Abstract
Background
This is an update of a Cochrane Review first published in 2005. Cervical dystonia is the most common form of focal dystonia and is a highly disabling movement disorder characterised by involuntary, usually painful, head posturing. Currently, botulinum toxin type A (BtA) is considered the first line therapy for this condition.
Objectives
To compare the efficacy, safety, and tolerability of botulinum toxin type A (BtA) versus placebo in people with cervical dystonia.
Search methods
To identify studies for this review we searched Cochrane Movement Disorders' Trials Register, CENTRAL, MEDLINE, Embase, reference lists of articles and conference proceedings. All elements of the search, with no language restrictions, were run in October 2016.
Selection criteria
Double‐blind, parallel, randomised, placebo‐controlled trials (RCTs) of BtA versus placebo in adults with cervical dystonia.
Data collection and analysis
Two review authors independently assessed records, selected included studies, extracted data using a paper pro forma, and evaluated the risk of bias. We resolved disagreements by consensus or by consulting a third review author. We performed meta‐analyses using a random‐effects model for the comparison of BtA versus placebo to estimate pooled effects and corresponding 95% confidence intervals (95% CI). In addition, we performed preplanned subgroup analyses according to BtA dose used, the BtA formulation used, and the use or not of guidance for BtA injection. The primary efficacy outcome was improvement in cervical dystonia‐specific impairment. The primary safety outcome was the proportion of participants with any adverse event.
Main results
We included eight RCTs of moderate overall risk of bias, including 1010 participants with cervical dystonia. Six studies excluded participants with poorer responses to BtA treatment, therefore including an enriched population with a higher probability of benefiting from this therapy. Only one trial was independently funded. All RCTs evaluated the effect of a single BtA treatment session, using doses from 150 U to 236 U of onabotulinumtoxinA (Botox), 120 U to 240 U of incobotulinumtoxinA (Xeomin), and 250 U to 1000 U of abobotulinumtoxinA (Dysport).
BtA was associated with a moderate‐to‐large improvement in the participant's baseline clinical status as assessed by investigators, with reduction of 8.06 points in the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS total score) at week 4 after injection (95% CI 6.08 to 10.05; I2 = 0%) compared to placebo, corresponding on average to a 18.7% improvement from baseline. The mean difference (MD) in TWSTRS pain subscore at week 4 was 2.11 (95% CI 1.38 to 2.83; I2 = 0%). Overall, both participants and clinicians reported an improvement of subjective clinical status. There were no differences between groups regarding withdrawals due to adverse events. However, BtA treatment was associated with an increased risk of experiencing an adverse event (risk ratio (RR) 1.19; 95% CI 1.03 to 1.36; I2 = 16%). Dysphagia (9%) and diffuse weakness/tiredness (10%) were the most common treatment‐related adverse events (dysphagia: RR 3.04; 95% CI 1.68 to 5.50; I2 = 0%; diffuse weakness/tiredness: RR 1.78; 95% CI 1.08 to 2.94; I2 = 0%). Treatment with BtA was associated with a decreased risk of participants withdrawing from trials. We have moderate certainty in the evidence across all of the aforementioned outcomes.
We found no evidence supporting the existence of a clear dose‐response relationship with BtA, nor a difference between BtA formulations, nor a difference with use of EMG‐guided injection.
Due to clinical heterogeneity, we did not pool data regarding health‐related quality of life, duration of clinical effect, or the development of secondary non‐responsiveness.
Authors' conclusions
We have moderate certainty in the evidence that a single BtA treatment session is associated with a significant and clinically relevant reduction of cervical dystonia‐specific impairment, including severity, disability, and pain, and that it is well tolerated, when compared with placebo. There is also moderate certainty in the evidence that people treated with BtA are at an increased risk of developing adverse events, most notably dysphagia and diffuse weakness. There are no data from RCTs evaluating the effectiveness and safety of repeated BtA injection cycles. There is no evidence from RCTs to allow us to draw definitive conclusions on the optimal treatment intervals and doses, usefulness of guidance techniques for injection, the impact on quality of life, or the duration of treatment effect.
Plain language summary
Treatment with botulinum toxin type A for people with involuntary posturing of the head, or cervical dystonia
The review question
We reviewed the evidence about the effect of botulinum toxin type A (BtA) in people with involuntary positioning of the head, or cervical dystonia. This is an update of a previous Cochrane Review and we assessed the effectiveness (reduction in severity, disability and pain) and safety of BtA versus placebo (a pretend medicine) in cervical dystonia.
Background
Cervical dystonia, also called spasmodic torticollis, is a disease that causes undesired, uncontrollable, often painful, abnormal placement of the head. It is a relatively uncommon condition (affecting 57 to 280 people per million) that can be very disabling and can affect a person's quality of life negatively. In most cases the cause is unknown and no cure exists. Since cervical dystonia is normally a long‐term disease it requires long‐term treatment.
Botulinum toxin is a powerful, natural chemical that can cause severe paralysis (an inability to move in the part of the body where it is applied) in animals and humans. It can also be used to treat many conditions, in particular those with involuntary muscle contractions, such as cervical dystonia. Botulinum toxin is delivered by injections into the muscles that contract to produce most of the disease symptoms. There are different types of botulinum toxin, not all are available for treating health conditions. BtA is typically considered the first treatment option in cervical dystonia.
Study characteristics
We performed a rigorous search of the medical literature in October 2016 and found eight studies that compared treatment with BtA versus placebo. These studies included a total of 1010 participants, with on average a moderate disease impairment. The participants remained in the majority of studies for a short period of time ‐ between 16 and 20 weeks after the treatment. The average age of people in the studies was 52.3 years, and they had had cervical dystonia for an average of 4.8 to 12.1 years before taking part in the trials. Most, 64%, of the people in the studies were women. Seven of the eight trials were funded by drug manufacturers with possible interests in the results of the studies.
Key results
The results show that a single treatment session improved cervical dystonia symptoms, including pain, and participant's self‐evaluations. However, the risk of having an unpleasant or undesirable event, particularly swallowing difficulties and tiredness, was also increased. Only three studies examined the impact of BtA on quality of life, suggesting some benefit from BtA.
Certainty in the evidence
The certainty in the evidence for overall and pain improvement, the risk of undesired events, self‐evaluation, the risk of swallowing difficulties, and the risk of participants not tolerating treatment, is moderate.
Nevertheless, to be included in the studies, participants had to have a history of successful treatment with BtA. People with certain types of cervical dystonia, in particular the types that make the head turn mostly backward or forward, were not allowed to participate in the studies, and it is known that these types respond less to botulinum toxin treatment. Therefore, the conclusions from this review may not apply to all people with cervical dystonia.
We can draw no conclusions regarding long‐term effects of BtA for this condition.
Summary of findings
Summary of findings for the main comparison. Botulinum toxin type A compared to placebo for cervical dystonia.
| Botulinum toxin type A compared to placebo for cervical dystonia | ||||||
| Patient or population: adults with cervical dystonia Setting: hospital‐based, movement disorders clinics Intervention: botulinum toxin type A Comparison: placebo | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty in the evidence (GRADE) | What happens | ||
| With placebo | With botulinum toxin type A | Difference | ||||
|
Cervical dystonia‐specific impairment
Number of participants: 522 (4 RCTs) Assessed 3 to 6 weeks post‐injection using TWSTRS total score |
‐ | 3.9 TWSTRS units decrease | 12.45 TWSTRS units decrease | The mean change from baseline was 8.06 TWSTRS units higher (6.08 higher to 10.05 higher) in the BtA group compared to the placebo group | ⊕⊕⊕⊝ Moderatea | BtA treatment probably improves cervical dystonia‐specific impairment |
|
Adverse events
Number of participants: 952 (7 RCTs) Assessed at any point during follow‐up |
RR 1.19 (1.03 to 1.36) | 46.5% | 55.3% (47.9 to 63.2) | 8.8% more (1.4 more to 16.7 more) | ⊕⊕⊕⊝ Moderatea | BtA treatment probably increases the risk of adverse events |
|
Subjective participant assessment
Number of participants: 624 (5 RCTs) Assessed 3 to 6 weeks post‐injection |
RR 2.30 (1.83 to 2.90) | 24.2% | 55.7% (44.3 to 70.2) | 31.5% more (20.1 more to 46 more) | ⊕⊕⊕⊝ Moderatea | BtA treatment probably increases the likelihood that patients will detect any form of improvement |
|
Pain relief
Number of participants: 429 (3 RCTs) Assessed 3 to 6 weeks post‐injection using TWSTRS pain subscore |
‐b | ‐b | ‐b | The mean change from baseline was 2.11 TWSTRS units higher (1.38 higher to 2.83 higher) in the BtA group compared to the placebo group | ⊕⊕⊕⊝ Moderatea | BtA treatment probably improves cervical dystonia‐related pain |
|
Tolerability
Number of participants: 574 (4 RCTs) Assessed at any point during follow‐up |
RR 0.38 (0.23 to 0.62) | 20.5% | 7.8% (4.7 to 12.7) |
12.7% fewer (15.8 to 7.8) |
⊕⊕⊕⊝ Moderatea | BtA treatment probably slightly decreases the risk of withdrawal of clinical trials |
|
Dysphagia
Number of participants: 1007 (8 RCTs) Assessed at any point during follow‐up |
RR 3.04 (1.68 to 5.50) | 3.0% | 9.2% (5.1 to 16.7) | 6.2% more (2.1 more to 13.7 more) | ⊕⊕⊕⊝ Moderatea | BtA treatment probably increases the risk of dysphagia |
|
Diffuse weakness/tiredness
Number of participants: 823 (6 RCTs) Assessed at any point during follow‐up |
RR 1.78 (1.08 to 2.94) | 5.6% | 10.1% (6.1 to 16.6) | 4.4% more (0.5 more to 11 more) | ⊕⊕⊕⊝ Moderatea | BtA treatment probably increases the risk of diffuse weakness/tiredness |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BtA: botulinum toxin type A; CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded one level due to serious study limitations, namely concerns with randomisation procedures and other biases such as 'for‐profit' bias. b Data were only available as between‐group differences.
Background
This review is an update of a review previously published in the Cochrane Database of Systematic Reviews 2005, Issue 1 (Costa 2005), evaluating the efficacy and safety of botulinum toxin type A (BtA) versus placebo in the treatment of cervical dystonia.
Description of the condition
See Table 3 for glossary of terms.
1. Glossary of terms.
| Term | Definition |
| BtA‐non‐responsive | People who do not experience the expected benefit from treatment with botulinum toxin type A |
| Cervical dystonia or spasmodic torticollis | A common movement disorder in which people have abnormal movements or postures of the head and neck that they cannot control. It is frequently accompanied by social embarrassment and pain. |
| Chemodenervation | The process by which botulinum toxin causes muscular paralysis. Although all the anatomical elements necessary for muscular control are intact (i.e. nerve, synapse and muscle), there is a chemical process that disables the transmission of the electrical signal from the nerve to the muscle. |
| Dysphagia | A discomfort or difficulty when swallowing. |
| Electromyography | An examination that displays the electrical activity of muscles using pieces of metal attached to the skin or inserted into the muscle. |
| Non‐naive | People who have been treated in the past with botulinum toxin. |
| Voluntary action | Movements that people are able to control, start and stop when they want to. |
Dystonia is the third most common movement disorder, after Parkinson’s disease and essential tremor, with an overall prevalence of 164 per million (Steeves 2012). Dystonia syndromes are a group of disabling, painful disorders characterised by involuntary sustained or intermittent muscle contractions causing abnormal, often repetitive, movements or postures of the face, neck, trunk or limbs (Albanese 2013). Dystonic movements are typically patterned or twisting, and are often initiated or worsened by voluntary action (Albanese 2013). These neurological disorders can be classified based on topographic distribution, including focal dystonia (one body region, e.g. cervical dystonia and blepharospasm), segmental dystonia (two or more adjacent regions), multifocal dystonia (two or more nonadjacent regions), hemidystonia (ipsilateral regions) and generalised dystonia (trunk and two or more other regions) (Albanese 2013; Tarsy 2006).
Focal dystonia is a highly disabling movement disorder, with serious functional and social impairment. Close to half of the patient population quits work by the age of forty or retires early due to dystonia, and 10 years later, only 25% of people are working compared to 62% of the general population (Zoons 2012). Moreover, health‐related quality of life is significantly diminished, mainly attributable to depression and anxiety, with scores comparable to people with multiple sclerosis, Parkinson’s disease or stroke (Zoons 2012).
Cervical dystonia, also called spasmodic torticollis, is the most common form of adult‐onset focal dystonia, with estimates from population studies ranging from 57 per million in Europe (ESDE 2000) to as high as 280 per million in the USA (Jankovic 2006). It typically has its onset in the fifth decade (Albanese 2013), and affects more women than men (Defazio 2013). This condition is characterised by abnormal movements of the head, neck, and shoulder, resulting in posturing of the head away from its normal central position (Foltz 1959). It may present predominantly with sustained abnormal posture, spasm, jerks, tremor, or a combination of these features. Neck or shoulder pain, or both, occur in more than 70% of individuals with cervical dystonia (Chan 1991; Tarsy 2006).
Cervical dystonia can be classified according to the dominant head position, with the most common type involving horizontal turning, the so‐called rotatory (or simple) torticollis (Chan 1991; Albanese 2013). Other common patterns include laterocollis (tilt to one side), retrocollis (tilt upwards resulting in neck extension) and anterocollis (tilt downwards resulting in neck flexion). Among all forms of cervical dystonia, complex torticollis, a combination of these abnormal patterns, is found relatively frequently in clinical practice.
The aetiology of most forms of dystonia is still not fully understood, with the exception of early‐onset dystonia, for which a hereditary aetiology is common (Balint 2015). In most cases of focal adult‐onset dystonia, such as cervical dystonia, the pathophysiology is generally considered to result from inhibition of the central nervous system (CNS) at multiple levels (Hallett 1998) resulting in abnormal sensorimotor integration. Cervical dystonia can also be secondary to brain injury, infections of the CNS, drugs (such as levodopa or antipsychotics), toxics, vascular or neoplastic disorders and may also be psychogenic (i.e. functional) (Albanese 2013). Although most cases of cervical dystonia are currently classified as idiopathic, it should be observed that some may come to be reclassified as inherited, since new gene discoveries are under investigation (Albanese 2013; Balint 2015).
The natural course of cervical dystonia remains unclear. It usually develops gradually and deteriorates over the initial years. The clinical presentation in adults seldom progresses to generalised dystonia, although it often extends to contiguous body regions. For most individuals, cervical dystonia is a life‐long disorder, with only about 10% undergoing spontaneous remissions (Jahnanshani 1990).
To date, no curative or disease‐modifying treatments are available for cervical dystonia.
Description of the intervention
Botulinum toxin is a powerful biological toxin produced by Clostridium botulinum. The active form of botulinum toxin is a di‐chain polypeptide composed of two chains: a heavy chain (100 kDa) and a light chain (50 kDa), and by associating with certain auxiliary proteins (haemagglutinins and non‐haemagglutinins), the toxin forms a non‐covalent multimeric complex of variable size (Simpson 2004). The nontoxic proteins aid the formation of neutralising antibodies, though beyond this their role is unclear (Frevert 2010). Botulinim toxin binds to peripheral cholinergic nerve terminals of the neuromuscular junction as well as sympathetic ganglionic, parasympathetic ganglionic, and postganglionic terminals (Simpson 2004). After binding to an acceptor protein, botulinum toxin is endocytosed at the presynaptic membrane of acetylcholine nerve terminals (Pellizzari 1999). By action of the N‐terminal on the heavy chain, a pore is formed on the endocytic membrane, which permits the release of the light chain into the cytosol. This light chain, which is a zinc protease, performs the key‐action of the botulinum toxin, by cleaving soluble N‐ethylmaleimide‐sensitive factor attachment receptor proteins (SNARE proteins) (Pellizzari 1999).
SNAREs are docking proteins for acetylcholine vesicles that allow for the release of acetylcholine into the synaptic cleft (Pellizzari 1999). The overall effect of botulinum toxin is a local chemodenervation by the temporary blockade of acetylcholine release at cholinergic synapses. Temporary synapses are consequently formed via the process of axonal sprouting (Duchen 1971; Holland 1981; Juzans 1996).
There are seven immunologically distinct botulinum toxin serotypes (labelled A to G). These different botulinum toxin serotypes cleave specific SNARE proteins. Serotype A cleaves SNARE protein SNAP 25, located on the inner membrane of nerve cells (Pellizzari 1999).
Botulinum toxin is injected into the muscles thought to be involved in dystonia, with or without guidance by either electromyography (EMG) or ultrasound. As a general rule, the number of muscles injected and the number of injection sites per muscle are tailored to the severity of the case in question and the mass of the muscle, respectively. Within roughly three months after injection of botulinum toxin into skeletal muscle, the nerve terminal resumes exocytosis, and the muscle returns to its baseline clinical function, showing a wearing‐off response from the botulinum toxin injection (Jankovic 2004). Eventually, the muscle paralysis subsides, and this is associated with the formation of new sprouts capable of neurotransmission. Over time, synaptic activity resumes in the original nerve terminals, leading to sprout regression (de Paiva 1999).
Currently there are two commercially available botulinum toxin serotypes ‐ botulinum toxin type A (BtA) and botulinum toxin type B (BtB). The following products are commonly available (three BtA and one BtB): onabotulinumtoxinA (Botox, Allergan Inc., Irvine, CA, USA), abobotulinumtoxinA (Dysport/Reloxin/Azzalure, Ipsen Pharma, Boulogne Billancourt, France), incobotulinumtoxinA (Xeomin/Bocoture Merz GmbH, Frankfurt, Germany), and rimabotulinumtoxinB (Myobloc/Neurobloc, Solstice Neurosciences Inc., Louisville, KY, USA). Other BtA formulations are available in more restricted markets and are yet to receive a generic name: Prosigne/Lantox (Lanzhou Institute of Biological Products, China), PurTox (Mentor Worldwide LLC, Santa Barbara, CA, USA), and Neuronox (Medy‐Tox Inc, South Korea) (Walker 2014).
How the intervention might work
The therapeutic potential of all botulinum toxin serotypes derives from their ability to inhibit the release of acetylcholine from the presynaptic nerve terminal into the synaptic cleft, causing local chemodenervation (Jankovic 2004). In addition to this, recent research has also suggested that botulinum toxin is active at multiple levels, namely sensory nerve terminals, and muscle spindles, which leads to a reduction in sensory input and fewer muscle contractions (Filippi 1993; Matak 2014; Rosales 1996; Rosales 2010).
It has been further suggested that cortical reorganisation may result from changes in the spinal cord, brainstem, and central nervous pathways (Palomar 2012). Animal research has shown the presence of supra‐therapeutic levels of botulinum toxin by way of retrograde axonal transport and penetration of the CNS (Antonucci 2008; Boroff 1975). However, botulinum toxin has not been shown to penetrate the blood‐brain barrier in humans.
Until recently, SNARE proteins were considered the only target‐molecules of botulinum toxin. Thus, it was widely accepted that the therapeutic and toxic actions of botulinum toxin were exclusively mediated by SNARE cleavage preventing the release of synaptic neurotransmitters. However, recent studies have suggested that a number of botulinum toxin actions might not be mediated by SNARE cleavage, specifically regarding neuroexocytosis, cell cycle and apoptosis, neuritogenesis and gene expression (Matak 2015). The existence of unknown botulinum toxin molecular targets and modulation of unknown signalling pathways is a possibility that may prove to be pharmacologically relevant.
Why it is important to do this review
BtA is the toxin serotype that has been most intensively studied and approved for the treatment of the large number of focal dystonias. BtA is considered the first line therapy for cervical dystonia (Albanese 2013), though BtB has been shown to be efficacious, though with a different safety profile (Marques 2016; Duarte 2016).
This is an update of a Cochrane Systematic Review that previously assessed the efficacy and safety of BtA in comparison to placebo in people with cervical dystonia. Since the release of the original review, three new trials have been published (Comella 2011; Poewe 2016; Truong 2010). Furthermore, Cochrane’s criteria for evaluating studies' risk of bias and the certainty in evidence have evolved and been updated. Therefore, the authors consider it important to update this review.
Objectives
To compare the efficacy, safety, and tolerability of botulinum toxin type A (BtA) versus placebo in people with cervical dystonia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), blinded, single or multiple dose, parallel‐designed, of any duration, assessing the efficacy or safety, or both, of BtA treatment versus placebo in people with cervical dystonia were eligible for inclusion in this review. We excluded non‐parallel study designs, namely cross‐over trials, from this updated version of the review, due to uncertainty about whether this type of study design was appropriate to study people with cervical dystonia, as well as methodological concerns with regards to detection and performance bias.
Types of participants
Adults (i.e. 18 years of age or older), in any setting, with a clinical diagnosis made by any physician, specialist or otherwise, of idiopathic cervical dystonia. We allowed trials enrolling participants with any form of cervical dystonia, and additional or more widespread dystonias, for inclusion. Participants could have had prior exposure to BtA or BtB, and could be taking any concomitant medications if on stable regimens.
There were no restrictions regarding the number of participants recruited to trials, or the number of recruitment centres.
Types of interventions
Intramuscular injections of BtA compared to placebo. We allowed all administration schedules and injection techniques, performed with or without guidance by either EMG or ultrasound.
Types of outcome measures
Primary outcomes
Cervical dystonia‐specific improvement
Overall improvement on any validated symptomatic rating scale, such as Cervical Dystonia Severity Scale (CDSS), Tsui scale, Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), and TWSTRS Severity and Disability subscales scores, measured between weeks 3 and 6.
Adverse events
The proportion of participants with any adverse event, measured at any point during study follow‐up. In this item we also evaluated adverse events of special interest, such as sore throat/dry mouth, neck weakness, dysphagia, injection site pain, voice change, and systemic complaints (e.g. diffuse muscle weakness, malaise, dizziness, and headache), measured at any point during study follow‐up.
Secondary outcomes
Subjective evaluation of clinical status
Evaluated by either participants, or clinicians, or both, as assessed with validated assessment tools such as Patient Subjective Assessment of Change, Patient Global Assessment of Improvement, Patient Evaluation of Global Response (PEGR), Patient and Physician Global Assessment of Change, Investigator Global Assessment of Efficacy (IGAE), Physician Global Assessment of Change (PGAC), and visual analogue scale (VAS) for symptom severity, measured between weeks 3 and 6.
Pain relief
As assessed with validated assessment tools such as Patient Assessment of Pain, TWSTRS Pain subscale score, and VAS pain score, measured between weeks 3 and 6.
Health‐related quality of life
As assessed with validated assessment tools such as Short Form 36 (SF‐36) Quality‐of‐Life questionnaire and Cervical Dystonia Impact Profile (CDIP)‐58 scale, measured at any point during study follow‐up.
Tolerability
We defined tolerability as the number of participant withdrawals due to adverse events, measured at any point during study follow‐up.
Duration of effect
As assessed by the number of days until need for reinjection or effect waning.
Search methods for identification of studies
For this update, we expanded the search strategy to capture all the search terms for BtA formulations that were currently available. The search strategy was designed to include other botulinum toxin formulations and other dystonic disorders that are also under current revision by our group.
Electronic searches
We ran the final search for the original version of this review in June 2003, based on the search strategy developed for Cochrane Movement Disorders to identify all papers since 1977, the first year that botulinum toxin was used therapeutically in any condition. The search for the current update was run for the last time in October 2016.
For the identification of studies considered for inclusion in this review, we developed detailed search strategies for each database searched. Please see Appendix 1 for the Cochrane Central Register of Controlled Trials (CENTRAL) strategy, Appendix 2 for the MEDLINE search strategy, and Appendix 3 for the Embase strategy.
We assessed non‐English language papers, translated them as necessary and evaluated them for inclusion.
We did not search trials registries.
Databases searched
Cochrane Movement Disorders' Trials Register (June 2003);
CENTRAL (2016, Issue 11) in the Cochrane Library;
MEDLINE (1977 to 6 October 2016);
Embase (1977 to 6 October 2016).
Searching other resources
The search strategy also included:
searches through reference lists of located trials and review articles concerning botulinum toxin;
handsearch of abstracts of international congresses relevant in the fields of movement disorders and botulinum toxins (American Academy of Neurology, Movement Disorders Society, International Association of Parkinsonism and Related Disorders, and International Neurotoxin Association (1985 to October 2016));
personal communication with other researchers in the field;
contact with drug manufacturers;
whenever necessary, we contacted authors of published trials for further information and unpublished data.
Data collection and analysis
Selection of studies
Two review authors independently screened all titles and abstracts identified from searches to determine which ones met the inclusion criteria. We retrieved in full text any papers identified as potentially relevant by at least one review author or those without an available abstract. Two review authors independently screened full‐text articles, with discrepancies resolved by discussion and by consulting a third review author where necessary to reach consensus. We collated duplicate publications and have presented these by individual study. We have outlined the screening and selection process in a PRISMA flow chart (Liberati 2009), see Figure 1.
1.

Study flow diagram
Data extraction and management
Two review authors extracted data independently from included studies using a piloted data extraction form. We resolved any discrepancies were by discussion until consensus was reached, or through consultation with a third review author where necessary. Data extracted included the following items from each study.
Participants: inclusion and exclusion criteria, demographics and clinical baseline characteristics, number and reasons for withdrawals, exclusions and loss to follow‐up, if any
Interventions: full description of intervention, duration of treatment period and follow‐up, providers, and co‐interventions, if any
Comparisons: number of randomised participants to each arm, compliance and dropouts, reasons for dropouts, and ability to perform an intention‐to‐treat analysis
Outcomes: definition of outcomes, use of validated measurement tools, time‐point measurements, change from baseline or post‐interventional measures, and missing outcomes, if any
Study design: interventional, randomised, controlled, double‐blind.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies according to the domains described in the Cochrane tool for assessing risk of bias (Higgins 2011a), and classified the risk of bias for each domain as high, unclear, or low, and the overall assessment as high or low. We assessed two further domains, which are described below: enriched population and independent funding. We used the following definitions for each domain in the 'Risk of bias' assessment.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated).
Allocation concealment (checking for possible selection bias). We assessed the method used to conceal allocation to interventions prior to assignment to determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated); high risk of bias (e.g. open list).
Blinding of participants and personnel (checking for possible performance bias). We assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, such as identical tablets matched in appearance or smell, or a double‐dummy technique); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how it was achieved). Studies that are not double‐blind will be considered to have high risk of bias.
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study has a clear statement that outcome assessors were unaware of treatment allocation, and ideally describes how this was achieved); unclear risk of bias (study states that outcome assessors were blind to treatment allocation but lacks a clear statement on how it was achieved). We considered studies where outcome assessment is not blinded as having a high risk of bias.
Selective reporting (checking for reporting bias). We assessed whether primary and secondary outcome measures were pre‐specified and whether these were consistent with those reported. We assessed selective reporting as: low risk of bias (studies reporting primary and secondary outcomes); high risk of bias (not all pre‐specified outcomes reported or only for certain data collection time points).
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (< 10% of participants did not complete the study and/or used ‘baseline observation carried forward’ analysis); unclear risk of bias (used 'last observation carried forward' analysis); high risk of bias (used 'completer' analysis).
In addition to these criteria, we considered the implications of baseline imbalances in prognostic factors affecting the trial outcomes, as these may lead to selection bias (Corbett 2014).
For‐profit bias
In order to assess the study source of funding, we added this domain in place of the ‘other bias’ domain.
Low risk of bias: the trial appears to be free of industry sponsorship or other type of for‐profit support that may introduce bias into trial design, conduct, or trial results.
Unclear risk of bias: the trial may or may not be free of for‐profit bias as the trial did not provide any information on clinical trial support or sponsorship.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Enriched population
Because the clinical effect of botulinum toxin treatment is easily perceived, botulinum toxin‐naive participants are likely to recognise the presence or absence of beneficial clinical effects, or frequent adverse events, or both, effectively revealing the respective allocation arm. It is also relevant that, by preferentially including responders to botulinum toxin or excluding non‐responders to botulinum toxin, there is an increased likelihood that these participants would respond more favourably to botulinum toxin than a naive population would. We opted to subdivide this domain in two: preferential enrolment of known positive responders to botulinum toxin; and exclusion of known poor responders to botulinum toxin.
Low risk of bias: at least 70% of trial participants were naive to treatment with botulinum toxin; the trial did not exclude any particular form of cervical dystonia including those associated with a poorer response to botulinum toxin (such as pure anterocollis and retrocollis).
Unclear risk of bias: the trial did not make explicit the percentage of participants who were known to be botulinum toxin naive.
High risk of bias: arbitrarily defined as more than 30% of participants non‐naive to botulinum toxin; explicit exclusion of people with forms of cervical dystonia associated with a poorer response to botulinum toxin.
Measures of treatment effect
We compared disease symptoms at baseline to disease symptoms in weeks 2 to 4 post‐injection in the BtA and placebo arms. We extracted continuous outcomes whenever possible,, pooled the data from the studies, where adequate, and used them for comparison.
Dichotomous data
We based analysis of these data on the number of events and the number of people assessed in the intervention and comparison groups. We used these to calculate the risk ratio (RR) and 95% confidence interval (CI).
Continuous data
We based analysis of these data on the mean, standard deviation (SD) and number of people assessed for both the intervention and comparison groups to calculate mean difference (MD) and 95% CI. Where the MD was reported without individual group data, we used this to report the study results. If more than one study measured the same outcome using different validated tools, we calculated the standardised mean difference (SMD), namely Hedges’ (adjusted) g (Hedges 1985), and 95% CI. For interpretation of effect sizes with SMDs, we used a rule of thumb to define a small effect (SMD = 0.2), a moderate effect (SMD = 0.5), or a large effect (SMD = 0.8) (Cohen 1988). If necessary for comparison, we dichotomised rating scales using each study author's own criteria for improvement or no improvement.
Time‐to‐event data
We planned to analyse these data based on log hazard ratios (HR) and standard errors obtained from results of Cox proportional hazards regression models. We had planned to use these in order to calculate a HR and 95% CI.
Unit of analysis issues
Whenever the included studies had multiple arms with different dosages of botulinum toxin, we combined all groups to create a single pair‐wise comparison, using the Review Manager 5 (RevMan 5) calculator (RevMan 2014), according to the methods suggested by Cochrane (Higgins 2011b). We also would have opted to create a single, pair‐wise comparison in case of multiple treatment groups using different interventions (e.g. onabotulinumtoxinA and abobotulinumtoxinA) if these had been compared to the same comparator.
This method combined all relevant experimental intervention groups of the study into a single group, and all relevant control intervention groups into a single control group. This approach avoided the duplication of the control group that would happen if multiple comparisons (e.g. BtA dose1 versus placebo; BtA dose2 versus placebo) were included in the meta‐analysis, as well as the loss of information if one dosage group was chosen to the detriment of the others. If applicable, we planned to explore the effect of dosage in subgroup analysis.
For dichotomous outcomes, we planned to sum both the sample sizes and the numbers of people with events across groups. For continuous outcomes, means and standard deviations could be combined using a pooled mean or SD (Higgins 2011b; Higgins 2011c).
Dealing with missing data
For missing outcome or summary data we used imputation methods to derive the missing data (where possible) and reported any assumptions in the review. In these cases we carried out sensitivity analyses to investigate the effects of any imputed data on pooled effect estimates.
As a first option we chose to use the available information (e.g. standard error (SE), 95% CI or exact P value) to recover the missing data algebraically (Higgins 2011b; Higgins 2011c; Wiebe 2006). When change from baseline SD was not reported or not possible to extract, we attempted to create a correlation coefficient based on another study in this review, and then used this correlation coefficient to impute a change from baseline SD (Abrams 2005; Follmann 1992; Higgins 2011b).
If this were to fail, and if there was at least one sufficiently large and similar study, we would use a method of single imputation (Furukawa 2006; Higgins 2011b).
Lastly, if there were a sufficient number of included studies with complete information, we would have used multiple imputation methods to derive missing data (Carpenter 2013; Rubin 1991).
If none of these methods proved successful, we would have conducted a narrative synthesis for the data in question.
Assessment of heterogeneity
We assessed whether studies were similar enough to allow pooling of data using meta‐analysis. Where data were pooled using meta‐analysis, we assessed the degree of heterogeneity by visual inspection of forest plots and by examining the Chi2 test for heterogeneity (Deeks 2011). We quantified heterogeneity using the I2 statistic (Higgins 2003). We considered an I2 value of 50% or more to represent substantial levels of heterogeneity, but interpreted this value in light of the size and direction of effects and the strength of the evidence for heterogeneity, based on the P value from the Chi2 test.
Assessment of reporting biases
We included too few studies in this review, namely fewer than 10, to allow construction of a funnel plot (Sterne 2001), and formal testing of asymmetry (Peters 2006), which may indicate publication bias. Should enough studies be included in future updates of this review, we plan to undertake these analyses.
Data synthesis
We performed the analyses with Review Manager 5 (RevMan 5) version 5.3 (RevMan 2014), Stata version 14 (Stata 2015) and Trial Sequential Analysis (TSA) (Thorlund 2011; TSA 2011).
Meta‐analysis
We based the decision whether or not to meta‐analyse data on an assessment of whether the interventions in the included trials were similar enough in terms of participants, settings, intervention, comparison and outcome measures to ensure meaningful conclusions from a statistically pooled result. We conducted data synthesis using a random‐effects model.
We pooled effect measures by applying the Mantel‐Haenszel method for dichotomous outcomes, and applying the inverse‐variance or generic inverse‐variance method for continuous outcomes. In addition, we had planned to pool time‐to‐event data using the generic inverse‐variance method. We presented all results with 95% CI.
We calculated the number of participants needed to treat for an additional beneficial outcome (NNTB) and for an additional harmful outcome (NNTH) from meta‐analysis estimates, rather than treating data as if they came from a single trial, as the latter approach is more prone to bias, especially when there are significant imbalances between groups within one or more trials in the meta‐analysis (Altman 2002). However, caution is needed in the interpretation of these findings since they may be misleading because of variation in the event rates in each trial, differences in the outcomes considered, and differences in clinical setting (Smeeth 1999).
Where there were no data that could be combined into a meta‐analysis we undertook a narrative approach to result synthesis.
Trial Sequential Analysis
In order to explore whether the cumulative data were of adequate power to evaluate the primary outcomes of this review, we performed a Trial Sequential Analysis (TSA) (Wetterslev 2008), and calculated a required information size (also known as the 'heterogeneity‐adjusted required information size') (Wetterslev 2009). TSA aims to evaluate whether statistically significant results of meta‐analysis are reliable by accounting for the required information size (i.e. the number of participants in the meta‐analysis required to accept or reject an intervention effect). The technique is analogous to sequential monitoring boundaries in single trials. TSA adjusts the threshold of statistical significance and has been shown to reduce the risk of random errors due to repetitive testing of accumulating data (Imberger 2016).
We calculated the required information size and computed the trial sequential monitoring boundaries using the O’Brien‐Fleming approach (O'Brien 1979). The required information size was based on the event proportion or standard deviation in the control group; assumption of a plausible relative risk reduction (RRR) of 10%; a 5% risk of type I error; a 20% risk of type II error (power = 80%); and the observed heterogeneity of the meta‐analysis (Jakobsen 2014; Wetterslev 2009).
Assessing the certainty in the evidence
As recommended by the GRADE Working Group methodology (Schünemann 2011), two review authors independently assessed all of the outcomes in the following domains: study limitations, inconsistency, indirectness, imprecision and publication bias. In case of disagreement the authors attempted to reach consensus, consulting an independent third review author if necessary. For this purpose, we used the GRADEpro GDT software tool (GRADEpro GDT 2015), which we then used to export a 'Summary of findings' table for inclusion in the review manuscript.
To ensure the consistency and reproducibility of GRADE judgements, we applied the following criteria to each domain for all key comparisons of the critical outcomes.
Study limitations: we downgraded once if more than 30% of participants were from studies classified as being at a high risk of bias across any domain, with the exception of 'for‐profit bias'.
Inconsistency: we downgraded once if heterogeneity was statistically significant or if the I2 value was more than 40%. When we did not perform a meta‐analysis we downgraded once if trials did not show effects in the same direction.
Indirectness: we downgraded once if more than 50% of the participants were outside the target group.
Imprecision: we downgraded once if the optimal information size criterion was not met or, alternatively, if it was met but the 95% CI failed to exclude important benefit or important harm (Guyatt 2011).
Publication bias: we downgraded once where there was direct evidence of publication bias or if estimates of effect were based on small scale, industry‐sponsored studies that raised a high index of suspicion of publication bias.
We applied the following definitions to the certainty in the evidence (Balshem 2011):
high certainty: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect;
very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
'Summary of findings' table
As has become standard practice in Cochrane Reviews, we have included a 'Summary of findings' table to present the main findings of this review in a simple tabular format, based on the results of the GRADE analysis.
Subgroup analysis and investigation of heterogeneity
We pre‐planned subgroup analyses for the following areas, independently of the presence or not of significant heterogeneity.
Different BtA formulations
Different BtA doses: high (Botox/Xeomin > 201 U; Dysport = 1000 U), medium (Botox/Xeomin 101 U to 200 U; Dysport = 500 U), and low (Botox/Xeomin < 100 U; Dysport = 250 U) doses, all defined arbitrarily
EMG‐guided versus non‐EMG‐guided botulinum toxin injection
Sensitivity analysis
We conducted sensitivity analyses for every study where we applied imputation methods.
Results
Description of studies
We identified three new studies for inclusion in this update: Comella 2011; Poewe 2016; Truong 2010.
We identified two studies (Charles 2012; Truong 2005) as duplicates of two previously included studies (Brashear 1998, Truong 2002, respectively). We chose to replace the original references as the current study reports contain more data. The older references can now be found as secondary references under the new studies.
Overall, we included eight parallel‐designed studies comparing BtA (different total treatment doses) with placebo in this update, with a total of 1010 participants with cervical dystonia.
Results of the search
See: Figure 1, flow diagram of study selection.
We last ran the electronic search in October 2016. The search returned 1646 records (208 through CENTRAL; 182 though MEDLINE; 1256 through Embase), resulting in 1599 records after removing all duplicates. After title and abstract screening, we assessed 24 articles for full‐text screening, with eight being included for both the qualitative and quantitative syntheses.
We excluded nine trials for having a cross‐over design; two due to not being randomised; two for including the wrong population; and another two for studying the wrong outcomes.
We did not retrieve any unpublished trials.
For this update, we contacted the author of Truong 2005 and Truong 2010 for clarification of whether the study population was the same in both trials. The author replied and confirmed that these were two different studies.
Included studies
We have listed all the included studies in this review in the Characteristics of included studies table.
See Table 4 for a summary of the clinical characteristics of included studies.
2. Summary of included studies ‐ participants and drug administration.
| Study ID | Number of participants | Total dropouts | Age mean, range (years) | Baseline CD impairment BtA/placebo | % participants naive to Bt | BtA formulation | Total dose per participant | EMG‐guidance | Study duration (weeks) |
| Charles 2012 | 170 | 35 (11 in BtA) |
55, 31‐76 |
9.2/9.3 (CDSS) |
0 | Botox (OnaBtA) | 236 | No | 10 |
| Comella 2011 | 233 | 14 (8 in BtA) |
53 | 42.4/41.8 (TWSTRS) |
39 | Xeomin (IncoBtA) | 120 or 240 | At discretion | 20 |
| Greene 1990 | 55 | 3 (3 in BtA) |
50 | 21% severe/ 41% severe |
100 | Botox (OnaBtA) | 150 to 165 | No | 12 |
| Poewe 1998 | 75 | 2 (2 in BtA) |
47, 26‐82 |
13.9/14.4 (Tsui scale) |
100 | Dysport (AboBtA) | 250, 500 or 1000 |
No | 8 |
| Poewe 2016 | 213 | N/A | 49 | 46/47 (TWSTRS) |
10 | Dysport (AboBtA) | 500 | N/A | 12 |
| Truong 2005 | 80 | 56 (22 in BtA) |
54, 27‐78 |
45.1/46.2 (TWSTRS) |
26 | Dysport (AboBtA) | 500 | At discretion | 20 |
| Truong 2010 | 116 | 33 (10 in BtA) |
53, 20‐79 |
43.8/45.8 (TWSTRS) |
17 | Dysport (AboBtA) | 500 | At discretion | 12 |
| Wissel 2001 | 68 | 0 | 48, 18‐75 |
11.1/11.5 (Tsui scale) |
31 | Dysport (AboBtA) | 500 | No | 16 |
Bt: botulinum toxin; CD: cervical dystonia; CDSS: Cervical Dystonia Severity Scale; EMG: electromyography; TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale
The eight included studies enrolled a total of 1010 adult participants, with a mean age of 52.3 years (range 18 to 82), 649 of whom were female (64%). Trial size varied from 55 to 233 participants. Seven studies were performed in a multicenter setting – two large trials (Charles 2012; Comella 2011) and one small study (Truong 2005) in North America, one medium‐sized study in the USA and Russia (Truong 2010), two trials enrolling up to 75 participants each in Europe (Poewe 1998; Wissel 2001), and one large study enrolling participants at 61 sites in 11 countries (Poewe 2016). The three larger studies (Charles 2012; Comella 2011; Poewe 2016) enrolled a total of 616 participants, accounting for 61% of the participants included in this review.
Participants' baseline characteristics differed between trials. The mean duration of cervical dystonia ranged from 4.8 to 12.1 years, though the distribution was generally equivalent between treatment and placebo arms in each trial, exceeding a three‐year difference in one study only (Greene 1990). The overall disease impairment at baseline was moderate‐to‐severe in all trials, with scores ranging from 41.8 to 46.2 on the TWSTRS scale, 13.9 to 14.4 on the Tsui scale, and 9.2 to 9.3 on the CDSS.
Only two studies exclusively enrolled participants who had never been exposed to botulinum toxin (Greene 1990; Poewe 1998). For all other trials, between 61% and 100% of participants had received prior treatment with botulinum toxin, with time since last injection before study entry ranging from 10 weeks to 16 weeks. All but one small trial (Greene 1990) excluded clinical forms of cervical dystonia associated with a poorer response to botulinum toxin, such as pure anterocollis and retrocollis. Overall, we assessed only one study (Greene 1990) as not having an enriched population. We deemed all other studies to be at high risk of bias for this domain. As a result, the population characteristics across studies did not allow for conducting a subgroup analysis for people naive and non‐naive to botulinum toxin.
The number of dropouts was generally small in most trials, although its interpretation needs to be adjusted to the time point reported in each study. Total dropouts from trials varied from 3% to 6% at week 8 (Poewe 1998; Comella 2011), to as high as 21% at week 10 (Charles 2012) and 29% at week 12 (Truong 2010). One study (Truong 2005), however, showed considerably higher rates of dropouts, ranging from 54% at as early as week 8, to 70% at week 12; reasons for discontinuation in this study were not reported by the trial authors.
Overall, the number of dropouts was higher among participants allocated to placebo arms: 27% (combined n = 90) of the participants allocated to placebo withdrew, compared to only 12% (combined n = 56) of participants allocated to BtA. In trials that reported the reasons for dropouts, lack of efficacy was the most frequent reason for participant discontinuation from the study, accounting for half (combined n = 45) of total dropouts in placebo arms and for 23% (combined n = 13) in BtA arms. In the intervention arms, adverse events were responsible for 7% (n = 4) of discontinuation across studies, as compared to 0% in the placebo arms (Comella 2011; Greene 1990).
Study design and interventions
Five trials used a fixed dose of 500 U of BtA formulation Dysport to compare with placebo (Poewe 2016; Poewe 1998; Truong 2005; Truong 2010; Wissel 2001; combined n = 515). In the same trial, Poewe 1998 further assessed low (250 U) and high (1000 U) doses of Dysport in two different arms (n = 37). Two previously included studies (Charles 2012; Greene 1990; combined n = 225) compared BtA formulation Botox with placebo, with doses varying from 95 U to 360 U. One new study (n = 233) evaluating the BtA formulation Xeomin versus placebo was identified in this update, using dosages of 120 U and 240 U (Comella 2011). All studies were designed to allow one single treatment session.
Most studies performed BtA injection without EMG guidance (Charles 2012; Greene 1990; Poewe 1998; Wissel 2001). However, for almost half of the participants included across studies (n = 429), EMG guidance was left at the discretion of the investigator performing the injection (Comella 2011, Truong 2005, Truong 2010).
The duration of trials ranged from 8 weeks to 20 weeks post‐injection. For most participants, study termination occurred between weeks 8 to 12 (Comella 2011; Truong 2005), as determined by the clinical need for reinjection, or study dropout.
All studies except two (Charles 2012; Poewe 1998) assessed efficacy and other primary outcomes using an intent‐to‐treat (ITT) analysis, which included all participants randomised to treatment. Some of these studies (Comella 2011; Greene 1990; Truong 2010) performed the safety assessment on a per‐protocol (PP) population, which included only participants who had received a dose of study medication.
Excluded studies
We have listed all the excluded studies in this review, together with reasons for their exclusion, in the Characteristics of excluded studies table.
Risk of bias in included studies
See Characteristics of included studies: 'Risk of bias' table.
We evaluated the included studies using a modified version of the Cochrane 'Risk of bias' tool. See Figure 2 and Figure 3 for the 'Risk of bias' summary graphs. These assessments were based on the information available in the primary report data.
2.

Risk of bias of included studies: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Overall, we considered no studies to be at low risk of bias across all domains. However, the only two domains that we considered to represent a high risk of bias were the 'for‐profit bias' and 'enriched population' domains. It is noteworthy that all trials but one had a high risk of both 'for‐profit bias' and 'enriched population'.
Allocation
Three studies (Comella 2011; Truong 2005; Truong 2010) clearly described the process of random sequence generation; we assessed the other five studies to be at unclear risk of bias for this criterion. Poewe 1998, Poewe 2016, and Truong 2005 described an adequate allocation concealment process and we rated them as being at a low risk. All but one of the included trials reported a higher disease impairment at baseline in the control arm although it is unclear whether these differences are either statistically significant or clinically relevant. This leads us to assess the overall risk of selection bias across the included trials to be a serious cause for concern, despite the fact that overall no one trial is uniquely responsible for this assessment.
Blinding
We judged the blinding of participants and personnel involved in the trial to be at a low risk of bias in the majority of studies included in this review; three trials (Charles 2012; Poewe 2016; Truong 2010) did not describe enough information to allow for a clear judgment. We considered that only Greene 1990 had adequately blinded participants and investigators measuring both objective and subjective outcomes. For the assessment of objective outcomes, we also judged Comella 2011 to be at low risk. We considered that the remaining studies were at an unclear risk of bias across this domain. We judged Poewe 1998 at low risk of performance bias, but at an unclear risk of both elements of detection bias.
Incomplete outcome data
Six out of eight studies summarised the reasons for missing data and used appropriate statistical tools to deal with it, and we rated them at low risk of bias. Greene 1990 reported missing data across the study visits so that we assessed this study at unclear risk of attrition bias. Truong 2005 reported a large number of dropouts in both intervention arms, though this was asymmetrical, with over 60% of participants withdrawing from the placebo arm by week 4. For this reason we rated Truong 2005 at a high risk of attrition bias.
Selective reporting
We considered all studies to be at low risk for reporting bias.
Other potential sources of bias
For‐profit bias
All trials but one (Greene 1990) declared funding or supply of study vials from industry sources, and we rated them at a high risk of bias for funding and potential conflicts of interest.
Enriched population
Three studies (Charles 2012; Poewe 2016; Truong 2010) preferentially enrolled participants known to have previously responded to BtA treatment, and were at high risk of bias for enriched population. On the other hand, all studies except one (Greene 1990) excluded forms of cervical dystonia known to have a poorer clinical response to BtA injection, and were also considered to be at a high risk of bias for this domain.
Publication bias
We intended to use funnel plots to explore publication bias. However, due to the small number of included studies, the power of this analysis was considered to be inadequate (Sterne 2011).
Effects of interventions
See: Table 1
The key results of this review can be found in Table 1.
Preceding data analysis
See Dealing with missing data.
Poewe 1998, Poewe 2016, and Truong 2005 reported no SD for the primary outcome, so we opted to impute these values from other studies in this review (Wissel 2001; Truong 2010; Truong 2010, respectively), which used the same scale, time point and error measurement for assessing the same outcome.
Charles 2012 did not report the absolute numerical values for the primary outcome at week 4. Therefore, we opted to have two review authors independently extract these data from the graph provided in the report. Since the two review authors reported very similar values, we imputed these data (mean values from the two authors) and used them for analysis.
Poewe 1998 and Comella 2011 presented data separately for each BtA dose, reporting sample sizes, means and SD for each intervention group. In order to conduct pooled analyses we combined the BtA groups using a pooled SD formula for paired data.
Primary outcomes
Cervical dystonia‐specific improvement
The included trials assessed the primary outcome at either week 4 (Charles 2012; Comella 2011; Poewe 1998; Poewe 2016; Truong 2005; Truong 2010; Wissel 2001) or week 6 (Greene 1990) following initial injection of study medication. Most trials reported the primary efficacy outcome as the change from baseline measurement using validated scales, namely the CDSS (Charles 2012), the Tsui scale (Poewe 1998; Wissel 2001) and the TWSTRS (Comella 2011; Poewe 2016; Truong 2005; Truong 2010). Greene 1990 reported no objective efficacy measurements. The CDSS uses a protractor and wall chart to rate the severity of the head's deviation. The Tsui scale (range, 0 to 25) grades severity of postural deviance, acknowledging the presence of tremor and the pattern of movements; it does not assess disability, pain or other subjective symptoms. TWSTRS (range, 0 to 85) is composed of three subscales that grade severity (range, 0 to 35), disability (range, 0 to 30), and pain (range, 0 to 20). Tarsy 1997 demonstrated that Tsui and TWSTRS score reduction rates after botulinum toxin therapy correlate significantly with each other.
Seven trials (Charles 2012; Comella 2011; Poewe 1998; Poewe 2016; Truong 2005; Truong 2010; Wissel 2001; combined n = 833) contributed data for this outcome. Overall, treatment with BtA was associated with a moderate‐to‐large cervical dystonia‐specific improvement (SMD 0.70, 95% CI 0.52 to 0.89; I2 = 36%; moderate certainty in the evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 1 Cervical dystonia‐specific improvement.
Four trials (Comella 2011; Poewe 2016; Truong 2005; Truong 2010; combined n = 522) used the TWSTRS total score to assess cervical dystonia‐specific improvement. Overall, treatment with BtA was associated with a MD of 8.06 in TWSTRS total score compared to placebo (95% CI 6.08 to 10.05; I2 = 0%; Analysis 1.2), representing a 18.7% improvement compared to the baseline clinical status (43.55 TWSTRS combined baseline score).
1.2. Analysis.
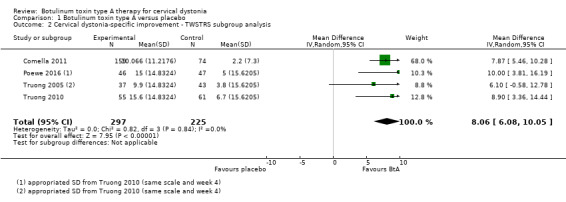
Comparison 1 Botulinum toxin type A versus placebo, Outcome 2 Cervical dystonia‐specific improvement ‐ TWSTRS subgroup analysis.
Three trials (Comella 2011; Truong 2005; Truong 2010; combined n = 429) contributed data regarding the TWSTRS subscales. Overall, treatment with BtA was associated with a MD of 3.13 in the TWSTRS severity subscale (95% CI 2.15 to 4.11; I2 = 0%; Analysis 1.3), and 2.52 in the TWSTRS disability subscale (95% CI 1.72 to 3.31; I2 = 23%; Analysis 1.4).
1.3. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 3 Cervical dystonia‐specific severity ‐ as assessed with TWSTRS subscale.
1.4. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 4 Cervical dystonia‐specific disability ‐ as assessed with TWSTRS subscale.
For the Trial Sequential Analysis we could not use the results of the overall improvement, since these data were only available as SMD (Thorlund 2011). Thus, we opted to use the data from trials that used the TWSTRS scale.
The required information size was based on the event proportion or standard deviation in the control group; assumption of a plausible relative risk reduction (RRR) of 10%; a 5% risk of type I error; a 20% risk of type II error (power = 80%); and the observed heterogeneity of the meta‐analysis. We assumed a baseline TWSTRS of 42 points and an SD of 10. Given these constraints, the cumulative evidence overcame the heterogeneity‐adjusted required information size ‐ 180 participants. We are able to conclude that the cumulative evidence is adequately‐powered to demonstrate the 8.16 TWSTRS point difference at week 4 between BtA and placebo.
1.1. Overall improvement with high versus medium versus low dose of BtA subgroup analysis
We carried out a preplanned subgroup analysis to assess overall improvement according to the BtA dosages used (see Subgroup analysis and investigation of heterogeneity). Considering the current evidence behind the potency equivalence between BtA formulations, we assigned arbitrary thresholds for high, medium, and low doses of BtA. Two trials (Comella 2011; Poewe 1998; combined n = 193) contributed data to the high‐dose subgroup; six trials (Comella 2011; Poewe 1998; Poewe 2016; Truong 2005; Truong 2010; Wissel 2001; combined n = 545) contributed data to the medium‐dose subgroup; and one trial (Poewe 1998; n = 39) contributed data to the low‐dose subgroup.
One study (Charles 2012) reported a range of BtA injection doses that crossed the arbitrary dose limits we defined. We therefore did not include it in this subgroup meta‐analysis.
All three dosages were efficacious against placebo (high dose: SMD 1.08, 95% CI 0.53 to 1.63; I2 = 52%; medium dose: SMD 0.76, 95% CI 0.59 to 0.94; I2 = 0%; low dose: SMD 1.24, 95% CI 0.55 to 1.94), though we did not find a difference in efficacy between the subgroups (P = 0.25; Analysis 1.5).
1.5. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 5 Cervical dystonia‐specific improvement ‐ doses subgroup analysis.
1.2. Overall improvement with Botox versus Dysport versus Xeomin subgroup analysis
We carried out a preplanned subgroup analysis to assess overall improvement according to BtA formulation (see Subgroup analysis and investigation of heterogeneity). One trial (Charles 2012; n = 170) contributed data to the Botox subgroup; five trials (Poewe 1998; Poewe 2016; Truong 2005; Truong 2010; Wissel 2001; combined n = 430) contributed data to the Dysport subgroup; and one trial (Comella 2011; n = 233) contributed data to the Xeomin subgroup.
All three formulation were efficacious against placebo (Botox: SMD 0.38, 95% CI 0.08 to 0.69; Dysport: SMD 0.75, 95% CI 0.54 to 0.96; I2 = 8%; Xeomin: SMD 0.82, 95% CI 0.53 to 1.10), though we did not find a difference in efficacy between the subgroups (P = 0.08; Analysis 1.6).
1.6. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 6 Cervical dystonia‐specific improvement ‐ BtA formulation subgroup analysis.
1.3 Overall improvement with EMG‐guided versus non‐EMG‐guided injection subgroup analysis
We carried out a preplanned subgroup analysis to assess the comparative efficacy of BtA in trials that used EMG versus trials that did not use EMG (see Subgroup analysis and investigation of heterogeneity). Four trials (Comella 2011; Poewe 2016; Truong 2005; Truong 2010; combined n = 522) contributed data to the EMG‐guided subgroup; and three trials (Charles 2012; Poewe 1998; Wissel 2001; combined n = 311) contributed data to the non‐EMG‐guided subgroup.
It is important to note that all four trials that contributed data to the EMG‐guided subgroup left the use of EMG at the discretion of the investigator.
Both comparisons were efficacious against placebo (EMG‐guided: SMD 0.71, 95% CI 0.52 to 0.89; I2 = 0%; non‐EMG‐guided: SMD 0.79; 95% CI 0.27 to 1.31; I2 = 75%), though we did not find a difference in efficacy between the subgroups (P = 0.76; Analysis 1.7).
1.7. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 7 Cervical dystonia‐specific improvement ‐ EMG‐guided versus non‐EMG‐guided subgroup analysis.
Adverse events
Seven trials (Charles 2012; Comella 2011; Poewe 1998; Poewe 2016; Truong 2005; Truong 2010; Wissel 2001: combined n = 952) contributed data for this outcome. Adverse events related to study treatment were reported in 55.3% of participants in the BtA groups, compared to 46.5% of participants in the placebo arms. Overall, treatment with BtA was associated with a 20% increase in the risk of adverse events, when compared with placebo (risk ratio (RR) 1.19; 95% CI 1.03 to 1.36; I2 = 16%; moderate certainty in the evidence; Analysis 1.8). The number needed to treat for an additional harmful outcome (NNTH) with a single BtA treatment was 9 (95% CI 5 to 31).
1.8. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 8 Adverse events.
Adverse events were measured as the proportion of trial participants who experienced any adverse event during any point in follow‐up.
The required information size was based on the event proportion or standard deviation in the control group; assumption of a plausible relative risk reduction (RRR) of 10%; a 5% risk of type I error; a 20% risk of type II error (power = 80%); and the observed heterogeneity of the meta‐analysis. We assumed a control event rate of 46%. Given these constraints, the cumulative evidence overcame the heterogeneity‐adjusted required information size ‐ 892 participants. We are able to conclude that the cumulative evidence is adequately‐powered to demonstrate the 23% risk difference in adverse events between BtA and placebo.
2.1 Adverse events with high versus medium versus low dose of BtA subgroup analysis
We carried out a preplanned subgroup analysis to assess the risk of adverse events according to the BtA dosages used (see Subgroup analysis and investigation of heterogeneity). Considering the current evidence behind the potency equivalence between BtA formulations, we assigned arbitrary thresholds for low, medium, and high doses of BtA. Two trials (Comella 2011; Poewe 1998; combined n = 193) contributed data to the high‐dose subgroup; six trials (Comella 2011; Poewe 1998; Poewe 2016; Truong 2005; Truong 2010; Wissel 2001; combined n = 664) contributed data to the medium‐dose subgroup; and one trial (Poewe 1998; n = 39) contributed data to the low‐dose subgroup.
We did not find a difference in the risk of adverse events between the subgroups (P = 0.66; Analysis 1.9).
1.9. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 9 Adverse events ‐ doses subgroup analysis.
2.2 Adverse events with Botox versus Dysport versus Xeomin subgroup analysis
We carried out a preplanned subgroup analysis to assess the risk of adverse events according to BtA formulation (see Subgroup analysis and investigation of heterogeneity). One trial (Charles 2012; n = 170) contributed data to the Botox subgroup; five trials (Poewe 1998; Poewe 2016; Truong 2005; Truong 2010; Wissel 2001; n = 549) contributed data to the Dysport subgroup; and one trial (Comella 2011; n = 233) contributed data to the Xeomin subgroup.
Overall, we did not find a difference in the risk of adverse events between these subgroups (P = 0.34; Analysis 1.10).
1.10. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 10 Adverse events ‐ BtA formulation subgroup analysis.
2.3 Adverse events with EMG‐guided versus non‐EMG‐guided injection subgroup analysis
We carried out a preplanned subgroup analysis to assess the risk of adverse events in trials that used EMG versus trials that did not use EMG (see Subgroup analysis and investigation of heterogeneity). Four trials (Comella 2011; Poewe 2016; Truong 2005; Truong 2010; n = 640) contributed data to the EMG‐guided subgroup; and three trials (Charles 2012; Poewe 1998; Wissel 2001; n = 312) contributed data to the non‐EMG‐guided subgroup.
It is important to note that all four trials that contributed data to the EMG‐guided subgroup left the use of EMG at the discretion of the investigator.
Overall, we did not find a difference in the risk of adverse events between these subgroups (P = 0.52; Analysis 1.11).
1.11. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 11 Adverse events ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis.
2.4 Adverse events of special interest
Treatment with BtA was only associated with an increased risk of two adverse events of special interest, namely dysphagia (RR 3.04, 95% CI 1.68 to 5.50; I2 = 0%; moderate certainty in the evidence; Analysis 1.12), and diffuse weakness/tiredness (RR 1.78, 95% CI 1.08 to 2.94; I2 = 0%; moderate certainty in the evidence; Analysis 1.13). The NNTH with a single BtA treatment for dysphagia was 16 (95% CI 7 to 49) and that for diffuse weakness/tiredness was 21 (95% CI 9 to 208).
1.12. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 12 Dysphagia.
1.13. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 13 Diffuse weakness/tiredness.
The remaining adverse events that were reported in the included trials included neck weakness (RR 3.23, 95% CI 0.95 to 10.91; I2 = 23%; Analysis 1.14), voice changes/hoarseness (RR 1.83, 95% CI 0.37 to 8.95; I2 = 26%; Analysis 1.15), sore throat/dry mouth (RR 1.66, 95% CI 0.78 to 3.51; I2 = 5%; Analysis 1.16), vertigo/dizziness (RR 1.47, 95% CI 0.38 to 5.73; I2=0%; Analysis 1.17), malaise/upper respiratory infection (RR 1.29, 95% CI 0.63 to 2.64; I2 = 45%; Analysis 1.18), injection site pain (RR 1.33, 95% CI 0.88 to 2.02; I2 = 0%; Analysis 1.19), and headache (RR 1.05, 95% CI 0.59 to 1.86; I2 = 0%; Analysis 1.20), though we did not find a difference between BtA and placebo regarding the risk of each one of these adverse events.
1.14. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 14 Neck weakness.
1.15. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 15 Voice change/hoarseness.
1.16. Analysis.
Comparison 1 Botulinum toxin type A versus placebo, Outcome 16 Sore throat/dry mouth.
1.17. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 17 Vertigo/dizziness.
1.18. Analysis.
Comparison 1 Botulinum toxin type A versus placebo, Outcome 18 Malaise/upper respiratory infection.
1.19. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 19 Local pain (injection site).
1.20. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 20 Headache.
Secondary outcomes
Subjective evaluation of clinical status
The included trials assessed subjective evaluation of overall improvement by both physicians and participants between weeks 3 to 6 after BtA injection. They used four scales to evaluate the amount of improvement: the Patient Evaluation of Global Response (PEGR), the Global Assessment of Change (GAS), the visual analogue scale (VAS), and the Clinical Global Rating. PERG and GAS (range, ‐4 to +4) are similar scales ranging from "Very marked worsening" (‐4) to "Complete resolution of cervical dystonia symptoms" (+4). VAS (range, 0 mm to 100 mm) assesses the change from baseline in symptom severity, where 0 mm indicates "Much worse", 50 mm "No change", and 100 mm "Symptom‐free". The Clinical Global Rating is a scale taking into account 6 grades of efficacy (excellent, good, moderate, slight improvement, no change, condition worse) and 4 grades of adverse events (none, mild, moderate, extreme).
The trials measured subjective assessments using these validated scales as the change from baseline to weeks 3 to 6.
We could not combine data from three studies (Greene 1990; Poewe 2016; Truong 2010) into the meta‐analysis for this outcome. Greene 1990 reported no data for the control group, Poewe 2016 reported a change from baseline for both study groups but did not report a measure of dispersion, and Truong 2010 did not report the change from baseline.
3.1. Subjective assessment by clinicians
Four trials (Charles 2012; Comella 2011; Poewe 1998; Wissel 2001; n = 544) contributed data for this outcome. Treatment with BtA was associated with an increased likelihood of clinical improvement when compared to placebo as assessed by physicians between weeks 4 and 20 after drug injection (RR 1.91, CI 1.47 to 2.49; I2 = 28%; Analysis 1.21). We calculated an NNTB of 3 (95% CI 2 to 6) with a single BtA treatment session.
1.21. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 21 Any improvement by subjective clinician assessment.
3.2. Subjective assessment by participants
Five trials (Charles 2012; Comella 2011; Poewe 1998; Truong 2005; Wissel 2001; n = 624) contributed data for this outcome. Treatment with BtA was associated with an increased likelihood of clinical improvement when compared to placebo as assessed by participants between weeks 4 and 20 after drug injection (RR 2.30, CI 1.83 to 2.90; I2 = 0%; moderate certainty in the evidence; Analysis 1.22). We calculated an NNTB of 3 (95% CI 2 to 5) with a single BtA treatment session.
1.22. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 22 Any improvement by subjective participant assessment.
Two trials (Poewe 1998; Truong 2005) considered only those participants reporting more than a half of improvement from baseline, and therefore these pooled results may underestimate the likelihood of participants reporting a subjective benefit with BtA treatment.
Pain relief
Six trials (Charles 2012; Comella 2011; Greene 1990; Truong 2005; Truong 2010; Wissel 2001; n = 722) contributed data to this outcome. They measured pain relief with validated pain scales as the change from baseline to weeks 3 to 6. Overall, treatment with BtA was associated with a moderately increased efficacy with regards to pain relief at weeks 4 to 6 (SMD 0.50, 95% CI 0.35 to 0.65; I2 = 0%; moderate certainty in the evidence; Analysis 1.26).
1.26. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 26 Cervical dystonia‐specific pain.
Three trials (Comella 2011; Truong 2005; Truong 2010; n = 429) contributed data measured on the TWSTRS pain subscale, and for this pooled result we found a MD of 2.11 TWSTRS points favouring participants allocated to BtA (95% CI 1.38 too 2.83; I2 = 0%; Analysis 1.27).
1.27. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 27 Cervical dystonia‐specific pain ‐ TWSTRS pain subscale subgroup analysis.
Health‐related quality of life
Three studies (Poewe 2016; Truong 2005; Truong 2010) assessed the impact of BtA on quality of life.
Poewe 2016 (n = 213) used the Cervical Dystonia Impact Profile (CDIP)‐58 scale (including eight subscales: head and neck symptoms, pain and discomfort, sleep, upper limb activities, walking, annoyance, mood and psychosocial functioning), and reported a significant improvement in total CDIP‐58 score (49.3 in BtA versus 59.4 in placebo; P < 0.0001), as well as in all eight CDIP‐58 subscales (P < 0.0003).
Truong 2005 (n = 80) and Truong 2010 (n = 116) reported an improvement from baseline to week 8 in the physical function domain of the SF‐36 (Truong 2005: odds ratio (OR) 1.60, P = 0.011; Truong 2010: MD 10.10, 95% CI 2.95 to 17.25; P = 0.018), but no benefit in social functioning when compared to placebo (Truong 2005: OR 0.30, 95% CI 0.23 to 0.82; P = 0.265; Truong 2010: MD 6.90, 95% CI 2.16 to 15.96; P = 0.125).
Tolerability
Adverse drug reactions (ADRs) can be a result of adverse events cause by the intervention (i.e. type A and/or type B ADRs), or lack of efficacy of the treatment (i.e. failure of therapy, a type F ADR) (Edwards 2000).
Four trials (Charles 2012; Comella 2011; Greene 1990; Truong 2010; n = 574) contributed data to this outcome. Overall, BtA was associated with a decreased likelihood of withdrawal from trials (RR 0.36, 95% CI 0.21 to 0.61; I2 = 0%; moderate certainty in the evidence; Analysis 1.30).
1.30. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 30 Tolerability ‐ withdrawals.
Three trials (Charles 2012; Comella 2011; Truong 2010; n = 519) contributed data regarding the withdrawals due to lack of efficacy. Overall, BtA was associated with a decreased likelihood of withdrawal from trials due to this reason (RR 0.30, 95% CI 0.17 to 0.53; I2 = 0%; Analysis 1.31).
1.31. Analysis.
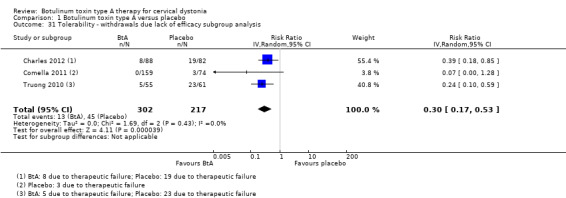
Comparison 1 Botulinum toxin type A versus placebo, Outcome 31 Tolerability ‐ withdrawals due lack of efficacy subgroup analysis.
Two trials (Comella 2011; Greene 1990; n = 288) contributed data regarding the withdrawals due to adverse events. Overall, we did not find a difference between BtA and placebo regarding withdrawals due to adverse events (RR 3.10; 95% CI 0.36 to 26.74; I2 = 0%; Analysis 1.32).
1.32. Analysis.
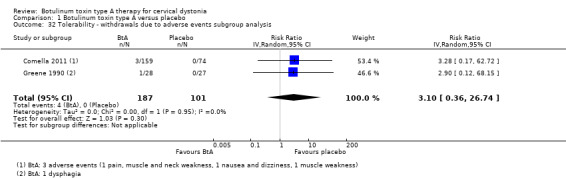
Comparison 1 Botulinum toxin type A versus placebo, Outcome 32 Tolerability ‐ withdrawals due to adverse events subgroup analysis.
Duration of effect
We did not pool results for this outcome due to the lack of combinable data.
Most studies assessed duration of effect on participants who showed some level of response to the assigned study treatment, either BtA or placebo. Poewe 1998 reported a dose‐dependent duration of effect, with request for re‐injection occurring at week 8 in 39%, 50% and 94% of participants treated with high‐, medium‐ to low‐dose, and placebo, respectively.
Truong 2005 reported a mean duration of effect of 22.8 weeks (SD = 12.5; range 9 to 46) until recurrence of symptoms, while Truong 2010 reported a mean time to re‐treatment of 14.4 weeks (range 4 to 30).
Poewe 2016 evaluated response to BtA over five treatment cycles. It reports that the duration of treatment effect for treatment responders was more than 85 days from cycles 2 to 5, irrespective of the dose used.
Discussion
Summary of main results
This updated review included eight randomised, parallel‐designed trials, that enrolled 1010 people with cervical dystonia, of whom 70% had been previously treated with botulinum toxin for their condition.
As can be seen in the Table 1, in comparison to placebo, BtA was probably more efficacious in reducing cervical dystonia‐associated impairment, including disease severity, disability, and associated pain. Treatment with BtA also increased the likelihood that participants and clinicians would detect any form of improvement. Uncertanity remains over the effect of BtA on other domains of people's quality of life, such as social functioning or mental health.
However, treatment with BtA increased the risk of experiencing an adverse event. In particular, BtA increased in the risk of two specific adverse events of special interest, namely dysphagia and diffuse weakness/tiredness. No fatalities or serious adverse events were considered related to BtA treatment in any trial. Finally, treatment with BtA slightly decreased the risk of withdrawal from the included clinical trials. Data for special subpopulations, such as children and pregnant women, were not available.
We found low to moderate statistical heterogeneity for most efficacy and safety outcome estimates.
BtA doses
All dosages were efficacious against placebo, but we found no clear‐cut evidence of a dose‐response gradient. It is, however, noteworthy that these trials were not dose‐response studies, they were not adequately powered to assess this question, and that we based this conclusion on arbitrarily defined dose‐subgroup analyses.
BtA formulations
Although none of the trials was designed or powered to evaluate the comparative utility of the three most widely used formulations of BtA (Botox (onabotulinumtoxinA), Dysport (abobotulinumtoxinA), and Xeomin (incobotulinumtoxinA)), we did not find differences between these subgroups in terms of overall efficacy or safety.
Use of EMG
Although none of the trials was designed to evaluate the comparative utility of injection technique with or without EMG, we did not find differences between trials that allowed and those that did not allow the use of EMG, in terms of overall efficacy or safety.
Duration of effect
Results from the few studies that addressed the duration of clinical effect of a treatment cycle in this review were not conclusive, with time to re‐treatment ranging greatly, from 1 to 11 months. We could not adequately evaluate long‐term duration of effect as all trials but one evaluated only a single treatment session.
Overall completeness and applicability of evidence
All included trials addressed the primary research question directly, using similar and validated assessment tools. However, they did not fully report data for all outcomes, and in some cases we could not pool results and compare them across studies. This limited the amount of data available and, consequently, the confidence in overall conclusions.
The participants included in the trials were not fully representative of the overall population of people with cervical dystonia. The effects of population enrichment and the moderate overall disease impairment (as assessed by the baseline TWSTRS scores) preclude definite conclusions concerning all people with this condition.
Four noteworthy factors challenge the implementation of the evidence in this review. Firstly, there was a considerably heterogeneous regional distribution, with most trials being conducted in North America and Europe. Differences in clinical practice, training of experts, and local guidelines in other regions of the world may present an obstacle to the application of the evidence here demonstrated. Secondly, sample size across included trials was relatively small and many subgroup analyses addressing clinically relevant questions for the main outcomes were underpowered. More studies are needed to provide robust evidence for these questions. Thirdly, the use of enriched populations in clinical trials limits applicability of results into clinical practice, as complex and potentially poorer responders are usually excluded in these trials. The fact that such individuals are common in clinical practice further complicates issues of generalisation. Fourthly, it is common for people with cervical dystonia to have concomitant medications for their condition, such as muscle relaxants and benzodiazepines. Reasonably, participants in trials are required to be on a stable dose of these medications for many weeks to avoid confounding factors. As a result, little is known at present about the impact of these drug regimens with regard to implementation of the evidence in this review.
Quality of the evidence
See Characteristics of included studies, 'Risk of bias' tables, 'Risk of bias' summary tables (Figure 2; Figure 3), and Table 1.
We considered all included trials but one to be at a high risk for both 'for‐profit bias' and having an enriched population. Only three of the included studies adequately described the randomisation or allocation methods, or both, with the remaining trials being assessed at an unclear risk of bias for these items. We considered most studies to be appropriately blinded in general. However, only a single trial provided a satisfactory description of blinding of objective outcome assessment, and we considered all but one possibly biased regarding subjective outcome assessment, as most studies predominantly enrolled participants with previous exposure to botulinum toxin. This represents a major methodological limitation that may have resulted in a biased assessment of the intervention effect, particularly with regards to subjective outcomes, namely pain assessment, subjective assessment by participants and clinicians, and quality of life assessments, which are highly susceptible to biased estimations.
We could not compare some outcomes across studies, as some studies did not report relevant data. We were unable to impute values for missing data due to imbalances between baseline characteristics of the participants and incomplete description of the variables, further reducing the amount of combinable data, and therefore our confidence in the results.
The included trials each enrolled between 55 and 233 participants, and although individually some of these trials were underpowered, the pooling of the trials permitted an adequate sample size for the majority of efficacy outcomes.
Taken together, as can be seen in Table 1, we consider that there is moderate certainty in the evidence that a single treatment session of BtA improves cervical dystonia‐associated impairment in certain types of cervical dystonia, including severity, disability, and pain. The certainty in the evidence supporting the higher occurrence of any adverse event, as well diffuse weakness/tiredness and dysphagia is moderate. The certainty in the evidence assessing the change in subjective evaluation of clinical status evaluated by participants is moderate. Finally, we have moderate certainty in the evidence that treatment with BtA increases the likelihood of participants not dropping out of clinical trials.
Potential biases in the review process
Although we followed the methods recommended by Cochrane in order to minimise bias in the review process, certain areas do deserve attention. In particular, we have not searched clinical trials registries. Although this opens the current review to the potential bias of having missed trials, we consider this possibility highly unlikely because we have extensively contacted other experts in this field and US and European trials in this area are well‐known.
An additional bias was that we could not obtain data for all outcomes in the included trials. A further limitation of this review is the small number of participants contributing data to each outcome, although Trial Sequential Analysis showed that both the primary efficacy and safety outcomes were adequately powered to demonstrate the difference that we observed.
Agreements and disagreements with other studies or reviews
Overall, the results of this updated review are in agreement with the conclusions of earlier versions (Costa 2005). The current clinical practice guidelines of the American Academy of Neurology and the European Academy of Neurology agree that BtA is "established as safe and effective for cervical dystonia treatment" (Simpson 2016) and that it is considered an "effective and safe treatment for cervical dystonia" (Albanese 2011). We now conclude that no claims can be made regarding a clear dose‐response relationship for efficacy outcomes. On the other hand, a clear relationship exists for the increased risk of treatment‐related adverse events of special interest, namely dysphagia and diffuse weakness/tiredness.
Authors' conclusions
Implications for practice.
In this updated Cochrane Review we found that a single treatment session of botulinum toxin type A (BtA) is effective and well‐tolerated in the treatment of moderately‐impaired adults with certain types of cervical dystonia. The clinical benefit includes moderate to large improvements across several objective disease domains, namely severity, disability, and pain. The benefit is also meaningful when subjectively assessed by patients. The evidence is less robust regarding health‐related quality of life improvements. Adverse events are frequent, but are not commonly associated with treatment discontinuation. In fact, since withdrawals were less frequent in the BtA group, we can assume that people with cervical dystonia find the risk‐benefit profile of BtA highly favourable. Dysphagia and diffuse weakness/tiredness are the most frequent treatment‐related adverse events of special interest. We are moderately certain about the conclusions based on the evidence.
The available evidence does not allow for firm conclusions regarding the existence of a clear dose‐benefit response, nor to support or not support routine use of EMG‐guided BtA injection. The current evidence does not allow conclusions regarding the comparative risk‐benefit profiles of the different BtA formulations available.
We can draw no conclusions regarding people with pure retrocollis or anterocollis, as these were predominantly excluded in the clinical trials.
Implications for research.
We have had access only to published research data from trials of BtA versus placebo in adults with certain types of cervical dystonia. The net benefit of a single BtA injection in the treatment of cervical dystonia has been clearly established in the published trials, making it difficult to determine which and how many resources should be invested in future research.
Nonetheless, further studies are needed to establish the relative effectiveness of different doses of BtA, assessing efficacy, safety, duration of effect, and quality of life across regimes, with repeated BtA treatment sessions, and assessed under conditions more closely resembling clinical practice (pragmatic clinical trials). Because therapy typically requires optimising a dose for each patient rather than administering a fixed dose of botulinum toxin, such a line of research would be important to support physicians' management of doses and allow for a more solid and safe individualisation of patient treatment. Also to be determined is the added value, if any, of guidance methods (e.g. EMG) in injecting botulinum toxin into the cervical muscles.
Future research concerning all formulations of botulinum neurotoxin should endeavour to establish clinical effectiveness not only based on changes from baseline, but also, preferably, based on validated measures of Minimal Clinically Important Difference/Change (Brożek 2006). Research is required in order to establish such a parameter for the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), currently the most widely used and disseminated clinical scale in the field. We are, however, aware of an effort to create a new clinical scale in dystonia ‐ the Comprehensive Cervical Dystonia Rating Scale (Comella 2015), which will include a revision of the TWSTRS, to be named TWSTRS‐2, with a Minimal Clinically Important Change validation being planned.
It is currently uncertain whether or not the clinical effectiveness of botulinum toxin decays over time, with repeated treatment sessions, and whether a possible loss of effectiveness occurs in all clinical domains. Future studies comparing any form of BtA should address the comparative proportion of participants who develop secondary non‐responsiveness to treatment.
Finally, in conducting this systematic review we were faced with the fact that there is no defined core outcome set in cervical dystonia research, as there are for other areas (Tugwell 2007). The definition of a set of core outcome measures to be included in future research, via well‐established methodology to determine the inclusion of patient‐reported outcomes (Macefield 2014), would be relevant to promote research in this field, as well as to support the clinical effectiveness of botulinum toxin.
Given the high degree of certainty in the results, and that the outcomes are mostly adequately‐powered and provide robust evidence of efficacy, future efforts to update this review may not be justified, unless Cochrane methodology changes or some of the research suggestions are published.
What's new
| Date | Event | Description |
|---|---|---|
| 14 November 2016 | New citation required but conclusions have not changed | New authorship, accumulation of changes, re‐assessment and rewriting according to new quality standards, addition of a 'Summary of findings' table |
| 6 October 2016 | New search has been performed | Three new trials enrolling 562 participants overall were included in the meta‐analysis and systematic review (Comella 2011; Poewe 2016; Truong 2010) |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 6 October 2008 | Amended | Converted to new review format. |
| 25 October 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Ema Roque (Cochrane Movement Disorders) and Daisy Abreu (Clinical Pharmacology Unit, Faculty of Medicine, University of Lisbon) sincerely for their contributions to this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Botulinum Toxins] explode all trees
#2 Botulinum Toxins, Type A
#3 (botul* near/2 tox*):ti,ab
#4 (botox or dysport or xeomin or myobloc or rimabotulinum* or abobotuli* or onabotulinum* or oculinum or purtox or CNBTX or Neuronox):ti,ab
#5 {or #1‐#4}
#6 MeSH descriptor: [Dystonic Disorders] explode all trees
#7 MeSH descriptor: [Dystonia] explode all trees
#8 MeSH descriptor: [Torticollis] explode all trees
#9 MeSH descriptor: [Blepharospasm] explode all trees
#10 MeSH descriptor: [Meige Syndrome] explode all trees
#11 MeSH descriptor: [Hemifacial Spasm] explode all trees
#12 (cervic* near/2 dysto*):ti,ab
#13 blepharosp*:ti,ab
#14 (hem* near/2 spasm*):ti,ab
#15 (meige and (dysto* or syndrom*)):ti,ab
#16 (crani* near/2 dysto*):ti,ab
#17 (foca* near/2 dysto*):ti,ab
#18 (write* and (cramp* or dysto*)):ti,ab
#19 torticol*:ti,ab
#20 {or #6‐#19}
#21 #5 and #20
#22 MeSH descriptor: [Animals] explode all trees
#23 MeSH descriptor: [Humans] explode all trees
#24 #22 not #23
#25 #21 not #24 in Trials
Appendix 2. MEDLINE search strategy
#1 randomized controlled trial.pt.
#2 controlled clinical trial.pt.
#3 randomized.ab.
#4 placebo.ab.
#5 clinical trials as topic.sh.
#6 randomly.ab.
#7 trial.ti.
#8 1 or 2 or 3 or 4 or 5 or 6 or 7
#9 exp botulinum toxins/
#10 exp botulinum toxins, type A/
#11 (botul$ adj2 tox$).ti,ab.
#12 (botox or dysport or xeomin or myobloc or rimabotulinum$ or abobotuli$ or onabotulinum$ or oculinum or purtox or CNBTX or Neuronox).ti,ab.
#13 9 or 10 or 11 or 12
#14 (cervic$ adj2 dysto$).ti,ab.
#15 blepharosp$.ti,ab.
#16 (hem$ adj2 spasm$).ti,ab.
#17 (meige and (dysto$ or syndrom$)).ti,ab.
#18 (crani$ adj2 dysto$).ti,ab.
#19 (foca$ adj2 dysto$).ti,ab.
#20 (write$ and (cramp$ or dysto$)).ti,ab.
#21 torticol$.ti,ab.
#22 exp dystonic disorders/
#23 exp dystonia/
#24 exp torticollis/
#25 exp blepharospasm/
#26 exp meige syndrome/
#27 exp hemifacial spasm/
#28 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
#29 8 and 3 and 28
#30 exp animals/ not humans/
#31 29 not 30
Appendix 3. Embase search strategy
#1 random$.tw.
#2 clinical trial:.mp.
#3 placebo$.mp.
#4 double‐blind$.tw.
#5 1 or 2 or 3 or 4
#6 exp Hemifacial Spasm/
#7 exp Meige Syndrome/
#8 exp blepharospasm/
#9 exp torticollis/
#10 exp Dystonia/
#11 exp Dystonic Disorders/
#12 (cervic$ adj2 dysto$).ti,ab.
#13 blepharosp$.ti,ab.
#14 (hem$ adj2 spasm$).ti,ab.
#15 (meige and (dysto$ or syndrom$)).ti,ab.
#16 (crani$ adj2 dysto$).ti,ab.
#17 (foca$ adj2 dysto$).ti,ab.
#18 (write$ and (cramp$ or dysto$)).ti,ab.
#19 torticol$.ti,ab.
#20 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
#21 exp Botulinum Toxins, Type A/
#22 exp Botulinum Toxins/
#23 (botul$ adj2 tox$).ti,ab.
#24 (botox or dysport or xeomin or myobloc or rimabotulinum$ or abobotuli$ or onabotulinum$ or oculinum or purtox or CNBTX or Neuronox).ti,ab.
#25 21 or 22 or 23 or 24
#26 19 and 20 and 25
#27 limit 26 to human
Data and analyses
Comparison 1. Botulinum toxin type A versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cervical dystonia‐specific improvement | 7 | 833 | Std. Mean Difference (Random, 95% CI) | 0.70 [0.52, 0.89] |
| 2 Cervical dystonia‐specific improvement ‐ TWSTRS subgroup analysis | 4 | 522 | Mean Difference (IV, Random, 95% CI) | 8.06 [6.08, 10.05] |
| 3 Cervical dystonia‐specific severity ‐ as assessed with TWSTRS subscale | 3 | 429 | Mean Difference (IV, Random, 95% CI) | 3.13 [2.15, 4.11] |
| 4 Cervical dystonia‐specific disability ‐ as assessed with TWSTRS subscale | 3 | 429 | Mean Difference (IV, Random, 95% CI) | 2.52 [1.72, 3.31] |
| 5 Cervical dystonia‐specific improvement ‐ doses subgroup analysis | 6 | 777 | Std. Mean Difference (Random, 95% CI) | 0.84 [0.68, 1.00] |
| 5.1 Low dose | 1 | 39 | Std. Mean Difference (Random, 95% CI) | 1.24 [0.55, 1.94] |
| 5.2 Medium dose | 6 | 545 | Std. Mean Difference (Random, 95% CI) | 0.76 [0.59, 0.94] |
| 5.3 High dose | 2 | 193 | Std. Mean Difference (Random, 95% CI) | 1.08 [0.53, 1.63] |
| 6 Cervical dystonia‐specific improvement ‐ BtA formulation subgroup analysis | 7 | 833 | Std. Mean Difference (Random, 95% CI) | 0.70 [0.52, 0.89] |
| 6.1 Botox | 1 | 170 | Std. Mean Difference (Random, 95% CI) | 0.38 [0.08, 0.69] |
| 6.2 Dysport | 5 | 430 | Std. Mean Difference (Random, 95% CI) | 0.75 [0.54, 0.96] |
| 6.3 Xeomin | 1 | 233 | Std. Mean Difference (Random, 95% CI) | 0.82 [0.53, 1.10] |
| 7 Cervical dystonia‐specific improvement ‐ EMG‐guided versus non‐EMG‐guided subgroup analysis | 7 | 833 | Std. Mean Difference (Random, 95% CI) | 0.70 [0.52, 0.89] |
| 7.1 EMG‐guided injection | 4 | 522 | Std. Mean Difference (Random, 95% CI) | 0.71 [0.52, 0.89] |
| 7.2 Non‐EMG‐guided injection | 3 | 311 | Std. Mean Difference (Random, 95% CI) | 0.79 [0.27, 1.31] |
| 8 Adverse events | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| 9 Adverse events ‐ doses subgroup analysis | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Low dose | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.56, 3.85] |
| 9.2 Medium dose | 6 | 664 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.06, 1.44] |
| 9.3 High dose | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.72, 5.02] |
| 10 Adverse events ‐ BtA formulation subgroup analysis | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| 10.1 Botox | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.30] |
| 10.2 Dysport | 5 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.02, 1.66] |
| 10.3 Xeomin | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.92, 1.62] |
| 11 Adverse events ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| 11.1 EMG‐guided injection | 4 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.36] |
| 11.2 Non‐EMG‐guided injection | 3 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.82, 2.50] |
| 12 Dysphagia | 8 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 3.04 [1.68, 5.50] |
| 13 Diffuse weakness/tiredness | 6 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.08, 2.94] |
| 14 Neck weakness | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 3.23 [0.95, 10.91] |
| 15 Voice change/hoarseness | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.37, 8.95] |
| 16 Sore throat/dry mouth | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.78, 3.51] |
| 17 Vertigo/dizziness | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.38, 5.73] |
| 18 Malaise/upper respiratory infection | 7 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.63, 2.64] |
| 19 Local pain (injection site) | 7 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.88, 2.02] |
| 20 Headache | 6 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.59, 1.86] |
| 21 Any improvement by subjective clinician assessment | 4 | 544 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.47, 2.49] |
| 22 Any improvement by subjective participant assessment | 5 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.83, 2.90] |
| 23 Any improvement by subjective participant assessment ‐ doses subgroup analysis | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23.1 Low dose | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.30, 8.43] |
| 23.2 Medium dose | 4 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.82, 3.25] |
| 23.3 High dose | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 3.39 [2.16, 5.33] |
| 24 Any improvement by subjective participant assessment ‐ BtA formulation subgroup analysis | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.1 Botox | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.34, 2.94] |
| 24.2 Dysport | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.49, 3.04] |
| 24.3 Xeomin | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 3.23 [2.03, 5.14] |
| 25 Any improvement by subjective participant assessment ‐ EMG guided vs non‐EMG‐guided subgroup analysis | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 25.1 EMG‐guided injection | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [1.99, 4.43] |
| 25.2 Non‐EMG‐guided injection | 3 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.53, 2.69] |
| 26 Cervical dystonia‐specific pain | 6 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.35, 0.65] | |
| 27 Cervical dystonia‐specific pain ‐ TWSTRS pain subscale subgroup analysis | 3 | Mean Difference (Random, 95% CI) | 2.11 [1.38, 2.83] | |
| 28 Cervical dystonia‐specific pain ‐ BtA formulation subgroup analysis | 6 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.35, 0.65] | |
| 28.1 Botox | 2 | Std. Mean Difference (Random, 95% CI) | 0.51 [0.01, 1.02] | |
| 28.2 Dysport | 3 | Std. Mean Difference (Random, 95% CI) | 0.52 [0.28, 0.77] | |
| 28.3 Xeomin | 1 | Std. Mean Difference (Random, 95% CI) | 0.55 [0.27, 0.83] | |
| 29 Cervical dystonia‐specific pain ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis | 6 | 654 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.35, 0.65] |
| 29.1 EMG‐guided injection | 3 | 429 | Std. Mean Difference (Random, 95% CI) | 0.53 [0.33, 0.73] |
| 29.2 Non‐EMG‐guided injection | 3 | 225 | Std. Mean Difference (Random, 95% CI) | 0.50 [0.20, 0.80] |
| 30 Tolerability ‐ withdrawals | 4 | 574 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.23, 0.62] |
| 31 Tolerability ‐ withdrawals due lack of efficacy subgroup analysis | 3 | 519 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.17, 0.53] |
| 32 Tolerability ‐ withdrawals due to adverse events subgroup analysis | 2 | 288 | Risk Ratio (IV, Random, 95% CI) | 3.10 [0.36, 26.74] |
1.23. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 23 Any improvement by subjective participant assessment ‐ doses subgroup analysis.
1.24. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 24 Any improvement by subjective participant assessment ‐ BtA formulation subgroup analysis.
1.25. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 25 Any improvement by subjective participant assessment ‐ EMG guided vs non‐EMG‐guided subgroup analysis.
1.28. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 28 Cervical dystonia‐specific pain ‐ BtA formulation subgroup analysis.
1.29. Analysis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 29 Cervical dystonia‐specific pain ‐ EMG‐guided vs non‐EMG‐guided subgroup analysis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Charles 2012.
| Methods | Randomised, double‐blind, parallel design Randomisation: carried out in blocks of four; method not described Setting: multicentre (22 centres in the USA, 1 in Canada) Duration: 10 weeks |
|
| Participants | 170 participants enrolled (BtA group = 88; placebo group = 82) % Female: BtA: 70%; placebo: 80% Mean age, range: BtA: 55 years; placebo: 55 years Mean CD duration: BtA: 11.2 years; placebo: 9.1 years Mean CD severity, SD (CDSS): BtA: 9.2, 4.8; placebo: 9.3, 4.2 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
BtA: Botox (onabotulinumtoxinA); 25 ng of neurotoxin complex protein per 100 U, diluted with 1 mL sterile solution Placebo: 0.5 mg of human serum albumin and 0.9 mg of sodium chloride Study drug preparation: BtA provided in vials by Allergan Muscles injected: the doses and muscles injected were determined by the physician based on clinical assessment EMG guidance: no BtA dose per participant: maximum: 360 U; mean, range: 236 U, 91 U‐360 U |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | This was a 2‐period clinical trial consisting of a 10‐week open‐label period followed by a 10‐week double‐blind period, with up to 6 weeks between periods. Participants who successfully completed the open phase (i.e. responded to BtA and were compliant with the study protocol) were enrolled into the blinded phase. 214 participants were enrolled in Period I, of whom 170 continued into Period II. We only considered the results of the blinded phase in this review. Study discontinuations, reasons: BtA: n = 11 (13%), lack of efficacy: n = 8, unrelated reasons: n = 3 Placebo: n = 24 (29%), lack of efficacy: n = 19, unrelated reasons: n = 5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: post‐randomisation exclusions were described as related to lack of efficacy or administrative reasons |
| Selective reporting (reporting bias) | Low risk | Comment: the expected outcomes that are usually evaluated in intervention trials for this condition were reported in this study |
| For‐profit bias | High risk | The study was supported by Allergan. |
| Enriched population – preferential enrolment of positive responders | High risk | Comment: to enrol in the study, participants had to have had at least 2 previous successful treatments with ≤ 360 U of Botox administered at 12‐ to 16‐week intervals. Also, all participants enrolled in phase II were compliant to treatment during phase I trial. |
| Enriched population – exclusion of poor responders | High risk | Quote: "Pure anterocollis and isolated head shift was exclusionary" |
Comella 2011.
| Methods | Randomised, double‐blind, parallel design Randomisation: block‐wise randomisation using a software‐generated code Setting: multicentre (37 centres in the USA) Duration: 8 weeks, follow‐up up to 20 weeks |
|
| Participants | 233 participants enrolled (BtA 120 U group = 78; BtA 240 U group = 81; placebo group = 74) % Female: BtA 120 U: 51%; BtA 240 U: 54%; placebo: 49% Mean age, SD: BtA 120 U: 52.8 years, 11.5; BtA 240 U: 53.2 years, 12.2; placebo: 52.4 years, 10.8 Mean CD duration: BtA 120 U: 9.3 years, 8.4; BtA 240U: 9.7 years, 9.0; placebo: 10.8 years, 9.0 Mean CD severity, SD (TWSTRS total): BtA 120 U: 42.6, 9.7; BtA 240 U: 42.1, 9.3; placebo: 41.8, 7.9 Inclusion criteria:
Exclusion criteria:
Other medications for focal dystonia were required to be on a stable dose for at least 3 months |
|
| Interventions |
BtA: Xeomin (incobotulinumtoxinA); 120 U or 240 U, diluted in 4.8 mL Placebo: reconstitution of powder with 0.9% NaCl diluted in 4.8 mL Study drug preparation: vials and providers not mentioned Muscles injected: the number of injection sites per muscle and the volume injected into each muscle were determined at the discretion of the investigator EMG guidance: left at discretion of the investigator BtA dose per participant: 120 U or 240 U |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Study discontinuations (at week 8), reasons: BtA 120 U: n = 3 (4%), adverse events: n = 1, consent withdrawal: n = 1, lost to F/U: n = 1 BtA 240 U: n = 5 (6%), adverse events: n = 2, consent withdrawal: n = 1, lost to F/U: n = 1, unrelated reasons: n = 1 Placebo: n = 6 (8%), lack of efficacy: n = 3, consent withdrawal: n = 1, lost to F/U: n = 1, unrelated reasons: n = 1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using RANCODE version 3.6 (IDV, Gauting). Block‐wise randomization by previous treatment with botulinum toxin ensured a balanced treatment assignment for each center for pretreated and treatment‐naïve patients" |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects, investigators, medical staff, (…) data managers and monitors were blind to subjects' treatment group" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Subjects, investigators, medical staff, biostatisticians responsible for data analysis, data managers and monitors were blind to subjects' treatment group" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: although placebo was identical to intervention, the fact that most of the participants had previously been treated with Bt could have led to a degree of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Missing subject data was provided and their absence was regarded according to an ITT protocol" Comment: post‐randomisation exclusions were low and roughly distributed evenly between groups (BtA 120 U group = 3; BtA 240 U group = 5; Placebo group = 6). The reasons for exclusions were described. |
| Selective reporting (reporting bias) | Low risk | Comment: the outcomes mentioned in the study protocol matched the outcomes reported in the study. |
| For‐profit bias | High risk | Comment: study funded by Merz Pharmaceuticals GmbH, Frankfurt |
| Enriched population – preferential enrolment of positive responders | High risk | Quote: "A total of 233 subjects were randomized (...) Of these, 143 were previously treated with botulinum toxin" |
| Enriched population – exclusion of poor responders | High risk | Quote: "Subjects were excluded if they had (…) predominant anterocollis or retrocollis" |
Greene 1990.
| Methods | Randomised, double‐blind, parallel design Randomisation: stratified by CD classification; method not described Setting: single‐centre (USA) Duration: 12 weeks |
|
| Participants | 55 participants enrolled (BtA group = 28; placebo group = 27) % Female: BtA: 61%; placebo: 67% Mean age: BtA: 46.8 years; placebo: 52.6 years Mean CD duration: BtA: 6.6 years; placebo: 9.8 years CD severity: BtA: 7% mild, 71% moderate, 21% severe; placebo: 11% mild, 48% moderate, 41% severe Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
BtA: Botox (onabotulinumtoxinA); diluted in saline solution to a concentration of 25 U per 1 mL Placebo: saline solution Study drug preparation: BtA provided in vials by Smith‐Kettlewell Eye Research Institute (USA) Muscles injected: the doses, muscles, and number of injected sites per muscle were determined by the physician based on clinical assessment and classification of CD EMG guidance: no BtA dose per participant: 150 U, rotational torticollis and torticollis plus retrocollis; 165 U, head tilt |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Study discontinuations, reasons: BtA: n = 3 (11%), adverse events: n = 1, unrelated reasons: n = 2 Placebo: n = 0 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were divided into 3 cells (A, pure rotational torticollis; B, torticollis plus retrocollis; and C, head tilt with or without torticollis and retrocollis). In order to ensure reasonable balance of Botox and placebo injections in each cell, randomization was stratified by cell type, which was completed for blocks of 4 sequentially enrolled patients in each cell type" Comment: insufficient information about the method of randomisation to permit judgement of low or high risk |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The blinded physicians then injected them with Botox or saline, using syringes filled by the unblinded physicians according to the protocol" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Two blinded physicians gave the injections, determined the degree of head turning and disability, and videotaped the patients; but they did not examine the strength or size of the neck muscles, so that the presence of muscle atrophy would not identify patients receiving active injection. Videotapes of each patient visit were rated by the 2 blinded observers independently" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Patients previously treated with Botox were excluded from the trial" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study authors stated that some data were lost, accounting for up to 13% of total data, and it is unclear whether this had an impact on the overall results. |
| Selective reporting (reporting bias) | Low risk | Comment: the expected outcomes that are usually evaluated in intervention trials for this condition were reported in this study. |
| For‐profit bias | Low risk | Study drug was provided by Dr. A. Scott, from Smith‐Kettlewell Eye Research Institute (USA). |
| Enriched population – preferential enrolment of positive responders | Low risk | Comment: participants who had previously received Botox injections were excluded. |
| Enriched population – exclusion of poor responders | Low risk | Comment: Exclusion criteria did not include forms of dystonia known to have poorer response to treatment |
Poewe 1998.
| Methods | Randomised, double‐blind, parallel design Randomisation: not described Setting: multicentre (Germany and Austria) Duration: 8 weeks |
|
| Participants | 75 participants enrolled (BtA 250 U group = 19; BtA 500 U group = 18; BtA 1000 U group = 18; placebo group = 20). % Female: all groups: 48% Mean age, SD: all groups: 47 years, 11.5 Mean CD duration, SD: all groups: 7.4 years, 6.7 CD severity (Tsui modified scale): BtA 250 U: 14.3; BtA 500 U: 13.1; BtA 1000 U: 14.5; placebo: 14.4 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
BtA: Dysport (abobotulinumtoxinA); vials of 500 U, diluted with 1 mL sterile solution Placebo: 0.125 mg of human serum albumin and 2.5 mg of lactose, diluted with 1 mL sterile solution Study drug preparation: BtA provided in vials by Speywood Pharmaceuticals Muscles injected: a total of 2.5 mL of the study drug or placebo was injected in each participant (0.75 mL into 2 sites in the sternocleidomastoid muscle, and 1.75 mL into 2 sites in the splenius capitis muscle) EMG guidance: no BtA dose per participant: 250 U, 500 U, or 1000 U |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Study discontinuations, reasons: BtA 250 U: n = 0 BtA 500 U: n = 2 (11%), lost to F/U: n = 2 BtA 1000 U: n = 0 Placebo: n = 0 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive treatment with placebo or total dose of 250, 500, or 1000 Dysport units of botulinum toxin type A in a double blind prospective study design" Comment: insufficient information about the sequence generation process to permit judgement of ‘low risk or high risk |
| Allocation concealment (selection bias) | Low risk | Comment: sequentially numbered drug containers of identical appearance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were randomly assigned to receive treatment with placebo or total dose of 250, 500, or 1000 Dysport units of botulinum toxin type A in a double blind prospective study design" Quote: "All three vials were identical in appearance" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: insufficient information to permit judgement of low risk or high risk |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: insufficient information to permit judgement of low risk or high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient in the 500 unit group was lost to follow up and had to be excluded from result analysis. A further case of 500 unit group had missed one follow up visit and was excluded from efficacy analysis but included in analysis of adverse events" Comment: reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Comment: the expected outcomes that are usually evaluated in intervention trials for this condition were reported in this study. |
| For‐profit bias | High risk | Quote: "Toxin and placebo preparations was supplied by Speywood Pharmaceuticals Ltd" |
| Enriched population – preferential enrolment of positive responders | Low risk | Comment: all participants were previously untreated with botulinum toxin type A |
| Enriched population – exclusion of poor responders | High risk | Quote: "Seventy five patients (...) with rotational torticollis and hyperactivity clinically confined to one splenius capitis and the contralateral sternomastoid muscles" |
Poewe 2016.
| Methods | Randomised, double‐blind, parallel design Randomisation: not adequately described Setting: multicentre (61 centres in 11 countries) Duration: 12 weeks |
|
| Participants | 369 participants enrolled overall 213 participants enrolled with data contributing to the current review (BtA group = 159; placebo group = 54) % Female: BtA: 64%; placebo: 63% Mean age: BtA: 49 years; placebo: 50 years Mean CD duration: BtA: 7 years; placebo: 6 years Mean CD severity, SD (TWSTRS‐total): BtA: 46, 9; placebo: 47, 9 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
BtA: Dysport (abobotulinumtoxinA) Placebo: supplied in a 1‐mL prefilled syringe indistinguishable from the active products Study drug preparation: provided as a freeze‐dried powder containing 500 U of BtA haemagglutinin complex together with 125 µg of human albumin and 2.5 mg of lactose. The powder was reconstituted with 1.1 mL sodium chloride for injection using a glass syringe Muscles injected: administered into 2‐4 neck muscles (levator scapulae, trapezius, sternocleidomastoid, splenius capitis, scalenus (medius and anterior), semispinalis capitis, or longissimus capitis) in a single dosing session according to the physicians’ clinical judgment of the individual’s pattern of dystonic activity EMG guidance: left at discretion of the investigator BtA dose per patient: 500 U |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To maintain blinding, all study treatments were identical in appearance and smell. All injections during the double‐blind phase were prepared by dedicated and trained site personnel who were independent from investigators and had no contact with the investigators performing study assessment or the trial patients" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "To maintain blinding, all study treatments were identical in appearance and smell. All injections during the double‐blind phase were prepared by dedicated and trained site personnel who were independent from investigators and had no contact with the investigators performing study assessment or the trial patients" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Inclusion of a considerable proportion of non‐naive participants, meaning they may have been able to foresee group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| For‐profit bias | High risk | Quote: "This study was sponsored by Ipsen" |
| Enriched population – preferential enrolment of positive responders | High risk | Inclusion of a considerable proportion of non‐naive participants, meaning they may have been able to foresee group allocation |
| Enriched population – exclusion of poor responders | High risk | Exclusion of nonresponsive phenotypes |
Truong 2005.
| Methods | Randomised, double‐blind, parallel design Randomisation: Block‐wise randomisation using a software‐generated code, stratification by centre Setting: multicentre (16 centres in USA) Duration: 4 weeks, follow‐up up to 20 weeks |
|
| Participants | 80 participants enrolled (BtA group = 37; placebo group = 43) % Female: BtA: 62%; placebo: 63% Mean age, SD: BtA: 53.4 years, 11.6; placebo: 53.6 years, 12.1 Mean CD duration, SD: BtA: 7.1 years, 7.1; placebo: 5.7 years, 5.2 Mean CD severity, SD (TWSTRS total): BtA: 45.1, 8.7; placebo: 46.2, 9.4 Inclusion criteria:
Exclusion criteria:
Medications such as muscle relaxants and benzodiazepines were required to be on a stable dose for ≥ 6 weeks |
|
| Interventions |
BtA: Dysport (abobotulinumtoxinA); 500 U Placebo: 0.125 mg of human serum albumin and 2.5 mg of lactose Study drug preparation: BtA provided in vials by Ipsen Ltd Muscles injected: the doses and number of injection sites per muscle were determined at the discretion of the investigator. EMG guidance: left at discretion of the investigator BtA dose per participant: 500 U |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Participants who showed no benefit at week 4 were terminated from the study. Those who had evidence of response at week 4 continued in the study until additional injections were needed. Study discontinuations (at week 4), reasons: BtA: n = 15 (41%), reasons not described Placebo: n = 27 (63%), reasons not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was in blocks of four and was stratified by center and according to whether or not the patient had been treated previously with botulinum toxin" Quote: "All patients were randomly assigned to treatment using a randomization code generated before the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "Dysport was provided in a clear glass vial as a freeze dried white pellet (…). Placebo was provided in identical clear glass vials (…)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo was provided in identical clear glass vials (…). Study medication was supplied in individual patient boxes, containing one vial of either Dysport or placebo. Subjects, investigators, medical staff, (…) data managers and monitors were blind to subjects' treatment group" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Quote: "Whenever possible, an investigator or research nurse other than the one performing the TWSTRS assessment who was blind to treatment condition performed the assessment for adverse events. All sites were asked to achieve as much consistency as possible with respect to assessors" Comment: insufficient information to permit judgement of low risk or high risk |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Quote: "At each post‐treatment visit, patients and investigators independently assessed the change from baseline" Comment: insufficient information to permit judgement of low risk or high risk. Although placebo was identical to intervention, the fact that most of the participants had previously been treated with Botox could have led to a degree of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: post‐randomisation exclusions at week 4 were high in both intervention arms, though this difference was asymmetrical, with more dropouts happening in the placebo arm |
| Selective reporting (reporting bias) | Low risk | Comment: the outcomes mentioned in the study protocol matched the outcomes reported in the study |
| For‐profit bias | High risk | Comment: study funded by Beauford Ipsen |
| Enriched population – preferential enrolment of positive responders | High risk | Comment: out of the 80 participants enrolled, 21 were de novo |
| Enriched population – exclusion of poor responders | High risk | Quote: "Patients with pure retrocollis were not permitted to participate" |
Truong 2010.
| Methods | Randomised, double‐blind, parallel design Randomisation: pre‐generated randomisation code Setting: multicentre (16 centres in USA, 4 in Russia) Duration: 12 weeks |
|
| Participants | 116 participants enrolled (BtA group = 55; placebo group = 61) % Female: BtA: 67%; placebo: 62% Mean age, SD: BtA: 51.9, 13.4; placebo: 53.9, 12.5 Mean CD duration, SD: BtA: 12.0 years, 8.8; placebo: 11.8 years, 8.8 Mean CD severity, SD (TWSTRS total): BtA: 43.8, 8.0; placebo: 45.8, 8.8 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
BtA: Dysport (abobotulinumtoxinA) Placebo: not described Study drug preparation: BtA provided in vials by Ipsen Muscles injected: the doses and number of injection sites per muscle were determined at the discretion of the investigator EMG guidance: left at discretion of the investigator BtA dose per participant: 500 U |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes |
Study discontinuations, reasons: BtA: n = 10 (18%), lack of efficacy: n = 5, consent withdrawal: n = 2, lost to F/U: n = 1, unrelated reasons: n = 2 Placebo: n = 23 (38%), lack of efficacy: n = 23 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In the double‐blind treatment phase, patients were randomized using a pre‐generated randomization code to receive intramuscular injection of either 500 units Dysport or placebo (1:1)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: blinding not specified although study described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Efficacy variables were assessed using intent‐to‐treat (ITT) analysis" Quote: "Safety assessments were based on the safety population, which included all patients who received at least one dose of study medication" Comment: the reasons for discontinuation were described |
| Selective reporting (reporting bias) | Low risk | Comment: the expected outcomes that are usually evaluated in intervention trials for this condition were reported in this study. |
| For‐profit bias | High risk | Quote: "This study was supported by the Ipsen Group. Editorial assistance for the preparation of this manuscript was provided by Ogilvy Healthworld Medical Education; funding was provided by Ipsen Limited, Slough, UK" |
| Enriched population – preferential enrolment of positive responders | High risk | Quote: "Patients were excluded if they had a (...) previous poor response to BoNT‐A or BoNT‐B treatments; current treatment with BoNT‐B due to lack of efficacy of BoNT‐A or the presence of neutralising antibodies to BoNT‐A" |
| Enriched population – exclusion of poor responders | High risk | Quote: "Patients were excluded if they had a diagnosis of pure anterocollis or retrocollis" |
Wissel 2001.
| Methods | Randomised, double‐blind, parallel design Randomisation: method not described Setting: multicentre (Austria and Czech Republic) Duration: 4 weeks, follow‐up up to 16 weeks |
|
| Participants | 68 participants enrolled (BtA group = 35; placebo group = 33) % Female: BtA: 46%; placebo: 56% Mean age, SD: BtA: 45.8 years, 13.2; placebo: 49.7 years, 9.6 Mean CD duration, SD: BtA: 6.5 years, 8.0; placebo: 4.8 years, 4.4 Mean CD severity, SD (Tsui scale): BtA: 11.1, 1.7; placebo: 11.5, 1.8 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
BtA: Dysport (abobotulinumtoxinA); 500 U, diluted with 1 mL 0.9% saline solution Placebo: 0.125 mg of human serum albumin and 2.5 mg of lactose, diluted with 1 mL 0.9% saline solution Study drug preparation: BtA and placebo provided in vials by Ipsen Muscles injected: based on clinical assessment 2 or 3 muscles were selected for injection: sternocleidomastoid (100 U‐200 U), splenius capitis (250 U‐350 U), trapezius (100 U‐200 U), and levator scapulae (100 U‐200 U). Each muscle was injected in 2 sites EMG guidance: no BtA dose per participant: 500 U |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Participants were withdrawn from the study if they were considered non‐responders at week 4. Participants with an ongoing response at weeks 4 and 8 continued until re‐treatment was required. Study discontinuations (at week 4), reasons: BtA: n = 0 Placebo: n = 0 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive either placebo or 500 units of Dysport" Comment: insufficient information about the method of randomisation to permit judgement of low risk or high risk |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement of low risk or high risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Blinded study medication was supplied (...) as identical vials containing either Dysport (...) or placebo" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: insufficient information to permit judgement of low or high risk |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: although placebo was identical to intervention, the fact that most of the participants had previously been treated with Bt could have led to a degree of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In order to remove the bias created by the withdrawal of the majority of placebo patients at week 4, a last observation carried forward technique was used for the week 8 analyses" |
| Selective reporting (reporting bias) | Low risk | Comment: the expected outcomes that are usually evaluated in intervention trials for this condition were reported in this study. |
| For‐profit bias | High risk | Quote: "Blinded study medication was supplied by Ipsen Ltd" |
| Enriched population – preferential enrolment of positive responders | High risk | Comment: out of the 68 participants enrolled, 47 had received BtA injections previously |
| Enriched population – exclusion of poor responders | High risk | Quote: "Patients with pure anterocollis were excluded" |
Bt: botulinum toxin BtA: botulinum toxin type A CD: cervical dystonia CDSS: Cervical Dystonia Severity Scale F/U: follow‐up GAS: Global Assessment Scale IGAE: Investigator Global Assessment of Efficacy PEGR: Patient Evaluation of Global Response TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blackie 1990 | Cross‐over design |
| Buchman 1994 | The primary outcome was not clinical; pharmacokinetic study |
| Gelb 1989 | Cross‐over design |
| Koller 1990 | Cross‐over design |
| Lange 1991 | This study has recruited part of the same population from Greene 1990; the primary outcome was not clinical |
| Lorentz 1991 | Cross‐over design |
| Lu 1995 | Cross‐over design |
| Maurri 1990 | Not randomised |
| Moore 1991 | Cross‐over design |
| Ostergaard 1994 | Cross‐over design |
| Perlmutter 1989 | Cross‐over design |
| Relja 1993 | Not randomised |
| Tsui 1986 | Cross‐over design |
| Tsui 1988 | The primary outcome was not clinical |
| Yoshimura 1990 | This study has recruited part of the same population from Gelb 1989 |
Differences between protocol and review
For this updated review the study designs accepted were restricted to parallel‐group studies, and we opted not to exclude based on allocation concealment. No changes were made in the type of participants included or in the interventions allowed.
Adverse events, which were originally a secondary outcome, were included in this updated review as a primary safety outcome. Also, in this safety analysis we considered the proportion of participants with the most frequent adverse events, which was not stated in the original protocol. An assessment of the duration of effect was included as a new secondary outcome measure.
We no longer consider immunogenicity to be an outcome to be studied in this systematic review, as we believe it does not enhance patient's, physician's, or policymaker's ability to make decisions regarding question of this review. At most, it is an inadequate surrogate measure of the risk of developing clinical non‐responsiveness.
We used new approaches to deal with missing data and unit of analysis issues.
We used the latest recommended Cochrane tool for assessing risk of bias in this review, which was expanded to include two additional criteria, added by the review authors. We opted to include the enriched population domains, since a known positive response to botulinum toxin type A and certain disease subtype are known to influence the magnitude of response to the intervention. As has been verified in a recent Cochrane methodology systematic review (Lundh 2017), industry‐sponsored trials display "the existence of an industry bias that cannot be explained by standard 'Risk of bias' assessments". We analysed blinding of outcome assessment in two new subcategories: subjective and objective assessment and also added a ‘Summary of findings' table. The search strategy was prolonged to October 2016.
Trial Sequential Analysis was not in the original review protocol.
Contributions of authors
Austen P Moore ‐ APM; Cristina Sampaio ‐ CS; Filipe Brogueira Rodrigues ‐ FBR; Gonçalo S Duarte ‐ GSD; João Costa ‐ JC; Joaquim Ferreira ‐ JJF; Mafalda Castelão ‐ MC; Raquel E Marques ‐ REM.
Concieving the review ‐ APM, CS, JC, JJF
Designing the review ‐ APM, CS, JC, JJF
Co‐ordinating the review ‐ JC
Designing search strategies – FBR, GSD, JC
Undertaking searches – FBR, GSD
Screening search results – FRB, GSD, MC, REM
Organising retrieval of papers ‐ FRB, GSD, JC, MF, REM
Screening retrieved papers against eligibility criteria ‐ FRB, GSD, MC, REM
Appraising quality of papers ‐ FRB, GSD, MC, REM
Extracting data from papers ‐ FRB, GSD, MC, REM
Writing to authors of papers for additional information – GSD, JC, REM
Data management for the review – FRB, GSD, MC, REM
Entering data into Review Manager 5 ‐ FRB, GSD, MC, REM
Analysis of data ‐ FRB, GSD, MC, REM
Interpretation of data ‐ APM, CS, FRB, GSD, JC, JJF, MC, REM
Writing the review ‐ FRB, GSD, JC, MC, REM
GRADE assessment ‐ GSD, FBR
Providing general advice on the review – APM, CS, JC, JJF
Performing previous work that was the foundation of the current review – Ana Borges, Claudia Espírito Santo, Miguel Coelho.
Sources of support
Internal sources
Cochrane Movement Disorders, Portugal.
The Walton Centre for Neurology and Neurosurgery, UK.
External sources
No sources of support supplied
Declarations of interest
JC, JJF, and CS were investigators in clinical trials in botulinum toxin A and B use in dystonia sponsored by Elan (manufacturer of botulinum toxin type B), Allergan (manufacturer of botulinum toxin type A), and Ipsen (manufacturer of botulinum toxin type A). Searching for studies, selection of studies, data extraction and analysis (including risk of bias), and GRADE assessment were performed by authors (FBR, GSD, MC, REM) who were not trialists. JJF and CS were speakers in symposiums promoted by Elan, Allergan, and Ipsen. APM has received royalties from Ipsen for the use 'LIVEchart' scoring system for botulinum toxin treatment efficacy. He has additionally received consulting fees from Ipsen, Merz (manufacturer of botulinum toxin type A), Eisai (manufacturer of botulinum toxin type B), and Allergan. The same companies have provided for support for travel to meetings for studies or other purposes.
These authors contributed equally to this work.
These authors contributed equally to this work.
These authors contributed equally to this work.
These authors contributed equally to this work.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Charles 2012 {published data only}
- Brashear A, Truong D, Charles D, Comella C, Hauser RA, Tsui J. A randomized double‐blind, placebo‐controlled study of intramuscular Botox for the treatment of cervical dystonia (CD). Movement Disorders 1998;13 Suppl 2:276 (P4.243). [Google Scholar]
- Charles D, Brashear A, Hauser RA, Li HI, Boo LM, Brin MF, CD 140 Study Group. Efficacy, tolerability, and immunogenicity of onabotulinumtoxinA in a randomized, double‐blind, placebo‐controlled trial for cervical dystonia. Clinical Neuropharmacology 2012;35(5):208‐14. [DOI] [PubMed] [Google Scholar]
- Comella C. Botox for cervical dystonia. Toxins'99 (www.wemove.org). 1999:8.
- Hauser RA, Comella C, Brashear A, Truong D, Charles PD, Tsui J. A randomized, multicenter, placebo‐controlled study of original otox (botulinum toxin type A) purified neurotoxin complex for the treatment of cervical dystonia. Movement Disorders 2000;15 Suppl 2:30. [Google Scholar]
Comella 2011 {published data only}
- Comella CL, Jankovic J, Truong DD, Hanschmann A, Grafeon S, on behalf of the U.S. Xeomin Cervical Dystonia Study Group. Efficacy and safety of incobotulinumtoxinA (NT 201, XEOMIN®, botulinumneurotoxin type A, without accessory proteins) in patients with cervical dystonia. Journal of the Neurological Sciences 2011;308(1‐2):103‐9. [DOI: 10.1016/j.jns.2011.05.041.] [DOI] [PubMed] [Google Scholar]
- Coulonn J‐M. Incobotulinumtoxin A (Xeomin) injected with flexible intervals is a well‐tolerated long‐term treatment of cervical dystonia. Annals of Physical and Rehabilitation Medicine 2013;56:e398. [Google Scholar]
- Evidente VG, Brashear A, Comella CL, Fernandez H, Grafe S, LeDoux MS, et al. IncobotulinumtoxinA (Xeomin; botulinumneurotoxin type A, free from accessory proteins): flexibility of dosing and injection intervals in cervical dystonia. PM&R 2011;3(10S1):S215 (P131). [Google Scholar]
- Evidente VG, Truong D, Jankovic J, Comella CL, Grafe S, Hanschmann A. IncobotulinumtoxinA (Xeomin®) injected for blepharospasm or cervical dystonia according to patient needs is well tolerated. Journal of the Neurological Sciences 2014;346(1‐2):116‐20. [DOI: 10.1016/j.jns.2014.08.004; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fernandez H, Truong D, Asmus F, Hanschmann A, LeDoux M. IncobotulinumtoxinA (Xeomin®) remains well tolerated across flexible inter‐injection intervals of 6‐20 weeks in the treatment of cervical dystonia. Neurology 2012;78(Meeting Abstracts 1):P01.219. [Google Scholar]
- Fernandez HH, Evidente VG, Truong D, Brodsky M, Hanschmann A, Comella CL, et al. Long‐term treatment of blepharospasm and cervical dystonia: incobotulinum toxin A is well tolerated when injected at flexible intervals based on patient needs. Journal of the Neurological Sciences 2013;333:109‐51 (P1070). [Google Scholar]
- Fernandez HH, Pappert EJ, Comella CL, Evidente VG, Truong DD, Verma A, et al. Efficacy and safety of incobotulinumtoxinA in subjects previously treated with botulinum toxin versus toxin‐naïve subjects with cervical dystonia. Tremor and Other Hyperkinetic Movements 2013;3:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe S, Comella C, Hanschmann A, Jankovic J, Truong D. Efficacy and safety of NT 201 (botulinum neurotoxin free from complexing proteins) in cervical dystonia. PM&R 2010;2(9S):S29 (P49). [Google Scholar]
- Singer C, Pappert E, Hanschmann A, Fernandez H. IncobotulinumtoxinA (NT 201, Xeomin) administered at flexible intervals of 6‐20 weeks in subjects with cervical dystonia. Movement Disorders 2012;27 Suppl.1:S365 (P1105). [Google Scholar]
- Truong D, Fernandez H, Grafe S. IncobotulinumtoxinA (NT‐201) injections are safe and effective in adult subjects with cervical dystonia across dosing intervals in a repeated dose‐study. Movement Disorders 2011;26 Suppl.2:S211 (P625). [Google Scholar]
Greene 1990 {published data only}
- Greene P, Kang U, Fahn S, Brin M, Moskowitz C, Flaster E. Double‐blind, placebo‐controlled study of botulinum toxin injection for torticollis. Neurology 1988;38 Suppl 1:244. [DOI] [PubMed] [Google Scholar]
- Greene P, Kang U, Fahn S, Brin M, Moskowitz C, Flaster E. Double‐blind, placebo‐controlled trial of botulinum toxin injections for the treatment of spasmodic torticollis. Neurology 1990;40(8):1213‐8. [DOI] [PubMed] [Google Scholar]
Poewe 1998 {published data only}
- Poewe W. Dysport for Cervical Dystonia. Toxins'99 (www.wemove.org). 1999:10.
- Poewe W, Brans J, Kessler K, Kanovsky P, Odergren T, Aramideh M, et al. Dysport for cervical dystonia. Movement Disorders 2000;15 Suppl 2:7. [Google Scholar]
- Poewe W, Deuschl G, Nebe A, Feifel E, Wissel J, Benecke R, et al. What is the optimal dose of botulinum toxin A in the treatment of cervical dystonia? Results of a double blind, placebo controlled, dose ranging study using Dysport. German Dystonia Study Group. Journal of Neurology, Neurosurgery and Psychiatry 1998;64(1):13‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poewe 2016 {published data only}
Truong 2005 {published data only}
- Coleman CM, Chang SF, Copley‐Merriman C, Hubble J, Masaquel C. Health‐related quality of life improvements with dysport in cervical dystonia. PM&R 2011;3 Suppl.1(10):S190 (P41). [Google Scholar]
- Hauser RA, Truong D, Hubble J, Coleman C, Beffy JL, Chang S, Picaut P. AbobotulinumtoxinA (Dysport) dosing in cervical dystonia: an exploratory analysis of two large open‐label extension studies. Journal of Neural Transmission 2013;120(2):299‐307. [DOI: 10.1007/s00702-012-0872-1; PUBMED: 22878514] [DOI] [PubMed] [Google Scholar]
- Jen M, Kurth H, Iheanacho I, Dinet J, Gabriel S, Wasiak R, et al. Improvement of SF‐36 scores in cervical dystonia patients ‐ is there a treatment effect when evaluating subscales?. Basal Ganglia 2014;126:1‐6. [Google Scholar]
- Truong D, Duane DD, Jankovic J, Singer C, Seeberger LC, Comella CL, et al. Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: results of the first US randomized, double‐blind, placebo‐controlled study. Movement Disorders 2005;20(7):783‐91. [DOI] [PubMed] [Google Scholar]
- Truong DD, Duane DD, Jankovic J, Singer C, Comella CL. The efficacy and safety of Dysport (botulinumtype A toxin) in cervical dystonia: results of the first US study. Movement Disorders 2002;17 Suppl 5:S306 (P1007). [DOI] [PubMed] [Google Scholar]
Truong 2010 {published data only}
- Mordin M, Masaquel C, Abbott C, Copley‐Merriman C. Factors affecting the health‐related quality of life of patients with cervical dystonia and impact of treatment with abobotulinumtoxinA (Dysport): results from a randomised, double‐blind, placebo‐controlled study . BMJ Open 2014;4(10):e005150. [DOI: 10.1136/bmjopen-2014-005150; PUBMED: 25324317] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong D, Brodsky M, Lew M, Brashear A, Jankovic J, Molho E, et al. Global Dysport Cervical Dystonia Study Group. Long‐term efficacy and safety of botulinum toxin type A (Dysport) in cervical dystonia. Parkinsonism & Related Disorders 2010;16(5):316‐23. [DOI: 10.1016/j.parkreldis.2010.03.002; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wissel 2001 {published data only}
- Kanovsky P, Ruzicka E, Jech R, Roth J, Schnider P, Auff E, et al. Standardized dose of 500 Mu of Dysport for cervical dystonia: results of a prospective, randomized, double‐blind, placebo‐controlled study. Movement Disorders. 2000; Vol. 15 Suppl 2:31.
- Poewe W. Dysport for cervical cystonia. Toxins'99 (www.wemove.org). 1999:10.
- Poewe W, Brans J, Kessler K, Kanovsky P, Odergren T, Aramideh M, et al. Dysport for cervical dystonia. Movement Disorders. 2000; Vol. 15 Suppl 2:7.
- Wissel J, Kanovsky P, Ruzicka E, Bares M, Hortova H, Streitova H, et al. Efficacy and safety of a standardised 500 unit dose of Dysport (clostridium botulinum toxin type A haemaglutinin complex) in a heterogeneous cervical dystonia population: results of a prospective, multicentre, randomised, double‐blind, placebo‐controlled, parallel group study. Journal of Neurology 2001;248 (12):1073‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Blackie 1990 {published data only}
- Blackie JD, Lees AJ. Botulinum toxin treatment in spasmodic torticollis. Journal of Neurology, Neurosurgery and Psychiatry 1990;53 (8):640‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjanski N, Blackie J, Purves A, Hambleton P, Lees AJ. Botulinus toxin in the treatment of spasmodic torticollis long‐term results. Journal of Neurology. 2nd Congress of European Neurological Society, 1990.
Buchman 1994 {published data only}
- Buchman AS, Comella CL, Stebbins GT, Weinstein SL. Determining a dose‐ effect curve for botulinum toxin in the sternocleidomastoid muscle in cervical dystonia. Clinical Neuropharmacology 1994;17(2):188‐95. [Google Scholar]
Gelb 1989 {published data only}
- Gelb DJ, Arbor A, Don M, Yoshimura RKO, Lowenstein DH, Aminoff MJ. Change in pattern of muscle activity following botulinum toxin injections for torticollis. Neurology 1989;39 Suppl 1:320. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Lowenstein DH, Aminoff MJ. Botulinum toxin in the treatment of spasmodic torticollis. Neurology. 1988; Vol. 38 Suppl 2:244. [DOI] [PubMed]
- Gelb DJ, Lowenstein DH, Aminoff MJ. Controlled trial of botulinum toxin injections in the treatment of spasmodic torticollis. Neurology 1989;39 (1):80‐4. [DOI] [PubMed] [Google Scholar]
Koller 1990 {published data only}
- Koller W, Vetere‐Overfield B, Gray C, Dubinsky R. Failure of fixed‐dose, fixed muscle injection of botulinum toxin in torticollis. Clinical Neuropharmacology 1990;13 (4):355‐8. [DOI] [PubMed] [Google Scholar]
Lange 1991 {published data only}
- Lange DJ, Rubin M, Greene PE, Kang J, Moskowitz CB, Brin MF, et al. Distant effects of locally injected botulinum toxin: a double‐blind study of single fiber EMG changes. Muscle and Nerve 1991;14:672‐5. [DOI] [PubMed] [Google Scholar]
Lorentz 1991 {published data only}
- Lorentz IT, Subramaniam SS, Yiannikas C. Treatment of idiopathic spasmodic torticollis with botulinum toxin A: a double‐blind study on twenty‐three patients. Movement Disorders 1991;6 (2):145‐50. [DOI] [PubMed] [Google Scholar]
Lu 1995 {published data only}
- Lu CS, Chen RS, Tsai CH. Double‐blind, placebo‐controlled study of botulinum toxin injections in the treatment of cervical dystonia. Journal of the Formosan Medical Association 1995;94 (4):189‐92. [PubMed] [Google Scholar]
Maurri 1990 {published data only}
- Maurri S, Brogelli S. Botulinum toxin therapy for blepharospasm, hemifacial spasm and spasmodic torticollis: clinical studies with a follow‐up of 40 months. Movement Disorders 1990;5 Suppl 1:73 (P262). [Google Scholar]
Moore 1991 {published data only}
- Moore AP, Blumhardt LD. A double blind trial of botulinum toxin "A" in torticollis, with one year follow up. Journal of Neurology, Neurosurgery and Psychiatry 1991;54(9):813‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AP, Blumhardt LD. Double blind study of botulinum toxin A in torticollis. Journal of Neurology 1991;54(9):813‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostergaard 1994 {published data only}
- Ostergaard L, Fuglsang‐Frederiksen A, Werdelin L, Sjo O, Winkel H. Quantitative EMG in botulinum toxin treatment of cervical dystonia. A double‐blind, placebo‐controlled study. Electroencephalography and Clinical Neurophysiology 1994;93(6):434‐9. [DOI] [PubMed] [Google Scholar]
Perlmutter 1989 {published data only}
- Perlmutter JS, Tempel LW, Burde R. Double‐blind, placebo‐controlled, crossover trial of botulinum‐A toxin for torticollis. Neurology 1989;39 Suppl 1:352. [Google Scholar]
Relja 1993 {published data only}
- Relja M, Petravic D. Botulinum toxin type A injections for cervical dystonia: a double‐blind, placebo controlled study. The Canadian Journal of Neurological Sciences 1993;20 Suppl 4:S185 (6‐17‐01). [Google Scholar]
Tsui 1986 {published data only}
- Tsui JK, Eisen A, Stoessl AJ, Calne S, Calne DB. Double‐blind study of botulinum toxin in spasmodic torticollis. Lancet 1996;2 (2):245‐7. [DOI] [PubMed] [Google Scholar]
Tsui 1988 {published data only}
- Tsui J, Eisen A, Calne DB. Botulinum toxin in spasmodic torticollis. Advances in Neurology. Stanley Fahn et al. Vol. 50: Dystonia 2, New York: Raven Press, 1988:593‐7. [PubMed] [Google Scholar]
Yoshimura 1990 {published data only}
- Yoshimura D, Aminoff M, Gelb D. Long term follow‐up of patients treated with botulinum toxin for spasmodic torticollis. Movement Disorders 1990;5 (Suppl 1):75 (P268). [Google Scholar]
Additional references
Abrams 2005
- Abrams KR, Gillies CL, Lambert PC. Meta‐analysis of heterogeneously reported trials assessing change from baseline. Statistics in Medicine 2005;24:3823‐44. [DOI] [PubMed] [Google Scholar]
Albanese 2011
- Albanese A, Asmus F, Bhatia KP, Elia AE, Elibol B, Filippini G, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. European Journal of Neurology 2011;18:5‐18. [DOI] [PubMed] [Google Scholar]
Albanese 2013
- Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Movement Disorders 2013;28(7):863‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 2002
- Altman DG, Deeks JJ. Meta‐analysis, Simpson's paradox, and the number needed to treat. BMC Medical Research Methodology 2002;2(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Antonucci 2008
- Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long‐distance retrograde effects of botulinum neurotoxin. Journal of Neuroscience 2008;28:3689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balint 2015
- Balint B, Bhatia KP. Isolated and combined dystonia syndromes ‐ an update on new genes and their phenotypes. European Journal of Neurology 2015;22(4):610‐7. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Boroff 1975
- Boroff DA, Chen GS. On the question of permeability of the blood–brain barrier to botulinum toxin. International Archives of Allergy and Applied Immunology 1975;48:495–504. [DOI] [PubMed] [Google Scholar]
Brożek 2006
- Brożek JL, Guyatt GH, Schünemann HJ. How a well‐grounded minimal important difference can enhance transparency of labelling claims and improve interpretation of a patient reported outcome measure. Health and Quality of Life Outcomes 2006;4:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 2013
- Carpenter J, Kenward M. Multiple Imputation and its Application. Wiley, 2013. [Google Scholar]
Chan 1991
- Chan J, Brin MF, Fanh S. Idiopathic cervical dystonia: clinical characteristics. Movement Disorders 1991;6:119‐26. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc , 1988. [Google Scholar]
Comella 2015
- Comella CL, Fox SH, Bhatia KP, Perlmutter JS, Jinnah HA, Zurowski M, et al. Development of the comprehensive cervical dystonia rating scale: methodology. Movement Disorders 2015;2(2):135‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corbett 2014
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5(1):79‐85. [DOI] [PubMed] [Google Scholar]
de Paiva 1999
- Paiva A, Meunier FA, Molgó J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proceedings of the National Academy of Sciences of the United States of America 1999;96:3200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Defazio 2013
- Defazio G, Jankovic J, Giel JL, Papapetropoulos S. Descriptive epidemiology of cervical dystonia. Tremor and Other Hyperkinetic Movements 2013;3:tre‐03‐193‐4374‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duarte 2016
- Duarte GS, Castelão M, Rodrigues FB, Marques RE, Ferreira J, Sampaio C, et al. Botulinum toxin type A versus botulinum toxin type B for cervical dystonia. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD004314.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duchen 1971
- Duchen LW. An electron microscopic study of the changes induced by botulinum toxin in the motor end‐plates of slow and fast skeletal muscle fibres of the mouse. Journal of the Neurological Sciences 1971;14:47‐60. [DOI] [PubMed] [Google Scholar]
Edwards 2000
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356(9237):1255‐9. [DOI] [PubMed] [Google Scholar]
ESDE 2000
- Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group. A prevalence study of primary dystonia in eight European countries. Journal of Neurology 2000;247:787‐92. [DOI] [PubMed] [Google Scholar]
Filippi 1993
- Filippi GM, Errico P, Santarelli R, Bagolini B, Manni E. Botulinum A toxin effects on rat jaw muscle spindles. Acta Oto‐laryngologica 1993;113:400–4. [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769‐73. [DOI] [PubMed] [Google Scholar]
Foltz 1959
- Foltz EL, Knopp LM, Ward AA. Experimental spasmodic torticollis. Journal of Neurosurgery 1959;16:55‐72. [DOI] [PubMed] [Google Scholar]
Frevert 2010
- Frevert J, Dressler D. Complexing proteins in botulinum toxin type A drugs: a help or a hindrance?. Biologics: Targets & Therapy 2010;4:325‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version assessed 3 January 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
Hallett 1998
- Hallett M. The neurophysiology of dystonia. Archives of Neurology 1998;55(5):601‐3. [DOI] [PubMed] [Google Scholar]
Hedges 1985
- Hedges LV, Olkin I. Statistical Methods for Meta‐Analysis. San Diego, California: Academic Press, Inc, 1985. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holland 1981
- Holland RL, Brown MC. Nerve growth in botulinum toxin poisoned muscles. Neuroscience 1981;6:1167–79. [DOI] [PubMed] [Google Scholar]
Imberger 2016
- Imberger G, Thorlund K, Gluud C, Wetterslev J. False‐positive findings in Cochrane meta‐analyses with and without application of Trial Sequential Analysis: an empirical review. BMJ Open 2016;6(8):e011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jahnanshani 1990
- Jahnanshani M, Marion M‐H, Marsden CD. Natural history of adult‐onset idiopathic torticollis. Archives of Neurology 1990;47:548‐52. [DOI] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jankovic 2004
- Jankovic J. Botulinum toxin in clinical practice. Journal of Neurology, Neurosurgery, and Psychiatry 2004;75:951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jankovic 2006
- Jankovic J, Tsui J, Bergeron C. Prevalence of cervical dystonia and spasmodic torticollis in the United States general population. Parkinsonism & Related Disorders 2007;13(7):411–6. [DOI] [PubMed] [Google Scholar]
Juzans 1996
- Juzans P, Comella J, Molgo J, Faille L, Angaut‐Petit D. Nerve terminal sprouting in botulinum type‐A treated mouse levator auris longus muscle. Neuromuscular Disorders : NMD 1996;6(3):177‐85. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macefield 2014
- Macefield RC, Jacobs M, Korfage IJ, Nicklin J, Whistance RN, Brookes ST, et al. Developing core outcomes sets: methods for identifying and including patient‐reported outcomes (PROs). Trials 2014;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marques 2016
- Marques RE, Duarte GS, Rodrigues FB, Castelão M, Ferreira J, Sampaio C, et al. Botulinum toxin type B for cervical dystonia. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD004315.pub3; PUBMED: 27176573] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matak 2014
- Matak I, Lacković Z. Botulinum toxin A, brain and pain. Progress in Neurobiology 2014;119‐120:39–59. [DOI] [PubMed] [Google Scholar]
Matak 2015
- Matak I, Lacković Z. Botulinum neurotoxin type A: actions beyond SNAP‐25?. Toxicology 2015;335:79‐84. [DOI] [PubMed] [Google Scholar]
O'Brien 1979
- O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549‐56. [PubMed] [Google Scholar]
Palomar 2012
- Palomar FJ, Mir P. Neurophysiological changes after intramuscular injection of botulinum toxin. Clinical Neurophysiology 2012;123(23):54–60. [DOI] [PubMed] [Google Scholar]
Pellizzari 1999
- Pellizzari R, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 1999;354:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters 2006
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta‐analysis. JAMA 2006;295(6):676‐80. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Centre, The Cochrane Collaboration, 2014.
Rosales 1996
- Rosales RL, Arimura K, Takenaka S, Osame M. Extrafusal and intrafusal muscle effects in experimental botulinum toxin‐A injection. Muscle & Nerve 1996;19:488–96. [DOI] [PubMed] [Google Scholar]
Rosales 2010
- Rosales RL, Dressler D. On muscle spindles, dystonia and botulinum toxin. European Journal of Neurology 2010;17:71–80. [DOI] [PubMed] [Google Scholar]
Rubin 1991
- Rubin DB, Schenker N. Multiple imputation in health‐care databases: an overview and some applications. Statistics in Medicine 1991;10:585–98. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Simpson 2004
- Simpson LL. Identification of the major steps in botulinum toxin action. Annual Review of Pharmacology and Toxicology 2004;44:167‐93. [DOI] [PubMed] [Google Scholar]
Simpson 2016
- Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016;10:1818‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smeeth 1999
- Smeeth L, Haines A, Ebrahim S. Numbers needed to treat derived from meta‐analysis ‐ sometimes informative, usually misleading. BMJ 1999;318(7197):1548‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stata 2015 [Computer program]
- StataCorp. Stata Statistical Software: release 14. Version accessed 3 January 2017. StataCorp, 2015.
Steeves 2012
- Steeves TD, Day L, Dykeman J, Jette N, Pringsheim T. The prevalence of primary dystonia: a systematic review and meta‐analysis. Movement Disorders 2012;27(14):1789‐96. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046‐55. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tarsy 1997
- Tarsy D. Comparison of clinical rating scales in treatment of cervical dystonia with botulinum toxin. Movement Disorders 1997;12(1):100‐2. [DOI] [PubMed] [Google Scholar]
Tarsy 2006
- Tarsy D, Simon DK. Dystonia. New England Journal of Medicine 2006;355:818‐29. [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa ˙manual.pdf 2011.
TSA 2011 [Computer program]
- Copenhagen Trial Unit. Trial Sequential Analysis. Version 0.9 Beta. Version accessed 3 January 2017. Copenhagen: Copenhagen Trial Unit, 2011.
Tugwell 2007
- Tugwell P, Boers M, Brooks P, Simon L, Strand V, Idzerda L. OMERACT: an international initiative to improve outcome measurement in rheumatology. Trials 2007;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2014
- Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. Journal of Clinical and Aesthetic Dermatology 2014;7(2):31‐9. [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiebe 2006
- Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ. A systematic review identifies a lack of standardization in methods for handling missing variance data. Journal of Clinical Epidemiology 2006;59(4):342‐53. [DOI] [PubMed] [Google Scholar]
Zoons 2012
- Zoons E, Dijkgraaf MG, Dijk JM, Schaik IN, Tijssen MA. Botulinum toxin as treatment for focal dystonia: a systematic review of the pharmaco‐therapeutic and pharmaco‐economic value. Journal of Neurology 2012;259(12):2519‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Costa 2000
- Costa J, Ferreira JJ, Sampaio C. Botulinum toxin type A for the treatment of cervical dystonia: a systematic review. Movement Disorders 2000;15 Suppl 3:29. [Google Scholar]
Costa 2005
- Costa J, Espírito‐Santo C, Borges A, Ferreira JJ, Coelho M, Moore P, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD003633.pub2] [DOI] [PubMed] [Google Scholar]
Ferreira 1999
- Ferreira JJ, Costa J, Sampaio C. Botulinum toxin type A for the treatment of cervical dystonia: a systematic review. European Journal of Neurology 1999;6 Suppl 3:76. [Google Scholar]


