
| 525 per 1000 | 698 per 1000 (488 to 1000) | |||||
| Moderate | ||||||
| 527 per 1000 | 701 per 1000 (490 to 1000) | |||||
| Infection Follow‐up: mean 1 years | Study population | RR 0.91 (0.71 to 1.16) | 50 (1) | ⊕⊕⊕⊝ moderate¹ | ||
| 880 per 1000 | 801 per 1000 (625 to 1000) | |||||
| Moderate | ||||||
| 880 per 1000 | 801 per 1000 (625 to 1000) | |||||
| Bronchiolitis obliterans syndrome Follow‐up: 1 to 2 years | Study population | RR 1.66 (0.42 to 6.53) | 76 (2) | ⊕⊕⊕⊝ moderate¹ | ||
| 75 per 1000 | 124 per 1000 (31 to 490) | |||||
| Moderate | ||||||
| 87 per 1000 | 144 per 1000 (37 to 568) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
¹ All studies were assessed to be at high risk of bias using the Cochrane risk of bias tool
Background
Description of the condition
Success in lung transplantation has led to wide acceptance of the procedure as a treatment for most end‐stage lung diseases. Over 30,000 lung transplantations have been reported to the International Society for Heart and Lung Transplantation (Christie 2011); and of the more than 2700 lung transplantations reported annually, one year survival is over 80%, and five year survival is 60% (Aurora 2009; Christie 2011).
Long‐term recipient survival after lung transplantation remains suboptimal, mainly due to occurrence of bronchiolitis obliterans syndrome. Bronchiolitis obliterans syndrome and late graft failure are responsible for more than 40% of deaths beyond the first year of transplantation (Christie 2011). Primary risks for developing bronchiolitis obliterans syndrome are acute rejection and lymphocytic bronchitis (Hollmen 2008; Verleden 2009). Lung transplant recipients are also at high risk of developing morbidities that inhibit long‐term survival. Major morbidities in lung transplant recipients five years after transplantation are hypertension (85%), hyperlipidaemia (55%), diabetes mellitus (37%), and kidney dysfunction (36%) (Christie 2011).
Description of the intervention
Immunological rejection of lungs means that transplant recipients are at risk of increased morbidity and reduced survival compared with the general population (Christie 2011; Lechler 2005; Verleden 2009). Finding the most effective immunosuppressive treatment strategy is essential to reduce morbidity and increase survival (Iversen 2009).
Optimally, lung transplant recipients should develop immunological tolerance for grafts without compromising general immunity (Chen 2006). Avoidance of adverse effects associated with immunosuppressive agents, such as kidney and cardiovascular diseases and malignancies enhance patients' survival (Flechner 2008; Hauptman 2005).
Maintenance immunosuppressive therapy for lung transplant recipients often involves three types of drugs directed against the T‐cell activation and proliferation cascade: antiproliferative agents (mycophenolate mofetil or azathioprine), calcineurin inhibitors (tacrolimus or cyclosporin), and steroids (prednisolone) (Iversen 2009). Mammalian target of rapamycin inhibitors (sirolimus or everolimus) may also be used as maintenance immunosuppression (Iversen 2009). The optimal combination and dose of these drugs has been the focus of much debate, especially given that calcineurin inhibitors are highly nephrotoxic, and the prolonged use of steroids causes several complications (Flechner 2008; Iversen 2009). No combination of these maintenance immunosuppressive agents has been completely successful in preventing acute and chronic rejection and graft failure without causing adverse reactions (Iversen 2009).
Antibodies specific for T‐cells ‐ induction therapy ‐ have also been used to prevent rejection (Hachem 2006). The aim of T‐cell specific antibody induction therapy is to deplete circulating T‐cells immediately after transplantation before the full effect of calcineurin inhibitor treatment is achieved, thus diminishing rates of acute rejection following transplantation (Iversen 2009). It has also been suggested that temporary immune system manipulation using antibody induction against T‐cells to enhance graft acceptance may pave the way for long‐term reduction of maintenance immunosuppressive treatment (Chatenoud 2008).
Induction is usually commenced before or at the time of maintenance immunosuppressive therapy, and is typically used for a short period of time to avoid risks of severe infection and sepsis. Induction therapy enables delayed introduction or dose reduction of calcineurin inhibitors (Iversen 2009; Rosenberg 2005).
Several T‐cell specific antibodies have been used. These include polyclonal antibodies of horse or rabbit (antithymocyte globulin (ATG) or antilymphocyte globulin (ALG)), or one of the monoclonal agents specific for the CD3 receptor (muromonab‐CD3), the CD52 surface protein (alemtuzumab), or interleukin‐2 receptor antagonists (IL‐2RA; daclizumab or basiliximab) (Hachem 2006; Hachem 2008; Iversen 2009).
ATG, ALG, muromonab‐CD3, and alemtuzumab tend to eradicate functional T‐cell population from the circulation causing profound immunosuppression. The monoclonal IL‐2RA have been developed to increase immunosuppression specificity, with the aim of potentially avoiding over‐immunosuppression toxicity. The IL‐2RA exerts their effects through binding to the alpha subunit of the interleukin‐2 receptor found only on activated T‐cells. Interleukin‐2 receptor blockade prevents interleukin‐2 receptor‐stimulated clonal expression of the T‐cell (Iversen 2009).
Why it is important to do this review
The International Society for Heart and Lung Transplantation has reported that 50% of all transplant centres use T‐cell antibody induction for lung transplant recipients (Christie 2011; Iversen 2009). Consensus on use of immunosuppressive antibody induction after lung transplantation has not yet been achieved (Hachem 2006; Iversen 2009). To enhance survival, it is essential to establish clear evidence to identify an optimal, safe and effective immunosuppressive treatment strategy for lung transplant recipients.
Objectives
We aimed to assess the benefits and harms of immunosuppressive T‐cell antibody induction with ATG, ALG, IL‐2RA, alemtuzumab, or muromonab‐CD3 for lung transplant recipients.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) assessing immunosuppressive induction with monoclonal or polyclonal antibodies against T‐cells for lung transplant recipients were sought. Quasi‐randomised and non‐randomised controlled studies that were identified were considered only for reporting of harms.
Types of participants
We included all patients who had received their first isolated single or double lung transplantation. Data for adult and paediatric patients were planned to be analysed and reported separately because immunological differences in paediatric patients were expected (Aurora 2009).
Types of interventions
Studies comparing any dose and duration of immunosuppressive antibody induction with ATG, ALG, alemtuzumab, muromonab‐CD3, or IL‐2RA versus placebo or no intervention.
One class of immunosuppressive T‐cell antibody induction versus another class of immunosuppressive T‐cell antibody induction (e.g. IL‐2RA versus ATG).
Immunosuppressive T‐cell antibody preparation versus different formulation of same class antibody preparation (e.g. basiliximab versus daclizumab).
Types of outcome measures
Primary outcomes
Mortality
Acute rejection (≥ A2) according to the classification of the International Society for Heart and Lung Transplantation (A0 (no rejection), A1 (minimal rejection), A2 (mild rejection), A3 (severe rejection); Stewart 2007). We did not plan to evaluate secondary types of rejection such as airway inflammation related to bronchioles
Adverse events. Serious adverse events were defined as any untoward medical occurrence that was life threatening, resulted in death, or persistent or significant disability, or any medical event which might have jeopardised the patient or required intervention/s to prevent it. All other adverse events (any medical occurrence not necessarily having a causal relationship with treatment) were considered as non‐serious (ICH GCP 1996).
Secondary outcomes
Quality of life
Infection
Bronchiolitis obliterans syndrome
Post‐transplantation lymphoproliferative disease (PTLD)
Cancer
Kidney injury requiring haemodialysis.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's specialised register to 4 March 2013 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
The Cochrane Renal Group’s specialised register contains studies identified from:
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of pulmonology and transplant textbooks, review articles, and relevant studies
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies
Bibliographies of relevant articles
US Food and Drug Administration (FDA) and European Medicines Agency (EMA) drug approval reviews
The Science Citation Index Expanded (1945 to August 2011) (Royle 2003).
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that could be relevant to the review. Three authors independently assessed study eligibility. Excluded studies were listed with the reason for exclusion. Disagreements were resolved by discussion or in consultation with a third author. Study authors were contacted if information about methodology or data was unclear or missing.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms (Higgins 2011; Moher 2009). Studies reported in non‐English language journals were planned to be translated before assessment. Where more than one publication of one study existed, publications were grouped together and the publication with the most complete data were used. Where relevant outcomes were only published in earlier versions we planned to use these data. Any discrepancy between published versions was planned to be highlighted. Any further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review. Disagreements were resolved by consultation among all authors.
We extracted the following information from each study: first author, country of origin, study design, inclusion and exclusion criteria, number of participants, patient characteristics, study drugs, dose, administration, additional immunosuppression, follow‐up period, primary and secondary outcomes, adverse events, and patients lost to follow‐up.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Studies assessed as adequately reporting generation of the allocation sequence, allocation concealment, blinding, outcome data reporting, with no evidence of selective outcome reporting, and without vested interests were considered to be at low risk of bias (Gluud 2006; Kjaergard 2001; Moher 1998; Schulz 1995; Wood 2008). Studies assessed to include one or more unclear or inadequate quality components were considered to be at high risk of bias (Moher 1998; Schulz 1995; Wood 2008). High inter‐rater agreement between blinded and unblinded assessments as well as between independent assessors has been found previously (Gluud 2006; Kjaergard 2001).
Measures of treatment effect
For dichotomous outcomes results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment, the mean difference (MD) were used, or the standardised mean difference (SMD) if different scales had been used (Higgins 2003; Thompson 2002).
Dealing with missing data
Contacted original investigators to request missing data.
Performed sensitivity analyses to assess how sensitive results were to reasonable changes in the assumptions that are made. We performed worst‐worst case scenario analyses, best‐best case scenario analyses, worst‐best case scenario analyses, and best‐worst case scenario analyses.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
We planned to construct funnel plots to explore bias if more than 10 studies were included in this review (Egger 1997; Macaskill 2001). However, the small number of included studies (6) meant that this could not be undertaken.
Data synthesis
Data were pooled using the random‐effects model and the fixed‐effect model was used to ensure data robustness.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were planned to be undertaken.
Individual antibody preparation compared with other classes of antibody preparation (e.g. ATG versus IL‐2RA)
Antibody preparation compared to different formulation of same class antibody preparation (e.g. basiliximab versus daclizumab)
Comparisons of studies at low risk of bias with studies at high risk of bias
Studies with early initiation of calcineurin inhibitor (at the time of transplantation) compared to studies with late initiation of calcineurin inhibitor (one to two weeks after transplantation).
Sensitivity analysis
Zero‐event trials
The principal analysis tool used for this review, Review Manager 5, was not designed to analyse studies with zero events when meta‐analyses are performed as relative risk or odds ratios. It seemed unjustified and unreasonable to exclude zero event studies (Keus 2009) that would potentially create a risk of inflating the magnitude of the pooled treatment effects. We therefore performed a random‐effects meta‐analysis with empirical continuity correction of 0.01 in studies with zero events (Sweeting 2004).
Trial sequential analysis
Trial sequential analysis was conducted because cumulative meta‐analyses carry risks of producing random errors due to sparse data and repetitive testing on accumulating data (Thorlund 2011; TSA 2011; Wetterslev 2008). We calculated the required information size, that is, the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect, to minimise random errors (Wetterslev 2008; Wetterslev 2009). Information size calculation should also account for heterogeneity in the meta‐analysis.
In our meta‐analyses, information size was based on an assumed plausible relative risk reduction of 20% or on the relative risk reduction observed in the included studies assessed as low risk of bias (Wetterslev 2008).
The underlying assumption of trial sequential analysis is that significance testing may be performed each time a new study is added to the meta‐analysis. We planned to add studies according to publication year, and if more than one study was published in a year, to add these in alphabetical order according to the family name of the first author.
The required information size was calculated and the trial sequential alpha‐spending and beta‐spending monitoring boundaries were constructed on the basis of the risk for type I (5%) and type II (20%) errors, nominated relative risk, proportion with the outcome in the control group, and observed heterogeneity (TSA 2011; Wetterslev 2008). These boundaries determined the statistical inference that may be drawn regarding cumulative meta‐analyses not achieving the required information size. If a trial sequential monitoring boundary is crossed before the required information size is reached in a cumulative meta‐analysis, firm evidence may have been established and further studies may be superfluous. Conversely, if the alpha‐ and beta‐spending boundaries are not surpassed, it is probably necessary to continue adding studies to detect or reject a certain intervention effect. Defaults used were: type I error, 5%; type II error, 20%; and adjusted information size for diversity unless otherwise stated (Thorlund 2011; Wetterslev 2008).
Results
Description of studies
Results of the search
The search strategy described identified 134 references, and we found another 12 references from other sources. After exclusions, six studies (nine publications; six peer‐reviewed journal articles, two conference abstracts, and one study from ClinicalTrials.gov) were included in our review. Figure 1 depicts the results of our search strategy.
1.
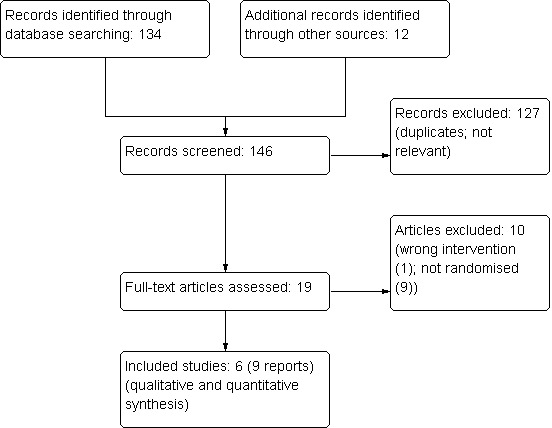
Study flow diagram
Included studies
We included six single‐centre studies that enrolled 278 participants (Brock 2001; Chaparro 1999; Conte 2010; Hartvig 2008; Mullen 2007; Senn 2001).
Chaparro 1999 (60 participants) compared ALG versus placebo; Hartvig 2008 (44 participants) compared ATG versus no intervention; Brock 2001 (64 participants) compared ATG versus muromonab‐CD3; Mullen 2007 (50 participants) compared ATG versus daclizumab; Senn 2001 (24 participants) compared ATG versus basiliximab; and Conte 2010 (36 participants) randomised participants to three groups: ATG, daclizumab, or no intervention.
We compared T‐cell antibody induction versus no T‐cell antibody induction (3 studies, 140 participants; Chaparro 1999; Conte 2010; Hartvig 2008); polyclonal T‐cell antibody induction versus no T‐cell antibody induction (3 studies, 125 participants; Chaparro 1999; Conte 2010; Hartvig 2008); polyclonal T‐cell antibody induction versus muromonab‐CD3 induction (1 study, 64 participants; Brock 2001); polyclonal T‐cell antibody induction versus IL‐2RA induction (3 studies, 100 participants; Conte 2010; Mullen 2007; Senn 2001); and IL‐2RA induction versus no T‐cell antibody induction (1 study, 25 participants; Conte 2010).
Participants in all included studies were adults. Mean age of the total study population was reported in four studies (range: 49 to 53 years) (Brock 2001; Conte 2010; Hartvig 2008; Mullen 2007). With one possible exception, mean ages of participants in single‐centre study treatment groups were similar (Brock 2001; Hartvig 2008; Mullen 2007). In Conte 2010, the mean ages of participants were 49 years in the daclizumab treatment arm; and 58 years and 53 years in the ATG and control groups respectively.
Five studies reported on numbers of single versus double lung transplant recipients (Brock 2001; Conte 2010; Hartvig 2008; Mullen 2007; Senn 2001). In three studies, more than half were single lung transplantations (Brock 2001; Conte 2010; Hartvig 2008). Mullen 2007 reported that more than 70% were double lung transplantations; all participants in Senn 2001 were double lung transplant recipients (100%). Numbers of single and double lung transplantations were similar among treatment groups in the single‐centre studies.
Brock 2001 and Mullen 2007 examined ATG derived from horse (ATGAM®), Conte 2010 and Hartvig 2008 investigated rabbit ATG (Thymoglobulin®); the type of ATG was unclear in Senn 2001.
Although maintenance immunosuppressive treatments were the same in all studies, immunosuppressive treatments varied. A triple immunosuppression regimen was used in five studies (Brock 2001; Conte 2010; Hartvig 2008; Mullen 2007; Senn 2001); Chaparro 1999 did not report maintenance immunosuppression. Participants in all studies received steroid therapy and calcineurin inhibitors; in four studies, the calcineurin inhibitor was cyclosporin (Brock 2001; Conte 2010; Hartvig 2008; Senn 2001); Mullen 2007 administered either tacrolimus or cyclosporin. Brock 2001 and Hartvig 2008 administered azathioprine as an antiproliferative agent; and mycophenolate mofetil was used in Conte 2010, Mullen 2007 and Senn 2001.
Follow‐up varied from six months (Senn 2001), 12 months (Mullen 2007), 2 years (Chaparro 1999; Conte 2010; Brock 2001) and 8 years (Hartvig 2008).
All included studies were published in English.
Excluded studies
See Characteristics of excluded studies.
We excluded 10 studies after full‐text assessment (AIRSAC Trial 2009; Barlow 2001; Borro 2005; Geldmacher 2001; Jaksch 2011; Lawrence 1989; Lischke 2007; Marom 2001; Meiser 1997; van Loenhout 2010). None of these studies assessed T‐cell antibody induction in randomised settings with the use of concomitant immunosuppression.
Risk of bias in included studies
Overall, study methodology was inadequately reported (Figure 2; Figure 3) and all included studies were assessed to be at high risk of bias (Brock 2001; Chaparro 1999; Conte 2010; Hartvig 2008; Mullen 2007; Senn 2001).
2.
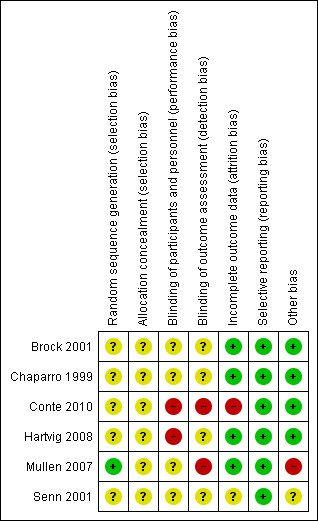
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.
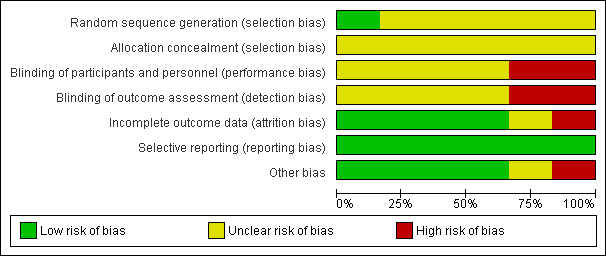
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Sequence generation allocation using computer‐generated randomisation was reported adequately by Mullen 2007; was unclear in Brock 2001, and was not reported in Chaparro 1999, Conte 2010, Hartvig 2008 or Senn 2001.
Allocation concealment was not reported in any of the included studies.
Blinding
Despite reporting that randomisation was double‐blinded, Chaparro 1999 provided no further information. Neither Brock 2001 nor Senn 2001 reported blinding; and Conte 2010 was not blinded. Two studies reported partial blinding: Hartvig 2008 reported that pathologists who examined transbronchial lung biopsy specimens were blinded to the study drug assignment; participants and outcome assessors were not blinded. Mullen 2007 reported that although study participants were blinded, personnel and outcome assessors were not.
Incomplete outcome data
Outcome data reporting was incomplete in five studies (Brock 2001; Chaparro 1999; Conte 2010; Hartvig 2008; Mullen 2007; Senn 2001), but in four of these, omissions did not put them at risk of bias (Brock 2001; Chaparro 1999; Hartvig 2008; Mullen 2007). Five patients died within 30 days following transplantation (group allocations not clear), and were excluded from the analysis by Conte 2010; hence, data analysis was per‐protocol. Incomplete data was not reported by Senn 2001.
Selective reporting
Although we had access to the Conte 2010 study protocol, we were unable to obtain protocols for the other included studies. However, all reported on expected clinical outcome measures (Brock 2001; Chaparro 1999; Hartvig 2008; Mullen 2007; Senn 2001).
Other potential sources of bias
Mullen 2007 was industry‐sponsored. We identified no other issues that could be construed as imposing risk of bias in four studies (Brock 2001; Chaparro 1999; Conte 2010; Hartvig 2008). Senn 2001 did not appear to have any other potential sources of bias, however this was an abstract‐only report (with no additional data provided) and therefore we have assessed the bias as unclear.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Polyclonal or monoclonal T‐cell antibody induction versus no induction
Chaparro 1999 (60 participants) compared ALG versus placebo; Hartvig 2008 (44 participants) compared ATG versus no intervention; Conte 2010 (36 participants) randomised patients to three groups: ATG, daclizumab, or no intervention.
Mortality
There was no significant difference in the number of deaths between patients treated with any kind of antibody induction compared with placebo or no induction (33/82 (40%) versus 26/58 (45%), (Analysis 1.1 (3 studies, 140 participants): RR 0.93, 95% CI 0.67 to 1.27; I² = 0%). Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 935 participants was not obtained (Figure 4).
1.1. Analysis.
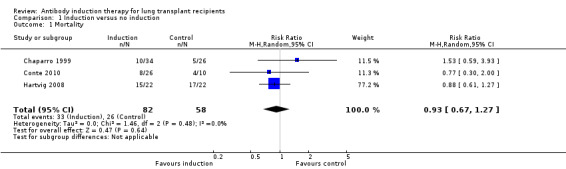
Comparison 1 Induction versus no induction, Outcome 1 Mortality.
4.
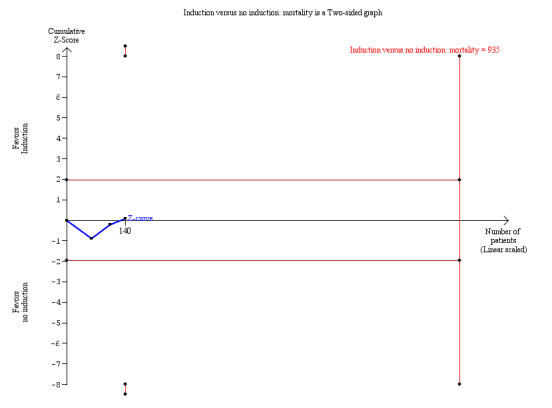
Any induction versus no induction; mortality: trial sequential analysis of the effect of induction versus no induction on mortality based on three studies (140 participants). The required information size of 935 patients was calculated based on type I error of 5%, type II error of 20%, risk reduction of 20%, and information size was adjusted for heterogeneity (I² = 0%)
Acute rejection
Acute rejection was defined as the number of patients who experienced at least one episode of rejection. There was no significant difference in the number of patients experiencing acute rejection between those treated with any kind of antibody induction compared with placebo or no induction (29/82 (35%) versus 28/58 (48%) (Analysis 1.2 (3 studies, 140 participants): RR 0.68, 95% CI 0.33 to 1.41; I² = 62%). Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 2200 participants was not obtained.
1.2. Analysis.
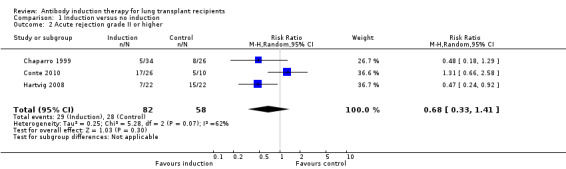
Comparison 1 Induction versus no induction, Outcome 2 Acute rejection grade II or higher.
Adverse events
Hartvig 2008 reported that no other adverse events occurred in any of the treatment groups (Analysis 1.3). Chaparro 1999 and Conte 2010 did not report adverse events. Therefore, only the zero event study (Hartvig 2008) contributed data for analysis, and consequently, a meta‐analysis using zero event correction was not conducted.
1.3. Analysis.
Comparison 1 Induction versus no induction, Outcome 3 Adverse events.
Quality of life
Quality of life measures were not reported.
Infection
Infection was defined as the number of patients who experienced at least one episode of infection. There was no significant difference in the number of infections between those treated with any kind of antibody induction compared to placebo or no intervention (36/56 (64%) versus 22/48 (46%) (Analysis 1.4 (2 studies, 104 participants): RR 1.40, 95% CI 0.97 to 2.01; I² = 0%).
1.4. Analysis.
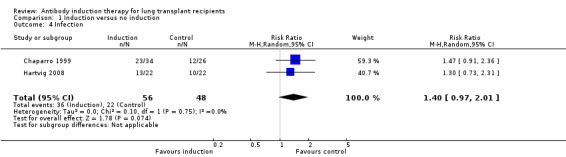
Comparison 1 Induction versus no induction, Outcome 4 Infection.
Pneumonia (viral, bacterial, or fungal)
Pneumonia was defined as the number of patients who experienced at least one episode of pneumonia. There was no significant difference in the number of patients with pneumonia between those treated with any kind of antibody induction compared with placebo or no induction (33/60 (55%) versus 14/36 (39%), (Analysis 1.5 (2 studies, 96 participants): RR 1.38, 95% CI 0.18 to 10.63; I² = 94%).
1.5. Analysis.
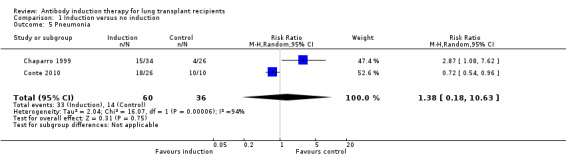
Comparison 1 Induction versus no induction, Outcome 5 Pneumonia.
Cytomegalovirus infection
There was no significant difference in the number of patients with cytomegalovirus infection between those treated with antibody induction compared with placebo or no induction (25/48 (52%) versus 15/32 (47%), (Analysis 1.6 (2 studies 80 participants): RR 1.14, 95% CI 0.73 to 1.80; I² = 0%).
1.6. Analysis.
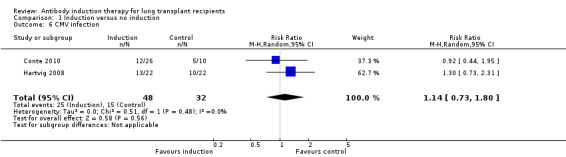
Comparison 1 Induction versus no induction, Outcome 6 CMV infection.
Bronchiolitis obliterans syndrome
There was no significant difference in the number of patients with bronchiolitis obliterans syndrome between those treated with antibody induction compared with placebo or no induction (30/82 (37%) versus 31/58 (53%), (Analysis 1.7 (3 studies, 140 participants): RR 0.74, 95% CI 0.51 to 1.07; I² = 10%).
1.7. Analysis.
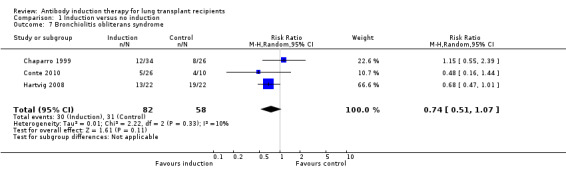
Comparison 1 Induction versus no induction, Outcome 7 Bronchiolitis obliterans syndrome.
Post‐transplantation lymphoproliferative disease
Hartvig 2008 reported no significant difference in the number of patients diagnosed with PTLD between those treated with any kind of antibody induction compared with no induction (1/22 (5%) versus 0/22 (0%), (Analysis 1.8 (1 study, 44 participants) RR 3.00, 95% CI 0.13 to 69.87). This was confirmed using Fisher's exact test (P = 1.0)
1.8. Analysis.
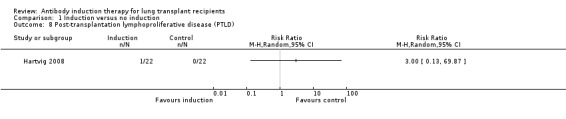
Comparison 1 Induction versus no induction, Outcome 8 Post‐transplantation lymphoproliferative disease (PTLD).
Cancer
Hartvig 2008 reported no significant difference in the number of patients diagnosed with cancer between the antibody induction group compared with the no induction group (8/22 (36%) versus 3/22 (14%), (Analysis 1.9 (1 study, 44 participants): RR 2.67, 95% CI 0.81 to 8.75). This was confirmed using Fisher's exact test (P = 0.16). The eight malignancies reported in antibody induction group included three non‐small cell lung cancers, one prostate cancer, one squamous cell nasopharynx cancer, and four skin cancers. There were three malignancies reported among control group: one non‐small cell lung cancer and two skin cancers.
1.9. Analysis.
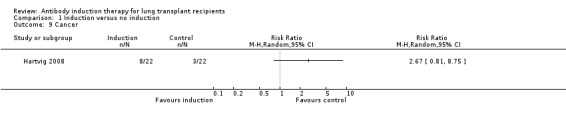
Comparison 1 Induction versus no induction, Outcome 9 Cancer.
Kidney injury requiring haemodialysis
Kidney injury requiring haemodialysis was not reported.
Polyclonal T‐cell antibody versus no induction
Chaparro 1999 (60 participants) compared ALG versus placebo, and Hartvig 2008 (44 participants) compared ATG versus no intervention. Conte 2010 (36 participants) randomised patients to three groups: ATG, daclizumab or no intervention. The 15 patients who received daclizumab in Conte 2010 were excluded from the analyses.
Mortality
There was no significant difference in the number of deaths between patients treated with polyclonal antibody induction compared with no induction (29/67 (43%) versus 26/58 (45%), (Analysis 2.1 (3 studies, 125 participants): RR 0.94, 95% CI 0.66 to 1.31; I² = 0%). Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 935 patients was not achieved.
2.1. Analysis.
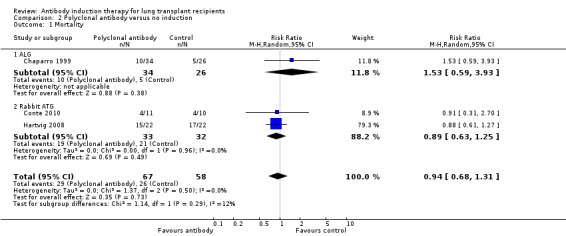
Comparison 2 Polyclonal antibody versus no induction, Outcome 1 Mortality.
Acute rejection
There was no significant difference in the number of patients experiencing acute rejection between those treated with polyclonal T‐cell antibody induction compared with no induction (21/67 (31%) versus 28/58 (48%), (Analysis 2.2 (3 studies, 125 participants): RR 0.68, 95% CI 0.44 to 1.04; I² = 75%). Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 3344 patients was not achieved.
2.2. Analysis.

Comparison 2 Polyclonal antibody versus no induction, Outcome 2 Acute rejection grade II or higher.
Adverse events
Hartvig 2008 reported that no other adverse events occurred in any of the treatment groups (Analysis 2.3). Chaparro 1999 and Conte 2010 did not report adverse events. Therefore, only the zero event study (Hartvig 2008) contributed data for analysis, and consequently, a meta‐analysis using zero event correction was not conducted.
2.3. Analysis.
Comparison 2 Polyclonal antibody versus no induction, Outcome 3 Adverse events.
Quality of life
Quality of life measures were not reported.
Infection
There was no significant difference in the number of infections between patients treated with polyclonal T‐cell antibody induction compared with no induction (36/56 (64%) versus 22/48 (46%), (Analysis 2.4 (2 studies, 104 participants): RR 1.40, 95% CI 0.97 to 2.01; I² = 0%).
2.4. Analysis.
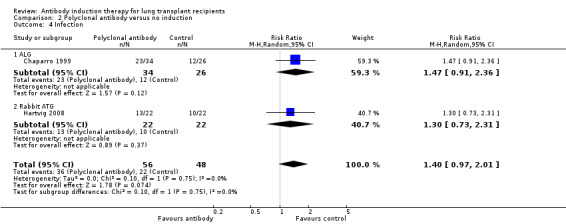
Comparison 2 Polyclonal antibody versus no induction, Outcome 4 Infection.
Pneumonia (viral, bacterial, and fungal)
There was no significant difference in the number of patients with pneumonia between those treated with polyclonal T‐cell antibody induction compared with no induction (23/45 (51%) versus 14/36 (39%), (Analysis 2.5 (2 studies, 81 participants): RR 1.40, 95% CI 0.97 to 2.01; I² = 92%).
2.5. Analysis.

Comparison 2 Polyclonal antibody versus no induction, Outcome 5 Pneumonia.
Cytomegalovirus infection
There was no significant difference in the number of patients with cytomegalovirus infection between those treated with polyclonal T‐cell antibody induction compared with no induction (19/33 (58%) versus 15/32 (47%), (Analysis 2.6 (2 studies, 65 participants): RR 1.23, 95% CI 0.77 to 1.97; I² = 0%).
2.6. Analysis.
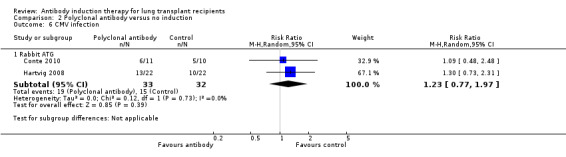
Comparison 2 Polyclonal antibody versus no induction, Outcome 6 CMV infection.
Bronchiolitis obliterans syndrome
There was no significant difference in the number of patients with bronchiolitis obliterans syndrome between those treated with polyclonal T‐cell antibody induction compared with no induction (28/67 (42%) versus 31/58 (53%), (Analysis 2.7 (3 studies, 125 participants): RR 0.76, 95% CI 0.56 to 1.05; I² = 0%).
2.7. Analysis.
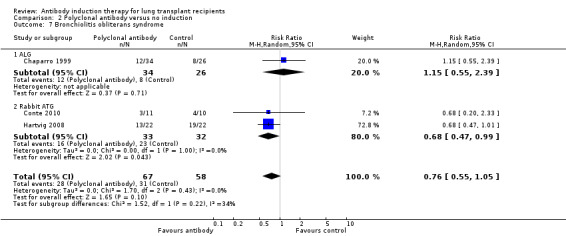
Comparison 2 Polyclonal antibody versus no induction, Outcome 7 Bronchiolitis obliterans syndrome.
Post‐transplantation lymphoproliferative disease
Hartvig 2008 reported no significant difference in the number of patients diagnosed with PTLD between those treated with polyclonal T‐cell antibody induction compared with no induction (1/22 (5%) versus 0/22 (0%), (Analysis 2.8 (1 study, 44 participants): RR 3.00, 95% CI 0.13 to 69.87). This was confirmed using Fisher's exact test (P = 1.0).
2.8. Analysis.
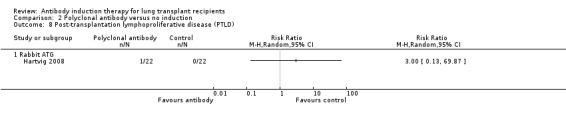
Comparison 2 Polyclonal antibody versus no induction, Outcome 8 Post‐transplantation lymphoproliferative disease (PTLD).
Cancer
Hartvig 2008 reported no significant difference in the number of patients diagnosed with cancer between the polyclonal T‐cell antibody induction group compared with no induction (8/22 (36%) versus 3/22 (14%), (Analysis 2.9 (1 study, 44 participants): RR 2.67, 95% CI 0.81 to 8.75). This was confirmed using Fisher's exact test (P = 0.16). The eight malignancies reported in antibody induction group included three non‐small cell lung cancers, one prostate cancer, one squamous cell nasopharynx cancer, and four skin cancers. There were three malignancies reported among control group that included one non‐small cell lung cancer and two skin cancers.
2.9. Analysis.
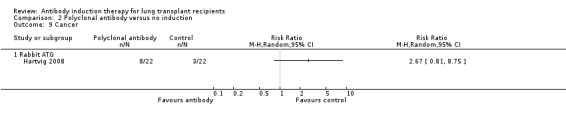
Comparison 2 Polyclonal antibody versus no induction, Outcome 9 Cancer.
Kidney injury requiring haemodialysis
Kidney injury requiring haemodialysis was not reported.
Interleukin‐2 receptor antagonist versus no induction
Conte 2010 (36 participants) randomised patients to three groups: ATG, daclizumab or no intervention. The 11 patients who received ATG were excluded from the analyses.
Mortality
Conte 2010 reported no significant difference in the number of deaths among patients who received IL‐2RA compared with those who received no induction therapy (4/15 (27%) versus 4/10 (40%), (Analysis 3.1 (1 study, 25 participants): RR 0.67, 95% CI 0.22 to 2.07). This was confirmed using Fisher's exact test (P = 0.67). Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 1131 patients was not achieved.
3.1. Analysis.
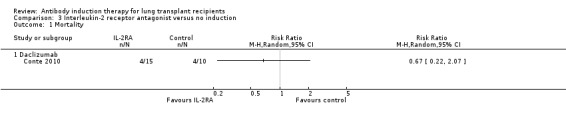
Comparison 3 Interleukin‐2 receptor antagonist versus no induction, Outcome 1 Mortality.
Acute rejection
Conte 2010 reported no significant difference in the number of patients experiencing at least one episode of rejection between those treated with IL‐2RA compared with no induction (8/15 (53%) versus 5/10 (50%), (Analysis 3.2 (1 study, 25 participants): RR 1.07, 95% CI 0.49 to 2.33). This was confirmed using Fisher's exact test (P = 1.0). Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 778 patients was not achieved.
3.2. Analysis.
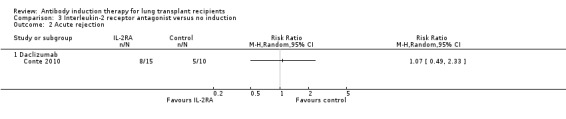
Comparison 3 Interleukin‐2 receptor antagonist versus no induction, Outcome 2 Acute rejection.
Adverse events
Adverse events were not reported.
Quality of life
Quality of life measures were not reported.
Infection
Infection rates were not reported.
Pneumonia (viral, bacterial, or fungal)
Conte 2010 reported no significant difference in the number of patients with pneumonia between those treated with IL‐2RA compared with no induction (10/15 (67%) versus 10/10 (100%), (Analysis 3.3 (1 study, 25 participants): RR 0.69, 95% CI 0.47 to 1.00). This was confirmed using Fisher's exact test (P = 0.06).
3.3. Analysis.
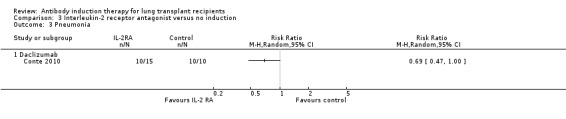
Comparison 3 Interleukin‐2 receptor antagonist versus no induction, Outcome 3 Pneumonia.
Cytomegalovirus infection
Conte 2010 reported no significant difference in the number of patients with cytomegalovirus infection between those treated with IL‐2RA compared with no induction (6/15 (40%) versus 5/10 (50%), (Analysis 3.4 (1 study, 25 participants): RR 0.80, 95% CI 0.33 to 1.92). This was confirmed using Fisher's exact test (P = 0.70).
3.4. Analysis.
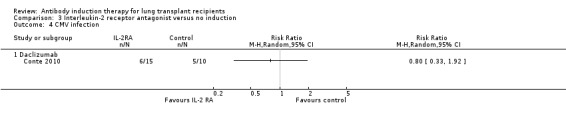
Comparison 3 Interleukin‐2 receptor antagonist versus no induction, Outcome 4 CMV infection.
Bronchiolitis obliterans syndrome
Conte 2010 reported no significant difference in the number of patients with bronchiolitis obliterans syndrome between those treated with IL‐2RA compared with no induction (2/15 (13%) versus 4/10 (40%), (Analysis 3.5 (1 study, 25 participants): RR 0.33, 95% CI 0.07 to 1.49). This was confirmed using Fisher's exact test (P = 0.18).
3.5. Analysis.
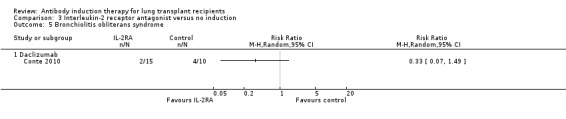
Comparison 3 Interleukin‐2 receptor antagonist versus no induction, Outcome 5 Bronchiolitis obliterans syndrome.
Post‐transplantation lymphoproliferative disease
PTLD was not reported.
Cancer
Cancers were not reported.
Kidney injury requiring haemodialysis
Kidney injury requiring haemodialysis was not reported.
Polyclonal T‐cell antibody versus muromonab‐CD3
Brock 2001 (64 participants) compared ATG versus muromonab‐CD3.
Mortality
Brock 2001 did not report sufficient mortality data to enable statistical analysis. Two year survival for the entire cohort was 68%, with no differences observed in survival among the three induction groups: the ATG and muromonab‐CD3 groups (both of which were randomised), and non‐randomised daclizumab group.
Acute rejection
Acute rejection was not sufficiently reported to enable statistical analysis. Brock 2001 reported that there was no difference in freedom from acute rejection episodes of grade A2 or greater among the three groups (randomised ATG and muromonab‐CD3 groups; non‐randomised daclizumab group).
Adverse events
Brock 2001 reported drug‐specific adverse effects were more common in the ATG group compared with the muromonab‐CD3 group (25/34 (74%) versus 12/30 (40%), (Analysis 4.1 (1 study, 64 participants): RR 1.84, 95% CI 1.13 to 2.98). This was confirmed using Fisher's exact test (P = 0.01). Cytokine release syndrome occurred in 12/30 (40%) patients in the muromonab‐CD3 group, among whom it was associated with hypoxia (5/30, 17%), hypotension (5/30, 17%), and rigor (1/30, 3%). Thrombocytopenia occurred in 25/34 (74%) patients in the ATG group.
4.1. Analysis.

Comparison 4 Polyclonal antibody versus muromonab‐CD3, Outcome 1 Adverse events.
Quality of life
Quality of life measures were not reported.
Infection
Brock 2001 reported no significant difference in the number of infections between patients treated with ATG compared with muromonab‐CD3 (25/34 (74%) versus 23/30 (77%), (Analysis 4.2 (1 study, 64 participants): RR 0.96, 95% CI 0.72 to 1.27). This was confirmed using Fisher's exact test (P = 1.0).
4.2. Analysis.
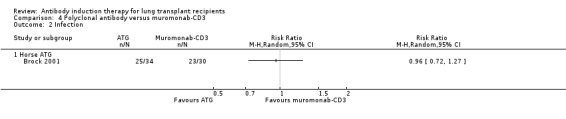
Comparison 4 Polyclonal antibody versus muromonab‐CD3, Outcome 2 Infection.
The incidences of pneumonia or cytomegalovirus infection were not reported.
Bronchiolitis obliterans syndrome
Brock 2001 reported no significant difference in the number of patients with bronchiolitis obliterans syndrome between those treated with ATG compared with muromonab‐CD3 (5/34 (15%) versus 7/30 (23%), (Analysis 4.3 (1 study, 64 participants): RR 0.63, 95% CI 0.22 to 1.78). This was confirmed using Fisher's exact test (P = 0.52).
4.3. Analysis.
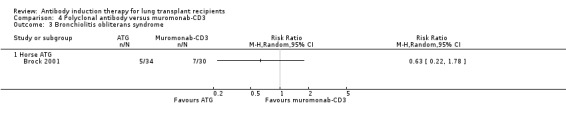
Comparison 4 Polyclonal antibody versus muromonab‐CD3, Outcome 3 Bronchiolitis obliterans syndrome.
Post‐transplantation lymphoproliferative disease
Brock 2001 reported no significant difference in the number of patients diagnosed with PTLD between those treated with ATG compared with muromonab‐CD3 (2/34 (6%) versus 2/30 (7%), (Analysis 4.4 (1 study, 64 participants): RR 0.88; 95% CI 0.13 to 5.88). This was confirmed using Fisher's exact test (P = 1.0).
4.4. Analysis.
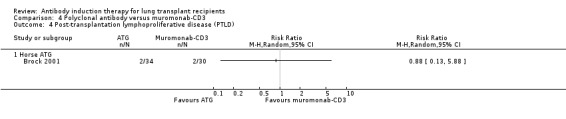
Comparison 4 Polyclonal antibody versus muromonab‐CD3, Outcome 4 Post‐transplantation lymphoproliferative disease (PTLD).
Cancer
Cancers were not reported.
Kidney failure requiring haemodialysis
Kidney failure requiring haemodialysis was not reported.
Polyclonal T‐cell antibody versus interleukin‐2 receptor antagonist
Mullen 2007 (50 participants) compared ATG versus daclizumab; Senn 2001 (24 participants) compared ATG versus basiliximab; and Conte 2010 (36 participants) randomised patients to three groups: ATG, daclizumab or no intervention. The 10 patients in Conte 2010 not receiving T‐cell antibody induction were excluded from the analyses.
Mortality
There was no significant differences in the number of deaths between patients treated with ATG induction compared with IL‐2RA induction (7/47 (15%) versus 6/53 (11%), (Analysis 5.1 (3 studies, 100 participants): RR 1.41, 95% CI 0.54 to 3.70; I² = 0%). Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 5787 patients was not obtained.
5.1. Analysis.
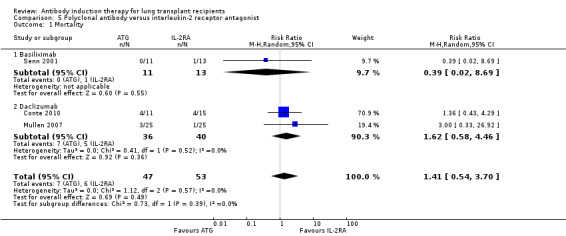
Comparison 5 Polyclonal antibody versus interleukin‐2 receptor antagonist, Outcome 1 Mortality.
Acute rejection
There was no significant difference in the number of patients experiencing acute rejection between those treated with polyclonal antibody induction compared with IL‐2RA (25/36 (69%) versus 21/40 (53%), (Analysis 5.2 (2 studies, 76 participants): RR 1.35, 95% CI 0.94 to 1.94; I² = 0%). Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 698 patients was not obtained.
5.2. Analysis.
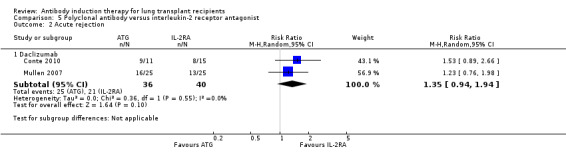
Comparison 5 Polyclonal antibody versus interleukin‐2 receptor antagonist, Outcome 2 Acute rejection.
Adverse events
Mullen 2007 reported that one drug‐related adverse event occurred in the ATG group that involved lymphopenia and thrombocytopenia (Analysis 5.3). ATG infusion was temporarily discontinued.
5.3. Analysis.
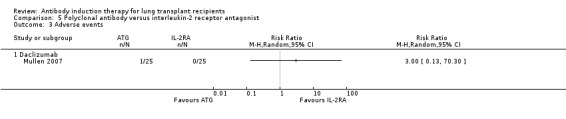
Comparison 5 Polyclonal antibody versus interleukin‐2 receptor antagonist, Outcome 3 Adverse events.
Quality of life
Quality of life measures were not reported.
Infection
Mullen 2007 reported no significant difference in the number of infections between patients treated with ATG compared with IL‐2RA (20/25 (80%) versus 22/25 (88%), (Analysis 5.4 (1 study, 50 participants): RR 0.91, 95% CI 0.71 to 1.16). This was confirmed using Fisher's exact test (P = 0.70).
5.4. Analysis.
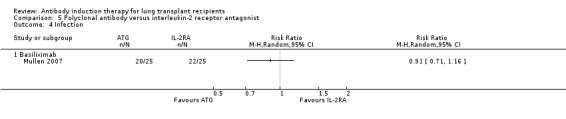
Comparison 5 Polyclonal antibody versus interleukin‐2 receptor antagonist, Outcome 4 Infection.
Pneumonia (viral, bacterial, or fungal)
Conte 2010 reported no significant difference in the number of patients with pneumonia between those treated with ATG compared with IL‐2RA (8/11 (73%) versus 10/15 (67%), (Analysis 5.5 (1 study, 26 participants): RR 1.09, 95% CI 0.66 to 1.81). This was confirmed using Fisher's exact test (P = 1.0).
5.5. Analysis.
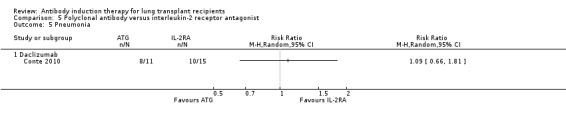
Comparison 5 Polyclonal antibody versus interleukin‐2 receptor antagonist, Outcome 5 Pneumonia.
Cytomegalovirus infection
There was no significant difference in the number of patients with cytomegalovirus infection between those treated with ATG compared with IL‐2RA (10/36 (28%) versus 18/40 (45%), (Analysis 5.6 (2 studies, 76 participants): RR 0.69, 95% CI 0.17 to 2.88; I² = 80%).
5.6. Analysis.
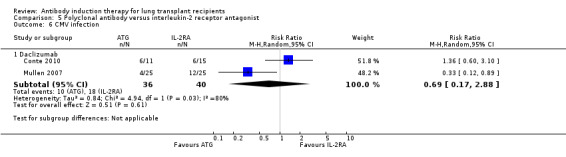
Comparison 5 Polyclonal antibody versus interleukin‐2 receptor antagonist, Outcome 6 CMV infection.
Bronchiolitis obliterans syndrome
There was no significant difference in the number of patients with bronchiolitis obliterans syndrome between those treated with ATG compared with IL‐2RA (4/36 (11%) versus 3/40 (8%), (Analysis 5.7 (2 studies, 76 participants): RR 1.70, 95% CI 0.42 to 6.79; I² = 0%).
5.7. Analysis.
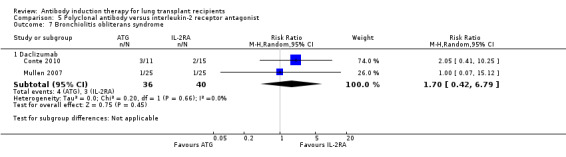
Comparison 5 Polyclonal antibody versus interleukin‐2 receptor antagonist, Outcome 7 Bronchiolitis obliterans syndrome.
Post‐transplantation lymphoproliferative disease
Mullen 2007 reported no significant difference in the number of patients diagnosed with PTLD between those treated with ATG induction and the IL‐2RA induction (1/25 (4%) versus 1/25 (4%), (Analysis 5.8 (1 study, 50 participants): RR 1.00, 95% CI 0.07 to 15.12). This was confirmed using Fisher's exact test (P = 1.0).
5.8. Analysis.
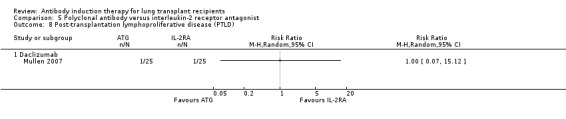
Comparison 5 Polyclonal antibody versus interleukin‐2 receptor antagonist, Outcome 8 Post‐transplantation lymphoproliferative disease (PTLD).
Cancer
Mullen 2007 reported no malignancies were seen in either the ATG induction or IL‐2RA induction groups. The only study contributing data for this outcome reported no events, and therefore, meta‐analysis was not undertaken.
Kidney failure requiring haemodialysis
Kidney failure requiring haemodialysis was not reported.
Subgroup analyses
We performed subgroup analyses on ATG type (rabbit versus horse) compared with IL‐2RA on several outcome measures: mortality, acute rejection, bronchiolitis obliterans syndrome, and cytomegalovirus infection. We found no significant differences between type of ATG when applying the test of interaction regarding mortality, acute rejection and bronchiolitis obliterans syndrome. Horse ATG may be better in preventing cytomegalovirus infection than rabbit ATG (P = 0.03).
We conducted subgroup analyses on IL‐2RA type (basiliximab versus daclizumab) compared with ATG on mortality, and found no significant differences between IL‐2RAs when applying the test of interaction.
Other subgroup analyses
We were unable to perform planned subgroup analyses on risk of bias (high versus low) because all included studies were assessed as high risk of bias. Likewise, subgroup analysis on timing (early versus late) calcineurin inhibitor initiation could not be undertaken because this outcome was not reported.
Assessment of harm in non‐randomised controlled studies
We identified eight non‐RCTs that were assessed for the risk of harm (Barlow 2001; Borro 2005; Burton 2006; Garrity 2001; Hachem 2005; Lischke 2007; van Loenhout 2010; Marom 2001) (Table 5). We assessed numbers of patients with infection, cytomegalovirus infection, PTLD, and other adverse effects. Overall, no clear harmful effects were identified regarding comparisons of types of induction therapies, or induction therapy type compared with controls.
1. Assessment of harm in non‐randomised controlled studies.
| Study | Participants | Study groups | PTLD | Infection | CMV | Other adverse events |
| Barlow 2001 | 63 | Muromonab‐CD3: 38 ATG: 25 |
Not reported | No difference between groups | No difference between groups | Not reported |
| Borro 2005 | 28 | Daclizumab: 15 Control: 13 |
Not observed |
Fungal Daclizumab: 1 (7%) Control: 2 (15%) Bacterial Daclizumab: 4 (27%) Control: 2 (15%) |
Daclizumab: 4 (27%) Control: 5 (38%) |
Not observed |
| Burton 2006 | 335 | ATG: 151 Daclizumab: 151 |
ATG: 8 (5%) Daclizumab: 2 (1%) |
Not reported | No difference between groups | Not reported |
| Garrity 2001 | 61 | Daclizumab: 27 Control: 34 |
Daclizumab: 1 (4%) Control: 1 (3%) |
Fungal Daclizumab: 5 (19%) Control: 5 (15%) |
Daclizumab: 5 (19%) Control: 8 (24%) |
Not reported |
| Hachem 2005 | 157 | ATG: 75 Basiliximab: 82 |
ATG: 0.53 cases/100 patient‐years Basiliximab: 3 cases/100 patient‐years |
Not reported |
CMV‐viraemia ATG: 15.1 episodes/100 patient‐months Basiliximab: 15.6 episodes/100 patient‐months |
Not reported |
| Lischke 2007 | 25 | ATG: 12 Daclizumab: 13 |
No PTLD | ATG: 10 (83%) Daclizumab: 6 (46%) |
No difference between groups | Thrombocytopenia ATG: 9 (75%) Daclizumab: 0 (0%) |
| Marom 2001 | 86 | Daclizumab: 43 Control: 43 |
Not observed | Not reported | Not reported | Not reported |
| van Loenhout 2010 | 40 | Alemtuzumab: 20 Control: 20 |
Not reported | No difference between groups | Not reported | Not observed |
ATG ‐ antithymocyte globulin; CMV ‐ cytomegalovirus; PTLD ‐ post‐transplant lymphoproliferative disease
Discussion
Summary of main results
This review identified six studies (278 participants) that assessed the effects of different types of T‐cell antibody induction in lung transplant recipients. All included studies were assessed at high risk of bias.
Overall our meta‐analyses did not find any statistically significant differences between any of the randomised groups regarding mortality, acute rejection, infection, pneumonia, cytomegalovirus infection, bronchiolitis obliterans syndrome, PTLD, or cancer.
The only study comparing ATG with muromonab‐CD3 seemed to show an increase in adverse events in the ATG group; however, trial sequential analysis could not exclude random error (Conte 2010).
None of the studies reported on quality of life or kidney failure requiring haemodialysis.
Findings were confirmed when the fixed‐effect model was applied to the meta‐analyses. The required information size was not obtained in any trial sequential analyses for the primary outcome measures. Absence of evidence however does not necessarily mean absence of effect.
Overall completeness and applicability of evidence
We examined the evidence from six RCTs that investigated the use of T‐cell antibody induction for lung transplant recipients. We were unable to obtain data relating to all pre‐defined outcome measures because they were not all reported in the included studies.
Overall, reporting in the included studies was suboptimal: five studies reported adequately on mortality; four on acute rejection; four on infection; and five on bronchiolitis obliterans syndrome. Only two studies reported on drug‐related adverse events. None of the studies reported on quality of life or kidney failure requiring haemodialysis.
Not all types of T‐cell antibody induction currently available have been studied in randomised studies. Alemtuzumab for induction after lung transplantation has been introduced during the last decade, and is now used for almost 10% of all lung transplant recipients. However, no evidence from randomised studies regarding alemtuzumab was identified. IL‐2RA were found to be the most commonly used type of induction therapy, used in over 40% of all lung transplant recipients. Nevertheless only one included randomised study with 25 patients investigated the use of IL‐2RA compared with no intervention. A study that compared IL‐2RA with no intervention has been completed, but contact with the investigators indicated that results were not yet available (Waddell 2006).
Quality of the evidence
We conducted this review in accordance with the requirements in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and conducted trial sequential analysis (Wetterslev 2008; TSA 2011).
The quality and quantity of available evidence limited our findings and interpretations. Very limited numbers of participants were included in the studies, and hence, risks of random errors potentially explain occasional positive findings in individual studies. Additionally, study participants may not be representative of the general patient population.
Follow‐up in five studies was between six months and two years; and eight years in one study. We therefore were unable to elicit evidence relating to longer‐term (greater than two years) effects of T‐cell antibody induction on outcome measures. Long‐term effects in terms of mortality, bronchiolitis obliterans syndrome, infection, and cancer would be particularly valuable.
We explored the presence of statistical heterogeneity using the Chi² test and measured heterogeneity using the I² test (Higgins 2003). The Chi² test is low powered in meta‐analyses where studies are small or few in number, as in this review. This means that while a statistically significant result may indicate a problem with heterogeneity, a non‐significant result must not be taken as evidence of homogeneity. To reflect our concern with heterogeneity, we looked at both fixed‐effect and random‐effects models to provide more conservative effect estimates. No differences were seen between fixed‐effect and random‐effects models for any of the primary outcome measures considered in this review.
Precision of our results was influenced because many outcomes planned for meta‐analyses includes few patients and events, and thus, have wide confidence intervals around the effect estimate.
Potential biases in the review process
Bias is known to impact on the estimated intervention effect, and studies assessed at high risk of bias tend to overestimate beneficial intervention effects (Kjaergard 2001; Moher 1998; Schulz 1995). Of the six included studies, reporting was suboptimal: allocation concealment was not reported in any of the included studies; adequate allocation sequence generation was reported by one study; participant blinding was reported by one study; one was blinded for the pathologist who examined transbronchial biopsies for rejection. Reporting of incomplete outcome data was adequate in four studies, and all reported on reasonably expected outcome measures. Four studies appeared to be free of other components that could put the study at risk of bias, and one was unclear. Accordingly, all studies were considered to be at high risk of bias, and estimated intervention effects may therefore be due to systematic errors.
The risk of random error is higher among data from small studies. Studies need to be sufficiently large to reduce the risk of random error and increase the chance of observing a true intervention effect (Wetterslev 2008). To address these issues, we also conducted trial sequential analysis. Trial sequential analysis is a statistical method that assesses risk of random error caused by sparse data and formal or informal repetitive testing of accumulated data. Trial sequential analysis of outcome measures in this review showed that the required information size was not reached. Hence, we were unable to determine whether there may be beneficial or harmful effects associated with the use of antibody induction or relative superiority of any antibody over another.
Agreements and disagreements with other studies or reviews
Data from the International Society for Heart and Lung Transplantation registry, which are based on nearly 11,000 lung recipients who received lung transplants between January 2000 and June 2009, showed that T‐cell antibody induction appeared to have a favourable effect on survival (Christie 2011). Due to the observational nature of these data, findings should be interpreted with caution because they were not adjusted for diagnosis category, age, centre, or other potentially confounding factors. The reduced mortality observed in the registry data could not be confirmed in our meta‐analyses. Furthermore, the registry data showed that compared with other types of induction therapy, IL‐2RA induction was associated with a lower incidence of acute rejection during the first year following transplantation (Christie 2011). The lower rejection rates among lung transplant recipients who were treated with IL‐2RA induction therapy appeared to be similar across age groups, despite apparent differences in rejection rates with other induction strategies by recipient age category (Christie 2011).
Using data from the same registry, Hachem 2008 found that IL‐2RA induction therapy for single and double lung transplant recipients and induction with ATG for double lung transplant recipients was associated with lower mortality. We were unable to confirm this reduction in mortality and acute rejection associated with the use of IL‐2RA in our meta‐analyses.
Large cohort studies conducted by Burton 2006 and Hachem 2005 compared ATG and IL‐2RA. Burton 2006 compared induction using rabbit ATG with daclizumab induction in 335 lung transplant recipients. Patients who received ATG had a statistically significant lower incidence of acute rejection compared with patients who were treated with daclizumab (Burton 2006). Hachem 2005 compared induction using horse ATG with basiliximab induction in 157 lung transplant recipients. Lung transplant recipients with severe ischaemia‐reperfusion injury after transplantation, and those who were serologically mismatched for cytomegalovirus, were excluded from ATG induction therapy (Hachem 2005). Acute rejection and bronchiolitis obliterans syndrome occurred less often in the ATG group compared with the basiliximab group (Hachem 2005).
Findings similar to those reported by Burton 2006 and Hachem 2005 showing less rejection associated with ATG compared with IL‐2RA were not found when data from the International Society for Heart and Lung Transplantation registry were analysed (Christie 2011). Likewise, our meta‐analyses could not confirm these findings.
Traditionally, immunosuppressive therapy for lung transplantation has gained much knowledge from the reported experiences of other types of organ transplantation. A Cochrane review by Webster 2010 that included 71 studies reporting data on 10,537 patients investigated the use of IL‐2RA for kidney transplant recipients. Webster 2010 found that compared with placebo, IL‐2RA reduced graft loss, including death, by 25% at six months and one year, but not beyond (Webster 2010). Furthermore, compared with placebo, IL‐2RA reduced biopsy‐proven acute rejection (RR 0.75, 95% CI 0.58 to 0.98) and cytomegalovirus disease (RR 0.81, 95% CI 0.68 to 0.97) in kidney transplant recipients (Webster 2010). Where IL‐2RA was compared with ATG in kidney transplant recipients, biopsy‐proven acute rejection at one year was increased in the IL‐2RA group by 30%, but incidence of malignancies (RR 0.25, 95% CI 0.07 to 0.87) and cytomegalovirus disease (RR 0.68, 95 % CI 0.50 to 0.97) were reduced when IL‐2RA was compared with ATG (Webster 2010).
In a recent Cochrane review (Penninga 2012a), we compared antibody induction versus no induction for liver transplant recipients in 17 studies with a total of 1951 patients. Antibody induction may reduce acute rejection when compared with no antibody induction. No other clear benefits or harms were associated with the use of antibody induction compared with no antibody induction (Penninga 2012a).
Furthermore, we reported the use of antibody induction therapy compared with corticosteroid for liver transplant recipients. Our review included 10 studies that presented data from 1589 participants (Penninga 2012b). Antibody induction seems to reduce diabetes mellitus and may reduce cytomegalovirus infection when compared with corticosteroid induction. No other clear benefits or harms were associated with the use of antibody induction compared with corticosteroid induction (Penninga 2012b).
Our earlier Cochrane review (Penninga 2010) investigated the use of antibody induction for heart transplant recipients. This review included 22 studies that presented data from 1427 participants. This review demonstrated that compared with placebo, IL‐2RA induction may reduce acute rejection when meta‐analysed using a fixed‐effect model; however, this effect did not occur using a random‐effects model. Polyclonal antibodies may be superior in reducing acute rejection compared with IL‐2RA for heart transplant recipients, but not for other outcomes. The review found no significant differences regarding mortality, infections, and malignancy (Penninga 2010).
Overall, potential advantages of some antibody types concerning certain outcome measures in other solid organ transplant recipients were not found in lung transplant recipients. Whether this is due to the limited numbers of patients and events, systematic errors, design errors, or organ‐specific differences is currently unclear.
Authors' conclusions
Implications for practice.
Recognising the limitations of the review relating to the size and nature of the included studies, our systematic review did not show any clear beneficial or harmful effects associated with the use of antibody induction therapy regarding mortality, acute rejection, infection, pneumonia, cytomegalovirus infection, bronchiolitis obliterans syndrome, PTLD or cancer. When trial sequential analyses were conducted on the primary outcome measures of mortality and acute rejection, the required information size was not obtained for any of the comparisons.
Implications for research.
Given the result of our analysis, it appears warranted that appropriately sized and powered randomised studies comparing T‐cell antibodies versus placebo in lung transplant patients using contemporarily adjunctive immunosuppression be undertaken. These studies should investigate interventions using basiliximab (currently the only IL‐2RA commercially available), ATG, or alemtuzumab. Such studies should be designed and conducted to achieve low risks of systematic error (bias) and random error (play of chance), and should follow the CONSORT guidelines.
Acknowledgements
We would like to thank the following people.
The Cochrane Renal Group for their support in preparing this review.
The referees for their comments and feedback during the preparation of the protocol and review.
The investigators and participants of the included randomised controlled trials. Without their initiative we would have nothing to review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. 1 Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Induction versus no induction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.67, 1.27] |
| 2 Acute rejection grade II or higher | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.33, 1.41] |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Infection | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.97, 2.01] |
| 5 Pneumonia | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.18, 10.63] |
| 6 CMV infection | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.73, 1.80] |
| 7 Bronchiolitis obliterans syndrome | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.07] |
| 8 Post‐transplantation lymphoproliferative disease (PTLD) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Cancer | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Polyclonal antibody versus no induction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.68, 1.31] |
| 1.1 ALG | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.59, 3.93] |
| 1.2 Rabbit ATG | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.63, 1.25] |
| 2 Acute rejection grade II or higher | 3 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.30, 1.79] |
| 2.1 ALG | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.18, 1.29] |
| 2.2 Rabbit ATG | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.25, 3.06] |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Rabbit ATG | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Infection | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.97, 2.01] |
| 4.1 ALG | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.91, 2.36] |
| 4.2 Rabbit ATG | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.3 [0.73, 2.31] |
| 5 Pneumonia | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 ALG | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.23, 8.48] |
| 6 CMV infection | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Rabbit ATG | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.77, 1.97] |
| 7 Bronchiolitis obliterans syndrome | 3 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.55, 1.05] |
| 7.1 ALG | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.55, 2.39] |
| 7.2 Rabbit ATG | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.47, 0.99] |
| 8 Post‐transplantation lymphoproliferative disease (PTLD) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Rabbit ATG | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Cancer | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Rabbit ATG | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Interleukin‐2 receptor antagonist versus no induction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Daclizumab | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Acute rejection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Daclizumab | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pneumonia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Daclizumab | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 CMV infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Daclizumab | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Bronchiolitis obliterans syndrome | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Daclizumab | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Polyclonal antibody versus muromonab‐CD3.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Horse ATG | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Horse ATG | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Bronchiolitis obliterans syndrome | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Horse ATG | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Post‐transplantation lymphoproliferative disease (PTLD) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Horse ATG | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Polyclonal antibody versus interleukin‐2 receptor antagonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.54, 3.70] |
| 1.1 Basiliximab | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.02, 8.69] |
| 1.2 Daclizumab | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.58, 4.46] |
| 2 Acute rejection | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Daclizumab | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.94, 1.94] |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Daclizumab | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Basiliximab | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pneumonia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Daclizumab | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 CMV infection | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Daclizumab | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.17, 2.88] |
| 7 Bronchiolitis obliterans syndrome | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Daclizumab | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.42, 6.79] |
| 8 Post‐transplantation lymphoproliferative disease (PTLD) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Daclizumab | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Cancer | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Daclizumab | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
5.9. Analysis.
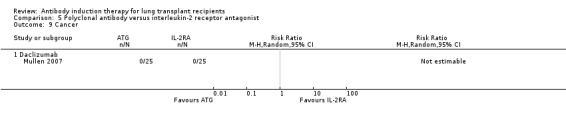
Comparison 5 Polyclonal antibody versus interleukin‐2 receptor antagonist, Outcome 9 Cancer.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brock 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Concomitant immunosuppressive treatment
Follow‐up: 2 years |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pair‐wise randomisation, not further specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol was available, but the study reported on mortality, rejection, infection, bronchiolitis obliterans syndrome, PTLD and adverse events |
| Other bias | Low risk | Study appears to be free of other bias components |
Chaparro 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomittant immunosuppression: unknown Follow‐up: 2 years |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind study, but no other information given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind study, but no other information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the study reported on mortality, rejection, infection, pneumonia, bronchiolitis obliterans syndrome, PTLD, and adverse events |
| Other bias | Low risk | Study appears to be free of other bias components |
Conte 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Concomitant immunosuppressive treatment
Follow‐up: 2 years |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 patients died within 30 days after transplantation (unclear in which group), and were excluded from the analysis by the authors, hence data analysis was per‐protocol |
| Selective reporting (reporting bias) | Low risk | A protocol was available (clinicaltrials.gov NCT00181142), and all pre‐specified outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other bias, additional data were obtained from the study authors |
Hartvig 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppressive treatment
Follow‐up: 8 years |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Partially blinding as only the pathologists who examined the transbronchial lung biopsy specimens were blinded to the study drug assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number and reasons for dropouts and withdrawals in all intervention groups were described |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the study reported on mortality, rejection, infection, CMV‐infection, bronchiolitis obliterans syndrome, PTLD, and adverse events |
| Other bias | Low risk | Study appeared to be free of other bias |
Mullen 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Concomitant immunosuppressive treatment
Follow‐up: 1 year |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Only patients were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up, and there were no patients who discontinued treatment |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | High risk | Study was industry sponsored |
Senn 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Concomitant immunosuppression
Follow‐up: 6 months |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on the number and reasons for dropouts and withdrawals in all intervention groups |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed. The study reported on all expected outcomes |
| Other bias | Unclear risk | Abstract‐only report. Study appeared to be free of other bias components, however not additional data were obtained from authors |
ALG ‐ antilymphocyte globulin, ATG ‐ antithymocyte globulin; CrCl ‐ creatinine clearance; ICU ‐ intensive care unit; IV ‐ intravenous; MMF ‐ mycophenolate mofetil; PEEP ‐ positive and expiratory pressure; PRA ‐ plasma renin activity; PTLD ‐ post‐transplant lymphoproliferative disorders
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AIRSAC Trial 2009 | Investigated azathioprine versus sirolimus, not antibodies |
| Barlow 2001 | OKT3 and rabbit ATG were given at random based on the availability of rabbit ATG |
| Borro 2005 | Comparative study, not randomised |
| Garrity 2001 | Retrospective study, not randomised |
| Geldmacher 2001 | Not randomised |
| Jaksch 2011 | Differences in concomitant immunosuppressive agents between the study groups |
| Lawrence 1989 | Investigated association between interleukin‐2 levels and risk for rejection and infection; no intervention groups and not randomised |
| Lischke 2007 | Comparative study, not randomised |
| Marom 2001 | Retrospective study, not randomised |
| Meiser 1997 | Not randomised |
| van Loenhout 2010 | Not randomised, but compared with historical control group |
ATG ‐ antithymocyte globulin
Characteristics of ongoing studies [ordered by study ID]
NCT00105183.
| Trial name or title | Study of EZ‐2053 in the prophylaxis of acute pulmonary allograft rejection |
| Methods | A double‐blind, placebo‐controlled, multicenter, dose‐ranging study of an anti‐human‐T‐lymphocyte immune globulin (EZ‐2053) in the prophylaxis of acute pulmonary allograft rejection |
| Participants | Adult recipients of primary pulmonary allograft(s) |
| Interventions | Patients randomised to receive one infusion of EZ‐2053 9 mg/kg or placebo through a central venous catheter, each day for 5 days following transplant surgery |
| Outcomes | Primary outcome: first occurrence of death, graft loss, acute rejection and/or loss to follow‐up between groups who receive 9 mg/kg or placebo within 12 months |
| Starting date | March 2005 |
| Contact information | Manager of Regulatory Affairs, Fresenius Biotech North America |
| Notes | Study completed January 2011. No published results available |
Saggar 2011.
| Trial name or title | Intraoperative versus postoperative thymoglobulin in lung transplantation |
| Methods | Prospective single centre double‐blind RCT |
| Participants | All patients eligible for bilateral lung transplantation between the ages of 18 to 65 years |
| Interventions | Intraoperative dosing of Thymoglobulin followed by 3 additional postoperative doses (the first of these 3 postoperative doses will be placebo) versus 3 postoperative doses of Thymoglobulin (the intraoperative dose will be placebo) |
| Outcomes | Primary graft dysfunction assessed at 24 hours and 48 hours post‐transplant |
| Starting date | January 2006 |
| Contact information | Rajan Saggar MD, Department of Pulmonology and Critical Care at David Geffen School of Medicine at UCLA |
| Notes | No results identified |
Waddell 2006.
| Trial name or title | Study comparing Simulect® (basiliximab) plus standard immunosuppression to standard immunosuppression alone for the prevention of acute rejection and bronchiolitis obliterans in lung transplant |
| Methods | A randomised, double‐blind, placebo‐controlled study |
| Participants | Recipients of a first double or single lung or lobar allograft |
| Interventions | Simulect® (basiliximab) versus placebo |
| Outcomes | The proportion of patients who experience one or more acute allograft rejections in the first six months of treatment |
| Starting date | May 2006 |
| Contact information | Dr Thomas Waddell, University Health Network, Toronto, Canada |
| Notes | Study has been completed. The principal investigator has been contacted, but results are not yet available |
Differences between protocol and review
At the Cochrane Colloquium, October 2010, Keystone, Colorado, USA, agreement was reached that baseline imbalance and early stopping in individual studies may cause bias in individual studies, but not necessarily in the meta‐analysis. We therefore removed baseline imbalance and early stopping as bias criteria.
Contributions of authors
Draft the protocol: LP, CM, EP, MI, DS, CG
Study selection: LP, CM, EP
Extract data from studies: LP, EP, CM
Enter data into RevMan: LP, EP
Carry out the analysis: LP, CM, EP
Interpret the analysis: LP, EP, CM, CG
Draft the final review: LP, CM, EP, CG
Critical revision of the draft of the review: CG, MI, DS
Disagreement resolution: CG
Update the review: LP, CM, MI, CG, DS
Sources of support
Internal sources
-
Rigshospitalet Research Council, Copenhagen, Denmark.
grant to LP
External sources
No sources of support supplied
Declarations of interest
MI received a fee for a lecture on induction therapy in Vienna, Austria in June 2008. Program by the German Transplantation Society, and the meeting was sponsored by Genzyme.
New
References
References to studies included in this review
Brock 2001 {published data only}
- Brock MV, Borja MC, Ferber L, Orens JB, Anzcek RA, Krishnan J, et al. Induction therapy in lung transplantation: a prospective, controlled clinical trial comparing OKT3, anti‐thymocyte globulin, and daclizumab. Journal of Heart & Lung Transplantation 2001;20(12):1282‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chaparro 1999 {published data only}
- Chaparro C, Gutierrez CA, Kesten S, Hutcheon M, Winton T, Snell G, et al. Antilymphocyte globulin (ALG) vs. placebo in immunosuppression in lung transplant [abstract]. Journal of Heart & Lung Transplantation 1999;18(1):46. [Google Scholar]
Conte 2010 {published data only}
- Conte JV. The use of daclizumab and anti‐thymocyte globulin in lung transplantation. www.clinicaltrials.gov/ct2/show/NCT00181142 (accessed 1 October 2013).
Hartvig 2008 {published data only}
- Hartwig MG, Snyder LD, Appel JZ 3rd, Cantu E 3rd, Lin SS, Palmer SM, et al. Rabbit anti‐thymocyte globulin induction therapy does not prolong survival after lung transplantation. Journal of Heart & Lung Transplantation 2008;27(5):547‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hartwig MG, Snyder LD, Appel JZ, Simsir S, Lin SS, Palmer SM, et al. Rabbit antithymocyte globulin induction therapy for lung transplantation does not affect long‐term allograft function or survival [abstract]. Journal of Heart & Lung Transplantation 2005;24(2):S81‐2. [Google Scholar]
- Palmer SM, Miralles AP, Lawrence CM, Gaynor JW, Davis RD, Tapson VF. Rabbit antithymocyte globulin decreases acute rejection after lung transplantation: results of a randomized, prospective study. Chest 1999;116(1):127‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mullen 2007 {published data only}
- Mullen JC, Dueck A, Bentley MJ, Modry DL, Stewart K, Lien DC, et al. A randomized controlled trial of daclizumab vs. anti‐thymocyte globulin induction for lung transplantation [abstract]. Journal of Heart & Lung Transplantation 2005;24(2):S81. [DOI] [PubMed] [Google Scholar]
- Mullen JC, Oreopoulos A, Lien DC, Bentley MJ, Modry DL, Stewart K, et al. A randomized, controlled trial of daclizumab vs anti‐thymocyte globulin induction for lung transplantation. Journal of Heart & Lung Transplantation 2007;26(5):504‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Senn 2001 {published data only}
- Senn B, Dutly A, Naef R, Speich R, Weder W, Boehler A. A single center prospective, randomised trial of basiliximab versus ATG induction therapy in lung transplantation [abstract]. European Respiratory Journal 2001;18(Suppl 33):179s. [Google Scholar]
References to studies excluded from this review
AIRSAC Trial 2009 {published data only}
- Bhorade SM, Ahya V, Kotloff R, Baz M, Valentine V, Arcasoy S, et al. Long term follow‐up in the AIRSAC Trial, a multicenter randomized clinical trial in lung transplant recipients [abstract]. Journal of Heart & Lung Transplantation 2009;28(2 Suppl 1):S119‐20. [EMBASE: 70140164] [Google Scholar]
- Shyu S, Dew MA, Vito Dabbs A, Crespo M, Zaldonis DB, Johnson BA, et al. Long term follow‐up in the AIRSAC Trial, a multicenter randomized clinical trial in lung transplant recipients [abstract]. Journal of Heart & Lung Transplantation 2009;28(2 Suppl 1):S68. [Google Scholar]
Barlow 2001 {published data only}
- Barlow CW, Moon MR, Green GR, Gamberg P, Theodore J, Reitz BA, et al. Rabbit antithymocyte globulin versus OKT3 induction therapy after heart‐lung and lung transplantation: Effect on survival, rejection, infection, and obliterative bronchiolitis. Transplant International 2001;14(4):234‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borro 2005 {published data only}
- Borro JM, Torre M, Miguelez C, Fernandez R, Gonzalez D, Lemos C. Comparative study of basiliximab treatment in lung transplantation. Transplantation Proceedings 2005;37(9):3996‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garrity 2001 {published data only}
- Garrity ER Jr, Villanueva J, Bhorade SM, Husain AN, Vigneswaran WT. Low rate of acute lung allograft rejection after the use of daclizumab, an interleukin 2 receptor antibody. Transplantation 2001;71(6):773‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Geldmacher 2001 {published data only}
- Geldmacher H, Hoper M, Blach M, Struber M, Wirsing M, Haverich A, et al. The effect of total nodal irradiation and ATG therapy in the treatment of obliterative bronchiolitis after lung transplantation [abstract]. Pneumologie 2001;55(SH1):S64. [Google Scholar]
Jaksch 2011 {published data only}
- Jaksch P, Nierlich P, Scheed A, Ernst MB, Augustin V, Aigner C, et al. Alemtuzumab induction in lung transplantation: efficacy and safety [abstract]. European Surgery ‐ Acta Chirurgica Austriaca 2011;43(Suppl 242):27. [EMBASE: 70582326] [Google Scholar]
- Jaksch P, Scheed A, Zweytick B, Ernst M, Augustin V, Klepetko W. Alemtuzumab induction in lung transplantation: efficacy and safety [abstract]. Journal of Heart & Lung Transplantation 2012;31(4 Suppl 1):S67. [EMBASE: 70734276] [Google Scholar]
Lawrence 1989 {published data only}
- Lawrence EC, Holland VA, Young JB, Windsor NT, Brousseau KP, Noon GP, et al. Dynamic changes in soluble interleukin‐2 receptor levels after lung or heart‐lung transplantation. American Review of Respiratory Disease 1989;140(3):789‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lischke 2007 {published data only}
- Lischke R, Simonek J, Davidova R, Schutzner J, Stolz AJ, Vojácek J, et al. Induction therapy in lung transplantation: initial single‐center experience comparing daclizumab and antithymocyte globulin. Transplantation Proceedings 2007;39(1):205‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marom 2001 {published data only}
- Marom EM, Choi YW, Palmer SM, DeLong DM, Stuart MD, McAdams HP. Reperfusion edema after lung transplantation: effect of daclizumab. Radiology 2001;221(2):508‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meiser 1997 {published data only}
- Meiser BM, Uberfuhr P, Schulze C, Fuchs A, Mair H, Reichenspurner H, et al. Tacrolimus (FK506) proves superior to OKT3 for treating episodes of persistent rejection following intrathoracic transplantation. Transplantation Proceedings 1997;29(1‐2):605‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Loenhout 2010 {published data only}
- Loenhout KC, Groves SC, Galazka M, Sherman B, Britt E, Garcia J, et al. Early outcomes using alemtuzumab induction in lung transplantation. Interactive Cardiovascular & Thoracic Surgery 2010;10(2):190‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00105183 {published data only}
- Trulock EP 3rd. Study of EZ‐2053 in the prophylaxis of acute pulmonary allograft rejection. www.clinicaltrials.gov/ct2/show/NCT00105183 (accessed 1 October 2013).
Saggar 2011 {published data only}
- Saggar R. Intraoperative versus postoperative thymoglobulin in lung transplantation. www.clinicaltrials.gov/ct2/show/NCT00592306 (accessed 1 October 2013).
Waddell 2006 {published data only}
- Waddell TK. A randomized, double blind, placebo controlled study comparing simulect plus standard immunosuppression to standard immunosuppression alone for the prevention of acute rejection and bronchiolitis obliterans syndrome in bilateral lung and single lung transplant patients. http://www.clinicaltrials.gov/ct2/show/NCT00188825 (accessed 1 October 2013).
- Waddell TK, Borro JM, Roman A, Carreno MV, Zurbano F, Santos F, et al. A double‐blind randomized trial comparing basiliximab to placebo in lung transplantation [abstract]. Journal of Heart & Lung Transplantation 2006;25(2 Suppl):S114. [Google Scholar]
Additional references
Aurora 2009
- Aurora P, Edwards LB, Christie JD, Dobbels F, Kirk R, Rahmel AO, et al. Registry of the international society for heart and lung transplantation: twelfth official pediatric lung and heart/lung transplantation report‐2009. Journal of Heart & Lung Transplantation 2009;28(10):1023‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burton 2006
- Burton CM, Andersen CB, Jensen AS, Iversen M, Milman N, Boesgaard S, et al. The incidence of acute cellular rejection after lung transplantation: a comparative study of anti‐thymocyte globulin and daclizumab. Journal of Heart & Lung Transplantation 2006;25(6):638‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chatenoud 2008
- Chatenoud L. The long and winding road towards induction of allograft tolerance in the clinic. Transplant International 2008;21(8):725‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2006
- Chen W, Zhang L. Regulatory T‐cell subsets and their roles in transplantation tolerance. Current Opinion in Organ Transplantation 2006;11(4):373‐8. [EMBASE: 2006354779] [Google Scholar]
Christie 2011
- Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty‐eighth Adult Lung and Heart‐Lung Transplant Report‐‐2011. Journal of Heart & Lung Transplantation 2011;30(10):1104‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flechner 2008
- Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor‐sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clinical Transplantation 2008;22(1):1‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gluud 2006
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163(6):493‐501. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hachem 2005
- Hachem RR, Chakinala MM, Yusen RD, Lynch JP, Aloush AA, Patterson GA, et al. A comparison of basiliximab and anti‐thymocyte globulin as induction agents after lung transplantation. Journal of Heart & Lung Transplantation 2005;24(9):1320‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hachem 2006
- Hachem RR. Induction immunosuppression after lung transplantation. Current Opinion in Organ Transplantation 2006;11(6):617‐20. [EMBASE: 2006573225] [Google Scholar]
Hachem 2008
- Hachem RR, Edwards LB, Yusen RD, Chakinala MM, Alexander Patterson G, Trulock EP. The impact of induction on survival after lung transplantation: an analysis of the International Society for Heart and Lung Transplantation Registry. Clinical Transplantation 2008;22(5):603‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hauptman 2005
- Hauptman PJ, Mehra MR. It is time to stop ignoring malignancy in heart transplantation: a call to arms. Journal of Heart & Lung Transplantation 2005;24(8):1111‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011], The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollmen 2008
- Hollmen M, Tikkanen JM, Nykanen AI, Koskinen PK, Lemstrom KB. Tacrolimus treatment effectively inhibits progression of obliterative airway disease even at later stages of disease development. Journal of Heart & Lung Transplantation 2008;27(8):856‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ICH GCP 1996
- ICH GCP. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Guideline for Good Clinical Practice. E6 (R1). http://ichgcp.net (accessed 1 October 2013).
Iversen 2009
- Iversen M, Corris P. Immunosuppression. In: Fisher AJ, Verleden G, Massard G editor(s). Lung transplantation. Plymouth, UK: European Respiratory Society Journals Ltd, 2009:147‐68. [Google Scholar]
Keus 2009
- Keus F, Wetterslev J, Gluud C, Gooszen HG, Laarhoven CJ. Robustness assessments are needed to reduce bias in meta‐analyses that include zero‐event randomized trials. American Journal of Gastroenterology 2009;104(3):546‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lechler 2005
- Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation‐‐how much of the promise has been realized?. Nature Medicine 2005;11(6):605‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Penninga 2010
- Penninga L, Møller CH, Gustafsson F, Gluud C, Steinbrüchel DA. Immunosuppressive T‐cell antibody induction therapy for heart transplant recipients. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penninga 2012a
- Penninga L, Wettergren A, Wilson CH, Chan AW, Steinbrüchel DA, Gluud C. Antibody induction versus placebo, no induction, or another type of antibody induction for liver transplant recipients. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penninga 2012b
- Penninga L, Wettergren A, Wilson CH, Chan A‐W, Steinbrüchel DA, Gluud C. Antibody induction versus corticosteroid induction for liver transplant recipients. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenberg 2005
- Rosenberg PB, Vriesendorp AE, Drazner MH, Dries DL, Kaiser PA, Hynan LS, et al. Induction therapy with basiliximab allows delayed initiation of cyclosporine and preserves renal function after cardiac transplantation. Journal of Heart & Lung Transplantation 2005;24(9):1327‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Waugh N. Literature searching for clinical and cost‐effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system. Health Technology Assessment (Winchester, England) 2003;7(34):iii, ix‐x, 1‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stewart 2007
- Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. Journal of Heart & Lung Transplantation 2007;26(12):1229‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thompson 2002
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis‐‐a simulation study. PLoS One [Electronic Resource] 2011;6(10):e25491. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark, 2011. http://www.ctu.dk/tsa/files/tsa_manual.pdf (accessed 1 October 2013).
Verleden 2009
- Verleden G, Fisher AJ, Boehler A, Estenne M. Bronchiolitis obliterans syndrome. In: Fisher AJ, Verleden G, Massard G editor(s). Lung transplantation. Plymouth, UK: European Respiratory Society Journals Ltd, 2009:197‐211. [Google Scholar]
Webster 2010
- Webster AC, Ruster LP, McGee R, Matheson SL, Higgins GY, Willis NS, et al. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003897.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Penninga 2011
- Penninga L, Møller CH, Penninga EI, Iversen M, Gluud C, Steinbrüchel DA. Antibody induction therapy for lung transplant recipients. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008927] [DOI] [PMC free article] [PubMed] [Google Scholar]


