Abstract
Background
Non‐surgical treatment, including ergonomic positioning or equipment, are sometimes offered to people experiencing mild to moderate symptoms from carpal tunnel syndrome (CTS). The effectiveness and duration of benefit from ergonomic positioning or equipment interventions for treating CTS are unknown.
Objectives
To assess the effects of ergonomic positioning or equipment compared with no treatment, a placebo or another non‐surgical intervention in people with CTS.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (14 June 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (2011, Issue 2, in The Cochrane Library), MEDLINE (1966 to June 2011), EMBASE (1980 to June 2011), CINAHL Plus (1937 to June 2011), and AMED (1985 to June 2011). We also reviewed the reference lists of randomised or quasi‐randomised trials identified from the electronic search.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing ergonomic positioning or equipment with no treatment, placebo or another non‐surgical intervention in people with CTS.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted data and assessed risk of bias of included studies. We calculated risk ratios (RR) and mean differences (MD) with 95% confidence intervals (CI) for the primary and secondary outcomes. We pooled results of clinically and statistically homogeneous trials, where possible, to provide estimates of the effect of ergonomic positioning or equipment.
Main results
We included two trials (105 participants) comparing ergonomic versus placebo keyboards. Neither trial assessed the primary outcome (short‐term overall improvement) or adverse effects of interventions. In one small trial (25 participants) an ergonomic keyboard significantly reduced pain after 12 weeks (MD ‐2.40; 95% CI ‐4.45 to ‐0.35) but not six weeks (MD ‐0.20; 95% CI ‐1.51 to 1.11). In this same study, there was no difference between ergonomic and standard keyboards in hand function at six or 12 weeks or palm‐wrist sensory latency at 12 weeks. The second trial (80 participants) reported no significant difference in pain severity after six months when using either of the three ergonomic keyboards versus a standard keyboard. No trials comparing (i) ergonomic positioning or equipment with no treatment, (ii) ergonomic positioning or equipment with another non‐surgical treatment, or (iii) different ergonomic positioning or equipment regimes, were found.
Authors' conclusions
There is insufficient evidence from randomised controlled trials to determine whether ergonomic positioning or equipment is beneficial or harmful for treating carpal tunnel syndrome.
Keywords: Humans, Computer Peripherals, Carpal Tunnel Syndrome, Carpal Tunnel Syndrome/therapy, Ergonomics, Ergonomics/instrumentation, Ergonomics/methods, Patient Positioning, Patient Positioning/methods, Randomized Controlled Trials as Topic, Time Factors, Treatment Outcome
Plain language summary
Ergonomic positioning or equipment for carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a condition where the median nerve, one of two main nerves to the hand, is compressed at the wrist, leading to pain in the hand, wrist and sometimes arm, and numbness and tingling especially in the thumb, index and middle finger. Weakness of the thumb muscles can also occur in severe cases. It affects approximately three per cent of the population, more commonly women.
Surgical treatment for CTS involves opening the carpal tunnel, the tunnel in which the median nerve passes through the wrist. Non‐surgical treatments include medications, exercises, splinting and ergonomic interventions. Ergonomic interventions, such as keyboard modification, allow the hand to be used while the wrist is positioned in a straight position (neither flexed, extended or deviated to either side). In this straight (or neutral) wrist position the tunnel through which the median nerve passes is at its most capacious. This position is expected theoretically to place the least pressure on the median nerve.
This review aimed to find out how effective ergonomic treatments were in treating CTS. Only two studies were found (involving 105 participants). Both were designed to minimise research biases, but neither was of high quality. Neither study assessed short‐term overall improvement, adverse effects or need for surgery as outcomes. One small study (25 participants) found an ergonomic keyboard reduced pain after 12 weeks but the second study reported no difference in pain severity between the keyboard groups at six months. Neither study found improvements in hand function or signs of CTS by people using ergonomic computer keyboards more than those experienced by people using standard keyboards. Based on the two studies in this review, which represent all the available evidence of sufficient quality for inclusion, there is no strong evidence for or against the use of ergonomic keyboards for the treatment of CTS.
Summary of findings
Summary of findings for the main comparison. Ergonomic keyboard compared with placebo for carpal tunnel syndrome.
| Ergonomic keyboard compared with placebo for carpal tunnel syndrome | ||||||
| Patient or population: people with carpal tunnel syndrome Settings: Intervention: ergonomic keyboard Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ergonomic keyboard | |||||
| Short‐term overall improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This clinically important outcome was not measured in any of the included studies |
| Adverse effects ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This clinically important outcome was not measured in any of the included studies |
|
Short‐term improvement in CTS symptoms (pain) (3 months or less) ‐ At end of 12 weeks treatment (Protouch Keyboard) Scale: 0 to 10 |
The mean improvement in CTS symptoms (pain) at the end of 12 weeks treatment (Protouch Keyboard) in the control group was 4.3 | The mean improvement in CTS symptoms (pain) at the end of 12 weeks treatment (Protouch Keyboard) in the intervention group was 2.4 lower (4.45 to 0.35 lower) compared with placebo | 20 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
|
Short‐term improvement in functional ability (3 months or less) (Protouch Keyboard) Scale: 13 to 65 |
The mean improvement in functional ability at the end of 12 weeks treatment (Protouch Keyboard) in the intervention group was 30.4 | The mean improvement in functional ability at the end of 12 weeks treatment (Protouch Keyboard) in the intervention group was 2.2 lower (11.57 lower to 7.17 higher) | 20 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
|
Long‐term improvement in CTS symptoms (pain ) from baseline to end of 6 months treatment (Apple Adjustable Keyboard) Scale: 0 to 10 |
The mean improvement in CTS symptoms (pain) from baseline to the end of 6 months treatment in the Apple Adjustable Keyboard was ‐0.29 |
The mean improvement in CTS symptoms (pain) from baseline to the end of 6 months treatment in the Apple Adjustable Keyboard was 0.70 lower (‐0.97 higher to 2.37 lower) |
‐ | ⊕⊝⊝⊝ very low1 | ||
| Long‐term improvement in functional ability (> 3 months) ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | This clinically important outcome was not measured in any of the included studies |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; CTS: carpal tunnel syndrome; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment was unclear. 2 Confidence intervals were relatively wide given the small sample.
3 Only one study with small sample (indirectness).
Background
Description of the condition
Carpal tunnel syndrome (CTS) is a condition in which the median nerve at the level of the carpal tunnel undergoes irritation, often attributed to compression (Kerwin 1996). Symptoms of CTS include pain in the wrist and hand which can radiate to the arm (Rempel 1998) and paraesthesiae (altered sensation, such as tingling) especially in the thumb, index, middle and radial half of the ring finger (Szabo 1994). Advanced stages of median nerve compression can result in thenar muscle weakness (Szabo 1994).
Median nerve compression in the carpal tunnel is the most common example of nerve compression in the body (Rosenthal 1987). Carpal tunnel syndrome is said to affect three per cent of the general population (Katz 1990; Levine 1993; Sadosky 2008) but higher rates have been identified in populations of certain occupations such as meat packers (Hagberg 1992) and those with medical conditions such as renal failure (Katims 1989). Newport 2000 suggests that the incidence of CTS is increasing, and that with age expectancy of 70 years, 3.5% of males and 11% of females will have been affected by CTS at some time during their lives. More recent data support this, in that adjusted annual incidence rates increased from 258 per 100,000 person‐years between 1981 and 1985 to 424 per 100,000 person‐years between 2000 and 2005 in Minnesota, USA (Gelfman 2009). Other studies have observed certain personal characteristics such as obesity to be associated with increased incidence of CTS (Atroshi 1999). Age and gender have also been found to have an effect upon the incidence of CTS. People aged less than 25 years accounted for only 2.4% of patients presenting to Australian general practices between 2000 and 2009 with CTS, compared with patients aged 45 to 64 years who accounted for 45.5% of these patients (Charles 2009). Sixty‐seven per cent of CTS encounters at Australian general practices were attributable to females (Charles 2009). Females in their fourth and fifth decades suffer CTS four times more commonly than males (Atroshi 1999).
Carpal tunnel syndrome does not follow a predictable course. Some patients experience a deterioration in hand function whilst others describe 'silent' periods and intermittent exacerbation of symptoms (Braun 1989).
Description of the intervention
The treatment of CTS can be categorised into surgical and non‐surgical. Surgical treatment is usually offered to those with severe CTS, who have constant symptoms, severe sensory disturbance or, in addition to, thenar motor weakness. Non‐surgical treatments are offered to those who have intermittent symptoms of mild to moderate CTS. Non‐surgical interventions may also be used as a temporary measure while awaiting carpal tunnel release (CTR).
Surgery for CTS involves open or endoscopic division of the flexor retinaculum in order to provide greater space for the contents of the carpal canal. Carpal tunnel release is the most common hand and wrist surgery in the USA, where more than 400,000 CTRs are performed annually (Concannon 2000). The average annual incidence of CTR surgery from 1981 to 2005 in Minnesota, USA, was 109 per 100,000 person‐years (Gelfman 2009). Surgical treatment options for patients with CTS have been examined in other Cochrane reviews: Surgical treatment options for carpal tunnel syndrome (Scholten 2007), and Surgical versus non‐surgical treatment for carpal tunnel syndrome (Verdugo 2008).
Non‐surgical options for the treatment of CTS include many different interventions such as ergonomic modification, including equipment or positioning (for example ergonomic keyboards and handles), splinting, therapeutic ultrasound, exercises, yoga, oral medication, vitamins and complementary therapies. Their effectiveness in the management of CTS remains uncertain. As stated above, surgical management of CTS offers relief of symptoms by creating greater space in the carpal tunnel. Non‐surgical treatments for CTS must address different pathophysiological aspects of CTS in order to be successful.
How the intervention might work
Ergonomic positioning or equipment for treating CTS aims to position the wrist in a neutral position to provide maximum space within the carpal tunnel, and to avoid repeated or prolonged positioning of the wrist in flexion or extension (Hagberg 1992). For example, this may be achieved by altering the angle of a tool handle or keyboard. Treatments may reduce or even prevent the hand being exposed to vibration, by using robotic control of vibrating equipment, or using insulating tool handles or gloves. Other ergonomic interventions involve the use of forearm support and workplace modification to prevent prolonged static holding of the weight of the arm, forearm and hand (Herbert 2000).
Occupations involving keyboard and mouse use are increasing worldwide, especially in computerised customer service industries. Despite cross‐sectional and prospective research, the relationship between CTS and keyboard and mouse use remains unclear (Thomsen 2008; Fagarasanu 2003). Research into the efficacy of ergonomic keyboards and arm rests for reducing forearm, wrist and hand pain has ensued (Rempel 2006). Such keyboards, armrest and computer mouse devices are up to three times the cost of standard equipment, and many cannot be individually adjusted to individual operators.
Why it is important to do this review
Following the publication of the original version of this review (O'Connor 2003), a number of new trials and systematic reviews on non‐surgical treatment for CTS have been conducted (Ashworth 2010; Gerritsen 2002; Goodyear‐Smith 2004; Huisstede 2010; Muller 2004; Ono 2010; Piazzini 2007). The most recent review (Huisstede 2010) searched for studies published until January 2010 and concluded that the evidence base for a number of interventions was still incomplete. Given the personal and financial impact of CTS and the number of proposed ergonomic solutions, it is important to ascertain the efficacy of ergonomic equipment and/or positioning for the treatment of CTS.
Objectives
The objective of this review was to compare the effectiveness of ergonomic positioning or equipment for CTS with no treatment, placebo or another non‐surgical treatment for improving clinical outcome.
This review replaces the part of the previous review titled Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome (O'Connor 2003) dealing with ergonomic interventions.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished studies using or attempting to use a randomised methodology were eligible for inclusion. We included studies comparing ergonomic positioning or equipment with no treatment, placebo, or another non‐surgical treatment. We also included studies comparing one ergonomic positioning or equipment regimen versus another regimen. We excluded studies comparing ergonomic positioning or equipment with surgical treatment. There were no language restrictions.
Types of participants
All participants with a diagnosis of CTS, as defined by the authors of each study. We excluded participants who had undergone previous surgery for CTS.
Types of interventions
All ergonomic positioning or equipment interventions. Comparison interventions included no treatment, placebo, and other non‐surgical interventions for CTS; we excluded surgical interventions as comparisons.
Types of outcome measures
The outcomes reported in this review have been modified from the original review (O'Connor 2003) to make them as consistent as possible with other Cochrane reviews on CTS (Scholten 2007; Marshall 2007; Verdugo 2008).
Primary outcomes
Short‐term overall improvement (any measure in which patients indicate the intensity of their complaints compared with baseline) (dichotomous outcome; three months or less).
Secondary outcomes
Adverse effects.
Short‐term improvement in CTS symptoms (for example, pain, paraesthesiae, nocturnal paraesthesiae) (three months or less).
Short‐term improvement in functional ability or health‐related quality of life (three months or less).
Short‐term improvement in neurophysiologic parameters (three months or less).
Long‐term improvement in CTS symptoms (greater than three months).
Long‐term improvement in functional ability or health‐related quality of life (greater than three months).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Neuromuscular Disease Group Specialized Register (13 June 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (2011, Issue 2, in The Cochrane Library), MEDLINE (1966 to June 2011), Embase (1980 to June 2011), CINAHL Plus (1937 to June 2011) and AMED (1985 to June 2011). The detailed search strategies are listed in the appendices: CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), CINAHL Plus (Appendix 4) and AMED (Appendix 5).
Searching other resources
We also reviewed the reference lists of randomised or quasi‐randomised trials identified from the electronic search to identify any potentially relevant studies for inclusion.
Data collection and analysis
The review authors followed the recommended strategies for data collection and analysis as documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
At least two review authors independently selected trials for possible inclusion against a predetermined checklist of inclusion criteria. We initially categorised studies into the following groups.
Possibly relevant ‐ studies that met the inclusion criteria and studies from which it was not possible to determine whether they met the criteria either from their title or abstract.
Excluded ‐ those clearly not meeting the inclusion criteria.
If a title, or abstract, appeared to meet the eligibility criteria for inclusion of the review, or we could not tell, we obtained a full text version of the article and two review authors independently assessed it in order to determine whether it met the inclusion criteria. We resolved discrepancies between the authors via discussion.
Data extraction and management
Two review authors independently extracted data using a standard data extraction form. We resolved any discrepancies between the authors by consensus. We pilot tested the data extraction form and modified it accordingly before use. In addition to risk of bias characteristics and study results, we recorded the following details.
Participant details, including demographic and inclusion/exclusion criteria.
Types of interventions used and their comparison.
Outcomes reported, including the type and timing of measures used.
One review author compiled all comparisons and entered outcome data into the Cochrane statistical software Review Manager (RevMan) 5. All authors cross‐checked data. For trials where the required data were not reported, one author requested further information. When unsuccessful, we included the study in the review and fully described it, but did not include it in any meta‐analysis. We made an entry of these data in the notes section of the 'Characteristics of included studies' table.
Assessment of risk of bias in included studies
In this updated review we used The Cochrane Collaboration's tool for assessing risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed risk of bias in this review by reporting the trial's conduct against the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data (defined separately for data measured at 3 months or less, and after 3 months).
Selective reporting.
Other sources of bias (e.g. inappropriate unit of analysis).
Each item was rated as being at 'Low risk', 'Unclear risk' or 'High risk' of bias. The authors resolved any discrepancies between their ratings through discussion.
Measures of treatment effect
We used RevMan 5 for data analysis. Results are expressed as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes and mean differences with 95% CIs for continuous outcomes if the same measurement tool was used to measure the same outcome across separate studies. Alternatively, we summarised continuous outcomes using the standardised mean difference (SMD) when studies measured the same outcome but employed different measurement tools.
Unit of analysis issues
We sought information about the unit of randomisation (participants or wrists) from the included studies, and if not reported, we contacted trialists for clarification.
Dealing with missing data
We sought relevant missing information about study design, outcome data, or attrition rates like dropouts, losses to follow‐up and withdrawn study participants from the authors of included studies, where possible.
Assessment of heterogeneity
We planned to assess clinical and statistical heterogeneity as follows. We would have assessed clinical heterogeneity by determining whether the characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across studies. We would have assessed statistical heterogeneity using the Chi‐square statistic and the I2 test (Higgins 2002). We would have interpreted the I2 statistic using the following as an approximate guide:
0 to 40% might not be important heterogeneity;
30 to 60% may represent moderate heterogeneity;
50 to 90% may represent substantial heterogeneity; and
75 to 100% may represent considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
To assess publication bias, we intended to generate funnel plots if at least 10 studies examining the same treatment comparison were included in the review (Higgins 2011). To assess outcome reporting bias, protocols of trials were searched on the clinical trials register that is maintained by the US National Institute of Health at http://clinicaltrials.gov, and we searched protocols of trials published after July 1st 2005 using the Clinical Trial Register at the International Clinical Trials Registry Platform of the World Health Organisation (http://apps.who.int/trialssearch), to compare with the corresponding published RCTs (Dwan 2008, Dwan 2011).
Data synthesis
We planned to combine the results of studies with similar characteristics (participants, interventions, outcome measures and timing of outcome measurement) to provide estimates of the efficacy of ergonomic positioning or equipment interventions for treating CTS. We planned to undertake meta‐analysis on pooled results using either a fixed‐effect or random‐effects model (depending on the level of clinical and methodological heterogeneity). We set statistical significance at P < 0.05 for primary and secondary outcome measures. Where data could not be combined, we presented a narrative synthesis of results.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses according to severity of CTS symptoms and sex, since these factors may cause variations in outcomes. We defined subgroups as follows.
Severity of CTS symptoms: early (E), intermediate (I) and advanced (A) symptoms (Szabo 1992).
Sex: male, female.
Sensitivity analysis
We planned sensitivity analyses for each element on the 'Risk of bias' table by excluding studies that had a high risk of bias. We also planned sensitivity analyses using the following filter.
Quality of diagnostic criteria: high (A), moderate (B) and low (C) quality (Rempel 1998).
Results
Description of studies
Results of the search
The search conducted up until June 2011 identified 763 records. Table 1 reports the number of hits retrieved by each search strategy. The number of records after removal of duplicates was 558. From these, 15 full text papers were retrieved for further examination. After screening the full text of the 15 selected papers for eligibility, two studies (Rempel 1999; Tittiranonda 1999) met the inclusion criteria. A flow diagram of the study selection process is presented in Figure 1.
1.
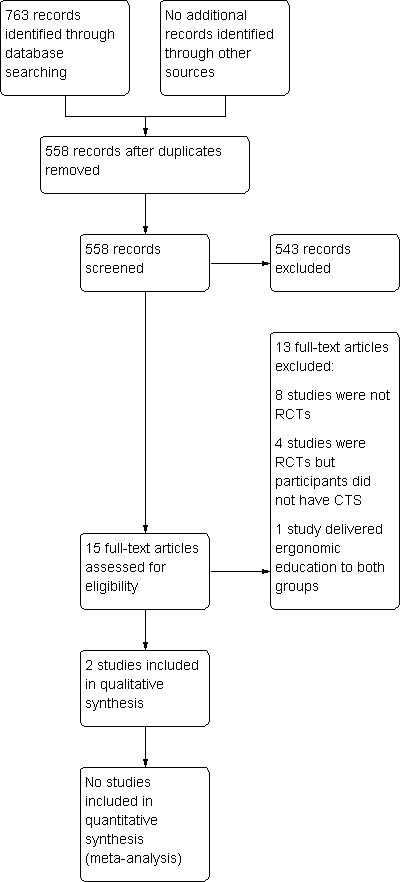
Study flow diagram.
Table 1
| Database | Period searched | Date searched | # hits |
| Cochrane Neuromuscular Disease Group Specialized Register | to 13 June 2011 | 13 June 2011 | 16 |
| CENTRAL | to 13 June 2011 | 13 June 2011 | 96 |
| MEDLINE | 1966 to June 2011 | 13 June 2011 | 260 |
| EMBASE | 1980 to June 2011 | 13 June 2011 | 176 |
| CINAHL Plus | 1937 to June 2011 | 13 June 2011 | 185 |
| AMED | 1985 to June 2011 | 13 June 2011 | 30 |
Included studies
Both of the included trials studied the effect of ergonomic equipment (keyboards) versus placebo (standard keyboard). Rempel 1999 compared an ergonomically adjusted keyboard, using altered force‐displacement key characteristics, with a standard keyboard for 12 weeks. Tittiranonda 1999 compared three ergonomic keyboard designs with a standard keyboard for six months.
Excluded studies
In total, 543 studies were excluded after screening of titles and abstracts, and 13 of 15 retrieved articles were excluded after review of the full publication. Reasons for exclusion of studies are given in the 'Characteristics of excluded studies' table. The main reasons for exclusion were non‐randomised study design and that interventions other than ergonomic positioning or equipment for CTS were under investigation.
Risk of bias in included studies
For details of risk of bias in the included studies, see the 'Characteristics of included studies' tables and Figure 2.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation was judged to be adequate in both trials. Rempel 1999 used a random number table, while Tittiranonda 1999 referred to using a using a random permuted block method.
Allocation concealment was unclear in both trials, as insufficient information was provided by the authors.
Blinding
Blinding of self‐reported outcomes was judged as likely to have been achieved for Rempel 1999, but not for Tittiranonda 1999.
Blinding of other outcomes was judged as likely to have been achieved for both trials.
Incomplete outcome data
Incomplete outcome data (three months or less) were judged to be adequately addressed in Rempel 1999 as the numbers and reasons for drop‐outs were clearly reported.
Incomplete data (both at three months or less and after three months) were judged as inadequately addressed by Tittiranonda 1999 as the time points at which participants withdrew and the number of participants withdrawing per subgroup were not reported.
Selective reporting
We judged selective outcome reporting to be present in both trials. In Rempel 1999, mean data for one of the outcomes (Phalen's test time) were only reported graphically, with no measure of variability, and no measure of variability was reported for the functional ability outcomes (though these data were provided by the authors on request). In Tittiranonda 1999, the authors state that all outcomes were analysed separately for the subgroups of patients with CTS and those with tendonitis but for the majority, the data reported were based on a combined analysis of subgroups. It is unclear if any additional outcomes were analysed but not reported, as no trial protocol or registry entry could be identified for these studies. Therefore, our assessment of reporting bias is limited.
Other potential sources of bias
Other potential threats to validity (such as bias related to the study design, analysis used or some other problem), we judged to be unclear for both studies.
Effects of interventions
See: Table 1
Ergonomic positioning or equipment versus no treatment
No trials found.
Ergonomic positioning or equipment versus placebo
Two trials (Rempel 1999; Tittiranonda 1999) compared ergonomic versus standard keyboards. Rempel 1999 compared Protouch keyboard versus standard keyboard in 25 participants. Tittiranonda 1999 compared Apple Adjustable keyboard versus Comfort Keyboard System versus Microsoft Natural Keyboard versus regular keyboard in 80 participants.
Primary outcomes
1) Short‐term overall improvement (three months or less)
Not reported as an outcome in either trial.
Secondary outcomes
1) Adverse effects
Not reported as an outcome in either trial.
2) Short‐term improvement in CTS symptoms (three months or less)
Reported as an outcome in Rempel 1999 but not Tittiranonda 1999.
Rempel 1999 measured pain on a visual analogue scale (VAS) (range 0 to 10) after six weeks of treatment and again at the end of the 12‐week treatment period using endpoint scores. The Protouch Keyboard was not statistically significantly favoured over a standard keyboard in terms of pain at six weeks (MD ‐0.20; 95% CI ‐1.51 to 1.11, Analysis 1.1). However, participants using the Protouch Keyboard had statistically significantly less pain at the end of the 12‐week treatment period (MD ‐2.40; 95% CI ‐4.45 to ‐0.35, Analysis 1.1) compared with the standard keyboard. Phalen test time (measured as the duration of time from which the wrist is bent to the onset of symptoms as reported by the patient) was also measured at 12 weeks (Analysis 1.2), but no statistically significant difference between groups was found in the right‐handed‐CTS afflicted participants (MD 14.20; 95% CI ‐3.20 to 31.60) or in the left‐handed‐CTS afflicted participants (MD 12.70; 95% CI ‐9.88 to 35.28).
1.1. Analysis.
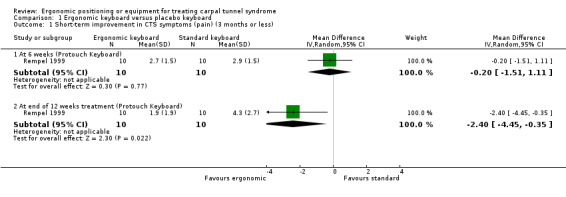
Comparison 1 Ergonomic keyboard versus placebo keyboard, Outcome 1 Short‐term improvement in CTS symptoms (pain) (3 months or less).
1.2. Analysis.
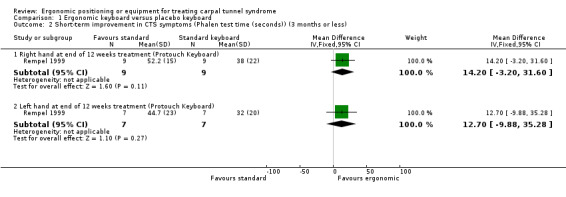
Comparison 1 Ergonomic keyboard versus placebo keyboard, Outcome 2 Short‐term improvement in CTS symptoms (Phalen test time (seconds)) (3 months or less).
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
Reported as an outcome in Rempel 1999 but not Tittiranonda 1999.
Self‐assessed hand function was assessed in Rempel 1999. There was no statistically significant difference between the ergonomic and standard keyboard groups in terms of the overall hand function score at six weeks (MD ‐1.20; 95% CI ‐10.01 to 7.61) and at the end of 12 weeks treatment (MD ‐2.20; 95% CI ‐11.57 to 7.17) (Analysis 1.3).
1.3. Analysis.
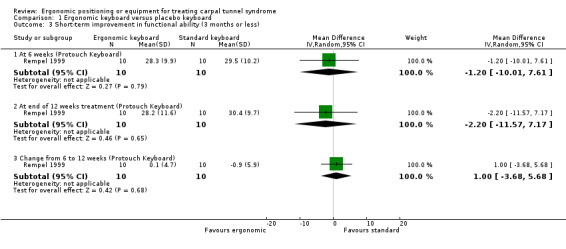
Comparison 1 Ergonomic keyboard versus placebo keyboard, Outcome 3 Short‐term improvement in functional ability (3 months or less).
4) Short‐term improvement in neurophysiologic parameters (three months or less)
Reported as an outcome in Rempel 1999 but not Tittiranonda 1999.
At the end of 12 weeks treatment, Rempel 1999 found no statistically significant difference between the Protouch Keyboard and standard keyboard groups in palm‐wrist sensory latency (ms) in right‐handed‐CTS afflicted participants (MD 0.28; 95% CI ‐0.09 to 0.65) or left‐handed‐CTS afflicted participants (MD ‐0.14; 95% CI ‐0.46 to 0.18) (Analysis 1.4).
1.4. Analysis.
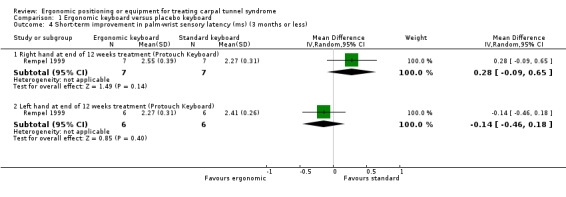
Comparison 1 Ergonomic keyboard versus placebo keyboard, Outcome 4 Short‐term improvement in palm‐wrist sensory latency (ms) (3 months or less).
5) Long‐term improvement in CTS symptoms (over three months)
Reported as an outcome in Tittiranonda 1999 but not Rempel 1999.
Tittiranonda 1999 measured pain using a VAS (range 0 to 10) and data were reported for CTS participants separately as mean change scores from baseline to six months. When compared with the standard keyboard group, there was no statistically significant change from baseline in pain severity in the Apple Adjustment Keyboard group (MD 0.70; 95% CI ‐0.97 to 2.37), in the Comfort Keyboard System group (MD 0.97; 95% CI ‐0.64 to 2.58) or in the Microsoft Natural Keyboard group (MD 0.79; 95% CI ‐1.53 to 3.11) (Analysis 1.5). Tittiranonda 1999 reported the number of participants who improved, worsened, or remained the same in overall pain severity at six months. However, these data were only reported graphically and with no measures of variability presented. Furthermore, the data were not reported separately for the subgroup of CTS patients in the trial (the total sample consisted of CTS and tendonitis patients). Tittiranonda 1999 reported the number of participants with worse, same, or better Phalen's and Tinel signs at the end of six months treatment, and Phalen test time (ms), but these data were not reported separately for CTS participants (data for CTS and tendonitis patients were combined).
1.5. Analysis.
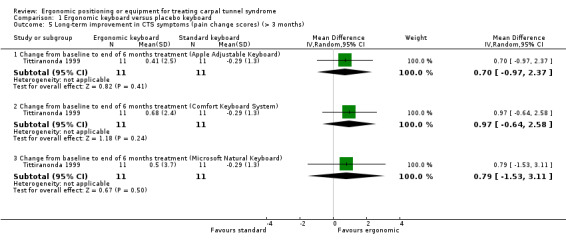
Comparison 1 Ergonomic keyboard versus placebo keyboard, Outcome 5 Long‐term improvement in CTS symptoms (pain change scores) (> 3 months).
6) Long‐term improvement in functional ability or health‐related quality of life (over three months)
Reported as an outcome in Tittiranonda 1999 but not Rempel 1999.
Tittiranonda 1999 measured self‐reported functional status and reported this as mean change from baseline to six months. However, the data were not reported separately for the subgroup of CTS patients (the total sample consisted of CTS and tendonitis patients).
Ergonomic positioning or equipment versus another non‐surgical treatment
No trials found.
Different ergonomic positioning or equipment regimens
One trial (Tittiranonda 1999) compared three ergonomic keyboards (Apple Adjustable keyboard, Comfort Keyboard System, Microsoft Natural Keyboard) versus regular keyboard in 80 participants.
Primary outcomes
1) Short‐term overall improvement (three months or less)
Not reported as an outcome.
Secondary outcomes
1) Adverse effects
Not reported as an outcome.
2) Short‐term improvement in CTS symptoms (three months or less)
Not reported as an outcome.
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
Not reported as an outcome.
4) Short‐term improvement in neurophysiologic parameters (three months or less)
Not reported as an outcome.
5) Long‐term improvement in CTS symptoms (over three months)
Tittiranonda 1999 measured pain using a VAS (range 0 to 10) and data were reported for CTS participants separately as mean change scores from baseline to six months. There was no statistically significant change from baseline in pain severity when the Apple Adjustment Keyboard group was compared with the Comfort Keyboard System group (MD ‐0.27; 95% CI ‐2.32 to 1.78), when the Apple Adjustment Keyboard was compared with the Microsoft Natural Keyboard (MD ‐0.09; 95% CI ‐2.73 to 2.55), or when the Comfort Keyboard System was compared with the Microsoft Natural Keyboard (MD 0.18; 95% CI ‐2.43 to 2.79) (Analysis 2.1). Tittiranonda 1999 reported the number of participants who improved, worsened, or remained the same in overall pain severity, Phalen's and Tinel signs and Phalen test time at six months. However, these data were not reported separately for the subgroup of CTS patients (the total sample consisted of CTS and tendonitis patients).
2.1. Analysis.
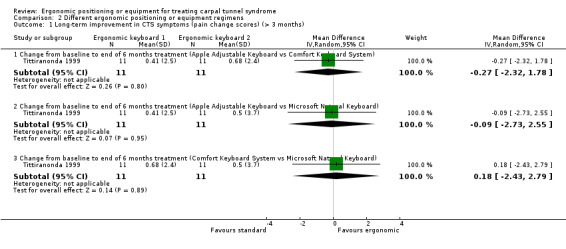
Comparison 2 Different ergonomic positioning or equipment regimens, Outcome 1 Long‐term improvement in CTS symptoms (pain change scores) (> 3 months).
6) Long‐term improvement in functional ability or health‐related quality of life (over 3 months)
Tittiranonda 1999 measured self‐reported functional status and reported this as mean change from baseline to six months. However, the data were not reported separately for the subgroup of CTS patients (the total sample consisted of CTS and tendonitis patients).
Discussion
Unfortunately, there has been limited investigation of ergonomic positioning or equipment for management of CTS. This is not necessarily unexpected since a clinical approach might typically first focus on reduction of symptoms, and changes to perpetuating factors such as work environment are less likely to be under the control of the patient and treating clinician. Recommendations regarding changes can be made by clinicians but the responsibility for them falls on the employer. Another factor that makes evaluating ergonomic changes for treatment of CTS more difficult is its timing: there are often delays in implementation in the workplace or prescription so that medical or surgical interventions are given in the meantime. The study by Tittiranonda 1999 highlights that ergonomic positioning or equipment changes are not just used for CTS. They are primary treatments for other concurrent disorders such as tendonitis and repetitive strain injury. This limited the contribution of their study to this review since data were not reported separately for the subgroup of CTS patients. Further study of ergonomic intervention for management of CTS is necessary in order to clarify its potential role in managing CTS.
Summary of main results
There is limited and very low quality evidence from two small trials comparing ergonomic versus placebo keyboard indicating that ergonomic keyboard may result in greater pain reduction than a standard keyboard. However, no primary outcome data and limited data for secondary outcomes were available and no pooling across studies was possible. There is no evidence to suggest that the use of an ergonomic keyboard rather than a standard one improves the other symptoms and signs of CTS. No trials were identified that compared ergonomic keyboards versus control, or ergonomic keyboards versus another non‐surgical treatment, or assessed the efficacy of other ergonomic positioning or equipment interventions.
Quality of the evidence
Overall the quality of the evidence was very low, as both trials had unclear allocation concealment, one trial did not blind participants and was at high risk of bias due to incomplete outcome data (Tittiranonda 1999), and both studies were at high risk of bias due to selective reporting of outcomes.
Potential biases in the review process
While our described methods attempted to minimise bias in the selection of studies, collection of published data and analysis for the review, our searches were limited to electronic databases and as a result, we have only included published studies. Further, assessment of selective outcome reporting was limited as no protocols trial registry entries for the studies were identified.
Agreements and disagreements with other studies or reviews
Only one of the other available systematic reviews on non‐surgical treatment for CTS has included the two studies looking at ergonomic equipment or positioning for CTS included in this review (Huisstede 2010). In agreement with this review Huisstede 2010 concludes that ergonomic keyboard may be superior to standard keyboard in terms of pain reduction, but more research on other outcomes important to patients is required.
Authors' conclusions
Implications for practice.
There is insufficient evidence regarding short‐ and long‐term improvement in symptoms, functional ability, health‐related quality of life, neurophysiologic parameters, need for surgery, adverse effects and cost to determine whether ergonomic positioning or equipment is superior to control, placebo or other non‐surgical treatment for carpal tunnel syndrome.
Implications for research.
Methodologically rigorous studies are required to determine the effect of non‐surgical interventions such as ergonomic positioning or equipment on outcomes such as overall, short‐ and long‐term improvement in CTS symptoms, functional ability, health‐related quality of life, neurophysiologic parameters, need for surgery and adverse effects, using validated scales.
Notes
This is one of six new reviews that will update the currently published review 'Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome' (O'Connor 2003). When all six reviews are published we will withdraw the original review from publication. This review includes a new search, revised review question and selection criteria, updated methodology and an updated review team.
Acknowledgements
We would like to thank Louisa Dunn, Kate Jewitt, Carolyn Reid, Angela Gunn and Rachel Barton from the Cochrane Neuromuscular Disease Group for their assistance in devising the search strategy, helping to locate people to translate the non‐English trials and ongoing support for this review. We thank Katherine Beringer from the School of Public Health and Preventive Medicine at Monash University for her assistance in retrieving studies relevant to the review. We thank David Rempel who corresponded with the principal review author to clarify additional information and/or provided additional data for the review. We thank Malgorzata Bala, Duray Seker, Usha Buenger and other colleagues for their assistance in translating abstracts and papers for the review.
We thank the following institutions for their support during the review:
School of Public Health and Preventive Medicine, Monash University, Melbourne, AUSTRALIA
School of Occupational Therapy, University of South Australia, Adelaide, AUSTRALIA
Institute for Rehabilitation Research and Development, Ottawa, CANADA
Editorial support from the Cochrane Neuromuscular Disease Group is funded by the MRC Centre for Neuromuscular Diseases.
Appendices
Appendix 1. CENTRAL search strategy
#1"carpal tunnel" or carpal‐tunnel or "carp*tunn*" or "carp*syndr*" #2"nerve entrapment" or "nerve compression" or "entrapment neuropath*" #3(#1 OR #2) #4work or workplace or workstation or position or tool* or keyboard* or terminal* or mouse or ergonomic* or "computer design" or engineering or glove* or armrest* or "arm support*" #5(#3 AND #4)
Appendix 2. MEDLINE (OvidSP) search strategy
1 randomized controlled trial.pt. (308386) 2 controlled clinical trial.pt. (82578) 3 randomized.ab. (214849) 4 placebo.ab. (125246) 5 drug therapy.fs. (1456618) 6 randomly.ab. (155580) 7 trial.ab. (221813) 8 groups.ab. (1034167) 9 or/1‐8 (2694405) 10 exp animals/ not humans.sh. (3598690) 11 9 not 10 (2284564) 12 carpal tunnel syndrome/ or carpal tunnel syndrome.tw. (7120) 13 (nerve entrapment or nerve compression or entrapment neuropath$).mp. (10471) 14 12 or 13 (16732) 15 (work or workplace or workstation or position or tool$1 or keyboard$ or terminal$ or mouse or ergonomic$ or computer design or engineering or glove$ or armrest$ or arm support).tw. (1563090) 16 11 and 14 and 15 (273) 17 remove duplicates from 16 (260)
Appendix 3. EMBASE (OvidSP) search strategy
1 crossover‐procedure/ (30474) 2 double‐blind procedure/ (100517) 3 randomized controlled trial/ (288881) 4 single‐blind procedure/ (13930) 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. (997092) 6 or/1‐5 (1067774) 7 exp animals/ (1660472) 8 exp humans/ (12427590) 9 7 not (7 and 8) (1259431) 10 6 not 9 (1033033) 11 limit 10 to embase (829998) 12 carpal tunnel syndrome/ or carpal tunnel syndrome.tw. (9665) 13 (nerve entrapment or nerve compression or entrapment neuropath$).mp. (11195) 14 12 or 13 (19448) 15 (work or workplace or workstation or position or tool$1 or keyboard$ or terminal$ or mouse or ergonomic$ or computer design or engineering or glove$ or armrest$ or arm support).tw. (1811052) 16 11 and 14 and 15 (177) 17 remove duplicates from 16 (176)
Appendix 4. CINAHL Plus (EBSCOhost) search strategy
S23 S18 and S21 and S22 S22 work or workplace or workstation or position or tool* or keyboard* or terminal* or mouse or ergonomic* or computer design or engineering or glove* or armrest* or arm support S21 S19 or S20 S20 nerve entrapment or nerve compression or entrapment neuropath* S19 carpal tunnel syndrome S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 S17 ABAB design* S16 TI random* or AB random* S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) S11 PT ("clinical trial" or "systematic review") S10 (MH "Factorial Design") S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") S8 (MH "Meta Analysis") S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") S6 (MH "Quasi‐Experimental Studies") S5 (MH "Placebos") S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") S3 (MH "Clinical Trials+") S2 (MH "Crossover Design") S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample")
Appendix 5. AMED (OvidSP) search strategy
1 Randomized controlled trials/ (1430) 2 Random allocation/ (292) 3 Double blind method/ (417) 4 Single‐Blind Method/ (12) 5 exp Clinical Trials/ (3072) 6 (clin$ adj25 trial$).tw. (5186) 7 ((singl$ or doubl$ or treb$ or trip$) adj25 (blind$ or mask$ or dummy)).tw. (2127) 8 placebos/ (514) 9 placebo$.tw. (2423) 10 random$.tw. (11923) 11 research design/ (1651) 12 Prospective Studies/ (351) 13 meta analysis/ (106) 14 (meta?analys$ or systematic review$).tw. (1586) 15 control$.tw. (26050) 16 (multicenter or multicentre).tw. (683) 17 ((study or studies or design$) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment$)).tw. (9144) 18 or/1‐17 (40195) 19 carpal tunnel syndrome/ or carpal tunnel syndrome.tw. (428) 20 (nerve entrapment or nerve compression or entrapment neuropath$).mp. (348) 21 19 or 20 (726) 22 (work or workplace or workstation or position or tool$1 or keyboard$ or terminal$ or mouse or ergonomic$ or computer design or engineering or glove$ or armrest$ or arm support).tw. (27317) 23 18 and 21 and 22 (30)
Data and analyses
Comparison 1. Ergonomic keyboard versus placebo keyboard.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (pain) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 At 6 weeks (Protouch Keyboard) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.51, 1.11] |
| 1.2 At end of 12 weeks treatment (Protouch Keyboard) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐2.4 [‐4.45, ‐0.35] |
| 2 Short‐term improvement in CTS symptoms (Phalen test time (seconds)) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Right hand at end of 12 weeks treatment (Protouch Keyboard) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 14.20 [‐3.20, 31.60] |
| 2.2 Left hand at end of 12 weeks treatment (Protouch Keyboard) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 12.70 [‐9.88, 35.28] |
| 3 Short‐term improvement in functional ability (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 At 6 weeks (Protouch Keyboard) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐10.01, 7.61] |
| 3.2 At end of 12 weeks treatment (Protouch Keyboard) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐11.57, 7.17] |
| 3.3 Change from 6 to 12 weeks (Protouch Keyboard) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐3.68, 5.68] |
| 4 Short‐term improvement in palm‐wrist sensory latency (ms) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Right hand at end of 12 weeks treatment (Protouch Keyboard) | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.09, 0.65] |
| 4.2 Left hand at end of 12 weeks treatment (Protouch Keyboard) | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.46, 0.18] |
| 5 Long‐term improvement in CTS symptoms (pain change scores) (> 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Change from baseline to end of 6 months treatment (Apple Adjustable Keyboard) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.7 [‐0.97, 2.37] |
| 5.2 Change from baseline to end of 6 months treatment (Comfort Keyboard System) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.97 [‐0.64, 2.58] |
| 5.3 Change from baseline to end of 6 months treatment (Microsoft Natural Keyboard) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐1.53, 3.11] |
Comparison 2. Different ergonomic positioning or equipment regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long‐term improvement in CTS symptoms (pain change scores) (> 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Change from baseline to end of 6 months treatment (Apple Adjustable Keyboard vs Comfort Keyboard System) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.32, 1.78] |
| 1.2 Change from baseline to end of 6 months treatment (Apple Adjustable Keyboard vs Microsoft Natural Keyboard) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐2.73, 2.55] |
| 1.3 Change from baseline to end of 6 months treatment (Comfort Keyboard System vs Microsoft Natural Keyboard) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐2.43, 2.79] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Rempel 1999.
| Methods | Randomised, triple‐blind, controlled trial Blinded subjects, treaters and assessors |
|
| Participants | Total n = 25 participants randomised
Intervention group* n = 10
Control group* n = 10 4 males; 16 females* Mean ± SD age: Intervention 45.3 ± 10.4 yrs* Control 39.9 ± 9.38 yrs* Inclusion criteria: 1. Clinical diagnosis of CTS based on history and physical examination 2. Paraesthesiae, numbness or tingling in at least 2 fingers of median nerve distribution 3. Positive Phalen's or Tinel's sign or thenar atrophy 4. Numbness, tingling or diminished sensation with use of hands or awkward posture 5. Keyboard used greater than or equal to 2 hours per day or greater/equal to 10 hours per week 6. Employed in current job for greater than or equal to 3 months Exclusion criteria: 1. Neck symptoms 2. Acute major trauma to arm or shoulder 3. Evidence of cervical root involvement, thoracic outlet syndrome or pronator teres syndrome on physical examination 4. Prior CTR or surgery to hands, wrists |
|
| Interventions | Intervention: Protouch Keyboard (ergonomically adjusted for force‐displacement characteristics of keys) for 12 weeks Control: MacPro Plus Keyboard (standard keyboard) for 12 weeks |
|
| Outcomes | Outcomes assessed at 6 and 12 weeks 1. Pain using visual analogue scale 2. Hand function using ordinal questionnaire (13 items modified from Levine/Pransky scored on ordinal scale 1‐5; summed to provide overall score) 3. Phalen test time (in seconds). This time point is calculated as the duration of time from which the wrist is bent to the onset of symptoms as reported by the patient. 4. Nerve conduction: right and left palm‐wrist median sensory latencies (in msec) (at 12 weeks only) Note: end points are reported for continuous outcomes |
|
| Notes | *Data only reported for participants completing treatment (n = 20) Peripheral nerve conduction and Phalen test time values for both hands are reported. Mean and SD data for Phalen test time endpoints were provided by the authors in a personal communication. SD data for the functional ability endpoints were provided by the authors in a personal communication. Funding: This study was funded by the manufacturer of the Protouch and MacPro Plus keyboards. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After matching, one of the partners of a pair was randomly assigned to keyboard A and the other partner was assigned to the control, keyboard B." Comment: while not stated in the publication, when contacted the authors reported that a random number table (block design) was used to generate the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were matched according to self‐reported average computer usage hours per week and to the hand involved. After matching, one of the partners of a pair was randomly assigned to keyboard A and the other partner was assigned to the control, keyboard B." Comment: not enough information to determine whether the allocation sequence was adequately concealed until interventions were assigned. |
| Blinding (performance bias and detection bias) Self‐reported outcomes | Low risk | Quote: "To the extent possible, patients were blinded to keyboard group. The keyboards could not be identified from external appearance; all identifying labels were masked. Patients were told that the study was to "evaluate the effectiveness of different keyboards" but were not provided with information about the differences between the keyboards nor the number of different keyboards evaluated." Comment: participants were probably blinded to treatment allocation although it is possible for participants to distinguish between keyboards based on 'feel' (different force‐displacement characteristics of keys). At unannounced times throughout the study, participant workstations were visited to ensure that the assigned keyboard was used. When contacted, the authors confirmed that participants were not told of treatment allocation. |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | Quote: "The medical personnel performing these measures were blinded to the patients' keyboard assignments." Quote: "A standardized physical examination of the upper extremities consisting of a timed Phalen's test was performed by a trained nurse practitioner who was also blinded to the questionnaire response." Quote: "The occupational therapist and neurologist were blinded to clinical findings, physical examination findings, and keyboard assignment." Comment: outcome assessors were probably blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "From the 25 eligible patients, 24 were matched into 12 pairs. During the first 2 weeks of the study, four subjects dropped out. The dropouts were all female: three had right hand symptoms and one had bilateral symptoms; three were administrative assistants and one was a technical writer/editor. Two reported that they could no longer participate in the study because of their heavy workloads. The other two withdrew because of worsening symptoms and discomfort (both assigned keyboard B). Of the remaining four subjects from the four broken pairs, one could not be rematched and was dropped, and three were rematched using the original matching criteria: two to each other and one to the back‐up subject. Therefore, 20 subjects (ten matched pairs) completed the study." Comment: numbers of drop‐outs and reasons for drop‐outs reported. |
| Selective reporting (reporting bias) | High risk | Comment: only mean data (no measures of variability) for the functional difficulty measure were reported numerically (these data were provided by the authors on request). Further, data for the Phalen's test time were only reported graphically, with no measure of variability presented. |
| Other bias | Low risk | No other sources of bias identified. |
Tittiranonda 1999.
| Methods | Randomised, single‐blind, placebo‐controlled trial of three ergonomic keyboard designs Blinded assessors Quality of diagnostic criteria = B |
|
| Participants | Total n = 80 participants randomised
Intervention group 1 n = 20 participants
Intervention group 2 n = 20 participants
Intervention group 3 n = 20 participants
Placebo group n = 20 participants 34 males; 46 females Mean ± SD age: Intervention group 1: 45 ± 8 yrs Intervention group 2: 41 ± 10 yrs Intervention group 3: 45 ± 7 yrs Placebo group: 44 ± 8 yrs Inclusion criteria: 1. Medical history and physical examination consistent with CTS 2. Paraesthesia, numbness or tingling on volar surface of digits 1‐3 3. Numbness, tingling or diminished sensation in hands with use or with awkward posture 4. Symptom duration of at least 1 week or having occurred at least 20 times in past year 5. Positive Phalen's or Tinel's sign 6. Full‐time employee in current job for > 3 months 7. Use computer keyboard greater than or equal to 4 hours per day or greater/equal to 20 hours per week Exclusion criteria: 1. Acute major trauma to hand, wrist or shoulder within last year 2. Thoracic outlet, cervical root or pronator teres syndromes on physical exam 3. Previous hand or wrist surgery 4. CTS diagnosis > 2 years prior to assessment date |
|
| Interventions | Intervention group 1: Apple Adjustable keyboard for 6 months Intervention group 2: Comfort Keyboard System for 6 months Intervention group 3: Microsoft Natural Keyboard for 6 months Placebo group: Regular keyboard for 6 months |
|
| Outcomes | Outcomes assessed at 6 months 1. Phalen's sign 2. Tinel's sign 3. Phalen test time (in seconds) 4. Pain using visual analogue scale (0 = no pain, 10 = worst pain) 5. Hand function using questionnaire (11 items modified from Levine/Pransky scored on visual analogue scale) |
|
| Notes | Attempts to clarify allocation method with authors were unsuccessful Change scores are reported for continuous outcomes. Negative values indicate worsening of symptoms or function. For the majority of the outcomes, data were reported only for the combined group of participants (i.e. data were rarely reported for the CTS subgroup only; instead, combined data on CTS and tendonitis patients were reported). Values for Phalen's sign and Tinel's sign are an aggregate of right and left hands. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible subjects were assigned one of the three alternative keyboard designs or a conventional placebo by using a random permuted block method [Pocock, 1991] and were stratified on the basis of disorder type (carpal tunnel syndrome and tendonitis or tendonitis only). Therefore, at the start of the study, there were 4 treatment groups with 20 subjects in each group." Comment: the randomisation sequence was probably adequately generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eligible subjects were assigned one of the three alternative keyboard designs or a conventional placebo by using a random permuted block method [Pocock, 1991] and were stratified on the basis of disorder type (carpal tunnel syndrome and tendonitis or tendonitis only). Therefore, at the start of the study, there were 4 treatment groups with 20 subjects in each group." Comment: not enough information to determine whether the allocation sequence was adequately concealed until interventions were assigned. |
| Blinding (performance bias and detection bias) Self‐reported outcomes | High risk | Quote: "This was a 6‐month, prospective, observer‐blinded, placebo‐controlled, randomized clinical trial comparing four keyboard treatments in 80 subjects with carpal tunnel syndrome and/or tendonitis." Comment: it is unlikely that participants were blinded to treatment allocation, especially given the nature of the interventions. Images of the four keyboards are displayed in Figure 2 of the publication and the ergonomic keyboards look different to the placebo keyboard. However, the investigators did make efforts to blind participants to treatment allocation (as per quote below). Quote: "For subjects who were randomized to the placebo group, their own keyboard was taken to the ergonomics laboratory one week prior to the trial. At the laboratory, dust particles were expelled from the inner mechanism and outer surface of the keyboards using compressed air. A label containing an alphanumeric identification number and a message that read "This keyboard has been modified as part of an interventional field trial" was attached to the left corner of the keyboard cover. An additional label which read "This keyboard has been internally modified as part of the LLNL keyboard field study. Please do not attempt to repair or open it. If technical problems arise, call for immediate assistance" was attached to the bottom of the keyboard. The screws underneath the keyboard platform were painted shut with blue‐coloured ink to prevent tampering. On the day of randomisation, the keyboards were returned to the subjects and they were told that their keyboard had been "modified" in some way and that the researchers were blinded to the modification to comply with the study protocol." |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | Quote: "Physical examination of the upper extremities was conducted for each participant at baseline and at the end of the 6‐month trial. The examination was standardized and consisted of inspection, palpation, passive and resisted movements, and a series of provocative tests that included timed Phalen’s, Tinel’s, and Finkelstein’s tests. The examiners were blinded to previous medical history and keyboard assignments." Comment: outcome assessors were probably blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) 3 months or less | High risk | Quote: "Eleven (14%) subjects withdrew from the study during the 6 months (Table IV). Withdrawals were most frequent in the kb2 group (n = 9); five of these were due to keyboard mechanical failure. One each withdrew from the other alternative keyboard groups. Reasons given for withdrawal were: frustration with their reduced productivity (n = 2, kb2) and increased discomfort (n = 1, kb2), inadequate workspace for use of detached numeric/function key pad (n = 1, kb1), and lack of time commitment (n = 1, kb3)." Comment: the specific time points at which participants withdrew, and the number of participants in the CTS subgroup who withdrew, were not reported (the number reported refers to the total sample). |
| Incomplete outcome data (attrition bias) After 3 months | High risk | Comment: See above |
| Selective reporting (reporting bias) | High risk | Quote: "All analyses were carried out for each limb for all subjects and then for each disorder type separately (CTS and tendonitis and tendonitis only)." Comment: for the majority of the outcomes, data were reported only for the combined group of participants (i.e., few data on the CTS subgroup were reported) |
| Other bias | Low risk | No other sources of bias identified. |
CTR: carpal tunnel release CTS: carpal tunnel syndrome SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersen 2003 | Not an RCT |
| Atroshi 2007 | Not an RCT |
| Bernaards 2006 | Protocol for RCT that will include participants with neck and upper limb symptoms, not CTS |
| Boyd 2005 | Not an RCT |
| Conlon 2009 | RCT but participants did not have CTS |
| Marangoni 2010 | Not an RCT and participants did not have CTS |
| Porrata 2007 | Not an RCT |
| Shafer Crane 2005 | Not an RCT |
| Simmer‐Beck 2006 | RCT but participants did not have CTS |
| Stevenson 2005 | Not an RCT |
| Thomsen 2008 | Not an RCT |
| Werner 2005a | RCT but both groups of CTS participants received ergonomic education (the intervention group also received splint) |
| Werner 2005b | Not an RCT |
CTS: carpal tunnel syndrome RCT: randomised controlled trial
Differences between protocol and review
This is a split review replacing the ergonomic positioning or equipment interventions included in the previous review titled Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome (O'Connor 2003).
In the review by O'Connor et al. (O'Connor 2003), types of outcome measures included in the review were as follows:
Primary outcome:
The primary outcome measure was improvement in clinical symptoms, such as pain and paraesthesiae, at least three months after the end of treatment.
Secondary outcome measures included: 1. improvement in functional status and/or health‐related quality of life parameters at least three months after treatment; 2. improvement in objective physical examination measures, such as grip, pinch strength, and sensory perception at least three months after treatment; 3. improvement in neurophysiological parameters after three months after treatment; 4. clinical improvement at less than three months of follow‐up; 5. clinical improvement at one year after treatment; 6. need for surgical release of the flexor retinaculum during follow‐up.
The outcomes reported in this review have been modified from the original review (O'Connor 2003) to make them as consistent as possible with other Cochrane reviews on carpal tunnel syndrome (Marshall 2007; Scholten 2007; Verdugo 2008).
Assessment for study risk of bias has been performed using The Cochrane Collaboration's 'Risk of bias' tool in this update of the review. We also included a 'Summary of findings' table.
Contributions of authors
DENISE O'CONNOR (DOC) co‐ordinated each stage of the review and was responsible for: design of the review (in collaboration with NMW and SM); developing the protocol (in collaboration with NMW and SM); developing the search strategy; undertaking the searches for studies; screening the search results (independently of, but in addition to MP, NMW and SM); organising retrieval of papers; screening retrieved papers against inclusion/exclusion criteria (independently of, but in addition to MP, NMW and SM); appraising the risk of bias of papers (independently of, but in addition to MP and SM); extracting data from papers (independently of, but in addition to MP, NMW and SM); writing to study investigators for additional information; providing additional data about papers; summarising the risk of bias of the studies (independently of, but in addition to MP); compiling the summary of comparisons, tables of included and excluded studies; entering data into RevMan (independently, but in addition to NMW) performing analysis of data; interpreting the findings; writing the review (with contribution from MP, NMW and SM); final approval of the version to be published.
MATTHEW PAGE (MP) was involved in the following stages of the review: screening the search results (independently of, but in addition to DOC, NMW and SM); screening retrieved papers against inclusion/exclusion criteria (independently of, but in addition to DOC); appraising the risk of bias of papers (independently of, but in addition to DOC and SM); extracting data from papers (independently of, but in addition to DOC, NMW and SM); summarising the risk of bias of the studies (independently, but in addition to DOC); compiling the summary of comparisons, tables of included and excluded studies; entering data into RevMan; performing analysis of data; contributing to the writing of the review (in collaboration with DOC, SM and NMW).
SHAWN MARSHALL (SM) was involved in the following stages of the review: design of the review (in collaboration with DOC and NMW); developing the protocol (in collaboration with DOC and NMW); screening the search results (independently of, but in addition to DOC, MP, and NMW); screening retrieved papers against inclusion/exclusion criteria (independently of, but in addition to DOC, MP and NMW); appraising the risk of bias of papers (independently of, but in addition to DOC and MP); extracting data from papers (independently of, but in addition to DOC and MP); contributing to the writing of the review (in collaboration with DOC, MP and NMW).
NICOLA MASSY‐WESTROPP (NMW) was involved in the following stages of the review: design of the review (in collaboration with DOC and SM); developing the protocol (in collaboration with DOC and SM); screening the search results (independently of, but in addition to DOC, MP, and SM); screening retrieved papers against inclusion/exclusion criteria (independently of, but in addition to DOC, MP and SM); contributing to the writing of the review (in collaboration with DOC, MP and SM).
Sources of support
Internal sources
School of Public Health and Preventive Medicine, Monash University, Australia.
Physical Medicine & Rehabilitation, University of Ottawa, Ottawa, Canada.
School of Health Sciences, University of South Australia, Adelaide, Australia.
DOC holds an Australian National Health and Medical Research Council Public Health Fellowship (606726), Australia.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Rempel 1999 {published data only}
- Rempel D, Tittiranonda P, Burastero S, Hudes M, So Y. Effect of keyboard keyswitch design on hand pain. Journal of Occupational and Environmental Medicine 1999;41(2):111‐9. [PUBMED: 10029956] [DOI] [PubMed] [Google Scholar]
Tittiranonda 1999 {published data only}
- Tittiranonda P, Rempel D, Armstrong T, Burastero S. Effect of four computer keyboards in computer users with upper extremity musculoskeletal disorders. American Journal of Industrial Medicine 1999;35(6):647‐61. [PUBMED: 10332518] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andersen 2003 {published data only}
- Andersen JH, Thomsen JF, Overgaard E, Lassen CF, Brandt LPA, Vilstrup I, et al. Computer use and carpal tunnel syndrome: a 1‐year follow‐up study. JAMA: Journal of the American Medical Association 2003;289(22):2963‐9. [DOI] [PubMed] [Google Scholar]
Atroshi 2007 {published data only}
- Atroshi I, Gummesson C, Ornstein E, Johnsson R, Ranstam J. Carpal tunnel syndrome and keyboard use at work: a population‐based study. Arthritis & Rheumatism 2007;56(11):3620‐5. [DOI] [PubMed] [Google Scholar]
Bernaards 2006 {published data only}
- Bernaards CM, Ariens GA, Hildebrandt VH. The (cost‐)effectiveness of a lifestyle physical activity intervention in addition to a work style intervention on the recovery from neck and upper limb symptoms in computer workers. BMC Musculoskeletal Disorders 2006;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyd 2005 {published data only}
- Boyd KU, Gan BS, Ross DC, Richards RS, Roth JH, MacDermid JC. Outcomes in carpal tunnel syndrome: symptom severity, conservative management and progression to surgery. Clinical & Investigative Medicine 2005;28(5):254‐60. [PubMed] [Google Scholar]
Conlon 2009 {published data only}
- Conlon CF, Krause N, Rempel DM. A randomized controlled trial evaluating an alternative mouse or forearm support on change in median and ulnar nerve motor latency at the wrist. American Journal of Industrial Medicine 2009;52(4):304‐10. [DOI] [PubMed] [Google Scholar]
Marangoni 2010 {published data only}
- Marangoni AH. Effects of intermittent stretching exercises at work on musculoskeletal pain associated with the use of a personal computer and the influence of media on outcomes. Work 2010;36(1):27‐37. [DOI] [PubMed] [Google Scholar]
Porrata 2007 {published data only}
- Porrata H, Porrata A, Sosner J. New carpal ligament traction device for the treatment of carpal tunnel syndrome unresponsive to conservative therapy. Journal of Hand Therapy 2007;20(1):20‐8. [DOI] [PubMed] [Google Scholar]
Shafer Crane 2005 {published data only}
- Shafer Crane GA, Meyer RA, Schlinger MC, Bennett DL, Robinson KK, Rechtien JJ. Effect of occupational keyboard typing on magnetic resonance imaging of the median nerve in subjects with and without symptoms of carpal tunnel syndrome. American Journal of Physical Medicine and Rehabilitation 2005;84(4):258‐66. [DOI] [PubMed] [Google Scholar]
Simmer‐Beck 2006 {published data only}
- Simmer‐Beck M, Bray KK, Branson B, Glaros A, Weeks J. Comparison of muscle activity associated with structural differences in dental hygiene mirrors. Journal of Dental Hygiene 2006;80:1. [PubMed] [Google Scholar]
Stevenson 2005 {published data only}
- Stevenson JR, Blake JM, Douglas TF, Kercheval DM. Does continuous passive motion during keyboarding affect hand blood flow and wrist function? A prospective case report. Work 2005;24(2):145‐55. [PubMed] [Google Scholar]
Thomsen 2008 {published data only}
- Thomsen JF, Gerr F, Atroshi I. Carpal tunnel syndrome and the use of computer mouse and keyboard: A systematic review. BMC Musculoskeletal Disorders 2008;9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Werner 2005a {published data only}
- Werner RA, Franzblau A, Gell N. Randomized controlled trial of nocturnal splinting for active workers with symptoms of carpal tunnel syndrome. Archives of Physical Medicine and Rehabilitation 2005;86(1):1‐7.. [DOI] [PubMed] [Google Scholar]
Werner 2005b {published data only}
- Werner RA, Franzblau A, Gell N, Hartigan AG, Ebersole M, Armstrong TJ. Incidence of carpal tunnel syndrome among automobile assembly workers and assessment of risk factors. Journal of Occupational & Environmental Medicine 2005;47(10):1044‐50. [DOI] [PubMed] [Google Scholar]
Additional references
Ashworth 2010
- Ashworth N. Carpal tunnel syndrome. Clinical Evidence 2010;03:1114. [PMC free article] [PubMed] [Google Scholar]
Atroshi 1999
- Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA: Journal of the American Medical Association 1999;282:153‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Braun 1989
- Braun RM, Davidson K, Doehr S. Provocative testing in the diagnosis of dynamic carpal tunnel syndrome. Journal of Hand Surgery (American Volume) 1989;14:195‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Charles 2009
- Charles J, Fahridin S, Britt H. Carpal tunnel syndrome. Australian Family Physician 2009;38(9):665. [PubMed] [Google Scholar]
Concannon 2000
- Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plastic & Reconstructive Surgery 2000;105:1662‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dwan 2008
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan A‐W, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS ONE 2008;3(8):e3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwan 2011
- Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database of Systematic Reviews 2011;Issue 1:Art. No.: MR000031.DOI: 10.1002/14651858.MR000031.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fagarasanu 2003
- Fagarasanu M, Kumar S. Carpal tunnel syndrome due to keyboarding and mouse tasks: a review. International Journal of Industrial Ergonomics 2003;31(2):119‐36. [Google Scholar]
Gelfman 2009
- Gelfman R, Melton LJ 3rd, Yawn BP, Wollan PC, Amadio PC, Stevens JC. Long‐term trends in carpal tunnel syndrome. Neurology 2009;72(1):33‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerritsen 2002
- Gerritsen AAM, Krom MCTFM, Struijs MA, Scholten RJPM, Vet HCW, Bouter LM. Conservative treatment options for carpal tunnel syndrome: a systematic review of randomised controlled trials. Journal of Neurology 2002;249:272‐80. [DOI] [PubMed] [Google Scholar]
Goodyear‐Smith 2004
- Goodyear‐Smith F, Arroll B. What can family physicians offer patients with carpal tunnel syndrome other than surgery? A systematic review of nonsurgical management. Annals of Family Medicine 2004;2:267‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hagberg 1992
- Hagberg M, Morgenstern H, Kelsh M. Impact of occupations and job tasks on the prevalence of carpal tunnel syndrome. Scandinavian Journal of Work, Environment & Health 1992;18:337‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herbert 2000
- Herbert R, Gerr F, Dropkin J. Clinical evaluation and management of work‐related carpal tunnel syndrome. American Journal of Industrial Medicine 2000;37:62‐74. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0. [Updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Huisstede 2010
- Huisstede BM, Hoogvliet P, Randsdorp MS, Glerum S, Middelkoop M, Koes BW. Carpal tunnel syndrome. Part 1: Effectiveness of nonsurgical treatments ‐ a systematic review. Archives of Physical Medicine and Rehabilitation 2010;91:981‐1004. [DOI] [PubMed] [Google Scholar]
Katims 1989
- Katims JJ, Rouvelas P, Sadler B, Weseley SA. Current perception threshold: Reproducibility and comparison with nerve conduction in evaluation of carpal tunnel syndrome. Transactions of the American Society for Artificial Internal Organs 1989;35:280‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Katz 1990
- Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self‐administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. Journal of Rheumatology 1990;17:1495‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Kerwin 1996
- Kerwin G, Williams CS, Seiler JG 3rd. The pathophysiology of carpal tunnel syndrome. Hand Clinics 1996;12:243‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Levine 1993
- Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self‐administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. Journal of Bone & Joint Surgery 1993;75:1585‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marshall 2007
- Marshall SC, Tardif G, Ashworth NL. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD001554.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller 2004
- Muller M, Tsui D, Schnurr R, Biddulph‐Deisroth L, Hard J. Effectiveness of hand therapy interventions in primary management of carpal tunnel syndrome: a systematic review. Journal of Hand Therapy 2004;17:210‐28. [DOI] [PubMed] [Google Scholar]
Newport 2000
- Newport ML. Upper extremity disorders in women. Clinical Orthopaedics & Related Research 2000;372:85‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ono 2010
- Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. International Journal of General Medicine 2010;3:255‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piazzini 2007
- Piazzini DB, Aprile I, Ferrera PE, Bertolini C, Tonali P, Maggi L, et al. A systematic review of conservative treatment of carpal tunnel syndrome. Clinical Rehabilitation 2007;21:299‐314. [DOI] [PubMed] [Google Scholar]
Rempel 1998
- Rempel D, Evanoff B, Amadio PC, Krom M, Franklin G, Franzblau A, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. American Journal of Public Health 1998;88:1447‐51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rempel 2006
- Rempel DM, Krause N, Goldberg R, Benner D, Hudes M, Goldener GU. A randomised controlled trial evaluating the effects of two workstation interventions on upper body pain and incident musculoskeletal disorders among computer operators. Occupational and Environmental Medicine 2006;63:300‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenthal 1987
- Rosenthal EA. Tenosynovitis: tendon and nerve entrapment. Hand Clinics 1987;3:585‐607. [MEDLINE: ] [PubMed] [Google Scholar]
Sadosky 2008
- Sadosky A, McDermott AM, Brandendburg NA, Stauss M. A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studies neuropathic pain conditions. Pain Practice 2008;8(1):45‐56. [DOI] [PubMed] [Google Scholar]
Scholten 2007
- Scholten RJPM, Molen AM, Uitdehaag BMJ, Bouter LM, Vet HCW. Surgical treatment options for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003905.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Szabo 1992
- Szabo RM, Madison M. Carpal tunnel syndrome. Orthopedic Clinics of North America 1992;23:103‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Szabo 1994
- Szabo RM, Steinberg DR. Nerve entrapment syndromes in the wrist. Journal of the American Academy of Orthopedic Surgeons 1994;2:115‐23. [DOI] [PubMed] [Google Scholar]
Verdugo 2008
- Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non‐surgical treatment for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD001552.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
O'Connor 2003
- O'Connor D, Marshall S, Massy‐Westropp N. Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003219] [DOI] [PMC free article] [PubMed] [Google Scholar]


