Abstract
Background
Healthcare professionals, including nurses, frequently advise people to improve their health by stopping smoking. Such advice may be brief, or part of more intensive interventions.
Objectives
To determine the effectiveness of nursing‐delivered smoking cessation interventions in adults. To establish whether nursing‐delivered smoking cessation interventions are more effective than no intervention; are more effective if the intervention is more intensive; differ in effectiveness with health state and setting of the participants; are more effective if they include follow‐ups; are more effective if they include aids that demonstrate the pathophysiological effect of smoking.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialized Register and CINAHL in January 2017.
Selection criteria
Randomized trials of smoking cessation interventions delivered by nurses or health visitors with follow‐up of at least six months.
Data collection and analysis
Two review authors extracted data independently. The main outcome measure was abstinence from smoking after at least six months of follow‐up. We used the most rigorous definition of abstinence for each trial, and biochemically‐validated rates if available. Where statistically and clinically appropriate, we pooled studies using a Mantel‐Haenszel fixed‐effect model and reported the outcome as a risk ratio (RR) with a 95% confidence interval (CI).
Main results
Fifty‐eight studies met the inclusion criteria, nine of which are new for this update. Pooling 44 studies (over 20,000 participants) comparing a nursing intervention to a control or to usual care, we found the intervention increased the likelihood of quitting (RR 1.29, 95% CI 1.21 to 1.38); however, statistical heterogeneity was moderate (I2 = 50%) and not explained by subgroup analysis. Because of this, we judged the quality of evidence to be moderate. Despite most studies being at unclear risk of bias in at least one domain, we did not downgrade the quality of evidence further, as restricting the main analysis to only those studies at low risk of bias did not significantly alter the effect estimate. Subgroup analyses found no evidence that high‐intensity interventions, interventions with additional follow‐up or interventions including aids that demonstrate the pathophysiological effect of smoking are more effective than lower intensity interventions, or interventions without additional follow‐up or aids. There was no evidence that the effect of support differed by patient group or across healthcare settings.
Authors' conclusions
There is moderate quality evidence that behavioural support to motivate and sustain smoking cessation delivered by nurses can lead to a modest increase in the number of people who achieve prolonged abstinence. There is insufficient evidence to assess whether more intensive interventions, those incorporating additional follow‐up, or those incorporating pathophysiological feedback are more effective than one‐off support. There was no evidence that the effect of support differed by patient group or across healthcare settings.
Plain language summary
Does support and intervention from nurses help people to stop smoking?
Background
Most smokers want to quit, and may be helped by advice and support from healthcare professionals. Nurses are the largest healthcare workforce, and are involved in virtually all levels of health care. The main aim of this review was to determine if nursing‐delivered interventions can help adult smokers to stop smoking.
Study characteristics
This review of clinical trials covered 58 studies in which nurses delivered a stop‐smoking intervention to smokers. More than 20,000 participants were included in the main analysis, including hospitalized adults and adults in the general community. The most recent search was conducted in January 2017. All studies reported whether or not participants had quit smoking at six months or longer.
Key Results
This review found moderate‐quality evidence that advice and support from nurses could increase people's success in quitting smoking, whether in hospitals or in community settings. Eleven studies compared different nurse‐delivered interventions and did not find that adding more components changed the effect.
Quality of evidence
The quality of evidence was moderate, meaning that further research may change our confidence in the result. This is because results were not consistent across all of the studies, and in some cases there were not very many studies contributing to comparisons.
Summary of findings
Summary of findings for the main comparison. Nursing interventions for smoking cessation.
| Nursing interventions for smoking cessation | ||||||
| Patient or population: Adult smokers Settings: Any Intervention: Cessation interventions delivered by nurses | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Nursing interventions | |||||
| Smoking cessation at longest follow‐up (high and low intensity) Follow‐up: 6+ months | 122 per 10001 | 157 per 1000 (147 to 168) | RR 1.29 (1.21 to 1.38) | 20,881 (44 studies) | ⊕⊕⊕⊝ moderate2, 3 | Pooled results from the two subgroups described below |
| Smoking cessation at longest follow‐up ‐ High intensity intervention Follow‐up: 6+ months | 141 per 10001 | 182 per 1000 (171 to 195) | RR 1.29 (1.21 to 1.38) | 16,865 (37 studies) | ⊕⊕⊕⊝ moderate2, 3 | High intensity = initial contact > 10 minutes, additional materials (e.g. manuals) and/or strategies other than simple leaflets, additional follow‐up visits |
| Smoking cessation at longest follow‐up ‐ Low intensity intervention Follow‐up: 6+ months | 51 per 10001 | 64 per 1000 (50 to 82) | RR 1.27 (0.99 to 1.62) | 4016 (7 studies) | ⊕⊕⊕⊝ moderate2, 4 | Low intensity = advice provided (with or without a leaflet) during single consultation lasting 10 minutes or less with up to one follow‐up visit |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Control group quit rate based on average across all included studies. 2Not downgraded for risk of bias: Sensitivity analyses excluding studies at high or unclear risk of bias did not significantly alter the effect size. 3Downgraded one level for inconsistency: Unexplained statistical heterogeneity present. 4Downgraded one level for imprecision: Total number of events < 300, confidence intervals include a significant effect and no effect.
Background
Tobacco‐related deaths and disabilities are on the increase worldwide because of continued use of tobacco (mainly cigarettes). Tobacco use has reached epidemic proportions in many low‐ and middle‐income countries, while steady use continues in high‐income nations like the USA (The Tobacco Atlas 2015; CDC 2016). According to the Centers for Disease Control, 68% of adult smokers in the USA want to quit and millions have tried (CDC 2017), with 70% of smokers visiting a healthcare professional each year (AHRQ 2008). Nurses, representing the largest number of healthcare providers worldwide, are involved in most of these visits, and therefore have the potential for a profound effect on the reduction of tobacco use (Youdan 2005).
Systematic reviews (e.g. Stead 2013) have confirmed the effectiveness of advice from physicians to stop smoking. The Agency for Health Care Research and Quality Clinical Practice Guideline (AHRQ 2008) lists nurses as one of the many providers from whom advice to stop smoking could increase quit rates, but identifies the effectiveness of advice to quit smoking given by clinicians other than physicians (including nurses) as an area requiring further research. The American Nurses Association (ANA 2012) state that nurses have tremendous potential to implement smoking cessation interventions effectively and advance tobacco use reduction goals proposed by Healthy People 2020, and note that nurses must be equipped to assist with smoking cessation, to prevent tobacco use, and to promote strategies to decrease exposure to second‐hand smoke. The American Nurses Association/American Nurses Foundation promotes the mission of Tobacco‐Free Nurses to the nation’s registered nurses through its constituent associations, members, and organizational affiliates (ANA 2012).
A review of nursing's specific role in smoking cessation is essential if the profession is to endorse the International Council of Nurses' (ICN) call to encourage nurses to "...integrate tobacco use prevention and cessation ... as part of their regular nursing practice" (ICN 2012).
The aim of this review is to examine and summarize randomized controlled trials where nurses provided smoking cessation interventions. The review therefore focuses on the nurse as the intervention provider, rather than on a particular type of intervention. We do not include smoking cessation interventions targeting pregnant women, because of the particular circumstances and motivations among this population. Interventions for pregnant smokers have been reviewed elsewhere (Chamberlain 2017; Coleman 2015).
Objectives
To determine the effectiveness of nursing‐delivered interventions on smoking behavior in adults. To establish whether nursing‐delivered smoking cessation interventions: (i) are more effective than no intervention; (ii) are more effective if the intervention is more intensive; (iii) differ in effectiveness with health state and setting of the participants; (iv) are more effective if they include follow‐ups; (v) are more effective if they include aids that demonstrate the pathophysiological effect of smoking.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria for studies were: (i) they had to have at least two treatment groups; (ii) allocation to treatment groups must have been stated to be 'random'. We excluded studies that used historical controls.
Types of participants
Participants were adult smokers, 18 years and older, of either gender and recruited in any type of healthcare or other setting. The only exceptions were studies that had exclusively recruited pregnant women. We included trials in which 'recent quitters' were classified as smokers, but conducted sensitivity analyses to determine whether they differed from trials that excluded such individuals.
Types of interventions
We define 'nursing intervention' as the provision of advice, counseling, and/or strategies to help people quit smoking. The review includes cessation studies that compared usual care with an intervention, brief advice with a more intensive smoking cessation intervention or different types of interventions. We included studies of smoking cessation interventions as a part of multifactorial lifestyle counseling or rehabilitation only if it was possible to discern the specific nature and timing of the intervention, and to extract data on the outcomes for those who were smokers at baseline. We define 'advice' as verbal instructions from the nurse to stop smoking, whether or not they provided information about the harmful effects of smoking. We grouped interventions into low and high intensity for comparison. We categorize as 'low intensity' those trials where advice was provided (with or without a leaflet) during a single consultation lasting 10 minutes or less, with up to one follow‐up visit. We categorize as 'high intensity' those trials where the initial contact lasted more than 10 minutes, there were additional materials (e.g. manuals) or strategies or both, other than simple leaflets, and usually participants had more than one follow‐up contact. We excluded studies where participants were randomized to receive advice versus advice plus some form of pharmacotherapy, since these were primarily comparisons of the effectiveness of pharmacotherapies rather than nursing interventions. These are covered in separate reviews (Cahill 2016; Hughes 2014; Stead 2012).
Types of outcome measures
The primary outcome was smoking cessation rather than a reduction in withdrawal symptoms or a reduction in the number of cigarettes smoked. Trials had to report follow‐up of at least six months for inclusion in the review. We excluded trials which did not include data on smoking cessation rates. We used the strictest available criteria to define abstinence in each study, e.g. sustained cessation rather than point prevalence. Where biochemical validation was used, we regarded only participants meeting the biochemical criteria for cessation as abstainers. We counted participants lost to follow‐up as continuing smokers (in intention‐to‐treat analyses).
Search methods for identification of studies
We searched the Tobacco Addiction Review Group Specialized Register for trials (most recent search 10 January 2017). This Register includes trials located from systematic searches of electronic databases and handsearching of specialist journals, conference proceedings, and reference lists of previous trials and overviews. At the time of the search the Register included the results of searches of:
Cochrane Central Register of Controlled trials (CENTRAL), in the Cochrane Library 2016, Issue 11;
MEDLINE (via OVID) to update 20161202;
Embase (via OVID) to week 201650;
PsycINFO (via OVID) to update 20160926.
See the Tobaco Addiction Group module in the Cochrane Library for full search strategies and a list of other resources searched. We checked all trials with 'nurse*' or 'nursing' or 'health visitor' in the title, abstract, or keywords for relevance. See Appendix 1 for the search strategy. We also searched the Cumulative Index to Nursing and Allied Health Literature (CINAHL) on OVID for 'nursing' and 'smoking cessation' from 1983 to January 2017.
Data collection and analysis
Selection of studies
For this update, two review authors independently screened titles and abstracts. Where there was uncertainty, we requested the full text. Two review authors checked the full text of articles flagged for inclusion, with discrepancies resolved by discussion or by referral to a third review author.
Data extraction and management
Two review authors independently extracted data from the published reports, contacting study authors where necessary, and resolving disagreements by referral to a third person. For each trial, we extracted the following data:
(i) Author(s) and year; (ii) Country of origin, study setting, and design; (iii) Number and characteristics of participants and definition of 'smoker'; (iv) Description of the intervention and designation of its intensity (high or low); (v) Outcomes and biochemical validation.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' tool to assess bias in four domains:
random sequence generation (a potential source of selection bias);
allocation concealment (also a potential source of selection bias);
incomplete outcome data (attrition bias);
other biases.
We did not judge the trials on the basis of blinding, as we tested behavioral interventions where blinding of participants and providers is not possible.
We judged each included study to be at high, unclear, or low risk of bias in each of the above domains, according to the guidelines in the Cochrane Handbook.
Measures of treatment effect
We use the risk ratio (RR) for summarizing individual trial outcomes and for the estimate of the pooled effect. Where we judged a group of studies to be sufficiently clinically and statistically homogeneous, we used the Mantel‐Haenszel fixed‐effect method (Greenland 1985) to calculate a weighted average of the risk ratios of the individual trials, with a 95% confidence interval.
Dealing with missing data
In trials where the details of the methodology were unclear or where the results were expressed in a form that did not allow for extraction of key data, we approached the original investigators for additional information. We treated participants lost to follow‐up as continuing smokers. We excluded from totals only those participants who died before follow‐up or were known to have moved to an untraceable address.
Assessment of heterogeneity
To assess statistical heterogeneity between trials we used the I2 statistic (Higgins 2003). This measures the percentage of total variation across studies due to heterogeneity rather than to chance. Values of I2 over 75% indicate a considerable level of heterogeneity (Chapter 8, Cochrane Handbook).
'Summary of findings' table
Following standard Cochrane methodology, we created a 'Summary of findings' table for our primary outcome, smoking cessation at longest follow‐up. This includes a GRADE evaluation of the quality of evidence, based on the five standard considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias).
Results
Description of studies
Included studies
Fifty‐eight trials met the inclusion criteria, of which nine are new for this update (Kim 2003; Jorstad 2013; Berndt 2014; Hornnes 2014; Gilbody 2015; Kadda 2015; Pardavila‐Belio 2015; Zwar 2015; Smit 2016). Trials were of nursing interventions for smoking cessation in adults who used tobacco (primarily cigarettes), published between 1987 and 2017. One trial (Sanders 1989a; Sanders 1989b) had two parts with randomization at each stage, so is treated here as two separate studies, making a total of 59 studies in the Characteristics of included studies table. Forty‐four studies contributed to the primary meta‐analysis that compared a nursing intervention to a usual‐care or minimal‐intervention control. Eleven studies included a comparison between two nursing interventions, involving different components or different numbers of contacts, and contribute to a secondary meta‐analysis. Six further studies did not contribute to a meta‐analysis and their results are described separately. Sample sizes of studies contributing to a meta‐analysis ranged from 25 to 2700, but were typically between 150 and 500. Figure 1 documents the flow of studies screened and included in this update.
1.
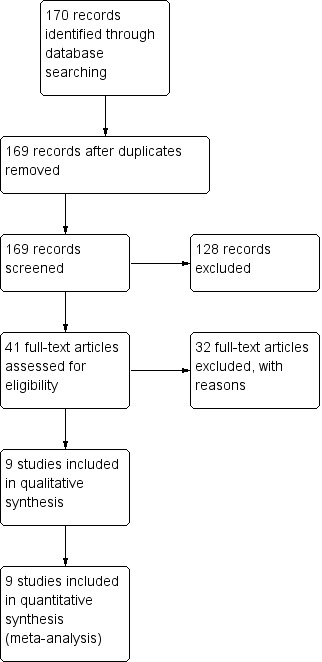
Study flow diagram for 2017 update
Seventeen trials took place in the USA, 11 in the UK, five in The Netherlands, four in Canada, three in Australia, Denmark, and Spain, and two each in China, Japan, Norway, and South Korea. One trial took place in Belgium, one in Greece, and one in Sweden. One multicenter study was conducted in multiple European countries.
Twenty‐two trials intervened with hospitalized participants (Taylor 1990; DeBusk 1994; Rigotti 1994; Lewis 1998; Allen 1996; Carlsson 1997; Miller 1997; Feeney 2001; Bolman 2002; Hajek 2002; Quist‐Paulsen 2003; Froelicher 2004; Hasuo 2004; Chouinard 2005; Hennrikus 2005; Nagle 2005; Hanssen 2007; Wood 2008; Meysman 2010; Cossette 2011; Berndt 2014; Hornnes 2014). Two trials (Rice 1994; Jorstad 2013) recruited hospitalized participants, but with follow‐up after discharge. Kadda 2015 recruited participants following discharge after open‐heart surgery. Twenty‐eight studies recruited from primary care or outpatient clinics (Sanders 1989b; Janz 1987; Vetter 1990; Sanders 1989a; Risser 1990; Hollis 1993; Nebot 1992; Family Heart 1994; OXCHECK 1994; Tønnesen 1996; Campbell 1998; Lancaster 1999; Steptoe 1999; Canga 2000; Aveyard 2003; Kim 2003; Ratner 2004; Hilberink 2005; Kim 2005; Duffy 2006; Sanz‐Pozo 2006; Tønnesen 2006; Aveyard 2007; Jiang 2007; Wood 2008; Chan 2012; Gilbody 2015; Zwar 2015; Smit 2016). In some trials, the recruitment took place during a clinic visit, whilst in others the invitation to enroll was made by letter. One study (Terazawa 2001) recruited employees during a workplace health check, two studies enrolled community‐based adults motivated to make a quit attempt (Davies 1992; Alterman 2001), one study recruited mothers taking their child to a pediatric clinic (Curry 2003), one study recruited people being visited by a home healthcare nurse (Borrelli 2005), and one study recruited university students on campus (Pardavila‐Belio 2015).
Eighteen studies focused on adults with diagnosed cardiovascular health problems (Taylor 1990; DeBusk 1994; Family Heart 1994; Rice 1994; Rigotti 1994; Allen 1996; Carlsson 1997; Miller 1997; Campbell 1998; Feeney 2001; Bolman 2002; Hajek 2002; Jiang 2007; Chan 2012 (subgroup with cardiovascular disease); Cossette 2011; Jorstad 2013; Berndt 2014; Hornnes 2014; Kadda 2015), two studies were in participants with respiratory diseases (Tønnesen 1996; Tønnesen 2006), one was in people with diabetes (Canga 2000), and one was in people with severe mental illness (Gilbody 2015). One study recruited participants either with diagnosed cardiovascular health problems or judged to be at high risk of developing heart disease (Wood 2008). Two studies recruited surgical patients: Ratner 2004 recruited people attending a surgical pre‐admission clinic and Meysman 2010 recruited people admitted to surgical wards. One study recruited head‐and‐neck‐cancer patients at four medical centres (Duffy 2006).
All studies included adults 18 years and older who used some form of tobacco. Allen 1996, Curry 2003 and Froelicher 2004 studied women only, and Terazawa 2001 and Kim 2003 studied men only. The definition of tobacco use varied and in some cases included recent quitters.
Nine studies examined a smoking cessation intervention as a component of multiple risk factor reduction interventions in adults with cardiovascular disease (DeBusk 1994; Allen 1996; Carlsson 1997; Campbell 1998; Hanssen 2007; Jiang 2007; Wood 2008; Jorstad 2013; Kadda 2015). In four studies, the smoking cessation component was clearly defined, of high intensity, and independently measurable (DeBusk 1994; Allen 1996; Carlsson 1997; Jiang 2007), whereas in the remaining five the smoking component was less clearly specified (Campbell 1998; Hanssen 2007; Wood 2008; Jorstad 2013; Kadda 2015).
Fourty‐four studies with a total of over 20,000 participants contributed to the main comparison of nursing intervention versus control. We classified 36 as high‐intensity on the basis of the planned intervention, although in some cases implementation may have been incomplete. In seven, we classified the intervention as low‐intensity (Janz 1987; Vetter 1990; Davies 1992; Nebot 1992; Tønnesen 1996; Aveyard 2003; Nagle 2005). All of these were conducted in outpatient, primary care or community settings. One further study (Hajek 2002) may be considered as a comparison between a low‐intensity intervention and usual care. Participants in the usual‐care control group received systematic brief advice and self‐help materials from the same nurses who provided the intervention. Unlike the other trials in the low‐intensity subgroup, this trial was conducted amongst inpatients with cardiovascular disease. Since the control group received a form of nursing intervention, we primarily classified the trial as a comparison of two intensities of nursing intervention. But since other studies had usual‐care groups that may have received advice from other healthcare professionals, we also report the sensitivity of the main analysis results to including it as a low‐intensity nursing intervention compared to usual‐care control.
Hajek 2002 and 10 other studies contributed to a second group comparing two interventions involving a nursing intervention. Three of these tested additional components as part of a session: demonstration of carbon monoxide (CO) levels to increase motivation to quit (Sanders 1989b); CO and spirometry feedback (Risser 1990); and CO feedback plus additional materials and an offer to find a support buddy (Hajek 2002). Five involved additional counseling sessions from a nurse (Alterman 2001; Feeney 2001; Tønnesen 2006; Aveyard 2007; Jiang 2007). One other study compared two interventions with a usual‐care control (Miller 1997). The minimal intervention condition included a counseling session and one telephone call after discharge from hospital. In the intensive condition, participants received three additional telephone calls, and those who relapsed were offered further face‐to‐face meetings, and nicotine replacement therapy if needed. We classified both interventions as intensive in the main meta‐analysis, but compared the intensive and minimal conditions in a separate analysis of the effect of additional follow‐up. Chouinard 2005 also assessed the effect of additional telephone support as an adjunct to an inpatient counseling session, so is pooled in a subgroup with Miller 1997. We included in the same subgroup a study that tested additional telephone follow‐up as a relapse prevention intervention for people who had inpatient counseling (Hasuo 2004).
Five studies (Family Heart 1994; OXCHECK 1994; Campbell 1998; Steptoe 1999; Wood 2008) were not included in any meta‐analysis and do not have results displayed graphically because their designs did not allow us to extract appropriate outcome data. The first part of a two‐stage intervention study is also included in this group (Sanders 1989a); the second part (Sanders 1989b) is included in one of the meta‐analyses. These studies are discussed separately in the Effects of interventions section below.
We determined whether the nurses delivering the intervention were providing it alongside clinical duties that were not smoking‐related, were working in health promotion roles, or were employed specifically as project nurses. Of the high‐intensity intervention studies, 21 used nurses for whom the intervention was a core component of their role (Hollis 1993; DeBusk 1994; Allen 1996; Carlsson 1997; Terazawa 2001; Kim 2003; Quist‐Paulsen 2003; Froelicher 2004; Duffy 2006; Aveyard 2007; Chan 2012; Meysman 2010; Cossette 2011; Jorstad 2013; Berndt 2014; Hornnes 2014; Gilbody 2015; Kadda 2015; Pardavila‐Belio 2015; Zwar 2015; Smit 2016). One study (Kim 2005) employed retired nurses who were trained to provide a brief intervention using the '5 As' framework. In only four studies were intensive interventions intended to be delivered by nurses for whom it was not a core task (Lancaster 1999; Bolman 2002; Curry 2003; Sanz‐Pozo 2006). Most of the low‐intensity interventions were delivered by primary care or outpatient clinic nurses. One low‐intensity inpatient intervention was delivered by a clinical nurse specialist (Nagle 2005).
Follow‐up periods for reinforcement and outcome measurements varied across studies, with a tendency for limited reinforcement and shorter follow‐up periods in the older studies. All trials had some contact with participants in the first three months of follow‐up for restatement of the intervention or point prevalence data collection or both. Eight of the studies had less than one year final outcome data collection (Janz 1987; Vetter 1990; Davies 1992; Lewis 1998; Canga 2000; Kim 2003; Berndt 2014; Pardavila‐Belio 2015). The rest had follow‐up at one year or beyond. The outcome used for the meta‐analysis was the longest follow‐up (six months and beyond), with the exception of Hanssen 2007, in which we preferred 12‐month over 18‐month data. The outcome in this study was point prevalence abstinence and we judged the 18‐month data to be too conservative, due to a rise in abstinent participants in the control group.
A brief description of the main components of each intervention is provided in the 'Characteristics of included studies' table.
Excluded studies
Sixty studies that we had identified as potentially relevant but subsequently excluded are listed in the Characteristics of excluded studies table, along with the reason for exclusion for each. The most common reasons for exclusion were: study design (not a randomized clinical trial); less than six months follow‐up; multicomponent studies with insufficient detail on smoking intervention/outcome; and studies in which the impact of the nursing intervention was confounded by additional pharmacological or behavioral treatment that was not provided to the control arm.
Risk of bias in included studies
As seen in Figure 2, we rated most studies at low or unclear risk of selection bias (random sequence generation and allocation concealment) and attrition bias (loss to follow‐up). As seen in Figure 3, we judged 16 studies to be at low risk of bias across all domains, and 16 at high risk of bias in at least one domain. The rest were at unclear risk of bias.
2.
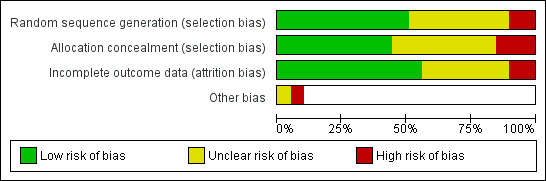
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
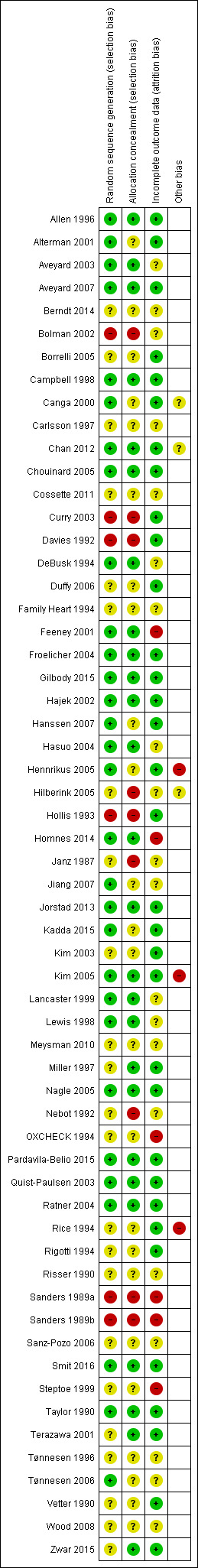
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Thirty studies provided details of a method of random sequence generation judged to be at low risk of bias, and a further 23 studies did not report how the sequence was generated and were hence rated as unclear for this domain. We judged five studies to be at high risk based on their reported methods of random sequence generation: Bolman 2002 was a cluster‐randomized study in which some hospitals picked their allocation; in Curry 2003 participants drew a colored ball from a bag; Davies 1992 allocated based on order of attendance; Hollis 1993 randomized participants based on health record number; and Sanders 1989a/Sanders 1989b randomized participants based on day of attendance. In addition to these five studies, we rated three further studies in which providers rather than participants were randomized at high risk of selection bias: Hilberink 2005 reported that self‐selection at practice level may have affected the results; in Janz 1987 allocation was determined by clinic session; and in Nebot 1992 the providers were also responsible for allocating participants, rendering allocation concealment impossible. Overall, we judged 26 studies to be at low risk of bias for allocation concealment, 24 studies had unclear risk of bias because concealments was unspecified, and we rated eight studies at high risk of bias for concealment. A sensitivity analysis including only the results of studies judged to be at low risk of selection bias did not alter the main conclusions.
Incomplete outcome data
We judged 33 studies that reported minimal to moderate loss to follow‐up and accounted for all participants in their reporting to be at low risk of attrition bias. A further 20 studies did not provide sufficient detail with which to judge the likelihood of attrition bias and hence we rated them as 'unclear' for this domain. We judged five studies to be at high risk of attrition bias: in Feeney 2001, 79% of usual‐care participants were not followed up; OXCHECK 1994 stated that their methods of accounting for missing participants may have overestimated the effect; in Sanders 1989a/Sanders 1989b only a subsample of participants from the control group was followed up; and in Steptoe 1999 and in Hornnes 2014 overall dropout rates were high and varied between intervention and control groups.
Other potential sources of bias
Definitions of abstinence ranged from single point prevalence to sustained abstinence (multiple point prevalence with self‐report of no slips or relapses). In one study (Miller 1997) we used validated abstinence at one year rather than continuous self‐reported abstinence, because only the former outcome was reported for disease diagnosis subgroups.
Of the 44 studies included in the primary meta‐analysis, 22 biochemically validated self‐reports of abstinence using either urinary/saliva cotinine or exhaled CO. One study tested CO levels only amongst people followed up in person (Curry 2003), and five studies used some validation but did not report rates based on biochemical validation of every self‐reported quitter (Nebot 1992; Rice 1994; Miller 1997; Froelicher 2004; Borrelli 2005). Fifteen studies did not use any biochemical validation and relied on self‐reported smoking cessation at a single follow‐up, although two warned participants that samples might be requested for testing (i.e. 'bogus pipeline'), and Jiang 2007 sought confirmation of smoking status from a family member.
We judged three studies (Rice 1994; Hennrikus 2005; Kim 2005) to be at high risk of other bias because of differences between intervention and control groups in validation rates for reported cessation. We judged two studies to be at unclear risk of bias. In Canga 2000, the same nurse conducted all interviews and follow‐up examinations, allowing the potential for observer bias, and in Chan 2012 there was potential for the study intervention to overlap with the standard care received by participants in the control group.
Effects of interventions
See: Table 1
Effects of intervention versus control/usual care
Smokers offered advice by a nursing professional had an increased likelihood of quitting compared to smokers without intervention, with evidence of moderate statistical heterogeneity between the results of the 44 studies contributing to this comparison (I2 = 50%). Heterogeneity was marginally more apparent in the subgroup of 37 high‐intensity trials (I2 = 53%). There was one trial with a significant negative effect for treatment (Rice 1994). This result may be explained by the fact that participants in both arms were advised to quit and more people in the control group had had coronary artery bypass graft surgery. Further, a multivariate analysis of one‐year follow‐up data revealed quitters were significantly more likely to be less than 48 years old, male, to have had individualized versus group or no cessation instruction, and to have had a high degree of perceived threat relative to their health state. In addition to this, three studies reported particularly large positive effects (Canga 2000; Terazawa 2001; Pardavila‐Belio 2015). Pooling all 44 studies using a fixed‐effect model gave a risk ratio (RR) of 1.29 with a 95% confidence interval (CI) 1.21 to 1.38 at the longest follow‐up (Figure 4; Analysis 1.1). Because of the heterogeneity we tested the sensitivity to pooling the studies using a random‐effects model. This did not materially alter the estimated effect size or greatly widen the confidence interval (RR 1.31, 95% CI 1.18 to 1.45, analysis not shown). A sensitivity analysis excluding the four outlying trials widened the CI but did not alter the point estimate whilst greatly reducing statistical heterogeneity in the high‐intensity subgroup (I2 = 15%). A further sensitivity analysis restricted to only those studies at low risk of bias across all domains also did not significantly alter the point estimate (RR 1.25, 95% CI 1.10 to 1.51, analysis not shown).
4.
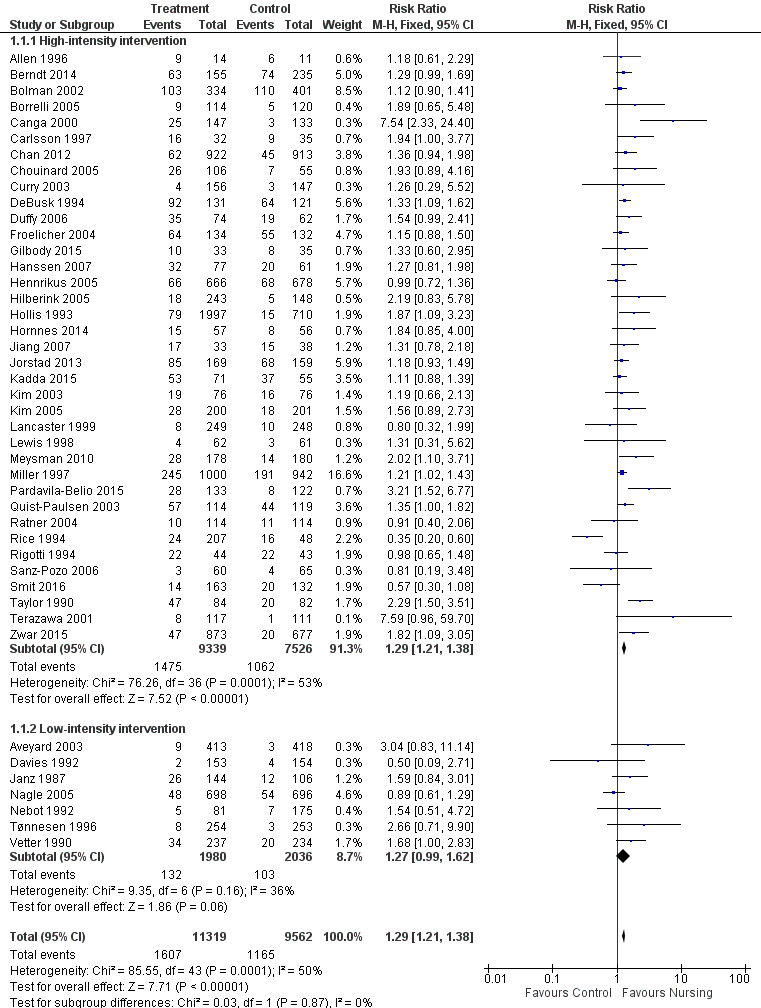
Trials of nursing intervention versus control grouped by intensity of intervention. Outcome: Smoking cessation at longest follow‐up.
1.1. Analysis.
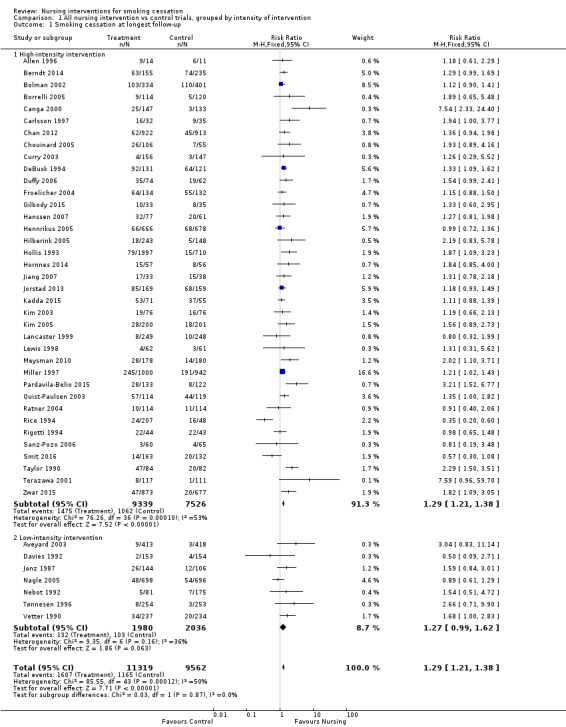
Comparison 1 All nursing intervention vs control trials, grouped by intensity of intervention, Outcome 1 Smoking cessation at longest follow‐up.
We also tested the sensitivity of these results to excluding studies that did not validate all reports of abstinence, and limiting the analysis to studies judged to be at low risk of selection bias. None of these altered the estimates to any great extent, although confidence intervals became wider due to the smaller number of studies. Excluding one study (Bolman 2002) for which we were not able to enter the numbers of quitters directly did not alter the results.
Some participants in Taylor 1990 had been encouraged to use nicotine replacement therapy (NRT). Exclusion of these people did not alter the significant effect of the intervention in this study. In Miller 1997 more people in the intervention conditions than the controls used NRT (44% of intensive and 39% of minimal intervention versus 29% of control). People who were prescribed NRT had lower quit rates than those who were not, but the relative differences in quit rates between the usual‐care and intervention groups were similar for the subgroups that did and did not use NRT. However, because of the different rates of use of NRT, it is probable that the increased use of NRT contributed to the effects of the nursing intervention. Use of NRT was also encouraged as part of the Canga 2000 intervention, with 17% of the intervention group accepting a prescription, and as part of the Duffy 2006 intervention, although at six months similar percentages in the intervention and control groups had used NRT over the course of the study.
Six further studies which compared a nursing intervention to control/usual care were not included in the meta‐analysis (Sanders 1989a; Family Heart 1994; OXCHECK 1994; Campbell 1998; Steptoe 1999; Wood 2008). Although they met the main inclusion criteria, in five trials the design did not allow data extraction for meta‐analysis in a comparable format to other studies, and in Sanders 1989a only a random sample of the control group was followed up.
Sanders 1989a, in which smokers visiting their family doctor were asked to make an appointment for cardiovascular health screening, reported that only 25.9% of the patients made and kept such an appointment. The percentage that had quit at one month and at one year and reported last smoking before the one‐month follow‐up was higher both in the attenders (4.7%) and the non‐attenders (3.3%) than in the usual‐care controls (0.9%). This suggests that the invitation to make an appointment for health screening could have been an anti‐smoking intervention in itself, and that the additional effect of the structured nursing intervention was small.
We do not have comparable data for OXCHECK 1994, which used similar health checks, because the households had been randomized to be offered the health check in different years. The authors compared the proportions of smokers in the intervention group who reported stopping smoking in the previous year to patients attending for their one‐year follow‐up, and to controls attending for their first health check. They found no difference in the proportions that reported stopping smoking in the previous year.
The Family Heart 1994 study offered nurse‐led cardiovascular screening for men aged 40 to 59 and for their partners, with smoking cessation as one of the recommended lifestyle changes. Cigarette smokers were invited to attend up to three further visits. Smoking prevalence was lower amongst those who returned for the one‐year follow up than amongst the control group screened at one year. This difference was reduced if non‐returners were assumed to have continued to smoke, and if CO‐validated quitting was used. In that case there was a reduction of only about one percentage point, with weak evidence of a true reduction.
Campbell 1998 invited people with a diagnosis of coronary heart disease to nurse‐run clinics promoting medical and lifestyle aspects of secondary prevention. There was no significant effect on smoking cessation. At one year the decline in smoking prevalence was greater in the control group than in the intervention group. Four‐year follow‐up did not alter the effect of a lack of benefit.
Steptoe 1999 recruited people at increased risk of coronary heart disease for a multicomponent intervention. The quit rate amongst smokers followed up after one year was not significantly higher in the intervention group (9.4%, 95% CI ‐9.6 to 28.3), and there was greater loss to follow‐up of smokers in the intervention group.
Wood 2008 recruited people with established or increased risk of coronary heart disease for a multicomponent lifestyle intervention, coordinated by nurses. The authors reported results separately for those participants recruited in hospital and those recruited in general practice. For coronary patients recruited in hospital who had smoked within one month at baseline, abstinence at one year favored the intervention group (58% versus 47%), but the difference was not significant (P = 0.06). For participants at high risk of coronary heart disease recruited in general practice, the prevalence of smoking fell from baseline but did not differ between conditions.
Effect of intervention intensity
We detected no evidence from our indirect comparison between subgroups that the trials we classified as using higher‐intensity interventions had larger treatment effects. In this update of the review the point estimate for the pooled effect of the seven lower‐intensity trials is effectively the same as for the 37 of higher intensity. For the low‐intensity group the confidence interval does not exclude 1, but there were fewer studies (high‐intensity subgroup RR 1.29, 95% CI 1.21 to 1.38, I2 = 53%, 16,865 participants, Analysis 1.1.1; low‐intensity subgroup RR 1.27, 95% CI 0.99 to 1.62, I2 = 36%, 4016 participants, Analysis 1.1.2). In a sensitivity analysis we included Hajek 2002, a study for which we were uncertain about the classification of the control group (as noted above in the Description of studies section), in the low‐intensity subgroup. Including this study in the low‐intensity subgroup reduced the point estimate and there was no evidence of a treatment effect (RR 1.09, 95% CI 0.92 to 1.29). Compared to the other trials in the low‐intensity subgroup, the Hajek 2002 trial was conducted amongst hospitalized participants with cardiovascular disease and the overall quit rates were high. The large number of events gave this trial a high weight in the meta‐analysis.
The distinction between low‐ and high‐intensity subgroups was based on our categorization of the intended intervention. We particularly noted low levels of implementation in the trial reports for Lancaster 1999, Bolman 2002 and Curry 2003, so we tested the effect of moving them from the high‐ to the low‐intensity subgroup. This reduced the point estimate of effect in the low‐intensity subgroup and increased it in the high‐intensity one. If these three studies and Hajek 2002 are included in the low‐intensity subgroup, the pooled estimate of effect is small and non‐significant (RR 1.09, 95% CI 0.96 to 1.25, 6056 participants, Analysis 4.1). We also assessed the sensitivity of the results to using additional participants in the control group for Aveyard 2003 (see Characteristics of included studies for details). This reduced the size of the effect in the low‐intensity subgroup but did not alter our conclusions.
4.1. Analysis.
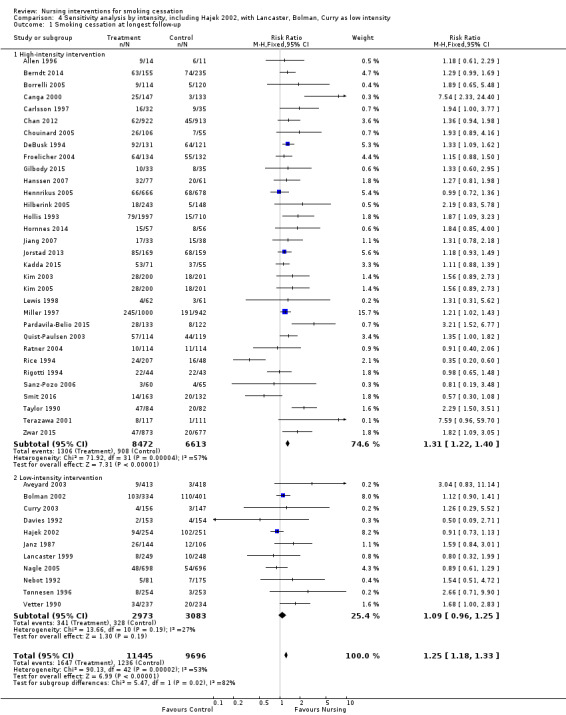
Comparison 4 Sensitivity analysis by intensity, including Hajek 2002, with Lancaster, Bolman, Curry as low intensity, Outcome 1 Smoking cessation at longest follow‐up.
Effects of differing health states and client settings
Trials in hospitals recruited participants with health problems, but some trials specifically recruited those with cardiovascular disease, and amongst these some interventions addressed multiple risks whilst most only addressed smoking. Trials in primary care generally did not select participants with a particular health problem. We combined setting and disease diagnosis in one set of subgroups (Analysis 2.1).
2.1. Analysis.
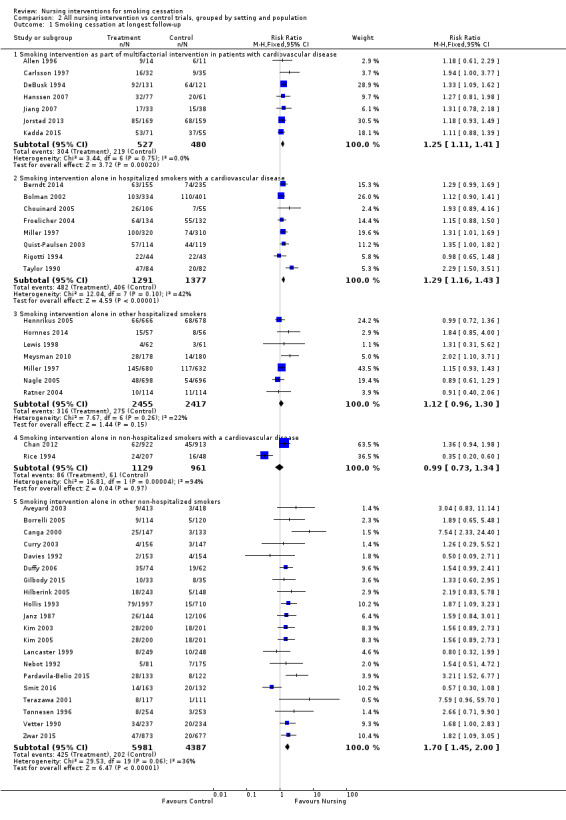
Comparison 2 All nursing intervention vs control trials, grouped by setting and population, Outcome 1 Smoking cessation at longest follow‐up.
Seven trials that included a smoking cessation intervention from a nurse as part of cardiac rehabilitation showed a significant pooled effect on smoking (RR 1.25, 95% CI 1.11 to 1.41, I2 = 0%, 1007 participants, Analysis 2.1.1). Six of these (Allen 1996; Carlsson 1997; Hanssen 2007; Jiang 2007; Jorstad 2013; Kadda 2015) did not use biochemical validation of quitting, and in the seventh (DeBusk 1994) we were unable to confirm the proportion of dropouts with the study authors.
There was some heterogeneity (I2 = 42%) among eight trials of smoking‐specific interventions in hospitalized smokers with cardiovascular disease, due to the strong intervention effect in one of the eight trials (Taylor 1990). The RR was 1.29 (95% CI 1.16 to 1.43, 2668 participants, Analysis 2.1.2) and the effect remained significant if we excluded Taylor 1990 (reducing the I2 to 0%) or if we applied a random‐effects model. A sensitivity analysis of the effect of including Hajek 2002 in this category increased the heterogeneity (I2 = 56%), and the pooled effect was significant whether we used a fixed‐effect or a random‐effects model (Analysis 5.1). Excluding Taylor 1990 again removed heterogeneity but the point estimate decreased (RR 1.14, 95% CI 1.02 to 1.27, analysis not shown).
5.1. Analysis.
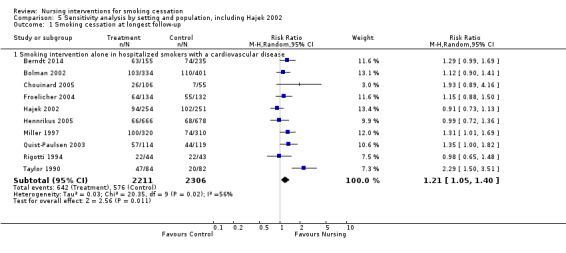
Comparison 5 Sensitivity analysis by setting and population, including Hajek 2002, Outcome 1 Smoking cessation at longest follow‐up.
Among the seven trials in non‐cardiac hospitalized smokers the risk ratio was small and the confidence interval did not exclude no effect (RR 1.12, 95% CI 0.96 to 1.30, 4872 participants, Analysis 2.1.3). We included in this subgroup one trial that began the intervention in a pre‐admission clinic for elective surgery patients (Ratner 2004).
Heterogeneity was high (I2 = 94%) between two trials of interventions delivered to non‐hospitalized adults with cardiovascular disease (Rice 1994; Chan 2012; Analysis 2.1.4). Subgroup analysis in Rice 1994, however, suggested that smokers who had experienced cardiovascular bypass surgery were more likely to quit, and these participants were over‐represented in the control group who received advice to quit but no structured intervention.
Pooling 20 trials of cessation interventions for other non‐hospitalized adults showed an increase in the success rates (RR 1.70, 95% CI 1.45 to 2.00, 10,368 participants, Analysis 2.1.5). A sensitivity analysis testing the effect of excluding those trials (Janz 1987; Vetter 1990; Curry 2003; Hilberink 2005) where a combination of a nursing intervention and advice from a physician was used did not substantially alter this.
Higher‐ versus lower‐intensity interventions
Effects of physiological feedback
Two trials (Sanders 1989b; Risser 1990) evaluated the effect of physiological feedback as an adjunct to a nursing intervention compared to nursing without physiological feedback. Neither found any evidence of an effect at maximum follow‐up (Analysis 3.1.1 (RR 1.06, 95% CI 0.55 to 2.02, 751 participants) and Analysis 3.1.2 (RR 0.33, 95% CI 0.10 to 1.15, 90 participants)).
3.1. Analysis.
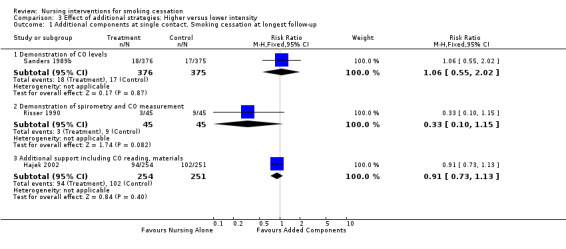
Comparison 3 Effect of additional strategies: Higher versus lower intensity, Outcome 1 Additional components at single contact. Smoking cessation at longest follow‐up.
Effects of other components at a single contact
One trial in hospitalized smokers with cardiovascular disease (Hajek 2002) found no evidence of a significant benefit of additional support from a nurse giving additional written materials, a written quiz, an offer of a support buddy, and CO measurement compared to controls receiving brief advice and a self‐help booklet (RR 0.91, 95% CI 0.73 to 1.13, 505 participants, Analysis 3.1.3).
Effects of additional telephone support
There was weak evidence from pooling three trials (Miller 1997; Hasuo 2004; Chouinard 2005) that additional telephone support increased cessation compared to less or no telephone support, as the lower limit of the confidence interval was at the boundary of no effect (RR 1.25, 95% CI 1.00 to 1.56, I2 = 0%; 1220 participants, Analysis 3.2.1).
3.2. Analysis.
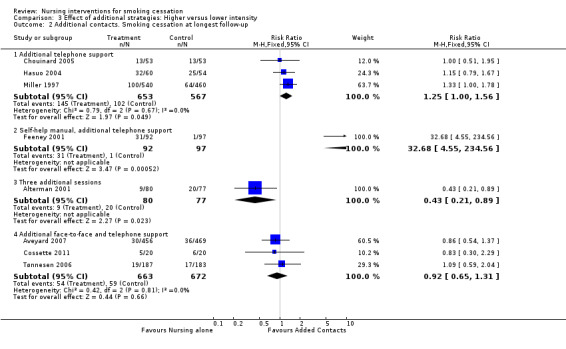
Comparison 3 Effect of additional strategies: Higher versus lower intensity, Outcome 2 Additional contacts. Smoking cessation at longest follow‐up.
Effects of additional face‐to‐face sessions
One trial of additional support from an alcohol and drug assessment unit nurse for people admitted to a coronary care unit (Feeney 2001) showed a very large benefit for the intervention (RR 32.68, 95% CI 4.55 to 234.56, 189 participants, Analysis 3.2.2). The cessation rate among the controls, however, was very low (1/97), and there was a large number of dropouts, particularly from the control group. This could have underestimated the control group quit rate. Another trial (Alterman 2001), offering four nurse sessions rather than one as an adjunct to nicotine patch, showed no benefit, with the control group having a significantly higher quit rate (RR 0.43, 95% CI 0.21 to 0.89, 157 participants, Analysis 3.2.3). No explanation was offered for the lower than expected quit rates in the intervention group.
Effects of additional face‐to‐face sessions and telephone support
Pooled results from three trials (Tønnesen 2006; Aveyard 2007; Cossette 2011) did not show an effect of providing additional clinic sessions and telephone support compared with fewer clinic sessions and less or no telephone support (RR 0.92, 95% CI 0.65 to 1.31, I2 = 0%, 1335 participants, Analysis 3.2.4).
Discussion
Summary of main results
The results of this meta‐analysis support a modest but positive effect for smoking cessation interventions by nurses, but we rated the quality of evidence as moderate due to unexplained statistical heterogeneity (see Table 1). A structured smoking cessation intervention delivered by a nurse was more effective than usual care on smoking abstinence at six months or longer from the start of treatment. The direction of effect was consistent in different intensities of intervention, in different settings, and in smokers with and without tobacco‐related illnesses. In a subgroup of low‐intensity studies the confidence interval did not exclude no effect, but the point estimate was effectively the same as that in the larger group of high‐intensity studies. We found insufficient evidence to assess whether more intensive interventions, those incorporating additional follow‐up, or those incorporating pathophysiological feedback are more effective than one‐off support. There was no evidence that the effect of support differed by patient group or across healthcare settings.
Overall completeness and applicability of evidence
Overall, these meta‐analysis findings need to be interpreted carefully in light of the methodological limitations of both the review and the clinical trials. In terms of the review, it is possible that there was a publication selection bias due to using only tabulated data derived from published works (Stewart 1993). Data from the unpublished or missed studies or both could have shown more or less favorable results, although a funnel plot for the main comparison did not suggest the presence of reporting bias. For recent updates, we have also searched clinical trials registries and 'grey' literature to identify relevant unpublished studies. Secondly, finding statistical heterogeneity between the incidences of cessation in different studies limits any assumption that interventions in any clinical setting and with any type of participant are equally effective.
The findings of this review, and in particular the estimated size of the treatment effect, have remained remarkably stable since its initial publication. In 1999, 15 studies contributed to the main analysis, with a pooled risk ratio of 1.30 (95% CI 1.16 to 1.44). Further studies have more than doubled the number of participants and thus narrowed the CIs, but have had very little impact on the point estimate, which in this most recent update is the same as it was in 1999.
Effectiveness by intervention characteristics and population
The effect estimates are similar for high‐ and low‐intensity smoking cessation interventions by nurses, as was found in a review of physicians' advice (Stead 2013). Presumably, the more components added to the intervention the more intensive the intervention. However, assessing the contribution of factors such as total contact time, number of contacts, and content of the intervention was difficult. Our distinction between high and low intensity, based on the length of initial contact and number of planned follow‐ups, may not have accurately distinguished among the key elements that could have contributed to greater efficacy. We found that the nature of the smoking cessation interventions varied from advice alone, to more intensive interventions with multiple components, and that the description of what constituted 'advice only' varied. In most trials, advice was given with an emphasis on stopping smoking because of some existing health problem. To make most interventions more intensive, verbal advice was supplemented with a variety of counseling messages, including benefits of and barriers to cessation (e.g. Taylor 1990) and effective coping strategies (e.g. Allen 1996). Manuals and printed self‐help materials were also added to many interventions, along with repeated follow‐up (Hollis 1993; Miller 1997). In some studies, the proposed intervention was not delivered consistently to all participants. In recent updates the evidence for the benefit of a low‐intensity intervention has become weaker than that for a more intensive intervention, and the estimated effect is sensitive to the inclusion of one additional study (Hajek 2002) and to the classification of intensity of three studies. Almost all the intensive interventions were delivered either by dedicated project staff or by nurses with a health promotion role. Most studies in which the intensive intervention was intended to be delivered by a nurse with other roles consistently reported problems in delivering the intervention. None showed a statistically significant benefit for the intervention. We found no studies of brief opportunistic advice that were directly analogous to the low‐intensity interventions used in physician advice trials (Stead 2013).
In two studies in the low‐intensity category (Janz 1987; Vetter 1990), advice from a physician was also part of the intervention and this almost certainly contributed to the overall effect. The most highly‐weighted study in the high‐intensity subgroup (Miller 1997) produced only relatively modest results. This was due in part to the effect of the minimal treatment condition that had just one follow‐up telephone call. However, using just the high‐intensity condition in the analysis did not materially alter the pooled estimate.
One study (Miller 1997) provided data on the effect of the same intervention in smokers with different types of illness and suggested a greater effect in cardiovascular patients. In these individuals the intervention increased the 12‐month quit rate from 24% to 34%. In other types of patients, the rates were increased from 18.5% to 21%. However, this hypothesis was not formally tested. In this study participants were eligible if they had smoked any tobacco in the month prior to hospitalization, but were excluded if they had no intention of quitting (although they were also excluded if they wanted to quit on their own). These criteria may have contributed to the relatively high quit rates achieved. Also, a higher proportion of participants in the intensive treatment arm than in the minimal or usual‐care intervention arms were prescribed nicotine replacement therapy (NRT). However, the intervention was also effective in those not prescribed NRT. Those given NRT were heavier smokers (with higher levels of addiction) who achieved lower cessation rates than those who did not use NRT.
This suggests that nursing professionals may have an important 'window of opportunity' to intervene with patients in the hospital setting, or at least to introduce the notion of not resuming tobacco use upon hospital discharge. The size of the effect may be dependent on the reason for hospitalization. The additional telephone support, with the possibility of another counseling session for people who relapsed after discharge, seemed to contribute to more favorable outcomes in the intensive intervention used by Miller 1997, although pooled results from three studies testing the addition of telephone counseling and further face‐to‐face contact did not detect an effect. A separate Cochrane Review of the efficacy of interventions for hospitalized patients (Rigotti 2012) supports the efficacy of interventions for this patient group, but only when the interventions included post‐discharge support for at least one month.
Providing additional physiological feedback in the form of spirometry and demonstrated CO level as an adjunct to nursing intervention did not appear to have an effect. Three studies in primary care or outpatient settings used this approach (Sanders 1989b; Risser 1990; Hollis 1993). It was also used as part of the enhanced intervention in a study with hospitalized patients (Hajek 2002). A separate Cochrane Review (Bize 2012) found little evidence about the effects of most types of biomedical tests for risk assessment on smoking cessation.
The identification of an effect for a nurse‐mediated intervention in smokers who were not hospitalized is based on 20 studies. The largest study (Hollis 1993) increased the quit rate from 2% in those who received only advice from a physician to 4% when a nurse delivered one of three additional interventions, including a video, written materials, and a follow‐up telephone call. Control group quit rates were less than 10% in almost all these studies, and more typically between 4% and 8%. The risk ratio in this group of studies (1.7) was a little higher than in some subgroups, but because of the low background quit rate the proportion of participants likely to become long‐term quitters as a result of a nursing intervention in these settings is likely to be small. However, because of the large number of people who could be reached by nurses, the effect would be important.
Combined efforts of many types of healthcare professionals are likely to be required. The US Public Health Service clinical practice guideline Treating Tobacco Use and Dependence (AHRQ 2008) used logistic regression to estimate efficacy for interventions delivered by different types of providers. Their analysis did not distinguish among the non‐physician medical healthcare providers, so that dentists, health counselors, and pharmacists were included with nurses. The guideline concluded that these providers were effective (Table 15, odds ratio (OR) 1.7, 95% CI 1.3 to 2.1). They also concluded that interventions by multiple clinician types were more effective (Table 16, OR 2.5, 95% CI 1.9 to 23.4). Although it was recognized that there could be confounding between the number of providers and the overall intensity of the intervention, the findings confirmed that a nursing intervention that reinforces or complements advice from physicians or other healthcare providers or both is likely to be an important component in helping smokers to quit.
Authors' conclusions
Implications for practice.
Support for smoking cessation by a nurse leads to a modest improvement in tobacco abstinence. Most of these interventions were delivered by nurses with a specialist health promotion function and there was insufficient evidence to know whether general nurses can achieve the same benefits. Commissioners and providers of smoking cessation services need to consider the quality of delivery of smoking cessation services if these are to be provided by general nurses.
Implications for research.
Further studies of nursing interventions are warranted, with more careful consideration of sample size, participant selection, refusals, dropouts, long‐term follow‐up, and biochemical verification. Additionally, controlled studies are needed that carefully examine the effects of 'brief advice by nursing', as this type of professional counseling may more accurately reflect the current standard of care. Work is now required to systematize interventions so that more rigorous comparisons can be made between studies. None of the trials reviewed was a replication study; this is a very important method to strengthen the science, and should be encouraged.
What's new
| Date | Event | Description |
|---|---|---|
| 26 October 2017 | New citation required but conclusions have not changed | Conclusions unchanged |
| 26 October 2017 | New search has been performed | Searches updated. Nine new included studies |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 14 June 2013 | New citation required but conclusions have not changed | Conclusions unchanged. |
| 14 June 2013 | New search has been performed | Searches updated. Seven new included studies, and new data (longer follow‐up) added for two already included studies. |
| 22 June 2011 | Amended | Additional table converted to appendix to correct pdf format |
| 29 October 2008 | Amended | Converted to new review format. |
| 21 October 2007 | New citation required and minor changes | Updated for issue 1 2008 with 12 new studies included; no major changes to results. The conclusions did not change. |
| 14 September 2003 | New citation required and conclusions have changed | Updated for issue 1 2004 with 7 new studies. Conclusions now give more emphasis to possible differences between high and low intensity interventions. |
| 14 October 2001 | New citation required and minor changes | Updated for issue 3 2001 with 3 new studies. The conclusions did not change substantially. |
Acknowledgements
Lindsay Stead was an author for previous versions of this review. Nicky Cullum and Tim Coleman for their helpful peer review comments on the original version of this review. Hitomi Kobayasha, a doctoral student, for assistance with Japanese translation of a study. Jong‐Wook Ban and Hyunjik Kim for Korean language assistance. Thank you to Olga Kadda for providing additional study data for Kadda 2015, and Harald Jorstad for providing additional study data for Jorstad 2013.
Appendices
Appendix 1. Register search strategy
Run using Cochrane Register of Studies (CRS) software
#1 (nurse* or nursing):TI,AB,XKY,MH,EMT,KY #2 (health visitor*):TI,AB,XKY,MH,EMT,KY #3 #1 OR #2
XKY, MH, EMT, KY are keyword fields. XKY field includes indexing terms added for the use of the tobacco addiction group.
Appendix 2. Glossary of terms
| Term | Definition |
| Abstinence | A period of being quit, i.e. stopping the use of cigarettes or other tobacco products, May be defined in various ways; see also: point prevalence abstinence; prolonged abstinence; continuous/sustained abstinence |
| Biochemical verification | Also called 'biochemical validation' or 'biochemical confirmation': A procedure for checking a tobacco user's report that he or she has not smoked or used tobacco. It can be measured by testing levels of nicotine or cotinine or other chemicals in blood, urine, or saliva, or by measuring levels of carbon monoxide in exhaled breath or in blood. |
| Bupropion | A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant) |
| Carbon monoxide (CO) | A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence. |
| Cessation | Also called 'quitting' The goal of treatment to help people achieve abstinence from smoking or other tobacco use, also used to describe the process of changing the behaviour |
| Continuous abstinence | Also called 'sustained abstinence' A measure of cessation often used in clinical trials involving avoidance of all tobacco use since the quit day until the time the assessment is made. The definition occasionally allows for lapses. This is the most rigorous measure of abstinence |
| 'Cold Turkey' | Quitting abruptly, and/or quitting without behavioural or pharmaceutical support. |
| Craving | A very intense urge or desire [to smoke]. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
| Dopamine | A neurotransmitter in the brain which regulates mood, attention, pleasure, reward, motivation and movement |
| Efficacy | Also called 'treatment effect' or 'effect size': The difference in outcome between the experimental and control groups |
| Harm reduction | Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco. |
| Lapse/slip | Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence, but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse. |
| nAChR | [neural nicotinic acetylcholine receptors]: Areas in the brain which are thought to respond to nicotine, forming the basis of nicotine addiction by stimulating the overflow of dopamine |
| Nicotine | An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking. |
| Nicotine Replacement Therapy (NRT) | A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco‐free The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges. |
| Outcome | Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial. |
| Pharmacotherapy | A treatment using pharmaceutical drugs, e.g. NRT, bupropion |
| Point prevalence abstinence (PPA) | A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long‐term quitters. cf. prolonged abstinence, continuous abstinence |
| Prolonged abstinence | A measure of cessation which typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. See: Hughes et al 'Measures of abstinence in clinical trials: issues and recommendations'; Nicotine & Tobacco Research, 2003: 5 (1); 13‐25 |
| Relapse | A return to regular smoking after a period of abstinence |
| Secondhand smoke | Also called passive smoking or environmental tobacco smoke [ETS] A mixture of smoke exhaled by smokers and smoke released from smouldering cigarettes, cigars, pipes, bidis, etc. The smoke mixture contains gases and particulates, including nicotine, carcinogens and toxins. |
| Self‐efficacy | The belief that one will be able to change one's behaviour, e.g. to quit smoking |
| SPC [Summary of Product Characteristics] | Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively. |
| Tapering | A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment |
| Tar | The toxic chemicals found in cigarettes. In solid form, it is the brown, tacky residue visible in a cigarette filter and deposited in the lungs of smokers. |
| Titration | A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects. |
| Withdrawal | A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
Data and analyses
Comparison 1. All nursing intervention vs control trials, grouped by intensity of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at longest follow‐up | 44 | 20881 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.21, 1.38] |
| 1.1 High‐intensity intervention | 37 | 16865 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.21, 1.38] |
| 1.2 Low‐intensity intervention | 7 | 4016 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.99, 1.62] |
Comparison 2. All nursing intervention vs control trials, grouped by setting and population.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at longest follow‐up | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Smoking intervention as part of multifactorial intervention in patients with cardiovascular disease | 7 | 1007 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.11, 1.41] |
| 1.2 Smoking intervention alone in hospitalized smokers with a cardiovascular disease | 8 | 2668 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.16, 1.43] |
| 1.3 Smoking intervention alone in other hospitalized smokers | 7 | 4872 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.96, 1.30] |
| 1.4 Smoking intervention alone in non‐hospitalized smokers with a cardiovascular disease | 2 | 2090 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.34] |
| 1.5 Smoking intervention alone in other non‐hospitalized smokers | 20 | 10368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.45, 2.00] |
Comparison 3. Effect of additional strategies: Higher versus lower intensity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Additional components at single contact. Smoking cessation at longest follow‐up | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Demonstration of CO levels | 1 | 751 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.55, 2.02] |
| 1.2 Demonstration of spirometry and CO measurement | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.10, 1.15] |
| 1.3 Additional support including CO reading, materials | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.73, 1.13] |
| 2 Additional contacts. Smoking cessation at longest follow‐up | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Additional telephone support | 3 | 1220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.00, 1.56] |
| 2.2 Self‐help manual, additional telephone support | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 32.68 [4.55, 234.56] |
| 2.3 Three additional sessions | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.21, 0.89] |
| 2.4 Additional face‐to‐face and telephone support | 3 | 1335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.31] |
Comparison 4. Sensitivity analysis by intensity, including Hajek 2002, with Lancaster, Bolman, Curry as low intensity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at longest follow‐up | 43 | 21141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.18, 1.33] |
| 1.1 High‐intensity intervention | 32 | 15085 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.22, 1.40] |
| 1.2 Low‐intensity intervention | 11 | 6056 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.25] |
Comparison 5. Sensitivity analysis by setting and population, including Hajek 2002.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at longest follow‐up | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Smoking intervention alone in hospitalized smokers with a cardiovascular disease | 10 | 4517 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.05, 1.40] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 1996.
| Methods | Country: USA (Maryland) Recruitment setting: hospital inpatients. Intervention: Prior to hospital discharge and 2 weeks post‐discharge | |
| Participants | 116 women post‐CABG. 25 smokers amongst them. Smoker defined by use of cigs in 6 months before admission Nurses provided intervention as part of their core role | |
| Interventions | 1. Multiple risk factor intervention, self‐efficacy programme: 3 sessions with nurse using AHA Active Partnership Program and a follow‐up call 2. Usual care (standard discharge teaching and physical therapy instructions) Intensity: High | |
| Outcomes | Abstinence at 12m ('current use') Validation: none | |
| Notes | Data on number of quitters derived from percentages. Likely to include some who stopped prior to intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Consenting patients were randomly assigned to receive special intervention or usual care by using a computerized schema that achieved a balanced allocation." |
| Allocation concealment (selection bias) | Low risk | "The nurse obtaining study consent and the participants were unaware of the group assignment at the time consent was obtained and baseline data were collected." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar number lost to follow‐up in both arms; dropouts counted as smokers |
Alterman 2001.
| Methods | Country: USA Recruitment setting: community volunteers, motivated to quit, cessation clinic | |
| Participants | 160 smokers (≥ 1 pack/day) in relevant arms | |
| Interventions | All received nicotine patch 21 mg 8 weeks incl weaning Medium Intensity: 4 sessions over 9 weeks, 15 ‐ 20 mins, advice and education from nurse practitioner Low Intensity: single 30‐min session with nurse, 3 videos | |
| Outcomes | Abstinence at 12 m, not defined Validation: CO < 9 ppm, urine cotinine < 50 ng/ml | |
| Notes | No control group so not in main analysis High intensity intervention not included in review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Urn randomization" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Rate of dropout similar in both groups; dropouts counted as smokers. Authors give 77 as ITT denominator for medium‐intensity group. N randomized of 80 used in MA. |
Aveyard 2003.
| Methods | Country: UK Recruitment setting: 65 general practices, invitation by letter | |
| Participants | 831 current smokers in relevant arms, volunteers but not selected by motivation (> 80% precontemplators) Intervention from practice nurses with 2 days training in Pro‐Change system | |
| Interventions | 1. In addition to tailored self‐help in 2., asked to make appointment to see practice nurse. Single postal reminder if no response. Up to 3 visits, at time of letters. Reinforced use of manual 2. Self‐help manual based on Transtheoretical model, maximum of 3 letters generated by expert system. No face‐to‐face contact Intensity: Low (Standard S‐H control and telephone counselling arms not used in review) | |
| Outcomes | Abstinence at 12 m, self‐reported sustained for 6m Validation: saliva cotinine < 14.2 ng/ml | |
| Notes | Low uptake of nurse component, 20% attended 1st visit, 6% 2nd and 2% 3rd, also more withdrawals (20%) Nursing arm discontinued part‐way through recruitment. We use only the Manual (control) group recruited during 4‐arm section of trial (3/418, data from author website www.publichealth.bham.ac.uk/berg/pdf/Addiction2003.pdf, compared to 15/683 for Manual group across the entire trial). This increases apparent benefit of nurse intervention. A sensitivity analysis did not alter any findings from the MA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Minimization was used to allocate individuals to arms to balance three predictors of smoking cessation success... Questionnaires were read optically and the data transferred automatically to the Access database that performed the minimization and controlled the contacts." |
| Allocation concealment (selection bias) | Low risk | "There was no reason and no way that the clerical assistant running the database could alter the questionnaire reading schedule." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Highest withdrawal rate in nursing group (20%). Participants with missing data counted as smokers |
Aveyard 2007.
| Methods | Country: UK Recruitment: Patients from 26 GP practices; 92% volunteers in response to mailing |
|
| Participants | 925 smokers 51% women, av. age 43, 50% smoked 11 ‐ 20 cpd Interventions from practice nurses trained to give NHS smoking cessation support and manage NRT | |
| Interventions | Both interventions included 8 wks 16 mg nicotine patch
1. Basic support; 1 visit (20 ‐ 40 mins) before quit attempt, phone call on TQD, visits/phone calls at 7 ‐ 14 days and at 21 ‐ 28 days (10 ‐ 20 mins)
2. Weekly support; as 1. plus additional call at 10 days and visits at 14 and 21 days Intensity: High (for both groups) |
|
| Outcomes | Abstinence at 12m (sustained at 1, 4, 12, 26 wks) Validation: CO < 10 ppm at treatment visits, saliva cotinine < 15 ng/ml at follow‐ups | |
| Notes | New for 2013 update. Both conditions included nurse support; Included in analysis of effects of additional strategies only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random number sequence and sealed number envelopes were generated by a statistician." |
| Allocation concealment (selection bias) | Low risk | "Nurses opened the envelopes in sequence following eligibility assessment and consent." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 288 (31%) lost to follow‐up, similar across groups, included in ITT analysis |
Berndt 2014.
| Methods | Country: Netherlands Recruitment setting: patients from 8 cardiac wards |
|
| Participants | 625 participants (intervention = 155, control = 235) 16 nurses were trained as counsellors on a 4‐day training course |
|
| Interventions | 1. Face‐to‐face counselling, usual care and NRT. The face‐to face counselling lasted 3 months, consisting of 6 face‐to‐face sessions of 30 ‐ 45 minutes and ending with a follow‐up call 5 weeks after the last session 2. Telephone counselling, usual care and NRT. This lasted for 3 months and consisted of 7 phone calls of 15 minutes duration 3. Usual care, which consisted of assessment of their smoking behaviour, the delivery of brief quit advice and occasionally the delivery of an informational brochure. This advice was usually given by cardiologists |
|
| Outcomes | 6m continued abstinence (defined as abstinence for at least 90 days, self‐reported). Up to 5 cigarettes allowed to still be considered abstinent. Not clear whether the 6‐month follow‐up was from the day of study intake or from when the interventions were completed | |
| Notes | New for 2017 update; previously listed as ongoing study (Bernt 2012 in previous version of this review) As the telephone counselling was not delivered by nurses, we have not included these data in the review Funding: supported by a research grant from ZonMw, the Dutch Organization for Health Research and Development (Grant Number: 50‐50110‐96‐524) Declarations of interest: Authors report no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified. Randomization done on the ward level by sequential cross‐over randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This study used an ITT scenario using all cases in which those participants who were lost to follow‐up were treated as smokers. Although the study reports no significant between‐group differences in loss to follow‐up, actual number lost to follow‐up not reported |
Bolman 2002.
| Methods | Country: Netherlands Recruitment setting: cardiac ward patients in 11 hospitals | |
| Participants | 789 smokers who had smoked in previous week Nurses had 2 hours training and delivered intervention alongside normal duties | |
| Interventions | 1. Cardiologist advice on ward and 1st check‐up, GP notified, nurse provided stage‐of‐change‐based counselling and provided a self‐help cessation manual and a brochure on smoking and CHD. Nurse assessed smoking behaviour, addiction, motivation, addressed pros and cons, barriers and self‐efficacy, encouraged a quit date 2. Usual care (nurses on control wards intended to be blind to status) Intensity: High (but not consistently delivered) | |
| Outcomes | Abstinence at 12 m (no smoking since hospital discharge) Validation: none ('bogus pipeline') | |
| Notes | Process analysis indicated some implementation failure Due to cluster‐randomization there were baseline differences between intervention and control participants. Raw numbers quit are misleading. Regression analyses suggest no significant effect on continuous abstinence at 12 m, so numbers quit in intervention group in MA adjusted to approximate the odds ratio and confidence interval from regression analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "The design was partially randomized: 4 of the 11 hospitals selected the experimental condition themselves... while the remaining 7 hospitals were randomly assigned." (Exclusion of the 4 hospitals who selected condition did not change results.) Baseline differences between intervention and control participants due to cluster randomization |
| Allocation concealment (selection bias) | High risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants lost to follow‐up counted as smokers, with the exception of 25 deaths, 38 refusals, and 64 missing baseline data which were excluded from analysis denominator. Significantly more loss to follow‐up in intervention group |
Borrelli 2005.
| Methods | Country: USA Recruitment/setting: Home healthcare nursing service | |
| Participants | 278 smoking patients of home healthcare nurses, not selected by motivation 54% women, av. age 57, av. cpd 21 Home healthcare nurses trained to deliver intervention during usual visits | |
| Interventions | 1. Motivational enhancement. 3 x 20 ‐ 30‐min sessions during nursing visits. 5‐min follow‐up call 2. Standard care control based on 5As model, single 5 ‐ 15‐min session with brief support at subsequent nursing visits, consistent with guidelines Intensity: High | |
| Outcomes | Abstinence at 12 m (no smoking since 6 m assessment) Validation: CO < 10 ppm obtained for 60%, informant report also used | |
| Notes | Nurses treated an average of 4 participants (range 1 ‐ 13). Within‐nurse correlation low, so multilevel models not reported 39 deaths and 5 who quit before intervention excluded from denominators. Included in high‐intensity subgroup. Control intervention was more than usual care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomized by nurse, method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70.5% retention rate; similar number of dropouts in both groups. Unclear if denominators used in published article include all lost to follow‐up; denominators for MA generated from data provided elsewhere in article |
Campbell 1998.
| Methods | Country: UK (Scotland) Recruitment setting: GP (Family Practice) Intervention: within 3 m of enrolment | |
| Participants | Approx 200 smokers amongst 1343 patients with CVD diagnosis | |
| Interventions | 1. Multiple risk factor intervention, at least 1 x 45‐min counselling session plus follow‐up visits 2. Usual care | |
| Outcomes | Abstinence at 12 m Validation: none | |
| Notes | Not included in MA. Data presented as odds ratio for non‐smoking | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used random numbers tables to centrally randomise patients (by individual after stratification for age, sex, and practice)." |
| Allocation concealment (selection bias) | Low risk | Eligibility and randomization determined centrally |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7% missing data at final follow‐up; counted as smokers. Similar numbers in both groups |
Canga 2000.
| Methods | Country: USA Recruitment: 15 primary care centres, 2 hospitals Intervention: After enrolment | |
| Participants | 280 smokers with diabetes (incl 16 recent quitters) Intervention delivered by a single research nurse | |
| Interventions | 1. Individual counselling based on NCI physician manual: 40 mins, follow‐up with phone call, 2 further visits, letter 2. Usual care Intensity: High | |
| Outcomes | Abstinence at 6 m for > 5 m Validation: urine cotinine | |
| Notes | NRT offered to 105 of intervention group but only accepted by 25. No reported use in control group Quit rate for NRT user subgroup not stated 6 in Int and 4 in control failed/refused validation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to experimental or control groups using a computer‐generated allocation method." |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope used but not specified to be numbered and opaque |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very low dropout rate: 1 loss to follow‐up and 1 death. Loss to follow‐up counted as smoker |
| Other bias | Unclear risk | "Although the same nurse conducted all interviews and follow‐up examinations with the potential for some degree of observer bias, the high incidence cessation ratio is very difficult to explain only on that basis." |
Carlsson 1997.
| Methods | Country: Sweden Recruitment setting: Hospital CCU. Intervention at home 4 weeks after discharge | |
| Participants | 168 survivors of AMI. 67 smokers amongst them, defined as present smoker by questionnaire Intervention delivered by a trained nurse rehabilitator | |
| Interventions | 1. Multiple risk factor intervention in secondary prevention unit, 1½ hrs smoking cessation component as part of 9 hours group/ individual counselling. 4 visits to nurse during 9 m 2. Usual care, follow‐up by general practitioners Intensity: High | |
| Outcomes | Abstinence at 12 m Validation: none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number and proportion of lost to follow‐up not specified; all participants missing data counted as smokers |
Chan 2012.
| Methods | Country: Hong Kong Recruitment setting: Cardiac outpatient clinics at 10 major hospitals |
|
| Participants | 1860 Chinese cardiac patients smoking ≥ 1 cig in past week. 91% men, av. age 58, av. cpd 12 Excluded from study if "too clinically ill." Intervention delivered by trained nurse counsellors |
|
| Interventions | 1. Intervention: At baseline, 30‐min individual face‐to‐face counselling matched to stage of readiness to quit. At 1 wk and 1 m: telephone calls from nurse counsellor, re‐assessment of stage and counselling to suit that stage, avg. phone call length 15 mins 2. Control: 15‐min, individual face‐to‐face counselling on healthy diet from nurse counsellor at baseline Pharmacotherapy: No smoking cessation drugs provided, but stage‐matched medication counselling on NRT was discussed with intervention participants "if deemed appropriate". Intensity: High |
|
| Outcomes | 7‐day PP at 12 m (30‐day PP at 12 m and 3 and 6 m outcomes also reported) Validation: CO ≤ 8 ppm, urinary cotinine < 100 ng/ml |
|
| Notes | New for 2013 update Validated rates used in MA; only about 25% of people self‐reporting abstinence were validated Participants in intervention group had higher stage of readiness to quit smoking than in the control group. Adjusted OR provided in text (unadjusted OR 1.35, 95% CI 0.91 to 2.00; adjusted OR 1.26, 95% CI 0.85 to 1.87); numbers used in MA are unadjusted No contamination observed 54% intervention received all counselling |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation sequence was generated sequentially by the project co‐ordinator based on simple random sampling procedure using MS Excel." |
| Allocation concealment (selection bias) | Low risk | "serially numbered sealed and opaque envelope" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of follow‐up in both groups at 12 m (85.5% intervention and 84.3% control). "No statistically significant difference was found between the two groups." ITT analysis conducted, 25 who died during study removed from denominators |
| Other bias | Unclear risk | "Some cardiac out‐patient clinics provided rehabilitation programmes which included health talks or brief advice on smoking cessation as their usual or standard care (but no stage‐matched counselling). It is plausible that our intervention might overlap with the usual standard care which patients from the control group received. As brief intervention in an out‐patient setting is effective, the usual care might have biased the effect towards null." |
Chouinard 2005.
| Methods | Country: Canada Recruitment setting: Inpatients with cardiovascular disease (MI, angina, CHF) or PVD, unselected by motivation | |
| Participants | 168 past‐month smokers Av. age 56 Intervention delivered by a research nurse | |
| Interventions | 1. Counselling by research nurse (1 x 10 ‐ 60 mins, av. 40 mins, based on Transtheoretical Model, included component to enhance social support from a significant family member), 23% used pharmacotherapy 2. As 1, plus telephone follow‐up, 6 calls over 2 m post‐discharge, 29% used pharmacotherapy 3. Control: cessation advice, 11% used pharmacotherapy. | |
| Outcomes | Abstinence at 6 m (sustained at 2 m and 6 m) Validation: Urine cotinine or CO | |
| Notes | 2 interventions combined versus control in high‐intensity subgroup. 1 versus 2 used in higher versus lower comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Cluster randomization was used... by first randomly assigning individuals to predetermined clusters of three to six subjects. The group assignment was then randomly assigned to each of these clusters." |
| Allocation concealment (selection bias) | Low risk | "Individuals not familiar with the study were in charge of the randomization procedure, which included inserting the information into envelopes that were sealed and would be opened by the investigator only at the time of treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 deaths and 3 not meeting follow‐up criteria excluded from MA; all other dropouts and those lost to follow‐up counted as smokers; similar numbers in all arms |
Cossette 2011.
| Methods | Country: Canada Recruitment setting: all smokers admitted to 1 specialist cardiac hospital were asked to participate by the study nurse. Willing to quit |
|
| Participants | 40 current daily smokers, 40% women, av. age 57 Intervention delivered by nurse specializing in smoking cessation |
|
| Interventions | 1. Intervention: usual care during hospitalization, consisting of 1 or more sessions with the study nurse. Follow‐up: 6 phone calls by study nurse at wk 1, 2, 3, 4, 8, 12 and then if needed additional phone calls could be arranged between 3 m and 6 m post‐discharge. At wk 3 appointment with the study nurse if asked by participant 2. Control: usual care during hospitalization, consisting of 1 or more sessions with the study nurse, referral to a national quitline or a community centre for smoking cessation Pharmacotherapy: NRT, bupropion or varenicline were suggested during hospitalization and follow‐up Intensity: High |
|
| Outcomes | Abstinence: self‐reported abstinence at 6 m Validation: only for 1 participant |
|
| Notes | New for 2013 update. Included in analysis 3.2 only (additional contact), as all participants received nurse counselling in hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified, but generated by a centre for randomized controlled trials |
| Allocation concealment (selection bias) | Unclear risk | Opaque sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data similar in both groups and analyses are ITT, participants lost to follow‐up considered smokers |
Curry 2003.
| Methods | Country: USA Recruitment setting: mothers attending 4 paediatric clinics, unselected by motivation | |
| Participants | 303 women (any smoking), 23% in precontemplation av. age 33, av cpd 12 Intervention delivered either by clinic nurses or a study interventionist. Nurses received 8 hours individual training in motivational interviewing | |
| Interventions | 1. Clinician advice based on 5As (1 ‐ 5 mins), self‐help materials targeted for mothers. Asked to meet a nurse or health educator who provided motivational interviewing during visit. Up to 3 phone calls over 3 m 2. No intervention Intensity: High (but implementation incomplete) | |
| Outcomes | Abstinence at 12 m (sustained at 3 m and 12 m. PP also reported) Validation: CO < 10 ppm, only for women followed up in person. Tabulated rates based on self‐report | |
| Notes | Intervention included physician advice. Not all participants received intervention. Based on counsellor records, 74% received face‐to‐face intervention, average length 13 mins, and 78% had at least 1 phone call. Nurses provided intervention as part of their normal duties | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants "determined their randomization group by choosing a Ping‐Pong ball out of a brown paper bag. The bag contained several Ping‐Pong balls that were either white or yellow, and the color of the selected ball indicated their study group." |
| Allocation concealment (selection bias) | High risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19% lost at final follow‐up; counted as smokers. Similar numbers lost to follow‐up in both groups |
Davies 1992.
| Methods | Country: Canada Recruitment setting: healthy adult community‐based volunteers | |
| Participants | 307 essentially healthy adult smokers of at least 5 cpd | |
| Interventions | 1. 'Time To Quit' programme delivered by a student nurse trained in programme 2. Visit by same student nurse prior to receiving training Intensity: Low | |
| Outcomes | Abstinence at 9 m Validation: Cotinine < 100 ng/mL | |
| Notes | Effect of training and manuals on nurse intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Each participating nurse visited a control participant first, then received training. Authors state: "While the study protocol introduced an order bias, it was deliberately selected for practical reasons." |
| Allocation concealment (selection bias) | High risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow‐up counted as smokers in outcome data (30 in experimental group, 20 in control group) |
DeBusk 1994.
| Methods | Country: USA (California) Recruitment setting: inpatients with AMI at 5 hospitals | |
| Participants | 131/293 intervention and 121/292 control participants were smokers, as defined by any use of tobacco in 6 m before admission Nurses provided intervention as part of their core role | |
| Interventions | 1. Multiple risk factor intervention case‐management system with smoking cessation, nutritional counselling, lipid‐lowering therapy and exercise therapy. Smoking cessation: 2‐min physician then nurse counselling with 8 repeated telephone follow‐ups. NRT offered only to highly‐addicted participants who relapsed post‐discharge 2. Usual care including physician counselling. Group cessation programmes available for USD 50 (2% enrolled) | |
| Outcomes | PP abstinence at 1 yr Validation: plasma cotinine < 10 ng/mL, or 11 ‐ 15 ng/mL with expired CO < 10 ppm | |
| Notes | Number of quitters derived from smoking cessation rates based on number of baseline smokers ‐ Author contacted for smoker dropout rates | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned using a computer program that achieved a balanced allocation to the two management conditions within each hospital." |
| Allocation concealment (selection bias) | Low risk | "Randomization was done centrally; nurses were notified of the assignments by telephone calls from the coordinating center staff." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear what percentage of smokers were lost to follow‐up. "Among participants who did not relapse before death or dropout, censoring occurred at the last point at which they reported not smoking." |
Duffy 2006.
| Methods | Country: USA Recruitment setting: Head‐and‐neck‐cancer patients at 4 medical centres (1 university, 3 VA) who smoked |
|
| Participants | 136 head‐and‐neck‐cancer patients who were current smokers or had quit smoking within the last 6 m 84% men, av. age 57 |
|
| Interventions | 1. CBT workbook, 9 ‐ 11 CBT sessions via telephone and pharmacologic management "as needed" Smokers offered NRT or bupropion or both. Nurses trained in CBT 2. Usual care (referred based on participant’s needs, insurance, and ability to pay, given handout of resources) Intensity: High |
|
| Outcomes | Smoking prevalence at 6 m Validation: none |
|
| Notes | New for 2013 update. Subgroup from trial of head‐and‐neck‐cancer patients who smoked, were depressed, or were alcohol‐dependent. Sex and age stats based on overall sample of 184 (not just smokers) Intervention group offered NRT or bupropion or both – at 6 m 21/74 in intervention group had used NRT and 14/62 in usual‐care group had used NRT |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All smokers lost to follow‐up counted as smokers in final outcome data. From the total group of 184 randomized: 4% died, 12% lost to follow‐up. "The loss to follow‐up was evenly distributed between the two randomized groups." |
Family Heart 1994.
| Methods | Country: UK Recruitment setting: Male general practice (family practice) patients aged 40 ‐ 59 and partners, identified by household | |
| Participants | 7460 male and 5012 female medical practice patients who reported ‘smoking’ on a questionnaire | |
| Interventions | 1. Screening for cardiovascular risk factors, risk‐related lifestyle intervention during a single 1½ hr visit 2. Delayed screening (at 1 year) for families in the same practice (internal control) and the paired practice (external control) | |
| Outcomes | Smoking prevalence at 1yr Validation: CO | |
| Notes | Not included in MA because outcome not directly comparable with cessation studies. Smoking prevalence was lower in the intervention participants at 1 yr than in either internal or external practice controls. But non‐returners in the intervention group had a higher smoking prevalence at baseline than returners | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized by practice (1 of a pair in each of 14 towns): "a pair of willing practices in each town with similar sociodemographic characteristics was randomized to either arm of the study." "Within each intervention practice, the list of men was randomly divided into two groups: intervention and an internal comparison group." Method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 87% attended final follow‐up; similar numbers lost in both groups. Those lost to follow‐up significantly more likely to be smokers at start of study |
Feeney 2001.
| Methods | Country: Australia Recruitment/setting: CCU, single hospital | |
| Participants | 198 smokers in previous week, unselected for motivation | |
| Interventions | 1. Stanford Heart Attack Staying Free programme. Review by Alcohol and Drug Assessment Unit (ADAU) physician. Self‐help manual, high relapse risk patients counselled on coping strategies, audiotapes. On discharge ADAU nurse contacted weekly for 4 weeks and 2, 3, 12 m 2. Verbal and written didactic advice, video, review by ADAU nurse, supportive counselling and follow‐up offered at 3, 6, 12 m | |
| Outcomes | Abstinence at 12 m, continuous and validated at 1 m and 3 m Validation: urine cotinine < 400 ng/ml at each ADAU clinic visit | |
| Notes | Both intervention and control included a nursing component so not in main analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random list of odd and even numbers was generated and a sequence of 200 sealed envelopes created." |
| Allocation concealment (selection bias) | Low risk | "With patient consent an envelope was opened and they were assigned to either programme." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants who attended basic ADAU follow‐up programme assessed, so large number of dropouts. More dropouts in group 2 (79%) than group 1 (51%), so treating dropouts as smokers may overestimate treatment effect. 9 deaths (4/5) excluded from denominator in analysis |
Froelicher 2004.
| Methods | Country: USA Recruitment/setting: Inpatients with CVD or PVD admitted to 10 hospitals | |
| Participants | 277 female current smokers or recent quitters (smoked in month before admission), willing to make serious quit attempt at discharge Av. age 61, av. cpd 18 ‐ 19 Intervention delivered by trained research nurses | |
| Interventions | 1. As usual care + nurse‐managed cessation and relapse prevention: 30 ‐ 45 mins individual counselling predischarge with multimedia materials. Up to 5 phone calls (5 ‐ 10 mins) at 2, 7, 21, 28, 90 days. Relapsers offered additional session 2. Usual care; brief physician counselling, Self‐help pamphlet, list of resources Patch or gum offered to selected women after discharge who had relapsed and wanted to try to quit (pharmacotherapy used by 20% of intervention and 23% of control group) Intensity: High | |
| Outcomes | Abstinence at 12 m (7‐day PP). Also followed at 24 m, 30 m but validation not attempted Validation: Saliva cotinine < 14 ng/ml or family/friend verification | |
| Notes | New for 2008/1 update 11 deaths at 12 m, excluded from cessation denominators. 73% of participants reached at all 4 follow‐ups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was by random permuted blocks, stratified by hospital, with an equal chance of assignment to the usual‐care group or the intervention group." |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 participants (13 intervention; 7 control) lost to follow‐up included in MA as smokers. 11 deaths excluded from MA |
Gilbody 2015.
| Methods | Country: United Kingdom Recruitment: smokers aged 18 or over with a severe mental illness who wanted to cut down or quit smoking |
|
| Participants | 97 participants were randomised (intervention = 33, control = 35), of whom 15 withdrew | |
| Interventions | 1. Bespoke Smoking Cessation service which was delivered by a mental health nurse trained to deliver smoking cessation behavioural support. This was an individually‐tailored smoking cessation service based on current guidelines but with enhanced levels of contact and support 2. Usual care where participants were advised to see their GP or consult with usual NHS quit smoking services |
|
| Outcomes | The primary outcome was CO‐verified smoking cessation at 12 months | |
| Notes | New for 2017 update Funding: National Institute of Health Research Health Technology Assessment Programme Declarations of interest: authors declare no competing interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomization was used following a computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Automated: "We used a secure telephone randomisation service run by the York Trials Unit to generate the random sequence and make the random allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 participant withdrawals in total (15%), 5 from the usual‐care arm and 10 from the intervention arm |
Hajek 2002.
| Methods | Country: UK Recruitment/setting: inpatients with MI or for CABG at 17 hospitals | |
| Participants | 540 smokers or recent quitters (26%) who had not smoked in hospital and motivated to quit. 26 deaths, 9 moved address excluded from denominator in analysis Intervention delivered by nurses alongside other duties | |
| Interventions | 1. As control, + CO reading, booklet on smoking and cardiac recovery, written quiz, offer to find support buddy, commitment, reminder in notes. Implemented by cardiac nurses during routine work, est time 20 mins 2. Verbal advice, Smoking and Your Heart booklet | |
| Outcomes | Abstinence at 12 m, sustained (no more than 5 cigs since enrolment and 7‐day PP) Validation: saliva cotinine < 20 ng/ml (CO used at 6‐week follow‐up and for visits at 12 m) | |
| Notes | Control meets criteria for a low‐intensity intervention, so not included in comparison 1, but included there and in inpatient CVD category in sensitivity analyses (Comparisons 4 and 5) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization method not described |
| Allocation concealment (selection bias) | Low risk | Nurses opened a "serially numbered, opaque, sealed envelope designating the patient's allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant differences in numbers lost to follow‐up or participants who had died or moved away. Those who had died or moved away excluded from outcome data; those lost to follow‐up counted as smokers |
Hanssen 2007.
| Methods | Country: Norway Recruitment/setting: inpatients with AMI | |
| Participants | 133 daily smokers amongst 288 participants. Not selected by motivation Demographics not given for smoking subgroup Intervention delivered by research nurses | |
| Interventions | 1. Structured but individualized telephone support addressing lifestyle issues including smoking, diet and exercise. Nurse‐initiated calls at 1, 2, 3, 4, 6, 8,12, 24 weeks post‐discharge. Smoking not explicitly addressed at each call. Reactive phone support line available 6 hrs/week 2. Usual care; outpatient visit at 6 ‐ 8 weeks and primary care follow‐up Intensity: High | |
| Outcomes | PP abstinence at 6, 12 and 18 m. Primary trial outcome was health‐related quality of life Validation: none | |
| Notes | New for 2008/1 (6m data only). Longer‐term data added 2013. 12 m data now used in analyses as 18 m data include rise in abstinence in control group; given outcome is PPA using 18 m data judged too conservative. Smoking was part of a multicomponent intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated list of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | "Group allocation in sealed opaque envelopes prepared by the researcher." However, fewer control group participants raises possibility of selection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | More lost to follow‐up in intervention group at all follow‐up points; participants with missing data at final follow‐up counted as smokers |
Hasuo 2004.
| Methods | Country: Japan Recruitment: Inpatients (all diagnoses) | |
| Participants | 120 current smokers or recent quitters (smoked in past month) who intended to be quit on day of discharge Diagnoses include cancer (n = 37), cardiac (n = 57) | |
| Interventions | 1. Intervention: nurse counselling (3 x 20‐min sessions). Telephone follow‐up with focus on relapse prevention at 7, 21, 42 days (5‐min/call) 2. Control: Same inpatient counselling but no follow‐up contact | |
| Outcomes | Abstinence at 12 m (not defined) Validation: urinary cotinine | |
| Notes | New for 2008/1 Both groups included inpatient counselling so not used in main comparison; effect of telephone follow‐up. Intervention was intended to prevent relapse | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization stratified by smoking status, FTND, and self‐efficacy |
| Allocation concealment (selection bias) | Low risk | Computerized programme randomly assigned individual participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | More control participants missing outcome data at 12 m than intervention group (9 versus 5). MA denominators exclude 6 deaths, but include 8 who were still smoking on day of discharge. This gives marginally larger relative effect |
Hennrikus 2005.
| Methods | Country: USA Recruitment/setting: Inpatients (all diagnoses) admitted to 4 hospitals Selected: Invited to participate | |
| Participants | 2095 current smokers (smoked in past week and considered self to be regular smoker for at least a month in past year) Not selected by motivation; approx 10% in each group confident they could quit Av. age 47 Intervention delivered by research nurses | |
| Interventions | 1. Brief advice: as control, plus labels in records to prompt advice from nurses and physicians 2. Brief advice and counselling: As 1. plus 1 bedside (or phone) session using motivational interviewing and relapse prevention approaches and 3 to 6 calls (2 ‐ 3 days, 1 wk, 2 ‐ 3 wks, 1 m, 6 m) 3. Control: modified usual care: smoking cessation booklet in hospital (not used in MA) Intensity: High Pharmacotherapy not offered | |
| Outcomes | Abstinence at 12 m (7‐day PP). Validation: saliva cotinine < 15 ng/ml | |
| Notes | Brief advice and counselling regarded as nurse intervention, compared to Brief advice. Including Usual Care in control as well would marginally increase RR but not change conclusion of no effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Research assistants... randomized [participants] to one of three treatment conditions by looking up the next available group assignment on a list on which the three conditions were randomly ordered within blocks of 30 assignments." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 78 deaths and ineligible (too ill) for follow‐up excluded from denominators; all other participants missing data at final follow‐up counted as smokers. Similar numbers lost to follow‐up in all groups |
| Other bias | High risk | High and differential levels of refusal to provide validation and of misreporting |
Hilberink 2005.
| Methods | Country: Netherlands Recruitment/setting: People with COPD identified by prescription and diagnosis codes in 43 general practices | |
| Participants | 392 current smokers with COPD. Not selected for motivation, ˜ 50% willing to quit within 6 m, different between groups 50% women, av. age 59 Parts of intervention delivered by practice nurses alongside other duties | |
| Interventions | 1. SMOCC intervention: booklet for COPD population and video. Stage‐based intervention: Precontemplators given information on advantages of quitting. Contemplators received self‐efficacy‐enhancing intervention, discussion of barriers, info on NRT if dependent and further visit at 2 weeks. Preparers had visit to GP to schedule quit date and max 2 follow‐ups, and max 3 phone calls from practice nurse/assistant 2. Usual care Intensity: High | |
| Outcomes | PP abstinence at 6, 12 m Validation: Urine cotinine < 50 ng/mL at 12 m | |
| Notes | First included in 2008. 1 year follow‐up reported in Hilberink 2011 ‐ data changed in 2013 update. Only the telephone follow‐up for people in preparation stage was explicitly provided by a nurse. Paper notes that practices differed in amount of tasks delegated to practice nurses Paper reports use of multilevel modelling. No adjustment to crude RR needed for clustering Denominators exclude 2 deaths and those for whom follow‐up not attempted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization took place on practice level." No information provided on sequence generation |
| Allocation concealment (selection bias) | High risk | "Self‐selection at practice level possibly affected the quit rate too." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/48 practices dropped out after randomization, leading to an imbalance in numbers of participants. Dropouts counted as smokers; deaths and participants for whom follow‐up was not attempted at 6 m excluded from outcome data |
Hollis 1993.
| Methods | Country: USA (Portland, OR) Recruitment: Internal medicine/family clinics | |
| Participants | 2691 internal medicine/family clinic adults who reported being a smoker on a questionnaire | |
| Interventions | 1. Brief physician advice (30 secs and pamphlet from nurse) 2. Brief physician message plus nurse who promoted self‐quit attempts ‐ advice, CO feedback, 10‐min video and manual (1 of 3 types) + follow‐up call and materials 3. Brief physician advice plus nurse‐promoted group programme ‐ advice, CO, + video‐ask to join group with schedule, coupon, etc, follow‐up calls 4. Brief physician advice, and nurse‐offered choice between self‐directed and group‐assisted quit ‐ shown both types of materials Intensity: High | |
| Outcomes | Abstinence at 1 yr (2 point prevalence) Validation: Saliva cotinine at 12 m | |
| Notes | All 3 nurse‐mediated interventions compared with 1. Saliva samples only obtained for approx half of reported quitters. Compliance and confirmation rates did not differ between groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Two random digits contained in the patient's health record number were used to assign patients to one of ... four interventions." |
| Allocation concealment (selection bias) | High risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Smoking status missing for 24% of participants at 12 m; "response rates did not differ significantly across treatment groups." Non‐respondents counted as smokers |
Hornnes 2014.
| Methods | Country: Denmark Recruitment: 2 university medical sites |
|
| Participants | 254 participants (intervention = 116, control = 138) with a history of smoking, an estimated 44% of these were motivated to quit. These participants were patients admitted with an acute stroke or transient ischaemic attack who met the inclusion criteria 38% women in intervention; 42% women in control Average age of 71 years in intervention; 70 years in control |
|
| Interventions | 1. Study nurses trained to perform motivational interview at home at 1, 4, 7 and 10 months after discharge from hospital. These visits usually lasted 1 hour with tailored advice on smoking cessation or continued refraining from smoking 2. The control group had the usual treatment of the stroke units, including lifestyle counselling which had 1 session with a nurse trained in motivational interviewing |
|
| Outcomes | Smoking status at 2 years. Unsure what the abstinence criteria were, likely self‐reported as no mention of biochemical verification | |
| Notes | New for 2017 update. Control group (usual care) did include some nurse contact so question over whether to include in primary analysis; have included, as control group was 'lifestyle' rather than smoking cessation‐specific. Sensitivity analysis removing Hornnes 2014 from primary analysis did not impact result. Denominator excludes deaths but also excludes those with severe dementia/disease (1 I, 3 C) as results not reported for those patients Funding: the study was supported by the Ludvig and Sara Elsass Foundation, the Lundbeck Foundation and The Danish Heart Foundation (Grant 07‐4‐B703‐A1378‐22384F). Declarations of interest: Klaus Groes Larsen is an employee at H. Lundbeck A/S. The other authors report no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | |
Janz 1987.
| Methods | Country: USA (Michigan) Recruitment setting: OP Dept Med Clinic (R.A.) | |
| Participants | Smokers (≥ 5 cpd) attending clinics | |
| Interventions | 1. Physician discussed personal susceptibility, self‐efficacy and concern, trained nurse counselled on problems and strategies 2. As 1, and self‐help manual Step‐by‐Step Quit Kit + 1 telephone call 3. Usual‐care control (from physicians not involved in study) Intensity: Low | |
| Outcomes | Abstinence at 6 m (self‐report by telephone) Validation: none | |
| Notes | 1 and 2 vs 3. Interventions included both physician and nurse components Data derived from graphs of percentages. Original data sought but not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each clinic site was divided into half‐day clinic units with each unit assigned to either experimental or control status....Smokers at the clinic on experimental half‐days were further randomized into one of two experimental groups." Method of sequence generation not specified |
| Allocation concealment (selection bias) | High risk | Allocation dependent on half‐day clinic attended |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if participants lost to follow‐up counted as smokers. Total dropout from baseline to 6 m = 15.6%. "Drop‐out rates did not vary significantly across study groups." |
Jiang 2007.
| Methods | Country: China Recruitment: Patients at 2 tertiary medical centres |
|
| Participants | 71 people with CHD who had used tobacco within 6 m before hospital admission (out of a larger sample of 167 CHD patients) 60% men, av. age 62 |
|
| Interventions | 1. Nurse‐led cardiac rehabilitation programme (addressing medication adherence, diet, exercise, and smoking where relevant). Individual teaching in hospital followed by 12 wks individual support post‐discharge from experienced cardiac nurse 2. Usual care Intensity: High |
|
| Outcomes | PP abstinence at 12 m Validation: no biochemical validation. Smoking status confirmed by family member |
|
| Notes | New for 2013 update. Demographic characteristics based on broader sample of 167, not broken down into smokers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generalized random table" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of smokers lost to follow‐up not specified. Participants lost to follow‐up who had been smoking at baseline counted as smokers |
Jorstad 2013.
| Methods | Country: Netherlands Recruitment: participants in 11 centres aged 18 ‐ 80 if they had been diagnosed with ACS within 8 weeks prior to the entry to the study |
|
| Participants | 754 participants (intervention = 375, control = 379) were randomized with 58 dropouts 73% women in intervention; 74% women in control Av. age 57.5 years in intervention; 57.8 years in control |
|
| Interventions | 1. 4 outpatient clinic visits to a cardiovascular nurse during the first 6 months after inclusion, at weeks 2, 7, 12 and 17 after baseline. The nurse programme was based on healthy lifestyles, biometric risk factors and medication adherence 2. Usual care included outpatient clinic visits to treating cardiologists and other relevant specialists and referral to cardiovascular rehabilitation programme in line with national guidelines for secondary prevention of cardiovascular disease |
|
| Outcomes | Self‐reported abstinence at 12 months | |
| Notes | New for 2017 update. Data included in MA were obtained through contacting study authors Funding: the study was sponsored by an unrestricted grant from AstraZeneca, The Netherlands. Authors state the sponsor had no role in the design, data collection, data analysis, data interpretation and writing of this report. Declarations of interest: authors declare no competing interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of pregenerated block‐stratified randomization protocol: "The online randomisation protocol consisted of a pregenerated block‐stratified randomisation protocol (http://www. responsestudie.nl). Study personnel entered patient’s initials, date of birth and gender, and participating individuals were assigned a study identification number along with their allocation to either the intervention group or control group " |
| Allocation concealment (selection bias) | Low risk | Automated, see above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a 7% dropout from the intervention arm and 10% from the control arm |
Kadda 2015.
| Methods | Country: Greece Recruitment: 500 participants who had undergone open heart surgery |
|
| Participants | 500 participants randomized (250 to control and 250 to intervention) Sex: 74% men in intervention; 75% men in control Average age: 65.8 years in intervention; 63.8 years in control |
|
| Interventions | 1. Intervention arm received in‐depth teaching on appropriate dietary, non‐smoking and exercise behaviours with follow‐up support. Each face‐to‐face session lasted for about 2 hours, and was on an individual patient basis delivered by experienced, trained clinical nurses 2. Control arm received usual care, including general instructions for adopting healthy diets, initiation of physical activity programmes, empowered to start walking and to quit smoking |
|
| Outcomes | Follow‐up at 1 year with self‐reported smoking status | |
| Notes | New for 2017 update. Data included in MA were obtained through contacting study authors Funding: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using a binary sequence of random numbers that was created in MSExcel using the rand() command |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The response rate at follow‐up was 100% |
Kim 2003.
| Methods | Country: South Korea Recruitment: 152 male smokers who visited family practice at 1 tertiary hospital between November 2001 and January 2002 |
|
| Participants | 152 men (intervention = 76, control = 76) Average age: 45.9 years in intervention; 46.0 years in control |
|
| Interventions | 1. Trained nurse provided telephone counselling to experimental group at 8th and 17th week of follow‐up 2. Control was self‐help material |
|
| Outcomes | Self‐reported smoking status at 25 weeks, no further detail provided | |
| Notes | New for 2017 update. Study in Korean so had to be translated for inclusion in this review. Brief report, so some detail lacking | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified: "… using random sequence, they were randomized to experimental group (76) and control group (76)…” |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 55/76 intervention and 57/76 control follow‐up at 25 weeks |
Kim 2005.
| Methods | Country: South Korea Recruitment/setting: Internal medicine outpatient department | |
| Participants | 401 daily smokers, 65% willing to quit within 1 m 92% men, av. age 52 | |
| Interventions | Test of 5As approach recommended by US AHCPR guideline. All participants Asked about smoking status and Advised to quit by physicians. Counsellors (retired nurses trained in cessation) Assessed willingness to quit, and enrolled and randomized participants 1. Intervention: Counsellors provided Assist and Arrange components to participants willing to quit within 1 m; set quit date, provided self‐help materials, supplied cigarette substitute. Culturally‐specific for Koreans. Other participants given 4Rs 2. Control: Counsellors told participants to quit without further assistance | |
| Outcomes | Abstinence at 5 m Validation: CO ≤ 7 ppm | |
| Notes | New for 2008/1 update Marginal to include because 5 m follow‐up and counsellors were retired nurses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly assigned the study participants to either the intervention or the control group. This was consummated according to a random list determined by fixed randomization with an allocation ration of 1:1, a block size of 6 and 12 allocation strata." |
| Allocation concealment (selection bias) | Low risk | "The treatment assignments based on each of the 12 stratum were placed in sealed opaque envelopes which the counselors opened at the formal enrollment of the study participants." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very low loss to follow‐up (7/401). All lost to follow‐up counted as smokers |
| Other bias | High risk | Differential validation rates (58.3% intervention; 69.2% control). |
Lancaster 1999.
| Methods | Country: UK Recruitment setting: General practice, recruitment during a visit or by letter. Smokers who completed a questionnaire about smoking habits. | |
| Participants | 497 smokers (av. cpd 17) | |
| Interventions | 1. Physician advice (face‐to‐face or in a letter) and a leaflet 2. As 1, plus invitation to contact a trained practice nurse for more intensive tailored counselling. Up to 5 follow‐up visits offered | |
| Outcomes | Abstinence at 12 m (sustained at 3 m and 12 m) Validation: saliva cotinine at 3 m and 12 m | |
| Notes | 2 vs 1. Only 30% took up offer of extended counselling. Included in high‐intensity subgroup based on intended intervention but sensitivity analysis for effect of treating as low intensity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent statistical advisor performed randomization from computer‐generated random numbers." |
| Allocation concealment (selection bias) | Low risk | "The allocations, in blocks of 20, were in sequential sealed, opaque envelopes opened by the research nurse at the time of recruitment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 75% completed follow‐up survey at 12 m; number in each group not specified Participants lost to follow‐up counted as smokers |
Lewis 1998.
| Methods | Country: USA Recruitment setting: hospital inpatients (excluding some cardiac conditions) | |
| Participants | 185 hospitalized adults; self‐reported ‘regular use’ for at least 1 year Counselling intervention delivered by research nurse | |
| Interventions | 1. Minimal care (MC): motivational message from physician to quit plus pamphlet 2. Counselling and nicotine patch (CAP) 3. Counselling and placebo patch (CPP) In addition groups 2 and 3 received a motivational message and instructions on patch use from physician, 4 sessions of telephone counselling by nurse based on CBT and motivational interviewing Intensity: High | |
| Outcomes | 7‐day PP abstinence at 6 m Validation: CO ≤ 10 ppm | |
| Notes | Compared 3 vs 1; Nurse counselling and placebo patch compared to minimal care to avoid confounding with effect of NRT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patient was randomized... using a predetermined computer‐generated randomization code." |
| Allocation concealment (selection bias) | Low risk | Randomization determined centrally |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants lost to follow‐up counted as smokers; exact numbers lost to follow‐up not specified |
Meysman 2010.
| Methods | Setting: Surgical wards in 4 Flemish hospitals Recruitment: inpatient smokers on surgical wards | |
| Participants | 358 adult smokers admitted for surgery; randomized to experimental (178) or control (180) groups 63% men, mean age 43.2, 39% smoked > 20 cpd, 61% 10 ‐ 20 cpd Motivation to quit not required | |
| Interventions | 1. Brief nurse‐led counselling session; SoC assessed, and appropriate advice given, i.e. precontemplators got risks of smoking and health gains after cessation, contemplators got barriers and pitfalls to quitting, + raising self efficacy, and preparers were either referred to a SC counsellor of agreed a cessation plan with the nurse
2. Standard booklet on smoking cessation Level of intensity: High |
|
| Outcomes | Self‐reported continuous abstinence on discharge and at 6 m post‐discharge Validation: None | |
| Notes | New for 2013 update. Intervention delivered by qualified smoking cessation nurse 4 in control group and 9 in experimental group used some form of pharmacological smoking cessation aid |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants stratified by SoC. Method of randomization not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants lost to follow‐up counted as smokers, exact numbers not provided |
Miller 1997.
| Methods | Country: USA (California) Recruitment setting: hospital inpatients | |
| Participants | 1942 hospitalized smokers (any tobacco use in week prior to admission) Counselling delivered by a research nurse | |
| Interventions | 1. Intensive: 30‐min inpatient counselling, video, workbook, relaxation tape + 4 phone calls after discharge 2. Minimal: 30‐min counselling etc. + 1 phone call 3. Usual care Intensity: High | |
| Outcomes | Abstinence at 12 m (PP, sustained abstinence also reported, but not by disease subgroup) Validation: plasma cotinine or family member corroboration at 12m | |
| Notes | 1+2 vs 3 in main analysis ‐ classifying both interventions as high intensity. Cardiovascular and other diagnoses separated in analysis by setting. 1 vs 2 in analysis of effect of additional telephone contact (sustained abstinence) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified |
| Allocation concealment (selection bias) | Low risk | "Nurses opened sealed envelopes in front of patients to determine patients' assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Deaths excluded from MA denominator; all others lost to follow‐up considered smokers; similar loss to follow‐up across all groups (10%) |
Nagle 2005.
| Methods | Country: Australia Recruitment/setting: Inpatients (all diagnoses) admitted to 1 teaching hospital (excluding intensive care units), invited to participate | |
| Participants | 1422 current smokers or quitters (including 331 who had quit in past 12 m). Not selected by motivation 40% men in intervention group, 33% men in controls Main part of intervention delivered by specialist | |
| Interventions | 1. Assessment and identification of smokers with the Smoking Cessation Clinical Pathway as chart reminder for ward nurses, Clinical Nurse Specialist provided 2 brief counselling sessions, offer of NRT, (3% provided) discharge letter 2. Usual care and assessment of smoking status, no standardized clinical assessment Intensity: Low (borderline) | |
| Outcomes | Abstinence at 12 m ( 7‐day validated PP, continuous self‐reported abstinence also given) Validation: Saliva cotinine ≤ 15 ng/ml | |
| Notes | New for 2008/1 update Study includes recent quitters; no difference in intervention effect. 85% of recent smokers received at least 1 counselling session, 38% received 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized. "Randomization was based on blocks of 20 patients being assigned to either control or intervention. Stratification into recent smoker and recent quitter categories occured prior to randomization." |
| Allocation concealment (selection bias) | Low risk | "Patients who reported smoking within the last 12 months were entered by the research assistant at the patient's bedside into the LAPSMOKE program on a laptop computer, which gave an immediate random allocation to either control or intervention that could not be changed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "At 12 months no difference for completed surveys or for loss to follow‐up existed between the intervention group and the control group." 28 deaths at 12 m excluded from denominator, all other participants missing data counted as smokers |
Nebot 1992.
| Methods | Country: Spain Recruitment: Primary Care Centre (participants not selected for motivation to quit) | |
| Participants | 425 smokers (at least 1 cpd in past wk) | |
| Interventions | 1. Physician advice 2. Physician advice and nicotine gum 3. Nurse counselling (up to 15 mins) Intensity: Low All received booklet and offer of follow‐up visit or call | |
| Outcomes | Abstinence at 12 m (sustained at 2 m and 12 m) Validation: 1/4 validated by expired CO at 2 m | |
| Notes | 3 vs 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each PCT was randomly allocated to perform the three different interventions successively. Each physician was assigned to a different intervention option every week, so that during that week they could not include any patient under an intervention option different from previously scheduled." Method of sequence generation not specified |
| Allocation concealment (selection bias) | High risk | Randomization does not allow blinding of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 76/425 participants lost to follow‐up; numbers lost to follow‐up not broken down by group; those lost to follow‐up at 2 m not included in final analysis |
OXCHECK 1994.
| Methods | Country: UK Recruitment: People aged 35 ‐ 64 in 5 urban general practices (family practice) who returned a baseline questionnaire | |
| Participants | 11,090 general practice patients | |
| Interventions | 1. Health check and risk factor counselling 2. Delayed intervention | |
| Outcomes | Smoking prevalence, and reported quitting in previous year | |
| Notes | Not included in MA because outcome not directly comparable with cessation studies. When all intervention participants (including non‐attenders) are compared to controls there was no significant difference in the proportion who had stopped smoking in previous year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were... randomized by household to be offered a health check during a specified year over the four year period... After a health check had been performed during the first two years of the study half the patients were randomly assigned to be re‐examined annually." Method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "We used values from the initial health check for non‐attenders... The difference between controls and attenders will over‐estimate the effectiveness of the health check because non‐attenders are likely to have been less compliant with advice than attenders." |
Pardavila‐Belio 2015.
| Methods | Country: Spain Recruitment was over 2 campuses and 14 college schools. Methods used to recruit participants included announcements on university signboards, newspapers, website and emails inviting all undergraduate and masters students to participate |
|
| Participants | 255 college student smokers (intervention = 133, control = 122) (age 18 ‐ 24 years, mean = 20.1 years intervention, 20.5 years control) 38% were men |
|
| Interventions | 1. Intervention arm received a 50‐minute motivational interview conducted by a nurse with online self‐help material. The follow‐up included a reinforcing email and group therapy 2. The control group received brief advice (5 ‐ 10 minutes) and a self‐help pamphlet, Stop Smoking |
|
| Outcomes | Self‐reported abstinence, with biochemical verification at 6 months (urine cotinine measurement) | |
| Notes | New for 2017 update 37.6% of the participants randomised to the intervention group did not receive the complete protocol Funding: funded by the María Egea Foundation, University of Navarra (Spain) Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation method. 1 member of the team generated a blocked random number sequence, using Epilnfo version 7.0.9.7, and prepared sealed opaque sequentially‐numbered enveloped (1 ‐ 255) with the corresponding condition written inside. After each student agreed to participate in the study, the envelope was opened, determining the group to which he or she would be assigned |
| Allocation concealment (selection bias) | Low risk | Nurse and student unaware of which arm participant would be assigned to until envelope opened |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11/122 control and 19/133 intervention lost to follow‐up |
Quist‐Paulsen 2003.
| Methods | Country: Norway Recruitment/setting: Inpatients admitted to cardiac ward of 1 general hospital, invited to participate | |
| Participants | 240 current smokers (smoked daily before symptoms began) Av. 15 cpd Intervention delivered by 3 cardiac nurses | |
| Interventions | 1. Intervention: Usual care plus 1 ‐ 2 sessions with nurse using booklet focusing on fear arousal and relapse prevention. 5 telephone follow‐ups at 2, 7, 21 days, 3 m, 5 m). Clinic visit to nurse at 6 wks Gum or patch encouraged for participants with strong urges to smoke in hospital 2. Control: usual care (advice to quit + booklet) Intensity: High | |
| Outcomes | PP abstinence at 12m Validation: urine cotinine < 2.0 mmol/mol creatinine | |
| Notes | New for 2008/1 update. Included in CVD subcategory | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was in blocks of varying sizes." |
| Allocation concealment (selection bias) | Low risk | "The nurses were given a serially numbered sealed envelope from a secretary who was otherwise uninvolved in the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | MA does not include 5 deaths, 2 participants who had moved away and 10 post‐randomization withdrawals due to change in diagnosis; all other losses to follow‐up considered to be smoking (18 in intervention group, 4 in control group) |
Ratner 2004.
| Methods | Country: Canada Recruitment: Patients having presurgical assessment | |
| Participants | 237 smokers (past 7 days) awaiting elective surgery 52% women Av. 12 cpd | |
| Interventions | 1. Pre‐admission clinic 15 mins counselling from trained research nurse, materials, nicotine gum, quit kit, hotline number. Post‐operative counselling in hospital. 9 follow‐up calls over 16 wks 2. Usual care Intensity: High | |
| Outcomes | PP abstinence at 12 m Validation: urine cotinine (NicoMeter) | |
| Notes | New for 2008/1 update Included in hospitalized patient subgroup for Comparison 2 although the initial intervention was delivered pre‐admission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated, randomly determined group allocation." |
| Allocation concealment (selection bias) | Low risk | Nurses opened a "sealed envelope" after recruiting participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who withdrew or were lost to follow‐up (28.7%) were counted as smokers. "There was no differential loss to follow‐up at 12 months." 9 deaths at 12 m excluded from denominators |
Rice 1994.
| Methods | Country: USA (Michigan) Recruitment: Previously hospitalized; self‐referral or by provider | |
| Participants | 255 smokers (≥ 10 cpd) with CVD | |
| Interventions | 1. Smokeless programme, individual delivery by nurse, 5 sessions 2. Same programme, 5 group sessions 3. Same programme, written self‐help format 4. Usual care control Intensity: High | |
| Outcomes | Abstinence at 12 m Validation: saliva thiocyanate measured, but self‐report used as outcome | |
| Notes | 1+2+3 vs 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were initially stratified by the singularity elements of 1) sex, 2) smoking history and 3) a health factor, and then randomized to one of four intervention groups." Sequence generation through table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8% did not provide data at final follow‐up and counted as smokers in final analysis; 12 died before follow‐up and were not included in final outcome figures |
| Other bias | High risk | Differential non‐participation by experimental group assignments |
Rigotti 1994.
| Methods | Country: USA (Boston) Setting/ Recruitment: Cardiac surgery unit | |
| Participants | 87 smokers (1+ pack of cigs in past 6 m) scheduled for CABG | |
| Interventions | 1. 3 sessions behavioural model with video tape and face‐to‐face counselling by registered nurse 2. Usual care control Intensity: High | |
| Outcomes | Sustained abstinence at 12 m Validation: saliva cotinine < 20 ng/mL | |
| Notes | Abstinence rates include some smokers who had quit prior to surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to control or intervention groups after surgery." Method not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 deaths not counted in final meta‐analysis. No other participants lost to follow‐up at 12m |
Risser 1990.
| Methods | Country: USA Setting: Nurse‐staffed health promotion clinic | |
| Participants | 90 smokers attending health promotion clinic for annual visit | |
| Interventions | 1. 50‐min session, self‐help materials, offer of training and counselling programme 2. as 1, plus 10‐min personalized motivational intervention with spirometry, CO measurement and discussion of symptoms | |
| Outcomes | PP abstinence at 1yr Validation: expired CO | |
| Notes | Not in main comparison: effect of additional components No group without intervention. (No true control group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/45 lost to follow‐up in control group, 13/45 lost to follow‐up in treatment group; counted as smokers in final analysis. "Although attrition was unevenly distributed in the two groups, the reasons for attrition were distributed similarly in both groups." |
Sanders 1989a.
| Methods | Country: UK Setting: Primary care clinics (11) | |
| Participants | 4210 primary care clinic attenders identified by questionnaire as smokers | |
| Interventions | 1. Asked by doctor (following advice to quit) to make appointment with nurse for health check. Advice, discussion, leaflet and offer of follow‐up by nursing 2. Usual care control Intensity: Low | |
| Outcomes | Sustained abstinence at 12 m (self‐report of not smoking at 1 m and 12 m and gave date on which they last smoked as before the 1 m follow‐up) Validation: urine cotinine | |
| Notes | Only a sample of usual‐care group followed up so not appropriate to use data in main MA. A significant effect of the intervention was apparent only for the sustained cessation outcome. 12 m PP abstinence rates were 11.2% for intervention, 10% for control (NS) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "...smokers identified were intended to be allocated to a control or intervention group on a one to two basis according to day of attendance... The designation of specific days was itself randomized across weeks and practices..." |
| Allocation concealment (selection bias) | High risk | Randomized according to day of attendance. "Although the doctors were given a desktop card to remind them which were control days and which intervention, 120 patients were allocated to the wrong group and were excluded from further analysis." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only a random sample of the control group was followed up. Non‐respondents counted as smokers. Response rate significantly higher in attenders (63.8% versus 54.4% in non‐attenders and 56.5% in control) |
Sanders 1989b.
| Methods | Country: UK Setting: Primary care clinics (11) | |
| Participants | 751 smokers who attended a health check (having been randomly allocated to an intervention offering a health check ‐ see Sanders 1989a) | |
| Interventions | 1. Health check from a practice nurse; advice, leaflet and offer of follow‐up 2. As 1, with demonstration of expired CO levels | |
| Outcomes | Sustained abstinence at 1 yr (self‐report of not smoking at 1 m and 12 m and who gave date on which they last smoked as before the 1 m follow‐up) Validation: urine cotinine in a sample of participants indicated a relatively high deception rate | |
| Notes | 2 vs 1 for effect of CO demonstration as an adjunct to nurse advice This was part of same study as Sanders 1989a, and randomized a subgroup of participants in the main study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | See above (Sanders 1989a) |
| Allocation concealment (selection bias) | High risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | See above |
Sanz‐Pozo 2006.
| Methods | Country: Spain Setting: Primary care clinic | |
| Participants | 125 daily smokers attending clinic, motivated to make a quit attempt but not interested in using pharmacotherapy Intervention 52% women, Control 62% women, av. age ˜ 40, av. cpd 19 | |
| Interventions | 1. Brief advice from doctor at recruitment, appointment with clinic nurse 7 days before TQD, on TQD, 1 wk, 1 m, 2 m, 3 m 2. Brief advice only No pharmacotherapy | |
| Outcomes | Sustained abstinence at 24 m (from 12 m) Validation: CO < 8 ppm | |
| Notes | New for 2008/1 Control group rates also higher at 12 m follow‐up. Some baseline differences but logistic regression did not alter conclusion of no effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients recruited were randomised, according to the clinic from which they came." Method not described |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants lost to follow‐up counted as smokers. Participants in intervention group who did not complete all sessions may have been automatically classified as smokers ‐ unclear what effect this may have had on results |
Smit 2016.
| Methods | Country: Netherlands Setting: General practices |
|
| Participants | 414 adult smokers with internet access, willing to quit within 6 months (intervention = 163, control = 132) 60% women, av. age 48, av. cpd NR, mean FTND 5.4 |
|
| Interventions | Participants recruited by practice nurses and asked to sign up for PAS via PAS website. Once signed up to PAS, randomized into 3 groups: 1. Multiple computerized tailoring and tailored counselling by a practice nurse (1 session face‐to‐face smoking cessation counselling by practice nurse at 6 wks (incl. discussion of medication in people who smoked > 10 cpd), plus telephone call at 6 m with offer of additional support + computer‐tailored cessation programme, based on I‐Change Model) 2. Multiple computerized tailoring (computer‐tailored programme alone, identical to above but without nurse counselling) ‐ note this is the control arm for our analyses 3. Usual care (not included in our analyses due to confounding) |
|
| Outcomes | Prolonged abstinence (since 6‐wk follow‐up) at 12 m Validation: saliva cotinine (cutoff not specified) |
|
| Notes | New for 2017 update, previously listed as ongoing (Dutch Trial Register NTR1351) Funded by KWF Kankerbestrijding (UM 2007‐3834) 1 vs 2 used in main analysis; 3 not included due to confounding Funding: supported by the Dutch Cancer Society (UM 2007‐3834) Declarations of interest: Hein de Vries is scientific director of Vision2Health, a company that licenses evidence‐based innovative computer‐tailored health communication tools. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization took place at respondent level and was conducted by means of a computer software randomization device” |
| Allocation concealment (selection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 93/163 I and 75/132 C followed up at 12 m. “Retention was unrelated to experimental condition” |
Steptoe 1999.
| Methods | Country: UK Setting: Primary care clinics (20) | |
| Participants | 404 smokers (from total of 883 people with modifiable CVD risk factors) | |
| Interventions | 1. Behavioural counselling using stages of change approach. 2 ‐ 3 20‐min sessions + 1 ‐ 2 phone contacts. NRT used if appropriate 2. Usual care | |
| Outcomes | Sustained abstinence at 12 m (4 m and 12 m) Validation: saliva cotinine | |
| Notes | Not included in MA. Used practice‐based analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "cluster randomized" using the "minimisation technique to balance groups for the Jarman score of social deprivation, ratio of patient to practice nurse hours per week, and fundholding status." Sequence generation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Participants who smoked... tended to drop out more in the intervention than control groups." Overall dropout rates high |
Taylor 1990.
| Methods | Country: USA (California) Recruitment setting: Hospital (patients with AMI) | |
| Participants | 173 smokers following AMI. Smoker defined as any use of tobacco | |
| Interventions | 1. Nurse counselling on self‐efficacy, benefits and risks, + manual coping with high‐risk situations Further telephone counselling as needed up to 6 m 2. Usual care control Intensity: High | |
| Outcomes | Abstinence at 12 m Validation: serum thiocyanate < 110 nmol/L, expired CO < 10 ppm | |
| Notes | Nurses averaged 3½ hours/participant including phone contact Slightly higher loss to follow‐up in control group. Nicotine gum was prescribed to 5 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random list of odd and even numbers was generated" |
| Allocation concealment (selection bias) | Low risk | "a sequence of numbers sealed in envelopes was created...the nurse assessing the intervention called the nurse coordinator who opened the next envelope to determine the condition to which the patient would be assigned" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants missing data counted as smokers. 14/86 participants in intervention group and 29/87 participants in control group missing data at 12‐m follow‐up |
Terazawa 2001.
| Methods | Country: Japan Recruitment setting: Workplace annual health check | |
| Participants | 228 male smokers, av. age 39, av. cpd 23 | |
| Interventions | 1. 15 ‐ 20‐min stage‐matched counselling by trained nurses. 4 follow‐up calls for those willing to set a quit date. 1 wk after intervention, 3 ‐ 4 days , 1 m, 3 m after cessation 2. Usual care | |
| Outcomes | Sustained abstinence at 12 m (> 6 m, validated at 6 m and 12 m) Validation: CO, urine | |
| Notes | 25 from intervention group set quit date More intervention group in preparation/contemplation II subgroups at baseline; 17 vs 7 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified |
| Allocation concealment (selection bias) | Low risk | "Randomly divided into two groups before contact with subject. Participants' employee ID numbers were used for assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported in either group |
Tønnesen 1996.
| Methods | Country: Denmark Recruitment setting: outpatient chest clinic | |
| Participants | 507 smokers of < 10 cpd or of > 10 cpd who had refused a trial of NRT. Age 20 ‐ 70 yrs Intervention delivered by clinic nurses given 8‐hr training and 3 problem‐solving meetings | |
| Interventions | 1. Motivational approach, 5 mins of benefits/risks, brochures on hazards and how to quit. 4 ‐ 6 wks letter sent 2. Control ‐ questionnaire and CO measurement. No advice to stop smoking Intensity: Low | |
| Outcomes | Sustained abstinence at 1 yr (stopped during intervention and no reported smoking during year) Validation: CO < 10 ppm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12% lost to follow‐up and counted as smokers; numbers lost in each group not specified |
Tønnesen 2006.
| Methods | Country: Denmark Recruitment setting: 7 outpatient chest clinics | |
| Participants | 370 smokers of > 1 cpd with COPD 52% women, av. age 61, av. cpd 20 | |
| Interventions | Factorial trial. Nicotine sublingual tablet and placebo arms collapsed in MA 1. High support: 7 x 20 ‐ 30‐min clinic visits (0, 2, 4, 8, 12 wks, 6 m, 12 m) and 5 x 10‐min phone calls (1, 6, 10 wks , 4½ m. 9 m), total contact time 4½ hrs 2. Low support: 4 clinic visits (0, 2 wks, 6 m, 12 m) and 6 phone calls (1, 4, 6, 9, 12 wks, 9 m), total time 2½ hrs | |
| Outcomes | Sustained abstinence at 12 m (validated at all visits from wk 2, PP also reported) Validation: CO < 10 ppm | |
| Notes | Not in main comparison; compares different intensities of nurse counselling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated to one of the four treatment groups using a block randomization list at each center." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 82/370 participants lost to follow‐up and counted as smokers. "One potential bias may have been the large early dropout of failures from the study. Consequently, these patients were not exposed to the possible effect of more intensive support." |
Vetter 1990.
| Methods | Country: UK (Wales) Recruitment setting: general practice (family practice) | |
| Participants | 226 smokers aged 60+ in general practice who completed a health questionnaire. Unselected by motivation to quit | |
| Interventions | 1. Letter asking participant to visit doctor who advised on importance of stopping smoking, opportunity to see practice nurse who gave advice on lifestyle factors concentrating on quitting smoking 2. No contact, completed questionnaire only Intensity: Low | |
| Outcomes | PP abstinence at 6 m Validation: expired CO (cut‐off point not stated) | |
| Notes | Intervention included nursing and physician advice | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% lost to follow‐up in intervention group; 9% lost to follow‐up in treatment group. Participants lost to follow‐up counted as smokers |
Wood 2008.
| Methods | Country: (multicentre) France, Italy, Poland, Spain, Sweden, UK, Netherlands, Denmark Recruitment setting: hospitals and general practices |
|
| Participants | 2554 smokers who were patients at participating GP practices (at high risk of developing CHD) or hospitals (with established CHD). Demographics not reported | |
| Interventions | 1. Nurse coordinated lifestyle intervention. Includes nurse counselling (at least 8 weekly sessions) covering smoking cessation where participants were self‐reported smokers at baseline 2. Usual care (not specified) |
|
| Outcomes | Abstinence at 12 m (definition not provided) Validation: CO < 6 ppm |
|
| Notes | New for 2013 Subset of participants from larger study which included smokers and non‐smokers. N provided above is smokers followed up at 12 m, larger study had 5405 participants. Baseline N smoking not clear, study does not contribute to MA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Cluster‐randomized, method of allocation concealment not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified for subgroup of smokers at baseline, not clear how smoking status of those smokers lost to follow‐up was reported |
Zwar 2015.
| Methods | Country: Australia Recruitment setting: general practices |
|
| Participants | 2390 daily or weekly smokers (intervention = 873, control = 677) aged 18+ presenting to general practices in Australia, recruited from 101 general practices 54% women, av. age 43, av. cpd 17 |
|
| Interventions | 1. GP encouraged all smokers to see Practice Nurse – face‐to‐face visit and then flexible package of ongoing support, incl. 3 further face‐to‐face visits and telephone support for participants who preferred that mode 2. Referral to quitline 3. GP usual care Participants in all 3 groups encouraged to use pharmacotherapy |
|
| Outcomes | 12‐m sustained abstinence (of at least 10 m) Validation: none |
|
| Notes | New for 2017 update, previously listed as ongoing (ACTRN12609001040257) Funded by Australian Government National Health and Medical Research Council (NHMRC) Main analysis includes 1 v 3; 2 not included in this review as not nurse‐based intervention Only 43% of participants in the PN intervention group attended to see the nurse. Pharmacotherapy use similar across all arms Funding: Australian National Health and Medical Research Council Project Grant (568617). Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | “Randomization of practices was performed after practice recruitment but prior to patient recruitment with allocation concealment by a researcher who took no further part in the study” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81.7% intervention and 82.3% control followed up at 12 m |
ACS = acute coronary syndrome AHCPR = Agency for Health Care Policy and Research CABG = Coronary Artery Bypass Graft CBT ‐ cognitive behavioural therapy CCU = Coronary Care Unit CHD = coronary heart disease CHF = congestive heart failure CO = carbon monoxide COPD = Chronic obstructive pulmonary disease cpd = cigarettes per day CVD = cardiovascular disease FTND: Fagerström Test for Nicotine Dependence ITT = intention‐to‐treat m = month(s) MA = meta‐analysis (A)MI = (Acute) Myocardial Infarction NRT = nicotine replacement therapy NS: not statistically significant PP = point prevalence PVD = peripheral vascular disease SMOCC = Smoking cessation for patients with COPD in general practice SoC = stage of change (precontemplation, contemplation, preparation, action) TQD = target quit date
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aertsen‐Van Der Kuip 2006 | Abstract only, 3 m follow‐up |
| Andrews 2007 | Cluster‐randomized trial with only 2 community clusters and baseline differences between participants. Nurse‐led counselling confounded with NRT availability and personal contact from a community health worker for the duration of the trial |
| Avanzini 2011 | Not a randomized trial, uses matched controls |
| Bredie 2011 | Only 3 m follow‐up |
| Browning 2000 | Not a randomized trial, uses historical control |
| Brunner‐Frandsen 2012 | Comparison of nurse‐delivered counselling with more intensive smoking cessation therapy. Intensive therapy confounded additional nurse‐delivered telephone support with specialist counselling and pharmacotherapy |
| Cabezas 2011 | Both nurses and/or physicians provided intervention |
| Carlsson 1998 | Describes 5 studies, only 1 reporting smoking cessation is included in review separately (Carlsson 1997) |
| Caslin 2006 | Abstract only, insufficient information for inclusion. Study of nurse intervention for smoking cessation in hospitalized inpatients |
| Chan 2005 | Pilot study of intervention directed at non‐smoking mothers with smoking partners; follow‐up < 6 m |
| Chan 2008 | Intervention directed at non‐smoking mothers with smoking partners |
| Duffy 2014 | Intervention delivered by multiple professionals, not just nurses (listed as Duffy 2012 in previous version, ongoing) |
| Efraimsson 2008 | Less than 6 m follow‐up |
| Fletcher 1987 | Number of quitters after 6 m not stated (Total of 20 participants) |
| French 2007 | Not RCT. Control and intervention ran sequentially. Study of a nurse‐delivered home‐visiting programme to prevent post‐partum relapse |
| Fritz 2008 | Adolescent smokers |
| Galvin 2001 | Only 3 m follow‐up (Total of 42 participants) |
| Gies 2008 | Only 3 m follow‐up. Non‐random assignment to nurse intervention and control. Randomly compared 1 and 4 phone follow‐ups |
| Griebel 1998 | Maximum follow‐up was 6 wks post‐hospital discharge |
| Haddock 1997 | No long‐term follow‐up. Randomization unclear |
| Hall 2007 | Follow‐up < 6 m |
| Happell 2014 | Paper only reports on intervention arm ‐ no control details available |
| Heath 2012 | Not randomized, evaluation |
| Hjalmarson 2007 | Not randomized, intervention allocated by treatment site |
| Jansink 2013 | Originally listed as ongoing study, now completed. Lifestyle intervention for Type 2 diabetes, smoking prevalence and smoking cessation results not reported |
| Jelley 1995 | Not RCT. Control and intervention ran sequentially |
| Johnson 1999 | Not RCT. No equivalent study groups, intervention allocated according to cardiac unit of admission |
| Johnson 2000 | Population and intervention not within scope. Recruited women who had stopped smoking during pregnancy for a relapse prevention intervention |
| Jonsdottir 2015 | Multi‐behaviour intervention, cannot separate out smoking components or smoking population |
| Katz 2012 | Not randomized; pre/post design |
| Kendrick 1995 | Intervention in pregnant smokers. See Chamberlain 2017 |
| Koelewijn‐van Loon 2009 | Signficant baseline difference in smoking behaviour between intervention and control groups. Data provided does not specify number quit in relation to those smoking at baseline |
| Kotz 2009 | Effects of nurse intervention (counselling) confounded with pharmacotherapy (nortriptyline) |
| Kruis 2014 | Intervention delivered by multiple professionals, not just nurses |
| Lakerveld 2010 | Abstract only, insufficient detail provided for inclusion |
| Lakerveld 2013 | Multibehaviour intervention, cannot separate out smoking components or smoking population |
| Lifrak 1997 | 4 advice sessions with a nurse practitioner compared with a more intensive intervention of 16 weekly therapy sessions. All also received nicotine patch therapy |
| Lou 2013 | Only (approximately) 40% of those delivering the intervention were nurses |
| McHugh 2001 | Multiple risk factor intervention with shared care. Cannot evaluate effect of nursing |
| Meulepas 2007 | Cluster‐randomized trial of intervention in people with COPD. Effect of nurse counselling confounded with effect of (GP‐initiated) COPD support service |
| O'Connor 1992 | Intervention in pregnant smokers. See Chamberlain 2017 |
| Persson 2006 | Study of nurse‐counselling for smoking cessation in general practice patients with diabetes. Practices assigned to intervention or control; practice assignment not randomized |
| Planer 2011 | Study of bupropion, both intervention and control groups received nurse support |
| Pozen 1977 | Intervention in post‐MI patients. Only 1 m follow‐up, and number of smokers at baseline not reported |
| Reeve 2000 | Follow‐up < 6 m |
| Reid 2003 | Stepped‐care intervention from nurse counsellor confounded with nicotine patch therapy (no evidence of effect of the combination) |
| Rigotti 1997 | Intervention not given by a nurse |
| Smith 2009 | Compares physician + nurse advice with nurse‐initiated counselling only |
| Stanislaw 1994 | Follow‐up < 6 m |
| Sun 2000 | Follow‐up < 6 m |
| Targhetta 2011 | Trial of training of medical staff, including nurses |
| Van Elderen 1994 | Multicomponent intervention, smoking cessation element not clear |
| Van Zuilen 2011 | Study of multifactoral lifestyle intervention for people with chronic kidney disease. Unable to extract data on baseline smokers only; insufficient detail on nature of stop smoking intervention to include |
| Wadland 1999 | Not randomized. The 2 groups were recruited by different means and given different interventions, both of which included telephone counselling by nurses or counsellors |
| Wadland 2001 | Follow‐up < 6 m (90 days). Nurses and counsellors provided telephone‐based intervention |
| Wewers 1994 | Follow up < 6 m |
| Wewers 2009 | Intervention led by lay health advisors (managed by nurses, but nurses never had contact with participants) |
| Wilson 2008 | Planned sample size of 303 not reached, only 91 participants randomized between 3 conditions. Adherence to interventions (5 hrs of individual or group support) was very low. No participants achieved complete cessation |
| Woollard 1995 | No data presented on number of smokers or quitting |
| Zakrisson 2011 | Evaluation of nurse‐led interdisciplinary intervention that involved physicians |
Characteristics of ongoing studies [ordered by study ID]
Lachman 2015.
| Trial name or title | Community‐based comprehensive lifestyle programs in people with coronary artery disease: Objectives, design and expected results of Randomized Evaluation of Secondary Prevention by Outpatient Nurse SpEcialists 2 trial (RESPONSE 2) |
| Methods | Multicentre randomized trial |
| Participants | Patients, 18 years or older, who have recently been hospitalized for CAD in the Netherlands |
| Interventions | In addition to usual care, patients in the intervention group are referred to ≥ 1 of 3 community‐based lifestyle programs, including the smoking cessation program: Luchtsignaal. |
| Outcomes | Proportion of participants not smoking (defined as urine cotinine concentration < 200 ng/mL) at 12 months |
| Starting date | April 2013 |
| Contact information | Ron J. G. Peters (r.j.peters@amc.nl) |
| Notes | Trial register no. NTR3937 Funding: The study was supported by unrestricted grants from Weight Watchers International, Inc, New York, NY, Philips Consumer Lifestyle, the Netherlands |
Mularski 2013.
| Trial name or title | Linking in‐hospital smoking cessation assistance with outpatient services and automated telephone follow‐up using dedicated nurse tobacco treatment specialists: a randomized controlled trial |
| Methods | Multicentre randomized controlled trial |
| Participants | Hospitalized adult (≥ 18 years) smokers who are interested in remaining abstinent after discharge |
| Interventions | Brief inpatient counseling with an assisted referral (AR) to existing outpatient counseling, discharge medications, and 4 interactive voice recognition (IVR) follow‐up calls 4 ‐ 49 days post‐discharge |
| Outcomes | Not stated |
| Starting date | Not stated |
| Contact information | R. A. Mularski (richard.a.mularski@kpchr.org) |
| Notes | Funded by: NHLBI 1U01HL1053231 (1 of 6 Consortium of Hospitals Advancing Research on Tobacco or CHART) |
NCT02106637.
| Trial name or title | Early in‐hospital initiation of pharmacotherapy for smoking cessation, concomitant with nurse‐led support, in patients after an acute coronary syndrome (ACS) |
| Methods | Prospective, double‐blind, randomized, placebo‐controlled, multicentre study |
| Participants | Adult smokers aged 21 years or older, who are in a stable clinical condition following a recent (< 10 days) ACS event |
| Interventions | Varenicline will be initiated during hospitalization and continued for 12 weeks following discharge |
| Outcomes | Continuous abstinence rate (CAR) from smoking 1 year after hospitalization, as determined by self‐reporting and verified by CO testing Non inferior serious adverse event (SAE) rate |
| Starting date | June 2014 |
| Contact information | Ilan Goldenberg (ilan.goldenberg@sheba.health.gov.il) |
| Notes | ClinicalTrials.gov Identifier: NCT02106637 |
Contributions of authors
VHR wrote the original review. For the 2017 update, VHR & LH screened studies and extracted data, and JHB and JLB performed data extraction and updated the text and meta‐analysis.
Sources of support
Internal sources
Wayne State University College of Nursing, Adult Health & Administration, USA.
Nuffield Department of Primary Care Health Sciences, University of Oxford, UK.
External sources
American Heart Association, USA.
National Institute of Health Research (NIHR), UK.
Declarations of interest
VHR was the principal investigator in one of the studies included in this review.
LH: None known
JLB: None known
JHB: None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Allen 1996 {published data only}
- Allen JK. Coronary risk factor modification in women after coronary artery bypass surgery. Nursing Research 1996;45(5):260‐5. [DOI] [PubMed] [Google Scholar]
Alterman 2001 {published data only}
- Alterman AI, Gariti P, Mulvaney F. Short‐ and long‐term smoking cessation for three levels of intensity of behavioral treatment. Psychology of Addictive Behaviors 2001;15(3):261‐4. [PubMed] [Google Scholar]
Aveyard 2003 {published data only}
- Aveyard P, Griffin C, Lawrence T, Cheng KK. A controlled trial of an expert system and self‐help manual intervention based on the stages of change versus standard self‐help materials in smoking cessation. Addiction 2003;98(3):345‐54. [DOI] [PubMed] [Google Scholar]
Aveyard 2007 {published data only}
- Aveyard P, Brown K, Saunders C, Alexander A, Johnstone E, Munafo MR, et al. Weekly versus basic smoking cessation support in primary care: a randomised controlled trial. Thorax 2007;62(10):898‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berndt 2014 {published data only}
- Berndt N, Bolman C, Froelicher ES, Mudde A, Candel M, Vries H, et al. Effectiveness of a telephone delivered and a face‐to‐face delivered counseling intervention for smoking cessation in patients with coronary heart disease: a 6‐month follow‐up. Journal of Behavioral Medicine 2014;37(4):709‐24. [DOI] [PubMed] [Google Scholar]
Bolman 2002 {published data only}
- Bolman C, Vries H, Breukelen G. A minimal‐contact intervention for cardiac inpatients: long‐term effects on smoking cessation. Preventive Medicine 2002;35(2):181‐92. [DOI] [PubMed] [Google Scholar]
- Bolman C, Vries H, Breukelen G. Evaluation of a nurse‐managed minimal‐contact smoking cessation intervention for cardiac inpatients. Health Education Research 2002;17(1):99‐116. [DOI] [PubMed] [Google Scholar]
- Segaar D, Willemsen MC, Bolman C, Vries H. Nurse adherence to a minimal‐contact smoking cessation intervention on cardiac wards. Research in Nursing & Health 2007;30(4):429‐44. [DOI] [PubMed] [Google Scholar]
Borrelli 2005 {published data only}
- Borrelli B, Hayes RB, Dunsiger S, Fava JL. Risk perception and smoking behavior in medically ill smokers: a prospective study. Addiction 2010;105(6):1100‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli B, Lee C, Novak S. Is provider training effective? Changes in attitudes towards smoking cessation counseling and counseling behaviors of home health care nurses. Preventive Medicine 2008;46(4):358‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli B, Novak S, Hecht J, Emmons K, Papandonatos G, Abrams D. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community‐nurse Assisted Research and Education on Smoking). Preventive Medicine 2005;41(5‐6):815‐21. [DOI] [PubMed] [Google Scholar]
- Hayes RB, Dunsiger S, Borrelli B. The influence of quality of life and depressed mood on smoking cessation among medically ill smokers. Journal of Behavioral Medicine 2010;33(3):209‐18. [DOI] [PubMed] [Google Scholar]
Campbell 1998 {published data only}
- Campbell NC, Ritchie LD, Thain J, Deans HG, Rawles JM, Squair JL. Secondary prevention in coronary heart disease: a randomised trial of nurse led clinics in primary care. Heart 1998;80(5):447‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NC, Thain J, Deans HG, Ritchie LD, Rawles JM, Squair JL. Secondary prevention clinics for coronary heart disease: randomised trial of effect on health. BMJ 1998;316(7142):1434‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie P, Campbell NC, Ritchie LD, Simpson JA, Thain J. Secondary prevention clinics for coronary heart disease: four year follow up of a randomised controlled trial in primary care. BMJ 2003;326(7380):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Canga 2000 {published data only}
- Canga N, Irala J, Vara E, Duaso MJ, Ferrer A, Martinez‐Gonzalez MA. Intervention study for smoking cessation in diabetic patients: a randomized controlled trial in both clinical and primary care settings. Diabetes Care 2000;23(10):1455‐60. [DOI] [PubMed] [Google Scholar]
Carlsson 1997 {published data only}
- Carlsson R, Lindberg G, Westin L, Israelsson B. Influence of coronary nursing management follow up on lifestyle after acute myocardial infarction. Heart 1997;77(3):256‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2012 {published data only}
- Chan SS, Leung DY, Lau C, Wong V, Lam T. Cost‐effectiveness analysis of a low intensity nurse‐led stage‐matched smoking cessation intervention to cardiac patients in Hong Kong. Circulation 2010;122(2):E87. [Google Scholar]
- Chan SS, Leung DY, Wong DC, Lau CP, Wong VT, Lam TH. A randomized controlled trial of stage‐matched intervention for smoking cessation in cardiac out‐patients. Addiction 2012;107(4):829‐37. [DOI] [PubMed] [Google Scholar]
- Chan SSC, Lam TH, Lau C‐P. The effectiveness of a nurse‐delivered smoking cessation intervention for cardiac patients: a randomised controlled trial. Nicotine & Tobacco Research 2005;7:692. [Google Scholar]
Chouinard 2005 {published data only}
- Chouinard MC, Robichaud‐Ekstrand S. The effectiveness of a nursing inpatient smoking cessation program in individuals with cardiovascular disease. Nursing Research 2005;54(4):243‐54. [DOI] [PubMed] [Google Scholar]
- Chouinard MC, .Robichaud‐Ekstrand S. Predictive value of the transtheoretical model to smoking cessation in hospitalized patients with cardiovascular disease. European Journal of Cardiovascular Prevention & Rehabilitation 2007;14(1):51‐8. [DOI] [PubMed] [Google Scholar]
Cossette 2011 {published data only}
- Cossette S, Frasure‐Smith N, Robert M, Chouinard MC, Juneau M, Guertin MC, et al. A pilot randomized trial of a smoking cessation nursing intervention in cardiac patients after hospital discharge. Canadian Journal of Cardiovascular Nursing 2012;22(4):16‐26. [PubMed] [Google Scholar]
- Cossette S, Frasure‐Smith N, Robert M, Chouinard MC, Juneau M, Guertin MC, et al. A pre‐assessment for nursing intervention to support tobacco cessation in patients hospitalized for cardiac problems: a pilot study (So‐Live). [Évaluation préliminaire d'une intervention infirmière de soutien à la cessation tabagique chez des patients hospitalisés pour un problème cardiaque: étude pilote (So‐Live)]. Recherche en Soins Infirmiers 2011;105:60‐75. [PubMed] [Google Scholar]
Curry 2003 {published data only}
- Curry SJ, Ludman EJ, Graham E, Stout J, Grothaus L, Lozano P. Pediatric‐based smoking cessation intervention for low‐income women: a randomized trial. Archives of Pediatric and Adolescent Medicine 2003;157(3):295‐302. [DOI] [PubMed] [Google Scholar]
Davies 1992 {published data only}
- Davies BL, Matte‐Lewis L, O'Connor AM, Dulberg CS, Drake ER. Evaluation of the "Time to Quit" self‐help smoking cessation program. Canadian Journal of Public Health 1992;83(1):19‐23. [PubMed] [Google Scholar]
DeBusk 1994 {published data only}
- DeBusk RF, Miller NH, Superko HR, Dennis CA, Thomas RJ, Lew HT, et al. A case‐management system for coronary risk factor modification after acute myocardial infarction. Annals of Internal Medicine 1994;120(9):721‐9. [DOI] [PubMed] [Google Scholar]
Duffy 2006 {published data only}
- Duffy SA, Ronis DL, Valenstein M, Lambert MT, Fowler KE, Gregory L, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiology, Biomarkers & Prevention 2006;15(11):2203‐08. [DOI] [PubMed] [Google Scholar]
- Duffy SA, Scheumann AL, Fowler KE, Darling‐Fisher C, Terrell JE. Perceived difficulty quitting predicts enrollment in a smoking‐cessation program for patients with head and neck cancer. Oncology Nursing Forum 2010;37(3):349‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Family Heart 1994 {published data only}
- Family Heart Study Group. Randomised controlled trial evaluating cardiovascular screening and intervention in general practice: principal results of British family heart study. BMJ 1994;308(6924):313‐20. [PMC free article] [PubMed] [Google Scholar]
- Wood DA, Kinmonth A‐L, Davies G, Thompson SG, Pyke SDM, Cramb R, et al. British family heart study: its design and method, and prevalence of cardiovascular risk factors. British Journal of General Practice 1994;44:62‐7. [PMC free article] [PubMed] [Google Scholar]
Feeney 2001 {published data only}
- Feeney GF, McPherson A, Connor JP, McAlister A, Young MR, Garrahy P. Randomized controlled trial of two cigarette quit programmes in coronary care patients after acute myocardial infarction. Internal Medicine Journal 2001;31(8):470‐5. [DOI] [PubMed] [Google Scholar]
Froelicher 2004 {published data only}
- Froelicher ES, Christopherson DJ. Women's Initiative for Nonsmoking (WINS) I: Design and methods. Heart & Lung 2000;29(6):429‐37. [DOI] [PubMed] [Google Scholar]
- Froelicher ES, Christopherson DJ, Miller NH, Martin K. Women's initiative for nonsmoking (WINS) IV: description of 277 women smokers hospitalized with cardiovascular disease. Heart & Lung 2002;31(1):3‐14. [DOI] [PubMed] [Google Scholar]
- Froelicher ES, Li WW, Mahrer‐Imhof R, Christopherson D, Stewart AL. Women's Initiative for Non‐Smoking (WINS) VI: Reliability and validity of health and psychosocial measures in women smokers with cardiovascular disease. Heart & Lung 2004;33(3):162‐75. [DOI] [PubMed] [Google Scholar]
- Froelicher ES, Miller NH, Christopherson DJ, Martin K, Parker KM, Amonetti M, et al. High rates of sustained smoking cessation in women hospitalized with cardiovascular disease: the Women's Initiative for Nonsmoking (WINS). Circulation 2004;109(5):587‐93. [DOI] [PubMed] [Google Scholar]
- Mahrer‐Imhof R, Froelicher ES, Li WW, Parker KM, Benowitz N. Women's Initiative for Nonsmoking (WINS V): under‐use of nicotine replacement therapy. Heart & Lung 2002;31(5):368‐73. [DOI] [PubMed] [Google Scholar]
- Martin K, Froelicher ES, Miller NH. Women's Initiative for Nonsmoking (WINS) II: The intervention. Heart & Lung 2000;29(6):438‐45. [DOI] [PubMed] [Google Scholar]
Gilbody 2015 {published data only}
- Gilbody S, Peckham E, Man M‐S, Mitchell N, Li J, Becque T, et al. Bespoke smoking cessation for people with severe mental ill health (SCIMITAR): A pilot randomised controlled trial. Lancet Psychiatry 2015;2(5):395‐402. [DOI] [PubMed] [Google Scholar]
Hajek 2002 {published data only}
- Hajek P, Taylor TZ, Mills P. Brief intervention during hospital admission to help patients to give up smoking after myocardial infarction and bypass surgery: randomised controlled trial. BMJ 2002;324(7329):87‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanssen 2007 {published data only}
- Hanssen TA, Nordrehaug JE, Eide GE, Hanestad BR. Does a telephone follow‐up intervention for patients discharged with acute myocardial infarction have long‐term effects on health‐related quality of life? A randomised controlled trial. Journal of Clinical Nursing 2009;18(9):1334‐45. [DOI] [PubMed] [Google Scholar]
- Hanssen TA, Nordrehaug JE, Eide GE, Hanestad BR. Improving outcomes after myocardial infarction: a randomized controlled trial evaluating effects of a telephone follow‐up intervention. European Journal of Cardiovascular Prevention and Rehabilitation 2007;14(3):429‐37. [DOI] [PubMed] [Google Scholar]
Hasuo 2004 {published data only}
- Hasuo S, Tanaka H, Oshima A. Efficacy of a smoking relapse prevention program by postdischarge telephone contacts: a randomized trial. Nippon Koshu Eisei Zasshi (Japanese Journal of Public Health) 2004;51(6):403‐12. [PubMed] [Google Scholar]
Hennrikus 2005 {published data only}
- Hennrikus D, Lando HA, McCarty MC, Vessey JT. The effectiveness of a systems approach to smoking cessation in hospital inpatients. Society for Research on Nicotine and Tobacco 7th Annual Meeting March 23‐23 Seattle Washington. 2001:47.
- Hennrikus DJ, Lando HA, McCarty MC, Klevan D, Holtan N, Huebsch JA, et al. The TEAM project: the effectiveness of smoking cessation interventions with hospital patients. Preventive Medicine 2005;40(3):249‐58. [DOI] [PubMed] [Google Scholar]
Hilberink 2005 {published data only}
- Hilberink SR, Jacobs JE, Bottema BJ, Vries H, Grol RP. Smoking cessation in patients with COPD in daily general practice (SMOCC): six months' results. Preventive Medicine 2005;41(5‐6):822‐7. [DOI] [PubMed] [Google Scholar]
- Hilberink SR, Jacobs JE, Breteler MH, Vries H, Grol RP. General practice counseling for patients with chronic obstructive pulmonary disease to quit smoking: Impact after 1 year of two complex interventions. Patient Education and Counseling 2011;83(1):120‐4. [DOI] [PubMed] [Google Scholar]
Hollis 1993 {published data only}
- Hollis JF, Lichtenstein E, Mount K, Vogt TM, Stevens VJ. Nurse‐assisted smoking counseling in medical settings: minimizing demands on physicians. Preventive Medicine 1991;20(4):497‐507. [DOI] [PubMed] [Google Scholar]
- Hollis JF, Lichtenstein E, Vogt TM, Stevens VJ, Biglan A. Nurse‐assisted counseling for smokers in primary care. Annals of Internal Medicine 1993;118(7):521‐5. [DOI] [PubMed] [Google Scholar]
Hornnes 2014 {published data only}
- Hornnes N, Larsen K, Brink‐Kjaer T, Boysen G. Specific antismoking advice after stroke. Danish Medical Journal 2014;61(4):A4816. [PubMed] [Google Scholar]
Janz 1987 {published data only}
- Janz NK, Becker MH, Kirscht JP, Eraker SA, Billi JE, Woolliscroft JO. Evaluation of a minimal‐contact smoking cessation intervention in an outpatient setting. American Journal of Public Health 1987;77:805‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2007 {published data only}
- Jiang X, Sit JW, Wong TK. A nurse‐led cardiac rehabilitation programme improves health behaviours and cardiac physiological risk parameters: evidence from Chengdu, China. Journal of Clinical Nursing 2007;16(10):1886‐97. [DOI] [PubMed] [Google Scholar]
Jorstad 2013 {published data only}
- Jorstad HT, Birgelen C, Alings AM, Liem A, Dantzig JM, Jaarsma W, et al. Effect of a nurse‐coordinated prevention programme on cardiovascular risk after an acute coronary syndrome: main results of the RESPONSE randomised trial. Heart 2013;99(19):1421‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kadda 2015 {published data only}
- Kadda O, Kotanidou A, Manginas A, Stavridis G, Nanas S, Panagiotakos DB. Lifestyle intervention and one‐year prognosis of patients following open heart surgery: a randomised clinical trial. Journal of Clinical Nursing 2015;24(11‐12):1611‐21. [DOI] [PubMed] [Google Scholar]
Kim 2003 {published data only}
- Kim YE, Song YM, Lee JK, Jung HS, Kang SC. A study evaluating the effect of telephone counselling on smoking performed by a nurse cessation: a preliminary, randomized, controlled trial. Journal of the Korean Academy of Family Medicine 2003;24(7):634‐41. [Google Scholar]
Kim 2005 {published data only}
- Kim JR, Lee MS, Hwang JY, Lee JD. Efficacy of a smoking cessation intervention using the AHCPR guideline tailored for Koreans: a randomized controlled trial. Health Promotion International 2005;20(1):51‐9. [DOI] [PubMed] [Google Scholar]
Lancaster 1999 {published data only}
- Lancaster T, Dobbie W, Vos K, Yudkin P, Murphy M, Fowler G. Randomised trial of nurse‐assisted strategies for smoking cessation in primary care. British Journal of General Practice 1999;49(440):191‐4. [PMC free article] [PubMed] [Google Scholar]
Lewis 1998 {published data only}
- Lewis SF, Piasecki TM, Fiore MC, Anderson JE, Baker TB. Transdermal nicotine replacement for hospitalized patients: A randomized clinical trial. Preventive Medicine 1998;27(2):296‐303. [DOI] [PubMed] [Google Scholar]
Meysman 2010 {published data only}
- Meysman M, Boudrez H, Nackaerts K, Dieriks B, Indemans R, Vermeire P. Smoking cessation rates after a nurse‐led inpatient smoking cessation intervention. Journal of Smoking Cessation 2010;5(1):69‐76. [Google Scholar]
Miller 1997 {published data only}
- Miller NH, Smith PM, DeBusk RF, Sobel DS, Taylor CB. Smoking cessation in hospitalized patients. Results of a randomized trial. Archives of Internal Medicine 1997;157(4):409‐15. [PubMed] [Google Scholar]
- Miller NH, Smith PM, Taylor CB, Sobel D, DeBusk RF. Smoking cessation in hospitalized patients ‐ results of a randomized trial. Circulation 1995;92(8):SS179 (abstract 855). [PubMed] [Google Scholar]
- Taylor CB, Miller NH, Herman S, Smith PM, Sobel D, Fisher L, et al. A nurse‐managed smoking cessation program for hospitalized smokers. American Journal of Public Health 1996;86(11):1557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagle 2005 {published data only}
- Hensley MJ, Nagle AL, Schofield MJ, Koschel A. Efficacy of a brief nurse provided nicotine management intervention for hospitalised smokers. Respirology 2002;7:A12. [Google Scholar]
- Nagle AL, Hensley MJ, Schofield MJ, Koschel AJ. A randomised controlled trial to evaluate the efficacy of a nurse‐provided intervention for hospitalised smokers. Australian and New Zealand Journal of Public Health 2005;29(3):285‐91. [DOI] [PubMed] [Google Scholar]
Nebot 1992 {published data only}
- Nebot M, Cabezas C. Does nurse counseling or offer of nicotine gum improve the effectiveness of physician smoking‐cessation advice?. Family Practice Research Journal 1992;12(3):263‐70. [PubMed] [Google Scholar]
OXCHECK 1994 {published data only}
- Imperial Cancer Research Fund OXCHECK Study Group. Effectiveness of health checks conducted by nurses in primary care: Results of the OXCHECK study after one year. BMJ 1994;308(6924):308‐12. [PMC free article] [PubMed] [Google Scholar]
- Imperial Cancer Research Fund OXCHECK Study Group. Effectiveness of health checks conducted by nurses in primary care: final results of the OXCHECK study. BMJ 1995;310(6987):1099‐104. [PMC free article] [PubMed] [Google Scholar]
Pardavila‐Belio 2015 {published data only}
- Pardavila‐Belio MI, Garcia‐Vivar C, Pimenta AM, Canga‐Armayor A, Pueyo‐Garrigues S, Canga‐Armayor N. Intervention study for smoking cessation in Spanish college students: Pragmatic randomized controlled trial. Addiction 2015;110(10):1676‐83. [DOI] [PubMed] [Google Scholar]
Quist‐Paulsen 2003 {published data only}
- Brown DW. Nurse‐led intervention increases smoking cessation among people with coronary heart disease. Evidence Based Healthcare 2004;8:128‐30. [Google Scholar]
- Quist‐Paulsen P, Bakke PS, Gallefoss F. Does smoking cessation improve quality of life in patients with coronary heart disease?. Scandinavian Cardiovascular Journal 2006;40(1):11‐16. [DOI] [PubMed] [Google Scholar]
- Quist‐Paulsen P, Bakke PS, Gallefoss F. Predictors of smoking cessation in patients admitted for acute coronary heart disease. European Journal of Cardiovascular Prevention and Rehabilitation 2005;12(5):472‐7. [DOI] [PubMed] [Google Scholar]
- Quist‐Paulsen P, Gallefoss F. Randomised controlled trial of smoking cessation intervention after admission for coronary heart disease. BMJ 2003;327(7426):1254‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist‐Paulsen P, Lydersen S, Bakke PS, Gallefoss F. Cost effectiveness of a smoking cessation program in patients admitted for coronary heart disease. European Journal of Cardiovascular Prevention and Rehabilitation 2006;13(2):274‐80. [DOI] [PubMed] [Google Scholar]
Ratner 2004 {published data only}
- Ratner PA, Johnson JL, Richardson CG, Bottorff JL, Moffat B, Mackay M, et al. Efficacy of a smoking‐cessation intervention for elective‐surgical patients. Research in Nursing and Health 2004;27(3):148‐61. [DOI] [PubMed] [Google Scholar]
Rice 1994 {published data only}
- Rice VH, Fox DH, Lepczyk M, Sieggreen M, Mullin M, Jarosz P, et al. A comparison of nursing interventions for smoking cessation in adults with cardiovascular health problems. Heart and Lung 1994;23(6):473‐86. [PubMed] [Google Scholar]
Rigotti 1994 {published data only}
- Rigotti NA, McKool KM, Shiffman S. Predictors of smoking cessation after coronary artery bypass graft surgery. Results of a randomized trial with 5‐year follow‐up. Annals of Internal Medicine 1994;120(4):287‐93. [DOI] [PubMed] [Google Scholar]
Risser 1990 {published data only}
- Risser NL, Belcher DW. Adding spirometry, carbon monoxide, and pulmonary symptom results to smoking cessation counseling: a randomized trial. Journal of General Internal Medicine 1990;5(1):16‐22. [DOI] [PubMed] [Google Scholar]
Sanders 1989a {published data only}
- Sanders D, Fowler G, Mant D, Fuller A, Jones L, Marzillier J. Randomized controlled trial of anti‐smoking advice by nurses in general practice. Journal of the Royal College of General Practitioners 1989;39(324):273‐6. [PMC free article] [PubMed] [Google Scholar]
Sanders 1989b {published data only}
- Sanders D, Fowler G, Mant D, Fuller A, Jones L, Marzillier J. Randomized controlled trial of anti‐smoking advice by nurses in general practice. Journal of the Royal College of General Practitioners 1989;39(324):273‐6. [PMC free article] [PubMed] [Google Scholar]
Sanz‐Pozo 2006 {published data only}
- Sanz Pozo B, Miguel Díaz J, Aragón Blanco M, González González AI, Cortes Catalán M, Vázquez I. Effectiveness of non‐pharmacological primary care methods for giving up tobacco dependency [Efectividad de los métodos no farmacológicos para la deshabituación tabáquica en atención primaria]. Atencion Primaria 2003;32(6):366‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz‐Pozo B, Miguel‐Díaz J, Aragón‐Blanco M, García‐Caballo M, Gomez‐Suarez E, Fernandez‐Dominguez JF. Effectiveness of a programme of intensive tobacco counselling by nursing professionals [Efectividad de un programa de consejo antitabaco intensivo realizado por profesionales de enfermería]. Atencion Primaria 2006;37:266‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smit 2016 {published data only}
- Smit ES, Vries H, Hoving C. Results of the PAS study: a randomized controlled trial evaluating the effectiveness of a web‐based multiple tailored smoking cessation program combined with tailored counseling by practice nurses. Health Communication 2016;31(9):1165‐73. [DOI: 10.1080/10410236.2015.1049727] [DOI] [PubMed] [Google Scholar]
- Smit ES, Vries H, Hoving C. The PAS study: a randomized controlled trial evaluating the effectiveness of a web‐based multiple tailored smoking cessation programme and tailored counselling by practice nurses. Contemporary Clinical Trials 2010;31(3):251‐8. [DOI] [PubMed] [Google Scholar]
Steptoe 1999 {published data only}
- Steptoe A, Doherty S, Rink E, Kerry S, Kendrick T, Hilton S. Behavioural counselling in general practice for the promotion of healthy behaviour among adults at increased risk of coronary heart disease: randomised trial. BMJ 1999;319(7215):943‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 1990 {published data only}
- Taylor CB, Houston‐Miller N, Killen JD, DeBusk RF. Smoking cessation after acute myocardial infarction: effects of a nurse‐managed intervention. Annals of Internal Medicine 1990;113(2):118‐23. [DOI] [PubMed] [Google Scholar]
Terazawa 2001 {published data only}
- Terazawa T, Mamiya T, Masui S, Nakamura M. The effect of smoking cessation counseling at health checkup. Sangyo Eiseigaku Zasshi 2001;43(6):207‐13. [DOI] [PubMed] [Google Scholar]
Tønnesen 1996 {published data only}
- Tønnesen P, Mikkelsen K, Markholst C, Ibsen A, Bendixen M, Pedersen L, et al. Nurse‐conducted smoking cessation with minimal intervention in a lung clinic: a randomized controlled study. European Respiratory Journal 1996;9(11):2351‐5. [DOI] [PubMed] [Google Scholar]
Tønnesen 2006 {published data only}
- Tønnesen P, Mikkelsen K, Bremann L. Nurse‐conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130(2):334‐42. [DOI] [PubMed] [Google Scholar]
Vetter 1990 {published data only}
- Vetter NJ, Ford D. Smoking prevention among people aged 60 and over: a randomized controlled trial. Age and Ageing 1990;19(3):164‐8. [DOI] [PubMed] [Google Scholar]
Wood 2008 {published data only}
- Wood D, Kotseva K, Connolly S, Jennings C, Mead A, Jones J, et al. Nurse‐coordinated multidisciplinary, family‐based cardiovascular disease prevention programme (EUROACTION) for patients with coronary heart disease and asymptomatic individuals at high risk of cardiovascular disease: a paired, cluster‐randomised controlled trial. Lancet 2008;371(9629):1999‐2012. [DOI] [PubMed] [Google Scholar]
Zwar 2015 {published data only}
- Zwar N, Richmond R, Halcomb E, Furler J, Smith J, Hermiz O, et al. Quit in general practice: a cluster randomised trial of enhanced in‐practice support for smoking cessation. BMC Family Practice 2010;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwar NA, Richmond RL, Halcomb EJ, Furler JS, Smith JP, Oshana Hermiz O, et al. Quit in general practice: a cluster randomized trial of enhanced in‐practice support for smoking cessation. Family Practice 2015;32(2):173‐180. [DOI: 10.1093/fampra/cmu089] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aertsen‐Van Der Kuip 2006 {published data only}
- Aertsen‐Van der Kuip M, Besselink RM, Vlugt MJ, Peters E, Fouwels AJ, Wollersheim Hal. Successful motivational interviewing for smoking cessation in a secondary cardiovascular prevention clinic. European Heart Journal 2006;27(Suppl 1):165. [Google Scholar]
Andrews 2007 {published data only}
- Andrews JO, Felton G, Wewers ME, Waller J, Tingen M. The effect of a multi‐component smoking cessation intervention in African American women residing in public housing. Research in Nursing & Health 2007;30(1):45‐60. [DOI] [PubMed] [Google Scholar]
Avanzini 2011 {published data only}
- Avanzini F, Di GP, Amodeo R, Baldo S, Bergna ML, Busi G, et al. Effectiveness of a nurse‐led educational intervention for patients admitted for acute coronary syndrome [Efficacia di un intervento educativo infermieristico in pazienti ricoverati per una sindrome coronarica acuta]. Assistenza Infermieristica e Ricerca:Air 2011;30:16‐23. [PubMed] [Google Scholar]
Bredie 2011 {published data only}
- Bredie SJ, Fouwels AJ, Wollersheim H, Schippers GM. Effectiveness of nurse based motivational interviewing for smoking cessation in high risk cardiovascular outpatients: A randomized trial. European Journal of Cardiovascular Nursing 2011;10(3):174‐9. [DOI] [PubMed] [Google Scholar]
Browning 2000 {published data only}
- Browning KK, Ahijevych KL, Ross P Jr, Wewers ME. Implementing the Agency for Health Care Policy and Research's Smoking Cessation Guideline in a lung cancer surgery clinic. Oncology Nursing Forum 2000;27(8):1248‐54. [PubMed] [Google Scholar]
Brunner‐Frandsen 2012 {published data only}
- Brunner Frandsen N, Sorensen M, Hyldahl TK, Henriksen RM, Bak S. Smoking cessation intervention after ischemic stroke or transient ischemic attack. A randomized controlled pilot trial. Nicotine & Tobacco Research 2012;14(4):443‐7. [DOI] [PubMed] [Google Scholar]
- Brunner‐Frandsen NS, Bak S. Smoking cessation intervention after stroke or transient ischemic attack. A randomised controlled trial. Cerebrovascular Diseases 2010;29(Suppl 2):323. [Google Scholar]
Cabezas 2011 {published data only}
- Cabezas C, Advani M, Puente D, Rodriguez T, Martin C, ISTAPS Study Group. Effectiveness of a stepped primary care smoking cessation intervention: cluster randomized clinical trial (ISTAPS study). Addiction 2011;106(9):1696‐706. [DOI] [PubMed] [Google Scholar]
- Cabezas C, Martin C, Granollers S, Morera C, Ballve JL, Zarza E, et al. Effectiveness of a stepped primary care smoking cessation intervention (ISTAPS study): design of a cluster randomised trial. BMC Public Health 2009;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente D, Cabezas C, Rodriguez‐Blanco T, Fernandez‐Alonso C, Cebrian T, Torrecilla M, et al. The role of gender in a smoking cessation intervention: a cluster randomized clinical trial. BMC Public Health 2011;11:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlsson 1998 {published data only}
- Carlsson R. Serum cholesterol, lifestyle, working capacity and quality of life in patients with coronary artery disease. Experiences from a hospital‐based secondary prevention programme. Scandinavian Cardiovascular Journal Supplement 1998;50:1‐20. [DOI] [PubMed] [Google Scholar]
Caslin 2006 {published data only}
- Caslin EK, Newhouse R, Zissimos J, Murray P, Thompson KM, Ashen D, et al. Smoking Cessation Intervention and Nurse Initiation (SCINI) Trial. 13th World Conference on Tobacco & Health. 2006.
- Thompson KM, Caslin EK, Murray P, Zissimos J, Newhouse R, Yung R. Barriers to implementing nurse initiated inpatient smoking cessation [Abstract].. Proceedings of the American Thoracic Society 2006: A294. 2006.
Chan 2005 {published data only}
- Chan SS, Lam TH, Salili F, Leung GM, Wong DC, Botelho RJ, et al. A randomized controlled trial of an individualized motivational intervention on smoking cessation for parents of sick children: a pilot study. Applied Nursing Research 2005;18(3):178‐81. [DOI] [PubMed] [Google Scholar]
Chan 2008 {published data only}
- Chan SS, Leung GM, Wong DC, Lam TH. Helping Chinese fathers quit smoking through educating their nonsmoking spouses: a randomized controlled trial. American Journal of Health Promotion 2008;23(1):31‐4. [DOI] [PubMed] [Google Scholar]
- Chan SS, Wong DC, Lam T‐H. Will mothers of sick children help their husbands to stop smoking after receiving a brief intervention from nurses? Secondary analysis of a randomised controlled trial. BMC Pediatrics 2013;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffy 2014 {published data only}
- Duffy SA, Ronis DL, Karvonen‐Gutierrez CA, Ewing LA, Dalack GW, Smith PM, et al. Effectiveness of the tobacco tactics program in the Department of Veterans Affairs. Annals of Behavioral Medicine 2014;48(2):265‐74. [DOI] [PubMed] [Google Scholar]
Efraimsson 2008 {published data only}
- Efraimsson EO, Hillervik C, Ehrenberg A. Effects of COPD self‐care management education at a nurse‐led primary health care clinic. Scandinavian Journal of Caring Sciences 2008;22(2):178‐85. [DOI] [PubMed] [Google Scholar]
Fletcher 1987 {published data only}
- Fletcher V. An individualized teaching programme following primary uncomplicated myocardial infarction. Journal of Advanced Nursing 1987;12(2):195‐200. [DOI] [PubMed] [Google Scholar]
French 2007 {published data only}
- French GM, Groner JA, Wewers ME, Ahijevych K. Staying smoke free: an intervention to prevent postpartum relapse. Nicotine & Tobacco Research 2007;9(6):663‐70. [DOI] [PubMed] [Google Scholar]
Fritz 2008 {published data only}
- Fritz DJ, Hardin SB, Gore PA Jr, Bram D. A computerized smoking cessation intervention for high school smokers. Pediatric Nursing 2008;34(1):13‐7. [PubMed] [Google Scholar]
Galvin 2001 {published data only}
- Galvin K, Webb C, Hillier V. Assessing the impact of a nurse‐led health education intervention for people with peripheral vascular disease who smoke: the use of physiological markers, nicotine dependence and withdrawal. International Journal of Nursing Studies 2001;38(1):91‐105. [DOI] [PubMed] [Google Scholar]
Gies 2008 {published data only}
- Gies CE, Buchman D, Robinson J, Smolen D. Effect of an inpatient nurse‐directed smoking cessation program. Western Journal of Nursing Research 2008;30(1):6‐19. [DOI] [PubMed] [Google Scholar]
Griebel 1998 {published data only}
- Griebel B, Wewers ME, Baker CA. The effectiveness of a nurse‐managed minimal smoking‐cessation intervention among hospitalized patients with cancer. Oncology Nursing Forum 1998;25(5):897‐902. [PubMed] [Google Scholar]
Haddock 1997 {published data only}
- Haddock J, Burrows C. The role of the nurse in health promotion: an evaluation of a smoking cessation programme in surgical pre‐admission clinics. Journal of Advanced Nursing 1997;26(6):1098‐110. [PubMed] [Google Scholar]
Hall 2007 {published data only}
- Hall S, Reid E, Ukoumunne OC, Weinman J, Marteau TM. Brief smoking cessation advice from practice nurses during routine cervical smear tests appointments: a cluster randomised controlled trial assessing feasibility, acceptability and potential effectiveness. British Journal of Cancer 2007;96(7):1057‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Happell 2014 {published data only}
- Happell B, Stanton R, Platania‐Phung C, McKenna B, Scott D. The cardiometabolic health nurse: physical health behaviour outcomes from a randomised controlled trial. Issues in Mental Health Nursing 2014;35(10):768‐75. [DOI] [PubMed] [Google Scholar]
Heath 2012 {published data only}
- Heath J, Inglett S, Young S, Joshua TV, Sakievich N, Hawkins J, et al. The impact of the Georgia Health Sciences University nursing faculty practice on tobacco cessation rates. Nursing Clinics of North America 2012;47(1):1‐12. [DOI] [PubMed] [Google Scholar]
Hjalmarson 2007 {published data only}
- Hjalmarson A, Boethius G. The effectiveness of brief advice and extended smoking cessation counseling programs when implemented routinely in hospitals. Preventive Medicine 2007;45(2‐3):202‐7. [DOI] [PubMed] [Google Scholar]
Jansink 2013 {published data only}
- Jansink R, Braspenning J, Keizer E, Weijden T, Elwyn G, Grol R. No identifiable Hb1Ac or lifestyle change after a comprehensive diabetes programme including motivational interviewing: A cluster randomized trial. Scandinavian Journal of Primary Health Care 2013;31(2):119‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansink R, Braspenning J, Weijden T, Niessen L, Elwyn G, Grol R. Nurse‐led motivational interviewing to change the lifestyle of patients with type 2 diabetes (MILD‐project): protocol for a cluster, randomized, controlled trial on implementing lifestyle recommendations. BMC Health Services Research 2009;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jelley 1995 {published data only}
- Jelley MJ, Prochazka AV. A smoking cessation intervention in family planning clinics. Journal of Women's Health 1995;4:555‐67. [Google Scholar]
Johnson 1999 {published data only}
- Johnson JL, Budz B, Mackay M, Miller C. Evaluation of a nurse‐delivered smoking cessation intervention for hospitalized patients with cardiac disease. Heart and Lung 1999;28(1):55‐64. [DOI] [PubMed] [Google Scholar]
Johnson 2000 {published data only}
- Johnson JL, Ratner PA, Bottorff JL, Hall W, Dahinten S. Preventing smoking relapse in postpartum women. Nursing Research 2000;49(1):44‐52. [DOI] [PubMed] [Google Scholar]
- Ratner PA, Johnson JL, Bottorff JL, Dahinten S, Hall W. Twelve‐month follow‐up of a smoking relapse prevention intervention for postpartum women. Addictive Behaviors 2000;25(1):81‐92. [DOI] [PubMed] [Google Scholar]
Jonsdottir 2015 {published data only}
- Jonsdottir H, Amundadottir OR, Gudmundsson G, Halldorsdottir BS, Hrafnkelsson B, Ingadottir TS, et al. Effectiveness of a partnership‐based self‐management programme for patients with mild and moderate chronic obstructive pulmonary disease: a pragmatic randomized controlled trial. Journal of Advanced Nursing 2015;71(11):2634‐49. [DOI] [PubMed] [Google Scholar]
Katz 2012 {published data only}
- Katz DA, Vander Weg MW, Holman J, Nugent A, Baker L, Johnson S, et al. The Emergency Department Action in Smoking Cessation (EDASC) trial: impact on delivery of smoking cessation counseling. Academic Emergency Medicine 2012;19(4):409‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kendrick 1995 {published data only}
- Kendrick JS, Zahniser SC, Miller N, Salas N, Stine J, Gargiullo PM, et al. Integrating smoking cessation into routine public prenatal care: the Smoking Cessation in Pregnancy project. American Journal of Public Health 1995;85(2):217‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koelewijn‐van Loon 2009 {published data only}
- Koelewijn‐van Loon MS, Weijden T, Steenkiste B, Ronda G, Winkens B, Severens JL, et al. Involving patients in cardiovascular risk management with nurse‐led clinics: a cluster randomized controlled trial. Canadian Medical Association Journal 2009;181(12):E267‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotz 2009 {published data only}
- Kotz D, Huibers MJH, West RJ, Wesseling G, Schayck OC. What mediates the effect of confrontational counselling on smoking cessation in smokers with COPD?. Patient Education and Counseling 2009;76(1):16‐24. [DOI] [PubMed] [Google Scholar]
- Kotz D, Wesseling G, Huibers MJ, Schayck OC. Efficacy of confrontational counselling for smoking cessation in smokers with previously undiagnosed mild to moderate airflow limitation: study protocol of a randomized controlled trial. BMC Public Health 2007;7:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz D, Wesseling G, Huibers MJ, Schayck OC. Efficacy of confronting smokers with airflow limitation for smoking cessation.[see comment]. European Respiratory Journal 2009;33(4):754‐62. [DOI] [PubMed] [Google Scholar]
Kruis 2014 {published data only}
- Kruis AL, Boland MR, Assendelft WJ, Gussekloo J, Tsiachristas A, Stijnen T, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: Results of cluster randomised trial. Nederlands Tijdschrift voor Geneeskunde 2015;159(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruis AL, Boland MR, Assendelft WJ, Gussekloo J, Tsiachristas A, Stijnen T, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. BMJ 2014;349:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lakerveld 2010 {published data only}
- Lakerveld J, Bot SD, Chinapaw MJ, Kostense PJ, Nijpels G. An individual lifestyle intervention program is not more effective in changing diabetes risk and lifestyle behaviors than providing health brochures: The Hoorn Prevention Study. Diabetologia 2010;53:S82. [Google Scholar]
Lakerveld 2013 {published data only}
- Lakerveld J, Bot SD, Chinapaw MJ, Tulder MW, Kostense PJ, Dekker JM, et al. Motivational interviewing and problem solving treatment to reduce type 2 diabetes and cardiovascular disease risk in real life: A randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2013;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lifrak 1997 {published data only}
- Lifrak P, Gariti P, Alterman AI, McKay J, Volpicelli J, Sparkman T, et al. Results of two levels of adjunctive treatment used with the nicotine patch. American Journal of Addiction 1997;6(2):93‐8. [PubMed] [Google Scholar]
Lou 2013 {published data only}
- Lou P, Zhu Y, Chen P, Zhang P, Yu J, Zhang N, et al. Supporting smoking cessation in chronic obstructive pulmonary disease with behavioral intervention: a randomized controlled trial. BMC Family Practice 2013;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
McHugh 2001 {published data only}
- McHugh F, Lindsay GM, Hanlon P, Hutton I, Brown MR, Morrison C, et al. Nurse led shared care for patients on the waiting list for coronary artery bypass surgery: a randomised controlled trial. Heart 2001;86(3):317‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meulepas 2007 {published data only}
- Meulepas MA, Jacobs JE, Smeenk FW, Smeele I, Lucas AE, Bottema BJ, et al. Effect of an integrated primary care model on the management of middle‐aged and old patients with obstructive lung diseases. Scandinavian Journal of Primary Health Care 2007;25(3):186‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 1992 {published data only}
- O'Connor AM, Davies BL, Dulberg CS, Buhler PL, Nadon C, McBride BH, et al. Effectiveness of a pregnancy smoking cessation program. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1992;21(5):385‐92. [DOI] [PubMed] [Google Scholar]
Persson 2006 {published data only}
- Persson LG, Hjalmarson A. Smoking cessation in patients with diabetes mellitus: results from a controlled study of an intervention programme in primary healthcare in Sweden. Scandinavian Journal of Primary Health Care 2006;24(2):75‐80. [DOI] [PubMed] [Google Scholar]
Planer 2011 {published data only}
- Planer D, Lev I, Elitzur Y, Sharon N, Ouzan E, Pugatsch T, et al. Bupropion for smoking cessation in patients with acute coronary syndrome. Archives of Internal Medicine 2011;171(12):1055‐60. [DOI] [PubMed] [Google Scholar]
Pozen 1977 {published data only}
- Pozen MW, Stechmiller JA, Harris W, Smith S, Fried DD, Voigt GC. A nurse rehabilitator's impact on patients with myocardial infarction. Medical Care 1977;15(10):830‐7. [DOI] [PubMed] [Google Scholar]
Reeve 2000 {published data only}
- Reeve K, Calabro K, Adams‐McNeill J. Tobacco cessation intervention in a nurse practitioner managed clinic. Journal of the American Academy of Nurse Practitioners 2000;12(5):163‐9. [DOI] [PubMed] [Google Scholar]
Reid 2003 {published data only}
- Reid R, Pipe A, Higginson L, Johnson K, D'Angelo MS, Cooke D, et al. Stepped care approach to smoking cessation in patients hospitalized for coronary artery disease. Journal of Cardiopulmonary Rehabilitation 2003;23(3):176‐82. [DOI] [PubMed] [Google Scholar]
Rigotti 1997 {published data only}
- Rigotti NA, Arnsten JH, McKool KM, Wood‐Reid KM, Pasternak RC, Singer DE. Efficacy of a smoking cessation program for hospital patients. Archives of Internal Medicine 1997;157(22):2653‐60. [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Smith PM, Burgess E. Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial. Canadian Medical Association Journal 2009;180(13):1297‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanislaw 1994 {published data only}
- Stanislaw AE, Wewers ME. A smoking cessation intervention with hospitalized surgical cancer patients: a pilot study. Cancer Nursing 1994;17(2):81‐6. [PubMed] [Google Scholar]
Sun 2000 {published data only}
- Sun C. Roles of psychological nursing played in the course of auricle point applying to help individuals giving up smoking. Shanxi Nursing Journal 2000;14(2):69‐70. [Google Scholar]
Targhetta 2011 {published data only}
- Targhetta R, Bernhard L, Sorokaty JM, Balmes JL, Nalpas B, Perney P. Intervention study to improve smoking cessation during hospitalization. Public Health 2011;125(7):457‐63. [DOI] [PubMed] [Google Scholar]
Van Elderen 1994 {published data only}
- Elderen‐van Kemenade T, Maes S, Broek Y. Effects of a health education programme with telephone follow‐up during cardiac rehabilitation. British Journal of Clinical Psychology 1994;33(3):367‐78. [DOI] [PubMed] [Google Scholar]
Van Zuilen 2011 {published data only}
- Zuilen AD, Blankestijn PJ, Buren M, Dam MA, Kaasjager KA, Ligtenberg G, et al. Nurse practitioners improve quality of care in chronic kidney disease: two‐year results of a randomised study. Netherlands Journal of Medicine 2011;69(11):517‐26. [PubMed] [Google Scholar]
- Zuilen AD, Bots ML, Dulger A, Tweel I, Buren M, Dam MA, et al. Multifactorial intervention with nurse practitioners does not change cardiovascular outcomes in patients with chronic kidney disease. Kidney International 2012;82(6):710‐7. [DOI] [PubMed] [Google Scholar]
Wadland 1999 {published data only}
- Wadland WC, Stoffelmayr B, Berger E, Crombach A, Ives K. Enhancing smoking cessation rates in primary care. Journal of Family Practice 1999;48(9):711‐8. [PubMed] [Google Scholar]
Wadland 2001 {published data only}
- Wadland WC, Stoffelmayr B, Ives K. Enhancing smoking cessation of low‐income smokers in managed care. Journal of Family Practice 2001;50(2):138‐44. [PubMed] [Google Scholar]
Wewers 1994 {published data only}
- Wewers ME, Bowen JM, Stanislaw AE, Desimone VB. A nurse‐delivered smoking cessation intervention among hospitalized postoperative patients‐‐influence of a smoking‐related diagnosis: a pilot study. Heart and Lung 1994;23(2):151‐6. [PubMed] [Google Scholar]
Wewers 2009 {published data only}
- Wewers ME, Ferketich AK, Harness J, Paskett ED. Effectiveness of a nurse‐managed, lay‐led tobacco cessation intervention among Ohio Appalachian women. Cancer Epidemiology, Biomarkers and Prevention 2009;18(12):3451‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2008 {published data only}
- Wilson JS, Elborn JS, Fitzsimons D, McCrum‐Gardner E. Do smokers with chronic obstructive pulmonary disease report their smoking status reliably? A comparison of self‐report and bio‐chemical validation. International Journal of Nursing Studies 2011;48(7):856‐62. [DOI] [PubMed] [Google Scholar]
- Wilson JS, Fitzsimons D, Bradbury I, Stuart EJ. Does additional support by nurses enhance the effect of a brief smoking cessation intervention in people with moderate to severe chronic obstructive pulmonary disease? A randomised controlled trial. International Journal of Nursing Studies 2008;45(4):508‐17. [DOI] [PubMed] [Google Scholar]
Woollard 1995 {published data only}
- Woollard J, Beilin LJ, Lord T, Puddey I, MacAdam D, Rouse I. A controlled trial of nurse counselling of life‐style change for hypertensives treated in general practice ‐preliminary results. Clinical and Experimental Pharmacology and Physiology 1995;22(6‐7):466‐8. [DOI] [PubMed] [Google Scholar]
Zakrisson 2011 {published data only}
- Zakrisson AB, Engfeldt P, Hägglund D, Odencrants S, Hasselgren M, Arne M, et al. Nurse‐led multidisciplinary programme for patients with COPD in primary health care: a controlled trial. Primary Care Respiratory Journal 2011;20(4):427‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Lachman 2015 {published data only}
- Lachman S, Minneboo M, Snaterse M, Jorstad HT, Ter Riet G, Scholte Op Reimer WJ, et al. Community‐based comprehensive lifestyle programs in patients with coronary artery disease: Objectives, design and expected results of Randomized Evaluation of Secondary Prevention by Outpatient Nurse SpEcialists 2 trial (RESPONSE 2). American Heart Journal 2015;170(2):216‐22. [DOI] [PubMed] [Google Scholar]
Mularski 2013 {published data only}
- Mularski RA, Leo MC, Waiwaiole LA, Carlson CA, Conn DE, Funkhouser KA, et al. Linking in‐hospital smoking cessation assistance with outpatient services and automated telephone follow‐up using dedicated nurse tobacco treatment specialists: A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A3905. [Google Scholar]
NCT02106637 {published data only}
- NCT02106637. Early in‐hospital Initiation of pharmacotherapy for smoking cessation, concomitant with nurse‐led support, in patients after an acute coronary syndrome (ACS). clinicaltrials.gov/show/NCT02106637 (first received 8 April 2014).
Additional references
AHRQ 2008
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Dept of Health and Human Services, 2008. [Google Scholar]
ANA 2012
- American Nurses Association. Tobacco Free Nurses. www.nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy‐Nurse/TobaccoFree.html (accessed 24 July 2013).
Bize 2012
- Bize R, Burnand B, Mueller Y, Rège‐Walther M, Camain JY, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD004705.pub4] [DOI] [PubMed] [Google Scholar]
Cahill 2016
- Cahill K, Lindson‐Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD006103.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2016
- Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2005‐2015. Morbidity and Mortality Weekly Report 2016;65(44):1205‐11. [DOI] [PubMed] [Google Scholar]
CDC 2017
- Centers for Disease Control and Prevention. Quitting smoking among adults—United States, 2000–2015. Morbidity and Mortality Weekly Report 2017;65(52):1457‐64. [DOI] [PubMed] [Google Scholar]
Chamberlain 2017
- Chamberlain C, O'Mara‐Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD001055.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane Handbook
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Coleman 2015
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi‐Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD010078.pub2] [DOI] [PubMed] [Google Scholar]
Greenland 1985
- Greenland S, Robins J. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41(1):55‐68. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2014
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000031.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICN 2012
- International Council of Nurses. Tobacco use and health: ICN position. www.icn.ch/publications/position‐statements/ (accessed 24 July 2013).
Rigotti 2012
- Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001837.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stead 2012
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
Stead 2013
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann‐Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 1993
- Stewart LA, Parmar MK. Meta‐analysis of the literature or of individual patient data: is there a difference?. Lancet 1993;341(8842):418‐22. [DOI] [PubMed] [Google Scholar]
The Tobacco Atlas 2015
- Eriksen M, Mackay J, Schluger N, Gomeshtapeh FI, Drope J. The Tobacco Atlas. www.tobaccoatlas.org. 5th. Atlanta, GA: American Cancer Society; New York, NY: World Lung Foundation, 2015; Vol. (accessed 22 November 2017).
Youdan 2005
- Youdan B, Queally B. Nurses' role in promoting and supporting smoking cessation. Nursing Times 2005;101(10):26. [PubMed] [Google Scholar]
References to other published versions of this review
Rice 1999a
- Rice VH, Stead LF. Nursing interventions for smoking cessation. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001188] [DOI] [PubMed] [Google Scholar]
Rice 1999b
- Rice VH. Nursing intervention and smoking cessation: a meta‐analysis. Heart and Lung 1999;28(6):438‐54. [DOI] [PubMed] [Google Scholar]
Rice 2001
- Rice VH, Stead LF. Nursing interventions for smoking cessation. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001188] [DOI] [PubMed] [Google Scholar]
Rice 2004
- Rice VH, Stead LF. Nursing interventions for smoking cessation. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001188.pub2] [DOI] [PubMed] [Google Scholar]
Rice 2006
- Rice VH, Stead L. Nursing intervention and smoking cessation: meta‐analysis update. Heart and Lung 2006;25(3):147‐63. [DOI] [PubMed] [Google Scholar]
Rice 2008
- Rice VH, Stead LF. Nursing interventions for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD001188.pub3] [DOI] [PubMed] [Google Scholar]
Rice 2013
- Rice VH, Hartmann‐Boyce J, Stead LF. Nursing interventions for smoking cessation. Cochrane Database of Systematic Reviews 2013. [DOI: 10.1002/14651858.CD001188.pub4] [DOI] [PubMed] [Google Scholar]


