Abstract
Background
Psoriasis is an immune‐mediated disease for which some people have a genetic predisposition. The condition manifests in inflammatory effects on either the skin or joints, or both, and it has a major impact on quality of life. Although there is currently no cure for psoriasis, various treatment strategies allow sustained control of disease signs and symptoms. Several randomised controlled trials (RCTs) have compared the efficacy of the different systemic treatments in psoriasis against placebo. However, the relative benefit of these treatments remains unclear due to the limited number of trials comparing them directly head to head, which is why we chose to conduct a network meta‐analysis.
Objectives
To compare the efficacy and safety of conventional systemic agents (acitretin, ciclosporin, fumaric acid esters, methotrexate), small molecules (apremilast, tofacitinib, ponesimod), anti‐TNF alpha (etanercept, infliximab, adalimumab, certolizumab), anti‐IL12/23 (ustekinumab), anti‐IL17 (secukinumab, ixekizumab, brodalumab), anti‐IL23 (guselkumab, tildrakizumab), and other biologics (alefacept, itolizumab) for patients with moderate to severe psoriasis and to provide a ranking of these treatments according to their efficacy and safety.
Search methods
We searched the following databases to December 2016: the Cochrane Skin Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and LILACS. We also searched five trials registers and the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) reports. We checked the reference lists of included and excluded studies for further references to relevant RCTs. We searched the trial results databases of a number of pharmaceutical companies and handsearched the conference proceedings of a number of dermatology meetings.
Selection criteria
Randomised controlled trials (RCTs) of systemic and biological treatments in adults (over 18 years of age) with moderate to severe plaque psoriasis or psoriatic arthritis whose skin had been clinically diagnosed with moderate to severe psoriasis, at any stage of treatment, in comparison to placebo or another active agent.
Data collection and analysis
Three groups of two review authors independently undertook study selection, data extraction, 'Risk of bias' assessment, and analyses. We synthesised the data using pair‐wise and network meta‐analysis (NMA) to compare the treatments of interest and rank them according to their effectiveness (as measured by the Psoriasis Area and Severity Index score (PASI) 90) and acceptability (the inverse of serious adverse effects). We assessed the certainty of the body of evidence from the NMA for the two primary outcomes, according to GRADE; we evaluated evidence as either very low, low, moderate, or high. We contacted study authors when data were unclear or missing.
Main results
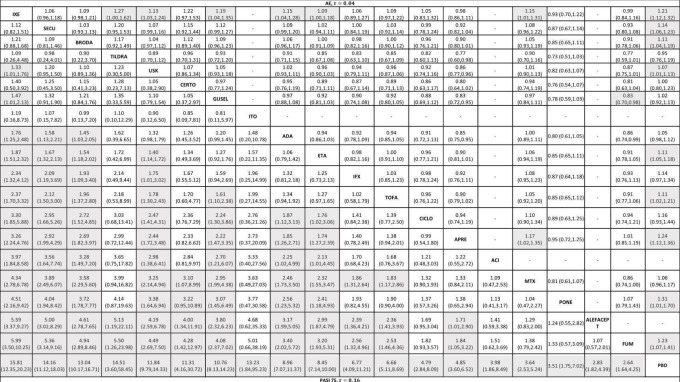
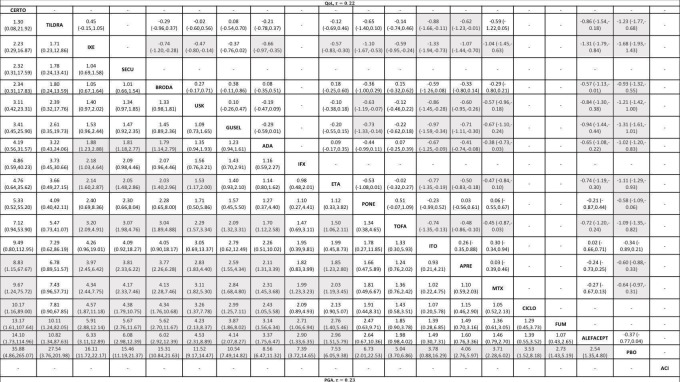
We included 109 studies in our review (39,882 randomised participants, 68% men, all recruited from a hospital). The overall average age was 44 years; the overall mean PASI score at baseline was 20 (range: 9.5 to 39). Most of these studies were placebo controlled (67%), 23% were head‐to‐head studies, and 10% were multi‐armed studies with both an active comparator and placebo. We have assessed all treatments listed in the objectives (19 in total). In all, 86 trials were multicentric trials (two to 231 centres). All of the trials included in this review were limited to the induction phase (assessment at less than 24 weeks after randomisation); in fact, all trials included in the network meta‐analysis were measured between 12 and 16 weeks after randomisation. We assessed the majority of studies (48/109) as being at high risk of bias; 38 were assessed as at an unclear risk, and 23, low risk.
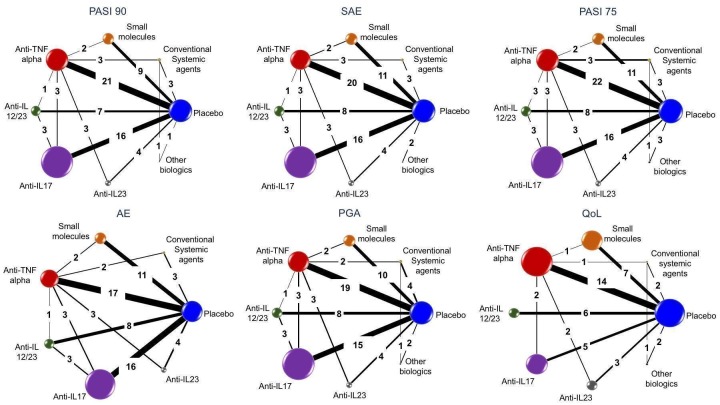
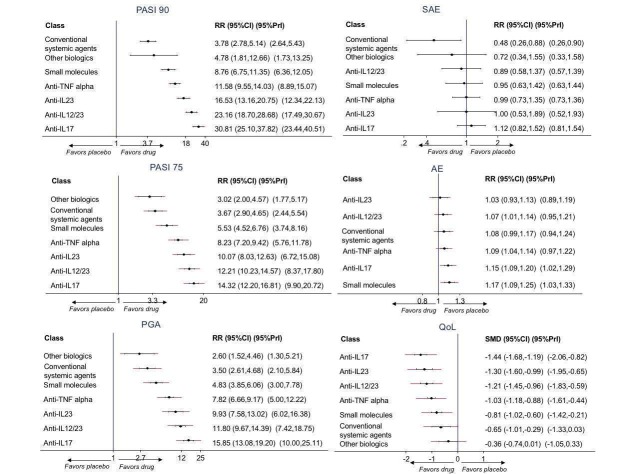
Network meta‐analysis at class level showed that all of the interventions (conventional systemic agents, small molecules, and biological treatments) were significantly more effective than placebo in terms of reaching PASI 90.
In terms of reaching PASI 90, the biologic treatments anti‐IL17, anti‐IL12/23, anti‐IL23, and anti‐TNF alpha were significantly more effective than the small molecules and the conventional systemic agents. Small molecules were associated with a higher chance of reaching PASI 90 compared to conventional systemic agents.
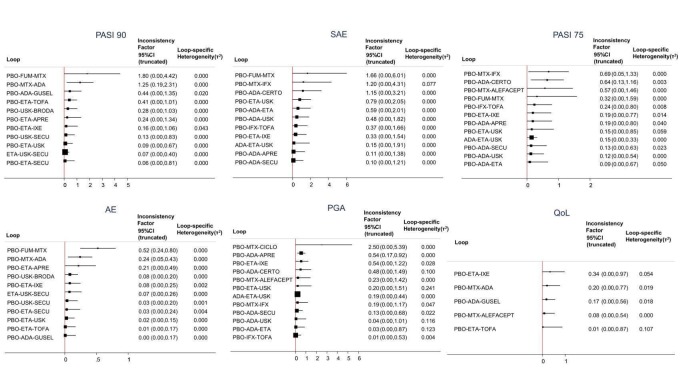
At drug level, in terms of reaching PASI 90, all of the anti‐IL17 agents and guselkumab (an anti‐IL23 drug) were significantly more effective than the anti‐TNF alpha agents infliximab, adalimumab, and etanercept, but not certolizumab. Ustekinumab was superior to etanercept. No clear difference was shown between infliximab, adalimumab, and etanercept. Only one trial assessed the efficacy of infliximab in this network; thus, these results have to be interpreted with caution. Tofacitinib was significantly superior to methotrexate, and no clear difference was shown between any of the other small molecules versus conventional treatments.
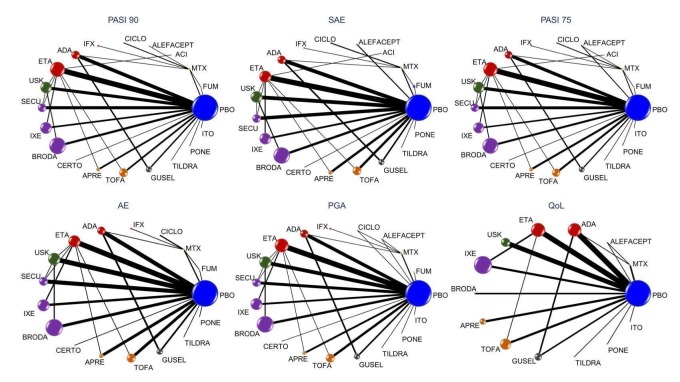
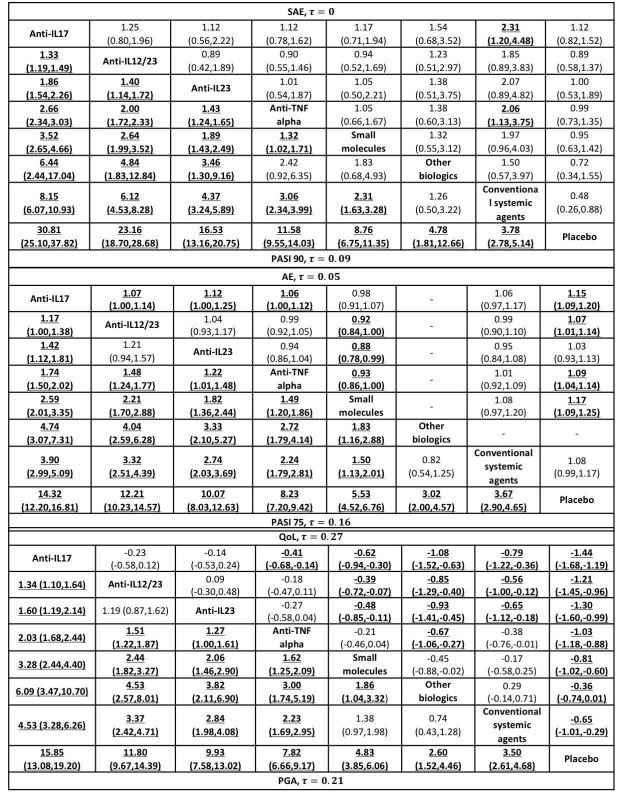
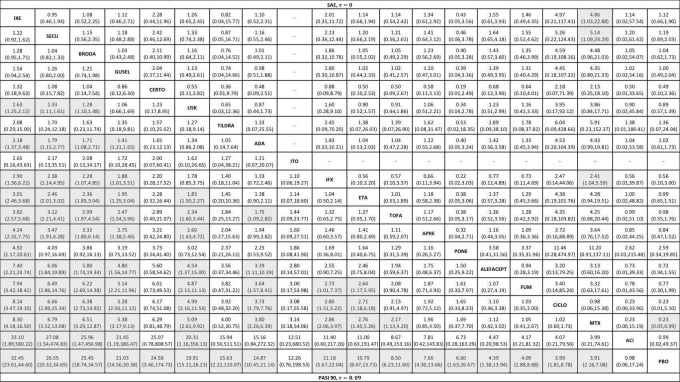
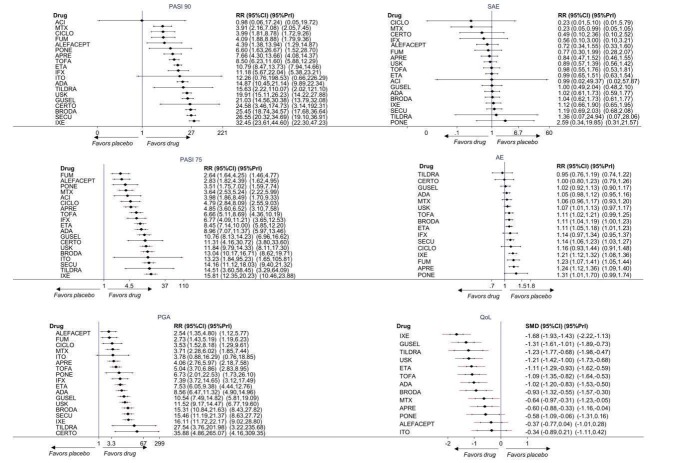
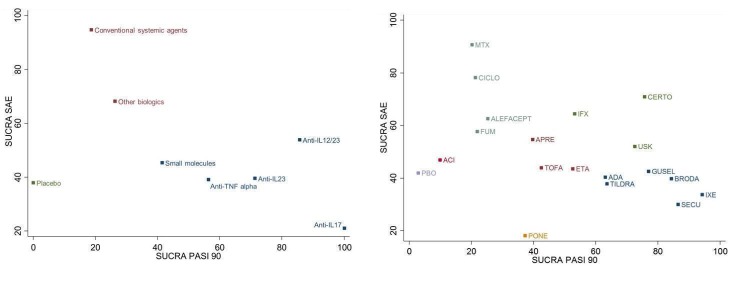
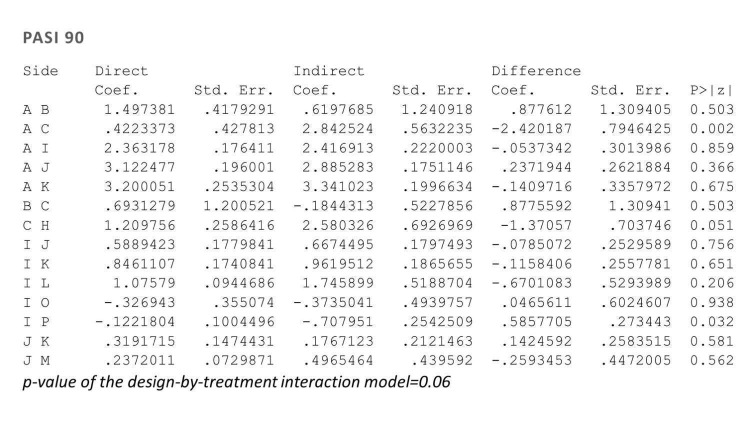
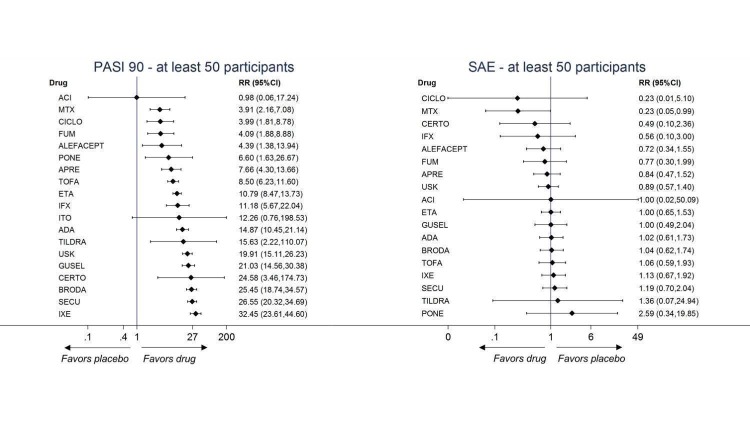
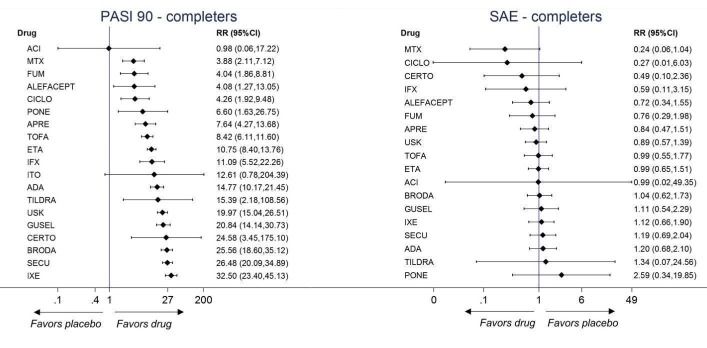
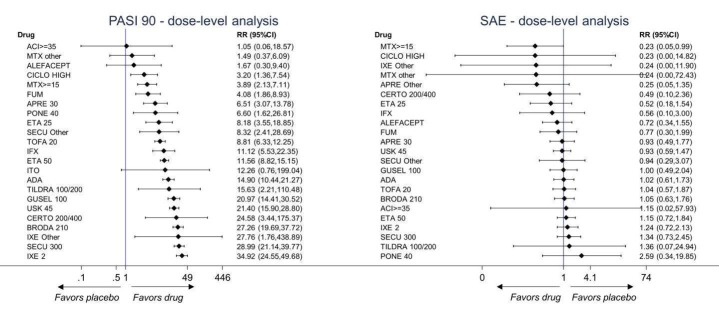
Network meta‐analysis also showed that ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab, and ustekinumab outperformed other drugs when compared to placebo in terms of reaching PASI 90: the most effective drug was ixekizumab (risk ratio (RR) 32.45, 95% confidence interval (CI) 23.61 to 44.60; Surface Under the Cumulative Ranking (SUCRA) = 94.3; high‐certainty evidence), followed by secukinumab (RR 26.55, 95% CI 20.32 to 34.69; SUCRA = 86.5; high‐certainty evidence), brodalumab (RR 25.45, 95% CI 18.74 to 34.57; SUCRA = 84.3; moderate‐certainty evidence), guselkumab (RR 21.03, 95% CI 14.56 to 30.38; SUCRA = 77; moderate‐certainty evidence), certolizumab (RR 24.58, 95% CI 3.46 to 174.73; SUCRA = 75.7; moderate‐certainty evidence), and ustekinumab (RR 19.91, 95% CI 15.11 to 26.23; SUCRA = 72.6; high‐certainty evidence).
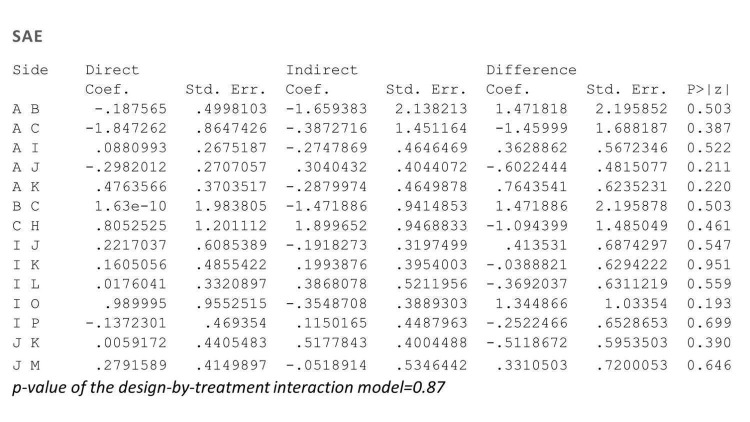
We found no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects (SAEs): the relative ranking strongly suggested that methotrexate was associated with the best safety profile regarding all of the SAEs (RR 0.23, 95% CI 0.05 to 0.99; SUCRA = 90.7; moderate‐certainty evidence), followed by ciclosporin (RR 0.23, 95% CI 0.01 to 5.10; SUCRA = 78.2; very low‐certainty evidence), certolizumab (RR 0.49, 95% CI 0.10 to 2.36; SUCRA = 70.9; moderate‐certainty evidence), infliximab (RR 0.56, 95% CI 0.10 to 3.00; SUCRA = 64.4; very low‐certainty evidence), alefacept (RR 0.72, 95% CI 0.34 to 1.55; SUCRA = 62.6; low‐certainty evidence), and fumaric acid esters (RR 0.77, 95% CI 0.30 to 1.99; SUCRA = 57.7; very low‐certainty evidence). Major adverse cardiac events, serious infections, or malignancies were reported in both the placebo and intervention groups. Nevertheless, the SAEs analyses were based on a very low number of events with low to very low certainty for just over half of the treatment estimates in total, moderate for the others. Thus, the results have to be considered with caution.
Considering both efficacy (PASI 90 outcome) and acceptability (SAEs outcome), highly effective treatments also had more SAEs compared to the other treatments, and ustekinumab, infliximab, and certolizumab appeared to have the better trade‐off between efficacy and acceptability.
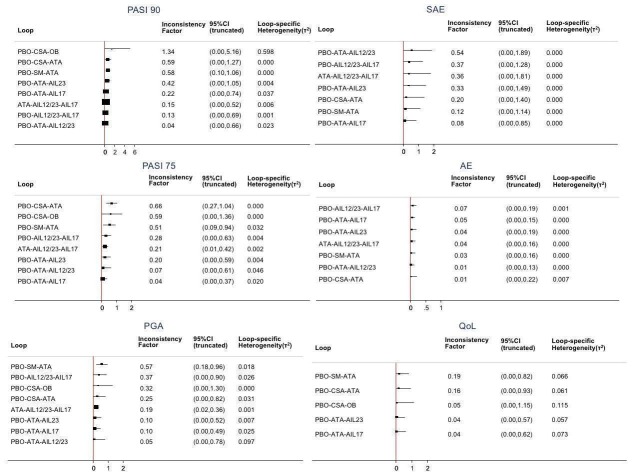
Regarding the other efficacy outcomes, PASI 75 and Physician Global Assessment (PGA) 0/1, the results were very similar to the results for PASI 90.
Information on quality of life was often poorly reported and was absent for a third of the interventions.
Authors' conclusions
Our review shows that compared to placebo, the biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab, and ustekinumab are the best choices for achieving PASI 90 in people with moderate to severe psoriasis on the basis of moderate‐ to high‐certainty evidence. At class level, the biologic treatments anti‐IL17, anti‐IL12/23, anti‐IL23, and anti‐TNF alpha were significantly more effective than the small molecules and the conventional systemic agents, too. This NMA evidence is limited to induction therapy (outcomes were measured between 12 to 16 weeks after randomisation) and is not sufficiently relevant for a chronic disease. Moreover, low numbers of studies were found for some of the interventions, and the young age (mean age of 44 years) and high level of disease severity (PASI 20 at baseline) may not be typical of patients seen in daily clinical practice.
Another major concern is that short‐term trials provide scanty and sometimes poorly reported safety data and thus do not provide useful evidence to create a reliable risk profile of treatments. Indeed, we found no significant difference in the assessed interventions and placebo in terms of SAEs. Methotrexate appeared to have the best safety profile, but as the evidence was of very low to moderate quality, we cannot be sure of the ranking. In order to provide long‐term information on the safety of the treatments included in this review, it will be necessary to evaluate non‐randomised studies and postmarketing reports released from regulatory agencies as well.
In terms of future research, randomised trials comparing directly active agents are necessary once high‐quality evidence of benefit against placebo is established, including head‐to‐head trials amongst and between conventional systemic and small molecules, and between biological agents (anti‐IL17 versus anti‐IL23, anti‐IL23 versus anti‐IL12/23, anti‐TNF alpha versus anti‐IL12/23). Future trials should also undertake systematic subgroup analyses (e.g. assessing biological‐naïve patients, baseline psoriasis severity, presence of psoriatic arthritis, etc.). Finally, outcome measure harmonisation is needed in psoriasis trials, and researchers should look at the medium‐ and long‐term benefit and safety of the interventions and the comparative safety of different agents.
Keywords: Adult; Humans; Network Meta‐Analysis; Antibodies, Monoclonal; Antibodies, Monoclonal/adverse effects; Antibodies, Monoclonal/therapeutic use; Chronic Disease; Immunosuppressive Agents; Immunosuppressive Agents/adverse effects; Immunosuppressive Agents/therapeutic use; Psoriasis; Psoriasis/drug therapy; Psoriasis/pathology; Randomized Controlled Trials as Topic; Remission Induction; Tumor Necrosis Factor‐alpha; Tumor Necrosis Factor‐alpha/antagonists & inhibitors
Systemic (oral or injected) medicines for psoriasis
What is the aim of this review?
The aim of this review was to compare different systemic medicines (oral or injected medicines that work throughout the entire body) used to treat chronic plaque psoriasis in adults (over 18 years of age), to find out which are the safest and most effective at clearing psoriasis. We wanted to rank the medicines in order of their safety and how well they work, to help the development of a treatment pathway for people with chronic plaque psoriasis. We collected and analysed all relevant studies to answer this question and found 109 studies.
Key messages
The results showed that a selection of treatments from the class of biological medicines appear to be the most effective systemic medicines for achieving a chronic plaque psoriasis score of PASI (Psoriasis Area and Severity Index) 90, which translates into a 90% improvement in psoriasis from the beginning of the study. We found no significant difference in serious adverse effects (SAEs) (i.e. serious side effects) when comparing any of the assessed treatments with placebo. However, as the evidence was of very low to moderate quality, we cannot be sure of these results.
For some of the interventions, we found low numbers of studies, so more research needs to be conducted to directly compare the systemic medicines with each other, rather than comparing them with placebo (an inactive substance) (once effect against placebo has been established by high‐quality evidence). In addition, longer‐term studies are needed to provide more evidence about the benefit and safety of systemic medicines and to compare their safety profiles. Indeed, the results of this review are limited to the induction treatment (i.e. outcomes were measured up to 24 weeks after participants were allocated to their treatment group), which is not an appropriate treatment option for a chronic disease.
We rated the certainty of the evidence as ranging from very low (mainly conventional medicines) to high (mainly biological medicines). We downgraded the certainty of the evidence due to risk of bias (concerns with the study methods) and then for either inconsistent results or imprecision (inaccuracy).
What was studied in the review?
Psoriasis is characterised by patches of red, flaky skin covered with scales (known as plaques) or other inflammatory effects that are seen on the skin or joints, or both. Psoriasis is caused by an abnormal response within the immune system in people who may have a genetic predisposition towards the condition.
Approximately 2% of the population have psoriasis, and 90% of those people have plaque psoriasis. Around 10% to 20% of people with chronic plaque psoriasis will need to have systemic treatments. Psoriasis impacts on quality of life, including a person's psychosocial life.
We compared 19 systemic medicines by identifying studies that compared one or more of these medicines with either placebo or with another medicine to treat moderate to severe forms of plaque psoriasis in adults who were at any stage of treatment. The medicines we assessed were conventional systemic treatments (a varied group of treatments that are the oldest treatments given to clear psoriasis), biologics (treatments that use substances made from living organisms, or synthetic versions, to target the immune system), and small molecules (which affect molecules inside immune cells). We included studies whose participants may also have had psoriatic arthritis. The main outcomes we were interested in were achievement of PASI 90 and any serious side effects that were thought to be associated with the medicines.
We combined all of the studies to allow indirect analysis of the treatments, so we could compare them with each other (network meta‐analysis).
What are the main results of the review?
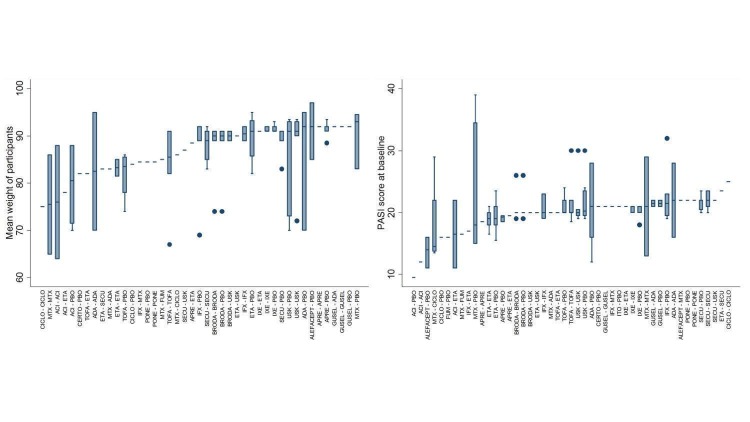
The 109 studies enrolled 39,882 people (all recruited from a hospital) with moderate to severe psoriasis: 26,902 men and 12,384 women; the overall average age was 44 years, the overall mean PASI score at the start of the study was 20 (range: 9.5 to 39), indicating a high level of disease severity. Most studies (n = 73) compared the systemic medicine with a placebo treatment, a total of 25 trials compared systemic treatments with other systemic treatments, and 11 trials compared systemic treatments with systemic treatments and placebo. Most studies were short‐term, and in all, 86 trials were multicentric trials (two to 231 centres).
The outcomes presented here were measured 12 to 16 weeks after the study participants were randomised.
The results showed that compared with placebo, all treatments (assessed in the following groupings: anti‐IL17, anti‐IL12/23, anti‐IL23, and anti‐TNF alpha (i.e. the treatments known as the biologics); small molecule treatments; other biologics; and conventional systemic agents) were more effective in treating psoriasis when assessed using an index that required 90% improvement (PASI 90).
In relation to the same outcome (PASI 90), the biologic treatments anti‐IL17, anti‐IL12/23, anti‐IL23, and anti‐TNF alpha appeared to work better than the small molecules and the conventional systemic agents; and small molecules were associated with a better outcome compared to conventional systemic agents. (IL is an abbreviation of interleukin; TNF is an abbreviation of tumour necrosis factor ‐ both are types of cytokine. A cytokine affects the behaviour of a cell.)
In terms of individual drugs, again when assessing the ability to reach PASI 90, all of the anti‐IL17 drugs and guselkumab (an anti‐IL23 drug) were more effective than the anti‐TNF alpha drugs infliximab, adalimumab, and etanercept, but not certolizumab. Ustekinumab (an IL‐12/‐23 drug) was better than etanercept. No clear difference was shown between infliximab, adalimumab, and etanercept. Tofacitinib (a small molecule) was superior to methotrexate (a conventional systemic agent), and no difference was shown between the other small molecules and the conventional drugs.
Judged against placebo, six biological medicines worked best at clearing psoriasis lesions. These medicines were ranked as follows (most effective first): ixekizumab, secukinumab (both based on high‐certainty evidence), brodalumab, guselkumab, certolizumab (all based on moderate‐certainty evidence), and ustekinumab (high‐certainty evidence). Regarding the outcomes PASI 75 and Physician Global Assessment (PGA) 0/1 (i.e. achieving 75% improvement and achieving a PGA score of 0 or 1), the results were very similar to the results for PASI 90.
For the risk of serious side effects, there were no clear differences between all of the systemic medicines compared with placebo treatment. Methotrexate had the best safety profile (based on moderate‐certainty evidence), followed by ciclosporin (very low‐certainty evidence), certolizumab (moderate‐certainty evidence), infliximab (very low‐certainty evidence), alefacept (low‐certainty evidence), and fumaric acid esters (very low‐certainty evidence) (all of these are conventional treatments except for certolizumab, infliximab (anti‐TNF alpha drugs), and alefacept (classed under 'other biologics'). Major adverse cardiac events, serious infections, or malignancies were reported in both placebo and intervention groups. However, the number of serious side effects was very low, and our conclusions are based on low to very low‐ (for just over half of the results) or moderate‐certainty evidence, so they should be interpreted with caution. The most effective treatments (in terms of reaching PASI 90) had the highest numbers of reported side effects; ustekinumab, infliximab, and certolizumab appeared to have the best compromise between effectiveness and side effects.
For all studies, little information was recorded about quality of life; one third of the medicines studied had no quality of life data.
How up‐to‐date is this review?
We searched for studies that had been published up to December 2016.
Summary of findings
Summary of findings for the main comparison.
Any systemic treatment compared to placebo for chronic plaque psoriasis
| Any systemic treatment compared to placebo for chronic plaque psoriasis (network meta‐analysis) | |||||||
|
Patient or population: people with chronic plaque psoriasis Intervention: any systemic treatment Comparison: placebo Setting: all the participants were recruited from a hospital setting Timescale: 12 to 16 weeks after randomisation | |||||||
| Intervention | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | SUCRA | № of participants (studies)b | Certainty of the evidence (GRADE) | Comments | |
| Risk with placeboa | Risk with any systemic treatment | ||||||
| PASI 90 | |||||||
| Ixekizumab | Moderate | RR 32.45 (23.61 to 44.60) | 94.3 | 3268 (4 RCTs) | ⊕⊕⊕⊕ High | ‐ | |
| 15 per 1000 | 487 per 1000 (354 to 669) | ||||||
| Secukinumab | Moderate | RR 26.55 (20.32 to 34.69) | 86.5 | 2707 (7 RCTs) | ⊕⊕⊕⊕ High | ‐ | |
| 15 per 1000 | 398 per 1000 (305 to 520) | ||||||
| Brodalumab | Moderate | RR 25.45 (18.74 to 34.57) | 84.3 | 4109 (5 RCTs) | ⊕⊕⊕⊝ Moderate | Reasons for downgrading by one level: three studies contributing to this estimate at high risk of bias in selective reporting domain | |
| 15 per 1000 | 382 per 1000 (281 to 520) | ||||||
| Guselkumab | Moderate | RR 21.03 (14.56 to 30.38) | 77 | 1502 (3 RCTs) | ⊕⊕⊕⊝ Moderate | Reasons for downgrading by one level: one study contributing to this estimate at high risk of bias in selective reporting domain | |
| 15 per 1000 | 315 per 1000 (218 to 456) | ||||||
| Certolizumab | Moderate | RR 24.58 (3.46 to 174.73) | 75.7 | 176 (1 RCT) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to imprecision: wide CIs | |
| 15 per 1000 | 369 per 1000 (52 to 1000) | ||||||
| Ustekinumab | Moderate | RR 19.91 (15.11 to 26.23) | 72.6 | 3832 (7 RCTs) | ⊕⊕⊕⊕ High | ‐ | |
| 15 per 1000 | 299 per 1000 (227 to 393) | ||||||
| Tildrakizumab | Moderate | RR 15.63 (2.22 to 110.07) | 63.6 | 355 (1 RCT) | ⊕⊕⊝⊝ Low | Downgraded one level due to risk of bias and one level due to imprecision. The single study contributing to this estimate at unclear risk of bias in both blinding domains; wide CIs | |
| 15 per 1000 | 234 per 1000 (33 to 1000) | ||||||
| Adalimumab | Moderate | RR 14.87 (10.45 to 21.14) | 63.1 | 3199 (8 RCTs) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to inconsistency ‐ inconsistent loops of evidence | |
| 15 per 1000 | 223 per 1000 (157 to 317) | ||||||
| Itolizumab | Moderate | RR 12.26 (0.76 to 198.53) | 56 | 225 (1 RCT) | ⊕⊕⊝⊝ Low | Downgraded one level due to imprecision (wide CIs) and one level due to risk of bias (moderate risk using credibility of evidence) | |
| 15 per 1000 | 184 per 1000 (12 to 1000) | ||||||
| Infliximab | Moderate | RR 11.18 (5.67 to 22.04) | 53.2 | (0 RCTs) | ⊕⊝⊝⊝ Very low | Downgraded one level due to risk of bias (credibility of risk), one level due to imprecision (wide CIs) and one level due to inconsistency (inconsistent loop of evidence) | |
| 15 per 1000 | 168 per 1000 (85 to 331) | ||||||
| Etanercept | Moderate | RR 10.79 (8.47 to 13.73) | 52.6 | 4954 (12 RCTs) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to inconsistency (global inconsistency ‐ side‐splitting approach) | |
| 15 per 1000 | 162 per 1000 (127 to 206) | ||||||
| Tofacitinib | Moderate | RR 8.50 (6.23 to 11.60) | 42.5 | 2826 (4 RCTs) | ⊕⊕⊝⊝ Low | Downgraded one level due to risk of bias: two studies at high risk of bias in incomplete outcome data domain; and downgraded one level due to inconsistency (global approach) | |
| 15 per 1000 | 128 per 1 000 (93 to 174) | ||||||
| Apremilast | Moderate | RR 7.66 (4.30 to 13.66) | 39.7 | 1775 (4 RCTs) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to risk of bias: one study had a slight risk of bias in selective reporting domain | |
| 15 per 1000 | 115 per 1000 (65 to 205) | ||||||
| Ponesimod | Moderate | RR 6.60 (1.63 to 26.67) | 37.3 | 326 (1 RCT) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to imprecision: wide CIs | |
| 15 per 1000 | 99 per 1000 (24 to 400) | ||||||
| Alefacept | Moderate | RR 4.39 (1.38 to 13.94) | 25.3 | (0 RCTs) | ⊕⊝⊝⊝ Very low | Downgraded two levels due to risk of bias and a further one level due to imprecision ‐ study indirectly contributing to the estimates at high risk of bias in selective reporting domain; wide CIs | |
| 15 per 1000 | 66 per 1000 (21 to 209) | ||||||
| Fumaric acid esters (FAEs) | Moderate | RR 4.09 (1.88 to 8.88) | 21.9 | 704 (1 RCT) | ⊕⊝⊝⊝ Very low | Downgraded two levels due to risk of bias, and one level due to imprecision ‐ the studies indirectly contributing to this estimate at high risk of bias in blinding domain; wide CIs | |
| 15 per 1000 | 61 per 1000 (28 to 133) | ||||||
| Ciclosporin | Moderate | RR 3.99 (1.81 to 8.78) | 21.3 | (0 RCTs) | ⊕⊝⊝⊝ Very low | Downgraded two levels due to risk of bias, and a further one level due to imprecision ‐ the single study indirectly contributing to this estimate at high risk of bias in blinding; wide CIs | |
| 15 per 1000 | 60 per 1000 (27 to 132) | ||||||
| Methotrexate | Moderate | RR 3.61 (2.01 to 6.48) | 20.2 | 282 (2 RCTs) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to inconsistency (inconsistent loop of evidence) | |
| 15 per 1000 | 59 per 1000 (32 to 106) | ||||||
| Acitretin | Moderate | RR 0.98 (0.06 to 17.24) | 9.9 | (0 RCTs) | ⊕⊝⊝⊝ Very low | Downgraded two levels due to risk of bias and a further one level due to imprecision. The single study contributing to this estimate at high risk of bias in incomplete outcome data and blinding domains; wide CIs | |
| 15 per 1000 | 15 per 1000 (1 to 259) | ||||||
| Serious adverse events | |||||||
| Methotrexate | Moderate | RR 0.23 (0.05 to 0.99) | 90.7 | 282 (2 RCTs) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to imprecision (wide CIs) | |
| 17 per 1000 | 4 per 1000 (1 to 17) | ||||||
| Ciclosporin | Moderate | RR 0.23 (0.01 to 5.10) | 78.2 | (0 RCTs) | ⊕⊝⊝⊝ Very low | Downgraded two levels due to risk of bias (credibility of evidence), and one level due to imprecision (wide CIs) | |
| 17 per 1000 | 4 per 1000 (0 to 87) | ||||||
| Certolizumab | Moderate | RR 0.49 (0.10 to 2.36) | 70.9 | 176 (1 RCT) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to imprecision (wide CIs) | |
| 17 per 1000 | 8 per 1000 (2 to 40) | ||||||
| Infliximab | Moderate | RR 0.56 (0.10 to 3.00) | 64.4 | (0 RCTs) | ⊕⊝⊝⊝ Very low | Downgraded two levels due to risk of bias, and one level due to imprecision: credibility of evidence; wide CIs | |
| 17 per 1000 | 10 per 1000 (2 to 51) | ||||||
| Alefacept | Moderate | RR 0.72 (0.34 to 1.55) | 62.6 | 736 (2 RCTs) | ⊕⊕⊝⊝ Low | Downgraded one level due to risk of bias (credibility of evidence), and one level due to imprecision (wide CIs) | |
| 17 per 1000 | 12 per 1000 (6 to 26) | ||||||
| Fumaric acid esters (FAEs) | Moderate | RR 0.77 (0.30 to 2.00) | 57.7 | 704 (1 RCT) | ⊕⊝⊝⊝ Very low | Downgraded by one level due to risk of bias and one level due to imprecision: credibility of evidence; wide CIs | |
| 17 per 1000 | 13 per 1000 (5 to 34) | ||||||
| Apremilast | Moderate | RR 0.84 (0.47 to 1.51) | 54.7 | 2036 (5 RCTs) | ⊕⊕⊝⊝ Low | Downgraded one level due to risk of bias and one level due to imprecision: credibility of evidence and wide CIs | |
| 17 per 1000 | 14 per 1000 (8 to 26) | ||||||
| Ustekinumab | Moderate | RR 0.89 (0.57 to 1.39) | 52 | 4154 (8 RCTs) | ⊕⊕⊝⊝ Low | Downgraded one level due to risk of bias and one level due to imprecision ‐ credibility of evidence; wide CIs | |
| 17 per 1000 | 15 per 1000 (10 to 24) | ||||||
| Acitretin | Moderate | RR 0.99 (0.02 to 49.37) | 46.9 | (0 RCTs) | ⊕⊝⊝⊝ Very low | Downgraded by two levels due to risk of bias and one level due to imprecision: credibility of evidence; wide CIs | |
| 17 per 1000 | 17 per 1000 (0 to 839) | ||||||
| Tofacitinib | Moderate | RR 0.98 (0.55 to 1.76) | 44 | 2838 (5 RCTs) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to imprecision (wide CIs) | |
| 17 per 1000 | 17 per 1000 (9 to 30) | ||||||
| Etanercept | Moderate | RR 0.99 (0.65 to 1.51) | 43.6 | 3783 (11 RCTs) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to imprecision (CIs including one) | |
| 17 per 1000 | 17 per 1000 (11 to 26) | ||||||
| Guselkumab | Moderate | RR 1.00 (0.49 to 2.04) | 42.6 | 1502 (3 RCTs) | ⊕⊕⊝⊝ Low | Downgraded one level due to risk of bias (credibility of evidence), and one level due to imprecision (CIs including one) | |
| 15 per 1000 | 15 per 1000 (7 to 31) | ||||||
| Adalimumab | Moderate | RR 1.02 (0.61 to 1.73) | 40.4 | 3199 (8 RCTs) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to imprecision (CIs including one) | |
| 18 per 1000 | 19 per 1000 (11 to 31) | ||||||
| Brodalumab | Moderate | RR 1.04 (0.62 to 1.73) | 39.8 | 4109 (5 RCTs) | ⊕⊕⊝⊝ Low | Downgraded one level due to risk of bias (credibility of evidence) and one level due to imprecision (CIs including 1) | |
| 17 per 1000 | 18 per 1000 (11 to 30) | ||||||
| Tildrakizumab | Moderate | RR 1.36 (0.07 to 24.94) | 37.8 | 355 (1 RCT) | ⊕⊕⊝⊝ Low | Downgraded one level due to risk of bias (credibility of evidence) and one level due to imprecision (CIs including 1) | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||||
| Ixekizumab | Moderate | RR 1.12 (0.66 to 1.90) | 33.7 | 3268 (4 RCTs) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to imprecision (CIs including one) | |
| 15 per 1000 | 16 per 1000 (10 to 28) | ||||||
| Secukinumab | Moderate | RR 1.19 (0.69 to 2.03) | 29.9 | 2707 (7 RCTs) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to imprecision (CIs including one) | |
| 10 per 1000 | 12 per 1000 (7 to 20) | ||||||
| Ponesimod | Moderate | RR 2.59 (0.34 to 19.85) | 18.1 | 326 (1 RCT) | ⊕⊕⊕⊝ Moderate | Downgraded one level due to imprecision (CIs including one) | |
| 15 per 1000 | 39 per 1000 (5 to 296) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PASIc: Psoriasis Area and Severity Index; RR: risk ratio; SUCRAd: Surface Under the Cumulative Ranking | |||||||
| GRADE Working Group grades of evidence High certainty/quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty/quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty/quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty/quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
a 'Risk with placebo' is the median placebo‐group risk value in the included studies for the assumed risk with placebo. b 'Number of studies (participants)' is from the direct comparisons.
c The Psoriasis Area and Severity Index combines the assessment of the severity of lesions and the area affected into a single score in the range of 0 (no disease) to 72 (maximal disease); PASI 90: 90% improvement in the PASI.
d SUCRA was expressed as a percentage between 0 (when a treatment is certain to be the worst) to 100% (when a treatment is certain to be the best).
Background
Please refer to our glossary (see Table 8).
Table 1.
Glossary
| Term | Definition |
| Antagonist | A substance that interferes with or inhibits the physiological action of another. |
| Antigen | A molecule capable of inducing an immune respons |
| Anti‐TNF alpha | A pharmaceutical drug that suppresses the physiologic response to tumor necorsis factor (TNF) |
| Biological agent | Therapeutic agents consisting of immune molecules such as soluble receptors, recombinant cytokines, and monoclonal antibodies that target effector molecules or cells of the immune system |
| CD6 | Cluster of differentiation (CD) 6 is a protein encoded by the CD6 gene |
| Cheilitis | An inflammation of the lips |
| Chimeric protein | A chimeric protein can be made by combining two different genes |
| Complex cyclophilin‐ciclosporin | Cyclophilins are a family of proteins that bind to ciclosporin, an immunosuppressant agent |
| Creatinine | A compound that is produced by metabolism of creatine and excreted in the urine |
| Cyclic adenosine monophosphate | It is a second messenger important in many biological processes |
| Cytokines | Small proteins produced by a broad range of cells that are important in cell signaling; they are immunomodulating agents |
| Dendritic cells | Antigen‐presenting cells of the immune system |
| Dermis | It is a layer of the skin |
| Epitope | It is a part of an antigen |
| Erythematous | Redness of the skin |
| Folic acid | B vitamin |
| Humanised antibody | Antibodies from non‐human species whose protein sequences have been modified to increase their similarity to antibody variants produced naturally in humans |
| IL‐17A | A pro‐inflammatory cytokine |
| IL‐23R | A cytokine receptor |
| Immune‐mediated | A group of diseases that are characterised by common inflammatory pathways leading to inflammation, and which may result from a dysregulation of the normal immune response |
| Immunogenicity | This is the ability of a particular substance, such as an antigen or epitope, to provoke an immune response in the body of a human or animal |
| Immunoglobulin 1 Fc | An antibody |
| Interferon (IFN)‐c | A protein released by cells, usually in response to a pathogen |
| Interleukin | A kind of cytokine |
| Janus kinase (JAK) inhibitors | A pharmaceutical drug that inhibits the activity of one or more of the Janus kinase family of enzymes |
| Keratinocytes | Epidermal cells that constitute 95% of the epidermis |
| Lymphocyte | A subtype of a white blood cell |
| Lymphoid organ | Part of the body that defends the body against invading pathogens that cause infections or the spread of tumours |
| Metalloproteinases | A protease enzyme |
| Monoclonal antibodies | Antibodies that are made by identical immune cells that are all clones of a unique parent cell |
| Murine sequence | Mouse genomic sequencing |
| Neutrophils | Type of white blood cell involved in the innate immune system |
| p40 | Subunit beta of interleukin 12 and 23 |
| Periumbilical | Around the navel |
| Pharmacological treatments | Drugs |
| Phase I | First‐in‐man studies |
| Phase II | Studies to assess how well the drug works, as well as to continue phase I safety assessments in a larger group of volunteers and participants |
| Phase III | Randomised controlled multicenter trials on large patient groups and are aimed at being the definitive assessment of how effective the drug is |
| Phase IV | Post‐marketing trials involve the safety surveillance |
| Phosphodiesterase 4 inhibitors | A pharmaceutical drug used to block the degradative action of phosphodiesterase 4 |
| Progressive multifocal leukoencephalopathy | A rare viral neurological disease characterised by progressive damage of the white matter of the brain at multiple locations |
| Receptor | A protein molecule that receives chemical signals from outside a cell |
| Small molecules | Chemically manufactured molecules (or SMOLs for short) |
| Sphingosine 1‐phosphate receptor agonists | A class of protein‐coupled receptors that are targets of the lipid signalling molecule Sphingosine‐1‐phosphate |
| T cells/CD4 T cells | A type of white blood cell that is of key importance to the immune system |
| Th1 and Tc1 cells | A type of T cell |
| Th17 and Tc17 cells | A type of T cell |
| TNF‐alpha | A protein that is part of the inflammatory response |
| Tumour necrosis factor antagonists | Class of biological agents |
| Umbilic | Navel |
| Xerosis | Dry skin |
Description of the condition
Psoriasis is an immune‐mediated disease for which a person can have genetic susceptibility, manifesting in chronic inflammatory effects on either the skin or joints, or both, with a prevalence ranging from 0.91% (United States) to 8.5% (Norway) (Boehncke 2015; Parisi 2013). The causes of psoriasis are not fully understood. There appears to be interaction between environmental factors and genetic susceptibility. Genome‐wide (or whole genome) association trials found several candidate genes relating to psoriasis (Elder 2010). Various environmental factors, including stress, injury, and infections, are suspected to trigger or aggravate the evolution of psoriasis. An inflammatory immune response involving dendritic cells, T cells, keratinocytes, neutrophils, and the cytokines released from immune cells initiates the pathophysiological process (Jariwala 2007; Lowes 2008; Wilson 2007; Zheng 2007).
Diagnosis is made based on clinical findings; skin biopsy is rarely used to diagnose the disease (Boehncke 2015). Several clinical types of psoriasis exist: plaque, pustular, inverse, and erythrodermic. Plaque psoriasis is the most common form, affecting 90% of people with psoriasis (Griffiths 2007). Plaque psoriasis typically appears as raised erythematous and well‐demarcated areas of inflamed skin covered with silvery white, scaly skin (Griffiths 2007). The location of the plaques is usually symmetrical on the elbows, knees, scalp, lower back, and the periumbilical region. For 5% to 25% of people with psoriatic rheumatic disease, their skin is also involved (Helliwell 2005; Zachariae 2003).
Severity
Chronicity characterises the natural history of plaque psoriasis; this means that severity varies over time, from minor localised patches to complete body coverage. The severity of the disease usually fluctuates around the same level for a particular person (Nijsten 2007), but for each person with this disease, the evolution and duration of remission is unpredictable. The psoriasis is declared clear when remission is complete.
More than a dozen outcome instruments are used to assess the severity of psoriasis and the efficacy of different treatments for psoriasis (Naldi 2010; Spuls 2010); the Psoriasis Area and Severity Index (PASI) score is one of these instruments (Schmitt 2005). The Psoriasis Area and Severity Index combines the assessment of the severity of lesions and the area affected into a single score in the range of 0 (no disease) to 72 (maximal disease). Recent clinical trials evaluating biological therapies that have received secondary marketing authorisation by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) used PASI 75, i.e. 75% improvement in the PASI score, as the primary end point. However, the PASI has substantial limitations, such as low‐response distribution, no consensus on interpretability, and low responsiveness in mild disease (Spuls 2010).
Impact and quality of life
Disease severity alone does not determine the burden of psoriasis. Multiple studies have described an impairment of the quality of life (QoL); others have focused on an evaluation of the stigma people experience; and others have studied the impact on psychosocial life (Kimball 2005).
Impairment of QoL in people with psoriasis, when measured with the 36‐item Short Form Health Survey (SF‐36) questionnaire has been found to be higher than that of people with hypertension, diabetes, or depression (Rapp 1999).
Many tools exist to measure the QoL of people with psoriasis and other skin disorders. These measures may be categorised as psoriasis‐specific (Psoriasis Index of Quality of Life (PSORIQoL), Psoriasis Disability Index (PDI)); skin‐specific (Dermatology Life Quality Index (DLQI), Skindex (a quality‐of‐life measure for patients with skin disease)); and generic QoL measures (SF‐36). However, methodological weaknesses exist in the use of QoL questionnaires, and there is poor reporting of QoL outcomes in randomised clinical trials (Le Cleach 2008). Several case‐control studies reported a higher risk of metabolic syndrome and cardiovascular comorbidities (Kremers 2007; Naldi 2005).
Description of the intervention
There is currently no cure for psoriasis, but various treatments can help to control the symptoms; thus, long‐term treatment is usually needed. In daily practice, a treatment strategy needs to be defined, and this usually involves an induction therapy, e.g. the remission of the psoriasis flare, and a maintenance therapy, e.g. increasing the period of remission.
The therapeutic approach to psoriasis includes topical treatments as a single strategy and a first‐line therapy in the management of minor forms (Mason 2013). Nevertheless, about 20% to 30% of people with psoriasis have a moderate to severe form requiring a second‐line therapy including phototherapy and conventional systemic agents, such as ciclosporin, methotrexate, or acitretin. Among the systemic agents, the choice of drug is not clear. The NICE 2012 clinical guidelines in the UK had proposed methotrexate as the first choice of systemic agent. Other countries, such as France, do not have any available guidelines. Systemic biological agents, such as the tumour necrosis factor (TNF) antagonists (infliximab, etanercept, adalimumab), the monoclonal antibody ustekinumab that targets interleukin‐12 and ‐23 (IL‐12/‐23), anti‐IL17 drugs (secukinumab or ixekizumab), and more recently new small molecules (apremilast) are "third‐line" therapies (Boehncke 2015). Indeed, there are mandatory reimbursement criteria that patients must meet before being considered for these treatments due to their high costs: moderate to severe psoriasis after failure, intolerance or contraindication to at least two conventional systemic agents (Nast 2015b).
We used the European S3 guidelines terminology to categorise the treatments (Nast 2015b).
Oral systemic treatments
Conventional systemic agents
Conventional systemic agents are a heterogeneous group of treatments that are the oldest interventions given to clear psoriasis.
The existing oral systemic pharmacological treatments available for psoriasis are ciclosporin, methotrexate, acitretin (which is the retinoid of choice for psoriasis), and fumaric acid esters (FAEs) which are licensed for psoriasis in Germany and used off‐licence in other countries (Atwan 2015).
Randomised controlled trials against placebo for both induction and maintenance therapies have demonstrated the efficacy of ciclosporin for psoriasis (Bigby 2004; Christophers 1992; Ellis 1991; Flytstrom 2008; Koo 1998; Heydendael 2003; Ho 1999; Mahrle 1995; Meffert 1997; Mrowietz 1995; Shupack 1997). In 2008, Saurat et al conducted the only randomised trial comparing the efficacy of methotrexate with placebo (Saurat CHAMPION, 2008). Randomised trials against placebo have demonstrated the efficacy of derivatives of vitamin A, the retinoids, in the treatment of plaque psoriasis (Pettit 1979). Fumaric acid esters are an alternative therapy for people with psoriasis, even though the mechanisms of action are not completely understood (Ormerod 2004). A Cochrane Review on FAEs for psoriasis was published in 2015 (Atwan 2015).
Small molecules
Small molecules affect molecules inside immune cells. Recently, small molecule drugs have been developed and show potential to treat psoriasis patients not responding to conventional treatments. These small molecule drugs include apremilast (Papp 2012b), tofacitinib (Bachelez 2015), and ponesimod (Vaclavkova 2014). Tofacitinib and ponesimod had not been approved for psoriasis at the time our analyses were done.
Biological therapies
Biological therapies use substances made from living organisms, or synthetic versions, to target the immune system. In the twentieth century, the development of biological treatments expanded the therapeutic spectrum of systemic treatments for psoriasis. All of the biologics have to be given by infusion or subcutaneous injection, and all have had at least one evaluation of their effectiveness against placebo: alefacept (Krueger 2002; Lebwohl 2003), etanercept (Leonardi 2003), infliximab (Chaudhari 2001), adalimumab (Menter REVEAL, 2008), certolizumab (Reich 2012), ustekinumab (Lebwohl 2010), secukinumab (Reich 2015), ixekizumab (Leonardi 2012), brodalumab (Papp 2012), guselkumab (Gordon X‐PLORE, 2015), tildrakizumab (Papp 2015a), and itolizumab (Krupashankar 2014). Certolizumab, tildrakizumab, and itolizumab had not been approved for psoriasis at the time our analyses were done.
How the intervention might work
Dysregulation of the immune system is a critical event in psoriasis, and the evolving knowledge of the role of the immune system in the disease has had a significant impact on treatment development.
Indeed, psoriatic plaque shows marked infiltration by activated T cells, especially CD4+ cells in the dermis. The activated T cells produce several important cytokines, namely, interferon (IFN)‐c, TNF alpha (by Th1 and Tc1 cells), IL‐17A, and IL‐23R (by Th17 and Tc17 cells) (Boehncke 2015).
Oral systemic treatments
Conventional systemic agents
Ciclosporin
Ciclosporin is an immunosuppressive agent (a drug that reduces the efficacy of the immune system); it acts by inhibiting the initial phase of the activation of CD4+ T cells, which leads to a block on the synthesis of interleukin 2 by the complex cyclophilin‐ciclosporin, thus, preventing T cell proliferation that is key to the pathogenesis of psoriasis (see above) (Ho 1996). This immunosuppression is rapid and reversible. Ciclosporin rapidly reduces the severity of the lesions (over one to three months), but the continuation of treatment is difficult after two years because of the development of adverse effects, such as elevated creatinine levels (Maza 2011). A dose of 5.0 mg/kg/day ciclosporin was significantly more effective than 2.5 mg/kg/day ciclosporin for induction of the remission of psoriasis; however, elevated creatinine was significantly more likely with 5.0 mg/kg/day ciclosporin than with 2.5 mg/kg/day ciclosporin (Christophers 1992).
Methotrexate
Methotrexate is an antimetabolite (an inhibitor of a chemical that is part of normal metabolism), which acts as an antagonist of folic acid (Montaudie 2011). Low doses of methotrexate exert anti‐inflammatory and immunomodulatory activities (Montaudie 2011). The efficacy of methotrexate cannot be assessed earlier than three months; its long‐term safety profile is good. In clinical practice, methotrexate is administered orally at 15 to 25 mg/week (Montaudie 2011).
Retinoids
Retinoids, including acitretin, are involved in the growth and differentiation of skin tissue; they bind to nuclear receptors that belong to the large family of steroid hormone receptors (Sbidian 2011). Retinoids modulate many types of proteins, including epidermal structural proteins, metalloproteinases, and cytokines (Sbidian 2011). The efficacy of retinoids is evaluated after two to three months of treatment, but skin side effects (e.g. xerosis, cheilitis) may limit the ability to increase the dose. Treatment with retinoids is best avoided in women of childbearing age because of risks to a developing foetus and the necessity of using contraception two years after discontinuation of treatment (Sbidian 2011). People receiving 50 mg/day to 75 mg/day acitretin have significantly improved psoriasis compared with those receiving 10 mg/day to 25 mg/day acitretin (Goldfarb 1988).
FAEs
FAEs are chemical compounds derived from the unsaturated dicarboxylic acid (Atwan 2015). Oral preparations of FAEs in psoriasis were developed containing dimethyl fumarate (DMF) and salts of monoethyl fumarate (MEF) as main compounds (Atwan 2015). FAEs produce anti‐inflammatory effects by preventing the proliferation of T cells (Atwan 2015).
FAEs are an effective therapy in people with psoriasis (50% to 70% achieve PASI 75 improvement within four months of treatment). Tolerance is limited by gastrointestinal side effects and flushing of the skin (Atwan 2015). Several case‐series described rare adverse events, such as progressive multifocal leukoencephalopathy (Balak 2016). In clinical practice, FAEs are administered orally. People receive this after a gradual dose incrementation the equivalent of 720 mg of DMF per day.
Small molecules
Small molecule drugs modulate proinflammatory cytokines and selectively inhibit signalling pathways: phosphodiesterase 4 inhibitors (apremilast), Janus kinase (JAK) inhibitors (tofacitinib), or sphingosine 1‐phosphate receptor agonists (ponesimod) (Torres 2015).
Apremilast
Apremilast belongs to the phosphodiesterase 4 (PDE4) inhibitors family (Torres 2015). By increasing cyclic adenosine monophosphate (cAMP) levels, PDE4 inhibitors reduce production of pro‐inflammatory TNF alpha and IFNγ in patients with psoriasis. Apremilast has recently been approved for psoriasis; its efficacy seems to be higher than conventional systemic therapy; however, no randomised controlled trials (RCTs) have assessed apremilast versus methotrexate or ciclosporin. The safety of the drug should be detailed in the near future with phase 4 studies. In clinical practice, apremilast is administered orally at 30 mg twice a day (Torres 2015).
Tofacitinib
Tofacitinib is a Janus kinase (JAK) inhibitor (Torres 2015). JAK inhibitors targets the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, which is pivotal for the downstream signaling of inflammatory cytokines involved in psoriasis. Tofacitinib had not been approved for psoriasis at the time our analyses were done (Torres 2015).
Ponesimod
Ponesimod is a sphingosine 1‐phosphate receptor agonist that causes dose‐dependent sequestration of lymphocytes in lymphoid organs, thus, preventing T cell proliferation, which is key to the pathogenesis of psoriasis. Ponesimod had not been approved for psoriasis at the time our analyses were done (Torres 2015).
Biological therapies
Biological therapies have been developed in recent years and target and prevent T cell proliferation (e.g. alefacept and itolizumab, a humanised IgG1 (immunoglobulin G1) monoclonal antibody, which selectively targets CD6) or target cytokines involved in psoriasis physiopathology (e.g. anti‐TNF alpha, anti‐IL12/23, anti‐IL23, anti‐IL17).
Alefacept
Alefacept is an immunosuppressive agent (a fusion protein that blocks the growth of some types of T cells). Alefacept (either 7.5 mg intravenously (IV) or 15 mg intramuscularly (IM) once a week) is used to control inflammation in moderate to severe psoriasis with plaque formation, where it interferes with lymphocyte activation. This drug was never approved for the European drug market. It was sold in North America, Switzerland, Israel, and Australia. In 2011, the manufacturers made a decision to cease sales of alefacept. This decision was not related to any specific safety concern nor the result of any FDA‐mandated or voluntary product recall (Heffernan 2010).
Anti‐TNF alpha
Two monoclonal antibodies against tumour necrosis factor alpha (TNF‐α) (infliximab, adalimumab) and one recombinant TNF‐α receptor (etanercept) have been developed to inhibit TNF‐α signalling, thus, preventing its inflammatory effects and are approved in psoriasis (Gisondi 2004). A third, certolizumab, is being assessed for psoriasis in phase 3 trials.
Etanercept is a recombinant TNF‐α receptor and weakly immunogenic (provokes only a mild immune response). Its efficacy is assessed at three months. A 50 mg dose of etanercept is administered subcutaneously twice weekly for three months during the induction phase (remission of the psoriasis flare) with 50 mg administered weekly as maintenance therapy (Gisondi 2004).
Infliximab is a chimeric antibody that neutralises the action of TNF‐α. Its efficacy is evaluated after six to eight weeks of treatment. A dose of 5.0 mg/kg infliximab is given as an intravenous (IV) induction regimen at 0, 2, and 6 weeks followed by a maintenance regimen of 5.0 mg/kg every 8 weeks. The presence of a murine sequence at recognition sites can lead to the development of anti‐infliximab antibodies that may impair the therapeutic effect (Gisondi 2004).
Adalimumab is a fully humanised antibody with very low immunogenicity. Its efficacy is estimated after eight and 12 weeks of treatment. One dose of 80 mg is administered subcutaneously, followed one week later by a 40 mg subcutaneous dose, which is administered every two weeks (Mossner 2009). Those receiving TNF‐α blockers are potentially exposed to a greater risk of infection and require regular monitoring (Tubach 2009).
Certolizumab is an anti‐TNF alpha with a unique structure that does not contain an Fc (fragment crystallisable) portion as adalimumab or infliximab does based on the human immunoglobulin G1 Fc. Therefore, certolizumab does not display Fc‐mediated effects (improving solubility, increasing drug stability, and decreasing immunogenicity). Certolizumab had not been approved for psoriasis at the time our analyses were done (Campanati 2017).
Anti‐IL12/23, Anti‐IL23, Anti‐IL17
Additional monoclonal antibodies have been developed against pro‐inflammatory cytokines: IL‐12, IL‐23, and IL‐17 inhibit the inflammatory pathway at a different point to the anti‐TNF alpha antibodies (Dong 2017).
Interleukin‐12 and IL‐23 share a common domain, p40, which is the target of ustekinumab (which the FDA has recently approved) (Savage 2015). A 45 mg subcutaneous dose is administered initially (90 mg if body weight is over 100 kg), then 45 mg (or 90 mg) subcutaneously four weeks later, and thereafter 45 mg (or 90 mg) subcutaneously every 12 weeks (Savage 2015). Interleukin‐23 plays an essential role in skin inflammation in psoriasis leading to the development of agents that selectively target the IL‐23p19 subunit (Dong 2017). Drugs targeting the p19 subunit of IL‐23 are guselkumab (a fully human IgG1k monoclonal IL‐23 antagonist), tildrakizumab (a humanised IgG1k monoclonal antibody), and risankizumab (high affinity humanised IgG1 monoclonal antibody) (Dong 2017). In July 2017, the FDA approved guselkumab for psoriasis. Guselkumab is given as a 100 mg subcutaneous injection every 8 weeks, following two starter doses at week 0 and week 4. Risankizumab was assessed after we began the systematic review and will be added in the next update.
Interleukin‐17 inhibitors include secukinumab (a recombinant fully human anti‐IL17A IgG1k monoclonal antibody), ixekizumab (a humanised anti‐IL17 immunoglobulin G4 monoclonal antibody), and brodalumab (a human IgG2 monoclonal antibody that decreases the downstream effect of IL‐17 by antagonisng the IL‐17RA receptor) (Dong 2017). The recommended dosage for secukinumab is 300 mg administered subcutaneously at weeks 0, 1, 2, 3, and 4, and then every 4 weeks thereafter. Ixekizumab is administered at 160 mg (2 x 80 mg injections) at weeks 0, 2, 4, 6, 8, 10, and 12, and then every 4 weeks thereafter (Dong 2017).
Why it is important to do this review
To determine the treatment pathway in psoriasis, the efficacy and safety of each systemic treatment must be determined relative to other therapies. Several randomised controlled trials (RCTs) have compared against placebo the efficacy of the different systemic treatments for psoriasis. However, there are few trials comparing conventional systemic therapies head‐to‐head, systemic therapies against biological therapies, or biological therapies head‐to‐head. Several previous meta‐analyses or indirect comparison meta‐analyses have been published (Bansback 2009; Brimhall 2008; Gomez‐Garcia 2017; Gospodarevskaya 2009; Lin 2012; Loveman 2009; Nast 2015; Nelson 2008; Reich 2008; Reich 2012a; Schmitt 2008; Signorovitch 2010; Signorovitch 2015; Spuls 1997; Strober 2006; Tan 2011; Turner 2009; Woolacott 2006). However, the number of studies included in these publications was low, the searches were not exhaustive, and several trials have been published since their search dates. Also, the publications did not evaluate some systemic and biological treatments.
A network meta‐analysis enables the best use of the direct and indirect information available to determine the relative efficacy of treatments. In other words, a network meta‐analysis will help to highlight the missing key comparisons that are needed to inform clinical practice.
The plans for this review were published as a protocol 'Systemic pharmacological treatments for chronic plaque psoriasis' (Sbidian 2015).
Objectives
To compare the efficacy and safety of conventional systemic agents (acitretin, ciclosporin, fumaric acid esters, methotrexate), small molecules (apremilast, tofacitinib, ponesimod), anti‐TNF alpha (etanercept, infliximab, adalimumab, certolizumab), anti‐IL12/23 (ustekinumab), anti‐IL17 (secukinumab, ixekizumab, brodalumab), anti‐IL23 (guselkumab, tildrakizumab), and other biologics (alefacept, itolizumab) for patients with moderate to severe psoriasis and to provide a ranking of these treatments according to their efficacy and safety.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Phase I trials were not eligible because participants, outcomes, dosages, and schema of administration of interventions are too different from phase II, III, and IV studies. Cross‐over trials were not eligible (because of the unpredictable evolution of psoriasis and risk of carry‐over bias). Non‐randomised studies, including follow‐up studies, were not eligible.
Types of participants
We considered trials that included adults (over 18 years of age) with moderate to severe plaque psoriasis (i.e. needed systemic treatment) or psoriatic arthritis whose skin had been clinically diagnosed with moderate to severe psoriasis and who were at any stage of treatment.
Types of interventions
We considered trials that assessed systemic and biological treatments, irrespective of the dose and duration of treatment, compared with placebo or with each other.
Systemic and biological treatments included the following:
-
Systemic conventional treatments
FAEs
Acitretin
Ciclosporin
Methotrexate
-
Small molecules
Apremilast
Tofacitinib
Ponesimod
-
Anti‐TNF alpha
Infliximab
Etanercept
Adalimumab
Certolizumab
-
Anti‐IL12/23
Ustekinumab
-
Anti‐IL17
Secukinumab
Brodalumab
Ixekizumab
-
Anti‐IL23
Tildrakizumab
Guselkumab
-
Other biologic treatments
Itolizumab
Alefacept
We were interested to compare both the different drugs (n = 19) and the different classes of drugs (n = 7).
A new anti‐IL23 molecule (BI 655066, risankizumab) appeared after we began this review and was not included in this systematic review. However, the ongoing studies of risankizumab have been reported in this review.
Active comparators included the following:
any of the aforementioned systemic and biological treatments; or
additional treatment not of primary interest but used for the network synthesis, such as topical treatment or phototherapy.
In multi‐arm trials, study groups assessing drugs other than those mentioned above were not eligible. In cases of multi‐dose trials, we grouped together all of the different dose groups as a single arm and performed sensitivity analysis at dose level.
In our Background section, we have referred to ongoing Cochrane Reviews that address some of the systemic treatments administered to adults with plaque psoriasis. We considered these treatments in our review, and we have liaised with each of these teams to harmonise our protocols. However, the Cochrane Review on FAEs, published in 2015, included people with all types of psoriasis and not only plaque‐type psoriasis (Atwan 2015).
Types of outcome measures
Psoriasis is a chronic disease; treatments are symptomatic often with a return to baseline after discontinuation. In the absence of an existing defined core outcome set (Spuls 2016), we chose the most relevant outcomes for patients (COMET). The Psoriasis Area and Severity Index score (PASI) 75 is the most common outcome measure used. However, confronted with a debilitating and a socially and psychologically highly visible disease, a completely "clear or almost clear" skin is a more stringent test in the induction phase (remission of the psoriasis flare).
Primary outcomes
The proportion of participants who achieved clear or almost clear skin, that is, at least PASI 90.
The proportion of participants with serious adverse effects (SAE). We used the definition of severe adverse effects from the International Conference of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, which includes death, life‐threatening events, initial or prolonged hospitalisation, and adverse events requiring intervention to prevent permanent impairment or damage.
Secondary outcomes
Proportion of participants who achieve PASI 75 at induction phase.
Proportion of participants who achieve a Physician Global Assessment (PGA) value of 0 or 1.
Quality of life measured by a specific scale. Available validated scales are the Dermatology Life Quality Index (DLQI), Skindex, Psoriasis Disability Index (PDI), or Psoriasis Symptom Inventory (PSI).
The proportions of participants with adverse effects (AE).
Proportion of participants with at least one relapse in the maintenance phase (between 52 to 104 weeks).
Timings
Where possible, we evaluated the outcomes at two different timings:
induction therapy (short‐term remission) (evaluation less than 24 weeks after the randomisation); and
maintenance therapy (long‐term remission) (evaluation between 52 and 104 weeks after the randomisation).
We did not include studies that had timings outside of these time ranges in our review. All of the outcomes except the proportion of participants with at least one relapse in the maintenance phase were recorded during the randomisation phase.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 15 December 2016:
the Cochrane Skin Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11) in the Cochrane Library using the strategy in Appendix 2;
MEDLINE Ovid (from 1946) using the strategy in Appendix 3;
Embase Ovid (from 1974) using the strategy in Appendix 4; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 5.
Trials registers
We searched the following trials registers up to 22 December 2016 with the following search terms: psoriasis AND one by one each drug name listed in Types of interventions:
World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/);
ISRCTN registry (www.isrctn.com);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov);
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au); and
EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
Previous meta‐analyses and systematic reviews
We looked at the search strategies of previous meta‐analyses to improve our search strategies.
References from other studies
We checked the bibliographies of included and excluded studies for further references to relevant trials.
Unpublished literature
We searched the trial results databases of various pharmaceutical companies to identify ongoing and unpublished trials. We made attempts to locate unpublished and ongoing trials through correspondence with authors and pharmaceutical companies (see Table 9).
Table 2.
Investigators contacted
| Contact | Requested Information | Contacted | Reply (last check 1/03/2017) | |
| Missing data | ||||
| Akcali 2014 | Prof. Akcali | Outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 8 and 21 November 2016 | No response |
| Al‐Hamamy 2014 | Prof. Al‐Hamamy | Outcomes: PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 8 and 21 November 2016 | No response |
| Asahina 2010 | Prof. Asahina | Outcome: PASI 90 | 8 November 2016 | Asahina 2010 detailed report |
| Asahina 2016 | Prof. Asahina Pfizer | Outcomes: AEs & SAEs | 3 and 12 January 2017 | Additional data to the publication not provided |
| Asawanonda 2006 | Prof. Asawanonda | Outcomes: PASI 75, PGA 0/1, AEs & SAEs | 21 November 2016 15 December 2016 |
Asawanonda 2006 sent detailed report for PASI 75 and AEs. PGA was not collected during this study. |
| Bissonnette 2015 | Prof. Bisonnette Innovaderm Recherches Inc. | Outcomes: PASI 90, PGA 0/1, AEs | 8 and 21 November 2016 | Additional data to the publication not provided |
| Blauvelt FEATURE, 2015 | Dr Blauvelt Novartis |
Outcome: QoL scale | 8 and 21 November 2016 | Additional data to the publication not provided |
| Caproni 2009 | Prof. Fabri | Outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 8 and 21 November 2016 | Caproni 2009 sent detailed report for PASI 90 and SAEs. Other outcomes (PGA, QoL and AEs) not collected during this study. |
| Dogra 2013 | Prof. Dogra | Outcomes: PGA 0/1, QoL scale, AEs & SAEs | 8 and 21 November 2016 | No response |
| Dogra 2012 | Prof. Dogra | Outcomes: PGA 0/1, QoL scale, AEs & SAEs | 8 November 2016 | PGA & QoL scale not collected during this study. AEs & SAEs not provided per arm |
| Fallah Arani 2011 | Dr Fallah Arani | Outcomes: PASI 90, PGA 0/1 and QoL scale | 8 and 21 November 2016 | Outcomes not collected during this study |
| Flytström 2008 | Prof. Flytstrom | Outcomes: PGA 0/1 | 12 and 19 January 2017 | Additional data to the publication not provided |
| Gisondi 2008 | Prof. Gisondi | Outcomes: PASI 90, PGA 0/1, QoL scale, AEs & SAEs | 8 November 2016 | Gisondi 2008 sent detailed report for the requested outcomes except for QoL (not assessed during the study) |
| Gordon 2006 | Prof. Gordon | Outcomes: PGA0/1, AEs | 3 and 12 January 2017 | No response |
| Gottlieb 2012 | Prof. Gottlieb Abbvie |
Outcomes: PASI 90 & QoL scale | 8 November 2016 | Gottlieb 2012 sent detailed report for the requested outcomes |
| Gottlieb 2011 | Prof. Gottlieb Amgen |
Outcomes: PASI 90, PGA 0/1, QoL scale, AEs & SAEs | 8 November 2016 | Gottlieb 2011 sent detailed report for the requested outcomes |
| Griffiths ACCEPT, 2010 | Prof. Griffiths Janssen |
Outcome: QoL scale | 16 December 2016 | QoL was not collected during this study |
| Jacobe 2008 | Prof. Jacobe | Outcomes: PASI 90, PGA 0/1, QoL scale, AEs & SAEs | 8 and 20 November 2016 | No response |
| Krueger 2016 | Pfizer | Outcomes: PASI 90, QoL scale | 3 and 12 January 2017 | No response |
| Krupashankar 2014 | Prof. Ganapathi R&D, Biocon Research Limited |
Outcomes: QoL scale, AEs & SAEs | 8 and 21 November 2016 | Krupashandar sent detailed report for the requested outcomes, however AEs and SAEs were only available for the entire trial and not at the time of the major outcome assessment |
| Lebwohl AMAGINE‐2, 2015 | Prof. Lebwohl Valeant Pharmaceuticals NA LLC |
Outcomes: PASI 90 & QoL scale | 8 and 21 November 2016 | Lebwohl AMAGINE‐2, 2015 sent detailed report for PASI 90, individual scores and median difference from baseline of QoL were not available |
| Lebwohl AMAGINE‐3, 2015 | Prof. Lebwohl Valeant Pharmaceuticals NA LLC |
Outcomes: PASI 90 & QoL scale | 8 and 21 November 2016 | Lebwohl AMAGINE‐3, 2015 sent detailed report for PASI 90, individual scores and median difference from baseline of QoL were not available |
| Leonardi 2012 | Prof. Leonardi | Outcomes: QoL scale & AEs | 8 and 21 November 2016 | No response |
| Mahajan 2010 | Prof. Kaur | Outcomes: PASI 90, PGA 0/1, QoL scale, AEs & SAEs | 8 and 21 November 2016 | No response |
| Menter REVEAL, 2008 | Prof. Menter | Outcome: PGA 0/1 | 8 and 21 November 2016 | No response |
| Menter EXPRESS‐II, 2007 | Prof. Menter | Outcome: PGA 0/1 | 8 and 21 November 2016 | No response |
| Mrowietz BRIDGE, 2016 | Prof. Mrowietz | Outcome: QoL scale | 3 and 12 January 2017 | Additional data to the publication not provided |
| Ortonne 2013 | Prof. Paul Novartis |
Outcome: PASI 90 | 3 January 2017 | Additional data to the publication not provided |
| Papp 2013a | Prof. Papp | Outcome: QoL scale | 22 November 2016 13 December 2016 | Additional data to the publication not provided |
| Papp AMAGINE‐1, 2016 | Prof. Papp | Outcome: QoL scale | 22 November 2016 13 December 2016 | Additional data to the publication not provided |
| Papp 2005 | Prof. Papp | Outcome: QoL scale, AEs & SAEs | 22 November 2016 13 December 2016 | Additional data to the publication not provided |
| Papp 2012a | Prof. Papp | Outcome: QoL scale | 22 November 2016 13 December 2016 | Additional data to the publication not provided |
| Papp 2013b | Prof. Papp | Outcome: PASI 90, PGA0/1, QoL scale | 3 January 2017 | Additional data to the publication not provided |
| Paul JUNCTURE, 2015 | Prof. Paul Novartis |
Outcome: QoL scale | 15 December 2016, 2 January 2017 | Additional data to the publication not provided |
| Reich 2015 | Prof. Reich Novartis |
Outcomes: PGA 0/1 & QoL scale | 8 November 2016, 16 December 2016 | Additional data to the publication not provided |
| Reich LIBERATE, 2017 | Prof. Reich PelotonAdvantage | Outcome: QoL scale | 4 January 2017 | Additional data to the publication not provided |
| Rich 2013 | Prof. Rich | Outcome: QoL scale | 22 November 2016, 13 December 2016 | No response |
| Sterry PRESTA, 2010 | Prof. Sterry | Outcomes: PASI 90 & QoL scale | 8 and 21 November 2016 | No response |
| Strober 2011 | Prof. Strober Abbvie |
Outcome: QoL scale | 8 November 2016 | Strober sent detailed report for the requested outcomes |
| Thaci CLEAR, 2015 | Prof. Thaçi Novartis |
Outcome: QoL scale | 8 and 21 November 2016 | Additional data to the publication not provided |
| Torii 2010 | Prof. Torii | Outcomes: PASI 90 & PGA0/1 | 21 November 2016 | Torii sent detailed report for the requested outcomes |
| Tyring 2006 | Prof. Tyring | Outcomes: PGA 0/1 & QoL scale | 8 and 21 November 2016 | No response |
| Van Bezooijen 2016 | Dr van Bezooijen | Outcomes: PASI 90, adverse effects | 4 and 12 January 2017 | Additional data to the publication not provided |
| Van de Kerkhof 2008 | Prof. van der Kherkhof Pfizer | Outcome: AEs | 8 and 21 November 2016 | Additional data to the publication not provided |
| Yan 2011 | No contact | Outcomes: AEs and SAEs | No | Authors' email not found |
| Zhu LOTUS, 2013 | No contact | Outcome: PASI 90 | No | Authors' email not found |
| Awaiting classification studies | ||||
| Elewski 2016 | Prof. Elewski Abbvie | Study's protocol and outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 3 and 12 January 2017 | Will be included when published |
| Khatri 2016 | Prof. Khattri | Study's protocol and outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 3 and 12 January 2017 | No response |
| Lee 2016 | Prof. Lee | Study's protocol and outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 3 and 12 January 2017 | No response |
| Reich 2016 | Prof. Reich | Study's protocol and outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 3 January 2017 | Will be included when published |
| Chow 2015 | Prof. Chow | outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 8 November 2016, 16 December 2016 | No response |
| Gurel 2015 | Prof. Gurel | Study's protocol and outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 17 and 24 January 2017 | Gurel 2015 sent detailed report for the requested outcomes. Finally Gurel study was classified in the included studies section. |
| Han 2007 | No contact | Outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | No | Authors' email not found |
| NCT01988103 | Dr Nogarales, MD Celgene Corporation | Asking for study protocol and efficacy/safety results | 12 and 19 January 2017 | Email response: "Thank you very much for your email and your interest in our study in Japanese subjects. May I please enquire as to the planned timing for publication for your meta‐analysis as we have just recently submitted our primary manuscript?" Will be included when published |
| NCT02248792 | Prof. Krishna | Asking for study protocol and efficacy/safety results | 5 and 12 January 2017 | No response |
| DRKS00000716 | Prof. Jacobi | Asking for study protocol and efficacy/safety results | 12 and 19 January 2017 | No response |
| CTRI/2015/05/005830 | Prof. Shah | Asking for study protocol and efficacy/safety results | 12 and 19 January 2017 | |
| Abstracts | ||||
| Yilmaz 2002 | Prof. Yilmaz | Outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 16 December 2016 | Yilmaz 2002 sent detailed report for the requested outcomes. Finally Yilmaz 2002 study was classified in the included studies section. |
| Mrowietz 2005 | Prof. Mrowietz | Study's protocol and outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 16 December 2016, 3 January 2017 | Additional data to the publication not provided. Finally Mrowietz study was classified in the awaiting classification section. |
| Reich 2004 | Prof. Reich | Study's protocol and outcomes: PASI 90, PASI 75, PGA 0/1, QoL scale, AEs & SAEs | 16 December 2016 | Additional data to the publication not provided. Finally Reich 2004 study was classified in the awaiting classification section. |
| Ongoing studies | ||||
| NCT01558310 | Dr Yamauchi Dr Patnaik, Director, Clinical Science Institute | Asking for study protocol and efficacy/safety results | 5 January 2017 | Email response: Dear Dr Sbidian, Thank you for your kind email, forwarded to me by Dr Paul Yamauchi, MD,PhD. Our " Study to Evaluate the Effectiveness of STELARA ™ (USTEKINUMAB) in the Treatment of Scalp Psoriasis (NCT 01558310)” completed enrolment in December 2016 and the last subject will complete in December 2017, as such we do not have the final data analysis. What is you absolute cut‐ off for publication data ? Would an interim analysis report be acceptable ? Best regards, Rickie Patnaik Director, Clinical Science Institute Will be included when published |
| EUCTR2013‐004918‐18‐NL | Prof. Spuls | Asking for study protocol and efficacy/safety results | 5 January 2017 | Email response "The study is currently ongoing and has not yet been analysed. Therefore, we are not able to provide data on efficacy or safety. We can provide you with the study protocol. Will this be helpful? Kind regards, Phyllis Spuls and Celine Busard " Will be included when published |
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: quality of life; SAE: serious adverse events
We searched reviews submitted to the U.S. Food and Drug Administration and the European Medicines Agency (EMA) for drug registration (using www.accessdata.fda.gov/scripts/cder/drugsatfda and www.ema.europa.eu/ema).
Conference proceedings
We handsearched the proceedings of the following conferences during the periods not included in the Cochrane Skin Specialised Register:
The American Academy of Dermatology (AAD) from 2008 to 2009 and from 2012 to 2013;
The Society for Investigative Dermatology (SID) from 2008 to 2009 and from 2012 to 2013; and
The European Academy of Dermatology and Venereology (EADV) from 2008 to 2013.
Adverse effects
We did not perform a separate search for rare or delayed adverse effects of the target interventions. However, we examined data on adverse effects from the included studies we identified.
Data collection and analysis
Selection of studies
Two groups of two review authors (LLC/ES or IGD/GD) independently examined each title and abstract to exclude irrelevant reports. These authors independently examined full‐text articles to determine eligibility. We contacted study authors for clarification when necessary and discussed disagreements to reach consensus. We list excluded studies and document the primary reason for exclusion.
Data extraction and management
Three groups of two review authors (LLC, GD, CH, IGD, CM, or ES) each extracted the data from published and unpublished reports independently using a standardised form. We pilot‐tested this form (Data Extraction Form) on a set of included trials. We extracted the data to populate the 'Characteristics of included studies' tables in RevMan Manager 5.3 (Revman 2014).
We extracted the data from the reports of the U.S. Food and Drug Administration (FDA) when available, if not from the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), and finally from the published reports.
Outcome data
We extracted (arm‐level data) from each included trial; hence, the total number of participants randomised to each intervention. For binary outcomes, we also extracted the number of participants (if available) who:
reached PASI 90, PASI 75, or PGA 0/1 during the induction phase;
had at least one relapse in the maintenance phase; and
had at least one SAE/one AE during the induction phase.
For quality of life, we extracted from each included trial the mean change score of the study specific scale from baseline to follow‐up.
When PASI 90 and PASI 75 outcomes were not reported and when the information was available, we extracted the PASI score at baseline and at the evaluation point (or the percentage reduction in PASI from baseline to follow up) to calculate the number of participants who reached PASI 75 and 90.
Regarding the assessment of quality of life, we recorded all specific quality of life (QoL) scales (Dermatology Life Quality Index (DLQI), Skindex, Psoriasis Disability Index (PDI), and Psoriasis Symptom Inventory (PSI)).
Data on potential effect modifiers
We extracted baseline demographic and clinical characteristics of participants that may have acted as effect modifiers (age, sex, body weight, duration of psoriasis, severity of psoriasis at baseline, previous psoriasis treatment). One review author (ES) checked and entered the data into the RevMan computer software. We contacted the authors of the trials to request missing data (see Table 9).
Assessment of risk of bias in included studies
We used Cochrane's 'Risk of bias' (RoB) tool to assess the risk of bias. Three groups of two review authors each (LLC, GD, CH, IGD, CM, or ES) independently assessed the risk of bias, and one author (LLC) resolved any disagreements. For each of the following domains and according to the general principles in section 8.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we graded the following 'Risk of bias' domains as 'low', 'high', or 'unclear'.
-
Selection bias
Was the allocation sequence adequately generated? We considered randomisation adequate (low risk of bias) if the allocation sequence was generated from a table of random numbers or was computer‐generated. We considered randomisation inadequate (high risk of bias) if sequences could be related to prognosis. We considered randomisation unclear if the paper stated that the trial was randomised, but did not describe the method.
Was allocation adequately concealed? We deemed allocation concealment as adequate if the report stated that it was undertaken by means of sequentially pre‐numbered sealed opaque envelopes or by a centralised system. We considered a double‐blind double‐dummy process as at low risk of bias even if the paper did not describe the method of allocation concealment.
-
Performance and detection bias
Was knowledge of the allocated intervention adequately prevented during the study? We evaluated the risk of bias separately for personnel and participants, outcomes assessors, and each outcome.
-
Attrition bias
Were incomplete outcome data adequately addressed? We examined if there was imbalance across intervention groups in numbers or reasons for missing data, type of measure undertaken to handle missing data, and whether the analysis was carried out on an intention‐to‐treat (ITT) basis. We assessed the use of strategies to handle missing data.
-
Reporting bias
Were reports of the study free of suggestion of selective outcome reporting? We evaluated if each outcome was measured, analysed, and reported. We compared outcomes specified in protocols (if available on the FDA website or ClinicalTrials.gov) and in material and methods with outcomes presented in the results section. We considered reporting bias inadequate if one specified outcome in protocols was lacking in the main report.
-
Other risk of bias
We did not fulfil the 'other risk of bias' item as we did not highlight particular circumstances leading to other risk of bias from particular trial designs, contamination between the experimental and control groups, and particular clinical settings.
Overall risk of bias
To summarise the quality of evidence and to interpret the network results, we used these six RoB criteria (random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessor, incomplete outcome data, and selective outcome reporting) in order to classify each trial.
We would classify the trial as having low risk of bias if we rated none of the domains above as high risk of bias and two or less as unclear risk.
We would classify the trial as having moderate risk of bias if we rated one domain as high risk of bias, one or less domains as unclear risk, or no domains as high risk of bias but three or less were rated as unclear risk.
All other cases were assumed to pertain to high risk of bias.
Measures of treatment effect
Relative treatment effects
For each pair‐wise comparison and each dichotomous outcome at each time point, we used risk ratios (RR) with 95% confidence intervals (CI) as a measure of treatment effect. For continuous variables (e.g. quality of life scale), we used the standardised mean difference (SMD) with 95% CI.
Relative treatment ranking ‐ network meta‐analysis
For every treatment, we estimated the ranking probabilities of being at each possible rank for all outcomes. We inferred on treatment hierarchy using the surface under the cumulative ranking curve (SUCRA) (Salanti 2011). SUCRA was expressed as a percentage between 0 (when it is certain a treatment is the worst) to 100% (when it is certain a treatment is the best).
Unit of analysis issues
The primary unit of analysis was the participant. We did not consider studies with non‐standard design features that would lead to clustering (e.g. cross‐over trials).
We treated comparisons from trials with multiple intervention groups as independent two‐arm studies in the pair‐wise meta‐analyses. At the network meta‐analysis stage, we properly accounted for the within‐trial correlation.
Dealing with missing data
We extracted, when possible, both the number of randomised and analysed participants in each study arm. We contacted trial authors or sponsors by email to request missing outcome data (numbers of events and numbers of participants for important dichotomous clinical outcomes) when these were not available in study reports that were less than 10 years old (See Table 9). For the main analysis, we assumed that any participant with missing outcome data did not experience clearance, whatever the group. In a sensitivity analysis, we also synthesised the data ignoring the missing participants (complete case analysis) assuming that they were missing at random (Mavridis 2014).
Assessment of heterogeneity
We undertook meta‐analyses only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar (section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011). Potential sources of heterogeneity included participants' baseline characteristics (weight, the duration of previous treatment, treatment doses, co‐interventions, and duration of treatment). When enough data were available, we investigated the distributions of these characteristics across studies and treatment comparisons. The latter allows assessing transitivity, i.e. whether there were important differences between the trials evaluating different comparisons other than the treatments being compared (Salanti 2014). To further reassure the plausibility of the transitivity assumption, we only included in our analyses trials not involving co‐interventions and with a timing of outcome assessment from 12 to 16 weeks.
In the classical meta‐analyses, we assessed statistical heterogeneity by visual inspection of the forest plots and using the Q‐test and the I² statistic. We interpreted the I² statistic according to the following thresholds (section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011): 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity.
In the network meta‐analysis, the assessment of statistical heterogeneity in the entire network was based on the estimated heterogeneity standard deviation parameter (τ) estimated from the network meta‐analysis models (Jackson 2014). We inferred on the presence or absence of important heterogeneity by comparing the magnitude of τ with the empirical distributions provided in Turner et al and Rhodes et al (Rhodes 2015; Turner 2012). We also estimated the prediction intervals to assess how much the estimated heterogeneity affects the relative effects with respect to the additional uncertainly anticipated in future studies (Riley 2011). Where feasible, we would have investigated the possible sources of heterogeneity in subgroup analyses and meta‐regression.
Although we restricted the risk of important heterogeneity in our data by considering eligible only studies with a follow‐up period between 12 and 16 weeks and without co‐interventions, we investigated differences in heterogeneity across the different analyses. Specifically, we observed whether splitting the nodes of the network and analysing each drug separately reduced the heterogeneity estimate. We also ran a series of sensitivity analyses (see Sensitivity analysis), and we monitored whether heterogeneity became smaller or larger compared to the primary analysis.
Assessment of reporting biases
To assess reporting biases, we used an adaptation of the funnel plot by subtracting from each study‐specific effect size the mean of meta‐analysis of the study‐specific comparison, which we plotted against the study standard error (Chaimani 2013). We employed this 'comparison‐adjusted funnel plot' for all comparisons of an active treatment against placebo. When we detected funnel plot asymmetry for the two primary outcomes, we investigated the presence of small‐study effects in the network meta‐regression (Chaimani 2012).
Data synthesis
We conducted pair‐wise meta‐analyses to synthesise trials comparing one of the treatments against placebo or two treatments against each other. We performed pair‐wise meta‐analyses for all outcomes and comparisons, provided that at least two studies were available, using a random‐effects model.
We then employed network meta‐analysis to estimate the relative effects for all possible comparisons between any pair of treatments. We provided a graphical depiction of the evidence network for all outcomes to illustrate the network geometry (Chaimani 2017). We ran network meta‐analysis using the approach of multivariate meta‐analysis, which treats the different comparisons that appear in studies as different outcomes (White 2012).
We interpreted a statistically non‐significant P value (e.g. larger than 0.05) as a finding of uncertainty unless confidence intervals were sufficiently narrow to rule out an important magnitude of effect.
We assessed inconsistency (i.e. the possible disagreement between the different pieces of evidence) locally and globally. Specifically, we used the loop‐specific approach (Bucher 1997) and the side‐splitting method (Dias 2010). We also fit the design by treatment interaction model to evaluate the presence of inconsistency in the entire network (Higgins 2012).
We conducted pair‐wise meta‐analyses using Review Manager 5 (RevMan 5) (Revman 2014), and we performed all other analyses in Stata 14 using the 'network' (www.stata‐journal.com/article.html?article=st0410) and 'network graphs' packages (www.stata‐journal.com/article.html?article=st0411).
Subgroup analysis and investigation of heterogeneity
We considered running subgroup analyses and meta‐regressions to investigate potential sources of heterogeneity or inconsistency (such as weight of participants, duration of psoriasis, baseline severity, previous systemic treatments), but no sufficient data on these characteristics were available to perform these additional analyses.
Sensitivity analysis
To assess the robustness of our results, we performed the following sensitivity analyses for the two primary outcomes: (1) running the analysis at dose‐level considering that each different drug dose is a different intervention; (2) excluding trials at high risk of bias; (3) excluding trials with a total sample size smaller than 50 randomised participants; and (4) analysing only the observed participants assuming that missing participants are missing at random.
'Summary of findings' table
We included a 'Summary of findings' table in our review. We downgraded evidence based on the five Grading of Recommendations, Assessment, Development and Evaluation (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) (Schunemann 2011). We assessed the confidence of the evidence estimates from network meta‐analysis, based on an extension of the standard GRADE approach which is based on the contributions of the direct comparisons to the estimation in the network meta‐analysis (Salanti 2014).
We included an overall grading of the evidence for the two main outcomes: • PASI 90 during the induction phase • Serious adverse effects during the induction phase
We assessed the study limitations by first evaluating the risk of bias of each direct estimate and then integrating these judgements with the contribution of each direct estimate to the network estimates.
We assessed inconsistency by considering the networks' heterogeneity (network meta‐analysis estimate of between‐study variance and prediction intervals) and using both local and global inconsistency in the networks.
We assessed imprecision by focusing on the CIs of the network meta‐analysis treatment effect estimates and by examining ranking probabilities (rankograms).
We assessed indirectness by evaluating the distribution of the potential effect modifiers (baseline demographic and clinical characteristics of participants).
We assessed publication bias by considering the comprehensive search strategy that we performed and the risk of publication bias in the specific field. The comparison‐adjusted funnel plots that test the presence of small‐study effects in the network assisted our judgement.
For each outcome, we chose the median placebo‐group risk value in the included studies for the assumed risk with placebo. According to the software GRADEpro 2008 (www.gradepro.org), we assigned four levels of certainty of evidence: high, moderate, low, or very low. We used this assessment, which two authors (LLC and ES) conducted, to inform the main text of the discussion section.
Results
Description of studies
Results of the search
The Electronic searches retrieved 4798 records after deduplication. The searches of other sources identified 622 records from trials registers and three further records from other sources. We had a total of 5422 records after removal of duplicates.
After reviewing the titles and abstracts, we discarded 4738 citations. We examined the full text of the remaining 684 citations: 410 did not meet the inclusion criteria. Within this group, 203 did not include participants with moderate to severe psoriasis and so did not meet our inclusion criteria. We have not created 'Characteristics of excluded studies' tables for this group. We had a further 207 excluded studies (see Characteristics of excluded studies). We identified 14 trials as studies awaiting classification (reported in 18 references) (see Characteristics of studies awaiting classification). We identified 34 studies as ongoing (see Characteristics of ongoing studies).
We included 109 studies, reported in 222 references. For a further description of our screening process, see the study flow diagram (Figure 1).
Figure 1.
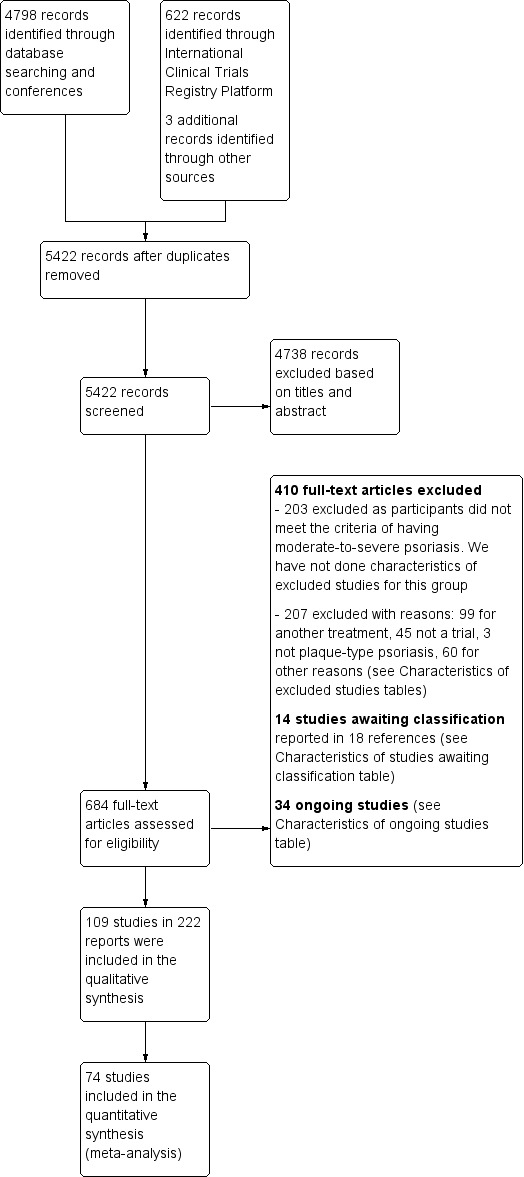
Study flow diagram
Included studies
Trial design
All trials used a parallel‐group design. The mean sample size was 366 (range: 10 to 1881). In all, 88 trials were multicentric trials (2 to 231 centres) and 15 were single‐centre trials (Akcali 2014; Al‐Hamamy 2014; Asawanonda 2006; Chaudhari 2001; Chladek 2005; Dogra 2013; Dogra 2012; Dubertret 1989; Ellis 1991; Gisondi 2008; Gurel 2015; Hunter 1963; Mahajan 2010; Shehzad 2004; Van Bezooijen 2016); for six trials, single‐centre or multicentric status was not clear (Caproni 2009; Engst 1994; Goldfarb 1988; Jacobe 2008; Olsen 1989; Yilmaz 2002). All of the trials recruited participants from a hospital setting. The trials took place worldwide (n = 37, 33.9%), in Europe (n = 28, 25.7%), in North America (n = 21, 19.3%), in Asia (n = 17, 15.6%), or in the Middle East (n = 1, 0.9%). The location was not stated for five trials (Caproni 2009; Engst 1994; Goldfarb 1988; Jacobe 2008; Olsen 1989).
In total, 55 trials out of 109 were multiarm; 40 multiarm trials assessed the same experimental drug at multiple dose levels; seven assessed at least two different drugs; eight assessed both the same experimental drug at multiple dose levels and different drugs.
In total, 15 trials (Al‐Hamamy 2014; Asawanonda 2006; Bissonnette 2013; Gottlieb 2012; Gurel 2015; Jacobe 2008; Lowe 1991; Mahajan 2010; Ruzicka 1990; Saurat 1988; Shehzad 2004; Sommerburg 1993; Tanew 1991; Van Bezooijen 2016; Yilmaz 2002) had a co‐intervention mainly with phototherapy. Only 14 studies were carried out before the year 2000 (Dubertret 1989; Ellis 1991; Engst 1994; Goldfarb 1988; Hunter 1963; Laburte 1994; Lowe 1991; Meffert 1997; Nugteren‐Huying 1990; Olsen 1989; Ruzicka 1990; Saurat 1988; Sommerburg 1993; Tanew 1991).
Characteristics of the participants
This review included 109 trials, with a total of 39,882 randomised participants. We summarise the characteristics of the participants in the Characteristics of included studies. The participants were reported to be between 27 and 56.5 years old, with an overall mean age of 44; there were more men (26,902) than women (12,384). Age and gender were unreported for, respectively, 743 and 596 participants (eight and nine studies). The overall mean weight was 85.6 (range: 64 to 97), and the overall mean Psoriasis Area and Severity Index (PASI) score at baseline was 20 (range: 9.5 to 39).
Characteristics of the comparisons
Trials with two parallel arms (the different dose groups were grouped together in one "arm")
Intervention versus placebo: 73 trials compared systemic treatments with placebo
Twenty‐one trials compared systemic conventional treatments versus placebo
Acitretin (n = 9) (Goldfarb 1988; Gurel 2015; Lowe 1991; Olsen 1989; Ruzicka 1990; Saurat 1988; Sommerburg 1993; Tanew 1991; Yilmaz 2002)
Fumaric acid esters (FAEs) (n = 3) (Nugteren‐Huying 1990; Mrowietz BRIDGE, 2016; Van Bezooijen 2016)
Ciclosporin (n = 2) (Ellis 1991; Meffert 1997)
Methotrexate (n = 7) (Al‐Hamamy 2014; Asawanonda 2006; Hunter 1963; Gottlieb 2012; Mahajan 2010; Shehzad 2004; Warren METOP, 2017)
Nine trials compared small molecule treatments versus placebo
Apremilast (n = 4) (Papp 2012b; Papp 2013b; Papp ESTEEM‐1, 2015; Paul ESTEEM‐2, 2015)
Tofacitinib (n = 4) (Krueger 2016; Papp 2012a; Papp OPT Pivotal‐1, 2015; Papp OPT Pivotal‐2, 2015)
Ponesimod (n = 1) (Vaclavkova 2014)
Forty‐three trials compared biological treatments versus placebo
Anti‐TNF alpha
Etanercept (n = 8) (Bagel 2012; Gottlieb 2003; Gottlieb 2011; Leonardi 2003; Papp 2005; Strober 2011; Tyring 2006; Van de Kerkhof 2008)
Adalimumab (n = 5) (Asahina 2010; Bissonnette 2013; Gordon 2006; Menter REVEAL, 2008; Cai 2016)
Infliximab (n = 6) (Chaudhari 2001; Gottlieb 2004; Reich EXPRESS, 2005; Torii 2010; Yang 2012; Menter EXPRESS‐II, 2007)
Certolizumab (n = 1) (Reich 2012)
-
Anti‐IL12/23
Ustekinumab (n = 6) (Igarashi 2012; Krueger 2007; Leonardi PHOENIX‐1, 2008; Papp PHOENIX‐2, 2008; Tsai PEARL, 2011; Zhu LOTUS, 2013)
-
-
Anti‐IL17
Secukinumab (n = 6) (Blauvelt FEATURE, 2015; Langley ERASURE, 2014; Papp 2013a; Paul JUNCTURE, 2015; Reich 2015; Rich 2013)
Ixekizumab (n = 2) (Gordon UNCOVER‐1, 2016; Leonardi 2012)
Brodalumab (n = 3) (Papp AMAGINE‐1, 2016; Papp 2012; Nakagawa 2016)
-
-
Anti‐IL23
Guselkumab (n = 0)
Tildrakizumab (n = 1) (Papp 2015a)
-
-
Other biologics
itolizumab (n = 1) (Krupashankar 2014)
Alefacept (n = 4) (Ellis 2001; Jacobe 2008; Krueger 2002; Lebwohl 2003)
-
Intervention versus active comparators: 25 trials compared systemic treatments with systemic treatments
Acitretin versus acitretin (n = 1) (Dogra 2013)
Acitretin versus ciclosporin (n = 1) (Akcali 2014)
Ciclosporin versus methotrexate (n = 4) (Flytström 2008; Heydendael 2003; Piskin 2003, Sandhu 2003)
Ciclosporin versus ciclosporin (n = 3) (Dubertret 1989; Engst 1994; Laburte 1994)
Methotrexate versus methotrexate (n = 2) (Chladek 2005; Dogra 2012)
Methotrexate versus FAEs (n = 1) (Fallah Arani 2011)
Methotrexate versus alefacept (n = 1) (Yan 2011)
Methotrexate versus infliximab (n = 1) (Barker RESTORE‐1, 2011)
Acitretine versus etanercept (n = 2) (Caproni 2009; Gisondi 2008)
Etanercept versus etanercept (n = 3) (Ortonne 2013; Sterry PRESTA, 2010; Strohal PRISTINE, 2013)
Etanercept versus infliximab (n = 1) (de Vries PIECE, 2016)
Etanercept versus ustekinumab (n = 1) (Griffiths ACCEPT, 2010)
Tofacitinib versus tofacitinib (n = 2) (Asahina 2016; Bissonnette 2015)
Secukinumab versus secukinumab (n = 1) (Mrowietz SCULPTURE, 2015)
Secukinumab versus ustekinumab (n = 1) (Thaci CLEAR, 2015)
Trials with three parallel arms (the different dose groups were grouped together in one "arm")
A total of 11 trials compared systemic treatments with systemic treatments and placebo.
Methotrexate versus adalimumab versus placebo (n = 1) (Saurat CHAMPION, 2008)
Etanercept versus ixekizumab versus placebo (n = 2) (Griffiths UNCOVER‐2, 2015; Griffiths UNCOVER‐3, 2015)
Etanercept versus secukinumab versus placebo (n = 1) (Langley FIXTURE, 2014)
Etanercept versus apremilast versus placebo (n = 1) (Reich LIBERATE, 2017)
Guselkumab versus adalimumab versus placebo (n = 3) (Blauvelt VOYAGE‐1, 2016; Gordon X‐PLORE, 2015; Reich VOYAGE‐2, 2017)
Brodalumab versus ustekinumab versus placebo (n = 2) (Lebwohl AMAGINE‐2, 2015; Lebwohl AMAGINE‐3, 2015)
Tofacitinib versus etanercept versus placebo (n = 1) (Bachelez 2015)
In total, the dataset consisted of 109 studies, which provide information on 204, 159, and 152 comparisons between 35 different drug doses, 20 different drugs, and 8 different drug classes, respectively (both including placebo). For the sensitivity analyses, the different drug doses were divided into the following:
methotrexate, taken orally, ≥ 15 or < 15 mg per week;
ciclosporin, taken orally, ≥ 3 or < 3 mg/Kg per day;
acitretin, taken orally, ≥ 35 or < 35 mg per day;
apremilast, taken orally, 30 mg twice a day or other dosages per day;
ponesimod, taken orally, 40 mg per day or other dosages per day;
tofacitinib, taken orally, 20 mg per day or other dosages per day;
etanercept, subcutaneous (S/C), 25 mg twice a week or etanercept 50 mg twice a week;
infliximab, intravenous, 5 mg/kg at week 0, 2, and 4 then every 6 weeks or other dosages;
adalimumab, S/C, 80 mg at week 0, 40 mg at week 1 then 40 mg every other week or other dosages;
secukinumab, S/C, 300 mg at week 0, 1, 2, 3, and 4 then every 4 weeks or other dosages;
ixekizumab, S/C, 80 mg every two weeks or other dosages;
brodalumab, S/C, 210 mg every two weeks or other dosages;
guselkumab, S/C, 100 mg at week 0 and 4 then every 16 weeks or other dosages.
Alefacept (S/C or intravenous (IV)), FAEs (taken orally), certolizumab (S/C), itolizumab (IV), ustekinumab (S/C 45 mg or 90 mg according to the weight) and tildrakizumab (S/C) were grouped in one dosage whatever the dosages.
For each study, we provide details of the dosage in Characteristics of included studies.
Characteristics of the outcomes
Regarding the efficacy outcomes during induction therapy (eight to 24 weeks), out of 109 trials, 82 reported PASI 90, 76 reported on Physician Global Assessment (PGA) 0/1, 93 reported PASI 75, and 54 trials reported assessment of change in quality of life. Fifty‐two studies used the dermatology‐specific instrument Dermatology Life Quality Index (DLQI); two studies used other specific skin instruments (Skindex). For all of these studies, the investigators provided citations to reports indicating that the tools had been previously validated.
Out of 109 trials, 73 reported the number of participants with adverse effects (different from the number of adverse effects), and 85 reported the number of serious adverse effects.
These outcomes were evaluated between eight and 24 weeks: eight weeks (five studies), 10 weeks (seven studies), 12 weeks (56 studies), 13 weeks (two studies), 14 weeks (two studies), 15 weeks (one study), 16 weeks (22 studies), and 24 weeks (10 studies). Timing of assessment was unknown or not clearly defined for four studies (Engst 1994; Hunter 1963; Saurat 1988; Shehzad 2004).
No trial assessed the outcome 'Proportion of participants with at least one relapse in the maintenance phase (between 52 to 104 weeks)'.
Funding
In all, 82 studies declared a source of funding, 79 studies declared a pharmaceutical company funding, four studies declared a unique institutional funding (Chladek 2005; de Vries PIECE, 2016; Flytström 2008; Heydendael 2003), five studies had no funding source (Akcali 2014; Asawanonda 2006; Fallah Arani 2011; Gurel 2015; Yan 2011), and 21 studies did not report the source of funding (Al‐Hamamy 2014; Caproni 2009; Dogra 2012; Dogra 2013; Dubertret 1989; Engst 1994; Gisondi 2008; Hunter 1963; Laburte 1994; Mahajan 2010; Meffert 1997; Nugteren‐Huying 1990; Piskin 2003; Ruzicka 1990; Sandhu 2003; Saurat 1988; Shehzad 2004; Sommerburg 1993; Torii 2010; Yang 2012; Yilmaz 2002).
Excluded studies
We excluded 410 full‐text reports. The main reason for exclusion was that the participants did not present with moderate to severe psoriasis (n = 203): these psoriasis participants were included in trials assessing the efficacy of our treatments of interest for psoriatic arthritis or had cutaneous lesions of psoriasis but not moderate to severe psoriasis. We detail the reason for exclusion of the 207 full‐text reports in Characteristics of excluded studies: we excluded 99 because they assessed another intervention, 45 were not a trial, three did not include plaque‐type psoriasis, and we excluded 60 for other reasons.
For six studies with three arms, one arm was not included as the intervention was not included in our search:
Saurat 1988: acitretin versus placebo versus etretinate (etretinate arm was not included);
Shehzad 2004: PUVA (psoralen and ultraviolet A) therapy versus methotrexate (methotrexate only was included);
Gottlieb 2011; Strober 2011: briakinumab versus etanercept versus placebo (briakinumab arm was not included);
Gisondi 2008: etanercept versus acitretin versus etanercept plus acitretin (etanercept plus acitretin arm was not included);
Al‐Hamamy 2014: narrowband ultraviolet B phototherapy plus methotrexate versus narrowband ultraviolet B alone and methotrexate alone (arm with methotrexate alone was not included).
Thaçi 2002 compared two different dosages of ciclosporin (a fixed dosage of 200 mg/day and a dosage corresponding to 2.5 mg/kg/day), and we were unable to classify the fixed dosage group either in the ciclosporin ≥ 3 mg/kg/day group nor in the ciclosporin < 3 mg/day group for the subgroup meta‐analysis.
Studies awaiting classification
We classified 14 trials reported in 18 references as studies awaiting classification. More details regarding the studies awaiting classification are available in Studies awaiting classification and Table 9.
Ongoing studies
We classified 34 trials as ongoing studies. More details are available in Characteristics of ongoing studies and Table 9. Most of the ongoing studies compare a biological treatments versus another biological treatment or versus placebo (n = 13 and n = 14, respectively). Three ongoing studies assess apremilast versus placebo, and four assess conventional systemic treatments versus conventional systemic treatments (n = 2) or placebo (n = 2).
Risk of bias in included studies
Figure 2 and Figure 3 summarise 'Risk of bias' assessments. Regarding the overall risk of bias across studies, 23 trials were at low risk of bias (Asahina 2016; Bachelez 2015; Blauvelt FEATURE, 2015; Blauvelt VOYAGE‐1, 2016; Cai 2016; Gordon UNCOVER‐1, 2016; Griffiths UNCOVER‐2, 2015; Griffiths UNCOVER‐3, 2015; Langley ERASURE, 2014; Langley FIXTURE, 2014; Leonardi 2012; Papp PHOENIX‐2, 2008; Papp 2012; Papp 2012a; Papp 2012b;Reich 2015;Reich 2012; Reich VOYAGE‐2, 2017; Rich 2013; Saurat CHAMPION, 2008; Thaci CLEAR, 2015; Vaclavkova 2014;Warren METOP, 2017). We categorised almost half of the studies (48/109) as at high risk of bias. Among the high‐risk group, five studies had only one high risk of bias domain with all the other dimensions at low risk (Bissonnette 2015; Lebwohl 2003; Papp 2013a; Papp OPT Pivotal‐1, 2015; Reich LIBERATE, 2017). We categorised the remaining 38 studies as unclear risk of bias because we assessed one or more criteria as unclear. Among the unclear 'Risk of bias' group, 11 studies had only one unclear risk of bias with all the other dimensions at low risk (Bagel 2012; Krueger 2016; Leonardi 2003; Leonardi PHOENIX‐1, 2008; Menter EXPRESS‐II, 2007; Menter REVEAL, 2008; Papp AMAGINE‐1, 2016; Paul JUNCTURE, 2015; Paul ESTEEM‐2, 2015; Reich EXPRESS, 2005; Tyring 2006). Further details of these assessments are available in the 'Risk of bias' table corresponding to each trial in the Characteristics of included studies.
Figure 2.
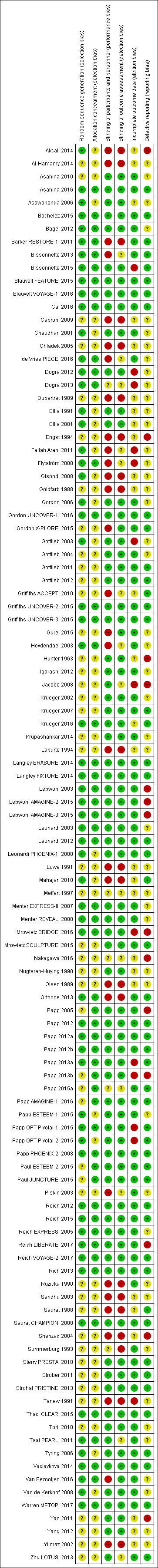
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Figure 3.
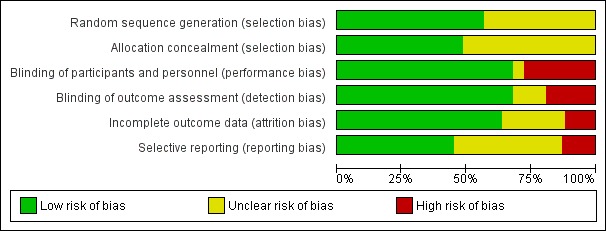
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
Allocation
The method of sequence generation was not described at all, or was at best unclear, in 48 trials. The remaining studies (n = 61) described the method used to generate the allocation sequence in sufficient detail; therefore, we judged this domain as low risk of bias for these studies. For allocation concealment, the majority of studies (n = 56) received a judgement of unclear risk of bias for this domain because of the absence of reporting the method used to guarantee concealment. We considered the risk low for the 53 remaining trials.
Blinding
Blinding of participants and personnel was achieved in 74 studies, whereas 30 studies were at high risk of performance bias. The remaining five studies were at unclear risk of performance bias. Blinding of outcome assessment was reported clearly in only 74 of the 109 included studies, whereas 21 studies were at high risk of detection bias. The risk of detection bias was unclear in the remaining 14 studies.
Incomplete outcome data
In more than half of the trials (69/109), incomplete outcome data appeared to have been adequately addressed, and any missing outcome data were reasonably well balanced across intervention groups, with similar reasons for missing data across the groups. However, in 13 studies the reporting of missing outcome data was largely inadequate because of one or more of the following reasons: the high number of withdrawn participants, an imbalance between groups in the number of withdrawn participants, an imbalance in reasons for missing outcomes, or no intention‐to‐treat (ITT) analysis provided. In 27 studies, this domain was as at unclear risk of bias due to one or more of the following reasons: the numbers of participants, reasons, or missing data methods were not reported.
Selective reporting
We considered 14 trials at high risk of selective outcome reporting because results for outcomes detailed in the methods section were not reported in the results section (Akcali 2014; Engst 1994; Hunter 1963; Jacobe 2008; Lebwohl 2003; Lebwohl AMAGINE‐2, 2015; Lebwohl AMAGINE‐3, 2015; Mrowietz BRIDGE, 2016; Nakagawa 2016;Papp 2013b; Papp 2005; Reich LIBERATE, 2017; Shehzad 2004; Yan 2011). In all, we considered 49 studies to be at low risk of bias for this domain as outcome details in the trial register and in the methods section were reported in the results section. For other trials (n = 46), we considered the risk of bias as unclear because we did not find these trials in any register.
Other potential sources of bias
As detailed in the Methods section, we did not fulfil the 'other risk of bias' item as we did not highlight particular circumstances leading to other risk of bias from particular trial designs, contamination between the experimental and control groups, and particular clinical settings.
Effects of interventions
See: Table 1
See: Table 1 The summary of findings for the main comparison provides overall estimates of treatment effects compared with placebo and the certainty of the available evidence for the two primary outcomes (PASI 90 and serious adverse effects during the induction phase), obtained through network meta‐analysis.
Seven trials provided no usable or retrievable data and did not contribute further to the results of this review (Akcali 2014; Chladek 2005; Engst 1994; Lowe 1991; Piskin 2003; Olsen 1989; Shehzad 2004; see Table 9). The main reason we could not use their data was that these studies addressed none of our outcomes. Fifteen studies, involving 1113 participants (2.8% of the participants in this review), had a co‐intervention and did not contribute further to the results of this review as we could not assess the specific intervention effect (Al‐Hamamy 2014; Asawanonda 2006; Bissonnette 2013; Gottlieb 2012; Gurel 2015; Jacobe 2008; Lowe 1991; Mahajan 2010; Ruzicka 1990; Saurat 1988; Shehzad 2004; Sommerburg 1993; Tanew 1991; Van Bezooijen 2016; Yilmaz 2002). Twenty‐six studies had an outcome assessment before 12 weeks (Akcali 2014; Chaudhari 2001; Goldfarb 1988; Gottlieb 2004; Hunter 1963; Menter EXPRESS‐II, 2007; Meffert 1997; Olsen 1989; Reich EXPRESS, 2005; Ruzicka 1990; Sommerburg 1993; Saurat 1988; Torii 2010; Yang 2012), or later than 16 weeks (Al‐Hamamy 2014; Asahina 2016; Asawanonda 2006; Bissonnette 2013; Bissonnette 2015; de Vries PIECE, 2016; Engst 1994; Gisondi 2008; Gottlieb 2012; Ortonne 2013; Strohal PRISTINE, 2013; Van Bezooijen 2016).
In total, 35 studies, involving 4433 participants, were not included in the classical or network meta‐analysis. The interventions of the 35 studies particularly concerned the following:
infliximab (n = 7) (Chaudhari 2001; de Vries PIECE, 2016; Gottlieb 2004; Menter EXPRESS‐II, 2007; Reich EXPRESS, 2005; Torii 2010; Yang 2012)
acitretin (n = 8) (Akcali 2014; Goldfarb 1988; Gisondi 2008; Gurel 2015; Lowe 1991; Ruzicka 1990; Saurat 1988; Sommerburg 1993)
methotrexate (n = 5) (Asawanonda 2006; Al‐Hamamy 2014; Gottlieb 2012; Mahajan 2010; Shehzad 2004)
ciclosporin (n = 1) (Meffert 1997)
We included a total of 74 studies, involving 35,454 participants (88.9% participants of this review), in the network meta‐analysis for at least one of the outcomes.
Figure 4 and Figure 5 show the network diagrams for all of the outcomes included in the review. The size of the nodes is proportional to the total number of participants allocated to each class level (Figure 4)/drug level (Figure 5) intervention, and the thickness of the lines is proportional to the number of trials evaluating each direct comparison.
Figure 4.
Network plot for all the outcomes at class‐level
The size of the nodes is proportional to the total number of participants allocated to each intervention and the thickness of the lines proportional to the number of studies evaluating each direct comparison.
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: quality of life; SAE: serious adverse events
Figure 5.
Network plot for all the outcomes at drug‐level
The size of the nodes is proportional to the total number of participants allocated to each intervention and the thickness of the lines proportional to the number of studies evaluating each direct comparison.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: quality of life; SAE: serious adverse events
Figure 6 shows the network meta‐analysis estimates of all of the outcomes for each comparisons at class level.
Figure 6.
Relative effects of the class‐level intervention as estimated from the network meta‐analysis model
Drugs are reported in order of primary benefit ranking. Each cell contains the risk ratio (RR) (for dichotomous outcomes: PASI 90, serious adverse events, PASI 75, PGA 0/1, adverse events) or the standardised mean difference (SMD) (for the quality‐of‐life outcome), plus the 95% confidence interval, of the class level in the respective column versus the class level in the respective row. RRs larger than 1 for the lower triangle and smaller than 1 (or SMDs smaller than zero) for the upper triangle favour the treatment on the left. Significant results are bolded and underscored.
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician's Global Assessment; QoL: quality of life; SAE: serious adverse events
Figure 7, Figure 8, and Figure 9 show the network meta‐analysis estimates of all the outcomes for each comparison at drug level.
Figure 7.
Relative effects of the intervention as estimated from the network meta‐analysis model for Psoriasis Area and Severity Index (PASI) 90 and serious adverse events (SAEs)
Drugs are reported in order of primary benefit ranking. Each cell contains the risk ratio (RR) and 95% confidence interval for the two primary outcomes (PASI 90 and SAEs) of the intervention in the respective column versus the class level in the respective row. RRs larger than 1 for the lower triangle and smaller than 1 for the upper triangle favour the treatment on the left. Significant results are highlighted in grey.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; τ (Tau): estimated heterogeneity standard deviation parameter; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
Figure 8.
Relative effects of the intervention as estimated from the network meta‐analysis model for Psoriasis Area and Severity Index (PASI 75) and adverse events (AEs)
Drugs are reported in order of primary benefit ranking. Each cell contains the Risk Ratio (RR) and 95% confidence interval for the two secondary outcomes (PASI 75 and adverse events) of the intervention in the respective column versus the comparator in the respective row. RRs larger than 1 for the lower triangle and smaller than 1 for the upper triangle favour the treatment on the left. Significant results are are highlighted in grey.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; τ (Tau): estimated heterogeneity standard deviation parameter; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
Figure 9.
Relative effects of the intervention as estimated from the network meta‐analysis model for Physician's Global Assessment (PGA 0/1) and quality of life (QoL)
Drugs are reported in order of primary benefit ranking. Each cell contains the risk ratio (RR) and 95% confidence interval (PGA 0/1) or standardized mean difference (quality of life) of the intervention in the respective column versus the comparator in the respective row. RRs larger than 1 for the lower triangle and smaller than 1 (or SMDs smaller than zero) for the upper triangle favour the treatment on the left. Significant results are are highlighted in grey.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; τ (Tau): estimated heterogeneity standard deviation parameter; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
Figure 10 and Figure 11 show all of the relative effects from the network meta‐analyses against placebo with their 95% confidence and prediction intervals at class and drug level.
Figure 10.
Interval plot. Network meta‐analysis estimates of class‐level versus placebo for all the outcomes
AE: adverse events; CI: confidence interval; PGA: Physician Global Assessment; PrI: predictive interval; QoL: Specific quality of life scale; RR: risk ratio; SAE: serious adverse events; SMD: standardised mean difference
Figure 11.
Interval plot. Network meta‐analysis estimates of the interventions versus placebo for all the outcomes
AE: adverse events; CI: confidence interval; PGA: Physician Global Assessment; PrI: predictive interval; QoL: Specific quality of life scale; RR: risk ratio; SAE: serious adverse events; SMD: standardised mean difference
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
Figure 12 shows a two‐dimensional ranking plot based on surface under the cumulative ranking curve (SUCRA) values for benefit (PASI 90) and acceptability (serious adverse events) at class and drug level. The different colours represent different groups of interventions considering their performance on both outcomes simultaneously. Interventions belonging to the same group were assumed to have a similar performance when the two primary outcomes were considered jointly (Chaimani 2013).
Figure 12.
Ranking plot. Ranking plot representing simultaneously the efficacy (x axis, PASI 90) and the acceptability (y axis, serious adverse events) of all the interventions (class and drug levels) for patients with moderate‐to‐severe psoriasis. Optimal treatment should be characterised by both high efficacy and acceptability and should be in the right upper corner of this graph.
The different colours represent different groups of interventions considering their performance on both outcomes simultaneously. Interventions belonging to the same group are assumed having a similar performance when the two primary outcomes are considered jointly
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
PASI: Psoriasis Area and Severity Index; SAE: serious adverse events; SUCRA: surface under the cumulative ranking curve
Figure 13 and Figure 14 show the ranking for all the outcomes at class and drug level, respectively.
Figure 13.
Ranking for all the outcomes at class level
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: quality of life; SAE: serious adverse events
Figure 14.
Ranking for all the outcomes at drug level
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
AE: adverse events; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: quality of life; SAE: serious adverse events
1. Primary outcomes
1.1 The proportion of participants who achieved clear or almost clear skin, e.g. PASI 90
DIRECT EVIDENCE
We report treatment estimates for pair‐wise meta‐analyses at class (see Figure 15) and drug level in Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; and Analysis 1.11, respectively.
Figure 15.
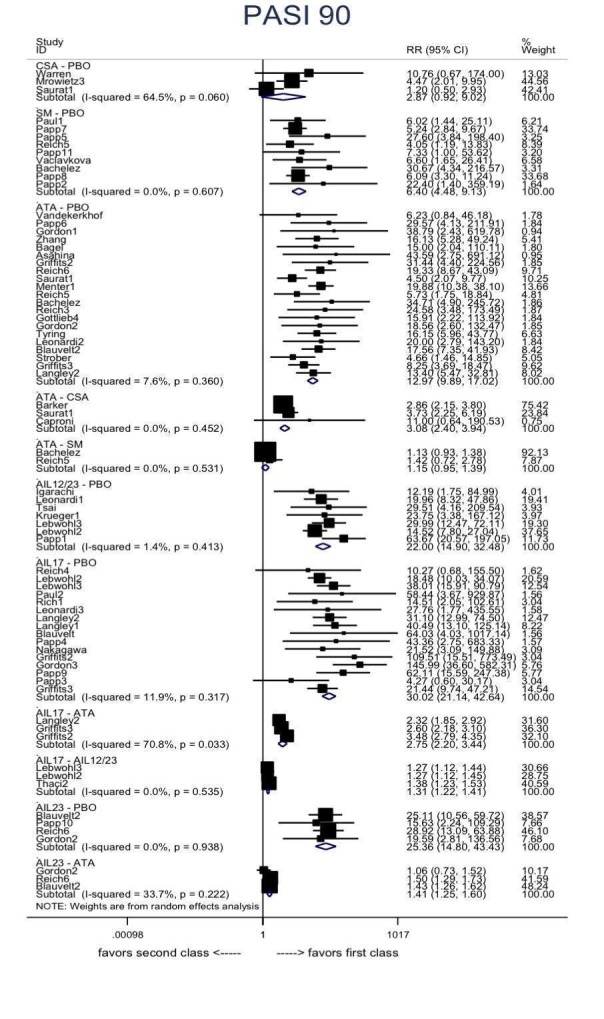
PASI 90: direct summary effects for comparisons including at least two studies at class level
AIL12/23: anti‐IL12/23; AIL17: anti‐IL17; AIL23: anti‐IL23, ATA: anti‐TNF alpha; CSA: conventional systemic agents; OB: other biologics; PBO: placebo; SM: small molecules
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio
Analysis 1.1.
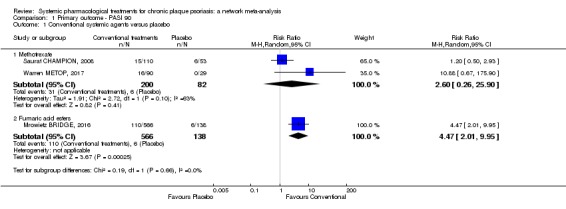
Comparison 1 Primary outcome ‐ PASI 90, Outcome 1 Conventional systemic agents versus placebo.
Analysis 1.2.
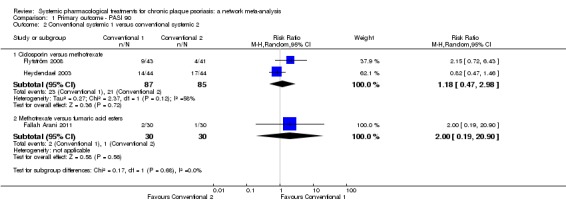
Comparison 1 Primary outcome ‐ PASI 90, Outcome 2 Conventional systemic 1 versus conventional systemic 2.
Analysis 1.3.
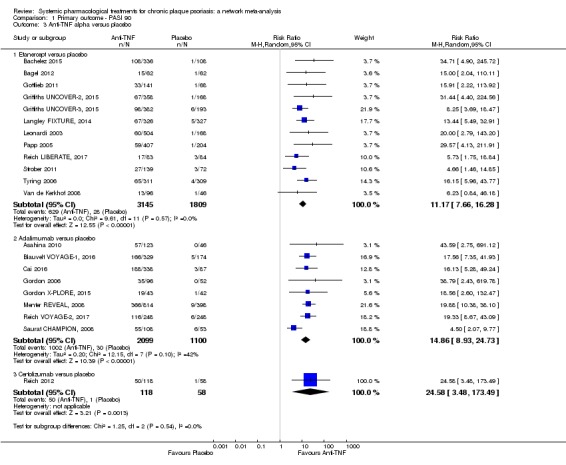
Comparison 1 Primary outcome ‐ PASI 90, Outcome 3 Anti‐TNF alpha versus placebo.
Analysis 1.4.
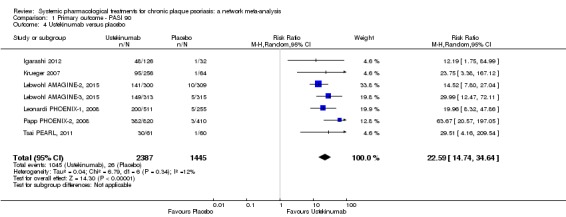
Comparison 1 Primary outcome ‐ PASI 90, Outcome 4 Ustekinumab versus placebo.
Analysis 1.5.
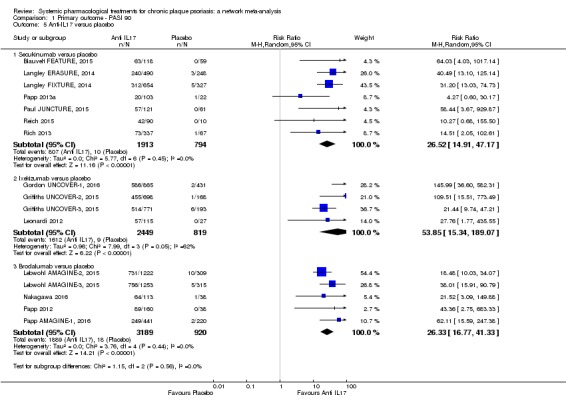
Comparison 1 Primary outcome ‐ PASI 90, Outcome 5 Anti‐IL17 versus placebo.
Analysis 1.6.
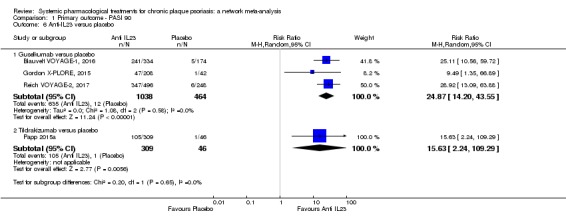
Comparison 1 Primary outcome ‐ PASI 90, Outcome 6 Anti‐IL23 versus placebo.
Analysis 1.7.
Comparison 1 Primary outcome ‐ PASI 90, Outcome 7 Other biologics.
Analysis 1.8.
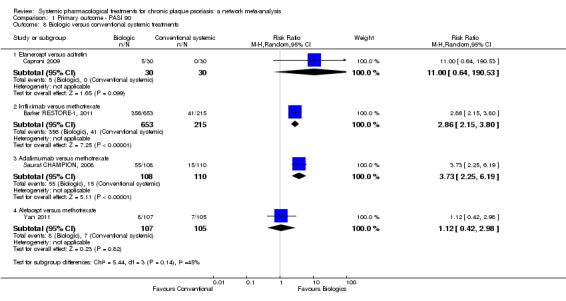
Comparison 1 Primary outcome ‐ PASI 90, Outcome 8 Biologic versus conventional systemic treatments.
Analysis 1.9.
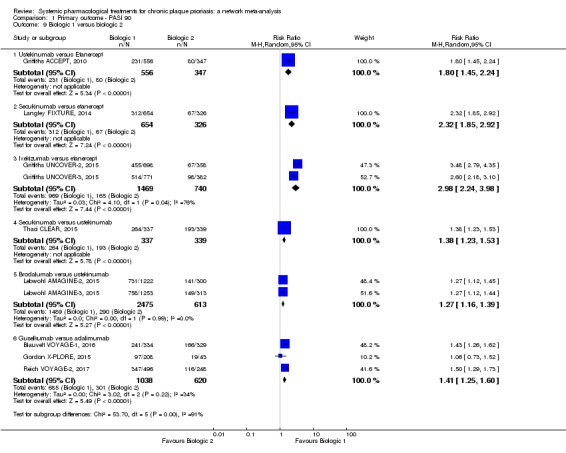
Comparison 1 Primary outcome ‐ PASI 90, Outcome 9 Biologic 1 versus biologic 2.
Analysis 1.10.
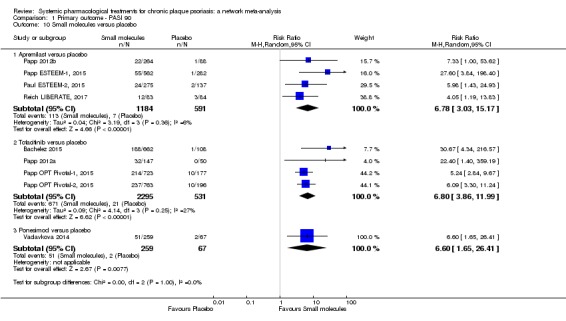
Comparison 1 Primary outcome ‐ PASI 90, Outcome 10 Small molecules versus placebo.
Analysis 1.11.
Comparison 1 Primary outcome ‐ PASI 90, Outcome 11 Biologic versus small molecules.
In terms of reaching PASI 90, anti‐IL17 treatments (secukinumab, ixekizumab, and brodalumab) were more effective than placebo (risk ratio at class level (RR) 30.02, 95% confidence interval (CI) 21.14 to 42.64). These findings were also confirmed for anti‐IL23 (guselkumab and tildrakizumab) (class‐level RR 25.36, 95% CI 14.80 to 43.43); ustekinumab (RR 22.00, 95% CI 14.90 to 32.48); anti‐TNF alpha (etanercept, adalimumab, and certolizumab) (class‐level RR 12.97, 95% CI 9.89 to 17.02); and small molecules (apremilast, tofacitinib, and ponesimod) (class‐level RR 6.40, 95% CI 4.48 to 9.13). Both infliximab and adalimumab were more effective than methotrexate (respectively: RR 2.86, 95% CI 2.15 to 3.80; and RR 3.73, 95% CI 2.25 to 6.19). Ustekinumab, secukinumab, and ixekizumab were more effective than etanercept; secukinumab and brodalumab were more effective than ustekinumab; and guselkumab was more effective than adalimumab. No significant difference was observed between etanercept and tofacitinib or apremilast in terms of this outcome (reaching PASI 90).
NETWORK META‐ANALYSES
The PASI 90 outcome was available in 58 trials, involving 31,176 participants (87.9% of the participants in the meta‐analysis). This outcome was reported in two other trials (Nugteren‐Huying 1990; Sandhu 2003); however, the number of randomised participants was not available. These trials were added in the complete case analyses. This outcome was also reported in three other trials (Dogra 2012; Dogra 2013; Mrowietz SCULPTURE, 2015), comparing different dosages of the same drug in each case. These trials were added to the sensitivity analysis at dose level. PASI 90 was not reported for the remaining nine trials, and we were not able to obtain missing information from the trial authors (Table 9). Thirty‐nine trials, involving 16,888 participants, were placebo‐controlled trials; seven studies, involving 2048 participants, were head‐to‐head comparisons; and 12 studies, involving 12,240 participants, had both a placebo and at least two active treatments arms.
See Figure 4; Figure 5; Figure 6; Figure 7; Figure 10; Figure 11; Figure 13; and Figure 14.
Table 10 summarises the main results of both the direct and indirect evidence and the network meta‐analysis for PASI 90 at 12 to 16 weeks. The summary relative effects from the network meta‐analysis are presented in league tables for both class‐level (Figure 6) and drug‐level (Figure 7) analyses.
Table 3.
Direct and indirect evidences and network meta‐analysis results summary table for PASI 90 at 12 to 16 weeks
| Network meta‐analysis | Direct evidence | Indirect evidence | |||||||
| Comparisons* | RR | LCI | UCI | RR | LCI | UCI | RR | LCI | UCI |
| FAEs vs placebo | 4.09 | 1.88 | 8.88 | 4.47 | 1.97 | 10.14 | 1.86 | 0.16 | 21.16 |
| Methotrexate vs placebo | 3.91 | 2.16 | 7.08 | 1.53 | 0.66 | 3.53 | 17.16 | 5.69 | 51.75 |
| Adalimumab vs placebo | 14.87 | 10.45 | 21.14 | 14.42 | 10.08 | 20.64 | 108.8 | 2.24 | 5287.86 |
| Etanercept vs placebo | 10.79 | 8.47 | 13.73 | 10.62 | 7.52 | 15.01 | 11.21 | 7.26 | 17.32 |
| Ustekinumab vs placebo | 19.91 | 15.11 | 26.23 | 22.7 | 15.46 | 33.34 | 17.91 | 12.71 | 25.24 |
| Secukinumab vs placebo | 26.55 | 20.32 | 34.69 | 24.53 | 14.93 | 40.32 | 28.25 | 19.1 | 41.78 |
| Ixekizumab vs placebo | 32.45 | 23.61 | 44.60 | 39.46 | 20.64 | 75.44 | 24.51 | 10.05 | 59.77 |
| Brodalumab vs placebo | 25.45 | 18.74 | 34.57 | 26.58 | 16.65 | 42.41 | 23.74 | 10.09 | 55.86 |
| Apremilast vs placebo | 7.66 | 4.30 | 13.66 | 6.72 | 3.07 | 14.69 | 10.83 | 2.43 | 48.31 |
| Tofacitinib vs placebo | 8.50 | 6.23 | 11.60 | 6.3 | 4.14 | 9.56 | 17.91 | 8.3 | 38.62 |
| Guselkumab vs placebo | 21.03 | 14.56 | 30.38 | 26.1 | 14.71 | 46.3 | 12.7 | 4.28 | 37.69 |
| Methotrexate vs FAEs | 0.96 | 0.38 | 2.44 | 2 | 0.19 | 21.03 | 0.83 | 0.3 | 2.32 |
| Alefacept vs methotrexate | 1.12 | 0.42 | 3.02 | 1.12 | 0.42 | 3.02 | |||
| Ciclosporin vs methotrexate | 1.02 | 0.60 | 1.73 | 1.02 | 0.6 | 1.73 | |||
| Infliximab vs methotrexate | 2.86 | 2.06 | 3.97 | 2.86 | 2.06 | 3.97 | |||
| Adalimumab vs methotrexate | 3.80 | 2.26 | 6.39 | 3.35 | 2.02 | 5.57 | 13.2 | 3.4 | 51.32 |
| Etanercept vs acitretin | 11.00 | 0.63 | 191.47 | 11 | 0.63 | 191.47 | |||
| Guselkumab vs adalimumab | 1.41 | 1.21 | 1.65 | 1.4 | 1.18 | 1.66 | 2.88 | 0.68 | 12.21 |
| Ustekinumab vs etanercept | 1.85 | 1.50 | 2.27 | 1.8 | 1.27 | 2.55 | 1.95 | 1.37 | 2.77 |
| Secukinumab vs etanercept | 2.46 | 2.01 | 3.02 | 2.33 | 1.66 | 3.28 | 2.62 | 1.82 | 3.77 |
| Ixekizumab vs etanercept | 3.01 | 2.46 | 3.68 | 2.93 | 2.44 | 3.53 | 5.73 | 2.07 | 15.85 |
| Apremilast vs etanercept | 0.71 | 0.40 | 1.25 | 0.72 | 0.36 | 1.45 | 0.69 | 0.26 | 1.81 |
| Tofacitinib vs etanercept | 0.79 | 0.59 | 1.06 | 0.88 | 0.73 | 1.08 | 0.49 | 0.3 | 0.81 |
| Secukinumab vs ustekinumab | 1.33 | 1.11 | 1.61 | 1.38 | 1.03 | 1.84 | 1.19 | 0.79 | 1.81 |
| Brodalumab vs ustekinumab | 1.28 | 1.10 | 1.48 | 1.27 | 1.1 | 1.46 | 1.64 | 0.69 | 3.89 |
FAES: fumaric acid esters; LCI: low confidence interval; RR: risk ratio; UCI: upper confidence interval; vs: versus,
*The comparisons listed in this table were included in at least one direct‐evidence analysis.
All of the interventions appeared superior to placebo in terms of reaching PASI 90. Anti‐IL17 treatment was associated with a higher chance of reaching PASI 90 compared to all of the interventions: versus anti‐IL12/23 (risk ratio (RR) 1.33, 95% confidence interval (CI) 1.19 to 1.49); versus anti‐IL23 (RR 1.86, 95% CI 1.54 to 2.26); versus anti‐TNF alpha (RR 2.66, 95% CI 2.34 to 3.03); versus small molecules (RR 3.52, 95% CI 2.65 to 4.66); versus other biologics (RR 6.44, 95% CI 2.44 to 17.04); versus conventional systemic agents (RR 8.15, 95% CI 6.07 to 10.93) (Figure 6). In terms of reaching PASI 90, all of the biologic interventions (anti‐IL17, anti‐IL12/23, anti‐IL23, anti‐TNF alpha) appeared significantly superior to the small molecule class of treatments and the conventional systemic class of treatments. Small molecules were associated with a higher chance of reaching PASI 90 compared to conventional systemic agents (RR 2.31, 95% CI 1.63 to 3.28).
Results of comparisons between each of the drugs are available in Figure 7. There was no significant difference between the three anti‐IL17 (brodalumab, ixekizumab, and secukinumab) and the two anti‐IL23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. All of the anti‐IL17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti‐IL23) were significantly more effective than three anti‐TNF alpha agents: infliximab, adalimumab, and etanercept. The direct comparison regarding certolizumab and tildrakizumab only included one trial each, so the interpretation of the results regarding certolizumab and tildrakizumab was difficult (related to wide CIs). Ustekinumab was superior to etanercept (RR 1.85, 95% CI 1.50 to 2.27). No significant difference was shown between the anti‐TNF alpha drugs. Tofacitinib was significantly superior to methotrexate (RR 2.17, 95% CI 1.13 to 4.20), and no significant difference was shown between apremilast and the conventional drugs (versus acitretin: RR 7.81, 95% CI 0.42 to 143.83; versus fumaric acid: RR 1.87, 95% CI 0.71 to 4.93; versus ciclosporin: RR 1.92, 95% CI 0.72 to 5.12; versus methotrexate: RR 1.96, 95% CI 0.85 to 4.50).
Ranking class‐level analysis (Figure 10; Figure 13; Table 11)
Table 4.
Ranking findings for all outcomes at class level
| Class‐level interventions | SUCRA PASI 90 | Rank PASI 90 | SUCRA SAE | Rank SAE | SUCRA PASI 75 | Rank PASI 75 | SUCRA AE | Rank AE | SUCRA PGA | Rank PGA | SUCRA QoL | Rank QoL |
| Anti‐IL12/23 | 85.7 | 2 | 53.9 | 3 | 85.0 | 2 | 57.0 | 3 | 83.8 | 2 | 75.7 | 3 |
| Anti‐IL17 | 100.0 | 1 | 21.0 | 8 | 99.6 | 1 | 14.1 | 6 | 99.9 | 1 | 95.4 | 1 |
| Anti‐IL23 | 71.3 | 3 | 39.6 | 5 | 72.2 | 3 | 78.7 | 2 | 73.1 | 3 | 83.4 | 2 |
| Anti‐TNF alpha | 56.4 | 4 | 39.2 | 6 | 57.4 | 4 | 47.5 | 5 | 57.5 | 4 | 58.4 | 4 |
| Other biologics | 26.3 | 6 | 68.2 | 2 | 17.0 | 7 | _ | _ | 16.6 | 7 | 15.5 | 7 |
| Small molecules | 41.5 | 5 | 45.4 | 4 | 42.7 | 5 | 7.9 | 7 | 42.0 | 5 | 40.4 | 5 |
| Conventional systemic treatments |
18.7 | 7 | 94.8 | 1 | 26.0 | 6 | 50.8 | 4 | 27.1 | 6 | 30.8 | 6 |
| Placebo | 0 | 8 | 38.0 | 7 | 0 | 8 | 94.0 | 1 | 0 | 8 | 0.4 | 8 |
AE: adverse events; FAEs: fumaric acid esters; PGA: Physician Global Assessment; QoL: Specific quality of life scale; SAE: serious adverse events
Ranking analysis for PASI 90 performed with SUCRA strongly suggested that anti‐IL17 was the best treatment at class level (versus placebo: RR 30.81, 95% confidence interval (CI) 25.10 to 37.82; SUCRA = 100; high‐certainty evidence), followed by anti‐IL12/23 (versus placebo: RR 23.16, 95% CI 18.70 to 28.68; SUCRA = 85.7; high‐certainty of evidence), anti‐IL23 (versus placebo: RR 16.53, 95% CI 13.16 to 20.75; SUCRA = 71.3; moderate‐certainty evidence), then anti‐TNF alpha (versus placebo: RR 11.58, 95% CI 9.55 to 14.03; SUCRA = 56.4; moderate‐certainty evidence). The heterogeneity τ for this network overall was 0.09, which we considered low heterogeneity.
Ranking drug‐level analysis (Figure 11; Figure 14; Table 12)
Table 5.
Ranking findings for all outcomes at drug level
| Drug | SUCRA PASI 90 | Rank PASI 90 | SUCRA SAE | Rank SAE | SUCRA PASI 75 | Rank PASI 75 | SUCRA AE | Rank AE | SUCRA PGA | Rank PGA | SUCRA QoL | Rank QoL |
| Acitretin | 9.9 | 19 | 46.9 | 9 | 26.0 | 15 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adalimumab | 63.1 | 8 | 40.4 | 14 | 60.2 | 9 | 70.1 | 5 | 56.9 | 8 | 57.6 | 7 |
| Alefacept | 25.3 | 15 | 62.6 | 5 | 12.6 | 18 | ‐ | ‐ | 13.1 | 18 | 15.9 | 13 |
| Apremilast | 39.7 | 13 | 54.7 | 7 | 33.2 | 14 | 14.3 | 16 | 27.9 | 14 | 28.6 | 10 |
| Brodalumab | 84.3 | 3 | 39.8 | 15 | 82.1 | 3 | 46.4 | 9 | 84.0 | 5 | 52.3 | 8 |
| Certolizumab | 75.7 | 5 | 70.9 | 3 | 71.6 | 6 | 78.0 | 4 | 90.1 | 1 | ‐ | ‐ |
| Ciclosporin | 21.3 | 17 | 78.2 | 2 | 33.2 | 13 | 36.8 | 12 | 24.0 | 16 | ‐ | ‐ |
| Etanercept | 52.6 | 11 | 43.6 | 11 | 57.7 | 10 | 45.9 | 10 | 51.7 | 10 | 67.6 | 5 |
| FAEs | 21.9 | 16 | 57.7 | 6 | 11.1 | 19 | 17.8 | 15 | 15.4 | 17 | ‐ | ‐ |
| Guselkumab | 77.0 | 4 | 42.6 | 12 | 71.6 | 7 | 78.2 | 3 | 67.5 | 7 | 84.3 | 2 |
| Infliximab | 53.2 | 10 | 64.4 | 4 | 48.0 | 11 | 40.1 | 11 | 52.4 | 9 | ‐ | ‐ |
| Itolizumab | 56.0 | 9 | ‐ | ‐ | 71.6 | 8 | ‐ | ‐ | 29.4 | 13 | 16.0 | 12 |
| Ixekizumab | 94.3 | 1 | 33.7 | 17 | 91.8 | 1 | 18.1 | 14 | 85.9 | 3 | 99.2 | 1 |
| Methotrexate | 20.2 | 18 | 90.7 | 1 | 21.3 | 16 | 68.4 | 6 | 24.9 | 15 | 31.5 | 9 |
| Placebo | 2.9 | 20 | 42.0 | 13 | 0.0 | 20 | 88.0 | 1 | 0.3 | 19 | 1.2 | 14 |
| Ponesimod | 37.3 | 14 | 18.1 | 19 | 21.3 | 17 | 14.0 | 17 | 48.7 | 11 | 28.1 | 11 |
| Secukinumab | 86.5 | 2 | 29.9 | 18 | 86.7 | 2 | 36.3 | 13 | 84.4 | 4 | ‐ | ‐ |
| Tildrakizumab | 63.6 | 7 | 37.8 | 16 | 78.3 | 4 | 86.1 | 2 | 86.3 | 2 | 74.9 | 4 |
| Tofacitinib | 42.5 | 12 | 44.0 | 10 | 46.2 | 12 | 47.3 | 8 | 36.6 | 12 | 65.1 | 6 |
| Ustekinumab | 72.6 | 6 | 52.0 | 8 | 75.2 | 5 | 64.3 | 7 | 70.4 | 6 | 77.4 | 3 |
AE: adverse events; FAEs: fumaric acid esters; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; QoL: specific quality of life scale; SAE: serious adverse events; SUCRA: Surface Under the Cumulative Ranking
Ranking analysis for PASI 90 performed with SUCRA strongly suggested that ixekizumab was the best treatment at drug level (versus placebo: RR 32.45, 95% CI 23.61 to 44.60; SUCRA = 94.3; high‐certainty evidence), followed by secukinumab (versus placebo: RR 26.55, 95% CI 20.32 to 34.69; SUCRA = 86.5; high certainty of evidence), brodalumab (versus placebo: RR 25.45, 95% CI 18.74 to 34.57; SUCRA = 84.3; moderate‐certainty evidence), guselkumab (versus placebo: RR 21.03, 95% CI 14.56 to 30.38; SUCRA = 77; moderate‐certainty evidence), certolizumab (versus placebo: RR 24.58, 95% CI 3.46 to 174.73; SUCRA = 75.7; moderate‐certainty evidence), then ustekinumab (versus placebo: RR 19.91, 95% CI 15.11 to 26.23; SUCRA = 72.6; high‐certainty evidence). The heterogeneity τ for this network overall was 0.09, which we considered low heterogeneity.
1.2 The proportion of participants with serious adverse effects
DIRECT EVIDENCE
We report treatment estimates for pair‐wise meta‐analyses at class (Figure 16) and drug level in Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.9; and Analysis 2.10, respectively. We provide details of the serious adverse effects in Table 13 (number of serious infections, number of malignancies, number of major adverse cardiac events per arm at class level).
Table 6.
Total number of serious adverse events during the induction phase at class‐level and most severe types
| Number of randomised participants | Number of serious adverse events | Number of serious infections | Number of malignancies | Number of MACE | ||||||
| Drug | Placebo | Drug | Placebo | Drug | Placebo | Drug | Placebo | Drug | Placebo | |
| Conventional systemic agents | 767 | 220 | 19 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anti‐TNF | 4508 | 2640 | 85 | 44 | 21 | 9 | 20 | 7 | 6 | 2 |
| Anti‐IL12/23 | 2547 | 1607 | 38 | 23 | 7 | 5 | 4 | 1 | 4 | 3 |
| Anti‐IL17 | 7551 | 2533 | 149 | 36 | 47 | 7 | 21 | 2 | 19 | 3 |
| Anti‐IL23 | 1347 | 510 | 23 | 7 | 4 | 1 | 0 | 0 | 1 | 0 |
| Other biologics | 509 | 227 | 15 | 10 | ‐ | ‐ | 2 | 1 | ‐ | ‐ |
| Small molecules | 3920 | 1280 | 89 | 28 | 15 | 5 | 14 | 0 | 5 | 1 |
MACE: Major adverse cardiac events
Figure 16.
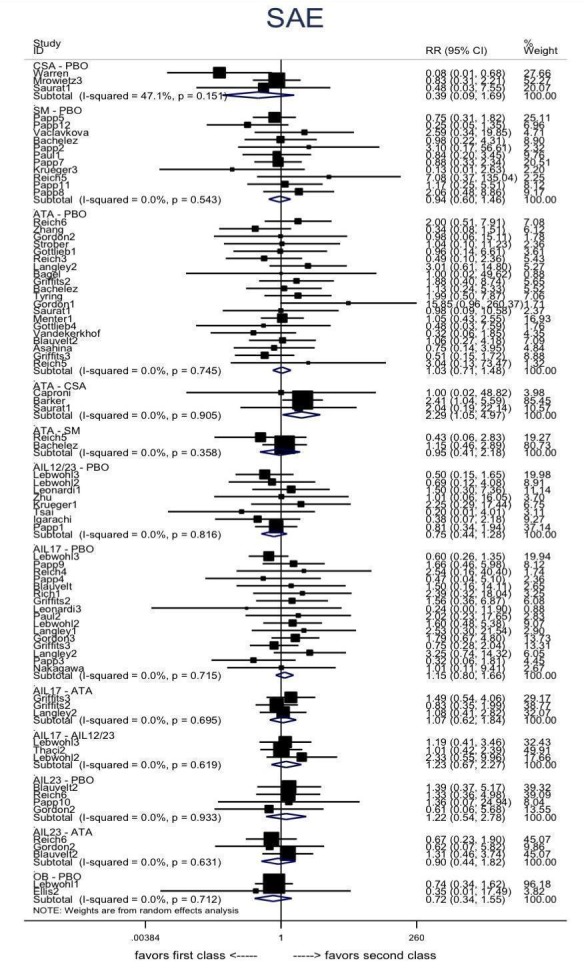
Serious adverse effects: direct summary effects for comparisons including at least two studies at class level
AIL12/23: anti‐IL12/23; AIL17: anti‐IL17; AIL23: anti‐IL23, ATA: anti‐TNF alpha; CSA: conventional systemic agents; OB: other biologics; PBO: placebo; SM: small molecules
CI: confidence interval; RR: risk ratio; SAE: serious adverse events
Analysis 2.1.
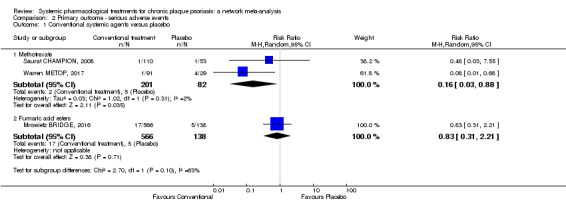
Comparison 2 Primary outcome ‐ serious adverse events, Outcome 1 Conventional systemic agents versus placebo.
Analysis 2.2.
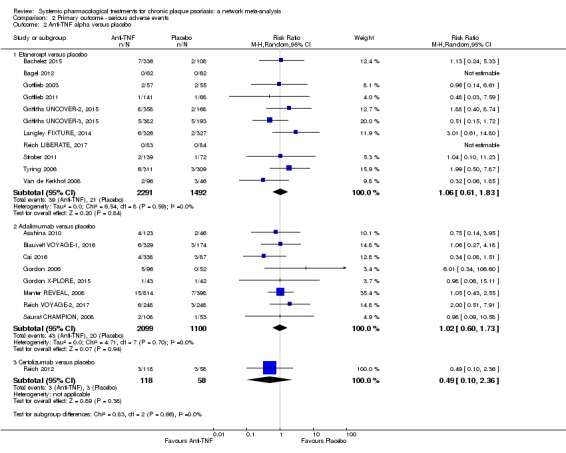
Comparison 2 Primary outcome ‐ serious adverse events, Outcome 2 Anti‐TNF alpha versus placebo.
Analysis 2.3.
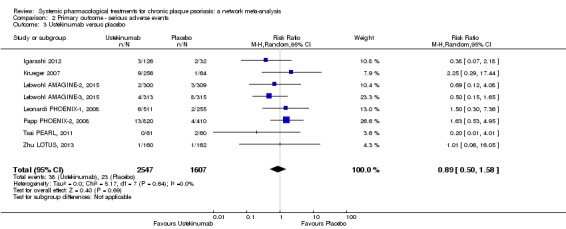
Comparison 2 Primary outcome ‐ serious adverse events, Outcome 3 Ustekinumab versus placebo.
Analysis 2.4.
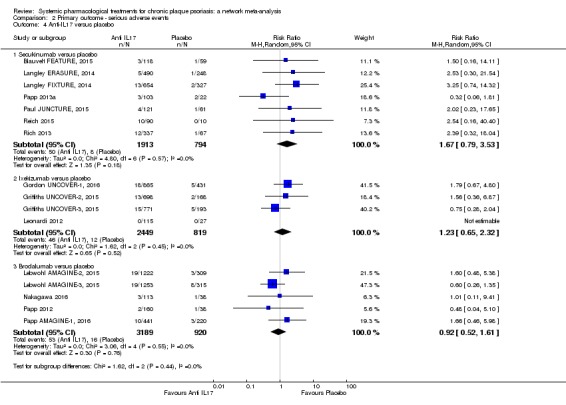
Comparison 2 Primary outcome ‐ serious adverse events, Outcome 4 Anti‐IL17 versus placebo.
Analysis 2.5.
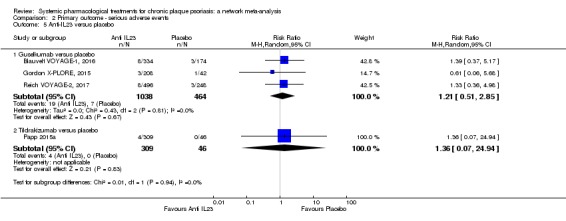
Comparison 2 Primary outcome ‐ serious adverse events, Outcome 5 Anti‐IL23 versus placebo.
Analysis 2.6.
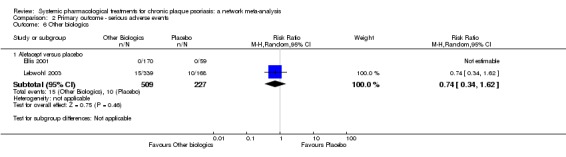
Comparison 2 Primary outcome ‐ serious adverse events, Outcome 6 Other biologics.
Analysis 2.7.
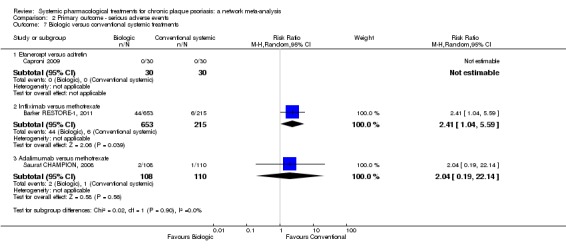
Comparison 2 Primary outcome ‐ serious adverse events, Outcome 7 Biologic versus conventional systemic treatments.
Analysis 2.8.
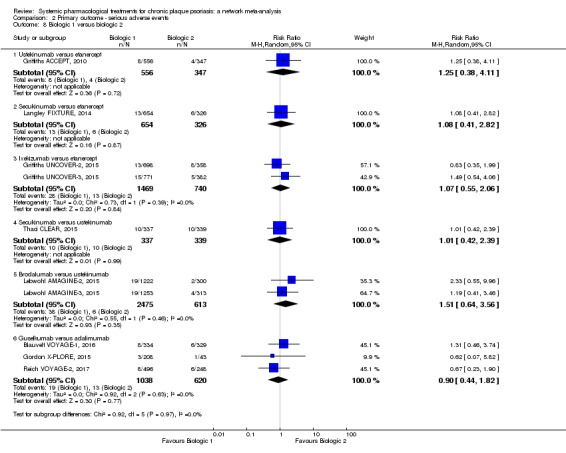
Comparison 2 Primary outcome ‐ serious adverse events, Outcome 8 Biologic 1 versus biologic 2.
Analysis 2.9.
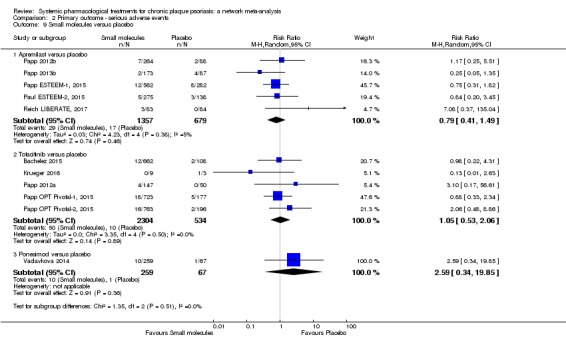
Comparison 2 Primary outcome ‐ serious adverse events, Outcome 9 Small molecules versus placebo.
Analysis 2.10.
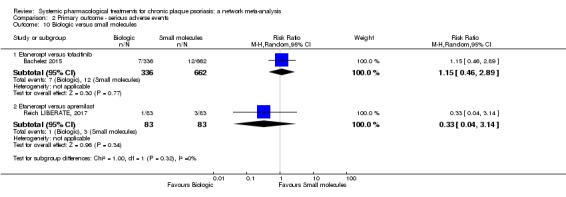
Comparison 2 Primary outcome ‐ serious adverse events, Outcome 10 Biologic versus small molecules.
No significant differences were observed between methotrexate, FAEs, etanercept, adalimumab, certolizumab, ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab, tildrakizumab, alefacept, apremilast, tofacitinib, ponesimod, and placebo in terms of the number of paticipants with serious adverse effects (SAEs). The risk of SAEs was significantly higher for participants on infliximab compared to methotrexate (RR 2.41, 95% CI 1.04 to 5.59).
There were zero SAEs in the following trials: Fallah Arani 2011 (comparing methotrexate with FAEs); Flytström 2008 (comparing ciclosporin with methotrexate); and Heydendael 2003 (comparing ciclosporin with methotrexate).
NETWORK META‐ANALYSES
The SAE outcome was available in 60 trials, involving 30,898 participants (87.1% of the participants in the meta‐analysis). This outcome was reported in one other trial (Sterry PRESTA, 2010); however, the number of randomised participants was not available. This trial was added to the complete‐cases analyses. This outcome was also reported in two other trials (Laburte 1994; Mrowietz SCULPTURE, 2015), comparing different dosages of the same drug in each case. These studies were added to the sensitivity analysis at dose level. SAEs were not reported for the 11 remaining trials, and we were not able to obtain missing information from the trial authors (Table 9). Forty‐two trials, involving 16,822 participants, were placebo‐controlled trials; six, involving 1836 participants, were head‐to‐head comparisons, and 12, involving 12,240 participants, had both a placebo and at least two active treatments arms.
See Figure 4; Figure 5; Figure 6; Figure 7; Figure 10; Figure 11; Figure 13; and Figure 14.
Table 14 summarised the main results of both direct and indirect evidences and the network meta‐analysis for SAEs at 12 to 16 weeks. We present the summary relative effects from the network meta‐analysis in league tables for both class‐level (Figure 6) and drug‐level (Figure 7) analyses. No significant difference was found between all of the interventions and the placebo regarding the risk of SAE. Two significant associations were found: anti‐IL17 agents and anti‐TNF alpha agents had a higher risk of SAE compared with conventional systemic agents (RR 2.31, 95%CI 1.20 to 4.48; RR 2.06, 95% CI 1.13 to 3.75, respectively). The results are available in Figure 7 for comparison between each drug. Ixekizumab, secukinumab, and infliximab were at higher risk of SAE than methotrexate (RR 4.86, 95%CI 1.03 to 22.88; RR 5.14, 95% CI 1.09 to 24.29; RR 2.41, 95% CI 1.04 to 5.59, respectively).
Table 7.
Direct and indirect evidence and network meta‐analysis results summary table for serious adverse events at 12 to 16 weeks
| Network meta‐analysis | Direct evidence | Indirect evidence | |||||||
| Comparisons* | RR | LCI | UCI | RR | LCI | UCI | RR | LCI | UCI |
| FAEs vs placebo | 0.77 | 0.30 | 1.99 | 0.83 | 0.31 | 2.21 | 0.19 | 0 | 12.57 |
| Methotrexate vs placebo | 0.23 | 0.05 | 0.99 | 0.16 | 0.03 | 0.86 | 0.68 | 0.04 | 11.67 |
| Adalimumab vs placebo | 1.02 | 0.61 | 1.73 | 1.05 | 0.62 | 1.78 | 0.07 | 0 | 26.92 |
| Etanercept vs placebo | 0.99 | 0.65 | 1.51 | 1.09 | 0.65 | 1.84 | 0.76 | 0.31 | 1.89 |
| Ustekinumab vs placebo | 0.89 | 0.57 | 1.39 | 0.74 | 0.44 | 1.26 | 1.36 | 0.61 | 2.99 |
| Secukinumab vs placebo | 1.19 | 0.69 | 2.03 | 1.61 | 0.78 | 3.33 | 0.75 | 0.3 | 1.87 |
| Ixekizumab vs placebo | 1.12 | 0.66 | 1.90 | 1.16 | 0.62 | 2.16 | 0.97 | 0.18 | 5.12 |
| Brodalumab vs placebo | 1.04 | 0.62 | 1.73 | 0.92 | 0.53 | 1.62 | 2.77 | 0.38 | 20.28 |
| Apremilast vs placebo | 0.84 | 0.47 | 1.52 | 0.78 | 0.42 | 1.44 | 4.33 | 0.09 | 201.27 |
| Tofacitinib vs placebo | 0.98 | 0.55 | 1.76 | 1.05 | 0.53 | 2.06 | 0.67 | 0.08 | 5.35 |
| Guselkumab vs placebo | 1.00 | 0.49 | 2.04 | 1.21 | 0.51 | 2.85 | 0.52 | 0.08 | 3.41 |
| Methotrexate vs FAEs | 0.30 | 0.06 | 1.59 | 1 | 0.02 | 48.83 | 0.23 | 0.04 | 1.45 |
| Ciclosporin vs methotrexate | 0.98 | 0.06 | 15.38 | 0.98 | 0.06 | 15.38 | ‐ | ‐ | ‐ |
| Infliximab vs methotrexate | 2.41 | 1.04 | 5.59 | 2.41 | 1.04 | 5.59 | ‐ | ‐ | ‐ |
| Adalimumab vs methotrexate | 4.43 | 0.99 | 19.81 | 2.24 | 0.21 | 23.56 | 6.68 | 1.04 | 42.76 |
| Etanercept vs acitretin | 1.00 | 0.02 | 48.82 | 1 | 0.02 | 48.83 | ‐ | ‐ | ‐ |
| Guselkumab vs adalimumab | 0.98 | 0.51 | 1.88 | 0.89 | 0.44 | 1.79 | 2.07 | 0.26 | 16.45 |
| Ustekinumab vs etanercept | 0.90 | 0.52 | 1.57 | 1.25 | 0.38 | 4.11 | 0.83 | 0.44 | 1.54 |
| Secukinumab vs etanercept | 1.20 | 0.66 | 2.19 | 1.17 | 0.45 | 3.04 | 1.22 | 0.56 | 2.65 |
| Ixekizumab vs etanercept | 1.14 | 0.66 | 1.94 | 1.02 | 0.53 | 1.95 | 1.47 | 0.53 | 4.09 |
| Apremilast vs etanercept | 0.85 | 0.42 | 1.72 | 2.69 | 0.41 | 17.5 | 0.7 | 0.33 | 1.5 |
| Tofacitinib vs etanercept | 0.99 | 0.53 | 1.87 | 0.87 | 0.35 | 2.19 | 1.12 | 0.47 | 2.7 |
| Secukinumab vs ustekinumab | 1.33 | 0.74 | 2.38 | 1.01 | 0.42 | 2.39 | 1.68 | 0.77 | 3.68 |
| Brodalumab vs ustekinumab | 1.16 | 0.64 | 2.11 | 1.32 | 0.59 | 2.98 | 0.95 | 0.33 | 2.71 |
FAES: fumaric acid esters; LCI: low confidence interval; RR: risk ratio; UCI: upper confidence interval
*The comparisons listed in this table were included in at least one direct‐evidence analysis.
Ranking class‐level analysis (Figure 10; Figure 13; Table 11)
Ranking analysis for SAE performed with SUCRA strongly suggested that conventional systemic treatment was associated with the best safety profile at class level in terms of serious adverse events (versus placebo: RR 0.48, 95% CI 0.26 to 0.88; SUCRA = 94.8), followed by other biologics (versus placebo: RR 0.72, 95% CI 0.34 to 1.55; SUCRA = 68.2), anti‐IL12/23 (versus placebo: RR 0.89, 95% CI 0.58 to 1.37; SUCRA = 53.9), and then small molecules (versus placebo: RR 0.95, 95% CI 0.63 to 1.42; SUCRA = 45.4). The heterogeneity τ for this network overall was 0, which we considered low heterogeneity.
Ranking drug‐level analysis (Figure 11; Figure 14; Table 12)
Ranking analysis for SAE performed with SUCRA strongly suggested that methotrexate was associated with the best safety profile at drug level in terms of serious adverse events (versus placebo: RR 0.23, 95% CI 0.05 to 0.99; SUCRA = 90.7; moderate‐certainty evidence), followed by ciclosporin (versus placebo: RR 0.23, 95% CI 0.01 to 5.10; SUCRA = 78.2; very low‐certainty evidence), certolizumab (versus placebo: RR 0.49, 95% CI 0.10 to 2.36; SUCRA = 70.9; moderate‐certainty evidence), infliximab (versus placebo: RR 0.56, 95% CI 0.10 to 3.00; SUCRA = 64.4; very low‐certainty evidence), alefacept (versus placebo: RR 0.72, 95% CI 0.34 to 1.55; SUCRA = 62.6; low‐certainty evidence), and then the FAEs (versus placebo: RR 0.77, 95% CI 0.30 to 1.99; SUCRA = 57.7; very low‐certainty evidence). The heterogeneity τ for this network overall was 0, which we considered low heterogeneity.
Placebo had a worse ranking for SAE than conventional systemic agents, other biologics, anti‐IL12/23, and small molecules (see Table 12). Nevertheless, analyses on serious adverse events were based on a very low number of events and were reduced to the short time frame of the trials. Table 13 gives details of the types of SAE; major adverse cardiac events, serious infections, or malignancies were reported in both placebo and intervention groups.
1.3 Relationship between PASI 90 and serious adverse events
See Figure 12.
These findings for both efficacy (PASI 90) and acceptability (serious adverse events) were combined together in a bivariate ranking plot, where serious adverse events was transformed into acceptability by using the inverse values of the corresponding RRs so that higher values indicate higher acceptability (due to lower SAE): accordingly, the ideal treatment (highest performance = best efficacy + best acceptability) should appear in the upper right corner of the plot.
At class level, the highly effective treatments had serious adverse events. However, the anti‐IL12/23 treatment group was the class with the better compromise between efficacy and acceptability.
At drug level, ustekinumab, certolizumab, and infliximab might be the overall best treatments considering both outcomes jointly. This result has to be considered with cautioun for certolizumab and infliximab as only one trial was available for this drug.
2. Secondary outcomes
2.1 Mean difference of quality of life measured by a specific scale
DIRECT EVIDENCE
We report treatment estimates for pair‐wise meta‐analyses at class (Figure 17) and drug level in Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7; Analysis 3.8; Analysis 3.9; Analysis 3.10; and Analysis 3.11 respectively.
Figure 17.
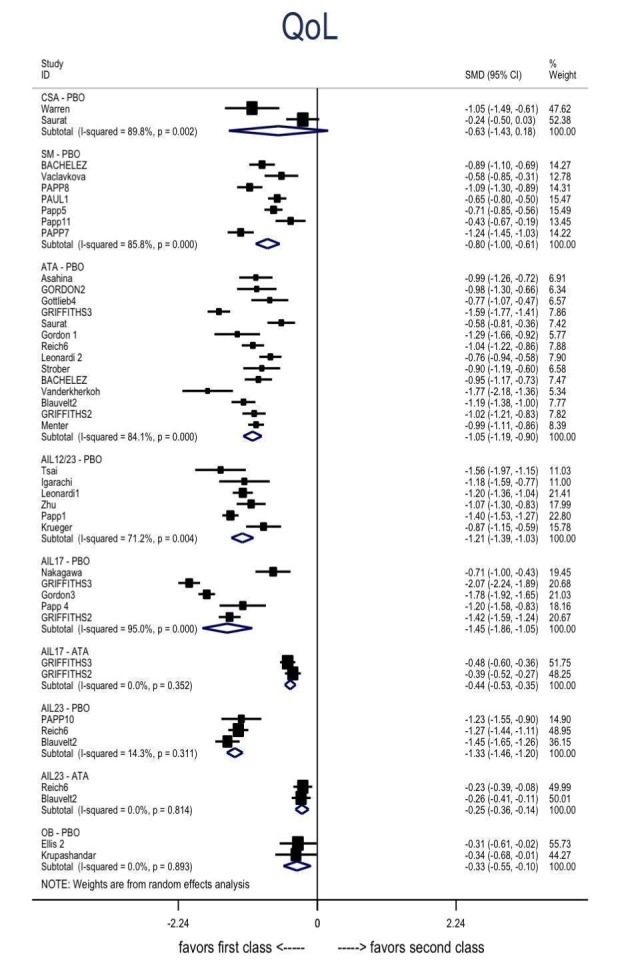
Specific quality of life scale: direct summary effects for comparisons including at least two studies at class level
AIL12/23: anti‐IL12/23; AIL17: anti‐IL17; AIL23: anti‐IL23, ATA: anti‐TNF alpha; CSA: conventional systemic agents; OB: other biologics; PBO: placebo; SM: small molecules
CI: confidence interval; QoL: specific quality of life scale; SMD: standardised mean difference
Analysis 3.1.
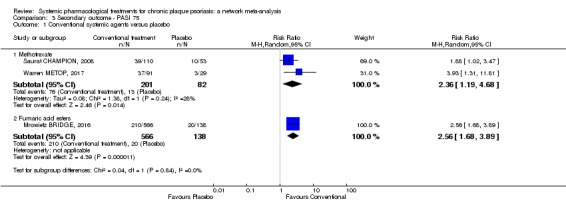
Comparison 3 Secondary outcome ‐ PASI 75, Outcome 1 Conventional systemic agents versus placebo.
Analysis 3.2.
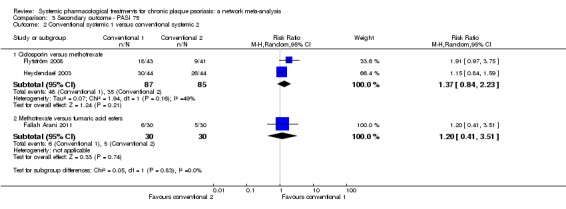
Comparison 3 Secondary outcome ‐ PASI 75, Outcome 2 Conventional systemic 1 versus conventional systemic 2.
Analysis 3.3.
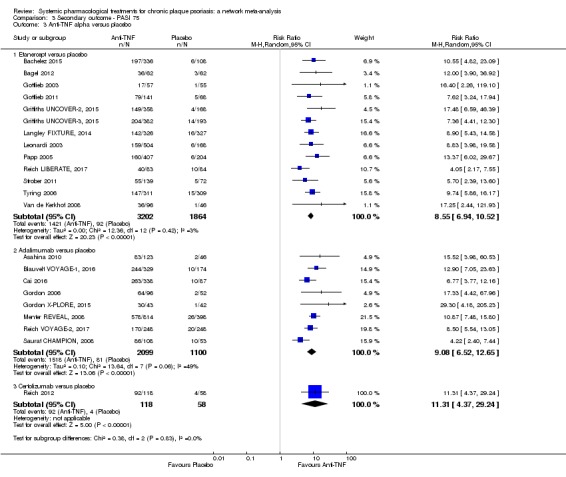
Comparison 3 Secondary outcome ‐ PASI 75, Outcome 3 Anti‐TNF alpha versus placebo.
Analysis 3.4.
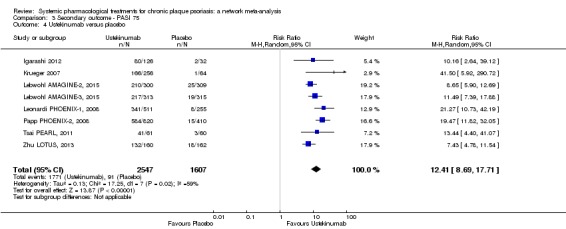
Comparison 3 Secondary outcome ‐ PASI 75, Outcome 4 Ustekinumab versus placebo.
Analysis 3.5.
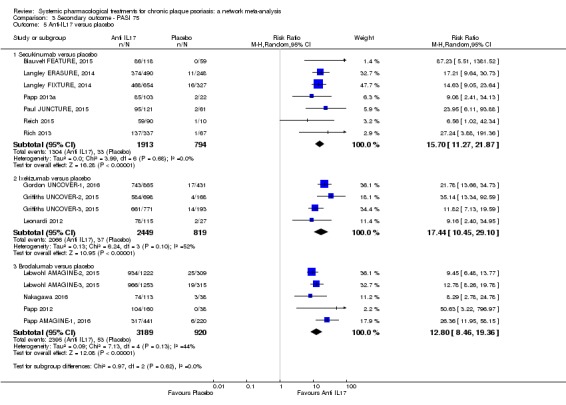
Comparison 3 Secondary outcome ‐ PASI 75, Outcome 5 Anti‐IL17 versus placebo.
Analysis 3.6.
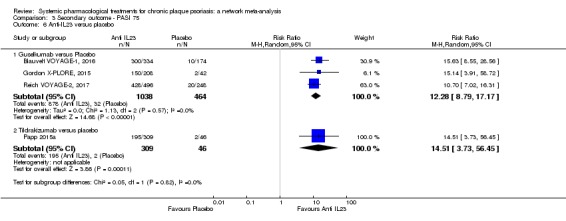
Comparison 3 Secondary outcome ‐ PASI 75, Outcome 6 Anti‐IL23 versus placebo.
Analysis 3.7.
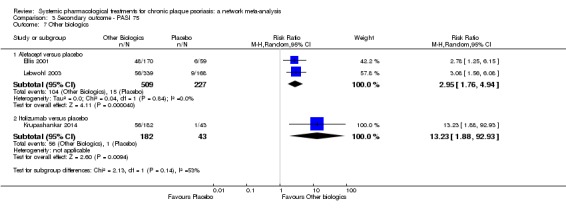
Comparison 3 Secondary outcome ‐ PASI 75, Outcome 7 Other biologics.
Analysis 3.8.
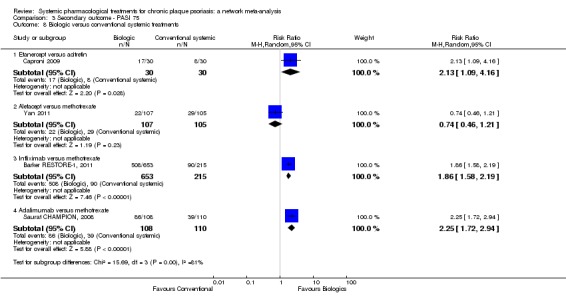
Comparison 3 Secondary outcome ‐ PASI 75, Outcome 8 Biologic versus conventional systemic treatments.
Analysis 3.9.
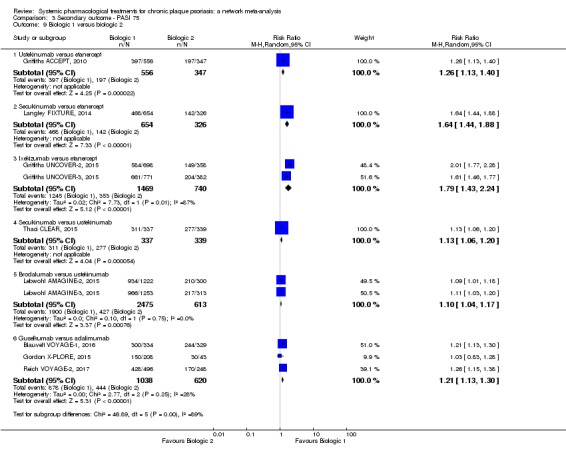
Comparison 3 Secondary outcome ‐ PASI 75, Outcome 9 Biologic 1 versus biologic 2.
Analysis 3.10.
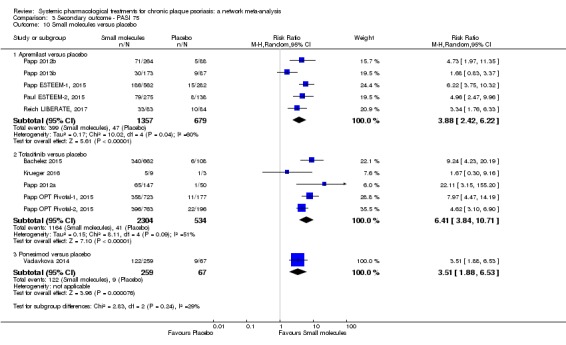
Comparison 3 Secondary outcome ‐ PASI 75, Outcome 10 Small molecules versus placebo.
Analysis 3.11.
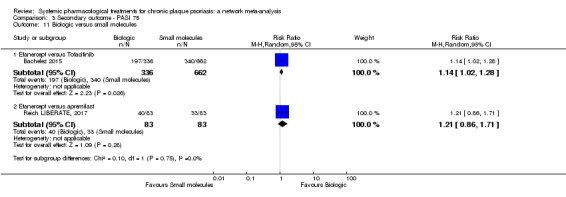
Comparison 3 Secondary outcome ‐ PASI 75, Outcome 11 Biologic versus small molecules.
NETWORK META‐ANALYSES
The quality of life outcome was available in 39 trials, involving 21,745 participants (61.3% of the participants in this review). This outcome was reported in one other trial (Krueger 2002); however, the number of randomised participants was not available. This trial were added to the complete case analyses. This outcome was also reported in another trial (Mrowietz SCULPTURE, 2015), comparing different dosages of the same drug. This trial, Mrowietz SCULPTURE, 2015, was added in the sensitivity analyses at dose level. The quality of life outcome was not reported for the 35 remaining trials, and we were not able to obtain missing information from the trial authors (Table 9). Twenty‐eight trials, involving 13,040 participants, were placebo‐controlled trials; two, involving 1080 participants, were head‐to‐head comparisons; and nine, involving 7625 participants, had both a placebo and at least two active treatments arms.
See Figure 4; Figure 5; Figure 6; Figure 9; Figure 10; Figure 11; Figure 13; and Figure 14.
We present the summary relative effects from the network meta‐analysis in league tables for both class‐level (Figure 6) and drug‐level (Figure 9) analyses. All of the interventions appeared superior to placebo in terms of showing significant improvement on a quality of life scale. Anti‐IL17, anti‐IL23, and anti‐IL12/23 were associated with a higher chance of improving quality of life compared to small molecules and conventional systemic agents (Figure 6). These differences were statistically significant for all of the classes. No significant difference was shown between the different biological agents except for anti‐IL17 and anti‐TNF alpha (anti‐IL17 was more favourable than anti‐TNF alpha). No significant differences were shown between the small molecules and the conventional agents. Results of comparisons between each of the drugs are available in Figure 9.
Ranking class‐level analysis (Figure 10; Figure 13; Table 11)
Ranking analysis for quality of life performed with SUCRA strongly suggested that anti‐IL17 was the best treatment at class level (versus placebo: standardised mean difference (SMD) ‐1.44, 95% confidence interval (CI) ‐1.68 to ‐1.19; SUCRA = 95.4), followed by anti‐IL23 (versus placebo: SMD ‐1.30 95% CI ‐1.60 to ‐0.99; SUCRA = 83.4), anti‐IL12/23 (versus placebo: SMD ‐1.21 95% CI ‐1.45 to ‐0.96; SUCRA = 75.7), then anti‐TNF alpha (versus placebo: SMD ‐1.03 95% CI ‐1.18 to ‐0.88 SUCRA = 58.4). The heterogeneity τ for this network overall was 0.27, which we considered moderate heterogeneity.
Ranking drug‐level analysis (Figure 11; Figure 14Table 12)
Ranking analysis for quality of life performed with SUCRA strongly suggested that ixekizumab was the best treatment at drug level (versus placebo: SMD ‐1.68 95% CI ‐1.93 to ‐1.43; SUCRA = 99.2), followed by guselkumab (versus placebo: SMD ‐1.31 95% CI ‐1.61 to ‐1.01; SUCRA = 84.3), ustekinumab (versus placebo: SMD ‐1.21 95% CI ‐1.42 to ‐1.00; SUCRA = 77.4), tildrakizumab (versus placebo: SMD ‐1.23 95% CI ‐1.77 to ‐0.68; SUCRA = 74.9), then etanercept (versus placebo: SMD ‐1.11 95% CI ‐1.29 to ‐0.93; SUCRA = 67.6). The heterogeneity τ for this network overall was 0.22, which we considered low to moderate heterogeneity. Moreover, six interventions (acitretin, certolizumab, ciclosporin, fumaric acid, infliximab, secukinumab) were not included in the ranking at drug level, due to no available data.
In total, available information on quality of life was poorly reported and lacking for a third of the interventions, so has to be considered with cautious.
2.2 Proportion of participants who achieve a Physician Global Assessment (PGA) value at 0 or 1
DIRECT EVIDENCE
We report treatment estimates for pair‐wise meta‐analyses at class (Figure 18) and drug level in Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.9; Analysis 4.10; and Analysis 4.11, respectively.
Figure 18.
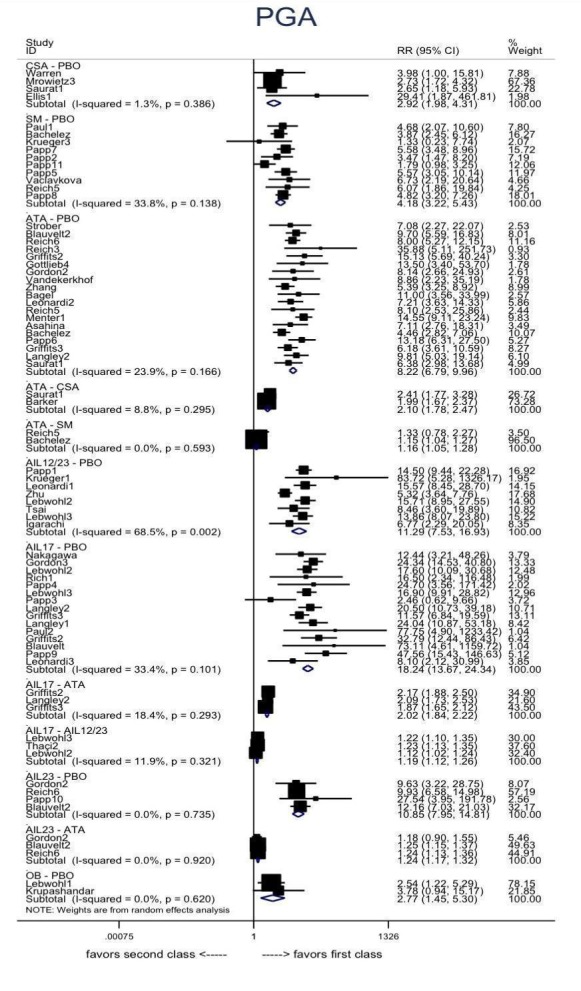
Physician Global Assessment 0/1: direct summary effects for comparisons including at least two studies at class‐level
AIL12/23: anti‐IL12/23; AIL17: anti‐IL17; AIL23: anti‐IL23, ATA: anti‐TNF alpha; CSA: conventional systemic agents; OB: other biologics; PBO: placebo; SM: small molecules
AE: adverse events; CI: confidence interval; PGA: Physician Global Assessment; RR: risk ratio
Analysis 4.1.
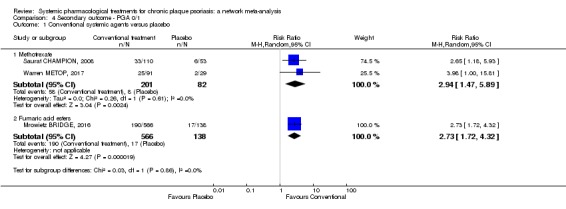
Comparison 4 Secondary outcome ‐ PGA 0/1, Outcome 1 Conventional systemic agents versus placebo.
Analysis 4.2.
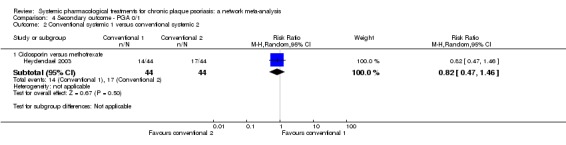
Comparison 4 Secondary outcome ‐ PGA 0/1, Outcome 2 Conventional systemic 1 versus conventional systemic 2.
Analysis 4.3.
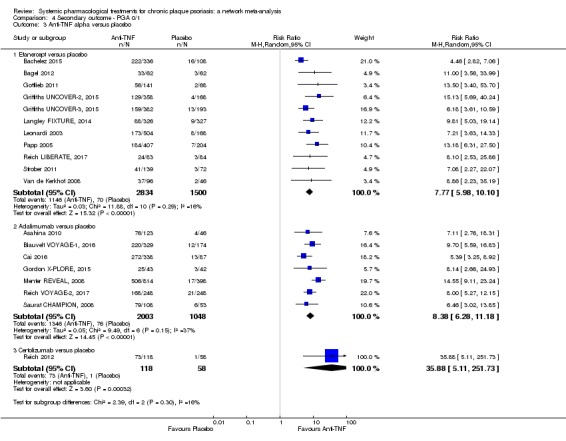
Comparison 4 Secondary outcome ‐ PGA 0/1, Outcome 3 Anti‐TNF alpha versus placebo.
Analysis 4.4.
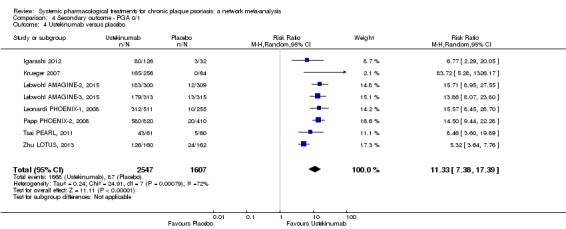
Comparison 4 Secondary outcome ‐ PGA 0/1, Outcome 4 Ustekinumab versus placebo.
Analysis 4.5.
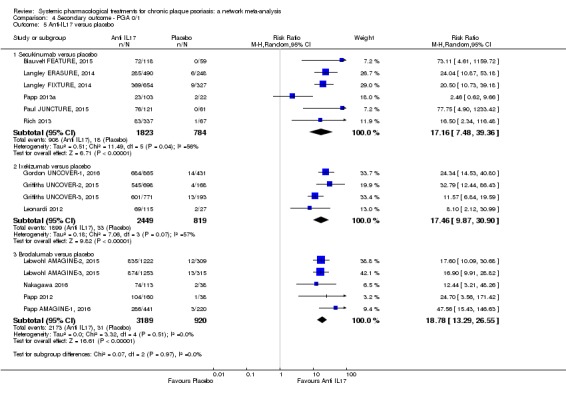
Comparison 4 Secondary outcome ‐ PGA 0/1, Outcome 5 Anti‐IL17 versus placebo.
Analysis 4.6.
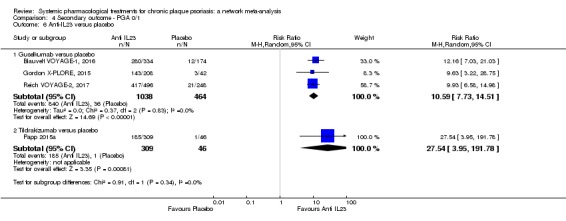
Comparison 4 Secondary outcome ‐ PGA 0/1, Outcome 6 Anti‐IL23 versus placebo.
Analysis 4.7.
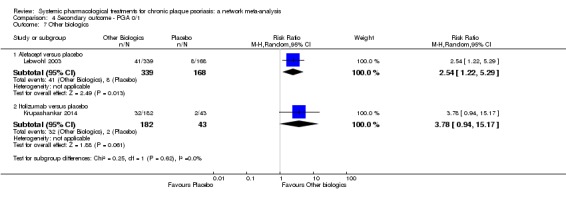
Comparison 4 Secondary outcome ‐ PGA 0/1, Outcome 7 Other biologics.
Analysis 4.8.
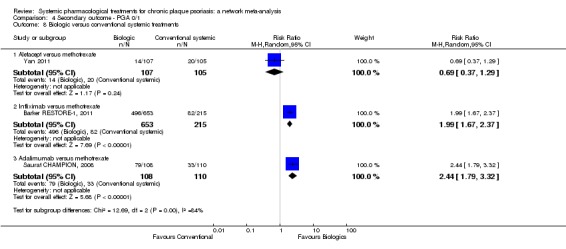
Comparison 4 Secondary outcome ‐ PGA 0/1, Outcome 8 Biologic versus conventional systemic treatments.
Analysis 4.9.
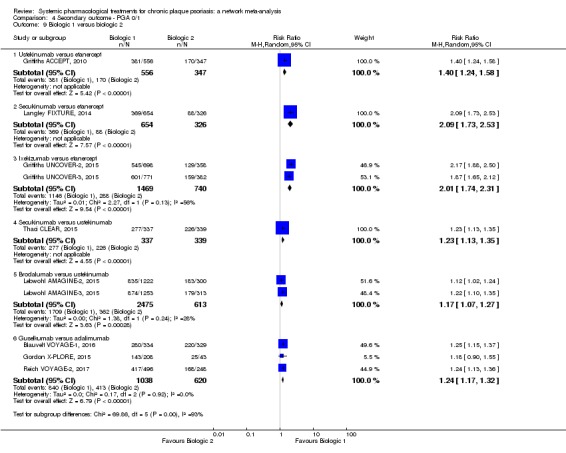
Comparison 4 Secondary outcome ‐ PGA 0/1, Outcome 9 Biologic 1 versus biologic 2.
Analysis 4.10.
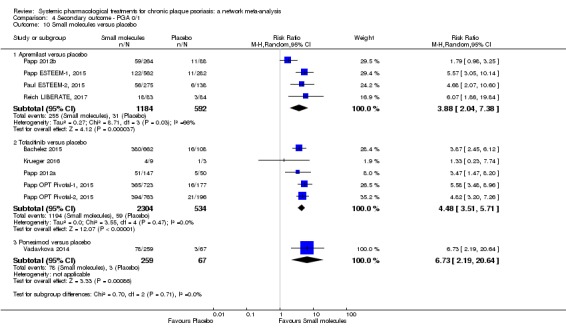
Comparison 4 Secondary outcome ‐ PGA 0/1, Outcome 10 Small molecules versus placebo.
Analysis 4.11.
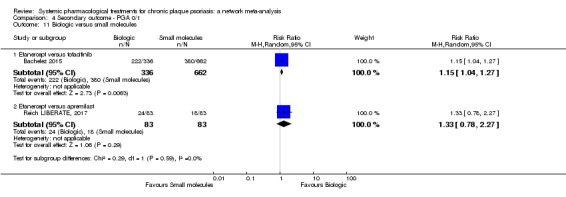
Comparison 4 Secondary outcome ‐ PGA 0/1, Outcome 11 Biologic versus small molecules.
NETWORK META‐ANALYSES
The PGA 0/1 outcome was available in 56 trials, involving 31,030 participants (87.5% of the participants in this review). This outcome was reported in four other studies (Krueger 2002; Nugteren‐Huying 1990; Sandhu 2003; Sterry PRESTA, 2010); however, the number of randomised participants was not available. These trials were added to the complete case analyses. This outcome was also reported in another trial (Mrowietz SCULPTURE, 2015), comparing different dosages of the same drug. These trials were added in the sensitivity analysis at dose level. PGA 0/1 was not reported for the 13 remaining trials, and we were not able to obtain missing information from the trial authors (Table 9). Forty trials, involving 16,946 participants, were placebo‐controlled trials; four, involving 1844 participants, were head‐to‐head comparisons; and 12, involving 12,240 participants, had both a placebo and at least two active treatments arms.
See Figure 4; Figure 5; Figure 6; Figure 9Figure 10; Figure 11; Figure 13; and Figure 14.
We presented the summary relative effects as estimated from the network meta‐analysis in league tables at class level (Figure 6) and drug level (Figure 9). All of the interventions appeared superior to placebo in terms of reaching PGA 0/1, and anti‐IL17 monoclonal antibodies were associated with a better chance in terms of this outcome compared to the other drug classes (Figure 6). These differences were statistically significant. All of the interventions (anti‐IL17, anti‐IL23, anti‐IL12/23, anti‐TNF alpha) appeared significantly superior to the small molecule class of treatments and the conventional systemic class of treatments. No significant difference was found between small molecule and conventional systemic agents. Results of comparisons between each of the drugs are available in Figure 9.
Ranking class‐level analysis (Figure 10; Figure 13; Table 11)
Ranking analysis for PGA 0/1 performed with SUCRA strongly suggested that anti‐IL17 was the best treatment at class level (versus placebo: RR 15.85, 95% confidence interval (CI) 13.08 to 19.20; SUCRA = 99.9), followed by anti‐IL12/23 (versus placebo: RR 11.80, 95% CI 9.67 to 14.39; SUCRA = 83.8), anti‐IL23 (versus placebo: RR 9.93, 95% CI 7.58 to 13.02; SUCRA = 73.1), then anti‐TNF alpha (versus placebo: RR 7.82, 95% CI 6.66 to 9.17; SUCRA = 57.5). The heterogeneity τ for this network overall was 0.21, which we considered low to moderate heterogeneity.
Ranking drug‐level analysis (Figure 11; Figure 14; Table 12)
Ranking analysis for PGA 0/1 performed with SUCRA strongly suggested that certolizumab was the best treatment at drug level (versus placebo: RR 35.88, 95% CI 4.86 to 265.07; SUCRA = 90.1), followed by tildrakizumab (versus placebo: RR 27.54, 95% CI 3.76 to 201.98; SUCRA = 86.3), ixekizumab (versus placebo: RR 16.11, 95% CI 11.72 to 22.17; SUCRA = 85.9), secukinumab (versus placebo: RR 15.46, 95% CI 11.19 to 21.37; SUCRA = 84.4), brodalumab (versus placebo: RR 15.31, 95% CI 10.84 to 21.63; SUCRA = 84), then ustekinumab (versus placebo: RR 11.52, 95% CI 9.17 to 14.4; SUCRA = 70.4). The heterogeneity τ for this network overall was 0.23, which we considered low to moderate heterogeneity.
2.3 Proportion of participants who achieve PASI 75
DIRECT EVIDENCE
We report treatment estimates for pair‐wise meta‐analyses at class (Figure 19) and drug level in Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7; Analysis 3.8; Analysis 3.9; Analysis 3.10; and Analysis 3.11, respectively.
Figure 19.
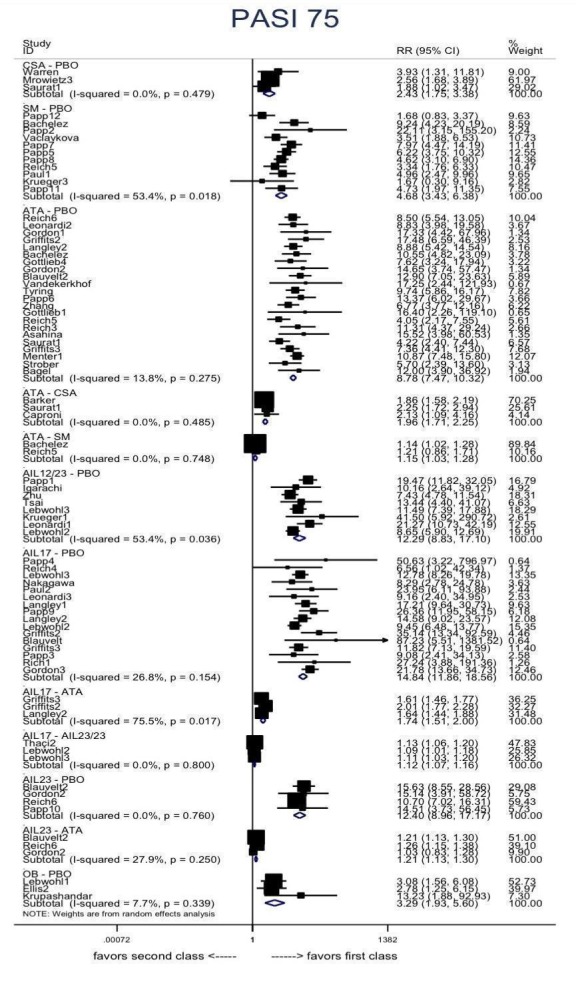
PASI 75: direct summary effects for comparisons including at least two studies at class level
AIL12/23: anti‐IL12/23; AIL17: anti‐IL17; AIL23: anti‐IL23, ATA: anti‐TNF alpha; CSA: conventional systemic agents; OB: other biologics; PBO: placebo; SM: small molecules
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio
NETWORK META‐ANALYSES
PASI 75 outcome was available in 64 trials, involving 32,518 participants (91.7% of the participants in this review). This outcome was reported in two other trials (Krueger 2002; Sterry PRESTA, 2010); however, the number of randomised participants was not available. These trials were added to the complete case analyses. This outcome was also reported in five other trials (Dogra 2012; Dogra 2013; Dubertret 1989; Laburte 1994; Mrowietz SCULPTURE, 2015), comparing different dosages of the same drug in each case. These trials were added in the sensitivity analysis at dose level. PASI 75 was not reported for the three remaining trials, and we were not able to obtain missing information from the trial authors (Table 9). Forty‐five trials, involving 18,330 participants, were placebo‐controlled trials; seven, involving 1948 participants, were head‐to‐head comparisons; and 12, involving 12,240 participants, had both a placebo and at least two active treatments arms.
See Figure 4; Figure 5; Figure 6; Figure 8; Figure 10; Figure 11; Figure 13; and Figure 14.
We present the summary relative effects from the network meta‐analysis in league tables for both class‐level (Figure 6) and drug‐level (Figure 8) analyses. All of the interventions appeared superior to placebo in terms of reaching PASI 75. The anti‐IL17 class of drugs was associated with a higher chance of reaching PASI 75 compared to the other classes (Figure 6). These differences were statistically significant for all of the classes. All of the interventions (anti‐IL17, anti‐IL23, anti‐IL12/23, anti‐TNF alpha) appeared significantly superior to the small molecule class and the conventional systemic class, and the small molecules appeared significantly superior to the conventional systemic agents. Results of comparisons between each of the drugs are available in Figure 8.
Ranking class‐level analysis (Figure 10; Figure 13; Table 11)
Ranking analysis for PASI 75 performed with SUCRA strongly suggested that anti‐IL17 was the best treatment at class level (versus placebo: RR 14.32, 95% CI 12.20 to 16.81; SUCRA = 99.6), followed by anti‐IL12/23 (versus placebo: RR 12.21, 95% CI 10.23 to 14.57; SUCRA = 85.0), anti‐IL23 (versus placebo: RR 10.07, 95% CI 8.03 to 12.63; SUCRA = 72.2), then anti‐TNF alpha (versus placebo: RR 8.23 95% CI 7.20 to 9.42; SUCRA = 57.4). The heterogeneity τ for this network overall was 0.16, which we considered low heterogeneity.
Ranking drug‐level analysis (Figure 11; Figure 14; Table 12)
Ranking analysis for PASI 75 performed with SUCRA strongly suggested that ixekizumab was the best treatment at drug level (versus placebo: RR 15.81, 95% CI 12.35 to 20.23; SUCRA = 91.8), followed by secukinumab (versus placebo: RR 14.16, 95% CI 11.12 to 18.03; SUCRA = 86.7), brodalumab (versus placebo: RR 13.04 95% CI 10.17 to 16.71; SUCRA = 82.1), tildrakizumab (versus placebo: RR 14.51, 95% CI 3.60 to 58.45; SUCRA = 78.3), then ustekinumab (versus placebo: RR 11.84, 95% CI 9.79 to 14.33; SUCRA = 75.2). The heterogeneity τ for this network overall was 0.16, which we considered low heterogeneity.
Focusing on efficacy outcomes (PASI 90, PASI 75, and PGA 0/1), the results were identical at class level (Figure 10) and very close at drug level (Figure 11).
2.4 The proportions of participants with adverse effects
DIRECT EVIDENCE
We report treatment estimates for pair‐wise meta‐analyses at class (Figure 20) and drug level in Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4; Analysis 6.5; Analysis 6.6; Analysis 6.7; Analysis 6.8; Analysis 6.9; and Analysis 6.10, respectively.
Figure 20.
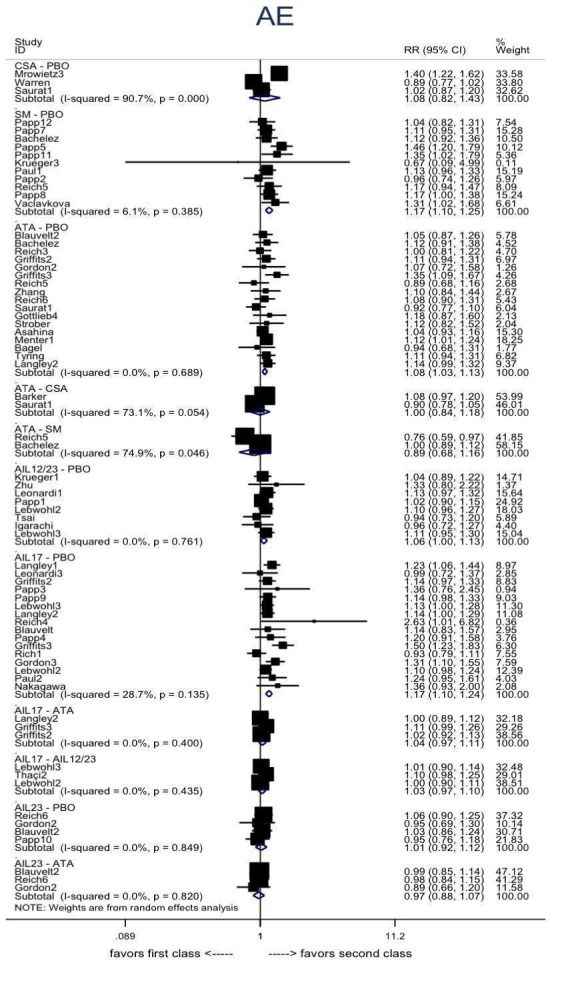
Adverse effects : direct summary effects for comparisons including at least two studies at class‐level
AIL12/23: anti‐IL12/23; AIL17: anti‐IL17; AIL23: anti‐IL23, ATA: anti‐TNF alpha; CSA: conventional systemic agents; OB: other biologics; PBO: placebo; SM: small molecules
AE: adverse events; CI: confidence interval; RR: risk ratio
Analysis 6.1.
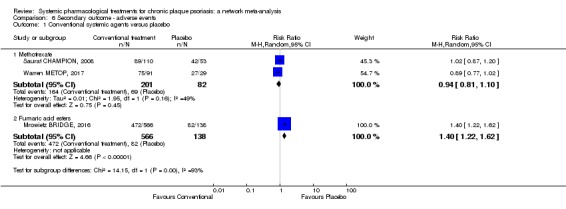
Comparison 6 Secondary outcome ‐ adverse events, Outcome 1 Conventional systemic agents versus placebo.
Analysis 6.2.
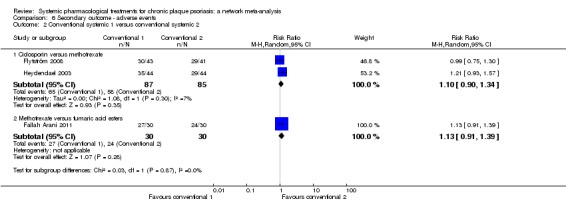
Comparison 6 Secondary outcome ‐ adverse events, Outcome 2 Conventional systemic 1 versus conventional systemic 2.
Analysis 6.3.
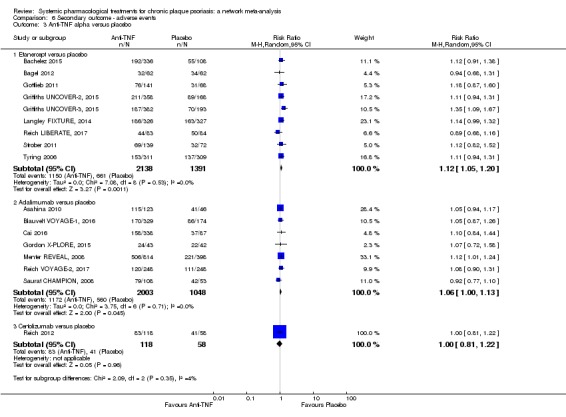
Comparison 6 Secondary outcome ‐ adverse events, Outcome 3 Anti‐TNF alpha versus placebo.
Analysis 6.4.
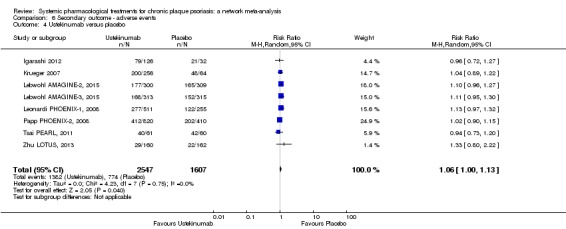
Comparison 6 Secondary outcome ‐ adverse events, Outcome 4 Ustekinumab versus placebo.
Analysis 6.5.
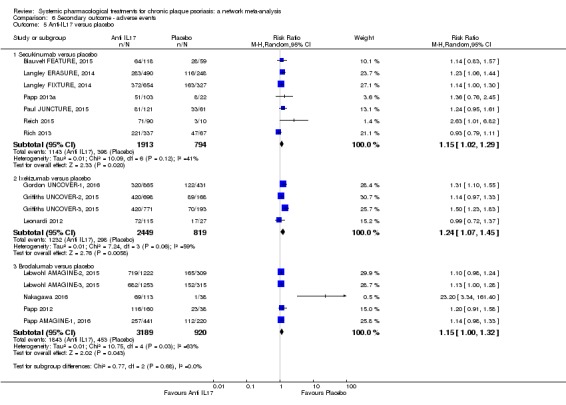
Comparison 6 Secondary outcome ‐ adverse events, Outcome 5 Anti‐IL17 versus placebo.
Analysis 6.6.
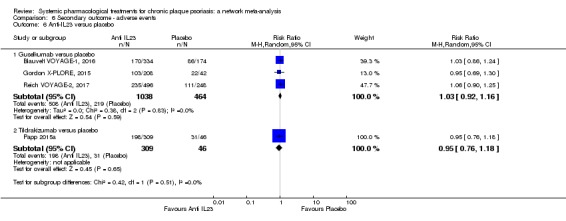
Comparison 6 Secondary outcome ‐ adverse events, Outcome 6 Anti‐IL23 versus placebo.
Analysis 6.7.
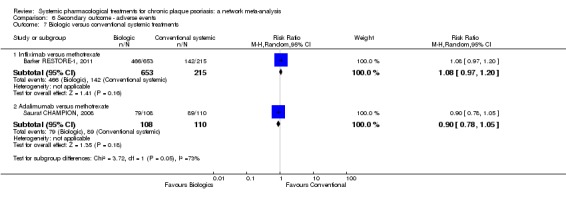
Comparison 6 Secondary outcome ‐ adverse events, Outcome 7 Biologic versus conventional systemic treatments.
Analysis 6.8.
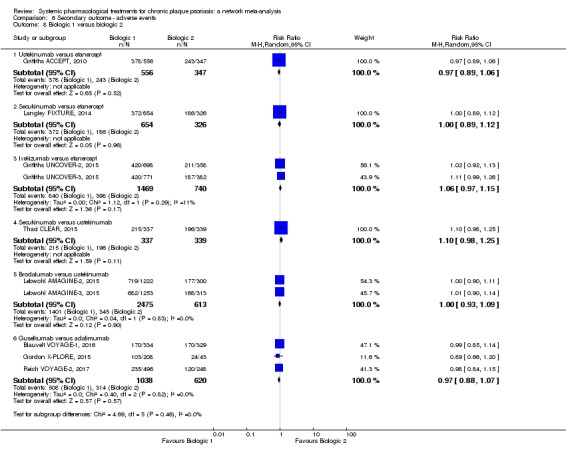
Comparison 6 Secondary outcome ‐ adverse events, Outcome 8 Biologic 1 versus biologic 2.
Analysis 6.9.
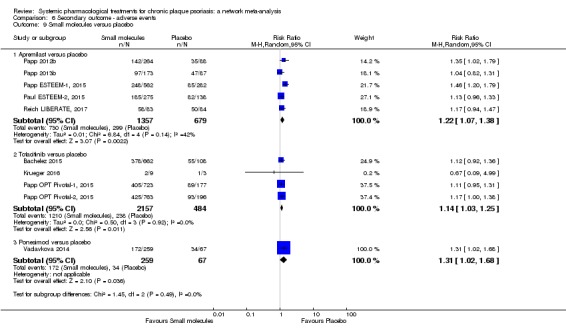
Comparison 6 Secondary outcome ‐ adverse events, Outcome 9 Small molecules versus placebo.
Analysis 6.10.
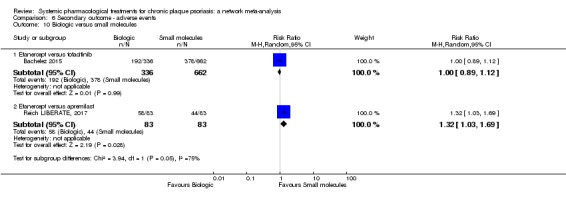
Comparison 6 Secondary outcome ‐ adverse events, Outcome 10 Biologic versus small molecules.
NETWORK META‐ANALYSES
Adverse events (AEs) outcome was available in 54 trials, involving 29,699 participants (83.8% of the participants in this review). AEs were not reported for the 36 remaining trials, and we were not able to obtain missing information from the trial authors (Table 9). This outcome was also reported in another trial (Mrowietz SCULPTURE, 2015), comparing different dosages of the same drug. Mrowietz SCULPTURE, 2015 was added to the sensitivity analyse at dose level. Thirty‐seven trials, involving 15,683 participants, were placebo‐controlled trials; five, involving 1,776 participants, were head‐to‐head comparisons; and 12, involving 12,240 participants, had both a placebo and at least two active treatments arms.
See Figure 4; Figure 5; Figure 6; Figure 8; Figure 10; Figure 11; Figure 13; and Figure 14.
We present the summary relative effects from the network meta‐analysis in league tables for both class‐level (Figure 6) and drug‐level (Figure 8) analyses. All of the interventions had a more significant risk of AEs compared to placebo. Significant associations were found: anti‐IL17 had a higher risk of AE compared with all the other interventions. Results of comparisons between each of the drugs are available in Figure 8.
Ranking class‐level analysis (Figure 10; Figure 13; Table 11)
Ranking analysis for AEs performed with SUCRA strongly suggested that placebo was associated with the best safety profile regarding all the adverse events (SUCRA 94.0). Anti‐IL23 was the best treatment at class level (versus placebo: RR 1.03, 95% CI 0.93 to 1.13; SUCRA = 78.7), followed by anti‐IL12/23 (versus placebo: RR 1.07, 95% CI 1.01 to 1.14; SUCRA = 57.0), then conventional systemic treatment (versus placebo: RR 1.08, 95% CI 0.99 to 1.17; SUCRA = 50.8). The heterogeneity τ for this network overall was 0.05, which we considered low heterogeneity.
Ranking drug‐level analysis (Figure 11; Figure 14; Table 12)
Ranking analysis for AE performed with SUCRA strongly suggested that placebo was associated with the best safety profile regarding all the adverse events (SUCRA = 88), then tildrakizumab (versus placebo: RR 0.95, 95% CI 0.76 to 1.19; SUCRA = 86.1), followed by guselkumab (versus placebo: RR 1.02, 95% CI 0.80 to 1.13; SUCRA = 78.2) and certolizumab (versus placebo: RR 1.00 95% CI 0.80 to 1.23; SUCRA = 78). The heterogeneity τ for this network overall was 0.04, which we considered low heterogeneity.
2.5. Participants with at least 1 relapse in the maintenance phase (between 52 to 104 weeks)
There were no data available for the maintenance phase.
3. Assessment of heterogeneity and inconsistency
We did not identify important heterogeneity neither in direct meta‐analyses nor in network meta‐analysis. The common outcome‐specified network heterogeneity and the prediction intervals suggested the presence of low heterogeneity for all outcomes except for quality of life, which appeared to have moderate heterogeneity. We investigated differences in heterogeneity between class‐ and drug‐level analysis, and we also investigated differences in heterogeneity between primary and sensitivity analyses for the primary outcomes (see 4. subgroup and sensitivity analyses). The results were very closed.
The distribution of some participant characteristics (age, sex ratio, weight, severity of psoriasis) did not give an indication of important differences in these characteristics across comparisons (see Figure 21; Figure 22).
Figure 21.
Distributions of age and sex ratio of participants across comparisons
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
Figure 22.
Distributions of weight of participants and PASI score at baseline across comparisons
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
At class‐level analysis, the global test for inconsistency was not significant for all of the outcomes except for PASI 75 (data not shown). At drug‐level analysis, the global test for inconsistency was not significant for all of the outcomes but only marginally non‐significant for PASI 90. Results of a global test for inconsistency, at drug level, are detailed in Figure 23 and Figure 24 for PASI 90 and SAEs, respectively. The loop‐specific and side‐splitting approaches indicated a handful of loops and comparisons with statistically significant inconsistency (Figure 25; Figure 26). This apparent inconsistency does not generally exceed however the expected level of inconsistency that has been suggested by empirical evidence (Veroniki 2013), which is about 10% of the total number of loops.
Figure 23.
Side‐splitting approach and design‐by‐treatment interaction model for inconsistency for Psoriasis Area and Severity Index (PASI) 90
Treatment codes: A = PBO, B = FUM, C = MTX, D = ACI, E = ALEFACEPT, F = CICLO, G = IFX, H = ADA, I = ETA, J = USK, K = SECU, L = IXE, M = BRODA, N = CERTO, O = APRE, P = TOFA, Q = GUSEL, R = TILDRA, S = PONE, T = ITO
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
Figure 24.
Side‐splitting approach and design‐by‐treatment interaction model for inconsistency for serious adverse events (SAEs)
Treatment codes: A = PBO, B = FUM, C = MTX, D = ACI, E = ALEFACEPT, F = CICLO, G = IFX, H = ADA, I = ETA, J = USK, K = SECU, L = IXE, M = BRODA, N = CERTO, O = APRE, P = TOFA, Q = GUSEL, R = TILDRA, S = PONE, T = ITO
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
Figure 25.
Inconsistency plots for all the outcomes at class‐level
Inconsistency factor (IF) is calculated as the risk ratio (RR)/standardised mean difference (SMD) for direct evidence over the RR/SMD for indirect evidence in the loop with its 95% confidence interval (CI). IF value close to 0 indicates the absence of evidence for disagreement between direct and indirect evidence.
AIL12/23: anti‐IL12/23; AIL17: anti‐IL17; AIL23: anti‐IL23, ATA: anti‐TNF alpha; CSA: conventional systemic agents; OB: other biologics; PBO: placebo; SM: small molecules
Figure 26.
Inconsistency plots for all the outcomes at drug level
Inconsistency factor (IF) is calculated as the risk ratio (RR)/standardised mean difference (SMD) for direct evidence over the RR/SMD for indirect evidence in the loop with its 95% confidence interval (CI). IF value close to 0 indicates the absence of evidence for disagreement between direct and indirect evidence.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
Possible explanation of this apparent inconsistency could be the differences between the previous treatment allowed across these trials: for example, participants enrolled in the Saurat CHAMPION, 2008 trial (adalimumab versus methotrexate versus placebo) were naïve to methotrexate and TNF alpha antagonists whereas participants enrolled in the Menter REVEAL, 2008 trial (adalimumab versus placebo) could have received previous systemic treatment including methotrexate.
4. Subgroup and sensitivity analyses
We had enough data for none of the aforementioned characteristics that may act as effect modifiers and therefore we were not able to run subgroup analyses and meta‐regressions to investigate their potential effect on the results.
Results of the sensitivity analyses involving the following were similar to those of the main analysis for the two primary outcomes:
excluding studies with less than 50 participants (Figure 27) (the heterogeneity τ for this subgroup network was 0.08 for PASI 90 and 0 for SAEs, which we considered low heterogeneity);
completers (Figure 28) (the heterogeneity τ for this subgroup network was 0.09 for PASI 90 and 0 for SAEs, which we considered low heterogeneity);
analyses at dose level (Figure 29) (the heterogeneity τ for this subgroup network was 0.10 for PASI 90 and 0 for SAEs, which we considered low heterogeneity); and
excluding studies at high risk of bias (Figure 30) (the heterogeneity τ for this subgroup network was 0.12 for PASI 90 and 0 for SAEs, which we considered low heterogeneity).
Figure 27.
Sensitivity analyses ‐ Interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events) for trials with at least 50 participants.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio; SAE: serious adverse events
Figure 28.
Sensitivity analyses ‐ Interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events) for the completers.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio; SAE: serious adverse events
Figure 29.
Sensitivity analyses ‐ Interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events) for all the interventions depending on the doses
MTX ≥ 15/MTX other: methotrexate ≥ 15 mg per week/methotrexate < 15 mg per week; ALEFACEPT: alefacept all dosages; CICLO High: ciclosporin ≥ 3 mg/kg/day; ACI ≥ 35: acitretin ≥ 35 mg per day; FUM: fumaric acid esters all dosages; APRE 30: apremilast 30 mg twice daily; PONE 40: ponesimod 40 mg per day; TOFA 20: tofacitinib 20 mg per day; ETA 25/ETA 50: etanercept 25 mg twice a week/etanercept 50 mg twice a week; IFX: infliximab 5 mg/kg week O, 2, 4 every 6 weeks; ADA: adalimumab 80 mg Week 0, 40 mg Week 1 then 40 mg every other week; CERTO 200/400: certolizumab all dosages; USK 45: ustekinumab 45 mg; SECU 300/SECU other: secukinumab 300 mg every injection/secukinumab other dosages; IXE 200/IXE other: ixekizumab 200 mg per injection/ixekizumab other dosages; TILDRA 100/200: tildrakizumab all dosages; GUSEL 100: guselkumab 100 mg per injection; BRODA 210: brodalumab 210 mg per injection
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio; SAE: serious adverse events
Figure 30.
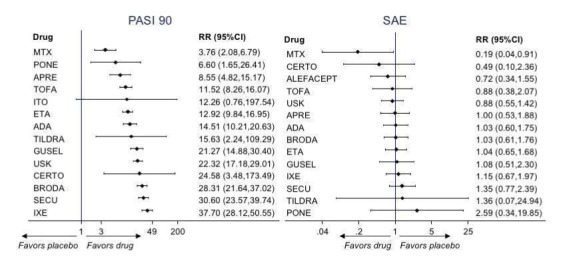
Sensitivity analyses ‐ Interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events) for all the interventions excluding studies at high risk of bias.
ACI: acitretin; ADA: adalimumab; APRE: apremilast; BRODA: brodalumab; CERTO: certolizumab; CICLO: ciclosporin; ETA: etanercept; FUM: fumaric acid; IFX: infliximab; ITO: itolizumab; IXE: ixekizumab; GUSEL: guselkumab; MTX: methotrexate; PBO: placebo; PONE: ponesimod; SECU: secukinumab; TILDRA: tildrakizumab; TOFA: tofacitinib; USK: ustekinumab
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio; SAE: serious adverse events
5. Reporting bias
The comparison‐adjusted funnel plots generally appeared symmetrical, and only the graph for quality of life presented some evidence of small‐study effects which might be caused by selective outcome reporting (Figure 31).
Figure 31.
Funnel plot for network meta‐analysis of all the outcomes
AE: adverse event; lnRR: Mean effect size; PASI: Psoriasis Area and Severity Index; QoL: Specific quality of life scale; RR: Risk ratio; SAE: serious adverse events; SMD: standardised mean difference
6. Grading of the evidence
We graded the evidence for the two primary outcomes, PASI 90 and serious adverse events, for all of the network intervention estimates according to the approach proposed by Salanti 2014. We considered five domains: study limitations (by first evaluating the risk of bias of each direct estimate (Figure 2) and then by integrating these judgements with the contribution of each direct estimate to the network estimates (Figure 32)), consistency of effect, imprecision, indirectness, and publication bias.
Figure 32.
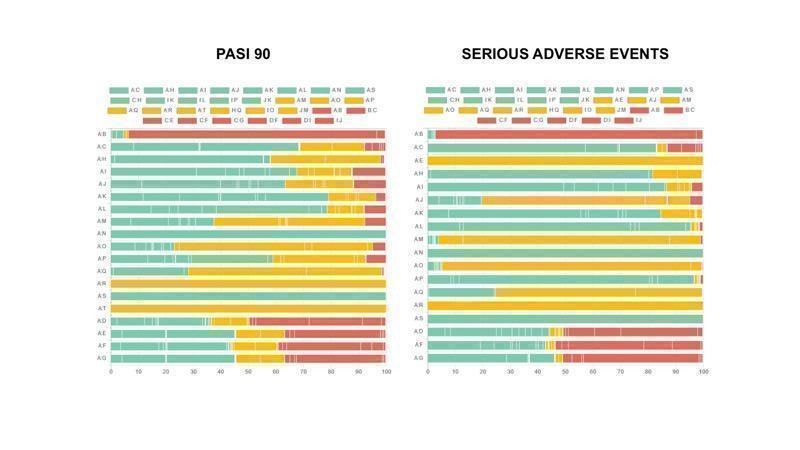
Study bias distribution for each primary outcome (PASI 90 and serious adverse events)
The following graphs show how much information (i.e. the percentage contribution of each direct comparison in the network estimates) comes from low (green), unclear/moderate (yellow) and high (red) risk of bias studies. Here we have all drugs versus placebo as it is difficult to have all comparisons due to space limitations. To evaluate the direct comparisons we used the mean level of bias of the included studies in each comparison.
We used the web application CINeMA (CINeMA 2017).
The codes of the treatments are A = Placebo, B = Fumaric acid esters, C = Methotrexate, D = Acitretin, E = Alefacept, F = Ciclosporin, G = Infliximab, H = Adalimumab, I = Etanercept, J = Ustekinumab, K = Secukinumab, L = Ixekizumab, M = Brodalumab, N = Certolizumab, O = Apremilast, P = Tofacitinib, Q = Guselkumab, R = Tildrakizumab, S = Ponesimod, T = Itolizumab
For PASI 90, we judged the confidence in the treatment estimate to be high for ixekizumab, secukinumab, and ustekinumab; moderate for brodalumab, guselkumab (reasons for downgrading: studies limitations), certolizumab (imprecision), adalimumab (inconsistency), etanercept (inconsistency), apremilast (study limitations), ponesimod (imprecision), and methotrexate (inconsistency); and low or very low for all of the other treatments (tildrakizumab, itolizumab, tofacitinib, infliximab, acitretin, ciclosporin, fumaric acid esters, alefacept). More detail on the reasons for downgrading are available in Table 1.
For serious adverse events, we judged the confidence in the treatment estimate to be low to very low for almost all of the treatment, except methotrexate, certolizumab, tofacitinib, etanercept, adalimumab, ixekizumab, secukinumab, and ponesimod, which we assessed as moderate certainty (downgrading linked to imprecision for all "moderate certainty" drugs). More detail on the reasons for downgrading are available in Table 1.
Discussion
Summary of main results
Our systematic review and meta‐analysis compared all drugs and drugs undergoing phase II/III trials used for moderate to severe psoriasis in 2017 except a new anti‐IL23 molecule (BI 655066, risankizumab).
In total, this review included 109 studies, involving 39,882 randomised adult participants, which assessed outcomes during the induction phase (less than 24 weeks after randomisation). In total, 55 trials were multiarm. Seventy‐three trials compared systematic treatment against placebo, 25 were head to head trials, and 11 had both active comparator and placebo. Fifteen trials had a co‐intervention mainly phototherapy. Finally, 79 studies declared pharmaceutical company funding, and 21 studies did not report the source of funding.
We included 74 studies (without co‐intervention and with a timing of outcome assessment from 12 to 16 weeks after randomisation (classed as induction therapy)), involving 35,454 participants (88.9% participants of this review), in the network meta‐analysis. Conventional systemic treatments, the oldest class‐level treatment (acitretin, ciclosporin, fumaric acid esters, methotrexate); anti‐TNF alpha treatments (etanercept, infliximab, adalimumab, certolizumab); an anti‐IL12/23 treatment (ustekinumab); and anti‐IL17 treatments (secukinumab, ixekizumab, brodalumab) have all been approved for psoriasis except certolizumab. And except for apremilast and alefacept, small molecule drugs (tofacitinib, ponesimod), anti‐IL23 treatments (guselkumab and tildrakizumab), and other biologics (itolizumab) had not been approved for psoriasis at the time we conducted our analyses.
All of the assessed interventions appeared superior to placebo in terms of reaching Psoriasis Area and Severity Index (PASI) 90.
At class level, network meta‐analysis showed that the biologics anti‐IL17, followed by anti‐IL12/23, anti‐IL23, and anti‐TNF alpha outperformed the small molecules and the conventional systemic agents in terms of reaching PASI 90 measured at the twelfth to the sixteenth week of treatment after randomisation, with small molecules producing a better outcome than conventional systemic agents.
The most effective drug for reaching PASI 90 when compared to placebo was ixekizumab (high‐certainty evidence), followed by secukinumab (high‐certainty evidence), brodalumab (moderate‐certainty evidence), guselkumab (moderate‐certainty evidence), certolizumab (moderate‐certainty evidence), then ustekinumab (high‐certainty evidence) (see Table 1).
At drug‐level, all of the anti‐IL17 agents and guselkumab (an anti‐IL23 drug) were significantly more effective in reaching PASI 90 than three anti‐TNF alpha agents (infliximab, adalimumab, and etanercept, but not certolizumab), and ustekinumab was superior to etanercept. No statistically significant difference was shown between infliximab, adalimumab, and etanercept. Only one trial assessed the efficacy of infliximab in this network; thus, the results involving infliximab have to be interpreted with caution. Tofacitinib was significantly superior to methotrexate, and no clear difference was shown between any of the other small molecules versus conventional treatments. The results were almost the same for the other efficacy outcome PASI 75.
No significant difference was found between all of the interventions and the placebo regarding the risk of serious adverse effects (SAEs). The relative ranking for SAEs strongly suggested that methotrexate was associated with the best safety profile regarding all the SAEs (moderate‐certainty evidence), followed by ciclosporin (very low‐certainty evidence), certolizumab (moderate‐certainty evidence), infliximab (very low‐certainty evidence), alefacept (low‐certainty evidence), then fumaric acid esters (FAEs) (very low‐certainty evidence). Major adverse cardiac events, serious infections, or malignancies (see Table 13) were reported in both placebo and intervention groups.
Information on quality of life was often poorly reported and was absent for a third of the interventions.
Finally, considering both efficacy (PASI 90 outcome) and acceptability (SAE outcome), highly effective treatments had also more SAE than the other treatments, and ustekinumab, infliximab, and certolizumab appeared to be the better compromise between efficacy and acceptability (bearing in mind the limitations that affect interpretation of the SAE results, such as the very low number of events on which the results were based, with just over half of the treatment estimates being based on low to very low certainty evidence (the rest moderate)).
Overall completeness and applicability of evidence
We were able to draw some conclusions on the effectiveness (and ranking) of the systemic treatment options for moderate to severe chronic plaque psoriasis during the induction phase. Long‐term efficacy and safety data are lacking. Specific details are listed below.
Participants
Participants in the included studies had a mean age of 44 years and had moderate to severe psoriasis with an overall mean PASI score at baseline of 20 (range: 9.5 to 39). This young age and the high level of disease severity may not be typical of patients seen in daily clinical practice especially for patients who need a first‐line systemic treatment. In addition, patients selected for randomised controlled trials (RCTs) generally have few major comorbidities. Almost all studies including one biological arm excluded patients with history of infectious diseases or malignancies and signs of severe renal, cardiac, hepatic, demyelinating, or other disorders. This may impact the generalisibility of these results for clinical practice. However, some participants characteristics (such as being overweight, imbalanced sex ratio in favour of males, presence of metabolic syndrome) were reflective of a moderate to severe psoriasis population, comparable to literature data (Wolkenstein 2009).
Interventions
Evidence on 19 treatments included in this review was derived from 74 trials (searched for up to December 2016). We included all interventions irrespective of the dose. Thus, we increased the number of available RCTs per intervention and had more power to assess SAEs and adverse events (AEs). The number of studies was still low for the following interventions: certolizumab, tildrakizumab, itolizumab, infliximab, ponesimod, acitretin, ciclosporin, alefacept, fumaric acid, and methotrexate, meaning we must be cautious of the conclusions drawn for these drugs. In terms of efficacy, the results from the subgroup analysis using a standard dose for each intervention was similar for PASI 90 (and SAE) compared to the main analyses, making us confident with the results of the main analysis.
For drugs just approved or not yet approved for psoriasis, ongoing studies are still investigating guselkumab, tildrakizumab, a third anti‐IL23 (risankizumab, which will be included in the next update of this review), certolizumab, tofacitinib, and itolizumab (Characteristics of ongoing studies). Ponesimod development in psoriasis is most uncertain and should be excluded from the next update of this review.
Comparisons
The majority of the studies included in the review were placebo‐controlled (around 70%) as were the identified ongoing studies. Once the benefit of a treatment has been established against placebo using high‐quality evidence, only head‐to‐head trials would be helpful to provide physicians with efficacy estimates between the different biologics based on stronger evidence than indirect comparisons.
Outcomes
Many of the trials included in this review provided evidence for the proportion of participants who reached PASI 90, PASI 75, or Physician Global Assessment (PGA) 0/1 or who experienced SAE or AE. On the other hand, patient‐reported outcome (PRO) data were scanty and poorly reported. Moreover, the heterogeneity of the scales used for PRO in psoriasis trials required using the standardised mean difference in the network. So, from a clinical point of view, the interpretation of the results was difficult: a significant result for PRO between two drugs did not mean that the result was clinically useful for the patients.
Timing
All of the included trials assessed the efficacy of the different treatments during the induction treatment phase (less than 24 weeks, with evidence in the network meta‐analysis (NMA) assessed 12 to 16 weeks after randomisation). This is an unwelcome finding for a chronic disease. The trials were designed to detect differences in the severity of psoriasis in response to therapy over short periods of treatment and are often underpowered and of insufficient duration to detect rare or long‐term adverse events. Therefore, it is of interest to conduct studies taking into account the induction of remission but also the long‐term management (long‐term remission) and the long‐term safety of the drug. In order to provide long‐term information on the safety of the treatments included in this review, it will be necessary to also evaluate non‐randomised studies and postmarketing reports released from regulatory agencies.
Quality of the evidence
Overall, we judged the confidence in the treatment estimate for PASI 90 to be high or moderate for anti‐IL17 agents, anti‐IL12/23 agents, anti‐IL23 agents, anti‐TNF alpha agents (except infliximab), methotrexate, and apremilast. We judged the confidence in treatment estimate for PASI 90 as low or very low certainty for most of the comparisons involving conventional systemic agents (except for methotrexate), infliximab, other biologics, and tofacitinib; we downgraded the certainty of the evidence due to risk of bias and then either for inconsistency or imprecision. We judged the confidence in the treatment estimate for SAEs to be low to very low certainty for half of the treatment estimates, moderate for the others; we downgraded the certainty of the evidence due to imprecision and risk of bias.
Risk of bias
The risk of bias in included studies appeared to be globally moderate to low (Figure 2; Figure 3). However, some limitations should be discussed.
There was variation in how well the studies took measures to blind investigators and participants: a third of trials in this systematic review were considered at high or unclear risk of performance bias (35 out of 109). This is an important point to highlight as the outcomes used for assessing efficacy were subjective. However, the proportion of trials at high risk of blinding used in the network meta‐analyses decreased to 15% (13 out of 74).
The reporting of missing outcome data was largely inadequate in a few studies. Since we chose a likely scenario that any participant with missing outcome data did not experience clearance for the overall analyses, the risk of overestimating efficacy due to how we reported missing data was minimised.
Finally, a few trials were considered at high risk of selective outcome reporting. However, we chose a stringent definition of studies at high risk of selective outcome reporting: we considered reporting bias inadequate if one specified outcome in protocols was lacking in the main report. A large proportion of included trials did not report the PRO outcomes in the main report but only in slicing publications (see Included studies). We extracted outcomes of interest both in main and slicing publications, but this disadvantaged trials that did not report all of the specified outcomes in the main report.
Indirect comparison and network meta‐analyses as standard pair‐wise meta‐analyses provide "observational" evidence since the treatments being compared have not been randomised across studies. However, we considered carefully the assumption underpinning the validity of indirect comparisons to reassure a sufficiently coherent evidence base (Cipriani 2013). The limitations of this review are reflected by the GRADE evaluations.
Heterogeneity (i.e. variation in effect modifiers within comparisons) and inconsistency (imbalance in effect modifiers between comparisons)
No evidence of the presence of heterogeneity either in direct comparisons or in the entire networks was found except in relation to the quality of life outcome (poorly reported, few studies per comparisons). There was no global inconsistency for the two primary outcomes, and the global test for inconsistency was significant only for PASI 75 at the class‐level analysis. According to the local tests, for each outcome, a handful of loops and comparisons, which does not exceed the expected level of inconsistency from empirical evidence (Veroniki 2013), appeared to have important inconsistency. Thus, we downgraded the strength of evidence for inconsistency for methotrexate, adalimumab, etanercept, infliximab, and tofacitinib.
Imprecision
The number of studies was low for the following interventions (one to two studies per interventions): certolizumab, tildrakizumab, itolizumab, infliximab, ponesimod, acitretin, ciclosporin, alefacept, fumaric acid, and methotrexate. We downgraded the strength of evidence for imprecision for all of these interventions for the two primary outcomes.
Indirectness or transitivity assumption
We did not find any evidence that important variables, such as age, sex, weight, and duration and severity of psoriasis, varied across comparisons (see Characteristics of included studies and Figure 31 and Figure 32). However, several comparisons had only one or two studies, and the lack of data did not allow us to check the distributions of previous treatments across comparisons; thus, transitivity cannot be assessed statistically properly.
Several participant characteristics have changed in newer trials, such as participants' exclusion criteria. However, most of the included trials were conducted after 2000, minimising the variability across trial participant characteristics. The location of the trial could also create some differences between participants as the response of treatment could be related to genetic background (Chiu 2014). To further reassure the plausibility of the transitivity assumption, we only included in our analyses trials not involving co‐interventions. Moreover, the trials were also fairly similar in terms of outcome assessment (less than 24 weeks). As a consequence, we excluded from the meta‐analyses most of the trials assessing infliximab efficacy. Indeed, timing of efficacy outcome assessments was from 8 to 10 weeks for infliximab trials. However, as differences in response rates of biologics for the treatment of psoriasis have been reported in several meta‐analyses published to date mainly related to the primary endpoint times, we assumed the importance of a similar timing of outcome assessment between trials (Puig 2014). Thus, the possibility of intransitivity seems to be unlikely even if it could not be totally excluded.
Publication bias
We assessed publication bias considering the comprehensive search strategy we performed and the risk for publication bias in the specific field. The comparison‐adjusted funnel plot for all placebo‐controlled trials for all the outcomes did not indicate any evident risk of publication bias for the two primary outcomes.
Potential biases in the review process
We performed a wide search for trials, including five trials registers and databases of each company when available, and we searched the U.S. Food and Drug Administration and the European Medicines Agency (EMA) databases and abstract proceedings of seven congresses up to a maximum of 10 years. We did not approach pharmaceutical companies for additional data when their databases were not open access, and it is possible that additional data from this source could contribute to this review. The probability that we missed a trial is low considering our wide search and is supported by the absence of small‐study effects (testing by the comparison‐adjusted funnel plots). However, the fact that 14 studies have not yet been incorporated may be a source of potential bias.
Study selection, data extraction, and 'Risk of bias' assessments were done in duplicate and independently, and we reached consensus by discussing any discrepancies. Some published trial reports did not provide enough details to extract outcomes and adequately assess risk of bias, especially studies performed before 2000 (e.g. before the International Committee of Medical Journal Editors issued the requirement of trial registration for publication). However, we contacted the authors of the trials to request missing data, but we cannot avoid some biased assessment in the review process due to incomplete reporting of trial details, results, or both.
We had some departures from the protocol plans (see Differences between protocol and review). After protocol publication, we added five biological drugs either approved for psoriasis or for which there were ongoing phase 3 trials. We chose to keep only PASI 90 as the primary efficacy outcome and not a composite outcome including PASI and Physician Global Assessment (PGA). Indeed, PASI 90 and PGA do not reflect the same measures (see Agreements and disagreements with other studies or reviews). To minimise inconsistency, we assessed the primary outcome between the twelth and the sixteenth week rather than less than 24 weeks.
Agreements and disagreements with other studies or reviews
We searched in MEDLINE Ovid (from 1946) using the strategy "Psoriasis" AND "Meta‐analysis" for already published network meta‐analyses. Seven network meta‐analyses were systematically reviewed and have compared the short‐term efficacy of treatments for moderate to severe psoriasis (Gomez‐Garcia 2017; Gupta 2014; Jabbar‐Lopez 2017; Lin 2012; Reich 2012a; Schmitt 2014;Signorovitch 2015).
We compared our findings with the four most recent network meta‐analyses (Gomez‐Garcia 2017; Jabbar‐Lopez 2017; Schmitt 2014;Signorovitch 2015). Schmitt 2014 included 48 trials, involving 16,696 participants, assessing both conventional systemic (ciclosporin, methotrexate, acitretin, FAEs) and biologic treatments (infliximab, adalimumab, etanercept, alefacept, and ustekinumab). Signorovitch 2015 included 15 trials, involving 7388 participants, assessing only anti‐TNF alpha agents (infliximab, adalimumab, etanercept) and anti‐IL12/23 drugs (ustekinumab). Gomez‐Garcia 2017 included 27 trials, involving 10,629 participants, assessing three anti‐TNF alpha agents (infliximab, etanercept, and adalimumab), one anti‐IL12/23 agent (ustekinumab), and one anti‐IL17 agent (secukinumab). Jabbar‐Lopez 2017 included 41 trials, involving 20,561 participants, assessing the same drugs as Gomez‐Garcia 2017, plus ixekizumab (another anti‐IL17 agent) and methotrexate.
Thus, compared to previous reviews, we included more interventions and consequently more trials (n = 109) and participants (n = 39,882). Regarding the overlapping period, we also included more trials than the other meta‐analyses. Indeed, we performed a larger search in terms of the number of databases used, including trials registers and other resources (unpublished literature), irrespective of the date or language limitations.
Schmitt 2014 and Signorovitch 2015 chose PASI 75 as primary outcome during the induction phase (less than 16 weeks); however, data on PASI 90 were also available. Gomez‐Garcia 2017 presented both PASI 75 and PASI 90 results. Finally, Jabbar‐Lopez 2017 chose a composite outcome: PASI 90 or Physician Global Assessment (PGA) 1. We chose PASI 90 as our primary efficacy outcome because complete clearance seems the less subjective outcome and the most relevant regarding patient expectation in short‐term assessment (induction phase). The composite outcome used by Jabbar‐Lopez 2017 did not reflect complete or almost complete clearance. Indeed, PGA 1 is highly correlated to PASI 75 and not PASI 90, which could lead to a classification bias (Robinson 2012).
Jabbar‐Lopez 2017 presented their results using number needed to treat (NNT). Although NNT is an easily understandable and very useful measure for patients and clinicians, it can be misleading in a network meta‐analysis since it requires the assumption of a common average control group risk applying to all studies. This is a rather strong assumption particularly in networks involving also head‐to‐head studies without a control group as here.
Infliximab was the best drug in terms of reaching PASI 75 in the network meta‐analyses of Schmitt 2014 and Signorovitch 2015. Adalimumab and ustekinumab were more likely to reach PASI 75 than etanercept. These last results are partly confirmed by our review: ustekinumab was more effective at reaching both PASI 75 and 90 than etanercept; however, no significant difference was shown between adalimumab and etanercept, as in the most recent network meta‐analyses from Gomez‐Garcia et al (Gomez‐Garcia 2017) and Jabbar‐Lopez et al (Jabbar‐Lopez 2017). Infliximab was also the most effective drug in Gomez‐Garcia 2017, without significant difference between infliximab and secukinumab. Infliximab was ranked in third place after ixekizumab and secukinumab in Jabbar‐Lopez 2017, without significant difference between infliximab and secukinumab. Our findings did not find such efficacy for infliximab. To prevent inconsistency in our network meta‐analaysis, we chose to include trials assessing outcomes between 12 to 16 weeks. Thus, only one trial, Barker RESTORE‐1, 2011, which compared infliximab versus methotrexate, was taken into account for this intervention. Regarding the four previous network meta‐analyses, two did not assess inconsistency (Schmitt 2014 ; Signorovitch 2015), and two reported significant global and local inconsistency for PASI 75, which preclude interpretation of their results (Gomez‐Garcia 2017; Jabbar‐Lopez 2017).
Authors' conclusions
In terms of achieving PASI 90 with induction therapy (evaluation between 12 to 16 weeks after the randomisation), we found the following results.
At class level, all of the assessed interventions (conventional systemic agents, small molecules, and biological treatments) showed significant superiority compared with placebo.
The biologic treatments anti‐IL17, anti‐IL12/23, anti‐IL23, and anti‐TNF alpha showed significant superiority compared with small molecules and the conventional systemic agents, with small molecules achieving better results than conventional systemic agents.
All of the anti‐IL17 agents and the anti‐IL23 guselkumab were significantly more effective than all of the anti‐TNF alpha agents except for certolizumab (i.e. infliximab, adalimumab, and etanercept), and the anti‐IL12/23 ustekinumab was superior to the anti‐TNF alpha etanercept.
When compared with placebo, in order of highest efficacy, the following biological agents are the best choices: ixekizumab (high‐certainty evidence), secukinumab (high‐certainty evidence), brodalumab (moderate‐certainty evidence), guselkumab (moderate‐certainty evidence), certolizumab (moderate‐certainty evidence), and ustekinumab (high‐certainty evidence).
Tofacitinib was superior to methotrexate, and no difference was shown between the other small molecules and the conventional drugs.
Regarding the other efficacy outcome (PASI 75), the results were very similar to the results for PASI 90.
In terms of serious adverse events, there was no significant difference between all of the assessed interventions and placebo. The surface under the cumulative ranking curve (SUCRA) strongly suggested that methotrexate had the best safety profile regarding the serious adverse events (SAEs) (moderate‐certainty evidence), followed by ciclosporin (very low‐certainty evidence), certolizumab (moderate‐certainty evidence), infliximab (very low‐certainty evidence), alefacept (low‐certainty evidence), and FAEs (very low‐certainty evidence). Major adverse cardiac events, serious infections, or malignancies were reported in both placebo and intervention groups. Nevertheless, analyses on SAE events were based on a very low number of events with a low to very low certainty for just over half of the treatment estimates in total, moderate for the others. Thus, the results have to be considered with caution.
Considering both efficacy (PASI 90 outcome) and acceptability (SAE outcome), highly effective treatments also had more SAEs than the other treatments: ustekinumab, infliximab, and certolizumab appeared to be the better compromise between efficacy and acceptability.
Information on quality of life was often poorly reported and was absent for a third of the interventions.
Conservative interpretation is warranted with regard to the results for conventional systemic agents, as well as ponesimod, tildrakizumab, infliximab, certolizumab, alefacept, and itolizumab as these drugs have been evaluated in few trials. The evidence is limited to a selected trial population (participants were young (mean age of 44 years) and had a high level of disease severity (with an overall mean score of PASI 20 at baseline)) and to the induction treatment phase (for the NMA results, measurement was done 12 to 16 weeks after randomisation, but all results were measured less than 24 weeks after randomisation), which is not relevant enough for a chronic disease, which would require long‐term treatment.
Our main results (i.e. superiority of efficacy of the biologic treatments anti‐IL17, anti‐IL12/23, anti‐IL23, and anti‐TNF alpha compared with small molecules and the conventional systemic agents, with small molecules achieving better results than conventional systemic agents) do not reflect the way patients are managed in "real‐life". Currently, biological treatments have been positioned as third‐line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents). Recently, the same restrictions were applied to apremilast. Such decisions were based on the lack of long‐term safety knowledge but also taking into account economic consideration. In this review, we found insufficient evidence to evaluate long‐term safety, and we did not address economic considerations; thus, the question of the choice of the first‐line treatment for moderate to severe psoriasis is still debated.
The first choice in conventional systemic agents is still in question as the limited number of trials assessing conventional systemic agents did not allow us to draw robust conclusions; this is also true for some small molecule treatments and biological treatments.
From a clinical point of view, we need drugs that can be administered long term to provide continuous effective control, because continued remission after successful treatment is as important as successful induction of remission. Moreover, treatment should be easy to use, well accepted by patients, have minimal drug to drug interactions, and should have minimal monitoring requirements because convenience is also an important issue when dealing with chronic diseases that require prolonged treatments. Finally, the cost of the drug should be affordable by most patients and by any national health service.
Specific questions and issues in the management of psoriasis still remain unmet:
Which conventional systemic agents have the best benefice/risk balance?
Which patients are candidates for small molecule treatment?
Which treatments work for subgroups of patients (age, psoriasis severity, previous treatment, psoriatic arthritis)?
Adjustment of therapy for patients with stable low disease activity.
Add‐on therapy or switching for patients who failed with a systemic treatment.
Long‐term safety data for all the treatments.
1. Future trials need to ensure the following.
Participants: enough information about participants is needed to enable systematic subgroup analyses for biological‐naïve patients (or conventional systemic agent‐naïve); future trials also need to provide an adequate description of data regarding other important potential effect modifiers such as previous systemic treatments, whether participants are overweight/obese, the duration of a participant's psoriasis, baseline psoriasis severity (efficacy differences could be expected for patients with PASI at 10 and patients with PASI at 40); and presence of psoriatic arthritis.
Interventions: high‐quality trials assessing the efficacy of conventional systemic agents are needed.
Comparators: once the benefit of a treatment has been established against placebo, only head‐to‐head trials would be helpful to provide physicians efficacy estimates between the different biologics with a stronger evidence than indirect comparisons. Thus, head‐to‐head comparisons are lacking between the conventional systemic agents and small molecules and against themselves. More head‐to‐head comparisons between biological agents are also needed (anti‐IL17 versus anti‐IL23, anti‐IL23 versus anti‐IL12/23, anti‐TNF alpha versus anti‐IL12/23).
Outcomes: outcome measure harmonisation is needed for psoriasis as it has been done for eczema by the COMET (Core Outcome Measures in Effectiveness Trials) Initiative.
Timing assessment strategy: all of the trials included in this review were limited to the induction phase (less than 24 weeks). Long‐term efficacy data is critical for chronic diseases. Placebo‐controlled long‐term trials would not be ethical due to the suffering it would entail for the people in the placebo group. However, long‐term studies comparing different drugs would be ethical and informative. Such long‐term trials could also assess the adjustment of therapy for patients with stable cleared psoriasis.
2. New trial designs are needed such as pragmatic trials that permitted dose adjustment once in remission, switching, and additional treatments (i.e. adding two or more systemic treatments) as per normal clinical practice. All of this unmet medical need evidence would improve the management of the condition.
3. Finally, evidence‐based decision making and management of chronic plaque psoriasis request both efficacy AND safety data. As we already know, the limitations of network meta‐analysis and in the same way the limitations of randomised clinical trials (included in these meta‐analyses) means we cannot reliably interpret the significance of rare events given their current design. Actually, these studies are designed to detect differences in the severity of psoriasis in response to therapy over short periods of treatment and are often underpowered and of insufficient duration to detect rare or long‐term adverse events. One way to counter this is to include observational cohort studies/registries in a network observational meta‐analysis.
Acknowledgements
The Cochrane Skin editorial base wishes to thank Gloria Sanclemente, who was the Cochrane Dermatology Editor for this review; Ben Carter, who was the Statistical Editor; the clinical referees, Tamar Nijstein and Steven Feldman; and Denise Mitchell who copy‐edited sections of this review.
We would like to thank Dr Ibrahim Yaylali from Cochrane Oral Health for his translation of Gurel 2015 from Turkish into English.
We would like to thank Professors Rintaro Mori and Erika Ota from St Luke's International University, Graduate School of Nursing Science, Tokyo, Japan, for their translation of Rinsho Iyaku 1991 from Japanese into English.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group, and was also supported by the Complex Reviews Support Unit, also funded by the National Institute for Health Research (project number 14/178/29).
Department of Health Disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, the Complex Reviews Support Unit, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. CRS/Cochrane Skin Group Specialised Register search strategy
(Psoria* or “palmoplantar* pustulosis” or “pustulosis palmaris et plantaris” or (pustulosis and palms and soles)) and (methotrexate* or amethopterin or mtx or mexate or fumar* or dimethylfumarate or fae or dmf or fumaderm or acitretin or tegison or soriatane or neotigason or ((oral or orally or systemic) and retinoid*) or isotretinoin or accutane or etretin* or ustekinumab or stelara or secukinumab or “CNTO 1275” or “cdp571” or etanercept* or enbrel or adalimumab* or d2e7 or humira or golimumab or simponi or briakinumab or “ABT‐874” or “psoralen uva” or ciclosporin or cyclosporine or cyclosporine or alefacept or brodalumab or ixekizumab or phototherap* or ultraviolet or PUVA or photochemotherap* or photodynamic or “light therap*” or photoradiation or “broad band uvb” or “broad band ultraviolet b” or “narrow band uvb” or “narrow band ultraviolet b” or BBUVB or NBUVB or BB‐UVB or NB‐UVB or infliximab* or “monoclonal antibod*” or remicade or interleukin* or “anti tumour necrosis factor” or “anti tumor necrosis factor” or “tumour necrosis factor antibod*” or “tumor necrosis factor antibod*” or “tnf antibod*” or “tnf alpha antibod*” or “anti tnf” or “immunoglobulin fab fragment*” or “p40 subunit” or “tumor necrosis factor*” or tnf or “antitumor necrosis factor*” or “antitumour necrosis factor*” or ampremilast or ponesimod or guselkumab or tofacitinib or itolizumab or certolizumab or tildrakizumab)
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Library, search strategy
#1MeSH descriptor: [Psoriasis] this term only #2psoria*:ti,ab,kw #3palmoplantar* pustulosis:ti,ab,kw #4pustulosis palmaris et plantaris:ti,ab,kw #5pustulosis and palms and soles:ti,ab,kw #6#1 or #2 or #3 or #4 or #5 #7MeSH descriptor: [Methotrexate] explode all trees #8MeSH descriptor: [Fumarates] explode all trees #9MeSH descriptor: [Etretinate] explode all trees #10MeSH descriptor: [Acitretin] explode all trees #11MeSH descriptor: [Isotretinoin] explode all trees #12MeSH descriptor: [Retinoids] explode all trees #13MeSH descriptor: [Antibodies, Monoclonal] explode all trees #14MeSH descriptor: [Interleukin‐12] explode all trees #15MeSH descriptor: [Interleukin‐23] explode all trees #16MeSH descriptor: [Interleukin‐12 Subunit p40] explode all trees #17MeSH descriptor: [Tumor Necrosis Factors] explode all trees #18MeSH descriptor: [Tumor Necrosis Factor‐alpha] explode all trees #19MeSH descriptor: [Receptors, Tumor Necrosis Factor, Type II] explode all trees #20MeSH descriptor: [Receptors, Tumor Necrosis Factor] explode all trees #21MeSH descriptor: [Receptors, Tumor Necrosis Factor, Type I] explode all trees #22MeSH descriptor: [TNF‐Related Apoptosis‐Inducing Ligand] explode all trees #23MeSH descriptor: [Antibodies, Monoclonal] explode all trees #24MeSH descriptor: [Immunoglobulin Fab Fragments] explode all trees #25MeSH descriptor: [Phototherapy] explode all trees #26MeSH descriptor: [Ultraviolet Therapy] explode all trees #27MeSH descriptor: [PUVA Therapy] explode all trees #28MeSH descriptor: [Photochemotherapy] explode all trees #29MeSH descriptor: [Cyclosporine] explode all trees #30(methotrexate* or amethopterin or mtx or mexate or fumar* or dimethylfumarate or fae or dmf or fumaderm or acitretin or tegison or soriatane or neotigason or ((oral or orally or systemic) and retinoid*) or isotretinoin or accutane or etretin* or ustekinumab or stelara or secukinumab or "CNTO 1275" or "cdp571" or etanercept* or enbrel or adalimumab* or "d2e7" or humira or golimumab or simponi or briakinumab or "ABT‐874" or "psoralen uva" or ciclosporin or cyclosporine or cyclosporine or alefacept or brodalumab or ixekizumab or phototherap* or ultraviolet or PUVA or photochemotherap* or photodynamic or "light therap*" or photoradiation or "broad band uvb" or "broad band ultraviolet b" or "narrow band uvb" or "narrow band ultraviolet b" or BBUVB or NBUVB or BB‐UVB or NB‐UVB or infliximab* or "monoclonal antibod*" or remicade or interleukin* or "anti tumour necrosis factor" or "anti tumor necrosis factor" or "tumour necrosis factor antibod*" or "tumor necrosis factor antibod*" or "tnf antibod*" or "tnf alpha antibod*" or "anti tnf" or "immunoglobulin fab fragment*" or "p40 subunit" or "tumor necrosis factor*" or tnf or "antitumor necrosis factor*" or "antitumour necrosis factor*" or ampremilast or ponesimod or guselkumab or tofacitinib or itolizumab certolizumab or tildrakizumab):ti,ab,kw #31{or #7‐#30} #32#6 and #31
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Psoriasis/ or psoria$.ti,ab. 2. palmoplantar$ pustulosis.ti,ab. 3. pustulosis palmaris et plantaris.ti,ab. 4. (pustulosis and palms and soles).ti,ab. 5. 1 or 2 or 3 or 4 6. exp Methotrexate/ 7. methotrexate$.mp. 8. amethopterin.mp. 9. mtx.ti,ab. 10. mexate.mp. 11. exp Fumarates/ 12. (fumar$ and esters).mp. 13. dimethylfumarate.mp. 14. fae.ti,ab. 15. dmf.ti,ab. 16. fumarate$1.mp. 17. fumaderm.mp. 18. Etretinate/ 19. Acitretin/ 20. Tegison.mp. 21. (Soriatane or Neotigason).mp. 22. ((oral or orally or systemic) and retinoid$).ti,ab. 23. Isotretinoin/ 24. Accutane.mp. 25. isotretinoin.ti,ab. 26. etretin$.mp. 27. acitretin.mp. 28. Retinoids/ 29. Ustekinumab.mp. 30. stelara.mp. 31. secukinumab.mp. 32. apremilast.mp. 33. ponesimod.mp. 34. guselkumab.mp. 35. tofacitinib.mp. 36. itolizumab.mp. 37. CNTO 1275.mp. 38. exp antibodies, monoclonal/ 39. monoclonal antibod$.mp. 40. exp Interleukin‐23/ or exp Interleukin‐12/ 41. exp Interleukin‐12 Subunit p40/ or p40 subunit.mp. 42. exp Tumor Necrosis Factors/ or exp Tumor Necrosis Factor‐alpha/ or exp Receptors, Tumor Necrosis Factor, Type II/ or exp Receptors, Tumor Necrosis Factor/ or exp Receptors, Tumor Necrosis Factor, Type I/ or exp TNF‐Related Apoptosis‐Inducing Ligand/ 43. (anti tumour necrosis factor or anti tumor necrosis factor).mp. 44. (tumor necrosis factor‐alpha or tumour necrosis factor‐alpha).mp. 45. anti tnf.mp. 46. (tnf antibod$ or tnf alpha antibod$).mp. 47. (tumour necrosis factor antibod$ or tumor necrosis factor antibod$).mp. 48. (antitumor necrosis factor or antitumour necrosis factor).mp. 49. exp Immunoglobulin Fab Fragments/ 50. (infliximab$ or monoclonal antibody cA2 or remicade).mp. 51. cdp571.mp. 52. (etanercept$ or enbrel).mp. 53. (adalimumab$ or d2e7 or humira).mp. 54. (golimumab or simponi).mp. 55. (Briakinumab or ABT‐874).mp. 56. exp Phototherapy/ 57. exp Ultraviolet Therapy/ 58. exp PUVA Therapy/ 59. exp Photochemotherapy/ 60. photodynamic therap$.mp. 61. phototherap$.mp. 62. photochemotherap$.mp. 63. puva.mp. 64. ultraviolet.mp. 65. light therap$.mp. 66. photoradiation therap$.mp. 67. BBUVB.mp. 68. NBUVB.mp. 69. BB‐UVB.mp. 70. NB‐UVB.mp. 71. broad band uvb.mp. 72. broad band ultraviolet b.mp. 73. narrow band uvb.mp. 74. narrow band ultraviolet b.mp. 75. psoralen ultraviolet a.mp. 76. psoralen uva.mp. 77. Cyclosporine/ 78. (Ciclosporin or cyclosporine or cyclosporin).mp. 79. alefacept.mp. 80. brodalumab.mp. 81. ixekizumab.mp. 82. certolizumab.mp. 83. tildrakizumab.mp. 84. or/6‐83 85. randomized controlled trial.pt. 86. controlled clinical trial.pt. 87. randomized.ab. 88. placebo.ab. 89. clinical trials as topic.sh. 90. randomly.ab. 91. trial.ti. 92. 85 or 86 or 87 or 88 or 89 or 90 or 91 93. exp animals/ not humans.sh. 94. 92 not 93 95. 5 and 84 and 94
[Lines 85‐94: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. exp PSORIASIS/ 2. psoria$.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] 3. palmoplantar$ pustulosis.mp. 4. pustulosis palmaris et plantaris.mp. 5. (pustulosis and palms and soles).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] 6. 1 or 2 or 3 or 4 or 5 7. methotrexate/ 8. methotrexate$.ti,ab. 9. amethopterin.ti,ab. 10. mtx.ti,ab. 11. mexate.ti,ab. 12. fumaric acid derivative/ 13. (fumar$ and esters).ti,ab. 14. dimethylfumarate.ti,ab. 15. fae.ti,ab. 16. dmf.ti,ab. 17. fumarate$1.ti,ab. 18. fumaderm.ti,ab. 19. etretinate/ 20. acitretin.ti,ab. 21. tegison.ti,ab. 22. (Soriatane or Neotigason).ti,ab. 23. ((oral or orally or systemic) and retinoid$).ti,ab. 24. isotretinoin/ 25. isotretinoin.ti,ab. 26. Accutane.ti,ab. 27. etretin$.ti,ab. 28. retinoid/ 29. ustekinumab.ti,ab. 30. ustekinumab/ 31. stelara.ti,ab. 32. secukinumab/ 33. secukinumab.ti,ab. 34. ampremilast.ti,ab. 35. ponesimod/ 36. ponesimod.ti,ab. 37. guselkumab/ 38. guselkumab.ti,ab. 39. tofacitinib/ 40. tofacitinib.ti,ab. 41. itolizumab/ 42. itolizumab.ti,ab. 43. "CNTO 1275".ti,ab. 44. monoclonal antibod$.ti,ab. 45. exp monoclonal antibody/ 46. interleukin 23/ 47. interleukin 12/ 48. interleukin 12p40/ 49. p40 subunit.ti,ab. 50. exp tumor necrosis factor/ 51. tumor necrosis factor alpha/ 52. tumor necrosis factor receptor 2/ 53. tumor necrosis factor receptor/ 54. tumor necrosis factor related apoptosis inducing ligand/ 55. (anti tumour necrosis factor or anti tumor necrosis factor).ti,ab. 56. (tumor necrosis factor‐alpha or tumour necrosis factor‐alpha).ti,ab. 57. anti tnf.ti,ab. 58. (tnf antibod$ or tnf alpha antibod$).ti,ab. 59. (tumour necrosis factor antibod$ or tumor necrosis factor antibod$).ti,ab. 60. (antitumor necrosis factor or antitumour necrosis factor).ti,ab. 61. "immunoglobulin F(ab) fragment"/ 62. (infliximab$ or monoclonal antibody cA2 or remicade).ti,ab. 63. cdp571.ti,ab. 64. (etanercept$ or enbrel).ti,ab. 65. (adalimumab$ or d2e7 or humira).ti,ab. 66. (golimumab or simponi).ti,ab. 67. (Briakinumab or ABT‐874).ti,ab. 68. exp phototherapy/ 69. PUVA/ 70. photochemotherapy/ 71. photodynamic therap$.ti,ab. 72. phototherap$.ti,ab. 73. photochemotherap$.ti,ab. 74. puva.ti,ab. 75. ultraviolet.ti,ab. 76. light therap$.ti,ab. 77. photoradiation therap$.ti,ab. 78. BBUVB.ti,ab. 79. NBUVB.ti,ab. 80. BB‐UVB.ti,ab. 81. NB‐UVB.ti,ab. 82. broad band uvb.ti,ab. 83. broad band ultraviolet b.ti,ab. 84. narrow band uvb.ti,ab. 85. narrow band ultraviolet b.ti,ab. 86. psoralen ultraviolet a.ti,ab. 87. psoralen uva.ti,ab. 88. cyclosporin/ 89. (Ciclosporin or cyclosporine or cyclosporin).ti,ab. 90. alefacept/ 91. alefacept.ti,ab. 92. brodalumab.ti,ab. 93. ixekizumab.ti,ab. 94. ixekizumab/ 95. brodalumab/ 96. certolizumab.mp. 97. tildrakizumab.mp. 98. or/7‐97 99. crossover procedure.sh. 100. double‐blind procedure.sh. 101. single‐blind procedure.sh. 102. (crossover$ or cross over$).tw. 103. placebo$.tw. 104. (doubl$ adj blind$).tw. 105. allocat$.tw. 106. trial.ti. 107. randomized controlled trial.sh. 108. random$.tw. 109. or/99‐108 110. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 111. human/ or normal human/ 112. 110 and 111 113. 110 not 112 114. 109 not 113 115. 6 and 98 and 114
Appendix 5. LILACS search strategy
psoria$
We searched using the term above and the Controlled clinical trials topic‐specific query filter.
Data and analyses
Comparison 1.
Primary outcome ‐ PASI 90
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Conventional systemic agents versus placebo | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Methotrexate | 2 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [0.26, 25.90] |
| 1.2 Fumaric acid esters | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 4.47 [2.01, 9.95] |
| 2 Conventional systemic 1 versus conventional systemic 2 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Ciclosporin versus methotrexate | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.47, 2.98] |
| 2.2 Methotrexate versus fumaric acid esters | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 20.90] |
| 3 Anti‐TNF alpha versus placebo | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Etanercept versus placebo | 12 | 4954 | Risk Ratio (M‐H, Random, 95% CI) | 11.17 [7.66, 16.28] |
| 3.2 Adalimumab versus placebo | 8 | 3199 | Risk Ratio (M‐H, Random, 95% CI) | 14.86 [8.93, 24.73] |
| 3.3 Certolizumab versus placebo | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 24.58 [3.48, 173.49] |
| 4 Ustekinumab versus placebo | 7 | 3832 | Risk Ratio (M‐H, Random, 95% CI) | 22.59 [14.74, 34.64] |
| 5 Anti‐IL17 versus placebo | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Secukinumab versus placebo | 7 | 2707 | Risk Ratio (M‐H, Random, 95% CI) | 26.52 [14.91, 47.17] |
| 5.2 Ixekizumab versus placebo | 4 | 3268 | Risk Ratio (M‐H, Random, 95% CI) | 53.85 [15.34, 189.07] |
| 5.3 Brodalumab versus placebo | 5 | 4109 | Risk Ratio (M‐H, Random, 95% CI) | 26.33 [16.77, 41.33] |
| 6 Anti‐IL23 versus placebo | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Guselkumab versus placebo | 3 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 24.87 [14.20, 43.55] |
| 6.2 Tildrakizumab versus placebo | 1 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 15.63 [2.24, 109.29] |
| 7 Other biologics | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 12.26 [0.76, 197.54] |
| 7.1 Itolizumab versus placebo | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 12.26 [0.76, 197.54] |
| 8 Biologic versus conventional systemic treatments | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Etanercept versus acitretin | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 11.00 [0.64, 190.53] |
| 8.2 Infliximab versus methotrexate | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [2.15, 3.80] |
| 8.3 Adalimumab versus methotrexate | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 3.73 [2.25, 6.19] |
| 8.4 Alefacept versus methotrexate | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.42, 2.98] |
| 9 Biologic 1 versus biologic 2 | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Ustekinumab versus Etanercept | 1 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [1.45, 2.24] |
| 9.2 Secukinumab versus etanercept | 1 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [1.85, 2.92] |
| 9.3 Ixekizumab versus etanercept | 2 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [2.24, 3.98] |
| 9.4 Secukinumab versus ustekinumab | 1 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.23, 1.53] |
| 9.5 Brodalumab versus ustekinumab | 2 | 3088 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.16, 1.39] |
| 9.6 Guselkumab versus adalimumab | 3 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.25, 1.60] |
| 10 Small molecules versus placebo | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Apremilast versus placebo | 4 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 6.78 [3.03, 15.17] |
| 10.2 Tofacitinib versus placebo | 4 | 2826 | Risk Ratio (M‐H, Random, 95% CI) | 6.80 [3.86, 11.99] |
| 10.3 Ponesimod versus placebo | 1 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 6.60 [1.65, 26.41] |
| 11 Biologic versus small molecules | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Etanercept versus Tofacitinib | 1 | 998 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.93, 1.38] |
| 11.2 Etanercept versus apremilast | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.72, 2.78] |
Comparison 2.
Primary outcome ‐ serious adverse events
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Conventional systemic agents versus placebo | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Methotrexate | 2 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.03, 0.88] |
| 1.2 Fumaric acid esters | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.31, 2.21] |
| 2 Anti‐TNF alpha versus placebo | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Etanercept versus placebo | 11 | 3783 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.61, 1.83] |
| 2.2 Adalimumab versus placebo | 8 | 3199 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.60, 1.73] |
| 2.3 Certolizumab versus placebo | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.10, 2.36] |
| 3 Ustekinumab versus placebo | 8 | 4154 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.50, 1.58] |
| 4 Anti‐IL17 versus placebo | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Secukinumab versus placebo | 7 | 2707 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.79, 3.53] |
| 4.2 Ixekizumab versus placebo | 4 | 3268 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.65, 2.32] |
| 4.3 Brodalumab versus placebo | 5 | 4109 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.52, 1.61] |
| 5 Anti‐IL23 versus placebo | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Guselkumab versus placebo | 3 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.51, 2.85] |
| 5.2 Tildrakizumab versus placebo | 1 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.07, 24.94] |
| 6 Other biologics | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Alefacept versus placebo | 2 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.34, 1.62] |
| 7 Biologic versus conventional systemic treatments | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Etanercept versus acitretin | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Infliximab versus methotrexate | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.04, 5.59] |
| 7.3 Adalimumab versus methotrexate | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.19, 22.14] |
| 8 Biologic 1 versus biologic 2 | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Ustekinumab versus etanercept | 1 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.38, 4.11] |
| 8.2 Secukinumab versus etanercept | 1 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.82] |
| 8.3 Ixekizumab versus etanercept | 2 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.55, 2.06] |
| 8.4 Secukinumab versus ustekinumab | 1 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.42, 2.39] |
| 8.5 Brodalumab versus ustekinumab | 2 | 3088 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.64, 3.56] |
| 8.6 Guselkumab versus adalimumab | 3 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.44, 1.82] |
| 9 Small molecules versus placebo | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Apremilast versus placebo | 5 | 2036 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.41, 1.49] |
| 9.2 Tofacitinib versus placebo | 5 | 2838 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.53, 2.06] |
| 9.3 Ponesimod versus placebo | 1 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [0.34, 19.85] |
| 10 Biologic versus small molecules | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Etanercept versus tofacitinib | 1 | 998 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.46, 2.89] |
| 10.2 Etanercept versus apremilast | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.14] |
Comparison 3.
Secondary outcome ‐ PASI 75
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Conventional systemic agents versus placebo | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Methotrexate | 2 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [1.19, 4.68] |
| 1.2 Fumaric acid esters | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [1.68, 3.89] |
| 2 Conventional systemic 1 versus conventional systemic 2 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Ciclosporin versus methotrexate | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.84, 2.23] |
| 2.2 Methotrexate versus fumaric acid esters | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.41, 3.51] |
| 3 Anti‐TNF alpha versus placebo | 22 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Etanercept versus placebo | 13 | 5066 | Risk Ratio (M‐H, Random, 95% CI) | 8.55 [6.94, 10.52] |
| 3.2 Adalimumab versus placebo | 8 | 3199 | Risk Ratio (M‐H, Random, 95% CI) | 9.08 [6.52, 12.65] |
| 3.3 Certolizumab versus placebo | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 11.31 [4.37, 29.24] |
| 4 Ustekinumab versus placebo | 8 | 4154 | Risk Ratio (M‐H, Random, 95% CI) | 12.41 [8.69, 17.71] |
| 5 Anti‐IL17 versus placebo | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Secukinumab versus placebo | 7 | 2707 | Risk Ratio (M‐H, Random, 95% CI) | 15.70 [11.27, 21.87] |
| 5.2 Ixekizumab versus placebo | 4 | 3268 | Risk Ratio (M‐H, Random, 95% CI) | 17.44 [10.45, 29.10] |
| 5.3 Brodalumab versus placebo | 5 | 4109 | Risk Ratio (M‐H, Random, 95% CI) | 12.80 [8.46, 19.36] |
| 6 Anti‐IL23 versus placebo | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Guselkumab versus Placebo | 3 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 12.28 [8.79, 17.17] |
| 6.2 Tildrakizumab versus placebo | 1 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 14.51 [3.73, 56.45] |
| 7 Other biologics | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Alefacept versus placebo | 2 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [1.76, 4.94] |
| 7.2 Itolizumab versus placebo | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 13.23 [1.88, 92.93] |
| 8 Biologic versus conventional systemic treatments | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Etanercept versus acitretin | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.09, 4.16] |
| 8.2 Alefacept versus methotrexate | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.46, 1.21] |
| 8.3 Infliximab versus methotrexate | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [1.58, 2.19] |
| 8.4 Adalimumab versus methotrexate | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.72, 2.94] |
| 9 Biologic 1 versus biologic 2 | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Ustekinumab versus etanercept | 1 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.13, 1.40] |
| 9.2 Secukinumab versus etanercept | 1 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.44, 1.88] |
| 9.3 Ixekizumab versus etanercept | 2 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.43, 2.24] |
| 9.4 Secukinumab versus ustekinumab | 1 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.06, 1.20] |
| 9.5 Brodalumab versus ustekinumab | 2 | 3088 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.04, 1.17] |
| 9.6 Guselkumab versus adalimumab | 3 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.13, 1.30] |
| 10 Small molecules versus placebo | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Apremilast versus placebo | 5 | 2036 | Risk Ratio (M‐H, Random, 95% CI) | 3.88 [2.42, 6.22] |
| 10.2 Tofacitinib versus placebo | 5 | 2838 | Risk Ratio (M‐H, Random, 95% CI) | 6.41 [3.84, 10.71] |
| 10.3 Ponesimod versus placebo | 1 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 3.51 [1.88, 6.53] |
| 11 Biologic versus small molecules | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Etanercept versus Tofacitinib | 1 | 998 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.02, 1.28] |
| 11.2 Etanercept versus apremilast | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.86, 1.71] |
Comparison 4.
Secondary outcome ‐ PGA 0/1
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Conventional systemic agents versus placebo | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Methotrexate | 2 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.47, 5.89] |
| 1.2 Fumaric acid esters | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [1.72, 4.32] |
| 2 Conventional systemic 1 versus conventional systemic 2 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Ciclosporin versus methotrexate | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.47, 1.46] |
| 3 Anti‐TNF alpha versus placebo | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Etanercept versus placebo | 11 | 4334 | Risk Ratio (M‐H, Random, 95% CI) | 7.77 [5.98, 10.10] |
| 3.2 Adalimumab versus placebo | 7 | 3051 | Risk Ratio (M‐H, Random, 95% CI) | 8.38 [6.28, 11.18] |
| 3.3 Certolizumab versus placebo | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 35.88 [5.11, 251.73] |
| 4 Ustekinumab versus placebo | 8 | 4154 | Risk Ratio (M‐H, Random, 95% CI) | 11.33 [7.38, 17.39] |
| 5 Anti‐IL17 versus placebo | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Secukinumab versus placebo | 6 | 2607 | Risk Ratio (M‐H, Random, 95% CI) | 17.16 [7.48, 39.36] |
| 5.2 Ixekizumab versus placebo | 4 | 3268 | Risk Ratio (M‐H, Random, 95% CI) | 17.46 [9.87, 30.90] |
| 5.3 Brodalumab versus placebo | 5 | 4109 | Risk Ratio (M‐H, Random, 95% CI) | 18.78 [13.29, 26.55] |
| 6 Anti‐IL23 versus placebo | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Guselkumab versus placebo | 3 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 10.59 [7.73, 14.51] |
| 6.2 Tildrakizumab versus placebo | 1 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 27.54 [3.95, 191.78] |
| 7 Other biologics | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Alefacept versus placebo | 1 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.22, 5.29] |
| 7.2 Itolizumab versus placebo | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 3.78 [0.94, 15.17] |
| 8 Biologic versus conventional systemic treatments | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Alefacept versus methotrexate | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.37, 1.29] |
| 8.2 Infliximab versus methotrexate | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.67, 2.37] |
| 8.3 Adalimumab versus methotrexate | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.79, 3.32] |
| 9 Biologic 1 versus biologic 2 | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Ustekinumab versus etanercept | 1 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.24, 1.58] |
| 9.2 Secukinumab versus etanercept | 1 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.73, 2.53] |
| 9.3 Ixekizumab versus etanercept | 2 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.74, 2.31] |
| 9.4 Secukinumab versus ustekinumab | 1 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.13, 1.35] |
| 9.5 Brodalumab versus ustekinumab | 2 | 3088 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.07, 1.27] |
| 9.6 Guselkumab versus adalimumab | 3 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.17, 1.32] |
| 10 Small molecules versus placebo | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Apremilast versus placebo | 4 | 1776 | Risk Ratio (M‐H, Random, 95% CI) | 3.88 [2.04, 7.38] |
| 10.2 Tofacitinib versus placebo | 5 | 2838 | Risk Ratio (M‐H, Random, 95% CI) | 4.48 [3.51, 5.71] |
| 10.3 Ponesimod versus placebo | 1 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 6.73 [2.19, 20.64] |
| 11 Biologic versus small molecules | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Etanercept versus tofacitinib | 1 | 998 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.04, 1.27] |
| 11.2 Etanercept versus apremilast | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.78, 2.27] |
Comparison 5.
Secondary outcome ‐ quality of life
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Conventional systemic agents versus placebo | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Methotrexate | 2 | 283 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.40, 0.06] |
| 2 Anti‐TNF alpha versus placebo | 14 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Etanercept versus placebo | 7 | 2779 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.37, ‐0.83] |
| 2.2 Adalimumab versus placebo | 7 | 2774 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.16, ‐0.88] |
| 3 Ustekinumab versus placebo | 6 | 2917 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐1.39, ‐1.03] |
| 4 Anti‐IL17 versus placebo | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Ixekizumab versus placebo | 3 | 3126 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.76 [‐2.09, ‐1.43] |
| 4.2 Brodalumab versus placebo | 2 | 349 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.44, ‐0.47] |
| 5 Anti‐IL23 versus placebo | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Guselkumab versus placebo | 2 | 1252 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐1.63, ‐1.14] |
| 5.2 Tildrakizumab versus placebo | 1 | 355 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐1.55, ‐0.91] |
| 6 Other biologics | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Alefacept versus placebo | 1 | 229 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.62, ‐0.02] |
| 6.2 Itolizumab versus placebo | 1 | 225 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.68, ‐0.01] |
| 7 Biologic versus conventional systemic treatments | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Alefacept versus methotrexate | 1 | 212 | Mean Difference (IV, Fixed, 95% CI) | 1.31 [‐0.28, 2.90] |
| 7.2 Adalimumab versus methotrexate | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.75, ‐1.05] |
| 8 Biologic 1 versus biologic 2 | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Ixekizumab versus etanercept | 2 | 2209 | Mean Difference (IV, Fixed, 95% CI) | ‐1.99 [‐2.39, ‐1.59] |
| 8.2 Guselkumab versus adalimumab | 2 | 1407 | Mean Difference (IV, Fixed, 95% CI) | ‐1.73 [‐2.50, ‐0.97] |
| 9 Small molecules versus placebo | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Apremilast versus placebo | 3 | 1609 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.77, ‐0.47] |
| 9.2 Tofacitinib versus placebo | 3 | 2629 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.28, ‐0.89] |
| 9.3 Ponesimod versus placebo | 1 | 326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.86, ‐0.31] |
| 10 Biologic versus small molecules | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Etanercept versus Tofacitinib | 1 | 998 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.19, 0.07] |
Analysis 5.1.
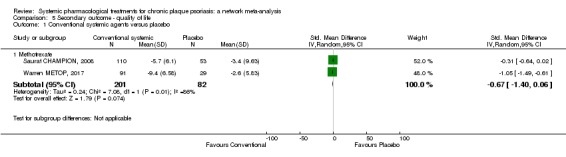
Comparison 5 Secondary outcome ‐ quality of life, Outcome 1 Conventional systemic agents versus placebo.
Analysis 5.2.
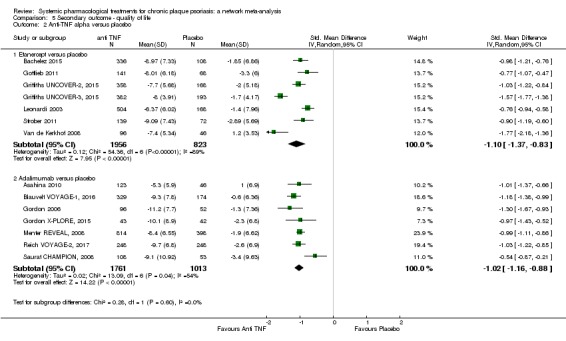
Comparison 5 Secondary outcome ‐ quality of life, Outcome 2 Anti‐TNF alpha versus placebo.
Analysis 5.3.
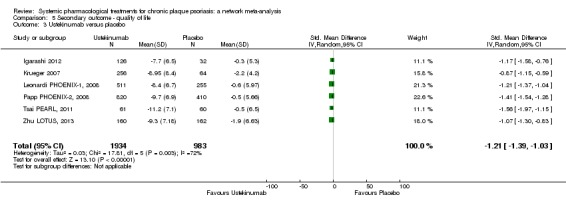
Comparison 5 Secondary outcome ‐ quality of life, Outcome 3 Ustekinumab versus placebo.
Analysis 5.4.
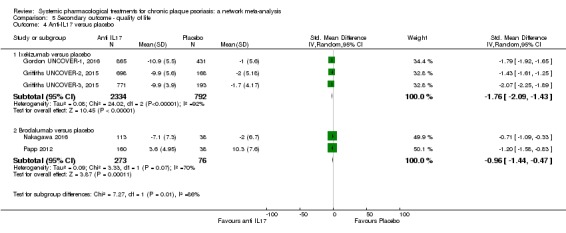
Comparison 5 Secondary outcome ‐ quality of life, Outcome 4 Anti‐IL17 versus placebo.
Analysis 5.5.
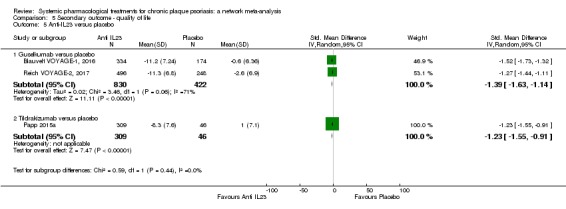
Comparison 5 Secondary outcome ‐ quality of life, Outcome 5 Anti‐IL23 versus placebo.
Analysis 5.6.
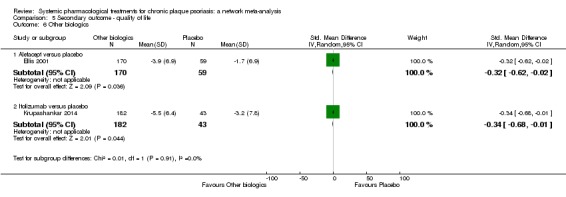
Comparison 5 Secondary outcome ‐ quality of life, Outcome 6 Other biologics.
Analysis 5.7.
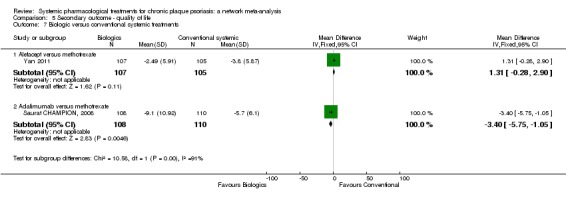
Comparison 5 Secondary outcome ‐ quality of life, Outcome 7 Biologic versus conventional systemic treatments.
Analysis 5.8.
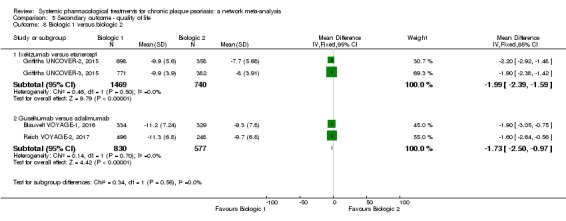
Comparison 5 Secondary outcome ‐ quality of life, Outcome 8 Biologic 1 versus biologic 2.
Analysis 5.9.
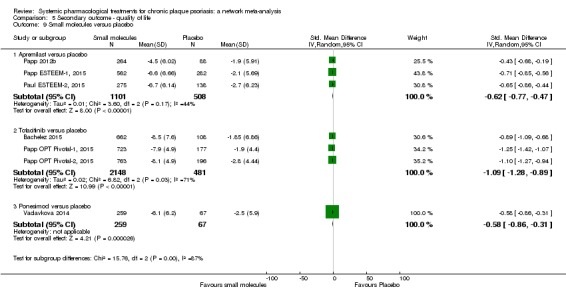
Comparison 5 Secondary outcome ‐ quality of life, Outcome 9 Small molecules versus placebo.
Analysis 5.10.
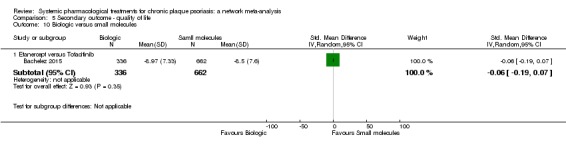
Comparison 5 Secondary outcome ‐ quality of life, Outcome 10 Biologic versus small molecules.
Comparison 6.
Secondary outcome ‐ adverse events
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Conventional systemic agents versus placebo | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Methotrexate | 2 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.81, 1.10] |
| 1.2 Fumaric acid esters | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.22, 1.62] |
| 2 Conventional systemic 1 versus conventional systemic 2 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Ciclosporin versus methotrexate | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.90, 1.34] |
| 2.2 Methotrexate versus fumaric acid esters | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.39] |
| 3 Anti‐TNF alpha versus placebo | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Etanercept versus placebo | 9 | 3529 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.05, 1.20] |
| 3.2 Adalimumab versus placebo | 7 | 3051 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.00, 1.13] |
| 3.3 Certolizumab versus placebo | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.22] |
| 4 Ustekinumab versus placebo | 8 | 4154 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.00, 1.13] |
| 5 Anti‐IL17 versus placebo | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Secukinumab versus placebo | 7 | 2707 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.02, 1.29] |
| 5.2 Ixekizumab versus placebo | 4 | 3268 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.07, 1.45] |
| 5.3 Brodalumab versus placebo | 5 | 4109 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.00, 1.32] |
| 6 Anti‐IL23 versus placebo | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Guselkumab versus placebo | 3 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.16] |
| 6.2 Tildrakizumab versus placebo | 1 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.18] |
| 7 Biologic versus conventional systemic treatments | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Infliximab versus methotrexate | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.97, 1.20] |
| 7.2 Adalimumab versus methotrexate | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.05] |
| 8 Biologic 1 versus biologic 2 | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Ustekinumab versus etanercept | 1 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.89, 1.06] |
| 8.2 Secukinumab versus etanercept | 1 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.12] |
| 8.3 Ixekizumab versus etanercept | 2 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.97, 1.15] |
| 8.4 Secukinumab versus ustekinumab | 1 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.98, 1.25] |
| 8.5 Brodalumab versus ustekinumab | 2 | 3088 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.93, 1.09] |
| 8.6 Guselkumab versus adalimumab | 3 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 9 Small molecules versus placebo | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Apremilast versus placebo | 5 | 2036 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.38] |
| 9.2 Tofacitinib versus placebo | 4 | 2641 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.03, 1.25] |
| 9.3 Ponesimod versus placebo | 1 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.02, 1.68] |
| 10 Biologic versus small molecules | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Etanercept versus tofacitinib | 1 | 998 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.12] |
| 10.2 Etanercept versus apremilast | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.03, 1.69] |
Differences between protocol and review
1. Between the first protocol submission (January 2014) and the first search (February 2015), we identified and added in the protocol new systemic therapeutics for psoriasis.
Background > Description of the intervention
Oral systemic treatment
Biological therapies
Background > How the intervention might work?
Oral systemic treatment
Biological therapies
Objectives
We expanded our objectives to clarify the types of systemic treatments for psoriasis. We changed: "To assess the effects of systemic pharmacological treatments for chronic plaque psoriasis" to "To compare the efficacy and safety of conventional systemic agents (acitretin, ciclosporin, fumaric acid esters, methotrexate), small molecules (apremilast, tofacitinib, ponesimod), anti‐TNF alpha (etanercept, infliximab, adalimumab, certolizumab), anti‐IL12/23 (ustekinumab), anti‐IL17 (secukinumab, ixekizumab, brodalumab), anti‐IL23 (guselkumab, tildrakizumab), and other biologics (alefacept, itolizumab) for patients with moderate to severe psoriasis and to provide a ranking of these treatments according to their efficacy and safety."
Methods > Types of intervention
We changed: "Systemic and biological treatments include the following: fumaric acid esters, retinoids (acitretin), ciclosporin, methotrexate, infliximab, etanercept, adalimumab, ustekinumab, briakinumab, alefacept, brodalumab, ixekizumab" to the following:
"Systemic and biological treatments included the following:
Systemic conventional treatments:
Fumaric acid esters
Acitretin
Ciclosporin
Methotrexate
Small molecules
Apremilast
Tofacitinib
Ponesimod
Anti‐TNF alpha
Infliximab
Etanercept
Adalimumab
Certolizumab
Anti‐IL12/23
Ustekinumab
Anti‐IL17
Secukinumab
Brodalumab
Ixekizumab
Anti‐IL23
Tildrakizumab
Guselkumab
Other biologic treatment
Itolizumab
Alefacept
A new anti‐IL23 molecule (BI 655066, risankizumab) appeared after we began this review and was not included in this systematic review. However, the ongoing studies of risankizumab have been reported in this review."
2. Background > Why it is important to do this review?
We updated the published literature regarding other systemic reviews and meta‐analyses.
3. Methods > Criteria for considering studies for this review
Selection of trials
We added: "Phase I trials were not eligible because participants, outcomes, dosages, and schema of administration of interventions are too different from phase II, III, and IV studies."
Outcomes
Primary outcome 1
In the Protocol, we wrote, "The proportion of participants who achieved clear or almost clear skin. (By clear or almost clear, we mean a Physician Global Assessment (PGA) value of 0 or 1 or a 90/100 PASI.)"
In the review, we changed this sentence to "The proportion of participants who achieved clear or almost clear skin, that is, at least PASI 90".
As PASI and PGA are two different scales, we preferred to assess them separately and added as a secondary outcome "Proportion of participants who achieve a Physician Global Assessment (PGA) value of 0 or 1".
Primary outcome 1
We also modified the sentence about serious adverse effects (SAEs) (in the protocol we said we would use the FDA's definition): "The proportion of participants with serious adverse effects (SAE). We used the definition of severe adverse effects from the International Conference of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, which includes death, life‐threatening events, initial or prolonged hospitalisation, and adverse events requiring intervention to prevent permanent impairment or damage." The definition remains the same.
Secondary outcome 3
For'Quality of life measured by a specific scale', we listed Dermatology Life Quality Index (DLQI), Skindex, Psoriasis Disability Index (PDI), or Psoriasis Symptom Inventory (PSI). It is not an exhaustive list. Moreover, we had PSI as a validated scale because it was used by some study authors.
Timings
We modified the period of the induction therapy assessment to less than 24 weeks after randomisation instead of 12 to 24 weeks because Nast et al defined the induction period as being with a duration less than 24 weeks (Nast 2015b).
To avoid duplicating text, we removed the text discussing timing for remission, as published in the protocol, and edited the timings for induction and maintenance therapy to include the relevant short‐ or long‐term remission classification. We also removed the timings given in the protocol for the quality of life outcome for the same reason (we felt the text was duplicative).
We clarified that our inclusion criteria was to only include studies that reported our timings of interest by editing as follows: "We did not include studies that had timings outside of these time ranges in our analyses" to "We did not include studies that had timings outside of these time ranges in our review."
4. Methods > Search methods for identification of studies
We removed the following two sentences from the review:
"We contacted key investigators and experts in the field to identify further published or unpublished data."
"We contacted pharmaceuticals companies producing fumaric acid esters, and retinoids (fumaric acid esters, retinoids (acitretin), ciclosporin, methotrexate, alefacept, infliximab, etanercept, adalimumab, certolizumab, ustekinumab, secukinumab, brodalumab, ixekizumab, tildrakizumab, guselkumab, Itolizumab, apremilast, tofacitinib, ponesimod."
We replaced them with the following:
"We searched in the trial results databases of each company to identify ongoing and unpublished trials."
5. Methods > Data extraction and management
We added some details regarding the data extraction (outcome data, other data) for greater clarity and added the sentence, "We extracted the data from the reports of the U.S. Food and Drug Administration (FDA) when available, if not from the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), and finally from the published reports."
6. Methods > Assessment of risk of bias in included studies
We added information regarding the network meta‐analysis 'Risk of bias' assessment (under "overall risk of bias").
Network meta‐analysis
"To summarise the quality of evidence and to interpret the network results, we used these six RoB criteria (random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessor, incomplete outcome data, and selective outcome reporting) in order to classify each trial.
We would classify the trial as having low risk of bias if we rated none of the domains above as high risk of bias and two or less as unclear risk.
We would classify the trial as having moderate risk of bias if we rated one domain as high risk of bias, one or less domains as unclear risk, or no domains as high risk of bias but three or less were rated as unclear risk.
All other cases were assumed to pertain to high risk of bias."
7. Methods > Measure of treatment effect
We added an explanation related to relative treatment ranking.
8. Methods > Dealing with missing data
We clarified who the authors or sponsors we contacted were: "We contacted trial authors or sponsors by email to request missing outcome data (numbers of events and numbers of participants for important dichotomous clinical outcomes) when these were not available in study reports that were less than 10 years old."
9. Methods > Assessment of reporting bias and Assessment of heterogeneity
We added an explanation regarding the network meta‐analysis:
"We undertook meta‐analyses only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar (section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011). Potential sources of heterogeneity included participants' baseline characteristics (weight, the duration of previous treatment, treatment doses, co‐interventions, and duration of treatment). When enough data were available, we investigated the distributions of these characteristics across studies and treatment comparisons. The latter allows assessing transitivity, i.e. whether there were important differences between the trials evaluating different comparisons other than the treatments being compared (Salanti 2014). To further reassure the plausibility of the transitivity assumption, we only included in our analyses trials not involving co‐interventions and with a timing of outcome assessment from 12 to 16 weeks.
In the classical meta‐analyses, we assessed statistical heterogeneity by visual inspection of the forest plots and using the Q‐test and the I² statistic. We interpreted the I² statistic according to the following thresholds (section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011): 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity.
In the network meta‐analysis, the assessment of statistical heterogeneity in the entire network was based on the estimated heterogeneity standard deviation parameter (τ) estimated from the network meta‐analysis models (Jackson 2014). We inferred on the presence or absence of important heterogeneity by comparing the magnitude of τ with the empirical distributions provided in Turner et al and Rhodes et al (Rhodes 2015; Turner 2012). We also estimated the prediction intervals to assess how much the estimated heterogeneity affects the relative effects with respect to the additional uncertainly anticipated in future studies (Riley 2011). Where feasible, we would have investigated the possible sources of heterogeneity in subgroup analyses and meta‐regression.
Although we restricted the risk of important heterogeneity in our data by considering eligible only studies with a follow‐up period between 12 and 16 weeks and without co‐interventions, we investigated differences in heterogeneity across the different analyses. Specifically, we observed whether splitting the nodes of the network and analysing each drug separately reduced the heterogeneity estimate. We also ran a series of sensitivity analyses (see Sensitivity analysis), and we monitored whether heterogeneity became smaller or larger compared to the primary analysis."
Assessment of reporting biases
To assess reporting biases, we used an adaptation of the funnel plot by subtracting from each study‐specific effect size the mean of meta‐analysis of the study‐specific comparison, which we plotted against the study standard error (Chaimani 2013). We employed this 'comparison‐adjusted funnel plot' for all comparisons of an active treatment against placebo. When we detected funnel plot asymmetry for the two primary outcomes, we investigated the presence of small‐study effects in the network meta‐regression (Chaimani 2012).
10. Methods > Data synthesis
We added the software used for the review: "We conducted pair‐wise meta‐analyses using Review Manager 5 (RevMan 5) (Revman 2014), and we performed all other analyses in Stata 14 using the 'network' (www.stata‐journal.com/article.html?article=st0410) and 'network graphs' packages (www.stata‐journal.com/article.html?article=st0411)."
11. Methods > Sensitivity analysis
We added "To assess the robustness of our results, we performed the following sensitivity analyses for the two primary outcomes: (1) running the analysis at dose‐level considering that each different drug dose is a different intervention; (2) excluding trials at high risk of bias; (3) excluding trials with a total sample size smaller than 50 randomised participants; and (4) analysing only the observed participants assuming that missing participants are missing at random."
12. Methods > 'Summary of findings' table
We added a section detailing the methods used to create the 'Summary of findings' tables; we also explained how we used GRADE to assess the certainty (quality/confidence) of the evidence.
13. Contributions of authors
We changed or added authors' contributions: LLC, GD, IGD, and ES screened papers against eligibility criteria. LLC, GD, IGD, CH, CM, CD, and ES appraised the quality of papers. LLC, GD, IGD, CH, CM, CD, and ES extracted data for the review and sought additional information about papers. AC responded to the methodological and statistical comments of the referees instead of LT (Ludovic Trinquard was no longer available and was replaced by Anna Chaimani). AC, LLC, and ES worked on the methods sections instead of LT, ES, and LLC (Ludovic Trinquard was no longer available and was replaced by Anna Chaimani).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT, active‐controlled, open‐label trial Date of study: January 2008‐January 2009 Location: Gaziantep, Turkey (1 centre) |
|
| Participants |
Randomised: 55 participants (mean age 39 years, 33 male) Inclusion criteria
Exclusion criteria None Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 25), orally, 0.3‐0.5 mg/kg/d Control intervention B. Cyclosporin (n = 21), orally, 3 mg/kg/d |
|
| Outcomes | Assessment at 8 weeks Primary outcome of the trial
Outcomes of the trial
|
|
| Notes | Funding source: Quote (p 1121): "No specific grant" Declarations of interest: Quote (p 1121): "The authors declare that there are no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p1119): "Patients were stratified into one of two groups via a computer‐generated randomisation schedule" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not stated that it was a blinded trial. Acitretin has visible side effects (muco cutaneous dryness) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no independent assessor. Not stated that it was a blind trial. Acitretin has visible side effects. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 55, analysed 46 Management of missing data: not stated |
| Selective reporting (reporting bias) | High risk | Comment: no primary or secondary outcomes stated. No protocol available |
| Methods | RCT, active‐placebo controlled, open‐label trial Date of study: February 2010‐October 2011 Location: Baghdad, Iraq (1 centre) |
|
| Participants |
Randomised: 120 participants (mean age 41 years, 41 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
No more statements regarding time and reasons of follow‐up |
|
| Interventions |
Intervention A. Methotrexate + NBUVB (n = 38), 20 mg/week + 45 mJ/cm2, 3 times/week Control intervention B. NBUVB (n = 38), 45 mJ/cm2, 3 times/week C. Methotrexate (n = 37), 20 mg/week |
|
| Outcomes | Assessment at 6 months Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding: not stated Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1531): "three groups randomly...” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: No description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not stated that it was a blind trial, probably not blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no independent assessor. Not stated that it was a blind trial, probably not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 120, analysed 113 Management of missing data: not stated |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. The outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active, placebo‐controlled, double blind Date of study: September 2005‐December 2006 Location: 42 centres in Japan |
|
| Participants |
Randomised: 169 participants (mean age 45 years, 143 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Adalimumab (n = 38), 40 mg, SC, eow B. Adalimumab (n = 43), 40 mg, SC, 2 injections, week 0, 1 injection eow (week 2) C. Adalimumab (n = 41), 80 mg, SC, eow Control D. Placebo (n = 46), 0.8 mL, SC, eow |
|
| Outcomes | Assessment at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding: support by Abbott (Quote p309) Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p301): "Patients were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p301): "Adalimumab 40mg/0.8mL and Placebo 0.8 mL were supplied two‐vial cartons (Adalimumab+Adalimumab, Adalimumab+placebo, Placebo+Placebo)" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no specific description of the method used to guarantee blinding of outcome assessment however considering that this was a placebo‐controlled trial with no known systematic AEs we considered the risk as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 169, analysed 169 Management of missing data: quote (p302): "Patients without evaluation at week 16 were considered non‐responders for the primary analysis" Comment: the report provided sufficient detail about the management of missing data to permit a clear judgment |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. The outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled, double blind Date of study: March 2012‐January 2014 Location: 16 centres in Japan |
|
| Participants |
Randomised: 95 participants, 94 treated (mean age 49 years, 78 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Tofacitinib (n = 43), orally, 5 mg twice daily Control intervention B. Tofacitinib (n = 44), orally, 10 mg twice daily |
|
| Outcomes | Assessment at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p878): "This study was sponsored by Pfizer Inc. Medical writing support under the guidance of the authors was provided by Kate Silverthorne, Ph.D., at Complete Medical Communications and was funded by Pfizer Inc" Declarations of interest: Quote (p878): "A. A., A. I., S. I., H. S. and M. O. have received consultancy fees from Pfizer Inc. Y. S., Y. T., S. T. and M. N. are employees of Pfizer Japan Inc. T. E. has nothing to disclose." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p870): "Patients were randomized 1:1 to tofacitinib 5 or 10 mg b.i.d. using a computer‐generated randomization schedule". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p870): "patients were registered by the investigator in a central randomized management system" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p870): "Tofacitinib was supplied as 5 mg tablets with a corresponding matching placebo. Patients and study staff were unable to determine from the packaging which treatment group the patient was assigned to. Patients, investigators, study teams, the contract research organization and the sponsor remained blinded throughout the study period " Comment: the report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p870): "Tofacitinib was supplied as 5 mg tablets with a corresponding matching placebo. Patients and study staff were unable to determine from the packaging which treatment group the patient was assigned to. Patients, investigators, study teams, the contract research organization and the sponsor remained blinded throughout the study period " Comment: the report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned n = 95, 94 received at least 1 dose of study drug, 87 had moderate‐severe psoriasis (study population) and 12 had psoriatic arthritis Management of missing data: Quote (page 871): "The full analysis set included all randomized patients who received one or more dose of study drug...Missing values were treated as non‐responders (non‐responder imputation)." Table 2: 87 analysed participants Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01519089). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active placebo‐controlled, double blind Date of study: not reported Location: Bangkok, Thailand, Asia |
|
| Participants |
Randomised: 24 participants (mean age 40 years (methotrexate) 48 years (placebo), 15 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 11), 15 mg/week, orally Control B. Placebo (n = 13), orally Co‐intervention: phototherapy UVB |
|
| Outcomes | Assessment at 24 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding: (quote 1013) no funding source Declarations of interest: (quote 1013) "None identified" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1014): "randomized by way of randomization cards" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1014): "to receive either MTX or placebo, which were identical in appearance" Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1015): "PASI scores were given by a investigator blinded to the treatment assignment" Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 24, analyzed 24 Management of missing data: Comment: no more precision regarding methods for dealing with missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. The outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active placebo‐controlled, double blind Date of study: 29 November 2010‐13 September 2012 Location: 122 worldwide excluding the USA and Canada |
|
| Participants |
Randomised: 1106 participants (mean age 46 years, 458 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Tofacitinib (n = 330), orally, 5 mg twice daily Control intervention B. Tofacitinib (n = 332) orally, 10 mg twice daily C. Etanercept (n = 336) SC, 50 mg twice weekly D. Placebo (n = 108) |
|
| Outcomes | Assessment at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 555): "This study was designed and funded by Pfizer Inc. Study investigators gathered the data, which were maintained in a database by Pfizer." Declarations of interest: Quote (p 560): "HB has provided consultancy services for AbbVie, Amgen, Boehringer, Celgene, Janssen, Leo Pharma, Lilly, Novartis, MSD, Pfizer, and Sandoz. He has also acted as an adviser for AbbVie, Amgen, Boehringer, Celgene, Janssen, Leo Pharma, Lilly, Novartis, Pfizer, and Sandoz; has served on speaker’s bureaus for AbbVie, Amgen, Celgene, Janssen, Leo Pharma, Lilly, Novartis, and Pfizer; and has received a research grant from Pfizer. PCMvdK has provided consultancy services for Celgene, Centocor, Almirall, Amgen, Pfizer, Philips, Abbott, Ely Lilly, Galderma, Novartis, JanssenCilag, Leo Pharma, Sandoz, and Mitsubishi. He has also done clinical trials for Basilea, Pfizer, Ely Lilly, Amgen, AbbVie, Philips Lighting, JanssenCilag, and Leo Pharma. RS has served on speaker’s bureaus for Pfizer, Schülke and Mayr, Lohmann & Rauscher, Meda Pharmaceuticals, Menarini Pharmaceuticals, Stockhausen, and Smith & Nephew; has had consulting agreements with Pfizer, Novartis, Lohmann & Rauscher, Urgo, Chemomedica, Schülke & Mayr, and Pantec Biotechnologies; and has received research and educational grants from Stockhausen, 3M‐Woundcare, Smith & Nephew, Lohmann & Rauscher, Enjo Commercials, Urgo, Chemomedica, and Schülke & Mayr. FV has been a principal investigator, member of a scientific advisory board, or speaker for AbbVie, Janssen, Eli Lilly, Merck, Novartis, and Pfizer. SC has been a consultant and/or speaker for Pfizer, AbbVie, Novartis, Merck, and Janssen‐Cilag. JPa, JPr, PG, HT, MT, HV, and RW are employees of Pfizer Inc. AK, J‐HL, and VY declare no competing interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 553): "A computer‐generated randomization schedule was used to assign patients to the treatment groups". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 553‐4): "The study site contacted an interactive voice response system or web‐based interactive response system..." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 553): "For this randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group phase 3 study" Comment: the report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 553): "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor), Patients and study personnel were masked to treatment assignment: the study drug packaging was labelled.... " Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1106, 1101 received at least 1 dose of study drug Management of missing data: Quote (page 554): "The primary analysis population for efficacy was the full analysis set, which was defined as all patients who received at least one dose of study drug... We judged patients with missing values for all binary endpoints to be non‐responders in efficacy assessments" Table 2: 1101 analysed participants Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01241591). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: not stated Location: North America |
|
| Participants |
Randomised: 124 participants (median age 39 years (etanercept) and 42 years (placebo), 69 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 62), SC, 50 mg, twice a week Control intervention B. Placebo (n = 62), SC, twice a week |
|
| Outcomes | Assessment at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding: Amgen Inc Declarations of interest (quote p86): "Dr Bagel receives a salary as founder of the Psoriasis Treatment Center of Central New Jersey. He has received speaker honoraria from Leo Pharma, Galderma, Centocor, Abbott, and Amgen. He has also been compensated as a consultant for Galderma and has served as an investigator for Centocor, Abbott, and Amgen. Dr Lynde has received research grants and honoraria from Amgen, Abbott, Merck, Ortho Biotech, Leo Pharma, and Galderma, for whom he has served as an advisory board member, consultant, and speaker. He has also served as an investigator for Amgen, Abbott, Merck, Ortho Biotech, and Leo Pharma. Dr Tyring has received a research grant and honoraria from Amgen, for whom he has served as a consultant, investigator, and speaker. He has also served as an investigator and/or speaker for Abbott, Leo Pharma, Galderma, GSK, Novartis, Merck, Epiphany, Inhibitex, AiCuris, and Pfizer. Dr Kricorian, Yifei Shi, and Dr Klekotka are employees of Amgen Inc. and have received Amgen stock/stock options." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p87): "Each patient provided written informed consent and received a unique identification number and randomised assignment from an Interactive Web Response System" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p87): "Each patient provided written informed consent and received a unique identification number and randomised assignment from an Interactive Web Response System" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p87): "patients and clinicians were blinded throughout the study as to treatment assignments." Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"patients and clinicians were blinded throughout the study as to treatment assignments." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 124, analysed 124 Dropouts and withdrawals
Quote (p89): "included in ITT efficacy analysis" Management of missing data: Quote (p88): "Last observation carried forward imputation was used for missing values" Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. The outcomes mentioned in the methods section appeared to have been reported except for QoL. |
| Methods | RCT, active‐controlled, open‐label trial Date of study: September 2005‐June 2008 Location: 106 centres in Europe |
|
| Participants |
Randomised: 868 participants (mean age 43 years, 586 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
Reasons not stated at week 16 |
|
| Interventions |
Intervention A. Infliximab (n = 653), IV, 5 mg/kg, weeks 0, 2, 6, 14, 22 Control intervention B. Methotrexate (n = 215), orally, 15 mg/week for 22 weeks |
|
| Outcomes | Assessment at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding: financial support for this study was provided by Schering‐Plough Research Institute, now Merck, Sharp & Dohme Corporation, Whitehouse Station, NJ, U.S.A. Declarations of interest: (Quote Appendix 1): "J.B. has served as a consultant and/or paid speaker for, and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis including Abbott, Celgene, Centocor, Janssen‐Cilag, Johnson and Johnson, Merck, Novartis, Pfizer, Schering‐Plough and Wyeth. M.H. has served as a consultant and/or paid speaker for, and/or has participated in clinical trials sponsored by Abbott, Amgen, Essex, Janssen, Leo, Medac, Novartis, Pfizer, Schering‐Plough and Wyeth. G.W. has no conflicts of interest to disclose. J.‐P.O. has been a consultant for Schering‐Plough, Abbott, Merck‐Serono, Centocor, Wyeth, Janssen‐Cilag, Meda‐Pharma, Pierre‐Fabre and Galderma. H.Z. is an employee of Merck, Sharp & Dohme. H.v.H. was an employee of Merck, Sharp & Dohme at the time of the RESTORE1 study and during the preparation of this manuscript. K.R. has served as a consultant and/or paid speaker for, and/or participated in clinical trials sponsored by Abbott, Celgene, Centocor, Janssen‐Cilag, Leo, Medac and Merck." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p1110): “At each eligible subject's baseline visit, study centres telephoned the Interactive Voice REsponse Syste .... for randomisation" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p1110): “At each eligible subject's baseline visit, study centres telephoned the Interactive Voice REsponse Syste .... for randomisation" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p1110): “open‐label trial” Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p1110): “open‐label trial” Comment: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 868, analysed 868 Quote (p1110‐11): "Primary and secondary efficacy analyses were based on the ITT population, the ITT population included all randomised patients. At week 16, patients who dropped out early or had missing data for PASI 75 ... were considered nonresponders" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00251641). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, single blind Date of study: May 2009‐June 2011 Location: Montréal, Quebec, Canada (5 centres) |
|
| Participants |
Randomised: 30 participants (median age 56 years (adalimumab) and 57 years (placebo), 23 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Adalimumab (n = 20), SC, 80/40 mg, eow Control intervention B. Topical treatment, phototherapy or no treatment (n = 10) |
|
| Outcomes | Assessment at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding: Abbott laboratories Declarations of interest: (quote p 89) "Dr Bissonnette and Dr Bolduc have been investigators, advisors and/ or consultants and received grants and/or honoraria from Abbott, Amgen, Astellas, Novartis, Janssen Ortho, Pfizer, Celgene, and Tribute. Drs Tardif, Harel, Pressacco, and Guertin have no conflicts of interest to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p84): "were randomised a concealed computer generated code created by the sponsor" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p84): "were randomised a concealed computer generated code created by the sponsor" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (83‐4): "single‐blind (cardiologist and all staff involved in vascular imaging and analysis were blinded to treatment assignment)" Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (83‐4): "single‐blind (cardiologist and all staff involved in vascular imaging and analysis were blinded to treatment assignment)" Comment: probably done; however, no statement regarding secondary outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 30, analysed 30 Quote (p84): "For all end points, the analysis was conducted on the ITT population, ... for the PASI 75 end point,... a nonresponder imputation method was used" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00940862). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: 20 August 2010‐14 May 2014 Location: 65 centres in Europe, North and South America, and Australia |
|
| Participants |
Randomised: 674 participants (mean age 46 years, 458 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Tofacitinib (n = 338), orally, 10 mg twice daily Control intervention B. Tofacitinib (n = 336), orally, 5 mg twice daily |
|
| Outcomes | Assessment at 24 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 1395 & 1400): "This study was sponsored by Pfizer Inc. Pfizer conducted the data analysis and the authors interpreted the data and collaborated in the manuscript preparation. All authors have access to the study data." Declaration of interest: (quote: Appendix 1): "R.B. has received honoraria, grants or worked as a consultant for AbbVie, Amgen, Apopharma, Astellas, Celgene, Eli Lilly, Incyte, Janssen, LEO Pharma, Merck, Novartis, Pfizer and Tribute. L.I. has served as a consultant and/or paid speaker for, and/or participated in clinical trials sponsored by, AbbVie, Almirall, Amgen, Celgene, Centocor, Eli Lilly, Janssen‐Cilag, LEO Pharma, MSD, Novartis, Pfizer and UCB. H.S. has served as a principal investigator and consultant for Pfizer, Celgene, Janssen, Amgen, Novartis, Eli Lilly and Merck. C.E.M.G has received grant/research support and/or received honoraria from AbbVie, Actelion, Biotest, Celgene, Eli Lilly, Incyte, Janssen, LEO Pharma, MSD, Novartis, Pfizer, Sandoz, Stiefel U.K., Trident, Zymogenetics and UCB. P.F. has served as a consultant for Galderma, LEO/Peplin, Ascent, Clinuvel, Aspen, Janssen‐Cilag, Eli Lilly, Australian Ultraviolet Services, Novartis, Wyeth/Pfizer, Mayne Pharma, MedyTox and Roche. He has also served on advisory boards/speaker’s bureaus and/or as a clinical trial investigator for CSL, Galderma, 3M/iNova/Valeant, LEO/Peplin, Ascent, Clinuvel, GSK/Stiefel, Abbott/AbbVie, BiogenIdec, Janssen‐Cilag, Merck Serono, ScheringPlough/MSD, Wyeth/Pfizer, Amgen, Novartis, Eli Lilly, Celgene, Roche, Aspen, Actelion, Sanofi Aventis, MedyTox, Shape and BMS. He has received travel grants from Galderma, LEO/ Peplin, BiogenIdec, Merck Serono, Ascent, Abbott/Abbvie, Schering‐Plough/MSD, Janssen–Cilag, Wyeth/Pfizer, Novartis and Roche. R.R. is a consultant, investigator and/or speaker for AbbVie, Eli Lilly, Galderma, Janssen‐Cilag, LEO Pharma, Novartis and Pfizer. M.B., S.T.R., H.T., J.P., H.V., L.M., P.G. and R.W. are employees of Pfizer Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1398): "A computer‐generated central randomisation schema was implemented using an automated web/telephone sytem." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 1398): "A computer‐generated central randomisation schema was implemented using an automated web/telephone sytem." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1398, Clinical.gov, NCT01186744): "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor) " Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1397): "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor) " Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 674, analysed 662 Dropouts and withdrawals: Tofacitinib 5 mg twice‐daily group (39), tofacitinib 10 mg twice‐daily group (41) Imbalanced numbers for withdrawal consent: tofacitinib 5 mg twice‐daily group (12), tofacitinib 10 mg twice‐daily group (0) Management of missing data: Quote (page 1398): "Efficacy analysis was performed on the full analysis set comprising patients who were randomised and received one or more doses of the study drug" (page 1400) "666 patients with moderate‐severe psoriasis were randomised to the initial period and received study medication". However only 662 patients were analysed for the outcomes. Comment: we judged this as a high risk of bias |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCTNCT01186744). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: May 2012‐January 2013 Location: 32 centres in the USA/Germany/France/Estonia/India/Switzerland |
|
| Participants |
Randomised: 177 participants (mean age 45 years (secukinumab 300 mg), 46 years (secukinumab 150 mg), 47 years (placebo), 117 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab (n = 59), SC, 300 mg, weeks 1, 2, 3, 4, 8, 12 B. Secukinumab (n = 59), SC, 150 mg, weeks 1, 2, 3, 4, 8, 12 Control intervention C. Placebo (n = 59), SC, weeks 1, 2, 3, 4, 8, 12 |
|
| Outcomes | Assessment at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
AEs |
|
| Notes | Funding: Novartis Pharmaceuticals, Basel, Switzerland. Declarations of interest (quote p 484): "A.B. has served as a scientific consultant and clinical study investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer and Sandoz. J.C.P. has served as a consultant, investigator, speaker or advisory board member for Abbott, Biogen‐Idec (formerly Biogen), Centocor, Essex Pharma, Galderma, Janssen‐Cilag/Janssen‐Ortho, Merck‐Serono (formerly Serono), MSD, Novartis, Pfizer and Wyeth, and has received unrestricted research grants from Biogen‐Idec and Wyeth. A.B.G. has served as scientific consultant and/or clinical study investigator for Abbott, Abbvie, Actelion, Akros Pharma, Amgen, Astellas Pharma, Beiersdorf, BMS, Canfite, Celgene, Coronado BioSciences, CSL Behring, GSK, Immune Control, Incyte, Janssen‐Ortho, Lerner Medical Devices, Lilly ICOS, Merck, Novartis, Novo Nordisk, Pfizer, Teva, UCB, Vertex Pharmaceuticals and Xenoport. K.K. has served as a study investigator for Celgene, Hexal, Mitsubishi and Novartis. H.S. has served as a study investigator, consultant and speaker for Novartis. M.R.‐M. has served as a study investigator for Novartis. V.S., R.P., C.P. and S.C. are full‐time employees of Novartis. C.P. and S.C. own stock in Novartis" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 486): “were randomised via interactive response technology to one of the treatment arms...using a validate system that automated the random assignment of subject numbers to randomisation numbers” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 486): “were randomised via interactive response technology to one of the treatment arms...using a validate system that automated the random assignment of subject numbers to randomisation numbers” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p486): “Subjects, study management team, investigator staff, persons performing the assessments and data analysts were blinded...” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p486): “Subjects, study management team, investigator staff, persons performing the assessments and data analysts were blinded...” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 177, analysed 177 Dropouts and withdrawals
Management of missing data: quote (supplemental appendix) "Missing values were imputed as non‐response for all efficacy analyses regardless of the reason of missing data" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01555125). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active placebo‐controlled, double blind Date of study: December 2014‐April 2016 Location: 101 centres worldwide |
|
| Participants |
Randomised: participants (mean age 44 years, 608 male) Inclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Guselkumab (n = 334), SC, 100 mg, weeks 0 and 4, then every 8 weeks Control intervention B. Adalimumab (n = 329), 80 mg week 0, then 40 mg week 1, and every 2 weeks C. Placebo (n = 174) |
|
| Outcomes | Assessment at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 405): "Supported by Janssen Research & Development LLC, Spring House, PA." DEclarations of interest Quote (p 405): "Dr Blauvelt has served as a scientific adviser and clinical study investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Genentech, GSK, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Regeneron, Sandoz, Sanofi‐Genzyme, Sun, UCB, and Valeant, and as a paid speaker for Eli Lilly. Dr Papp has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for AbbVie, Akesis, Akros, Allergan, Alza, Amgen, Anacor, Artax, Astellas, AstraZeneca, Baxalta, Baxter, Biogen, Boehringer Ingelheim, Bristol‐Myers Squibb, CanFite, Celgene, Celtic, Cipher, Dermira, Dow Pharmaceuticals, Eli Lilly, Ferring Pharmaceuticals, Formycon, Forward Pharma, Funxional Therapeutics, Fujisawa, Galderma, Genentech, Genexion, Genzyme, Gilead, GSK, Janssen, Kyowa Hakko Kirin, Leo, Lypanosys, Medimmune, Meiji Seika Pharma, Merck (MSD), Merck‐Serono, Mitsubishi Pharma, Mylan, Novartis, NovImmune, Pan Genetics, Pfizer, Regeneron, Roche, Sanofi‐Aventis, Stiefel, Takeda, UCB, Vertex, and Valeant. Dr Griffiths has received honoraria and/or grants as an investigator, speaker, and/or advisory board member for AbbVie, Eli Lilly, Janssen, Leo, Novartis, Pfizer, Sandoz, and Sun Pharma. Dr Kimball has received honoraria as a consultant for AbbVie, BMS, Dermira, Eli Lilly ICOS LLC, Merck, and Novartis; and received grants and/or funding for research or the residency/fellowship program as a principal investigator for AbbVie, Amgen, Boehringer Ingelheim, Dermira, Janssen, Merck, and Novartis. Drs Randazzo, Wasfi, Shen, and Li are all employees of Janssen Research & Development LLC (subsidiary of Johnson & Johnson) and own stock in Johnson & Johnson." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p3): "Patients were randomised using a permuted block method Central randomisation was implemented using an interactive World Wide Web response system (Perceptive Informatics, East Windsor, NJ)." Comment: clearly defined |
| Allocation concealment (selection bias) | Low risk | Quote (p3): "Central randomisation was implemented using an interactive World Wide Web response system (Perceptive Informatics, East Windsor, NJ)." Comment: clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "To maintain the blind, matching placebos were used." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p3): "To maintain the blind, matching placebos were used." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 837, 837 analysed Management of missing data: quote (page 3): "Patients who discontinued study agent because of lack of efficacy or anAE of psoriasis worsening or who started a protocol‐prohibited psoriasis treatment were considered nonresponders (binary end points) or had baseline values carried over (continuous end points). Other patients with missing data were considered nonresponders for binary end points (nonresponder imputation) and had last observation carried forward for continuous end points (and all PSSD end points)." Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02207231). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: 14 August 2012‐21 December 2013 Location: China |
|
| Participants |
Randomised: 425 participants (mean age 43 years, 310 men) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Adalimumab (n = 338), SC, 40 mg, week 0, 2 injections, eow 1 injection Control intervention B. Placebo (n = 87), SC |
|
| Outcomes | Assessment at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 2): "Abbvie Inc participated in the study design, study research, collection, analysis and interpretation of data" Declarations of interest: Quote (p 2): "L Cai, J Gu, J Zheng, M Zheng, G Wang, L‐Y Xi, F Hao, X‐M Liu, Q‐N Sun, Y Wang, W Lai, H Fang, Y‐T Tu, Q Sun, J Chen and X‐H Gao were investigators for this study, and J‐Z Zhang was the principal investigator for this study; all declare no financial, professional or personal relationships that might be perceived as a conflict of interest. Y Gu and HD Teixeira receive a salary as employees of AbbVie and may also receive stock, stock options and/or stock grants. MM Okun is a former AbbVie employee." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2 & Appendix): "The randomisation schedule was prepared by the Statistics Department of AbbVie, US. Randomization was performed using an adequate block size.“ Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2 & Appendix): “An interactive voice/web response system determined patient randomisation. The randomisation schedule was prepared by the Statistics Department of AbbVie, US. Randomization was performed using an adequate block size.“ Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 2 & Appendix): “Patients in Period A were randomised 4:1 to receive adalimumab 40 mg every‐other‐week (following a single 80 mg dose), or matching placebo...All AbbVie personnel with direct oversight of the conduct and management of the trial (with the exception of the drug supply team), the investigator, study‐site personnel and the patient remained blinded to each patient’s treatment throughout the 12 week blinded period of the study." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 2 & Appendix): “Patients in Period A were randomised 4 : 1 to receive adalimumab 40 mg every‐other‐week (following a single 80 mg dose), or matching placebo...All AbbVie personnel with direct oversight of the conduct and management of the trial (with the exception of the drug supply team), the investigator, study‐site personnel and the patient remained blinded to each patient’s treatment throughout the 12 week blinded period of the study.“ Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned: 425, analysed 425 (ITT) Quote (p 3): "Efficacy was analysed in Period A for all randomised patients [intent‐to‐treat (ITT_A Population)]... Missing data were handled using non‐responder imputation (NRI) for categorical variables and last‐observation‐carried‐forward (LOCF) for continuous variables." Comment: ITT analyses |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01646073). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled Date of study: not stated Location: not stated |
|
| Participants |
Randomised: 60 participants (age range 28‐67 years (etanercept), 32‐65 years (acitretin), 24 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 30), SC, 50 mg, twice a week, 12 weeks Control intervention B. Acitretin (n = 30), orally, 0.4 mg/kg/day, 12 weeks |
|
| Outcomes | Assessment at 12 weeks Primary and secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 211): "Patients were randomly assigned to one of the two groups" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: probably open‐label trial, term "blind" not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably open‐label trial, term "blind" not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of the method used to manage the missing data. No ITT analyses mentioned |
| Selective reporting (reporting bias) | Unclear risk | Comment: no primary or secondary outcomes stated |
| Methods | RCT, placebo‐controlled, double blind Date of study: not stated Location: single centre, New Jersey, USA |
|
| Participants |
Randomised: 33 participants (age mean 35 years (infliximab 10), 51 years (infliximab 5), 45 years (placebo), 23 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Infliximab (n = 11), IV, 5 mg/kg , weeks 0, 2, 6, 10 Control intervention B. Infliximab (n = 11), IV, 10 mg/kg , weeks 0, 2, 6, 10 C. Placebo (n = 11), IV, 20 mL, weeks 0, 2, 6, 10 |
|
| Outcomes | Assessment at 10 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding : Y Johnson and Johnson, Centocor Inc Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1843): "...were randomly assigned... by means of a lock‐of‐six randomisation scheme" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1843): "Placebo was supplied in a identical manner except that it did not contain IFX...The infliximab infusion solution was given by investigators unaware of treatment assignment..." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1843): "All assessments were done in a masked manner" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 33, analysed 33 Dropouts and withdrawals
Management of missing data: quote (p 1844): "The primary analysis was done according to ITT, all randomised patients were included Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled Date of study: not stated Location: Prague, Czech Republic |
|
| Participants |
Randomised: 41 participants (mean age 50 years (A), 46 years (B), 44 years (C), 41 years (D), 24 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrextate (n = 12), 7.5 mg/week, 2.5‐2.5‐2.5 at 12 h, for 13 weeks Control intervention B. Methotrextate (n = 12), 15 mg/week, 5‐5‐5 at 12 h, 13w C. Methotrextate (n = 7), 7.5 mg/week, once a week, for 13 weeks D. Methotrextate (n = 10), 15 mg/week, once a week, 13 weeks |
|
| Outcomes | Assessment at 13 weeks Primary or secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding: Czech Ministry of Education Declarations if interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 247): "were randomly assigned" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 247): "were randomly assigned" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: probably open‐label trial, term "blind" not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably open‐label trial, term "blind" not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of the method used to manage the missing data. No ITT analyses mentioned |
| Selective reporting (reporting bias) | Unclear risk | Comment: no primary or secondary outcomes stated |
| Methods | RCT, active‐controlled, Date of study: April 2009 and June 2011 Location: 5 centres in The Netherlands |
|
| Participants |
Randomised: 50 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention (n = 48) A. Infliximab (n = 25), IV, 5 mg/kg, weeks 0, 2, 6, 15, 22 Control intervention B. Etanercept (n = 23), SC, 50 mg twice weekly |
|
| Outcomes | Assessment at 24 weeks Primary outcomes of the trial PASI 75 Secondary outcomes of the trial QoL scale, Global assessment, treatment satisfaction |
|
| Notes | Funding source quote (p 1): "study was funded by a program grant from the Netherlands Organization for Scientific Research‐Medical Sciences (NWO‐MW; project 152001006)." Declaration of interest: "A.C.Q. de Vries: none reported; H.B. Thio: has been a consultant and invited speaker for Biogen/Idec, Janssen, Abbvie, Pfizer, MSD, Leopharma, Teva and Novartis. He has received educational grants from Abbvie, Janssen, Pfizer and Biogen/Idec.; W.J.A. de Kort: medical advisor for Novartis; B.C. Opmeer: none reported; H.M. van der Stok: Involved in performing clinical trials with Abbvie, Pfizer, Novartis, Janssen, BioClinic, AMGEN and LeoPharma.; E.M.G.J. de Jong: received research grants for the independent research fund of the department of dermatology of University Medical Centre St Radboud Nijmegen, the Netherlands from AbbVie, Pfizer, and Janssen. Has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Janssen, MSD, and Pfizer.; B. Horvath: Unrestricted Educational Grant from AbbVie, IIS Studies by Janssen, AbbVie, Performing clinical trial Novartis, Solenne B.V., Consultancies: Abbvie, Janssen, Philips, Galderma.; J.J.V.Busschbach: none reported; T.E.C. Nijsten: received research grants for the independent research fund of the department of dermatology of Erasmus MC, Rotterdam, the Netherlands from AbbVie, Leo Pharma, MSD, Pfizer, and Janssen. Has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Leo Pharma, Galderma, Janssen, MSD, and Pfizer. ; Ph.I. Spuls: consultancies in the past for Leopharma, AbbVie and Novartis. In the past an independent research grant from Schering Plough and from Leopharma. Involved in performing clinical trials with many pharmaceutical industries that manufacture drugs used for the treatment of psoriasis." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 4 & 8): “...was a multi‐centre, single‐blind, investigator initiated, randomised controlled trial comparing infliximab and etanercept in the treatment of moderate to severe chronic plaque type psoriasis... Adequate generation of an unpredictable allocation sequence and concealment of allocation was achieved by using a secure online internet facility (the TEN‐ALEA Clinical Trial Data Management System, provided by the Trans European Network http://www.tenalea.com/) performed in the coordinating centre by the main investigators. The sequence was generated in random block sizes of two and four to ensure it was unknown and not predictable by the investigators involved in randomising participants." Comment: done |
| Allocation concealment (selection bias) | Low risk | Quote (p 4 & 8): “...was a multi‐centre, single‐blind, investigator initiated, randomised controlled trial comparing infliximab and etanercept in the treatment of moderate to severe chronic plaque type psoriasis... Adequate generation of an unpredictable allocation sequence and concealment of allocation was achieved by using a secure online internet facility (the TEN‐ALEA Clinical Trial Data Management System, provided by the Trans European Network http://www.tenalea.com/) performed in the coordinating centre by the main investigators. The sequence was generated in random block sizes of two and four to ensure it was unknown and not predictable by the investigators involved in randomising participants." Comment: done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 4 & 8): “...was a multi‐centre, single‐blind, investigator initiated, randomised controlled trial comparing infliximab and etanercept in the treatment of moderate to severe chronic plaque type psoriasis..." Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 8): "Efficacy outcomes were carried out by trained assessors who were blinded to treatment allocation." Comment: no clear description of measures taken to guarantee the blinding of investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 50, analysed 48 Quote (p 8 & 9): "Missing data on primary endpoint were imputed using last observation carried forward. Analyses were carried out according to intention‐to‐treat (ITT) principle, apart from the longer term data where a per protocol analysis (PPA) was performed" Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | The trial was prospectively registered on the Dutch Trial Register: www.trialregister.nl/trialreg/index.asp; NTR 1559 The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled, double blind Date of study: August 2008‐September 2009 Location: Chandigarh, India |
|
| Participants |
Randomised: 60 participants (mean age 37 years, 48 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 30), orally, 10 mg/week, for 12 weeks Control intervention B. Methotrexate (n = 30), orally, 25 mg/week, for 12 weeks |
|
| Outcomes | Assessment at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding: none declared Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 730): “The randomisation list was generated using a random number table, and the code was kept by an investigator who was not directly involved in the study” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 730): “The randomisation list was generated using a random number table, and the code was kept by an investigator who was not directly involved in the study. All tablets were supplied in sealed envelopes bearing the code for any particular patient according to the randomisation list” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 730‐31): “Double blind study, …, the 10 mg group was also given an oral placebo tablet in addition to the MTX to give an equal number of tablets in both groups. The placebo tablets were identical in appearance to the MTX tablets in colour, texture, size, shape and markings. All tablets were supplied in sealed envelopes bearing the code for any particular patient according to the randomisation list” Comment: clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 730‐31): “Double blind study, …, the 10 mg group was also given an oral placebo tablet in addition to the MTX to give an equal number of tablets in both groups. The placebo tablets were identical in appearance to the MTX tablets in colour, texture, size, shape and markings. All tablets were supplied in sealed envelopes bearing the code for any particular patient according to the randomisation list” Comment: clearly described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 60, analysed 51 Dropouts and withdrawals
Management of missing data: no ITT analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled, double blind Date of study: March 2008‐March 2009 Location: Chandigarh, India |
|
| Participants |
Randomised: 61 participants (mean age 37 years, 51 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 20), orally, 25 mg/day, for 12 weeks Control intervention B. Acitretin (n = 20), orally, 35 mg/day, for 12 weeks C. Acitretin (n = 21), orally, 50 mg/day, for 12 weeks |
|
| Outcomes | Assessment at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding (quote e305): none declared Declarations of interest (quote e305): none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p e306): “Randomization list was generated using random number table and code was kept with a study coordinator who was not directly involved in assessment of endpoint” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p e306): “Randomization list was generated using random number table and code was kept with a study coordinator who was not directly involved in assessment of endpoint” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p e306): “double blind” Comment: no description of the method used to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p e306): “double blind” “Randomization list was generated using random number table and code was kept with a study coordinator who was not directly involved in assessment of endpoint” Comment: no description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 61, analysed 48 Dropouts and withdrawals:
Not ITT analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled Date of study: July 1987‐January 1988 Location: Paris |
|
| Participants |
Randomised: 37 participants (mean age, sex ratio: not stated) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Cyclosporin (n = 18), orally, 2.5 mg/kg/d Control intervention B. Cyclosporin (n = 19), orally, 5 mg/kg/d |
|
| Outcomes | Time to Assessment for the primary outcome: not stated Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding: not stated however one out of four authors was a staff member of Sandoz pharmaceutical company Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 136): "The patients were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 136): "The patients were randomised..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not specified as blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not specified as blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 37, analysed 37 Dropouts and withdrawals Not stated Management of missing data: no description of the method used to guarantee management of missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, active, controlled, double blind Date of study: not stated Location: single‐centre (University of Michigan Medical Center, Ann Arbor) |
|
| Participants |
Randomised: 85 participants (mean age 46 years (cyclosporine 3), 42 years (cyclosporine 5), 46 years (cyclosporine 7.5), 43 years (placebo), 66 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ciclosporin (Sandimmun) (n = 15), orally, 7.5 mg/kg, 8 weeks Control intervention B. Ciclosporin (Sandimmun) (n = 20), orally, 5 mg/kg, 8 weeks C. Ciclosporin (Sandimmun) (n = 25), orally, 3 mg/kg, 8 weeks D. Vehicle (Sandimmun oral olive oil) (n = 25), orally, 8 weeks |
|
| Outcomes | Assessment at 8 weeks Primary or secondary outcomes not stated Outcomes
|
|
| Notes | Funding (p 277): Sandoz research Institute, the Babcock Dermatologic Endowment (Ann Arbor) and a Clinical research centre grant (M01‐RR‐00042) from the National Institutes of Health Declarations of interest: not stated (p 277) "Drs Ellis and Voorhees are consultants to Sandoz Pharmaceuticals corporation (the manufacturer of cyclosporine). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 278): "patients were assigned numbers in consecutive order; each number had been preassigned to one of four treatments groups by means of a computer generated random code in blocks 17" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 278): "The preparation of cyclosporine and vehicle were identical …patients were blinded to their treatment" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 278): "Other physician who were blinded to group assignment and laboratory findings evaluated the patient" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 85, analysed not stated Dropouts and withdrawals not stated Quote (p 279): "In the primary, intention‐to‐treat analysis" Management of missing data: no description of the method used to guarantee management of missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, active placebo‐controlled, double blind Date of study: 14 May 1998‐22 February 1999 Location: 22 centres in USA |
|
| Participants |
Randomised: 229 participants (mean age 45 years, 163 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Alefacept (n = 57), IV, 0.025 mg/kg, once a week, 12 weeks Control intervention B. Alefacept (n = 55), IV, 0.075 mg/kg, once a week, 12 weeks C. Alefacept (n = 58), IV, 0.150 mg/kg, once a week, 12 weeks D. Placebo (n = 59), IV, once a week, 12 weeks |
|
| Outcomes | Assessments at 14 weeks Primary or secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding (p 254) : "supported by Biogen and a grant from the National Institutes of Health ... at the university of Utah" Declarations of interest (p 254): "A patent on the use of alefacept (LFA3TIP) for the treatment of psoriasis has been assigned to Biogen and the University of Michigan; neither Dr. Ellis nor Dr. Krueger has a financial interest in the patent. Dr. Ellis and Dr. Krueger are consultants to Biogen, as well as to other companies that manufacture treatments for psoriasis." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 249): “Randomization scheme was generated before the study, with a block size of four at each center” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 249): “Randomization scheme was generated before the study, with a block size of four at each center” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 249): "Double blind... all preparations were identical in appearance" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no specific description of the method used to guarantee blinding of outcome assessment however considering that this is a placebo‐controlled trial with no known systematic AEs we considered the risk as low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote (p 250): "were conducted according to the intention‐to‐treat principle" Dropouts and withdrawals
Comment: no description of the method used to guarantee missing data management, number of participants analysed not stated |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, active‐controlled, open‐label trial Date of study: not stated Location: not stated |
|
| Participants |
Randomised: 22 participants (mean age 45.9 years, 18 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ciclosporin A (n = 10), orally, 1.25 mg/kg/d (increase to 2.5 if PASI > 50% of initial PASI), 12 months Control intervention B. Ciclosporin A, (n = 12), orally, 2.5 mg/kg/d (increase to 5 if PASI > 50% of initial PASI), 12 months |
|
| Outcomes | Assessment period: not stated but longer than 16 weeks Primary or secondary outcomes of the trial: not stated Outcomes of the trial
|
|
| Notes | Funding: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 189): "Patients enrolled in the study were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 189): "Patients enrolled in the study were randomised..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded (open‐label) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blinded (open‐label) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Dropouts and withdrawals
Management of missing data: no description of the method used to guarantee management of missing data, ITT analyses not mentioned |
| Selective reporting (reporting bias) | High risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section were not reported in results section |
| Methods | RCT, active‐controlled, open‐label trial Date of study: October 2006‐February 2009 Location: Rotterdam/Eindhoven |
|
| Participants |
Randomised: 60 participants (mean age 41 years (methotrexate) and 43 years (fumarate), 36 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 30), orally, 15 mg/week, Weinstein schema 15 mg weekly in 3 equal doses of 5 mg each 12 h apart, 16 weeks Control intervention B. Fumarate (n = 30), orally, 720 mg, 30 mg followed by 120 mg and max 720 mg after week 9, 16 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source (p 855): none Declarations of interest (p 855): "none declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 856): “patients were randomly assigned ... randomisation was performed centrally according to a computered‐generated randomisation list” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 856): “Only the research nurse, who had no contact with the patients before randomisation had insight into the allocation schedule” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 856): “could not be blinded because treatment intake differed in both groups” Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 857): “by the same trained assessors (one trained physician and a research nurse in consensus in each site)” Comment: not specified whether "trained assessors" were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 60, analysed 51 Management of missing data: Quote (p 857): “Analysis was by Intention‐to‐treat...” Comment: ITT analysis not performed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, active‐controlled, open‐label trial Date of study: Feb 2002‐Feb 2005 Location: multicentre (n = 5), Sweden |
|
| Participants |
Randomised: 84 participants (mean age: 48 years (methotrexate), 46 years (ciclosporin); 55 male) Inclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate + folic acid (n = 41), orally, 7.5 mg/kg /week (5 mg folic acid except days of methotrexate), 12 weeks Control intervention B. Ciclosporin (n = 43), orally, 3 mg/kg, divided into 2 doses, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding (p121): "Financial support from the Swedish Psoriasis Association and the Welander foundation" Declarations of interest (p116): "none declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 117): "Randomization was performed with the use of computer‐generated random numbers, numbers by calling a central telephone number" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 117): "Randomization was performed with the use of computer‐generated random numbers, numbers by calling a central telephone number" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 117): "Blinded assessors performed the PASI at baseline and monthly thereafter" Comment: no description of method used to guarantee no communication between care givers or participants and assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 84, analysed 68 Management of missing data: not ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, active‐controlled, investigator‐blinded pilot trial Date of study: Feb 2002‐Feb 2005 Location: Verona, Italy |
|
| Participants |
Randomised: 60 participants (mean age 55 years (acitretin); 55 years (etanercept), 53 years (acitretin + etanercept), 33 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (25mg) and acitretin (0.4 mg/kg) (n = 18), SC (etanercept) and orally (acitretin), twice a week (etanercept) and once a day (acitretin), 24 weeks Control intervention B. Acitretin (n = 20), orally, 0.4 mg/kg, once a day, 24 weeks C. Etanercept (n = 22), SC, 25 mg, twice a week, 24 weeks |
|
| Outcomes | Assessments at 24 weeks Primary outcomes of the trial ≥ PASI 75 improvement from baseline Secondary outcomes of the trial
|
|
| Notes | Funding: not stated Declarations of interest (p 1345): "PG has received lecture fees from Merck‐Serono, Schering‐Plough, Wyeth. GG has received consultation and lecture fees from Abbott, Janssen‐Cilag, Merck‐Serono, Schering‐Plough, Wyeth." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1346): "Randomization was performed with the use of computer‐generated random numbers and block size of four patients" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 1346): "Randomization was performed with the use of computer‐generated random numbers and block size of four patients" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 1346): "The PASI assessor was blinded concerning the group allocation of the patient" Comment: acitretin provide visible AEs |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 60, analysed 60 Management of missing data, quote (p 1346): "An ITT analysis was performed" Comment: no description of the method used to manage the missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, placebo‐controlled, double blind Date of study: not stated Location: not stated |
|
| Participants |
Randomised: 38 participants (mean age 45‐48 years, 31 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 10), orally, 10‐25 mg/day, 8 weeks B. Acitretin (n = 16), orally, 50‐75 mg/day, 8 weeks Control intervention C. Placebo (n = 12), orally, daily, 8 weeks |
|
| Outcomes | Assessments at 8 weeks Primary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding sources, quote (p 655): "Supported in part by Hoffman‐La Roche Inc., Nutley, NJ, and the Babcock Dermatologic Endowment" Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 656): "21 patients were randomly and equally divided into 4 groups" Comment: no description of the method used to generate the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 656): "21 patients were randomly and equally divided into 4 groups" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 656): "we have studied 38 patients in a double‐blind fashion" Comment: visible side effect of acitretin |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 656): "we have studied 38 patients in a double‐blind fashion" Comment: visible side effect of acitretin |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 38, analysed 38 No mention of how the missing data were managed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: March 2003‐June 2004 Location: Multicentre (n = 18) in USA, Canada |
|
| Participants |
Randomised: 148 participants (mean age 44 years, 99 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Adalimumab (n = 46), SC, 40 mg, 12 weeks, week 0: 2 injections, 1 injection eow B. Adalimumab, (n = 50), SC, 40 mg, 12 weeks, week 0, week 1: 2 injections, 1 injection weekly Control intervention C. Placebo (n = 52), SC, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding, quote (p 598): "Supported by Abbott Laboratories" Declarations of interest (p 598): "Dr Gordon has received research support and honoraria and is a consultant for Abbott. Dr Langley is an investigator and has received research funding to conduct research studies with Abbott. Dr Leonardi is a consultant and speaker for Abbott. Dr Menter has received honoraria and is a consultant for Abbott. Dr Kang is an ad‐hoc consultant for Abbott. Dr Heffernan is a consultant for and has received research funding from Abbott. Drs Zhong, Hoffman, and Okun and Ms Lim are full‐time employees of Abbott." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 699): "Patients were centrally randomised..." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 699): "Patients were centrally randomised..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 599): "To maintain blinding, prefilled syringes were identically labelled and all patients received the same number of injections at the same time points" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 599): "To maintain blinding, prefilled syringes were identically labelled and all patients received the same number of injections at the same time points" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 148, analysed 147 Dropouts and withdrawals
Management of missing data, quote (p 601): "modified intent‐to‐treat analysis... a patient with missing data was counted as a nonresponder at that visit" Comment: few lost to follow‐up, well‐balanced number and reasons between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, placebo‐controlled, double blind Date of study: November 2011‐June 2014 Location: multicentre (104) in Europe, Australia, North America |
|
| Participants |
Randomised: 1296 participants (mean age 45 years, 883 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ixekizumab (n = 432), SC, 80 mg, 2 injections week 0, 1 injection monthly Control intervention B. Ixekizumab (n = 433), SC, 80 mg, 2 injections week 0, 1 injection eow C. Placebo (n = 431), SC |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 346): “The trials were sponsored by Eli Lilly and were designed by the scientific steering committee and Eli Lilly personnel. The site investigators collected the data, Eli Lilly personnel performed the data analyses, and all the authors had access to the data.” Declarations of interest (p 355): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Gordon received grants and personal fees from Abbvie, Amgen, Celgene, Eli Lilly, Novartis; and personal fees from Pfizer and Medac. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (supplemental appendix): “Patients were assigned to treatment groups as determined by a computer‐generated random sequence .." Comment: clearly defined |
| Allocation concealment (selection bias) | Low risk | Quote (supplemental appendix): “Patients were assigned to treatment groups as determined by a computer‐generated random sequence using an interactive voice response system (IVRS). Site personnel confirmed that they had located the correct assigned investigational product package by entering a confirmation number found on the package into the IVRS” Comment: clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 346): “double‐blind, placebo‐controlled” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 346): “double‐blind, placebo‐controlled” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1296, analysed 1296 Management of missing data: Quote (p 348): “Unless otherwise specified, all analyses of efficacy during the induction period were performed according to the intention‐to‐treat principle. Missing values for the PASI and the sPGA score were imputed conservatively as nonresponses, regardless of the reason for the missing data” Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01474512). The pre‐specified outcomes mentioned in the protocol and in the methods section appeared to have been reported. |
| Methods | RCT, active placebo‐controlled, double blind Date of study: October 2011‐August 2013 Location: multicentre (n = 31), Europe & North America |
|
| Participants |
Randomised: 293 participants (mean age 47 years, 207 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Guselkumab (n = 41), SC, 5 mg weeks 0, 4, 16 Control intervention B. Guselkumab (n = 41), SC, 15 mg weeks 0, 4, 16 C. Guselkumab(n = 42), SC, 50 mg weeks 0, 4, 16 D. Guselkumab (n = 42), SC, 100 mg weeks 0, 4, 16 E. Guselkumab (n = 42), SC, 200 mg weeks 0, 4, 16 F. Adalimumab (n = 43), SC, 40 mg 2 injections week 0, 1 injection week 1, 1 injection eow G Placebo (n = 42), SC (100 mg weeks 0, 4, 16) |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 137): “This study was sponsored by Janssen Research and Development. Janssen supplied the study agents and collected and analysed the data. All the authors had full access to the data”. Declarations of interest (p 144): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Gordon received grants and personal fees from Abbvie, Amgen, Celgene, Eli Lilly, Novartis; and personal fees from Pfizer and Medac. Reich received personal fees from Celgene, Centocor/Janssen, Forward Pharma, GSK, Janssen Cilag, LEO Pharma, Lilly Medoc, MSD, Novartis, Ocean Pharma, Pfizer, Regeneron, Takeda, Vertex. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 137): “patients were randomised…” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 137): “patients were randomised…” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 137, p 143): “double‐blind… Adalimumab was not administered in a blinded, placebo‐controlled manner”, “Another potential issue was to use of a blinded efficacy evaluator at each site instead of the administration of ADA in a blinded manner” Quote (p 553‐4): "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor ), Patients and study personnel were masked to treatment assignment: the study drug packaging was labelled.... " Comment: adalimumab group was not double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 137): “to ensure objectivity, all efficacy assessment were performed by an evaluator at each study site who was unaware of the study group” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 293, analysed 293 Dropouts and withdrawals
Management of missing data: Quote (p 138): “Patients with missing PGA or PASI score at week 16 were categorized as not having had a response” Comment: low number of withdrawals, balanced number and reasons between groups |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01483599). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: August 2000‐January 2001 Location: multicentre (locations not specified) |
|
| Participants |
Randomised: 112 participants (mean age 47 years, 70 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 57), SC, auto‐administered, 25 mg twice a week, 24 weeks Control intervention B. Placebo (n = 55), SC, auto‐administered, twice a week, 24 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial At 4, 8, 12, 24 weeks
|
|
| Notes | Funding source, quote (p 1631): "This study was sponsored by Immunex Corp, a subsidiary of Amgem, Inc.) Declarations of interest not stated except "Dr Zitnik is an employee of Amgen" (p1627) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1628): "Patients ... were to be randomised in block of 6 with equal allocation between the treatment group...Patients were assigned numbers based on randomisation tables verified by Immunex Pharmaceutical Planning" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 1628): "Patients ... were to be randomised in block of 6 with equal allocation between the treatment group...Patients were assigned numbers based on randomisation tables verified by Immunex Pharmaceutical Planning, after which the Immunex Clinical Distribution Department shaped blind‐labelled vials of study drug to the pharmacies". Comment: we don't know whether the investigators were blinded or the numbers of participants per block. This probably was a centralized randomisation; however, it's not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1628): "... performed blinded labelling and packaging of the study drug. ... multicenter, randomised, double‐blind" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1628): "... performed blinded labelling and packaging of the study drug. ... multicenter, randomised, double‐blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 112, 112 participants analysed for the primary endpoint Dropouts and withdrawals
Management of missing data: Quote (1628): "Patients were analysed on an intent‐to‐treat basis... If a patient discontinued treatment before the end of the study, the last observation was carried forward for efficacy analyses" Comment: high rate of withdrawal in placebo group and imbalanced reasons for withdrawal |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: 2001‐2003 Location: 24 centres in USA |
|
| Participants |
Randomised: 249 participants (mean age 44 years, 174 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals after a 30‐week study period
Reasons
|
|
| Interventions |
Intervention A. Infliximab (n = 99), IV, 3 mg/kg, weeks 0, 2, 6, for 10 weeks Control intervention B. Infliximab (n = 99), IV, 5 mg/kg, weeks 0, 2, 6, for 10 weeks C. Placebo (n = 51), IV, equivalent, weeks 0, 2, 6, for 10 weeks |
|
| Outcomes | Assessments at 10 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (534): "Supported by Centocor Inc" Declarations of interest (p 534): "Drs Gottlieb and Menter have received research support from and served as consultants for Centocor Inc. Drs Baker, Bala, Dooley, Evans, Guzzo, and Marano, and Ms Li, are employees of Centocor Inc. " |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 535): "Randomisation was carried out using adaptive treatment allocation and was stratified by the investigational site". Comment: no description of the method used to generate random sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 535): "Randomissation was carried out using adaptive treatment allocation and was stratified by the investigational site". Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 535): "Patients and investigators were unaware of treatment assignments. Double blind was achieved and maintained by using an independent pharmacist or staff member to prepare all study infusion" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 535): "Patients and investigators were unaware of treatment assignments. Double blind was achieved and maintained by using an independent pharmacist or staff member to prepare all study infusion" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 249 randomised, 249 analysed Methods for dealing with missing data: Quote (p 536): "All randomised patients were included in the efficacy analysis at week 10... Patients who discontinued... were considered to have not achieved the dichotomous end points or were assigned the baseline value for continuous end points after the event occurrence" Comment: done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: June 2008‐March 2009 Location: 33 centres in the USA |
|
| Participants |
Randomised: 209 participants (mean age 43.5 years, 145 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 141), SC, auto‐administered, 50 mg twice a week, 11 weeks Control intervention B. Placebo (n = 68), SC, auto‐administered, twice a week |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial At 4, 8, 12 weeks
|
|
| Notes | Funding source, quote (Appendix 1): "Abbott Laboratories funded this study and participated in the study design, data collection, data management, data analysis and preparation of the manuscript. All of the authors had full access to the data and were involved in the analysis of data, development and revision of the manuscript, and decision to submit the manuscript for publication. The corresponding author takes responsibility for the integrity of the data and the accuracy of the data analysis..)" Declarations of interest, quote (Appendix 1): "A.B.G. has been a consultant or served on an advisory board for Amgen, Centocor, Celgene, Bristol Myers Squibb, Beiersdorf, Abbott, TEVA, Actelion, UCB, Novo Nordisk, Immune Control, DermiPsor, Incyte, PureTech, Magen Biosciences, Cytokine Pharmasciences, Alnylam, Ono, Pfizer, Schering, Canfite, Schering, UCB, BIND Biosciences and Merck, and has received research/educational grants (paid to Tufts Medical Center) from Centocor, Amgen, Immune Control, Abbott, Novo Nordisk, UCB and Novartis. C.L. has been an investigator for Abbott, Allergan, Altana, Alza, Amgen, Astellas, Celgene, Centocor, Genentech, Bristol Myers, Eli Lilly, Galderma, Genzyme, Pfizer, Incyte, CombinatoRx, 3M Pharmaceuticals, Perrigo Israel Pharmaceutical, ScheringPlough, RTL, Novartis, Vitae and Wyeth; has served on an advisory board and has been a speaker for Abbott, Amgen and Centocor; and has been a consultant for Abbott, Amgen, Centocor and Pfizer. F.K. has been an investigator for Abbott, Centocor, Amgen, Wyeth, Novartis and Merck; and has served on an advisory board and has been a speaker for Abbott, Centocor, Amgen, Eisai, Astellas and Wyeth. S.M. has been an investigator for Abbott, Amgen, Celgene, Centocor, Graceway and Novo Nordisk; and has been a speaker for Abbott. M.O. and D.A.W. are employees of Abbott." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 653): "Patients were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 653): "Patients were randomised" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 653): “Patients enrolled in the placebo arm received SC injections matching active treatment to maintain the blind. To maintain the blind, all patients received two SC injections at weeks 0 and 4 and one SC injection at week 8, consisting of either briakinumab or matching placebo, depending on the treatment arm. In addition, each patient also received two SC injections biweekly, 3 days apart, week 0 through week 11, consisting of either etanercept or matching placebo, depending on the treatment arm.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 653): “Patients enrolled in the placebo arm received SC injections matching active treatment to maintain the blind. To maintain the blind, all patients received two SC injections at weeks 0 and 4 and one SC injection at week 8, consisting of either briakinumab or matching placebo, depending on the treatment arm. In addition, each patient also received two SC injections biweekly, 3 days apart, week 0 through week 11, consisting of either etanercept or matching placebo, depending on the treatment arm.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 209, analysed 209 Management of missing data: Quote (p 654): “The primary efficacy analysis consisted of four comparisons performed in the intent‐to‐treat population (i.e. all randomised patients), …, Nonresponder imputation was used to handle missing data.” Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00691964). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: November 2010–December 2011 Location: Multicentre in Boston/USA |
|
| Participants |
Randomised: 478 participants (methotrexate: mean age 43 years & 153 male; placebo: mean age 45 years & 167 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 239), orally, 15 mg/week 7.5 mg‐10 mg to a maximum of 15 mg, 24 weeks + etanercept, SC, 50 mg x 2/weeks, S1‐S12 and 50 mg/week, S12‐S24, 24 weeks Control intervention B. Placebo (n = 239), orally, 24 weeks + etanercept, SC, 50 mg x 2/weeks, S1‐S12 and 50 mg/week, S12‐S24, 24 weeks |
|
| Outcomes | Assessments at 24 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 649): "This study was funded by Immunex Corporation, a wholly owned subsidiary of Amgen Inc, and by Wyeth, which was acquired by Pfizer..." Declarations of interest (Appendix): "A.B.G. is a consultant and/or advisory board member for Abbott, Actelion, Amgen, Astellas, Beiersdorf, Bristol‐Myers Squibb, Can‐Fite, Celgene, Centocor (Janssen), Dermipsor, Incyte, Lilly, Merck, Novartis, Novo Nordisk, Pfizer, TEVA, and UCB and is a recipient of research/educational grants paid to Tufts Medical Center by Abbott, Amgen, Celgene, Centocor (Janssen), Immune Control, Novartis, Novo Nordisk, Pfizer, and UCB. R.G.L. has served as an investigator, on the scientific advisory board, and speaker for Abbott, Amgen, Centocor, and Pfizer, and as an advisor and investigator for Celgene, Novartis, and Johnson & Johnson. B.E.S. has served as an advisor, consultant, investigator, and speaker for Abbott, Amgen, and Centocor, and as an advisor, consultant, and investigator for Celgene, Novartis, Maruho, and Pfizer. K.A.P. has been a consultant, advisory board member, and investigator for Abbott, Amgen, Celgene, Centocor, Janssen‐Ortho, MedImmune, Merck, Pfizer, Schering‐Plough, and Wyeth (Wyeth was acquired by Pfizer in October 2009); has consulted for Astellas and UCB; and has served as a speaker for Abbott, Amgen, Celgene, Janssen‐Ortho, Pfizer, Schering‐Plough, and Wyeth. P.K., K.C., E.H.Z.T., M.H., and G.K. are employees and stockholders of Amgen Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 650): "This was a randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 650): "This was a randomised...study" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 650): “double‐blinded placebo‐controlled” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 650): “double‐blinded placebo‐controlled” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 478, analysed 478 Management of missing data: Quote (p 651): “Efficacy analyses were performed using the ITT set (all randomised patients)... Missing postbaseline data were imputed using last observation carried forward for primary analyses of all efficacy endpoints...” Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01001208). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled, open‐label trial Date of study: 26 March 2007‐15 January 2009 Location: 67 centres in Manchester/UK |
|
| Participants |
Randomised: 903 participants (mean age 45 years, 613 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ustekinumab (n = 209), SC, 45 mg, weeks 0‐4, 4 weeks Control intervention B. Ustekinumab (n = 347), SC, 90 mg, weeks 0‐4, 4 weeks C. Etanercept (n = 347), SC, 50 mg x 2/weeks, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding, quote (p 127): "Supported by Centocor Research and Development." Declarations of interest (p 127) "Dr. Griffiths reports receiving consulting and lecture fees from Abbott, Janssen‐Cilag, Merck Serono, Novartis, Schering‐Plough, and Wyeth and grant support from Merck Serono; Dr. Strober, receiving consulting and lecture fees from Centocor, Johnson & Johnson, Amgen, and Abbott Laboratories and grant support from Amgen and Abbott Laboratories; Dr. van de Kerkhof, receiving consulting fees from Schering‐Plough, Celgene, Centocor, Almirall, UCB, Wyeth, Pfizer, Soffinova, Abbott, Actelion, Galderma, Novartis, Janssen‐Cilag, and Leo Pharma; Dr. Ho, receiving advisory‐board and lecture fees from Schering, Abbott, Janssen‐Ortho, Pfizer, Amgen, and Wyeth and grant support from Centocor, Abbott, Amgen, and Wyeth; Dr. Menter, receiving advisory‐board, consulting, and lecture fees from Abbott, Amgen, Astellas, Biogen Idec, Celgene, Centocor, Genentech, Warner Chilcott, and Wyeth; Drs. Yeilding, Guzzo, Xia, and Dooley and Ms. Li, being employees of Johnson & Johnson and having equity and holding stock options in Johnson & Johnson; Dr. Zhou, being an employee of Johnson & Johnson, having equity and holding stock options in Johnson & Johnson, and having equity in Wyeth; Dr. Fidelus‐Gort, being a former employee of Johnson & Johnson and having equity and holding stock options in Johnson & Johnson; and Dr. Goldstein, receiving consulting fees from Centocor. No other potential conflict of interest relevant to this article was reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 119): “We randomly assigned...” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 119): “We randomly assigned...” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 119): “Patients were aware of their treatment assignment”, ... “All study personnel, except those who dispensed or administered a study agent remained unaware of the treatment assignments" Comment: high risk for participants and unclear risk for personnel (no description of means used to avoid communication between participants and personnel and very difficult to avoid) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 119): “All study personnel, except those who dispensed or administered a study agent remained unaware of the treatment assignments" Comment: no description of the method used to assess the primary outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 903 participants underwent randomisation, 903 were analysed Comment: methods for dealing with missing data not specified |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00454584). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active, placebo‐controlled, double blind Date of study: 10 May 2012‐7 May 2015 Location: 118 centres in Europe, Australia, North America |
|
| Participants |
Randomised: 1224 participants (mean age 45 years, 821 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ixekizumab (n = 347), SC, 80 mg, 2 injections week 0, 1 injection monthly Control intervention B. Ixekizumab (n = 351), SC, 80 mg, 2 injections week 0, 1 injection eow C. Etanercept (n = 358), SC, 50 mg 1 injection twice weekly D. Placebo (n = 168), SC |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 543): “The funder Eli Lilly. Data were collected by investigators, gathered by Parexel International, and analysed by the funder”. agents and collected and analysed the data. All the authors had full access to the data”. Declarations of interest, Quote (p 550‐551): "CEMG has received grants and personal fees from Eli Lilly, Abbvie, Janssen, Novartis, Sandoz, Pfizer, and GlaxoSmithKline; personal fees from Actelion, Amgen, and UCB Pharma; grants from LEO Pharma and Merck Sharp & Dohme; and is president of the International Psoriasis Council. KR has received personal fees from AbbVie, Amgen, Biogen, Celgene, Forward Pharma, Janssen‐Cilag, LEO Pharma, Eli Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, and Takeda. ML is an employee of the Mount Sinai Medical Center which receives research funds from AbGenomics, AbbVie, Amgen, Anacor, Aqua, Canfite Biopharma, Celgene, Clinuvel, Coronado Biosciences, Ferndale, Lilly, Janssen Biotech, LEO Pharmaceuticals, Merz, Novartis, Pfizer, Sandoz, and Valeant. PvdK has received grants from Celgene, Centocor, Allmiral, Pfizer, Philips, AbbVie, Eli Lilly, Galderma, Novartis, Janssen Cilag, and Leo Pharma; and has served as a speaker for Amgen, a consultant for Sandoz and Mitisibishu, and a speaker and consultant for Celgene, AbbVie, Eli Lilly, Galderma, Novartis, Janssen Cilag, and Leo Pharma. CP has received grants and personal fees from Amgen, Abbvie, Celgene, Eli Lilly, Novartis, Janssen, Pfizer, and Leo Pharma. KP has received honoraria as consultant and/or scientific officer and/or advisory board and/or steering committee member and/or acted as a paid speaker and/ or participated in clinical trials and/or received clinical research grants sponsored by 3M, Abbott/AbbVie, Akesis, Akros, Allergan, Alza, Amgen, Anacor, Apotex, Astellas, Baxter, Berlex, Biogen, Boehringer Ingelheim, Celgene, Celtic, Centocor, Cipher, Dermira, Dow Pharma, Eli Lilly, Forward Pharma, Fujisawa, Funxional Therapeutics, Galderma, Genentech, Genexion, GlaxoSmithKline, Isotechnika, Janssen, Janssen Biotech, Johnson & Johnson, Kataka, Kirin, Kyowa, Leo Pharma, Lypanosys, Medical Minds, Medimmune, Merck, Mitsubishi, Novartis, NovImmune, Pan Genetics, Pfizer, Roche, Regneron, Merck‐Serono, Stiefel, Takeda, UCB, Vertex, Wyeth/Pfizer, and Xoma. AM has served as an advisory board member and/or consultant and/or investigator and/or speaker and/or received compensation in the form of grants and/or honoraria from AbbVie, Allergan, Amgen, ApoPharma, Boehringer Ingelheim, Celgene, Convoy Therapeutics, Eli Lilly, Genentech, Janssen Biotech, LEO Pharma, Merck, Novartis, Pfizer, Symbio and Maruho, Syntrix, Wyeth, and XenoPort. GSC, JE, LZ, RJS, SB, DKB, OOO, MPH, and BJN were employees of and hold stock in Eli Lilly & Co during the conduct of this study. " |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 542): “randomly assigned”, “An interactive voice response system" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 542): “An interactive voice response system was used to assign double‐blind investigational product to every patient. Site personnel confirmed that they had located the correct assigned investigational product package by entering a confirmation number found in the package into to IVRS” Comment: clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 542): “Patients, investigators and study personnel were masked to the treatment allocation. A double‐dummy design was used” Comment: clearly defined |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 542): “Patients, investigators and study personnel were masked to the treatment allocation. A double‐dummy design was used” Comment: clearly defined |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1224, analysed 1224 Management of missing data: Quote (p 543): “All missing data were imputed using non‐responder imputation (NRI)” Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01597245). One pre‐specified outcome in the protocol missing from the results section (assessment of efficacy at 60 weeks); however, as we assessed outcomes at induction phase (between 8‐24 weeks), we judged that the risk of selective reporting was low. |
| Methods | RCT, active, placebo‐controlled, double blind Date of study: 18 July 2012‐18 January 2016 Location: 101 in Europe, Asia, North and South America |
|
| Participants |
Randomised: 1346 participants (mean age 46 years, 918 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ixekizumab (n = 386), SC, 80 mg, 2 injections week 0, 1 injection monthly Control intervention B. Ixekizumab (n = 385), SC, 80 mg, 2 injections week 0, 1 injection eow C. Etanercept (n = 382), SC, 50 mg 1 injection twice weekly D. Placebo (n = 193), SC |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: quote (p 543): “The funder Eli Lilly. Data were collected by investigators, gathered by Parexel International, and analysed by the funder”. agents and collected and analysed the data. All the authors had full access to the data”. Declarations of interest: quote (p 550‐551): "CEMG has received grants and personal fees from Eli Lilly, Abbvie, Janssen, Novartis, Sandoz, Pfizer, and GlaxoSmithKline; personal fees from Actelion, Amgen, and UCB Pharma; grants from LEO Pharma and Merck Sharp & Dohme; and is president of the International Psoriasis Council. KR has received personal fees from AbbVie, Amgen, Biogen, Celgene, Forward Pharma, Janssen‐Cilag, LEO Pharma, Eli Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, and Takeda. ML is an employee of the Mount Sinai Medical Center which receives research funds from AbGenomics, AbbVie, Amgen, Anacor, Aqua, Canfite Biopharma, Celgene, Clinuvel, Coronado Biosciences, Ferndale, Lilly, Janssen Biotech, LEO Pharmaceuticals, Merz, Novartis, Pfizer, Sandoz, and Valeant. PvdK has received grants from Celgene, Centocor, Allmiral, Pfizer, Philips, AbbVie, Eli Lilly, Galderma, Novartis, Janssen Cilag, and Leo Pharma; and has served as a speaker for Amgen, a consultant for Sandoz and Mitisibishu, and a speaker and consultant for Celgene, AbbVie, Eli Lilly, Galderma, Novartis, Janssen Cilag, and Leo Pharma. CP has received grants and personal fees from Amgen, Abbvie, Celgene, Eli Lilly, Novartis, Janssen, Pfizer, and Leo Pharma. KP has received honoraria as consultant and/or scientific officer and/or advisory board and/or steering committee member and/or acted as a paid speaker and/ or participated in clinical trials and/or received clinical research grants sponsored by 3M, Abbott/AbbVie, Akesis, Akros, Allergan, Alza, Amgen, Anacor, Apotex, Astellas, Baxter, Berlex, Biogen, Boehringer Ingelheim, Celgene, Celtic, Centocor, Cipher, Dermira, Dow Pharma, Eli Lilly, Forward Pharma, Fujisawa, Funxional Therapeutics, Galderma, Genentech, Genexion, GlaxoSmithKline, Isotechnika, Janssen, Janssen Biotech, Johnson & Johnson, Kataka, Kirin, Kyowa, Leo Pharma, Lypanosys, Medical Minds, Medimmune, Merck, Mitsubishi, Novartis, NovImmune, Pan Genetics, Pfizer, Roche, Regneron, Merck‐Serono, Stiefel, Takeda, UCB, Vertex, Wyeth/Pfizer, and Xoma. AM has served as an advisory board member and/or consultant and/or investigator and/or speaker and/or received compensation in the form of grants and/or honoraria from AbbVie, Allergan, Amgen, ApoPharma, Boehringer Ingelheim, Celgene, Convoy Therapeutics, Eli Lilly, Genentech, Janssen Biotech, LEO Pharma, Merck, Novartis, Pfizer, Symbio and Maruho, Syntrix, Wyeth, and XenoPort. GSC, JE, LZ, RJS, SB, DKB, OOO, MPH, and BJN were employees of and hold stock in Eli Lilly & Co during the conduct of this study. " |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 542): “randomly assigned” "An interactive voice response system" Comment: probably done" |
| Allocation concealment (selection bias) | Low risk | Quote (p 542): “An interactive voice response system was used to assign double‐blind investigational product to every patient. Site personnel confirmed that they had located the correct assigned investigational product package by entering a confirmation number found in the package into to IVRS” Comment: clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 542): “Patients, investigators and study personnel were masked to the treatment allocation. A double‐dummy design was used” Comment: clearly defined |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 542): “Patients, investigators and study personnel were masked to the treatment allocation. A double‐dummy design was used” Comment: clearly defined |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1346, analysed 1346 Management of missing data: Quote (p 543): “All missing data were imputed using non‐responder imputation (NRI)” Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01646177). One pre‐specified outcome in the protocol missing from the results section (assessment of efficacy at 60 weeks); however, as we assessed outcomes at induction phase (between 8‐24 weeks), we judged that the risk of selective reporting was low. |
| Methods | RCT, placebo‐controlled, single blind Date of study: not stated Location: one centre, Turkey |
|
| Participants |
Randomised: 50 participants (mean age 43 years, 25 male) Inclusion criteria
Exclusion criteria
Dropouts No participants lost to follow‐up |
|
| Interventions |
Intervention Acitretine (0.3‐0.5 mg/kg/day, 25 mg) (n = 25) Control intervention Placebo (n = 25) Co‐invervention NBUVB |
|
| Outcomes | Assessment at 12 weeks Primary outcome
Outcomes:
|
|
| Notes | Funding: none Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 3): "The physicians were not blinded" Comment: high risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "An independent assessor who is not from the team performed the outcome assessment." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomized 50, analysed 50, no loss to follow‐up during the 12 weeks Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, active‐controlled, open‐label trial Date of study: October 1998‐June 2000 Location: multicentre (> 1) in Amsterdam/the Netherlands |
|
| Participants |
Randomised: 88 participants, mean age 40 years, 57 male Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 44), orally, 15 mg/week until 4 weeks then increase up to 22.5 mg if reduction from baseline PASI < 25%, 3 divided doses with 12‐h interval, 12 weeks Control intervention B. Ciclosporin (n = 44), orally, 3 mg/kg until 4 weeks then increase up to 5 mg/kg if reduction from baseline PASI < 25%, 2 divided doses, 12 weeks |
|
| Outcomes | Assessments at weeks 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding sources, quote (p 664): "Supported by a grant (OG 97‐009) from the Dutch Health Authorities" Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 660): "Randomisation was performed centrally with the use of computer‐generated random numbers and block size of eight patients" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 660): "Randomisation was performed centrally with the use of computer‐generated random numbers and block size of eight patients" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 660): "The score of the PASI ... was determined... by trained assessors who were unaware of the treatment assignment" Comment: no description of method used to guarantee no communication between care givers or participants and assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88 randomised, 85 analysed Quote (p 660‐1): "If a patient missed a visit, we used the score from the previous visit". Comment: few lost to follow‐up, well‐balanced number and reasons between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: not stated Location: 1 centre in London |
|
| Participants |
Randomised: 41 participants (no description of the study population) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 19), orally, 2.5 mg every day for 1 week and 1 week after Control intervention B. Placebo (n = 17), orally, every day for 1 week and 1 week after |
|
| Outcomes | Assessments not clearly stated (reported at 4 weeks) Primary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee random sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1 & 2): “Control tablet of identical appearance... thus neither physician, patient nor pharmacist was aware whether drug or control had been dispensed” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1 & 2): “Control tablet of identical appearance... thus neither physician, patient nor pharmacist was aware whether drug or control had been dispensed” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 41 randomised participants and 38 analysed Comment: no description of the method used to manage missing data Not ITT analyses |
| Selective reporting (reporting bias) | High risk | No pre‐specified outcomes mentioned in the methods section |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: March 2008‐March 2010 Location: 35 centres in Japan |
|
| Participants |
Randomised: 160 participants (age median 45 years, 126 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ustekinumab (n = 64), SC, 45 mg, weeks 0‐4, every 12 weeks, 64 weeks Control intervention B. Ustekinumab (n = 62), SC, 90 mg, weeks 0‐4, every 12 weeks, 64 weeks C. Placebo (n = 32), SC , weeks 0‐4, every 12 weeks, 64 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 242): "This study was supported by Janssen pharmaceutical KK, a part of the Johnson & Johnson family of companies. Declarations of interest (p 242): "Igarashi has served as a consultant and speaker for Janssen Pharmaceutical K.K.; H. Nakagawa has served as a consultant for Abbott Japan and Tanabe Mitsubishi, and as a consultant and speaker for Janssen Pharmaceutical K.K.; M. Song is an employee of Centocor Research & Development, Inc., a division of Johnson & Johnson Pharmaceutical Research & Development, L.L.C., and owns stock in Johnson & Johnson; T. Kato and M. Kato are employees of Janssen Pharmaceutical K.K. and own stock in Johnson & Johnson." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 244): “randomised” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 244): “randomised” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 243): “double‐blind placebo‐control” Comment: used a placebo without visible side effect |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 243): “double‐blind placebo‐control” Comment: used a placebo without visible side effect |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 160 randomised, 157 analysed (2 did not received a dose of the drug and 1 was excluded in the placebo group due to lack of efficacy data after receiving a single dose) Methods for dealing with missing data Quote (p 244): “Efficacy analyses were based on all randomised patients with efficacy data after randomisation... Patients who discontinued the study... were considered as treatment failures” Comment: few lost of follow‐up, well‐balanced number and reasons between groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: not stated Location: not stated |
|
| Participants |
Randomised: 16 participants (mean age 50 years, 11 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Alefacept (n = 8), IM, 15 mg, once a week, 12 weeks Control intervention B. Placebo (n = 8), IM, once a week, 12 weeks Co‐intervention: UVB 3 times/week, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary or secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding source (p 1068): "Biogen/IDEC provided alefacept and NB UVB for this study" Declarations of interest (p 1068): "none reported" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 1067): "randomised... study" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 1067): "randomised... study" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1067) 'double‐blind study... placebo" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomized (16), analysed (15) Comment: no description of the method used to manage the missing data, no ITT analyses |
| Selective reporting (reporting bias) | High risk | Comment: no pre‐specified outcomes mentioned in the methods section except PASI score. In the results section, safety, biological data are reported |
| Methods | RCT, placebo‐controlled, double blind Date of study: 21 November 1999‐22 March 2001 Location: 51 centres in USA/Canada |
|
| Participants |
Randomised: 553 participants analysed (mean age 45 years, 387 male) out of 569 patients randomised Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Alefacept (n = 367), IV, 7.5 mg, once a week, 12 weeks Control intervention B. Placebo (n = 186), IV, once a week, 12 weeks |
|
| Outcomes | Assessments at 14 weeks Primary or secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding source, quote (p 821): "This study was funded by Biogen, Inc, Cambridge, Massachusetts" Declarations of interest p 821): "A patent on the use of alefacept for the treatment of psoriasis has been assigned to Biogen and the University of Michigan. None of the authors have a financial interest in the patent. Gerald G. Krueger, MD, Kim A. Papp, MD, and Charles N. Ellis, MD, are consultants to Biogen, as well as to other companies that have and are developing treatments for psoriasis." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 822): " This was a randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 822): " This was a randomised..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 823): “... The active drug and placebo were visually indistinguishable from each other, and the injection volume was consistent. Physician who had no contact with the patient, … A pharmacist who had no contact with the patient…. The physician and the pharmacist were instructed not to communicate any information to the examining physicians….” Comment: use of a placebo with no major side effects |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 823): "An examining physician administered the study drug" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 569 included/557 analysed (comment: 16 participants excluded, 7 because of poor compliance and 9 were not dosed) Quote (p 825): "Analyses of efficacy end points ... were based on the intent‐to‐treat population, which included patients who were randomised had a baseline assessment and had at least 1 injection" Methods for dealing with missing data: not stated Comment: not modified ITT (7 participants excluded because of poor compliance) however 7/557 low rate |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: June 2003–March 2005 Location: 46 centres in Utah, USA |
|
| Participants |
Randomised: 320 participants Ustekinumab 12/23 45 mg (64) (mean age 46 years; 38 male) Ustekinumab 12/23 90 mg (64) (mean age 46 years; 47 male) Ustekinumab 12/23 45 mg 4‐weekly (64) (mean age 45 years; 39 male) Ustekinumab 12/23 90 mg 4‐weekly (64) (mean age 44 years; 52 male) Placebo (64) (mean age 44 years; 46 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ustekinumab 12/23 (n = 64), SC, 45 mg, 45 mg 1 dose, 1 week Control intervention B. Ustekinumab 12/23 (n = 64), SC, 90 mg, 45 mg 1 dose, 1 week C. Ustekinumab 12/23 (n = 64), SC, 45 mg, 45 mg/week, 4 weeks D. Ustekinumab 12/23 (n = 64), SC, 90 mg, 45 mg/week, 4 weeks E. Placebo (n = 64), SC |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source (p 590): "Supported by Centocore, Malvern, PA" Declarations of interest (p 590‐1): "Dr. Krueger reports receiving fees as a consultant or advisory board member for Abbott, Almirall, Alza, Amgen, Astellas, Boehringer Ingelheim, Barrier Therapeutics, Bristol‐Myers Squibb, Centocor, Connetics, and Genentech; Dr. Langley, for Centocor, Abbott, and Amgen/Wyeth; Dr. Leonardi, for Abbott, Amgen, Centocor, and Genentech; and Dr. Lebwohl, for Abbott, Amgen, Astellas, Centocor, Connetics, Galderma, Genentech, Novartis, PharmaDerm, and Warner Chilcott. Dr. Krueger reports receiving lecture fees from Abbott, Amgen, Boehringer Ingelheim, Centocor, and Connetics; Dr. Langley, from Abbott and Amgen/ Wyeth; Dr. Leonardi, from Abbott, Amgen, Centocor, and Genentech; and Dr. Lebwohl, from Abbott, Astellas, Amgen, Centocor, Connetics, Galderma, Genentech, PharmaDerm, and Warner Chilcott. Dr. Krueger reports receiving stipends for a clinical research fellowship from Amgen and Centocor; Dr. Langley, grant support from Centocor, Abbott, and Amgen/Wyeth; Dr. Leonardi, educational grants from Amgen and Genentech; and Dr. Lebwohl, grants from Abbott, Amgen, Astellas, Centocor, Connetics, Galderma, Genentech, PharmaDerm, and Warner Chilcott. Drs. Yeilding, Guzzo, Wang, and Dooley report being employees of Centocor. Dr. Krueger reports owning stock options from ZARS Pharma; Drs. Yeilding, Guzzo, and Dooley report holding stock and stock options in Johnson & Johnson; and Dr. Wang reports being a stockholder in Johnson & Johnson. No other potential conflict of interest relevant to this article was reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 581): “Patients ... were randomly assigned” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 581): “Patients ... were randomly assigned” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 581): “This placebo‐controlled, double‐blind...phase 2 study” Comment: placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 581): “This placebo‐controlled, double‐blind...phase 2 study” Comment: no specific description of the method used to guarantee blinding of outcome assessment however considering that this is a placebo‐controlled trial with no known systematic AEs we considered the risk as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 320 included/320 analysed Quote (p 582): "Efficacy data from all patients who underwent randomisation were analysed... Missing values at week 12 were replaced with the most recently available values for all efficacy variables, missing data at other time points were not imputed" Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00320216). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: March 2013‐November 2013 Location: 6 centres in the USA |
|
| Participants |
Randomised: 12 participants (mean age 45.5 years, 8 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Tofacitinib (n = 9), orally, 10 mg twice daily Control intervention B. Placebo (n = 3) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 1079): “This study was sponsored by Pfizer Inc. Both Pfizer Inc and non‐Pfizer Inc authors have participated in the study design, data collection, data analysis, and open scientific discussion of the data; its interpretation; and the development of the associated manuscript. The views and opinions expressed within the manuscript are those of all authors and do not necessarily represent those of the funding organization. Medical writing support was funded by Pfizer Inc.”. Declarations of interest (p 1079) : "J. Krueger received research funding from Novartis, Pfizer Inc, Janssen, Lilly, Merck, Kadmon, Dermira, Boehringer, BMS, and Paraxel during the conduct of the study; grants paid to institutions from Amgen, Innovaderm and Kyowa; and personal fees from Serono, BiogenIdec, Delenex, AbbVie, Sanofi, Baxter, Xenoport, and Kineta. M. Suárez‐Fariñas receives research funding and speakers' fees from Pfizer. J. D. Clark, H. Tan, R. Wolk, S. T. Rottinghaus, M. Z. Whitley, H. Valdez, D. von Schack, S. P. O'Neil, P. S. Reddy, and S. Tatulych are employees of Pfizer Inc. The rest of the authors declare that they have no relevant conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1079): “Patients were randomised 3:1 to receive 10 mg of oral tofacitinib or placebo twice daily for 12 weeks by using an automated Web or telephone randomization system” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1079): “Patients were randomised 3:1 to receive 10 mg of oral tofacitinib or placebo twice daily for 12 weeks by using an automated Web or telephone randomisation system” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1079): “This was a phase 2, randomised, placebo‐controlled, double‐blind study carried out in 6 centers” Comment: placebo‐controlled, probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1079): “This was a phase 2, randomised, placebo‐controlled, double‐blind study carried out in 6 centers” Comment: placebo controlled, probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 12, analysed 11 Management of missing data: Quote: not mentioned |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01710046). The pre‐specified outcomes in the protocol or those mentioned in the methods section have been reported in the results section. |
| Methods | RCT, placebo‐controlled, double blind Date of study: not stated Location: 20 centres in India |
|
| Participants |
Randomised: 331 participants (mean age 37 years, 65 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Itolizumab (n = 91,) IV, 0.4 mg/kg for 4 weeks, then 1.6 mg/kg once a week for 4 weeks, then eow to 12 weeks Control intervention B. Itolizumab (n = 91), IV, 1.6 mg/kg for 4 weeks, then 1.6 mg/kg once a week for 4 weeks, then eow to 12 weeks C. Placebo (n = 43), IV |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source (p 484): "Biocon limited funded this study and supplied the drug". Comment: Biocon Limited designed, performed, analysed and wrote the paper (medical writing) Declarations of interest (p 484): "Drs Krupashankar, Dogra, Kura, Saraswat, Leelavathy, Sumathy, Shah, Gopal, Narayana Rao, Srinivas, Bhat, Shetty, Manmohan, Sai Krishna, Padmaja, Pratap, Garg, Gupta, Pandey, and Khopkar were investigators who conducted the clinical trial described in this manuscript and collected the trial data; they received honoraria from the study sponsor. Dr Montero is a part‐time employee of Biocon Research Limited. Drs Ramakrishnan, Nair, and Ganapathi are employees of Biocon Research Limited." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 485): “This was a 52‐week phase 3, double‐blind, randomised, placebo‐controlled...” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 485): “This was a 52‐week phase 3, double‐blind, randomised, placebo‐controlled...” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 485): "To maintain blind dummy infusion was given" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 485): "To maintain blind dummy infusion was given" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 225 randomised, 220 analysed (not dosed (2), withdrawn the day after the first infusion (2), lost to follow‐up (1)) Quote (p 486): "The full analysis set intent‐to‐treat population contained randomised patients who received at least 1 infusion and had at least 1 visit after enrollement, ..., Missing values were imputed using last observation carried forward" Comment: few lost to follow‐up, well‐balanced number and reasons between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled, open‐label trial Date of study: not stated Location: 27 centres worldwide |
|
| Participants |
Randomised: 251 participants (mean age 41 years, 176 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ciclosporin A (n = 119), orally, 2.5 mg/kg/d, 12 weeks Control intervention B. Ciclosporin A (n = 132), orally, 5 mg/kg/d, 12 weeks |
|
| Outcomes | Period assessments: 12 weeks Primary or secondary outcomes of the trial:
Outcmes of the trial
|
|
| Notes | Funding and declarations of interest: not stated but the first author was employed by Sandoz Pharma Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 367): "... was an open randomised study in parallel group" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 367): "... was an open randomised study in parallel group" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 367): "... was an open randomised study in parallel group" Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 367): "... was an open randomised study in parallel group" Comment: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Management of missing data: no description of the method used to guarantee management of missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: June 2011‐April 2013 Location: 88 centres worldwide (Erasure) |
|
| Participants |
Randomised: 738 participants mean age 45 years, 509 male Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab 300 (n = 245), SC, 300 mg, weeks 0, 1, 2, 3, 4 and every 4 weeks, 12 weeks Control intervention B. Secukinumab 150 (n = 245), SC, 150 mg, weeks 0, 1, 2, 3, 4 and every 4 weeks, 12 weeks C. Placebo (n = 248), SC, weeks 0, 1, 2, 3, 4 and every 4 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 326): "funded by Novartis Pharmaceuticals" Declarations of interest (p 337): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Langley received personal fees from Eli Lilly, Leo, Novartis, Janssen, Amgen, AbbVie, Celgene, Merck, Pfizer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol and Appendix): "Randomization numbers were generated by the Interactive Response Technology (IRT) provider using a validated system, which automated the random assignment of subject numbers to randomisation numbers..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol and Appendix): "Randomization numbers were generated by the Interactive Response Technology (IRT) provider using a validated system, which automated the random assignment of subject numbers to randomisation numbers..." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol and Appendix): “Subjects, investigator staff, persons performing the assessments, and data analysts were blinded to the identity of the treatment from the time of randomisation until primary objective analyses" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol and Appendix): “Subjects, investigator staff, persons performing the assessments, and data analysts were blinded to the identity of the treatment from the time of randomisation until primary objective analyses" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 738 included/738 analysed Quote (p 329): "The analyses of the efficacy end points included all the patients who underwent randomisation according to the treatment assigned at randomisation... Missing values ... were conservatively imputed as nonresponses, regardless the reason of missing data" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01365455). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active, placebo‐controlled, double‐blind trial Date of study: June 2011‐June 2013 Location: 231 centres worldwide (Fixture) |
|
| Participants |
Randomised: 1306 participants, mean age 44 years, 929 male Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Sekunimab 300 (n = 327), SC , 300 mg, weeks 0, 1, 2, 3, 4 and every 4 weeks, 12 weeks Control intervention B. Sekunimab 150 (n = 327), SC, 150 mg, weeks 0, 1, 2, 3, 4 and every 4 weeks, 12 weeks C. Etanercept 50 (n = 326), SC, 50 mg/week twice a week, 12 weeks D. Placebo (n = 326), SC, weeks 0, 1, 2, 3, 4 and every 4 weeks, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 326): "funded by Novartis Pharmaceuticals" Declarations of interest (p 337): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Langley received personal fees from Eli Lilly, Leo, Novartis, Janssen, Amgen, AbbVie, Celgene, Merck, Pfizer." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol and Appendix): "Randomization numbers were generated by the Interactive Response Technology (IRT) provider using a validated system, which automated the random assignment of subject numbers to randomisation numbers..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol and Appendix): “Subjects, investigator staff, persons performing the assessments, and data analysts were blinded to the identity of the treatment from the time of randomisation until primary objective analyses" "Randomization numbers were generated by the Interactive Response Technology (IRT) provider" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol and Appendix): “Subjects, investigator staff, persons performing the assessments, and data analysts were blinded to the identity of the treatment from the time of randomisation until primary objective analyses Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol and Appendix): “Subjects, investigator staff, persons performing the assessments, and data analysts were blinded to the identity of the treatment from the time of randomisation until primary objective analyses Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (p 329): "The analyses of the efficacy end points included all the patients who underwent randomisation according to the treatment assigned at randomisation... Missing values ... were conservatively imputed as nonresponses, regardless the reason of missing data" 1306 included/1306 analysed Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01358578). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: 23 March 2000‐05 January 2001 Location: 64 centres in Europe, USA and Canada |
|
| Participants |
Randomised: 507 participants (mean age 45 years, 333 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Alefacept 10 (n = 173), IM, 10 mg once a week, 12 weeks Control intervention B. Alefacept 15 (n = 166), IM, 15 mg once a week, 12 weeks C. Placebo (n = 168), IM, 0.9 mL once a week, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary or secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding source, quote (p 725): "Support for this research and data monitoring and analysis were provided by Biogen Inc." Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 720): "The present multicenter, randomised...ICT Inc..., a contract research organization, was responsible for patient randomisation and tracking and study inventory" Comment: unlikely to introduce selection bias |
| Allocation concealment (selection bias) | Low risk | Quote: "a contract research organization, was responsible for patient randomisation and tracking and study inventory" "Unblinded pharmacist prepared, coded… and maintained drug accountability” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 720): "Unblinded pharmacist prepared, coded… and maintained drug accountability” Comment: placebo‐controlled, probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 720): "All efficacy measures were assessed ... by a dermatologist blinded to treatment" Comment: placebo‐controlled, probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 507 included/507 analysed Quote (p 721): "Statistical analyses for efficacy measures were based on the intent‐to‐treat population composed of those patients who were randomised, had at least 1 injection and had a baseline assessment" Comment: no description of the method used to manage missing data however number and reasons for withdrawal well‐balanced between groups |
| Selective reporting (reporting bias) | High risk | Comment: no protocol was available. No pre‐specified primary outcome |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: August 2012‐September 2014 Location: 142 centres worldwide |
|
| Participants |
Randomised: 1831 participants (mean age 45 years, 1258 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Brodalumab (n = 610), SC, 140 mg (2 injections week 0, 1 injection eow) Control intervention B. Brodalumab (n = 612), SC, 210 mg (2 injections week 0, 1 injection eow) C. Ustekinumab (n = 300), SC, 45/90 mg (week 0, week 4 and every 12 weeks) D. Placebo (n = 309), orally (same drug administration) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 1319) “Amgen funded both studies. ... and Amgen conducted the data analyses. All the authors interpreted the data” Declarations of interest (p 1327): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Dr. Lebwohl reports grant support from Amgen, AbbVie, Janssen Biotech, UCB Pharma, Pfizer, Celgene, Eli Lilly, and Novartis outside the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol): “The randomisation lists will be generated by Amgen using a permuted block design within each strata...via an interactive voice response system” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol): “The randomisation lists will be generated by Amgen using a permuted block design within each strata...via an interactive voice response system” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol, cf 6. Treatment procedure): “This is a double dummy procedure...” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol, cf 6. Treatment procedure): “This is a double dummy procedure...” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1831, analysed 1831 Dealing with missing data Quote (protocol & p 1321) "...with missing data imputed as indicating no response" Comment: well described |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT0178603). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported except for participant‐reported outcome |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: September 2012‐August 2014 Location: 142 centres worldwide (no sites that were included in the AMAGINE‐2 study) |
|
| Participants |
Randomised: 1881 participants (mean age 45 years, 1288 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Brodalumab (n = 629), SC, 140 mg (2 injections week 0, 1 injection eow) Control intervention B. Brodalumab (n = 624), SC, 210 mg (2 injections week 0, 1 injection eow) C. Ustekinumab (n = 313), SC, 45/90 mg (week 0, week 4 and every 12 weeks) D. Placebo (n = 315), orally (same drug administration) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 1319) “Amgen funded both studies. ... and Amgen conducted the data analyses. All the authors interpreted the data” Declarations of interest (p 1327): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Dr. Lebwohl reports grant support from Amgen, AbbVie, Janssen Biotech, UCB Pharma, Pfizer, Celgene, Eli Lilly, and Novartis outside the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol): “The randomisation lists will be generated by Amgen using a permuted block design within each strata... Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol): “The randomisation lists will be generated by Amgen using a permuted block design within each strata...via an interactive voice response system” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol, cf 6. Treatment procedure): “This is a double dummy procedure...” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol, cf 6. Treatment procedure): "This is a double dummy procedure...” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 1881, analysed 1881 Dealing with missing data Quote (protocol & p 1321) "...with missing data imputed as indicating no response" Comment: well described |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01708629). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported except for participant‐reported outcome |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: December 2001‐April 2002 Location: 47 centres in USA |
|
| Participants |
Randomised: 672 participants (mean age 45 years, 672 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept LD (n = 169), SC auto‐administered, 25 mg, once/week, 12 weeks Control intervention B. Etanercept MD (n = 167), SC auto‐administered, 25 mg, twice/week, 12 weeks C. Etanercept HD (n = 168), SC auto‐administered, 50 mg, twice/week, 12 weeks D. Placebo (n = 168), SC, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 2021): "Supported by Immunex, Seattle, a wholly‐owned subsidiary of Agen, Thousand Oaks, Calif" Declarations of interest (p 2021): "Drs. Leonardi, Powers, Goffe, and Gottlieb report having served as consultants for Amgen, and Drs. Leonardi, Goffe, and Gottlieb report having served as paid lecturers for Amgen. Dr. Gottlieb reports having served as a consultant and paid lecturer for Johnson & Johnson, Genentech, and Biogen; Dr. Leonardi reports having served as a consultant and paid lecturer for Johnson & Johnson and Genentech; Dr. Powers reports having served as a consultant for Genentech and Biogen; and Dr. Goffe reports having served as a consultant and paid lecturer for Biogen. Dr. Zitnik and Ms. Wang report owning equity in Amgen." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2016): "Patients underwent central randomisation with the use of a permuted block randomisation list, with equal allocation to each of the four treatment groups" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: no description of the method used to guarantee the allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 2015): "Double‐blind... Etanercept ... was supplied to patients in syringes, each containing the contents of one reconstituted vial of etanercept or matching placebo...All patients received two injections per dose of study" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 2015): "Double‐blind... Etanercept ... was supplied to patients in syringes, each containing the contents of one reconstituted vial of etanercept or matching placebo...All patients received two injections per dose of study" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 672 randomised participants, 652 analysed (20 participants did not receive the treatment and were excluded from the analyses) Comment: modified ITT but number of participants not receiving treatment and not included in the analysis low and comparable between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: April 2010‐May 2011 Location: 23 centres internationally |
|
| Participants |
Randomised: 142 participants (mean age 46 years, 81 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Placebo (n = 27), SC, 0, 2, 4, 8, 12, 16 weeks, 16 weeks Control intervention B. Ixekizumab (n = 28), SC, 10 mg, 0, 2, 4, 8, 12, 16 weeks, 16 weeks C. Ixekizumab (n = 30), SC, 25 mg, 0, 2, 4, 8, 12, 16 weeks, 16 weeks C. Ixekizumab (n = 29), SC, 75 mg, 0, 2, 4, 8, 12, 16 weeks, 16 weeks C. Ixekizumab (n = 28), SC, 150 mg, 0, 2, 4, 8, 12, 16 weeks, 16 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 1190): "Funded by Eli Lilly" Declarations of interest (p 1198): "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Leonardi received personal fees from Abbott, Amgen, Certocor, Eli Lilly and Pfizer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol p 44): “... from the central randomisation center using an IVRS” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol p 44): “... from the central randomisation center using an IVRS” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol p 22): “The investigators and patients are blinded while the sponsor is unblinded to study assignment” Comment: placebo‐controlled trial, no systematic AE for the drug, probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol p 22): “The investigators and patients are blinded while the sponsor is unblinded to study assignment” Comment: placebo‐controlled trial, no systematic AE for the drug, probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Included 142/141 analysed (1 in the placebo group who did not have any post‐baseline assessment) Quote (protocol p 62 & p 1192): "All efficacy and health outcome analyses will be conducted on all patients who received any amount of study drug and have any post‐baseline efficacy assessment....Missing data for the primary timepoint at week 12 will be imputed by the last observation carried forward method" Comment: m‐ITT and 1 participant out of 142 was not included in the analyses |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01107457). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: December 2005–September 2007 Location: 48 centres in USA, Canada, Belgium |
|
| Participants |
Randomised: 766 participants (mean age 45 years, 531 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ustekinumab (n = 255), SC, 45 mg, weeks 0‐4 and every 12 weeks, 40 weeks Control intervention B. Ustekinumab (n = 256), SC, 90 mg, weeks 0‐4 and every 12 weeks, 40 weeks C. Placebo (n = 255), SC, weeks 0‐4, 40 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 1665): Centocor Inc. Declarations of interest (p 1673): "CLL has served as a consultant for Abbott, Amgen, Centocor, and Genentech, as an investigator for Abbott, Allergan, Altana, Alza, Amgen, Astellas, Celgene, Centocor, Genentech, Bristol Myers, Eli Lilly, Fujisawa, Galderma, CombinatoRx, 3M Pharmaceuticals, Perrigo Isreal Pharamceutical, ScheringPlough, Serono, RTL, Novartis, Vitae, and Wyeth, and as a speaker for Abbott, Amgen, Centocor, Genentech, and Warner Chilcott. ABK has served as an investigator and consultant for Abbott, Amgen, and Centocor and has been a study steering committee member, speaker, and fellowship funding recipient from Centocor. KAP has served as a consultant and advisory board member for Abbott, Alza, Amgen, Celgene, Centocor, Johnson and Johnson, Isotechnika, Janssen Ortho Biotech, Medimmune, MerckSerono, and Wyeth. KBG has served as a consultant for Abbott, Amgen, Astellas, Centocor, and Genentech and has received grant support from Abbott, Astellas, and Centocor. NY, CG, YW, SL, and LTD are employees of Centocor and own stock in Johnson and Johnson." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1667‐68): “...via a centralised interactive voice response system” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 1667‐68): “...via a centralised interactive voice response system” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1666‐67): “This phase 3, double‐blind, placebo‐controlled... Patients received placebo injections as needed to preserve the blind. The study sponsor was unblinded to treatment... Site monitors, investigators, site personnel involved in the study conduct, and patients remained blinded until week 76” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1666‐67): “This phase 3, double‐blind, placebo‐controlled... Patients received placebo injections as needed to preserve the blind. The study sponsor was unblinded to treatment... Site monitors, investigators, site personnel involved in the study conduct, and patients remained blinded until week 76” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Included 255/256/255 Analysed 255/256/255 Quote (p 1668): "Efficacy data from all randomised patients were analysed according to the assigned treatment group.... Patients who discontinued study treatment... were deemed to be treatment failures" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00267969). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: not stated Location: 2 centres in Santa Monica and New York City, USA |
|
| Participants |
Randomised: 34 participants, age range 20‐75 years, 24 male Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 16), orally, 50 mg, daily, 12 weeks Control intervention B. Placebo (n = 18), orally, daily, 12 weeks Co‐intervention: UVB (phototherapy) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source (p 591): "Supported by Roche dermatologics, Nutley, New Jersey and the Skin Research Foundation of California, Santa Monica, California" Declarations of interest; not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 592): "Patients receiving UVB phototherapy were randomly assigned" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 592): "Patients receiving UVB phototherapy were randomly assigned" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 592): "were randomly assigned to either acitretin or placebo" Comment: no more precision however adverse effects of acitretin such as cheilitis are visible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 592): "were randomly assigned to either acitretin or placebo... the same observer who was unaware of patient group examined the patients throughout the investigation" Comment: no more precision however adverse effects of acitretin such as cheilitis are visible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 34 included / 34 analysed (Table 2) Comment: no description of the method used to manage the missing data or to perform the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: January 2007–September 2007 Location: 1 centre in Chandighar, India |
|
| Participants |
Randomised: 40 participants (mean age 37 years, 29 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate 0.5 mg/kg + folic acid, (n = 20), orally 5 mg/d Day‐1; Day+1 + NBUVB 3/week max 1200 mJ/cm2 Control intervention B. Placebo + folic acid (n = 20), orally, 5 mg/d Day‐1; Day+1 + NBUVB 3/week max 1200 mJ/cm2 |
|
| Outcomes | Assessments at 6 months Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: not stated Declarations of interest (p 595): "not declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 596): “... were randomised by way of random number table” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 596): “... were randomised by way of random number table” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 596): “patient‐blinded study” Comment: not double blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 596): “patient‐blinded study” Comment: not double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20/20 included; 20/20 analysed Quote (p 596):“Intention to treat principle was followed for the analysis of the observations” Comment: no description of the method used to manage the missing data |
| Selective reporting (reporting bias) | Low risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, placebo‐controlled, double blind Date of study: not stated Location: 17 centres in Germany |
|
| Participants |
Randomised: 128 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ciclosporin (n = 43), orally, 1.25 mg/kg/d, 10 weeks Control intervention B. Ciclosporin (n = 41), orally, 2.5 mg/kg/d, 10 weeks C. Placebo (n = 44), orally, 10 weeks |
|
| Outcomes | Assessments at 10 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source not stated however 3 out of 4 authors from Sandoz pharmaceuticals Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 77): "patients were randomised" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 77): "double blind study period" Comment: no description of the method used to guarantee blinding regarding the need of hypertension and renal function surveillance and modification in ciclosporin groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 77): "double blind study period" Comment: no description of the method used to guarantee blinding, regarding the need of hypertension and renal function surveillance and modification in ciclosporin groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 128 included/120 analysed Comment: methods for dealing with missing data not specified, not ITT analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: not stated Location: 63 centres in Europe, USA, Canada |
|
| Participants |
Randomised: 835 participants (mean age 44 years, 551 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Infliximab (n = 313), IV, 3 mg/kg, weeks 0, 2, 6; 10 weeks Control intervention B. Infliximab (n = 314), IV, 5 mg/kg, weeks 0, 2, 6; 10 weeks C. Placebo (n = 208), IV, weeks 0, 2, 6; 10 weeks |
|
| Outcomes | Assessments at 10 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding (p 31. e1) by Centocor, Inc, Malvern, Penn, and Schering‐Plough, Kenilworth, NJ. Declarations of interest (appendix): "Dr Menter has received consulting, research, and/or speaking support from Abbott Laboratories, Allergan Inc, Allermed, Amgen Inc, Astralis Inc, Berlex Inc, Biogen Idec Inc, Centocor Inc, Cephalon, Collagenex Pharmaceuticals, CombinatoRx, Connetics Corp, Corixa Corporation, Dermik Laboratories, Doak Dermatologics, Dow, Ferndale Laboratories Inc, Fujisawa Healthcare Inc, Galderma, Genentech Inc, Genzyme, GlaxoSmithKline, Ligand Pharmaceuticals, Medicis, Med‐Immune Inc, Novartis Pharmaceuticals, Otsuka Pharmaceutical Inc, Protein Design Labs, QLT USA, Regeneration Pharma AG, Roche Laboratories, Serono, Sinclair, Synta Pharma, Thermosurgery, 3M Pharmaceuticals, Vertex, XOMA, and Zars Inc. Dr Feldman has received consulting, research, and/or speaking support from Amgen, Centocor, and Biogen. Dr Papp's support is as follows: Abbott: Investigator, Consultant; Amgen: Investigator, Consultant, Speaker, Advisory Boards; Centocor: Investigator, Consultant, Speaker, Senior Medical Officer for Canada (non‐compensatory), Advisory Boards; Genentech: Investigator, Consultant, Speaker, Senior Medical Officer for Canada (non‐compensatory), Advisory Boards; Serono: Investigator, Consultant, Speaker, Advisory Boards; Schering: Investigatory, Consultant, Speaker, Advisory Boards; and Wyeth: Speaker, Advisory Boards. Dr Weinstein has received consulting, research, and/or speaking support from Allergan, Amgen, Centocor, Biogen, Genentech, Valeant, Collagenex, CombinatoRx, Fujisawa, Abbott, and QLT. Dr Gottlieb has received research support from and/or is a consultant and/or speaker for Amgen, Inc, BiogenIdec, Inc, Centocor, Inc, Genentech, Inc, Abbott Labs, Ligand Pharmaceuticals, Inc, Beiersdorf, Inc, Fujisawa Healthcare, Inc, Celgene Corp, Bristol Myers Squibb, Inc, Warner Chilcott, Paradigm, Wyeth Pharmaceuticals, Schering‐Plough Corp, Eisai, Roche, Sankyo, Medarex, Kemia, Celera, TEVA, Actelion, and Amarill. At the time of the study, Dr Gottlieb was affiliated with the Clinical Research Center, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, New Brunswick, NJ. Dr Guzzo, Dr Dooley, Ms Li, and Ms Arnold are employees of Centocor, Inc. Mr Evans was an employee of Centocor, Inc at the time this study was conducted and is currently an employee of Scios, Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 31; e2): "Randomizations were performed by ClinPhone (Lawrenceville, NJ), allocating patients using a minimization algorithm with a biased coin assignment by means of an interactive voice response system" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 31; e2): "Randomizations were performed by ClinPhone (Lawrenceville, NJ), allocating patients using a minimization algorithm with a biased coin assignment by means of an interactive voice response system" "Patients, investigators, and all study staff except pharmacists were blinded to treatment assignments" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 31. e2): "Patients, investigators, and all study staff except pharmacists were blinded to treatment assignments... to receive IFX 3 mg/Kg or 5mg/Kg or placebo"" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 31. e2): "Patients, investigators, and all study staff except pharmacists were blinded to treatment assignments... to receive IFX 3 mg/Kg or 5mg/Kg or placebo"" Comment: placebo‐controlled, probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 835 included / 835 analysed Quote (p 31.e3/4): "For patients who discontinued... these patients were considered as not meeting the respective end‐points regardless of the observed data" Comment: ITT |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: December 2004‐August 2007 Setting: 81 centres (67+14)) in USA, Canada |
|
| Participants |
Randomised: 1212 participants (mean age 44 years, 803 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Adalimumab (n = 814), SC, 40 mg, week 0: 2 injections, week 1: eow, 16 weeks Control intervention B. Placebo, SC (n = 398), week 0: 2 injections/week 1: eow, 16 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source quote (p 106): "Supported by Abbott Laboratories Declarations of interest (p 106): "Dr Menter has received research support and/or lecture honoraria from Abbott, Amgen, Astellas, Biogen, Centocor, Genentech, and Wyeth. Dr Tyring has received research support from, has consulted for, and is part of the speakers’ bureaus for Abbott. Dr Gordon has received research support and honoraria from Abbott, Amgen, and Centocor. Dr Kimball is an investigator, speaker, and consultant for Abbott, Amgen, Biogen, Centocor, and Genentech. Dr Leonardi is a consultant for Abbott, Amgen, Centocor, and Genentech and is an investigator for Abbott, Allergan, Altana, Amgen, Astellas, Biogen, Bristol Myers, Centocor, Fujisawa, Galderma, Genentech, Serono, CombinatoRx, 3M Pharmaceuticals, Schering Plough, RTL, and Vitae; he also received an educational grant from Amgen and Genentech, and is part of the speakers’ bureaus for Abbott, Amgen, Centocor, Genentech, and Warner Chilcott. Dr Langley is a scientific advisory board member, investigator, and speaker for Abbott, Amgen, Astellas, Centocor, Norvartis, and Wyeth. Dr Strober serves on the advisory boards of, has received honoraria from, and is an investigator for Abbott, Amgen, Astellas, Centocor, Genentech, and Wyeth, and is part of the speakers’ bureaus for Abbott, Amgen, Astellas, Genentech, and Wyeth. Dr Kaul, Ms Gu, and Dr Okun are employees of Abbott Laboratories. Dr Papp is a consultant for and has received honoraria and travel grants from Abbott, Alza, Amgen, Astellas, Celgene, Centocor, Genentech, Isotechnika, Johnson and Johnson, Serono, Schering‐Plough, and UCB." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 107): ”Randomization schedules were generated by one of our data management departments before study inception” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 107): ”Patients were randomised by centre via an interactive voice response system". "ADA and placebo‐filled syringes were identically labelled and packaged, and self‐administrated by patients" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 107): "Double‐blind, placebo‐controlled... ADA and placebo‐filled syringes were identically labelled and packaged, and self‐administrated by patients" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 107): "Double‐blind, placebo‐controlled... ADA and placebo‐filled syringes were identically labelled and packaged, and self‐administrated by patients" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1212 included/1212 analysed Quote (p 109): "The primary efficacy analyses were conducted on ITT population... a patient with missing data for a visit... had the last observation carried forward" Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT002377887). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported except for participant‐reported outcome |
| Methods | RCT, active‐controlled, double blind Date of study: November 2012‐November 2015 Setting: 57 centres in Austria, Germany, the Netherlands and Poland |
|
| Participants |
Randomised: participants (mean age, male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Dimethyl fumarate (DMF) (n = 280), orally, maximum daily dose of 720 mg DMF Control intervention B. DMF + salt of monoethyl fumarate (n = 286), orally, maximum daily dose of 720 mg DMF C. Placebo (n = 138) |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 1) “This research was funded by Almirall S.A.”. Declarations of interest (p 1): "U.M. has been an advisor and/or received speaker honoraria and/or received grants and/or participated in clinical trials for the following companies: Abbott/AbbVie, Almirall Hermal, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Foamix, Forward Pharma, Galderma, Janssen, LEO Pharma, Lilly, Medac, Miltenyi Biotec, MSD, Novartis, Pfizer, Teva, UCB, VBL and XenoPort. J.C.S. receives advisory board/consulting fees from AbbVie, Biogen, Biogenetica International Laboratories, Egis Pharmaceuticals, Fresenius, LEO Pharma, Lilly, Novartis, Pierre Fabre, Polpharma, Sandoz and Toray Corporation; and receives speaker fees from AbbVie, Actavis, Adamed, Astellas, Berlin‐Chemie Menarini, Fresenius, Janssen‐Cilag, LEO Pharma, Mitsubishi Tanabe Pharma, Novartis, Pierre Fabre, Takeda and Vichy, and clinical trial funding from AbbVie, Actelion, Almirall, Amgen, GlaxoSmithKline, Janssen‐Cilag, Merck, Mitsubishi Tanabe Pharma, Novartis, Regeneron and Takeda. P.V.K. declares consultancy fees for Celgene, Centocor, Almirall, Amgen, Pfizer, Philips, Abbott, Lilly, Galderma, Novartis, Janssen‐Cilag, LEO Pharma, Sandoz and Mitsubishi Tanabe Pharma and carries out clinical trials for Basilea, Pfizer, Lilly, Amgen, AbbVie, Philips Lighting, Janssen‐Cilag and LEO Pharma. R.L." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2): “Randomisation was performed by the investigators using an interactive web‐based response system.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2): “Randomisation was performed by the investigators using an interactive web‐based response system. The randomisation sequence was kept concealed from the investigators during the trial.” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 2): “Treatment was uptitrated over the first 9 weeks, with placebo or up to a maximum daily dose of 720 mg DMF in the LAS41008 or Fumaderm® groups” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 2): “Treatment was uptitrated over the first 9 weeks, with placebo or up to a maximum daily dose of 720 mg DMF in the LAS41008 or Fumaderm® groups” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 704, analysed 671 Management of missing data: Quote (p 4): “All statistical analyses were based on the full analysis set (FAS) and the per protocol set (PPS). As the results of both were consistent, data are presented here only for the FAS. A last‐observation‐carried‐forward approach was used to handle missing data for the PASI‐ and PGA‐derived end points.” DMF/DMF + MEF/placebo Randomized 280/286/138 Safety set analysis 279/283/137 (not treated patients excluded) Full set analysis 267/273/131 (not explained) Comment: not ITT analysis |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01726933). Some pre‐specified outcomes and those mentioned in the methods section as DLQI had not been reported. |
| Methods | RCT, active‐controlled, double blind Date of study: August 2011–March 2013 Setting: 133 centres in North and South America, Europe and Asia |
|
| Participants |
Randomised: 966 participants (mean age 46 years, 635 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab (n = 482), SC, 150 mg weeks 0, 1, 2, 3 then monthly Control intervention B. Secukinumab (n = 484), SC, 300 mg weeks 0, 1, 2, 3 then monthly |
|
| Outcomes | Assessments at 52 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: quote (p 27) “Study funded by Novartis Pharma...Novartis conducted data analyses, and all authors had access to data”. Declarations of interest (p 27): "The authors received writing and editorial support from Barry Weichman and Jinling Wu in the preparation of the manuscript from BioScience Communications, New York, NY, supported by Novartis. Dr Mrowietz has served as advisor and/or received speaker honoraria and/or received grants and/or participated in clinical trials for Abbott/AbbVie, Almirall, Amgen, BASF, Biogen Idec, Celgene, Centocor, Eli Lilly, Forward Pharma, Galderma, Janssen, Leo Pharma, Medac, MSD, Miltenyi Biotech, Novartis, Pfizer, Teva, VBL, and Xenoport. Dr Leonardi has served as consultant and/or investigator and/or participated in a speaker’s bureau for AbbVie, Amgen, Celgene, Dermira, Eli Lilly, Galderma, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Sandoz, Stiefel, and UCB. Dr Girolomoni has received advisory/speaker honoraria and/or research funding from AbbVie, Almirall, Boehringer Ingelheim, Celgene, Dompe, Eli Lilly, Galderma, Janssen, Leo Pharma, Merck Serono, Maruho, MSD, Novartis, and Pfizer. Dr Toth has served as investigator for Novartis, Amgen, Eli Lilly, Johnson & Johnson, Abbott, Celgene, Merck, Galderma, and Leo Pharma. Dr Morita has served as consultant and/or paid speaker for and/or participated in psoriasis clinical trials sponsored by AbbVie, Mitsubishi Tanabe, Janssen, Novartis, Eli Lilly, Kyowa‐Kirin, Leo Pharma, Maruho, and MSD. Dr Szepietowski has served as advisor and/or received speakers honoraria and/or participated in clinical trials for Abbott/AbbVie, Actavis, Amgen, BASF, Astellas, Berlin‐Chemie/Menarini, Biogenetica International Laboratories, Centocor, Fresenius, Janssen, Leo Pharma, Mitsubishi Tanabe, Novartis, Pierre‐Fabre, Takeda, Toray Corporation, and Vichy. Dr Regnault, Ms Thurston, and Dr Papavassilis are employees of and/or own stock in Novartis. Dr Balki has no conflicts of interest to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 28): “were randomised” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 28): “administered via 2 150‐mg SC injections or one 150‐mg SC and one placebo SC injection respectively” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 28): "administered via 2 150‐mg SC injections or one 150‐mg SC and one placebo SC injection respectively" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 966, analysed 966 Management of missing data: Quote (p 29): “Missing values for PASI or IGA 2011 modified version responses were imputed as non response regardless of the reason for missing data” Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01406938). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: October 2012– March 2013 Setting: multicentre (56) in Japan |
|
| Participants |
Randomised: 151 participants (mean age 45 years, 120 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Brodalumab (n = 39), SC, 70 mg, 2 injections week 0, 1 injection eow Control intervention B. Brodalumab (n = 37), SC, 140 mg, 2 injections week 0, 1 injection eow C. Brodalumab (n = 37), SC, 210 mg, 2 injections week 0, 1 injection eow D. Placebo (n = 38), orally (same drug administration) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 51) “The study was supported by Kyowa Hakko Kirin Co., Ltd.” Declarations of interest (p 51): "H. Nakagawa is a consultant and/or received research grants and/or speaker honoraria from for Kyowa Hakko Kirin Co., Ltd., AbbVie, Mitsubishi‐Tanabe Pharma, Janssen Pharmaceutical K.K., Novartis Pharma K.K., Eli Lilly Japan K.K., LEO Pharma Maruho Corporation Limited and MSD K.K.H. Niiro has no conflict of interest to declare. K. Ootaki is an employee of Kyowa Hakko Kirin Co., Ltd." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 45): “were randomised to receive…” Comment: not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 45): “were randomised to receive…” Comment: not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 51): “double‐blind…” Comment: not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 151, analysed 151 Comment: no supplementary explanation regarding the management of missing data |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01748539). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported except for participant‐reported outcome |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: not stated Setting: multicentre in the Netherlands |
|
| Participants |
Randomised: 39 participants (mean age 44 years, 27 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Dimethylfumarate (n = 12), orally, 120 mg, gradual increase 1‐6 tablets, once a day, 16 weeks Control intervention B. Octyl hydrogen fumarate (n = 10), orally, 284 mg, gradual increase 1‐6 tablets, once a day, 16 weeks C. Placebo (n = 12), orally, once a day, 16 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 331): "The patients were randomly assigned..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 331): "The patients were randomly assigned..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 331): “The double‐blind treatment lasted 16 weeks for each patients... All tablets (provided by Fumapharm AG, Muri, Switzerland) had the same appearance, size and colour” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 331): “The double‐blind treatment lasted 16 weeks for each patients...All tablets (provided by Fumapharm AG, Muri, Switzerland) had the same appearance, size and color” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 39, analysed 34 Comment: no description of the method used to perform analyses of the primary outcome and to manage missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: not stated Setting: not stated |
|
| Participants |
Randomised: 15 participants, age range 23‐72 years, 11 male Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 10), orally, 25/50 mg, daily, 8 weeks Control intervention B. Placebo (n = 5), orally, daily, 8 weeks |
|
| Outcomes | Assessments at 8 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding by Hoffman‐La Roche Inc Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 681): "Patients were assigned to... in a random, double‐blind fashion" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 681): "Patients were assigned to... in a random, double‐blind fashion" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 681): "Patients were assigned to... in a random, double‐blind fashion" Comment: visible adverse effects of acitretin such as cheilitis were visible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 681): "Patients were assigned to... in a random, double‐blind fashion" Comment: visible adverse effects of acitretin such as cheilitis were visible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 included / Number of patients analysed not stated Comment: no description of the methods used to perform the efficacy analyses and to manage the missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section were reported. |
| Methods | RCT, active‐controlled, open‐label study Date of study: 21 September 2007‐August 2009 Setting: 17 centres in Austria, France, Greece and Italy |
|
| Participants |
Randomised: 72 participants randomised, 69 analysed (mean age 46 years, 50 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept twice‐a‐week/once‐a‐week group (n = 38), 50 mg SC twice a week for 12 weeks then 50 mg once a week to week 24 Control intervention B. Etanercept once‐a‐week/once‐a‐week group (n = 34), 50 mg SC injections once a week for the full 24‐week treatment period |
|
| Outcomes | Assessments at 24 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 1080): "TWyeth Research, which was acquired by Pfizer in October 2009, sponsored this clinical trial and was responsible for the collection and analysis of data. Editorial ⁄medical writing assistance was funded by Pfizer Inc." Declarations of interest (p 1080):" J.P.O. has been an investigator or consultant for Schering‐Plough, Abbott, Merck‐Serono, Centocor, Pfizer, Janssen‐Cilag, Meda‐Pharma, Pierre‐Fabre, Galderma and Leo‐Pharma. C.P. has been an investigator or consultant for Abbott, Amgen, Celgene, Janssen Cilag, Leo Pharma, Novartis and Pfizer Inc. E.B. has no conflicts of interest. V.M., G.G., Y.B. and J.M.G. are employees of Pfizer Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1081): “Patients were randomised by the investigator or other authorized person using an automatic online enrolment system in a 1:1 ratio” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1081): “Patients were randomised by the investigator or other authorized person using an automatic online enrolment system in a 1:1 ratio” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 1081): “This was a multicenter, multinational, randomised, open‐label study” Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 1081): “This was a multicenter, multinational, randomised, open‐label study” Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72 included/69 analysed Quote (p 1082): "All efficacy analyses were based on the modified intent‐to treat (mITT) population, which was defined as all patients who had received one or more doses of ETN and had baseline and post baseline data...The MMRM and GEE models have been developed for the analysis of longitudinal categorical data and to handle missing data without any imputation; this kind of model is preferred to the last‐observation carried forward approach for analysis of longitudinal data" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: The protocol for the study was available on ClinicalTrials.gov (NCT00581100). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: not stated Location: 50 centres in USA, Canada & Western Europe |
|
| Participants |
Randomised: 611 participants (mean age 45 years, male 382 out of 583 participants who received 1 dose) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 204), SC, 25 mg twice a week, 12 weeks Control intervention B. Etanercept (n = 203), SC , 50 mg twice a week, 12 weeks C. Placebo (n = 204), SC, twice a week, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 1304): "This study was supported by Immunex Corporation (Seattle, WA, U.S.A)" Declarations of interest: (p 1304) S.T. has received research support from Amgen; C.E.M.G. has been a paid consultant for Wyeth and Amgen; A.M.N and R.Z. are both full‐time employees of Amgen." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 1305): "Patients were randomly assigned (using an Interactive Voice Response system) to receive placebo or etanercept) Comment: not stated |
| Allocation concealment (selection bias) | Low risk | Quote (p 1305): "Patients were randomly assigned (using an Interactive Voice Response system) to receive placebo or etanercept) Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1305): “ the patients, study site personnel and all sponsor representatives remained blinded to the initial randomisation treatment groups...” Comment: placebo‐controlled, probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1305): “ the patients, study site personnel and all sponsor representatives remained blinded to the initial randomisation treatment groups...” Comment: placebo‐controlled, probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 611 randomised participants, 583 analysed (28 participants did not receive the treatment and were excluded from the analyses). Sensitivity analysis (Table 2) were performed with the 611 randomised participants. Management of missing data: quote "In the analyses, missing post baseline efficacy data were imputed using last observation carried forward. In addition, a sensitivity analysis was performed on the binary efficacy endpoints to evaluate the robustness of the primary analysis. This sensitivity analysis included all randomised patients. In addition, rather than using LOCF imputation patients with missing data at a given visit were assumed to have not met the response criteria for that endpoint". Comment: the main result (primary outcome) was not an ITT analysis |
| Selective reporting (reporting bias) | High risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported except for the results of participant‐reported endpoints summarized in a separate publication. |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: December 2009–April 2010 Location: 23 centres worldwide |
|
| Participants |
Randomised: 198 participants (mean age 42 years, 107 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Brodalumab 70 (n = 39), SC, 70 mg, day 1‐weeks 1, 2, 4, 6, 8, 10, 10 weeks Control intervention B. Brodalumab 140 (n = 39), SC, 140 mg, day 1 and weeks 1, 2, 4, 6, 8, 10, 10 weeks C. Brodalumab 210 (n = 40), SC, 210 mg, day 1 and weeks 1, 2, 4, 6, 8, 10, 10 weeks D. Brodalumab 280 (n = 42), SC, 280 mg, day 1 and weeks 1, 2, 4, 6, 8, 10, 10 weeks E. Placebo (n = 38), SC, day 1 and weeks 1, 2, 4, 6, 8, 10, 10 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 1182): "The study was funded by Amgen" Declarations of interest (p 1188‐89): "Dr. Papp reports receiving consulting fees from Abbott, Amgen, Astellas, Celgene, Centocor, Eli Lilly, Galderma, Graceway Pharmaceuticals, Janssen, Johnson & Johnson, Merck, Norvartis, Pfizer, and UCB, lecture fees from Abbott, Amgen, Astellas, Celgene, Centocor, Galderma, Janssen, LEO Pharma, Merck, Novartis, Pfizer, and Stiefel, and grant support from Abbott, Amgen, Astellas, Celgene, Centocor, Eli Lilly, Galderma, Glaxo‐SmithKline, Graceway Pharmaceuticals, Janssen, Johnson & Johnson, Medimmune, Merck, Novartis, Pfizer, Stiefel, and UCB; Dr. Leonardi, receiving consulting fees from Abbott, Amgen, Centocor, Eli Lilly, and Pfizer, lecture fees from Abbott and Amgen, and investigator fees from Abbott, Amgen, Celgene, Centocor, Galderma, GlaxoSmithKline, Incyte, Maruho, Novartis, Novo Nordisk, Pfizer, Schering‐Plough (now Merck), Sirtris, Stiefel, Vascular Biogenics, and Wyeth (now Pfizer); Dr. Menter, receiving consulting fees from Abbott, Amgen, Astellas, Centocor, Galderma, Genentech, and Wyeth, lecture fees from Abbott, Amgen, Centocor, Galderma, and Wyeth, and fees for expert testimony from Galderma; Dr. Krueger, receiving consulting fees from Centocor, Eli Lilly, and Pfizer and grant support from Amgen, Centocor, Eli Lilly, Merck, and Pfizer; and Drs. Krikorian, Aras, Li, Russell, Thompson, and Baumgartner being full‐time employees of Amgen. No other potential conflict of interest was relevant to this article was reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (protocol p 30): “Randomization: IVRS will be used to randomise subjects into the study. The randomisation list will be generated by Amgen using a permuted block design within each of 4 strata based on BMI at baseline, and participation in the PK study” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (protocol p 30): “Randomization: IVRS will be used to randomise subjects into the study. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (protocol p 24 & 50): “double‐blind placebo controlled... Subjects randomised to active drug will receive additional placebo injections as necessary to maintain the blind” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (protocol p 39): "PASI assessments will be performed by a blinded assessor. The blinded assessor will be a healthcare professional who has been certified as trained with the standard PASI" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 198 included/198 analysed Quote (p 1183): "The analyses of efficacy endpoints were performed on data from all patients who underwent randomisation (full set analysis), according to the intention‐to‐treat principle... Missing data were handled by means of the baseline‐value‐carried‐forward method or the imputation of no response" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00307437). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: July 2008‐August 2009 Location: 42 centres in USA, Canada |
|
| Participants |
Randomised: 197 participants (tofacitinib 2 mg (49) mean age 46 years, 29 male; tofacitinib 5 (49) mean age 44 years, 29 male; tofacitinib 15 (49) mean age 44 years, 31 male; placebo (n = 50) mean age 44 years, 36 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Tofacitinib (n = 49), orally, 2 mg, twice a day, 12 weeks Control intervention B. Tofacitinib, (n = 49), orally, 5 mg, twice a day, 12 weeks C. Tofacitinib (n = 49), orally, 15 mg, twice a day, 12 weeks D. Placebo (n = 50), orally, twice a day, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 668): "This study was funded by Pfizer Inc" Declarations of interest (appendix): "K.A.P. has been a principal investigator, an advisor or consultant, a Scientific Officer, member of a Scientific Advisory Board and a speaker for the following groups: Abbott, Amgen, Astellas, Celgene, Centocor‐Ortho Biotech, Incyte, Isotechnika, Janssen, Lilly, Medimmune, Merck, Pfizer Inc. and Novartis. A.M. has been on the Advisory Board, been a consultant to, been an investigator for, been a speaker for, obtained a research grant from, or obtained honoraria from the following groups: Abbott, Allergan, Amgen, Astellas, Asubio, Celgene, Centocor, DUSA, Eli Lilly, Galderma, Genentech, Novartis, Novo Nordisk, Pfizer Inc., Promius, Stiefel, Syntrix Biosystems, Warner Chilcott and Wyeth. B.S. has been a principal investigator, an advisor or consultant, or a speaker for the following groups: Abbot, Amgen, Celgene, Centocor‐Ortho Biotech, Janssen, Pfizer Inc., Maruho and Novartis. R.G.L. has been an investigator, served as a principal investigator or on the Advisory Board, or been a speaker for the following groups: Abbott, Amgen, Centocor⁄Ortho Biotech, Pfizer Inc., Novartis and Celgene. R.W., S.K., H.T., P.G. and M.B. are employees of Pfizer Inc. J.A.H. was a full‐time employee of Pfizer Inc. during the conduct and reporting of the study and now works at Novartis Pharma AG, Basel, Switzerland. " |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 669): "A computer‐generated central randomisation schema was implemented in an automated web ⁄telephone system." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 669): "A computer‐generated central randomisation schema was implemented in an automatedTreatment identification was concealed by use of study drugs that were identical in labelling, packaging, appearance and odour" web ⁄telephone system." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 669): "Patients, investigational site staff, the Pfizer study team and data analysts were blinded to treatment from the time of randomisation until database lock... Treatment identification was concealed by use of study drugs that were identical in labelling, packaging, appearance and odour" Comment: probably one |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 669): "Patients, investigational site staff, the Pfizer study team and data analysts were blinded to treatment from the time of randomisation until database lock... Treatment identification was concealed by use of study drugs that were identical in labelling, packaging, appearance and odour" Comment: probably one |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 197 included / 195 analysed Quote (p 670): "The full analysis set included all randomised patients who received one or more doses of investigational drug...This population ... represents a modified intent‐to‐treat analysis... Patients with missing values had the missing values imputed but last observation carried forward.... As a sensitivity analysis the patients [with missing values] were also considered nonresponders (NRI)" Comment: mITT and two patients out of 197 not analysed |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00678210). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: September 2008‐October 2009 Location: 35 centres in Canada and USA |
|
| Participants |
Randomised: 352 participants (mean age 44 years, 221 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Apremilast (n = 88), orally, 30 mg, twice a day, 16 weeks Control intervention B. Apremilast (n = 176), orally, 10‐20 mg twice a day, 16 weeks C. Placebo (n = 88), orally, twice a day 16 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source quote (p 738): "Funding Celgene Corporation" Declarations of interest quote (p 745): "KP has served as an investigator for Abbott, Amgen, Celgene, Centocor, Galderma, Incyte, Isotechnika, Janssen, Lilly, Medimmune, Merck, Novartis, and Pfizer; an adviser for Abbott, Amgen, Astellas, BMS, Celgene, Centocor, Galderma, Incyte, Isotechnika, Janssen, Johnson & Johnson, Lilly, Medimmune, Merck, Novartis, Pfizer, and UCB; and a speaker for Abbott, Amgen, Astellas, Celgene, Centocor, Isotechnika, Janssen, Novartis, and Pfizer. JCC has served as an investigator for Celgene, Centocor, Novartis, and Pfizer; as a speaker for Centocor and Abbott; and as an adviser for Pfizer, Abbott, and Novartis. LR has been a paid investigator for doing clinical trials for Amgen, Genentech, Abbott, Centocor, Basilea, Leo, Isotechnika, Stiefel, GSK, Galderma, 3‐M, Serono, Novartis, Astellas, UCB, Celgene, Johnson & Johnson, and Pfizer. HS has served as an investigator for Abbott, Centocor, Celgene, Amgen, and Pfizer; as a speaker for Abbott and Centocor; and as an adviser for Centocor. RGL has served as an investigator for Abbott, Centocor, Celgene, Amgen, Pfizer, Johnson & Johnson/Ortho Biotech, and Novartis; as a speaker for Abbott, Centocor, Amgen, Pfizer, Johnson & Johnson/Ortho Biotech, and Novartis; and as an adviser for Abbott, Centocor, Celgene, Amgen, Pfizer, Johnson & Johnson/Ortho Biotech, and Novartis. RTM has served as an investigator for Abbott, Centocor, Celgene, Amgen, Novartis, Lilly, Pfizer, Allergan, and Galderma; as a speaker for Centocor and Amgen; and as an adviser for Centocor. CH and RMD are employees of Celgene Corporation." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 739): "Eligible patients were randomly assigned in a 1:1:1:1 ratio to oral apremilast 10 mg twice daily, apremilast 20 mg twice daily, apremilast 30 mg twice daily, or placebo, with a permuted‐block randomisation list generated by an interactive voice response system (ClinPhone, East Windsor, NJ, USA)." Comment: clearly described |
| Allocation concealment (selection bias) | Low risk | Quote (p 739): "Eligible patients were randomly assigned in a 1:1:1:1 ratio to oral apremilast 10 mg twice daily, apremilast 20 mg twice daily, apremilast 30 mg twice daily, or placebo, with a permuted‐block randomisation list generated by an interactive voice response system (ClinPhone, East Windsor, NJ, USA)." Comment: clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 739): "Treatment was double‐blind for the first 16 weeks of the 24‐week treatment phase." Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 739): "Treatment was double‐blind for the first 16 weeks of the 24‐week treatment phase." Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 352 included / 352 analysed Quote (p 740): "Efficacy data were assessed by intention to treat. Missing data were handled with the last‐observation carried‐forward method." Comment: number of lost to follow‐up and reasons comparable across group |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00773734). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind trial Date of study: March 2010‐February 2011 Location: 19 international centres |
|
| Participants |
Randomised: 125 participants (mean age 46 years, 91 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab (n = 29), SC, 25 mg, 0, 4, 8 weeks, 12 weeks Control intervention B. Secukinumab (n = 26), SC, 3 x 25 mg, 0, 4, 8 weeks, 12 weeks C. Secukinumab (n = 21), SC, 3 x 75 mg, 0, 4, 8 weeks, 12 weeks D. Secukinumab (n = 27), SC, 3 x 150 mg, 0, 4, 8 weeks, 12 weeks E. Placebo (n = 22), SC, 0, 4, 8 weeks, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source (p412): "Novartis Pharm AG, Basel, Switzerland" Declarations of interest (Appendix): "K.A.P. has received honoraria for lecturing at industry‐sponsored meetings and has received industry funding for presentations and consultation at national and international meetings; he has also received research grants from and been a paid consultant to Novartis and other pharmaceutical companies; has served as a scientific officer for pharmaceutical and biotechnology corporations; and is a participant on clinical, scientific and corporate advisory boards. R.G.L. has been a member of scientific advisory boards and served as a clinical investigator for Abbott, Amgen, Celgene, Centocor⁄Johnson & Johnson, Eli Lilly, Fujisawa, Novartis and Pfizer, and has served as a speaker for Abbott, Amgen, Centocor⁄Johnson & Johnson, Fujisawa and Novartis. B.S. has consulted for Novartis and several other pharmaceutical companies; he has been a member of an advisory board for Novartis and several other pharmaceutical companies. S.H., H.J.T., C.P. and H.B.R. are full‐time employees of and own stock in Novartis. M.A., D.R.B. and P.K. declare no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 414): “The randomisation numbers were generated by an interactive voice response provider using a validated automated system” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 414): “The randomisation numbers were generated by an interactive voice response provider using a validated automated system” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 413 & 414): “Double‐blind, placebo controlled...Patients, investigator staff, persons performing the assessments and data analysts were blinded ... remained blind until final database lock” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 413 & 414): “Double‐blind, placebo controlled...Patients, investigator staff, persons performing the assessments and data analysts were blinded ... remained blind until final database lock” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 125 included/125 analysed Quote (p 415): "The full analysis set consisted of all patients who were randomised... The missing score was imputed by carrying forward the last non missing post baseline PASI" Comment: very high number of withdrawals (38%) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01071252). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: April 2006‐May 2007 Location: multicentre (30) in Canada, the Czech Republic, and Germany |
|
| Participants |
Randomised: 260 participants (mean age 46 years, 163 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Apremilast (n = 173), orally, 10‐20 mg, twice a day, 12 weeks Control intervention B. Placebo (n = 87) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source quote (p 27): "This study was sponsored by Celgene Corporation" Declarations of interest (p27): "Dr Papp is a consultant and investigator for Celgene Corporation, Abbott, Amgen, Centocor, Janssen‐Ortho, Merck, Novartis and Pfizer and an investigator for Astellas, Leo Pharma and Galderma, receiving honoraria and grants. Dr Kaufmann is an investigator for Abbott, Centocor, Leo, Novartis, Wyeth and Celgene Corporation, but has not received financial compensation. The Department of Dermatology received investigator fees for performing the clinical trials. He served as a speaker for Basilea and Allmiral and received honoraria from each. Dr Thac ¸ i is on the advisory board of and is a consultant, investigator and speaker for Abbott, Leo, Novartis, Pfizer, Biogen‐Idec, Janssen‐Cilag and MSD, and received honoraria from each. He is also an investigator for Celgene Corporation. The Department of Dermatology received honoraria ⁄ compensation for conducting studies; no direct compensation was received. Ms Hu receives a salary as an employee of Celgene Corporation. Ms Sutherland receives a salary, stocks and stock options as an employee of Celgene Corporation. Dr Rohane received a salary and stock options as a former employee of Celgene Corporation. " |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 377): " investigators randomised subjects 1 : 1 : 1 to double‐blind treatments for 12 weeks with placebo, apremilast 20 mg QD or apremilast 20 mg twice daily" Comment: no description of the method to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote (p 377): "Using an interactive voice response system, investigators randomised subjects 1 : 1 : 1 to double‐blind treatments" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 377): "One capsule of placebo or apremilast was taken orally in the morning before meals, and one capsule of placebo or apremilast was taken in the evening" Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 377): "One capsule of placebo or apremilast was taken orally in the morning before meals, and one capsule of placebo or apremilast was taken in the evening" Comment: probably done, placebo controlled |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 260 included / 260 analysed Management of missing data were not stated and substantial number lost to follow‐up (18%) |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00606450). The pre‐specified outcomes listed on ClinicalTrials.gov were not detailed, the choice of the primary outcome was not clearly defined. In the methods section, PASI 75 was defined as the primary outcome, no QoL outcomes were listed in the methods section although they were in the protocol on clinicaltrilas.gov |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: November 2010‐June 2012 Location: 64 centres in Europe, Asia & North America |
|
| Participants |
Randomised: 355 participants (mean age 45 years, 270 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Tildrakizumab (n = 42), SC, 5 mg weeks 0, 4, every 12 weeks Control intervention B. Tildrakizumab (n = 92), SC, 15 mg weeks 0, 4, every 12 weeks C. Tildrakizumab (n = 89), SC, 50 mg weeks 0, 4, every 12 weeks D. Tildrakizumab (n = 86), SC, 100 mg weeks 0, 4, every 12 weeks E. Tildrakizumab (n = 46), SC, 200 mg weeks 0, 4, every 12 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 930): “This study was funded by Merck & Co, nc., Kenilworth, NJ, USA”. Declarations of interest (Appendix 1): "E.P.B., A.M., Q.L., Y.Z. and R.S. are current or former employees of Merck & Co., Inc. K.P. has served as a consultant, advisory board member and/or investigator for Abbott (AbbVie), Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Foreward Pharma, Galderma, Genentech, Incyte, Isotechnika, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Medimmune, Merck Sharp Dome, Merck Serono, Novartis, Regeneron, Stiefel, Takeda, Pfizer and USB. D.T. has served as a consultant, advisory board member and/or investigator for Abbott (AbbVie), Almiral, Amgen, Astellas, Biogen Idec, Boehringer Ingelheim, Celgene, Dignity, Forward Pharma, Galderma, GlaxoSmithKline, Isotechnika, Janssen‐Cilag, LEO Pharma, Lilly, Maruho, Medac, Medimmune, Merck Sharp Dome, Merck Serono, Novartis, Regeneron, Sandoz, Sanofi‐Aventis, Takeda and Pfizer. K.R. has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Amgen, Biogen Idec, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Lilly, Medac, Merck, Novartis, Pfizer, Vertex and Takeda. E.R. has received travel support and nonfinancial support for histology study report preparation from Merck & Co., Inc., and has received speaker’s fees and travel support, or served on advisory boards for Abb‐ Vie, Novartis, Pfizer, Janssen and Amgen. R.G.L. has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Celgene, Centocor, Janssen‐Cilag, LEO Pharma, Merck, MSD (formerly Essex, Schering‐Plough), Novartis and Pfizer (formerly Wyeth). J.G.K. has received personal fees (consulting and/or speaking fees) and grants paid to his institution from Novartis, Pfizer, Janssen, Lilly, Merck, Kadmon, Dermira, Boehringer and BMS; Amgen, Innovaderm, Paraxel and Kyowa have paid grants to J.G.K.’s institution; J.G.K. has also received personal fees from Serono, Biogen Idec, Delenex, AbbVie, Sanofi, Baxter, Xenoport and Kineta. A.B.G. has current consulting/advisory board agreements with Amgen Inc., Astellas, Akros, Centocor (Janssen) Inc., Celgene Corp., Bristol Myers Squibb Co., Beiersdorf Inc., Abbott Labs (AbbVie), TEVA, Actelion, UCB, Novo Nordisk, Novartis, Dermipsor Ltd, Incyte, Pfizer, Canfite, Lilly, Coronado, Vertex, Karyopharm, CSL Behring Biotherapies for Life, GlaxoSmithKline, Xenoport, Catabasis Meiji Seika Pharma Co., Ltd, Takeda, Mitsubishi Tanabe Pharma Development America, Inc, and has received research/educational grants (paid to Tufts Medical Center) from Centocor (Janssen), Amgen, Abbott (Abb‐ Vie), Novartis, Celgene, Pfizer, Lilly, Coronado, Levia, Merck and Xenoport. H.N. has received consultancy/speaker honoraria and/or grants from Novartis, Tanabe Mitsubishi, Maruho, Abbott/AbbVie, Eli Lilly, Merck Sharp & Dohme, Janssen and LEO Pharma." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 931): “Randomisation of treatment and allocation was done centrally by means of an interactive web response system… Comment: no description of the method used to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote (p 931): “Randomisation of treatment and allocation was done centrally by means of an interactive web response system…” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 931): “double‐blind" Comment: no description of the method used to guarantee blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 932): “double‐blind" Comment: no description of the method used to guarantee blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 355, analysed 352 Management of missing data: Quote (p 932): “The primary analysis was performed on all randomised participants who received at least one or more doses of treatment. Participants who discontinued treatment prior to week 16... were considered to not have achieved PASI 75 at week 16" Comment: low number lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01225731). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: 29 August 2012‐12 March 2014 Location: 73 centres worldwide (Europe, USA & Canada) |
|
| Participants |
Randomised: 661 participants (mean age 46 years, 484 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Brodalumab (n = 222), SC, 210 mg every 2 weeks Control intervention B. Brodalumab (n = 219), SC, 140 mg every 2 weeks C. Placebo (n = 220) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 1): "This study was funded by Amgen Inc. & AstraZeneca/MedImmune." Declarations of interest (p 13‐14): "K.A.P. has served as a consultant, investigator and/or speaker for AbbVie, Amgen Inc., Astellas Pharma, Bayer AG, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Forward Pharma, Galderma, Janssen Biotech Inc., LEO Pharma, Merck, Novartis, Pfizer, Roche and UCB Pharma. K.R. has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Amgen Inc., Biogen‐Idec, Celgene, Centocor, Covagen, Forward Pharma, GSK, Janssen‐Cilag, LEO Pharma, Lilly, Medac, MSD, Novartis, Pfizer, Takeda and Vertex. C.P. has served as a consultant and investigator for Amgen Inc., AbbVie, Boehringer, Janssen‐Cilag, LEO Pharma, Lilly, Novartis and Pfizer. A.B. has served as a consultant and investigator for AbbVie, Amgen Inc., Anacor, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Genentech, Janssen, Merck, Novartis, Pfizer, Regeneron and Sandoz." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 2 & 3): "Patients were randomized... IP supply was controlled by interactive voice response system and box numbers were assigned at each visit" Comment: no description of the method used to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote (p 2 & 3): "Patients were randomized...IP supply was controlled by interactive voice response system and box numbers were assigned at each visit". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "Randomizations remained blinded to all patients and investigators... Throughout the study, patients received placebo as needed to maintain the blind until it was broken. " Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "Randomizations remained blinded to all patients and investigators... Throughout the study, patients received placebo as needed to maintain the blind until it was broken. " Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 661, 661 analysed Management of missing data: quote (p 4 & 5): "The full analysis set included all randomised patients... Mutiple imputations for missing data" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01708590; AMAGINE‐1). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: September 2010‐December 2012 Location: 72 centres in USA, Canada, Australia, Belgium, France, UK, Italy, Germany |
|
| Participants |
Randomised: 844 participants (apremilast (562) mean age 46 years, 379 male; placebo (282) mean age 47 years, 194 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Apremilast (n = 562), orally, 30 mg, twice a day, 16 weeks Control intervention B. Placebo (n = 282), orally, twice a day, 16 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source quote (p 37): "This study was sponsored by Celgene Corporation" Declarations of interest (p 48): "Dr Papp has served as an investigator for Abbott (AbbVie), Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Galderma, Genentech, Incyte, Isotechnika, Janssen, LEO Pharma, Lilly, MedImmune, Merck Sharp & Dohme, Merck‐Serono, Novartis, Pfizer, Stiefel, and Wyeth; a consultant for Abbott, Amgen, Astellas, Biogen Idec, Boehringer Ingelheim, BMS, Celgene, Centocor, Forward Pharma, Galderma, Genentech, Incyte, Isotechnika, Janssen, Johnson &Johnson, Kyowa Kirin, LEO Pharma, Lilly, MedImmune, Merck Sharp & Dohme, Merck‐Serono, Novartis, Pfizer, Takeda Pharmaceuticals, UCB, and Wyeth; and a speaker for Abbott, Amgen, Astellas, Celgene, Centocor, Isotechnika, Janssen, Novartis, and Pfizer. Dr Reich has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Amgen, Biogen Idec, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, Takeda, and Vertex. Dr Leonardi has served on the advisory board and as an investigator and/or speaker for Abbott, Amgen, Celgene,Centocor, Galderma, Genentech, GlaxoSmithKline, Lilly, Novartis, Novo Nordisk, Pfizer, Sirtris, Stiefel, Vascular Biogenics, and Wyeth." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 38): "ESTEEM 1 was.. multicenter, randomised, double‐blind, placebo controlled study". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 38): "ESTEEM 1 was.. multicenter, randomised, double‐blind, placebo controlled study" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 38): "ESTEEM 1 was.. multicenter, randomised, double‐blind, placebo controlled study... Blinding was maintained until all patients discontinued or completed the week 52 visit" Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 38 & 39): "ESTEEM 1 was.. multicenter, randomised, double‐blind, placebo controlled study... Blinding was maintained until all patients discontinued or completed the week 52 visit" Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 844included/844 analysed Quote (p 39): "Efficacy data were assessed for the full analysis set (all randomised patients)...Missing data were handled with the last‐observation‐carried‐forward methodology" Comment: done |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01194219). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported except the number of participants with a psoriasis flare or rebound during placebo‐controlled phase |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: 12 January 2012–18 September 2014 Location: multicentre (74) in USA, Canda, Colombia, Germany, Japan, Hungary, Serbia, Taiwan, Ukraine |
|
| Participants |
Randomised: 901 participants (mean age 46 years, 643 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Tofacitinib (n = 363), orally, 5 mg twice daily Control intervention B. Tofacitinib (n = 360), orally, 10 mg twice daily B. Placebo (n = 177), orally (same drug administration) |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 949): "Pfitzer Inc" Declarations of interest (appendix): "K.A.P. has participated in advisory boards or panels for AbbVie, Amgen, Astellas, Baxter, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, LEO Pharma, Merck, Novartis, Pfizer Inc. and UCB. He has been an investigator for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Genentech, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Takeda and UCB. He has acted as a consultant for AbbVie, Amgen, Astellas, Baxter, Boehringer Ingelheim, Celgene, Eli Lilly, Forward Pharma, Janssen, Merck, Novartis, Pfizer Inc., Takeda and UCB. He has been a speaker for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Genentech, Janssen, LEO Pharma, Merck, Novartis, Pfizer and UCB. M.A.M. has participated in advisory boards or panels for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Genentech, Janssen Biotech, LEO Pharma and Pfizer Inc. He has served as a consultant for AbbVie, Allergan, Amgen, Convoy Therapeutics, Eli Lilly, Janssen Biotech, LEO Pharma, Novartis, Pfizer Inc., Syntrix, Wyeth and XenoPort. He has been an Investigator for AbbVie, Allergan, Amgen, ApoPharma, Boehringer Ingelheim, Celgene, Convoy Technologies, Eli Lilly, Genentech, Janssen Biotech, LEO Pharma, Merck, Novartis, Pfizer Inc., Symbio/Maruho, Syntrix and Wyeth. He has been a speaker for AbbVie, Amgen, Janssen Biotech, LEO Pharma and Wyeth. He has received grants from AbbVie, Allergan, Amgen, ApoPharma, Boehringer Ingelheim, Celgene, Convoy Technologies, Genentech, Janssen Biotech, LEO Pharma, Merck, Pfizer Inc., Symbio/Maruho and Syntrix. He has received honoraria from AbbVie, Allergan, Amgen, Boehringer Ingelheim, Convoy Technologies, Eli Lilly, Genentech, Janssen Biotech, LEO Pharma, Novartis, Pfizer Inc., Syntrix, Wyeth and XenoPort." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 951): “Randomization using an automated web/telephone randomization system at the study site ensured patient, investigator and sponsor blinding ” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 951): “Randomization using an automated web/telephone randomization system at the study site ensured patient, investigator and sponsor blinding ” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 951): “Investigator and sponsor blinding… with placebo tablets according to the treatment group, appropriately labelled to avoid treatment‐group conflict. All patients took a total of two tablets for each dose” Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 951): “Investigator and sponsor blinding… with placebo tablets according to the treatment group, appropriately labelled to avoid treatment‐group conflict. All patients took a total of two tablets for each dose” Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 901, analysed 900 Management of missing data: Quote (p 951): "The full analysis set included all patients who were randomised and received at least one dose of the study drug...Nonresponder imputation was used to manage missing values." Comment: withdrawal for lack of efficacy: tofacitinib 5 group 5% (20/363), tofacitinib 10 group 4% (15/360), placebo group 14% (25/177) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01276639). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: 4 March 2011–18 September 2014 Location: multicentre (94) in Mexico, Poland, Puerto Rico, Serbia, Taiwan, Ukraine |
|
| Participants |
Randomised: 960 participants (mean age 46 years, 648 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Tofacitinib (n = 382), orally, 5 mg twice daily Control intervention B. Tofacitinib (n = 381), orally, 10 mg twice daily C. Placebo (n = 196), orally (same drug administration) |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 949): "Pfitzer Inc" Declarations of interest (appendix) : "K.A.P. has participated in advisory boards or panels for AbbVie, Amgen, Astellas, Baxter, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, LEO Pharma, Merck, Novartis, Pfizer Inc. and UCB. He has been an investigator for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Genentech, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Takeda and UCB. He has acted as a consultant for AbbVie, Amgen, Astellas, Baxter, Boehringer Ingelheim, Celgene, Eli Lilly, Forward Pharma, Janssen, Merck, Novartis, Pfizer Inc., Takeda and UCB. He has been a speaker for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Genentech, Janssen, LEO Pharma, Merck, Novartis, Pfizer and UCB. M.A.M. has participated in advisory boards or panels for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Genentech, Janssen Biotech, LEO Pharma and Pfizer Inc. He has served as a consultant for AbbVie, Allergan, Amgen, Convoy Therapeutics, Eli Lilly, Janssen Biotech, LEO Pharma, Novartis, Pfizer Inc., Syntrix, Wyeth and XenoPort. He has been an Investigator for AbbVie, Allergan, Amgen, ApoPharma, Boehringer Ingelheim, Celgene, Convoy Technologies, Eli Lilly, Genentech, Janssen Biotech, LEO Pharma, Merck, Novartis, Pfizer Inc., Symbio/Maruho, Syntrix and Wyeth. He has been a speaker for AbbVie, Amgen, Janssen Biotech, LEO Pharma and Wyeth. He has received grants from AbbVie, Allergan, Amgen, ApoPharma, Boehringer Ingelheim, Celgene, Convoy Technologies, Genentech, Janssen Biotech, LEO Pharma, Merck, Pfizer Inc., Symbio/Maruho and Syntrix. He has received honoraria from AbbVie, Allergan, Amgen, Boehringer Ingelheim, Convoy Technologies, Eli Lilly, Genentech, Janssen Biotech, LEO Pharma, Novartis, Pfizer Inc., Syntrix, Wyeth and XenoPort." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 951): “Randomization using an automated web/telephone randomization system at the study site ensured patient, investigator and sponsor blinding ” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 951): “Randomization using an automated web/telephone randomization system at the study site ensured patient, investigator and sponsor blinding ” Comment: no description of the method to guarantee the allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 951): “Investigator and sponsor blinding… with placebo tablets according to the treatment group, appropriately labelled to avoid treatment‐group conflict. All patients took a total of two tablets for each dose” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 951): “Investigator and sponsor blinding… with placebo tablets according to the treatment group, appropriately labelled to avoid treatment‐group conflict. All patients took a total of two tablets for each dose” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 960, analysed 959 Management of missing data: Quote (p 951): "The full analysis set included all patients who were randomised and received at least one dose of the study drug...Nonresponder imputation was used to manage missing values." Comment: imbalance of withdrawal between groups: lack of efficacy: tofacitinib 5 group (15), tofacitinib 10 group (2), placebo group (24) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01276639). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: March 2006–September 2007 Location: 70 centres in Europe and North America |
|
| Participants |
Randomised: 1230 participants (mean age 45 years, 840 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ustekinumab (n = 409), SC, 45 mg, weeks 0‐4 and every 12 weeks, 52 weeks Control intervention B. Ustekinumab (n = 411), SC, 90 mg, weeks 0‐4 and every 12 weeks, 52 weeks C. Placebo (n = 410), SC, weeks 0‐4, 4 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding Centocor Inc (p1675) Declaration of interest (p1684): "KP has served as a consultant and advisory board member for Abbott, Alza, Amgen, Celgene, Centocor, Isotechnika, Janssen Ortho Biotech, Johnson & Johnson, Medimmune, MerckSerono, and Wyeth. RGL has received research grants, served on scientific advisory boards, and has been a speaker for Amgen, Biogen‐Idec, Centocor, Genentech, Novartis, Schering‐Plough, and Serono. ML has received honoraria, served as a speaker and advisory board member for Abbott, Amgen, Centocor, Genentech, and Stiefel, and has served as an advisory board member for Astellas and a consultant for UCB. GK has received fees as a consultant or advisory board member for Abbott, Almirall, Alza, Amgen, Anacor, Astellas, Barrier Therapeutics, Boehringer Ingleheim, Bristol Myers Squibb, Centocor, CombinatoRx, Exelixis, Genentech, Genzyme, Isis, L’Oreal, Lupin Limited, Magen Biosciencs, MedaCorp, Medicis, Novartis, Nova Nordisc, Schering‐Plough, Somagenics, theDerm.org, Synvista, Warner Chilcot, UCB, USANA Health Sciences, and ZARS, owns equities and stock in ZARS, and has received lecture fees from Abbott, Amgen, Astellas, Boehringer Ingleheim, Centocor, Connetics, National Psoriasis Foundation, The Foundation for Better Health Care, and Warner Chilcot, and has received partial stipend support for a clinical research fellowship from Abbott, Amgen, and Centocor. KR has received honoraria as a consultant and advisory board member and acted as a paid speaker for Abbot, Biogen‐Idec, Centocor, Janssen‐Cilag, Schering‐Plough, MerckSerono, UCB, and Wyeth. PS, NY, CG, M‐CH, YW, SL, and LTD are employees of Centocor. PS, NY, CG, YW, SL, and LTD own stock in Johnson and Johnson." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1676): “Patients were randomly assigned... with biais coin assignment via a centralised interactive voice response system (ClinPhone, East Windsor, NJ, USA)” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1676): “Patients were randomly assigned... with biais coin assignment via a centralised interactive voice response system (ClinPhone, East Windsor, NJ, USA)” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1676 & 1977): “Double‐blind,..., placebo‐controlled...Site monitors investigators personels involved in the study conduct,and patients remained blinded... until W52” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1676 & 1977): “Double‐blind,..., placebo‐controlled...Site monitors investigators personels involved in the study conduct,and patients remained blinded... until W52” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1230 included/ 1230 analysed Quote (p 1679): "Efficacy data were analysed by the assigned treatment group... Non‐responder status was assigned for binary variables ... for those patients who discontinued study treatment ..." Comment: ITT analyses |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00307437). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: 29 October 2012–25 March 2016 Location: 40 centres in Europe & USA |
|
| Participants |
Randomised: 413 participants (mean age 45 years, 276 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Apremilast (n = 275), orally, 30 mg twice a day until week 32 Control intervention B. Placebo (n = 138), orally (same drug administration) |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 1387): "This study was sponsored by Celgene Corporation" Declarations of interest (Appendix): C.P. has served as an investigator and consultant for AbbVie, Amgen, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis and Pfizer. J. Cather has been an investigator for Amgen, Celgene, Galderma, Merck, Novartis and Pfizer, and has served on advisory boards for AbbVie, Janssen, OrthoBiotech and Medac. M.G. has been an investigator for AbbVie, Allergan, Celgene, Dermira, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Janssen Pharmaceutical, Kythera, Kyowa Hakko Kirin Pharma, LEO Pharma, Merck, Novartis, Pfizer, Regeneron and Takeda, and has served as a speaker for AbbVie, Actelion, Amgen, Astellas, Galderma, Janssen Pharmaceutical, LEO Pharma, Novartis and Pfizer. Y.P. has been an investigator for AbbVie, Amgen, Astellas, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Centocor/Janssen, Eli Lilly, Galderma, Isotechnika, LEO Pharma, Merck, Novartis, Pfizer, Pharmascience, Regeneron, Schering and Stiefel/GSK, and has served as a speaker for AbbVie, Amgen, Galderma, Janssen, LEO Pharma and Novartis. U.M. has been an advisor for and/or received speaker honoraria from and/or received grants from and/or participated in clinical trials for Abbott/AbbVie, Almirall‐Hermal, Amgen, BASF, Biogen Idec, Celgene, Centocor, Eli Lilly, Forward Pharma, Galderma, Janssen, LEO Pharma, Medac, MSD, Miltenyi Biotech, Novartis, Pfizer, Teva, VBL and XenoPort. C.F. has served on the advisory board for and/or received speaker honoraria from Celgene, Novartis, Janssen and AbbVie. J. Crowley has been an investigator for AbbVie, Amgen, AstraZeneca, Celgene, Janssen, Maruho, Merck, Pfizer and Regeneron; has served on the advisory board for AbbVie, Amgen, Celgene and Lilly; and has been a speaker for AbbVie and Amgen." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 1388): "Patient were randomised (2:1) via an interactive voice response system..." Comment: no description of the method used to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote (p 1388): "Patient were randomised (2:1) via an interactive voice response system..." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1388) "identically matching placebo tablets twice daily during the placebo controlled phase" Comment: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1388): "double‐blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 413, analysed 411 Management of missing data: Quote (p 1389‐90): "Efficacy assessments were conducted for the modified intention‐to‐treat population... The last‐observation‐carried‐forward methodology was used...." Comment: we judged this as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00235820). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: 7 June 2012– 4 January 2013 Location: 38 centres worldwide |
|
| Participants |
Randomised: 182 participants (mean age 45 years, 125 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab (n = 61), SC, 150 mg weeks 0, 1, 2, 3 then monthly Control intervention B. Secukinumab (n = 60), SC, 300 mg weeks 0, 1, 2, 3 then monthly C. Placebo (n = 61), (same drug administration) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (supplemental file) “The study was sponsored by Novartis Pharma and designed by the scientific steering committee and Novartis personnel. Novartis conducted the data analysis, and all authors had access to the data”. Declarations of interest (p 29): "Dr Paul has served as a consultant for AbbVie Pharmaceuticals, Amgen, Celgene Corporation, Eli Lilly and Company, Janssen Pharmaceuticals, LEO Pharma, Novartis Pharmaceuticals Corporation, Pfizer Inc and Pierre Fabre. Dr Lacour has participated in clinical trials sponsored by Novartis and has received honoraria as a coordinator of clinical trials sponsored by Novartis. Dr Kreutzer has received honoraria for giving speeches for, has received travel grants from, and conducts clinical trials for AbbVie Pharmaceuticals, Biogen, Novartis and Janssen‐Cilag. Dr Jazayeri has served as investigator for and received grants from Novartis. Dr Adams has served as investigator for and received grants from Amgen, Eli Lilly and Company and Novartis. Ms Guindon and Dr Papavassilis are full‐time employees of and own stock in Novartis. Mr You is a full‐time employee of Novartis. Dr Tedremets has no conflicts of interest to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 28 and supplemental file): “were randomly allocated”, “Randomization was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject to a treatment arm and specified unique medication pack number" Comment: no description of the method used to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject to a treatment arm and specified unique medication pack number" Comment: well described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1083): “During the induction period, subjects…in the secu 150 mg group were administrated one 150 mg injection and one placebo,….,in the placebo group…2 placebo autoinjections” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1083): “During the induction period, subjects … in the secu 150 mg group were administrated one 150 mg injection and one placebo, …., in the placebo group … 2 placebo autoinjections” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 182, analysed 181 Management of missing data: Quote (Supplemental file): “Missing values with respect to response variables based on PASI score or IGA mod 2011 score were imputed as nonresponse regardless of the reason for missing data” Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01636687). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled, open‐label trial Date of study: not stated Location: Amsterdam and throughout the Netherlands, number not stated |
|
| Participants |
Randomised: 10 participants (ciclosporin (5), mean age 41 years, 4 male; Methotrexate (5), mean age 45 years, 3 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ciclosporin (n = 5), orally, 3 mg/kg/d, 16 weeks Control intervention B. Methotrexate (n = 5), orally, 15 mg/kg/week, 16 weeks |
|
| Outcomes | Assessments at 12 weeks Primary and secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding not declared Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 559): "Patients were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 559): "Patients were randomised..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 559): “Laboratory results were obtained in a blinded fashion before randomisation and at week 12 of therapy. The code was broken only after all definitive results were obtained from all participating patients." Comment: open‐label trial, no double dummy used to guarantee blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 included/10 analysed Comment: no statistical analyses section; however, the results were available for the 10 participants initially randomised. Methods for dealing with missing data: not applicable |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: October 2005‐November 2006 Location: 15 centres in France and Germany |
|
| Participants |
Randomised: 176 participants, mean age 43 years, 213 male Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Certolizumab (n = 59), SC, 200 mg, Certolizumab pegol (CZP) 400 mg week 0 – certolizumab 200 mg weeks 1‐10, 10 weeks Control intervention B. Certolizumab (n = 58), SC, 400 mg, certolizumab 400 mg week 0 – certolizumab 400 mg weeks 1‐10, 10 weeks C. Placebo (n = 59), SC, certolizumab 400 mg week 0 – placebo weeks 1‐10, 10 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source quote (p 180): "This study was funded by UCB Pharma, Brussels, Belgium" Declarations of interest (p 180): "K.R. has served as consultant and ⁄ or paid speaker for and ⁄ or has participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including Abbott, Biogen Idec, Celgene, Centocor, Janssen‐Cilag, Leo, Medac, Merck, MSD (formerly Essex, Schering‐Plough), Novartis and Pfizer (formerly Wyeth). J.‐P.O. is a consultant for Abbott, Centocor, Galderma, Janssen‐ Cilag, Leo, Meda Pharma, Merck Serono and UCB Pharma. A.B.G. has current consulting ⁄ advisory board agreements with Amgen, Astellas, Centocor (Janssen), Celgene, Bristol‐Myers Squibb, Beiersdorf, Abbott, TEVA, Actelion, UCB Pharma, Novo Nordisk, Novartis, Dermipsor, Incyte, Pfizer, Canfite, Merck and Lilly. Research ⁄ educational grants paid to Tufts Medical Center: Centocor (Janssen), Amgen, Immune Control, Abbott, Novo Nordisk, UCB Pharma, Novartis, Celgene and Pfizer. I.J.T. and G.C. are full‐time employees of UCB Pharma. C.T. is a former employee of UCB Pharma. P.M. has served as consultant and ⁄ or paid speaker for and has received grants, consulting and ⁄ or speaker fees from Abott Amgen, Biogen Idec, Bristol‐Myers Squibb, Celgene, Janssen, Novartis, Merck, Pfizer and UCB Pharma." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 181): "Eligible patients were randomised to receive... Randomization was centralized using a dynamic allocation procedure... Treatment was assigned using an interactive voice‐response system"“Randomization was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject treatment arm and specified unique medication pack number Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 181): "Eligible patients were randomised to receive... Randomization was centralized using a dynamic allocation procedure... Treatment was assigned using an interactive voice‐response system" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 181): "CZP... or matching placebo in liquid formulation for subcutaneous injection... Study doses of CZP or placebo were prepared containing the same volume and labelled in the same manner by designed unblinded pharmacists" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 181): "CZP... or matching placebo in liquid formulation for subcutaneous injection... Study doses of CZP or placebo were prepared containing the same volume and labelled in the same manner by designed unblinded pharmacists" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 176 included/176 analysed Quote (p 182): "Co‐primary efficacy assessments were performed on the intention‐to‐treat population... Nonresponder imputations for missing values were used for the primary analysis" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00245765). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported except for pharmacokinetic profile of CDP870. |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: December 2008‐July 2009 Location: 14 centres in the USA and Canada |
|
| Participants |
Randomised: 100 participants (mean age 44 years, 100 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab (n = 30), SC, 3 mg/kg, 1 infusion (day 1) Control intervention B. Secukinumab (n = 29), SC, 10 mg/kg, 1 infusion (day 1) C. Secukinumab (n = 31), SC, 10 mg/kg, 3 infusions (says 1, 15, 29) D. Placebo (n = 10) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 534): "This trial and publication were found by Novartis Pharma AG, Basel, Switzerland." Declarations of interest (p 534): " KR has served as a consultant or paid speaker for, or participated in clinical trials sponsored by, AbbVie, Amgen, Biogen‐Idec, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, Leo, Lilly, Medac, MSD, Novartis, Pfizer, Takeda and Vertex. KAP has received grants and has consulted and served as an investigator for AbbVie, Amgen, Astellas, Biogen‐Idec, Celgene, Centocor, Eli Lilly, Forward Pharma, Fujisawa, GlaxoSmithKline, Janssen, Kyowa‐Kirin, Leo, MSD, Novartis (outside the submitted work), Pfizer and Takeda. RTM has received grants/clinical trial stipends from Novartis. JHT served as a clinical investigator for Novartis during conduct of this study. RB received grants from Novartis during the conduct of this study and has received grants, personal fees and non‐ financial support from AbbVie, Amgen, Astellas, Celgene, Eli Lilly, Janssen, Pfizer and Tribute. MB has served as a clinical trial sponsor for Amgen, Eli Lilly and Novartis. DG has served as a clinical trial investigator for Novartis. RAK is a member of an advisory board for Novartis and several other pharmaceutical companies. YP has received grants from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Merck, Pfizer and Novartis (outside the submitted work). LAR, WMB, TMF and NAB‐S declare no conflict of interests. GS has received grants/clinical trial payments from Janssen, MSD and Novartis (unrelated to secukinumab). JMS, US, TP, EK, GAW, FK and CCB are full‐time employees of Novartis. WH and DML are full‐time employees of and own stock in Novartis. MMS was a full‐time employee of Novartis at the time the study was conducted and the manuscript" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (supplemental appendix): "The randomisation scheme was generated by Novartis Drug Supply Management using a validated system. The randomisation scheme was reviewed and approved by the Biostatistics Quality Assurance group of Novartis and was locked after approval. Subjects were assigned randomisation numbers, according to the randomisation schedule. Each site, upon evaluation of a qualified subject for the trial, faxed the enrolment sheet to the clinical trial leader (CTL) at the fax number provided. The CTL then assigned the randomisation number in a sequential manner and faxed it back to the unblinded pharmacist or qualified site personnel at the site, who then prepared and provided the study medication for the clinic in a blinded fashion." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (supplemental appendix): "Each site, upon evaluation of a qualified subject for the trial, faxed the enrolment sheet to the clinical trial leader (CTL) at the fax number provided. The CTL then assigned the randomisation number in a sequential manner and faxed it back to the unblinded pharmacist or qualified site personnel at the site, who then prepared and provided the study medication for the clinic in a blinded fashion... Treatment allocation and clinical assessment of the subjects were blinded. For preparation of the study medication from bulk supplies, treatment allocation cards were sent to the pharmacist or qualified site personnel at the investigator’s site." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (supporting information): "To maintain the blind of the study, the appearance of placebo infusion bags, ready to administer to the subject, was identical to that of active drug infusion bags. Placebo and active medication were prepared by an unblinded pharmacist or qualified site personnel assigned at each site." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (supporting information): "To maintain data integrity, no subject‐level data were circulated; therefore, blinding was maintained at the individual subject level" Comment probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100 randomised participants, 94 analysed for PASI 75 or 90, 87 analysed for primary outcome (change in PASI) Quote (p 530): "Efficacy and pharmacodynamic parameters were evaluated in all subjects who received ≥ 1 dose of study medication and had a major protocol deviations... Subjects lost to follow‐up were considered relapsed on the day of th first visit without available PASI data" Comment: low rate of loss to follow‐up and reasons comparable between groups |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00805480). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: not stated Location: 32 centres in Europe and Canada |
|
| Participants |
Randomised: 378 participants (mean age 43 years, 268 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals (week 24)
|
|
| Interventions |
Intervention A. Infliximab (n = 301), IV, 5 mg/kg weeks 0, 2, 6 and every 8 weeks, 10 weeks Control intervention B. Placebo (n = 77), IV, equivalent, weeks 0, 2, 6 and every 8 weeks, 10 weeks |
|
| Outcomes | Assessments at 10 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source (p 386): This study was funded by Centocor, and Schering‐Plough, Kenilworth, NJ, USA" Declarations of interest (p 386): "Consultancies: Dr Reich (Abbott, Biogen Idec, Centocor, Schring‐Plough, Essex, Serano, Wyeth), Dr Nestle (Biogen Idec, Centocor, Schring‐Plough, Genentech, Galderma)..." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1368): "A adaptative treatment allocation was used... The treatment assignment was stored electronically and the stored data were used to allocate future patients in such a way that the imbalance between treatment groups was kept to a minimum" “Randomization was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject to a treatment arm and specified unique medication pack number" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 1368): "A adaptative treatment allocation was used... The treatment assignment was stored electronically and the stored data were used to allocate future patients in such a way that the imbalance between treatment groups was kept to a minimum" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1368): "The investigators, study site personnel, and patients remained blinded until the database lock at week 50... placebo group" Comment: probably done, placebo controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1368): "The investigators, study site personnel, and patients remained blinded until the database lock at week 50... placebo group" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 378 included / 378 analysed Quote (p 1368): "The primary endpoint ... as well as.. were analysed on an intention‐to‐treat basis... In patients who discontinued the study agent ... the patients were regarded as not achieving the endpoints for binary responses" Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: October 2012‐April 2016 Location: 82 centres worldwide (USA, Europe, Australia) |
|
| Participants |
Randomised: 250 participants (mean age 45 years, 157 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Apremilast (n = 83), orally, 30 mg twice daily Control intervention B. Etanercept (n = 83), SC, 50 mg weekly D. Placebo (n = 84) |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 2): "This study was sponsored by Celgene Corporation." Declarations of interest (p1): "K. Reich has received honoraria as a consultant and/or advisory board member and/or acted as a paid speaker and/or participated in clinical trials sponsored by AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene Corporation, Centocor, Covagen, Eli Lilly, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Medac, Merck Sharp & Dohme Corp., Novartis, Ocean Pharma, Pfizer, Regeneron, Takeda, UCB Pharma and XenoPort. M. Gooderham has received honoraria, grants and/or research funding as a speaker, investigator, advisory board member, data safety monitoring board member and/or consultant for AbbVie, Actelion, Amgen, Astellas Pharma US, Boehringer Ingelheim, Celgene Corporation, Dermira, Eli Lilly, Galderma, Janssen, Kyowa Hakko Kirin Pharma, LEO Pharma, MedImmune, Merck & Co., Inc., Novartis, Pfizer, Regeneron, Roche" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 3): "Eligible patients were randomised (1 : 1 : 1) via an interactive voice response system to placebo; apremilast oral tablet, 30 mg twice daily; or etanercept subcutaneous injection, 50 mg QW".“Randomization was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject to a treatment arm and specified unique medication pack number" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 3): "Eligible patients were randomised (1 : 1 : 1) via an interactive voice response system to placebo; apremilast oral tablet, 30 mg twice daily; or etanercept subcutaneous injection, 50 mg QW". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "Per the double dummy design, patients received oral tablets (apremilast 30 mg or placebo) twice daily and two subcutaneous injections (etanercept 25 mg each dose or saline placebo) QW." Comment: clearly defined |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "Per the double dummy design, patients received oral tablets (apremilast 30 mg or placebo) twice daily and two subcutaneous injections (etanercept 25 mg each dose or saline placebo) QW." Comment: clearly defined |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 250, 250 analysed Management of missing data: quote (p 3): "Efficacy assessments were conducted for the modified intent‐to treat (mITT) population (all randomised patients who received ≥1 dose of study medication and had both baseline PASI and ≥1 post‐treatment PASI evaluations)... Last‐observation‐carried‐forward (LOCF) methodology was used to impute missing efficacy measurements." Comment: done |
| Selective reporting (reporting bias) | High risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01241591). The pre‐specified outcomes and those mentioned in the methods section have not been reported as DLQI |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: November 2014‐May 2016 Location: 115 centres worldwide |
|
| Participants |
Randomised: 992 participants (mean age 44 years, 692 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Guselkumab (n = 496), SC, 100 mg, weeks 0 and 4, then every 8 weeks Control intervention B. Adalimumab (n = 248), 80 mg week 0, then 40 mg week 1, and every 2 weeks C. Placebo (n = 248) |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 1): "Supported by Janssen Research & Development, LLC." Declarations of interest (p 1): "Dr Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Amgen, Biogen, Boehringer Ingelheim Pharma, Celgene, Covagen, Eli Lilly, Forward Pharma, GlaxoSmithKline, Janssen, Leo, Medac, Merck Sharp & Dohme, Novartis, Ocean Pharma, Pfizer, Regeneron, Takeda, UCB Pharma, and Xenoport. Dr Armstrong has served as investigator and/or advisor/consultant for AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Merck, Novartis, and Pfizer. Dr Foley has served as a consultant, investigator, speaker, and/or advisor for and/or received travel grants from 3M/iNova/Valeant, Abbott/AbbVie, Amgen, Biogen Idec, BMS, Boehringer Ingelheim, Celtaxsys, Celgene, Cutanea, Eli Lilly, Galderma, GSK/Stiefel, Janssen, LEO/Peplin, Novartis, Regeneron, Schering‐Plough/MSD, UCB, and Wyeth/Pfizer. Dr Gordon has received research support from AbbVie, Amgen, Boeringher Ingelheim, Eli Lilly, and Janssen, and consultant/ honoraria from AbbVie, Amgen, Boeringher Ingelheim, Celgene, Eli Lilly, Janssen, Novartis, and Pfizer. Drs Song, Wasfi, Randazzo, Li, and Shen are all employees of Janssen Research & Development, LLC (subsidiary of Johnson & Johnson) and own stock in Johnson & Johnson." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 3): "Patients were randomized 2:1:1 using a permuted block method at baseline to guselkumab 100 mg at weeks 0, 4, 12, and 20; placebo at weeks 0, 4, and 12, then guselkumab at weeks 16 and 20; or adalimumab 80 mg at week 0, 40 mg at week 1, and every 2 weeks thereafter through week 23 (Fig 1). Central randomization occurred using an interactive web based response system (Perceptive Informatics, East Windsor, NJ)." Comment: clearly defined |
| Allocation concealment (selection bias) | Low risk | Quote (p 3): "Patients were randomized using a permuted block method at baseline in a 2:1:2 ratio to guselkumab 100 mg at weeks 0, 4, 12, and every 8 weeks through week 44; placebo at weeks 0, 4, and 12 followed by guselkumab 100 mg at weeks 16 and 20, and every 8 weeks through week 44; or adalimumab 80 mg at week 0, 40 mg at week 1, and 40 mg every 2 weeks through week 47. Central randomization was implemented using an interactive World Wide Web response system (Perceptive Informatics, East Windsor, NJ)." Comment: clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "double‐blind, placebo‐ and adalimumab comparator controlled study" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "double‐blind, placebo‐ and adalimumab comparator controlled study" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 992, 992 analyzed Management of missing data: quote (p 3): "All randomized patients were included in the primary analysis and some secondary efficacy analyses according to their assigned treatment group.... Patients who discontinued treatment due to lack of efficacy or an adverse event [AE] of worsening of psoriasis, or started a protocol‐prohibited medication/therapy to improve psoriasis were considered treatment failures." Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02207244). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: July 2009‐December 2010 Location: 60 centres in Portland, USA |
|
| Participants |
Randomised: 404 participants Secukinimab A (66) (mean age 43 years, 53 male) Secukinimab B (138) (mean age 44 years, 104 male) Secukinimab C (133) (mean age 45 years, 105 male) Placebo (67) (mean age 44 years, 44 male) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention A. Secukinumab (n = 66), SC, 150 mg, week 0, 12 weeks Control intervention B. Secukinumab (n = 138), SC, 150 mg, weeks 0, 4, 8, 12 weeks C. Secukinumab (n = 133), SC, 150 mg, weeks 0, 1, 2, 4, 12 weeks D. Placebo (n = 67), SC, weeks 0, 1, 2, 4, 8, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding support quote (p 402): "Novartis Pharma AG, Basel, Switzerland" Declarations of interest (appendix): "P.R. has received honoraria for lecturing in industry‐sponsored meetings and has received research grants from pharmaceutical companies as an investigator. B.S. has consulted for Novartis and several other pharmaceutical companies; he has served on an advisory board for Novartis and several other pharmaceutical companies. D.T. has served as a speaker and served on advisory boards for Abbott, Biogen‐Idec, Janssen‐Cilag, Leo, MSD, Novartis and Pfizer. C. Paul has received honoraria from and has been a paid consultant to Abbott, Amgen, Celgene, Janssen‐Cilag, Novartis and Pierre Fabre. K.R., E.H., A.G., M.M. and C. Papavassilis are full‐time employees of, and own stock in Novartis. J.‐P.O., A.M. and R.E.S. declare no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 404): “Randomization numbers were generated by the interactive response technology provider using a validated system that automated the random assignment of patients numbers to randomisation numbers” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 404): “Randomization numbers were generated by the interactive response technology provider using a validated system that automated the random assignment of patients numbers to randomisation numbers” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 404): “Patients, investigator staff, persons performing the assessments and data analysts were blinded to the identity of treatment from the time of randomisation until primary outcome analysis” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 404): “Patients, investigator staff, persons performing the assessment and data analysts were blinded to the identity of treatment from the time of randomisation until primary outcome analysis” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 404 included/404 analysed Quote (p 405): "Following th intent‐to‐treat principle, data were analysed... Missing values were replaced using the last‐observation‐carried‐forward approach" Comment: ITT analyses |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00941031). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: December 1986‐March 1988 Location: 7 centres in Germany |
|
| Participants |
Randomised: 82 participants (mean age 44 years, 55 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin, orally, 35 mg, daily, 8 weeks (n=42) Control intervention B. Placebo, orally, daily, 8 weeks (n=40) |
|
| Outcomes | Assessments at 8 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding sources: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 483): "The study was designed as a randomized, double‐blind, placebo‐controlled parallel group trial" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 483): "The study was designed as a randomized, double‐blind, placebo‐controlled parallel group trial" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 483): "The study was designed as a randomized, double‐blind, placebo‐controlled parallel group trial" Comment: no description of the method used to guarantee blinding as visible side effects are related to acitretin |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 483): "The study was designed as a randomized, double‐blind, placebo‐controlled parallel group trial... the investigators blinded to treatment assignment" Comment: no description of the method used to guarantee blinding of outcome assessment as visible side effects are related to acitretin |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 82 included/78 analysed Quote (p 483): "... according to the intention‐to‐treat principle.. Dropout data were evaluated on the date of dropout" Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled, open label Date of study: not stated Location: multicentric (number not stated) in North India |
|
| Participants |
Randomised: 30 participants (methotrexate: mean age 39 years, 12 male; ciclosporin: mean age 46 years, 13 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 15), orally, 0.5 mg/kg dose tapered after PASI 75 obtained Control intervention B. Ciclosporin (n = 15), orally, 3 mg/kg increased to 4 if no change or rise of dose tapered after PASI 75 obtained |
|
| Outcomes | Assessments at 12 weeks Primary or secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 459): "Patients were randomly assigned to either..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 459): "Patients were randomly assigned to either..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30 included/30 analysed Methods for dealing with missing data: not stated |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported.No primary outcome declared |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: not stated Location: 6 centres in France and Switzerland |
|
| Participants |
Randomised: 42 participants (placebo (22) mean age 43 years, 16 male; acitretin (20), mean age 46 years, 16 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 20), orally, 2 x 25/d 2 weeks and 25/d + UVA 3/weeks, daily, 10 weeks Control intervention C. Placebo, orally (n = 22), daily, 10 weeks Co‐intervention: UVA 3/week, 10 weeks |
|
| Outcomes | Assessments not clearly stated (reported at 8 weeks) Primary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 219): "This multicenter study was performed in a double‐blind, parallel fashion... The patients were randomly allocated to ..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 219): "This multicenter study was performed in a double‐blind, parallel fashion... The patients were randomly allocated to ..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p 219): "This multicenter study was performed in a double‐blind, parallel fashion...All patients initially received 2 capsules of test medication (placebo, acitretin 2x25 mg, ...." Comment: no description of the method used to guarantee blinding of outcome assessment with visible AE in both acitretin and etretinate groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no description of the method used to guarantee blinding of outcome assessment with visible AE in both acitretin and etretinate groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote (p 220): "Patients who left the study ... were not included in the evaluation of efficacy" Comment: not ITT analyses (number lost to follow‐up unknown) |
| Selective reporting (reporting bias) | Low risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: unreported Location: multicentre (n = 28) in Europe & Canada |
|
| Participants |
Randomised: 271 participants (mean age 42, 178 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Adalimumab (n = 108), SC, 80 mg at week 0, 40 mg at week 1 and 40 mg eow Control intervention B. Methotrexate (n = 110), orally, 7.5‐25 mg weekly C. Placebo (n = 53), SC and orally (same drug administration) |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 561): "Abbott Laboratories funded this study and participated in the study design, data collection, data management, data analysis and preparation of the manuscript" Declarations of interest (p 558): "J.‐H.S., G.S., L.D., K.P. and J.‐P.O. have served as consultants for Abbott Laboratories. In addition, they have participated in continuing medical education events supported by unrestricted educational grants from Abbott. R.G.L. reports receiving fees as a consultant or advisory board member for Abbott, Amgen, Astellas, Boehringer‐ Ingelheim, Barrier Therapeutics and Genentech; he has received lecture fees from Abbott, Amgen/ Wyeth and Biogen‐Idec, and has been the principal investigator and received grants from Abbott, Amgen, Astellas, Centocor, Galderma and Genentech. K.U., M.K. and A.C. are employees of Abbott. " |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 559):"Randomisation was completed through a central computer‐generated scheme stratified by centre, with block sizes of four" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 559): "Patient numbers were centrally assigned by an interactive voice‐response system in consecutive order". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 559): "Adalimumab (Humira; Abbott Laboratories) or matching placebo for SC injection was provided as sterile preservative‐free solution in prefilled syringes. Oral methotrexate tablets were supplied by Wyeth Pharma (Münster, Germany), and placebo tablets were supplied by Abbott GmbH & Co. KG (Ludwigshafen, Germany). Both the methotrexate and placebo tablets were administered as capsules (encapsulated tablets) as a single weekly dose. The capsules for both methotrexate and placebo were supplied by Fisher Clinical Services (Basel, Switzerland)." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 559): "Adalimumab (Humira; Abbott Laboratories) or matching placebo for SC injection was provided as sterile preservative‐free solution in prefilled syringes. Oral methotrexate tablets were supplied by Wyeth Pharma (Münster, Germany), and placebo tablets were supplied by Abbott GmbH & Co. KG (Ludwigshafen, Germany). Both the methotrexate and placebo tablets were administered as capsules (encapsulated tablets) as a single weekly dose. The capsules for both methotrexate and placebo were supplied by Fisher Clinical Services (Basel, Switzerland)." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 271, analysed 271 Management of missing data: quote (p 562): "Data for 16 patients with missing week 16 assessments for PASI, including the 15 patients who discontinued and one additional patient in the methotrexate group, were imputed as nonresponse." Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00235820). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported except for DLQI that was published in a second study. |
| Methods | RCT, active‐controlled, open label Date of study: March 2001‐November 2001 Location: 1 centre in Karachi, Pakistan |
|
| Participants |
Randomised: 40 participants (age from 18‐50 years, 60 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. PUVA therapy (+ psoralen) (n = 20), 4 times/week Control intervention B. Methothrexate (n = 20), orally, 10 mg/week, 5 mg Saturday + Sunday |
|
| Outcomes | Time of assessments: not stated Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Immunex Corporation Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (in the method section): “The selected patients ... randomly allocated to...” Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (in the method section): “The selected patients ... randomly allocated to...” Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of the methods used to manage missing data, no description of the methods used to assess the primary outcome (ITT, PP...) |
| Selective reporting (reporting bias) | High risk | Comment: no protocol was available. The outcomes mentioned in the results section were not specified in the methods section. |
| Methods | RCT, placebo‐controlled, double blind Date of study: 1986‐1988 Location: 7 centres in Germany |
|
| Participants |
Randomised: 88 participants (mean age 45 years, 68 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 44), orally, 50 mg (15 days) then 25 mg, daily, 8 weeks Control intervention B. Placebo (n = 44), orally, daily, 8 weeks Co‐intervention PUVA (8‐methoxypsoralen), orally 0.6 mg/kg, 3‐5/week, 8 weeks |
|
| Outcomes | Assessments at 8 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 310): "The study was designed as a randomised, double‐blind, parallel groups trial... Both investigators and biostatisticians were blinded" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 310): "The study was designed as a randomised, double‐blind, parallel groups trial... Both investigators and biostatisticians were blinded" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (p 310 & 311): "The study was designed as a randomised, double‐blind, parallel group trial... Both investigators and biostatisticians were blinded… however due to well know side effect pattern of acitretin, ..., the possibility of an investigator bias cannot be excluded" Comment: visible AE in acitretin groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p 310 & 311): " The study was designed as a randomised, double‐blind, parallel group trial... Both investigators and biostatisticians were blinded… however due to well know side effect pattern of acitretin, ..., the possibility of an investigator bias cannot be excluded" Comment: visible AE in acitretin groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88 included/83 analysed Quote (p 311): "Patients who discontinued the trial prematurely were evaluated on the date of discontinuation of therapy" Comment: not ITT, low number of dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled, double blind Date of study: December 2005‐May 2008 Location: centres (n = 98) worldwide |
|
| Participants |
Randomised: 754 participants (mean age 46 years, 473 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept, SC, 50 mg, twice a week, 12 weeks Control intervention B. Etanercept, SC, 50 mg, once a week, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary and secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding, quote (p 8): "Wyeth Research, which was acquired by Pfizer in October 2009, sponsored this clinical trial and was responsible for the collection and analysis of data..." Declarations of interest (p 8): "WS has received fees for speaking/consulting from Abbott, Schering‐Plough, Wyeth, and Janssen‐Cilag. J‐PO has received fees for speaking/conferences/consulting from Schering‐Plough, Abbott, Merck‐Serono, Centocor, Wyeth, Janssen‐Cilag, MedPharma, Laboratorios Pierre‐Fabre, Galderma Laboratories, and Leo Pharma. BK has served on advisory boards for Schering‐Plough and Roche; has received funds for research/travel/conferences from Wyeth, Centocor, Abbott, Schering‐Plough, Roche, and Bristol‐Myers Squibb; and has served on a speaker panel for Bristol‐Myers Squibb. OB has received fees from Wyeth, Schering‐Plough, Abbott, Roche, Chugai, and Bristol‐Myers Squibb. DR, RDP, JE, CM, and BF are all employees of Pfizer." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 3): "We randomly assigned participants to ..." Comment: no description of the method used to generate random sequences |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 3): "We randomly assigned participants to ..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "In the double blind period..." Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "In the double blind period..." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 754 included/752 analysed Quote (p 4): "The modified intention‐to‐treat (ITT) population included all randomised participants who took at least one dose of the test drug and at least one post baseline efficacy evaluation... Efficacy analyses used the last observation carried forward method for imputation of missing data" Comment: mITT and only 2 of 754 participants not included in the analysis of the primary outcome |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00245960). The pre‐specified outcomes mentioned in the methods section appeared to have been reported except for the results of participant‐reported endpoints summarized in a separate publication. |
| Methods | RCT, placebo‐controlled, double blind Date of study: July 2008‐April 2009 Location: 41 centres in the USA |
|
| Participants |
Randomised: 211 participants (mean age 45 years, 131 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 139), SC auto‐administered, 50 mg twice a week, 11 weeks Control intervention B. Placebo (n = 72), SC auto‐administered, twice a week |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial At 4, 8, 12 weeks
|
|
| Notes | Funding source, quote (Appendix 1): "Abbott Laboratories funded this study and participated in the study design, data collection, data management, data analysis and preparation of the manuscript. All of the authors had full access to the data and were involved in the analysis of data, development and revision of the manuscript, and decision to submit the manuscript for publication. The corresponding author takes responsibility for the integrity of the data and the accuracy of the data analysis." Declarations of interest (appendix 1): "B.E.S. has been an investigator, consultant, speaker, and served on an advisory board for Amgen, Abbott and Centocor; and has also been a speaker for Astellas. J.J.C. has received research support from Abbott, Amgen, Centocor, Celgene and Eli Lilly; has been a consultant for Abbott, Amgen and Centocor; and has been a speaker for Abbott. P.S.Y. has served as a consultant, principle investigator, speaker or advisory board member for Abbott, Amgen, Astellas and Centocor. M.O. and D.A.W. are employees of Abbott." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 662): "Patients were randomised..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 662): "Patients were randomised" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 662): “Patients enrolled in the placebo arm received SC injections matching active treatment to maintain the blind. To maintain the blind, all patients received two SC injections at weeks 0 and 4 and one SC injection at week 8, consisting of either briakinumab or matching placebo, depending on the treatment arm. In addition, each patient also received two SC injections biweekly, 3 days apart, week 0 through week 11, consisting of either etanercept or matching placebo, depending on the treatment arm.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 662): “Patients enrolled in the placebo arm received SC injections matching active treatment to maintain the blind. To maintain the blind, all patients received two SC injections at weeks 0 and 4 and one SC injection at week 8, consisting of either briakinumab or matching placebo, depending on the treatment arm. In addition, each patient also received two SC injections biweekly, 3 days apart, week 0 through week 11, consisting of either etanercept or matching placebo, depending on the treatment arm.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 211, analysed 211 Management of missing data: Quote (p 663): “The primary efficacy analysis consisted of four comparisons performed in the intent‐to‐treat population (i.e. all randomised patients), …, Nonresponder imputation was used to handle missing data.” Comment: done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00710580). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled, double blind Date of study: April 2008‐March 2012 Location: 32 centres in Europe, Latin America and Asia |
|
| Participants |
Randomised: 273 participants (mean age 44 years, 190 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 137), SC, 50 mg, once a week, 24 weeks Control intervention B. Etanercept (n = 136), SC, 50 mg, twice a week, 24 weeks |
|
| Outcomes | Assessments at 24 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source, quote (p 177): "The PRISTINE trial was sponsored by Pfizer Inc..." Declarations of interest (p 177‐178): "Robert Strohal has been a paid consultant of and has received research grants from Pfizer Inc, which provided funding for the PRISTINE study. He is also a member of the Pfizer European Expert Board and of the Pfizer Speakers Bureau. Luis Puig has been a paid consultant of and has received research grants from Pfizer; he has served on Pfizer advisory boards and the Speakers Bureau. Edgardo Chouela is a paid consultant and speaker for Pfizer Inc and Galderma and has conducted clinical studies for Novartis, Jannssen, Pfizer and Roche. Tsen‐Fang Tsai has been a paid consultant of Pfizer Inc; he has served as an investigator and received honoraria for serving as an advisor and speaker for Pfizer. Jeffrey Melin, Bruce Freundlich and Charles Molta were previous employees of Wyeth and Pfizer Inc. Joanne Fuiman, Ronald Pedersen and Deborah Robertson are current employees of Pfizer Inc." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 170): "Subjects were randomly assigned to one of the 2 etanercept treatment groups... in 1:1 treatment allocation" Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 170): "Subjects were randomly assigned to one of the 2 etanercept treatment groups... in 1:1 treatment allocation" Comment: not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 170): "The study consisted of a 12‐week double‐blind treatment period" Comment: probably done, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 170): "The study consisted of a 12‐week double‐blind treatment period" Comment: probably done, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 273 enrolled and randomised and 270 analysed Quote (p 171): "All efficacy analyses were performed using the modified intent‐to‐treat population which included all randomised subjects who received at least one dose of etanercept and had both baseline and on therapy PASI evaluations. The last observation‐carried‐forward method was used for the imputation of missing data..." Comment: mITT and only 3 of 273 participants not included in the analyses of the primary outcome |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00663052). The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: not stated Location: 2 centres in Austria (Vienna, Innsbruck) |
|
| Participants |
Randomised: 60 participants (mean age 40 years (acitretin), 49 years (placebo); 42 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 30), orally, 1 mg/kg, daily, 12 weeks or until complete clearing Control intervention B. Placebo (n = 30), orally, daily, 12 weeks Co‐intervention PUVA, phototherapy, 4 times/week, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary and secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding: supported by a grant from Hoffma La Roche & Co Ltd Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 682): "Only patients ... were included and assigned randomly..." Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 682): "Only patients ... were included and assigned randomly..." Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (p682): "Acitretin ... or placebo..." Comment: no description of the method used to guarantee blinding of participants and personnel as acitretin leads to visible adverse effects (cheilitis) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (p682): "Acitretin ... or placebo..." Comment: no description of the method used to guarantee blinding of participants and personnel as acitretin leads to visible adverse effects (cheilitis) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomly assigned 60, analysed 48 Quote (p 683): "Of the 60 patients, 48 completed the study and were included in the statistical analysis" Comment: not ITT |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, no outcomes defined in the method section |
| Methods | RCT, active‐controlled, double blind Date of study: 27 February 2014–11 May 2015 Location: 137 centres in Europe, Australia and Asia |
|
| Participants |
Randomised: 676 participants (mean age 46 years, 481 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Secukinumab (n = 334), SC, 300 mg weeks 0, 1, 2, 3 then monthly Control intervention B. Ustekinumab (n = 335), SC, 45/90 mg weeks 0, 4 then every 12 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 400): "Novartis Pharma supported this study" Declarations of interest (p 400): "Dr Thaçi has served as a consultant, served as an advisory board member, and/or received honoraria for lecturing for AbbVie, Amgen, Biogen‐Idec, Celgene, Eli Lilly, Janssen‐Cilag, Leo Pharma, MSD, Novartis, Pfizer, Regeneron, and Sanofi. Dr Blauvelt has served as a scientific consultant and clinical study investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen Ortho Biotech, Merck, Novartis, Pfizer, and Sandoz. Dr Reich has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Amgen, Biogen‐Idec, Celgene, Centocor, Covagen, Eli Lilly, Forward Pharma, GSK, Janssen‐Cilag, Leo Pharma, Medac, MSD, Novartis, Pfizer, Vertex, Takeda, and Xenoport..." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 402): “were randomised via an interactive response technology system" Randomization was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject to a treatment arm and specified unique medication pack number Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p402): “were randomised via an interactive response technology system “ Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p402) : “To maintain blinding, placebo injections matching the secukinumab regimen were given in the ustekinumab group” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p402) : “To maintain blinding, placebo injections matching the secukinumab regimen were given in the ustekinumab group” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 676, analysed 669 Management of missing data: Quote (p 403): “Missing values with respect to response variables based on PASI and IGA mod 2011 scores were imputed as nonresponse (nonresponder imputation)." Comment: however it was not an ITT analysis as 7 participants were not taken into account but low rate of dropout |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02074982). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: not stated Location: 28 centres in Japan |
|
| Participants |
Randomised: 54 participants (mean age 46 years, 36 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Infliximab (n = 35), IV, 5 mg/kg, weeks 0, 2, 6; 10 weeks Control intervention B. Placebo (n = 19), IV, weeks 0, 2, 6; 10 weeks |
|
| Outcomes | Assessments at 10 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 41): "Eligible patients were randomised in a 2:1 ratio to either... using the dynamic allocation method" Comment: no description of the methods used to guarantee the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p41): "Eligible patients were randomised in a 2:1 ratio to either... using the dynamic allocation method" Comment: no description of the methods used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p41): "The induction phase of th treatment was .. double‐blind placebo controlled trial... Infliximab or placebo was administered by IV drip infusion over a period of at least 2h ... Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p41): "The induction phase of th treatment was .. double‐blind placebo controlled trial... Infliximab or placebo was administered by intravenous drip infusion over a period of at least 2h ... Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 54, analysed 54 Quote (p42): "This primary endpoint analysis was performed on an "intent‐to‐treat" basis...Patients who discontinued the study treatment ... were handled as "not improved" in the assessment" Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: December 2008‐March 2010 Location: 13 centres in Taiwan and Korea |
|
| Participants |
Randomised: 121 participants (mean age 41 years, 103 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ustekinumab, SC, 45 mg, weeks 0, 4, 16 + placebo week 12, 16 weeks Control intervention B. Placebo, SC, weeks 0‐4 + ustekinumab 45 mg weeks 12‐16 |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source quote (p 162): "This study was supported by Centocore, Inc" Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 155): “Patients were enrolled in this multicenter..., double‐blind, placebo‐controlled study... Randomization was performed via an interactive voice response system based on minimization with bias‐coin assignment...”“Randomization was conducted via Interactive Response Technology, which assigned a randomisation number that linked the subject to a treatment arm and specified unique medication pack number" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p155): “Patients were enrolled in this multicenter..., double‐blind, placebo‐controlled study... Randomization was performed via an interactive voice response system based on minimization with bias‐coin assignment...” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p155): “Patients were enrolled in this multicenter..., double‐blind, placebo‐controlled study... Comment: placebo trial, probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p155): “Patients were enrolled in this multicenter..., double‐blind, placebo‐controlled study... Comment: placebo trial, probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 121, analysed 121 Quote (p 156): “For all efficacy analyses, patients were analysed by assigned treatment groups...Data were analysed by intent‐to‐treat for the primary endpoint... Patients who discontinued study treatment... were judged as non‐responders for binary endpoints” Comment: ITT analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: June 2003–January 2004 Location: 39 centres in Houston, USA and Canada |
|
| Participants |
Randomised: 620 participants (mean age 46 years, 419 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept (n = 311), 50 mg, SC, twice weekly, 12 weeks Control intervention B. Placebo (n = 309), SC, twice weekly, 12 weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding, quote (p 361): "The study was designed by Immunex, S Tyring, and other members of the Etanercept Psoriasis study group (The complete data set was held at the central data‐processing facility at Amgen) Declarations of interest (p 367‐368): "S Tyring has received research support from Amgen. A Gottlieb is a consultant for several companies (Amgen, BiogenIdec, CellGate, Centocor, Genentech, Novartis AG, Wyeth Pharmaceuticals, Schering‐Plough Corporation, Eisai, Celgene, Bristol Myers Squibb, Beiersdorf, Warner Chilcott, Abbott Labs, Allergan, Kemia, Roche, Sankyo, Medarex, Celera, TEVA, Actelion, and Advanced ImmuniT) and is on the speaker’s bureau for Amgen, BiogenIdec, and Wyeth Pharmaceuticals. She has also received research funding from Amgen, BiogenIdec, Centocor, Genentech, Abbott Labs, Ligand Pharmaceuticals, Beiersdorf, Fujisawa Healthcare, Celgene Corp, Synta, Bristol Myers Squibb, Warner‐Chilcott, and Paradigm. K Papp is a consultant, has received research funding, and has served as a speaker for Amgen, BiogenIdec, Centocor, Genentech, Novartis, Wyeth, Schering‐Plough, Abbott, Allergan, Medimmune, Serono, Xoma, Isotechnica, and GlaxoSmithKline. He has also served as a medical or scientific officer for Amgen, Centocor, Genentech, and Serono. K Gordon has received research support and honoraria from Abbott, Amgen, Biogen‐IDEC, Centocor, Genentech, and Synta. C Leonardi is: a consultant, investigator, and speaker for Amgen and Genentech and has received educational grants from these companies; a consultant, investigator, and speaker for Centocor; a consultant and investigator for Serono; and a consultant, investigator, and speaker for Abbott..." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 30): “Randomisation code lists were generated in the Biostatistics Department at Amgen by a designed person with no other association with the study” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 30): “Randomisation code lists were generated in the Biostatistics Department at Amgen by a designed person with no other association with the study” Comment: no precision |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 30): "All patients received 2 injections per dose of investigational product”, Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 30): “To prevent study assessors from being influenced by the presence of an injection site reaction, patients applied dressings to the last three injection sites and to any erythematous injection sites before each psoriasis evaluation” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 620, analysed 617 for the primary outcome Management of missing data: quote (p 31): “The primary analyses for all efficacy endpoints included all randomised patients who received at least one dose of investigational product. Missing values were imputed using last observation carried forward” Comment: only 2 participants did not receive at least one dose, 618 participants should be involved in the mITT, however 617 participants were analysed for the primary outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT00111449). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported |
| Methods | RCT, active/placebo‐controlled, double blind Date of study: 22 September 2010‐24 October 2012 Location: 58 centres in Europe and Russia |
|
| Participants |
Randomised: 326 participants (mean age 40 years, 245 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ponesimod (n = 126), orally, 10 mg for 7 days then 20 mg, 16 weeks B. Ponesimod (n = 133), orally, 10 mg for 7 days then 20 mg days 8 ‐15 then 40 mg, 16 weeks Control intervention C. Placebo (n = 67), orally, 16 weeks |
|
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding (p 2044): "This study was sponsored by Actelions Pharmaceuticals" Declarations of interest (p 2044): "AV, MB, and DD’A were employees and stockholders of Actelion Pharmaceuticals when the study was done. SC has been lecturer, consultant, or both, for AbbVie, Janssen‐Cilag, Leo‐Pharma, Merck, Novartis, and Pfizer. MS has received personal fees for statistical consultancy from Actelion Pharmaceuticals and SDE Research. PA, PH, and P‐GS declare that they have no competing interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 2037): "A unique seven‐digit randomisation number was assigned to each patient by an independent service provider (ICON Clinical Research, Dublin, Ireland) via an interactive voice or internet‐based response system" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 2037): "A unique seven‐digit randomisation number was assigned to each patient by an independent service provider (ICON Clinical Research, Dublin, Ireland) via an interactive voice or internet‐based response system" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 2037): "The investigational drug and matching placebo were identical in appearance and packaging...The primary investigator, care providers, patients, and sponsor were unaware of study treatment assignment and lymphocyte count. An independent physician monitored patients after the first dose was administered or increased until the end of the study." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 2037): "The investigational drug and matching placebo were identical in appearance and packaging...The primary investigator, care providers, patients, and sponsor were unaware of study treatment assignment and lymphocyte count. An independent physician monitored patients after the first dose was administered or increased until the end of the study." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 326, analysed 326 Quote (p 2037): "All randomised patients were assessed by intention‐to‐treat for the primary, secondary, and all efficacy endpoints in the induction period... Missing or invalid values were handled with nonresponder imputation" Comment: ITT analyses |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01208090). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported |
| Methods | RCT, placebo‐controlled, double blind Date of study: 2013 and June 2015 Location: single centre in the Netherlands |
|
| Participants |
Randomised: 33 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Fumaric acid (n = 18), from 215 mg once daily up to a maximum of 215 mg 4 times a day, 24 weeks Control intervention B. Placebo Co‐intervention Etanercept (n = 14) (50 mg SC twice weekly for 12 weeks followed by 50 mg once weekly for an additional 12 weeks) |
|
| Outcomes | Assessments at 24 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding, quote (supplemental appendix): "This investigator‐initiated study was supported by a grant of Pfizer Pharmaceuticals. Pfizer was not involved in any study procedure, but Pfizer was granted the right to read, but not to edit, the manuscript prior to submission for publication." Declarations of interest (p 413): "Investigator‐initiated project grant from Pfizer. E. Prens has acted as a consultant for AbbVie, Amgen, Astra‐Zeneca, Baxter, Eli Lilly, Galderma, Janssen‐Cilag, Novartis and Pfizer and has received investigator‐initiated research grants (paid to Erasmus MC) from Pfizer, Janssen‐Cilag and AbbVie. M.B.A. van Doorn has acted as a consultant for Abbott, Janssen, LEO Pharma, MSD and Pfizer, and has been an investigator for Eli Lilly, Idera Pharmaceu‐ticals, Cutanea and Novartis. T. van Gelder has been on the speakers’ bureau or worked as consultant for Sandoz, Novartis, Teva, Chiesi, Astellas and Roche". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (supplemental appendix): “Using a computer‐generated randomisation list, patients were randomised at baseline to a 1:1 ratio to receive either etanercept combined with oral fumarates (combination group) or etanercept only (monotherapy group). ” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (supplemental appendix): “Using a computer‐generated randomisation list, patients were randomised at baseline to a 1:1 ratio to receive either etanercept combined with oral fumarates (combination group) or etanercept only (monotherapy group).” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (supplemental appendix): "Patients and the study physicians were not blinded for the allocated treatment group.” Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (supplemental appendix): “The independent PASI assessor (E.P.P.) was blinded to treatment throughout the course of the study.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 33, analysed 33 for the primary outcome Management of missing data: quote (supplemental appendix): “Patients lost to follow‐up were not included in the PASI 75 response and PGA score analyses. ” Comment: not ITT analyses however all randomised participants reached the primary outcome assessment |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol for the study was available on European Clinical Trials Database (EudraCT) (EudraCT No. 2011‐005685‐38) (not found). The pre‐specified results mentioned in the methods section appeared to have been reported. |
| Methods | RCT, placebo‐controlled, double blind Date of study: Jun 2006‐May 2007 Location: multicentre (numbers of centres not stated) in Belgium, France, Germany, Hungary, Italy, Netherlands, Poland, Romania, Spain |
|
| Participants |
Randomised: 143 participants (mean age 45 years, 84 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Etanercept, 50 mg, self‐administered SC, once a week, 12 weeks (n=96) Control intervention B. Placebo, self‐administered SC, once a week, 12 weeks (n=46) |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source (p 1184): "This study was supported financially by Wyeth Pharmaceuticals, Collegeville, PA, USA)" Comments: 3 authors were employed by Wyeth pharmaceuticals which supported this study financially Declarations of interest (p 1177): "C.Z., M.P.B., L.P. and J.W. are employed by Wyeth Pharmaceuticals, which supported this study financially. " |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 1178): "Patients were randomly assigned (using the Clinical Operations Randomization Environment system) ... according to a 2:1 treatment allocation" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (p1178): "Patients were randomly assigned (using the Clinical Operations Randomization Environment system) ... according to a 2:1 treatment allocation" Comment: not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 1178): "In both the double blind controlled study..., etanercept was supplied as a sterile lyophilised powder. All study drugs were self‐administrated QW by the patient by subcutaneous injections" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 1178): "In both the double blind controlled study..., etanercept was supplied as a sterile lyophilised powder. All study drugs were self‐administrated QW by the patient by subcutaneous injections" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 142, analysed 142 Management of missing data, quote (p 1179): "The primary population for efficacy and safety analyses ... was the modified intent‐to‐treat population. The last observations were carried forward in cases of missing efficacy data" Comment: done |
| Selective reporting (reporting bias) | Unclear risk | Comment: the specified outcomes mentioned in the methods section appeared to have been reported however no protocol was available, |
| Methods | RCT, placebo‐controlled Date of study: 22 February 2013‐13 May 2015 Location: 13 centres in Europe |
|
| Participants |
Randomised: 120 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Methotrexate (n = 91), SC, IM, 17.5‐22.5 mg/week, 12 weeks Control intervention B. Placebo (n = 29) |
|
| Outcomes | 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: Quote (p 528) "Funding source: Medac. The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and all authors had final responsibility for the decision to submit for publication" Declarations of interest (p 536): "RBW has received personal fees from AbbVie, Almirall, Amgen, Boehringer Ingelheim Pharma, Celgene, Janssen‐Cilag, Leo, Lilly, Novartis, Pfizer, and Xenoport outside the submitted work. UM has been an advisor to, received speakers honoraria or grants from, or participated in clinical for Abbott/AbbVie, Almirall Hermal, Amgen, BASF, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Foamix, Forward Pharma, Galderma, Janssen, Leo Pharma, Medac, MSD, Miltenyi Biotech, Novartis, Pfizer, Teva, VBL, and Xenoport. RvK has been an investigator, consultant, advisor, or speaker for Abbvie, Almirall, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Eli Lilly, GSK, Leo, Janssen‐Cilag, MSD, Novartis, Pfizer, UCB, and VBL Pharma. JN has received grants from Amgen, Novartis, Janssen‐Cilag, LEO, Lilly, Medac, Regeneron, and Dermapharm, outside the submitted work. DW‐T has been an advisor to, received speakers honoraria or grants from, or participated in clinical for Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim Pharma, Celgene, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, Leo, Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, UCB Pharma, and VBL. KG has been an advisor to, received speakers honoraria or grants from, or participated in clinical for Abbott/AbbVie, Almirall, Biogen, Boehringer Ingelheim, Celgene, Delenex, Eli Lilly, Galderma, Janssen, Medac, MSD, Novartis, and Pfizer. KR has received personal fees from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, Leo, Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Takeda, UCB Pharma, and Xenoport, outside the submitted work. IZ, TMF, and NB‐S declare no competing interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (p 3): "Eligible patients were randomly assigned (3:1), via computer‐generated random numbers (RandList 1.2) in an ascending order, to receive either methotrexate or placebo injections for the first 16 weeks of the study (phase 1)." Comments: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (p 3): "Eligible patients were randomly assigned (3:1), via computer‐generated random numbers (RandList 1.2) in an ascending order, to receive either methotrexate or placebo injections for the first 16 weeks of the study (phase 1)." Comments: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 3): "Study phase 1 was done in a double‐blind manner, with group allocation concealed from participants and investigators from the time of randomisation until an interim database lock at week 16...The syringes for placebo and active drug were not distinguishable and were fully coated to prevent identification of colour differences between injections" Comments: clearly defined |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 3): "Study phase 1 was done in a double‐blind manner, with group allocation concealed from participants and investigators from the time of randomisation until an interim database lock at week 16...The syringes for placebo and active drug were not distinguishable and were fully coated to prevent identification of colour differences between injections" Comments: clearly defined |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of randomised participants, n = 120, 120 analysed Quote (p 4): "All outcomes were analysed in the modified intention to‐treat population of patients who had received at least one injection of study drug, with missing data treated as indicating no response (non‐responder imputation)." Comment: probably done |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT02902861). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. |
| Methods | RCT, active‐controlled, Date of study: April 2007‐January 2009 Setting: 7 centres in China |
|
| Participants |
Randomised: 212 participants Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Alefacept + placebo oral methotrexate (n = 107), IM, 15 mg/week, 12 weeks Control intervention B. Methotrexate + placebo (n = 105), IM, alefacept, orally, 7.5 mg/week, 12 weeks |
|
| Outcomes | Time to evaluate assessment: not stated Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source (p 742): none reported Declarations of interest (p 742): none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 738): "Each patient was assigned a random number in a chronological order" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 738): " The present study,..., double‐blind, double dummy... Patients in the experimental group were intramuscularly injected with... and orally administered with the bank dummy methotrexate... and the patients in the control group..." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p 738): "The present study,..., double‐blind, double dummy... Patients in the experimental group were intramuscularly injected with... and orally administered with the bank dummy methotrexate... and the patients in the control group..." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote (p 739): "Full set analysis was assessed" Comment: no description of the methods used for dealing with missing data |
| Selective reporting (reporting bias) | High risk | Comment: no protocol available. No primary outcome clearly identified |
| Methods | RCT, placebo‐controlled, double blind Date of study: February 2009‐February 2010 Location: 9 centres in China |
|
| Participants |
Randomised: 129 participants (mean age 39 years (infliximab) and 40 years (placebo), 95 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Infliximab (n = 84), IV, 5 mg/kg, weeks 0, 2, 6, 14, 22; 22 weeks Control intervention B. Placebo (n = 45), IV, weeks 0, 2, 6 then infliximab 5 mg/kg weeks 10, 12, 16 |
|
| Outcomes | Assessments at 10 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p1846): "This randomised, double‐blind, placebo controlled trial... Eligible patients were randomly assigned in a 1:2 ratio to the placebo and infliximab" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p1846): "This randomised, double‐blind, placebo controlled trial... Eligible patients were randomly assigned in a 1:2 ratio to the placebo and infliximab" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p1846): "This randomised, double‐blind, placebo controlled trial... Eligible patients were randomly assigned in a 1:2 ratio to the placebo and infliximab... Infliximab 5 mg/kg or placebo was administered by intravenous drip infusion over a period of at least 2 hours on the starting day of treatment (week 0) and at weeks 2 and 6 (induction)". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (p1846): "This randomised, double‐blind, placebo controlled trial... Eligible patients were randomly assigned in a 1:2 ratio to the placebo and infliximab... Infliximab 5 mg/kg or placebo was administered by intravenous drip infusion over a period of at least 2 hours on the starting day of treatment (week 0) and at weeks 2 and 6 (induction)". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 129, 129 Analysed Quote: "In the primary efficacy analysis, data from all randomised subjects were analysed according to their assigned treatment group..." Comment: no description of the method used to manage the missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. The pre‐specified outcomes mentioned in the methods section appeared to have been reported |
| Methods | RCT, placebo‐controlled, open‐label trial Date of study: unreported Location: Turkey |
|
| Participants |
Randomised: 50 participants (no description of the study population) Inclusion/exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Acitretin (n = 50), orally, 0.5‐0.7 mg/kg, daily Control intervention B. Placebo (n = 50) Co‐intervention PUVA, twice weekly, 8‐MOP at a dosage of 0.4‐0.6 g/kg, 2h before UVA exposure |
|
| Outcomes | Time of assessments not stated Primary or secondary outcomes of the trial
Outcomes of the trial
|
|
| Notes | Funding source: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (abstract): "The patients were equally allocated to treatment groups in the study" Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (abstract): "The patients were equally allocated to treatment groups in the study" Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (abstract): "We performed an open, controlled study..." Comment: not blinded, subjective outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (abstract): "We performed an open, controlled study..." Comment: not blinded, subjective outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomly assigned 50 Comment: no description of the number of participants analysed, no description of the method used to manage missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: only an abstract available |
| Methods | RCT, placebo‐controlled, double blind (LOTUS) Date of study: 23 October 2009‐07 July 2011 Location: 14 centres in China |
|
| Participants |
Randomised: 322 participants (mean age 40 years, 248 male) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
|
| Interventions |
Intervention A. Ustekinumab (n = 160), SC, 45 mg, week 0, week 4, 4weeks Control intervention B. Placebo (n = 162), SC, week 0, week 4, 4weeks |
|
| Outcomes | Assessments at 12 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding source quote (p 173): "This study was supported by Janssen Research & Development" Declarations of interest (p 173): "Drs Zhu, Zang and Wand served as investigators for this Janssen RD‐sponsored study..." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (p 167): "The LOTUS study is a phase 3, multicenter, randomized, double blind, placebo‐controlled... Comment: no description of the method used to guarantee random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote (p 167): Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (p 167): "The LOTUS study is a phase 3, multicenter, randomized, double blind, placebo‐controlled... Comment: placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (p 167): "The LOTUS study is a phase 3, multicenter, randomized, double blind, placebo‐controlled... Comment: no description of the method used to guarantee blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly assigned 322, analyzed 322 Quote (p 167): "For efficacy analyses, all randomized patients were included... Patients who discontinued study treatment... were considered treatment failures" Comment: ITT analyses |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for the study was available on ClinicalTrials.gov (NCT01008995). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported |
AEs: adverse events; ACR: American College of Rheumatology; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BSA: Body Surface Area; eow: every other week; CIN: cervical intraepithelial neoplasia; DLQI: Dermatology Life Quality Index; ECG: electrocardiogram; HD: high dose; IGA: Investigator’s Global Assessment; IM: intramuscular; ITT: intention‐to‐treat; IV: intravenous; LD: low dose; m‐ITT: modified ITT; MD: medium dose; NAPSI: Nail psoriasis severity index; NBUVB: narrow‐band UVB; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; PP: per protocol; PSI: Psoriasis Severity Index; PSSI: Psoriasis Scalp Severity Index; PUVA: psoralen plus ultraviolet A; QoL: quality of life; RCT: randomised controlled trial; SC: subcutaneous; SF36: 36‐item Short Form Health Survey; SIAQ: Self‐ Injection Assessment Questionnaire; TB: tuberculosis; TBR: target background ratio; UVB: ultraviolet B; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abufarag 2010 | Other treatment |
| Akhyani 2010 | Other treatment |
| Altmeyer 1994 | Not plaque‐type psoriasis |
| Angsten 2007 | Not a trial |
| Anonymous 2005 | Not a trial |
| Anonymous 2008 | Not a trial |
| Arifov 1998 | Not a randomized trial |
| Armati 1972 | Other treatment |
| Avgerinou 2011 | Not randomized trial |
| Bagot 1994 | Other treatment |
| Bartlett 2008 | Not a trial |
| Barzegari 2004 | Other treatment |
| Batchelor 2009 | Not a trial |
| Bayerl 1992 | Other treatment |
| Beissert 2009 | Other treatment |
| Berbis 1989 | Assessment < 8 weeks |
| Bhuiyan 2010 | Other treatment |
| Bigby 2004 | Not a trial |
| Bissonnette 2006 | Other treatment |
| Bissonnette 2010 | Other treatment |
| Bjerke 1989 | Other treatment |
| Callis Duffin 2017 | Comparison of the same drug with the same dosages |
| Cassano 2006 | Identical dosing regimens |
| Cassano 2010 | Not a trial |
| Cather 2006 | Dose ranging after remission |
| Chládek 2002 | Basic science (aim of study: to understand the physiopathology of the disease) |
| Chodorowska 1999a | Not a trial |
| Chodorowska 1999b | Not a trial |
| de Jong 2003 | Other treatment |
| Dubiel 1972 | Not a trial |
| Duffin 2016 | Comparison of 2 different ways of drug injection for the same drug and the same dosage |
| Ecker‐Schlipf 2009 | Other treatment |
| Elewski 2007 | Pooled trials |
| Ellis 1986 | Assessement < 8 weeks |
| Ellis 2002 | Medico‐economic study |
| Ellis 2012 | Other treatment |
| Engst 1989 | Assessment < 8 weeks |
| Erkko 1997 | Basic science (aim of study: to understand the physiopathology of the disease) |
| Ezquerra 2007 | Other treatment |
| Fernandes 2013 | Not a trial |
| Finzi 1993 | Other treatment |
| Fleischer 2005 | Other treatment |
| Fredriksson 1971 | Other treatment |
| Fredriksson 1978 | Other treatment |
| Friedrich 2001 | Other treatment |
| Gambichler 2011 | Other treatment |
| Ganguly 2004 | Pooled trials |
| Gil 2003 | Not a randomized trial |
| Goerz 1978 | Not a trial |
| Gollnick 1988 | Other treatment |
| Gollnick 1993 | Other treatment |
| Gollnick 2002 | Other treatment |
| Gottlieb 2002c | Other treatment |
| Gottlieb 2003a | Other Treatment |
| Gottlieb 2004b | Pooled trials |
| Gottlieb 2005a | Other treatment |
| Gottlieb 2010 | Cross‐over trial |
| Goupille 1995 | Not a randomized trial |
| Griffiths 1998 | Other treatment |
| Griffiths 2002a | Pooled trials |
| Griffiths 2002b | Pooled trials |
| Griffiths 2005 | Pooled trials |
| Grim 2000 | Basic science (aim of study: to understand the physiopathology of the disease) |
| Grossman 1994 | Other treatment |
| Gulliver 1996 | Not a trial |
| Gupta 2005 | Other treatment |
| Gupta 2007 | Other treatment |
| Gupta 2008 | Other treatment |
| Han 2013 | Other treatment |
| Hashizume 2007 | Comparison of 2 methods of administration |
| Heule 1988 | Assessment < 8 weeks |
| Ho 2010 | Other treatment |
| Hunter 1972 | Other treatment |
| Iest 1989 | Not randomized trial |
| Kavanaugh 2009 | Not a randomized trial |
| Kimball 2008 | Drug withdrawn for safety reasons |
| Kimball 2011 | Drug withdrawn for safety reasons |
| Koo 1998 | Other treatment |
| Kopp 2015 | Phase 1 trial |
| Kragballe 1989 | Other treatment |
| Krishnan 2005 | Pooled trials |
| Krueger 1980 | Other treatment |
| Krueger 2002b | Not a trial |
| Krueger 2003 | Not a trial |
| Krueger 2012 | Phase 1 trial |
| Krueger 2015 | Phase 1 trial |
| Kuijpers 1998 | Other treatment |
| Lajevardi 2015 | Other treatment |
| Langewouters 2005 | Other treatment |
| Langley 2006 | Other treatment |
| Langley 2010 | Other treatment |
| Langner 2004 | Not plaque‐type psoriasis |
| Lauharanta 1989 | Other treatment |
| Lawrence 1983 | Other treatment |
| Leavell 1970 | Other treatment |
| Lebwohl 2003a | Pooled trials |
| Lebwohl 2009 | Pooled trials |
| Lebwohl 2012 | Other treatment |
| Lebwohl 2013 | Other treatment |
| Ledo 1988 | Other treatment |
| Legat 2005 | Other treatment |
| Leonardi 2010a | Pooled trials |
| Leonardi 2010b | Not a randomized trial |
| Leonardi 2010c | Pooled trials |
| Leonardi 2011a | Not plaque‐type psoriasis |
| Levell 1995 | Other treatment |
| Liang 1995 | Assessment < 8 weeks |
| Lui 2011 | Other treatment |
| Lui 2012 | Other treatment |
| Lynde 2012 | Other treatment |
| Macdonald 1972 | Not a randomized trial |
| Mahrle 1995 | Other treatment |
| Malik 2010 | Other treatment |
| Marecki 2004 | Other treatment |
| Marks 1986 | Not a randomized trial |
| McInnes 2013 | Pooled trials |
| Mease 2011 | Drug withdrawn for safety reason |
| Meffert 1989 | Other treatment |
| Menon 2012 | Basic science (aim of study: to understand the physiopathology of the disease) |
| Menter 2007a | Pooled trials |
| Menter 2014 | Drug withdrawn for safety reasons |
| Meyer 2011 | Other treatment |
| Mittal 2009 | Other treatment |
| Moller 2009 | Other treatment |
| Monk 1986 | Not a randomized trial |
| Montgomery 1993 | Other treatment |
| Mrowietz 1991 | The two study arms compared the same molecule with the same dosage |
| Mrowietz 2012 | Pooled trials |
| Narang 2012 | Other treatment |
| Nieboer 1990 | Other treatment |
| Nijsten 2008 | Not a trial |
| Noda 2011 | Not a randomized trial |
| Novotny 1973 | Not a trial |
| Nyfors 1978 | Not a trial |
| Orfanos 1978 | Other treatment |
| Orfanos 1979 | Other treatment |
| Ortonne 2008 | Comparison of 2 schemes of administration |
| Ortonne 2011 | Other treatment |
| Osamu 2014 | Phase 1 trial |
| Papp 2001 | Other treatment |
| Papp 2006 | Other treatment |
| Papp 2008a | Other treatment |
| Papp 2009 | Pooled data |
| Papp 2011a | Pooled trials |
| Papp 2011b | Drug withdrawn for safety reasons |
| Papp 2011c | Drug withdrawn for safety reasons |
| Papp 2012c | Phase 1 trial |
| Papp 2012d | Pooled trials |
| Park 2013 | Other treatment |
| Paul 2012 | Other treatment |
| Paul 2014 | Other treatment |
| Pettit 1979 | Assessment < 8 weeks |
| Petzelbauer 1990 | Not a randomized trial |
| Piascik 2003 | Not a trial |
| Ports 2013 | Other treatment |
| Punwani 2012 | Other treatment |
| Rabasseda 2012 | Not a trial |
| Radmanesh 2011 | Comparison of 2 schemes of administration |
| Raman 1998 | Other treatment |
| Reich 2011 | Pooled trials |
| Reich 2014 | Other treatment |
| Reitamo 1999 | Other treatment |
| Reitamo 2001 | Other treatment |
| Rim 2003 | Other treatment |
| Rinsho Iyaku 1991 | Other treatment |
| Ritchlin 2006a | Not a randomized trial |
| Ritchlin 2006b | Not a randomized trial |
| Ritchlin 2006c | Not a randomized trial |
| Salim 2006 | Other treatment |
| Scholl 1981 | Other treatment |
| Schopf 1998 | Other treatment |
| Schulze 1991 | Other treatment |
| Shintani 2011 | Comparison of 2 schemes of administration |
| Shiohara 1992 | Not a trial |
| Shupack 1997 | Not a trial |
| Simonova 2005 | Other treatment |
| Sofen 2011 | Basic science (aim of study: to understand the physiopathology of the disease) |
| Sofen 2014 | Phase 1 trial |
| Spadaro 2008 | Not a trial |
| Spuls 2012 | Not a trial |
| Sticherling 1994 | Not a trial |
| Strober 2004 | Not a trial |
| Strober 2012 | Not a randomized trial |
| Sweetser 2006 | Cross over trial |
| Talwar 1992 | Not a randomized trial |
| Tejasvi 2012 | Other treatment |
| Thaçi 2002 | The two study arms compared the same molecule with the same dosage |
| Thaçi 2010 | Other treatment |
| Tong 2008 | Other treatment |
| Van Joost 1988 | Assessment shorter than 8 weeks |
| Vena 2005 | Comparison of 2 schemes of administration |
| Vena 2012 | Other treatment |
| Viglioglia 1978 | Not a trial |
| Witkamp 1995 | Other treatment |
| Wolf 2012 | Other treatment |
| Wright 1966 | Not a randomized trial |
| Wu 2015 | Other treatment |
| Yesudian 2013 | Other treatment |
| Yoon 2007 | Dose‐escalation study |
| Zachariae 2008 | Other treatment |
| Zhang 2007 | Other treatment |
| Zhang 2009a | Other treatment |
| Zhang 2009b | Other treatment |
| Zhang 2012 | Other treatment |
| Zhu 2009 | Pooled trials |
| Zhuang 2016 | Phase 1 trial |
| Zobel 1987 | Not a trial |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT, active/placebo‐controlled, double blind Date of study: not stated Location: Canada, Germany and Poland |
| Participants |
Randomised: 455 participants (mean age 43, 313 male) Inclusion criteria
Exclusion criteria
|
| Interventions |
Interventions (n = 355) Drug: voclosporin 0.8 mg/kg/day Drug: ciclosporin 3.0 mg/kg/day Control intervention (n = 89) Drug: placebo |
| Outcomes |
At week 24, Primary outcome measures
Secondary outcome measures
|
| Notes | Randomised, placebo and ciclosporin controlled study of ISA247 in plaque psoriasis patients (ESSENCE), NCT00408187 Participants in the voclosporin and ciclosporin arms (n = 355) were treated for 24 weeks; these participants were combined into a ‘24‐week treatment group'. In the placebo group, 89 participants were included. As the authors presented their results grouping ciclosporin and voclosporin together, we asked them to provide the results for the subgroup of participants with ciclosporin treatment arm. Two emails were sent without response (8 November 2016, 16 December 2016) |
| Methods | Randomised, parallel group, multiple arm trial Date of study: 10/12/2013 (starting date) Location: India |
| Participants |
Total sample size: 120 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention 1: acitretin: orally, 25‐50 mg/day, daily single dose Total duration: 90 days Intervention 2: ciclosporin: orally 2.5‐5 mg/kg/day, daily in 2 divided doses Total duration: 90 days Intervention 3: methotrexate: orally 7.5‐15 mg/week in 3 divided doses Total duration: 90 days Control Intervention 1: palmoplantar psoriasis: variant of psoriasis in which only palms and soles are affected Control Intervention 2: psoriasis vulgaris: variant of psoriasis in which lesions appear on body skin |
| Outcomes |
At 90 days
|
| Notes | Starting date: 10‐12‐2013. Recruitment status: open to recruitment We sent an email to Prof. Shah (5 and 12 January 2017) without response |
| Methods | Randomised, active‐controlled, parallel‐group, simple blind Date of study: 03/06/2008 (starting date) Location: Germany |
| Participants |
Total sample size: 50 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1: adalimumab (Humira), SC, 80 mg initially and 40 mg eow for 24 weeks Intervention 2: etanercept (Enbrel), SC, 50 mg twice a week for 12 weeks and 25 mg twice a week subsequently for another 12 weeks. Intervention 3: FAEs (Fumaderm), orally, up to 6 doses/day for 24 weeks |
| Outcomes |
Week 8:
Week 24:
|
| Notes | Starting date: 03/06/2008, Prof. Arnd Jacobi, Klinik für Dermatologie und Allergologie Philipps‐Universität Marburg Recruitment status on ICTRP search portal: complete: follow‐up complete We emailed Prof. Jacobi (5 January 2017) without response |
| Methods | Randomised, placebo‐controlled, double‐blind trial date: January 2014 to April 2016 Location: worldwide |
| Participants |
Total sample size: 217 Inclusion criteria
Exclusion Criteria
|
| Interventions |
Intervention Adalimumab, SC, 40 mg, eow for 25 weeks starting 1 week after initial loading dose of 80 mg Control intervention Placebo |
| Outcomes |
At week 12 mNAPSI 75, PGA of fingernails of clear or minimal PASI 75/90/100 for participants with baseline PASI at 5 |
| Notes |
NCT02016482
Abstract
No original paper published. We emailed the study authors (3 and 12 January 2017) for the protocol and results. Abbvie response: "As this data has not been published in a manuscript at this time I am providing the link below for you to submit a request for this data. Please let me know if you have any further questions". Will be included when published |
| Methods | Randomised, double‐blind, active‐controlled trial Date: not stated Location: China |
| Participants | No statement except a total number of participants (n = 144) |
| Interventions |
Intervention Recombinant human tumour necrosis factor receptor (50 mg/week) Control intervention Methotrexate (7.5 mg/week) |
| Outcomes |
At 12 weeks Proportion of PASI 50, PASI 75, PASI 90 |
| Notes |
Abstract in Journal of Clinical Dermatology 2007 (730‐2) HAN Ling, FANG Xu, HUANG Qiong, YANG Qin‐ping, FU Wen‐wen, ZHENG Zhi‐zhong, GU Jun, SUN Jiao‐fang, XU Ai‐e (Department of Dermatology,Huashan Hospital, Fudan University, Shanghai 200040, China) Objective: To evaluate the effect of recombinant human tumour necrosis factor receptor (rhTNFR:Fc) in the treatment of moderate to severe plaque psoriasis on psoriasis area and severity index (PASI). Methods: Using randomised, double‐blind and double‐simulated, parallel‐controlled with positive drug, multicenter, clinical trial was employed to investigate 144 cases of patients with moderate to severe plaque psoriasis, of which there were 72 cases in both trial group and the control group respectively, to evaluate the effect on PASI. Results: 124 cases of patients had accomplished the 12‐week clinical trial. After 12 weeks the rate of PASI50, PASI75, PASI90 were significantly higher than those of the control group (P < 0.01). The therapeutic effects on trunk and limbs of the trial group were also much better. Conclusion: The effect of rhTNFR:Fc is more quick and significant, especially assessed by PASI sore. Abstract not available at the BIUM and United States NLM libraries. No email address for the authors available When we searched Google, we found another abstract of the same study. "Chinese Journal of Dermatology 2007, 40(11) 655‐658" http://manu41.magtech.com.cn/Jwk_cmazp/EN/abstract/abstract11844.shtml#), which had no supplemental information to enable contacting the authors: Abstract "Objective To investigate the efficacy and tolerability of a recombinant human tumour necrosis factor:Fc fusion protein (rhTNFR:Fc,with a trade name of Yisaipu) in the treatment of moderate to severe psoriasis vulgaris. Methods A multicentre,randomised,double blind,and parallel‐controlled trial was performed. One hundred and forty‐four patients with moderate to severe psoriasis vulgaris from four centres were randomly assigned and treated with either once‐weekly subcutaneous injection of rhTNFR:Fc (50 mg) or oral methotrexate (methotrexate)(7.5 mg) for 12 weeks.Patients were followed up at 2,4,8,12 weeks after the treatment. Results One hundred and twenty‐four patients finished the 12‐week course of treatment. At 12 weeks after the treatment,a 50%, 75%, 90% improvement in psoriasis area and severity index (PASI) was achieved by 86.11%, 76.39%, 52.78% respectively of rhTNFR:Fc‐treated patients,and by 63.89%, 44.44%, 22.22% respectively in methotrexate‐treated patients,and all the three improvement rates were of significant difference between the two groups of patients (all P<0.01).Physician global assessment (PGA), dermatology life quality index (DLQI) and 10‐cm visual analogue scale (VAS) all reduced more significantly, and more patients were cured or approximately cured in rhTNFR:Fc‐treated patients than in MTX‐treated patients (all P<0.05).Adverse reactions,mainly including decrease of leucocytes or neutrophils,infection, dysfunction of liver, edema and pruritus at the injection site,etc,occurred in 26.39% of rhTNFR:Fc‐treated patients and 29.17% of MTX‐treated patients (P>0.05). Conclusion Compared with MTX,rhTNFR:Fc acts more quickly with a higher cure rate and less toxic reactions in the treatment of psoriasis vulgaris." No contact with the authors as Authors' emails were not found |
| Methods | Randomised, double‐blind, active‐controlled trial Date: April 2015‐August 2016 Location: USA |
| Participants |
Total sample size: 12 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: Ixekizumab once every 2 weeks, SC, 160 mg 2 injections at week 0 followed by 80 mg ixekizumab given as a single SC injection once every 2 weeks through week 12. After week 12 participants will receive 80 mg ixekizumab every 4 weeks through week 44 Control intervention: Ixekizumab once every 4 weeks, SC, 160 mg, 2 injections at week 0 followed by 80 mg ixekizumab given as a single SC injection once every 4 weeks through week 44 |
| Outcomes |
At week 12, Primary outcome
Secondary outcomes
|
| Notes |
NCT02387801 Study start date: April 2015 Study completion date: August 2016 Published abstract entitled “Early onset of clinical improvement with ixekizumab in patients with moderate‐severe plaque psoriasis” published in 2016 in Journal of the European Academy of Dermatology and Venereology, Vol 30 No original paper published. We emailed the study authors (3 and 12 January 2017) for the protocol and results but have not had a response. |
| Methods | RCT, placebo‐controlled, open‐label trial Date of study: July 2009‐April 2011 Setting: Korea |
| Participants |
Total sample size: 60 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Etanercept + acitretin (combination of etanercept, 25 mg twice a week and acitretin 10 mg twice a day for 24 weeks) Control intervention Etanercept, 50 mg twice a week for 12 weeks followed by 25 mg twice a week for 12 weeks Acitretin, 10 mg twice a day for 24 weeks |
| Outcomes |
At week 24 Primary outcome
Secondary outcomes
|
| Notes |
NCT00936065 (Study evaluating the efficacy and safety of etanercept and acitretin in Korean patient with moderate to severe psoriasis) Study start date: July 2009 Study completion date: April 2011 Abstracts:
We emailed the study authors (3 and 12 January 2017) for the protocol and results as additional information request for risk of bias assessment but have not had a response. |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: not stated Setting: not stated |
| Participants |
Randomised: 175 participants (characteristics not stated) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
| Interventions |
Intervention A. Dimethyl fumarate (n = 105), orally, 240 mg, 3 times/day; 16 weeks Control Intervention B. Placebo (n = 70), orally, 2 capsules , 3 times/day; 16 weeks |
| Outcomes | Assessments at 16 weeks Primary outcomes of the trial
Secondary outcomes of the trial
|
| Notes | Funding, quote (abstract) by Biogen Idec, Inc and Fumapharm Abstracts: “Results of a phase III study of a novel oral formulation of dimethyl fumarate in the treatment of moderate to severe plaque psoriasis: efficacy, safety, and quality of life effects” published in 2005 in the JEADV, Suppl. 2 (Poster P/06.97). We asked the study authors to provide the protocol and results by email. Additional data to the publication not provided. Finally, as the 'Risk of bias' tool assessment was not possible and there were missing data for the results, Mrowietz 2005 was included in "awaiting classification". |
| Methods | RCT, active‐controlled, double‐blind trial (SIGNATURE) Date of study: October 2013‐July 2016 Location: UK |
| Participants |
Randomised: 230 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Biological: secukinumab 150 mg at day 0 (initiation of study drug) and at weeks 1, 2, 3 & 4 Control Intervention Biological: secukinumab 300 mg at day 0 (initiation of study drug) and at weeks 1, 2, 3 & 4 |
| Outcomes |
At 16 weeks Primary outcome
Secondary outcomes
|
| Notes | On www.clinicaltrials.gov Study completion date: July 2016 Ongoing study |
| Methods | RCT, placebo‐controlled, double‐blind trial, phase 2 Date of study: July 2013‐December 2015 Location: Japan |
| Participants |
Randomised: 254 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: A. Apremilast (30 mg tablet twice a day for 68 weeks) Control intervention: B. Apremilast (20 mg tablet twice a day for 68 weeks) C. Placebo |
| Outcomes |
At week 16 Primary outcome:
Secondary outcomes:
|
| Notes | Study completed, but not yet published Enrollment: 254 Study start date: July 2013 Study completion date: December 2015 Sent e‐mail to Dr Nogarales, MD Celgene Corporation (12 January 2017) Will be included when published as the article has just been submitted |
| Methods | RCT, placebo‐controlled, double‐blind trial, phase 2 Date of study: February 2014‐July 2015 Location: world‐wide |
| Participants |
Randomised: 166 participants Inclusion criteria
Exclusion criteria
With regards to TB the following applies:
|
| Interventions |
Intervention A. Drug: BI 655066 (low dose) (18 mg BI 655066 administered by SC injection plus 2 placebo‐matching BI 655066 injections at week 0, followed by 2 placebo‐matching BI 655066 injections each at weeks 4 and 16.) Control intervention B. Drug: BI 655066 (median dose) (90 mg BI 655066 administered by SC injection plus 2 placebo‐matching BI 655066 injections at week 0, followed 90 mg BI 655066 plus 1 placebo‐matching BI 655066 injection at weeks 4 and 16.) C. Drug: BI 655066 (high dose) (180 mg BI 655066 administered by SC injection as 2 injections plus a placebo‐matching BI 655066 injection at week 0, followed 180 mg BI 655066 administered as 2 injections at 2eeks 4 and 16.) D. Drug: ustekinumab (Stelara administered by SC injection plus 2 saline injections at week 0, Stelara injection plus 1 saline injection at weeks 4 and 16. Stelara dose was 45 mg for participants with body weight ≤ 100 kg at randomisation or 90 mg for participants with body weight > 100 kg at randomisation.) |
| Outcomes |
At week 12 Primary outcome
Secondary outcomes
|
| Notes | Study completed July 2015 and the results are available on ClinicalTrials.gov BI 655066 is a new anti‐IL23, not included in the initial search. It will be in the Cochrane Review update, so the trial will be included too |
| Methods | RCT, active‐controlled, double‐blind trial, phase 3 Date of study: November 2013‐January 2015 Location: India |
| Participants |
Randomised: 50 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Methotrexate 10 mg/week Control intervention Methotrexate 25 mg/week |
| Outcomes |
At week 12 Primary outcome
Secondary outcomes
|
| Notes | On ClinicalTrials.gov, Estimated Enrollment: 50 Study start date: November 2013 Estimated primary completion date: January 2015 (final data collection date for primary outcome measure) Emails sent to Prof. Krishna (5 and 12 January 2017) |
| Methods | RCT, placebo‐controlled, double blind Date of study: not stated Setting: multicenter (locations not stated) |
| Participants |
Randomised: 195 participants (no description of the study population) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals
|
| Interventions |
Intervention A. Alefacept (n = 130), IM, 15 mg, once a week, 12 weeks Control Intervention B. Placebo (n = 65), IM, once a week, 12 weeks |
| Outcomes | Assessments at 14 weeks Primary or secondary outcomes of the trial
Outcomes of the trial
|
| Notes | Funding: not stated Abstracts: “Alefacept in the treatment of psoriasis for whom conventional therapies are ineffective or inappropriate” published in 2004 in the Journal of the European Academy of Dermatology and Venereology, Poster P105 We asked the study authors to provide the protocol and results by email. The had no acces to the data ("I do not have access to the data you require") Finally, as the 'Risk of bias' tool assessment was not possible and there were missing data for the results, Reich 2004 was included in "awaiting classification". |
| Methods | RCT, active‐controlled, double‐blind trial, phase 3 Date of study: November 2013‐January 2015 Location: India |
| Participants |
Randomised: 198 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Biological: secukinumab 150 mg weekly for 5 weeks, then once every 4 weeks up to and including Week 128 Control Intervention Biological: secukinumab 300 mg weekly for 5 weeks, then once every 4 weeks up to and including Week 128 Biological: Placebo |
| Outcomes |
At week 16 Primary outcome
Secondary outcomes
|
| Notes |
NCT01807520 Enrollment: 198 Study start date: June 2013 Estimated study completion date: January 2017 Abstracts
We asked the study authors to provide the protocol and results by email (3 January 2017) As there is no original publication available, the authors and the firm could not send us such details. Will be included when published. |
AEs: adverse effects; BMI: body mass index; BSA: body surface area;DLQI: Dermatology Life Quality Index; ECG: electrocardiogram; eow: every other week; FAEs: fumaric acid esters; IGA: Investigator's Global Assessment; IM: intramuscular; IV: intravenous; NAPSI: Nail Psoriasis Severity Index; PASI: Psoriasis Area and Severity Index; PGA: Physician's Global Assessment; PUVA: psoralen plus ultraviolet A; RCT: randomised controlled trial; SC: subcutaneous; SF36: short‐form 36; SPGA: static physician global assessment; TB: tuberculosis; UVB: ultraviolet B; VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Acitretin plus methotrexate in the treatment of moderate to severe psoriasis vulgaris |
| Methods | Phase 4 RCT, active/placebo‐controlled, double‐blind trial Date of study: January 2016‐December 2016 Location: China |
| Participants |
Randomised: 350 participants Inclusion criteria
Main exclusion criteria
|
| Interventions |
Intervention group A. Acitretin plus methotrexate group (n = 100) Control intervention group B. Acitretin Capsules (n = 100), 1 pill, twice a day C. Methotrexate (n = 100), 7.5 mg/week, and then 25 mg/week D. Blank group (n = 50), none |
| Outcomes |
Time point outcome measured: not stated Primary outcome
Secondary outcome
|
| Starting date | January 2016 |
| Contact information | Prof. Xia Yumin; xiayumin1202@163.com |
| Notes | Ongoing study |
| Trial name or title | A randomised, double‐blind, placebo‐controlled, comparative, prospective, multicentre trial to assess efficacy and safety of apremilast tablets in subjects with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy |
| Methods | Phase 3 RCT, placebo‐controlled, double‐blind trial Date of study: October 2016 ‐ Location: India |
| Participants |
Randomised: 231 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Apremilast 30 mg tablets: administered 1 tablet twice daily for 16 weeks Control intervention Placebo tablets: administered 1 tablet twice daily for 16 weeks |
| Outcomes |
At week 16 Primary outcome
Secondary outcomes
|
| Starting date | 20 October 016 |
| Contact information | Dr Piyush Agarwal, DrPiyush.Agarwal@glenmarkpharma.com |
| Notes | Ongoing study |
| Trial name or title | Optimising adalimumab treatment in psoriasis with concomitant methotrexate ‐ OPTIMAP |
| Methods | Phase 4 RCT, placebo‐controlled, open‐label trial Date of study: February 2014 ‐ Location: the Netherlands |
| Participants |
Randomised: number of participants not stated Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Adalimumab with methotrexate Control intervention Adalimumab monotherapy Dosage and frequency of adalimumab and methotrexate: not stated |
| Outcomes |
Primary end point(s)
Timepoint(s) of evaluation of this end point: week 49 Secondary end point(s)
Timepoint(s) of evaluation of this end point: week 13, 25, 37 and 49 |
| Starting date | 12 December 2013 |
| Contact information | Pr Phyllis Spuls Department of Dermatology Academic Medical Center Meibergdreef 9 1105AZ Amsterdam Netherlands |
| Notes | Recruitment status (ICTRP search portal): authorised‐recruitment may be ongoing or finished Target sample: not specified We emailed Prof. Phyllis Spuls (5 January 2017) Email response "The study is currently ongoing and has not yet been analysed. Therefore, we are not able to provide data on efficacy or safety. We can provide you with the study protocol. Will this be helpful? Kind regards, Phyllis Spuls and Celine Busard " Will be included when published |
| Trial name or title | A randomised, multicenter Study to evaluate the Effect of secukinumab 300 mg s.c. administered during 52 weeks to patients suffering from new‐onset moderate to severe plaque Psoriasis as early Intervention compared to standard treatment with narrow band UVB (STEPin study) ‐ STEPin |
| Methods | RCT, placebo‐controlled, open‐label trial Date of study: November 2016 ‐ Location: Europe Phase 4 |
| Participants |
Randomised: 196 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Secukinumab 300 mg SC. versus narrowband‐UVB Dosage and frequency not stated |
| Outcomes |
At 52 weeks, Primary outcome
Secondary outcomes
|
| Starting date | 8 November 2016 |
| Contact information | Novartis medical information service, novartis.laakeinformaatio@novartis.com |
| Notes | Ongoing study |
| Trial name or title | BI 655066 (risankizumab) versus adalimumab in a randomised, double blind, parallel group trial in moderate to severe plaque psoriasis to assess safety and efficacy after 16 weeks of treatment and after inadequate adalimumab treatment response (IMMvent) ‐ BI 655066 (risankizumab) versus adalimumab |
| Methods | RCT, active/placebo‐controlled, double‐blind trial Date of study: February 2016 ‐ Location: worldwide Phase 3 |
| Participants |
Randomised: 600 participants planned Inclusion criteria
Exclusion criteria Patients with
|
| Interventions |
Intervention Product Name: BI 655066 Product Code: BI 655066 90 mg/mL Pharmaceutical Form: solution for injection in pre‐filled syringe INN or proposed INN: risankizumab Control intervention Humira® (adalimumab) solution for Injection 40 mg/0.8 mL in a single‐use, pre‐filled syringe |
| Outcomes |
At week 16 Primary outcome
Secondary outcomes
|
| Starting date | 17 May 17 2016 |
| Contact information | Boehringer Ingelheim Pharma GmbH & Co. KG |
| Notes | Ongoing study BI 655066 (a new anti‐IL23) will be included |
| Trial name or title | Vascular inflammation in psoriasis trial (The VIP Trial) (VIP) |
| Methods | RCT, active/placebo‐controlled, double blind trial Date of study: February 2012 ‐ Location: USA Phase 4 |
| Participants |
Randomised: 96 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Adalimumab (Humira). Humira will be given at an initial dose of 80 mg followed by 40 mg the 2nd week, subsequent doses will be given at 40 mg and follow FDA dosing schedule Control intervention B. NB‐UVB phototherapy. Phototherapy will be given 3 times per week according to the Fitzpatrick scale for skin types C. Placebo injection will be given according to the same dose and schedule as the active comparator |
| Outcomes |
At weeks 4 and 12 Primary outcome measures
Secondary outcome measures:
|
| Starting date | 14 February 2012 |
| Contact information | Joel Gelfand, MD MSCE (Principal investigator) |
| Notes | On ClinicalTrials.gov, Estimated Primary Completion Date: July 2017 Ongoing study |
| Trial name or title | A study to evaluate the effectiveness of Stelara™ (ustekinumab) in the treatment of scalp psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: March 2012 ‐ Location: USA Phase 4 |
| Participants |
Randomised: 30 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Ustekinumab (at weeks 0, 4, 16, 28, and week 40 and placebo at weeks 12 and 52. The participants when assigned to ustekinumab, depending on body weight, will receive either 45 mg or 9 mg ustekinumab doses) Control intervention Placebo |
| Outcomes |
At week 12, Primary outcome
Secondary outcomes
|
| Starting date | August 2012 |
| Contact information | Paul Steven Yamauchi, MD, PhD |
| Notes | On ClinicalTrials.gov Estimated enrolment: 30 Study start date: August 2012 Estimated study completion date: December 2013 We emailed Dr Yamauchi (5 and 12 January 2017) Email response: Dear Dr Sbidian, Thank you for your kind email, forwarded to me by Dr Paul Yamauchi, MD,PhD. Our " Study to Evaluate the Effectiveness of STELARA ™ (USTEKINUMAB) in the Treatment of Scalp Psoriasis (NCT 01558310)” completed enrolment in December 2016 and the last subject will complete in December 2017, as such we do not have the final data analysis. What is you absolute cut‐ off for publication data ? Would an interim analysis report be acceptable ? Best regards, Rickie Patnaik Director, Clinical Science Institute Will be included when published |
| Trial name or title | A study to evaluate the efficacy and safety of subcutaneous MK‐3222, followed by an optional long‐term safety extension study, in participants with moderate‐severe chronic plaque psoriasis (MK‐3222‐010) |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: December 2012 ‐ Location: not stated Phase 3 |
| Participants |
Randomised: 772 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Drug: MK‐3222 200 mg (SC, 200 mg at week 0 and week 4, and then every 12 weeks until study end or participant discontinuation.) Control intervention Drug: MK‐3222 100 mg (SC at a dose of 100 mg at week 0 and week 4, and then every 12 weeks until study end or participant discontinuation.) Drug: matching placebo (SC at week 0 and week 4) |
| Outcomes |
At week 12 Primary outcome (composite outcome)
Secondary outcomes
|
| Starting date | December 2012 |
| Contact information | Medical Director: Merck Sharp & Dohme Corp. |
| Notes | On ClinicalTrials.gov Estimated study completion date: October 2019 Still ongoing |
| Trial name or title | A study to evaluate the efficacy and safety/tolerability of subcutaneous tildrakizumab (SCH 900222/MK‐3222) in participants with moderate‐to‐severe chronic plaque psoriasis followed by a long‐term extension study (MK‐3222‐011) |
| Methods | RCT, active/placebo‐controlled, double‐blind trial Date of study: February 2013 ‐ Location: not stated Phase 3 |
| Participants |
Randomised: 1090 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Tildrakizumab 200 mg (SC on weeks 0, 4, 16, 28, 40 and 52) Control interventions Tildrakizumab 100 mg (SC on weeks 0, 4, 16, 28, 40 and 52) Etanercept 50 mg (twice weekly until week 12 and once weekly from week 12 to week 28) Placebo |
| Outcomes |
At week 12 Primary outcome (composite outcome)
Secondary outcomes
|
| Starting date | February 2013 |
| Contact information | Medical director: Merck Sharp & Dohme Corp |
| Notes | On ClinicalTrials.gov Estimated Study Completion Date: June 2019 Ongoing study |
| Trial name or title | A study of guselkumab in participants with moderate to severe plaque‐type psoriasis and an inadequate response to ustekinumab (NAVIGATE) |
| Methods | RCT, active/placebo‐controlled, double‐blind trial Date of study: October 2014 ‐ Location: world‐wide Phase 3 |
| Participants |
Randomised: 872 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Ustekinumab (45 mg or 90 mg given by SC injection at weeks 0 and 4 for all participants, Participants with an IGA score of 0 or 1 at week 16 will also receive ustekinumab every 12 weeks from week 16 to week 40) Control intervention Guselkumab (100 mg given by SC injection at weeks 16 and 20 and every 8 weeks) Placebo |
| Outcomes |
At week 12 Primary outcome (composite outcome)
Secondary outcomes
|
| Starting date | October 2014 Final completion date: May 2016 |
| Contact information | Janssen Research & Development, LLC Clinical Trial |
| Notes | Ongoing study |
| Trial name or title | Safety and efficacy of etanercept in patients with psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: October 2014 ‐ Location: China Phase 4 |
| Participants |
Randomised: 80 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Etanercept (participants under the treatment of 50 mg Etanercept) Control intervention Placebo |
| Outcomes |
At week 24 Primary outcome
Secondary outcomes
|
| Starting date | May 2014 |
| Contact information | Yang Min, Ph.D, Chengdu PLA General Hospital |
| Notes | On ClinicalTrials.gov Estimated Primary Completion Date: December 2016 Ongoing study |
| Trial name or title | Optimizing psoriasis treatment of etanercept combined methotrexate |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: December 2014 ‐ Location: China Phase 4 |
| Participants |
Randomised: 488 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Methotrexate (dosage not stated) Control intervention Co‐intervention: etanercept (dosage not stated) |
| Outcomes |
At week 24 Primary outcome
Secondary outcomes
|
| Starting date | November 2014 |
| Contact information | Min Zheng, director of dermatology, Zhejiang University |
| Notes | On ClinicalTrials.gov, Estimated Primary Completion Date: October 2016 (Final data collection date for primary outcome measure) Still ongoing |
| Trial name or title | An efficacy and safety of CNTO 1959 (guselkumab) in participants with moderate to severe plaque‐type psoriasis |
| Methods | RCT, active/placebo‐controlled, double‐blind trial Date of study: December 2014 ‐ Location: Japan Phase 3 |
| Participants |
Randomised: 226 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention CNTO 1959 50 mg (50 mg at weeks 0, 4 and then every 8 weeks thereafter) Control interventions CTNO 1959 100 mg (100 mg at weeks 0, 4 and then every 8 weeks thereafter) Placebo |
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
| Starting date | Study start date: December 2014 Final completion date: September 2018 |
| Contact information | Janssen Pharmaceutical K.K. |
| Notes | Ongoing study |
| Trial name or title | A study to evaluate the efficacy and safety of two dose levels of certolizumab pegol (CZP) in subjects with plaque psoriasis (PSO) (CIMPASI‐2) |
| Methods | RCT, active/placebo‐controlled, double‐blind trial Date of study: December 2014 ‐ Location: World‐wide Phase 3 |
| Participants |
Randomised: 227 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Certolizumab pegol (400 mg at weeks 0, 2, 4, followed by certolizumab pegol 200 mg every 2 weeks from week 6 to week 14) Control intervention Certolizumab pegol (certolizumab pegol 400 mg every 2 weeks through week 14) Placebo |
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
| Starting date | Starting study date: 22 December 2014 Study completion date: September 2018 |
| Contact information | UCB Biopharma S.P.R.L. |
| Notes | Ongoing study |
| Trial name or title | Efficacy and safety study of certolizumab pegol (CZP) versus active comparator and placebo in subjects with plaque psoriasis (PSO) (CIMPACT) |
| Methods | RCT, active/placebo‐controlled, double‐blind trial Date of study: January 2015 ‐ Location: worldwide Phase 3 |
| Participants |
Randomised: 559 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Certolizumab pegol (SC injection 400 mg at weeks 0, 2, 4, followed by certolizumab pegol 200 mg every 2 weeks from week 6 to week 14) Control intervention Certolizumab pegol (SC injection 400 mg every 2 weeks through week 14) Etanercept (SC injection 50 mg twice weekly through week 12) Placebo |
| Outcomes |
At week 12 Primary outcome
Secondary outcomes
|
| Starting date | Start study date: 20 January 2015 Study completion date: January 2019 |
| Contact information | UCB Biopharma S.P.R.L. |
| Notes | Ongoing study |
| Trial name or title | A phase 4 study of efficacy and safety of apremilast in subjects with moderate plaque psoriasis (UNVEIL) |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: April 2015 ‐ Location: USA Phase 4 |
| Participants |
Randomised: 221 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Apremilast (30 mg tablets orally twice daily weeks 0‐52) Control intervention Drug: placebo |
| Outcomes |
At week 16, Primary composite outcome
Secondary outcomes
|
| Starting date | Study starting date: 3 April 2015 Study completion date: November 2016 |
| Contact information | Joana Goncalves, MD Celgene Corporation |
| Notes | Ongoing study |
| Trial name or title | Study of secukinumab compared to Fumaderm® in adults with moderate to severe psoriasis (PRIME) |
| Methods | RCT, active‐controlled, open‐label study Date of study: June 2015 ‐ Location: USA Phase 3 |
| Participants |
Randomised: 202 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Secukinumab (300 mg at weeks 0, 1, 2, 3, 4, 8, 12, 16 and 20) Control intervention Fumaderm® (week 0: 1 tablet of Fumaderm® INITIAL in the evening Week 1: 1 tablet Fumaderm® INITIAL, in the morning and evening Week 2: 1 tablet Fumaderm® INITIAL in the morning, at noon and in the evening until the last tablet of a 40‐tablet‐blister is consumed Week 2‐3: At the day after the last tablet of the Fumaderm® INITIAL 40‐tablet‐blister is consumed and through week 3, 1 tablet of Fumaderm® in the evening Week 4: 1 tablet Fumaderm® in the morning and evening Week 5: 1 tablet Fumaderm® in the morning, at noon and in the evening Week 6: 1 tablet of Fumaderm® in the morning and at noon, 2 tablets of Fumaderm® in the evening Week 7: 2 tablets of Fumaderm® in the morning, 1 tablet of Fumaderm® at noon, 2 tablets of Fumaderm® in the evening Weeks 8‐24: 2 tablets of Fumaderm® in the morning, at noon and in the evening) |
| Outcomes |
At week 24 Primary outcome
Secondary outcomes
|
| Starting date | Study starting date: April 2015 Study completion date: June 2016 |
| Contact information | Novartis Pharmaceuticals |
| Notes | Ongoing study |
| Trial name or title | A study comparing different dosing regimens of ixekizumab (LY2439821) in participants with moderate to severe plaque psoriasis (IXORA‐P) |
| Methods | RCT, active/placebo‐controlled, double‐blind trial Date of study: July 2015 ‐ Location: worldwide Phase 3 |
| Participants |
Randomised: 1227 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Ixekizumab (160 mg ixekizumab given as 2 SC injections at baseline and then 80 mg ixekizumab given as 1 SC injection every 2 weeks to week 52) Control interventions Ixekizumab (160 mg ixekizumab given as 2 SC injections at baseline and then 80 mg ixekizumab given as 1 SC injection every 4 weeks to week 52) Placebo |
| Outcomes |
At week 52 Primary composite outcome
Secondary outcomes
|
| Starting date | Study starting date: August 2015 Study completion date: October 2017 |
| Contact information | Call 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559 Mon ‐ Fri 9 AM ‐ 5 PM Eastern time (UTC/GMT ‐ 5 hours, EST) |
| Notes | Ongoing study |
| Trial name or title | Study of the efficacy and safety Of apremilast (CC‐10004), in subjects with moderate plaque psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: April 2015 ‐ Location: USA Phase 4 |
| Participants |
Randomised: 221 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Apremilast (30 mg tablets orally twice daily weeks 0‐52) Control intervention Placebo |
| Outcomes |
At week 16, Primary composite outcome
Secondary outcomes
|
| Starting date | Study starting date: 18 September 2015 Study completion date: November 2016 |
| Contact information | Joana Goncalves, MD Celgene Corporation |
| Notes | Ongoing study |
| Trial name or title | Evaluation of cardiovascular risk markers in psoriasis patients treated with secukinumab (CARIMA) |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: September 2015 ‐ Location: Germany Phase 4 |
| Participants |
Randomised: 151 participants Key inclusion criteria
Key exclusion criteria
|
| Interventions |
Intervention Secukinumab 300 (300 mg every week for 4 weeks followed by 300 mg secukinumab every 4 weeks until week 48) Control interventions Secukinumab 150 (150 mg every week for 4 weeks followed by 300 mg secukinumab every 4 weeks until week 48) Placebo |
| Outcomes |
At week 12, Primary outcome
Secondary outcomes
|
| Starting date | April 2014 |
| Contact information | Novartis Pharmaceuticals |
| Notes | On ClinicalTrials.gov, Primary completion date: April 2016 (final data collection date for primary outcome measure) Still ongoing |
| Trial name or title | A study of ixekizumab (LY2439821) in participants with moderate‐to‐severe plaque psoriasis (IXORA‐S) |
| Methods | RCT, active‐controlled, open‐label study Date of study: September 2015 ‐ Location: USA Phase 3 |
| Participants |
Randomised: 300 participants Inclusion criteria:
Exclusion criteria
|
| Interventions |
Intervention Ixekizumab (160 mg ixekizumab given as 2 SC injections at baseline followed by 80 mg ixekizumab given as a single SC injection once every 2 weeks from week 2 through week 12. After week 12 participants will receive 80 mg ixekizumab every 4 weeks through week 52) Control intervention Ustekinumab (45 mg ustekinumab given as SC injection for participants ≤ 100 kg and 90 mg SC injection for participants > 100 kg at weeks 0, 4, 16, 28, and 40) |
| Outcomes |
At week 12, Primary outcome
Secondary outcomes
|
| Starting date | Start study date: October 2015 Completion study date: May 2017 |
| Contact information | Call 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559 Mon ‐ Fri 9 AM ‐ 5 PM Eastern time (UTC/GMT ‐ 5 hours, EST) |
| Notes | Ongoing study |
| Trial name or title | A study of ixekizumab (LY2439821) in participants with moderate‐to‐severe plaque psoriasis naive to systemic treatment |
| Methods | RCT, active‐controlled, single‐blind study Date of study: December 2015 ‐ Location: Germany Phase 3 |
| Participants |
Randomised: 162 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Ixekizumab (60 mg ixekizumab given as 2 SC injections followed by 80 mg ixekizumab given SC every 2 weeks until week 12 and then 80 mg ixekizumab given SC every 4 weeks until week 24) Control interventions FAEs (105 mg FAE given orally followed by 215 mg FAE given orally 1‐3 times/day until week 24) Methotrexate (7.5 mg starting dose up to 30 mg methotrexate given orally once a week until week 24) |
| Outcomes |
At week 24 Primary outcome
Secondary outcome
|
| Starting date | Study start date: January 2016 Study completion date: November 2017 |
| Contact information | Call 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559 Mon ‐ Fri 9 AM ‐ 5 PM Eastern time (UTC/GMT ‐ 5 hours, EST) |
| Notes | Ongoing study |
| Trial name or title | Comparison study of psoriasis severity assessment tools |
| Methods | RCT, placebo‐controlled, open‐label study Date of study: September 2015 ‐ Location: Korea Phase 4 |
| Participants |
Randomised: 34 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Ciclosporin A (men 200 mg/day, women 150 mg/day for 16 weeks) Control intervention Methotrexate (initial dose 10 mg/week, increasing 2.5 mg every 2 weeks up to 15 mg/week) |
| Outcomes |
At week 16 Primary outcome
Secondary outcome
|
| Starting date | Study start date: August 2014 Study completion date: March 2016 |
| Contact information | Sang Woong Youn, Associate Professor, Seoul National University Hospital |
| Notes | Ongoing study |
| Trial name or title | BI 655066/ABBV‐066 (risankizumab) in moderate to severe plaque psoriasis with randomized withdrawal and re‐treatment |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: February 2016 ‐ Location: worldwide Phase 3 |
| Participants |
Randomised: 500 participants Inclusion criteria
Exclusion criteria:
|
| Interventions |
Intervention ABBV‐066 (SC injection, dosage not stated) Control intervention Placebo |
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
| Starting date | February 2016 |
| Contact information | Boehringer Ingelheim |
| Notes | Ongoing study BI 655066 will be included |
| Trial name or title | BI 655066 compared to placebo & active comparator (ustekinumab) in patients with moderate to severe chronic plaque psoriasis |
| Methods | RCT, placebo/active‐controlled, double‐blind study Date of study: September 2017 ‐ Location: worldwide Phase 3 |
| Participants |
Randomised: 500 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention ABBV‐066 (SC, dosage not stated) Control interventions Ustekinumab (dosage not stated)) Placebo |
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
| Starting date | February 2016 |
| Contact information | Boehringer Ingelheim |
| Notes | Ongoing study BI 655066 will be included |
| Trial name or title | BI 655066/ABBV‐066 (risankizumab) versus ustekinumab and placebo comparators in a randomized double blind trIal for maintenance use in moderate to severe plaque type psoriasis (UltIMMa‐1) |
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: February 2016 ‐ Location: worldwide Phase 3 |
| Participants |
Randomised: 500 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention ABBV‐066 (SC, dosage not stated) Control interventions Ustekinumab (dosage not stated)) Placebo |
| Outcomes |
At week 16 Primary composite outcome
Secondary outcomes
|
| Starting date | February 2016 |
| Contact information | Boehringer Ingelheim |
| Notes | Ongoing study BI 655066 will be included |
| Trial name or title | Study to evaluate the effect of secukinumab compared to placebo on aortic vascular inflammation in subjects with moderate to severe plaque psoriasis (VIP‐S) |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: February 2016 ‐ Location: USA Phase 4 |
| Participants |
Randomised: 84 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Secukinumab 300 (300 mg once weekly at baseline, weeks 1, 2, 3 and 4 followed by monthly dosing starting at week 8 through week 48 inclusive) Control intervention Placebo |
| Outcomes |
At week 12 Primary outcome
Secondary outcomes
|
| Starting date | Study start date: February 2016 Study completion date: March 2018 |
| Contact information | Novartis Pharmaceuticals, 1‐888‐669‐6682 |
| Notes | Ongoing study |
| Trial name or title | Study of secukinumab with 2 mL pre‐filled syringes (ALLURE) |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: April 2016 ‐ Location: worldwide Phase 4 |
| Participants |
Randomised: 210 participants Inclusion criteria Subjects eligible for inclusion in this study must fulfil all of the following criteria:
Exclusion criteria
|
| Interventions |
Intervention Secukinumab 2 mL form (secukinumab 300 mg/2 mL + 2 x 1 mL placebo SC. at randomization, weeks 1 , 3, 4, thereafter 4‐weekly until week 48) Control interventions Secukinumab 1 mL form (secukinumab 150 mg/1 mL x 2 + 2 mL placebo SC. at randomization, weeks 1 , 3, 4, thereafter 4‐weekly until Week 48) Placebo (2 mL + 2 x 1 mL placebo SC at randomization, weeks 1, 3, and 4, thereafter 4‐weekly until week 48) |
| Outcomes |
At week 12 Primary composite outcome
Secondary outcome
|
| Starting date | Study start date: 8 March 2016 Study completion date: September 2018 |
| Contact information | Novartis Pharmaceuticals, 1‐888‐669‐6682, +41613241111 |
| Notes | Ongoing study |
| Trial name or title | Study of secukinumab compared to ustekinumab in subjects with plaque psoriasis (CLARITY) |
| Methods | RCT, active‐controlled, double‐blind study Date of study: July 2016 ‐ Location: worldwide Phase 3 |
| Participants |
Randomised: 1100 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Secukinumab 300 (300 mg, SC at randomization, weeks 1, 2 and 3 and thereafter 4‐weekly till week 48) Control intervention Ustekinumab 45/90 (45 mg or 90 mg SC based on participant's weight (at randomization visit) to be administered at randomization, week 4, 16, 28 and 40) |
| Outcomes |
At week 12 Primary composite outcome
Secondary outcomes
|
| Starting date | Study start date: June 2016 Study completion date: August 2018 |
| Contact information | Novartis Pharmaceuticals, 1‐888‐669‐6682, +41613241111 |
| Notes | Ongoing study |
| Trial name or title | Efficacy and safety study of guselkumab in the treatment of participants with moderate to severe plaque‐type psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: September 2016 ‐ Location: worldwide Phase 3 |
| Participants |
Randomised: 78 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Guselkumab (100 mg guselkumab administered as a 100 mg/mL solution in a single‐use prefilled syringe (PFS) assembled in a self‐dose device at weeks 0, 4, 12, 20, and 28) Control intervention Placebo |
| Outcomes |
At week 16 Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 14 September 2016 Study completion date: July 2018 |
| Contact information | Janssen Research & Development, LLC Clinical Trial |
| Notes | Ongoing study |
| Trial name or title | A study to compare the efficacy of guselkumab to FAEs for the treatment of participants with moderate to severe plaque psoriasis (POLARIS) |
| Methods | RCT, active‐controlled, open‐label study Date of study: November 2016 ‐ Location: Germany Phase 3 |
| Participants |
Randomised: 119 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Guselkumab (100 mg administered as 100 mg/mL solution SC by single‐use prefilled syringe (PFS) at weeks 0, 4, 12 and 20) Control intervention FAEs (to this aim, FAE doses will be slowly increased beginning with increasing doses of Fumaderm initial (containing 30 mg dimethylfumarate) over the first 3 weeks. Thereafter, participants will be switched to Fumaderm tablets (containing 120 mg dimethylfumarate) starting with 1 tablet per day. Fumaderm dose may be increased to a maximum of 3x2 tablets per day) |
| Outcomes |
At week 24 Primary outcome
Secondary outcomes
|
| Starting date | Study start date: December 2016 Study completion date: December 2017 |
| Contact information | Janssen‐Cilag G.m.b.H, Germany Clinical Trial |
| Notes | Ongoing study |
| Trial name or title | A Study of KHK4827 (brodalumab) in subjects with moderate to severe psoriasis in Korea |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: December 2016 ‐ Location: Korea Phase 3 |
| Participants |
Randomised: 60 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention KHK4827 (SC, dosage not stated) Control intervention Placebo |
| Outcomes |
At week 12 Primary composite outcome
Secondary outcomes
|
| Starting date | Study start date: 1 December 2016 Study completion date: December 2018 |
| Contact information | Kyowa Hakko Kirin Korea Co., Ltd |
| Notes | Ongoing study |
| Trial name or title | Randomized controlled double blind trial to study safety and efficaccy of itolizumab (antiCD6) in moderate‐to‐severe psoriasis |
| Methods | RCT, placebo‐controlled, double‐blind trial Date of study: February 2016 ‐ Location: worldwide Phase 3 |
| Participants |
Randomised: 144 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Itolizumab: itolizumab 1.6 mg/kg body weight bi‐weekly administered IV by 12 weeks (weeks 0‐8), and every 4 weeks by 24 weeks (Week 12‐36). Control intervention Placebo 1.6 mg/kg body weight bi‐weekly administered intravenously by 12 weeks (weeks 0‐8), then 1.6 mg/kg body weight biweekly administered intravenously by 12 weeks (weeks 12‐20), and every 4 weeks by 12 weeks (weeks 24‐36) |
| Outcomes |
At week 12 Primary outcome
Secondary outcomes
|
| Starting date | Start study date: 15/10/2015 Study completion date: not specified |
| Contact information | Dr Gray Lovio, ogray@infomed.sld.cu |
| Notes | Ongoing study |
| Trial name or title | A randomised, double‐blind, placebo controlled, multicenter study of subcutaneous secukinumab, to demonstrate efficacy after twelve weeks of treatment and to assess safety, tolerability and long‐term efficacy up to one year in subjects with moderate to severe chronic plaque‐type psoriasis with or without psoriatic arthritis comorbidity |
| Methods | RCT, active/placebo‐controlled, double‐blind trial Date of study: February 2017 ‐ Location: Thailand |
| Participants |
Randomised: 40 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention A. Secukinumab 300 mg SC (administration not specified) Control intervention B. Secukinumab 150 mg SC (administration not specified) C. Placebo |
| Outcomes |
At week 12 Primary outcome (composite)
Secondary outcomes
|
| Starting date | 28 February 2017 |
| Contact information | Kerstin Letzelter, kerstin.letzelter@novartis.com |
| Notes | Ongoing study |
BMI: body mass index; BSA: Body Surface Area; ECG: electrocardiogram; FAEs: fumaric acid esters; IV: intravenous; NAPSI: Nail Psoriasis Severity Index; PASI: Psoriasis Area and Severity Index; PGA: Physician's Global Assessment; QoL: quality of life; RCT: randomised controlled trial: SC: subcutaneous; sPGA: static physician global assessment; TB: tuberculosis; UVA/B: ultraviolet A/B
Contributions of authors
ES and LLC were the contacts with the editorial base. ES co‐ordinated contributions from the co‐authors and wrote the final draft of the review. LLC, GD, IGD, and ES screened papers against eligibility criteria. ES obtained data on ongoing and unpublished studies. LLC, GD, IGD, CH, CM, CD, and ES appraised the quality of papers. LLC, GD, IGD, CH, CM, CD, and ES extracted data for the review and sought additional information about papers. ES entered data into RevMan. AC analysed and interpreted data. AC, LLC, and ES worked on the methods sections. ES and LLC drafted the clinical sections of the background and responded to the clinical comments of the referees. AC responded to the methodology and statistical comments of the referees. CH was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. ES is the guarantor of the update.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
-
The French Society of Dermatology (SFD), France; French Ministry of Health, France.
Grant support was from the Programme Hospitalier de Recherche Clinique (DGOS n°14‐0322). The funding agencies have no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation and review of the manuscript.
Declarations of interest
Emilie Sbidian: grant support came from the French Society of Dermatology and the French Ministry of Health, France, the Programme Hospitalier de Recherche Clinique (DGOS no.14‐0322). The funding agencies have no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation and review of the manuscript. Anna Chaimani: nothing to declare. Ignacio Garcia‐Doval: nothing to declare. Giao Do: nothing to declare. Camille Hua: nothing to declare. Canelle Mazaud: nothing to declare. Catherine Droitcourt: nothing to declare. Carolyn Hughes: nothing to declare. John R Ingram: Dr Ingram is Deputy Editor of the British Journal of Dermatology. Luigi Naldi: I received compensation for consultancy or participating in advisory board meetings from the following pharmaceutical companies: AbbVie, Almirall, Janssen‐Cilag, Novartis, Sanofi, L'Oreal. My institution also received an unrestricted grant from AbbVie. The money did not fund the review. Olivier Chosidow: nothing to declare. Laurence Le Cleach: two grants were obtained to support this review work, one from the French Ministry of Health, France (Programme Hospitalier de Recherche Clinique (DGOS no.14‐0322), and one from the French Society of Dermatology (SFD).
Key Editor Gloria Sanclemente: "I have not been involved in any study included in this review, but in the last three years, I have received sponsoring for attending scientific meetings or congresses by Janssen‐Cilag, Novartis, and AbbVie. I also declare that I am currently co‐ordinating a Diploma in Evidence‐Based Dermatology in which attendees have been sponsored by Pfizer, AbbVie and Novartis laboratories."
Clinical referee Steven Feldman: "I have received research, speaking and/or consulting support from a variety of companies including Galderma, GSK/Stiefel, Almirall, Leo Pharma, Baxter, Boeringer Ingelheim, Mylan, Celgene, Pfizer, Valeant, AbbVie, Cosmederm, Anacor, Astellas, Janssen, Lilly, Merck, Merz, Novartis, Qurient, National Biological Corporation, Caremark, Advance Medical, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. I am founder and majority owner of www.DrScore.com. I am a founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment."
New
References
References to studies included in this review
- Akcali C, Guven EH, Kirtak N, Inaloz HS, Ozgoztasi O, Guvenc U. Serum concentrations of interleukin‐2 and tumour necrosis factor‐alpha under cyclosporine versus acitretin treatment in plaque‐type psoriasis. Journal of International Medical Research 2014;42(5):1118‐22. [CENTRAL: CN‐01114333; PUBMED: 25143337] [DOI] [PubMed] [Google Scholar]
- Al‐Hamamy HR, Al‐Mashhadani SA, Mustafa IN. Comparative study of the effect of narrowband ultraviolet B phototherapy plus methotrexate vs. narrowband ultraviolet B alone and methotrexate alone in the treatment of plaque‐type psoriasis. International Journal of Dermatology 2014;53(12):1531‐5. [CENTRAL: CN‐01089920; PUBMED: 24738793] [DOI] [PubMed] [Google Scholar]
- Asahina A, Nakagawa H, Etoh T, Ohtsuki M, Adalimumab M04‐688 Study Group. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. Journal of Dermatology 2010;37(4):299‐310. [CENTRAL: CN‐00762123; PUBMED: 20507398] [DOI] [PubMed] [Google Scholar]
- Asahina A, Etoh T, Igarashi A, Imafuku S, Saeki H, Shibasaki Y, et al. Oral tofacitinib efficacy, safety and tolerability in Japanese patients with moderate to severe plaque psoriasis and psoriatic arthritis: A randomized, double‐blind, phase 3 study. Journal of Dermatology 2016;43(8):869‐80. [PUBMED: 26875540] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asawanonda P, Nateetongrungsak Y. Methotrexate plus narrowband UVB phototherapy versus narrowband UVB phototherapy alone in the treatment of plaque‐type psoriasis: a randomized, placebo‐controlled study. Journal of the American Academy of Dermatology 2006;54(6):1013‐8. [CENTRAL: CN‐00556468; PUBMED: 16713455] [DOI] [PubMed] [Google Scholar]
- Bachelez H, Kerkhof PC, Strohal R, Kubanov A, Valenzuela F, Lee JH, et al. Tofacitinib versus etanercept or placebo in moderate‐to‐severe chronic plaque psoriasis: a phase 3 randomised non‐inferiority trial. Lancet 2015;386(9993):552‐61. [CENTRAL: CN‐01091031; PUBMED: 26051365] [DOI] [PubMed] [Google Scholar]; Valenzuela F, Paul C, Mallbris L, Tan H, Papacharalambous J, Valdez H, et al. Tofacitinib versus etanercept or placebo in patients with moderate to severe chronic plaque psoriasis: patient‐reported outcomes from a Phase 3 study. Journal of the European Academy of Dermatology and Venereology : JEADV 2016;30(10):1753‐1759. [CENTRAL: CN‐01368560; PUBMED: 27271195] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagel J, Kricorian G, Klekotka P, Tyring S. Etanercept therapy for moderate to severe plaque psoriasis with involvement of the scalp. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB150. [CENTRAL: CN‐00843659] [DOI] [PubMed] [Google Scholar]; Bagel J, Lynde C, Tyring S, Kricorian G, Shi Y, Klekotka P. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double‐blind, placebo‐controlled study of etanercept. Journal of the American Academy of Dermatology 2012;67(1):86‐92. [CENTRAL: CN‐00870940; PUBMED: 22014541] [DOI] [PubMed] [Google Scholar]
- Barker J, Hoffmann M, Wozel G, Ortonne J P, Zheng H, Hoogstraten H, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate‐to‐severe plaque psoriasis: results of an open‐label, active‐controlled, randomized trial (RESTORE1). British Journal of Dermatology 2011;165(5):1109‐17. [CENTRAL: CN‐00805921; PUBMED: 21910713] [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Tardif J‐C, Harel F, Pressacco J, Bolduc C, Guertin MC. Effects of the tumor necrosis factor‐alpha antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: Results of a randomized controlled trial. Circulation: Cardiovascular Imaging 2013;6(1):83‐90. [CENTRAL: CN‐00906599; EMBASE: 2013325307] [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Iversen L, Sofen H, Griffiths CE, Foley P, Romiti R, et al. Tofacitinib withdrawal and retreatment in moderate‐to‐severe chronic plaque psoriasis: A randomized controlled trial. British Journal of Dermatology 2015;172(5):1395‐406. [CENTRAL: CN‐01254758; PUBMED: 25418186] [DOI] [PubMed] [Google Scholar]
- Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer‐Mulard M, et al. Secukinumab administration by pre‐filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). British Journal of Dermatology 2015;172(2):484‐93. [CENTRAL: CN‐01052626; PUBMED: 25132411] [DOI] [PubMed] [Google Scholar]
- Blauvelt A, Papp KA, Griffiths CEM, Randazzo B, Wasfi Y, Shen Y‐K, et al. Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. Journal of the American Academy of Dermatology 2016;76(3):405‐417. [CENTRAL: CN‐01341398; PUBMED: 28057360] [DOI] [PubMed] [Google Scholar]
- Cai L, Gu J, Zheng J, Zheng M, Wang G, Xi LY, et al. Efficacy and safety of adalimumab in Chinese patients with moderate‐to‐severe plaque psoriasis: results from a phase 3, randomized, placebo‐controlled, double‐blind study. Journal of the European Academy of Dermatology and Venereology : JEADV 2016;31(1):89‐95. [CENTRAL: CN‐01248561; PUBMED: 27504914] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caproni M, Antiga E, Melani L, Volpi W, Bianco E, Fabbri P. Serum levels of IL‐17 and IL‐22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized‐controlled trial. Journal of Clinical Immunology 2009;29(2):210‐4. [CENTRAL: CN‐00685566; PUBMED: 18763027] [DOI] [PubMed] [Google Scholar]
- Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque‐type psoriasis: a randomised trial. Lancet 2001;357(9271):1842‐7. [CENTRAL: CN‐00348743; PUBMED: 11410193] [DOI] [PubMed] [Google Scholar]; Gottlieb AB, Chaudhari U, Mulcahy LD, Li S, Dooley LT, Baker DG. Infliximab monotherapy provides rapid and sustained benefit for plaque‐type psoriasis. Journal of the American Academy of Dermatology 2003;48(6):829‐35. [CENTRAL: CN‐00438058; PUBMED: 12789171] [DOI] [PubMed] [Google Scholar]; Gottlieb AB, Masud S, Ramamurthi R, Abdulghani A, Romano P, Chaudhari U, et al. Pharmacodynamic and pharmacokinetic response to anti‐tumor necrosis factor‐alpha monoclonal antibody (infliximab) treatment of moderate to severe psoriasis vulgaris. Journal of the American Academy of Dermatology 2003;48(1):68‐75. [CENTRAL: CN‐00466030; PUBMED: 12522373] [DOI] [PubMed] [Google Scholar]
- Chladek J, Grim J, Martinkova J, Simkova M, Vaneckova J. Low‐dose methotrexate pharmacokinetics and pharmacodynamics in the therapy of severe psoriasis. Basic & Clinical Pharmacology & Toxicology 2005;96(3):247‐8. [CENTRAL: CN‐00513064; PUBMED: 15733224] [DOI] [PubMed] [Google Scholar]
- Vries AC, Thio HB, Kort WJ, Opmeer BC, Stok HM, Jong EM, et al. A prospective randomized controlled trial comparing infliximab and etanercept in patients with moderate‐to‐severe chronic plaque‐type psoriasis: the Psoriasis Infliximab vs. Etanercept Comparison Evaluation (PIECE) study. British Journal of Dermatology 2016;176(3):624‐633. [CENTRAL: CN‐01336979; PUBMED: 27416891] [DOI] [PubMed] [Google Scholar]
- Dogra S, Krishna V, Kanwar AJ. Efficacy and safety of systemic methotrexate in two fixed doses of 10 mg or 25 mg orally once weekly in adult patients with severe plaque‐type psoriasis: a prospective, randomized, double‐blind, dose‐ranging study. Clinical and Experimental Dermatology 2012;37(7):729‐34. [CENTRAL: CN‐00879485; PUBMED: 22830389] [DOI] [PubMed] [Google Scholar]
- Dogra S, Jain A, Kanwar AJ. Efficacy and safety of acitretin in three fixed doses of 25, 35 and 50 mg in adult patients with severe plaque type psoriasis: A randomized, double blind, parallel group, dose ranging study. Journal of the European Academy of Dermatology and Venereology : JEADV 2013;27(3):e305‐11. [CENTRAL: CN‐00911790; EMBASE: 2013118368] [DOI] [PubMed] [Google Scholar]
- Dubertret L, Perussel M, Robiola O, Feutren G. Cyclosporin in psoriasis. A long‐term randomized study on 37 patients. Acta Dermato‐Venereologica, Supplement 1989;69(146):136. [CENTRAL: CN‐00064909; PUBMED: 2692368] [PubMed] [Google Scholar]
- Ellis CN, Fradin MS, Messana JM, Brown MD, Siegel MT, Hartley AH, et al. Cyclosporine for plaque‐type psoriasis. Results of a multidose, double‐blind trial. New England Journal of Medicine 1991;324(5):277‐84. [CENTRAL: CN‐00072304; PUBMED: 1986287] [DOI] [PubMed] [Google Scholar]
- Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. The New England journal of medicine 2001;345(4):248‐55. [PUBMED: 11474662] [DOI] [PubMed] [Google Scholar]; Ellis CN, Mordin MM, Adler EY. Effects of alefacept on health‐related quality of life in patients with psoriasis: results from a randomized, placebo‐controlled phase II trial. American Journal of Clinical Dermatology 2003;4(2):131‐9. [CENTRAL: CN‐00435105; PUBMED: 12553852] [DOI] [PubMed] [Google Scholar]
- Engst RH, Bubl R, Huber J, Schober C, Jessberger B. Long‐term cyclosporin A for psoriasis. Acta Dermatovenerologica Alpina, Panonica et Adriatica 1994;3(4):188‐92. [CENTRAL: CN‐00178929; EMBASE: 1995061459] [Google Scholar]
- Fallah Arani S, Neumann H, Hop WC, Thio HB. Fumarates vs. Methotrexate in moderate to severe chronic plaque psoriasis: a multicentre prospective randomized controlled clinical trial. British Journal of Dermatology 2011;164(4):855‐61. [CENTRAL: CN‐00785701; PUBMED: 21175564] [DOI] [PubMed] [Google Scholar]
- Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. British Journal of Dermatology 2008;158(1):116‐21. [CENTRAL: CN‐00628309; PUBMED: 17986302] [DOI] [PubMed] [Google Scholar]
- Gisondi P, Giglio M, Cotena C, Girolomoni G. Combining etanercept and acitretin in the therapy of chronic plaque psoriasis: a 24‐week, randomized, controlled, investigator‐blinded pilot trial. British Journal of Dermatology 2008;158(6):1345‐9. [CENTRAL: CN‐00638916; PUBMED: 18410408] [DOI] [PubMed] [Google Scholar]
- Goldfarb MT, Ellis CN, Gupta AK, Tincoff T, Hamilton TA, Voorhees JJ. Acitretin improves psoriasis in a dose‐dependent fashion. Journal of the American Academy of Dermatology 1988;18(4 Pt 1):655‐62. [CENTRAL: CN‐00053926; PUBMED: 2967310] [DOI] [PubMed] [Google Scholar]; Gupta AK, Goldfarb MT, Ellis CN, Voorhees JJ. Side‐effect profile of acitretin therapy in psoriasis. Journal of the American Academy of Dermatology 1989;20(6):1088‐93. [CENTRAL: CN‐00061373; PUBMED: 2526824] [DOI] [PubMed] [Google Scholar]
- Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double‐blind, randomized controlled trial and open‐label extension study. Journal of the American Academy of Dermatology 2006;55(4):598‐606. [CENTRAL: CN‐00568251; PUBMED: 17010738] [DOI] [PubMed] [Google Scholar]; Shikiar R, Heffernan M, Langley RG, Willian MK, Okun MM, Revicki DA. Adalimumab treatment is associated with improvement in health‐related quality of life in psoriasis: patient‐reported outcomes from a phase II randomized controlled trial. Journal of Dermatological Treatment 2007;18(1):25‐31. [CENTRAL: CN‐00579275; PUBMED: 17365264] [DOI] [PubMed] [Google Scholar]; Shikiar R, Willian MK, Okun MM, Thompson CS, Revicki DA. The validity and responsiveness of three quality of life measures in the assessment of psoriasis patients: Results of a phase II study. Health and Quality of Life Outcomes 2006;27(4):71. [CENTRAL: CN‐00576575; PUBMED: 17005043] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AW, Lynde CW, McBride SR, Stahle M, Edson‐Heredia E, Zhu B, et al. Effect of Ixekizumab treatment on work productivity for patients with moderate‐to‐severe plaque psoriasis: Analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatology 2016;152(6):661‐9. [PUBMED: 26953848] [DOI] [PubMed] [Google Scholar]; Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 Trials of Ixekizumab in Moderate‐to‐Severe Plaque Psoriasis. New England Journal of Medicine 2016;375(4):345‐56. [CENTRAL: CN‐01167902; PUBMED: 27299809] [DOI] [PubMed] [Google Scholar]
- Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. New England Journal of Medicine 2015;373(2):136‐44. [CENTRAL: CN‐01076768; PUBMED: 26154787] [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, et al. A randomized trial of etanercept as monotherapy for psoriasis. Archives of Dermatology 2003;139(12):1627‐32. [CENTRAL: CN‐00459604; PUBMED: 14676082] [DOI] [PubMed] [Google Scholar]
- Feldman SR, Gordon KB, Bala M, Evans R, Li S, Dooley LT, et al. Infliximab treatment results in significant improvement in the quality of life of patients with severe psoriasis: a double‐blind placebo‐controlled trial. British Journal of Dermatology 2005;152(5):954‐60. [CENTRAL: CN‐00513174; PUBMED: 15888152] [DOI] [PubMed] [Google Scholar]; Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque‐type psoriasis: a randomized, double‐blind, placebo‐controlled trial. Journal of the American Academy of Dermatology 2004;51(4):534‐42. [CENTRAL: CN‐00501751; PUBMED: 15389187] [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Leonardi C, Kerdel F, Mehlis S, Olds M, Williams DA. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. British Journal of Dermatology 2011;165(3):652‐60. [CENTRAL: CN‐00811739; PUBMED: 21574983] [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Langley RG, Strober BE, Papp KA, Klekotka P, Creamer K, et al. A randomized, double‐blind, placebo‐controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. British Journal of Dermatology 2012;167(3):649‐57. [CENTRAL: CN‐00842357; 22533447] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CE, Strober BE, Kerkhof P, Ho V, Fidelus‐Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate‐to‐severe psoriasis. New England Journal of Medicine 2010;362(2):118‐28. [CENTRAL: CN‐00734856; PUBMED: 20071701] [DOI] [PubMed] [Google Scholar]; Young MS, Horn EJ, Cather JC. The ACCEPT study: ustekinumab versus etanercept in moderate‐to‐severe psoriasis patients. Expert Review of Clinical Immunology 2011;7(1):9‐13. [CENTRAL: CN‐00780322; PUBMED: 21162644] [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Reich K, Lebwohl M, Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015;386(9993):541‐51. [CENTRAL: CN‐01091029; PUBMED: 26072109] [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Reich K, Lebwohl M, Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015;386(9993):541‐51. [CENTRAL: CN‐01091029; PUBMED: 26072109] [DOI] [PubMed] [Google Scholar]; Kerkhof P, Guenther L, Gottlieb AB, Sebastian M, Wu JJ, Foley P, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate‐to‐severe psoriasis: results from the randomized, controlled and open‐label phases of UNCOVER‐3. Journal of the European Academy of Dermatology and Venereology : JEADV 2017;31(3):477‐482. [CENTRAL: CN‐01287590; PUBMED: 27910156] [DOI] [PubMed] [Google Scholar]
- Gurel G, Saracotlu ZN, Aksu AEK. A single‐blind study comparing acitretin and narrow‐band UVB with the combination of placebo and narrow‐band UVB in the treatment of plaque‐type psoriasis [Plak tip psoriasis tedavisinde asitretin ve dar bant UVB ile plasebo ve dar bant UVB kombinasyonunun karsilastirilditi tek kor calisma]. Türkderm 2015;49(1):2‐6. [CENTRAL: CN‐01102298; EMBASE: 2015064189] [Google Scholar]
- Heydendael VM, Spuls PI, Opmeer BC, Borgie CA, Reitsma JB, Goldschmidt WF, et al. Methotrexate versus cyclosporine in moderate‐to‐severe chronic plaque psoriasis. New England Journal of Medicine 2003;349(7):658‐65. [CENTRAL: CN‐00439969; PUBMED: 12917302] [DOI] [PubMed] [Google Scholar]; Opmeer BC, Heydendael VM, Borgie CA, Spuls PI, Bossuyt PM, Bos JD, et al. Costs of treatment in patients with moderate to severe plaque psoriasis: economic analysis in a randomized controlled comparison of methotrexate and cyclosporine. Archives of Dermatology 2004;140(6):685‐90. [CENTRAL: CN‐00468098; PUBMED: 15210458] [DOI] [PubMed] [Google Scholar]
- Hunter GA, Turner AN. Methotrexate in the treatment of psoriasis: a controlled clinical trial. Australasian Journal of Dermatology 1963;7(2):91‐2. [PUBMED: 14148789] [DOI] [PubMed] [Google Scholar]
- Igarashi A, Kato T, Kato M, Song M, Nakagawa H, Japanese Ustekinumab Study Group. Efficacy and safety of ustekinumab in Japanese patients with moderate‐to‐severe plaque‐type psoriasis: Long‐term results from a phase 2/3 clinical trial. Journal of Dermatology 2012;39(3):242‐52. [CENTRAL: CN‐00860708; PUBMED: 21955098] [DOI] [PubMed] [Google Scholar]; Nakagawa H, Schenkel B, Kato M, Kato T, Igarashi A, Japanese Ustekinumab Study Group. Impact of ustekinumab on health‐related quality of life in Japanese patients with moderate‐to‐severe plaque psoriasis: results from a randomized, double‐blind, placebo‐controlled phase 2/3 trial. Journal of Dermatology 2012;39(9):761‐9. [CENTRAL: CN‐00860068; PUBMED: 22409383] [DOI] [PubMed] [Google Scholar]
- Jacobe H, Winterfield L, Kim F, Huet‐Adams B, Cayce R. The role of narrowband UV‐B plus alefacept combination therapy in the treatment of psoriasis. Archives of Dermatology 2008;144(8):1067‐8; author reply 1068‐9. [CENTRAL: CN‐00650560; PUBMED: 18711092] [DOI] [PubMed] [Google Scholar]
- Feldman SR, Menter A, Koo JY. Improved health‐related quality of life following a randomized controlled trial of alefacept treatment in patients with chronic plaque psoriasis. British Journal of Dermatology 2004;150(2):317‐26. [CENTRAL: CN‐00471158; PUBMED: 14996104] [DOI] [PubMed] [Google Scholar]; Krueger GG. Clinical response to alefacept: Results of a phase 3 study of intravenous administration of alefacept in patients with chronic plague psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2003;17(Suppl 2):17‐24. [CENTRAL: CN‐00456895; PUBMED: 12795771] [DOI] [PubMed] [Google Scholar]; Krueger GG, Papp KA, Stough DB, Loven KH, Gulliver WP, Ellis CN, et al. A randomized, double‐blind, placebo‐controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. Journal of the American Academy of Dermatology 2002;47(6):821‐33. [CENTRAL: CN‐00411712; PUBMED: 12451365] [DOI] [PubMed] [Google Scholar]; Menter A, Cather JC, Baker D, Farber HF, Lebwohl M, Darif M. The efficacy of multiple courses of alefacept in patients with moderate to severe chronic plaque psoriasis. Journal of the American Academy of Dermatology 2006;54(1):61‐3. [CENTRAL: CN‐00622887; EMBASE: 2005584777] [DOI] [PubMed] [Google Scholar]
- Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human interleukin‐12/23 monoclonal antibody for the treatment of psoriasis. New England Journal of Medicine 2007;356(6):580‐92. [CENTRAL: CN‐00575216; PUBMED: 17287478] [DOI] [PubMed] [Google Scholar]
- Krueger J, Clark JD, Suarez‐Farinas M, Fuentes‐Duculan J, Cueto I, Wang CQ, et al. Tofacitinib attenuates pathologic immune pathways in patients with psoriasis: A randomized phase 2 study. Journal of Allergy and Clinical Immunology 2016;137(4):1079‐90. [CENTRAL: CN‐01153710; PUBMED: 27059729] [DOI] [PubMed] [Google Scholar]
- Dogra S, Krupashankar DS, Budamakuntla L, Srinivas CR, Khopkar U, Gupta S, et al. Long‐term efficacy and safety of itolizumab in patients with moderate‐to‐severe chronic plaque psoriasis: A double‐blind, randomized‐withdrawal, placebo‐controlled study. Journal of the American Academy of Dermatology 2015;73(2):331‐3.e1. [CENTRAL: CN‐01108850; PUBMED: 26183983] [DOI] [PubMed] [Google Scholar]; Krupashankar DS, Dogra S, Kura M, Saraswat A, Budamakuntla L, Sumathy TK, et al. Efficacy and safety of itolizumab, a novel anti‐CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: Results of a double‐blind, randomized, placebo‐controlled, phase‐III study. Journal of the American Academy of Dermatology 2014;71(3):484‐92. [CENTRAL: CN‐01002561; PUBMED: 24703722] [DOI] [PubMed] [Google Scholar]
- Laburte C, Grossman R, Abi‐Rached J, Abeywickrama KH, Dubertret L. Efficacy and safety of oral cyclosporin A (CyA; Sandimmun) for long‐term treatment of chronic severe plaque psoriasis. British Journal of Dermatology 1994;130(3):366‐75. [CENTRAL: CN‐00100273; PUBMED: 8148280] [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Langley RG, Philipp S, Sigurgeirsson B, Blauvelt A, Martin R, et al. Secukinumab improves physical function in subjects with plaque psoriasis and psoriatic arthritis: Results from two randomized, phase 3 trials. Journal of Drugs in Dermatology 2015;14(8):821‐33. [CENTRAL: CN‐01132993; PUBMED: 26267726] [PubMed] [Google Scholar]; Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis‐‐results of two phase 3 trials. New England Journal of Medicine 2014;371(4):326‐38. [CENTRAL: CN‐00999505; PUBMED: 25007392] [DOI] [PubMed] [Google Scholar]; Ohtsuki M, Morita A, Abe M, Takahashi H, Seko N, Karpov A, et al. Secukinumab efficacy and safety in Japanese patients with moderate‐to‐severe plaque psoriasis: Subanalysis from ERASURE, a randomized, placebo‐controlled, phase 3 study. Journal of Dermatology 2014;41(12):1039‐46. [CENTRAL: CN‐01037251; PUBMED: 25354738] [DOI] [PubMed] [Google Scholar]
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis‐‐results of two phase 3 trials. The New England Journal of Medicine 2014;371(4):326‐38. [CENTRAL: CN‐00999505; PUBMED: 25007392] [DOI] [PubMed] [Google Scholar]
- Finlay AY, Salek MS, Haney J, Alefacept Clinical Study Group. Intramuscular alefacept improves health‐related quality of life in patients with chronic plaque psoriasis. Dermatology (Basel, Switzerland) 2003;206(4):307‐15. [CENTRAL: CN‐00437818; PUBMED: 12771471] [DOI] [PubMed] [Google Scholar]; Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE, et al. An international, randomized, double‐blind, placebo‐controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Archives of Dermatology 2003;139(6):719‐27. [CENTRAL: CN‐00438439; PUBMED: 12810502] [DOI] [PubMed] [Google Scholar]; Ortonne JP. Clinical response to alefacept: Results of a phase 3 study of intramuscular administration of alefacept in patient with chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2003;17(Suppl 2):12‐6. [CENTRAL: CN‐00456894; PUBMED: 12795770] [DOI] [PubMed] [Google Scholar]; Ortonne JP, Lebwohl M, Griffiths CEM. Alefacept‐induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. European Journal of Dermatology 2003;13(2):117‐23. [CENTRAL: CN‐00436640; PUBMED: 12695125] [PubMed] [Google Scholar]
- Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. New England Journal of Medicine 2015;373(14):1318‐28. [CENTRAL: CN‐01089800; PUBMED: 26422722] [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. New England Journal of Medicine 2015;373(14):1318‐28. [CENTRAL: CN‐01089800; PUBMED: 26422722] [DOI] [PubMed] [Google Scholar]
- Feldman SR, Kimball AB, Krueger GG, Woolley JM, Lalla D, Jahreis A. Etanercept improves the health‐related quality of life of patients with psoriasis: results of a phase III randomized clinical trial. Journal of the American Academy of Dermatology 2005;53(5):887‐9. [CENTRAL: CN‐00561361; PUBMED: 16243150] [DOI] [PubMed] [Google Scholar]; Gordon KB, Gottlieb AB, Leonardi CL, Elewski BE, Wang A, Jahreis A, et al. Clinical response in psoriasis patients discontinued from and then reinitiated on etanercept therapy.[Erratum appears in J Dermatolog Treat. 2006;17(3):192]. Journal of Dermatological Treatment 2006;17(1):9‐17. [CENTRAL: CN‐00555056; PUBMED: 16467018] [DOI] [PubMed] [Google Scholar]; Krueger GG, Elewski B, Papp K, Wang A, Zitnik R, Jahreis A. Patients with psoriasis respond to continuous open‐label etanercept treatment after initial incomplete response in a randomized, placebo‐controlled trial. Journal of the American Academy of Dermatology 2006;54(3 Suppl 2):S112‐9. [PUBMED: 16488321] [DOI] [PubMed] [Google Scholar]; Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. New England Journal of Medicine 2003;349(21):2014‐22. [CENTRAL: CN‐00459025; 14627786] [DOI] [PubMed] [Google Scholar]
- Edson‐Heredia E, Banerjee S, Zhu B, Maeda‐Chubachi T, Cameron GS, Shen W, et al. A high level of clinical response is associated with improved patient‐reported outcomes in psoriasis: analyses from a phase 2 study in patients treated with ixekizumab. Journal of the European Academy of Dermatology and Venereology : JEADV 2016;30(5):864‐5. [PUBMED: 25773781 ] [DOI] [PubMed] [Google Scholar]; Gordon KB, Leonardi CL, Lebwohl M, Blauvelt A, Cameron GS, Braun D, et al. A 52‐week, open‐label study of the efficacy and safety of ixekizumab, an anti‐interleukin‐17A monoclonal antibody, in patients with chronic plaque psoriasis. Journal of the American Academy of Dermatology 2014;71(6):1176‐82. [CENTRAL: CN‐01091643; PUBMED: 25242558] [DOI] [PubMed] [Google Scholar]; Langley RG, Rich P, Menter A, Krueger G, Goldblum O, Dutronc Y, et al. Improvement of scalp and nail lesions with ixekizumab in a phase 2 trial in patients with chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2015;29(9):1763‐70. [CENTRAL: CN‐01089988; PUBMED: 25693783] [DOI] [PubMed] [Google Scholar]; Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson‐Heredia E, et al. Anti‐interleukin‐17 monoclonal antibody ixekizumab in chronic plaque psoriasis. New England Journal of Medicine 2012;366(13):1190‐9. [CENTRAL: CN‐00814008; PUBMED: 22455413] [DOI] [PubMed] [Google Scholar]; Tham LS, Tang CC, Choi SL, Satterwhite JH, Cameron GS, Banerjee S. Population exposure‐response model to support dosing evaluation of ixekizumab in patients with chronic plaque psoriasis. Journal of Clinical Pharmacology 2014;54(10):1117‐24. [CENTRAL: CN‐01048421; PUBMED: 24752880] [DOI] [PubMed] [Google Scholar]; Zhu B, Edson‐Heredia E, Cameron GS, Shen W, Erickson J, Shrom D, et al. Early clinical response as a predictor of subsequent response to ixekizumab treatment: results from a phase II study of patients with moderate‐to‐severe plaque psoriasis. British Journal of Dermatology 2013;169(9):1337‐41. [CENTRAL: CN‐01121535; PUBMED: 24032554] [DOI] [PubMed] [Google Scholar]; Zhu B, Edson‐Heredia E, Guo J, Maeda‐Chubachi T, Shen W, Kimball AB. Itching is a significant problem and a mediator between disease severity and quality of life for patients with psoriasis: Results from a randomized controlled trial. British Journal of Dermatology 2014;171(5):1215‐9. [CENTRAL: CN‐01036585; PUBMED: 24749812] [DOI] [PubMed] [Google Scholar]
- Guenther L, Han C, Szapary P, Schenkel B, Poulin Y, Bourcier M, et al. Impact of ustekinumab on health‐related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. Journal of the European Academy of Dermatology and Venereology : JEADV 2011;25(7):851‐7. [CENTRAL: CN‐00887488; EMBASE: 2011360521] [DOI] [PubMed] [Google Scholar]; Hu C, Szapary PO, Yeilding N, Zhou H. Informative dropout modeling of longitudinal ordered categorical data and model validation: application to exposure‐response modeling of physician's global assessment score for ustekinumab in patients with psoriasis. Journal of Pharmacokinetics and Pharmacodynamics 2011;38(2):237‐60. [CENTRAL: CN‐00787444; PUBMED: 21327538] [DOI] [PubMed] [Google Scholar]; Kimball AB, Gordon KB, Fakharzadeh S, Yeilding N, Szapary PO, Schenkel B, et al. Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. British Journal of Dermatology 2012;166(4):861‐72. [CENTRAL: CN‐00841277; PUBMED: 22356258] [DOI] [PubMed] [Google Scholar]; Kimball AB, Papp KA, Wasfi Y, Chan D, Bissonnette R, Sofen H, et al. Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. Journal of the European Academy of Dermatology and Venereology : JEADV 2013;27(12):1535‐45. [CENTRAL: CN‐00915003; PUBMED: 23279003] [DOI] [PubMed] [Google Scholar]; Lebwohl M, Leonardi C, Griffiths CE, Prinz JC, Szapary PO, Yeilding N, et al. Long‐term safety experience of ustekinumab in patients with moderate‐to‐severe psoriasis (Part I of II): results from analyses of general safety parameters from pooled Phase 2 and 3 clinical trials. Journal of the American Academy of Dermatology 2012;66(5):731‐41. [CENTRAL: CN‐00860736; PUBMED: 21930328] [DOI] [PubMed] [Google Scholar]; Lebwohl M, Papp K, Han C, Schenkel B, Yeilding N, Wang Y, et al. Ustekinumab improves health‐related quality of life in patients with moderate‐to‐severe psoriasis: results from the PHOENIX 1 trial. British Journal of Dermatology 2010;162(1):137‐46. [CENTRAL: CN‐00743778; PUBMED: 19903183] [DOI] [PubMed] [Google Scholar]; Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 76‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 1). Lancet 2008;371(9625):1665‐74. [CENTRAL: CN‐00631485; 18486739] [DOI] [PubMed] [Google Scholar]; Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, et al. Long‐term safety of ustekinumab in patients with moderate‐to‐severe psoriasis: final results from 5 years of follow‐up. British Journal of Dermatology 2013;168(4):844‐54. [PUBMED: 23301632] [DOI] [PubMed] [Google Scholar]; Reich K, Papp KA, Griffiths CE, Szapary PO, Yeilding N, Wasfi Y, et al. An update on the long‐term safety experience of ustekinumab: results from the psoriasis clinical development program with up to four years of follow‐up. Journal of Drugs in Dermatology 2012;11(3):300‐12. [CENTRAL: CN‐00860086; PUBMED: 22395580] [PubMed] [Google Scholar]; Rich P, Bourcier M, Sofen H, Fakharzadeh S, Wasfi Y, Wang Y, et al. Ustekinumab improves nail disease in patients with moderate‐to‐severe psoriasis: Results from PHOENIX 1. British Journal of Dermatology 2014;170(2):398‐407. [CENTRAL: CN‐00982377; PUBMED: 24117389] [DOI] [PubMed] [Google Scholar]; Zhou H, Hu C, Zhu Y, Lu M, Liao S, Yeilding N, et al. Population‐based exposure‐efficacy modeling of ustekinumab in patients with moderate to severe plaque psoriasis. Journal of Clinical Pharmacology 2010;50(3):257‐67. [CENTRAL: CN‐00752537; PUBMED: 19934030] [DOI] [PubMed] [Google Scholar]; Zhu Y, Hu C, Lu M, Liao S, Marini JC, Yohrling J, et al. Population pharmacokinetic modeling of ustekinumab, a human monoclonal antibody targeting IL‐12/23p40, in patients with moderate to severe plaque psoriasis. Journal of Clinical Pharmacology 2009;49(2):162‐75. [PUBMED: 19179295] [DOI] [PubMed] [Google Scholar]
- Lowe NJ, Prystowsky JH, Bourget T, Edelstein J, Nychay S, Armstrong R. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. Journal of the American Academy of Dermatology 1991;24(4):591‐4. [CENTRAL: CN‐00075422; PUBMED: 1827799] [DOI] [PubMed] [Google Scholar]
- Mahajan R, Kaur I, Kanwar AJ. Methotrexate/narrowband UVB phototherapy combination vs. narrowband UVB phototherapy in the treatment of chronic plaque‐type psoriasis‐‐a randomized single‐blinded placebo‐controlled study. Journal of the European Academy of Dermatology and Venereology : JEADV 2010;24(5):595‐600. [CENTRAL: CN‐00759274; PUBMED: 20015056] [DOI] [PubMed] [Google Scholar]
- Meffert H, Bräutigam M, Färber L, Weidinger G. Low‐dose (1.25 mg/kg) cyclosporin A: treatment of psoriasis and investigation of the influence on lipid profile. Acta Dermato‐Venereologica 1997;77(2):137‐41. [CENTRAL: CN‐00138820; PUBMED: 9111826] [DOI] [PubMed] [Google Scholar]
- Feldman SR, Gottlieb AB, Bala M, Wu Y, Eisenberg D, Guzzo C, et al. Infliximab improves health‐related quality of life in the presence of comorbidities among patients with moderate‐to‐severe psoriasis. British Journal of Dermatology 2008;159(3):704‐10. [CENTRAL: CN‐00668311; PUBMED: 18627375] [DOI] [PubMed] [Google Scholar]; Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate‐to‐severe plaque psoriasis. Journal of the American Academy of Dermatology 2007;56(1):31.e1‐15. [CENTRAL: CN‐00576883; PUBMED: 17097378] [DOI] [PubMed] [Google Scholar]; Reich K, Nestle F O, Wu Y, Bala M, Eisenberg D, Guzzo C, et al. Infliximab treatment improves productivity among patients with moderate‐to‐severe psoriasis. European Journal of Dermatology 2007;17:381‐6. [DOI] [PubMed] [Google Scholar]; Reich K, Ortonne JP, Kerkmann U, Wang Y, Saurat JH, Papp K, et al. Skin and nail responses after 1 year of infliximab therapy in patients with moderate‐to‐severe psoriasis: A retrospective analysis of the EXPRESS trial. Dermatology 2010;221(2):172‐8. [PUBMED: 20628238] [DOI] [PubMed] [Google Scholar]
- Armstrong AW, Villanueva Quintero DG, Echeverria CM, Gu Y, Karunaratne M, Reyes Servin O. Body region involvement and quality of life in psoriasis: analysis of a randomized controlled trial of adalimumab. American Journal of Clinical Dermatology 2016;17(6):691‐699. [CENTRAL: CN‐01286209; PUBMED: 27815915 ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Gordon K, Papp K, Poulin Y, Gu Y, Rozzo S, Sasso EH. Long‐term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open‐label extension study for patients from REVEAL. Journal of the American Academy of Dermatology 2012;66(2):241‐51. [CENTRAL: CN‐00860821; PUBMED: 21752491] [DOI] [PubMed] [Google Scholar]; Kimball AB, Bensimon AG, Guerin A, Yu AP, Wu EQ, Okun MM, et al. Efficacy and safety of adalimumab among patients with moderate to severe psoriasis with co‐morbidities: Subanalysis of results from a randomized, double‐blind, placebo‐controlled, phase III trial. American Journal of Clinical Dermatology 2011;12(1):51‐62. [CENTRAL: CN‐00779604; PUBMED: 21110526] [DOI] [PubMed] [Google Scholar]; Menter A, Augustin M, Signorovitch J, Yu AP, Wu EQ, Gupta SR, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. Journal of the American Academy of Dermatology 2010;62(5):812‐8. [CENTRAL: CN‐00743358; PUBMED: 20219265] [DOI] [PubMed] [Google Scholar]; Menter A, Gordon KB, Leonardi CL, Gu Y, Goldblum OM. Efficacy and safety of adalimumab across subgroups of patients with moderate to severe psoriasis. Journal of the American Academy of Dermatology 2010;63(3):448‐56. [CENTRAL: CN‐00761638; PUBMED: 20605254] [DOI] [PubMed] [Google Scholar]; Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. Journal of the American Academy of Dermatology 2008;58(1):106‐15. [CENTRAL: CN‐00703914; PUBMED: 17936411] [DOI] [PubMed] [Google Scholar]; Papp K, Menter A, Poulin Y, Gu Y, Sasso EH. Long‐term outcomes of interruption and retreatment vs. continuous therapy with adalimumab for psoriasis: subanalysis of REVEAL and the open‐label extension study. Journal of the European Academy of Dermatology and Venereology : JEADV 2013;27(5):634‐42. [CENTRAL: CN‐00970158; PUBMED: 22429586] [DOI] [PubMed] [Google Scholar]; Revicki DA, Willian MK, Menter A, Gordon KB, Kimball AB, Leonardi CL, et al. Impact of adalimumab treatment on patient‐reported outcomes: results from a Phase III clinical trial in patients with moderate to severe plaque psoriasis. Journal of Dermatological Treatment 2007;18(6):341‐50. [CENTRAL: CN‐00628656; PUBMED: 18058494] [DOI] [PubMed] [Google Scholar]
- Mrowietz U, Szepietowski JC, Loewe R, Kerkhof P, Lamarca R, Ocker WG, et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate‐to‐severe chronic plaque psoriasis: a randomized, double‐blind, Fumaderm® ‐ and placebo‐controlled trial (BRIDGE). British Journal of Dermatology 2017;176(3):615‐623. [CENTRAL: CN‐01336917; PUBMED: 27515097] [DOI] [PubMed] [Google Scholar]
- Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, et al. Secukinumab retreatment‐as‐needed versus fixed‐interval maintenance regimen for moderate to severe plaque psoriasis: A randomized, double‐blind, noninferiority trial (SCULPTURE). Journal of the American Academy of Dermatology 2015;73(1):27‐36.e1. [CENTRAL: CN‐01109352; PUBMED: 25982539] [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Niiro H, Ootaki K, Japanese brodalumab study group. Brodalumab, a human anti‐interleukin‐17‐receptor antibody in the treatment of Japanese patients with moderate‐to‐severe plaque psoriasis: Efficacy and safety results from a phase II randomized controlled study. Journal of Dermatological Science 2016;81(1):44‐52. [CENTRAL: CN‐01133729; PUBMED: 26547109] [DOI] [PubMed] [Google Scholar]; Umezawa Y, Nakagawa H, Niiro H, Ootaki K, Japanese Brodalumab Study Group. Long‐term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate‐to‐severe plaque psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2016;30(11):1957‐1960. [PUBMED: 27358210] [DOI] [PubMed] [Google Scholar]
- Nugteren‐Huying W M, Schroeff J G, Hermans J, Suurmond D. Fumaric acid therapy for psoriasis: a randomized, double‐blind, placebo‐controlled study. Journal of the American Academy of Dermatology 1990;22:311‐2. [DOI] [PubMed] [Google Scholar]; Nugteren‐Huying WM, Schroeff JG, Hermans J, Suurmond D. Fumaric acid therapy in psoriasis; a double‐blind, placebo‐controlled study [Fumaarzuurtherapie tegen psoriasis; een dubbelblind, placebo‐gecontroleerd onderzoek]. Nederlands Tijdschrift voor Geneeskunde 1990;134(49):2387‐91. [CENTRAL: CN‐00072165; PUBMED: 2263264] [PubMed] [Google Scholar]
- Olsen EA, Weed WW, Meyer CJ, Cobo LM. A double‐blind, placebo‐controlled trial of acitretin for the treatment of psoriasis. Journal of the American Academy of Dermatology 1989;21(4 Pt 1):681‐6. [CENTRAL: CN‐00063370; PUBMED: 2530251] [DOI] [PubMed] [Google Scholar]
- Ortonne JP, Paul C, Berardesca E, Marino V, Gallo G, Brault Y, et al. A 24‐week randomized clinical trial investigating the efficacy and safety of two doses of etanercept in nail psoriasis. British Journal of Dermatology 2013;168(5):1080‐7. [CENTRAL: CN‐00967538; PUBMED: 23013207] [DOI] [PubMed] [Google Scholar]
- Krueger GG, Langley RG, Finlay AY, Griffiths CE, Woolley JM, Lalla D, et al. Patient‐reported outcomes of psoriasis improvement with etanercept therapy: results of a randomized phase III trial. British Journal of Dermatology 2005;153(6):1192‐9. [CENTRAL: CN‐00553127; PUBMED: 16307657] [DOI] [PubMed] [Google Scholar]; Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. British Journal of Dermatology 2005;152(6):1304‐12. [CENTRAL: CN‐00522450; PUBMED: 15948997] [DOI] [PubMed] [Google Scholar]
- Gordon KB, Kimball AB, Chau D, Viswanathan HN, Li J, Revicki DA, et al. Impact of brodalumab treatment on psoriasis symptoms and health‐related quality of life: Use of a novel patient‐reported outcome measure, the Psoriasis Symptom Inventory. British Journal of Dermatology 2014;170(3):705‐15. [CENTRAL: CN‐00981224; PUBMED: 24079852] [DOI] [PMC free article] [PubMed] [Google Scholar]; Papp K, Leonardi C, Menter A, Thompson EH, Milmont CE, Kricorian G, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. Journal of the American Academy of Dermatology 2014;71(6):1183‐1190.e3. [CENTRAL: CN‐01107365; EMBASE: 2015237135] [DOI] [PubMed] [Google Scholar]; Papp K, Menter A, Strober B, Kricorian G, Thompson EH, Milmont CE, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult‐to‐treat moderate‐to‐severe plaque psoriasis. Journal of the American Academy of Dermatology 2015;72(3):436‐439.e1. [CENTRAL: CN‐01111298; PUBMED: 25553889] [DOI] [PubMed] [Google Scholar]; Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti‐interleukin‐17‐receptor antibody for psoriasis. The New England Journal of Medicine 2012;366(13):1181‐9. [CENTRAL: CN‐00814009; PUBMED: 22455412] [DOI] [PubMed] [Google Scholar]
- Bushmakin AG, Mamolo C, Cappelleri JC, Stewart M. The relationship between pruritus and the clinical signs of psoriasis in patients receiving tofacitinib. Journal of Dermatological Treatment 2015;26(1):19‐22. [CENTRAL: CN‐01051702; PUBMED: 24289224] [DOI] [PubMed] [Google Scholar]; Mamolo C, Harness J, Tan H, Menter A. Tofacitinib (CP‐690,550), an oral Janus kinase inhibitor, improves patient‐reported outcomes in a phase 2b, randomized, double‐blind, placebo‐controlled study in patients with moderate‐to‐severe psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2014;28(2):192‐203. [CENTRAL: CN‐00959043; PUBMED: 23294276] [DOI] [PubMed] [Google Scholar]; Mamolo CM, Bushmakin AG, Cappelleri JC. Application of the Itch Severity Score in patients with moderate‐to‐severe plaque psoriasis: Clinically important difference and responder analyses. Journal of Dermatological Treatment 2015;26(2):121‐3. [CENTRAL: CN‐01083900; PUBMED: 24716586] [DOI] [PubMed] [Google Scholar]; Papp KA, Menter A, Strober B, Langley RG, Buonanno M, Wolk R, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo‐controlled dose‐ranging study. British Journal of Dermatology 2012;167(3):668‐77. [CENTRAL: CN‐00856433; PUBMED: 22924949] [DOI] [PubMed] [Google Scholar]; Strober B, Buonanno M, Clark JD, Kawabata T, Tan H, Wolk R, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. British Journal of Dermatology 2013;169(5):992‐9. [CENTRAL: CN‐01122547; PUBMED: 23855761] [DOI] [PubMed] [Google Scholar]; Valenzuela F, Papp KA, Pariser D, Tyring SK, Wolk R, Buonanno M, et al. Effects of tofacitinib on lymphocyte sub‐populations, CMV and EBV viral load in patients with plaque psoriasis. BMC Dermatology 2015;15:8. [CENTRAL: CN‐01109432; PUBMED: 25951857] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet 2012;380(9843):738‐46. [CENTRAL: CN‐00859723; PUBMED: 22748702] [DOI] [PubMed] [Google Scholar]; Strand V, Fiorentino D, Hu C, Day RM, Stevens RM, Papp KA. Improvements in patient‐reported outcomes with apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of moderate to severe psoriasis: results from a phase IIb randomized, controlled study. Health and Quality of Life Outcomes 2013;10(11):82. [CENTRAL: CN‐00876574; PUBMED: 23663752] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, et al. Efficacy and safety of secukinumab in the treatment of moderate‐to‐severe plaque psoriasis: a randomized, double‐blind, placebo‐controlled phase II dose‐ranging study. British Journal of Dermatology 2013;168(2):412‐21. [CENTRAL: CN‐00967073; PUBMED: 23106107] [DOI] [PubMed] [Google Scholar]; Sigurgeirsson B, Kircik L, Nemoto O, Mikazans I, Haemmerle S, Thurston HJ, et al. Secukinumab improves the signs and symptoms of moderate‐to‐severe plaque psoriasis in subjects with involvement of hands and/or feet: Subanalysis of a randomized, double‐blind, placebo‐controlled, phase 2 dose‐ranging study. Journal of the European Academy of Dermatology and Venereology : JEADV 2014;28(8):1127‐9. [CENTRAL: CN‐01041806; PUBMED: 24330415] [DOI] [PubMed] [Google Scholar]
- Papp KA, Kaufmann R, Thaci D, Hu C, Sutherland D, Rohane P. Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, dose‐comparison study. Journal of the European Academy of Dermatology and Venereology : JEADV 2013;27(3):e376‐83. [CENTRAL: CN‐01124587; PUBMED: 23030767] [DOI] [PubMed] [Google Scholar]
- Papp K, Thaci D, Reich K, Riedl E, Langley RG, Krueger JG, et al. Tildrakizumab (MK‐3222), an anti‐interleukin‐23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo‐controlled trial. British Journal of Dermatology 2015;173(4):930‐9. [CENTRAL: CN‐01105188; PUBMED: 26042589] [DOI] [PubMed] [Google Scholar]
- Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized,double‐blind, placebo‐controlled study of brodalumab in patients withmoderate‐to‐severe plaque psoriasis. British Journal of Dermatology 2016;175(2):273‐86. [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Pariser DM, Wasel NR, Goncalves J, Day RM, Chen R, et al. Apremilast, an oral phosphodiesterase‐4 inhibitor, in the treatment of palmoplantar psoriasis: Results of a pooled analysis from phase II PSOR‐005 and phase III Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) clinical trials in patients with moderate to severe psoriasis. Journal of the American Academy of Dermatology 2016;75(1):99‐105. [PUBMED: 27021239] [DOI] [PubMed] [Google Scholar]; Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). Journal of the American Academy of Dermatology 2015;73(1):37‐49. [CENTRAL: CN‐01085116; PUBMED: 26089047] [DOI] [PubMed] [Google Scholar]; Rich P, Gooderham M, Bachelez H, Goncalves J, Day RM, Chen R, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult‐to‐treat nail and scalp psoriasis: Results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2).. Journal of the American Academy of Dermatology 2016;74(1):134‐42. [CENTRAL: CN‐01127546; PUBMED: 26549249] [DOI] [PubMed] [Google Scholar]
- Papp KA, Menter MA, Abe M, Elewski B, Feldman SR, Gottlieb AB, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo‐controlled, phase III trials. British Journal of Dermatology 2015;173(4):949‐61. [CENTRAL: CN‐01105187; PUBMED: 26149717] [DOI] [PubMed] [Google Scholar]
- Papp KA, Menter MA, Abe M, Elewski B, Feldman SR, Gottlieb AB, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo‐controlled, phase III trials. British Journal of Dermatology 2015;173(4):949‐61. [CENTRAL: CN‐01105187; PUBMED: 26149717] [DOI] [PubMed] [Google Scholar]
- Langley RG, Feldman SR, Han C, Schenkel B, Szapary P, Hsu MC, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin‐related quality of life in patients with moderate‐to‐severe psoriasis: results from a randomized, double‐blind, placebo‐controlled phase iii trial. Journal of the American Academy of Dermatology 2010;63(3):457‐65. [CENTRAL: CN‐00761719; PUBMED: 20462664] [DOI] [PubMed] [Google Scholar]; Langley RG, Lebwohl M, Krueger GG, Szapary PO, Wasfi Y, Chan D, et al. Long‐term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate‐to‐severe psoriasis: Results from the PHOENIX 2 study through 5 years of follow‐up. British Journal of Dermatology 2015;172(5):1371‐83. [CENTRAL: CN‐01254538; PUBMED: 25307931] [DOI] [PubMed] [Google Scholar]; Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 52‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 2). Lancet 2008;371(9625):1675‐84. [CENTRAL: CN‐00631486; PUBMED: 18486740] [DOI] [PubMed] [Google Scholar]; Reich K, Schenkel B, Zhao N, Szapary P, Augustin M, Bourcier M, et al. Ustekinumab decreases work limitations, improves work productivity, and reduces work days missed in patients with moderate‐to‐severe psoriasis: results from PHOENIX 2. Journal of Dermatological Treatment 2011;22(6):337‐47. [CENTRAL: CN‐00860939; PUBMED: 21034290] [DOI] [PubMed] [Google Scholar]
- Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). British Journal of Dermatology 2015;173(6):1387‐99. [CENTRAL: CN‐01133855; PUBMED: 26357944] [DOI] [PubMed] [Google Scholar]
- Lacour JP, Paul C, Jazayeri S, Papanastasiou P, Xu C, Nyirady J, et al. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. Journal of the European Academy of Dermatology and Venereology : JEADV 2017;31(5):847‐56. [CENTRAL: CN‐01342022; PUBMED: 28111801] [DOI] [PubMed] [Google Scholar]; Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: A randomized, controlled trial (JUNCTURE). Journal of the European Academy of Dermatology and Venereology : JEADV 2015;29(6):1082‐90. [CENTRAL: CN‐01043227; PUBMED: 25243910] [DOI] [PubMed] [Google Scholar]
- Piskin G, Heydendael VM, Rie MA, Bos JD, Teunissen MB. Cyclosporin A and methotrexate are equally effective in reducing T cell numbers in psoriatic skin lesions but have no consistent effect on IFN‐gamma and IL‐4 expression in psoriatic skin in situ. Archives of Dermatological Research 2003;294(12):559‐62. [CENTRAL: CN‐00456554; PUBMED: 12624782] [DOI] [PubMed] [Google Scholar]
- Reich K, Ortonne JP, Gottlieb AB, Terpstra IJ, Coteur G, Tasset C, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab' certolizumab pegol: results of a phase II randomized, placebo‐controlled trial with a re‐treatment extension. British Journal of Dermatology 2012;167(1):180‐90. [CENTRAL: CN‐00856435; PUBMED: 22413944] [DOI] [PubMed] [Google Scholar]
- Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M, et al. Evidence that a neutrophil‐keratinocyte crosstalk is an early target of IL‐17A inhibition in psoriasis. Experimental Dermatology 2015;24(7):529‐35. [CENTRAL: CN‐01171151; PUBMED: 25828362] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet 2005;366(9494):1367‐74. [CENTRAL: CN‐00531178; PUBMED: 16226614] [DOI] [PubMed] [Google Scholar]; Reich K, Nestle FO, Papp K, Ortonne JP, Wu Y, Bala M, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate‐to‐severe psoriasis: a randomized controlled trial. British Journal of Dermatology 2006;154(6):1161‐8. [CENTRAL: CN‐00565410; PUBMED: 16704649] [DOI] [PubMed] [Google Scholar]
- Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept, and placebo, in patients with moderate to severe plaque psoriasis: 52‐week results from a phase 3b, randomized, placebo‐controlled trial (LIBERATE). Journal of the European Academy of Dermatology and Venereology : JEADV 2017;31(3):507‐517. [CENTRAL: CN‐01285623; PUBMED: 27768242] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. Journal of the American Academy of Dermatology 2017;76(3):418‐431. [CENTRAL: CN‐01341399; PUBMED: 28057361] [DOI] [PubMed] [Google Scholar]
- Augustin M, Abeysinghe S, Mallya U, Qureshi A, Roskell N, McBride D, et al. Secukinumab treatment of plaque psoriasis shows early improvement in DLQI response ‐ results of a phase II regimen‐finding trial. Journal of the European Academy of Dermatology and Venereology : JEADV 2016;30(4):645‐9. [CENTRAL: CN‐01265142; PUBMED: 26660143] [DOI] [PMC free article] [PubMed] [Google Scholar]; Paul C, Reich K, Gottlieb A B, Mrowietz U, Philipp S, Nakayama J, et al. Secukinumab improves hand, foot and nail lesions in moderate‐to‐severe plaque psoriasis: Subanalysis of a randomized, double‐blind, placebo‐controlled, regimen‐finding phase 2 trial. Journal of the European Academy of Dermatology and Venereology : JEADV 2014;28(12):1670‐5. [CENTRAL: CN‐01119275; PUBMED: 24393602] [DOI] [PubMed] [Google Scholar]; Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, et al. Secukinumab induction and maintenance therapy in moderate‐to‐severe plaque psoriasis: a randomized, double‐blind, placebo‐controlled, phase II regimen‐finding study. British Journal of Dermatology 2013;168(2):402‐11. [CENTRAL: CN‐00965685; PUBMED: 23362969] [DOI] [PubMed] [Google Scholar]
- Ruzicka T, Sommerburg C, Braun‐Falco O, Köster W, Lengen W, Lensing W, et al. Efficiency of acitretin in combination with UV‐B in the treatment of severe psoriasis. Archives of Dermatology 1990;126(4):482‐6. [CENTRAL: CN‐00066767; PUBMED: 2138875] [PubMed] [Google Scholar]
- Sandhu K, Kaur I, Kumar B, Saraswat A. Efficacy and safety of cyclosporine versus methotrexate in severe psoriasis: a study from north India. Journal of Dermatology 2003;30(6):458‐63. [CENTRAL: CN‐00456950; PUBMED: 12810993] [DOI] [PubMed] [Google Scholar]
- Saurat JH, Geiger JM, Amblard P, Beani JC, Boulanger A, Claudy A, et al. Randomized double‐blind multicenter study comparing acitretin‐PUVA, etritinate‐PUVA and placebo‐PUVA in the treatment of severe psoriasis. Dermatologica 1988;177(4):218‐24. [CENTRAL: CN‐00058056; PUBMED: 2976000] [DOI] [PubMed] [Google Scholar]
- Navarini AA, Poulin Y, Menter A, Gu Y, Teixeira HD. Analysis of body regions and components of PASI scores during adalimumab or methotrexate treatment for patients with moderate‐to‐severe psoriasis. Journal of Drugs in Dermatology 2014;13(5):554‐62. [CENTRAL: CN‐00993155; PUBMED: 24809878] [PubMed] [Google Scholar]; Prussick R, Unnebrink K, Valdecantos WC. Efficacy of adalimumab compared with methotrexate or placebo stratified by baseline BMI in a randomized placebo‐ controlled trial in patients with psoriasis. Journal of Drugs in Dermatology 2015;14(8):864‐8. [CENTRAL: CN‐01132989; PUBMED: 26267731] [PubMed] [Google Scholar]; Reich K, Signorovitch J, Ramakrishnan K, Yu AP, Wu EQ, Gupta SR, et al. Benefit‐risk analysis of adalimumab versus methotrexate and placebo in the treatment of moderate to severe psoriasis: comparison of adverse event‐free response days in the CHAMPION trial. Journal of the American Academy of Dermatology 2010;63(6):1011‐8. [CENTRAL: CN‐00791143; PUBMED: 20933301] [DOI] [PubMed] [Google Scholar]; Revicki D, Willian MK, Saurat JH, Papp KA, Ortonne JP, Sexton C, et al. Impact of adalimumab treatment on health‐related quality of life and other patient‐reported outcomes: results from a 16‐week randomized controlled trial in patients with moderate to severe plaque psoriasis. British Journal of Dermatology 2008;158(3):549‐57. [CENTRAL: CN‐00628564; PUBMED: 18047521] [DOI] [PubMed] [Google Scholar]; Saurat JH, Langley RG, Reich K, Unnebrink K, Sasso EH, Kampman W. Relationship between methotrexate dosing and clinical response in patients with moderate to severe psoriasis: subanalysis of the CHAMPION study. British Journal of Dermatology 2011;165(2):399‐406. [CENTRAL: CN‐00800067; PUBMED: 21564071] [DOI] [PubMed] [Google Scholar]; Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). British Journal of Dermatology 2008;158(3):558‐66. [CENTRAL: CN‐00628565; PUBMED: 18047523] [DOI] [PubMed] [Google Scholar]
- Shehzad T, Dar NR, Zakria M. Efficacy of concomitant use of puva and methotrexate in disease clearance time in plaque type psoriasis. Journal of the Pakistan Medical Association 2004;54(9):453‐5. [CENTRAL: CN‐00727152; PUBMED: 15518366] [PubMed] [Google Scholar]
- Sommerburg C, Kietzmann H, Eichelberg D, Goos M, Heese A, Holzle E, et al. Acitretin in combination with PUVA: A randomized double‐blind placebo‐ controlled study in severe psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 1993;2(4):308‐17. [CENTRAL: CN‐00180920; EMBASE: 1993350796] [Google Scholar]
- Gniadecki R, Robertson D, Molta CT, Freundlich B, Pedersen R, Li W, et al. Self‐reported health outcomes in patients with psoriasis and psoriatic arthritis randomized to two etanercept regimens. Journal of the European Academy of Dermatology and Venereology : JEADV 2012;26(11):1436‐43. [CENTRAL: CN‐00971660; PUBMED: 22035157] [DOI] [PubMed] [Google Scholar]; Griffiths CE, Sterry W, Brock F, Dilleen M, Stefanidis D, Germain JM, et al. Pattern of response in patients with moderate‐to‐severe psoriasis treated with etanercept. British Journal of Dermatology 2015;172(1):230‐8. [CENTRAL: CN‐01116415; PUBMED: 24861696] [DOI] [PubMed] [Google Scholar]; Kirkham B, Vlam K, Li W, Boggs R, Mallbris L, Nab HW, et al. Early treatment of psoriatic arthritis is associated with improved patient‐reported outcomes: findings from the etanercept PRESTA trial. Clinical and Experimental Rheumatology 2015;33(1):11‐19. [CENTRAL: CN‐01090489; PUBMED: 25535650] [PubMed] [Google Scholar]; Prinz JC, Fitzgerald O, Boggs RI, Foehl J, Robertson D, Pedersen R, et al. Combination of skin, joint and quality of life outcomes with etanercept in psoriasis and psoriatic arthritis in the PRESTA trial. Journal of the European Academy of Dermatology and Venereology : JEADV 2011;25(5):559‐64. [CENTRAL: CN‐00802619; PUBMED: 20840349] [DOI] [PubMed] [Google Scholar]; Sterry W, Ortonne JP, Kirkham B, Brocq O, Robertson D, Pedersen RD, et al. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ (Clinical Research Ed.) 2010;340:c147. [CENTRAL: CN‐00734727; PUBMED: 20124563] [DOI] [PubMed] [Google Scholar]
- Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. British Journal of Dermatology 2011;165(3):661‐8. [CENTRAL: CN‐00811738; PUBMED: 21574984] [DOI] [PubMed] [Google Scholar]
- Puig L, Strohal R, Husni ME, Tsai TF, Noppakun N, Szumski A, et al. Cardiometabolic profile, clinical features, quality of life and treatment outcomes in patients with moderate‐to‐severe psoriasis and psoriatic arthritis. Journal of Dermatological Treatment 2015;26(1):7‐15. [CENTRAL: CN‐01051703; PUBMED: 24283931] [DOI] [PubMed] [Google Scholar]; Strohal R, Puig L, Chouela E, Tsai TF, Melin J, Freundlich B, et al. The efficacy and safety of etanercept when used with as‐needed adjunctive topical therapy in a randomised, double‐blind study in subjects with moderate‐to‐severe psoriasis (the PRISTINE trial). Journal of Dermatological Treatment 2013;24(3):169‐78. [CENTRAL: CN‐00881482; PUBMED: 22251226] [DOI] [PubMed] [Google Scholar]; Thaci D, Galimberti R, Amaya‐Guerra M, Rosenbach T, Robertson D, Pedersen R, et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate‐to‐severe psoriasis: results from the PRISTINE trial. Journal of the European Academy of Dermatology & Venereology : JEADV 2014;28(7):900‐6. [CENTRAL: CN‐01041533; PUBMED: 23848989] [DOI] [PubMed] [Google Scholar]
- Tanew A, Guggenbichler A, Hönigsmann H, Geiger JM, Fritsch P. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double‐blind comparison study. Journal of the American Academy of Dermatology 1991;25(4):682‐4. [CENTRAL: CN‐00612571; PUBMED: 1838750] [DOI] [PubMed] [Google Scholar]
- Thaci D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. Journal of the American Academy of Dermatology 2015;73(3):400‐9. [CENTRAL: CN‐01090628; PUBMED: 26092291] [DOI] [PubMed] [Google Scholar]
- Torii H, Nakagawa H, Japanese Infliximab Study investigators. Infliximab monotherapy in Japanese patients with moderate‐to‐severe plaque psoriasis and psoriatic arthritis. A randomized, double‐blind, placebo‐controlled multicenter trial. Journal of Dermatological Science 2010;59(1):40‐9. [CENTRAL: CN‐00760986; PUBMED: 20547039] [DOI] [PubMed] [Google Scholar]
- Tsai TF, Ho JC, Song M, Szapary P, Guzzo C, Shen YK, et al. Efficacy and safety of ustekinumab for the treatment of moderate‐to‐severe psoriasis: a phase III, randomized, placebo‐controlled trial in Taiwanese and Korean patients (PEARL). Journal of Dermatological Science 2011;63(3):154‐63. [CENTRAL: CN‐00810821; PUBMED: 21741220] [DOI] [PubMed] [Google Scholar]; Tsai TF, Song M, Shen YK, Schenkel B, Choe YB, Kim NI, et al. Ustekinumab improves health‐related quality of life in Korean and Taiwanese patients with moderate to severe psoriasis: results from the PEARL trial. Journal of Drugs in Dermatology 2012;11(8):943‐9. [CENTRAL: CN‐00859638; PUBMED: 22859239] [PubMed] [Google Scholar]
- Krishnan R, Cella D, Leonardi C, Papp K, Gottlieb AB, Dunn M, et al. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. British Journal of Dermatology 2007;157(6):1275‐7. [CENTRAL: CN‐00627972; PUBMED: 17916204] [DOI] [PubMed] [Google Scholar]; Tyring S, Bagel J, Lynde C, Klekotka P, Thompson EH, Gandra SR, et al. Patient‐reported outcomes in moderate‐to‐severe plaque psoriasis with scalp involvement: results from a randomized, double‐blind, placebo‐controlled study of etanercept. Journal of the European Academy of Dermatology and Venereology : JEADV 2013;27(1):125‐8. [CENTRAL: CN‐00971136; PUBMED: 22188302] [DOI] [PubMed] [Google Scholar]; Tyring S, Gordon KB, Poulin Y, Langley RG, Gottlieb AB, Dunn M, et al. Long‐term safety and efficacy of 50 mg of etanercept twice weekly in patients with psoriasis. Archives of Dermatology 2007;143(6):719‐26. [CENTRAL: CN‐00589825; PUBMED: 17576937] [DOI] [PubMed] [Google Scholar]; Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double‐blind placebo‐controlled randomised phase III trial. Lancet 2006;367(9504):29‐35. [CENTRAL: CN‐00532672; PUBMED: 16399150] [DOI] [PubMed] [Google Scholar]
- Chimenti S, Arenberger P, Karpati S, Sator PG, Vaclavkova A, Burcklen M, et al. A phase II study of ponesimod, an oral, selective sphingosine 1‐phosphate receptor‐1 modulator, in chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2013;27(Suppl 4):22. [CENTRAL: CN‐01025267; PUBMED: 23822555]23822555 [Google Scholar]; Kemeny L, Yankova R, Talamonti M, Vaclavkova A, Burcklen M, Thomas G, et al. A phase II study of ponesimod in chronic plaque psoriasis: Improvements in patient‐reported outcomes. Journal of the European Academy of Dermatology and Venereology : JEADV 2013;27(Suppl 4):21. [CENTRAL: CN‐01025268; PUBMED: 23822555]23822555 [Google Scholar]; Vaclavkova A, Chimenti S, Arenberger P, Hollo P, Sator PG, Burcklen M, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double‐blind, placebo‐controlled phase 2 trial. Lancet 2014;384(9959):2036‐45. [CENTRAL: CN‐01040467; PUBMED: 25127208] [DOI] [PubMed] [Google Scholar]
- Bezooijen JS, Balak DMW, Doorn MBA, Looman CW, Schreurs MW, Koch BC, et al. Combination therapy of etanercept and fumarates versus etanercept monotherapy in psoriasis: a randomized exploratory study. Dermatology 2016;232(4):407‐14. [PUBMED: 27576483] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich K, Segaert S, Kerkhof P, Durian C, Boussuge MP, Paolozzi L, et al. Once‐weekly administration of etanercept 50 mg improves patient‐reported outcomes in patients with moderate‐to‐severe plaque psoriasis. Dermatology (Basel, Switzerland) 2009;219(3):239‐49. [CENTRAL: CN‐00730853; PUBMED: 19752505] [DOI] [PubMed] [Google Scholar]; Kerkhof PC, Segaert S, Lahfa M, Luger TA, Karolyi Z, Kaszuba A, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate‐to‐severe plaque psoriasis: a randomized controlled trial with open‐label extension. British Journal of Dermatology 2008;159(5):1177‐85. [CENTRAL: CN‐00681015; PUBMED: 18673365] [DOI] [PubMed] [Google Scholar]
- Warren RB, Mrowietz U, Kiedrowski R, Niesmann J, Wilsmann‐Theis D, Ghoreschi K, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque‐type psoriasis (METOP): a 52 week, multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet (London, England) 2017;389(10068):528‐537. [CENTRAL: CN‐01330160; PUBMED: 28012564] [DOI] [PubMed] [Google Scholar]
- Yan H, Tang M, You Y, Yu JB, Zhang JA, Li XH, et al. Treatment of psoriasis with recombinant human LFA3‐antibody fusion protein: a multi‐center, randomized, double‐blind trial in a Chinese population. European Journal of Dermatology 2011;21(5):737‐43. [CENTRAL: CN‐00810848; PUBMED: 21737373] [DOI] [PubMed] [Google Scholar]
- Yang HZ, Wang K, Jin HZ, Gao TW, Xiao SX, Xu JH, et al. Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: a randomized, double‐blind, placebo‐controlled multicenter trial. Chinese Medical Journal 2012;125(11):1845‐51. [CENTRAL: CN‐00904898; PUBMED: 22884040] [PubMed] [Google Scholar]
- Yilmaz E, Yilmaz F, Yerebakan O. Re‐PUVA therapy for psoriasis vulgaris: an effective choice. Journal of the European Academy of Dermatology and Venereology : JEADV 2002;16(Suppl s1):258. [CENTRAL: CN‐00478820] [Google Scholar]; Yılmaz E, Yılmaz F, Yerebakan O. Re‐PUVA therapy for psoriasis vulgaris: an effective choice [Psoriazis vulgaris tedavisinde etkili bir seçenek: asitretin ile PUVA kombinasyonu (Re‐PUVA)]. Türkiye Klinikleri Dermatoloji Dergisi 2002;12(4):204‐208. [Google Scholar]
- Zheng M, Zhu X‐J, Song M, Shen Y‐K, Wang B‐X. A randomized, double‐blind, placebo‐controlled study of ustekinumab in Chinese patients with moderate to severe plaque psoriasis: LOTUS trial results. Journal of Dermatology 2012;39(s1):238‐9. [CENTRAL: CN‐01032271; EMBASE: 70801108] [Google Scholar]; Zhu X, Zheng M, Song M, Shen YK, Chan D, Szapary PO, et al. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque‐type psoriasis: results from a phase 3 clinical trial (LOTUS). Journal of Drugs in Dermatology 2013;12(2):166‐74. [CENTRAL: CN‐00965604; PUBMED: 23377389] [PubMed] [Google Scholar]; Zhu X‐J, Zheng M, Song M, Han C, Chan D, Shen Y‐K, et al. Ustekinumab improves health‐related quality of life in Chinese patients with moderate‐to‐severe plaque psoriasis: Results from the LOTUS trial and curative effect observation. Journal of Clinical Dermatology 2014;43(9):521‐6. [CENTRAL: CN‐01037236; EMBASE: 2014949827] [Google Scholar]
References to studies excluded from this review
- Abufarag A, Aigner S, Czeloth N, Dalken B, Koch H, Niemann G, et al. Selective activation of naturally occurring regulatory T cells (Tregs) by the monoclonal antibody BT‐061 as a novel therapeutic opportunity in psoriasis: Early clinical results after single doses. Journal of Investigative Dermatology 2010;130(Suppl 2):S64. [CENTRAL: CN‐00795758; EMBASE: 70263767] [Google Scholar]
- Akhyani M, Chams‐Davatchi C, Hemami MR, Fateh S. Efficacy and safety of mycophenolate mofetil vs. methotrexate for the treatment of chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2010;24(12):1447‐51. [CENTRAL: CN‐00771805; PUBMED: 20384673] [DOI] [PubMed] [Google Scholar]
- Altmeyer PJ, Matthes U, Pawlak F, Hoffmann K, Frosch PJ, Ruppert P, et al. Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double‐blind study in 100 patients. Journal of the American Academy of Dermatology 1994;30(6):977‐81. [CENTRAL: CN‐00101455; PUBMED: 8188891] [DOI] [PubMed] [Google Scholar]
- Angsten M, Schopf RE. Anti‐TNF‐alpha‐therapy of psoriasis with Infliximab or Etanercept. Clinical, histological and immunohistochemical course. Aktuelle Dermatologie 2007;33(8‐9):310‐6. [CENTRAL: CN‐00726884; EMBASE: 2007503340] [Google Scholar]
- Anonymous. Aldalimumab in psoriatic arthritis and as the initial therapy in rheumatoid arthritis [Adalimumab bei Psoriasis‐Arthritis und zur Initialtherapie bei rheumatoider Arthritis]. Krankenpflege Journal 2005;43(7‐10):244. [PUBMED: 16515313] [PubMed] [Google Scholar]
- Anonymous. Trial watch: novel biologic for psoriasis shows superiority over current best‐seller. Nature Reviews. Drug Discovery 2008;7(11):880‐1. [PUBMED: 18974743] [DOI] [PubMed] [Google Scholar]
- Arifov S, Vaisov A, Ismagilov A, Abidova Z. Acitretin (neotigason) in the treatment of psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 1998;11(Suppl 2):S290. [CENTRAL: CN‐00465900] [Google Scholar]
- Armati RP. Retinoic acid for psoriasis. Australasian Journal of Dermatology 1972;13(2):79‐83. [CENTRAL: CN‐00008047; PUBMED: 4566493] [DOI] [PubMed] [Google Scholar]
- Avgerinou G, Tousoulis D, Siasos G, Oikonomou E, Maniatis K, Papageorgiou N, et al. Anti‐tumor necrosis factor alpha treatment with adalimumab improves significantly endothelial function and decreases inflammatory process in patients with chronic psoriasis. International Journal of Cardiology 2011;151(3):382‐3. [CENTRAL: CN‐00860819; PUBMED: 21764467] [DOI] [PubMed] [Google Scholar]
- Bagot M, Grossman R, Pamphile R, Binderup L, Charue D, Revuz J, et al. Additive effects of calcipotriol and cyclosporine A: from in vitro experiments to in vivo applications in the treatment of severe psoriasis. Comptes rendus de l'Académie des sciences. Série III, Sciences de la vie 1994;317(3):282‐6. [CENTRAL: CN‐00107966; PUBMED: 7994616] [PubMed] [Google Scholar]
- Bartlett BL, Tyring SK. Ustekinumab for chronic plaque psoriasis. Lancet 2008;371(9625):1639‐40. [CENTRAL: CN‐00631484; PUBMED: 18486724] [DOI] [PubMed] [Google Scholar]
- Barzegari M, Ghaninejad H, Shizarpoor M. Comparison of bath PUVA and acitretin in treatment of psoriatic patients. Iranian journal of dermatology 2004;7(4):31‐5. [CENTRAL: CN‐00509588] [Google Scholar]
- Batchelor JM, Ingram JR, Williams H. Adalimumab vs methotrexate for the treatment of chronic plaque psoriasis. Archives of Dermatology 2009;145(6):704‐6. [EMBASE: 2009300916] [DOI] [PubMed] [Google Scholar]
- Bayerl C. Treatment of psoriasis vulgaris with etretinate versus cyclosporin A. Report on a study. [Parallelgruppenvergleich Etretinat versus Cyclosporin A bei Psoriasis vulgaris]. Aktuelle Dermatologie 1992;18(1‐2):27‐31. [CENTRAL: CN‐00197510; EMBASE: 1992096910] [Google Scholar]
- Beissert S, Pauser S, Sticherling M, Frieling U, Loske KD, Frosch PJ, et al. A comparison of mycophenolate mofetil with ciclosporine for the treatment of chronic plaque‐type psoriasis. Dermatology (Basel, Switzerland) 2009;219(2):126‐32. [CENTRAL: CN‐00729115; PUBMED: 19546522] [DOI] [PubMed] [Google Scholar]
- Berbis P, Geiger JM, Vaisse C, Rognin C, Privat Y. Benefit of progressively increasing doses during the initial treatment with acitretin in psoriasis. Dermatologica 1989;178(2):88‐92. [CENTRAL: CN‐00058682; PUBMED: 2522405] [DOI] [PubMed] [Google Scholar]
- Bhuiyan MSI, Sikder MdA, Rashid MM, Rabin F. Role of oral colchicine in plaque type psoriasis. A randomized clinical trial comparing with oral methotrexate. Journal of Pakistan Association of Dermatologists 2010;20(3):146‐51. [CENTRAL: CN‐00789712; EMBASE: 2010613015] [Google Scholar]
- Bigby M. A randomized controlled trial of methotrexate and cyclosporine in the treatment of psoriasis. Archives of Dermatology 2004;140(3):347‐8. [CENTRAL: CN‐00515754; EMBASE: 2004120959] [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Papp K, Poulin Y, Lauzon G, Aspeslet L, Huizinga R, et al. A randomized, multicenter, double‐blind, placebo‐controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. Journal of the American Academy of Dermatology 2006;54(3):472‐8. [CENTRAL: CN‐00555245; PUBMED: 16488299] [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Papp K, Maari C, Yao Y, Robbie G, White WI, et al. A randomized, double‐blind, placebo‐controlled, phase i study of medi‐545, an anti‐interferon‐alfa monoclonal antibody, in subjects with chronic psoriasis. Journal of the American Academy of Dermatology 2010;62(3):427‐36. [CENTRAL: CN‐00734807; PUBMED: 20159310] [DOI] [PubMed] [Google Scholar]
- Bjerke JR, Geiger JM. Acitretin versus etretinate in severe psoriasis. A double‐blind randomized Nordic multicenter study in 168 patients. Acta Dermato‐Venereologica. Supplementum 1989;146:206‐7. [CENTRAL: CN‐00064911; PUBMED: 2532847] [PubMed] [Google Scholar]
- Callis Duffin K, Bagel J, Bukhalo M, Mercado Clement IJ, Choi SL, Zhao F, et al. Phase 3, open‐label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate‐to‐severe plaque psoriasis (UNCOVER‐A). Journal of the European Academy of Dermatology and Venereology : JEADV 2017;31(1):107‐113. [CENTRAL: CN‐01368719; PUBMED: 27500949] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano N, Loconsole F, Galluccio A, Miracapillo A, Pezza M, Vena GA. Once‐weekly administration of high‐dosage Etanercept in patients with plaque psoriasis: results of a pilot experience (power study). International Journal of Immunopathology and Pharmacology 2006;19(1):225‐9. [CENTRAL: CN‐00563725] [PubMed] [Google Scholar]
- Cassano N, Loconsole F, Miracapillo A, Travaglini M, Digiuseppe MD, Congedo M, et al. Treatment of psoriasis with different dosage regimens of etanercept: preliminary results from the Talpharanta Plastic Study Group. International Journal of Immunopathology and Pharmacology 2010;23(3):797‐802. [PUBMED: 20943050] [DOI] [PubMed] [Google Scholar]
- Cather J, Krueger G, Jackson M, Samstov A. Efficacy and safety of low‐dose acitretin for the treatment of moderate to severe plaque‐type psoriasis. Abstract P2877. American Academy of Dermatology 64th Annual Meeting March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB217. [CENTRAL: CN‐00602420] [Google Scholar]
- Chládek J, Grim J, Martínková J, Simková M, Vanìèková J, Koudelková V, et al. Pharmacokinetics and pharmacodynamics of low‐dose methotrexate in the treatment of psoriasis. British Journal of Clinical Pharmacology 2002;54(2):147‐56. [CENTRAL: CN‐00409768] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodorowska G, Czelej D, Juszkiewicz‐Borowiec M, Pietrzak A, Wojnowska D, Krasowska D. Selected cytokines and acute phase proteins in psoriatic patients treated with cyclosporin A or Re‐PUVA methods. Annales Universitatis Mariae Curie‐Sklodowska. Section D: Medicina 1999;54:173‐80. [CENTRAL: CN‐00325819] [PubMed] [Google Scholar]
- Chodorowska G, et al. Plasma levels of selected cytokines and acute phase proteins in 2 groups of psoriatic patients treated with cyclosporine A or RE‐PUVA method. Abstract P‐662. The 8th Congress of the European Academy of Dermatology and Venereology. Amsterdam, The Netherlands 29th Sept‐3rd October 1999. Journal of the European Academy of Dermatology and Venereology : JEADV 1999;12(Suppl 2):S330. [CENTRAL: CN‐00478492] [Google Scholar]
- Jong EM, Mork NJ, Seijger MM, Brassine M, Lauharanta J, Jansen CT, et al. The combination of calcipotriol and methotrexate compared with methotrexate and vehicle in psoriasis: results of a multicentre placebo‐controlled randomized trial. British Journal of Dermatology 2003;148(2):318‐25. [CENTRAL: CN‐00422725] [DOI] [PubMed] [Google Scholar]
- Dubiel W, Happle R. Experimental treatment with fumaric acid monoethylester in psoriasis vulgaris [Behandlungsversuch mit Fumarsauremonoathylester bei Psoriasis vulgaris:]. Zeitschrift fur Haut‐ und Geschlechtskrankheiten 1972;47(13):545‐50. [PUBMED: 4265800] [PubMed] [Google Scholar]
- Duffin KC, Bagel J, Bukhalo M, Clement IJM, Zhao F, Gill A, et al. Comparison of the pharmacokinetics of ixekizumab following subcutaneous administration using a prefilled syringe versus an autoinjector in patients with moderate‐to‐severe psoriasis. Journal of the American Academy of Dermatology 2016;74(5 Suppl 1):AB242. [EMBASE: 72275926] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker‐Schlipf B. Psoriasis vulgaris: How effective and safe is the calcineurin inhibitor voclosporin? [Psoriasis vulgaris: Wie wirksam und sicher ist der calcineurin‐ inhibitor voclosporin?]. Arzneimitteltherapie 2009;27(3):97‐8. [EMBASE: 2009143373] [Google Scholar]
- Elewski B, Leonardi C, Gottlieb AB, Strober BE, Simiens MA, Dunn M, et al. Comparison of clinical and pharmacokinetic profiles of etanercept 25 mg twice weekly and 50 mg once weekly in patients with psoriasis. British Journal of Dermatology 2007;156(1):138‐42. [CENTRAL: CN‐00577519] [DOI] [PubMed] [Google Scholar]
- Ellis CN, Gorsulowsky DC, Hamilton TA, Billings JK, Brown MD, Headington JT, et al. Cyclosporine improves psoriasis in a double‐blind study. JAMA 1986;256(22):3110‐6. [CENTRAL: CN‐00045553] [PubMed] [Google Scholar]
- Ellis CN, Reiter KL, Bandekar RR, Fendrick AM. Cost‐effectiveness comparison of therapy for psoriasis with a methotrexate‐based regimen versus a rotation regimen of modified cyclosporine and methotrexate. Journal of the American Academy of Dermatology 2002;46(2):242‐50. [PUBMED: 11807436] [DOI] [PubMed] [Google Scholar]
- Chow C, Zhang Z, Goldfarb MT, Simpson MJ, Ellis CN. Evaluation of Psoriasis Area and Severity Index, Static Physician's Global Assessment, and Lattice System ‐ Physician's Global Assessment for assessing severity of psoriasis. Journal of the American Academy of Dermatology 2012;131(Suppl 1):S81. [CENTRAL: CN‐00843715] [Google Scholar]
- Engst R, Huber J. Results of cyclosporin treatment of severe, chronical psoriasis vulgaris. Hautarzt 1989;40(8):486‐9. [EMBASE: 1989202264] [PubMed] [Google Scholar]
- Erkko P, Granlund H, Nuutinen M, Reitamo S. Comparison of cyclosporin A pharmacokinetics of a new microemulsion formulation and standard oral preparation in patients with psoriasis. British Journal of Dermatology 1997;136(1):82‐8. [PUBMED: 9039300] [PubMed] [Google Scholar]
- Ezquerra GM, Regana MS, Millet PU. Combination of acitretin and oral calcitriol for treatment of plaque‐type psoriasis. Acta Dermato‐Venereologica 2007;87(5):449‐50. [CENTRAL: CN‐00619032] [DOI] [PubMed] [Google Scholar]
- Fernandes IC, Torres T, Selores M. Maintenance treatment of psoriasis with cyclosporine A: comparison between continuous and weekend therapy. Journal of the American Academy of Dermatology 2013;68(2):341‐2. [CENTRAL: CN‐00841435] [DOI] [PubMed] [Google Scholar]
- Italian Multicenter Study Group on Cyclosporin in Psoriasis. Cyclosporin versus etretinate: Italian multicenter comparative trial in severe plaque‐form psoriasis.. Dermatology (Basel, Switzerland) 1993;187(Suppl 1):8‐18. [CENTRAL: CN‐00095652] [DOI] [PubMed] [Google Scholar]
- Fleischer AB, Carroll C, Hartle JE, Krejci‐Manwaring J, McCarty MA, Feldman SR. A randomized, double‐blind, right/left comparative study of the efficacy of acitretin with and without the co‐administration of 0.1 percent tacrolimus ointment in the treatment of moderate to severe psoriasis. Journal of Investigative Dermatology 2005;124(4 Suppl):A46. [CENTRAL: CN‐00550944] [Google Scholar]
- Fredriksson T. Antipsoriatic activity of retinoic acid (vitamin A acid). Dermatologica 1971;142(3):133‐6. [CENTRAL: CN‐00006362] [DOI] [PubMed] [Google Scholar]
- Fredriksson T, Pettersson U. Severe psoriasis‐‐oral therapy with a new retinoid. Dermatologica 1978;157(4):238‐44. [CENTRAL: CN‐00382865] [DOI] [PubMed] [Google Scholar]
- Friedrich M, Sterry W, Klein A, Ruckert R, Docke WD, Asadullah K. Addition of pentoxifylline could reduce the side effects of fumaric acid esters in the treatment of psoriasis. Acta Dermato‐Venereologica 2001;81(6):429‐30. [CENTRAL: CN‐00388555] [DOI] [PubMed] [Google Scholar]
- Gambichler T, Tigges C, Scola N, Weber J, Skrygan M, Bechara FG, et al. Etanercept plus narrowband ultraviolet b phototherapy of psoriasis is more effective than etanercept monotherapy at 6 weeks. British Journal of Dermatology 2011;164(6):1383‐6. [CENTRAL: CN‐00812147] [DOI] [PubMed] [Google Scholar]
- Ganguly R, et al. Etanercept therapy provides clinically meaningful improvement in dermatology quality of life index in patients with chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology: JEADV 2004;18(6):807. [CENTRAL: CN‐00550919] [Google Scholar]
- Gil JM, Sanchez‐Regana M, PaIazon DB, Cuchillero RO, Ezquerra GM, Millet PU. Association between calcitriol per os and acitretinoin in the treatment of psoriasis. Journal of the European Academy of Dermatology and Venereology: JEADV 2003;17(Suppl 3):383. [CENTRAL: CN‐00478546]12834446 [Google Scholar]
- Goerz G, Orfanos CE. Systemic treatment of psoriasis with a new aromatic retinoid. Preliminary evaluation of a multicenter controlled study in the Federal Republic of Germany. Dermatologica 1978;157(Suppl 1):38‐44. [PUBMED: 357217] [PubMed] [Google Scholar]
- Gollnick H, Bauer R, Brindley C, Orfanos CE, Plewig G, Wokalek H, et al. Acitretin versus etretinate in psoriasis. Clinical and pharmacokinetic results of a German multicenter study. Journal of the American Academy of Dermatology 1988;19(3):458‐68. [CENTRAL: CN‐00055942] [DOI] [PubMed] [Google Scholar]
- Gollnick HPM, Zaun H, Ruzicka T, Sommerburg C, Loew S, Mahrle G, et al. Relapse rate of severe generalized psoriasis after treatment with acitretin or etretinate. Results of the first randomized double‐blind multicenter half‐year follow‐up study. European Journal of Dermatology 1993;3(6):442‐6. [CENTRAL: CN‐00181049] [Google Scholar]
- Gollnick H, Altmeyer P, Kaufmann R, Ring J, Christophers E, Pavel S, et al. Topical calcipotriol plus oral fumaric acid is more effective and faster acting than oral fumaric acid monotherapy in the treatment of severe chronic plaque psoriasis vulgaris. Dermatology (Basel, Switzerland) 2002;205(1):46‐53. [CENTRAL: CN‐00397743] [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Vaishnaw K, Rizova E. Alefacept (AMEVIVETM) Does Not Blunt Primary or Secondary Immune Responses. Journal of Investigative Dermatology 2002;118(6):1098. [CENTRAL: CN‐00790440] [Google Scholar]
- Gottlieb AB, Casale TB, Frankel E, Goffe B, Lowe N, Ochs HD, et al. CD4+ T‐cell‐directed antibody responses are maintained in patients with psoriasis receiving alefacept: results of a randomized study. Journal of the American Academy of Dermatology 2003;49(5):816‐25. [CENTRAL: CN‐00474727] [DOI] [PubMed] [Google Scholar]
- Gottlieb B, Goffe B, Veith J, Stevens S, Nakanishi A. Safety of etanercept in an integrated multistudy database of patients with psoriasis. Journal of Investigative Dermatology 2004;122(3):A55. [CENTRAL: CN‐00509648] [Google Scholar]
- Gottlieb AB, Griffiths CEM, Ho VC, Lahfa M, Mrowietz U, Murrell DF, et al. Oral pimecrolimus in the treatment of moderate to severe chronic plaque‐type psoriasis: A double‐blind, multicentre, randomized, dose‐finding trial. British Journal of Dermatology 2005;152(6):1219‐27. [CENTRAL: CN‐00522446] [DOI] [PubMed] [Google Scholar]
- Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, Fretzin S, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: Randomised, double‐blind, placebo‐controlled, crossover trial. Lancet 2009;373:633‐40. [DOI] [PubMed] [Google Scholar]
- Goupille P, Valat JP. Is methotrexate really effective in patients with psoriatic arthritis?. Journal of Rheumatology 1995;22(12):2369‐70. [PUBMED: 8965271] [PubMed] [Google Scholar]
- Griffiths CM, Boffa M, Wishart J, Adam C, Inglesias L, Kerkhof P, et al. A double‐blind, randomised trial to compare the effects of oral liarozole with acitretin in the treatment of chronic plaque psoriasis. British Journal of Dermatology 1998;139(Suppl 51):19. [Google Scholar]
- Griffiths CEM, Humbert P, Koo J, Ortonne JP, Christophers E. Relationship between clinical response and quality of life in psoriasis patients treated with alefacept. Journal of the European Academy of Dermatology and Venereology : JEADV 2002;16:292. [CENTRAL: CN‐00478562]12195579 [Google Scholar]
- Griffiths CEM, Ortonne JP, Christophers E. Effect of alefacept based on patients' response to prior therapy for psoriasis. British Journal of Dermatology 2002;147(Suppl 62):45. [CENTRAL: CN‐00406976] [Google Scholar]
- Griffiths CEM. A higher treatment standard for patients with moderate to severe psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2005;19(Suppl 2):7. [CENTRAL: CN‐00602410]16274405 [Google Scholar]
- Grim J, Chladek J, Martinkova J, Simkova M, Vaneckova J, Koudelkova V. Pharmacokinetics (PK) and pharmacodynamics (PD) of low dose methotrexate (LDMTX) in the treatment of psoriasis. British Journal of Clinical Pharmacology 2000;50(4):390‐1. [EMBASE: 2000362454] [Google Scholar]
- Grossman RM, Thivolet J, Claudy A, Souteyrand P, Guilhou JJ, Thomas P, et al. A novel therapeutic approach to psoriasis with combination calcipotriol ointment and very low‐dose cyclosporine: results of a multicenter placebo‐controlled study. Journal of the American Academy of Dermatology 1994;31(1):68‐74. [CENTRAL: CN‐00102638] [DOI] [PubMed] [Google Scholar]
- Gulliver WP, Murphy GF, Hannaford VA, Primmett DRN. Increased bioavailability and improved efficacy, in severe psoriasis, of a new microemulsion formulation of cyclosporin. British Journal of Dermatology, Supplement 1996;135(48):35‐9. [EMBASE: 1996272533] [DOI] [PubMed] [Google Scholar]
- Gupta SK, Dogra A, Kaur G. Comparative efficacy of methotrexate and hydroxyurea in treatment of psoriasis. Journal of Pakistan Association of Dermatologists 2005;15(3):247‐51. [CENTRAL: 2005580293] [Google Scholar]
- Gupta R, Gupta S. Methotrexate‐betamethasone weekly oral pulse in psoriasis. Journal of Dermatological Treatment 2007;18(5):291‐4. [CENTRAL: CN‐00619338] [DOI] [PubMed] [Google Scholar]
- Gupta AK, Langley RG, Lynde C, Barber K, Gulliver W, Lauzon G, et al. ISA247: quality of life results from a phase II, randomized, placebo‐controlled study. Journal of Cutaneous Medicine and Surgery 2008;12(6):268‐75. [CENTRAL: CN‐00683900] [DOI] [PubMed] [Google Scholar]
- Han C, Kavanaugh A, Genovese MC, Hsu B, Deodhar AA, Hsia EC. Sustained improvement in health‐related quality of life, work productivity, employability, and reduced healthcare resource utilization of patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis treated with golimumab: 5‐year results from 3 phase iii studies. Arthritis and Rheumatism 2013;65:S137. [CENTRAL: CN‐01062782] [Google Scholar]
- Hashizume H, Ito T, Yagi H, Takigawa M, Kageyama H, Furukawa F, et al. Efficacy and safety of preprandial versus postprandial administration of low‐dose cyclosporin microemulsion (Neoral) in patients with psoriasis vulgaris. Journal of Dermatology 2007;34(7):430‐4. [CENTRAL: CN‐00610280] [DOI] [PubMed] [Google Scholar]
- Heule F, Meinardi MM, vanJoost T, Bos JD. Low‐dose cyclosporine effective in severe psoriasis: a double‐blind study. Transplantation Proceedings 1988;20(3 Suppl 4):32‐41. [CENTRAL: CN‐00054273] [PubMed] [Google Scholar]
- Ho SG, Yeung CK, Chan HH. Methotrexate versus traditional Chinese medicine in psoriasis: a randomized, placebo‐controlled trial to determine efficacy, safety and quality of life. Clinical and Experimental Dermatology 2010;35(7):717‐22. [CENTRAL: CN‐00761451] [DOI] [PubMed] [Google Scholar]
- Hunter GA, Simmons IJ, Thomas BM. A clinical trial of hydroxyurea for psoriasis. Australasian Journal of Dermatology 1972;13(3):93‐9. [CENTRAL: CN‐00008809] [DOI] [PubMed] [Google Scholar]
- Iest J, Boer J. Combined treatment of psoriasis with acitretin and UVB phototherapy compared with acitretin alone and UVB alone. British Journal of Dermatology 1989;120(5):665‐70. [CENTRAL: CN‐00568569] [DOI] [PubMed] [Google Scholar]
- Kavanaugh A. The efficacy of ustekinumab on the articular and dermatologic manifestations of psoriatic arthritis. Current Rheumatology Reports 2009;11(4):233‐4. [CENTRAL: CN‐00958869] [DOI] [PubMed] [Google Scholar]
- Kimball AB, Gordon KB, Langley RG, Menter A, Chartash EK, Valdes J, et al. Safety and efficacy of ABT‐874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo‐controlled, phase 2 trial. Archives of Dermatology 2008;144(2):200‐7. [CENTRAL: CN‐00630180] [DOI] [PubMed] [Google Scholar]
- Kimball AB, Gordon KB, Langley RG, Menter A, Perdok RJ, Valdes J. Efficacy and safety of ABT‐874, a monoclonal anti‐interleukin 12/23 antibody, for the treatment of chronic plaque psoriasis: 36‐week observation/retreatment and 60‐week open‐label extension phases of a randomized phase II trial. Journal of the American Academy of Dermatology 2011;64(2):263‐74. [CENTRAL: CN‐00770794] [DOI] [PubMed] [Google Scholar]
- Koo J. A randomized, double‐blind study comparing the efficacy, safety and optimal dose of two formulations of cyclosporin, Neoral and Sandimmun, in patients with severe psoriasis. OLP302 Study Group. British Journal of Dermatology 1998;139(1):88‐95. [CENTRAL: CN‐00155452] [DOI] [PubMed] [Google Scholar]
- Kopp T, Riedl E, Bangert C, Bowman EP, Greisenegger E, Horowitz A, et al. Clinical improvement in psoriasis with specific targeting of interleukin‐23. Nature2015; Vol. 521, issue 7551:222‐6. [CENTRAL: CN‐01074739] [DOI] [PubMed]
- Kragballe K, Jansén CT, Geiger JM, Bjerke JR, Falk ES, Gip L, et al. A double‐blind comparison of acitretin and etretinate in the treatment of severe psoriasis. Results of a Nordic multicentre study. Acta Dermato‐Venereologica 1989;69(1):35‐40. [CENTRAL: CN‐00057941] [PubMed] [Google Scholar]
- Krishnan KR, Cella D, Woolley M, Lalla D, Zitnik R, Brajac D. Etanercept improves symptoms of depression and fatigue in patients with psoriasis. 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26; Atlanta, GA. 2005; Vol. 158:NR293. [CENTRAL: CN‐00595903] [Google Scholar]
- Krueger GG, Shelby NJ, Hansen CD, Taylor MB. Comparison of labelling indices of skin involved and uninvolved with psoriasis: Placebo and oral retinoid RO 10‐9359 vs. time. Clinical Research 1980;28(1):21A. [CENTRAL: CN‐00192437] [Google Scholar]
- Krueger G, Vaishnaw A, Rizova E. Pharmacodynamic effects of IM or IV Alefacept: Selective reductions in memory‐ effector (CD45RO+) cells are related to clinical improvement in psoriasis. Journal of Investigative Dermatology 2002;118(6):1098. [CENTRAL: CN‐00795004] [Google Scholar]
- Krueger GG, Gordon KB, Kerkhof P, Sterry W. Repeated courses of IM alefacept in psoriasis: rationale and design of an international study that mimics the clinical practice setting. Journal of Investigative Dermatology 2003;121(2):57. [CENTRAL: CN‐00550788] [Google Scholar]
- Krueger JG, Fretzin S, Suarez‐Farinas M, Haslett PA, Phipps KM, Cameron GS, et al. IL‐17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. Journal of Allergy and Clinical Immunology 2012;130(1):145‐54.e9. [CENTRAL: CN‐00832721; PUBMED: 22677045] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JG, Ferris LK, Menter A, Wagner F, White A, Visvanathan S, et al. Anti‐IL‐23A mAb BI 655066 for treatment of moderate‐to‐severe psoriasis: Safety, efficacy, pharmacokinetics, and biomarker results of a single‐rising‐dose, randomized, double‐blind, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 2015;136(1):116‐124.e7. [CENTRAL: CN‐01110170; PUBMED: 25769911] [DOI] [PubMed] [Google Scholar]
- Kuijpers AL, Pelt JP, Bergers M, Boegheim PJ, Bakker JE, Siegenthaler G, et al. The effects of oral liarozole on epidermal proliferation and differentiation in severe plaque psoriasis are comparable with those of acitretin. British Journal of Dermatology 1998;139(3):380‐9. [CENTRAL: CN‐00159672] [DOI] [PubMed] [Google Scholar]
- Lajevardi V, Hallaji Z, Daklan S, Abedini R, Goodarzi A, Abdolreza M. The efficacy of methotrexate plus pioglitazone vs. methotrexate alone in the management of patients with plaque‐type psoriasis: a single‐blinded randomized controlled trial. International Journal of Dermatology 2015;54(1):95‐101. [CENTRAL: CN‐01052011] [DOI] [PubMed] [Google Scholar]
- Langewouters AMG, Bovenschen HJ, Jong EMGJ, Erp PJM, Kerkhof PCM. The effect of topical corticosteroids in combination with alefacept on circulating T‐cell subsets in psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2005;19(Suppl 2):240. [CENTRAL: CN‐00602421]15752302 [Google Scholar]
- Langley R, Leondardi C, Okun M. Long‐term safety and efficacy of adalimumab in psoriasis. 4th European Association of Dermatology & Venereology (EADV) Spring Symposium Saariselka, Lapland, Finland. February 9‐12th, 2006. 2006; Vol. Suppl:P‐021. [CENTRAL: CN‐00602234] [Google Scholar]
- Langley RG, Papp K, Bissonnette R, Toth D, Matheson R, Hultquist M, et al. Safety profile of intravenous and subcutaneous siplizumab, an anti‐CD2 monoclonal antibody, for the treatment of plaque psoriasis: results of two randomized, double‐blind, placebo‐controlled studies. International Journal of Dermatology 2010;49(7):818‐28. [CENTRAL: CN‐00761682] [DOI] [PubMed] [Google Scholar]
- Langner A, Roszkiewicz J, Baran E, Placek W. Results of a phase II study of a novel oral fumarate, BG‐12, in the treatment of severe psoriasis.. Journal of the European Academy of Dermatology and Venereology : JEADV 2004;18(6):798. [CENTRAL: CN‐00550917] [Google Scholar]
- Lauharanta J, Geiger JM. A double‐blind comparison of acitretin and etretinate in combination with bath PUVA in the treatment of extensive psoriasis. British Journal of Dermatology 1989;121(1):107‐12. [CENTRAL: CN‐00061540] [DOI] [PubMed] [Google Scholar]
- Lawrence CM, Marks J, Shuster S. Addition of retinoids to PUVA for psoriasis. Lancet 1983;1(8326 Pt 1):706. [CENTRAL: CN‐00030597] [DOI] [PubMed] [Google Scholar]
- Leavell UW, Yarbro JW. Hydroxyurea. A new treatment for psoriasis. Archives of Dermatology 1970;102(2):144‐50. [CENTRAL: CN‐00004668] [DOI] [PubMed] [Google Scholar]
- Lebwohl M. The effect of psoriasis and its treatments on circulating T‐cell subsets: results of alefacept studies. Journal of the European Academy of Dermatology and Venereology : JEADV 2003;17(Suppl 3):377. [CENTRAL: CN‐00478640]12834444 [Google Scholar]
- Lebwohl M, Kimball A, Gordon K, Szapary P. Comparable efficacy and safety of ustekinumab in moderate to severe psoriasis patients previously treated with systemic therapies and treatment‐naive patients. Journal of the American Academy of Dermatology. 2009; Vol. 67th Annual Meeting of the American Academy of Dermatology, AAD San Francisco, CA United States:Supplemental. [Google Scholar]
- Lebwohl MG, Kircik L, Callis Duffin K, Pariser D, Hooper M, Wenkert D, et al. Safety and efficacy of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. Dermatology and Therapy 2012;2:S39. [CENTRAL: CN‐01027857] [DOI] [PubMed] [Google Scholar]
- Lebwohl MG, Kircik L, Callis Duffin K, Pariser D, Hooper M, Wenkert D, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. Journal of the American Academy of Dermatology 2013;69(3):385‐92. [CENTRAL: CN‐00964028] [DOI] [PubMed] [Google Scholar]
- Ledo A, Martin M, Geiger JM, Marron JM. Acitretin (Ro 10‐1670) in the treatment of severe psoriasis. A randomized double‐blind parallel study comparing acitretin and etretinate. International Journal of Dermatology 1988;27(9):656‐60. [CENTRAL: CN‐00058382] [DOI] [PubMed] [Google Scholar]
- Legat LJ, Hofer A, Wackernagel A, Salmhofer W, Kerl H, Wolf P. Alefacept plus 311 nm narrowband ultraviolet B (NB‐UVB) phototherapy in the treatment of psoriasis. Journal of Investigative Dermatology 2005;125(1):A4. [CENTRAL: CN‐00550842] [Google Scholar]
- Leonardi C, Guenther L, Wasel N, Yeilding N, Szapary PO, Hsu MC, et al. Characterization of infections associated with ustekinumab in moderate to severe psoriasis patients. Journal of the European Academy of Dermatology and Venereology 2010;24:22. [EMBASE: 70238720]20050290 [Google Scholar]
- Leonardi C, Menter A, Gu Y, Okun M. Efficacy and safety of weekly adalimumab in psoriasis patients with a less than PASI 50 response to 40 mg every other week: Results from an open‐label extension study. Journal of the European Academy of Dermatology and Venereology 2010;24:9‐10. [EMBASE: 70238693] [Google Scholar]
- Leonardi C, Papp K, Asahina A, Gu Y, Rozzo S. Long‐term safety of adalimumab for psoriasis: An analysis of all adalimumab exposure in all global clinical trials. Journal of the European Academy of Dermatology and Venereology 2010;24:26. [CENTRAL: 70238729] [Google Scholar]
- Leonardi C, Langley RG, Papp K, Tyring SK, Wasel N, Vender R, et al. Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: efficacy and safety results from REACH, a randomized, placebo‐controlled, double‐blind trial. Archives of Dermatology 2011;147(4):429‐36. [CENTRAL: CN‐00785710] [DOI] [PubMed] [Google Scholar]
- Levell N J, Shuster S, Munro CS, Friedmann PS. Remission of ordinary psoriasis following a short clearance course of cyclosporin. Acta Dermato‐Venereologica 1995;75(1):65‐9. [CENTRAL: CN‐00113972] [DOI] [PubMed] [Google Scholar]
- Liang GS, Kerdel FA. Combination therapy and the use of an initial dose of intramuscular methotrexate in patients hospitalized for psoriasis. Journal of Dermatological Treatment 1995;6(2):73‐6. [CENTRAL: CN‐00171665] [Google Scholar]
- Lui H, Tan J, Shear N, Bissonnette R, Gulliver W. Efficacy and safety of alefacept in combination with narrowband uvb compared to alefacept alone in subjects with moderate to severe psoriasis: results of the Canadian alefacept phototherapy psoriasis study. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB150. [CENTRAL: CN‐00843846] [Google Scholar]
- Lui H, Gulliver W, Tan J, Hong CH, Hull P, Shear NH, et al. A randomized controlled study of combination therapy with alefacept and narrow band UVB phototherapy (UVB) for moderate to severe psoriasis: efficacy, onset, and duration of response. Journal of Drugs in Dermatology 2012;11(8):929‐37. [CENTRAL: CN‐01164684] [PubMed] [Google Scholar]
- Lynde CW, Gupta AK, Guenther L, Poulin Y, Levesque A, Bissonnette R. A randomized study comparing the combination of nbUVB and etanercept to etanercept monotherapy in patients with psoriasis who do not exhibit an excellent response after 12 weeks of etanercept. Journal of Dermatological Treatment 2012;23(4):261‐7. [PUBMED: 21797805] [DOI] [PubMed] [Google Scholar]
- Macdonald A, Fry L. Retinoic acid in the treatment of psoriasis. British Journal of Dermatology 1972;86(5):524‐7. [CENTRAL: CN‐00007340] [DOI] [PubMed] [Google Scholar]
- Mahrle G, Schulze HJ, Farber L, Weidinger G, Steigleder GK. Low‐dose short‐term cyclosporine versus etretinate in psoriasis: improvement of skin, nail, and joint involvement. Journal of the American Academy of Dermatology 1995;32(1):78‐88. [CENTRAL: CN‐00109143] [DOI] [PubMed] [Google Scholar]
- Malik T, Ejaz A. Comparison of methotrexate and azathioprine in the treatment of psoriasis: A randomized controlled trial. Journal of Pakistan Association of Dermatologists 2010;20(3):152‐7. [CENTRAL: CN‐00789615] [Google Scholar]
- Marecki S, Kirkpatrick P. Efalizumab. Nature Reviews. Drug Discovery 2004;3(6):473‐4. [PUBMED: 15214332] [DOI] [PubMed] [Google Scholar]
- Marks JM. Cyclosporin A treatment of severe psoriasis. British Journal of Dermatology 1986;115(6):745‐6. [PUBMED: 3542010] [DOI] [PubMed] [Google Scholar]
- McInnes IB, Papp K, Puig L, Reich K, Ritchlin CT, Strober B, et al. Safety of ustekinumab from the placebo‐controlled periods of psoriatic arthritis and psoriasis clinical developmental programs. Arthritis and Rheumatism 2013;72:Suppl. [CENTRAL: CN‐01058553] [Google Scholar]
- Mease P, Genovese MC, Gladstein G, Kivitz AJ, Ritchlin C, Tak PP, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six‐month, multicenter, randomized, double‐blind, placebo‐controlled, phase II trial. Arthritis and Rheumatism 2011;63(4):939‐48. [CENTRAL: CN‐00779390] [DOI] [PubMed] [Google Scholar]
- Meffert H, Sönnichsen N. Acitretin in the treatment of severe psoriasis: a randomized double‐blind study comparing acitretin and etretinate. Acta Dermato‐Venereologica. Supplementum 1989;146:176‐7. [CENTRAL: CN‐00064910] [PubMed] [Google Scholar]
- Menon S, Boy MG, Wang C, Wilkinson BE, Zwillich SH, Chan G, et al. Single and multiple‐dose pharmacokinetics of tofacitinib (CP‐690,550) from a double‐blind, placebo‐controlled, dose‐escalation study in medically stable subjects with psoriasis. Clinical Pharmacology and Therapeutics 2012;91:S33. [CENTRAL: CN‐01034861] [Google Scholar]
- Menter A, Guzzo C, Li S, Gottlieb AB. Efficacy of infliximab in patients with severe psoriasis: Subgroup analysis from clinical trials. Journal of the American Academy of Dermatology 2007;56(2):AB174. [CENTRAL: CN‐00615988] [Google Scholar]
- Menter A, Papp KA, Tan H, Tyring S, Wolk R, Buonanno M. Efficacy of tofacitinib, an oral janus kinase inhibitor, on clinical signs of moderate‐to‐severe plaque psoriasis in different body regions. Journal of Drugs in Dermatology 2014;13(3):252‐6. [CENTRAL: CN‐00985274] [PubMed] [Google Scholar]
- Meyer MW, Zachariae C, Bendtzen K, Skov L. Immunogenicity of tumour necrosis factor inhibitors in patients with psoriasis receiving long‐term treatment. British Journal of Dermatology 2011;165(6):e17. [EMBASE: 70610785] [Google Scholar]
- Mittal R, Malhotra S, Pandhi P, Kaur I, Dogra S. Efficacy and safety of combination Acitretin and Pioglitazone therapy in patients with moderate to severe chronic plaque‐type psoriasis: a randomized, double‐blind, placebo‐controlled clinical trial. Archives of Dermatology 2009;145(4):387‐93. [CENTRAL: CN‐00682023] [DOI] [PubMed] [Google Scholar]
- Moller I. Efficacy of leflunomide in patients with psoriatic arthritis [Eficacia del tratamiento con leflunomida en pacientes con artritis psoriasica]. Seminarios de la Fundacion Espanola de Reumatologia 2009;10(2):48‐52. [PUBMED: 2009314647] [Google Scholar]
- Monk BE. Cyclosporin A and psoriasis. British Journal of Dermatology 1986;115(2):249‐50. [PUBMED: 3741788] [DOI] [PubMed] [Google Scholar]
- Montgomery JA, Snyder HW Jr, Walsh DA, Walsh GM. BCX‐34. Purine nucleoside phosphorylase (PNP) inhibitor. Drugs of the Future 1993;18(10):887‐90. [EMBASE: 1994025323] [Google Scholar]
- Mrowietz U, Christophers E. Low‐dose ciclosporin A (Sandimmun) in psoriasis: A multicenter dose‐finding study. Zeitschrift fur Hautkrankheiten 1991;66(Suupl 1):25‐9. [CENTRAL: CN‐00182853] [Google Scholar]
- Mrowietz U, Reich K, Rozzo S, Gu Y. Achievement of European Consensus Programme treatment goals in three clinical trials of adalimumab in moderate‐to‐severe psoriasis. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB183. [EMBASE: 70704582] [Google Scholar]
- Narang T, Dogra S, Handa S. Serendipity opens new avenues: A pilot study to evaluate the efficacy of saxagliptin in combination with cyclosporine and acitretin in diabetic psoriasis patients. Dermatology and Therapy 2012;2:S36‐7. [CENTRAL: CN‐01027858] [Google Scholar]
- Nieboer C, Hoop D, Langendijk PN, Loenen AC, Gubbels J. Fumaric acid therapy in psoriasis: a double‐blind comparison between fumaric acid compound therapy and monotherapy with dimethylfumaric acid ester. Dermatologica 1990;181(1):33‐7. [CENTRAL: CN‐00351435] [DOI] [PubMed] [Google Scholar]
- Nijsten T, Spuls P, Stern RS. STROBE: a Beacon for observational studies. Archives of Dermatology 2008;144(9):1200‐4. [PUBMED: 18794467] [DOI] [PubMed] [Google Scholar]
- Noda S, Mizuno K, Adachi M. Treatment effect of adalimumab and infliximab in Japanese psoriasis patients: Results in a single community‐based hospital. Journal of Investigative Dermatology 2011;131:S38. [EMBASE: 70520994] [DOI] [PubMed] [Google Scholar]
- Novotny F. Use of methotrexate in psoriasis. Ceskoslovenska Dermatologie 1973;48(5):301‐5. [PUBMED: 4586960] [PubMed] [Google Scholar]
- Nyfors A. Benefits and adverse drug experiences during long‐term methotrexate treatment of 248 psoriatics. Danish Medical Bulletin 1978;25(5):208‐11. [PUBMED: 359259] [PubMed] [Google Scholar]
- Orfanos CE, Goerz G. Oral psoriasis treatment with a new aromatic retinoid (Ro 10‐9359): a multi‐centre controlled study of 291 patients (preliminary results). Deutsche Medizinische Wochenschrift 1978;103(5):195‐9. [CENTRAL: CN‐00017768] [DOI] [PubMed] [Google Scholar]
- Orfanos CE, Steigleder GK, Pullmann H, Bloch PH. Oral retinoid and UVB radiation: a new, alternative treatment for psoriasis on an out‐patient basis. Acta Dermato‐Venereologica 1979;59(3):241‐4. [CENTRAL: CN‐00020394] [PubMed] [Google Scholar]
- Ortonne JP, Griffiths CEM, Dauden E, Strohal R, Robertson D, Pedersen R, et al. Efficacy and safety of continuous versus paused etanercept teatment in patients with moderate‐to‐severe psoriasis over 54 weeks: the CRYSTEL Study. Expert Review of Dermatology 2008;3(6):657‐65. [CENTRAL: CN‐00754922] [Google Scholar]
- Ortonne JP, Chimenti S, Reich K, Gniadecki R, Sprøgel P, Unnebrink K, et al. Efficacy and safety of adalimumab in patients with psoriasis previously treated with anti‐tumour necrosis factor agents: subanalysis of BELIEVE. Journal of the European Academy of Dermatology and Venereology : JEADV 2011;25(9):1012‐20. [CENTRAL: CN‐00812830] [DOI] [PubMed] [Google Scholar]
- Osamu N, Hirotaka N, Koji S, Kenji T. Clinical pharmacology of the anti‐IL‐17 receptor antibody brodalumab (KHK4827) in Japanese normal healthy volunteers and Japanese subjects with moderate to severe psoriasis: A randomized, dose‐escalation, placebo‐controlled study. Journal of Dermatological Science 2014;75(3):201‐4. [CENTRAL: CN‐00999213] [DOI] [PubMed] [Google Scholar]
- Papp K, Bissonnette R, Krueger JG, Carey W, Gratton D, Gulliver WP, et al. The treatment of moderate to severe psoriasis with a new anti‐CD11a monoclonal antibody. Journal of the American Academy of Dermatology 2001;45(5):665‐74. [CENTRAL: CN‐00374574] [DOI] [PubMed] [Google Scholar]
- Papp K, Langley R, Bissonnette R, Rosoph L. A Phase III, randomized, multicenter, double‐blind, placebo‐controlled. Journal of the American Academy of Dermatology 2006;54:AB9. [DOI] [PubMed] [Google Scholar]
- Papp K, Bissonnette R, Rosoph L, Wasel N, Lynde CW, Searles G, et al. Efficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double‐blind, placebo‐controlled phase III study. Lancet 2008;371(9521):1337‐42. [CENTRAL: CN‐00631348] [DOI] [PubMed] [Google Scholar]
- Papp K, Okun M, Vender R. Adalimumab in the treatment of psoriasis: Pooled efficacy and safety results from three pivotal studies. Journal of Cutaneous Medicine and Surgery 2009;13(Suppl 2):S58‐66. [PUBMED: 19799828] [DOI] [PubMed] [Google Scholar]
- Papp K, Signorovitch J, Mulani P, Bao Y. Comparison of psoriasis sign and symptom reduction and complete clearance with adalimumab versus etanercept. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB153. [CENTRAL: CN‐00843873] [Google Scholar]
- Papp K, Signorovitch J, Sundaram M, Bao Y. Effects of abt‐874 treatment on health‐related quality of life and work productivity and activity impairment in patients with psoriasis. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB155. [CENTRAL: CN‐00843874] [Google Scholar]
- Papp K, Yu A, Sundaram M, Bao Y. Achieving long‐term sustained response is associated with improvements in patient‐reported outcomes in patients with psoriasis treated with abt‐874. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB160. [CENTRAL: CN‐00843875] [Google Scholar]
- Papp KA, Reid C, Foley P, Sinclair R, Salinger DH, Williams G, et al. Anti‐IL‐17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: results from a phase I, randomized, placebo‐controlled trial. Journal of Investigative Dermatology 2012;132(10):2466‐9. [CENTRAL: CN‐00854479] [DOI] [PubMed] [Google Scholar]
- Papp KA, Poulin Y, Bissonnette R, Bourcier M, Toth D, Rosoph L, et al. Assessment of the long‐term safety and effectiveness of etanercept for the treatment of psoriasis in an adult population. Journal of the American Academy of Dermatology 2012;66(2):e33‐45. [CENTRAL: CN‐00883092] [DOI] [PubMed] [Google Scholar]
- Park KK, Wu JJ, Koo J. A randomized, 'head‐to‐head' pilot study comparing the effects of etanercept monotherapy vs. etanercept and narrowband ultraviolet B (NB‐UVB) phototherapy in obese psoriasis patients. Journal of the European Academy of Dermatology and Venereology : JEADV 2013;27(7):899‐906. [CENTRAL: CN‐00969013] [DOI] [PubMed] [Google Scholar]
- Paul C, Kerkhof P, Puig L, Unnebrink K, Goldblum O, Thaçi D. Influence of psoriatic arthritis on the efficacy of adalimumab and on the treatment response of other markers of psoriasis burden: subanalysis of the BELIEVE study. European Journal of Dermatology 2012;22(6):762‐9. [CENTRAL: CN‐00966715] [DOI] [PubMed] [Google Scholar]
- Paul C, Puig L, Kragballe K, Luger T, Lambert J, Chimenti S, et al. Transition to ustekinumab in patients with moderate‐to‐severe psoriasis and inadequate response to methotrexate: A randomized clinical trial (TRANSIT). British Journal of Dermatology 2014;170(2):425‐34. [CENTRAL: CN‐00982375] [DOI] [PubMed] [Google Scholar]
- Pettit JH. Oral retinoid for psoriasis. A report of a double blind study. Acta Dermato‐Venereologica. Supplementum 1979;59(85):133‐6. [CENTRAL: CN‐00021931; MEDLINE: ] [PubMed] [Google Scholar]
- Petzelbauer P, Honigsmann H, Langer K, Anegg B, Strohal R, Tanew A, et al. Cyclosporin A in combination with photochemotherapy (PUVA) in the treatment of psoriasis. British Journal of Dermatology 1990;123(5):641‐7. [CENTRAL: CN‐00351600] [DOI] [PubMed] [Google Scholar]
- Piascik P. Alefacept, first biologic agent approved for treatment of psoriasis. Journal of the American Pharmacists Association 2003;43(5):649‐50. [PUBMED: 14626761] [DOI] [PubMed] [Google Scholar]
- Ports WC, Khan S, Lan S, Lamba M, Bolduc C, Bissonnette R, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. British Journal of Dermatology 2013;169(1):137‐45. [CENTRAL: CN‐00920320] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punwani N, Scherle P, Flores R, Shi J, Liang J, Yeleswaram S, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. Journal of the American Academy of Dermatology 2012;67(4):658‐64. [CENTRAL: CN‐00881387] [DOI] [PubMed] [Google Scholar]
- Rabasseda X. A report from the American Academy of Dermatology 70th Annual Meeting (March 16‐20, 2012 ‐ San Diego, California, USA). Drugs of Today 2012;48(5):367‐73. [PUBMED: 22645724] [DOI] [PubMed] [Google Scholar]
- Radmanesh M, Rafiei B, Moosavi ZB, Sina N. Weekly vs. daily administration of oral methotrexate (MTX) for generalized plaque psoriasis: a randomized controlled clinical trial. International Journal of Dermatology 2011;50(10):1291‐3. [CENTRAL: CN‐00805615] [DOI] [PubMed] [Google Scholar]
- Raman GV, Campbell SK, Farrer A, Albano JD, Cook J. Modifying effects of amlodipine on cyclosporin A‐induced changes in renal function in patients with psoriasis. Journal of Hypertension. Supplement 1998;16(4):S39‐41. [CENTRAL: CN‐00308573] [PubMed] [Google Scholar]
- Reich K, Hoogstraten BJF, Wozel G, Zheng H, Flint L. Long‐term efficacy and safety of maintenance versus intermittent infliximab therapy for moderate to severe plaque‐type psoriasis: the restore2 trial. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB150. [CENTRAL: CN‐00843879] [Google Scholar]
- Reich K, Puig L, Paul C, Kragballe K, Luger T, Lambert J, et al. One‐year safety and efficacy of ustekinumab and results of dose adjustment after switching from inadequate methotrexate treatment: the TRANSIT randomized trial in moderate‐to‐severe plaque psoriasis. British Journal of Dermatology 2014;170(2):435‐44. [CENTRAL: CN‐00982376] [DOI] [PubMed] [Google Scholar]
- Reitamo S, Spuls P, Sassolas B, Lahfa M, Claudy A, Griffiths C, et al. A double‐blind study in patients with severe psoriasis to assess the clinical activity and safety of Rapamycin (sirolimus) alone or in association with a reduced dose of cyclosporine. British Journal of Dermatology 1999;141(5):978‐9. [CENTRAL: CN‐00428747] [Google Scholar]
- Reitamo S, Spuls P, Sassolas B, Lahfa M, Claudy A, Griffiths CE, et al. Efficacy of sirolimus (rapamycin) administered concomitantly with a subtherapeutic dose of cyclosporin in the treatment of severe psoriasis: a randomized controlled trial. British Journal of Dermatology 2001;145(3):438‐45. [CENTRAL: CN‐00356242] [DOI] [PubMed] [Google Scholar]
- Rim JH, Park JY, Choe YB, Youn JI. The efficacy of calcipotriol + acitretin combination therapy for psoriasis: comparison with acitretin monotherapy. American Journal of Clinical Dermatology 2003;4(7):507‐10. [CENTRAL: CN‐00450180] [DOI] [PubMed] [Google Scholar]
- Clinical Study Group for Ciclosporin. Clinical efficacy of ciclosporin in the treatment of psoriasis: multicenter double blind study. Rincho Iyaku (Journal of Clinical Therapeutics and Medicines) 1991;7(3):617‐33. [CENTRAL: CN‐00545330] [Google Scholar]
- Ritchlin C. Efficacy and safety of infliximab for the treatment of psoriatic arthritis. Nature Clinical Practice. Rheumatology 2006;2(6):300‐1. [CENTRAL: CN‐00567618] [DOI] [PubMed] [Google Scholar]
- Ritchlin CT. The efficacy and safety of adalimumab in psoriatic arthritis. Current Rheumatology Reports 2006;8(5):329. [CENTRAL: CN‐00898612] [DOI] [PubMed] [Google Scholar]
- Ritchlin CT. The efficacy and safety of alefacept in psoriatic arthritis. Current Rheumatology Reports 2006;8(5):330‐1. [CENTRAL: CN‐00898611] [DOI] [PubMed] [Google Scholar]
- Salim A, Tan E, Ilchyshyn A, Berth‐Jones J. Folic acid supplementation during treatment of psoriasis with methotrexate: a randomized, double‐blind, placebo‐controlled trial. British Journal of Dermatology 2006;154(6):1169‐74. [CENTRAL: CN‐00565411] [DOI] [PubMed] [Google Scholar]
- Scholl E. Treatment of psoriasis on an outpatient‐base using UVB‐radiations, oral retinoid and ten percent saline baths. Schweizerische Rundschau für Medizin Praxis 1981;70(41):1806‐16. [CENTRAL: CN‐00026632] [PubMed] [Google Scholar]
- Schopf RE, Hultsch T, Lotz J, Brautigam M. Eosinophils, pruritus and psoriasis: effects of treatment with etretinate or cyclosporin‐A. Journal of the European Academy of Dermatology and Venereology : JEADV 1998;11(3):234‐9. [PUBMED: 9883435] [PubMed] [Google Scholar]
- Schulze HJ. Comparative trial of Sandimmune and etretinate for plaque‐type psoriasis. Zeitschrift fur Hautkrankheiten 1991;66(Suppl 1):33‐8. [CENTRAL: CN‐00180765] [Google Scholar]
- Shintani Y, Kaneko N, Furuhashi T, Saito C, Morita A. Safety and efficacy of a fixed‐dose cyclosporin microemulsion (100 mg) for the treatment of psoriasis. Journal of Dermatology 2011;38(10):966‐72. [CENTRAL: CN‐00811861] [DOI] [PubMed] [Google Scholar]
- Shiohara T, Imanishi K, Sagawa Y, Nagashima M. Differential effects of cyclosporine and etretinate on serum cytokine levels in patients with psoriasis. Journal of the American Academy of Dermatology 1992;27(4):568‐74. [CENTRAL: CN‐00361111] [DOI] [PubMed] [Google Scholar]
- Shupack J, Abel E, Bauer E, Brown M, Drake L, Freinkel R, et al. Cyclosporine as maintenance therapy in patients with severe psoriasis. Journal of the American Academy of Dermatology 1997;36(3 pt 1):423‐32. [CENTRAL: CN‐00137758] [DOI] [PubMed] [Google Scholar]
- Simonova OV, Nemtsov BF. Psoriatic arthritis: combined treatment with prospidin and methotrexate. Terapevticheskii Arkhiv 2005;77(8):60‐4. [CENTRAL: CN‐00530903] [PubMed] [Google Scholar]
- Sofen H, Smith S, Matheson R, Leonardi C, Calderon C, Bouman‐Thio E, et al. Results of a single ascending dose study to assess the safety and tolerability of CNTO 1959 following intravenous or subcutaneous administration in healthy subjects and in subjects with moderate to severe psoriasis. British Journal of Dermatology 2011;165(6):e10. [CENTRAL: CN‐01020427] [Google Scholar]
- Sofen H, Smith S, Matheson RT, Leonardi CL, Calderon C, Brodmerkel C, et al. Guselkumab (an IL‐23‐specific mAb) demonstrates clinical and molecular response in patients with moderate‐to‐severe psoriasis. Journal of Allergy and Clinical Immunology 2014;133(4):1032‐40. [CENTRAL: CN‐00984656] [DOI] [PubMed] [Google Scholar]
- Spadaro A, Ceccarelli F, Scrivo R, Valesini G. Life‐table analysis of etanercept with or without methotrexate in patients with psoriatic arthritis. Annals of the Rheumatic Diseases 2008;67(11):1650‐1. [CENTRAL: CN‐00651403] [DOI] [PubMed] [Google Scholar]
- Spuls P I, Hooft L. Brodalumab and ixekizumab, anti‐interleukin‐17‐receptor antibodies for psoriasis: a critical appraisal. British Journal of Dermatology 2012;167(4):710‐3; discussion 714‐5. [PUBMED: 23013312] [DOI] [PubMed] [Google Scholar]
- Sticherling M. Symposium report: "Therapy of severe psoriasis with Sandimmune". Symposium of Nurnberg Sandoz AG 13 February 1993, Nurnberg. Hautarzt 1994;45(1):50‐2. [PUBMED: 8150621] [PubMed] [Google Scholar]
- Strober BE, Clarke S. Etanercept for the treatment of psoriasis: combination therapy with other modalities. Journal of Drugs in Dermatology 2004;3(3):270‐2. [PUBMED: 15176161] [PubMed] [Google Scholar]
- Strober BE, Sobell JM, Duffin KC, Bao Y, Guerin A, Yang H, et al. Sleep quality and other patient‐reported outcomes improve after patients with psoriasis with suboptimal response to other systemic therapies are switched to adalimumab: results from PROGRESS, an open‐label Phase IIIB trial. British Journal of Dermatology 2012;167(6):1374‐81. [PUBMED: 22897348] [DOI] [PubMed] [Google Scholar]
- Sweetser M, Ticho B, Swan S. Subcutaneous administration of alefacept is bioequivalent to intramuscular administration: Results of a randomized, open‐label, crossover study in healthy volunteers. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB224. [CENTRAL: CN‐00602513] [PubMed] [Google Scholar]
- Talwar S. Methotrexate‐puvasol combination in treatment of psoriasis. Indian Journal of Dermatology, Venereology and Leprology 1992;58(1):15‐9. [CENTRAL: CN‐00663131] [Google Scholar]
- Tejasvi T, Chow C, Simpson MJ, Ellis CN. Use of clinical trial data to compare psoriasis area and severity index, static physician's global assessment, and lattice system‐physician's global assessment in assessing severity of psoriasis. Dermatology and Therapy 2012;2:S55. [CENTRAL: 71025691] [Google Scholar]
- Thaçi D, Bräutigam M, Kaufmann R, Weidinger G, Paul C, Christophers E. Body‐weight‐independent dosing of cyclosporine micro‐emulsion and three times weekly maintenance regimen in severe psoriasis. A randomised study. Dermatology (Basel, Switzerland) 2002;205(4):383‐8. [CENTRAL: CN‐00411587] [DOI] [PubMed] [Google Scholar]
- Thaçi D, Ortonne JP, Chimenti S, Ghislain PD, Arenberger P, Kragballe K, et al. A phase IIIb, multicentre, randomized, double‐blind, vehicle‐controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. British Journal of Dermatology 2010;163(2):402‐11. [CENTRAL: CN‐00771848] [DOI] [PubMed] [Google Scholar]
- Tong PZ, Si RL. Effectiveness observation on acitretin capsule for plaque psoriasis. Modern Journal of Integrated Traditional Chinese and Western Medicine [xian Dai Zhong Xi Yi Jie He za Zhi] 2008;17(3):364‐5. [CENTRAL: CN‐00792764] [Google Scholar]
- Joost T, Bos JD, Heule F, Meinardi MM. Low‐dose cyclosporin A in severe psorasis. A double‐blind study. British Journal of Dermatology 1988;118(2):183‐90. [PUBMED: 3280000] [DOI] [PubMed] [Google Scholar]
- Vena GA, Cassano N, Galluccio A, Loconsole F, Coviello C, Fai D, et al. Evaluation of the efficacy and tolerability of a new intermittent treatment regimen with cyclosporin A in severe psoriasis. Giornale Italiano di Dermatologia e Venereologia 2005;140(5):575‐82. [EMBASE: 2006301575] [Google Scholar]
- Vena GA, Galluccio A, Pezza M, Vestita M, Cassano N. Combined treatment with low‐dose cyclosporine and calcipotriol/betamethasone dipropionate ointment for moderate‐to‐severe plaque psoriasis: a randomized controlled open‐label study. Journal of Dermatological Treatment 2012;23(4):255‐60. [CENTRAL: CN‐00882673] [DOI] [PubMed] [Google Scholar]
- Viglioglia PA, Barclay A. Oral retinoids and psoriasis. Dermatologica 1978;157(Suppl 1):32‐7. [PUBMED: 357216] [DOI] [PubMed] [Google Scholar]
- Witkamp L, Zonneveld IM, Jung EG, Schopf RE, Christophers E, Grossman R, et al. Efficacy and tolerability of multiple‐dose SDZ IMM 125 in patients with severe psoriasis. British Journal of Dermatology 1995;133(1):95‐103. [CENTRAL: CN‐00118050] [DOI] [PubMed] [Google Scholar]
- Wolf P, Weger W, Legat FJ, Posch‐Fabian T, Gruber‐Wackernagel A, Inzinger M, et al. Treatment with 311‐nm ultraviolet B enhanced response of psoriatic lesions in ustekinumab‐treated patients: a randomized intraindividual trial. British Journal of Dermatology 2012;166(1):147‐53. [CENTRAL: CN‐00841290] [DOI] [PubMed] [Google Scholar]
- Wright ET, Wolborsky M, Hamer EE. Human low‐dosage parenteral methotrexate therapy. A controlled toxicity study. Archives of Dermatology 1966;93(6):731‐6. [PUBMED: 4222659] [PubMed] [Google Scholar]
- Wu C, Jin HZ, Shu D, Li F, He CX, Qiao J, et al. Efficacy and Safety of Tripterygium wilfordii Hook F Versus Acitretin in Moderate to Severe Psoriasis Vulgaris: A Randomized Clinical Trial. Chinese Medical Journal 2015;128(4):443‐9. [CENTRAL: CN‐01047537] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesudian PD, Hashim N, Bharati A, Alkali A, Warren RB, Cox T, et al. A prospective, double‐blind, randomized controlled trial of folic acid supplementation vs. Placebo in patients with chronic plaque psoriasis treated with methotrexate and effects on serum homocysteine. British Journal of Dermatology 2013;169(Suppl 1):59. [CENTRAL: CN‐00873113] [Google Scholar]
- Yoon HS, Youn JI. A comparison of two cyclosporine dosage regimens for the treatment of severe psoriasis. Journal of Dermatological Treatment 2007;18(5):286‐90. [CENTRAL: CN‐00619337] [DOI] [PubMed] [Google Scholar]
- Zachariae C, Mork NJ, Reunala T, Lorentzen H, Falk E, Karvonen SL, et al. The combination of etanercept and methotrexate increases the effectiveness of treatment in active psoriasis despite inadequate effect of methotrexate therapy. Acta Dermato‐Venereologica 2008;88(5):495‐501. [CENTRAL: CN‐00669633] [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhang Y‐Z, Wang S. The effect of acitretin on moderate to severe plaque psoriasis. Journal of Clinical Dermatology 2007;36(9):592‐3. [CENTRAL: CN‐00642111] [Google Scholar]
- Zhang GL, Huang F, Zhang JL, Li XF. A clinical study of leflunomide and methotrexate therapy in psoriatic arthritis. Chung‐Hua Nei Ko Tsa Chih (Chinese Journal of Internal Medicine) 2009;48(7):570‐4. [CENTRAL: CN‐00732533] [PubMed] [Google Scholar]
- Zhang LX, Bai YP, Song PH, You LP, Yang DQ. Effect of Chinese herbal medicine combined with acitretin capsule in treating psoriasis of blood‐heat syndrome type. Chinese Journal of Integrative Medicine 2009;15(2):141‐4. [CENTRAL: CN‐00700202] [DOI] [PubMed] [Google Scholar]
- Zhang GL, Huang F, Zhang JL, Li XF. A clinical study of leflunomide and methotrexate therapy in psoriatic arthritis. Zhonghua Nei Ke za Zhi [Chinese Journal of Internal Medicine] 2012;48(7):570‐4. [CENTRAL: CN‐00732533] [PubMed] [Google Scholar]
- Zhu Y, Hu C, Lu M, Liao S, Marini JC, Yohrling J, et al. Population pharmacokinetic modeling of ustekinumab, a human monoclonal antibody targeting IL‐12/23p40, in patients with moderate to severe plaque psoriasis. Journal of Clinical Pharmacology 2009;49(2):162‐75. [PUBMED: 19179295] [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Calderon C, Marciniak Jr SJ, et al. Pharmacokinetics and safety of guselkumab, an anti‐IL‐23 monoclonal antibody, in healthy subjects and patients with moderate to severe psoriasis in a first‐in‐human study. European Journal of Clinical Pharmacology2016; Vol. 72, issue 11:1303‐10. [DOI] [PubMed]
- Zobel AF. Cyclosporin is being tested for treatment of psoriasis. American Druggist 1987;195(3):102. [EMBASE: 1987106040] [Google Scholar]
References to studies awaiting assessment
- Chow C, Simpson MJ, Luger TA, Chubb H, Ellis CN. Comparison of three methods for measuring psoriasis severity in clinical studies (Part 1 of 2): Change during therapy in Psoriasis Area and Severity Index, Static Physician's Global Assessment and Lattice System Physician's Global Assessment. Journal of the European Academy of Dermatology and Venereology : JEADV 2015;29(7):1406‐14. [PUBMED: 25917315] [DOI] [PubMed] [Google Scholar]; Chow C, Simpson MJ, Zang Z, Goldfarb MT, Tejasvi T, Ellis CN. Longitudinal effects of active therapy in a clinical trial on Psoriasis Area and Severity Index, Static Physician's Global assessment and Lattice System‐ Physician's Global assessment for assessing severity of psoriasis. British Journal of Dermatology 2011;165(6):e30. [EMBASE: 70610815] [Google Scholar]; Simpson MJ, Chow C, Morgenstern H, Luger TA, Ellis CN. Comparison of three methods for measuring psoriasis severity in clinical studies (Part 2 of 2): use of quality of life to assess construct validity of the Lattice System Physician's Global Assessment, Psoriasis Area and Severity Index and Static Physician's Global Assessment. Journal of the European Academy of Dermatology and Venereology : JEADV 2015;29(7):1415‐20. [PUBMED: 25917214] [DOI] [PubMed] [Google Scholar]
- CTRI/2015/05/005830. Role of oral methotrexate, cyclosporine and acitretin in treatment of palmoplantar psoriasis (redcoloured, painful, itchy, fissured lesions on hands and feet) and psoriasis vulgaris (red coloured,scaly, itchy, elevated lesions on skin over body). ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=10246&EncHid=&modid=&compid=%27,%2710246det%27 Date first received: 29 May 2015.
- DRKS00000716. Regulatory T‐cell function in psoriasis vulgaris. drks‐neu.uniklinik‐freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00000716 Date first received: 2011/02/09.
- Elewski BE, Rich PA, Okun MM, Papp K, Baker CS, Crowley JJ, et al. Adalimumab for nail psoriasis: Efficacy and safety from the first 26 weeks of a Phase‐3, randomized, placebo‐controlled trial. Journal of the European Academy of Dermatology and Venereology: JEADV 2016;30:65. [EMBASE: 611235503] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Fang X, Huang Q, Yang QP, Fu WW, Zheng ZZ, et al. Analysis of the effect of recombinant human tumor necrosis factor receptor in the treatment of moderate to severe plaque psoriasis on PASI score. Journal of Clinical Dermatology 2007;36(11):730‐2. [CENTRAL: CN‐00708092] [Google Scholar]
- Khatri S, Amir Y, Min M, Goldblum O, Solotkin K, Yang F, et al. Early onset of clinical improvement with ixekizumab in patients with moderate‐to‐severe plaque psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2016;30:73‐4. [EMBASE: 611235569] [Google Scholar]
- Lee JH, Youn JI, Kim TY, Choi JH, Park CJ, Choe YB, et al. A multicenter, randomized, open‐label pilot trial assessing the efficacy and safety of etanercept 50 mg twice weekly followed by etanercept 25 mg twice weekly, the combination of etanercept 25 mg twice weekly and acitretin, and acitretin alone in patients with moderate to severe psoriasis. BMC Dermatology2016; Vol. 16, issue 1:11. [CENTRAL: CN‐01177229; PUBMED: 27455955] [DOI] [PMC free article] [PubMed]
- Mrowietz U, Spellman M. Dimethyl Fumarate (BG00012) as an oral therapy for moderate to severe psoriasis: results of a multicenter, randomized, double‐blind, placebo‐controlled trial. Journal of Investigative Dermatology 2005;125(3 Suppl):A69. [CENTRAL: CN‐00792615] [Google Scholar]; Mrowietz U, Spellman MC. Results of a phase III study of a novel oral formulation of dimethylfumarate in the treatment of moderate to severe plaque psoriasis: efficacy, safety, and quality of life effects. Journal of the European Academy of Dermatology and Venereology : JEADV 2005;19(Suppl 2):187. [CENTRAL: CN‐00602493]15752288 [Google Scholar]; Ortonne JP, Kerkhof P, Mrowietz U. A novel oral agent improves quality of life (QOL) in patients with plaque psoriasis. 4th European Association of Dermatology & Venereology (eadv) Spring Symposium Saariselka, Lapland, Finland. 2006; Vol. S:P‐013. [CENTRAL: CN‐00602214] [Google Scholar]
- NCT01961609. Secukinumab in TNF‐IR psoriasis patients. (SIGNATURE). clinicaltrials.gov/ct2/show/NCT01961609 Date first received: 10 October 2013.
- NCT01988103. Efficacy and safety study of two doses of Apremilast (CC‐10004) in Japanese subjects with moderate‐to‐severe plaque‐type psoriasis. clinicaltrials.gov/ct2/show/NCT01988103 Date first received: 24 May 2013.
- NCT02054481. BI 655066 dose ranging in psoriasis, active comparator ustekinumab. clinicaltrials.gov/ct2/show/NCT02054481 Date first received: 3 February 2014.
- NCT02248792. Quality of life of patients with psoriasis treated with methotrexate: prospective, randomized, double‐blind, parallel group study. clinicaltrials.gov/ct2/show/NCT02248792 Date first received: 22 September 2014.
- Reich K. Alefacept in the treatment of psoriasis for whom conventional therapies are ineffective or inappropriate. Journal of the European Academy of Dermatology and Venereology : JEADV 2004;18(6):808. [CENTRAL: CN‐00550795] [Google Scholar]; Sclessinger J, Pariser R, Park S, Wierz G. Evaluation of the efficacy and safety of alefacept in patients for whom conventional psoriasis therapies are ineffective or inappropriate. Journal of the American Academy of Dermatology 2007;56(2):AB192. [CENTRAL: CN‐00616055] [Google Scholar]
- Reich K, Sullivan J, Arenberger P, Mrowietz, U, Jazayeri, S, Augustin, M, et al. Secukinumab shows significant efficacy in nail psoriasis: Week 32 results from the transfigure study. Annals of the Rheumatic Diseases 2016;75:603‐4. [Google Scholar]
References to ongoing studies
- ChiCTR‐INR‐16009710. Acitretin plus methotrexate in the treatment of moderate to severe psoriasis vulgaris [The role of keratin 17 in the pathogenesis of psoriasis through PI3K/Akt signal pathways]. www.chictr.org.cn/showprojen.aspx?proj=16444/ChiCTR‐INR‐16009710 Date first received: 2 November 2016.
- CTRI/2016/10/007345. A Randomized, Double‐Blind, Placebo‐Controlled, Comparative, Prospective, Multicentre Trial to Assess Efficacy and Safety of Apremilast Tablets in Subjects with Moderate to Severe Plaque Psoriasis who are Candidates for Phototherapy or Systemic Therapy. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=16164&EncHid=&modid=&compid=%27,%2716164det%27/CTRI/2016/10/007345 Date first received: 20 October 2016.
- EUCTR2013‐004918‐18‐NL. Optimising adalimumab treatment in psoriasis with concomitant methotrexate ‐ OPTIMAP [Optimising adalimumab treatment in psoriasis with concomitant methotrexate]. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐004918‐18‐NL/EUCTR2013‐004918‐18‐NL Date first received: 12 December 2013.
- EUCTR2015‐002423‐26‐FI. Study of the efficacy of early intervention with secukinumab 300 mg s.c. compared to narrow‐band UVB in patients with new‐onset moderate to severe plaque psoriasis. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015‐002423‐26‐FI/EUCTR2015‐002423‐26‐FI Date first received: 21 October 2016.
- EUCTR2015‐003623‐65. BI 655066 (risankizumab) versus adalimumab in a randomised, double blind, parallel group trial in moderate to severe plaque psoriasis to assess safety and efficacy after 16 weeks of treatment and after inadequate adalimumab treatment response (IMMvent) ‐ BI 655066 (risankizumab) versus adalimumab. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number%3A2015‐003623‐65/EUCTR2015‐003623‐65 Date first received: 17 May 2016.
- NCT01553058. Vascular Inflammation in Psoriasis Trial (The VIP Trial) (VIP). clinicaltrials.gov/ct2/show/NCT01553058 Date first received; 14 February 2012.
- NCT01558310. A Study to evaluate the effectiveness of STELARA ™ (USTEKINUMAB) in the treatment of scalp psoriasis. clinicaltrials.gov/ct2/show/NCT01558310 Date first received: 13 March 2012.
- NCT01722331. A study to evaluate the efficacy and safety of subcutaneous MK‐3222, followed by an optional long‐term safety extension study, in participants with moderate‐to‐severe chronic plaque psoriasis (MK‐3222‐010). clinicaltrials.gov/ct2/show/NCT01722331 Date first received: 16 March 2017.
- NCT01729754. A study to evaluate the efficacy and safety/tolerability of subcutaneous tildrakizumab (SCH 900222/MK‐3222) in participants with moderate‐to‐severe chronic plaque psoriasis followed by a long‐term extension study (MK‐3222‐011). clinicaltrials.gov/ct2/show/NCT01729754 Date first received: 13 November 2012.
- NCT02203032. A study of guselkumab in participants with moderate to severe plaque‐type psoriasis and an Inadequate response to ustekinumab (NAVIGATE). clinicaltrials.gov/ct2/show/NCT02203032Date first received: October 2014.
- NCT02258282. Safety and Efficacy of Etanercept in Patients With Psoriasis. clinicaltrials.gov/ct2/show/NCT02258282Date first received: March 2014.
- NCT02313922. Optimizing Psoriasis Treatment of Etanercept Combined Methotrexate. clinicaltrials.gov/ct2/show/NCT02313922 Date first received: 8 December 2014.
- NCT02325219. An efficacy and safety of CNTO 1959 (Guselkumab) in participants with moderate to severe plaque‐type psoriasis. clinicaltrials.gov/ct2/show/NCT02325219Date first received: December 2014.
- NCT02326272. A study to evaluate the efficacy and safety of two dose levels of certolizumab pegol (CZP) in subjects with plaque psoriasis (PSO) (CIMPASI‐2). clinicaltrials.gov/ct2/show/NCT02326272 Date first received: 22 December 2014.
- NCT02346240. Efficacy and safety study of certolizumab pegol (CZP) versus active comparator and placebo in subjects with plaque psoriasis (PSO) (CIMPACT). clinicaltrials.gov/ct2/show/NCT02346240 Date first received: 20 January 2015.
- NCT02425826. A phase 4 study of efficacy and safety of apremilast in subjects with moderate plaque psoriasis (UNVEIL). clinicaltrials.gov/ct2/show/NCT02425826 Date first received: 3 April 2015.
- NCT02474082. Study of secukinumab compared to fumaderm® in adults with moderate to severe psoriasis. (PRIME). clinicaltrials.gov/ct2/show/NCT02474082 Date first received: 16 April 2015.
- NCT02513550. A study comparing different dosing regimens of ixekizumab (LY2439821) in participants with moderate to severe plaque psoriasis (IXORA‐P). clinicaltrials.gov/ct2/show/NCT02513550 Date first received: 30 July 2015.
- NCT02555826. A phase 4 study of efficacy and safety of apremilast in subjects with moderate plaque psoriasis. (UNVEIL). clinicaltrials.gov/ct2/show/NCT02325219 Date first received: 3 April 3 2015.
- NCT02559622. Evaluation of cardiovascular risk markers in psoriasis patients treated with secukinumab (CARIMA). clinicaltrials.gov/ct2/show/NCT02559622 Date first received: 4 August 2015.
- NCT02561806. A study of Ixekizumab (LY2439821) in participants with moderate‐to‐severe plaque psoriasis. clinicaltrials.gov/ct2/show/NCT02325219 Date first received: 25 September 2015.
- NCT02634801. A study of Ixekizumab (LY2439821) in participants with moderate‐to‐severe plaque psoriasis naive to systemic treatment [clinicaltrials.gov/ct2/show/NCT02325219]. Date first received: 16 December 2015.
- NCT02655705. Comparison study of psoriasis severity assessment tools [clinicaltrials.gov/ct2/show/NCT02655705]. Date first received: 4 January 2016.
- NCT02672852. BI 655066 / ABBV‐066 (Risankizumab) in moderate to severe plaque psoriasis with randomized withdrawal and re‐treatment [clinicaltrials.gov/ct2/show/NCT02672852]. Date first received: 1 February 2016.
- NCT02684357. BI 655066 compared to placebo & active comparator (Ustekinumab) in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/ct2/show/NCT02684357 Date first received: 16 February 2016.
- NCT02684370. BI 655066 (Risankizumab) compared to placebo and active comparator (Ustekinumab) in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/ct2/show/NCT02684370 Date first received: 16 February 2016.
- NCT02690701. Study to evaluate the effect of secukinumab compared to placebo on aortic vascular inflammation in subjects with moderate to severe plaque psoriasis (VIP‐S) [clinicaltrials.gov/ct2/show/NCT02690701]. Date first received: 19 February 2016.
- NCT02748863. Study of secukinumab with 2 mL pre‐filled syringes (ALLURE). clinicaltrials.gov/ct2/show/NCT02748863 Date first received: 8 March 2016.
- NCT02826603. Study of secukinumab compared to ustekinumab in subjects with plaque psoriasis (CLARITY). clinicaltrials.gov/ct2/show/NCT02826603 Date first received: 30 November 2015.
- NCT02905331. Efficacy and safety study of guselkumab in the treatment of participants with moderate to severe plaque‐type psoriasis. clinicaltrials.gov/ct2/show/NCT02905331 Date first received: 14 September 2016.
- NCT02951533. A study to compare the efficacy of guselkumab to fumaric acid esters for the treatment of participants with moderate to severe plaque psoriasis (POLARIS) [clinicaltrials.gov/ct2/show/NCT02951533]. Date first received: 28 October 2016.
- NCT02982005. A study of KHK4827 (Brodalumab) in subjects with moderate to severe psoriasis in Korea. clinicaltrials.gov/ct2/show/NCT02982005 Date first received: 1 December 2016.
- RPCEC00000201. Randomized controlled double blind trial to study Safety and Efficaccy of itolizumab (antiCD6) in Moderate‐to‐Severe Psoriasis.. registroclinico.sld.cu/trials/RPCEC00000201‐En Date first received: 15 October 2015.
- TCTR20161028001. A randomised, double‐blind, placebo controlled, multicenter study of subcutaneous secukinumab, to demonstrate efficacy after twelve weeks of treatment and to assess safety, tolerability and long‐term efficacy up to one year in subjects with moderate to severe chronic plaque‐type psoriasis with or without psoriatic arthritis comorbidity. clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=2148 Date first received: 28 February 2017.
Additional references
- Atwan A, Ingram JR, Abbott R, Kelson MJ, Pickles T, Bauer A, et al. Oral fumaric acid esters for psoriasis. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD010497.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balak DM, Fallah Arani S, Hajdarbegovic E, Hagemans CA, Bramer WM, Thio HB, et al. Efficacy, effectiveness and safety of fumaric acid esters in the treatment of psoriasis: a systematic review of randomized and observational studies. British Journal of Dermatology 2016;175(2):250‐62. [PUBMED: 26919824] [DOI] [PubMed] [Google Scholar]
- Bansback N, Sizto S, Sun H, Feldman S, Willian MK, Anis A. Efficacy of systemic treatments for moderate to severe plaque psoriasis: systematic review and meta‐analysis. Dermatology 2009;219(3):209‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Boehncke WH, Schon MP. Psoriasis. Lancet 2015;386(9997):983‐94. [PUBMED: 26025581] [DOI] [PubMed] [Google Scholar]
- Brimhall AK, King LN, Licciardone JC, Jacobe H, Menter A. Safety and efficacy of alefacept, efalizumab, etanercept and infliximab in treating moderate to severe plaque psoriasis: a meta‐analysis of randomized controlled trials. British Journal of Dermatology 2008;159(2):274‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of clinical epidemiology 1997;50(6):683‐91. [PUBMED: 9250266] [DOI] [PubMed] [Google Scholar]
- Campanati A, Benfaremo D, Luchetti MM, Ganzetti G, Gabrielli A, Offidani A. Certolizumab pegol for the treatment of psoriasis. Expert Opinion on Biological Therapy 2017;17(3):387‐94. [PUBMED: 28165828] [DOI] [PubMed] [Google Scholar]
- Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161‐76. [PUBMED: 26062088] [DOI] [PubMed] [Google Scholar]
- Chaimani A, Vasiliadis HS, Pandis N, Schmid CH, Welton NJ, Salanti G. Effects of study precision and risk of bias in networks of interventions: a network meta‐epidemiological study. International Journal of Epidemiology 2013;42(4):1120‐31. [PUBMED: 23811232] [DOI] [PubMed] [Google Scholar]
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;16(S0895):30775‐2. [PUBMED: 28088593] [DOI] [PubMed] [Google Scholar]
- Chiu HY, Wang TS, Chan CC, Cheng YP, Lin SJ, Tsai TF. Human leucocyte antigen‐Cw6 as a predictor for clinical response to ustekinumab, an interleukin‐12/23 blocker, in Chinese patients with psoriasis: a retrospective analysis. British Journal of Dermatology 2014;171(5):1181‐8. [PUBMED: 24734995] [DOI] [PubMed] [Google Scholar]
- Christophers E, Mrowietz U, Henneicke HH, Farber L, Welzel D. Cyclosporine in psoriasis: a multicenter dose‐finding study in severe plaque psoriasis. The German Multicenter Study. Journal of the American Academy of Dermatology 1992;26(1):86‐90. [PUBMED: 1732342] [DOI] [PubMed] [Google Scholar]
- Institute of Social and Preventive Medicine, University of Bern. CINeMA: Confidence in Network Meta‐Analysis. Bern: Institute of Social and Preventive Medicine, University of Bern, 2017.
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta‐analysis. Annals of Internal Medicine 2013;159(2):130‐7. [PUBMED: 23856683] [DOI] [PubMed] [Google Scholar]
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in medicine 2010;29(7‐8):932‐44. [PUBMED: 20213715] [DOI] [PubMed] [Google Scholar]
- Dong J, Goldenberg G. New biologics in psoriasis: an update on IL‐23 and IL‐17 inhibitors. Cutis 2017;99(2):123‐7. [PUBMED: 28319618] [PubMed] [Google Scholar]
- Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, et al. Molecular dissection of psoriasis: integrating genetics and biology. Journal of Investigative Dermatology 2010;130(5):1213‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flytstrom I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. British Journal of Dermatology 2008;158(1):116‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gisondi P, Gubinelli E, Cocuroccia B, Girolomoni G. Targeting tumor necrosis factor‐alpha in the therapy of psoriasis. Current Drug Targets. Inflammation and Allergy 2004;3(2):175‐83. [PUBMED: 15180471] [DOI] [PubMed] [Google Scholar]
- Gomez‐Garcia F, Epstein D, Isla‐Tejera B, Lorente A, Velez Garcia‐Nieto A, Ruano J. Short‐term efficacy and safety of new biological agents targeting the interleukin‐23‐T helper 17 pathway for moderate‐to‐severe plaque psoriasis: a systematic review and network meta‐analysis. British Journal of Dermatology 2017;176(3):594‐603. [PUBMED: 27292159] [DOI] [PubMed] [Google Scholar]
- Gospodarevskaya E, Picot J, Cooper K, Loveman E, Takeda A. Ustekinumab for the treatment of moderate to severe psoriasis. Health Technology Assessment 2009;13(Suppl 3):61‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370(9583):263‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gupta AK, Daigle D, Lyons DC. Network meta‐analysis of treatments for chronic plaque psoriasis in Canada. Journal of Cutaneous Medicine and Surgery 2014;18(6):371‐8. [PUBMED: 25348757] [DOI] [PubMed] [Google Scholar]
- Heffernan MP, Leonardi CL. Alefacept for psoriasis. Seminars in Cutaneous Medicine and Surgery 2010;29(1):53‐5. [PUBMED: 20430308] [DOI] [PubMed] [Google Scholar]
- Helliwell PS, Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Annals of the Rheumatic Diseases 2005;64(Suppl 2):ii3‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [PUBMED: 26062084] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, et al. The mechanism of action of cyclosporin A and FK506. Clinical Immunology & Immunopathology 1996;80(3 Pt 2):S40‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ho VC, Griffiths CE, Albrecht G, Vanaclocha F, Leon‐Dorantes G, Atakan N, et al. Intermittent short courses of cyclosporin (Neoral(R)) for psoriasis unresponsive to topical therapy: a 1‐year multicentre, randomized study. The PISCES Study Group. British Journal of Dermatology 1999;141(2):283‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jabbar‐Lopez ZK, Yiu ZZN, Ward V, Exton LS, Mohd Mustapa MF, Samarasekera E, et al. Quantitative evaluation of biologic therapy options for psoriasis: a systematic review and network meta‐analysis. Journal of Investigative Dermatology 2017;137(8):1646‐54. [PUBMED: 28457908] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Barrett JK, Rice S, White IR, Higgins JP. A design‐by‐treatment interaction model for network meta‐analysis with random inconsistency effects. Statistics in Medicine 2014;33(21):3639‐54. [PUBMED: 24777711] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwala SP. The role of dendritic cells in the immunopathogenesis of psoriasis. Archives of Dermatological Research 2007;299(8):359‐66. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. American Journal of Clinical Dermatology 2005;6(6):383‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kremers HM, McEvoy MT, Dann FJ, Gabriel SE. Heart disease in psoriasis. Journal of the American Academy of Dermatology 2007;57(2):347‐54. [PUBMED: 17433490] [DOI] [PubMed] [Google Scholar]
- Cleach L, Chassany O, Levy A, Wolkenstein P, Chosidow O. Poor reporting of quality of life outcomes in dermatology randomized controlled clinical trials. Dermatology 2008;216(1):46‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Papp K, Han C, Schenkel B, Yeilding N, Wang Y, et al. Ustekinumab improves health‐related quality of life in patients with moderate‐to‐severe psoriasis: results from the PHOENIX 1 trial. British Journal of Dermatology 2010;162(1):137‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lin VW, Ringold S, Devine EB. Comparison of ustekinumab with other biological agents for the treatment of moderate to severe plaque psoriasis: a Bayesian Network meta‐analysis. Archives of Dermatology 2012;148(12):1403‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Loveman E, Turner D, Hartwell D, Cooper K, Clegg A. Infliximab for the treatment of adults with psoriasis. Health Technology Assessment 2009;13(Suppl 1):55‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes‐Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. Journal of Investigative Dermatology 2008;128(5):1207‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mason AR, Mason J, Cork M, Dooley G, Hancock H. Topical treatments for chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD005028.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavridis D, Chaimani A, Efthimiou O, Leucht S, Salanti G. Addressing missing outcome data in meta‐analysis. Evidence‐based mental health 2014;17(3):85‐9. [PUBMED: 25009175] [DOI] [PubMed] [Google Scholar]
- Maza A, Montaudie H, Sbidian E, Gallini A, Aractingi S, Aubin F, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non‐plaque psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2011;25 Suppl 2:19‐27. [PUBMED: 21388455] [DOI] [PubMed] [Google Scholar]
- Montaudie H, Sbidian E, Paul C, Maza A, Gallini A, Aractingi S, et al. Methotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. Journal of the European Academy of Dermatology and Venereology : JEADV 2011;25 Suppl 2:12‐8. [PUBMED: 21388454] [DOI] [PubMed] [Google Scholar]
- Mossner R, Reich K. Management of severe psoriasis with TNF antagonists. Adalimumab, etanercept and infliximab. Current Problems in Dermatology 2009;38:107‐36. [PUBMED: 19710553] [DOI] [PubMed] [Google Scholar]
- Mrowietz U, Farber L, Henneicke‐von Zepelin HH, Bachmann H, Welzel D, Christophers E. Long‐term maintenance therapy with cyclosporine and posttreatment survey in severe psoriasis: results of a multicenter study. German Multicenter Study. Journal of the American Academy of Dermatology 1995;33(3):470‐5. [PUBMED: 7657870] [DOI] [PubMed] [Google Scholar]
- Naldi L, Chatenoud L, Linder D, Belloni Fortina A, Peserico A, Virgili AR, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case‐control study. Journal of Investigative Dermatology 2005;125(1):61‐7. [PUBMED: 15982303] [DOI] [PubMed] [Google Scholar]
- Naldi L. Scoring and monitoring the severity of psoriasis. What is the preferred method? What is the ideal method? Is PASI passe? facts and controversies. Clinics in Dermatology 2010;28(1):67‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nast A, Jacobs A, Rosumeck S, Werner RN. Efficacy and safety of systemic long‐term treatments for moderate‐to‐severe psoriasis: a systematic review and meta‐analysis. Journal of Investigative Dermatology 2015;135(11):2641‐8. [PUBMED: 26046458] [DOI] [PubMed] [Google Scholar]
- Nast A, Jacobs A, Rosumeck S, Werner RN. Methods Report: European S3‐Guidelines on the systemic treatment of psoriasis vulgaris‐‐update 2015‐‐EDF in cooperation with EADV and IPC. Journal of the European Academy of Dermatology and Venereology 2015 Oct 15 [Epub ahead of print]; Vol. 29, issue 12:e1‐22. [DOI: 10.1111/jdv.13353] [DOI] [PubMed]
- Nelson AA, Pearce DJ, Fleischer AB Jr, Balkrishnan R, Feldman SR. Cost‐effectiveness of biologic treatments for psoriasis based on subjective and objective efficacy measures assessed over a 12‐week treatment period. Journal of the American Academy of Dermatology 2008;58(1):125‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nijsten T, Looman CW, Stern RS. Clinical severity of psoriasis in last 20 years of PUVA study. Archives of Dermatology 2007;143(9):1113‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ormerod AD, Mrowietz U. Fumaric acid esters, their place in the treatment of psoriasis. British Journal of Dermatology 2004;150(4):630‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. Journal of Investigative Dermatology 2013;133(2):377‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Puig L, Lopez A, Vilarrasa E, Garcia I. Efficacy of biologics in the treatment of moderate‐to‐severe plaque psoriasis: a systematic review and meta‐analysis of randomized controlled trials with different time points. Journal of the European Academy of Dermatology and Venereology: JEADV 2014;28(12):1633‐53. [PUBMED: 24033851] [DOI] [PubMed] [Google Scholar]
- Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. Journal of the American Academy of Dermatology 1999;41(3 Pt 1):401‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Reich K, Sinclair R, Roberts G, Griffiths CE, Tabberer M, Barker J. Comparative effects of biological therapies on the severity of skin symptoms and health‐related quality of life in patients with plaque‐type psoriasis: a meta‐analysis. Current Medical Research & Opinion 2008;24(5):1237‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Reich K, Burden AD, Eaton JN, Hawkins NS. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta‐analysis of randomized controlled trials. British Journal of Dermatology 2012;166(1):179‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rhodes KM, Turner RM, Higgins JP. Predictive distributions were developed for the extent of heterogeneity in meta‐analyses of continuous outcome data. Journal of Clinical Epidemiology 2015;68(1):52‐60. [PUBMED: 25304503] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ (Clinical research ed.) 2011;342:d549. [PUBMED: 21310794] [DOI] [PubMed] [Google Scholar]
- Robinson A, Kardos M, Kimball AB. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. Journal of the American Academy of Dermatology 2012;66(3):369‐75. [PUBMED: 22041254] [DOI] [PubMed] [Google Scholar]
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salanti G, Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta‐analysis. PloS One 2014;9(7):e99682. [PUBMED: 24992266] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LJ, Wittmann M, McGonagle D, Helliwell PS. Ustekinumab in the treatment of psoriasis and psoriatic arthritis. Rheumatology and Therapy 2015;2(1):1‐16. [PUBMED: 27747495] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbidian E, Maza A, Montaudie H, Gallini A, Aractingi S, Aubin F, et al. Efficacy and safety of oral retinoids in different psoriasis subtypes: a systematic literature review. Journal of the European Academy of Dermatology and Venereology : JEADV 2011;25 Suppl 2:28‐33. [PUBMED: 21388456] [DOI] [PubMed] [Google Scholar]
- Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque‐type psoriasis. Dermatology 2005;210(3):194‐9. [PUBMED: 15785046] [DOI] [PubMed] [Google Scholar]
- Schmitt J, Zhang Z, Wozel G, Meurer M, Kirch W. Efficacy and tolerability of biologic and nonbiologic systemic treatments for moderate‐to‐severe psoriasis: meta‐analysis of randomized controlled trials. British Journal of Dermatology 2008;159(3):513‐26. [PUBMED: 18627372] [DOI] [PubMed] [Google Scholar]
- Schmitt J, Rosumeck S, Thomaschewski G, Sporbeck B, Haufe E, Nast A. Efficacy and safety of systemic treatments for moderate‐to‐severe psoriasis: meta‐analysis of randomized controlled trials. British Journal of Dermatology 2014;170(2):274‐303. [PUBMED: 24131260] [DOI] [PubMed] [Google Scholar]
- Schunemann H, Hill S, Guyatt G, Akl EA, Ahmed F. The GRADE approach and Bradford Hill's criteria for causation. Journal of Epidemiology and Community Health 2011;65(5):392‐5. [PUBMED: 20947872] [DOI] [PubMed] [Google Scholar]
- Signorovitch JE, Wu EQ, Yu AP, Gerrits CM, Kantor E, Bao Y, et al. Comparative effectiveness without head‐to‐head trials: a method for matching‐adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics 2010;28(10):935‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Signorovitch JE, Betts KA, Yan YS, LeReun C, Sundaram M, Wu EQ, et al. Comparative efficacy of biological treatments for moderate‐to‐severe psoriasis: a network meta‐analysis adjusting for cross‐trial differences in reference arm response. British Journal of Dermatology 2015;172(2):504‐12. [PUBMED: 25288183] [DOI] [PubMed] [Google Scholar]
- Spuls PI, Witkamp L, Bossuyt PM, Bos JD. A systematic review of five systemic treatments for severe psoriasis. British Journal of Dermatology 1997;137(6):943‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Spuls PI, Lecluse LL, Poulsen ML, Bos JD, Stern RS, Nijsten T. How good are clinical severity and outcome measures for psoriasis?: quantitative evaluation in a systematic review. Journal of Investigative Dermatology 2010;130(4):933‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Spuls PI, Gerbens LA, Simpson E, Apfelbacher CJ, Chalmers JR, Thomas KS, et al. Patient‐Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. British Journal of Dermatology 2016;176(4):979‐84. [PUBMED: 27858989] [DOI] [PubMed] [Google Scholar]
- Strober BE, Siu K, Menon K. Conventional systemic agents for psoriasis. A systematic review. Journal of Rheumatology 2006;33(7):1442‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Tan JY, Li S, Yang K, Ma B, Chen W, Zha C, et al. Ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: a meta‐analysis. Journal of Dermatological Treatment 2011;22(6):323‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Torres T, Filipe P. Small molecules in the treatment of psoriasis. Drug Development Research 2015;76(5):215‐27. [PUBMED: 26255795] [DOI] [PubMed] [Google Scholar]
- Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Breban M, et al. Risk of tuberculosis is higher with anti‐tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: The three‐year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis & Rheumatism 2009;60(7):1884‐94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D, Picot J, Cooper K, Loveman E. Adalimumab for the treatment of psoriasis. Health Technology Assessment 2009;13(Suppl 2):49‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [PUBMED: 22461129] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. International journal of epidemiology 2013;42(1):332‐45. [PUBMED: 23508418] [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Research Synthesis Methods 2012;3(2):111‐25. [PUBMED: 26062085] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17‐producing helper T cells. Nature Immunology 2007;8(9):950‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wolkenstein P, Revuz J, Roujeau JC, Bonnelye G, Grob JJ, Bastuji‐Garin S. Psoriasis in France and associated risk factors: results of a case‐control study based on a large community survey. Dermatology (Basel, Switzerland) 2009;218(2):103‐9. [PUBMED: 19060463] [DOI] [PubMed] [Google Scholar]
- Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Vergel YB, et al. Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technology Assessment 2006;10(46):1‐233, i‐iv. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. American Journal of Clinical Dermatology 2003;4(7):441‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham‐Anderson J, Wu J, et al. Interleukin‐22, a T(H)17 cytokine, mediates IL‐23‐induced dermal inflammation and acanthosis. Nature 2007;445(7128):648‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Sbidian E, Cleach L, Trinquart L, Do G, Hughes C, Naldi L, et al. Systemic pharmacological treatments for chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011535] [DOI] [Google Scholar]