Abstract
Background
Beta blockers are commonly used to treat hypertension. The blood pressure reading is the primary tool for physicians and patients to assess the efficacy of the treatment. The blood pressure lowering effect of beta‐1 selective blockers is not known.
Objectives
To quantify the dose‐related effects of various doses and types of beta‐1 selective adrenergic receptor blockers on systolic and diastolic blood pressure versus placebo in people with primary hypertension.
Search methods
We searched the Database of Abstracts of Reviews of Effectiveness (DARE) for related reviews.
We searched the following databases for primary studies: the Cochrane Hypertension Specialised Register (All years to 15 October 2015), CENTRAL via the Cochrane Register of Studies Online (2015, Issue 10), Ovid MEDLINE (1946 to 15 October 2015), Ovid EMBASE (1974 to 15 October 2015) and ClinicalTrials.gov (all years to 15 October 2015).
The Hypertension Group Specialised Register includes controlled trials from searches of CAB Abstracts, CINAHL, Cochrane Central Register of Controlled Trials, EMBASE, Food Science and Technology Abstracts (FSTA), Global Health, LILACS, MEDLINE, ProQuest Dissertations & Theses, PsycINFO, Web of Science and the WHO International Clinical Trials Registry Platform (ICTRP).
Electronic databases were searched using a strategy combining the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision) with selected MeSH terms and free text terms. No language restrictions were used. The MEDLINE search strategy was translated into CENTRAL, EMBASE, the Hypertension Group Specialised Register and ClinicalTrials.gov using the appropriate controlled vocabulary as applicable. Full strategies are in Appendix 1.
Selection criteria
Randomised, double‐blind, placebo‐controlled parallel or cross‐over trials. Studies had to contain a beta blocker monotherapy arm with fixed dose. People enrolled into the studies had to have primary hypertension at baseline. Duration of studies had to be between 3 weeks to 12 weeks. Drugs in this class of beta blockers are atenolol, betaxolol, bevantolol, bisoprolol, esmolol, metoprolol, nebivolol, pafenolol, practolol.
Data collection and analysis
Two authors confirmed the inclusion of studies and extracted the data independently. Review Manager (RevMan) 5.3.5 was used to synthesise data.
Main results
We identified 56 RCTs (randomised controlled trials) that examined the blood pressure (BP) lowering efficacy of beta‐1 selective blockers (beta‐1 blocker) in 7812 primary hypertensive patients. Among the included trials, 26 RCTs were parallel studies and 30 RCTs were cross‐over studies, examining eight beta‐1 blockers. Overall, the majority of beta‐1 blockers studied significantly lowered systolic blood pressure (SBP) and diastolic blood pressure (DBP). In people with mild to moderate hypertension, beta‐1 selective blockers lowered BP by an average of ‐10/‐8 mmHg and reduced heart rate by 11 beats per minute. The maximum BP reduction of beta‐1 blockers occurred at twice the starting dose. Individual beta‐1 blockers did not exhibit a graded dose‐response effect on SBP and DBP over the recommended dose range.
Most beta‐1 blockers tested significantly lowered heart rate. A graded dose‐response of beta‐1 blockers on heart rate was evident. Higher dose beta‐1 blockers lowered heart rate more than lower doses. Individually and overall beta‐1 blockers did not affect pulse pressure, which distinguishes them from other classes of drugs.
Authors' conclusions
This review provides low quality evidence that in people with mild to moderate hypertension, beta‐1 selective blockers lowered BP by an average of ‐10/‐8 mmHg and reduced heart rate by 11 beats per minute as compared to placebo. The effect of beta‐1 blockers at peak hours, ‐12/‐9 mmHg, was greater than the reduction at trough hours, ‐8/‐7 mmHg. Beta‐1 selective blockers lowered BP by a greater magnitude than dual receptor beta‐blockers and partial agonist beta‐blockers, lowered BP similarly to nonselective beta‐blockers. Beta‐1 selective blockers lowered SBP by a similar degree and lowered DBP by a greater degree than diuretics, angiotensin converting enzyme inhibitors and angiotensin receptor blockers. Because DBP is lowered by a similar extent to SBP, beta‐1 selective blockers do not reduce pulse pressure.
Keywords: Humans, Adrenergic beta‐Antagonists, Adrenergic beta‐Antagonists/administration & dosage, Adrenergic beta‐Antagonists/therapeutic use, Antihypertensive Agents, Antihypertensive Agents/administration & dosage, Antihypertensive Agents/therapeutic use, Blood Pressure, Blood Pressure/drug effects, Diastole, Diastole/drug effects, Essential Hypertension, Heart Rate, Heart Rate/drug effects, Hypertension, Hypertension/drug therapy, Randomized Controlled Trials as Topic, Systole, Systole/drug effects
Plain language summary
Beta‐1 selective blockers for treatment of high blood pressure
Background
Beta‐1 selective blockers are a subclass of beta blockers that are commonly used to treat high blood pressure. Drugs in this class include atenolol (Tenormin), metoprolol (Lopressor), nebivolol (Bystolic) and bisoprolol (Zebeta, Monocor). We developed a comprehensive methodology to examine how different doses and drugs in this class of drugs lower blood pressure.
Characteristics of included studies
We found and included 56 clinical trials examining the blood pressure lowering effect of eight beta‐1 blockers in 7812 people with high blood pressure. These participants were randomly assigned to receive a fixed dose of beta‐1 blocker treatment or placebo for 3 weeks to 12 weeks.
Key results
On average, beta‐1 blockers lowered BP by ‐10 points of systolic and ‐8 points of diastolic pressure in people with mild to moderate high blood pressure. In general, higher doses of beta‐1 blockers did not show greater reduction of blood pressure compared to lower doses. The maximum blood pressure reduction was exhibited at twice the recommended starting dose.
Higher doses of beta‐1 blockers lowered heart rate more than the lower doses, therefore are more likely to cause the common side effect of slowed heart rate. Beta‐1 selective blockers lower systolic and diastolic BP to a similar degree, as is the case for the other subclasses of beta blockers, and thus have little or no effect on pulse pressure. This is different from other classes of antihypertensive drugs, such as thiazide diuretics, angiotensin converting enzyme inhibitors and angiotensin receptor blockers.
Quality of evidence
The quality of the evidence was judged to be low due to various types of bias that could exaggerate the effect. A low quality of evidence means future research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Summary of findings
for the main comparison.
| Blood pressure lowering effects of beta‐1 blockers compared with placebo for primary hypertension | |||
|
Patient or population: People with primary hypertension Intervention: Beta‐1 selective blockers Comparison: Placebo | |||
| Outcomes | mean estimates of combining 1x and 2x starting dose (95% CI) | No of Participants (No. of RCT) | Quality of the evidence (GRADE) |
| Systolic blood pressure | ‐10.4 (‐11.1, ‐9.7)1,2,3 | 5246 (47) | Low4,5 |
| Diastolic blood pressure | ‐8.3 (‐8.7, ‐7.8)1,2,3 | 5316 (48) | Low4,5 |
| Heart rate | ‐10.9 (‐11.5, ‐10.4)1,2 | 3407 (33) | Low4,5 |
| Pulse pressure | ‐1.8 [‐2.3, ‐1.2]1,2 | 5246 (47) | Very low4,5,6 |
| WDAE | 0.9 (0.5, 1.5) | 2618 (3) | Low7,8 |
| 95% CI: 95% confident interval; WDAE: Withdrawal due to adverse effect. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
| Footnotes | |||
| |||
Background
Description of the condition
Elevated blood pressure (hypertension) is a highly prevalent condition that is associated with an increased risk of adverse cardiovascular events including stroke, myocardial infarction, congestive heart failure and renal failure. Antihypertensive drug treatment has been shown to reduce the incidence of these adverse events. There are a number of classes of antihypertensive drugs used to treat elevated blood pressure. Beta adrenergic receptor blockers (beta‐blockers) are one such class of drug.
Description of the intervention
Beta‐blockers were originally marketed and used to treat angina. During their use in people with angina, it was discovered that they also lowered blood pressure. Since then, they have received clinical attention because of their established effectiveness for certain arrhythmias and their known preventative action in people who have had a myocardial infarction.
Five previous systematic reviews are relevant to this proposed review. Wright 2000 assessed the mortality and morbidity associated with different types of beta blockers. He found that patients treated with non‐selective beta blockers, post myocardial infarction, significantly reduced total mortality as compared to placebo, whereas beta‐1 selective beta blockers or partial agonist beta‐blockers did not. A recent review assessed the effects of beta adrenergic blocking agents on morbidity and mortality in adults with hypertension (Wiysonge 2012). This review concluded that beta blockers were not the best class of drugs to use as first‐line antihypertensive therapy. However, it is possible that this related to beta‐1 selective beta blockers, as atenolol was the beta‐blocker used in 75% of the trials.
Three systematic reviews have assessed the effects of beta‐blockers on blood pressure. A Cochrane systematic review on beta blockers in hypertension during pregnancy (Magee 2003) showed that oral beta‐blockers decreased the incidence of severe hypertension and the need for additional antihypertensive therapy. A systematic review of the dose‐response blood pressure lowering effect of beta blocker drugs and other antihypertensive drugs (Law 2005) did not differentiate between the different classes of beta‐blockers. Finally, a systematic review of the blood pressure lowering efficacy of beta‐blockers given as a second‐line drug did not differentiate between the different classes of beta‐blockers (Chen 2010).
How the intervention might work
Beta adrenergic receptors are present in many peripheral body systems including the heart, blood vessels, kidneys, and nervous system. At the present time, the mechanism whereby beta‐blockers lower blood pressure is not known, though many hypothetical mechanisms have been proposed. Beta blockers could lower blood pressure by decreasing cardiac output, reducing renin production, modulating the sympathetic nervous system or other mechanisms. It is likely to be a combination of mechanisms that lead to the blood pressure lowering effect.
Beta‐blockers were designed to competitively inhibit beta‐receptors and thus modulate sympathetic nervous system activity. There are three main classes of beta‐receptors: beta‐1, beta‐2 and beta‐3. Beta‐1 (cardioselective) receptor blockers have a greater specificity to block beta‐1 receptors than beta‐2 receptors. However, this selectivity diminishes as the dose of the beta‐blocker increases. Drugs in this class have no intrinsic sympathomimetic activity (partial agonist) or alpha blocking effects. Beta‐1 adrenergic receptors are the predominant adrenergic receptor in the heart. Activation of beta‐1 receptors opens L‐type calcium channels through the cAMP/protein kinase A pathway. Opening of L‐type calcium channels allows calcium ions to flow into the cells and produce a positive inotropic and chronotropic effect (Kamp 2000). Blocking of beta‐1 receptors decreases heart rate and cardiac contractility. This effect could contribute to the antihypertensive effect of beta‐1 blockers (Westfall 2011).
Why it is important to do this review
Since it is probable that beta‐blockers with different mechanisms of action have different effects to reduce morbidity and mortality, it is crucial to determine whether they have different abilities to lower blood pressure. No published review has compared the blood pressure lowering effect of beta blockers based on their mechanism of action. If beta‐blockers with different beta receptor selectivity lower blood pressure differently, it would provide useful information towards understanding the mechanism by which they lower blood pressure.
Furthermore since blood pressure measurement is used on a daily basis by physicians managing people with high blood pressure, it is important to accurately assess the average magnitude of blood pressure lowering effects of beta blockers both individually and as sub‐classes. The findings of this review will be compared to the results of the Cochrane reviews of the other the subclasses of beta blockers: non‐selective beta blockers (Wong 2014a), partial agonist beta blockers (Wong 2014b) dual alpha and beta blockers (Wong 2015). The information found in this review will be useful for clinicians, researchers designing future drug trials and authors of other systematic reviews.
Objectives
Primary objective
To quantify the dose‐related effects of various doses and types of beta‐1 selective adrenergic receptor blockers on systolic and diastolic blood pressure versus placebo in people with primary hypertension.
Secondary objectives
To determine the effects of beta‐1 selective adrenergic receptor blockers on variability of blood pressure.
To determine the effects of beta‐1 selective adrenergic receptor blockers on pulse pressure.
To quantify the dose‐related effects of beta‐1 selective adrenergic receptor blockers on heart rate.
To quantify the effects of beta‐1 selective adrenergic receptor blockers at various doses on withdrawals due to adverse events.
Methods
Criteria for considering studies for this review
Types of studies
Study design must meet the following criteria:
placebo‐controlled;
random allocation to beta adrenergic receptor blocker group and placebo group;
parallel or cross‐over design;
double‐blinded;
duration of follow‐up of at least three weeks;
blood pressure measurements at baseline (following washout) and at one or more time points between 3 weeks to 12 weeks after starting treatment.
Types of participants
Participants had to have a baseline blood pressure of at least 140 mmHg systolic or a diastolic blood pressure of at least 90 mmHg, or both, measured in a standard way. Participants must not have had creatinine levels greater than 1.5 times the normal level. Participants were not restricted by age, gender, baseline risk or any other co‐morbid conditions.
Types of interventions
Monotherapy with any beta‐1 selective adrenergic receptor blocker, including atenolol, betaxolol, bevantolol, bisoprolol, esmolol, metoprolol and nebivolol, pafenolol, practolol.
Data from trials in which titration to a higher dose was based on blood pressure response were not eligible.
Types of outcome measures
Primary outcomes
Change in trough (13 hours to 26 hours after the dose) or peak (1 hour to 12 hours after the dose), or both, systolic and diastolic blood pressure compared to placebo. If blood pressure measurements were available at more than one time within the acceptable window, we used the mean differences (MD) of blood pressures taken in the 3 week to 12 week range.
Secondary outcomes
Change in standard deviation compared to placebo.
Change in pulse pressure compared to placebo.
Change in heart rate compared to placebo.
Number of participants who withdrew due to adverse events compared to placebo.
Search methods for identification of studies
Electronic searches
We searched the Hypertension Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (2015 Issue 9), Ovid MEDLINE (1946 to October 2015), Ovid EMBASE (1974 to October 2015) and ClinicalTrials.gov (all years to October 2015) for randomised controlled trials. We searched the Database of Abstracts of Reviews of Effects (DARE) for related reviews (October 2015). No language restrictions were applied. The WHO International Clinical Trials Registry Platform (ICTRP) is searched for inclusion in the Group's Specialised Register.
We used a modified, expanded version of the standard search strategy of the Hypertension Group with additional terms related to beta adrenergic receptor blockers in general and all the specific drugs listed above to identify the relevant articles. The MEDLINE strategy was translated into CENTRAL, EMBASE, ClinicalTrials.gov, and the Hypertension Group Specialised Register (Appendix 1) .
Searching other resources
We used previously published meta‐analyses on dose‐response of beta adrenergic receptor blockers to help identify references to trials.
We searched the bibliographies of pertinent articles, reviews and texts for additional citations.
Data collection and analysis
Selection of studies
We performed the initial search of all the databases to identify citations with potential relevance. The initial screen of these abstracts excluded articles whose titles or abstracts, or both were clearly irrelevant. We retrieved the full text of the remaining articles and translated them into English where required. We imported the references and abstracts identified by our search into Reference Manager 11 software. Two independent reviewers assessed the eligibility of the trials using a trial selection form based on the criteria listed above. A third reviewer resolved discrepancies.
Data extraction and management
Two review authors independently extracted data using a standard form and then cross‐checked them. A second person confirmed all numeric calculations and graphic interpolations.
Assessment of risk of bias in included studies
We assessed the risk of bias with the standard Cochrane 'Risk of bias' tool (Higgins 2011a). The domains assessed included allocation sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, completeness of participant follow‐up, handling of incomplete outcome data and protection against selective outcome reporting.
Measures of treatment effect
The position of the patient during blood pressure measurement might affect the blood pressure reading or true lowering effect. However, in order not to lose valuable data, we used data reported from any single position, regardless of the position. When blood pressure measurement data were available from more than one position, sitting blood pressure was the first preference. If both standing and supine were available, we used standing blood pressure. We reported effect measures as the mean difference (MD) in BP, heart rate and pulse pressure between the treatment and placebo groups with 95% confidence interval (CI). We reported risk ratio (RR) for withdrawal due to adverse effects.
Dealing with missing data
In the case of missing information in the included studies, we contacted the investigators (using email, letter, fax or a combination) to obtain the missing information.
In the case of missing standard deviations (SD) of the change in blood pressure, we imputed the standard deviation, based on the information in the same study or from other studies using the same drug. We used the following hierarchy (listed from high to low preference) to impute standard deviation values:
standard deviation of change in blood pressure taken in a different position than that of the blood pressure data used;
standard deviation of blood pressure at the end of treatment;
standard deviation of blood pressure at the end of treatment measured in a different position than that of the blood pressure data used;
standard deviation of blood pressure at baseline (unless this measure was used for entry criteria);
mean standard deviation of change in blood pressure from other studies using the same drug.
Assessment of heterogeneity
We tested for heterogeneity of treatment effect between the trials using a standard Chi2 statistic for heterogeneity. We applied the fixed‐effect model to obtain summary statistics of pooled trials, unless significant between‐study heterogeneity was present, in which case we used the random‐effects model (Deeks 2011).
Data synthesis
We carried out data synthesis and analysis using the Cochrane Review Manager software, RevMan 5.3.5 (RevMan 2014).
We combined data for changes in blood pressure and heart rate using mean difference (MD). We analysed drop‐outs due to side effects by using risk ratio (RR). When we found statistically significant RR, we calculated risk difference (RD), and number needed to treat for an additional harmful outcome (NNTH).
When we used the generic inverse variance method to incorporate cross‐over studies into meta‐analysis, we used the formula listed in the Cochrane Handbook for Systematic Reviews of Interventions, section 16.4.6.1 (Higgins 2011b) to calculate the standard deviation of the difference between treatment and placebo. In order to minimise the loss of statistical power for the estimates we did not adjust the standard error and sample sizes shown in the 'Data and analyses' tables for subgroup comparisons. To avoid double counting of participants when comparing multiple subgroups or combining two subgroups for overall estimates, we adjusted the standard error or the sample size in analyses for studies containing multiple dosage subgroups.
Subgroup analysis and investigation of heterogeneity
If possible, subgroup analyses included:
different regimens of the same active chemical entity;
gender, age and race;
co‐morbid conditions: ischaemic heart disease, peripheral vascular disease, diabetes;
baseline severity of hypertension: mild, moderate, severe.
Sensitivity analysis
We tested the robustness of the results using several sensitivity analyses, including:
trials that were industry‐sponsored versus non‐industry sponsored;
trials with blood pressure data measured in the sitting position versus other measurement positions;
trials with reported standard deviations of blood pressure change versus imputed standard deviations.
Results
Description of studies
Results of the search
In order to save time and effort, the trial search coordinator from Cochrane Hypertension group developed a comprehensive search strategy (Appendix 1) so that all four subclasses of beta blockers were searched simultaneously. We used the same study inclusion criteria for the other three beta blocker reviews (Wong 2014a; Wong 2014b; Wong 2015). Citations were then sorted according to their respective subclasses afterward. Please refer to Figure 1 for the flow diagram of study selection.
1.
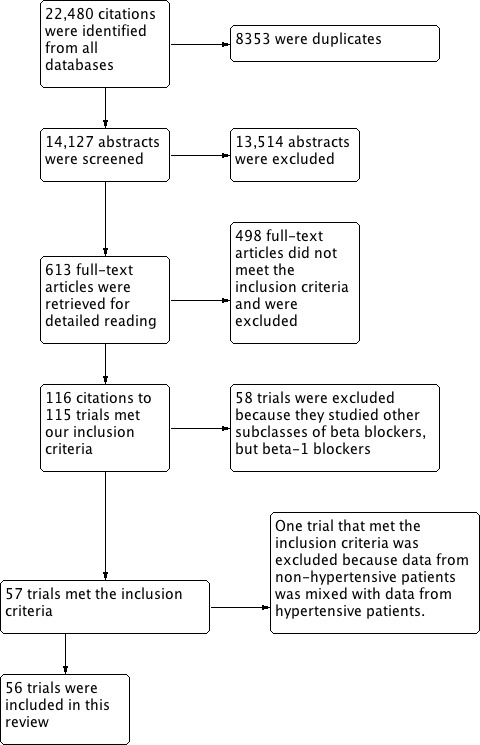
PRISMA study flow diagram
The search was first run in May 2010, and subsequent searches have been performed up to October 2015. A total of 22,480 citations have been identified in all searches since May 2010, of which 8353 were confirmed to be duplicates. The reviewers then screened 14,127 titles and abstracts, of which 13,514 citations were excluded. We judged 613 citations to potentially meet the inclusion criteria based on title and abstract and retrieved them for detailed review. We excluded 498 full text articles which did not meet our inclusion criteria. One hundred and fifteen trials met our inclusion criteria for all four subclasses. In total, 56 trials were included in this review.
Included studies
We included 56 RCTs that examined the BP lowering efficacy of eight beta‐1 blockers in 7812 primary hypertensive patients. Twenty six RCTs were parallel studies and the other 30 RCTs were cross‐over studies with duration of treatment ranging from 3 weeks to 16 weeks. When study duration was longer than 12 weeks, we used only the data obtained between 3 weeks to 12 weeks. The mean baseline BP of the randomised participants in the studies was 155.6/101.1 mmHg. Please refer to Characteristics of included studies for details of included studies.
Excluded studies
We excluded one RCT that met the inclusion criteria because it reported data from non‐hypertensive participants mixed with hypertensive participants. This RCT was listed in the Characteristics of excluded studies.
Risk of bias in included studies
Figure 2 shows the 'Risk of bias' summary of each included study.
2.
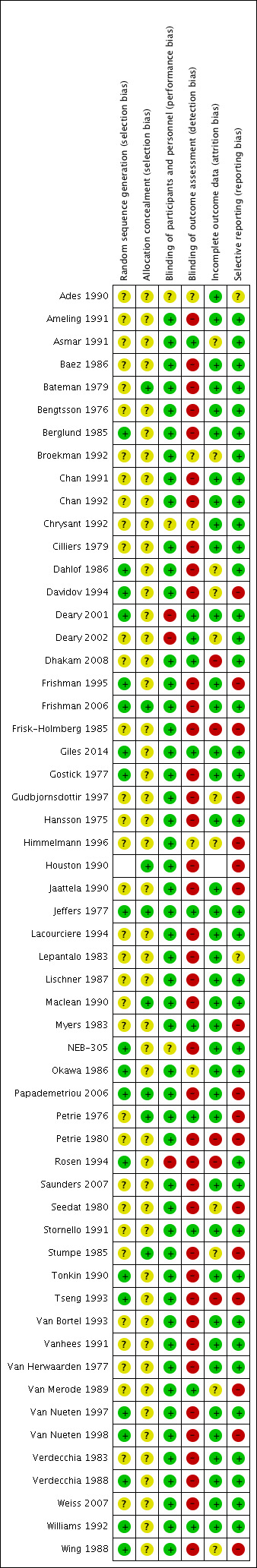
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Details regarding random sequence generation and allocation concealment were poorly reported in many of the included studies. This information was important in determining the potential of selection bias in this review. Because of poor reporting, it was difficult to judge the potential for selection bias. In the parallel studies, we examined the baseline characteristics of participants in each intervention group. We did not find any significant difference in baseline characteristics between the intervention groups which would raise concerns regarding selection bias. Hence, we did not find any evidence that suggested a high risk of selection bias in the parallel studies. It was impossible to assess whether randomisation was conducted properly in cross‐over studies, thus there is an unclear risk of selection bias in cross‐over trials.
Blinding
Beta blockers are well known for their ability to lower heart rate. The assessor could detect the difference in heart rate when measuring blood pressure. Using an automated machine to measure BP would mitigate this risk of detection bias. However this was not done in most of the included trials in this review. Therefore, the risk of detection and performance bias is high in this review.
Incomplete outcome data
Most of the studies reported the method by which they handled dropouts. The dropout rates were low and most of studies used an ITT analysis. Therefore, we judged that the risk of attrition bias in this review was low.
Selective reporting
All the studies included reported the SBP, DBP and heart rate as outcomes of the participants. Withdrawal due to adverse effects is an important outcome in clinical trials. Only two studies reported useful data on withdrawal due to adverse effects. The risk of reporting bias was high because most of the studies failed to report withdrawal due to adverse effects.
Other potential sources of bias
Funnel plots of the pooled data showed a paucity of small and less effective studies and positive outliers. The asymmetry of funnel plots indicated the high potential for publication bias (Sterne 2011). Some of the extreme outliers were large studies with a large effect that was questionable. It was not possible to prove that these trials were flawed, therefore we kept them in the review, however, they add to the suspicion of a high risk of bias. As a result, BP lowering estimates of the group combining the starting dose and twice the starting dose are likely to be overestimated.
Effects of interventions
See: Table 1
The primary outcomes and some key secondary outcomes are summarized in the Table 1.
The results below are presented in descending order according to the sample size.
Blood pressure lowering efficacy of nebivolol
Nebivolol is a beta‐1 selective blocker which has been marketed in Canada since early 2013. Nebivolol is indicated for treatment of hypertension in Canada and the United States of America (USA) (eCPS; FDA). The recommended doses of nebivolol are 5 mg to 20 mg daily (eCPS). Twelve RCTs examining the blood pressure lowering efficacy of 1 mg to 40 mg per day nebivolol in 3209 hypertensive participants were included in this review. Eight of them were parallel studies and the other four were cross‐over studies. In addition, NEB‐305 was an unpublished RCT from an FDA report. The mean baseline BP of the participants in the included studies was 154.6/100.4 mmHg.
Please refer to Analysis 1.1, Analysis 1.2, Analysis 1.3 and Analysis 1.4 for the results of nebivolol. All nebivolol doses significantly lowered trough SBP and DBP compared to placebo. The test for subgroup differences by direct comparison included five large RCTs with multiple dosage subgroups. There was no dose‐related effect within the recommended dose range (starting dose, twice the starting dose, four times the starting dose) for SBP (P = 0.47) and DBP (P = 0.52). The maximum BP lowering effect was seen at 5 mg/day (starting dose). Since there was no dose response within the recommended doses, we pooled the three dosages into subgroups (5 mg, 10 mg and 20 mg) to calculate the estimates for blood pressure lowering efficacy. The estimate for blood pressure lowering efficacy for nebivolol is ‐8/‐6 mmHg. Heterogeneity was significant in these subgroups and we discuss the validity of this estimate further in the Discussion.
1.1. Analysis.
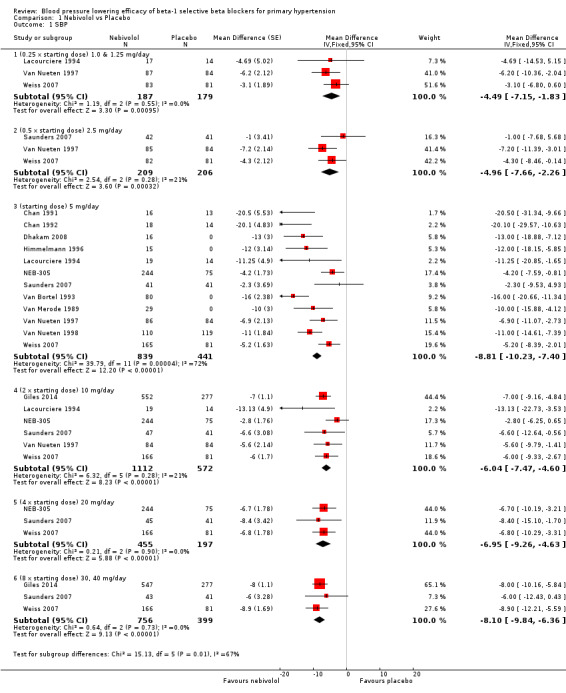
Comparison 1 Nebivolol vs Placebo, Outcome 1 SBP.
1.2. Analysis.
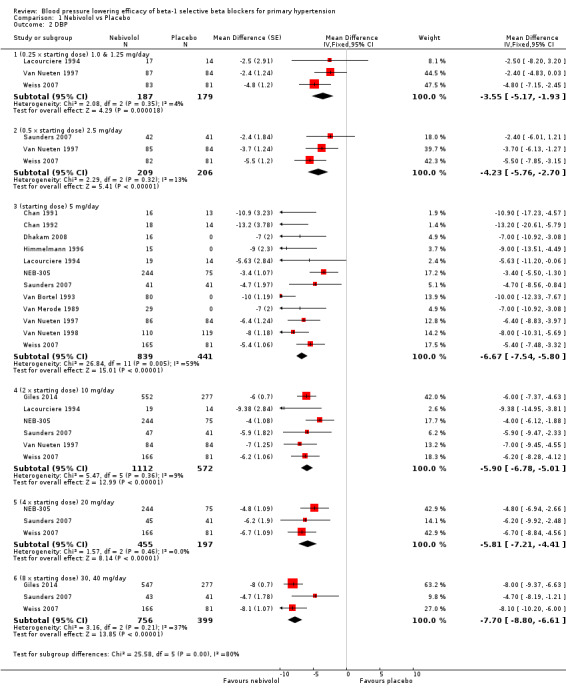
Comparison 1 Nebivolol vs Placebo, Outcome 2 DBP.
1.3. Analysis.
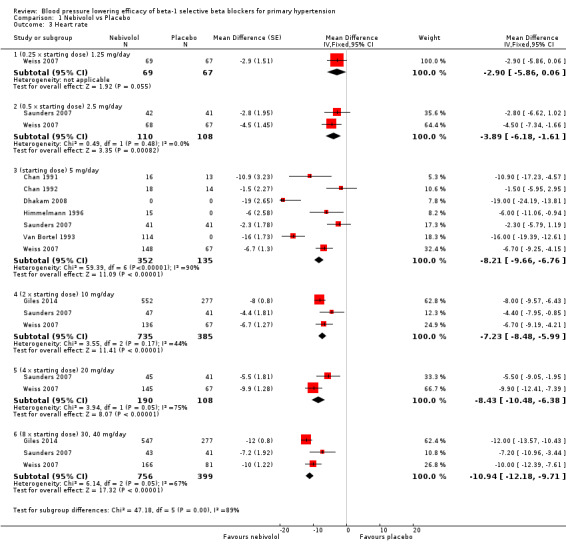
Comparison 1 Nebivolol vs Placebo, Outcome 3 Heart rate.
1.4. Analysis.
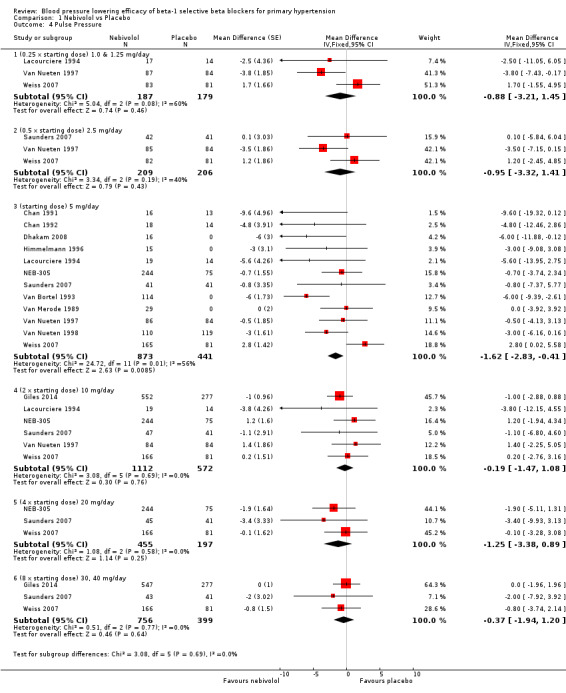
Comparison 1 Nebivolol vs Placebo, Outcome 4 Pulse Pressure.
Nebivolol 1.25 mg/day did not significantly lower heart rate. Starting from 2.5 mg/day, nebivolol significantly lowered heart rate compared to placebo. Heterogeneity in the 5 mg/day subgroup was significant. The test of subgroup differences by direct comparison was significant (P = 0.0004) for heart rate. Only nebivolol 5 mg/day significantly lowered pulse pressure. However, the 5 mg/day subgroup was also the only subgroup in which heterogeneity was significant.
Four RCTs, NEB‐305; Saunders 2007; Van Nueten 1997; Weiss 2007, provided both peak and trough data in the same participants. We compared the difference in peak and trough effect for SBP and DBP. Peak measurements were not significantly different from trough measurements in SBP (Analysis 1.5). The BP lowering effect at peak was significantly greater than trough measurements for DBP, averaging 2 mmHg difference (Analysis 1.6).
1.5. Analysis.
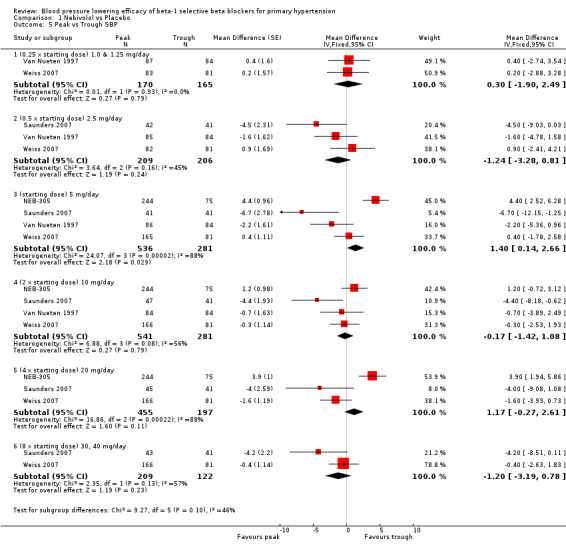
Comparison 1 Nebivolol vs Placebo, Outcome 5 Peak vs Trough SBP.
1.6. Analysis.
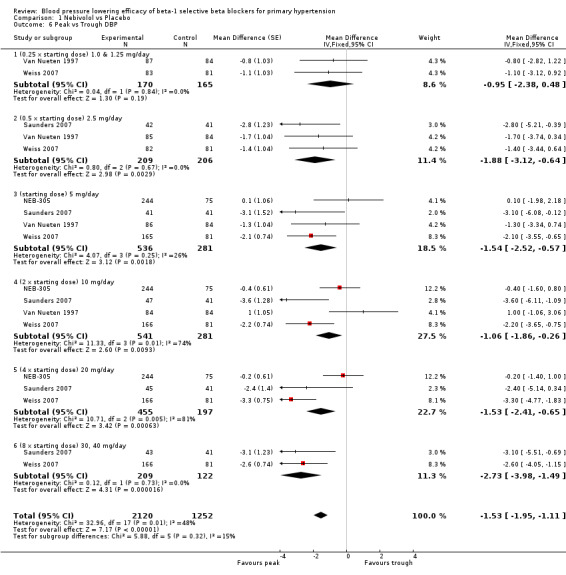
Comparison 1 Nebivolol vs Placebo, Outcome 6 Peak vs Trough DBP.
The blood pressure variability was not significantly different between nebivolol and placebo for SBP (P = 0.61) and DBP (P = 0.52).
Blood pressure lowering efficacy of atenolol
Atenolol is indicated for the treatment of hypertension and angina in Canada and the USA (eCPS; FDA). The recommended doses for hypertension are 50 mg to 100 mg daily (FDA). Twenty‐three RCTs examining the blood pressure lowering efficacy of 25 mg to 200 mg per day atenolol in 1119 hypertensive participants were included in this review. Seven of the included studies were parallel studies and the other 16 were cross‐over studies. The mean baseline BP for the atenolol studies was 162.3/104.2 mmHg.
Please refer to Analysis 2.1, Analysis 2.2, Analysis 2.3 and Analysis 2.4 for the atenolol results. All atenolol doses significantly lowered SBP and DBP compared to placebo. The maximum BP lowering effect was shown at 100 mg/day (twice the starting dose). The test for subgroup differences within the recommended dose range (starting dose, twice the starting dose and four times the starting dose) by direct comparison was not significant for SBP (P = 0.56) and DBP (P = 0.22). However, only two small studies provided information for direct comparison. The test for subgroup differences by indirect comparison was not significant for SBP (P = 0.31) but significant for DBP (P = 0.04). Given this inconsistency, the evidence remains inconclusive that atenolol exhibits a dose‐response effect.
2.1. Analysis.
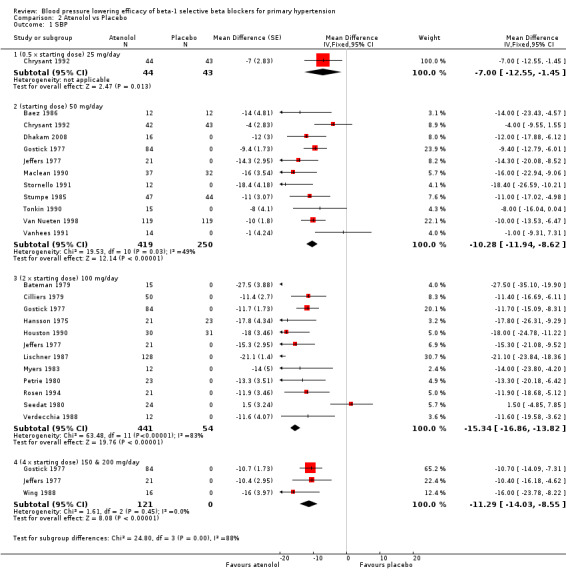
Comparison 2 Atenolol vs Placebo, Outcome 1 SBP.
2.2. Analysis.
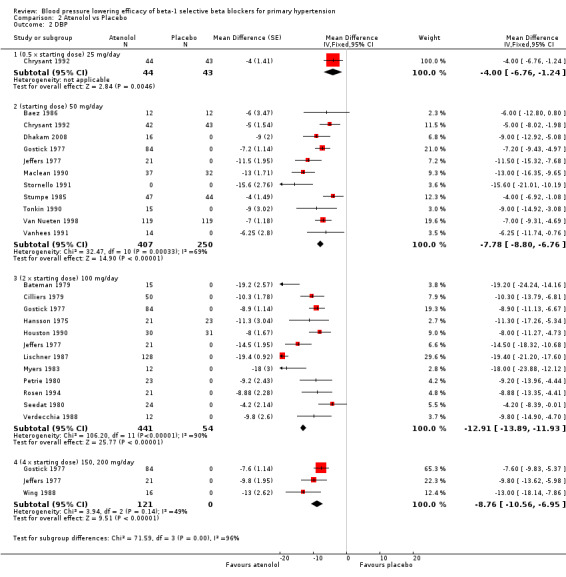
Comparison 2 Atenolol vs Placebo, Outcome 2 DBP.
2.3. Analysis.
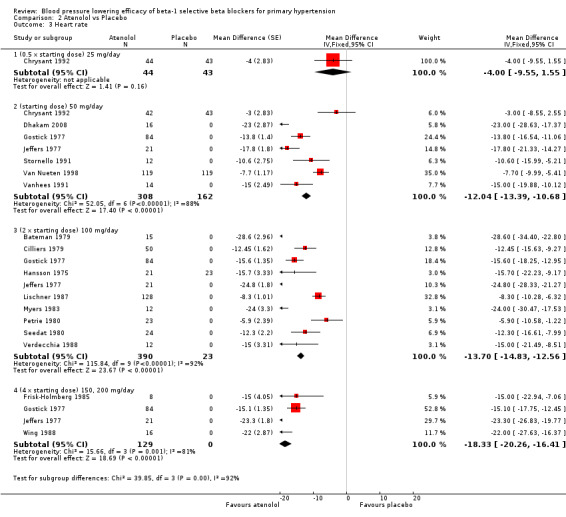
Comparison 2 Atenolol vs Placebo, Outcome 3 Heart rate.
2.4. Analysis.
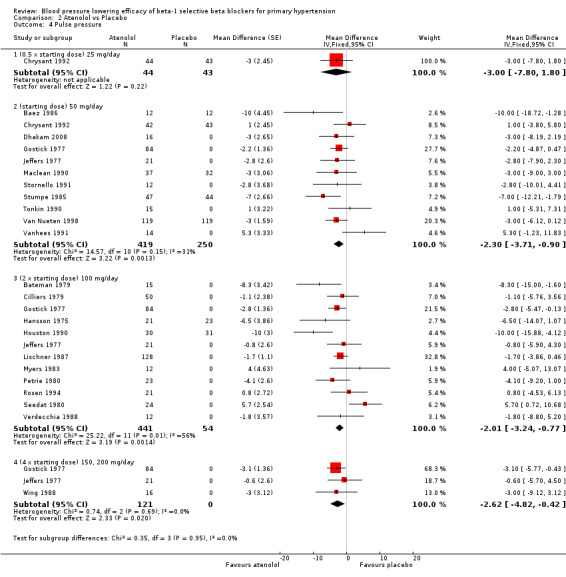
Comparison 2 Atenolol vs Placebo, Outcome 4 Pulse pressure.
We found significant heterogeneity in both 50 mg/day and 100 mg/day subgroups in SBP and DBP. We discuss the probable reasons for heterogeneity in the Discussion. These two subgroups contained the largest sample size. The estimate of blood pressure lowering efficacy combining the starting dose and twice the starting dose was ‐13/‐11 mmHg.
Atenolol 25 mg/day did not significantly lower heart rate. Starting from 50 mg/day and higher, atenolol significantly lowered heart rate compared to placebo. The test for subgroup differences in the recommended dose range by direct comparison was significant for heart rate (P = 0.008).
Atenolol 50 mg/day and higher significantly lowered pulse pressure compared to placebo. There were no differences between 50 mg/day, 100 mg/day and 200 mg/day by direct comparison for pulse pressure.
Blood pressure variability was not significantly different between the treatment and placebo groups for SBP (P = 0.3) and DBP (P = 0.13).
Blood pressure lowering efficacy of metoprolol
Metoprolol is indicated for the treatment of hypertension, angina and stable acute myocardial infarction (MI) in Canada and the USA. The recommended doses for hypertension are 100 mg to 200 mg daily in Canada and 100 mg to 450 mg daily in the USA (eCPS; FDA). Nine RCTs examining the blood pressure lowering efficacy of 25 mg to 400 mg per day metoprolol in 1004 hypertensive participants were included in this review. Four of the included studies were parallel studies and the other five were cross‐over studies. The mean baseline BP of the metoprolol studies was 154.4/100.3 mmHg.
Please refer to Analysis 3.1, Analysis 3.2, Analysis 3.3 and Analysis 3.4 for the metoprolol results. All metoprolol doses significantly lowered SBP and DBP compared to placebo. The maximum BP lowering effect was seen at 200 mg/day (twice the starting dose). The test for subgroup differences in the recommended dose range (100 mg/day to 400 mg/day) by direct comparison was not significant for both SBP (P = 0.12) and DBP (P = 0.12). Significant heterogeneity was present in the SBP 400 mg/day and DBP 200 mg/day subgroups. Since there was no definitive dose‐response within the recommended range, we pooled all the recommended doses, 100 mg, 200 mg/day and 400 mg/day, to estimate the blood pressure lowering effects. The estimate of blood pressure lowering effect of metoprolol was ‐9/‐8 mmHg.
3.1. Analysis.
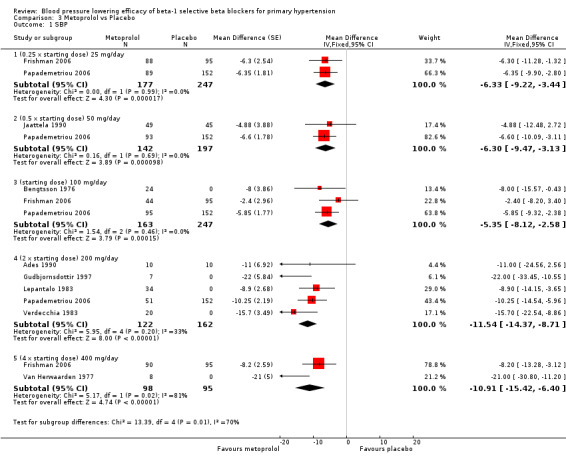
Comparison 3 Metoprolol vs Placebo, Outcome 1 SBP.
3.2. Analysis.
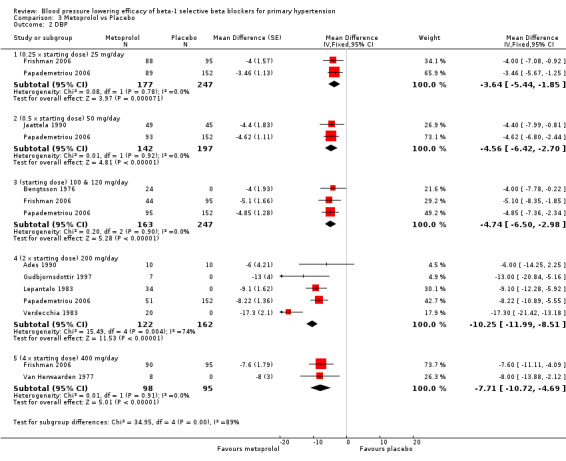
Comparison 3 Metoprolol vs Placebo, Outcome 2 DBP.
3.3. Analysis.
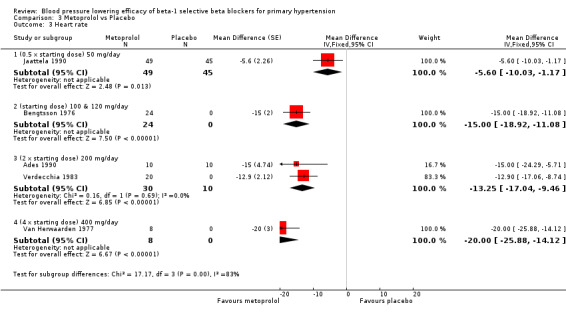
Comparison 3 Metoprolol vs Placebo, Outcome 3 Heart rate.
3.4. Analysis.
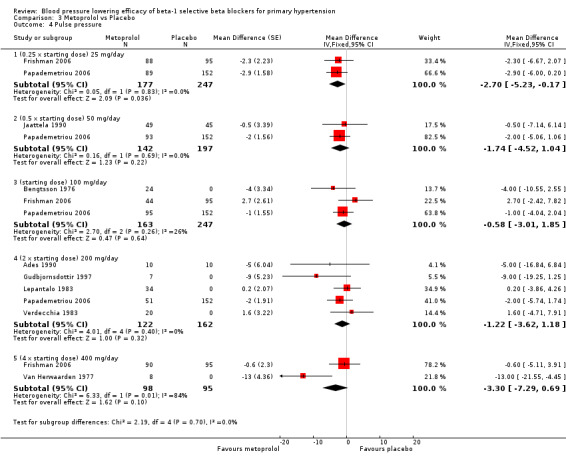
Comparison 3 Metoprolol vs Placebo, Outcome 4 Pulse pressure.
Only five RCTs reported heart rate, therefore the sample size for heart rate was fairly small. However, all metoprolol doses significantly lowered heart rate compared to placebo. The test for subgroup differences by indirect comparison was significant in heart rate (P = 0.0007).
Metoprolol did not significantly change pulse pressure.
Metoprolol did not significantly change blood pressure variability compared to placebo for SBP (P = 0.56) or DBP (P = 0.86).
Blood pressure lowering efficacy of betaxolol
Please refer to the Analysis 5.1 to Analysis 5.4 for the results for betaxolol. Betaxolol is indicated for treatment of hypertension in the USA (FDA). The recommended daily doses of betaxolol are 10 mg/day to 20 mg/day. Two RCTs examined the BP lowering efficacy of betaxolol at dosage of 5 mg/day to 20 mg/day in hypertensive 627 participants were included in this review. The duration of both studies was four weeks. Ameling 1991 was a cross‐over study and Williams 1992 was a parallel study. Both studies measured BP using a mercury sphygmomanometer. Williams 1992 reported that they measured BP 24 hours after the last dose (trough). Ameling 1991 did not report the time of the measurement. The mean baseline BP of the included studies was 158.9/102.8 mmHg.
5.1. Analysis.
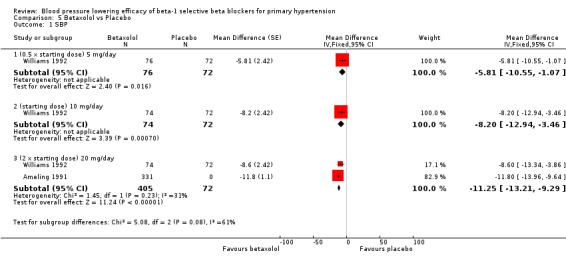
Comparison 5 Betaxolol vs Placebo, Outcome 1 SBP.
5.4. Analysis.
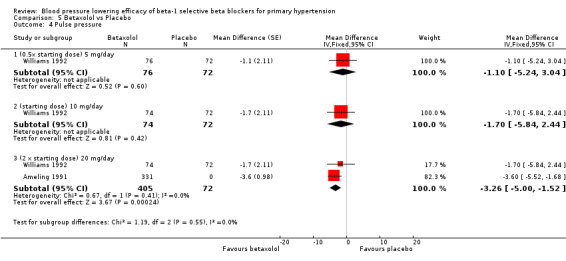
Comparison 5 Betaxolol vs Placebo, Outcome 4 Pulse pressure.
All doses of betaxolol significantly lowered SBP, DBP and heart rate. Williams 1992 provided data for direct comparison between the dose subgroup. There was no significant difference in BP lowering effect between the dose subgroups. Since there was no significant difference between the dosages, we pooled all three doses to estimate the mean effect. The estimated BP lowering effect of betaxolol is ‐11/‐8 mmHg.
Only 20 mg/day betaxolol also significantly lowered pulse pressure.
We were not able to perform analysis on BP variability because neither of the two included studies reported SD.
Blood pressure lowering efficacy of bisoprolol
Bisoprolol is indicated for the treatment of hypertension in Canada and the USA. The recommended doses for hypertension are 5 mg to 20 mg/day in Canada and the USA (eCPS; FDA). Seven RCTs examining the blood pressure lowering efficacy of 5 mg to 20 mg/day bisoprolol in 622 people with hypertension were included in this review. Three of the included studies were parallel design and the other four were cross‐over design. The mean baseline BP was 151.2/100.1 mmHg.
Please refer to Analysis 4.1, Analysis 4.2, Analysis 4.3 and Analysis 4.4 for the results of bisoprolol. All doses of bisoprolol significantly lowered SBP and DBP compared to placebo. There was significant heterogeneity for 5 mg/day (starting dose) subgroup for both outcomes. The test for subgroup differences in the recommended dose range by direct comparison was not significant in SBP (P = 0.76) and DBP (P = 0.32). Since there was no significant difference between the subgroups, we combined the three subgroups to obtain the estimate of BP lowering of bisoprolol, ‐10/‐8 mmHg.
4.1. Analysis.
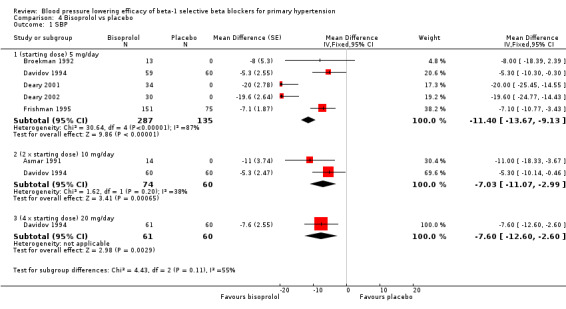
Comparison 4 Bisoprolol vs placebo, Outcome 1 SBP.
4.2. Analysis.
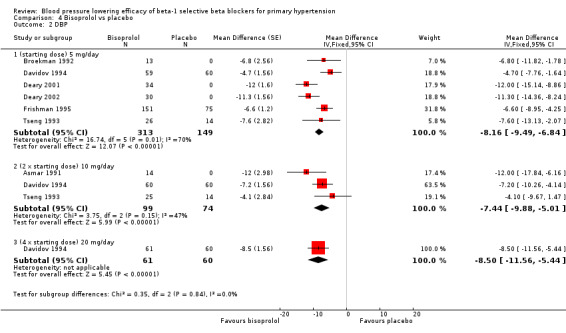
Comparison 4 Bisoprolol vs placebo, Outcome 2 DBP.
4.3. Analysis.
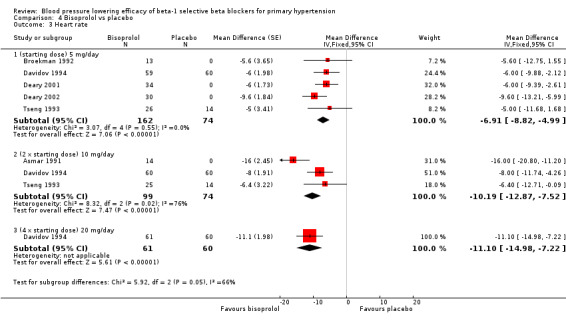
Comparison 4 Bisoprolol vs placebo, Outcome 3 Heart rate.
4.4. Analysis.
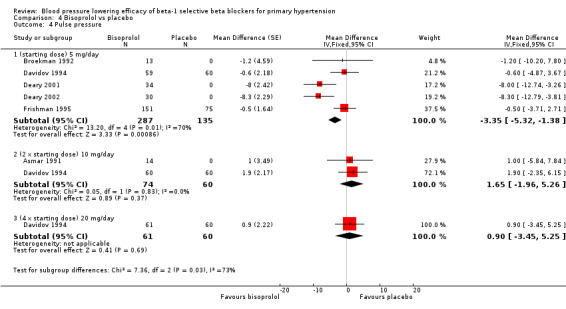
Comparison 4 Bisoprolol vs placebo, Outcome 4 Pulse pressure.
All doses of bisoprolol significantly lowered heart rate compared to placebo. The test for subgroup differences by direct comparison was not significant in heart rate (P = 0.12).
Only 5 mg/day (starting dose) bisoprolol significantly lowered pulse pressure. This effect was not seen in other subgroups.
There was no significant difference in blood pressure variability between treatment and placebo for SBP (P = 0.66) or DBP (P = 0.96).
Blood pressure lowering efficacy of bevantolol
Please refer to Analysis 6.1, Analysis 6.2, Analysis 6.3, and Analysis 6.4 for the results for bevantolol. Bevantolol is not available in Canada, the USA or the European Union (EU). We did not find the product monograph or recommended starting dose for bevantolol from these government agencies. We included one parallel study (Okawa 1986) examining the BP lowering efficacy of 100 mg/day to 400 mg/day bevantolol in 139 hypertensive patients for six weeks in this review.
6.1. Analysis.
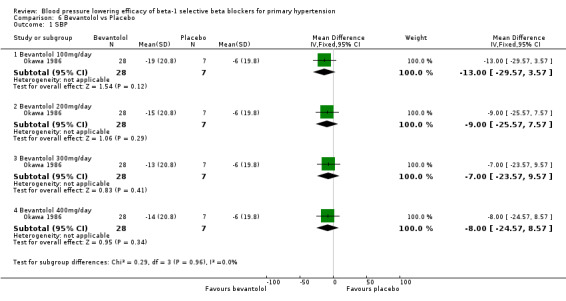
Comparison 6 Bevantolol vs Placebo, Outcome 1 SBP.
6.2. Analysis.
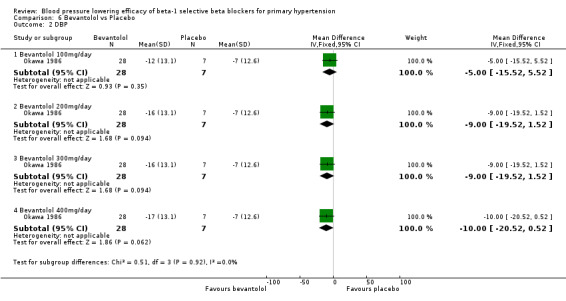
Comparison 6 Bevantolol vs Placebo, Outcome 2 DBP.
6.3. Analysis.
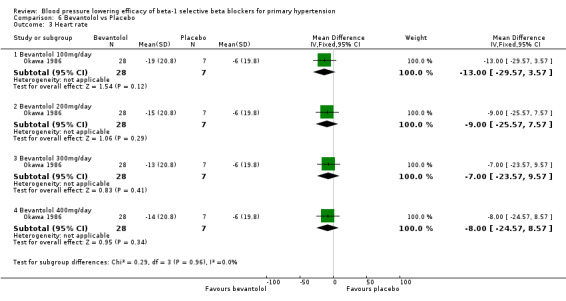
Comparison 6 Bevantolol vs Placebo, Outcome 3 Heart rate.
6.4. Analysis.
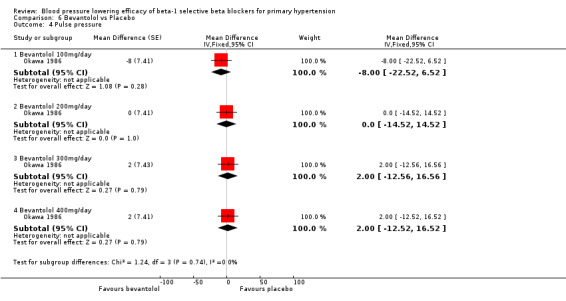
Comparison 6 Bevantolol vs Placebo, Outcome 4 Pulse pressure.
Bevantolol did not significantly lower SBP, DBP, heart rate or pulse pressure compared to placebo. Bevantolol did not significantly change BP variability. The end treatment SD for SBP was 20.8 for the bevantolol group and 19.8 for the placebo group. The end treatment SD for DBP was 13.1 for the bevantolol group and 12.6 for the placebo group.
Blood pressure lowering efficacy of pafenolol
Please refer to Analysis 7.1, Analysis 7.2, Analysis 7.3 and Analysis 7.4 for the results of pafenolol. Pafenolol is not available in Canada, the USA or the EU. We did not find the product monograph or recommended starting dose for pafenolol on the websites of these government agencies. Two RCTs examining the blood pressure lowering efficacy of 25 mg/day to 100 mg/day pafenolol in 161 hypertensive participants were included. Both studies were parallel studies with treatment periods of four weeks. The mean baseline BP was 161.1/109.6 mmHg.
7.1. Analysis.
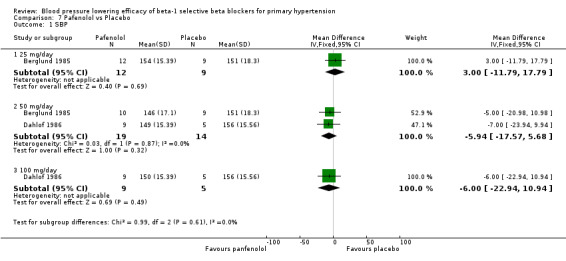
Comparison 7 Pafenolol vs Placebo, Outcome 1 SBP.
7.2. Analysis.
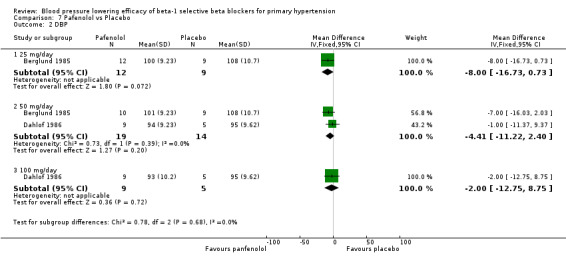
Comparison 7 Pafenolol vs Placebo, Outcome 2 DBP.
7.3. Analysis.
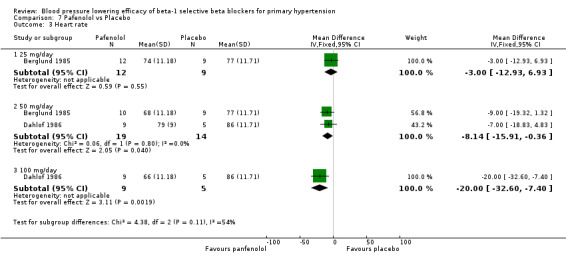
Comparison 7 Pafenolol vs Placebo, Outcome 3 Heart rate.
7.4. Analysis.
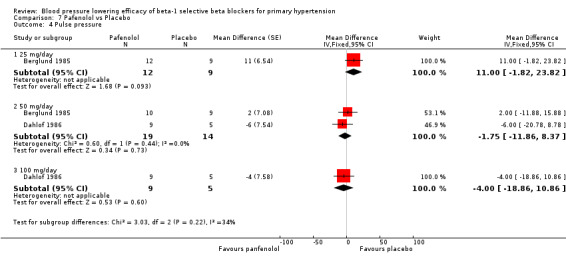
Comparison 7 Pafenolol vs Placebo, Outcome 4 Pulse pressure.
Pafenolol did not significantly lower SBP, DBP or pulse pressure. Pafenolol 50 mg/day and 100 mg/day significantly lowered heart rate.
Blood pressure lowering efficacy of practolol
Please refer to Analysis 8.1, Analysis 8.2, Analysis 8.3 and Analysis 8.4 for the results of practolol. Practolol is not available in Canada, the USA or the E.U. We did not find the product monograph or recommended starting dose for practolol on the websites of these government agencies. We included one cross‐over study examining the blood pressure lowering effect of 600 mg/day practolol in 24 hypertensive participants for four weeks in this review. The baseline BP was 182.9/123.3 mmHg.
8.1. Analysis.
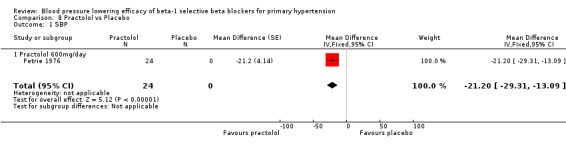
Comparison 8 Practolol vs Placebo, Outcome 1 SBP.
8.2. Analysis.
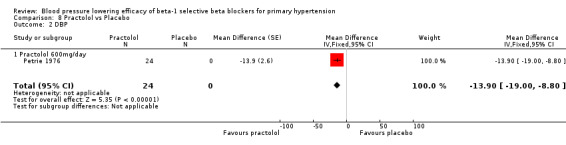
Comparison 8 Practolol vs Placebo, Outcome 2 DBP.
8.3. Analysis.
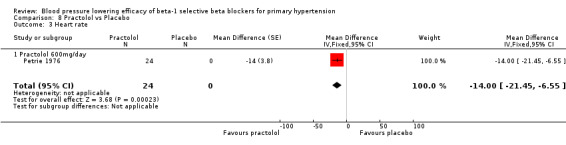
Comparison 8 Practolol vs Placebo, Outcome 3 Heart rate.
8.4. Analysis.
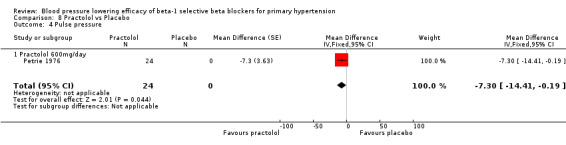
Comparison 8 Practolol vs Placebo, Outcome 4 Pulse pressure.
Practolol 600 mg/day significantly lowered SBP, DBP, heart rate and pulse pressure compared to placebo.
Pooled haemodynamic effect of beta‐1 selective blockers
We pooled the data for all available beta‐1 selective blockers, based on the recommended starting doses. This allowed us to estimate the blood pressure lowering effect of beta‐1 selective blockers as a whole subclass, as well as to compare it to other classes of antihypertensive drugs. Please refer to Analysis 9.1, Analysis 9.2, Analysis 9.3 and Analysis 9.4 for the results of beta‐1 blockers.
9.1. Analysis.
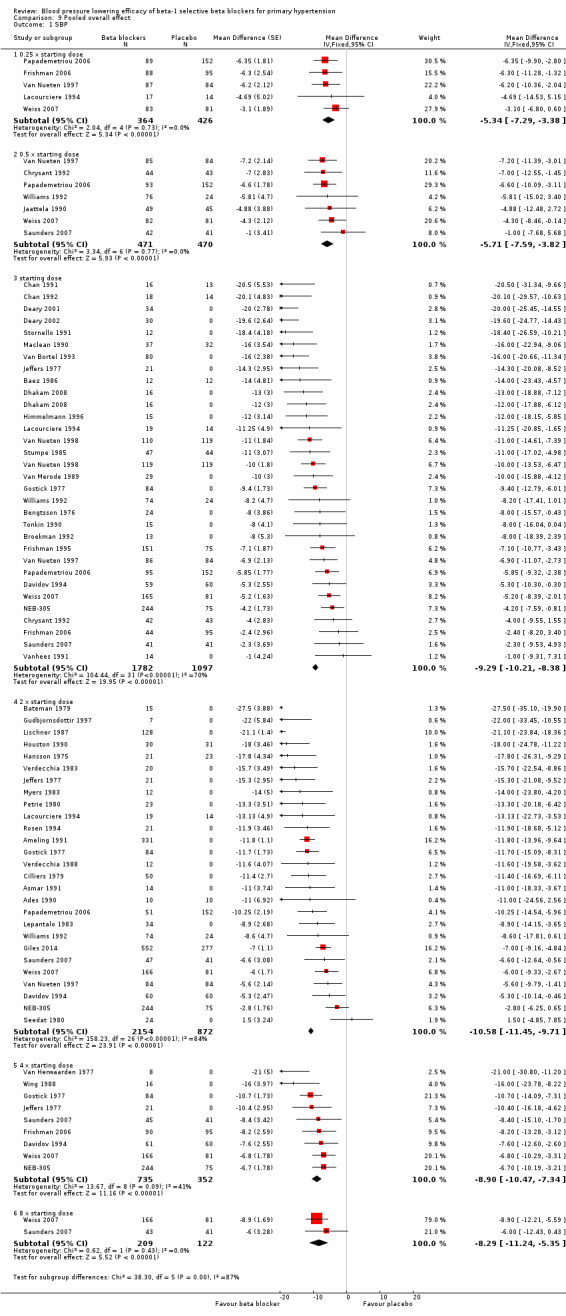
Comparison 9 Pooled overall effect, Outcome 1 SBP.
9.2. Analysis.
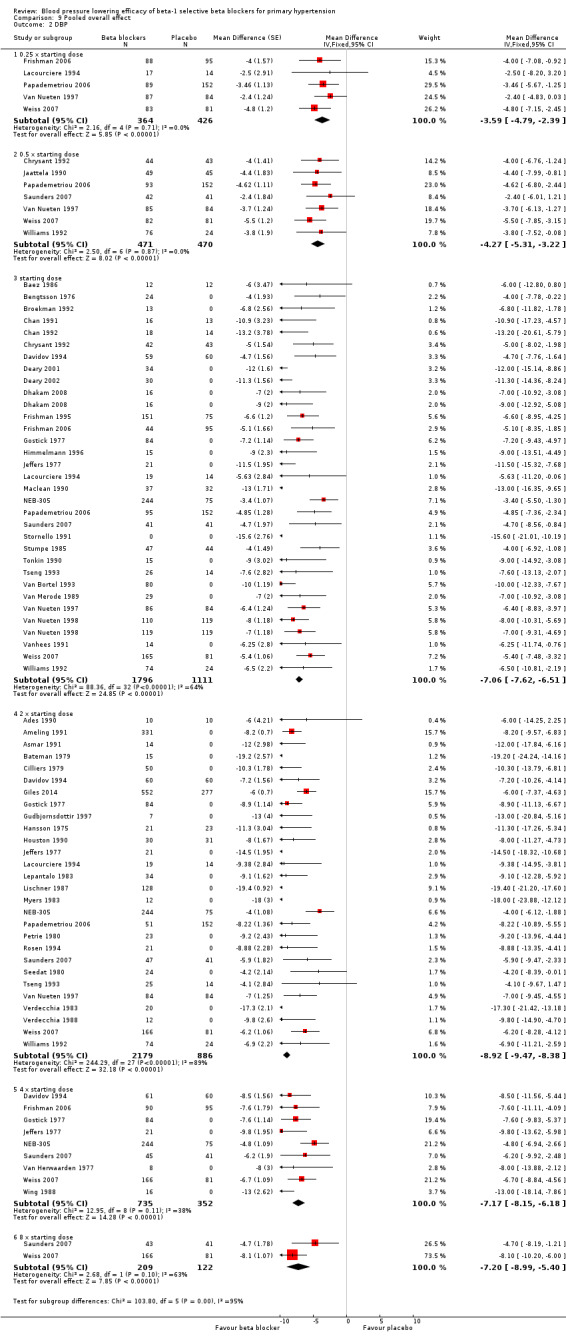
Comparison 9 Pooled overall effect, Outcome 2 DBP.
9.3. Analysis.
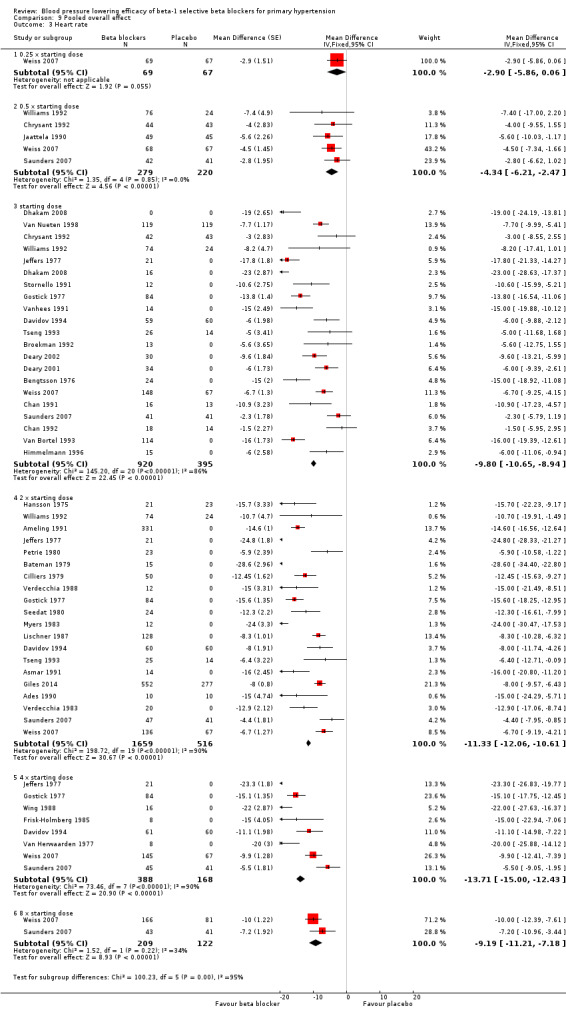
Comparison 9 Pooled overall effect, Outcome 3 Heart rate.
9.4. Analysis.
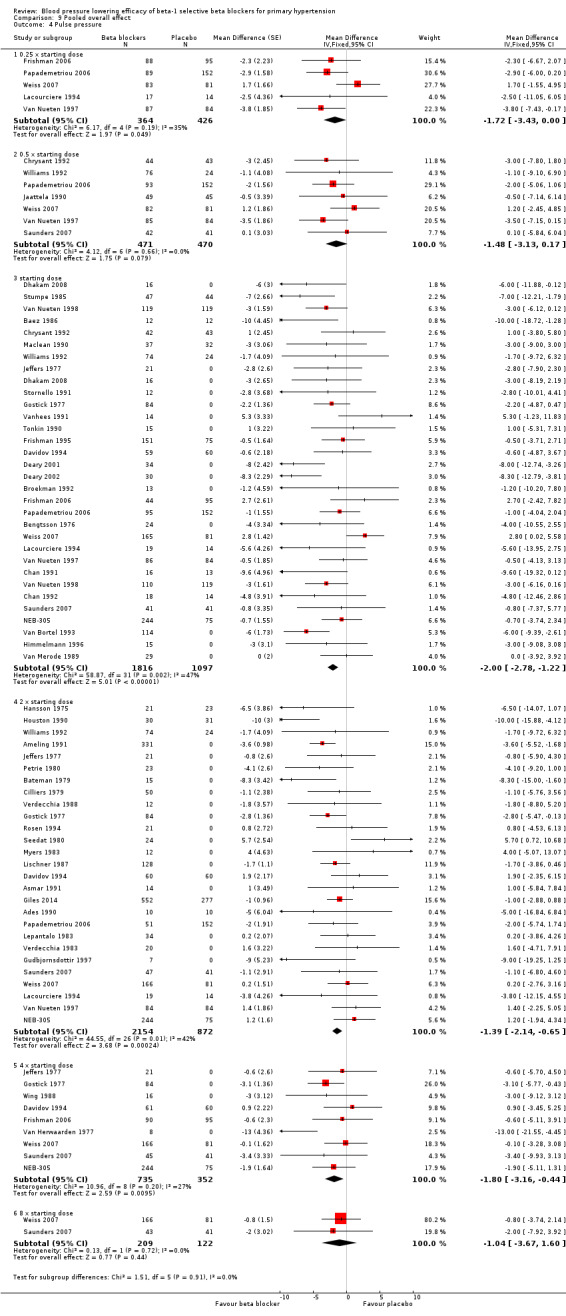
Comparison 9 Pooled overall effect, Outcome 4 Pulse pressure.
All pooled beta‐1 blocker doses significantly lowered SBP and DBP compared to placebo. The starting dose and twice the starting dose subgroups each contained over 2000 participants, which provided good estimates to represent this subclass of beta blockers. Heterogeneity was significant in these two subgroups. The source of heterogeneity is explored in the Discussion. The test for subgroup differences in the starting dose, twice the starting dose, four times the starting dose and eight times the starting dose subgroups by direct comparison was not significant for SBP (P = 0.23) and DBP (P = 0.11). The BP lowering estimate (SBP/DBP) combining the starting dose and twice the starting dose subgroups was ‐10/‐8 mmHg.
All doses of beta‐1 blockers significantly lowered heart rate. Beta‐1 selective blockers significantly lowered pulse pressure at the starting dose, twice the starting dose and four times the starting dose compared to placebo. The test for subgroup differences by direct comparison in pulse pressure was not significant.
Blood pressure variability
We tested the overall effect of beta‐1 blockers on BP variability using an unpaired t‐test. We extracted end treatment SD from parallel studies for the beta‐1 blocker group and the placebo group. Beta‐1 blockers did not significantly change BP variability in SBP (P = 0.83) or DBP (P = 0.52). The overall mean of end treatment SD for beta‐1 blockers (SBP/DBP) was 14.5/8.6 and placebo group was 14.9/8.5.
Withdrawal due to adverse effects
We pooled the data for withdrawal due to adverse effects (Analysis 9.5). Out of the 56 RCTs, only three RCTs reported withdrawal due to adverse effects data that could be used for the analysis. In these three RCTs, there was no significant difference in withdrawal due to adverse effects between treatment and placebo (RR 0.85 [0.50, 1.45]).
9.5. Analysis.
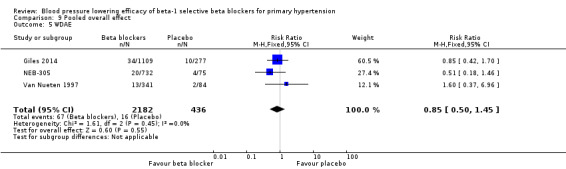
Comparison 9 Pooled overall effect, Outcome 5 WDAE.
Discussion
Summary of main results
Nebivolol
The maximum blood pressure lowering effect of nebivolol occurred at 5 mg/day. Doses higher than 5 mg/day did not provide additional BP lowering effects. The data from 5 mg/day, 10 mg/day and 20 mg/day (starting dose, twice the starting dose and four times the starting dose) were pooled to estimate the average BP lowering effect of nebivolol, which was ‐8/‐6 mmHg. The funnel plots for nebivolol showed a paucity of small negative studies (Figure 3; Figure 4). This suggested that small negative studies were not published. The estimate above was likely an over estimation of the true effect. The funnel plots also showed several outliers on the left hand side of the graph. These positive outliers would exaggerate the effect.
3.
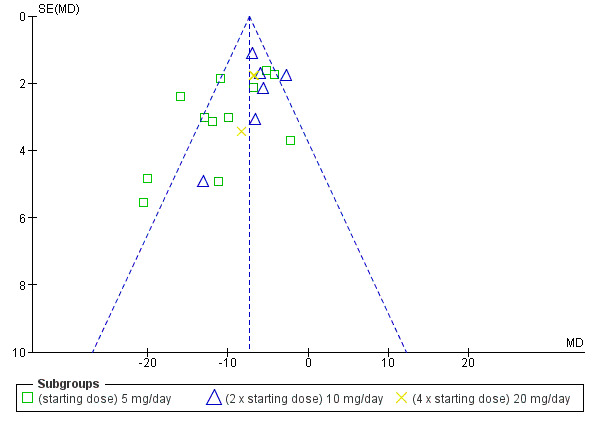
Funnel plot of nebivolol 5 mg to 20 mg (SBP)
4.
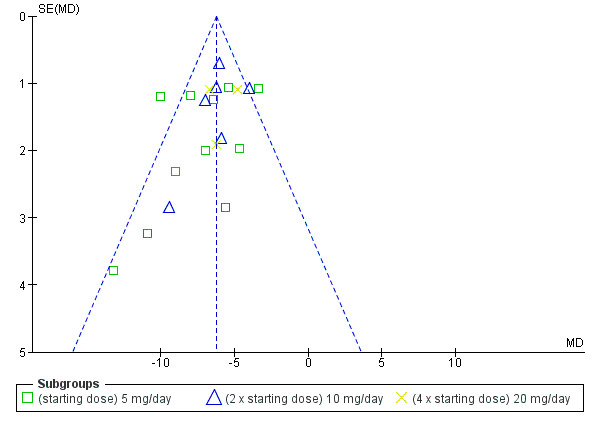
Funnel plot of nebivolol 5 mg to 20 mg (DBP)
Nebivolol lowered systolic and diastolic BP to a similar degree, and therefore it had only a small effect on pulse pressure. Heterogeneity in the 5 mg/day subgroup suggested that the statistically significant effect on pulse pressure could be caused by variation, as no other dose of nebivolol showed a similar effect.
Peak to trough difference
Only four nebivolol studies reported both peak and trough measurements. If there was a greater effect at peak (seen with some diastolic BP measurements) the effect was small (2 mmHg).
Atenolol
Both the recommended starting dose (50 mg daily) subgroup and twice the starting dose (100 mg daily) subgroup contained a large number of subjects and provided a good estimate of the blood pressure lowering efficacy of atenolol. Funnel plots were used to identify extreme outliers and assessment of bias. The funnel plots of the starting dose did not show any extreme outliers or provide evidence of asymmetry which would suggest publication bias.
In the twice the starting dose subgroup, the funnel plot identified Lischner 1987 as an extreme outlier for both SBP and DBP. Another RCT (Ravid 1985) from the same laboratory was also identified as an extreme outlier in the Cochrane review on non‐selective beta blockers (Wong 2014a). These data are therefore of questionable validity. This represented a common problem in performing the systematic review and meta‐analysis, as such studies lead to an overestimation of the magnitude of blood pressure lowering effect. If we removed the extreme outlier, the estimate of twice the starting dose decreased from ‐15/‐13 mmHg to ‐12/‐10 mmHg. Removing it would also decrease the overall estimate of the pooled atenolol starting dose and twice the starting dose subgroups from ‐13/‐11 mmHg to ‐11/‐9 mmHg, which was closer to the overall estimates of the class.
Atenolol was the only beta‐1 blocker that significantly lowered pulse pressure across several doses. The combined estimate of the pulse pressure effect of starting dose, twice and four times the starting dose atenolol was small (‐2 mmHg [1‐3]). It did not show a dose‐response effect and is unlikely to be clinically significant.
Metoprolol
Most of the data for metoprolol came from two studies which tested the dose‐response effect of the drug at several doses (Frishman 2006, Papademetriou 2006). These two RCTs showed a greater BP lowering in the recommended dose range compared to lower doses (25 mg/day and 50 mg/day). However, the different doses within the recommended dose range did not show a significant dose response effect. The mean BP lowering effect of the 100 mg/day, 200 mg/day and 400 mg/day (starting dose, twice and four times the starting dose) was ‐9/‐8 mmHg.
Bisoprolol
No additional BP lowering effect was seen for doses higher than the recommended starting dose of bisoprolol. The mean BP lowering effect of the 5 mg/day, 10 mg/day and 20 mg/day (starting dose, twice the starting dose and four times the starting dose) was ‐11/‐8 mmHg. This was likely to be exaggerated because of the presence of two extreme positive outliers (Deary 2001, Deary 2002) in the data.
Betaxolol, bevantolol, pafenolol and practolol
The sample sizes of these four beta‐1 blockers were small. They contributed little weight to the overall pooled estimates. Their estimates are reported in the Main results.
Overall pooled blood pressure lowering effect of beta‐1 blockers
The sample size for the beta‐1 selective blockers was the largest of the four subclasses of beta blockers. The pooled data included 6313 participants from 51 RCTs and multiple dosages. The data set provided the best opportunity to explore whether there was a graded dose‐response effect. The findings showed a similar and smaller BP lowering effect at a quarter of the starting dose and half the starting dose, but then a flat and similar BP lowering for the starting dose, twice, four times and eight times the starting dose (see Analysis 9.1; Analysis 9.2.). Twice the starting dose subgroups had the most data and exhibited considerable heterogeneity, which we explore below. The lack of a dose‐response effect above twice the starting dose suggests that higher doses of beta‐1 blockers were not more effective in lowering BP.
In Analysis 9.10 and Analysis 9.11, we demonstrated that the primary source of heterogeneity in the twice the starting dose subgroup was the difference in BP lowering effect between the individual beta‐1 blockers. Atenolol showed the largest effect size among the five beta‐1 blockers. In the discussion for atenolol, we explained that the estimate of the twice the starting dose subgroup of atenolol could be exaggerated due to an extreme outlier. The effect size of this atenolol subgroup would change from ‐15/‐13 mmHg to ‐12/‐10 mmHg if we removed the extreme outlier from the analysis. We also considered the fact that mean baseline BP of atenolol studies (162/104 mmHg) was higher than the other beta‐1 blockers. In addition, most studies for atenolol, metoprolol and betaxolol measured BP at peak hours. Peak measurements would also contribute to a greater effect size.
9.10. Analysis.
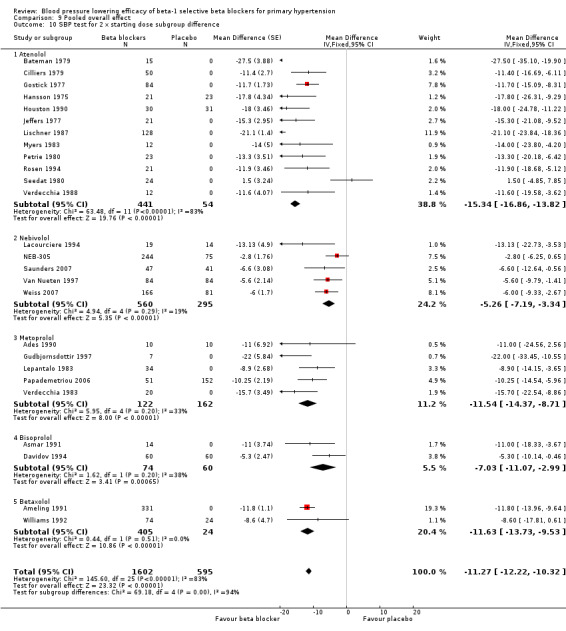
Comparison 9 Pooled overall effect, Outcome 10 SBP test for 2 x starting dose subgroup difference.
9.11. Analysis.
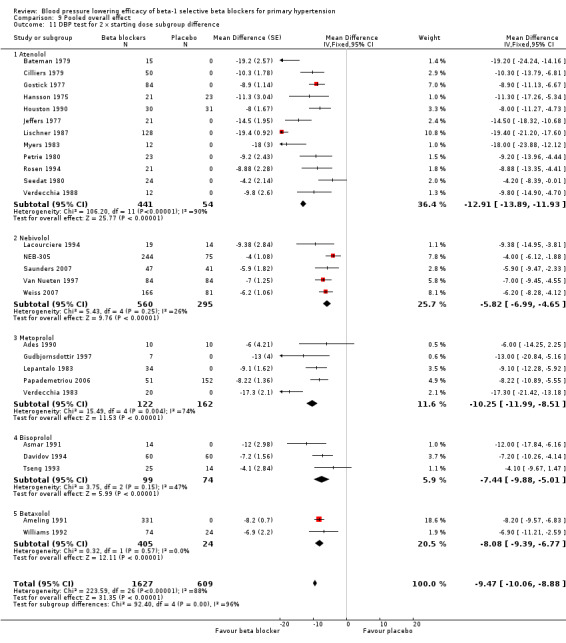
Comparison 9 Pooled overall effect, Outcome 11 DBP test for 2 x starting dose subgroup difference.
Difference in pharmacodynamic properties could also have contributed to the difference in BP lowering efficacy. Heart rate reduction during exercise was used for many beta‐1 blockers to test the potency of beta‐1 blockade. Bisoprolol 10 mg (at twice the starting dose) had equivalent exercise heart rate percentage change from baseline compared to 50 mg atenolol (starting dose) and 100 mg metoprolol (starting dose) (Lancaster 1988). Five mg nebivolol (starting dose) had an equivalent effect compared to 100 mg atenolol (twice the starting dose) (Veverka 2006). If beta‐1 blockade was the dominant mechanism by which blood pressure was lowered by beta blockers, nebivolol should have lowered BP the most and bisoprolol the least. As this did not fit with the data, potency of beta‐1 blocking ability did not explain the difference in blood pressure lowering effect.
Beta‐1 selectivity also might explain the differences. Both nebivolol and bisoprolol are highly beta‐1 selective. The beta‐1/beta‐2 selectivity ratios were 321 fold for nebivolol and 100 fold for bisoprolol (Lancaster 1988; Veverka 2006). The selectivity ratios for atenolol, metoprolol and betaxolol were much less, at 35 fold, 40 fold and 20 fold respectively (Lancaster 1988). In this case, it appeared that beta blockers with less beta‐1 selectivity lower BP by a greater magnitude.
The mechanism by which beta blockers lower blood pressure is the most likely explanation for differences in BP lowering effect. However, studying the pharmacodynamic properties of beta blockers is notoriously difficult. The methods to test pharmacodynamic properties vary between research groups. The outcomes are often not comparable to each other (Fitzgerald 1991). It is thus difficult to fully explain the observed differences in BP lowering effect between different beta‐1 blockers.
The starting dose and twice the starting dose subgroups contained the largest sample size. The best estimate of BP lowering efficacy for beta‐1 blockers was determined by combining the results from all the starting dose and twice the starting doses. It was ‐10/‐8 mmHg.
Pulse pressure
Atenolol was the only beta‐1 blocker that consistently lowered pulse pressure at different doses. This effect was also seen in the pooled analysis. Since no other beta‐1 blocker exhibited a similar effect on pulse pressure it is more likely that the atenolol data are incorrect.
Overall completeness and applicability of evidence
This review provides the most up‐to‐date assessment of the blood pressure lowering efficacy of beta‐1 selective blockers. All the studies included had the same primary objective which was to compare the blood pressure effect of beta‐1 blockers and placebo. This review contains a fairly large sample size for the continuous outcomes that we examined.
Quality of the evidence
Table 1 summarises the important findings based on combining the data from the starting dose and twice the starting dose of beta‐1 selective blockers, and incorporates the quality of evidence in this review.
We have included 56 RCTs examining the BP lowering efficacy of eight beta‐1 blockers in 7812 people with hypertension in this review. The large sample size provided adequate power to draw robust conclusions. The risk of detection bias was of concern in beta blocker studies. Most of the included studies did not use automated machines when measuring BP, which would be the best way to mitigate the risk of detection bias. Therefore, the risk of detection bias by loss of blinding was high. Nebivolol and atenolol contributed a large portion of data to our pooled analyses. Both of the beta‐1 blockers showed high risk of publication bias either due to skewed funnel plots or the presence of extreme outliers.
Pulse pressure data was seldom reported in the trials. We calculated pulse pressure by subtracting the reduction in DBP from the reduction in SBP. Indirectness of this outcome was the reason the evidence was further downgraded for pulse pressure. For these reasons, the quality of evidence was judged to be low to very low in the 'Summary of findings' table.
Potential biases in the review process
The rigid methodology and comprehensive search strategy minimised the potential for bias in selection of included studies. The search of citations in the three databases was performed by the Cochrane Hypertension Information Specialist. Once we had the set of citations, we sorted through them following the inclusion criteria. This process minimised any grey areas that may have introduced bias.
On the occasions that data had to be obtained from figures, two reviewers extracted data independently and then the average of the two values was used. This minimised the potential of human error during the data extraction process. We are confident that the potential for bias during the review process was low.
Agreements and disagreements with other studies or reviews
Two published reviews (Chen 2010; Law 2005) reported the blood pressure lowering efficacy of beta blockers. Chen 2010 examined the additional effect of beta blockers in combination with other antihypertensive drugs. Law 2005 examined the BP lowering effect of antihypertensive drugs, including beta‐blockers, as monotherapy. Both reviews pooled all the subclasses of beta blockers and doses together, assuming that they had similar effects. We were able to show that beta‐1 selective blockers could have significantly different BP lowering effects from the other sub‐classes of beta blockers. Our review is unique in the sense that we did not assume that all beta blockers lowered blood pressure similarly.
When compared to the other subclasses, beta‐1 selective blockers appeared to lower BP more than dual receptor blockers (Wong 2015) and partial agonists (Wong 2014b). Nonselective beta blockers and beta‐1 selective blockers appeared to lower blood pressure similarly (Wong 2014a), however most trials included in the nonselective beta blocker review measured BP at peak effect. These are indirect comparisons, but represent fairly strong evidence that the BP lowering efficacy of different subclasses of beta blockers are not the same. Direct head‐to‐head RCTs would be the best way to determine whether the differences between the subclasses is real.
There are a number of reasons why the pooled estimate of BP lowering for beta‐1 selective beta blockers (‐10/8 mmHg) is greater than the real effect. One of these reasons is that about half of the trials measured the effect at peak rather than trough. The estimate of BP lowering at trough (‐8/‐7 mmHg) is less than the overall pooled estimate and is the number that is best compared to other drug classes.
Other Cochrane reviews have compared antihypertensive drug classes with placebo and used similar inclusion/exclusion criteria to this review. Based on indirect comparison, the pooled trough SBP lowering effect of beta‐1 selective blockers (‐8 mmHg) is similar to the average SBP reduction for thiazide diuretics (‐9 mmHg) (Musini 2014), angiotensin converting enzyme (ACE) inhibitors (‐8 mmHg) (Heran 2008a), angiotension receptor blockers (ARBs) (‐9 mmHg) (Heran 2008b) and renin inhibitors (‐8 mmHg) (Musini 2008). In contrast the trough DBP lowering of beta‐1 selective blockers (‐7 mmHg) is greater than that for thiazide diuretics (‐4 mmHg) (Musini 2014), ACE inhibitors (‐5 mmHg) (Heran 2008a), ARBs (‐5 mmHg) (Heran 2008b) and renin inhibitors (‐5 mmHg) (Musini 2008). This provides fairly strong evidence that the BP lowering effect of different classes of drugs is not the same as has been assumed by others (Law 2005).
The property of beta‐blockers to lower DBP to a similar extent to SBP explains the fact that beta‐1 selective blockers have no effect on pulse pressure. This finding is similar to the other beta blocker subclasses and is substantially less than the average reduction of pulse pressure seen with thiazides (‐5 mmHg) (Musini 2014) and drugs that inhibit renin angiotensin system (‐3 mmHg) (Heran 2008a; Heran 2008b; Musini 2008). This finding is important and might be the explanation as to why first‐line beta blockers (Wiysonge 2012) do not reduce mortality and morbidity as much as first‐line thiazides; Wright 2000; Wright 2009), first‐line calcium channel blockers (Chen 2010b), and first‐line drugs inhibiting the renin angiotensin system (Xue 2015).
Authors' conclusions
Implications for practice.
This review provides low quality evidence that in people with mild to moderate hypertension, beta‐1 selective blockers lowered BP by an average of ‐10/‐8 mmHg and reduced heart rate by 11 beats per minute as compared to placebo. The effect of beta‐1 blockers at peak hours, ‐12/‐9 mmHg, was greater than the reduction at trough hours, ‐8/‐7 mmHg. Beta‐1 blockers lowered BP maximally at twice the recommended starting doses. Individual beta‐1 blockers did not exhibit a significant graded dose‐response effect on SBP and DBP over the recommended dose range. This suggests that higher doses of beta‐1 blockers might not provide additional BP lowering effects, however might cause more side effects.
A graded dose‐response of beta‐1 blockers on heart rate was evident. Higher dose beta‐1 blockers lowered heart rate to a greater amount when compared to lower doses. Beta‐1 selective beta blockers did not reduce pulse pressure with the exception of atenolol, which reduced it by 2 mmHg.
Implications for research.
Better reporting of method of randomisation and allocation concealment in future RCTs is required.
Beta blocker studies should use automated machines to measure blood pressure in order to minimise detection bias.
All RCTs measuring blood pressure lowering effects must be published.
Future blood pressure lowering trials must report: resting blood pressure and heart rate measured in a standard sitting position; the time after administration of the drug that the BP was measured; the standard deviation or standard error of mean of all measurements; and withdrawals due to adverse effects in the treatment and placebo arms.
Future systematic reviews should focus on comparing the BP lowering effects of different beta blockers and sub‐classes of beta blockers in head‐to‐head trials using automated BP measurements.
Feedback
Comment, 17 March 2016
Summary
Thank you for an immense amount of painstaking work on this. I was also pleasantly surprised to see that access to the data was so easy. I had not realised this was possible, and will take it up with our ME to see how to do this with our own reviews. I was surprised to see that despite over 5,000 participants in many studies for SPB, for instance, your GRADE assessment was lo, indicating a huge degree of uncertainty. After all, this indicates that: "Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate." So despite dozens of trials and thousands of participants, we don't know if these drugs drop BP. Does that seem likely? To my mind it seemed unlikely. I did wonder at the small size of some of the studies (lowest 8 participants). Half the studies with a treatment size below 50 could be lost, and still retain 90% of participants. The Drop in BP is still 8.3 (7.7 to 8.9) with 4659 participants. So small size isn't an issue, or not a big one, in this case in terms of overestimating treatment effect. But you do give a lot of weight to asymmetric funnel plots and ascribe this to possible publication bias. Now a systematic review of methods to detect publication bias (J Clin Epidemiol. 2000 Feb;53(2):207‐16.) and didn't really indicate that we can rely on these tests to positively detect publication bias. And to overcome results from 5,000 participants with a mean drop in BP of over 9 mm in your analysis would require a massive amount of unpublished null or negative data ‐ quite beyond the realms of possibility. It would be a massive scandal. I would suggest that your use of GRADE is possibly wrong. For the most part the studies, and especially the larger ones, looked relatively unbiased, or at least without flags for high risk of bias. The problem with GRADE is that it asks us to downgrade studies for all sorts of reasons, some of which may even have some evidence of a small effect on the estimate of efficacy, but many of which do not. Other major issues (small size being one) are ignored. Anyway, it would be helpful to this reader to know whether or not you really think there is a chance that the possible bias is so great as to suggest that these drugs don't work.
Reply
Thank you for your comment. We agree with you that this review does prove that beta‐1 selective beta blockers do lower BP. We down GRADED the evidence to low because we think that the current overall effect estimate (‐10/‐8 mmHg) is likely to be exaggerated. As explained in the discussion the pooled effect of trials measuring the BP at trough was ‐8/‐7 mmHg and in a separate analysis the pooled effect of the parallel trials, which are larger and of better quality, was ‐7/‐6 mmHg [1]. We think that these estimates are closer to the true BP lowering effect of beta‐1 selective blockers. We recognize the limitation of the GRADE assessment tool. However, we believe that our downgrade to low is reasonable given the limitations of the tool. Both SBP and DBP analyses contain multiple outliers that exaggerate the effect estimate, and there is an inherent risk of loss of blinding in all beta‐blocker trials due to the effect on heart rate. We also have reason to believe that trials in which the BP lowering effect was absent or of small magnitude are unlikely to be published leading to a high risk of publication bias. We have discussed this to a greater extent in the non‐selective beta blocker review [2]. References: 1. Wong GW, Wright JM. Are the estimates of blood pressure (BP) lowering effect the same in parallel and cross‐over trials [abstract]? In: the 23rd Cochrane Colloquium; 2015 Oct 3‐7; Vienna, Austria. John Wiley & Sons, Ltd.; 2015. Abstract LRO 5.4.
2. Wong GWK, Wright JM. Blood pressure lowering efficacy of nonselective beta‐blockers for primary hypertension. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No.: CD007452. DOI: 10.1002/14651858.CD007452.pub2.
Contributors
Name: Andrew Moore Email Address: andrew.moore@ndcn.ox.ac.uk Affiliation: University of Oxford, UK
What's new
| Date | Event | Description |
|---|---|---|
| 23 April 2016 | Feedback has been incorporated | New feedback and authors' reply incorporated |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 3, 2016
| Date | Event | Description |
|---|---|---|
| 18 June 2010 | Amended | Protocol was amended: added a second author, clarified inclusion criteria to accept cross‐over studies and double‐blinded studies. Updated background section. Removed acebutolol from the beta‐1 selective list as it belongs to the partial agonist group. |
Acknowledgements
The authors would like to acknowledge the help provided by the Cochrane Hypertension Group. We would like to thank Stephen Adams for retrieving the articles.
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 15 October 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp adrenergic beta‐antagonists/ (79534) 2 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. (90056) 3 acebutolol.mp. (1071) 4 exp alprenolol/ (2182) 5 alprenolol.mp. (1560) 6 amosulalol.mp. (45) 7 arotinolol.mp. (83) 8 atenolol.mp. (7428) 9 befunolol.mp. (113) 10 betaxolol.mp. (944) 11 bevantolol.mp. (81) 12 exp bisoprolol/ (889) 13 bisoprolol.mp. (1271) 14 bopindolol.mp. (136) 15 bucindolol.mp. (173) 16 bucumolol.mp. (6) 17 bufetolol.mp. (12) 18 bufuralol.mp. (396) 19 bunitrolol.mp. (101) 20 exp bupranolol/ (196) 21 bupranolol.mp. (328) 22 butofilolol.mp. (10) 23 carazolol.mp. (145) 24 exp carteolol/ (319) 25 carteolol.mp. (428) 26 carvedilol.mp. (2755) 27 exp celiprolol/ (388) 28 celiprolol.mp. (492) 29 cetamolol.mp. (15) 30 cloranolol.mp. (2) 31 cyanopindolol.mp. (635) 32 deacetylmetipranolol.mp. (5) 33 dihydroalprenolol.mp. (1687) 34 dilevalol.mp. (131) 35 epanolol.mp. (58) 36 esmolol.mp. (1098) 37 indenolol.mp. (50) 38 iodocyanopindolol.mp. (1048) 39 exp labetalol/ (1748) 40 labetalol.mp. (2229) 41 landiolol.mp. (236) 42 exp levobunolol/ (228) 43 levobunolol.mp. (277) 44 mepindolol.mp. (86) 45 exp metoprolol/ (5024) 46 metoprolol.mp. (6976) 47 exp metipranolol/ (262) 48 metipranolol.mp. (315) 49 moprolol.mp. (17) 50 exp nadolol/ (783) 51 nadolol.mp. (1207) 52 nadoxolol.mp. (8) 53 nebivolol.mp. (761) 54 nifenalol.mp. (13) 55 nipradilol.mp. (162) 56 oxprenolol.mp. (1340) 57 exp penbutolol/ (176) 58 penbutolol.mp. (259) 59 exp pindolol/ (3715) 60 pindolol.mp. (4618) 61 exp practolol/ (1546) 62 practolol.mp. (2157) 63 pronethalol.mp. (211) 64 exp propranolol/ (31625) 65 propranolol.mp. (42477) 66 proxodolol.mp. (23) 67 exp sotalol/ (1975) 68 sotalol.mp. (2930) 69 sulfinalol.mp. (5) 70 talinolol.mp. (240) 71 tertatolol.mp. (175) 72 tilisolol.mp. (28) 73 exp timolol/ (3463) 74 timolol.mp. (4378) 75 toliprolol.mp. (16) 76 xibenolol.mp. (4) 77 or/1‐76 (145421) 78 hypertension/ (202792) 79 hypertens$.tw. (322888) 80 exp blood pressure/ (258033) 81 (blood pressure or bloodpressure).mp. (368499) 82 or/78‐81 (620920) 83 randomized controlled trial.pt. (413758) 84 controlled clinical trial.pt. (91901) 85 randomized.ab. (305710) 86 placebo.ab. (158376) 87 clinical trials as topic/ (179345) 88 randomly.ab. (216422) 89 trial.ti. (135204) 90 or/83‐89 (941528) 91 animals/ not (humans/ and animals/) (4036142) 92 90 not 91 (863690) 93 77 and 82 and 92 (8822) 94 93 and (2014$ or 2015$).ed. (236) 95 remove duplicates from 94 (213) *************************** Database: Cochrane Central Register of Controlled Trials <Issue 10, 2015> via Cochrane Register of Studies Online Search date: 15 October 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR Adrenergic beta‐Antagonists EXPLODE ALL TREES 9197 #2 (acebutolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevanolol or bisoprolol or bopindolol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bupranolol or butofilolol or carazolol or carteolol or carvedilol or celiprolol or cetamolol or cloranolol or cyanopindolol or deacetylmetipranolol or dihydroalprenolol or dilevalol or epanolol or esmolol) 5792 #3 (indenolol or iodocyanopindolol or labetalol or levobunolol or mepindolol or metipranolol or metoprolol or nadolol or nadoxolol or nebivolol or nifenalol or nipradilol or oxprenolol or penbutolol or pindolol or practolol or pronethalol or propranolol or sotalol or sulfinalol or talinolol or tertatolol or tilisolol or timolol or toliprolol or xibenolol) 10487 #4 #1 OR #2 OR #3
#5 MESH DESCRIPTOR Hypertension 13058 #6 hypertens*:TI,AB 30044 #7 MESH DESCRIPTOR Blood Pressure EXPLODE ALL TREES 22928 #8 blood pressure*:TI,AB 39350 #9 #5 OR #6 OR #7 OR #862225 #10 #4 AND #9 7961 *************************** Database: Embase <1980 to 2015 October 14> Search Date: 15 October 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp beta adrenergic receptor blocking agent/ (233618) 2 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. (101743) 3 acebutolol.mp. (4473) 4 exp alprenolol/ (3668) 5 alprenolol.mp. (3863) 6 amosulalol.mp. (122) 7 arotinolol.mp. (239) 8 atenolol.mp. (28052) 9 befunolol.mp. (306) 10 betaxolol.mp. (3108) 11 bevantolol.mp. (221) 12 exp bisoprolol/ (6963) 13 bisoprolol.mp. (7444) 14 bopindolol.mp. (293) 15 bucindolol.mp. (995) 16 bucumolol.mp. (26) 17 bufetolol.mp. (39) 18 bufuralol.mp. (776) 19 bunitrolol.mp. (271) 20 exp bupranolol/ (747) 21 bupranolol.mp. (777) 22 butofilolol.mp. (33) 23 carazolol.mp. (474) 24 exp carteolol/ (1392) 25 carteolol.mp. (1416) 26 carvedilol.mp. (11838) 27 exp celiprolol/ (1435) 28 celiprolol.mp. (1462) 29 cetamolol.mp. (41) 30 cloranolol.mp. (45) 31 cyanopindolol.mp. (1506) 32 deacetylmetipranolol.mp. (28) 33 dihydroalprenolol.mp. (2794) 34 dilevalol.mp. (373) 35 epanolol.mp. (104) 36 esmolol.mp. (4190) 37 indenolol.mp. (135) 38 iodocyanopindolol.mp. (675) 39 exp labetalol/ (8871) 40 labetalol.mp. (9028) 41 landiolol.mp. (403) 42 exp levobunolol/ (909) 43 levobunolol.mp. (919) 44 mepindolol.mp. (346) 45 exp metoprolol/ (27178) 46 metoprolol.mp. (30056) 47 exp metipranolol/ (936) 48 metipranolol.mp. (951) 49 moprolol.mp. (50) 50 exp nadolol/ (4932) 51 nadolol.mp. (5044) 52 nadoxolol.mp. (27) 53 nebivolol.mp. (2959) 54 nifenalol.mp. (95) 55 nipradilol.mp. (365) 56 oxprenolol.mp. (4172) 57 exp penbutolol/ (792) 58 penbutolol.mp. (817) 59 exp pindolol/ (8423) 60 pindolol.mp. (8696) 61 exp practolol/ (2859) 62 practolol.mp. (3073) 63 pronethalol.mp. (105) 64 exp propranolol/ (75390) 65 propranolol.mp. (80073) 66 proxodolol.mp. (30) 67 exp sotalol/ (10836) 68 sotalol.mp. (11108) 69 sulfinalol.mp. (16) 70 talinolol.mp. (607) 71 tertatolol.mp. (330) 72 tilisolol.mp. (150) 73 exp timolol/ (9884) 74 timolol.mp. (12468) 75 toliprolol.mp. (133) 76 xibenolol.mp. (98) 77 or/1‐76 (284190) 78 exp hypertension/ (531015) 79 hypertens$.tw. (462678) 80 (blood pressure or bloodpressure).mp. (463482) 81 or/78‐80 (939766) 82 randomized controlled trial/ (386041) 83 crossover procedure/ (44726) 84 double‐blind procedure/ (124191) 85 (randomized or randomly).ab. (716198) 86 (crossover$ or cross‐over$).tw. (76212) 87 placebo$.ab. (214767) 88 (doubl$ adj blind$).tw. (155583) 89 or/82‐88 (991842) 90 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5388776) 91 89 not 90 (877051) 92 77 and 81 and 91 (11565) *************************** Database: Hypertension Group Specialised Register Search Date: 15 October 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MeSH DESCRIPTOR Adrenergic beta‐Antagonists EXPLODE ALL TREES (1480) #2 ((acebutolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevanolol or bisoprolol or bopindolol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bupranolol or butofilolol or carazolol or carteolol or carvedilol or celiprolol or cetamolol or cloranolol or cyanopindolol or deacetylmetipranolol or dihydroalprenolol or dilevalol or epanolol or esmolol)) (2507) #3 ((indenolol or iodocyanopindolol or labetalol or levobunolol or mepindolol or metipranolol or metoprolol or nadolol or nadoxolol or nebivolol or nifenalol or nipradilol or oxprenolol or penbutolol or pindolol or practolol or pronethalol or propranolol or sotalol or sulfinalol or talinolol or tertatolol or tilisolol or timolol or toliprolol or xibenolol)) (325) #4 #1 OR #2 OR #3 (5951) #5 (RCT):DE OR (Review OR Meta‐Analysis):MISC2 (21968) #6 #4 AND #5 (3725) *************************** Database: ClinicalTrials.gov (via Cochrane Register of Studies) Search Date: 15 October 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Study type: Interventional Studies Conditions: hypertension Interventions: (beta blocker) or (adrenergic beta‐antagonist) Outcome Measures: blood pressure Search terms: randomized ***************************
Data and analyses
Comparison 1. Nebivolol vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 13 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 (0.25 x starting dose) 1.0 & 1.25 mg/day | 3 | 366 | Mean Difference (Fixed, 95% CI) | ‐4.49 [‐7.15, ‐1.83] |
| 1.2 (0.5 x starting dose) 2.5 mg/day | 3 | 415 | Mean Difference (Fixed, 95% CI) | ‐4.96 [‐7.66, ‐2.26] |
| 1.3 (starting dose) 5 mg/day | 12 | 1280 | Mean Difference (Fixed, 95% CI) | ‐8.81 [‐10.23, ‐7.40] |
| 1.4 (2 x starting dose) 10 mg/day | 6 | 1684 | Mean Difference (Fixed, 95% CI) | ‐6.04 [‐7.47, ‐4.60] |
| 1.5 (4 x starting dose) 20 mg/day | 3 | 652 | Mean Difference (Fixed, 95% CI) | ‐6.95 [‐9.26, ‐4.63] |
| 1.6 (8 x starting dose) 30, 40 mg/day | 3 | 1155 | Mean Difference (Fixed, 95% CI) | ‐8.10 [‐9.84, ‐6.36] |
| 2 DBP | 13 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 (0.25 x starting dose) 1.0 & 1.25 mg/day | 3 | 366 | Mean Difference (Fixed, 95% CI) | ‐3.55 [‐5.17, ‐1.93] |
| 2.2 (0.5 x starting dose) 2.5 mg/day | 3 | 415 | Mean Difference (Fixed, 95% CI) | ‐4.23 [‐5.76, ‐2.70] |
| 2.3 (starting dose) 5 mg/day | 12 | 1280 | Mean Difference (Fixed, 95% CI) | ‐6.67 [‐7.54, ‐5.80] |
| 2.4 (2 x starting dose) 10 mg/day | 6 | 1684 | Mean Difference (Fixed, 95% CI) | ‐5.90 [‐6.78, ‐5.01] |
| 2.5 (4 x starting dose) 20 mg/day | 3 | 652 | Mean Difference (Fixed, 95% CI) | ‐5.81 [‐7.21, ‐4.41] |
| 2.6 (8 x starting dose) 30, 40 mg/day | 3 | 1155 | Mean Difference (Fixed, 95% CI) | ‐7.70 [‐8.80, ‐6.61] |
| 3 Heart rate | 8 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 (0.25 x starting dose) 1.25 mg/day | 1 | 136 | Mean Difference (Fixed, 95% CI) | ‐2.9 [‐5.86, 0.06] |
| 3.2 (0.5 x starting dose) 2.5 mg/day | 2 | 218 | Mean Difference (Fixed, 95% CI) | ‐3.89 [‐6.18, ‐1.61] |
| 3.3 (starting dose) 5 mg/day | 7 | 487 | Mean Difference (Fixed, 95% CI) | ‐8.21 [‐9.66, ‐6.76] |
| 3.4 (2 x starting dose) 10 mg/day | 3 | 1120 | Mean Difference (Fixed, 95% CI) | ‐7.23 [‐8.48, ‐5.99] |
| 3.5 (4 x starting dose) 20 mg/day | 2 | 298 | Mean Difference (Fixed, 95% CI) | ‐8.43 [‐10.48, ‐6.38] |
| 3.6 (8 x starting dose) 30, 40 mg/day | 3 | 1155 | Mean Difference (Fixed, 95% CI) | ‐10.94 [‐12.18, ‐9.71] |
| 4 Pulse Pressure | 13 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 (0.25 x starting dose) 1.0 & 1.25 mg/day | 3 | 366 | Mean Difference (Fixed, 95% CI) | ‐0.88 [‐3.21, 1.45] |
| 4.2 (0.5 x starting dose) 2.5 mg/day | 3 | 415 | Mean Difference (Fixed, 95% CI) | ‐0.95 [‐3.32, 1.41] |
| 4.3 (starting dose) 5 mg/day | 12 | 1314 | Mean Difference (Fixed, 95% CI) | ‐1.62 [‐2.83, ‐0.41] |
| 4.4 (2 x starting dose) 10 mg/day | 6 | 1684 | Mean Difference (Fixed, 95% CI) | ‐0.19 [‐1.47, 1.08] |
| 4.5 (4 x starting dose) 20 mg/day | 3 | 652 | Mean Difference (Fixed, 95% CI) | ‐1.25 [‐3.38, 0.89] |
| 4.6 (8 x starting dose) 30, 40 mg/day | 3 | 1155 | Mean Difference (Fixed, 95% CI) | ‐0.37 [‐1.94, 1.20] |
| 5 Peak vs Trough SBP | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 (0.25 x starting dose) 1.0 & 1.25 mg/day | 2 | 335 | Mean Difference (Fixed, 95% CI) | 0.30 [‐1.90, 2.49] |
| 5.2 (0.5 x starting dose) 2.5 mg/day | 3 | 415 | Mean Difference (Fixed, 95% CI) | ‐1.24 [‐3.28, 0.81] |
| 5.3 (starting dose) 5 mg/day | 4 | 817 | Mean Difference (Fixed, 95% CI) | 1.40 [0.14, 2.66] |
| 5.4 (2 x starting dose) 10 mg/day | 4 | 822 | Mean Difference (Fixed, 95% CI) | ‐0.17 [‐1.42, 1.08] |
| 5.5 (4 x starting dose) 20 mg/day | 3 | 652 | Mean Difference (Fixed, 95% CI) | 1.17 [‐0.27, 2.61] |
| 5.6 (8 x starting dose) 30, 40 mg/day | 2 | 331 | Mean Difference (Fixed, 95% CI) | ‐1.20 [‐3.19, 0.78] |
| 6 Peak vs Trough DBP | 4 | 3372 | Mean Difference (Fixed, 95% CI) | ‐1.53 [‐1.95, ‐1.11] |
| 6.1 (0.25 x starting dose) 1.0 & 1.25 mg/day | 2 | 335 | Mean Difference (Fixed, 95% CI) | ‐0.95 [‐2.38, 0.48] |
| 6.2 (0.5 x starting dose) 2.5 mg/day | 3 | 415 | Mean Difference (Fixed, 95% CI) | ‐1.88 [‐3.12, ‐0.64] |
| 6.3 (starting dose) 5 mg/day | 4 | 817 | Mean Difference (Fixed, 95% CI) | ‐1.54 [‐2.52, ‐0.57] |
| 6.4 (2 x starting dose) 10 mg/day | 4 | 822 | Mean Difference (Fixed, 95% CI) | ‐1.06 [‐1.86, ‐0.26] |
| 6.5 (4 x starting dose) 20 mg/day | 3 | 652 | Mean Difference (Fixed, 95% CI) | ‐1.53 [‐2.41, ‐0.65] |
| 6.6 (8 x starting dose) 30, 40 mg/day | 2 | 331 | Mean Difference (Fixed, 95% CI) | ‐2.73 [‐3.98, ‐1.49] |
Comparison 2. Atenolol vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 22 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 (0.5 x starting dose) 25 mg/day | 1 | 87 | Mean Difference (Fixed, 95% CI) | ‐7.0 [‐12.55, ‐1.45] |
| 1.2 (starting dose) 50 mg/day | 11 | 669 | Mean Difference (Fixed, 95% CI) | ‐10.28 [‐11.94, ‐8.62] |
| 1.3 (2 x starting dose) 100 mg/day | 12 | 495 | Mean Difference (Fixed, 95% CI) | ‐15.34 [‐16.86, ‐13.82] |
| 1.4 (4 x starting dose) 150 & 200 mg/day | 3 | 121 | Mean Difference (Fixed, 95% CI) | ‐11.29 [‐14.03, ‐8.55] |
| 2 DBP | 22 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 (0.5 x starting dose) 25 mg/day | 1 | 87 | Mean Difference (Fixed, 95% CI) | ‐4.0 [‐6.76, ‐1.24] |
| 2.2 (starting dose) 50 mg/day | 11 | 657 | Mean Difference (Fixed, 95% CI) | ‐7.78 [‐8.80, ‐6.76] |
| 2.3 (2 x starting dose) 100 mg/day | 12 | 495 | Mean Difference (Fixed, 95% CI) | ‐12.91 [‐13.89, ‐11.93] |
| 2.4 (4 x starting dose) 150, 200 mg/day | 3 | 121 | Mean Difference (Fixed, 95% CI) | ‐8.76 [‐10.56, ‐6.95] |
| 3 Heart rate | 17 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 (0.5 x starting dose) 25 mg/day | 1 | 87 | Mean Difference (Fixed, 95% CI) | ‐4.0 [‐9.55, 1.55] |
| 3.2 (starting dose) 50 mg/day | 7 | 470 | Mean Difference (Fixed, 95% CI) | ‐12.04 [‐13.39, ‐10.68] |
| 3.3 (2 x starting dose) 100 mg/day | 10 | 413 | Mean Difference (Fixed, 95% CI) | ‐13.70 [‐14.83, ‐12.56] |
| 3.4 (4 x starting dose) 150, 200 mg/day | 4 | 129 | Mean Difference (Fixed, 95% CI) | ‐18.33 [‐20.26, ‐16.41] |
| 4 Pulse pressure | 22 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 (0.5 x starting dose) 25 mg/day | 1 | 87 | Mean Difference (Fixed, 95% CI) | ‐3.0 [‐7.80, 1.80] |
| 4.2 (starting dose) 50 mg/day | 11 | 669 | Mean Difference (Fixed, 95% CI) | ‐2.30 [‐3.71, ‐0.90] |
| 4.3 (2 x starting dose) 100 mg/day | 12 | 495 | Mean Difference (Fixed, 95% CI) | ‐2.01 [‐3.24, ‐0.77] |
| 4.4 (4 x starting dose) 150, 200 mg/day | 3 | 121 | Mean Difference (Fixed, 95% CI) | ‐2.62 [‐4.82, ‐0.42] |
Comparison 3. Metoprolol vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 9 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 (0.25 x starting dose) 25 mg/day | 2 | 424 | Mean Difference (Fixed, 95% CI) | ‐6.33 [‐9.22, ‐3.44] |
| 1.2 (0.5 x starting dose) 50 mg/day | 2 | 339 | Mean Difference (Fixed, 95% CI) | ‐6.30 [‐9.47, ‐3.13] |
| 1.3 (starting dose) 100 mg/day | 3 | 410 | Mean Difference (Fixed, 95% CI) | ‐5.35 [‐8.12, ‐2.58] |
| 1.4 (2 x starting dose) 200 mg/day | 5 | 284 | Mean Difference (Fixed, 95% CI) | ‐11.54 [‐14.37, ‐8.71] |
| 1.5 (4 x starting dose) 400 mg/day | 2 | 193 | Mean Difference (Fixed, 95% CI) | ‐10.91 [‐15.42, ‐6.40] |
| 2 DBP | 9 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 (0.25 x starting dose) 25 mg/day | 2 | 424 | Mean Difference (Fixed, 95% CI) | ‐3.64 [‐5.44, ‐1.85] |
| 2.2 (0.5 x starting dose) 50 mg/day | 2 | 339 | Mean Difference (Fixed, 95% CI) | ‐4.56 [‐6.42, ‐2.70] |
| 2.3 (starting dose) 100 & 120 mg/day | 3 | 410 | Mean Difference (Fixed, 95% CI) | ‐4.74 [‐6.50, ‐2.98] |
| 2.4 (2 x starting dose) 200 mg/day | 5 | 284 | Mean Difference (Fixed, 95% CI) | ‐10.25 [‐11.99, ‐8.51] |
| 2.5 (4 x starting dose) 400 mg/day | 2 | 193 | Mean Difference (Fixed, 95% CI) | ‐7.71 [‐10.72, ‐4.69] |
| 3 Heart rate | 5 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 (0.5 x starting dose) 50 mg/day | 1 | 94 | Mean Difference (Fixed, 95% CI) | ‐5.6 [‐10.03, ‐1.17] |
| 3.2 (starting dose) 100 & 120 mg/day | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐15.0 [‐18.92, ‐11.08] |
| 3.3 (2 x starting dose) 200 mg/day | 2 | 40 | Mean Difference (Fixed, 95% CI) | ‐13.25 [‐17.04, ‐9.46] |
| 3.4 (4 x starting dose) 400 mg/day | 1 | 8 | Mean Difference (Fixed, 95% CI) | ‐20.00 [‐25.88, ‐14.12] |
| 4 Pulse pressure | 9 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 (0.25 x starting dose) 25 mg/day | 2 | 424 | Mean Difference (Fixed, 95% CI) | ‐2.70 [‐5.23, ‐0.17] |
| 4.2 (0.5 x starting dose) 50 mg/day | 2 | 339 | Mean Difference (Fixed, 95% CI) | ‐1.74 [‐4.52, 1.04] |
| 4.3 (starting dose) 100 mg/day | 3 | 410 | Mean Difference (Fixed, 95% CI) | ‐0.58 [‐3.01, 1.85] |
| 4.4 (2 x starting dose) 200 mg/day | 5 | 284 | Mean Difference (Fixed, 95% CI) | ‐1.22 [‐3.62, 1.18] |
| 4.5 (4 x starting dose) 400 mg/day | 2 | 193 | Mean Difference (Fixed, 95% CI) | ‐3.30 [‐7.29, 0.69] |
Comparison 4. Bisoprolol vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 6 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 (starting dose) 5 mg/day | 5 | 422 | Mean Difference (Fixed, 95% CI) | ‐11.40 [‐13.67, ‐9.13] |
| 1.2 (2 x starting dose) 10 mg/day | 2 | 134 | Mean Difference (Fixed, 95% CI) | ‐7.03 [‐11.07, ‐2.99] |
| 1.3 (4 x starting dose) 20 mg/day | 1 | 121 | Mean Difference (Fixed, 95% CI) | ‐7.6 [‐12.60, ‐2.60] |
| 2 DBP | 7 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 (starting dose) 5 mg/day | 6 | 462 | Mean Difference (Fixed, 95% CI) | ‐8.16 [‐9.49, ‐6.84] |
| 2.2 (2 x starting dose) 10 mg/day | 3 | 173 | Mean Difference (Fixed, 95% CI) | ‐7.44 [‐9.88, ‐5.01] |
| 2.3 (4 x starting dose) 20 mg/day | 1 | 121 | Mean Difference (Fixed, 95% CI) | ‐8.5 [‐11.56, ‐5.44] |
| 3 Heart rate | 6 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 (starting dose) 5 mg/day | 5 | 236 | Mean Difference (Fixed, 95% CI) | ‐6.91 [‐8.82, ‐4.99] |
| 3.2 (2 x starting dose) 10 mg/day | 3 | 173 | Mean Difference (Fixed, 95% CI) | ‐10.19 [‐12.87, ‐7.52] |
| 3.3 (4 x starting dose) 20 mg/day | 1 | 121 | Mean Difference (Fixed, 95% CI) | ‐11.1 [‐14.98, ‐7.22] |
| 4 Pulse pressure | 6 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 (starting dose) 5 mg/day | 5 | 422 | Mean Difference (Fixed, 95% CI) | ‐3.35 [‐5.32, ‐1.38] |
| 4.2 (2 x starting dose) 10 mg/day | 2 | 134 | Mean Difference (Fixed, 95% CI) | 1.65 [‐1.96, 5.26] |
| 4.3 (4 x starting dose) 20 mg/day | 1 | 121 | Mean Difference (Fixed, 95% CI) | 0.9 [‐3.45, 5.25] |
Comparison 5. Betaxolol vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 (0.5 x starting dose) 5 mg/day | 1 | 148 | Mean Difference (Fixed, 95% CI) | ‐5.81 [‐10.55, ‐1.07] |
| 1.2 (starting dose) 10 mg/day | 1 | 146 | Mean Difference (Fixed, 95% CI) | ‐8.2 [‐12.94, ‐3.46] |
| 1.3 (2 x starting dose) 20 mg/day | 2 | 477 | Mean Difference (Fixed, 95% CI) | ‐11.25 [‐13.21, ‐9.29] |
| 2 DBP | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 (0.5 x starting dose) 5 mg/day | 1 | 148 | Mean Difference (Fixed, 95% CI) | ‐3.8 [‐6.54, ‐1.06] |
| 2.2 (starting dose) 10 mg/day | 1 | 146 | Mean Difference (Fixed, 95% CI) | ‐6.5 [‐9.24, ‐3.76] |
| 2.3 (2 x starting dose) 20 mg/day | 2 | 477 | Mean Difference (Fixed, 95% CI) | ‐7.94 [‐9.17, ‐6.71] |
| 3 Heart rate | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 (0.5 x starting dose) 5 mg/day | 1 | 148 | Mean Difference (Fixed, 95% CI) | ‐7.4 [‐10.34, ‐4.46] |
| 3.2 (starting dose) 10 mg/day | 1 | 146 | Mean Difference (Fixed, 95% CI) | ‐8.2 [‐11.14, ‐5.26] |
| 3.3 (2 x starting dose) 20 mg/day | 2 | 477 | Mean Difference (Fixed, 95% CI) | ‐13.40 [‐15.03, ‐11.77] |
| 4 Pulse pressure | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 (0.5x starting dose) 5 mg/day | 1 | 148 | Mean Difference (Fixed, 95% CI) | ‐1.1 [‐5.24, 3.04] |
| 4.2 (starting dose) 10 mg/day | 1 | 146 | Mean Difference (Fixed, 95% CI) | ‐1.7 [‐5.84, 2.44] |
| 4.3 (2 x starting dose) 20 mg/day | 2 | 477 | Mean Difference (Fixed, 95% CI) | ‐3.26 [‐5.00, ‐1.52] |
5.2. Analysis.
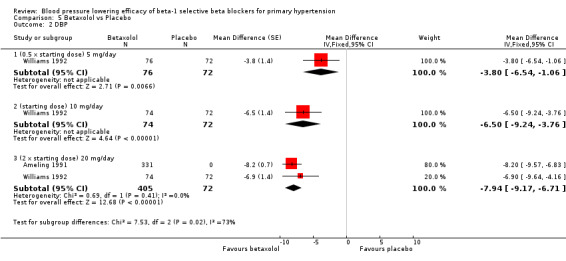
Comparison 5 Betaxolol vs Placebo, Outcome 2 DBP.
5.3. Analysis.
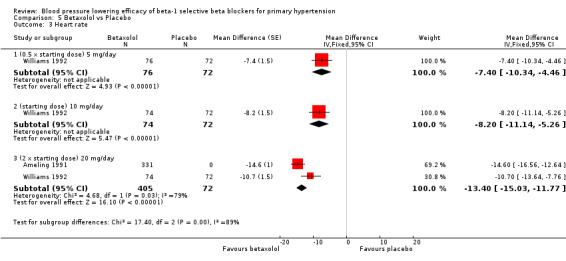
Comparison 5 Betaxolol vs Placebo, Outcome 3 Heart rate.
Comparison 6. Bevantolol vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Bevantolol 100mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐29.57, 3.57] |
| 1.2 Bevantolol 200mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐25.57, 7.57] |
| 1.3 Bevantolol 300mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐23.57, 9.57] |
| 1.4 Bevantolol 400mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐24.57, 8.57] |
| 2 DBP | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Bevantolol 100mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐15.52, 5.52] |
| 2.2 Bevantolol 200mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐19.52, 1.52] |
| 2.3 Bevantolol 300mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐19.52, 1.52] |
| 2.4 Bevantolol 400mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐20.52, 0.52] |
| 3 Heart rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Bevantolol 100mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐29.57, 3.57] |
| 3.2 Bevantolol 200mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐25.57, 7.57] |
| 3.3 Bevantolol 300mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐23.57, 9.57] |
| 3.4 Bevantolol 400mg/day | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐24.57, 8.57] |
| 4 Pulse pressure | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 Bevantolol 100mg/day | 1 | Mean Difference (Fixed, 95% CI) | ‐8.0 [‐22.52, 6.52] | |
| 4.2 Bevantolol 200mg/day | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [‐14.52, 14.52] | |
| 4.3 Bevantolol 300mg/day | 1 | Mean Difference (Fixed, 95% CI) | 2.0 [‐12.56, 16.56] | |
| 4.4 Bevantolol 400mg/day | 1 | Mean Difference (Fixed, 95% CI) | 2.0 [‐12.52, 16.52] |
Comparison 7. Pafenolol vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 25 mg/day | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐11.79, 17.79] |
| 1.2 50 mg/day | 2 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐5.94 [‐17.57, 5.68] |
| 1.3 100 mg/day | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐22.94, 10.94] |
| 2 DBP | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 25 mg/day | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐16.73, 0.73] |
| 2.2 50 mg/day | 2 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐4.41 [‐11.22, 2.40] |
| 2.3 100 mg/day | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐12.75, 8.75] |
| 3 Heart rate | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 25 mg/day | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐12.93, 6.93] |
| 3.2 50 mg/day | 2 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐8.14 [‐15.91, ‐0.36] |
| 3.3 100 mg/day | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐32.60, ‐7.40] |
| 4 Pulse pressure | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 25 mg/day | 1 | 21 | Mean Difference (Fixed, 95% CI) | 11.0 [‐1.82, 23.82] |
| 4.2 50 mg/day | 2 | 33 | Mean Difference (Fixed, 95% CI) | ‐1.75 [‐11.86, 8.37] |
| 4.3 100 mg/day | 1 | 14 | Mean Difference (Fixed, 95% CI) | ‐4.0 [‐18.86, 10.86] |
Comparison 8. Practolol vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐21.2 [‐29.31, ‐13.09] |
| 1.1 Practolol 600mg/day | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐21.2 [‐29.31, ‐13.09] |
| 2 DBP | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐13.9 [‐17.00, ‐8.80] |
| 2.1 Practolol 600mg/day | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐13.9 [‐17.00, ‐8.80] |
| 3 Heart rate | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐14.0 [‐21.45, ‐6.55] |
| 3.1 Practolol 600mg/day | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐14.0 [‐21.45, ‐6.55] |
| 4 Pulse pressure | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐7.30 [‐14.41, ‐0.19] |
| 4.1 Practolol 600mg/day | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐7.30 [‐14.41, ‐0.19] |
Comparison 9. Pooled overall effect.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 50 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 0.25 x starting dose | 5 | 790 | Mean Difference (Fixed, 95% CI) | ‐5.34 [‐7.29, ‐3.38] |
| 1.2 0.5 x starting dose | 7 | 941 | Mean Difference (Fixed, 95% CI) | ‐5.71 [‐7.59, ‐3.82] |
| 1.3 starting dose | 30 | 2879 | Mean Difference (Fixed, 95% CI) | ‐9.29 [‐10.21, ‐8.38] |
| 1.4 2 x starting dose | 27 | 3026 | Mean Difference (Fixed, 95% CI) | ‐10.58 [‐11.45, ‐9.71] |
| 1.5 4 x starting dose | 9 | 1087 | Mean Difference (Fixed, 95% CI) | ‐8.90 [‐10.47, ‐7.34] |
| 1.6 8 x starting dose | 2 | 331 | Mean Difference (Fixed, 95% CI) | ‐8.29 [‐11.24, ‐5.35] |
| 2 DBP | 51 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 0.25 x starting dose | 5 | 790 | Mean Difference (Fixed, 95% CI) | ‐3.59 [‐4.79, ‐2.39] |
| 2.2 0.5 x starting dose | 7 | 941 | Mean Difference (Fixed, 95% CI) | ‐4.27 [‐5.31, ‐3.22] |
| 2.3 starting dose | 31 | 2907 | Mean Difference (Fixed, 95% CI) | ‐7.06 [‐7.62, ‐6.51] |
| 2.4 2 x starting dose | 28 | 3065 | Mean Difference (Fixed, 95% CI) | ‐8.92 [‐9.47, ‐8.38] |
| 2.5 4 x starting dose | 9 | 1087 | Mean Difference (Fixed, 95% CI) | ‐7.17 [‐8.15, ‐6.18] |
| 2.6 8 x starting dose | 2 | 331 | Mean Difference (Fixed, 95% CI) | ‐7.20 [‐8.99, ‐5.40] |
| 3 Heart rate | 37 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 0.25 x starting dose | 1 | 136 | Mean Difference (Fixed, 95% CI) | ‐2.9 [‐5.86, 0.06] |
| 3.2 0.5 x starting dose | 5 | 499 | Mean Difference (Fixed, 95% CI) | ‐4.34 [‐6.21, ‐2.47] |
| 3.3 starting dose | 20 | 1315 | Mean Difference (Fixed, 95% CI) | ‐9.80 [‐10.65, ‐8.94] |
| 3.4 2 x starting dose | 20 | 2175 | Mean Difference (Fixed, 95% CI) | ‐11.33 [‐12.06, ‐10.61] |
| 3.5 4 x starting dose | 8 | 556 | Mean Difference (Fixed, 95% CI) | ‐13.71 [‐15.00, ‐12.43] |
| 3.6 8 x starting dose | 2 | 331 | Mean Difference (Fixed, 95% CI) | ‐9.19 [‐11.21, ‐7.18] |
| 4 Pulse pressure | 50 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 0.25 x starting dose | 5 | 790 | Mean Difference (Fixed, 95% CI) | ‐1.72 [‐3.43, ‐0.00] |
| 4.2 0.5 x starting dose | 7 | 941 | Mean Difference (Fixed, 95% CI) | ‐1.48 [‐3.13, 0.17] |
| 4.3 starting dose | 30 | 2913 | Mean Difference (Fixed, 95% CI) | 0.00 [‐2.78, ‐1.22] |
| 4.4 2 x starting dose | 27 | 3026 | Mean Difference (Fixed, 95% CI) | ‐1.39 [‐2.14, ‐0.65] |
| 4.5 4 x starting dose | 9 | 1087 | Mean Difference (Fixed, 95% CI) | ‐1.80 [‐3.16, ‐0.44] |
| 4.6 8 x starting dose | 2 | 331 | Mean Difference (Fixed, 95% CI) | ‐1.04 [‐3.67, 1.60] |
| 5 WDAE | 3 | 2618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.50, 1.45] |
| 6 SBP combined starting dose and 2 x starting dose | 47 | 5246 | Mean Difference (Fixed, 95% CI) | ‐10.42 [‐11.11, ‐9.72] |
| 6.1 starting dose | 30 | 2509 | Mean Difference (Fixed, 95% CI) | ‐9.71 [‐10.75, ‐8.67] |
| 6.2 2 x starting dose | 27 | 2737 | Mean Difference (Fixed, 95% CI) | ‐10.99 [‐11.93, ‐10.06] |
| 7 DBP combined starting dose and 2 x starting dose | 48 | 5316 | Mean Difference (Fixed, 95% CI) | ‐8.27 [‐8.69, ‐7.84] |
| 7.1 starting dose | 31 | 2540 | Mean Difference (Fixed, 95% CI) | ‐7.23 [‐7.85, ‐6.60] |
| 7.2 2x starting dose | 28 | 2776 | Mean Difference (Fixed, 95% CI) | ‐9.16 [‐9.74, ‐8.58] |
| 8 Heart rate combined starting dose and 2 x starting dose | 33 | 3407 | Mean Difference (Fixed, 95% CI) | ‐10.93 [‐11.48, ‐10.37] |
| 8.1 starting dose | 20 | 1268 | Mean Difference (Fixed, 95% CI) | ‐10.03 [‐10.90, ‐9.17] |
| 8.2 2 x starting dose | 20 | 2139 | Mean Difference (Fixed, 95% CI) | ‐11.57 [‐12.30, ‐10.84] |
| 9 Pulse pressure combined starting dose and 2 x starting dose | 47 | 5246 | Mean Difference (Fixed, 95% CI) | ‐1.76 [‐2.34, ‐1.19] |
| 9.1 starting dose | 30 | 2509 | Mean Difference (Fixed, 95% CI) | ‐2.09 [‐2.94, ‐1.23] |
| 9.2 2 x starting dose | 27 | 2737 | Mean Difference (Fixed, 95% CI) | ‐1.49 [‐2.27, ‐0.71] |
| 10 SBP test for 2 x starting dose subgroup difference | 26 | 2197 | Mean Difference (Fixed, 95% CI) | ‐11.27 [‐12.22, ‐10.32] |
| 10.1 Atenolol | 12 | 495 | Mean Difference (Fixed, 95% CI) | ‐15.34 [‐16.86, ‐13.82] |
| 10.2 Nebivolol | 5 | 855 | Mean Difference (Fixed, 95% CI) | ‐5.26 [‐7.19, ‐3.34] |
| 10.3 Metoprolol | 5 | 284 | Mean Difference (Fixed, 95% CI) | ‐11.54 [‐14.37, ‐8.71] |
| 10.4 Bisoprolol | 2 | 134 | Mean Difference (Fixed, 95% CI) | ‐7.03 [‐11.07, ‐2.99] |
| 10.5 Betaxolol | 2 | 429 | Mean Difference (Fixed, 95% CI) | ‐11.63 [‐13.73, ‐9.53] |
| 11 DBP test for 2 x starting dose subgroup difference | 27 | 2236 | Mean Difference (Fixed, 95% CI) | ‐9.47 [‐10.06, ‐8.88] |
| 11.1 Atenolol | 12 | 495 | Mean Difference (Fixed, 95% CI) | ‐12.91 [‐13.89, ‐11.93] |
| 11.2 Nebivolol | 5 | 855 | Mean Difference (Fixed, 95% CI) | ‐5.82 [‐6.99, ‐4.65] |
| 11.3 Metoprolol | 5 | 284 | Mean Difference (Fixed, 95% CI) | ‐10.25 [‐11.99, ‐8.51] |
| 11.4 Bisoprolol | 3 | 173 | Mean Difference (Fixed, 95% CI) | ‐7.44 [‐9.88, ‐5.01] |
| 11.5 Betaxolol | 2 | 429 | Mean Difference (Fixed, 95% CI) | ‐8.08 [‐9.39, ‐6.77] |
| 12 Heart rate test for 2 x starting dose subgroup difference | 19 | 1346 | Mean Difference (Fixed, 95% CI) | ‐12.24 [‐13.05, ‐11.42] |
| 12.1 Atenolol | 10 | 413 | Mean Difference (Fixed, 95% CI) | ‐13.70 [‐14.83, ‐12.56] |
| 12.2 Nebivolol | 2 | 291 | Mean Difference (Fixed, 95% CI) | ‐5.94 [‐7.98, ‐3.90] |
| 12.3 Metoprolol | 2 | 40 | Mean Difference (Fixed, 95% CI) | ‐13.25 [‐17.04, ‐9.46] |
| 12.4 Bisoprolol | 3 | 173 | Mean Difference (Fixed, 95% CI) | ‐10.19 [‐12.87, ‐7.52] |
| 12.5 Betaxolol | 2 | 429 | Mean Difference (Fixed, 95% CI) | ‐14.43 [‐16.35, ‐12.51] |
9.6. Analysis.
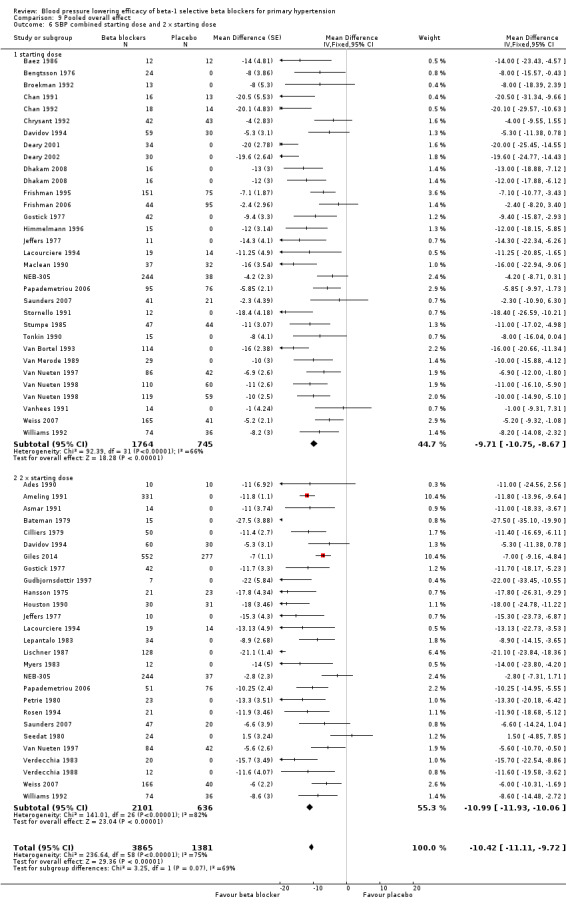
Comparison 9 Pooled overall effect, Outcome 6 SBP combined starting dose and 2 x starting dose.
9.7. Analysis.
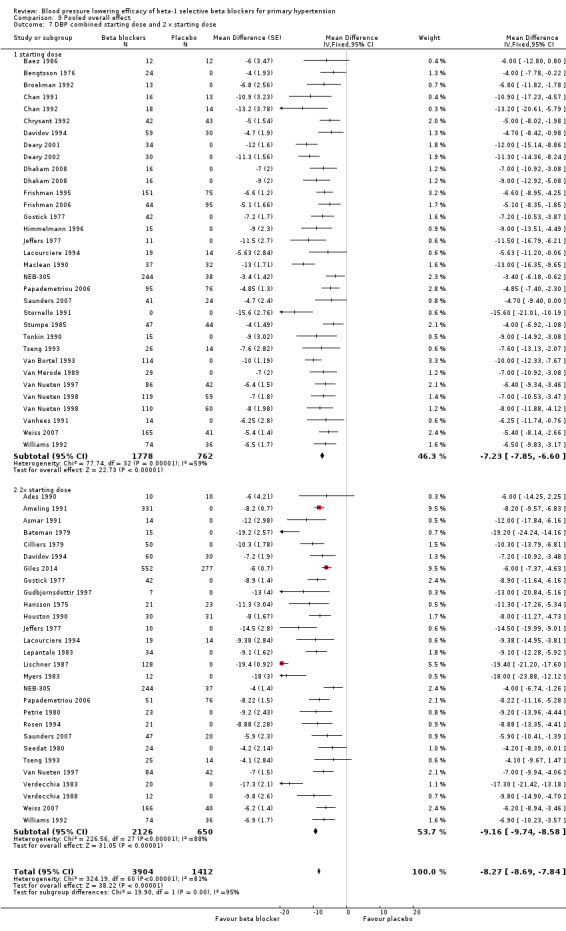
Comparison 9 Pooled overall effect, Outcome 7 DBP combined starting dose and 2 x starting dose.
9.8. Analysis.
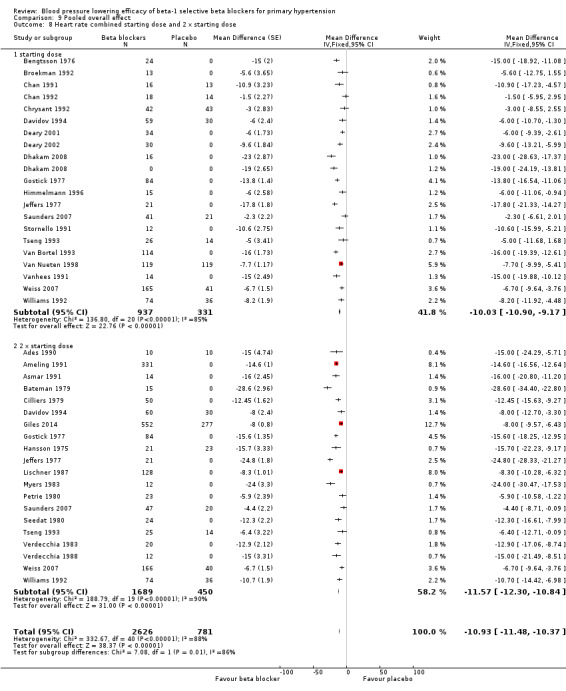
Comparison 9 Pooled overall effect, Outcome 8 Heart rate combined starting dose and 2 x starting dose.
9.9. Analysis.
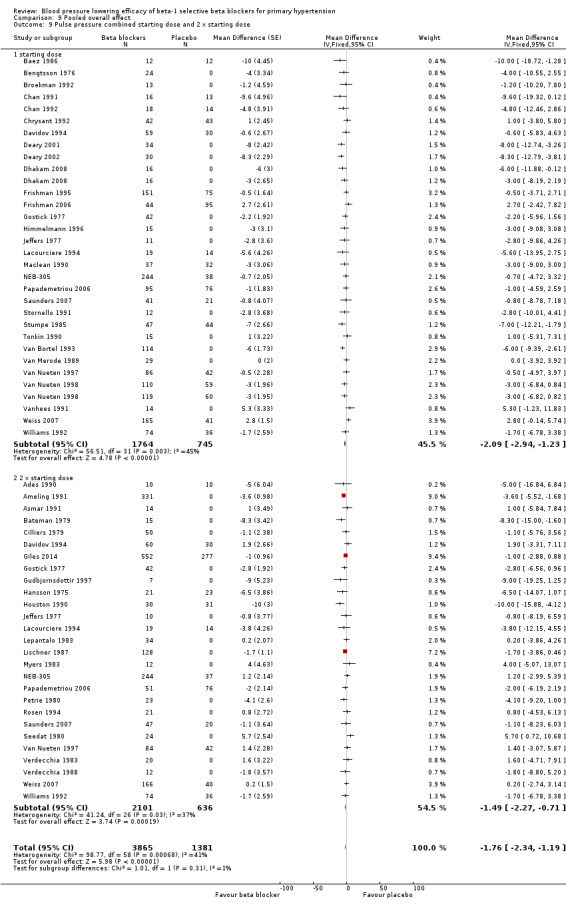
Comparison 9 Pooled overall effect, Outcome 9 Pulse pressure combined starting dose and 2 x starting dose.
9.12. Analysis.
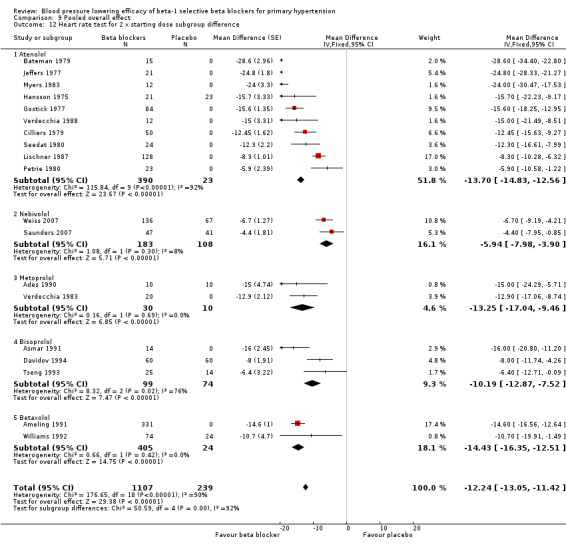
Comparison 9 Pooled overall effect, Outcome 12 Heart rate test for 2 x starting dose subgroup difference.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ades 1990.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study, 11‐week treatment period | |
| Participants | N = 30, 22 men, mean age = 47 years, mean baseline BP 145/94 mmHg, primary hypertension | |
| Interventions | Metoprolol 100 mg once daily, propranolol 80 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate, exercise training, cardiac output, echocardiogram, muscle biopsy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Did not report withdrawal due to adverse effects |
Ameling 1991.
| Methods | Randomised, double‐blinded, placebo‐controlled, cross‐over study. Patients were seen for the first two weeks and BP was measured in every visit. Patients who were eligible, were randomised after week 2. Each treatment period lasted for four weeks and then patients crossed‐over to another treatment for another four weeks. BP was measured in two‐week intervals during the treatment period | |
| Participants | Previously undiagnosed patients who had mean office DBP > 95 mmHg in two office visits, during the first two weeks, were included in the study. The study randomised 331 patients (188, 56.8% male) to betaxolol or placebo. Mean age = 50 years. Baseline SBP = 167.4 mmHg, DBP = 104.7 mmHg, heart rate = 80.3 bpm Exclusion criteria: age < 20 years or > 70 years; SBP > 190 mmHg or DBP > 125 mmHg; use of any medication that may affect BP; pregnancy; use of oral contraceptive; heart failure; bradycardia; chronic hepatic, renal or metabolic diseases; bronchial asthma; chronic pulmonary diseases |
|
| Interventions | Participants were randomised to betaxolol 20 mg once daily fixed dose (N = 163) or placebo (N = 168) for four weeks, and then crossed over. | |
| Outcomes | Quality of life SBP, DBP, heart rate Withdrawal due to adverse effects |
|
| Notes | SD of BP measurements were not reported. SD were imputed using the weighted mean SD of studies in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Subjects who were withdrawn from the study had no systematic follow up of their Quality of life assessment." There was no information about the follow up on SBP, DBP or heart rate data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal due to adverse effects was reported |
| Selective reporting (reporting bias) | Low risk | SBP, DBP, heart rate were reported without SD |
Asmar 1991.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study. Four‐week treatment period | |
| Participants | N = 14, 7 men, mean age = 53, baseline BP 156/101, primary hypertensive patients | |
| Interventions | Bisoprolol 10 mg once daily, placebo | |
| Outcomes | Resting BP, heart rate, haemodynamic parameters, pulse wave velocity determinations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and automated machine was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Automated machine was used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Baez 1986.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study. Three‐week run in, 10‐week treatment periods | |
| Participants | N = 36, 24 men, age 33 years to 68 years Exclusion: haematological, renal, hepatic, gastrointestinal, autoimmune or cardiac diseases |
|
| Interventions | Atenolol 50 mg, 100 mg once daily (only 50 mg data used), doxazosin 16 mg once daily, placebo | |
| Outcomes | SBP, DBP, lab analysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Bateman 1979.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, four‐week treatment periods | |
| Participants | N = 15, 11 men, age range 31 years to 64 years. Primary hypertension, without history of gout, chronic obstructive pulmonary disease, diabetes, ischaemic heart disease | |
| Interventions | Atenolol 100 mg once daily, chlorthalidone 25 mg once daily, combination of two drugs, placebo | |
| Outcomes | SBP, DBP, ambulatory blood pressure monitoring, biochemical analysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | All tablets were identical in appearance and taste |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Bengtsson 1976.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, four‐week treatment period | |
| Participants | N = 24, all women, mean age is 56 years, mean baseline BP 183/107 mmHg. Primary hypertension with normal renal function | |
| Interventions | Metoprolol 40 mg thrice daily or placebo | |
| Outcomes | SBP, DBP, heart rate, lab parameters | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts, 96% of tablets were taken |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Berglund 1985.
| Methods | Randomised, double‐blinded, parallel study, four‐week wash out, four‐week treatment | |
| Participants | N = 31, 24 male, mean age = 51.4 years, BP range 150‐200/100‐115 mmHg | |
| Interventions | Pafenolol 25mg or 50 mg once daily, placebo | |
| Outcomes | Casual BP, heart rate, 24 hour BP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information, but baseline was balanced |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used, but no description on placebo tablet |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Did not mention withdrawal due to adverse effects as outcome, however, no patients dropped out |
Broekman 1992.
| Methods | Randomised, double‐blinded, cross‐over study. six‐week treatment period | |
| Participants | N = 13, all men, mean age 51.2 years | |
| Interventions | Bisoprolol 5 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate, sexual information | |
| Notes | Group 1 only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Low risk | Did not report baseline. Other outcomes were reported |
Chan 1991.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study. four‐week run in, week four‐treatment period | |
| Participants | N = 29, seven men, mean age = 46 years, mean baseline BP 163/106 mmHg, primary hypertension, normal renal function, without insulin dependent diabetes and secondary hypertension | |
| Interventions | Nebivolol 5 mg once daily, placebo | |
| Outcomes | BP, heart rate biochemistry, chest X‐ray, electrocardiography, quality of life | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Chan 1992.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study. four‐week run‐in, four‐week treatment period | |
| Participants | N = 32, eight men, mean age = 47 years, mean baseline BP = 164/105.8 mmHg. Primary hypertension, without renal disease, diabetes, secondary hypertension | |
| Interventions | Nebivolol 5 mg once daily, placebo | |
| Outcomes | Rest SBP, DBP, heart rate, plasma biochemistry, plasma renin and hormone level | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching placebo tablet was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Chrysant 1992.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study, three‐week run in, four‐week treatment period | |
| Participants | N = 256, 120 men, mean age 53 years, mean baseline BP 152.5/103 mmHg. Primary hypertension without renal failure, congestive heart failure, lung diseases, liver diseases or blood diseases. Women of child bearing age were excluded | |
| Interventions | Atenolol 25 mg, 50 mg once daily, hydrochlorothiazide 25 mg/triamterene 50 mg once daily, their combinations, placebo | |
| Outcomes | SBP, DBP, heart rate, withdrawal due to adverse effects | |
| Notes | Withdrawal due to adverse effects not reported according to treatment groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight withdrawals in total |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Cilliers 1979.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, four‐week treatment periods | |
| Participants | N = 50, 29 men, mean age 47 years, mean baseline BP 165.8/107.7 mmHg. Primary hypertension, never‐been‐treated patients | |
| Interventions | Atenolol 100 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate, withdrawal due to adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not used automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nine dropouts, three withdrawal due to adverse effects |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Dahlof 1986.
| Methods | Multicenter, randomised, double‐blinded, parallel studies, four‐week run in, four‐week treatment | |
| Participants | N = 23, 12 men, mean age = 49 years, primary hypertensive, mean BP 164/109 mmHg | |
| Interventions | Pafenolol 50 mg or 100 mg once daily, placebo | |
| Outcomes | Rest and exercise BP, heart rate, dropouts | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information, but baseline groups were balanced |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used. But no description on the placebo tablet |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patients were accounted for. Did not mention the procedure to process dropout patients |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Davidov 1994.
| Methods | Multi‐center, randomised, double‐blinded, placebo‐controlled parallel study. Four‐week to six‐week run in period, four‐week treatment period | |
| Participants | N = 276, 70% men, mean age = 53 years, mean baseline BP 149/100 mmHg, primary hypertension without any condition that might influence blood pressure | |
| Interventions | Bisoprolol 5 mg, 10 mg, 20 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information but baseline was balanced |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on withdrawal |
| Selective reporting (reporting bias) | High risk | Did not report withdrawals due to adverse effects |
Deary 2001.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, two‐week run in, six‐week treatment | |
| Participants | N = 34, 25 men, mean age= 47 years, DBP between 95 and 110 mmHg in three readings in 3 months. | |
| Interventions | Bisoprolol 5 mg once daily, amlodipine 5 mg once daily, doxazosin 1 mg to 4 mg once daily, lisinopril 2.5 mg to 10 mg once daily, bendrofluazide 2.5 mg once daily, placebo | |
| Outcomes | Resting BP, quality of life, ambulatory blood pressure monitoring, left ventricular function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized with Latin square" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Physician determined which "best" treatment was repeated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Automated machine was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Deary 2002.
| Methods | Randomised, double‐blinded, placebo‐controlled, cross‐over study. Two‐week run‐in, six‐week treatment | |
| Participants | N = 30, 22 men, DBP > 95 mmHg after run in | |
| Interventions | Bisoprolol 5 mg once daily, amlodipine 5 mg once daily, doxazosin 4 mg once daily, lisinopril 10 mg once daily, bendrofluazide 2.5 mg once daily, placebo | |
| Outcomes | BP, heart rate, central BP, plasma atrial natriuretic peptide, pulse wave analysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The best treatment was repeated after rotation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Automated machine was used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Dhakam 2008.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, two‐week run in, five‐week treatment period | |
| Participants | N = 16, 10 men, mean age 70 years, mean baseline BP 158/84 mmHg. Never‐treated subjects without secondary hypertension, diabetes, renal impairment | |
| Interventions | Atenolol 50 mg once daily, nebivolol 5 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate, aortic augmentation index, biochemical analysis | |
| Notes | Isolated systolic hypertension | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Automated machine was used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Did not mention dropouts |
| Selective reporting (reporting bias) | Low risk | Did not report withdrawal due to adverse effects |
Frishman 1995.
| Methods | Multi‐centre, randomised, double‐blinded placebo‐controlled parallel study. Four‐week to six‐week run in period, four‐week treatment period | |
| Participants | N = 509, 245 men, mean baseline BP = 151/100 mmHg, primary hypertension, no more than 10% below or 35% above ideal weight | |
| Interventions | Bisoprolol 5 mg once daily, hydrochlorothiazide 25 mg once daily, B5/H25 combination, placebo | |
| Outcomes | SBP, DBP, heart rate, withdrawal due to adverse effects | |
| Notes | It was unclear to which group participants who withdrew due to adverse effects belonged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information, but baseline was balanced |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo tablet was used at the same frequency |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Did not report how they dealt with dropouts, but dropout rate was low |
| Selective reporting (reporting bias) | High risk | Withdrawal due to adverse effects was not reported based on treatment group |
Frishman 2006.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study, four‐week to five‐week run in, nine‐week treatment period | |
| Participants | N = 1092, 57% men, mean age = 54, mean baseline BP 152.6/99.9 mmHg, primary hypertension without previous cardiovascular event or contraindication to beta blockers | |
| Interventions | Metoprolol 25 mg, 100 mg, 400 mg once daily, felodipine 2.5 mg, 10 mg, 20 mg, any of the combination of metoprolol and felodipine listed or placebo | |
| Outcomes | Rest trough SBP, DBP, safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Each centre has blocks of patients randomised to a group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% of all patients dropouts. ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported, did not report withdrawal due to adverse effects according to treatment groups |
Frisk‐Holmberg 1985.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, three‐week run in, six‐week treatment periods | |
| Participants | N = 8, all men, primary hypertension | |
| Interventions | Atenolol 100 mg twice daily, placebo | |
| Outcomes | Exercise/rest haemodynamic, metabolic effect | |
| Notes | Did not report SBP, DBP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Did not mention dropouts |
| Selective reporting (reporting bias) | High risk | Did not report SBP, DBP, withdrawal due to adverse effects |
Giles 2014.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study, six‐week run in, eight‐week treatment period | |
| Participants | Mean SBP/DBP is 155/100 mmHg, mean age 51 years, mild to moderate hypertensive patients. 4161 randomized, 1386 to monotherapy groups | |
| Interventions | Nebivolol 10 mg/day and 40 mg/day, valsartan 160 mg/day and 320 mg/day, nebivolol and valsartan combination 10/160 mg/day, 10/320 mg/day, 20/320 mg/day and placebo | |
| Outcomes | Change of SBP, DBP and heart rate, withdrawal due to adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation codes were generated by the Statistical Programming Department at the Forest Research Institute (Jersey City, NJ, USA) and implemented (including the maintenance of masking) by a 24‐h interactive web response system." |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Tablets were identical in appearance, taste, and smell, and participants and research staff were masked to study drug treatment for the duration of the trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Automated machine was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was less than 10% |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
Gostick 1977.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, 12‐week run in, four‐week treatment period each | |
| Participants | N = 98, 84 were available for analysis, mean age 50.1 years, mean baseline BP 165.6/101.9 mmHg. Mild to moderate hypertension | |
| Interventions | Atenolol 50 mg, 100 mg, 200 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate, blood urea, uric acid, serum electrolytes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Latin square design |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 dropouts, all patients accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Gudbjornsdottir 1997.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, three‐week run in, six‐week treatment period | |
| Participants | N = 7, four men, mean age = 52 years, mean baseline BP 154/97. Primary hypertension diagnosed for at least one year, normal ECG, blood count, serum electrolyte, normal liver and renal function | |
| Interventions | Metoprolol 100 mg twice daily, placebo | |
| Outcomes | SBP, DBP, blood glucose, hormone | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | High risk | Did not report dropouts |
Hansson 1975.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study, 16‐week treatment periods (data for weeks 4, 8 and 12 were reported and used in analysis) | |
| Participants | N = 45, 26 men, mean age 46 years, mean baseline BP 169/106 mmHg. Primary hypertension | |
| Interventions | Atenolol 50 mg twice daily, placebo | |
| Outcomes | SBP, DBP, heart rate, withdrawal due to adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient withdrew |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Himmelmann 1996.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study. Four‐week run in, four‐week treatment period | |
| Participants | N = 14. 12 men and 3 women, mean age 50 years, 95 < DBP < 110 mmHg, primary hypertension | |
| Interventions | Nebivolol 5 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate, ambulatory blood pressure monitoring, blood chemistry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Did not use machine |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not mention dropouts |
| Selective reporting (reporting bias) | High risk | Withdrawal due to adverse effects was not reported |
Houston 1990.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study, two‐week to four‐week run in, eight‐week treatment period | |
| Participants | N = 92, 61% men, mean age 48 years, mean baseline BP 140/96.5 mmHg. Primary hypertension without renal, hepatic disease, neoplastic or peripheral vascular diseases | |
| Interventions | Atenolol 25 mg, 50 mg twice daily (only 50 mg twice daily result was available), clonidine 0.1 mg, 0.2 mg twice daily, placebo | |
| Outcomes | SBP, DBP, serum lipid | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Selective reporting (reporting bias) | High risk | |
Jaattela 1990.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study, two‐week run in, four‐week treatment period. This publication contained two studies with two sets of patients, we combined their data as one RCT | |
| Participants | N = 99, 45 men, mean age = 59 years, mean baseline BP 169/101 mmHg, primary hypertension, without heart failure, diabetes and asthma | |
| Interventions | Metoprolol 50 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate, response rate, safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Did not report dropouts, but only three patients' data were missing at the end |
| Selective reporting (reporting bias) | High risk | Did not report withdrawal due to adverse effects |
Jeffers 1977.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, four‐week run in, four‐week treatment period | |
| Participants | N = 21, eight men, mean age 53 years, mean baseline BP 161.7/111.5 mmHg | |
| Interventions | Atenolol 50 mg, 100 mg, 200 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order of administration was determined by a random code |
| Allocation concealment (selection bias) | Low risk | Participant received pre‐packed container |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Automated machine was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants dropped out |
| Selective reporting (reporting bias) | Low risk | Withdrawal due to adverse effects was reported but not according to treatment group |
Lacourciere 1994.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study. Eight‐week run‐in, 12‐week treatment period | |
| Participants | N = 240 (226 completed), 95 < DBP < 110 mmHg, mild to moderate primary hypertension | |
| Interventions | Nebivolol 1 mg, 5 mg, 10 mg once daily, Hydrochlorothiazide 12.5 mg, 25, mg once daily, every possible combination of the two drugs, placebo | |
| Outcomes | SBP, DBP, lipids, lipoprotein, apolipoprotein, routine lab parameters | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Did not report the procedure to deal with dropout data. But only 6% patients dropped out |
| Selective reporting (reporting bias) | Low risk | Did not report withdrawal due to adverse effects or heart rate |
Lepantalo 1983.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study. Four‐week run in, four‐week treatment period | |
| Participants | N = 34, 17 men, mean age = 54.1 years, mean baseline BP 160/100, primary hypertension | |
| Interventions | Metoprolol 100 mg twice daily, propranolol 80 mg twice daily, placebo | |
| Outcomes | SBP, DBP, peripheral vascular resistance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Did not report withdrawal due to adverse effects |
Lischner 1987.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, two‐week run in, four‐week treatment periods | |
| Participants | N = 128, 60 men, mean age 54 years for women, 52 years for men, mean baseline BP 167/111.3 mmHg. Primary hypertension without hepatic or renal diseases, obesity | |
| Interventions | Atenolol 100 mg once daily, amiloride 5 mg/hydrochlorothiazide 50 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate | |
| Notes | Data for women and men were reported separately. We combined the data according to the recommendation of the Cochrane Handbook for Systematic Reviews of Interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data of withdrawn patients were not included |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Maclean 1990.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study, three‐week run in, six‐week treatment period | |
| Participants | N = 136, primary hypertensive patients. Exclusion: renal or hepatic impairment, COPD, diabetes, peripheral vascular diseases, heart failure, heart block, myocardial infarction | |
| Interventions | Atenolol 50 mg once daily, nitrendipine 20 mg once daily, atenolol 50 mg/nitrendipine 20 mg, placebo | |
| Outcomes | SBP, DBP, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Double dummy design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 patients dropped out. All reasons were stated |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Myers 1983.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, six‐week treatment period | |
| Participants | N = 12, eight men, mean age 52.8 years, mean baseline BP 173/109 mmHg. Primary hypertension, without atrioventricular block, history of hepatic or renal diseases, diabetes, alcoholism, asthma, myocardial infarction, stroke, congestive heart failure, bradycardia. | |
| Interventions | Atenolol 100 mg once daily, hydrochlorothiazide 50 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate, biochemical test | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients dropped out, their data were not included in analysis |
| Selective reporting (reporting bias) | High risk | Dropouts were reported, but did not provide reason |
NEB‐305.
| Methods | Multi‐center, randomised, double‐blinded, placebo‐controlled, parallel study, 12‐week treatment period | |
| Participants | N = 807, 53.5% men, mean age = 53.4 years, mean baseline BP 151/99 mmHg. Primary hypertension, without asthma and COPD, renal and liver diseases, insulin dependent diabetes | |
| Interventions | Nebivolol 5 mg, 10 mg, 20 mg once daily, placebo | |
| Outcomes | Rest peak/trough SBP, DBP, withdrawal due to adverse effects, response rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information, however, the baseline demographic was similar in each group |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for. Analysis was ITT LOCF |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Okawa 1986.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study. After four weeks of placebo washout, patients were randomised to treatment. During the first two weeks of treatment, patients randomised to high dose were force titrated to respective dosage. After that, doses were fixed for the duration of next six weeks | |
| Participants | N = 139 (87 male) 100 mmHg < DBP < 120 mmHg |
|
| Interventions | Bevantolol 50 mg twice daily, 100 mg twice daily, 150 mg twice daily, 200 mg twice daily or placebo | |
| Outcomes | SBP, DBP, heart rate, withdrawal due to adverse effects, adverse effect, clinical lab | |
| Notes | Three withdrawalw due to adverse effects in bevantolol, none in placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information, but baseline was balanced |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebos were given at the same frequency as active treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on follow up |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal due to adverse effects were reported |
| Selective reporting (reporting bias) | Low risk | Haemodynamic data were primary outcomes, withdrawal due to adverse effects were reported in detail |
Papademetriou 2006.
| Methods | Multicenter, randomised, double‐blinded, placebo‐controlled, parallel study. Four‐week to five‐week run in, eight‐week treatment period | |
| Participants | N = 1571, 51% men, mean age = 53 years, mean baseline BP 151/100 mmHg. Primary hypertension without heart failure, recent MI, contraindication for beta blockers | |
| Interventions | Metoprolol 25 mg, 50 mg, 100 mg, 200 mg once daily, Hydrochlorothiazide 6.25 mg, 12.5 mg, 25 mg once daily, any combination of the two drugs, placebo | |
| Outcomes | Trough SBP, DBP, haematology, lipid, urinalysis, ECG | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central, computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Interactive voice response system allocated patients to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2.9% withdrawal due to adverse effects, ITT analysis |
| Selective reporting (reporting bias) | High risk | Peak pressure was mentioned in protocol but not reported, withdrawal due to adverse effects was not reported according to treatment groups |
Petrie 1976.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study. After 14 days of washout period, patients were randomised to a sequence of four‐week treatments | |
| Participants | N = 24 (13 male) 105 < DBP < 125 mmHg Exclusion: recent MI, evidence of heart failure, heart block, gross ischaemia, grade III or IV retinopathy, diabetes, gout, impaired liver function, or creatinine clearance less than 60 ml/min or if they were on any other drug treatment |
|
| Interventions | Methyldopa 250 mg thrice daily, practolol 200 mg thrice daily, propranolol 80 mg thrice daily, methyldopa 250 mg and propranolol 80 mg thrice daily, methyldopa 250 mg and practolol 200 mg thrice daily or placebo | |
| Outcomes | SBP, DBP, heart rate, quality of life | |
| Notes | SD were imputed using the average of other studies that provided SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Drug packages were pre‐packed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants took the same number of pills |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | SD were not reported |
Petrie 1980.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, four‐week run in, four‐week treatment periods | |
| Participants | N = 23, 12 men, mean age 40.9 years, mean baseline BP 155/109.4 mmHg. | |
| Interventions | Atenolol 100 mg once daily, oxprenolol 160 mg once daily, propranolol 160 mg once daily, placebo | |
| Outcomes | SBP, DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Did not report dropouts |
| Selective reporting (reporting bias) | High risk | Did not report withdrawal due to adverse effects |
Rosen 1994.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, four‐week run in, four‐week treatment periods | |
| Participants | N = 21, all men, age range 31 years to 70 years, mean baseline BP 157/101.9 mmHg | |
| Interventions | Atenolol 100 mg once daily, propranolol 80 mg twice daily, alpha‐methyldopa 500 mg twice daily, hydrochlorothiazide/triamterene 50 mg/25 mg twice daily, placebo | |
| Outcomes | SBP, DBP, sleep assessment, sexual function questionnaire | |
| Notes | 8 of 21 patients withdrew from the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Modified Latin square |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No indication that placebo was used at same frequency |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40% of patient dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Saunders 2007.
| Methods | Multi‐center, randomised, double‐blinded, placebo‐controlled, parallel study. Four‐week run‐in, 12‐week treatment period | |
| Participants | N = 300, 45.3% men, mean age = 50.9 years, mean baseline BP 152.2/100.2 mmHg. Primary hypertensive, BMI < 40, no cardiovascular condition or diabetes | |
| Interventions | Nebivolol 2.5 mg, 5 mg, 10 mg, 20 mg, 40 mg once daily, placebo | |
| Outcomes | Rest peak/trough SBP, DBP, heart rate, ECG, lab parameters | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT LOCF was used for analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Seedat 1980.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, four‐week treatment period | |
| Participants | N = 24, Africans with primary hypertension. Exclusion criteria: heart failure, asthma, MI, heart block, pregnancy, impaired renal and hepatic function | |
| Interventions | Atenolol 100 mg once daily, chlorthalidone 25 mg once daily, combination of two drugs, placebo | |
| Outcomes | SBP, DBP, heart rate, plasma renin activity, serum potassium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not mention dropouts |
| Selective reporting (reporting bias) | High risk | Withdrawal due to adverse effects was not mentioned |
Stornello 1991.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, three‐week run in, six‐week treatment periods | |
| Participants | N = 12, four men, mean age 51.9 years, primary hypertension with non–insulin‐dependent diabetes mellitus, persistent aluminuria, no evidence of urinary tract infection, other kidney disease | |
| Interventions | Atenolol 50 mg once daily, enalapril 20 mg once daily, chlorthalidone 12.5 mg once daily, placebo | |
| Outcomes | SBP, DBP, Heart rate, renal vascular resistance, filtration fraction, blood chemistry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Automated machine was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Stumpe 1985.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study, four‐week run in, four‐week treatment periods | |
| Participants | N = 232. Exclusion: history of heart disease or asthma | |
| Interventions | Atenolol 50 mg once daily, celiprolol 200 mg, 400 mg, 600 mg once daily, placebo | |
| Outcomes | SBP, DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Double dummy design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not report dropout rate |
| Selective reporting (reporting bias) | High risk | Did not report withdrawal due to adverse effects |
Tonkin 1990.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, four‐week run in, four‐week treatment periods | |
| Participants | N = 15, seven men, median age 61 years, primary hypertension patients. Exclusion criteria: diabetes, cardiac disease, COPD, impaired renal or hepatic function | |
| Interventions | Atenolol 50 mg once daily, diltiazem 60 mg once daily, combination of two drugs, placebo | |
| Outcomes | SBP, DBP, ECG, haematology, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 4 x 4 Latin square design |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Tseng 1993.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study. Two‐ week to four‐week run in period, three‐week treatment period | |
| Participants | N = 65, 35 men, mean age = 51 years, mean baseline BP 160/101 mmHg, primary hypertension without any co‐morbidity | |
| Interventions | Bisoprolol 5 mg, 10 mg once daily or placebo | |
| Outcomes | DBP, heart rate, response rate, biochemical haematological change, ECG | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information, but baseline was balanced |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Did not report dropouts |
| Selective reporting (reporting bias) | High risk | Did not report SBP |
Van Bortel 1993.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study. Four‐week run in, four‐week treatment period | |
| Participants | N = 114, 76 men, mean age = 54, 95 < DBP < 120 mmHg, primary hypertension, normal liver and renal function, no history of heart failure, severe vascular disease, uncontrolled diabetes | |
| Interventions | Nebivolol 5 mg once daily, placebo | |
| Outcomes | BP, heart rate, quality of life, ECG, lab parameters | |
| Notes | Only 80 patients randomised to cross‐over group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Van Herwaarden 1977.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, four‐week treatment period | |
| Participants | N = 8, mean age = 34 years | |
| Interventions | Metoprolol 100 mg thrice daily, propranolol 80 mg thrice daily, placebo | |
| Outcomes | Rest and adrenaline infused SBP, DBP, heart rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Van Merode 1989.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study. Four‐week run in, four‐week treatment period | |
| Participants | N = 29, 21 men, primary hypertension with normal kidney and liver functions | |
| Interventions | Nebivolol 5 mg once daily, placebo | |
| Outcomes | SBP, DBP, pulse pressure, carotid diameter and compliance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Automated machine was used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not mention dropout |
| Selective reporting (reporting bias) | High risk | Did not report withdrawal due to adverse effects |
Van Nueten 1997.
| Methods | Multi‐centered, randomised, double‐blinded, placebo‐controlled, parallel study. Four‐week run in, four‐week treatment period | |
| Participants | N = 509, 53% men, baseline mean BP 158.4/102.7 mmHg. Exclusion criteria: secondary hypertension, asthma and COPD, bradycardia, AV block, insulin dependent diabetes, MI or stroke in past six months, heart failure, renal hepatic disease, 50% over weight base on BMI | |
| Interventions | Nebivolol 0.5 mg, 1 mg, 2.5 mg, 5 mg, 10 mg once daily, placebo | |
| Outcomes | Rest peak/trough BP, ECG, lab parameters, symptom score, withdrawal due to adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo tablets were identical in appearance and taste |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All dropouts reported, dropout rate was low |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Van Nueten 1998.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study, four‐week run in, four‐week treatment period | |
| Participants | N = 364, 191 men, mean age = 55 years, mean baseline BP 166.5/104 mmHg. Primary hypertension, previously untreated or responded poorly to previous treatment | |
| Interventions | Nebivolol 5 mg once daily, Atenolol 50 mg once daily, placebo | |
| Outcomes | SBP, DBP, adverse event, ECG, blood chemistry, haematology, urine test | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All tablets were identical |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 patients were lost to follow‐up at the end |
| Selective reporting (reporting bias) | High risk | Did not report withdrawal due to adverse effects |
Vanhees 1991.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, three‐week run in, three‐week treatment periods | |
| Participants | N = 15, all men, mean age 39.4 years, mean baseline BP 166/93 | |
| Interventions | Atenolol 50 mg once daily, verapamil 240 mg once daily, enalapril 10 mg once daily, matching placebo | |
| Outcomes | Exercise tolerance, rest SBP, DBP, exercise parameters, urine and blood analysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Verdecchia 1983.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, two‐week placebo, four‐week treatment period | |
| Participants | N = 20, 14 men, mean age = 44 years, mean baseline BP 165.5/108.3, primary hypertension with normal ECG | |
| Interventions | Metoprolol 200 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate, systolic time interval | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Verdecchia 1988.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, three‐week run in, four‐week treatment periods | |
| Participants | N = 12, five men, mean age 48.6 years, primary hypertension | |
| Interventions | Atenolol 100 mg once daily, placebo | |
| Outcomes | SBP, DBP, heart rate, ambulatory blood pressure monitoring, withdrawal due to adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 4 x 4 Latin square design |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Weiss 2007.
| Methods | Multi‐center, randomised, double‐blinded, placebo‐controlled, parallel study. Two‐week run‐in, 12‐week treatment period | |
| Participants | N = 909, 57% men, mean age = 54.7 years, mean baseline BP 153.1/99.5 mmHg. Primary hypertensive, BMI < 35, no history of cardiovascular disease or diabetes | |
| Interventions | Nebivolol 1.25 mg, 2.5mg , 5 mg, 10 mg, 20 mg, 40 mg once daily, placebo | |
| Outcomes | Rest peak/trough SBP, DBP, heart rate, safety | |
| Notes | Not all patients in 40 mg group took 40 mg, therefore we excluded this group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data was presented as ITT LOCF |
| Selective reporting (reporting bias) | Low risk | All data reported |
Williams 1992.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study. Participants entered a three‐week to four‐week placebo run in period, during which they were instructed to take the capsule at 10 am every morning. Patients who met the entry criteria were randomised to one of the 4 treatment groups for another five weeks. After the fixed dose period, there was a one‐week follow‐up period | |
| Participants | N = 317 Patients with mean DBP between 95 mmHg and 110 mmHg during the run in period were eligible for randomization |
|
| Interventions | Betaxolol 5 mg, 10 mg, 20 mg once daily or placebo | |
| Outcomes | SBP, DBP, heart rate, withdrawal, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information, but baseline was balanced |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo capsules were used in same frequency |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Withdrawn were accounted for and not included in analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reasons for withdrawal were reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Wing 1988.
| Methods | Randomised, double‐blinded, placebo‐controlled cross‐over study, four‐week run in, four‐week treatment periods | |
| Participants | N = 16, six men, mean age 59 years. Primary hypertension without target organ damage | |
| Interventions | Atenolol 50 mg once daily, enalapril 20 mg once daily, atenolol/enalapril combination, placebo | |
| Outcomes | SBP, DBP, heart rate, blood chemical analysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 4 x 4 Latin square design |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not use automated machine |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on dropouts |
| Selective reporting (reporting bias) | High risk | Did not report withdrawal due to adverse effects |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Wald 2008 | Non‐hypertensive and hypertensive participants were mixed and randomised. The data of hypertensive patients were not reported separately |
Differences between protocol and review
There are no differences between the protocol and review.
Contributions of authors
Gavin Wong took the lead role in searching, identifying and assessing studies, in data extraction and analyses, and in interpreting the data and writing the review.
Heidi Boyda aided in the identifying of the included studies, in verifying the data extraction, and reviewing the final draft of the manuscript.
James Wright formulated the idea for the review, developed the basis for the protocol and participated in the interpretation and writing of the review.
Sources of support
Internal sources
University of British Columbia, Department of Anesthesiology, Pharmacology & Therapeutics, Canada.
External sources
Canadian Institutes of Health Research, Canada.
Declarations of interest
Gavin Wong: nothing to declare Heide Boyda: nothing to declare James Wright: nothing to declare.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Ades 1990 {published data only}
- Ades PA, Gunther PG, Meyer WL, Gibson TC, Maddalena J, Orfeo T, et al. Cardiac and skeletal muscle adaptations to training in systemic hypertension and effect of beta blockade (metoprolol or propranolol). American Journal of Cardiology 1990;66(5):591‐6. [DOI] [PubMed] [Google Scholar]
Ameling 1991 {published data only}
- Ameling EH, Korte DF, Man in 't Veld A. Impact of diagnosis and treatment of hypertension on quality of life: a double‐blind, randomized, placebo‐controlled, cross‐over study of betaxolol. Journal of Cardiovascular Pharmacology 1991;18(5):752‐60. [DOI] [PubMed] [Google Scholar]
Asmar 1991 {published data only}
- Asmar RG, Kerihuel JC, Girerd XJ, Safar ME. Effect of bisoprolol on blood pressure and arterial hemodynamics in systemic hypertension. American Journal of Cardiology 1991;68(1):61‐4. [DOI] [PubMed] [Google Scholar]
Baez 1986 {published data only}
- Baez MA, Garg DC, Jallad NS, Weidler DJ. Antihypertensive effect of doxazosin in hypertensive patients: comparison with atenolol. British Journal of Clinical Pharmacology 1986;21(Suppl 1):63s‐67s. [PUBMED: 2939869] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bateman 1979 {published data only}
- Bateman DN, Dean CR, Mucklow JC, Bulpitt CJ, Dollery CT. Atenolol and chlorthalidone in combination for hypertension. British Journal of Clinical Pharmacology 1979;7(4):357‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bengtsson 1976 {published data only}
- Bengtsson C. The effect of metoprolol ‐ a new selective adrenergic beta1‐receptor blocking agent‐‐ in mild hypertension. Acta Medica Scandinavica 1976;199(1‐2):65‐70. [DOI] [PubMed] [Google Scholar]
Berglund 1985 {published data only}
- Berglund G, Faire U, Castenfors J, Andersson G, Hartford M, Liedholm H, et al. Monitoring 24‐hour blood pressure in a drug trial. Evaluation of a noninvasive device. Hypertension 1985;7:688‐94. [DOI] [PubMed] [Google Scholar]
Broekman 1992 {published data only}
- Broekman CP, Haensel SM, Ven LLM, Slob AK. Bisoprolol and hypertension: effects on sexual functioning in men. Journal of Sex & Marital Therapy 1992;18(4):325‐31. [DOI] [PubMed] [Google Scholar]
Chan 1991 {published data only}
- Chan TYK, Woo KS, Critchley JAJH, Swaminathan R, Nicholls MG. Effects of nebivolol on blood pressure, hormones and cardiac function in Chinese subjects with essential hypertension. Drug investigations 1991;3:98‐104. [Google Scholar]
Chan 1992 {published data only}
- Chan TY, Woo KS, Nicholls MG. The application of nebivolol in essential hypertension: a double‐blind, randomized, placebo‐controlled study. International Journal of Cardiology 1992;35(3):387‐95. [DOI] [PubMed] [Google Scholar]
Chrysant 1992 {published data only}
- Chrysant SG, Chappel C, Farnham DJ, Levin B, Lueg M, McCluskey D, et al. Antihypertensive and metabolic effects of single and combined atenolol regimens. Journal of Clinical Pharmacology 1992;32(1):61‐5. [DOI] [PubMed] [Google Scholar]
Cilliers 1979 {published data only}
- Cilliers AJ. Atenolol as primary therapy in previously untreated hypertensives and as an adjuvant to other therapy. A South African multicentre study. South African Medical Journal 1979;Suid‐Afrikaanse Tydskrif Vir Geneeskunde. 55(9):321‐4. [PubMed] [Google Scholar]
Dahlof 1986 {published data only}
- Dahlof B, Sjoberg KH, Flygt C, Jern S, Hansen S, Hansson L, et al. Pafenolol in hypertension: a double‐blind randomized trial of a new beta 1‐selective adrenoceptor blocker. Journal of Cardiovascular Pharmacology 1986;8(1):55‐9. [DOI] [PubMed] [Google Scholar]
Davidov 1994 {published data only}
- Davidov ME, Singh SP, Vlachakis ND, Blumenthal JB, Simon JS, Bryzinski BS, et al. Bisoprolol, a once‐a‐day beta‐blocking agent for patients with mild to moderate hypertension. Clinical Cardiology 1994;17(5):263‐8. [DOI] [PubMed] [Google Scholar]
Deary 2001 {published data only}
- Deary AJ, Schumann AL, Murfet H, Haydock SF, Foo RSY, Brown MJ. Double‐blind, placebo‐controlled crossover comparison of five classes of antihypertensive drugs. Journal of Hypertension 2002;20:771‐7. [DOI] [PubMed] [Google Scholar]
Deary 2002 {published data only}
- Deary AJ, Schumann AL, Murfet H, Haydock S, Foo RS, Brown MJ. Influence of drugs and gender on the arterial pulse wave and natriuretic peptide secretion in untreated patients with essential hypertension.[see comment]. Clinical Science 2002;103(5):493‐9. [DOI] [PubMed] [Google Scholar]
Dhakam 2008 {published data only}
- Dhakam Z, Yasmin, McEniery CM, Burton T, Brown MJ, Wilkinson IB. A comparison of atenolol and nebivolol in isolated systolic hypertension. Journal of Hypertension 2008;26:351‐6. [DOI] [PubMed] [Google Scholar]
Frishman 1995 {published data only}
- Frishman WH, Burris JF, Mroczek WJ, Weir MR, Alemayehu D, Simon JS, et al. First‐line therapy option with low‐dose bisoprolol fumarate and low‐dose hydrochlorothiazide in patients with stage I and stage II systemic hypertension. Journal of Clinical Pharmacology 1995;35(2):182‐8. [DOI] [PubMed] [Google Scholar]
Frishman 2006 {published data only}
- Frishman WH, Hainer JW, Sugg J, M‐FACT Study Group. A factorial study of combination hypertension treatment with metoprolol succinate extended release and felodipine extended release results of the Metoprolol Succinate‐Felodipine Antihypertension Combination Trial (M‐FACT). American Journal of Hypertension 2006;19(4):388‐95. [DOI] [PubMed] [Google Scholar]
Frisk‐Holmberg 1985 {published data only}
- Frisk‐Holmberg M, Juhlin‐Dannfelt A, Astrom H. Haemodynamic and metabolic responses to prolonged exercise after chronic beta 1‐adrenoceptor blockade in hypertensive man. Clinical Physiology 1985;5(3):231‐42. [PubMed] [Google Scholar]
Giles 2014 {published data only}
- Giles TD, Weber MA, Basile J, et al. Efficacy and safety of nebivolol and valsartan as fixed‐dose combination in hypertension: a randomised, multicentre study. Lancet 2014;383:1889‐98. [DOI] [PubMed] [Google Scholar]
Gostick 1977 {published data only}
- Gostick NK, Mayhew SR, Million R, Sagar D, Suxena SR, Ingram DF, et al. A dose‐response study of atenolol in mild to moderate hypertension in general practice. Current Medical Research & Opinion 1977;5(2):179‐84. [DOI] [PubMed] [Google Scholar]
Gudbjornsdottir 1997 {published data only}
- Gudbjornsdottir S, Fowelin J, Elam M, Attvall S, Bengtsson BA, Marin P, et al. The effect of metoprolol treatment on insulin sensitivity and diurnal plasma hormone levels in hypertensive subjects. European Journal of Clinical Investigation 1997;27(1):29‐35. [DOI] [PubMed] [Google Scholar]
Hansson 1975 {published data only}
- Hansson L, Aberg H, Karlberg BE, Westerlund A. Controlled study of atenolol in treatment of hypertension. British Medical Journal 1975;2(5967):367‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Himmelmann 1996 {published data only}
- Himmelmann A, Hedner T, Snoeck E, Lundgren B, Hedner J. Haemodynamic effects and pharmacokinetics of oral d‐ and l‐nebivolol in hypertensive patients. European Journal of Clinical Pharmacology 1996;51(3‐4):259‐64. [DOI] [PubMed] [Google Scholar]
Houston 1990 {published data only}
- Houston MC, Burger C, Hays JT, Nadeau J, Swift L, Bradley CA, et al. The effects of clonidine hydrochloride versus atenolol monotherapy on serum lipids, lipid subfractions, and apolipoproteins in mild hypertension. American Heart Journal 1990;120(1):172‐9. [DOI] [PubMed] [Google Scholar]
Jaattela 1990 {published data only}
- Jaattela A, Baandrup S, Houtzagers J, Westergren G. The efficacy of low dose metoprolol CR/ZOK in mild hypertension and in elderly patients with mild to moderate hypertension. Journal of Clinical Pharmacology 1990;30(2 Suppl):S66‐71. [DOI] [PubMed] [Google Scholar]
Jeffers 1977 {published data only}
- Jeffers TA, Webster J, Petrie JC, Barker NP. Atenolol once‐daily in hypertension. British Journal of Clinical Pharmacology 1977;4(5):523‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacourciere 1994 {published data only}
- Lacourciere Y, Arnott W. Placebo‐controlled comparison of the effects of nebivolol and low‐dose hydrochlorothiazide as monotherapies and in combination on blood pressure and lipid profile in hypertensive patients. Journal of Human Hypertension 1994;8(4):283‐8. [PubMed] [Google Scholar]
Lepantalo 1983 {published data only}
- Lepantalo M, Totterman KJ. Effect of long‐term beta‐adrenergic‐blockade on calf blood flow in hypertensive patients. Clinical Physiology 1983;3(1):35‐42. [DOI] [PubMed] [Google Scholar]
- Lepantalo MJ, Totterman KJ. Lower limb haemodynamics during antihypertensive treatment with metoprolol and propranolol. International Angiology 1985;4(2):225‐8. [PubMed] [Google Scholar]
Lischner 1987 {published data only}
- Lischner M, Lang R, Jutrin I, Ravid M. Atenolol vs. amiloride‐hydrochlorothiazide in the treatment of mild to moderate hypertension: a double‐blind, crossover, placebo‐controlled study. Drug Intelligence & Clinical Pharmacy 1987;21(1 Pt 1):43‐6. [DOI] [PubMed] [Google Scholar]
Maclean 1990 {published data only}
- Maclean D, Mitchell ET, Lewis R, Irvine N, McLay JS, McEwen J, et al. Comparison of once daily atenolol, nitrendipine and their combination in mild to moderate essential hypertension. British Journal of Clinical Pharmacology 1990;29(4):455‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 1983 {published data only}
- Myers MG, Champlain J. Effects of atenolol and hydrochlorothiazide on blood pressure and plasma catecholamines in essential hypertension. Hypertension 1983;5(4):591‐6. [DOI] [PubMed] [Google Scholar]
NEB‐305 {unpublished data only}
- Mylan Bertek Pharmaceuticals. A study of the safety and efficacy of nebivolol in hypertensive patients. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/021742s000TOC.cfm (accessed Oct 2011).
Okawa 1986 {published data only}
- Okawa KK. Dose response studies of bevantolol in hypertensive patients. Angiology 1986;37:233‐8. [DOI] [PubMed] [Google Scholar]
Papademetriou 2006 {published data only}
- Papademetriou V, Hainer JW, Sugg J, Munzer D, ATTACH Study Group. Factorial antihypertensive study of an extended‐release metoprolol and hydrochlorothiazide combination. American Journal of Hypertension 2006;19(12):1217‐25. [DOI] [PubMed] [Google Scholar]
Petrie 1976 {published data only}
- Petrie JC, Galloway DB, Jeffers TA, Millar HR, Smith MC, Wood RA, et al. Methyldopa and propranolol or practolol in moderate hypertension. British Medical Journal 1976;2(6028):137‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrie 1980 {published data only}
- Petrie JC, Jeffers TA, Robb OJ, Scott AK, Webster J. Atenolol, sustained‐release oxprenolol, and long‐acting propranolol in hypertension. British Medical Journal 1980;280(6231):1573‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosen 1994 {published data only}
- Rosen RC, Kostis JB, Jekelis A, Taska LS. Sexual sequelae of antihypertensive drugs: treatment effects on self‐report and physiological measures in middle‐aged male hypertensives. Archives of Sexual Behavior 1994;23(2):135‐52. [DOI] [PubMed] [Google Scholar]
Saunders 2007 {published data only}
- Saunders E, Smith WB, DeSalvo KB, Sullivan WA. The efficacy and tolerability of nebivolol in hypertensive African American patients. Journal of Clinical Hypertension 2007;9(11):866‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seedat 1980 {published data only}
- Seedat YK. Trial of atenolol and chlorthalidone for hypertension in black South Africans. British Medical Journal 1980;281(6250):1241‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stornello 1991 {published data only}
- Stornello M, Valvo EV, Scapellato L. Comparative effects of enalapril, atenolol and chlorthalidone on blood pressure and kidney function of diabetic patients affected by arterial hypertension and persistent proteinuria. Nephron 1991;58(1):52‐7. [DOI] [PubMed] [Google Scholar]
Stumpe 1985 {published data only}
- Stumpe K, Kolloch R, Mathieu M, Capone P. A comparison of celiprolol and atenolol in the treatment of hypertension: a placebo controlled double blind study. British Journal of Clinical Practice 1985;Supplement. 40:73‐5. [PubMed] [Google Scholar]
Tonkin 1990 {published data only}
- Tonkin AL, Wing LM, Russell AE, West MJ, Bune AJ, Morris MJ, et al. Diltiazem and atenolol in essential hypertension: additivity of effects on blood pressure and cardiac conduction with combination therapy. Journal of Hypertension 1990;8(11):1015‐9. [DOI] [PubMed] [Google Scholar]
Tseng 1993 {published data only}
- Tseng C‐D, Chiang F‐T, Hsu K‐L, Tseng Y‐Z, Hu W‐H, Chen J‐S, et al. Short‐term efficacy and safety of bisoprolol in treatment of patients with mild‐to‐moderate essential hypertension ‐ a two‐center, double‐blind study in Taiwan. Acta Cardiologica Sinica 1993;9(3):155‐60. [Google Scholar]
Van Bortel 1993 {published data only}
- Bortel LM, Breed JG, Joosten J, Kragten JA, Lustermans FA, Mooij JM, et al. Nebivolol in hypertension: a double‐blind placebo‐controlled multicenter study assessing its antihypertensive efficacy and impact on quality of life. Journal of Cardiovascular Pharmacology 1993;21(6):856‐62. [PubMed] [Google Scholar]
Vanhees 1991 {published data only}
- Vanhees L, Fagard R, Lijnen P, Amery A. Effect of antihypertensive medication on endurance exercise capacity in hypertensive sportsmen. Journal of Hypertension 1991;9(11):1063‐8. [DOI] [PubMed] [Google Scholar]
Van Herwaarden 1977 {published data only}
- Herwaarden CL, Fennis JF, Binkhorst RA, Van't Laar A. Haemodynamic effects of adrenaline during treatment of hypertensive patients with propranolol and metoprolol. European Journal of Clinical Pharmacology 1977;12(6):397‐402. [DOI] [PubMed] [Google Scholar]
Van Merode 1989 {published data only}
- Merode T, Bortel LM, Smeets FA, Mooij JM, Bohm RO, Rahn KH, et al. Verapamil and nebivolol improve carotid artery distensibility in hypertensive patients. Journal of Hypertension ‐ Supplement 1989;7(6):S262‐3. [DOI] [PubMed] [Google Scholar]
Van Nueten 1997 {published data only}
- Nueten L, Dupont AG, Vertommen C, Goyvaerts H, Robertson JI. A dose‐response trial of nebivolol in essential hypertension. Journal of Human Hypertension 1997;11(2):139‐44. [DOI] [PubMed] [Google Scholar]
Van Nueten 1998 {published data only}
- Nueten L, Taylor FR, Robertson JI. Nebivolol vs atenolol and placebo in essential hypertension: a double‐blind randomised trial. Journal of Human Hypertension 1998;12(2):135‐40. [DOI] [PubMed] [Google Scholar]
Verdecchia 1983 {published data only}
- Verdecchia P, Brignole M, Delfino G, Queirolo C, Marchi G, Bertulla A, et al. Systolic time intervals as possible predictors of pressure response to sustained beta‐adrenergic blockade in arterial hypertension. A within‐patient, placebo‐controlled study. Hypertension 1983;5(1):140‐6. [DOI] [PubMed] [Google Scholar]
Verdecchia 1988 {published data only}
- Verdecchia P, Gatteschi C, Benemio G, Boldrini F, Guerrieri M, Porcellati C, et al. Duration of the antihypertensive action of atenolol, enalapril and placebo: a randomized within‐patient study using ambulatory blood pressure monitoring. International Journal of Clinical Pharmacology, Therapy, & Toxicology 1988;26(11):570‐4. [PubMed] [Google Scholar]
Weiss 2007 {published data only}
- (2007). Erratum. The Journal of Clinical Hypertension 2007;9:806. [DOI: 10.1111/j.1751-7176.2007.tb00010.x] [DOI] [Google Scholar]
- Weiss RJ, Weber MA, Carr AA, Sullivan WA. A randomized, double‐blind, placebo‐controlled parallel‐group study to assess the efficacy and safety of nebivolol, a novel beta‐blocker, in patients with mild to moderate hypertension. Journal of Clinical Hypertension 2007;9(9):667‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1992 {published data only}
- William RL, Goyle KK, Herman TS, Rofman BA, Ruoff GE, Hogan LB. Dose‐dependent effect of betaxolol in hypertension: A double‐blind, multicenter study. Journal of Clinical Pharmacology 1992;32:360‐7. [DOI] [PubMed] [Google Scholar]
Wing 1988 {published data only}
- Wing LM, Chalmers JP, West MJ, Russell AE, Morris MJ, Cain MD, et al. Enalapril and atenolol in essential hypertension: attenuation of hypotensive effects in combination. Clinical & Experimental Hypertension ‐ Part A, Theory & Practice 1988;10(1):119‐33. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Wald 2008 {published data only}
- Wald DS, Law M, Mills S, Bestwick JP, Morris JK, Wald NJ. A 16‐week, randomized, double‐blind, placebo‐controlled, crossover trial to quantify the combined effect of an angiotensin‐converting enzyme inhibitor and a beta‐blocker on blood pressure reduction. Clinical Therapeutics 2008;30:2030‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Chen 2010
- Chen JMH, Heran BS, Perez MI, Wright JM. Blood pressure lowering efficacy of beta‐blockers as second‐line therapy for primary hypertension. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007185.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2010b
- Chen N, Zhou M, Yang M, Guo J, Zhu C, Yang J, et al. Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD003654.pub4] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
eCPS
- Canadian Pharmacist Association. https://www.e‐therapeutics.ca/cps.showMonograph.action Accessed October 2012.
FDA
- FDA Online Label Repository. http://labels.fda.gov/ Accessed May 2013.
Fitzgerald 1991
- Fitzgerald JD. The applied pharmacology of beta‐adrenoceptor antagonists (beta blockers) in relation to clinical outcomes. Cardiovascular drugs and therapy 1991;5:561‐576. [DOI] [PubMed] [Google Scholar]
Heran 2008a
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2008b
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003822.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kamp 2000
- Kamp TJ, Hell JW. Regulation of Cardiac L‐Type Calcium Channels by Protein Kinase A and Protein Kinase C. Circulation Research. 2000;87:1095‐102. [DOI] [PubMed] [Google Scholar]
Lancaster 1988
- Lancaster SG, Sorkin EM. Bisoprolol: a preliminary review of its pharmacodynamic and pharmacokinetics properties and therapeutics efficacy in hypertension and angina pectoris. Drugs 1988;36:256‐85. [DOI] [PubMed] [Google Scholar]
Law 2005
- Law M, Morris JK, Jordan R, Wald N. Headaches and the treatment of blood pressure results from a meta‐analysis of 94 randomized placebo‐controlled trials with 24 000 participants. Circulation 2005;112:2301‐2306. [DOI] [PubMed] [Google Scholar]
Magee 2003
- Magee LA, Duley L. Oral beta‐blockers for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002863] [DOI] [PubMed] [Google Scholar]
Musini 2008
- Musini VM, Fortin PM, Bassett K, Wright JM. Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007066.pub2] [DOI] [PubMed] [Google Scholar]
Musini 2014
- Musini VM, Nazer M, Bassett K, Wright JM. Blood pressure‐lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD003824.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ravid 1985
- Ravid M, Lang R, Jutrin I. The relative antihypertensive potency of propranolol, oxpranolol, atenolol and metoprolol given once daily. Archive Internal Medicine 1985;145:1321‐3. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Veverka 2006
- Veverka A, Nuzum DS, Jolly JL. Nebivolol: A third generation beta adrenergic blocker. Annals of Pharmacotherapy 2006 Jul‐Aug;40(7‐8):1353‐60. [PUBMED: 16822893] [DOI] [PubMed] [Google Scholar]
Westfall 2011
- Westfall TC, Westfall DP. Ch. 8 Neurotransmission: The autonomic and somatic motor nervous systems. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12. The McGraw‐Hill Companies, Inc , 2011. [Google Scholar]
Wiysonge 2012
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta‐blockers for hypertension. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD002003.pub4] [DOI] [PubMed] [Google Scholar]
Wong 2014a
- Wong GWK, Wright JM. Blood pressure lowering efficacy of nonselective beta‐blockers for primary hypertension. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD007452.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2014b
- Wong GWK, Boyda HN, Wright JM. Blood pressure lowering efficacy of partial agonist beta blocker monotherapy for primary hypertension. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD007450.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2015
- Wong GWK, Laugerotte A, Wright JM. Blood pressure lowering efficacy of dual alpha and beta blockers for primary hypertension. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD007449.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2000
- Wright JM. Choosing a first‐line drug in the management of elevated blood pressure: What is the evidence? 2: β‐Blockers. Canadian Medical Association Journal 2000;163(2):188‐192. [PMC free article] [PubMed] [Google Scholar]
Wright 2009
- Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001841.pub2] [DOI] [PubMed] [Google Scholar]
Xue 2015
- Xue H, Lu Z, Tang WL, Pang LW, Wang GM, Wong GWK, Wright JM. First‐line drugs inhibiting the renin angiotensin system versus other first‐line antihypertensive drug classes for hypertension. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD008170.pub2] [DOI] [PubMed] [Google Scholar]


