Abstract
Background
The projected rise in the incidence of type 2 diabetes mellitus (T2DM) could develop into a substantial health problem worldwide. Whether diet, physical activity or both can prevent or delay T2DM and its associated complications in at‐risk people is unknown.
Objectives
To assess the effects of diet, physical activity or both on the prevention or delay of T2DM and its associated complications in people at increased risk of developing T2DM.
Search methods
This is an update of the Cochrane Review published in 2008. We searched the CENTRAL, MEDLINE, Embase, ClinicalTrials.gov, ICTRP Search Portal and reference lists of systematic reviews, articles and health technology assessment reports. The date of the last search of all databases was January 2017. We continuously used a MEDLINE email alert service to identify newly published studies using the same search strategy as described for MEDLINE up to September 2017.
Selection criteria
We included randomised controlled trials (RCTs) with a duration of two years or more.
Data collection and analysis
We used standard Cochrane methodology for data collection and analysis. We assessed the overall quality of the evidence using GRADE.
Main results
We included 12 RCTs randomising 5238 people. One trial contributed 41% of all participants. The duration of the interventions varied from two to six years. We judged none of the included trials at low risk of bias for all 'Risk of bias' domains.
Eleven trials compared diet plus physical activity with standard or no treatment. Nine RCTs included participants with impaired glucose tolerance (IGT), one RCT included participants with IGT, impaired fasting blood glucose (IFG) or both, and one RCT included people with fasting glucose levels between 5.3 to 6.9 mmol/L. A total of 12 deaths occurred in 2049 participants in the diet plus physical activity groups compared with 10 in 2050 participants in the comparator groups (RR 1.12, 95% CI 0.50 to 2.50; 95% prediction interval 0.44 to 2.88; 4099 participants, 10 trials; very low‐quality evidence). The definition of T2DM incidence varied among the included trials. Altogether 315 of 2122 diet plus physical activity participants (14.8%) developed T2DM compared with 614 of 2389 comparator participants (25.7%) (RR 0.57, 95% CI 0.50 to 0.64; 95% prediction interval 0.50 to 0.65; 4511 participants, 11 trials; moderate‐quality evidence). Two trials reported serious adverse events. In one trial no adverse events occurred. In the other trial one of 51 diet plus physical activity participants compared with none of 51 comparator participants experienced a serious adverse event (low‐quality evidence). Cardiovascular mortality was rarely reported (four of 1626 diet plus physical activity participants and four of 1637 comparator participants (the RR ranged between 0.94 and 3.16; 3263 participants, 7 trials; very low‐quality evidence). Only one trial reported that no non‐fatal myocardial infarction or non‐fatal stroke had occurred (low‐quality evidence). Two trials reported that none of the participants had experienced hypoglycaemia. One trial investigated health‐related quality of life in 2144 participants and noted that a minimal important difference between intervention groups was not reached (very low‐quality evidence). Three trials evaluated costs of the interventions in 2755 participants. The largest trial of these reported an analysis of costs from the health system perspective and society perspective reflecting USD 31,500 and USD 51,600 per quality‐adjusted life year (QALY) with diet plus physical activity, respectively (low‐quality evidence). There were no data on blindness or end‐stage renal disease.
One trial compared a diet‐only intervention with a physical‐activity intervention or standard treatment. The participants had IGT. Three of 130 participants in the diet group compared with none of the 141 participants in the physical activity group died (very low‐quality evidence). None of the participants died because of cardiovascular disease (very low‐quality evidence). Altogether 57 of 130 diet participants (43.8%) compared with 58 of 141 physical activity participants (41.1%) group developed T2DM (very low‐quality evidence). No adverse events were recorded (very low‐quality evidence). There were no data on non‐fatal myocardial infarction, non‐fatal stroke, blindness, end‐stage renal disease, health‐related quality of life or socioeconomic effects.
Two trials compared physical activity with standard treatment in 397 participants. One trial included participants with IGT, the other trial included participants with IGT, IFG or both. One trial reported that none of the 141 physical activity participants compared with three of 133 control participants died. The other trial reported that three of 84 physical activity participants and one of 39 control participants died (very low‐quality evidence). In one trial T2DM developed in 58 of 141 physical activity participants (41.1%) compared with 90 of 133 control participants (67.7%). In the other trial 10 of 84 physical activity participants (11.9%) compared with seven of 39 control participants (18%) developed T2DM (very low‐quality evidence). Serious adverse events were rarely reported (one trial noted no events, one trial described events in three of 66 physical activity participants compared with one of 39 control participants ‐ very low‐quality evidence). Only one trial reported on cardiovascular mortality (none of 274 participants died ‐ very low‐quality evidence). Non‐fatal myocardial infarction or stroke were rarely observed in the one trial randomising 123 participants (very low‐quality evidence). One trial reported that none of the participants in the trial experienced hypoglycaemia. One trial investigating health‐related quality of life in 123 participants showed no substantial differences between intervention groups (very low‐quality evidence). There were no data on blindness or socioeconomic effects.
Authors' conclusions
There is no firm evidence that diet alone or physical activity alone compared to standard treatment influences the risk of T2DM and especially its associated complications in people at increased risk of developing T2DM. However, diet plus physical activity reduces or delays the incidence of T2DM in people with IGT. Data are lacking for the effect of diet plus physical activity for people with intermediate hyperglycaemia defined by other glycaemic variables. Most RCTs did not investigate patient‐important outcomes.
Keywords: Humans; Diet; Exercise; Cause of Death; Combined Modality Therapy; Combined Modality Therapy/methods; Diabetes Complications; Diabetes Complications/prevention & control; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/complications; Diabetes Mellitus, Type 2/epidemiology; Diabetes Mellitus, Type 2/prevention & control; Diet, Diabetic; Fasting; Fasting/blood; Glucose Tolerance Test; Incidence; Randomized Controlled Trials as Topic; Risk
Plain language summary
Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk
Review question
Are diet or physical activity, or both able to prevent or delay the development of type 2 diabetes and its associated complications in at‐risk people?
Background
People with moderately elevated blood glucose (often referred to as 'prediabetes') are said to be at an increased risk of developing type 2 diabetes. It is currently recommended that all people with increased risk of developing type 2 diabetes should adjust their eating habits and physical activity levels. We wanted to find out whether these changes in diet, physical activity or both could prevent or delay type 2 diabetes in people at increased risk. We also wanted to know the effects on patient‐important outcomes, such as complications of diabetes (e.g. kidney and eye disease, heart attack, stroke), death from any cause, health‐related quality of life (a measure of a person’s satisfaction with their life and health) and side‐effects.
Study characteristics
Participants had to have blood glucose levels higher than considered normal, but below the glucose levels that are used to diagnose type 2 diabetes mellitus. We found 12 randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) with 5238 participants. The duration of the treatments varied from two years to six years. Most trials included people defined as being at increased risk of type 2 diabetes based on glucose levels measured two hours after ingestion of 75 g of glucose (i.e. 'impaired glucose tolerance' (IGT) after an oral glucose tolerance test).
This evidence is up to date as of January 2017. We used a MEDLINE email alert service to identify newly published studies up to September 2017.
Key results
One study compared diet only with physical activity only. Fifty‐seven of 130 participants (44%) in the diet‐only group compared with 58 of 141 participants (41%) in the physical activity‐only group developed type 2 diabetes. Two studies compared physical activity with standard treatment; in one study 58 of 141 participants (41%) in the physical activity group compared with 90 of 133 participants (68%) in the control group developed type 2 diabetes; in the other study 10 of 84 participants (12%) in the physical activity group compared with seven out of 39 participants (18%) in the control group developed type 2 diabetes. Eleven studies compared diet plus physical activity with standard or no treatment. Diet plus physical activity decreased the risk of developing type 2 diabetes, which occurred in 315 of 2122 participants (15%) in the diet plus physical activity group compared with 614 of 2389 participants (26%) in the standard treatment group.
We detected neither an advantage nor a disadvantage of diet, physical activity or both with regard to heart attacks or strokes. Our included studies did not report on complications of diabetes such as kidney or eye disease. The effects on health‐related quality of life were inconclusive. Very few participants died in the course of the studies and side‐effects were also rare. Future long‐term studies should investigate more patient‐important outcomes like complications of diabetes, because we do not know for sure whether ’prediabetes’ is just a condition arbitrarily defined by a laboratory measurement or is, in fact, a real risk factor for type 2 diabetes mellitus and whether treatment of this condition translates into better patient‐important outcomes.
Quality of the evidence
All included trials had deficiencies in the way that they were conducted or how key items were reported. For diet plus physical activity compared with standard treatment, we found rather good evidence that the development of new type 2 diabetes was reduced or delayed. For the other comparisons the number of participants was small, resulting in a high risk of random errors (play of chance).
Summary of findings
Summary of findings for the main comparison. Diet plus physical activity versus standard treatment.
| Diet plus physical activity versus standard treatment for prevention or delay of type 2 diabetes mellitus | ||||||
|
Population: people at increased risk of developing type 2 diabetes mellitus Settings: outpatients Intervention: diet plus physical activity Comparison: standard treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Standard treatment | Diet plus physical activity | |||||
|
All‐cause mortality Follow‐up: up to 6 years (mean duration 3.6 years) |
5 per 1000 | 5 per 1000 (2 to 12) | RR 1.12 (0.50 to 2.50) | 4099 (10) | ⊕⊝⊝⊝ very lowa | The 95% prediction interval ranged from 0.44 to 2.88 TSA showed that 0.61% of the diversity‐adjusted information size was accrued to detect or reject a 10% RRR |
|
Incidence of type 2 diabetes mellitus Diagnostic criteria:
Follow‐up: up to 6 years (mean duration 3.8 years) |
257 per 1000 | 146 per 1000 (129 to 164) | RR 0.57 (0.50 to 0.64) | 4511 (11) | ⊕⊕⊕⊝ moderateb | The 95% prediction interval ranged from 0.50 to 0.65 TSA showed firm evidence for a 10% RRR in favour of diet plus physical activity |
|
Serious adverse events (SAE) Follow‐up: up to 6 years |
See comment | See comment | See comment | 250 (2) | ⊕⊕⊝⊝ lowc | In 1 trial 1/51 participants in the diet plus physical activity group compared with 0/51 participants in the standard treatment group experienced a SAE (EDIPS 2009) 1 trial reported that no adverse occurred (Da Qing 1997). In 4 other trials it was clearly described that SAE data had been collected but data were not presented (DPP 2002; HELP PD 2011; IDPP 2006; JDPP 2013) |
|
Cardiovascular mortality Follow‐up: up to 6 years (mean duration 3.1 years) |
2 per 1000 | 2 per 1000 (1 to 9) | RR 0.94 (0.24 to 3.65) | 3263 (7) | ⊕⊝⊝⊝ very lowa | TSA showed that 0.13% of the diversity‐adjusted information size was accrued to detect or reject a 10% RRR |
| Non‐fatal myocardial infarction/stroke Follow‐up: 3.11 years | See comment | See comment | See comment | 102 (1) | ⊕⊝⊝⊝ lowd | 1 trial reported that none of the participants experienced a non‐fatal myocardial infarction or non‐fatal stroke (EDIPS 2009) |
|
Health‐related quality of life Description: SF‐36 to evaluate the SF‐6D, PCS and MCS MID was defined as difference in scores between groups of at least 3% Follow‐up: 3.2 years |
See comment | See comment | See comment | 2144 (1) | ⊕⊝⊝⊝ very lowe | SF‐6D and PCS improved in the diet plus physical activity group (DPP 2002), MID was not achieved MCS improved in the placebo group (DPP 2002), MID was not achieved |
|
Socioeconomic effects
Description: direct medical costs of the interventions Follow‐up: up to 3 years |
The mean direct medical costs of the intervention ranged across control groups from USD 61 to USD 184 | The mean direct medical costs in the intervention groups ranged across diet plus physical activity group from USD 225 to USD 3625 | ‐ | 2775 (4) | ⊕⊕⊝⊝ lowf | 1 trial reported on the health system/society perspective: USD 31,500/USD 51,600 per QALY with diet plus physical activity (DPP 2002) 1 trial reported total extra 3‐year mean costs for the diet plus physical activity group of GBP 1126, with GBP 615 being dietitian costs, and more outpatient visits in the intervention group than in the control group costing GBP 327 more (PODOSA 2014). 1 trial reported direct medical costs for each participant in the diet plus physical activity group of USD 850 compared with USD 142 in the control group; direct costs of care outside the trial were USD 5177 for the diet plus physical activity group compared with USD 7454 for the control group (HELP PD 2011) 1 trial reported direct medical costs of interventions over the 3‐year trial period of USD 61 per participant in the control group compared with USD 225 in the diet plus physical activity group (IDPP 2006) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADA: American Diabetes Association; CI: confidence interval; DPP: Diabetes Prevention Program; FGL: fasting glucose levels; FPG: fasting plasma glucose; MCS: mental component summaries; MID: minimal important difference; OGTT: oral glucose tolerance test; PCS: physical component summaries;PG: plasma glucose; QUALY: quality‐adjusted life years; RR: risk ratio; RRR: relative risk reduction; SAE: serious adverse event; SF‐36: 36‐Item Short‐Form; SF‐6D: health utility index (SF‐6D); T2DM: type 2 diabetes mellitus; TSA: trial sequential analysis; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
*Assumed risk was derived from the event rates in the comparator groups
aDowngraded by three levels because of risk of bias including possible publication and other bias, inconsistency and imprecision ‐ see Appendix 14. bDowngraded by one level because of other bias (early termination of three trials due to benefit providing the majority of data) ‐ see Appendix 14. cDowngraded by two levels because of reporting bias and imprecision ‐ see Appendix 14. dDowngraded by two levels because of serious imprecision (very sparse data) ‐ see Appendix 14. eDowngraded by three levels because of serious risk of bias (performance bias, detection bias, other bias) and imprecision ‐ see Appendix 14. fDowngraded by two levels because of risk of bias (trial stopped early for benefit providing the majority of data) and imprecision ‐ see Appendix 14.
Summary of findings 2. Diet versus physical activity or standard treatment.
| Diet versus physical activity or standard treatment for prevention or delay of type 2 diabetes mellitus | ||||||
|
Population: people at increased risk of developing type 2 diabetes mellitus Settings: outpatients Intervention: dietary intervention Comparison: physical activity or standard treatment | ||||||
| Outcomes | Physical activity | Diet or standard treatment | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments |
|
All‐cause mortality Follow‐up: 6 years |
See comment | See comment | See comment | 530 (1) | ⊕⊝⊝⊝ very lowa | 3/130 participants died in the diet group vs 0/141 participants in the physical activity group 3/133 participants died in the standard treatment group |
|
Incidence of type 2 diabetes mellitus Definition: WHO 1985 criteria Follow‐up: 6 years |
See comment | See comment | See comment | 530 (1) | ⊕⊝⊝⊝ very lowa | 57/130 participants developed T2DM in the diet group vs 58/141 participants in the physical activity group 90/133 participants developed T2DM in the standard treatment group |
|
Serious adverse events Follow‐up: 6 years |
See comment | See comment | See comment | 530 (1) | ⊕⊝⊝⊝ very lowa | No (serious) adverse events occurred in any group |
|
Cardiovascular mortality Follow‐up: 6 years |
See comment | See comment | See comment | 530 (1) | ⊕⊝⊝⊝ very lowa | No participants in any group died of cardiovascular reasons |
| Non‐fatal myocardial infarction/stroke | Not reported | |||||
| Health‐related quality of life | Not reported | |||||
| Socioeconomic effects | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; T2DM: type 2 diabetes mellitus; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
*Assumed risk was derived from the event rates in the comparator groups
aDowngraded by three levels because of risk of reporting and other bias and serious imprecision (very sparse data) ‐ see Appendix 15
Summary of findings 3. Physical activity versus standard treatment.
| Physical activity versus standard treatment for prevention or delay of type 2 diabetes mellitus | ||||||
|
Population: people at increased risk of developing type 2 diabetes mellitus Settings: outpatients Intervention: physical activity Comparison: standard treatment | ||||||
| Outcomes | Standard treatment | Physical activity | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments |
|
All‐cause mortality Follow‐up: 3 and 6 years |
See comment | See comment | See comment | 397 (2) | ⊕⊝⊝⊝ very lowa | 1 trial reported that 0/141 participants in the physical activity group compared with 3/133 participants in the standard treatment group died (Da Qing 1997) 1 trial reported that 3/84 participants in the physical activity group and 1/39 participants in the standard treatment group died (Hellgren 2016 ‐ data provided by trial authors) |
|
Incidence of type 2 diabetes mellitus Definition:
Follow‐up: 3 and 6 years |
See comment | See comment | See comment | 397 (2) | ⊕⊝⊝⊝ very lowa | 1 trial reported that 58/141 participants in the physical activity group compared with 90/133 participants in the standard treatment group developed T2DM (Da Qing 1997) 1 trial reported that 10/84 participants in the physical activity group compared with 7/39 participants in the standard treatment group developed T2DM (Hellgren 2016) |
|
Serious adverse events Follow‐up: 3 and 6 years |
See comment | See comment | See comment | 397 (2) | ⊕⊝⊝⊝ very lowa | 1 trial reported no (serious) adverse events occurred (Da Qing 1997). 1 trial reported that 3/66 participants in the physical activity group compared with 1/39 participants in the standard treatment group experienced a serious adverse event (Hellgren 2016 ‐ data provided by trial authors) |
|
Cardiovascular mortality Follow‐up: 6 years |
See comment | See comment | See comment | 274 (1) | ⊕⊝⊝⊝ very lowa | No participants in any group died of cardiovascular reasons |
|
Non‐fatal myocardial infarction/stroke
Description: non‐fatal myocardial infarction/stroke Follow‐up: 3 years |
See comment | See comment | See comment | 123 (1) | ⊕⊝⊝⊝ very lowa | 1 trial reported that 0/66 participants in the physical activity group compared with 3/31 participants in the standard treatment group experienced a non‐fatal myocardial infarction (Hellgren 2016 ‐ data provided by trial authors) 1 trial reported that 1/66 participants in the physical activity group compared with 1/31 participants in the standard treatment group experienced a non‐fatal stroke (Hellgren 2016 ‐ data provided by trial authors) |
|
Health‐related quality of life Definition: measured by two questions (grading total physical and mental health from 1 to 7; grading general health from 1 (best) to 5 (very bad)) Follow‐up: 3 years |
See comment | See comment | See comment | 123 (1) | ⊕⊝⊝⊝ very lowa | 27%, 43% and 29% of participants in the physical activity group experienced worse, unchanged and better health‐related quality of life, respectively (Hellgren 2016 ‐ data provided by trial authors) 35%, 43% and 22% in the standard treatment group experienced worse, unchanged and better health‐related quality of life, respectively (Hellgren 2016 ‐ data provided by trial authors) |
| Socioeconomic effects | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FPG: fasting plasma glucose; OGTT: oral glucose tolerance test; RR: risk ratio; T2DM: type 2 diabetes mellitus | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
*Assumed risk was derived from the event rates in the comparator groups
aDowngraded by three levels because of risk of reporting and other bias and serious imprecision (very sparse data) ‐ see Appendix 16
Background
Description of the condition
'Prediabetes', 'borderline diabetes', the 'prediabetic stage', 'high risk of diabetes' or 'intermediate hyperglycaemia' (WHO/IDF 2006) are often characterised by various measurements of elevated blood glucose concentrations (such as isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT), isolated elevated glycosylated haemoglobin A1c (HbA1c) or combinations thereof). These elevated blood glucose levels indicating hyperglycaemia are considered too high to be normal but below the diagnostic threshold for type 2 diabetes mellitus (T2DM). Therefore, because of the continuous spectrum from the normal to the diabetic stage, a sound evidence base is needed to define thresholds for conditions of 'sub‐diabetes'. It is obvious that the different terms used to describe various stages of hyperglycaemia might induce different emotional reactions. For example, the term 'prediabetes' may imply (at least for the lay person) that diabetes is unavoidable whereas (high) risk of diabetes has the positive connotation that the disease may be avoided altogether. We will use all of the above‐mentioned terms throughout this systematic review, however a we will focus on 'prediabetes' because many people associate this label with dire consequences ‐ despite the disputable construct of intermediate health states termed prediseases (Viera 2011). On the other side, any diagnosis of 'prediabetes' might be an opportunity to review, for example, eating habits and physical activity levels, thus enabling affected individuals to actively change their way of life.
The most commonly used criteria to define people with a high risk of developing T2DM were established by the American Diabetes Association (ADA) and the World Health Organization (WHO). The first glycaemic measurement used to define the prediabetic stage by the US National Diabetes Data Group was IGT (NDDG 1979). IGT is based on the measurement of plasma glucose two hours after ingestion of 75 g glucose. The prediabetic range is defined as a plasma glucose level between 7.8 to 11.1 mmol/L (140 to 200 mg/dL) two hours after the glucose load. Studies have indicated that IGT is caused by insulin resistance and defective insulin secretion (Abdul‐Ghani 2006). In 1997 the ADA and later on the WHO introduced the IFG concept to define 'prediabetes' (ADA 1997; WHO 1999). The initial definition of IFG was 6.1 to 6.9 mmol/L (110 to 125 mg/dL). Later on, the ADA reduced the lower threshold for defining IFG to 5.6 mmol/L (100 mg/dL) (ADA 2003). However, this lower cut‐off point for IFG to define 'prediabetes' was not endorsed by the WHO (WHO/IDF 2006). IFG seems to be associated with ß‐cell dysfunction (impaired insulin secretion) and an increase of the hepatic glucose output (DeFronzo 1989). More recently, HbA1c has been introduced for identifying people with a high risk of developing T2DM. In 2009, the International Expert Committee (IEC) suggested the HbA1c to identify people with a high risk of T2DM. People with HbA1c measurements between 6.0% to 6.4% fulfilled this criterion (IEC 2009). Shortly after, the ADA redefined this HbA1c level as 5.7% to 6.4% to identify people with a high risk of developing T2DM (ADA 2010). Unlike IFG and IGT, HbA1c reflects longer‐term glycaemic control, that is, how the blood glucose levels have been during the previous two to three months (Inzucchi 2012).
In 2010, the International Diabetes Federation (IDF) estimated the prevalence of IGT to be 343 million, and this number is predicted to increase to 471 million by 2035 (IDF 2013). Studies have shown poor correlations between HbA1c and IFG/IGT (Gosmanov 2014; Selvin 2011). Moreover, the various glycaemic tests do not seem to identify the same people (Gosmanov 2014; Selvin 2011). The risk of progression from 'prediabetes' to T2DM depends on the diagnostic criteria used to identify 'prediabetes'. Some people diagnosed with 'prediabetes' will never develop T2DM, and some will return to normoglycaemia. IGT is often accepted as the best glycaemic variable for 'prediabetes' to predict progression to T2DM. However, studies indicate that less than half of the people defined as prediabetic by means of IGT will develop T2DM in the following 10 years. IFG and HbA1c are both thought to predict a different risk spectrum for developing T2DM (Cheng 2006; Morris 2013). Most importantly, 'prediabetes' is commonly an asymptomatic condition, and naturally often remains 'undiagnosed' (CDC 2015). Consequently, 'prediabetes' may exist before the diagnosis of T2DM is established.
Currently, ADA recommends reduced calorie intake and increased physical activity for people with increased risk of T2DM (ADA 2017). It is still not clarified if any particular intervention, especially glucose‐lowering drugs, should be recommended for people with 'prediabetes' (Yudkin 2014). Trials have indicated that the progression from 'prediabetes' to T2DM is reduced, or maybe just delayed with 'lifestyle' interventions (increased physical activity, dietary changes or both) (Diabetes Prevention Program 2002; Diabetes Prevention Program FU 2009; Finnish Diabetes Prevention Study Group 2001). A recent meta‐analysis of 22 studies with behaviour‐changing interventions in people at high risk of T2DM concluded that the effect of lifestyle interventions on longer‐term diabetes prevention had not been clarified (Dunkley 2014).
The prescription of pharmacological glucose‐lowering interventions for the prevention of T2DM is not generally accepted among international diabetes associations and clinicians. Several groups of pharmacological glucose‐lowering interventions have been investigated in people with 'prediabetes'. Some findings indicate that the progression from 'prediabetes' to T2DM is reduced or maybe just delayed (Diabetes Prevention Program 2002; Diabetes Prevention Program FU 2009). However, the ADA recommends metformin for people with 'prediabetes' and a body mass index more than 35 kg/m², aged less than 60 years, and women with prior gestational diabetes mellitus (ADA 2015).
Description of the intervention
Interventional as well as observational studies have shown a reduction in the incidence of T2DM with reduced calorie intake and increased physical activity in people at risk of T2DM (Da Qing 1997; DPP 2002; Helmrich 1991; Smith 2016). It has been shown that diet plus physical activity reduces cardiovascular risk factors, thereby indicating a potential beneficial effect on mortality and cardiovascular outcomes (Balk 2015).
Diet plus physical activity is recommended as an initial intervention not only for people with intermediate hyperglycaemia but also for people with T2DM (ADA 2017). However, one large‐scale, randomised, controlled trial in people overweight or obese people with T2DM did not show a substantial effect on mortality and cardiovascular outcomes with intensive diet plus physical activity compared with control after 9.6 years (Look AHEAD 2013).
Nutritional advice usually consists of caloric restriction in overweight people, low total fat content (especially saturated fat) and high (predominantly unrefined) carbohydrate content. Physical activity advice usually consists of an intervention programme.
Adverse effects of the intervention
Physical activity or diet interventions are not generally considered to be associated with any serious adverse event. However, physical activity may cause traumatic injuries of variable severity depending on the type and intensity of physical activity. Additionally, exercising may produce adverse effects on the cardiovascular system in those people with insufficient training or unfavourable cardiovascular fitness (even cardiovascular events and death may potentially occur while exercising). Also, the implementation of dietary measures may produce several deficiencies in nutritional status if restrictive low‐calorie diets are used. Further, dieting may reduce quality of life of people under this treatment. Unfortunately, very little information on these issues is available from randomised controlled trials.
How the intervention might work
There are prospective cohort studies that have shown that increased physical activity, independent of other risk factors, has a protective effect against the development of T2DM (Helmrich 1991; Manson 1992). These epidemiological prospective studies demonstrated that various levels of regular physical activity once to several times a week were associated with a decreased incidence of the disease at long‐term follow‐up (14 years and five years respectively) (Helmrich 1991; Manson 1992).
Three large clinical trials in people with IGT have shown a relative risk reduction of about 50% in the progression to T2DM with restricted diet and increased physical activity (Da Qing 1997; DPP 2002; DPS 2001). However, whether and how diet, physical activity or both influence the risk of complications associated with T2DM is still not clarified.
Why it is important to do this review
There has been an increased focus on the prevention or delay of T2DM with non‐pharmacological interventions and glucose‐lowering medications. Recently, several systematic reviews and health technology assessment reports have been performed in people with elevated risk of T2DM (Aguiar 2014; Ali 2012; Ashra 2015; Balk 2015; Cardona‐Morrell 2010; Dunkley 2014; Gillett 2012; Gillies 2007; Glechner 2015; Gong 2015; Hopper 2011; ICER 2016; Merlotti 2014a; Merlotti 2014b; Modesti 2016; Norris 2005; Santaguida 2005; Schellenberg 2013; Selph 2015; Stevens 2015; Yamaoka 2005; Yates 2007; Yoon 2013; Yuen 2010; Zhang 2017; Zheng 2016).
Sixteen of these systematic reviews included exclusively people with intermediate hyperglycaemia (Aguiar 2014; Gillett 2012; Gillies 2007; Glechner 2015; Gong 2015; Hopper 2011; ICER 2016; Norris 2005; Santaguida 2005; Selph 2015; Stevens 2015; Yamaoka 2005; Yates 2007; Yoon 2013; Yuen 2010; Zheng 2016); 10 systematic reviews included people with an increased risk of T2DM defined by additional variables with intermediate hyperglycaemia being one risk factor only (e.g. obesity, metabolic risk factors, family history of diabetes etc) (Ali 2012; Ashra 2015; Balk 2015; Cardona‐Morrell 2010; Dunkley 2014; Merlotti 2014a; Merlotti 2014b; Modesti 2016; Schellenberg 2013; Zhang 2017).
This review is an update of the Cochrane Review published in 2008 (Orozco 2008). In this update we have implemented new methodology and changed the priority of outcomes, focusing on patient‐important outcome measures. Also, we have only included trials where intermediate hyperglycaemia was measured at baseline as an indicator of increased risk for the development of T2DM.
Objectives
To assess the effects of diet, physical activity or both for the prevention or delay of T2DM and its associated complications in people at increased risk of developing T2DM.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials (RCTs).
Types of participants
Nondiabetic individuals at increased risk of developing T2DM, that is, diagnosed with intermediate hyperglycaemia or 'prediabetes'.
Diagnostic criteria for 'prediabetes'
To be consistent with changes in the classification of and diagnostic criteria for 'prediabetes' (impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and elevated glycosylated haemoglobin A1c (HbA1c)) over the years, the diagnosis had to be established using the standard criteria valid at the time of the trial commencing (for example ADA 1997; ADA 2010; NDDG 1979; WHO 1999). Ideally, the diagnostic criteria should have been described. If necessary, we used the trial authors' definition of 'prediabetes' but contacted trial authors for additional information. Differences of glycaemic measurements used to define 'prediabetes' may introduce substantial heterogeneity. We therefore planned to subject diagnostic criteria to a subgroup analysis.
Types of interventions
We planned to investigate the following comparisons of intervention versus comparator.
Intervention
Diet
Physical activity
Diet plus physical activity
Comparator
Standard treatment
No intervention
Comparison of interventions
Diet versus physical activity
Other concomitant interventions had to be the same in the intervention and comparator groups to establish fair comparisons.
Minimum duration of intervention
We included trials with a minimum duration of intervention of two years.
Summary of exclusion criteria
We excluded the following.
Trials where the intervention or comparator group comprised the administration of any pharmacological agent.
People diagnosed with 'metabolic syndrome' because this is a special cohort of doubtful clinical usefulness and uncertain distinct disease entity (a composite of risk indicators such as elevated blood lipids, insulin resistance, obesity, high blood pressure).
Trials applying diet advice through single‐food or supplement dietary changes (e.g. zinc supplement).
Trials with identical diet or physical activity interventions, or both, applied with different approaches (e.g. same advice applied by means of individual or group sessions).
We did not exclude trials because one or several of our primary or secondary outcome measures were not reported in the publication. In case none of our primary or secondary outcomes were reported, we included the trial and contacted the corresponding author for supplementary data. If no additional data were available we planned to provide some basic information in a supplementary table.
Types of outcome measures
Primary outcomes
All‐cause mortality
Incidence of T2DM
Serious adverse events
Secondary outcomes
Cardiovascular mortality
Non‐fatal myocardial infarction
Non‐fatal stroke
Amputation of lower extremity
Blindness or severe vision loss
End‐stage renal disease
Non‐serious adverse events
Hypoglycaemia
Health‐related quality of life
Time to progression to T2DM
Measures of blood glucose control
Socioeconomic effects
Method of outcome measurement
All‐cause mortality: defined as death from any cause
Incidence of T2DM and time to progression to T2DM: defined according to diagnostic criteria valid at the time the diagnosis was established, using the standard criteria valid at the time of the trial commencing (e.g. ADA 2008; WHO 1998). If necessary, we used the trial authors' definition of T2DM.
Serious adverse events: defined according to the International Conference on Harmonization Guidelines as any event that led to death, that was life‐threatening, required in‐patient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability, and any important medical event that may have had jeopardised the participant or required intervention to prevent it (ICH 1997) or as reported in trials.
Cardiovascular mortality, non‐fatal myocardial infarction, non‐fatal stroke, amputation of lower extremity, blindness or severe vision loss, hypoglycaemia (mild, moderate, severe/serious): defined as reported in trials.
End‐stage renal disease: defined as dialysis, renal transplantation or death due to renal disease.
Non‐serious adverse events: defined as number of participants with any untoward medical occurrence not necessarily having a causal relationship with the intervention.
Health‐related quality of life: defined as mental and physical health‐related quality of life, separate and combined, evaluated by a validated instrument such as Short‐Form 36.
Measures of blood glucose control: fasting blood glucose, blood glucose two hours after ingestion of 75 g glucose and HbA1c measurements.
Socioeconomic effects: for example costs of the intervention, absence from work, medication consumption.
Timing of outcome measurement
Measured at the end of the intervention and the end of follow‐up: all‐cause mortality, cardiovascular mortality, blindness or severe vision loss, hypoglycaemia (mild, moderate, severe/serious), end‐stage renal disease, non‐serious adverse events; health‐related quality of life, measures of blood glucose control, socioeconomic effects
Measured at the end of the intervention and the longest reported end of follow‐up: incidence of T2DM
Measured at any time of the intervention and during follow‐up: serious adverse events
Search methods for identification of studies
Electronic searches
We based this review update on different search techniques.
First, we extracted the included trials of two systematic reviews targeting people at increased risk for T2DM. The first review was funded by the Agency for Healthcare Research and Quality (AHRQ) and evaluated lifestyle interventions (Schellenberg 2013). The second review was funded by the Centers for Disease Control and Prevention Community Preventive Services Task Force and evaluated combined diet and physical activity programs (Balk 2015). Both of these reviews included extensive and highly sensitive search strategies conducted in several databases up to June 2013 (Schellenberg 2013) and up to February 2015 (Balk 2015). In addition to evaluating these two systematic reviews, we checked the reference lists of a further 28 systematic reviews and extracted 145 potentially relevant trials in total. This snowballing search technique is reflected in the upper right part of the trial flow diagram.
Second, we identified further trials using a revised search strategy from 2014 to the specified date. We did not place restrictions on the language of publication, and searched the following literature databases.
Cochrane Central Register of Controlled Trials (CENTRAL) via Cochrane Register of Studies Online (CRSO) (searched 17 January 2017).
MEDLINE Ovid (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)) (from 1946 to present, searched 17 January 2017).
Embase Ovid (from 1974 to 2017 Week 3, searched 17 January 2017)
Additionally we searched the following trials registers from inception to the specified date.
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 17 January 2017).
WHO International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/) searched 17 January 2017)
For detailed search strategies, see Appendix 1. We continuously applied an email alert service for MEDLINE via OvidSP to identify newly published trials using the search strategy detailed in Appendix 1 up to September 2017.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved, included trials, (systematic) reviews, meta‐analyses and health technology assessment reports.
Data collection and analysis
Selection of studies
Two review authors (BH and BR) independently scanned the abstract or title, or both, of every record we retrieved, to determine which trials should be assessed further. We investigated the full‐text articles of all potentially relevant articles. We resolved discrepancies through consensus or by recourse to a third review author (MM). We prepared a flow diagram of the number of trials identified and excluded at each stage in accordance with the PRISMA flow diagram of trial selection (Liberati 2009; Figure 1).
1.

Trial flow diagram
Data extraction and management
For trials that fulfilled the inclusion criteria, two review authors (BH and GG) independently extracted outcome data and assessed the risk of bias. One review author (BH) extracted key characteristics of participants and interventions and another (GG) checked them. We reported data on efficacy outcomes and adverse events using standard data extraction sheets from Cochrane Metabolic and Endocrine Disorders. We resolved any disagreements by discussion or, if required, by consultation with a third review author (BR) (for details, see Characteristics of included studies; Table 4; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13; Appendix 14; Appendix 15: Appendix 16; Appendix 17; Appendix 18; Appendix 19).
1. Overview of trial populations.
| Trial (design) | Intervention(s) and comparator(s) | Description of power and sample size calculation | Screened/eligible (N) | Randomised (N) | ITT (N) | Analysed (N) | Finishing trial (N) | Randomised finishing trial (%) | Follow‐up (extended follow‐up)a |
|
Da Qing 1997a (cluster‐RCT) |
Intervention 1: diet | "Power calculations were done for the original 6‐year intervention trial. For the present study we estimated minimal detectable differences. With an α of 0·05, we estimated that there was an 80% chance of detecting a 43% reduction in all‐cause mortality and a 63% reduction in cardiovascular disease mortality when comparing the control group with the combined lifestyle intervention groups" | 110,660 | ‐ | 130 | 130 | 130 | ‐ | 6 years (23 years) |
| Intervention 2: physical activity | ‐ | 141 | 141 | 141 | ‐ | ||||
| Intervention 3: physical activity + diet | ‐ | 126 | 126 | 126 | ‐ | ||||
| Comparator: standard treatment | ‐ | 133 | 133 | 133 | ‐ | ||||
| total: | 577 | 530 | 530 | 530 | 91.9b | ||||
|
DPP 2002 (parallel RCT) |
Intervention: physical activity + diet | "The principal analyses of primary and secondary outcomes will employ the "intent‐to‐treat" approach ... The intent‐to‐treat analyses will include all randomized participants with all participants included in their randomly assigned treatment group; treatment group assignment will not be altered based on the participant’s adherence to the assigned treatment regimen. All statistical tests will be two‐sided. The overall significance level of the primary outcome will be α = 0.05. However, because interim analyses will be conducted throughout the DPP, the significance levels used in the interim and final analyses of the primary outcome will be adjusted to account for the multiplicity of interim analyses" | 153,183 | 1079 | 1079 | 1079 | 1052 | 97.5 | 2.8 years (15 years) |
| Comparator: placebo + standard treatment | 1082 | 1082 | 1082 | 1042 | 96.3 | ||||
| total: | 2161c | 2161 | 2161 | 2094 | 96.9 | ||||
|
DPS 2001d (parallel RCT) |
Intervention: physical activity + diet | "The DPS is designed to be large enough to detect a 35% reduction in diabetes incidence with an intensive diet and exercise intervention with 80% power (beta = 20%) at the two‐tailed 5% significance level (alpha = 5%) ..." | ‐ | 265 | 265 | 265 | 241e | 91 | Median 4 years (10.6 years) |
| Comparator: standard treatment | 257 | 257 | 257 | 239e | 93 | ||||
| total: | 522 | 522 | 522 | 480e | 92 | ||||
|
EDIPS 2009 (parallel RCT) |
Intervention: physical activity + diet | "EDIPS‐Newcastle was designed to contribute to the European study. We aimed for a sample size of 100 participants (50 in each arm), contributing to a planned total of 750 participants across Europe" | 482 | 51 | 51 | 21f | 21 | 41.2 | 3.11 years (3.11 years) |
| 51 | 51 | 21 | 21 | 41.2 | |||||
| Comparator: standard treatment | total: | 102 | 102 | 42 | 42 | 41.2 | |||
|
Hellgren 2016 (parallel RCT) |
Intervention: physical activity | ‐ | 9734 | ‐ | 66 | 66 | 66 | ‐ | 3 years |
| ‐ | 30 | 30 | 30 | ‐ | |||||
| Comparator: standard treatment | total: | 123g | 96 | 96 | 96 | 78 | |||
|
HELP PD 2011 (parallel RCT) |
Intervention: physical activity + diet | "Based on a longitudinal correlation of r = 0.20, this sample was projected to provide 94% power to detect a net intervention effect of 3.5 mg/dL (two‐sided alpha of 0.05) and 86% power to detect an effect size of 3 mg/dL. These estimates include allowance for a 5% loss to follow‐up rate every 6 months" | 1818 | 151 | 151 | 127h | 127h | 84.1 | 2 years |
| 150 | 150 | 134 | 134 | 89.3 | |||||
| Comparator: standard treatment | total: | 301 | 301 | 261 | 261 | 86.7 | |||
|
IDPP 2006 (parallel RCT) |
Intervention: physical activity + diet | "It was assumed that the cumulative incidence of diabetes in 3 years would be approximately 30% in the control group and that there would be a 50% reduction with the intervention methods. The sample size required in each of the four subgroups was 134 with a type 1 error of 5%, 80% power, and allowing for a dropout rate of 10%" | 10,839 | 133 | 120 | 120 | 120 | 91 | 3 years |
| 136 | 133 | 133 | 133 | 98.5 | |||||
| Comparator: standard treatment | total: | 269 | 253 | 253 | 253 | 94.1 | |||
|
JDPP 2013 (parallel RCT) |
Intervention: physical activity + diet | "According to prospective studies on the Japanese population, the yearly incidence of diabetes among subjects with IGT varies between 1 and 5% ... Therefore, it was assumed that the 6‐year cumulative incidence of diabetes would be 30% in the control group. The present study was designed to detect a 50% reduction in the incidence by the intervention. Thus the sample size required was 313 with a type 1 error of 5%, with 80% power (beta = 20%) at the two‐tailed 5% significance level, and allowing for a withdrawal rate of 30%" | 1279 | 152 | 146 | 103 | 103 | 67.8 | 3 years |
| Comparator: standard treatment | 152 | 150 | 110 | 110 | 72.4 | ||||
| total: | 304 | 296 | 213 | 213 | 70.1 | ||||
|
Kosaka 2005 (parallel RCT) |
Intervention: physical activity + diet | ‐ (Number of randomised participants was calculated based on the following information: "The rate of drop‐out during the 1‐year observation was 5.6% in the control group and 4.7% in the intensive intervention group, respectively") |
‐ | 107 | 102 | 102 | 95 | 88.8 | 4 years |
| 376 | 356 | 356 | 324 | 86.2 | |||||
| Comparator: standard treatment | total: | 483 | 458 | 458 | 419 | 86.7 | |||
|
Oldroyd 2005 (parallel RCT) |
Intervention: physical activity + diet | "We calculated that a sample size of 100 individuals (50 in each arm) was necessary to detect a 0.6 mmol/l difference in mean fasting plasma glucose and a 20% difference in the proportion with glucose intolerance, both with 90% power at the 5% significance level" | 498 | 39 | 39 | 30i | 30 | 76.9 | 2 years |
| 39 | 39 | 24 | 24 | 61.5 | |||||
| Comparator: no intervention | total: | 78 | 78 | 54 | 54 | 69.2 | |||
|
PODOSA 2014 (cluster‐RCT) |
Intervention: physical activity + diet | "When the protocol was amended in 2009, we knew that the number of families with more than one person recruited with impaired fasting glucose or impaired glucose tolerance was small, so the new power calculation did not take clustering into account. A sample of 150 people assessed at 3 years gave 86% power to detect a mean difference in weight of 2.5 kg between the two groups, assuming an SD of 5 kg with a two‐sided 5% significance level." | 1319 | 85j | 84 | 84 | 84 | 98.8 | 3 years |
| 86 | 83 | 83 | 83 | 96.5 | |||||
| Comparator: standard treatment | total: | 171 | 167 | 167 | 167 | 97.7 | |||
|
SLIM 2003 (parallel RCT) |
Intervention: physical activity + diet | "It was calculated that, based on the results of the Finnish Diabetes Prevention Study (DPS), 50–60 subjects per group would be sufficient to detect a 1.0 mmol/l difference in 2‐h glucose concentration between groups ..." | 2820 | 74 | 74 | 52k | 35 | 47.3 | 4.1 years (range 3 to 6 years) |
| Comparator: standard treatment | 73 | 73 | 54 | 35 | 47.9 | ||||
| total: | 147 | 147 | 106 | 70 | 47.6 | ||||
| Grand total | All interventions | 2136 | |||||||
| All comparators | 3091 | ||||||||
| All interventions and comparators | 5238l | ||||||||
"‐" denotes not reported
aRandomised numbers in each group not specified. bAfter 6 years of intervention, during the subsequent 17‐year follow‐up period after the intervention had stopped 6 participants were lost to follow‐up. cOf the 1082 participants assigned to placebo, 1052 were available for the DPPOS (see DPP 2002); of these 935 were enrolled in the DPPOS. Of the 1079 assigned to the behaviour changing intervention 1042 were available for the DPPOS; of these 915 were enrolled in the DPPOS. dTrial authors state that ITT analysis was performed but data are presented as a per‐protocol analysis. eData for the end of intervention. Participants included in the extension period were 200 in the intervention group and 166 in the control group. fNumber of analysed participants varied during the trial (intervention group: 39 at 1 year; 35 at 2 years; 27 at 3 years; 28 at 4 years; 21 at 5 years; control group: 43 at 1 year; 37 at 2 years; 33 at 3 years; 28 at 4 years; 21 at 5 years). g123 participants were eligible for the trial, 4 died and 10 developed T2DM and did not complete the final examination. One received a gastric bypass; 17 refused follow‐up. Not specified to which intervention groups these people were randomised. hNumber of analysed participants varied during the trial (intervention group: 139 at 6 months, 135 at 12 months, 125 at 18 months and 127 at 24 months; control group: 141 at 6 months, 138 at 12 months, 132 at 18 months and 134 at 24 months). iNumber of analysed participants varied during the trial (intervention group: 37 at 6 months, 32 at 12 months and 30 at 24 months; control group: 32 at 6 months, 30 at 12 months and 24 at 24 months). j78 families with 85 participants and 55 family volunteers were allocated to the intervention, 78 families with 86 participants and 69 family volunteers were allocated to the control. kNumber of analysed participants varied during the trial (intervention group: 52 at 3 years, 51 at 4 years, 34 at 5 years and 35 (one that was missing at 6 years attended 6‐year follow‐up) at 6 years; control group: 54 at 3 years, 43 at 4 years, 29 at 5 years and 35 (6 that were missing at five years attended 6‐year follow‐up) at 6 years. l2 trials did not report the number of randomised participants per intervention group. Therefore, numbers do not add up accurately.
DPP: Diabetes Prevention Program; DPPOS: Diabetes Prevention Program Outcome Study; EDIPS: European Diabetes Prevention Study; HELP PD: Healthy Living Partnerships to Prevent Diabetes; IDPP: Indian Diabetes Prevention Programmes; ITT: intention‐to‐treat; JDPP: Japan Diabetes Prevention Program; PODOSA: Prevention of Diabetes and Obesity in South Asians; RCT: randomised controlled trial; SLIM: Study on Lifestyle‐intervention and Impaired glucose tolerance Maastricht
We provided information about potentially relevant ongoing trials, including trial identifier, in the 'Characteristics of ongoing studies' table and in a joint appendix 'Matrix of study endpoints (publications and trial documents)' (Appendix 6). For each included trial, we tried to retrieve the protocol and planned to report primary, secondary and other outcomes in comparison with data in publications in a joint appendix. If not available from the search of the databases, reference screening or Internet searches, we asked trial authors to provide a copy of the protocol.
We emailed all authors of the included trials to enquire whether they would be willing to answer questions regarding their trials. We presented the results of this survey in Appendix 13. We sought relevant missing information on the trial from the primary author(s) of the article, if possible.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary trial, we maximised the information yield by collating all available data, and we used the most complete data set aggregated across all known publications. We listed duplicate publications, companion documents, multiple reports of a primary trial, and trial documents of included trials (such as trial registry information) as secondary references under the study ID of the included trial. Furthermore, we also listed duplicate publications, companion documents, multiple reports of a trial, and trial documents of excluded trials (such as trials registry information) as secondary references under the study ID of the excluded trial.
Data from clinical trials registers
If data from included trials were available as study results in clinical trials registers, such as ClinicalTrials.gov or similar sources, we made full use of this information and extracted the data. If there was also a full publication of the trial, we collated and critically appraised all available data. If an included trial was marked as a completed study in a clinical trials register but no additional information (study results, publication or both) was available, we added this trial to the table 'Characteristics of studies awaiting classification'.
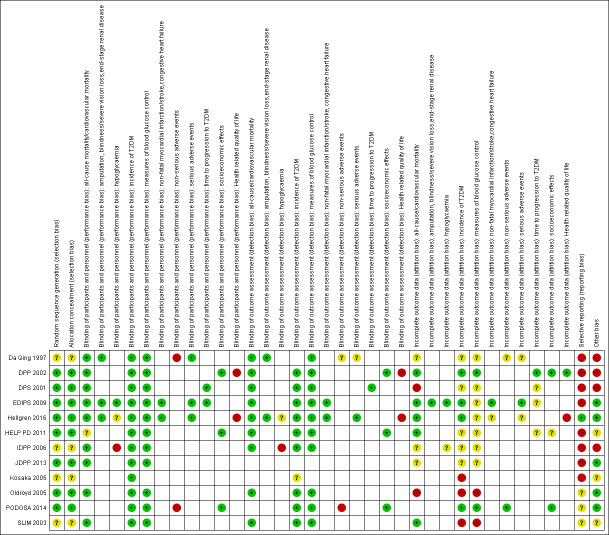
Assessment of risk of bias in included studies
Two review authors (BH and GG) independently assessed the risk of bias of each included trial. We resolved any disagreements by consensus, or by consultation with a third review author (BR). If adequate information was not available from the trial publication, trial protocol or both, we contacted trial authors for missing data on 'Risk of bias' items.
We used the Cochrane 'Risk of bias' assessment tool (Higgins 2011a) and judged risk of bias criteria as either low, high, or unclear and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) where any of the specified criteria for a judgement on low, unclear or high risk of bias justified the associated categorisation.
Random sequence generation
Selection bias due to inadequate generation of a randomised sequence ‐ assessment at trial level
We described for each included trial the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing of lots, tossing a coin, shuffling cards or envelopes, and throwing dice are adequate if performed by an independent person not otherwise involved in the trial. Use of the minimisation technique will be considered as equivalent to being random.
Unclear risk of bias: insufficient information about the sequence generation process.
High risk of bias: the sequence generation method was non‐random (e.g. sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number; allocation by judgement of the clinician; allocation by preference of the participant; allocation based on the results of a laboratory test or a series of tests; allocation by availability of the intervention). We excluded such trials.
Allocation concealment
Selection bias due to inadequate concealment of allocations prior to assignment ‐ assessment at trial level
We described for each included trial the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
Low risk of bias: central allocation (including telephone, interactive voice‐recorder, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
Unclear risk of bias: insufficient information about the allocation concealment.
High risk of bias: using an open random allocation schedule (e.g. a list of random numbers); using assignment envelopes without appropriate safeguards; alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. We excluded such trials.
Blinding of participants and study personnel
Performance bias due to knowledge of the allocated interventions by participants and personnel during the trial ‐ assessment at outcome level
We evaluated the risk of performance bias separately for each outcome (Hróbjartsson 2013). We noted whether outcomes were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Low risk of bias: blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken; no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information about the blinding of participants and study personnel; the trial did not address this outcome.
High risk of bias: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; blinding of trial participants and key personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinding of outcome assessment
Detection bias due to knowledge of the allocated interventions by outcome assessment ‐ assessment at outcome level
We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether outcomes were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Low risk of bias: blinding of outcome assessment ensured, and unlikely that the blinding could have been broken; no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information about the blinding of outcome assessors; the trial did not address this outcome.
High risk of bias: no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Attrition bias due to amount, nature or handling of incomplete outcome data ‐ assessment at outcome level
We described for each included trial, and for each outcome, the completeness of data including attrition and exclusions from the analysis. We investigated whether attrition and exclusions were reported and the number included in the analysis at each stage (compared with the number of randomised participants per intervention/comparator groups), if reasons for attrition or exclusion were reported, and whether missing data were balanced across groups or were related to outcomes. We considered the implications of missing outcome data per outcome, such as high dropout rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between trial arms).
Low risk of bias: no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; appropriate methods, such as multiple imputation, were used to handle missing data.
Unclear risk of bias: insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias; the trial did not address this outcome.
High risk of bias: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ or similar analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation.
Selective reporting
Reporting bias due to selective outcome reporting ‐ assessment at trial level
We assessed outcome reporting bias by integrating the results of Appendix 6, 'Matrix of trial endpoints (publications and trial documents)' (Mathieu 2009), with those of Appendix 7 'High risk of outcome reporting bias according to ORBIT classification' (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting.
Low risk of bias: the trial protocol was available and all of the trial’s pre‐specified (primary and secondary) outcomes that were of interest in the review were reported in the pre‐specified way; the study protocol was not available but it was clear that the published reports included all expected outcomes (ORBIT classification).
Unclear risk of bias: insufficient information about selective reporting.
High risk of bias: not all of the trial’s pre‐specified primary outcomes were reported; one or more primary outcomes was reported using measurements, analysis methods or subsets of the data (e.g. subscales) that had not been pre‐specified; one or more reported primary outcomes had not been pre‐specified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); one or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis; the trial report failed to include results for a key outcome that would be expected to have been reported for such a trial (ORBIT classification).
Other bias
Bias due to problems not covered elsewhere ‐ assessment at trial level
Other risk of bias reflects other circumstances that may threaten the validity of the trials, for example, funding bias and academic bias (Lundh 2012).
Low risk of bias: the trial appeared to be free of other sources of bias.
Unclear risk of bias: insufficient information to assess whether an important risk of bias existed; insufficient rationale or evidence that an identified problem introduced bias.
High risk of bias: had a potential source of bias related to the specific trial design used; has been claimed to have been fraudulent; had some other serious problem.
We presented a 'Risk of bias' graph and a 'Risk of bias' summary figure.
We distinguished between self‐reported, investigator‐assessed and adjudicated outcome measures.
We considered the following self‐reported outcomes.
Non‐serious adverse events
Hypoglycaemia, if reported by participants
Health‐related quality of life
Blood glucose control, if measured by trial participants
We considered the following investigator‐assessed outcomes
All‐cause mortality
Incidence of T2DM
Time to progression to T2DM
Serious adverse events
Cardiovascular mortality
Non‐fatal myocardial infarction
Non‐fatal stroke
Amputation of lower extremity
Blindness or severe vision loss
End‐stage renal disease
Hypoglycaemia, if measured by trial personnel
Blood glucose control, if measured by trial personnel
Socioeconomic effects.
Summary assessment of risk of bias
Risk of bias for a trial across outcomes
Some risk of bias domains like selection bias (sequence generation and allocation sequence concealment) affected the risk of bias across all outcome measures in a trial. Otherwise, we did not perform a summary assessment of the risk of bias across all outcomes for a trial. In case of high risk of selection bias, we excluded the trial.
Risk of bias for an outcome within a trial and across domains
We assessed the risk of bias for an outcome measure including all of the entries relevant to that outcome, that is, both trial‐level entries and outcome‐specific entries. Low risk of bias was defined as low risk of bias for all key domains, unclear risk of bias as unclear risk of bias for one or more key domains and high risk of bias as high risk of bias for one or more key domains.
Risk of bias for an outcome across trials and across domains
These were our main summary assessments that were be incorporated in our judgements about the quality of evidence in the 'Summary of finding' table(s). Low risk of bias was defined as most information coming from trials at low risk of bias, unclear risk of bias as most information coming from trials at low or unclear risk of bias and high risk of bias as a sufficient proportion of information coming from trials at high risk of bias.
Measures of treatment effect
When at least two trials were available for a comparison of a given outcome we expressed dichotomous data as risk ratio (RR) with 95% confidence intervals (CIs) and with trial sequential analysis (TSA)‐adjusted CIs if the diversity‐adjusted required information size was not reached. We planned to calculate time‐to‐event data as hazard ratio (HR) with 95% CI with the generic inverse variance method. We planned to use unadjusted HRs for preference, as adjustment could differ among the included trials. We expressed continuous data reported on the same scale as mean difference (MD) with 95% CIs and with TSA‐adjusted CIs if the diversity‐adjusted required information size was not reached. For trials addressing the same outcome but using different outcome measure scales we planned to use standardised mean differences (SMD) with 95% CI. For outcomes meta‐analysed as SMD and the generic inverse variance method, we are presently unable to conduct TSA and adjust the 95% CIs. The scales measuring health‐related quality of life (HRQoL) may go in different directions. In some scales, values increase with improved HRQoL, whereas in other scales, values decrease with improved HRQoL. To adjust for the different directions of the scales, we planned to multiply the scales that reported better HRQoL with decreasing values by ‐1.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. If more than one comparison from the same trial was eligible for inclusion in the same meta‐analysis, we either combined groups to create a single, pair‐wise comparison or appropriately reduced the sample size so that the same participants did not contribute multiply (splitting the 'shared' group into two or more groups). While the latter approach offers some solution to adjusting the precision of the comparison, it does not account for correlation arising from the same set of participants being in multiple comparisons (Deeks 2011).
We planned to reanalyse cluster‐randomised trials that did not appropriately adjust for potential clustering of participants within clusters in their analysis. The variance of the intervention effects would have been inflated by a design effect (DEFF). Calculation of a DEFF involves estimation of an intra‐cluster correlation (ICC). We planned to obtain estimates of ICCs through contact with trial authors, or by imputing them using estimates from other included studies that reported ICCs, or using external estimates from empirical research (e.g. Bell 2013). We planned to examine the impact of clustering using sensitivity analyses.
Dealing with missing data
We tried to obtain missing data from trial authors and carefully evaluated important numerical data such as screened, randomly assigned participants as well as intention‐to‐treat, and as‐treated and per‐protocol populations.
We investigated attrition rates (e.g. dropouts, losses to follow‐up, withdrawals), and critically appraised issues concerning missing data and imputation methods (e.g. last observation carried forward).
Where means and standard deviations (SDs) for outcomes were not reported and we could not get the information that we needed from trial authors, we imputed these values by assuming the SDs of the missing outcome to be the average of the SDs from those trials in which this information was reported.
We planned to investigate the impact of imputation on meta‐analyses by performing sensitivity analyses.
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we planned not to report trial results as the pooled effect estimate in a meta‐analysis.
We identified heterogeneity (inconsistency) by visually inspecting the forest plots and by using a standard Chi² test with a significance level of α = 0.1. In view of the low power of this test, we also considered the I² statistic (Higgins 2003), which quantifies inconsistency across trials to assess the impact of heterogeneity on the meta‐analysis (Deeks 2011; Higgins 2002).
Assessment of reporting biases
If we included 10 or more trials investigating a particular outcome, we used funnel plots to assess small‐trial effects. Several explanations may account for funnel plot asymmetry, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. We therefore interpreted results carefully (Sterne 2011).
Data synthesis
We planned to undertake (or display) a meta‐analysis only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar to ensure an answer that would be clinically meaningful. Unless good evidence showed homogeneous effects across trials of different methodological quality, we primarily summarised low risk of bias data using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration to the whole distribution of effects and presented a 95% prediction interval (Borenstein 2017a; Borenstein 2017b; Higgins 2009). A prediction interval needs at least three trials to be calculated and specifies a predicted range for the true treatment effect in an individual trial (Riley 2011). For rare events such as event rates below 1%, we planned to use the Peto's odds ratio method, provided that there was no substantial imbalance between intervention and comparator group sizes and intervention effects were not exceptionally large. In addition, we performed statistical analyses according to the statistical guidelines presented in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Trial sequential analyses
In a single trial, sparse data and interim analyses increase the risk of type I and type II errors. To avoid type I errors, group sequential monitoring boundaries are applied to decide whether a trial could be terminated early because of a sufficiently small P value, that is the cumulative Z‐curve crosses the monitoring boundaries (Lan 1983). Likewise, before reaching the planned sample size of a trial, the trial may be stopped due to futility if the cumulative Z‐score crosses the futility monitoring boundaries. Sequential monitoring boundaries for benefit, harm, or futility can be applied to meta‐analyses as well, called trial sequential monitoring boundaries (Higgins 2010; Wetterslev 2008). In TSA, the addition of each trial in a cumulative meta‐analysis is regarded as an interim meta‐analysis and helps to clarify if significance or futility is reached or whether additional trials are needed (Wetterslev 2008).
TSA combines a calculation of the diversity‐adjusted required information size (cumulated meta‐analysis sample size to detect or reject a specific relative intervention effect) for meta‐analysis with the threshold of data associated with statistics. We planned to perform TSA on all outcomes included in the 'Summary of findings' table (Brok 2009; Pogue 1997; Wetterslev 2008).
The idea in TSA is that if the cumulative Z‐curve crosses the boundary for benefit or harm before a diversity‐adjusted required information size is reached, a sufficient level of evidence for the anticipated intervention effect has been reached with the assumed type I error and no further trials may be needed. If the cumulative Z‐curve crosses the boundary for futility before a diversity‐adjusted required information size is reached, the assumed intervention effect can be rejected with the assumed type II error and no further trials may be needed. If the Z‐curve does not cross any boundary, then there is insufficient evidence to reach a conclusion. To construct the trial sequential monitoring boundaries, the required information size is needed and is calculated as the least number of participants needed in a well‐powered single trial and subsequently adjusted for diversity among the included trials in the meta‐analysis (Brok 2009; Wetterslev 2008). We applied TSA as it decreases the risk of type I and II errors due to sparse data and multiple updating in a cumulative meta‐analysis, and it provides us with important information in order to estimate the risks of imprecision when the required information size is not reached. Additionally, TSA provides important information regarding the need for additional trials and the required information size of such trials (Wetterslev 2008).
We applied trial sequential monitoring boundaries according to an estimated clinically important effect. We based the required information size on an a priori effect corresponding to a 10% relative risk reduction (RRR) for beneficial effects of the interventions and a 30% relative risk increase for harmful effects of the interventions.
For continuous outcomes we performed TSA with MDs, by using the trials applying the same scale to calculate the required sample size. For continuous outcomes, we tested the evidence for the achieved differences in the cumulative meta‐analyses.
For adjustment of heterogeneity of the required information size we used the diversity (D²) estimated in the meta‐analyses of included trials. When diversity was zero in a meta‐analysis, we performed a sensitivity analysis using an assumed diversity of 20%.
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity and performed subgroup analyses to investigate interactions.
Trials with a long duration (four years and longer) versus trials with a short duration (less than four years).
Diagnostic 'prediabetes' criteria (IFG, IGT, HbA1c)
Age, depending on data
Sex
Ethnicity, depending on data
Comorbid conditions, such as hypertension or obesity
Participants with previous gestational diabetes mellitus
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes by restricting the analysis to the following.
Published trials.
Taking into account risk of bias, as specified in the 'Assessment of risk of bias in included studies' section.
Trials using the following filters: imputation, language of publication, source of funding (industry versus other), or country.
We also planned to test the robustness of results by repeating the analysis using different measures of effect size (RR, OR, etc.) and different statistical models (fixed‐effect and random‐effects models).
Quality of evidence
We presented the overall quality of the evidence for each outcome according to the GRADE approach, which takes into account issues relating not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results. Two review authors (BH and BR) rated the quality of evidence for each outcome. We presented a summary of the evidence in 'Summary of findings' tables ( Table 1; Table 2; Table 3). These tables provide key information about the best estimate of the magnitude of the effect of interventions, in relative terms and as absolute differences, the numbers of participants and trials addressing each important outcome, and rate the overall confidence in effect estimates for each outcome. We created the ’Summary of findings’ tables on the basis of methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011) by means of the table editor in Review Manager 5 (RevMan 2014), and included three appendices (Appendix 14; Appendix 15; Appendix 16) providing checklists as guides to the consistency and reproducibility of GRADE assessments (Meader 2014) to help with the standardisation of the 'Summary of findings' tables. Alternatively, we would have used the GRADEproGDT software (GRADEproGDT 2015) and would have presented evidence profile tables as an appendix. We presented results for the outcomes as described in the Types of outcome measures section. If meta‐analysis was not possible, we presented the results in a narrative format in the 'Summary of findings' tables. We justified all decisions to downgrade the quality of the evidence using footnotes, and we made comments to aid the reader’s understanding of the review where necessary.
We presented a 'Summary of findings' table to report the following outcomes, listed according to priority.
All‐cause mortality
Incidence of T2DM
Serious adverse events
Cardiovascular mortality
Non‐fatal myocardial infarction/stroke
Health‐related quality of life
Socioeconomic effects
Results
Description of studies
For a detailed description of studies, see the 'Characteristics of included studies', 'Characteristics of excluded studies', 'Characteristics of studies awaiting classification' and 'Characteristics of ongoing studies' sections.
Studies awaiting classification
We classified five trials in six references as studies awaiting classification (see 'Characteristics of studies awaiting classification' table).
Ongoing studies
We found three ongoing randomised controlled trials (RCTs) in nine references (NCT01530165; PREVIEW; PROPELS). We estimate the ongoing trials to include 23,808 participants. The definition of intermediate hyperglycaemia varied among the ongoing trials; one trial included participants with impaired glucose tolerance (IGT) (NCT01530165), one trial included participants with IGT, impaired fasting glucose (IFG) or both (PREVIEW), and one trial included participants with IFG or moderately elevated glycosylated haemoglobin A1c (HbA1c) (PROPELS). All the ongoing trials assessed one or more outcomes of interest for our review. Two of the ongoing trials' predefined outcomes stated that they would assess one of our primary outcomes (incidence of T2DM) (NCT01530165; PREVIEW). Future updates will include all ongoing trials, if possible.
Results of the search
The original Cochrane Review published in 2008 included eight RCTs. In this update we excluded two of these trials as they did not have intermediate hyperglycaemia as an inclusion criterion (Bo 2007; Wing 1998). Thus, six trials of the original review remained (Da Qing 1997; DPP 2002; DPS 2001; IDPP 2006; Kosaka 2005; Oldroyd 2005). The updated search of the databases identified 2072 records after duplicates were removed (Figure 1). We excluded most of the references on the basis of their titles and abstracts because they clearly did not meet the inclusion criteria. We evaluated 181 references further. In this updated search we identified a total of 56 additional references for four of the already included trials of the 2008 review (Da Qing 1997; DPP 2002; DPS 2001; IDPP 2006). After screening the full‐texts and evaluating the existing references from the 2008 review, 12 RCTs published in 127 records met our inclusion criteria. We excluded a total of 62 references from the updated search after full‐text evaluation. Therefore, in addition to the six included trials of the Cochrane Review published in 2008 we included six more trials (EDIPS 2009; Hellgren 2016; HELP PD 2011; JDPP 2013; PODOSA 2014; SLIM 2003).
We continuously used a MEDLINE email alert service to identify newly published studies using the same search strategy as described for MEDLINE up to September 2017.
Included studies
A detailed description of the characteristics of included studies is presented elsewhere (see Characteristics of included studies; Table 4 and Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12). The following is a succinct overview:
Source of data
We contacted all trial authors or investigators through email (see Appendix 13). When important information was lacking on ongoing studies and excluded studies, we contacted investigators for clarification.
Overview of trial populations
Ten trials reported the number of participants screened (Da Qing 1997; DPP 2002; EDIPS 2009; Hellgren 2016; HELP PD 2011; IDPP 2006; JDPP 2013; Oldroyd 2005; PODOSA 2014; SLIM 2003). Two trials did not report the number of participants randomised to each intervention group upon trial initiation (Da Qing 1997; Hellgren 2016). A total of 5238 participants were randomised; 11 trials evaluated diet plus physical activity (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; Kosaka 2005; Oldroyd 2005; PODOSA 2014; SLIM 2003). The two trial randomising participants to physical activity only in one of their treatment arms did not report the number of participants randomised to the intervention groups (Da Qing 1997; Hellgren 2016); the same was the case for the one trial arm randomising to diet only (Da Qing 1997).
Ten trials provided information about sample size and power calculations (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; Oldroyd 2005; PODOSA 2014; SLIM 2003).
The proportion of participants finishing the trial varied from 48% (SLIM 2003) to 98% (PODOSA 2014).
Trial design
Ten included trials were parallel RCTs and two trials had a cluster‐randomised design (Da Qing 1997; PODOSA 2014). In Da Qing each clinic was randomised to carry out the intervention on each of the eligible participants attending the clinic (Da Qing 1997). A total of 33 health care clinics were included (Da Qing 1997). No intra‐cluster correlation (ICC) coefficient was reported. However, we estimated it to be 0.05. The design effect was therefore estimated to be 1+ (average cluster size ‐ 1) x 0.05 = 1 + ((530/33) ‐ 1) x 0.05 = 1.75. For dichotomous data, we divided both the number of participants experiencing an event as well as the number of participants by the design effect. For continuous data we only adjusted the sample size for the design effect. Means and standard deviations remained unchanged. In the PODOSA 2014 trial participants constituted 156 family clusters that were randomised (78 families with 85 participants were allocated to the intervention group and 78 families with 86 participants were allocated to the control group). First degree relatives living in the same city were not randomised separately. The ICC coefficient was assumed to be zero.
The number of participants varied from 78 (Oldroyd 2005) to 2161 (DPP 2002). One trial contributed 41% of the total number of all randomised participants (DPP 2002). Four trials were multi‐centre trials (Da Qing 1997; DPP 2002; DPS 2001; JDPP 2013), four trials were single‐centre trials (EDIPS 2009; Hellgren 2016; HELP PD 2011; Oldroyd 2005), and four trials did not provide any centre description (IDPP 2006; Kosaka 2005; PODOSA 2014; SLIM 2003). All trials were performed in outpatient settings.
None of the trials reported blinding of the participants and investigators. One trial had a run‐in period of three weeks where participants had to fill out a diary and take placebo pills (DPP 2002). The duration of the intervention in the included trials varied from two to six years. Three trials had an extended follow‐up period after the intervention period had stopped (Da Qing 1997; DPP 2002; DPS 2001). The extended follow‐up period varied from 5 to 17 years. One trial based the sample size calculation on an anticipated incidence of T2DM during six years of follow‐up. Trial authors said that they had planned six years' follow‐up, but only reported three years of follow‐up (JDPP 2013). Another trial originally planned a follow‐up of three years, but was extended to six years in the course of the trial (SLIM 2003).
Six trials were performed in European countries (DPS 2001; EDIPS 2009; Hellgren 2016; Oldroyd 2005; PODOSA 2014; SLIM 2003); two trials in the USA (DPP 2002; HELP PD 2011) and four trials in Asian countries (Da Qing 1997; IDPP 2006; JDPP 2013; Kosaka 2005).
Two trials included one or more intervention arms with metformin (DPP 2002; IDPP 2006). Results from these intervention arms will be reported elsewhere (Lü 2010). One of the trials originally included a troglitazone arm, which was discontinued due to liver toxicity of the drug (DPP 2002).
Three of the included trials stated that they had received grants from a pharmaceutical company (DPP 2002; DPS 2001; IDPP 2006). Eight trials reported non‐commercial funding (Da Qing 1997; EDIPS 2009; Hellgren 2016; HELP PD 2011; JDPP 2013; Oldroyd 2005; PODOSA 2014; SLIM 2003). One trial did not report the funding source (Kosaka 2005).
Three trials were terminated early due to beneficial effects in the intervention group (DPP 2002; DPS 2001; IDPP 2006).
Participants
Four trials included only Asian people (Da Qing 1997; IDPP 2006; JDPP 2013; Kosaka 2005): two of these trials included Japanese people (JDPP 2013; Kosaka 2005), one trial included Asian Indians (IDPP 2006) and one trial included Chinese people (Da Qing 1997). One trial performed in the UK only included people of Indian or Pakistini origin (PODOSA 2014). Two trials included only white people (Oldroyd 2005; SLIM 2003). Two trials included people with different ethnicity (DPP 2002; HELP PD 2011): in one of the trials more than 50% of the included participants were white and about 20% African American (DPP 2002), the other trial reported about 75% were white and 25% African American (HELP PD 2011). Three trials did not report the ethnicity of the participants (DPS 2001; EDIPS 2009; Hellgren 2016): two of the trials might have included mainly white participants as they were conducted in Sweden and Finland (DPS 2001; Hellgren 2016).
All trial authors provided information on gender. One trial included only men (Kosaka 2005), the remaining trials included both men and women. All trials except one had a balanced distribution of women and men in the intervention and comparator groups, this trial included 54% women in the intervention group compared with 31% in the comparator group (Oldroyd 2005).
The age of the included participants varied from 45 to 63 years.
All trials except one reported fasting glucose values at baseline (Hellgren 2016). Mean fasting glucose values at baseline varied from 5.5 mmol/L to 6.2 mmol/L. Nine trials reported two‐hour glucose values after a glucose‐load at baseline and ranged from 8.2 mmol/L to 9.2 mmol/L (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; IDPP 2006; JDPP 2013; Oldroyd 2005; PODOSA 2014; SLIM 2003). Glycosylated haemoglobin A1c (HbA1c) values were reported at baseline in six trials and ranged from 5.7% to 6.2% (DPP 2002; DPS 2001; IDPP 2006; JDPP 2013; Oldroyd 2005; SLIM 2003). One trial did not report any glycaemic baseline values (Hellgren 2016).
All trials reported body mass index (BMI) at baseline. Three of the included trials required a minimum BMI for all eligible participants (DPP 2002; EDIPS 2009; HELP PD 2011). In two trials the mean BMI at baseline was less than 25 kg/m2 (JDPP 2013; Kosaka 2005). Four trials had a mean BMI at baseline between 25 kg/m2 to 30 kg/m2 (Da Qing 1997; Hellgren 2016; IDPP 2006; SLIM 2003). Six trials reported a mean BMI at baseline above 30 kg/m2 (DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; Oldroyd 2005; PODOSA 2014). None of the trials had a mean BMI at baseline above 35 kg/m2. Most trials excluded participants with other endocrine conditions, hepatic or kidney disease.
Diagnosis of intermediate hyperglycaemia
The diagnosis applied in the included trials for identifying intermediate hyperglycaemia varied. Three trials applied the World Health Organization (WHO) 1985 diagnostic criteria for the definition of IGT (fasting plasma glucose (FPG) < 7.8 mmol/L and two‐hour plasma glucose after oral glucose tolerance test (OGTT) between ≥ 7.8 mmol/L and < 11.1 mmol/L) (Da Qing 1997; DPS 2001; Oldroyd 2005). Three trials applied the WHO 1999 criteria for the definition of IGT (fasting plasma glucose (FPG) < 7.0 mmol/L and two‐hour plasma glucose after OGTT between ≥ 7.8 mmol/L and < 11.1 mmol/L) (EDIPS 2009; IDPP 2006; JDPP 2013). For one of these trials it was stated that the diagnosis of IGT was based on the OGTT and trial authors did not clearly describe any measurement of FPG (EDIPS 2009). In the main publication it was described that intermediate hyperglycaemia was defined according to the WHO 1999 criteria, but the reference for the diagnostic criteria was the WHO 1985 reference (EDIPS 2009; WHO 1985). The investigators confirmed that the diagnosis of T2DM was established by FPG or two‐hour plasma glucose (EDIPS 2009). Therefore, plasma glucose must have been measured (EDIPS 2009). One trial applied the WHO 1999 criteria for the definition of IFG (FPG ≥ 6.1 and < 7.8 mmol/L and two‐hour glucose < 7.8 mmol/L) and/or IGT (FPG < 7.0 mmol/L and two‐hour plasma glucose after OGTT between ≥ 7.8 mmol/L and < 11.1 mmol/L) (PODOSA 2014). One trial included people with IGT defined by FPG < 7.8 mmol/L and two‐hour plasma glucose after 100 g OGTT between 8.9 mmol/L and 13.3 mmol/L, which roughly corresponds to 7.8 mmol/L to 11 mmol/L after a 75 g OGTT (Kosaka 2005). Therefore, the criterion was almost identical to the WHO 1980 criteria for IGT (FPG < 8.0 mmol/L and two‐hour plasma glucose after OGTT between ≥ 8.0 mmol/L and < 11.0 mmol/L) (WHO 1980). One trial included people with fasting glucose levels between 5.3 mmol/L and 6.9 mmol/L (HELP PD 2011). This definition did not adhere to the definition by the WHO or American Diabetes Association (ADA), however, the definition was very close to the glycaemic levels recommended by ADA 2003 (FPG 5.6 mmol/L to 6.9 mmol/L) (ADA 2003). One trial applied the diagnostic criteria for impaired glucose defined by ADA 1997 (FPG 5.3 mmol/L to 6.9 mmol/L and two‐hour plasma glucose after OGTT between 7.8 and 11.0 mmol/L) (ADA 1997) (DPP 2002). For the Native American Indian clinics FPG less then 6.9 mmol/L with no lower limit applied. Before June 1997, the criterion for FPG was 5.6 to 7.7 mmol/L, or less than 7.7 mmol/L in the Native American Indian clinics (DPP 2002). A total of 54 participants (total in all three intervention groups) included in the Diabetes Prevention Program (DPP) had FPG above 7.0 mmol/L at baseline (DPP 2002). Thirteen percent of the participants included in the DPP had HbA1c ≥ 6.5% at baseline (DPP 2002). One trial defined IGT by FPG < 7.8 mmol/L and two‐hour plasma glucose after OGTT between ≥ 7.8 mmol/L and < 12.5 mmol/L (SLIM 2003). No medical associations recommend these limits to diagnose IGT (SLIM 2003). Twenty‐two participants of the initially randomised 147 participants had a two‐hour glucose value above 11 mmol/L in this trial (SLIM 2003). One trial defined IFG as FPG > 6.0 mmol/L to < 7.0 mmol/L with two‐hour plasma glucose after OGTT < 8.9 mmol/L, while IGT was defined as two‐hour plasma glucose after OGTT between 8.8 mmol/L and < 12.2 mmol/L, and fasting glucose < 7.0 mmol/L (Hellgren 2016).
Interventions
All the participants in the included trials were treatment‐naive with regard to pharmacological glucose‐lowering interventions. Two of the included trials had more than one intervention group of relevance for this review (Da Qing 1997; Hellgren 2016). Hellgren 2016 initially analysed data from the two intervention groups separately. As the treatment effect was almost identical in both groups, they analysed the two groups together and thereafter designated them as the combined intervention group. Both the intervention arms in Hellgren 2016 had a more intense physical activity strategy than the comparator group. Diet advice was identical in both intervention groups and the comparator group. Da Qing 1997 had four intervention arms: physical activity only, diet only, physical activity plus diet and control group. We combined data for the physical activity only, diet only and physical activity plus diet group as one intervention group in the post‐intervention follow‐up period.
Ten trials aimed for weight reduction for all participants allocated to the intervention groups or only for participants with a BMI above a certain limit (this BMI limit varied among the included trials) (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; JDPP 2013; Kosaka 2005; Oldroyd 2005; PODOSA 2014; SLIM 2003). Participants with BMI above 25 kg/m2 allocated to the diet‐only group in Da Qing 1997 were encouraged to reduce their calorie intake, so they lost 0.5 kg to 1.0 kg per month until they achieved a BMI of 23 kg/m2. For the other intervention groups in Da Qing 1997, the intervention intensity did not change according to BMI. Three trials aimed for a BMI less than 25 kg/m2 in the diet plus physical activity group (DPS 2001; EDIPS 2009; Oldroyd 2005). One trial advised participants with a BMI above 22 kg/m2 allocated to the diet plus physical activity group to lose weight (Kosaka 2005). One trial recommended a weight reduction of at least 7% in participants in the diet plus physical activity group (DPP 2002); two trials recommended a weight loss of 5% to 7% (HELP PD 2011; SLIM 2003); one trial aimed at 5% weight reduction in obese or overweight participants allocated to diet plus physical activity (JDPP 2013). One trial recommended a weight loss goal of 2.5 kg more in the diet plus physical activity group compared with the control group (PODOSA 2014).
The physical activity interventions differed largely among the trials. Some trials did not aim for a certain amount of minutes of activity, but just an increase in existing physical activity levels for the participants in the intervention group (Da Qing 1997; DPS 2001; Hellgren 2016). Other trials recommended physical activity of different intensity with a minimum number of minutes per day or week (DPP 2002; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; Kosaka 2005; Oldroyd 2005; PODOSA 2014; SLIM 2003). Some defined physical activity levels in the intervention groups, which corresponded to the physical activity recommended in the control groups in other trials. Some trials promoted physical activity (Da Qing 1997; EDIPS 2009; HELP PD 2011; JDPP 2013Kosaka 2005; Oldroyd 2005; PODOSA 2014) and others offered physical activity programmes (DPP 2002; DPS 2001; Hellgren 2016; SLIM 2003). Most programmes included walking and cycling.
Only one trial focused exclusively on physical activity interventions for the prevention of T2DM (Hellgren 2016).
The diet interventions were mainly based on caloric restriction, reduced fat intake and increased fibre intake (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; Kosaka 2005; Oldroyd 2005; PODOSA 2014; SLIM 2003).
The number of contacts with the participants in the intervention groups ranged from 3 to 46 (Hellgren 2016; IDPP 2006). The intervention was applied to the participants in groups or on an individual basis. Nine of the trials applied both individual and group sessions for the participants in the intervention group (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; PODOSA 2014; SLIM 2003); three trials provided individual sessions only (Hellgren 2016; Kosaka 2005; Oldroyd 2005). In most of the trials the intervention facilitators were a physiotherapist, an exercise physiologist and a dietitian.
In one of the included trials, the control group did not receive any intervention (Oldroyd 2005). In the remaining included trials, the control group received recommendations, advice or education on how to increase physical activity and reduce calorie intake.
Three trials had an extended follow‐up period after the intervention period had stopped (Da Qing 1997; DPP 2002; DPS 2001). In the Da Qing 1997 and DPS 2001 follow‐up studies, the participants did not receive any kind of diet or physical activity advice from the investigators. In the DPP 2002 follow‐up study (Diabetes Prevention Program Outcome Study (DPPOS)) sessions on diet plus physical activity were offered to all participants every third month. The participants initially randomised to the DPP diet plus exercise group were also offered sessions in order to reinvigorate their self‐management behaviours for weight loss (DPP 2002). In the Da Qing 1997 the participants were re‐examined after 20 years and 23 years of follow‐up. In the DPP 2002 and DPS 2001 follow‐up studies the participants were examined yearly.
Outcomes
All included trials reported one or more of the primary outcomes for this review. All included trials, except one (Oldroyd 2005), explicitly specified the primary outcome. Nine trials predefined a primary outcome with interest for this review (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; Kosaka 2005; SLIM 2003). Six trials had registered a protocol in a trials register (DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; JDPP 2013; PODOSA 2014). One trial changed its primary outcome from incidence of T2DM to weight change during the trial in order to ensure statistical power (PODOSA 2014). Weight change had originally been defined as a secondary outcome. Reporting on adverse events, macrovascular and microvascular complications was sparse.
Excluded studies
We excluded 41 trials after full‐text evaluation (for details see Characteristics of excluded studies). We excluded 16 trials published in 26 references as the duration of the intervention was less than two years (D‐CLIP; DH!AAN; Hesselink 2013; Huang 2007; J‐DOIT; Kawahara 2008; Kinmonth 2008; Lindahl 1999; Marrero 2016; Page 1992; Ramachandran 2013; Sathish 2017; Savoye 2007; Thompson 2008; Villareal 2006; Yates 2011). We excluded 11 trials published in 12 references as they did not have intermediate hyperglycaemia as an inclusion criterion or the people with intermediate hyperglycaemia were analysed together with people of another glycaemic status, and separate data were not available (APHRODITE; Bo 2007; E‐LITE; Eriksson 2006; NCT02374788; Rosas 2016; Schmidt 2016; SHINE; The Fasting Hyperglycaemia Study 1997a; Wing 1998; Yates 2012). We excluded three trials published in three references as they did not allocate the participants to diet, physical activity or both by randomisation (De la Rosa 2007; Eriksson 1991; Tao 2004). We excluded one trial due to inadequate description of the intervention group (Sartor 1980). We excluded 10 trials in 18 references as they did not compare the interventions of interest for this review (Grey 2004; Jarrett 1979; Let's Prevent; Liao 2002; Nanditha 2014; NCT02250066; PULSE; Saito 2011; Wein 1999; Wong 2013). Of these, seven trials in 14 references compared the same intensity of the diet plus physical activity intervention, but applied the advice differently (e.g. one visit per year versus four visits per year) (Grey 2004; Let's Prevent; Nanditha 2014; PULSE; Saito 2011; Wein 1999; Wong 2013). We furthermore excluded 16 systematic reviews (Aguiar 2014; Gillett 2012; Gillies 2007; Glechner 2015; Gong 2015; Hopper 2011; ICER 2016; Norris 2005; Santaguida 2005; Selph 2015; Stevens 2015; Yamaoka 2005; Yates 2007; Yoon 2013; Yuen 2010; Zheng 2016).
Risk of bias in included studies
For details on the risk of bias of the included trials see Characteristics of included studies.
For an overview of review authors' judgements about each risk of bias item for individual trials and across all trials see Figure 2 and Figure 3. No trial was free from risk of bias in all 'Risk of bias' domains.
2.
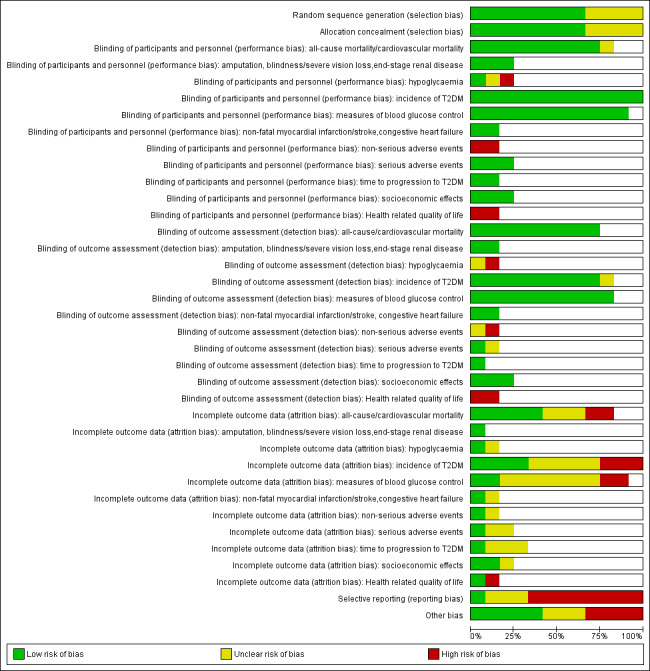
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies (blank cells indicate that the particular outcome was not investigated in some studies).
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study (blank cells indicate that the study did not report that particular outcome).
Allocation
We judged eight of the trials to be at low risk of selection bias with regard to the method of randomisation and allocation concealment (DPP 2002; DPS 2001; EDIPS 2009; Hellgren 2016; HELP PD 2011; JDPP 2013; Oldroyd 2005; PODOSA 2014).
The remaining trials reported that the participants were randomised but provided no further description (Da Qing 1997; IDPP 2006; Kosaka 2005; SLIM 2003).
We evaluated trial baseline data for our predefined prognostic baseline variables. In one trial a significantly larger proportion of control participants reported engaging in regular physical activity at least once a week compared with intervention participants (53% versus 24%) and there were fewer women (10/32 (32%)) than men (22/32 (69%)) in the control group compared with the diet plus physical activity group (Oldroyd 2005). Another trial reported that physical activity was significantly higher at baseline in the diet plus physical activity group than in the control group (Da Qing 1997).
Blinding
Double‐blinding was not possible or practical in the included trials due to the type of intervention.
One trial mentioned that an independent outcome committee evaluated the incidence of T2DM (DPS 2001). None of the remaining trials reported that a blinded outcome committee was instituted to assess outcomes during the intervention period.
Where measured, all primary outcomes of this review were investigator assessed and we judged these to be at low risk of performance and detection bias. All the included trials reported blood glucose measurements performed by the investigators and we judged these outcomes measures to be at low risk of performance and detection bias. Two trials explicitly stated that glycaemic measures were masked for the investigators and participants until diabetes was confirmed (DPP 2002; DPS 2001). Non‐serious adverse events and mild hypoglycaemia were partly or exclusively self‐reported in all trials. Overall, we considered the risk of performance bias and detection bias to be low or unclear for our secondary outcomes.
Incomplete outcome data
We considered overall risk of attrition bias to be unclear for most of our outcomes. Ten trials reported the number of participants randomised and finishing the trial for each intervention group (DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; Kosaka 2005; Oldroyd 2005; PODOSA 2014; SLIM 2003). Two trials did not describe how many participants were initially randomised to each intervention group but reported the number analysed (Da Qing 1997; Hellgren 2016). Three trials provided details on the participants not completing the trial (DPS 2001; JDPP 2013; PODOSA 2014).
Selective reporting
Six of the trials had a published protocol (DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; JDPP 2013; PODOSA 2014). We judged one trial as low risk of selective outcome reporting bias after the investigators provided additional information (Hellgren 2016). We judged eight of the included trials to be at high risk of reporting bias on one or more of the outcomes of relevance for our review (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; Kosaka 2005). Three trials had an unclear risk of selective outcome reporting bias (Oldroyd 2005; PODOSA 2014; SLIM 2003). Two of the judgments of unclear risk of selective outcome reporting bias were based on lack of trial protocol (Oldroyd 2005; SLIM 2003) and one trial because the primary outcome was changed during the trial (PODOSA 2014). For more details see Appendix 6 and Appendix 7. One trial had a low risk of selective outcome reporting bias (Hellgren 2016).
Other potential sources of bias
We judged five trials to be at low risk of other bias (EDIPS 2009; Hellgren 2016; JDPP 2013; Oldroyd 2005; PODOSA 2014). Three trials had an unclear risk of other bias (HELP PD 2011; Kosaka 2005; SLIM 2003). We judged four trials to be at high risk of other bias, either because the trial was terminated early for benefit (DPP 2002; DPS 2001; IDPP 2006) or because trial authors did not report an ICC coefficient in their cluster‐RCT making it necessary to estimate this coefficient (Da Qing 1997).
Four of the included trials had received grants from a pharmaceutical company and we judged these as unclear risk of other bias (DPP 2002; DPS 2001; HELP PD 2011; IDPP 2006). One trial did not report funding source (Kosaka 2005). It is known that receiving funding or provision of free drugs or devices from a pharmaceutical company leads to more favourable results and conclusions than sponsorship from other sources (Lundh 2017). One trial prolonged the intervention period without any explanation (SLIM 2003).
Effects of interventions
See: Table 1; Table 2; Table 3
Baseline characteristics
For details of baseline characteristics, see Appendix 3; Appendix 4 and Appendix 5.
Diet versus comparator
One trial compared diet with one or more comparators (Da Qing 1997). This trial had four intervention arms: diet only, physical activity only, diet plus physical activity and standard treatment (Da Qing 1997). The trial was cluster randomised. The trial had an extended follow‐up period of 17 years. No ICC coefficient was reported. Based on an anticipated ICC coefficient of 0.05 we assumed a design effect of 1.75 (Higgins 2011). In the post‐interventional follow‐up period the trialists combined the three intervention groups into one composite intervention group. Data for the extended follow‐up period for the combined intervention groups are described in the section 'Diet plus physical activity versus standard treatment'.
Diet versus physical activity
Only one trial compared diet with physical activity in one of its trial arms (Da Qing 1997). The overall quality of evidence was very low because of risk of reporting and other bias and serious imprecision (very sparse data)
Primary outcomes
All‐cause mortality
Three out of 130 participants in the diet‐only trial arm compared with none out of 141 participants in the physical activity‐only trial arm died during the intervention period (when adjusting for cluster‐RCT design: 2/74 versus 0/81). None of the participants died due to cardiovascular disease. None of the participants who died had developed T2DM before death.
Incidence of T2DM
The incidence of T2DM was the primary outcome. T2DM was defined according to the WHO 1985 criteria (either a FPG ≥ 140 mg/dL (7.8 mmol/L) or higher or a two‐hour plasma glucose ≥ 200 mg/dL (11.1 mmol/L) after 75 g OGTT) and confirmed with a repeated test 7 to 14 days after the first test or from a report of physician‐diagnosed diabetes with evidence in the medical record of high glucose concentrations, or use of glucose‐lowering drugs. Fifty‐seven out of 130 participants in the diet‐only group compared with 58 participants out of 141 in the physical activity only group developed T2DM during the intervention period (when adjusting for cluster‐RCTdesign: 33/74 versus 33/81). The cumulative incidence of T2DM at six years was 43.8% (95% CI 35.3 to 52.3) in the diet only group compared with 41.1% (95% CI 33.4 to 49.4) in the physical activity only group (not adjusted for clustering). In the diet only group the incidence rate of T2DM was 10.0/100 person years (95% CI 7.5 to 12.5) compared with 8.3/100 person years (95% CI 6.4 to 10.3) in the physical activity only group (not adjusted for clustering). When analysing the incidence according to FPG 7.8 mmol/L or above, the rate was 3.7/100 person years (95% CI 2.1 to 5.3) in the diet only group compared with 5.3/100 person years (95% CI 3.6 to 7.0) in the physical activity only group (not adjusted for clustering).
Due to the differences in dietary advice according to BMI, Da Qing 1997 evaluated separately the incidence of T2DM in those who had a BMI at baseline less than 25 kg/m2 or 25 kg/m2 or higher. Of the lean participants (BMI at baseline < 25 kg/m2), 21 (38.2%) out of 55 participants in the diet only compared with 15 (26.5%) out of 57 participants in the physical activity only group developed T2DM during the intervention period (when adjusting for clustering 12/31 versus 9/33). Of the overweight participants (BMI at baseline ≥ 25 kg/m2), 36 (48%) out of 75 participants in the diet only compared with 43 (51.2%) out of 84 participants in the physical activity group developed T2DM during the intervention period (when adjusting for clustering 21/43 versus 26/46).
Of the 263 diabetes diagnoses made in all the intervention groups during the six years of intervention, 55 (21%) diagnoses were initially made by local physicians and confirmed by the city hospital with an OGTT; 208 (79%) were made as a result of the systematic OGTT during the trial.
Serious adverse events
No (serious) adverse events occurred in both groups.
Secondary outcomes
Cardiovascular mortality
None of the participants in either group died of cardiovascular reasons.
Non‐fatal myocardial infarction
Not reported, however from a published abstract it was apparent that cardiovascular events were collected during the trial.
Non‐fatal stroke
Not reported, however from a published abstract it was apparent that cardiovascular events were collected during the trial.
Amputation of lower extremity
Not reported.
Blindness or severe vision loss
Da Qing 1997 reported the incidence of severe retinopathy among the participants originally assigned to diet only (4.5/1000 person years (95% CI 1.8 to 7.1)) compared with physical activity only (5.3/1000 person years (95% CI 2.5 to 8.0)) after 20 years of follow‐up (i.e. 14 years after the end of intervention). This difference was not statistically significant.
End‐stage renal disease
Not reported.
Non‐serious adverse events
No adverse events occurred in either group.
Hypoglycaemia
Not reported.
Health‐related quality of life
Not reported.
Time to progression to T2DM
Not reported.
Measures of blood glucose control
Fasting glucose
At the end of follow‐up after six years of intervention, FPG in the diet only group was 7.0 mmol/L (SD 4.4) based on 130 participants (74 participants when adjusting for clustering) compared with 6.8 mmol/L (SD 2.2) in 141 participants (81 participants when adjusting for clustering) in the physical activity group.
Glucose two hours after an oral glucose load
At the end of follow‐up after six years of intervention, two‐hour glucose after an oral glucose load in the diet only group was 10.5 mmol/L (SD 4.9) based on 130 participants (74 participants when adjusting for clustering) compared with 10.5 mmol/L (SD 3.9) in 141 participants (81 participants when adjusting for clustering) in the physical activity group.
HbA1c
Not reported.
Socioeconomic effects
Not reported.
Diet versus standard treatment
Only one trial compared diet with standard treatment in one of its trial arms (Da Qing 1997). The overall quality of evidence was very low because of risk of reporting and other bias and serious imprecision (very sparse data)
Primary outcomes
All‐cause mortality
Three out of 130 participants in the diet only group compared with three participants out of 133 in the standard treatment group died during the intervention period (when adjusting for cluster‐design: 2/74 versus 2/76). None of the participants died because of cardiovascular disease. None of the participants who died had developed T2DM before death. The HR (adjusted for age and clustering) when combining diet only, physical activity only and diet plus physical activity compared with standard treatment was 1.33 (95% CI 0.45 to 3.92) at the end of the intervention period.
Incidence of T2DM
Fifty‐seven out of 130 participants in the diet only group compared with 90 participants out of 133 in the standard treatment group developed T2DM during the intervention period (when adjusting for cluster design: 33/74 versus 51/76).
The cumulative incidence of T2DM at six years was 43.8% (95% CI 35.3 to 52.3) in the diet only group compared with 67.7% (95% CI 59.8 to 75.2) in the standard treatment group (not adjusted for clustering). In the diet only group, the incidence rate of T2DM (defined according to WHO 1985) was 10.0/100 person years (95% CI 7.5 to 12.5) compared with 15.7/100 person years (95% CI 12.7 to 18.7) in the standard treatment group (not adjusted for clustering). When analysing the incidence according to FPG 7.8 mmol/L or higher, the rate was 3.7/100 person years (95% CI 2.1 to 5.3) in the diet only group compared with 9.6/100 person years (95% CI 7.2 to 12.0) in the standard treatment group (not adjusted for clustering).
Due to the differences in dietary advice according to BMI, the trialists evaluated separately incidence of T2DM in those who had BMI at baseline less than 25 kg/m2 or 25 kg/m2 or higher. Of the lean participants (BMI at baseline < 25 kg/m2) 21 (38.2%) out of 55 participants in the diet only group compared with 30 (60%) out of 50 participants in the standard treatment group developed T2DM during the intervention period (when adjusting for clustering 12/31 versus 17/29). Of the obese participants (BMI at baseline ≥ 25 kg/m2) 36 (48%) out of 75 participants in the diet only group compared with 60 (72.3%) out of 83 participants in the standard treatment group developed T2DM during the intervention period (when adjusting for clustering 21/43 versus 34/47).
After 20 years of follow‐up (i.e. 14 years after the intervention had stopped) the HR (adjusted for age and clustering) for the diet only group versus standard treatment group was 0.58 (95% CI 0.38 to 0.89).
Serious adverse events
No (serious) adverse events occurred in either group.
Secondary outcomes
Cardiovascular mortality
None of the participants in either group died of cardiovascular reasons.
Non‐fatal myocardial infarction
Not reported, however from a published abstract it was apparent that cardiovascular events were collected during the trial.
Non‐fatal stroke
Not reported, however from a published abstract it was apparent that cardiovascular events were collected during the trial.
Amputation of lower extremity
Not reported.
Blindness er severe vision loss
Not reported.
End‐stage renal disease
Not reported.
Non‐serious adverse events
No adverse events occurred in either group.
Hypoglycaemia
Not reported.
Health‐related quality of life
Not reported.
Time to progression to T2DM
Not reported.
Measures of blood glucose control
Fasting glucose
FPG at the end of follow‐up after six years of intervention in the diet only group was 7.0 mmol/L (SD 4.4) based on 130 participants (74 participants when adjusting for clustering) compared with 7.6 mmol/L (SD 2.6) in 133 participants (76 participants when adjusting for clustering) in the standard treatment group.
Glucose two hours after an oral glucose load
Glucose two hours after an oral glucose load at the end of follow‐up after six years of intervention in the diet only group was 10.5 mmol/L (SD 4.9) based on 130 participants (74 participants when adjusting for clustering) compared with 12.4 mmol/L (SD 4.2) in 133 participants (76 participants when adjusting for clustering) in the standard treatment group.
HbA1c
Not reported.
Socioeconomic effects
Not reported.
Physical activity versus standard treatment
Two trials compared physical activity with one or more controls (Da Qing 1997; Hellgren 2016). Da Qing 1997 had four intervention arms: diet only, physical activity only, diet plus physical activity and standard treatment (Da Qing 1997). The trial was cluster randomised. No ICC coefficient was reported. Based on an anticipated ICC coefficient of 0.05 we assumed a design effect of 1.75 (Higgins 2011). In the post‐interventional follow‐up period, Da Qing 1997 combined the three intervention groups into one composite intervention group. Data for the extended follow‐up period for the combined intervention group are described in the section 'Diet plus physical activity versus standard treatment'.
One trial had two physical activity groups (Hellgren 2016), and initially analysed data from these two groups separately (Hellgren 2016). As the outcomes proved to be essentially the same in both groups, they analysed the physical activity groups together and thereafter designated them as the combined physical activity group. Both intervention arms in Hellgren 2016 had a more intense physical activity strategy than the comparator group.The diet advice was identical in the physical activity groups and the standard treatment group.
The two trials comparing physical activity with standard treatment varied according to several important prognostic baseline characteristics (e.g. Da Qing 1997 included only Asian Chinese and the average age of the participants was about 45 years compared with Hellgren 2016 including presumably only white people with an average age of participants of 63 years). Da Qing 1997 included people with IGT according to the WHO 1985 criteria. Hellgren 2016 included participants with IGT, IFG or both. Da Qing 1997 had a duration of the intervention of six years compared with three years in Hellgren 2016.
The overall quality of evidence was very low mainly because of risk of bias and imprecision (sparse data)
Primary outcomes
All‐cause mortality
One trial reported that none of the 141 participants in the physical activity only group compared with three participants out of 133 in the standard treatment group died during the intervention period (when adjusting for cluster‐RCT design: 0/81 versus 2/76) (Da Qing 1997). None of the participants who died had developed T2DM before death. The HR (adjusted for age and clustering) when combining diet only, physical activity only and diet plus physical activity compared with standard treatment was 1.33 (95% CI 0.45 to 3.92) at the end of the intervention period (Da Qing 1997). The other trial reported that four participants died; three out of 84 in the physical activity group and one out of 39 in the standard treatment group. It was not possible to judge to which group the deceased participants had originally been randomised, however, the investigators provided this additional information (Hellgren 2016) (Analysis 1.1).
1.1. Analysis.
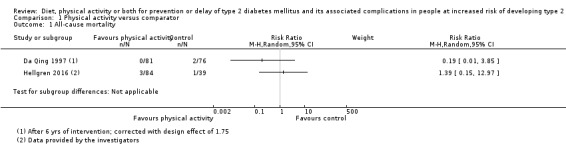
Comparison 1 Physical activity versus comparator, Outcome 1 All‐cause mortality.
Incidence of type 2 diabetes
Da Qing 1997's primary outcome was the incidence of T2DM, which they defined according to the WHO 1985 criteria (either a FPG ≥ 7.8 mmol/L or higher or a two‐hour plasma glucose ≥ 11.1 mmol/L after a 75 gram OGTT) and confirmed with a repeat test 7 to 14 days after the first test or from a report of physician‐diagnosed diabetes with evidence in the medical record of high glucose concentrations, or use of glucose‐lowering drugs. Fifty‐eight out of 141 participants in the physical activity only group compared with 90 participants out of 133 in the standard treatment group developed T2DM during the intervention period (when adjusting for cluster design: 33/84 versus 51/76) ‐ Analysis 1.2.
1.2. Analysis.

Comparison 1 Physical activity versus comparator, Outcome 2 Incidence of type 2 diabetes.
The cumulative incidence of T2DM at six years was 41.1% (95% CI 33.4 to 49.4) in the physical activity only group compared with 67.7% (95% CI 59.8 to 75.2) in the standard treatment group (not adjusted for clustering). In the physical activity only group the incidence of T2DM (defined according to WHO 1985) was 8.3/100 person years (95% CI 6.4 to 10.3) compared with 15.7/100 person years (95% CI 12.7 to 18.7) in the standard treatment group (not adjusted for clustering). When analysing the incidence according to FPG 7.8 mmol/L or higher, the incidence of T2DM was 5.3/100 person years (95% CI 3.6 to 7.0) in the physical activity only group compared with 9.6/100 person years (95% CI 7.2 to 12.0) in the standard treatment group (not adjusted for clustering) (Da Qing 1997).
Of the lean participants (BMI at baseline < 25 kg/m2), 15 (26.3%) out of 57 participants in the physical activity only group compared with 30 (60%) out of 50 in the standard treatment group developed T2DM during the intervention period (when adjusting for clustering 9/33 versus 17/29). Of the overweight participants (BMI at baseline ≥ 25 kg/m2), 43 (51.2%) out of 84 participants in the physical activity only group compared with 60 (72.3%) out of 83 participants in the standard treatment group developed T2DM during the intervention period (when adjusting for clustering 25/48 versus 34/47) (Da Qing 1997).
For all the 263 diabetes diagnoses made in all the intervention groups during the six years of intervention in Da Qing 1997, 55 diagnoses (21%) were initially made by local physicians and confirmed by the city hospital with an OGTT; 208 (79%) were made as a result of the systematic OGTT during the trial.
The other trial reporting T2DM defined the condition by FPG more than 6.9 mmol/L and/or two‐hour plasma glucose concentration more than 12.1 mmol/L (Hellgren 2016). Ten (11.9%) out of 84 participants in the physical activity group compared with seven (17.9%) out of 39 participants in the standard treatment group developed T2DM during the intervention period (Analysis 1.2).
In the physical activity group 32 participants had IGT only; 38 had IFG only and 11 had IGT and IFG combined. Of the 32 participants with IGT at baseline in the physical activity group, seven (22%) developed T2DM; of the 38 with IFG at baseline, three (7.8%) developed T2DM; of the 11 with combined IFG and IGT, two (18.2%) developed T2DM. In the standard treatment group 15 participants had IGT only; 21 had IFG only and six had IGT and IFG combined. Of the 15 participants with IGT at baseline in the standard treatment group five (33%) developed T2DM; of the 21 with IFG at baseline, two (7.8%) developed T2DM; of the six with combined IFG and IGT, four (66.7%) developed T2DM (Hellgren 2016). The number of the participants included in the subgroups of different glycaemic definitions of intermediate hyperglycaemia did not add up to the total number of participants developing T2DM provided by the investigators of the trial (physical activity 81 versus 84 and standard treatment 32 versus 39) (Hellgren 2016). We contacted the investigators about the participants who did not participate in the final follow‐up and asked if these people had developed T2DM (Hellgren 2016).
One trial had an extended follow‐up period (Da Qing 1997). The HR for the incidence of T2DM (adjusted for age and clustering) of physical activity versus standard treatment after 20 years of follow‐up was 0.51 (95% CI 0.31 to 0.83) (Da Qing 1997).
Serious adverse events
One trial reported that they did not observe any adverse events. However, this seems unlikely, because the publications indicated that serious adverse events were experienced (e.g. cardiovascular events) (Da Qing 1997). The other trial did not publish any data on serious adverse events, but these were provided by the investigators (Hellgren 2016). No further definition of the outcome was provided. Three (4.5%) out of 66 participants in the physical activity group versus one (2.6%) out of 39 participants in the standard treatment group experienced a serious adverse event (Hellgren 2016) (Analysis 1.3).
1.3. Analysis.

Comparison 1 Physical activity versus comparator, Outcome 3 Serious adverse events.
Secondary outcomes
Cardiovascular mortality
None of the participants in the groups died of cardiovascular reasons.
Non‐fatal myocardial infarction
One trial reported that none of the 66 participants in the physical activity group compared with three (9.7%) of 31 participants in the standard treatment group experienced a non‐fatal myocardial infarction. No further definition of the outcome was provided. These data were provided by the investigators (Hellgren 2016).
Non‐fatal stroke
One trial reported that one (1.5%) out of 66 participants in the physical activity group compared with one (3.2%) out of 31 participants in the standard treatment group experienced a non‐fatal stroke. No further definition of the outcome was provided. These data were provided by the investigators (Hellgren 2016).
Amputation of lower extremity
One trial reported that none of the participants in the trial experienced an amputation of the lower extremity. No further definition of the outcome was provided. These data were provided by the investigators (Hellgren 2016).
Blindness or severe vision loss
Not reported
End‐stage renal disease
Not reported.
Non‐serious adverse events
One trial reported that no adverse event was observed (Da Qing 1997).
Hypoglycaemia
One trial reported that none of the participants in the trial experienced hypoglycaemia. No further definition of the outcome was provided. These data were provided by the investigators (Hellgren 2016).
Health‐related quality of life
One of the trials comparing physical activity with standard treatment reported health‐related quality of life (HrQoL) (Hellgren 2016). Information on this outcome was provided by the investigators. It is unclear whether they assessed HrQoL with a validated instrument. It was measured by two questions, where participants graded their total physical and mental health from 1 to 7. Participants also graded their general health from 1 (best) to 5 (very bad). There were questions about sleep and energy. The investigators reported that 13 participants (27%) in the physical activity group reported that HrQoL from baseline to end of follow‐up worsened, in 21 participants (43%) it was unchanged and in 14 participants (29%) it increased ‐ the total number of participants in the physical activity group included in the analyses was 48 participants. The investigators reported that eight participants (35%) in the standard treatment group rated themselves worsened, 10 participants (43%) remained unchanged and five participants (22%) improved ‐ the total number of participants in the standard treatment group included in the analyses was 23 (Hellgren 2016). These data were provided by the investigators.
Time to progression to type 2 diabetes
Not reported.
Measures of blood glucose control
Fasting glucose
FPG after six years of intervention in the physical activity group was 6.8 mmol/L (SD 2.2) based on 141 participants (81 participants when adjusting for clustering) compared with 7.6 mmol/L (SD 2.6) in 133 participants (76 participants when adjusting for clustering) in the standard treatment group (Da Qing 1997). The investigators of Hellgren 2016 provided data on change in fasting plasma glucose on request (Analysis 1.4).
1.4. Analysis.

Comparison 1 Physical activity versus comparator, Outcome 4 Fasting plasma glucose.
Glucose two hours after an oral glucose load
One trial reported glycaemic values after six years of intervention. The two‐hour glucose after an oral glucose load in the physical activity group was 10.5 mmol/L (SD 3.9) based on 141 participants (81 participants when adjusting for clustering) compared with 12.4 mmol/L (SD 4.2) in 133 participants (76 participants when adjusting for clustering) in the standard treatment group (Da Qing 1997). The investigators of Hellgren 2016 provided data on change in glucose two hours after an OGTT on request (Analysis 1.5).
1.5. Analysis.

Comparison 1 Physical activity versus comparator, Outcome 5 2 hour glucose values.
HbA1c
Not reported.
Socioeconomic effects
Not reported.
Diet plus physical activity versus standard treatment
Eleven trials compared the combination of diet plus physical activity with standard treatment or no intervention (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; Kosaka 2005; Oldroyd 2005; PODOSA 2014; SLIM 2003). Da Qing 1997 had four intervention arms: diet only, physical activity only, diet plus physical activity and standard treatment. The trial was cluster randomised. The authors reported that HRs at 20 and 23 years of follow‐up were adjusted for clustering, but no information of adjustment for clustering was provided at the end of intervention. No ICC coefficient was reported (Da Qing 1997). Based on an anticipated ICC coefficient of 0.05 we assumed a design effect of 1.75 (Higgins 2011). In the post‐intervention follow‐up period Da Qing 1997 combined the three intervention groups (diet only, physical activity only, diet plus physical activity) into one intervention group. Two other trials also reported data with relevance to this review after the intervention had been stopped (DPP 2002; DPS 2001).
Da Qing 1997 included people with IGT according to the WHO 1985 criteria (two‐hour plasma glucose ≥ 6.7 mmol/L and < 11.0 mmol/L after an OGTT).
Nine trials exclusively included people with IGT (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; IDPP 2006; JDPP 2013; Kosaka 2005; Oldroyd 2005; SLIM 2003). One trial included people with IGT, IFG or both (PODOSA 2014). One trial included people with FPG levels between 5.3 to 6.9 mmol/L (HELP PD 2011).
Primary outcomes
All‐cause mortality
Ten trials reported data on all‐cause mortality (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; Oldroyd 2005; PODOSA 2014; SLIM 2003). None of these trials had predefined all‐cause mortality as a primary outcome (see Appendix 6). In Da Qing 1997 five out of 126 participants died in the diet plus physical activity group versus three out of 133 participants in the standard treatment group (when adjusted for clustering; diet plus physical activity three out of 72 participants versus standard treatment two out of 76 participants). Most of the trials reporting death were from trials with low risk of selection bias (DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; JDPP 2013; Oldroyd 2005; PODOSA 2014).
A total of 12 deaths were reported in 2049 participants in the diet plus physical activity group versus 10 out of 2050 participants in the comparator group (RR 1.12, 95% CI 0.50 to 2.50; P = 0.86; 4099 participants, 10 trials; very low‐quality evidence). Funnel plot asymmetry was not present. The 95% prediction interval ranged from 0.44 to 2.88 (Analysis 2.1).
2.1. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 1 All‐cause mortality.
Trial sequential analysis (TSA) showed that 0.61% of the diversity‐adjusted information size was accrued so far to detect or reject a 10% relative risk reduction (RRR). Diversity was zero, but we applied a diversity of 20% when calculating the diversity‐required information size, as heterogeneity is likely to increase when future trials are included. As only a minor fraction of the diversity‐adjusted required information size to detect or reject a 10% RRR was accrued, we could not calculate the TSA‐adjusted 95% CIs with diversity at 20%. If a diversity of 0% was applied, then 0.77% of the diversity‐adjusted information size was accrued to detect or reject a 10% RRR. Still, we could not calculate the TSA‐adjusted 95% CIs.
Subgroup analyses according to the duration of the intervention ((≥ 4 years) versus trials with short duration (< 4 years)), diagnostic criteria (IGT versus other); age (≥ 50 years versus < 50 years); ethnicity (Asian only versus other and mixed ethnicities); morbidity (BMI ≥ 30 kg/m2 versus < 30 kg/m2) showed no statistical significant interactions (Appendix 18). We could not perform subgroup analyses according to sex and previous gestational diabetes due to lack of data.
Sensitivity analyses including only trials with low risk of selection bias (DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; JDPP 2013; Oldroyd 2005; PODOSA 2014) did not substantially change the effect estimate (RR 1.14, 95% CI 0.42 to 3.12; P = 0.79).
One trial reported a HR (adjusted for age and clustering) when combining diet only, physical activity only and diet plus physical activity compared with control at the end of intervention (HR 1.33, 95% CI 0.45 to 3.92) (Da Qing 1997).
Three trials comparing diet plus physical activity had extended follow‐up periods (Da Qing 1997; DPP 2002; DPS 2001). Two of these reported mortality data during the follow‐up period (Da Qing 1997; DPS 2001).
One trial reported that six out of 257 participants originally randomised to diet plus physical activity group died compared with 10 out of 248 participants in the control arm after a median follow‐up of 10.6 years (i.e. 6.6 years after the intervention had stopped) (DPS 2001). Mortality in the former diet plus physical activity group was 2.2/1000 person years (95% CI 1.0 to 0.35) compared with 3.8/1000 person years (95% CI 2.0 to 7.0) in the former control group. The HR was 0.57 (95% CI 0.21 to 1.58) (unknown adjusting) (DPS 2001).
One trial reported a HR (adjusted for age and clustering) when combining diet only, physical activity only and diet plus physical activity compared with control to be 0.96 (95% CI 0.65 to 1.41) after 20 years of follow‐up (i.e. 14 years after the intervention had stopped) (Da Qing 1997). The same trial reported 23 years of follow‐up (i.e. 17 years after the intervention had stopped); 121 out of 430 participants (after cluster‐adjustment: 69/246) allocated to the former intervention group (combined diet only, physical activity only and diet plus physical activity) versus 53 out of 138 (after cluster‐adjustment 30/79) in the former control group died (Da Qing 1997). The cumulative incidence of all‐cause mortality was 28.1% (95% CI 23.9 to 32.4) in the combined former intervention group and 38.4% (95% CI 30.3 to 46.5) in the former comparator group, with a HR (adjusted for cluster‐randomisation) of 0.71 (95% CI 0.51 to 0.99; P = 0.049). Deaths per 1000 person years were 14.3 (95% CI 11.8 to 16.9) in the combined former intervention group compared with 19.9 (95% CI 14.5 to 25.2) in the former comparator group.
Post‐hoc subgroup analyses after 23 years of follow‐up showed that, among women, the cumulative incidence of all‐cause mortality in the former comparator group was elevated compared with the combined former intervention group (HR 0.46, 95% CI 0.24 to 0.87; P = 0.02). The number of deaths included in this post‐hoc analysis was 30 deaths in 200 women in the combined former intervention group compared with 17 deaths out of 59 women in the former comparator group (Da Qing 1997). For men the intervention did not show statistically significant differences (91 deaths among 230 male participants in the combined former intervention group compared with 36 deaths out of 79 participants in the former comparator group; HR 0.97 (95% CI 0.97 to 1.46). We performed multivariable analyses in order to test if different baseline characteristics could explain the differences (e.g. smoking, age), but the difference between men and women persisted. However, when the time to onset of T2DM was included in the multivariable models, then the intervention variable was no longer significant (Da Qing 1997).
Incidence of type 2 diabetes
Eleven trials reported data on the incidence of T2DM. The definition of T2DM varied among the included trials. Three trials applied the WHO 1985 diagnostic criteria (either FPG ≥ 7.8 mmol/L or higher or a two‐hour plasma glucose ≥ 11.1 mmol/L after a 75 g OGTT) (Da Qing 1997; DPS 2001; Oldroyd 2005); four trials applied the WHO 1999 criteria to establish the diagnosis of T2DM (either FPG ≥ 7.0 mmol/L and/or a two‐hour plasma glucose concentration ≥ 11.1 mmol/L after a 75 g OGTT) (EDIPS 2009; IDPP 2006; JDPP 2013; SLIM 2003). One trial established the diagnosis of T2DM based on the ADA 1997 diagnostic criteria (FPG ≥ 7.0 mmol/L or a two‐hour plasma glucose ≥ 11.1 mmol/L after a 75 g OGTT (i.e. identical to WHO 1999 criteria)) (DPP 2002). One trial applied FPG levels above 7.0 mmol/L to establish the diagnosis of T2DM (HELP PD 2011). One trial applied FPG levels above 7.8 mmol/L to establish the diagnosis of T2DM (Kosaka 2005). One trial established the diagnosis of T2DM based on two‐hour plasma glucose of 11.1 mmol/L or higher after a 75 g OGTT (PODOSA 2014). Furthermore, four trials also defined the diagnosis of T2DM as reported by a physician or the use of glucose‐lowering drugs (Da Qing 1997; EDIPS 2009; HELP PD 2011; PODOSA 2014).
A total of 315 out of 2122 participants developed T2DM in the diet plus physical activity group versus 614 out of 2389 participants in the comparator group (RR 0.57, 95% CI 0.50 to 0.64; P < 0.00001; 4511 participants, 11 trials; moderate quality evidence). The 95% prediction interval ranged from 0.50 to 0.65. TSA showed firm evidence for a 10% RRR in favour of diet plus physical activity. Funnel plot asymmetry was not present (Analysis 2.8).
2.8. Analysis.
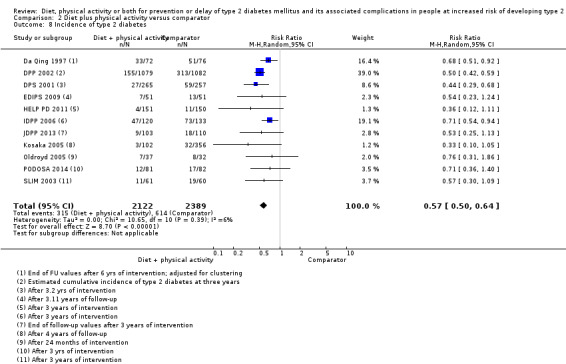
Comparison 2 Diet plus physical activity versus comparator, Outcome 8 Incidence of type 2 diabetes.
Of the 12 participants in PODOSA 2014 diagnosed with T2DM in the diet plus physical activity group, only two participants were diagnosed with OGTT at the third year of the trial. The remaining 10 participants who developed T2DM in the diet plus physical activity group during the trial were diagnosed by a physician. In the control group 10 participants were diagnosed with T2DM, based on a physicians' diagnosis and seven participants were diagnosed with the OGTT at the third year of the trial (PODOSA 2014).
The incidence rate of T2DM in the Japan Diabetes Prevention Program (JDPP 2013) trial was 2.7/100 person years in the diet plus physical activity group compared with 5.1/100 person years in the control group. The JDPP trial included both lean and obese participants with a BMI ranging from 16.8 to 39.6 kg/m2. Additional analyses were made according to BMI at baseline. T2DM developed in five out of 52 participants in the lowest quartile (two from the control group and three from the intervention group) during the three years. An effect of the behaviour‐changing intervention was therefore not apparent in this lowest BMI quartile. However, the cut‐off point for the lower quartile was not available from the publication (JDPP 2013). Subgroup analysis for the participants with BMI more than 22.5 kg/m2 revealed a significant decrease in the cumulative incidence of T2DM with the intervention (P = 0.027). The cumulative incidence of T2DM was significantly lower in the diet plus physical activity group compared with controls among participants with baseline HbA1c levels of 5.7% or higher, while this was not found among participants with baseline HbA1c levels less than 5.7% (JDPP 2013).
The Indian Diabetes Prevention Programmes (IDPP 2006) trial reported a cumulative incidence of T2DM at 39.3% (95% CI 30.4 to 48.5) in the diet plus physical activity group compared with 55.0% (95% CI 46 to 63.5) in the control group (IDPP 2006). The number of participants needed to be treated to prevent one T2DM case was 6.4. The HR for developing T2DM (adjusted for sex, age, family history of diabetes, BMI, waist circumference, baseline fasting and two‐hour glucose and corresponding insulin values, hypertension and smoking) for the diet plus physical activity group compared with the control group was 0.62 (95% CI 0.23 to 1.02; P = 0.018) (IDPP 2006). Plasma glucose two‐hour after an OGTT, fasting and two‐hour insulin showed independent influence on the development of T2DM (IDPP 2006).
In Da Qing 1997 the incidence rate of T2DM in the diet plus physical activity group was 9.6/100 person years (95% CI 7.2 to 12.0) compared with 15.7/100 person years (95% CI 12.7 to 18.7) in the control group (not adjusted for clustering). When analysing the incidence rate of T2DM according to fasting plasma glucose of 7.8 mmol/L or higher, the incidence rate of T2DM was 5.5/100 person years (95% CI 3.7 to 7.3) in the diet plus physical activity group compared with 9.6/100 person years (95% CI 7.2 to 12.0) in the control group (not adjusted for clustering) (Da Qing 1997). The HR (adjusted for age and clustering) when combining diet only, physical activity only and diet plus physical activity compared with control was 0.49 (95% CI 0.33 to 0.73).
Of the lean participants (BMI at baseline < 25 kg/m2), 16 (34.8%) out of 46 participants in the diet plus physical activity group compared with 30 (60%) out of 50 in the control group developed T2DM during the intervention period (when adjusting for clustering 9/26 versus 17/29). Of the obese participants (BMI at baseline ≥ 25 kg/m2), 42 (52.5%) out of 80 participants in the diet plus physical activity group compared with 60 (72.3%) out of 83 in the control group developed T2DM during the intervention period (when adjusting for clustering 24/46 versus 34/47) (Da Qing 1997).
A total of 263 participants were diagnosed with T2DM during the six years of intervention; 55 diagnoses (21%) were initially made by local physicians and confirmed by the city hospital with an OGTT; 208 (79%) were made as a result of the systematic OGTTs during the trial (Da Qing 1997).
In DPS 2001 the incidence rate of T2DM after a median of four years' intervention was 4.2/100 person years in the diet plus physical activity group compared with 7.4/100 person years in the control group. The HR (adjusted for sex, age, BMI, waist circumference, fasting and two‐hour glucose, insulin, homeostatic model assessment ‐ insulin resistance (HOMA‐IR), estimated risk with The Finnish Diabetes Risk Score (FINDRISC)) was 0.54 (95% CI 0.37 to 0.78) in favour of the diet plus physical activity group. Incidence and HRs were present for several baseline variables (sex, age, BMI, waist circumference; fasting plasma glucose; two‐hour glucose; fasting insulin; HOMA‐IR and FINDRISC) (Appendix 19). The interaction between age at baseline as a continuous variable and intervention effect was statistically significant (P = 0.0130) (Appendix 19). Baseline glycaemic (fasting and two‐hour glucose) status was directly associated with diabetes incidence in the diet plus physical activity group and the control group. However, the effect of intervention was independent of glycaemic status (Appendix 19).
The trial authors performed several subgroup analyses according to different prognostic baseline variables in DPP 2002 (Appendix 19). Subgroup analyses found that treatment effects did not differ substantially according to sex, race or ethnic group; however, the effect of the behaviour‐changing intervention was greater among participants with lower baseline glucose concentrations two hours after a glucose load.
One publication presented a composite analysis including three of the included trials (DPS 2001; EDIPS 2009; SLIM 2003). A total of 749 participants were included in the analyses with a mean follow‐up of 3.1 years (Penn 2013). The HR for the incidence of T2DM was 0.42, (95% CI 0.29 to 0.60; P < 0.001) in favour of diet plus physical activity. On average 7.4 people had to undergo diet plus physical activity for a mean of 3.1 years to prevent one case of T2DM (number needed to treat for an additional beneficial outcome 22.9 for one year).
Subgroup analysis: analysing trials according to the duration of the intervention and diagnostic criteria showed no interaction between the subgroups (P = 0.88 and P = 0.42) (Analysis 2.9; Analysis 2.10) (Appendix 18). Subgroup analyses stratifying the included trials according to age, ethnicity and obesity showed statistically significant interactions (Appendix 18) (Analysis 2.11; Analysis 2.12; Analysis 2.13). However, the CIs in these analyses overlap to a small degree. As a caveat, these observations should be regarded as hypothesis‐generating only. We could not perform subgroup analyses according to sex and previous gestational diabetes due to lack of data.
2.9. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 9 Incidence of type 2 diabetes: duration of the intervention.
2.10. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 10 Incidence of type 2 diabetes: diagnostic criteria.
2.11. Analysis.
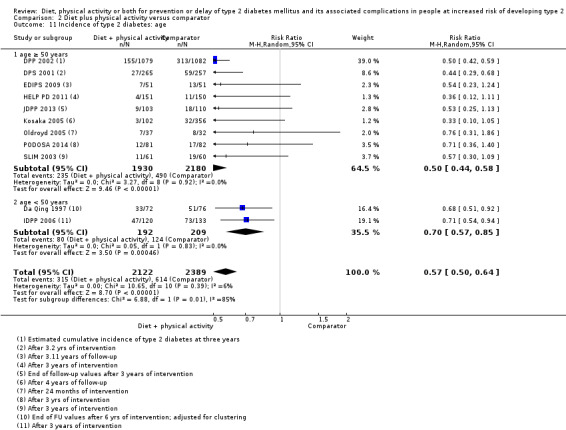
Comparison 2 Diet plus physical activity versus comparator, Outcome 11 Incidence of type 2 diabetes: age.
2.12. Analysis.
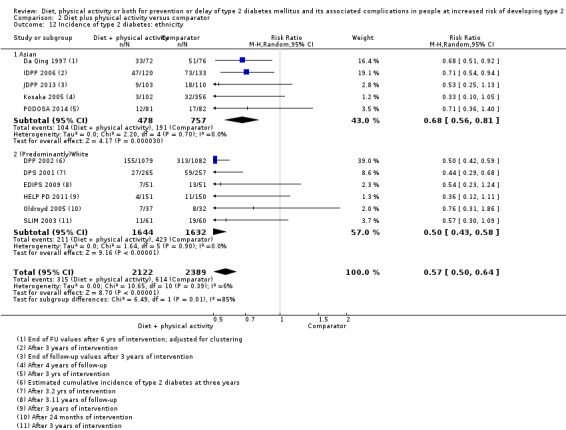
Comparison 2 Diet plus physical activity versus comparator, Outcome 12 Incidence of type 2 diabetes: ethnicity.
2.13. Analysis.
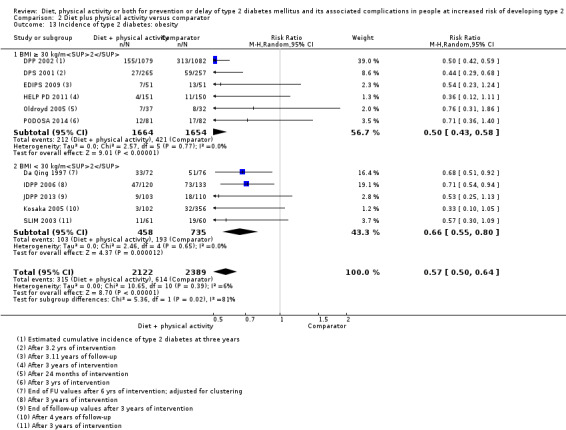
Comparison 2 Diet plus physical activity versus comparator, Outcome 13 Incidence of type 2 diabetes: obesity.
Sensitivity analysis: we could not perform sensitivity analysis according to publication status (all included trials were published) and language of publication (all included trials were published in English). Sensitivity analysis restricted to only trials with low risk of selection bias showed a RR of 0.50 (95% CI 0.44 to 0.58; P < 0.00001) (DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; JDPP 2013; Oldroyd 2005; PODOSA 2014), that is, it did not show substantial differences to including all trials. Analysing trials performed in Asia showed a RR of 0.67 (95% CI 0.56 to 0.82; P <0.0001) (Da Qing 1997; IDPP 2006; JDPP 2013; Kosaka 2005); for trials performed in Europe, the RR was 0.54 (95% CI 0.41 to 0.72; P < 0.0001) (DPS 2001; EDIPS 2009; Oldroyd 2005; PODOSA 2014; SLIM 2003).
Three trials reported the incidence of T2DM after an extended follow‐up period (Da Qing 1997; DPP 2002; DPS 2001). One trial reported the incidence of T2DM after nine years (i.e. five years after the end of the intervention period). A total of 106 in the diet plus physical activity group out of 265 participants versus 140 out of 257 participants in the control group were diagnosed with T2DM (DPS 2001). The incidence of T2DM per 100 person years was 4.5 (95% CI 3.8 to 5.5) in the diet plus physical activity group versus 7.2 (95% CI 6.1 to 8.5) in the control group; the HR (adjusted for sex, age, BMI and two‐hour glucose) was 0.61 (95% CI 0.48 to 0.79; P < 0.001) and the absolute risk reduction was 19%. The number needed to treat to prevent one case of T2DM was 5.2. In the post‐intervention follow‐up (median seven years), 62 out of 200 participants in the former diet plus physical activity group, compared with 68 out of 166 participants in the former control group were newly diagnosed with T2DM. The incidence of T2DM in the former diet plus physical activity group was 4.9 (95% CI 3.8 to 6.3) compared with 7.0 (95% CI 5.5 to 8.9) in the control group (DPS 2001); the HR (adjusted for sex, age, BMI and two‐hour glucose) was 0.67 (95% CI 0.48 to 0.95; P = 0.023). There was a 32% relative risk reduction and a 15% absolute risk reduction during the post‐intervention follow‐up period in favour of the former diet plus physical activity group (DPS 2001).
Da Qing 1997 reported the incidence of T2DM after 20 and 23 years of follow‐up. The HR (adjusted for age and clustering) of the former diet plus physical activity group (diet only, physical activity only, diet plus physical activity) versus control after 20 years of follow‐up was 0.57 (95% CI 0.41 to 0.81). After 23 years of follow‐up 312 out of 430 participants of the combined former intervention group versus 124 out of 138 participants in the former control group developed T2DM. The HR (adjusted for clustering) was 0.55 (95% CI 0.40 to 0.76; P = 0.001). The median delay to the onset of T2DM was 3.6 years after 20 years of follow‐up.
During the first seven years the incidence of T2DM in the Diabetes Prevention Program Outcomes Study (DPPOS) compared with the incidence of T2DM in the Diabetes Prevention Program (DPP) decreased in the control group (‐42%) (HR 0.58, 95% CI 0.48 to 0.69) compared with the incidence in the former diet plus physical activity group (31%) group (HR 1.31, 95% CI 1.07 to 1.61) (see DPP 2002). After 15 years of follow‐up in the DPPOS the incidence of T2DM was reduced by 27% in the former diet plus physical activity group (HR 0.73, 95% CI 0.65 to 0.83; P < 0.0001) (DPP 2002). Over the 15 years of follow‐up the average annual T2DM incidence rate was 5.2% in the former diet plus physical activity group and 7.0% in the former control group. At year 15, the cumulative incidence of T2DM was 562 participants (62%) in the former control group versus 480 participants (55%) in the former diet plus physical activity group (DPP 2002). The median delay to onset of T2DM after 10 years was about four years by diet plus physical activity compared with control. After 10 years of follow‐up (i.e. about seven years after the intervention period had stopped) 23% in the former diet plus physical activity group compared with 19% in the former control group had become normoglycaemic (fasting glucose < 6.1 mmol/L, two‐hour glucose < 7.8 mmol/L, and no previous diagnosis of T2DM) (DPP 2002). The annual incidence of T2DM in the diet plus physical activity group rose slowly through year 4 after randomisation, then declined to steady levels at year 7. The control group had the highest incidence of T2DM early in the DPP, with a relatively steady decline through the end of the DPP and into the DPPOS, and levelling late in the DPPOS. Post hoc analysis defining T2DM by HbA1c at 6.5% or higher showed that the incidence rate was reduced with diet plus physical activity compared with control during the DPP (4.6 cases/100 person years versus 8.8 cases/100 person years) and at 10 years of follow‐up (3.5 cases/100 person years versus 5.0 cases/100 person years) (DPP 2002). Only participants with HbA1c less than 6.5% at baseline were included in this post hoc analysis (diet plus physical activity N = 932 at baseline; control N = 922 at baseline) (DPP 2002). During the total follow‐up period there were differences among ethnic groups in the incidence of diabetes defined as HbA1c 6.5% or higher or FPG 7.0 mmol/L or higher and/or two‐hour plasma glucose 11.1 mmol/L or higher (Appendix 19). Only 26% of the participants diagnosed with T2DM according to FPG or glucose values after an OGTT had previous or simultaneous HbA1 6.5% or higher. On the other hand, 55% of those first attaining an HbA1c 6.5% or higher had current or previous diagnosis of T2DM defined according to FPG 7.0 mmol/L or higher and/or two‐hour plasma glucose 11.1 mmol/L or higher (DPP 2002).
Serious adverse events
Two trials reported serious adverse events (Da Qing 1997; EDIPS 2009). In one trial the investigators provided information that one out of 51 participants in the diet plus physical activity group compared with none out of 51 participants in the control group experienced a serious adverse event (EDIPS 2009). The other trial reported that no adverse events were experienced in the intervention arms (low‐quality evidence) (Da Qing 1997) (Analysis 2.29)). Four other trials clearly described recording serious adverse events but they did not present any data (DPP 2002; HELP PD 2011; IDPP 2006; JDPP 2013). In DPP 2002 only serious adverse events related to metformin were reported for each trial arm. We could not perform any meta‐analysis or subgroup analysis due to lack of data.
2.29. Analysis.
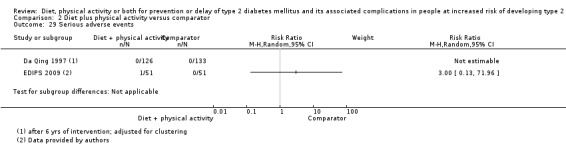
Comparison 2 Diet plus physical activity versus comparator, Outcome 29 Serious adverse events.
Secondary outcomes
Cardiovascular mortality
Seven trials reported data on cardiovascular mortality (Da Qing 1997; DPP 2002; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; Oldroyd 2005). Four of the trials reported that none of the participants died due to cardiovascular disease (EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013). The number of participants who died due to cardiovascular disease was low (four out of 1626 participants in the diet plus physical activity group compared with four out of 1637 participants in the control group) (RR 0.94, 95% CI 0.24 to 3.65; P = 0.93; 3263 participants, 7 trials; very low‐quality evidence). The 95% prediction interval was not meaningful (Analysis 2.14).
2.14. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 14 Cardiovascular mortality.
TSA showed that 0.13% of the diversity‐adjusted information size was accrued to detect or reject a 10% RRR. Diversity was zero, but we applied a diversity of 20% when calculating the diversity‐required information size as heterogeneity is likely to increase when future trials are included. As only a minor fraction of the diversity‐adjusted required information size to detect or reject a 10% RRR was accrued, we could not calculate the TSA‐adjusted 95% CIs with a diversity at 20%. Applying diversity at 0%, 0.16% of the diversity‐adjusted information size was accrued to detect or reject a 10% RRR. Still, we could not calculate the TSA‐adjusted 95% CIs.
We did not perform subgroup or sensitivity analyses due to lack of data.
One trial reported cardiovascular mortality after the intervention period had stopped (Da Qing 1997). The trial reported the HR (adjusted for age and clustering) after a total follow‐up period of 20 years (i.e. 14 years after the intervention had stopped) for the combined former diet only, physical activity only and diet plus physical activity group versus former control to be 0.83 (95% CI 0.48 to 1.05). After 23 years of follow‐up 78 participants died as a result of cardiovascular disease; 51 in the combined former intervention groups versus 27 in the former control group (when adjusting for clustering 29 versus 15). Cumulative incidences were 11.9% (95% CI 8.8 to 15.0) versus 19.6% (95% CI 12.9 to 26.3; the HR (adjusted for age and clustering) was 0.59 (95% CI 0.36 to 0.96; P = 0.03). Post hoc analyses comparing women with men after 23 years of follow‐up showed a HR of 0.28 (95% CI to 0.11 to 0.71; P = 0.01) and 0.91 (95% CI 0.50 to 1.65), respectively (Da Qing 1997).
Non‐fatal myocardial infarction
None of the trials reported non‐fatal myocardial infarction at the end of the intervention period. However, the investigators of one of the included trials reported that none of the participants in the diet plus physical activity group or the standard treatment group (51 participants in each intervention group) had a non‐fatal myocardial infarction (EDIPS 2009) ‐ low quality evidence.
The DPP reported the total number of non‐fatal cardiovascular events at the end of the intervention. Non‐fatal cardiovascular events occurred in 24 out of 1079 participants (2.2%) (incidence rate 9.7 events per 1000 patient‐years) in the diet plus physical activity group compared with 18 out of 1082 participants (1.7%) with an incidence rate of 7.3 events per 1000 patient‐years in the control group. No substantial differences were seen in the diet plus physical activity group compared with standard treatment (DPP 2002). The excess of events in the diet plus physical activity group was due to hospitalisations because of cardiovascular disease and revascularisation procedures (DPP 2002).
The IDPP also only reported the total number of cardiovascular events with four (3.3%) out of 120 participants in the diet plus physical activity group compared with two (1.5%) out of 133 participants in the control group (IDPP 2006).
Da Qing 1997 reported a cumulative incidence of any first cardiovascular event to be 5.2 (95% CI 3.0 to 7.3) in the combined intervention group (diet only, physical activity only, diet plus physical activity) compared with 5.4 (95% CI 1.5 to 9.2) in the control group during the intervention period. The incidence rate per 100 person years was 0.9 (95% CI 0.2 to 1.6) in the intervention group compared with 0.9 (95% CI 0.5 to 1.3) in the control group during the intervention period. The HR adjusted for age and clustering was 0.96 (95% CI 0.76 to 1.44).
From one trial it was apparent that cardiovascular event data were collected during the intervention period, but no data were reported (DPS 2001).
Two trials reported cardiovascular complications after the intervention period had stopped (Da Qing 1997; DPS 2001). One trial reported composite cardiovascular morbidity after a total follow‐up of 10.6 years (i.e. about 6.6 years after the end of intervention); 57 cardiovascular events were reported in 257 participants of the former diet plus physical activity group compared with 54 events in 248 participants in the former control group (DPS 2001). The incidence rate of cardiovascular morbidity was 22.9 in the former diet plus physical activity group versus 22.0 per 1000 person years in the former control group; the HR was 1.04( 95% CI 0.72 to 1.51). Men and women had the same incidence in the two intervention groups (DPS 2001). Da Qing 1997 reported the cumulative incidence of any first cardiovascular event to be 40.9 (95% CI 36.0 to 45.9) in the combined former intervention group (diet only, physical activity only, diet plus physical activity) compared with 44.1 (95% CI 35.3 to 53.0) in the former control group after a total follow‐up period of 20 years (i.e. 14 years after the intervention had stopped). The incidence rate per 100 person years was 2.3 (95% CI 1.9 to 2.7) in the intervention group compared with 2.5 (95% CI 1.9 to 3.2) in the control group after 20 years of follow‐up. The HR adjusted for age and clustering was 0.98 (95% CI 0.71 to 1.37) (Da Qing 1997).
Non‐fatal stroke
None of the trials reported non‐fatal stroke at the end of the intervention period. However, the investigators of one of the included trials reported that none of the participants in the diet plus physical activity or the control group (51 participants in each intervention group) had a non‐fatal stroke (EDIPS 2009) ‐ low quality evidence.
Several trials reported data on composite cardiovascular events (see above) (DPP 2002; Da Qing 1997; IDPP 2006).
Amputation of lower extremity
None of the trials reported amputation of the lower extremity at the end of the intervention period. However, the investigators of one of the included trials reported that none of the participants in the diet plus physical activity or the control group (51 participants in each intervention group) had an amputation of the lower extremity (EDIPS 2009).
Several trials reported data on composite cardiovascular events (see above) (DPP 2002; Da Qing 1997; IDPP 2006).
Blindness or severe vision loss
We did not identify trials with data on blindness or severe vision loss for this comparison.
One trial reported severe retinopathy after 20 years of follow‐up. Severe retinopathy occurred in 31 participants out of 238 in the intervention group (cumulative incidence 9.2%) compared with 17 out of 93 in the control group (cumulative incidence 16.2%) (Da Qing 1997). The HR (adjusted for clustering and age) was 0.53 (95% CI 0.29 to 0.99; P = 0.048) in favour of the intervention (Da Qing 1997). All participants with severe retinopathy had developed T2DM by the time the retinopathy was recognised (Da Qing 1997).
One trial reported an aggregate outcome of microvascular disease (nephropathy, retinopathy and neuropathy) after 15 years of follow‐up (DPP 2002). The prevalence of microvascular outcomes after 15 years did not differ substantially between the intervention and control group despite group differences in the incidence of T2DM (former diet plus physical activity group 11.3% (95% CI 10.1 to 12.7) compared with former control 12.4% (95% CI 11.1 to 13.8)). The women but not the men in the former diet plus physical activity group experienced a reduction in microvascular disease (RR 0.79, 95% CI 0.64 to 0.98) compared with the former control group. There were no substantial differences in the treatment effects on aggregate microvascular complications in subgroups defined by age or ethnicity, except that Hispanic Americans had a lower microvascular disease prevalence in the former diet plus physical activity group than in the former control group (RR 0.43, 95% CI 0.20 to 0.91) (DPP 2002).
End‐stage renal disease
None of the trials reported end‐stage renal disease at the end of the intervention period.
One trial reported renal replacement therapy or death due to kidney failure after 20 years of follow‐up (i.e. 14 years after the intervention had stopped) . Seven out of 441 participants (when adjusting for clustering: 4 out of 252 participants) of the combined former intervention group compared with two out of 136 participants (when adjusting for clustering 1 out of 78 participants) in the former control group developed end‐stage renal disease (Da Qing 1997).
Non‐serious adverse events
One trial reported that no non‐serious adverse events were reported (diet plus physical activity 0/126 (adjusted for clustering 0/72 versus control 0/76)) (Da Qing 1997). Another trial reported three (3.6%) out of 84 participants in the diet plus physical activity group compared with four (4.8%) out of 83 participants in the control group experienced a non‐serious adverse event (PODOSA 2014).
Hypoglycaemia
Two trials reported that none of the participants experienced hypoglycaemia (IDPP 2006: diet plus physical activity 0/133 compared with control 0/136; EDIPS 2009: diet plus physical activity 0/51 compared with control 0/51).
Health‐related quality of life
The DPP trial applied the 36‐item Short‐Form (SF‐36) to evaluate the health utility index (SF‐6D), physical component summaries (PCS) and mental component summaries (MCS). Minimal important difference (MID) was defined as a difference in scores between groups of at least 3% (DPP 2002). A total of 1070 participants from the diet plus physical activity group and 1074 participants from the standard treatment group were included (DPP 2002). In both the diet plus physical activity arm as well as the control arm HrQoL summary scores worsened during the trial, but the decline for SF‐6D (P < 0.05) and PCS (P < 0.01) was slower in diet plus physical activity participants compared to the changes in the control group; however, none reached the MID of 3%. After a mean of 3.2 years of follow‐up there were improvements in the SF‐6D (0.008; P = 0.04) in the diet plus physical activity group compared with control, however, the MID of 3% between the intervention groups was not achieved. The PCS (1.57; P < 0.0001) also improved in the diet plus physical activity group compared with control (DPP 2002). Again, the MID between the intervention groups was not achieved. MCS improved in the placebo group compared with the diet plus physical activity group during the intervention period with 0.28 (SD 0.32). No exact P‐value was provided, the value was more than 5%. The MID was not achieved (DPP 2002). The overall quality of evidence for this outcome measure was very low.
The investigators from the PODOSA 2014 reported that the participants were asked how healthy they were during the trial. The replies were not analysed at the end of the intervention.
One trial stated in the design article that HrQoL measured with the SF‐36 would be assessed. However, no data were available (HELP PD 2011). One publication stated that HrQoL was measured, but no data were reported (EDIPS 2009). According to the investigators HrQoL was not analysed (EDIPS 2009).
Time to progression to type 2 diabetes
One trial reported data on the time to progression to T2DM in the post‐interventional follow‐up period (median of seven years). Among the participants who developed T2DM the median time to the onset of T2DM was 15 years (95% CI 13 to 17) in the former intervention group compared with 10 years (95% CI 8 to 12 years) in the former control group (95% CI 8 to 12 years) (DPS 2001).
Measures of blood glucose control
Fasting glucose
Ten trials reported FPG (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; HELP PD 2011; IDPP 2006; JDPP 2013; Oldroyd 2005; PODOSA 2014; SLIM 2003). Effect‐estimates in both random‐effects and fixed‐effect models showed differences (random MD ‐0.17 mmol/L, 95% CI ‐0.27 to ‐0.06; P = 0.003; fixed MD ‐0.21 mmol/L, 95% CI ‐0.27 to ‐0.15; P < 0.00001; 10 trials; 3530 participants; Analysis 2.18). The 95% prediction interval ranged between ‐0.43 mmol/L to 0.09 mmol/L.
2.18. Analysis.
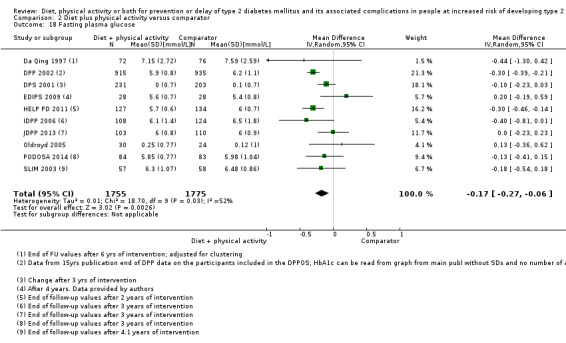
Comparison 2 Diet plus physical activity versus comparator, Outcome 18 Fasting plasma glucose.
TSA showed that diversity‐adjusted information size was accrued to detect or reject a difference in fasting plasma glucose at ‐0.17 mmol/L in favour of the diet plus physical activity group. Diversity was 70%.
Subgroup analyses: according to duration of the intervention ((≥ 4 years) versus trials with short duration (< 4 years)), diagnostic criteria of participants with IGT versus other; age (included participants ≥ 50 years versus < 50 years); ethnicity (Asian versus predominantly White); morbidity (BMI ≥ 30 kg/m2 versus < 30 kg/m2); showed no statistically significant interactions (Appendix 18). We could not perform subgroup analyses according to sex and previous gestational diabetes due to lack of data.
Sensitivity analysis: we could not perform sensitivity analyses according to publication status and language of publication as all included trials were published in English. Sensitivity analysis restricted to trials with low risk of selection bias showed a MD of ‐0.14 mmol/L (95% CI ‐0.26 to ‐0.01; P = 0.03) (DPP 2002; DPS 2001; EDIPS 2009;HELP PD 2011; JDPP 2013; Oldroyd 2005; PODOSA 2014). Analysing trials according to geographies for trials performed in Asia showed a MD of ‐0.18 mmol/L (95% CI ‐0.49 to 0.13; P = 0.15) (Da Qing 1997; IDPP 2006; JDPP 2013). Trials performed in Europe showed a MD of ‐0.08 mmol/L (95% CI ‐0.80 to 0.08; P = 0.15) (DPS 2001; EDIPS 2009; Oldroyd 2005; PODOSA 2014; SLIM 2003). One trial reported FPG after 20 years of follow‐up (Da Qing 1997). The mean FPG was 7.9 (SD 3.2) in 260 participants (149 participants when adjusting for clustering) in the combined former intervention groups compared with 8.7 (SD 3.1) in 80 participants (46 participants when adjusting for clustering) in the former control group (Da Qing 1997). Another trial reported FPG after 15 years of follow‐up. FPG was 6.8 mmol/L (SD 2.0) in 751 participants in the former diet plus physical activity group compared with 6.8 mmol/L (SD 1.9) in 780 participants in the former control group (DPP 2002).
Glucose two hours after an oral glucose load
Nine trials reported glucose values two hours after an oral glucose load (Da Qing 1997; DPP 2002; DPS 2001; EDIPS 2009; IDPP 2006; JDPP 2013; Oldroyd 2005; PODOSA 2014; SLIM 2003). Two hours after an oral glucose load glucose values were higher in the control group compared with the diet plus physical activity group (random MD ‐0.46 mmol/L, 95% CI ‐0.79 to ‐0.12; P = 0.008; fixed MD ‐0.29 mmol/L, 95% CI ‐0.44 to ‐0.15; P < 0.00001; 9 trials; 3261 participants; Analysis 2.24). The 95% prediction interval ranged between ‐1.41 mmol/L to 0.49 mmol/L.
2.24. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 24 2h plasma glucose.
TSA showed that diversity‐adjusted information size was accrued to detect or reject a difference in two‐hour plasma glucose at ‐0.46 mmol/L in favour of the diet plus physical activity group. Diversity was 82%.
Subgroup analyses: according to duration of the intervention ((≥ 4 years) versus trials with short duration (< 4 years)), ethnicity (Asian versus predominantly white); morbidity (BMI ≥ 30 kg/m2 versus < 30 kg/m2); showed no statistically significant interactions (Appendix 18). The subgroups of trials according to age (≥ 50 years versus < 50 years) showed significant interaction between subgroups (P = 0.003). However, the CIs in these analyses overlap to a small degree. As a caveat, these observations should be regarded as hypothesis‐generating only. We could not perform subgroup analyses according to sex, diagnostic criteria of participants with IGT versus other and previous gestational diabetes due to lack of data.
Sensitivity analysis: we could not perform sensitivity analysis according to publication status (all included trials were published) and language of publication (all included trials were published in English). Sensitivity analysis restricted to trials with low risk of selection bias showed a MD of ‐0.14 mmol/L (95% CI ‐0.26 to ‐0.01; P = 0.03) (DPP 2002; DPS 2001; EDIPS 2009; JDPP 2013; Oldroyd 2005; PODOSA 2014). Analysing trials performed in Asia showed a MD of ‐1.10 mmol/L (95% CI ‐2.3 to 0.11; P = 0.07) (Da Qing 1997; IDPP 2006; JDPP 2013). Trials performed in Europe showed a MD of ‐0.35 mmol/L (95% CI ‐0.78 to 0.08; P = 0.11) (DPS 2001; EDIPS 2009; Oldroyd 2005; PODOSA 2014).
One trial reported glucose values two hours after an OGTT after 20 years of follow‐up (Da Qing 1997). The mean two‐hour glucose value was 11.5 mmol/L (SD 5.0) in 100 participants (57 participants when adjusting for clustering) in the combined former intervention groups compared with 13.8 mmol/L (SD 5.8) in 28 participants (16 participants when adjusting for clustering) in the former control group (Da Qing 1997).
HbA1c
Four trials reported data on HbA1c (DPP 2002; DPS 2001; EDIPS 2009; SLIM 2003).
In the random‐effects model the MD was ‐0.11% (95% CI ‐0.23 to 0.02; P = 0.09; 4 trials; 2453 participants) (Analysis 2.31), in the fixed‐effect model the MD was ‐0.18%, 95% CI ‐0.23 to ‐0.13; P < 0.00001). The 95% prediction interval ranged between ‐1.64% and 1.42%.
2.31. Analysis.
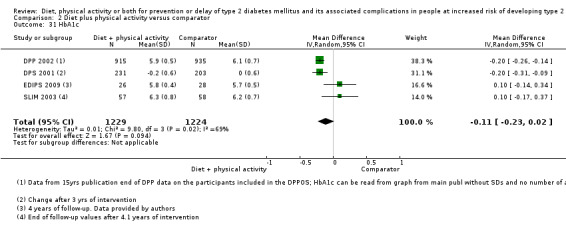
Comparison 2 Diet plus physical activity versus comparator, Outcome 31 HbA1c.
TSA showed that 36.5% diversity‐adjusted information size was accrued to detect or reject a HbA1c difference of ‐0.11% between the intervention groups. Alfa‐spending‐adjusted 95% CI was ‐0.34 to 0.12. Diversity was 86%.
Subgroup analysis: analysing trials according to the duration of the intervention (≥ 4 years) versus trials with short duration (< 4 years) showed no interaction between subgroups (P = 0.99) (Analysis 2.34; Appendix 18). Analysing trials according to the BMI ≥ 30 kg/m2 versus < 30 kg/m2 showed no interaction between subgroups (P = 0.10) (Analysis 2.35). All trials reporting HbA1c included people with IGT, all included participants aged 50 years or more, and all included only white or mainly white people (DPP 2002; DPS 2001; EDIPS 2009; SLIM 2003).
2.34. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 34 HbA1c: duration of the intervention.
2.35. Analysis.
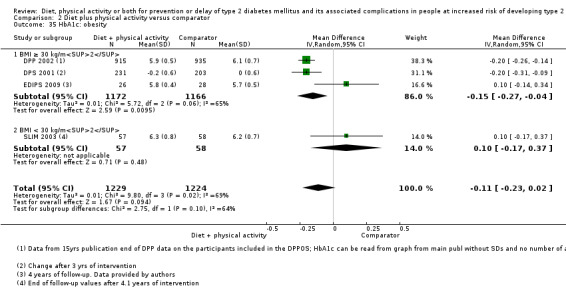
Comparison 2 Diet plus physical activity versus comparator, Outcome 35 HbA1c: obesity.
Sensitivity analysis: we could not perform sensitivity analysis according to publication status (all included trials were published) and language of publication (all included trials were published in English). Sensitivity analysis restricted to only trials with low risk of selection bias showed MD ‐0.15%, 95% CI ‐0.27 to ‐0.04; P = 0.009 (DPP 2002; DPS 2001; EDIPS 2009). Analysing trials according to geographies in trials performed in Europe showed a MD of ‐0.02%, 95% CI ‐0.26 to 0.21; P = 0.84 (DPS 2001; EDIPS 2009; SLIM 2003).
One trial reported the HbA1c after 20 years of follow‐up (Da Qing 1997). The mean HbA1c was 7.34% (SD 1.7) in 271 participants (155 participants when adjusting for clustering) in the combined former intervention group compared with 7.83% (SD 2.0) in 81 participants (46 participants when adjusting for clustering) in the former control group (Da Qing 1997). Another trial reported the HbA1c after 15 years of follow‐up. The mean HbA1c was 6.2% (SD 1.2) in 751 participants in the former diet plus physical activity group compared with 6.3% (SD 1.2) in 780 participants in the former control group (DPP 2002).
Socioeconomic effects
During DPP 2002, the diet plus physical activity groups were substantially more expensive than the standard treatment intervention. Direct medical costs of the interventions during the DPP were estimated to be USD 3628 for the diet plus physical activity group versus USD 184 for the control group. Direct medical costs (hospital days, emergency room visits, urgent care visits, outpatient visits, calls to providers, supplies, laboratory tests, and prescription medications within the intervention groups) outside the DPP were estimated to be USD 5182 for the diet plus physical activity group compared with USD 5680 for the control group. However, due to the high direct medical costs of T2DM, the diet plus physical activity intervention was estimated to be cost‐effective. The quality of evidence was low. From the perspective of the health system (direct medical costs of the interventions plus direct medical costs of care outside the trial) the cost was USD 31,500 per quality‐adjusted life years (QALY) gained with diet plus physical activity compared with control. From the perspective of society (direct medical costs plus non medical costs (expenditure on medical treatment but not involving purchase of medical services or products) plus indirect costs (costs to society due to morbidity and mortality, e.g. absent from work due to medical treatment)) the cost was USD 51,600 per QALY gained with diet plus physical activity compared with control.
PODOSA 2014 reported that the extra mean cost for the diet plus physical activity group was GBP 1126 (95% CI –2414 to 4666) after three years, with GBP 615 of the difference being dietitian costs. Primary‐care visits and costs did not differ between groups, but there were more outpatient visits in the intervention group (costing GBP 327 more than in the control group).
IDPP 2006 estimated direct medical costs of interventions over the three‐year trial period to be USD 61 per participant in the control group compared with USD 225 in the diet plus physical activity group. The cost‐effectiveness to prevent one case of diabetes with diet plus physical activity was USD 1052.
In HELP PD 2011, direct medical costs for each participant in the diet plus physical activity group were USD 850 compared with USD 142 in the control group. Direct costs of care outside the trial were USD 5177 for the diet plus physical activity group compared with USD 7454 for the control group.
The overall quality of evidence was low for this outcome measure.
Discussion
Summary of main results
This Cochrane Review investigated the effects of diet or physical activity, or both in people at increased risk of developingT2DM. We included 12 trials with a total of 5238 participants. We judged all trials as at unclear or high risk of bias in one or more 'Risk of bias' domains. The amount of evidence on patient‐important outcomes was limited. The meta‐analysis comparing diet plus physical activity with standard or no treatment showed moderate‐quality evidence of a reduced incidence of T2DM after the end of the intervention. The diversity‐adjusted required information size of TSA to confirm a 10% RRR was reached. The reporting on mortality and macrovascular as well as microvascular diabetes complications was insufficient and we judged the quality of evidence for these outcome measures as low or very low.
Overall completeness and applicability of evidence
The diagnosis of intermediate hyperglycaemia varied among trials and some trials used a definition that may have included participants judged to be euglycaemic or having T2DM. However, all trials except one had IGT as an inclusion criterion (HELP PD 2011). The results of this review might therefore not be applicable to people defined by other glycaemic categories of intermediate hyperglycaemia.
Detailed information about the participants was lacking in most trials. The included trials applied different intensities of diet and physical activity. In our review, all programmes were conducted in adults. Therefore, our results may not apply to children and adolescents. A potential selection bias exists as more healthy and motivated people may participate in a clinical trial. However, a Cochrane Review observed that clinical outcomes in people participating in RCTs are comparable to similar individuals outside trials (Vist 2008). However, the implementation of diet and physical activity programmes outside a clinical trial often shows less pronounced effects in surrogate markers (e.g. glycaemic measures) (Ashra 2015).
Three of the included trials contributed about 70% of all data (Da Qing 1997; DPP 2002; DPS 2001). Reporting of complications associated with T2DM during the intervention period was lacking. However, after 23 years of follow‐up, all‐cause mortality and cardiovascular mortality were reduced in one study (Da Qing 1997). This effect was only found in women. However, the trial found a delay in the onset of T2DM of 3.6 years (Da Qing 1997). The only data on microvascular complications were available after an extended follow‐up period. There was discrepancy in the long‐term effects of the diet plus physical activity intervention in the DPP 2002 and the Da Qing 1997 follow‐up. Da Qing 1997 reported a reduction of severe retinopathy after 20 years of follow‐up in the former intervention group (HR 0.53, 95% CI 0.29 to 0.99; P = 0.048). In DPP 2002 there was no influence on microvascular outcomes after 15 years of follow‐up.
The three major trials all included people with IGT. Unfortunately, similar trials have not been performed in people with IFG or moderately elevated HbA1c. One of the excluded trials included 379 overweight Japanese people with IFG only. However, the intervention group and the control group received similar diet and physical activity advice, but with less frequent visits in the control group, and was consequently not included in this review (Saito 2011). The trial did not find an impact on the risk of T2DM in participants with isolated IFG after 36 months of intervention (HR 1.17, 95% CI 0.50 to 2.74) (Saito 2011). However, because of the technical complexities of performing an OGTT compared with measuring fasting glucose or HbA1c, most people with intermediate hyperglycaemia are expected to be diagnosed with these modalities (International Expert Committee 2009). It is therefore unclear whether similar effects in reducing or delaying T2DM incidence will be found in the majority of people with intermediate hyperglycaemia.
The number of participants diagnosed with T2DM in the control groups in the included trials was higher than that estimated from observational studies (Cheng 2006; Morris 2013). This might be explained by the regular glycaemic testing of people participating in a RCT. Therefore, many of those diagnosed with T2DM in a RCT may not be diagnosed in a 'real‐world' setting.
Most trials included participants aged above 50 years. In both DPP 2002 and DPS 2001, diet plus physical activity was most effective in reducing T2DM incidence in people aged 60 years or more. A person aged 65 years or more with newly diagnosed T2DM with a HbA1c of 7% has a theoretical life‐time risk of blindness or end‐stage renal disease of less than 0.5% (Vijan 1997). The reason for the lack of reliable data on diabetic complications T2DM in our review might be explained by the low rate of these complications in the people with the largest intervention effect and the time it takes to develop these complications (Vijan 1997).
In all trials reporting the direct costs of the diet and physical activity programmes, the intervention was significantly more expensive than control (DPP 2002; EDIPS 2009; HELP PD 2011; IDPP 2006). However, the diet plus physical activity intervention programmes were estimated to be cost‐effective due to the reduction of T2DM with these programmes (DPP 2002). On the other hand, the interventions in the included trials would be challenging and costly to implement in daily life. In general, the adherence to diet and lifestyle advice was low in the included trials, and might even be lower in a non‐trial setting. A more cost‐effective and long‐lasting solution could be to enable people to undertake physical activity as part of their everyday life, and regulation of food costs.
Not only people with intermediate hyperglycaemia but also people with manifest T2DM are recommended to increase physical activity and lower calorie intake (ADA 2017). However, the only long‐term RCT assessing the effects of diet plus physical activity in people with T2DM was stopped early due to futility after a median follow‐up of 9.6 years (Look AHEAD 2013). In addition, long‐term follow‐up data for DPP 2002 have not shown any substantial effect on mortality or diabetes‐related complications after 15 years of follow‐up, even though the incidence of T2DM was reduced with the diet plus physical activity intervention.
Quality of the evidence
For all trials, we contacted one or more trial authors to obtain supplemental information on baseline data, bias domains and outcomes. In addition, we asked investigators to confirm our extracted outcomes. Unfortunately only four investigators (33%) of the included trials either just confirmed a question or provided additional data that could be implemented for the 'Risk of bias' assessment or the meta‐analyses of outcomes (EDIPS 2009; Hellgren 2016; IDPP 2006; PODOSA 2014).
We included trials with an intervention duration of two years or more. Trials with shorter duration could have been included, but as we were focusing on patient‐important outcomes we did not include such short‐term studies (D‐CLIP; DH!AAN; Hesselink 2013; Huang 2007; J‐DOIT; Kawahara 2008; Kinmonth 2008; Lindahl 1999; Marrero 2016; Page 1992; Ramachandran 2013; Sathish 2017; Savoye 2007; Thompson 2008; Villareal 2006; Yates 2011).
None of the 11 included trials in our review was classified as having low risk of bias in all 'Risk of bias' domains. The description of randomisation and allocation was insufficient in 33% of the included trials (Da Qing 1997; IDPP 2006; Kosaka 2005; SLIM 2003). Only one trial was classified as having low risk of bias for selective outcome reporting (Hellgren 2016). The remaining trials had insufficient reporting of one or more outcomes of relevance to our review. However, we were able to assess one or more of our predefined outcomes in all of the included trials.
For the comparisons 'diet only versus comparator' and 'physical activity only versus comparator', we judged the quality of evidence to be very low because of sparse data and various risks of bias. For the comparison 'diet plus physical activity' more data were available. However, again most outcome measures were associated with low‐ or very low‐quality evidence.
Potential biases in the review process
We were unable to draw funnel plots to assess small‐study bias due to lack of data for most outcomes. However, for one of our primary outcomes ‐ the incidence of T2DM ‐ we were able to draw a funnel plot, which did not indicate publication bias. If more data had been available on the patient‐important outcomes of our review, we would have performed more meta‐analyses. Many of the included trials were not designed or powered to detect our predefined patient‐important outcomes. For the performed meta‐analyses we investigated heterogeneity and the potential reasons for it through subgroup and sensitivity analyses. We were dealing with a substantially heterogeneous group of trials. Our meta‐analyses were limited by the inability to use individual participant data to assess whether distinct clinical characteristics may have influenced the effect estimates of the interventions. To reduce the risk of random errors, we conducted TSA on all predefined outcomes and calculated prediction intervals, whenever possible. We contacted all trial authors for clarification if one of the bias domains was not adequately reported. Several trials were published in more than one publication, which for some trials made it difficult to separate the primary publication from companion papers. We excluded trials including participants with IGT due to other conditions (e.g. cystic fibrosis or glucocorticoid treatment). We included trials with a minimum duration of two years in order to detect clinically relevant differences for the predefined outcomes. Even though we focused on long‐term trials, the reporting of clinical outcomes in the included trials was poor. Two review authors carried out data extraction. However, the review authors extracting the data were not blinded as to which trial they were extracting data from.
Agreements and disagreements with other studies or reviews
We conducted an extensive search for trials, including publications in all languages and tried to obtain additional data on all trials. Investigators of three trials provided additional information (EDIPS 2009; Hellgren 2016; PODOSA 2014). We looked for additional trials and cross‐checked our data with other meta‐analyses and Cochrane Reviews of relevance (Aguiar 2014; Ali 2012; Ashra 2015; Balk 2015; Cardona‐Morrell 2010; Dunkley 2014; Gillett 2012; Gillies 2007; Glechner 2015; Gong 2015; Hopper 2011; ICER 2016; Merlotti 2014a; Merlotti 2014b; Modesti 2016; Norris 2005; Santaguida 2005; Schellenberg 2013; Selph 2015; Stevens 2015; Yamaoka 2005; Yates 2007; Yoon 2013; Yuen 2010; Zhang 2017; Zheng 2016). However, several publications defined the increased risk of T2DM development to be associated with additional covariates, with intermediate hyperglycaemia being only one risk factor (e.g. obesity, metabolic risk factors, family history of diabetes ‐ Ali 2012; Ashra 2015; Balk 2015; Cardona‐Morrell 2010; Dunkley 2014; Merlotti 2014a; Merlotti 2014b; Modesti 2016; Schellenberg 2013; Zhang 2017). Furthermore, these systematic reviews excluded trials with the same intensity in diet and physical activity, where the only difference between the intervention arms was the approach on how to motivate the participants. We excluded these trials (e.g. evaluating mobile text messages versus individual sessions, or one visit a year versus four visits a year) because this setting addresses another research question, and it is important to clarify whether the interventions work as such. Also, our review is the first focusing on patient‐important outcomes. Therefore, we only included trials with an intervention duration of two years or more. Even though we focused on longer‐term trials, the reporting on patient‐important outcomes was still lacking. Observational data of trials with extended follow‐up periods could also not prove long‐term beneficial effects regarding patient‐important outcomes (DPP 2002; DPS 2001). The only trial reporting long‐term benefits was Da Qing 1997, however, few participants were included in the analyses. In addition, these observational extension periods of interventional trials need to be interpreted with caution because the long‐term cohort may not be comparable with the originally randomised participants.
Authors' conclusions
Implications for practice.
There is moderate‐quality evidence that diet plus physical activity reduces or delays the risk of type 2 diabetes mellitus in people with impaired glucose tolerance. Whether there is the same intervention effect in people with increased risk defined by other glycaemic variables, such as impaired fasting glucose or elevated glycosylated haemoglobin A1c (HbA1c) levels, needs to be clarified. There is no clear evidence whether diet alone or physical activity alone influences the risk of type 2 diabetes mellitus. Data on patient‐important outcomes such as mortality, macrovascular and microvascular diabetic complications and health‐related quality of life are sparse.
Implications for research.
It remains to be clarified whether the reduction in the incidence of type 2 diabetes mellitus with diet plus physical activity in people with impaired glucose tolerance could decrease the long‐term risk of complications associated with type 2 diabetes mellitus. Future trials should also investigate the effect of diet plus physical activity in people with impaired fasting glucose or moderately elevated HbA1c and focus on patient‐important outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 21 November 2017 | New citation required and conclusions have changed | Update: diet plus physical activity reduces the risk of type 2 diabetes mellitus in people with impaired glucose tolerance. Data are lacking for the effect of diet plus physical activity for people with intermediate hyperglycaemia defined by other glycaemic variables |
| 21 November 2017 | New search has been performed | This review is an update of the review published in issue 3, 2008. Six trials of the original review were used for our review update. Also, we found six additional new trials and therefore established a database of 12 included trials. |
Notes
We have based parts of the Methods and Appendix 1 sections of this Cochrane Protocol on a standard template established by the CMED Group.
Acknowledgements
We thank Erika Ota for evaluating the Japanese reference of the Japan Diabetes Prevention Program (JDPP 2013). We thank Anne Douglas for confirming and providing data on the PODOSA 2014 trial, Linda Penn for confirming and providing data for the EDIPS 2009 trial and Margaretha Hellgren for confirming and providing data for the Hellgren 2016 trial.
Appendices
Appendix 1. Search strategies
| Search strategy overview |
| 1. Population block (prediabetes, diabetes risk, diagnostic criteria (IFG, IGT, HbA1c)) AND 2. Intervention (exercise, diet, lifestyle) AND 3. Outcomes (diabetes complications, micro/macro vascular, mortality, diabetes incidence) AND 4. RCTs (in MEDLINE additionally filter for systematic reviews and meta‐analyses) |
| Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Register of Studies Online) |
| 1. MESH DESCRIPTOR Prediabetic state 2. MESH DESCRIPTOR Glucose Intolerance 3. (prediabet* or pre diabet*):TI,AB,KY 4. (intermediate hyperglyc?emi*):TI,AB,KY 5. ((impaired fasting ADJ2 glucose) or IFG or impaired FPG):TI,AB,KY 6. glucose intolerance:TI,AB,KY 7. ((impaired glucose ADJ (tolerance or metabolism)) or IGT):TI,AB,KY 8. ("HbA(1c)" or HbA1 or HbA1c or "HbA 1c" or ((glycosylated or glycated) ADJ h?emoglobin)):TI,AB,KY 9. (risk ADJ3 ("type 2" or "type II" or diabetes or T2D* or NIDDM)):TI,AB,KY 10. MESH DESCRIPTOR Diabetes mellitus WITH QUALIFIERS PC 11. MESH DESCRIPTOR Diabetes mellitus, Type 2 WITH QUALIFIERS PC 12. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 13. MESH DESCRIPTOR Life Style 14. MESH DESCRIPTOR Exercise EXPLODE ALL TREES 15. MESH DESCRIPTOR Exercise Therapy EXPLODE ALL TREES 16. MESH DESCRIPTOR Diet EXPLODE ALL TREES 17. MESH DESCRIPTOR Diet Therapy EXPLODE ALL TREES 18. ((lifestyle or life style) ADJ3 (intervention? or change* or modif* or program or programme)):TI,AB,KY 19. diet*:TI,AB,KY 20. (nutrition* ADJ3 (intervention? or change* or modif* or program or programme)):TI,AB,KY 21. exercis*:TI,AB,KY 22. physical activit*:TI,AB,KY 23. resistance training:TI,AB,KY 24. #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 25. #12 AND #24 26. (diabetes prevention ADJ (program* or stud* or trial?)):TI,AB,KY 27. #25 OR #26 28. complication?:TI,AB,KY 29. mortality:TI,AB,KY 30. (CHD or CVD):TI,AB,KY 31. (coronary ADJ2 disease):TI,AB,KY 32. (coronar* ADJ (event? or syndrome?)):TI,AB,KY 33. (heart ADJ (failure or disease? or attack? or infarct*)):TI,AB,KY 34. (myocardial ADJ (infarct* or isch?emi*)):TI,AB,KY 35. cardiac failure:TI,AB,KY 36. angina:TI,AB,KY 37. revasculari*:TI,AB,KY 38. (stroke or strokes):TI,AB,KY 39. cerebrovascular:TI,AB,KY 40. ((brain* or cerebr*) ADJ (infarct* or isch?emi*)):TI,AB,KY 41. apoplexy:TI,AB,KY 42. ((vascular or peripheral arter*) ADJ disease?):TI,AB,KY 43. cardiovascular:TI,AB,KY 44. (neuropath* or polyneuropath*):TI,AB,KY 45. (retinopath* or maculopath*):TI,AB,KY 46. (nephropath* or nephrotic or proteinuri* or albuminuri*):TI,AB,KY 47. ((kidney or renal) ADJ (disease? or failure or transplant*)):TI,AB,KY 48. ((chronic or endstage or end stage) ADJ (renal or kidney)):TI,AB,KY 49. (CRD or CRF or CKF or CRF or CKD or ESKD or ESKF or ESRD or ESRF):TI,AB,KY 50. (microvascular or macrovascular or ((micro or macro) ADJ vascular)):TI,AB,KY 51. (cancer or carcino* or neoplas* or tumo?r?):TI,AB,KY 52. (amputation? or ulcer* or foot or feet or wound*):TI,AB,KY 53. ((risk or progress* or prevent* or inciden* or conversion or develop* or delay*) ADJ4 (diabetes or T2D* or NIDDM or "type 2" or "type II")):TI,AB,KY 54. #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 55. #27 AND #54 56. (2014 OR 2015 OR 2016 OR 2017):PD 57. #55 AND #56 |
| MEDLINE (Ovid SP) |
| 1. Prediabetic state/ 2. Glucose Intolerance/ 3. (prediabet* or pre diabet*).tw. 4. intermediate hyperglyc?emi*.tw. 5. ((impaired fasting adj2 glucose) or IFG or impaired FPG).tw. 6. glucose intolerance.tw. 7. ((impaired glucose adj (tolerance or metabolism)) or IGT).tw. 8. ("HbA(1c)" or HbA1 or HbA1c or "HbA 1c" or ((glycosylated or glycated) adj h?emoglobin)).tw. 9. (risk adj3 ("type 2" or "type II" or diabetes or T2D* or NIDDM)).tw. 10. *Diabetes mellitus/pc 11. *Diabetes mellitus, Type 2/pc 12. or/1‐11 13. Life Style/ 14. exp Exercise/ 15. exp Exercise Therapy/ 16. exp Diet/ 17. exp Diet Therapy/ 18. ((lifestyle or life style) adj3 (intervention? or change* or modif* or program or programme)).tw. 19. diet*.tw. 20. (nutrition* adj3 (intervention? or change* or modif* or program or programme)).tw. 21. exercis*.tw. 22. physical activit*.tw. 23. resistance training.tw. 24. or/13‐23 25. 12 and 24 26. (diabetes prevention adj (program* or stud* or trial?)).tw. 27. 25 or 26 28. complication?.tw. 29. mortality.tw. 30. (CHD or CVD).tw. 31. (coronary adj2 disease).tw. 32. (coronar* adj (event? or syndrome?)).tw. 33. (heart adj (failure or disease? or attack? or infarct*)).tw. 34. (myocardial adj (infarct* or isch?emi*)).tw. 35. cardiac failure.tw. 36. angina.tw. 37. revasculari*.tw. 38. (stroke or strokes).tw. 39. cerebrovascular.tw. 40. ((brain* or cerebr*) adj (infarct* or isch?emi*)).tw. 41. apoplexy.tw. 42. ((vascular or peripheral arter*) adj disease?).tw. 43. cardiovascular.tw. 44. (neuropath* or polyneuropath*).tw. 45. (retinopath* or maculopath*).tw. 46. (nephropath* or nephrotic or proteinuri* or albuminuri*).tw. 47. ((kidney or renal) adj (disease? or failure or transplant*)).tw. 48. ((chronic or endstage or end stage) adj (renal or kidney)).tw. 49. (CRD or CRF or CKF or CRF or CKD or ESKD or ESKF or ESRD or ESRF).tw. 50. (microvascular or macrovascular or ((micro or macro) adj vascular)).tw. 51. (cancer or carcino* or neoplas* or tumo?r?).tw. 52. (amputation? or ulcer* or foot or feet or wound*).tw. 53. ((risk or progress* or prevent* or inciden* or conversion or develop* or delay*) adj4 (diabetes or T2D* or NIDDM or "type 2" or "type II")).tw. 54. or/28‐53 55. 27 and 54 [56‐65: Cochrane Handbook 2008 RCT filter – sens/prec version] 56. randomized controlled trial.pt. 57. controlled clinical trial.pt. 58. randomi?ed.ab. 59. placebo.ab. 60. clinical trials as topic/ 61. randomly.ab. 62. trial.ti. 63. or/56‐62 64. exp animals/ not humans/ 65. 63 not 64 66. 55 and 65 [67: Wong 2006a – systematic reviews filter – spec version] 67. cochrane database of systematic reviews.jn. or search*.tw. or meta analysis.pt. or medline.tw. or systematic review.tw. 68. 55 and 67 69. 66 or 68 70. (2014* or 2015* or 2016* or 2017*).dc. 71. 69 and 70 72. remove duplicates from 71 |
| Embase (Ovid SP) |
| 1. (prediabet* or pre diabet*).tw. 2. intermediate hyperglyc?emi*.tw. 3. ((impaired fasting adj2 glucose) or IFG or impaired FPG).tw. 4. glucose intolerance.tw. 5. ((impaired glucose adj (tolerance or metabolism)) or IGT).tw. 6. ("HbA(1c)" or HbA1 or HbA1c or "HbA 1c" or ((glycosylated or glycated) adj h?emoglobin)).tw. 7. (risk adj3 ("type 2" or "type II" or diabetes or T2D* or NIDDM)).tw. 8. or/1‐7 9. ((lifestyle or life style) adj3 (intervention? or change* or modif* or program or programme)).tw. 10. diet*.tw. 11. (nutrition* adj3 (intervention? or change* or modif* or program or programme)).tw. 12. exercis*.tw. 13. physical activit*.tw. 14. resistance training.tw. 15. or/9‐14 16. 8 and 15 17. (diabetes prevention adj (program* or stud* or trial?)).tw. 18. 16 or 17 19. complication?.tw. 20. mortality.tw. 21. (CHD or CVD).tw. 22. (coronary adj2 disease).tw. 23. (coronar* adj (event? or syndrome?)).tw. 24. (heart adj (failure or disease? or attack? or infarct*)).tw. 25. (myocardial adj (infarct* or isch?emi*)).tw. 26. cardiac failure.tw. 27. angina.tw. 28. revasculari*.tw. 29. (stroke or strokes).tw. 30. cerebrovascular.tw. 31. ((brain* or cerebr*) adj (infarct* or isch?emi*)).tw. 32. apoplexy.tw. 33. ((vascular or peripheral arter*) adj disease?).tw. 34. cardiovascular.tw. 35. (neuropath* or polyneuropath*).tw. 36. (retinopath* or maculopath*).tw. 37. (nephropath* or nephrotic or proteinuri* or albuminuri*).tw. 38. ((kidney or renal) adj (disease? or failure or transplant*)).tw. 39. ((chronic or endstage or end stage) adj (renal or kidney)).tw. 40. (CRD or CRF or CKF or CRF or CKD or ESKD or ESKF or ESRD or ESRF).tw. 41. (microvascular or macrovascular or ((micro or macro) adj vascular)).tw. 42. (cancer or carcino* or neoplas* or tumo?r?).tw. 43. (amputation? or ulcer* or foot or feet or wound*).tw. 44. ((risk or progress* or prevent* or inciden* or conversion or develop* or delay*) adj4 (diabetes or T2D* or NIDDM or "type 2" or "type II")).tw. 45. or/19‐44 46. 18 and 45 [47: Wong 2006b "sound treatment studies" filter ‐ SDSSGS version] 47. random*.tw. or clinical trial*.mp. or exp treatment outcome/ 48. 46 and 47 [49‐52: TSC portal filter for exclusion of animal references] 49. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 50. human/ or normal human/ or human cell/ 51. 49 and 50 52. 49 not 51 53. 48 not 52 54. conference.pt. 55. 53 not 54 56. limit 55 to embase 57. remove duplicates from 56 58. (2014* or 2015* or 2016* or 2017*).dc. 59. 57 and 58 |
| ICTRP Search Portal (Standard search) |
| prediabet* AND lifestyle OR prediabet* AND style OR prediabet* AND exercis* OR prediabet* AND activity OR prediabet* AND diet* OR diabet* AND prevent* AND lifestyle OR diabet* AND prevent* AND style OR diabet* AND prevent* AND exercis* OR diabet* AND prevent* AND activity OR diabet* AND prevent* AND diet* OR diabet* AND incidence AND lifestyle OR diabet* AND incidence AND style OR diabet* AND incidence AND exercis* OR diabet* AND incidence AND activity OR diabet* AND incidence AND diet* |
| ClinicalTrials.gov (Expert search) |
| EXACT "Interventional" [STUDY‐TYPES] AND ( prediabetes OR prediabetic OR "pre diabetes" OR "pre diabetic" OR hyperglycemia OR hyperglycaemia OR hyperglycemic OR hyperglycaemic OR "impaired glucose tolerance" OR "impaired fasting glucose" OR "glucose intolerance" OR IGT OR IFG OR "HbA(1c)" OR HbA1 OR HbA1c OR "HbA 1c" or glycosylated hemoglobin OR glycosylated haemoglobin OR glycated hemoglobin OR glycated haemoglobin OR "risk for diabetes" OR "risk of diabetes" OR "risk for type 2" OR "risk for type II" OR "risk of type 2" OR "risk of type II" ) [DISEASE] AND ( exercise OR exercises OR training OR lifestyle OR "life style" OR activity OR activities OR physical OR diet OR dietary OR diets OR nutrition OR nutritional OR "diabetes prevention" OR "diabetes mellitus prevention" OR "type 2 prevention" OR "type II prevention" ) [TREATMENT] AND ( complication OR complications OR mortality OR coronary OR heart OR myocardial OR infarct OR infarction OR infarcts OR infarctions OR ischemia OR ischemic OR ischaemia OR ischaemic OR failure OR angina OR revascularization OR revascularisation OR revascularizations OR revascularisations OR stroke OR strokes OR cerebrovascular OR apoplexy OR vascular or peripheral OR cardiovascular OR neuropathy OR neuropathies OR polyneuropathy OR polyneuropathies OR retinopathy OR retinopathies OR maculopathy OR maculopathies OR nephropathy OR nephropathies OR nephrotic OR proteinuria OR proteinuric OR albuminuria OR kidney OR renal OR microvascular OR macrovascular OR "micro vascular" OR "macro vascular" OR cancer OR carcinoma OR neoplasm OR neoplasms OR tumor OR tumors OR tumour OR tumours OR amputation OR amputations OR ulcer OR foot OR feet OR wounds OR ((diabetes OR "type 2" OR "type II" OR T2D OR T2DM) AND (risk OR progress OR progression OR progressed OR incident OR incidence OR conversion OR developed OR development OR develop OR delay OR delayed OR prevention OR prevent OR prevented)) ) [OUTCOME] |
Appendix 2. Description of interventions
| Trial ID | Intervention(s) | Intervention(s) appropriate as applied in a clinical practice settinga (description) | Comparator(s) | Comparator(s) appropriate as applied in a clinical practice settinga (description) |
| Da Qing 1997 |
I1: diet: participants with BMI < 25 kg/m2 were prescribed a diet containing 25‐30 kcal/kg body wt (105‐126 kj/kg), 55%‐65% carbohydrate, 10%‐15% protein, and 25%‐30% fat. These participants were encouraged to consume more vegetables, control their intake of alcohol, and reduce their intake of simple sugars.
Participants with BMI > 25 kg/m2 were encouraged to reduce their calorie intake so as to gradually lose weight at a rate of 0.5‐1.0 kg per month until they achieved a BMI of 23 kg/m2 Group/individual: both Medium: group Facilitator: physician Frequency: weekly for one month, monthly for three months, and then once every third month for the reminder of the six years' intervention period (30 visits) Extended FU period: no intervention provided. Examination after 20 years and 23 years of follow‐up |
Intensified diet and/or physical activity is an appropriate comparator |
Standard recommendation: received general information about diabetes and impaired glucose tolerance. Were provided brochures with general instructions for diet and physical activities. Group/individual: no formal group or individual counselling were performed Medium: group Facilitator: not specified Frequency: no formal sessions arranged Extended FU period: no intervention provided. Examination after 20 years and 23 years of follow‐up |
Standard diet and physical activity recommendation is an appropriate comparator |
|
I2: physical activity: participants in clinics assigned to the physical activity group were taught and encouraged to increase the amount of their leisure physical activity by at least 1 unit/day (mild‐moderate‐strenuous and very strenuous) and by 2 units/day if possible for those < 50 years of age with no evidence of cardiovascular disease or arthritis. Group/individual: both Medium: group Facilitator: not specified Frequency: weekly for one month, monthly for three months, and then once every third month for the reminder of the six years' intervention period (30 visits) Extended FU period: no intervention provided. Examination after 20 years and 23 years of follow‐up | ||||
|
I3: diet plus physical activity: diet as described for the diet group (I1) combined with physical activity (I2) Group/individual: both Medium: group Facilitator: physician Frequency: weekly for one month, monthly for three months, and then once every third month for the reminder of the six years' intervention period (30 visits) Extended FU period: no intervention provided. Examination after 20 years and 23 years of follow‐up | ||||
| DPP 2002 |
Diet plus physical activity Dietary intervention: healthy, low‐calorie, low‐fat diet Physical activity intervention: moderate intensity for at least 150 minutes per week The aim was to achieve and maintain a weight reduction of at least 7% of initial body weight through physical activity and diet. A 16‐lesson programme covering diet, physical activity, and behaviour modification was applied during the first 24 weeks after enrolment was flexible and individualised. Subsequent individual sessions (usually monthly) were designed to reinforce the behavioural changes. Group/individual: both Medium: in person Facilitator: case manager ("lifestyle coach"), usually a dietitian Frequency: 40 (16 lessons in first 24 weeks, then monthly) Extended FU period: lifestyle sessions were offered to all participants every 3rd month with educational material and reinforcement of original weight loss and physical activity goals. DPP lifestyle participants were also offered two group classes compromising four sessions every year in order to reinvigorate their self‐management behaviours for weight loss. Yearly visits |
Intensified diet and physical activity is an appropriate comparator |
Placebo + standard recommendation Standard diet intervention: culturally sensitive materials and motivational strategies Standard physical activity intervention: participants were encouraged to increase their activity gradually and to try to reach the goal of at least 30 minutes of physical activity (such as walking or biking) on 5 days each week Written information and annual 30 min individual session on healthy lifestyles. Changes in dietary and physical activity recommended for weight loss for overweight and obese participants. Group/individual: both (At the first visit, the staff will spend approximately 20 ‐ 30 minutes with each participant individually) Medium: in person Facilitator: staff (not described further) Frequency: annually (three times) Extended FU period: lifestyle sessions were offered to all participants every 3rd month with educational material and reinforcement of original weight loss and physical activity goals. Yearly visits |
Standard diet and physical activity recommendation is an appropriate comparator |
| DPS 2001 |
Diet plus physical activity Dietary intervention: baseline three‐day food record was completed before first appointment. The participants were advised to consume a diet with more than 50% of daily calories from carbohydrates; less than 10% from saturated fat and 20% from mono‐ and polyunsaturated fat, or up to 25% if the surplus is from monounsaturated fat; cholesterol less than 300 mg/day; and approximately 1.0 g protein per kg ideal body weight per day. The increase in the intake of dietary fibre to 15 g per 1000 kcal or more was encouraged. It was aimed to reduce the intake of saturated fat and participants are encouraged to use low‐fat milk and milk products, low‐fat meat and meat products, soft margarines and vegetable oil rich in monounsaturated fatty acids (primarily rapeseed oil). If weight loss was not achieved during the first 6‐12 months and the BMI was over 30 kg/m2, a very low‐calorie diet was considered. Physical activity intervention: guided to increase their physical activity. Exercise programmes differed among study centres according to local situation and facilities. Endurance exercise (walking, jogging, swimming, aerobic ball games, skiing) was recommended to increase aerobic capacity and cardiorespiratory fitness. Supervised, progressive, individually tailored circuit‐type resistance training sessions were organised, if possible, twice a week. The moderate intensity and medium‐ to high‐volume programmes were designed to improve the functional capacity and strength of the large muscle groups of the upper and lower body. The aim was a BMI of less than 25 kg/m2 but, in practice, a weight loss of 5 to 10 kg depending on degree of obesity was the target for many study participants. Group/individual: both (the person primarily in charge of preparing meals in the family, if different from the study participant, was also informed about the study aims and invited to join in the sessions with the nutritionist or the group meetings) Medium: in person Facilitator: nutritionist, physician Frequency: seven sessions with a clinical nutritionist during the first year of the study and then one session every 3 months. No of contacts: 15 Extended FU period: yearly visit by a nurse, no specific diet or physical activity counselling was provided |
Intensified diet and physical activity is an appropriate comparator |
Standard recommendation Standard diet intervention: adjust total energy intake in order to reduce BMI below 25 kg/m2 and to keep to a diet with less than 30% of daily energy from fat. Advised to reduce alcohol intake and to stop smoking as appropriate. The dietary advice is provided by verbal and written information. Standard physical activity intervention: verbal general information about the health effects of recreational physical activity was provided but no specific individual propositions and programmes were given Group/individual: both Medium: in person Facilitator: nutritionist Frequency: advice given initially and in annual follow‐up visits No of contacts: four Extended FU period: yearly visit by a nurse, no specific diet or physical activity counselling was provided |
Standard diet and physical activity recommendation is an appropriate comparator |
| EDIPS 2009 |
Diet plus physical activity both intervention and control groups were offered standard health promotion advice including widely available contemporary written leaflets on healthy eating and physical activity. Received quarterly newsletter Dietary intervention: advice and counselling to develop an individual plan for behaviour change, with the aim of achieving: > 50% total dietary energy intake from carbohydrate, reduced total and saturated fat intake with < 30% total dietary energy from fat, increased fibre intake, and weight loss to achieve BMI < 25 kg/m2; invited to cook and eat sessions. Analysis of participants' three‐day food diaries, collected quarterly, and regular weight and waist measurements were used to tailor individual dietary advice. Physical activity intervention: encourage participation in increased physical activity equivalent to accumulating 30 minutes of moderate aerobic physical activity per day. Analysis of participants' three‐day activity diaries, collected quarterly, was used in motivational feedback and to tailor goals for increasing physical activity, which were negotiated at each visit In addition to individual and group activities, participants received an information pack detailing facilities and opportunities for physical activity in Newcastle upon Tyne, a City Card (a discount scheme run by Newcastle Leisure Services offering up to 80% discount on access to physical activity facilities) and the opportunity to meet with a trainer at a local leisure centre and take part in an induction session. Group/individual: both Medium: in person Facilitator: dietician and physiotherapist trained in motivational interviewing Frequency: immediately following randomisation and two weeks later, then monthly for the first three months and every three months thereafter up to five years. No. of contacts: 17 |
Intensified diet and physical activity is an appropriate comparator |
Standard recommendation both intervention and control groups were offered standard health promotion advice including widely available contemporary written leaflets on healthy eating and physical activity. Control group participants were otherwise offered 'usual care' by their primary care physician. Group/individual: individual Medium: in person Facilitator: no personal involved in the trial (advised to contact primary care physician) Frequency: none No. of contacts: none |
Standard diet and physical activity recommendation is an appropriate comparator |
| Hellgren 2016 |
I1: physical activity (basic intervention) Information and brochures were distributed. In addition the participants were: 1. offered the possibility of cost‐free blood glucose assessments at the health care unit; 2. provided with a telephone number to a personal nurse for support (available 8:00 a.m. to 5:00 p.m., daily); 3. given a prescription for physical activity. The prescription was used as a referral to a physiotherapist, who gave individualised advice about physical activity. This routine was the same as in ordinary practice; 4. given a step‐counter. After two years, the participants received a letter with questions concerning physical activity and an offer of a renewal of the prescription for physical activity. Group/individual: individual Medium: in person Facilitator: physiotherapist, personal nurse Frequency: NS No. of contacts: NS I2: physical activity (intensive intervention) Information and brochures were distributed. In addition the participants were: 1. offered the possibility of cost‐free blood glucose assessments at the health care unit; 2. provided with a telephone number to a personal nurse for support (available 8:00 a.m. to 5:00 p.m., daily); 3. given a prescription for physical activity. The prescription was used as a referral to a physiotherapist, who gave individualised advice about physical activity. This routine was the same as in ordinary practice; 4. given a step‐counter. 5. invitation to participate in eight group sessions focusing on physical activity. After two years, the participants received a letter with questions concerning physical activity and an offer of a renewal of the prescription for physical activity. Group/individual: both Medium: in person Facilitator: lifestyle coach (nurse), a nutritionist and a physiotherapist Frequency: six sessions were held during the first six months and another two sessions during the following six months. During the second year, the participants were invited for two additional group sessions, at six‐month intervals. During the third year, the participants received a telephone call every third month, with a focus on general well‐being, a reminder to be physically active and an offer of a new prescription for physical activity. No. of contacts: 14 |
Intensified diet and physical activity is an appropriate comparator |
Standard recommendation Information about the metabolic condition (IGT or IFG, or both) was given orally and in writing. Brochures with information about recommended diet and physical activity were distributed. Group/individual: NS Medium: in person Facilitator: NS Frequency: baseline, then yearly No. of contacts: 3 |
Standard diet and physical activity recommendation is an appropriate comparator |
| HELP PD 2011 |
Diet plus physical activity The goal was weight loss 5% to 7% The intervention was divided into two phases First phase dietary intervention (month 1‐6): reduction of intake by 500‐1000 kcal per day; reduction in total fats to 25%‐30%, saturated fats to 7%, and protein to 15% of intake; increase in fruit and vegetable consumption to 5 servings per day; intake of ≥ 3 whole grain servings per day The first phase physical activity intervention (month 1‐6): gradual progression to 180 minutes of moderate intensity physical activity per week (e.g. 30 min/day of walking, 6 days/week) Second phase dietary intervention (month 7‐24): isocaloric intake tailored to maintenance of lost weight; maintenance of 25%‐30% energy intake from total fats, 7% from saturated fat, and 15% from protein; continued daily intake of 5 fruits and vegetables and ≥ 3 whole grain servings The second phase physical activity intervention (month 7‐24): maintenance of 180 min of moderate intensity physical activity per week; coping with injuries and other barriers to the maintenance of physical activity Group/individual: both Medium: in person Facilitator: community health workers, dietician Frequency: in the first intervention phase then 1 group session per week (for 6 months). In addition, all participants receive three personalised consultations with a dietician. During phase 2 (months 7‐24), participants had 2 scheduled contacts with the community health worker each month, one group session and one phone contact. No. of contacts: 46 |
Intensified diet and physical activity is an appropriate comparator |
Standard recommendation Information about healthy eating and activity to support weight loss, and discuss existing community resources that may fit the individual needs of comparison participants as they pursue dietary change, increased physical activity and weight loss. Group/individual: individual Medium: in person Facilitator: dietician Frequency: two individual sessions with a nutritionist during the first 3 months, In addition, comparison participants receive a quarterly newsletter with topics related to healthy lifestyle. No. of contacts: 6 |
Standard diet and physical activity recommendation is an appropriate comparator |
| IDPP 2006 |
Diet plus physical activity Dietary intervention: diet modification with reduction in total calories, refined carbohydrates and fats, avoidance of sugar and inclusion of fibre‐rich food Physical activity intervention: participants who were involved in physical labour or who had to walk or cycle for > 30 min/day or were performing physical activities regularly were asked to continue their routine activities. Participants engaged in sedentary or light physical activity were advised and regularly motivated to walk briskly for at least 30 min each day. The intervention was explained individually at the time of randomisation, then again by phone or letter after 2 weeks; thereafter monthly telephonic contacts were maintained for continued motivation. Personal sessions were conducted at 6‐monthly intervals. Group/individual: both Medium: in person Facilitator: physician dietician and social worker Frequency: every 6 months No. of contacts: 6 |
Intensified diet and physical activity is an appropriate comparator |
Standard recommendation standard health care advice Group/individual: both Medium: in person Facilitator: physician dietician and social worker Frequency: once a year No. of contacts: 3 |
Standard diet and physical activity recommendation is an appropriate comparator |
| JDPP 2013 |
Diet plus physical activity The goals of intervention were: 1) to reduce initial body weight by 5% in overweight and obese participants, and 2) to increase energy expenditure due to leisure time physical activity by 700 kcal per week. The interventions were carried out by the study nurse in each collaborative centre in the form of both group and individual sessions, using the guideline, curriculum, and educational materials provided by the committee of the study group. When needed, the study nurse could ask a part‐time dietician for diet counselling. A 27‐page booklet titled "Change Your Lifestyle to Prevent Diabetes" was given to each participant as a guide. Data on dietary intake and physical activities were assessed by the study group and the results were sent back to study nurses at each collaborative center. Dietary intervention: participants were advised to take the proper amount of calories, decrease the mean percent of energy derived from dietary fat to less than 25%, and restrict daily alcohol consumption to less than 160 kcal. They were also advised to eat three meals a day and avoid eating late at night. Physical activity intervention: personalised goals, such as a minimum of 20 minutes' moderate walking each day, were set. Group/individual: both Medium: in person Facilitator: public health nurses or dietician Frequency: during the initial six months, four group sessions were conducted. The individual sessions were conducted biannually during the three years with each session lasting 20‐40 minutes. To reinforce the intervention, between‐visit contact by fax was also made monthly during the initial twelve months. No. of contacts: 10 (excluding fax contact) |
Intensified diet and physical activity is an appropriate comparator |
Standard recommendation general verbal and written information on a healthy lifestyle and diabetes in one group session Group/individual: group Medium: in person Facilitator: public health nurses or dietician Frequency: one time No. of contacts: 1 |
Standard diet and physical activity recommendation is an appropriate comparator |
| Kosaka 2005 |
Diet plus physical activity Participants with a BMI ≥ 22 kg/m2 were advised to lose weight. participants with BMI less than 22 kg/m2 were advised to maintain their present weight. Family members were participating in the education of the participants. Dietary intervention: eating smaller meals (reduce amount about 10%), consume large amount of vegetables, reduce consumption of fat‐rich foods Physical activity intervention: physical activity: walking 30‐40 min/day; besides advice on how to increase physical activity during the day (e.g. taking staircase instead of an escalator etc.) Frequency: every 3‐4 months No. of contacts: 16 Group/individual: individual Medium: in person Facilitator: ‐ |
Intensified diet and physical activity is an appropriate comparator |
Standard recommendation Standard diet intervention: participants with a BMI ≥ 24 kg/m2 were advised to take 5%–10% smaller meals than they had been taking, and to increase their physical activity. They were encouraged to lose weight. Participants with a BMI < 24 kg/m2 were told to avoid gaining weight by dieting and physical activity. Standard physical activity intervention: participants with a BMI ≥ 24 kg/m2 were advised to increase physical activity. They were encouraged to lose weight. Participants with a BMI < 24 kg/m2 were told to avoid gaining weight by dieting and physical activity. Frequency: every 6 months No. of contacts: 8 Group/individual: individual Medium: in person Facilitator: ‐ |
Standard diet and physical activity recommendation is an appropriate comparator |
| Oldroyd 2005 |
Diet plus physical activity Dietary intervention: participants were encouraged to eat regular meals, eat more fruit and vegetables, reduce the fat content of foods, reduce sugar intake and eat adequate dietary fibre. The goal was to reduce BMI to < 25 kg/m2 in those who were overweight or obese, to achieve a dietary fat intake of 30% of total energy intake, a polyunsaturated to saturated fat ratio of 1.0, 50% of energy from carbohydrate and a dietary fibre intake of 20 g per 4.2 MJ. All participants in the intervention group were given written nutrition education material Physical activity intervention: a graded physical activity plan, tailored to the participant’s lifestyle and designed to enable them to achieve 20–30 min of aerobic activity at least once a week. The type of physical activity was tailored to the participant’s interests, lifestyle and physical abilities. physical activities such as walking, cycling, swimming, dancing and playing golf were encouraged. Information leaflets about physical activity facilities available in Newcastle were provided as appropriate. A CiTY CARD (a scheme offering up to 80% discount on use of all public leisure facilities in the city) was offered to all participants Frequency: first 6 months 3 appointments at 2‐weekly intervals, followed by 3 at monthly intervals. One after 9 months and 5 at 2‐monthly intervals between 12 and 24 months No. of contacts: 12 Group/Individual: individual Medium: in person Facilitator: dietitian and physiotherapist |
Intensified diet and physical activity is an appropriate comparator |
No intervention Participants in the control group were offered no dietary or physical activity advice for the duration of the study Frequency: none No. of contacts: none Group/Individual: NA Medium: NA Facilitator: none |
No intervention is an appropriate comparator |
| PODOSA 2014 |
Diet plus physical activity Weight loss goal of 2.5 kg more in the intervention than control group. Dietary intervention: weight loss through a calorie‐deficit diet (not specific in nutritions) using culturally adapted and translated resources Physical activity intervention: weight loss through physical activity of at least 30 min daily brisk walking, using culturally adapted and translated resources Frequency: baseline, monthly for the first 3 months, then every 3 months No. of contacts: 15 Group/Individual: both Medium: family Facilitator: dietician. In both the intervention and control groups family volunteers were asked to follow the advice given and to help the participants to follow it. |
Intensified diet and physical activity is an appropriate comparator |
Standard recommendation Standard dietary intervention: standard written and verbal advice on healthy eating Standard physical intervention: standard written and verbal advice on promotion of physical activity Frequency: baseline, then annually No. of contacts: 4 Group/Individual: both Medium: family Facilitator: dietician. In both the intervention and control groups family volunteers were asked to follow the advice given and to help the participants to follow it. |
Standard diet and physical activity recommendation is an appropriate comparator |
| SLIM 2003 |
I: Diet plus physical activity Dietary intervention: dietary recommendations were based on the Dutch guidelines for a healthy diet (about 55% energy from carbohydrates (maximum 15%‐25% energy mono‐ and disaccharides); 30%‐35% of energy from fat (≤ 10% energy saturated fatty acids; < 33 mg/MJ cholesterol, maximal 300 mg a day); 10%‐15% of energy from protein; Fibre more than 3 g/MJ a day. A bodyweight loss of 5%‐7% was the objective. If participants did not lose weight on this regimen during the first year, mild energy restriction was prescribed during the second year. No very‐low calorie diet or dietary products were used to encourage weight loss. Physical activity intervention: participants were encouraged to increase their level of physical activity to at least 30 min of moderate physical activity a day for at least 5 days a week. Individual advice was given on how to increase daily physical activity (walking, cycling, swimming), and goals were set. Furthermore, participants were encouraged to participate in a physical activity programme, especially designed for the trial, including components of aerobic training and components of resistance training. Participants had free access to these training sessions, and were stimulated to participate for at least 1 hour a week. Participation in the physical activity sessions was recorded. Frequency: The participants were seen at a baseline visit, after 4‐6 weeks and thereafter every third month. No. of contacts: 14 visits during the planned three years of intervention. However, the duration of the trial was extended, so there have probably been more visits even though not stated. After three months the participants were seen every third month. Group/Individual: both Medium: in person Facilitator: dietician |
Intensified diet and physical activity is an appropriate comparator |
C: Standard recommendation Participants in the control group were given, oral and written information, about the beneficial effects of a healthy diet, weight loss and increased physical activity, whereas no individual advice or programmes were provided. No additional appointments were scheduled apart from annual follow‐up visits Frequency: one (at baseline ‐ thereafter annual follow‐up visits with measurement of outcome variables, but no advice on diet or physical activity was given) No. of contacts: one Group/Individual: individual Medium: in person Facilitator: not reported |
Standard diet and physical activity recommendation is an appropriate comparator |
|
aThe term 'clinical practice setting' refers to the specification of the intervention/comparator as used in the course of a standard medical treatment (such as dose, dose escalation, dosing scheme, provision for contraindications and other important features) BMI: body mass index; C: comparator; FU: follow‐up; I: intervention; N/CPS: no specification of clinical practice setting possible | ||||
Appendix 3. Baseline characteristics (I)
| Trial ID | Intervention(s) and comparator(s) | Duration of intervention (duration of follow‐up) | Description of participants | Trial period (year to year) | Country | Setting | Ethnic groups (%) | Duration of being at risk for T2DM (mean years (SD)) |
| Da Qing 1997 | I1: diet | 6 years (6 years) | Impaired glucose tolerance, Asian | 1986‐1992: intervention period | Chinese community | Outpatient | Asian: 100 | ‐ |
| I2: physical activity | Asian: 100 | |||||||
| I3: diet plus physical activity | Asian: 100 | |||||||
| C: standard recommendation | Asian: 100 | |||||||
| DPP 2002 | I: diet plus physical activity | Mean 2.8 years (mean 15 years) | Impaired glucose tolerance and elevated fasting glucose Being overweight or obese |
1996‐1999 (recruitment period) July 2001 (end of treatment period) followed up in the DPP Outcomes Study (DPPOS 2002, to 2014) |
USA | Outpatient | White: 54 African American: 19 Hispanic: 17 American Indian: 6 Asian: 5 | ‐ |
| C: placebo + standard treatment | White: 54 African American: 20 Hispanic: 16 American Indian: 6 Asian: 5 | |||||||
| DPS 2001 | I: diet plus physical activity | Mean 3.2 years (3.2 years) | Impaired glucose tolerance Being overweight |
1993 to 1998 (recruitment period) The intervention period lasted until end of 2001 |
Finland | Outpatient | ‐ | ‐ |
| C: standard treatment | ||||||||
| EDIPS 2009 | I: diet plus physical activity | Mean 3.11 years (3.11 years) | Impaired glucose tolerance Being overweight |
2000 to 2003 (recruitment period) | UK | Outpatient | ‐ | ‐ |
| C: standard treatment | ||||||||
| Hellgren 2016 | I: physical activity | 3 years (3 years) | Impaired fasting glucose and/or impaired glucose tolerance | ‐ | Sweden | Outpatient | NR, assume nearly 100% were white | ‐ |
| C: standard treatment | ||||||||
| HELP PD 2011 | I: diet plus physical activity | 2 years (2 years) | Impared fasting glucose Being overweight |
Recruitment from 2007 to 2009. Data collection during 2007 to 2011. Analyses performed in 2011 to 2012 |
USA | Outpatient | African American: 25.8 White: 73.5 Other: 0.7 |
‐ |
| C: standard treatment | African American: 23.3 White: 74.0 Other: 2.7 |
|||||||
| IDPP 2006 | I: diet plus physical activity | 3 years (3 years) | Impaired glucose tolerance. Asian Indian | ‐ | India | Outpatient | Asian Indian: 100 | ‐ |
| C: standard treatment | Asian Indian: 100 | |||||||
| JDPP 2013 | I: diet plus physical activity | 3 years (6 years) | Impaired glucose tolerance | Recruitment started in March 1999 and was completed in December 2002 Follow‐up of participants started 1999 and the last completed 3 years' follow‐up in 2008 |
Japan | Outpatient | Asian Japanese: 100 |
‐ |
| C: standard treatment | Asian Japanese: 100 |
|||||||
| Kosaka 2005 | I: diet plus physical activity | 4 years (4 years) | Impaired glucose tolerance | ‐ | Japan | Outpatient | Asian Japanese: 100 | ‐ |
| C: standard recommendation | Asian Japanese: 100 | |||||||
| Oldroyd 2005 | I: diet plus physical activity | 24 months (24 months) | Impaired glucose tolerance | 1994 to 1998 | UK | Outpatient | White: 100 | ‐ |
| C: no intervention | White: 100 | |||||||
| PODOSA 2014 | I: diet plus physical activity | 3 years (3 years) | Impaired glucose tolerance or impaired fasting glucose | Recruitment 2006 to 2011 | UK | Outpatient | Indian: 34 Pakistani: 66 |
‐ |
| C: standard treatment | Indian: 33 Pakistani: 67 |
|||||||
| SLIM 2003 | I: diet plus physical activity | 4.1 years (4.1 years) | Impaired glucose tolerance | Recruitment from 1999 to 2000. In 2002 a second screening period was made. Trial was completed 2006 |
The Netherlands | Outpatient | White:100 | ‐ |
| C: standard treatment | White:100 | |||||||
| ‐ denotes not reported C: comparator; I: intervention; SD: standard deviation | ||||||||
Appendix 4. Baseline characteristics (II)
| Trial ID | Intervention(s) and comparator(s) | Indicator of increased risk: IFG (mean mmol/L (SD)) | Indicator of increased risk: 2h‐PPG (mean mmol/L (SD)) | Indicator of increased risk: elevated HbA1c (mean % (SD)) | Comorbidities | Comedications/Co‐interventions |
| Da Qing 1997 | I1: diet | 5.56 (0.81) | 9.03 (0.94) | ‐ | ‐ | ‐ |
| I2: physical activity | 5.56 (0.83) | 8.83 (0.79) | ‐ | ‐ | ‐ | |
| I3: diet plus physical activity | 5.67 (0.80) | 9.11 (0.93) | ‐ | ‐ | ‐ | |
| C: standard treatment | 5.52 (0.82) | 9.03 (0.89) | ‐ | ‐ | ‐ | |
| DPP 2002 | I: diet plus physical activity | 5.90 (0.45) | 9.13 (0.93) | 5.91 (0.51) | 16% of the women in both group had previously had gestational diabetes Overall 29.6% had a history of hypertension. 34% had a history of stroke. 16% had a history of revascularisation. 32% had a history of myocardial infarction |
17% in all treatment groups had antihypertensive treatment at baseline. 5.2% of participants reported taking pharmacologic therapy for dyslipidaemia at entry to the trial |
| C: placebo + standard treatment | 5.92 (0.47) | 9.13 (0.95) | 5.91 (0.50) | |||
| DPS 2001 | I: diet plus physical activity | 6.05 (0.78) | 8.82 (1.50) | 5.7 (0.6) | Cardiovascular disease: 8.2% Lipid‐lowering intervention: 4.3% Antihypertensive intervention: 27.7% |
29% had antihypertensive treatment at baseline 5% had lipid‐lowering treatment at baseline |
| C: standard treatment | 6.11 (0.72) | 8.83 (1.44) | 5.6 (0.6) | Cardiovascular disease: 8.1% Lipid‐lowering intervention: 6.1 Antihypertensive intervention: 31.5 |
31% had antihypertensive treatment at baseline 7% had lipid‐lowering treatment at baseline |
|
| EDIPS 2009 | I: diet plus physical activity | 5.7 (0.6) | 8.7 (1.1) | ‐ | ‐ | ‐ |
| C: standard treatment | 5.8 (0.5) | 8.9 (1.3) | ‐ | ‐ | ‐ | |
| Hellgren 2016 | I: physical activity | ‐a | ‐ | ‐ | Only reported for all intervention groups together: 16% were diagnosed with cardiovascular disease; 26% with hyperlipidaemia; 49% with hypertension | ‐ |
| C: standard treatment | ‐ | ‐ | ‐ | ‐ | ||
| HELP PD 2011 | I: diet plus physical activity | 5.8 (0.7) | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 5.9 (0.6) | ‐ | ‐ | ‐ | ‐ | |
| IDPP 2006 | I: diet plus physical activity | 5.4 (0.7) | 8.5 (0.7) | 6.1 (0.5) | 31.6 % had hypertension at baseline | ‐ |
| C: standard treatment | 5.5 (0.8) | 8.6 (0.7) | 6.2 (0.5) | 32.4% had hypertension at baseline | ‐ | |
| JDPP 2013 | I: diet plus physical activity | 5.9 (0.5)b | 9.2 (0.9) | 5.7 (0.4)c | ‐ | ‐ |
| C: standard treatment | 6.1 (0.5) | 9.0 (0.9) | 5,8 (0,4) | ‐ | ‐ | |
| Kosaka 2005 | I: diet plus physical activity | 6.27 (0.42) | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 6.22 (0.47) | ‐ | ‐ | |||
| Oldroyd 2005 | I: diet plus physical activity | 6.05 (0.89) | 9.15 (0.89) | 5.8 (0.7) | ‐ | ‐ |
| C: no intervention | 6.16 (0.89) | 9.22 (0.92) | 5.9 (0.5) | |||
| PODOSA 2014 | I: diet plus physical activity | 5.8 (0.6) | 8.2 (1.6) | ‐ | ‐ | Cholesterol‐lowering: 16% Antihypertensive: 25% |
| C: standard treatment | 5.8 (0.6) | 8.3 (1.5) | ‐ | ‐ | Cholesterol‐lowering: 29% Antihypertensive: 31% |
|
| SLIM 2003 | I: diet plus physical activity | 6.0 (0.87) | 8.59 (1.55) | 5.6 (0.5) | ‐ | 21% of the participants were receiving blood pressure‐lowering medication at baseline |
| C: standard treatment | 5.9 (0.70) | 8.46 (1.84) | 5.8 (0.5) | ‐ | 18% of the participants were receiving blood pressure‐lowering medication at baseline | |
| ‐ denotes not reported C: comparator; FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; 2h‐PPG: 2‐hour postprandial plasma glucose; I: intervention; SD: standard deviation aIn the combined intervention Hellgren 2016 had 56.1% impaired fasting glucose; 43.9% impaired glucose tolerance and 18.2% impaired fasting glucose and impaired glucose tolerance combined; the usual‐care group had 56.1% impaired fasting glucose; 63.3% impaired glucose tolerance and 6.7% impaired fasting glucose and impaired glucose tolerance combined. bBaseline data reported for 123 participants in the intervention group and 131 participants in the control group. cBaseline data reported according to HbA1c at baseline ‐ data were therefore calculated by combining the groups for each intervention arm. | ||||||
Appendix 5. Baseline characteristics (III)
| Trial ID | Intervention(s) and comparator(s) | Sex (female %) | Age (mean years (SD)) | Systolic/diastolic blood pressure (mean mmHg (SD)) | BMI (mean kg/m² (SD)) | Weight (mean kg (SD)) |
| Da Qing 1997 | I1: diet | 55 | 44.7 (9.4) | 132 (23.5)/87.3 (14.5) | 25.3 (3.8) | ‐ |
| I2: physical activity | 43 | 44.2 (8.7) | 132 (23.5)/87.3 (14.5) | 25.4 (3.7) | ‐ | |
| I3: diet plus physical activity | 44 | 44.4 (9.2) | 132 (23.5)/87.3 (14.5) | 26.3 (3.9) | ‐ | |
| C: standard treatment | 45 | 46.5 (9.3) | 134.4 (23.4)/88.5 (13.5) | 26.2 (3.9) | ‐ | |
| DPP 2002 | I: diet plus physical activity | 68 | 50.6 (11.3) | 123.7 (14.8)/78.6 (9.2) | 33.9 (6.8) | 94.1 (20.8) |
| C: placebo + standard treatment | 69 | 50.3 (10.4) | 123.5 (14.4)/78.0 (9.2) | 34.2 (6.7) | 94.3 (20.2) | |
| DPS 2001 | I: diet plus physical activity | 66 | 55 (7) | 140 (18)/86 (9) | 31.3 (4.6) | 86.7 (14.0) |
| C: standard treatment | 68 | 55 (7) | 136 (17)/86 (10) | 31.0 (4.5) | 85.5 (14.4) | |
| EDIPS 2009 | I: diet plus physical activity | 59 | 56.8 (40‐72)a | ‐ | 34.1 (5.5) | 93.4 (16.0) |
| C: standard treatment | 61 | 57.4 (38‐74) | ‐ | 33.5 (4.6) | 90.6 (12.5) | |
| Hellgren 2016 | I: physical activity | 61 | 63 (9) | 146 (22)/83 (11) | 30.1 (4.6) | ‐ |
| C: standard treatment | 53 | 63 (9) | 143 (15)/83 (9) | 29.7 (4.8) | ‐ | |
| HELP PD 2011 | I: diet plus physical activity | 58 | 57.3 (10.1) | ‐ Reported for the whole study population: 127.2 (14.1)/73.2 (9.4) | 32.8 (3.9) | 94.4 (14.7) |
| C: standard treatment | 57 | 58.5 (9.0) | ‐ Reported for the whole study population: 127.2 (14.1)/73.2 (9.4) | 32.6 (4.1) | 93.0 (16.2) | |
| IDPP 2006 | I: diet plus physical activity | 22 | 46.1 (5.7) | 121.5 (14.4)/74.4 (8.1) | 25.7 (3.3) | ‐ |
| C: standard treatment | 24 | 45.2 (5.7) | 124.1 (16.0)/76.2 (8.6) | 26.3 (3.7) | ‐ | |
| JDPP 2013 | I: diet plus physical activity | 48b | 51.1 (6.5)c | ‐ | 24.8 (3.6) | 64.9 (12.9) |
| C: standard treatment | 50 | 51.7 (6.1) | ‐ | 24.5 (3.2)JDPP | 63.9 (11.7) | |
| Kosaka 2005 | I: diet plus physical activity | 0 | 30s: 5.2% 40s: 32.9% 50s: 53.9% 60s: 8.1% | 123 (18)/78 (13) | 24.0 (2.3) | ‐ |
| C: standard treatment | 0 | 30s: 3.9% 40s: 32.3% 50s: 56.9% 60s: 6.9% | 124 (17)/79 (11) | 23.8 (2.1) | ‐ | |
| Oldroyd 2005 | I: diet plus physical activity | 54 | 58.1 (52.1) | 137.2 (19.9)/77.0 (12.6) | 30.4 (5.6) | 85.3 (17.9) |
| C: no intervention | 31 | 57.5 (44.7) | 132.8 (16.4)/75.5 (9.8) | 29.9 (4.9) | 85.5 (14.2) | |
| PODOSA 2014 | I: diet plus physical activity | 54 | 52.8 (10.2) | 136.9 (21.8)/82.7 (12.5) | 30.6 (5.0) | 79.8 (16.2) |
| C: standard treatment | 55 | 52.2 (10.3) | 137.0 (19.7)/83.5 (10.7) | 30.5 (4.6) | 80.7 (15.0) | |
| SLIM 2003 | I: diet plus physical activity | 46 | 54.2 (5.8) | 142 (16)/90 (9) | 29.6 (3.8) | 87.5 (13.7) |
| C: standard treatment | 44 | 58.4 (6.8) | 145 (14)/88 (7) | 29.2 (3.3) | 83.0 (11.7) | |
| ‐ denotes not reported BMI: body mass index; C: comparator; EDIPS: European Diabetes Prevention Study (EDIPS) ‐ Newcastle; SD: standard deviation aData are expressed as mean (range). bBaseline data reported for 123 participants in the intervention group and 131 participants in the control group. cBaseline data reported according to HbA1c at baseline ‐ data were therefore calculated by combining the groups for each intervention arm. | ||||||
Appendix 6. Matrix of study endpoints (publications and trial documents)
| Trial ID | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c | Trial results available in trials register Yes/No | Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c |
| Da Qing 1997 | N/T | Primary outcome measure(s): incidence of T2DM after 6 years | Primary outcome measure(s): incidence of T2DM after 6 years | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): composite cardiovascular events after 6 years | |||
| Other outcome measure(s): mortality, fasting glucose, 2‐hour glucose values, insulin resistance and insulin secretion | Other outcome measure(s): mortality, insulin resistance and insulin secretion | |||
| DPP 2002 |
Source:NCT00004992; design article and protocol available from website (DPP 2002) Primary outcome measure(s): incidence of T2DM |
No | Primary outcome measure(s): incidence of T2DM | Primary outcome measure(s): incidence of T2DM |
| Secondary outcome measure(s): HbA1c; insulin and glucose; electrocardiogram; cardiovascular symptom assessment; blood pressure; carotid ultrasound; lipoproteins; fibrinolysis and clotting factors; albumin excretion; physical measurements; physical activity; nutrient intake; health‐related quality of life; resource utilisation; safety | Secondary outcome measure(s): HbA1c; insulin and glucose; electrocardiogram; cardiovascular symptom assessment; blood pressure; lipoproteins; fibrinolysis and clotting factors; albumin excretion; physical measurements; physical activity; nutrient intake; health‐related quality of life; resource utilisation; safety | Secondary outcome measure(s): insulin; cardiovascular symptom assessment; blood pressure; lipoproteins; fibrinolysis and clotting factors; albumin excretion; physical measurements; nutrient intake; health‐related quality of life; resource utilization | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| DPS 2001 |
Source: design article and NCT00279240 Primary outcome measure(s): incidence of T2DM |
No | Primary outcome measure(s): diabetes | Primary outcome measure(s): diabetes |
| Secondary outcome measure(s): changes in plasma glucose, insulin and HbA1c, changes in physical activity and diet | Secondary outcome measure(s): changes in plasma glucose, insulin, HbA1c, changes in physical activity and diet | Secondary outcome measure(s): changes in glucose, insulin, changes in physical activity and diet | ||
| Other outcome measure(s): cardiovascular risk factors. Cardiovascular mortality and morbidity | Other outcome measure(s): cardiovascular risk factors, cognition | Other outcome measure(s): cardiovascular risk factors, cognition | ||
| EDIPS 2009 |
Source:ISRCTN15670600 Primary outcome measure(s): incidence of diabetes confirmed by two OGTTs (between one and 12 weeks apart). |
No | Primary outcome measure(s): incidence of T2DM | Primary outcome measure(s): incidence of T2DM |
|
Secondary outcome measure(s): Current secondary outcome measures as of 26/01/2009: Changes in: weight; physical activity; dietary fibre intake; carbohydrate intake as a percentage of total dietary energy; fat intake as a percentage of total dietary energy Previous secondary outcome measures: proportion of energy consumed from fat, protein, carbohydrates and saturated, monounsaturated, polyunsaturated fatty acids, fibre and cholesterol; physical activity; glucose tolerance; insulin sensitivity; cardiovascular risk factors; cardiovascular morbidity and mortality; quality of life |
Secondary outcome measure(s): the proportion of energy consumed from fat, protein, carbohydrates and saturated, monounsaturated, polyunsaturated fatty acids, fibre and cholesterol, body weight, physical activity, mortality | Secondary outcome measure(s): | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| Hellgren 2016 |
Source: study protocol (provided by the investigators) and main publication Primary outcome measure(s): incidence of T2DM |
No | Primary outcome measure(s): incidence of T2DM | Primary outcome measure(s): incidence of T2DM |
| Secondary outcome measure(s): physical activity, LDL, triglycerides, HbA1c | Secondary outcome measure(s): physical activity, LDL, triglycerides, HbA1c | Secondary outcome measure(s): physical activity, LDL, triglycerides, HbA1c | ||
| Other outcome measure(s): insulin resistance | Other outcome measure(s): blood pressure, incidence of T2DM, weight, waist circumference, insulin resistance | Other outcome measure(s): diastolic blood pressure, incidence of T2DM, insulin resistance | ||
| HELP PD 2011 |
Source:NCT00631345 and design article Primary outcome measure(s): fasting glucose |
No | Primary outcome measure(s): fasting glucose | Primary outcome measure(s): fasting glucose |
| Secondary outcome measure(s): weight loss, waist circumference, dietary intake, physical activity, economic evaluation of the program | Secondary outcome measure(s): weight loss, waist circumference, dietary intake, physical activity, economic evaluation of the program | Secondary outcome measure(s): weight loss, waist circumference, economic evaluation of the program | ||
| Other outcome measure(s): homeostasis model of insulin resistance, triglycerides, HDL‐C, blood pressure, the metabolic syndrome, health‐related quality of life and behavioral constructs, incidence of T2DM, serious adverse events | Other outcome measure(s): homeostasis model of insulin resistance, incidence of T2DM | Other outcome measure(s): homeostasis model of insulin resistance, triglycerides, HDL‐C | ||
| IDPP 2006 |
Source: main publication Primary outcome measure(s): incidence of T2DM |
No | Primary outcome measure(s): incidence of T2DM | Primary outcome measure(s): incidence of T2DM |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | ||
| Other outcome measure(s): compliance, weight, plasma glucose, adverse events, insulin secretion, waist circumference, blood pressure, BMI, cholesterol levels, glycaemic measures | Other outcome measure(s): compliance, weight, plasma glucose, adverse events, insulin secretion, waist circumference, blood pressure, BMI, cholesterol levels, glycaemic measures | Other outcome measure(s): insulin secretion, cholesterol levels | ||
| JDPP 2013 |
Source:UMIN000003136 Primary outcome measure(s): incidence of diabetes |
No | Primary outcome measure(s): incidence of diabetes | Primary outcome measure(s): incidence of diabetes |
| Secondary outcome measure(s): changes of body weight, BMI, waist circumference, blood glucose, insulin, HbA1c, blood pressure, lipids, liver function, and health behavior | Secondary outcome measure(s): changes of body weight, BMI, waist circumference, blood glucose, insulin, blood pressure, lipids, liver function, and health behavior | Secondary outcome measure(s): changes in body, weight, insulin | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| Kosaka 2005 |
Source: main publication Primary outcome measure(s): development of diabetes |
No | Primary outcome measure(s): development of diabetes | Primary outcome measure(s): development of diabetes |
| Secondary outcome measure(s): improvement of glucose tolerance | Secondary outcome measure(s): improvement of glucose tolerance | Secondary outcome measure(s): improvement of glucose tolerance | ||
| Other outcome measure(s): changes in body weight | Other outcome measure(s): changes in body weight | Other outcome measure(s): changes in body weight | ||
| Oldroyd 2005 |
Source: main article Primary outcome measure(s): ‐ |
No | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | ||
| Other outcome measure(s): described as main outcome measures; change from baseline nutrient intake, physical activity, anthropometry, glucose tolerance and insulin sensitivity | Other outcome measure(s): change in nutrient intake, mortality, physical activity, BMI, body weight, lipids, insulin sensitivity, glycaemic measures, incidence of T2DM | Other outcome measure(s): change in nutrient intake, mortality, physical activity, BMI, body weight, lipids, insulin sensitivity | ||
| PODOSA 2014 |
Source:ISRCTN25729565 Primary outcome measure(s): weight change |
No | Primary outcome measure(s): weight change | Primary outcome measure(s): weight change |
| Secondary outcome measure(s): fasting and 2‐hour glucose; progression to T2DM; BMI; waist circumference and hip circumference; cost effectiveness | Secondary outcome measure(s): fasting and 2‐hour glucose; progression to T2DM; BMI; waist circumference and hip circumference; cost effectiveness | Secondary outcome measure(s): ‐ | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): blood pressure, adverse events, physical activity | Other outcome measure(s): ‐ | ||
| SLIM 2003 |
Source: Mensink et al. Diabetes Research and Clinical Practice 2003 Primary outcome measure(s): change in glucose tolerance, defined as the 2‐hour blood glucose concentration during the OGTT |
No | Primary outcome measure(s): change in glucose tolerance, defined as the 2‐hour blood glucose concentration during the OGTT | Primary outcome measure(s): change in glucose tolerance, defined as the 2‐hour blood glucose concentration during the OGTT |
| Secondary outcome measure(s): changes in fasting plasma glucose concentration, changes in plasma insulin concentration, changes in insulin resistance (as indicated by the HOMA index) and changes in HbA1c | Secondary outcome measure(s): changes in fasting plasma glucose concentration, changes in plasma insulin concentration, changes in insulin resistance (as indicated by the HOMA index) and changes in HbA1c | Secondary outcome measure(s): changes in fasting plasma glucose concentration, changes in plasma insulin concentration, changes in insulin resistance (as indicated by the HOMA index) and changes in HbA1c | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): diet intake, physical activity, body weight, free fatty acid, blood pressure | Other outcome measure(s): diet intake, physical activity, body weight, free fatty acid, blood pressure | ||
| BMI: body mass index; EMA: European Medicines Agency; FDA: Food and Drug Administration (US); HbA1c: glycosylated A1c; HOMA: homeostatic model assessment; N/A: not applicable; N/T: no trial document available; OGTT: oral glucose tolerance test; T2DM: type 2 diabetes mellitus aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers). bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary trial). cPrimary and secondary outcomes refer to verbatim specifications in publication/records. Other outcome measures refer to all outcomes not specified as primary or secondary outcome measures. | ||||
Appendix 7. High risk of outcome reporting bias according to ORBIT (Outcome Reporting Bias In Trials) classification
| Trial ID | Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d |
| Da Qing 1997 | Non‐fatal myocardial infarction | N/D | Yes (abstract mentions it was assessed) | N/D | N/D |
| Non‐fatal stroke | Yes (abstract mentions it was assessed) | ||||
| Amputation of lower extremity | Yes (abstract mentions it was assessed) | ||||
| DPP 2002 | Serious adverse events | Yes | |||
| Non‐fatal myocardial infarction | N/D | Yes | |||
| Non‐fatal stroke | Yes | ||||
| Non‐serious adverse events | Yes | N/D | |||
| Hypoglycaemia | Yes | ||||
| DPS 2001 | Cardiovascular mortality | Yes | |||
| Non‐fatal myocardial infarction | Yes | ||||
| Non‐fatal stroke | Yes | ||||
| EDIPS 2009 | Health‐related quality of life | Yes | |||
| Hellgren 2016 | N/D | ||||
| HELP PD 2011 | Serious adverse events | N/D | Yes | N/D | |
| Non‐serious adverse events | Yes | ||||
| Health‐related quality of life | Yes | ||||
| Time to progression to T2DM | Yes | ||||
| IDPP 2006 | Serious adverse events | Yes | |||
| Non‐fatal myocardial infarction | Yes | ||||
| Non‐fatal stroke | Yes | ||||
| Non‐serious adverse events | N/D | Yes | N/D | ||
| JDPP 2013 | Incidence of T2DM | Yes (long‐term (6 years) follow‐up of diabetes incidence) | N/D | ||
| Serious adverse events | Yes | N/D | |||
| Non‐serious adverse events | Yes | ||||
| Measures of blood glucose control | Yes | ||||
| Kosaka 2005 | All‐cause mortality | N/D | Yes | ||
| Serious adverse events | Yes | ||||
| Cardiovascular mortality | Yes | ||||
| Non‐serious adverse events | Yes | ||||
| Measures of blood glucose control | N/D | Yes | N/D | ||
| Oldroyd 2005 | N/D | ||||
| PODOSA 2014 | N/D | ||||
| SLIM 2003 | N/D | ||||
| N/A: not applicable; N/D: none detected aClear that outcome was measured and analysed; trial report states that outcome was analysed but reports only that result was not significant (Classification 'A', table 2, Kirkham 2010). bClear that outcome was measured and analysed; trial report states that outcome was analysed but reports no results (Classification 'D', table 2, Kirkham 2010). cClear that outcome was measured but was not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results (Classification 'E', table 2, Kirkham 2010). dUnclear whether outcome was measured; not mentioned, but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results (Classification 'G', table 2, Kirkham 2010). | |||||
Appendix 8. Definition of endpoint measurement and assessor (I)
| Trial ID | All‐cause mortality | Development of type 2 diabetes mellitus | Serious adverse events | Cardiovascular mortality | Non‐fatal myocardial infarction | Non‐fatal stroke | Amputation of lower extremity |
| Da Qing 1997 | IO | WHO 1985 criteria (either a fasting plasma glucose ≥ 140 mg/dL (7.8 mmol/L) or higher or a 2‐hour plasma glucose ≥ 200 mg/dL (11.1 mmol/L) after a 75 g OGTT and confirmed with a repeat test 7‐14 days after) or from a report of physician‐diagnosed diabetes with evidence in the medical record of high glucose concentrations, or use of glucose‐lowering drugs AO |
"No adverse events were recorded" SO, IO |
"..cardiovascular disease death (defined as death attributed to coronary heart disease, stroke, or sudden death)" IO |
N/I | N/I | N/I |
| DPP 2002 | IO | ADA criteria (fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L) or 2‐hour plasma glucose ≥ 200 mg/dL (11.1 mmol/L) after a 75 g OGTT, and confirmed with a repeated test) IO |
"Serious adverse events have been defined to include any adverse experience occurring at any dose that results in any of the following outcomes: death; a life‐threatening adverse experience; inpatient hospitalisation or prolongation of hospitalisation; a persistent or significant disability/incapacity; or a congenital anomaly/birth defect" IO |
"CVD‐related deaths" IO |
N/I | N/I | N/I |
| DPS 2001 | IO | WHO 1985 (either a fasting plasma glucose concentration of ≥ 140 mg/dL (7.8 mmol/L) or higher or a 2‐hour plasma glucose ≥ 200 mg/dL (11.1 mmol/L) after a 75 g OGTT, and confirmed with a repeat test) AO |
Serious adverse events IO |
IO | N/I | N/I | N/I |
| EDIPS 2009 | One patient died with no reason explained; one patient died due to colon cancer; one died due to lung cancer IO |
WHO 1999 (either a fasting plasma glucose ≥ 7.0 mmol/L
(≥ 126 mg/dL) and/or a 2‐hour plasma glucose concentration of
≥ 11.1 mmol/L (≥ 200 mg/dL), and confirmed with a repeat test) IO |
N/I | N/I | N/I | N/I | N/I |
| Hellgren 2016 | All‐cause mortality IO |
Fasting plasma glucose > 6.9 mmol/L and/or 2‐hour plasma glucose concentration > 12.1 mmol/L IO |
"Serious adverse events" IO |
N/I | Non‐fatal myocardial infarction IO |
Non‐fatal stroke IO |
amputation of lower extremity IO |
| HELP PD 2011 | No participants died IO |
Fasting glucose ≥ 126 mg/dL (ADA 2004) or using diabetes medication at the visit IO |
"Serious adverse events" IO |
No participants died IO |
N/I | N/I | N/I |
| IDPP 2006 | IO | WHO 1999 (either a fasting plasma glucose ≥ 7.0 mmol/L
(≥ 126 mg/dL) and/or a 2‐hour plasma glucose concentration
≥ 11.1 mmol/L (≥ 200 mg/dL), and confirmed with a repeat test) IO |
IO | N/I | N/I | N/I | N/I |
| JDPP 2013 | IO | WHO 1998 (either a fasting plasma glucose ≥ 7.0 mmol/L
(≥ 126 mg/dL) and/or a 2‐hour plasma glucose concentration
≥ 11.1 mmol/L (≥ 200 mg/dL), and confirmed with a repeat test) IO |
N/I | N/I | N/I | N/I | N/I |
| Kosaka 2005 | N/I | Diabetes was determined by fasting plasma glucose and it was judged to have developed when fasting plasma glucose reached or exceeded 140 mg/dL (7.8 mmol/L) on two consecutive tests performed at an interval of 2 weeks or less IO |
N/I | N/I | N/I | N/I | N/I |
| Oldroyd 2005 | IO | WHO 1985 (either a fasting plasma glucose concentration ≥ 140 mg/dL (7.8 mmol/L) or higher or a 2‐hour plasma glucose ≥ 200 mg/dL (11.1 mmol/L) after a 75 g OGTT) IO |
N/I | One participant died after stroke IO |
N/I | N/I | N/I |
| PODOSA 2014 | N/I | Based on 2‐hour plasma glucose concentration ≥ 11.1 mmol/L (≥ 200 mg/dL), and confirmed with a repeat test at year three, or diagnosed by physician IO |
Serious adverse events IO |
N/I | N/I | N/I | N/I |
| SLIM 2003 | IO | WHO 1999 (either a fasting plasma glucose ≥ 7.0 mmol/L
(≥ 126 mg/dL) and/or a 2‐hour plasma glucose concentration of
≥ 11.1 mmol/L (≥ 200 mg/dL); single OGTT was performed IO |
N/I | N/I | N/I | N/I | N/I |
| ADA: American Diabetes Association; AO: adjudicated outcome measurement; HbA1c: glycosylated haemoglobin A1c; IO: investigator‐assessed outcome measurement; N/D: not defined; N/I: not investigated; OGTT: oral glucose tolerance test; SO: self‐reported outcome measurement; WHO: World Health Organization | |||||||
Appendix 9. Definition of endpoint measurement and assessor (II)
| Trial ID | Blindness or severe vision loss | End‐stage renal disease | Nonserious adverse events | Hypoglycaemic events | Health‐related quality of life | Time to progression to T2DM | Measures of blood glucose control | Socioeconomic effects |
| Da Qing 1997 | N/I | "Nephropathy was defined as a history of renal dialysis or transplantation, death from nephropathy or end‐stage renal disease (ESRD), or among living participants as ACR ≥ 300 mg/g (to convert values to mg/mmol multiply by 0.113) or serum creatinine ≥ 177 μmol/l (2 mg/dl). Severe nephropathy was defined as that which led to renal replacement therapy or death from nephropathy or ESRD." IO |
"No adverse events were recorded" SO, IO |
N/I | N/I | N/I | 2 hour OGTT; FPG IO |
N/I |
| DPP 2002 | N/I | N/I | N/I | N/I | 36‐Item Short‐
Form (SF‐36) health survey SO |
N/I | 2 hour OGTT; HbA1c; FPG IO |
"The direct costs of medical care received outside the study and indirect costs were determined annually from patient self‐report. Direct non‐medical costs were assessed once during DPP and once during DPPOS, and costs were annualized. All costs were adjusted to 2000 or 2010 U.S. Dollars using the Consumer Price Index and the Medical Consumer Price Index." IO |
| DPS 2001 | N/I | N/I | N/I | N/I | N/I | N/I | 2 hour OGTT; HbA1c; FPG IO |
N/I |
| EDIPS 2009 | N/I | N/I | N/I | N/I | N/I | N/I | N/I | N/I |
| Hellgren 2016 | N/I | End‐stage renal disease IO |
N/I | Hypoglycaemia SO, IO |
Health‐related quality of life SO |
N/I | 2 hour OGTT; HbA1c; FPG IO |
N/I |
| HELP PD 2011 | N/I | N/I | N/I | N/I | 36‐Item Short‐
Form (SF‐36) health survey SO |
"The distribution of times until the development of T2DM (measured from the date of randomisation to the date of the clinical visit or report triggering the diagnosis)" IO |
FPG IO |
Cost analysis, cost effectiveness analysis (CEA) and cost utility analysis (CUA) IO |
| IDPP 2006 | N/I | N/I | N/I | Hypoglycaemia SO, IO |
N/I | N/I | 2 hour OGTT; FPG IO |
N/I |
| JDPP 2013 | N/I | N/I | N/I | N/I | N/I | N/I | 2 hour OGTT; FPG IO |
N/I |
| Kosaka 2005 | N/I | N/I | N/I | N/I | N/I | N/I | 2 hour OGTT; HbA1c; FPG IO |
N/I |
| Oldroyd 2005 | N/I | N/I | N/I | N/I | N/I | N/I | 2 hour OGTT; FPG IO |
N/I |
| PODOSA 2014 | N/I | N/I | Mild or moderate adverse events SO, IO |
N/I | N/I | N/I | 2 hour OGTT; FPG IO |
"The time and costs related to dietitians, costs related to general practitioner and hospital outpatients, and participants’ opportunity costs were described (without inferential statistics) by year and for the 3 years combined as appropriate." IO |
| SLIM 2003 | N/I | N/I | N/I | N/I | N/I | N/I | 2 hour OGTT; HbA1c; FPG IO |
N/I |
| AO: adjudicated outcome measurement; DPP: Diabetes Prevention Programme; DPPOS: Diabetes Prevention Program Outcomes Study; FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; IO: investigator‐assessed outcome measurement; N/I: not investigated; OGTT: oral glucose tolerance test; SO: self‐reported outcome measurement | ||||||||
Appendix 10. Adverse events (I)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Deaths (N) | Deaths (% of participants) | Participants with at least one adverse event (N) | Participants with at least one adverse event (%) | Participants with at least one severe/serious adverse event (N) | Participants with at least one severe/serious adverse event (%) |
| Da Qing 1997 | I1: diet | 130 | 3 | 2.3 | 0 | 0 | 0 | 0 |
| I2: physical activity | 131 | 0 | 0 | 0 | 0 | 0 | 0 | |
| I3: diet plus physical activity | 126 | 5 | 4 | 0 | 0 | 0 | 0 | |
| C: standard treatment | 133 | 3 | 2.3 | 0 | 0 | 0 | 0 | |
| DPP 2002 | I: diet plus physical activity | 1079 | 3 | 0.3 | Musculoskeletal symptoms: 728 Gastrointestinal symptoms: 390 |
Musculoskeletal symptoms: 67 Gastrointestinal symptoms: 36 |
‐ | ‐ |
| C: placebo + standard treatment | 1082 | 5 | 0.5 | Musculoskeletal symptoms: 639 Gastrointestinal symptoms: 930 |
Musculoskeletal symptoms: 59 Gastrointestinal symptoms: 86 | |||
| DPS 2001 | I: diet plus physical activity | 265 | 1 | 0.4 | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 257 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| EDIPS 2009 | I: diet plus physical activity | 51 | 2 | 3.9 | ‐ | ‐ | 1 | 2.0 |
| C: standard treatment | 51 | 0 | 0 | ‐ | ‐ | 0 | 0 | |
| Hellgren 2016 | I: physical activity | 66 (mortality: 84) | 3 | 3.6 | ‐ | ‐ | 3 | 4.5 |
| C: standard treatment | 31 (mortality: 39) | 1 | 2.6 | ‐ | ‐ | 1 | 3.2 | |
| HELP PD 2011 | I: diet plus physical activity | 151 | 0 | 0 | 11a | 7.3 | 5a | 3.3 |
| C: standard treatment | 150 | 0 | 0 | 15 | 10 | 5 | 3.3 | |
| IDPP 2006 | I: diet plus physical activity | 120 | 1 | 0.8 | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 133 | 1 | 0.8 | ‐ | ‐ | ‐ | ‐ | |
| JDPP 2013 | I: diet plus physical activity | 103 | 1 | 1.0 | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 110 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Kosaka 2005 | I: diet plus physical activity | 102 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 356 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Oldroyd 2005 | I: diet plus physical activity | 37 at 6 months 32 at 12 months 30 at 24 months |
1 | 2.7 | ‐ | ‐ | ‐ | ‐ |
| C: no intervention | 32 at 6 months 30 at 12 months 24 at 24 months |
0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| PODOSA 2014 | I: diet plus physical activity | 84 | ‐ | ‐ | 3 | 3.6 | ‐ | ‐ |
| C: standard treatment | 83 | ‐ | ‐ | 4 | 4.8 | ‐ | ‐ | |
| SLIM 2003 | I: diet plus physical activity | 74 at baseline
52 at 3 years
51 at 4 years
34 at 5 years 35 (one that was missing at five years attended 6‐year follow‐up) at 6 years |
0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 73 at baseline 54 at 3 years 43 at 4 years 29 at 5 years 35 (six that were missing at five years attended 6‐year follow‐up) at 6 years |
1 | 1.4 | ‐ | ‐ | ‐ | ‐ | |
| ‐ denotes not reported
C: comparator; I: intervention aData only available after one year of intervention. | ||||||||
Appendix 11. Adverse events (II)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants discontinuing trial due to an adverse event (N) | Participants discontinuing trial due to an adverse event (%) | Participants with at least one hospitalisation (N) | Participants with at least one hospitalisation (%) | Participants with at least one outpatient treatment (N) | Participants with at least one outpatient treatment (%) |
| Da Qing 1997 | I1: diet | 130 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| I2: physical activity | 131 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| I3: diet plus physical activity | 126 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| C: standard treatment | 133 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| DPP 2002 | I: diet plus physical activity | 1079 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo + standard treatment | 1082 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| DPS 2001 | I: diet plus physical activity | 265 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 257 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| EDIPS 2009 | I: diet plus physical activity | 51 | 1 | 2.0 | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 51 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Hellgren 2016 | I: physical activity | 84 | 1 | 1.1 | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 39 | 1 | 2.5 | ‐ | ‐ | ‐ | ‐ | |
| HELP PD 2011 | I: diet plus physical activity | 151 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 150 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| IDPP 2006 | I: diet plus physical activity | 120 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 133 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| JDPP 2013 | I: diet plus physical activity | 103 | 2 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 110 | 2 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Kosaka 2005 | I: diet plus physical activity | 102 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 356 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Oldroyd 2005 | I: diet plus physical activity | 37 at 6 months 32 at 12 months 30 at 24 months |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: no intervention | 32 at 6 months 30 at 12 months 24 at 24 months |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| PODOSA 2014 | I: diet plus physical activity | 85 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 86 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| SLIM 2003 | I: diet plus physical activity | 74 at baseline
52 at 3 years
51 at 4 years
34 at 5 years 35 (one that was missing at five years attended 6‐year follow‐up) at 6 years |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: standard treatment | 73 at baseline 54 at 3 years 43 at 4 years 29 at 5 years 35 (six that were missing at five years attended 6‐year follow‐up) at 6 years |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ denotes not reported C: comparator; I: intervention | ||||||||
Appendix 12. Adverse events (III)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants with a specific adverse event (description) | Participants with at least one specific adverse events (N) | Participants with at least one specific adverse event (%) |
| Da Qing 1997 | I1: diet | 130 | ‐ | ‐ | ‐ |
| I2: physical activity | 131 | ‐ | ‐ | ‐ | |
| I3: diet plus physical activity | 126 | ‐ | ‐ | ‐ | |
| C: standard treatment | 133 | ‐ | ‐ | ‐ | |
| DPP 2002 | I: diet plus physical activity | 1079 | 1. Gastrointestinal symptoms 2. Muskoskeletal symptoms 3. Hospitalisation |
1. 140 2. 259 3. 168 |
1. 12.9 2. 24.0 3. 15.5 |
| C: placebo + standard treatment | 1082 | 1. Gastrointestinal symptoms 2. Muskoskeletal symptoms 3. Hospitalisation |
1. 331 2. 228 3. 174 |
1. 30.6 2. 21.1 3. 16.3 |
|
| DPS 2001 | I: diet plus physical activity | 265 | ‐ | ‐ | ‐ |
| C: standard treatment | 257 | ‐ | ‐ | ‐ | |
| EDIPS 2009 | I: diet plus physical activity | 51 | ‐ | ‐ | ‐ |
| C: standard treatment | 51 | ‐ | ‐ | ‐ | |
| Hellgren 2016 | I: physical activity | 66 | ‐ | ‐ | ‐ |
| C: standard treatment | 30 | ‐ | ‐ | ‐ | |
| HELP PD 2011 | I: diet plus physical activity | 151 | ‐ | ‐ | ‐ |
| C: standard treatment | 150 | ‐ | ‐ | ‐ | |
| IDPP 2006 | I: diet plus physical activity | 120 | ‐ | ‐ | ‐ |
| C: standard treatment | 133 | ‐ | ‐ | ‐ | |
| JDPP 2013 | I: diet plus physical activity | 103 | ‐ | ‐ | ‐ |
| C: standard treatment | 110 | ‐ | ‐ | ‐ | |
| Kosaka 2005 | I: diet plus physical activity | 102 | ‐ | ‐ | ‐ |
| C: standard treatment | 356 | ‐ | ‐ | ‐ | |
| Oldroyd 2005 | I: diet plus physical activity | 37 at 6 months 32 at 12 months 30 at 24 months |
‐ | ‐ | ‐ |
| C: no intervention | 32 at 6 months 30 at 12 months 24 at 24 months |
‐ | ‐ | ‐ | |
| PODOSA 2014 | I: diet plus physical activity | 85 | ‐ | ‐ | ‐ |
| C: standard treatment | 86 | ‐ | ‐ | ‐ | |
| SLIM 2003 | I: diet plus physical activity | 74 at baseline
52 at 3 years
51 at 4 years
34 at 5 years 35 (one that was missing at five years attended 6‐year follow‐up) at 6 years |
‐ | ‐ | ‐ |
| I2: standard treatment | 73 at baseline 54 at 3 years 43 at 4 years 29 at 5 years 35 (six that were missing at five years attended 6‐year follow‐up) at 6 years |
‐ | ‐ | ‐ | |
| ‐ denotes not reported C: comparator; I: intervention | |||||
Appendix 13. Survey of study investigators providing information on trials
| Date trial author contacted | Date trial author replied | Date trial author was asked for additional information (short summary) | Date trial author provided data (short summary) | |
| 130750‐201504‐HR‐020 | 5 April 2017 | No reply | Asked for the duration of the intervention | N/A |
| ChiCTR‐PRC‐13003267 | 3 April 2017 | No reply | Asked for the duration of the intervention | N/A |
| Da Qing 1997 | 9 March 2017 | No reply | Authors were asked to confirm outcomes and asked for additional information, therein a study protocol | N/A |
| DPP 2002 | 9 March 2017 | No reply | Authors were asked to confirm outcomes and asked for additional information | N/A |
| DPS 2001 | 17 March 2017 | No reply | Authors were asked to confirm outcomes and asked for additional information | N/A |
| EDIPS 2009 | 27 March 2017 | 20 April 2017 | Authors were asked to confirm outcomes and asked for additional information | Provided additional information on outcomes |
| Hellgren 2016 | 29 March 2017 | 26 April 2017 | Authors were asked to confirm outcomes and asked for additional information, therein a study protocol | Provided additional information on outcomes and a study protocol |
| HELP PD 2011 | 20 March 2017 | No reply | Authors were asked to confirm outcomes and asked for additional information | N/A |
| IDPP 2006 | 15 March 2017 | 19 March 2017 Did not reply to the questions, but just confirmed the already‐extracted data |
Authors were asked to confirm outcomes and asked for additional information, therein a study protocol | N/A |
| Kosaka 2005 | 16 March 2017 | No reply | Authors were asked to confirm outcomes and asked for additional information, therein a study protocol | N/A |
| Oldroyd 2005 | 16 March 2017 | No reply | Authors were asked to confirm outcomes and asked for additional information, therein a study protocol | N/A |
| NCT01530165 | 3 April 2017 | 5 April 2017 | Principal investigator asked if the trial is completed and published | The principal investigator replied that the trial was going to be completed in June 2017 |
| PODOSA 2014 | 22 March 2017 | 23 March 2017 The request was forwarded to a research fellow |
Authors were asked to confirm outcomes and asked for additional information | 3 April 2017 |
| SLIM 2003 | 17 March 2017 | No reply | Authors were asked to confirm outcomes and asked for additional information, therein a study protocol | N/A |
| N/A: not applicable | ||||
Appendix 14. Checklist to aid consistency and reproducibility of GRADE assessments: diet plus physical activity versus standard treatment
| (1) All‐cause mortality | (2) Incidence of T2DM | (3) Serious adverse events | (4) Cardiovascular mortality | (5) Non‐fatal myocardial infarction/stroke | (6) Health‐related quality of life | (7) Socioeconomic effects | ||
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | |
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | Yes | No (↓) | Yes | |
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | Yes | No (↓) | Yes | |
| Was an objective outcome used? | Yes | Yes | Yes | Yes | Yes | No (↓) | Yes | |
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Unclear | Yes | N/A | Unclear | Yes | |
| No other biases reported (i.e. no potential of other bias)? | No (↓) | No (↓) | No (↓) | No (↓) | Yes | No (↓) | Yes | |
| Did the trials end up as scheduled (i.e. not stopped early)? | No (↓) | No (↓) | Yes | No (↓) | Yes | No (↓) | No (↓) | |
| Inconsistencyc | Point estimates did not vary widely? | Yes | Yes | N/A | No (↓) | N/A | N/A | N/A |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Substantial | Substantial | N/A | Substantial | N/A | N/A | N/A | |
| Was the direction of effect consistent? | No (↓) | Yes | N/A | No (↓) | N/A | N/A | N/A | |
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40%‐60%), high I² > 60%)? | Low | Low | N/A | Low | N/A | N/A | N/A | |
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | Not statistically significant | N/A | Not statistically significant | N/A | N/A | N/A | |
| Indirectnessa | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable |
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | |
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Imprecisiond | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | No (↓) | Yes | N/A | No (↓) | N/A | N/A | N/A |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100‐300 participants, low: < 100 participants)?b | High | High | Intermediate | High | Low (↓) | High | High | |
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5‐10 studies, small: < 5 studies)?b | Moderate | Large | Small (↓) | Moderate | Small (↓) | Small (↓) | Small (↓) | |
| Was the outcome a common event (e.g. occurs more than 1/100)? | Yes | Yes | Yes | Yes | Yes | Not applicable | Not applicable | |
| Publication biase | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was grey literature searched? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| There was no industry influence on studies included in the review? | No (↓) | No (↓) | Yes | No (↓) | Yes | No (↓) | No (↓) | |
| There was no evidence of funnel plot asymmetry? | Yes | Yes | N/A | Unclear | N/A | N/A | N/A | |
| There was no discrepancy in findings between published and unpublished trials? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| (↓): key item for possible downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); N/A: not applicable aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials. bDepends on the context of the systematic review area. cQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. eQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials | ||||||||
Appendix 15. Checklist to aid consistency and reproducibility of GRADE assessments: diet versus physical activity or standard treatment
| (1) All‐cause mortality | (2) Incidence of T2DM | (3) Serious adverse events | (4) Cardiovascular mortality | (5) Non‐fatal myocardial infarction/stroke | (6) Health‐related quality of life | (7) Socioeconomic effects | ||
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | N/A | N/A | N/A |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | ||||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | ||||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | ||||
| Was an objective outcome used? | Yes | Yes | Yes | Yes | ||||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?b | Yes | Yes | Yes | Yes | ||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Yes | Yes | ||||
| No other biases reported (i.e. no potential of other bias)? | No (↓) | No (↓) | No (↓) | No (↓) | ||||
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | ||||
| Inconsistencyc | Point estimates did not vary widely? | N/A | N/A | N/A | N/A | |||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | N/A | N/A | N/A | N/A | ||||
| Was the direction of effect consistent? | N/A | N/A | N/A | N/A | ||||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40%‐60%), high I² > 60%)? | N/A | N/A | N/A | N/A | ||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | N/A | N/A | N/A | N/A | ||||
| Indirectnessa | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | ||||
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Yes | ||||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | ||||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | ||||
| Imprecisiond | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | N/A | N/A | N/A | N/A | |||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100‐300 participants, low: < 100 participants)?b | Low (↓) | Low (↓) | Low (↓) | Low (↓) | ||||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5‐10 studies, small: < 5 studies)?b | Small (↓) | Small (↓) | Small (↓) | Small (↓) | ||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | N/A | Yes | N/A | N/A | ||||
| Publication biase | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | |||
| Was grey literature searched? | Yes | Yes | Yes | Yes | ||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | ||||
| There was no industry influence on studies included in the review? | Yes | Yes | Yes | Yes | ||||
| There was no evidence of funnel plot asymmetry? | N/A | N/A | N/A | N/A | ||||
| There was no discrepancy in findings between published and unpublished trials? | N/A | N/A | N/A | N/A | ||||
| (↓): key item for possible downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); N/A: not applicable aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials. bDepends on the context of the systematic review area. cQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. eQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials | ||||||||
Appendix 16. Checklist to aid consistency and reproducibility of GRADE assessments: physical activity versus standard treatment
| (1) All‐cause mortality | (2) Incidence of T2DM | (3) Serious adverse events | (4) Cardiovascular mortality | (5) Non‐fatal myocardial infarction/stroke | (6) Health‐related quality of life | (7) Socioeconomic effects | ||
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Yes | Yes | N/A |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Yes | Yes | ||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | Yes | No (↓) | ||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | Yes | No (↓) | ||
| Was an objective outcome used? | Yes | Yes | Yes | Yes | Yes | No (↓) | ||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?b | Yes | Yes | Yes | Yes | No (↓) | No (↓) | ||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | No (↓) | No (↓) | No (↓) | Yes | ||
| No other biases reported (i.e. no potential of other bias)? | No (↓) | No (↓) | No (↓) | No (↓) | No (↓) | Yes | ||
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Inconsistencyc | Point estimates did not vary widely? | N/A | N/A | N/A | N/A | N/A | N/A | |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Was the direction of effect consistent? | N/A | N/A | N/A | N/A | N/A | N/A | ||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40%‐60%), high I² > 60%)? | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Was the test for heterogeneity statistically significant (P < 0.1)? | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Indirectnessa | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | ||
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Yes | Yes | Unclear | ||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | ||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Imprecisiond | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | N/A | N/A | N/A | N/A | N/A | N/A | |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100‐300 participants, low: < 100 participants)?b | Low (↓) | Low (↓) | Low (↓) | Low (↓) | Low (↓) | Low (↓) | ||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5‐10 studies, small: < 5 studies)?b | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | ||
| Was the outcome a common event (e.g. occurs more than 1/100)? | Yes | Yes | Yes | Yes | Yes | Not applicable | ||
| Publication biase | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was grey literature searched? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| There was no industry influence on studies included in the review? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| There was no evidence of funnel plot asymmetry? | N/A | N/A | N/A | N/A | N/A | N/A | ||
| There was no discrepancy in findings between published and unpublished trials? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | ||
| (↓): key item for possible downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); N/A: not applicable aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials. bDepends on the context of the systematic review area. cQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. eQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials | ||||||||
Appendix 17. Health‐related quality of life: instruments
| Trial ID | Instrument | Dimensions (subscales) (no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | Minimal important difference |
| DPP 2002 | SF‐36 (G) | Physical functioning (10) Role‐physical (4) Bodily pain (2) General health) (5) Vitality (4) Social functioning (2) Role‐emotional (3) Mental health (5) | Yes | Likert‐scale | Scores for dimensions
Physical component summary (PCS) Mental component summary (MCS) |
Minimum scores: 0 Maximum scores:100 |
No | Higher values mean better assessment | Minimal important difference was defined as HRQoL scores between groups differed by at least 3 %; In other publication (Marrero et al) minimal important difference is defined as two points on either PCS or MCS. |
| HELP PD 2011 | SF‐36 (G) | Physical functioning (10) Role‐physical (4) Bodily pain (2) General health (5) Vitality (4) Social functioning (2) Role‐emotional (3) Mental health (5) | Yes | Likert‐scale | Scores for dimensions
Physical component summary (PCS) Mental component summary (MCS) |
Minimum scores: 0 Maximum scores:100 |
No | Higher values mean better assessment | NR |
| G: generic; S: specific; SF: short‐form health survey | |||||||||
Appendix 18. Subgroup analyses: diet plus physical activity versus control
| Outcome | Trials with long duration (≥ 4 years) versus trials with short duration (< 4 years) (P value for test of interaction | Impaired glucose tolerance versus other diagnostic criteria (P value for test of interaction) | Younger versus older participants (see text) (P value for test of interaction) | Female versus male (see text) (P value for test of interaction) | Ethnicity (see text) (P value for test of interaction) | Comorbidity (see text) (P value for test of interaction) | Participants with previous gestational diabetes mellitus |
| All‐cause mortality | 0.78 | Not possible due to lack of data | 0.73 | Not possible due to lack of data | 0.79 | 0.79 | Not possible due to lack of data |
| Incidence of type 2 diabetes mellitus | 0.88 | 0.42 | 0.009 | Not possible due to lack of data | 0.01 | 0.02 | Not possible due to lack of data |
| Fasting plasma glucose | 0.60 | 0.11 | 0.19 | Not possible due to lack of data | 0.71 | 0.88 | Not possible due to lack of data |
| 2‐hour plasma glucose | 0.22 | Not possible due to lack of data | 0.003 | Not possible due to lack of data | 0.12 | 0.12 | Not possible due to lack of data |
| HbA1c | 0.99 | Not possible due to lack of data | Not possible due to lack of data | Not possible due to lack of data | Not possible due to lack of data | 0.10 | Not possible due to lack of data |
Appendix 19. Reported subgroup analyses of diabetes incidence in trials
| Trial ID | Reported subgroup | Publication of study (secondary reference of primary reference) |
| Da Qing 1997 |
BMI < 25 kg/m2 versus 25 kg/m2 or more The relative decrease in the rate of development of T2DM in the physical activity compared with control was similar in overweight and lean at the end of intervention |
Pan 1997 |
| DPP 2002 |
Age (N not provided for each subgroup in the intervention and control group of age) 25‐44 years; incidence (cases/100 person years); I: 6.2 ; C: 11.6; reduction in incidence diet plus physical activity versus control 48 (95% CI 27 to 63) 45‐59 years; incidence (cases/100 person years); I: 4.7; C: 10.8; reduction in incidence diet plus physical activity versus control 59 (95% CI 44 to 70) ≥ 60 years; incidence (cases/100 person years); I: 3.1; C: 10.8; reduction in incidence diet plus physical activity versus control 71 (95% CI 51 to 83) Sex Male; incidence (cases/100 person years); I: 4.6 (N = 345); C: 12.5 (N = 335); reduction in incidence diet plus physical versus control 65 (95% CI 49 to 76) Female; incidence (cases/100 person years); I: 5.0 (N = 734); C: 10.3 (N = 747); reduction in incidence diet plus physical activity versus control 54 (95% CI 40 to 64) Race or ethnic group White: incidence (cases/100 person years); I: 5.2 (N = 580); C: 10.3 (N = 586); reduction in incidence diet plus physical activity versus control 51 (95% CI 35 to 63) African; incidence (cases/100 person years); I: 5.1 (N = 204); C: 12.4 (N = 220); reduction in incidence diet plus physical activity versus control 61 (95% CI 37 to 76) Hispanic; incidence (cases/100 person years); I: 4.2 (N = 178); C: 11.7 (N = 168); reduction in incidence diet plus physical activity versus control 66 (95% CI 41 to 80) American Indian; incidence (cases/100 person years); I: 4.7 (N = 60); C: 12.9 (N = 59); reduction in incidence diet plus physical activity versus control 65 (95% CI 7 to 87) Asian; incidence (cases/100 person years); I: 3.8 (N = 57); C: 12.1 (N = 49); reduction in incidence diet plus physical activity versus control 71 (95% CI 24 to 89) BMI (N not provided for each subgroup in the intervention and control group of BMI) 22 to < 30 kg/m2; incidence (cases/100 person years); I: 3.3; C: 9.0; reduction in incidence diet plus physical activity versus control 65 (95% CI 46 to 77) 30 to < 35 kg/m2; incidence (cases/100 person years); I: 3.7; C: 8.9; reduction in incidence diet plus physical activity versus control 61 (95% CI 40 to 75) 35 kg/m2 or above; incidence (cases/100 person years); I: 7.3; C: 14.3; reduction in incidence diet plus physical activity versus control 51 (95% CI 34 to 63) Fasting plasma glucose (N not provided for each subgroup in the intervention and control group of fasting plasma glucose) 5.3‐6.0 mmol/L; incidence (cases/100 person years); I: 2.9; C: 6.4; reduction in incidence diet plus physical activity versus control 55 (95% CI 38 to 68) 6.1‐6.9 mmol/L; incidence (cases/100 person years); I: 3.7; C: 8.9; reduction in incidence diet plus physical activity versus control 63 (95% CI 51 to 72) Plasma glucose 2 hours after an oral glucose load (N not provided for each subgroup in the intervention and control group of glucose values) 7.7‐8.5 mmol/L; incidence (cases/100 person years); I: 1.8; C: 7.1; reduction in incidence diet plus physical activity versus control 76 (95% CI 58 to 86) 8.6‐9.5 mmol/L; incidence (cases/100 person years); I: 4.4; C: 10.3; reduction in incidence diet plus physical activity versus control 60 (95% CI 41 to 72) 9.6‐11.0 mmol/L; incidence (cases/100 person years); I: 8.5; C: 16.1; reduction in incidence diet plus physical activity versus control 50 (95% CI 33 to 63) P < 0.05 for the test of heterogeneity across strata |
Knowler 2002 |
|
DPP 2002 (only participants with FPG < 7 mmol/L and HbA1c < 6.5% at baseline) |
Diabetes incidence defined by FPG ≥ 7.0 mmol/L and/or 2‐hour plasma glucose ≥ 11.1 mmol/L according to ethnicity at the end of intervention All participants; diet plus physical activity 4.3/100 person years (N = 932); control 8.6/100 person years (N = 922) White participants; diet plus physical activity 4.7/100 person years (N = 539); control 8.5/100 person years (N = 534) African American; diet plus physical activity 4.1/100 person years (N = 161); control 8.0/100 person years (N = 147) Hispanic; diet plus physical activity 3.6/100 person years (N = 140); control 9.1/100 person years (N = 135) American Indian; diet plus physical activity 3.9/100 person years (N = 50); control 7.9/100 person years (N = 53) Asian American; diet plus physical activity 3.7/100 person years (N = 42); control 11.4/100 person years (N = 53) Diabetes incidence defined by FPG ≥ 7.0 mmol/L and/or 2‐hour plasma glucose ≥ 11.1 mmol/L according to ethnicity after a follow‐up period of 9.9 years All participants; diet plus physical activity 4.9/100 person years (N = 932); control 6.8/100 person years (N = 922) White participants; diet plus physical activity 4.7/100 person years (N = 539); control 6.1/100 person years (N = 534) African American; diet plus physical activity 6.1/100 person years (N = 161); control 8.8/100 person years (N = 147) Hispanic; diet plus physical activity 5.3/100 person years (N = 140); control 7.0/100 person years (N = 135) American Indian; diet plus physical activity 4.2/100 person years (N = 50); control 6.5/100 person years (N = 53) Asian American; diet plus physical activity 3.8/100 person years (N = 42); control 10.2/100 person years (N = 53) Diabetes incidence defined by HbA1c ≥ 6.5% according to ethnicity at the end of intervention All participants; diet plus physical activity 4.6/100 person years (N = 932); control 8.8/100 person years (N = 922) White participants; diet plus physical activity 3.8/100 person years (N = 539); control 6.7/100 person years (N = 534) African American; diet plus physical activity 10.0/100 person years (N = 161); control 18.3/100 person years (N = 147) Hispanic; diet plus physical activity 2.9/100 person years (N = 140); control 7.2/100 person years (N = 135) American Indian; diet plus physical activity 6.2/100 person years (N = 50); control 11.5/100 person years (N = 53) Asian American; diet plus physical activity 3.3/100 person years (N = 42); control 11.7/100 person years (N = 53) Diabetes incidence defined by HbA1c ≥ 6.5% according to ethnicity after a follow‐up period of 9.9 years All participants; diet plus physical activity 3.5/100 person years (N = 932); control 5.0/100 person years (N = 922) White participants; diet plus physical activity 3.1/100 person years (N = 539); control 4.1/100 person years (N = 534) African American; diet plus physical activity 5.8/100 person years (N = 161); control 9.2/100 person years (N = 147) Hispanic; diet plus physical activity 3.0/100 person years (N = 140); control 4.6/100 person years (N = 135) American Indian; diet plus physical activity 4.6/100 person years (N = 50); control 5.9/100 person years (N = 53) Asian American; diet plus physical activity 3.5/100 person years (N = 42); control 6.3/100 person years (N = 53) Diabetes incidence defined by FPG ≥ 7.0 mmol/L and/or 2‐hour plasma glucose ≥ 11.1 mmol/L according to HbA1c at baseline at the end of intervention (read from figure) HbA1c < 5.5%; diet plus physical activity 3.3/100 person years (N = 182); control 5.3/100 person years (N = 186) HbA1c between 5.5% ‐5.9%; diet plus physical activity 3.3/100 person years (N = 394); control 8/100 person years (N = 385) HbA1c between 6.0% ‐6.4%; diet plus physical activity 3.2/100 person years (N = 346); control 11.7/100 person years (N = 361) Diabetes defined by FPG ≥ 7.0 mmol/L and/or 2‐hour plasma glucose ≥ 11.1 mmol/L according to HbA1c at baseline after a follow‐up period of 9.9 years (read from figure) HbA1c < 5.5%; diet plus physical activity 4.7/100 person years (N = 182); control 4.7/100 person years (N = 186) HbA1c between 5.5% ‐5.9%; diet plus physical activity 4.0/100 person years (N = 394); control 6.8/100 person years (N = 385) HbA1c between 6.0%‐6.4%; diet plus physical activity 6.7/100 person years (N = 346); control 9.0/100 person years (N = 361) Diabetes incidence defined by HbA1c ≥ 6.5% according to HbA1c at baseline at the end of intervention (read from figure) HbA1c < 5.5%; diet plus physical activity 0.40/100 person years (N = 182); control 0.50/100 person years (N = 186) HbA1c between 5.5%‐5.9%; diet plus physical activity 2.3/100 person years (N = 394); control 4.6/100 person years (N = 385) HbA1c between 6.0%6.4%; diet plus physical activity 9.2/100 person years (N = 346); control 21.5/100 person years (N = 361) T2DM incidence defined by HbA1c ≥ 6.5% according to HbA1c at baseline after a follow‐up period of 9.9 years (read from figure) HbA1c < 5.5%; diet plus physical activity 1.6/100 person years (N = 182); control 0.8/100 person years (N = 186) HbA1c between 5.5%‐5.9%; diet plus physical activity 2.7/100 person years (N = 394); control 3.5/100 person years (N = 385) HbA1c between 6.0%‐6.4%; diet plus physical activity 6.2/100 person years (N = 346); control 11.5/100 person years (N = 361) T2DM incidence defined by FPG ≥ 7.0 mmol/L and/or 2‐hour plasma glucose ≥ 11.1 mmol/L according to sex and age at baseline at the end of intervention (read from figure) Men < 45 years; diet plus physical activity 3.0/100 person years (N = unknown); control 9.5/100 person years (N = unknown) Men 45‐59 years; diet plus physical activity 3.5/100 person years (N = unknown); control 7.5/100 person years (N = unknown) Men ≥ 60 years; diet plus physical activity 2.5/100 person years (N = unknown); control 10.0/100 person years (N = unknown) Women < 45 years; diet plus physical activity 5.5/100 person years (N = unknown); control 8.8/100 person years (N = unknown) Women 45‐59 years; diet plus physical activity 4.0/100 person years (N = unknown); control 7.5/100 person years (N = unknown) Women ≥ 60 years; diet plus physical activity 2.3/100 person years (N = unknown); control 4.5/100 person years (N = unknown) T2DM incidence defined by FPG ≥ 7.0 mmol/L and/or 2 hour plasma glucose ≥ 11.1 mmol/L according to sex and age at baseline after a follow‐up period of 9.9 years (read from figure) Men < 45 years; diet plus physical activity 5.3/100 person years (N = unknown); control 8.0/100 person years (N = unknown) Men 45‐59 years; diet plus physical activity 5.0/100 person years (N = unknown); control 6.8/100 person years (N = unknown) Men ≥ 60 years; diet plus physical activity 3.8/100 person years (N = unknown); control 8.0/100 person years (N = unknown) Women < 45 years; diet plus physical activity 6.3/100 person years (N = unknown); control 7.5/100 person years (N = unknown) Women 45‐59 years; diet plus physical activity 4.5/100 person years (N = unknown); control 7.0/100 person years (N = unknown) Women ≥ 60 years; diet plus physical activity 2.3/100 person years (N = unknown); control 4.5/100 person years (N = unknown) T2DM incidence defined by HbA1c ≥ 6.5% according to sex and age at baseline at the end of intervention (read from figure) Men < 45 years; diet plus physical activity 3.5/100 person years (N = unknown); control 9.0/100 person years (N = unknown) Men 45‐59 years; diet plus physical activity 3.0/100 person years (N = unknown); control 8.5/100 person years (N = unknown) Men ≥ 60 years; diet plus physical activity 1.5/100 person years (N = unknown); control 10.0/100 person years (N = unknown) Women < 45 years; diet plus physical activity 5.5/100 person years (N = unknown); control 8.0/100 person years (N = unknown) Women 45‐59 years; diet plus physical activity 5.0/100 person years (N = unknown); control 8.5/100 person years (N = unknown) Women ≥ 60 years; diet plus physical activity 3.3/100 person years (N = unknown); control 7.5/100 person years (N = unknown) T2DM incidence defined by HbA1c ≥ 6.5% according to sex and age at baseline after a follow‐up period of 9.9 years (read from figure) Men < 45 years; diet plus physical activity 4.3/100 person years (N = unknown); control 6.0/100 person years (N = unknown) Men 45‐59 years; diet plus physical activity 2.5/100 person years (N = unknown); control 5.0/100 person years (N = unknown) Men ≥ 60 years; diet plus physical activity 1.5/100 person years (N = unknown); control 5.5/100 person years (N = unknown) Women < 45 years; diet plus physical activity 5.0/100 person years (N = unknown); control 5.0/100 person years (N = unknown) Women 45‐59 years; diet plus physical activity 3.3/100 person years (N = unknown); control 4.0/100 person years (N = unknown) Women ≥ 60 years; diet plus physical activity 1.8/100 person years (N = unknown); control 3.5/100 person years (N = unknown) |
Knowler 2014 |
| DPS 2001 |
Sex Male (N = 172); incidence (cases/100 person years); I: 3.7 (95% CI 2.2 to 6.2); C: 6.8 (95% CI 5.8 to 12.6); HR diet plus physical activity versus control 0.43 (95% CI 0.22 to 0.81) Female (N = 350); incidence (cases/100 person years); I: 4.3 (95% CI 3.0 to 6.2); C: 6.9 (95% CI 5.2 to 9.2); HR diet plus physical activity versus control 0.61 (95% CI 0.39 to 0.97) P interaction 0.33 Age < 51 years; incidence (cases/100 person years); I: 6.0 (95% CI 3.9 to 9.2); C: 7.6 (95% CI 5.1 to 11.2); HR diet plus physical activity versus control 0.77 (95% CI 0.44 to 1.38) 51‐61 years; incidence (cases/100 person years); I: 4.0 (95% CI 2.3 to 6.7); C: 8.0 (95% CI 5.5 to 11.5); HR diet plus physical activity versus control 0.49 (95% CI 0.26 to 0.93) > 61 years; incidence (cases/100 person years); I: 2.4 (95% CI 1.3 to 4.7); C: 6.6 (95% CI 4.2 to 10.3); HR diet plus physical activity versus control 0.36 (95% CI 0.17 to 0.80) P interaction 0.0130 BMI < 28.7 kg/m2; incidence (cases/100 person years); I: 1.7 (95% CI 0.8 to 3.7); C: 5.2 (95% CI 3.3 to 8.1); HR diet plus physical activity versus control 0.32 (95% CI 0.13 to 0.79) 28.7‐32.3 kg/m2; incidence (cases/100 person years); I: 4.8 (95% CI 3.0 to 7.7); C: 7.9 (95% CI 5.3 to 11.9); HR diet plus physical activity versus control 0.59 (95% CI 0.32 to 1.10) > 32.3 kg/m2; incidence (cases/100 person years); I: 5.8 (95% CI 3.8 to 8.9); C: 9.6 (95% CI 6.7 to 13.8); HR diet plus physical activity versus control 0.60 (95% CI 0.34 to 1.04) P interaction 0.75 Fasting plasma glucose < 5.8 mmol/L; incidence (cases/100 person years); I: 2.4 (95% CI 1.3 to 4.5); C: 3.8 (95% CI 2.2 to 6.6); HR diet plus physical activity versus control 0.63 (95% CI 0.28 to 1.45) 5.8‐6.4 mmol/L; incidence (cases/100 person years); I: 2.7 (95% CI 1.4 to 5.1); C: 6.9 (95% CI 4.6 to 10.4); HR diet plus physical activity versus control 0.37 (95% CI 0.18 to 0.79) > 6.4 mmol/L; incidence (cases/100 person years); I: 7.7 (95% CI 5.2 to 11.5); C: 12.2 (95% CI 8.8 to 17.0); HR diet plus physical activity versus control 0.62 (95% CI 0.37 to 1.03) P interaction 0.68 Plasma glucose 2 hours after an oral glucose load < 8.2 mmol/L; incidence (cases/100 person years); I: 1.3 (95% CI 0.5 to 3.0); C: 5.3 (95% CI 3.4 to 8.4); HR diet plus physical activity versus control 0.23 (95% CI 0.09 to 0.61) 8.2‐9.3 mmol/L; incidence (cases/100 person years); I: 4.2 (95% CI 2.5 to 6.9); C: 5.8 (95% CI 3.7 to 9.1); HR diet plus physical activity versus control 0.70 (95% CI 0.36 to 1.37) > 9.3 mmol/L; incidence (cases/100 person years); I: 7.6 (95% CI 5.1 to 11.3); C: 12.0 (95% CI 8.6 to 16.8); HR diet plus physical activity versus control 0.62 (95% CI 0.37 to 1.04) P interaction 0.69 Number of participants in each tertile was roughly 174 |
Lindström 2008 |
BMI: body mass index; FPG: fasting plasma glucose; HR: hazard ratio; HbA1c: glycosylated haemoglobin A1c; T2DM: type 2 diabetes mellitus
Data and analyses
Comparison 1. Physical activity versus comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Incidence of type 2 diabetes | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Serious adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Fasting plasma glucose | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 2 hour glucose values | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 2. Diet plus physical activity versus comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 10 | 4099 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.50, 2.50] |
| 2 All‐cause mortality: duration of intervention | 10 | 4099 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.50, 2.50] |
| 2.1 ≥ 4 years | 3 | 817 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.33, 5.28] |
| 2.2 < 4 years | 7 | 3282 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.38, 2.77] |
| 3 All‐cause mortality: diagnostic criteria | 10 | 4099 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.50, 2.50] |
| 3.1 IGT | 9 | 3798 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.50, 2.50] |
| 3.2 Other criteria | 1 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 All‐cause mortality: age | 10 | 4099 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.50, 2.50] |
| 4.1 Age ≥ 50 years | 8 | 3682 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.39, 2.66] |
| 4.2 Age < 50 years | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.32, 6.16] |
| 5 All‐cause mortality: sex | 10 | 4099 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.50, 2.50] |
| 6 All‐cause mortality: ethnicity | 10 | 4099 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.50, 2.50] |
| 6.1 Asian | 4 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.37, 4.39] |
| 6.2 (Predominantly)White | 6 | 3302 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.36, 2.94] |
| 7 All‐cause mortality: obesity | 10 | 4099 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.50, 2.50] |
| 7.1 BMI ≥ 30 | 6 | 3322 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.36, 2.94] |
| 7.2 BMI < 30 | 4 | 777 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.37, 4.39] |
| 8 Incidence of type 2 diabetes | 11 | 4511 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.50, 0.64] |
| 9 Incidence of type 2 diabetes: duration of the intervention | 11 | 4511 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.50, 0.64] |
| 9.1 Long duration (≥ 4 years) | 4 | 1249 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.74] |
| 9.2 Short duration (< 4 years) | 7 | 3262 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.48, 0.67] |
| 10 Incidence of type 2 diabetes: diagnostic criteria | 11 | 4511 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.50, 0.64] |
| 10.1 IGT | 10 | 4210 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.50, 0.66] |
| 10.2 Other criteria | 1 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.11] |
| 11 Incidence of type 2 diabetes: age | 11 | 4511 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.50, 0.64] |
| 11.1 age ≥ 50 years | 9 | 4110 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.44, 0.58] |
| 11.2 age < 50 years | 2 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.57, 0.85] |
| 12 Incidence of type 2 diabetes: ethnicity | 11 | 4511 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.50, 0.64] |
| 12.1 Asian | 5 | 1235 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.56, 0.81] |
| 12.2 (Predominantly)White | 6 | 3276 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.43, 0.58] |
| 13 Incidence of type 2 diabetes: obesity | 11 | 4511 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.50, 0.64] |
| 13.1 BMI ≥ 30 kg/m2 | 6 | 3318 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.43, 0.58] |
| 13.2 BMI < 30 kg/m2 | 5 | 1193 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.55, 0.80] |
| 14 Cardiovascular mortality | 7 | 3263 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.24, 3.65] |
| 15 Non‐fatal stroke | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16 Non‐serious adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17 Amputation of lower extremity | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18 Fasting plasma glucose | 10 | 3530 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.27, ‐0.06] |
| 19 Fasting plasma glucose: duration of intervention | 10 | 3530 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.27, ‐0.06] |
| 19.1 Long duration (≥ 4 years) | 3 | 697 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.24, 0.01] |
| 19.2 Short duration (< 4 years) | 7 | 2833 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.30, ‐0.03] |
| 20 Fasting plasma glucose: diagnostic criteria | 10 | 3530 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.27, ‐0.06] |
| 20.1 IGT | 9 | 3269 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.26, ‐0.01] |
| 20.2 Other criteria | 1 | 261 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] |
| 21 Fasting plasma glucose: age | 10 | 3530 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.27, ‐0.06] |
| 21.1 Age ≥ 50 years | 8 | 3150 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.26, ‐0.03] |
| 21.2 Age < 50 years | 2 | 380 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.78, ‐0.04] |
| 22 Fasting plasma glucose: ethnicity | 10 | 3530 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.27, ‐0.06] |
| 22.1 Asian | 4 | 760 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.30, 0.05] |
| 22.2 (Predominantly) White | 6 | 2770 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.30, ‐0.04] |
| 23 Fasting plasma glucose: obesity | 10 | 3530 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.27, ‐0.06] |
| 23.1 BMI ≥ 30 kg/m2 | 6 | 2822 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.30, ‐0.03] |
| 23.2 < 30 kg/m2 | 4 | 708 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.34, 0.05] |
| 24 2h plasma glucose | 9 | 3261 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.79, ‐0.12] |
| 25 2 hour plasma glucose: duration of the intervention | 9 | 3261 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.79, ‐0.12] |
| 25.1 Long duration (≥ 4 years) | 3 | 697 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.77, ‐0.05] |
| 25.2 Short duration (< 4 years) | 6 | 2564 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.70, 0.08] |
| 26 2 hour plasma glucose: age | 9 | 3261 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.79, ‐0.12] |
| 26.1 Age ≥ 50 years | 7 | 2881 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.49, ‐0.05] |
| 26.2 Age < 50 years | 2 | 380 | Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐2.49, ‐0.76] |
| 27 2 hour plasma glucose: ethnicity | 9 | 3261 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.79, ‐0.12] |
| 27.1 Asian | 4 | 760 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.72, ‐0.14] |
| 27.2 (Predominantly) White | 5 | 2501 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.55, 0.03] |
| 28 2 hour plasma glucose: obesity | 9 | 3261 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.79, ‐0.12] |
| 28.1 BMI ≥ 30 kg/m2 | 5 | 2553 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.55, 0.02] |
| 28.2 BMI < 30 kg/m2 | 4 | 708 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.77, ‐0.13] |
| 29 Serious adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 30 Hypoglycaemia | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 31 HbA1c | 4 | 2453 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.23, 0.02] |
| 32 Non‐fatal myocardial infarction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 33 End‐stage renal disease | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 34 HbA1c: duration of the intervention | 4 | 2453 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.23, 0.02] |
| 34.1 ≥ 4 years | 2 | 549 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.37, 0.21] |
| 34.2 < 4 years | 2 | 1904 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.36, 0.22] |
| 35 HbA1c: obesity | 4 | 2453 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.23, 0.02] |
| 35.1 BMI ≥ 30 kg/m2 | 3 | 2338 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.27, ‐0.04] |
| 35.2 BMI < 30 kg/m2 | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.17, 0.37] |
2.2. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 2 All‐cause mortality: duration of intervention.
2.3. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 3 All‐cause mortality: diagnostic criteria.
2.4. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 4 All‐cause mortality: age.
2.5. Analysis.
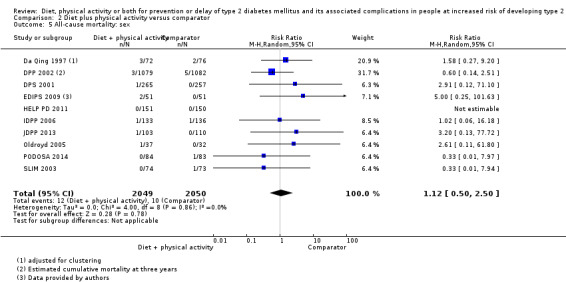
Comparison 2 Diet plus physical activity versus comparator, Outcome 5 All‐cause mortality: sex.
2.6. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 6 All‐cause mortality: ethnicity.
2.7. Analysis.
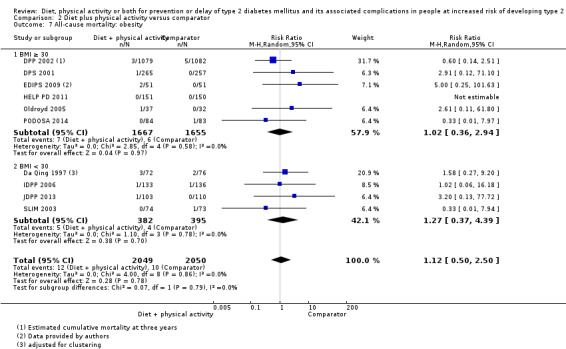
Comparison 2 Diet plus physical activity versus comparator, Outcome 7 All‐cause mortality: obesity.
2.15. Analysis.
Comparison 2 Diet plus physical activity versus comparator, Outcome 15 Non‐fatal stroke.
2.16. Analysis.
Comparison 2 Diet plus physical activity versus comparator, Outcome 16 Non‐serious adverse events.
2.17. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 17 Amputation of lower extremity.
2.19. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 19 Fasting plasma glucose: duration of intervention.
2.20. Analysis.
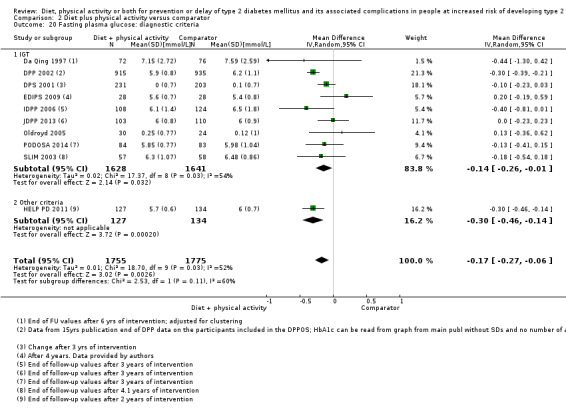
Comparison 2 Diet plus physical activity versus comparator, Outcome 20 Fasting plasma glucose: diagnostic criteria.
2.21. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 21 Fasting plasma glucose: age.
2.22. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 22 Fasting plasma glucose: ethnicity.
2.23. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 23 Fasting plasma glucose: obesity.
2.25. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 25 2 hour plasma glucose: duration of the intervention.
2.26. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 26 2 hour plasma glucose: age.
2.27. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 27 2 hour plasma glucose: ethnicity.
2.28. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 28 2 hour plasma glucose: obesity.
2.30. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 30 Hypoglycaemia.
2.32. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 32 Non‐fatal myocardial infarction.
2.33. Analysis.

Comparison 2 Diet plus physical activity versus comparator, Outcome 33 End‐stage renal disease.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Da Qing 1997.
| Methods | Cluster‐randomised, controlled, clinical trial, randomisation ratio 1:1:1:1 | |
| Participants |
Inclusion criteria: IGT (WHO 1985) Exclusion criteria: ‐ Diagnostic criteria: 2‐h plasma glucose after an OGTT ≥ 7.8 mmol/L and < 11.1 mmol/L (WHO 1985). No explicit mention of FPG measurements, but FPG should be < 7.8 mmol/L according to the criteria suggested by the trial authors |
|
| Interventions |
Number of study centres: 33 healthcare clinics Treatment before study: none Run‐in period: none Extension period: yes, extended follow‐up 17 years after the end of intervention |
|
| Outcomes | Composite outcome measures reported: yes (Quote from publication: "CVD events were defined as the first nonfatal or fatal cardiovascular events including myocardial infarction, sudden death, stroke, or amputation") | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English and Chinese Funding: non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "The trial was designed as a controlled clinical trial in which subjects were randomized by clinic to investigate the incidence of diabetes in people with IGT" | |
| Notes | Each clinic, rather than each participant, was randomised to carry out the intervention on each of the eligible subjects attending that clinic according to one of the 4 specified intervention protocols | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "subjects were randomised by clinic" Comment: insufficient information about the allocation concealment |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information. Physical activity (expressed in units/d) was significantly higher at baseline in the DPPA group than in the control group |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk | Comment: no blinding but judged that the outcome was not likely to be influenced by lack of blinding, investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) amputation, blindness/severe vision loss,end‐stage renal disease | Low risk |
Quote from publication: "Investigators who assessed the outcomes at follow‐up were masked to treatment allocation. Patients and other investigators were not masked." Comment: sufficient blinding of outcome assessment, investigator‐assessed outcome measurement. Not assessed during the intervention period |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "Investigators who assessed the outcomes at follow‐up were masked to treatment allocation. Patients and other investigators were not masked." Comment: sufficient blinding of outcome assessment after the end of intervention. Assume investigators were not blinded during the trial due to the design of the study. Investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: investigator‐assessed outcome measurement. Assume investigators were not blinded during the trial due to the design of the trial. Outcome unlikely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) non‐serious adverse events | High risk | Comment: investigator‐assessed and self‐reported outcome measurement. Assume participants and investigators were not blinded during the trial due to the design of the study. The outcome could be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) serious adverse events | Low risk | Comment: investigator‐assessed outcome measurement. Assume investigators were not blinded during the trial due to the design of the study. Outcome unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause/cardiovascular mortality | Low risk |
Quote from publication: "Investigators who assessed the outcomes at follow‐up were masked to treatment allocation. Patients and other investigators were not masked." and: "From a review of this information, two physicians, blinded to the participant’s intervention, independently determined and assigned the underlying cause of death. A third physician (also blinded to the intervention) settled any disagreements. Only a general classification of cause of death was used (stroke, heart disease or any other CVD, cancer, injuries, diabetes or renal, and other)." Comment: sufficient blinding of outcome assessment after the end of intervention. Assume investigators were not blinded during the trial due to the design of the study. Investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) amputation, blindness/severe vision loss,end‐stage renal disease | Low risk |
Quote from publication: "Investigators who assessed the outcomes at follow‐up were masked to treatment allocation. Patients and other investigators were not masked." Comment: sufficient blinding of outcome assessment, investigator‐assessed outcome measurement. Not assessed during the intervention period |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: Investigator‐assessed outcome measurement. Assume investigators were not blinded during the trial due to the design of the study. Outcome unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) non‐serious adverse events | Unclear risk | Comment: Investigator‐assessed and self‐reported outcome measurement. Assume participants and investigators were not blinded during the trial due to the design of the study. The outcome could be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) serious adverse events | Unclear risk | Comment: Investigator‐assessed outcome measurement. Assume investigators were not blinded during the trial due to the design of the study. Outcome unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause/cardiovascular mortality | Unclear risk |
Quote from publication (extended follow‐up period): "We tried to follow up all the original study participants to establish their vital status. For deceased participants, we collected date and cause of death from death certificates, reviews of medical records, and interviews with proxy informants. We asked proxy informants about the date, place, and circumstances of death along with information about hospitals or physicians from whom the participant had received care around the time of death. We obtained medical records and death certificates and, together with the informant interviews, they were reviewed and adjudicated independently by two doctors (JW and YA) to establish the underlying cause of death." Quote from main publication: "Of the 577 subjects with IGT who were randomized, 530 completed the study. Of the remainder, 7 people refused follow‐up, 29 left Da Qing in 1988 (mostly because of the establishment of a new oil field elsewhere), and 11 died during the course of the study." Comment: not stated if they did the same to establish mortality status at the end of intervention period. Not reported to which group the 29 participants who left the trial were randomised |
| Incomplete outcome data (attrition bias) incidence of T2DM | Unclear risk |
Quote from publication: "Of the 577 subjects with IGT who were randomized, 530 completed the study. Of the remainder, 7 people refused follow‐up,
29 left Da Qing in 1988 (mostly because of the establishment of a new oil field elsewhere), and 11 died during the course of the study." Comment: not reported to which group the 29 participants who left the trial were randomised |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk |
Quote from publication: "Of the 577 subjects with IGT who were randomized, 530 completed the study. Of the remainder, 7 people refused follow‐up,
29 left Da Qing in 1988 (mostly because of the establishment of a new oil field elsewhere), and 11 died during the course of the study." Comment: not reported to which group the 29 participants who left the trial were randomised |
| Incomplete outcome data (attrition bias) non‐serious adverse events | Unclear risk |
Quote from publication: "Of the 577 subjects with IGT who were randomized, 530 completed the study. Of the remainder, 7 people refused follow‐up,
29 left Da Qing in 1988 (mostly because of the establishment of a new oil field elsewhere), and 11 died during the course of the study." Comment: not reported to which group the 29 participants who left the trial were randomised |
| Incomplete outcome data (attrition bias) serious adverse events | Unclear risk |
Quote from publication: "Of the 577 subjects with IGT who were randomized, 530 completed the study. Of the remainder, 7 people refused follow‐up,
29 left Da Qing in 1988 (mostly because of the establishment of a new oil field elsewhere), and 11 died during the course of the study." Comment: not reported to which group the 29 participants who left the trial were randomised |
| Selective reporting (reporting bias) | High risk | Comment: It is apparent from an abstract of the trial that cardiovascular outcomes were collected, but they were not reported in a format suitable for meta‐analyses. One of the articles reported that HRQoL and use of health care was evaluated, but there were no data |
| Other bias | High risk | Comment: the study was cluster‐randomised, and no ICC was reported. Therefore, all data in the meta‐analyses are based on an assumed intra‐cluster coefficient |
DPP 2002.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: ≥ 25 years, BMI ≥ 24 kg/m2 in Asians BMI ≥ 22 kg/m2, FPG 95‐125 mg/dl (5.3‐6.9 mmol/L) and 2 hour plasma glucose after an OGTT 140‐199 mg/dL (7.8‐11.0 mmol/L). Because of the relative higher rate of progression from impaired glucose tolerance to diabetes in Native Americans and the small size of the population, the glucose requirement for eligibility in the Southwest American Indian Center will be fasting glucose < 126 mg/dL (7.0 mmol/L) and 2 hour plasma glucose after an OGTT 140‐199 mg/dL (7.8‐11.0 mmol/L). Exclusion criteria: T2DM, participants taking medicines known to alter glucose tolerance, ever used glucose‐lowering drugs during pregnancy, illnesses that could seriously reduce their life expectancy or their ability to participate in the trial, cardiovascular disease (hospitalisation for treatment of heart disease in past 6 months; NYHA class > 2; left bundle branch block or third degree atrioventricular block; aortic stenosis; SBP > 180 mmHg or DBP > 105 mmHg); cancer requiring treatment in the past five years (unless prognosis is considered good); renal disease; gastrointestinal disease; anaemia (hematocrit < 36.0% in men or < 33.0% in women); electrolyte abnormality (serum potassium < 3.2 or > 5.5 mmol/L). Diagnostic criteria: impaired glucose tolerance (2 hour plasma glucose after an OGTT 140‐199 mg/dl (7.8‐11.0 mmol/L)) and elevated fasting glucose (FPG 95‐125 mg/dl (5.3‐6.9 mmol/L)) (ADA 1997). |
|
| Interventions |
Number of study centres: 27 Treatment before study: none Run‐in period: 3 weeks; during the run‐in period the participants had to fill out a daily dairy and placebo pills according to a schedule Extension period: yes, an additional follow‐up with a median of 5.7 years (IQR 5.5–5.8) after end of the intervention period |
|
| Outcomes | Composite outcome measures reported: yes (Quote from publication: ".....a composite microvascular‐neuropathic outcome for diabetic retinopathy, nephropathy, or reduced light touch sensation in the feet. Secondary outcomes include the individual components of the composite primary outcome, cardiovascular disease, further development of diabetes, measures of glycaemia, insulin secretion, insulin sensitivity, cardiovascular disease risk factors, physical activity, nutrition, bodyweight, health‐related quality of life, and economic assessments.") | |
| Study details | Trial terminated early: yes, the trial was stopped one year earlier than originally planned due to larger intervention effect of diet and physical activity than anticipated. | |
| Publication details |
Language of publication: English Funding: commercial funding (Lipha (Merck‐Sante) provided medicines, and LifeScan donated materials)/non‐commercial funding (the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the Office of Women’s Health, the National Center for Minority Health and Human Disease, the Centers for Disease Control and Prevention, and the American Diabetes Association) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "The principal objective of the DPP is to prevent or delay the development of NIDDM in those persons who are at high risk for its development by virtue of having impaired glucose tolerance" | |
| Notes | Individuals who meet only one of the glucose inclusion criteria was re‐screened after 6 months. Because of the relative higher rate of progression from impaired glucose tolerance to T2DM in Native Americans and the small size of the population, the glucose requirement for eligibility in the Southwest American Indian Center differed (see above) The trial included initially four intervention groups. The metformin group is not included in this review. The troglitazone group was discontinued in 1998 because of potential liver toxicity. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "A sequence of randomization numbers within a clinical center will be constructed of the form XXYZZZ, where XX is the clinical center number, Y is a number that indicates assignment to either the intensive lifestyle intervention or pharmacological treatment, and ZZZ is a three digit sequence number within each XXY combination. The DPP Coordinating Center will prepare the master randomization list with assignments to the three treatment groups within a clinical center using the standard urn design.
The sequence of pharmacological randomization numbers within a clinical center with the specific pharmacological treatment assignment (i.e., metformin or placebo) will be forwarded, in confidence, to the drug distribution center for drug labelling and distribution. Pharmacological treatment assignment to the sequence of pharmacological randomization numbers will be known only by the staff of the DPP Coordinating Center and the drug distribution center." Comment: adequate generation of random sequence ensured |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "A sequence of randomization numbers within a clinical center will be constructed of the form XXYZZZ, where XX is the clinical center number, Y is a number that indicates assignment to either the intensive lifestyle intervention or pharmacological treatment, and ZZZ is a three digit sequence number within each XXY combination. The DPP Coordinating Center will prepare the master randomization list with assignments to the three treatment groups within a clinical center using the standard urn design.
The sequence of pharmacological randomization numbers within a clinical center with the specific pharmacological treatment assignment (i.e., metformin or placebo) will be forwarded, in confidence, to the drug distribution center for drug labelling and distribution. Pharmacological treatment assignment to the sequence of pharmacological randomization numbers will be known only by the staff of the DPP Coordinating Center and the drug distribution center." Comment: adequate allocation concealment ensured |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." Comment: no blinding but judged that the outcome is not likely to be influenced by lack of blinding, investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Primary outcome data (OGTT and FPG results) measured centrally will remain masked to the investigators and to the participants until confirmed progression from IGT to diabetes" Comment: assessed centrally unblinded, the outcome is not likely to be influenced by lack of blinding. Participants and investigators blinded to until progression to T2DM |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Primary outcome data (OGTT and FPG results) measured centrally will remain masked to the investigators and to the participants until confirmed progression from IGT to diabetes" and Plasma lipid levels and HbA1c measured
centrally will remain masked to the investigators and to the participants during the study." Comment: assessed centrally unblinded, the outcome is not likely to be influenced by lack of blinding. Participants and investigators blinded to until progression to T2DM |
| Blinding of participants and personnel (performance bias) socioeconomic effects | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." Comment: no blinding but judged that the outcome is not likely to be influenced by lack of blinding, investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) Health related quality of life | High risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." Comment: no blinding and the outcome is likely to be influenced by lack of blinding, self‐reported outcome measurement |
| Blinding of outcome assessment (detection bias) all‐cause/cardiovascular mortality | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." Comment: no blinding but judged that the outcome is not likely to be influenced by lack of blinding, investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Primary outcome data (OGTT and FPG results) measured centrally will remain masked to the investigators and to the participants until confirmed progression from IGT to diabetes" Comment: assessed centrally unblinded, the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Primary outcome data (OGTT and FPG results) measured centrally will remain masked to the investigators and to the participants until confirmed progression from IGT to diabetes" and "Plasma lipid levels and HbA1c measured
centrally will remain masked to the investigators and to the participants during the study." Comment: assessed centrally unblinded, the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) socioeconomic effects | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." Comment: no blinding but judged that the outcome is not likely to be influenced by lack of blinding, investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) Health related quality of life | High risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." Comment: no blinding and the outcome is likely to be influenced by lack of blinding, self‐reported outcome measurement |
| Incomplete outcome data (attrition bias) all‐cause/cardiovascular mortality | Low risk |
Quote from publication: "At the close of the study, 99.6 percent of the participants were alive, of whom 92.5 percent had attended a scheduled visit within the previous five months" Comment: not stated how many participants who had known vital status in each intervention group at the end of follow‐up for the DPP trial. However, at inception of the number with unknown mortality status are relatively low. At inception of the DPPOS the number between the intervention groups we balanced. |
| Incomplete outcome data (attrition bias) incidence of T2DM | Low risk |
Quote from publication: "At the close of the study, 99.6 percent of the participants were alive, of whom 92.5 percent had attended a scheduled visit within the previous five months" Comment: not stated how many participants who had known vital status at the end of follow‐up for the DPP trial. However, at inception of the DPPOS a relatively low and balanced number of participants in the intervention groups could not be included. |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Low risk | Comment: not stated how many participants who had known vital status at the end of follow‐up for the DPP trial. However, at inception of the DPPOS a relatively low and balanced number between the intervention groups could not be included. |
| Incomplete outcome data (attrition bias) time to progression to T2DM | Low risk | Comment: "At the close of the study, 99.6 percent of the participants were alive, of whom 92.5 percent had attended a scheduled visit within the previous five months" |
| Incomplete outcome data (attrition bias) socioeconomic effects | Low risk | Comment: not clearly described how many participants included in the costs analyses, but as the study have a high follow‐up rate, we assume that nearly all participants are included. |
| Incomplete outcome data (attrition bias) Health related quality of life | Low risk |
Quote from publication: The current report and analyses includes 3,234 participants seen at baseline, who were randomly assigned to one of the three treatment arms investigated. Comment: article reporting health related quality of life do not report the number of participants with available data at follow‐up |
| Selective reporting (reporting bias) | High risk | Comment: several outcome are likely to be measured and analysed, but not reported, e.g. hypoglycaemia, non‐serious adverse events. outcomes published in many different publications. Many outcomes are reported incompletely so that they cannot be entered in a meta‐analysis |
| Other bias | High risk |
Comment: trial terminated early for benefit Comment: received funding from a pharmaceutical company |
DPS 2001.
| Methods | Parallel, randomised, controlled, clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: BMI ≥ 25 kg/m2; IGT (2‐h plasma glucose after an OGTT 140‐200 mg/dL (7.8‐11.0 mmol/L)) and FPG < 140 mg/dL (7.8 mmol/L) (WHO 1985), 40‐65 years Exclusion criteria: diagnosis of diabetes mellitus, involvement of people with regularly vigorous physical activity, chronic disease, diseases likely to interfere with glucose metabolism (e.g. liver disease), psychological or physical disabilities deemed likely to interfere with participation in the study Diagnostic criteria: IGT (2‐h plasma glucose after an OGTT 140‐200 mg/dL (7.8‐11.0 mmol/L)) and FPG < 140 mg/dL (7.8 mmol/L) (WHO 1985) |
|
| Interventions |
Number of study centres: 5 Treatment before study: none Titration period: none |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated early: yes (the trial was prematurely terminated in March 2000 by an independent end point committee, since the incidence of diabetes in the intervention group was highly significantly lower than in the control group) | |
| Publication details |
Language of publication: English Funding: commercial funding (Novo Nordisk Foundation)/non‐commercial funding (Finnish Academy, Ministry of Education, Yrjö Jahnsson Foundation, and the Finnish Diabetes Research Foundation) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "The aim of the Diabetes Prevention Study is to assess the efficacy of an intensive diet‐exercise programme in preventing or delaying Type II (non‐insulin‐dependent) diabetes mellitus in subjects with impaired glucose tolerance, to evaluate the effects of the intervention programme on cardiovascular risk factors and to assess the determinants for the progression to diabetes in persons with impaired glucose tolerance." | |
| Notes | After the first screening OGTT, a repeat OGTT was carried out in participants with IGT and the mean value of the two 2‐h glucose concentrations was used as the criterion for inclusion in the study. The inclusion criteria were developed during the recruitment period but before the final criteria based on the two OGTTs were decided. After randomisation, study visits were scheduled for 1‐2 weeks, 5‐6 weeks, 3, 4 and 6 months from the beginning of the study and thereafter every 3 months. Every 3 months, 3‐d food records were completed throughout the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Subjects who enrolled in the study were randomly assigned to the intervention group or the control group by the study physician, with the use of a randomization list..." and "For the DPS and EDIPS‐Newcastle (but not SLIM) the randomisation lists were generated and supplied by the coordinating centre in Helsinki and staff who made baseline measurements had no access to the randomisation lists." Comment: adequate generation of random sequence ensured |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Subjects who enrolled in the study were randomly assigned to the intervention group or the control group by the study physician, with the use of a randomization list,.." and "For the DPS and EDIPS‐Newcastle (but not SLIM) the randomisation lists were generated and supplied by the coordinating centre in Helsinki and staff who made baseline measurements had no access to the randomisation lists." Comment: adequate allocation concealment ensured |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "The nurses who scheduled the study visits did not have access to the randomization list. However, the staff members involved in the intervention had to be aware of the group assignment; thus, the study was only partly blinded. Laboratory staff did not know the subjects’ group assignments, and the subjects were not informed of their plasma glucose concentrations during follow‐up unless diabetes was diagnosed." Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "The nurses who scheduled the study visits did not have access to the randomization list. However, the staff members involved in the intervention had to be aware of the group assignment; thus, the study was only partly blinded. Laboratory staff did not know the subjects’ group assignments, and the subjects were not informed of their plasma glucose concentrations during follow‐up unless diabetes was diagnosed." "The independent end‐points committee confirmed all newly diagnosed cases of diabetes" Comment: outcome evaluated by an independent outcome committee. Not described whether this committee was blinded. We assume that the outcome committee was blinded, however the outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk |
Quote from publication: "The nurses who scheduled the study visits did not have access to the randomization list. However, the staff members involved in the intervention had to be aware of the group assignment; thus, the study was only partly blinded. Laboratory staff did not know the subjects’ group assignments, and the subjects were not informed of their plasma glucose concentrations during follow‐up unless diabetes was diagnosed." Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) time to progression to T2DM | Low risk |
Quote from publication: "The nurses who scheduled the study visits did not have access to the randomization list. However, the staff members involved in the intervention had to be aware of the group assignment; thus, the study was only partly blinded. Laboratory staff did not know the subjects’ group assignments, and the subjects were not informed of their plasma glucose concentrations during follow‐up unless diabetes was diagnosed." "The independent end‐points committee confirmed all newly diagnosed cases of diabetes" Comment: outcome evaluated by an independent outcome committee. Not described whether this committee was blinded. We assume that the outcome committee was blinded, however the outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause/cardiovascular mortality | Low risk |
Quote from publication: "The nurses who scheduled the study visits did not have access to the randomization list. However, the staff members involved in the intervention had to be aware of the group assignment; thus, the study was only partly blinded. Laboratory staff did not know the subjects’ group assignments, and the subjects were not informed of their plasma glucose concentrations during follow‐up unless diabetes was diagnosed." Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk |
Quote from publication: "The nurses who scheduled the study visits did not have access to the randomization list. However, the staff members involved in the intervention had to be aware of the group assignment; thus, the study was only partly blinded. Laboratory staff did not know the subjects’ group assignments, and the subjects were not informed of their plasma glucose concentrations during follow‐up unless diabetes was diagnosed." "The independent end‐points committee confirmed all newly diagnosed cases of diabetes" Comment: outcome evaluated by an independent outcome committee. Not described whether this committee was blinded. We assume that the outcome committee was blinded, and the outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk |
Quote from publication: "The nurses who scheduled the study visits did not have access to the randomization list. However, the staff members involved in the intervention had to be aware of the group assignment; thus, the study was only partly blinded. Laboratory staff did not know the subjects’ group assignments, and the subjects were not informed of their plasma glucose concentrations during follow‐up unless diabetes was diagnosed." Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) time to progression to T2DM | Low risk |
Quote from publication: "The nurses who scheduled the study visits did not have access to the randomization list. However, the staff members involved in the intervention had to be aware of the group assignment; thus, the study was only partly blinded. Laboratory staff did not know the subjects’ group assignments, and the subjects were not informed of their plasma glucose concentrations during follow‐up unless diabetes was diagnosed." "The independent end‐points committee confirmed all newly diagnosed cases of diabetes" Comment: outcome evaluated by an independent outcome committee. Not described whether this committee was blinded. We assume that the outcome committee was blinded, and the outcome was not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause/cardiovascular mortality | High risk |
Quote from publication: "During the post‐intervention follow‐up, 36 additional participants withdrew and ten died without a verified diabetes diagnosis" Comment: participants diagnosed with T2DM not included in the mortality analyses |
| Incomplete outcome data (attrition bias) incidence of T2DM | Unclear risk |
Quote from publication: "During the study, 40 subjects (8 percent) withdrew — 23 in the intervention group and 17 in the control group. Of these subjects, 9 could not be contacted, 3 withdrew due to severe illness, 1 died, and 27 withdrew for personal reasons." "Subjects who withdrew from the study were considered to be at risk for diabetes until their last oral glucose tolerance test, at which point data were censored." 9 yrs publication: "The last‐observation carried‐forward method was applied to all measurements for those participants who developed diabetes or who were lost to follow‐up". Quote from 9 years publication: "Altogether, 86 participants were lost to follow up without a diabetes diagnosis: 49 in the intervention group and 37 in the control group. The baseline characteristics of the dropouts were similar between the groups." Comment: inappropriate method of imputation |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk | Comment: not explicitly stated how many participants were included in the analyses |
| Incomplete outcome data (attrition bias) time to progression to T2DM | Unclear risk |
Quote from publication: "During the study, 40 subjects (8 percent) withdrew — 23 in the intervention group and 17 in the control group. Of these subjects, 9 could not be contacted, 3 withdrew due to severe illness, 1 died, and 27 withdrew for personal reasons." "Subjects who withdrew from the study were considered to be at risk for diabetes until their last oral glucose tolerance test, at which point data were censored." 9 yrs publication: "The last‐observation carried‐forward method was applied to all measurements for those participants who developed diabetes or who were lost to follow‐up". and Quote from 9 years publication: "Altogether, 86 participants were lost to follow up without a diabetes diagnosis: 49 in the intervention group and 37 in the control group. The baseline characteristics of the dropouts were similar between the groups." Comment: inappropriate method of imputation |
| Selective reporting (reporting bias) | High risk | Comment: morbidity was described as being reported in design article, but no data available |
| Other bias | High risk |
Comment: trial terminated early for benefit Comment: received funding from the Novo Nordisk Foundation |
EDIPS 2009.
| Methods | Parallel, randomised, controlled, clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: IGT (mean 2‐h plasma glucose > 7.8 and < 11.1 mmol/L (WHO 1999) from 2 OGTTs, the 2nd conducted 1‐12 weeks after the 1st); aged 40‐74 years; BMI > 25 kg/m2 Exclusion criteria: previous diagnosis of T2DM according to WHO 1999 criteria; previous intensive treatment for IGT; previous participation in a programme of vigorous physical activity Diagnostic criteria: IGT (mean 2‐h plasma glucose after OGTTs > 7.8 and < 11.1 mmol/L) (WHO 1999) No explicit mention of FPG measurements but FPG should be < 7.0 mmol/L according to the criteria suggested by the trial authors |
|
| Interventions |
Number of study centres: 1 Run‐in period: none Extension period: no |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English Funding: non‐commercial funding (Wellcome Trust) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "The EDIPS in Newcastle upon Tyne, UK (EDIPS‐Newcastle) was designed to contribute to the evidence for diabetes prevention by lifestyle modification in people with IGT." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Eligible participants (with IGT) were randomly allocated to the Intervention (I) or Control (C) group using randomisation lists, prepared independently by the EDIPS co‐ordinating centre in Helsinki." Comment: adequate generation of random sequence ensured |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Eligible participants (with IGT) were randomly allocated to the Intervention (I) or Control (C) group using randomisation lists, prepared independently by the EDIPS co‐ordinating centre in Helsinki." Comment: adequate allocation concealment ensured |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "Blinding of participants and intervention staff was not possible. Data collection staff were blinded to the extent that this was possible given participants' knowledge of their allocation." Comment: investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) amputation, blindness/severe vision loss,end‐stage renal disease | Low risk | Comment: information provided by the trial authors. Investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) hypoglycaemia | Low risk | Comment: information provided by the trial authors. Investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "Blinding of participants and intervention staff was not possible. Data collection staff were blinded to the extent that this was possible given participants' knowledge of their allocation." Comment: investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: information provided by the trial authors. Investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) non‐fatal myocardial infarction/stroke,congestive heart failure | Low risk | Comment: information provided by the trial authors. Investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) serious adverse events | Low risk | Comment: information provided by the trial authors. Investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) time to progression to T2DM | Low risk | Comment: information provided by the trial authors. Investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause/cardiovascular mortality | Low risk |
Quote from publication: "Blinding of participants and intervention staff was not possible. Data collection staff were blinded to the extent that this was possible given participants' knowledge of their allocation." Comment: investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk |
Quote from publication: "Blinding of participants and intervention staff was not possible. Data collection staff were blinded to the extent that this was possible given participants' knowledge of their allocation." Comment: investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: information provided by the trial authors. Investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) non‐fatal myocardial infarction/stroke, congestive heart failure | Low risk | Comment: information provided by the trial authors. Investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause/cardiovascular mortality | Low risk | Comment: 2 in the control group and 4 in the intervention group had unknown mortality status; missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; the proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) amputation, blindness/severe vision loss,end‐stage renal disease | Low risk | Comment: information provided by the trial authors. All participants were included in the analysis |
| Incomplete outcome data (attrition bias) hypoglycaemia | Low risk | Comment: information provided by the trial authors. All participants were included in the analysis |
| Incomplete outcome data (attrition bias) incidence of T2DM | Low risk |
Quote from publication: "If participants who left the trial and were later reported to have developed T2D by their physician were included in the analysis as having developed diabetes (rather than having left), then the number of cases of diabetes becomes 20 (I = 7, C = 13) and the relative risk of diabetes incidence becomes 0.54 (95% CI:0.2 to 1.2)." Comment: the investigators performed analyses including all participants and the participants who left the trial. The statistical significance of the effect estimate did not differ |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk | Comment: information provided by the trial authors. Unknown reason for missingness and how missing data were handled |
| Incomplete outcome data (attrition bias) non‐fatal myocardial infarction/stroke,congestive heart failure | Low risk | Comment: information provided by the trial authors. Investigator‐assessed outcome measurement. The outcome was not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) serious adverse events | Low risk | Comment: information provided by the trial authors. All randomised participants were included in the analysis |
| Incomplete outcome data (attrition bias) time to progression to T2DM | Unclear risk | Comment: information provided by the trial authors. Unknown reason for missingness and how missing data were handled |
| Selective reporting (reporting bias) | High risk | Comment: more outcomes of relevance to this review defined but not published (e.g. glycaemic measures). However, these data were provided by the investigators on request, except for HRQoL, which was listed as an outcome, but no additional data were provided |
| Other bias | Low risk | Comment: no other sources of bias identified |
Hellgren 2016.
| Methods | Parallel, randomised, controlled, clinical trial, randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: 35‐75 years; IGT and/or IFG Exclusion criteria: T2DM Diagnostic criteria: IFG was defined as FPG > 6.0 < 7.0 mmol/L with a 2‐h glucose < 8.9 mmol/L, while IGT was defined as 2‐h glucose 8.8 mmol/L and < 12.2 mmol/L, and an FPG < 7.0 mmol/L |
|
| Interventions |
Number of study centres: 1 Run‐in period: none Extension period: no |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English Funding: non‐commercial funding (Swedish Research Council) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "We hypothesised that the expected increase in insulin resistance over three years’ time in individuals with impaired glucose tolerance (IGT) and/or impaired fasting glucose could be attenuated by an intervention with focus on physical activity in ordinary primary care" | |
| Notes | Data from the two intervention groups were initially analysed separately. As the outcomes proved to be essentially the same in both groups, the two groups were then analysed together and thereafter designated as the combined intervention group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "All eligible participants were randomised to one of two different interventions, or to ‘care as usual’, using a specially designed computer system." Comment: adequate generation of random sequence ensured |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "All eligible participants were randomised to one of two different interventions, or to ‘care as usual’, using a specially designed computer system." Comment: adequate generation of allocation concealment ensured |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) amputation, blindness/severe vision loss,end‐stage renal disease | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) hypoglycaemia | Unclear risk | Comment: could be investigator‐assessed or self‐reported outcome measure depending on the severity. No blinding described |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) non‐fatal myocardial infarction/stroke,congestive heart failure | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) serious adverse events | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Health related quality of life | High risk | Comment: self‐reported outcome measure. No blinding. The outcome was likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause/cardiovascular mortality | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) amputation, blindness/severe vision loss,end‐stage renal disease | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) hypoglycaemia | Unclear risk | Comment: could be investigator‐assessed or self‐reported outcome measure depending on the severity. No blinding described |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) non‐fatal myocardial infarction/stroke, congestive heart failure | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) serious adverse events | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Health related quality of life | High risk | Comment: self‐reported outcome measure. No blinding. The outcome was likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause/cardiovascular mortality | Low risk | Comment: reply from the investigators that mortality status was known on all randomised participants |
| Incomplete outcome data (attrition bias) incidence of T2DM | Low risk |
Quote from publication: "Four people died during the first year from reasons not associated with the study, and 10 of the participants developed Type 2 diabetes and refrained from the final examination. Another 13 participants dropped out of the study for various reasons (e.g. worsening general health, difficulties attending the group sessions and other social reasons). Most of the dropouts were in the intervention group (N = 5 in the IIG and N = 6 in the BIG)." Comment: a total of 13 participants dropped out, but were contacted and asked at the end of the trial and asked if they had developed T2DM. 11 of these were from the intervention group; 2 of these from the standard group. The reason for dropout was not explained. The proportion of missing outcomes was enough to induce clinically relevant bias in intervention effect estimate |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk | Comment: data provided by the investigators. Only about 70% of the randomised participants were included in the analyses. Unclear how missing data were handled. |
| Incomplete outcome data (attrition bias) non‐fatal myocardial infarction/stroke,congestive heart failure | Unclear risk | Comment: data provided by the investigators. Only about 80% were included in the analyses. Not clear how missing data were handled |
| Incomplete outcome data (attrition bias) serious adverse events | Unclear risk | Comment: information on number provided by the trial authors. Participants lost to follow‐up not included in the analysis |
| Incomplete outcome data (attrition bias) Health related quality of life | High risk | Comment: data provided by the investigators. Only about 60% of the randomised participants were included in the analyses. Not clear how missing data were handled |
| Selective reporting (reporting bias) | Low risk | Comment: trial protocol provided by the investigator. The trial assessed several outcomes of interest to the review but these were either not reported in publications or reported in a format that made them unsuitable for meta‐analysis. However, the investigators provided the additional information |
| Other bias | Low risk | Comment: no other sources of bias identified |
HELP PD 2011.
| Methods | Parallel, randomised, controlled, clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: able to read/understand English at or above a level sufficient to comprehend recruitment and intervention materials; BMI ≥ 25 kg/m2 but < 40 kg/m2; fasting blood glucose 95‐125 mg/dL (inclusive); ≥ 21 years Exclusion criteria: currently involved in a supervised programme for weight loss; history of DM, or newly diagnosed DM at screening; history of CVD occurring within the past 6 months, including myocardial infarction, angina, coronary revascularisation, stroke, transient ischaemic attack, carotid revascularisation, peripheral arterial disease, and congestive heart failure; uncontrolled high blood pressure (> 160/100 mmHg); pregnancy, breast feeding, or planning pregnancy within 2 years; other chronic disease likely to limit lifespan to < 2‐3 years, including any cancer requiring treatment in past 5 years except non‐melanoma skin cancer; chronic use of medicine known to significantly affect glucose metabolism, e.g. corticosteroids; conditions/criteria likely to interfere with participation and acceptance of randomised assignment, including the following: inability/unwillingness to give informed consent, another household member already randomised to HELP PD, major psychiatric or cognitive problems (schizophrenia, dementia, self‐reported active illegal substance or alcohol abuse), and participation in another research study that would interfere with HELP PD Diagnostic criteria: fasting blood glucose 95‐125 mg/dL (5.3‐6.9 mmol/L) (inclusive) |
|
| Interventions |
Number of study centres: 1 Run‐in period: none Extension period: according to clinicaltrial.com then an extension period is planned |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English Funding: non‐commercial funding (National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)). Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To examine the impact of a 24‐month, community‐based diabetes prevention program on fasting blood glucose, insulin, insulin resistance as well as body weight, waist circumference, and BMI in the second year of follow‐up" | |
| Notes | Some of the publications were supported by Joslin Diabetes Center and Novo Nordisk. According to ClinicalTrials.gov an extended follow‐up period up to 72 months after randomisation was planned. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Eligible participants were randomly assigned, if equal probability, to either the lifestyle intervention or the enhanced usual care arm using a web‐based data management system that verifies eligibility" Comment: adequate generation of random sequence ensured |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Eligible participants were randomly assigned, if equal probability, to either the lifestyle intervention or the enhanced usual care arm using a web‐based data management system that verifies eligibility" Comment: adequate allocation concealment ensured |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Unclear risk |
Quote from publication: "Outcomes are masked and are collected every 6 months" "Although neither the participants nor interventionists were masked to treatment assignment, the primary outcome, fasting blood glucose, was chosen to be highly objective." Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "Outcomes are masked and are collected every 6 months" "Although neither the participants nor interventionists were masked to treatment assignment, the primary outcome, fasting blood glucose, was chosen to be highly objective." Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk |
Quote from publication: "Outcomes are masked and are collected every 6 months" "Although neither the participants nor interventionists were masked to treatment assignment, the primary outcome, fasting blood glucose, was chosen to be highly objective." Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) socioeconomic effects | Low risk | Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause/cardiovascular mortality | Low risk |
Quote from publication: "Outcomes are masked and are collected every 6 months" "Although neither the participants nor interventionists were masked to treatment assignment, the primary outcome, fasting blood glucose, was chosen to be highly objective." Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk |
Quote from publication: "Outcomes are masked and are collected every 6 months" "Although neither the participants nor interventionists were masked to treatment assignment, the primary outcome, fasting blood glucose, was chosen to be highly objective." Comment: investigator assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk |
Quote from publication: "Outcomes are masked and are collected every 6 months" "Although neither the participants nor interventionists were masked to treatment assignment, the primary outcome, fasting blood glucose, was chosen to be highly objective." Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) socioeconomic effects | Low risk | Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause/cardiovascular mortality | Low risk |
Quote from publication: "An intention‐to‐treat approach was used and included all postrandomized values according to the group they were assigned" Comment: 1% of the randomised participants refused or withdrew by 24 months' assessment in the intervention group; 3% of the randomised participants in the control group refused or withdrew by 24 months' follow‐up. |
| Incomplete outcome data (attrition bias) incidence of T2DM | Unclear risk |
Quote from publication: "An intention‐to‐treat approach was used and included all postrandomized values according to the group they were assigned" Comment: 84% of the randomised participants completed 24‐month assessment in the intervention group; 89% of the randomised participants in the control group completed 24 months' follow‐up. Insufficient information to assess whether missing data were likely to induce bias |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk |
Quote from publication: "An intention‐to‐treat approach was used and included all postrandomized values according to the group they were assigned" Comment: 84% of the randomised participants completed 24‐month assessment in the intervention group; 89% of the randomised participants in the control group completed 24 months' follow‐up. Insufficient information to assess whether missing data were likely to induce bias |
| Incomplete outcome data (attrition bias) time to progression to T2DM | Unclear risk |
Quote from publication: "The distribution of times until the development of DM and the metabolic syndrome (measured from the date of randomization to the date of the clinical visit or report triggering the diagnosis) will be described using Kaplan‐Meier plots ..., with censoring taken to occur at the time of the last contact with participants" Comment: data were censored at the last time of contact with the participants, and not the time of diagnosis which could exaggerate a potential intervention effect. |
| Incomplete outcome data (attrition bias) socioeconomic effects | Unclear risk |
Quote from publication: "An intention‐to‐treat approach was used and included all postrandomized values according to the group they were assigned" Comment: 84% of the randomised participants completed 24‐month assessment in the intervention group; 89% of the randomised participants in the control group completed 24 months' follow‐up. Insufficient information to assess whether missing data were likely to induce bias |
| Selective reporting (reporting bias) | High risk | Comment: several outcomes of interest for this review were measured but not reported (see table Appendix 7) |
| Other bias | Unclear risk | Comment: some of the publications were supported by Novo Nordisk. According to ClinicalTrials.gov an extended follow‐up period to 72 months after randomisation was planned. Data from the extension period are still not available |
IDPP 2006.
| Methods | Parallel, randomised, controlled, clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: IGT (mean 2‐h plasma glucose after OGTT 140‐199 mg/dL (7.8‐11.0 mmol/L) and FPG < 126 mg/dL (7.0 mmol/L)) (WHO 1999); no major illness; 35‐55 years Exclusion criteria: diagnosis of DM during recruitment; pregnancy Diagnostic criteria: IGT (WHO 1999) |
|
| Interventions |
Number of study centres: ‐ Treatment before study: none Titration period: none |
|
| Outcomes | Composite outcome measures reported: yes (CVD) | |
| Study details | Trial terminated early: yes; Quote from publication: "After a median follow‐up period of 30 months, because there were significant differences in the outcome measure between the control and intervention groups, the committee recommended the termination of the study in December 2004" | |
| Publication details |
Language of publication: English Funding: commercial (M/S US Vitamins) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "In a prospective community‐based study, we tested whether the progression to diabetes could be influenced by interventions in native Asian Indians with IGT who were younger, leaner and more insulin resistant than the above populations" | |
| Notes | Two more intervention groups existed that were not included in this review; 1 ) metformin and 2) diet plus physical activity combined with metformin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "A randomised, controlled clinical trial was performed in subjects who were......" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "Masking: Open Label" and "However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: outcome evaluated by an independent outcome committee. No blinding |
| Blinding of participants and personnel (performance bias) hypoglycaemia | High risk |
Quote from publication: "Masking: Open Label" and "However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: self‐reported and investigator‐assessed outcome measure. No blinding. The outcome could have been influenced by lack of blinding. No hypoglycaemic events in the groups of interest for this review were reported |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "Masking: Open Label" "However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: outcome evaluated by an independent outcome committee and investigator‐assessed outcome measure |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk |
Quote from publication: "Masking: Open Label" and ""However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: investigator‐assessed outcome measure, unclear if this outcome also was assessed by the blinded independent outcome committee. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause/cardiovascular mortality | Low risk |
Quote from publication: "However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: outcome evaluated by an independent outcome committee |
| Blinding of outcome assessment (detection bias) hypoglycaemia | High risk | Comment: self‐reported and investigator‐assessed outcome measure. No blinding. The outcome could have been influenced by lack of blinding. No hypoglycaemic events in the groups of interest for this review were reported |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk |
Quote from publication: "However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: outcome evaluated by an independent outcome committee and investigator‐assessed outcome measure |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk |
Quote from publication: "However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: investigator‐assessed outcome measure, unclear if this outcome also was assessed by the blinded independent outcome committee. The outcome was not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause/cardiovascular mortality | Unclear risk | Comment: unknown whether mortality status known for participants lost to follow‐up. 7 participants in the DPPA group and 2 participants in the control group were lost to follow‐up; 5 participants in the DPPA group were mentioned as not willing. However, the proportion of missing outcomes compared with observed event risk may have had a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) hypoglycaemia | Unclear risk | Comment: 7 participants in the DPPA group and 2 participants in the control group were lost to follow‐up; 5 participants in the DPPA group were mentioned as not willing. The proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) incidence of T2DM | Unclear risk | Comment: 7 participants in the DPPA group and 2 participants in the control group were lost to follow‐up; 5 participants in the DPPA group were mentioned as not willing. The proportion of missing outcomes compared with observed event risk was not enough to have had a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk | Comment: only 79% of the participants randomised to the intervention group and 91% of the participants randomised to the control group were included in the analysis of glycaemic measures. Insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias |
| Selective reporting (reporting bias) | High risk |
Quote from publication: "An internal safety committee monitored the adverse events and safety of study protocol. The data and final outcome measures were monitored by the international monitoring committee who had looked at the results three times, i.e. when 500 subjects had completed the follow‐up assessments at 12, 24 and 30 months. The principal investigators were blinded to the interim results." Comment: several outcomes with relevance for this review not reported or only reported in a format that made them unsuitable for meta‐analyses, e.g. adverse events |
| Other bias | High risk |
Comment: trial terminated early for benefit Comment: role of funding source not described |
JDPP 2013.
| Methods | Parallel, randomised, controlled, clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: aged 30‐60 years; IGT based on WHO 1999 criteria (mean 2‐ho plasma glucose after OGTT 7.8‐11.0 mmol/L and FPG < 7.0 mmol/L) Exclusion criteria: previous diagnosis of DM other than gestational diabetes; a history of gastrectomy; physical conditions such as ischaemic heart disease, heart failure, exercise‐induced asthma and orthopedic problems where physical activity was not allowed by a doctor; liver and kidney diseases; autoimmune diseases; habit of heavy alcohol drinking, already having vigorous physical activity Diagnostic criteria: several diagnostic criteria for impaired glucose tolerance, therein the WHO 1998 |
|
| Interventions |
Number of study centres: 32 Treatment before study: ‐ Titration period: ‐ |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English and Japanese Funding: non‐commercial funding (Ministry of Health, Labour and Welfare, Japan) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "A randomized control trial was performed to test whether a lifestyle intervention program, carried out in a primary healthcare setting using existing resources, can reduce the incidence of type 2 diabetes in Japanese with impaired glucose tolerance (IGT)." | |
| Notes | This trial was excluded from the original Cochrane Review as it was classified as not randomised. The sample size calculation of the trial was based on an anticipated incidence of T2DM during 6 years of follow‐up. It was described that six years follow‐up was planned, but only three years follow‐up was reported. Quote from publication: "Participants with IGT, aged 30–60 years, were recruited through health checkups conducted at each collaborative center. The recruitment started in March 1999 and was completed in December 2002. A two‐step strategy was adopted for identifying participants with IGT as described previously. The definition of IGT using 75 g oral glucose tolerance test (OGTT) was based on the WHO’s criteria" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Participants were randomly allocated (allocation ratio 1:1) to the ILG or the UCG, using a computer‐generated randomization. The Taves method of minimization13 was used to ensure that the groups were balanced for public health centers, gender, age groups" Comment: adequate generation of random sequence ensured |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Participants were randomly allocated (allocation ratio 1:1) to the ILG or the UCG, using a computer‐generated randomization. The Taves method of minimization13 was used to ensure that the groups were balanced for public health centers, gender, age groups" Comment: adequate allocation concealment ensured |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "The result of the randomization was unmasked to the participants, those administering the interventions, and those assessing the data" Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "The result of the randomization was unmasked to the participants, those administering the interventions, and those assessing the data" Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk |
Quote from publication: "The result of the randomization was unmasked to the participants, those administering the interventions, and those assessing the data" Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause/cardiovascular mortality | Unclear risk |
Quote from publication: "We randomly assigned the 304 subjects with IGT to two groups and analyzed the data for 296 individuals (150 in the control group and 146 in the intervention group) (Figure 1). A total of 83 subjects (28%) withdrew from the study before the 3‐year mark (40 in the control group and 43 in the intervention group). The withdrawals were due to personal reasons (moving etc) in 18 cases, medical reasons in 5, and loss of contact in 40. Twenty subjects were not able to continue the study for reasons related to the collaborative centers themselves, such as the closure of a center. The rate of withdrawal was higher among men than women (36.9% vs. 19.0%, p < 0.01)." Comment: reason for loss to follow‐up in intervention group: personal reasons (N = 11); medical reasons (N = 3); loss of contact (N = 21); closure of centres (N = 8); reason for loss to follow‐up in the comparator group: personal reasons (N = 7); medical reasons (N = 2); loss of contact (N = 19); closure of centres (N = 12). A relatively large number of participants did not complete the trial. However, the number and reasons for missingness were balanced between the intervention groups |
| Incomplete outcome data (attrition bias) incidence of T2DM | Unclear risk |
Quote from publication: "We randomly assigned the 304 subjects with IGT to two groups and analyzed the data for 296 individuals (150 in the control group and 146 in the intervention group) (Figure 1). A total of 83 subjects (28%) withdrew from the study before the 3‐year mark (40 in the control group and 43 in the intervention group). The withdrawals were due to personal reasons (moving etc) in 18 cases, medical reasons in 5, and loss of contact in 40. Twenty subjects were not able to continue the study for reasons related to the collaborative centres themselves, such as the closure of a center. The rate of withdrawal was higher among men than women (36.9% vs. 19.0%, p < 0.01)." Comment: reason for loss to follow‐up in intervention group: personal reasons (N = 11); medical reasons (N = 3); loss of contact (N = 21); closure of centres (N = 8); reason for loss to follow‐up in comparator group: personal reasons (N = 7); medical reasons (N = 2); loss of contact (N = 19); closure of centres (N = 12). A relatively large number of participants did not complete the trial. However, the number and reasons for missingness were balanced between the intervention groups |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk |
Quote from publication: "We randomly assigned the 304 subjects with IGT to two groups and analyzed the data for 296 individuals (150 in the control group and 146 in the intervention group) (Figure 1). A total of 83 subjects (28%) withdrew from the study before the 3‐year mark (40 in the control group and 43 in the intervention group). The withdrawals were due to personal reasons (moving etc) in 18 cases, medical reasons in 5, and loss of contact in 40. Twenty subjects were not able to continue the study for reasons related to the collaborative centers themselves, such as the closure of a center. The rate of withdrawal was higher among men than women (36.9% vs. 19.0%, p < 0.01)." Comment: reason for loss to follow‐up in intervention group: personal reasons (N = 11); medical reasons (N = 3); loss of contact (N = 21); closure of centres (N = 8); reason for loss to follow‐up in comparator group: personal reasons (N = 7); medical reasons (N = 2); loss of contact (N = 19); closure of centres (N = 12). A relatively large number of participants did not complete the trial. However, the number and reasons for missingness were balanced between the intervention groups |
| Selective reporting (reporting bias) | High risk | Comment: it is clear from publication that adverse events were collected and HbA1c analysed, but data could not be entered in the meta‐analysis |
| Other bias | Low risk | Comment: no other sources of bias identified |
Kosaka 2005.
| Methods | Parallel, randomised, controlled, clinical trial, randomisation ratio 1:4 | |
| Participants |
Inclusion criteria: impaired glucose tolerance (FPG < 140 mg/dL (7.8 mmol/L) and a 2‐h plasma glucose value between 160‐239 mg/dL (8.9‐13.3 mmol/L) on 100 g OGTTs; which roughly corresponds to 140‐199 mg/dL on 75 g OGTT); men Exclusion criteria: known DM, diagnosed or suspected malignant neoplasm, diagnosed or suspected disease of the liver, pancreas, endocrine organs, or kidney; ischaemic heart disease or cerebrovascular disease or a history of such disease Diagnostic criteria: IGT roughly according to the WHO 1980 criteria (WHO 1980) |
|
| Interventions |
Number of study centres: ‐ Treatment before study: ‐ Titration period: none |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English Funding: NR Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "In this paper, we report that the development of diabetes can be significantly prevented by intervention in lifestyle designed to achieve and maintain the ideal body weight of each individual during an observation period of 4 years in subjects with IGT." | |
| Notes | Only men were included as trial authors expected a higher dropout rate among women | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "One of every five subjects was randomly selected for allocation the intensive intervention group, and the others were assigned to the standard intervention (control) group." Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: investigator‐assessed outcome measure. No blinding of participants described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Unclear risk | Comment: investigator‐assessed outcome measure. No blinding of outcome assessors described. The outcome was not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) incidence of T2DM | High risk | Comment: 88.8% of the originally randomised participants in the intervention group and 86.2% of the originally randomised participants in the control group had complete data for the diabetes outcome. Method of handling missing data as well as explanation of dropouts was not stated in the paper |
| Selective reporting (reporting bias) | High risk | Comment: no trial protocol available. Glycaemic measures analysed but no data reported |
| Other bias | Unclear risk | Comment: no funding source provided |
Oldroyd 2005.
| Methods | Parallel, randomised, controlled, clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: IGT (2‐h plasma glucose after an OGTT 140‐200 mg/dL (7.8‐11.0 mmol/L)) (WHO 1985); 24‐75 years; European origin Exclusion criteria: pregnant individuals, on therapeutic diets or whose medical condition prevented them from undertaking moderate physical activity Diagnostic criteria: IGT (2‐h plasma glucose after an OGTT 140‐200 mg/dL (7.8‐11.0 mmol/L)) (WHO 1985) |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Titration period: none |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English Funding: non‐commercial funding (British Heart Foundation, Northern & Yorkshire NHS Research and Development and the Royal College of General Practitioners) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To evaluate the effectiveness of lifestyle interventions in people with impaired glucose tolerance (IGT)." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "...using a random number table to the intervention or control group at the first appointment." Comment: adequate generation of random sequence ensured |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Researchers performing the randomisation were blinded to the group allocation." "There were fewer women (10/32 (32%)) than men (22/32 (69%)) in the control group compared with the intervention group..." Comment: adequate allocation concealment ensured |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk | Comment: investigator‐assessed outcome measure. No blinding of participants described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: investigator‐assessed outcome measure. No blinding of participants. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: investigator‐assessed outcome measure. No blinding of participants described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause/cardiovascular mortality | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: investigator‐assessed outcome measure. No blinding described. The outcome was not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause/cardiovascular mortality | High risk |
Quote from publication: "Fourteen participants (five intervention, nine control) withdrew from the study over 24 months follow‐up. Reasons for withdrawing were family problems, work commitments or ill health." and "Nine participants (three intervention, six control) failed to attend assessments over 24 months follow‐up. In addition, one intervention participant died after a stroke between 12 and 24 months. Complete results are presented here for 69 participants after 6 months, 62 participants after 12 months and 54 participants after 24 months follow‐up (Fig. 1)." Comment: missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. Detailed flow chart provided in publication. However, only 69% of the participants completed the study. This proportion of missing outcomes could have induced clinically relevant bias in intervention effect estimate |
| Incomplete outcome data (attrition bias) incidence of T2DM | High risk |
Quote from publication: "Fourteen participants (five intervention, nine control) withdrew from the study over 24 months follow‐up. Reasons for withdrawing were family problems, work commitments or ill health." and "Nine participants (three intervention, six control) failed to attend assessments over 24 months follow‐up. In addition, one intervention participant died after a stroke between 12 and 24 months. Complete results are presented here for 69 participants after 6 months, 62 participants after 12 months and 54 participants after 24 months follow‐up (Fig. 1)." Comment: missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. Detailed flow chart provided in publication. However, only 69% of the participants completed the study. This proportion of missing outcomes could have induced clinically relevant bias in intervention effect estimate |
| Incomplete outcome data (attrition bias) measures of blood glucose control | High risk |
Quote from publication: "Fourteen participants (five intervention, nine control) withdrew from the study over 24 months follow‐up. Reasons for withdrawing were family problems, work commitments or ill health." and "Nine participants (three intervention, six control) failed to attend assessments over 24 months follow‐up. In addition, one intervention participant died after a stroke between 12 and 24 months. Complete results are presented here for 69 participants after 6 months, 62 participants after 12 months and 54 participants after 24 months follow‐up (Fig. 1)." Comment: missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. Detailed flow chart provided in publication. However, only 69% of the participants completed the study. This proportion of missing outcomes could have induced clinically relevant bias in intervention effect estimate |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available |
| Other bias | Low risk | Comment: no other sources of bias identified |
PODOSA 2014.
| Methods | Cluster‐randomised, controlled, clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: Indian and Pakistani origin; normal place of residence is in Greater Glasgow & Clyde or Lothian Health Board areas; given informed consent; IGT on OGTT at least once or IFG; age ≥ 35 years; waist size of > 90 cm (men) or > 80 cm (women); no confirmed medical history of diabetes (other than gestational diabetes) Exclusion criteria: unwilling to give consent to co‐operate; T2DM on the OGTT during the screening phase of the study; other disease where adherence to the intervention is contraindicated or improbable e.g. terminal illness or psychological or physical illnesses; alcohol dependency; planned or actual pregnancy; use of prescribed drugs that affect the primary outcome; expectation, reported by participants or the general practitioner, that the person will be emigrating or dying before the conclusion of the trial; failure to make a commitment to stay in the study until, at least, the 3‐year follow‐up examination Diagnostic criteria: IGT (mean 2‐h post prandial plasma glucose 7.8‐11.0 mmol/L and FPG < 7.0 mmol/L or IFG (FPG 6.1‐6.9 mmol/L)) (WHO 1999) |
|
| Interventions |
Number of study centres: ‐ Treatment before study: none Titration period: none |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English Funding: non‐commercial (National Prevention Research Initiative) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To evaluate whether a 3‐year family based programme combining weight loss and physical activity can reduce the incidence of type 2 diabetes in South Asians with impaired glucose tolerance." | |
| Notes | Cluster‐randomised trial: family clusters (78 families with 85 participants were allocated to the intervention group and 78 families with 86 participants were allocated to the control group. Adult relatives (known as family volunteers) to support participants in behaviour change. Eligible family volunteers were aged ≥ 18 years and reported interacting with participants at least weekly. 53% of the families in the intervention group had family volunteers and 56% of the families in the control group. Investigators confirmed data and provided information on which outcomes were not assessed in the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Families were randomised (using a random number generator program, with permuted blocks of random size, stratified by location [Edinburgh or Glasgow], ethnic group [Indian or Pakistani], and number of participants in the family [one vs more than one]) to intervention or control. Participants in the same family were not randomised separately" Comment: adequate generation of random sequence ensured |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Families were randomised (using a random number generator program, with permuted blocks of random size, stratified by location [Edinburgh or Glasgow], ethnic group [Indian or Pakistani], and number of participants in the family [one vs more than one]) to intervention or control. Participants in the same family were not randomised separately" Comment: adequate allocation concealment ensured |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "We did this non‐blinded trial in two National Health Service (NHS) regions in Scotland (UK)." and "There was no masking of group status except for the 3‐year measure of weight, waist size, and hip size by independent research nurses" Comment: investigator‐assessed outcome measure. No blinding of participants or investigators. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk |
Quote from publication: "We did this non‐blinded trial in two National Health Service (NHS) regions in Scotland (UK)." and "There was no masking of group status except for the 3‐year measure of weight, waist size, and hip size by independent research nurses" Comment: investigator‐assessed outcome measure. No blinding of participants or investigators. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) non‐serious adverse events | High risk |
Quote from publication: "We did this non‐blinded trial in two National Health Service (NHS) regions in Scotland (UK)." and "There was no masking of group status except for the 3‐year measure of weight, waist size, and hip size by independent research nurses" Comment: self‐reported and investigator‐assessed outcome measure. No blinding of participants or investigators. The outcome could have been influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) socioeconomic effects | Low risk |
Quote from publication: "We did this non‐blinded trial in two National Health Service (NHS) regions in Scotland (UK)." and "There was no masking of group status except for the 3‐year measure of weight, waist size, and hip size by independent research nurses" Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk |
Quote from publication: "We did this non‐blinded trial in two National Health Service (NHS) regions in Scotland (UK)." and "There was no masking of group status except for the 3‐year measure of weight, waist size, and hip size by independent research nurses" Comment: investigator‐assessed outcome measure. No blinding. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk |
Quote from publication: "We did this non‐blinded trial in two National Health Service (NHS) regions in Scotland (UK)." and "There was no masking of group status except for the 3‐year measure of weight, waist size, and hip size by independent research nurses" Comment: investigator‐assessed outcome measure. No blinding. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) non‐serious adverse events | High risk |
Quote from publication: "We did this non‐blinded trial in two National Health Service (NHS) regions in Scotland (UK)." and "There was no masking of group status except for the 3‐year measure of weight, waist size, and hip size by independent research nurses" Comment: investigator‐assessed outcome measure. No blinding. The outcome could have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) socioeconomic effects | Low risk |
Quote from publication: "We did this non‐blinded trial in two National Health Service (NHS) regions in Scotland (UK)." and "There was no masking of group status except for the 3‐year measure of weight, waist size, and hip size by independent research nurses" Comment: investigator‐assessed outcome measure. The outcome was not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) incidence of T2DM | Low risk |
Quote from publication: "Analyses were by modified intention to treat, excluding participants who died or were lost to follow‐up" Comment: 95% of the participants in each intervention group had complete glycaemic data after 3 years of intervention. Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. The proportion of missing outcomes was not enough to have had a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Low risk |
Quote from publication: "Analyses were by modified intention to treat, excluding participants who died or were lost to follow‐up" Comment: 95% of the participants in each intervention group had complete glycaemic data after 3 years of intervention. Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. The proportion of missing outcomes was not enough to have had a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) non‐serious adverse events | Low risk |
Quote from publication: "Analyses were by modified intention to treat, excluding participants who died or were lost to follow‐up" Comment: missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. The proportion of missing outcomes was not enough to have had a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) socioeconomic effects | Low risk |
Quote from publication: "Analyses were by modified intention to treat, excluding participants who died or were lost to follow‐up" Comment: missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. The proportion of missing outcomes was not enough to have had a clinically relevant impact on the intervention effect estimate |
| Selective reporting (reporting bias) | Unclear risk |
Quote from publication: "The primary outcome of the trial was, therefore, altered on June 29, 2009, to change in weight at 3 years to ensure sufficient statistical power, in agreement with the Trial Steering Committee, Data Monitoring and Ethics Committee, and funders. Weight change at 3 years was included in the original protocol as a secondary outcome." Comment: the investigator replied that none of the outcomes that had relevance to this review were unpublished. Outcomes that were not published had not been collected (e.g. mortality) |
| Other bias | Low risk | Comment: no other sources of bias identified. The ICC was negative |
SLIM 2003.
| Methods | Parallel, randomised, controlled, clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: mean 2‐h blood glucose ≥ 7.8 and ≤ 12.5 mmol/L; mean fasting blood glucose ≤ 7.8 mmol/L; white; age 40‐70 years Exclusion criteria: known DM; mean 2‐hour blood glucose > 12.5 mmol/L; mean fasting blood glucose > 7.8 mmol/L; any chronic illness that made 5 years' survival improbable, or that interfered with glucose tolerance, or that made participation in a lifestyle‐intervention impossible; medication known to interfere with glucose tolerance; participation in a regular vigorous physical activity and/or diet programme Diagnostic criteria: impaired glucose tolerance (mean 2‐h blood glucose ≥ 7.8 and ≤ 12.5 mmol/L; mean fasting blood glucose ≤ 7.8 mmol/L) |
|
| Interventions |
Number of study centres: ‐ Treatment before study: none Titration period: none |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English Funding: non‐commercial funding (Netherlands Organisation for Scientific Research (ZonMW: 940‐35‐034) and the Dutch Diabetes Research Foundation (DFN: 98.901) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "The high prevalence of disturbances in glucose homeostasis observed in the preliminary screening underscore the importance of early (lifestyle) interventions in those at risk for developing diabetes. SLIM will address this topic in the Dutch population." | |
| Notes | Glycacemic measures in the inclusion criteria are expressed as venous blood glucose and not as plasma glucose. Originally, the trial follow‐up was planned to have a duration of the intervention for 3 years, but was extended to 6 years during the study. In 2002, a 2nd screening was performed and additional 33 participants were included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Randomisation was carried out with stratification for sex and mean 2 h‐plasma glucose concentration." Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk | Comment: investigator‐assessed outcome measure. No blinding of participants or investigators described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: investigator‐assessed outcome measure. No blinding of participants or investigators described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: investigator‐assessed outcome measure. No blinding of participants or investigators described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause/cardiovascular mortality | Low risk | Comment: investigator‐assessed outcome measure. No blinding of investigators described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: investigator‐assessed outcome measure. No blinding of investigators described. The outcome was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: investigator‐assessed outcome measure. No blinding of investigators described. The outcome was not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause/cardiovascular mortality | Low risk | Comment: mortality status was unknown in 1 person in each intervention group (Figure 1; Roumen et al. European Journal of Clinical Nutrition (2011)). This proportion of missing outcomes was not enough to have had a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) incidence of T2DM | High risk | Comment: 47% in the intervention group and 48% in the control group completed 6 years' follow‐up. Not described how the missing data for this variable was handled. The proportion of missing data was large enough to have had a clinically relevant impact on the intervention effect estimate. Besides, it was clear from the publication that the participants who dropped out were more obese, had higher glucose values on a 2‐h OGTT and had lower economic status at baseline |
| Incomplete outcome data (attrition bias) measures of blood glucose control | High risk |
Quote from publication: "Changes over time between groups were assessed using mixed model analysis on intention‐to‐treat, which included all available observations, including those from later dropouts." Comment: 77% of the participants initially randomised to the intervention group and 79% in the control group were included in the analyses of glycaemic measures. This proportion of missing data was large enough to have had a clinically relevant impact on the intervention effect estimate. Besides, it was clear from the publication that the participants who dropped out at baseline were more obese, had higher glucose values on a 2‐h OGTT and had lower economic status |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available |
| Other bias | Unclear risk | Comment: not reported in the publications why the duration of the intervention was prolonged. No data available after 6 years of intervention. |
Note: where the judgement is 'Unclear' and the description is blank, the study did not report that particular outcome.
— denotes not reported
ADA: American Diabetes Association; BMI: body mass index; CVD: cardiovascular disease; DBP: diastolic blood pressure; DM: diabetes mellitus; DPP: Diabetes Prevention Program; DPPA: diet plus physical activity; DPPOS: Diabetes Prevention Program Outcome Study; DPS: Diabetes Prevention Study; EDIPS: European Diabetes Prevention Study; FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; HELP PD: Healthy Living Partnerships to Prevent Diabetes; HRQoL: health‐related quality of life; ICC: intra‐cluster coefficient; IDPP: Indian Diabetes Prevention Programmes; IFG: impaired fasting glucose; IGT: impaired glucose tolerance; JDPP: Japan Diabetes Prevention Program; NYHA: New York Heart Association; OGTT: oral glucose tolerance test; PODOSA: Prevention of Diabetes and Obesity in South Asians; SBP: systolic blood pressure; SLIM: Study on Lifestyle‐intervention and Impaired glucose tolerance Maastricht; T2DM: type 2 diabetes mellitus; WHO: World health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| APHRODITE | Included normoglycaemic people and people with intermediate hyperglycaemia. Participants have a high FIND‐RISK score, but not necessarily intermediate hyperglycaemia |
| Bo 2007 | The trial did not have intermediate hyperglycaemia as an inclusion criterion |
| D‐CLIP | Duration of the intervention < 2 years |
| De la Rosa 2007 | Not a randomised trial |
| DH!AAN | Outcomes only reported after 1 year of intervention Quote from publication: "At the time of registration of the trial, the intended primary outcome was the 3‐year incidence of type 2 diabetes. However, as the initial response rate was lower than expected, the recruitment period had to be extended to 2 years. Due to a fixed end date of the study (grant restrictions), the follow‐up time was reduced to 2 years. As this period was too short to properly investigate differences in incidence of type 2 diabetes between the control and intervention group, we changed the primary outcome to the more proximal outcomes, namely changes in weight and other weight‐related measurements (body mass index, waist circumference, and fat mass) after 1 year. Secondary outcomes were changes in glucose metabolism, blood pressure, and lipid profile after 1 year." |
| E‐LITE | Included participants with metabolic syndrome or intermediate hyperglycaemia |
| Eriksson 1991 | Inadequate randomisation, the control group was not randomised |
| Eriksson 2006 | Included participants with T2DM |
| Grey 2004 | Both experimental and control groups received the same nutritional education and physical activity training |
| Hesselink 2013 | Duration of the intervention < 2 years |
| Huang 2007 | Duration of the intervention < 2 years |
| J‐DOIT | Duration of the intervention < 2 years |
| Jarrett 1979 | Not comparing intervention of interest (carbohydrate 120 g/day + placebo versus 'limit sucrose (i.e. table sugar) intake' + placebo) |
| Kawahara 2008 | Duration of the intervention < 2 years |
| Kinmonth 2008 | Included participants being overweight and having a parental history of diabetes ‐ the number of participants with intermediate hyperglycaemia was not available. Duration of the intervention < 2 years |
| Let's Prevent | Same intensity of diet and physical activity was applied in the intervention and comparator group |
| Liao 2002 | Not comparing intervention of interest (diet plus endurance physical activity versus diet plus stretching) |
| Lindahl 1999 | Duration of the intervention < 2 years (intervention performed after 1 year was a telephone call at 24 months) |
| Marrero 2016 | Duration of the intervention < 2 years |
| Nanditha 2014 | Same intensity of diet and physical activity was applied in the intervention and comparator group |
| NCT02250066 | Did not compare interventions of interest (mono‐saturated fat versus high carbohydrate diet) |
| NCT02374788 | Included participants with T2DM |
| Page 1992 | Duration of the intervention < 2 years |
| PULSE | Same intensity of diet and physical activity in the intervention arms |
| Ramachandran 2013 | Duration of the intervention < 2 years |
| Rosas 2016 | Included participants with metabolic syndrome |
| Saito 2011 | Same intensity of diet and physical activity was applied in the intervention and comparator group |
| Sartor 1980 | No description of the diet‐only group |
| Sathish 2017 | Duration of the intervention < 2 years |
| Savoye 2007 | Duration of the intervention < 2 years |
| Schmidt 2016 | Included participants with gestational diabetes |
| SHINE | Included participants with metabolic syndrome |
| Tao 2004 | Inadequate randomisation: quasi‐randomised participants |
| The Fasting Hyperglycaemia Study 1997a | Not possible to get separate data on the participants with impaired glucose tolerance (Rury Holman was asked when request was made in another review (Hemmingsen 2016)). This article describes 37% of participants with normal glucose tolerance, 26% with T2DM and 37% with impaired glucose tolerance |
| Thompson 2008 | Duration of the intervention < 2 years |
| Villareal 2006 | Duration of the intervention < 2 years |
| Wein 1999 | Same intensity of diet and physical activity was applied in the intervention and comparator group |
| Wing 1998 | Included participants being overweight and a parental history of diabetes ‐ the number of participants with intermediate hyperglycaemia was not available |
| Wong 2013 | Same intensity of diet and physical activity was applied in the intervention and comparator group |
| Yates 2011 | Duration of the intervention < 2 years |
| Yates 2012 | Intermediate hyperglycaemia was not an inclusion criterion |
APHRODITE: Active Prevention in High Risk individuals Of DIabetes Type 2 in Eindhoven; D‐CLIP: Diabetes Community Lifestyle Improvement Program; FIND‐RISK: Finish Diabetes RIsk Score; J‐DOIT1: Japan Diabetes Outcome Intervention Trial‐1; T2DM: type 2 diabetes mellitus
Characteristics of studies awaiting assessment [ordered by study ID]
130750‐201504‐HR‐020.
| Methods |
Parallel, randomised, controlled, clinical trial (RCT) Randomisation ratio: 1:1:1 |
| Participants |
Condition: IFG (5.6‐6.9 mmol/L) or moderately elevated HbA1c (5.7%‐6.4%) Enrollment: 1200 Inclusion criteria: IFG or moderately elevated HbA1c, 30‐70 years |
| Interventions |
Intervention 1: concealing of lifestyle modification aimed at reducing weight, total intake of carbohydrate, fat and saturated fat, increasing intake of fibre and physical activity level Intervention 2: periodic health examination during a 3‐month period for monitoring fasting blood glucose and HbA1c Comparator: periodic health examination during a 1‐year period for checking health status about T2DM Duration of the intervention: not clearly described |
| Outcomes |
Primary outcome(s): HbA1c Secondary outcome(s): fasting blood glucose Other outcome(s): ‐ |
| Notes | Study protocol for an ongoing trial. Not possible to estimate the duration of the intervention from the study protocol. Investigators were asked, but no reply |
ChiCTR‐PRC‐13003267.
| Methods |
Parallel, randomised, controlled, clinical trial (RCT) Randomisation ratio: 1:1 |
| Participants |
Condition: IGT and/or IFG Enrollment: not reported Inclusion criteria: Chinese, 40–69 years, IGT and/or IFG and < 150 min/week of moderate/vigorous physical activity |
| Interventions |
Intervention: individualised physical activity plan Comparator: not specified Duration of the intervention: not clearly described |
| Outcomes |
Primary outcome(s): ‐ Secondary outcome(s): ‐ Other outcome(s): physical activity level and blood glucose level |
| Notes | Study protocol for an ongoing trial. Not possible to estimate the duration of the intervention from the study protocol. Priority of outcomes not described in protocol. Investigators were asked, but no reply |
iHealth‐T2D.
| Methods |
Parallel, randomised, controlled, clinical trial (RCT) Randomisation ratio: 1:1 |
| Participants |
Condition: waist circumference ≥ 100 cm or HbA1c ≥ 6.0% Enrollment: 3600 Inclusion criteria: waist circumference ≥ 100 cm or HbA1c ≥ 6.0%; South Asian, 40‐70 years |
| Interventions |
Intervention: intensive lifestyle modification Comparator: usual care Duration of the intervention: assume 1 year |
| Outcomes |
Primary outcome(s): incidence of T2DM and > 7% reduction in weight Secondary outcome(s): reduction of ≥ 5 cm waist circumference and health gains in family members Other outcome(s): identifying social, demographic and environmental factors influencing primary and secondary outcomes |
| Notes | Protocol for an ongoing trial. Unknown if data would be presented for the participants included based on moderately elevated HbA1c. Duration of the intervention might be too short for inclusion in this review |
NDPS.
| Methods |
Parallel, randomised, controlled, clinical trial (RCT) Randomisation ratio: 1:1 |
| Participants |
Condition: HbA1c ≥ 6.0 to < 6.5% and a normal fasting plasma glucose (< 5.6 mmol/L) Enrollment: ‐ Inclusion criteria: HbA1c ≥ 6.0 to < 6.5% |
| Interventions |
Intervention: intensive diet plus physical activity advice Comparator: standard control Duration of the intervention: 40 months |
| Outcomes |
Primary outcome(s): HbA1c Secondary outcome(s): homeostasis model assessment, physical activity levels, dietary intake, weight, body fat mass, visceral fat, BMI and waist circumference and health status Other outcome(s): ‐ |
| Notes | A fraction of participants in an ongoing randomised clinical trial |
Zong 2015.
| Methods |
Parallel, randomised, controlled, clinical trial (RCT) Randomisation ratio: 1:1 |
| Participants |
Condition: IFG (6.1‐6.9 mmol/L) and/or IGT (2‐h OGTT 7.8‐11.0 mmol/L) Enrollment: 214 Inclusion criteria: IFG and/or IGT |
| Interventions |
Intervention: not described Comparator: not described Duration of the intervention: 2 years |
| Outcomes |
Primary outcome(s): HbA1c Secondary outcome(s): homeostasis model assessment, physical activity levels, dietary intake, weight, body fat mass, visceral fat, BMI and waist circumference and health status Other outcome(s): ‐ |
| Notes | We are currently searching for Chinese authors to help clarify the intervention |
HbA1c: glycosylated haemoglobin A1c; IFG: impaired fasting glucose; IGT: impaired glucose tolerance; OGTT: oral glucose tolerance test; PDPP: Pakistan Diabetes Prevention Program; T2DM: type 2 diabetes mellitus
Characteristics of ongoing studies [ordered by study ID]
NCT01530165.
| Trial name or title | Acronym: PDPP |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open‐label Primary purpose: prevention |
| Participants |
Condition: impaired glucose tolerance (WHO) Enrollment: 20,000 Inclusion criteria: IGT; 30‐64 yrs Exclusion criteria: T1DM; T2DM; pregnancy; presence of chronic disease rendering survival for 3 years unlikely; any psychological or physical disability to interfere with participation in the trial; ischaemic heart disease |
| Interventions |
Intervention(s): aggressive lifestyle intervention consisting of nutritional and physical activity advice Comparator(s): standard advice Duration of the intervention: 2 years |
| Outcomes |
Primary outcome(s): incidence of T2DM Secondary outcome(s): cost effectiveness; components of the metabolic syndrome; the impact of city planning on prevalence of obesity and T2DM Other outcome(s): ‐ |
| Starting date |
Study start date: 2011 Study completion date: June 2017 |
| Contact information | Responsible party/principal investigator: Asma Ahmed, Aga Khan University |
| Study identifier | NCT number: NCT01530165 |
| Official title | Pakistan Diabetes Prevention Program |
| Stated purpose of study | Quote from publication: "The Karachi‐based Pakistan Diabetes Prevention Study aims to address key issues in the prevention of type 2 diabetes " |
| Notes | The study completion date is provided by the principal investigator. Conducted in Pakistan |
PREVIEW.
| Trial name or title | Acronym: PREVIEW |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open‐label Primary purpose: prevention |
| Participants |
Condition: IFG or IGT (IFG: fasting venous plasma glucose concentration 5.6‐6.9 mmol/L or IGT: venous plasma glucose concentration of 7.8‐11.0 mmol/L at 2‐h after oral administration of 75 g glucose with fasting plasma glucose less than 7.0 mmol/L). Enrollment: 2500 Inclusion criteria: age 25‐70 years; overweight or obesity (BMI > 25 kg/m2); IFG or IGT; informed consent required; provided participants have not recently (within 1 month) changed habits; motivation and willingness to be randomised to any of the groups and to do his/her best to follow the given protocol Exclusion criteria: diabetes mellitus (other than gestational diabetes mellitus); significant cardiovascular disease including current angina; myocardial infarction or stroke within the past 6 months; heart failure; symptomatic peripheral vascular disease; systolic blood pressure above 160 mmHg and/or diastolic blood pressure above 100 mmHg whether on or off treatment for hypertension. If being treated, no change in drug treatment within last 3 months; advanced chronic renal impairment; significant liver disease e.g. cirrhosis (fatty liver disease allowed); malignancy which is currently active or in remission for < 5 years after last treatment (local basal and squamous cell skin cancer allowed); active inflammatory bowel disease, celiac disease, chronic pancreatitis or other disorder potentially causing malabsorption; previous bariatric surgery; chronic respiratory, neurological, musculoskeletal or other disorders where, in the judgement of the investigator, participants would have unacceptable risk or difficulty in complying with the protocol (e.g. physical activity programme); a recent surgical procedure until after full convalescence (investigators judgement); transmissible blood‐borne diseases; psychiatric illness (e.g. major depression, bipolar disorder); use currently or within the previous 3 months of prescription medication that has the potential of affecting body weight or glucose metabolism such as glucocorticoids (but excluding inhaled and topical steroids; bronchodilators are allowed); psychoactive medication, epileptic medication, or weight loss medications (either prescription, over the counter or herbal). Low‐dose antidepressants are allowed if they, in the judgement of the investigator, do not affect weight or participation to the study protocol. Levothyroxine for treatment of hypothyroidism is allowed if the participant has been on a stable dose for at least 3 months; engagement in competitive sports; self‐reported weight change of > 5% (increase or decrease) within 2 months prior to screening; special diets within 2 months prior to study start; severe food intolerance expected to interfere with the study; regularly drinking > 21 alcoholic units/week (men), or > 14 alcoholic units/week (women); use of drugs of abuse within the previous 12 months; blood donation or transfusion within the past 1 month before baseline; self‐reported eating disorders; pregnancy or lactation, including plans to become pregnant within the next 36 months; no access to either phone or Internet (this is necessary when being contacted by the instructors during the maintenance phase); adequate understanding of national language; psychological or behavioural problems which, in the judgement of the investigator, would lead to difficulty in complying with the protocol; haemoglobin concentration below local laboratory reference values (i.e. anaemia); creatinine > 1.5 times Upper Limit of Normal (local laboratory reference values); alanine transaminase and/or aspartate transaminase > 3 times the Upper Limit of Normal (local laboratory reference values) or any other significant abnormality on these tests which in the investigators opinion may be clinically significant and require further assessment; electrocardiography. Any abnormality which in the opinion of the investigator might indicate undiagnosed cardiac disease requiring further assessment (e.g. significant conduction disorder, arrhythmia, pathological Q waves). This is done in adults 55‐70 years of age. |
| Interventions |
Intervention(1): high protein/high‐intensity physical activity Intervention(2): high protein/moderate‐intensity physical activity Intervention(3): moderate protein/high‐intensity physical activity Intervention(4): moderate protein/moderate‐intensity physical activity Comparator(s): participants follow a moderate protein diet and moderate‐intensity physical activity intervention Duration of the intervention: 3 years |
| Outcomes |
Primary outcome(s): incidence of T2DM Secondary outcome(s): HbA1c; change in body weight and waist, hip and thigh circumference; change in body fat mass (kg, proportion of body weight); proportion of participants maintaining at least 0%, 5% or 10% weight loss (relative to initial body weight); insulin sensitivity; risk factors for cardiovascular disease, with at least the following measures: blood pressure, lipids (triglycerides, total, low‐density lipoprotein and high‐density lipoprotein cholesterol), C‐reactive protein, and liver enzymes; changes in perceived quality of life and work ability, habitual well‐being, sleep and chronic stress, subjective appetite sensations, and habitual physical activity; the effects of stature (height; proportion leg‐length/height) in adults and changes in stature in children and adolescents, on the changes in relationship between reduction in body weight, body fat and insulin sensitivity Other outcome(s): ‐ |
| Starting date |
Study start date: June 2013 Study completion date: December 2018 |
| Contact information | Responsible party/principal investigator: Anne Birgitte Raben, Professor, University of Copenhagen |
| Study identifier | NCT number: NCT01777893 |
| Official title | PREVention of Diabetes Through Lifestyle Intervention and Population Studies in Europe and Around the World |
| Stated purpose of study | Quote: "Our hypothesis is that a high‐protein, low‐GI diet will be superior in preventing type‐2 diabetes, compared with a moderate protein, moderate GI diet, and that high‐intensity physical activity will be superior compared to moderate‐intensity physical activity" |
| Notes | ‐ |
PROPELS.
| Trial name or title | Acronym: PROPELS |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open‐label Primary purpose: prevention |
| Participants |
Condition: intermediate hyperglycaemia Enrollment: 1308 Inclusion criteria: 40–74 years old for white European, or aged 25–74 years old for South Asian; previously recorded plasma glucose or HbA1c value in the prediabetes range within the last 5 years; have access to a mobile phone, and willing to use it as part of the study Exclusion criteria: due to the nature of the intervention those unable to undertake ambulatory‐based activity will be excluded; T2DM; screen‐detected diabetes at baseline; pregnancy; normoglycaemia with no previous record of intermediate hyperglycaemia in the previous 5 years |
| Interventions |
Intervention(s): diet as the comparator group, but additional physical activity provided Comparator(s): receive a booklet detailing information on risk factors for T2DM and cardiovascular disease and how physical activity can be used to prevent T2DM and cardiovascular disease. Duration of the intervention: 48 months |
| Outcomes |
Primary outcome(s): change in ambulatory activity Secondary outcome(s): time spent in sedentary, light, moderate and vigorous intensity physical activity assessed by accelerometer and self report; website use and text messages sent/received (intervention group 3 only); fasting and 2‐h post‐challenge glucose and HbA1c; fasting lipid profile, fasting insulin, highly sensitive C‐reactive protein, key adipokines (interleukin 6 and tumour necrosis factor alpha), urea and electrolytes (sodium, potassium, urea, creatinine) and liver function tests; markers of chronic inflammation and adipokines; vitamin C and D; genetic analysis; urine sample; height; body weight; BMI; body fat percentage; waist circumference; arm and leg length; blood pressure; medication status; smoking status; family history of disease; muscular/skeletal injury; illness perceptions; self efficacy; self regulation; quality of life; depression and anxiety; diet; sleep; body composition Other outcome(s):‐ |
| Starting date |
Study start date: August 2013 Study completion date: August 2018 |
| Contact information | Responsible party/principal investigator: University of Leicester, UK |
| Study identifier | ISRCTN: 83465245 |
| Official title | The PRomotion Of Physical activity through structured Education with differing Levels of ongoing Support for those with pre‐diabetes |
| Stated purpose of study | Quote: "Can an intervention to support physical activity behaviour change lead to sustained increases in physical activity over four years in those with a high risk of type 2 diabetes." |
| Notes | ‐ |
BMI: body mass index; HbA1c: glycosylated haemoglobin A1c; IFG: impaired fasting glucose; IGT: impaired glucose tolerance; T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus
Differences between protocol and review
This is an update of a previous Cochrane Review (Orozco 2008). The original review included trials with other definitions of increased risk of type 2 diabetes mellitus. The current review only defines increased risk of type 2 diabetes mellitus with glycaemic measures. The original review included trials with a duration of one year or more. The current review includes trials with a duration of two years or more. The original review excluded trials not reporting the primary outcome. This review includes trials irrespective of the outcomes reported. The primary and secondary outcomes of the review have been changed, so the focus is on patient‐important outcomes.
Contributions of authors
All protocol authors read and approved the final review update draft.
Bianca Hemmingsen (BH): review draft, acquisition of trial reports, trial selection, data extraction of all trials, data analysis, contact trial authors, data interpretation, GRADE assessment and writing of review drafts.
Gabriel Gimenez‐Perez (GG): data extraction of all trials and review of drafts.
Didac Mauricio (DM): data interpretation and writing of review drafts.
Marta Roque i Figuls (MRF): data interpretation and writing of review drafts.
Maria‐Inti Metzendorf (MIM): search strategy development, acquisition of trial reports and review of drafts.
Bernd Richter (BR): review draft, search strategy development, acquisition of trial reports, trial selection, data analysis, data interpretation, GRADE assessment and writing of review drafts.
Sources of support
Internal sources
No sources of support supplied
External sources
-
WHO, Other.
This review update has been partially funded by WHO.
Declarations of interest
BH: none known.
GG: none known.
DM: has received consulting, educational activities or speaker's fees from Astra Zeneca, Eli Lilly, Ferrer, GlaxoSmithKline, Janssen, Menarini, Merck Sharp Dhome, Novartis, Novonordisk and Sanofi.
MRF: none known.
MIM: none known.
BR: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Da Qing 1997 {published data only}
- Chen Y, Wang J, An Y, Gong Q, He Y, Zhang B, et al. Effect of lifestyle interventions on reduction of cardiovascular disease events and its mortality in pre‐diabetic patients:long‐term follow‐up of Da Qing Diabetes Prevention Study. Zhonghua Nei Ke Za Zhi 2015;54(1):13‐7. [PUBMED: 25877139] [PubMed] [Google Scholar]
- Gong Q. Both fasting and 2‐hour post load glycemic progression predicts subsequent cardiovascular events in persons with impaired glucose tolerance: long‐term follow‐up of the Da Qing Diabetes Prevention Study. app.core‐apps.com/tristar_ada14/abstract/e3048dedfd3d5fbcc516bc86387992ec (accessed 24 April 2017).
- Gong Q, Gregg EW, Wang J, An Y, Zhang P, Yang W, et al. Long‐term effects of a randomised trial of a 6‐year lifestyle intervention in impaired glucose tolerance on diabetes‐related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54(2):300‐7. [PUBMED: 21046360] [DOI] [PubMed] [Google Scholar]
- Gong Q, Zhang P, Wang J, An Y, Gregg EW, Li H, et al. Changes in mortality in people with IGT before and after the onset of diabetes during the 23‐year follow‐up of the Da Qing diabetes prevention study. Diabetes Care 2016;39(9):1550‐5. [PUBMED: 27411697] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all‐cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23‐year follow‐up study. The Lancet Diabetes & Endocrinology 2014;2(6):474‐80. [PUBMED: 24731674] [DOI] [PubMed] [Google Scholar]
- Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long‐term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20‐year follow‐up study. Lancet 2008;371(9626):1783‐9. [PUBMED: 18502303] [DOI] [PubMed] [Google Scholar]
- Li GW, Hu YH, Yang WY, Jiang YY, Wang JP, Xiao JZ, et al. Effects of insulin resistance and insulin secretion on the efficacy of interventions to retard development of type 2 diabetes mellitus: the Da Qing IGT and Diabetes Study. Diabetes Research and Clinical Practice 2002;58:193‐200. [DOI] [PubMed] [Google Scholar]
- Li H, Wang J, Hu Y, Chen Y, An Y, Zhang P, et al. Both fasting and 2‐hour post load glycemic progression predicts subsequent cardiovascular events in persons with impaired glucose tolerance: long‐term follow‐up of the Da Qing Diabetes Prevention Study. Diabetes 2014; Vol. 63, issue 0:A350.
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance.The Da Qing IGT and diabetes study. Diabetes Care 1997;20:537‐44. [DOI] [PubMed] [Google Scholar]
DPP 2002 {published data only}
- Diabetes Prevention Program. clinicaltrials.gov/ct2/show/NCT00004992 (accessed 23 January 2017).
- Diabetes Prevention Program Outcomes Study (DPPOS). clinicaltrials.gov/ct2/show/NCT00038727 (accessed 23 January 2017).
- Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett‐Connor E, Fowler S, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2006;61(10):1075‐81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program. The 10‐year cost‐effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent‐to‐treat analysis of the DPP/DPPOS. Diabetes Care 2012;35(4):723‐30. [PUBMED: 22442395] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Group. Impact of lifestyle intervention and metformin on health‐related quality of life: the diabetes prevention program randomized trial. Journal of General Internal Medicine 2012;27(12):1594‐601. [PUBMED: 22692637] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Group. Long‐term prevalence and predictors of urinary incontinence among women in the Diabetes Prevention Program Outcomes Study. International Journal of Urology 2015;22(2):206‐12. [PUBMED: 25352018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Group. Metabolic syndrome components and their response to lifestyle and metformin interventions are associated with differences in diabetes risk in persons with impaired glucose tolerance. Diabetes, Obesity & Metabolism 2014;16(4):326‐33. [PUBMED: 24118860] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Group. Protocol for the Diabetes Prevention Program (DPP). dppos.bsc.gwu.edu/documents/1124073/1127212/DPPPROTOCOL.PDF/807eddd1‐d9bf‐497d‐89f0‐5de15fc43d79 (accessed 23 January 2017).
- Diabetes Prevention Program (DPP) Group. Protocol for the Diabetes prevention Program Outcomes Study (DPPOS). dppos.bsc.gwu.edu/documents/1124073/1127212/Version+4.2+May+1%2C+2016/14e782f2‐2da9‐47d1‐a501‐6004bcaee74e (accessed 31 January 2017).
- Diabetes Prevention Program (DPP) Group. The cost‐effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Annals of Internal Medicine 2005;142(5):323‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. 10‐year follow‐up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374(9707):2054. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. Factors affecting the decline in incidence of diabetes in the Diabetes Prevention Program Outcomes Study (DPPOS). Diabetes 2015;64(3):989‐98. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: a randomized clinical trial. Diabetes Care 2015;38(1):51‐8. [PUBMED: 25336746] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. Lifestyle and metformin interventions have a durable effect to lower CRP and tPA levels in the diabetes prevention program except in those who develop diabetes. Diabetes Care 2014;37(8):2253‐60. [PUBMED: 24824548] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. Long‐term changes in dietary and food intake behaviour in the Diabetes Prevention Program Outcomes Study. Diabetic Medicine 2014;31(12):1631‐42. [PUBMED: 24824893] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25(12):2165‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care 2000;23:1619‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program: recruitment methods and results. Controlled Clinical Trials 2002;23:157‐71. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10‐year follow‐up. Journal Clinical Endocrinology and Metabolism 2015;100(4):1646‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. Within‐trial cost‐effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care 2003;26(9):2518‐23. [PUBMED: 12941712] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Lifestyle and metformin ameliorate insulin sensitivity independently of the genetic burden of established insulin resistance variants in Diabetes Prevention Program participants. Diabetes 2016;65(2):520‐6. [PUBMED: 26525880] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Long‐term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15‐year follow‐up: the Diabetes Prevention Program Outcomes Study. Lancet. Diabetes & Endocrinology 2015;3(11):866‐75. [PUBMED: 26377054] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman WH. The cost‐effectiveness of diabetes prevention: results from the Diabetes Prevention Program and the Diabetes Prevention Program Outcomes Study. Clinical Diabetes and Endocrinology 2015; Vol. 1, issue 9:pp. [DOI] [PMC free article] [PubMed]
- Herman WH, Edelstein SL, Ratner RE, Montez MG, Ackermann RT, Orchard TJ, et al. Effectiveness and cost‐effectiveness of diabetes prevention among adherent participants. The American Journal of Managed Care 2013;19(3):194‐202. [PUBMED: 23544761] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Barrett‐Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Kriska AM, Golden SH, Bray GA, Luchsinger JA, Crandall J, et al. Physical function decline in the diabetes prevention program outcome study. Diabetes 2014;63(0):A344. [Google Scholar]
- Marrero D, Pan Q, Barrett‐Connor E, Groot M, Zhang P, Percy C, et al. DPPOS Research Group. Impact of diagnosis of diabetes on health‐related quality of life among high risk individuals: the Diabetes Prevention Program Outcomes Study. Quality of Life Research 2014;23(1):75‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MJ, Whitaker RC, Yu D, Ackermann RT. The comparative efficacy of lifestyle intervention and metformin by educational attainment in the Diabetes Prevention Program. Preventive Medicine 2015;77:125‐30. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault L, Ma Y, Dagogo‐Jack S, Horton E, Marrero D, Crandall J, et al. Sex differences in diabetes risk and the effect of intensive lifestyle modification in the Diabetes Prevention Program. Diabetes Care 2008;31(7):1416‐21. [PUBMED: 18356403] [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group. Costs associated with the primary prevention of type 2 diabetes mellitus in the Diabetes Prevention Program. Diabetes Care 2003;26:36‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care 2005;28:888‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 2005;54:1566‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group. Long‐term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35(4):731‐7. [PUBMED: 22442396] [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group. Within‐trial cost‐effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care 2003;26:2518‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring, Md.) 2008;16(6):1413‐20. [PUBMED: 18421273] [DOI] [PubMed] [Google Scholar]
- Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obesity Research 2004;12(9):1426‐34. [PUBMED: 15483207] [DOI] [PMC free article] [PubMed] [Google Scholar]
DPS 2001 {published data only}
- NCT00518167. clinicaltrials.gov/ct2/show/NCT00518167 (accessed 12 January 2017).
- Eriksson J, Lindström J, Valle T, Aunola S, Hämäläinen H, Ilsnne‐Parikka P, et al. Prevention of type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland. Study design and 1‐year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999;42:793‐801. [DOI] [PubMed] [Google Scholar]
- Hamalainen H, Ronnemaa T, Virtanen A, Lindstrom J, Eriksson JG, Valle TT, et al. Improved fibrinolysis by an intensive lifestyle intervention in subjects with impaired glucose tolerance. The Finnish Diabetes Prevention Study. Diabetologia 2005;48(11):2248‐53. [PUBMED: 16205886] [DOI] [PubMed] [Google Scholar]
- Herder C, Peltonen M, Koenig W, Kraft I, Muller‐Scholze S, Martin S, et al. Systemic immune mediators and lifestyle changes in the prevention of type 2 diabetes: results from the Finnish Diabetes Prevention Study. Diabetes 2006;55(8):2340‐6. [PUBMED: 16873699] [DOI] [PubMed] [Google Scholar]
- Ilanne‐Parikka P, Eriksson JG, Lindstrom J, Peltonen M, Aunola S, Hamalainen H, et al. Effect of lifestyle intervention on the occurrence of metabolic syndrome and its components in the Finnish Diabetes Prevention Study. Diabetes Care 2008;31(4):805‐7. [PUBMED: 18184907] [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Lindstrom J, Lakka TA, Eriksson JG, Niskanen L, Wikstrom K, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes 2005;54(1):158‐65. [PUBMED: 15616024] [DOI] [PubMed] [Google Scholar]
- Lehtisalo J, Lindstrom J, Ngandu T, Kivipelto M, Ahtiluoto S, Ilanne‐Parikka P, et al. Association of long‐term dietary fat intake, exercise, and weight with later cognitive function in the Finnish Diabetes Prevention Study. The Journal of Nutrition, Health & Aging 2016;20(2):146‐54. [PUBMED: 26812510] [DOI] [PubMed] [Google Scholar]
- Lehtisalo J, Lindstrom J, Ngandu T, Kivipelto M, Ahtiluoto S, Ilanne‐Parikka P, et al. Diabetes, glycaemia, and cognition‐a secondary analysis of the Finnish Diabetes Prevention Study. Diabetes/Metabolism Research and Reviews 2016;32(1):102‐10. [PUBMED: 26172529] [DOI] [PubMed] [Google Scholar]
- Lindström J, Eriksson JG, Valle TT, Aunola S, Cepaitis Z, Hakumaki M, et al. Prevention of diabetes mellitus in subjects with impaired glucose tolerance in the Finnish Diabetes Prevention Study: results from a randomized clinical trial. Journal of the American Society of Nephrology : JASN 2003;14(7 Suppl 2):S108‐13. [PUBMED: 12819313] [DOI] [PubMed] [Google Scholar]
- Lindström J, Ilanne‐Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow‐up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673‐9. [DOI] [PubMed] [Google Scholar]
- Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson G, et al. The Finnish Diabetes Prevention Study (DPS). Diabetes Care 2003;26:3230‐6. [DOI] [PubMed] [Google Scholar]
- Lindström J, Peltonen M, Eriksson JG, Aunola S, Hamalainen H, Ilanne‐Parikka P, et al. Determinants for the effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Care 2008;31(5):857‐62. [PUBMED: 18252900] [DOI] [PubMed] [Google Scholar]
- Lindström J, Peltonen M, Eriksson JG, Ilanne‐Parikka P, Aunola S, Keinanen‐Kiukaanniemi S, et al. Improved lifestyle and decreased diabetes risk over 13 years: long‐term follow‐up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia 2013;56(2):284‐93. [PUBMED: 23093136] [DOI] [PubMed] [Google Scholar]
- Lindström J, Peltonen M, Eriksson JG, Louheranta A, Fogelholm M, Uusitupa M, et al. High‐fibre, low‐fat diet predicts long‐term weight loss and decreased type 2 diabetes risk: the Finnish Diabetes Prevention Study. Diabetologia 2006;49(5):912‐20. [PUBMED: 16541277] [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Lehtisalo J, Lindstrom J, Ngandu T, Kivipelto M, Ahtiluoto S, et al. Cognition in the Finnish diabetes prevention study. Diabetes Research and Clinical Practice 2015;108(3):e63‐6. [PUBMED: 25799080] [DOI] [PubMed] [Google Scholar]
- Ottelin AM, Lindstrom J, Peltonen M, Martikainen J, Uusitupa M, Gylling H, et al. Costs of a self‐selected, health‐promoting diet among the participants of the Finnish Diabetes Prevention Study. Diabetes Care 2007;30(5):1275‐7. [PUBMED: 17325262] [DOI] [PubMed] [Google Scholar]
- Penn L, White M, Lindstrom J, Boer AT, Blaak E, Eriksson JG, et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European Diabetes Prevention Study RCT. PloS One 2013;8(2):e57143. [PUBMED: 23451166] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne‐Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344(18):1343‐50. [DOI] [PubMed] [Google Scholar]
- Uusitupa M, Lindi V, Louheranta A, Salopuro T, Lindström J, Tuomilehto J. Long term improvement in insulin sensitivity by changing lifestyles of people with impaired glucose tolerance; 4‐year results from the Finnish Diabetes Prevention Study. Diabetes 2003;52:2532‐8. [DOI] [PubMed] [Google Scholar]
- Uusitupa M, Louheranta A, Lindström J, Valle T, Sundvall J, Eriksson J, et al. The Finnish Diabetes Prevention Study. British Journal of Nutrition 2000;83(Suppl 1):S137‐52. [DOI] [PubMed] [Google Scholar]
- Uusitupa M, Peltonen M, Lindstrom J, Aunola S, Ilanne‐Parikka P, Keinanen‐Kiukaanniemi S, et al. Ten‐year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study‐‐secondary analysis of the randomized trial. PloS One 2009;4(5):e5656. [PUBMED: 19479072] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello VD, Lindstrom J, Eriksson JG, Ilanne‐Parikka P, Keinanen‐Kiukaanniemi S, Pihlajamaki J, et al. Markers of cholesterol metabolism as biomarkers in predicting diabetes in the Finnish Diabetes Prevention Study. Nutrition, Metabolism, and Cardiovascular Diseases : NMCD 2015;25(7):635‐42. [PUBMED: 25921846] [DOI] [PubMed] [Google Scholar]
EDIPS 2009 {published data only}
- ISRCTN15670600. www.isrctn.com/ISRCTN15670600 (accessed 22 March 2017).
- Penn L, Moffatt SM, White M. Participants' perspective on maintaining behaviour change: a qualitative study within the European Diabetes Prevention Study. BMC Public Health 2008;8:235. [PUBMED: 18616797] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn L, White M, Lindstrom J, Boer AT, Blaak E, Eriksson JG, et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European Diabetes Prevention Study RCT. PloS One 2013;8(2):e57143. [PUBMED: 23451166] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn L, White M, Oldroyd J, Walker M, Alberti KG, Mathers JC. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health 2009;9:342. [PUBMED: 19758428] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hellgren 2016 {published data only}
- Hellgren MI, Jansson PA, Wedel H, Lindblad U. A lifestyle intervention in primary care prevents deterioration of insulin resistance in patients with impaired glucose tolerance: a randomised controlled trial. Scandinavian Journal of Public Health 2016;‐:‐. [PUBMED: 27550085] [DOI] [PubMed] [Google Scholar]
- Hellgren MI, Petzold M, Beteus‐Forslund H, Wedel H, Jansson PA, Lindblad U. Feasibility of a randomized controlled intervention with physical activity in participants with impaired glucose tolerance recruited by FINDRISC: a pilot study. Scandinavian Journal of Public Health 2014;42(5):463‐70. [PUBMED: 24867622] [DOI] [PubMed] [Google Scholar]
HELP PD 2011 {published data only}
- Blackwell CS, Foster KA, Isom S, Katula JA, Vitolins MZ, Rosenberger EL, et al. Healthy Living Partnerships to Prevent Diabetes: recruitment and baseline characteristics. Contemporary Clinical Trials 2011;32(1):40‐9. [PUBMED: 20974289] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katula JA, Vitolins MZ, Rosenberger EL, Blackwell C, Espeland MA, Lawlor MS, et al. Healthy Living Partnerships to Prevent Diabetes (HELP PD): design and methods. Contemporary Clinical Trials 2010;31(1):71‐81. [PUBMED: 19758580] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katula JA, Vitolins MZ, Rosenberger EL, Blackwell CS, Morgan TM, Lawlor MS, et al. One‐year results of a community‐based translation of the Diabetes Prevention Program: Healthy‐Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care 2011;34(7):1451‐7. [PUBMED: 21593290] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor MS, Blackwell CS, Isom SP, Katula JA, Vitolins MZ, Morgan TM, et al. Cost of a group translation of the Diabetes Prevention Program: healthy living partnerships to prevent diabetes. American Journal of Preventive Medicine 2013;44(4 Suppl 4):S381‐9. [PUBMED: 23498303] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00631345. Healthy living partnership to prevent diabetes (HELPPD). clinicaltrials.gov/ct2/show/NCT00631345 (accessed 17 March 2017).
- Vitolins MZ, Isom SP, Blackwell CS, Kernodle D, Sydell JM, Pedley CF, et al. The healthy living partnerships to prevent diabetes and the diabetes prevention program: a comparison of year 1 and 2 intervention results. Translational Behavioral Medicine 2016 [Epub ahead of print]. [PUBMED: 27796775] [DOI] [PMC free article] [PubMed]
IDPP 2006 {published data only}
- NCT00279240. clinicaltrials.gov/show/NCT00279240 (accessed 3 April 2017).
- Ramachandran A, Arun N, Shetty AS, Snehalatha C. Efficacy of primary prevention interventions when fasting and postglucose dysglycemia coexist: analysis of the Indian Diabetes Prevention Programmes (IDPP‐1 and IDPP‐2). Diabetes Care 2010;33(10):2164‐8. [PUBMED: 20519663] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP‐1). Diabetologia 2006;49:289‐97. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Metabolic syndrome does not increase the risk of conversion of impaired glucose tolerance to diabetes in Asian Indians‐‐Result of Indian diabetes prevention programme. Diabetes Research and Clinical Practice 2007;76(2):215‐8. [PUBMED: 16982107] [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Snehalatha C, Yamuna A, Mary S, Ping Z. Cost‐effectiveness of the interventions in the primary prevention of diabetes among Asian Indians. Within‐trial results of the Indian Diabetes Prevention Programme (IDPP). Diabetes Care 2007;30:2548‐52. [DOI] [PubMed] [Google Scholar]
- Snehalatha C, Mary S, Joshi VV, Ramachandran A. Beneficial effects of strategies for primary prevention of diabetes on cardiovascular risk factors: results of the Indian Diabetes Prevention Programme. Diabetes & Vascular Disease Research 2008;5(1):25‐9. [PUBMED: 18398809] [DOI] [PubMed] [Google Scholar]
- Snehalatha C, Mary S, Selvam S, Sathish Kumar CK, Shetty SB, Nanditha A, et al. Changes in insulin secretion and insulin sensitivity in relation to the glycemic outcomes in subjects with impaired glucose tolerance in the Indian Diabetes Prevention Programme‐1 (IDPP‐1). Diabetes Care 2009;32(10):1796‐801. [PUBMED: 19587369] [DOI] [PMC free article] [PubMed] [Google Scholar]
JDPP 2013 {published data only}
- Japan Diabetes Prevention Program. Lifestyle intervention using existing human resources on the development of type 2 diabetes. upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000003799&language=E (accessed 27 January 2017).
- Sakane N. Japan Diabetes Prevention Program. Nippon Rinsho 2005;63(Suppl 2):488‐92. [PubMed] [Google Scholar]
- Sakane N, Sato J, Tsushita K, Tsujii S, Kotani K, Tominaga M, et al. Effect of baseline HbA1c level on the development of diabetes by lifestyle intervention in primary healthcare settings: insights from subanalysis of the Japan Diabetes Prevention Program. BMJ Open Diabetes Research & Care 2014;2(1):e000003. [PUBMED: 25452854] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane N, Sato J, Tsushita K, Tsujii S, Kotani K, Tominaga M, et al. Effects of lifestyle intervention on weight and metabolic parameters in patients with impaired glucose tolerance related to beta‐3 adrenergic receptor gene polymorphism Trp64Arg(C/T): results from the Japan Diabetes Prevention Program. Journal of Diabetes Investigation 2016;7(3):338‐42. [PUBMED: 27330719] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane N, Sato J, Tsushita K, Tsujii S, Kotani K, Tsuzaki K, et al. Prevention of type 2 diabetes in a primary healthcare setting: three‐year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health 2011;11(1):40. [PUBMED: 21235825] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kosaka 2005 {published data only}
- Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Research and Clinical Practice 2005;67:152‐62. [DOI] [PubMed] [Google Scholar]
Oldroyd 2005 {published data only}
- Oldroyd JC, Unwin NC, White M, Imrie K, Mathers JC, Alberti KGMM. Randomised controlled trial evaluating the effectiveness of behavioural interventions to modify cardiovascular risk factors in men and women with impaired glucose tolerance: outcomes at 6 months. Diabetes Research and Clinical Practice 2001;52:29‐43. [DOI] [PubMed] [Google Scholar]
- Oldroyd JC, Unwin NC, White M, Mathers JC, Alberti KGMM. Randomised controlled trial evaluating lifestyle interventions in people with impaired glucose tolerance. Diabetes Research and Clinical Practice 2006;72:117‐27. [DOI] [PubMed] [Google Scholar]
PODOSA 2014 {published data only}
- ISRCTN25729565. www.isrctn.com/ISRCTN25729565 (accessed 20 March 2017).
- Bhopal RS, Douglas A, Wallia S, Forbes JF, Lean ME, Gill JM, et al. Effect of a lifestyle intervention on weight change in South Asian individuals in the UK at high risk of type 2 diabetes: a family‐cluster randomised controlled trial. Lancet. Diabetes & Endocrinology 2014;2(3):218‐27. [PUBMED: 24622752] [DOI] [PubMed] [Google Scholar]
- Douglas A, Bhopal RS, Bhopal R, Forbes JF, Gill JM, McKnight J, et al. Design and baseline characteristics of the PODOSA (Prevention of Diabetes & Obesity in South Asians) trial: a cluster, randomised lifestyle intervention in Indian and Pakistani adults with impaired glycaemia at high risk of developing type 2 diabetes. BMJ Open 2013;3(2):e002226. [PUBMED: 23435795] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh P, Cezard G, Gill JM, Wallia S, Douglas A, Sheikh A, et al. Associations between weight change and biomarkers of cardiometabolic risk in South Asians: secondary analyses of the PODOSA trial. International Journal of Obesity 2016;40(6):1005‐11. [PUBMED: 26927315] [DOI] [PMC free article] [PubMed] [Google Scholar]
SLIM 2003 {published data only}
- Corpeleijn E, Feskens EJ, Jansen EH, Mensink M, Saris WH, Blaak EE. Lifestyle intervention and adipokine levels in subjects at high risk for type 2 diabetes: the Study on Lifestyle intervention and Impaired glucose tolerance Maastricht (SLIM). Diabetes Care 2007;30(12):3125‐7. [PUBMED: 17890316] [DOI] [PubMed] [Google Scholar]
- Corpeleijn E, Feskens EJ, Jansen EH, Mensink M, Saris WH, Bruin TW, et al. Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: the SLIM study. Diabetologia 2006;49(10):2392‐401. [PUBMED: 16896932] [DOI] [PubMed] [Google Scholar]
- Mensink M, Blaak EE, Corpeleijn E, Saris WH, Bruin TW, Feskens EJ. Lifestyle intervention according to general recommendation improves glucose tolerance. Obesity Research 2003;11(12):1588‐96. [DOI] [PubMed] [Google Scholar]
- Mensink M, Corpeleijn E, Feskens EJM, Kruijshoop M, Saris WHM, Bruin TWA, et al. Study on lifestyle‐intervention and impaired glucose tolerance Maastricht (SLIM): design and screening results. Diabetes Research and Clinical Practice 2003;61:49‐58. [DOI] [PubMed] [Google Scholar]
- Mensink M, Feskens EJ, Kruijshoop M, Bruin TW, Saris WH, Blaak EE. Subscapular skinfold thickness distinguishes between transient and persistent impaired glucose tolerance: Study on Lifestyle‐intervention and Impaired glucose tolerance Maastricht (SLIM). Diabetic medicine : a Journal of the British Diabetic Association 2003;20(7):552‐7. [PUBMED: 12823236] [DOI] [PubMed] [Google Scholar]
- Mensink M, Feskens EJ, Saris WH, Bruin TW, Blaak EE. Study on lifestyle intervention and impaired glucose tolerance Maastricht (SLIM): preliminary results after one year. International Journal of Obesity and Related Metabolic Disorders 2003;27(3):377‐84. [PUBMED: 12629566] [DOI] [PubMed] [Google Scholar]
- Penn L, White M, Lindstrom J, Boer AT, Blaak E, Eriksson JG, et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European Diabetes Prevention Study RCT. PloS One 2013;8(2):e57143. [PUBMED: 23451166] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumen C, Corpeleijn E, Feskens EJ, Mensink M, Saris WH, Blaak EE. Impact of 3‐year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabetic Medicine : a Journal of the British Diabetic Association 2008;25(5):597‐605. [PUBMED: 18445174] [DOI] [PubMed] [Google Scholar]
- Roumen C, Feskens EJ, Corpeleijn E, Mensink M, Saris WH, Blaak EE. Predictors of lifestyle intervention outcome and dropout: the SLIM study. European Journal of Clinical Nutrition 2011;65(10):1141‐7. [PUBMED: 21587283] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
APHRODITE {published data only}
- Study protocol. www.trialregister.nl/trialreg/admin/rctview.asp?TC=1082 (accessed 3 April 2017).
- Vermunt PW, Milder IE, Wielaard F, Oers JA, Westert GP. An active strategy to identify individuals eligible for type 2 diabetes prevention by lifestyle intervention in Dutch primary care: the APHRODITE study. Family Practice 2010;27(3):312‐9. [PUBMED: 20089573] [DOI] [PubMed] [Google Scholar]
Bo 2007 {published data only}
- Bo S, Ciccone G, Baldi C, Benini L, Dusio F, Forastiere G, et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. Journal of General Internal Medicine 2007;22(12):1695‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
D‐CLIP {published data only}
- Study protocol. clinicaltrials.gov/show/NCT01283308 (accessed 17 September 2017).
- Weber MB, Ranjani H, Meyers GC, Mohan V, Narayan KM. A model of translational research for diabetes prevention in low and middle‐income countries: The Diabetes Community Lifestyle Improvement Program (D‐CLIP) trial. Primary Care Diabetes 2012;6(1):3‐9. [PUBMED: 21616737] [DOI] [PubMed] [Google Scholar]
De la Rosa 2007 {published data only}
- Rosa A, Fogelfeld L, Kolish J, Sanghani R, Pikelny I, Stronger JH. Detecting and managing metabolic syndrome: preliminary results. Ethnicity and Disease 2007;17(S5):24‐5. [Google Scholar]
DH!AAN {published data only}
- Study protocol. www.trialregister.nl/trialreg/admin/rctview.asp?TC=1499 (accessed 29 March 2017).
- Vlaar EM, Valkengoed IG, Nierkens V, Nicolaou M, Middelkoop BJ, Stronks K. Feasibility and effectiveness of a targeted diabetes prevention program for 18 to 60‐year‐old South Asian migrants: design and methods of the DH!AAN study. BMC Public Health 2012;12:371. [PUBMED: 22621376] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaar EM, Valkengoed IG, Nierkens V, Nicolaou M, Middelkoop BJ, Stronks K. Feasibility and effectiveness of a targeted diabetes prevention program for 18 to 60‐year‐old South Asian migrants: design and methods of the DH!AAN study. BMC Public Health 2012;12:371. [PUBMED: 22621376] [DOI] [PMC free article] [PubMed] [Google Scholar]
E‐LITE {published data only}
- Ma J, King AC, Wilson SR, Xiao L, Stafford RS. Evaluation of lifestyle interventions to treat elevated cardiometabolic risk in primary care (E‐LITE): a randomized controlled trial. BMC Family Practice 2009;10:71. [PUBMED: 19909549] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eriksson 1991 {published data only}
- Eriksson KF, Lindgarde F. Prevention of type 2 (non‐insulin‐dependent) diabetes mellitus by diet and physical exercise. The 6‐year Malmo feasibility study. Diabetologia 1991;34:891‐8. [DOI] [PubMed] [Google Scholar]
Eriksson 2006 {published data only}
- Eriksson KM, Westborg CJ, Eliasson MCE. A randomized trial of lifestyle intervention in primary healthcare for the modification of cardiovascular risk factors. The Björknäs study. Scandinavian Journal of Public Health 2006;34:453‐61. [DOI] [PubMed] [Google Scholar]
Grey 2004 {published data only}
- Grey M, Berry D, Davidson M, Galasso P, Gustafson E, Melkus GD. Preliminary testing of a program to prevent type 2 diabetes among high risk youth. The Journal of School Health 2004;74(1):10‐5. [DOI] [PubMed] [Google Scholar]
Hesselink 2013 {published data only}
- Hesselink AE, Bilo HJ, Jonkers R, Martens M, Weerdt I, Rutten GE. A cluster‐randomized controlled trial to study the effectiveness of a protocol‐based lifestyle program to prevent type 2 diabetes in people with impaired fasting glucose. BMC Family Practice 2013;14:184. [PUBMED: 24295397] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2007 {published data only}
- Huang SH, Weng KP, Hsieh KS, Ou SF, Lin CC, Chien KJ, et al. Effects of a classroom‐based weight‐control intervention on cardiovascular disease in elementary‐school obese children. Acta Paediatrica Taiwanica 2007;48:201‐6. [PubMed] [Google Scholar]
Jarrett 1979 {published data only}
- Jarrett RJ, Keen H, Fuller JH, McCartney M. Worsening to diabetes in men with impaired glucose tolerance ("borderline diabetes"). Diabetologia 1979;16(1):25‐30. [PUBMED: 761734] [DOI] [PubMed] [Google Scholar]
J‐DOIT {published data only}
- Study protocol. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000000798 (accessed 3 April 2017).
- Sakane N, Kotani K, Takahashi K, Sano Y, Tsuzaki K, Okazaki K, et al. Japan Diabetes Outcome Intervention Trial‐1 (J‐DOIT1), a nationwide cluster randomized trial of type 2 diabetes prevention by telephone‐delivered lifestyle support for high‐risk subjects detected at health checkups: rationale, design, and recruitment. BMC Public Health 2013;13:81. [PUBMED: 23356246] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawahara 2008 {published data only}
- Kawahara T, Takahashi K, Inazu T, Arao T, Kawahara C, Tabata T, et al. Reduced progression to type 2 diabetes from impaired glucose tolerance after a 2‐day in‐hospital diabetes educational program: the Joetsu Diabetes Prevention Trial. Diabetes Care 2008;31(10):1949‐54. [PUBMED: 18591401] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinmonth 2008 {published data only}
- Kinmonth AL, Wareham NJ, Hardeman W, Sutton S, Prevost AT, Fanshawe T, et al. Efficacy of a theory‐based behavioural intervention to increase physical activity in an at‐risk group in primary care (ProActive UK): a randomised trial. Lancet 2008;371:41‐8. [DOI] [PubMed] [Google Scholar]
Let's Prevent {published data only}
- ISRCTN80605705. www.isrctn.com/ISRCTN80605705 (accessed 27 March 2017).
- NCT00677937. clinicaltrials.gov/ct2/show/NCT00677937 (accessed 27 March 2017).
- Davies MJ, Gray LJ, Ahrabian D, Carey M, Farooqi A, Gray A, et al. A community‐based primary prevention programme for type 2 diabetes mellitus integrating identification and lifestyle intervention for prevention: a cluster randomised controlled trial. Programme Grants Applied Research 2017;5(2):‐. [PubMed] [Google Scholar]
- Davies MJ, Gray LJ, Troughton J, Gray A, Tuomilehto J, Farooqi A, et al. A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: the Let's Prevent Diabetes cluster randomised controlled trial. Preventive Medicine 2016;84:48‐56. [PUBMED: 26740346] [DOI] [PubMed] [Google Scholar]
- Gray LJ, Khunti K, Williams S, Goldby S, Troughton J, Yates T, et al. Let's prevent diabetes: study protocol for a cluster randomised controlled trial of an educational intervention in a multi‐ethnic UK population with screen detected impaired glucose regulation. Cardiovascular Diabetology 2012;11:56. [PUBMED: 22607160] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LJ, Yates T, Troughton J, Khunti K, Davies MJ. Engagement, retention, and progression to type 2 diabetes: a retrospective analysis of the cluster‐randomised "Let's Prevent Diabetes" trial. PLoS Medicine 2016;13(7):e1002078. [PUBMED: 27404094] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal J, Ahrabian D, Davies MJ, Gray LJ, Khunti K, Yates T, et al. Cost‐effectiveness of a pragmatic structured education intervention for the prevention of type 2 diabetes: economic evaluation of data from the Let's Prevent Diabetes cluster‐randomised controlled trial. BMJ Open 2017;7(1):e013592. [PUBMED: 28069625] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2002 {published data only}
- Carr DB, Utzschneider KM, Boyko EJ, Asberry PJ, Hull RL, Kodama K, et al. A reduced‐fat diet and aerobic exercise in Japanese Americans with impaired glucose tolerance decreases intra‐abdominal fat and improves insulin sensitivity but not beta‐cell function. Diabetes 2005;54(2):340‐7. [DOI] [PubMed] [Google Scholar]
- Liao D, Asberry PJ, Shofer JB, Callahan H, Matthys C, Boyko EJ, et al. Improvement of BMI, body composition, and body fat distribution with lifestyle modification in Japanese Americans with impaired glucose tolerance. Diabetes Care 2002;25(9):1504‐10. [DOI] [PubMed] [Google Scholar]
Lindahl 1999 {published data only}
- Lindahl B, Nilsson TK, Borch‐Johnsen K, Roder ME, Soderberg S, Widman L, et al. A randomized lifestyle intervention with 5‐year follow‐up in subjects with impaired glucose tolerance: pronounced short‐term impact but long‐term adherence problems. Scandinavian Journal of Public Health 2009;37(4):434‐42. [PUBMED: 19181821] [DOI] [PubMed] [Google Scholar]
- Lindahl B, Nilsson TK, Jansson JH, Asplund K, Hallmans G. Improved fibrinolysis by intense lifestyle intervention. A randomized trial in subjects with impaired glucose tolerance. Journal of Internal Medicine 1999;246:105‐12. [DOI] [PubMed] [Google Scholar]
Marrero 2016 {published data only}
- NCT02000024. clinicaltrials.gov/ct2/show/NCT02000024 (accessed 10 April 2017).
- Marrero DG, Palmer KN, Phillips EO, Miller‐Kovach K, Foster GD, Saha CK. Comparison of commercial and self‐initiated weight loss programs in people with prediabetes: a randomized control trial. American Journal of Public Health 2016;106(5):949‐56. [PUBMED: 26890171] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nanditha 2014 {published data only}
- Nanditha A, Ram J, Snehalatha C, Selvam S, Priscilla S, Shetty AS, et al. Early improvement predicts reduced risk of incident diabetes and improved cardiovascular risk in prediabetic Asian Indian men participating in a 2‐year lifestyle intervention program. Diabetes Care 2014;37(11):3009‐15. [PUBMED: 25216506] [DOI] [PubMed] [Google Scholar]
NCT02250066 {published data only}
- NCT02250066. clinicaltrials.gov/ct2/show/NCT02250066 (accessed 10 April 2017).
NCT02374788 {published data only}
- NCT02374788. clinicaltrials.gov/ct2/show/NCT02374788 (accessed 27 March 2017).
Page 1992 {published data only}
- Page RC, Harnden KE, Walravens NK, Onslow C, Sutton P, Levy JC, et al. 'Healthy living' and sulphonylurea therapy have different effects on glucose tolerance and risk factors for vascular disease in subjects with impaired glucose tolerance. The Quarterly Journal of Medicine 1993;86(3):145‐54. [PUBMED: 8483989] [PubMed] [Google Scholar]
- Page RCL, Harnden, Cook JTE, Turner RC. Can life‐styles of subjects with impaired glucose tolerance be changed? A feasibility study. Diabetic Medicine 1992;9:562‐6. [DOI] [PubMed] [Google Scholar]
PULSE {published data only}
- Study protocol. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4149 (accessed 3 April 2017).
Ramachandran 2013 {published data only}
- NCT00819455. clinicaltrials.gov/ct2/show/NCT00819455 (accessed 10 April 2017).
- Ram J, Selvam S, Snehalatha C, Nanditha A, Simon M, Shetty AS, et al. Improvement in diet habits, independent of physical activity helps to reduce incident diabetes among prediabetic Asian Indian men. Diabetes Research and Clinical Practice 2014;106(3):491‐5. [PUBMED: 25458326] [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Snehalatha C, Ram J, Selvam S, Simon M, Nanditha A, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel‐group, randomised controlled trial. Lancet. Diabetes & Endocrinology 2013;1(3):191‐8. [PUBMED: 24622367] [DOI] [PubMed] [Google Scholar]
Rosas 2016 {published data only}
- Rosas LG, Lv N, Xiao L, Lewis MA, Zavella P, Kramer MK, et al. Evaluation of a culturally‐adapted lifestyle intervention to treat elevated cardiometabolic risk of Latino adults in primary care (Vida Sana): a randomized controlled trial. Contemporary Clinical Trials 2016;48:30‐40. [PUBMED: 26995280] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saito 2011 {published data only}
- Study protocol. rctportal.niph.go.jp/en/detail?trial_id=UMIN000001959 (accessed 29 March 2017).
- Saito T, Watanabe M, Nishida J, Izumi T, Omura M, Takagi T, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Archives of Internal Medicine 2011;171(15):1352‐60. [PUBMED: 21824948] [DOI] [PubMed] [Google Scholar]
Sartor 1980 {published data only}
- Sartor G, Schersten B, Carlstrom S, Melander A, Norden A, Persson G. Ten‐year follow‐up of subjects with impaired glucose tolerance: prevention of diabetes by tolbutamide and diet regulation. Diabetes 1980;29(1):41‐9. [PUBMED: 7380107] [DOI] [PubMed] [Google Scholar]
Sathish 2017 {published data only}
- Study protocol. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000262909 (assessed 23 March 2017).
- Sathish T, Oldenburg B, Tapp RJ, Shaw JE, Wolfe R, Sajitha B, et al. Baseline characteristics of participants in the Kerala Diabetes Prevention Program: a cluster randomized controlled trial of lifestyle intervention in Asian Indians. Diabetic Medicine 2017;34(5):647‐53. [PUBMED: 27279083] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savoye 2007 {published data only}
- Savoye M, Shaw M, Dziura J, Tamborlane WV, Rose P, Guandalini C, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children. A randomized controlled trial. JAMA 2007;297(24):2697‐704. [DOI] [PubMed] [Google Scholar]
Schmidt 2016 {published data only}
- Schmidt MI, Duncan BB, Castilhos C, Wendland EM, Hallal PC, Schaan BD, et al. Lifestyle INtervention for Diabetes prevention After pregnancy (LINDA‐Brasil): study protocol for a multicenter randomized controlled trial. BMC Pregnancy and Childbirth 2016;16:68. [PUBMED: 27029489] [DOI] [PMC free article] [PubMed] [Google Scholar]
SHINE {published data only}
- Trief PM, Cibula D, Delahanty LM, Weinstock RS. Self‐determination theory and weight loss in a Diabetes Prevention Program translation trial. Journal of Behavioral Medicine 2016;Epub Dec:Epub. [PUBMED: 28004335] [DOI] [PubMed] [Google Scholar]
Tao 2004 {published data only}
- Tao LL, Deng YB, Fan XB, Bao QD. Effect of exercise training in patients with impaired glucose tolerance. Zhongguo Linchuang Kangfu 2004;8:2912‐3. [Google Scholar]
The Fasting Hyperglycaemia Study 1997a {published data only}
- Dyson PA, Hammersley MS, Morris RJ, Holman RR, Turner RC. The Fasting Hyperglycaemia Study: II. Randomized controlled trial of reinforced healthy‐living advice in subjects with increased but not diabetic fasting plasma glucose. Metabolism: Clinical and Experimental 1997;46(12 Suppl 1):50‐5. [PUBMED: 9439560] [DOI] [PubMed] [Google Scholar]
- Hammersley MS, Meyer LC, Morris RJ, Manley SE, Turner RC, Holman RR. The Fasting Hyperglycaemia Study: I. Subject identification and recruitment for a non‐insulin‐dependent diabetes prevention trial. Metabolism: Clinical and Experimental 1997;46(12 Suppl 1):44‐9. [PUBMED: 9439559] [DOI] [PubMed] [Google Scholar]
- Karunakaran S, Hammersley MS, Morris RJ, Turner RC, Holman RR. The Fasting Hyperglycaemia Study: III. Randomized controlled trial of sulfonylurea therapy in subjects with increased but not diabetic fasting plasma glucose. Metabolism: Clinical and Experimental 1997;46(12 Suppl 1):56‐60. [PUBMED: 9439561] [DOI] [PubMed] [Google Scholar]
Thompson 2008 {published data only}
- Thompson JL, Allen P, Helitzer DL, Qualls C, Whyte AN, Wolfe VK, et al. Reducing diabetes risk in American Indian women. American Journal of Preventive Medicine 2008;34(3):192‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villareal 2006 {published data only}
- Villareal DT, Miller III BV, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. American Journal of Clinical Nutrition 2006;84:1317‐23. [DOI] [PubMed] [Google Scholar]
Wein 1999 {published data only}
- Wein P, Beischer N, Harris C, Permezel M. A trial of simple versus intensified dietary modification for prevention of progression to diabetes mellitus in women with impaired glucose tolerance. The Australian & New Zealand Journal of Obstetrics & Gynaecology 1999;39(2):162‐6. [PUBMED: 10755770] [DOI] [PubMed] [Google Scholar]
Wing 1998 {published data only}
- Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individual with a family history of diabetes. Diabetes Care 1998;21:350‐9. [DOI] [PubMed] [Google Scholar]
Wong 2013 {published data only}
- Wong CK, Fung CS, Siu SC, Lo YY, Wong KW, Fong DY, et al. A short message service (SMS) intervention to prevent diabetes in Chinese professional drivers with pre‐diabetes: a pilot single‐blinded randomized controlled trial. Diabetes Research and Clinical Practice 2013;102(3):158‐66. [PUBMED: 24466598] [DOI] [PubMed] [Google Scholar]
Yates 2011 {published data only}
- Yates T, Davies MJ, Sehmi S, Gorely T, Khunti K. The Pre‐diabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) programme study: are improvements in glucose regulation sustained at 2 years?. Diabetic Medicine : a Journal of the British Diabetic Association 2011;28(10):1268‐71. [PUBMED: 21672008] [DOI] [PubMed] [Google Scholar]
Yates 2012 {published data only}
- Yates T, Davies MJ, Henson J, Troughton J, Edwardson C, Gray LJ, et al. Walking away from type 2 diabetes: trial protocol of a cluster randomised controlled trial evaluating a structured education programme in those at high risk of developing type 2 diabetes. BMC Family Practice 2012;13:46. [PUBMED: 22642610] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
130750‐201504‐HR‐020 {published data only}
- Study protocol. cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=6480 (accessed 3 April 2017).
ChiCTR‐PRC‐13003267 {published data only}
- Study protocol. www.chictr.org.cn/showproj.aspx?proj=6292 (accessed 3 April 2017).
iHealth‐T2D {published data only}
- NCT02949739. clinicaltrials.gov/show/NCT02949739 (accessed 3 April 2017).
NDPS {published data only}
- ISRCTN34805606. www.isrctn.com/ISRCTN34805606?q=&filters=conditionCategory:Nutritional%5C,%20Metabolic%5C,%20Endocrine&sort=&offset=27&totalResults=1239&page=3&pageSize=10&searchType=basic‐search (accessed 27 March 2017).
- Pascale M, Murray N, Bachmann M, Barton G, Clark A, Howe A, et al. Study protocol: The Norfolk Diabetes Prevention Study [NDPS]: a 46 month multi ‐ centre, randomised, controlled parallel group trial of a lifestyle intervention [with or without additional support from lay lifestyle mentors with type 2 diabetes] to prevent transition to type 2 diabetes in high risk groups with non ‐ diabetic hyperglycaemia, or impaired fasting glucose. BMC Public Health 2017;17(1):31. [PUBMED: 28056894] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zong 2015 {published data only}
- Zong Y, Duan P, Ding X, Si L, Liu J, Tu P. Effects of lifestyle and quantitative nutrition interventions on individuals with prediabetes. Zhonghua Yi Xue Za Zhi 2015;95(40):3293‐6. [PUBMED: 26815351] [PubMed] [Google Scholar]
References to ongoing studies
NCT01530165 {published data only}
- NCT01530165. apps.who.int/trialsearch/Trial3.aspx?trialid=NCT01530165 (first received 4 April 2017).
- NCT01530165. clinicaltrials.gov/show/NCT01530165 (first received 3 April 2017).
PREVIEW {published data only}
- ACTRN12613000857707. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000857707 (first received 3 April 2017).
- NCT01777893. clinicaltrials.gov/ct2/show/NCT01777893 (first received 3 April 2017).
- Fogelholm M, Larsen TM, Westerterp‐Plantenga M, MacDonald I, Martínez AJ, Handjieva‐Darlenska T, et al. PREVIEW‐Design, methods and baseline participant description of an international intervention to prevent type‐2 diabetes. Annals of Nutrition and Metabolism 2015;67:414. [Google Scholar]
- Raben A, Fogelholm M, Larsen TM, Drummens M, Poppitt S, Formiguera JA, et al. PREVIEW: Prevention of diabetes through lifestyle intervention and population studies in Europe and a round the world. Over 2,000 volunteers randomized to the 3‐y RCT. Obesity Facts. Conference: 22nd Congress of the European Congress on Obesity, ECO 2015 Prague Czech Republic, 2015; Vol. 8, issue 0:126.
PROPELS {published data only}
- ISRCTN83465245. www.isrctn.com/ISRCTN83465245 (first received 27 March 2017).
- Morton K, Sutton S, Hardeman W, Troughton J, Yates T, Griffin S, et al. A text‐messaging and pedometer program to promote physical activity in people at high risk of type 2 diabetes: the development of the PROPELS follow‐on support program. JMIR mHealth and uHealth 2015;3(4):e105. [PUBMED: 26678750] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates T, Griffin S, Bodicoat DH, Brierly G, Dallosso H, Davies MJ, et al. Promotion of physical activity through structured education with differing levels of ongoing support for people at high risk of type 2 diabetes (PROPELS): study protocol for a randomized controlled trial. Trials 2015;16:289. [PUBMED: 26130075] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abdul‐Ghani 2006
- Abdul‐Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55(5):1430‐5. [PUBMED: 16644701] [DOI] [PubMed] [Google Scholar]
ADA 1997
- American Diabetes Association. Report on the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20(Suppl 1):S5‐20. [DOI] [PubMed] [Google Scholar]
ADA 2003
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow‐up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26(11):3160‐7. [DOI] [PubMed] [Google Scholar]
ADA 2004
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27(Suppl 1):s5‐s10. [DOI: 10.2337/diacare.27.2007] [DOI] [PubMed] [Google Scholar]
ADA 2008
- American Diabetes Association. Standards of medical care in diabetes ‐ 2008. Diabetes Care 2008;31(Suppl 1):S12‐54. [PUBMED: 18165335] [DOI] [PubMed] [Google Scholar]
ADA 2010
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl 1):S62‐9. [PUBMED: 20042775] [DOI] [PMC free article] [PubMed] [Google Scholar]
ADA 2015
- American Diabetes Association. Standards of medical care in diabetes ‐ 2015. Diabetes Care 2015;38(Suppl 1):S1‐93. [PUBMED: 24357209] [PubMed] [Google Scholar]
ADA 2017
- American Diabetes Association. Standards of medical care in diabetes ‐ 2017. Diabetes Care 2017;40:(Suppl 1). [Google Scholar]
Aguiar 2014
- Aguiar EJ, Morgan PJ, Collins CE, Plotnikoff RC, Callister R. Efficacy of interventions that include diet, aerobic and resistance training components for type 2 diabetes prevention: a systematic review with meta‐analysis. The International Journal of Behavioral Nutrition and Physical Activity 2014;11:2. [PUBMED: 24423095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ali 2012
- Ali MK, Echouffo‐Tcheugui J, Williamson DF. How effective were lifestyle interventions in real‐world settings that were modelled on the Diabetes Prevention Program?. Health Affairs (Project Hope) 2012;31(1):67‐75. [PUBMED: 22232096] [DOI] [PubMed] [Google Scholar]
Ashra 2015
- Ashra NB, Spong R, Carter P, Davies MJ, Dunkley A, Gillies C, et al. A systematic review and meta‐analysis assessing the effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes mellitus in routine practice. www.gov.uk/government/uploads/system/uploads/attachment_data/file/456147/PHE_Evidence_Review_of_diabetes_prevention_programmes‐_FINAL.pdf (accessed 17 March 2017).
Balk 2015
- Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Annals of Internal Medicine 2015;163(6):437‐51. [PUBMED: 26167912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 2013
- Bell ML, McKenzie JE. Designing psycho‐oncology randomised trials and cluster randomised trials: variance components and intra‐cluster correlation of commonly used psychosocial measures. Psychooncology 2013;22:1738‐47. [DOI] [PubMed] [Google Scholar]
Borenstein 2017a
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta‐analysis: I² is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5‐18. [DOI] [PubMed] [Google Scholar]
Borenstein 2017b
- Borenstein M. Prediction intervals. www.meta‐analysis.com/prediction (accessed 3 July 2017).
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial Sequential Analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Cardona‐Morrell 2010
- Cardona‐Morrell M, Rychetnik L, Morrell SL, Espinel PT, Bauman A. Reduction of diabetes risk in routine clinical practice: are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta‐analysis. BMC Public Health 2010;10:653. [PUBMED: 21029469] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2015
- Centers for Disease Control and Prevention (CDC). www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html (accessed October 2015).
Cheng 2006
- Cheng C, Kushner H, Falkner BE. The utility of fasting glucose for detection of prediabetes. Metabolism: Clinical and Experimental 2006;55(4):434‐8. [PUBMED: 16546472] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DeFronzo 1989
- DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non‐insulin‐dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism: Clinical and Experimental 1989;38(4):387‐95. [PUBMED: 2657323] [DOI] [PubMed] [Google Scholar]
Diabetes Prevention Program 2002
- Knowler WC, Barrett‐Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346(6):393‐403. [PUBMED: 11832527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diabetes Prevention Program FU 2009
- Diabetes Prevention Program Research Group. 10‐year follow‐up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374(9702):1677‐86. [DOI: 10.1016/S0140-6736(09)61457-4; PUBMED: 19878986] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunkley 2014
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta‐analysis. Diabetes Care 2014;37(4):922‐33. [PUBMED: 24652723] [DOI] [PubMed] [Google Scholar]
Finnish Diabetes Prevention Study Group 2001
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne‐Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344(18):1343‐50. [PUBMED: 11333990] [DOI] [PubMed] [Google Scholar]
Gillett 2012
- Gillett M, Royle P, Snaith A, Scotland G, Poobalan A, Imamura M, et al. Non‐pharmacological interventions to reduce the risk of diabetes in people with impaired glucose regulation: a systematic review and economic evaluation. Health Technology Assessment (Winchester, England) 2012;16(33):1‐236, iii‐iv. [PUBMED: 22935084] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillies 2007
- Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta‐analysis. BMJ 2007;334:299. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glechner 2015
- Glechner A, Harreiter J, Gartlehner G, Rohleder S, Kautzky A, Tuomilehto J, et al. Sex‐specific differences in diabetes prevention: a systematic review and meta‐analysis. Diabetologia 2015;58(2):242‐54. [PUBMED: 25465437] [DOI] [PubMed] [Google Scholar]
Gong 2015
- Gong QH, Kang JF, Ying YY, Li H, Zhang XH, Wu YH, et al. Lifestyle interventions for adults with impaired glucose tolerance: a systematic review and meta‐analysis of the effects on glycemic control. Internal Medicine (Tokyo, Japan) 2015;54(3):303‐10. [PUBMED: 25748739] [DOI] [PubMed] [Google Scholar]
Gosmanov 2014
- Gosmanov AR, Wan J. Low positive predictive value of hemoglobin A1c for diagnosis of prediabetes in clinical practice. The American Journal of the Medical Sciences 2014;348(3):191‐4. [PUBMED: 24556928] [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEproGDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Helmrich 1991
- Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS Jr. Physical activity and reduced occurrence of non‐insulin‐dependent diabetes mellitus. New England Journal of Medicine 1991;325:147‐52. [DOI] [PubMed] [Google Scholar]
Hemmingsen 2016
- Hemmingsen B, Sonne DP, Metzendorf MI, Richter B. Insulin secretagogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. The Cochrane Database of Systematic Reviews 2016;10:CD012151. [PUBMED: 27749986] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2010
- Higgins JP, Whitehead A, Simmonds M. Sequential methods for random‐effects meta‐analysis. Statistics in Medicine 2011;30(9):903‐21. [PUBMED: 21190240] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hopper 2011
- Hopper I, Billah B, Skiba M, Krum H. Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta‐analysis of randomised controlled clinical trials. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(6):813‐23. [PUBMED: 21878448] [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICER 2016
- Institute for Clinical and Economic Review. Diabetes prevention programs: effectiveness and value. Final Evidence Report and Meeting Summary July 2016.
ICH 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice //1997 CFR & ICH Guidelines. PA 19063‐2043. USA: Barnett International/PAREXEL, 1997.
IDF 2013
- International Diabetes Federation (IDF). IDF Diabetes Atlas. 6th Edition. Brussels: International Diabetes Federation, 2013. [Google Scholar]
IEC 2009
- International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32(7):1327‐34. [PUBMED: 19502545] [DOI] [PMC free article] [PubMed] [Google Scholar]
Inzucchi 2012
- Inzucchi SE. Clinical practice. Diagnosis of diabetes. New England Journal of Medicine 2012;367(6):542‐50. [PUBMED: 22873534] [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Lan 1983
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659‐63. [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Look AHEAD 2013
- Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New England Journal of Medicine 2013;369(2):145‐54. [DOI: 10.1056/NEJMoa1212914] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012;Issue 12:Art. No.: MR000033. DOI: 10.1002/14651858.MR000033.pub2. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. The Cochrane Database of Systematic Reviews 2017;2:MR000033. [PUBMED: 28207928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lü 2010
- Lü Q, Ke LQ, Tong N, Cao L, Wu T, Zhang J. Metformin for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD008558] [DOI] [Google Scholar]
Manson 1992
- Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among U.S. male physicians. JAMA 1992;268(1):63‐7. [PubMed] [Google Scholar]
Mathieu 2009
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977‐84. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merlotti 2014a
- Merlotti C, Morabito A, Ceriani V, Pontiroli AE. Prevention of type 2 diabetes in obese at‐risk subjects: a systematic review and meta‐analysis. Acta Diabetologica 2014;51(5):853‐63. [PUBMED: 25085464] [DOI] [PubMed] [Google Scholar]
Merlotti 2014b
- Merlotti C, Morabito A, Pontiroli AE. Prevention of type 2 diabetes; a systematic review and meta‐analysis of different intervention strategies. Diabetes, Obesity & Metabolism 2014;16(8):719‐27. [PUBMED: 24476122] [DOI] [PubMed] [Google Scholar]
Modesti 2016
- Modesti PA, Galanti G, Cala' P, Calabrese M. Lifestyle interventions in preventing new type 2 diabetes in Asian populations. Internal and Emergency Medicine 2016;11(3):375‐84. [PUBMED: 26475162] [DOI] [PubMed] [Google Scholar]
Morris 2013
- Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, et al. Progression rates from HbA1c 6.0‐6.4% and other prediabetes definitions to type 2 diabetes: a meta‐analysis. Diabetologia 2013;56(7):1489‐93. [PUBMED: 23584433] [DOI] [PubMed] [Google Scholar]
NDDG 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039‐57. [DOI] [PubMed] [Google Scholar]
Norris 2005
- SL Norris, X Zhang, A Avenell, E Gregg, CH Schmid, Lau J. Long‐term non‐pharmacological weight loss interventions for adults with prediabetes. Cochrane Database of Systematic Reviews 2005;Issue 2:CD005270. [DOI: 10.1002/14651858.CD005270] [DOI] [PubMed] [Google Scholar]
Penn 2013
- Penn L, White M, Lindstrom J, Boer AT, Blaak E, Eriksson JG, et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European Diabetes Prevention Study RCT. PloS One 2013;8(2):e57143. [PUBMED: 23451166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93; discussion 661‐6. [PUBMED: 9408720] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Santaguida 2005
- Santaguida PL, Balion C, Hunt D, Morrison K, Gerstein H, Raina P, et al. Diagnosis, prognosis, and treatment of impaired glucose tolerance and impaired fasting glucose. Evidence Report/Technology Assessment (Summary) 2005;128:1‐11. [PUBMED: 16194123] [PMC free article] [PubMed] [Google Scholar]
Schellenberg 2013
- Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta‐analysis. Annals of Internal Medicine 2013;159(8):543‐51. [PUBMED: 24126648] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Selph 2015
- Selph S, Dana T, Bougatsos C, Blazina I, Patel H, Chou R. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 U.S. Preventive Services Task Force Recommendation. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews 2015; Vol. Report no.:13‐05190‐EF‐1. [PUBMED: 25973510] [PubMed]
Selvin 2011
- Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross‐sectional analysis of community‐based data. Annals of Internal Medicine 2011;154(5):303‐9. [PUBMED: 21357907] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2016
- Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose‐response meta‐analysis of prospective cohort studies. Diabetologia 2016;59(12):2527‐45. [PUBMED: 27747395] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Stevens 2015
- Stevens JW, Khunti K, Harvey R, Johnson M, Preston L, Woods HB, et al. Preventing the progression to type 2 diabetes mellitus in adults at high risk: a systematic review and network meta‐analysis of lifestyle, pharmacological and surgical interventions. Diabetes Research and Clinical Practice 2015;107(3):320‐31. [PUBMED: 25638454] [DOI] [PubMed] [Google Scholar]
Viera 2011
- Viera AJ. Predisease: when does it make sense?. Epidemiologic Reviews 2011;33:122‐34. [DOI] [PubMed] [Google Scholar]
Vijan 1997
- Vijan S, Hofer TP, Hayward RA. Estimated benefits of glycemic control in microvascular complications in type 2 diabetes. Annals of Internal Medicine 1997;127(9):788‐95. [PUBMED: 9382399] [DOI] [PubMed] [Google Scholar]
Vist 2008
- Vist GE, Bryant D, Somerville L, Birminghem T, Oxman AD. Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate. Cochrane Database of Systematic Reviews (Online) 2008;3:MR000009. [PUBMED: 18677782] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
WHO 1980
- World Health Organization. WHO Expert Committee on diabetes mellitus. apps.who.int/iris/bitstream/10665/41399/1/WHO_TRS_646.pdf (accessed 30 November 2017).
WHO 1985
- World Health Organization. Diabetes Mellitus: Report of a WHO Study Group. apps.who.int/iris/bitstream/10665/39592/1/WHO_TRS_727.pdf (accessed 30 November 2017).
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. Part 1: diagnosis and classification of diabetes mellitus. Report of a World Health Organization Consultation. Geneva: World Health Organization, 1999:1‐59. [Google Scholar]
WHO/IDF 2006
- World Health Organization/ International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf 2006; Vol. (accessed 17 March 2017).
Wong 2006a
- Wong SS, Wilczynski NL, Haynes RB. Comparison of top‐performing search strategies for detecting clinically sound treatment studies and systematic reviews in MEDLINE and Embase. Journal of the Medical Library Association 2006;94(4):451‐5. [PMC free article] [PubMed] [Google Scholar]
Wong 2006b
- Wong SSL, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in Embase. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamaoka 2005
- Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes. A meta‐analysis of randomized controlled trials. Diabetes Care 2005;28:2780‐6. [DOI] [PubMed] [Google Scholar]
Yates 2007
- Yates T, Khunti K, Bull F, Gorely T, Davies MJ. The role of physical activity in the management of impaired glucose tolerance: a systematic review. Diabetologia 2007;50(6):1116‐26. [PUBMED: 17415549] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 2013
- Yoon U, Kwok LL, Magkidis A. Efficacy of lifestyle interventions in reducing diabetes incidence in patients with impaired glucose tolerance: a systematic review of randomized controlled trials. Metabolism: Clinical and Experimental 2013;62(2):303‐14. [PUBMED: 22959500] [DOI] [PubMed] [Google Scholar]
Yudkin 2014
- Yudkin JS, Montori VM. The epidemic of pre‐diabetes: the medicine and the politics. BMJ 2014;349:g4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuen 2010
- Yuen A, Sugeng Y, Weiland TJ, Jelinek GA. Lifestyle and medication interventions for the prevention or delay of type 2 diabetes mellitus in prediabetes: a systematic review of randomised controlled trials. Australian and New Zealand Journal of Public Health 2010;34(2):172‐8. [PUBMED: 23331362] [DOI] [PubMed] [Google Scholar]
Zhang 2017
- Zhang X, Imperatore G, Thomas W, Cheng YJ, Lobelo F, Norris K, et al. Effect of lifestyle interventions on glucose regulation among adults without impaired glucose tolerance or diabetes: a systematic review and meta‐analysis. Diabetes Research and Clinical Practice 2017;123:149‐64. [PUBMED: 28024276] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2016
- Zheng L, Wu J, Wang G, Persuitte G, Ma Y, Zou L, et al. Comparison of control fasting plasma glucose of exercise‐only versus exercise‐diet among a pre‐diabetic population: a meta‐analysis. European Journal of Clinical Nutrition 2016;70(4):424‐30. [PUBMED: 26330149] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Orozco 2008
- Orozco LJ, Buchleitner AM, Gimenez‐Perez G, Roque I Figuls M, Richter B, Mauricio D. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD003054.pub3; PUBMED: 18646086] [DOI] [PubMed] [Google Scholar]