Abstract
Background
Pneumonia is a common and potentially serious illness. Corticosteroids have been suggested for the treatment of different types of infection, however their role in the treatment of pneumonia remains unclear. This is an update of a review published in 2011.
Objectives
To assess the efficacy and safety of corticosteroids in the treatment of pneumonia.
Search methods
We searched the Cochrane Acute Respiratory Infections Group's Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS on 3 March 2017, together with relevant conference proceedings and references of identified trials. We also searched three trials registers for ongoing and unpublished trials.
Selection criteria
We included randomised controlled trials (RCTs) that assessed systemic corticosteroid therapy, given as adjunct to antibiotic treatment, versus placebo or no corticosteroids for adults and children with pneumonia.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently assessed risk of bias and extracted data. We contacted study authors for additional information. We estimated risk ratios (RR) with 95% confidence intervals (CI) and pooled data using the Mantel–Haenszel fixed‐effect model when possible.
Main results
We included 17 RCTs comprising a total of 2264 participants; 13 RCTs included 1954 adult participants, and four RCTs included 310 children. This update included 12 new studies, excluded one previously included study, and excluded five new trials. One trial awaits classification.
All trials limited inclusion to inpatients with community‐acquired pneumonia (CAP), with or without healthcare‐associated pneumonia (HCAP). We assessed the risk of selection bias and attrition bias as low or unclear overall. We assessed performance bias risk as low for nine trials, unclear for one trial, and high for seven trials. We assessed reporting bias risk as low for three trials and high for the remaining 14 trials.
Corticosteroids significantly reduced mortality in adults with severe pneumonia (RR 0.58, 95% CI 0.40 to 0.84; moderate‐quality evidence), but not in adults with non‐severe pneumonia (RR 0.95, 95% CI 0.45 to 2.00). Early clinical failure rates (defined as death from any cause, radiographic progression, or clinical instability at day 5 to 8) were significantly reduced with corticosteroids in people with severe and non‐severe pneumonia (RR 0.32, 95% CI 0.15 to 0.7; and RR 0.68, 95% CI 0.56 to 0.83, respectively; high‐quality evidence). Corstocosteroids reduced time to clinical cure, length of hospital and intensive care unit stays, development of respiratory failure or shock not present at pneumonia onset, and rates of pneumonia complications.
Among children with bacterial pneumonia, corticosteroids reduced early clinical failure rates (defined as for adults, RR 0.41, 95% CI 0.24 to 0.70; high‐quality evidence) based on two small, clinically heterogeneous trials, and reduced time to clinical cure.
Hyperglycaemia was significantly more common in adults treated with corticosteroids (RR 1.72, 95% CI 1.38 to 2.14). There were no significant differences between corticosteroid‐treated people and controls for other adverse events or secondary infections (RR 1.19, 95% CI 0.73 to 1.93).
Authors' conclusions
Corticosteroid therapy reduced mortality and morbidity in adults with severe CAP; the number needed to treat for an additional beneficial outcome was 18 patients (95% CI 12 to 49) to prevent one death. Corticosteroid therapy reduced morbidity, but not mortality, for adults and children with non‐severe CAP. Corticosteroid therapy was associated with more adverse events, especially hyperglycaemia, but the harms did not seem to outweigh the benefits.
Keywords: Humans, Adrenal Cortex Hormones, Adrenal Cortex Hormones/adverse effects, Adrenal Cortex Hormones/therapeutic use, Ampicillin, Ampicillin/adverse effects, Ampicillin/therapeutic use, Anti‐Bacterial Agents, Anti‐Bacterial Agents/therapeutic use, Budesonide, Budesonide/adverse effects, Budesonide/therapeutic use, Dexamethasone, Dexamethasone/adverse effects, Dexamethasone/therapeutic use, Hydrocortisone, Hydrocortisone/adverse effects, Hydrocortisone/therapeutic use, Pneumonia, Pneumonia/drug therapy, Pneumonia/mortality, Prednisolone, Prednisolone/adverse effects, Prednisolone/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Is treatment with corticosteroids beneficial and safe for people with pneumonia?
Review question
We looked at the effects of treating people with pneumonia using corticosteroids (also called steroids or glucocorticoids) on numbers of deaths, response to treatment, treatment complications, and side effects. We compared treatment with corticosteroids in addition to antibiotics with placebo or no treatment.
Background
Acute pneumonia is a lung infection treated with antibiotics that target the bacteria that caused the infection. Pneumonia is quite common, and despite adequate antibiotic treatment, complications and sometimes death can occur.
Corticosteroids are hormones produced naturally in the adrenal gland. Corticosteroids have been found to be beneficial in the treatment of some infections. However, their beneficial effects are often offset by serious side effects, mainly when used at high doses and over the long term. This is an update of a review published in 2011.
Search date
The evidence is current to 3 March 2017.
Study characteristics
We included 17 studies evaluating systemic corticosteroid therapy (given intravenously or by tablets) for people with pneumonia (2264 participants; 1954 adults and 310 children). We included 12 new studies in this update and excluded one previously included study. All included studies evaluated people who had acquired pneumonia in the community (community‐acquired pneumonia (CAP)) being treated in the hospital; no studies assessed people who had developed pneumonia while in hospital or who were on breathing machines (mechanically ventilated).
Study funding sources
Eight trials did not report funding sources; seven were funded by academic sponsors; one was funded by a pharmaceutical company; and one reported receiving no funding.
Key results
Corticosteroids reduced deaths in adults with severe CAP, but not in people with non‐severe CAP. Eighteen adults with severe CAP need to be treated with corticosteroids to prevent one death.
People with CAP treated with corticosteroids had lower clinical failure rates (death, worsening of imaging studies, or no clinical improvement), shorter time to cure, a shorter hospital stay, and fewer complications. We found good‐quality evidence that corticosteroids reduced clinical failure rates in children with pneumonia, but the data were based on a small number of children with different types of pneumonia.
People treated with corticosteroids had higher blood glucose levels (hyperglycaemia) than those not treated with corticosteroids. Corticosteroid treatment was not associated with increased rates of other serious adverse events.
Corticosteroids were beneficial for adults with severe CAP. People with non‐severe CAP may also benefit from corticosteroid therapy, but with no survival advantage.
Quality of the evidence
We downgraded the quality of the evidence due to issues with study design, unclear results, or results that were not similar across studies. For the outcomes of death and clinical failure in adults, we graded the quality of the evidence as moderate. For the outcomes of clinical failure in people with severe CAP, non‐severe CAP, and in children, we graded the quality of the evidence as high.
Summary of findings
Summary of findings for the main comparison. Corticosteroids compared to control for pneumonia.
| Corticosteroids compared to control for pneumonia | ||||||
| Patient or population: people with community‐acquired pneumonia Setting: hospitalised patients Intervention: corticosteroids Comparison: control (placebo or no treatment) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with corticosteroids | |||||
| Mortality ‐ adults | Study population | RR 0.66 (0.47 to 0.92) | 1863 (11 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 82 per 1000 | 53 per 1000 (38 to 74) | |||||
| Mortality ‐ adults ‐ severe CAP | Study population | RR 0.58 (0.40 to 0.84) | 995 (9 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 131 per 1000 | 76 per 1000 (52 to 110) | |||||
| Mortality ‐ adults ‐ non‐severe CAP | Study population | RR 0.95 (0.45 to 2.00) | 868 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | ||
| 29 per 1000 | 28 per 1000 (13 to 58) | |||||
| Early clinical failure ‐ adults | Study population | RR 0.40 (0.23 to 0.70) | 1324 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 3 4 | ||
| 373 per 1000 | 149 per 1000 (86 to 261) | |||||
| Early clinical failure ‐ adults ‐ severe CAP | Study population | RR 0.32 (0.15 to 0.70) | 419 (5 RCTs) | ⊕⊕⊕⊕ HIGH 5 | ||
| 422 per 1000 | 135 per 1000 (63 to 296) | |||||
| Early clinical failure ‐ adults ‐ non‐severe CAP | Study population | RR 0.68 (0.56 to 0.83) | 905 (2 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 352 per 1000 | 240 per 1000 (197 to 292) | |||||
| Early clinical failure ‐ children | Study population | RR 0.41 (0.24 to 0.70) | 88 (2 RCTs) | ⊕⊕⊕⊕ HIGH 6 7 | ||
| 659 per 1000 | 270 per 1000 (158 to 461) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CAP: community‐acquired pneumonia; CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
1Some trials had unclear allocation concealment, which seemed to exaggerate the effect of corticosteroids. 2We downgraded quality for imprecision because the 95% CIs overlap no effect and the RR ranged from ‐55% to 100%. 3We downgraded quality for inconsistency (I² = 89.3% for this outcome). 4Some trials had unclear allocation concealment, however we did not downgrade quality because the effect of intervention remained significant in trials with low and unclear allocation concealment. 5We downgraded quality for inconsistency (I² = 74% for this outcome) and upgraded one level for large effect (RR = 0.32). 6We downgraded quality for risk of bias (analysis includes two low‐quality trials). 7We upgraded quality one level for large effect (RR = 0.41).
Background
Description of the condition
Acute pneumonia is a common and potentially serious illness. Despite significant advances in its aetiological investigation, antimicrobial therapy, and improvements in supportive care, acute pneumonia is still among the top 10 most common causes of death among all age groups. In the USA in 2013, pneumonia was the sixth‐leading cause of death in people aged 65 years and over, and the single most common cause of infection‐related mortality (Xu 2016).
In the past, acute pneumonia was classified as community‐acquired pneumonia (CAP) and hospital‐acquired pneumonia (HAP) including ventilator‐associated pneumonia (VAP), based on differences in aetiologic agents and antibiotic susceptibilities among these entities. Following increases in healthcare delivery shifting to outpatient settings, an additional pneumonia category, healthcare‐associated pneumonia (HCAP), has been defined. Healthcare‐associated pneumonia occurs in non‐hospitalised people who have had extensive healthcare contact, defined as including one or more of: intravenous therapy, wound care, or intravenous chemotherapy over the previous 30 days; residing in a nursing home or other long‐term care facility; hospitalisation in an acute care hospital for two or more days over the previous 90 days; or attending a hospital or haemodialysis clinic over the previous 30 days (Friedman 2002).
The aetiology of CAP varies by geographic region. However, Streptococcus pneumoniae is the most common cause worldwide (File 2010). The overall incidence of CAP in adults is approximately 5.16 to 6.11 cases per 1000 persons per year and increases with age. There is seasonal variation, with more cases of pneumonia occurring during the winter months. About 20% of all people with CAP are admitted to hospital (Niederman 2007), and approximately 10% to 20% of patients require admission to intensive care units (ICU) (Marrie 2007; Restrepo 2008). Thirty‐day mortality in people who are hospitalised for CAP is approximately 10% to 12% and is higher in people admitted to the ICU (Musher 2014).
HAP, VAP, and HCAP may be caused by a broad variety of pathogens and can be polymicrobial. Common pathogens include aerobic gram‐negative bacilli and gram‐positive cocci (e.g. Staphylococcus aureus, including methicillin‐resistant S aureus (MRSA) and Streptococcus spp). Hospital‐acquired pneumonia due to viruses or fungi is significantly less common, except in people who are immunocompromised (Jones 2010).
Precise incidence rates of HAP and HCAP are difficult to determine because of differences in local epidemiology and infection control measures. Estimates of HAP incidence range from 5 to more than 20 episodes per 1000 hospitalisations (Chawla 2008), accounting for up to 25% of all nosocomial (hospital‐acquired) infections (Magill 2014; Torres 2010). Hospital‐acquired pneumonia is the leading cause of death among hospital‐acquired infections, with estimates of HAP‐associated mortality ranging from 20% to 50% (Chawla 2008; Rosenthal 2012). The rate of VAP development is between 10% and 30% of patients receiving more than 48 hours of mechanical ventilation (Rosenthal 2012; Torres 2010). The occurrence of VAP has a significant impact on patient outcomes; it is associated with substantial morbidity, significantly longer ICU stays, and a two‐fold mortality rate compared with similar patients who do not have VAP (Safdar 2005).
Description of the intervention
Corticosteroids (glucocorticoids, steroids) include steroid hormones that are naturally produced in the adrenal cortex of vertebrates and their synthetic analogues. Corticosteroids are involved in many physiological processes, including stress response, immune response and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels and behaviour. Synthetic derivatives of natural steroids include prednisone, prednisolone, methylprednisolone, betamethasone, dexamethasone, triamcinolone, and hydrocortisone. Corticosteroids are important components in the treatment of many inflammatory, allergic, immunologic, and malignant disorders. Corticosteroids are administered by inhalation, orally, or intravenously (Rhen 2005).
Unfortunately, the therapeutic effects of corticosteroids are often accompanied by clinically significant side effects, most of which are related to the dose and duration of therapy (Rhen 2005). Many adverse effects occur only with prolonged administration; most short‐term adverse events are reversible when the drug is discontinued. Side effects observed with long‐term, high‐dose treatment include obesity with a special fat distribution pattern (e.g. face swelling), immune depression, delayed wound healing, growth retardation in children, hirsutism, diabetes, depressive disorders, Cushing’s syndrome, and osteoporosis (Oray 2016). Short‐term use of corticosteroids may be associated with hyperglycaemia, fluid retention, and hypertension, which are typically transient (Schäcke 2002). Neuropsychiatric side effects, ranging from insomnia and irritability to mania, psychosis, and delirium, are commonly seen with short‐term corticosteroid use (Warrington 2006).
How the intervention might work
Corticosteroids have been suggested for the treatment of different types of infections, including meningitis, tuberculosis, pneumocystis pneumonia, other bacterial pneumonia, and septic shock. The theoretical advantages of corticosteroids differ for each infection type (Chalmers 2010; Kalil 2016; Salluh 2008).
In septic shock, a condition of extreme physiological stress, suboptimal cortisol production has been termed critical illness‐related corticosteroid insufficiency (CIRCI) (Marik 2008). The major theoretical purpose of corticosteroids in sepsis is to restore balance to the altered hypothalamic‐pituitary‐adrenal axis (Annane 2004; Mandell 2015). People with pneumonia might present with CIRCI, requiring corticosteroid support. In addition, corticosteroids might have a local effect at the site of infection in pneumonia. In pulmonary infections, the release of cytokines and other inflammatory mediators from alveolar macrophages serves as a useful mechanism to eliminate invading pathogens. However, excessive release can potentially be harmful to the lung and the host. Corticosteroids might reduce pulmonary inflammation in severe pneumonia, preventing respiratory failure (Mandell 2015). Several in vitro studies have demonstrated that corticosteroids decrease cytokine expression in human cells and inhibit migration of phagocytic cells (Rhen 2005). It has been shown that corticosteroids also diminish the release of cytokines (mainly interleukin‐6 (IL‐6) in serum and broncho‐alveolar lavage) in vivo, and that C‐reactive protein (an acute‐phase protein related to IL‐6) and neutrophil counts in broncho‐alveolar aspirates were decreased in people receiving corticosteroids. Possible explanations for the latter include the effect of corticosteroids on neutrophil migration or accelerated neutrophil apoptosis in a cytokine‐depleted milieu, or both (Montón 1999). Another potential benefit of corticosteroids in pneumonia is by blocking a Jarisch‐Herxheimer–like reaction to initiation of antibiotics in patients with high bacterial load. The Jarisch‐Herxheimer–like reaction is thought to be due to high concentrations of cytokines shortly after initiation of antibiotics, possibly through release of endotoxin or other bacterial mediators in people with a high bacterial burden (Wunderink 2015). This is the probable mechanism underlying the benefit of corticosteroids in meningococcal meningitis (Brouwer 2015).
The possible benefits of corticosteroid therapy must be balanced against their potential adverse effects. Hyperglycaemia is known to be associated with poor clinical outcomes in critically ill patients. Several trials have shown that hyperglycaemic critically ill patients have higher mortality rates than those who are normoglycaemic, although a causal relationship has not been proved (Krinsley 2003). Fluid retention as an adverse effect of corticosteroid therapy with resulting pulmonary congestion may also have deleterious effects in people with pneumonia, especially those with severe pneumonia or acute respiratory distress syndrome (ARDS). Other common adverse effects of short‐term corticosteroid therapy may be more relevant to milder cases of pneumonia, for example development of delirium may result in prolongation of hospital stay, and insomnia might affect patients' quality of life. Systemic glucocorticoid therapy is associated with a dose‐dependent increase in the risk of infection, especially with common bacterial, viral, and fungal pathogens. Specifically, in high‐dose glucocorticoid therapy there is an immediate risk of infection due to inhibitory effects on phagocytic cell function. Most clinical trials evaluating the role of corticosteroids in pneumonia used short courses of relatively low‐dose corticosteroids, which are not expected to pose a significant infection risk.
Why it is important to do this review
In clinical practice, use of corticosteroids for people with pneumonia remains variable. Current guidelines do not address corticosteroids in the standard management of people with CAP, HAP, HCAP, or VAP (Kalil 2016; Mandell 2007). An exception is the British guidelines, which state that "... steroids are not recommended in the routine treatment of high severity CAP" (Lim 2009).
The 2011 version of this Cochrane Review showed that corticosteroids were beneficial for accelerating the time to resolution of symptoms with no effect on mortality for most people with pneumonia (Chen 2011). Since then, several relatively large randomised controlled trials assessing the role of corticosteroids for people with pneumonia have been published. Combining data from all relevant trials may lead to more definitive conclusions, particularly whether the effects of corticosteroids could be patient‐specific. Compiling all existing studies might allow for the assessment of corticosteroid effects for specific patient subgroups with pneumonia. For this update we revised the protocol, inclusion criteria, and analyses and re‐extracted all data. We have reported deviations from the original review, Chen 2011, in the Differences between protocol and review section of this update.
Objectives
To assess the efficacy and safety of corticosteroids in the treatment of pneumonia. In particular, we aimed to answer whether systemic steroid treatment:
reduces all‐cause mortality among people with pneumonia;
reduces morbidity among people with pneumonia;
increases complication rates among people with pneumonia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials assessing the effectiveness of corticosteroids for pneumonia were eligible for inclusion.
Types of participants
Adults or children with radiographically confirmed pneumonia, including community‐acquired pneumonia (CAP) treated in the community, hospitals, or intensive care unit (ICU); healthcare‐associated pneumonia (HCAP); hospital‐acquired pneumonia (HAP); and ventilator‐associated pneumonia (VAP).
We excluded studies including only neonates, people with Pneumocystis pneumonia, and people with HIV.
Types of interventions
Systemic corticosteroid treatment, given as adjunct to antibiotic treatment versus antibiotics alone or antibiotics with placebo. Corticosteroids may have been given at any dose, mode, and for any duration. We included the following corticosteroids: prednisone, prednisolone, methylprednisolone, betamethasone, dexamethasone, triamcinolone, and hydrocortisone.
Types of outcome measures
Primary outcomes
All‐cause mortality within 30 days after randomisation. If not reported at day 30, we extracted the outcome closest to 30 days.
Secondary outcomes
Early clinical failure (clinical failure at 5 to 7 days), defined as death from any cause, radiographic progression, or clinical instability, as defined in the study. We accepted the study definitions and assessed their compatibility with the outcome suggested by the Infectious Diseases Society of America (IDSA), that is at least one of the following: temperature higher than 37.8 °C, heart rate higher than 100 beats per minute, respiratory rate higher than 24 breaths per minute, systolic blood pressure lower than 90 mm Hg or need for vasopressor support, altered mental status, inability for oral intake, or inadequate oxygenation at room temperature (PaO₂ < 60 mm Hg or pulse oximetry < 90%) (Mandell 2007).
Time to clinical cure, defined at least by no fever, haemodynamic stability, and return to baseline respiratory condition. We accepted the study definitions for clinical cure, recorded them, and assessed their compatibility with the IDSA outcome definitions.
Development of respiratory failure not present initially, defined as need for non‐invasive or invasive mechanical ventilation that was not present at onset of pneumonia.
Development of shock not present initially.
Transfer to ICU among participants not admitted initially to the ICU.
Duration of hospital stay for hospitalised participants.
Duration of ICU stay for participants admitted to the ICU.
Pneumonia complications not present initially, including empyema, lung abscess, pneumothorax (as defined in study).
Secondary infections, including any superinfection diagnosed ≥ 72 hours from randomisation.
-
Adverse events:
any adverse event;
hyperglycaemia, preferably defined as new need for insulin treatment;
neuropsychiatric events, including delirium;
gastrointestinal bleeding; and
adverse cardiac events, including arrhythmia, congestive heart failure exacerbation, or acute coronary event.
Search methods for identification of studies
Electronic searches
The previous version of this review included trials identified from searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the China National Knowledge Infrastructure (CNKI) and VIP databases up to 2010 (Chen 2011).
For this update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) which includes the Cochrane Acute Respiratory Infections Group's Specialised Register (searched 2 March 2017), MEDLINE Ovid (1946 to 2 March 2017), Embase (Elsevier) (1974 to 3 March 2017), and LILACS (Latin American and Caribbean Health Sciences Literature) (BIREME) (1982 to 3 March 2017). We did not search the China National Knowledge Infrastructure (CNKI) and VIP databases due to lack of search experts with Chinese language knowledge, but we did consider articles identified in the previous search.
We used the search strategy described in Appendix 1 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search Embase (Appendix 2) and LILACS (Appendix 3).
Searching other resources
We handsearched references of all included studies for more trials. We reviewed trials included in recent systematic reviews assessing steroids for pneumonia for inclusion eligibility (Horita 2015; Marti 2015; Siemieniuk 2015; Wan 2016).
We also searched the following conference proceedings: European Congress of Clinical Microbiology and Infectious Diseases (2001 to 2016) and ICAAC/Annual Meeting of the Infectious Diseases Society of America (IDSA) (2001 to 2016) and intensive care conference proceedings.
We searched trial registers for ongoing and unpublished trials: WHO International Clinical Trials Registry Platform (ICTRP) (who.int/ictrp/en, searched 3 March 2017), ISRCTN registry (www.isrctn.com, searched 3 March 2017), and ClinicalTrials.gov (www.clinicaltrials.gov, searched 3 March 2017).
Data collection and analysis
Selection of studies
Two review authors (AS, KS) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search.
We retrieved full‐text study reports, and two review authors (AS, KS) independently screened the full texts to identify studies for inclusion and recorded reasons for exclusion of the ineligible studies. Any disagreements were resolved by discussion or by consulting a third review author (MP). We identified and excluded duplicates and planned to collate multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We did not use any language or publication status restrictions. We recorded the selection process in a PRISMA flow diagram and Characteristics of excluded studies tables (Moher 2009).
Data extraction and management
We used a data collection form for study characteristics and outcome data that had been piloted. Two review authors (AS and KS or EC) independently extracted study characteristics from the included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: N, mean age, severity of condition, comorbidities, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant antibiotic therapy, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (AS, KS or EC) independently extracted outcome data from the included studies. We planned to note in the Characteristics of included studies table if outcome data were not reported in a usable way. Any disagreements were resolved by consensus or by involving a third review author (MP or LL). One review author (AS) transferred data into the Review Manager 5 file (Review Manager 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (MP) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (AS, KS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion or by involving another review author (MP). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided quotes from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
We entered outcome data for each study into the data tables in Review Manager 5 to calculate the treatment effects (Review Manager 2014). We used risk ratios for dichotomous outcomes and absolute mean differences for continuous outcomes (time to and durations). When means and standard deviations were not provided for the continuous outcomes in the primary studies, we estimated the means and standard deviations from the reported figures (medians, interquartile ranges) to enable meta‐analysis, using the methods described by Wan 2014.
Dealing with missing data
We contacted investigators to verify key study characteristics and obtain missing numerical outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including these studies in the overall assessment of results by a sensitivity analysis.
Where numerical outcome data were missing and could not be obtained from the authors, we calculated data from other available statistics such as P values according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. Where we identified substantial heterogeneity, we reported this with details from our exploration of possible causes according to prespecified subgroup analysis.
Assessment of reporting biases
We planned that if we were able to pool more than 10 trials, we would create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We undertook meta‐analyses where more than one study provided usable data in any single comparison, only where this was meaningful, that is if the treatments, participants, and the underlying clinical question were sufficiently similar and clinically homogeneous for pooling to make sense. We pooled risk ratios (RRs) and mean differences (MDs) with 95% confidence intervals (CIs). As we observed no events in all trials for the analysis of mortality in children, we pooled risk differences (RDs) to estimate the 95% CIs of no difference between arms. We assumed clinical heterogeneity, and initially applied the random‐effects model to all meta‐analyses. However, as results of the fixed‐effect and random‐effects models were similar, we used a fixed‐effect meta‐analysis to summarise the best estimate of the intervention effect, given that most meta‐analyses included few studies and so as not exaggerate the contribution of small studies (Higgins 2011).
We performed data analysis using Review Manager 5 software (Review Manager 2014).
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: mortality and early clinical failure. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2015). We downgraded the evidence from 'high quality' by one level for serious, and by two levels for very serious study limitations or risk of bias, indirectness of evidence, inconsistency across the included studies, lack of precision of effect estimates, or potential publication bias.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses a priori for adults and children. We used the Chi² test to test for subgroup interactions in Review Manager 5 software (Review Manager 2014). We expected further heterogeneity in the effect estimates for mortality and clinical failure by the following factors and conducted subgroup analysis when data provided in the primary studies allowed. When outcome data were not provided by the studies for the specific subgroup, we conducted meta‐regression.
Pneumonia and sepsis severity: analysed by a trial‐level or participant‐level subgroup analysis of severe versus non‐severe pneumonia. Severe pneumonia was assessed using the Pneumonia Severity Index (PSI) and defined as ≥ 4 or equivalent. We regarded trials that did not subgroup participants according to pneumonia severity as including people with severe pneumonia if the mortality rate in the control arm was 9% or more, based on PSI IV mortality rate of 9.3% (Fine 1997). We also analysed this factor at the study level using meta‐regression of the percentage of participants in the study with hypotension at presentation (systolic blood pressure < 90 mm Hg).
Place and mode of acquisition of the infection (CAP + HCAP/HAP/VAP): analysed by subgroup analyses.
Underlying chronic obstructive pulmonary disease (COPD): analysed using meta‐regression of the percentage of participants with COPD in the trial.
Pathogen causing the pneumonia: analysed through meta‐regression of the percentage of participants with S pneumoniae, Legionella spp, Chlamydophila pneumoniae, and Mycoplasma pneumoniae infections.
We performed meta‐regression using Comprehensive Meta‐Analysis V3.
Sensitivity analysis
We examined the effect of risk of bias on the results for mortality through sensitivity analysis on allocation concealment, as the most likely factor potentially affecting effect estimates for all‐cause mortality (Wood 2008).
Results
Description of studies
Results of the search
The searches yielded 5545 distinct references. We removed 1272 records because they were duplicates. We assessed the titles of 4273 and the abstracts of 88 distinct records. After removing irrelevant and clearly ineligible studies, we assessed the full texts of 24 studies for eligibility. We included 17 studies in the review and meta‐analysis, excluded six studies, and identified one study as awaiting classification because of unclear design and inconsistency in results (Figure 1).
1.
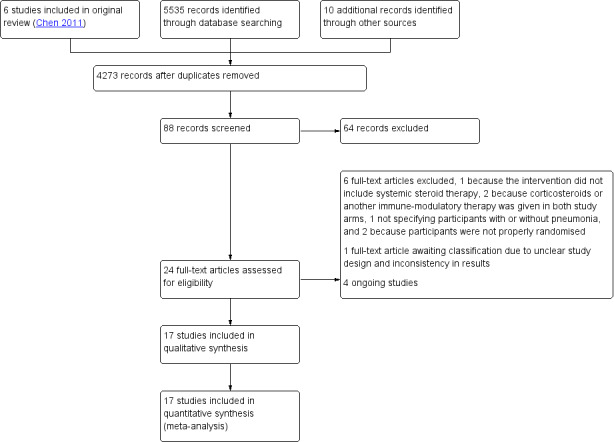
Study flow diagram.
Included studies
Details of the included studies are summarised in the Characteristics of included studies table.
We included 17 randomised controlled trials in this update, five of which were included in the 2011 review (Chen 2011). The studies were performed between 1966 and 2014, but Marik 1993 did not specify recruitment dates. Six of the studies were multicentre trials including from two to seven centres, all in a single country (Blum 2015; Confalonieri 2005; Meijvis 2011; Sabry 2011; Torres 2015; Van Woensel 2003), and 11 studies were single‐centre trials. The trials were conducted worldwide: eight in Europe, four in China or Japan, three in the Middle East, and one each in South Africa and Australia.
All included trials limited inclusion to participants with CAP, with or without HCAP, who were treated as inpatients. None of the trials included people with CAP who were treated in the community, HAP, or VAP. Inclusion was not limited by CAP pathogens in all adult trials. Van Woensel 2003 included only young children who were mechanically ventilated due to respiratory syncytial virus lower respiratory tract infections (82 infants, of whom 41 had pneumonia). Two trials limited inclusion to children with M pneumoniae (Luo 2014; Wu 2014).
A total of 2264 participants were randomised (1122 to the intervention arm), of whom 1954 (86%) were adults (n = 13 trials, mean age 69.8 years) and 307 (14%) were children (n = 4 trials, mean age 5.6 years, Luo 2014; Nagy 2013; Van Woensel 2003; Wu 2014). Of the adult trials, three reported that no people with chronic lung disease were included (Mikami 2007; Nafae 2013; Sabry 2011); in six trials reporting data between 11.2% and 35.9% of participants had chronic lung disease. A single trial reported that people with diabetes mellitus were not included (McHardy 1972), and in five trials between 10.3% and 19.7% of participants had diabetes.
Six trials included only adults with severe pneumonia. Four trials defined severe pneumonia according to the Infectious Diseases Society of America and American Thoracic Society guidelines (ATS/IDSA guidelines) for severe pneumonia, Mandell 2007, or earlier versions of these guidelines (Confalonieri 2005; El‐Ghamrawy 2006; Sabry 2011; Torres 2015). Marik 1993 defined severe pneumonia as presentation of three or more British Thoracic Society (BTS) criteria for severe pneumonia (BTS guidelines 1987). Fernández‐Serrano 2011 defined pneumonia as the presence of respiratory failure and extensive radiologically confirmed consolidations.
The intervention included oral prednisone in three trials (Blum 2015; Luo 2014; McHardy 1972), and intravenous dexamethasone, hydrocortisone, or methylprednisolone in 13 trials. One trial used prednisone without limiting the administration route (Snijders 2010). The duration of corticosteroid treatment was 10 days in one trial (Fernández‐Serrano 2011), seven to 10 days in one trial (Wu 2014), seven days in seven trials (Blum 2015; Confalonieri 2005; El‐Ghamrawy 2006; McHardy 1972; Nafae 2013; Sabry 2011; Snijders 2010), five days in three trials (Luo 2014; Nagy 2013; Torres 2015), and two to four days in four trials (Hatakeyama 1995; Meijvis 2011; Mikami 2007; Van Woensel 2003). In one trial only one dose of corticosteroids was given (Marik 1993). Most adult trials used steroid doses equivalent to 40 mg to 50 mg of prednisone per day. The comparator for corticosteroids was placebo in 11 trials and no treatment in six trials (Confalonieri 2005; Luo 2014; McHardy 1972; Mikami 2007; Sabry 2011; Wu 2014).
Seven trials were funded by academic sponsors (Blum 2015; Confalonieri 2005; Fernández‐Serrano 2011; Meijvis 2011; Nagy 2013; Torres 2015; Van Woensel 2003), one trial was funded by a pharmaceutical company (Snijders 2010), and one trial reported receiving no funding (Sabry 2011). The remaining eight trials did not report their funding source. We sought additional data for all trials, which two authors supplied (Blum 2015; Torres 2015).
Excluded studies
We excluded six studies (see Characteristics of excluded studies table). One trial that was included in the 2011 review was excluded because it examined the use of inhaled corticosteroids (Cao 2007). Other reasons for exclusion were: not limited to people with pneumonia (Van Woensel 2011), corticosteroids given to both treatment arms (Huang 2014), participants in the control group were subsequently given either corticosteroids or intravenous immunoglobulin therapy (Shan 2017), quasi‐randomisation (Wagner 1956), and one non‐randomised trial (Montón 1999).
Studies awaiting classification
One trial that assessed children with refractory M pneumoniae pneumonia is awaiting classification (Lan 2015). We contacted the authors for clarification about study design and inconsistencies in reported results.
Risk of bias in included studies
Risk of bias for all trials is summarised in Figure 2 and Figure 3.
2.
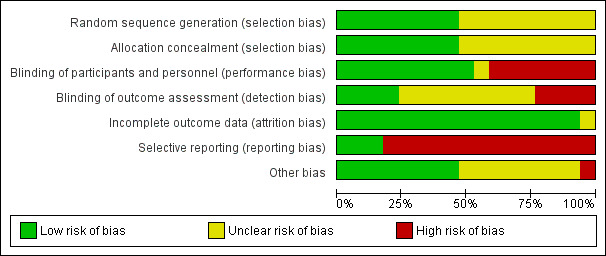
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
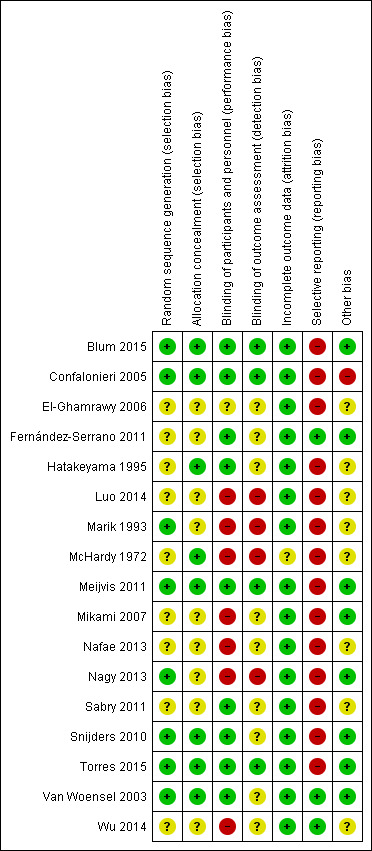
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed generation of the randomisation sequencing as low risk of bias in eight trials (Blum 2015; Confalonieri 2005; Marik 1993; Meijvis 2011; Nagy 2013; Snijders 2010; Torres 2015; Van Woensel 2003). The risk of bias was unclear in nine trials. We assessed the risk of bias for allocation concealment as low in eight trials (Blum 2015; Confalonieri 2005; Hatakeyama 1995; McHardy 1972; Meijvis 2011; Snijders 2010; Torres 2015; Van Woensel 2003), and unclear in nine trials.
Blinding
We assessed nine double‐blinded, placebo‐controlled trials as at low risk of bias (Blum 2015; Confalonieri 2005; Fernández‐Serrano 2011; Hatakeyama 1995; Meijvis 2011; Sabry 2011; Snijders 2010; Torres 2015; Van Woensel 2003). We assessed seven open‐label trials as at high risk of bias (Luo 2014; Marik 1993; McHardy 1972; Mikami 2007; Nafae 2013; Nagy 2013; Wu 2014). El‐Ghamrawy 2006 used a placebo, but did not describe blinding and was therefore assessed as at unclear risk of bias. We assessed four trials in which outcome assessors were blinded as at low risk of bias (Blum 2015; Confalonieri 2005; Meijvis 2011; Torres 2015). Nine trials did not specify if outcome assessors were blinded and were assessed as at unclear risk of bias (El‐Ghamrawy 2006; Fernández‐Serrano 2011; Hatakeyama 1995; Mikami 2007; Nafae 2013; Sabry 2011; Snijders 2010; Van Woensel 2003; Wu 2014). We assessed four trials in which outcome assessors were not blinded as at high risk of bias (Luo 2014; Marik 1993; McHardy 1972; Nagy 2013).
Incomplete outcome data
Eleven trials provided full intention‐to‐treat analysis (all randomly assigned participants were included in the analysis for mortality). In five trials there was postrandomisation exclusion of participants, but numbers of dropouts were given per study arm and reasons for exclusion were provided (Blum 2015; Confalonieri 2005; Fernández‐Serrano 2011; Torres 2015; Van Woensel 2003), therefore we judged these trials as at low risk of attrition bias. McHardy 1972 reported postrandomisation exclusions, but did not provide the number and study arm distribution of these participants and was therefore judged as at unclear risk of bias.
Selective reporting
Trial registries were available for seven studies conducted from 2010 onwards (Blum 2015; Fernández‐Serrano 2011; Meijvis 2011; Nagy 2013; Sabry 2011; Snijders 2010; Torres 2015). Only one of these seven studies prespecified all study outcomes in the registry (Fernández‐Serrano 2011), therefore we judged the remaining studies as at high risk of reporting bias. Of the trials that did not have a registry, most were conducted before mandatory trial registry, but three were relatively new trials and were therefore judged as being at high risk of reporting bias as well (Luo 2014; Nafae 2013; Wu 2014). Of the older trials without a registry, only Van Woensel 2003 had full agreement in outcomes between methods and results, hence we judged the remaining studies as at high risk of reporting bias.
Other potential sources of bias
Eight studies reported on sample size calculations and were classified as being at low risk of other potential sources of bias (Blum 2015; Fernández‐Serrano 2011; Meijvis 2011; Mikami 2007; Nagy 2013; Snijders 2010; Torres 2015; Van Woensel 2003). Confalonieri 2005 reported on sample size calculation but also reported on having an early stop for benefit, when the upper stopping boundary defined for stopping was crossed for the outcome of PaO₂:FiO₂, and was thus classified as being at high risk of other potential sources of bias. We classified the remaining eight studies as being at unclear risk of bias for this domain.
Effects of interventions
See: Table 1
Primary outcome
1. All‐cause mortality
Adults
All‐cause mortality was reported in 11 of the 13 trials that included adults (N = 1863). Corticosteroid therapy was associated with a significantly lower rate of all‐cause mortality compared to control (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.47 to 0.92; I² = 0%, fixed‐effect model). Despite the lack of statistical heterogeneity in this analysis, the benefit was larger and statistically significant in studies with unclear allocation concealment methods (RR 0.36, 95% CI 0.19 to 0.71) compared to studies using low‐risk methods (RR 0.80, 95% CI 0.54 to 1.19; Analysis 1.1; moderate‐quality evidence) (Table 1). No small‐studies effect was demonstrated in the funnel plot analysis (Figure 4).
1.1. Analysis.
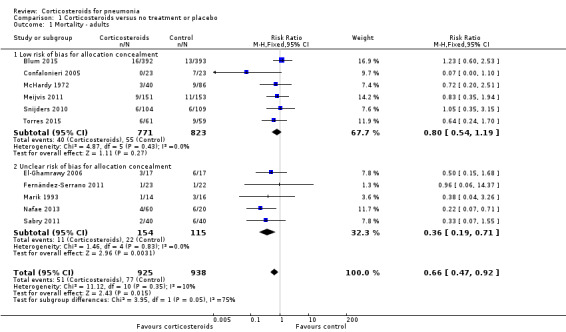
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 1 Mortality ‐ adults.
4.
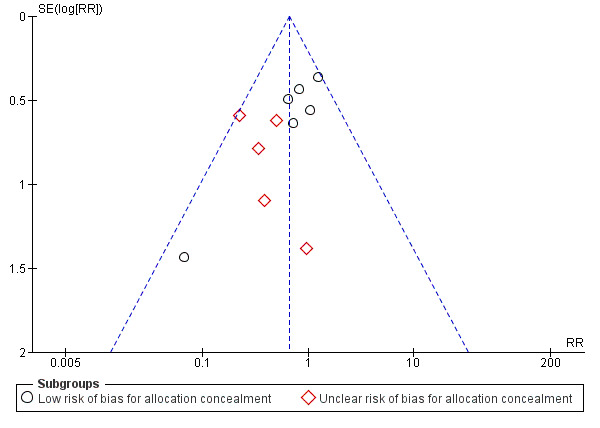
Funnel plot of comparison: 1 Corticosteroids versus no treatment or placebo, outcome: 1.1 Mortality ‐ adults.
Subgroup analyses
Nine studies (995 participants) provided data on participants with severe pneumonia. All‐cause mortality was significantly lower for participants receiving corticosteroids compared to control (RR 0.58, 95% CI 0.40 to 0.84; I² = 12%, fixed‐effect model). We judged the quality of the evidence as moderate due to differences in effect estimates between low and unclear risk of bias (Analysis 1.2). Excluding Confalonieri 2005, which was stopped early due to significant difference between the groups based on predefined stopping rules, with 0 deaths in the steroid arm, did not significantly change the overall result (RR 0.65, 95% CI 0.44 to 0.95), but brought the difference between the trials at low risk or bias and unclear risk of bias to statistical significance (P = 0.03 for subgroup differences). Four trials reported mortality in participants with non‐severe pneumonia (868 participants). There was no significant difference between corticosteroid therapy and control (RR 0.95, 95% CI 0.45 to 2.00; fixed‐effect model). We graded the quality of the evidence as moderate due the large confidence intervals leading to uncertainty in the true effect estimate. In the meta‐regression analysis, higher mortality in the control arm was significantly associated with a larger effect of corticosteroids on mortality, with the ratio of log RRs decreasing by 0.217 for every 1% increase in the mortality rate of the control arm (P = 0.007, Figure 5).
1.2. Analysis.
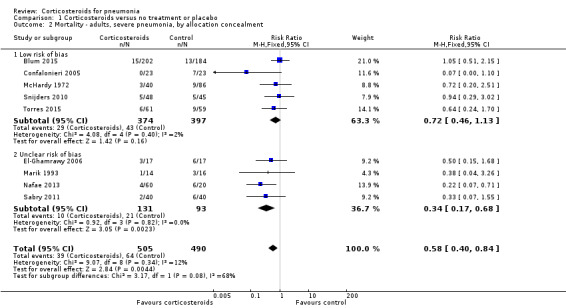
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 2 Mortality ‐ adults, severe pneumonia, by allocation concealment.
5.
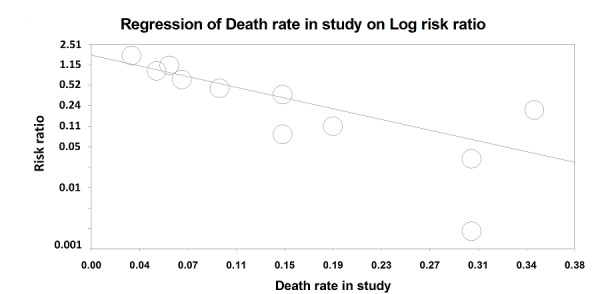
Meta‐regression of the log risk ratios for the effect of steroids on mortality against the mortality rate (%) in the control group.
Most adult trials reporting on mortality used corticosteroid doses equivalent to 40 mg to 50 mg of prednisone per day, for 5 to 10 days. All studies included participants with CAP or HCAP. Participants with HAP or VAP were excluded, therefore subgroup analysis by place of acquisition was not possible. None of the studies provided mortality data for the subgroups of septic shock versus no septic shock and by specific pathogens, and only one study reported mortality in the subgroup of participants with chronic obstructive pulmonary disease (COPD) (Blum 2015). In meta‐regression analyses for these factors, no significant association was found between the risk ratios in individual trials and percentage of participants with septic shock (six studies), COPD (nine studies, three of which excluded participants with COPD), or pathogens causing pneumonia (analysing the percentage of participants with documented Pneumococcal pneumonia, pneumonia caused by atypical bacteria or isolation of a virus). The percentage of participants with no microbiological documented infection was inversely associated with the effect of corticosteroids, but this association was not statistically significant. In a post hoc meta‐regression analysis according to mean age of study population, the effect was significantly smaller with increasing age, with the ratio of log RRs increasing by 0.05 for every 1% increase in the mean age (P = 0.018, Figure 6).
6.

Meta‐regression of the log risk ratios for the effect of steroids on mortality against the mean age in the study population.
Children
There were no reported deaths in the four trials that included children (risk difference (RD) 0.00, 95% CI ‐0.03 to 0.03; 4 trials, 266 children, Analysis 1.4).
1.4. Analysis.
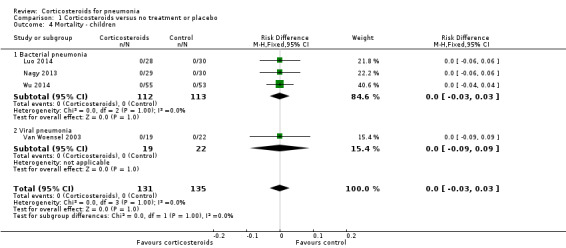
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 4 Mortality ‐ children.
Secondary outcomes
1. Early clinical failure
Adults
Six trials reported the outcome of early clinical failure (1324 participants). The definitions used in the trials varied and are reported in Table 2. There was a significantly lower rate of early clinical failure in participants treated with corticosteroids compared to controls (RR 0.40, 95% CI 0.23 to 0.7; random‐effects model). We found substantial heterogeneity in this analysis (Analysis 1.5, I² = 72%). We downgraded the quality of the data for this outcome to moderate due to inconsistency, despite the large effect.
1. Study definitions for clinical failure and time to cure.
| Study name | Clinical failure definition | Time to clinical cure |
| Blum 2015 | Number of participants not reaching clinical stability on day 5. Clinical stability defined as stable vital signs for ≥ 24 hours. Stable vital signs were temperature of ≤ 37.8 °C, heart rate of ≤ 100 beats per min, spontaneous respiratory rate of ≤ 24 breaths per min, systolic blood pressure of ≥ 90 mm Hg (≥ 100 mm Hg for participants with hypertension) without vasopressor support, mental status back to level before occurrence of community‐acquired pneumonia, ability for oral intake, and adequate oxygenation on room air (PaO₂ ≥ 60 mm Hg or pulse oximetry ≥ 90%). |
Time to clinical stability defined as stable vital signs for ≥ 24 hours. Stable vital signs were temperature of ≤ 37.8 °C, heart rate of ≤ 100 beats per min, spontaneous respiratory rate of ≤ 24 breaths per min, systolic blood pressure of ≥ 90 mm Hg (≥ 100 mm Hg for participants with hypertension) without vasopressor support, mental status back to level before occurrence of community‐acquired pneumonia, ability for oral intake, and adequate oxygenation on room air (PaO₂ ≥ 60 mm Hg or pulse oximetry ≥ 90%). |
| Confalonieri 2005 | Number of participants not achieving PaO₂:FiO₂ improvement ≥ 100 mm Hg compared to study entry, evaluated at day 8 | Time to weaning from mechanical ventilation |
| El‐Ghamrawy 2006 | Not evaluated | Time to weaning from mechanical ventilation |
| Fernández‐Serrano 2011 | Not evaluated | Time to resolution of morbidity score, a semi‐quantitative score combining clinical and radiological variables (not detailed in the manuscript but reference provided) |
| Luo 2014 | Number of participants with no infiltrate resolution at day 7 | Time to resolution of hypoxaemia |
| Nafae 2013 | Number of participants with no improvement at day 7 (improvement definition not provided) | Time to weaning from mechanical ventilation |
| Nagy 2013 | Number of participants not improving based on clinical and radiological status on day 7 | Time to fever resolution |
| Mikami 2007 | Not evaluated | Time to fever resolution |
| Sabry 2011 | Number of participants not achieving PaO₂:FiO₂ improvement ≥ 100 mm Hg compared to study entry, evaluated at day 8 | Time to weaning from mechanical ventilation |
| Snijders 2010 | Number of participants with clinical failure at day 7. Clinical failure defined as: persistence or progression of all signs and symptoms that developed during the acute disease episode after randomisation, or the development of a new pulmonary or extrapulmonary infection, or the deterioration of chest radiography after randomisation, or death due to pneumonia, or the inability to complete the study owing to adverse events. |
Time to clinical stability defined as when all 4 of the following criteria were met: improvement of cough and shortness of breath, temperature < 37.8 °C for at least 8 hours, declining serum C‐reactive protein levels, and adequate oral intake and gastrointestinal absorption. |
| Torres 2015 | Number of participants with treatment failure between 72 hours and 120 hours after treatment initiation. Treatment failure defined as radiographic progression (increase of ≥ 50% of pulmonary infiltrates compared with baseline), persistence of severe respiratory failure (PaO₂:FiO₂ < 200 mm Hg, with respiratory rate ≥ 30 breaths/min in participants not intubated), development of shock, need for invasive mechanical ventilation not present at baseline, or death. | Time to clinical stability defined as when all of the following criteria were met: temperature ≤ 37.2 °C, heart rate ≤ 100 beats/min, systolic blood pressure ≥ 90 mm Hg, and arterial oxygen tension ≥ 60 mm Hg when the participant was not receiving supplemental oxygen. In participants receiving oxygen therapy at home, stability was considered to be achieved when oxygen needs were the same as before admission. |
| Van Woensel 2003 | Not evaluated | Duration of supplemental oxygen need |
| Wu 2014 | Not evaluated | Time to fever resolution |
FiO₂ = inspired oxygen concentration PaO₂ = partial pressure of oxygen in arterial blood
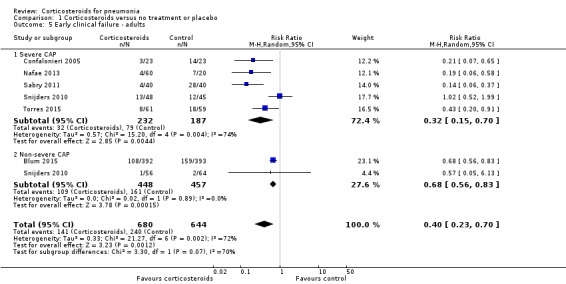
1.5. Analysis.
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 5 Early clinical failure ‐ adults.
Subgroup analyses
Five trials including 419 participants provided data regarding early clinical failure in participants with severe pneumonia, and two trials including 905 participants reported this outcome in participants with non‐severe pneumonia. In both subgroups the rate of early clinical failure was significantly lower in the corticosteroid arm compared to control (RR 0.32, 95% CI 0.15 to 0.7; I² = 74% for severe pneumonia, and RR 0.68, 95% CI 0.56 to 0.83; I² = 0% for non‐severe pneumonia, random‐effects model; Analysis 1.5). Heterogeneity in the subgroup of severe pneumonia resulted from different magnitudes of benefit rather than from opposing direct of effects. We graded the evidence for both analyses as of high quality.
The dose of corticosteroid used in most adult trials reporting on early clinical failure was equivalent to 40 mg to 50 mg of prednisone per day, for 5 to 10 days. Only Confalonieri 2005 reported clinical failure in the subgroup of participants with septic shock. No trials reported on this outcome by pathogen or among participants with and without COPD. Meta‐regression analyses could not be fitted due to the paucity of studies reporting on the outcome and participant characteristics.
Children
The outcome of early clinical failure was reported in two studies (88 children) with definitions provided in Table 2, and was significantly lower in the corticosteroid group compared to the control group (RR 0.41, 95% CI 0.24 to 0.70; I² = 25%, fixed‐effect model; Analysis 1.6). We graded this analysis as high quality.
1.6. Analysis.

Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 6 Early clinical failure ‐ children.
In an analysis by allocation concealment among adults and children combined, early clinical failure was significantly lower in the corticosteroid arm both for trials with low risk of bias (RR 0.59, 95% CI 0.37 to 0.94) and trials with unclear risk RR 0.27, 95% CI 0.14 to 0.52; random‐effects model; Analysis 1.7). The number of studies was too small for a funnel plot analysis.
1.7. Analysis.
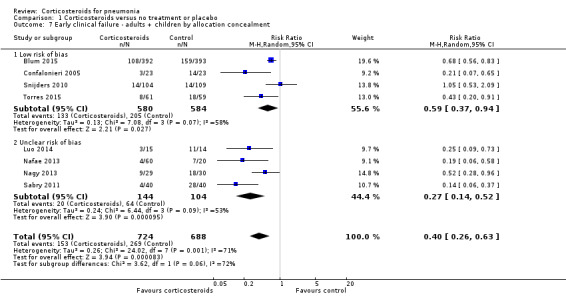
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 7 Early clinical failure ‐ adults + children by allocation concealment.
2. Time to clinical cure
Adults
Nine trials reported time to clinical cure (1322 participants). We accepted the definitions provided in all studies (Table 2). Three trials reported this outcome as median with interquartile range (IQR) (Blum 2015; Fernández‐Serrano 2011; Torres 2015), and one trial reported median with range (Confalonieri 2005). For these trials, means and standard deviations (SDs) were estimated from the median value provided. The time to clinical cure was significantly shorter in the corticosteroid group compared to placebo, but we found substantial heterogeneity in this analysis (mean difference (MD) ‐1.83 days, 95% CI ‐2.45 to ‐1.21; I² = 83%, random‐effects model; Analysis 1.8). In a sensitivity analysis, excluding the trials that did not meet our predefined criteria for clinical cure did not significantly affect results.
1.8. Analysis.
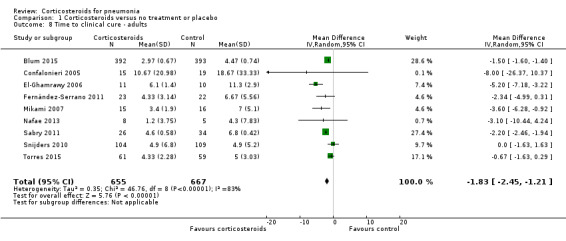
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 8 Time to clinical cure ‐ adults.
Children
Four trials reported time to clinical cure, of which three studies (225 children) evaluated bacterial pneumonia and one included 41 children with respiratory syncytial virus pneumonia. In one trial (Nagy 2013), mean and SD were estimated from the median and IQR. In the three trials evaluating bacterial pneumonia, time to clinical cure was significantly shorter in the corticosteroid arm compared to the control arm (MD ‐1.57 days, 95% CI ‐2.55 to ‐0.60 days; I² = 80%, random‐effects model; Analysis 1.9). Van Woensel 2003, which evaluated viral pneumonia, showed no effect of corticosteroids on time to clinical cure. Here we also accepted the study definitions for time to clinical cure, which were not compatible with our protocol definitions (Table 2).
1.9. Analysis.
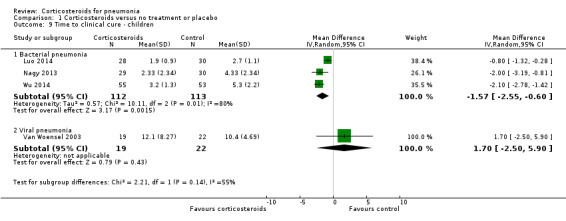
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 9 Time to clinical cure ‐ children.
3. Development of respiratory failure not present initially
The need for new non‐invasive or invasive mechanical ventilation, not present at onset of pneumonia, was reported in four adult trials (1030 participants) and was significantly lower in the corticosteroid arm compared to the control arm without heterogeneity (RR 0.40, 95% CI 0.20 to 0.77; fixed‐effect model; Analysis 1.10).
1.10. Analysis.
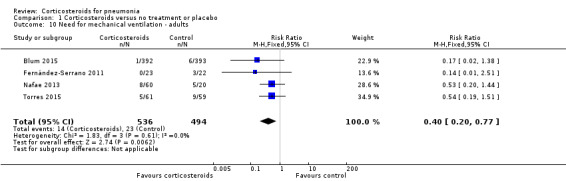
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 10 Need for mechanical ventilation ‐ adults.
4. Development of shock not present initially
The development of shock not present initially was reported in six adult trials (five including participants with severe pneumonia) and was significantly lower in the corticosteroid arm without heterogeneity (RR 0.18, 95% CI 0.09 to 0.34; fixed‐effect model; Analysis 1.11).
1.11. Analysis.
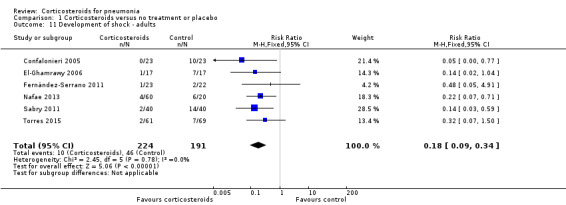
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 11 Development of shock ‐ adults.
5. Transfer to ICU among participants not admitted initially to the ICU
In the four adult trials (1164 participants) reporting the need for ICU transfer (2 included participants with non‐severe pneumonia), we found no significant difference between the corticosteroids and control arms (RR 0.73, 95% CI 0.45 to 1.18; I² = 0%, fixed‐effect model; Analysis 1.12).
1.12. Analysis.
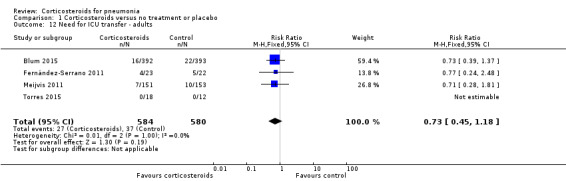
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 12 Need for ICU transfer ‐ adults.
6. Duration of hospital stay for hospitalised participants
Adults
The length of hospital stay was significantly shorter in the corticosteroid group compared to control (MD ‐2.91 days, 95% CI ‐4.92 to ‐0.89; 9 trials, 1658 participants; Analysis 1.13). We found considerable heterogeneity for this analysis (I² = 91%), analysed using the random‐effects model. Five of the trials provided median (range/IQR) values for this outcome, and means with standard deviations were calculated or requested from authors.
1.13. Analysis.
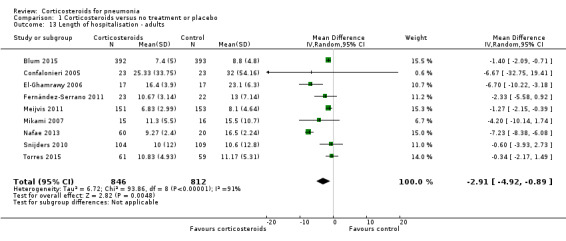
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 13 Length of hospitalisation ‐ adults.
Children
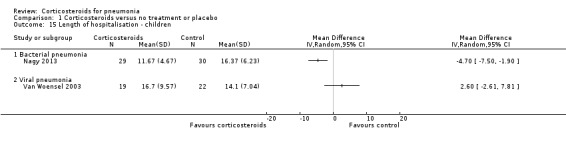
One trial including children with bacterial pneumonia, Nagy 2013, and one trial including children with viral pneumonia, Van Woensel 2003, reported lengths of hospital stay. We did not pool results due to significant heterogeneity, with a significant benefit to steroids in the trial on bacterial pneumonia (MD ‐4.70 days with steroids, 95% CI ‐7.50 to ‐1.90) and no difference in the trial on viral pneumonia (Analysis 1.15).
1.15. Analysis.
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 15 Length of hospitalisation ‐ children.
7. Duration of ICU stay for participants admitted to the ICU
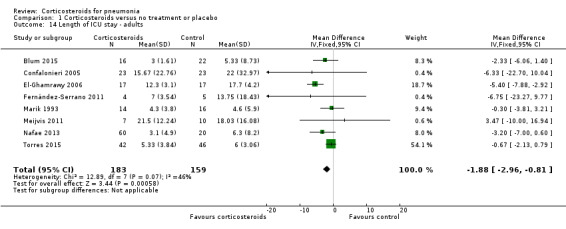
For adults who were admitted to the ICU (342 participants from 8 studies), ICU stay was significantly shorter in the corticosteroid group compared to control (MD ‐1.88 days, 95% CI ‐2.96 to ‐0.81; I² = 46%, fixed‐effect model; Analysis 1.14). Five trials provided median values (range/IQR) for this outcome, and means (SD) were calculated from these values.
1.14. Analysis.
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 14 Length of ICU stay ‐ adults.
8. Pneumonia complications not present initially
Nine trials reported pneumonia complications, including eight adult trials (1573 participants) and one trial in children (59 children). Most trials defined this outcome as a combination of lung abscess, empyema or pleural effusion. One trial defined complications as septic shock or acute respiratory distress syndrome (ARDS) (Nafae 2013), and two trials evaluated this outcome without providing a definition. The rate of pneumonia complications was significantly lower for the corticosteroid arm when compared to controls (RR 0.58, 95% CI 0.40 to 0.84; I² = 43%, fixed‐effect model; Analysis 1.16).
1.16. Analysis.
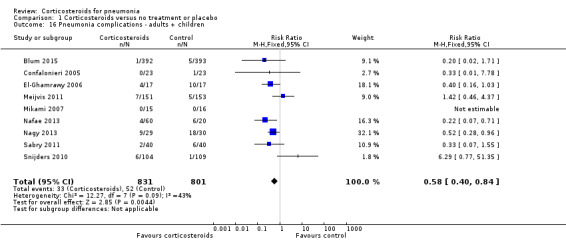
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 16 Pneumonia complications ‐ adults + children.
9. Secondary infections
For adults, we found no difference in the rate of secondary infections between the corticosteroid group and the control group (RR 1.19, 95% CI 0.73 to 1.93; 7 trials, 1533 participants, I² = 0%, fixed‐effect model; Analysis 1.17). All trials addressing this outcome in children had no reported cases of secondary infections (RD 0.00, 95% CI ‐0.03 to 0.03; 3 trials, 225 children; Analysis 1.18).
1.17. Analysis.
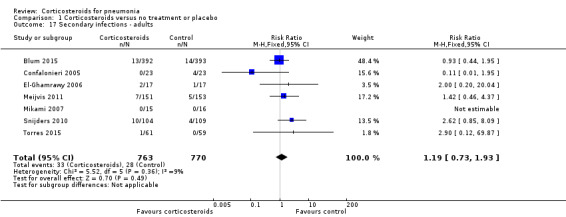
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 17 Secondary infections ‐ adults.
1.18. Analysis.
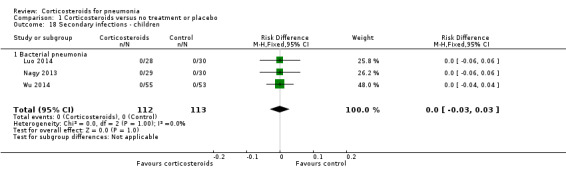
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 18 Secondary infections ‐ children.
10. Adverse events
Adults
Three trials reported on any adverse event (1028 participants). There was a trend towards more adverse events in the corticosteroid arm compared to control, but with no statistical significance (RR 1.21, 95% CI 0.99 to 1.47; fixed‐effect model; Analysis 1.19). Hyperglycaemia developed significantly more frequently in the corticosteroid arm (RR 1.72, 95% CI 1.38 to 2.14; 7 trials, 1578 participants, I² = 0%, fixed‐effect model; Analysis 1.20). We found no significant difference between the two arms for gastrointestinal bleeding (RR 0.91, 95% CI 0.40 to 2.05; 7 trials, 1190 participants, fixed‐effect model; Analysis 1.21), neuropsychiatric adverse events (RR 1.95, 95% CI 0.70 to 5.42; 4 trials, 1149 participants, fixed‐effect model; Analysis 1.22), or adverse cardiac events (RR 0.6, 95% CI 0.32 to 1.13; 5 trials, 1249 participants, fixed‐effect model; Analysis 1.23).
1.19. Analysis.
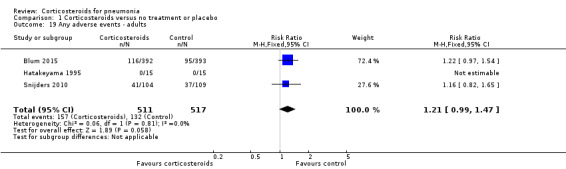
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 19 Any adverse events ‐ adults.
1.20. Analysis.
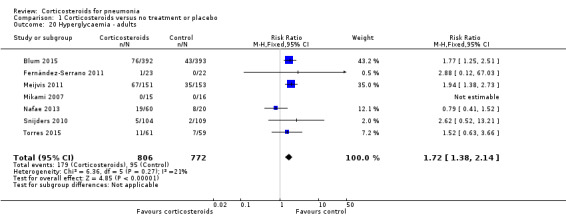
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 20 Hyperglycaemia ‐ adults.
1.21. Analysis.
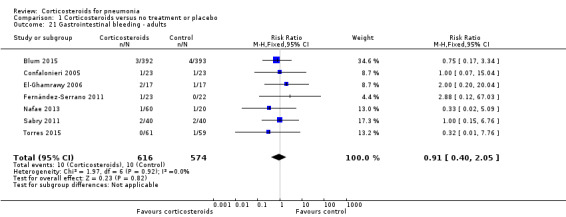
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 21 Gastrointestinal bleeding ‐ adults.
1.22. Analysis.
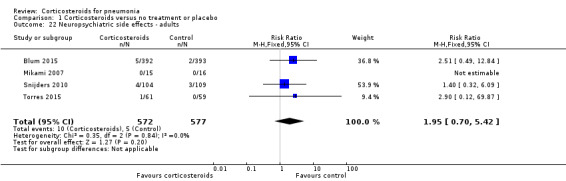
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 22 Neuropsychiatric side effects ‐ adults.
1.23. Analysis.
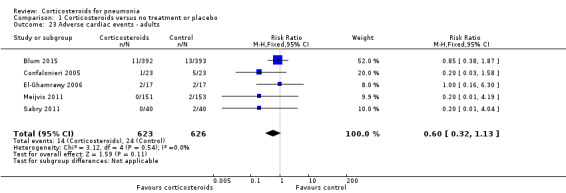
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 23 Adverse cardiac events ‐ adults.
Children
No adverse events were reported in trials in children. Two trials reported no adverse event in either arm (Analysis 1.24), and one trial reported no cases of hyperglycaemia.
1.24. Analysis.
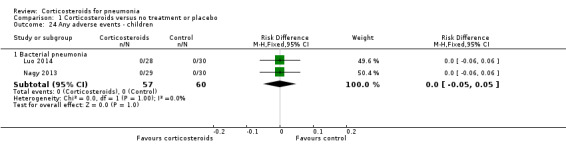
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 24 Any adverse events ‐ children.
Discussion
Summary of main results
In this review, we summarised the evidence on corticosteroid therapy, in addition to antibiotics, for pneumonia. Throughout the review, we separated the evidence for adults and children.
For adults, corticosteroids significantly reduced mortality for participants with severe pneumonia (RR 0.58, 95% CI 0.40 to 0.84), number needed to treat for an additional beneficial outcome (NNTB) 18 patients (95% CI 12 to 49 patients). We assessed the quality of the evidence as moderate because unclear allocation concealment methods exaggerated effect estimates. There was no benefit or harm for steroids with non‐severe pneumonia (RR 0.95, 95% CI 0.45 to 2.00). Overall, we found an association between the mean age of study population and the effect of corticosteroids such that the effect was significantly smaller with increasing age (P = 0.018). Early clinical failure, defined as lack of clinical or radiological improvement, or both, between days five and eight, was significantly reduced with corticosteroids for participants with severe and non‐severe pneumonia, but the benefit was larger and the NNTB was smaller for severe pneumonia (RR 0.32, 95% CI 0.15 to 0.7; NNTB 4, 95% CI 3 to 8 patients versus RR 0.68, 95% CI 0.56 to 0.83; NNTB 8, 95% CI 6 to 16 patients, respectively; high‐quality evidence). Corticosteroids reduced the time to clinical cure, length of hospital stay, and length of ICU stay for participants admitted to the ICU. Similarly, the development of respiratory failure necessitating mechanical ventilation, the development of shock not present at pneumonia onset, and the rate of pneumonia complications, mostly pyogenic (RR 0.58, 95% CI 0.40 to 0.84), were reduced among corticosteroid‐treated participants. Of the adverse events, only hyperglycaemia was significantly more common among corticosteroids‐treated participants (RR 1.72, 95% CI 1.38 to 2.14). There was no significant difference between groups for gastrointestinal, neuropsychiatric, and cardiac adverse events and superinfections (RR 1.19, 95% CI 0.73 to 1.93).
The paediatric trials were few and heterogenous, two including only children with M pneumoniae pneumonia (Luo 2014; Wu 2014), one children with any pneumonia (Nagy 2013), and one children with severe respiratory syncytial virus pneumonitis/pneumonia (Van Woensel 2011). There were no deaths in these trials. In the bacterial pneumonia trials, corticosteroids reduced early clinical failure rates (RR 0.41, 95% CI 0.24 to 0.70; high‐quality evidence) and shortened the time to clinical cure. In the single trial assessing children with respiratory syncytial virus, no benefit with regard to time to clinical cure was observed with corticosteroids. No adverse events were reported.
Overall completeness and applicability of evidence
The inclusion criteria for this review were designed to include all types of pneumonia; the search was not limited by participant age, place of acquisition of pneumonia, or the type of pathogen causing it. However, the completeness of the results is affected by the trials found and types of pneumonia studied. The results of this review apply only to hospitalised CAP and HCAP. We included adult and paediatric trials, but separated the analyses due to the differences between them in terms of outcomes. The reported rates of death from CAP in children are as low as 0.01% to 0.001% (Lee 2010), compared to 10% to 12% in adults (Musher 2014). Indeed, none of the paediatric trials included in our review reported deaths or complications as defined in this review.
Among adults, there is an overall advantage to corticosteroid therapy. The question remains as to whom this benefit applies: who are the patients that will gain from corticosteroid therapy when hospitalised with CAP/HCAP? The first clear group to emerge is those with severe pneumonia defined using ATS/IDSA or BTS criteria (BTS guidelines 1987; Mandell 2007). Among patients with severe CAP/HCAP, 18 patients need to be treated to prevent one death and four to prevent one clinical failure at five to eight days. The corticosteroid regimen most commonly used in these trials was an intravenous (IV) formulation equivalent to 40 mg to 50 mg of prednisone per day for five to 10 days. Few trials used higher dosing, up to 90 mg of prednisone‐equivalents a day, and one old trial used a single dose of IV hydrocortisone 10 mg/kg (Marik 1993). Corticosteroid therapy has been shown to reduce mortality rates in people with septic shock (Annane 2009), and have conflicting effects on people with ARDS (Ruan 2014). The Surviving Sepsis Campaign Guidelines recommend IV hydrocortisone at a dose of 200 mg per day to people with septic shock not restoring haemodynamic stability after adequate fluid resuscitation and vasopressor therapy (Rhodes 2017). It is not possible to determine to what extent the effect of corticosteroids on mortality in severely ill patients is explained by its effect on septic shock or ARDS. However, meta‐regression did not show an association between the percentage of patients with septic shock and the effect of corticosteroid therapy on mortality, pointing at the possible contribution of corticosteroid in pneumonia independent of septic shock. People with pneumonia and septic shock have, by definition, severe pneumonia, and the same steroid regimen applies to both, except that with septic shock the recommendations are to continue steroids until haemodynamic stability is reached followed by tapering off.
Some people with pneumonia may be affected by corticosteroid therapy differently than others. Corticosteroids have been shown to significantly reduce failure among people with COPD exacerbation, but not mortality (Walters 2014), thus people with COPD exacerbation and CAP might gain more from corticosteroid therapy than other patients. We could not perform a subgroup analysis to determine if there is a difference in the effect of corticosteroids in patients with or without COPD due to the paucity of trials providing relevant data. A meta‐regression analysis did not show an association between the percentage of participants with COPD in the trials and corticosteroid effects on mortality. To note, three trials excluded people with COPD.
People with diabetes mellitus may be adversely affected by corticosteroid therapy by being more prone to hyperglycaemia and its detrimental effect on survival in acutely ill patients (Krinsley 2003). We did not perform subgroup analyses according to this confounder due to the paucity of outcome data. Overall, hyperglycaemia was significantly more common with corticosteroids.
Elderly patients with pneumonia have higher rates of treatment failure, need for intensive care, and mortality (Kaplan 2002). The trials included in this review were not limited by age definitions, but as previously shown, participants' mean age in these randomised controlled trials was lower than that of people treated for CAP in clinical practice (Avni 2015). There were no outcome data by age subgroup, but by meta‐regression we observed decreasing effects of corticosteroid therapy on mortality with increasing mean age of the trials' population, denoting a smaller survival benefit with aging. This finding could be linked to more diabetes with increasing age, the neuropsychiatric effects of corticosteroids (although not shown in the overall analysis), or different inflammatory response in elderly patients.
The pathogenesis of pneumonia may be different with different causative pathogens. The inflammatory response caused by bacterial pneumonia is thus different from that caused by viral pneumonia, and the radiographic presentation of 'atypical' bacteria is different from that of S pneumoniae or other bacteria causing pneumonia (Virkki 2002). None of the adult trials included in this review restricted the inclusion to specific pathogens, and none reported relevant outcome data by pathogen. Meta‐regression analyses did not show associations between corticosteroid effects and the percentage of participants in the trials with the different pathogens. However, these analyses were also limited by the fact that the bacterial aetiology of CAP/HCAP is frequently unknown, and data were frequently not reported. Some of the cases of pneumonia with no microbiological documented infection may actually represent pulmonary infiltrates of a non‐infectious, inflammatory aetiology (e.g. organising pneumonia). A possible theory might be that the effect of corticosteroids in pneumonia is at least partially driven by its effect on the subset of patients with non‐infectious pneumonia. Our results do not support this hypothesis.
The heterogeneity in the paediatric trials precludes strong conclusions for children with CAP. Overall, a significant benefit was demonstrated with regard to early clinical failure, pointing at the potential of this intervention. However, we were unable to define the children likely to benefit from corticosteroids and the dose and duration from the available trials.
Quality of the evidence
The results for the main outcomes and the quality of evidence assessments are summarised in Table 1. For the primary outcome of mortality, we assessed all analyses as of moderate quality. For the outcome of early clinical failure, we assessed the quality of the main analysis as moderate. However, we judged the analyses of severe pneumonia, non‐severe pneumonia, and paediatric participants as high quality. Several reasons led us to downgrade the quality of the evidence. Some of the trials included in the review had unclear allocation concealment. We downgraded the evidence quality for risk of bias when a sensitivity analysis implied that unclear allocation concealment exaggerated the effect of corticosteroids, but not when such an effect was not evident. We downgraded quality for imprecision whenever the 95% CI overlapped no effect with a wide confidence interval so that harm caused by corticosteroids could not have been ruled out, and for inconsistency whenever considerable heterogeneity was found. In cases of a risk ratio lower than 0.5, we upgraded the quality of the evidence by one level.
Potential biases in the review process
This review has a few limitations that should be noted. For most predefined subgroups, lack of data precluded us from performing subgroup analyses. Only two trials provided mortality data per study arm in the subgroups of severe and non‐severe pneumonia (Blum 2015; Snijders 2010). For the remaining trials we classified the trial as including severe or non‐severe pneumonia participants according to the mortality rate in the control arm. Hence, for most trials the classification is at the trial level and not at the participant level. Furthermore, mortality, although reflecting the severity of pneumonia, may be influenced by other parameters such as the antibiotic regimens used or the standard of medical care given in the specific centre. Nevertheless, we found a good correlation between the trials' percentage of participants with Pneumonia Severity Index four to five and our severity classification based on mortality (data not shown). To note, one of the trials that included people with severe pneumonia by its own definition was actually classified as non‐severe pneumonia according to the mortality rate in the control arm (Fernández‐Serrano 2011).
In place of subgroup analyses, we performed meta‐regression. The primary outcome of mortality was reported in 11 out of the 13 adult trials, thus meta‐regression analyses could have been done for this outcome, but not for the secondary outcomes, which were reported in fewer trials. Meta‐regression analyses may contribute to the understanding of the association between specific confounders and the effect, but they should be regarded as observational, and provide only trial‐level evidence as opposed to the patient‐level evidence provided by randomised controlled trials.
We chose all‐cause mortality as the primary outcome, assuming it to be the most objective outcome and the most relevant outcome to patients. This is true for the adult population, but not for the paediatric trials, where mortality cannot be analysed because of the very low event rate.
For the outcomes of early clinical failure and time to clinical cure, we allowed some diversity in the outcome definitions and accepted definitions that did not meet the strict predefined criteria of the review. This may lead to an indirectness bias. For example, we accepted fever resolution as a surrogate for clinical cure. It is true that a patient would not be held as cured while still febrile, but the opposite might not be true, as a patient may be afebrile but not cured.
For some continuous outcomes, we had to estimate means from medians to perform meta‐analysis. This is imprecise, since these outcomes were reported as medians due to their skewed distribution. These analyses should thus be viewed with caution.
The dose and schedule of corticosteroids administered varied, as shown in the Characteristics of included studies table. We did not account for this variability in our analysis, assuming a common effect of different corticosteroid regimens.
Agreements and disagreements with other studies or reviews
The previous version of this review showed that corticosteroid therapy hastens the resolution of symptoms for people with pneumonia, but with no effect on mortality (Chen 2011). In 2015, two relatively large, high‐quality randomised controlled trials evaluating corticosteroid for people with CAP were published. The first trial included people with severe pneumonia hospitalised mostly in the ICU and showed reduced clinical failure rates with corticosteroid therapy (Torres 2015). The second trial included people with severe and non‐severe pneumonia (and provided separate outcome data) and showed shortened time to clinical stability with corticosteroids (Blum 2015). Neither of the trials showed an effect on mortality. Since then, a few reviews and meta‐analyses assessing corticosteroid therapy in pneumonia have been published (Horita 2015; Marti 2015; Siemieniuk 2015; Wan 2016), all showing benefit to corticosteroid therapy but to different extent. Our review adds several pieces of knowledge over previous reviews. First, we have shown the importance of allocation concealment even for the objective outcome of mortality. Trials that did not describe adequate methods of allocation concealment showed greater mortality reductions with corticosteroids, affecting the overall analysis of mortality. While Siemieniuk 2015 graded the quality of the evidence for mortality as moderate, we added a GRADE appraisal of the evidence for the important subgroup of patients with severe pneumonia who benefit from corticosteroid therapy. The former reviews did not assess the outcome of early clinical failure. We showed that corticosteroids significantly reduce early clinical failure regardless of the pneumonia severity classification, but that the effect is larger in the subgroup of patients with severe pneumonia, a finding that strengthens the result of the mortality analysis. This is in opposition to Siemieniuk 2015, who assessed the need for mechanical ventilation as a secondary outcome and found a lower magnitude of effect in people with severe pneumonia.
Authors' conclusions
Implications for practice.
The results of this review support the use of corticosteroids in adults with severe community‐acquired pneumonia (CAP) using either the Infectious Diseases Society of America and American Thoracic Society guidelines or British Thoracic Society criteria for severe CAP. Among these patients, corticosteroids reduce mortality, clinical failure, complication rates, length of hospitalisation, and time to clinical cure. People with non‐severe CAP may benefit from corticosteroid therapy as well, but with no survival advantage. Regarding the latter, caution is required for people with diabetes due to the effect of corticosteroids on glucose control. The quality of the evidence for these conclusions is mostly moderate. Corticosteroid therapy is associated with more adverse events, especially hyperglycaemia, but the harm does not seem to outweigh the benefits.
Regarding paediatric patients, it seems there is some advantage for corticosteroid therapy, but the low event rate of complications and the paucity of data preclude recommendation of its regular use.
Implications for research.
Further research is needed to clarify the role of corticosteroid therapy in specific patient populations. There are no randomised controlled trials (RCTs) assessing the effects of corticosteroids in people with hospital‐acquired pneumonia or ventilator‐associated pneumonia, and too few RCTs have evaluated this question in paediatric patients. Other populations in which more study is required are diabetic patients, elderly patients, and those with viral pneumonia. Future trials should also evaluate the recommended dose and duration of corticosteroid therapy.
What's new
| Date | Event | Description |
|---|---|---|
| 3 March 2017 | New search has been performed | A new team of authors updated this review. |
| 3 March 2017 | New citation required and conclusions have changed | We included 12 new studies in this update (Blum 2015; El‐Ghamrawy 2006; Fernández‐Serrano 2011; Hatakeyama 1995; Luo 2014; Meijvis 2011; Nafae 2013; Nagy 2013; Sabry 2011; Snijders 2010; Torres 2015; Wu 2014). We excluded one previously included study, Cao 2007, and excluded five new trials (Huang 2014; Montón 1999; Shan 2017; Van Woensel 2011; Wagner 1956). One trial is awaiting classification (Lan 2015). We found that corticosteroids reduce mortality and morbidity in adults with severe community‐acquired pneumonia and morbidity in adults and children with non‐severe community‐acquired pneumonia. |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 3, 2011
| Date | Event | Description |
|---|---|---|
| 5 July 2012 | Amended | Correction made to Analysis 1.5 graph label. |
Acknowledgements
We acknowledge the contributions of Yuanjing Chen, Ka Li, Hongshan Pu, and Taixiang Wu, authors of the 2011 version of this review (Chen 2011).
The review authors wish to thank Elizabeth Dooley (Managing Editor), Ann Jones (Assistant Managing Editor), and Justin Clark (Information Specialist) of the Cochrane Acute Respiratory Infections Group. We would also like to thank the following people for commenting on the protocol: Anne Lyddiatt, Chanpen Choprapawon, Marco Confalonieri, Sree Nair, and Allen Cheng; and Amanda Young, Marco Confalonieri, Robert Ware, and Allen Cheng for commenting on the draft review. Finally, we wish to thank the following people for commenting on this update: Amanda Young, Marco Confalonieri, Teresa Neeman, and Allen Cheng.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1. exp Pneumonia/ 2. pneumon*.tw. 3. bronchopneumon*.tw. 4. pleuropneumon*.tw. 5. CAP.tw. 6. HAP.tw. 7. Respiratory Distress Syndrome, Adult/ 8. adult respiratory distress syndrome.tw. 9. acute respiratory distress syndrome.tw. 10. ARDS.tw. 11. or/1‐10 12. exp Steroids/ 13. steroid*.tw,nm. 14. exp Adrenal Cortex Hormones/ 15. adrenal cortex hormone*.tw,nm. 16. corticosteroid*.tw,nm. 17. corticoid*.tw,nm. 18. glucocorticoid*.tw,nm. 19. glucocorticosteroid*.tw,nm. 20. pregnenedione*.tw,nm. 21. pregnenolone*.tw,nm. 22. hydrocortisone.tw,nm. 23. hydroxypregnenolone.tw,nm. 24. hydroxycorticosteroid*.tw,nm. 25. tetrahydrocortisol.tw,nm. 26. cortodoxone.tw,nm. 27. cortisone.tw,nm. 28. fludrocortisone.tw,nm. 29. corticosterone.tw,nm. 30. triamcinolone.tw,nm. 31. prednisone.tw,nm. 32. prednisolone.tw,nm. 33. paramethasone.tw,nm. 34. methylprednisolone.tw,nm. 35. dexamethasone.tw,nm. 36. clobetasol.tw,nm. 37. beclomethasone.tw,nm. 38. betamethasone.tw,nm. 39. budesonide.tw,nm. 40. (efcortesol or hydrocortone or solu‐cortef).tw,nm. 41. (betnelan or betnesol).tw,nm. 42. (deflazacort or calcort).tw,nm. 43. (medrone or solu‐medrone or depo‐medrone).tw,nm. 44. kenalog.tw,nm. 45. (novolizer or pulmicort or symbicort).tw,nm. 46. (beclometasone or aerobec or asmabec or beclazone or becodisks or becotide or clenil modulite or qvar or becloforte).tw,nm. 47. cortisol.tw,nm. 48. or/12‐47 49. 11 and 48
Appendix 2. Embase (Elsevier) search strategy
#55 #51 AND #54 #54 #52 OR #53 #53 random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR ((singl* OR doubl*) NEAR/2 (blind* OR mask*)):ab,ti #52 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #51 #11 AND #50 #50 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 #49 cortisol:ti,ab #48 beclometasone:ti,ab OR aerobec:ti,ab OR asmabec:ti,ab OR beclazone:ti,ab OR becodisks:ti,ab OR becotide:ti,ab OR clenil:ti,ab AND modulite:ti,ab OR qvar:ti,ab OR becloforte:ti,ab #47 novolizer:ti,ab OR pulmicort:ti,ab OR symbicort:ti,ab #46 kenalog:ti,ab #45 medrone:ti,ab OR 'solu medrone':ti,ab OR 'depo medrone':ti,ab #44 deflazacort:ti,ab OR calcort:ti,ab #43 betnelan:ti,ab OR betnesol:ti,ab #42 efcortesol:ti,ab OR hydrocortone:ti,ab OR 'solu cortef':ti,ab #41 budesonide:ti,ab #40 betamethasone:ti,ab #39 beclomethasone:ti,ab #38 clobetasol:ti,ab #37 dexamethasone:ti,ab #36 methylprednisolone:ti,ab #35 paramethasone:ti,ab #34 prednisolone:ti,ab #33 prednisone:ti,ab #32 triamcinolone:ti,ab #31 corticosterone:ti,ab #30 fludrocortisone:ti,ab #29 cortisone:ti,ab #28 'cortodoxone'/de #27 cortodoxone:ti,ab #26 tetrahydrocortisol:ti,ab #25 hydroxycorticosteroid*:ti,ab #24 hydroxypregnenolone:ti,ab #23 hydrocortisone:ti,ab #22 pregnenolone*:ti,ab #21 pregnenedione*:ti,ab #20 'pregnane derivative'/de #19 glucocorticosteroid*:ti,ab #18 glucocorticoid*:ti,ab #17 corticoid*:ti,ab #16 corticosteroid*:ti,ab #15 'adrenal cortex hormone*':ti,ab #14 'corticosteroid'/exp #13 steroid*:ti,ab #12 'steroid'/exp #11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 #10 ards:ti,ab #9 acute AND respiratory AND distress AND syndrome:ti,ab #8 adult AND respiratory AND distress AND syndrome:ti,ab #7 'adult respiratory distress syndrome'/de #6 hap:ti,ab #5 cap:ti,ab #4 pleuropneumon*:ti,ab #3 bronchopneumon*:ti,ab #2 pneumon*:ti,ab #1 'pneumonia'/exp
Appendix 3. LILACS (BIREME) search strategy
(MH:Pneumonia OR MH:C08.381.677$ OR MH:C08.730.610$ OR pneumon$ OR Neumonía OR bronchopneumon$ OR pleuropneumon$ OR CAP OR HAP OR MH:"Respiratory Distress Syndrome, Adult" OR "adult respiratory distress syndrome" OR "acute respiratory distress syndrome" OR "Síndrome de Dificultad Respiratoria del Adulto" OR "Síndrome do Desconforto Respiratório do Adulto" OR ARDS) AND (MH: Steroids OR MH:D04.808$ OR steroid$ OR Esteroide$ OR Esteroide$ OR MH:"Adrenal Cortex Hormones" OR MH: D06.472.040$ OR adrenal cortex hormone$ OR corticosteroid$ OR Corticoesteroide$ OR corticoid$ OR glucocorticoid$ OR glucocorticosteroid$ OR pregnenedione$ OR pregnenolone$ OR hydrocortisone OR hydroxypregnenolone OR hydroxycorticosteroid$ OR tetrahydrocortisol OR cortodoxone OR cortisone OR fludrocortisone OR corticosterone OR triamcinolone OR prednisone OR prednisolone OR paramethasone OR methylprednisolone OR dexamethasone OR clobetasol OR beclomethasone OR betamethasone OR budesonide OR efcortesol OR hydrocortone OR solu‐cortef OR betnelan OR betnesol OR deflazacort OR calcort OR medrone OR solu‐medrone OR depo‐medrone OR kenalog OR novolizer OR pulmicort OR symbicort OR beclometasone OR aerobec OR asmabec OR beclazone OR becodisks OR becotide OR "clenil modulite" OR qvar OR becloforte OR cortisol)
Data and analyses
Comparison 1. Corticosteroids versus no treatment or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality ‐ adults | 11 | 1863 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.47, 0.92] |
| 1.1 Low risk of bias for allocation concealment | 6 | 1594 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.54, 1.19] |
| 1.2 Unclear risk of bias for allocation concealment | 5 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.19, 0.71] |
| 2 Mortality ‐ adults, severe pneumonia, by allocation concealment | 9 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.40, 0.84] |
| 2.1 Low risk of bias | 5 | 771 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.46, 1.13] |
| 2.2 Unclear risk of bias | 4 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.68] |
| 3 Mortality ‐ adults, non‐severe pneumonia, by allocation concealment | 4 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.45, 2.00] |
| 3.1 Low risk of bias | 3 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.44, 2.06] |
| 3.2 Unclear risk of bias | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.06, 14.37] |
| 4 Mortality ‐ children | 4 | 266 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 4.1 Bacterial pneumonia | 3 | 225 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 4.2 Viral pneumonia | 1 | 41 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 5 Early clinical failure ‐ adults | 6 | 1324 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.23, 0.70] |
| 5.1 Severe CAP | 5 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.15, 0.70] |
| 5.2 Non‐severe CAP | 2 | 905 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.56, 0.83] |
| 6 Early clinical failure ‐ children | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.24, 0.70] |
| 6.1 Bacterial pneumonia | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.24, 0.70] |
| 7 Early clinical failure ‐ adults + children by allocation concealment | 8 | 1412 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.26, 0.63] |
| 7.1 Low risk of bias | 4 | 1164 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.94] |
| 7.2 Unclear risk of bias | 4 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.14, 0.52] |
| 8 Time to clinical cure ‐ adults | 9 | 1322 | Mean Difference (IV, Random, 95% CI) | ‐1.83 [‐2.45, ‐1.21] |
| 9 Time to clinical cure ‐ children | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Bacterial pneumonia | 3 | 225 | Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐2.55, ‐0.60] |
| 9.2 Viral pneumonia | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐2.50, 5.90] |
| 10 Need for mechanical ventilation ‐ adults | 4 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.20, 0.77] |
| 11 Development of shock ‐ adults | 6 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.09, 0.34] |
| 12 Need for ICU transfer ‐ adults | 4 | 1164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.45, 1.18] |
| 13 Length of hospitalisation ‐ adults | 9 | 1658 | Mean Difference (IV, Random, 95% CI) | ‐2.91 [‐4.92, ‐0.89] |
| 14 Length of ICU stay ‐ adults | 8 | 342 | Mean Difference (IV, Fixed, 95% CI) | ‐1.88 [‐2.96, ‐0.81] |
| 15 Length of hospitalisation ‐ children | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.1 Bacterial pneumonia | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Viral pneumonia | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Pneumonia complications ‐ adults + children | 9 | 1632 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.40, 0.84] |
| 17 Secondary infections ‐ adults | 7 | 1533 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.73, 1.93] |
| 18 Secondary infections ‐ children | 3 | 225 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 18.1 Bacterial pneumonia | 3 | 225 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 19 Any adverse events ‐ adults | 3 | 1028 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.99, 1.47] |
| 20 Hyperglycaemia ‐ adults | 7 | 1578 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.38, 2.14] |
| 21 Gastrointestinal bleeding ‐ adults | 7 | 1190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.40, 2.05] |
| 22 Neuropsychiatric side effects ‐ adults | 4 | 1149 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.70, 5.42] |
| 23 Adverse cardiac events ‐ adults | 5 | 1249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.32, 1.13] |
| 24 Any adverse events ‐ children | 2 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 Bacterial pneumonia | 2 | 117 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
1.3. Analysis.
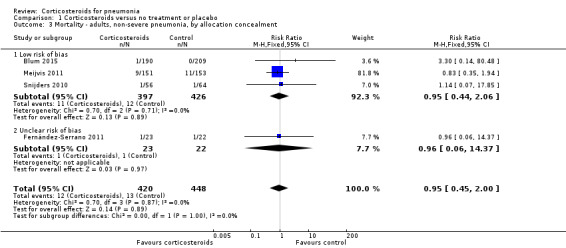
Comparison 1 Corticosteroids versus no treatment or placebo, Outcome 3 Mortality ‐ adults, non‐severe pneumonia, by allocation concealment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blum 2015.
| Methods |
Study design: Randomised, double‐blind, placebo‐controlled trial Study duration: December 2009 to May 2014 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: according to ERS/ESCMID guidelines |
|
| Interventions |
|
|
| Outcomes | Primary outcome: time to clinical stability defined as time (days) until stable vital signs for 24 hours Mortality outcome definition: 30‐day all‐cause mortality Other relevant outcomes:
Full agreement between outcomes in registry vs results: only primary outcome defined in registry Full agreement between outcomes in methods vs results: yes, except for timing of CAP scores defined only in results |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed with a prespecified computer‐generated randomisation list kept centrally at the pharmacy of the main study centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat data presented. |
| Selective reporting (reporting bias) | High risk | Only primary outcome reported in registry, differences in outcome description between methods and results. |
| Other bias | Low risk | Sample size calculation without early stop |
Confalonieri 2005.
| Methods |
Study design: Randomised, double‐blind, placebo‐controlled trial Study duration: July 2000 to March 2003 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: according to ATS guidelines |
|
| Interventions |
|
|
| Outcomes | Primary outcome: improvement in PaO₂:FiO₂ (PaO₂:FiO₂ 300 or 100 increase from study entry) and mortality Mortality outcome definition: 28 days, in hospital, and 60‐days all‐cause mortality Other relevant outcomes:
Full agreement between outcomes in registry vs results: no registry Full agreement between outcomes in methods vs results: no |
|
| Notes | Funding source: academic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schemes were generated in blocks of 10 for each participating site by a central randomisation centre. |
| Allocation concealment (selection bias) | Low risk | The randomisation assignment provided to the recruiting centre in sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, except 2 participants who met the exclusion criteria after randomisation. |
| Selective reporting (reporting bias) | High risk | No agreement between outcomes reported in methods and in results |
| Other bias | High risk | No sample size calculation, interim analysis every 20 participants. Stopped after 46 patients due to significant difference between the groups, based on predefined stopping rules. |
El‐Ghamrawy 2006.
| Methods |
Study design: Randomised, placebo‐controlled trial Study duration: February 2005 to February 2006 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: according to national guidelines for CAP treatment |
|
| Interventions |
|
|
| Outcomes | Primary outcome: not defined Mortality outcome definition: in hospital Other relevant outcomes:
Full agreement between outcomes in registry vs results: no registry Full agreement between outcomes in methods vs results: no, more outcomes in results |
|
| Notes | Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo use without mention of blinding and no description of the placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomisation dropouts |
| Selective reporting (reporting bias) | High risk | No agreement in outcomes between methods and results |
| Other bias | Unclear risk | No sample size calculation, no report on early stop |
Fernández‐Serrano 2011.
| Methods |
Study design: Randomised, double‐blind, placebo‐controlled trial Study duration: January 2000 to December 2002 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: 1 g/day of ceftriaxone for 9 days and 500 mg/day of levofloxacin for 25 days |
|
| Interventions |
|
|
| Outcomes | Primary outcome: presence of respiratory failure requiring conventional MV or non‐invasive PPV Mortality outcome definition: not specified Other relevant outcomes:
Full agreement between outcomes in registry vs results: yes Full agreement between outcomes in methods vs results: yes |
|
| Notes | Funding source: academic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat data presented. |
| Selective reporting (reporting bias) | Low risk | Full agreement between outcomes in registry, methods, and results |
| Other bias | Low risk | Sample size calculation without early stop |
Hatakeyama 1995.
| Methods |
Study design: Randomised, double‐blind, placebo‐controlled trial Study duration: January 1992 to April 1993 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: clindamycin 600 mg x 2/day |
|
| Interventions |
|
|
| Outcomes | Primary outcome: not defined Mortality outcome definition: mortality not reported Other relevant outcomes:
Full agreement between outcomes in registry vs results: no registry Full agreement between outcomes in methods vs results: no, primary outcome not defined |
|
| Notes | Funding source: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Centrally concealed at pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomisation dropouts |
| Selective reporting (reporting bias) | High risk | No registry, outcomes not defined in methods. |
| Other bias | Unclear risk | No sample size calculation. No report on early stop |
Luo 2014.
| Methods |
Study design: Randomised, open‐label trial Study duration: May 2007 to May 2010 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: IV azithromycin |
|
| Interventions |
|
|
| Outcomes | Primary outcome: not specifically defined, implied: duration of dyspnoea and hypoxia Mortality outcome definition: 7‐days all‐cause mortality Other relevant outcomes:
Full agreement between outcomes in registry vs results: no registry Full agreement between outcomes in methods vs results: no |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomisation dropouts |
| Selective reporting (reporting bias) | High risk | No agreement in outcomes between methods and results |
| Other bias | Unclear risk | No sample size calculation. No report on early stop |
Marik 1993.
| Methods |
Study design: Randomised, open‐label, placebo‐controlled trial Study duration: Not specified |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: IV cefoxitin 1 g x 4/d (some received additional therapy according to Gram stain) |
|
| Interventions |
|
|
| Outcomes | Primary outcome: TNF‐α level Mortality outcome definition: in‐ICU mortality Other relevant outcomes:
Full agreement between outcomes in registry vs results: no registry Full agreement between outcomes in methods vs results: outcomes not defined in methods |
|
| Notes | Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by a random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomisation dropouts |
| Selective reporting (reporting bias) | High risk | Outcomes not defined in methods, no registry. |
| Other bias | Unclear risk | No sample size calculation. No report on early stop |
McHardy 1972.
| Methods |
Study design: Randomised, open‐label trial Study duration: January 1966 to June 1970 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: 4 groups, 2 received IV ampicillin 250 mg x 4/d (1 intervention and 1 control) and 2 received IV ampicillin 500 mg x 4/d (1 intervention and 1 control) |
|
| Interventions |
|
|
| Outcomes | Primary outcome: not specifically defined, implied: mortality rate Mortality outcome definition: mortality at 3 months Other relevant outcomes:
Full agreement between outcomes in registry vs results: no registry Full agreement between outcomes in methods vs results: outcomes not defined in methods |
|
| Notes | Funding source: anonymous | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not clearly specified. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by sealed and numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were postrandomisation dropouts, but numbers are not provided. Number randomised provided, but number evaluated not clear. |
| Selective reporting (reporting bias) | High risk | Outcomes not defined in methods, no registry. |
| Other bias | Unclear risk | No sample size calculation. No report on early stop |
Meijvis 2011.
| Methods |
Study design: Randomised, double‐blind, placebo‐controlled trial Study duration: November 2007 to September 2010 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: according to national guidelines |
|
| Interventions |
|
|
| Outcomes | Primary outcome: length of hospital stay in days until hospital discharge or death Mortality outcome definition: 30‐day all‐cause mortality Other relevant outcomes:
Full agreement between outcomes in registry vs results: no, more outcomes in results Full agreement between outcomes in methods vs results: yes |
|
| Notes | Funding source: academic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Use of pre‐numbered boxes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomisation dropouts |
| Selective reporting (reporting bias) | High risk | Not all outcomes defined in registry |
| Other bias | Low risk | Sample size calculation without early stop |
Mikami 2007.
| Methods |
Study design: Randomised, open‐label trial Study duration: September 2003 to February 2004 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: as decided by treating physician |
|
| Interventions |
|
|
| Outcomes | Primary outcome: length of hospital stay Mortality outcome definition: not specified Other relevant outcomes:
Full agreement between outcomes in registry vs results: no registry Full agreement between outcomes in methods vs results: no |
|
| Notes | Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomisation dropouts |
| Selective reporting (reporting bias) | High risk | No registry, no agreement between outcomes in methods and results |
| Other bias | Low risk | Sample size calculation without early stop |
Nafae 2013.
| Methods |
Study design: Randomised, single‐blind (participants only), placebo‐controlled trial Study duration: September 2010 to September 2012 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: according to ATS/IDSA guidelines |
|
| Interventions |
|
|
| Outcomes | Primary outcome: clinical recovery Mortality outcome definition: 7‐days mortality Other relevant outcomes:
Full agreement between outcomes in registry versus results: no registry Full agreement between outcomes in methods versus results: no, outcomes not detailed in methods |
|
| Notes | Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind; only participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomisation dropouts |
| Selective reporting (reporting bias) | High risk | No registry, outcomes not specified in methods. |
| Other bias | Unclear risk | No sample size calculation. No report on early stop |
Nagy 2013.
| Methods |
Study design: Randomised, open‐label, placebo‐controlled trial Study duration: June 2007 to September 2009 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: IV imipenem |
|
| Interventions |
|
|
| Outcomes | Primary outcome: clinical improvement on day 7 Mortality outcome definition: 14‐days all‐cause mortality Other relevant outcomes:
Full agreement between outcomes in registry vs results: outcomes not defined in registry Full agreement between outcomes in methods vs results: yes |
|
| Notes | Funding source: academic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not clearly specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The control group was given placebo, but according to the methods description there was no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomisation dropouts |
| Selective reporting (reporting bias) | High risk | Outcomes not defined in registry. |
| Other bias | Low risk | Sample size calculation without early stop |
Sabry 2011.
| Methods |
Study design: Randomised, double‐blind, placebo‐controlled trial Study duration: July 2010 to January 2011 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: maximal conventional therapy (not further defined) |
|
| Interventions |
|
|
| Outcomes | Primary outcome: improvement in PaO₂:FiO₂ (PaO₂:FiO₂ > 300 or ≥ 100 increase from study entry) and Sepsis‐related Organ Failure Assessment (SOFA) score by day 8 and the development of delayed septic shock Mortality outcome definition: 8 days Other relevant outcomes:
Full agreement between outcomes in registry vs results: no, more outcomes in results Full agreement between outcomes in methods vs results: yes |
|
| Notes | Funding source: no funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomisation dropouts |
| Selective reporting (reporting bias) | High risk | Not all outcomes presented in results were defined in registry. |
| Other bias | Unclear risk | No sample size calculation. Early stop not reported. |
Snijders 2010.
| Methods |
Study design: Randomised, double‐blind, placebo‐controlled trial Study duration: August 2005 to July 2008 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: according to national guidelines, amoxicillin for mild‐moderate CAP, moxifloxacin for moderate‐severe CAP or when atypical pneumonia suspected |
|
| Interventions |
|
|
| Outcomes | Primary outcome: clinical outcome at day 7 Mortality outcome definition: 30‐days all‐cause mortality Other relevant outcomes:
Full agreement between outcomes in registry vs results: no, more outcomes in results Full agreement between outcomes in methods vs results: yes |
|
| Notes | Funding source: pharmaceutical company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Concealment by pre‐numbered containers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat data presented. |
| Selective reporting (reporting bias) | High risk | Not all outcomes defined in registry. |
| Other bias | Low risk | Sample size calculation without early stop |
Torres 2015.
| Methods |
Study design: Randomised, double‐blind, placebo‐controlled trial Study duration: June 2004 to February 2012 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: according to international guidelines |
|
| Interventions |
|
|
| Outcomes | Primary outcome: treatment failure (composite outcome of early treatment failure ‐ within 72 hours, late treatment failure between 72 and 120 hours, or both early and late treatment failure) Mortality outcome definition: in‐hospital mortality Other relevant outcomes:
Full agreement between outcomes in registry vs results: only primary outcome defined in registry Full agreement between outcomes in methods vs results: yes |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Allocation concealed in pre‐numbered boxes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat data presented. |
| Selective reporting (reporting bias) | High risk | Not all outcomes defined in registry. |
| Other bias | Low risk | Sample size calculation without early stop |
Van Woensel 2003.
| Methods |
Study design: Randomised, double‐blind, placebo‐controlled trial Study duration: December 1997 to March 2001 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: not restricted |
|
| Interventions |
|
|
| Outcomes | Primary outcome: duration of mechanical ventilation Mortality outcome definition: not clearly specified, implied: in‐hospital mortality Other relevant outcomes:
Full agreement between outcomes in registry vs results: no registry Full agreement between outcomes in methods vs results: yes |
|
| Notes | Funding source: academic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation in groups of 10 |
| Allocation concealment (selection bias) | Low risk | Trial medication prepared in advance in the pharmacy centre, where the concealed randomisation list was kept until the study was completed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 children excluded postrandomisation, but exclusion was justified. |
| Selective reporting (reporting bias) | Low risk | No registry, full agreement between outcomes in methods vs results |
| Other bias | Low risk | Sample size calculation without early stop |
Wu 2014.
| Methods |
Study design: Randomised, open‐label trial Study duration: January 2010 to December 2012 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Antibiotic therapy: azithromycin |
|
| Interventions |
|
|
| Outcomes | Primary outcome: not clearly defined, implied: clinical improvement Mortality outcome definition: not specified Other relevant outcomes:
Full agreement between outcomes in registry vs results: no registry Full agreement between outcomes in methods vs results: yes |
|
| Notes | Funding source: academic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomisation dropouts |
| Selective reporting (reporting bias) | Low risk | No registry, full agreement between methods and results |
| Other bias | Unclear risk | No sample size calculation. Early stop not reported. |
ARDS = acute respiratory distress syndrome ATS = American Thoracic Society BP = blood pressure CAP = community‐acquired pneumonia CHF = congestive heart failure COPD = chronic obstructive pulmonary disease CRP = C‐reactive protein CVD = cerebrovascular disease ERS/ESCMID = European Respiratory Society/European Society of Clinical Microbiology and Infectious Diseases FiO₂ = inspired oxygen concentration GI = gastrointestinal GIB = gastrointestinal bleed GIT = gastrointestinal tract HAP = hospital‐acquired pneumonia HCAP = healthcare‐associated pneumonia IDSA = Infectious Diseases Society of America ICU = intensive care unit IQR = interquartile range IV = intravenous MV = mechanical ventilation PaO₂ = partial pressure of oxygen in arterial blood PSI = pneumonia severity index PO = per oral PPV = positive pressure ventilation RSV = respiratory syncytial virus SD = standard deviation TB = tuberculosis TNF = tumour necrosis factor VAP = ventilator‐associated pneumonia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cao 2007 | Examined the use of inhaled corticosteroids, not systemic corticosteroid treatment as specified in our inclusion criteria |
| Huang 2014 | Corticosteroids administered to both study groups. |
| Montón 1999 | Prospective, non‐randomised trial (confirmed through author contact) |
| Shan 2017 | Participants in the control group were subsequently given either corticosteroids or intravenous immunoglobulin therapy. |
| Van Woensel 2011 | Population included people with and without pneumonia, and outcomes were not reported separately for participants with pneumonia. |
| Wagner 1956 | Quasi‐randomised controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
Lan 2015.
| Methods | Unclear, presented as randomised, however the methods section described selection of children for different management strategies |
| Participants | Children with refractory Mycoplasma pneumoniae pneumonia |
| Interventions | IV methylprednisolone 2 mg/kg/d versus no treatment |
| Outcomes | Treatment failure reported, however results in abstract and in text are inconsistent. |
| Notes | Awaiting clarification from authors; if they are unreachable, we will exclude the study. |
Characteristics of ongoing studies [ordered by study ID]
NCT01283009.
| Trial name or title | Extended steroid in CAP(e) (ESCAPe) |
| Methods | Randomised, double‐blind |
| Participants | Adults with severe CAP |
| Interventions | Methylprednisolone |
| Outcomes | Primary outcome: all‐cause 60‐day mortality Secondary outcomes:
|
| Starting date | (first received 21 January 2011) |
| Contact information | Gianfranco Umberto Meduri, MD |
| Notes | Completed |
NCT02618057.
| Trial name or title | Effects of oral steroid in Mycoplasma pneumoniae pneumonia |
| Methods | Randomised, open‐label |
| Participants | Children with M pneumoniae pneumonia |
| Interventions | Prednisolone and levofloxacin |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | (first received 8 November 2015) |
| Contact information | Dr Ki Wook Yun; pedwilly@gmail.com |
| Notes | Recruiting (August 2017) |
NCT02735707.
| Trial name or title | Randomized, embedded, multifactorial adaptive platform trial for community‐acquired pneumonia (REMAP‐CAP) |
| Methods | Randomised, open‐label |
| Participants | Adults with severe CAP admitted to ICU |
| Interventions | Comparison of third‐generation cephalosporins plus macrolide versus a respiratory quinolone |
| Outcomes | Primary outcomes:
|
| Starting date | (first received 11 December 2015) |
| Contact information | Prof Marc Bonten; M.J.M.Bonten@umcutrecht.nl |
| Notes | Recruiting (August 2017) |
NCT03121690.
| Trial name or title | The applicability of different scoring systems and use of steroids in the treatment of hospital acquired pneumonia |
| Methods | Randomised |
| Participants | Adults with HAP |
| Interventions | Prednisone |
| Outcomes | Assessment of the prognostic value of different pneumonia severity scores |
| Starting date | (first received 10 April 2017) |
| Contact information | Rabab Hamed Hassan, Assiut University |
| Notes | Not yet recruiting (August 2017) |
CAP = community‐acquired pneumonia HAP = hospital‐acquired pneumonia ICU = intensive care unit
Differences between protocol and review
This review is an update of the Chen 2011 review. However, in light of new evidence, changes made in recommendations for treatment of pneumonia, and changing questions surrounding the evidence on pneumonia management, the review has changed in many aspects from the original review. We list the essential differences between the 2011 protocol and our update.
We prospectively rewrote a protocol for this update, before starting the update.
We defined new objectives for this review not identical to the 2011 review objectives. Specifically, we broadened the efficacy assessment to include mortality and morbidity, and we did not assess relative adrenal insufficiency and dose‐effect relationships, which were defined as objectives in the original review.
The inclusion and exclusion criteria for this update are similar to the original 2011 review with a few differences. Differing from the 2011 review we did not exclude studies including participants with immunosuppression, tuberculosis, acute schistosomiasis, fungal or parasitic infections, or chemotherapy and radiotherapy, as we believed these can be pooled together with other pneumonia patients. We excluded studies including neonates and people with HIV and Pneumocystis pneumonia, as we believed these represent different entities and require separate consideration.
There were several differences in the types of interventions assessed in this update compared to the 2011 review. First, we specified corticosteroid therapy to include only systemic administration and exclude inhaled corticosteroids. The mechanisms of action of these two interventions are different and cannot be pooled. We included only trials comparing corticosteroids to placebo or no treatment and excluded trials in which corticosteroids were given to both treatment arms, as the question of the review is on the efficacy of corticosteroid therapy.
We assessed all‐cause mortality as our primary outcome, as this is the currently recommended outcome for assessment in severe infections and the only outcome that will change practice. We added several secondary outcomes that were not collected in the 2011 review, including early clinical failure, length of hospitalisation, and pneumonia complications. We also specified the outcome of adverse effects and added outcomes of specific adverse effects including superinfections, adverse effects requiring discontinuation of corticosteroids, hyperglycaemia, gastrointestinal bleeding, and neuropsychiatric and cardiac adverse events. The outcomes assessed in our review are aligned with contemporary guidance for outcome assessment in clinical trials of pneumonia (FDA CAP industry guidance; FDA HAP/VAP industry guidance; Mandell 2007), and we believe that evidence summaries should address these relevant outcomes. We changed the outcome definition of 'mortality' to 'all‐cause mortality' because we believe that 'all‐cause mortality' more clearly specifies that we collected all death cases including pneumonia‐related cases and cases not related to pneumonia. To note, we revised the outcomes during the writing of the protocol and before starting the update.
We changed subgroup analyses to address the contemporary relevant clinical questions. We performed subgroup analyses based on different patient characteristics (pneumonia severity, comorbidities, pathogen, etc.) and added meta‐regression analyses when subgroup analysis was not applicable.
We re‐applied inclusion and exclusion criteria and re‐extracted all data in duplicate, since we did not agree with the study selection and outcome extraction in a sample of tested articles, and since 'Risk of bias' methodology has developed since the 2011 review.
Contributions of authors
Concept and design ‐ all authors
Search ‐ Anat Stern, Keren Skalsky, Tomer Avni
Data extraction ‐ Anat Stern, Keren Skalsky, Elena Carrara
Data entry into Review Manager 5 and review ‐ Anat Stern, Keren Skalsky, Mical Paul
Data analysis ‐ Anat Stern, Keren Skalsky, Mical Paul
Wrote the first version of the review ‐ Anat Stern, Mical Paul
Reviewed and comments on the review ‐ all authors
Sources of support
Internal sources
No sources of support supplied
External sources
Cochrane Acute Respiratory Infections Group, Australia.
Declarations of interest
Anat Stern ‐ None known.
Keren Skalsky ‐ None known.
Tomer Avni ‐ None known.
Elena Carrara ‐ None known.
Leonard Leibovici ‐ None known.
Mical Paul ‐ None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Blum 2015 {published and unpublished data}
- Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter‐Widmer I, et al. Adjunct prednisone therapy for patients with community‐acquired pneumonia: a multicentre, double‐blind, randomised, placebo‐controlled trial. Lancet 2015;385(9977):1511‐8. [DOI] [PubMed] [Google Scholar]
Confalonieri 2005 {published data only}
- Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G. Hydrocortisone infusion for severe community‐acquired pneumonia: a preliminary randomised study. American Journal of Respiratory and Critical Care Medicine 2005;171(3):242‐8. [DOI] [PubMed] [Google Scholar]
El‐Ghamrawy 2006 {published data only}
- El‐Ghamrawy AH, Shokier MH, Esmat AA. Effects of low‐dose hydrocortisone in ICU patients with severe community‐acquired pneumonia. Egyptian Journal of Chest Diseases and Tuberculosis 2006;55:91‐9. [Google Scholar]
Fernández‐Serrano 2011 {published data only}
- Fernández‐Serrano S, Dorca J, Garcia‐Vidal C, Fernández‐Sabé N, Carratalà J, Fernández‐Agüera A, et al. Effect of corticosteroids on the clinical course of community‐acquired pneumonia: a randomized controlled trial. Critical Care 2011;15(2):R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatakeyama 1995 {published data only}
- Hatakeyama S, Tachibana A, Suzuki K, Okano H. Treatment of aspiration pneumonia with low‐dose methylprednisolone and antibiotics. Japanese Journal of Thoracic Diseases 1998;33(1):51‐6. [PubMed] [Google Scholar]
Luo 2014 {published data only}
- Luo Z, Luo J, Liu E, Xu X, Liu Y, Zeng F, et al. Effects of prednisolone on refractory Mycoplasma pneumoniae pneumonia in children. Pediatric Pulmonology 2014;49(4):377‐80. [DOI] [PubMed] [Google Scholar]
Marik 1993 {published data only}
- Marik P, Kraus P, Sribante J, Havlik I, Lipman J, Johnson DW. Hydrocortisone and tumour necrosis factor in severe community‐acquired pneumonia. A randomised controlled study. Chest 1993;104(2):389‐92. [DOI] [PubMed] [Google Scholar]
McHardy 1972 {published data only}
- McHardy VU, Schonell ME. Ampicillin dosage and use of prednisolone in treatment of pneumonia: co‐operative controlled trial. British Medical Journal 1972;4(5840):569‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meijvis 2011 {published data only}
- Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, Velzen‐Blad H, et al. Dexamethasone and length of hospital stay in patients with community‐acquired pneumonia: a randomised, double‐blind, placebo‐controlled trial. Lancet 2011;377(9782):2023‐30. [DOI] [PubMed] [Google Scholar]
Mikami 2007 {published data only}
- Mikami K, Suzuki M, Kitagawa H, Kawakami M, Hirota N, Yamaguchi H. Efficacy of corticosteroids in the treatment of community‐acquired pneumonia requiring hospitalisation. Lung 2007;185(5):249‐55. [DOI] [PubMed] [Google Scholar]
Nafae 2013 {published data only}
- Nafae RM, Ragab MI, Amany FM, Rashed SB. Adjuvant role of corticosteroids in the treatment of community‐acquired pneumonia. Egyptian Journal of Chest Diseases and Tuberculosis 2013;62(3):439–45. [Google Scholar]
Nagy 2013 {published data only}
- Nagy B, Gaspar I, Papp A, Bene Z, Nagy B Jr, Voko Z, et al. Efficacy of methylprednisolone in children with severe community acquired pneumonia. Pediatric Pulmonology 2013;48(2):168‐75. [DOI] [PubMed] [Google Scholar]
Sabry 2011 {published data only}
- Sabry NA, Omar EE. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacology & Pharmacy 2011;2:73‐81. [DOI: 10.4236/pp.2011.22009] [DOI] [Google Scholar]
Snijders 2010 {published data only}
- Snijders D, Daniels JM, Graaff CS, Werf TS, Boersma WG. Efficacy of corticosteroids in community‐acquired pneumonia: a randomized double‐blinded clinical trial. American Journal of Respiratory and Critical Care Medicine 2010;181(9):975‐82. [DOI] [PubMed] [Google Scholar]
Torres 2015 {published and unpublished data}
- Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community‐acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313(7):677‐86. [DOI] [PubMed] [Google Scholar]
Van Woensel 2003 {published data only}
- Woensel JB, Aalderen WM, Weerd W, Jansen NJ. Dexamethasone for treatment of patients mechanically ventilated for lower respiratory tract infection caused by respiratory syncytial virus. Thorax 2003;58(5):383‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2014 {published data only}
- Wu YJ, Sun J, Zhang JH, Feng LL. Clinical efficacy of adjuvant therapy with glucocorticoids in children with lobar pneumonia caused by Mycoplasma pneumoniae. Zhongguo Dang Dai Er Ke Za Zhi [Chinese Journal of Contemporary Pediatrics] 2014;16(4):401‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Cao 2007 {published data only}
- Cao LF, Lu YM, Ma HG, Ma M. Budesonide inhaling auxiliary therapy after Mycoplasma pneumoniae infection of children. International Journal of Respiration 2007;27(8):567‐9. [Google Scholar]
Huang 2014 {published data only}
- Huang L, Gao X, Chen M. Early treatment with corticosteroids in patients with Mycoplasma pneumoniae pneumonia: a randomized clinical trial. Journal of Tropical Pediatrics 2014;60(5):338‐42. [DOI] [PubMed] [Google Scholar]
Montón 1999 {published data only}
- Montón C, Ewig S, Torres A, El‐Ebiary M, Filella X, Rañó A, et al. Role of glucocorticoids on inflammatory response in nonimmunosuppressed patients with pneumonia: a pilot study. European Respiratory Journal 1999;14(1):218‐20. [DOI] [PubMed] [Google Scholar]
Shan 2017 {published data only}
- Shan LS, Liu X, Kang XY, Wang F, Han XH, Shang YX. Effects of methylprednisolone or immunoglobulin when added to standard treatment with intravenous azithromycin for refractory Mycoplasma pneumoniae pneumonia in children. World Journal of Pediatrics 2017 Jan 27 [Epub ahead of print]. [DOI: 10.1007/s12519-017-0014-9] [DOI] [PubMed]
Van Woensel 2011 {published data only}
- Woensel JB, Vyas H, STAR Trial Group. Dexamethasone in children mechanically ventilated for lower respiratory tract infection caused by respiratory syncytial virus: a randomized controlled trial. Critical Care Medicine 2011;39(7):1779‐83. [DOI] [PubMed] [Google Scholar]
Wagner 1956 {published data only}
- Wagner HN Jr, Bennett IL Jr, Lasagna L, Cluff LE, Rosenthal MB, Mirick GS. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bulletin of the Johns Hopkins Hospital 1956;98(3):197‐215. [PubMed] [Google Scholar]
References to studies awaiting assessment
Lan 2015 {published data only}
- Lan Y, Yang D, Chen Z, Tang L, Xu Y, Cheng Y. Effectiveness of methylprednisolone in treatment of children with refractory Mycoplasma pneumoniae pneumonia and its relationship with bronchoalveolar lavage cytokine levels. Zhonghua Er Ke Za Zhi 2015;53(10):779‐83. [PubMed] [Google Scholar]
References to ongoing studies
NCT01283009 {published and unpublished data}
- NCT01283009. Extended Steroid in CAP(e) (ESCAPe). clinicaltrials.gov/ct2/show/NCT01283009 (first received 21 January 2011).
NCT02618057 {published and unpublished data}
- NCT02618057. Effects of oral steroid in Mycoplasma pneumoniae pneumonia. clinicaltrials.gov/ct2/show/NCT02618057 (first received 8 November 2015).
NCT02735707 {published and unpublished data}
- NCT02735707. Randomized, embedded, multifactorial adaptive platform trial for community‐acquired pneumonia (REMAP‐CAP). clinicaltrials.gov/ct2/show/NCT02735707 (first received 11 December 2015).
NCT03121690 {published and unpublished data}
- NCT03121690. The applicability of different scoring systems and use of steroids in the treatment of hospital acquired pneumonia. clinicaltrials.gov/ct2/show/NCT03121690 (first received 10 April 2017).
Additional references
Annane 2004
- Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis. BMJ 2004;329(7464):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Annane 2009
- Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, Gaudio R, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 2009;301(22):2362. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avni 2015
- Avni T, Shiver‐Ofer S, Leibovici L, Tacconelli E, DeAngelis G, Cookson B, et al. Participation of elderly adults in randomized controlled trials addressing antibiotic treatment of pneumonia. Journal of the American Geriatrics Society 2015;63(2):233‐43. [DOI] [PubMed] [Google Scholar]
Brouwer 2015
- Brouwer MC, McIntyre P, Prasad K, Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD004405.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS guidelines 1987
- British Thoracic Society. Community‐acquired pneumonia in adults in British hospitals in 1982‐1983: a survey of aetiology, mortality, prognostic factors and outcome. The British Thoracic Society and the Public Health Laboratory Service. Quarterly Journal of Medicine 1987;62(239):195‐220. [PubMed] [Google Scholar]
Chalmers 2010
- Chalmers JD, Singanayagam A, Akram AR, Mandal P, Short PM, Choudhury G, et al. Severity assessment tools for predicting mortality in hospitalised patients with community‐acquired pneumonia. Systematic review and meta‐analysis. Thorax 2010;65(10):878‐83. [DOI] [PubMed] [Google Scholar]
Chawla 2008
- Chawla R. Epidemiology, etiology, and diagnosis of hospital‐acquired pneumonia and ventilator‐associated pneumonia in Asian countries. American Journal of Infection Control 2008;36(4 Suppl):S93‐100. [DOI] [PubMed] [Google Scholar]
Comprehensive Meta‐Analysis V3 [Computer program]
- Biostat. Comprehensive Meta‐Analysis V3.3.07. Version (accessed prior to 30 August 2017). Englewood (NJ): Biostat, 2014.
FDA CAP industry guidance
- FDA. Guidance for industry. Community‐acquired pneumonia ‐ developing antimicrobial drugs for treatment. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM123686.pdf (accessed prior to 11 August 2017).
FDA HAP/VAP industry guidance
- FDA. Guidance for industry. Hospital‐acquired bacterial pneumonia and ventilator‐associated bacterial pneumonia: developing drugs for treatment. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM234907.pdf (accessed prior to 11 August 2017).
File 2010
- File TM Jr, Marrie TJ. Burden of community‐acquired pneumonia in North American adults. Postgraduate Medicine 2010;122(2):130‐41. [DOI] [PubMed] [Google Scholar]
Fine 1997
- Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. New England Journal of Medicine 1997;336(4):243‐50. [DOI] [PubMed] [Google Scholar]
Friedman 2002
- Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care‐associated bloodstream infections in adults: a reason to change the accepted definition of community‐acquired infections. Annals of Internal Medicine 2002;137(10):791‐7. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed prior to 30 August 2017). Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horita 2015
- Horita N, Otsuka T, Haranaga S, Namkoong H, Miki M, Miyashita N, et al. Adjunctive systemic corticosteroids for hospitalized community‐acquired pneumonia: systematic review and meta‐analysis 2015 update. Scientific Reports 2015;5:14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2010
- Jones RN. Microbial etiologies of hospital‐acquired bacterial pneumonia and ventilator‐associated bacterial pneumonia. Clinical Infectious Diseases 2010;51(Suppl 1):S81‐7. [DOI] [PubMed] [Google Scholar]
Kalil 2016
- Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital‐acquired and ventilator‐associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clinical Infectious Diseases 2016;63(5):e61‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 2002
- Kaplan V, Angus DC, Griffin MF, Clermont G, Scott Watson R, Linde‐Zwirble WT. Hospitalized community‐acquired pneumonia in the elderly: age‐ and sex‐related patterns of care and outcome in the United States. American Journal of Respiratory and Critical Care Medicine 2002;165(6):766‐72. [DOI] [PubMed] [Google Scholar]
Krinsley 2003
- Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clinic Proceedings 2003;78(12):1471. [DOI] [PubMed] [Google Scholar]
Lee 2010
- Lee GE, Lorch SA, Sheffler‐Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics 2010;126(2):204‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Google Scholar]
Lim 2009
- Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Jeune I, et al. Pneumonia Guidelines Committee of the BTS Standards of Care Committee. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64(Suppl 3):iii1. [DOI] [PubMed] [Google Scholar]
Magill 2014
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Emerging Infections Program Healthcare‐Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point‐prevalence survey of health care‐associated infections. New England Journal of Medicine 2014;370(13):1198‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandell 2007
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clinical Infectious Diseases 2007;44(Suppl 2):S27‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandell 2015
- Ellison RT 3rd, Donowitz GR. Acute pneumonia. In: Mandell GL, Bennett JE editor(s). Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 8th Edition. Vol. 1, London: Churchill Livingstone, 2015:823‐46. [Google Scholar]
Marik 2008
- Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. American College of Critical Care Medicine. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Critical Care Medicine 2008;36(6):1937‐49. [DOI] [PubMed] [Google Scholar]
Marrie 2007
- Marrie TJ, Shariatzadeh MR. Community‐acquired pneumonia requiring admission to an intensive care unit: a descriptive study. Medicine 2007;86(2):103‐11. [DOI] [PubMed] [Google Scholar]
Marti 2015
- Marti C, Grosgurin O, Harbarth S, Combescure C, Abbas M, Rutschmann O, et al. Adjunctive corticotherapy for community acquired pneumonia: a systematic review and meta‐analysis. PLoS ONE 2015;10(12):e0144032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264‐9. [DOI] [PubMed] [Google Scholar]
Musher 2014
- Musher DM, Thorner AR. Community‐acquired pneumonia. New England Journal of Medicine 2014;271(17):1619‐28. [DOI] [PubMed] [Google Scholar]
Niederman 2007
- Niederman MS. Recent advances in community‐acquired pneumonia: inpatient and outpatient. Chest 2007;131(4):1205‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oray 2016
- Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long‐term side effects of glucocorticoids. Expert Opinion on Drug Safety 2016;15(4):457‐65. [DOI] [PubMed] [Google Scholar]
Restrepo 2008
- Restrepo MI, Mortensen EM, Velez JA, Frei C, Anzueto A. A comparative study of community‐acquired pneumonia patients admitted to the ward and the ICU. Chest 2008;133(3):610‐7. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhen 2005
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids ‐ new mechanisms for old drugs. New England Journal of Medicine 2005;353(16):1711‐23. [DOI] [PubMed] [Google Scholar]
Rhodes 2017
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Critical Care Medicine 2017;45(3):486‐552. [DOI] [PubMed] [Google Scholar]
Rosenthal 2012
- Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004‐2009. American Journal of Infection Control 2012;40(5):396‐407. [DOI] [PubMed] [Google Scholar]
Ruan 2014
- Ruan SY, Lin HH, Huang CT, Kuo PH, Wu HD, Yu CJ. Exploring the heterogeneity of effects of corticosteroids on acute respiratory distress syndrome: a systematic review and meta‐analysis. Critical Care 2014;18(2):R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Safdar 2005
- Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator‐associated pneumonia: a systematic review. Critical Care Medicine 2005;33(10):2184‐93. [DOI] [PubMed] [Google Scholar]
Salluh 2008
- Salluh JI, Póvoa P, Soares M, Castro‐Faria‐Neto HC, Bozza FA, Bozza PT. The role of corticosteroids in severe community‐acquired pneumonia: a systematic review. Critical Care 2008;12(3):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schäcke 2002
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacology & Therapeutics 2002;96(1):23‐43. [DOI] [PubMed] [Google Scholar]
Siemieniuk 2015
- Siemieniuk RA, Meade MO, Alonso‐Coello P, Briel M, Evaniew N, Prasad M, et al. Corticosteroid therapy for patients hospitalized with community‐acquired pneumonia: a systematic review and meta‐analysis. Annals of Internal Medicine 2015;163(7):519‐28. [DOI] [PubMed] [Google Scholar]
Torres 2010
- Torres A, Ferrer M, Badia JR. Treatment guidelines and outcomes of hospital‐acquired and ventilator‐associated pneumonia. Clinical Infectious Diseases 2010;51(Suppl 1):S48‐53. [DOI] [PubMed] [Google Scholar]
Virkki 2002
- Virkki R, Juven T, Rikalainen H, Svedström E, Mertsola J, Ruuskanen O. Differentiation of bacterial and viral pneumonia in children. Thorax 2002;57(5):438‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2014
- Walters JA, Tan DJ, White CJ, Gibson PG, Wood‐Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD001288.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [DOI: 10.1186/1471-2288-14-135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2016
- Wan YD, Sun TW, Liu ZQ, Zhang SG, Wang LX, Kan QC. Efficacy and safety of corticosteroids for community‐acquired pneumonia: a systematic review and meta‐analysis. Chest 2016;149(1):209‐19. [DOI] [PubMed] [Google Scholar]
Warrington 2006
- Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clinic Proceedings 2006;81(10):1361‐7. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wunderink 2015
- Wunderink RG. Corticosteroids for severe community‐acquired pneumonia: not for everyone. JAMA 2015;313(7):673‐4. [DOI] [PubMed] [Google Scholar]
Xu 2016
- Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: Final Data for 2013. National Vital Statistics Reports 2016;64(2):1‐119. [PubMed] [Google Scholar]
References to other published versions of this review
Chen 2011
- Chen Y, Li K, Pu H, Wu T. Corticosteroids for pneumonia. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD007720.pub2] [DOI] [PubMed] [Google Scholar]


