Abstract
Background
Hypertension is a major public health problem that increases the risk of cardiovascular and kidney diseases. Several studies have shown an inverse association between calcium intake and blood pressure. As small reductions in blood pressure have been shown to produce rapid reductions in vascular disease risk even in individuals with normal blood pressure ranges, this review intends to evaluate the effect of calcium supplementation in normotensive individuals as a preventive health measure.
Objectives
To assess the efficacy and safety of calcium supplementation versus placebo or control for reducing blood pressure in normotensive people.
Search methods
We searched the Cochrane Hypertension Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In‐Process, EMBASE and ClinicalTrials.gov for randomised controlled trials up to October 2014. The WHO International Clinical Trials Registry Platform (ICTRP) is searched for inclusion in the Group's Specialised Register. We also reviewed reference lists from retrieved studies and contacted authors of relevant papers. We applied no language restrictions.
Selection criteria
We selected trials that randomised normotensive people to dietary calcium interventions such as supplementation or food fortification versus placebo or control. We excluded quasi‐random designs. The primary outcomes were hypertension (defined as blood pressure ≥ 140/90 mmHg) and blood pressure measures.
Data collection and analysis
Two review authors independently selected trials for inclusion, abstracted the data and assessed the risks of bias.
Main results
We included 16 trials with 3048 participants. None of the studies reported hypertension as a dichotomous outcome. The effect on systolic and diastolic blood pressure was mean difference (MD) ‐1.43 mmHg (95% confidence interval (CI) ‐2.15 to ‐0.72) and ‐0.98 mmHg (95%CI ‐1.46 to ‐0.50) respectively. The effect on systolic and diastolic blood pressure for those younger than 35 years (7 trials with 399 participants) was ‐2.11 mmHg (95%CI ‐3.58 to ‐0.64) / ‐2.61 mmHg (95% CI ‐3.74, ‐1.49). The effect on systolic and diastolic blood pressure for those 35 years or more (9 trials with 2649 participants) was ‐0.96 mmHg (95%CI ‐1.83 to ‐0.09) / ‐0.59 mmHg (95%CI ‐1.13 to ‐0.06). The effect on systolic and diastolic blood pressure for women (6 trials with 1823 participants) was ‐1.45 mmHg (95% CI ‐2.78 to ‐0.12) / ‐0.92 mmHg (95% CI ‐1.71 to ‐0.14). The effect on systolic and diastolic blood pressure for men (5 trials with 617 participants) was ‐2.07 (95%CI ‐3.56 to ‐0.59] / ‐1.91 (95%CI ‐2.80 to ‐1.02).The quality of evidence for each of these outcomes was high. The effect is consistent in both genders regardless of baseline calcium intake.
The effect on systolic blood pressure was 0.08 mmHg (95% CI ‐2.16 to 2.32) with doses less than 1000 mg, ‐1.14 mmHg (95% CI ‐2.01 to ‐0.27) with 1000 ‐ 1500 mg, and ‐2.79 mmHg (95% CI ‐4.71 to ‐0.86) with more than 1500 mg. The effect on diastolic blood pressure was ‐0.54 mmHg (95% CI ‐2.23 to 1.15), ‐0.71 mmHg (95% CI ‐1.37 to ‐0.06) and ‐1.43 mmHg (95% CI ‐2.22 to ‐0.64) respectively. The quality of evidence for each of these outcomes was high.
None of the studies reported adverse events.
Authors' conclusions
An increase in calcium intake slightly reduces both systolic and diastolic blood pressure in normotensive people, particularly in young people, suggesting a role in the prevention of hypertension. These results should be interpreted with caution, since the proposed biological mechanism explaining the relationship between calcium and blood pressure has not been fully confirmed. The effect across multiple prespecified subgroups and a possible dose response effect reinforce this conclusion. Even small reductions in blood pressure could have important health implications for reducing vascular disease.
There is a great need for adequately‐powered clinical trials randomising young people. Subgroup analysis should involve basal calcium intake, age, sex, basal blood pressure, and body mass index. We also require assessment of side effects, optimal doses and the best strategy to improve calcium intake.
Keywords: Adult; Female; Humans; Male; Middle Aged; Dietary Supplements; Age Factors; Calcium, Dietary; Calcium, Dietary/administration & dosage; Diastole; Essential Hypertension; Hypertension; Hypertension/prevention & control; Randomized Controlled Trials as Topic; Sex Factors; Systole
Extra calcium to prevent high blood pressure
Review question
We wanted to find out the effects of calcium intake on blood pressure in people with normal blood pressure.
Background
Hypertension is a serious health problem that increases the risk of heart and kidney diseases. Several studies have shown that increasing calcium intake lowers blood pressure even in individuals within a normal blood pressure range. Increasing calcium intake also has benefits for pregnancy outcomes, effects which are thought to be mediated also by blood pressure reduction. High blood pressure has been identified as a major risk factor for mortality and even small reductions in blood pressure can decrease the occurrence of coronary artery disease, stroke and death.
Study characteristics
We selected studies that assessed the effect of dietary calcium interventions such as supplementation or food fortification on blood pressure in normotensive people of all ages. The last search date was October 2014.
Key findings
This review analysed information from 16 trials (3048 participants). We found that an increase in calcium intake slightly reduces both systolic and diastolic blood pressure 1.43 mmHg lower and 0.98 mmHg lower respectively. This effect was higher with doses of calcium above 1000 mg/day. Systolic blood pressure was reduced by 1.14 mmHg with doses of calcium 1000 to 1500 mg/day and by 2.79 mmHg with doses of calcium equal to or over 1500 mg/day.
We noted a reduction in blood pressure in both men and women and at ages from 11 to 82 years old, but the reduction was greater among younger people. Systolic blood pressure was reduced by 2.11 mmHg among those less than 35 years and by 0.96 mmHg among those 35 years or older.
None of the studies reported adverse events. We need further research to determine the ideal dosage of supplementation and whether it is more effective and safer as part of the diet or as a supplement.
Quality of the evidence
We found high quality of evidence for systolic and diastolic blood pressure in both men and women. The quality of evidence was also high for participants 35 years or older and moderate for younger people.
The quality of evidence was high for doses of calcium of 1000 to 1500 mg/day and was moderate for lower or higher doses.
Five of the 16 trials were industry funded.
Summary of findings
Summary of findings for the main comparison.
Calcium supplementation/fortification compared to control for prevention of primary hypertension
| Calcium supplementation/fortification compared to control for prevention of primary hypertension | ||||
| Patient or population: People who may be at risk for primary hypertension Settings: US (8), New Zealand (3), and one each in The Netherlands, Belgium and Denmark, Guatemala and Iran Intervention: Calcium supplementation/fortification Comparison: Placebo | ||||
| Outcomes | Illustrative blood pressure in control groupb | Mean Difference in mmHg (95% CIa) | No of Participants (studies) | Quality of the evidence (GRADE)c |
| Systolic blood pressure | 115.64 | 1.43 lower (2.15 lower to 0.72 lower) | 3048 (16 studies) | ⊕⊕⊕⊕ high |
| Diastolic blood pressure | 78.20 | 0.98 lower (1.46 lower to 0.50 lower) | 2947 (15 studies) | ⊕⊕⊕⊕ high |
| Systolic blood pressure. Dose less than 1000 mg a day | 101.60 | 0.08 higher (2.16 lower to 2.32 higher) | 263 (2 studies) | ⊕⊕⊕⊝ moderate1 |
| Systolic blood pressure. Dose between 1000 mg a day and less than 1500 mg a day | 122.43 | 1.14 lower (2.01 lower to 0.27 lower) | 2435 (8 studies) | ⊕⊕⊕⊕ high |
| Systolic blood pressure. Dose 1500 mg a day or more | 112.85 | 2.79 lower (4.71 lower to 0.86 lower) | 350 (7 studies) | ⊕⊕⊕⊝ moderate1 |
| Systolic blood pressure. Less than 35 years of age | 113.25 | 2.11 lower (3.58 lower to 0.64 lower) | 399 (7 studies) | ⊕⊕⊕⊝ moderate1 |
| Systolic blood pressure. 35 years or older | 121.43 | 0.96 lower (1.83 lower to 0.09 lower) | 2649 (9 studies) | ⊕⊕⊕⊕ high |
| aCI: Confidence interval; bEstimated using Comprehensive Meta‐Analysis Software Software; cGRADE Working Group grades of evidence | ||||
| High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1. Small number of participants and studies.
Background
Description of the condition
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure defines "hypertension" as blood pressure above 139 mmHg systolic and/or diastolic above 89 mmHg . It also defines blood pressure ranging from 120–139 mmHg systolic and/or 80–89 mmHg diastolic as “prehypertension” in order to identify those individuals in whom early intervention by adoption of healthy lifestyles could reduce blood pressure, decrease the rate of progression of blood pressure to hypertensive levels with age, or prevent hypertension entirely (Chobanian 2003).
Primary hypertension may develop as a result of environmental or genetic causes. Secondary hypertension has multiple etiologies, such as renal, vascular, and endocrine causes. Primary or essential hypertension accounts for 90‐95% of adult cases and secondary hypertension accounts for 2‐10% of cases (Carretero 2000).
Hypertension is a major public health problem that increases the risk of cardiovascular and kidney diseases in both the developed and the developing world. The global prevalence of hypertension and high blood pressure are estimated to be 30% and 26% respectively (Kearney 2004) and high blood pressure has been estimated to increase to 29% by the year 2025 (Kearney 2005).
High blood pressure has been identified as the leading risk factor for mortality and the third leading risk factor for disease burden globally (Ezzati 2002). In the year 2001, 7.6 million (13.5%) of all deaths were attributable to high blood pressure (Lawes 2008).
While the prevalence of hypertension seems to be stabilising or decreasing in the developed world, it is increasing in developing countries (Kearney 2004). Low‐income and middle‐income regions contribute up to 80% of the attributable burden of disease, affecting the younger age groups more than in high‐income countries (Lawes 2008). While chronic diseases have increased in these countries, problems related to undernutrition such as micronutrient deficiencies persist, causing a double burden of disease (Llanos 2008). These present a challenge to developing interventions, as excess and deficit nutritional problems have to be tackled within the same population and frequently within the same home (Garrett 2005).
Description of the intervention
Several studies have shown an inverse association between calcium intake and blood pressure or hypertension. The hypothesis originated with the observation that indigenous Guatemalan women have a low incidence of oedema‐, proteinuria‐, and hypertension‐gestosis associated with a high calcium intake due to the Mayan habit of treating corn with lime water (Belizan 1980). Based on this hypothesis, a series of studies has been conducted mainly in pregnant women, but also in children, as well as in young and older adults (Belizan 1980; Belizan 1983).
A recent World Health Organization (WHO) review of observational epidemiological and ecological studies found an inverse (protective) association between cardiovascular disease mortality and increased water hardness (measured by calcium carbonate or another hardness parameter and/or the calcium and magnesium content of water). (WHO 2009).
A Cochrane review in 2006 found that calcium supplementation in hypertensive people elicited a small but statistically significant reduction in systolic blood pressure (SBP) (mean difference: ‐2.5 mmHg, 95% confidence interval (CI) ‐4.5 to ‐0.6), but not in diastolic blood pressure (DBP) (mean difference: ‐0.8 mmHg, 95% CI ‐2.1 to 0.4) (Dickinson 2006).
Several reviews have shown an association between calcium intake and blood pressure (Allender 1996; Griffith 1999; Van Mierlo 2006). The most recent review in 2006 found that calcium supplementation (mean daily dose: 1200 mg) reduced SBP by 1.86 mmHg (95% CI 2.91 to 0.81) and DBP by 0.99 mmHg (95% CI 1.61 to 0.37) (Van Mierlo 2006). In people with a relatively low calcium intake (less than 800 mg per day) higher blood pressure estimates were obtained, at 2.63 (95% CI 4.03 to 1.24) for SBP and 1.30 (95% CI 2.13 to 0.47) for DBP.
Furthermore, a Cochrane review has shown that calcium supplementation has an effect on reducing pregnancy hypertensive diseases (Hofmeyr 2014).
How the intervention might work
The mechanisms by which calcium could influence blood pressure are not well understood. One hypothesis is that low calcium intake would lead to changes in vitamin D and parathormone levels triggering a chain of reactions resulting in an increase in intracellular calcium and consequently increased vascular smooth muscle reactivity, which would result in increased peripheral vascular resistance and thus increased blood pressure (Belizan 1988; Webb 2003). In this way, high blood pressure could be a collateral effect of hormones that are released to compensate for low calcium levels in the organism (Heaney 2006). More basic and clinical studies are required to understand the mechanisms involved in the relationship between calcium intake and blood pressure.
Why it is important to do this review
Small reductions in blood pressure were predicted to have important health implications as they were shown to produce rapid reductions in vascular disease risk even in individuals with normal blood pressure ranges (Lewington 2002). A 2 mmHg‐lower systolic blood pressure is predicted to produce about 10% lower stroke mortality and about 7% lower mortality from ischaemic heart disease, while a 5 mmHg reduction in SBP at the population level is predicted to result in a 14% reduction in stroke death, a 9% reduction in coronary artery disease‐related death and a 7% reduction in total mortality (Whelton 2002). In the same way, a 2 mmHg reduction in SBP in adults is estimated to have the potential to save about 12,000 lives a year in the United States (Stamler 1991).
Due to the high frequency of hypertension, population‐based strategies to reduce blood pressure are more cost effective than individual strategies (Kearney 2005).
Calcium supplementation or food fortification are affordable interventions that, if proven effective in reducing blood pressure even by small levels, could have considerable impact at a population level. The effects on children and young people are of particular importance, as blood pressure tends to track into adulthood (Williams 2011).
This review explores the efficacy and safety of calcium supplementation or food fortification in preventing hypertensive‐related problems in normotensive people of different ages. It looks at the effect in reducing blood pressure in each population group and in preventing, rather than treating, hypertensive‐related problems. It also provides more information on the effect of increasing calcium intake on blood pressure in non‐pregnant women of reproductive age. Reviewing the effect of calcium in a normotensive population is valuable in assessing whether it could allow women to reach pregnancy with a lower range of blood pressure and a lower risk of developing pre‐eclampsia or eclampsia.
As there have been some concerns about adverse events of calcium supplementation (Bolland 2008; Curhan 2004; Harris 2002), there is a need to assess adverse events such as renal tract stone formation, impaired absorption of other minerals and increased cardiovascular events.
Excess calcium in the body had been implicated as a risk factor for kidney stone formation; however, data suggest that free calcium in the body does not increase the risk and that high calcium intake may actually be a protective factor against the formation of kidney stones (Curhan 2004; Heaney 2006; Jackson 2006; Williams 2001).
The effect of calcium supplementation on cardiovascular events is unclear, as there are currently conflicting data, studies have not been powered to significantly detect cardiac events, and the methodology does not allow the results to be generalisable to a broader population. Two studies that were conducted in cohorts of older women have reported a higher incidence of cardiovascular events such as myocardial infarction and the composite end point of myocardial infarction, stroke, or sudden death in the experimental groups. However, these differences were not statistically significant (Bolland 2008; Sabbagh 2009).
Calcium has been shown to interfere with iron absorption in the short term; however, research has also shown that prolonged calcium supplementation has no effect on iron absorption over time (Harris 2002; Ilich‐Ernst 1998; Kalkwarf 1998; Sokoll 1992).
Objectives
To assess the efficacy and safety of calcium supplementation versus placebo or control for reducing blood pressure in normotensive people.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing trials with random allocation to dietary calcium intervention such as supplementation or food fortification versus placebo or control. We exclude quasi‐random designs.
Types of participants
Participants include normotensive people of different ages, but excluding pregnant women.
We planed to analyse groups by age, sex, baseline calcium intake, dose received, duration of intervention, type of intervention, ethnicity, baseline blood pressure, and intake of other minerals involved in blood pressure regulation, such as sodium, magnesium, potassium and fat intake.
Types of interventions
We include calcium interventions such as supplementation using pills, tablets or sprinkle powder, or any food or beverage fortification, compared to placebo or control.
Calcium fortification could include salt of calcium carbonate, sulphate, citrate, citrate malate, chloride, hydroxyapatite, phosphate, acetate, lactate, glycerophosphate, gluconate, oxide, or hydroxide. Calcium content in these salts varies from 9% to 70% (Allen 2006).
We exclude studies with no placebo or control. We also exclude interventions where calcium was combined with other macro‐ or micronutrient to assess the effects of both.
Types of outcome measures
Primary outcomes
Hypertension, defined as blood pressure ≥ 140/90 mmHg
Systolic and diastolic blood pressure
Secondary outcomes
Any adverse event
Withdrawals due to adverse events
Kidney stone formation
Iron deficiency anaemia
Anaemia
Total mortality
Cardiovascular events
Myocardial infarction
Stroke
Sudden death
Search methods for identification of studies
Electronic searches
We searched the Database of Abstracts of Reviews of Effects (DARE) and the Cochrane Database of Systematic Reviews for related reviews.
We searched the following databases for primary studies: the Cochrane Hypertension Group Specialised Register (all years to October 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (2014 Issue 9), MEDLINE (1946 ‐ October 2014), EMBASE (1974 ‐ October 2014) and ClinicalTrials.gov (all years to October 2014). The Cochrane Hypertension Group Specialised Register includes controlled trials from searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, AGRICOLA, Allied and Complementary Medicine (AMED), BIOSIS, CAB Abstracts, CINAHL, Food Science and Technology Abstracts (FSTA), Global Health, International Pharmaceutical Abstracts (IPA), LILACS, ProQuest Dissertations & Theses, PsycINFO, SCIRUS, Web of Science and the WHO International Clinical Trials Registry Platform (ICTRP).
We searched electronic databases using a strategy combining the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) with selected MeSH terms and free‐text terms relating to calcium. The MEDLINE search strategy (Appendix 1) was translated into CENTRAL (Appendix 2), EMBASE (Appendix 3), the Cochrane Hypertension Group Specialised Register (Appendix 4), LILACS (Appendix 5), and ClinicalTrials.gov (Appendix 6) using the appropriate controlled vocabulary as applicable. We applied no language restrictions.
We also searched systematic reviews and meta‐analyses from these databases to check their reference lists, as well as those of randomised controlled trials included in the review.
Searching other resources
Other sources:
Reference lists of all papers and relevant reviews identified
We contacted authors of relevant papers regarding any further published or unpublished work
We contacted authors of trials reporting incomplete information to provide the missing information
We searched ISI Web of Science for papers citing studies included in the review
Data collection and analysis
Pairs of review authors independently assessed the methodological quality and other inclusion criteria of the identified trials, resolving disagreements by consensus.
Selection of studies
We imported references and abstracts of searched results to Early Reviewer Organizing Software (EROS) (Ciapponi 2011; Glujovsky 2010), basing selection of studies on the criteria listed above.
Data extraction and management
Two review authors independently extracted data, using a standard form, and then cross checked them. A third person confirmed all numeric calculations and graphic interpolations.
Descriptive data include authors, year of publication, country, time span of the trial, gender, type of placebo, baseline dietary calcium intake, type, dose and duration of calcium‐related intervention, compliance, co‐interventions, trial quality assessments, and numbers randomised and analysed.
The position of the participant during blood pressure measurement may affect the blood pressure‐lowering effect. However, in order to not lose valuable data if only one position was reported, we collected data from that position. When blood pressure measurement data were available in more than one position, sitting blood pressure is the first preference. If both standing and supine measurements were available, we used standing blood pressure.
Assessment of risk of bias in included studies
GC and MLC independently assessed risks of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement through discussion with the whole team. We made explicit judgements about whether studies had high risk of bias, according to the criteria described below. We assessed the magnitude and direction of the bias and whether we considered it was likely to impact on the findings through sensitivity analysis. SeeSensitivity analysis below.
(1) Sequence generation (checking for possible selection bias)
We describe the method used to generate the allocation sequence for each included study in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as:
| • | low risk of bias (any truly random process, e.g. random number table; computer random number generator) |
| • | high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number) |
| • | unclear risk of bias |
(2) Allocation concealment (checking for possible selection bias)
We describe the method used to conceal the allocation sequence for each included study and determine whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as:
| • | low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes) |
| • | high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth) |
| • | unclear risk of bias |
(3) Blinding (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We consider studies at low risk of bias if they are blinded, or if we judge that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
| • | low, high or unclear risk of bias for participants |
| • | low, high or unclear risk of bias for personnel |
| • | low, high or unclear risk of bias for outcome assessors |
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations).
We describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the re‐analyses.
We assessed methods as:
| • | low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups) |
| • | high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation) |
| • | unclear risk of bias |
(5) Selective reporting bias
We describe for the included study how we investigated the possibility of selective outcome reporting bias and our findings.
We assessed the methods as:
| • | low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported) |
| • | high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported) |
| • | unclear risk of bias |
(6) Other sources of bias
We describe any important concerns we have about other possible sources of bias for each included study.
We assessed whether the study was free of other problems that could put it at risk of bias and record our judgement as:
| • | low risk of bias (the study appears to be free of other sources of bias) |
| • | high risk of bias (potential source of bias related to the specific study design used; or has been claimed to have been fraudulent; or had some other problem) |
| • | unclear risk of bias |
Measures of treatment effect
For continuous data, we used the mean difference (MD) if outcomes were measured in the same way between trials. We used the standardised mean difference (SMD) to combine trials that measure the same outcome but used different methods.
Unit of analysis issues
In the case of studies with more than one treatment comparison we divided the control groups by the number of subgroups.
Dealing with missing data
In the case of missing information in the included studies, we contacted investigators (using email, letter and/or fax) to obtain the missing information. In the case of missing standard deviation of blood pressure change, we imputed the standard deviation based on the information in the same trial or from other trials which assessed calcium‐related interventions. We used the following hierarchy (listed from high to low preference) to impute standard deviation values:
| 1. | standard deviation of change in blood pressure taken in a different position from that of the blood pressure data used |
| 2. | standard deviation of blood pressure at the end of treatment |
| 3. | standard deviation of blood pressure at the end of treatment measured in a different position from that of the blood pressure data used |
| 4. | standard deviation of blood pressure at baseline (except if this measure is used as an entry criterion) |
| 5. | mean standard deviation of change in blood pressure from other trials assessing calcium‐related interventions |
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics (Higgins 2003; Higgins 2011). We regarded heterogeneity as moderate if T² was greater than zero and either I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. I² values greater than 50% indicate high levels of heterogeneity.
Assessment of reporting biases
We investigated reporting biases (such as publication bias) by producing funnel plots. We assessed funnel plot asymmetry visually. In case of asymmetry suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2014). For continuous data, we used the mean difference (MD) and its 95% confidence interval (CI) if outcomes were measured in the same way between trials. We used the standardised mean difference (SMD) to combine trials that measure the same outcome but using different methods. We compared categorical data using risk ratios (RRs) and their 95% CIs. We tested for statistical heterogeneity among trials using the I² statistic. We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and we judged the trials’ populations and methods to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary where we considered an average treatment effect across trials was clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful we did not combine trials.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses:
We analysed sex and age using recommended nutrient intake age groups (1 to less than 4 years; 4 to less than 6 years; 6 to less than 10 years; 10 to less than 19 years; 19 to less than 50 years; 50 and over), for men and women.
Ethnicity
Duration of calcium intervention
Dose received
Intake of other minerals: where possible we analysed groups according to intakes of minerals involved in blood pressure regulation such as sodium, magnesium, potassium
Fat intake
Baseline calcium intake: we divided population groups into low or adequate calcium intake, according to WHO Food and Agriculture Organization (FAO) recommendations by age group
Baseline blood pressure: high blood pressure as defined by trial authors. Ideally, high blood pressure would be defined as diastolic blood pressure ≥ 90 mmHg (or systolic blood pressure ≥ 140 mmHg).
Sensitivity analysis
We planned sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor‐quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result. We tested the robustness of the results using several sensitivity analyses, including:
| 1. | Trials that are industry‐sponsored versus non‐industry sponsored |
| 2. | Trials with blood pressure data measured in the sitting position versus other measurement positions |
| 3. | Trials with reported standard deviations of blood pressure change versus imputed standard deviations |
| 4. | Risk of bias items |
In order to explore the robustness of the results, we performed four post hoc sensitivity analyses. The first sensitivity analysis was by mean difference and standardised mean difference in those cases when the result combined final blood pressure values and blood pressure change from baseline. We decided to present the results as mean differences, as they are easier to interpret; however in order to be more accurate we compared the mean difference results with the standardised mean differences. We based the other analyses on duration of intervention, on blood pressure methodology (auscultatory and oscillometric method) and on clinic blood pressure measurements and automated ambulatory blood pressure.
Results
Description of studies
Results of the search
We retrieved 1627 references by the electronic searches and considered 88 as potentially eligible after screening. See Figure 1.
Figure 1.
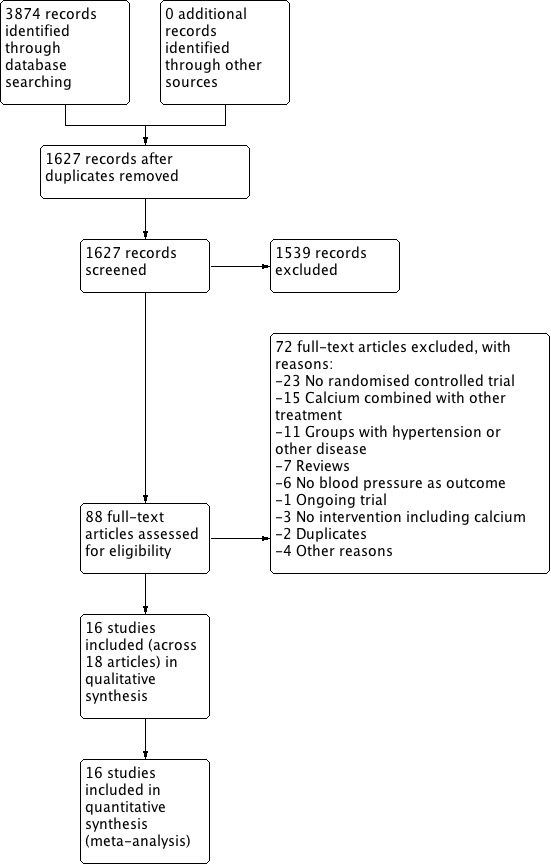
Study flow diagram.
Included studies
We included 16 randomised trials coming from 18 articles (there were two secondary references for Lyle 1987 and Lijnen 1995). See Characteristics of included studies.
Participants
Most of the studies were performed in adults; four studies were performed in older men and women (Reid 2005; Reid 2010; Thomsen 1987; Van Beresteyn 1986), one study in teenagers (Davis 1996) and one in 11‐year‐old children (Gillman 1995).
We found 11 studies (Belizan 1983; Cutler 1992; Gillman 1995; Hilary Green 2000; Johnson 1985; Lyle 1992; Reid 2005; Reid 2010; Sacks 1998; Shidfar 2010; Van Beresteyn 1986) reporting baseline mean calcium intake with values ranging from around 400 mg to 1120 mg a day in adult groups. Using this range, we organised the studies into three categories: less than 600 mg a day, 600 to less than 800 mg a day, and 800 mg a day or more, as none of the studies in adult populations had intakes above the FAO Recommended Dietary Intake of 1000 mg per day for people between 19 and 50 years of age.
We found five studies that only included women (Johnson 1985; Reid 2005; Sacks 1998; Thomsen 1987; Van Beresteyn 1986) and four studies that only included men (Lijnen 1995; Lyle 1987; Reid 2010; Shidfar 2010).
Sample sizes
For most studies the sample size was less than 100 participants; three studies had a sample size between 100 and 200 participants and the two largest studies were Cutler 1992 with 471 participants and Reid 2005 with 1471 participants.
Settings
Most studies were performed in higher‐income countries, with eight set in the USA (Cutler 1992; Davis 1996; Gillman 1995; Johnson 1985; Lyle 1987; Lyle 1992; McCarron 1985; Sacks 1998), three in New Zeland (Hilary Green 2000; Reid 2005; Reid 2010), three in Europe (Lijnen 1995 in Belgium; Thomsen 1987 in Denmark; Van Beresteyn 1986 in the Netherlands). Two studies were set in middle‐income countries: Belizan 1983 in Guatemala and Shidfar 2010 in Iran.
Interventions
The intervention consisted of a supplement tablet in 13 studies, while one study (Hilary Green 2000) evaluated the effect of two servings per day of high‐calcium skim milk versus ordinary skim milk (control), and two studies used a fortified juice (Gillman 1995; Van Beresteyn 1986).
For most studies the intervention was 1000 to 2000 mg of elemental calcium per day. Two studies had an intervention group with 600 mg of calcium a day (Gillman 1995; Reid 2010) and another study compared a high‐calcium skim milk containing 1075 mg to 720 mg of the non‐fortified skim milk (Hilary Green 2000).
Seven studies used calcium carbonate for the intervention (Cutler 1992; Johnson 1985; Lyle 1992; Lyle 1987; Shidfar 2010; Sacks 1998; Van Beresteyn 1986); three studies used calcium citrate (Gillman 1995; McCarron 1985; Reid 2005), one study used gluconate (Lijnen 1995) and two studies used a combination of calcium salts (Belizan 1983; Thomsen 1987). Three did not report the salt used (Davis 1996; Hilary Green 2000; Reid 2010).
We did not specify a minimum intervention time in order to include studies. However the included studies had a median follow‐up intervention period of 3.5 months. After initiation of calcium supplementation, blood pressure seemed to stabilise at between 1.5 and 2.5 months (Belizan 1983). Four studies had interventions that lasted a year or more: Thomsen 1987 one year, Reid 2010 two years, Reid 2005 two‐and‐a‐half years and Johnson 1985 four years.
Excluded studies
We excluded four studies for not having a randomised controlled trial (RCT) design, three studies for not reporting the number of participants (Dwyer 1998; Morris 1988; Smith 1987), two studies had a co‐intervention that could affect the blood pressure result (Eftekhari 2009; Shalileh 2010), two studies included hypertensive people (Pan 1993; Bostick 2000) and in two studies the outcome was not change in blood pressure (Karanja 1987; Pan 2000). See Characteristics of excluded studies.
Risk of bias in included studies
See Figure 2; Figure 3. Some information to assess risk of bias was not available for 10 published papers. We found contact details for eight of those studies and obtained the required information from five (Cutler 1992; Gillman 1995; Lyle 1987; Lyle 1992; Sacks 1998).
Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Allocation concealment risk of bias was low for seven of the 16 included studies, and unclear or not described or the remaining nine studies. For the seven studies classified as low risk, allocation was made by a centralised unit or packets were of identical appearance and were numbered at randomisation.
Blinding
Blinding bias was low for 11 of the 16 studies and unclear or not described for the remaining five studies. For the 11 studies classified as low risk of performance bias, blinding of participants and personnel was ensured by a double‐blind design and identical appearance of the food or supplement provided.
Studies at low risk of detection bias used a random‐baseline sphygmomanometer, a blood pressure machine that automatically entered the blood pressure data on computer tape, an ambulatory blood pressure monitor, or trained personnel who were blinded to the allocation groups.
Incomplete outcome data
Attrition bias was low for 10 of the 16 studies, while we classified two studies at high risk (Belizan 1983; Johnson 1985), as they had more than 10% dropouts. For the remaining four studies the information was unclear or not described.
Selective reporting
We classified all studies at low risk of reporting bias, as all primary outcomes were addressed or there was no evidence of selective reporting bias.
Other potential sources of bias
We detected no other bias for 12 of the 16 studies. Davis 1996 did not present baseline characteristics of the population so we rated it as at unclear risk. We rated three studies at high risk of bias: baseline characteristics of intervention and placebo groups presented small differences (in different directions) in Hilary Green 2000; in Lyle 1992 the treatment group presented at baseline more men than the placebo group, although blood pressure values showed no difference; and in Thomsen 1987 placebo participants had higher initial weight and lower systolic blood pressure.
Effects of interventions
See: Table 1
Primary outcomes
Hypertension defined as blood pressure ≥ 140/90 mmHg. None of the studies reported hypertension as a dichotomous outcome.
Systolic and diastolic blood pressure
Effect considering all the studies reporting change or final value of blood pressure
There was a reduction in blood pressure with calcium supplementation/fortification compared with control. The overall effect on systolic blood pressure was a mean difference (MD) of ‐1.43 mmHg (95% confidence interval (CI) ‐2.15 to ‐0.72) reported in 16 trials (N = 3048) with low heterogeneity (P = 0.68; I² = 0%) (Analysis 1.1) and the effect on diastolic blood pressure was ‐0.98 mmHg (95% CI ‐1.46 to ‐0.50) in 15 trials (N = 2947) with moderate heterogeneity (P = 0.01; I² = 49%) (Analysis 1.2).
Analysis 1.1.
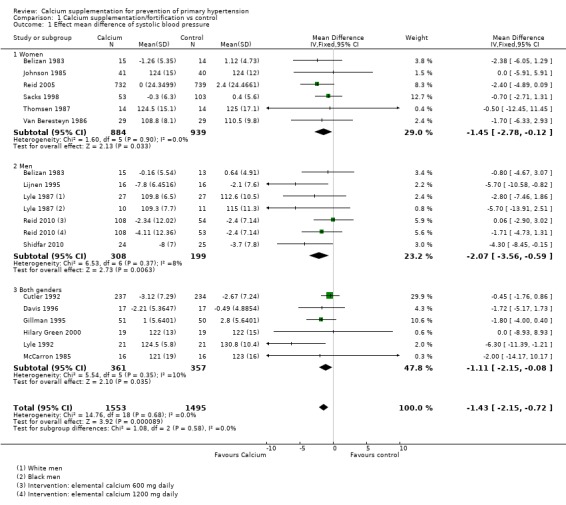
Comparison 1 Calcium supplementation/fortification vs control, Outcome 1 Effect mean difference of systolic blood pressure.
Analysis 1.2.
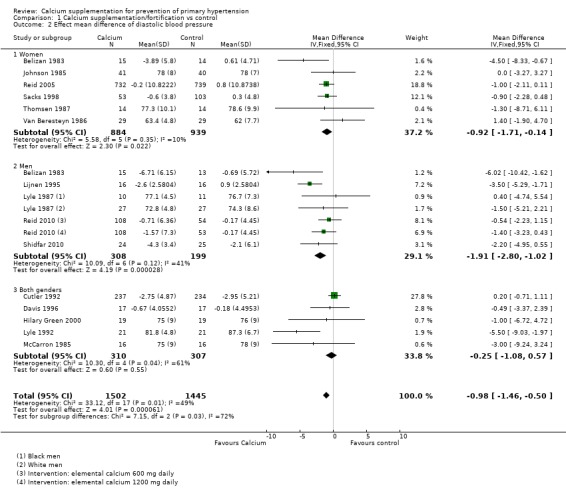
Comparison 1 Calcium supplementation/fortification vs control, Outcome 2 Effect mean difference of diastolic blood pressure.
Effect considering only the studies reporting change in blood pressure
The estimated effect on change in systolic pressure was ‐1.28 mmHg (95% CI ‐2.04 to ‐0.52), reported in nine trial subgroups (N = 2694) (Analysis 1.3). The estimated effect on change in diastolic pressure was ‐0.96 mmHg (95% CI ‐1.47 to ‐0.45) reported in eight trials (N = 2593) (Analysis 1.4). Heterogeneity was low for systolic blood pressure (P = 0.50; I² = 0%) and high for diastolic (P = 0.005; I² = 62%).
Analysis 1.3.
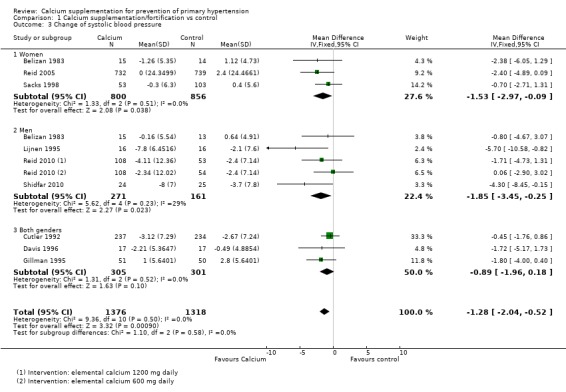
Comparison 1 Calcium supplementation/fortification vs control, Outcome 3 Change of systolic blood pressure.
Analysis 1.4.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 4 Change of diastolic blood pressure.
Effect considering only the studies reporting final values of blood pressure
The estimated effect on final systolic blood pressure was ‐2.19 mmHg (95% CI ‐3.84 to ‐0.54), reported in 10 trials (N = 538) (Analysis 1.5) and on diastolic blood pressure ‐ 1.22 mmHg (95% CI ‐2.52 to ‐0.08), reported in nine trials (N = 437) (Analysis 1.6). Heterogeneity was low for both systolic (P = 0.27; I² = 18%) and diastolic blood pressure (P = 0.22; I² = 24%).
Analysis 1.5.
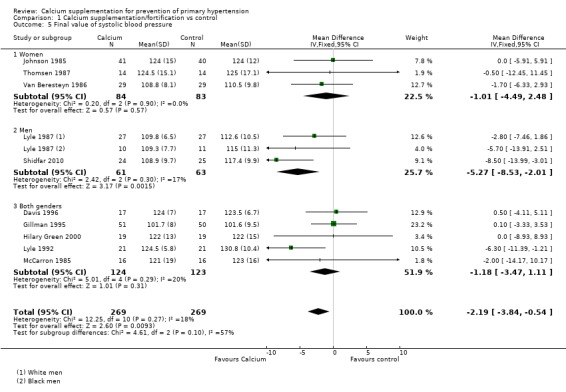
Comparison 1 Calcium supplementation/fortification vs control, Outcome 5 Final value of systolic blood pressure.
Analysis 1.6.
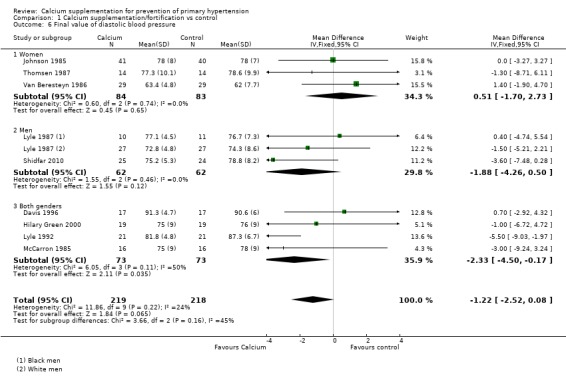
Comparison 1 Calcium supplementation/fortification vs control, Outcome 6 Final value of diastolic blood pressure.
Subgroup analyses
We report tests for subgroup differences only when P values were less than 0.1.
Analysis by sex
Of the 16 studies included, 10 studies (Belizan 1983; Johnson 1985; Lijnen 1995; Lyle 1987; Reid 2005; Reid 2010; Sacks 1998; Shidfar 2010; Thomsen 1987; Van Beresteyn 1986) presented the results by sex.
Effect considering all the studies reporting change or final value of blood pressure
The overall effect on systolic blood pressure was ‐1.45 mmHg (95% CI ‐2.78 to ‐0.12) for women, six studies (N = 1823) with low heterogeneity (P = 0.90; I² = 0%) and ‐2.07 mmHg (95% CI ‐3.56 to ‐0.59) for men, five studies (N = 507) with low heterogeneity (P = 0.37; I² = 8%) (Analysis 1.1). The effect on diastolic blood pressure was ‐0.92 mmHg (95% CI ‐1.71 to ‐0.14) for women, six studies (N = 1823) with low heterogeneity (P = 0.35; I² = 10%) and ‐1.91 mmHg (95% CI ‐2.80 to ‐1.02) in men, five studies (N = 617) with moderate heterogeneity (P = 0.12; I² = 41%) (Analysis 1.2) (Test for subgroup differences: Chi² = 7.15, df = 2 (P = 0.03), I² = 72.0%).
Effect considering only the studies reporting change in blood pressure
For those studies showing change in systolic blood pressure the effect was ‐1.53 mmHg (95% CI ‐2.97 to ‐0.09) for women, three studies (N = 1656) with low heterogeneity (P = 0.51; I² = 0%) and ‐1.85 mmHg (95% CI ‐3.45 to ‐0.25) for men, four studies (N = 432) with low heterogeneity (P = 0.23; I² = 29%) (Analysis 1.3). The effect on diastolic was ‐1.13 mmHg (95% CI ‐1.98 to ‐0.29) for women, three studies (N = 1656) with moderate heterogeneity (P = 0.21; I² = 36%) and ‐2.01 mmHg (95% CI ‐2.94 to ‐1.08) for men, four studies (N = 432) with high heterogeneity (P = 0.06; I² = 57%) (Analysis 1.4).
Effect considering only the studies reporting final values of blood pressure
In those studies reporting final values the effect on systolic blood pressure was ‐1.01 mmHg (95% CI ‐4.49 to 2.48) for women, three studies (N = 167) with low heterogeneity (P = 0.90; I² = 0%) and ‐5.27 mmHg (95% CI ‐8.53 to ‐2.01) for men, two studies (N = 124) with low heterogeneity (P = 0.30; I² = 17%) (Analysis 1.5). On diastolic blood pressure the effect was 0.51 mmHg (95% CI ‐1.70 to 2.73) in women, three studies (N = 167) with low heterogeneity (P = 0.74; I² = 0%) and ‐1.88 mmHg (95% CI ‐4.26 to 0.50) in men, two studies (N = 124) with low heterogeneity (P = 0.46; I² = 0%) (Analysis 1.6).
Analysis by age
Although all studies reported the age groups of the population, most of them did not present their results by age group, so it was not possible to do the analysis using the groups originally planned. We divided studies into those that presented a mean age of less than 35 years and those with a mean age of 35 or more.
Effect considering all the studies reporting change or final value of blood pressure
The overall effect on systolic blood pressure was ‐2.11 mmHg (95% CI ‐3.58 to ‐0.64) for those younger than 35 years, seven studies (N = 399) with moderate heterogeneity (P = 0.36; I² = 9%) and ‐0.96 mmHg (95% CI ‐1.83 to ‐0.09) for those aged 35 years or more, nine studies (N = 2649) with low heterogeneity (P = 0.81; I² = 0%) (Analysis 1.7). The overall effect on diastolic blood pressure was ‐2.61 mmHg (95% CI ‐3.74 to ‐1.49) for those younger than 35 years, six studies (N = 298) with high heterogeneity (P = 0.02; I² = 60%) and ‐0.59 mmHg (95% CI ‐1.13 to ‐0.06) for those aged 35 years or more, nine studies (N = 2649) with low heterogeneity (P = 0.70; I² = 0%) (Analysis 1.8) (Test for subgroup differences: Chi² = 10.09, df = 1; P = 0.001, I² = 90.1%).
Analysis 1.7.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 7 Effect mean difference of systolic blood pressure by age.
Analysis 1.8.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 8 Effect mean difference of diastolic blood pressure by age.
Effect considering only the studies reporting change in blood pressure
For those studies showing change in systolic blood pressure the effect was ‐2.56 mmHg (95% CI ‐4.90 to ‐0.23) for those younger than 35 years, two studies (N = 89) with low heterogeneity (P = 0.30; I² = 16%) and ‐0.98 mmHg (95% CI ‐1.87 to ‐0.10) for those aged 35 years or more, five studies (N = 2470) with low heterogeneity (P = 0.40; I² = 2%) (Analysis 1.9). The effect on diastolic blood pressure was ‐3.96 mmHg (95% CI ‐5.48 to ‐2.44) for those younger than 35 years, two studies (N = 89) with low heterogeneity (P = 0.56; I² = 0%) and ‐0.58 mmHg (95% CI ‐1.13 to ‐0.04) for those aged 35 years or more, five studies (N = 2470) with low heterogeneity (P = 0.34; I² = 12%) (Analysis 1.10).
Analysis 1.9.
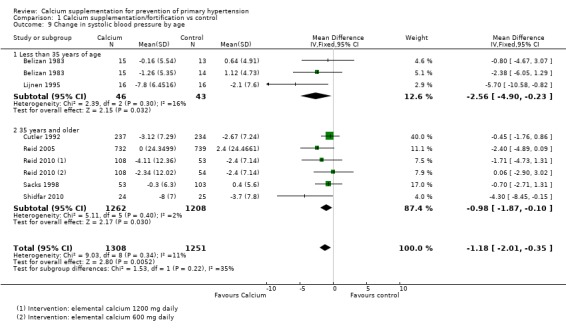
Comparison 1 Calcium supplementation/fortification vs control, Outcome 9 Change in systolic blood pressure by age.
Analysis 1.10.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 10 Change in diastolic blood pressure by age.
Effect considering all the studies reporting change or final value of blood pressure
In those studies reporting final values the effect for systolic blood pressure was ‐1.81 mmHg (95% CI ‐3.71 to 0.09) for those younger than 35 years, five studies (N = 310) with low heterogeneity (P = 0.29; I² = 19%) and ‐3.39 mmHg (95% CI ‐6.76 to ‐0.03) for those aged 35 years or more, five studies (N = 228) with low heterogeneity (P = 0.25; I² = 26%) (Analysis 1.11); diastolic blood pressure was ‐0.99 mmHg (95% CI ‐2.66 to 0.68) in those younger than 35 years, four studies (N = 209) with high heterogeneity (P = 0.05; I² = 58%) and ‐1.58 mmHg (95% CI ‐3.65 to 0.49) in those aged 35 years or more, five studies (N = 228) with low heterogeneity (P = 0.70; I² = 0%) (Analysis 1.12).
Analysis 1.11.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 11 Final value in systolic blood pressure by age.
Analysis 1.12.
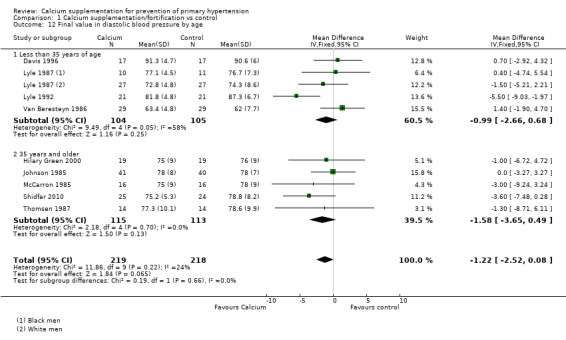
Comparison 1 Calcium supplementation/fortification vs control, Outcome 12 Final value in diastolic blood pressure by age.
Analysis by basal calcium intake
Of the 16 studies included, 11 studies presented the results by basal calcium intake. See Description of studies. However one study (Gillman 1995) was carried out in children, so we excluded it from the analysis as the nutrient recommendations for children are different, and another study (Lyle 1992) gave a range of intakes and could not be classified for this analysis.
Effect considering all the studies reporting change or final value of blood pressure
The effect on systolic blood pressure was ‐1.70 mmHg (95% CI ‐6.33 to 2.33) for those that were consuming on average less than 600 mg, one study (N = 58); ‐0.87 mmHg (‐0.1.88 to 0.13) for those that consumed between 600 and 800 mg of calcium per day, five studies (N = 786) without heterogeneity (P = 0.44; I² = 0%); and ‐01.34 mmHg (95% CI ‐2..80 to 0.13) for those consuming more than 800 mg of calcium per day, four studies (N = 1860) (Analysis 1.13). The overall effect on diastolic blood pressure was 1.40 mmHg (95% CI ‐1.90 to 4.70) for those that were consuming on average less than 600 mg of calcium per day, one study (N = 58); ‐0.41 mmHg (95% CI ‐1.11 to 0.29) for those that consumed between 600 and 800 mg of calcium per day, five studies (N = 786) with high heterogeneity (P = 0.08; I² = 52%); and ‐1.14 mmHg (95% CI ‐1.96 to ‐0.33) for those consuming more than 800 mg of calcium per day, three studies (N = 1822) with low heterogeneity (P = 0.25; I² = 25%) (Analysis 1.14).
Analysis 1.13.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 13 Effect mean difference in systolic blood pressure by basal calcium intake.
Analysis 1.14.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 14 Effect mean difference in diastolic blood pressure by basal calcium intake.
Effect considering only the studies reporting change in blood pressure
None of the studies showing basal calcium intake and reporting change in blood pressure had a group with calcium intake less than 600 mg/day. For those studies showing change in systolic blood pressure the effect was ‐0.90 mmHg (95% CI ‐1.92 to 0.12) for those who consumed between 600 and 800 mg of calcium per day, four studies (N = 705) with low heterogeneity (P = 0.30; I² = 19%) and ‐1.37 mmHg (95% CI ‐2.86 to 0.12) for those consuming more than 800 mg of calcium per day, three studies (N = 1822) with low heterogeneity (P = 0.64; I² = 0%) (Analysis 1.15). The effect on diastolic blood pressure was ‐0.43 mmHg (95% CI ‐1.15 to 0.29) for those who consumed between 600 and 800 mg of calcium per day, four studies (N = 705) with high heterogeneity (P = 0.04; I² = 63%) and ‐1.14 mmHg (95% CI ‐1.96 to ‐0.33) for those consuming more than 800 mg of calcium per day, three studies (N = 1822) with moderate heterogeneity (P = 0.15; I² = 44%) (Analysis 1.16).
Analysis 1.15.
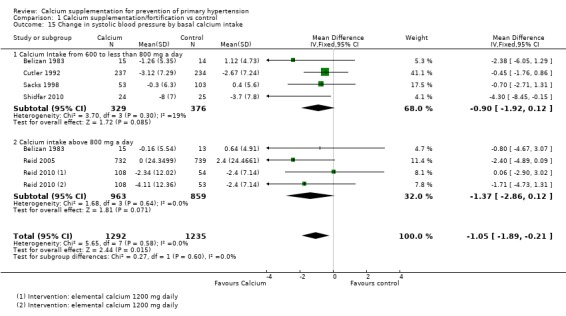
Comparison 1 Calcium supplementation/fortification vs control, Outcome 15 Change in systolic blood pressure by basal calcium intake.
Analysis 1.16.
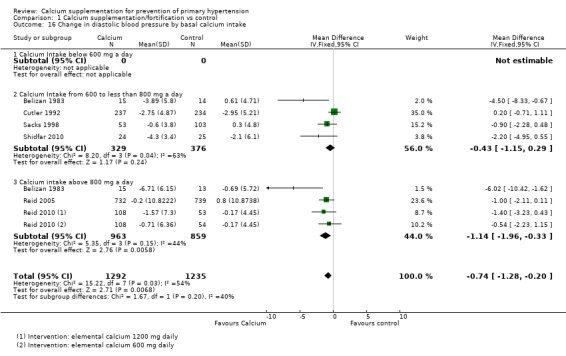
Comparison 1 Calcium supplementation/fortification vs control, Outcome 16 Change in diastolic blood pressure by basal calcium intake.
Effect considering all the studies reporting final value of blood pressure
In those studies reporting final values the effect on systolic blood pressure was ‐1.70 mmHg (95% CI ‐6.33 to 2.93) for those consuming less than 600 mg a day, one study (N = 58); ‐4.56 mmHg (95% CI ‐8.58 to ‐0.54) for those who consumed between 600 and 800 mg of calcium per day, two studies (N = 130) with high heterogeneity (P = 0.04; I² = 77%) and 0.00 mmHg (95% CI ‐8.93 to 8.93) for those consuming more than 800 mg of calcium per day, one study (N = 38) (Analysis 1.17). The effect on diastolic blood pressure was 1.40 mmHg (95% CI ‐1.90 to 4.70) for those consuming less than 600 mg a day, one study (N = 58); ‐1.49 mmHg (95% CI ‐4.00 to 1.01) for those who consumed between 600 and 800 mg of calcium per day, two studies (N = 130) with moderate heterogeneity (P = 0.16; I² = 48%) and ‐1.00 mmHg (95% CI ‐6.72 to 4.72) for those consuming more than 800 mg of calcium per day, one study (N = 38) (Analysis 1.18).
Analysis 1.17.
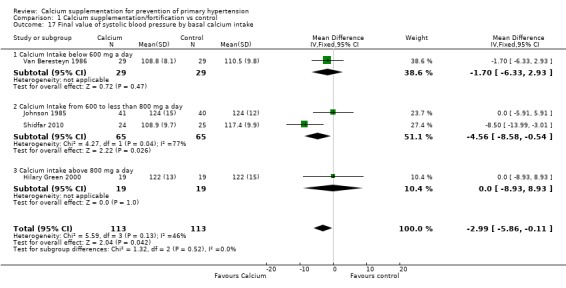
Comparison 1 Calcium supplementation/fortification vs control, Outcome 17 Final value of systolic blood pressure by basal calcium intake.
Analysis 1.18.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 18 Final value of diastolic blood pressure by basal calcium intake.
Analysis by dose
Effect considering all the studies reporting change or final value of blood pressure
The overall effect on systolic blood pressure was 0.08 mmHg (95% CI ‐2.16 to 2.32) for the group with doses less than 1000 mg, two studies (N = 263) with low heterogeneity (P = 0.99; I² = 0%), ‐1.14 mmHg (95% CI ‐2.01 to ‐0.27) with doses between 1000 and 1500 mg, eight studies (N = 2435) with low heterogeneity (P = 0.74; I² = 0%) and ‐2.79 mmHg (95% CI ‐4.71 to ‐0.86) with doses more than 1500 mg, seven studies (N = 350) with low heterogeneity (P = 0.45; I² = 0%) (Analysis 1.19).
Analysis 1.19.
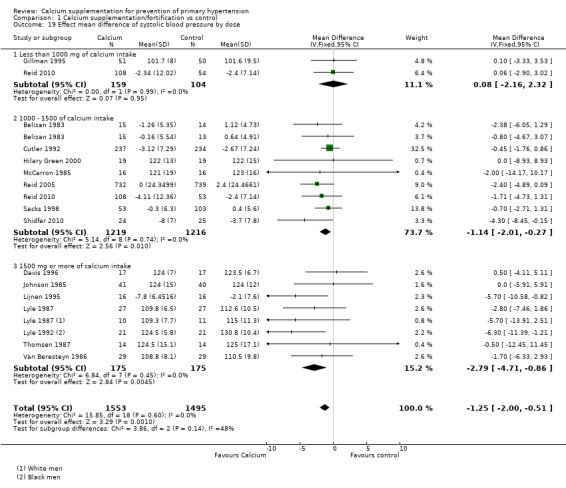
Comparison 1 Calcium supplementation/fortification vs control, Outcome 19 Effect mean difference of systolic blood pressure by dose.
The overall effect on diastolic blood pressure was ‐0.54 mmHg (95% CI ‐2.23 to 1.15) for the group with doses less than 1000 mg, one study (N = 162), ‐0.71 mmHg (95% CI ‐1.37 to ‐0.06) with doses between 1000 and 1500 mg, seven studies (N = 964) with high heterogeneity (P = 0.03; I² = 55%) and ‐1.43 mmHg (95% CI ‐2.22 to ‐0.64) with doses more than 1500 mg, eight studies (N = 1821) with high heterogeneity (P = 0.04; I² = 51%) (Analysis 1.20).
Analysis 1.20.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 20 Effect mean difference of diastolic blood pressure by dose.
Effect considering all the studies reporting change or final value of blood pressure
For those studies showing change in systolic blood pressure the effect was 0.06 (95%CI ‐2.90 to 3.02) with less than 1000 mg of calcium intake one study (N = 162), ‐1.15 (95%CI ‐2.02 to ‐0.27) with 1000 ‐ 1500 of calcium intake, six studies (N = 2365) and ‐5.70 (95%CI ‐10.58 to ‐0.82) with 1500 mg or more of calcium intake, one study (N= 32) (Analysis 1.22)
Analysis 1.22.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 22 Change in systolic blood pressure by dose.
For those studies showing change in diastolic blood pressure the effect was ‐0.54 (95%CI ‐2.23 to 1.15) with less than 1000 mg of calcium intake one study (N = 162), ‐0.68 (95%CI ‐1.35 to ‐0.02) with 1000 ‐ 1500 of calcium intake, five studies (N = 894) and ‐1.69 (95%CI ‐2.64 to ‐0.75) with 1500 mg or more of calcium intake, two studies (N= 1503) (Analysis 1.21)
Analysis 1.21.
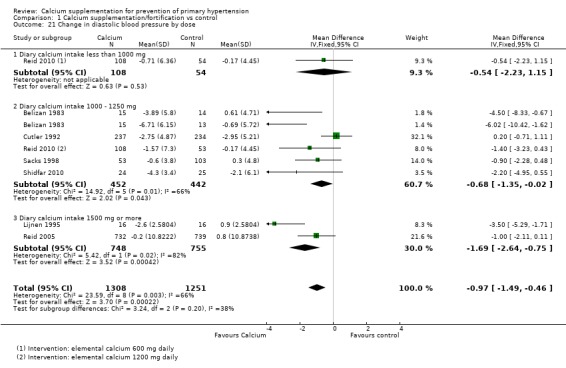
Comparison 1 Calcium supplementation/fortification vs control, Outcome 21 Change in diastolic blood pressure by dose.
Effect considering all the studies reporting final value of blood pressure
For those studies reporting final values in systolic blood pressure the effect was 0.10 (95%CI ‐3.33 to 3.53) with less than 1000 mg of calcium intake one study (N = 101),‐0.70 (95%CI ‐7.90 to 6.50) with 1000 ‐ 1500 of calcium intake, two studies (N = 70) and ‐2.25 (95%CI ‐4.34 to ‐0.16) with 1500 mg or more of calcium intake, six studies (N= 318) (Analysis 1.23)
Analysis 1.23.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 23 Final value in systolic blood pressure by dose.
For those studies reporting final values in diastolic blood pressure the effect was ‐1.91 (95%CI ‐6.13 to 2.30) with 1000 ‐ 1250 of calcium intake, two studies (N = 70) and ‐0.80 (95%CI ‐2.26 to 0.65) with 1500 mg or more of calcium intake, six studies (N= 318) (Analysis 1.24).
Analysis 1.24.
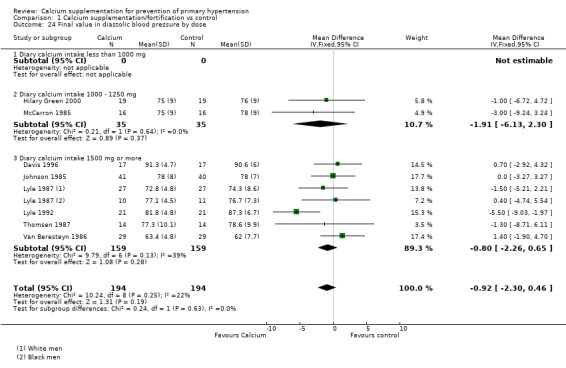
Comparison 1 Calcium supplementation/fortification vs control, Outcome 24 Final value in diastolic blood pressure by dose.
Analysis by intervention duration
The overall effect on systolic blood pressure was ‐1.79 mmHg (95% CI ‐2.92 to ‐0.67) where the intervention lasted less than six months, 11 studies (N = 674) with low heterogeneity (P = 0.47; I² = 0%) and ‐0.83 mmHg (95% CI ‐1.83 to 0.17) where the intervention lasted six months or more, five studies (N = 2374) with low heterogeneity (P = 0.76; I² = 0%) (Analysis 1.25). The overall effect on diastolic blood pressure was ‐1.95 mmHg (95% CI ‐2.77 to ‐1.14) where the intervention lasted less than six months, 10 studies (N = 573) with moderate heterogeneity (P = 0.03; I² = 48%) and ‐0.43 mmHg (95% CI ‐1.03 to 0.17) where the intervention lasted six months or more, five studies (N = 2374) with low heterogeneity (P = 0.54; I² = 0%) (Analysis 1.26) (Test for subgroup differences: Chi² = 8.65, df = 1, P = 0.003, I² = 88.4%).
Analysis 1.25.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 25 Effect mean difference of systolic blood pressure by duration.
Analysis 1.26.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 26 Effect mean difference of diastolic blood pressure by duration.
Analysis by intervention type (fortification and supplementation)
The overall effect on systolic blood pressure was ‐1.33 mmHg (95% CI ‐2.10 to ‐0.56) where the intervention was food supplementation, 14 studies (N = 2909) with low heterogeneity (P = 0.51; I² = 0%) and 0.09 mmHg (95% CI ‐3.11 to 3.29) where the intervention was food fortification, two studies (N = 139) with low heterogeneity (P = 0.98; I² = 0%) (Analysis 1.27). The overall effect on diastolic blood pressure was ‐0.97 mmHg (95% CI ‐1.45 to, ‐0.48) where the intervention was food supplementation, 14 studies (N = 2909) with high heterogeneity (P = 0.006; I² = 53%) and ‐1.00 mmHg (95% CI ‐6.72 to 4.72) where the intervention was food fortification, one study (N = 38) (Analysis 1.28).
Analysis 1.27.
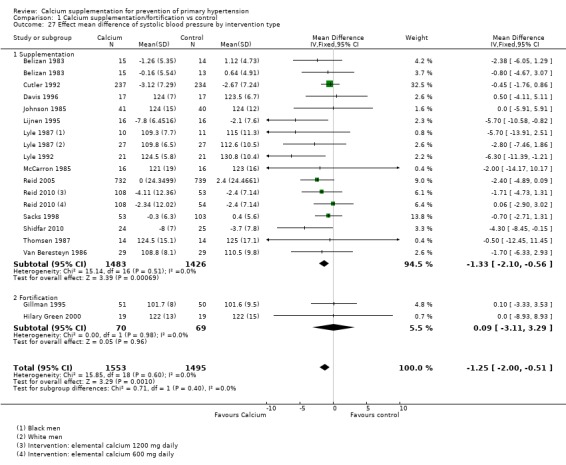
Comparison 1 Calcium supplementation/fortification vs control, Outcome 27 Effect mean difference of systolic blood pressure by intervention type.
Analysis 1.28.

Comparison 1 Calcium supplementation/fortification vs control, Outcome 28 Effect mean difference of diastolic blood pressure by intervention type.
Analysis by ethnicity, fat intake, other minerals
It was not possible to do this analysis presented in the protocol, as the information was not available.
Planned sensitivity analysis results:
1‐ Sensitivity analysis of risk of bias
Figure 2 shows risks of bias classification of studies.
Mean effect on systolic blood pressure in 16 studies (N = 3048) (mean difference in all cases) was ‐1.43 mmHg (‐2.15 to ‐0.72). When we restricted the analyses to only those studies with low risk of bias the results still showed a significant effect:
1‐ Random sequence: ‐1.26 mmHg (‐2.04 to ‐0.49) in 10 studies (N = 1730)
2‐ Allocation concealment: ‐1.20 mmHg (‐2.09 to ‐0.31) in 7 studies (N = 1193)
3‐ Blinding of participants: ‐1.35 mmHg (‐2.11 to ‐0.59) in 12 studies (N = 2788)
4‐ Blinding of outcome assessment: ‐1.34 mmHg (‐2.10 to ‐0.58) in 11 studies (N = 2800)
5‐ Incomplete outcome data: ‐1.46 mmHg (‐2.35 to ‐0.57) in 10 studies (N =1211)
Mean effect on diastolic blood pressure in 15 studies (N = 2947) was ‐0.98 mmHg (‐1.46 to ‐0.50). When we restricted the analyses to only those studies with low risk of bias the results still showed a significant effect:
1‐ Random sequence: ‐0.87 mmHg 9‐1.40 to ‐0.34) in 9 studies (N =1629)
2‐ Allocation concealment: ‐0.73 mmHg (‐1.40 to ‐0.05) in 6 studies (N =1092)
3‐ Blinding of participants: ‐1.04 mmHg (‐1.55 to ‐0.53) in 11 studies (N = 2687)
4‐ Blinding of outcome assessment: ‐0.81 mmHg (‐1.33 to ‐0.29) in 10 studies (N = 2799)
5‐ Incomplete outcome data: ‐0.87 mmHg (‐1.52 to ‐0.22) in 9 studies (N =1110)
2‐ Sensitivy analysis for industry‐funded studies
We performed a sensitivity analysis excluding five studies that we believed to be industry‐funded (Gillman 1995; Hilary Green 2000; Johnson 1985; Lijnen 1995; Reid 2010).
Mean difference of the effect on systolic blood pressure excluding industry‐funded studies was ‐1.40 mmHg (95% CI ‐2.23 to ‐0.56) 11 studies (N = 1473), whereas for the industry‐funded studies the mean difference was ‐1.54 mmHg (95% CI ‐2.94 to ‐0.15) 5 studies (N=1575).
Mean difference of the effect on diastolic blood pressure excluding industry‐funded studies was ‐0.78 mmHg (95% CI ‐1.33 to ‐0.22) 11 studies (N = 1473), whereas for the industry‐funded studies the mean difference was ‐1.59 mmHg (95% CI ‐2.55 to ‐0.64) 4 studies (N=1474).
3‐ Sensitivity analysis by position of the participant during blood pressure measurement
| Systolic blood pressure | |
| Sitting position (Belizan 1983; Gillman 1995; Johnson 1985) | ‐1.60 mmHg (95% CI ‐3.23 to 0.03), 3 studies (N=299) |
| Standing (Lijnen 1995) | ‐5.70 mmHg (95% CI ‐10.58 to ‐0.82), 1 study (N=32) |
| Supine (McCarron 1985; Thomsen 1987) | ‐1.24 mmHg (95% CI ‐9.76 to 7.29), 2 studies (N=60) |
| Diastolic blood pressure | |
| Sitting (Belizan 1983; Johnson 1985) | ‐2.90 mmHg (95% CI ‐5.06 to ‐0.73), 2 studies (N=138) |
| Standing (Lijnen 1995) | ‐3.50 mmHg (95% CI ‐5.29 to ‐1.71), 1 study (N=32) |
| Supine (McCarron 1985; Thomsen 1987) | ‐2.29 mmHg (95% CI ‐7.07 to 2.48), 2 studies (N=60) |
4‐ Sensitivity analysis for trials with imputed standard deviations
We did not impute any standard deviation in the data used from these 16 trials.
Post hoc sensitivity analyses
Sensitivity analysis comparing mean difference and standardised mean difference results
We did a sensitivity analysis comparing mean difference (MD) and standardised mean difference (SMD) results for all 24 outcomes reported in data analysis. Even though the mean difference results were in the same direction, of the 24 analyses performed, seven presented confidence intervals with different statistical significance between MD and SMD results, suggesting that we should be more cautious in interpreting these results. The following list shows cases where the confidence interval crosses the line of no effect on one measurement method but not on the other:
The mean difference effect on change of systolic blood pressure for men was ‐1.85 mmHg (95% CI ‐3.45 to ‐0.25), whereas the standardised mean difference was ‐0.19 mmHg (95% CI ‐0.39 to 0.01) Analysis 1.3.
The mean difference effect on the final value of diastolic blood pressure for both genders was ‐2.33 mmHg (95% CI ‐4.50 to ‐0.17), whereas the standardised mean difference was ‐0.32 mmHg (95% CI ‐0.65 to 0.01) Analysis 1.6.
The mean difference effect on systolic blood pressure in the group with intakes higher than 800 mg a day was ‐1.34 mmHg (95% CI ‐2.80 to 0.13), whereas the standardised mean difference was ‐0.09 mmHg (95% CI ‐0.19 to ‐0.00) Analysis 1.13.
The mean difference effect on change of systolic blood pressure in the group with intakes higher than 800 mg a day was ‐1.37 mmHg (95% CI ‐2.86 to 0.12), whereas the standardised mean difference was ‐0.10 mmHg (95% CI ‐0.19 to ‐0.00) Analysis 1.15.
The mean difference effect on the final value of systolic blood pressure in the group with intakes between 600 and 800 mg a day was ‐4.56 mmHg (95% CI ‐8.58 to ‐0.54), whereas the standardised mean difference was ‐0.30 mmHg (95% CI ‐0.65 to 0.05) Analysis 1.17.
The overall mean difference effect on the final value of systolic blood pressure by basal calcium intake was ‐2.99 mmHg (95% CI ‐5.86 to ‐0.11), whereas the standardised mean difference was ‐0.22 mmHg (95% CI ‐0.48 to 0.04) Analysis 1.17.
The mean difference effect on systolic blood pressure for those with interventions longer than six months was ‐0.83 mmHg (95% CI ‐1.83 to 0.17), whereas the standardised mean difference was ‐0.08 mmHg (95% CI ‐0.16 to ‐0.00) Analysis 1.25.
When we analysed the results in units of standard deviation (SMDs), each study weight was modified; if the weight increased in those studies showing more effect, the final result using this method showed a higher effect. Correspondingly, when the weights were increased in the studies with no effect, the final result tended to show a weaker global effect.
Sensitivity analysis excluding studies with less than 3.5 months of intervention
Of the 16 included studies, eight (Belizan 1983; Cutler 1992; Johnson 1985; Sacks 1998; Reid 2005; Reid 2010; Lijnen 1995; Thomsen 1987) presented interventions lasting more than3.5 months (N=2619).
The mean effect in systolic blood pressure was ‐1.43 mmHg (‐2.15 to ‐0.72) (Analysis 1.1). When we performed a sensitivity analysis only including the studies with interventions lasting more than 3.5 months the results were still significant: ‐1.03 mmHg (‐1.87 to ‐0.19).
The mean effect in diastolic blood pressure was ‐0.98 mmHg (‐1.46 to ‐0.50) (Analysis 1.2). When we performed a sensitivity analysis only including the studies with interventions lasting more than 3.5 months the results were still significant: ‐0.91 mmHg (95% CI ‐1.42 to ‐0.39).
Sensitivity analysis by blood pressure methodology
Blood pressure was measured using an auscultatory method in seven studies (Belizan 1983;Cutler 1992; Johnson 1985;Lyle 1992;Lyle 1987;McCarron 1985;Thomsen 1987; [N=786])., and using an oscillometric method in six studies (Gillman 1995 (only systolic blood pressure, [N=101]);Hilary Green 2000; Davis 1996;Reid 2005; Reid 2010;Sacks 1998 [N=2123]).
| Systolic blood pressure | |
| Auscultatory | ‐1.12 mmHg (95% CI ‐2.19 to ‐0.04) |
| Oscillometric | ‐1.34 mmHg (95% CI ‐2.38 to ‐0.31) |
| Diastolic blood pressure | |
| Auscultatory | ‐0.61 mmHg (95% CI ‐1.39 to 0.16) |
| Oscillometric | ‐0.88 mmHg (95% CI ‐1.75 to ‐0.01) |
Sensitivity analysis by studies reporting clinic blood pressure measurements and automated ambulatory blood pressure
Blood pressure was measured at a clinic in eight studies (Belizan 1983; Gillman 1995; Cutler 1992; Johnson 1985;Lyle 1992;Lyle 1987;McCarron 1985;Thomsen 1987; [N=887]) and using automated ambulatory measurements in three studies (Hilary Green 2000; Davis 1996; Sacks 1998; [N=228]).
We did not find any study using ambulatory measurements reported by the participant. Those studies reporting ambulatory measurement were conducted with automated devices.
| Systolic blood pleasure | |
| Clinic measurements | ‐1.25 mmHg (95% CI ‐2.22 to ‐0.28) |
| Automated ambulatory measurements | ‐0.92 mmHg (95% CI ‐2.63 to 0.78) |
| Diastolic blood pressure | |
| Clinic | ‐0.61 mmHg (95% CI ‐1.39 to 0.16) |
| Automated ambulatory measurements | ‐0.83 mmHg (95% CI ‐2.05 to 0.39) |
Assessment of potential reporting biases (such as publication bias)
Funnel plot visual analysis revealed no asymmetry (Figure 4; Figure 5)
Figure 4.
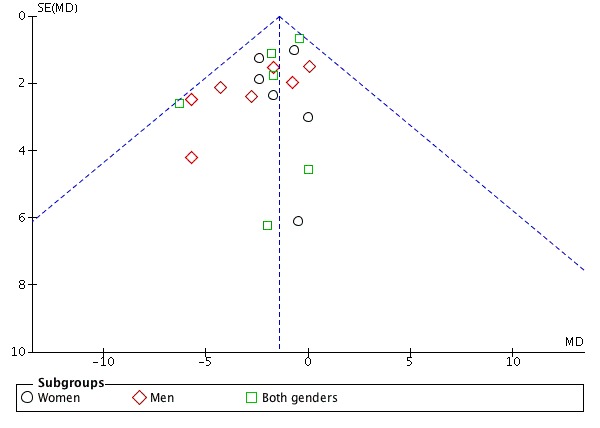
Funnel plot of comparison: 1 Calcium supplementation/fortification vs control, outcome: 1.1 Effect mean difference of systolic blood pressure.
Figure 5.
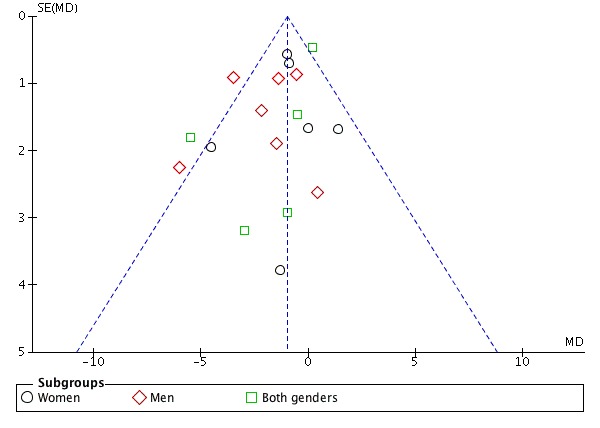
Funnel plot of comparison: 1 calcium supplementation/fortification vs control, Effect Mean difference of Diastolic blood pressure.
Secondary Outcomes
Cutler 1992 is the only article evaluating side effects, but reported none. A further two study reports (Lyle 1987; McCarron 1985) mentioned that the supplements were well tolerated and that no participants required withdrawal from the trial after randomisation.
No trials reported any incidence of kidney stone formation, iron deficiency anaemia, anaemia, cardiovascular events, myocardial infarction, stroke or mortality.
Discussion
The aim of this review was to evaluate the effectiveness of calcium supplementation, as a single nutrient, for the prevention of primary hypertension. We analysed the effect of calcium according to sex, intervention dose, intervention duration, age of participants and basal calcium intake.
Summary of main results
There was a reduction in both systolic and diastolic blood pressure in the groups receiving calcium compared to those receiving placebo or control. The effect of calcium on diastolic blood pressure was higher in men than in women. We found a lower effect in those studies that did not discriminate between the results by sex, and in at least one of those studies (Lyle 1992) a sex imbalance at randomisation was reported as a possible explanation.
The effect was confirmed in multiple prespecified subgroups. We detected a dose‐response effect trend, both in systolic and in diastolic blood pressure, that could reinforce the efficacy of the intervention. Those studies with interventions of 1500 mg of calcium a day or higher showed a higher decrease in systolic and diastolic blood pressure than those studies with interventions less than 1000 mg a day. For those studies with interventions of less than 1000 mg we found no effect, although in this last group there were very few studies from which to draw any conclusion.
When we evaluated the overall effect and change of blood pressure before and after the intervention with calcium, those studies performed in younger people tended to show higher reductions in systolic and diastolic blood pressure than those in older people.
There was no difference in the effect by baseline calcium intake, reported in nine of the 16 included studies. This can be due to different methods used in assessing calcium intake among the studies. The information provided in this review therefore does not contradict the possibility of a higher effect in populations with low calcium intake, as has been suggested before (Belizan 1980; Belizan 1983; WHO 2009). Only two of the selected studies were performed in low‐ or middle‐income countries.
It is difficult to assess the effect of differences in the forms of calcium interventions, such as diet, fortification or supplements, since 14 of the 16 included studies used supplementation as the intervention.
Our data show a greater effect in those studies lasting less than six months. There is some suggestion that the effect might be lost over time in populations with adequate calcium intake, as some studies showed no effect after 30 months (Reid 2005) and one year (Thomsen 1987).
Overall completeness and applicability of evidence
We found a substantial number of studies to address the objectives of the review, with no evidence of publication bias, although some population groups such as children and teenagers might not be well represented. Only one study was performed in children (Gillman 1995), and one in teenagers (Davis 1996).
The effect was higher in two studies from low‐ and middle‐income countries (Belizan 1983; Shidfar 2010) (MD ‐2.41 mmHG, 95% CI ‐4.65 to ‐0.17); however, we also found an effect on blood pressure reduction in high‐income countries, 14 studies (‐1.27 mmHg, 95% CI ‐2.02 to ‐0.53).
The effect on diastolic blood pressure was higher in men, in those younger than 35 years old and in those receiving intervention for less than six months (Test for subgroup differences: P values 0.03, 0.004 and 0.003 respectively).
The other subgroup analyses look underpowered and therefore need to be interpreted very cautiously. For example, we observed a trend to higher effect with increasing doses; however the test for subgroup differences P values was not statistically significant (0.14 and 0.34 for systolic and diastolic blood pressure respectively).
The findings of this review support the importance of an adequate calcium intake for the prevention of high blood pressure and the need to explore interventions to increase calcium intake in both men and women. For cardiovascular risk prevention, a small decrease in blood pressure outweighs a larger decrease only among hypertensive groups (Gillman 1995). Additionally, small reductions in blood pressure of general population are predicted to have important health implications, as they are shown to produce rapid reductions in vascular disease risk even in individuals with normal blood pressure ranges (Lewington 2002). Population‐wide decreases in blood pressure of 2 – 3 mmHg could decrease the prevalence of hypertension by 17%, the risk of coronary artery disease by 6% and the risk of stroke by 15% (Cook 1995). A 2 mmHg lower systolic blood pressure is predicted to produce about 10% lower stroke mortality and about 7% lower mortality from ischaemic heart disease, and a 5 mmHg reduction in systolic blood pressure at the population level is predicted to result in a 14% reduction in stroke death, 9% reduction in coronary artery disease‐related death and a 7% reduction in total mortality (Whelton 2002). In the same way a 2 mmHg reduction in systolic blood pressure in adults is estimated to have the potential to save about 12,000 lives a year in the United States (Stamler 1991) and to generate an increase in life expectancy of 1.8 months in men and 1.4 months in women (Selmer 2000).
Quality of the evidence
We included 16 trials, with 3048 participants, providing high quality evidence (Guyatt 2011) of the effect calcium supplementation on systolic and diastolic blood pressure (Table 1).
Risks of bias for random sequence generation and incomplete outcome data were low for 63% of the studies; allocation concealment risk of bias was low for 44% of the studies and unclear for the remainder; blinding of participants and personnel and risk of detection bias were low for 69% of the studies, and we rated all the studies at low risk of reporting bias.
Potential biases in the review process
We restricted this review to clinical trials in which the intervention was calcium supplementation as a single ingredient, which limited the number of studies we could include. On the other hand, we used an exhaustive search strategy to avoid publication selection bias. Two review authors independently assessed the articles and double‐checked data extraction to minimise errors.
Many of the studies were old and even though in those cases where published information was not enough to assess risk of bias, we attempted to contact authors, although the response was limited. Nevertheless, there was generally a low risk of bias.
Agreements and disagreements with other studies or reviews
Our results are in line with the most recent review by Van Mierlo 2006 that includes a meta‐analysis of 40 randomised controlled trials (RCTs) with normotensive and hypertensive people, showing that supplementation with around 1 gm of calcium per day significantly reduces systolic blood pressure by 1.9 mmHg and diastolic blood pressure by 1.0 mmHg. This review also found a higher effect in populations with low basal calcium intake. In a previous meta‐analysis involving 42 trials in normotensive and hypertensive people, the pooled analysis shows a reduction in systolic blood pressure of ‐1.44 mmHg (95% CI ‐2.20 to ‐0.68; P < .001) and in diastolic blood pressure of ‐0.84 mmHg (95% CI ‐1.44 to ‐0.24; P < .001) (Griffith 1999).
Our results are in the same direction as the Dickinson 2006 review in hypertensive people. Although this shows a statistically significantly larger reduction in blood pressure in the calcium group, the authors interpret this as more likely reflecting a bias due to poor quality trials than a real effect. We performed a sensitivity analysis excluding studies classified at high and moderate risk of bias. All studies were classified at low risk of selective reporting bias, so we could conduct no analysis for this domain. For the remaining five domains evaluated, the effect persisted after removing studies classified as being at high or moderate risk.
Calcium intake also showed effects on different populations. A Cochrane review (Hofmeyr 2014) showed that a good calcium intake has benefits for pregnancy outcomes, effects which are thought to be mediated by blood pressure reduction. Preliminary observations show that calcium supplementation during pregnancy could also have effects on reducing the blood pressure of the progeny (Belizan 1988, Hatton 2003). Consequently, calcium intake could play a role in the prevention of hypertension, particularly at a young age where small changes in blood pressure could have a higher effect. It has been shown that lowering blood pressure at younger ages is relevant, since the relative risk of cardiovascular diseases with blood pressure decreases with age and no significant deviations from linearity occurred in the associations of either systolic or diastolic blood pressure (Rapsomaniki 2014).
Authors' conclusions
An increase in calcium intake slightly reduces both systolic and diastolic blood pressure in normotensive people. The effect was confirmed in multiple prespecified subgroups, including a possible dose‐response effect, reinforcing the efficacy of the intervention. The effects can be observed after only 3.5 months of intervention. Although the effect is small, an adequate calcium intake should be an objective to be reached in the population.
Randomised controlled trials (RCT) are needed with high power in the early stages of life for a long period of time (at least one year), randomising young people of both sexes to attain a daily calcium intake of at least 1 gm in comparison with a control group. Subgroup analyses should be prespecified and powered to assess outcomes on systolic and diastolic blood pressure related to basal calcium intake, age, sex, basal blood pressure, and body mass index (BMI).
There is a need for studies exploring the mechanisms of calcium intake on blood pressure. This will allow the identification of early markers of individuals that could be more susceptible to calcium intake. It would also be of interest to assess whether there is a causal relationship between a given polymorphism and the effect of calcium on blood pressure. Research into the mechanisms could be nested within the RCT suggested above, to see if, as hypothesised, calcium could have an effect on vasoconstriction, the first stage in the further development of hypertension, particularly in young people.
More research is needed to assess the dose required and the best strategy to improve calcium intake, comparing the effect of dietary calcium with a supplemental version. Furthermore, if the effect of calcium intake on blood pressure is confirmed, studies of calcium fortification will be desirable to include populations with low calcium intake involving a universal effect on blood pressure.
Any future research on calcium intake must report adverse events, particularly in older people.
Acknowledgements
The authors would like to acknowledge the contribution of Daniel Comandé to the search strategy.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 16 October 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 dietary supplements/ 2 calcium, dietary/ 3 calcium carbonate/ 4 (calcium adj8 (acetate$ or add$ or boost$ or carbonate$ or consum$ or daily or day or diet$ or fed or feed$ or food or fortif$ or intake$ or suppl$)).tw. 5 (calcium adj8 (capsul$ or compound$ or liquid$ or oral$ or pill$ or powder$ or tab$)).tw. 6 (apocal or aragonite or biocal or calcimix or calcite or calsan or calsup or caltrate or maxicalc or mega cal or os cal or oscal or vaterite).tw. 7 or/1‐6 8 hypertension/ 9 hypertens$.tw. 10 exp blood pressure/ 11 (blood pressure or bloodpressure).tw. 12 ((arterial or diastolic or systolic) adj2 pressure).tw. 13 or/8‐12 14 randomized controlled trial.pt. 15 controlled clinical trial.pt. 16 randomized.ab. 17 placebo.ab. 18 clinical trials as topic/ 19 randomly.ab. 20 trial.ti. 21 or/14‐20 22 animals/ not (humans/ and animals/) 23 (eclampsia or preeclampsia).ti. 24 21 not (22 or 23) 25 7 and 13 and 24 26 remove duplicates from 25
Appendix 2. CENTRAL search strategy
Database: Cochrane Central Register of Controlled Trials < Issue 9 2014 > Search Date: 16 October 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ ID Search #1 MeSH descriptor: [Dietary Supplements] this term only #2 MeSH descriptor: [Calcium, Dietary] this term only #3 MeSH descriptor: [Calcium Carbonate] this term only #4 (calcium near/10 (acetate* or add* or boost* or carbonate* or consum* or daily or day or diet* or fed or feed or food or fortif* or intake$ or suppl*)):ti,ab #5 (calcium near/10 (capsul* or compound* or liquid* or oral* or pill* or powder* or tab*)):ti,ab #6 (apocal or aragonite or biocal or calcimix or calcite or calsan or calsup or caltrate or maxicalc or mega cal or os cal or oscal or vaterite):ti,ab #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Hypertension] explode all trees #9 hypertens*:ti,ab #10 MeSH descriptor: [Blood Pressure] explode all trees #11 ("blood pressure" or bloodpressure):ti,ab #12 ((arterial or diastolic or systolic) near/2 pressure):ti,ab in Trials #13 #8 or #9 or #10 or #11 or #12 #14 #7 and #13
Appendix 3. EMBASE search strategy
Database: Embase <1974 to 2014 Week 41> Search date: 16 October 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 diet supplementation/ 2 calcium intake/ 3 calcium carbonate/ 4 (calcium adj8 (acetate$ or add$ or boost$ or carbonate$ or consum$ or daily or day or diet$ or fed or feed or food or fortif$ or intake$ or suppl$)).tw. 5 (calcium adj8 (capsul$ or compound$ or liquid$ or oral$ or pill$ or powder$ or tab$)).tw. 6 (apocal or aragonite or biocal or calcimix or calcite or calsan or calsup or caltrate or maxicalc or mega cal or os cal or oscal or vaterite).tw. 7 or/1‐6 8 exp hypertension/ 9 hypertens$.tw. 10 exp blood pressure/ 11 (blood pressure or bloodpressure).tw. 12 ((arterial or diastolic or systolic) adj2 pressure).tw. 13 or/8‐12 14 randomized controlled trial/ 15 crossover procedure/ 16 double‐blind procedure/ 17 (randomi$ed or randomly).tw. 18 (crossover$ or cross‐over$).tw. 19 placebo.ab. 20 (doubl$ adj blind$).tw. 21 assign$.ab. 22 allocat$.ab. 23 or/14‐22 24 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 25 23 not 24 26 7 and 13 and 25 27 remove duplicates from 26
Appendix 4. Hypertension Group Specialised Register search strategy
Database: Hypertension Group Specialised Register Search date: 16 October 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (calcium:TI AND (hypertension) NOT (channel):TI NOT (antagonist*):TI) AND ( INREGISTER) 2 RCT 3 #1 AND #2
Appendix 5. LILACS search strategy
Search Date: 17 October 2014
(MH Hipertensión Inducida en el Embarazo OR Transient Hypertension OR Hipertensión Gestacional OR Hipertensão Gestacional OR Gestational Hypertension OR Pregnancy‐Induced Hypertension OR Gestosis OR Eclampsia$ OR Pre Eclampsia$ OR Pregnancy Toxemia$ OR Preeclampsia$) AND (MH Intervalo entre Nacimientos OR Birth Spac$ OR Birth Interval$ OR Delivery Interval$ OR Delivery Spac$ OR Intergenesic Interval$ OR Reproductive Pattern$ OR Subsequent Pregnan$ OR Espaçamento entre Nascimentos OR Padrão Reprodutivo OR Gravidez Subseqüente OR Patrón Reproductivo OR Timing OR Intervalo Intergenésico OR Interpregnancy Spac$ OR Interpregnancy Interval$) [Palabras]
Appendix 6. ClinicalTrials.gov search strategy
Database: ClinicalTrials.gov (via Cochrane Register of Studies) Search date: 16 October 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Study type: Interventional Studies Interventions: calcium NOT channel Outcome Measures: blood pressure Search terms: randomized
Data and analyses
Comparison 1.
Calcium supplementation/fortification vs control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effect mean difference of systolic blood pressure | 16 | 3048 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐2.15, ‐0.72] |
| 1.1 Women | 6 | 1823 | Mean Difference (IV, Fixed, 95% CI) | ‐1.45 [‐2.78, ‐0.12] |
| 1.2 Men | 5 | 507 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐3.56, ‐0.59] |
| 1.3 Both genders | 6 | 718 | Mean Difference (IV, Fixed, 95% CI) | ‐1.11 [‐2.15, ‐0.08] |
| 2 Effect mean difference of diastolic blood pressure | 15 | 2947 | Mean Difference (IV, Fixed, 95% CI) | ‐0.98 [‐1.46, ‐0.50] |
| 2.1 Women | 6 | 1823 | Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐1.71, ‐0.14] |
| 2.2 Men | 5 | 507 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐2.80, ‐1.02] |
| 2.3 Both genders | 5 | 617 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.08, 0.57] |
| 3 Change of systolic blood pressure | 9 | 2694 | Mean Difference (IV, Fixed, 95% CI) | ‐1.28 [‐2.04, ‐0.52] |
| 3.1 Women | 3 | 1656 | Mean Difference (IV, Fixed, 95% CI) | ‐1.53 [‐2.97, ‐0.09] |
| 3.2 Men | 4 | 432 | Mean Difference (IV, Fixed, 95% CI) | ‐1.85 [‐3.45, ‐0.25] |
| 3.3 Both genders | 3 | 606 | Mean Difference (IV, Fixed, 95% CI) | ‐0.89 [‐1.96, 0.18] |
| 4 Change of diastolic blood pressure | 8 | 2593 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.47, ‐0.45] |
| 4.1 Women | 3 | 1656 | Mean Difference (IV, Fixed, 95% CI) | ‐1.13 [‐1.98, ‐0.29] |
| 4.2 Men | 4 | 432 | Mean Difference (IV, Fixed, 95% CI) | ‐2.01 [‐2.94, ‐1.08] |
| 4.3 Both genders | 2 | 505 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.73, 1.01] |
| 5 Final value of systolic blood pressure | 10 | 538 | Mean Difference (IV, Fixed, 95% CI) | ‐2.19 [‐3.84, ‐0.54] |
| 5.1 Women | 3 | 167 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐4.49, 2.48] |
| 5.2 Men | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐5.27 [‐8.53, ‐2.01] |
| 5.3 Both genders | 5 | 247 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐3.47, 1.11] |
| 6 Final value of diastolic blood pressure | 9 | 437 | Mean Difference (IV, Fixed, 95% CI) | ‐1.22 [‐2.52, 0.08] |
| 6.1 Women | 3 | 167 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐1.70, 2.73] |
| 6.2 Men | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐1.88 [‐4.26, 0.50] |
| 6.3 Both genders | 4 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐2.33 [‐4.50, ‐0.17] |
| 7 Effect mean difference of systolic blood pressure by age | 16 | 3048 | Mean Difference (IV, Fixed, 95% CI) | ‐1.25 [‐2.00, ‐0.51] |
| 7.1 Less than 35 years of age | 7 | 399 | Mean Difference (IV, Fixed, 95% CI) | ‐2.11 [‐3.58, ‐0.64] |
| 7.2 35 years and older | 9 | 2649 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.83, ‐0.09] |
| 8 Effect mean difference of diastolic blood pressure by age | 15 | 2947 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.45, ‐0.48] |
| 8.1 Less than 35 years of age | 6 | 298 | Mean Difference (IV, Fixed, 95% CI) | ‐2.61 [‐3.74, ‐1.49] |
| 8.2 35 years and older | 9 | 2649 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐1.13, ‐0.06] |
| 9 Change in systolic blood pressure by age | 7 | 2559 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐2.01, ‐0.35] |
| 9.1 Less than 35 years of age | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐2.56 [‐4.90, ‐0.23] |
| 9.2 35 years and older | 5 | 2470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.98 [‐1.87, ‐0.10] |
| 10 Change in diastolic blood pressure by age | 7 | 2559 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.49, ‐0.46] |
| 10.1 Less than 35 years of age | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐3.96 [‐5.48, ‐2.44] |
| 10.2 35 years and older | 5 | 2470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.13, ‐0.04] |
| 11 Final value in systolic blood pressure by age | 10 | 538 | Mean Difference (IV, Fixed, 95% CI) | ‐2.19 [‐3.84, ‐0.54] |
| 11.1 Less than 35 years of age | 5 | 310 | Mean Difference (IV, Fixed, 95% CI) | ‐1.81 [‐3.71, 0.09] |
| 11.2 35 years and older | 5 | 228 | Mean Difference (IV, Fixed, 95% CI) | ‐3.39 [‐6.76, ‐0.03] |
| 12 Final value in diastolic blood pressure by age | 9 | 437 | Mean Difference (IV, Fixed, 95% CI) | ‐1.22 [‐2.52, 0.08] |
| 12.1 Less than 35 years of age | 4 | 209 | Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐2.66, 0.68] |
| 12.2 35 years and older | 5 | 228 | Mean Difference (IV, Fixed, 95% CI) | ‐1.58 [‐3.65, 0.49] |
| 13 Effect mean difference in systolic blood pressure by basal calcium intake | 9 | 2704 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.86, ‐0.22] |
| 13.1 Calcium Intake below 600 mg a day | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐6.33, 2.93] |
| 13.2 Calcium Intake from 600 to less than 800 mg a day | 5 | 786 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐1.88, 0.13] |
| 13.3 Calcium intake above 800 mg a day | 4 | 1860 | Mean Difference (IV, Fixed, 95% CI) | ‐1.34 [‐2.80, 0.13] |
| 14 Effect mean difference in diastolic blood pressure by basal calcium intake | 9 | 2704 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.20, ‐0.15] |
| 14.1 Calcium Intake below 600 mg a day | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐1.90, 4.70] |
| 14.2 Calcium Intake from 600 to less than 800 mg a day | 5 | 786 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.11, 0.29] |
| 14.3 Calcium intake above 800 mg a day | 4 | 1860 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐1.94, ‐0.34] |
| 15 Change in systolic blood pressure by basal calcium intake | 6 | 2527 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐1.89, ‐0.21] |
| 15.1 Calcium Intake from 600 to less than 800 mg a day | 4 | 705 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.92, 0.12] |
| 15.2 Calcium intake above 800 mg a day | 3 | 1822 | Mean Difference (IV, Fixed, 95% CI) | ‐1.37 [‐2.86, 0.12] |
| 16 Change in diastolic blood pressure by basal calcium intake | 6 | 2527 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐1.28, ‐0.20] |
| 16.1 Calcium Intake below 600 mg a day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Calcium Intake from 600 to less than 800 mg a day | 4 | 705 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.15, 0.29] |
| 16.3 Calcium intake above 800 mg a day | 3 | 1822 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐1.96, ‐0.33] |
| 17 Final value of systolic blood pressure by basal calcium intake | 4 | 226 | Mean Difference (IV, Fixed, 95% CI) | ‐2.99 [‐5.86, ‐0.11] |
| 17.1 Calcium Intake below 600 mg a day | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐6.33, 2.93] |
| 17.2 Calcium Intake from 600 to less than 800 mg a day | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐4.56 [‐8.58, ‐0.54] |
| 17.3 Calcium intake above 800 mg a day | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐8.93, 8.93] |
| 18 Final value of diastolic blood pressure by basal calcium intake | 4 | 226 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐2.38, 1.38] |
| 18.1 Calcium Intake below 600 mg a day | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐1.90, 4.70] |
| 18.2 Calcium Intake from 600 to less than 800 mg a day | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐2.00, 1.01] |
| 18.3 Calcium intake above 800 mg a day | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.72, 4.72] |
| 19 Effect mean difference of systolic blood pressure by dose | 16 | 3048 | Mean Difference (IV, Fixed, 95% CI) | ‐1.25 [‐2.00, ‐0.51] |
| 19.1 Less than 1000 mg of calcium intake | 2 | 263 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐2.16, 2.32] |
| 19.2 1000 ‐ 1500 of calcium intake | 8 | 2435 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐2.01, ‐0.27] |
| 19.3 1500 mg or more of calcium intake | 7 | 350 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐4.71, ‐0.86] |
| 20 Effect mean difference of diastolic blood pressure by dose | 15 | 2947 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.45, ‐0.48] |
| 20.1 Diary calcium intake < less than 1000 mg | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐2.23, 1.15] |
| 20.2 Diary calcium intake 1000 ‐ 1250 mg | 7 | 964 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.37, ‐0.06] |
| 20.3 Diary calcium intake 1500 mg or more | 8 | 1821 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐2.22, ‐0.64] |
| 21 Change in diastolic blood pressure by dose | 7 | 2559 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.49, ‐0.46] |
| 21.1 Diary calcium intake less than 1000 mg | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐2.23, 1.15] |
| 21.2 Diary calcium intake 1000 ‐ 1250 mg | 5 | 894 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐1.35, ‐0.02] |
| 21.3 Diary calcium intake 1500 mg or more | 2 | 1503 | Mean Difference (IV, Fixed, 95% CI) | ‐1.69 [‐2.64, ‐0.75] |
| 22 Change in systolic blood pressure by dose | 7 | 2559 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐2.01, ‐0.35] |
| 22.1 Less than 1000 mg of calcium intake | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐2.90, 3.02] |
| 22.2 1000 ‐ 1500 of calcium intake | 6 | 2365 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐2.02, ‐0.27] |
| 22.3 1500 mg or more of calcium intake | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐10.58, ‐0.82] |
| 23 Final value in systolic blood pressure by dose | 9 | 489 | Mean Difference (IV, Fixed, 95% CI) | ‐1.56 [‐3.29, 0.17] |
| 23.1 Less than 1000 mg of calcium intake | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐3.33, 3.53] |
| 23.2 1000 ‐ 1500 of calcium intake | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐7.90, 6.50] |
| 23.3 1500 mg or more of calcium intake | 6 | 318 | Mean Difference (IV, Fixed, 95% CI) | ‐2.25 [‐4.34, ‐0.16] |
| 24 Final value in diastolic blood pressure by dose | 8 | 388 | Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐2.30, 0.46] |
| 24.1 Diary calcium intake less than 1000 mg | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Diary calcium intake 1000 ‐ 1250 mg | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐6.13, 2.30] |
| 24.3 Diary calcium intake 1500 mg or more | 6 | 318 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐2.26, 0.65] |
| 25 Effect mean difference of systolic blood pressure by duration | 16 | 3048 | Mean Difference (IV, Fixed, 95% CI) | ‐1.25 [‐2.00, ‐0.51] |
| 25.1 Less than 6 month | 11 | 674 | Mean Difference (IV, Fixed, 95% CI) | ‐1.79 [‐2.92, ‐0.67] |
| 25.2 6 months or more | 5 | 2374 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐1.83, 0.17] |
| 26 Effect mean difference of diastolic blood pressure by duration | 15 | 2947 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.45, ‐0.48] |
| 26.1 Less than 6 month | 10 | 573 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐2.77, ‐1.14] |
| 26.2 6 month or more | 5 | 2374 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.03, 0.17] |
| 27 Effect mean difference of systolic blood pressure by intervention type | 16 | 3048 | Mean Difference (IV, Fixed, 95% CI) | ‐1.25 [‐2.00, ‐0.51] |
| 27.1 Supplementation | 14 | 2909 | Mean Difference (IV, Fixed, 95% CI) | ‐1.33 [‐2.10, ‐0.56] |
| 27.2 Fortification | 2 | 139 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐3.11, 3.29] |
| 28 Effect mean difference of diastolic blood pressure by intervention type | 15 | 2947 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.45, ‐0.48] |
| 28.1 Supplementation | 14 | 2909 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.45, ‐0.48] |
| 28.2 Fortification | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.72, 4.72] |
Differences between protocol and review
Agustina Mazzoni was listed in the published protocol with the role of extracting data but María Luisa Cafferata and Gabriela Cormick did this work.
We planned to include cluster‐randomised trials in the analyses along with individually randomised trials. We planned to adjust their sample sizes using the methods described in the Cochrane Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we used ICCs from other sources, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster‐randomised trials and individually randomised trials, we planned to synthesise the relevant information. We considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and if the choice of randomisation unit was considered to be unlikely. We planned also to acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit. We did not find any cluster randomised trials that met our eligibility criteria.
For dichotomous data, we planned to present results as summary risk ratios with 95% confidence intervals. None of the studies reported hypertension as a dichotomous outcome.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised double‐blind clinical trial. " The trial was conducted in Guatemala. |
|
| Participants | 57 subjects (28 men and 29 women). Age:18 and 35 years. Healthy subjects not receiving medical treatment, women were not using hormonal contraceptives. Subjects .. "were free of diseases as assessed by a comprehensive clinical examination and blood and urine tests." |
|
| Interventions | Calcium supplementation vs placebo tablets. Intervention group: daily oral tablet containing 0.8 gms of calcium carbonate and 5.23 gms of calcium lactate gluconate (Calcium‐Sandoz, 1,000 mg), representing 1 gm of elemental calcium. Placebo group: daily oral tablet of the same weight, size, and organoleptic characteristics as the calcium tablet. Trial duration: 22 weeks. |
|
| Outcomes | Systolic blood pressure: read when the appearance of the first Korotkoff's sound occurred; Diastolic blood pressure: taken at the disappearance of the fifth Korotkoff's sound. The final value and SD were calculated from the reported basal blood pressure values and the percent changes between basal values and stable period (weeks 9 through 23) reported in the article. Blood levels of total calcium and magnesium by atomic absorption spectrophotometry Blood levels of inorganic phosphate by spectrophotometry Blood levels of albumin by dye‐binding bromocresol purpose Total calcium intake: basal dietary intake measured by 24 hr. food record plus compliance with supplementation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer random number generator was used. Participants were randomly assigned to 2 treatment groups. "Separate randomisation schedules were used for sex and age groups (18 ‐ 23 years and 24 ‐ 35 years)." |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered containers were similar for both types of tablets, and a key number indicated the composition |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The placebo group received a daily tablet of the same weight, size, and organoleptic characteristics as the calcium tablet." The treatment assignment was made double‐blind. The composition of the tablet unknown to participants or to the professional in charge of the examinations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Composition of the tablet was unknown to participants or to professional in charge of the examinations or BP measurements. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 28 men and 29 women were randomised to the study groups and 23 men and 20 women completed the study. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes addressed. |
| Other bias | Low risk | No differences between groups were found in the variables collected during the baseline period except for systolic BP in the dorsal position among the men. |
| Methods | Randomised double‐blind clinical trial. | |
| Participants | Healthy subjects. with high‐normal diastolic blood pressure, not taking antihypertensive drugs, not grossly obese (BMI < 36.15 kg/m*), and not consuming more than 21 alcohol‐containing drinks weekly. Intervention group: 237 participants assigned to receive calcium. Control group: 234 participants assigned to receive placebo. Gender: Men and women. Age: 30 to 54 years. Exclusion criteria included pre‐existing cardiovascular or life‐threatening conditions, conditions requiring or contraindicating any of the study interventions, and intent to become pregnant during the study period. Age average: 43 years; 69% were men, 86% were white, and 51% had completed college. Baseline blood pressures averaged 125/84 mm Hg and BMI averaged 27.3 kg/m2. Dietary calcium intake: average 970 mg. |
|
| Interventions | Calcium supplementation vs placebo tablets. Intervention group: calcium carbonate representing calcium, 25 mmol or 1.0 g (2 pills per day). Control group: placebo tablet. Trial duration: 6 months. |
|
| Outcomes | Primary: "change in diastolic blood pressure from baseline to final follow‐up." Secondary: "changes in systolic blood pressure and intervention compliance measures." |
|
| Notes | Dietary calcium intakes according to the food frequency questionnaire data averaged 970 mg. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation lists were computer‐generated at the TOHP Data Coordinating Center. |
| Allocation concealment (selection bias) | Low risk | Randomisation assignments were obtained from the coordinating centre by telephone when possible, otherwise sealed opaque envelopes were used to convey the treatment assignment. Adherence to the appropriate assignment sequence was monitored by the coordinating centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind fashion, with placebo controls |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Trained, certified observers who were blinded to participants' treatments. Blood pressure was measured with a Hawksley random‐zero sphygmomanometer." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Blood pressure data were complete for 95% of participants at 3 months and 93% at 6 months. Pill counts were obtained for 91% at 6 weeks, 90% at 3 months, and 84% at 6 months. |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective reporting. |
| Other bias | Low risk | Baseline characteristics were similar. |
| Methods | Randomised control trial. The trial was conducted in the United States of America. |
|
| Participants | 34 healthy, normotensive adolescents. Ethnicity: African‐American. Age:14 ‐ 19 years. |
|
| Interventions | Intervention: 1.5 grams of calcium per day. Control group: daily placebo tablets. Trial duration: 4 weeks. |
|
| Outcomes | Ambulatory systolic blood pressure and diastolic blood pressure. | |
| Notes | There is no information of calcium intake reported. Participants were recruited from a high school in Los Angeles. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors reported that participants were randomly assigned to the treatment or control group. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The ambulatory blood pressure unit measured the blood pressure every 30 minutes during the day. "Unit was placed on each participant for 24 hours." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported from how many participants gave data for the results |
| Selective reporting (reporting bias) | Low risk | The planned outcome was reported. |
| Other bias | Unclear risk | No information of baseline characteristics was reported. |
| Methods | Randomised, double‐blind , placebo‐controlled trial. The trial was conducted in the United States of America. |
|
| Participants | 101 5th‐grade students. Gender: 50 girls and 51 boys; Ethnicity: 61 were black. Setting: inner city school. |
|
| Interventions | Intervention: 480 ml of juice containing 600 mg calcium (as calcium citrate malate) daily Control: Same juice with no calcium. Trial duration: 12 weeks |
|
| Outcomes | "Blood pressure 4 times on each of 3 weekly sittings at baseline and at follow‐up" | |
| Notes | Nutrient data from 3 sets of 2‐day food records on each participant. Funding: Procter and Gamble Co. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation was performed by a centralised unit with the ID numbers that researchers provided. ID labels were affixed to each 'juice box', and sent to researchers who were completely blinded to treatment assignment. |
| Allocation concealment (selection bias) | Low risk | Random allocation was performed by a centralised unit with the ID numbers that researchers provided. ID labels were affixed to each 'juice box' and sent to researchers who were completely blinded to treatment assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators and participants were masked to treatment assignment throughout the intervention period. "The intervention and placebo beverages were formulated to look and taste the same." "Single‐serving containers ("juice boxes") and labelled with the subject's name and study identification number." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Automated device (Dinamap Vital Signs Monitor model 845‐A, Critikon, Inc., Tampa, Fla.)." "Blood pressure data were automatically recorded on a floppy disk; investigators and participants were masked to these data until the end of the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 106 participants randomised, 5 moved from the school and the analyses included 101 participants. Age, sex, and race of non‐participants and those who dropped out before intervention were similar. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were small differences (in different directions) between intervention and placebo participants in baseline systolic blood pressure, hours of television watched, and amount of dietary calcium. |
| Methods | Double‐blind, randomised, controlled cross‐over study The trial was conducted in New Zealand |
|
| Participants | 38 healthy volunteers. Age: over 40 years |
|
| Interventions | Intervention: high‐calcium skim powder milk. Control: replacement of usual liquid milk with 2 servings a day of skim non‐fortified powder milk. Trial duration: 4 weeks, with a minimum of 4 weeks of wash‐out between interventions. |
|
| Outcomes | Systolic blood pressure and ambulatory blood pressure | |
| Notes | "For many people in the trial, the control skim milk provided additional calcium to the diet. This may explain the small reduction in office".. standing systolic blood pressure observed in the control group. Calcium intake was calculated using 24 hour food recalls. This study was supported by The New Zealand Dairy Board. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated "Randomized double‐blind controlled trial'. Double‐blind, randomised, controlled cross‐over study. "Each volunteer consumed each of the milks in randomised order." "The milk was provided to the volunteers as a dry powder." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Ambulatory blood pressure monitor. Automated oscillometric blood pressure monitor (A&D, Model UA‐751; A&D Medical Division, Milpitas, California, USA)." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not reported |
| Selective reporting (reporting bias) | Low risk | All primary outcomes were reported. |
| Other bias | High risk | There were small differences (in different directions) between intervention and placebo participants in baseline office and ambulatory blood pressure, except for baseline systolic blood pressure: Skim milk 121 ± 14 and high‐calcium skim milk 125 ± 19. Controls may have accidentally received a calcium boost from the placebo milk that should be treated as a potential bias. |
| Methods | Randomised double‐blind clinical trial. . Women were divided into a control and an experimental group in a The trial was conducted in the United States of America. |
|
| Participants | 81 normotensive and 34 medicated hypertensive women. Age: between 35 and 65 years. |
|
| Interventions | Intervention group: 3 daily tablets of a calcium carbonate supplement containing 500 mg calcium‐tablet . Control group: placebo tablets. Trial duration: 4‐year. |
|
| Outcomes | Bone mineral content and blood pressure | |
| Notes | Most of the women were using thiazides "Dietary calcium of all women was determined using a precoded food record form, which had been tested for validity against weighed food intakes." This study was supported by Wisconsin Milk Marketing Board, Inc, Marion Laboratories, Kansas City, MO |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The original group of women, including the hypertensives, was divided into a control and an experimental group in a double‐blind design." However methods were not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Blood pressure was measured from the right arm of seated participants using a standard mercury sphygmomanometer." Not reported if outcome assessors were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 44 participants were randomised to the intervention and 41 were analysed. 51 participants were randomized to placebo and 40 were analysed. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes were reported |
| Other bias | Low risk | Baseline characteristics were similar between the groups. |
| Methods | Double‐blind, placebo‐controlled parallel‐group cross‐over study. The trial was conducted in Belgium. |
|
| Participants | 32 male participants. Age: 24 ± 1 (range 20 ‐ 44 years) and weight 75.9 ± 1.3 kg. |
|
| Interventions | Intervention group: 1 g elemental calcium as calcium gluconate powder twice a day (morning and evening). Control group: placebo with the same orange flavour as intervention. Trial duration: 16 weeks. |
|
| Outcomes | Blood pressure recorded in standing position Intracellular cationic concentrations Transmembrane cation transport systems Plasma total and ionised calcium Calciotropic hormones |
|
| Notes | This study was supported by Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind placebo‐control parallel‐group. The calcium supplement and placebo were both orange flavour. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were included in the results. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes results were reported. |
| Other bias | Low risk | Baseline characteristics were similar between calcium and placebo groups. |
| Methods | Randomised, double‐blind, placebo‐controlled trial. The trial was conducted in the United States of America. |
|
| Participants | Normotensive male participants. Ethnicity: Black (n = 21) and white (n = 54)." Age: 19 to 52 years. |
|
| Interventions | Internvention group: calcium, 1500 mg a day. Control group: placebo. "Participants were randomly assigned within racial groups to either a treatment". Trial duration: 12‐week period. |
|
| Outcomes | Blood pressure Serum levels of total and ionised calcium Total inorganic phosphorus Parathyroid hormone Overnight urinary electrolyte values |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | The supplements were strip‐wrapped individually and coded by someone not involved in the research study. The participants did not know which group they were assigned to, and the researcher(s) who collected other information also were not aware of the group assignment. Early analyses were completed prior to revealing the assigned groups as well. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Assignment was double‐blind. "Indistinguishable placebo tablets were composed of microcrystalline methylcellulose and starch." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Use of a random‐baseline sphygmomanometer and blinded observers to eliminate bias during blood pressure measurement, documentation of nutrient intake other than the supplement, and control for body weight and other possible confounders." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were included in the results. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | The groups were similar at baseline. |
| Methods | Randomised, double‐blind, placebo‐controlled trial The trial was conducted in the United States of America. |
|
| Participants | 42 adults. Gender: men and women. High normal or mildly hypertensive levels of blood pressure. |
|
| Interventions | Intervention group: 500 mg of elemental calcium as calcium carbonate tablets. Control group: placebo tablets. Trial duration: 8 weeks. |
|
| Outcomes | Blood pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were given a random number of calcium or placebo tablets |
| Allocation concealment (selection bias) | Low risk | The supplements were strip‐wrapped individually and coded by someone not involved in the research study. The participants did not know which group they were assigned to, and the researcher(s) who collected other information also were not aware of the group assignment. Early analyses were completed prior to revealing the assigned groups as well. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Experimental group assessment was double‐blind. Tablets contained 500 mg of elemental calcium in the form of calcium carbonate; Indistinguishable placebo tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Measurements were taken with random zero sphygmomanometer at least 1 minute apart. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 44 men and women participants were randomised, 2 participants withdrew due to appointment conflicts and 42 participants completed the study. Missing outcome data balanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | In the treatment group there were more men than in the placebo group. 8 of the 10 women were allocated to the placebo group. However, blood pressure measurements showed not statistically significant differences between groups. |
| Methods | Randomised, double‐blind, placebo‐ controlled, cross‐over trial The trial was conducted in the United States of America. |
|
| Participants | 32 normotensive subjects. Healthy volunteers with no signs of secondary hypertension. Age: between 21 and 70 years. |
|
| Interventions | Intervention group: 1000 mg a day of elemental calcium as the carbonate or citrate salt. Control group: placebo tablets. Trial duration: 8 weeks, or placebo. |
|
| Outcomes | Change in blood pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment of participants was done separately in blocks by computer. |
| Allocation concealment (selection bias) | Low risk | Medications were pre‐packaged by randomisation number for each participant and dispensed every 2 weeks. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Placebo tablets consisted of microcrystalline cellulose and starch and were identical in taste and appearance to the calcium carbonate tablets. Subjects and members of the investigative staff were blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A Hawksley random‐zero sphygmomanometer (Hawksley & Sons, Ltd., Lancing, England) was used for measurement of blood pressure after the participant was supine for 5 minutes and after standing for 2 minutes." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 32 normotensive participants were reported in the results. |
| Selective reporting (reporting bias) | Low risk | Primary outcome result reported |
| Other bias | Low risk | The baseline characteristics were similar between the groups. |
| Methods | Double‐blind, randomised, controlled trial. The trial was conducted in New Zealand. |
|
| Participants | Healthy postmenopausal women more than 5 years from postmenopause. Age: more than 55 years (mean age, 74 years; Mean baseline weight: 67 kg; Mean baseline blood pressure:134/70 mmHg. Exclusion criteria: participants receiving therapy for osteoporosis or taking calcium supplements, major ongoing disease including serum creatinine greater than 1.8 mg/dl (0.2 mmol/litre), untreated hypo‐ or hyperthyroidism, liver disease, serum 25‐hydroxyvitamin D below 10 g/litre (25 nmol/litre), malignancy, or metabolic bone disease, users of hormone replacement therapy, anabolic steroids, glucocorticoids, or bisphosphonate in the previous 1 year. |
|
| Interventions | Intervention group: calcium as calcium citrate (1 gm of elemental calcium a day; n = 732). Control group: identical placebo (n = 739). Trial duration 30 months. |
|
| Outcomes | Primary outcome: fracture incidence Secondary analysis: ‐ Body weight ‐ Blood pressure |
|
| Notes | Dietary calcium intake was assessed using a validated food frequency questionnaire. Calcium was provided by Citracal, Mission Pharmacal, San Antonio TX. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatments were allocated randomly using a minimisation algorithm balancing for current thiazide use, age, and the occurrence of fractures resulting from minimal trauma after the age of 40 years." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study. "Subjects received 1 g elemental calcium daily as citrate (Citracal, Mission Pharmacal, San Antonio TX) or an identical placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blood pressure was measured using a Dinamap automatic monitor (Johnson & Johnson, Tampa, FL) at each visit." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts were not reported. |
| Selective reporting (reporting bias) | Low risk | A variety of preplanned models were run: an intention‐to‐treat analysis, with and without imputation (maximum likelihood) of missing values, and with and without adjustment for compliance; a per protocol analysis; and an analysis of the change in blood pressure, excluding those taking blood pressure‐lowering medication. |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
| Methods | Randomised controlled trial. The trial was conducted in New Zealand. |
|
| Participants | 323 healthy men. Age: over 40 years. |
|
| Interventions | Intervention groups: group 1: 600 mg calcium a day or group 2: 1200 mg calcium a day as calcium citrate. Control group: placebo. Trial duration: 2 years. |
|
| Outcomes | Primary endpoint: change in the ratio of HDL to LDL cholesterol Secondary endpoints: changes in cholesterol fractions, triglycerides, blood pressure, and body composition |
|
| Notes | This study was supported by Mission Pharmacal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatments were allocated randomly by using computer‐generated random numbers (Microsoft Excel 2003; Microsoft, Redmond, WA) within blocks of random sizes in multiples of 3." |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed before the study began by the study statistician and was conveyed to a staff member who dispensed the study medication into numbered containers. This individual had no direct contact with other study staff nor with trial participants. Subjects were allocated a study number according to the sequence of their enrolment." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study staff were blinded to treatment allocation throughout the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blood pressure was measured by using a Dinamap automatic monitor (Johnson & Johnson, Tampa, FL)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Complete follow‐up was achieved in 96% of the participants, and the proportions of those randomly assigned still receiving the trial medication at study end were as follows: 93% in the placebo group, 91% in the Ca600 group, and 86%in the Ca1200 group (P = 0.19 for between‐group comparisons)." |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were small differences (in different directions) between intervention and placebo participants. |
| Methods | Randomised, double blind parallel group trial. The trial was conducted in the United States of America. |
|
| Participants | 321 participants 93% completed baseline and midpoint measurements. "Exclusion criteria included reported diastolic blood pressure 65 mm Hg; hypertension; BMI > 32 kg/m²; insulin‐dependent diabetes; cardiovascular disease; renal failure; medications that affect blood pressure, weight loss diets, use of nutritional supplements of calcium, magnesium, or potassium (including antacid preparations)." |
|
| Interventions | Intervention group: calcium carbonate 1200 mg daily (Caltrate 600 mg twice daily, Lederle Laboratories). Control group:identical placebo. Trial duration: 16 weeks. "The placebo group received twice the number of participants as the four treatment groups to improve statistical power." |
|
| Outcomes | Ambulatory 24‐hour blood pressure 24‐hour urine Body weight Health and side effects questionnaire Pill counts |
|
| Notes | Participants who had baseline systolic blood pressure above 160 mmHg or diastolic blood pressure above 95 mm Hg were excluded and advised to see their physicians. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed by a computer programme directed by the statistician on the project. The statistician had no contact with the data collectors or the participants. (Information provided by the author) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were under double‐blind conditions for 16 weeks but methods not describe. The participants were not informed about their specific supplement group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The blood pressure machine automatically entered the blood pressure data on computer tape that was later converted to an ASCII file at the study office. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 321 participants were randomised. 300 participants were available for follow‐up measurements and 290 completed the study measurements. |
| Selective reporting (reporting bias) | Low risk | Primary outcome reported. |
| Other bias | Low risk | Baseline characteristics were similar. |
| Methods | Randomised, double‐blind clinical trial. The trial was conducted in Iran. | |
| Participants | 49 overweight men (BMI > 25 kg/m² , BMI = 27.5 ± 1.7) Age: 34.4 ± 4.8 years. |
|
| Interventions | Intervention group: carbonate calcium (1250 mg elemental calcium daily) Control group: Placebo. Trial duration: 8 weeks. |
|
| Outcomes | Blood pressure Serum lipid profile |
|
| Notes | Diet was assess with a 24‐hour dietary recall questionnaires at baseline, 4th week, and end of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were divided randomly (by random number tables) into case and placebo groups |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind clinical trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants used low‐calorie diets and we had to exclude them from the study (fewer than 10%) |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Baseline characteristics were similar between the 2 groups |
| Methods | Double‐blind randomised placebo‐controlled trial. The trial was conducted in Denmark. |
|
| Participants | 28 healthy women with early menopause (6 months to 3 years earlier). Overweight was not an exclusion criterion. | |
| Interventions | Intervention group:2000 mg calcium per day (14 participants). Control group: identical‐looking placebo tablets (14 participants). Trial duration: 1 year. |
|
| Outcomes | Blood pressure. BP was measured by mercury manometer after 10 min of supine rest | |
| Notes | Tablets were provided by Sandoz | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated into 2 groups according to random sampling numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial. Participants received identical‐looking tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were evaluated at the end of the study. |
| Selective reporting (reporting bias) | Low risk | The primary outcome was reported. |
| Other bias | High risk | Placebo participants had higher initial weight and lower systolic blood pressure. |
| Methods | Double‐blind, placebo‐controlled trial. Participants were assigned to 2 groups according to a randomised block design that accounted for habitual calcium intake and BMI. The trial was conducted in the Netherlands. |
|
| Participants | 58 normotensive healthy female dietetic students, not receiving any medical treatment at the time of recruitment. Age: 20 ‐ 23 year. Weight:49 ‐ 76 kg. |
|
| Interventions | Intervention group: Daily lemonade or apple juice with powder containing 1500 mg Calcium ‐ calcium carbonate (1. 251 g), citric acid (2. 168 g), sodium‐hydrogen carbonate (0.5 g), and dextrose (2.88 g). Control group: Daily lemonade or apple juice placebo powder with citric acid (0.85 g), sodium‐hydrogen carbonate (0.5 g), dextrose (4.5 g), and corn‐flour (0. 1 g). Both groups received a low‐calcium diet (500 mg Calcium a day) restricting intake of dairy products. Trial duration: 6 weeks. |
|
| Outcomes | Difference for each individual between baseline blood pressure and final blood pressure Individual change in blood pressure during the experiment as indicated by the regression coefficient (slope) obtained from linear regression analysis of blood pressure versus time during the experimental period |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned to 2 groups according to a randomised block design that accounted for habitual calcium intake and body mass index. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled trial but methods not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported in the results |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were similar between the 2 groups. |
ABP: ambulatory blood pressure BMI: body mass index BP: blood pressure DBP: diastolic blood pressure HDL: high‐density lipids LDL: low‐density lipids SBP: systolic blood pressure SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bostick 2000 | There was no discrimination in the outcomes for hypertensive or normotensive participants. "Persons with or without hypertension or hypercholesterolaemia, or taking or not taking antihypertensive or cholesterol‐lowering medications were eligible to participate except as specified below." |
| Dwyer 1998 | Number of cases in each cross‐over step were not reported. |
| Eftekhari 2009 | Low fat, high fibre diet was a co‐ intervention. |
| Karanja 1987 | Blood pressure is not an outcome of the study. |
| Luft 1986 | Quasi‐randomised trial |
| Morris 1988 | No details of number of participants in calcium placebo groups |
| Pan 1993 | Most participants (63%) were taking antihypertensive drugs. |
| Pan 2000 | Blood pressure is not an outcome of the study. |
| Shalileh 2010 | Energy‐restricted diet was a co‐intervention. |
| Smith 1987 | Quasi‐randomised study. It used even and odd number from a table of random numbers |
| Weinberge 1993 | Salt‐sensitive or salt‐resistant participants. No details of number of participants in calcium placebo groups |
Contributions of authors
| Roles and responsibilities | |
| TASK | WHO HAS UNDERTAKEN THE TASK |
| Drafted the protocol | Gabriela Cormick/Agustín Ciapponi/ José M Belizán |
| Developed a search strategy | Gabriela Cormick/Agustín Ciapponi |
| Selected which trials to include (2 people + 1 arbiter in the event of dispute) | Gabriela Cormick/María Luisa Cafferata/Agustín Ciapponi |
| Extracted data from trials (3 people) | Gabriela Cormick/María Luisa Cafferata/Agustín Ciapponi |
| Entered data into RevMan (Cochrane software) | Gabriela Cormick/Agustín Ciapponi |
| Carried out the analysis | Gabriela Cormick/Agustín Ciapponi |
| Interpreted the analysis | Gabriela Cormick/Agustín Ciapponi/ María Luisa Cafferata/José M Belizán |
| Drafted the final review | Gabriela Cormick/Agustín Ciapponi/María Luisa Cafferata/José M Belizán |
| Responsible to keep the review up to date | Gabriela Cormick/Agustín Ciapponi/ María Luisa Cafferata |
Sources of support
Internal sources
-
Institute for Clinical Effectiveness and Health Policy, Argentina.
www.iecs.org.ar
External sources
No sources of support supplied
Declarations of interest
Gabriela Cormick: Nothing to declare.
Agustín Ciapponi: Nothing to declare.
María Luisa Cafferata: Nothing to declare.
José M Belizán: Nothing to declare.
New
References
References to studies included in this review
- Belizan JM, Villar J, Pineda O, Gonzalez AE, Sainz E, Garrera G, et al. Reduction of blood pressure with calcium supplementation in young adults. JAMA 1983;249(9):1161‐5. [PubMed] [Google Scholar]
- Cutler JA, Whelton PK, Appel L, Charleston J, Dalcin AT, Ewart C, et al. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels: Results of the trials of hypertension prevention, phase I. JAMA 1992;4(267):1213‐20. [DOI] [PubMed] [Google Scholar]; Yamamoto ME, Applegate WB, Klag MJ, Borhani NO, Cohen JD, Kirchner KA, et al. Lack of blood pressure effect with calcium and magnesium supplementation in adults with high‐normal blood pressure. Results from Phase I of the Trials of Hypertension Prevention (TOHP). Trials of Hypertension Prevention (TOHP) Collaborative Research Group. Annals of Epidemiology 1995;5(2):96‐107. [DOI] [PubMed] [Google Scholar]
- Davis IJ, Grim C, Dwyer K, Nicholson L, Dwyer J. The effects of calcium supplementation on ambulatory blood pressure in African‐American adolescents. Journal of the National Medical Association 1996;88(12):774‐8. [PMC free article] [PubMed] [Google Scholar]
- Gillman MW, Hood MY, Moore LL, Nguyen US, Singer MR, Andon MB. Effect of calcium supplementation on blood pressure in children. Journal of Pediatrics 1995;127(2):186‐92. [DOI] [PubMed] [Google Scholar]
- Hilary Green J, Richards JK, Bunning RL. Blood pressure responses to high‐calcium skim milk and potassium‐enriched high‐calcium skim milk. Journal of Hypertension 2000;18(9):1331‐9. [DOI] [PubMed] [Google Scholar]
- Johnson NE, Smith EL, Freudenheim JL. Effects on blood pressure of calcium supplementation of women. American Journal of Clinical Nutrition 1985;42(1):12‐7. [DOI] [PubMed] [Google Scholar]
- Lijnen P, Petrov V. Dietary calcium, blood pressure and cell membrane cation transport systems in males. Journal of Hypertension 1995;13(8):875‐82. [DOI] [PubMed] [Google Scholar]; Petrov V, Lijnen P. Modification of intracellular calcium and plasma renin by dietary calcium in men. American Journal of Hypertension 1999;12(12 Pt 1‐2):1217‐24. [DOI] [PubMed] [Google Scholar]
- Lyle RM, Melby CL, Hyner GC. Metabolic differences between subjects whose blood pressure did or did not respond to oral calcium supplementation. American Journal of Clinical Nutrition 1988;47(6):1030‐5. [DOI] [PubMed] [Google Scholar]; Lyle RM, Melby CL, Hyner GC, Edmondson JW, Miller JZ, Weinberger MH. Blood pressure and metabolic effects of calcium supplementation in normotensive white and black men. JAMA 1987;257(13):1772‐6. [PubMed] [Google Scholar]
- Lyle RM. Does baseline serum total calcium level influence the blood pressure response to calcium supplementation? A double‐blind study. Netherlands Journal of Medicine 1992;41(1‐2):48‐55. [PubMed] [Google Scholar]
- McCarron DA, Morris CD. Blood pressure response to oral calcium in persons with mild to moderate hypertension. A randomized, double‐blind, placebo‐controlled, crossover trial. Annals of Internal Medicine 1985;103(6 ( Pt 1)):825‐31. [DOI] [PubMed] [Google Scholar]
- Reid IR, Horne A, Mason B, Ames R, Bava U, Gamble GD. Effects of calcium supplementation on body weight and blood pressure in normal older women: a randomized controlled trial. Journal of Clinical Endocrinology &Metabolism 2005;90(7):3824‐9. [DOI] [PubMed] [Google Scholar]
- Reid IR, Ames R, Mason B, Bolland MJ, Bacon CJ, Reid HE, et al. Effects of calcium supplementation on lipids, blood pressure, and body composition in healthy older men: a randomized controlled trial. American Journal of Clinical Nutrition 2010;91(1):131‐9. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Willett WC, Smith A, Brown LE, Rosner B, Moore TJ. Effect on blood pressure of potassium, calcium, and magnesium in women with low habitual intake. Hypertension 1998;31(1):131‐8. [DOI] [PubMed] [Google Scholar]
- Shidfar F, Moghayedi M, Kerman SR, Hosseini S, Shidfar S. Effects of a calcium supplement on serum lipoproteins, apolipoprotein B, and blood pressure in overweight men. International Journal of Endocrinology and Metabolism 2010;8(4):194‐200. [Google Scholar]
- Thomsen K, Nilas L, Christiansen C. Dietary calcium intake and blood pressure in normotensive subjects. Acta Medica Scandinavica 1987;222(1):51‐6. [DOI] [PubMed] [Google Scholar]
- Beresteyn EC, Schaafsma G, Waard H. Oral calcium and blood pressure: a controlled intervention trial. American Journal of Clinical Nutrition 1986;44(6):883‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Bostick RM, Fosdick L, Grandits GA, Grambsch P, Gross M, Louis TA. Effect of calcium supplementation on serum cholesterol and blood pressure. A randomized, double‐blind, placebo‐controlled, clinical trial. Archives of Family Medicine 2000;9(1):31‐8; Discussion 39. [DOI] [PubMed] [Google Scholar]
- Dwyer JH, Dwyer KM, Scribner RA, Sun P, Li L, Nicholson LM, et al. Dietary calcium, calcium supplementation, and blood pressure in African American adolescents. American Journal of Clinical Nutrition 1998;68(3):648‐55. [DOI] [PubMed] [Google Scholar]
- Eftekhari MH, Rajaeifard AR, Ahmadi A, Kashfi SM, Khajeh Rahim AA. Effect of two isocaloric diets, low fat‐ high calcium and low fat‐ high fiber on weight reduction, lipid profile, and blood pressure. International Cardiovascular Research Journal 2009;3(4):200‐6. [Google Scholar]
- Karanja N, Morris CD, Illingworth DR, McCarron DA. Plasma lipids and hypertension: response to calcium supplementation. American Journal of Clinical Nutrition 1987;45(1):60‐5. [DOI] [PubMed] [Google Scholar]
- Luft FC, Aronoff GR, Sloan RS, Fineberg NS, Weinberger MH. Short‐term augmented calcium intake has no effect on sodium homeostasis. Clinical Pharmacology and Therapeutics 1986;39(4):414‐9. [DOI] [PubMed] [Google Scholar]
- Morris CD, Karaja N, McCarron DA. Dietary vs supplemental calcium to reduce blood pressure. Western Section Hypertension. 1988:139A. [Google Scholar]
- Pan WH, Wang CY, Li LA, Kao LS, Yeh SH. No significant effect of calcium and vitamin D supplementation on blood pressure and calcium metabolism in elderly Chinese. Chinese Journal of Physiology 1993;36(2):85‐94. [PubMed] [Google Scholar]
- Pan Z, Zhao L, Guo D, Yang R, Xu C, Wu X. Effects of oral calcium supplementation on blood pressure in population. Zhonghua Yu Fang Yi Xue Za Zhi 2000;34(2):109‐12. [PubMed] [Google Scholar]
- Shalileh M, Shidfar F, Haghani H, Eghtesadi S, Heydari I. The influence of calcium supplement on body composition, weight loss and insulin resistance in obese adults receiving low calorie diet. Journal of Research in Medical Sciences 2010;15(4):191‐201. [PMC free article] [PubMed] [Google Scholar]
- Smith AN. The Effect of Calcium Supplementation on Blood Pressure of Black Women. Chappel Hill: University of North Carolina, 1987. [Google Scholar]
- Weinberger MH, Wagner UL, Fineberg NS. The blood pressure effects of calcium supplementation in humans of known sodium responsiveness. American Journal of Hypertension 1993;6(9):799‐805. [DOI] [PubMed] [Google Scholar]
Additional references
- Allen L, Benoist B, Dary O, Hurrel R. Guidelines on food fortification with micronutrients (WHO/FAO). //www.who.int/nutrition/publications/micronutrients/9241594012/en/. WHO/FAO, (accessed 16th May 2015).
- Allender PS, Cutler JA, Follmann D, Cappuccio FP, Pryer J, Elliott P. Dietary calcium and blood pressure: a meta‐analysis of randomized clinical trials. Annals of Internal Medicine 1996;124(9):825‐31. [DOI] [PubMed] [Google Scholar]
- Belizan JM, Villar J. The relationship between calcium intake and edema‐, proteinuria‐, and hypertension‐gestosis: an hypothesis. American Journal of Clinical Nutrition 1980;33(10):2202‐10. [DOI] [PubMed] [Google Scholar]
- Belizan JM, Villar J, Repke J. The relationship between calcium intake and pregnancy‐induced hypertension: up‐to‐date evidence. American Journal of Obstetrics and Gynecology 1988;158(4):898‐902. [DOI] [PubMed] [Google Scholar]
- Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008;336(7638):262‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero Oscar A, Oparil Suzanne. Essential Hypertension: Part I: Definition and Etiology. Circulation 2000;101:329‐35. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42(6):1206‐52. [PUBMED: 14656957] [DOI] [PubMed] [Google Scholar]
- Ciapponi A, Glujovsky D, Bardach A, García Martí S, Comande D. EROS: a new software for early stage of systematic reviews. HTAi 2011 Conference. Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Archives of Internal Medicine 1995;155(7):701‐9. [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses' Health Study II. Archives of Internal Medicine 2004;164(8):885‐91. [DOI] [PubMed] [Google Scholar]
- Dickinson HO, Nicolson D, Cook JV, Campbell F, Beyer FR, Ford GA, et al. Calcium supplementation for the management of primary hypertension in adults. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004639.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet 2002;360(9343):1347‐60. [PUBMED: 12423980] [DOI] [PubMed] [Google Scholar]
- Garrett J, Ruel MT. The coexistence of child undernutrition and maternal overweight: prevalence, hypotheses, and programme and policy implications. Maternal and Child Nutrition 2005;1(3):185‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glujovsky D, Bardach A, García Martí S, Comande D, Ciapponi A. New software for early stage of systematic reviews. XVIII Cochrane Colloquium. The Joint Colloquium of the Cochrane & Campbell Collaborations. Keystone Resort, Colorado, USA, 2010. [Google Scholar]
- Griffith LE, Guyatt GH, Cook RJ, Bucher HC, Cook DJ. The influence of dietary and nondietary calcium supplementation on blood pressure. American Journal of Hypertension 1999;12(1):92. [DOI] [PubMed] [Google Scholar]
- Guyatt, G, Oxman, A. D, Akl, E. A, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology2011; Vol. 64, issue 4:383‐94. [DOI] [PubMed]
- Harris SS. The effect of calcium consumption on iron absorption and iron status. Nutrition in Clinical Care 2002;5(5):231‐5. [DOI] [PubMed] [Google Scholar]
- Hatton DC, Harrison‐Hohner J, Coste S, Reller M, McCarron D. Gestational calcium supplementation and blood pressure in the offspring. American journal of hypertension 2003;16(10):801‐5. [PUBMED: 14553957] [DOI] [PubMed] [Google Scholar]
- Heaney RP. Calcium intake and disease prevention. Arquivos Brasileiros de Endocrinologia e Metabologia 2006;50(4):685‐93. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from cochrane‐handbook.org.
- Hofmeyr GJ, Lawrie TA, Atallah ÁN, Duley L, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001059.pub4; CD001059] [DOI] [PubMed] [Google Scholar]
- Ilich‐Ernst JZ, McKenna AA, Badenhop NE, Clairmont AC, Andon MB, Nahhas RW, et al. Iron status, menarche, and calcium supplementation in adolescent girls. American Journal of Clinical Nutrition1998; Vol. 68, issue 4:880‐7. [DOI] [PubMed]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Women's Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. New England Journal of Medicine2006; Vol. 354, issue 7:669‐83. [DOI] [PubMed]
- Kalkwarf HJ, Harrast SD. Effects of calcium supplementation and lactation on iron status. American Journal of Clinical Nutrition 1998 ;67(6):1244‐9. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. Journal of Hypertension2004 ; Vol. 22, issue 1:11‐9. [DOI] [PubMed]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365(9455):217‐23. [DOI] [PubMed] [Google Scholar]
- Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood‐pressure‐related disease 2001. Lancet2008; Vol. 371, issue 9623:1513‐8. [DOI] [PubMed]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360(9349):1903‐13. [DOI] [PubMed] [Google Scholar]
- Llanos A, Oyarzún MT, Bonvecchio A, Rivera JA, Uauy R. Are research priorities in Latin America in line with the nutritional problems of the population?. Public Health Nutrition 2008;11(5):466‐77. [DOI] [PubMed] [Google Scholar]
- Rapsomaniki E, Timmis A, George J, Pujades‐Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1·25 million people. Lancet 2014;383(9932):1899‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Sabbagh Z, Vatanparast H. Is calcium supplementation a risk factor for cardiovascular diseases in older women?. Nutrition Reviews 2009;67(2):105‐8. [DOI] [PubMed] [Google Scholar]
- Selmer RM, Kristiansen IS, Haglerod A, Graff‐Iversen S, Larsen HK, Meyer HE, et al. Cost and health consequences of reducing the population intake of salt. Journal of Epidemiology and Community Health 2000;54(9):697‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoll LJ, Dawson‐Hughes B. Calcium supplementation and plasma ferritin concentrations in premenopausal women. American Journal of Clinical Nutrition 1992;56(6):1045‐8. [DOI] [PubMed] [Google Scholar]
- Stamler J. Blood pressure and high blood pressure. Aspects of risk. Hypertension1991; Vol. 18, issue 3 Suppl:I95‐107. [DOI] [PubMed]
- Mierlo LA, Arends LR, Streppel MT, Zeegers MP, Kok FJ, Grobbee DE, et al. Blood pressure response to calcium supplementation: a meta‐analysis of randomized controlled trials. Journal of Human Hypertension 2006;20(8):571‐80. [DOI] [PubMed] [Google Scholar]
- Webb RC. Smooth muscle contraction and relaxation. Advances in Physiology Education2003; Vol. 27, issue 1‐4:201‐6. [DOI] [PubMed]
- Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 2002;288(15):1882‐8. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Calcium and Magnesium in Drinking water. Geneva: World Health Organization, 2009. [Google Scholar]
- Williams CP, Child DF, Hudson PR, Davies GK, Davies MG, John R, et al. Why oral calcium supplements may reduce renal stone disease: report of a clinical pilot study. Journal of Clinical Pathology 2001 ;54(1):54‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. High blood pressure in young people and premature death. BMJ 2011;22:342:d1104. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Cormick Gabriela, Ciapponi Agustín, Mazzoni Agustina, Belizán José M, Cafferata María Luisa. Calcium supplementation for prevention of primary hypertension. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD010037] [DOI] [PMC free article] [PubMed] [Google Scholar]


