Abstract
Background
Raynaud's phenomenon is a vasospastic disease characterized by digital pallor, cyanosis, and extremity pain. Primary Raynaud's phenomenon is not associated with underlying disease, but secondary Raynaud's phenomenon is associated with connective tissue disorders such as systemic sclerosis, systemic lupus erythematosus, and mixed connective tissue disease. Calcium channel blockers promote vasodilation and are commonly used when drug treatment for Raynaud's phenomenon is required.
Objectives
To assess the benefits and harms of calcium channel blockers (CCBs) versus placebo for treatment of individuals with Raynaud's phenomenon with respect to Raynaud's type (primary vs secondary) and type and dose of CCBs.
Search methods
We searched the Cochrane Central Register of Controlled Trials (May 19, 2017), MEDLINE (1946 to May 19, 2017), Embase (1947 to May 19, 2017), clinicaltrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Portal. We applied no language restrictions. We also searched bibliographies of retrieved articles and contacted key experts for additional and unpublished data.
Selection criteria
All randomized controlled trials (RCTs) comparing calcium channel blockers versus placebo.
Data collection and analysis
Two review authors independently assessed search results and risk of bias and extracted trial data. We used the GRADE approach to assess the quality of evidence.
Main results
This review contains 38 RCTs (33 cross‐over RCTs) with an average duration of 7.4 weeks and 982 participants; however, not all trials reported all outcomes of interest. Nine of the identified trials studied patients with primary Raynaud's phenomenon (N = 365), five studied patients with secondary Raynaud's phenomenon (N = 63), and the rest examined a mixture of patients with primary and secondary Raynaud's phenomenon (N = 554). The most frequently encountered risk of bias types were incomplete outcome data and poor reporting of randomization and allocation methods.
When researchers considered both primary and secondary Raynaud's phenomenon, evidence of moderate quality (downgraded for inconsistency) from 23 trials with 528 participants indicates that calcium channel blockers (CCBs) were superior to placebo in reducing the frequency of attacks. CCBs reduced the average number of attacks per week by six ( weighted mean difference (WMD) ‐6.13, 95% confidence interval (CI) ‐6.60 to ‐ 5.67; I² = 98%) compared with 13.7 attacks per week with placebo. When review authors excluded Kahan 1985C, a trial showing a very large reduction in the frequency of attacks, data showed that CCBs reduced attack frequency by 2.93 per week (95% CI ‐3.44 to ‐2.43; I² = 77%).
Low‐quality evidence (downgraded for imprecision and inconsistency) from six trials with 69 participants suggests that the average duration of attacks did not differ in a statistically significant or clinically meaningful way between CCBs and placebo (WMD ‐1.67 minutes, 95% CI ‐3.29 to 0); this is equivalent to a ‐9% difference (95% CI ‐18% to 0%).
Moderate‐quality evidence (downgraded for inconsistency) based on 16 trials and 415 participants showed that CCBs reduced attack severity by 0.62 cm (95% CI ‐0.72 to ‐ 0.51) on a 10‐cm visual analogue scale (lower scores indicate less severity); this was equivalent to absolute and relative percent reductions of 6% (95% CI ‐11% to ‐8%) and 9% (95% CI ‐11% to ‐8%), respectively, which may not be clinically meaningful.
Improvement in Raynaud's pain (low‐quality evidence; downgraded for imprecision and inconsistency) and in disability as measured by a patient global assessment (moderate‐quality evidence; downgraded for imprecision) favored CCBs (pain: WMD ‐1.47 cm, 95% CI ‐2.21 to ‐0.74; patient global: WMD ‐0.37 cm, 95% CI ‐0.73 to 0, when assessed on a 0 to 10 cm visual analogue scale, with lower scores indicating less pain and less disability). However, these effect estimates were likely underpowered, as they were based on limited numbers of participants, respectively, 62 and 92. For pain assessment, absolute and relative percent improvements were 15% (95% ‐22% to ‐7%) and 47% (95% CI ‐71% to ‐24%), respectively. For patient global assessment, absolute and relative percent improvements were 4% (95% CI ‐7% to 0%) and 9% (95% CI ‐19% to 0%), respectively.
Subgroup analyses by Raynaud's type, CCB class, and CCB dose suggest that dihydropyridine CCBs in higher doses may be more effective for primary Raynaud's than for secondary Raynaud's, and CCBs likely have a greater effect in primary than in secondary Raynaud's. However, differences were small and were not found for all outcomes. Dihydropyridine CCBs were studied as they are the subgroup of CCBs that are not cardioselective and are traditionally used in RP treatment whereas other CCBs such as verapamil are not routinely used and diltiazem is not used as first line subtype of CCBs. Most trial data pertained to nifedipine.
Withdrawals from studies due to adverse effects were inconclusive owing to a wide CI (risk ratio [RR] 1.30, 95% CI 0.51 to 3.33) from two parallel studies with 63 participants (low‐quality evidence downgraded owing to imprecision and a high attrition rate); absolute and relative percent differences in withdrawals were 6% (95% CI ‐14% to 26%) and 30% (95% CI ‐49% to 233%), respectively. In cross‐over trials, although a meta‐analysis was not performed, withdrawals were more common with CCBs than with placebo. The most common side effects were headache, dizziness, nausea, palpitations, and ankle edema. However, in all trials, no serious adverse events (death or hospitalization) were reported.
Authors' conclusions
Randomized controlled trials with evidence of low to moderate quality showed that CCBs (especially the dihydropyridine class) may be useful in reducing the frequency, duration, severity of attacks, pain and disability associated with Raynaud's phenomenon. Higher doses may be more effective than lower doses and these CCBs may be more effective in primary RP. Although there were more withdrawals due to adverse events in the treatment groups, no serious adverse events were reported.
Plain language summary
Calcium channel blockers for treatment of patients with Raynaud’s phenomenon
Raynaud's phenomenon (RP) is a disorder that results in decreased blood flow to the fingers and toes as the result of vasospasm. Symptoms include discoloration (such as a fingertip turning white, then blue and/or red), pain, and, in severe cases, open sores of the digits. Cold, stress, and emotional discomfort are the most common triggers of a Raynaud's attack. No underlying disease is associated with primary RP. Secondary RP is associated with underlying conditions such as systemic sclerosis.
This review assessed the benefits and harms of calcium channel blockers (CCBs) compared with placebo (a substance that appears the same as the active drug but has no active ingredient) for treatment of patients with RP, based on studies published up to May 19, 2017. CCBs are drugs that increase blood flow to the digits and usually are used as first‐line treatment for patients with RP. The objective of this review was to determine the benefits and harms of CCBs overall, by dose and type of drug and by type of RP (primary vs secondary).
Study characteristics
We identified and included 38 studies with 982 people 18 years old and over with disease of various duration and severity. Nine studies included patients with primary RP, five included patients with secondary RP, and the rest examined patients with both types of RP. Trial duration ranged from 2 to 20 weeks.
What did this review discover about the use of CCBs versus placebo for RP?
Reviewers found that:
• CCBs probably reduce slightly the frequency, severity, and overall patient assessment of Raynaud's attacks (moderate‐quality evidence downgraded for concerns of imprecision or inconsistency);
• CCBs may improve slightly the duration and pain of Raynaud's attacks (low‐quality evidence downgraded for imprecision and inconsistency);
• because of lack of data and high dropout rates, effects of CCBs on risk of dropout due to treatment side effects remain uncertain;
• the most common side effects were headache, dizziness, nausea, palpitations, and ankle edema; and
• serious adverse events (death or hospitalization) were not reported.
Best estimates of what happens to people with RP who take CCBs for 2 to 20 weeks
When investigators considered both primary and secondary RP, they reported that 528 people who took CCBs experienced six fewer attacks per week than those who took placebo. People who took a CCB had an average of 8 attacks per week, compared with 14 attacks per week among those taking placebo.
Duration of attacks (in minutes) was about the same for people taking CCBs or placebo. However, this finding was based on a small number of people.
Severity of attacks measured on a 10‐cm scale (lower scores indicate less severe attacks) was 0.62 cm lower with CCBs; this was equal to a 6% reduction. People who took a CCB rated the severity of an attack as 6.1 cm, compared with 6.7 cm for those taking placebo.
Pain was reduced by 1.5 points on a 0 to 10 scale (15% absolute reduction, lower score means less pain) with CCBs compared with placebo. People who took a CCB reported a pain score of 1.6 points, compared with 3.1 points for those taking placebo.
Overall disability was reduced by 0.4 points on a 0 to 10 scale (4% absolute reduction, lower score means less disability) among people who took CCBs compared with placebo. People who took a CCB reported a disability score of 3.5 points, compared with 3.9 points for those taking placebo.
Six more people out of 100 who took a CCB withdrew from the study owing to adverse events (6% more withdrawals). Out of 100 people taking a CCB, 25 withdrew from the study, compared with 19 out of 100 taking placebo.
This review suggests that CCBs (particularly drugs in the dihydropyridine class such as nifedipine) in higher doses may be beneficial for the management of RP, particularly primary RP. Although slightly more participants taking CCBs withdrew as the result of treatment side effects, no reported side effects were serious.
Summary of findings
Summary of findings for the main comparison. Calcium channel blockers compared with placebo for treatment of Raynaud's phenomenon.
| Calcium channel blockers (CCBs) compared with placebo for treatment of Raynaud's phenomenon | ||||||
| Patient or population: patients with Raynaud's phenomenon Settings: outpatient settings Intervention: calcium channel blockers (CCBs) (all) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | CCBs (all) | |||||
| Frequency of attacks Average number of attacks/week Follow‐up: 4 to 20 weeks | Mean frequency of attacks in control groups: 13.7 attacksa |
Mean frequency of attacks in intervention groups: 6.13 lower (6.60 to 5.67 lower)b |
528 (23 studies) | ⊕⊕⊕⊝ moderatec |
Note: Excluding a study with a very large reduction in frequency of attacks changed the mean difference to ‐2.93 per week (95% CI ‐3.44 to ‐2.43).
NNTB: N/Ad Absolute risk difference: N/Ad Relative percent change: ‐44% (95% CI ‐48% to ‐41%) |
|
|
Duration of attacks Average duration per attack measured in minutes Follow‐up: 2 to 20 weeks |
Mean duration of attacks in control groups: 18.8 minutesa | Mean duration of attacks in intervention groups:
1.67 fewer minutes (‐3.29 to 0) |
69 (6 studies) | ⊕⊕⊝⊝ lowc,e |
NNTB: N/Ad Absolute risk difference: N/Ad Relative percent change: ‐9% (95% CI ‐18% to 0%) |
|
| Severity of attacks Average severity per attack assessed on a 10‐cm visual analogue scale (0 = no symptoms, 10 = maximal severity) Follow‐up: 2 to 12 weeks | Mean severity of attacks in control groups: 6.7 cma | Mean severity of attacks in intervention groups: 0.62 lower (0.72 to 0.51 lower) | 415 (18 studies) | ⊕⊕⊕⊝ moderatec |
NNTB: N/Ad Absolute risk difference: ‐6% (95% CI ‐7% to ‐5%) Relative percent change: ‐9% (95% CI ‐11% to ‐8%) |
|
|
Pain Average pain per attack, measured on a 10‐cm visual analogue scale (0 = no pain, 10 = maximal pain) Follow‐up: 2 to 10 weeks |
Mean pain in control groups: 3.13 cma | Mean pain in intervention groups: 1.47 lower (2.21 to 0.74 lower) | 62 (4 studies) | ⊕⊕⊝⊝ lowc,e |
N/Ad Absolute risk difference: ‐15% (95% CI ‐22% to ‐7%) Relative percent change: ‐47% (95% CI ‐71% to ‐24%) |
|
|
Patient global
Disability due to Raynaud's assessed on a 10‐cm visual analogue scale (0 = no disability, 10 = maximal disability) Follow‐up: 5 weeks |
Mean patient global in control group: 3.9 cma |
Mean patient global in intervention groups: 0.37 lower (0.73 lower to 0) | 92 (2) | ⊕⊕⊕⊝ moderatee |
NNTB: N/Af Absolute risk difference: ‐4% (95% CI ‐7% to 0%) Relative percent change: ‐9% (95% CI ‐19% to 0%) |
|
| Number of withdrawals due to adverse events Number of participants who dropped out of studies owing to adverse treatment effects Follow‐up: 2 to 20 weeks | 194 per 1000 | 252 per 1000 (99 to 645) | RR 1.30 (0.51 to 3.33) | 63 (2 studies) | ⊕⊕⊝⊝ lowe,g |
NNTH: N/Af Absolute risk reduction: 6% (95% CI ‐14% to 26%) Relative percent change: 30% (95% CI ‐49% to 233%) |
| Serious adverse events Number of participants who died or withdrew and were hospitalized as a result of adverse effects of treatment | See comment. | See comment. | Not estimable | 0 (0) | See comment. | No serious adverse events reported |
| *The basis for the assumed risk (eg, median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CCB: calcium channel blocker; CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aFinal value: weighted mean of scores in placebo group across studies in the meta‐analysis. bWith exclusion of Kahan 1985c, CCBs leading to reduced frequency of attacks per week by 2.93 (95% CI ‐3.44 to ‐2.43). cDowngraded 1 level for significant statistical heterogeneity (I² > 50%). dN/A value not calculated. eDowngraded 1 level for imprecision (total population size < 400 for continuous outcomes; total number of events < 300 for dichotomous outcomes). fNNTB (number needed to benefit) and NNTH (number needed to harm) calculated only for statistically significant outcomes. gDowngraded 1 level for inclusion of studies with high risk of bias due to attrition.
Background
Description of the condition
Raynaud's phenomenon (RP) is defined as vasospasms or "attacks" of the arteries or arterioles of the extremities (and rarely other areas) causing pallor and at least one other color change upon reperfusion, such as cyanosis or rubor (Herrick 2005; Wigley 2002). During an RP attack, blood flow to the extremities is restricted with subsequent pallor and/or pain. Each attack is characterized by its frequency, duration, and level of pain. Primary RP is idiopathic and occurs in the absence of other underlying causes such as connective tissue disease. Secondary RP occurs in people with underlying diseases affecting the blood vessels, especially systemic sclerosis (also called SSc, scleroderma) and systemic lupus erythematosus (Wigley 2002). RP may also be accompanied by digital ulcers, which may occur secondary to severe ischemia (loss of blood to the digits) (Wigley 2002). In most cases (> 80%), exposure to cold temperatures triggers this physiologic response, but emotional stress has been documented as another trigger (García‐Carrasco 2008; Wigley 2002). Primary RP has an earlier onset (median age at onset is around 14 years) and is characterized by milder symptoms. Secondary RP often has a later onset (usually after age 40) with more severe symptoms and may be associated with complications such as tissue loss, ulcers, and amputation (Wigley 2002). Irreversible digital ischemia may develop in patients whose RP occurs secondary to a systemic sclerosis spectrum disorder (Herrick 2005).
The prevalence of this condition is based on climate conditions so is geographically variable but has been estimated at around 3% to 5% in the general population, with most cases (80% to 90%) diagnosed as primary RP (Maundrell 2015; Silman 1990). Secondary RP accounts for about 10% to 20% of RP prevalence, but this depends on the underlying cause. More than 90% of patients with SSc have RP (Levien 2010; Maundrell 2015). SSc is an autoimmune connective tissue disease characterized by fibrosis of the skin and internal organs, including the gastrointestinal tract, lungs, kidney, and heart, along with significant vasculopathy and pulmonary arterial hypertension (Ortonne 1989). In general, RP is much more common among women (prevalence of primary RP has been estimated at between 2% and 20% in women compared with 1% to 12% among men), and primary RP has a genetic component (about 50% of patients with primary RP have a first‐degree relative with the disease) (Maundrell 2015). Diagnosis of primary RP is based on patient history (i.e., sensitivity to cold exposure with pallor, then rubor or cyanosis of the fingers and toes after cold exposure) and a thorough evaluation to rule out the presence of underlying causes. Diagnosis of secondary Raynaud's phenomenon may be associated with older age at onset (i.e., after age 40), often with more severe symptoms, as well as positive laboratory tests, suggesting an underlying connective tissue disease (i.e., positive antinuclear antibodies, positive rheumatoid factor, and the presence of specific autoantibodies), and magnification of the nail folds, indicating the presence of a microvascular disease (Wigley 2002).
The pathogenesis of Raynaud's phenomenon is not clearly understood, but a general hypothesis is that the major underlying cause is an imbalance of vasoconstrictors and vasodilators (with imbalance more toward the prevalence of vasoconstrictors) (Herrick 2005). Existing evidence suggests that causes of the underlying pathogenesis of Raynaud's phenomenon likely include abnormalities in the blood vessels (i.e., smooth muscle and endothelium), in neural control of vascular tone, and in intravascular mediators, including those produced by platelet activation and oxidative stress (Herrick 2005). However, vascular abnormalities may be minimal in primary Raynaud's phenomenon and more severe in secondary Raynaud's phenomenon (Herrick 2005; Wigley 2002). This might explain why Raynaud's phenomenon secondary to systemic sclerosis spectrum disorders but not primary Raynaud's phenomenon often leads to irreversible digital ischemia and is much more severe (Herrick 2005). In addition, secondary Raynaud's phenomenon is often associated with structural abnormalities in the microvascular system and arteries. Given that Raynaud's phenomenon is more common among women, some have hypothesized that hormonal factors may be involved (Herrick 2005). It has been estimated that 14% to 37% of cases of primary Raynaud's phenomenon eventually progress to secondary Raynaud's phenomenon (Maundrell 2015).
For most people with Raynaud's phenomenon (the vast majority who have primary Raynaud's phenomenon), treatment is conservative (i.e., avoiding cold temperatures and emotional stress, keeping warm, stopping smoking); however, for RP requiring a pharmacological intervention (usually secondary RP), many different drugs may be beneficial (García‐Carrasco 2008; Goundry 2012; Herrick 2005; Wigley 1987).
Description of the intervention
Many randomized controlled trials have examined treatments for both primary and secondary RP. Secondary RP has been studied mostly in SSc and other connective tissue diseases. Conservative treatments and older treatments (i.e. ganglion blockers, alpha blockers) have been superseded by a variety of drugs considered to be more efficacious with lower side effect profiles (Hansteen 1976). These include calcium channel blockers (CCBs), prostacyclin analogues, angiotensin‐converting enzyme inhibitors, and phosphodiesterase inhibitors, among others (García‐Carrasco 2008). CCBs have become the first line of pharmacological treatment for RP owing to their effectiveness and tolerability (García‐Carrasco 2008).
How the intervention might work
Calcium channel blockers (CCBs) are calcium channel antagonists that bind to voltage‐gated calcium channels to prevent influx of calcium ions into smooth and cardiac muscle cells, thereby promoting vasodilation (Sturgill 1998). The most common class of CCBs is the dihydropyridines, which include nifedipine, nicardipine, amlodipine, and felodipine. Non‐dihydropyridine classes of CCBs include benzothiazepine (i.e., diltiazem), phenylalkylamine (i.e., verapamil), and others. Dihydropyridines are more effective for RP, as they are highly selective for vascular smooth muscle in the walls of arteries. They are fast acting and thus are used more often (Sturgill 1998).
Why it is important to do this review
Multiple studies have shown that CCBs have some efficacy in treating individuals with RP. Previous meta‐analyses showed efficacy in the treatment of patients with primary RP and RP secondary to SSc (Ennis 2016; Thompson 2001; Thompson 2005). However, the vascular dysfunction that underlies RP is not clearly understood, and variable patient responses to treatment are based on RP type and severity. As such, no specific guidelines outlining the most efficacious drug interventions have been developed (Dziadzio 1999; Goundry 2012). Moreover, the nuances of treatment have not been well studied. No previous meta‐analyses have examined effects of dose or CCB type, or differences in response dependent on the subtype of RP. This review is different from previous reviews in that review authors analyzed effect of CCBs in both primary and secondary RP and by CCB type and dose.
This review is based on the generic protocol for drug interventions for Raynaud's phenomenon (Pope 2015).
Objectives
To assess the benefits and harms of calcium channel blockers (CCBs) versus placebo for treatment of individuals with Raynaud’s phenomenon (RP) with respect to Raynaud's type (primary vs secondary) and type and dose of CCBs.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and cross‐over RCTs that lasted one week or longer. We included studies reported as full text, those published as abstract only, and unpublished data. We applied no language restrictions.
Types of participants
We used no standardized definition of RP. Studies included primary RP and/or RP secondary to systemic sclerosis or other connective tissue diseases (LeRoy 1992; Masi 1980) and enrolled participants with RP at any stage.
In the absence of an accepted definition for RP, we included all participants reported to have RP and noted the criteria that authors used to define RP.
Types of interventions
Interventions of interest were CCBs and placebo. We applied no restrictions on interventions and comparators, such as delivery, dose, duration, and intensity. We allowed all co‐interventions.
Types of outcome measures
We considered outcomes for trials one week or longer in duration. Outcome measurements included the following.
Major outcomes
Frequency of attacks (average attacks/week)
Duration of attacks (average duration per attack in minutes)
Severity of attacks
Pain (i.e. visual analogue scale [VAS], numerical rating scale [NRS])
Patient global assessment (measured on various scales, i.e. 0 to 10 VAS)
Withdrawals (due to treatment adverse effects)
Serious adverse events (treatment adverse effects leading to death or withdrawal from study and hospitalization)
Minor outcomes
Function
Raynaud's condition score (RCS; Merkel 2002)
Physician's global assessment
Change in digital ulceration
Treatment preference
General improvement
Side effects
Search methods for identification of studies
Electronic searches
We designed a sensitive search strategy to retrieve RCTs from electronic bibliographic databases. We identified items from the following databases on May 19, 2017.
Cochrane Library via Wiley (May 19, 2017) including the Cochrane Central Register of Controlled Trials (CENTRAL), the Database of Reviews of Effects (DARE), Health Technology Assessment (HTA), and the Economic Evaluations Database (EED).
MEDLINE via OVID (1946 to May 19, 2017).
Embase via OVID (1947 to May 19, 2017).
Clinicaltrials.gov (all years).
World Health Organization (WHO) International Clinical Trials Portal (all years).
We applied no language restrictions. We used the randomized controlled trials filter from Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions. We devised the search strategy for the Cochrane Library Web interface, then adapted it for use with other databases. We have presented the search strategy in Appendix 1.
Searching other resources
We searched all bibliographies of retrieved articles and contacted key experts for additional and unpublished data.
For safety assessments, we searched the websites of regulatory agencies including US Food and Drug Administration‐MedWatch (http://www.fda.gov/Safety/MedWatch/default.htm), European Medicines Evaluation Agency (http://www.ema.europa.eu), Australian Adverse Drug Reactions Bulletin (http://www.tga.gov.au/safety/ews‐monitoring.htm), and UK Medicines and Healthcare products Regulatory Agency (MHRA) pharmacovigilance and drug safety updates (http://www.mhra.gov.uk/Safetyinformation/index.htm), using the keywords "nifedipine," "nicardipine," "nisoldipine," "diltiazem," "verapamil," "amlodipine," "isradipine," and "BAY K 9320," on July 23, 2017.
On July 19, 2017, we searched PubMed for errata or retractions from included studies published in full text (www.ncbi.nlm.nih.gov/pubmed) and found none.
Data collection and analysis
Selection of studies
We included only randomized trials that compared CCBs versus placebo. We included trials comparing CCBs versus other active treatments if they also included a placebo group. We excluded trials comparing CCBs versus other forms of treatment (i.e., natural herbal methods and surgical methods). Two review authors (PTi, SH, or FR) independently reviewed references retrieved through the search and identified studies that met the inclusion criteria. JP resolved differences regarding selection.
Data extraction and management
We recorded study characteristics and outcome data on a data collection form that had been piloted on at least one study in the review. One review author (FR) extracted study characteristics from included studies, and a second review author (LJM or ETG) spot‐checked study characteristics for accuracy against the trial report. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any "run‐in" period, washout period, number of study centers and locations, study setting, withdrawals, and date of study.
Participants: N, mean age, age range, sex, disease duration, severity of condition, diagnostic criteria, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Characteristics of trial design: as outlined below in the Assessment of risk of bias in included studies section.
Notes: funding for trial and notable declarations of interest of trial authors.
We extracted the number of events and the number of participants per treatment group for dichotomous outcomes, and means and standard deviations and number of participants per treatment group for continuous outcomes. We converted the standard deviation (SD) to a standard error (SE) for entry into the generic inverse variance method in RevMan version 5 (2014), using the formula SE = SD/(square root) (N).
We noted in the Characteristics of included studies table if outcome data were not reported in a usable way, and when data were transformed or estimated from a graph. We resolved disagreements by consensus or by consultation with a third person (JP or GAW). One review author (FR) transferred data into the Review Manager RevMan version 5 (2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review against data in the study reports.
For all outcomes, we extracted data from studies and reported as follows:
If both final values and change from baseline values were reported for the same outcome, we preferentially extracted changes from baseline.
If both unadjusted and adjusted values were reported for the same outcome, we extracted the adjusted values.
If data are analyzed based on intention‐to‐treat (ITT) and another sample (i.e. per‐protocol, as‐treated), we extracted both but noted these differences.
If data were available for multiple time points, we used the data that corresponded most closely with those of other RCTs.
For cross‐over studies, we extracted changes in each arm (placebo and treatment) by comparing them with baseline values for each arm when available, or by noting differences between final treatment and placebo.
Assessment of risk of bias in included studies
Two review authors (SH, PTi, or FR) independently assessed the risk of bias of each included study according to the domain‐based evaluation outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We compared assessments, identified inconsistencies, and reached consensus.
For each included study, we rated the following domains as "low risk," "high risk," or "unclear risk" for "risk of bias" assessments.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias: We considered cross‐over effects and baseline characteristics.
We reported an assessment of bias for each included study in a "Risk of bias" table within the Characteristics of included studies section.
Measures of treatment effect
We estimated treatment effects for continuous outcomes using weighted mean difference (WMD) or standardized mean difference (SMD). When investigators used different scales to measure the same conceptual outcome (i.e. pain), we calculated SMDs instead, along with corresponding 95% confidence intervals (CIs). We back‐translated the SMD to a typical scale (i.e. 0 to 10 for pain) by multiplying the SMD by a typical among‐person standard deviation (i.e. standard deviation at baseline of the control group from the most representative trial) (as per Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions) (Schünemann 2011).
We estimated dichotomous outcomes using risk ratios (RRs) by dividing the proportion of events in the treatment group by the proportion of events in the control group. We reported 95% CIs with each outcome estimate.
In the Effects of interventions section under Results and in the "Comments" column of Table 1, we provided absolute percent difference, relative percent change from baseline, and number needed to treat for an additional beneficial outcome (NNTB) (we provided NNTB only when the outcome showed a statistically significant difference).
For dichotomous outcomes, we calculated the NNTB from the control group event rate and the risk ratio using the Visual Rx NNT calculator (Cates 2008). We calculated the NNTB using the Wells calculator (available at the Cochrane Musculoskeletal Group [CMSG] Editorial Office).
For dichotomous outcomes, we calculated absolute risk difference using the risk difference statistic in RevMan version 5 (2014) and expressed the result as a percentage. For continuous outcomes, we calculated absolute benefit as improvement in the intervention group minus improvement in the control group, in original units, expressed as a percentage.
We calculated relative percent change for dichotomous data as "Risk ratio ‐ 1" and expressed this as a percentage. For continuous outcomes, we calculated relative difference in changes from baseline as absolute benefit divided by the baseline mean of the control group, expressed as a percentage.
Unit of analysis issues
The participant was the unit of analysis for each outcome. For cross‐over trials, calculation of standard errors and use of the generic inverse variance method accounted for the fact that observations were paired.
When a single trial reported multiple trial arms, we included only the relevant arms. If two comparisons were combined in the same meta‐analysis (i.e. different doses, different drugs), we halved the control group to avoid double‐counting.
Dealing with missing data
We made all possible efforts to obtain any missing data. We had foreign language studies translated when possible, and we used the Cochrane network to try to obtain articles that were not available at the national libraries in Ottawa and Washington, if they also were not available at local universities. When feasible, we estimated missing standard deviations (using standard errors, P values, confidence intervals, error bars in graphs, range and sample size, etc., if available).
Assessment of heterogeneity
We assessed heterogeneity by using Chi² and I² tests and by visually inspecting forest plots for outliers.
As recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), an I² value of 0% to 40% might "not be important"; 30% to 60% may represent "moderate" heterogeneity; 50% to 90% may represent "substantial" heterogeneity; and 75% to 100% represents "considerable" heterogeneity. As noted in the Cochrane Handbook for Systematic Reviews of Interventions, we will keep in mind that the importance of I² depends on the magnitude and direction of effects; and on the strength of evidence for heterogeneity.
For the Chi² test, a P value ≤ 0.10 indicates evidence of statistical heterogeneity.
We reported the presence of substantial heterogeneity and investigated possible causes by following the recommendations provided in Section 9.6 of the Cochrane Handbook for Systematic Reviews of Interventions.
Assessment of reporting biases
For outcomes when more than 10 studies were present, we examined funnel plots to assess publication bias (Egger 1997; Sterne 2011). We used this approach to assess frequency of attacks and severity of attacks.
Data synthesis
We undertook meta‐analyses only when this was meaningful (i.e., when treatments, participants, and the underlying clinical question were similar enough for pooling to make sense). We used the generic inverse variance method for the meta‐analysis by entering the estimate of treatment effect and the standard error for each study. For RTSI 2000, we reported a geometric mean and found that the P value for attack frequency was not given with enough precision for calculation of a standard error for inclusion in the meta‐analysis.
We used a fixed‐effect model and performed a sensitivity analysis using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
In addition to examining all CCBs versus placebo for any subset of RP, we performed the following subgroup analyses when possible.
Dihydropyridine type CCBs versus placebo. Dihydropyridine CCBs were studied as they are the subgroup of CCBs that are not cardioselective and are traditionally used in RP treatment whereas other CCBs such as verapamil are not routinely used and diltiazem is not used as first line subtype of CCBs.
CCBs versus placebo by dose (i.e., low dose, medium, medium/high dose). Low, medium and high doses for CCBs were defined as specified in the guideline in Appendix 2. Since only three studies used high doses of CCBs, we combined trials using high doses CCBs with the medium dose CCBs for outcomes where data for analysis was available. We had most data with respect to dose for nifedipine. Clinical impression is to start with lower doses and that if tolerated, higher doses may yield more benefit.
CCBs versus placebo by actual CCB drug (mainly nifedipine, nicardipine, and nisoldipine, as these CCBs were used most frequently in included trials).
CCBs versus placebo by Raynaud's type (primary or secondary).
Nifedipine versus placebo by disease type (primary or secondary RP).
For major outcomes with significant heterogeneity (I² > 50%), we tested robustness of results derived from the fixed effect by repeating the analysis using a random‐effects model. Because results from the two models were similar (i.e., in direction of effect), we reported fixed‐effect results throughout.
Sensitivity analysis
To address the presence of significant heterogeneity (I² > 50%; Higgins 2003), we performed post hoc sensitivity analyses. We repeated analyses of major outcomes with significant heterogeneity by omitting the study or studies believed to be responsible for the heterogeneity. Reasons for omission included the following.
Study used a scale of measure different from that used in other studies.
Study used study duration different from that used in other studies.
Grading the evidence
In addition to providing tools that can be used to assess risk of study bias, we used the GRADE approach in evaluating the overall quality of evidence for reported outcomes (Grade 2008). Through this approach, we assessed the quality of evidence as follows: (1) high quality from RCTs, (2) moderate quality downgraded by one level owing to a study limitation, (3) low quality double‐downgraded for study limitations, and, last, (4) very low quality triple‐downgraded owing to multiple study limitations. We downgraded evidence using the following GRADE approach.
Limitations in the design and implementation of available studies, suggesting high likelihood of bias.
Indirectness of evidence (indirect population, intervention, control, outcomes) (not applicable to this meta‐analysis, as only RCTs were analyzed).
Unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses).
Imprecision of results (wide confidence intervals).
High probability of publication bias.
"Summary of findings" table
We have summarized the major outcomes of this review ‐ frequency of attack, duration of attack, severity of attack, pain, patient global assessment, withdrawals due to adverse events, and serious adverse events ‐ in Table 1. This table contains intervention effect estimates, comparators, and details on quality of evidence.
Results
Description of studies
Refer to Characteristics of included studies,Characteristics of excluded studies, and Characteristics of studies awaiting classification for descriptions of individual studies.
Results of the search
The search performed on December 2, 2015, and updated on May 19, 2017, yielded 3389 hits (1061 from the Cochrane Library, 1027 from Medline, 1251 from Embase, and 50 from clinicaltrials.gov). After we screened out duplicates, 2337 articles remained. Further screening of these publications yielded 305 articles. When we examined the 305 articles further, we identified 77 articles, from which we excluded 30 with reasons and found that seven trials were awaiting classification, two trials were ongoing, and 38 trials met the inclusion criteria of this review. Figure 1 provides further details on our research results in a flow diagram format.
1.
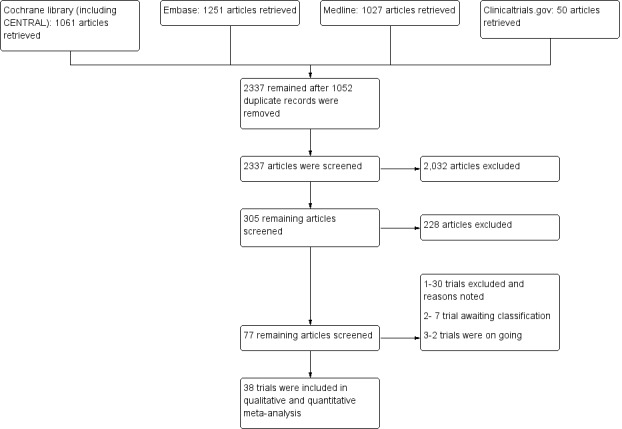
Flow diagram of study.
Included studies
We included 38 trials that investigated effects of CCBs versus placebo in 982 participants with RP (Aldoori 1986; Bravard 1983; Challenor 1987; Challenor 1989; Constantini 1987; Corbin 1986; Ettinger 1984; Ferri 1992; Finch 1988; French Co‐op 1991; Gjorup 1986a; Gjorup 1986b; Hawkins 1985; Kahan 1985a; Kahan 1985b; Kahan 1985c; Kahan 1987; Kallenberg 1987; Kinney 1982; Kirch 1987; La Civita 1997; Leppert 1989; Malamet 1984; Meyrick Thomas 1987; Muller‐Buhl 1983; Nilsson 1987; Rhedda 1985; Rodeheffer 1983; RTSI 2000; Rupp 1987; Sarkozi 1986; Sauza 1984; Smith 1982; Teixeira da Costa; Waller 1986; White 1986; Wigley 1987; Wollersheim 1991). See Characteristics of included studies.
Only nine of these studies exclusively studied patients with primary RP (N = 365 participants), five studied patients with secondary RP (N = 63 participants), and remaining studies (N = 554 participants) examined a mixture of patients with primary RP and secondary RP.
Of the 38 trials included in this systematic review, 36 were double‐blind and two were single‐blind; 33 were of cross‐over design and five were of parallel design. All studies were randomized, or appeared to be randomized. The duration of studies ranged from 2 to 20 weeks, with an average of 7.4 weeks and a median of 3 weeks per arm, with publication years ranging from 1982 to 2000.
Of the 38 included studies, 21 used low‐dose CCBs, 13 used medium‐dose CCBs, and three used high‐dose CCBs (see Appendix 2 for dosage ranges). Twenty‐two RCTs compared nifedipine versus placebo (Aldoori 1986; Bravard 1983; Challenor 1989; Constantini 1987; Corbin 1986; Finch 1988; Gjorup 1986b; Hawkins 1985; Kahan 1985a; Kahan 1985c; Kallenberg 1987; Kirch 1987; Malamet 1984; Meyrick Thomas 1987; Nilsson 1987; Rodeheffer 1983; RTSI 2000; Sarkozi 1986; Sauza 1984; Smith 1982; Waller 1986; White 1986). The daily dose of nifedipine ranged from 10 to 80 mg/d, the mean dose was 45 mg/d, and the median dose was 40 mg/d. Six trials compared nicardipine versus placebo (Ferri 1992; French Co‐op 1991; Kahan 1987; Rupp 1987; Wigley 1987; Wollersheim 1991a). Daily doses of nicardipine ranged from 30 to 100 mg, with a mean of 68 mg/d. Two trials compared nisoldipine versus placebo (Challenor 1987; Gjorup 1986a), providing daily dosages of 10 mg and 20 mg, respectively. Three trials compared diltiazem versus placebo (Kahan 1985b; Rhedda 1985; Teixeira da Costa), giving a mean dosage of 240 mg/d (dosages ranged from 180 mg to 360 mg, with a median dose of 180 mg/d). Individual studies compared Bay K 9320, amlodipine, isradipine, or verapamil (Muller‐Buhl 1983; La Civita 1997; Leppert 1989; Kinney 1982, respectively) versus placebo.
Excluded studies
We excluded 30 additional articles (see Characteristics of excluded studies). We excluded 10 studies because they lacked a placebo (Della Bella 1997; Dziadzio 1999; Leppert 1993; Myrdal 1994; Park 2013; Rademaker 1989; Rademaker 1992; Ringqvist 1993; Varela‐Aguilar 1997Wu 2008); another four studies because they did not present placebo data (Codella 1989; La Civita 1996; Rademaker 1989; Varela‐Aguilar 1997); nine trials because they were not randomized (Creager 1984; Garcia Hernandez2004; Joseph 1988; Kallenberg 1991; Lewis 1987; Pisenti 1984; Smith 1985; Vayssairat 1989; Wollersheim 1987); one trial because researchers gave participants placebo and treatment simultaneously (Schmidt 1989); two studies because they were not of adequate duration (duration < 1 week: Weber 1990; Kahan 1983b); one study because study authors reported an insufficient washout duration of one day (Winston 1983); and three were excluded because they were meta‐analyzes (Ennis 2016; Thompson 2001; Thompson 2005).
We have not currently included seven studies because we were unable to locate the articles or full data (EUCTR2009‐018194‐31‐GB; Kahan 1982; Kahan 1983a; Redondo 1986; van Heereveld 1988; Wasir 1983; Wise 1987). See Studies awaiting classification.
As of July 2017, we found two studies in Clinicaltrials.gov that were ongoing: One study compared 10% nifedipine versus 5% sildenafil (and placebo) (Vera‐Kellet 2017); the other compared diltiazem versus nitroglycerin and placebo (Nazarinia 2016).
Risk of bias in included studies
The most commonly encountered biases were lack of random sequence generation (in 78% of studies) and concealment of allocation (in 70% of studies), followed by performance, attrition, and selective reporting biases, respectively. See Characteristics of included studies, Figure 2, and Figure 3 for additional details on risk of bias of included studies.
2.
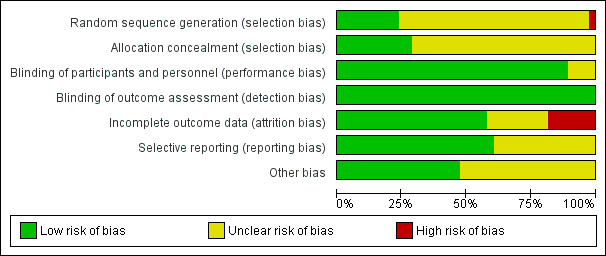
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
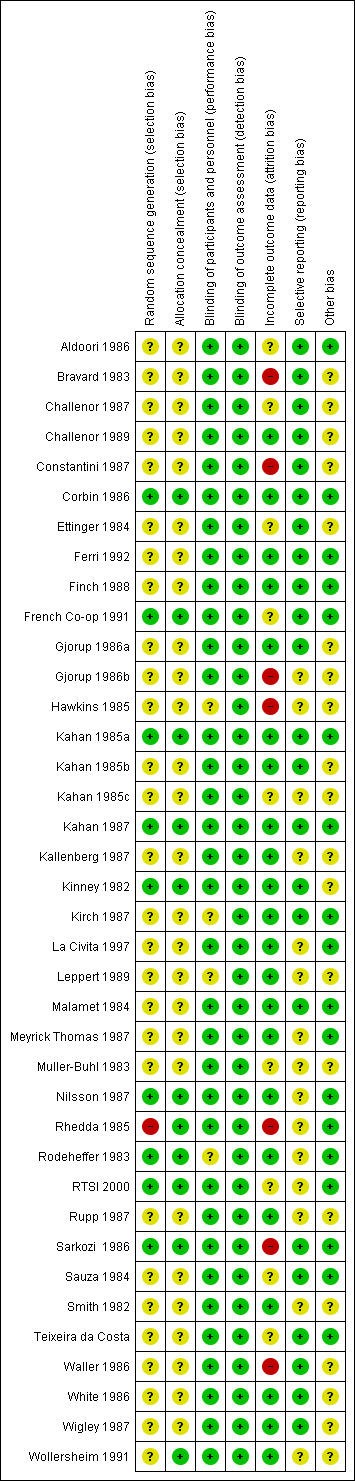
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged allocation sequence generation as "high risk" for Rhedda 1985. See Characteristics of included studies. Although an independent collaborator performed randomization by using computer‐generated random numbers, randomization did not produce groups with equal baseline characteristics. At completion of the trial, researchers reported that the control group had more severe RP than the active treatment group. Nine of the 38 included studies adequately described allocation sequence and concealment, and we rated them as having "low risk" (Corbin 1986; French Co‐op 1991; Kahan 1985a; Kahan 1987; Nilsson 1987; Rhedda 1985; Rodeheffer 1983; RTSI 2000; Sarkozi 1986). Methods of concealment used by these trials included automated computer generation, tables of random numbers, block randomization, and randomization by a third party co‐ordinating center. The remainder of these studies failed to adequately describe how the allocation sequence and concealment were generated; we rated them as having "unclear risk of bias."
Blinding
Investigators stated that most (34/38; 89%) of the included RCTs were "double‐blind"; we assumed that these RCTs had adequate blinding and classified them as "low risk." Four studies (Hawkins 1985; Kirch 1987; Leppert 1989; Rodeheffer 1983) were "single‐blind," at least for some part of the trial. Therefore, we rated performance bias for these trials as showing "unclear risk", as seen in Figure 3.
With regard to blinding of outcome assessments, in some studies participants kept diaries for assessment of outcomes of interest (frequency, severity and duration of RP). Some studies used patient global assessments. Outcomes assessments appeared to be adequately blinded as all studies were blinded and 89% were double blind.
Incomplete outcome data
We found incomplete outcome data due to attrition in approximately 40% of the included trials. We assessed risk of bias due to incomplete outcome data as "unclear" in eight studies (Aldoori 1986; Challenor 1987; Constantini 1987; French Co‐op 1991; Muller‐Buhl 1983; RTSI 2000; Sauza 1984; Teixeira da Costa). These studies reported attrition with unclear effects on outcomes (i.e., it was clear whether attrition occurred in treatment or placebo phase in cross‐over studies, losses from groups were equal in small trials, dropout was uneven in larger trials). We classified eight trials as having "high risk" of bias with regard to attrition (Bravard 1983; Constantini 1987; Gjorup 1986b; Hawkins 1985; Rhedda 1985; RTSI 2000; Sarkozi 1986; Waller 1986). These trials reported high dropout rates, unequal dropout between treatment and placebo groups, and exclusion from analyses (due to missing data from subjective diary assessments or non‐compliance of participants). We classified the remaining 20 trials as having "low risk" of bias due to attrition; they had minimal or equal loss to follow‐up and treated participants on an intention‐to‐treat (ITT) basis.
Selective reporting
We rated selective reporting bias as "low risk" for most trials. Two trials did not report all proposed outcomes (Hawkins 1985; RTSI 2000). Hawkins 1985 noted that the severity of attacks on a 5‐point Likert scale was parallel to that on a 10‐cm VAS scale but did not report actual results; RTSI 2000 did not report all minor outcomes mentioned in the methods. We classified risk of reporting bias as "unclear" for these two trials.
Other potential sources of bias
Other potential risks of bias considered were possible carryover effects in cross‐over trials, and similarity of baseline characteristics in parallel trials. Overall, we judged these sources of bias as "low risk" for 18 trials (Aldoori 1986; Corbin 1986; Ferri 1992; Finch 1988; French Co‐op 1991; Kahan 1985a; Kirch 1987; La Civita 1997; Malamet 1984; Meyrick Thomas 1987; Muller‐Buhl 1983; Nilsson 1987; Rhedda 1985; Rodeheffer 1983; RTSI 2000; Sarkozi 1986; Sauza 1984; Teixeira da Costa). See Characteristics of included studies. For remaining included studies, we judged risk of bias for this domain as "unclear" owing to lack of sufficient information. Carryover bias was determined to be present if: there was no washout between treatments in a crossover trial (inadequate washout) and/or the baseline RP characteristics were dissimilar at the second treatment or the baseline characteristics for RP were not provided. CCBs are fast in their onset and rapidly washout when discontinued so the washout did not have to be for very long (such as one to two weeks) for patients to be assumed to be in steady state.
Effects of interventions
See: Table 1
Primary outcomes
Comparison 1. All calcium channel blockers (CCBs) versus placebo for all subsets of Raynaud's phenomenon (RP)
See Table 1.
Frequency of attacks
Average number of attacks/week in 528 participants from 23 studies
See Aldoori 1986; Challenor 1987; Challenor 1989; Corbin 1986; Ettinger 1984; Ferri 1992; Finch 1988; French Co‐op 1991; Gjorup 1986a; Hawkins 1985; Kahan 1985a; Kahan 1985b; Kahan 1985c; Kahan 1987; Kirch 1987; Malamet 1984; Meyrick Thomas 1987; Rodeheffer 1983; Rupp 1987; Sarkozi 1986; Smith 1982; Waller 1986; and Wigley 1987 (Analysis 1.1).
1.1. Analysis.
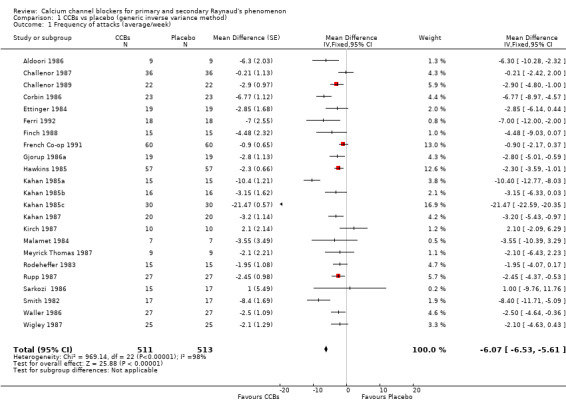
Comparison 1 CCBs vs placebo (generic inverse variance method), Outcome 1 Frequency of attacks (average/week).
When we considered all trials regardless of the class or type of CCB, dose, or RP type, we found that the weighted mean difference (WMD) for the frequency of attacks per week was ‐6.07 (95% confidence interval [CI] ‐ 6.53 to ‐5.61). The negative sign in the WMD indicates that the intervention (CCBs) reduced the number of Raynaud's attacks per week when compared with placebo. This was equivalent to a relative reduction of 44% (95% CI ‐48% to ‐41%) in the frequency of attacks per week with CCBs. However, significant statistical heterogeneity (I² = 98%) was present. Excluding Kahan 1985c ‐ a short trial of two weeks per arm that showed a very large effect for CCBs compared with the remaining trials ‐ reduced heterogeneity to I² = 77. As a result, the new WMD was ‐2.93 (95% CI ‐3.44 to ‐2.43) (Analysis 1.2).
1.2. Analysis.
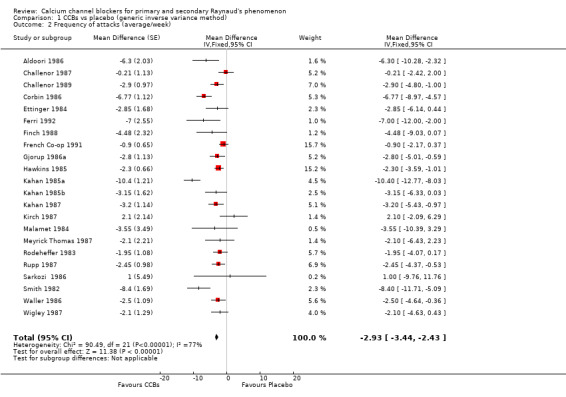
Comparison 1 CCBs vs placebo (generic inverse variance method), Outcome 2 Frequency of attacks (average/week).
We did not include in the analysis RTSI 2000, which used a different scale of measure (geometric mean), provided an imprecise P value, and was much larger and longer than the other trials. This parallel trial compared effects of nifedipine versus placebo (77 participants took nifedipine, and 81 took placebo) in participants with primary RP and found a 66% reduction in frequency of attacks with nifedipine (P < 0.001) after one year. Additionally, participants taking nifedipine reported greater improvement in their RP symptoms compared with participants taking placebo (P < 0.001). Overall, effects of nifedipine on the frequency of attacks reported in this trial were consistent with the overall conclusion of this meta‐analysis.
Examination of the funnel plot of the frequency of attacks (Figure 4) showed an asymmetrical distribution of treatment effects around the mean estimate of effect (Egger 1997), with more studies showing benefit for CCBs.
4.
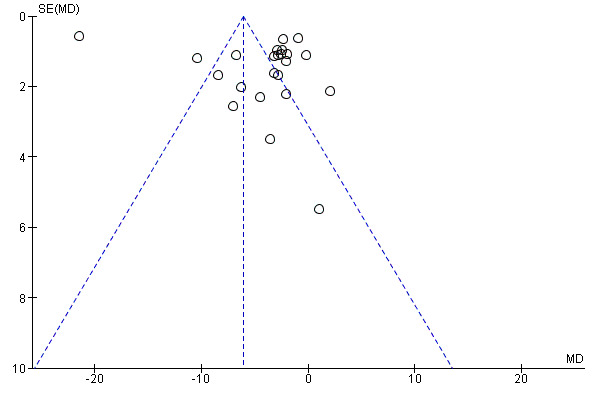
Funnel plot of comparison: 1 CCBs vs placebo (generic inverse variance method), outcome: 1.1 Frequency of attacks (average/week).
Duration of attacks
Average duration per attack measured in minutes in 69 participants from six studies
See Aldoori 1986; Ettinger 1984; Meyrick Thomas 1987; Finch 1988; Kirch 1987; and Malamet 1984 (Analysis 1.3).
1.3. Analysis.
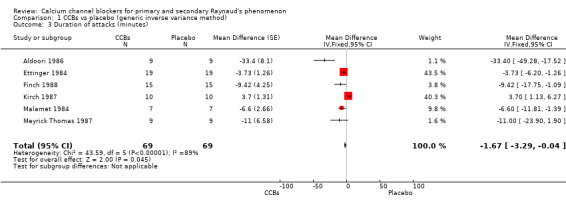
Comparison 1 CCBs vs placebo (generic inverse variance method), Outcome 3 Duration of attacks (minutes).
The WMD for the average duration of attacks in the six trials was ‐1.67 minutes (95% CI ‐3.29 to 0) with considerable heterogeneity (I² = 89). This was equal to a small relative percent reduction of 9% (95% CI ‐18% to 0%).
Severity of attacks
Average severity per attack assessed on a 10‐cm visual analogue scale (0 = no symptoms, 10 = maximal severity) in 374 participants from 16 studies
See Challenor 1989; Ettinger 1984; Ferri 1992; Finch 1988; French Co‐op 1991; Gjorup 1986a; Hawkins 1985; Kahan 1985a; Kahan 1985b; Kahan 1985c; Kahan 1987; Kirch 1987; Malamet 1984; Rupp 1987; Smith 1982; and Wigley 1987 (Analysis 1.4).
1.4. Analysis.
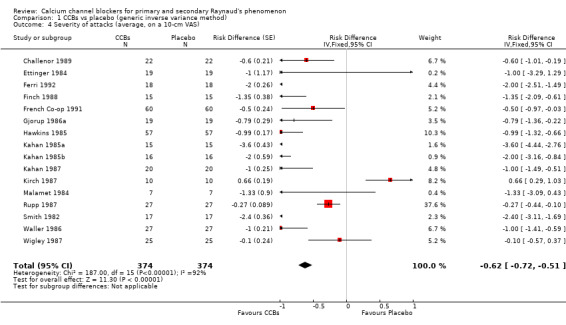
Comparison 1 CCBs vs placebo (generic inverse variance method), Outcome 4 Severity of attacks (average, on a 10‐cm VAS).
The pooled WMD was ‐0.62 cm (95% CI ‐0.72 to ‐ 0.51) with significant heterogeneity (I² = 92%). The negative sign in the WMD indicates that the severity of attacks was less among the active treatment group than the placebo group. Absolute risk difference was ‐6% (95% CI ‐11% to ‐8%) and relative percent change was ‐9% (95% CI ‐11% to ‐8%).
Examination of the funnel plot (Figure 5) for severity of attacks showed some asymmetry with a few outliers in the direction of effect.
5.
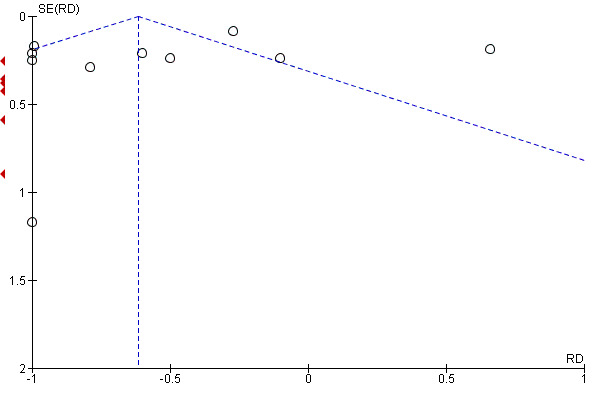
Funnel plot of comparison: 11 CCBs vs placebo (generic inverse variance method), outcome: 11.4 Severity of attacks (average, on a 10‐cm VAS).
Pain
Average pain per attack measured on a 10‐cm visual analogue scale (0 = no pain, 10 = maximal pain) in 62 participants from 4 studies
See Aldoori 1986; Ettinger 1984; Malamet 1984; and Rupp 1987 (Analysis 1.5).
1.5. Analysis.
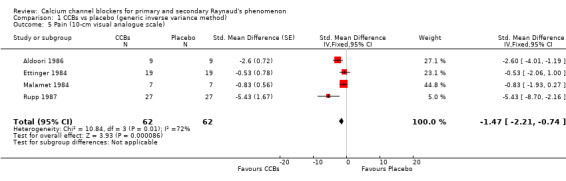
Comparison 1 CCBs vs placebo (generic inverse variance method), Outcome 5 Pain (10‐cm visual analogue scale).
The pooled summary WMD for pain in these studies was ‐1.47 cm (95% CI ‐2.21 to ‐0.74). The negative sign indicates that pain was less in the active treatment group. Absolute risk reduction was ‐15% (95% CI ‐22 % to ‐7%) and relative percent change was ‐47% (95% CI ‐71% to ‐24%). Heterogeneity was moderate (I² = 77%).
Patient global
Disability due to Raynaud's assessed on a 10‐cm visual analogue scale (0 = no disability, 10 = maximal disability) in 96 participants from two studies
See Challenor 1987 and French Co‐op 1991 (Analysis 1.6).
1.6. Analysis.
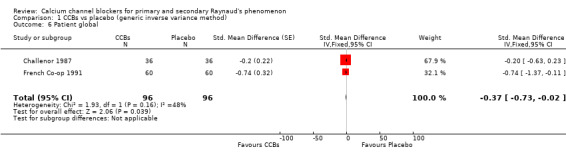
Comparison 1 CCBs vs placebo (generic inverse variance method), Outcome 6 Patient global.
The WMD for patient global was ‐0.37 (95% CI ‐0.73 to 0). We did not calculate the number needed to treat for an additional beneficial outcome (NNTB) for this non‐significant result, but absolute risk difference was ‐4% (95% CI ‐7% to 0%) and relative percent change was ‐9% (95% CI ‐19% to 0%).
Withdrawals
Numbers of participants who dropped out of studies owing to adverse treatment effects among a total of 63 participants from two studies
See Constantini 1987 and Sarkozi 1986 (Analysis 1.7).
1.7. Analysis.
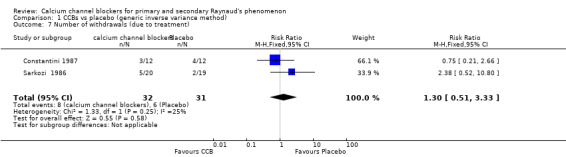
Comparison 1 CCBs vs placebo (generic inverse variance method), Outcome 7 Number of withdrawals (due to treatment).
The pooled summary risk ratio (RR) of withdrawals from two parallel trials that reported this outcome was 1.32 (95% CI 0.51 to 3.33); 8 out of 32 withdrew from active treatment compared with 6 out of 31 from placebo treatment. Absolute risk difference was 6% (95% CI ‐14% to 26%) and relative percent change was 30% (95% CI ‐49% to 233%). We did not calculate the number needed to treat for an additional harmful outcome (NNTH) because withdrawals did not differ statistically between the two groups.
We did not analyze withdrawals for cross‐over trials but did notice that overall withdrawals were more frequent with CCBs than with placebo. From 10 cross‐over trials with 281 participants that reported withdrawals, 39 participants withdrew while on active treatment compared with 15 while taking placebo (see Appendix 3).
Serious adverse events
Number of participants who died or withdrew and were hospitalized as a result of adverse effects of treatment
Investigators reported no serious adverse events
Note:Wollersheim 1991 met the inclusion criteria of this review, but the only outcome that could be included in the meta‐analysis was withdrawals. For the other reported outcomes ‐ frequency, duration, and severity of attacks ‐ trial authors reported no statistically significant differences between groups but provided no estimates of variance. This trial used medium‐dose nicardipine and included a mixture of participants with primary and secondary RP. If we had been able to include in our meta‐analysis trial data on frequency, duration, and severity of attacks, we expect that the non‐significant results would decrease observed effects of CCBs on these outcomes. Also, we did not include RTSI 2000 in the meta‐analysis, as this trial reported geometric mean differences and did not report the P value with enough precision for calculation of a standard error.
Subgroup analyses included the following comparisons.
Comparison 2. CCBs versus placebo by RP type (primary vs secondary)
Frequency of attacks
Average number of attacks/week in 528 participants from 12 studies
See Challenor 1989; Corbin 1986; Ettinger 1984; Kahan 1985a; Kahan 1985b; Kahan 1987; Kirch 1987; Malamet 1984; Meyrick Thomas 1987; Rodeheffer 1983; Rupp 1987; and Sarkozi 1986 (Analysis 2.1).
2.1. Analysis.
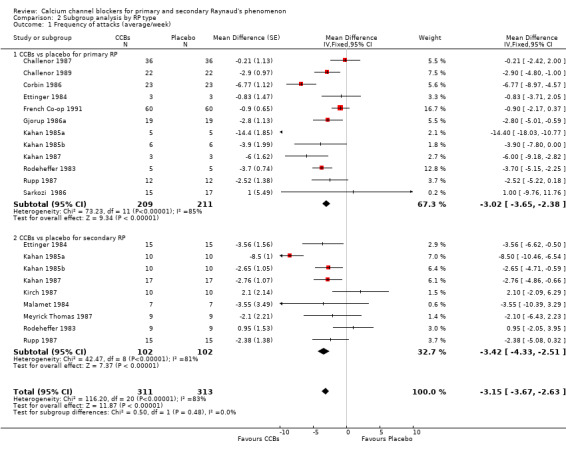
Comparison 2 Subgroup analysis by RP type, Outcome 1 Frequency of attacks (average/week).
In 226 people with primary RP, CCBs reduced the frequency of attacks per week by 3.02 (95% CI ‐3.65 to ‐2.38) compared with placebo. In 102 people with secondary RP, CCBs reduced the average number of attacks over a one‐week period by 3.42 (95% CI ‐4.33 to ‐2.51) compared with placebo. These differences between groups were not statistically significant (P = 0.48; I² = 48%).
Severity of attacks
Average severity per attack assessed on a 10‐cm visual analogue scale (0 = no symptoms, 10 = maximal severity) in 253 participants from 10 studies
See Challenor 1989; French Co‐op 1991; Gjorup 1986a; Hawkins 1985; Kahan 1985a; Kahan 1985b; Kahan 1987; Kirch 1987; Malamet 1984; and Rupp 1987 (Analysis 2.2).
2.2. Analysis.
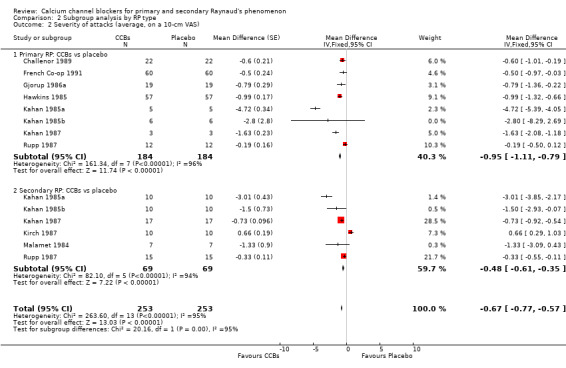
Comparison 2 Subgroup analysis by RP type, Outcome 2 Severity of attacks (average, on a 10‐cm VAS).
Compared with placebo, CCBs reduced the severity of attacks by 0.95 on a 10‐cm scale (95% CI ‐1.11 to ‐0.79; I² = 96%) in 184 people with primary Raynaud's. In 69 people with secondary RP, CCBs reduced the severity of attacks by 0.48 (95% CI ‐0.61 to ‐0.35; I² = 94%) compared with placebo.
The difference between these subgroups was statistically significant (P < 0.0001; I² = 95%).
Comparison 3. Nifedipine versus placebo by RP type (primary vs secondary)
Frequency of attacks
Average number of attacks/week in 233 participants from nine studies
See Challenor 1989; Corbin 1986; Ettinger 1984; Kahan 1985a; Kirch 1987; Malamet 1984; Meyrick Thomas 1987; Rodeheffer 1983 and Sarkozi 1986 (Analysis 3.1).
3.1. Analysis.
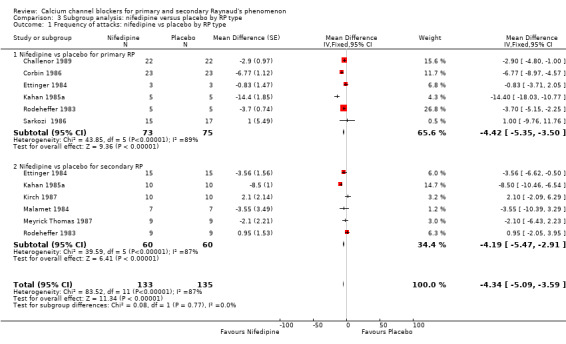
Comparison 3 Subgroup analysis: nifedipine versus placebo by RP type, Outcome 1 Frequency of attacks: nifedipine vs placebo by RP type.
Among 90 participants with primary RP, nifedipine reduced the frequency of attacks by 4.42 (95% CI ‐5.35 to ‐3.50) compared with placebo. In 60 participants with secondary RP, nifedipine reduced the frequency of attacks by 4.19 (95% CI ‐5.47 to ‐2.91) compared with placebo. However, heterogeneity was substantial (I² = 98% and I² = 87%, respectively).
The difference between these subgroups was not statistically significant (P = 0.77).
Severity of attacks
Average severity per attack assessed on a 10‐cm visual analogue scale (0 = no symptoms, 10 = maximal severity) in 39 participants from four trials
See Challenor 1989; Kahan 1985a; Kirch 1987; and Malamet 1984 (Analysis 3.2).
3.2. Analysis.
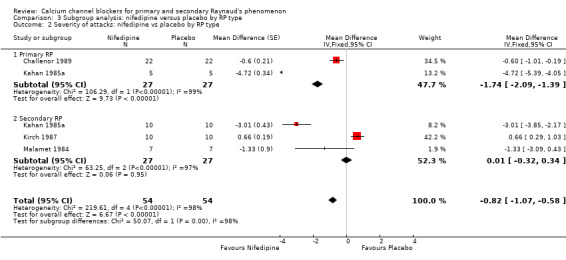
Comparison 3 Subgroup analysis: nifedipine versus placebo by RP type, Outcome 2 Severity of attacks: nifedipine vs placebo by RP type.
In 27 participants with primary RP, nifedipine when compared with placebo reduced the severity of attacks (WMD 1.74, 95% CI ‐2.09 to ‐1.39). In 27 participants with secondary RP, nifedipine did not appear to be any more beneficial than placebo (WMD 0.01, 95% CI ‐0.32 to 0.34). However, heterogeneity was substantial (I² = 98% and I² = 96%, respectively).
The difference between these subgroups was statistically significant (P < 0.0001; I² = 97%).
Comparison 4. CCBs versus placebo for primary and secondary RP by CCB class (dihydropyridine class vs non‐dihydropyridine class)
For frequency and severity of attacks, we had enough data to perform a subgroup analysis by CCB class (dihydropyridine vs non‐dihydropyridine). We further analyzed the data for these outcomes by type of dihydropyridine CCB (i.e., nifedipine vs placebo, nicardipine vs placebo, and nisoldipine vs placebo).
Frequency of attacks (average/week)
Average number of attacks/week in 528 participants from 23 studies
See Aldoori 1986; Challenor 1987; Challenor 1989; Corbin 1986; Ettinger 1984; Ferri 1992; Finch 1988; French Co‐op 1991; Gjorup 1986a; Hawkins 1985; Kahan 1985a; Kahan 1985b; Kahan 1985c; Kahan 1987; Kirch 1987; Malamet 1984; Meyrick Thomas 1987; Rodeheffer 1983; Rupp 1987; Sarkozi 1986; Smith 1982; Waller 1986; and Wigley 1987 (Analysis 4.1).
4.1. Analysis.
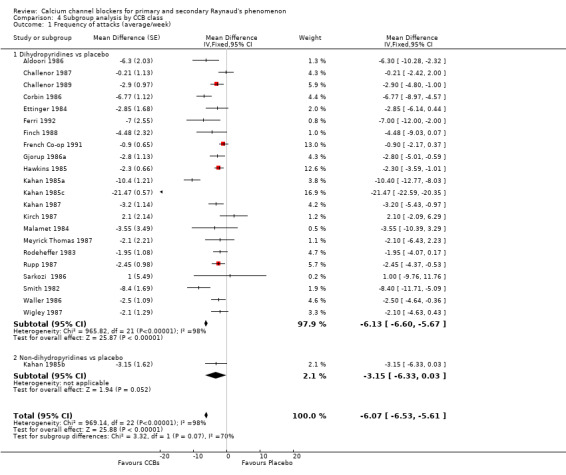
Comparison 4 Subgroup analysis by CCB class, Outcome 1 Frequency of attacks (average/week).
Of the 23 studies included in this analysis, 22 used the dihydropyridine class of CCBs, and only one used the non‐dihydropyridine class (Kahan 1985b). The WMD for frequency of attacks in the dihydropyridine class of CCBs compared with placebo was ‐6.13 (95% CI ‐6.60 to ‐5.67).
We further analyzed the data for frequency of attacks by dihydropyridine CCB type. Among 22 studies using the dihydropyridine class, 15 used nifedipine, 4 nicardipine, and 2 nisoldipine. In trials comparing nifedipine versus placebo, nifedipine reduced the number of attacks by 8.62 (95% CI ‐9.20 to ‐8.03; I² = 98%) in 290 people; nicardipine reduced the frequency of attacks by 1.92 (95% CI ‐2.80 to ‐1.04; I² = 50%) in 150 people; and nisoldipine reduced the frequency of attacks by 3.00 per week (95% CI ‐4.57 to ‐1.43; I² = 0%) in 39 people (Analysis 4.2).
4.2. Analysis.
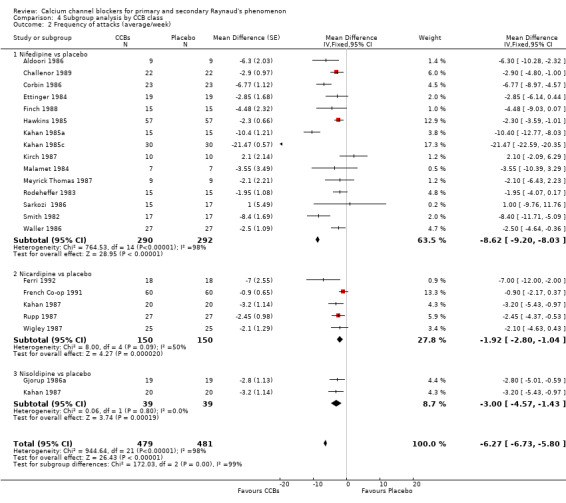
Comparison 4 Subgroup analysis by CCB class, Outcome 2 Frequency of attacks (average/week).
Severity of attacks
Average severity per attack assessed on a 10‐cm visual analogue scale (0 = no symptoms, 10 = maximal severity) in 374 participants from 16 studies
See Challenor 1989; Ettinger 1984; Ferri 1992; Finch 1988; French Co‐op 1991; Gjorup 1986a; Hawkins 1985; Kahan 1985a; Kahan 1985b; Kahan 1985c; Kahan 1987; Kirch 1987; Malamet 1984; Rupp 1987; Smith 1982; and Wigley 1987 (Analysis 4.3).
4.3. Analysis.
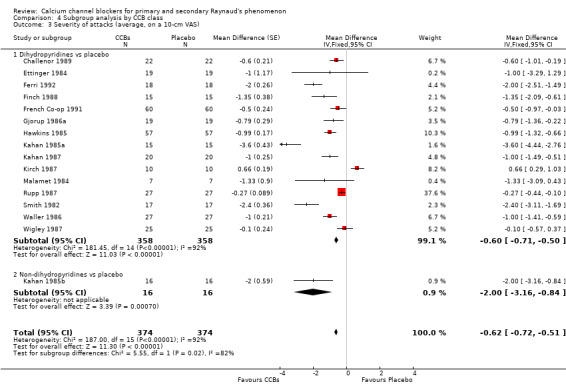
Comparison 4 Subgroup analysis by CCB class, Outcome 3 Severity of attacks (average, on a 10‐cm VAS).
Only 1 of the 16 trials providing data on severity used the non‐dihydropyridine class, and 15 used the dihydropyridine class. The WMD for severity of attacks in the 15 trials comparing dihydropyridine CCBs versus placebo was ‐0.60 (95% CI ‐0.71 to ‐0.50). Heterogeneity was substantial (I² = 92%).
Further analysis of data by dihydropyridine CCB type shows that when compared with placebo, nifedipine reduced the severity of attacks by 0.79 (95% CI ‐0.96 to ‐0.61; I² = 94%) in 189 people; nicardipine reduced the severity of attacks by 0.47 (95% CI ‐0.61 to ‐0.33; I² = 91%) in 150 people; and nisoldipine reduced the severity of attacks by 0.79 (95% CI ‐1.36 to ‐0.22) in 19 people (Analysis 4.4).
4.4. Analysis.
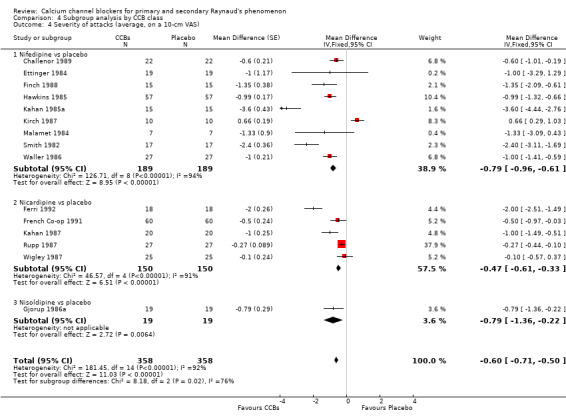
Comparison 4 Subgroup analysis by CCB class, Outcome 4 Severity of attacks (average, on a 10‐cm VAS).
Comparison 5. CCBs versus placebo in comparison by CCB dose for RP (both primary and secondary RP included)
Most of the included trials used low‐dose CCBs (refer to Appendix 2 for dosage ranges), and few used high‐dose CCBs. Hence, for this subgroup analysis, we examined low‐dose CCBs versus placebo, and medium/high‐dose CCBs versus placebo.
Frequency of attacks
Average number of attacks/week in 528 participants from 23 studies
See Aldoori 1986; Challenor 1987; Challenor 1989; Corbin 1986; Ettinger 1984; Ferri 1992; Finch 1988; French Co‐op 1991; Gjorup 1986a; Hawkins 1985; Kahan 1985a; Kahan 1985b; Kahan 1985c; Kahan 1987; Kirch 1987; Malamet 1984; Meyrick Thomas 1987; Rodeheffer 1983; Rupp 1987; Sarkozi 1986; Smith 1982; Waller 1986; and Wigley 1987 (Analysis 5.1).
5.1. Analysis.
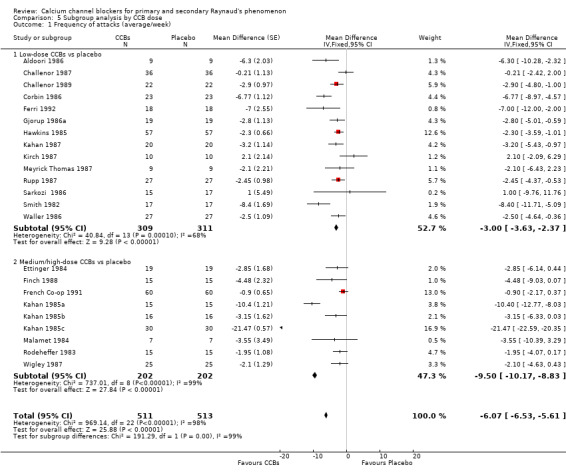
Comparison 5 Subgroup analysis by CCB dose, Outcome 1 Frequency of attacks (average/week).
When we examined the frequency of attacks by CCB dose versus placebo, we found that the pooled WMD for frequency of attacks with low‐dose CCBs was ‐3.00 (95% CI ‐3.63 to ‐2.37; I² = 68%) compared with ‐9.50 (95% CI ‐10.17 to ‐8.83; I² = 92%) with medium/high‐dose CCBs. However, medium/high‐dose CCBs showed substantial heterogeneity (I² = 99%). A sensitivity analysis of medium/high‐dose CCBs versus placebo that excluded Kahan 1985a and Kahan 1985c, whose treatment effect was much larger than that noted in the remaining studies, eliminated heterogeneity and revealed that the WMD for frequency of attacks between CCBs and placebo was ‐1.74 (95% CI ‐2.63 to ‐0.85; I² = 0%).
Differences between subgroups by dosage were statistically significant (P < 0.0001; I² = 98%).
Duration of attacks
Average duration per attack measured in minutes in 69 participants from six studies
See Aldoori 1986; Ettinger 1984; Finch 1988; Kirch 1987; Malamet 1984; and Meyrick Thomas 1987 (Analysis 5.2).
5.2. Analysis.
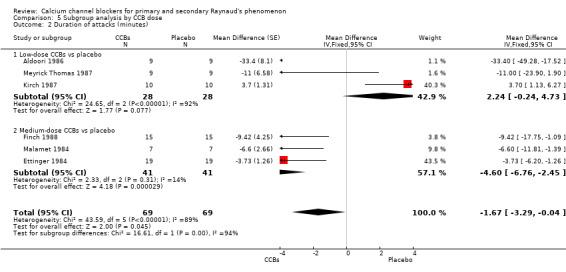
Comparison 5 Subgroup analysis by CCB dose, Outcome 2 Duration of attacks (minutes).
The pooled WMD for the duration of attacks with low‐dose CCBs was 2.24 (95% CI ‐0.24 to 4.73) compared with placebo for 56 participants. However, heterogeneity was substantial (I² = 92%). The WMD for the duration of attacks with medium‐dose CCBs was ‐4.60 (95% CI ‐6.76 to ‐2.45) compared with placebo for 82 participants.
Differences between these subgroups were statistically significant (P < 0.0001; I² = 94%).
Severity of attacks
Average severity per attack assessed on a 10‐cm visual analogue scale (0 = no symptoms, 10 = maximal severity) in 374 participants from 16 studies
See Challenor 1989; Ettinger 1984; Ferri 1992; Finch 1988; French Co‐op 1991; Gjorup 1986a; Hawkins 1985; Kahan 1985a; Kahan 1985b; Kahan 1985c; Kahan 1987; Kirch 1987; Malamet 1984; Rupp 1987; Smith 1982; and Wigley 1987 (Analysis 5.3).
5.3. Analysis.
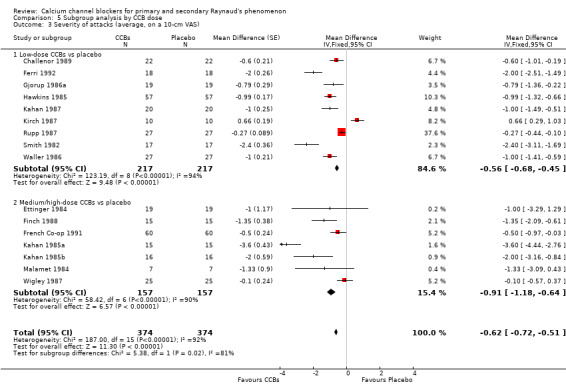
Comparison 5 Subgroup analysis by CCB dose, Outcome 3 Severity of attacks (average, on a 10‐cm VAS).
The WMD for severity of attacks was ‐0.56 (95% CI ‐0.68 to ‐0.45; I² = 94%) when low‐dose CCBs were compared with placebo in 217 people, and ‐0.91 (95% CI ‐1.18 to ‐0.64; I² = 90%) when medium/high‐dose CCBs were compared with placebo in 157 people.
Differences between these subgroups were statistically significant (P = 0.02; I² = 81%).
Pain
Average pain per attack measured on a 10‐point visual analogue scale (0 = no pain, 10 = maximal pain) in 62 participants from four studies
See Aldoori 1986; Ettinger 1984; Malamet 1984; and Rupp 1987 (Analysis 5.4).
5.4. Analysis.
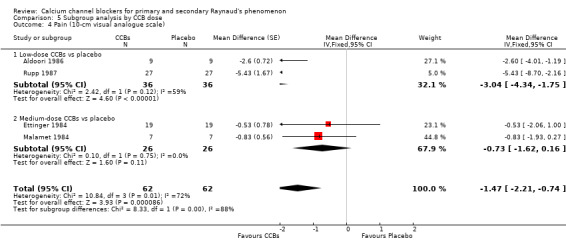
Comparison 5 Subgroup analysis by CCB dose, Outcome 4 Pain (10‐cm visual analogue scale).
The WMD for pain was ‐3.04 (95% CI ‐4.34 to ‐1.75; I² = 59%) for 36 participants with low‐dose CCBs, and ‐0.73 (95% CI ‐1.62 to 0.16) for 26 participants with medium‐dose CCBs when each was compared with placebo.
Again, differences between these subgroups were statistically significant (P = 0.04; I² = 88%).
Patient global
Disability due to Raynaud's assessed on a 10‐cm visual analogue scale (0 = no disability, 10 = maximal disability) in 96 participants from two studies
See Challenor 1987 and French Co‐op 1991 (Analysis 5.5).
5.5. Analysis.
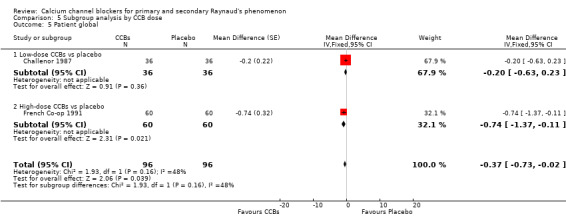
Comparison 5 Subgroup analysis by CCB dose, Outcome 5 Patient global.
Challenor 1987 compared a low‐dose CCB versus placebo in 36 people and reported that the WMD for patient global was ‐0.20 (95% CI ‐0.63 to 0.23). French Co‐op 1991 compared a high‐dose CCB versus placebo in 60 people and reported that the WMD for patient global was ‐0.74 (95% CI ‐1.37 to ‐0.11). Differences between these two trials were not statistically significant (P = 0.16; I² = 48%).
Minor outcomes
See Analysis 6.1 to Analysis 6.2 (Appendix 3).
6.1. Analysis.
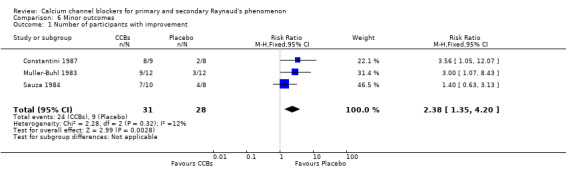
Comparison 6 Minor outcomes, Outcome 1 Number of participants with improvement.
6.2. Analysis.
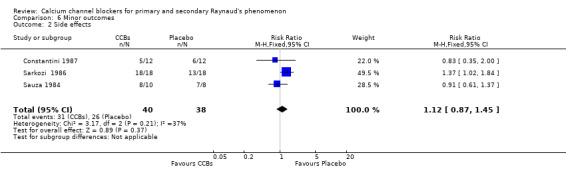
Comparison 6 Minor outcomes, Outcome 2 Side effects.
For our minor outcomes, trials reported general improvement, treatment preference, changes in digital ulcers, and side effects.
General improvement
Three parallel RCTs reported on and analyzed general improvement (Constantini 1987; Sarkozi 1986; Sauza 1984). For these trials, the risk ratio of improvement was 2.38 (95% CI 1.35 to 4.20; I² = 12%). Data from cross‐over trials were not analyzed; however, more participants from these trials reported improvement while receiving active treatment over placebo (nine cross‐over trials with 109 participants reported 76 cases of general improvement on active treatment vs 29 cases on placebo).
Treatment preference
Four cross‐over trials with a total of 89 participants reported treatment preference (Corbin 1986; Gjorup 1986a; Gjorup 1986b; Rupp 1987). Of the these 89 participants, 61 preferred active treatment and 17 indicated a preference for placebo.
Changes in digital ulcers
Only one study considered changes in digital ulceration (Meyrick Thomas 1987). This study reported 18 new digital ulcers in six patients taking placebo and nine new ulcers among three patients taking nifedipine.
Side effects
Three parallel trials reported side effects and thus were meta‐analyzed for this outcome (Constantini 1987; Sarkozi 1986; Sauza 1984). The RR for side effects was 1.12 (95% CI 0.87 to 1.45). Hence, side effects were more common with CCBs than with placebo. This was also true for the cross‐over trials: Of 573 participants from 26 cross‐over trials, 265 experienced side effects on active treatment compared with 85 on placebo.
Results from the search of regulatory websites
On March 8, 2016, we searched for black box warnings on websites for FDA MedWatch, European Medicines Evaluation Agency, Australian Adverse Drug Reactions Bulletin, and UK Medicines and Healthcare products Regulatory agencies. We found no black box warnings for nifedipine, nicardipine, nisoldipine, diltiazem, verapamil, amlodipine, isradipine, or BAY K 9320.
Discussion
Summary of main results
For this review, we identified 38 studies with 982 participants and considered all subsets of Raynaud's phenomenon (RP) (both primary and secondary). However, not all studies reported all outcomes of interest. We used the Cochrane risk of bias assessment tool as well as the GRADE approach to evaluate the quality of evidence. In addition, we examined effects of calcium channel blockers (CCBs) by class, type, and dose, as well as by Raynaud's type (primary vs secondary). The most frequently encountered risk of bias types were incomplete outcome data and lack of reporting of methods used for randomization generation and allocation. For most outcomes, we downgraded evidence for small sample sizes because included trials did not always report all outcomes. Overall, evidence quality ranged from low to moderate (three moderate quality and three low quality).
Major outcomes
CCBs (all) versus placebo for all RP subsets
When we examined CCBs compared with placebo for all RP subsets, we found that CCBs (given for between 2 and 20 weeks) were superior in reducing the frequency, severity, and pain of attacks, as seen in Table 1. Reductions in frequency and severity of Raynaud's attacks were small to moderate and were based on evidence of moderate quality derived from 23 trials with 528 participants, and from 18 trials with 415 participants, respectively. Reduced frequency of attacks with CCBs remained after a post hoc sensitivity analysis was performed to address some of the observed heterogeneity. Other plausible explanations for the remainder of the heterogeneity noted for these outcomes included variations in the duration of trials, in CCB dose and type, and in methodological rigor.
Evidence of low quality from four studies with 62 participants shows that CCBs were better than placebo for reducing pain associated with Raynaud's attacks. The impact of CCBs on the duration of attacks and on patient global, although favoring CCBs, was uncertain because evidence on small numbers of participants was obtained from a limited number of trials measuring and reporting these outcomes.
Two parallel studies with 69 participants analyzed withdrawals due to adverse events and found that withdrawals were more common with CCBs than with placebo; however, this evidence was of low quality and differences may not have been important. For cross‐over trials, we did not analyze withdrawals (because of the challenges involved in analyzing dichotomous outcomes from cross‐over trials), but we noted that overall, more withdrawals were reported with CCBs than with placebo. These studies did not report serious adverse events (leading to withdrawal followed by hospitalization or death) directly attributed to treatment.
CCBs versus placebo by RP type (primary vs secondary)
Examination of all CCBs versus placebo by RP type revealed that CCBs (when compared with placebo) had a similar effect in both subgroups with regard to reducing the frequency of attacks (a reduction of three attacks/week). However, the number of participants with primary RP was much larger than the number with secondary RP. Hence, this difference in sample sizes may have affected observed effect estimates. Another comparison revealed that CCBs were superior to placebo in primary RP with regard to reducing the severity of attacks (‐0.91 cm vs ‐0.48 cm, respectively). The smaller treatment effect sizes noted in secondary RP with regard to RP severity are consistent with those reported in clinical practice owing to the more severe nature of the disease in this subpopulation.
Nifedipine versus placebo by RP type (primary or secondary)
Comparison of nifedipine versus placebo by RP type showed that nifedipine was slightly better in reducing the frequency of attacks in primary RP (‐4.42 vs ‐4.19, respectively); and nifedipine was much more effective in reducing the severity of attacks in primary than in secondary RP (‐1.74 cm vs 0.01 cm, respectively). However, sample sizes for these subgroup analyses were very small, likely making analyses underpowered to detect the true effects of treatment. In general, improvement in secondary RP may be less than in primary RP, as the condition in the former group is likely more severe and potentially less reversible with fixed vascular changes ‐ not just vasospasm.
Subgroup analysis by CCB class: dihydropyridines (most common class of CCBs) versus placebo for all RP subsets
Comparison of only the dihydropyridine class of CCBs versus placebo for all RP subsets yielded similar results to those obtained when all CCBs were compared with placebo; this was expected because all trials but one used the dihydropyridine class of CCBs.
Nifedipine, nicardipine, and nisoldipine were analyzed separately versus placebo for frequency and severity of attacks. All three CCBs were superior to placebo in decreasing both the frequency and the severity of attacks. Nifedipine was used by most of the included trials and led to the largest reduction in frequency of attacks. Nifedipine also had a larger effect estimate with regard to severity of attacks when compared with nicardipine ‐ the second most commonly used CCB. Nisoldipine was used by only two trials and had a larger effect size on severity of attacks, but this effect estimate was likely imprecise owing to the small sample size. Overall, nifedipine appeared to be the most beneficial treatment with regard to reducing the frequency and severity of attacks.
CCBs (by dose) versus placebo for all RP subsets
Comparison of CCBs versus placebo by CCB dose (low, medium/high) revealed that higher doses were superior to lower doses in reducing the frequency, duration, and severity of attacks, as well as in improving patient global. In addition, although most trials used low‐dose CCBs, and fewer used medium to high doses, results show that higher doses had larger treatment effects and hence appeared more beneficial with regard to the frequency, duration, and severity of attacks, as well as improved patient global. Although significant heterogeneity was evident, low‐dose CCBs reduced the frequency of attacks per week by 3.0 and the severity of attacks by 0.56 cm (on a scale of 0 to 10 cm), and medium/high‐dose CCBs reduced the frequency of attacks by 9.5 and severity by 0.91 cm. In addition, medium/high‐dose CCBs were superior to placebo in reducing the average duration of attacks (mean difference [WMD] ‐4.60, 95% CI ‐6.76 to ‐2.45), but low‐dose CCBs compared with placebo did not produce this effect (WMD 2.24, 95% CI ‐0.24 to 4.73). Higher doses of CCBs were better in reducing disability due to RP (patient global): A study comparing a high‐dose CCB versus placebo in 60 participants found a reduction of 0.74 cm (95% CI ‐1.37 to ‐0.11) with CCBs, but another trial using a low‐dose CCB versus placebo in 36 participants found a reduction of 0.20 cm (95% CI ‐0.63 to 0.23).
Researchers found a larger reduction in pain with low‐dose CCBs versus placebo than with higher‐dose CCBs versus placebo. However, overall sample sizes were small, and a larger number of trial participants used low‐dose CCBs compared with placebo.
Overall, higher doses of CCBs compared with placebo appear to be more beneficial with regard to the primary outcomes examined. Larger treatment effect estimates of CCBs at higher doses are consistent with findings reported in clinical practice, but dosages generally are based on patient tolerability.
Heterogeneity and publication bias
We explored explanations for the presence of heterogeneity in treatment effects by performing subgroup and sensitivity analyses. Overall, we observed heterogeneity with most outcomes that was partially explained by differences in the type and dose of CCBs, as well as by differences in disease type (primary vs secondary RP) and study duration, with most trials being of very short duration. Other plausible factors that may have contributed to heterogeneity but were not explored include differences in inclusion criteria of trials, rigor in trial methods, trial type (cross‐over vs parallel), and differences in the time of year trials were performed. To our knowledge, only one study reported that concomitant medications were continued during the trial. Hence, we do not believe that use of concomitant vasodilator medications was a significant confounder in the observed results.
For major outcomes reported in at least 10 trials (frequency and severity of attacks), we examined funnel plots to assess for publication bias. Both funnel plots showed some asymmetry around the mean effect estimate but did not indicate publication bias as a major concern. Plausible explanations for the presence of asymmetry in these funnel plots include the presence of significant heterogeneity, differences in the quality and size of trials, differences in methods (i.e., parallel vs cross‐over design), and possibly selective reporting.
Minor outcomes
With regard to our minor outcomes, analysis of three parallel trials showed that CCBs were associated with more general improvement in patients' Raynaud's condition. We were unable to meta‐analyze dichotomous outcomes from cross‐over trials, but we did examine overall trends. Cross‐over trials also reported that more participants reported improvement while taking CCBs than placebo, and more participants preferred active treatment over placebo. One trial reporting changes in digital ulceration found that nine participants developed 18 new digital ulcers while taking placebo, and three participants developed six new digital ulcers while taking nifedipine. Results from this trial suggest that CCBs such as nifedipine may be useful for prevention of digital ulcers. However, participants who took CCBs reported more side effects than were reported by those who took placebo, but none of these reported adverse effects were serious.
Conclusions from this review and meta‐analysis
Overall, although they were associated with more non‐serious side effects, CCBs were preferred to placebo and were better in reducing the frequency, duration, and severity of Raynaud's attacks when given over 2 to 20 weeks. In addition, CCBs produced a potentially clinically important mean improvement in pain, and more participants taking CCBs reported general improvement. In particular, CCBs showed greater benefit at higher doses in reducing the frequency and severity of attacks for patients with primary RP. It is important to note that researchers reported no serious adverse events directly related to treatment with CCBs. These findings indicate that CCBs, particularly those in the dihydropyridine class (i.e., nifedipine and nicardipine), given at higher doses may be beneficial for the management of RP (especially among patients with primary RP) requiring pharmacological intervention (although most people with RP, especially primary RP, do not require pharmacological treatment). Some clinically important outcomes of interest (i.e., changes in average duration of attacks, pain, and patient global) were measured or reported by only a few trials. Hence, effect estimates for these outcomes were imprecise but appeared to favor CCBs. The findings of this review may be generalizable to other members of the dihydropyridine class of CCBs, such as amlodipine, bepridil, felodipine, isradipine, and nisoldipine.
Overall completeness and applicability of evidence
Most of the trials included in this review were small and included a mixture of patients with primary and secondary RP. Fewer trials enrolled patients with secondary RP, which makes it more difficult to generalize the results of this review to these patients. Review results indicate that CCBs (particularly those in the dihydropyridine class) may be beneficial in the management of RP, especially primary RP. Furthermore, both nifedipine and nicardipine ‐ the most commonly used dihydropyridines ‐ proved superior to placebo in reducing the frequency and severity of RP attacks. However, several concerns must be addressed to guide clinicians in using or interpreting the findings of this review.
The overall quality of evidence ranged from low to moderate, with evidence for three outcomes being of low quality and three of moderate quality. Many trials included in the meta‐analyses were not methodologically rigorous. This variation in the quality of the evidence requires cautious interpretation and use of review findings, especially in clinical practice.
Most trials had small sample sizes. As a result, we were unable to conduct subgroup analysis for all major outcomes of this review. The overall small sample size was a problem, especially for some outcomes such as duration and patient global, which were not measured or reported by many trials. For outcomes with small sample sizes, results often have large confidence intervals and are imprecise.
Most included trials were of the cross‐over design. Potential carryover effects between active treatment and placebo are possible with this method. We were unable to estimate a period effect. Results may be biased.
The dosage of CCBs used was highly variable, with most trials using low‐dose CCBs. We found evidence of higher efficacy for medium/high versus.low doses of CCBs in RP treatment. Hence, suboptimal doses in most included trials may be responsible for the non‐significant findings observed for some outcomes. Clinicians may start with low doses of CCBs and may increase dosage to achieve a desired effect (if not obtained with low dose), as long as the patient can tolerate it and no serious side effects develop.
Most included trials include a mixture of patients with primary and secondary RP. Only seven trials exclusively included patients with primary RP, and even fewer included only those with secondary RP. Consistent with the findings of this study, patients with primary RP often respond better to medication, possibly as a result of the milder nature of their disease. Only a few studies including patients with secondary RP reported the important outcomes of interest. Most trials did not stratify randomization between primary and secondary RP (or within secondary RP between systemic sclerosis, which is the most severe form of secondary RP, and other connective tissue disorders). This could have introduced bias, as groups may not have been equal with respect to the proportion of primary and secondary RP between treatment arms (for parallel designed studies).
Another problem could be the variability of treatment duration between trials. However, CCBs are fast acting, and most patients respond to treatment quickly. Thus efficacy of CCBs would be expected in short trials, but longer trials may report increased dropout and greater regression to the mean and/or attenuation of benefit for other reasons.
Included trials showed high variability in outcome measurements and scales used. To allow for ease of interpretation, we converted all results to the most commonly used scales. For instance, for severity of attacks, we converted the results of all trials to a 10‐cm visual analogue scale score. This variability in the scales of measure likely contributed to some of the observed heterogeneity.
Last, significant statistical heterogeneity was seen with most outcomes. Some heterogeneity was partially reduced by subgroup and sensitivity analyses. However, heterogeneity often remained and is likely a result of the different factors discussed above.
Quality of the evidence
We assessed the quality of evidence from trials included in this review using the GRADE approach (Grade 2008). Many trials, particularly older ones from the 1980s (when reporting was less standardized and protocols were not registered) failed to fully describe their methods. The deficiency that we most commonly encountered when assessing risk of bias in these trials was lack of full descriptions of random sequence generation and allocation concealment. Most publications noted that the study was "randomized" and "double‐blind" without providing further details.
We downgraded outcomes of frequency of attacks and severity of attacks to moderate quality owing to concerns about inconsistency of results across trials. We downgraded evidence on patient global assessment to moderate quality for concerns about imprecision due to the small sample size. We downgraded evidence on duration of attacks and pain to low quality for concerns about both inconsistency and imprecision. We downgraded evidence quality to low for withdrawals due to adverse events, given the small sample size (imprecision) and high attrition rate noted in the two studies that reported this outcome.
Given the moderate to low quality of evidence on major outcomes, one should use caution, but trial results do mirror what is seen in clinical practice; hence they have face validity despite methodological flaws.
Potential biases in the review process
We performed an extensive and sensitive electronic search of all important databases to ensure retrieval of as many studies as possible. In addition, at least two review authors performed most of the steps of the review and consulted with a third review author regarding disagreements.
Some subgroup analyses were post hoc which may have introduced bias as many trials did not stratify randomization on the analyses of interest. We did plan to study: primary vs. secondary RP and RP from systemic sclerosis (as the scleroderma patients are more difficult to treat and have more severe and complicated RP), we planned to study subsets of CCBs (dihydropyridines and nifedipine in particular as we knew the bulk of the data was from nifedipine), but studying some other subgroups post hoc may have been biased. Overall, we did not identify any major biases in the review process.
Agreements and disagreements with other studies or reviews
The findings of this review, although broader in scope, are similar to those of our 2005 meta‐analysis ‐ Thompson 2005 ‐ and of a recent meta‐analysis ‐ Ennis 2016 ‐ on the use of oral vasodilators (including CCBs) for primary RP. Both of these meta‐analyses noted reduced frequency of attacks among patients with primary RP when treated with CCBs. Thompson 2005 found that CCBs decreased the frequency of attacks in patients with primary RP by a mean of 2.8 to 5 attacks per week, and Ennis 2016 noted a decrease of 0.6 to 2.8 attacks per week. In the current review, when review authors considered both primary and secondary RP, we found an average decrease in the frequency of six attacks per week (and an average decrease of three attacks per week with exclusion of Kahan 1985c). When considering only primary RP, we found an average decrease of three attacks per week. This difference between our study and Ennis 2016 may be due to the fact that Ennis 2016 included all trials examining CCBs versus placebo or other treatments. We limited our review to trials examining only CCBs versus placebo and no other oral vasodilators from other classes of medications.
The Thompson 2001 meta‐analysis shows that CCBs significantly reduced both the frequency and the severity of attacks in patients with systemic sclerosis. This review did not find that CCBs significantly reduced any of the reported major outcomes in patients with secondary RP. Differences between these reviews are likely due to the fact that review authors for Thompson 2001 included a larger number of trials (any trials with over 75% of trial participants with systemic sclerosis) and performed subset analyses of data from trials that included participants with systemic sclerosis, if data could be extracted. In our current review of secondary RP, we included only trials in which all participants had secondary RP of any origin, resulting in very small numbers of trials and participants. We included any trial of participants with secondary RP in which nearly all had connective tissue disease.
Authors' conclusions
Implications for practice.
This review is the largest and most comprehensive systematic review conducted to investigate the efficacy of calcium channel blockers (CCBs) for the management of Raynaud's phenomenon (RP). Evidence of low to moderate quality assembled for this review indicates that CCBs (such as nifedipine) may be effective in reducing the frequency and severity of Raynaud's attacks, especially in primary RP. In addition, results of this review show that higher doses may be more useful than lower doses (although in clinical practice, dose is based on desired outcome and patient tolerability). Adverse effects were more common with CCBs, but none were considered serious. Longer‐term treatment effects of CCBs for RP are unknown, as most trials have had a very short duration. However, CCBs are used routinely over the long term for patients with RP who require drug treatment with ongoing effectiveness for long periods of time, often as needed or on a regular basis. Harms include side effects such as hypotension, flushing, and peripheral edema which can lead to discontinuation of treatment in some patients. However, serious adverse events within the trials (most of which were relatively short duration) were rare and did not differ from placebo, or not reported.
Implications for research.
In many randomized controlled trials undertaken to study RP, the placebo effect may be great, so the sample size may need to be adjusted upward.
Most of the included studies were older and smaller studies that had methodological deficiencies. Larger studies that are more methodologically robust would allow for better estimation of the true effect of CCBs, but likely no further trials are needed to examine current use of CCBs in patients with RP. Head‐to‐head comparisons of CCBs and other treatments for RP could clarify the relative positioning of other agents and/or reveal relative effects of various RP treatments via a network meta‐analysis. In addition, not all included trials reported all outcomes of interest. Future trials should use standardized outcome measurements (a minimum core set) and similar time frames (i.e. frequency of RP attacks over one week); these trials need to be registered and should report all outcomes studied within the trial to yield more accurate estimations of treatment effect.
Additionally, most included trials were of the cross‐over design with potential carryover effects between placebo and active treatment, which could introduce bias against the active treatment effect. Differences in the number of RP attacks between individuals can be very skewed, so an advantage can be gained by using cross‐over trials with fast‐acting and fast‐washing out medications for RP treatment; also, a sample size (cost and feasibility) advantage and having each patient act as his or her own control can reduce variability between patients in the number of RP attacks.
Response to treatment differs between patients with primary and secondary RP. Secondary RP is more difficult to manage, as the disease is more severe. When possible, researchers should report the results of primary and secondary RP subsets separately. More important, future studies should include use of the validated Raynaud's condition score (RCS) to allow for more consistent comparison between trials (Khanna 2009; Merkel 2002). Use of various scales for measurement of outcomes likely contributes to heterogeneity.
History
Protocol first published: Issue 1, 1996 Review first published: Issue 12, 2017
| Date | Event | Description |
|---|---|---|
| 5 September 2008 | Amended | Converted to new review format |
Acknowledgements
We are grateful to Ingrid Tows and the German Cochrane Centre for their services in translating a German study.
We also thank our reviewers for their valuable feedback and contributions to this review.
Appendices
Appendix 1. Appendix 1: search methods
Appendix 1: search methods
| Raynaud's phenomenon | |||
| Database and coverage | Search date | Number of references retrieved | With duplicates removed |
| The Cochrane Library
Cochrane Reviews
Issue 12 of 12, December 2015
Other Reviews (DARE)
Issue 2 of 4, April 2014 CENTRAL Methods studies Issue 3 of 4, July 2012 Technology assessments Issue 2 of 4, April 2014 Economic evaluation |
December 2, 2015 | 14
26 1011 5 2 3 |
8
26 358 2 3 3 |
| Ovid MEDLINE(R) 1946‐present | 1027 | 1005 | |
| Ovid Embase Classic + Embase 1947‐present | 1251 | 884 | |
| Clinicaltrials.gov | 50 | 48 | |
| WHO portal (who.int/trialsearch, all years) | 0 | 0 | |
| Totals | 3389 | 2337 | |
Cochrane Library – Issue 6, 2014 Search name: Raynaud's Last saved: 02/12/2015 13:58:12.787 Description:
ID search:
#1 MeSH descriptor: [Raynaud Disease] explode all trees
#2 raynaud*:ti,ab,kw
#3 vasospasm:ti,ab,kw
#4 #1 or #2 or #3
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to present>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Raynaud Disease/ (5811)
2 Vasospasm.ti,ab. (9022)
3 raynaud$.tiw. (5879)
4 or/1‐3 (16735)
5 randomized controlled trial.pt. (376269)
6 controlled clinical trial.pt. (88551)
7 randomized.ab. (296657)
8 placebo.ab. (154994)
9 clinical trials as topic.sh. (170411)
10 randomly.ab. (214652)
11 trial.ti. (127683)
12 or/5‐11 (909747)
13 exp animals/ not humans.sh. (3951755)
14 12 not 13 (839533)
15 4 and 14 (1027)
Database: Embase Classic + Embase <1947 to June 20, 2014>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Raynaud phenomenon/ (11646)
2 vasospasm/ (7831)
3 raynaud$.tiw. (8895)
4 or/1‐3 (20897)
5 random$.tiw. (900126)
6 factorial$.tiw. (23754)
7 crossover$.tiw. (50156)
8 cross over.tw. (22510)
9 cross‐over.tw. (22510)
10 placebo$.tiw. (207490)
11 (doubl$ adj blind$).tiw. (150810)
12 (singl$ adj blind$).tiw. (14649)
13 assign$.tiw. (244100)
14 allocate$.tiw. (85325)
15 volunteer$.tiw. (186103)
16 crossover procedure/ (39529)
17 double blind procedure/ (118455)
18 randomized controlled trial/ (346367)
19 single blind procedure/ (18431)
20 or/5‐19 (1457107)
21 4 and 20 (1251)
Clinicaltrials.gov Advanced search screen
Condition=raynaud
Updated search performed on May 19, 2017
| Raynaud's phenomenon | |||
| Database and coverage | Search date | Number of references retrieved | With duplicates removed |
| EBM Reviews ‐ CENTRAL (via OVID) | May 19, 2017 | 101 | |
| Ovid Medline(R) 1946‐present | May 19, 2017 | 50 | |
| Ovid Embase Classic + Embase 1947‐present | May 19, 2017 | 104 | |
| Totals | 255 | ||
CENTRAL Search name: Raynaud's Last saved: May 19, 2017 Description:
ID search:
C1 ‐ Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <April 2017>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Raynaud Disease/ (267)
2 Vasospasm.ti,ab. (570)
3 raynaud$.tiw. (524)
4 or/1‐3 (1084)
5 limit 4 to yr="2015 ‐Current" (101)
C1 ‐ Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to present>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Raynaud Disease/ (6394)
2 Vasospasm.ti,ab. (10215)
3 raynaud$.tiw. (6728)
4 or/1‐3 (18859)
5 randomized controlled trial.pt. (462560)
6 controlled clinical trial.pt. (94063)
7 randomized.ab. (395329)
8 placebo.ab. (186432)
9 clinical trials as topic.sh. (185904)
10 randomly.ab. (275397)
11 trial.ti. (177271)
12 or/5‐11 (1120634)
13 exp animals/ not humans.sh. (4399234)
14 12 not 13 (1032602)
15 4 and 14 (1163)
16 limit 15 to ed=20151202‐20170519 (50)
C1 ‐ Database: Embase <1974 to May 18, 2017>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Raynaud phenomenon/ (11924)
2 vasospasm/ (7993)
3 raynaud$.tiw. (9084)
4 or/1‐3 (21611)
5 random$.tiw. (1188308)
6 factorial$.tiw. (30059)
7 crossover$.tiw. (61652)
8 cross over.tw. (27084)
9 cross‐over.tw. (27084)
10 placebo$.tiw. (254043)
11 (doubl$ adj blind$).tiw. (177952)
12 (singl$ adj blind$).tiw. (19292)
13 assign$.tiw. (311284)
14 allocate$.tiw. (115199)
15 volunteer$.tiw. (219603)
16 crossover procedure/ (51208)
17 double blind procedure/ (138220)
18 randomized controlled trial/ (449401)
19 single blind procedure/ (26863)
20 or/5‐19 (1850737)
21 4 and 20 (1495)
22 Raynaud phenomenon/ (11924)
23 vasospasm/ (7993)
24 raynaud$.tiw. (9084)
25 or/22‐24 (21611)
26 random$.tiw. (1188308)
27 factorial$.tiw. (30059)
28 crossover$.tiw. (61652)
29 cross over.tw. (27084)
30 cross‐over.tw. (27084)
31 placebo$.tiw. (254043)
32 (doubl$ adj blind$).tiw. (177952)
33 (singl$ adj blind$).tiw. (19292)
34 assign$.tiw. (311284)
35 allocate$.tiw. (115199)
36 volunteer$.tiw. (219603)
37 crossover procedure/ (51208)
38 double blind procedure/ (138220)
39 randomized controlled trial/ (449401)
40 single blind procedure/ (26863)
41 or/26‐40 (1850737)
42 25 and 41 (1495)
43 limit 42 to dd=20151202‐20170519 (104)
Appendix 2. Appendix 2: dosage guidelines
Dosage guidelines*
| Calcium channel blocker | Low dose (daily dose) |
Medium dose (daily dose) |
High dose (daily dose) |
| Amlodipine | 2.5 mg | 5 mg | 10 mg |
| Isradipine | 5 mg | 10 mg | ‐ |
| Nicardipine | 60 mg | 90 mg | 120 mg |
| Nifedipine | < or = 30 mg | >30 mg | 90 mg |
| Nisoldipine | 20 mg | 30 mg | 60 mg |
| Diltiazem | 180 mg | 240 mg | 360 mg |
| Verapamil | 180 mg | 240 mg | 360 mg |
*Dosage based on Greater Rochester Independent Practice Association (GRIPA) guidelines.
Appendix 3. Appendix 3: Dichotomous outcomes from cross‐over trials
| Study | General improvement | Treatment preference | Side effects | Withdrawals | ||||||||||||
| Treatment | Placebo | Treatment | Placebo | Treatment | Placebo | Treatment | Placebo | |||||||||
| Events | Total | Events | Total | Events | Total | Events | Total | Events | Total | Events | Total | Events | Total | Events | Total | |
| Aldoori 1986 | 10 | 13 | 4 | 13 | ||||||||||||
| Bravard 1983 | 5 | 5 | 3 | 5 | ||||||||||||
| Challenor 1989 | 16 | 24 | 5 | 24 | 2 | 24 | 0 | 24 | ||||||||
| Corbin 1986 | 15 | 22 | 2 | 22 | 14 | 23 | 2 | 23 | 3 | 22 | 0 | 22 | ||||
| Ettinger 1984 | 15 | 19 | 12 | 19 | 12 | 22 | 1 | 22 | ||||||||
| Ferri 1992 | 2 | 21 | 0 | 21 | ||||||||||||
| French Co‐op 1991 | 19 | 69 | 7 | 69 | 2 | 69 | 5 | 69 | ||||||||
| Gjorup 1986a | 12 | 19 | 4 | 19 | 5 | 19 | 0 | 19 | ||||||||
| Gjorup 1986b | 19 | 21 | 0 | 21 | 16 | 21 | 0 | 21 | 4 | 26 | 0 | 26 | ||||
| Hawkins 1985 | 19 | 57 | 6 | 57 | 7 | 57 | 1 | 57 | ||||||||
| Kahan 1985a | 12 | 15 | 1 | 15 | 6 | 15 | 2 | 15 | ||||||||
| Kahan 1985b | 9 | 16 | 3 | 16 | 6 | 16 | 2 | 16 | ||||||||
| Kahan 1985c | 9 | 30 | 3 | 30 | ||||||||||||
| Kahan 1987 | 7 | 20 | 2 | 20 | 7 | 20 | 2 | 20 | ||||||||
| Kallenberg 1987 | 14 | 15 | 0 | 15 | ||||||||||||
| Kinney 1982 | 3 | 14 | 10 | 14 | ||||||||||||
| Kirch 1987 | 8 | 10 | 2 | 10 | 2 | 10 | 2 | 10 | ||||||||
| La Civita 1997 | 11 | 20 | 0 | 20 | ||||||||||||
| Leppert 1989 | 5 | 10 | 3 | 10 | ||||||||||||
| Meyrick Thomas 1987 | 1 | 9 | 2 | 9 | 1 | 10 | 0 | 10 | ||||||||
| Nilsson 1987 | 20 | 28 | 8 | 28 | 1 | 28 | 0 | 28 | ||||||||
| Rhedda 1985 | 2 | 19 | 1 | 19 | ||||||||||||
| Rodeheffer 1983 | 9 | 15 | 2 | 15 | 15 | 27 | 11 | 27 | 12 | 15 | 3 | 15 | ||||
| Rupp 1987 | 15 | 27 | 9 | 27 | ||||||||||||
| Smith 1982 | 15 | 17 | 1 | 17 | ||||||||||||
| Taixeira Da Costa 1987 | 4 | 14 | 6 | 14 | ||||||||||||
| Waller 1986 | 26 | 29 | 5 | 29 | ||||||||||||
| Wigley 1987 | 12 | 25 | 7 | 25 | 12 | 25 | 7 | 25 | ||||||||
| White 1982 | 9 | 11 | 1 | 11 | ||||||||||||
| Wollersheim 1991 | 2 | 25 | 1 | 25 | ||||||||||||
| Total | 76 | 109 | 29 | 109 | 61 | 89 | 17 | 89 | 265 | 573 | 85 | 573 | 39 | 281 | 15 | 281 |
Data and analyses
Comparison 1. CCBs vs placebo (generic inverse variance method).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of attacks (average/week) | 23 | 1024 | Mean Difference (Fixed, 95% CI) | ‐6.07 [‐6.53, ‐5.61] |
| 2 Frequency of attacks (average/week) | 22 | Mean Difference (Fixed, 95% CI) | ‐2.93 [‐3.44, ‐2.43] | |
| 3 Duration of attacks (minutes) | 6 | 138 | Mean Difference (Fixed, 95% CI) | ‐1.67 [‐3.29, ‐0.04] |
| 4 Severity of attacks (average, on a 10‐cm VAS) | 16 | 748 | Risk Difference (Fixed, 95% CI) | ‐0.62 [‐0.72, ‐0.51] |
| 5 Pain (10‐cm visual analogue scale) | 4 | 124 | Std. Mean Difference (Fixed, 95% CI) | ‐1.47 [‐2.21, ‐0.74] |
| 6 Patient global | 2 | 192 | Std. Mean Difference (Fixed, 95% CI) | ‐0.37 [‐0.73, ‐0.02] |
| 7 Number of withdrawals (due to treatment) | 2 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.51, 3.33] |
Comparison 2. Subgroup analysis by RP type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of attacks (average/week) | 15 | 624 | Mean Difference (Fixed, 95% CI) | ‐3.15 [‐3.67, ‐2.63] |
| 1.1 CCBs vs placebo for primary RP | 12 | 420 | Mean Difference (Fixed, 95% CI) | ‐3.02 [‐3.65, ‐2.38] |
| 1.2 CCBs vs placebo for secondary RP | 9 | 204 | Mean Difference (Fixed, 95% CI) | ‐3.42 [‐4.33, ‐2.51] |
| 2 Severity of attacks (average, on a 10‐cm VAS) | 10 | 506 | Mean Difference (Fixed, 95% CI) | ‐0.67 [‐0.77, ‐0.57] |
| 2.1 Primary RP: CCBs vs placebo | 8 | 368 | Mean Difference (Fixed, 95% CI) | ‐0.95 [‐1.11, ‐0.79] |
| 2.2 Secondary RP: CCBs vs placebo | 6 | 138 | Mean Difference (Fixed, 95% CI) | ‐0.48 [‐0.61, ‐0.35] |
Comparison 3. Subgroup analysis: nifedipine versus placebo by RP type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of attacks: nifedipine vs placebo by RP type | 9 | 268 | Mean Difference (Fixed, 95% CI) | ‐4.34 [‐5.09, ‐3.59] |
| 1.1 Nifedipine vs placebo for primary RP | 6 | 148 | Mean Difference (Fixed, 95% CI) | ‐4.42 [‐5.35, ‐3.50] |
| 1.2 Nifedipine vs placebo for secondary RP | 6 | 120 | Mean Difference (Fixed, 95% CI) | ‐4.19 [‐5.47, ‐2.91] |
| 2 Severity of attacks: nifedipine vs placebo by RP type | 4 | 108 | Mean Difference (Fixed, 95% CI) | ‐0.82 [‐1.07, ‐0.58] |
| 2.1 Primary RP | 2 | 54 | Mean Difference (Fixed, 95% CI) | ‐1.74 [‐2.09, ‐1.39] |
| 2.2 Secondary RP | 3 | 54 | Mean Difference (Fixed, 95% CI) | 0.01 [‐0.32, 0.34] |
Comparison 4. Subgroup analysis by CCB class.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of attacks (average/week) | 23 | Mean Difference (Fixed, 95% CI) | ‐6.07 [‐6.53, ‐5.61] | |
| 1.1 Dihydropyridines vs placebo | 22 | Mean Difference (Fixed, 95% CI) | ‐6.13 [‐6.60, ‐5.67] | |
| 1.2 Non‐dihydropyridines vs placebo | 1 | Mean Difference (Fixed, 95% CI) | ‐3.15 [‐6.33, 0.03] | |
| 2 Frequency of attacks (average/week) | 21 | 960 | Mean Difference (Fixed, 95% CI) | ‐6.27 [‐6.73, ‐5.80] |
| 2.1 Nifedipine vs placebo | 15 | 582 | Mean Difference (Fixed, 95% CI) | ‐8.62 [‐9.20, ‐8.03] |
| 2.2 Nicardipine vs placebo | 5 | 300 | Mean Difference (Fixed, 95% CI) | ‐1.92 [‐2.80, ‐1.04] |
| 2.3 Nisoldipine vs placebo | 2 | 78 | Mean Difference (Fixed, 95% CI) | ‐1.00 [‐4.57, ‐1.43] |
| 3 Severity of attacks (average, on a 10‐cm VAS) | 16 | 748 | Mean Difference (Fixed, 95% CI) | ‐0.62 [‐0.72, ‐0.51] |
| 3.1 Dihydropyridines vs placebo | 15 | 716 | Mean Difference (Fixed, 95% CI) | ‐0.60 [‐0.71, ‐0.50] |
| 3.2 Non‐dihydropyridines vs placebo | 1 | 32 | Mean Difference (Fixed, 95% CI) | ‐2.0 [‐3.16, ‐0.84] |
| 4 Severity of attacks (average, on a 10‐cm VAS) | 15 | 716 | Mean Difference (Fixed, 95% CI) | ‐0.60 [‐0.71, ‐0.50] |
| 4.1 Nifedipine vs placebo | 9 | 378 | Mean Difference (Fixed, 95% CI) | ‐0.79 [‐0.96, ‐0.61] |
| 4.2 Nicardipine vs placebo | 5 | 300 | Mean Difference (Fixed, 95% CI) | ‐0.47 [‐0.61, ‐0.33] |
| 4.3 Nisoldipine vs placebo | 1 | 38 | Mean Difference (Fixed, 95% CI) | ‐0.79 [‐1.36, ‐0.22] |
Comparison 5. Subgroup analysis by CCB dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of attacks (average/week) | 23 | 1024 | Mean Difference (Fixed, 95% CI) | ‐6.07 [‐6.53, ‐5.61] |
| 1.1 Low‐dose CCBs vs placebo | 14 | 620 | Mean Difference (Fixed, 95% CI) | ‐1.00 [‐3.63, ‐2.37] |
| 1.2 Medium/high‐dose CCBs vs placebo | 9 | 404 | Mean Difference (Fixed, 95% CI) | ‐9.50 [‐10.17, ‐8.83] |
| 2 Duration of attacks (minutes) | 6 | 138 | Mean Difference (Fixed, 95% CI) | ‐1.67 [‐3.29, ‐0.04] |
| 2.1 Low‐dose CCBs vs placebo | 3 | 56 | Mean Difference (Fixed, 95% CI) | 2.24 [‐0.24, 4.73] |
| 2.2 Medium‐dose CCBs vs placebo | 3 | 82 | Mean Difference (Fixed, 95% CI) | ‐4.60 [‐6.76, ‐2.45] |
| 3 Severity of attacks (average, on a 10‐cm VAS) | 16 | 748 | Mean Difference (Fixed, 95% CI) | ‐0.62 [‐0.72, ‐0.51] |
| 3.1 Low‐dose CCBs vs placebo | 9 | 434 | Mean Difference (Fixed, 95% CI) | ‐0.56 [‐0.68, ‐0.45] |
| 3.2 Medium/high‐dose CCBs vs placebo | 7 | 314 | Mean Difference (Fixed, 95% CI) | ‐0.91 [‐1.18, ‐0.64] |
| 4 Pain (10‐cm visual analogue scale) | 4 | 124 | Mean Difference (Fixed, 95% CI) | ‐1.47 [‐2.21, ‐0.74] |
| 4.1 Low‐dose CCBs vs placebo | 2 | 72 | Mean Difference (Fixed, 95% CI) | ‐3.04 [‐4.34, ‐1.75] |
| 4.2 Medium‐dose CCBs vs placebo | 2 | 52 | Mean Difference (Fixed, 95% CI) | ‐0.73 [‐1.62, 0.16] |
| 5 Patient global | 2 | 192 | Mean Difference (Fixed, 95% CI) | ‐0.37 [‐0.73, ‐0.02] |
| 5.1 Low‐dose CCBs vs placebo | 1 | 72 | Mean Difference (Fixed, 95% CI) | ‐0.2 [‐0.63, 0.23] |
| 5.2 High‐dose CCBs vs placebo | 1 | 120 | Mean Difference (Fixed, 95% CI) | ‐0.74 [‐1.37, ‐0.11] |
Comparison 6. Minor outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with improvement | 3 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.35, 4.20] |
| 2 Side effects | 3 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aldoori 1986.
| Methods | Randomized double‐blind controlled trial | |
| Participants | 13 patients (10 women and 3 men) Median age 50 (range 25 to 68) 10 patients with diagnosis of Raynaud's, 3 with evidence of scleroderma Mean duration of symptoms 9 years (range 2 to 30) All but 2 treated previously for disease. |
|
| Interventions | Each received nifedipine 20 mg initial dose or identical placebo for 3 weeks followed by cross‐over to 10 mg nifedipine/8 hours or placebo. Two courses of treatment were separated by 1 week of washover. Duration 7 weeks (two 3‐week treatment periods and 1 week of washover) |
|
| Outcomes | Mean number and duration of attacks Severity of attacks (mild/moderate/severe) Severity of pain (10‐cm analogue scale) Overall grading of symptoms (mild/moderate/severe) Patient and observer opinions on treatment Side effects Patient preference for each period of treatment (1 or 2) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind cross‐over study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Diary used by participants to record subjective assessments during treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four participants who reported < 2 attacks during first course of treatment excluded from mean and severity analysis of number of attacks but included in other analyses |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | Washover period included |
Bravard 1983.
| Methods | Randomized double‐blind placebo‐controlled cross‐over study | |
| Participants | 9 patients with multiple sclerosis | |
| Interventions | 10 mg nifedipine 3× daily or matching placebo | |
| Outcomes | Numbers of attacks, outings; preference | |
| Notes | 2/9 excluded owing to incomplete diary data, and 2 others owing to different temperatures between treatment periods; thus results for 5/9 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as a double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used for subjective assessments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/9 participants excluded owing to incomplete diary or to differences in temperature between 2 study periods |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | Evidence insufficient for judgement of risk |
Challenor 1987.
| Methods | Randomized placebo‐controlled double‐blind trial 3‐Way cross‐over design |
|
| Participants | 36 participants with primary Raynaud's (32 female, 4 male) Mean age 47 years (range 19 to 67) Mean Raynaud's phenomenon duration 26.2 years (range 2 to 60 years) Vasoactive drugs discontinued before entry and other concomitant drug discontinued in 19 patients |
|
| Interventions | Nisoldipine 5 mg, 10 mg daily or matching placebo before food 3‐Way cross‐over each with duration of 4 weeks Total study length 3 months |
|
| Outcomes | Daily Raynaud's phenomenon attacks Severity Number of times outdoors Subjective response to treatment on 1 to 5 scale (1 = much better, 2 = better, 3 = same, 4 = worse, 5 = much worse) and 10‐cm visual analogue scale (from 0 = much better to 10 = much worse) |
|
| Notes | Length of washover not clear Mean ambient temperature similar during all treatment periods |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used to record number of attacks, severity, subjective improvement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4/36 dropouts |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | Length of washover not clear |
Challenor 1989.
| Methods | Double‐blind randomized placebo‐controlled (double‐dummy) trial Cross‐over design | |
| Participants | N = 24 patients with primary Raynaud's phenomenon ‐ 22 female, 2 male Mean age 37.7 ± 2.9 years, range 17 to 61 Mean duration of disease 21.0 ± 3.3 years 3 smokers 4 dropouts | |
| Interventions | Continuation of concurrent treatments unchanged Patients randomized to receive nifedipine or placebo Dose increased after 3 weeks After a further 3 weeks, patients crossed over to the opposite treatment for 6 weeks, with dose increasing as before | |
| Outcomes | Subjective and objective assessments Daily participant record of episodes, number of attacks, duration, and severity, as well as number of visits outdoors, unwanted effects, and improvement Vibration perception, skin temperature, and blood plasma measurements | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy RCT: "Patients were randomized to receive either a 10‐mg sustained‐release formulation of nifedipine twice daily before food or a matching placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used to record episodes of Raynaud’s phenomenon, noting number of attacks, duration, and severity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participant withdrawals from the study for personal reasons unrelated to treatment Two participant treatment discontinuations after 5 and 7 days of 40 mg sustained‐release nifedipine due to unwanted effects ‐ the latter included in the analysis |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Evidence insufficient for judgement of risk |
Constantini 1987.
| Methods | Randomized double‐blind placebo‐controlled trial Parallel design | |
| Participants | N = 24 patients with Raynaud's phenomenon ‐ 19 women and 5 men Mean age 41 years, range 19 to 63 Mean disease duration 3 years 6 idiopathic Raynaud's phenomenon, 6 connective tissue disease, 1 AVD, 1 IA 7 dropouts | |
| Interventions | 2 groups of 12 participants 1 group treated with nifedipine 20 mg for a month, the other group treated with matching placebo for a month | |
| Outcomes | Clinical and instrumental parameters evaluated at beginning and end of treatment Entity and frequency of ischemic attacks Skin trophism stage and degree of pain Capillaroscopy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind investigation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low riskUnclear riskHigh risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/24 dropouts: 3 from treatment group (2 for side effects and 1 for incorrect application of treatment) and 4 from placebo group (for side effects) |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | Evidence insufficient for judgement of risk |
Corbin 1986.
| Methods | Double‐blind placebo‐controlled randomized Cross‐over design | |
| Participants | N = 24 patients, all referred to peripheral vascular clinic, Royal Infirmary of Edinburgh Mean age 31 years, range 17 to 49 All with Raynaud's phenomenon for mean of 9.5 years 5 smokers 2 dropouts | |
| Interventions | Participants given a supply of nifedipine capsules or an identical supply of placebo for first week. Dose increased weekly for 3 weeks, then held constant Doses decreased if participants could not tolerate higher dose Participants randomized in blocks of 4 to receive either nifedipine for 4 weeks followed by placebo for 4 weeks, or vice versa Total study duration 8 weeks | |
| Outcomes | Participant diary used to record attacks Efficacy, FSP, side effects, drug compliance, and ambient daily temperature also recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomized in blocks of 4 to receive either nifedipine for 4 weeks followed by placebo for 4 weeks, or vice versa |
| Allocation concealment (selection bias) | Low risk | Block randomization concealing allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind: "Patients were given a supply of 5 mg nifedipine capsules or an identical placebo (supplied by Bayer UK Limited)." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used to record daily attacks of Raynaud's phenomenon. Participants asked to assess their response to each treatment in relation to pretrial status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant with unrelated illness during first week of trial and withdrawn; youngest participant,tolerating placebo in full doses and unable to tolerate even the starting dose of nifedipine; thus, only 22 participants completing 4 weeks of treatment with nifedipine and placebo |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | Baseline characteristics similar between groups |
Ettinger 1984.
| Methods | Randomized double‐blind placebo‐controlled cross‐over study | |
| Participants | 25 patients with Raynaud's Mean age 36.7 ± 2.5 years, range 21 to 60 Mean disease duration 9.5 ± 1.4 years 6 primary Raynaud's phenomenon 16 secondary Raynaud's phenomenon |
|
| Interventions | Nifedipine Dazoxiben Placebo |
|
| Outcomes | Participant diary used to record Raynaud's phenomenon with number, duration, severity, pain, overall response to treatment, and side effects | |
| Notes | Intervention with 3 arms; number of participants with placebo divided by 2 to avoid double‐counting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information insufficient for judgement of low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient for judgement of low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐blind" study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 19/25 completing the study |
| Selective reporting (reporting bias) | Low risk | All outcome data reported |
| Other bias | Unclear risk | Information insufficient for judgement of low or high risk of bias |
Ferri 1992.
| Methods | Randomized double‐blind placebo‐controlled trial Cross‐over design |
|
| Participants | 21 participants ‐ 18 women and 3 men Mean age 46 ± 12 years, range 22 to 65 All patients with active Raynaud's phenomenon From outpatient rheumatology unit of the University of Pisa All patients with typical Raynaud's phenomenon in both hands, and at least 1 attack daily during 2 weeks preceding the study 3 dropouts |
|
| Interventions | During month preceding the trial, no treatment with NSAIDs, dipyridamole vasodilators, and other drugs interfering with sympathetic nervous system functions Entry into 8‐week study; given placebo or nicardipine twice daily for 3 weeks followed by 2‐week washout period and cross‐over for the next 3 weeks |
|
| Outcomes | Participants given an instruction booklet and a clinical diary for recording subjective symptom variations and other parameters such as body weight, blood pressure, and side effects Complete history and careful physical examination performed at entry followed by clinical screening
Participant diary used to record number of attacks each day, severity of discomfort by VAS, and degree of hand disability Changes in digital blood flow evaluated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind cross‐over" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used to record subjective assessments, i.e., number of attacks, severity, etc |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/21 lost to follow‐up (1 from placebo group for non‐compliance, 2 from treatment group for side effects) |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | Washout of 2 weeks included between cross‐overs |
Finch 1988.
| Methods | Randomized double‐blind placebo‐controlled prospective study Cross‐over design | |
| Participants | N = 15 patients ‐ 11 female, 4 male Mean age 53, ranging from 15 to 74 Mean disease duration 8.3 years 3 patients with scleroderma, 1 with systemic lupus erythematosus, 1 with limited scleroderma (CREST), 8 with primary Raynaud's phenomenon No dropouts | |
| Interventions | Study duration 7 weeks For 1 week, all participants given placebo, followed by random assignment to placebo or nifedipine for 2 weeks Then 2‐week washout period and final 2‐week cross‐over period | |
| Outcomes | Daily record kept by participant of number, duration, and severity of attacks, as well as side effects During assessments, heart rate, finger blood flow, blood work, digital systolic pressure, and blood pressure recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind cross‐over |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients kept a daily diary of number, duration and severity of attacks." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | Washout period of 2 weeks included between cross‐overs |
French Co‐op 1991.
| Methods | Randomized multicenter double‐blind placebo‐controlled Cross‐over design | |
| Participants | N = 69 patients ‐ 18 men and 51 women Average age 38 ± 15 years, ranging from 18 to 64 Mean disease duration 15 ± 11 years 9 dropouts | |
| Interventions | Prospective study protocol
4 treatment periods, 5 hospital visits
Period 1: single‐blind placebo period (patient eligibility assessed) Period 2: participants randomly assigned to receive either nicardipine or placebo Period 3: single‐blind washout period with placebo Period 4: double‐blind cross‐over from period 2 Periods 2 and 4 each for 2 weeks; periods 1 and 3 each for 1 week Total study duration 6 weeks |
|
| Outcomes | Number of crises, intensity, and overall disability of each incident recorded in diary | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned....enters were unaware of randomization code." |
| Allocation concealment (selection bias) | Low risk | Assignment by third party through random generation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind cross‐over with identical appearing treatment and placebo tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "recorded by patients number of crises..intensity and overall disability" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 weeks: 9 dropouts (5 from placebo group and 2 from treatment group for side effects; 2 for personal reasons) |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | Washover period of 1 week included |
Gjorup 1986a.
| Methods | Double‐blind placebo‐controlled randomized trial Cross‐over design | |
| Participants | N = 19 patients ‐ 15 female, 4 male Median age 40 years, range 22 to 60 All with typical Raynaud's phenomenon Selected from files of general practitioners in hospital area Study completed by all patients |
|
| Interventions | 3‐Week periods of active and placebo treatment Participants assigned to receive nisoldipine or placebo during first period Study conducted during March and April Total study duration 6 weeks |
|
| Outcomes | Participant diary used to record daily number of attacks, grade of the most severe attack (on a scale from 1 to 10), whether the day had been better or worse than expected, and overall evaluation of preferred treatment Side effects also recorded |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind cross‐over study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients recorded number of attacks, severity, and efficacy." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | No mention of a washout period between cross‐over phases |
Gjorup 1986b.
| Methods | Double‐blind placebo‐controlled randomized trial Cross‐over design | |
| Participants | N = 26 patients ‐ 21 women, 5 men Median age 38 years, range 22 to 61 Patients selected from files of general practitioners in hospital area All with typical Raynaud's phenomenon Median duration of symptoms 14 years 12 tobacco users 5 dropouts | |
| Interventions | Cross‐over trial comprising 2 periods of treatment each for 2 weeks Participants randomly allocated to receive either nifedipine or placebo and crossed over for the second treatment period Total study duration 4 weeks | |
| Outcomes | Daily record of number of attacks Most severe attack graded Days compared with participant expectations Subjective overall evaluation at completion of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" ‐ "randomly allocated to receive either 20mg nifedipine or placebo in equivalent numbers" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant‐recorded number and severity of attacks and evaluation of treatment efficacy |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 lost to follow‐up (4 from treatment group for side effects) |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Other bias | Unclear risk | No mention of a washout period between cross‐over phases |
Hawkins 1985.
| Methods | Randomized controlled trial Double‐blinding not explicitly stated but given benefit of the doubt |
|
| Participants | 71 entering the study and 57 completing the study ‐ 49 female, 8 male At least 2 attacks during last 2 weeks before enrollment Mean age 49 years, range 17 to 78 Mean duration of Raynaud's 14.7 years, range 1 to 52 20 with idiopathic Raynaud's 25 with systemic sclerosis 4 with seropositive rheumatoid arthritis 3 with mixed connective tissue disease 3 with systemic lupus erythematosus 1 with Sjogren's syndrome and seropositive polyarthritis |
|
| Interventions | Following 2‐week run‐in period without therapy Randomized to either 10 mg nifedipine 4×/d or placebo Underwent 4 consecutive periods of treatment with alternating nifedipine and placebo |
|
| Outcomes | Number of attacks per week Severity on a 10‐cm visual analogue scale and on a 5‐point scale (none, mild, moderate, severe, and very severe) BP, presence of digital ulcers Thermal stress test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Does not explicitly state double‐blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Subjective participant assessments of number and severity of attacks |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14/71 dropouts ‐ 6 due to intercurrent illness, 8 due to drug side effects (7 from nifedipine and 1 from placebo) |
| Selective reporting (reporting bias) | Unclear risk | Severity of attacks not reported on a 5‐point Likert scale as proposed |
| Other bias | Unclear risk | No mention of washout period between cross‐over phases |
Kahan 1985a.
| Methods | Placebo‐controlled double‐blind randomized trial Cross‐over design | |
| Participants | N = 15 patients ‐ 13 women and 2 men Mean age 41 With symptomatic bilateral Raynaud's phenomenon: 7 systemic sclerosis, 1 rheumatoid arthritis, 2 systemic lupus erythematosus, 5 Raynaud's phenomenon 0 dropouts |
|
| Interventions | Each participant given placebo 3 times daily for 1 week, then matched capsules of nifedipine 3 times daily for 1 week, then placebo 3 times daily for another week, then prazosin 3 times daily for 1 week, and finally placebo yet again 3 times daily for 1 week During the study, no other vasoactive medication taken Total study duration 5 weeks |
|
| Outcomes | Participants diary used to record vasospastic attacks ‐ frequency, severity (10‐cm visual analogue scale), and subjective assessments | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization sequence was determined using a table of random numbers." |
| Allocation concealment (selection bias) | Low risk | Likely occurred |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind cross‐over study; dependent variables quantified before the code was broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used to record subjective assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None detected |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | Cross‐over phases separated by 1‐week placebo period |
Kahan 1985b.
| Methods | Double‐blind randomized placebo‐controlled prospective study Cross‐over design | |
| Participants | N = 16 patients ‐ 14 women and 2 men Mean age 41.5 years, range 18 to 57 7 with systemic sclerosis, 2 rheumatoid arthritis, 1 systemic lupus erythematosus, 6 idiopathic Raynaud's phenomenon Mean disease duration 12.8 years No dropouts | |
| Interventions | Each participant given placebo in a single‐blind fashion for 1 week Participants randomized to receive placebo or diltiazem in a double‐blind fashion for 2 weeks, then crossed over for the last 2 weeks Total study duration 5 weeks | |
| Outcomes | Participant diary used to record frequency, severity, and number of attacks Participants asked about overall effectiveness of treatments Side effects recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized double cross‐over protocol" Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind cross‐over study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients kept a record to attacks...severity, and effectiveness." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None detected |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | No mention of a washout period between cross‐over phases |
Kahan 1985c.
| Methods | Double‐blind randomized placebo‐controlled trial Cross‐over design | |
| Participants | N = 30 patients ‐ 26 female and 4 male Mean age 42.9 years All with Raynaud's phenomenon ‐ 10 associated with progressive systemic sclerosis, 5 systemic lupus erythematosus, 3 rheumatoid arthritis, 12 idiopathic Raynaud's phenomenon No dropouts | |
| Interventions | For 2 consecutive weeks, participants given 20 mg nifedipine and placebo 3× daily in random order | |
| Outcomes | Participant diary used to record weekly frequency of attacks and side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Number of attacks reported by participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Other bias | Unclear risk | No mention of a washout period between cross‐over phases |
Kahan 1987.
| Methods | Double‐blind randomized placebo‐controlled trial Cross‐over design | |
| Participants | 20 patients ‐ 16 women and 4 men Age 18 to 68 years 15 with progressive systemic sclerosis, 2 with rheumatoid arthritis, 3 with idiopathic Raynaud's phenomenon 0 dropouts |
|
| Interventions | No vasoactive medication during the study Initial 1‐week baseline observation period of no therapy, followed by 2 treatment periods, each of 2‐week duration, separated by a 1‐week baseline observation period Total study duration 6 weeks | |
| Outcomes | During each period, standardized participant diary used to record digital vasospastic attacks Severity of Raynaud's phenomenon assessed at the end of each period on a 4‐point clinical scale Last day of each period ‐ inquiries made about side effects related to treatment; blood pressure, and pulse rate recorded; complete blood counts, erythrocyte sedimentation rate, serum creatinine, serum aspartate, aminotransferase, alkaline phosphatase, and serum total protein measured before the study and on last day of 2 treatment periods |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on a table of random numbers |
| Allocation concealment (selection bias) | Low risk | "Randomization sequence was determined with a table of random numbers." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind cross‐over study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients kept a record of their digital vasospastic attacks...quantified before the drug code was broken." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | Treatment periods separated by 1‐week baseline observation (washout) periods |
Kallenberg 1987.
| Methods | Double‐blind randomized cross‐over trial | |
| Participants | 16 patients 8 with primary Raynaud's ‐ 6 female and 2 male Mean age 34 years, range 25 to 47 Duration of disease 20 years, range 3 to 37 8 with secondary Raynaud's ‐ 5 female and 3 male Mean age 44 years, range 28 to 63 Duration of disease 17 years, range 9 to 24 |
|
| Interventions | Nifedipine or placebo | |
| Outcomes | Side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used to record subjective assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1/16 dropouts |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Other bias | Unclear risk | Evidence insufficient for judgement of risk |
Kinney 1982.
| Methods | Double‐blind randomized placebo‐controlled trial Cross‐over design | |
| Participants | 16 patients ‐ 10 progressive systemic sclerosis, 2 mixed connective tissue disease, 2 with systemic lupus erythematosus, 2 with Raynaud's disease 2 dropouts | |
| Interventions | No vasoactive drug other than the one being tested (verapamil) All study participants non‐smokers except for 1 participant who continued to smoke 4 cigarettes a day throughout the duration of the experiment Concomitant medications not changed during this study Verapamil administered orally for 3 weeks followed by increased dosage for 3 weeks Last 6 weeks of study ‐ treatment groups crossed over Total study duration 12 weeks |
|
| Outcomes | Participants interviewed and examined at start of the study and every 3 weeks thereafter Diary analysis to ascertain whether participants had at least 1 episode of Raynaud's phenomenon on a given day Pulse volume, total blood flow in the tip of the finger, skin temperature, and cold pressor testing measured |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | Likely occurred on the basis of computer generation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study was double‐blinded." "Placebo‐treated patients received equivalent numbers of capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used to record subjective assessments and to check compliance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/16 participants with completed diary excluded from analysis |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | Evidence insufficient for judgement of risk |
Kirch 1987.
| Methods | Single‐blind placebo‐controlled randomized trial Cross‐over design | |
| Participants | N = 10 patients ‐ 5 male, 5 female Age 18 to 60 years Typical Raynaud's phenomenon and related connective tissue disorders No dropouts |
|
| Interventions | Participants given no drugs other than ketanserin, nifedipine, or placebo Initially received placebo orally during washout period of 4 weeks, then randomly assigned to receive either nifedipine or ketanserin orally for 4 weeks Then, a 2‐week lasting placebo phase interconnected with a cross‐over to the final 4‐week lasting therapy phase Total study duration 14 weeks | |
| Outcomes | Participant diary used to record possible adverse effects and frequency, duration, and severity of attacks Severity assessed on a 3‐point scale Patient treatment rating on a 3‐point scale Laboratory parameters performed including blood cell count, erythrocyte sedimentation rate, and biochemical analysis of renal and hepatic function Skin temperature recordings and videomicroscopy performed, thereby monitoring flowmetry, a standard cold provocation test, and typical morphological changes in skin capillaries | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind study ‐ patients unaware of their treatment arm |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used to record attack frequency and duration and adverse effects |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | Washout period of 2 weeks separating cross‐over periods |
La Civita 1997.
| Methods | Does not explicitly say "randomized" but appears to be given the benefit of the doubt Double‐blind placebo‐controlled trial Cross‐over design | |
| Participants | N = 24 patients ‐ 20 women, 4 men Age 22 to 63 years, mean age 45 15 with primary Raynaud's phenomenon, 9 with suspected secondary Raynaud's phenomenon Active Raynaud's phenomenon in all patients, with mean duration of 3.5 years 4 dropouts | |
| Interventions | Trial lasting 7 weeks
Participants given placebo or amlodipine for 3 weeks, followed by a 1‐week washout period, then crossed over to alternate treatment for 3 weeks Total treatment duration 7 weeks |
|
| Outcomes | Participant diary used to record subjective symptoms, blood pressure, number and severity of daily episodes, and adverse events Blood flow and radial blood flow examined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used for subjective assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/24 dropouts (2 for inadequate compliance, 2 for intercurrent disease) |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Other bias | Low risk | Washout period of 1 week separating cross‐over periods |
Leppert 1989.
| Methods | Single‐blind dose response study Cross‐over design | |
| Participants | 10 female patients Mean age 43 ± 7 years All with disabling primary Raynaud's phenomenon All with finger cytosolic pressure at 10°C below 50% of the value at 30°C All with severe Raynaud's phenomenon for a mean period of 12 ± 6 years 0 dropouts |
|
| Interventions | Total duration of treatment 9 weeks After an initial placebo run‐in period of 3 weeks, participants given isradipine capsules at a dose of 1.25 mg twice daily for 3 weeks, followed by 2.5 mg for a further 3 weeks No vasoactive compounds other than isradipine taken |
|
| Outcomes | Finger systolic pressure measured
At the end of each period, finger systolic blood pressure measured and participants questioned regarding side effects Participant assessment of effectiveness of therapy on a 0 to 100 mm visual analogue scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind study; participants unaware of their treatment arm |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Subjective participant assessment of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Other bias | Unclear risk | Evidence insufficient for judgement of risk |
Malamet 1984.
| Methods | Double‐blind cross‐over randomized placebo‐controlled trial Placebo, nifedipine (2 10‐mg capsules 3× daily), and dazoxiben (100 mg 4× daily) 10‐Week study with 6 treatment periods 2‐Week single‐blind placebo run‐in period 3 2‐week double‐blind cross‐over treatment periods (separated by 1‐week single‐blind washout periods) |
|
| Participants | 13 patients ‐ 7 female and 2 male Mean age 34.6 ± 4.0 years, range 21 to 55 Duration of Raynaud's 11.4 ± 2.6 years, range 1 to 22 8 patients with secondary Raynaud's (associated with connective tissue disease), 1 with primary Raynaud's |
|
| Interventions | Placebo Nifedipine (2 10‐mg capsules 3× daily) Daxoziben (100 mg 4× daily) |
|
| Outcomes | Number of Raynaud's attacks Severity of attacks (3‐point scale) Pain intensity (VAS scale) Duration of episodes (minutes) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used to record subjective assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | 1‐Week single‐blind washout period between cross‐over phases |
Meyrick Thomas 1987.
| Methods | Double‐blind placebo‐controlled randomized trial Cross‐over design | |
| Participants | 10 patients with diagnosis of systemic sclerosis; 1 dropout | |
| Interventions | Participants randomly allocated to group A or B. Following a 4‐week run‐in period during which they received no medication, participants given either oral nifedipine or placebo for 6 weeks. Following a 4‐week drug‐free washout period, participants crossed over to alternative treatment for 6 weeks after completion, with final 4‐week drug‐free washout period Total study duration 20 weeks | |
| Outcomes | Clinical and laboratory assessments performed at regular intervals throughout the trial; in addition, participants completed diary cards recording duration, severity, pain, and date of each attack and any side effects of treatment. Clinical assessment including evaluating compliance, recording lying and standing blood pressures and new digital ulcers, presenting inquiry regarding side effects, and checking completion of diary cards. Venous blood flow taken | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random order, cross‐over design" Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | "Patient[s] were randomly allocated to Group A or B." Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind..received either nifedipine or identical placebo capsules 3× daily (Group A or B)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patient recorded duration, severity, and date of each attack." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Other bias | Low risk | Cross‐over phases separated by a 4‐week drug‐free washout period |
Muller‐Buhl 1983.
| Methods | Randomized double‐blind placebo‐controlled trial Parallel design | |
| Participants | N = 24 patients ‐ 10 women, 14 men 3 with collagen disease, 21 with primary Raynaud's phenomenon Mean age 43.5 for drug, 43 for placebo No dropouts |
|
| Interventions | Treatment with calcium antagonist Bay K 9320 or placebo lasting 3 weeks | |
| Outcomes | Effectiveness, severity, and frequency recorded Subjective improvement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | No information provided for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided for judgement of risk |
| Selective reporting (reporting bias) | Unclear risk | No information provided for judgement of risk |
| Other bias | Unclear risk | No information provided for judgement of risk |
Nilsson 1987.
| Methods | Randomized double‐blind placebo‐controlled trial | |
| Participants | 28 patients ‐ 24 female and 4 male Average age 47.5 years Mean duration of symptoms 17.5 years |
|
| Interventions | Nifedipine 10 mg for 1 week and 20 mg (2 10‐mg capsules/d) for the next week or placebo 3×/d for 2 weeks during first treatment period, followed by 1‐week washover period Second period ‐ participants crossed over to opposite treatment |
|
| Outcomes | Changes in symptoms Side effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was made in blocks of 6." |
| Allocation concealment (selection bias) | Low risk | Blind allocation based on blocking |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind cross‐over study; code broken only after conclusion of treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant assessment of changes in symptoms and side effects |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/28 (1 participant on nifedipine with dropout due to side effects) |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Other bias | Low risk | Cross‐over phases separated by 1‐week washout period |
Rhedda 1985.
| Methods | Double‐blind placebo‐controlled randomized trial Cross‐over design | |
| Participants | 30 patients ‐ 7 male and 23 female All with typical features of Raynaud's All with primary disease and ANF‐negative without signs or symptoms to suggest connective tissue disorder 11 with secondary disease 8 dropouts |
|
| Interventions | Study including a 2‐week drug‐free baseline period, a 4‐week treatment period when participants received diltiazem or placebo, a 4‐week observation period when no drugs were taken, and finally a 4‐week treatment period when patients were crossed‐over to receive the opposite drug from the first treatment period, and finally, a terminal 2‐week observation period Total study duration 16 weeks | |
| Outcomes | Participant diary used to record number of episodes of vasospasm per day and duration of each attack Side effects reported At end of study, participants asked to identify period of active drug therapy Temperatures and barometric pressures recorded throughout the study |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization via computer‐generated random numbers by an independent collaborator However, findings showing that 1 group had more severe disease than the other, despite this randomization |
| Allocation concealment (selection bias) | Low risk | Participants unaware of randomization group, which was confirmed at the conclusion of the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study; blinding likely successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used to record subjective assessments (frequency of attacks, duration, side effects) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/30 participants with no analyzable data or dropping out |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Other bias | Low risk | A 4‐week observation period with no drugs taken used to separate cross‐over phases |
Rodeheffer 1983.
| Methods | Prospective randomized double‐blind placebo‐controlled trial Cross‐over design | |
| Participants | N = 15 patients ‐ 3 men and 12 women from outpatient rheumatology clinics at Johns Hopkins Hospital, the Good Samaritan Hospital, and the University of Maryland Hospital Mean age 34.1 years, range 20 to 52 1 patient with systemic lupus erythematosus, 9 with systemic sclerosis, the rest with symptomatic Raynaud's phenomenon related to cold or stress | |
| Interventions | Study protocol 7 weeks First 2‐week period ‐ all given placebo, then randomly assigned to nifedipine or placebo for next 2 weeks, then placebo for 1 week, finally crossed‐over to the other treatment for last 2 weeks |
|
| Outcomes | Diary used by participants to keep record of vasospastic attacks Digital artery systolic pressure measured Overall effectiveness assessed Frequency, severity, and duration recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Table of random numbers used" for randomization |
| Allocation concealment (selection bias) | Low risk | Likely occurred on basis of table of random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind.....received a supply of placebo capsules or matching 10mg nifedipine" Last few weeks single‐blind, with participants unaware of the their treatment arm |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Diary used by participants to record subjective assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Other bias | Low risk | Placebo period of 1 week used to separate cross‐over phases |
RTSI 2000.
| Methods | Randomized controlled trial Double‐masked for nifedipine and placebo 77 participants randomized to nifedipine, 81 to placebo |
|
| Participants | With primary Raynaud's phenomenon and history of 2 or more attacks the previous cold season | |
| Interventions | Nifedipine 30 to 60 mg per day or matching placebo | |
| Outcomes | Primary outcome: self‐reported color chart‐verified Raynaud's phenomenon attacks during 1 winter month after 1 year of treatment Secondary outcomes: verified attacks at 2 months, all attacks at 2 months and 1 year, quality of life |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Co‐ordinating center generated a set of allocations for each clinical center using an Autonomated Telephone Randomization System." |
| Allocation concealment (selection bias) | Low risk | Allocations generated in block sizes varying randomly to balance the number of participants allocated to each treatment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reports of participants with color charts to identify attacks |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Siimilar dropout rates between treatment and placebo. Complete outcome data provided for 73% and 75% on nifedipine and placebo, respectively At 2 months, 27/157 dropouts |
| Selective reporting (reporting bias) | Unclear risk | Not all minor outcomes reported |
| Other bias | Low risk | Two groups with similar baseline characteristics |
Rupp 1987.
| Methods | Double‐blind randomized trial Cross‐over design | |
| Participants | N = 30 patients ‐ 28 women and 2 men 28 participants at start of study Source: Outpatient Rheumatology Clinic at the University of Iowa Hospitals and Clinics Patients with systemic Raynaud's vasospasms, at least 3 attacks per week during the month preceding start of the study 2 patients excluded before the trial began; 1 dropout |
|
| Interventions | Complete history and examination for participants, who received placebo or nicardipine for 4 weeks followed by a cross‐over for the next 4 weeks Total study duration 8 weeks | |
| Outcomes | Participant diary used to record vasospastic attacks and side effects. Severity rated on an ordinal scale from 0 to 4. Pain associated with vasospasm recorded on a 15‐cm visual analogue scale. Daily number of episodes recorded. Variables recorded on a diary sheet; after completion of each 4‐week trial, participants evaluated in the human physiology laboratory. At study end, participants asked to give subjective evaluation of treatment periods. Used 15‐cm horizontal visual analogue scale to rate degree of pain with cold challenge with immersion of the right hand in ice for 90 seconds | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind cross‐over study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant assessment of attacks, severity, and side effects |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/28 dropouts while taking placebo for side effects |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Other bias | Unclear risk | No mention of a washover period |
Sarkozi 1986.
| Methods | Randomized double‐blind placebo‐controlled trial | |
| Participants | 39 patients ‐ 29 female and 10 male With idiopathic Raynaud's syndrome At least 2 attacks/week in the previous 3 months Mean disease duration 8.86 years Mean age 39.9 years |
|
| Interventions | Nifedipine 10 mg 3×/d or matching placebo | |
| Outcomes | Frequency of attacks Severity of attacks Change in pulse amplitude of digital blood flow and time to return to baseline Side effects Treatment compliance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization sequence determined from a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Allocation blind, based on a table of random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind RCT, with blinding likely maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Subjective assessments by participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/20 lost from nifedipine arm (1 loss to follow‐up, 1 dropout due to severe side effects, 2 exclusions owing to non‐compliance) 2/19 lost from placebo arm (1 pregnancy, 1 exclusion for non‐compliance) |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | Similar baseline characteristics among groups |
Sauza 1984.
| Methods | Parallel double‐blind randomized controlled trial | |
| Participants | 25 patients entering the trial, 18 completing the 10‐week study 17/18 completing the trial female Mean age 41 years, range 24 to 71 Only 1 with primary Raynaud's, 17 with secondary Raynaud's, 10 with recurrent digital pitting scars, none given vasodilators for at least 2 weeks before start of the study |
|
| Interventions | 10 given nifedipine (30 mg to 60 mg/d) 8 given matching placebo |
|
| Outcomes | Number of Raynaud's attacks during previous 2 weeks Intensity of attacks Side effects Improvements |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant subjective assessments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 18/25 completing the 10‐week trial (7 exclusions due to compliance issues, failure to attend follow‐up appointments, or modifications in treatment programs) |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | Two groups with similar baseline characteristics |
Smith 1982.
| Methods | Double‐blind randomized placebo‐controlled trial Cross‐over design | |
| Participants | N = 17 patients, all female Mean age 41 years, range 16 to 67 All with typical Raynaud's phenomenon with mean duration of 9.9 years 5 with idiopathic Raynaud's phenomenon, Raynaud's phenomenon secondary to systemic sclerosis, and RP secondary to systemic lupus erythematosus (in 1 patient) | |
| Interventions | After 2 weeks of baseline, participants randomized to receive placebo or nifedipine for 2 weeks followed by a cross‐over for 2 weeks Total study duration 6 weeks | |
| Outcomes | Frequency, severity (10‐cm visual analogue scale), drug effectiveness (10‐cm visual analogue scale) recorded in a participant diary Blood pressure, heart rate, side effects, and skin temperature recovery times measured during visits | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used to record daily attacks, severity, side effects, and drug effectiveness |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Other bias | Unclear risk | No mention of a washover period |
Teixeira da Costa.
| Methods | Double‐blind randomized placebo‐controlled trial Cross‐over study | |
| Participants | N = 15 patients, 14 with associated connective tissue disorder 1 dropout, 2 exclusions due to incomplete data | |
| Interventions | Study carried out in 2 treatment periods, each of 2 weeks separated by a 1‐week washout period from beginning of February to end of March Participants given diltiazem or placebo. During second treatment period, each group crossed‐over to opposite treatment Total study duration 5 weeks | |
| Outcomes | Subjective criteria: weekly frequency of vasospastic attacks recorded daily by participant; participant evaluation of effectiveness of study agent on 5‐point clinical scale, taking frequency, severity, and duration into account Objective: digital rheography recorded after 5 minutes of immersion of the hands in water at 30 degrees Celsius, and after 5 minutes of immersion at 5 degrees Celsius. Digital rheography performed immediately before start of the study, on the last day of first treatment, and on the last day of second treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Subjective participant assessment of frequency, severity, and duration of attacks and overall effectiveness of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/15 participants excluded (1 dropout due to side effects, 2 others with incomplete diary records excluded from analyses) |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | Washout period of 1 week separating cross‐over phases |
Waller 1986.
| Methods | Double‐blind randomized placebo‐controlled trial Cross‐over design | |
| Participants | N = 34 patients ‐ 8 male and 26 female
28 with primary Raynaud's phenomenon, 4 with Raynaud's phenomenon secondary to systemic sclerosis, 2 with Raynaud's phenomenon secondary to RA
Mean age 45 years, range 14 to 77 Mean duration of Raynaud's disease 14.7 years 10 smokers 5 dropouts |
|
| Interventions | Concurrent medication unchanged in 16 participants
Participants randomized after 2‐week run‐in period to receive a new biphasic release formulation of nifedipine or placebo Each period 4 weeks with 2‐week washout period between Total study duration 12 weeks |
|
| Outcomes | Participant diary used to record episodes, number of attacks, severity, side effects, and improvement Nifedipine concentration in blood plasma also recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients were requested to keep a daily record of episodes of Raynaud's phenomenon noting the number of attacks and their severity and evaluate overall disease." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/34 ‐ "Five patients (three primary, two secondary) withdrew from the study because of unwanted effects on starting active treatment." |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | Washout period of 2 weeks separating cross‐over phases |
White 1986.
| Methods | Double‐blind randomized placebo‐controlled trial Cross‐over design | |
| Participants | N = 11 patients ‐ 8 women, 3 men
Age range from 28 to 81 Also included 21 healthy control participants Primary Raynaud's phenomenon in 6 participants, Raynaud's phenomenon secondary to systemic disease in 5 participants 0 dropouts |
|
| Interventions | Participants given nifedipine or placebo for 1 week, then crossed over to receive opposite treatment for 1 week Total study duration 2 weeks | |
| Outcomes | Baseline digital temperature, pill counts, and subjective change in symptoms measured | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants asked to assessed treatment efficacy subjectively |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | No mention of a washout period |
Wigley 1987.
| Methods | Randomized double‐blind placebo‐controlled trial Cross‐over design | |
| Participants | N = 25 patients ‐ 21 female, 4 male Mean age 39 ± 2.6 years, range 22 to 65 19 of Caucasian descent Average disease duration 4.75 ± 0.8 years 10 with primary Raynaud's phenomenon, 15 with Raynaud's phenomenon secondary to connective tissue disorder No dropouts during cross‐over phase, 2 dropouts during parallel phase |
|
| Interventions | Participants randomly assigned to receive nicardipine or placebo for first 2 weeks followed by a second 2‐week period with agent not taken Continuation of agent from the second treatment period for an additional 4 weeks in a parallel design Total study duration 4 weeks | |
| Outcomes | Participant diary used to record frequency and severity Finger systolic pressure and venous sampling performed Beta‐thromboglobulin and platelet factor 4 measured | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Evidence insufficient for judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Subjects kept a daily diary of the frequency and severity of attacks." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts during cross‐over period of the study |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | Evidence insufficient for judgement of risk |
Wollersheim 1991.
| Methods | Randomized double‐blind placebo‐controlled cross‐over trial Washout period of 2 weeks separating cross‐over phases |
|
| Participants | 25 patients ‐ 10 male and 15 female Mean age 41.2 ± 10.7 years 16 with primary and 9 with secondary Raynaud's phenomenon Mean duration of disease 6.5 ± 6.1 years 10 smokers Mean attack rate in the winter 7.2 ± 2.9/d, range 2 to 14 attacks/d 8 with skin lesions, 3 who had undergone sympathectomy |
|
| Interventions | Nicardipine 30 mg 3× per day or matching placebo capsules for 3 weeks, then cross‐over to opposite treatment after 2‐week washout period for another 3 weeks Trial preceded by 3‐week baseline period without medication |
|
| Outcomes | Frequency, duration, and severity of attacks Treatment preference Drug effectiveness (rated on a 5‐point scale: much worse, worse, no difference, better, or much better) Ischemic attack changes Side effects Objective measurements: weight, systolic blood pressure, temperature, diastolic blood pressure, heart rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information insufficient for judgement of risk |
| Allocation concealment (selection bias) | Low risk | "A sheet detailing the assignment of all periods for all medication sets was provided in sealed envelopes to be opened only in the event of a serious adverse event." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; matching treatment and placebo capsules given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant diary used for subjective assessments; participants unaware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 dropouts; no data provided (2 from treatment group and 1 from placebo group) |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Washout period of 2 weeks separating cross‐over phases |
ANF: XXX.
AVD: atrioventricular dissociation.
BP: blood pressure.
CREST: calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia.
FSP: XXX.
IA: intracranial aneurysm.
NSAIDs: non‐steroidal anti‐inflammatory drugs.
RA: rheumatoid arthritis.
VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Codella 1989 | No placebo |
| Creager 1984 | Not a randomized controlled trial |
| Della Bella 1997 | No placebo |
| Dziadzio 1999 | No placebo |
| Ennis 2016 | A meta‐analysis |
| Garcia Hernandez2004 | Not a randomized controlled trial |
| Joseph 1988 | Not a randomized controlled trial |
| Kahan 1983b | Duration < 1 week |
| Kallenberg 1991 | Not a randomized dose‐finding study |
| La Civita 1996 | No placebo data presented |
| Leppert 1993 | No placebo |
| Lewis 1987 | Not a randomized controlled trial |
| Myrdal 1994 | No placebo |
| Nilsson 1990 | Not a randomized controlled trial |
| Park 2013 | No placebo |
| Pisenti 1984 | Not a randomized controlled trial |
| Rademaker 1989 | No placebo data presented |
| Rademaker 1992 | No placebo |
| Ringqvist 1993 | No placebo |
| Schmidt 1989 | No separation of placebo and treatment data |
| Shcherbakov 1987 | Open‐label study |
| Smith 1985 | Not a randomized controlled trial |
| Thompson 2001 | Meta‐analysis performed |
| Thompson 2005 | Meta‐analysis performed |
| Varela‐Aguilar 1997 | No placebo data presented |
| Vayssairat 1989 | Not a randomized controlled trial |
| Weber 1990 | Duration < 1 week |
| Winston 1983 | Insufficient "washout" period of 1 day |
| Wollersheim 1987 | Not a randomized controlled trial |
| Wu 2008 | Non‐randomized, no placebo |
Characteristics of studies awaiting assessment [ordered by study ID]
EUCTR2009‐018194‐31‐GB.
| Methods | Open‐label clinical trial |
| Participants | Patients with PRP (primary Raynaud's phenomenon) with Raynaud’s phenomenon for at least 2 years |
| Interventions | Amlodipine gel or placebo |
| Outcomes | Main objective: to determine the efficacy of amlodipine maleate gel in improving digital blood flow Primary endpoint: to improve symptoms by increasing blood flow to finger extremities Secondary objective: to ascertain the practicality of amlodipine maleate gel application; to establish short‐term side effect profile |
| Notes |
Kahan 1982.
| Methods | Double‐blind placebo‐controlled study |
| Participants | 46 with Raynaud's phenomenon |
| Interventions | Nifedipine or placebo |
| Outcomes | Frequency of attacks |
| Notes | Abstract only; data not available |
Kahan 1983a.
| Methods | Unknown |
| Participants | Patients with scleroderma |
| Interventions | Nifedipine |
| Outcomes | Unknown; abstract not available |
| Notes | Unable to obtain data |
Redondo 1986.
| Methods | Double‐blind controlled clinical study |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Unable to locate Spanish study |
van Heereveld 1988.
| Methods | Double‐blind placebo‐controlled cross‐over study |
| Participants | 12 Raynaud patients, 12 healthy volunteers |
| Interventions | Intravenously administered Nicardipine (5 mg/h during 85 minutes) |
| Outcomes | Blood pressure Skin temperature Heart rate |
| Notes | No report on any of the outcomes of interest for this review |
Wasir 1983.
| Methods | Double‐blind randomised controlled trial |
| Participants | 52 patients with primary or secondary Raynaud's phenomenon |
| Interventions | Intra‐arterial reserpine injection or sublingually administered nifedipine, orally administered trifluoperazine or dipyridamole, weekly on a long‐term basis |
| Outcomes | Besides the usual biochemical and immunological investigations, special tests including skin temperature, recovery time after a cold stress test, measurement of digital blood flow by strain‐gauge digital plethysmography, and radioisotopically determined glomerular filtration rate. Digital blood flow and skin temperature recovery times studied after 45 minutes, 2 hours, 6 hours, and 24 hours of intra‐arterial reserpine injection or sublingually administered nifedipine, orally administered trifluoperazine or dipyridamole; and weekly on a long‐term basis Raynaud's frequency, severity, and duration of attacks |
| Notes | Abstract only; data unavailable |
Wise 1987.
| Methods | Double‐blind placebo‐controlled trial |
| Participants | 21 participants with Raynaud's phenomenon |
| Interventions | Nifedipne or placebo |
| Outcomes | Perfusion pressure and digital blood flow |
| Notes | Outcomes of interest not reported |
Characteristics of ongoing studies [ordered by study ID]
Nazarinia 2016.
| Trial name or title | Assessing and Comparing the Effect of Diltiazem Gel Versus Nitroglycerin Ointment in Healing Process of Scleroderma Digital Ulcers |
| Methods | Randomized parallel assignment |
| Participants | 90 patients with scleroderma |
| Interventions | About 60 patients will be considered to be in control group receiving vaseline ointment as the placebo applying 2 times daily on their ulcers for 8 weeks. Experimental: Diltiazem Gel 2% About 30 patients will receive Diltiazem Gel 2% applying 2 times daily for 8 weeks on their digital ulcers. Experimental: Nitroglycerin Ointment 2% About 30 patients will receive nitroglycerin 2% applying 2 times daily for 8 weeks on their digital ulcers. |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | June 2016 |
| Contact information | Rheumatology Reseach Center, Shiraz University of Medical Sciences, Shiraz, Iran.Shiraz, Fars, Iran, Islamic Republic of |
| Notes |
Vera‐Kellet 2017.
| Trial name or title | Color Doppler Ultrasound Comparison of Topical 10% Nifedipine Versus 5% Sildenafil in Secondary Raynaud: A Randomized, Double‐blind, Placebo‐controlled Pilot Study |
| Methods | A randomized, double‐blind, placebo‐controlled pilot study took place in 10 patients with secondary RP. Topical 10% nifedipine on one hand and 5% sildenafil on the other hand were applied. The thumbs didn't receive any cream and served as a control group. The primary outcome was the improvement of blood flow and vessel diameter of the digital arteries measured by high frequency color Doppler ultrasound before and 1 hour after treatment. |
| Participants | 10 patients with clinical diagnosis of secondary Raynaud´s phenomenon associated with a connective tissue disease |
| Interventions | Experimental: 10% nifedipine cream Patient hands (right versus left) were randomized to treatment with topical sildenafil or nifedipine cream. The thumbs of both hands didn't receive any cream so that each subject served as her own control. Subjects were instructed to apply 5 grams of 10% nifedipine cream in one hand and 5 grams of 5% sildenafil cream to the opposite hand. Vinyl gloves were supplied to improve the absorption of the cream into the hand, leaving the thumb of both hands out of the glove without any cream. Active Comparator: 5% sildenafil cream Patient hands (right versus left) were randomized to treatment with topical sildenafil or nifedipine cream. The thumbs of both hands didn't receive any cream so that each subject served as her own control. Subjects were instructed to apply 5 grams of topical10% nifedipine cream in one hand and 5 grams of topical 5% sildenafil cream to the opposite hand. Vinyl gloves were supplied to improve the absorption of the cream into the hand, leaving the thumb of both hands out of the glove without any cream. |
| Outcomes | Primary Outcome Measures:Improvement of blood flow in the digital arteries (peak systolic velocity ) of the dorsal arterial arch of the proximal nail fold of the index, middle and thumb fingers of both hands [ Time Frame: Outcome measure will be assessed the same day of the study and the data will be presented after the data is analyzed (12 weeks) ]The peak systolic velocity peak is measured with Doppler sonography in centimeters/second Secondary Outcome Measures:Improvement of the diameter (mm) of the dorsal arterial arch of the proximal nail fold of the of the index, middle and thumb fingers of both hands. [ Time Frame: Outcome measure will be assessed the same day of the study and the data will be presented after the data is analyzed (12 weeks) ]The diameter is measured with Doppler sonography in millimeters |
| Starting date | August 2016 |
| Contact information | Responsible Party:Pontificia Universidad Catolica de Chile |
| Notes |
Differences between protocol and review
We used a generic protocol for pharmacological interventions for Raynaud's (Pope 2015). We have adjusted the text to reflect the specific interventions and controls assessed in this review. We added some post hoc analyses in this review that were not included in the registered protocol. We did plan to study: primary vs. secondary RP and RP from systemic sclerosis (as the scleroderma patients are more difficult to treat and have more severe and complicated RP), we planned to study subsets of CCBs (dihydropyridines and nifedipine in particular as we knew the bulk of the data was from nifedipine). All other subgroup analysis were post‐hoc.
Contributions of authors
Pope J ‐ Guarantor, coordinator; conceiving the review, designing search strategies, screening search results, obtaining and screening data on unpublished studies, providing general advice on the review, securing funding for the review, performing previous work that was the foundation of the current review.
Rirash F‐ collected data, assisted in designing the review, undertook searches, organized retrieval of papers, screened retrieved papers against inclusion criteria, appraised quality of papers, extracted data from papers, obtained and screened data on unpublished studies, managed data, entered data into RevMan version 5 (2014), analyzed and interpreted data, participated in writing the review.
Tingey P ‐ collected data, assisted in designing the review, undertook searches, organized retrieval of papers, screened retrieved papers against inclusion criteria, appraised quality of papers, extracted data from papers, obtained and screened data on unpublished studies, managed data, entered data into RevMan version 5 (2014), analyzed and interpreted data, participated in writing the review.
Harding S ‐ collected data, assisted in designing the review, undertook searches, organized retrieval of papers, screened retrieved papers against inclusion criteria, appraised quality of papers, extracted data from papers, obtained and screened data on unpublished studies, managed data, entered data into RevMan version 5 (2014), analyzed and interpreted data, participated in writing the review.
Maxwell L ‐ advised on and assisted with methods, data extraction, and calculations; commented on draft versions of the review.
Ghogomu E ‐ advised on and assisted with methods, data extraction, and calculations; commented on draft versions of the review.
Wells GA ‐ advised on methods, commented on draft versions of the review.
Tugwell P ‐ advised on methods, interpreted data, commented on draft versions of the review.
Sources of support
Internal sources
-
St. Joseph's Hospital, London, Ontario, Canada.
Pope Research Corporation
External sources
No sources of support supplied
Declarations of interest
JP: has consulted for Actelion, Mediquest, Pfizer, and United Therapeutics in the area of Raynaud's phenomenon and/or digital ulcers.
PTu: grants/honoraria from Bristol Myers and UCB.
FR: none known.
PTi: none known.
SH: none known.
LM: none known.
EG: none known.
GW: none known.
New
References
References to studies included in this review
Aldoori 1986 {published data only}
- Aldoori M, Campebell WB, Dieppe PA. Nifedipine in the treatment of Raynaud's syndrome. Cardiovascualr Research 1986;20(6):466‐70. [DOI] [PubMed] [Google Scholar]
Bravard 1983 {published data only}
- Bravard P, Moore N. Nifedipine for Raynaud's phenomenon. Lancet 1983;1(8316):130‐1. [PubMed] [Google Scholar]
Challenor 1987 {published data only}
- Challenor VF, Waller DG, Francis DA, Francis JL, Mani R, Roath S. Nisodipine in primary Raynaud's phenomenon. European Journal of Clinical Pharmacology 1987;33(1):27‐30. [DOI] [PubMed] [Google Scholar]
Challenor 1989 {published data only}
- Challenor VF, Waller DG, Hayward RA, Griffin MJ, Roath OS. Vibrotactile sensation and response to nifedipine dose titration in primary Raynaud's phenomenon. Angiology 1989;40(2):120‐8. [DOI] [PubMed] [Google Scholar]
Constantini 1987 {published data only}
- Constantini A, Martelli E, Bavera P, Agus GB. Slow release nifedipine in the treatment of Raynaud's phenomenon. International Journal of Angiology 1987;6(4):359‐63. [PubMed] [Google Scholar]
Corbin 1986 {published data only}
- Corbin DOC, Wood DA, Macintyre CCA, Housley E. A randomized double blind cross‐over trial of nifedipine in the treatment of primary Raynaud's phenomenon. European Heart Journal 1986;7(2):165‐70. [DOI] [PubMed] [Google Scholar]
Ettinger 1984 {published data only}
- Ettinger WH, Wise RA, Schaffhauser D, Wigley FM. Controlled double‐blind trial of dazoxiben and nifedipine in the treatment of Raynaud's phenomenon. American Journal of Medicine 1984;77(3):451‐6. [DOI] [PubMed] [Google Scholar]
Ferri 1992 {published data only}
- Ferri C, Cecchetti R, Cini G, Gambini I, Civita L, Bernini L, et al. Slow‐releasing nicardipine in the treatment of Raynaud's phenomena without underlying diseases. Clinical Rheumatology 1992;11(1):76‐80. [DOI] [PubMed] [Google Scholar]
Finch 1988 {published data only}
- Finch MB, Copland S, Passmore AP, Johnston GD. A double‐blind cross‐over study of nifedipine retard in patients with Raynaud's phenomenon. Clinical Rheumatology 1988;7(3):359‐65. [DOI] [PubMed] [Google Scholar]
French Co‐op 1991 {published data only}
- French Cooperative Multicenter Group for Raynaud's Phenomenon* Paris France. Controlled multicenter double‐blind trial of nicardipine in the treatment of primary Raynaud phenomenon. American Heart Journal 1991;122(1):352‐5. [DOI] [PubMed] [Google Scholar]
Gjorup 1986a {published data only}
- Gjorup T, Hartling OJ, Kelbaek H, Nielsen SL. Controlled double blind trial of nisoldipine in the treatment of idiopathic Raynaud's phenomenon. European Journal of Clinical Pharmacology 1986;31(4):387‐9. [DOI] [PubMed] [Google Scholar]
Gjorup 1986b {published data only}
- Gjorup T, Kelbaek H, Hartling OJ, Nielsen SL. Controlled double‐blind trial of the clinical effect of nifedipine in the treatment of idiopathic Raynaud's phenomenon. American Heart Journal 1986;111(4):742‐5. [DOI] [PubMed] [Google Scholar]
Hawkins 1985 {published data only}
- Hawkins SJ, Black CM, Hall ND, McGregor A, Ring EF, Maddison PJ. Clinical and laboratory effects of nifedipine in Raynaud's phenomenon. Rheumatology International 1986;6(2):85‐8. [DOI] [PubMed] [Google Scholar]
Kahan 1985a {published data only}
- Kahan A, Foult JM, Weber S, Amor B, Menkes CJ, Degeorges M. Nifedipine and alpha‐adrenergic blockade in Raynaud's phenomenon. European Heart Journal 1985;6(8):702‐5. [DOI] [PubMed] [Google Scholar]
Kahan 1985b {published data only}
- Kahan A, Amor B, Menkes CJ. A randomised double‐blind trial of diltiazem in the treatment of Raynaud's phenomenon. Annals of Rheumatic Diseases 1985;44(1):30‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahan 1985c {published data only}
- Kahan A, Weber S, Amor B, Menkes CJ, Hodara M, Degeorges M. Nifedipine and Raynaud's phenomenon associated with connective tissue diseases. International Journal of Angiology 1985;4(2):221‐3. [PubMed] [Google Scholar]
Kahan 1987 {published data only}
- Kahan A, Amor B, Menkes CJ, Weber S, Guerin F, Degeorges M. Nicardipine in the treatment of Raynaud's phenomenon: a randomized double‐blind trial. Angiology 1987;38(4):333‐7. [DOI] [PubMed] [Google Scholar]
Kallenberg 1987 {published data only}
- Kallenberg Cees GM, Wouda AA, Kuitert JJ, Tijssen J, Wesseling H. Nifedipine in Raynaud's phenomenon: relationship between immediate, short term and long term effects. Journal of Rheumatology 1986;14(2):284‐90. [PubMed] [Google Scholar]
Kinney 1982 {published data only}
- Kinney EL, Nicholas GG, Gallow J, Pontoriero C, Zelis R. The treatment of severe Raynaud's phenomenon with verapamil. Journal of Clinical Pharmacology 1982;22(1):74‐6. [DOI] [PubMed] [Google Scholar]
Kirch 1987 {published data only}
- Kirch W, Linder HR, Hutt HJ, Ohnhaus EE, Mahler F. Ketanserin versus nifedipine in secondary Raynaud's phenomenon. VASA 1987;16(1):77‐80. [PubMed] [Google Scholar]
La Civita 1997 {published data only}
- Civita L, Pitaro N, Rossi M, Giuggioli D, Gambini I, Cini G, et al. Amlodipine in the treatment of Raynaud's phenomenon: a double‐blind placebo‐controlled crossover study. Clinical Drug Investigation 1997;23(Suppl 1):126‐31. [Google Scholar]
Leppert 1989 {published data only}
- Leppert J, Jonasson T, Nilsson H, Ringqvist I. The effect of isradipine, a new calcium‐channel antagonist, in patients with primary Raynaud's phenomenon: a single‐blind dose response study. Cardiovascular Drugs and Therapy 1989;3(3):397‐401. [DOI] [PubMed] [Google Scholar]
Malamet 1984 {published data only}
- Malamet R, Wise RA, Ettinger WH, Wigley FM. Nifedipine in the treatment of Rayaud's phenomenon: evidence for platelet activation. American Journal of Medicine 1985;78(4):602‐8. [DOI] [PubMed] [Google Scholar]
Meyrick Thomas 1987 {published data only}
- Meyrick‐Thomas RH, Rademaker M, Grimes SM, Mackay A, Kovacs IB, Cook ED, et al. Nifedipine in the treatment of Raynaud's phenomenon in patients with systemic sclerosis. British Journal of Dermatology 1987;117(2):237‐41. [DOI] [PubMed] [Google Scholar]
Muller‐Buhl 1983 {published data only}
- Muller‐Buhl U, Diehm C, Scheuermann W, Morl H. Calcium antagonists in the treatment of Raynaud's phenomenon [Calciumantagonisten zur Behandlung des Raynaud‐phenomens]. Deutsche Medicinische Wochenschrift 1983;108(47):1795‐7. [DOI] [PubMed] [Google Scholar]
Nilsson 1987 {published data only}
- Nilsson H, Jonason T, Leppert J, Ringqvist I. The effect of the calcium‐entry blocker nifedipine on cold‐induced digital vasospasm. A double‐blind crossover study versus placebo. Acta Medica Scandinavica 1987;221(1):53‐60. [DOI] [PubMed] [Google Scholar]
Rhedda 1985 {published data only}
- Rhedda A, McCans J, Willan AR, Ford PM. A double blind placebo controlled crossover randomized trial of diltiazem in Raynaud's phenomenon. Journal of Rheumatology 1985;12(4):724‐7. [PubMed] [Google Scholar]
Rodeheffer 1983 {published data only}
- Rodeheffer RJ, Rommer JA, Wigley F, Smith CR. Controlled double‐blind trial of nifedipine in the treatment of Raynaud's phenomenon. New England Journal of Medicine 1983;308(15):880‐3. [DOI] [PubMed] [Google Scholar]
RTSI 2000 {published data only}
- Ranayd's Treatment Study Investigators (RTSI). Comparison of sustained‐release nifedipine and temperature biofeedback treatment of primary Raynaud phenomenon. Archives of Internal Medicine 2000;160(8):1101‐8. [DOI] [PubMed] [Google Scholar]
Rupp 1987 {published data only}
- Rupp PAF, Mellinger S, Kohler J, Dorsey JK, Furst DE. Nicardipine for the treatment of Raynaud's phenomena: a double blind crossover trial of a new calcium entry blocker. Journal of Rheumatology 1987;14(4):745‐50. [PubMed] [Google Scholar]
Sarkozi 1986 {published data only}
- Sarkozi J, Bookman AA, Mahon W, Ramsay C, Detsky AS, Keystone AC. Nifedipine in the treatment of idiopathic Raynaud's syndrome. Journal of Rheumatology 1986;13(2):331‐6. [PubMed] [Google Scholar]
Sauza 1984 {published data only}
- Sauza J, Kraus A, Gonzalez‐Amaro R, Alarcon‐Segovia D. Effect of calcium channel blocker Nifedipine on Raynaud's phenomenon: a controlled double blind trial. Journal of Rheumatology 1984;11(3):362‐4. [PubMed] [Google Scholar]
Smith 1982 {published data only}
- Smith CD, McKendry RJR. Controlled trial of nifedipine in the treatment of Raynaud's phenomenon. The Lancet 1982;2(8311):1299‐301. [DOI] [PubMed] [Google Scholar]
Teixeira da Costa {published data only}
- Teixeira da Costa J, Melo Gomez JA, Espirito Santo J, Viana Queiros M. Inefficacy of diltiazem in the treatment of Raynaud's phenomenon with associated connective tissue disease: a double blind placebo controlled study. Journal of Rheumatology 1987;14(4):858‐9. [PubMed] [Google Scholar]
Waller 1986 {published data only}
- Waller DG, Challenor VF, Francis DA, Roath OS. Clinical and rheological effects of nifedipine in Raynaud's phenomenon. British Journal of Clinical Pharmacology 1986;22(4):449‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 1986 {published data only}
- White CJ, Phillips WA, Abrahams LA, Watson TD, Singleton PT. Objective benefit of nifedipine in the treatment of Raynaud's phenomenon. American Journal of Medicine 1986;80(4):263‐5. [DOI] [PubMed] [Google Scholar]
Wigley 1987 {published data only}
- Wigley FM, Wise RA, Malamet R, Scott TE. Nicardipine in the treatment of Raynaud's phenomenon ‐ dissociation of platelet activation from vasospasm. Arthritis and Rheumatism 1987;30(3):281‐6. [DOI] [PubMed] [Google Scholar]
Wollersheim 1991 {published data only}
- Wollersheim H, Thien T. Double‐blind placebo‐controlled crossover study of oral nicardipine in the treatment of Raynaud's phenomenon. Journal of Cardiovascular Pharmacology 1991;18(6):813‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Codella 1989 {published and unpublished data}
- Codella O, Caramaschi P, Olivieri O, Perbellini L, Perbellini A, Bambara LM, Corrocher R, et al. Controlled comparison of ketanserin and nifedipine in Raynaud's phenomenon. Angiology 1989;40(2):114‐21. [DOI] [PubMed] [Google Scholar]
Creager 1984 {published data only}
- Codella O, Caramaschi P, Olivieri O, Perbellini L, Perbellini A, Bambara LM, et al. Controlled comparison of ketanserin and nifedipine in Raynaud's phenomenon. Angiology 1989;40(2):114‐21. [DOI] [PubMed] [Google Scholar]
- Creager MA, Pariser KM, Winston EM, Basmussen HM, Miller KB, Coffman JD. Nifedipine‐induced fingertip vasodilation in patients with Raynaud's phenomenon. American Heart Journal 1984;108:370‐3. [DOI] [PubMed] [Google Scholar]
Della Bella 1997 {published data only}
- Della Bella S, Molteni M, Mascagni B, Zulian C, Compasso S, Scorza R. Cytokine production in scleroderma patients: effects of therapy with either iloprost or nifedipine. Clinical and Experimental Rheumatology 1997;15:135‐41. [PubMed] [Google Scholar]
Dziadzio 1999 {published data only}
- Dziadzio M, Denton CP, Smith R, Howell K, Blann A, Bowers E, et al. Losartan therapy for Raynaud's phenomenon and scleroderma: clinical and biochemical findings in a fifteen week, randomized, parallel‐group, controlled trial. Arthritis & Rheumatology 1999;42(12):2646‐55. [DOI] [PubMed] [Google Scholar]
Ennis 2016 {published data only}
- Ennis H, Anderson ME, Wilkinson J, Herrick AL. Calcium channel blockers for primary Raynaud’s phenomenon. Cochrane Database of Systematic Reviews 2014 2014;Jan 30(1):Art. No.: CD002069. DOI: 10.1002/14651858.CD002069.pub4. [DOI] [PubMed] [Google Scholar]
Garcia Hernandez2004 {published data only}
- Garcia Hernandez FJ, Ocana Medina C, Mateos Romero L, Molinillo Lopez J, Arias Zambrano A, Gonzalez Leon R, Sanchez Roman J. Iloprost for severe Raynaud's phenomenon and ischaemic ulcers related with systemic diseases [Iloprost en el fenomeno de Raynaud grave y las ulceras isquemicas de las enfermedades sistemicas]. Medicina Clinica 2004;122(13):501‐4. [DOI] [PubMed] [Google Scholar]
Joseph 1988 {published data only}
- Joseph BZ, Organek HW, Grant A, Axelrod DA. Effects of nifedipine therapy on pulmonary Raynaud's in primary Sjogren's syndrome. Clinical and Experimental Rheumatology 1988;6:409‐10. [PubMed] [Google Scholar]
Kahan 1983b {published data only}
- Kahan A, Weber S, Amor B, Menkes CJ, Saporta L, Hodara M, et al. Calcium entry blocking agents in digital vasospasm (Raynaud's phenomenon). European Heart Journal 1983;4(Suppl C):123‐9. [DOI] [PubMed] [Google Scholar]
Kallenberg 1991 {published data only}
- Kallenberg CGM, Wouda AA, Meems L, Wesseling H. Once daily felodipine in patients with primary Raynaud's phenomenon. European Journal of Clinical Pharmacology 1991;40:313‐5. [DOI] [PubMed] [Google Scholar]
La Civita 1996 {published data only}
- Civita L, Giuggioli D, Grazia Del Chicca M, Longombardo G, Pasero G, Ferri C. Effect of isradipine on endothelin‐1 plasma concentrations in patients with Raynaud's phenomenon. Annals of Rheumatic Diseases 1996;55:331‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leppert 1993 {published data only}
- Leppert J, Nilsson H, Myrdal U, Edvinsson L, Hedner T, Ringqvist I. Sympathetic activation after two weeks of nifedipine treatment in primary Raynaud's patients and controls. Cardiovascular Drugs and Therapy 1993;7:901‐7. [DOI] [PubMed] [Google Scholar]
Lewis 1987 {published data only}
- Lewis P, Psaila JV, Morgan RH, Davies WT, Woodcock JP. Nifedipine in patients with Raynaud's syndrome ‐ effects on radial artery blood flow. European Heart Journal 1987;8(Suppl K):83‐6. [DOI] [PubMed] [Google Scholar]
Myrdal 1994 {published data only}
- Myrdal U, Leppert J, Edvinsson L, Ekman R, Hednar T, Nilsson H, et al. Magnesium sulphate infusion decreases circulating calcitonin gene‐related peptide (CG Raynaud's phenomenon) in women with primary Raynaud's phenomenon. Clinical Physiology 1994;14:539‐46. [DOI] [PubMed] [Google Scholar]
Nilsson 1990 {published data only}
- Nilsson H, Blychert E, Jonasson T, Leppert J, Ringqvist I. The effect of felodipine on cold‐induced digital vasospasm. Journal of Cardiovascular Pharmacology 1990;15(Suppl.4):S108‐10. [DOI] [PubMed] [Google Scholar]
Park 2013 {published data only}
Pisenti 1984 {published data only}
- Pisenti L, Hart LL. DIAS rounds. Nifedipine and prazosin in Raynaud's phenomenon. Drug Intelligence & Clinical Pharmacy 1984;8(13):213‐4. [PubMed] [Google Scholar]
Rademaker 1989 {published data only}
- Rademaker M, Cooke ED, Almond NE, Beacham JA, Smith RE, Mant TGK, et al. Comparison of intravenous infusions of iloprost and oral nifedipine in treatment of Raynaud's phenomenon in patients with systemic sclerosis: a double blind randomised study. British Medical Journal 1989;298:561‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rademaker 1992 {published data only}
- Rademaker M, Meyrick‐Thomas RH, Kirby JD, Kovacs IB. The anti‐platelet effect of nifedipine in patients with systemic sclerosis. Clinical and Experimental Rheumatology 1992;10:57‐62. [PubMed] [Google Scholar]
Ringqvist 1993 {published data only}
- Ringqvist I, Hedner T, Leppert J, Niklasson U, Edvinsson L. Effects of cold pressor test on circulating arterial natriuretic peptide 99‐126 (ANP) in patients with Raynaud's phenomenon and influence of treatment with magnesium sulphate and nifedipine. Clinical Physiology 1993;13:271‐80. [DOI] [PubMed] [Google Scholar]
Schmidt 1989 {published data only}
- Schmidt JF, Valentin N, Levin Nielsen S. The clinical effect of felodipine and nifedipine in Raynaud's phenomenon. European Journal of Clinical Pharmacology 1989;37:191‐2. [DOI] [PubMed] [Google Scholar]
Shcherbakov 1987 {published data only}
- Shcherbakov AB, Guseva NG, Mach ES. Treatment of Raynaud's syndrome with calcium entry blockers. Terapevticheskii Arkhiv 1987;59(4):89‐92. [PubMed] [Google Scholar]
Smith 1985 {published data only}
- Smith CR, Rodeheffer RJ. Treatment of Raynaud's phenomenon with calcium channel blockers. American Journal of Medicine 1985;78(Suppl 2b):39‐42. [DOI] [PubMed] [Google Scholar]
Thompson 2001 {published data only}
- Thompson AE 1, Shea B, Welch V, Fenlon D, Pope JE. Calcium‐channel blockers for Raynaud’s phenomenon in systemic sclerosis. Arthritis & Rheumatology 2011;44(8):1841‐7. [DOI] [PubMed] [Google Scholar]
Thompson 2005 {published data only}
- Thompson AE, Pope JE. Calcium channel blockers for primary Raynaud’s phenomenon: a meta‐analysis. Rheumatology 2005;44(2):145‐50. [DOI] [PubMed] [Google Scholar]
Varela‐Aguilar 1997 {published data only}
- Verela‐Aguilar JM, Sanchez‐Roman J, Talegon Melendez A, Castillo Palma J. Comparative study of misoprostol and nifedipine for the treatment of Raynaud's phenomenon secondary to systemic diseases. Doppler‐duplex hemodynamic evaluation [Estudio comparativo de misoprostol y nifedipino en el tratamiento del fenomeno de Raynaud secundario a enfermedades sistemacis. Valoracion hemodinamica mediante Doppler‐duplex]. Revista Clinica Espanol 1997;197(2):77‐83. [PubMed] [Google Scholar]
Vayssairat 1989 {published data only}
- Vayssairat M, Blaison N, Baudot N, Evenou P, Gilard M, Mathieu J‐F. Effect of nifedipine on cold stress post‐ischemic reactive hyperemia in Raynaud's phenomenon [Action de la nifedipine sur la reaction d'hyperhemie post‐ischemique au froid dans le phenomene de Raynaud]. Journal des Maladies Vasculaires 1989;14(4):299‐302. [PubMed] [Google Scholar]
Weber 1990 {published data only}
- Weber A, Bounameaux H. Effects of low‐dose nifedipine on a cold provocation test in patients with Raynaud's disease. Journal of Cardiovascular Pharmacology 1990;15:853‐5. [DOI] [PubMed] [Google Scholar]
Winston 1983 {published data only}
- Winston EL, Pariser MK, Miller KB, Salem SN, Creager MA. Nifedipine as a therapeutic modality for Raynaud's phenomenon. Arthritis & Rheumatology 1983;26(10):1177‐80. [DOI] [PubMed] [Google Scholar]
Wollersheim 1987 {published data only}
- Wollersheim H, Thien T, van't Laar A. Nifedipine in primary Raynaud's phenomenon and in scleroderma: oral versus sublingual hemodynamic effects. Journal of Clinical Pharmacology 1987;27:907‐13. [DOI] [PubMed] [Google Scholar]
Wu 2008 {published data only}
- Wu YJ, Luo SF, Yang SH, Chen JY, Yu KH, See LC. Vascular response of Raynaud's phenomenon to nifedipine or herbal (Duhuo‐Tisheng Tang with Danggui‐Sini Tang): a preliminary study. Chan Gung Medicine Journal 2008;31(5):492‐501. [PubMed] [Google Scholar]
References to studies awaiting assessment
EUCTR2009‐018194‐31‐GB {published data only}
Kahan 1982 {published data only}
- Kahan A, Weber S, Amor B, Saporta L, Hodara M, Degeorges M. Controlled study of nifedipine in the treatment of Raynaud's phenomenon [Etude controllee de la nifedipine dans le traitement du phenomene de Raynaud]. Revue du Rhumatism 1982;49(5):337‐43. [PubMed] [Google Scholar]
Kahan 1983a {published data only}
Redondo 1986 {published data only}
- Redondo FR, Noguerado A, Asensio JP, Bote L, Alvaro‐Gracia JL, Castellanos M. Nifedipine treatment of Raynaud's phenomenon: a double blind controlled clinical study. Revista Espanola de Reumatologia 1986;13:121‐3. [Google Scholar]
van Heereveld 1988 {published data only}
Wasir 1983 {published data only}
- Wasir HS, Singh G, Kaul R, Sachdeva, Kahan WA, Chhina GS, et al. A comparative study of trifluoperazine a calmodulin inhibitor, nifedipine, dipyridamole and intraarterial reserpine in the treatment of raynaud's phenomenon: a double blind randomised controlled trial [abstract]. Indian Heart Journal 1983;35(5):306. [Google Scholar]
Wise 1987 {published data only}
- Wise RA, Malamet R, Wigley FM. Acute effects of nifedipine on digital blood flow in human subjects with Raynaud's phenomenon: a double blind placebo controlled trial. Journal of Rheumatology 1987;14(2):278‐83. [PubMed] [Google Scholar]
References to ongoing studies
Nazarinia 2016 {published data only}
- Nazarinia MA. Assessing and Comparing the Effect of Diltiazem Gel Versus Nitroglycerin Ointment in Healing Process of Scleroderma Digital Ulcers. Clinicaltrials.gov 2017. [Google Scholar]
Vera‐Kellet 2017 {published data only (unpublished sought but not used)}
- Vera‐Kellet C. Color Doppler Ultrasound Comparison of Topical 10% Nifedipine Versus 5% Sildenafil in Secondary Raynaud: A Randomized, Double‐blind, Placebo‐controlled Pilot Study. Clinicaltrials.gov 2017.
Additional references
Cates 2008 [Computer program]
- Cates, C, MD. Visual Rx. 2016.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, editors. Chapter 9: Analysing data and undertaking meta‐analyses. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Egger 1997
- Egger M, Zellweger‐Zahner T, Scheider M, Junker C, Lengeler Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;350(907):326‐9. [DOI] [PubMed] [Google Scholar]
García‐Carrasco 2008
- García‐Carrasco M, Jiménez‐Hernández M, Escárcega RO, Mendoza‐Pinto C, Pardo‐Santos R, Levy R. Treatment of Raynaud's phenomenon. Autoimmunity Reviews 2008;8(1):62‐8. [DOI] [PubMed] [Google Scholar]
Goundry 2012
- Goundry B, Bell L, Langtree M, Moorthy A. Diagnosis and management of Raynaud’s phenomenon. British Medical Journal 2012;344(e289):doi: 10.1136/bmj.e289.. [DOI] [PubMed] [Google Scholar]
Grade 2008
- Grading Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2008;336(7653):1106‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansteen 1976
- Hansteen V. Medical treatment in Raynaud's disease. Acta Chirurgica Scandinavica Supplementum 1976;465:87‐91. [PubMed] [Google Scholar]
Herrick 2005
- Herrick AL. Pathogenesis of Raynaud's phenomenon. Rheumatology 2005;44(5):587‐96. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Khanna 2009
- Khanna PP, Maranian P, Gregory J, Khanna D. The minimally important difference and patient acceptable symptom state for the Raynaud’s condition score in patients with Raynaud’s phenomenon in a large randomized controlled clinical trial. Annals of the Rheumatic Diseases 2009;69(3):588‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
LeRoy 1992
- LeRoy EC, Medsger TA. Raynaud's phenomenon: a proposal for classification. Clinical and Experimental Rheumatology 1992;10(5):485‐8. [PubMed] [Google Scholar]
Levien 2010
- Levien TL. Advances in the treatment of Raynaud's phenomenon. Dove Press Journal: Vascular Health and Risk Management 2010 2010;24(6):167‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masi 1980
- Masi AT, Rodnan GP, Medsger TA Jr, et al. ACR Preliminary criteria for the classification of systemic sclerosis (scleroderma). Special article. Arthritis & Rheumatism 1980;23:581‐90. [DOI] [PubMed] [Google Scholar]
Maundrell 2015
- Maundrell A, Proudman SM. Epidemiology of Raynaud’s Phenomenon. Epidemiology of Raynaud's Phenomenon. 1st Edition. New York, NY: Springer, 2015:21‐35. [Google Scholar]
Merkel 2002
- Merkel PA, Herlyn K, Martin RW, Anderson JJ, Mayes MD, Bell P, .et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud's phenomenon. Arthritis & Rheumatism 2002;46(9):2410‐20. [DOI] [PubMed] [Google Scholar]
Ortonne 1989
- Ortonne JP, Torzuoli C, Dujardin P, Fraitag B. Ketanserin in the treatment of systemic sclerosis: a double‐blind controlled trial. British Journal of Dermatology 1989;120(2):261‐6. [DOI] [PubMed] [Google Scholar]
Pope 2015
- Pope J, Rirash F, Tingey PC, Harding SE, Maxwell LJ, Tanjong GE, et al. Drug interventions versus placebo for the treatment of Raynaud’s phenomenon: generic protocol. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011813] [DOI] [Google Scholar]
Schünemann 2011
- Schünemann H, Oxman AD, Vist GE, Higgins JBT, Deeks JJ, et al. editors. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Silman 1990
- Silman A, Holligan S, Brennan P, Maddison P. Prevalence of symptoms of Raynaud’s phenomenon in general practice. British Medical Journal 1190;301(6752):590‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, et al. Reccomendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. British Medical Journal 2011;343(d4002):doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
Sturgill 1998
- Sturgill MG, Seibold JR. Rational use of calcium‐channel antagonists in Raynaud’s phenomenon. Current Opinion in Rheumatology 1998;10(6):584–8. [DOI] [PubMed] [Google Scholar]
The Cochrane Collaboration 2014. [Computer program]
- Cochrane Collaboration 2014. Review Manager 5 (RevMan 5).Version 5.3. Copenhagen: Nordic Cochrane Centre.
Wigley 2002
- Wigley FM. Raynaud's phenomenon. New England Journal of Medicine 2002;347(13):1001‐8. [DOI] [PubMed] [Google Scholar]


