Abstract
Background
Approximately 600 million children of preschool and school age are anaemic worldwide. It is estimated that at least half of the cases are due to iron deficiency. Point‐of‐use fortification of foods with micronutrient powders (MNP) has been proposed as a feasible intervention to prevent and treat anaemia. It refers to the addition of iron alone or in combination with other vitamins and minerals in powder form, to energy‐containing foods (excluding beverages) at home or in any other place where meals are to be consumed. MNPs can be added to foods either during or after cooking or immediately before consumption without the explicit purpose of improving the flavour or colour.
Objectives
To assess the effects of point‐of‐use fortification of foods with iron‐containing MNP alone, or in combination with other vitamins and minerals on nutrition, health and development among children at preschool (24 to 59 months) and school (five to 12 years) age, compared with no intervention, a placebo or iron‐containing supplements.
Search methods
In December 2016, we searched the following databases: CENTRAL, MEDLINE, Embase, BIOSIS, Science Citation Index, Social Science Citation Index, CINAHL, LILACS, IBECS, Popline and SciELO. We also searched two trials registers in April 2017, and contacted relevant organisations to identify ongoing and unpublished trials.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs trials with either individual or cluster randomisation. Participants were children aged between 24 months and 12 years at the time of intervention. For trials with children outside this age range, we included studies where we were able to disaggregate the data for children aged 24 months to 12 years, or when more than half of the participants were within the requisite age range. We included trials with apparently healthy children; however, we included studies carried out in settings where anaemia and iron deficiency are prevalent, and thus participants may have had these conditions at baseline.
Data collection and analysis
Two review authors independently assessed the eligibility of trials against the inclusion criteria, extracted data from included trials, assessed the risk of bias of the included trials and graded the quality of the evidence.
Main results
We included 13 studies involving 5810 participants from Latin America, Africa and Asia. We excluded 38 studies and identified six ongoing/unpublished trials. All trials compared the provision of MNP for point‐of‐use fortification with no intervention or placebo. No trials compared the effects of MNP versus iron‐containing supplements (as drops, tablets or syrup).
The sample sizes in the included trials ranged from 90 to 2193 participants. Six trials included participants younger than 59 months of age only, four included only children aged 60 months or older, and three trials included children both younger and older than 59 months of age.
MNPs contained from two to 18 vitamins and minerals. The iron doses varied from 2.5 mg to 30 mg of elemental iron. Four trials reported giving 10 mg of elemental iron as sodium iron ethylenediaminetetraacetic acid (NaFeEDTA), chelated ferrous sulphate or microencapsulated ferrous fumarate. Three trials gave 12.5 mg of elemental iron as microencapsulated ferrous fumarate. Three trials gave 2.5 mg or 2.86 mg of elemental iron as NaFeEDTA. One trial gave 30 mg and one trial provided 14 mg of elemental iron as microencapsulated ferrous fumarate, while one trial gave 28 mg of iron as ferrous glycine phosphate.
In comparison with receiving no intervention or a placebo, children receiving iron‐containing MNP for point‐of‐use fortification of foods had lower risk of anaemia prevalence ratio (PR) 0.66, 95% confidence interval (CI) 0.49 to 0.88, 10 trials, 2448 children; moderate‐quality evidence) and iron deficiency (PR 0.35, 95% CI 0.27 to 0.47, 5 trials, 1364 children; moderate‐quality evidence) and had higher haemoglobin (mean difference (MD) 3.37 g/L, 95% CI 0.94 to 5.80, 11 trials, 2746 children; low‐quality evidence).
Only one trial with 115 children reported on all‐cause mortality (zero cases; low‐quality evidence). There was no effect on diarrhoea (risk ratio (RR) 0.97, 95% CI 0.53 to 1.78, 2 trials, 366 children; low‐quality evidence).
Authors' conclusions
Point‐of‐use fortification of foods with MNPs containing iron reduces anaemia and iron deficiency in preschool‐ and school‐age children. However, information on mortality, morbidity, developmental outcomes and adverse effects is still scarce.
Plain language summary
Powdered vitamins and minerals added to foods at the point‐of‐use reduces anaemia and iron deficiency in preschool‐ and school‐age children
Background to the question
Approximately one billion people worldwide are deficient in at least one vitamin or mineral (also known of micronutrients). Iron, vitamin A, zinc and iodine deficiencies are very frequent among children of preschool (aged 24 months to less than 5 years) and school age (5 to 12 years of age), limiting their health and daily physical performance. Anaemia, the condition in which red blood cells have limited capacity to carry oxygen, frequently results after prolonged iron deficiency.
Point‐of‐use fortification with powdered vitamins and minerals has been proposed as a public health intervention to reduce micronutrient deficiencies in children. In this process, a powdered premix containing iron, and possibly other vitamins and minerals, is added to foods either during or after cooking, or immediately before consumption to improve their nutritious value but not their flavour or colour. In some cases, point‐of‐use fortification is also known as home fortification.
Review question
What are the effects of point‐of‐use fortification of foods with iron‐containing micronutrient powders (MNP) alone, or in combination with other vitamins and minerals, on nutrition, health and development among children of preschool and school age (24 months to 12 years of age) compared with no intervention, a placebo (dummy pill) or regular iron‐containing supplements (as drops, tablets or syrup)?
Study characteristics
This review included 13 trials with 5810 participants from Latin America, Africa and Asia. All trials compared the provision of MNP for point‐of‐use fortification with no intervention or placebo. Six trials included participants younger than 59 months of age only, four included only children aged 60 months of age or older, and three trials included children both younger and older than 59 months of age. MNPs contained from two to 18 vitamins and minerals. We searched existing clinical trials in December 2016 and ongoing trials in April 2017. We also contacted relevant institutions for additional information upon publication of the protocol and in April 2017.
Key results
The review found that children receiving iron‐containing MNP for point‐of‐use fortification of foods were at significantly lower risk of having anaemia and iron deficiency and had higher haemoglobin concentrations. We did not find any positive or negative effect on diarrhoea or mortality, but the data on these two outcomes were very limited.
Quality of the evidence
We rated the overall quality of the evidence for the provision of multiple MNP versus no intervention or placebo as moderate for anaemia, iron deficiency and adverse effects. We judged the evidence to be of low quality for haemoglobin, mortality and diarrhoea, and to be very low‐quality for ferritin. In general, the most common risk of bias in the studies was the lack of blinding for participants, personnel and outcome assessors.
Authors' conclusions
Point‐of‐use fortification of foods with MNPs containing iron reduces anaemia and iron deficiency in preschool‐ and school‐age children and seems feasible for public health purposes. However, future research should aim to increase the body of evidence on mortality, morbidity, developmental outcomes and adverse effects. Due to the lack of trials, we were unable to determine at this time if this intervention has comparable effects to those observed with iron supplements (provided as drops, tablets or syrup).
Summary of findings
Summary of findings for the main comparison. Point‐of‐use fortification of foods with micronutrients powders (MNP) compared to no intervention or placebo in preschool and school‐age children.
| Point‐of‐use fortification of foods with MNP compared to no intervention or placebo in preschool and school‐age children | ||||||
|
Patient or population: preschool and school‐age children Setting: all settings Intervention: point‐of‐use fortification of foods with MNP Comparison: no intervention or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no intervention or placebo | Risk with point‐of‐use fortification of foods with MNP | |||||
| Anaemia (defined as haemoglobin < 110 g/L for children aged 24‐59 months and < 115 g/L for children aged 5‐11.9 years, adjusted by altitude where appropriate)* | Study population | RR 0.66 (0.49 to 0.88) | 2448 (10 RCTs) | ⊕⊕⊕⊝ Moderatea | Included studies: Inayati 2012 (C); Kemmer 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Ogunlade 2011; Osei 2008 (C); Troesch 2011b; Varma 2007 (C); Vinodkumar 2009 (C). | |
| 375 per 1000 | 247 per 1000 (184 to 330) | |||||
| Haemoglobin | The mean haemoglobin score in control groups ranged from 103.50 g/L to 128.00 g/L | The mean haemoglobin score in intervention groups was, on average, 3.37 g/L higher (0.94 g/L higher to 5.80 g/L higher) | ‐ | 2746 (11 RCTs) | ⊕⊕⊝⊝ Lowb | Included studies: Inayati 2012 (C); Kemmer 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Ogunlade 2011; Osei 2008 (C); Sharieff 2006 (C); Varma 2007 (C); Vinodkumar 2006 (C); Vinodkumar 2009 (C). |
| Iron deficiency (defined by using ferritin concentrations less than 15 µg/L) | Study population | RR 0.35 (0.27 to 0.47) | 1364 (5 RCTs) | ⊕⊕⊕⊝ Moderatec | Included studies: Macharia‐Mutie 2012; Osei 2008 (C); Sharieff 2006 (C); Troesch 2011b; Varma 2007 (C). | |
| 220 per 1000 | 77 per 1000 (59 to 104) | |||||
| Ferritin | 0 | The standardised mean ferritin score in intervention groups was, on average, 0.42 μg/L higher (4.36 μg/L lower to 5.19 μg/L higher) | ‐ | 1066 (3 RCTs) | ⊕⊝⊝⊝ Very lowd,e | Included studies: Osei 2008 (C); Sharieff 2006 (C); Varma 2007 (C). |
| All‐cause mortality (number of deaths during trial) | Study population | Not estimable | 115 (1 RCT) | ⊕⊕⊝⊝ Lowf | Included study: Inayati 2012 (C). | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Diarrhoea (≥ 3 liquid stools per day) | Study population | RR 0.97 (0.53 to 1.78) | 366 (2 RCTs) | ⊕⊕⊝⊝ Lowg | Included studies: Inayati 2012 (C); Osei 2008 (C). | |
| 96 per 1000 | 93 per 1000 (51 to 170) | |||||
| Adverse effects (any, as defined by trialists) | Study population | RR 1.09 (0.16 to 7.42) | 90 (1 RCT) | ⊕⊕⊕⊝ Moderate | Included study: Orozco 2015 (C). | |
| 43 per 1000 | 46 per 1000 (7 to 316) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; MNP: micronutrient powder; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aMost studies had no blinding. High heterogeneity (72%) with most studies showing a positive effect of MNP. bMost studies had no blinding. High heterogeneity (93%) with most studies showing a positive effect of MNP. cMost studies had no blinding. No heterogeneity with most studies showing a positive effect of MNP. dAll the studies had no or unclear blinding. e100% heterogeneity with most inconsistency in direction of effect. fOnly one low‐risk trial reported all‐cause mortality. gTwo low‐risk trials reported diarrhoea. No heterogeneity with both studies showing no difference between intervention and comparison group.
Background
Description of the condition
Vitamin and mineral deficiencies are highly prevalent among children of preschool (usually 24 to 59 months of age) and school‐age (five to 12 years of age), limiting their health and daily physical performance. In addition to anaemia, frequently reported micronutrient deficiencies in these age groups are those of iron, vitamin A, zinc and iodine.
Anaemia is a condition characterised by a reduction in the body's oxygen‐carrying capacity. It is estimated that over 1.6 billion people, or a quarter of the world's population, are anaemic. The prevalence of anaemia globally is 43% (range 38% to 47%) in children aged six to 59 months (Stevens 2013). Most anaemia occurs in low‐ and middle‐income countries (WHO 2015b). Causes of anaemia include iron, folate, vitamin B12 and vitamin A deficiencies; chronic inflammation; parasitic infestations and inherited blood disorders (Jimenez 2010; WHO 2001). The proportion of all anaemia amenable to iron is estimated to be 42% in children (WHO 2015b). The proportions of severe anaemia amenable to iron is estimated to be 50% for children (Stevens 2013). It is assumed to be higher (about 60%) in malaria‐free areas (Rastogi 2002; Stoltzfus 2004; WHO 2009). Iron deficiency can be caused by chronic poor dietary iron intake (in quantity and quality), together with increased iron requirements resulting from growth and from losses due to intestinal parasitic infestations and menstruation in postmenarchal girls (WHO 2001). Anaemia in children is diagnosed when the haemoglobin (Hb) concentration in the blood is below a predefined cut‐off value, which varies with age and the residential elevation (WHO 2011a). Iron deficiency anaemia is diagnosed by the combined presence of anaemia and iron deficiency, measured by ferritin or any other biomarker of iron status such as serum transferrin receptors or zinc protoporphyrin (WHO 2011b).
Iron deficiency anaemia in children of preschool and school‐age is associated with considerable morbidity. This condition appears to be associated with potentially irreversible impairment of cognitive development in preschool‐age children and with reduced learning and educational performance in school‐age children (Lozoff 2007). Iron deficiency has been estimated to contribute to 0.2% of deaths in children under five years of age, and every year approximately 2.2 million years are lost due to iron‐induced disability worldwide (measured in disability‐adjusted life years (DALYs), a measure of overall disease burden, expressed as the number of years lost due to ill‐health, disability or early death) (Black 2008; Stoltzfus 2004; WHO 2009).
Vitamin A is an essential nutrient that comprises a group of unsaturated organic compounds such as retinol, retinal and retinoic acid. Provitamin A carotenoids, which are produced in plants, are also a primary dietary source of vitamin A (Tanumihardjo 2016). When the intake, absorption or utilisation of vitamin A or provitamin A carotenoids is inadequate, vitamin A deficiency can occur. Vitamin A deficiency disorders include xerophthalmia and increased risk of death from infectious diseases, especially among preschool children (Tanumihardjo 2016). Vitamin A deficiency is a risk factor for blindness and for mortality from measles and diarrhoea in children (Stevens 2015). In 2013, the prevalence of vitamin A deficiency was 29% in children, mostly from in low‐ and middle‐income countries (Stevens 2015).
Zinc deficiency affects overall body metabolism and can be associated with poor growth, stunting and wasting; increased infections and the appearance of skin lesions. In countries where zinc intakes are inadequate, zinc deficiency may present as poor physical growth, impaired immune competence, reproductive dysfunction and sub optimal neurobehavioral development. This can increase risk of child morbidity and mortality, and preterm births (Brown 2009; King 2016). Some authors have estimated that zinc deficiency is responsible for about 4% of child mortality (Black 2008), and that supplementing children with this nutrient may help reduce deaths related to diarrhoea and pneumonia (Yakoob 2011). It is estimated that 29.8% (95% CI 29.4% to 30.1%) of school‐age children (approximately 241 million) have insufficient iodine intakes.
Iodine deficiency affects more than one‐third of school‐age children worldwide and results in developmental delays and other health problems (Andersson 2012). Vitamin D deficiency and folate insufficiency may also be a concern during childhood.
Folic acid, another form of which is known as folate, is one of the B vitamins. Inadequate folate intake is the main cause of folate deficiency. The use of antifolate drugs used in treatment and prophylaxis for Pneumocystis jirovecii pneumonia, malaria and toxoplasmosis selectively inhibit folate's actions in microbial organisms such as bacteria, protozoa and fungi. In some low‐ and middle‐income countries, malabsorptive conditions, such as tropical sprue, can also cause folate deficiency. Although essential throughout life, folate is particularly critical during early stages of human development. Folate deficiency may result in megaloblastic anaemia in which low numbers of large red blood cells occur (Bailey 2015). Among other micronutrients, current micronutrient powders (MNP) formulations generally include folic acid and there is ongoing debate on risks and benefits of the provision of supplemental folic acid through point‐of‐use fortification, especially in children living in sub‐Saharan Africa where malaria is endemic (Kupka 2015).
Vitamin D has a key role in bone metabolism (Winzenberg 2011), while adequate folate and folic acid intake is particularly important for pubescent girls who are capable of reproduction, as poor maternal folate status around the time of conception increases the risk of neural tube and other defects at birth (Mulinare 1988). Unfortunately, to date, there are insufficient data to estimate the global magnitude of inadequate folate or vitamin D status among any populations, including children.
In low‐income countries, some nutritional risk factors increase the incidence or severity of infectious diseases and contribute to a high number of deaths and loss of healthy years. Micronutrient deficiencies (iron, vitamin A and zinc), in combination with childhood underweight and sub optimal breastfeeding, cause 7% of deaths and 10% of total disease burden (WHO 2009). In 2010, globally, an estimated 27% (171 million) of children younger than five years of age were stunted and 16% (104 million) were underweight. Africa and Asia have more severe burdens of undernutrition, but the problem persists in some Latin American countries (Lutter 2011). Underweight and undernutrition particularly increase child death and disability. Due to overlapping effects, these risk factors are responsible for an estimated 3.9 million deaths (35% of total deaths) and 33% of total DALYs in children younger than five years of age. Their combined contribution to specific causes of death is highest for diarrhoeal diseases (73%) and close to 50% for pneumonia, measles and severe neonatal infections (WHO 2009).
Description of the intervention
Public health strategies to address micronutrient malnutrition include prevention of parasitic infestations and other infections; dietary diversification to improve the consumption of foods with highly absorbable vitamins and minerals; industrial fortification of staple foods; provision of supplementary foods; and provision of supplements in the form of liquids, pills and tablets (Bhutta 2008), with the latter being a widespread intervention.
There are few essential nutrition actions for preschool‐ and school‐age children (WHO 2013). The World Health Organization (WHO) recommends a supplemental provision of 2 mg of elemental iron per kilogram bodyweight per day for three months in children less than six years of age born at term. Children of school‐age and older should receive 30 mg of iron and 250 μg (0.25 mg) of folic acid daily for three months, particularly in populations where anaemia prevalence is greater than 40% (WHO 2001). The intermittent use of iron supplements is also recommended as a public health strategy for these age groups in settings where anaemia prevalence is higher than 20% (WHO 2011c). Both supplementation regimens have proven to be effective in reducing the risk of having anaemia and iron deficiency (De‐Regil 2011a; Gera 2007). Though the current recommendations only include iron alone or with folic acid, it has been suggested that administration of additional micronutrients may prevent or reverse anaemia derived from other nutritional deficiencies (Bhutta 2008), and also have positive effects on length or height and weight, serum zinc, serum retinol and motor development (Allen 2009). However, the long regimen duration, bad taste of liquid iron drops and syrups, adverse effects associated with daily iron supplementation (e.g. gastrointestinal discomfort, constipation and teeth staining with drops or syrups) and the limited implementation of large‐scale, intermittent iron supplementation programmes have triggered the development of new approaches to provide iron and other nutrients.
Point‐of‐use fortification of foods with MNP has been proposed as an alternative to oral supplements and industrially fortified foods to provide micronutrients to different age groups (Zlotkin 2001; Zlotkin 2005) and is currently recommended by the World Health Organization for infants, young children and children aged 2–12 years living in populations where anaemia is a public health problem (WHO 2016). It refers to the addition of vitamins and minerals in powder form to energy‐containing foods at home or in any other place where meals are to be consumed, such as schools, nurseries and refugee camps. MNPs can be added to foods either during or after cooking, or immediately before consumption without the explicit purpose of improving the flavour or colour. In some cases, point‐of‐use fortification is also known as home fortification.
Point‐of‐use fortification with MNP can be described as a hybrid intervention between industrial fortification of staple foods or condiments and targeted vitamin and mineral supplementation. The uniqueness of this intervention results from the mixture of advantages and disadvantages inherited from both parent interventions. Point‐of‐use fortification is similar to industrial fortification of staple foods because the vitamins and minerals are added to meals or condiments regularly consumed and usually does not require additional changes to dietary intake behaviours. Point‐of‐use fortification entails the fortification of foods immediately before consumption at home or at another point of use such as schools or child‐care facilities, and there is no long‐term interaction between micronutrients and the food that can diminish their shelf life. In industrial fortification, conversely, micronutrients are added to staple foods or condiments during industrial processing and are more prone to the potential undesirable chemical interactions over time that affect the food sensory properties as well as the bioavailability of some micronutrients (WHO/FAO 2006).
Like micronutrient supplementation, point‐of‐use fortification with MNP is targeted to specific populations so that the number and quantity of micronutrients can be tailored to meet the target groups' needs without increasing the risk of overload among other population groups. Also typical of vitamin and mineral supplementation, point‐of‐use fortification allows for flexibility in the provision regimen (e.g. daily, intermittently) (Hyder 2007), and can be adapted according to the selected delivery channel (e.g. health or school systems or social protection programmes) or context.
Both micronutrient supplementation and point‐of‐use fortification of foods with MNP require active participation from the target population to achieve and sustain high coverage and regular and appropriate use, with the possible exception being the use of MNP in institutional settings, where the powder is added to the meals prior to serving them to the participants, and a lesser degree of 'active participation' may potentially be expected of participants in some settings. However, in comparison to supplementation, the addition of MNP to foods or meals may result in a higher acceptance and use among children and care‐takers as a result of the lower number of adverse effects (Zlotkin 2005), and tastelessness of the product when prepared correctly. It is still unclear whether the absorption of MNP mimics that of supplements or that of industrial fortification of staple foods.
Point‐of‐use fortification of foods with MNP containing at least iron, vitamin A and zinc is recommended to improve iron status and reduce anaemia among infants and children aged six to 23 months of age (WHO 2016).
How the intervention might work
MNPs were initially conceived as a way to deliver a novel iron compound, encapsulated ferrous fumarate, an iron salt covered by a thin lipid layer aimed at preventing the interaction of iron with foods. The encapsulation minimises changes caused by iron to the taste or colour in the food to which it is added (Liyanage 2002). Other iron compounds have also been tested. Micronised ferric pyrophosphate has produced a similar haematological response in children aged six to 23 months in comparison to encapsulated ferrous fumarate (Christofides 2006; Hirve 2007). More recently, sodium iron ethylenediaminetetraacetic acid (NaFeEDTA) has been proposed as a more efficacious fortificant that, given in a low dose, could produce similar effects on Hb as those observed with ferrous sulphate among school‐age children, particularly when added to cereal‐based foods that are rich in inhibitors of iron absorption (Troesch 2011a). Independently of the source, the iron is frequently accompanied with other micronutrients such as zinc, vitamin A, vitamin C, vitamin D or folic acid, and in some cases, MNP formulations may include up to 15 vitamins and minerals. These formulations are currently developed by various manufacturers (De Pee 2008; De Pee 2009). From the packaging perspective, MNP were initially delivered in single‐dose sachets, which are lightweight and relatively simple to store and transport, and allow easier dosage control (De Pee 2008; SGHI 2008); although the disposal of non‐degradable sachets has raised some environmental concerns. Currently, the package of the MNP has been broadened to the use of bulk, multi‐serving packages from which powders are added over the meals by using measuring spoons.
The use of MNP by infants and young children aged six to 23 months has been reported to reduce the risk of anaemia and iron deficiency in settings where anaemia prevalence is higher than 20%; an effect apparently similar to that achieved by oral iron and folic acid supplements (De‐Regil 2011b; Dewey 2008). Although most of the trials have examined the provision of MNP on a daily basis, other trials suggest that providing this intervention in a flexible or intermittent regimen, and hence a lower overall monthly dose, produces the similar haematological response as daily use of MNP (Hyder 2007; Ip 2009; Sharieff 2006). The intermittent provision of iron was proposed in the 1990s as a feasible public health strategy to supplement children's and women's diets and to reduce anaemia, as it is supposed to maximise absorption by provision of iron in synchrony with the turnover of the mucosal cells (Beaton 1999; Berger 1997; Viteri 1997).
An important consideration when providing supplemental iron to children is the presence of malaria because the malaria parasite requires iron for growth, mainly circulating‐free iron (Okebe 2011). Approximately 40% of the world population is exposed to the parasite, and it is endemic in over 100 countries (WHO 2009; WHO 2010a). There were an estimated 839,000 malaria deaths worldwide in 2015 (uncertainty interval, 653,000 to 1.1 million). Of the estimated deaths, most occur in the WHO African Region (88%), followed by the WHO South‐East Asia Region (10%) and the WHO Eastern Mediterranean Region (2%) (WHO 2015a). Nonetheless, large reductions in the number of malaria cases and deaths have been documented between 2000 and 2015. Of all the complications associated with malaria, severe anaemia is the most common and causes the highest number of malaria‐related deaths. Although the mechanisms by which additional iron can benefit the parasite are far from clear (Prentice 2007), it has been hypothesised that the provision of iron along with foods or low doses of iron, either as encapsulated ferrous fumarate or NaFeEDTA, might help to prevent anaemia at the time of infection if it reduces the quantity of free iron (non‐transferrin‐bound iron) available to the parasite (Hurrell 2010).
In addition to malaria, another safety concern related to the use of MNPs is their possible effect on diarrhoea. One systematic review reported a slight, significant increase in the incidence of diarrhoea with MNP intake, with no significant increases in recurrent diarrhoea or upper respiratory infection (Salam 2013). Some trials have reported an increase in the number of diarrhoeal episodes after initiating the intervention, followed by a decrease in the frequency of liquid stools after few days (De‐Regil 2011b). As a preventive measure, some organisations have advocated the widespread distribution of information on the prompt detection and treatment of diarrhoea (WFP/DSM 2010). Despite these possible caveats, the use of MNP has been considered by some scientists as one of the most cost‐effective strategies to prevent vitamin and mineral malnutrition (Horton 2010).
Why it is important to do this review
The use of MNP for home or point‐of‐use fortification of complementary foods among infants and young children aged six to 23 months of age has been shown to be effective in reducing anaemia and iron deficiency in young children (De‐Regil 2011b). The initial success of this intervention has encouraged its use in other vulnerable populations, such as children of preschool (usually 24 months to less than 5 years of age) and school‐age (usually five to 12 years of age), as the distribution of MNP can potentially build on and enhance existing school‐feeding programmes in addition to other existing community‐based platforms. In 2012 to 2013, the World Food Programme (WFP) was in the process of planning or implementing MNP programmes to reach approximately 1.5 million school‐age children by adding MNP to school lunches (Martini 2013). Overall, MNP interventions have been pilot‐tested in all regions of the world for various population groups, but most often for children aged six to 23 months or six to 59 months; in 2013, 61 MNP projects were being implemented in 43 countries, and there were 16 national‐scale MNP programmes (UNICEF 2014). Projects distributing MNP in 2013 reported that they projected to reach more than 14 million participants in 2014 (UNICEF 2014). Likewise, it is also important to assess any adverse effects and potential adverse effects on health with this intervention in this age group.
To date, there is no systematic assessment of the effects of MNP provision among preschool‐ and school‐aged children to inform policy‐making.
This review will complement the findings of other systematic reviews, which explored the effects of point‐of‐use fortification with multiple MNP in children younger than two years of age (De‐Regil 2011b) and among pregnant women (Suchdev 2015).
Objectives
To assess the effects of point‐of‐use fortification of foods with iron‐containing MNP alone, or in combination with other vitamins and minerals on nutrition, health and development among children at preschool (24 to 59 months) and school (five to 12 years) age, compared with no intervention, a placebo or iron‐containing supplements.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs with randomisation at either individual or cluster level. Quasi‐RCTs are trials that use systematic methods to allocate participants to treatment groups such as alternation, assignment based on date of birth or case record number (Lefebvre 2011). We found no RCTs that met our inclusion criteria (Criteria for considering studies for this review). We included no other types of evidence (e.g. cohort or case‐control studies) in this review, but we considered such evidence in the Discussion, where relevant.
Types of participants
We included trials aimed at children aged 24 months (two years) to 59 months (less than five years of age) and five to 12 years of age at the time of receiving the intervention with the MNP. We did not include trials specifically targeting hospitalised children with clinical conditions, HIV‐associated infections or enterally fed children.
We defined school age as that between five and 12 years of age. Although many children do not attend schools, these ages are compulsory school years in most settings, providing with it an entry point to address the nutritional needs of this age group (Commission on Ending Childhood Obesity 2016). We acknowledge the overlap between this age range and the one used for adolescent age, 10 to 19 years of age (WHO 2003), and made a pragmatic decision based on the most suitable delivery platform.
We included trials carried out in settings where anaemia and iron deficiency were prevalent; thus participants could be anaemic or not. We also included trials for which the results for children aged between 24 and less than five years of age and five to 12 years of age could be extracted separately, or in which more than half of the participants fulfilled this criterion (we performed a Sensitivity analysis if marginal decisions were made).
Types of interventions
Interventions involved the provision of MNP for point‐of‐use fortification given at any dose, frequency and duration. The comparison groups included no intervention, placebo or usual supplementation. Specifically, we assessed the evidence on the following comparisons.
Point‐of‐use fortification of foods with MNP versus no intervention or placebo.
Point‐of‐use fortification of foods with MNP versus iron‐only supplement.
Point‐of‐use fortification of foods with MNP versus iron and folic acid supplements.
Point‐of‐use fortification of foods with MNP versus same micronutrients in supplements.
We included interventions that combined MNP with co‐interventions, such as education, vitamin A supplementation programmes, zinc for the treatment of diarrhoea or other approaches, but only if the co‐interventions were the same in both the intervention and comparison groups. We excluded trials examining supplementary food‐based interventions (lipid‐based supplements, chewable tablets, fortified complementary foods and other industrially fortified foods).
Types of outcome measures
Primary outcomes
Anaemia (defined as Hb lower than 110 g/L for children aged 24 to 59 months and lower than 115 g/L for children aged five to 11.9 years, adjusted by altitude where appropriate).*
Hb (in grams per litre).*
Iron deficiency (defined by using ferritin concentrations less than 15 µg/L).*
Ferritin (in micrograms per litre).*
All‐cause mortality (number of deaths during the trial).*
Diarrhoea (three liquid stools or more per day).*
Adverse effects (any, as defined by trialists).
We considered outcomes marked by an asterisk (*) as critical for decision making by a panel of experts and included them in the 'Summary of findings' tables (Guyatt 2011; Peña‐Rosas 2012).
Secondary outcomes
Iron deficiency anaemia (defined by the presence of anaemia plus iron deficiency, diagnosed with an indicator of iron status as selected by trialists).
Cognitive development and school performance (as defined by trialists).
Motor development and physical capacity (as defined by trialists).
All‐cause morbidity (number of participants with at least one episode of any disease during the trial).
Acute respiratory infection (as defined by trialists; e.g. pneumonia, bronchiolitis or bronchitis).
Growth (height‐for‐age Z‐score (HAZ)).
Growth (weight‐for‐age Z‐score (WAZ)).
Growth (weight‐for‐height Z‐score (WHZ)).
Adherence (percentage of children who consumed more than 70% of the expected doses over the intervention period).
Red blood cell folate (in milligrams per decilitre).
Serum/plasma retinol (in millimoles per litre).
Serum/plasma zinc concentrations (in millimoles per litre).
We intended to group the outcomes by time points as follows, if these follow‐up data were reported for the above outcomes: immediately postintervention, one to six months postintervention, and seven to 12 months postintervention.
Search methods for identification of studies
Electronic searches
We searched the electronic databases and trial registers listed below in December 2016 and April 2017. MNP formulations were introduced after 2000, therefore, we limited the searches by publication year from 2000 onwards. There were no language limits.
Cochrane Central Register of Controlled Trials CRSO (CENTRAL; searched 7 December 2016).
MEDLINE Ovid (2000 to 6 December 2016).
Embase Ovid (2000 to 6 December 2016).
BIOSIS ISI (2000 to 6 December 2016).
Social Citation Index Web of Science (SCI; 2000 to 7 December 2016).
Social Science Citation Index Web of Science (SSCI; 2000 to 7 December 2016).
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 2000 to 7 December 2016).
LILACS (Latin American and Caribbean Health Science Information database; lilacs.bvsalud.org/en; 2000 to 15 December 2016).
IBECS (ibecs.isciii.es; 2000 to 15 December 2016).
POPLINE (www.popline.org; 2000 to 15 December 2016).
SciELO (Scientific Library Online; www.scielo.br; 2000 to 15 December 2016).
ClinicalTrials.gov (clinicaltrials.gov; searched 19 April 2017).
WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/en; searched 13 April 2017).
Searching other resources
We contacted authors and known experts for assistance in identifying ongoing studies or unpublished data on 21 June 2014 and again on 19 April 2017. We also contacted the regional offices of WHO; the WHO Departments of Nutrition for Health and Development, Division of Nutrition, Physical Activity, and Obesity of the Centers for Disease Control and Prevention (CDC); the United Nations Children's Fund (UNICEF); the WFP; Nutrition International (formerly Micronutrient Initiative); Helen Keller International (HKI); Home Fortification Technical Advisory Group (HF‐TAG); the Global Alliance for Improved Nutrition (GAIN); the US Agency for International Development; and Sight and Life.
Data collection and analysis
Selection of studies
Two review authors (LD‐R and MJ) independently screened all titles and abstracts retrieved by the electronic searches for eligibility. One review author (LD‐R) searched the additional sources. Each review author independently assessed two‐thirds of the full‐text reports for inclusion according to the above criteria (Criteria for considering studies for this review); we assessed each paper in duplicate, resolving any disagreements through discussion.
If trials were published only as abstracts, or study reports contained little information on methods, we attempted to contact the authors to obtain further details of study design and results.
We recorded our decisions in a study flow diagram (Moher 2009).
Data extraction and management
For eligible trials, two review authors (MJ and JP‐R) independently extracted data using a form designed for this review. The data collection form was adapted from a similar review in a different population group (De‐Regil 2011b), and piloted by MJ and JP‐R on two included studies, before finalised for use in this review. MJ extracted data from half of the trials and JP‐R extracted data from the other half. LD‐R extracted data from all the trials. LD‐R entered data into Review Manager 5 (RevMan 2014), and the review author who extracted the data in duplicate checked LD‐R's data entry for accuracy. We resolved any discrepancies through discussion and documented the process.
We completed the data collection form electronically and recorded information on the following.
Trial methods
Study design.
Unit and method of allocation.
Method of sequence generation.
Masking of participants, personnel and outcome assessors.
Participants
Location of the study.
Sample size.
Age.
Sex.
Socioeconomic status (as defined by trialists and where such information was available).
Baseline prevalence of anaemia.
Baseline prevalence of soil helminths.
Baseline malaria prevalence.
Inclusion and exclusion criteria.
Intervention
Dose.
Type of iron compound.
Provision of MNP regimen.
Duration of the intervention.
Cointervention.
Comparison group
No intervention.
Placebo.
Provision of iron supplements.
Outcomes
Primary and secondary outcomes outlined under Types of outcome measures.
Exclusion of participants after randomisation and proportion of losses at follow‐up.
We recorded both prespecified and non‐prespecified outcomes, although we did not use the latter to underpin the conclusions of the review.
When information regarding any of the trials was unclear, we attempted to contact authors of the original reports to provide further details. If there was insufficient information for us to be able to assess risk of bias, we categorised trials as awaiting assessment, until further information is published or made available to us.
Assessment of risk of bias in included studies
Two review authors (LD‐R and MJ) independently assessed the risk of bias of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Both review authors assigned each study a rating of low, high or unclear risk of bias, for the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other potential sources of bias; we also assessed the overall risk of bias. We resolved any disagreement by discussion or by involving a third review author (JP‐R).
Random sequence generation (checking for selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it produced comparable groups.
Low risk of bias: any truly random process; for example, random number table, computer random number generator.
High risk of bias: any non‐random process; for example, odd or even date of birth, hospital or clinic record number.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Allocation concealment (checking for possible selection bias)
We described the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment.
Low risk of bias: for example, telephone or central randomisation; consecutively numbered, sealed, opaque envelopes.
High risk of bias: open random allocation, unsealed or non‐opaque envelopes.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Blinding of participants and personnel (checking for possible performance bias)
We described all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received.
Low risk of bias: neither participants nor personnel giving the intervention were aware of the intervention.
High risk of bias: either participants or personnel were aware of the intervention.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Blinding of outcome assessment (checking for possible detection bias)
We described all measures used, if any, to blind outcome assessors from knowledge as to which intervention a participant received.
Low risk of bias: blinding of outcomes, which is unlikely to have been broken.
High risk of bias: for example, no blinding of outcome assessment where measurement is likely to be influenced by lack of blinding, or where blinding could have been broken.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed outcomes in each included study as follows.
Low risk of bias: either there were no missing outcome data or the missing outcome data were unlikely to bias the results based on the following considerations: study authors provided transparent documentation of participant flow throughout the study, the proportion of missing data was similar in the intervention and control groups, the reasons for missing data were provided and balanced across intervention and control groups, the reasons for missing data were not likely to bias the results (for example, moving house).
High risk of bias: missing outcome data were likely to bias the results. Trials also received this rating if an 'as‐treated (per protocol)' analysis was performed with substantial differences between the intervention received and that assigned at randomisation, or if potentially inappropriate methods for imputation had been used.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Selective reporting (checking for possible reporting bias)
We stated how the possibility of selective outcome reporting was examined and what was found.
Low risk of bias: where it was clear that all the study's prespecified outcomes and all expected outcomes of interest to the review were reported.
High risk of bias: where not all the study's prespecified outcomes were reported, one or more reported primary outcomes were not prespecified, outcomes of interest were reported incompletely and so could not be used, the study failed to include results of a key outcome that would have been expected to have been reported.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Other bias (checking for other potential sources of bias not covered by the domains above)
We assessed if the study was free of other potential bias as follows.
Low risk of bias: where there was similarity between the outcome measure at baseline, similarity between potential confounding variables at baseline, or adequate protection of study arms against contamination.
High risk of bias: where there was no similarity between outcome measure at baseline, similarity between potential confounding variables at baseline or adequate protection of study arms against contamination.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Overall risk of bias
We summarised the overall risk of bias at two levels: within trials (across domains) and across trials.
Overall risk of bias within trials
For the assessment within trials, we assessed the likely magnitude and direction of the bias in each of the 'Risk of bias' domains and whether we considered they were likely to impact on the findings. We considered trials at high risk of bias if they had poor or unclear allocation concealment and either inadequate blinding of both participants and personnel or high/imbalanced losses to follow‐up. We explored the impact of the level of bias through a Sensitivity analysis.
Overall risk of bias between trials
For the assessment across trials, we set out the main findings of the review in the 'Summary of findings' table, prepared using GRADEpro software (GRADEpro GDT 2015). We listed the primary outcomes for each comparison, with estimates of relative effects, along with the number of participants and trials contributing data for those outcomes. For each individual outcome, we assessed the quality of the evidence using the GRADE approach (Balshem 2011), which involved consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias, and resulted in one out of four levels of quality (high, moderate, low or very low). This assessment was limited only to the trials included in this review.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as prevalence ratio (PR) or risk ratio (RR) with 95% confidence intervals (95% CI).
Continuous data
For continuous data, we used the mean difference (MD) with 95% CI if outcomes were measured in the same way between trials.
We used the standardised mean difference (SMD) with 95% CI to combine trials that measured the same outcome but used different measurement methods.
Rates
For rates, if they represented events that could have occurred more than once per participant, we reported the rate difference using the methodologies described in Deeks 2011.
Unit of analysis issues
Cluster‐randomised trials
Where we identified both cluster‐randomised trials and individually randomised trials reporting data for the same outcome, we considered that it was reasonable to combine the results from both if there was little heterogeneity between the study designs, and the interaction between the effect of intervention and the choice of randomisation unit was considered unlikely.
Cluster‐randomised trials are labelled with a '(C)'. Inayati 2012 (C) took into account the clustering effect by using a mixed‐effects model.
Where possible, we estimated the intra cluster correlation coefficient (ICC) from trials' original data sets and reported the design effect. On the basis of this information, we used the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions to calculate the adjusted sample sizes (Higgins 2011b). We estimated the ICC from original data provided by Lundeen 2010 (C) and imputed the ICC in seven other trials (Kemmer 2012 (C); Kounnavong 2011 (C); Osei 2008 (C); Sharieff 2006 (C); Varma 2007 (C); Vinodkumar 2006 (C); Vinodkumar 2009 (C)), and then calculated each trial's effective sample size.
Trials with more than two treatment groups
For trials with more than two intervention groups (multi‐arm trials), we included the directly relevant arms only. If we identified trials with various relevant arms, we combined the groups into a single, pair‐wise comparison (Higgins 2011b), and included the disaggregated data in the corresponding subgroup category. If the control group was shared by two or more study arms, we divided the control group (events and total population) equally by the number of relevant subgroup categories to avoid double counting the participants. We noted the details in the Characteristics of included studies tables.
Two trials included additional arms in the comparisons as they provided weekly and daily regimens (Kounnavong 2011 (C); Sharieff 2006 (C)).
Cross‐over trials
There were no cross‐over trials that met our inclusion criteria (Criteria for considering studies for this review). See Table 2 and our protocol (De‐Regil 2012), for methods for managing cross‐over trials archived for use in future updates of this review.
1. Unused methods archived for use in future updates of this review.
| Method | Approach |
| Unit of analysis issues |
Cross‐over trials We planned to only include the first period of a randomised cross‐over trial prior to the washout period or to a change in the sequence of treatments. We planned to treat them as parallel randomised controlled trials. |
Dealing with missing data
Missing participants
We noted dropout for each included study. We noted attrition on the 'Risk of bias' form and included it in the 'Risk of bias' summary. We conducted analysis on an available‐case analysis basis: we included data from those participants whose results were known. We considered attrition as a potential source of heterogeneity.
We attempted to include all participants randomised to each group in the analyses. We contacted the authors and asked them to provide additional information and we performed an available‐case analysis and discussed the extent to which the missing data could alter the results or conclusions (or both) of the review. We noted in the Description of studies when authors provided additional information.
Missing data
Where key data (e.g. standard deviations (SD)) were missing from the report, we attempted to contact the corresponding authors (or other authors if necessary) of the included trials to request the unreported data. If this information was not achievable, we did not impute it and noted that the study did not provide data for that particular outcome.
Assessment of heterogeneity
We assessed methodological heterogeneity by examining the methodological characteristics and risk of bias of the trials, and clinical heterogeneity by examining the similarity between the types of participants, interventions and outcomes.
For statistical heterogeneity, we examined the forest plots from meta‐analyses to look for heterogeneity among trials and used the I2 statistic, Tau2 and Chi2 test to quantify the level of heterogeneity among the trials in each analysis. If we identified moderate or substantial heterogeneity, we explored it by prespecified subgroup analysis (see Subgroup analysis and investigation of heterogeneity). We considered an I2 statistic greater than 50% to indicate substantial heterogeneity. We advise caution in the interpretation of analyses with high degrees of heterogeneity.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' under Assessment of risk of bias in included studies), we attempted to contact study authors, asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such trials in the overall assessment of results by conducting a sensitivity analysis (see Sensitivity analysis).
If more than 10 trials contributed data to the primary outcomes, we presented a funnel plot to evaluate asymmetry, and hence a possible indication of publication bias for primary outcomes. Any identified asymmetry could be due to publication bias, but could also be attributable to a real relationship between trial size and effect size (e.g. larger trials may have poorer participant supervision and thus compliance to supplementation, which may, in turn, influence effect size). In such a case, we included, in the Discussion, a section on the possible causes of the observed asymmetry, including descriptions of reported compliance in the larger trials as compared with smaller trials.
Data synthesis
We conducted a meta‐analysis to obtain an overall estimate of the effect of the treatment when more than one study had examined similar interventions using similar methods, been conducted in similar populations and measured similar (comparable) outcomes.
We accounted for heterogeneity using a random‐effects meta‐analysis for combining data, as we anticipated that there may be natural heterogeneity between trials attributable to the different doses, durations, populations and implementation or delivery strategies. We carried out the meta‐analysis using the inverse‐variance statistical method for continuous variables and the Mantel‐Haenszel statistical method for dichotomous variables (RevMan 2014).
We did not combine outcomes expressed as continuous or dichotomous measures.
Subgroup analysis and investigation of heterogeneity
Where data were available, we carried out the following subgroup analyses:
anaemic status of participants at the start of intervention (anaemia defined as Hb values less than 110 g/L or less than 115 g/L, adjusted by altitude if appropriate): anaemic, non‐anaemic, mixed/unknown;
age of children at the start of the intervention: 24 to 59 months, five to 12 years;
refugee status: yes, no;
malaria status of the study site at the time of the trial: yes, no, not reported;
frequency: daily, weekly, flexible;
duration of intervention: less than three months, three months or more;
iron content of product: 12.5 mg or less, more than 12.5 mg;
type of iron compound: as reported by the trialists; and
number of nutrients accompanying iron: one to four, five to nine, 10 or more.
We conducted the following, post hoc subgroup analysis:
micronutrient composition: iron alone, at least iron plus vitamin A plus zinc, other combinations without bundling iron plus vitamin A plus zinc.
We used only the primary outcomes in the subgroup analysis (Primary outcomes). We did not conduct subgroup analyses for outcomes with three or fewer trials. We explored the forest plots visually and identified where CI did not overlap to identify differences between subgroup categories. We also formally investigated differences between two or more subgroups (Borenstein 2009).
Sensitivity analysis
We carried out a sensitivity analysis to explore:
the effects of removing trials at high risk of bias (trials with poor or unclear allocation concealment and blinding on either domain (i.e. both participants and personnel) or high/imbalanced loss to follow‐up) from the analysis;
the effects of different ICC values for cluster trials (where these were included, see Unit of analysis issues); and
trials with mixed populations in which marginal decisions were made (i.e. when trials included populations with an age range broader than that of our inclusion criterion: Criteria for considering studies for this review).
We conducted the following, post‐hoc sensitivity analysis:
the effects of combining studies comparing the intervention versus no intervention or placebo.
Results
Description of studies
Results of the search
Figure 1 depicts the process for assessing and selecting trials for inclusion in this review. The search strategy identified 12,437 records for possible inclusion and 10,477 were available after duplicate records were removed. We assessed 81 full‐text reports corresponding to 57 studies for eligibility. We included 13 trials (17 reports) in the review, excluded 38 trials (57 reports) with reasons (Characteristics of excluded studies table), and identified six ongoing or unpublished trials (ACTRN12616001245482; NCT01917032; NCT02280330; NCT02302729; NCT02422953; PACTR201607001693286).
1.

Study flow diagram.
Included studies
We included 13 trials with 5810 participants. All included trials contributed data to the review but some trials randomised participants to intervention arms that were not relevant to the comparisons we assessed. We have indicated in the Characteristics of included studies tables if we did not include any randomised arms in the analyses. Nine out of 12 trials were randomised at cluster level (labelled with a '(C)'), and for them, we have only included the estimated effective sample size in the analysis, after adjusting the data to account for the clustering effect.
In addition to the published papers, abstracts and reports identified by the search, one trial author provided us with the original data sets, for us to analyse the results for children aged 24 months and older only (Lundeen 2010 (C). Additionally, we obtained additional information on the studies for various included studies (Inayati 2012 (C); Lundeen 2010 (C); Ogunlade 2011; Sharieff 2006 (C); Troesch 2011b), and obtained useful information for the description of the study or the 'Risk of bias' assessment. This is presented in the Characteristics of included studies table.
Settings
All 13 trials were published after 2005, with four taking place in India (Osei 2008 (C); Varma 2007 (C); Vinodkumar 2006 (C); Vinodkumar 2009 (C)) and four in other parts of Asia: Indonesia (Inayati 2012 (C)), Lao People's Democratic Republic (Kounnavong 2011 (C)), Kyrgyz Republic (Lundeen 2010 (C)), and China (Sharieff 2006 (C)). Two trials each were carried out in South Africa (Ogunlade 2011; Troesch 2011b) and Kenya (Macharia‐Mutie 2012), while one study each was conducted in Honduras (Kemmer 2012 (C)) and Colombia (Orozco 2015 (C)).
Nine trials took place in institutional settings with eight occurring in schools (Ogunlade 2011; Orozco 2015 (C); Osei 2008 (C); Sharieff 2006 (C); Troesch 2011b; Varma 2007 (C); Vinodkumar 2006 (C); Vinodkumar 2009 (C)) and one in a feeding centre (Macharia‐Mutie 2012). The remaining trials took place in communities.
Three trials reported to be conducted in areas where malaria is endemic (Kounnavong 2011 (C); Macharia‐Mutie 2012; Varma 2007 (C)).
We have indicated in the Characteristics of included studies table the reported baseline prevalence of anaemia, which varied substantially across the trials that provided this information (range 7.3% to 92% among the nine trials reporting these data that did not exclude participants with anaemia).
Participants
Among the 13 trials, the sample sizes ranged between 90 and 2193 participants, and included children from six months up to 15 years of age. Six trials only included children up to 59 months of age (Inayati 2012 (C); Kemmer 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Orozco 2015 (C)), while four trials included only children aged five years or older (Osei 2008 (C); Troesch 2011b; Vinodkumar 2006 (C); Vinodkumar 2009 (C)), and three trials included children both younger and older than 59 months of age (Ogunlade 2011; Sharieff 2006 (C); Varma 2007 (C)). Eleven trials included boys and girls; two trials did not report the sex of the participants (Vinodkumar 2006 (C); Vinodkumar 2009 (C)).
Seven trials excluded children with low Hb values (cut‐offs varied and ranged from 70 g/L to 110 g/L or lower) (Kounnavong 2011 (C); Lundeen 2010 (C); Orozco 2015 (C); Troesch 2011b; Varma 2007 (C); Vinodkumar 2006 (C); Vinodkumar 2009 (C)), while the study in Honduras included only non‐anaemic children (Kemmer 2012 (C)). Overall, the participants in these trials came from low‐ and middle‐income populations, with the authors of seven trials reporting that participants were of low socioeconomic status (Inayati 2012 (C); Lundeen 2010 (C); Ogunlade 2011; Orozco 2015 (C); Troesch 2011b; Vinodkumar 2006 (C); Vinodkumar 2009 (C)), and three trials describing the participants as living in rural agricultural communities (Kemmer 2012 (C); Macharia‐Mutie 2012; Osei 2008 (C)). In two trials, the authors mentioned that some (Kounnavong 2011 (C)) or all (Sharieff 2006 (C)) participants were relatively wealthy, based on household characteristics such as being able to pay school fees or having electricity, an improved water source and a latrine.
Comparisons
All included trials compared the provision of MNP versus no intervention or placebo and are therefore included in comparison 1. None of the trials contributed data to any other comparison.
Micronutrient powder composition, iron dose and regimen
As described in the Characteristics of included studies tables, there was little consistency in MNP composition across most trials. Three trials reported a formulation of 14 vitamins and minerals (Inayati 2012 (C); Orozco 2015 (C); Osei 2008 (C)), two trials reported a formulation with six vitamins and minerals (Kemmer 2012 (C); Sharieff 2006 (C)), and each of the remaining trials reported a different formulation (range of two to 18 vitamin and minerals).
The iron dose and type of iron compound also varied across trials. Four trials reported giving 10 mg of elemental iron either as NaFeEDTA (Osei 2008 (C)), chelated ferrous sulphate (Vinodkumar 2006 (C)), or microencapsulated ferrous fumarate (Inayati 2012 (C); Kounnavong 2011 (C)). Three trials gave 12.5 mg of elemental iron as microencapsulated ferrous fumarate (Kemmer 2012 (C); Lundeen 2010 (C); Orozco 2015 (C)). Three trials gave 2.5 mg of elemental iron (Macharia‐Mutie 2012; Troesch 2011b) or 2.86 mg of elemental iron (Ogunlade 2011) as NaFeEDTA. Two trials gave 30 mg of elemental iron (Sharieff 2006 (C)) or 14 mg of elemental iron (Varma 2007 (C)) as microencapsulated ferrous fumarate, while one trial gave 28 mg of elemental iron as ferrous glycine phosphate (Vinodkumar 2009 (C)). Overall, seven trials used encapsulated ferrous fumarate (Inayati 2012 (C); Kemmer 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C), Orozco 2015 (C), Sharieff 2006 (C), Varma 2007 (C)); four trials used iron as ferric sodium ethylenediaminetetraacetate (NaFeEDTA) (Macharia‐Mutie 2012; Ogunlade 2011, Osei 2008 (C), Troesch 2011b), one trial used chelated ferrous sulphate (Vinodkumar 2006 (C)), one trial used ferrous glycine phosphate (Vinodkumar 2009 (C)).
All trials included daily provision of MNP in at least one arm of the study. However, for seven trials carried out in school settings, daily was defined variably as five or six times a week when school was in session (Ogunlade 2011; Orozco 2015 (C); Osei 2008 (C); Sharieff 2006 (C); Troesch 2011b; Varma 2007 (C); Vinodkumar 2009 (C)). Two trials also included arms with intermittent use, providing MNPs either once or twice a week (Kounnavong 2011 (C); Sharieff 2006 (C)). The study duration ranged from eight weeks to 12 months, with four trials reporting a duration of 23 or 24 weeks (six months) (Kounnavong 2011 (C); Troesch 2011b; Varma 2007 (C); Vinodkumar 2009 (C)), and two trials reporting a duration of four months (Kemmer 2012 (C); Macharia‐Mutie 2012), with the remaining trials each reporting a different duration. For one trial, duration varied for each participant depending on whether they achieved a WHZ score of ‐1.0 or greater during the intervention period and were discharged (Inayati 2012 (C)). The most common co‐intervention was antihelminthic treatment, reported by nine trials (Kemmer 2012 (C); Kounnavong 2011 (C); Macharia‐Mutie 2012; Ogunlade 2011; Orozco 2015 (C); Osei 2008 (C); Troesch 2011b; Vinodkumar 2006 (C); Vinodkumar 2009 (C)). One trial also gave vitamin A supplementation (Kounnavong 2011 (C)), and one trial also provided a sweet or juice after carrying out assessments (Osei 2008 (C)).
Excluded studies
We excluded 38 studies (57 reports and two personal communications). The age of the participants was outside the scope of this review in 21 trials (Aboud 2011; Ahmed 2003; Geltman 2009; Hirve 2007; Ip 2009; Jack 2012; Jaeggi 2015; Khan 2014; Menon 2007; Neufeld 2008; Samadpour 2011; Smuts 2005; Soofi 2013; Suchdev 2007 (C); Teshome 2017; Troesch 2009; Wijaya‐Erhardt 2007; Zlotkin 2001; Zlotkin 2003a; Zlotkin 2003b; Zlotkin 2013). In 18 trials, the participants were less than 24 months old (Aboud 2011; Geltman 2009; Hirve 2007; Ip 2009; Jack 2012; Jaeggi 2015; Khan 2014; Menon 2007; Neufeld 2008; Samadpour 2011; Smuts 2005; Soofi 2013; Suchdev 2007 (C); Wijaya‐Erhardt 2007; Zlotkin 2001; Zlotkin 2003a; Zlotkin 2003b; Zlotkin 2013). In two trials, children were aged 12 to 59 months but 51% of the participants were aged between 12 and 23 months (Ahmed 2003; Teshome 2017). One trial was conducted on healthy, non‐pregnant, non‐lactating young women (Troesch 2009).
In eight trials, the interventions did not evaluate MNP‐containing iron. One trial evaluated the effect of iron drops and not powders for point‐of‐use fortification (Bagni 2009 (C)), while two trials involved a fortified condiment or seasoning in powder form and not a MNP for point‐of‐use fortification (Chen 2008; Manger 2008). Three trials evaluated the effects of zinc and placebo tablets dissolved in freshly prepared fruit juice and administered to the children every day (Kikafunda 1998); the effects of four powdered fortificants added to a base powder containing protein 8 g, sugar 12 g and maltodextrin 4 g to be dissolved in 100 mL of a soy‐based fruit drink (Osendarp 2007); or the effects of synthetic β‐carotene powder added to rice (Vuong 2002). Two trials were of ineligible interventions (Menon 2016; Rim 2008). From these, one study assessed the effects of behaviour‐change interventions in Bangladesh, where half of the sample were offered MNP sachets containing iron, folic acid, zinc, and vitamins A and C for sale to mothers (Menon 2016).
Six studies had types of study design other than RCT (Angdembe 2015; Clarke 2015; De Pee 2007; Huamán‐Espino 2012; Paganini 2016; Rah 2012). One trial described the post‐tsunami experience with distribution of Vitalita sprinkles in Aceh and Nias, Indonesia, and did not have a control group (De Pee 2007), and two involved cross‐sectional surveys (Angdembe 2015; Clarke 2015). One of these references was a literature review of the effects of iron‐fortified foods, including in‐home iron fortification of complementary foods using MNP in gut microbiome (Paganini 2016).
One registered study aimed to find out whether adding a small quantity of powdered beef liver to day‐care meals of Brazilian preschool children from Salvador for 12 months could prevent anaemia and micronutrient deficiencies, and improve their growth, health and development in the same way (or better), than adding a small quantity of MNP (Sprinkles) (Gibson 2010). However, the study was not conducted because the baseline micronutrient survey data showed no evidence of micronutrient deficiencies among the preschool‐age children.
Two studies had ineligible comparison types (Sampaio 2013; Selva Suárez 2011).
See the Characteristics of excluded studies table for a detailed description of the trials and the reasons for their exclusion.
Ongoing studies
We identified six ongoing studies (ACTRN12616001245482; NCT01917032; NCT02280330; NCT02302729; NCT02422953; PACTR201607001693286). The studies are being conducted in Colombia (NCT01917032), Guatemala (NCT02302729), Pakistan (NCT02422953), Philippines (NCT02280330), Tanzania (PACTR201607001693286), and Vietnam (ACTRN12616001245482). Most studies are being conducted in children of different age ranges: six to 12 months of age and preschool‐age children (36 to 48 months of age) (NCT02302729); healthy boys and girls aged four to six years (NCT02280330), children aged six to 59 months with moderate anaemia (Hb concentration 70 g/L to 100 g/L) (PACTR201607001693286), non‐anaemic children aged five to 59 months (NCT01917032), and healthy primary school boys and girls aged six to nine years (ACTRN12616001245482). One study is on pregnant women, lactating mothers and children aged six to 59 months (NCT02422953). See Characteristics of ongoing studies table for details.
Risk of bias in included studies
Overall, study methods were not well described in many of the included trials. However, we contacted all study authors and obtained a very high response rate: 83.3% of study authors provided additional information that improved the quality of our assessment (see Figure 2 and Figure 3). All provided clarifications are noted in the Characteristics of included studies table.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
In total, we judged nine out of 13 studies at low risk of bias (Inayati 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Ogunlade 2011; Orozco 2015 (C); Osei 2008 (C); Varma 2007 (C); Vinodkumar 2006 (C)).
Allocation
Sequence generation
In four trials, the method for generation of random sequence was unclear (Kemmer 2012 (C); Sharieff 2006 (C); Troesch 2011b; Vinodkumar 2009 (C)). We judged the remaining nine trials at low risk of bias on this domain. Inayati 2012 (C); Varma 2007 (C); and Vinodkumar 2006 (C) used random tables for sequence generation. Kounnavong 2011 (C) and Ogunlade 2011 used computer‐generated random numbers. Lundeen 2010 (C) and Osei 2008 (C) used shuffling cards to generate the random sequence. Orozco 2015 (C) reported using random blocks of variable length. Macharia‐Mutie 2012 used block randomisation by age and sex generated with Excel (Microsoft) by one investigator not involved in recruitment and data collection.
Allocation concealment
In one trial, allocation concealment was unclear (Sharieff 2006 (C)), while another did not conceal allocation and thus was rated at high risk of bias (Kounnavong 2011 (C)). We judged the remaining 11 trials at low risk of bias (Inayati 2012 (C); Kemmer 2012 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Ogunlade 2011; Orozco 2015 (C); Osei 2008 (C); Troesch 2011b; Varma 2007 (C); Vinodkumar 2006 (C); Vinodkumar 2009 (C))
Blinding
Blinding of participants and personnel
One trial had unclear blinding of participants (Orozco 2015 (C). We rated eight trials at high risk of bias as they did not blind the intervention to the participants and personnel (Inayati 2012 (C); Kemmer 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Sharieff 2006 (C); Vinodkumar 2006 (C); Vinodkumar 2009 (C)). We judged the remaining four trials at low risk of bias (Ogunlade 2011: Osei 2008 (C); Troesch 2011b; Varma 2007 (C)).
Blinding of outcome assessors
Five trials had unclear blinding of outcome assessment (Kemmer 2012 (C); Osei 2008 (C); Troesch 2011b; Varma 2007 (C); Vinodkumar 2009 (C)). Five trials did not blind the intervention and were at high risk of bias (Inayati 2012 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Sharieff 2006 (C); Vinodkumar 2006 (C)). We judged the three remaining trials at low risk of detection bias (Kounnavong 2011 (C); Ogunlade 2011; Orozco 2015 (C)).
Incomplete outcome data
We judged that trials with more than 20% loss to follow‐up, or with imbalanced loss to follow‐up in different arms of trials, were inadequate in terms of completeness of outcome data. Three trials were at high levels of attrition, or loss was not balanced across groups and may have occurred for reasons associated with treatment (Kemmer 2012 (C); Ogunlade 2011; Varma 2007 (C)). With Kemmer 2012 (C), 31% of participants did not have Hb measurements. In Ogunlade 2011, attrition of the intervention group was 17.1% while attrition in the control group was 9.3%. For Varma 2007 (C), both intervention and control arms had 20% or more loss to follow‐up.
We judged the remaining 10 trials at low risk of attrition bias (Inayati 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Orozco 2015 (C); Osei 2008 (C); Sharieff 2006 (C); Troesch 2011b; Vinodkumar 2006 (C); Vinodkumar 2009 (C)).
Selective reporting
In 11 trials, it was impossible to judge selective reporting (Kemmer 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Orozco 2015 (C), Osei 2008 (C); Sharieff 2006 (C); Troesch 2011b; Varma 2007 (C); Vinodkumar 2006 (C); Vinodkumar 2009 (C)). One trial had a high risk of bias because they excluded from analysis children with adherence lower than 60% (Ogunlade 2011). We judged one study at low risk of reporting bias (Inayati 2012 (C)).
Other potential sources of bias
We rated three studies at high risk of other potential sources of bias (Kounnavong 2011 (C); Orozco 2015 (C); Vinodkumar 2009 (C)). Kounnavong 2011 (C) and Vinodkumar 2009 (C) had imbalanced Hb levels among intervention and control arms at baseline. Orozco 2015 (C) reported that, at the start of the study, the overall mean age of preschool‐age children was 4.8 years (SD 0.3), with a minimum age of 3.8 years and a maximum age of 5.2 years, with statistical differences between the two groups. Also, 71.1% of participants presented an adequate nutritional status, compared to 25.6% who had malnutrition due to excess (15.6% were overweight and 10% were obese). There were significant differences in the nutritional status between the groups at the beginning of the study.
With the remainder of the trials, there was insufficient information to permit judgement (Inayati 2012 (C); Kemmer 2012 (C); Macharia‐Mutie 2012; Osei 2008 (C); Sharieff 2006 (C)), or other potential bias was unlikely (Lundeen 2010 (C); Ogunlade 2011; Troesch 2011b; Varma 2007 (C); Vinodkumar 2006 (C)).
Effects of interventions
See: Table 1
We included data from 13 trials, involving 5810 participants, in this review; for trials that included more than two treatment arms, we may not have included all arms in our analyses. We have organised the summary of results by comparisons and by primary and secondary outcomes. Most of the included trials focused on haematological indices and few reported on any of the other outcomes prespecified in the review protocol (De‐Regil 2012). Many of the findings showed heterogeneity that could not be explained by standard sensitivity analyses or subgroup analysis, including quality assessment, and so we used a random‐effects model to analyse the results.
See the Data and analyses section for detailed results on primary and secondary outcomes.
For those outcomes that included data from cluster‐randomised trials, the number included is the effective sample size; that is, sample sizes and event rates have been adjusted for cluster‐trials to take account of the design effect.
Comparison 1. Point‐of‐use fortification of foods with MNP versus no intervention or placebo
We included 13 trials that compared point‐of‐use fortification of foods with MNP versus no intervention/placebo.
Primary outcomes
Anaemia
Ten trials involving 2448 children evaluated anaemia. Children receiving multiple MNP for point‐of‐use fortification of foods were significantly less likely to have anaemia at follow‐up than children receiving no intervention or placebo (RR 0.66, 95% CI 0.49 to 0.88, P = 0.005; Tau2 = 0.13, Chi2 = 32.91, I2 = 73%; Analysis 1.1). We rated the quality of this evidence as moderate; most studies showed a positive effect of MNP but had no blinding and high heterogeneity. See Table 1. Among the trials, nine were conducted with participants with unreported or mixed anaemia status at the start of the intervention and of these, one excluded participants with severe anaemia (Hb less than 70 g/L) at participant selection (Kounnavong 2011 (C)). One study was conducted among non‐anaemic children (Kemmer 2012 (C)). See Analysis 1.2.
1.1. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 1 Anaemia.
1.2. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 2 Anaemia by anaemic status of participants at start of intervention.
The subgroup analysis test showed that there were no significant differences in the effect by age at the start of intervention (i.e. the effect was no longer significant for those participants aged 60 months or older) (Analysis 1.3; test for subgroup differences P = 0.67); frequency of provision (Analysis 1.5; test for subgroup differences P = 0.15); duration of the intervention (Analysis 1.6; test for subgroup differences P = 0.21); number of nutrients in addition to iron (Analysis 1.9; test for subgroup differences P = 0.07; ); but there were significant differences for malaria status of study site (Analysis 1.4; test for subgroup differences P = 0.02), iron content (Analysis 1.7; test for subgroup differences P = 0.002), type of iron compound (Analysis 1.8; test for subgroup differences P = 0.03) and micronutrient composition (Analysis 1.10; test for subgroup differences P = 0.002). However, these results must be interpreted with caution as heterogeneity was high.
1.3. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 3 Anaemia by age of children at start of intervention.
1.5. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 5 Anaemia by frequency.
1.6. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 6 Anaemia by duration of intervention.
1.9. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 9 Anaemia by number of nutrients accompanying iron.
1.4. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 4 Anaemia by malaria status of study site at time of trial.
1.7. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 7 Anaemia by iron content of product.
1.8. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 8 Anaemia by type of iron compound.
1.10. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 10 Anaemia by micronutrient composition.
Haemoglobin
Eleven included trials with 2746 children evaluated Hb. Compared to children receiving no intervention or placebo, children receiving multiple MNP had more Hb at follow‐up (MD 3.37 g/L, 95% CI 0.94 to 5.80, P = 0.006; Tau2 = 14.42, Chi2 = 134.09, I2 = 93%; Analysis 1.11). We rated the quality of this evidence as low; most studies showed a positive effect of MNP but had no blinding and high heterogeneity. See Table 1.
1.11. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 11 Haemoglobin (g/L).
The analysis by subgroups by anaemia status at the start of the intervention was not estimable as all studies contributing data were of mixed anaemia status (Analysis 1.12). There were differences for subgroups by frequency of provision (Analysis 1.15; test for subgroup differences P = 0.04), type of iron compound (Analysis 1.18; test for subgroup differences P < 0.00001), number of nutrients in addition to iron (Analysis 1.19; test for subgroup differences P = 0.003) and micronutrient composition (Analysis 1.20; test for subgroup differences P = 0.02).
1.12. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 12 Haemoglobin by anaemic status of participants at start of intervention (g/L).
1.15. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 15 Haemoglobin by frequency (g/L).
1.18. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 18 Haemoglobin by type of iron compound (g/L).
1.19. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 19 Haemoglobin by number of nutrients accompanying iron (g/L).
1.20. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 20 Haemoglobin by micronutrient composition (g/L).
There were no subgroup differences by age of the participants (Analysis 1.13; test for subgroup differences P = 0.21), malaria status of the study site (Analysis 1.14; test for subgroup differences P = 0.56), duration of the intervention (Analysis 1.16; test for subgroup differences P = 0.59) and iron content of the product (Analysis 1.17; test for subgroup differences P = 0.54).
1.13. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 13 Haemoglobin by age of children at start of intervention.
1.14. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 14 Haemoglobin by malaria status of study site at time of trial.
1.16. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 16 Haemoglobin by duration of intervention.
1.17. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 17 Haemoglobin by iron content of product.
There was no indication of publication bias through assessment of the funnel plot (Figure 4). When we analysed the results only with studies that compared provision of MNP versus placebo (i.e. we excluded those providing no intervention), the estimate changed to MD 1.29 g/L (95% CI ‐0.69 to 3.28, 4 studies, 1086 participants; analyses not shown).
4.
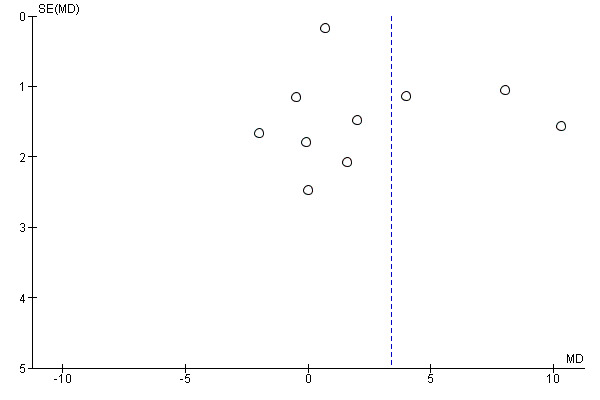
Funnel plot of comparison: 1 Point‐of‐use fortification of foods with micronutrients powders versus no intervention/placebo, outcome: 1.11 Haemoglobin (g/L).
Iron deficiency
Five trials with 1364 children evaluated iron deficiency (Macharia‐Mutie 2012; Osei 2008 (C); Sharieff 2006 (C); Troesch 2011b; Varma 2007 (C)). Children receiving multiple MNP for point‐of‐use fortification of foods were significantly less likely to have iron deficiency at follow‐up than those children receiving no intervention or placebo (RR 0.35, 95% CI 0.27 to 0.47, P = 0.001; Tau2 = 0.00, Chi2 = 0.65, I2 = 0%; Analysis 1.21). We rated the quality of this evidence as moderate; there was no heterogeneity and most studies showed a positive effect of MNP but they lacked blinding. See Table 1.
1.21. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 21 Iron deficiency.
All five trials were conducted with participants with unreported or mixed anaemia status at start of the intervention. The subgroups analysis tests were either not significant or not estimable for the characteristics evaluated (see Analysis 1.22; Analysis 1.23; Analysis 1.24; Analysis 1.25; Analysis 1.26; Analysis 1.27; Analysis 1.28; Analysis 1.29; Analysis 1.30).
1.22. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 22 Iron deficiency by anaemia status at start of intervention.
1.23. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 23 Iron deficiency by age of children at start of intervention.
1.24. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 24 Iron deficiency by malaria status of study site at time of trial.
1.25. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 25 Iron deficiency by frequency.
1.26. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 26 Iron deficiency by duration of intervention.
1.27. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 27 Iron deficiency by iron content of product.
1.28. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 28 Iron deficiency by type of iron compound.
1.29. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 29 Iron deficiency by number of nutrients accompanying iron.
1.30. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 30 Iron deficiency by micronutrient composition.
Ferritin
Three trials, involving 1066 children, provided information on ferritin concentrations (reported as arithmetic means) (Osei 2008 (C); Sharieff 2006 (C); Varma 2007 (C)). On average, it was uncertain whether children receiving multiple MNP had any difference in ferritin at follow‐up compared to children receiving no intervention or placebo (SMD 0.42 μg/L, 95% CI ‐4.36 to 5.19, Tau2 = 17.76, Chi2 = 1139.30, I2 = 100%; Analysis 1.31), as the quality of the evidence has been assessed as very low. Most studies had no or unclear blinding, and there was 100% heterogeneity with most inconsistency in the direction of the effect. See Table 1.
1.31. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 31 Ferritin (μg/L).
All‐cause mortality: single study results
One trial with 115 participants reported on all‐cause mortality (Inayati 2012 (C)). There were no deaths reported during this trial (Analysis 1.32). We rated the quality of this evidence as low as only one low‐risk trial reported all‐cause mortality. See Table 1.
1.32. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 32 All‐cause mortality.
Diarrhoea
Two trials with 366 participants reported data on diarrhoea (Inayati 2012 (C); Osei 2008 (C). Children receiving multiple MNP for point‐of‐use fortification of foods were as likely to have diarrhoea at follow‐up than those children receiving no intervention or placebo (RR 0.97, 95% CI 0.53 to 1.78, P = 0.93; Tau2 = 0.00, Chi2 = 0.42, I2 = 0%; Analysis 1.33). We rated the quality of this evidence as low; two low‐risk trials reported diarrhoea and there was no heterogeneity, with both studies showing no difference between the intervention and comparison groups. See Table 1.
1.33. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 33 Diarrhoea.
Adverse effects: single study results
One trial involving 90 children reported adverse effects (Orozco 2015 (C)). In this study, authors reported that, overall, four children (4.4%) reported adverse effects that consisted of abdominal pain and nausea. Two preschool‐age children (4.7%) in the MNP group and two children (4.3%) in the control group presented with nausea and abdominal pain (RR 1.09, 95% CI 0.16 to 7.42; Analysis 1.34). It is important to note that none of the participants required medical attention for the adverse effects attributed to the intervention; for this reason, none were withdrawn from the study. We rated the quality of this evidence as moderate. See Table 1.
1.34. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 34 Adverse effects.
Secondary outcomes
Iron deficiency anaemia
Three trials with 918 participants reported on iron deficiency anaemia (Macharia‐Mutie 2012; Osei 2008 (C); Varma 2007 (C). Children receiving multiple MNP for point‐of‐use fortification of foods were as likely to have iron deficiency anaemia as children receiving no intervention or placebo (RR 0.28, 95% CI 0.07 to 1.10, P = 0.07; Tau2 = 0.97, Chi2 = 5.90, I2 = 66%; Analysis 1.35).
1.35. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 35 Iron deficiency anaemia.
Cognitive development and school performance: single study results
One trial reported on cognitive development and school performance, but the information could not be extracted (Ogunlade 2011).
Motor development and physical capacity
No trials reported motor development and physical capacity.
All‐cause morbidity
Three trials with 538 participants reported on all‐cause morbidity (Inayati 2012 (C); Kounnavong 2011 (C); Osei 2008 (C)). Children receiving multiple MNP for point‐of‐use fortification of foods were as likely to have all‐cause morbidity as children receiving no intervention or placebo (RR 0.96, 95% CI 0.74 to 1.23, P = 0.72; Tau2 = 0.00, Chi2 = 0.83, I2 = 0%; Analysis 1.36).
1.36. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 36 All‐cause morbidity.
Acute respiratory infection: single study results
Only one trial with 115 participants reported on acute respiratory infection (Inayati 2012 (C)). Participants who received the iron‐containing MNP for point‐of‐use fortification were less likely to have an acute respiratory infection than participants receiving no intervention or placebo (RR 0.58, 95% CI 0.37 to 0.90, P = 0.01; see the illustrative forest plot in Analysis 1.37).
1.37. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 37 Acute respiratory infection.
Growth (height‐for‐age Z‐score)
Four trials with 617 participants reported on growth (HAZ) (Inayati 2012 (C); Kemmer 2012 (C); Kounnavong 2011 (C); Ogunlade 2011). There were no significant differences between children receiving MNPs and children receiving no intervention or placebo, although children receiving MNP were slightly shorter at follow‐up (MD ‐0.02, 95% CI ‐0.20 to 0.17, P = 0.86; Tau2 = 0.00, Chi2 = 0.25, I2 = 0%; Analysis 1.38).
1.38. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 38 Growth (height‐for‐age Z‐score).
Growth (weight‐for‐age Z‐score)
Three trials with 502 participants reported on growth (WAZ) (Kemmer 2012 (C); Kounnavong 2011 (C); Ogunlade 2011). There were no significant differences between children receiving MNPs and children receiving no intervention or placebo, although children receiving MNP were slightly thinner for their age at follow‐up (MD ‐0.01, 95% CI ‐0.09 to 0.07 P = 0.85; Tau2 = 0.00, Chi2 = 0.89, I2 = 0%; Analysis 1.39).
1.39. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 39 Growth (weight‐for‐age Z‐score).
Growth (weight‐for‐height Z‐score)
Two trials with 287 participants reported on growth (WHZ) (Inayati 2012 (C); Kounnavong 2011 (C)). Children receiving multiple MNP were heavier for their height at follow‐up in comparison to children receiving no intervention or placebo (MD 0.09, 95% CI 0.00 to 0.19, P = 0.04; Tau2 = 0.00, Chi2 = 0.15, I2 = 0%; Analysis 1.40).
1.40. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 40 Growth (weight‐for‐height Z‐score).
Adherence: single study results
One trial with 131 participants reported on adherence (Ogunlade 2011). Children receiving multiple MNP for point‐of‐use fortification of foods were as likely to adhere to the intervention as children receiving no intervention or placebo (RR 1.00, 95% CI 0.87 to 1.16, P = 0.95; see the illustrative forest plot in Analysis 1.41).
1.41. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 41 Adherence.
Red blood cell folate
No trials reported red blood cell folate.
Serum/plasma retinol
Two trials with 547 participants reported serum/plasma retinol (Varma 2007 (C); Vinodkumar 2006 (C)). It was uncertain whether the intervention made any change in serum/plasma retinol concentration at follow‐up (MD 10.08 mmol/L, 95% CI ‐10.72 to 30.88, P = 0.34; Tau2 = 214.16, Chi2 = 19.44, I2 = 95%; Analysis 1.42).
1.42. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 42 Serum/plasma retinol (mmol/L).
Serum/plasma zinc concentrations: single study results
One trial with 288 participants reported serum/plasma zinc concentrations (Osei 2008 (C)). There were no differences between groups. It was uncertain whether the intervention made any difference in serum/plasma zinc concentration at follow‐up (MD ‐0.10 mmol/L, 95% CI ‐0.79 to 0.59, P = 0.78; see the illustrative forest plot in Analysis 1.43).
1.43. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 43 Serum/plasma zinc concentrations (mmol/L).
Non‐prespecified outcomes
Studies reported several non‐prespecified outcomes for indicators of vitamin and mineral status, growth and other health outcomes.
Iron status: single study results
One study with 288 children reported total iron binding capacity (MD 22.90 µmol/kg, 95% CI 22.55 to 23.25, P < 0.001; Analysis 1.44) and serum transferrin receptors (MD ‐0.10 mg/L, 95% CI ‐0.44 to 0.24, P = 0.56; see the illustrative forest plot in Analysis 1.45) (Osei 2008 (C)). However, we are uncertain whether the intervention makes any difference in these outcomes.
1.44. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 44 Iron status (iron‐binding capacity) (non‐prespecified) (µmol/kg).
1.45. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 45 Iron status (serum‐transferrin receptors; non‐prespecified) (mg/L).
Serum vitamin E: single study results
One study with 66 children reported serum vitamin E (Vinodkumar 2006 (C)). It found no evidence of effect of multiple MNP in comparison with a placebo (MD 27.45 µg/dL, 95% CI ‐213.28 to 268.18, P = 0.82; see the illustrative forest plot in Analysis 1.46).
1.46. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 46 Serum vitamin E (non‐prespecified) (µg/dL).
Serum vitamin B12
Two studies with 329 children reported serum vitamin B12 (Osei 2008 (C); Vinodkumar 2006 (C)). There was no evidence of effect among children who consumed multiple MNP in comparison with children who received no intervention or placebo (MD 241.16 pg/mL, 95% CI ‐258.70 to 741.02, P = 0.34; Tau2 = 99511.92, Chi2 = 3.27, I2 = 69%; Analysis 1.47).
1.47. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 47 Serum vitamin B12 (non‐prespecified) (pg/mL).
Zinc deficiency: single study results
One study with 192 children reported zinc deficiency (Troesch 2011b). It found that multiple MNP significantly reduced zinc deficiency (serum zinc less than 6.5 µg/L) compared to no intervention or placebo (RR 0.64, 95% CI 0.45 to 0.93, P = 0.02; see the illustrative forest plot in Analysis 1.48).
1.48. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 48 Zinc deficiency (non‐prespecified).
Vitamin A deficiency: single study results
One study with 481 children reported vitamin A deficiency (serum retinol less than 0.70 µg/L) (Varma 2007 (C)). We were uncertain of effect among children who consumed multiple MNP in comparison with children who received no intervention or placebo (RR 1.31, 95% CI 0.69 to 2.48, P = 0.41; see the illustrative forest plot in Analysis 1.49).
1.49. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 49 Vitamin A deficiency (non‐prespecified).
Serum folate
Two studies with 329 children reported serum folate (Osei 2008 (C); Vinodkumar 2006 (C)). Children who consumed multiple MNP had higher serum folate concentrations in comparison with children who received no intervention or placebo (MD 2.16 ng/mL, 95% CI 0.76 to 3.56, P = 0.002; Tau2 = 0.36, Chi2 = 1.45, I2 = 31%; Analysis 1.50).
1.50. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 50 Serum folate concentration (ng/mL).
Height
Two studies with 182 children reported height (Inayati 2012 (C); Vinodkumar 2006 (C)). There was no evidence of effect among children who consumed multiple MNP in comparison with children who received no intervention or placebo (MD 0.05 cm, 95% CI ‐3.71 to 3.82, P = 0.98; Tau2 = 3.45, Chi2 = 1.86, I2 = 46%; Analysis 1.51).
1.51. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 51 Height (non‐prespecified) (cm).
Weight
Three studies with 313 children reported weight (Inayati 2012 (C); Ogunlade 2011; Vinodkumar 2006 (C)). There was no evidence of an effect among children who consumed multiple MNP in comparison with children who received no intervention or placebo (MD ‐0.02 kg, 95% CI ‐0.59 to 0.55, P = 0.94; Tau2 = 0.00, Chi2 = 1.85, I2 = 0%; Analysis 1.52).
1.52. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 52 Weight (non‐prespecified) (kg).
Fever: single study results
One study with 115 children reported fever (Inayati 2012 (C)). We were uncertain of any effect among children who consumed multiple MNP in comparison with children who received no intervention or placebo (RR 0.82, 95% CI 0.45 to 1.48, P = 0.50; Analysis 1.53).
1.53. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 53 Fever (non‐prespecified).
Stunting
Two studies with 654 children reported stunting (HAZ‐2 SD) (Kemmer 2012 (C); Osei 2008 (C)). There was no evidence of an effect among children who consumed multiple MNP in comparison with children who received no intervention or placebo (RR 0.91, 95% CI 0.66 to 1.25, P = 0.56; Tau2 = 0.04, Chi2 = 3.90, I2 = 74%; Analysis 1.54).
1.54. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 54 Stunting (non‐prespecified).
Angular stomatitis
Two studies with 236 children reported angular stomatitis (Vinodkumar 2006 (C); Vinodkumar 2009 (C)). Children who received placebo had a higher risk of having angular stomatitis compared with children who received multiple MNP (RR 0.04, 95% CI 0.01 to 0.29, P = 0.001; Tau2 = 0.00, Chi2 = 0.39, I2 = 0%; Analysis 1.55).
1.55. Analysis.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 55 Angular stomatitis (non‐prespecified).
Comparison 2. Point‐of‐use fortification of foods with MNP versus iron‐only supplement
We found no trials comparing point‐of‐use fortification of foods with MNP versus iron‐only supplement.
Comparison 3. Point‐of‐use fortification of foods with MNP versus iron and folic acid supplements
We found no trials comparing point‐of‐use fortification of foods with MNP versus iron and folic acid supplements.
Comparison 4. Point‐of‐use fortification of foods with MNP versus same micronutrients in supplements
We found no trials comparing point‐of‐use fortification of foods with MNP versus same micronutrients in supplements.
Discussion
Summary of main results
We included 13 trials, involving 5810 participants from nine countries in Latin America, Africa and Asia, in this review. We excluded 38 trials and six trials are ongoing or are unpublished. All trials assessed the provision of MNP for point‐of‐use fortification to no intervention or placebo. None of the trials evaluated the other comparisons of interest.
The sample sizes in the included trials ranged from 90 to 2193 participants. The age range of the participants was six months to 15 years. Six trials included participants aged six to 59 months, four included children aged 60 months or older and three trials included children both younger and older than 59 months of age. Two trials did not report the sex of the participants. The composition of the MNP in the trials varied: three trials used a formulation with 14 vitamins and minerals, two trials used a formulation with six vitamin and minerals, and the other eight trials provided MNP with different formulations ranging from two to 18 vitamins and minerals.
The iron compound and dose also varied in the included trials: one trial provided 30 mg of elemental iron, one trial provided 28 mg of elemental iron, one trial provided 14 mg of elemental iron, three trials provided 12.5 mg of elemental iron, four trials provided 10 mg of elemental iron, one trial provided 2.86 mg of elemental iron and two trials provided 2.5 mg of elemental iron. Seven trials used encapsulated ferrous fumarate (Inayati 2012 (C); Kemmer 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C), Orozco 2015 (C), Sharieff 2006 (C), Varma 2007 (C)); four trials used iron as NaFeEDTA (Macharia‐Mutie 2012; Ogunlade 2011, Osei 2008 (C), Troesch 2011b), one trial used chelated ferrous sulphate (Vinodkumar 2006 (C)), one trial used ferrous glycine phosphate (Vinodkumar 2009 (C)).
In comparison with receiving no intervention or placebo, children receiving iron‐containing MNP for point‐of‐use fortification of foods had a lower risk of anaemia and iron deficiency and a higher Hb concentration. We were unable to ascertain any effect on all‐cause mortality, as only one study with no reported deaths contributed data for this outcome. Two trials reported diarrhoea, with mixed results.
Nine of the 13 trials reported that participants had mixed anaemia status at baseline; this limited our ability to examine risk among non‐anaemic or anaemic populations. The effect on anaemia did not vary significantly by age of participants at the start of the intervention, frequency of provision or duration of intervention. There appears to be differences in the subgroups by the number of additional nutrients added, although the effect differed by the malaria risk in the trial setting, iron compound and iron content in the formulation. The magnitude of effects on Hb concentrations differed according to the frequency of the intervention, type of iron compound and number of nutrients, although heterogeneity in the subgroups was high.
As no included trials compared MNP to iron supplementation, which is the standard of care, we were unable to examine whether MNPs were as efficacious or safer than iron supplements (as drops, tablets or syrup).
Overall completeness and applicability of evidence
This review included 13 trials with 5810 children and all the trials were published after 2005. We only included RCTs and quasi‐RCTs in this review. This may have limited the inclusion of programme evaluation trials without control groups, which could have potentially provided additional evidence about the effectiveness of this intervention in programme settings (Hirve 2013; World Vision Mongolia 2005). The included trials only examined MNP versus no intervention or placebo and did not examine other comparisons of interest, including MNP versus iron‐only supplements, versus iron and folic acid supplements and versus the same micronutrients in supplements. The current review cannot compare the efficacy, adherence or adverse effects for these three interventions, which may be important for decision‐making by country programme managers and policy makers. The lack of trials comparing MNP to iron‐containing supplements is an important gap, as existing WHO guidelines currently recommend either daily or intermittent supplementation with iron for children younger than 12 years of age (WHO 2001; WHO 2011c), and researchers suggest that MNP consumed with food is safer than iron supplements (Hurrell 2010). With the exception of anaemia, Hb and iron status outcomes, there were insufficient trials to examine most of the primary and secondary outcomes of interest and by subgroups.
Eight of the 13 trials took place in Asia (Inayati 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C); Osei 2008 (C); Sharieff 2006 (C); Varma 2007 (C); Vinodkumar 2006 (C); Vinodkumar 2009 (C)), three in Africa (Macharia‐Mutie 2012; Ogunlade 2011; Troesch 2011b), and two in Latin America and the Caribbean (Kemmer 2012 (C); Orozco 2015 (C)). A 2011 global assessment of home fortification interventions identified 14 projects currently distributing MNP in Latin America and the Caribbean, including five programmes delivering MNP through early childhood development (ECD) centres (UNICEF/CDC 2013), but no trials included in this review were from ECD or school settings in Latin America and the Caribbean. Furthermore, none of the 13 trials took place in high‐income countries, and it is unknown if the results of trials would differ in high‐income countries. One trial conducted in the US among low‐income infants aged five to seven months documented lower intake adherence with MNP compared to iron drops (Geltman 2009; excluded as age range outside the scope of this review).
Most trials included both boys and girls. Some of the trials included children younger than 24 months of age. One trial provided the original data sets so the information included in this review could be limited to the participants aged 24 months or older (Lundeen 2010 (C)), but other trials were included because at least 50% of the sample was older than 24 months of age (Inayati 2012 (C); Kounnavong 2011 (C); Macharia‐Mutie 2012). As the prevalence of anaemia, and iron and other micronutrient deficiencies tend to be higher among children younger than 24 months compared to children older than 24 months, and there is typically an inverse relationship between deficiency and response to treatment, the inclusion of these younger children in the review might suggest a stronger effect of MNP programmes than would occur in programme settings with older children.
One trial excluded anaemic participants at baseline (Kemmer 2012 (C)) and two trials did not report anaemia prevalence at baseline (Sharieff 2006 (C); Vinodkumar 2006 (C)). All other trials included populations with different anaemia prevalence ranging from 7.25% to 92.1% across trials (or arms) at baseline. The predominance of trials with mixed anaemia status at baseline makes it impossible to examine risk among non‐anaemic populations or anaemic populations, and thus we cannot assess the efficacy of MNP with iron for the prevention or treatment of anaemia, exclusively.
For implementation projects or programmes, MNP are delivered to participants using multiple distribution platforms, including health systems through routine services and scheduled health facility events, community‐based distribution, general food distribution, ECD centre meals, school meals and market‐based approaches (UNICEF/CDC 2013). The 13 trials in this review also used multiple approaches to deliver MNP, including distributions through schools, feeding centres and communities, although these did not reflect all the delivery platforms currently in use in some settings. Nine of the trials were delivered through institutional settings (schools and a feeding centre), and with one exception (Sharieff 2006 (C)), the MNP was added at a central level to the meals prior to serving them to the children. This provides evidence for the emerging use of MNP in settings where the caretakers or participants do not necessarily have an active role in the point‐of‐use fortification, with preparing and adding the MNP to food prior to consumption, which could result in higher micronutrient intakes among children who regularly attend these institutions. Increased intake adherence and lower adverse effects are two of the proposed benefits for the use of MNP instead of iron supplements, but trials did not report adherence and adverse effects consistently and some trials did not report them. Lack of information about adverse effects and potential morbidities is an important gap, as some studies have suggested a small but significant increase in the incidence of diarrhoea with MNP intake among very young children (Soofi 2013), and documented changes in the gut microbiome among Kenyan children aged six months consuming MNP with iron (Jaeggi 2015).
Most of the 13 trials distributed MNP for daily intake (or daily when school was in session). The exceptions involved two trials with arms recommending intake twice a week (Kounnavong 2011 (C)) or weekly (Sharieff 2006 (C)). Other micronutrient intake schedules reported in projects include every other day, five days a week and flexible intake (with no suggested intake schedule other than no more than one dose a day and consuming all the MNP within a specific time frame) (UNICEF/CDC 2013). The micronutrient formulation, quantity of iron and iron compound varied across the 13 trials. The number of nutrients included in MNP formulations across the trials ranged from two to 18. One trial also included amylase‐rich, light malted barley flour with the micronutrient mix (Ogunlade 2011), and one trial included phytase (Troesch 2011b). Most existing programmes use a standard formulation of five or 15 micronutrients (UNICEF/CDC 2013). The trials included iron in the form of encapsulated ferrous fumarate, NaFeEDTA, chelated ferrous sulphate and ferrous glycine phosphate, and the iron doses ranged from 10 mg to 30 mg for ferrous fumarate, 2.5 mg to 10 mg for NaFeEDTA, 10 mg for chelated ferrous sulphate and 28 mg for ferrous glycine phosphate.
Some additional information about organoleptic properties of MNP mixed into foods (such as the food changed colour or consistency) came from direct communications with the authors (Macharia‐Mutie 2012; Ogunlade 2011). It is unknown if other trials experienced organoleptic issues not included in the final published documents. These changes could potentially influence the blinding of trials, or acceptability of the intervention to participants.
While the trials routinely reported Hb, anaemia and iron status indicators, most did not assess the status of other micronutrients in MNP or functional outcomes. The lack of information about functional outcomes is an especially important gap as these are the expected longer‐term health benefits that justify investment in this intervention. Similarly, there appears to be paucity of data on potential adverse health effects, which is important considering ongoing debates.
Quality of the evidence
We considered nine of the 13 trials at low risk of bias (Inayati 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Ogunlade 2011; Orozco 2015 (C); Osei 2008 (C); Varma 2007 (C); Vinodkumar 2006 (C)). In most studies, the main limitation was the lack of blinding at all levels: participants, personnel and outcome assessors. In one study, authors attempted to blind the intervention to the participants and the personnel, but the powders changed the consistency of the porridge and made it slightly darker and lighter compared to the original maize porridge, unmasking the intervention (Macharia‐Mutie 2012).
We judged the overall quality of the evidence for the provision of multiple MNP versus no intervention or placebo as moderate for anaemia, iron deficiency and adverse effects. We judged the evidence as low quality for Hb, all‐cause mortality and diarrhoea, and very low‐quality for ferritin (See Table 1).
Potential biases in the review process
We were aware of the possibility of introducing bias at every stage of the reviewing process. In this review, we tried to minimise bias in a number of ways; two review authors assessed eligibility for inclusion, carried out data extraction and assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements. Further, the process of reviewing research trials is known to be affected by prior beliefs and attitudes. It is difficult to control for this type of bias in the reviewing process.
While we designed our search strategies to be as inclusive as possible (Appendix 1), the literature that we identified was predominantly written in English and published in North American and European journals. Although we did attempt to assess reporting bias, constraints of time meant that this assessment largely relied on information available in the published trial reports and thus, reporting bias was not usually apparent.
We included a 'Summary of findings' table in this review (Table 1). Assessing the quality of the evidence relating to specific outcomes is a difficult process, but we attempted to produce the table using a transparent process. Two review authors (LD‐R, MJ) independently assessed the evidence for each outcome for each quality domain and discussed any disagreements.
Agreements and disagreements with other studies or reviews
One non‐Cochrane review assessed the effects of MNP in women and children (Salam 2013). Two reviews examined the effects and safety of MNP among younger children (aged six to 23 months) in the complementary feeding period (De‐Regil 2011b; Dewey 2009).
Salam 2013 carried out a systematic review of 16 MNP trials that took place in Asia, Africa and Haiti. Trials were included if they had MNP formulations with a minimum of two micronutrients, and otherwise could be consumed in the home or other settings, at any dosage and duration. While they aimed to examine the impact of MNP on women and children, they did not identify any studies among women that met their inclusion criteria. The final trials included in the review were all from low‐ and middle‐income countries and compared to no intervention or control. All the trial formulations included iron and the children were aged between six months and 11 years. Salam 2013 found MNP reduced the prevalence of anaemia, iron deficiency anaemia and vitamin A deficiency, and improved Hb concentrations. There were no significant effects on ferritin concentrations, zinc deficiency or anthropometric indicators. Four studies with morbidity outcome data showed a small but significant increase in the incidence of diarrhoea, and no significant effects on recurrent diarrhoea or upper respiratory illness.
De‐Regil 2011b included eight RCTs and quasi‐RCTs of apparently healthy children (aged six to 23 months) from Asia, Africa and the Caribbean. The review only included trials using MNP formulations with at least iron, zinc and vitamin A. The results of six trials compared MNP to no intervention or placebo, and two trials compared MNP to iron supplements. MNPs were found to reduce anaemia and iron deficiency when compared to no intervention or placebo, but had no effects on growth. These findings were consistent across the trials regardless of intervention duration or the prevalences of anaemia and malaria at baseline. The two trials that compared MNP to iron supplementation drops suggested there were no differences for anaemia or Hb concentrations, but the authors advised caution due to the limited number of trials included in this comparison. There was insufficient information to assess adverse effects or other functional outcomes.
Dewey 2009 carried out a systematic review and meta‐analysis of 16 trials of home fortification of complementary foods using MNP, crushable tablets, and lipid‐based or soy‐based products. Of the 16 included trials, five were defined as treatment and all involved MNP. Of the remaining 11 trials defined as prevention, eight included arms with MNP. Dewey 2009 did not specify any minimum formulation criteria for the MNP (or other home fortification products), but all trials with MNP contained iron. Dewey 2009 also included a trial not eligible for inclusion in the current review, which only compared MNP with iron to MNP with iron and zinc. The treatment trials showed no differences in the effects on Hb and ferritin between MNP and iron supplementation drops, which we were unable to assess in the current review due to a lack of trials examining this comparison. For the prevention trials, the overall effect size for ferritin and Hb concentrations include trials involving all the home fortification products and were not limited to MNP; they showed that home fortification improved ferritin and Hb concentrations reducing iron deficiency and anaemia.
There were several differences between our review and the above‐mentioned reviews. Compared to De‐Regil 2011b; Dewey 2009; and Salam 2013, our review only required the MNP formulation to include at least iron, whereas Salam 2013 required a minimum of two micronutrients, De‐Regil 2011b required iron, zinc and vitamin A, and Dewey 2009 had no minimum formulation criteria. Compared to this review, which focused on children aged 24 months to 12 years and included trials with children up to 15 years of age, De‐Regil 2011b and Dewey 2009 focused on children younger than 24 months of age. Salam 2013 included children up to 11 years of age, but 10 trials included only children younger than 36 months of age and four included children older than five years of age; in this review, seven trials included children aged five years or older. None of the trials included in De‐Regil 2011b or Dewey 2009 involved distribution and use of MNP through schools or other institutional settings. Salam 2013 included trials distributing outside the home but did not report which studies did so, and, in the current review eight of the 13 trials distributed through school or other institutional settings. Our review also had little information on intake adherence, which is a primary rationale for the use of MNP over iron supplementation.
Similar to Salam 2013 and De‐Regil 2011b, the current review found MNP reduced anaemia and iron deficiency, and increased mean Hb values when compared to no intervention or placebo. Salam 2013 reported three morbidity outcomes related to diarrhoea and upper respiratory infection, but, similar to De‐Regil 2011b and Dewey 2009, data in the current review were limited for adverse effects and functional outcomes, and variable outcome definitions limited some analyses.
Authors' conclusions
Implications for practice.
The findings of this review show that point‐of‐use fortification of foods with iron‐containing micronutrient powder (MNP) reduce anaemia and iron deficiency and improve haemoglobin concentrations among children aged 24 months to 12 years with mixed anaemia status (ranging from mild to severe public health problems) and primarily from low‐income populations. These findings are consistent across duration of supplementation.
No trials compared point‐of‐use fortification of foods with iron‐containing MNP to iron supplementation, to iron and folic acid supplements or multiple micronutrient supplements. Thus, it is impossible to compare the relative efficacy, health effects or safety compared to these interventions.
The trials included in this review provided MNP with diverse formulations ranging from two to 18 vitamins and minerals, iron doses, additional micronutrients composition and compounds. One expressed benefit of point‐of‐use fortification of foods with multiple MNP is that the formulation can be tailored to the target population's micronutrient intake gaps. While in theory this appears as a sensible approach, in many settings there is no current dietary intake or micronutrient status information in the population to tailor the formulation to a given country or community. In disadvantaged populations in low‐ and middle‐income countries, plant‐based diets with little access to animal source foods or fortified staple foods may be common. In addition to a limited dietary diversity, low bioavailability of vitamins and minerals in available staple foods due to dietary iron inhibitors (fibre, phytates, tannins), and poor vitamin and mineral content of the foods may contribute to the increased vulnerability to micronutrient malnutrition (HFTAG 2011). This has resulted in the pragmatic selection of formulations with about 15 vitamins and minerals to address multiple micronutrient deficiencies in addition to anaemia and iron deficiency in many projects being implemented. However, there are not enough studies to evaluate whether 15 vitamins and minerals (or any other grouping) is a more effective combination for health and functional outcomes for point‐of‐use fortification of foods with iron‐containing MNP.
Some of the included trials reported the addition of the iron‐containing MNP before, during or after cooking, which might facilitate the incorporation of this intervention as part of school feeding programmes. For the trials conducted through institutional settings (schools, feeding centre), most trials added the product in the kitchen prior to serving the meals to the participants. Kitchen and other food service staff require training to add the product safely to the foods during meal preparation. The needed training, support and behaviour change interventions can vary considerably by context, particularly in settings where school feeding programmes are carried out by volunteers or parents as opposed to more skilled, professional, food service staff. The use of multi‐serv MNP packaging in institutional settings may lower the cost per dose compared to individual packaged doses, due to reduced packaging costs (HFTAG 2011), an important benefit for the implementation of point‐of‐use fortification of foods with iron‐containing MNP in larger scale.
Integration of point‐of‐use fortification of foods with iron‐containing MNP into other programmes or sectors might improve the sustainability or feasibility to scale up when building on established or growing programme platforms, and could be an entry point for established co‐interventions in other public health initiatives. The opportunities for synergistically enhanced health and functional outcomes with integrated programmes are appealing but need to be documented as little evidence of these effects currently exists. It is important to monitor and understand effects, challenges and lessons learned when integrating point‐of‐use fortification of foods with iron‐containing MNP into multi‐sectorial programmes.
All the trials included at least one arm with daily MNP intake (or daily while school was in session, typically five or six days a week for about 10 months of the year), and Kounnavong 2011 (C) and Sharieff 2006 (C) each included intervention arms with either once or twice a week intake. There is also some interest in flexible regimens, where the MNP sachets are to be consumed at the preference of the individual or caretaker within a given period, with the caveat of not consuming more than one sachet a day. Based on programmatic experiences, some interest groups suggest that a reasonable and logistically feasible intake schedule is to consume one individual serving sachet two or three times a week (or every other day) (HFTAG 2011). Intermittent use can also be applied to institutional feeding programmes where the MNP is added a few days a week to the meals instead of daily. When recommending any intake schedule (daily, intermittent or flexible), it is important to suggest to participants that they develop a routine to help support the intake of all the individual MNP sachets. Further evaluation of these different intake regimens is needed.
Implications for research.
It is necessary to generate evidence of the benefits that nutrients other than iron have on children's health and nutritional status, to justify the added value of powder formulations containing multiple micronutrients.
Information on adverse effects is limited and frequently reported in an uneven manner. More data are needed to understand safety better and inform policy makers. It is hypothesised that MNP are safer than iron supplements, thus this is an important gap.
Intake adherence data are also similarly limited and reported inconsistently; as one of the main rationales for this intervention is that it will result in higher intake adherence compared to iron supplementation, this is also a significant gap.
In programmes that include point‐of‐use fortification of foods with MNP, a behaviour change intervention and communication strategy is a required intervention component when distributing individual MNP sachets directly to the participants, their families and caretakers (HFTAG 2011; Timmer 2013). Further research may be warranted to assess the relevance of behaviour change strategies targeted to either the children or families/caretakers in institutional feeding programmes where the involvement of children and families or caretakers on the use of MNP to the meal is not an individual decision (similar to what can occur with industrial fortification of staple foods in some contexts). The identification of potential implementation barriers may be required.
Examining the integration of MNP into existing programme platforms to deliver MNP through daycares, ECD centres, school feeding and other feeding centre programmes, which might be lower cost, result in increased vitamin and mineral intake, and improved health and functional outcomes among children who regularly attend these institutions is an important research gap.
Continued research to improve the bioavailability and absorption of iron and other micronutrients is needed, including the work already started with adding amylase and phytase reported in this review. Resolving sensory changes and organoleptic issues associated with amylase and phytase (e.g. food changed colour or consistency) are priorities.
Additional important research gaps include understanding the efficacy and interactions of MNP in the context of high burdens of malaria, HIV and other diseases.
Understanding the cost effectiveness of MNP interventions taking into consideration the entire intervention package, including the cost of the product, behaviour change intervention and communication and support, transportation, monitoring, training and refresher training, and how these costs vary across different delivery platforms and at increasing scale will provide useful guidance to the programmatic community, donors and other stakeholders when making investment decisions with scarce resources.
Acknowledgements
We would like to thank Ms Onnalee Gomez at the Division of Library Sciences & Services (DLSS), US Centers for Disease Control and Prevention (CDC), for her help in devising an early search strategy and searching the documents. We would like to thank the authors who provided additional data for this review. We would also like to thank Joanne Abbott for her support in the update, adaptation and run of the final search strategy in 2016.
The World Health Organization (WHO) and Luz Maria De‐Regil and Maria Elena Jefferds retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
We are also grateful to the editorial office of the Cochrane Developmental, Psychosocial and Learning Problems Group (CDPLPG) for their support in the preparation of this review. As part of the prepublication editorial process, the protocol and the review were commented on by three peer reviewers (an editor and two referees who were external to the editorial team) and one of CDPLPG's statistical editors.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials CRSO (CENTRAL)
(micronutrient* or multinutrient* or micro‐nutrient* or multi‐nutrient* or multimineral* or multi‐mineral or multimicronutrient* or multimicro‐nutrient* or trace element* or trace mineral* or trace nutrient* or iron or zinc or zn or fe or ferrous or ferric or retinol or vitamin a or multimineral* or multi‐mineral*):ti,ab,kw and (fortified food* or food fortif* or fortif* food or dietary supplement* or specialized food* or specialised food* or food* speciali*ed or enrich* or fortif* or supplement* or point of use or mix* or powder* or sachet* or sprinkles or packet* or shakti or rahama or anuka or chispitas or babyfer or bebe vanyan or supplefer or supplefem or mnp):ti,ab,kw and (baby or babies or infant* or child* or toddler* or preschool* or pre‐school* or school age*):ti,ab,kw,
MEDLINE Ovid
1. micronutrients/ 2. iron/ or zinc/ or vitamin A/ 3. (micronutrient$ or micro‐nutrient$).tw. 4. (multinutrient$ or multi‐nutrient$ or multi$ nutrient$).tw. 5. (multimicro‐nutrient$ or multimicronutrient$).tw. 6. (multivitamin$ or multi‐vitamin$).tw. 7. (multimineral$ or multi‐mineral$).tw. 8. (trace adj (element$ or mineral$ or nutrient$)).tw. 9. iron, dietary/ 10. ferric compounds/ or ferrous compounds/ 11. (iron or Fe or ferric$ or ferrous$ or zinc or Zn or vit$ A or retinol$).mp. 12. or/1‐11 13. food, fortified/ 14. dietary supplements/ 15. food, specialized/ 16. ((food$ or meal$ or drink$ or beverage$ or diet$ or snack$ or breakfast$ or break‐fast$ or lunch$ or dinner$) adj5 (fortif$ or enrich$ or supplement$)).tw. 17. "point of use".tw. 18. (home adj5 fortif$).tw. 19. ((in‐home or at‐home or school or child care or nursery) adj5 fortif$).tw. 20. (mix$ or powder$ or supplement$ or sachet$ or packet$ or powder$).tw. 21. (Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem or MNP).tw. 22. or/13‐21 23. (baby or babies or infant$ or toddler$ or preschool$ or pre‐school$ or child$ or school aged).tw. 24. exp child/ or exp infant/ 25. 23 or 24 26. 12 and 22 and 25 27. limit 26 to yr="1990 ‐Current" 28. limit 27 to (humans and "all child (0 to 18 years)") 29. exp Clinical Trial, Phase I/ or exp Controlled Clinical Trial/ or exp Clinical Trial/ or exp Clinical Trial, Phase III/ or exp Clinical Trial, Phase II/ or Randomized Controlled Trial/ 30. 28 and 29 31. (trial or trials).mp. 32. 28 and 31 33. 30 or 32
Embase Ovid
1. micronutrients/ 2. iron/ or zinc/ or vitamin A/ 3. (micronutrient$ or micro‐nutrient$).tw. 4. (multinutrient$ or multi‐nutrient$ or multi$ nutrient$).tw. 5. (multimicro‐nutrient$ or multimicronutrient$).tw. 6. (multivitamin$ or multi‐vitamin$).tw. 7. (multimineral$ or multi‐mineral$).tw. 8. (trace adj (element$ or mineral$ or nutrient$)).tw. 9. iron, dietary/ 10. ferric compounds/ or ferrous compounds/ 11. (iron or Fe or ferric$ or ferrous$ or zinc or Zn or vit$ A or retinol$).mp. 12. or/1‐11 13. food, fortified/ 14. dietary supplements/ 15. food, specialized/ 16. ((food$ or meal$ or drink$ or beverage$ or diet$ or snack$ or breakfast$ or break‐fast$ or lunch$ or dinner$) adj5 (fortif$ or enrich$ or supplement$)).tw. 17. "point of use".tw. 18. (home adj5 fortif$).tw. 19. ((in‐home or at‐home or school or child care or nursery) adj5 fortif$).tw. 20. (mix$ or powder$ or supplement$ or sachet$ or packet$ or powder$).tw. 21. (Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem or MNP).tw. 22. or/13‐21 23. (baby or babies or infant$ or toddler$ or preschool$ or pre‐school$ or child$ or school aged).tw. 24. exp child/ or exp infant/ 25. 23 or 24 26. 12 and 22 and 25 27. limit 26 to yr="1990 ‐Current" 28. limit 27 to (humans and "all child (0 to 18 years)") [Limit not valid in Embase; records were retained] 29. exp Clinical Trial, Phase I/ or exp Controlled Clinical Trial/ or exp Clinical Trial/ or exp Clinical Trial, Phase III/ or exp Clinical Trial, Phase II/ or Randomized Controlled Trial/ 30. 28 and 29 31. (trial or trials).mp. 32. 28 and 31 33. 30 or 32
BIOSIS ISI
1. TS=(micronutrient* or micro‐nutrient* or iron or zinc or multinutrient* or multi*‐nutrient* or multimicro‐nutrient* or multimicronutrient* or multivitamin* or multi‐vitamin* or multimineral* or multi‐mineral*) 2. TS=(vitamin‐a or¬ trace‐element* or trace‐mineral or trace‐nutrient* or ferric‐compound* or ferrous‐compound* or fe or ferric* or ferrous* or zn or retinol or vit*‐a) 3. TS=(dietary‐iron or iron‐dietary) 4. #1 OR #2 OR #3 5. TS=(point‐of‐use or food*‐fortified or fortified‐food* or dietary‐supplement* or food*‐specialzed or specialized‐food* or food*‐specialised or specialised‐food*) 6. TS=(home near/5 fortif*) or TS=(school near/5 fortif*)¬ or TS=(nursery near/5 fortif*) or TS=(childcare near/5 fortif*) or TS=("child‐care" near/5 fortif*) 7. TS=(food* near/5 supplement*) or ts=(meal* near/5 supplement*) or ts=(drink* near/5 supplement*) or ts=(beverage* near/5 supplement*) or ts=(diet* near/5 supplement*) or ts=(snack* near/5 supplement*) or ts=(breakfast* near/5 supplement*) or ts=("break‐fast*"near/5 supplement*) or ts=(lunch* near/5 supplement*) or ts=(dinner near/5 supplement*) 8. TS=(food* near/5 enrich*) or ts=(meal* near/5 enrich*) or ts=(drink* near/5 enrich*) or ts=(beverage* near/5 enrich*) or ts=(diet* near/5 enrich*) or ts=(snack* near/5 enrich*) or ts=(breakfast* near/5 enrich*) or ts=("break‐fast*"near/5 enrich*) or ts=(lunch* near/5 enrich*) or ts=(dinner near/5 enrich*) 9. TS=(mix* or powder* or supplement* or sachet* or packet* or powder*) 10. TS=(sprinkles or vita‐shakti or rahama or anuka or chispitas or babyfer or bebe‐vanyan or supplefer or supplefem or mnp) 11. (#5 OR #6 OR #7 OR #8 OR #9 OR #10) 12. TS=(baby or babies or infant* or toddler* or preschool* or pre‐school* or child* or school‐age*) 13. (#11 AND #12 AND #4) 14. TS=(trial or trials or random* or control*) 15. #13 AND #14
Science Citation Index Web of Science (SCI) and Social Science Citation Index Web of Science (SSCI)
#1 TS=(micronutrient* or micro‐nutrient* or iron or zinc or multinutrient* or multi*‐nutrient* or multimicro‐nutrient* or multimicronutrient* or multivitamin* or multi‐vitamin* or multimineral* or multi‐mineral*) #2 TS=(vitamin‐a or trace‐element* or trace‐mineral or trace‐nutrient* or ferric‐compound* or ferrous‐compound* or fe or ferric* or ferrous* or zn or retinol or vit*‐a) #3 TS=(dietary‐iron or iron‐dietary) #4 #1 OR #2 OR #3 #5 TS=(point‐of‐use or food*‐fortified or fortified‐food* or dietary‐supplement* or food*‐specialzed or specialized‐food* or food*‐specialised or specialised‐food*) #6 TS=(home near/5 fortif*) or TS=(school near/5 fortif*) or TS=(nursery near/5 fortif*) or TS=(childcare near/5 fortif*) or TS=("child‐care" near/5 fortif*) #7 TS=(food* near/5 supplement*) or ts=(meal* near/5 supplement*) or ts=(drink* near/5 supplement*) or ts=(beverage* near/5 supplement*) or ts=(diet* near/5 supplement*) or ts=(snack* near/5 supplement*) or ts=(breakfast* near/5 supplement*) or ts=("break‐fast*"near/5 supplement*) or ts=(lunch* near/5 supplement*) or ts=(dinner near/5 supplement*) #8 TS=(food* near/5 enrich*) or ts=(meal* near/5 enrich*) or ts=(drink* near/5 enrich*) or ts=(beverage* near/5 enrich*) or ts=(diet* near/5 enrich*) or ts=(snack* near/5 enrich*) or ts=(breakfast* near/5 enrich*) or ts=("break‐fast*"near/5 enrich*) or ts=(lunch* near/5 enrich*) or ts=(dinner near/5 enrich*) #9 TS=(mix* or powder* or supplement* or sachet* or packet* or powder*) #10 TS=(sprinkles or vita‐shakti or rahama or anuka or chispitas or babyfer or bebe‐vanyan or supplefer or supplefem or mnp) #11(#5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 TS=(baby or babies or infant* or toddler* or preschool* or pre‐school* or child* or school‐age*) #13 (#11 AND #12 AND #4) #14 TS=(trial or trials or random* or control*) #15 (#13 AND #14)
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S1.(MH "Micronutrients") S2. (MH "Iron") S3. (MH "Zinc") S4. "dietary iron" S5. (MH Vitamin A) S6. (MH "Ferric Compounds") S7. (MH "Ferrous Compounds") S8. micronutrient* OR micro‐nutrient* OR multinutrient* OR (multi* W1 nutrient*) OR multi‐nutrient* OR multimicro‐nutrient* OR multimicronutrient* OR multivitamin* OR multi*‐vitamin*OR multi*‐mineral* OR multimineral* S9. (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8) S10. “trace element*” OR “trace mineral*” OR “trace nutrient*” OR iron OR fe OR ferric* OR ferrous* OR zinc OR zn OR “vit* a” OR retinol S11. (MH "Food, Fortified") OR (MH "Dietary Supplements") OR "fortified food*" OR "specialized food*" OR "specialised food*" S12. "point‐of‐use" S13. (food* N5 fortif*) OR (meal* N5 fortif*) OR (drink* N5 fortif*) OR (beverage* N5 fortif*) OR (diet* N5 fortif*) OR (snack* N5 fortif*) OR (breakfast* N5 fortif*) OR (break‐fast* N5 fortif*) OR (lunch* N5 fortif*) OR (dinner* N5 fortif*) S14. (food* N5 enrich*) OR (meal* N5 enrich*) OR (drink* N5 enrich*) OR (beverage* N5 enrich*) OR (diet* N5 enrich*) OR (snack* N5 enrich*) OR (breakfast* N5 enrich*) OR (break‐fast* N5 enrich*) OR (lunch* N5 enrich*) OR (dinner* N5 enrich*) S15. (food* N5 supplement*) OR (meal* N5 supplement*) OR (drink* N5 supplement*) OR (beverage* N5 supplement*) OR (diet* N5 supplement*) OR (snack* N5 supplement*) OR (breakfast* N5 supplement*) OR (break‐fast* N5 supplement*) OR (lunch* N5 supplement*) OR (dinner* N5 supplement*) S16. home N5 fortif* S17. ("in home" N5 fortif*) OR ("at home" N5 fortif*) OR (school N5 fortif*) OR (childcare N5 fortif*) OR ("child care" N5 fortif*) OR (nursery N5 fortif*) S18. mix* or powder* or supplement* or sachet* or packet* S19. sprinkles or "vita shakti" or rahama OR anuka OR chispitas OR babyfer OR "bene vanyan" or supplefer or supplefem or mnp S20. (S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19) S21. baby or babies or infant* or toddler* or preschool* or pre‐school* or child* or "school age* S22. (MH "Child+") OR (MH "Infant+") S23. (S21 OR S22) S24. (S9 AND S20 AND S23) LIMIT TO 1990 ‐ 2016
LILACS (Latin American and Caribbean Health Science Information database)
(lilacs.bvsalud.org/en)
Micronutrients and (child or children or baby or babies or infant or infants or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(Iron or zinc or fe or zn or multinutrient or multinutrients¬ or multi‐nutrients or multivitamins or mult‐vitamins or multiminerals or multi‐minerals or multimicronutrients or multimicro‐nutrients or vitamin a or retinol or trace elements or trace minerals or trace nutrients or ferric or ferrous) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(Fortified food or fortified foods or specialized foods or specialized food or dietary supplements or dietary supplements) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(sprinkles or vita shakti or rahama or anuka or chispitas or babyfer or bebe vanyan or supplefer or supplefem or mnp) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(food or food or meal or meals or drink or drinks or beverage or beverages or diet or diets or dietary or snack or snacks or breakfast or breakfasts or break‐fast or break‐fasts or lunch or lunches or dinner or dinners or home or school or nursery or nurseries or childcare or child care) and (fortify or fortified or fortification or enrich or enriched or supplement or supplements or supplemental) AND (trial or trials)
(point of use or mix or mixes or mixed or powder or powders or supplement or supplements or supplemental or sachet or sachets or packet or packets) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
IBECS
(ibecs.isciii.es)
Micronutrients and (child or children or baby or babies or infant or infants or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged)
(Iron or zinc or fe or zn or multinutrient or multinutrients¬ or multi‐nutrients or multivitamins or mult‐vitamins or multiminerals or multi‐minerals or multimicronutrients or multimicro‐nutrients or vitamin a or retinol or trace elements or trace minerals or trace nutrients or ferric or ferrous) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged)
(Fortified food or fortified foods or specialized foods or specialized food or dietary supplements or dietary supplements) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged)
(sprinkles or vita shakti or rahama or anuka or chispitas or babyfer or bebe vanyan or supplefer or supplefem or mnp) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged)
(food or food or meal or meals or drink or drinks or beverage or beverages or diet or diets or dietary or snack or snacks or breakfast or breakfasts or break‐fast or break‐fasts or lunch or lunches or dinner or dinners or home or school or nursery or nurseries or childcare or child care) and (fortify or fortified or fortification or enrich or enriched or supplement or supplements or supplemental)
(point of use or mix or mixes or mixed or powder or powders or supplement or supplements or supplemental or sachet or sachets or packet or packets) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged)
POPLINE
Micronutrients and (child or children or baby or babies or infant or infants or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(Iron or zinc or fe or zn or multinutrient or multinutrients or multi‐nutrients or multivitamins or mult‐vitamins or multiminerals or multi‐minerals or multimicronutrients or multimicro‐nutrients or vitamin a or retinol or trace elements or trace minerals or trace nutrients or ferric or ferrous) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(Fortified food or fortified foods or specialized foods or specialized food or dietary supplements or dietary supplements) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(sprinkles or vita shakti or rahama or anuka or chispitas or babyfer or bebe vanyan or supplefer or supplefem or mnp) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(food or food or meal or meals or drink or drinks or beverage or beverages or diet or diets or dietary or snack or snacks or breakfast or breakfasts or break‐fast or break‐fasts or lunch or lunches or dinner or dinners or home or school or nursery or nurseries or childcare or child care) and (fortify or fortified or fortification or enrich or enriched or supplement or supplements or supplemental) AND (trial or trials)
(point of use or mix or mixes or mixed or powder or powders or supplement or supplements or supplemental or sachet or sachets or packet or packets) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
SciELO (Scientific Library Online)
(micronutrients AND (child OR children OR baby OR babies OR infant OR infants OR toddler OR toddlers OR preschool OR preschools OR preschooler OR preschoolers OR school age OR school aged) AND (trial OR trials)) OR ((iron OR zinc OR fe OR zn OR multinutrient OR multinutrients¬ OR multi‐nutrients OR multivitamins OR mult‐vitamins OR multiminerals OR multi‐minerals OR multimicronutrients OR multimicro‐nutrients OR vitamin a OR retinol OR trace elements OR trace minerals OR trace nutrients OR ferric OR ferrous) AND (child OR children OR baby OR babies OR toddler OR toddlers OR preschool OR preschools OR preschooler OR preschoolers OR school age OR school aged) AND (trial OR trials)) OR ((fortified food OR fortified foods OR specialized foods OR specialized food OR dietary supplements OR dietary supplements) AND (child OR children OR baby OR babies OR toddler OR toddlers OR preschool OR preschools OR preschooler OR preschoolers OR school age OR school aged) AND (trial OR trials)) OR ((sprinkles OR vita shakti OR rahama OR anuka OR chispitas OR babyfer OR bebe vanyan OR supplefer OR supplefem OR mnp) AND (child OR children OR baby OR babies OR toddler OR toddlers OR preschool OR preschools OR preschooler OR preschoolers OR school age OR school aged) AND (trial OR trials)) OR ((food OR food OR meal OR meals OR drink OR drinks OR beverage OR beverages OR diet OR diets OR dietary OR snack OR snacks OR breakfast OR breakfasts OR break‐fast OR break‐fasts OR lunch OR lunches OR dinner OR dinners OR home OR school OR nursery OR nurseries OR childcare OR child care) AND (fortify OR fortified OR fortification OR enrich OR enriched OR supplement OR supplements OR supplemental) AND (trial OR trials)) OR ((point of use OR mix OR mixes OR mixed OR powder OR powders OR supplement OR supplements OR supplemental OR sachet OR sachets OR packet OR packets) AND (child OR children OR baby OR babies OR toddler OR toddlers OR preschool OR preschools OR preschooler OR preschoolers OR school age OR school aged) AND (trial OR trials))
ClinicalTrials.gov
(clinicaltrials.gov)
"micronutrient powder" OR "sprinkle" OR "home fortification" OR "point‐of‐use fortification" OR "chispitas". Duplicates were removed.
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)
(apps.who.int/trialsearch)
"micronutrient powder"; "sprinkle"; "home fortification"; "point‐of‐use fortification"; and "chispitas". Duplicates were removed.
Data and analyses
Comparison 1. Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia | 10 | 2448 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.49, 0.88] |
| 2 Anaemia by anaemic status of participants at start of intervention | 10 | 2448 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.49, 0.88] |
| 2.1 Anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Non‐anaemic | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.74, 3.02] |
| 2.3 Mixed/unknown | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 3 Anaemia by age of children at start of intervention | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 3.1 Aged 24‐59 months | 6 | 1706 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.44, 0.93] |
| 3.2 Aged 60 months or older | 3 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.25, 1.12] |
| 4 Anaemia by malaria status of study site at time of trial | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 4.1 Yes | 4 | 934 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.29, 1.14] |
| 4.2 No | 4 | 1252 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.62, 0.85] |
| 4.3 Not reported | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.21, 0.56] |
| 5 Anaemia by frequency | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.46, 0.82] |
| 5.1 Daily | 9 | 2163 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.80] |
| 5.2 Weekly | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.50, 2.37] |
| 5.3 Flexible | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Anaemia by duration of intervention | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 6.1 Less than 3 months | 3 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.63, 0.80] |
| 6.2 3 months or longer | 6 | 1382 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.31, 0.84] |
| 7 Anaemia by iron content of product | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 7.1 12.5 mg elemental iron or less | 7 | 1705 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.65, 0.80] |
| 7.2 More than 12.5 mg elemental iron | 2 | 544 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.14, 0.50] |
| 8 Anaemia by type of iron compound | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 8.1 Iron EDTA | 4 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.56, 1.02] |
| 8.2 Encapsulated ferrous fumarate | 4 | 1389 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.35, 1.04] |
| 8.3 Other | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.21, 0.56] |
| 9 Anaemia by number of nutrients accompanying iron | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 9.1 1‐4 | 3 | 1185 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.16, 0.86] |
| 9.2 5‐9 | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 10‐15 | 6 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.03] |
| 10 Anaemia by micronutrient composition | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 10.1 Iron alone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Iron + vitamin A + zinc | 7 | 1705 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.65, 0.80] |
| 10.3 Iron + other combinations | 2 | 544 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.14, 0.50] |
| 11 Haemoglobin (g/L) | 11 | 2746 | Mean Difference (IV, Random, 95% CI) | 3.37 [0.94, 5.80] |
| 12 Haemoglobin by anaemic status of participants at start of intervention (g/L) | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 12.1 Anaemic | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Non‐anaemic | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Mixed/unknown | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 13 Haemoglobin by age of children at start of intervention | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 13.1 Aged 24‐59 months | 7 | 2023 | Mean Difference (IV, Random, 95% CI) | 2.02 [‐0.87, 4.92] |
| 13.2 Aged 60 months or older | 3 | 524 | Mean Difference (IV, Random, 95% CI) | 7.86 [‐0.76, 16.49] |
| 14 Haemoglobin by malaria status of study site at time of trial | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 14.1 Yes | 4 | 954 | Mean Difference (IV, Random, 95% CI) | 2.68 [1.15, 4.22] |
| 14.2 No | 3 | 1060 | Mean Difference (IV, Random, 95% CI) | 2.31 [‐2.84, 7.46] |
| 14.3 Not reported | 3 | 533 | Mean Difference (IV, Random, 95% CI) | 7.51 [‐1.22, 16.24] |
| 15 Haemoglobin by frequency (g/L) | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.27 [0.84, 5.70] |
| 15.1 Daily | 10 | 2315 | Mean Difference (IV, Random, 95% CI) | 3.84 [1.07, 6.61] |
| 15.2 Weekly | 2 | 232 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐3.07, 2.56] |
| 15.3 Flexible | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Haemoglobin by duration of intervention | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 16.1 Less than 3 months | 3 | 887 | Mean Difference (IV, Random, 95% CI) | 2.14 [‐5.04, 9.32] |
| 16.2 3 months or longer | 7 | 1660 | Mean Difference (IV, Random, 95% CI) | 4.26 [1.23, 7.29] |
| 17 Haemoglobin by iron content of product | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 17.1 12.5 mg elemental iron or less | 7 | 1706 | Mean Difference (IV, Random, 95% CI) | 3.05 [‐0.20, 6.29] |
| 17.2 More than 12.5 mg elemental iron | 3 | 841 | Mean Difference (IV, Random, 95% CI) | 5.33 [‐1.23, 11.88] |
| 18 Haemoglobin by type of iron compound (g/L) | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 18.1 Iron EDTA | 3 | 605 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.99, 2.02] |
| 18.2 Encapsulated ferrous fumarate | 5 | 1706 | Mean Difference (IV, Random, 95% CI) | 2.81 [‐0.77, 6.38] |
| 18.3 Other | 2 | 236 | Mean Difference (IV, Random, 95% CI) | 11.42 [8.81, 14.03] |
| 19 Haemoglobin by number of nutrients accompanying iron (g/L) | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 19.1 + 1‐4 micronutrients | 3 | 1185 | Mean Difference (IV, Random, 95% CI) | 8.11 [3.70, 12.52] |
| 19.2 + 5‐9 micronutrients | 2 | 470 | Mean Difference (IV, Random, 95% CI) | 4.85 [‐5.73, 15.43] |
| 19.3 + 10‐15 micronutrients | 5 | 892 | Mean Difference (IV, Random, 95% CI) | 0.69 [0.35, 1.03] |
| 20 Haemoglobin by micronutrient composition (g/L) | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 20.1 Iron alone | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Iron + vitamin A + zinc | 7 | 1830 | Mean Difference (IV, Random, 95% CI) | 1.53 [‐0.88, 3.95] |
| 20.3 Iron + other combinations | 3 | 717 | Mean Difference (IV, Random, 95% CI) | 8.95 [3.42, 14.49] |
| 21 Iron deficiency | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 22 Iron deficiency by anaemia status at start of intervention | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 22.1 Anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Non‐anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Mixed/unknown | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 23 Iron deficiency by age of children at start of intervention | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 23.1 Aged 24‐59 months | 3 | 884 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.22, 0.48] |
| 23.2 Aged 60 months or older | 2 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.26, 0.61] |
| 24 Iron deficiency by malaria status of study site at time of trial | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 24.1 Yes | 2 | 667 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.22, 0.48] |
| 24.2 No | 2 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.26, 0.61] |
| 24.3 Not reported | 1 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.18] |
| 25 Iron deficiency by frequency | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 25.1 Daily | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Weekly | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 25.3 Flexible | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Iron deficiency by duration of intervention | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 26.1 Less than 3 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 3 months or longer | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 27 Iron deficiency by iron content of product | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 27.1 12.5 mg elemental iron or less | 3 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.26, 0.56] |
| 27.2 More than 12.5 mg elemental iron | 2 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.21, 0.50] |
| 28 Iron deficiency by type of iron compound | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 28.1 Iron EDTA | 3 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.26, 0.56] |
| 28.2 Encapsulated ferrous fumarate | 2 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.21, 0.50] |
| 28.3 Other | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Iron deficiency by number of nutrients accompanying iron | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 29.1 + 1‐4 micronutrients | 1 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.21, 0.51] |
| 29.2 + 5‐9 micronutrients | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 + 10‐15 micronutrients | 4 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.25, 0.55] |
| 30 Iron deficiency by micronutrient composition | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 30.1 Iron alone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Iron + vitamin A + zinc | 4 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.25, 0.55] |
| 30.3 Iron + other combinations | 1 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.21, 0.51] |
| 31 Ferritin (μg/L) | 3 | 1066 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐4.36, 5.19] |
| 32 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 33 Diarrhoea | 2 | 366 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.53, 1.78] |
| 34 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 35 Iron deficiency anaemia | 3 | 918 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.07, 1.10] |
| 36 All‐cause morbidity | 3 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.74, 1.23] |
| 37 Acute respiratory infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 38 Growth (height‐for‐age Z‐score) | 4 | 617 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.20, 0.17] |
| 39 Growth (weight‐for‐age Z‐score) | 3 | 502 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 40 Growth (weight‐for‐height Z‐score) | 2 | 287 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.00, 0.19] |
| 41 Adherence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 42 Serum/plasma retinol (mmol/L) | 2 | 547 | Mean Difference (IV, Random, 95% CI) | 10.08 [‐10.72, 30.88] |
| 43 Serum/plasma zinc concentrations (mmol/L) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 44 Iron status (iron‐binding capacity) (non‐prespecified) (µmol/kg) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 45 Iron status (serum‐transferrin receptors; non‐prespecified) (mg/L) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 46 Serum vitamin E (non‐prespecified) (µg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 47 Serum vitamin B12 (non‐prespecified) (pg/mL) | 2 | 329 | Mean Difference (IV, Random, 95% CI) | 241.16 [‐258.70, 741.02] |
| 48 Zinc deficiency (non‐prespecified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 49 Vitamin A deficiency (non‐prespecified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 50 Serum folate concentration (ng/mL) | 2 | 329 | Mean Difference (IV, Random, 95% CI) | 2.16 [0.76, 3.56] |
| 51 Height (non‐prespecified) (cm) | 2 | 182 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐3.71, 3.82] |
| 52 Weight (non‐prespecified) (kg) | 3 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.59, 0.55] |
| 53 Fever (non‐prespecified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 54 Stunting (non‐prespecified) | 2 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.66, 1.25] |
| 55 Angular stomatitis (non‐prespecified) | 2 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.29] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Inayati 2012 (C).
| Methods |
Study design: randomised cluster trial; 4‐arm design. Only groups 1 and 2 were randomised. Unit of allocation: villages (n = 29). |
|
| Participants |
Location of the study: the archipelago of Nias in North Sumatra, Indonesia. Selection of participants: in project area from all children who attended the monthly growth monitoring activities implemented by the Church World Service or the Government of Indonesia, or both. Selection criteria: mildly wasted children (< ‐1.0 to ≥ ‐1.5 SD, according to NCHS reference data). Children were individually discharged when they reached WHZ ≥ ‐1.0 or when intervention period ended if they did not achieve WHZ ≥ ‐1.0 during study. Sample size: 215 children. Age: ≥ 6 to < 60 months (mean age about 35 months). Sex: both (approximately 43% girls). SES: 90% of children belonged to families with low SES. Baseline prevalence of anaemia: 61%. Baseline prevalence of soil helminths: not reported. Refugee status: no. Malaria endemicity: yes: malaria control programme was implemented in project area, and therefore provision of impregnated bed‐nets and artemisinin‐based combination therapy for the treatment of malaria was provided. At time of the study, endemic malaria situation on Nias Island was stable. Several activities performed to minimise risks of iron supplementation. Continuous collection of classic symptoms of malaria (cyclical occurrence of sudden coldness and then fever occurring every 2 or 3 days) via morbidity record. Conducted culturally appropriate information, education and communication programme with regard to malaria prevention, its signs and symptoms, and its appropriate treatment. However, there were no index children with malaria during study period (information provided by author). |
|
| Interventions | Villages were assigned to 1 of the following groups (only groups 1 and 2 were randomly allocated).
Iron dose: 10 mg of elemental iron. Type of iron compound: microencapsulated ferrous fumarate (information provided by author). Other nutrients: vitamin A 375 µg RE (1250 IU), zinc 5 mg, folic acid 150 μg (0.15 mg), iodine 50 µg, vitamin C (ascorbic acid) 35 mg, thiamine 0.5 mg, riboflavin 0.5 mg, niacin 6 mg, vitamin B12 0.9 mg, vitamin B6 0.5 mg, vitamin D 5 µg (200 IU), vitamin E 4 mg, copper 0.6 mg. Provision of MNP regimen: daily (7 sachets provided weekly). Duration of intervention: varied as children were individually discharged when they reached WHZ ≥ ‐1.0 or failed to achieve WHZ ≥ ‐1.0 at the end of intervention period. Length of stay of eligible children 55 ± 34 days in group 1, 35 ± 14 days in group 2, 85 ± 19 days in group 3 and 83 ± 19 days in group 4. Final values adjusted by duration of stay in programme. Co‐intervention: intensive nutrition education. For the purposes of this review, only groups 1 and 2 were compared. Groups 3 and 4 were not randomly allocated (information provided by author). |
|
| Outcomes | Weight, weight gain, height/length, WHZ, HAZ, MUAC, Hb, anaemia, reached discharge criterion, did not reach discharge criterion and adherence. Information on adverse effects (mortality, diarrhoea, acute respiratory infections, fever and all‐cause morbidity) were provided by author. | |
| Notes | Analysis took into account the clustering effect. Used mixed model to analyse data; villages were fitted as random effect. Following variables that were not normally distributed were log‐transformed: age of mother, number of children, income per capita, HAZ, weight gain per kg bodyweight per day, height gain per day, MUAC gain per day, weight gain per day. Mixed model included fixed and random effects. Type of programme (groups 1‐4) used as fixed effect and village as random factor. Source of funding: Neys‐van Hoogstraten Foundation (Netherlands), Eiselen Foundation (Germany), DSM Nutritional Products, and CWS (Church World Service) Indonesia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Manually by random tables, only groups 1 and 2 were randomised (information provided by author). For groups 3 and 4, the agency co‐ordinating study opened new project sites which were distanced out of daily communication range with the first 2 groups' villages to avoid spread of nutrition‐related knowledge. These groups were not included in this analysis. |
| Allocation concealment (selection bias) | Low risk | Villages in existing project areas randomly allocated to groups 1 and 2. Since intervention was allocated at village level, it was unlikely there was a selection bias at the individual level. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel aware of intervention and no placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors aware of intervention (information provided by author). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Since Hb was measured at discharge, 16.3% of participants had no data, balanced across groups. |
| Selective reporting (reporting bias) | Low risk | Data for adverse effects not reported in publication but were provided by author. |
| Other bias | Unclear risk | Data not adjusted by length of intervention (information confirmed by author). |
Kemmer 2012 (C).
| Methods |
Study design: randomised controlled trial; 2‐arm design. Unit of allocation: households. |
|
| Participants |
Location of the study: rural Honduras. Selection of participants: using immunisation records at local health clinics, at least 10% of the children 6‐60 months of age in each health clinic were identified and randomised at household level. Immunisation records at health clinic used because 98% of 1‐year‐old children were immunised in Honduras against hepatitis B; measles; diphtheria, pertussis and tetanus; and tuberculosis. Selection criteria: children were excluded if anaemic (Hb < 110 g/L). Sample size: 199 children. Age: 6‐60 months of age (mean (SD) age 34.66 ± 15.31 months). Sex: both (45% girls). SES: not reported, rural Honduras. Baseline prevalence of anaemia: 0%. Baseline prevalence of soil helminths: not reported. Refugee status: no. Malaria endemicity: not reported. |
|
| Interventions | Participants were randomly assigned to 1 of 2 groups.
Iron dose: 12.5 mg of elemental iron. Type of iron compound: microencapsulated ferrous fumarate. Other nutrients: zinc 5 mg, folic acid 150 µg (0.15 mg), vitamin A 480 µg RE (1600 IU) , vitamin C 50 mg, vitamin D 7.5 µg (300 IU). Provision of MNP regimen: daily (120 sachets). Duration of intervention: 4 months with follow‐up assessment at 4 and 8 months after initiating intervention. Co‐intervention: albendazole for helminths infestation every 4 months at each visit. |
|
| Outcomes | Anaemia, Hb, iron deficiency, serum TfRs, iron deficiency anaemia, HAZ, WHZ, WAZ, adherence and acceptability. | |
| Notes |
Cost per sachet: USD 0.025. 4‐month supply of 120 MNP sachets and pictorial and verbal instructions for use provided for each child assigned to group 1. Children withdrawn from study if anaemic after 4 months of follow‐up. Based on parental responses and counting of returned empty MNP packets, children who received MNP used a mean 108/120 (90%) packets. Number of packets consumed ranged from 24 to 120. Of children who received MNP, 55% used all 120 packets, and 86% used > 100 packets. Parents reported that only 3 children (2.75%) disliked food with MNP added, 1 child had diarrhoea, and 1 had difficulty in administering the MNP. Rice, beans and soup were the foods most commonly mixed with the MNP. 54.1% of children did not notice MNP in food, 32.1% liked food better with MNP and 13.8% did not like food with them. Impossible to account for the clustering because cluster size was not available. Source of funding: Wilford Hall Medical Center, San Antonio (USA); South Dakota State University Agricultural Experiment Station, Brookings (USA); and the Center for Disaster and Humanitarian Assistance Medicine, Uniformed Services University of the Health Sciences, Bethesda (USA). Micronutrient sprinkles were provided by Heinz Company, Canada. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Households of non‐anaemic children randomly assigned to group 1 or 2. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Households randomly selected at health clinic level. Although a selection bias at individual level was unlikely, number of children allocated to each group randomised differed for group 1 (n = 114) and group 2 (n = 85). Minimum of 10% of children within each health centre were randomly selected for participation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel aware of intervention as there was no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At 4‐month assessment, only 3.5% (n = 7) loss to follow‐up or refused Hb measurement. 35.5% were anaemic at either the 4‐month assessment (20.3%) or the 8‐month assessment (15.2%); those who were anaemic were removed from study and given iron treatment. Reported that 31% of participants do not have Hb measurements. Unclear whether losses were balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Study did not adjust for household clustering (unclear whether there was only 1 eligible child per house). Children in group 1 were at a higher mean altitude (5134 feet) than children in group 2 (4876 feet). |
Kounnavong 2011 (C).
| Methods |
Study design: randomised controlled trial; 3‐arm design. Unit of allocation: households. |
|
| Participants |
Location of the study: Lahanam zone, Songkhone District, Savannakheth Province, 600 km south of the capital city, Vientiane, Lao People's Democratic Republic. Selection of participants: in 2004, entire population in study area was registered in HDSS of the National Institute of Public Health. From the HDSS database, 367 eligible preschool‐age children were identified and invited to participate if they fitted all inclusion criteria. All eligible children in each household were enrolled and followed same intervention randomly assigned to household. Selection criteria: apparently healthy infants and children 6‐53 months of age at time of recruitment; willing to participate and receive complementary food in addition to breast milk. Exclusion criteria: fever or any illnesses on the day of enrolment; baseline Hb < 70 g/L; currently taking iron supplements. Of the 367 children who met criteria, 17 were absent day of enrolment and 14 excluded for fever/illness. Sample size: 336 children. Age: 6‐52 months (mean 32 months). Sex: both (58% girls). SES: each household was categorised into 1 of 2 SES groups: high (with electricity, improved water source and latrine) and low (lacking 1 or all these). Baseline prevalence of anaemia: 48.9%. Baseline prevalence of soil helminths: not reported. Refugee status: no. Malaria endemicity: not reported but a malaria control programme was successfully executed in all villages in 10 years prior. |
|
| Interventions | Participants randomly assigned to 1 of 3 groups.
Iron dose: 10 mg of elemental iron. Type of iron compound: microencapsulated ferrous fumarate (information provided by manufacturer). Other nutrients: vitamin A 400 μg RE (1330 IU), zinc 4.1 mg, vitamin D3 5 μg (200 IU), TE vitamin E 5 mg, vitamin B1 0.5 mg, vitamin B2 0.5 mg, vitamin B6 0.5 mg, folic acid 150 μg (0.15 mg), niacin 6 mg, vitamin B12 0.9 μg, vitamin C 30 mg, selenium 17 μg, copper 0.56 mg, iodine 90 μg. Provision of MNP regimen: daily (total 168 MNP sachets) and twice weekly (total 48 MNP sachets). Duration of intervention: 24 weeks. Co‐intervention: single high‐dose vitamin A every 6 months, and children aged ≥ 24 months received single dose of mebendazole for deworming in 2 months prior to study. Children who had not received mebendazole received it during baseline survey. For the purposes of this review, the results from groups 1 and 2 were combined and only reported separately in the subgroup assessing the scheme. |
|
| Outcomes | Hb, anaemia (measured at baseline, week 12 and week 24). HAZ, WAZ, WHZ (taken every 4 weeks). | |
| Notes | Analyses in this review included the estimated effective sample size only, after adjusting data to account for clustering effect. Authors provided mean cluster size and intra cluster correlation coefficient for anaemia. All children in group 2 consumed 2 sachets of MNP per week, giving 100% compliance for this group. In group 1, 72.7% of children consumed ≥ 5 sachets of MNP per week and 43.6% of the children consumed all 7 sachets per week for all 24 weeks. Most common reason for not taking powder in group 1 was illness, such as diarrhoea (n = 20), cough (n = 10) and forgetting to take supplements (n = 32). About 42% (93/221) of mothers reported that MNP changed colour of food and 97/221 reported an unpleasant smell or taste. Some mothers mixed MNP in liquids such as juice or milk. Many mothers felt the MNP had increased their child's appetite (31.7%) and playfulness (48.4%). Source of funding: Eco‐Health Project of the Research Institute for Humanity and Nature, Kyoto (Japan), in collaboration with the National Institute of Public Health, Ministry of Health (MOH) (Lao People’s Democratic Republic (PDR)). MNP supplements were provided by UNICEF through Hygiene and Prevention Department of Ministry of Health, Lao PDR. One author received funding from the Asian Health & Education Fund, Tokyo (Japan), and partial funding through the Institute of Tropical Medicine, Nagasaki University (NEKKEN) Fellowship (Japan). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number. |
| Allocation concealment (selection bias) | High risk | No method to conceal allocation (confirmed by author). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel aware of intervention and there was no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Anthropometrists and trained technicians who collected Hb data unaware of participant group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 98.5% of participants completed study without imbalance between groups. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | High risk | Although it was unintended, Hb concentrations were significantly different at baseline between control compared with the 2 intervention groups. Children in control group had, on average, a higher mean Hb concentration and thus a lower incidence of anaemia compared with the children in the 2 supplementation groups. Baseline anaemia prevalence varied by group: daily MNP (53.6%), twice weekly MNP (58.6%) and control (34.5%). Village health volunteers monitored adherence and were aware of allocation of participants. They visited households every week and recorded the number of sachets consumed by children in the 2 intervention groups, any adverse effects and any illnesses that occurred during the study period. |
Lundeen 2010 (C).
| Methods |
Study design: cluster‐randomised, community‐based effectiveness trial; 2 arm design. Unit of allocation: villages (24). |
|
| Participants |
Location of the study: Kyrgyz Republic. Selection of participants: study took place in 2 rural areas of the Kyrgyz Republic, the Ak‐Talaa District (rayon) of the Naryn Region (oblast) and the Karabura District of the Talas Region, and in Ak‐Bosogo, on the outskirts of Bishkek, the nation’s capital. In all 3 areas, there was an intervention and a control group. In each of 3 study areas, local primary healthcare clinics provided lists of all children 6‐36 months of age. Selection criteria: residents from 1 of the above areas. Age‐eligible children required parental consent, to be consuming semi‐solid food, not currently taking iron supplements, baseline Hb ≥ 70 g/L and no severe illnesses. Sample size: 2193 children (695 children aged ≥ 24 months). Age: 6‐36 months (only data for children aged ≥ 24 months used in this review, mean 30 months). Sex: both. SES: low. Baseline prevalence of anaemia: 73%. Baseline prevalence of soil helminths: hookworm infestations were not prevalent. Refugee status: no. Malaria endemicity: no. |
|
| Interventions | Villages with children aged ≥ 24 months randomly assigned to 1 of 2 groups.
Iron dose: 12.5 mg of elemental iron. Type of iron compound: microencapsulated ferrous fumarate. Other nutrients: vitamin A (retinol acetate) 300 μg RE, zinc (zinc gluconate) 5 mg, vitamin C (ascorbic acid) 30 mg, folic acid 160 μg (0.16 mg). Provision of MNP regimen: daily. Duration of intervention: 2 months. Co‐intervention: not reported. |
|
| Outcomes | Hb, anaemia morbidity, adherence and adverse effects. | |
| Notes | Results included in this review corresponded only to children aged ≥ 24 months. Data set provided by author. Analysis adjusted by clustering effect. Source of funding: Kyrgyz‐Swiss‐Swedish Health Project, which is financed by the Swiss Agency for Development and Cooperation (SDC) and the Swedish International Development Cooperation Agency (Sida) and implemented by the Swiss Red Cross. The study was conducted through an academic collaboration between the Sprinkles Group at Sick Kids, the Research Institute of the Hospital for Sick Children in Toronto, and the Kyrgyz‐Swiss‐Swedish Health Project in Kyrgyzstan. One author, Dr. Stanley Zlotkin owns the intellectual property rights to micronutrient Sprinkles. Any profit from the licensing of Sprinkles production, after expenses, is donated to the Hospital for Sick Children Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Villages and district centre parts randomly allocated to intervention and control groups using stratified randomisation to balance size of clusters. Sequence generated by shuffling cards (in envelopes) (information provided by author). |
| Allocation concealment (selection bias) | Low risk | Not described. Since intervention was allocated at village level, unlikely there was selection bias at individual level. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel aware of intervention and there was no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors aware of intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 156/1103 (14%) children lost to follow‐up in MNP group and 168/1090 (14.4%) children in control group, mainly because they could not be located or contacted to participate in follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Low risk | Study appeared free of other sources of bias. |
Macharia‐Mutie 2012.
| Methods |
Study design: randomised, partially blinded, controlled trial; 2‐arm design. Unit of allocation: individual. |
|
| Participants |
Location of the study: Migwani and Nzauni administrative locations within the Migwani Division, Mwingi District, Kenya. Migwani and Nzauni were 2 of 6 possible administrative locations in Migwani Division that were randomly selected. Selection of participants: random walk method. Selection criteria: aged 12‐59 months, apparently healthy, and lived in village for ≥ 6 months prior to intervention and continuing to live there for next year. Sample size: 279 children. Age: 12‐59 months (mean 37 months). Sex: both (52% girls). SES: not reported but study location was in agro‐ecological zone in a semi‐arid area that experienced food shortage for most of year. Baseline prevalence of anaemia: 35.5%. Baseline prevalence of soil helminths: not reported. Refugee status: no. Malaria endemicity: yes. |
|
| Interventions | Participants were randomly assigned to 1 of 3 groups and were fed at feeding centres.
Iron dose: 2.5 mg of elemental iron. Type of iron compound: NaFeEDTA. Other nutrients: vitamin A (retinyl palmitate) 100 µg RE, zinc 2.5 mg, folic acid 90 µg (0.09 mg), vitamin C 60 mg, vitamin D3 (cholecalciferol) 5 µg, TE vitamin E (1‐a tocopheryl acetate) 5 mg, niacin 6 mg, copper 0.34 mg, iodine 30 µg, thiamine 0.5 mg, riboflavin 0.5 mg, vitamin B6 0.5 mg, vitamin B12 0.9 µg, calcium 200 µg; pantothenic 2 mg, vitamin K (phylloquinone) 30 mg, selenium 17 µg. Provision of MNP regimen: daily. Duration of intervention: 16 weeks. Co‐intervention: children who had not been dewormed in 3 months prior to start of study were dewormed. Children aged ≥ 2 years received albendazole 400 mg, whereas children aged < 2 years received albendazole 200 mg. For the purpose of this review, only groups 2 and 3 were compared. |
|
| Outcomes | Stunting, underweight or wasting (defined by Z‐score < 2 SD for anthropometric indices WHO growth standards as reference), Hb, malaria parasitaemia, ferritin, serum TfR, CRP. | |
| Notes | Target daily intake 350 mL of porridge for all children, considered an amount that they could comfortably consume in 1 session. Food prepared and MNP added prior to serving. Porridge served between 8.00 a.m. and 11.00 a.m. No malaria at baseline, whereas at endpoint, microscopy showed that 3.8% of children had malaria, which did not differ across groups. Attendance and leftovers recorded daily. Elevated acute phase protein defined as CRP > 5 mg/L and a correction factor of 0.67 for children with elevated CRP used to adjust plasma ferritin concentration. Source of funding: Nevin Scrimshaw International Nutrition Foundation/Ellison Medical Foundation (USA), the Nutricia Research Foundation (Netherlands), and the Foundation Van Dam Nutrition Plan (Netherlands). One author received support for this research from the Nestle Foundation. DSM Nutritional Products (Switzerland) provided the multiple micronutrient powders. One author also received a study fellowship from Wageningen University (Netherlands). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by age and sex generated with Excel (Microsoft) by 1 investigator not involved in recruitment and data collection. |
| Allocation concealment (selection bias) | Low risk | All serving bowls labelled with child's name and identification number. Similar serving cups equivalent to 350 mL of porridge used to serve in all centres. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants fed at feeding centres and participants were unaware of intervention. The 2 types of porridge were cooked at 3 different cooking centres from where they were distributed in thermo flasks to 7 additional centres for feeding. All serving bowls were labelled with child's name and identification number at cooking centres before distribution to feeding centres. Feeding personnel aware of intervention because amaranth porridge was slightly darker in colour and thinner in consistency compared to the maize porridge (information provided by author). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors aware of intervention (information provided by author). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 239 children completed study, equivalent to 86% of children randomised at baseline. Endpoint measurement for biochemical indicators not done for 19 children, because either their veins could not be detected (n = 5) or their caretakers declined (n = 14). 9 children were absent for end measurement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Missing data values of Hb, ferritin, and TfR imputed before primary analysis using multiple imputations. Data imputed 5 times using fully conditional specification method with default PASW Statistics initialisation value. Treatment group, number of days attended, sex, age, baseline, and postintervention weight, height, Hb, plasma ferritin and TfR concentrations used as predictors in imputation model. Pooled estimates from imputed data reported. |
Ogunlade 2011.
| Methods |
Study design: randomised, parallel‐controlled single‐blind intervention; 2‐arm design. Unit of allocation: children. |
|
| Participants |
Location of the study: North West Province, South Africa. Selection of participants: attending 1 of 8 privately owned preschools serving a low SES community. Selection criteria: age eligible participants attended 1 of 8 schools; parental consent, Hb ≥ 125 g/L; no major chronic illnesses; no recent consumption of micronutrient supplements. Sample size: 151 children. Age: 36‐79 months (mean 58 months). Sex: both (50% girls). SES: low. Baseline prevalence of anaemia: 29%. Baseline prevalence of soil helminths: not reported. Refugee status: no. Malaria endemicity: no. |
|
| Interventions | Participants randomly assigned to 1 of 2 groups.
Iron dose: 2.86 mg of elemental iron. Type of iron compound: NaFeEDTA. Other nutrients: zinc 2.86 mg, vitamin A 457 μg RE, iodine 34.3 μg, calcium 457 mg, vitamin C 68.6 mg, vitamin E 5.71 mg, vitamin B12 1.03 μg, thiamine 0.57 mg, niacin 6.86 mg, riboflavin 0.57 mg, folic acid 103 μg (0.10 mg), vitamin B6 0.57 mg. Provision of MNP regimen: daily (5 days per week). Duration of intervention: 52 school days (11 weeks). Co‐intervention: mebendazole 500 mg (antihelminthic) before start of intervention. |
|
| Outcomes | Hb, height, weight, MUAC, triceps‐skinfold thickness, cognitive function, dietary intake. recruitment, dose delivered, dose received, context and fidelity, early childhood development, adherence. | |
| Notes | Authors provided data set. Pilot study assessing feasibility of implementing a point‐of‐use micronutrient fortification. 6‐21 kg (depending on the school population) of raw maize‐meal flour provided to preschools per week to ensure that all children received standard portion sizes of porridge. Porridge for intervention group had to be stiff before addition of amylase‐rich MNP. Study assistants prepared separate breakfast meals containing 2 types of porridge daily. Source of funding: National Research Foundation (South Africa). DSM Nutritional Products South Africa (Pty) Ltd provided the micronutrient powders. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers (information provided by author). |
| Allocation concealment (selection bias) | Low risk | Authors paired groups of 2 children per school based on Hb level and randomly allocated 1 in each pair to treatment or placebo. Code was only broken when data analysis was completed (information provided by author). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and schools were blinded to intervention. Maize porridge breakfast meals served in colour‐coded plastic plates. Supplement caused the porridge to become more fluid so it was not possible to conceal it to the children or caretakers; they were able to observe a difference but did not know which was treatment and which placebo (information provided by author). Quote: 'The intervention group (n = 76) received stiff maize meal porridge with added micronutrient powder (<8 g) (containing amylase‐rich light malted barley flour), while those in the control group (n = 75) received soft maize‐meal porridge with added placebo powder (<8 g) containing only maize maltodextrin. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cognitive assessors and research team did not know which child was allocated to which treatment and code was only broken once analysis of data was completed (information provided by author). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall attrition of 13.2%. Attrition of 17.1% in intervention group and 9.3% in control group. 12 children who completed follow‐up were excluded from final analyses because of recurrent absenteeism (< 60% adherence to study regimen). |
| Selective reporting (reporting bias) | High risk | 12 children excluded from analysis due to low adherence to study regimen (< 60%). |
| Other bias | Low risk | Study appeared free of other sources of bias. |
Orozco 2015 (C).
| Methods |
Study design: cluster‐randomised, triple‐blind, placebo‐controlled trial; 2 arms. Unit and method of allocation: children's centres (cluster) using random blocks of variable length. |
|
| Participants |
Location of the study: Fundación de Atención a la Niñez in Medellín, Colombia during 2013. Selection of participants: children with full attendance (8 hours). Selection criteria: no anaemia or severe acute malnutrition, registered in 2 children's centres of a non‐governmental organisation. Sample size: 90 children. Age: 2‐5 years (inclusive), mean (SD) age: 4.8 ± 0.3 years. Sex: both (47.8% girls). SES: most resided in houses classified as stratum 1, 2 and 3; mainly extended or joint families who lived with people of different generations in same residence and with a majority proportion of parents with high‐school studies. Baseline prevalence of anaemia: non‐anaemic. Baseline prevalence of soil helminths: not encountered. However, 51.1% of participants presented intestinal parasitic infestations such as Blastocystis Hominis, cysts of Endolimax Nana, Giardia Duodenalis, Entamoeba Coli, Entamoeba Histolytica and Iodamoeba Büstschlii. Refugee status: no. Malaria endemicity: not reported. |
|
| Interventions | Participants from the 2 different centres randomly assigned to 1 of 2 groups.
Iron dose: 12.5 mg of elemental iron. Type of iron compound: microencapsulated ferrous fumarate. Other nutrients: vitamin A (retinyl acetate) 400 µg RE, zinc (zinc gluconate) 4.1 mg, folic acid 150 μg, iodine (potassium iodide) 90 µg, vitamin C (ascorbic acid) 30 mg, thiamine 0.5 mg, riboflavin 0.5 mg, niacin (as nicotinamide) 6 mg, vitamin B12 0.9 µg, vitamin B6 0.5 mg, vitamin D (ergocalciferol) 5 µg, vitamin E (all‐rac‐α‐tocopherol) 5 mg, copper 0.56 mg. Provision of MNP regimen: daily (5 days per week). Duration of intervention: 9 weeks. Co‐intervention: children in placebo group received powder matrix with maltodextrins, corresponding to vehicle used in children of intervention group. All children received 10 mL of albendazole in syrup at beginning of study. |
|
| Outcomes | Anaemia, Hb, serum ferritin, serum transferrin, serum folate, weight, height, body mass index, adverse effects (nausea, abdominal pain, other). | |
| Notes | Source of funding: Universidad CES, Medellín, Colombia and Nutreo SAS (private food company). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as randomly allocated using random blocks of variable length. |
| Allocation concealment (selection bias) | Low risk | As children were assigned by centres with placebo and blinded, it was unlikely that intervention allocations could have been foreseen. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel blinded to intervention and placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded to intervention and placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants lost to follow‐up, 2 in each group. Authors indicated that losses were not related to variables of interest of study, but to social and economic situation of preschool‐age children's families. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess as there was no report of registration of a protocol. |
| Other bias | High risk | At start of study, mean (SD) age of preschool children was 4.8 ± 0.3 years, with a minimum age of 3.8 years and a maximum of 5.2 years, with statistical differences between the 2 groups. In addition, 71.1% of participants presented an adequate nutritional status, compared to 25.6% who had malnutrition due to excess (15.6% were overweight and 10% were obese). Significant differences in nutritional status between groups at beginning of study. |
Osei 2008 (C).
| Methods |
Study design: single‐blind, placebo‐controlled, cluster‐randomised; 2‐arm design. Unit of allocation: public primary schools (20). |
|
| Participants |
Location of the study: Tehri Garhwal, a hilly agrarian community located in mid‐Himalayan ranges of Uttarakhand State, 250 km from New Delhi, India. Selection of participants: children in grades 1‐4 in public primary schools. 20 schools selected for study from across 9 blocks (sub districts) in Tehri Garhwal district using a stratified random sampling procedure. 2 schools picked randomly from each stratum to participate and 1 school was randomly assigned to intervention and other to control arm. Within each selected school, names of all children were obtained and 25 were randomly selected for anthropometric, biochemical, and parasitological assessments. Selection criteria: parental consent; not severely anaemic (Hb < 70 g/L); no sickle cell disease, HIV or tuberculosis. Sample size: 499 children. Age: 6‐10 years (mean (SD) age 7.0 ± 1.0 years). Sex: both (52.1% girls). SES: not reported, but local population engaged primarily in subsistence agriculture. Baseline prevalence of anaemia: 36.7%. Baseline prevalence of soil helminths: 7.6%. Refugee status: no. Malaria endemicity: malaria is not endemic in the Tehri Garhwal district. |
|
| Interventions | 2 schools from each strata randomly assigned to 1 of 2 groups.
Iron dose: 10 mg of elemental iron. Type of iron compound: NaFeEDTA. Other nutrients: vitamin A (retinyl acetate) 375 µg RE, zinc (zinc gluconate) 4.2 mg, folic acid 225 μg, iodine (potassium iodide) 90 µg, vitamin C (ascorbic acid) 26.25 mg, thiamine (thiamine mononitrate) 0.68 mg, riboflavin (as riboflavin 5‐phosphate sodium) 0.68 mg, niacin (as nicotinamide) 9 mg, vitamin B12 (1% on mannitol, as carrier) 1.35 µg, vitamin B6 (pyridoxine hydrochloride) 0.75 mg, vitamin D (ergocalciferol) 3.75 µg, vitamin E (all‐rac‐α‐tocopherol) 5.25 mg, copper 0.45 mg. Provision of MNP regimen: daily (6 days per week). Duration of intervention: 8 months (1 school year). Co‐intervention: all children involved in anthropometric, biochemical and parasitological assessments (n = 25/school) given sweets or fruit juice (or both) after assessments and administered albendazole 500 mg orally before beginning fortification. |
|
| Outcomes | Anaemia, Hb, serum ferritin, serum retinol, zinc, folate, vitamin B12, serum TfR, total body iron, prevalence of anaemia, iron deficiency anaemia, adherence, HAZ, WAZ, WHZ, weight, height, diarrhoea, fever, cough, runny nose, vomiting, intestinal helminth infections. | |
| Notes | Analyses in this review included the estimated effective sample size only, after adjusting data to account for clustering effect. Premix provided as 500‐g packs accompanied by 2 sets of standardised plastic spoons that measured 0.5 g (for 2 children) and 2.5 g (for 10 children) of premix. Schools provided with 1 monthly supply of premix and dark brown plastic containers for storage after opening packet to prevent breakdown of light‐sensitive micronutrients. After meal preparation, school cook measured appropriate number of spoons of premix (based on number of children present), which was mixed thoroughly with small quantity of water and then added to food at room temperature. At baseline, children in the 2 groups did not differ in age; gender; anthropometric indices; intestinal parasite infection; recent morbidities; and circulating concentrations of Hb, ferritin, retinol, zinc, folate and vitamin B12. Study enumerators visited twice weekly to study schools to assess registers to ensure correct procedures were followed. On such visits, enumerators observed premix addition to meals, provided schools with additional premix if needed and collected packaging material for used premix. Blood spots made on filter paper cards for laboratory determination of serum retinol. Source of funding: Micronutrient Initiative (Canada), World Food Programme (Italy), Tufts University (USA), and International Nutrition Foundation (USA). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Names of schools were written on identical pieces of paper that were folded and shuffled and used to allocate intervention. |
| Allocation concealment (selection bias) | Low risk | Only study co‐ordinator and senior World Food Program officials in Delhi had access to codes to premix assignment. In addition, as this was a cluster trial, it was unlikely that there was selection bias at individual level. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were unaware of intervention. Micronutrient and placebo premix were in identical packets, which had no information easily identifying the contents. Only study co‐ordinator and senior World Food Program officials in Delhi had access to codes to premix assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study co‐ordinator had access to codes to premix assignment; unclear whether they assessed outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After intervention, 9% (n = 44) of participants were lost to follow‐up (11.6% in intervention group and 6% in control group). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allow judgement. |
| Other bias | Unclear risk | 2 selected schools were replaced using the same sampling procedure because the paths to these schools considered dangerous due to risk of wild animal attack. Data not adjusted for clustering. |
Sharieff 2006 (C).
| Methods |
Study design: cluster randomised clinical trial; 3‐arm design. Unit of allocation: classrooms (16). |
|
| Participants |
Location of the study: Baotou City, Neimenggu Autonomous Region, Northern China. Selection of participants: children attending Xin‐shi‐dai kindergarten (16 classroom clusters) in Baotou City. 4 classrooms for each group of 3‐year olds, 4‐year olds, 5‐year olds and 6‐year olds. Total 16 classrooms to select from, 6 assigned to daily MNP, 5 assigned to weekly MNP and 5 assigned to control. Selection criteria: enrolled in the school. Written parental consent. Sample size: 415 children. Age: 36‐60 months old (mean 57 months). Sex: both (approximately 45% girls). SES: parents had to pay a fee to admit their children to school; thus this was a relatively wealthy subgroup of the regional population. Baseline prevalence of anaemia: not reported. Baseline prevalence of soil helminths: not reported. Refugee status: not reported. Malaria endemicity: not reported. |
|
| Interventions | 1 classroom (cluster) in each age group randomly assigned to 1 of 3 groups.
Iron dose: 30 mg of elemental iron. Type of iron compound: microencapsulated ferrous fumarate. Other nutrients: zinc (gluconate) 5 mg, vitamin A 300 μg RE, vitamin C 50 mg, vitamin D3 7.5 μg, folic acid 150 μg. Provision of MNP regimen: daily (65 sachets) (group 1) and weekly (13 sachets) (group 2). Duration of intervention: 13 weeks. Co‐intervention: not described. For the purposes of this review groups 1 and 2 were combined and compared with group 3. They were only split for the subgroup analysis by scheme. |
|
| Outcomes | Number of MNP sachets consumed per child over the 13‐week period, serum ferritin concentration, free erythrocyte protoporphyrin and Hb concentration at end of study, HAZ, WAZ (data not reported), WHZ (data not reported), staining of teeth, metallic taste, stomach upset or any other adverse effects, compliance. | |
| Notes | Analyses in this review included the estimated effective sample size only, after adjusting data to account for clustering effect. Supplement mixed with standardised semi‐solid meal of rice porridge or congee. Control group received same meal but without MNP. Children added MNP sachet to their food under supervision by school teachers. Mean MNP consumption rate per child was 86% (daily group; SD 12%) and 87% (weekly group; SD 16%) adherence. On measures of anthropometric indices, we did not observe any significant differences among groups (data not shown). There were no reported adverse effects such as staining of teeth, metallic taste or stomach upset. Direct observations by research staff suggested that children did not comment on any change in taste of MNP‐served congee and accepted it well. Source of funding: Canadian Institutes of Health Research (Canada) and the HJ Heinz Foundation (USA). One of the authors owns the intellectual property rights to micronutrient Sprinkles®. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used for generation of random sequence not described. For each of the 4 age groups (3, 4, 5 and 6 year olds), 1 classroom randomly assigned to each of 3 arms (total 12 classrooms/clusters); no description of how remaining 4 of original 16 classrooms assigned to the 3 arms. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. Since interventions were allocated at classroom level selection bias at individual level was unlikely. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and students aware of intervention. After some acclimatisation period, children added MNP sachet contents to their congee (under supervision of the teachers). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors aware of intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants enrolled in each group completed study but venous blood samples were available for 86% of participants in group 1 (daily), 84% in group 2 (weekly) and 85% in control group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Analyses in this review included the estimated effective sample size only, after adjusting data to account for clustering effect. No Hb samples taken at baseline for control group (group 3) for ethical reasons. At baseline, except for sex, the demographic characteristics were similar across groups (daily 39% boys, weekly 63% boys and control 62% boys). |
Troesch 2011b.
| Methods |
Study design: randomised double‐blind placebo‐control trial; 2‐arm design. Unit of allocation: individual. |
|
| Participants |
Location of the study: Kimberley, Northern Cape, South Africa. Selection of participants: children attending preschool through grade 5 in either of 2 participating schools. Selection criteria: serum ferritin < 20 μg/L or serum TfR > 8.2 pg/L; Hb > 90 g/L; age 5‐11 years; no serious chronic medical problems; not taking nutritional supplements containing iron; parental consent. Sample size: 200 children. Age: 5‐11 years. Sex: both (45% girls). SES: low. Baseline prevalence of anaemia: 7.25% (6.3% intervention and 8.2% control). Baseline prevalence of soil helminths: not reported. Refugee status: no. Malaria endemicity: region free of malaria. |
|
| Interventions | Participants randomly assigned to 1 of 2 groups.
Iron dose: 2.5 mg of elemental iron. Type of iron compound: NaFeEDTA. Other nutrients: zinc (as zinc oxide) 2.5 mg, ascorbic acid 60 mg, vitamin A (retinyl palmitate) 400 µg RE, folic acid 90 µg (0.09 mg), vitamin D3 5 µg, vitamin E 5 mg, niacin 6 mg, copper 340 µg, iodine 30 µg, thiamine 0.5 mg, riboflavin 0.5 mg, vitamin B6 (pyridoxine) 0.5 mg, vitamin B12 0.9 µg, calcium 200 µg; vitamin B5 (pantothenic acid) 2 mg, selenium 17 µg, phytase. Provision of MNP regimen: daily (5 days per week) (total 113 days). Duration of intervention: 23 weeks. Co‐intervention: all participating children received antihelminthic treatment at baseline with mebendazole 500 mg orally. |
|
| Outcomes |
Primary outcomes: iron and zinc status. Other outcomes: height, weight, HAZ, WAZ, WHZ, triceps skin fold, subscapular skin fold, MUAC and adherence. |
|
| Notes | All participants consumed a bowl of 250 g of sweetened high‐phytate maize porridge prepared by trained field workers each morning with partially degermed, unfortified maize flour, water and a small quantity of sucrose. Porridge provided in addition to lunch meal of existing lunch feeding programme. No differences in the prevalence of elevated CRP (inflammation) between groups at baseline or 6 months, so because of low prevalence, children with elevated values were included in analysis, which had no substantial effect on results. Source of funding: Foundation Nutrition Industry (Switzerland), established by DSM Nutritional Products Ltd, ETH (Eidgenössische Technische Hochschule) Zurich (Switzerland); Medical Research Council, (South Africa); and North‐West University, Potchefstroom, (South Africa). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence unclear. |
| Allocation concealment (selection bias) | Low risk | Cooked porridge was served in colour‐coded bowls. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and caretakers blinded to interventions. Control group received an identical‐appearing powder consisting of unfortified carrier. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% did not complete study (5 participants in intervention group and 3 participants in control group). |
| Selective reporting (reporting bias) | Unclear risk | Data on adverse effects not reported. |
| Other bias | Low risk | Sex ratio varied at baseline between groups but was not different statistically P > 0.05 (boy:girl ratio: intervention 61:39; control 50:50). Analyses were adjusted for sex. |
Varma 2007 (C).
| Methods |
Study design: cluster randomised double‐blind trial; 2‐arm design. Unit of allocation: Anganwandi (daycare) centres (30). |
|
| Participants |
Location of the study: Mahestala block in South 24 Parganas, West Bengal, India. Selection of participants: 1 Anganwadi centre per 1000 people in general population and a mean of 20‐30 children per centre. To be eligible, a centre had to have 20 regularly attending children and a regular supply of rice and lentils from Integrated Child Development Service, and Anganwadi personnel needed to be willing to participate. Selection criteria: attending village‐based Integrated Child Development Service Anganwadi (daycare centres). Excluded if they had severe anaemia (Hb 80 g/L) and history of not attending the Anganwadi centre 5 times/week during the past 6 months. Sample size: 684 children. Age: 36‐66 months (mean 3.9 years). Sex: both. SES: not explicitly reported but likely low as children participating in the Integrated Child Development Service and attending the Anganwadi (daycare) centres received food supplements to improve health and nutritional status and relieve short‐term hunger. Baseline prevalence of anaemia: 25%. Baseline prevalence of soil helminths: prevalence of hookworm and other intestinal parasites low in this section of West Bengal. Refugee status: no. Malaria endemicity: low. |
|
| Interventions | Participating centres randomly assigned to 1 of 2 groups.
Iron dose: 14 mg of elemental iron. Type of iron compound: microencapsulated ferrous fumarate. Other nutrients: vitamin A (retinyl acetate) 150 µg RE (500 IU), folic acid 50 μg (0.05 mg). Provision of MNP regimen: daily (6 times/week). Duration of intervention: 24 weeks. Co‐intervention: none. |
|
| Outcomes | Hb, serum ferritin, serum retinol, prevalence rates of anaemia, iron deficiency, vitamin A deficiency, low vitamin A status, fever, abdominal pain, blood in stools, coughing. | |
| Notes | Analyses in this review included the estimated effective sample size only, after adjusting data to account for clustering effect. All attending children received a single 200 g portion of the khichdi treatment assigned to their Anganwadi centre. Anganwadi workers were taught proper storage procedures for the fortified premix to ensure that the premix was not exposed to excessive light or high humidity. Anganwadi workers were also taught proper preparation techniques (i.e. they were instructed to thoroughly mix the premix with the khichdi after the khichdi had cooled for 10 minutes to ensure a homogeneous mixture). Both premixes packed in resealable polyethylene bags in 500‐g increments. Each selected Anganwadi centre received 500‐g premix at baseline and after 3 months of intervention. Source of funding: Molecular Diagnostics (India) and the Child in Need Institute (India), Micronutrient Initiative (Canada) and Tufts University Friedman School of Nutrition Science and Policy (USA). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated with a random number table. |
| Allocation concealment (selection bias) | Low risk | Since interventions were allocated at Agarwandi level, selection bias at individual level unlikely. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded to interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the children enrolled, 168 (24.5%) were lost to follow‐up before the 24‐week assessment, with some imbalance between groups. 98/342 (28.6%) participants withdrew or were lost to follow‐up in the fortified khichdi, while 73/342 (21.3%) participants withdrew or were lost to follow‐up in the non‐fortified khichdi. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allow judgement. |
| Other bias | Low risk | None apparent. |
Vinodkumar 2006 (C).
| Methods |
Study design: pre‐ and post‐test with intervention and control schools randomly selected. Unit of allocation: school (5). |
|
| Participants |
Location of study: Chennai, Tamilnadu, South India. Selection of participants: from a survey of residential schools, study schools were selected prior to randomisation because these children had the lowest intake of outside (unfortified) cooked foods and schools had fewest holidays where children were allowed to go home, which would cause less disruption in study. Selection criteria: children in control group (might also be intervention children but not written clearly) with severe anaemia and vitamin A deficiency were treated and excluded. Intervention and control group children were selected after establishing their homogeneity in terms of age and SES. Sample size: 413 children. Age: 5‐15 years. Sex: both (57% girls, information provided by author). SES: low, families of all children had a monthly income < INR 1500 (USD 30). Baseline prevalence of anaemia: not reported. Baseline prevalence of soil helminths: likely high prevalence as all children were dewormed at baseline, after 4 months and after 9 months. Refugee status: no. Malaria endemicity: not reported. |
|
| Interventions | In intervention schools, there was a dosage of 1 g per child per day so that every month the required quantity for all children was pre measured, packed, sealed and delivered to the central kitchen at the schools so that 1 packet could be cut open every day and added to food during cooking. Supplement was dissolved in water and added to liquid food in the final stages of cooking, and it was sprinkles onto solid foods. All children ate in a central dining room.
Iron dose: 10 mg of elemental iron. Type of iron compound: chelated ferrous sulphate (along with malic acid as a biopromoter). Other nutrients: (per 1 g) vitamin A 900 µg RE (3000 IU), vitamin B2 1 mg, calcium pantothenate 1 mg, niacin 15 mg, vitamin B6 1 mg, vitamin E 30 IU, vitamin C 30 mg, lysine 250 mg, 13.75% of weight calcium. Provision of MNP regimen: daily. Duration of intervention: 9 months. Co‐intervention: deworming at baseline, after 4 months, and at endpoint (9 months). |
|
| Outcomes | Hb, anaemia, serum vitamin A, vitamin E, vitamin B12, folate, clinical signs of vitamin A deficiency (Bitot's spots, xerosis), angular stomatitis, height, weight. | |
| Notes | Analyses in this review included the estimated effective sample size only, after adjusting data to account for clustering effect. Vitamin A, folic acid, vitamin B12 and vitamin E were only analysed in certain participants, such as those with clinical signs of vitamin A deficiency or low Hb values. Generally observed that no waste of food prepared in schools and all prepared food was consumed. Children served themselves desired quantities and usually no food was left over on plate. No adverse effects reported (information provided by author). Source of funding: Sundar Serendipity Foundation (India) and MS Swaminathan Research Foundation (India). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study schools chosen prior to randomisation, which was done by computer‐generated random table (latter information provided by author). |
| Allocation concealment (selection bias) | Low risk | Since interventions were allocated at school level, selection bias at individual level unlikely. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel aware of treatment and used no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | School aware of intervention (information provided by author). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All children completed study. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allow judgement. |
| Other bias | Low risk | None apparent. |
Vinodkumar 2009 (C).
| Methods |
Study design: pre‐ and post‐test design with randomly selected intervention and control groups. Unit of allocation: schools (2). |
|
| Participants |
Location of the study: Chennai, Tamilnadu, India. Selection of participants: randomly selected an intervention and control school from a list of schools in Chennai that provide a noon meal. All parents gave informed consent. Selection criteria: children with severe anaemia (Hb < 8 g/dL) treated and excluded from both intervention and control schools. Sample size: 136 children. Age: 5‐9 years of age. Sex: both. SES: low. Families had monthly income < INR 2000 (USD 50). Baseline prevalence of anaemia: 60% intervention and 92.1% control groups. Baseline prevalence of soil helminths: not reported. Refugee status: no. Malaria endemicity: not reported. |
|
| Interventions | Intervention school provided with powder to add to school lunches. Participants randomly assigned to 1 of 2 groups.
Iron dose: 28 mg of elemental iron. Type of iron compound: ferrous glycine phosphate. Other nutrients: riboflavin 1 mg. Provision of MNP regimen: daily (5 times/week) (total 144 days). Duration of intervention: 6 months (July to December). Co‐intervention: children in both groups were de wormed with albendazole (400 mg) at baseline and end of study. |
|
| Outcomes | Hb, anaemia, clinical assessment of angular stomatitis. | |
| Notes | Analyses in this review include the estimated effective sample size only, after adjusting data to account for clustering effect. Children were homogenous in terms of age and SES. Mean attendance during the 6 months of study was > 90%. Source of funding: Sundar Serendipity Foundation (India) and MS Swaminathan Research Foundation (India). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The 2 schools were randomly selected. Method used to allocate intervention not described. |
| Allocation concealment (selection bias) | Low risk | Since interventions were allocated at school level selection bias at individual level unlikely. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel aware of intervention and used no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All children completed study. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allow judgement. |
| Other bias | High risk | Serious imbalance between groups for baseline Hb and anaemia. |
CRP: C‐reactive protein; HAZ: height‐for‐age Z‐score; Hb: haemoglobin; HDSS: Health and Demographic Surveillance System; MNP: micronutrient powder; MUAC: mid‐upper arm circumference; n: number of children; NaFeEDTA: sodium iron ethylenediaminetetraacetic acid; NCHS: National Center for Health Statistics; RE: retinol equivalent; SD: standard deviation; SES: socioeconomic status; TE: tocopherol equivalent; TfR: transferrin receptor; UNICEF: The United Nations Children's Fund; WAZ: weight‐for‐age Z‐score; WHO: World Health Organization; WHZ: weight‐for‐height Z‐score.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aboud 2011 | 302 mothers and children aged 8‐20 months from 45 rural villages of Khansama sub district of northern Bangladesh randomly assigned according to village (cluster) to 1 of 3 groups: group 1 (16 villages): participating mothers received 12 informational sessions on health and nutrition; group 2 (15 villages): participants received an additional 6 sessions delivered by peer educators who included modelling and coached practice in self‐feeding and verbal responsiveness with the child during play; group 3 (14 villages): along with the sessions, 6 months of a food powder fortified with minerals and vitamins. Sachets contained 12.5 mg of elemental iron, vitamin A 300 μg, folic acid 150 μg, vitamin C 50 mg, zinc 5 mg. Developmental outcomes included Home Observation for Measurement of the Environment Inventory, mother‐child responsive talk and language development. Nutritional outcomes included weight, height, self‐feeding and mouthfuls eaten. Age of children outside scope of review. |
| Ahmed 2003 | Consecutive 200 children aged 12‐59 months with diagnosis of iron deficiency anaemia based on history, physical examination, CBC and serum ferritin levels attending the Combined Military Hospital, Multan, Punjab Province of Pakistan for any health problem and their healthy siblings randomly assigned to 1 of 2 groups: group 1: iron in syrup at 6 mg/kg of elemental iron per day divided into 3 doses; group 2: equivalent doses of iron powder sprinkled over food. Iron powder obtained by crushing ferrous sulphate tablets. Tablet divided into 4 fractions and subsequently crushed and dispensed in plastic sachet of 0.25 tablet. Iron powder was sprinkled over rice, potatoes and porridge. Participants were followed up with Hb estimation and reticulocyte response at 2, 4 and 6 weeks. CBC and serum ferritin repeated at 6 weeks. 51% of participants were 12‐24 months of age. Type of participants did not meet the inclusion criteria for this review. |
| Angdembe 2015 | Cross‐sectional study carried out in Saturia Upazilla (sub district) of Manikganj District in rural Bangladesh among mothers of children aged 6‐59 months who received multiple MNP (Pushtikona) containing per sachet: vitamin A 0.4 mg, vitamin C 30 mg, vitamin D 0.005 mg, vitamin E 5 mg, vitamin B1 0.5 mg, vitamin B2 0.5 mg, niacin 6 mg, pyridoxine 0.5 mg, vitamin B12 0.0009 mg, folic acid 0.15 mg, elemental iron 10 mg, zinc 4.1 mg, copper 0.56 mg, selenium, iodine 0.09 mg sprinkled onto any semi‐solid food supplied by BRAC South Sudan in the past 60 days to assess adherence to MNP and associated factors. Study design outside scope of review. |
| Bagni 2009 (C) | 360 children aged 12‐60 months attending 4 public daycare centres in Rio de Janeiro, Brazil, were randomly assigned to 1 of 2 groups: group 1 (n = 180): daily meal prepared with iron‐fortified rice (with iron bisglicinate); group 2 (n = 174): non‐fortified placebo rice. Rice fortified once a week for 16 weeks with iron 4.2 mg for every 100 g of food ready in supplemented group. On days of fortification, solution added as iron drops to rice by researcher during assembly of the dishes from lunch. If the child requested an additional portion, the fortification solution (iron drops) was also administered to that additional portion, in a similar proportion to the amount of rice offered previously. Type of intervention is point‐of‐use fortification with iron drops and was outside the scope of this review. |
| Chen 2008 | 226 apparently healthy preschool children (24‐60 years old) from 15 nurseries or kindergartens in the Banan District of Chongqing, China, were randomly assigned to 1 of 3 groups for 6 months: group 1 (n = 61): fortified powder containing vitamin A (as acetate) 500 μg; group 2 (n = 71): fortified powder containing vitamin A (as acetate) 500 μg + 12 mg of elemental iron (as ferric sodium edentate); group 3 (n = 94): fortified powder containing vitamin A (as acetate) 500 μg + 12 mg of elemental iron (as ferric sodium edentate) + zinc (as zinc oxide) 12 mg, thiamine (as thiamine mononitrate) 0.7 mg, riboflavin 0.7 mg, folic acid 200 μg, niacinamide 7 mg, calcium (as calcium carbonate) 800 mg. Powders were sprinkled over porridge, soy milk, soup or noodles after cooking and were indistinguishable in taste, colour and packaging. Foods prepared with powders were delivered to each child at lunchtime or afternoon snack time 5 days a week. Type of intervention involved a fortified condiment or seasoning in powder form and not an MNP for point‐of‐use fortification. |
| Clarke 2015 | Cluster‐randomised controlled trial conducted in 60 rural communities with community‐based preschools in southern Mali. Children aged < 5 years living in 30 intervention communities received 2 rounds of seasonal malaria chemoprevention in October and November 2013, followed by home fortification with MNP for 4 months from January to April 2014. Delivery of interventions at community‐level organised by preschool management committees. Combined impact of interventions evaluated in May 2014 through cross‐sectional surveys to compare malaria infection, nutritional indices and cognitive performance. This was a before‐and‐after study without control assessing the combination of 2 interventions. The abstract contained limited additional information. |
| De Pee 2007 | Post‐tsunami experience with distribution of Vitalita sprinkles in Aceh and Nias, Indonesia and analysis data on knowledge, recognition of package, consumption and acceptability by mothers and children. Intervention did not have control group and was a descriptive article. |
| Geltman 2009 | 150 healthy 5‐ to 7‐month‐old infants randomly assigned to 1 of 2 groups: group 1 (n = 74): daily packet of MNP (Supplefer®) sprinkles (Sprinkles Global Health Initiative, Toronto, Ontario) containing 12.5 mg of elemental iron (as encapsulated iron) + vitamin A 480 µg RE (1600 IU), vitamin C 30 mg, folic acid 160 μg (0.16 mg), zinc 5 mg; group 2 (n = 76): multiple micronutrient drops (Tri‐Vi‐Sol with Iron®) (Mead Johnson and Company, Evansville, Indiana) containing 10 mg of elemental iron (as sulphate heptahydrate), vitamin A 450 µg RE (1500 IU), vitamin D 400 IU, and vitamin C 35 mg. Follow‐up included alternating telephone and home visits twice weekly for 3 months. Adherence was primary outcome and adverse effects and caretaker's attitude about supplements were secondary outcomes. Use of ferrous fumarate powder rather than traditional ferrous sulphate drops did not improve adherence with daily iron supplementation in low‐income infants. The study compares provision of micronutrients in powders to be added to food versus the provision of micronutrient in drops. Participants outside age range defined for inclusion in this review. Type of participants and type of comparisons are outside scope of this review. |
| Gibson 2010 | Trial aimed to find out whether adding a small quantity of powdered beef liver to daycare meals of Brazilian preschool children from Salvador for 12 months could prevent anaemia and micronutrient deficiencies, improve growth, health and development in the same way or better than adding a small quantity of micronutrients in powder form (Sprinkles). Trial not conducted because baseline micronutrient survey data showed no evidence of micronutrient deficiencies among the preschool children (personal communication). |
| Hirve 2007 | 432 anaemic (Hb 70‐100 g/L) children aged 6‐18 months, both sexes, living in Maharashtra, India, during 2004 and 2005, taking semi‐solid or solid weaning foods, not taking hematitic, likely to remain within study area for 2 months, with no major illness and non‐severe anaemia (Hb < 70 g/L). The 21 villages (n = 432) were randomised into 5 groups: group 1 (n = 84): daily MNP containing 12.5 mg of elemental iron (as microencapsulated ferrous fumarate), zinc (as gluconate) 5 mg, vitamin A 300 μg RE, and ascorbic acid 30 mg, folic acid 160 μg; group 2 (n = 83): daily MNP with 20 mg of elemental iron (as microencapsulated ferrous fumarate), zinc (as gluconate) 5 mg, vitamin A (as acetate) 300 μg RE, ascorbic acid 30 mg, folic acid 160 μg (0.16 mg); group 3 (n = 101): daily MNP with 30 mg of elemental iron (as microencapsulated ferrous fumarate), zinc (as gluconate) 5 mg, vitamin A 300 μg RE and ascorbic acid (as acetate) 30 mg, folic acid 160 μg; group 4 (n = 82): MNP daily containing 20 mg of elemental iron (as micronized ferric pyrophosphate), zinc (as gluconate) 5 mg, vitamin A (as acetate) 300 μg RE, ascorbic acid 30 mg, folic acid 160 μg; group 5 (n = 83): iron drops containing 20 mg of elemental iron (as ferrous glycine sulphate drops) daily. Type participants outside age range defined for inclusion in this review. |
| Huamán‐Espino 2012 | 714 infants and young children 6‐35 months of age participating in a pilot project carried out between December 2009 and August 2010 in regions of Apurimac, Ayacucho and Huancavelica, Peru. Intervention provided to all infants and young children in these communities. Protocol indicated that infants and children should be provided with at least 15 sachets of multiple MNP per month during 6‐month period. Multiple MNP provided contained 12.5 mg of elemental iron (as ferrous fumarate), zinc 5 mg, ascorbic acid 30 mg, vitamin A ˜300 µg RE (999 IU), folic acid 0.16 mg. MNP sachets were distributed as part of a grant from the World Food Programme to the Government of Peru. Cross‐sectional study conducted between October and November 2010 in 6 of 7 provinces in the Apurimac region to assess implementation of the universal "Chispitas®" multiple micronutrient supplement programme by determining the quantity and quality of sachets consumed and their connection with anaemia. Type of study design and comparisons outside scope defined for inclusion in this review. |
| Ip 2009 | 362 children (Hb ≥ 70 g/L) aged 6‐24 months living in 16 villages in Kaliganj sub district of Gazipur district in Bangladesh in this cluster‐randomised design were assigned to 1 of 3 groups: group 1 (5 villages, n = 120): 60 sachets of MNP daily over 2 months; group 2 (6 villages, n = 120): MNP flexibly over 3 months; group 3 (5 villages, n = 122): MNP flexibly over 4 months. Content of Sprinkles sachets was identical for all groups and included 12.5 mg of elemental iron (as microencapsulated ferrous fumarate), zinc (as zinc gluconate) 5 mg, vitamin A as retinol acetate 300 µg RE, folic acid 160 μg (0.16 mg), vitamin C 50 mg. Study conducted from May to September 2004. With a flexible regimen, mothers/caretakers decided how frequently to use MNP without exceeding 1 sachet per day. Outcomes postintervention included adherence, acceptability and haematological status, which also was evaluated at 6 months postintervention. The adherence, acceptability and haematological response to flexible administration over 4 months were preferable to daily. Participants outside age range defined for inclusion in this review. |
| Jack 2012 | 3112 infants aged 6‐7 months residing in Svay Rieng Operational Health District, Cambodia who were identified through listings of infants at health centre and village levels. This district was representative of rural Cambodia with a reasonably well‐functioning government health system and a low malaria incidence rate (< 1 case/1000 population). Cluster‐randomised trial with health centre catchment area as unit of randomisation. Clusters randomly assigned to 1 of 2 interventions: group 1 (10 centres, n = 1579): infant and young child feeding education only; group 2 (10 centres, n = 1533): infant and young child feeding education and daily Sprinkles in single‐dose sachets, delivered monthly to their homes by government village health workers. Sprinkles were mixed with the infant's meal immediately before serving. MNPs contained 12.5 mg of elemental iron (as microencapsulated ferrous fumarate), zinc (as gluconate) 10 mg, vitamin A (as retinol acetate) 300 μg RE, iodine 90 μg, vitamin B1 0.5 mg, vitamin B2 0.5 mg, vitamin B6 0.5 mg, vitamin B12 0.9 μg, niacin 6 mg, folic acid 160 μg (0.16 mg), ascorbic acid 30 mg, copper 0.3 mg, vitamin D 5 μg and vitamin E 6 IU. Adherence assessed monthly by count of unused sachets from each household. The infant and young child feeding education provided to caretakers of both groups in verbal, written and pictorial form together with cooking demonstrations, focusing on frequency, quantity, consistency and an increased consumption of animal‐source foods. Immunisations, biannual vitamin A capsules and mebendazole tablets (for deworming) provided to all children according to Cambodia Ministry of Health guidelines. Infants followed up to 24 months of age and outcomes measured at 6, 12, 18 and 24 months of age. Participants outside age range defined for inclusion in this review. |
| Jaeggi 2015 | 115 Kenyan infants aged 6 months in Msambweni County, in southern coastal Kenya, a malaria‐endemic area, consumed home‐fortified maize porridge daily for 4 months. 2 studies conducted. In study 1 (n = 80), infants randomly assigned to receive MNP containing 2.5 mg of elemental iron (as NaFeEDTA) (NaFeEDTA ± 2.5 mg of FeMNP, MixMe, DSM Nutritional Products Europe, Basel, Switzerland) or MNP without iron. In study 2 (n=80), they received a different MNP containing 12.5 mg of elemental iron (as ferrous fumarate) (± 12.5 mg of FeMNP, Sprinkles, Hexagon Nutrition, Mumbai) or MNP without iron. For 4 months, 7 MNP sachets and 2 kg of maize flour (Dola, Kitui Flour Mills, Mombasa, Kenya) were provided directly to participating mothers from 6 distribution points. Primary outcome was gut microbiome composition analysed by 16S pyro‐sequencing and targeted real‐time polymer chain reaction. Secondary outcomes included faecal calprotectin (marker of intestinal inflammation) and incidence of diarrhoea. Participants outside age range defined for inclusion in this review. |
| Khan 2014 | 100 infants aged 6‐11 months living in 26 rural villages in the Kaliganj sub district of Gazipur, Bangladesh randomised to 1 of 2 groups containing MNP with or without calcium for 2 months. Group 1: MNP contained 12.5 mg of elemental iron (as ferrous fumarate), zinc 5 mg, folic acid 160 μg, vitamin A 300 μg RE, vitamin C 30 mg. Group 2: MNP formulation + calcium 400 mg. Primary outcomes were Hb concentrations, adherence and adverse effects. Type of comparisons and participants outside scope of this review. |
| Kikafunda 1998 | 153 children mean age (± SD) 55.8 ± 11.2 months from 3 randomly selected nursery schools of medium, low and very low socioeconomic status in a suburb of Kampala, Uganda. Participants received either Zn (as zinc sulphate) 10 mg (n = 79) or placebo (n = 76) daily in freshly prepared fruit juice, Monday to Friday inclusive for 6 months. Type of intervention outside scope of this review. |
| Manger 2008 | 569 children aged 5.5‐13.4 years from 10 schools in sub district of Trakan Phutphon, Ubon Ratchathani province, in northeast Thailand, were randomly assigned to receive a seasoning powder (monosodium glutamate, salt, sugar, hydrolysed vegetable protein and dried meat powder) fortified with zinc 5 mg, 5 mg of elemental iron, vitamin A 270 μg RE and iodine 50 μg (per serving) or an unfortified seasoning powder with no micronutrients. Seasoning incorporated into a school lunch prepared centrally and delivered 5 days per week for 31 weeks. Type of intervention involved a fortified condiment or seasoning in powder form and not an MNP for home fortification. |
| Menon 2007 | 415 children of both sexes aged 9‐24 months at start of 2 months' intervention, with no severe anaemia (Hb < 70 g/L), not receiving wheat‐soy‐blend, living in rural Haiti and who were accompanied by their mother. Prevalence of anaemia at start was 46%. Randomisation was into 2 groups at food distribution point: group 1 (6 food distribution points, n = 254): daily MNP with 12.5 mg of elemental iron (as fumarate), zinc (as gluconate) 5 mg, vitamin A 400 μg RE, folic acid 160 μg (0.16 mg), vitamin C 30 mg; group 2 (4 food distribution points, n = 161): control group. Both groups received 8 kg of wheat‐soy‐blend, 2.5 kg oil (vitamin A fortified) and indirect ration of 10 kg soy‐fortified bulgur, and 2.5 kg brown lentils. The MNP were distributed once a month with the fortified wheat‐soy‐blend, each time 30 sachets with pictorial instructions were given to intervention group. Control group received the wheat‐soy‐blend. Type of participants outside age range defined for inclusion in this review. |
| Menon 2016 | Cluster‐randomised, non‐blinded evaluation with uncontrolled before‐and‐after cross‐sectional surveys to assess the impact of providing intensified interpersonal counselling + mass media + community mobilisation (intensive) compared with standard nutrition counselling + less intensive mass media + community mobilisation (non‐intensive) on complementary feeding practices and anthropometric measurements. In half the sample, randomly allocated in both the intensive and non‐intensive areas, the Shasthya Sebika offered MNP sachets containing iron, folic acid, zinc, and vitamins A and C for sale to mothers and received a small commission from the sales. Type of intervention and study design outside scope of this review. |
| Neufeld 2008 | 927 children aged 6‐12 months, beneficiaries of the Oportunidades programme, a conditional cash transfer programme implemented in rural areas in 1997 and urban areas in 2002 with authorisation of Oportunidades officials at the federal, state and local level, National Institute of Public Health Ethics Commission, in Mexico from communities (18 per supplement) randomly assigned to receive a fortified food, syrup or multiple MNP Sprinkles. Supplements delivered daily (6 months). Communities were randomly assigned (18 communities per supplement) to 1 of 3 interventions: group 1 (n = 265): 44 g of daily supplement Nutrisano (fortified food) containing 10 mg of elemental iron (as ferrous gluconate), vitamin A 400 μg RE, zinc 10 mg, vitamin C 50 mg, folic acid 50 μg (0.05 mg), vitamin E 6 mg, vitamin B2 0.8 mg and vitamin B12 0.7 μg and also provided energy, protein, lipids, carbohydrates and sodium; group 2 (n = 323): 5 mL of syrup daily containing 10 mg of elemental iron (as ferrous gluconate), vitamin A 400 μg RE, zinc 10 mg, vitamin C 50 mg, folic acid 50 μg (0.05 mg), vitamin E 6 mg, vitamin B2 0.8 mg, vitamin B12 0.7 μg; group 3 (n = 339): MNP 1 g (Sprinkles) containing 10 mg of elemental iron (as ferrous fumarate), vitamin A 400 μg RE, zinc 10 mg, vitamin C 50 mg, folic acid 50 μg (0.05 mg), vitamin E 6 mg, vitamin B2 0.8 mg, vitamin B12 0.7 μg. Child growth, development and micronutrient status measured at baseline. Hb concentration, anaemia after 4 and 10 months of supplementation and at 24 and 30 months of age. Preliminary results indicated that after 4 months' supplementation, the prevalence of anaemia was significantly (P < 0.05) higher in children receiving the Nutrisano (fortified food) in comparison with the multiple MNP and syrup. At 24 months of age, anaemia had decreased in all 3 groups (P < 0.001), but remained slightly higher in the Nutrisano (fortified food) group (fortified food: 12.3%, syrup: 8.8%, multiple MNP: 9.2%). The large decrease and the low prevalence at 24 months suggested that all supplements were similarly efficacious to prevent and cure anaemia; with the effect observed slower in children who received fortified food. Attrition was 20%, no difference between groups, no differences in characteristics of children lost to follow‐up and those who completed trial. Main reasons for attrition were: dislike or perceived reacted to supplements (43%) and migrated out of community (18%); but were not different between groups. The type of participants outside age range defined for inclusion in this review. |
| Osendarp 2007 | 396 children aged 6‐10 years home‐based in Adelaide, South Australia, and 384 children at 6 primary schools in Jakarta, Indonesia randomly allocated to 1 of 4 groups: group 1 (n = 106): drink containing 10 mg of elemental iron (as NaFeEDTA), zinc (as zinc sulphate) 5 mg, RE vitamin A (as retinol acetate) 400 µg, folic acid 150 µg, vitamin B6 1 mg, vitamin B12 1.5 µg, vitamin C 45 mg; group 2 (n = 96): docosahexanoic acid 88 mg + eicosapentaenoic acid 22 mg; group 3 (n = 92): 10 mg of elemental iron (as NaFeEDTA), zinc (as zinc sulphate) 5 mg, vitamin A (as retinol acetate) 400 µg RE, folic acid 150 µg (0.15 mg), vitamin B6 1 mg, vitamin B12 1.5 µg, vitamin C 45 mg, docosahexanoic acid 88 mg and eicosapentaenoic acid 22 mg; group 4 (n = 102): placebo. Fruit‐flavoured drinks (soy 0.6%) used 6 days a week for 12 months as vehicle for all treatments, which were added as powders. Trials conducted from August 2003 to April 2005. Intervention products used consisted of 4 powdered fortificants that were added to a base powder containing protein 8 g, sugar 12 g and maltodextrin 4 g to be dissolved in 100 mL of a soy‐based fruit drink in a plastic shaker with a screw top and then shaken for ≥ 20 seconds. Type of intervention outside scope of this review. |
| Paganini 2016 | Review on studies assessing the effects of iron‐fortified foods on the gut microbiome, gut inflammation and diarrhoea for infants and children. Not an intervention study. |
| Rah 2012 | Summary of the projects implemented as part of the World Food Programme and DSM partnership that have been implemented with the United Nations High Commissioner for Refugees either in context of refugee camps or emergency response. Several studies have been nested in these projects to assess potential impact of MNP on nutrition and health status of beneficiaries. MNP programmes in Bangladesh have been conducted as part of the Cyclone Sidr response targeting 101,000 children aged under 5 years and 59,000 pregnant and lactating women, food‐ and cash‐for‐work activities implemented in response to high food prices (targeting 14,500 children aged 6‐24 months and 6000 pregnant and lactating women, and programming in the Rohinga refugee camps. 2 projects providing multiple MNP are being carried out in Nepal: 1 in Bhutanese refugee camps (targeting 8500 children aged 6‐59 months) and another as part of high‐food price emergency response (targeting > 114,000 children aged 6‐59 months). In Kenya, one project at the Kakuma camp targeted 55,000 refugees of all ages. Study design varied from a cohort of children followed prospectively to pre‐ and postintervention population‐based representative cross‐sectional surveys to assess the impact of MNP. Main outcome assessed was Hb concentration as a proxy indicator of micronutrient deficiencies. Type of study design outside scope of this review. |
| Rim 2008 | 234 infants aged 6‐12 months recruited from 36 nurseries in the Democratic People's Republic of Korea and randomly divided into 1 of 2 groups: group 1: rice porridge fortified with 10 mg of iron (as ferrous sulphate) per day, added to the water in which rice was cooked; group 2: non‐fortified cereal for 6 months. Types of intervention outside scope of this review. |
| Samadpour 2011 | 362 eligible children aged 6‐18 months with Hb ≥ 70 g/L, with no clinical signs of acute or chronic illness who were receiving at least 1 complementary food and whose mothers were permanent residents of Hashtgerd, Iran were randomly assigned to 1 of 3 groups: group 1 (n = 120): Sprinkles, containing iron (as ferrous fumarate) 10 mg, zinc (as gluconate) 5 mg, vitamin A 375 µg, vitamin D 5 µg, vitamin E 6 mg, vitamin C 35 mg, vitamin B1 0.5 mg, vitamin B2 0.5 mg, vitamin B6 0.5 mg, vitamin B12 0.9 µg, niacin 6 mg, folic acid 150 µg, copper (as gluconate) 0.6 mg, iodine 59 µg; group 2 (n = 121): FoodLETS containing iron (as ferrous fumarate) 12.5 mg, zinc (as gluconate) 5 mg, vitamin A 300 µg, vitamin D 5 µg, vitamin E 6 mg, vitamin C 30 mg, vitamin B1 0.5 mg, vitamin B2 0.5 mg, vitamin B6 0.5 mg, vitamin B12 0.9 µg, niacin 6 mg, folic acid 160 µg, copper (as gluconate) 0.3 mg, iodine 590 µg; group 3 (n = 121): multiple micronutrients drops containing 9 micronutrients including iron (as ferrous sulphate) 10 mg, vitamin A 450 µg, vitamin D 10 µg, vitamin E, vitamin C 35 mg, vitamin B1 0.5 mg, vitamin B2 0.6 mg, vitamin B6 0.4 mg and niacin 8 mg. Intervention lasted 4 months. Assessed Hb, serum ferritin, serum retinol, serum zinc, 25(OH) D concentration and anthropometry at baseline and 4 months. Participants outside age range defined for inclusion in this review. |
| Sampaio 2013 | 143 healthy institutionalised infants and children aged 6‐48 months of both sexes living in Salvador, Bahia, Brazil in 2009 randomly assigned to 1 of 2 groups: group 1 (n = 75): daily sachet of sprinkles containing iron (as ferrous fumarate) 12.5 mg, zinc (as gluconate) 5 mg, vitamin A 375 µg RE, vitamin E 6 mg, vitamin C 35 mg, vitamin B1 0.5 mg, vitamin B2 0.5 mg, vitamin B6 0.5 mg, vitamin B12 0.9 µg, vitamin C 30 mg, vitamin D3 5 µg, niacin 6 mg, copper (as gluconate) 0.6 mg, and iodine (as potassium iodate) 50 µg; group 2 (n = 68): iron (as ferrous fumarate) 12.5 mg, vitamin A 375 µg RE, vitamin E 6 mg, vitamin C 35 mg, vitamin B1 0.5 mg, vitamin B2 0.5 mg, vitamin B6 0.5 mg, vitamin B12 0.9 µg, vitamin C 30 mg, vitamin D3 5 µg, niacin 6 mg, copper (as gluconate) 0.6 mg, iodine (as potassium iodate) 50 µg with no zinc. Both groups received multiple MNP for point‐of‐use fortification of foods for 90 days. MNPs were mixed with different foods by trained nutritionists who were not blinded to intervention provided as sachets were identified as having or not having zinc. Outcomes assessed were diarrhoea and acute respiratory infection. Type of comparisons outside scope of this review. |
| Selva Suárez 2011 | Paper summarises 3 projects with social marketing approach that were jointly carried out by Cuban government and United Nations agencies, aiming to reduce anaemia in boys and girls aged up to 5 years. Quantitative and qualitative methods and the triangulation of their results were used. All projects included training of key actors in healthy child feeding, nutrition and prevention of anaemia as well as extensive education to the families. 2 projects delivered an iron‐fortified foodstuff and the other 1 distributed multiple micronutrients powders for anaemia control and prevention among young children 12‐24 months of age living at "Calixto García" municipality Holguín province, 2009‐2011. No data available. Type of comparisons, study design and type of participants outside scope of this review. |
| Smuts 2005 | 290 term infants aged 6‐12 months recruited through health posts of Valley of a Thousand Hills, Durban in the KwaZulu‐Natal Province, South Africa and enrolled in study and randomly assigned to 1 of 4 groups: group 1: daily supplement containing 1 daily allowance of multiple micronutrients for young children; group 2: daily placebo supplement containing no micronutrients; group 3: weekly supplement containing 2 daily allowances of multiple micronutrients for young children and a placebo supplement on the other days of the week; group 4: daily supplement containing 10 mg of elemental iron. Micronutrient supplements provided were large chewable tablets or foodLETS. Type of participants outside age range defined for inclusion in this review. |
| Soofi 2013 | 2746 children aged 6‐18 months from urban and rural sites in Sindh, Pakistan from 256 clusters determined by a baseline census (111 clusters in Bilal colony and 145 clusters in Matiari), were randomly assigned to 1 of 3 groups: group 1 (n = 889): 14‐day supply of MNP in individual sachets to be given daily containing iron (as microencapsulated ferrous fumarate) 12.5 mg, vitamin C (as ascorbic acid) 50 mg, vitamin A (as retinol acetate) 300 μg, vitamin D (as vitamin D3) 5 μg, folic acid 150 μg, zinc (as zinc gluconate) 10 mg; group 2 (n = 910): 14‐day supply of MNP in individual sachets to be given daily containing iron (as microencapsulated ferrous fumarate) 12.5 mg, vitamin C (as ascorbic acid) 50 mg, vitamin A (as retinol acetate) 300 μg, vitamin D (as vitamin D3) 5 μg, folic acid 150 μg, no zinc; group 3 (n = 947): no sachets. All groups received basic infant and young child feeding messages based on UNICEF/WHO recommendations, namely promotion of exclusive breastfeeding up to 6 months of age and continued breastfeeding with appropriate complementary feeding with locally available foods thereafter. Type of participants and type of comparisons outside scope of this review. |
| Suchdev 2007 (C) | 703 children aged 6‐23 months at time of enrolment living in rural western Kenya, Nyando Division. 575 children followed for duration of intervention and follow‐up period. Exclusion criteria: unavailable for enrolment on 3 separate household visits and parental refusal to give informed consent. Children with Hb < 70 g/L referred for treatment, but still included in analysis. Type of participants outside scope of this review. |
| Teshome 2017 | Children aged 12‐36 months living in Kisumu West District, Kenya expected to remain resident in study area for duration of intervention and follow‐up; no known or reported allergy to premedication drugs; not severely malnourished (weight‐for‐height Z‐score, with no fever or reported or suspected systemic disorders (e.g. HIV infection, tuberculosis, sickle cell disease) and Hb ≥ 70 g/L randomly assigned to receive 1 of 3 interventions: group 1 (n = 112): daily home fortification for 30 days with sachets containing 3 mg of elemental iron (as NaFeEDTA); group 2 (n = 114): daily home fortification for 30 days with sachets containing 12.5 mg of elemental iron (as encapsulated ferrous fumarate); group 3 (n = 112): daily home fortification for 30 days with sachets containing no iron (placebo). All powders contained vitamin A 300 μg RE, zinc 5 mg, vitamin D 5 µg, vitamin E 5 mg, vitamin C 30 mg, thiamine (vitamin B1) 0.5 mg, riboflavin (vitamin B2) 0.5 mg, niacin (vitamin B3) 6 mg, vitamin B6 (pyridoxine) 0.5 mg, vitamin B12 (cobalamin) 0.9 µg, copper 0.56 mg, selenium 17 µg and iodine 90 µg. All participants received treatment 3 days before randomisation. The medications received under supervision included: 1. dihydroartemisinin‐piperaquine (SigmaTau, Rome, Italy; tablets of dihydroartemisinin 40 mg and piperaquine 320 mg), for 3 days at daily target dose of 4 mg/kg bodyweight; 2. albendazole (Indoco Remedies, Mumbai, India), for 3 days at daily target dose of 200 mg for children aged 12‐24 months or 400 mg for children aged > 24 to 36 months; 3. praziquantel 600 mg tablets (Cosmos, Nairobi, Kenya), as single dose at a target dose of 40 mg/kg bodyweight. Age of children was 12‐36 months (mean 23.6 months) with 54.5% being younger than 2 years. Type of participants outside scope of this review. |
| Troesch 2009 | 101 apparently healthy, non‐pregnant, non‐lactating young women studying or working at Institute of Food Science and Nutrition, Swiss Federal Institute of Technology in Zurich, Switzerland and the University of Zurich between January and April 2008 were randomly assigned to 1 of 6 groups receiving a maize porridge fortified with an MNP‐containing stable isotope‐labelled elemental iron as either ferrous sulphate or iron EDTA (NaFeEDTA) and different combinations of inhibitors and enhancers (ascorbic acid, calcium, phytase, l‐alpha‐glycerophosphocholine). They each consumed 2 meals in a cross‐over design for determination of iron absorption. Objective was to maximise iron absorption from a low‐iron MNPs by testing combinations of iron as NaFeEDTA, ascorbic acid and a microbial phytase active at gut pH, as well as the role of L‐α‐glycerophosphocholine. Types of participants and types of interventions not within scope of this review. |
| Vuong 2002 | 185 preschool‐age children aged 31‐70 months with low Hb concentrations from 2 communes in Thanh‐Mien district, Hai‐Hung province of northern Vietnam were assigned to 1 of 3 groups: group 1: xoi gac (fruit) that contained β‐carotene 3.5 mg per serving; group 2: rice mixed with synthetic β‐carotene 5.0 mg powder; group 3: rice without fortification. Each participant received about 110‐120 g cooked rice per day. No other foods or beverages provided. Fruit and powder preparations designed to contain β‐carotene 5.0 mg per serving on basis of recommended dietary allowance for retinol of 500 retinol equivalents for children aged 3‐6 years. Type of interventions did not include provision of iron and thus outside scope of this review. |
| Wijaya‐Erhardt 2007 | 284 children aged 6‐12 months from 12 villages in the Salam sub district and 6 villages in Ngluwar sub district, both located in Magelang district in centre of Java. Study took place from June to December 2000. Participants randomly assigned to 1 of 4 groups: group 1 (n = 72): daily multiple‐micronutrient food‐like tablets (foodLETS); group 2 (n = 70): weekly multiple‐micronutrient food‐like tablets (foodLETS); group 3 (n = 70): daily iron food‐like tablets (foodLETs); group 4 (n = 70): daily placebo. FoodLETS given as daily elemental iron (as ferrous sulphate), daily multiple micronutrients (14 nutrients: vitamins A, D, E, K, C, thiamine, riboflavin, vitamin B12, niacin, folate, iron, zinc, copper, iodine) and weekly multiple micronutrient (same 14 nutrients). Multiple micronutrient supplement and placebo produced in form of foodLETS and were provided in blister packs of 7 tablets. Results showed an increase in iron stores in daily iron and daily multiple micronutrients group, but not in weekly multiple micronutrients group. Adverse effects observed were vomiting and diarrhoea, with no significant difference between intervention groups. Type of participants outside age range defined for inclusion in this review. |
| Zlotkin 2001 | 557 anaemic children aged 6‐18 months from field study area for Kintampo Health Research Centre, located in Kintampo district of rural Ghana. This is a malaria endemic area where principal complementary food is a maize‐based porridge. Participants were randomly assigned to 1 of 2 groups: group 1 (n = 246): home fortification with MNP containing 80 mg of elemental iron (as microencapsulated ferrous fumarate) and ascorbic acid 50 mg added to weaning foods (after it was cooked); group 2 (n = 247): iron drops containing 40 mg of elemental iron given 3 times per day for 2 months. Study took place during May and August 1999. Dosage of iron in the Sprinkles sachet was double that in the ferrous sulphate drops. Outcomes included anaemia, ferritin, serum zinc and growth concentration. Anaemia was successfully treated in the 2 groups in 58% and 56% of children. There were no significant differences in adverse effects between groups. Diarrhoea was reported in 14.5% of participants receiving drops and 12.8% of participants receiving MNP. Type of participants outside age range defined for inclusion in this review. |
| Zlotkin 2003a | 437 Ghanaian non‐anaemic children aged 8‐20 months, who were ingesting a weaning food in addition to breast milk were randomised individually to 1 of 4 groups: group 1 (n = 110): MNP (with iron only) containing 40 mg of elemental iron (as microencapsulated ferrous fumarate) daily; group 2 (n = 107): MNP (with iron and vitamin A) containing 40 mg of elemental iron (as microencapsulated ferrous fumarate) + retinol equivalents (as retinyl acetate) 600 μg daily; group 3 (n = 112): 12.5 mg of elemental iron (as ferrous sulphate iron drops) daily; group 4 (n = 108): placebo in powder form. Primary outcome measures were change in Hb and anaemic status at baseline and end of study. Prophylactic supplementation provided to children for 6 months (October 1999 to March 2000) and children who maintained Hb of ≥ 100 g/L at end of intervention were reassessed at 12 months' postintervention. Acceptability of powders was better in comparison to iron drops. No significant changes were seen in mean Hb, ferritin or serum retinol values from baseline to end of supplementation period among groups. Study area considered a setting where intestinal parasites, malaria and infectious diarrhoea are common. Supplementation period began at end of rainy season and had finished by end of dry season when the burden of malaria is lower. Iron and haematological status maintained equally well among all groups, including MNP and placebo. Type of participants outside age range defined for inclusion in this review. |
| Zlotkin 2003b | 304 anaemic children aged 6‐18 months from rural Ghana. Research took place between February and May 2000, at end of dry season, in field study area of the Kintampo Health Research Centre, located in Brong Ahafo region of Ghana. This is a malaria endemic area in which principal complementary food is a maize‐based porridge, low in bioavailable iron and zinc. Participants were randomly assigned to 1 of 2 groups: group 1 (n = 144): home fortification with "multiple micronutrient powders" including 80 mg of elemental iron (as microencapsulated ferrous fumarate) + ascorbic acid 50 mg; group 2 (n = 160): home fortification with powders containing 80 mg of elemental iron (as microencapsulated ferrous fumarate) + zinc (as gluconate) 5 mg over 2 months. Outcomes included anaemia, ferritin, serum zinc and growth concentration. Both formulations were successful in treating anaemia. There was no effect on zinc status and growth. Type of participants outside age range defined for inclusion in this review. |
| Zlotkin 2013 | Objective was to determine effect of providing an MNP with or without iron on incidence of malaria among children living in a high malaria‐burden area. Double‐blind, cluster‐randomised trial of children aged 6‐35 months (mean 19.4 ± 8.6 months) conducted over 6 months in 2010 in a rural community setting in central Ghana, West Africa (n = 1958 living in 1552 clusters). Children randomised by cluster to receive an MNP with iron (iron group; iron 12.5 mg/day) or without iron (no iron group). The MNP with and without iron were added to semi‐liquid home‐prepared foods daily for 5 months followed by 1 month of further monitoring. Insecticide‐treated bed nets were provided at enrolment, as well as malaria treatment when indicated. Malaria incidence overall significantly lower in iron group compared with no iron group (76.1 episodes/100 child‐years with iron and 86.1 episodes/100 child‐years with no iron; RR 0.87, 95% CI 0.79 to 0.97), and during intervention period (79.4 episodes/100 child‐years with iron and 90.7 episodes/100 child‐years with no iron; RR 0.87, 95% CI 0.78 to 0.96). In secondary analyses, these differences were no longer statistically significant after adjusting for baseline iron deficiency and anaemia status overall (adjusted RR 0.87, 95% CI 0.75 to 1.01) and during intervention period (adjusted RR 0.86, 95% CI 0.74 to 1.00). Type of participants outside age range defined for inclusion in this review (more than half the children did not meet inclusion criteria). |
CBC: complete blood count; CI: confidence interval; Hb: haemoglobin; MNP: micronutrient powder; n: number of children; NaFeEDTA: sodium iron ethylenediaminetetraacetic acid; SD: standard deviation; RR: risk ratio; UNICEF: United Nations Children's Fund; WHO: World Health Organization.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616001245482.
| Trial name or title | Food Fortification Powder for Improving Micronutrient Status in Underweight and Overweight/Obese Primary School Children. |
| Methods | Randomised controlled trial. |
| Participants | 348 apparently healthy primary school boys and girls aged 6‐9 years, with haemoglobin concentration < 110 g/L in Vietnam. Exclusion criteria: haemoglobin concentration < 70 g/L, currently taking or planning to take vitamin and mineral supplementation, chronic disease or infection, congenital anomalies or severe malnutrition. |
| Interventions | Participants will be assigned to 1 of 2 groups.
|
| Outcomes | Haemoglobin concentration, iron status (measured by serum ferritin), zinc status (measured by serum zinc), body composition (by triceps skinfold thickness, mid‐upper arm circumference and waist circumference), cost‐benefit analysis, dietary intake, growth (assessed by height, weight and Z‐scores), morbidity and health status. |
| Starting date | 20 September 2016. |
| Contact information | Dr Ewa Szymlek‐Gay. Institute for Physical Activity and Nutrition (IPAN) School of Exercise and Nutrition Sciences 221 Burwood Highway Burwood VIC 3125, Australia. Telephone: +61 3 9244 5404. Email: ewa.szymlekgay@deakin.edu.au. Dr Hoang Thi Duc Ngan. National Institute of Nutrition 48B Tang Bat Ho Street Hai Ba Trung District Hanoi, Vietnam. Telephone: +84 43 9713088. Email: hoangthiducngan@dinhduong.org.vn. |
| Notes | Source of funding: National Institute of Nutrition, Vietnam, and Deakin University, Australia. |
NCT01917032.
| Trial name or title | Effect of Home‐Fortification with Sprinkles in Haematologic and Nutritional Status in Preschool Children in Medellín. |
| Methods | Randomised controlled trial, triple‐blind and placebo‐controlled. |
| Participants | 100 children aged 5‐59 months, not anaemic or with severe malnourished attending 2 child care centres in Medellín, Colombia. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups.
Interventions will be provided during weekdays for 11 weeks. |
| Outcomes |
Primary: haemoglobin at 10 weeks (g/dL). Secondary: transferrin at 10 weeks (mg/dL). Other outcomes: child growth Indicators. |
| Starting date | September 2013. |
| Contact information | Cristian Vargas, MD. Lic Viviana Ramírez, Nutritionist. Fundación de Atención a la Niñez (FAN), Medellin, Anqtioquia, Colombia. Telephone: +57 30 06099044. Email: crivargas24@hotmail.com and julianaorozcocano@hotmail.com. |
| Notes | Source of funding: CES University. |
NCT02280330.
| Trial name or title | Iodine Intake & Status of Preschoolers Given MNP for 6 Months. |
| Methods | Randomised controlled trial. |
| Participants | 396 apparently healthy boys and girls aged 4‐6 years attending La Trinidad Benguet daycare centres, in Benguet, Philippines who are permanent residents in the barangay or municipality, with informed written parental consent and whose mothers are willing to devote time for survey. Exclusion criteria: severely underweight, obvious clinical problem such as goitre, congenital heart disease or plans of transferring residence outside municipality within next 6 months. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups.
MNP contains 15 vitamins and minerals, including vitamin A, D, E, C, B1, B2, B6 and B12, niacin, folic acid, iron, zinc, copper, selenium, iodine. Iodine 90 µg per sachet of 1 g of MNP. |
| Outcomes | Urinary iodine at 6 months, weight‐for‐age, height‐for‐age, likability of a communication material on knowledge of mothers. |
| Starting date | November 2014. |
| Contact information | Imelda O Degay. University of the Philippines, Los Banos, Philippines. |
| Notes | Source of funding: Nutrition Center of the Philippines. |
NCT02302729.
| Trial name or title | Early Child Development and Nutrition in Guatemala. |
| Methods | Cluster‐randomised controlled trial. |
| Participants | 3000 infants aged 6‐12 months and preschool‐age children aged 36‐48 months, Spanish speakers, undernourished (length and height for age < ‐1 Z‐score), without any obvious health or developmental problems that would interfere with growth, nutrition or development, who are planning to remain in area for subsequent year and whose parents or legal guardians of child is aged ≥ 18 years, speak and understand Spanish and live with child in study community. Exclusion criteria: severe stunting (length and height for age < ‐3 Z‐scores), identified conditions that could interfere with their development and growth, severely anaemic (haemoglobin < 70 g/L). |
| Interventions | The children will be assigned through neighbourhood clusters randomised into 4 groups.
MNP contains zinc 9 mg, copper 0.3 mg, elemental iron 12.5 mg, vitamin D3 5 mg, folic acid 160 µg (0.16 mg), vitamin E 5 mg, iodine 90 µg, calcium 200 mg, vitamin A 250 µg RE, phosphorus 150 mg, vitamin C 40 mg, magnesium 40 mg, vitamin B12 0.9 µg, selenium 17 µg, thiamine 0.5 mg, manganese 0.17 mg, niacin 0.5 mg, biotin 8 mg, riboflavin 6 mg, vitamin B5 1.8 mg, vitamin B6 0.5 mg. Investigators will focus on preschool‐age children (aged 36‐48 months) to examine if micronutrients + a school readiness intervention can improve children's school readiness skills. |
| Outcomes | Cognitive, motor, social‐emotional development from baseline to 12 months; weight, length/height, micronutrient status from baseline to 12 months. |
| Starting date | December 2014. |
| Contact information | Dr Maureen M Black. University of Maryland. Telephone: +1 410 7062136. Email: mblack@peds.umaryland.edu. |
| Notes | Source of funding: University of Maryland and Association for the Study and Prevention of HIV/AIDS. |
NCT02422953.
| Trial name or title | Stunting Prevention Project in Thatta and Sajawal Districts, Sindh Province, Pakistan. |
| Methods | 29 Union Councils with best performance and highly covered Lady Health Workers catchment areas will be selected as intervention clusters. Of the 29 Union Councils, 10 Union Councils will be selected using simple random sampling. 5 Union Councils in each group, giving a total sample size of 5000 participants. Participants will be recruited and followed monthly for compliance of food‐based supplements, dietary diversity, pregnancy outcomes, and maternal and child morbidity and mortality. |
| Participants | 5000 pregnant women, lactating mothers and children aged 6‐59 months. Blood samples will be collected twice from a subset of 200 children (100 in each group). |
| Interventions | Interventions will focus on food‐based supplementation and non‐food‐based interventions delivered through Lady Health Workers. A blanket approach will be used for the distribution of food‐based supplementation, consisting of locally produced lipid‐based nutrient supplements for children aged 6‐23 months, MNP for children aged 24‐59 months and wheat soy blend for pregnant and lactating women. Control group will receive routine public and private health services available within the area. Infants and children will receive an MNP sachet to obtain RDA of 15 micronutrients on alternate days. |
| Outcomes | Height, weight and mid upper arm circumference. For children, length/height‐for‐age, weight‐for‐age and weight‐for‐length/height, anaemia, haemoglobin concentrations. |
| Starting date | January 2014. |
| Contact information | Dr Sajid Bashir Soofi. Department of Paediatrics and Child Health, Aga Khan University, Karachi, Pakistan. Email: sajid.soofi@aku.edu. |
| Notes | Sources of funding: Aga Khan University; United Nations World Food Programme, Islamabad, Pakistan, and Pakistan Ministry of Health. |
PACTR201607001693286.
| Trial name or title | The Efficacy of Multiple MNP Supplementation in Children Under 5 Years in Arusha District. |
| Methods | Randomised controlled trial. |
| Participants | 436 children aged 6‐59 months with moderate anaemia (Hb concentration 70‐100 g/L) whose families reside within study villages in Arusha, Tanzania. Exclusion criteria: chronic or acute disease, sickle‐cell anaemia or consuming multi‐vitamin‐mineral supplements on a regular basis. |
| Interventions | Participants will be randomly assigned to 1 of 4 groups.
|
| Outcomes | Haemoglobin concentration, vitamin A concentration and nutritional status. |
| Starting date | 7 September 2015. |
| Contact information | Dr Martin Kimanya. Telephone: +2557596284. Email: kejod@nm‐aist.ac.tz. |
| Notes | Source of funding: COSTECH, Dar‐es‐Salaam, and The Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania. |
COSTECH: Commission for Science and Technology; MNP: multiple micronutrient powder; RDA: recommended dietary allowance.
Differences between protocol and review
We updated the Background section.
We specified the age range for inclusion for children of preschool and school‐age under Types of participants.
We included adverse effects as part of the primary outcomes (Primary outcomes), instead of being reported as a secondary outcome as stated in our protocol (De‐Regil 2012).
We planned to search metaRegister of Controlled Trials (mRCT) but this service was not available at the time of the search and we decided to withdraw from the search strategy. Similarly, we intended to include other databases in our updated search strategy, but these were not completed due to lack of access (Biosis Previews, WorldCat, Networked Digital Library of Theses and Dissertations, DART‐Europe E‐theses Portal, Australasian Digital Theses Program, Theses Canada Portal, and ProQuest‐Dissertations and Theses).
We specified that we recorded our decisions in a study flow diagram (see Selection of studies).
We specified that we also extracted data on baseline malaria prevalence (see Data extraction and management).
We did not conduct analyses using the generic inverse‐variance method, as specified in our protocol (De‐Regil 2012), but used the inverse‐variance method for continuous variables and Mantel‐Haenszel statistical method for dichotomous variables (see Data synthesis).
We conducted a post‐hoc subgroup analysis to explore the effect of micronutrient composition (iron alone, at least iron plus vitamin A plus zinc, other combinations without bundling iron plus vitamin A plus zinc) (see Subgroup analysis and investigation of heterogeneity).
We conducted a post‐hoc sensitivity analysis to explore the effects of combining studies comparing the intervention versus no intervention or placebo (see Sensitivity analysis).
We included source of funding in all included studies.
Contributions of authors
Protocol development
LD‐R drafted the 'Background.'
LD‐R and JP‐R drafted the 'Methods.'
MJ provided feedback on the draft protocol (De‐Regil 2012).
Review development
All authors contributed to screening, extraction and assessment of data, as described in the Methods. The final manuscript was written and approved by all review authors.
LD‐R is the guarantor for the review.
Sources of support
Internal sources
-
Centers for Disease Control and Prevention (CDC), USA.
MJ works at the International Micronutrient Malnutrition Prevention and Control Programme (IMMPaCt).
-
Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization (WHO), Switzerland.
JP‐R is staff of the Department of Nutrition for Health and Development at the WHO.
External sources
-
Nutrition International (formerly Micronutrient Initiative), Canada.
WHO acknowledges Nutrition International (formerly Micronutrient Initiative) for their financial support for conducting systematic reviews on micronutrients interventions.
-
The Bill & Melinda Gates Foundation, USA.
WHO gratefully acknowledges the financial support from The Bill & Melinda Gates Foundation for the development of systematic reviews of the evidence on the effects of nutrition interventions.
-
International Micronutrient Malnutrition Prevention and Control (IMMPaCt) programme, US Centers for Disease Control and Prevention (CDC), USA.
WHO gratefully acknowledges the financial and technical support of CDC for this work.
Declarations of interest
LD‐R is a full‐time staff member of Nutrition International (formerly Micronutrient Initiative), an international non‐for‐profit organisation that delivers multiple micronutrient powders (MNP) to children, women of reproductive age and pregnant women. Nutrition International supports the implementation of large‐scale research projects that provide multiple MPN to children aged six to 23 months. None of them met the inclusion criteria of this review (Criteria for considering studies for this review). Nutrition International is a partner of the Home Fortification Technical Advisory Group and receives funds from the Canadian Department of Foreign Affairs. LD‐R was an Editor for the Cochrane Developmental, Psychosocial and Learning Problems Group.
MJ participated in UNICEF/Centers for Disease Control and Prevention (CDC) regional workshops on scaling up MNP interventions for young children aged six to 23 months; is coauthor on two publications included in a September 2013 Sign and Life supplement on MNP interventions and was an editor of the supplement. MJ is the lead author or coauthor of multiple journal articles on MNP programmes. She was an investigator on the first global assessment of home fortification interventions (UNICEF/CDC 2013) and provided technical assistance on the design, analysis and dissemination of the results of the 2013 and later MNP global assessment (UNICEF 2014). With colleagues from CDC and UNICEF, MJ provides technical assistance in the development of a home fortification toolkit and webinar series, which have a heavy focus on MNP. She also co‐ordinated and wrote the Monitoring Manual for Home Fortification Interventions, including MNP, (HF‐TAG 2013) for the Home Fortification Technical Advisory Group (HF‐TAG). MJ has participated in executive committee meetings and strategic planning of the HF‐TAG, and became a member of the executive committee in 2016. She is a coauthor on a Cochrane Review of MNP interventions in children six to 23 months (De‐Regil 2011b).
JP‐R co‐ordinates the Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, which received financial resources for the biennium 2016 to 2017 from the Bill & Melinda Gates Foundation (2013 to 2019); US CDC (2014 to 2019); Nutrition International (formerly Micronutrient Initiative) (2014 to 2017) and United States Agency for International Development (USAID; 2014 to 2017). Donors do not fund specific guidelines and do not participate in any decision related to the guideline development process, including the composition of policy questions, membership of the guideline groups, the conduct and interpretation of systematic reviews, or the formulation of recommendations.
Disclaimer: Luz Maria De‐Regil is a full‐time staff member of Nutrition International (formerly Micronutrient Initiative), Maria Elena del Socorro Jefferds is full‐time staff member of the US CDC, and Juan Pablo Peña‐Rosas is full‐time staff member of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the official position, decisions, policy or views of these organisations.
New
References
References to studies included in this review
Inayati 2012 (C) {published data only}
- Inayati DA, Scherbaum V, Purwestri RC, Wirawan NN, Suryantan J, Hartono S, et al. Combined intensive nutrition education and micronutrient powder supplementation improved nutritional status of mildly wasted children on Nias Island, Indonesia. Asia Pacific Journal of Clinical Nutrition 2012;21(3):361‐73. [PUBMED: 22705425] [PubMed] [Google Scholar]
- Inayati DA, Scherbaum V, Purwestri RC, Wirawan NN, Suryantan J, Hartono S, et al. Improved nutrition knowledge and practice through intensive nutrition education: a study among caregivers of mildly wasted children on Nias Island, Indonesia. Food and Nutrition Bulletin 2012;33(2):117‐27. [DOI: 10.1177/156482651203300205; PUBMED: 22908693] [DOI] [PubMed] [Google Scholar]
- Purwestri RC, Scherbaum V, Inayati DA, Wirawan NN, Suryantan J, Bloem MA, et al. Supplementary feeding with locally‐produced ready‐to‐use food (RUF) for mildly wasted children on Nias Island, Indonesia: comparison of daily and weekly programme outcomes. Asia Pacific Journal of Clinical Nutrition 2012;21(3):374‐9. [PUBMED: 22705426] [PubMed] [Google Scholar]
- Scherbaum V [pers comm]. Your paper on MNP. Email to: LM De‐Regil 5 February 2013.
Kemmer 2012 (C) {published data only}
- Kemmer TM, Omer PS, Gidvani‐Diaz VK, Coello M. Acceptance and effect of ferrous fumarate containing micronutrient sprinkles on anemia, iron deficiency and anthropometrics in Honduran children. In: Silverberg DS editor(s). Anemia. Rijeka (Croatia): InTech, 2012. [DOI: 10.5772/30987] [DOI] [Google Scholar]
Kounnavong 2011 (C) {published data only}
- Kounnavong S, Sunahara T, Mascie‐Taylor CG, Hashizume M, Okumura J, Moji K, et al. Effect of daily versus weekly home fortification with multiple micronutrient powder on haemoglobin concentration of young children in a rural area, Lao People's Democratic Republic: a randomised trial. Nutrition Journal 2011;10(129):1‐11. [DOI: 10.1186/1475-2891-10-129; PMC3266642; PUBMED: 22111770] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundeen 2010 (C) {published and unpublished data}
- Lundeen E [pers comm]. Kyrgyz Republic MNP data for children 24‐36 months. Email to: M Jefferds 3 November 2011.
- Lundeen E, Schueth T, Toktobaev N, Zlotkin S, Hyder SM, Houser R. Daily use of Sprinkles micronutrient powder for 2 months reduces anemia among children 6 to 36 months of age in the Kyrgyz Republic: a cluster‐randomized trial. Food and Nutrition Bulletin 2010;31(3):446‐60. [DOI: 10.1177/156482651003100307; PUBMED: 20973465] [DOI] [PubMed] [Google Scholar]
Macharia‐Mutie 2012 {published data only}
- Macharia‐Mutie CW, Moretti D, Briel N, Omusundi AM, Mwangi AM, Kok FJ, et al. Maize porridge enriched with a micronutrient powder containing low‐dose iron as NaFeEDTA but not amaranth grain flour reduces anemia and iron deficiency in Kenyan preschool children. Journal of Nutrition 2012;142(9):1756‐63. [DOI: 10.3945/jn.112.157578; PUBMED: 22810982] [DOI] [PubMed] [Google Scholar]
- Macharia‐Mutie CW, Omusundi AM, Mwai JM, Mwangi AM, Brouwer ID. Simulation of the effect of maize porridge fortified with grain amaranth or micronutrient powder containing NaFeEDTA on iron intake and status in Kenyan children. Public Health Nutrition 2013;16(9):1605‐13. [DOI: 10.1017/S1368980012005174; PUBMED: 23218415] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogunlade 2011 {published and unpublished data}
- Jerling J [pers comm]. Regards and question on Ogundale et al 2011 vir Cochrane review. Email to: JP Pena‐Rosas 3 November 2011.
- Ogunlade AO, Kruger HS, Jerling JC, Smuts CM, Covic N, Hanekom SM, et al. Point‐of‐use micronutrient fortification: lessons learned in implementing a preschool‐based pilot trial in South Africa. International Journal of Food Sciences and Nutrition 2011;62(1):1‐16. [DOI: 10.3109/09637486.2010.495710; PUBMED: 20701549] [DOI] [PubMed] [Google Scholar]
Orozco 2015 (C) {published data only}
- Orozco J, Vargas C, Rojas ML, Herrera AM, Montoya L, Sánchez JG, et al. The effect of powdered micronutrients on the hematologic values and nutritional status of healthy preschoolers. Medellín, 2013 [Efecto de los micronutrientes en polvo en el estado nutricional y en los valores hemáticos de preescolares sanos. Medellín, 2013]. Revista Facultad Nacional de Salud Pública 2015;33(2):161‐70. [DOI: 10.17533/udea.rfnsp.v33n2a03] [DOI] [Google Scholar]
Osei 2008 (C) {published data only}
- Osei AK, Houser RF, Bulusu S, Hamer DH. Acceptability of micronutrient fortified school meals by schoolchildren in rural Himalayan villages of India. Journal of Food Science 2008;73(7):S354‐8. [DOI: 10.1111/j.1750-3841.2008.00878.x; PUBMED: 18803728] [DOI] [PubMed] [Google Scholar]
- Osei AK, Rosenberg IH, Houser RF, Bulusu S, Mathews M, Hamer DH. Community‐level micronutrient fortification of school lunch meals improved vitamin A, folate, and iron status of schoolchildren in Himalayan villages of India. Journal of Nutrition 2010;140(6):1146‐54. [DOI: 10.3945/jn.109.114751; PUBMED: 20410083] [DOI] [PubMed] [Google Scholar]
Sharieff 2006 (C) {published data only}
- Sharieff W, Yin SA, Wu M, Yang Q, Schauer C, Tomlinson G, et al. Short‐term daily or weekly administration of micronutrient Sprinkles has high compliance and does not cause iron overload in Chinese schoolchildren: a cluster‐randomized trial. Public Health Nutrition 2006;9(3):336‐44. [PUBMED: 16684385] [DOI] [PubMed] [Google Scholar]
- Zlotkin S [pers comm]. Regards and question ‐ Sharieff 2006. Email to: JP Peña‐Rosas 2 November 2011.
Troesch 2011b {published data only}
- Troesch B [pers comm]. Regards and question on Troesch et al 2011. Email to: JP Peña‐Rosas 1 November 2011.
- Troesch B, Stuijvenberg ME, Smuts CM, Kruger HS, Biebinger R, Hurrell RF, et al. A micronutrient powder with low doses of highly absorbable iron and zinc reduces iron and zinc deficiency and improves weight‐for‐age Z‐scores in South African children. Journal of Nutrition 2011;141(2):237‐42. [DOI: 10.3945/jn.110.129247; PUBMED: 21178093] [DOI] [PubMed] [Google Scholar]
Varma 2007 (C) {published data only}
- Varma JL, Das S, Sankar R, Mannar MG, Levinson FJ, Hamer DH. Community‐level micronutrient fortification of a food supplement in India: a controlled trial in preschool children aged 36‐66 mo. American Journal of Clinical Nutrition 2007;85(4):1127‐33. [PUBMED: 17413115] [DOI] [PubMed] [Google Scholar]
Vinodkumar 2006 (C) {published data only}
- Vinodkumar M, Rajagopalan S. Impact of a multiple‐micronutrient food supplement on the nutritional status of schoolchildren. Food and Nutrition Bulletin 2006;27(3):203‐10. [DOI: 10.1177/156482650602700302; PUBMED: 17542110] [DOI] [PubMed] [Google Scholar]
Vinodkumar 2009 (C) {published data only}
- Vinodkumar M, Rajagopalan S. Efficacy of fortification of school meals with ferrous glycine phosphate and riboflavin against anemia and angular stomatitis in schoolchildren. Food and Nutrition Bulletin 2009;30(3):260‐4. [DOI: 10.1177/156482650903000307; PUBMED: 19927606] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aboud 2011 {published data only}
- Aboud FE, Akhter S. A cluster‐randomized evaluation of a responsive stimulation and feeding intervention in Bangladesh. Pediatrics 2011;127(5):e1191‐7. [DOI: 10.1542/peds.2010-2160; PUBMED: 21502222] [DOI] [PubMed] [Google Scholar]
- Aboud FE, Moore AC, Akhter S. Effectiveness of a community‐based responsive feeding programme in rural Bangladesh: a cluster randomized field trial. Maternal and Child Nutrition 2008;4(4):275‐86. [DOI: 10.1111/j.1740-8709.2008.00146.x; PUBMED: 18811792] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboud FE, Shafique S, Akhter S. A responsive feeding intervention increases children’s self‐feeding and maternal responsiveness but not weight gain. Journal of Nutrition 2009;139(9):1738‐43. [DOI: 10.3945/jn.109.104885; PUBMED: 19587124] [DOI] [PubMed] [Google Scholar]
Ahmed 2003 {published data only}
- Ahmed P, Mahmood A, Aziz S, Azim W. Comparison of response between food supplemented with powdered iron and iron in syrup form for iron deficiency anemia. Journal of the College of Physicians and Surgeons Pakistan 2003;13(7):402‐4. [DOI: ; PUBMED: 12887843] [PubMed] [Google Scholar]
Angdembe 2015 {published data only}
- Angdembe MR, Choudhury N, Haque MR, Ahmed T. Adherence to multiple micronutrient powder among young children in rural Bangladesh: a cross‐sectional study. BMC Public Health 2015;15(440):1‐10. [DOI: 10.1186/s12889-015-1752-z; PMC4424501; PUBMED: 25925874] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bagni 2009 (C) {published data only}
- Bagni UV, Baião MR, Souza MM, Luiz RR, Veiga GV. Effect of weekly rice fortification with iron on anaemia prevalence and haemoglobin concentration among children attending public daycare centres in Rio de Janeiro, Brazil [Efeito da fortificaçao semanel do arroz com ferro quelato sobre a frequencia de anemia e concentraçao de hemoglobina em crianças de creches municipais de Rio de Janeiro, Brasil]. Cadernos de Saude Publica 2009;25(2):291‐302. [PUBMED: 19219236] [DOI] [PubMed] [Google Scholar]
Chen 2008 {published and unpublished data}
- Chen K, Li TY, Chen L, Qu P, Liu YX. Effects of vitamin A, vitamin A plus iron and multiple micronutrient‐fortified seasoning powder on preschool children in a suburb of Chongqing, China. Journal of Nutritional Science and Vitaminology 2008;54(6):440‐7. [PUBMED: 19155581] [DOI] [PubMed] [Google Scholar]
- Chen K, Zhang X, Li TY, Chen L, Wei XP, Qu P, et al. Effect of vitamin A, vitamin A plus iron and multiple micronutrient‐fortified seasoning powder on infectious morbidity of preschool children. Nutrition 2011;27(4):428‐34. [DOI: 10.1016/j.nut.2010.04.004; PUBMED: 20605698] [DOI] [PubMed] [Google Scholar]
- Dr Chen [per comm]. Regards and question on study: effect of vitamin A, vitamin A plus iron and multiple micronutrient‐fortified seasoning powder on infectious morbidity of preschool children. Email to: JP Pena‐Rosas 1 November 2011. [DOI] [PubMed]
Clarke 2015 {published data only}
- Clarke SE, Sacko M, Roschnik N, Dicko Y, Diarra S, Thera P, et al. Seasonal malaria chemoprevention combined with micronutrient supplementation delivered through community preschools: findings from a cluster randomized trial in Mali. Tropical Medicine & International Health 2015;20(Suppl 1):25‐6. [Google Scholar]
De Pee 2007 {published data only}
- Pee S, Moench‐Pfanner R, Martini E, Zlotkin SH, Darnton‐Hill I, Bloem MW. Home fortification in emergency response and transition programming: experiences in Aceh and Nias, Indonesia. Food and Nutrition Bulletin 2007;28(2):189‐97. [DOI: 10.1177/156482650702800208; PUBMED: 24683678] [DOI] [PubMed] [Google Scholar]
Geltman 2009 {published data only}
- Geltman PL, Hironaka LK, Mehta SD, Padilla P, Rodrigues P, Meyers AF, et al. Iron supplementation of low‐income infants: a randomized clinical trial of adherence with ferrous fumarate sprinkles versus ferrous sulfate drops. Journal of Pediatrics 2009;154(5):738‐43. [DOI] [PubMed] [Google Scholar]
Gibson 2010 {published data only}
- Gibson R. Fortifying day‐care meals with desiccated beef liver or micronutrient sprinkles to combat anaemia and micronutrient deficiencies in Brazillian pre‐school children. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000646044 Date first registered: 9 August 2010.
- Gibson R [pers comm]. Regards and question. Email to: JP Peña‐Rosas 9 February 2017.
Hirve 2007 {published and unpublished data}
- Hirve S, Bhave S, Bavdekar A, Naik S, Pandit A, Schauer C, et al. Low dose 'Sprinkles' ‐ an innovative approach to treat iron deficiency anemia in infants and young children. Indian Pediatrics 2007;44(2):91‐100. [PUBMED: 17351300] [PubMed] [Google Scholar]
Huamán‐Espino 2012 {published data only}
- Huamán‐Espino L, Aparco JP, Nuñez‐Robles E, Gonzáles E, Pillaca J, Mayta‐Tristán P. Consumption of chispitas® multimicronutrient supplements and anemia in 6–35‐month‐old children: cross‐cut study in the context of a populational health intervention in Apurimac, Peru [Consumo de suplementos con multimicronutrientes Chispitas® y anemia en niños de 6 a 35 meses: estudio transversal en el contexto de una intervención poblacional en Apurímac, Perú]. Revista Peruana de Medicina Experimental y Salud Publica 2012;29(3):314‐23. [PUBMED: 23085791] [DOI] [PubMed] [Google Scholar]
- Munayco CV, Ulloa‐Rea ME, Medina‐Osis J, Lozano‐Revollar CR, Tejada V, Castro‐Salazar C, et al. Evaluation of the impact of multiple micronutrient powders on children anemia in three Andean regions in Peru [Evaluación del impacto de los multimicronutrientes en polvo sobre la anemia infantil en tres regiones andinas del Perú]. Revista Peruana de Medicina Experimental y Salud Publica 2013;30(2):229‐34. [PUBMED: 23949507] [PubMed] [Google Scholar]
Ip 2009 {published data only}
- Ip H, Hyder SM, Haseen F, Rahman M, Zlotkin SH. Improved adherence and anaemia cure rates with flexible administration of micronutrient Sprinkles: a new public health approach to anaemia control. European Journal of Clinical Nutrition 2009;63(2):165‐72. [DOI: 10.1038/sj.ejcn.1602917; PUBMED: 17895911] [DOI] [PubMed] [Google Scholar]
Jack 2012 {published data only}
- Jack SJ, Ou K, Chea M, Chhin L, Devenish R, Dunbar M, et al. Effect of micronutrient sprinkles on reducing anemia: a cluster‐randomized effectiveness trial. Archives of Pediatrics & Adolescent Medicine 2012;166(9):842‐50. [DOI: 10.1001/archpediatrics.2012.1003; PUBMED: 22801933] [DOI] [PubMed] [Google Scholar]
Jaeggi 2015 {published data only}
- Barth‐Jaeggi T, Moretti D, Kvalsvig J, Holding PA, Njenga J, Mwangi A, et al. In‐home fortification with 2.5 mg iron as NaFeEDTA does not reduce anaemia but increases weight gain: a randomised controlled trial in Kenyan infants. Maternal & Child Nutrition 2015;11(Suppl 4):151‐62. [DOI: 10.1111/mcn.12163; PUBMED: 25420455] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015;64(5):731‐42. [DOI: 10.1136/gutjnl-2014-307720; PUBMED: 25143342] [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Kvalsvig J. Changes in microbiota and iron status after iron fortification of complementary foods. clinicaltrials.gov/ct2/show/NCT01111864 Date first received: 23 April 2010.
Khan 2014 {published data only}
- Khan WU, Shafique S, Shikder H, Shakur YA, Sellen DW, Chowdhury JS, et al. Home fortification with calcium reduces Hb response to iron among anaemic Bangladeshi infants consuming a new multi‐micronutrient powder formulation. Public Health Nutrition 2014; Vol. 17, issue 7:1578‐86. [DOI: 10.1017/S1368980013001742; PUBMED: 23816321] [DOI] [PMC free article] [PubMed]
Kikafunda 1998 {published data only}
- Kikafunda JK, Walker AF, Allan EF, Tumwine JK. Effect of zinc supplementation on growth and body composition of Ugandan preschool children: a randomized, controlled, intervention trial. American Journal of Clinical Nutrition 1998;68(6):1261‐6. [PUBMED: 9846856] [DOI] [PubMed] [Google Scholar]
Manger 2008 {published data only}
- Manger MS, McKenzie JE, Winichagoon P, Gray A, Chavasit V, Pongcharoen T, et al. A micronutrient‐fortified seasoning powder reduces morbidity and improves short‐term cognitive function, but has no effect on anthropometric measures in primary school children in northeast Thailand: a randomized controlled trial. American Journal of Clinical Nutrition 2008;87(6):1715‐22. [PUBMED: 18541560] [DOI] [PubMed] [Google Scholar]
- Winichagoon P, McKenzie JE, Chavasit V, Pongcharoen T, Gowachirapant S, Boonpraderm A, et al. A multimicronutrient‐fortified seasoning powder enhances the hemoglobin, zinc, and iodine status of primary school children in North East Thailand: a randomized controlled trial of efficacy. Journal of Nutrition 2006;136(6):1617‐23. [PUBMED: 16702330] [DOI] [PubMed] [Google Scholar]
Menon 2007 {published and unpublished data}
- Loechl CU, Menon P, Arimond M, Ruel MT, Pelto G, Habicht JP, et al. Using programme theory to assess the feasibility of delivering micronutrient Sprinkles through a food‐assisted maternal and child health and nutrition programme in rural Haiti. Maternal and Child Nutrition 2009;5(1):33‐48. [DOI: 10.1111/j.1740-8709.2008.00154.x; PUBMED: 19161543] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon P, Ruel MT, Loechl CU, Arimond M, Habicht JP, Pelto G, et al. Micronutrient Sprinkles reduce anemia among 9‐ to 24‐mo‐old children when delivered through an integrated health and nutrition program in rural Haiti. Journal of Nutrition 2007;137(4):1023‐30. [PUBMED: 17374671] [DOI] [PubMed] [Google Scholar]
Menon 2016 {published data only}
- Frongillo EA, Nguyen PH, Saha KK, Sanghvi T, Afsana K, Hague R, et al. Large‐scale behavior‐change initiative for infant and young child feeding advanced language and motor development in a cluster‐randomized program evaluation in Bangladesh. Journal of Nutrition 2017;147(2):256‐63. [DOI: 10.3945/jn.116.240861; PUBMED: 28031374] [DOI] [PubMed] [Google Scholar]
- Menon P, Nguyen PH, Saha KK, Khaled A, Kennedy A, Tran LM, et al. Impacts on breastfeeding practices of at‐scale strategies that combine intensive interpersonal counseling, mass media, and community mobilization: results of cluster‐randomized program evaluations in Bangladesh and Viet Nam. PLoS Medicine 2016;13(10):e1002159. [DOI: 10.1371/journal.pmed.1002159; PUBMED: 27780198] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon P, Nguyen PH, Saha KK, Khaled A, Sanghvi T, Baker J, et al. Combining intensive counseling by frontline workers with a nationwide mass media campaign has large differential impacts on complementary feeding practices but not on child growth: results of a cluster‐randomized program evaluation in Bangladesh. Journal of Nutrition 2016;146(10):2075‐84. [DOI: 10.3945/jn.116.232314; PUBMED: 27581575] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon P, Rawat R. Impact of behavior change communications and market‐based approach to delivering micronutrient powders on stunting, infant feeding practices and anemia in Bangladesh. clinicaltrials.gov/ct2/show/NCT01678716 Date first received: 29 August 2012.
Neufeld 2008 {published and unpublished data}
- Aburto NJ, Ramirez‐Zea M, Neufeld LM, Flores‐Ayala R. The effect of nutritional supplementation on physical activity and exploratory behavior of Mexican infants aged 8‐12 months. European Journal of Clinical Nutrition 2010;64(6):644‐51. [DOI: 10.1038/ejcn.2010.52; PUBMED: 20354559] [DOI] [PubMed] [Google Scholar]
- Colchero A, Neufeld LM. Cost estimations of different types of micronutrient supplements for children and pregnant women (poster presentation). FASEB Journal 2008; Vol. 22:Abstract 678.21.
- García‐Guerra A, Neufeld LM, Domínguez CP, García‐Feregrino R, Hernández‐Cabrera A. Effect of three supplements with identical micronutrient content on anemia in Mexican children (poster presentation). FASEB Journal 2008; Vol. 22:Abstract 677.5.
Osendarp 2007 {published data only}
- Osendarp SJ, Baghurst KI, Bryan J, Calvaresi E, Hughes D, Hussaini M, et al. Effect of a 12‐mo micronutrient intervention on learning and memory in well‐nourished and marginally nourished school‐aged children: 2 parallel, randomized, placebo‐controlled studies in Australia and Indonesia. American Journal of Clinical Nutrition 2007;86(4):1082‐93. [PUBMED: 17921387] [DOI] [PubMed] [Google Scholar]
Paganini 2016 {published data only}
- Paganini D, Uyoga MA, Zimmermann MB. Iron fortification of foods for infants and children in low‐income countries: effects on the gut microbiome, gut inflammation, and diarrhea. Nutrients 2016;8(8):1‐11. [DOI: 10.3390/nu8080494; PMC4997407; PUBMED: 27529276] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rah 2012 {published data only}
- Rah JH, Pee S, Kraemer K, Steiger G, Bloem MW, Spiegel P, et al. Program experience with micronutrient powders and current evidence. Journal of Nutrition 2012;142(1):191‐6S. [DOI: 10.3945/jn.111.140004; PUBMED: 22131547] [DOI] [PubMed] [Google Scholar]
Rim 2008 {published data only}
- Rim H, Kim S, Sim B, Gang H, Kim H, Kim Y, et al. Effect of iron fortification of nursery complementary food on iron status of infants in the DPR Korea. Asia Pacific Journal of Clinical Nutrition 2008;17(2):264‐9. [PUBMED: 18586646] [PubMed] [Google Scholar]
Samadpour 2011 {published data only}
- Samadpour K, Long KZ, Hayatbakhsh R, Marks GC. Randomised comparison of the effects of Sprinkles and Foodlets with the currently recommended supplement (Drops) on micronutrient status and growth in Iranian children. European Journal of Clinical Nutrition 2011;65(12):1287‐94. [DOI: 10.1038/ejcn.2011.124; PUBMED: 21750564] [DOI] [PubMed] [Google Scholar]
Sampaio 2013 {published data only}
- NCT00967551. The impact of the use of zinc supplementation and other micronutrients on the occurrence of diarrhea diseases and respiratory infections in children of daycare centers. clinicaltrials.gov/show/NCT00967551 Date first received: 27 August 2009.
- Sampaio DL, Mattos AP, Ribeiro TC, Leite ME, Cole CR, Costa‐Ribeiro H Jr. Zinc and other micronutrients supplementation through the use of sprinkles: impact on the occurrence of diarrhoea and respiratory infections in institutionalized children [Suplementação de zinco e outros micronutrientes através do uso de sprinkles: impacto na ocorrência de doença diarreica e infecções respiratórias em crianças institucionalizadas]. Jornal de Pediatria 2013;89(3):286‐93. [DOI: 10.1016/j.jped.2012.11.004; PUBMED: 23664200] [DOI] [PubMed] [Google Scholar]
Selva Suárez 2011 {published data only}
- Selva Suárez LN, Ochoa Alonso AA. Actions for prevention and control of iron‐deficiency anemia in up to five‐years old children [Acciones para la prevención y control de la anemia por deficiencia de hierro en niños hasta cinco años]. Revista Cubana de Salud Pública 2011;37(3):200‐6. [DOI: 10.1590/S0864-34662011000300003] [DOI] [Google Scholar]
Smuts 2005 {published data only}
- Smuts CM, Dhansay MA, Faber M, Stuijvenberg ME, Swanevelder S, Gross R, et al. Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, and growth in South African infants. Journal of Nutrition 2005;135(3):653‐9S. [PUBMED: 15735110] [DOI] [PubMed] [Google Scholar]
Soofi 2013 {published data only}
- Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, et al. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster‐randomised trial. Lancet 2013;382(9886):29‐40. [DOI: 10.1016/S0140-6736(13)60437-7; PUBMED: 23602230] [DOI] [PubMed] [Google Scholar]
Suchdev 2007 (C) {published and unpublished data}
- Centers for Disease Control and Prevention. Baseline data from the Nyando Integrated Child Health and Education Project ‐ Kenya, 2007. Morbidity and Mortality Weekly Report 2007;56(42):1109‐13. [PubMed] [Google Scholar]
- Jefferds ME, Ogange L, Owuor M, Cruz K, Person B, Obure A, et al. Formative research exploring acceptability, utilization, and promotion in order to develop a micronutrient powder (Sprinkles) intervention among Luo families in western Kenya. Food and Nutrition Bulletin 2010;31(2 Suppl):S179‐85. [DOI: 10.1177/15648265100312S210; PUBMED: 20715602] [DOI] [PubMed] [Google Scholar]
- Suchdev PS, Ruth LJ, Woodruff BA, Mbakaya C, Mandava U, Flores‐Ayala R, et al. Selling Sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in Western Kenya: a cluster‐randomized controlled trial. American Journal of Clinical Nutrition 2012;95(5):1223‐30. [DOI: 10.3945/ajcn.111.030072; PMC4697950; PUBMED: 22492366] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchdev PS, Shah A, Jefferds ME, Eleveld A, Patel M, Stein AD, et al. Sustainability of market‐based community distribution of Sprinkles in western Kenya. Maternal and Child Nutrition 2013;9 Suppl 1:78‐88. [DOI: 10.1111/j.1740-8709.2012.00450.x; PUBMED: 23167586] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teshome 2017 {published data only}
- NCT02073149. Safe and efficacious iron for children in Kenya (SEICK) [Comparison of home fortification with two iron formulations in Kenyan children protected against malaria by artemisinin‐based combination therapy: a placebo‐controlled non‐inferiority trial]. clinicaltrials.gov/ct2/show/record/NCT02073149 Date first received: 25 February 2014.
- Teshome EM, Andang'o PEA, Osoti V, Terwel SR, Otieno W, Demir AY, et al. Daily home fortification with iron as ferrous fumarate versus NaFeEDTA: a randomised, placebo‐controlled, non‐inferiority trial in Kenyan children. BMC Medicine 2017;15:89. [doi.org/10.1186/s12916‐017‐0839‐z; PUBMED: 28449690 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Troesch 2009 {published data only}
- Troesch B. Optimized micronutrient powder containing low levels of highly bioavailable iron and zinc together with EDTA, phytase and ascorbic acid improves the nutritional status of children. Sight and Life Magazine 2010, issue 3:9‐14.
- Troesch B, Egli I, Zeder C, Hurrell RF, Pee S, Zimmermann MB. Optimization of a phytase‐containing micronutrient powder with low amounts of highly bioavailable iron for in‐home fortification of complementary foods. American Journal of Clinical Nutrition 2009;89(2):539‐44. [DOI: 10.3945/ajcn.2008.27026; PUBMED: 19106242] [DOI] [PubMed] [Google Scholar]
Vuong 2002 {published data only}
- Vuong le T, Dueker SR, Murphy SP. Plasma beta‐carotene and retinol concentrations of children increase after a 30‐d supplementation with the fruit Momordica cochinchinensis (gac). American Journal of Clinical Nutrition 2002;75(5):872‐9. [PUBMED: 11976161] [DOI] [PubMed] [Google Scholar]
Wijaya‐Erhardt 2007 {published data only}
- Wijaya‐Erhardt M, Erhardt JG, Untoro J, Karyadi E, Wibowo L, Gross R. Effects of daily or weekly multiple‐micronutrient and iron foodlike tablets on body iron stores of Indonesian infants aged 6‐12 mo: a double‐blind, randomized, placebo‐controlled trial. American Journal of Clinical Nutrition 2007;86(6):1680‐6. [PUBMED: 18065586] [DOI] [PubMed] [Google Scholar]
Zlotkin 2001 {published data only}
- Zlotkin S, Arthur P, Antwi KY, Yeung G. Treatment of anemia with microencapsulated ferrous fumarate plus ascorbic acid supplied as sprinkles to complementary (weaning) foods. American Journal of Clinical Nutrition 2001;74(6):791‐5. [PUBMED: 11722961] [DOI] [PubMed] [Google Scholar]
Zlotkin 2003a {published data only}
- Zlotkin S, Antwi KY, Schauer C, Yeung G. Use of microencapsulated iron (II) fumarate sprinkles to prevent recurrence of anaemia in infants and young children at high risk. Bulletin of the World Health Organization 2003;81(2):108‐15. [PMC2572396; PUBMED: 12756979] [PMC free article] [PubMed] [Google Scholar]
Zlotkin 2003b {published data only}
- Zlotkin S, Arthur P, Schauer C, Antwi KY, Yeung G, Piekarz A. Home‐fortification with iron and zinc Sprinkles of iron Sprinkles alone successfully treats anemia in infants and young children. Journal of Nutrition 2003;133(4):1075‐80. [PUBMED: 12672922] [DOI] [PubMed] [Google Scholar]
Zlotkin 2013 {published data only}
- Zlotkin S, Newton S, Aimone AM, Azindow I, Amenga‐Etego S, Tchum K, et al. Effect of iron fortification on malaria incidence in infants and young children in Ghana: a randomized trial. JAMA 2013;310(9):938‐47. [DOI: 10.1001/jama.2013.277129; PUBMED: 24002280] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616001245482 {published data only}
- ACTRN12616001245482. Food fortification powder for improving micronutrient status in underweight and overweight/obese primary school children [The effect of a multiple micronutrient powder for point‐of‐use fortification of foods on the micronutrient status of underweight and overweight/obese primary school children in Vietnam ‐ a randomised controlled trial]. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12616001245482 Date first registered: 6 September 2016.
NCT01917032 {published data only}
- NCT01917032. Effect of home‐fortification with micronutrient powder sprinkles in hematologic and nutritional status in preschool children in Medellín: a randomized clinical trial. clinicaltrials.gov/show/NCT01917032 Date first received: 2 August 2013.
NCT02280330 {published data only}
- NCT02280330. Iodine intake & status of preschoolers given micronutrient powder for 6 months. clinicaltrials.gov/ct2/show/NCT02280330 Date first received: 27 October 2014.
NCT02302729 {published data only}
- NCT02302729. Early child development and nutrition in Guatemala. clinicaltrials.gov/ct2/show/study/NCT02302729 Date first received: 31 October 2014.
NCT02422953 {published data only}
- Kureishy S, Khan GN, Arrif S, Ashraf K, Cespedes A, Habib MA, et al. A mixed methods study to assess the effectiveness of food‐based interventions to prevent stunting among children under‐five years in Districts Thatta and Sujawal, Sindh Province, Pakistan: study protocol. BMC Public Health 2017;17(1):1‐6. [DOI: 10.1186/s12889-016-3976-y; PUBMED: 28056945] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02422953. Effectiveness of food/nutrient based interventions to prevent stunting among children under five in Thatta and Sajawal districts, Sindh province, Pakistan. clinicaltrials.gov/ct2/show/record/NCT02422953 Date first received: 15 April 2015.
PACTR201607001693286 {published data only}
- PACTR201607001693286. The efficacy of multiple micronutrient powder supplementation in children under five years in Arusha District. apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201607001693286 Date first registered: 24 June 2016.
Additional references
Allen 2009
- Allen LH, Peerson JM, Olney DK. Provision of multiple rather than two or fewer micronutrients more effectively improves growth and other outcomes in micronutrient‐deficient children and adults. Journal of Nutrition 2009;139(5):1022‐30. [DOI: 10.3945/jn.107.086199; PUBMED: 19321586] [DOI] [PubMed] [Google Scholar]
Andersson 2012
- Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. Journal of Nutrition 2012;142(4):744‐50. [DOI: 10.3945/jn.111.149393; PUBMED: 22378324] [DOI] [PubMed] [Google Scholar]
Bailey 2015
- Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF 3rd, Mills JL, et al. Biomarkers of nutrition for development‐folate review. Journal of Nutrition 2015;145(7):1636S‐80S. [DOI: 10.3945/jn.114.206599; PMC4478945; PUBMED: 26451605] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI: 10.1016/j.jclinepi.2010.07.015; PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Beaton 1999
- Beaton GH, McCabe GP. Efficacy of intermittent iron supplementation in the control of iron deficiency anaemia in developing countries. The Micronutrient Initiative. Ottawa (Canada): The Micronutrient Initiative, 1999. [Google Scholar]
Berger 1997
- Berger J, Aguayo VM, Téllez W, Luján C, Traissac P, San Miguel JL. Weekly iron supplementation is as effective as 5 day per week iron supplementation in Bolivian school children living at high altitude. European Journal of Clinical Nutrition 1997;51(6):381‐6. [PUBMED: 9192196] [DOI] [PubMed] [Google Scholar]
Bhutta 2008
- Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. Maternal and Child Undernutrition Study Group. What works? interventions for maternal and child undernutrition and survival. Lancet 2008;371(9610):417‐40. [DOI: 10.1016/S0140-6736(07)61693-6; PUBMED: 18206226] [DOI] [PubMed] [Google Scholar]
Black 2008
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. [DOI: 10.1016/S0140-6736(07)61690-0; PUBMED: 18207566] [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. Oxford (UK): John Wiley & Sons, 2009. [Google Scholar]
Brown 2009
- Brown KH, Peerson JM, Baker SK, Hess SY. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food and Nutrition Bulletin 2009;30(Suppl 1):S12‐40. [DOI] [PubMed] [Google Scholar]
Christofides 2006
- Christofides A, Asante KP, Schauer C, Sharieff W, Owusu‐Agyei S, Zlotkin S. Multi‐micronutrient sprinkles including a low dose of iron provided as microencapsulated ferrous fumarate improves haematologic indices in anaemic children: a randomized clinical trial. Maternal and Child Nutrition 2006;2(3):169‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Commission on Ending Childhood Obesity 2016
- Commission on Ending Childhood Obesity. Report of the Commission on Ending Childhood Obesity. Geneva (Switzerland): World Health Organization, 2016. [Google Scholar]
De Pee 2008
- Pee S, Kraemer K, Briel T, Boy E, Grasset C, Moench‐Pfanner R, et al. World Food Programme, Sprinkles Global Health Initiative. Quality criteria for micronutrient powder products: report of a meeting organized by the World Food Programme and Sprinkles Global Health Initiative. Food and Nutrition Bulletin 2008;29(3):232‐41. [DOI: 10.1177/156482650802900309; PUBMED: 18947036] [DOI] [PubMed] [Google Scholar]
De Pee 2009
- Pee S, Bloem MW. Current and potential role of specially formulated foods and food supplements for preventing malnutrition among 6‐ to 23‐month‐old children and for treating moderate malnutrition among 6‐ to 59‐month‐old children. Food and Nutrition Bulletin 2009;30(Suppl 3):S434‐63. [DOI: 10.1177/15648265090303S305; PUBMED: 19998866] [DOI] [PubMed] [Google Scholar]
De‐Regil 2011a
- De‐Regil LM, Jefferds MED, Sylvetsky AC, Dowswell T. Intermittent iron supplementation for improving nutrition and development in children under 12 years of age. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009085.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2011b
- De‐Regil LM, Suchdev PS, Vist GE, Walleser S, Peña‐Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008959.pub2] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dewey 2008
- Dewey KG, Adu‐Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & Child Nutrition 2008;4(Suppl 1):24‐85. [DOI: 10.1111/j.1740-8709.2007.00124.x; PUBMED: 18289157] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dewey 2009
- Dewey KG, Yang Z, Boy E. Systematic review and meta‐analysis of home fortification of complementary foods. Maternal & Child Nutrition 2009;5(4):283‐321. [DOI: 10.1111/j.1740-8709.2009.00190.x] [DOI] [Google Scholar]
Gera 2007
- Gera T, Sachdev HP, Nestel P, Sachdev SS. Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials. Journal of Pediatric Gastroenterology and Nutrition 2007;44(4):468‐86. [DOI: 10.1097/01.mpg.0000243440.85452.38; PUBMED: 17414146] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 14 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [DOI: 10.1016/j.jclinepi.2010.09.012; PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
HFTAG 2011
- Home Fortification Technical Advisory Group (HFTAG). Programmatic guidance brief on use of micronutrient powders (MNP) for home fortification. Geneva (Switzerland): Home Fortification Technical Advisory Group, 2011:1‐8. [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hirve 2013
- Hirve S, Martini E, Juvekar SK, Agarwal D, Bavdekar A, Sari M, et al. Delivering Sprinkles Plus through the integrated child development services (ICDS) to reduce anemia in pre‐school children in India. Indian Journal of Pediatrics 2013;80(12):990‐5. [DOI: 10.1007/s12098-013-1063-2; PUBMED: 23723079] [DOI] [PubMed] [Google Scholar]
Horton 2010
- Horton S, Shekar M, McDonald C, Mahal A, Krystene‐Brooks J. Scaling up Nutrition. What will it cost?. Washington (DC): The International Bank for Reconstruction and Development/The World Bank, 2010. [Google Scholar]
Hurrell 2010
- Hurrell R. Iron and malaria: absorption, efficacy and safety. International Journal of Vitamin Nutrition Research 2010;80(4‐5):279‐92. [DOI: 10.1024/0300-9831/a000035; PUBMED: 21462111] [DOI] [PubMed] [Google Scholar]
Hyder 2007
- Hyder SM, Haseen F, Rahman M, Tondeur M, Zlotkin SH. Effect of daily versus once‐weekly home fortification with micronutrient Sprinkles on hemoglobin and iron status among young children in rural Bangladesh. Food and Nutrition Bulletin 2007;28(2):156‐64. [DOI: 10.1177/156482650702800204; PUBMED: 24683674] [DOI] [PubMed] [Google Scholar]
Jimenez 2010
- Jimenez C, Leets I, Puche R, Anzola E, Montilla R, Parra C, et al. A single dose of vitamin A improves haemoglobin concentration, retinol status and phagocytic function of neutrophils in preschool children. British Journal of Nutrition 2010;103(6):798‐802. [DOI: 10.1017/S0007114509992765; PUBMED: 20003622] [DOI] [PubMed] [Google Scholar]
King 2016
- King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, et al. Biomarkers of Nutrition for Development (BOND) ‐ zinc review. Journal of Nutrition 2016;146(4):858S‐885S. [DOI: 10.3945/jn.115.220079; PMC4807640; PUBMED: 26962190] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kupka 2015
- Kupka R. The role of folate in malaria ‐ implications for home fortification programmes among children aged 6‐59 months. Maternal & Child Nutrition 2015;11(Suppl 4):1‐15. [DOI: 10.1111/mcn.12102; PUBMED: 26756732] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liyanage 2002
- Liyanage C, Zlotkin S. Bioavailability of iron from micro‐encapsulated iron sprinkle supplement. Food and Nutrition Bulletin 2002;23(Suppl 3):133‐7. [PUBMED: 12362781] [PubMed] [Google Scholar]
Lozoff 2007
- Lozoff B. Iron deficiency and child development. Food and Nutrition Bulletin 2007;28(Suppl 4):S560‐71. [DOI: 10.1177/15648265070284S409; PUBMED: 18297894] [DOI] [PubMed] [Google Scholar]
Lutter 2011
- Lutter CK, Daelmans BM, Onis M, Kothari MT, Ruel MT, Arimond M, et al. Undernutrition, poor feeding practices, and low coverage of key nutrition interventions. Pediatrics 2011;128(6):1418‐27. [DOI: 10.1542/peds.2011-1392; PUBMED: 22065267] [DOI] [PubMed] [Google Scholar]
Martini 2013
- Martini E. Providing micronutrient powder (MNP) through school feeding to improve health and nutritional status of school children. Presentation at the UNICEF/CDC Regional Workshop on improving the nutritional quality of complementary foods for children 6‐23 months through home fortification in Central and Eastern Europe and Middle East and North Africa; 2013 May 29; Antalya, Turkey 2013.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulinare 1988
- Mulinare J, Cordero JF, Erickson JD, Berry RJ. Periconceptional use of multivitamins and the occurrence of neural tube defects. JAMA 1988;260(21):3141‐5. [PUBMED: 3184392] [PubMed] [Google Scholar]
Okebe 2011
- Okebe JU, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria‐endemic areas. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD006589.pub3] [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2012
- Peña‐Rosas JP, De‐Regil LM, Rogers LM, Bopardikar A, Panisset U. Translating research into action: WHO evidence‐informed guidelines for safe and effective micronutrient interventions. Journal of Nutrition 2012;142(1):197‐204S. [DOI: 10.3945/jn.111.138834; PUBMED: 22113868] [DOI] [PubMed] [Google Scholar]
Prentice 2007
- Prentice AM, Ghattas H, Doherty C, Cox SE. Iron metabolism and malaria. Food and Nutrition Bulletin 2007;28(Suppl 4):S524‐39. [DOI: 10.1177/15648265070284S406; PUBMED: 18297891] [DOI] [PubMed] [Google Scholar]
Rastogi 2002
- Rastogi R, Mathers CD. Global burden of iron deficiency anaemia in the year 2000. www.who.int/healthinfo/statistics/bod_irondeficiencyanaemia.pdf (accessed 27 July 2010).
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salam 2013
- Salam RA, MacPhail C, Das JK, Bhutta ZA. Effectiveness of micronutrient powders (MNP) in women and children. BMC Public Health 2013;13 Suppl 3:S22. [DOI: 10.1186/1471-2458-13-S3-S22] [DOI] [PMC free article] [PubMed] [Google Scholar]
SGHI 2008
- Sprinkles Global Health Initiative. Micronutrient Sprinkles for use in infants and young children: guidelines on recommendations for use and program monitoring and evaluation. www.sghi.org/resource_centre/GuidelinesGen2008.pdf (accessed on 10 September 2010).
Sharieff 2006
- Sharieff W, Bhutta Z, Schauer C, Tomlinson G, Zlotkin S. Micronutrients (including zinc) reduce diarrhoea in children: the Pakistan Sprinkles Diarrhoea Study. Archives of Disease in Childhood 2006;91(7):573‐9. [DOI: 10.1136/adc.2005.086199] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2013
- Stevens GA, Finucane MM, De‐Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995‐2011: a systematic analysis of population ‐ representative data. Lancet Global Health 2013;1(1):e16‐25. [10.1016/S2214‐109X(13)70001‐9; PMC4547326] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2015
- Stevens GA, Bennett JE, Hennocq Q, Lu Y, De‐Regil LM, Rogers L, et al. Trends and mortality effects of vitamin A deficiency in children in 138 low‐income and middle‐income countries between 1991 and 2013: a pooled analysis of population‐based surveys. Lancet Global Health 2015;3(9):e528‐36. [DOI: 10.1016/S2214-109X(15)00039-X; PUBMED: 26275329] [DOI] [PubMed] [Google Scholar]
Stoltzfus 2004
- Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anaemia. In: Ezzati M, Lopez AD, Rodgers A, Murray CLJ editor(s). Comparative Quantification of Health Risks: Global and Regional Burden of Disease. Geneva (Switzerland): World Health Organization, 2004:163‐209. [Google Scholar]
Suchdev 2015
- Suchdev PS, Peña‐Rosas JP, De‐Regil. Multiple micronutrient powders for home (point‐of‐use) fortification of foods in pregnant women. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011158.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanumihardjo 2016
- Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, et al. Biomarkers of nutrition for development (BOND) ‐ vitamin A review. Journal of Nutrition 2016;146(9):1816S‐48S. [DOI: 10.3945/jn.115.229708; PMC4997277; PUBMED: 27511929] [DOI] [PMC free article] [PubMed] [Google Scholar]
Timmer 2013
- Timmer A, Irizarry L, Tripp K, Flores‐Ayala R, Jefferds ME. Regional workshops to scale up home fortification to improve complementary feeding: collaboration between UNICEF, CDC and Partners. Sight and Life Special Supplement 2013;Sept:42‐50. [Google Scholar]
Troesch 2011a
- Troesch B, Stuijvenberg ME, Smuts CM, Kruger HS, Biebinger R, Hurrell RF, et al. A micronutrient powder with low doses of highly absorbable iron and zinc reduces iron and zinc deficiency and improves weight‐for‐age Z‐scores in South African children. Journal of Nutrition 2011;141(2):237‐42. [DOI: 10.3945/jn.110.129247; PUBMED: 21178093] [DOI] [PubMed] [Google Scholar]
UNICEF 2014
- UNICEF. Nutridash 2013: Global report of the pilot year. UNICEF 2014.
UNICEF/CDC 2013
- UNICEF/CDC. Global Assessment of Home Fortification Interventions 2011. Geneva (Switzerland): Home Fortification Technical Advisory Group, 2013. [Google Scholar]
Viteri 1997
- Viteri FE. Iron supplementation for the control of iron deficiency in populations at risk. Nutrition Reviews 1997;55(6):195‐209. [PUBMED: 9279056] [DOI] [PubMed] [Google Scholar]
WFP/DSM 2010
- World Food Programme/DSM. Dealing with diarrhoea in children in refugee, emergency and development situations in the context of micronutrient powder use. Technical Brief 2010; Vol. 1:3.
WHO 2001
- World Health Organization. Iron Deficiency Anaemia Assessment Prevention and Control: a Guide for Programme Managers. Geneva (Switzerland): World Health Organization, 2001:132. [Google Scholar]
WHO 2003
- World Health Organization. Strategic directions for improving the health and development of children and adolescents. Geneva (Switzerland): World Health Organization, 2003. [Google Scholar]
WHO 2009
- World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva (Switzerland): World Health Organization, 2009. [Google Scholar]
WHO 2010a
- World Health Organization. Malaria. In: Poumerol G, Wilder‐Smith A editor(s). Malaria. International Travel and Health. Situation as on 1 January 2010. Geneva (Switzerland): World Health Organization, 2010. [Google Scholar]
WHO 2011a
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva (Switzerland): World Health Organization, 2011. [Google Scholar]
WHO 2011b
- World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva (Switzerland): World Health Organization, 2011. [Google Scholar]
WHO 2011c
- World Health Organization. Guideline: intermittent iron supplementation in preschool and school‐age children. Geneva (Switzerland): World Health Organization, 2011. [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Essential nutrition actions: improving maternal, newborn, infant and young child health and nutrition. Geneva (Switzerland): World Health Organization, 2013. [PubMed] [Google Scholar]
WHO 2015a
- World Health Organization. World Malaria Report 2015. Geneva (Switzerland): World Health Organization, 2015. [Google Scholar]
WHO 2015b
- World Health Organization. The Global Prevalence of Anaemia in 2011. Geneva (Switzerland): World Health Organization, 2015. [Google Scholar]
WHO 2016
- World Health Organization. WHO guideline: Use of multiple micronutrient powders for point‐of‐use fortification of foods consumed by infants and young children aged 6–23 months and children aged 2–12 years. Geneva: World Health Organization, 2016. [PubMed] [Google Scholar]
WHO/FAO 2006
- World Health Organization, Food, Agricultural Organization of the United Nations. Guidelines on food fortification with micronutrients. Geneva (Switzerland): WHO, 2006. [Google Scholar]
Winzenberg 2011
- Winzenberg T, Powell S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta‐analysis. BMJ 2011;342:c7254. [PMC3026600; PUBMED: 21266418] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Vision Mongolia 2005
- World Vision Mongolia. Effectiveness of home‐based fortification with Sprinkles in an integrated nutrition program to address rickets and anemia. Ulaanbaatar, Mongolia: World Vision Mongolia 2005.
Yakoob 2011
- Yakoob MY, Theodoratou E, Jabeen A, Imdad A, Eisele TP, Ferguson J, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011;11(Suppl 3):S23. [DOI: 10.1186/1471-2458-11-S3-S23; PMC3231897; PUBMED: 21501441] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zlotkin 2005
- Zlotkin SH, Schauer C, Christofides A, Sharieff W, Tondeur MC, Hyder SM. Micronutrient sprinkles to control childhood anaemia. PLoS Medicine 2005;2(1):e1. [DOI: 10.1371/journal.pmed.0020001; PMC545194] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
De‐Regil 2012
- De‐Regil LM, Jefferds MED, Peña‐Rosas JP. Point‐of‐use fortification of foods with micronutrient powders containing iron in children of preschool and school age. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009666] [DOI] [PMC free article] [PubMed] [Google Scholar]


