Abstract
Background
Diverticular disease is a common condition in Western industrialised countries. Most individuals remain asymptomatic throughout life; however, 25% experience acute diverticulitis. The standard treatment for acute diverticulitis is open surgery. Laparoscopic surgery ‐ a minimal‐access procedure ‐ offers an alternative approach to open surgery, as it is characterised by reduced operative stress that may translate into shorter hospitalisation and more rapid recovery, as well as improved quality of life.
Objectives
To evaluate the effectiveness of laparoscopic surgical resection compared with open surgical resection for individuals with acute sigmoid diverticulitis.
Search methods
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) in the Cochrane Library; Ovid MEDLINE (1946 to 23 February 2017); Ovid Embase (1974 to 23 February 2017); clinicaltrials.gov (February 2017); and the World Health Organization (WHO) International Clinical Trials Registry (February 2017). We reviewed the bibliographies of identified trials to search for additional studies.
Selection criteria
We included randomised controlled trials comparing elective or emergency laparoscopic sigmoid resection versus open surgical resection for acute sigmoid diverticulitis.
Data collection and analysis
Two review authors independently selected studies, assessed the domains of risk of bias from each included trial, and extracted data. For dichotomous outcomes, we calculated risk ratios (RRs) with 95% confidence intervals (CIs). For continuous outcomes, we planned to calculate mean differences (MDs) with 95% CIs for outcomes such as hospital stay, and standardised mean differences (SMDs) with 95% CIs for quality of life and global rating scales, if researchers used different scales.
Main results
Three trials with 392 participants met the inclusion criteria. Studies were conducted in three European countries (Switzerland, Netherlands, and Germany). The median age of participants ranged from 62 to 66 years; 53% to 64% were female. Inclusion criteria differed among studies. One trial included participants with Hinchey I characteristics as well as those who underwent Hartmann’s procedure; the second trial included only participants with "a proven stage II/III disease according to the classification of Stock and Hansen"; the third trial considered for inclusion patients with "diverticular disease of sigmoid colon documented by colonoscopy and 2 episodes of uncomplicated diverticulitis, one at least being documented with CT scan, 1 episode of complicated diverticulitis, with a pericolic abscess (Hinchey stage I) or pelvic abscess (Hinchey stage II) requiring percutaneous drainage."
We determined that two studies were at low risk of selection bias; two that reported considerable dropouts were at high risk of attrition bias; none reported blinding of outcome assessors (unclear detection bias); and all were exposed to performance bias owing to the nature of the intervention.
Available low‐quality evidence suggests that laparoscopic surgical resection may lead to little or no difference in mean hospital stay compared with open surgical resection (3 studies, 360 participants; MD ‐0.62 (days), 95% CI ‐2.49 to 1.25; I² = 0%).
Low‐quality evidence suggests that operating time was longer in the laparoscopic surgery group than in the open surgery group (3 studies, 360 participants; MD 49.28 (minutes), 95% CI 40.64 to 57.93; I² = 0%).
We are uncertain whether laparoscopic surgery improves postoperative pain between day 1 and day 3 more effectively than open surgery. Low‐quality evidence suggests that laparoscopic surgery may improve postoperative pain at the fourth postoperative day more effectively than open surgery (2 studies, 250 participants; MD = ‐0.65, 95% CI ‐1.04 to ‐0.25).
Researchers reported quality of life differently across trials, hindering the possibility of meta‐analysis. Low‐quality evidence from one trial using the Short Form (SF)‐36 questionnaire six weeks after surgery suggests that laparoscopic intervention may improve quality of life, whereas evidence from two other trials using the European Organization for Research and Treatment of Cancer core quality of life questionnaire (EORTC QLQ‐C30) v3 and the Gastrointestinal Quality of Life Index score, respectively, suggests that laparoscopic surgery may make little or no difference in improving quality of life compared with open surgery.
We are uncertain whether laparoscopic surgery improves the following outcomes: 30‐day postoperative mortality, early overall morbidity, major and minor complications, surgical complications, postoperative times to liquid and solid diets, and reoperations due to anastomotic leak.
Authors' conclusions
Results from the present comprehensive review indicate that evidence to support or refute the safety and effectiveness of laparoscopic surgery versus open surgical resection for treatment of patients with acute diverticular disease is insufficient. Well‐designed trials with adequate sample size are needed to investigate the efficacy of laparoscopic surgery towards important patient‐oriented (e.g. postoperative pain) and health system‐oriented outcomes (e.g. mean hospital stay).
Keywords: Aged, Female, Humans, Male, Middle Aged, Acute Disease, Diverticulitis, Diverticulitis/surgery, Laparoscopy, Laparoscopy/adverse effects, Laparoscopy/methods, Laparoscopy/mortality, Length of Stay, Postoperative Complications, Postoperative Complications/epidemiology, Postoperative Complications/mortality, Quality of Life, Randomized Controlled Trials as Topic, Reoperation, Reoperation/statistics & numerical data, Sigmoid Diseases, Sigmoid Diseases/surgery
Plain language summary
Laparoscopic surgery versus open surgery for acute sigmoid diverticulitis
Background
Diverticular disease is a condition in which the inner layer of the intestinal wall (mucosa) protrudes through weak points in the muscular layer of the wall, forming small pouches (diverticula) that bulge out of the large bowel. The inflammation of diverticula is defined as diverticulitis. Diverticulitis is more common in the sigmoid colon than in the other tracts of the large bowel. In Western countries, diverticular disease is very common, affecting about 60% of the population over 70 years of age. Most individuals with diverticular disease have no symptoms or experience only mild pain in the lower abdomen, accompanied by a slight change in bowel habits. Individuals with acute diverticulitis may experience pain in the lower abdomen and other symptoms such as fever, nausea, vomiting, and shivering. Diverticulitis generally is treated medically with antibiotics and diet. However, for individuals who experience recurrent abdominal pain or complications, surgical resection of the affected bowel segment is required; this can be performed through conventional open or laparoscopic surgery techniques.
In open surgery, a large abdominal incision is made at the midline to gain access to the abdominal cavity, but via laparoscopy, only small parietal incisions (usually 5 to 12 mm long) are made through the abdominal wall, allowing positioning of gas laparoscopic parietal cannulas (tubes that are inserted into the body) that provide access to the abdominal cavity with long‐handled dedicated surgical instruments used under vision of an endoscopic camera. A laparoscopic parietal cannula is a sharp‐pointed surgical instrument that is fitted with a tight cannula and is used to insert the tight cannula into a body cavity.
This review addresses the question of whether laparoscopy is more effective and/or safer than open surgery in the treatment of individuals with diverticulitis of the sigmoid colon who require a surgical resection.
Study characteristics
We identified three trials that compared the efficacy of laparoscopic surgery and open surgery. These studies included 392 participants (195 in the laparoscopic group vs 197 in the open surgery group). The method used to allocate participants based on randomisation, that is, the choice of treatment that participants received, was determined by a method similar to coin tossing, so the two groups were as similar as possible.
Key findings
We found that laparoscopic surgical resection may lead to little or no difference in mean hospital stay when compared with open surgical resection. Operating time was longer in the laparoscopic group by an average of 49 minutes. No important differences were observed in terms of 30‐day postoperative mortality, early overall morbidity, major and minor complications, surgical complications, postoperative times to liquid and solid diets, and reoperations due to anastomotic leak. To assess quality of life, researchers used different scales at different periods of time. Although one trial reported that patients who received laparoscopic surgery had better quality of life, the other two trials showed no benefit favouring either laparoscopic surgery or open surgery.
Quality of the evidence
The quality of the evidence varied from low to very low owing to risk of bias (i.e. conclusions may overestimate benefits or underestimate harms because of biased study design and conduct) and limitations in the patient population sample. Well‐designed trials are necessary to obtain a more accurate estimate of the benefits and safety of laparoscopic surgery over open surgery.
Summary of findings
Summary of findings for the main comparison. Laparoscopic vs open surgery for acute sigmoid diverticulitis.
| Primary outcomes for sigmoid diverticulitis | |||||
|
Patient or population: patients with sigmoid diverticulitis
Settings: hospital
Intervention: laparoscopic surgery for acute sigmoid diverticulitis Control: open surgical resection | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Open surgical resection | Laparoscopic surgical resection | ||||
| Mean hospital stay | Mean hospital stay in the control group (derived from all 3 included studies) was 7.9 days. | Mean hospital stay in the intervention groups was 0.62 days lower (2.49 days lower to 1.25 days higher). | 360 (3 studies) | ⊕⊕⊝⊝ lowa | |
| 30‐Day postoperative mortality | 17 per 1000 | 4 per 1000 (1 to 36) | RR 0.24 (0.03 to 2.07) | 360 (3 studies) | ⊕⊕⊝⊝ lowb |
|
Overall surgical complications (follow‐up range 6 to 12 months) |
276 per 1000 |
231 per 1000 (229 to 326) |
RR 0.84 (0.60 to 1.19) |
360 (3 studies) |
⊕⊕⊝⊝ lowc |
|
Early overall morbidity (30 postoperative days) |
92 per 1000 |
136 per 1000 (47 to 386) |
RR 1.46 (0.51 to 4.20) |
113 (1 study) |
⊕⊝⊝⊝ very lowd |
|
Late overall morbidity (after the first 30 postoperative days: within 6 months) |
255 per 1000 |
153 per 1000 (66 to 352) |
RR 0.60 (0.26 to 1.38) |
93 (1 study) |
⊕⊝⊝⊝ very lowe |
|
Late overall mortality (after the first 30 postoperative days) |
21 per 1000 |
44 per 1000 (4 to 457) |
RR 2.04 (0.19 to 21.77) |
93 (1 study) |
⊕⊝⊝⊝ very lowf |
|
Major complications (follow‐up range 6 to 12 months) |
149 per 1000 |
106 per 1000 (61 to 181) |
RR 0.74 (0.43 to 1.25) |
360 (3 studies) | ⊕⊕⊝⊝ lowg |
|
Minor complications (follow‐up range 6 to 12 months) |
All included trials reported this outcome. Types of minor complications reported differed across trials, hence we did not pool the data. However, data for each trial show no evidence of differences between the two groups in terms of minor complications (Table 5). | 360 (3 studies) |
⊕⊝⊝⊝ very lowh | ||
|
Operative time (minutes) |
Mean operative time in the control group was 127 minutes. | Mean operative time in the intervention group was 49.28 minutes longer (40.64 to 57.93 higher). | 360 (3 studies) | ⊕⊕⊝⊝ lowi | |
|
Intraoperative blood loss (mL) |
Mean intraoperative blood loss was 20 mL. | Mean intraoperative blood loss in the intervention group was 10.0 mL less (32.1 lower to 12.1 higher). |
104 (1 study) |
⊕⊝⊝⊝ very lowj | |
|
Postoperative pain (at day 4) measured with visual analogue scale (VAS) |
Mean postoperative pain at day 4 was 2.3 VAS units. | Mean score for postoperative pain at day 4 in the intervention groups was 0.65 units lower (1.04 to 0.25 lower). | 250 (2 studies) | ⊕⊝⊝⊝ very lowk | |
|
Postoperative time to liquid diets (days) |
Mean postoperative time to liquid diet was 0.5 days. | Mean difference in postoperative time to liquid diet in the intervention group compared to control was 0 days (0.28 days shorter to 0.28 days longer). |
247 (2 studies) |
⊕⊝⊝⊝ very lowl | |
|
Postoperative time to solid diets (days) |
Mean postoperative time to solid diet was 2.5 days. | Mean difference postoperative time to solid diet in the intervention group compared to control was 0 days (0.42 days shorter to 0.42 days longer). |
247 (2 studies) |
⊕⊝⊝⊝ very lowm | |
|
Reoperation rate due to anastomotic leak (follow‐up range 6 to 12 months) |
53 per 1000 |
39 per 1000 (15 to 104) |
RR 0.75 (0.29 to 1.95) |
349 (3 studies) |
⊕⊕⊝⊝ lown |
|
Anastomotic stricture (follow‐up 6 months) |
19 per 1000 |
19 per 1000 (1 to 296) |
RR 1.00 (0.06 to 15.57) |
104 (1 study) |
⊕⊝⊝⊝ very lowo |
|
Small‐bowel obstruction (follow‐up 6 months) |
77 per 1000 |
77 per 1000 (2 to 166) |
RR 0.25 (0.03 to 2.16) |
104 (1 study) |
⊕⊝⊝⊝ very lowp |
|
Quality of life Scales used: (1) SF‐36 questionnaire; (2) Gastrointestinal Quality of Life Index score; (3) EORTC QLQ‐C30 (follow‐up range 6 to 12 months) |
Raue 2011 assessed global health status using the EORTC QLQ‐C30 v3 questionnaire and found no significant differences between laparoscopic surgery and open surgery groups at 7, 30, and 90 days, and 12 months postoperatively (each P > 0.05). Sigma Trial 2009 used the SF‐36 questionnaire 6 weeks after surgery and found that participants who underwent laparoscopic surgery scored significantly better than those who underwent open surgery in terms of role limitations due to physical health (PRF) (P = 0.039) and role limitations due to emotional problems (ERF) (P = 0.024), social functioning (SF) (P = 0.015), and pain (PN) (P = 0.032). Gervaz 2010 used the Gastrointestinal Quality of Life Index and reported that the median score was 115 in the open group vs 110 in the laparoscopic group (P = 0.17). |
⊕⊕⊝⊝ lowq | |||
|
Recurrent diverticulitis rate (follow‐up 6 months) |
One trial ‐ Gervaz 2010 ‐ reported this outcome and provided no evidence of differences in the diverticulitis recurrence rate between laparoscopic (1.9%) and open surgery groups (3.8%) (P = 0.56). In a second trial, 2 participants (1 in each group) developed recurrent diverticulitis treated with antibiotics. This outcome therefore was not subjected to meta‐analysis. | ⊕⊝⊝⊝ very lowr | |||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EORTC QLQ‐C30: European Organization for Research and Treatment of Cancer core quality of life questionnaire; ERF: role limitations due to emotional problems; PN: pain; PRF: role limitations due to physical health; RR: risk ratio; SF: social functioning; SF‐36: Short Form‐36. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aOne of the three trials had unclear allocation concealment; participants and personnel could not be blinded owing to the nature of the interventions; it is unclear whether outcome assessors were blinded across all outcomes. Because the outcome of interest was objective, we did not downgrade for performance or detection bias. However, two of the three trials were exposed to attrition bias, so risk of bias was downgraded by one level. We downgraded further by another level owing to imprecision because the optimal information size (OIS) criterion was not met.
bOne of three trials had unclear allocation concealment; participants and personnel could not be blinded owing to the nature of the interventions; it is unclear whether outcome assessors were blinded across all outcomes. Because the outcome of interest was objective, we did not downgrade for performance or detection bias. However, two of the three trials were exposed to attrition bias, so risk of bias was downgraded by one level. We downgraded further by another level owing to imprecision because the OIS criterion was not met.
cIt is unclear whether the outcome assessor was blinded, and because the outcome of interest was subjective, we downgraded the evidence by one level owing to serious concern about detection risk of bias. We downgraded by another level owing to serious concern regarding imprecision, as the OIS criterion was not met.
dIt is unclear whether the outcome assessor was blinded, and because the outcome of interest was subjective, we downgraded the evidence by one level owing to serious concern about detection risk of bias. We downgraded by two other levels owing to very serious concern regarding imprecision: too small sample size and very large confidence interval.
eIt is unclear whether the outcome assessor was blinded, and because the outcome of interest was subjective, we downgraded the evidence by one level owing to serious concern about detection risk of bias. We downgraded by two other levels owing to very serious concern regarding imprecision: too small sample size and very large confidence interval.
fIt is unclear whether the outcome assessor was blinded, and because the outcome of interest was subjective, we downgraded the evidence by one level owing to serious concern about detection risk of bias. We downgraded by two other levels owing to very serious concern regarding imprecision: too small sample size and very large confidence interval.
gOne of three trials had unclear allocation concealment; participants and personnel could not be blinded owing to the nature of the interventions; it is unclear whether outcome assessors were blinded across all outcomes. Because the outcome of interest was objective, we did not downgrade for performance or detection bias. However, two of the three trials were exposed to attrition bias, so risk of bias was downgraded by one level. We downgraded further by another level owing to imprecision because the OIS criterion was not met.
hOne of three trials had unclear allocation concealment; participants and personnel could not be blinded owing to the nature of the interventions; it is unclear whether outcome assessors were blinded across all outcomes. Because the outcome of interest was objective, we did not downgrade for performance or detection bias. However, two of the three trials were exposed to attrition bias, so risk of bias was downgraded by one level. We downgraded by two other levels owing to very serious concern regarding imprecision: too small sample size and very large confidence interval.
iOne of three trials had unclear allocation concealment; participants and personnel could not be blinded owing to the nature of the interventions; it is unclear whether outcome assessors were blinded across all outcomes. Because the outcome of interest was objective, we did not downgrade for performance or detection bias. However, two of the three trials were exposed to attrition bias, so risk of bias was downgraded by one level. We downgraded further by another level owing to imprecision because the OIS criterion was not met.
jIt is unclear whether the outcome assessor was blinded, and because the outcome of interest was subjective, we downgraded the evidence by one level owing to serious concern about detection risk of bias. We downgraded by two other levels owing to very serious concern regarding imprecision: too small sample size and very large confidence interval.
kOne of two trials had unclear allocation concealment; participants and personnel could not be blinded owing to the nature of the interventions; it is unclear whether the outcome assessor was blinded. Because the outcome of interest was subjective and one of the three two trials was exposed to attrition bias, we downgraded the evidence by one level owing to serious concern for risk of bias. In addition, we downgraded by two other levels owing to very serious concern regarding imprecision: too small sample size and very large confidence interval.
lOne of two trials had unclear allocation concealment; participants and personnel could not be blinded owing to the nature of the interventions; it is unclear whether the outcome assessor was blinded. Because the outcome of interest was subjective and one of the three two trials was exposed to attrition bias, we downgraded the evidence by one level owing to serious concern for risk of bias. In addition, we downgraded by two other levels owing to very serious concern regarding imprecision: too small sample size and very large confidence interval.
mOne of two trials had unclear allocation concealment; participants and personnel could not be blinded owing to the nature of the interventions; it is unclear whether the outcome assessor was blinded. Because the outcome of interest was subjective and one of the three two trials was exposed to attrition bias, we downgraded the evidence by one level owing to serious concern for risk of bias. In addition, we downgraded by two other levels owing to very serious concern regarding imprecision: too small sample size and very large confidence interval.
nOne of three trials had unclear allocation concealment; participants and personnel could not be blinded owing to the nature of the interventions; it is unclear whether outcome assessors were blinded across all outcomes. Because the outcome of interest was objective, we did not downgrade for performance or detection bias. However, two of the three trials were exposed to attrition bias, so risk of bias was downgraded by one level. We downgraded further by another level owing to imprecision because the OIS criterion was not met.
oIt is unclear whether the outcome assessor was blinded, and because the outcome of interest was subjective, we downgraded the evidence by one level owing to serious concern about detection risk of bias. We downgraded by two other levels owing to very serious concern regarding imprecision: too small sample size and very large confidence interval.
pIt is unclear whether the outcome assessor was blinded, and because the outcome of interest was subjective, we downgraded the evidence by one level owing to serious concern about detection risk of bias. We downgraded by two other levels owing to very serious concern regarding imprecision: too small sample size and very large confidence interval.
qOne of three trials had unclear allocation concealment; participants and personnel could not be blinded owing to the nature of the interventions; it is unclear whether outcome assessors were blinded across all outcomes. Because the outcome of interest was objective, we did not downgrade for performance or detection bias. However, two of the three trials were exposed to attrition bias, so risk of bias was downgraded by one level. We downgraded further by another level owing to inconsistency due to differences in the magnitude and direction of the effect of treatment across trials.
rOne of two trials had unclear allocation concealment; participants and personnel could not be blinded owing to the nature of the interventions; it is unclear whether the outcome assessor was blinded. Because the outcome of interest was subjective, and one of the three two trials was exposed to attrition bias, we downgraded the evidence by one level owing to serious concern for risk of bias. In addition, we downgraded by two other levels owing to very serious concern regarding imprecision: too small sample size and very large confidence interval.
4. Minor complications.
| Author | Klarenbeek | Gervaz 2010 | Raue 2011 | |||
| Trial | Sigma Trial 2009 | LAPDIV‐CAMIC trial | ||||
| Type of treatment | LSR | OSR | LSR | OSR | LSR | OSR |
| No. of participants | 52 | 52 | 27 | 30 | 75 | 68 |
| Types of complications | ||||||
| Postoperative fever (38.5°C) | 2 | 0 | ||||
| Prolonged ileus | 0 | 1 | 3 | 2 | ||
| Pneumonia | 2 | 1 | 0 | 1 | 1 | 4 |
| Wound infection | 8 | 9 | 0 | 1 | 15 | 15 |
| Renal complications | 0 | 2 | ||||
| Cardiac complications | 0 | 1 | ||||
| Anastomotic bleeding | 1 | 0 | ||||
| Portal vein thrombosis | 1 | 0 | ||||
| Urinary tract infection | 5 | 1 | 4 | 1 | ||
| Other | 3 | 5 | 1 | 1 | 3 | 5 |
LSR: laparoscopic surgical resection.
OSR: open surgical resection.
Background
Description of the condition
Diverticular disease is a common condition in Western industrialised countries. The term 'diverticular disease' refers to a wide spectrum of symptoms and signs that may include lower gastrointestinal haemorrhage, inflammation, pain, abscess formation, fistula, strictures, and perforations (Rodkey 1984; Weizman 2011).
Epidemiological studies report that the incidence of diverticular disease is steadily increasing in the United States, Western Europe, and Australia, and has reached 50% in the population older than 60 years (Painter 1971; Warner 2007; Golder 2011). Autopsy studies from the early part of the 20th century reported colonic diverticula rates of 2% to 10% (Painter 1971). These rates have increased dramatically over the years. More recent data (Warner 2007; Weizman 2011) suggest that up to 50% of individuals older than 60 years of age have colonic diverticula, with 10% to 25% developing complications such as diverticulitis. Conversely, the disease is rare in sub‐Saharan countries, where it occurs in individuals 40 years of age or older (Tanase 2015).
Diverticular disease affects mainly the sigmoid colon; its occurrence increases with age, and it has similar prevalence in individuals of both sexes (Jacobs 2007). Although most patients remain asymptomatic throughout life, up to a quarter experience acute diverticulitis (Jacobs 2007; Warner 2007).
According to an American study, the rate of admission for diverticular disease increased by 26% between 1998 and 2005, mainly among groups up to 64 years of age, while it remained stable in individuals 65 to 74 years of age (Etzioni 2009) and decreased in those older than 74 years (Salem 2007). Similar trends have been observed in Canadian and European data over the same time period (Kang 2003; Warner 2007).
Following successful conservative medical therapy for a first attack of diverticulitis, approximately one‐third of patients remain asymptomatic, one‐third have episodic abdominal cramps without frank diverticulitis, and one‐third experience a second attack of diverticulitis (Larson 1976; Rodkey 1984; Rege 1989; Hall 2011).
For several years, elective colonic resection has been recommended for patients who have had a second recurrence of diverticulitis (Kreis 2006) owing to a high probability of recurrent attacks with less chance of response to medical treatment (Parks 1969; Farmakis 1994). Indeed, 13.3% of patients had a recurrence after the first episode of acute diverticulitis, and 29.3% after the second episode (Broderick‐Villa 2005). Furthermore, 56% of patients with diverticulitis undergo emergency surgery owing to complications (phlegmon, perforation, bleeding, stricture, and fistula) (Hussain 2008). More recent studies have questioned these indications, primarily because the long‐term risk of relapse is quite low (Anaya 2005; Broderick‐Villa 2005; Eglinton 2010; Binda 2012a; Li 2014); recurrent episodes of uncomplicated diverticulitis do not lead to failure of conservative treatment or to increased risk of poor outcomes if patients develop complicated diverticulitis (Chapman 2005; Pittet 2009); and, most of all, long‐term risks of subsequent emergent surgery and of death and stoma formation are low (Salem 2004; Shaikh 2007; Eglinton 2010; Frileux 2010; Ritz 2011; Binda 2012a; Binda 2012b). Several national guidelines suggest that the indication for elective surgery has to be decided on a case‐by‐case basis (Rafferty 2006; Andersen 2012; Binda 2015). Also, indications for and techniques of surgery for complicated diverticulitis in an emergency setting are widely debated among those favouring a more conservative approach (i.e. for diverticular abscess, non‐resective techniques; for purulent peritonitis, laparoscopic lavage).
Sigmoidectomy, with or without a subsequent anastomosis, is the procedure most frequently performed, traditionally through laparotomic access. For years, the debate concerning technical details was based on the level of proximal and distal resection, the level of vascular ligation (Cirocchi 2012b), and the opportunity for a synchronous anastomosis (Cirocchi 2013).
After laparoscopic colectomy was first reported in 1991 (Jacobs 1991), its use was proposed for treatment of diverticular disease in an elective or emergency setting in several reports showing its feasibility (Bruce 1996; Kohler 1998; Dwivedi 2002; Lauro 2002; Senagore 2002; De Chaisemartin 2003; Lawrence 2003; Alves 2005; Neri 2006; Massomi 2011; Anania 2014).
A few studies have proposed laparoscopy as a more prudent approach to emergent treatment of patients with complicated diverticular disease (Cirocchi 2014; Cirocchi 2015; Cirocchi 2017).
Description of the intervention
Surgical treatment of patients with sigmoid diverticulitis consists of an operation that involves surgical removal of the sigmoid tract, with preservation of the rectum distally and all or part of the descending colon proximally (El 2010). This depends on the site of proximal resection, which is influenced by the height of vessel ligation and by the need for resection in an area of pliable colon without hypertrophy or inflammation. The distal margin should extend to the upper third of the rectum, where the taenia coalesces. A standard procedure is identified as sigmoid colectomy, and a procedure with wider resection as left hemicolectomy.
Bowel resection may be done in the traditional way or by laparoscopic surgery. The traditional bowel resection is performed through an open surgical approach. The patient is placed under general anaesthesia. When surgery is performed under open conditions, the peritoneal cavity is entered through a midline incision, extended above the umbilicus down to the pubis bone, or, more rarely, through a transverse incision. The descending colon and the sigmoid colon are dissected laterally for mobilisation. The left ureter is identified at the crossing of the iliac vessels, with attention to keeping the Gerota fascia intact. The trunks of the sigmoid arteries are ligated before cutting in the case of sigmoid colectomy, and the inferior mesenteric artery is ligated in the case of left hemicolectomy (Tocchi 2001; Andersen 2012; Ambrosetti 2014). The parietal peritoneum is divided up to the splenic flexure in the case of sigmoid colectomy, but this dissection is extended to the left transverse mesocolon in the case of left hemicolectomy (Ambrosetti 2014). Complete mobilisation of the splenic flexure is optional, is left to the discretion of the surgeon (Fozard 2011; Ambrosetti 2014), and varies with the height of the resection, which is influenced by the need to resect an area of pliable colon without hypertrophy or inflammation. The rectosigmoid junction is identified, and the upper rectum divided with a standard stapler or a purse‐string. A side‐to‐end or end‐to‐end colorectal anastomosis is performed after transanal insertion of a circular stapler (Sigma Trial 2009).
When resection is performed laparoscopically, four or five trocars are used and the patient is placed in a modified Trendelenburg position (Sigma Trial 2009; Gervaz 2010; Raue 2011; Ambrosetti 2014). Despite the minimally invasive technique, surgery follows the steps described above. Once the rectosigmoid junction is identified, the upper rectum is divided with an endoscopic stapler or purse‐string. The sigmoid colon is then extracted through a mini‐Pfannenstiel incision or a vertical suprapubic incision no longer than 6 cm, and specimen resection is completed extracorporeally at the proximal site by a purse‐string. The anvil of a circular stapling device is secured in the left colon with the purse‐string suture, or with the proximal part of a biofragmental ring. The colon is then reintroduced within the peritoneal cavity, the pneumoperitoneum is re‐established, and a side‐to‐end or end‐to‐end colorectal anastomosis is performed after transanal insertion of a circular stapler (Sigma Trial 2009; Gervaz 2010; Raue 2011).
In cases for which a direct anastomosis is not considered safe, a non‐restorative colon resection may be performed by producing a loop ileostomy proximal to the anastomosis (Binda 2012), or by performing an end colostomy with the interrupted descending colon (Gervaz 2009; Andersen 2012). A colostomy or ileostomy may be performed when the bowel has to be relieved of its normal digestive work as it heals. Both procedures involve creating a temporary opening of the colon or ileum on the skin surface through the abdominal wall and attaching a removable bag to it. Waste will be collected in the bag.
How the intervention might work
Video laparoscopic surgery, also known as conventional laparoscopy, or coelioscopy, is a surgical technique that provides access to the abdominal cavity through one or more small incisions (5 to 15 mm). Through the incision, an optical drive surgical instrument is inserted while connected to a camera, which displays images onto a monitor. Carbon dioxide gas is used to inflate the abdominal cavity (pneumoperitoneum) to create the space needed to allow manipulation of abdominal organs with surgical instruments, which may vary in number from one to five, depending on the type of intervention provided (Pouliquen 2009).
The laparoscopic technique was introduced more than two decades ago, and it has represented a landmark evolution of surgical treatment toward the minimal‐access approach. The first abdominal laparoscopic interventions included adhesiolysis (Cirocchi 2010), cholecystectomy (Cuschieri 1991), and hysterectomy (Summitt 1992), or removal of adnexal masses (Mettler 1997). Today, laparoscopic management is applied for almost all types of abdominal surgical interventions involving many organs including the colon‐rectum (Kwon 2012), the stomach (Chen 2015), the liver (Bhojani 2012), and the pancreas (de Rooij 2015).
As a minimally invasive technique, compared with conventional open surgery, laparoscopic intervention is characterised by reduced operative stress (Buunen 2004); this may translate into shorter hospitalisation and more rapid recovery (Acar 2014), as well as improved quality of life (Radosa 2014).
One of the first and most common uses of the laparoscopic approach to colon surgery was removal of polyps with a large, broad base and those that are inaccessible for endoscopic removal (Franklin 2000). A Cochrane review that evaluated short‐term benefits of laparoscopic versus conventional colorectal resection in 25 trials reported that the laparoscopic approach offers several advantages in terms of operative time, postoperative pain, and duration of postoperative ileus, as well as total morbidity and surgical morbidity (Schwenk 2005). Another Cochrane review that compared the efficacy of laparoscopic versus conventional surgery for acute appendicitis described a reduction in the incidence of wound infection (Sauerland 2010).
Limitations of laparoscopic colorectal surgery include a longer learning curve (Miskovic 2012), difficulty in executing complex tasks that require advanced psychomotor skills (Uchal 2005), and the interposition of a video camera (Alaraimi 2014).
Based on the findings of several studies that have confirmed its feasibility and advantages compared with the traditional open approach, laparoscopic colectomy has become an established treatment option for individuals with inflammatory bowel disease and colorectal cancer (Abraham 2007; Bonjer 2007; Kuhry 2008; Polle 2009). Laparoscopic sigmoid colectomy could offer similar advantages to individuals with diverticulitis, as this is also a minimally invasive technique.
Why it is important to do this review
Physicians and surgeons are aware of and are able to readily diagnose, treat, and prevent diverticular disease. The frequency of surgical resection for diverticulitis has increased with respect to the relative increase in urgent surgical resections (Massomi 2011). According to data from two retrospective databases in the United States, open surgery is the most frequently used treatment for patients with diverticulitis (Massomi 2011; Mbadiwe 2013). A new observational study based on the national database in the United States showed that the trend has changed in favour of laparoscopic surgery for most patients with acute diverticulitis (Moghadamyeghaneh 2015). Some trial authors have reported that laparoscopic surgery has been feasible for treatment of patients with moderate acute diverticulitis, and is equivalent to open surgery in terms of safety and efficacy (Garrett 2008). Other study authors have highlighted that the feasibility and safety of laparoscopic surgery can be extended in cases of severe acute diverticulitis (De Magistris 2013).
However, most of the published literature regarding the effectiveness of laparoscopic surgery for diverticulitis is based on evidence derived from non‐randomised studies (Purkayastha 2006; Siddiqui 2010; Cirocchi 2012a). The most recent of these reviews included 11 observational studies that comprised 1430 participants (570 undergoing laparoscopic surgery, and 860 open surgery) with complicated and uncomplicated diverticular disease of the sigmoid colon (Cirocchi 2012a). Review authors found no differences in postoperative mortality (odds ratio (OR) 1.15, 95% confidence interval (CI) 0.24 to 5.57) or in major postoperative complication rates (OR 0.41, 95% CI 0.34 to 1.54). Conversely, review results showed less overall morbidity (OR 0.46, 95% CI 0.25 to 0.84) and a lower minor postoperative complication rate (OR 0.37, 95% CI 0.18 to 0.78).
Given the non‐randomised nature of available evidence, we believe it is important to systematically gather evidence based on randomised clinical trials on the efficacy of laparoscopic surgery in diverticulitis.
Objectives
To evaluate the efficacy and safety of laparoscopic surgical resection in comparison with open surgical resection for individuals with acute sigmoid diverticulitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), including cluster‐RCTs, recruiting individuals treated for sigmoid diverticulitis and comparing elective or emergency laparoscopic sigmoid resection versus open surgery.
Types of participants
All individuals with a diagnosis of sigmoid colonic diverticulitis with no limits on age. We also considered for inclusion trials enrolling participants with both benign and malignant disease, if data on individuals with sigmoid diverticulitis could be extracted separately.
Types of interventions
Elective or emergency laparoscopic sigmoid resection versus open surgical sigmoid resection.
Types of outcome measures
Primary outcomes
Mean length of hospital stay (days)
Secondary outcomes
30‐Day postoperative mortality
-
Postoperative complications:
Overall surgical postoperative complications
Early overall postoperative morbidity (any postoperative complications (e.g. anastomotic leakage, infection) during the first 30 postoperative days)
Late overall postoperative morbidity (any postoperative complications (e.g. incisional hernia, abdominal adhesions caused by intestinal obstruction, postoperative anastomotic colorectal stenosis) after the first 30 postoperative days)
Late overall postoperative mortality (after the first 30 postoperative days)
Major complications (e.g. anastomotic leakage, intra‐abdominal abscess, ureteral injury, accidental enterotomy, postoperative small‐bowel obstruction, anastomotic bleeding, incisional hernia, enterocutaneous fistula, intra‐abdominal abscess)
Minor complications (e.g. wound infection, prolonged postoperative ileus)
Operative time (minutes)
Intraoperative blood loss (mL)
Postoperative pain (visual analogue scale (VAS) 0 to 10 or 0 to 100)
Postoperative time to liquid diet, solid diet, passing flatus, and bowel movement (days)
Reoperation rate for anastomotic leaks
-
Other adverse outcomes:
Anastomotic stricture
Small‐bowel obstruction
Quality of life (validated scales presented in primary studies)
Recurrent diverticulitis
Search methods for identification of studies
We searched for all published and unpublished RCTs. We did not restrict searches by language, date, or publication status.
Electronic searches
We searched the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) in the Cochrane Library (Appendix 1).
Ovid MEDLINE (1946 to 23 February 2017) (Appendix 2).
Ovid Embase (1974 to 23 February 2017) (Appendix 3).
Searching other resources
Clinicaltrials.gov (March 2017) (Appendix 4)
World Health Organization (WHO) International Clinical Trials Registry (March 2017) (Appendix 5)
We also searched the bibliographies of identified trials for additional studies.
When resources become available, we plan to search conference proceedings and grey literature databases for future updates.
Data collection and analysis
We conducted the review according to the recommendations of theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Colorectal Cancer Group (CCCG). We used Review Manager (RevMan) 5.3 (Review Manager 2014) software in conducting this review.
Selection of studies
Two review authors (RC, IA) assessed titles or abstracts of all studies identified by the initial search and excluded those that were clearly not relevant. We obtained the full text of potentially relevant studies. Review authors independently assessed these studies to determine whether they met the inclusion criteria for this review. We resolved disagreements on inclusion by reaching consensus with a third review author (AA). We based final decisions about inclusion or exclusion of studies on assessment of full texts and listed the excluded studies with reasons for their exclusion in the Characteristics of excluded studies table. To visually show selection of studies, we generated a PRISMA flow chart (Figure 1).
1.

PRISMA flow diagram.
Data extraction and management
Two review authors (RC, IA) independently extracted from each study data on the study population, interventions, and outcomes. We extracted the following data onto a preformatted data extraction form.
Year and language of publication.
Country of conduct of the trial.
Year of conduct of the trial.
Description of participants (by group).
Sample size.
Inclusion and exclusion criteria.
Description of interventions.
Technical details of laparoscopic surgery (Table 2).
Technical details of open surgery (Table 3).
Outcome data for primary and secondary outcomes.
Postoperative management in included studies (Table 4).
Duration of follow‐up.
Numbers of withdrawals (by group).
Complications.
Funding source.
Trials registration data.
Assessment of risk of bias (as reported below).
1. Technical details of LSR in included trials.
| Author | Trial | No. of trocars | Laparoscopic approach to colectomy | Vascular tie | Mobilisation of splenic flexure | Exteriorisation of bowel transected | Colorectal anastomosis |
| Klarenbeek | Sigma Trial 2009 | 4 to 5 trocars | NR | Sigmoid vessels were divided. | if needed | Suprapubic incision | "Intracorporeal double‐stapled anastomosis was created after closure of the suprapubic wound and reestablishment of the pneumoperitoneum" |
| Gervaz 2010 | 5 trocars | Medial to lateral | Trunks of the sigmoid vessels were divided. | if needed | Mini‐Pfannenstiel incision | "Intracorporeal side‐to‐end or end‐to‐end colorectal anastomosis was created after closure of the suprapubic wound and reestablishment of the pneumoperitoneum" | |
| Raue 2011 | LAPDIV‐CAMIC trial | 4 to 5 trocars | NR | NR | NR | Suprapubic or left‐sided transverse incision | "Circular stapler or a biofragmentable ring" |
LSR: laparoscopic surgical resection.
NR: not reported.
2. Technical details of OSR in included trials.
| Author | Trial | Laparotomic incision | Mobilisation of splenic flexure | Colorectal anastomosis |
| Klarenbeek | Sigma Trial 2009 | Midline laparotomy | if needed | Double‐stapled anastomosis |
| Gervaz 2010 | Midline laparotomy | if needed | Double‐stapled anastomosis | |
| Raue 2011 | LAPDIV‐CAMIC trial | Midline or transverse laparotomy | NR | Circular stapler or biofragmentable ring |
OSR: open surgical resection.
NR: not reported.
3. Postoperative management in included studies.
| Author | Trial | Postoperative analgesia | Nasogastric tube | Bladder catheter | Postoperative diet | Mobilisation |
Participants discharged |
| Klarenbeek | Sigma Trial 2009 | "After surgery all patients were started on intravenous patient controlled analgesia (PCApump) with morphine (0.02 mg/kg, max. 6 times/h) up to maximum postoperative day 3. Oral analgesia (Paracetamol 1 g/24 hours qid) was started on postoperative day 2" | "Nasogastric tubes were removed at the end of the operation" |
"Bladder catheters were removed on postoperative day 1" | "Noncarbonated liquids were offered the evening after the surgery. If oral liquids were tolerated, diet was advanced to soft, and thereafter, solid food was given" |
"Early mobilization was encouraged and implemented starting on the first postoperative day" |
"After having had a bowel movement, tolerating solid food, able to walk properly, and feeling comfortable with oral analgesia" |
| Gervaz 2010 | "Pain management was achieved with paracetamol i.v. 500 mg q.i.d. and ketorolac i.v. 30 mg t.i.d. for the first 48 hours, and then switched to paracetamol p.o. 500 mg. q.i.d. and ibuprofen p.o. 600 mg t.i.d. In addition, the patients were instructed to ask the nurse for opiates administration in case of intractable pain. Morphine was injected subcutaneously whether needed at a dose of 0.1 mg/kg, with a maximum daily dose of 0.6 mg/kg" |
NR | NR | "On postoperative day 1, all patients were free to drink as much fluid as tolerated, and were started on a solid diet on postoperative day 2" |
"They were encouraged to walk as soon as possible" |
NR | |
| Raue 2011 | LAPDIV‐CAMIC trial | NR | NR | NR | NR | NR | NR |
i.v.: in‐vein.
mg: milligrams.
NR: not reported.
p.o.: peroral.
q.i.d. : quadris in die.
t.i.d. : tris in die.
Assessment of risk of bias in included studies
IA and RC independently assessed the methodological quality of trials, without masking trial names. These review authors assessed the risk of bias of each study by following instructions and applying items given in Chapter 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (Appendix 6).
We addressed the following domains.
Sequence generation: For each included study, we assessed and described the method used to generate the allocation sequence. We judged studies as having (1) low risk of selection bias when the method used was a truly random process (e.g. random number table; computer random number generator); (2) high risk of selection bias when the method used was non‐random (e.g. hospital or clinic record number); or (3) unclear risk of selection bias when information was insufficient to permit a judgement (Savovic 2012).
Allocation concealment: For each study, we assessed and described whether it was possible to foresee the next assignment during the process of allocation. We judged studies as having (1) low risk of selection bias when the method used permitted allocation concealment (e.g. central randomisation; consecutively numbered sealed opaque envelopes); or (2) high risk of bias when a proper allocation tool was not used (e.g. alternation, unsealed or non‐opaque envelopes, date of birth); or (3) unclear risk of selection bias when information was insufficient to permit a judgement (Savovic 2012).
Blinding of participants, surgeons, and outcome assessors: Owing to the nature of the intervention, it was not possible to mask participants and investigators, and we considered all studies to be at high risk of performance bias by default. However, for overall risk of bias, we gave more weight to blinding of outcome assessors. We judged studies as having (1) low risk of bias when blinding was inadequate; (2) high risk of detection bias when the outcome measurement was likely to be influenced by absence of blinding; or (3) unclear risk when information was insufficient. In addition, in our assessment, we considered whether the outcome of interest was subjective or objective. We considered the main outcome to be an objective outcome (Savovic 2012).
Incomplete outcome data: For each included study, we assessed and described whether attrition bias could influence study results. We judged studies as having (1) low risk of attrition bias when no outcome data were missing, or when missing outcome data were of low incidence and were balanced across groups; (2) high risk of attrition bias when relevant data were missing (e.g. numbers or reasons for imbalance of missing data across groups), or when analysis deviated from intention‐to‐treat, or (3) unclear risk when we could provide no judgement owing to lack of information (Abraha 2015).
Selective reporting: For each included study, we assessed the possibility of selective outcome reporting bias. We judged included studies as having (1) low risk of bias when all expected outcomes were assessed and sufficiently reported; or (2) high risk of bias when we found evidence of omission or change in the prespecified outcomes, or incomplete reporting of outcome; or (3) unclear risk of selection reporting when information was insufficient to permit a judgement (Chan 2004; Macura 2010).
We graded all above domains for each trial and detailed our reasons and supporting evidence in the Characteristics of included studies table. We summarised the risk of bias for each study with consideration of adequate allocation concealment and the presence of attrition bias. We considered the primary outcome (mean hospital stay) as an objective outcome; therefore, we did not take into account performance or detection bias in evaluating quality.
Measures of treatment effect
For dichotomous outcomes, we calculated risk ratios (RRs) with respective 95% confidence intervals (CIs). For continuous outcomes, we calculated mean differences (MDs) with 95% CIs for outcomes such as length of hospital stay, and standardised mean differences (SMDs) with 95% CIs for quality of life and global rating scales, if investigators used different scales.
Unit of analysis issues
We checked included studies for unit of analysis errors. The unit of analysis was the individual participant. We identified no cluster‐randomised trials. If we need to identify cluster‐RCTs for future updates, we will seek expert statistical advice on how to pool data and how to take into account potential clustering effects on study results. In addition, we will consider the limitations of including cluster‐randomised studies owing to differences in surgeons and centres.
Dealing with missing data
Missing data and postrandomisation exclusions are common in RCTs and reviews (Abraha 2017). These elements may compromise the prognostic balance between allocated participants obtained through the process of randomisation and may introduce bias. In the event of missing data, we contacted trial authors to request the relevant information. When intention‐to‐treat analysis was not possible, we planned to conduct analyses using 'available cases' data (i.e. only those whose outcomes are known).
For continuous data (e.g. length of hospital stay), we presented available data from study reports/study authors and did not anticipate imputing missing data.
Assessment of heterogeneity
We planned to consider clinical and methodological heterogeneity, that is, the degree to which included studies varied in terms of participants, interventions, outcomes, characteristics such as length of follow‐up, and methodological quality (i.e. risk of bias).
We examined statistical heterogeneity using both the I² statistic and the Chi² test. For the Chi² test, we judged heterogeneity to be present for P values less than 0.10. Our interpretation of I² was guided by the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 9.5.2; Higgins 2011) as follows: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% represents considerable heterogeneity.
Assessment of reporting biases
In this review, we identified fewer than 10 RCTs that hindered the possibility of evaluating publication bias. In future updates, we plan to use visual asymmetry on a funnel plot to explore reporting bias (Egger 1997).
Data synthesis
We performed statistical analyses by using Review Manager 5.3 software (Review Manager 2014). We used a fixed‐effect analysis model when, in the judgement of the review authors, minimal clinical heterogeneity was supported by a statistically non‐significant Chi² value and I² less than 50% (Kontopantelis 2013). In all remaining circumstances, we used the random‐effects model.
If studies reported continuous variables as medians with ranges, which are not useable in a meta‐analysis, we assumed that the mean value was equal to the median value and estimated the standard deviation (SD) as follows: range/4 (sample size ≤ 70) or range/6 (sample size > 70) (Hozo 2005). To calculate the range, we used the formula (r = b ‐ a), where a is the smallest value (minimum) and b is the largest value (maximum). If neither range nor any other measure of dispersion was reported and it was impossible to estimate the mean and the SD on the basis of published data, we excluded corresponding continuous variables from the meta‐analysis.
We presented the results of meta‐analyses for each outcome graphically as forest plots.
Subgroup analysis and investigation of heterogeneity
In this review, we did not identify a sufficient number of trials and adequate data to perform subgroup analyses. For future updates, we plan to perform the following subgroup analyses.
Trials with complicated diverticulitis versus trials with uncomplicated diverticulitis.
Trials that performed sigmoidectomy versus trials that performed high anterior resection of the rectum.
Sensitivity analysis
In this review, we did not identify a sufficient number of trials to perform sensitivity analysis to assess the consistency and robustness of results of meta‐analyses. For future updates, we plan to perform sensitivity analysis based on:
including only studies with all domains at low risk of bias; and
repeating analyses using a fixed‐effect model for data synthesis.
'Summary of findings' table
We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach to assess the quality of evidence for each estimate of treatment effect (Schünemann 2011a; Schünemann 2011b). We could downgrade the quality of evidence by one (serious concern) or two (very serious concern) for the following reasons: risk of bias, imprecision (wide confidence interval), inconsistency (unexplained heterogeneity, inconsistency of results), indirectness (indirect population, intervention, control, outcomes), and publication bias (Balshem 2011; Abraha 2016).
We evaluated the quality of evidence for all outcomes assessed by the included studies. For each outcome, we judged quality as follows.
High quality evidence: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality evidence: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality evidence: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Results
Description of studies
Results of the search
We presented in Figure 1 the PRISMA flow diagram showing selection of studies for this review.
We identified a total of 635 publications using the literature search strategy described in the Appendices. After excluding 221 duplicates and 393 clearly irrelevant articles by reading titles and abstracts, we determined that 21 articles remained eligible for full‐text evaluation. After full‐text assessment, we excluded 15 publications and identified six publications of three trials that fulfilled the inclusion criteria (Sigma Trial 2009; Gervaz 2010; Raue 2011). See Characteristics of excluded studies tables and Characteristics of included studies tables. Searches for ongoing trials in ClinicalTrial.gov or in the WHO International Clinical Trials Registry revealed no trials relevant to this topic.
Included studies
The three included trials had a total of 392 participants (195 in the laparoscopic group and 197 in the open surgery group).
Country
All trials were conducted in central Europe. Gervaz 2010 was conducted in Switzerland, Sigma Trial 2009 was conducted in the Netherlands, and Raue 2011 recruited participants in Germany.
Participants
The number of recruited participants ranged from 104 in Sigma Trial 2009 to 156 in Raue 2011. Median age ranged from 62 to 66, whereas percentage of female participants ranged from 53% in Gervaz 2010 to 64% in Raue 2011. See Characteristics of included studies.
Inclusion criteria and the definition of diverticulitis differed considerably among included studies. Sigma Trial 2009 provided a description of the stage of diverticulitis according to the classification of Hinchey and included participants with Hinchey I (i.e. symptomatic diverticulitis of the sigmoid colon in which individuals had two or more recurrent attacks of acute diverticulitis with "or without pericolic abscess necessitating hospitalisation with intravenous antibiotics and nil per os"); those with Hinchey IIa, who had "previous recurrent attacks of acute diverticulitis with percutaneously drainable distant abscess necessitating CT‐guided drainage"; and participants with Hinchey IIb, who had the "presence of internal fistula between the sigmoid colon and a hollow organ with abscess or without". In addition, trialists included participants with the "presence of symptomatic stricture of the sigmoid colon with no evidence of cancer" or with "recurrent severe diverticular bleeding requiring blood transfusions verified at colonoscopy and/or arteriogram," along with participants who underwent Hartmann’s procedure. Raue 2011 included only participants with "a proven stage II/III disease (stage II: pericolic inflammation with or without local abscess; stage III: recurrent disease with stenosis, fistula, or bleeding) according to the classification of Stock and Hansen" (Hansen 1999). Gervaz 2010 considered for inclusion participants with "diverticular disease of sigmoid colon documented by colonoscopy and 2 episodes of uncomplicated diverticulitis, one at least being documented with CT scan, 1 episode of complicated diverticulitis, with a pericolic abscess (Hinchey stage I) or pelvic abscess (Hinchey stage II) requiring percutaneous drainage."
Intervention
All trials compared laparoscopy versus open surgery for sigmoid resection. Table 2 and Table 3 describe technical details of laparoscopic surgery and open surgery, respectively, as reported by each of the included studies.
Experimental group
Sigma Trial 2009 is the only study reporting that a learning curve of 15 laparoscopic and 15 open surgical resections for symptomatic diverticulitis had to be completed by surgeons before they could participate in the trial. We provided details of laparoscopic and open surgical procedures in Table 2 and Table 3, respectively.
Sigma Trial 2009 and Raue 2011 performed laparoscopic surgical resection through four to five ports, and Gervaz 2010 through five ports. Only Gervaz 2010 described trocar placement: Under direct vision, a 12‐mm optic trocar was inserted above the umbilicus, and two 5‐mm trocars were placed in the left and right paraumbilical areas; the operating 10‐mm trocar was located in the right iliac fossa, and a fifth trocar was positioned in the suprapubic midline. Only Gervaz 2010 reported the surgical technique used for the laparoscopic colectomy, which was the medial‐to‐lateral dissection.
In the laparoscopic group, exteriorisation of the transected bowel was performed through a midline suprapubic incision (Sigma Trial 2009), a mini‐Pfannenstiel incision (Gervaz 2010), or a suprapubic or left‐sided transverse incision (Raue 2011).
In terms of colorectal anastomosis, Gervaz 2010 performed an intracorporeal side‐to‐end or end‐to‐end colorectal anastomosis after closure of the suprapubic wound and re‐establishment of the pneumoperitoneum; Sigma Trial 2009 created an intracorporeal double‐stapled anastomosis after closure of the suprapubic wound and re‐establishment of the pneumoperitoneum, but it was unclear whether this was performed end‐to‐end or end‐to‐side; and Raue 2011 limited the description of the device used (a circular stapler or a biofragmentable ring) without describing how the anastomosis was performed.
Control group
Gervaz 2010 and Sigma Trial 2009 performed the open surgical resection through a midline incision, followed by a double‐stapled anastomosis; if considered necessary, investigators performed mobilisation of the splenic flexure intraoperatively and preserved the inferior mesenteric artery (IMA), dividing the sigmoid vessels only. Raue 2011 performed a midline or a transverse laparotomy followed by use of a circular stapler or a biofragmentable ring (however, study authors did not comment on the need for splenic flexure mobilisation and vascular ligation).
Sigma Trial 2009 and Raue 2011 fashioned a covering loop ileostomy in case of extensive inflammatory changes or compromised blood supply at the time of resection.
Postoperative outcomes
(Details are reported in Table 4.)
Postoperative analgesia: Only Sigma Trial 2009 and Gervaz 2010 reported the type of postoperative analgesia: intravenous patient‐controlled analgesia (PCA) pump with morphine until day three, and oral paracetamol from day two (Sigma Trial 2009); paracetamol and ketorolac for the first 48 hours, switched to paracetamol and ibuprofen and morphine, as required, in cases of severe pain (Gervaz 2010).
Postoperative pain:Sigma Trial 2009 used a visual analogue scale (VAS) to measure pain preoperatively and daily after surgery up to postoperative day four but did not report data in detail; we contacted trial authors to obtain data but without success. In addition, Sigma Trial 2009 measured postoperative pain at six weeks using the Short Form (SF)‐36 questionnaire. Gervaz 2010 reported data and compared VAS postoperative pain scores (0 to 10) obtained on days 1 to 4. Raue 2011 presented a figure showing the mean VAS pain score (0 to 100) without SD.
Postoperative nasogastric tube and bladder catheter: Only Sigma Trial 2009 reported postoperative management of the nasogastric tube and the bladder catheter: The nasogastric tube was removed at the end of the operation; the bladder catheter was removed on day 1.
Postoperative diet: In Sigma Trial 2009 and Gervaz 2010, participants were allowed to drink freely (non‐carbonated liquids in Sigma Trial 2009) and were started on a solid diet on postoperative day 2. Sigma Trial 2009 and Raue 2011 reported data on times to solid and liquid diets but expressed values only as medians and ranges for continuous variables. Gervaz 2010 did not report data on this outcome.
Early overall morbidity: Only Gervaz 2010 reported on complications during the first 30 postoperative days. Sigma Trial 2009 reported considered major and minor complications but did not report the time frame. Similarly, Raue 2011 reported postoperative morbidity but provided no details about the time frame.
Late overall morbidity:Gervaz 2010 and Raue 2011 reported this outcome but did not report the time frame. Sigma Trial 2009 defined the outcome as "late clinical outcomes" at the "6‐month follow‐up period."
Major complications: The three studies interpreted these differently. Raue 2011 considered the following complications in the methods: anastomotic leakage reoperation, intra‐abdominal/pelvic abscess, postoperative bleeding, and other reasons for reoperation (for the latter element, study authors did not provide a definition); Sigma Trial 2009 included evisceration, small‐bowel perforation, Richter hernia, myocardial infarction, and pulmonary embolism, in addition to anastomotic leakage reoperation, intra‐abdominal/pelvic abscess, and postoperative bleeding; and Gervaz 2010 defined all major complications that required reoperation, including small‐bowel perforation, small‐bowel obstruction, and intra‐abdominal abscess.
Minor complications: All three trials reported this information differently. Raue 2011 considered wound infection, deep vein thrombosis, urinary tract infection, and delayed bowel motility without the need for reoperation; Sigma Trial 2009 considered urinary tract infection, wound infection, pneumonia, and others; Gervaz 2010 included postoperative fever (at least 38.5°C), prolonged ileus, pneumonia, wound infection, portal vein thrombosis, anastomotic bleeding that did not require surgical reoperation, and others.
Overall surgical complications:Raue 2011 reported this outcome without providing any specific description; Gervaz 2010 reported surgical complications, such as wound infection, anastomotic leakage, and bleeding; and Sigma Trial 2009 did not describe overall surgical complications but reported anastomotic leakage reoperation, intra‐abdominal/pelvic abscess, postoperative bleeding, evisceration, small‐bowel perforation, wound infection, and Richter hernia.
Postoperative quality of life:Sigma Trial 2009 assessed quality of life by administering the SF‐36 questionnaire preoperatively and six weeks after surgery; in contrast, Raue 2011 assessed global health status using the European Organization for Research and Treatment of Cancer core quality of life questionnaire (EORTC QLQ‐C30 v3); and Gervaz 2010 utilised the median Gastrointestinal Quality of Life Index score for examination of this outcome.
Re‐admissions due to recurrence of diverticulitis:Sigma Trial 2009 and Gervaz 2010 assessed recurrence of diverticulitis. Sigma Trial 2009 analysed hospital re‐admissions at six months, whereas Gervaz 2010 reported data at 30 months after surgery. Raue 2011 did not analyse this outcome.
Excluded studies
We excluded 15 studies for the following reasons: 11 were observational studies that compared laparoscopic versus open surgical resection (Bruce 1996; Tuech 2000; Dwivedi 2002; Senagore 2002; De Chaisemartin 2003; Lawrence 2003; Thaler 2003; Simon 2005; Neri 2006; Massomi 2011; Anania 2014); two were non‐randomised controlled clinical trials (Kohler 1998; Alves 2005); and two publications reported results of a randomised trial that compared laparoscopic lavage versus colon resection and stoma (Angenete 2015; Angenete 2016).
Risk of bias in included studies
Overall, we considered studies to have high risk of bias mainly when we noted attrition bias (Figure 2; Figure 3).
2.
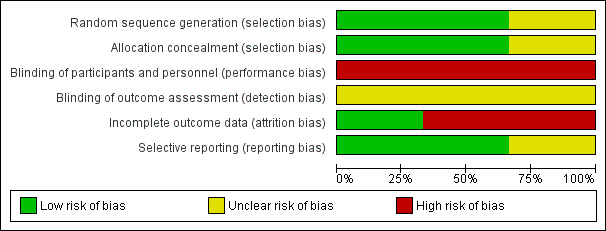
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Two studies used an appropriate randomisation process and allocation concealment methods (Sigma Trial 2009; Gervaz 2010). Gervaz 2010 generated randomisation by using a computer; investigators kept the sequence concealed, and an independent statistician communicated with the investigator via telephone. Sigma Trial 2009 generated the sequence via computer, and investigators used a secure website to obtain an automated randomised allocation number. Raue 2011 did not clearly report the method used to conceal allocation.
Blinding
In Gervaz 2010 and Sigma Trial 2009, investigators attempted to blind participants with respect to surgical treatment received by positioning a large dressing over the abdomen. Sigma Trial 2009 used the same blinding method for hospital staff, including physicians in charge of participant discharge, but Gervaz 2010 used this method only for nursing staff and families of participants. However, investigators could not be blinded, given the type of intervention provided, and we considered all studies to be at high risk of performance bias (Sigma Trial 2009; Gervaz 2010; Raue 2011).
In addition, we considered all three trials to be at unclear risk of detection bias, given that trial authors provided no clear information regarding blinding of outcome assessors. However, because the primary outcome was considered objective, we did not take the bias into account during the GRADE assessment for downgrading of evidence.
Incomplete outcome data
All trials declared the use of intention‐to‐treat analysis. However, we considered two trials to be at high risk of bias (Gervaz 2010; Raue 2011). Gervaz 2010 excluded five and eight participants allocated, respectively, to laparoscopic (four withdrew consent, one developed ovarian cancer) and open (seven withdrew consent, one developed open foramen ovale) groups because they did not receive the predefined treatment. Furthermore, investigators excluded from the final analysis two (one protocol violation, one undergoing Hartmann's procedure) and four (three protocol violations, one undergoing a diverting colostomy) participants in the laparoscopic and open groups, respectively, because of protocol violations or violations of the planned surgical procedure. Hence, the proportion of exclusions (14%) suggested high risk of attrition bias in this study. In Raue 2011, two participants in the laparoscopic group and 11 in the open surgery group did not receive the allocated treatment, and study authors did not clearly state the reasons. After providing treatment, investigators excluded two participants in the laparoscopic group and four in the open surgery group, but did not state the reasons for this. Finally, during follow‐up, two participants in the open surgery group were lost (died) and researchers did not analyse these data for the outcomes of interest. Imbalance between the two groups in the numbers of participants excluded from analysis and the fact that reasons for exclusion are not clearly reported have exposed this trial to risk of attrition bias.
Sigma Trial 2009 analysed all participants allocated to treatment groups for the primary outcomes; 18 and 17 participants in laparoscopic and open surgery groups, respectively, did not complete the SF‐36 questionnaire for evaluation of quality of life.
Selective reporting
For all included trials, the respective study protocol was available, and trial authors reported all prespecified (primary and secondary) outcomes of interest in the review. However, we deemed that Sigma Trial 2009 was at unclear risk of selective reporting bias, given that results for postoperative pain were not adequately reported; trial authors reported differences (daily on average VAS 1.6 points less) without reporting mean values for both groups at baseline and post baseline and did not indicate the time point at which the post‐baseline value was estimated.
Effects of interventions
See: Table 1
1. Primary outcome
1.1 Mean length of hospital stay
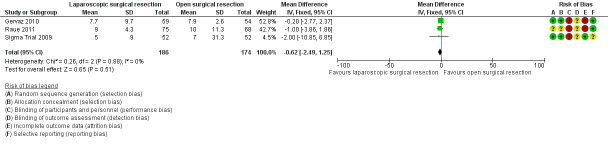
All included trials reported this outcome, including a total of 360 participants in the meta‐analysis (Sigma Trial 2009; Gervaz 2010; Raue 2011). Evidence was insufficient for review authors to determine whether the two interventions showed differences in terms of hospital stay (mean difference (MD) ‐0.62, 95% confidence interval (CI) ‐2.49 to 1.25; I² = 0%; low‐quality evidence) (Analysis 1.1; Figure 4).
1.1. Analysis.
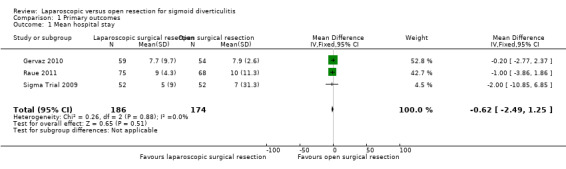
Comparison 1 Primary outcomes, Outcome 1 Mean hospital stay.
4.
Forest plot of comparison: 1 Primary outcomes, outcome: 1.1 Mean hospital stay.
2. Secondary outcomes
2.1 30‐Day postoperative mortality
All included trials evaluated this outcome (Sigma Trial 2009; Gervaz 2010; Raue 2011). The rate of 30‐day postoperative mortality was lower in the laparoscopic group (0%) than in the open resection group (1.7%), but trial results show no evidence of differences between interventions (risk ratio (RR) 0.24, 95% CI 0.03 to 2.07; I² = 0%; low‐quality evidence) (Analysis 2.1).
2.1. Analysis.
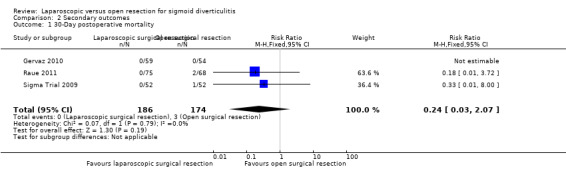
Comparison 2 Secondary outcomes, Outcome 1 30‐Day postoperative mortality.
2.2 Postoperative complications
2.2.1 Overall surgical complications
All three included trials reported this outcome (Sigma Trial 2009; Gervaz 2010; Raue 2011), including a total of 360 participants in the meta‐analysis. The rate of overall surgical complications was lower in the laparoscopic group than in the open surgery group, but data show no evidence of differences between groups (RR 0.84, 95% CI 0.60 to 1.19; I² = 0%; low quality evidence) (Figure 5; Analysis 2.2).
5.
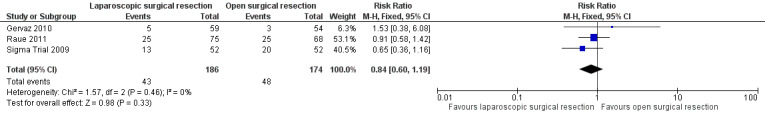
Forest plot of comparison: 2 Secondary outcomes, outcome: 2.2 Surgical complications.
2.2. Analysis.
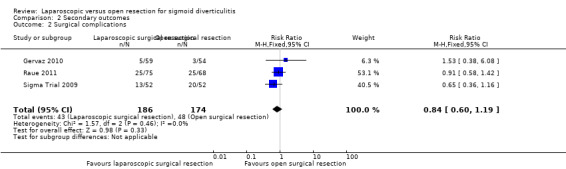
Comparison 2 Secondary outcomes, Outcome 2 Surgical complications.
2.2.1 Early overall morbidity
One trial including a total of 113 participants reported this outcome (Gervaz 2010). The proportion of participants with early overall morbidity was higher in the laparoscopic group (13.6%) than in the open surgery group (9.3%), but data show no evidence of differences (RR 1.46, 95% CI 0.51 to 4.20; very low quality evidence) (Analysis 2.3).
2.3. Analysis.
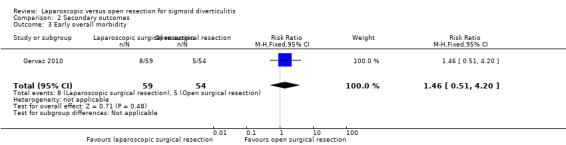
Comparison 2 Secondary outcomes, Outcome 3 Early overall morbidity.
2.2.3 Late overall morbidity
One trial including a total of 83 participants reported this outcome (Sigma Trial 2009). After the first 30 postoperative days, investigators noted no evidence of differences between the two interventions in terms of morbidity (7 in laparoscopic surgery (13.5%) vs 12 in open surgery (23.1%); RR 0.60, 95% CI 0.26 to 1.38; very low quality evidence) (Analysis 2.4).
2.4. Analysis.
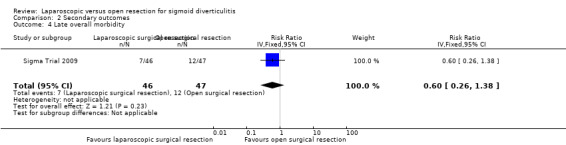
Comparison 2 Secondary outcomes, Outcome 4 Late overall morbidity.
2.2.4 Late overall mortality
One trial including a total of 83 participants reported this outcome (Sigma Trial 2009). In this trial, after the first 30 postoperative days, data show no evidence of differences between the two interventions in terms of mortality (2 laparoscopic surgery (3.8%) vs 0 open surgery (1.53%); RR 2.04, 95% CI 0.19 to 21.77; very low quality evidence) (Analysis 2.4).
2.2.5 Major complications
All three included trials reported this outcome and included a total of 360 participants in the meta‐analysis (Sigma Trial 2009; Gervaz 2010; Raue 2011). Although types of major complications were not uniform across studies, some types (i.e. complications requiring reoperation, intra‐abdominal/pelvic abscess) were common to all studies. On the basis of these components, we performed a meta‐analysis and found no evidence of differences between the two treatment groups (RR 0.74, 95% CI 0.43 to 1.25; I² = 0%; low quality evidence) (Figure 6; Analysis 2.6).
6.
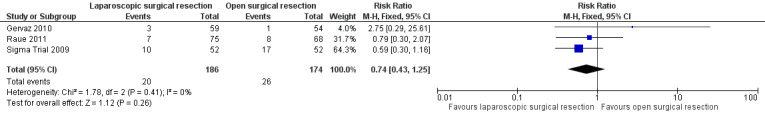
Forest plot of comparison: 2 Secondary outcomes, outcome: 2.6 Major complications.
2.6. Analysis.
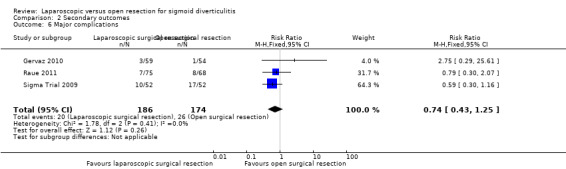
Comparison 2 Secondary outcomes, Outcome 6 Major complications.
2.2.6 Minor complications
All three included trials including a total of 360 participants reported this outcome (Sigma Trial 2009; Gervaz 2010; Raue 2011). Types of minor complications reported differed across trials, hence we did not pool trial data. However, we noted no evidence of differences between trial groups in terms of minor complications (thus judged low quality evidence; Table 5).
2.3 Operative time
All three included trials including a total of 360 participants in the meta‐analysis reported this outcome (Sigma Trial 2009; Gervaz 2010; Raue 2011). Low quality evidence shows that laparoscopic surgery can be associated with longer operative time compared with open surgery (MD 49.28 minutes, 95% CI 40.64 to 57.93; I² = 0%) (Analysis 2.7).
2.7. Analysis.
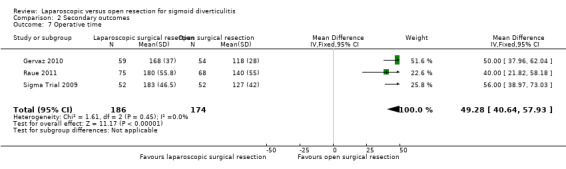
Comparison 2 Secondary outcomes, Outcome 7 Operative time.
2.4 Intraoperative blood loss
One trial comprising a total of 104 participants reported this outcome (Sigma Trial 2009). Intraoperative estimated blood loss was lower in the laparoscopic group than in the open surgery group, although data show no evidence of differences between groups (laparoscopic surgery: 100 mL, range 0 to 2000 mL; open surgery: 200 mL, range 10 to 2500 mL; MD ‐10.0 mL, 95% CI ‐32.1 to 12.1; very low quality evidence) (Analysis 2.8).
2.8. Analysis.
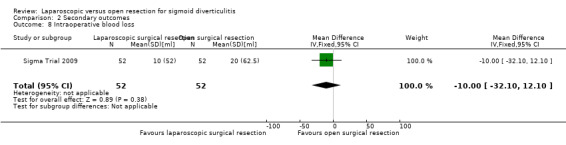
Comparison 2 Secondary outcomes, Outcome 8 Intraoperative blood loss.
2.5 Postoperative pain
All included trials including a total of 360 participants in the meta‐analysis reported this outcome (Sigma Trial 2009; Gervaz 2010; Raue 2011). All three trials used the same scale to measure postoperative pain (VAS scale 0 to 10 or 0 to 100); results were similar and showed an improvement of about 1 point. We performed a meta‐analysis on postoperative pain using data from two trials including a total of 250 participants at day 1, day 2, day 3, and day 4 (Gervaz 2010; Raue 2011). Sigma Trial 2009 reported that laparoscopic surgical resection was associated with less pain than open surgery, with an average difference of 1.6 points per day, as determined by VAS score (P = 0.003), but trial authors did not report mean values for both groups at baseline and post‐baseline assessment, hindering inclusion of this trial in the meta‐analysis. In this trial, the mean postoperative pain score based on the SF‐36 questionnaire was 61.5 in the laparoscopic group versus 62.0 in the open surgery group at baseline, whereas mean postoperative pain score at six weeks was 76.0 for the laparoscopic group and 64.0 for the open surgery group (P = 0.032) (data extracted from Figure 3 in Sigma Trial 2009). Similarly, in the Gervaz 2010 trial, the median VAS score for maximal pain was 4 (range 1 to 10) in the laparoscopic group and 5 (range 1 to 10) in the open surgery group (P = 0.05). In Raue 2011, median VAS score was 27 (range 1 to 99) in the laparoscopic group and 30 (range 1 to 96) in the open surgery group.
In the meta‐analysis including Gervaz 2010 and Raue 2011, low‐quality evidence shows that laparoscopic surgery may reduce pain at the fourth postoperative day (MD ‐0.65, 95% CI ‐1.04 to ‐0.25; I² = 0%; very low‐quality evidence) (Analysis 2.9).
2.9. Analysis.
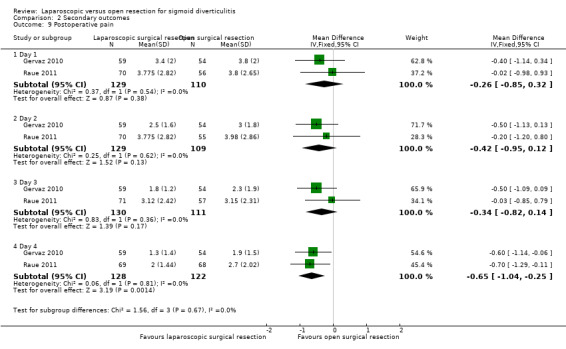
Comparison 2 Secondary outcomes, Outcome 9 Postoperative pain.
2.6 Postoperative time to liquid, solid diets, passing flatus, and bowel movement
Two trials including a total of 247 participants in both meta‐analyses reported time to liquid and solid diets (Sigma Trial 2009; Raue 2011). Results show no evidence of differences between intervention groups in terms of postoperative time to liquid diet (MD 0.00, 95% CI ‐0.28 to 0.28; I² = 0%; very low quality evidence) or solid diet (MD 0.00, 95% CI ‐0.42 to 0.42; I² = 0%; very low quality evidence) (Analysis 2.10; Analysis 2.11).
2.10. Analysis.
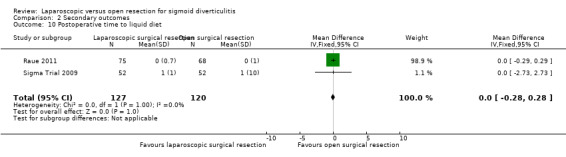
Comparison 2 Secondary outcomes, Outcome 10 Postoperative time to liquid diet.
2.11. Analysis.
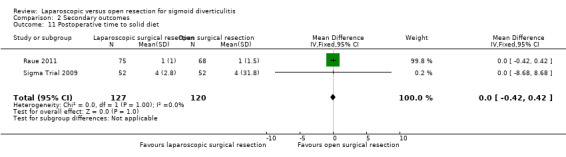
Comparison 2 Secondary outcomes, Outcome 11 Postoperative time to solid diet.
None of the included trials reported data on time to passing flatus or bowel movement.
2.7 Reoperation rate due to anastomotic leak
Three trials including a total of 349 participants in the meta‐analysis reported this outcome (Sigma Trial 2009; Gervaz 2010; Raue 2011). However, Gervaz 2010 did not contribute any events. We found no evidence of differences between intervention groups in reoperation rate due to anastomotic leak (3.9% in the laparoscopic group vs 5.3% in the open surgery group) (RR 0.75, 95% CI 0.29 to 1.95; I² = 0%, low‐quality evidence) (Analysis 2.12).
2.12. Analysis.
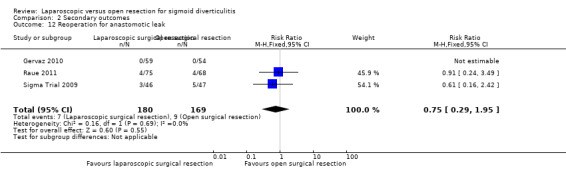
Comparison 2 Secondary outcomes, Outcome 12 Reoperation for anastomotic leak.
2.8 Other adverse outcomes: anastomotic stricture and small‐bowel obstruction
Only Sigma Trial 2009 including a total of 104 participants reported these outcomes. Available evidence of very low quality suggests that laparoscopic or open surgery may reduce the rate of anastomotic stricture (laparoscopic 1.9% vs open surgery 1.9%;
RR 1.00 (95% CI 0.06 to 15.57) P = 1.00) or small‐bowel obstruction (laparoscopic 1.9% vs open surgery 7.7%; RR 0.25 (95% CI 0.03 to 2.16)
P = 0.169) (Analysis 2.13). 1.00 [0.06, 15.57]
2.13. Analysis.
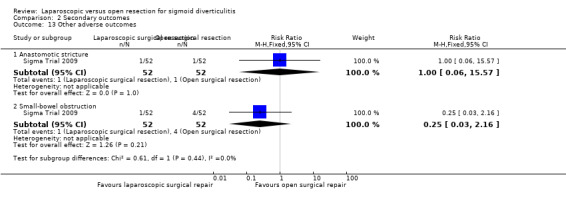
Comparison 2 Secondary outcomes, Outcome 13 Other adverse outcomes.
2.9 Quality of life
Included trials used different scales to report quality of life. Raue 2011 assessed global health status using the EORTC QLQ‐C30 v3 questionnaire, noting no significant differences between laparoscopic and open surgery groups at 7, 30, and 90 days, and 12 months postoperatively (each P > 0.05). Conversely, Sigma Trial 2009 analysed quality of life using the SF‐36 questionnaire six weeks after surgery and found that participants who underwent laparoscopic surgery scored significantly better than those who underwent open surgery in terms of role limitations due to physical health (PRF) (P = 0.039), role limitations due to emotional problems (ERF) (P = 0.024), social functioning (SF) (P = 0.015), and pain (PN) (P = 0.032). Gervaz 2010 used the Gastrointestinal Quality of Life Index score to measure quality of life, and reported that the median score was 115 in the open group versus 110 in the laparoscopic group (P = 0.17). We judged the quality of evidence as low.
2.10 Recurrent diverticulitis
One trial reported this outcome (Sigma Trial 2009). Data show no evidence of differences in the diverticulitis recurrence rate between laparoscopic (1.9%) and open surgery groups (3.8%) (P = 0.56). In Gervaz 2010, two participants (one in each group) developed recurrent diverticulitis treated with antibiotics. Therefore, this outcome was not subjected to meta‐analysis. We judged the quality of evidence to be very low.
Discussion
Summary of main results
Based on data from published literature, this review summarised evidence concerning the efficacy of laparoscopic surgery versus open surgery for patients with diverticular disease (see Table 1). Overall, we identified three randomised trials that evaluated a total of 360 participants (of 392 enrolled) with diverticulitis requiring surgical treatment. We rated the overall quality as low because of serious concern about risk of bias and imprecision. The main outcome was mean hospital stay; we can conclude that we found no evidence to support or refute the use of laparoscopic surgery versus open surgery to reduce mean hospital stay. We also evaluated several postoperative outcomes including surgical complications, mortality, postoperative pain, and operative time. Evidence of low quality shows that operative time was significantly longer in the laparoscopic group.
Evidence was insufficient to permit us to conclude that either treatment was associated with reduction in postoperative pain. Although at day 4, a significant reduction in postoperative pain occurred, it is difficult to recommend laparoscopic surgery ‐ not only because evidence is of low quality, but also because observed mean differences within groups and between groups have very limited clinical significance (Myles 2017).
We found insufficient evidence in favour of one of the interventions for the following outcomes: major complications, postoperative times to liquid and solid diets, and reoperation rate due to anastomotic leak. Researchers reported quality of life differently across trials, hindering the possibility of meta‐analysis. One trial used the Short Form (SF)‐36 questionnaire six weeks after surgery and reported results favouring laparoscopic intervention; however, the two trials that used the European Organization for Research and Treatment of Cancer core quality of life questionnaire (EORTC QLQ‐C30 v3) and the Gastrointestinal Quality of Life Index score, respectively, provided no evidence favouring either of the allocated treatments.
Data regarding secondary endpoints (i.e. early overall morbidity, late morbidity, minor complications, overall surgical complications, anastomotic stricture, enterocutaneous fistula, incisional hernia, intra‐abdominal abscess, small‐bowel obstruction, and recurrent diverticulitis after surgical resection) were limited and heterogeneous for meta‐analysis. However, revising the results reported by each of the primary studies revealed no statistically significant differences between groups.
Overall completeness and applicability of evidence
Despite our comprehensive search strategy, we identified only three studies for inclusion. These included trials provided different descriptions of inclusion criteria. However, common characteristics of participants in the three trials included pericolic inflammation with or without local abscess ‐ although Raue 2011 did not specify the number of episodes of symptomatic diverticulitis necessitating hospitalisation with intravenous antibiotics and 'nil per os' ‐ and previous recurrent attacks of acute diverticulitis with abscess, necessitating percutaneous computed tomography (CT)‐guided drainage. In addition, it is worth noting that Gervaz 2010 excluded patients with stenosis and bleeding.
In terms of applied techniques, two trials did not report laparoscopic approaches to colectomy (Sigma Trial 2009; Raue 2011). One trial did not report the level of vascular tie. The overall approach was similar across trials in terms of the number of trocars used and the abdominal site for exteriorisation of bowel transected.
Overall, applicability of review findings should be limited to stages II and III, according to Hansen 1999, or to stages Hinchey I and II.
Quality of the evidence
All included studies were randomised controlled trials, but we judged one trial as having unclear risk in terms of selection bias, as investigators did not provide a sufficient description regarding allocation concealment. The three trials could not avoid performance bias, given that the type of intervention provided could not allow blinding of participants and personnel, although some studies attempted a sort of blinding. Conversely, trials could avoid detection bias, but none of the included trials provided a clear description of blinding of the outcome assessor. Notwithstanding, we considered the main outcome (length of hospital stay) as an objective outcome, and we concluded that detection bias could not influence final results.
In addition to possible selection bias, we had serious concern about the risk of of attrition bias noted in two trials (Gervaz 2010; Raue 2011). We did not consider the presence of performance bias and potentially of detection bias when the outcome of interest was objective. Overall, we downgraded the quality of the evidence by two levels owing to risk of bias and imprecision because the result of the primary outcome included a large confidence interval. In the final analysis, we rated the overall quality of available evidence for this intervention as low.
The same concerns (risk of bias and imprecision) brought downgrading of evidence quality to low for the following outcomes: mean hospital stay, 30‐day postoperative mortality, major complications, and operative time for reoperation due to anastomotic leakage. We further downgraded the evidence for the remaining outcomes owing to additional serious concern regarding imprecision either because the sample size was small or the events were too few which determined a large confidence interval of the estimate of treatment effect.
Potential biases in the review process
Review authors performed a comprehensive search for trials of interventions across several major electronic databases. Two review authors independently screened titles and abstracts, assessed full texts of potentially relevant primary studies and reviews, and abstracted data. Review authors also screened reference lists of relevant systematic reviews and contacted trial authors to request further data. One review author performed analyses, and a second review author checked data for consistency.
Agreements and disagreements with other studies or reviews
Ignjatovic 2004 was the first systematic review on the treatment of individuals with sigmoid diverticulitis. Review authors presented inconclusive findings because the review included few studies and the quality of evidence was low.
In 2006, a meta‐analysis of non‐randomised studies comparing laparoscopic versus open surgery for the treatment of individuals with diverticular disease was published (Purkayastha 2006). Review authors included 12 studies published between 1996 and 2004, which analysed 19,608 participants (1192 who underwent laparoscopic surgery and 18,416 who underwent open surgery). Results favoured the laparoscopic approach, as data show significantly fewer infective (P = 0.01), pulmonary (P < 0.001), gastrointestinal tract (P = 0.03), and cardiovascular (P < 0.001) complications in the laparoscopic group than in the open group. Contrary to the findings of our review, length of hospital stay was significantly shorter (P < 0.0001) in the laparoscopic group. However, regarding operative time, meta‐analysis led to the same conclusion presented here ‐ that use of laparoscopic surgery extends operative time over that required for open surgery (odds ratio (OR) 67.59, 95 confidence interval (CI) 3.79 to 131.39; P = 0.04), albeit with significant heterogeneity (P < 0.001).
Siddiqui 2010 conducted a meta‐analysis to compare elective open versus laparoscopic sigmoid colectomy for diverticular disease, which included 19 studies and 2383 participants from the randomised trial Sigma Trial 2009 and from 10 prospective and eight retrospective studies. Review authors did not evaluate length of hospital stay but found that operative time was longer in the laparoscopic resection group than in the open surgery group (standardised mean difference (SMD) 1.94, 95% CI 1.14 to 2.74; P < 0.001). In addition, review authors reported significantly lower rates of wound infection (fixed‐effect risk ratio (RR) 0.54, 95% CI 0.36 to 0.80; 15 studies), blood transfusion (fixed‐effect RR 0.25, 95% CI 0.10 to 0.60; 4 studies), ileus (fixed‐effect RR 0.37, 95% CI 0.20 to 0.66; 8 studies), and incisional hernia (fixed‐effect RR 0.27, 95% CI 0.12 to 0.64; 7 studies) in the laparoscopic group than in the open surgery group. They did not find significant between‐group differences for the other outcomes analysed: anastomotic leak and stricture, bowel perforation and enterotomy, intra‐abdominal bleeding and abscess formation, small‐bowel obstruction, wound dehiscence, myocardial infarction, pneumonia, pulmonary embolus, urinary tract infection, need for rehospitalisation, and need for reoperation.
The Cirocchi 2012a meta‐analysis included one randomised trial ‐ Sigma Trial 2009 ‐ and 11 non‐randomised studies, of which three were prospective (437 participants) and eight retrospective studies (993 participants). The quality score for these studies, assessed on the modified Newcastle‐Ottawa Scale (Wells 2000), ranged from 3 to 7 out of 10. Non‐randomised studies did not assess mean hospital stay, operative time, and postoperative pain. Overall, postoperative mortality was low and did not differ significantly between laparoscopic and open surgery groups (2/530 vs 4/736; odds ratio (OR) 1.15, 95% CI 0.24 to 5.57; 10 studies). Although major complication rates were comparable between groups (9.5% vs 13.5%; OR 0.73, 95% CI 0.34 to 1.54; 10 studies; I² = 66%), contrary to our review, rates of overall morbidity (17% vs 27%; OR 0.46, 95% CI 0.25 to 0.84; 11 studies; I² = 74%) and minor complications (9% vs 18%; OR 0.37, 95% CI 0.18 to 0.78; nine studies; I² = 55%) were lower in the laparoscopic group than in the open group.
Reported advantages favouring laparoscopic surgery across the above cited reviews or meta‐analyses were derived mainly from non‐randomised studies. These studies include retrospective or prospective cohort studies, case‐control studies, and controlled clinical trials. Combining these studies resulted in an adequate sample size; however, these studies were highly exposed to selection bias.
The Gaertner 2013 review assessed nine studies, three of which were included in our review and involved 931 participants (Sigma Trial 2009; Gervaz 2010; Raue 2011). The Gaertner review did not perform meta‐analysis and concluded that laparoscopic colectomy for diverticular disease is associated with decreased postoperative morbidity and shorter hospital stay.
Authors' conclusions
Implications for practice.
Trial authors have presented uncertainty as to whether laparoscopic surgery provides any substantial advantages over open surgery with regard to surgical management of acute diverticular disease.
Implications for research.
This systematic review emphasises the need for results from well‐designed, adequately powered trials before conclusions can be drawn on the superiority of laparoscopic surgery over open surgical intervention. These trials will need to include adequate blinding of outcome assessors and will have to avoid post‐randomisation exclusions, so intention‐to‐treat analysis can be performed. When evidence confirms that laparoscopic surgery is longer than open surgery, the benefit of replacing laparoscopic surgery for open surgery will need to be demonstrated in future studies, and costs considered.
What's new
| Date | Event | Description |
|---|---|---|
| 27 November 2017 | Amended | Contact details updated. |
Acknowledgements
We thank Sara Hallum and Henning Keinke Andersen for revising the review and providing relevant suggestions that improved the review. We thank very much Sys Johnsen and Sara Hallum for performing and updating the search strategies.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Central Register of Controlled Trials (CENTRAL) (2017, Issue 2) #1 MeSH descriptor: [Diverticulitis] explode all trees #2 MeSH descriptor: [Diverticulitis, Colonic] explode all trees #3 diverticulit* #4 (#1 or #2 or #3) #5 MeSH descriptor: [Colorectal Surgery] explode all trees #6 surger* #7 MeSH descriptor: [Laparoscopy] explode all trees #8 laparoscop* #9 (#5 or #6 or #7 or #8) #10 (#4 and #9)
Appendix 2. MEDLINE search strategy
Ovid MEDLINE 1950 to 23.02.2017 1. exp Diverticulitis, Colonic/ or exp Diverticulitis/ 2. diverticulit*.mp. 3. 1 or 2 4. exp Colorectal Surgery/ 5. surger*.mp. 6. exp Laparoscopy/ 7. laparoscop*.mp. 8. 4 or 5 or 6 or 7 9. 3 and 8 10. randomized controlled trial.pt. 11. controlled clinical trial.pt. 12. randomized.ab. 13. placebo.ab. 14. clinical trial.sh. 15. randomly.ab. 16. trial.ti. 17. 10 or 11 or 12 or 13 or 14 or 15 or 16 18. humans.sh. 19. 17 and 18 20. 9 and 19
Appendix 3. Embase search strategy
Ovid Embase 1974 to 23.02.2017 1. exp colon diverticulosis/ or exp diverticulitis/ 2. diverticulit*.mp. 3. 1 or 2 4. exp colorectal surgery/ 5. surger*.mp. 6. exp Laparoscopy/ 7. laparoscop*.mp. 8. 4 or 5 or 6 or 7 9. 3 and 8 10. CROSSOVER PROCEDURE.sh.
11. DOUBLE‐BLIND PROCEDURE.sh.
12. SINGLE‐BLIND PROCEDURE.sh. 13. (crossover* or cross over*).ti,ab. 14. placebo*.ti,ab. 15. (doubl* adj blind*).ti,ab. 16. allocat*.ti,ab. 17. trial.ti. 18. RANDOMIZED CONTROLLED TRIAL.sh. 19. random*.ti,ab. 20. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.) 22. 20 not 21 23. 9 and 22
Appendix 4. Clinicaltrials.gov search strategy
randomised trial AND diverticulitis AND laparoscopic
Appendix 5. WHO International Clinical Trials Registry search strategy
randomised trial AND diverticulitis AND laparoscopic
Appendix 6. Criteria for judging risk of bias in the 'Risk of bias' assessment tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. | |
| Criteria for a judgement of ‘Low risk’ of bias | The investigators describe a random component in the sequence generation process such as:
*Minimising may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for a judgement of ‘High risk’ of bias | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: 1. sequence generated by odd or even date of birth; 2. sequence generated by some rule based on date (or day) of admission; 3. sequence generated by some rule based on hospital or clinic record number. 4. other non‐random approaches happening much less frequently than the systematic approaches mentioned above and tending to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example: 1. allocation by judgement of the clinician; 2. allocation by preference of the participant; 3. allocation based on the results of a laboratory test or a series of tests; and 4. allocation by availability of the intervention. |
| Criteria for a judgement of ‘Unclear risk’ of bias | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment | |
| Criteria for a judgement of ‘Low risk’ of bias | Participants and investigators enrolling participants could not foresee assignment because 1 of the following, or an equivalent method, was used to conceal allocation. 1. Central allocation (including telephone, Web‐based, and pharmacy‐controlled randomisation). 2. Sequentially numbered drug containers of identical appearance. 3. Sequentially numbered, opaque, sealed envelopes. |
| Criteria for a judgement of ‘High risk’ of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: 1. use if an open random allocation schedule (e.g. a list of random numbers); 2. use of assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); 3. alternation or rotation; 4. date of birth; 5. case record number; or 6. any other explicitly unconcealed procedure. |
| Criteria for a judgement of ‘Unclear risk’ of bias | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. This is usually the case if the method of concealment is not described or is not described in sufficient detail to allow a definitive judgement – for example, if use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque, and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of allocated interventions by participants and personnel during the study | |
| Criteria for a judgement of ‘Low risk’ of bias | Any 1 of the following. 1. No blinding or incomplete blinding, but review authors judge that the outcome is not likely to be influenced by lack of blinding. 2. Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Criteria for a judgement of ‘High risk’ of bias | Any 1 of the following. 1. No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding. 2. Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
| Criteria for a judgement of ‘Unclear risk’ of bias | Any 1 of the following. 1. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. 2. The study did not address this outcome. |
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of allocated interventions by outcome assessors | |
| Criteria for a judgement of ‘Low risk’ of bias | Any 1 of the following. 1. No blinding of outcome assessment, but review authors judge that the outcome measurement is not likely to be influenced by lack of blinding. 2. Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Criteria for a judgement of ‘High risk’ of bias | Any 1 of the following. 1. No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding. 2. Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
| Criteria for a judgement of ‘Unclear risk’ of bias | Any 1 of the following. 1. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. 2. The study did not address this outcome. |
|
INCOMPLETE OUTCOME DATA Attrition bias due to quantity, nature, or handling of incomplete outcome data | |
| Criteria for a judgement of ‘Low risk’ of bias | Any 1 of the following. 1. No missing outcome data. 2. Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias). 3. Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. 4. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. 5. For continuous outcome data, plausible effect size (differences in means or standardised differences in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size. 6. Missing data imputed using appropriate methods. |
| Criteria for a judgement of ‘High risk’ of bias | Any 1 of the following: 1. Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. 2. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate. 3. For continuous outcome data, plausible effect size (differences in means or standardised differences in means) among missing outcomes enough to induce clinically relevant bias in observed effect size. 4. ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation. 5. Potentially inappropriate application of simple imputation. |
| Criteria for a judgement of ‘Unclear risk’ of bias | Any 1 of the following. 1. Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ (e.g. number randomised not stated, no reasons for missing data provided). 2. The study did not address this outcome. |
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting | |
| Criteria for a judgement of ‘Low risk’ of bias | Any 1 of the following. 1. The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. 2. The study protocol is not available but it is clear that published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| Criteria for a judgement of ‘High risk’ of bias | Any 1 of the following. 1. Not all of the study’s prespecified primary outcomes have been reported. 2. One or more primary outcomes is reported by measurements, analysis methods, or subsets of data (e.g. subscales) that were not prespecified. 3. One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect). 4. One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. 5. The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Criteria for a judgement of ‘Unclear risk’ of bias | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. It is likely that most studies will fall into this category. |
|
OTHER BIAS Bias due to problems not covered elsewhere in the table | |
| Criteria for a judgement of ‘Low risk’ of bias | The study appears to be free of other sources of bias. |
| Criteria for a judgement of ‘High risk’ of bias | There is at least 1 important risk of bias. For example, the study: 1. had a potential source of bias related to the specific study design used; 2. has been claimed to have been fraudulent; or 3. had some other problem. |
| Criteria for a judgement of ‘Unclear risk’ of bias | There may be risk of bias, but either: 1. information is insufficient for assessment of whether an important risk of bias exists; or 2. rationale or evidence that an identified problem will introduce bias is insufficient. |
Data and analyses
Comparison 1. Primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean hospital stay | 3 | 360 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐2.49, 1.25] |
Comparison 2. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 30‐Day postoperative mortality | 3 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.07] |
| 2 Surgical complications | 3 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.60, 1.19] |
| 3 Early overall morbidity | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.51, 4.20] |
| 4 Late overall morbidity | 1 | 93 | Risk Ratio (IV, Fixed, 95% CI) | 0.60 [0.26, 1.38] |
| 5 Late overall mortality | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Major complications | 3 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.43, 1.25] |
| 7 Operative time | 3 | 360 | Mean Difference (IV, Fixed, 95% CI) | 49.28 [40.64, 57.93] |
| 8 Intraoperative blood loss | 1 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐32.10, 12.10] |
| 9 Postoperative pain | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Day 1 | 2 | 239 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.85, 0.32] |
| 9.2 Day 2 | 2 | 238 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.95, 0.12] |
| 9.3 Day 3 | 2 | 241 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.82, 0.14] |
| 9.4 Day 4 | 2 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.04, ‐0.25] |
| 10 Postoperative time to liquid diet | 2 | 247 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 11 Postoperative time to solid diet | 2 | 247 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.42, 0.42] |
| 12 Reoperation for anastomotic leak | 3 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.29, 1.95] |
| 13 Other adverse outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Anastomotic stricture | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.57] |
| 13.2 Small‐bowel obstruction | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
2.5. Analysis.
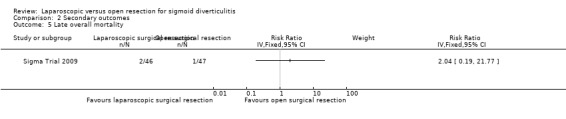
Comparison 2 Secondary outcomes, Outcome 5 Late overall mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gervaz 2010.
| Methods | Randomised controlled trial Single centre (University Hospital of Geneva, Switzerland) |
|
| Participants | Enrollment occurred between 2005 and February 2009. A total of 132 participants were randomised: 66 to the LSR group and 66 to the OSR group. Only 113 of the 132 randomised participants were operated on: 59 participants (median age 62.5, range 38 to 84) underwent LSR surgery and 54 (median age 57, range 29 to 82 years) underwent OSR surgery. 53% of these were female. Inclusion criteria: Participants between 18 and 85 years with diverticular disease defined as follows: "Diverticular disease of sigmoid colon documented by colonoscopy and 2 episodes of uncomplicated diverticulitis, 1 at least being documented with CT scan or 1 episode of complicated diverticulitis, with a pericolic abscess (Hinchey stage I) or pelvic abscess (Hinchey stage II) requiring percutaneous drainage." Exclusion criteria:"Associated colon cancer or any condition requiring extended colectomy, BMI 40, emergency procedure, use of opiates and/or analgesics within 48 h preceding the surgical procedure, any cognitive impairment (psychiatric disorder, Alzheimer’s disease . . .)." |
|
| Interventions | Laparoscopic sigmoid colectomy:"A 5‐trocar technique was used with the patient in a modified Trendelenburg position. Under direct vision, a 12‐mm optic trocar was inserted above the umbilicus, two 5‐mm trocars were placed in the left and right paraumbilical areas; the operating 10‐mm trocar was located in the right iliac fossa; a fifth trocar was positioned in the suprapubic midline, and this incision was extended (6–7 cm) for specimen extraction. The dissection began by identification, then coagulation with LigaSure of the trunk of the sigmoid arteries after visualization of the left ureter. The mesenteric attachments were freed widely, and the parietal peritoneum was divided up to the splenic flexure. Complete mobilization of the splenic flexure was optional and left to the discretion of the surgeon, but was required in most cases to create a tension‐free colorectal anastomosis. The rectosigmoid junction was identified, and the upper rectum was divided with the 45‐mm endoGIA stapler (Ethicon EndoSurgery, Spreitenbach, Switzerland). The sigmoid colon was extracted through the mini‐Pfannenstiel incision and specimen resection was completed extracorporeally. The anvil of the circular stapling device was secured in the left colon with a purse‐string suture, the colon was reintroduced within the peritoneal cavity, pneumoperitoneum was re‐established, and a side‐to‐end or end‐to‐end colorectal anastomosis was performed after transanal insertion of the 29‐mm circular stapler." Open sigmoid colectomy:"The peritoneal cavity was entered through a midline incision, which was extended above the umbilicus. Left and sigmoid colon were freed from their peritoneal attachments, and the left ureter was identified. The rectosigmoid junction was identified and the upper rectum was divided using a linear 60‐mm stapler after clearing off the mesorectum fatty tissue. The proximal dissection was completed with transection at the junction of the descending and sigmoid colon using a linear 75‐mm stapler. After insertion of the anvil of the 29‐mm circular stapler in the proximal colon, a double‐stapled colorectal anastomosis was performed, and the abdomen was closed in a standard manner." | |
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Funding sources. Any declaration of interest | Pascal Gervaz and Ihsan Inan were employed as consultants in 2008 and 2009 and have received honoraria from Covidien (formerly Tyco Healthcare) for a total sum of 4000 Euros each per year. | |
| Trial registry entries | The protocol was approved by the research ethics committee at Geneva University Hospitals, and was registered at ClinicalTrials.gov (Identifier NCT00453830). | |
| Notes | This trial reports long‐term follow‐up results for participants enrolled in the Gervaz 2010 RCT included in the present systematic review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence: "On the day before surgery, patients were admitted to hospital, and group allocation was determined by a computer‐generated random sequence." |
| Allocation concealment (selection bias) | Low risk | The randomised generated sequence was kept concealed and was shared with the investigator by an independent statistician via a telephone communication: "The random sequence, which was kept concealed, was communicated to the investigator by an independent statistician during a telephone communication." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given the type of intervention provided, it was not possible to blind investigators. However, investigators attempted to blind participants: "At the end of the procedure, a sterile, opaque dressing was applied over the lower abdomen, and left in place for 4 days, to conceal the type of incision. In addition, no precision regarding the surgical access route was made in all medical documents, as well as nursing Kardex. The patient, his family, and the nursing staff only knew that a sigmoid resection was performed until dressing removal on postoperative day 4." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Nineteen (14%) randomised participants were excluded after randomisation: "In 2 patients, a diverting colostomy and a Hartmann procedure were performed because of unexpected deep pelvic abscesses. The following four protocol violations occurred: 1 patient had an epidural catheter placed by another anaesthetist, and in 3 patients, blinding was not respected because of placement of an inadequate wound dressing; 11 patients withdrew consent the evening before the procedure; 1 patient had an open foramen ovale, which was considered a contraindication for laparoscopy; finally, 1 patient was diagnosed with ovarian cancer at the time of laparotomy." |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. The protocol of the trial was registered in a public registry: ClinicalTrials.gov Identifier NCT00453830 (Gervaz 2009). |
Raue 2011.
| Methods | Randomised controlled trial Multi‐centric (12 centres), Germany |
|
| Participants | Participants were enrolled between February 2005 and June 2008. A total of 156 participants were randomised: 77 in the LRS group and 79 in the OSR group. Only 75 participants underwent LSR (median age 64, range 27 to 85) vs 68 participants who underwent OSR (median age 68, range 38 to 84). 66% of these participants were female. Inclusion criteria: participants with a proven stage II/III disease (stage II: pericolic inflammation with or without local abscess; stage III: recurrent disease with stenosis, fistula, or bleeding) according to the classification of Stock and Hansen (Hansen 1999) Exclusion criteria: urgent surgery; fistulas to adjunct organs; body mass index > 34 kg/m²; manifest coagulopathy |
|
| Interventions | Study interventions:"Left colonic flexure was only mobilized if needed. The distal resection margin was the upper rectum below any visible taenia coli. The proximal resection margin was placed in the non‐inflamed descending colon. The unfixed specimen should measure at least 25 cm. Anastomosis was created using a circular stapler or a biofragmentable ring. OSR was performed via a midline or a traverse laparotomy. LSR was performed in a 4–5 trocar technique. The specimen was removed via a suprapubic or a left‐sided traverse incision. If conversion was necessary, LSR was converted to OSR." | |
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Funding sources. Any declaration of interest | This trial was supported by the Charité–University Medicine Berlin. No funding was received from other organisations. | |
| Trial registry entries | The trial was registered with ISRTCN: Identification number 55776829. | |
| Notes | Recurrent severe violations of the protocol were discovered at only 1 participating centre during recruitment and analysis (defiance of the randomisation result). All patients from this hospital (6 randomised patients and 17 patients operated on according to their preferred technique) were excluded from further analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The random sequence generation was not clearly reported. "The randomisation was performed using the method of minimisation and stratifying for participating clinic, operating surgeon, sex, age (< 50, 50 to 75, and > 75 years), body mass index (< 26 and ≥ 26 kg/m2), and stage of disease (stage II, stage III)." |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not clearly reported. Allocation of participants was performed centrally by telephone calls (as reported by the corresponding author). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given the type of intervention provided, it was not possible to blind investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two participants in the laparoscopic group and 11 in the open group did not receive the allocated treatment, and study authors did not clearly state the reasons. After treatment, 2 participants in the laparoscopic group and 4 in the open group were excluded from the analysis, but the reasons for this are not stated by investigators. Finally, 2 participants in the open group were lost (died) to follow‐up and were not analysed for the outcomes of interest. According to the intention‐to‐treat principle, converted cases were analysed in the laparoscopic group. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way (Schwenk 2004). |
Sigma Trial 2009.
| Methods | Randomised controlled trial Multi‐centric (5 centres), Netherlands |
|
| Participants | Participants were enrolled between February 2002 and December 2006. A total of 104 participants were randomised: 52 participants underwent LSR (median age 62, range 32 to 85) and 52 participants underwent OSR (median age 63, range 30 to 84). 57% of participants were female. Inclusion criteria:"Symptomatic diverticulitis of the sigmoid colon: previous 2 or more recurrent attacks of acute diverticulitis with (Hinchey I) or without pericolic abscess necessitating hospitalization with intravenous antibiotics and nil per os; previous recurrent attacks of acute diverticulitis with percutaneously drainable distant abscess necessitating CT‐guided drainage (Hinchey IIa); presence of internal fistula between the sigmoid colon and a hollow organ with abscess (Hinchey IIb) or without; presence of symptomatic stricture of the sigmoid colon with no evidence of cancer; recurrent severe diverticular bleeding requiring blood transfusions verified at colonoscopy and/or arteriogram. Surgery was performed at least 3 months after the last attack of diverticulitis." Exclusion criteria:"failure to sign informed consent, previous colorectal resectional surgery, previous laparotomy other than for gynaecologic or obstetrical surgery, and perforated diverticulitis with peritonitis (Hinchey III or IV)" |
|
| Interventions |
Laparoscopic sigmoid colectomy:"LSR was performed through 4 or 5 ports. The splenic flexure was mobilized if needed. The sigmoid colon was mobilized. The left ureter was identified. The sigmoid vessels were divided. The rectosigmoid junction was identified by the ab sence of taenia coli and transected at the level of the promontory using a laparoscopic stapler. The oral end of the transacted bowel was exteriorized through a suprapubic incision. The proximal resection margin was placed on supple, normal appearing descending colon with no signs of inflammation or induration of the mesentery and serosal surface. The specimen was retrieved and a purse string suture was fashioned at the oral bowel end. An intracorporeal double‐stapled anastomosis was created after closure of the suprapubic wound and reestablishment of the pneumoperitoneum. Care was taken to achieve a truly tension‐free anastomosis. If conversion was necessary, LSR was converted to a hand‐assisted LSR or to OSR. The hand‐port device was placed at the suprapubic incision site. The left hand of the surgeon was introduced through the hand‐port and assisted in improving exposure of the surgical field. All wounds were closed layer by layer." Open sigmoid colectomy:"OSR was carried out through a midline laparotomy. The splenic flexure was mobilized if needed. The sigmoid colon was mobilized. The left ureter was identified. The sigmoid vessels were divided. The rectosigmoid junction was identified by the absence of taenia coli and transected at the level of the promontory with a stapler. The proximal resection margin was placed on supple, normal appearing descending colon with no signs of inflammation or induration of the mesentery and serosal surface. The specimen was retrieved and a purse string suture was fashioned at the oral bowel end. A double‐stapled anastomosis was performed. Care was taken to achieve a truly tension‐free anastomosis. The midline wound was closed layer by layer." |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Funding sources. Any declaration of interest | Not reported | |
| Trial registry entries | The trial was registered in Current Controlled Trials (ISRCTN 43911188) and Nederlands Trial Register (NTR 928); the protocol was published in BMC Surgery (Klarenbeek 2007). | |
| Notes | Economic outcome assessment, postoperative pain score, and postoperative general health status were performed in only 57 patients recruited at the Department of Surgery of VU University Medical Center, Amsterdam. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation process. Participating institutions enrolled eligible participants by logging on to a secure website that provided an automated assignment and randomisation number for each participant (www.sigmatrial.nl). |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by assigning identity numbers to enrolled participants and by separating the generator of the allocation from the executor. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given the type of intervention provided, it was not possible to blind investigators. However, investigators attempted to blind participants: "Participants and hospital staff, including physicians in charge of participant discharge, were blinded. Blinding was provided by the of use identical opaque dressings covering the entire abdomen." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly stated in the manuscript whether outcome assessors were or were not blinded to treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant did not complete follow‐up because of death from myocardial infarction. According to the intention‐to‐treat principle, converted cases were analysed in the laparoscopic group. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available, and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way (Klarenbeek 2007). However, results for postoperative pain were not adequately reported: Study authors reported differences (daily on average VAS 1.6 point less) without reporting mean values for both groups at baseline and post baseline, without providing the point in time at which the post‐baseline value was estimated. |
BMI: body mass index.
CT: computed tomography.
LSR: laparoscopic surgical resection.
OSR: open surgical resection.
RCT: randomised controlled trial.
VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alves 2005 | Prospective multi‐centre CCT: 163 LSR vs 169 OSR. Individuals undergoing elective surgery for sigmoid diverticular disease |
| Anania 2014 | Observational study in which 33 participants underwent elective LSR for sigmoid diverticulitis |
| Angenete 2015 | Randomised trial in which laparoscopic lavage ‐ not laparoscopic resection ‐ was compared with open surgery |
| Angenete 2016 | Randomised trial in which laparoscopic lavage ‐ not laparoscopic resection ‐ was compared with open surgery |
| Bruce 1996 | Observational study of 25 LSR vs 17 OSR for individuals undergoing elective surgery for sigmoid diverticulitis |
| De Chaisemartin 2003 | Observational study of 29 LSR vs 32 OSR for individuals undergoing elective surgery for sigmoid diverticular disease |
| Dwivedi 2002 | Observational study of 66 LSR vs 88 OSR for individuals with 2 or more documented episodes of diverticulitis |
| Kohler 1998 | Prospective CCT of 25 LSR vs 34 OSR for all individuals with colonic diverticulitis who were electively treated with surgery |
| Lawrence 2003 | Observational study of 56 LSR vs 215 OSR for individuals undergoing elective surgery for sigmoid diverticulitis |
| Massomi 2011 | Retrospective analysis of the National Inpatient Sample database; 124,734 individuals underwent elective surgery for diverticulitis: 110,172 (88.3%) LSR vs 14,562 (11.7%) OSR |
| Neri 2006 | Observational study of 5 LSR vs 4 OSR for individuals admitted for a second time for acute and uncomplicated diverticulitis |
| Senagore 2002 | Observational study of 61 LSR vs 71 OSR for individuals undergoing elective resection of the sigmoid colon for diverticular disease |
| Simon 2005 | Observational study of 40 LSR vs 126 OSR for individuals undergoing elective surgery for sigmoid uncomplicated and complicated diverticulitis |
| Thaler 2003 | Observational study of 79 LSR vs 79 OSR for individuals undergoing elective surgery for sigmoid diverticular disease |
| Tuech 2000 | Prospective study of 22 LSR vs 24 OSR for individuals older than 75 years undergoing elective colectomy for diverticulitis |
CCT: controlled clinical trial. LSR: laparoscopic surgical resection. OSR: open surgical resection.
Differences between protocol and review
In the protocol for this review, the main outcome was 30‐day postoperative mortality (Cirocchi 2011). In our era, the outcome of mortality in populations with diverticulitis is not a critical outcome. Consequently, we considered it as a secondary outcome. Instead, we examined mean hospital stay as a primary outcome. We have also added several new secondary outcomes: postoperative pain, quality of life, anastomotic leak, anastomotic stricture, incisional hernia, enterocutaneous fistula, intra‐abdominal abscess, and small‐bowel obstruction.
Contributions of authors
Co‐ordinating and drafting the review: Iosief Abraha, Roberto Cirocchi.
Collecting data for the review: Iosief Abraha, Roberto Cirocchi, Alessandro Montedori.
Managing data for the review: Iosief Abraha, Alberto Arezzo, Gian Luigi Binda, Roberto Cirocchi, Alessandro Montedori.
Analysing data: Iosief Abraha, Roberto Cirocchi.
Interpreting data: Iosief Abraha, Alberto Arezzo, Gian Luigi Binda, Roberto Cirocchi, Alessandro Montedori.
Writing the review: Iosief Abraha, Alberto Arezzo, Gian Luigi Binda, Roberto Cirocchi, Alessandro Montedori.
Providing general advice on the review: Alberto Arezzo, Gian Luigi Binda, Roberto Cirocchi.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Gervaz 2010 {published data only}
- Gervaz P, Inan I, Perneger T, Schiffer E, Morel P. A prospective, randomized, single‐blind comparison of laparoscopic versus open sigmoid colectomy for diverticulitis. Annals of Surgery 2010;252:3‐8. [DOI] [PubMed] [Google Scholar]
- Gervaz P, Mugnier‐Konrad B, Morel P, Huber O, Inan I. Laparoscopic versus open sigmoid resection for diverticulitis: long‐term results of a prospective, randomized trial. Surgical Endoscopy 2011;25:3373–8. [DOI] [PubMed] [Google Scholar]
Raue 2011 {published data only}
- Raue W, Paolucci V, Asperger W, Albrecht R, Büchler MW, Schwenk W, et al. Laparoscopic sigmoid resection for diverticular disease has no advantages over open approach: midterm results of a randomized controlled trial. Langenbeck's Archives of Surgery 2011;396:973‐80. [DOI] [PubMed] [Google Scholar]
Sigma Trial 2009 {published data only}
- Klarenbeek BR, Bergamaschi R, Veenhof AA, Peet DL, Broek WT, Lange ES, et al. Laparoscopic versus open sigmoid resection for diverticular disease: follow‐up assessment of the randomized control Sigma trial. Surgical Endoscopy 2011;25:1121‐6. [DOI] [PubMed] [Google Scholar]
- Klarenbeek BR, Coupé VM, Peet DL, Cuesta MA. The cost effectiveness of elective laparoscopic sigmoid resection for symptomatic diverticular disease: financial outcome of the randomized control Sigma trial. Surgical Endoscopy 2011;25:776‐83. [DOI] [PubMed] [Google Scholar]
- Klarenbeek BR, Veenhof AA, Bergamaschi R, Peet DL, Broek WT, Lange ES, et al. Laparoscopic sigmoid resection for diverticulitis decreases major morbidity rates: a randomized control trial. Short‐term results of the Sigma trial. Annals of Surgery 2009;249:39‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alves 2005 {published data only}
- Alves A, Panis Y, Slim K, Heyd B, Kwiatkowski F, Mantion G, et al. French multicentre prospective observational study of laparoscopic versus open colectomy for sigmoid diverticular disease. British Journal of Surgery 2005;92:1520–5. [DOI] [PubMed] [Google Scholar]
Anania 2014 {published data only}
- Anania G, Vedana L, Santini M, Scagliarini L, Giaccari S, Resta G, et al. Complications of diverticular disease: surgical laparoscopic treatment. Il Giornale di Chirurgia 2014;35:126‐8. [PMC free article] [PubMed] [Google Scholar]
Angenete 2015 {published data only}
- Angenete E, Thornell A, Burcharth J, Pommergaard HC, Skullman S, Bisgaard T, et al. Laparoscopic lavage is feasible and safe for the treatment of perforated diverticulitis with purulent peritonitis: the first results from the randomized controlled trial DILALA. Annals of Surgery 2015;263:117‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Angenete 2016 {published data only}
- Angenete E, Thornell A, Burcharth J, Pommergaard HC, Skullman S, Bisgaard T, et al. Laparoscopic lavage is feasible and safe for the treatment of perforated diverticulitis with purulent peritonitis: the first results from the randomized controlled trial DILALA. Annals of Surgery 2016;263(1):117‐22. [PUBMED: 25489672] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruce 1996 {published data only}
- Bruce CJ, Coller JA, Murray JJ, Schoetz DJ Jr, Roberts PL, Rusin LC. Laparoscopic resection for diverticular disease. Diseases of the Colon and Rectum 1996;39(10 Suppl):S1‐6. [DOI] [PubMed] [Google Scholar]
De Chaisemartin 2003 {published data only}
- Chaisemartin C, Panis Y, Mognol P, Valleur P. Laparoscopic sigmoid resection for diverticulitis: is learning phase associated with increased morbidity?. Annales de Chirurgie 2003;128:81‐7. [DOI] [PubMed] [Google Scholar]
Dwivedi 2002 {published data only}
- Dwivedi A, Chahin F, Agrawal S, Chau WY, Tootla A, Tootla F, et al. Laparoscopic colectomy vs open colectomy for sigmoid diverticular disease. Diseases of the Colon and Rectum 2002;45:1309‐14. [DOI] [PubMed] [Google Scholar]
Kohler 1998 {published data only}
- Kohler L, Rixen D, Troidl H. Laparoscopic colorectal resection for diverticulitis. International Journal of Colorectal Disease 1998;13:43‐7. [DOI] [PubMed] [Google Scholar]
Lawrence 2003 {published data only}
- Lawrence DM, Pasquale MD, Wasser TE. Laparoscopic versus open sigmoid colectomy for diverticulitis. American Surgeon 2003;69:499‐503. [PubMed] [Google Scholar]
Massomi 2011 {published data only}
- Masoomi H, Buchberg B, Nguyen B, Tung V, Stamos MJ, Mills S. Outcomes of laparoscopic versus open colectomy in elective surgery for diverticulitis. World Journal of Surgery 2011;39:2143‐8. [DOI] [PubMed] [Google Scholar]
Neri 2006 {published data only}
- Neri V, Ambrosi A, Lauro G, Valentino TP. Elective laparoscopic‐assisted colectomy for sigmoid diverticulitis. Journal of the Society of Laparoendoscopic Surgeons 2006;10:66‐9. [PMC free article] [PubMed] [Google Scholar]
Senagore 2002 {published data only}
- Senagore AJ, Duepree HJ, Delaney CP, Dissanaike S, Brady KM, Fazio VW. Cost structure of laparoscopic and open sigmoid colectomy for diverticular disease: similarities and differences. Diseases of the Colon and Rectum 2002;45:485‐90. [DOI] [PubMed] [Google Scholar]
Simon 2005 {published data only}
- Simon T, Orangio GR, Ambroze WL, Armstrong DN, Schertzer ME, Choat D, et al. Factors associated with complications of open versus laparoscopic sigmoid resection for diverticulitis. Journal of the Society of Laparoendoscopic Surgeons 2005;9:63‐7. [PMC free article] [PubMed] [Google Scholar]
Thaler 2003 {published data only}
- Thaler K, Weiss EG, Nogueras JJ, Arnaud JP, Wexner SD, Bergamaschi R. Recurrence rates at minimum 5‐year follow‐up: laparoscopic versus open sigmoid resection for uncomplicated diverticulitis. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2003;13:325‐7. [DOI] [PubMed] [Google Scholar]
Tuech 2000 {published data only}
- Tuech JJ, Pessaux P, Rouge C, Regenet N, Bergamaschi R, Arnaud JP. Laparoscopic vs open colectomy for sigmoid diverticulitis: a prospective comparative study in the elderly. Surgical Endoscopy 2000;14:1031‐3. [DOI] [PubMed] [Google Scholar]
Additional references
Abraha 2015
- Abraha I, Cherubini A, Cozzolino F, Florio R, Luchetta ML, Rimland JM, et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta‐epidemiological study. BMJ 2015;350:h2445. [PUBMED: 26016488] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abraha 2016
- Abraha I, Rimland JM, Trotta F, Pierini V, Cruz‐Jentoft A, Soiza R, et al. Non‐pharmacological interventions to prevent or treat delirium in older patients: clinical practice recommendations: the SENATOR‐ONTOP series. Journal of Nutrition, Health & Aging 2016;20(9):927‐36. [PUBMED: 27791223] [DOI] [PubMed] [Google Scholar]
Abraha 2017
- Abraha I, Cozzolino F, Orso M, Marchesi M, Germani A, Lombardo G, et al. A systematic review found that deviations from intention‐to‐treat are common in randomized trials and systematic reviews. Journal of Clinical Epidemiology 2017;84:37‐46. [PUBMED: 28088592] [DOI] [PubMed] [Google Scholar]
Abraham 2007
- Abraham NS, Byrne CM, Young JM, Solomon MJ. Meta‐analysis of non‐randomized comparative studies of the short‐term outcomes of laparoscopic resection for colorectal cancer. Australian and New Zealand Journal of Surgery 2007;77:508‐16. [DOI] [PubMed] [Google Scholar]
Acar 2014
- Acar C, Bilen C, Bayazit Y, Aslan G, Koni A, Basok E, et al. Quality of life survey following laparoscopic and open radical nephrectomy. Urology Journal 2014;11(6):1944‐50. [PUBMED: 25433472] [PubMed] [Google Scholar]
Alaraimi 2014
- Alaraimi B, Bakbak W, Sarker S, Makkiyah S, Al‐Marzouq A, Goriparthi R, et al. A randomized prospective study comparing acquisition of laparoscopic skills in three‐dimensional (3D) vs. two‐dimensional (2D) laparoscopy. World Journal of Surgery 2014;38(11):2746‐52. [PUBMED: 25002241] [DOI] [PubMed] [Google Scholar]
Ambrosetti 2014
- Ambrosetti P, Gervaz P. Laparoscopic elective sigmoidectomy for diverticular disease: a plea for standardization of the procedure. Colorectal Disease 2014;16(2):90‐4. [PUBMED: 24128302] [DOI] [PubMed] [Google Scholar]
Anaya 2005
- Anaya DA, Flum DR. Risk of emergency colectomy and colostomy in patients with diverticular disease. Archives of Surgery 2005;140(7):681‐5. [PUBMED: 16027334] [DOI] [PubMed] [Google Scholar]
Andersen 2012
- Andersen JC, Bundgaard L, Elbrond H, Laurberg S, Walker LR, Stovring J. Danish national guidelines for treatment of diverticular disease. Danish Medical Journal 2012;59(5):C4453. [PUBMED: 22549495] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Bhojani 2012
- Bhojani FD, Fox A, Pitzul K, Gallinger S, Wei A, Moulton CA, et al. Clinical and economic comparison of laparoscopic to open liver resections using a 2‐to‐1 matched pair analysis: an institutional experience. Journal of the American College of Surgeons 2012;214(2):184‐95. [PUBMED: 22192894] [DOI] [PubMed] [Google Scholar]
Binda 2012
- Binda GA, Karas JR, Serventi A, Sokmen S, Amato A, Hydo L, et al. Primary anastomosis vs nonrestorative resection for perforated diverticulitis with peritonitis: a prematurely terminated randomized controlled trial. Colorectal Disease 2012;14(11):1403‐10. [PUBMED: 22672447] [DOI] [PubMed] [Google Scholar]
Binda 2012a
- Binda GA, Arezzo A, Serventi A, Bonelli L, Facchini M, Prandi M, et al. Multicentre observational study of the natural history of left‐sided acute diverticulitis. British Journal of Surgery 2012;99(2):276‐85. [PUBMED: 22105809] [DOI] [PubMed] [Google Scholar]
Binda 2012b
- Binda GA, Amato A, Serventi A, Arezzo A. Clinical presentation and risks. Digestive Diseases (Basel, Switzerland) 2012;30(1):100‐7. [PUBMED: 22572695] [DOI] [PubMed] [Google Scholar]
Binda 2015
- Binda GA, Cuomo R, Laghi A, Nascimbeni R, Serventi A, Bellini D, et al. Practice parameters for the treatment of colonic diverticular disease: Italian Society of Colon and Rectal Surgery (SICCR) guidelines. Techniques in Coloproctology 2015;19(10):615‐26. [PUBMED: 26377584] [DOI] [PubMed] [Google Scholar]
Bonjer 2007
- Bonjer HJ, Hop WC, Nelson H, Sargent DJ, Lacy AM, Castells A, et al. Laparoscopically assisted vs open colectomy for colon cancer: a meta‐analysis. Archives of Surgery 2007;142:298‐303. [DOI] [PubMed] [Google Scholar]
Broderick‐Villa 2005
- Broderick‐Villa G, Burchette RJ, Collins JC, Abbas MA, Haigh PI. Hospitalization for acute diverticulitis does not mandate routine elective colectomy. Archives of Surgery 2005;140:576‐81. [DOI] [PubMed] [Google Scholar]
Buunen 2004
- Buunen M, Gholghesaei M, Veldkamp R, Meijer DW, Bonjer HJ, Bouvy ND. Stress response to laparoscopic surgery: a review. Surgical Endoscopy 2004;18(7):1022‐8. [PUBMED: 15136930] [DOI] [PubMed] [Google Scholar]
Chan 2004
- Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [PUBMED: 15161896] [DOI] [PubMed] [Google Scholar]
Chapman 2005
- Chapman J, Davies M, Wolff B, Dozois E, Tessier D, Harrington J, et al. Complicated diverticulitis: is it time to rethink the rules?. Annals of Surgery 2005;242(4):576‐81; discussion 581‐3. [PUBMED: 16192818] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2015
- Chen XZ, Wen L, Rui YY, Liu CX, Zhao QC, Zhou ZG, et al. Long‐term survival outcomes of laparoscopic versus open gastrectomy for gastric cancer: a systematic review and meta‐analysis. Medicine 2015;94(4):e454. [PUBMED: 25634185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cirocchi 2010
- Cirocchi R, Abraha I, Farinella E, Montedori A, Sciannameo F. Laparoscopic versus open surgery in small bowel obstruction. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD007511.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cirocchi 2012a
- Cirocchi R, Farinella E, Trastulli S, Sciannameo F, Audisio RA. Elective sigmoid colectomy for diverticular disease. Laparoscopic vs open surgery: a systematic review. Colorectal Disease 2012;14:671‐83. [DOI] [PubMed] [Google Scholar]
Cirocchi 2012b
- Cirocchi R, Trastulli S, Farinella E, Desiderio J, Listorti C, Parisi A, et al. Is inferior mesenteric artery ligation during sigmoid colectomy for diverticular disease associated with increased anastomotic leakage? A meta‐analysis of randomized and non‐randomized clinical trials. Colorectal Disease 2012;14:e521‐9. [DOI] [PubMed] [Google Scholar]
Cirocchi 2013
- Cirocchi R, Trastulli S, Desiderio J, Listorti C, Boselli C, Parisi A, et al. Treatment of Hinchey stage III‐IV diverticulitis: a systematic review and meta‐analysis. International Journal of Colorectal Disease 2013;28(4):447‐57. [PUBMED: 23242271] [DOI] [PubMed] [Google Scholar]
Cirocchi 2014
- Cirocchi R, Arezzo A, Vettoretto N, Cavaliere D, Farinella E, Renzi C, et al. Role of damage control surgery in the treatment of Hinchey III and IV sigmoid diverticulitis: a tailored strategy. Medicine 2014;93(25):e184. [PUBMED: 25437034] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cirocchi 2015
- Cirocchi R, Trastulli S, Vettoretto N, Milani D, Cavaliere D, Renzi C, et al. Laparoscopic peritoneal lavage: a definitive treatment for diverticular peritonitis or a "bridge" to elective laparoscopic sigmoidectomy? A systematic review. Medicine 2015;94(1):e334. [PUBMED: 25569649] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cirocchi 2017
- Cirocchi R, Saverio S, Weber DG, Tabola R, Abraha I, Randolph J, et al. Laparoscopic lavage versus surgical resection for acute diverticulitis with generalised peritonitis: a systematic review and meta‐analysis. Techniques in Coloproctology 2017;21(2):93‐110. [PUBMED: 28197792] [DOI] [PubMed] [Google Scholar]
Cuschieri 1991
- Cuschieri A, Dubois F, Mouiel J, Mouret P, Becker H, Buess G, et al. The European experience with laparoscopic cholecystectomy. American Journal of Surgery 1991;161(3):385‐7. [PUBMED: 1825763] [DOI] [PubMed] [Google Scholar]
De Magistris 2013
- Magistris L, Azagra JS, Goergen M, Blasi V, Arru L, Facy O. Laparoscopic sigmoidectomy in moderate and severe diverticulitis: analysis of short‐term outcomes in a continuous series of 121 patients. Surgical Endoscopy 2013;27(5):1766‐71. [PUBMED: 23436080] [DOI] [PubMed] [Google Scholar]
de Rooij 2015
- Rooij T, Jilesen AP, Boerma D, Bonsing BA, Bosscha K, Dam RM, et al. A nationwide comparison of laparoscopic and open distal pancreatectomy for benign and malignant disease. Journal of the American College of Surgeons 2015;220(3):263‐270.e1. [PUBMED: 25600974] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eglinton 2010
- Eglinton T, Nguyen T, Raniga S, Dixon L, Dobbs B, Frizelle FA. Patterns of recurrence in patients with acute diverticulitis. British Jurnal of Surgery 2010;97(6):952‐7. [PUBMED: 20474006] [DOI] [PubMed] [Google Scholar]
El 2010
- Zarrok Elgazwi K, Baca I, Grzybowski L, Jaacks A. Laparoscopic sigmoidectomy for diverticulitis: a prospective study. Journal of the Society of Laparoendoscopic Surgeons / Society of Laparoendoscopic Surgeons 2010;14(4):469‐75. [PUBMED: 21605507] [DOI] [PMC free article] [PubMed] [Google Scholar]
Etzioni 2009
- Etzioni DA, Mack TM, Beart RW Jr, Kaiser AM. Diverticulitis in the United States: 1998‐2005: changing patterns of disease and treatment. Annals of Surgery 2009;249:210‐7. [DOI] [PubMed] [Google Scholar]
Farmakis 1994
- Farmakis N, Tudor RG, Keighley MR. The 5‐year natural history of complicated diverticular disease. British Journal of Surgery 1994;81(5):733‐5. [PUBMED: 8044566] [DOI] [PubMed] [Google Scholar]
Fozard 2011
- Fozard JB, Armitage NC, Schofield JB, Jones OM. ACPGBI position statement on elective resection for diverticulitis. Colorectal Disease 2011;13 Suppl 3:1‐11. [PUBMED: 21366820] [DOI] [PubMed] [Google Scholar]
Franklin 2000
- Franklin ME Jr, Diaz‐E JA, Abrego D, Parra‐Davila E, Glass JL. Laparoscopic‐assisted colonoscopic polypectomy: the Texas Endosurgery Institute experience. Diseases of the Colon and Rectum 2000;43(9):1246‐9. [PUBMED: 11005491] [DOI] [PubMed] [Google Scholar]
Frileux 2010
- Frileux P, Dubrez J, Burdy G, Roullet‐Audy JC, Dalban‐Sillas B, Bonnaventure F, et al. Sigmoid diverticulitis. Longitudinal analysis of 222 patients with a minimal follow up of 5 years. Colorectal Disease 2010;12(7):674‐80. [PUBMED: 19486099] [DOI] [PubMed] [Google Scholar]
Gaertner 2013
- Gaertner WB, Kwaan MR, Madoff RD, Willis D, Belzer GE, Rothenberger DA, et al. The evolving role of laparoscopy in colonic diverticular disease: a systematic review. World Journal of Surgery 2013;37:629‐38. [DOI] [PubMed] [Google Scholar]
Garrett 2008
- Garrett KA, Champagne BJ, Valerian BT, Peterson D, Lee EC. A single training center's experience with 200 consecutive cases of diverticulitis: can all patients be approached laparoscopically?. Surgical Endoscopy 2008;22(11):2503‐8. [PUBMED: 18347863] [DOI] [PubMed] [Google Scholar]
Gervaz 2009
- Gervaz P. Laparoscopic versus open sigmoid colectomy for diverticular disease. Clinical Trials.gov NCT00453830 2009.
Golder 2011
- Golder M, Ster IC, Babu P, Sharma A, Bayat M, Farah A. Demographic determinants of risk, colon distribution and density scores of diverticular disease. World Journal of Gastroenterology 2011;17(8):1009‐17. [PUBMED: 21448352] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2011
- Hall JF, Roberts PL, Ricciardi R, Read T, Scheirey C, Wald C, et al. Long‐term follow‐up after an initial episode of diverticulitis: what are the predictors of recurrence?. Diseases of the Colon and Rectum 2011;54(3):283‐8. [PUBMED: 21304297] [DOI] [PubMed] [Google Scholar]
Hansen 1999
- Hansen O, Stock W. Prophylaktische Operation bei der Divertikelkrankheit des Kolons ‐ Stufenkonzept durch exakte Stadieneinteilung. Langenbeck's Archives of Surgery 1999; Vol. (Suppl II):1257–60.
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. ..
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hussain 2008
- Hussain A, Mahmood H, Subhas G, EL‐Hasani S. Complicated diverticular disease of the colon: do we need to change the classical approach? A retrospective study of 110 patients in southeast England. World Journal of Emergency Surgery 2008;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ignjatovic 2004
- Ignjatoviċ D, Zivanoviċ V, Vasiċ G, Iliċ I. Meta‐analysis on minimally invasive surgical therapy of sigmoid diverticulitis. Acta Chirurgica Iugoslavica 2004;51:25‐8. [DOI] [PubMed] [Google Scholar]
Jacobs 1991
- Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 1991;1:144‐50. [PubMed] [Google Scholar]
Jacobs 2007
- Jacobs DO. Clinical practice. Diverticulitis. New England Journal of Medicine 2007;357(20):2057‐66. [PUBMED: 18003962] [DOI] [PubMed] [Google Scholar]
Kang 2003
- Kang JY, Hoare J, Tinto A, Subramanian S, Ellis C, Majeed A, et al. Diverticular disease of the colon ‐ on the rise: a study of hospital admissions in England between 1989/1990 and 1999/2000. Alimentary Pharmacology & Therapeutics 2003;17(9):1189‐95. [PUBMED: 12752356] [DOI] [PubMed] [Google Scholar]
Klarenbeek 2007
- Klarenbeek BR, Veenhof AA, Lange ES, Bemelman WA, Bergamaschi R, Heres P, et al. The Sigma‐trial protocol: a prospective double‐blind multi‐centre comparison of laparoscopic versus open elective sigmoid resection in patients with symptomatic diverticulitis. BMC Surgery 2007;7:16. [PUBMED: 17683563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kontopantelis 2013
- Kontopantelis E, Springate DA, Reeves D. A re‐analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta‐analyses. PloS One 2013;8(7):e69930. [PUBMED: 23922860] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kreis 2006
- Kreis Me, Jauch KW. Diverticular disease ‐ Update 2006. In: Neugebauer EAM, Sauerland S, Fingerhut A, Millat B, Buess GF editor(s). EAES Guidelines for Endoscopic Surgery. New York, New York, USA: Springer, 2006:157–60. [Google Scholar]
Kuhry 2008
- Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J. Long‐term results of laparoscopic colorectal cancer resection. Cancer Treatment Reviews 2008;34:498‐504. [DOI] [PubMed] [Google Scholar]
Kwon 2012
- Kwon S, Billingham R, Farrokhi E, Florence M, Herzig D, Horvath K, et al. Adoption of laparoscopy for elective colorectal resection: a report from the Surgical Care and Outcomes Assessment Program. Journal of the American College of Surgeons 2012;214(6):909‐18.e1. [PUBMED: 22533998] [DOI] [PMC free article] [PubMed] [Google Scholar]
Larson 1976
- Larson DM, Masters SS, Spiro HM. Medical and surgical therapy in diverticular disease: a comparative study. Gastroenterology 1976;71(5):734‐7. [PUBMED: 964566] [PubMed] [Google Scholar]
Lauro 2002
- Lauro A, Alonso Poza A, Cirocchi R, Doria C, Gruttadauria S, Giustozzi G, et al. Laparoscopic surgery for colon diverticulitis. Minerva Chirurgica 2002;57:1‐5. [PubMed] [Google Scholar]
Li 2014
- Li D, Baxter NN, McLeod RS, Moineddin R, Wilton AS, Nathens AB. Evolving practice patterns in the management of acute colonic diverticulitis: a population‐based analysis. Diseases of the Colon and Rectum 2014;57(12):1397‐405. [PUBMED: 25380006] [DOI] [PubMed] [Google Scholar]
Macura 2010
- Macura A, Abraha I, Kirkham J, Gensini GF, Moja L, Iorio A. Selective outcome reporting: telling and detecting true lies. The state of the science. Internal and Emergency Medicine 2010;5(2):151‐5. [PUBMED: 20300879] [DOI] [PubMed] [Google Scholar]
Mbadiwe 2013
- Mbadiwe T, Obirieze AC, Cornwell EE 3rd, Turner P, Fullum TM. Surgical management of complicated diverticulitis: a comparison of the laparoscopic and open approaches. Journal of the American College of Surgeons 2013;216(4):782‐8; discussion 788‐90. [PUBMED: 23521963] [DOI] [PubMed] [Google Scholar]
Mettler 1997
- Mettler L, Semm K, Shive K. Endoscopic management of adnexal masses. Journal of the Society of Laparoendoscopic Surgeons / Society of Laparoendoscopic Surgeons 1997;1(2):103‐12. [PUBMED: 9876656] [PMC free article] [PubMed] [Google Scholar]
Miskovic 2012
- Miskovic D, Ni M, Wyles SM, Tekkis P, Hanna GB. Learning curve and case selection in laparoscopic colorectal surgery: systematic review and international multicenter analysis of 4852 cases. Diseases of the Colon and Rectum 2012;55(12):1300‐10. [PUBMED: 23135590] [DOI] [PubMed] [Google Scholar]
Moghadamyeghaneh 2015
- Moghadamyeghaneh Z, Carmichael JC, Smith BR, Mills S, Pigazzi A, Nguyen NT, et al. A comparison of outcomes of emergent, urgent, and elective surgical treatment of diverticulitis. American Journal of Surgery 2015;210(5):838‐45. [PUBMED: 26116319] [DOI] [PubMed] [Google Scholar]
Myles 2017
- Myles PS, Myles DB, Galagher W, Boyd D, Chew C, MacDonald N, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. British Journal of Anaesthesia 2017;118(3):424‐9. [PUBMED: 28186223] [DOI] [PubMed] [Google Scholar]
Painter 1971
- Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. BMJ 1971;2(5759):450‐4. [PUBMED: 4930390] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parks 1969
- Parks TG. Natural history of diverticular disease of the colon. A review of 521 cases. BMJ 1969;4(5684):639‐42. [PUBMED: 5359917] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pittet 2009
- Pittet O, Kotzampassakis N, Schmidt S, Denys A, Demartines N, Calmes JM. Recurrent left colonic diverticulitis episodes: more severe than the initial diverticulitis?. World Journal of Surgery 2009;33(3):547‐52. [PUBMED: 19148697] [DOI] [PubMed] [Google Scholar]
Polle 2009
- Polle SW, Koperen PJ, Berge Henegouwen MI, Slors JF, Stokkers PC, Bemelman WA. Laparoscopic surgery for inflammatory bowel disease: an update. Nederlands Tijdschrift voor Geneeskunde 2009;153:B284. [PubMed] [Google Scholar]
Pouliquen 2009
- Pouliquen X. Gestes de base en chirurgie laparoscopique de l'adulte. Techniques Chirurgicales – Appareil Digestif Appareil Digestif. 2009;n.a.:1.26. [Google Scholar]
Purkayastha 2006
- Purkayastha S, Constantinides VA, Tekkis PP, Athanasiou T, Aziz O, Tilney H, et al. Laparoscopic vs. open surgery for diverticular disease: a meta‐analysis of non randomized studies. Diseases of Colon and Rectum 2006;49:446‐63. [DOI] [PubMed] [Google Scholar]
Radosa 2014
- Radosa JC, Meyberg‐Solomayer G, Kastl C, Radosa CG, Mavrova R, Graber S, et al. Influences of different hysterectomy techniques on patients' postoperative sexual function and quality of life. Journal of Sexual Medicine 2014;11(9):2342‐50. [PUBMED: 25042204] [DOI] [PubMed] [Google Scholar]
Rafferty 2006
- Rafferty J, Shellito P, Hyman NH, Buie WD. Practice parameters for sigmoid diverticulitis. Diseases of the Colon and Rectum 2006;49(7):939‐44. [PUBMED: 16741596] [DOI] [PubMed] [Google Scholar]
Rege 1989
- Rege RV, Nahrwold DL. Diverticular disease. Current Problems in Surgery 1989;26(3):133‐89. [PUBMED: 2651018] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ritz 2011
- Ritz JP, Lehmann KS, Stroux A, Buhr HJ, Holmer C. Sigmoid diverticulitis in young patients ‐ a more aggressive disease than in older patients?. Journal of Gastrointestinal Surgery 2011;15(4):667‐74. [PUBMED: 21318443] [DOI] [PubMed] [Google Scholar]
Rodkey 1984
- Rodkey GV, Welch CE. Changing patterns in the surgical treatment of diverticular disease. Annals of Surgery 1984;200(4):466‐78. [PUBMED: 6333217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salem 2004
- Salem L, Veenstra DL, Sullivan SD, Flum DR. The timing of elective colectomy in diverticulitis: a decision analysis. Journal of the American College of Surgeons 2004;199(6):904‐12. [PUBMED: 15555974] [DOI] [PubMed] [Google Scholar]
Salem 2007
- Salem TA, Molloy RG, O'Dwyer PJ. Prospective, five‐year follow‐up study of patients with symptomatic uncomplicated diverticular disease. Diseases of the Colon and Rectum 2007;50(9):1460‐4. [PUBMED: 17431721] [DOI] [PubMed] [Google Scholar]
Sauerland 2010
- Sauerland S, Jaschinski T, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD001546.pub3] [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savovic J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment (Winchester, England) 2012;16(35):1‐82. [PUBMED: 22989478] [DOI] [PubMed] [Google Scholar]
Schwenk 2004
- Schwenk W. [The LAPDIV‐CAMIC Study. Multicenter prospective randomized study of short‐term and intermediate‐term outcome of laparoscopic and conventional sigmoid resection in diverticular disease] [Die LAPDIV‐CAMIC‐Studie. Multizentrische prospektiv‐randomisierte Studie zu den kurz‐ und mittelfristigen Unterschieden nach laparoskopischer und konventioneller Sigmaresektion bei Divertikelerkrankung]. Der Chirurg; Zeitschrift fur alle Gebiete der Operativen Medizen 2004;75(7):706‐7. [PUBMED: 15098095] [DOI] [PubMed] [Google Scholar]
Schwenk 2005
- Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003145.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11. Presenting results and ‘Summary of findings' tables. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org (accessed March 2015).
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editors. Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org (accessed March 2015).
Shaikh 2007
- Shaikh S, Krukowski ZH. Outcome of a conservative policy for managing acute sigmoid diverticulitis. British Journal of Surgery 2007;94(7):876‐9. [PUBMED: 17380481] [DOI] [PubMed] [Google Scholar]
Siddiqui 2010
- Siddiqui MRS, Sajid MS, Khatri K, Cheek E, Baig MK. Elective open versus laparoscopic sigmoid colectomy for diverticular disease: a meta‐analysis with the Sigma trial. World Journal of Surgery 2010;34:2883‐901. [DOI] [PubMed] [Google Scholar]
Summitt 1992
- Summitt RL Jr, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopy‐assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstetrics and Gynecology 1992;80(6):895‐901. [PUBMED: 1448255] [PubMed] [Google Scholar]
Tanase 2015
- Tanase I, Paun S, Stoica B, Negoi I, Gaspar B, Beuran M. Epidemiology of diverticular disease ‐ systematic review of the literature. Chirurgia 2015;110(1):9‐14. [PUBMED: 25800310] [PubMed] [Google Scholar]
Tocchi 2001
- Tocchi A, Mazzoni G, Fornasari V, Miccini M, Daddi G, Tagliacozzo S. Preservation of the inferior mesenteric artery in colorectal resection for complicated diverticular disease. American Journal of Surgery 2001;182(2):162‐7. [PUBMED: 11574089] [DOI] [PubMed] [Google Scholar]
Uchal 2005
- Uchal M, Raftopoulos Y, Tjugum J, Bergamaschi R. Validation of a six‐task simulation model in minimally invasive surgery. Surgical Endoscopy 2005;19(1):109‐16. [PUBMED: 15531971] [DOI] [PubMed] [Google Scholar]
Warner 2007
- Warner E, Crighton EJ, Moineddin R, Mamdani M, Upshur R. Fourteen‐year study of hospital admissions for diverticular disease in Ontario. Canadian Journal of Gastroenterology (Journal canadien de gastroenterologie) 2007;21(2):97‐9. [PUBMED: 17299613] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weizman 2011
- Weizman Adam V, Nguyen Geoffrey C. Diverticular disease: epidemiology and management. Canadian Journal of Gastroenterology 2011;25(7):385‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2000
- Wells GA, Shea B, O’Connell D. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. Paper presented at 3rd Symposium on systematic reviews: behind the basics, Oxford. 2000.
References to other published versions of this review
Cirocchi 2011
- Cirocchi R, Farinella E, Trastulli S, Boselli C, Montedori A, Gullà N, et al. Laparoscopic versus open surgery for colonic diverticulitis. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD009277; CD009277] [DOI] [Google Scholar]