Abstract
Background
Observational evidence suggests a potential benefit with several anti‐adhesion therapies in women undergoing operative hysteroscopy (e.g. insertion of an intrauterine device or balloon, hormonal treatment, barrier gels or human amniotic membrane grafting) for decreasing intrauterine adhesions (IUAs).
Objectives
To assess the effectiveness of anti‐adhesion therapies versus placebo, no treatment or any other anti‐adhesion therapy, following operative hysteroscopy for treatment of female subfertility.
Search methods
We searched the following databases from inception to June 2017: the Cochrane Gynaecology and Fertility Group Specialised Register; the Cochrane Central Register of Studies (CRSO); MEDLINE; Embase; CINAHL and other electronic sources of trials, including trial registers, sources of unpublished literature and reference lists. We handsearched the Journal of Minimally Invasive Gynecology, and we contacted experts in the field. We also searched reference lists of appropriate papers.
Selection criteria
Randomised controlled trials (RCTs) of anti‐adhesion therapies versus placebo, no treatment or any other anti‐adhesion therapy following operative hysteroscopy in subfertile women. The primary outcome was live birth. Secondary outcomes were clinical pregnancy, miscarriage and IUAs present at second‐look hysteroscopy, along with mean adhesion scores and severity of IUAs.
Data collection and analysis
Two review authors independently selected studies, assessed risk of bias, extracted data and evaluated quality of evidence using the GRADE method.
Main results
The overall quality of the evidence was low to very low. The main limitations were serious risk of bias related to blinding of participants and personnel, indirectness and imprecision. We identified 16 RCTs comparing a device versus no treatment (two studies; 90 women), hormonal treatment versus no treatment or placebo (two studies; 136 women), device combined with hormonal treatment versus no treatment (one study; 20 women), barrier gel versus no treatment (five studies; 464 women), device with graft versus device without graft (three studies; 190 women), one type of device versus another device (one study; 201 women), gel combined with hormonal treatment and antibiotics versus hormonal treatment with antibiotics (one study; 52 women) and device combined with gel versus device (one study; 120 women). The total number of participants was 1273, but data on 1133 women were available for analysis. Only two of 16 studies included 100% infertile women; in all other studies, the proportion was variable or unknown.
No study reported live birth, but some (five studies) reported outcomes that were used as surrogate outcomes for live birth (term delivery or ongoing pregnancy).
Anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy.
There was insufficient evidence to determine whether there was a difference between the use of a device or hormonal treatment compared to no treatment or placebo with respect to term delivery or ongoing pregnancy rates (odds ratio (OR) 0.94, 95% confidence interval (CI) 0.42 to 2.12; 107 women; 2 studies; I² = 0%; very‐low‐quality evidence).
There were fewer IUAs at second‐look hysteroscopy using a device with or without hormonal treatment or hormonal treatment or barrier gels compared with no treatment or placebo (OR 0.35, 95% CI 0.21 to 0.60; 560 women; 8 studies; I² = 0%; low‐quality evidence). The number needed to treat for an additional beneficial outcome (NNTB) was 9 (95% CI 5 to 17).
Comparisons of different anti‐adhesion therapies following operative hysteroscopy
It was unclear whether there was a difference between the use of a device combined with graft versus device only for the outcome of ongoing pregnancy (OR 1.48, 95% CI 0.57 to 3.83; 180 women; 3 studies; I² = 0%; low‐quality evidence). There were fewer IUAs at second‐look hysteroscopy using a device with or without graft/gel or gel combined with hormonal treatment and antibiotics compared with using a device only or hormonal treatment combined with antibiotics, but the findings of this meta‐analysis were affected by evidence quality (OR 0.55, 95% CI 0.36 to 0.83; 451 women; 5 studies; I² = 0%; low‐quality evidence).
Authors' conclusions
Implications for clinical practice
The quality of the evidence ranged from very low to low. The effectiveness of anti‐adhesion treatment for improving key reproductive outcomes or for decreasing IUAs following operative hysteroscopy in subfertile women remains uncertain.
Implications for research
More research is needed to assess the comparative safety and (cost‐)effectiveness of different anti‐adhesion treatments compared to no treatment or other interventions for improving key reproductive outcomes in subfertile women.
Plain language summary
Anti‐adhesion treatment after hysteroscopy for women having difficulty becoming pregnant
Review question
To assess the effects of treatments for prevention of scar tissue (called adhesions) anti‐adhesion treatment) inside the womb after surgical treatment in women having difficulty becoming pregnant.
Background
Abdominal adhesions are web‐like structures where two normally separate surfaces in the tummy (abdomen) stick together due to damage to the lining of the abdomen. They commonly form after surgery to the abdomen. They can cause multiple conditions such as chronic pelvic pain and infertility. The present practice is based on tradition or observational studies.
Study characteristics
We searched for studies that randomly compared any treatment versus no treatment, placebo (pretend treatment) or any other intervention. Outcomes were live birth, clinical pregnancy, miscarriage and presence or severity of scar tissue at the second‐look procedure.
Key results
We found 16 studies. Treatments included using a device versus no treatment (two studies; 90 women), hormonal treatment versus no treatment or placebo (two studies; 136 women), device combined with hormonal treatment versus no treatment (one study; 20 women), barrier gel versus no treatment (five studies; 464 women), device with the use of membranes of the afterbirth of newborn babies versus device without membranes (three studies; 190 women), one type of device versus another device (one study; 201 women), gel combined with hormonal treatment and antibiotics versus hormonal treatment with antibiotics (one study; 52 women) or device combined with gel versus device (one study; 120 women). From 1273 randomly assigned women, data on 1133 women were available for analysis.
In only two studies, all women had difficulty becoming pregnant. Most studies (14/16) were at high risk of bias for at least one reason. As no study reported live births, we also included data on term delivery or ongoing pregnancy, which five studies reported.
It was unclear whether there was a difference between anti‐adhesion treatment compared to no treatment (two studies; 107 women) or to other treatment (three studies; 180 women) for increasing the chance of a liveborn baby, a term delivery or an ongoing pregnancy. The use of some anti‐adhesion therapies (device with or without hormonal treatment or hormonal treatment or gels) (eight studies; 560 women) may diminish the risk of scar tissue formation compared to no treatment. We would expect that out of 1000 women treated by surgery, between 153 and 365 women would develop scar tissue after using gels, compared with 545 women when no treatment was used. The evidence was current to 6 June 2017.
Quality of the evidence
The overall quality of the study evidence ranged from very low to low. There were limitations to the studies, for example, a serious risk of bias related to participants and investigators knowing what treatment was given.
More research is needed before anti‐adhesion treatment can be offered in everyday clinical practice after surgery of the womb in women having difficulty becoming pregnant.
Summary of findings
Summary of findings for the main comparison. Any anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy.
| Any anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy | ||||||
|
Patient or population: women treated by operative hysteroscopy for uterine pathology associated with subfertility or adverse pregnancy outcome Settings: single centre, Hysteroscopy Unit or Department of Obstetrics and Gynaecology of a university or non‐university tertiary care hospital Intervention: any anti‐adhesion therapy Comparison: no treatment or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment or placebo | Anti‐adhesion therapy | |||||
| Live birth a | No treatment or placebo | Device or hormonal treatment |
OR 0.94 (0.42 to 2.12) |
107 (2 RCTs) | ⊕⊝⊝⊝ Very low c,d,e | ‐ |
| Mean‐risk populationb | ||||||
| 407 per 1000 | 399 per 1000 (261 to 603) | |||||
|
Presence of intrauterine adhesions at second‐look hysteroscopy (second‐look hysteroscopy at 4‐12 weeks after operative hysteroscopy) |
No treatment or placebo | Device ± hormonal treatment or hormonal treatment or barrier gel | OR 0.35 g (0.21 to 0.60) | 560 (8 RCTs) |
⊕⊕⊝⊝ Low h,i | ‐ |
| Low‐risk populationf | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population f | ||||||
| 545 per 1000 | 234 per 1000 (153 to 365) | |||||
| High‐risk population f | ||||||
| 875 per 1000 | 376 per 1000 (245 to 586) | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a The two included studies reported term delivery (Abu Rafea 2013) or ongoing pregnancy (Roy 2014), which we used as a surrogate outcome for live birth.
b The assumed risk for the mean‐risk population was the pooled risk of all live births in control groups of the two included studies.
c Downgraded one level for serious risk of bias: one study was at high risk of bias in several domains, including allocation concealment.
d Downgraded one level for serious imprecision; only 43 events in total.
e Downgraded one level for serious indirectness, because only 30% (35/118) of all randomised women in this analysis were subfertile.
f The assumed risk for low‐, medium‐ and high‐risk population based on presence of intrauterine adhesions following hysteroscopic removal of endometrial polyps/following removal of submucous fibroids and intrauterine adhesions (mean of both)/removal of uterine septum, respectively, based on findings of a prospective cohort study (Yang 2013).
G Two studies reported no events (Lin 2015a; Vercellini 1989).
h Downgraded one level for serious risk of bias: all eight studies had several limitations but none was at high risk for selection bias related to random sequence generation or allocation concealment.
i Downgraded one level for serious indirectness, because in four of eight studies less than 50% of participants were subfertile and in four of eight studies it was unclear whether subfertile women were included.
Summary of findings 2. Any anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy.
| Any anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy | ||||||
|
Patient or population: women treated by operative hysteroscopy for uterine pathology Settings: multicentric, Hysteroscopy Unit of Department of Obstetrics and Gynaecology of a university, university‐affiliated or non‐university tertiary care hospital Intervention: anti‐adhesion therapy A Comparison: anti‐adhesion therapy B | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Anti‐adhesion therapy B | Anti‐adhesion therapy A | |||||
| Live birth a | Device | Device + graft |
OR 1.48 (0.57 to 3.83) |
180 (3 RCTs) |
⊕⊕⊝⊝ Low c,d |
‐ |
| 98 per 1000 b |
138 per 1000 (60 to 315) |
|||||
|
Presence of intrauterine adhesions at second‐look hysteroscopy (6‐12 weeks) |
Device or hormonal treatment with antibiotics | Device ± graft/gel or gel + hormonal treatment + and antibiotics | OR 0.55 (0.36 to 0.83) | 451 (5 RCTs) |
⊕⊕⊝⊝ Low f,g | ‐ |
| Low‐risk population e | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population e | ||||||
| 545 per 1000 | 403 per 1000 (327 to 496) | |||||
| High‐risk population e | ||||||
| 875 per 1000 | 647 per 1000 (525 to 796) | |||||
| *The basis for the assumed risk is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a The three included studies reported term delivery (Wang 2016) or ongoing pregnancy (Amer 2010; Gan 2017; Wang 2016), which we used as a surrogate outcome for live birth.
b The assumed risk for the average‐risk population is the pooled risk of all the live births in the control groups of the three included studies.
c Downgraded one level for serious risk of bias: despite several limitations none of the studies was at high risk for selection bias related to random sequence generation or allocation concealment.
d Downgraded one level for serious imprecision‐ only 21 events in total.
e The assumed risk for low/medium/high‐risk population is based on the presence of intrauterine adhesions following hysteroscopic removal of endometrial polyps/following removal of submucous fibroids and IUAs (mean of both)/removal of uterine septum, respectively, based on findings of a prospective cohort study (Yang 2013).
f Downgraded one level for serious risk of bias: despite several limitations none of the studies was at high risk for selection bias related to random sequence generation or allocation concealment.
g Downgraded one level for serious indirectness because, in two of five studies, less than 50% of participants were subfertile; in one of five studies, it was unclear if subfertile women were included and in two of five studies, the proportion of infertile women was not reported.
Background
Description of the condition
Intrauterine adhesions (IUAs) are fibrous strings at opposing walls of the uterus. The spectrum of severity of IUAs ranges from minimal to complete obliteration of the uterine cavity. Any trauma to the endometrium (the inner layer of the uterus) can lead to formation of IUAs; in daily clinical practice, nearly 90% of all IUAs are associated with postpartum or postabortion dilatation and curettage (Nappi 2007). The aetiological role of infection in the formation of IUAs is controversial, with the exception of genital tuberculosis (Deans 2010). IUA formation is the major long‐term complication of hysteroscopic surgery in women of reproductive age.
Several intrauterine anomalies have been linked with female subfertility (Bosteels 2015a). Endometrial polyps are benign, endometrial, stalk‐like masses protruding into the uterine cavity. Fibroids are excessive growths originating from the muscular portion of the uterine cavity. A septate uterus is a congenital malformation in which the longitudinal band separating left and right Müllerian ducts, which form the uterus in the human female foetus, has not been entirely resorbed. Hysteroscopy allows direct visualisation of the uterine cavity through a rigid, semi‐rigid or flexible endoscope. The hysteroscope consists of a rigid telescope with a proximal eyepiece and a distal objective lens that may be angled at 0 degrees to allow direct viewing, or offset at various angles to provide a fore‐oblique view. Operative hysteroscopy requires adequate visualisation through continuous fluid circulation using inflow and outflow channels. The sheath system of the operative hysteroscope contains one or two 1.6‐ to 2.0‐mm working channels for insertion of a small grasping or biopsy forceps, scissors, myoma fixation instruments, retraction loops, morcellator (surgical instruments used to divide and remove tissue during endoscopic surgery) and aspiration cannulae or unipolar or bipolar electrodiathermy instruments. Operative hysteroscopic procedures require a complex instrumentation setup, special training of the surgeon, and appropriate knowledge and management of complications. Removal of endometrial polyps by an endoscope is called hysteroscopic polypectomy. Hysteroscopic myomectomy is the procedure by which a fibroid is removed by hysteroscopy. Removal of a uterine septum is termed hysteroscopic septoplasty or septum resection. Removal of IUAs is called hysteroscopic adhesiolysis. A diagnostic or operative hysteroscopy following an operative hysteroscopy is termed a second‐look hysteroscopy.
One randomised controlled trial (RCT) reported the following numbers for the incidence of postsurgical IUAs at second‐look hysteroscopy: 3.6% after polypectomy, 6.7% after resection of uterine septa, 31.3% after removal of a solitary myoma and 45.5% after resection of multiple myomas (Taskin 2000). Mechanisms of tissue repair in the human endometrium are poorly understood (Revaux 2008) despite several hypotheses on the origin of cells for endometrial regeneration (Okulicz 2002). Endometrial stem or progenitor cells, present in women and rodents, may have an important function for endometrial regeneration in normal menstrual cycles and after delivery; this holds promise for new treatments for subfertility associated with IUAs or Asherman's syndrome (Deane 2013). The duration of endometrial wound healing depends on the type of pathology present, according to one prospective cohort study of 163 women undergoing operative hysteroscopy (Yang 2013); these investigators reported that the time needed for complete recovery of the endometrium ranges from one month following hysteroscopic removal of endometrial polyps to three months for the hysteroscopic treatment of submucous fibroids.
IUAs are associated with poor reproductive outcomes. This is due in part to infertility, with a prevalence as high as 43% (922/2151 women) according to one large review of observational studies (Schenker 1982). Poor outcomes also result from the clinical problem of recurrent miscarriage, ranging from 5% to 39% in women with IUAs, according to one review of observational studies (Kodaman 2007), and from major, and at times devastating, obstetrical complications, for example, placenta accreta or increta, as well as higher risks for preterm delivery, uterine rupture and peripartum hysterectomy as the endpoint of a successful hysteroscopic treatment for severe IUAs (Deans 2010).
Description of the intervention
Several observational studies have suggested different anti‐adhesion strategies for preventing IUAs following operative hysteroscopy.
Intrauterine device
An intrauterine device (IUD) may provide a physical barrier between the uterine walls, separating the endometrial layers after lysis of IUAs. At least 13 observational studies have recommended insertion of an IUD as an adjunct therapy for the prevention of IUAs (Deans 2010). Eight observational studies reported the use of a Foley catheter balloon as an alternative for similar purposes (Deans 2010).
Hormonal therapy
In 1964, Wood and Pena suggested use of oestrogen therapy to stimulate regeneration of the endometrium after surgical treatment for IUAs (Wood 1964).
Barrier gels
Hyaluronic acid (HA) or hyaluronan is a water‐soluble polysaccharide that consists of multiple disaccharide units of glucuronic acid and N‐acetylglucosamine bound together by a β1‐3‐type glucoside bond. Solutions of HA have viscoelastic properties that have led to interest in developing applications of HA in surgical procedures, for example, during eye surgery, and for prevention of postsurgical adhesions. However, HA may not be the ideal substance for all procedures because of its limited residence time when applied to a surgical site. It quickly enters the systemic circulation, then is cleared rapidly by catabolic pathways. Attempts to use HA for prevention of postsurgical adhesions have therefore resulted in variable success. Chemically modified derivatives of HA have been developed to circumvent the disadvantages of HA. One such derivative is auto‐cross‐linked polysaccharide (ACP), which is formed by cross‐linking of HA via direct formation of covalent ester bonds between hydroxyl and carboxyl groups of the HA molecule. ACP can be prepared through various degrees of cross‐linking: this allows tailoring of the viscosity properties of ACP gels (Renier 2005). Carboxymethylcellulose (CMC) is a high‐molecular‐weight polysaccharide that has greater viscosity than dextran 70. CMC can be used for adhesion prevention as a membrane barrier, or as a gel attained by mixing chemically derivative sodium hyaluronate and carboxymethylcellulose gel (HA‐CMC) (Leach 1998).
Human amniotic membrane grafting
Since the late 1990s, the surgical community has become more aware of the increasing potential of human amniotic membrane (HAM) as an adjunctive anti‐adhesion intervention. Human whole foetal membranes or amnion alone has been used in surgery to aid the repair of surface epithelial defects in the skin, eye, abdominal wall and peritoneum. HAM grafting has not been very popular in the field of obstetrics and gynaecology; its clinical use is limited as a graft in forming an artificial vagina, as a barrier in preventing postoperative intra‐abdominal adhesion formation and, finally, as a biological dressing following radical vulvectomy or groin dissection (Amer 2006).
How the intervention might work
Hypothetical underlying mechanisms of subfertility associated with IUAs include obstruction of sperm transport into the cervix, impaired embryo migration within the uterine cavity and failure of embryo implantation due to endometrial insufficiency (Deans 2010). Ideal anti‐adhesion adjunctive therapy following operative hysteroscopy would include application of a biologically active mechanical separator that achieves suppression of IUA formation and promotes healing of the endometrium. The bulk of evidence on how different interventions might work has been derived from observational or animal studies, largely in rodents and regrettably not in animal models validated for the study of human reproduction, such as primates (D'Hooghe 2009).
Intrauterine device
Use of an IUD (13 observational studies) or a Foley catheter balloon (eight observational studies) is often recommended following hysteroscopic treatment of IUAs or septoplasty, to act as a physical barrier separating opposing walls of the uterine cavity (Deans 2010). The type of IUD selected may be important; copper‐containing IUDs provoke an inflammatory reaction, probably with detrimental effects, whereas T‐shaped IUDs might provide too small a surface area to be truly effective in providing an efficient physical barrier. The loop IUD (e.g. the Lippes loop) is generally considered the IUD of choice for treatment of IUAs; however, it is no longer available in many countries (Kodaman 2007). One clinical controlled trial (CCT) compared use of a Foley catheter balloon for 10 days (59 women) versus insertion of an IUD for a three‐month period (51 women); fertility rates were poor in both the IUD group (20/59 women, or 34%) and the Foley catheter balloon group (14/51 women, or 28%) (Orhue 2003).
Hormonal therapy
Many studies recommend use of a cyclical oestrogen and progestogen treatment regimen following hysteroscopic treatment of IUAs to promote regeneration of the endometrium (Deans 2010). Various regimens consisting of oestrogen (e.g. conjugated equine oestrogen 2.5 mg twice daily for 30 days) with or without a progestogen (e.g. medroxyprogesterone acetate 10 mg for 10 days) have been proposed (Kodaman 2007). There are no comparative studies that examine dosage, administration or combinations of hormones (Deans 2010). In one RCT, 60 women undergoing dilatation and curettage during the first trimester of pregnancy were allocated to receive oestrogen combined with progestogen or no treatment (Farhi 1993). Women in the intervention group had a significantly thicker endometrium compared with women in the control group (8.4 with intervention vs 6.7 mm with no treatment; P = 0.02). Study authors concluded that postoperative hormonal treatment may be beneficial for IUA prevention following surgical trauma to the uterine cavity. Nevertheless, they provided no data on pregnancy outcomes or IUA recurrence (Farhi 1993). One systematic review of 26 observational studies concluded that hormonal therapy, particularly oestrogen treatment, may be beneficial for women with IUAs, but as adjunctive therapy combined with other anti‐adhesion strategies (Johary 2014).
Barrier gels
Use of biodegradable gel surgical barriers is based on the principle of keeping adjacent wound surfaces mechanically separate (Renier 2005). Several preclinical studies in various animal models demonstrated the effectiveness of ACP (Belluco 2001; Binda 2007; Binda 2009; Binda 2010; De Iaco 1998; Koçak 1999; Shamiyeh 2007; Wallwiener 2006), and HA‐CMC gels (Leach 1998; Schonman 2008), or of HA‐CMC membranes (Kelekci 2004; Rajab 2010), for preventing postsurgical adhesions. Other preclinical studies in animal models suggest that HA gel remains in situ longer than five to six days (Laurent 1992; Nimrod 1992). Similarly, animal studies demonstrated the persistence of HA‐CMC for about seven days after its application (Diamond 1988). The exact mechanisms by which ACP and HA‐CMC are able to reduce adhesion reformation are not well known but may be related to 'hydroflotation' or 'siliconising' effects. One French CCT (54 women) compared application of ACP gel (30 women) versus no gel (24 women) at the end of an operative hysteroscopic procedure performed to treat myomas, polyps, uterine septa or IUAs; investigators reported no statistically significant differences between comparison groups in the rate of adhesion formation, or in mean adhesion scores and severity of adhesions (Ducarme 2006). They provided no data on reproductive outcomes.
Human amniotic membrane grafting
Preclinical data on the effectiveness of HAM grafting in different animal models presented conflicting results. One trial demonstrated a beneficial effect in preventing de novo (new) adhesions (Szabo 2002), whereas two other animal studies reported that HAM grafting failed to prevent IUAs (Arora 1994; Badawy 1989). One observational study provided data on use of a fresh amniotic graft over an inflated Foley catheter balloon to prevent recurrence of IUAs after hysteroscopic lysis in 25 women with moderate‐to‐severe Asherman's syndrome. There was minimal adhesion reformation in 48% of study participants with severe adhesions. Study authors concluded that HAM grafting might be promising as adjunctive therapy following hysteroscopic adhesiolysis; it acts as a biologically active mechanical barrier to suppress adhesion formation while promoting endometrial healing (Amer 2006). A fresh HAM graft preserves its viability for 21 days following application in the pelvic cavity (Trelford Sauder 1977). In addition to serving as an anatomical barrier, HAM may promote the regeneration of epithelium by acting as a basement membrane substrate; HAM may also facilitate migration of epithelial cells, reinforce adhesion of the basal epithelium, promote epithelial cell differentiation (Meller 1999), and prevent cellular apoptosis (Hori 2006). Human amniotic epithelial cells produce factors or create a microenvironment for effective tissue repair and endometrial regeneration, possibly by stimulating endogenous stem cells (Padykula 1991).
Why it is important to do this review
At present, whether anti‐adhesion therapies after operative hysteroscopy might be beneficial for the outcome of pregnancy or live birth is unknown, and there are no relevant clinical guidelines. Providing a summary and critical appraisal of existing evidence on the effectiveness of different anti‐adhesion treatments in subfertile women after operative hysteroscopy is the main objective of this Cochrane Review. Moreover, little is known about the relative contributions of different anti‐adhesion strategies towards increasing reproductive benefit in women wishing to conceive following operative hysteroscopy; performing this head‐to‐head comparison of alternative anti‐adhesion interventions is a secondary objective of the present review.
Adhesions may cause infertility, abdominal pain or bowel obstruction. The healthcare burden associated with these three clinical problems is substantial (DeCherney 1997; diZerega 1994; Renier 2005). The total cost of adhesion‐related morbidity for the US health care system exceeds USD1 billion annually (Baakdah 2005). One trial in the domain of gynaecological oncology evaluated the cost‐effectiveness of an HA‐CMC anti‐adhesion barrier versus routine care, during which no adhesion prevention measures were taken, by applying a decision analysis model in the setting of women undergoing radical hysterectomy and pelvic lymphadenectomy for stage IB cervical cancer (Bristow 2007). Study authors concluded that given a conservative set of clinical and economic assumptions, an adhesion prevention strategy utilising an HA‐CMC barrier in women undergoing radical hysterectomy for stage IB cervical cancer might be cost‐effective from the perspective of society and from the view of a third‐party payer. To the best of our knowledge, no cost‐effectiveness studies have explored adhesion prevention after operative hysteroscopy in an infertile population; evidence retrieved through the present research could serve as the basis for economical studies of different anti‐adhesion treatments. This is another secondary objective of the present review.
Infertility, defined as the inability to conceive after a defined period of unprotected intercourse, is an often neglected aspect of reproductive health worldwide. Official ways of providing assistance for reproductive health care and family planning are few worldwide, despite an increasing absolute number of couples affected by infertility from 42.0 million in 1990 to 48.5 million in 2010 (Mascarenhas 2012). Reproductive health has long been recognised by the World Health Organization (WHO) as a priority global health topic (WHO: Reproductive Health).
Objectives
To assess the effectiveness of anti‐adhesion therapies versus placebo, no treatment or any other anti‐adhesion therapy following operative hysteroscopy for treatment of female subfertility.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished parallel‐group RCTs were eligible for inclusion. We excluded non‐randomised studies (e.g. studies with evidence of inadequate sequence generation such as alternate days, participant numbers), as they are associated with high risk of bias. We planned to include cross‐over trials if individually randomly assigned women were the unit of analysis; we aimed to include data from the first phase only in the meta‐analyses, as the cross‐over trial is not a valid study design in the context of subfertility.
Types of participants
Women of reproductive age undergoing operative hysteroscopy for subfertility associated with suspected or unsuspected intrauterine pathology before spontaneous conception or any subfertility treatment. Studies in which at least a proportion of women were undergoing operative hysteroscopy for subfertility were eligible. Studies excluding women wishing to conceive were not eligible.
Types of interventions
We included the following randomly assigned comparisons.
Anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy.
Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy.
Types of outcome measures
Primary outcomes
Live birth.
Live birth was defined as the delivery of at least one live foetus after 20 weeks of gestation that resulted in at least one live baby; we counted the delivery of singleton, twin or multiple pregnancies as one live birth.
In studies that failed to report live birth, we used the following measures as primary effectiveness outcomes:
Ongoing pregnancy, defined as pregnancy surpassing the first trimester or 12 weeks of pregnancy and was used as a surrogate outcome for live birth.
Term delivery, defined as birth at any time between three weeks before and two weeks after the expected date of delivery (37 to 42 weeks of gestation) was also used as a surrogate outcome for live birth.
Secondary outcomes
Clinical pregnancy, defined as pregnancy diagnosed by ultrasonographic visualisation of one or more gestational sacs or definitive clinical signs of pregnancy; this included ectopic pregnancy. We counted multiple gestational sacs as one clinical pregnancy.
Miscarriage, defined as spontaneous loss of a clinical pregnancy that occurred before 20 completed weeks of gestation (18 weeks' postfertilisation) or, if gestational age was unknown, loss of an embryo or foetus of bodyweight less than 400 g.
Presence of IUAs at second‐look hysteroscopy.
Mean adhesion scores at second‐look hysteroscopy.
Severity of adhesions at second‐look hysteroscopy.
We did not exclude studies on the basis of their reported outcome measures. We reviewed all potentially eligible studies that could have measured the outcomes of interest; we aimed to report any lack of data for the key outcomes in the final review.
We adhered as much as possible to terminology of the International Committee for Monitoring Assisted Reproductive Technology (ICMART) (ICMART) for key reproductive outcomes (live birth, pregnancy and miscarriage) (Zegers‐Hochschild 2009); we contacted primary study authors for clarification in cases of unclear definitions. We reported discrepancies or uncertainties in the final review.
At present, seven classification systems are reported for scoring the extent or severity of IUAs. None of these systems has been validated or universally accepted (Deans 2010). Therefore, we avoided pooling data from studies using different scoring systems, and we asked for clarification from primary study authors, when there was any uncertainty on the classification system used in the primary research.
According to a prospective cohort study, the duration of endometrial wound healing may differ according to the type of pathology; study authors concluded that recovery of the endometrium may vary from one month (after hysteroscopic removal of polyps) to three months (following hysteroscopic myomectomy) (Yang 2013). We planned to pool studies when assessment of IUAs by second‐look hysteroscopy was done between four and 12 weeks after operative hysteroscopy.
Search methods for identification of studies
We searched for all published and unpublished RCTs of anti‐adhesion therapies following operative hysteroscopy in subfertile women, with no language restrictions and in consultation with the Information Specialist of the Cochrane Gynaecology and Fertility Group (CGFG).
Electronic searches
We searched the following electronic databases, trial registers and websites using the search strategies provided in the appropriate appendices: the CGFG Specialised Register (6 June 2017) (Appendix 1), the Cochrane Central Register of Studies Online (CENTRAL) (2017, Issue 6) (Appendix 2), MEDLINE using PubMed (1950 to 6 June 2017) (Appendix 3) and Embase using Embase.com (1974 to 6 June 2017) (Appendix 4).
The search strategy combined both index and free‐text terms.
Our MEDLINE search included the Cochrane highly sensitive search strategy for identifying randomised trials as it appears in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Our Embase search included the trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN).
Electronic sources of trials included the following.
CENTRAL.
Cochrane Database of Systematic Reviews (CDSR) (2017, Issue 6).
Database of Abstracts of Reviews of Effectiveness (DARE) and the Health Technology Assessment Database (HTA Database) through the Centre for Reviews and Dissemination (www.crd.york.ac.uk) (from inception to 6 June 2017).
National Guideline Clearinghouse (www.guideline.gov/) for evidence‐based guidelines (from inception to 6 June 2017).
Citations, conference abstracts and proceedings in the Institute for Scientific Information (ISI) Web of Science (WOS) core collection, Biosis Previews and Biosis Citation Index through WOS (wcs.webofknowledge.com.kuleuven.ezproxy.kuleuven.be) (from inception to 6 June 2017) (Appendix 5) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (web.b.ebscohost.com.kuleuven.ezproxy.kuleuven.be) (from inception to 6 June 2017) (Appendix 6) through EBSCOhost, available at the Biomedical Library Gasthuisberg of the Catholic University of Leuven.
Trial registers for ongoing and registered trials: ISRCTN Registry (www.isrctn.com/) and WHO International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/) (from inception to 6 June 2017).
Latin American Caribbean Health Sciences Literature (LILACS) database, which is a source of trials from the Spanish and Portuguese speaking countries (lilacs.bvsalud.org/en/) (from inception to 6 June 2017).
European grey literature through the Open Grey database (www.opengrey.eu/) (from inception to 6 June 2017).
General search engines Turning Research Into Practice (TRIP) database (www.tripdatabase.com/), Google Scholar (scholar.google.com/) and Scopus, available at the Biomedical Library Gasthuisberg of the KU Leuven‐ University of Leuven, Leuven, Belgium (www‐scopus‐com.kuleuven.ezproxy.kuleuven.be) (from inception to 6 June 2017).
Searching other resources
Two review authors (JB and SJC) examined reference lists of articles retrieved by the search and contacted experts in the field to request additional data. We contacted the first or corresponding authors of included studies to ascertain whether they were aware of any ongoing or unpublished trials. We handsearched the Journal of Minimally Invasive Gynecology (from inception to 6 June 2017) to look for conference abstracts that were not covered in the CGFG Specialised Register, in liaison with the Information Specialist of the CGFG. We also searched reference lists of appropriate papers. We documented the search process in a PRISMA flow diagram in the final review.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search, we retrieved the full texts of all potentially eligible studies. Two review authors (JB and SJC) independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We corresponded with study investigators, as required, to clarify study eligibility. We resolved disagreements as to study eligibility by discussion or by consultation with a third review author (BWM). We classified the study as 'awaiting classification' if disagreements between review authors were not resolved, and we reported disagreements in the final review.
Data extraction and management
At least two review authors (JB for all included studies and HT/SW/SJC each for some studies) independently extracted data from all eligible studies using a data extraction form designed and pilot‐tested by the review authors. We resolved disagreements by discussion or by consultation with a third review author (BWM). Extracted data included study characteristics and outcome data (Appendix 7). When studies had multiple publications, we collated multiple reports on the same study, so that each study, rather than each report, was the unit of interest in the review, and we assigned such studies a single study identity with multiple references. We used the main trial report as the reference and derived additional details from secondary papers. We corresponded with study investigators to request further data on methods and results, as required. We included studies irrespective of whether outcomes were reported in a 'usable' way. In multiarm studies, we excluded data from arms that did not meet the eligibility criteria.
Assessment of risk of bias in included studies
At least two review authors (JB for all included studies and HT/SW/SJC each for some studies) independently assessed included studies for risk of bias using the Cochrane 'Risk of bias' tool (Higgins 2011). We assessed the following seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting and other potential sources of bias. We resolved disagreements by discussion or by consultation with a third review author (BWM). We fully described all judgements and presented our conclusions in the 'Risk of bias' table, which we incorporated into our interpretation of review findings by conducting sensitivity analyses.
Selective reporting is a type of reporting bias that affects the internal validity of an individual study (see Table 10.1A in the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011). This term refers to selective reporting of some outcomes (e.g. positive outcomes) and failure to report others (e.g. adverse events). We took care to search for within‐trial selective reporting, such as trials failing to report obvious outcomes, or failing to report them in insufficient detail to allow inclusion. We looked for published protocols and compared outcomes between the protocol and the final published study. When identified studies did not report the primary outcome of live birth but did report interim outcomes such as pregnancy, we planned to undertake informal assessment as to whether the interim values (e.g. pregnancy rates) were similar to those reported in studies that also reported live births.
If any outcomes were defined in the protocol or the study report, and data were insufficient to allow inclusion, we sought to mention this lack of data along with the suggestion that additional clinical trials need to be conducted to clarify these knowledge gaps.
Measures of treatment effect
For dichotomous data (e.g. live births, clinical pregnancy rates), we used the numbers of events in control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs). We treated ordinal data (e.g. adhesion scores) as continuous data. For continuous data, if all studies reported exactly the same outcomes, we calculated mean differences (MDs) between treatment groups. If similar outcomes were reported on different scoring scales, we did not calculate standardised mean differences (SMDs) because the seven different adhesion score classifications had not been validated. We aimed to reverse the direction of effect of individual studies, if required, to ensure consistency across trials. We presented 95% confidence intervals (CIs) for all outcomes and contacted corresponding or first authors of all included trials that reported data in a form that was not suitable for meta‐analysis. We reported data from reports that did not present additional data that could be analysed under 'other data.' When data were not available for calculating ORs or MDs, we planned to utilise the most detailed numerical data provided that might facilitate similar analyses of included studies (e.g. test statistics, P values). We compared the magnitude and direction of effect reported by studies with how they were presented in the review, while taking account of legitimate differences.
Unit of analysis issues
We performed the primary analysis per woman randomly assigned; however, we included per‐pregnancy data for one secondary outcome (miscarriage). If studies had reported only per‐cycle data, we would have contacted primary study authors to request per‐woman data. If these had been available, we would have briefly summarised per‐cycle data in an additional table without performing a meta‐analysis. We would have counted multiple live births (e.g. twins, triplets) as one live birth event only. We would have included only first‐phase data from cross‐over trials if relevant cross‐over trials had been found eligible.
Dealing with missing data
We analysed data on an intention‐to‐treat (ITT) basis; if data had been available, we would have attempted to obtain all missing data from the original researchers. If this had been impossible, we would have undertaken imputation of individual values for the beneficial primary outcome only (live birth); we would have assumed that live births did not occur in women without a reported outcome. For all other outcomes, we would have analysed only available data. We would have subjected any imputation undertaken for missing data for the primary outcome to sensitivity analysis. (See Sensitivity analysis.) If studies had reported sufficient detail to calculate MDs but had not information on associated standard deviations (SDs), we would have assumed that the outcome had an SD equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by measuring the I² statistic. We took an I² statistic greater than 50% to indicate substantial heterogeneity (Higgins 2003).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we minimised their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If we had included 10 or more studies in an analysis, we would have used a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
One review author (JB) entered the data and carried out all statistical analyses of the data in Review Manager 5 (RevMan 2014). When studies were sufficiently similar and substantial statistical heterogeneity could be confidently ruled out, we combined data derived from primary studies in a meta‐analysis using Review Manager 5 (RevMan 2014). We have used summary Mantel‐Haenszel ORs and a fixed‐effect model for the following comparisons.
Anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy.
Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy.
We considered outcomes of 'live birth' and 'clinical pregnancy' as positive outcomes of effectiveness and, as a consequence, higher numbers of these two outcomes as a benefit. We considered 'miscarriage,' 'presence of IUAs,' 'mean adhesion scores' or 'severity of adhesions' at second‐look hysteroscopy as negative outcomes of safety and interpreted higher numbers as harmful. An increase in the odds of a particular outcome that was beneficial (e.g. live birth) or detrimental (e.g. IUAs) was displayed graphically in the meta‐analyses to the right of the centre line, and a decrease in the odds of an outcome to the left of the centre line.
We defined analyses that were comprehensive and mutually exclusive, so that all eligible study results could be slotted into one stratum for each comparison, and that trials within the same stratum could be sensibly pooled. Stratification was not a requirement, but it allowed consideration of effects within each stratum as well as, or instead of, an overall estimate for the comparison. If we had retrieved no RCTs for some comparisons, we would have indicated their absence in the review to reveal knowledge gaps for which further research is needed. We would have presented a narrative overview if meta‐analysis had not been appropriate.
Subgroup analysis and investigation of heterogeneity
Where data were available, we conducted subgroup analyses to identify separate evidence within the following subgroup:
studies with HA gel versus studies with another type of gel for the primary outcome and the presence of IUAs at second‐look hysteroscopy.
We interpreted the findings of subgroup analyses cautiously, even when sufficient data were available; subgroup analysis is by itself observational in nature and the interpretation of formal statistical tests to detect differences between subgroups is problematic.
If we detected substantial heterogeneity, we explored possible explanations in the subgroup analyses (e.g. differing populations) or sensitivity analyses (e.g. differing risk of bias), or both. We took any statistical heterogeneity into account when interpreting the results.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcome to determine whether conclusions were robust to arbitrary decisions made regarding eligibility and analysis of studies. These analyses included consideration of whether review conclusions would have differed if:
only studies were included reporting the primary outcome (live birth) versus all studies reporting live birth or a surrogate outcome;
eligibility had been restricted to studies without high risk of bias;
study used only a random‐effects model;
alternative imputation strategies had been implemented;
summary effect measure had been risk ratio (RR) rather than OR.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared two 'Summary of findings' tables using GRADEpro GDT and Cochrane methods (Higgins 2011). These 'Summary of findings' tables evaluated the overall quality of the body of evidence for the two most important review outcomes (live birth as the primary outcome of effectiveness and presence of IUAs at second‐look hysteroscopy as the primary outcome of safety) for the two main review comparisons (i.e. anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy; anti‐adhesion therapy following operative hysteroscopy versus another anti‐adhesion therapy). We restricted the content of the 'Summary of findings' tables to these two main review outcomes in the interest of readability of the review. We presented the evidence for all other secondary outcomes in the text of the review. We assessed the quality of the evidence using GRADE criteria, including risk of bias, consistency of effect, imprecision, indirectness and publication bias. Two review authors independently made judgements about evidence quality (high, moderate, low or very low), with disagreements resolved by discussion. Judgements were justified, documented and incorporated into reporting of results for each outcome.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies tables.
Results of the search
Our original search retrieved 11 studies which were included in the original published version of this review in 2015. In the updated search in 2017, we identified 342 records by searching the following databases: CGFG Specialised Register (11 records), CENTRAL (14), MEDLINE (21), Embase (32), WoS (11), CINAHL (1), CRD (26), National Guideline Clearinghouse (3), ISRCTN Register of Controlled Trials (13), WHO ICTRP (22), LILACS (75), Open Grey (107) and Scopus (6). We retrieved 629 additional records through other sources: handsearch of the Journal of Minimally Invasive Gynecology (8) and handsearch of related articles on included studies (621).
After combining 342 records identified through electronic searches with 629 additional records obtained by searching other sources, we screened 971 records for duplicates using specialised software (www.myendnoteweb.com). We removed 899 duplicates. We screened 72 records for titles and abstracts: we excluded 50 records for being obviously irrelevant and six records for being duplicates. We assessed 16 full‐text articles for eligibility: we excluded 11 full‐text articles for various reasons. We identified five potentially eligible studies for the updated search. We included 16 studies in the present Cochrane Review for quantitative synthesis and critical appraisal (Characteristics of included studies table); two trials are ongoing (Characteristics of ongoing studies table) and one trial is awaiting classification (Characteristics of studies awaiting classification table).
See the PRISMA flow chart for a summary of studies retrieved by our search, including both our original search (from inception to 1 March 2015) and an updated search (from 1 March 2015 until 1 June 2017) (Figure 1).
1.
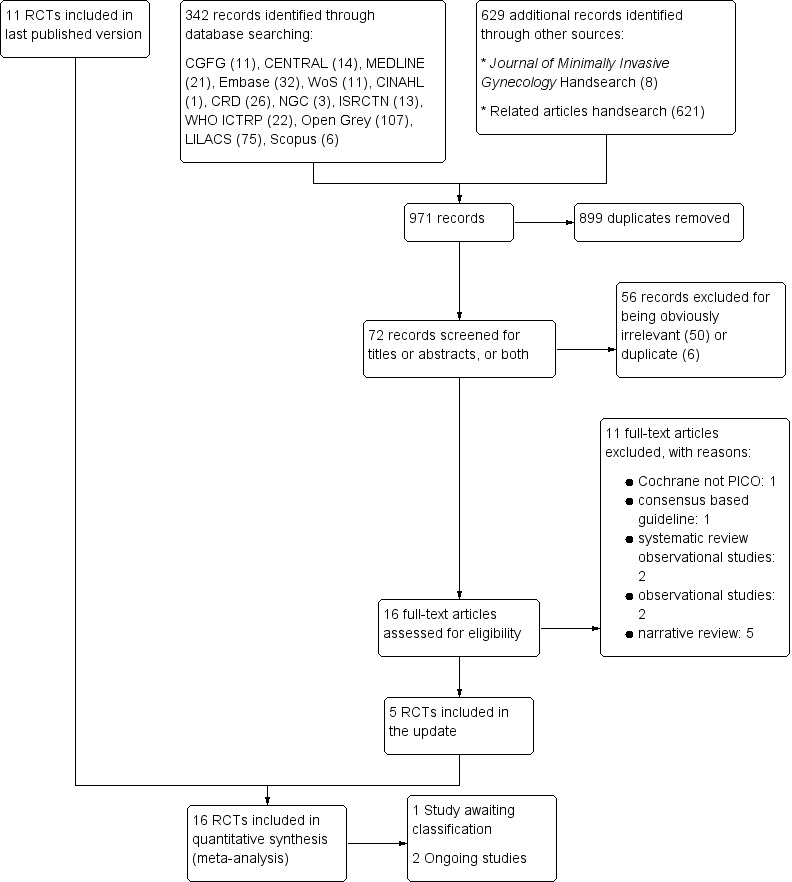
Study flow diagram: summary of searches since 2015. PICO: population, intervention, comparator, outcome; RCT: randomised controlled trial.
Included studies
Study design and setting
We included 16 parallel‐design RCTs: 15 studies used two comparison groups (Abu Rafea 2013; Acunzo 2003; Dabir‐Ashrafi 1996; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Fuchs 2014; Gan 2017; Guida 2004; Lin 2015a; Lin 2015b; Roy 2014; Vercellini 1989; Wang 2016; Xiao 2015), and one study used three comparison groups (Amer 2010). All but one (Xiao 2015) were single‐centre studies: six from Italy (Acunzo 2003; De Iaco 2003; Guida 2004; Di Spiezio Sardo 2011; Guida 2004; Vercellini 1989), four from China (Gan 2017; Lin 2015b; Wang 2016; Xiao 2015), one from Egypt (Amer 2010), one from Saudi Arabia (Abu Rafea 2013), one from Iran (Dabir‐Ashrafi 1996), one from India (Roy 2014), one from Taiwan (Lin 2015a), and one from South Korea (Do 2005).
Funding sources
See Characteristics of included studies table.
In six of 16 studies, primary authors stated that they had obtained no external funding (Amer 2010; De Iaco 2003; Di Spiezio Sardo 2011; Fuchs 2014; Guida 2004; Roy 2014). In seven of 16 studies, reporting of external funding was unclear; we failed to obtain clarification from corresponding authors of the primary study report despite several queries (Abu Rafea 2013; Acunzo 2003; Dabir‐Ashrafi 1996; Do 2005; Lin 2015a; Vercellini 1989; Xiao 2015). Three studies reported external funding by the Chinese Government (Gan 2017; Lin 2015b; Wang 2016).
Potential conflicts of interest
In nine of 16 studies, primary authors declared no potential conflicts of interest (Amer 2010; De Iaco 2003; Di Spiezio Sardo 2011; Fuchs 2014; Gan 2017; Guida 2004; Lin 2015b; Roy 2014; Wang 2016). In seven of 16 studies, reporting of potential conflicts of interest was unclear despite several queries to the corresponding authors (Abu Rafea 2013; Acunzo 2003; Dabir‐Ashrafi 1996; Do 2005; Lin 2015a; Vercellini 1989; Xiao 2015).
Participants
See Characteristics of included studies table for a detailed description of the main participant characteristics.
Abu Rafea 2013 randomly assigned 28 women diagnosed with an intrauterine septum with from infertility or adverse pregnancy outcomes, or both.
Acunzo 2003 included 92 women with irregular menses and IUAs treated by hysteroscopy.
Amer 2010 included 45 women with severe IUAs, all with subfertility, bound to undergo operative hysteroscopy.
Dabir‐Ashrafi 1996 randomly assigned 46 participants with subfertility and recurrent miscarriage with a fundal defect on hysterosalpingography (HSG).
De Iaco 2003 included 60 women bound to undergo endometrial ablation or hysteroscopic removal of submucosal fibroids, endometrial polyps, septate uterus or intrauterine synechiae.
Di Spiezio Sardo 2011 included 110 women diagnosed at clinic diagnostic hysteroscopy with single or multiple lesions suitable for surgical treatment or with resistant dysfunctional uterine bleeding requiring endometrial ablation.
Do 2005 included 64 women who underwent intrauterine surgery.
Fuchs 2014 included 52 women of confirmed fertility who underwent hysteroscopic surgery because of suspected retained products of conception.
Gan 2017 included 88 women with infertility or at least one spontaneous miscarriage and severe IUAs following hysteroscopic adhesiolysis.
Guida 2004 included 138 women with surgically treatable single lesions (fibroids, polyps and uterine septa, subgroups I to III) at diagnostic hysteroscopy.
Lin 2015a included 62 women undergoing hysteroscopy.
Lin 2015b included 201 women with moderate‐to‐severe IUAs (no prioritisation of the outcomes reported. or greater) after hysteroscopic adhesiolysis.
Roy 2014 included 90 women with septate uterus with a history of miscarriage or subfertility.
Vercellini 1989 included 20 women with two or more unexplained spontaneous miscarriages with a uterine septum.
Wang 2016 included 57 women following hysteroscopic adhesiolysis for severe IUAs.
Xiao 2015 included 120 women that underwent hysteroscopic adhesiolysis for moderate‐to‐severe IUAs.
The proportion of subfertile women was as follows:
0% (two studies; 72 women; Fuchs 2014; Vercellini 1989);
less than 50% (six studies; 567 women; Abu Rafea 2013; Acunzo 2003; Dabir‐Ashrafi 1996; Di Spiezio Sardo 2011; Lin 2015b; Roy 2014);
unknown (six studies; 532 women; De Iaco 2003; Do 2005; Gan 2017; Guida 2004; Lin 2015a; Xiao 2015).
Interventions and comparators
See Characteristics of included studies table.
1. Any intervention versus no treatment or placebo
Device versus no treatment (Abu Rafea 2013; Lin 2015a).
Hormonal treatment versus no treatment or placebo (Dabir‐Ashrafi 1996; Roy 2014).
Device combined with hormonal treatment versus no treatment (Vercellini 1989).
Barrier gel versus no treatment (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004).
2. Any intervention versus any other intervention
Device with graft versus device without graft (Amer 2010; Gan 2017; Wang 2016).
One type of device versus another type of device (Lin 2015b).
Gel combined with hormonal treatment and antibiotics versus hormonal treatment combined with antibiotics (Fuchs 2014).
Device combined with gel versus device (Xiao 2015).
In the previous version of this review Amer 2010 and Fuchs 2014 were erroneously classified under the comparison "Any therapy versus no treatment or placebo".
Outcomes
See Characteristics of included studies table.
-
Primary outcome
Secondary outcomes.
Clinical pregnancy (Abu Rafea 2013; Amer 2010; Fuchs 2014). Three studies reported pregnancy, not further defined which we used as a surrogate outcome for clinical pregnancy (Gan 2017; Roy 2014; Wang 2016).
Miscarriage (Abu Rafea 2013; Amer 2010; Gan 2017; Roy 2014; Wang 2016).
Presence of IUAs at second‐look hysteroscopy (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Fuchs 2014; Gan 2017; Guida 2004; Lin 2015a; Lin 2015b; Roy 2014; Vercellini 1989; Wang 2016; Xiao 2015).
Adhesion scores of IUAs at second‐look hysteroscopy (Acunzo 2003; Amer 2010; Gan 2017; Guida 2004; Lin 2015b; Wang 2016; Xiao 2015).
Severity of IUAs at second‐look hysteroscopy (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Fuchs 2014; Guida 2004; Roy 2014; Xiao 2015).
While several studies measured outcomes other than the key outcomes prespecified in our Cochrane Review's protocol (Amer 2010; Di Spiezio Sardo 2011; Do 2005; Gan 2017; Lin 2015b; Roy 2014; Vercellini 1989; Wang 2016; Xiao 2015), two studies reported none of the outcomes relevant for the quantitative synthesis and critical appraisal (Dabir‐Ashrafi 1996; Lin 2015a).
Excluded studies
See Characteristics of excluded studies table.
We excluded 15 potentially eligible studies for the following reasons.
Five were observational studies (Chen 2017; Hu 2014a; Hu 2014b; Liu 2016; NCT02328742).
Two were quasi‐randomised studies (Pabuccu 2008; Tonguc 2010).
Seven did not answer the PICO (population, intervention, comparator, outcome) research questions of this Cochrane Review (Bednarek 2011; Cheong 2016; Johns 2001; Kurtz 2002; NTR3120; Tsapanos 2002; Yaşar 2004).
One study explicitly excluded subfertile women from participation in the trial (Kim 2012).
Risk of bias in included studies
See the 'Risk of bias' summary for the review authors' judgements about each risk of bias item in the included study (Figure 2).
2.
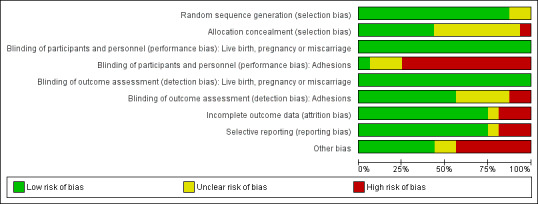
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
See the 'Risk of bias' graph for the review authors' judgements about each risk of bias item presented as percentages across the 16 included studies (Figure 3).
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged 14 of 16 studies at low risk of selection bias in relation to random sequence generation because all used computer‐generated randomisation lists (Abu Rafea 2013; Acunzo 2003; Amer 2010; De Iaco 2003; Di Spiezio Sardo 2011; Fuchs 2014; Gan 2017; Guida 2004; Lin 2015a; Lin 2015b; Roy 2014; Vercellini 1989; Wang 2016; Xiao 2015). We judged two studies at unclear risk of selection bias in relation to random sequence generation: the study reports claim that both trials were RCTs but did not describe the method of randomisation (Dabir‐Ashrafi 1996; Do 2005). We obtained no clarification from the authors of the primary studies despite several mailings. None of the included studies were at high risk of selection bias in relation to random sequence generation.
We judged seven of 16 studies at low risk of selection bias in relation to allocation concealment because investigators used sequentially numbered opaque sealed envelopes containing the allocated treatment (Amer 2010; Di Spiezio Sardo 2011; Fuchs 2014; Guida 2004; Lin 2015a; Vercellini 1989), or a code referring to the allocated treatment (Roy 2014). We judged eight of 16 studies at unclear risk of selection bias in relation to allocation concealment because study authors did not describe the method of allocation concealment and did not provide clarification as requested (Acunzo 2003; Dabir‐Ashrafi 1996; Do 2005; Lin 2015b; Wang 2016; Xiao 2015), or provided insufficient information (De Iaco 2003; Gan 2017). We judged one study at high risk of selection bias in relation to allocation concealment: randomisation was based on a computer‐generated list of numbers, but study authors reported that the allocation was unconcealed (Abu Rafea 2013).
Blinding
Performance bias
Five of 16 studies reported live births (or ongoing pregnancy or term delivery as surrogate outcomes for live birth) (Abu Rafea 2013; Amer 2010; Gan 2017; Roy 2014; Wang 2016), and six of 16 studies reported clinical pregnancy (or pregnancy not further specified a surrogate outcome) (Abu Rafea 2013; Amer 2010; Fuchs 2014; Gan 2017; Roy 2014; Wang 2016). We judged all six studies at low risk of performance bias in relation to blinding of participants and personnel because live birth and clinical pregnancy are unequivocal outcomes (Abu Rafea 2013; Amer 2010; Fuchs 2014; Gan 2017; Roy 2014; Wang 2016). We judged the remaining 10 studies at low risk as none reported live birth or clinical pregnancy (or a surrogate for these predefined outcomes). See Figure 3.
We judged only one of 16 studies at low risk of performance bias in relation to blinding of participants and personnel for the key outcome of adhesions as placebo pills containing folic acid were used for blinding participants and personnel (Roy 2014). We judged three studies at unclear risk of performance bias in relation to blinding of participants and personnel for the outcome of adhesions because the method of blinding of participants and personnel was not described (Dabir‐Ashrafi 1996; Gan 2017), or was not sufficiently clarified after contact with the study authors (De Iaco 2003). We judged 12 of 16 studies at high risk of performance bias in relation to blinding of participants and personnel for the outcome of presence of IUAs, as personnel (Amer 2010; Di Spiezio Sardo 2011; Do 2005; Fuchs 2014; Guida 2004; Lin 2015b; Wang 2016; Xiao 2015), or both participants and personnel (Abu Rafea 2013; Acunzo 2003; Lin 2015a; Vercellini 1989), were not blinded.
Detection bias
Five of 16 studies reported live births (or ongoing pregnancy or term delivery as surrogate outcomes for live birth) (Abu Rafea 2013; Amer 2010; Gan 2017; Roy 2014; Wang 2016), and six of 16 studies reported clinical pregnancy (or pregnancy not further specified a surrogate outcome) (Abu Rafea 2013; Amer 2010; Fuchs 2014; Gan 2017; Roy 2014; Wang 2016). We judged all six studies at low risk of detection bias in relation to blinding of outcome assessors because live birth and clinical pregnancy are unequivocal outcomes (Abu Rafea 2013; Amer 2010; Fuchs 2014; Gan 2017; Roy 2014; Wang 2016). We judged the remaining 10 studies at low risk as none reported live birth or clinical pregnancy (or a surrogate for these predefined outcomes). See Figure 3.
We judged nine of 16 studies at low risk of detection bias for the key outcome of adhesions because outcome assessors were independent observers blinded to treatment allocation (Amer 2010; De Iaco 2003; Di Spiezio Sardo 2011; Fuchs 2014; Gan 2017; Guida 2004; Lin 2015b; Roy 2014; Xiao 2015). We judged five of 16 studies to be at unclear risk of detection bias in relation to blinding of outcome assessors for the key outcome of adhesion formation because the method of blinding was not reported and clarification could not be obtained from the authors of the primary study (Abu Rafea 2013; Acunzo 2003; Do 2005; Wang 2016). We judged one study at unclear risk of performance and detection bias in relation to blinding of participants, personnel and outcome assessors for a subjective outcome not prespecified in this Cochrane Review: the method was unclear, and we obtained no clarification from the authors (Dabir‐Ashrafi 1996). Two studies were at high risk of detection bias in relation to blinding of outcome assessors for the outcome of adhesion formation: the outcome assessors in these two trials were not blinded (Lin 2015a; Vercellini 1989).
Incomplete outcome data
We judged 12 of 16 studies at low risk of attrition bias because all participants with relevant outcome data were included in the final data analysis (Abu Rafea 2013; Di Spiezio Sardo 2011; Vercellini 1989; Wang 2016), or loss to follow‐up was small (less than 10%) without imbalance across comparison groups for numbers or reasons for loss to follow‐up (Acunzo 2003; Amer 2010; Do 2005; Gan 2017; Guida 2004; Lin 2015a; Roy 2014; Xiao 2015). We judged one study at unclear risk of attrition bias because four of 50 (8%) participants were excluded and distribution among comparison groups was not reported: we obtained no clarification from the study authors (Dabir‐Ashrafi 1996). We judged three of 16 studies at high risk of attrition bias (De Iaco 2003; Fuchs 2014; Lin 2015b). In one study, loss to follow‐up after randomisation involved 20/60 included participants (De Iaco 2003). The second study excluded five of 26 participants in the intervention group and six of 26 participants in the control group after randomisation from the analysis (11/52 or 21%): reasons for discontinuation of the trial were not clarified (Fuchs 2014). Loss to follow‐up in the third trial was 19% (Lin 2015b).
Selective reporting
We judged 12 of 16 studies at low risk of reporting bias in relation to selective outcome reporting (Abu Rafea 2013; Acunzo 2003; Amer 2010; Dabir‐Ashrafi 1996; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004; Roy 2014; Vercellini 1989; Wang 2016; Xiao 2015). We judged one study at unclear risk of selective outcome reporting because we noted discrepancies between outcomes prespecified in the registered study protocol NCT01167296 and results reported in the abstract and in the results section (Lin 2015a). We judged three of 16 studies at high risk of reporting bias in relation to selective outcome reporting (Fuchs 2014; Gan 2017; Lin 2015b). One study failed to report data for the primary outcome of live birth despite a study duration of 27 months (Fuchs 2014). In the study protocol of Gan 2017, registered as NCT02496052, all secondary outcomes mentioned in the final study report were not predefined. A third study failed to report data for pregnancy rates in the published report of the study, although pregnancy was prespecified as a main outcome in the study protocol ISRCTN69690272 (Lin 2015b).
Other potential sources of bias
We judged seven of 16 studies at low risk of other potential sources of bias (Acunzo 2003; Dabir‐Ashrafi 1996; Di Spiezio Sardo 2011; Guida 2004; Lin 2015b; Roy 2014; Xiao 2015). We judged two of 16 studies to be at unclear risk of other potential sources of bias (Vercellini 1989; Wang 2016). Vercellini 1989 did not report the baseline characteristics of both comparison groups. In two women in the intervention group, the IUD was removed early and in one woman of the control group had a Foley balloon catheter inserted for persistent heavy bleeding. These three women should have been excluded from the analysis because these interventions could have affected the outcomes. We did not do sensitivity analyses comparing all data versus data excluding these three participants: the study was completed almost 30 years ago and it was no longer possible to retrieve data for individual participant data analysis (IPD). Wang 2016 offered cotreatment with artificial fertility treatment but it was unclear if comparable proportions of women received similar treatments in both comparison groups. We judged seven of 16 studies at high risk of other potential sources of bias (Abu Rafea 2013; Amer 2010; De Iaco 2003; Do 2005; Fuchs 2014; Gan 2017; Lin 2015a). One study excluded four of 28 participants (14%) from the final analysis after randomisation because they were not trying to conceive (Abu Rafea 2013). The reason for this postrandomisation exclusion was a lack of explicit inclusion and exclusion criteria. Analysis of study results showed that poor inclusion and exclusion criteria may lead to increased risk of bias. Moreover, researchers measured outcomes in this study over 12 to 18 months: this could have affected final pregnancy results if imbalance occurred across comparison groups for the time points at which this key outcome was measured. Finally, although there were no evident statistically significant differences in mean age of participants in both comparison groups, the MD was three years, and more women of a younger age were included in the intervention group. This baseline imbalance between comparison groups is clinically relevant, irrespective of P values. Amer 2010 provided evidence of baseline imbalance among participant characteristics in relation to differences in the prevalence of prior caesarean section as a cause of IUAs. Moreover, investigators provided cotreatment with laparoscopy and in vitro fertilisation (IVF) for some women but failed to reported data on the distribution in numbers among comparison groups. De Iaco 2003 recalculated data for the outcomes of presence of IUAs at second look and severity of IUAs and reported no statistically significant differences between comparison groups, although study authors concluded that the use of anti‐adhesion barrier gel improved outcomes of hysteroscopic surgery. This conclusion was not based on the available evidence. Investigators did not report baseline characteristics of both comparison groups. Do 2005 is at high risk of selection bias because there were clinically relevant differences in baseline characteristics between both comparison groups for age, parity and the number of miscarriages. Moreover, it was unclear if micro‐hysteroscopy or transvaginal ultrasound was used for outcome assessment of IUAs. Therefore, it is unclear if this study was at risk for information bias. Fuchs 2014 at follow‐up hysteroscopy offered cotreatment with hysteroscopic adhesiolysis to women with AFS grade II or III IUAs. They offered cotreatment to three of 20 (14%) women in the control group and to one of 21 (4%) women in the intervention group. This may have affected the magnitude and direction of the treatment effect. For Gan 2017, we had some concerns for performance bias related to cotreatments with IVF and laparoscopy whose proportions in both treatment arms were not reported. There was no fixed endpoint for measuring the secondary outcomes: the total duration of follow‐up via direct contact or telephone every three months lasted between six and 12 months. The longer the follow‐up period, the higher the cumulative pregnancy rate. Therefore, we judged this study at high risk for detection bias. We have some concern for imbalance in baseline characteristics between the two comparison groups of Lin 2015a: the number of participants with IUAs in the intervention group (17/31 women) was nearly doubled compared to the control group (10/31 women).
Effects of interventions
1. Anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy
We identified 10 studies any intervention versus no treatment or placebo (Abu Rafea 2013; Acunzo 2003; Dabir‐Ashrafi 1996; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004; Lin 2015a; Roy 2014; Vercellini 1989).
1.1. Live birth
No study reported live birth, but two studies reported outcomes that were used as surrogate outcomes for live birth (term delivery or ongoing pregnancy) (Abu Rafea 2013; Roy 2014). Based on a pooling of these two small studies, there was insufficient evidence to determine whether there was a difference in surrogate outcomes for live birth rate between the use of any intervention compared to no treatment or placebo (OR 0.94, 95% CI 0.42 to 2.12; 107 women; 2 studies; I² = 0%; very‐low‐quality evidence; Analysis 1.1). We stratified data according to device versus no treatment or placebo and hormonal treatment versus no treatment or placebo.
1.1. Analysis.
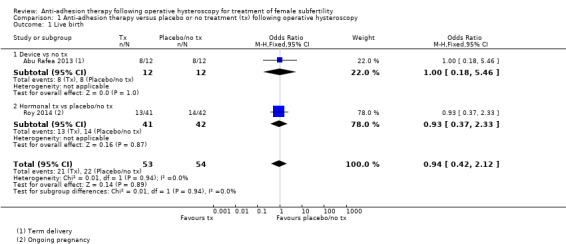
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 1 Live birth.
1.1.1. Device versus no treatment and hormonal treatment versus placebo or no treatment
One study reported data for the outcome of term delivery at 12 to 18 months (Abu Rafea 2013). There was insufficient evidence to determine whether there was a difference in term delivery rate at 12 to 18 months between the use of an intrauterine Foley catheter balloon and no treatment following hysteroscopic septum division (OR 1.00, 95% CI 0.18 to 5.46; 24 women; 1 study; Analysis 1.1).
1.1.2. Hormonal treatment versus placebo or no treatment
Roy 2014 reported data on ongoing pregnancy. We used these data as a surrogate for live birth. It was unclear whether there was a difference between treatment with oestradiol valerate 2 mg daily versus folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division (OR 0.93, 95% CI 0.37 to 2.33; 83 women; 1 study; Analysis 1.1).
Sensitivity analysis
We conducted a sensitivity analysis for Analysis 1.1. The choice to include two studies regardless of study quality (Abu Rafea 2013; Roy 2014), or to include only one study at low risk for selection bias related to random sequence generation and allocation concealment (Roy 2014), did not affect the direction/magnitude of the summary effect estimate or the statistical significance tests.
Sensitivity analyses on the choice of the summary effect measure (OR versus RR) or the analysis model (fixed‐effect versus random‐effects model) demonstrated no differences of the direction of the treatment effect or the statistical significance tests.
In Abu Rafea 2013, some women (4/28 (14%)) were not trying to conceive after treatment, although they had been randomly assigned (1/13 women in the intervention group and 3/15 women in the control group). As prespecified in the protocol under 'Dealing with missing data,' we conducted a sensitivity analysis on the choice to use an available data analysis rather than an ITT analysis with the imputation that no live births would have occurred in women without a reported outcome. There was no impact on the direction/magnitude of the effect size or on the statistical significance tests.
1.2. Clinical pregnancy
According to a meta‐analysis of Abu Rafea 2013 and Roy 2014, there was insufficient evidence to determine whether there was a difference in clinical pregnancy rates between the use of any intervention compared to no treatment or placebo (OR 0.86, 95% CI 0.37 to 2.01; 107 women; 2 studies; I² = 0%; Analysis 1.2). We stratified data according to device versus no treatment or placebo and hormonal treatment versus no treatment or placebo.
1.2. Analysis.
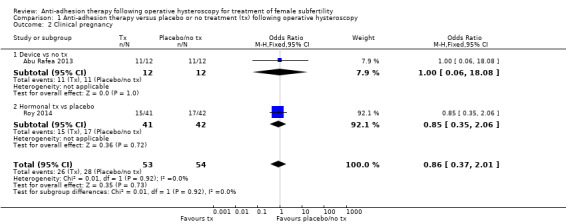
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 2 Clinical pregnancy.
1.2.1. Device versus placebo or no treatment
Abu Rafea 2013 did not define the outcome of pregnancy, and we obtain no clarification from study authors. Moreover, some women could have had more than one pregnancy during the follow‐up period of 12 to 18 months ‐ a point that could not be clarified. It was unclear whether there was a difference between the use of an intrauterine Foley catheter balloon versus no treatment following hysteroscopic septum division (OR 1.00, 95% CI 0.06 to 18.08; 24 women; 1 study; Analysis 1.2).
1.2.2. Hormonal treatment versus placebo or no treatment
According to Roy 2014, there was insufficient evidence to determine whether there was a difference between treatment with oestradiol valerate 2 mg daily versus folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division (OR 0.85, 95% CI 0.35 to 2.06; 83 women; 1 study; Analysis 1.2).
1.3. Miscarriage
According to a meta‐analysis of Abu Rafea 2013 and Roy 2014, there was insufficient evidence to determine whether there was a difference in miscarriage rates between the use of any intervention compared to no treatment or placebo (OR 0.68, 95% CI 0.18 to 2.57; 54 women; 2 studies; I² = 0%; Analysis 1.3). We stratified data according to device versus no treatment or placebo and hormonal treatment versus no treatment or placebo.
1.3. Analysis.
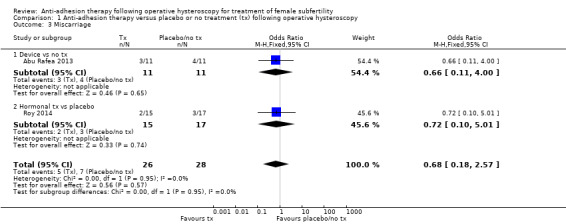
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 3 Miscarriage.
1.3.1. Device versus placebo or no treatment
According to Abu Rafea 2013, there was insufficient evidence to determine whether there was a difference between the use of an intrauterine Foley catheter balloon versus no treatment following hysteroscopic septum division (OR 0.66, 95% CI 0.11 to 4.00; 24 women; 22 clinical pregnancies; 1 study; Analysis 1.3).
1.3.2. Hormonal treatment versus placebo or no treatment
According to Roy 2014, there was insufficient evidence to determine whether there was a difference between treatment with oestradiol valerate 2 mg daily versus folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division (OR 0.72, 95% CI 0.10 to 5.01; 83 women; 32 clinical pregnancies; 1 study; Analysis 1.3).
1.4. Presence of intrauterine adhesions at second‐look hysteroscopy
According to a meta‐analysis of eight studies, anti‐adhesion treatment decreases the occurrence of IUAs at second‐look hysteroscopy compared to no treatment or placebo (OR 0.35, 95% CI 0.21 to 0.60; 560 women; 8 studies; I² = 0%; low‐quality evidence; Analysis 1.4) (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004; Lin 2015a; Roy 2014; Vercellini 1989).
1.4. Analysis.
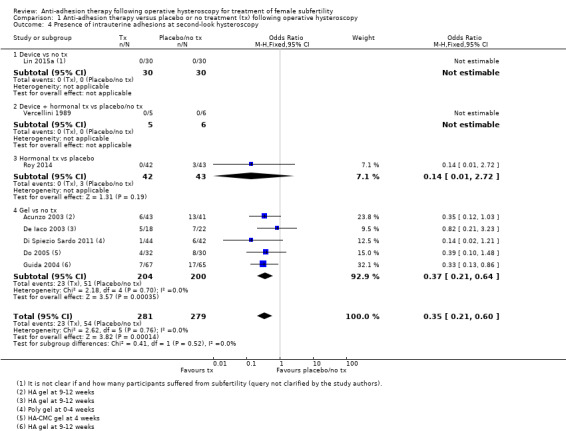
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 4 Presence of intrauterine adhesions at second‐look hysteroscopy.
The number needed to treat for an additional beneficial outcome (NNTB) was 9 (95% CI 5 to 17). We stratified data according to device versus no treatment or placebo, device plus hormonal treatment versus no treatment or placebo, hormonal treatment versus no treatment or placebo and gel versus no treatment or placebo.
1.4.1. Device versus placebo or no treatment
There was insufficient evidence from Lin 2015a to determine whether there was a difference between inserting an intrauterine balloon stent compared with no treatment following operative hysteroscopy for decreasing the occurrence of IUAs: there were no events in both treatment arms (OR not estimable; 60 women; 1 study; Analysis 1.4).
1.4.2. Device plus hormonal treatment versus placebo or no treatment
There was insufficient evidence from Vercellini 1989 to determine whether there was a difference between the insertion of an IUD followed by combined oestrogen‐progestin treatment for 30 days (intervention) versus no treatment (control) following hysteroscopic metroplasty for septate uterus in 20 women with two or more unexplained spontaneous miscarriages. A follow‐up HSG was done to detect uterine cavity abnormalities (residual fundal notch 1 cm or greater) and hysteroscopy was done in women with a residual notch (five women in intervention group and six women in control group). There were no IUAs detected in these 11 women: the effect size was, therefore, not determined (OR not estimable; 20 women; 1 study; Analysis 1.4).
1.4.3. Hormonal treatment versus placebo or no treatment
Based on the findings of Roy 2014, there is insufficient evidence to determine whether there is a difference between treatment with oestradiol valerate 2 mg daily versus folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division (OR 0.14, 95% CI 0.01 to 2.72; 85 women; 1 study; Analysis 1.4).
1.4.4. Gel versus placebo or no treatment
Based on the pooled data of five studies, the use of gel decreases the occurrence of IUAs at second‐look hysteroscopy compared to no treatment or placebo (OR 0.37, 95% CI 0.21 to 0.64; 404; 5 studies; I² = 0%; Analysis 1.4) (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004).
The NNTB was 7 (95% CI 4 to 14).
Subgroup analyses
A subgroup analysis according to the type of gel used demonstrated a consistent decrease of the occurrence of IUAs at second‐look hysteroscopy in favour of the use of ACP gel, CMC gel or HA‐CMC gel compared to no gel. There was no evidence for subgroup differences (Chi² = 0.88, degrees of freedom (df) = 2; P = 0.65; I² = 0%). Data from this subgroup analysis should be treated with caution as subgroup analysis by itself is observational in nature, and statistical interpretation of results is not without problems.
1.5. Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions
We aimed to pool the findings of Acunzo 2003 and Guida 2004 to estimate a summary effect size for the outcome of mean adhesion scores at second‐look hysteroscopy at 12 weeks in women treated by operative hysteroscopy for any intrauterine pathology after use of HA gel compared with no treatment. Statistical heterogeneity beyond chance was very high (I² = 99%) suggesting highly inconsistent findings across studies. The reason for this statistical heterogeneity was obvious: the prevalence of the outcome of interest (IUAs) at baseline in Guida 2004 was 0% as opposed to a 100% prevalence at baseline in Acunzo 2003. The populations were very different with respect to the risk of the adverse outcomes and the potential benefit on the adhesion scores. Therefore, we decided to report data for the mean adhesion scores at second‐look hysteroscopy in women not treated for IUAs and women treated for IUAs separately.
1.5.1. Gel versus placebo or no treatment
Guida 2004 demonstrated lower mean adhesion scores at second‐look hysteroscopy after the use of gel compared to no treatment in women treated for fibroids, endometrial polyps or uterine septa (MD in adhesion score ‐1.46, 95% CI ‐1.64 to ‐1.29; 132 women; 3 studies; I² = 0%; Analysis 1.5).
1.5. Analysis.
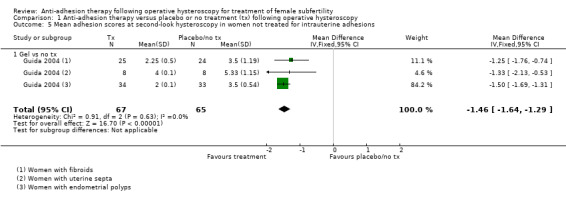
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 5 Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions.
1.6. Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions
1.6.1. Gel versus placebo or no treatment
Acunzo 2003 reported lower mean adhesion scores at second‐look hysteroscopy after the use of HA gel compared to no gel in women treated for IUAs (MD in adhesion score ‐3.30, 95% CI ‐3.37 to ‐3.23, 84 women; 1 study; Analysis 1.6).
1.6. Analysis.
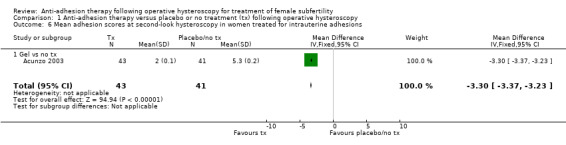
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 6 Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions.
1.7. Severity of adhesions at second‐look hysteroscopy: mild
Based on a pooling of six studies, there was no clear evidence of a difference between any anti‐adhesion treatment compared to no treatment or placebo for the occurrence of mild adhesions at second‐look hysteroscopy (OR 1.33, 95% CI 0.68 to 2.61; 494 women; 6 studies; I² = 0%; Analysis 1.7). We stratified the data for Analysis 1.7 according to hormonal treatment versus no treatment or placebo and gel versus no treatment or placebo.
1.7. Analysis.
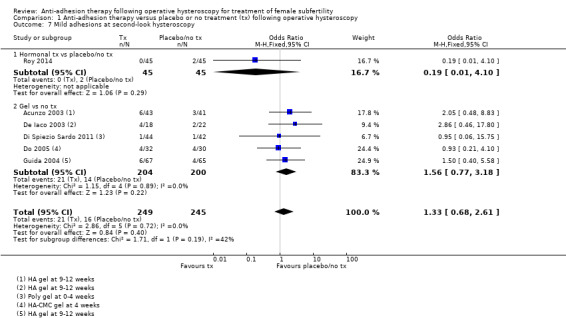
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 7 Mild adhesions at second‐look hysteroscopy.
1.7.1. Hormonal treatment versus placebo or no treatment
Based on the results of Roy 2014, there was insufficient evidence to determine whether there was a difference between treatment with oestradiol valerate 2 mg daily compared to the intake of folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division for the occurrence of mild adhesions at second‐look hysteroscopy (OR 0.19, 95% CI 0.01 to 4.10; 90 women; 1 study; Analysis 1.7).
1.7.2. Gel versus placebo or no treatment
Based on the pooled findings of five RCTs, there was no clear evidence of a difference between the use of any gel versus no gel for the occurrence of mild adhesions at any second‐look hysteroscopy (OR 1.56, 95% CI 0.77 to 3.18; 404 women; 5 studies; I² = 0%; Analysis 1.7) (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004).
Subgroup analyses
According to a subgroup analysis for Analysis 1.7, there was no clear evidence of a difference in the type of gel used in the occurrence of mild IUAs at second‐look hysteroscopy between the use of ACP gel, CMC gel or HA‐CMC gel compared to no gel. There was no evidence for subgroup differences (Chi² = 0.83, df = 2; P = 0.66; I² = 0%). Data from this subgroup analysis should be treated with caution as subgroup analysis by itself is observational in nature, and statistical interpretation of results is not without problems.
1.8. Severity of adhesions at second‐look hysteroscopy: moderate or severe
Based on the statistical pooling of the findings of six studies, the use of anti‐adhesion treatment decreases the occurrence of moderate or severe adhesions compared to no treatment or placebo (OR 0.08, 95% CI 0.03 to 0.24; 494 women; 6 studies; I² = 0%; Analysis 1.8) (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004; Roy 2014).
1.8. Analysis.
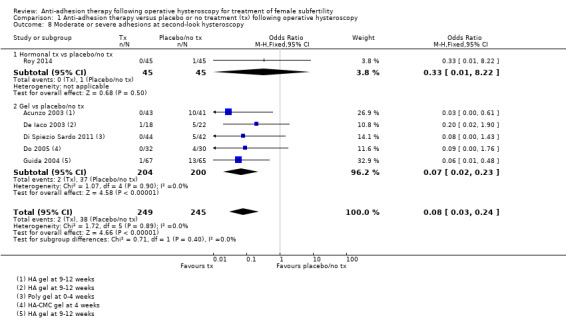
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 8 Moderate or severe adhesions at second‐look hysteroscopy.
The NNTB was 6 (95% CI 5 to 10). We stratified data according to hormonal treatment versus no treatment or placebo and gel versus no treatment or placebo.
1.8.1. Hormonal treatment versus placebo or no treatment
Based on the results of Roy 2014, there was insufficient evidence to determine whether there was a difference between treatment with oestradiol valerate 2 mg daily compared to the intake of folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division for the occurrence of moderate or severe adhesions at second‐look hysteroscopy (OR 0.33, 95% CI 0.01 to 8.22; 90 women; 1 study; Analysis 1.8).
1.8.2. Gel versus placebo or no treatment
Based on the pooled findings of five RCTs, the use of any anti‐adhesion barrier gel decreased the occurrence of moderate or severe adhesions at second‐look hysteroscopy (OR 0.07, 95% CI 0.02 to 0.23; 404 women; 5 studies; I² = 0%; Analysis 1.8) (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004).
The NNTB was 6 (95% CI 4 to 9).
Subgroup analyses
According to a subgroup analysis for Analysis 1.8, there was a consistent effect in favour of the use of ACP gel, CMC gel or HA‐CMC gel compared to no gel for decreasing the occurrence of moderate or severe IUAS at second‐look hysteroscopy. The subgroup interaction test did not identify any between‐group differences.
2. Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy
We identified six studies comparing any intervention versus any other intervention (Amer 2010; Fuchs 2014; Gan 2017; Lin 2015b; Wang 2016; Xiao 2015).
2.1. Live birth
2.1.1. Device plus graft versus device
No study reported live birth, but three studies reported outcomes that we used as surrogate outcomes for live birth (Amer 2010; Gan 2017; Wang 2016). The three studies compared the insertion of a Foley catheter balloon wrapped with fresh or freeze‐dried amniotic graft versus a Foley catheter balloon without graft for one to two weeks following hysteroscopic adhesiolysis in women with severe IUAs. Amer 2010 reported data on ongoing pregnancies or delivered at term. Gan 2017 reported data on ongoing pregnancies beyond 12 weeks of gestational age. Wang 2016 reported data on term delivery and ongoing pregnancy (but not yet delivered at the time of the survey) separately. For reasons of consistency throughout the review, we extracted data for term delivery and ongoing pregnancy and used these data as a surrogate for live birth. There was no clear evidence of a difference between inserting a Foley catheter balloon with fresh or freeze‐dried HAM graft compared to inserting a Foley catheter balloon only (OR 1.48, 95% CI 0.57 to 3.83; 180 women; 3 studies; I² = 0%; low‐quality evidence; Analysis 2.1).
2.1. Analysis.
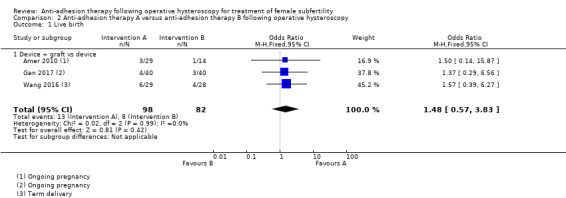
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 1 Live birth.
Sensitivity analysis
We conducted a sensitivity analysis for Analysis 2.1. The choice to include all studies regardless of study quality or to include only one study at low risk for selection bias related to random sequence generation and allocation concealment did not affect the statistical significance tests. Sensitivity analyses on the choice of the summary effect measure (OR versus RR) or the analysis model (fixed‐effect versus random‐effects model) did not demonstrate differences of the direction of the treatment effect or the statistical significance tests.
2.2. Clinical pregnancy
There was no clear evidence of a difference between treatment A versus treatment B for improving clinical pregnancy rates (OR 1.72, 95% CI 0.89 to 3.33; 221 women; 4 studies; I² = 0%; Analysis 2.2). We stratified data according to device plus graft versus device and gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics.
2.2. Analysis.
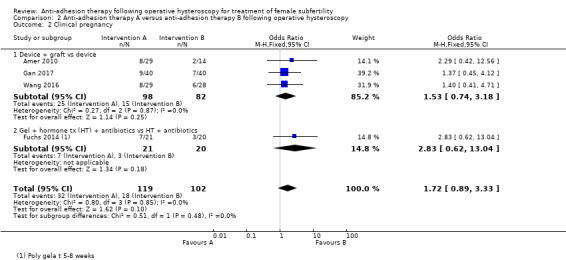
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 2 Clinical pregnancy.
2.2.1. Device plus graft versus device
Three studies compared the insertion of a Foley catheter balloon wrapped with fresh or freeze‐dried amniotic graft versus a Foley catheter balloon without graft following hysteroscopic adhesiolysis in women with severe IUAs (Amer 2010; Gan 2017; Wang 2016). There was no clear evidence of a difference between inserting a Foley catheter balloon wrapped with fresh or freeze‐fried HAM graft compared to inserting a Foley catheter for increasing the chance for a clinical pregnancy (OR 1.53, 95% CI 0.74 to 3.18; 180 women; 3 studies; I² = 0%; Analysis 2.2).
2.2.2. Gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics
One study compared the application of Oxiplex gel with sequential hormonal treatment for three weeks and antibiotic therapy for one week to sequential hormonal treatment and antibiotic therapy only in women with confirmed fertility undergoing operative hysteroscopy for retained products of conception (Fuchs 2014). This study reported data on pregnancy without further specification. We used these data as a surrogate outcome for clinical pregnancy. There was insufficient evidence from Fuchs 2014 to determine whether there was a difference between the application of Oxiplex gel combined with sequential hormonal treatment and antibiotics compared to sequential hormonal treatment combined with antibiotics (OR 2.83, 95% CI 0.62 to 13.04; 41 women; 1 study; I² = 0%; Analysis 2.2).
2.3. Miscarriage
2.3.1. Device plus graft versus device
According to a pooled analysis of data from three studies, there was no clear evidence of a difference between the insertion of a Foley catheter balloon wrapped with fresh or freeze‐dried amniotic graft versus a Foley catheter balloon without graft following hysteroscopic adhesiolysis in women with severe IUAs for the outcome miscarriage (OR 0.80, 95% CI 0.20 to 3.19; 180 women; 40 clinical pregnancies; 3 studies; I² = 0%; Analysis 2.3) (Amer 2010; Gan 2017; Wang 2016).
2.3. Analysis.
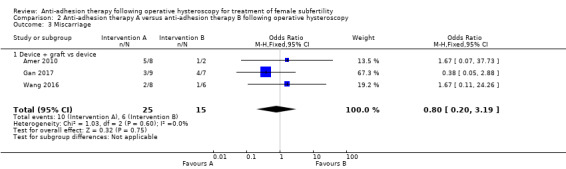
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 3 Miscarriage.
2.4. Presence of intrauterine adhesions at second‐look hysteroscopy
A pooled analysis of five RCTs demonstrated a decrease in the occurrence of IUAS with anti‐adhesion treatment consisting of barrier gel or intrauterine balloon with or without gel or graft compared to IUD plus balloon only or hormonal treatment plus antibiotics (OR 0.55, 95% CI 0.36 to 0.83; 451 women; 5 studies; I² = 0%; low‐quality evidence; Analysis 2.4) (Fuchs 2014; Gan 2017; Lin 2015b; Wang 2016; Xiao 2015).
2.4. Analysis.
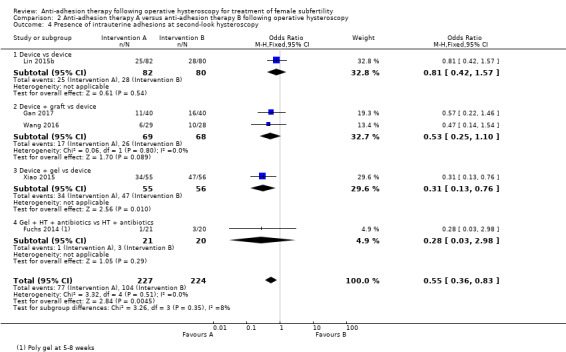
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 4 Presence of intrauterine adhesions at second‐look hysteroscopy.
The NNTB was 8 (95% CI 5 to 25). We stratified data according to device versus device, device plus graft versus device, device plus gel versus device and gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics.
2.4.1. Device versus device
There was insufficient evidence from Lin 2015b to determine whether there was a difference between inserting a specially designed intrauterine balloon compared to the Yantai Contraceptive Instrument, a heart‐shaped copper IUD with thread knitted tail for decreasing the occurrence of IUAs (OR 0.81, 95% CI 0.42 to 1.57; 162 women; 1 study; I² = 0%; Analysis 2.4).
2.4.2. Device plus graft versus device
Gan 2017 studied the rate of IUA reformation in women undergoing hysteroscopic adhesiolysis for severe IUAs: a clear definition of adhesion reformation was not given and further clarification could not be obtained from the study authors. Wang 2016 presented data on the recurrence of IUAs grade 5 or greater according to the 1988 AFS classification as evidence of adhesion reformation in women treated with hysteroscopic adhesiolysis for moderate or severe IUAs. Based on a pooling of the findings of two studies, it was unclear whether there was a difference between inserting a Foley catheter balloon wrapped with HAM versus a Foley catheter balloon without graft following hysteroscopic adhesiolysis in women with severe IUAs (OR 0.53, 95% CI 0.25 to 1.10; 137 women; 2 studies; I² = 0%; Analysis 2.4) (Gan 2017; Wang 2016).
2.4.3. Device plus gel versus device
Based on the findings of Xiao 2015 the injection of 2 mL of medical self‐cross‐linking sodium hyaluronate gel from the lumen of a Foley balloon catheter left in situ for 72 hours decreased the occurrence of IUAs compared to a Foley balloon catheter only following operative hysteroscopy in women with severe IUAs (OR 0.31, 95% CI 0.13 to 0.76; 111 people; 1 study; I² = 0%; Analysis 2.4).
The NNTB is 5 (95% CI 2 to 17).
2.4.4. Gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics
Fuchs 2014 compared the application of Oxiplex gel with sequential hormonal treatment for three weeks and antibiotic therapy for one week to sequential hormonal treatment and antibiotic therapy only in women with confirmed fertility undergoing operative hysteroscopy for retained products of conception. There was insufficient evidence to determine whether there was a difference between groups for decreasing the occurrence of IUAs (OR 0.28, 95% CI 0.03 to 2.98; 41 women; 1 study; I² = 0%; Analysis 2.4).
2.5. Mean adhesion scores at second‐look hysteroscopy
We aimed to pool three studies randomly comparing two anti‐adhesion treatments head‐to‐head measuring mean adhesion scores at second‐look hysteroscopy (Lin 2015b; Wang 2016; Xiao 2015). Statistical heterogeneity beyond chance was very high (I² = 92.1%) suggesting highly inconsistent findings across studies. The reason for this statistical heterogeneity was obvious: the interventions were clinically too diverse to allow statistical pooling. We stratified data according to device versus device, device plus graft versus device and device plus gel versus device.
2.5.1. Device versus device
Lin 2015b reported the median adhesion scores in both comparison groups before the operation and the median reduction of AFS scores in both groups. According to this study, it was unclear whether there was a difference in favour of the insertion of a specially designed intrauterine balloon compared to the Yantai Contraceptive Instrument for the median adhesion scores at second‐look hysteroscopy (Table 3). We considered converting the medians to means and the 95% CI to SD but the method for conversion is not robust.
1. Median American Fertility Society (AFS) scores Lin 2015b.
| Outcome |
Balloon group (intervention: n = 82) |
IUD group (control: n = 80) |
P value |
| AFS score before surgery (median, 95% CI) | 8 (5 to 12) | 8 (5 to 12) | 1.00 |
| Median reduction in AFS score | 7 (2 to 12) | 7 (0 to 12) | 1.00 |
IUD: intrauterine device; n: number of participants.
2.5.2. Device plus graft versus device
Two studies reported data on the median adhesion scores and their interquartile ranges (IQR) (Amer 2010; Gan 2017).
We considered converting the medians to means and the 95% CI to SD but the method for conversion is not robust.
Amer 2010 reported similar median adhesion scores and IQRs at second‐look hysteroscopy across the three intervention arms (Table 4). In contrast, Gan 2017 demonstrated lower median adhesion scores at second‐look hysteroscopy with the use of amniotic membrane graft compared to inserting a balloon catheter only without amnion graft (Table 5).
2. Median American Fertility Society (AFS) scores Amer 2010.
| Statistic | Fresh amnion graft (group 2: n = 14) | Dried amnion graft (group 3: n = 15) | No amnion graft (group 1: n = 14) | P value |
| Median | 1.5 | 2 | 2 | ‐ |
| IQR | 1 to 2 | 1 to 2 | 1 to 2 | 0.27 |
IQR: interquartile range; n: number of participants.
3. Median American Fertility Society (AFS) scores Gan 2017.
| Statistic |
Amnion graft (intervention: n = 40) |
No graft (control: n = 40) |
P value |
| Median | 2 | 4 | ‐ |
| IQR | 2 to 5 | 2 to 6 | 0.03 |
IQR: interquartile range; n: number of participants.
According to Wang 2016, the mean adhesion scores after inserting a balloon catheter with amniotic graft were significantly lower compared to inserting a balloon catheter alone without amniotic graft following hysteroscopic adhesiolysis in women with moderate or severe IUAs (MD in adhesion score ‐3.10, 95% CI ‐4.17 to ‐2.03; 57 women; 1 study; Analysis 2.5).
2.5. Analysis.
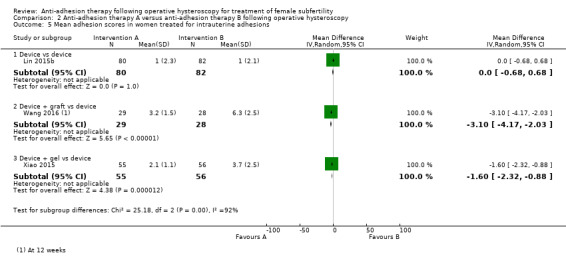
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 5 Mean adhesion scores in women treated for intrauterine adhesions.
2.5.3. Device plus gel versus device
According to Xiao 2015, the injection of 2 mL of medical self‐cross‐linking sodium hyaluronate gel from the lumen of a Foley balloon catheter left in situ for 72 hours was associated with lower mean adhesion scores at second‐look hysteroscopy compared to a Foley balloon catheter only following operative hysteroscopy in women with severe IUAs (MD in adhesion score ‐1.60, 95% CI ‐2.32 to ‐0.88; 111 women; 1 study; Analysis 2.5).
2.6. Severity of adhesions at second‐look hysteroscopy: mild
2.6.1. Device plus gel versus device
There was insufficient evidence from Xiao 2015 to determine whether there was a difference between the injection of 2 mL of medical self‐cross‐linking sodium hyaluronate gel from the lumen of a Foley balloon catheter left in situ for 72 hours compared to a Foley balloon catheter only following operative hysteroscopy in women with severe IUAs for the occurrence of mild adhesions at second‐look hysteroscopy (OR 1.11, 95% CI 0.53 to 2.34; 111 women; 1 study; Analysis 2.6).
2.6. Analysis.
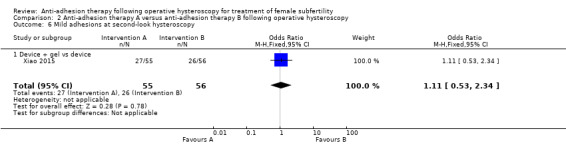
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 6 Mild adhesions at second‐look hysteroscopy.
2.7. Severity of adhesions at second‐look hysteroscopy: moderate or severe
According to a pooling of the findings of two studies, the application of a combined anti‐adhesion treatment consisting of barrier gel decreased the occurrence of moderate or severe IUAs following operative hysteroscopy compared to anti‐adhesion treatment not consisting of barrier gel (OR 0.25, 95% CI 0.10 to 0.61; 152 women; 2 studies; I² = 0%; Analysis 2.7) (Fuchs 2014; Xiao 2015).
2.7. Analysis.
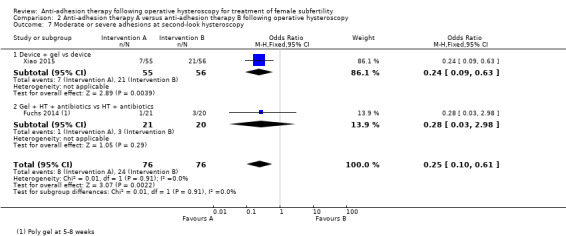
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 7 Moderate or severe adhesions at second‐look hysteroscopy.
The NNTB was 5 (95% CI 3 to 12). We stratified data according to device plus gel versus device and gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics.
2.7.1. Device plus gel versus device
Based on the findings of Xiao 2015, the injection of 2 mL of medical self‐cross‐linking sodium hyaluronate gel from the lumen of a Foley balloon catheter left in situ for 72 hours decreased the occurrence of moderate or severe IUAs compared to a Foley balloon catheter only following operative hysteroscopy in women with severe IUAs (OR 0.24, 95% CI 0.09 to 0.63; 111 women; 1 study; I² = 0%; Analysis 2.7).
The NNTB was 4 (95% CI 2 to 12).
2.7.2. Gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics
There was insufficient evidence from Fuchs 2014 to determine whether there was a difference between the application of Oxiplex gel with sequential hormonal treatment for three weeks and antibiotic therapy for one week to sequential hormonal treatment and antibiotic therapy only in women with confirmed fertility undergoing operative hysteroscopy for retained products of conception (OR 0.28, 95% CI 0.03 to 2.98; 41 women; 1 study; Analysis 2.7).
Discussion
Summary of main results
This systematic review aimed to investigate whether the use of anti‐adhesion therapy following operative hysteroscopy made a difference in the main outcomes of live birth or ongoing pregnancy, clinical pregnancy and miscarriage, or in the prevalence, extent or severity of IUAs in women with subfertility.
We searched for studies randomly comparing any anti‐adhesion therapy versus no treatment or placebo or any other anti‐adhesion treatment in subfertile women following operative hysteroscopy.
We retrieved 16 studies involving 1273 women randomly comparing the use of a device versus no treatment (two studies; 90 women), hormonal treatment versus no treatment or placebo (two studies; 136 women), device combined with hormonal treatment versus no treatment (one study; 20 women), barrier gel versus no treatment (five studies; 464 women), device with graft versus device without graft (three studies; 190 women), one type of device versus another device (one study; 201 women), gel combined with hormonal treatment and antibiotics versus hormonal treatment with antibiotics (one study; 52 women) or device combined with gel versus device (one study; 120 women). Only two of 16 studies included 100% infertile women; in all other studies, the proportion of infertile women was variable or unknown. Most studies (14/16) had at least one item at high risk of bias, and nine of 16 studies had two or more items at high risk of bias. Seven studies were at low risk for selection bias related to random sequence generation and allocation concealment (Amer 2010; Di Spiezio Sardo 2011; Fuchs 2014; Guida 2004; Lin 2015a; Roy 2014; Vercellini 1989). Only one study had all items at low risk of bias (Roy 2014).
Anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy
Based on a pooled analysis of the results from two studies there was insufficient evidence to determine whether there was a difference between inserting a device in the uterine cavity or starting hormonal treatment compared to no treatment or placebo for increasing the chance for term delivery or ongoing pregnancy (Figure 4).
4.
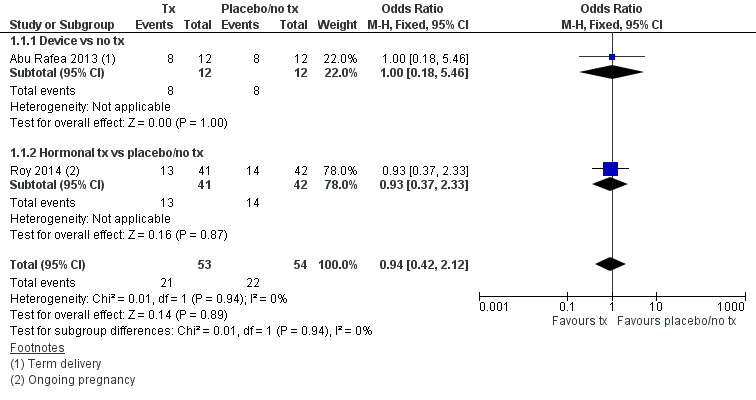
Forest plot of comparison: 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, outcome: 1.1 Live birth.
The pooled findings from eight studies demonstrated a summary effect in favour of the insertion of a device with or without hormonal treatment or hormonal treatment or anti‐adhesion barrier gels compared to no treatment or placebo for decreasing the occurrence of IUAs at any second‐look hysteroscopy (Figure 5). For the use of anti‐adhesion treatment in a medium‐risk population, we would expect that out of 1000 women treated by operative hysteroscopy, between 153 and 365 women would develop IUAs, compared with 545 women when no anti‐adhesion treatment was used (Figure 6).
5.
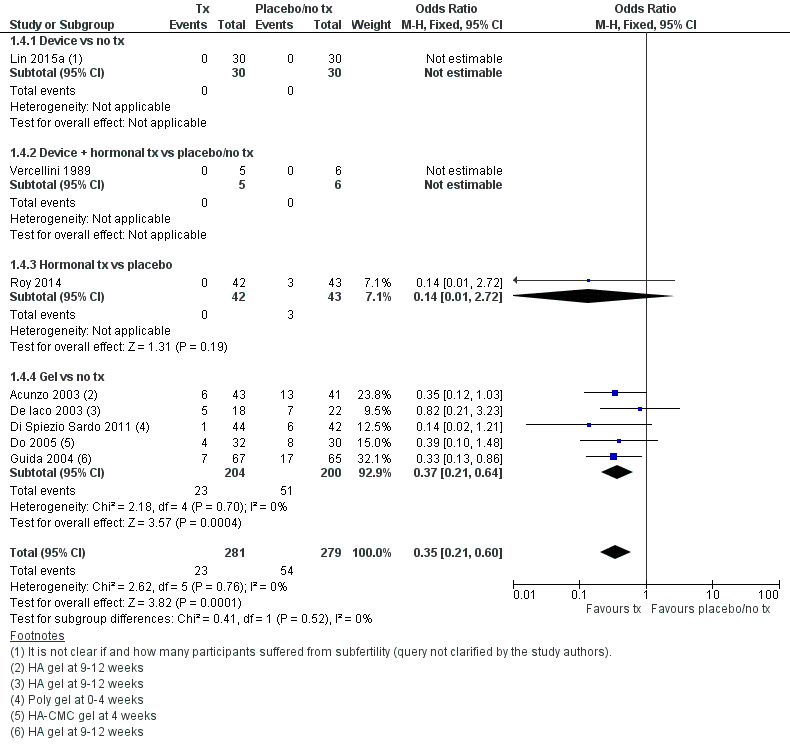
Forest plot of comparison: 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, outcome: 1.4 Presence of intrauterine adhesions at second‐look hysteroscopy.
6.
Cates' plot of numbers needed to treat for an additional beneficial outcome (NNTB) for Analysis 1.4 assuming medium risk of 545 women per 1000 with intrauterine adhesions at second‐look hysteroscopy in the control group (no treatment or placebo). Randomly compared to control, the use of device with or without hormonal treatment or hormonal treatment or barrier gels (intervention) decreased the number of women with intrauterine adhesions at second‐look hysteroscopy to 234 women per 1000 (95% confidence interval 153 to 365 women per 1000). Figure drawn using www.nntonline.net.
Anti‐adhesion therapy versus any other therapy following operative hysteroscopy
According to the pooled findings of three studies there was no clear evidence of a difference between the insertion of a Foley catheter balloon wrapped in amniotic membrane versus the insertion of a Foley catheter balloon without amniotic membrane for improving the ongoing pregnancy rates.
A meta‐analysis of the findings of five trials demonstrated differences head‐to‐head between the use of a device with or without graft/gel or gel plus hormonal treatment plus antibiotics randomly compared to the use of a device only or hormonal treatment plus antibiotics for decreasing the occurrence of IUAs at second‐look hysteroscopy. The findings of this meta‐analysis were not robust and highly affected by evidence quality.
Overall completeness and applicability of evidence
We retrieved only one small study that randomly compared the insertion of an IUD versus no treatment (Vercellini 1989). In everyday clinical practice, worldwide an IUD is very often inserted following the hysteroscopic treatment of IUAs or the resection of an intrauterine septum.
Only five of 16 studies reported data on the primary outcome of live birth but all five used surrogate outcomes. Only five of 16 studies reported data on an adverse reproductive outcome (miscarriage). Thirteen of 16 trials reported the secondary outcomes of prevalence, mean adhesion scores and severity of IUAs at second‐look hysteroscopy.
Only eight of 16 studies reported data on the proportion of women with subfertility. Out of 682 participants from these eight studies, only 247 women had subfertility (36%). Therefore, the evidence retrieved in this Cochrane Review is indirect for the target population of subfertile women undergoing operative hysteroscopy.
There were differences in the HA anti‐adhesion gel used by Acunzo 2003 and Guida 2004 compared to Xiao 2015. Xiao 2015 suggested that the use of their gel (a newly developed highly viscous and elastic self‐cross‐linking sodium hyaluronate gel, which uses fermentation technology on natural sodium hyaluronate gel) may be more advantageous: animal‐derived HA may stimulate immunological rejection and provoke inflammation.
We did not find any cost‐effectiveness studies on the use of anti‐adhesion treatment following operative hysteroscopy in a subfertile population.
In conclusion, we judged that the body of evidence retrieved was insufficient to address all research questions that were predefined for this Cochrane Review.
Quality of the evidence
Several limitations at study and outcome levels were related to performance bias, other potential sources of bias, attrition bias, and reporting and selection bias in decreasing order of frequency. Reasons for risk of bias at the study level and across studies are discussed in detail in the Risk of bias in included studies section and are graphically presented in Figure 2 and Figure 3.
Anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy
See Table 1.
We graded the overall quality of the evidence as very low for the outcome of live birth. The main limitations were serious risk for selection bias related to allocation concealment, serious imprecision and serious indirectness.
For the outcome of IUAs at second‐look hysteroscopy, we graded the overall quality of the evidence as low. The main limitations were high risk of performance bias related to blinding of participants/personnel and serious indirectness.
Anti‐adhesion therapy versus any other therapy following operative hysteroscopy
See Table 2.
We graded the overall quality of the evidence as low for the outcome of live birth. The main limitations were high risk of bias for selective outcome reporting and serious imprecision.
For the outcome of IUAs at second‐look hysteroscopy, we graded the overall quality of the evidence as very low. The main limitations were high risk of bias for selective outcome reporting and serious indirectness.
Potential biases in the review process
Limitations at the review level include the following.
We conducted no formal study of reporting bias because we retrieved a limited number of studies (fewer than 10 studies) for each randomised comparison. Nevertheless, we aimed to minimise the potential impact of reporting and publication bias by conducting a comprehensive search for all potentially eligible studies, and by staying alert for duplication of data as predefined in the protocol of this Cochrane Review (Bosteels 2013a). We consistently searched for related articles in published and secondary reports of included studies. We contacted all authors of included studies to ask if they were aware of any published or ongoing trials; we also contacted experts in the field.
We rigorously subjected to sensitivity analyses all choices to include only studies at low risk of bias versus all studies, to use available data analyses rather than ITT analyses or to exclude participants who were treated by an intervention not indicated for treating subfertility; we considered any observed substantial changes when interpreting results.
We used surrogate outcomes for the primary outcome of live birth: term delivery at 12 to 18 months for Abu Rafea 2013, ongoing pregnancies or delivered at term for Amer 2010, ongoing pregnancies beyond 12 weeks of gestational age for Gan 2017, and ongoing pregnancy for Roy 2014 and Wang 2016.
We used surrogate outcomes for the secondary outcome of clinical pregnancy: pregnancy without clear definition for Amer 2010, Fuchs 2014, Gan 2017, and Wang 2016.
At least two review authors independently and simultaneously extracted all data for the previous version of this Cochrane Review: JB extracted data from all studies, and TD/FB/JK/SW divided all studies between them, and each extracted data from only a portion of all the finally included studies. For the present update (five additional studies retrieved), JB extracted data from all five additional studies while independently and simultaneously HT (three), SW (three) and SJC (two) extracted data from some studies divided between them. In case of disagreement, BWM acted as a third review author for arbitration. This implies that JB may have had a larger influence than any one of all the authors involved in data extraction on the final decisions concerning this part of the review.
One of the Cochrane authors (SJC) translated two Chinese articles into English to allow data extraction and assessment of the risk of bias (Wang 2016; Xiao 2015). Queries in English and Chinese were sent to Lin 2015b, Wang 2016, and Xiao 2015. Despite several queries, we were able to obtain answers from the authors of primary study reports in six of 15 included studies (40%). For nine of 15 (60%) included studies, several queries remained unanswered. As predefined in the study protocol, we classified these items as 'unclear evidence' or 'unclear risk of bias.'
Agreements and disagreements with other studies or reviews
We found six systematic reviews that have summarised and critically appraised the available evidence on the effectiveness of anti‐adhesion therapy.
One Cochrane Review included 18 RCTs in 1262 women undergoing gynaecological pelvic surgery by laparoscopy (eight RCTs) or laparotomy (10 RCTs) (Ahmad 2015). The authors found no evidence on the effects of barrier agents used during pelvic surgery on either pain or fertility outcomes in women of reproductive age. The quality of the evidence ranged from very low to moderate. The most common limitations were imprecision and poor reporting of study methods. Most studies were commercially funded, and publication bias could not be ruled out.
Mais 2012 was a systematic review with a meta‐analysis performed to study the effectiveness of ACP gel for adhesion prevention in laparoscopic and hysteroscopic surgery. Data from three RCTs included in the Cochrane Review were pooled (Acunzo 2003; De Iaco 2003; Guida 2004): the proportion of women with adhesions at second look was significantly lower in women who received ACP gel than in the control group (RR 0.50, 95 % CI 0.31 to 0.85; P = 0.009; 3 studies; 256 women). Mais 2012 used the Jadad scale (an older and less valid tool for assessing the validity of intervention studies) and not the Cochrane 'Risk of bias' tool leading to 'high quality' for the three studies in Mais 2012 as opposed to the grading of the available evidence in the present Cochrane Review for the outcome of IUAs at second‐look hysteroscopy as 'low quality.'
Healy 2015 was a systematic review partially sponsored by a grant from the Intramural research program of the Program in Reproductive and Adult Endocrinology, National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH). The review included 13 studies. Seven studies that compared similar treatment methods were statistically pooled. The authors concluded that the use of HA gel or Intercoat gel after operative hysteroscopy may decrease IUA formation. According to their meta‐analysis, the data does not support the use of oestrogen therapy. Additional quality RCTs are needed to further establish better preventive measures of IUA formation.
Healy 2016 was a systematic review including 12 studies. Nine studies included by Healy 2016 were also included in this Cochrane Review (Acunzo 2003; Amer 2010; Dabir‐Ashrafi 1996; De Iaco 2003; Di Spiezio Sardo 2011; Fuchs 2014; Guida 2004; Roy 2014; Vercellini 1989); three studies included by Healy 2016 were excluded from this Cochrane review for being non‐randomised (Pabuccu 2008; Tonguc 2010) or excluding subfertile women (Kim 2012). Three studies demonstrating a benefit with the gels in preventing adhesion formation were all conducted by the same research group (Acunzo 2003; Di Spiezio Sardo 2011; Guida 2004); according to Healy 2016 these beneficial results have not been confirmed by other research groups. The final conclusion of Healy 2016 stated that there was a lack of definitive evidence to conclude that any treatment was effective in preventing IUAs following operative hysteroscopy. The available literature "has significant heterogeneity and a high risk of bias, making any definitive conclusions difficult."
Di Spiezio Sardo 2016 was a systematic review including 29 studies. Eight studies included by Di Spiezio Sardo 2016 were included in this Cochrane Review (Acunzo 2003; Amer 2010; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Fuchs 2014; Guida 2004; Roy 2014). Three studies included by Di Spiezio Sardo 2016 were excluded from this Cochrane Review for being non‐randomised (Pabuccu 2008; Tonguc 2010) or excluding subfertile women (Kim 2012). Eighteen studies were observational studies that were not eligible for this Cochrane Review. Di Spiezio Sardo 2016 concluded that "robust and high quality randomized trials to assess the effectiveness of different anti‐adhesion therapies are still needed before one or more of these strategies may be strongly recommended for improving clinical outcomes in women treated by operative hysteroscopy."
One systematic review by Salma 2014 including 28 observational studies of 1806 women with meta‐analysis of five studies and qualitative assessment of 23 studies reported a clinical benefit with the insertion of an IUD for all women with IUAs regardless of their severity. In the opinion of the authors of this review, use of IUDs should be combined with other anti‐adhesion therapies "to obtain maximal outcomes, in particular in patients with moderate to severe IUAs." This review had several methodological limitations, including the lack of a formal assessment of risk of bias, lack of appreciation of the role of confounding variables, lack of adjustment for confounders in data calculation for pooled analyses, evidence of substantial statistical heterogeneity for pooled analyses of the five included studies and lack of formal assessment of reporting bias.
Authors' conclusions
Implications for practice.
The quality of the body of evidence retrieved for all outcomes was low to very low and no studies reported live birth. For daily clinical practice, there is no clear evidence on the safety and effectiveness of anti‐adhesion treatment for improving rates of term delivery or ongoing pregnancy, or for decreasing intrauterine adhesions following operative hysteroscopy in subfertile women.
Implications for research.
More research is needed to assess the comparative safety and (cost)effectiveness of different anti‐adhesion treatments compared to no treatment or other interventions for improving key live birth and pregnancy rates in subfertile women.
What's new
| Date | Event | Description |
|---|---|---|
| 2 September 2017 | New citation required but conclusions have not changed | The addition of 5 new studies and additional data from one further study have not led to a change in the conclusions of this review |
| 2 September 2017 | New search has been performed | New searches from 1 March 2015 to 1 June 2017 identified 5 additional studies (Do 2005; Gan 2017; Vercellini 1989; Wang 2016; Xiao 2015). We updated the data of Lin 2015b which were partially presented in the previous review as Lin 2013. Amer 2010 and Fuchs 2014 were re‐classified under the comparison "Any therapy versus any other therapy" in the updated version. |
Acknowledgements
Cochrane Gynaecology and Fertility Group (CGFG). We wish to thank Professor Cindy Farquhar, CGF Co‐ordinating Editor, Ms Helen Nagels, CGF Managing Editor and Ms Jane Marjoribanks for editorial review. Ms Marian Showell, CGF Information Specialist, assisted in developing several of the search strategies used in the present review. We also thank the referees for their remarks and criticisms during the peer review process. The efforts of all these people combined have increased the validity of the present updated Cochrane Review.
Dr Jenneke Kasius coauthored the protocol for the 'Background' section, assisted in the search for and selection of studies and was involved in data extraction and risk of bias assessment of the first version of this Cochrane review. She has moved from the Department of Reproductive Medicine and Gynecology of the University Medical Center Utrecht to Radboud University Medical Centre Nijmegen, the Netherlands for postgraduate training as a gynaecological oncologist.
Ms Elizabeth Bosselaers (Managing Secretary CEBAM, Cochrane Belgium) for logistical support, language correction and assistance with the plain language summary. Ms Sofie De Wit (Managing Secretary Department of Obstetrics and Gynaecology, Imeldahospital Bonheiden, Belgium) for providing comments and criticisms on the content of the 'Plain language summary' section.
Ms Roos Colman, Biostatistician of the Biostatistics Unit, Campus UZ Gent, De Pintelaan 185, 4K3, Entrance 42, B‐9000 Ghent, Belgium for statistical advice.
Dr Pierandrea De Iaco (Italy), Dr Murali Subbaiah (India), Prof Dr Attilio DiSpiezio Sardo (Italy), Prof Dr Paolo Vercellini (Italy), Dr Mohamed Amer (Egypt) and Dr Moty Pansky (Israel) for answering queries.
Appendices
Appendix 1. CGF Specialised Register search strategy
Procite platform
Keywords CONTAINS "hysteroscopy" or "hysteroscopy pain" or "hysteroscopy pain ‐surgical" or "hysteroscopy, techniques" or "hysteroscope " or "office hysteroscopy" or "operative hysteroscopy" or Title CONTAINS "hysteroscopy" or "hysteroscopy pain" or "hysteroscopy pain ‐surgical" or "hysteroscopy, techniques" or "hysteroscope " or "office hysteroscopy" or "operative hysteroscopy" AND Keywords CONTAINS "adhesiolysis" or "adhesion" or "adhesions" or "adhesions outcome" or "adhesion prevention" or "adhesion formation" or "pelvic adhesions" or "Sepracoat" or "icodextrin" or "hydrogel" or "hydrotubation" or "Seprafilm" or "intergel" or "Barrier Membrane" or "hyaluronan" or "hyaluronic acid" or "hyaluronidase" or "Promethazine" or "dextran" or "SprayGel" or "adhesion barrier" or "adhesion barriers" or "post‐operative adhesions" or "gynecologic surgical procedure" or "pelvic adhesions" or "amnion graft" or "antibiotics" or "*Estrogens" or "Estrogen" or "oestrogen" or "intrauterine device" or "Intrauterine Devices, Medicated" or "Intrauterine Releasing Devices" or Title CONTAINS "adhesiolysis" or "adhesion" or "adhesions" or "adhesions outcome" or "adhesion prevention" or "adhesion formation" or "pelvic adhesions" or "Sepracoat" or "icodextrin" or "hydrogel" or "hydrotubation" or "Seprafilm" or "intergel" or "Barrier Membrane" or "hyaluronan" (
11 records
Database: Search strategy for JB1900 in the Cochrane Gynaecology and Fertility specialised register was rerun and date limited from 01.01.15 (last search) to 07.06.17 Most recent update: 7 June 2017
Appendix 2. CENTRAL search strategy
#1MeSH descriptor: [Hysteroscopy] explode all trees (403) #2hysteroscopic surgery (309) #3operative hysteroscopy (212) #4synechiolysis (6) #5#1 or #2 or #3 or #4 (639) #6barrier agent (816) #7hyaluronic acid gel (218) #8intrauterine balloon (116) #9amnion graft (52) #10estrogen treatment (6,178) #11MeSH descriptor: [Intrauterine Devices] explode all trees (616) #12MeSH descriptor: [Anti‐Bacterial Agents] explode all trees (10,749) #13#6 or #7 or #7 or #8 or #9 or #10 or #11 or #12 (18,605) #14intrauterine adhesions (119) #15adhesion score (566) #16reproductive outcome (4,098) #17#14 or #15 or #16 (4,665) #18#5 and #13 and #17 Publication Year from 2015 to 2017 (14) Cochrane reviews (7) Other reviews (0) Trials (7)
14 records
Database: Cochrane Database of Systematic Reviews: Issue 6 of 12, June 2017
Most recent update: 6 June 2017
Appendix 3. MEDLINE search strategy (PubMed)
(((((randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR "drug therapy"[Subheading] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms]))) AND ((((reproductive outcome) OR adhesion score) OR intrauterine adhesions) OR "Gynatresia"[Majr])) AND (((((((((("Anti‐Bacterial Agents"[Majr]) OR "Intrauterine Devices"[Mesh]) OR estrogen treatment) OR amnion graft) OR intrauterine balloon) OR gel) OR hyaluronan) OR hyaluronic acid gel) OR barrier agents) OR adhesion prevention)) AND (((((synechiolysis) OR operative hysteroscopy) OR "Gynecologic Surgical Procedures"[Majr]) OR hysteroscopic surgery) OR "Hysteroscopy"[Majr])
21 records
Database: MEDLINE using PubMed
Most recent update: 6 June 2017
Appendix 4. Embase search strategy (Embase.com)
#1'hysteroscopy'/exp OR 'hysteroscopy'(10,800) #2hysteroscopic AND 'surgery' (3,504) #3gynaecological AND 'surgery' (15,150) #4operative AND 'hysteroscopy'(1,775) #5synechiolysis (82) #6#1 OR #2 OR #3 OR #4 OR #5 (25,733) #7'adhesion'/exp AND 'prevention' (2,460) #8barrier AND agents (11,435) #9hyaluronic AND 'acid'/exp AND 'gel'/exp (28) #10'hyaluronan'/exp (33,533) #11'gel'/exp (55,900) #12'intrauterine'/exp AND 'balloon'/exp (602) #13'amnion'/exp AND graft (735) #14'estrogen'/exp AND treatment (79,899) #15'intrauterine'/exp AND 'device'/exp (22,666) #16'antibiotics'/exp (1,175,044) #17#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 (1,365,328) #18'intrauterine'/exp AND 'adhesions'/exp (465) #19'adhesion'/exp AND score (937) #20reproductive AND outcome (44,546) #21#18 OR #19 OR #20 (45,864) #22#6 AND #17 AND #21 (342) #23'clinical trial'/exp (1,201,041) #24'randomized controlled trial'/exp (447,991) #25'randomization'/exp (73,693) #26'single blind procedure'/exp (27,124) #27'double blind procedure'/exp (137,917) #28'crossover procedure'/exp (51,040) #29'placebo'/exp (305,105) #30randomi?ed AND controlled AND trial* AND [embase]/lim (554,250) #31rct AND [embase]/lim (23,901) #32'random allocation'/exp AND [embase]/lim (46,023) #33'randomly allocated' AND [embase]/lim (23,118) #34'allocated randomly' AND [embase]/lim (1,917) #35allocated NEAR/2 random AND [embase]/lim (766) #36'single blind$' AND [embase]/lim (29,481) #37'double blind$' AND [embase]/lim (193,062) #38(treble OR triple) NEAR/2 blind$ AND [embase]/lim (591) #39placebo$ AND [embase]/lim (364,742) #40'prospective study'/exp (372,790) #41#23 OR #24 OR 25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 (1,883,550) #42'case study'/exp (46,539) #43'case report'/exp AND [embase]/lim (1,627,297) #44'abstract report'/exp (89,710) #45'letter'/exp (926,515) #46#42 OR #43 OR #44 OR #45 (2,538,528) #47#41 NOT #46 (1,819,026) #48'animal'/exp (23,131,376) #49'human'/exp (18,279,043) #50#48 NOT #49 (4,852,333) #51#47 NOT #50 (1,756,205) #52#22 AND #51 (85) #53#22 AND #51 AND [1‐3‐2015]/sd NOT [1‐6‐2017]/sd (32)
32 records
Database: Embase using Embase.com
Most recent update: 6 June 2017
Appendix 5. Web of Science search strategy
# 1TS = (hysteroscopy) (509) # 2TS = (hysteroscopic surgery) (156) # 3TS = (operative hysteroscopy) (122) # 4TS = (synechiolysis) (8) # 5#1 OR #2 OR #3 OR #4 (585) # 6TS = (barrier agent)(3,157) # 7TS =(hyaluronic acid gel)(482) # 8TS = (intrauterine balloon)(70) # 9TS = (amnion graft)(35) # 10TS = (estrogen treatment) (5,460) # 11TS = (intrauterine device) (701) # 12TS = (antibiotics) (39,645) # 13#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 (49,338) # 14TS =(intrauterine adhesions) (125) # 15TS =(adhesion score) (690) # 16TS = (reproductive outcome)(4,515) # 17#14 OR #15 OR #16 (5,293) # 18#5 AND #13 AND #17 (28) # 19 TS =(randomized controlled trial) (82,310) # 20 #18 AND #19 (11) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=2015‐2017
11 records
Database: Web of Science (WoS)
Most recent update: 6 June 2017
Appendix 6. CINAHL search strategy (EBSCOhost)
S1 TX hysteroscopy (466) S2 TX hysteroscopic surgery (25) S3 TX operative hysteroscopy (28) S4 TX synechiolysis (2) S5 S1 OR S2 OR S3 OR S4 (473) S6 ""barrier agent"" (24,118) S7 TX hyaluronic acid gel (26) S8 TX intrauterine balloon (29) S9 TX amnion graft (4) S10 TX estrogen treatment (522) S11 TX intrauterine device (1,577) S12 TX antibiotics (35,078) S13 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 (37,486) S14 TX intrauterine adhesions (15) S15 TX adhesion score (29) S16 TX reproductive outcome (590) S17 S14 OR S15 OR S16 (632) S18 S5 AND S13 AND S17 (5) S19 (MH "Clinical Trials") (87,486) S20 PT clinical trial* (52,906) S21 (MH "Randomized Controlled Trials") (29,785) S22 PT randomized controlled trial* (30,863) S23 (MH "Random Assignment") (34,135) S24 TX Randomi*ation (5,181) S25 TX single blind* (8,515) S26 TX double blind* (707,324) S27 TX triple blind* (137) S28 ""TX treble blind*"" (38,806) S29 TX Placebo* (31,331) S30 TX prospective stud* (204,534) S31 S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 (975,773) S32 S18 AND S31 (1)
1 record
Database: CINAHL using EBSCOHOST
Most recent update: 6 June 2017
Appendix 7. Items of the pilot‐tested data extraction form
1. Source
Study ID.
Report ID.
Review author ID.
Citation and contact details.
2. Eligibility
Confirm eligibility for review.
Reason for exclusion.
3. Trial characteristics
Study design
Random sequence generation.
Participant recruitment.
Participant inclusion and exclusion criteria.
Allocation concealment.
Blinding of participants, personnel and outcome assessors.
Completeness of outcome data.
Selective outcome reporting.
Other potential sources of bias.
Follow‐up
Duration of follow‐up.
Type of follow‐up.
Size of study
Number of women recruited.
Number of women randomly assigned.
Number of women excluded.
Number of women withdrawn and lost to follow‐up.
Number of women analysed.
Study setting
Single‐ or multicentre.
Location.
Timing and duration.
Diagnostic criteria
Screening by transvaginal sonography (TVS).
Screening by hysterosalpingography (HSG).
Screening by TVS and HSG.
Screening by other ultrasound diagnostic procedures, e.g. saline infusion sonography or gel instillation sonography.
Screening by hysteroscopy.
Diagnosis confirmed by hysteroscopy and biopsy.
4. Characteristics of study participants
Baseline characteristics
Age.
Primary or secondary subfertility.
Duration of subfertility.
Diagnostic workup: baseline follicle‐stimulating hormone, semen analysis, diagnosis of tubal pathology, confirmatory test of ovulation.
Other contributory causes to subfertility than uterine factor.
Previous treatments, e.g. in vitro fertilisation (IVF), intrauterine insemination (IUI) or other treatments.
Treatment characteristics
IUI natural cycle.
IUI controlled ovarian stimulation with anti‐oestrogens or gonadotropins.
IVF protocol and number of embryos transferred.
Intracytoplasmic sperm injection protocol and number of embryos transferred.
Detailed description of hysteroscopic procedure.
Detailed description of anti‐adhesion therapy.
5. Interventions
Total number of intervention groups
Absence of other interventions in treatment and control groups
For each intervention and comparison group of interest:
specific intervention;
intervention details;
timing of the intervention.
6. Outcomes
Outcomes and time points reported
Definition and unit of measurement for each of the following outcomes.
Primary outcome
Live birth.
Presence of intrauterine adhesions at second‐look hysteroscopy.
Secondary outcomes
Pregnancy.
Miscarriage.
Mean adhesion scores at second‐look hysteroscopy.
Severity of adhesions at second‐look hysteroscopy.
For each outcome of interest:
sample size;
missing participants;
summary data for each intervention group in 2 × 2 table;
estimate of effect with 95% confidence interval;
subgroup analyses.
7. Miscellaneous
Funding source.
Key conclusions of study authors.
Miscellaneous comments from study authors.
References to other relevant studies.
Correspondence required.
Miscellaneous comments by review authors.
Data and analyses
Comparison 1. Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.42, 2.12] |
| 1.1 Device vs no tx | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.18, 5.46] |
| 1.2 Hormonal tx vs placebo/no tx | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.33] |
| 2 Clinical pregnancy | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.37, 2.01] |
| 2.1 Device vs no tx | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 18.08] |
| 2.2 Hormonal tx vs placebo | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.35, 2.06] |
| 3 Miscarriage | 2 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.18, 2.57] |
| 3.1 Device vs no tx | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 4.00] |
| 3.2 Hormonal tx vs placebo | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.10, 5.01] |
| 4 Presence of intrauterine adhesions at second‐look hysteroscopy | 8 | 560 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.21, 0.60] |
| 4.1 Device vs no tx | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Device + hormonal tx vs placebo/no tx | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Hormonal tx vs placebo | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.72] |
| 4.4 Gel vs no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.21, 0.64] |
| 5 Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐1.64, ‐1.29] |
| 5.1 Gel vs no tx | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐1.64, ‐1.29] |
| 6 Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐3.37, ‐3.23] |
| 6.1 Gel vs no tx | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐3.37, ‐3.23] |
| 7 Mild adhesions at second‐look hysteroscopy | 6 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.68, 2.61] |
| 7.1 Hormonal tx vs placebo/no tx | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.10] |
| 7.2 Gel vs no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.77, 3.18] |
| 8 Moderate or severe adhesions at second‐look hysteroscopy | 6 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.03, 0.24] |
| 8.1 Hormonal tx vs placebo/no tx | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.22] |
| 8.2 Gel vs placebo/no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.23] |
Comparison 2. Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.57, 3.83] |
| 1.1 Device + graft vs device | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.57, 3.83] |
| 2 Clinical pregnancy | 4 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.89, 3.33] |
| 2.1 Device + graft vs device | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.74, 3.18] |
| 2.2 Gel + hormone tx (HT) + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.62, 13.04] |
| 3 Miscarriage | 3 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.19] |
| 3.1 Device + graft vs device | 3 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.19] |
| 4 Presence of intrauterine adhesions at second‐look hysteroscopy | 5 | 451 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.36, 0.83] |
| 4.1 Device vs device | 1 | 162 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.42, 1.57] |
| 4.2 Device + graft vs device | 2 | 137 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.10] |
| 4.3 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.13, 0.76] |
| 4.4 Gel + HT + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.98] |
| 5 Mean adhesion scores in women treated for intrauterine adhesions | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Device vs device | 1 | 162 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.68, 0.68] |
| 5.2 Device + graft vs device | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐4.17, ‐2.03] |
| 5.3 Device + gel vs device | 1 | 111 | Mean Difference (IV, Random, 95% CI) | ‐1.6 [‐2.32, ‐0.88] |
| 6 Mild adhesions at second‐look hysteroscopy | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.34] |
| 6.1 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.34] |
| 7 Moderate or severe adhesions at second‐look hysteroscopy | 2 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.10, 0.61] |
| 7.1 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.09, 0.63] |
| 7.2 Gel + HT + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abu Rafea 2013.
| Methods | Parallel‐group randomised controlled trial. Single centre, Obstetrics and Gynecology Department, King Saud University, Riyadh, Saudi Arabia. Protocol approved by IRB: yes. Unclear whether statistical power calculation done (query not answered). Unclear about funding and conflicts of interest (query not answered). |
|
| Participants | Number recruited: not stated. Number randomly assigned: 28 women. Number excluded after randomisation: 4 women. Number analysed: 24 women. Women with infertility, adverse pregnancy outcomes (diagnosed with intrauterine septum by HSG, sonohysterography, hysteroscopy or a combination of these), or both. Inclusion and exclusion criteria: ill defined. Some women (1 in intervention group; 3 in control group) not trying to conceive after treatment, indicating poor definition of inclusion and exclusion criteria. Mean age and range (years): 29 (23‐38) years in intervention group; 32 (22‐40) years in control group. Study duration: not reported (query not answered). Number of subfertile women: 3 in intervention group; 2 in control group; most women had history of adverse pregnancy outcomes (miscarriage or preterm delivery). |
|
| Interventions |
Paediatric Foley catheter balloon for 5 days (intervention: n = 13) vs no catheter/balloon (control: n = 15) Cervix dilated to 10 mm, and all uterine septa divided using 26 French (9 mm diameter) resectoscope and a 30‐degree lens (Karl Storz, Tuttlingen, Germany) with monopolar electrode utilising 1.5% glycine as distension medium via an electronic fluid management system (Endomat, Karl Storz, Tuttlingen, Germany) and 120 Watts low‐voltage (cutting current mode) waveform delivered by an ICC 350 Erbe electrosurgical unit (Erbe, Tuttlingen, Germany). Resectoscopic metroplasty carried out using a Collin (Karl Storz, Tuttlingen, Germany) monopolar knife electrode at 90 degrees. All women had general anaesthesia and concomitant laparoscopy and treatment of pelvic pathology including adhesiolysis or reduction/excision of endometriosis, or both, when indicated using a CO2 laser or electrosurgery, or both. No‐one received preoperative endometrial thinning, antibiotic prophylaxis or adjuvant postoperative hormonal therapy. No specific timing was used to perform the surgery with regards to the menstrual cycle. Although reported that 2 women in intervention group and 1 in control group conceived after ART, whether other fertility treatments were offered and how these cotreatments were distributed among comparison groups (query not answered) remained unclear. |
|
| Outcomes | Length of residual septum: measured by HSG 12 weeks after operative hysteroscopy. First‐trimester loss, second‐trimester loss, preterm delivery, term delivery, ectopic pregnancy: measured at 12‐18 months after operative hysteroscopy. |
|
| Notes | No distinction between primary and secondary outcomes. Whether reproductive outcomes were measured at 1 or > 1 time points unclear; variation in time points at which reproductive outcomes measured was 6 months. Some women (1 in intervention group; 3 in control group) were not trying to conceive after treatment; they should have been excluded from analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on a computer generated list of numbers (unconcealed)." Comment: probably done. |
| Allocation concealment (selection bias) | High risk | Quote: "Randomization was based on a computer generated list of numbers (unconcealed)." Comment: no allocation concealment. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Quote: "After ethics approval and informed consent, 28 women were randomized in the operating room into having a no. 14 paediatric Foley catheter/balloon for five days (N = 13) versus no catheter/balloon (N = 15) following resectoscopic septum division. The Foley balloon was inflated with 5 mL of normal saline solution." Quote: "All patients were discharged the same day, and the patients with the Foley catheter/balloon were instructed to cut with scissors the end of the catheter at 5 days at home and remove the catheter themselves." Comment: physicians and personnel not blinded to intervention. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "They were also instructed to avoid pregnancy until their first assessment in 3 months by HSG, and they were reassessed at 6 and 12 to 18 months for pregnancy outcomes." Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) Adhesions | Unclear risk | Quote: "They were also instructed to avoid pregnancy until their first assessment in 3 months by HSG, and they were reassessed at 6 and 12 to 18 months for pregnancy outcomes." Quote: "We could not be certain that the < 1 cm septum, reported by the radiologist, in the balloon group was a recurrence or incomplete division at the time of metroplasty, but in the intention‐to‐treat (ITT) analysis, we considered this cavity as normal." Comment: no blinding of outcome assessors reported; unclear who did the assessment (query not answered). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "We could not be certain that the < 1 cm septum, reported by the radiologist, in the balloon group was a recurrence or incomplete division at the time of metroplasty but in the intention‐to‐treat (ITT) analysis, we considered this cavity as normal." Comment: no incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | High risk | Quote: "Fertility and pregnancy outcomes at 12 to 18 months post metroplasty are shown in Table 4." Comment: reproductive outcomes measured over considerable time period rather than at 1 predefined time point. Unclear whether more measurements were taken at 18 months in 1 of the comparison groups. Comment: although it reported that 2 women in intervention group and 1 in control group conceived after ART, whether other fertility treatments were provided and how these cotreatments were distributed among comparison groups was unclear. Some women (1 in intervention group; 3 in control group) were not trying to conceive after treatment; they should have been excluded from final analysis because conducting an ITT on the basis of poor inclusion and exclusion criteria can increase risk of bias. Comment: according to Table 1 of publication, mean age (range) in intervention was 29 (23‐38) years and control was 32 (22‐40) years with P = 0.59. Mean age difference should not be considered clinically irrelevant. We judged that some evidence suggested baseline imbalance between comparison groups. Comment: high risk of selection, performance and detection bias. |
Acunzo 2003.
| Methods | Parallel‐group randomised controlled trial. Single centre, Hysteroscopic Unit at the University of Naples Frederico II, Naples, Italy. Protocol approved by IRB: yes. Unclear whether statistical power calculation was done (query not answered). Funding and conflicts of interest not reported (query not answered). |
|
| Participants | Number recruited: 92 women. Number randomly assigned: 92 women. Number lost to follow‐up: 8 women. Number analysed: 84 women. 92 women with irregular menses and IUAs at diagnostic hysteroscopy. Inclusion criterion:
Exclusion criteria:
Study duration: 15 months (June 2001 to September 2002). Mean age (± SD): 30.1 (± 3.5) years. Number of subfertile women: 18 in intervention group; 16 in control group. |
|
| Interventions |
ACP gel (intervention: n = 46) vs no application of ACP gel (control: n = 46) Intervention group: received intrauterine application of 10 mL of ACP gel (Hyalobarrier Gel; Baxter, Pisa, Italy) under hysteroscopic view after operative hysteroscopy. Control group: only received hysteroscopic resection of IUAs. Diagnostic hysteroscopy performed with a 3.5‐mm instrument (Gynecare Versascope; Gynecare, Ethicon Inc., Somerville, NJ, USA) with normal saline solution (sodium chloride 0.9%) used as distension medium. Operative hysteroscopy was performed with a rigid resectoscope (Karl Storz, Tuttlingen, Germany) with a 12‐degree fore‐oblique telescope and a hook‐shaped monopolar electrode. Women in both groups received oral antibiotics (cefixime 400 mg/day) (Cefixoral; Menarini, Firenze, Italy) for 3 days after surgery. |
|
| Outcomes | Incidence of de novo adhesions, mean adhesion score and severity of adhesions according to the 1988 AFS classification system; all outcomes measured after 3 months. | |
| Notes | Individual data on subfertile women not presented separately (query not answered). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Following diagnostic hysteroscopy, patients were randomized into two groups: group A (N = 46), the treatment group, and group B (N = 46), the control group, using a computer‐generated randomisation list." Comment: probably done, as the same team of investigators published data from similar randomised trial. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method not described (query not answered). |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Quote: "Ultrasound scans were performed in each patient from group A immediately after ACP gel application and after 24, 48 and 72 hours. The gel‐related hyperechoic thickness that seemed to separate endometrial walls was the mean evaluated parameter." Comment: no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) Adhesions | Unclear risk | Quote: "Both the initial diagnostic hysteroscopy and the 3‐month follow‐up diagnostic hysteroscopy were performed by the same operator (G.A.). G.A. evaluated the adhesion score for each patient and was blind for patients' randomized allocation, whilst operative hysteroscopies and application of ACP gel were performed by a different operator (M.G.)." Comment: method not described (query not answered). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Eight women (three from group A [intervention] and five from group B [control]) did not attend for follow‐up hysteroscopy." Comment: unlikely to cause substantial attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | Low risk | Comment: no evidence of imbalance in baseline participant characteristics ‐ no cotreatment. |
Amer 2010.
| Methods | Parallel‐group randomised controlled trial with 3 comparison groups. Single centre, Department of Obstetrics and Gynecology of the Ain Shams Medical School, Cairo, Egypt. Protocol approved by IRB: yes. No statistical power calculation (query clarified by Dr Mohamed Amer). No external funding and no conflicts of interest (query clarified by Dr Mohamed Amer). |
|
| Participants | Number recruited: 45 women. Number randomly assigned: 45 women. Number lost to follow‐up: 2 women. Number analysed: 43 women Inclusion criteria:
Exclusion criteria:
Study duration: 62 months (from June 2004 to August 2009) Median age (range): 30.4 (26‐40) years. |
|
| Interventions |
Intrauterine balloon without amniotic graft (group 1; n=15) vs intrauterine balloon with fresh amnion (group 2; n=15) vs intrauterine balloon with dried amnion (group 3; n=15) 2 × misoprostol 200 mg tablets inserted vaginally the night before operation to facilitate cervical dilation. Operative hysteroscopy performed under general anaesthesia in follicular phase of menstrual cycle; however, for women with amenorrhoea, no special time was chosen. Simultaneous laparoscopy performed in women with infertility if they had not undergone a laparoscopy before, in women with previous complications of hysteroscopy such as uterine perforation and in women in whom uterine perforation occurred during the present procedure. Hysterometry with uterine sounding was followed by lysis of IUAs using 5‐French pointed tip semirigid scissors in 5‐mm rigid clinic hysteroscope, based on a 2.9‐mm telescope (Karl Storz GmbH & Co. KB). In women with thick fibrous adhesions, adhesiolysis performed using 9‐mm working element along with sheath and 4‐mm 30‐degree telescope (Karl Storz GmbH & Co. KB) equipped with a hysteroscopic monopolar knife (Collin operating knife) after cervical dilation to Hegar 9. Visualised adhesions incised with 50‐ to 100‐W cutting current, adjusted according to visual tissue effects, from an isolated electrosurgical generator (Valleylab SSE2L; Valleylab, Inc., Boulder, CO, USA). Glycine 1.5% (Glycocolle 1.5%; Aguettant Laboratory, Lyon, France) used as distension medium, with intrauterine pressure 120‐150 mmHg, automatically controlled using a Hamou Hysteromat (Karl Storz GmbH & Co. KB) with termination of procedure if fluid deficit exceeded 1 L. Freeze‐dried amniotic membrane hydrated using normal saline solution in a pan for 10 minutes before use. Previously prepared fresh amniotic graft was washed several times with sterile normal saline solution before application. Amniotic graft was cut to form a 5 × 5‐cm piece. This was spread on the balloon end of an 8‐French paediatric Foley catheter, so that the epithelial or basement membrane surface would be on top facing outwards, where the inflated balloon acts as a mould for the amnion. The catheter tip with the amnion on its surface was then introduced into inside of uterine cavity with aid of straight artery forceps. Balloon inflated with 3 mL to 5 mL of saline solution. A loose knot was made in catheter stem, which was then slipped upwards to just below the inflated balloon, then was tightened with aid of artery forceps, and catheter stem was cut with scissors just below knot after catheter stem was stretched so that balloon with graft on its surface was kept intrauterine. In women with a patulous cervix that would not keep the inflated balloon inside uterus, a cervical cerclage using braided polyester tape (Matrix Health Care SAE, Ameco, Egypt) was applied; it was removed later with the balloon. Postoperatively, ethinyl oestradiol 50 μg/day tablets (Laboratoires Cassenne, Puteaux, France) administered for 50 days. 2 weeks postoperatively, balloon was removed transcervically with crocodile forceps and with participant under paracervical anaesthesia (lidocaine 2%, 6 mL, plus atropine 0.5 mg in the same syringe), as an outpatient procedure without cervical dilation. In women who had cervical cerclage, tape was removed at time of balloon extraction. Second‐look hysteroscopy performed 2‐4 months postoperatively by independent observer blinded to method. Outcome measures included improvement in adhesion grade, improvement in menstruation, increased uterine length at sounding and complications. Subsequently, follow‐up provided via direct contact or telephone every 3 months for a mean (range) of 28 (6‐60) months for menstrual pattern and fertility. |
|
| Outcomes | Ongoing pregnancy rate, clinical pregnancy rate, adhesion score, duration of menstruation, improvement in menstruation, uterine length, uterine length increase, adhesion score improvement; some outcomes (improvement in adhesion grade, improvement in menstruation, increased uterine length at sounding and complications) assessed 2‐4 months after surgery, whereas other outcomes assessed via direct contact or telephone every 3 months for a mean (range) of 28 (6‐60) months for menstrual pattern and fertility. | |
| Notes | * Correspondence with authors on 4 January 2015. Dear Dr. Jan Bosteels, Thanks for your e‐mail and being interested in intrauterine adhesions management. 1. The first study is a pilot study and not a randomized study (Amer MI, Abd‐El‐Maeboud KH. Amnion graft following hysteroscopic lysis of intrauterine adhesions J Obstet Gynaecol Res 2006; 32(6): 559‐66). 2. I confirm that these two studies are different and no patients in the second study were involved in the first study. 3. It was a single‐blinded; only the first surgeon knew if the graft was used or not and which type; also the patient, but the assessor, did not know which group of patients he is assessing. 4. Analyses were conducted using commercially available software (SPSS for Windows, release 15.0; SPSS, Inc., Chicago, IL). All P values refer to 2‐tailed tests of significance, with P <0.05 considered significant. Data are given as count and percentage for categorical variables. Groups were compared using the c2 test and Fisher's exact test for categorized variables. For comparison of menstruation, uterine length and adhesion score, the Kruskal‐Wallis test was used. Data are given as median (interquartile range [IQR]; 25th to 75th percentile). Pairwise comparison was performed using the Mann‐Whitney test with Bonferroni correction. The critical level of significance was <0.02). 5. There was no funding for the present study. 6. There was no conflict of interest. 7. To my knowledge, I do not know that there are new anti‐adhesion therapy following operative hysteroscopy. With my best wishes. Dr. Mohamed I Amer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized preoperatively using a computer‐generated randomisation sheet into 3 groups of 15 women each." Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation to any group was concealed in an opaque envelope, which was opened at the time of operation." Comment: probably done. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Quote: "It was a single blinded, only the first surgeon that know if the graft used or not and which type also the patient, but the assessor did not know which group of patients he is assessing" (query clarified by Dr Mohamed Amer). Comment: method of blinding of participants and personnel not described. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "This was a pilot, randomized, comparative study with blinded independent evaluation of changes in adhesion grade, menstruation, uterine length, number of operations needed to achieve a functional uterine cavity, reproductive outcome, and complications." Quote: "A second‐look hysteroscopy was performed 2 to 4 months postoperative by an independent observer blinded to the method." Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) Adhesions | Low risk | Quote: "This was a pilot, randomized, comparative study with blinded independent evaluation of changes in adhesion grade, menstruation, uterine length, number of operations needed to achieve a functional uterine cavity, reproductive outcome, and complications." Quote: "A second‐look hysteroscopy was performed 2 to 4 months postoperative by an independent observer blinded to the method." Quote: "It was single blinded ‐ only the first surgeon knew if the graft was used or not and which type, also the patient, but the assessor did not know which group of patients he was assessing" (query clarified by Dr Mohamed Amer). Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 45 patients included in the study, 2 were lost to follow‐up (1 each in groups 1 and 2) and were excluded from analysis." Comment: unlikely to cause attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | High risk | Baseline imbalance in participant characteristics concerning caesarean section likely, as cause of IUAs. Quote: "Simultaneous laparoscopy was performed in women with infertility if they did not undergo laparoscopy before, in those with previous complications of hysteroscopy such as uterine perforation or if uterine perforation occurred during the present procedure." Comment: cotreatment by laparoscopy and distribution in numbers among comparison groups not stated. Quote: "All pregnancies were spontaneous except 3 that were achieved after in vitro fertilization (IVF). One pregnancy was terminated at 7 weeks' gestation because of a blighted ovum. Two patients underwent IVF treatment twice, but did not conceive. The other patients could not afford the cost of IVF." Comment: cotreatment with IVF in some women, resulting in 3 pregnancies; no available data on distribution of cotreatment among the 3 comparison groups. Potential for performance bias. |
Dabir‐Ashrafi 1996.
| Methods | Parallel‐group randomised controlled trial. Single centre, national referral university hospital in Tehran, Iran. Protocol approved by IRB: not reported (query not answered). Unclear whether statistical power calculation done (query not answered). Funding and conflicts of interest not reported (query not answered). |
|
| Participants | Number recruited: 59 women. Number excluded before randomisation: 13 women (9 women had abnormal findings at workup; 4 women excluded because angle between cervix and corpus could not be corrected). Number randomly assigned: 46 women. Number lost to follow‐up: 0 women. Number analysed: 46 women. Women with subfertility (15 women) and habitual abortion (44 women) with fundal defect on HSG. Underwent workup that included sperm analysis, assessment for infectious diseases (toxoplasmosis, Listeria monocytogenes, Mycoplasma hominis, syphilis), karyotyping, hormone profile (thyroxine, tri‐iodothyronine, thyroid‐stimulating hormone, T3 resin uptake, prolactin) and mid‐luteal progesterone assay. The 50 women whose examinations were normal and in whom diagnosis of septate uterus was confirmed by laparoscopy participated. Study duration: start and end dates not reported. Age: 26.7 ± 6.5 years in intervention group; 28.4 ± 4.5 years in control; note: not reported whether these numbers are means or medians with SDs. |
|
| Interventions |
Oestrogen (intervention: n = 23) vs no oestrogen (control: n =23) All women underwent hysteroscopic incision of septum with mini‐scissors by 1 surgeon who was unaware of treatment group. Ampicillin 1 g injected 1 hour before operations performed under general endotracheal anaesthesia. Distending medium 5% dextrose in water. Blood pressure cuff wrapped around plastic bottle to raise pressure of medium. Procedures performed with 7‐mm hysteroscope under laparoscopic guidance. Intervention group: conjugated oestrogen 1.25 mg/day 30 days beginning on day of operation. For last 7 days, they also took medroxyprogesterone acetate tablet 2 × 5‐mg/day. Control group: no hormone. Neither group used a splint. |
|
| Outcomes | Difference between ratios of length of septum to length of uterus in HSGs obtained preoperatively and postoperatively, directly measured on HSG on cessation of menstruation 1 month after procedure. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomized into two groups of 23 women each." Comment: method not stated (query not answered). |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All septal incisions were performed by one surgeon, who was unaware of the group to which a patient had been assigned." Comment: method not stated (query not answered). |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome; no live birth or pregnancy rates reported. |
| Blinding of participants and personnel (performance bias) Adhesions | Unclear risk | Quote: "The patients were randomized into two groups of 23 women each. Group 1 [intervention] received conjugated oestrogen 1.25 mg/d 30 days beginning on the day of the operation. For the last 7 days, they also took medroxyprogesterone acetate two 5‐mg tablets/d. Group 2 [control] received no hormone." Comment: unclear whether placebo pills used to blind participants and personnel (query not answered). |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome; no live birth or pregnancy rates reported. |
| Blinding of outcome assessment (detection bias) Adhesions | Unclear risk | Quote: "Directly on cessation of menstruation 1 month after the procedure, HSG was done and the results were compared with those of the preoperative HSG." Comment: outcome assessors not identified in report, method of blinding not reported (query not answered). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Four were omitted from the analysis because the angle between the cervix and the uterine corpus could not be corrected, as shown by HSG." Comment: 4/50 (8%) women were excluded; distribution among comparison groups not reported (query not answered). |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | Low risk | No evidence of baseline imbalance in participant characteristics. |
De Iaco 2003.
| Methods | Parallel‐group randomised controlled trial. Single centre, Department of Obstetrics and Gynecology of the University of Bologna, Bologna, Italy. Protocol approved by IRB: yes. No statistical power calculation for all outcomes (query clarified by Dr Pierandrea De Iaco). No external funding and no conflicts of interest (query clarified by Dr Pierandrea De Iaco). |
|
| Participants | Number recruited: 60 women. Number randomly assigned: 60 women. Number lost to follow‐up: 20 women. Number analysed: 40 women. Quote: "Women were eligible for inclusion if they were undergoing endometrial ablation or hysteroscopic removal of submucosal fibroids, endometrial polyps, septate uterus or intrauterine synechiae." Comment: source population not adequately described in numbers and characteristics. Quote: "Despite this, newly induced synechiae were less severe in the Hyalobarrier gel treated patients, thus reducing the risk of pregnancy morbidity and improving the outcomes of hysteroscopic surgery." Comment: not mentioned whether women were infertile, and if so, how many; some subfertile women might have been included. Study duration: 36 months: 1998 to 2001 (query clarified by Dr Pierandrea De Iaco). Age: 18‐65 years. |
|
| Interventions |
Application of Hyalobarrier gel (intervention: n = 18 women analysed) vs no adhesion prevention (control: n = 22 women analysed) Number of women randomly assigned to each group not reported and not clarified by study authors. Intervention group: gel applied with 20‐cm cannula with 5‐mm diameter to cover entire uterine cavity. Mean (± SD) volume 10.5 ± 5.5 mL Hyalobarrier gel (range 5 to 20 mL) applied in uterine cavity. Control group: no adhesion prevention measures. An 8‐mm hysteroscopic resectoscope (Storz, Tuttlingen, Germany) with electrosurgical tips used. In all cases, sorbitol‐mannitol (Clear‐Flex, Baxter SA, Lessines, Belgium) used as distension medium; fluid intake and output continuously monitored (Hysteromat, Storz). Second‐look hysteroscopy undertaken 9 weeks after initial procedure by blinded investigator after insertion into uterine cavity with a 5‐mm hysteroscope (Storz) with CO2 distension. |
|
| Outcomes | Incidence of de novo adhesions and severity of adhesions according to ASRM* modified scoring system: all outcomes measured after 9 weeks. | |
| Notes | *ASRM modified scoring system distinguishes only between stage I (mild) and stage II (severe) adhesions (different from the AFS 1988 classification system for IUAs). Correspondence with authors on 9 December 2014. Dear Dr. Jan Bosteels I have to admit that I have some difficulties in finding the data you are asking about research details. Anyway, these are my answers: 1. no statistical power had been used before the trial. 2. no funding, nor conflict of interest were present. 3. I have some difficulties in telling the precise period. I say: 1998‐2001. 4. patients were randomly allocated using a random table (from literature). 5. Dr. De Iaco performed the hysteroscopic surgery, while Dr. Muzzupapa performed the second‐look hysteroscopy without knowing the group of treatment. 6. I am not aware of ongoing studies about the same issue. Sincerely yours Pierandrea Dr. Pierandrea De Iaco Responsabile SSD Oncologia Ginecologica Policlinico Sant'Orsola‐Malpighi Via Massarenti 13 ‐ 40138 Bologna Fax 0516364392 Cell. 3356666354 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After completion of the surgical procedure, the patients who met the inclusion criteria were randomly assigned either to the treatment with Hyalobarrier gel or to the control group, according to a computer‐generated randomisation schedule." Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated using a random table" (query clarified by Dr Pierandrea De Iaco). Comment: method of allocation concealment not described. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) Adhesions | Unclear risk | Quote: "Dr. De Iaco performed the hysteroscopic surgery, while Dr. Muzzupapa performed the second‐look hysteroscopy without knowing the group of treatment" (query clarified by Dr Pierandrea De Iaco). Comment: method of blinding of participants and personnel not described. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) Adhesions | Low risk | Quote: "Second look hysteroscopy was undertaken nine weeks after the initial procedure by a blinded investigator after insertion in the uterine cavity of a 5 mm hysteroscope (Storz) with CO2 distension." Quote: "Dr. De Iaco performed the hysteroscopic surgery, while Dr. Muzzupapa performed the second‐look hysteroscopy without knowing the group of treatment" (query clarified by Dr Pierandrea De Iaco). Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Sixty patients aged from 18 to 65 years old were enrolled in the study and written, informed consent was obtained from each patient." Quote: "A total of 40 patients attended the postoperative diagnostic hysteroscopy, 18 in the intervention and 22 in the control group." Comment: loss to follow‐up of 20/60 enrolled participants, very likely to cause substantial attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | High risk | Quote: "In conclusion, the authors recognize that the data reported lack statistical significance given the small sample size of the population evaluated. Despite this, newly induced synechiae were less severe in the Hyalobarrier gel treated patients, thus reducing the risk of pregnancy morbidity and improving the outcomes of hysteroscopic surgery." Comment: our own recalculation demonstrated that differences were not statistically significant; primary study authors' conclusions were not based on results. Baseline characteristics in both comparison groups not explicitly presented; P values not given. |
Di Spiezio Sardo 2011.
| Methods | Parallel‐group randomised controlled trial. Single centre, Hysteroscopic Unit of the University of Naples Frederico II, Naples, Italy. Protocol approved by IRB: yes. Statistical power calculation for primary outcome of incidence of de novo adhesions (query clarified by Dr Attilio DiSpiezio Sardo). No external funding and no conflicts of interest (query clarified by Dr Attilio DiSpiezio Sardo). |
|
| Participants | Number recruited: 136 women. Number excluded before randomisation: 26 women (8 women declined after explanation of study protocol; 18 women excluded because they were unwilling to undergo surgery). Number randomly assigned: 110 women. Number lost to follow‐up: 0 women. Number excluded after randomisation: 24 women. In intervention group, 11/55 women, and in control group, 13/55 women, treated with endometrial ablation for resistant dysfunctional bleeding; these 24 participants were excluded from analyses, as endometrial ablation/resection is not indicated as a fertility‐enhancing surgical intervention. This judgement was subjected to several sensitivity analyses. Number analysed: 86 women. Premenopausal women diagnosed at clinic diagnostic hysteroscopy (n = 136) with single or multiple lesions suitable for surgical treatment or with resistant dysfunctional uterine bleeding requiring endometrial ablation invited to participate. Of 26 women who declined, 8 declined after explanation of study protocol, and 18 were excluded because they were unwilling to undergo surgery. Between September 2008 and June 2009, 110 premenopausal women were enrolled in study. Exclusion criteria:
Number of subfertile women with or without abnormal uterine bleeding: 12 in intervention group; 9 in control group; not possible to obtain individual outcome data for this small subgroup of subfertile women for IPD analysis (query clarified by Dr Attilio DiSpiezio Sardo). Duration of study: 10 months: September 2008 to June 2009. Mean age (± SD) in intervention group: 37 (± 3.1) years. Mean age (± SD) in control group: 36 (± 2.9) years. |
|
| Interventions |
Intercoat gel (intervention: n = 55) vs no gel (control: n = 55) Intervention group: after surgery, women underwent intrauterine application of 10 mL Intercoat gel under hysteroscopic guidance through inflow channel of resectoscope while operator gradually moved resectoscope from fundus of uterus back to external uterine ostium to apply gel throughout cavity and cervical canal. Procedure considered complete when, under hysteroscopic visualisation, gel seemed to have replaced all liquid medium, and cavity appeared completely filled by gel from tubal ostia to external uterine orifice. Control group: hysteroscopic surgery alone. Clinic diagnostic hysteroscopy performed with 5‐mm continuous‐flow hysteroscope with oval profile, a 30‐degree fore‐oblique telescope and a 5‐F operating channel (Karl Storz GmbH & Co. KG, Tuttlingen, Germany). Sodium chloride 0.9% solution used as distension medium and administered through electronic system of irrigation/aspiration (Endomat; Karl Storz GmbH & Co. KG). Operative hysteroscopy performed with rigid 27‐F resectoscope with 30‐degree fore‐oblique telescope with various bipolar loops and a bipolar energy source (Versapoint; Gynecare, division of Ethicon, Inc.). Sodium chloride 0.9% solution used as distension medium. Administration of antibiotics not reported. |
|
| Outcomes | Incidence of de novo adhesions, severity of adhesions according to 1988 AFS classification system and improvement of degree of patency of internal uterine ostium; all outcomes measured after 4 weeks (during early proliferating phase of following menstrual cycle). | |
| Notes | * Correspondence with authors on 27 December 2014: 1. Which method was used for a statistical power calculation before the trial? Our primary outcome was measured by the incidence of de novo IUA. On the basis of data previously published by our group [Guida M, Acunzo G, Di Spiezio Sardo A, Bifulco G, Piccoli R, Pellicano M, Cerrota G, Cirillo D, Nappi C. Effectiveness of auto‐cross‐linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a prospective randomized, controlled study. Hum Reprod 2004;19:1461‐1464; Acunzo G, Guida M, Pellicano M, Tommaselli GA, Di Spiezio Sardo A, Bifulco G, Cirillo D, Taylor A, Nappi C. Effectiveness of auto‐cross‐linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a prospective randomized, controlled study. Hum Reprod 2003;18:1918‐1921], we expected the incidence of adhesions at follow‐up in patients undergoing hysteroscopic procedures with the application of the gel to be 10%, and without to be 28%, respectively. These figures are consistent with current literature, which shows a mean incidence of IUA of 25% after common resectoscopic procedures (polypectomy, myomectomy and metroplasty) if adjusted by taking into account that our study was meant to include more adhesiogenic procedures such as endometrial ablation. For the probability of a type 1 statistical error to be less than 0.05, we calculated that a sample of 55 patients per group would provide 80% of statistical power. 2. Was there any funding for the present study? Was there any conflict of interest? The study was not funded by an external source. All authors had no conflict of interest regarding this study at that time. 3. Is it possible to provide the outcome data of the infertile women included in this study to be able to analyse them on an individual level? Unfortunately it is not possible. However the infertile patients were only a small proportion (12 Group 1 [intervention]; 9 Group 2 [control]). 4. Which method was used to conceal the allocation to one of the two interventions? The allocation sequence was concealed from the researchers (S.M, B.M, S.M.) who enrolled and assessed the participants and attached a sequentially numbered, opaque, sealed and stapled envelope containing the allocated treatment to the clinical record of the patient after having signed the informed consent. The envelope was opened immediately after the surgical removal of the intrauterine removal of the removal of the intrauterine lesion, in order for the surgeon (A.D.S.S.) to either inject the gel (group 1 [intervention]) or not (group 2 [control]). Patients were blinded to the procedure until the end of the study. This single‐blind study design was adopted to reduce bias derived from the patient's knowledge of which procedure she underwent. 5. How were the study participants, the treating physicians and the outcome assessors blinded? Who did the outcome assessments? Finally, are you aware of any ongoing research on anti‐adhesion therapy following operative hysteroscopy? Patients were blinded since they underwent operative hysteroscopy in general anaesthesia or loco‐regional anaesthesia (they were awake but couldn't see the monitor) and were kept blinded until the three months follow‐up visit. The treating physician (A.D.S.S.) was blinded until removal of the intrauterine lesion or after endometrial ablation, when he was informed whether to inject or not the intrauterine gel. The assessor (M.G.) was blinded since he performed the baseline and the follow‐up hysteroscopies and did not participate to the operative hysteroscopies, so he was completely unaware of the allocation of patients. This single‐blind study design was adopted to reduce bias derived from the patient's knowledge of which procedure she underwent. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After diagnostic hysteroscopy, patients were randomized via computer‐generated randomisation list into group 1 (treatment group: operative hysteroscopy plus intrauterine application of Intercoat gel; N = 55) and group 2 (control group: operative hysteroscopy alone; N = 55)." Comment: probably done, as the same team of investigators has published data on a similar randomised trial |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was concealed from the researchers (S.M, B.M, S.M.) who enrolled and assessed the participants and attached a sequentially numbered, opaque, sealed, and stapled envelope containing the allocated treatment to the clinical record of the patient after having signed the informed consent" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: probably done. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Quote: "Patients were blinded since they underwent operative hysteroscopy in general anaesthesia or loco‐regional anaesthesia (they were awake but couldn't see the monitor) and were kept blinded until the three months follow‐up visit" (query clarified by Dr Attilio DiSpiezio Sardo). Quote: "The envelope was opened immediately after the surgical removal of the intrauterine removal of the removal of the intrauterine lesion, in order for the surgeon (A.D.S.S.) to either inject the gel (group 1) or not (group 2)" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: personnel not blinded; participants blinded (query clarified by Dr Attilio DiSpiezio Sardo). |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) Adhesions | Low risk | Quote: "Both the initial and follow‐up diagnostic hysteroscopy were performed by the same surgeon (M.G.), who, blinded to patients' randomized allocation, also evaluated the rate and severity of adhesions in each patient." Quote: "The assessor (M.G.) was blinded since he performed the baseline and the follow‐up hysteroscopy and did not participate to the operative hysteroscopy, so he was completely unaware of the allocation of patients" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Intention‐to‐treat was the analysis method used; however, there were no deviations from random allocation." Comment: probably done; unlikely to cause attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | Low risk | Comment: no evidence of imbalance in baseline participant characteristics; no cotreatment. |
Do 2005.
| Methods | Parallel‐group randomised controlled trial. Single centre, Hallym University Kangdong Sacred Heart Hospital, Seoul, South Korea. Protocol approved by IRB: not reported; no contact possible due to absence of contact details. Statistical power calculation not reported; no contact possible due to absence of contact details. External funding and conflicts of interest not reported; no contact possible due to absence of contact details. |
|
| Participants | Number recruited: 64 women. Number randomly assigned: 64 women. Number excluded: 2 women, reason for exclusion not reported. Number lost to follow‐up: 0 women. Number analysed: 62 women. Inclusion criterion:
Exclusion criteria:
Proportion of women with infertility: unclear if infertile women were included or excluded. Study duration of study: 10 months. Mean age (range): 28 (22‐43) years. Mean age in intervention group: 26 years. Mean age in control group: 31 years. |
|
| Interventions |
HA/CMC gel (intervention: n = 32) vs saline (control: n = 30) Intervention group: after intrauterine surgery, 10 mL of HA + CMC applied on uterine cavity. Control group: 10 mL of saline applied. After surgery, antibiotics injected for 1 day, and then oral antibiotics administered for 3 days. Women who underwent dilatation and curettage were discharged on 1st postoperative day, and women who underwent hysteroscopy were discharged on 2nd postoperative. |
|
| Outcomes | Frequency and severity of IUAs compared by microhysteroscopy on 4th postoperative week, severity of IUAs classified in accordance with AFS 1988 guidelines. | |
| Notes | No contact data of the primary study authors reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized to 32 patients of study group (group A) and 32 patients of control group (group B) each." Comment: method not described; unclear if stratified randomisation was used; no contact possible. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described; no contact possible. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "After intrauterine surgery, in group A [intervention], 10ml of Hyaluronic acid + Sodium Carboxymethyl Cellulose (HA + CMC) was applied on uterine cavity, and in group B [control], 10ml of saline was applied." Comment: surgeons not blinded; easy to distinguish saline from gel. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Quote: "After intrauterine surgery, in group A, 10ml of Hyaluronic acid + Sodium Carboxymethyl Cellulose (HA + CMC) was applied on uterine cavity, and in group B, 10ml of saline was applied." Comment: surgeons not blinded; easy to distinguish saline from gel. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Not reported if gynaecologists who performed second‐look procedure 4 weeks after surgery were blinded or not; no contact possible. |
| Blinding of outcome assessment (detection bias) Adhesions | Unclear risk | Not reported if gynaecologists who performed second‐look procedure 4 weeks after surgery were blinded or not; no contact possible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In total 64 patients, 62 patients were followed up postoperatively. Group A was 32 patients, Group B was 30 patients, and 2 patients were excluded during study." Comment: reasons for postrandomisation exclusion not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | High risk | Quote: "See table of baseline characteristics Age in years group A [intervention]: 26 Age in years group B [control]: 31 Parity in group A: 0.8 Parity in group B: 1.5 Abortion in group A: 1.0 Abortion in group B: 1.8." Comment: high risk of selection bias. Quote: "As a result of transvaginal sonography, intrauterine adhesion was observed at 4 patients (13%) out of 32 patients in group A and had mild intrauterine adhesions." Comment: unclear if micro‐hysteroscopy or transvaginal ultrasound used for outcome assessment of IUAs. High risk of information bias. |
Fuchs 2014.
| Methods | Parallel‐group randomised controlled trial. Single centre, gynaecologic endoscopy unit of a tertiary care medical centre in Zerifin, Israel. Protocol approved by IRB: yes. Post hoc statistical power calculation; non‐inferiority design. No external funding and no conflicts of interest (query clarified by Dr Moty Pansky). |
|
| Participants | Number recruited: 110 women. Number excluded before randomisation: 58 women (14 did not meet inclusion criteria; 37 declined to participate; 7 excluded for other reasons). Number randomly assigned: 52 women. Number lost to follow‐up: 11 women. Number analysed: 41 women. Women who underwent hysteroscopic surgery because of suspected RPOC between September 2009 and June 2012 invited to participate in study, and enrollees gave signed informed consent. Inclusion criteria:
Study duration: 34 months; September 2009 to June 2012. Mean age (± SD) in intervention group: 29.5 (± 5.1) years. Mean age (± SD) in control group: 31.4 (± 6.5) years. Quote: "The study didn't include women with primary subfertility" (query clarified by Dr Moty Pansky). Comment: only women with confirmed fertility included in study. |
|
| Interventions |
Oxiplex gel (intervention: n = 21) vs no gel (control: n = 20) All hysteroscopic procedures performed under general anaesthesia. Pelvic bimanual examination performed under anaesthesia, and findings recorded in the medical records. Uterus considered enlarged when uterine fundus was palpated above pelvic brim. Sodium chloride 0.9% solution used as distension medium. Suspected RPOC removed via blunt dissection, with 4‐mm loop resectoscope (Stryker Corp., Kalamazoo, MI, USA) as a curette and under direct hysteroscopic view. All specimens sent for pathological analysis. Intervention group: after completion of hysteroscopic dissection, Oxiplex gel inserted into uterine cavity, up to complete filling of the cavity or up to 10 mL gel, whichever occurred first. All women discharged from the hospital several hours after procedure. Control group: no gel. Both intervention and control groups received sequential hormonal treatment (oestradiol valerate 2 mg/day for 11 days, followed by oestradiol valerate 2 mg/day + norgestrel 0.5 mg/day for 10 days) and antibiotic therapy (amoxicillin‐clavulanic acid, 875 mg, twice daily for 7 days). All women underwent diagnostic clinic hysteroscopy at 6‐8 weeks after operative procedure, performed by a surgeon blinded to treatment group. |
|
| Outcomes | Intraoperative and postoperative complication rates, incidence of moderate or severe adhesions and pregnancy defined as a positive heartbeat (query clarified by Dr Moty Pansky). Comment: primary and secondary outcomes not determined. |
|
| Notes | Quote: "Because this was a pilot study using a non‐inferiority design, post hoc power analysis was performed. This calculation showed that the power for detection of a statistically significant difference in rates of intrauterine adhesions between the 2 groups was 24%." Comment: study was substantially underpowered for the outcome of incidence of moderate or severe IUAs. * Correspondence with authors on 19 January 2015: 1. The first citation is an interim analysis that included 30 women, and was presented at AAGL [American Association of Gynecologic Laparoscopists] on 2011. The second citation is the final analysis that was published in JMIG [Journal of Minimally Invasive Gynecology] 2014 and included 52 women. The study population of the second citation includes all 30 women from the first one and 22 additional women. 2. Allocation was based on a computer‐generated randomisation scheme that was prepared in advance by the study coordinator. Sealed envelopes containing allocation were opened only following consent by the treating physician. The study coordinator documented the allocation on a password protected computer. 3. The control group received NS [normal saline] at the end of the procedure. The participants didn't know which group they were allocated to, nor did the outcome assessors. Naturally, the treating physician at time of procedure was aware of the treatment. Treating physicians' identity was documented and the study coordinator made sure that different physicians performed the treatment and the assessment per patient. 4. The gel was provided by J&J [Johnson & Johnson]. There was no funding for the study. There was no conflict of interest. 5. The study didn't include women with primary subfertility. 6. This was a pilot study designed to assess safety, hence there was no distinction between primary and secondary outcomes. 7. Pregnancy was defined as a positive heartbeat. 8. We are not aware of any ongoing research on anti‐adhesion therapy following operative hysteroscopy." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study entrants, in blocks of 12, were randomly allocated via a computer‐generated randomisation schedule, using institutional computer software, to treatment with (study group) or without (control group) Oxiplex gel." Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was based on a computer generated randomisation scheme that was prepared in advance by the study coordinator. Sealed envelopes containing allocation were opened only following consent by the treating physician. The study coordinator documented the allocation on a password protected computer" (query clarified by Dr Moty Pansky). Comment: probably done. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Quote: "Different surgeons performed the operative hysteroscopy and the follow‐up diagnostic hysteroscopy. Both the patients and the surgeons who performed the follow‐up studies were unaware of patient group assignment." Quote: "The participants didn't know which group they were allocated to, nor did the outcome assessors. Naturally, the treating physician at time of procedure was aware of the treatment. Treating physicians' identity was documented and the study coordinator made sure that different physicians performed the treatment and the assessment per patient" (query clarified by Dr Moty Pansky). Comment: participants probably blinded, as they were under general anaesthesia, but treating physicians not blinded. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) Adhesions | Low risk | Quote: "Different surgeons performed the operative hysteroscopy and the follow‐up diagnostic hysteroscopy." Quote: "All patients underwent diagnostic office hysteroscopy at 6 to 8 weeks after the operative procedure, performed by a surgeon who was blinded to the treatment group." Quote: "The participants didn't know which group they were allocated to, nor did the outcome assessors. Naturally, the treating physician at time of procedure was aware of the treatment. Treating physicians' identity was documented and the study coordinator made sure that different physicians performed the treatment and the assessment per patient" (query clarified by Dr Moty Pansky). Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from the figure 1 CONSORT flow diagram of the study report: "In the intervention group five women were excluded from analysis after randomisation: the intervention was discontinued but no further clarification was given." Quote from the figure 1 CONSORT flow diagram of the study report: "In the control group six women were excluded from analysis after randomisation: lost to follow‐up (3) and discontinuation of the intervention (3) without further clarification." Comment: likely to cause attrition bias. |
| Selective reporting (reporting bias) | High risk | Comment: at high risk of selective outcome reporting, as live birth rates not reported for a study from September 2009 to June 2012, and publication of the final study report in 2014. |
| Other bias | High risk | Quote: "Patients with a diagnosis of adhesions (AFS grade 1) were offered an additional procedure for adhesiolysis." Quote: "At follow‐up hysteroscopy, 3 patients in the control group (14%) had AFS stage 2 or 3 (moderate to severe) intrauterine adhesions, compared with 1 woman in the study group (4%), who had AFS stage 3 intrauterine adhesions (P = 0.30)." Comment: imbalance between groups for a cointervention. |
Gan 2017.
| Methods | Parallel‐group randomised controlled trial. Single centre, Department of Minimally Invasive Gynecologic Center, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, China. Protocol approved by IRB: yes. Study protocol registered as NCT02496052 in ClinicalTrials.gov. Statistical power calculation reported; sample size determined based on findings of a pilot study. External funding: supported by grants from Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (Grant No. ZYLX201406), Capital Health Research and Development of Special (Grant No. 2014‐1‐2112) and National Science and Technology Infrastructure Program (Grant No. 2014BAI05B03). Conflicts of interest reported: authors had no conflicts of interest. |
|
| Participants | Number recruited: 88 women. Number randomly assigned: 88 women. Number excluded: 2 women; 1 per treatment arm; reason: protocol violation. Number lost to follow‐up: 6 women. Intervention group: 3 women lost to follow‐up; 2 not undergo second hysteroscopy and 1 had incomplete data collection for defaulted follow‐up. Control group: 3 women lost to follow‐up; 3 did not undergo second hysteroscopy. Number analysed: 80 women. Consecutive series of women who fulfilled the recruitment criteria were invited to participate in the study until the enrolment target was met. All women had severe IUAs confirmed by outpatient diagnostic hysteroscopy and AFS IUA score ≥ 8. Inclusion criteria:
Exclusion criteria:
Precise proportion of women with infertility not reported in this mixed population of women with infertility or ≥ 1 spontaneous miscarriage. Study duration: 12 months. Mean age (± SD) in intervention group: 29.6 (± 3.7) years. Mean age (± SD) in control group: 30.8 (± 3.7) years. |
|
| Interventions |
Freeze‐dried amnion graft using a modified Foley catheter balloon as a scaffold (intervention: n = 40) vs Foley catheter balloon without amniotic grafting (control: n = 40) Hysteroscopic adhesiolysis performed under general anaesthesia by 1 experienced hysteroscopic operator. 2 × misoprostol 200 μg tablets administered vaginally the night before surgery for cervical priming. A bipolar resectoscope with a 9‐mm sheath and a 4‐mm 12‐degree telescope (Olympus Optical Company, Tokyo, Japan) used after cervical dilation with a 10 Hegar cervix dilator. Ultrasonographic guidance routinely used during procedure. Laparoscopy used to inspect pelvis and rule out pathology, such as endometriosis, and to verify tubal patency at end of hysteroscopic surgery. Normal saline used as distention medium and delivered through automated hysteroscopic distension pump at 260 mL/minute, under 100 mmHg of intrauterine pressure. Once location, extent and severity of IUAs had been assessed, they were resected using a needle or loop diathermy with electrosurgical generator voltage set at 320 W for the cutting mode and 160 W for the coagulation mode. Fluid volume recorded using modified automated fluid management system. Operating surgeon assessed when complete adhesiolysis had been achieved for all participants during surgery, and this was verified using normal panoramic view of uterine cavity under direct hysteroscopic visualisation; adhesiolysis characterised by adequate uterine cavity, no evidence of IUA and visible bilateral uterine horn, with or without tubal ostium. Following surgery, a 20 Foley catheter, with tip distal to balloon cut away, used as a scaffold for insertion of the amnion graft into uterine cavity. Intervention: balloon portion of Foley catheter covered with sterilised freeze‐dried amnion graft (Jiangxi Rui Ji Biotechnology, Jiangxi, China) and hydrated in sterile normal saline for 10 minutes before use. Size of each amnion graft 30 × 20 mm. 2 amnion grafts applied to Foley catheter, with epithelial amnion membrane surface facing outwards. Foley catheter was inserted into uterine cavity under ultrasonographic guidance. Balloon was initially inflated with 8‐10 mL of normal saline for 2‐3 minutes to ensure that amnion graft fully adhered to uterine cavity. Afterwards, 3‐5 mL of normal saline solution was withdrawn, leaving a mean of 5 mL within balloon. Control: protocol for insertion of Foley catheter and inflation of balloon was same as that used in intervention group; however, amnion grafting was not used. All images were digitally captured for further review and comparison using an integrated operating room (Karl Storz, Tuttlingen, Germany). Foley catheter remained in place for 1 week, after which time balloon was deflated and catheter removed as an outpatient procedure. All participants treated with daily dose of intravenous cefmetazole sodium 2 g for 7 days until the Foley catheter was removed. They also received cyclical postoperative therapy with oestrogens and progestogens as standard. Hormone therapy comprised oral oestradiol valerate 4 mg, which was administered daily for 21 days, with the addition of oral dydrogesterone 20 mg daily on days 12‐21 of menstrual cycle. |
|
| Outcomes | Primary outcome: AFS IUA score at follow‐up hysteroscopy 3 months after surgery. Secondary outcomes: changes in menstruation measured by PBAC score, IUA reformation rate, pregnancy rate. Follow‐up of the secondary outcomes conducted via direct contact or telephone contact every 3 months to assess menstrual pattern and reproductive outcomes. Total duration of follow‐up: 6‐12 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before surgery, the participants were randomly assigned to either the amnion group or the control group in a 1:1 ratio using a computer‐generated randomisation sheet." Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Group allocation was concealed using sealed opaque envelopes that were opened at the time of operation by the coordinator." Comment: probably done‐unclear if sequentially numbered opaque sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "Group assignment was not masked; however, the surgeons who performed the follow‐up hysteroscopy were blinded to both randomisation and allocation." Comment: surgeons and personnel not blinded, unclear if participants were blinded or not; query not answered. |
| Blinding of participants and personnel (performance bias) Adhesions | Unclear risk | Quote: "Group assignment was not masked; however, the surgeons who performed the follow‐up hysteroscopy were blinded to both randomisation and allocation." Comment: surgeons and personnel not blinded, unclear if participants were blinded or not; query not answered. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "Group assignment was not masked; however, the surgeons who performed the follow‐up hysteroscopy were blinded to both randomisation and allocation." Comment: outcome assessors blinded. |
| Blinding of outcome assessment (detection bias) Adhesions | Low risk | Quote: "Group assignment was not masked; however, the surgeons who performed the follow‐up hysteroscopy were blinded to both randomisation and allocation." Comment: outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 88 women recruited, 80 were included in the final analysis (40 in each group)." Comment: intervention group: 4 women excluded or lost to follow‐up; 2 did not undergo second hysteroscopy, 1 protocol violation and 1 incomplete data collection for defaulted follow‐up. Control group: 4 women excluded or lost to follow‐up; 3 did not undergo second hysteroscopy and 1 protocol violation. |
| Selective reporting (reporting bias) | High risk | Quote: "The primary efficacy outcome was the AFS IUA score at follow‐up hysteroscopy. This outpatient procedure was performed under local anaesthesia at 3 months after surgery using 4.5‐mm continuous perfusion hysteroscopy (30°) with settings, intrauterine pressure, and irrigation rates similar to those used during the initial hysteroscopic surgery. The extent and severity of any reformed IUAs was recorded. Lesions were scored according to the AFS system." Secondary outcomes were changes in menstruation, which were evaluated according to PBAC score, IUA reformation rate and pregnancy rate." Comment: in study protocol registered as NCT02496052 all secondary outcomes mentioned in the published study report were not predefined. |
| Other bias | High risk | Quote: "During the follow‐up period, nine women in the amnion group achieved pregnancy; six of these pregnancies occurred naturally, whereas three occurred following in vitro fertilization and embryo transfer. Spontaneous abortion during the first trimester was reported among three of the nine pregnancies in the amnion group. The remaining six pregnancies were ongoing at the time of final follow‐up (two pregnancies at <12 weeks and four pregnancies at >24 weeks). Seven pregnancies were reported in the control group: five had occurred naturally and two had occurred following in vitro fertilization and embryo transfer. Among these seven pregnancies, four spontaneous abortions were reported during the first trimester, whereas the remaining three pregnancies were ongoing (>18 weeks) at the time of final follow‐up. As shown in Table 1, the pregnancy rate in the amnion group (23%) was not statistically different from that the control group (18%)." Comment: it is unclear if all women of this mixed population infertility/spontaneous miscarriage were trying to conceive. Proportions of women treated with IVF/embryo transfer vs natural conception not reported; query not answered. Quote: "Laparoscopy was used to inspect the pelvis and rule out pathology, such as endometriosis, and to verify tubal patency at the end of the hysteroscopic surgery." Comment: differences in proportions of cotreatment with laparoscopy not reported; query not answered. Quote: "Secondary outcomes were changes in menstruation, which were evaluated according to pictorial blood‐loss assessment chart (PBAC) score the IUA reformation rate, and the pregnancy rate. Follow‐up was conducted via direct contact or telephone contact every 3 months to assess menstrual pattern and reproductive outcomes. The total duration of follow‐up was 6‐12 months." Comment: at high risk of detection bias if not all women were followed up until 12 months given that there was no fixed endpoint to measure secondary outcomes. |
Guida 2004.
| Methods | Parallel‐group randomised controlled trial after stratification according to type of pathology. Single centre, Hysteroscopic Unit of University of Naples Frederico II, Naples, Italy. Protocol approved by IRB: yes. Statistical power calculation for primary outcome of incidence of de novo adhesions (query clarified by Dr Attilio DiSpiezio Sardo). No external funding and no conflicts of interest (query clarified by Dr Attilio DiSpiezio Sardo). |
|
| Participants | Number recruited: 164 women. Number excluded before randomisation: 26 women (18 refused to undergo operative hysteroscopy; 8 refused to participate after explanation of the study protocol). Number randomly assigned: 138 women. Number lost to follow‐up: 6 women. Number analysed: 132 women. All participants with surgically remediable single lesions (myomas, polyps and uterine septa, subgroups I‐III) at diagnostic hysteroscopy were invited to participate. Between September 2002 and June 2003, 164 women met the study's inclusion criteria and were invited to participate. Of these, 26 did not participate: 18 refused to undergo operative hysteroscopy, and 8 refused after explanation of the study protocol. Inclusion criterion:
Exclusion criteria:
Study duration: 10 months (September 2002 to June 2003). Mean age (± SD) in intervention group: 37 (± 3.2) years. Mean age (± SD) in control group: 36 (± 2.8) years. Number of subfertile participants and individual outcome data not available for further IPD analysis (query clarified by Dr Attilio DiSpiezio Sardo). |
|
| Interventions |
ACP gel (intervention: n = 69) vs no treatment (control: n = 69) After diagnostic hysteroscopy and after written consent form was signed, women from each pathology subgroup (submucous myomas, endometrial polyps, septa) were randomly assigned to 2 groups using a computer‐generated randomisation list. Intervention group: intrauterine application of 10 mL of ACP gel (Hyalobarrier Gel; Baxter, Pisa, Italy) under hysteroscopic view after operative hysteroscopy. Control group: hysteroscopic surgery alone. Diagnostic hysteroscopy performed with a 3.5‐mm instrument (Gynecare Versascope; Gynecare, Ethicon Inc., Somerville, NJ, USA) and sodium chloride 0.9% solution as distension medium. Operative hysteroscopy performed using a rigid resectoscope (Karl Storz, Tuttlingen, Germany) with 12‐degree fore‐oblique telescope with hook‐shaped monopolar electrode. Both groups received oral antibiotics (cefixime 400 mg/day) (Cefixoral; Menarini, Firenze, Italy) for 3 days after surgery. |
|
| Outcomes | Incidence of de novo adhesions, mean adhesion score and severity of adhesions according to 1988 AFS classification system; all outcomes measured after 3 months. | |
| Notes | * Correspondence with authors on 27 December 2014: 1. Which method was used for a statistical power calculation before the trial? Primary outcome was the incidence of adhesion formation at three month follow‐up in the two groups (hysteroscopy plus gel vs. hysteroscopy only). We assumed that difference between the two groups in term of de novo intrauterine adhesion formation would be 15% with an incidence of de novo adhesion formation in the hysteroscopy only group of 25% (Taskin et al. J Am Assoc Gynecol Laparosc 2000; 7: 351‐354). For the probability of a type I error to be less than .05, we calculated that a sample of 136 patients (68 per group) would provide 80% statistical power. In the study, 138 patients were enrolled and unfortunately, 6 dropped out, leaving 67 patients in the hysteroscopy plus gel group and 65 in the hysteroscopy only group. For this reason, 80% power of the study using the per‐protocol sample size analysis was not reached. Nevertheless, the post‐hoc power analysis revealed that the study reached an 80% power. 2. Was there any funding for the present study? Was there any conflict of interest? The study was not funded by an external source. All authors had no conflict of interest regarding this study at that time. 3. Is it possible to provide the outcome data of the infertile women included in this study to be able to analyse them separately? Unfortunately it is not possible. 4. Which method was used to conceal the allocation to one of the two interventions? The allocation sequence was concealed from the researchers (G.A., G.B., R.P., M.P.), who enrolled and assessed the participants and attached a sequentially numbered, opaque, sealed, and stapled envelope containing the allocated treatment to the clinical record of the patient after having signed the informed consent. The envelope was opened immediately after the surgical removal of the intrauterine removal of the removal of the intrauterine lesion, in order for the surgeon (M.G.) to either inject the gel (group A) or not (group B). Patients were blinded to the procedure until the end of the study. This single‐blind study design was adopted to reduce bias derived from the patient's knowledge of which procedure she underwent. 5. How were the outcome assessors blinded? Finally, are you aware of any ongoing research on anti‐adhesion therapy following operative hysteroscopy? The researcher who assessed the de novo formation of intrauterine adhesion (G.A.) was the one who performed the baseline diagnostic hysteroscopy and, successively, performed the 3 month follow‐up hysteroscopy. He did not participate to any of the operative hysteroscopies, when the patients were allocated to group A or B and, thus, he was completely unaware to which group the patients were allocated. We are not aware of any ongoing research on anti‐adhesion therapy following operative hysteroscopy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After diagnostic hysteroscopy and after the written consent form was signed, patients from each pathology subgroup (submucous myomas, endometrial polyps, septa) were randomized into two groups, group A (treatment [intervention] group) (N = 69) and group B (control group) (N = 69), using a computer‐generated randomisation list." Comment: probably done, as the same team of investigators published data on a similar randomised trial. |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was concealed from the researchers (G.A., G.B., R.P., M.P.), who enrolled and assessed the participants and attached a sequentially numbered, opaque, sealed, and stapled envelope containing the allocated treatment to the clinical record of the patient after having signed the informed consent" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: probably done. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Quote: "The envelope was opened immediately after the surgical removal of the intrauterine removal of the removal of the intrauterine lesion, in order for the surgeon (M.G.) to either inject the gel (group A) or not (group B). Patients were blinded to the procedure until the end of the study. This single blind study design was adopted to reduce bias derived from the patient's knowledge of which procedure she underwent" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: personnel not blinded; participants blinded. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) Adhesions | Low risk | Quote: "Both the initial diagnostic hysteroscopy and the follow‐up diagnostic hysteroscopy were performed by the same operator (G.A.). G.A. evaluated the adhesion score for each patient and was blind for patients' randomized allocation, whilst operative hysteroscopies and application of ACP gel were performed by a different operator (M.G.)." Quote: "The researcher who assessed the de novo formation of intrauterine adhesion (G.A.) was the one who performed the baseline diagnostic hysteroscopy and, successively, performed the 3 month follow‐up hysteroscopy. He did not participate to any of the operative hysteroscopies, when the patients were allocated to group A [intervention] or B [control] and, thus, he was completely unaware to which group the patients were allocated" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Six women (two from group A [intervention] and four from group B [control]) did not attend for follow‐up hysteroscopy." Comment: unlikely to cause substantial attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | Low risk | Comment: no evidence of imbalance in baseline participant characteristics; no cotreatment. |
Lin 2015a.
| Methods | Parallel‐group randomised controlled trial. Single centre, tertiary medical centre, Shin Kong Wu Ho‐Su Memorial Hospital, Taipei, Taiwan. Protocol approved by IRB: yes. Study protocol registered as NCT01167296 in ClinicalTrials.gov. Statistical power calculation done before start of trial. No conflicts of interest declared by study authors. External funding not reported. |
|
| Participants | Number recruited: 68 women. Number excluded before randomisation: 6 women (5 refused to participate; 1 had history of PID). Number randomly assigned: 62 women. Number lost to follow‐up: 2 women. Number analysed: 60 women. Inclusion criterion:
Exclusion criteria:
Study duration: 8 months; trial recruited from July 2010 to April 2011. Mean age (± SD) in intervention group: 33.4 (± 4.8) years. Mean age (± SD) in control group: 35.4 years (± 7.2) years. Unclear whether participants had subfertility, and if so, how many (query not clarified by study authors). |
|
| Interventions |
Balloon uterine stent (intervention: n = 31) vs no stent (control: n = 31) Randomisation based on a 1:1 computer‐generated scheme in balanced blocks of 4. Randomisation codes sealed in sequentially numbered opaque envelopes by study co‐ordinator. Immediately before surgery, co‐ordinator opened envelope and assigned participants to receive balloon uterine stent insertion (intervention) or not (control). Intervention group: uterine stent present for 30 days after surgery. Endometrium swabbed before and 30 days after surgery, and stent removed and sent for bacterial culture. Control group: endometrial swabbing done before and 30 days after surgery, but no stent was inserted. Co‐ordinator, participants and gynaecologists were not blinded to intervention after assignment. Per routine practice, women self‐administered misoprostol 400 μg (Cytotec; Pharmacia) into vagina 24 hours and 12 hours before surgery to prime cervix. After anaesthesia, perineum and vagina disinfected and draped. Cervix and vagina subsequently thoroughly disinfected with povidone‐iodine, as in vaginal surgery. Applicator swab (Copan Venturi Transystem; Copan Italia) then inserted into uterine cavity, with care taken to avoid contact with vaginal wall. Whole endometrium swabbed from fundus to cervix. Applicator swab placed in a transport tube and sent to laboratory immediately for bacterial culture. Operative hysteroscopies performed with 22‐F resectoscope (Karl Storz) and 5% glucose solution for uterine distension and irrigation. For women in intervention group, stent was inserted into uterine cavity at conclusion of hysteroscopy, and balloon inflated with 8 mL sterile water. Postoperatively, women were prescribed 3 days of diclofenac (Cataflam; Novartis Farma) for pain relief. Prophylactic antibiotics were not given. 1 surgeon performed all operative procedures and swabbing. Women instructed to return if any symptoms of PID developed 30 days after surgery, all participants returned to hospital for bacterial culture and second‐look hysteroscopy. After disinfection of vagina and cervix with povidone‐iodine, endometrium was swabbed. For intervention group, after balloon was deflated, stent was removed carefully without touching the vaginal wall. Balloon was cut from stem and placed in a sterile jar. Then endometrium was swabbed and balloon and swab sent to laboratory immediately for bacterial culture. After cultures were collected, all participants underwent second‐look hysteroscopy for assessment of endometrium. |
|
| Outcomes | Primary outcome: incidence of bacterial colonisation of the uterus. Secondary outcomes: pain intensity on VAS scale used to record worst pain score from 3 days to 30 days following surgery; species of colonising bacteria. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on a 1:1 computer generated scheme in balanced blocks of four." Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization codes were sealed in sequentially numbered opaque envelopes by the study coordinator." Comment: probably done. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "The coordinator, patients, and gynaecologists were not blinded to intervention after assignment." Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Quote: "The coordinator, patients, and gynaecologists were not blinded to intervention after assignment." Comment: no blinding of participants, personnel and outcome assessors. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "The coordinator, patients, and gynaecologists were not blinded to intervention after assignment." Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) Adhesions | High risk | Quote: "The coordinator, patients, and gynaecologists were not blinded to intervention after assignment." Comment: no blinding of participants, personnel and outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 62 women were included in the study, and 31 women were assigned to each group. The balloon uterine stent fell out after a week in one woman in the stent group, and one woman in the control group was lost to follow‐up. Both of these patients were excluded from analysis. Data for 60 women were analysed." Comment: unlikely to cause substantial attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Main outcome measure(s): The primary outcome was the incidence of bacterial colonization of the uterus. Secondary outcomes were pain intensity and species of colonizing bacteria." Quote: "All second‐look hysteroscopies revealed a normal endometrium. No woman had IUAs." Comment: according to registered protocol, predefined outcomes were:
Published report stated in results section that no participant had IUAs at second‐look hysteroscopy, but this important finding was not explicitly stated in abstract. |
| Other bias | High risk | Number of participants with IUAs twice as high in intervention group (17/31) vs control group (10/31). Comment: imbalance in baseline characteristics between comparison groups. |
Lin 2015b.
| Methods | Parallel‐group randomised controlled trial. Single centre, university referral centre, Reproductive Medicine Centre of the Sir Run Run Shaw Hospital, Hangzhou, China. Protocol approved by IRB: yes. Study protocol registered as ISRCTN69690272 in ISRCTN Registry. Statistical power calculation done before start of trial. No conflicts of interest declared by authors. External funding: National Science Foundation of China (81270657), Zhejiang Public Welfare Technology Application Research Project (2013C33236), and Zhejiang Key Science and Technology Innovation Team Project (2011R50013‐26). |
|
| Participants | Number recruited: 207 women. Number excluded before randomisation: 6 women (3 surgical complications; 3 declined to participate). Number randomly assigned: 201 women. Number excluded after randomisation: 5 women (1 intervention group; 4 control group) with reason: protocol violation. Number lost to follow‐up: 34 women (16 intervention group; 18 control group) with reason: no second‐look hysteroscopy in time. Number analysed: 162 women (82 intervention group, 80 control group). Inclusion criteria:
Exclusion criteria:
Mean age (± SD) in intervention group: 29.7 (± 4.3) years. Mean age (± SD) in control group: 30.1 (± 5.1) years. Proportion of women with infertility in intervention group: 21/82 (26%). Proportion of women with infertility in control group: 18/80 (22%). Primary vs secondary infertility: not reported. Study duration: 20 months. |
|
| Interventions |
Intrauterine balloon (intervention: n = 82) vs IUD (control: n = 80) Hysteroscopic adhesiolysis carried out by 1 of 2 experienced hysteroscopic surgeons with use of 4.5‐mm rigid hysteroscope (Storz) with 5% mannitol perfusion under 100 mmHg pressure. Procedure performed under general anaesthesia in a day surgery unit. Ultrasonographic guidance routinely used; in some cases, laparoscopy was also performed either in exceptionally difficult cases or when there was a need to inspect pelvic organs to rule out pathology such as endometriosis or to verify tubal patency. Once the extent and severity of uterine adhesion had been assessed, adhesions were divided with use of hysteroscopic scissors until normal uterine anatomy was achieved. Intervention group: immediately following operative hysteroscopy specially designed intrauterine balloon (Cook Medical) inflated with 3‐5 mL normal saline fitted into uterine cavity. Control group: immediately following operative hysteroscopy heart‐shaped copper IUD (Yantai Contraceptive Instrument) with thread knitted tail fitted into uterine cavity. Both devices were removed after 1 week in outpatient department. All participants were treated with oral cefuroxime combined with metronidazole for 7 days. In all cases, hormone therapy was also begun from the day of operation, consisting of oestradiol valerate 6 mg/day for 21 days, with medroxyprogesterone acetate 6 mg/day for the last 7 days of the oestrogen therapy. After withdrawal bleed, hormone therapy repeated for another cycle. Second‐look hysteroscopy carried out in early proliferating phase, 1‐2 months after initial operation. After assessment of extent and severity of any reformed adhesions, hysteroscopic adhesiolysis was also carried out at time of second‐look procedure, if adhesions had recurred. |
|
| Outcomes | Primary outcomes: adhesion reformation, measured by second‐look hysteroscopy 1‐2 months after surgery. Power calculation done before start of trial; reduction in adhesion scores, measured by second‐look hysteroscopy 1‐2 months after surgery. Severity and extent of IUAs scored according to AFS 1988 classification. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After the completion of hysteroscopic adhesiolysis, recruited patients were randomized to one of the two treatment groups by computer‐generated numbers…" Comment: probably done; low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. Comment: unclear risk of bias; query not answered. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Blinding of surgeons impossible since balloon and IUD were easily recognised as being different. Blinding of participants not reported but device removed after 1 week at the outpatient department. Comment: probably no blinding of participants and personnel. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Blinding of surgeons impossible since balloon and IUD were easily recognised as being different. Blinding of participants not reported but device removed after 1 week at the outpatient department. Comment: probably no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "The surgeon who later performed the second‐look hysteroscopy was blinded to the randomisation." Comment: probably done; low risk of bias. |
| Blinding of outcome assessment (detection bias) Adhesions | Low risk | Quote: "The surgeon who later performed the second‐look hysteroscopy was blinded to the randomisation." Comment: probably done; low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "There were 39 women who were subsequently excluded from the study for the following reasons. In the balloon group, 1 woman was excluded because of protocol violation, in addition to 16 withdrawals because they did not proceed to second‐look hysteroscopy within the specified time frame. In the IUD group, 4 women were excluded because of protocol violation, in addition to 18 withdrawals because they did not proceed to second‐look hysteroscopy within the specified time frame." Comment: proportion of women lost follow‐up 34/201 (17%) women; high risk of attrition bias. |
| Selective reporting (reporting bias) | High risk | According to study protocol registered as ISRCTN69690272, secondary outcome was pregnancy rate after surgery. Not reported in study report. Comment: at high risk of selective outcome reporting since duration of study was 20 months and no data reported in final review of secondary outcome predefined in registered study protocol. |
| Other bias | Low risk | Quote: "No difference in baseline characteristics. Co‐treatment with antibiotics and hormone therapy in both comparison groups." Comment: no evidence for other potential sources of bias. |
Roy 2014.
| Methods | Parallel‐group randomised controlled trial. Single centre, Department of Obstetrics and Gynecology, All India Institute of Medical Sciences, New Delhi, India. Protocol approved by IRB: yes. No statistical power calculation (query clarified by Dr Murali Subbaiah). No funding (query clarified by Dr Murali Subbaiah). No conflict of interest (query clarified by Dr Murali Subbaiah). |
|
| Participants | Number recruited: 100 women. Number excluded before randomisation: 10 women. Number randomly assigned: 90 women. Number lost to follow‐up: 5 women did not attend for second‐look hysteroscopy and were excluded from analysis of second‐look hysteroscopy findings; 2 women did attend for second‐look hysteroscopy but were lost to follow‐up for assessment of reproductive outcome. Number analysed: 85 women for second‐look hysteroscopy findings; 83 women for reproductive outcomes. Inclusion criteria:
Exclusion criteria:
90 original participants aged 20‐35 years with history of infertility (n = 31) or abortion (n = 59); of these, 40 had first‐trimester and 19 had second‐trimester spontaneous abortions. Study duration: 12 months; January 2011 to December 2011. Mean duration of infertility (± SD) in intervention group: 5.9 (± 1.8) years. Mean age (± SD) in intervention group: 28.7 (± 4.8) years. Mean duration of infertility (± SD) in control group: 6.2 (± 1.1) years. Mean age (± SD) in control group: 27.3 (± 3.9) years. Comment: mixed population of primary/secondary subfertility and miscarriage. Clarified by Dr Murali Subbaiah, quoting: "only 30 infertile patients were included ‐ the rest had abortions." |
|
| Interventions |
Oestrogen therapy (intervention: n = 42) vs placebo (control: n = 43) Hysteroscopic resection of septum under general anaesthesia by single operator in early proliferating phase of menstrual cycle. Operative hysteroscopy by rigid resectoscope (Karl Storz Endoskope, Germany) with 30‐degree telescope, equipped with a hysteroscopic monopolar (Collin's) knife. Cutting current set at 60 Watts. After 10‐mm cervical dilation achieved using Hegar's dilator, uterine cavity distended by glycine solution (1.5%). Intervention group: after septal resection, oestradiol valerate 2 mg once daily for 30 days. Control group: folic acid 5 mg tablet for 30 days. Second‐look hysteroscopy performed by same operator after 2 months to check for residual septum and uterine cavity adhesions. Performed as an outpatient procedure with a 4‐mm, 30‐degree angled lens. |
|
| Outcomes | IUAs at second‐look hysteroscopy after 2 months, classified according to AFS classification; remnant septum defined as septum > 1 cm at second‐look hysteroscopy after 2 months; pregnancy, ongoing pregnancy and miscarriage measured after contact by telephone on a 3‐month basis during 12‐ to 24‐month period of follow‐up. | |
| Notes | Answers to queries on 6 December 2014: "Respected Sir, I would like to apologize for the delay in response. This was a small study and only 30 infertile patients were included (The rest had abortions). Fertility outcome after septal resection in infertile women was not separately analysed (Numbers are too small and the period of follow up is also less). Power calculation was not done for this study. There was no funding or conflict of interest. The two groups were coded as A and B and were concealed in separate covers. A third person who was not involved in the study was asked to choose one of the concealed covers randomly, and this was assigned. The investigators and patients were blinded to treatment allotment. I am not aware of any ongoing research on anti‐adhesion therapy following operative hysteroscopy. Yours sincerely, Dr. Murali Subbaiah" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were prospectively randomized into two groups, group A (treatment group) (N = 45) and group B (control group) (N = 45), using a computer‐generated randomisation list." Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "The investigators and patients were blinded to treatment allotment." Comment: "The two groups were coded as A and B and were concealed in separate covers. A third person who was not involved in the study was asked to choose one of the concealed covers randomly, and this was assigned. The investigators and patients were blinded to treatment allotment" (method clarified by Dr Murali Subbaiah). |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) Adhesions | Low risk | Quote: "The investigators and patients were blinded to treatment allotment." Quote: "After septal resection, the treatment group received 2 mg of oestradiol valerate, once daily for 30 days; in the control group, folic acid tablet (5 mg) was given as a placebo for 30 days." Comment: probably done. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) Adhesions | Low risk | Quote: "The investigators and patients were blinded to treatment allotment." Quote: "After septal resection, the treatment group received 2 mg of oestradiol valerate, once daily for 30 days; in the control group, folic acid tablet (5 mg) was given as a placebo for 30 days." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Five women (three from group A and two from group B) did not attend for follow‐up hysteroscopy and were excluded from the study. Further, two patients (one from each group) were lost to follow up." Comment: no ITT analysis, but numbers of women excluded after randomisation or lost to follow‐up and reasons were balanced between comparison groups. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective reporting. |
| Other bias | Low risk | No evidence of baseline imbalance. |
Vercellini 1989.
| Methods | Parallel‐group randomised controlled trial. Single centre, university referral centre, 1.a. Clinica Ostetrica e Ginecologica "L. Mangiagalli" dell Università di Milano, Milan, Italy. Ethical board/IRB approval: Council of the Institute of the First Obstetrics and Gynecologic Department of the Università degli Studi, Milan (query clarified by Paolo Vercellini). Study protocol registered in a clinical trial register: not registered (query clarified by Paolo Vercellini). Statistical power calculation before start of the trial: pilot study without preplanned power calculation (query clarified by Paolo Vercellini). Conflicts of interest: none (query clarified by Paolo Vercellini). External funding: investigator‐driven non‐commercial study (query clarified by Paolo Vercellini). |
|
| Participants | Number recruited: 20 women. Number randomly assigned: 20 women. Number excluded after randomisation: 0 women. Number lost to follow‐up: 0 women. Number analysed: 20 women (intervention: IUD + hormone treatment: n = 10; control: no IUD or hormone treatment: n = 10). Inclusion criteria:
Exclusion criteria: not reported. Mean age (range): 29 (25‐36) years. Proportion of women with infertility: all (query clarified by Paolo Vercellini). Primary vs secondary infertility: not applicable. Study duration: 24 months; January 1986 to December 1987. |
|
| Interventions |
IUD + hormone treatment (intervention: n = 10) vs no additional treatment (control: n = 10) Hysteroscopic incision in uterine septum scheduled for the early proliferating phase of the menstrual cycle. Participants allocated randomly to 2 groups. Intervention group: IUD (ML CU 205, Multilan S.A., Pfäffikon, Switzerland) inserted postoperatively + conjugated oestrogen 1.25 mg twice daily for 30 days + medroxyprogesterone acetate 10 mg/day on days 26‐30. Follow‐up HSG scheduled after withdrawal bleeding and IUD removal. Control group: no other therapeutic measures. In both groups a follow‐up HSG was scheduled after the first spontaneous menstrual period with repeat hysteroscopy in the next cycle in the case of abnormal HSG findings. |
|
| Outcomes | Main outcomes: residual fundal notch ≥ 5 cm, incidence of IUAs. No prioritisation of outcomes reported. |
|
| Notes | * Correspondence with authors on 17 March 2017: Dear Professor Bosteels, 1. Can you describe the method used to randomly allocate the study participants to one of both treatment groups? Computer generated randomisation list. 2. Can you describe the method that you used to make the surgeons unaware of the treatment allocation? Did you use sequentially numbered opaque sealed envelopes? Did you phone to a central randomisation trial office? Other method? Sequentially numbered opaque sealed envelops. 3. Were the outcome assessor who evaluated the HSG or did the second look hysteroscopy in case of abnormal HSG the same surgeons that performed the septum resection? Yes, they were the same surgeons that performed the septum resection. 4. Do you have any baseline characteristics data of both comparison groups e.g. mean age of women in either group, length of septum, etc…? Unfortunately I am unable to retrieve these data. The study was completed almost 30 years ago. 5. Two women had their IUD removed early in the intervention group and in 1 woman in the control group a balloon catheter was left in situ for 24 hours because of bleeding. What was the outcome regarding normality of the cavity in these 3 women? See reply to point 4. 6. Is it correct that this is a single centre study conducted at 1.a. Clinica Ostetrica e Ginecologica "L. Mangiagalli” dell Università di Milano? Yes, it is correct. 7. Were the study participants all women of proven fertility or did the study also include women with infertility with two miscarriages? Do you have data on the proportions of infertile women in both comparison groups? The study participants were all fertile. 8. Was there IRB/Ethical committee approval for this clinical trial? Yes, the study was approved by the Council of the Institute of the First Obstetrics and Gynecologic Department of the Università degli Studi, Milan. 9. Was the study funded by a research grant or was it an investigator‐driven non‐ commercial study? It was an investigator‐driven non‐commercial study. 10. Was a power calculation done before the conduct of the study? It was a pilot study and no pre‐planned power calculation was performed. Thank you for your interest in our study and best wishes for your work. Paolo Vercellini Department of Clinical Sciences and Community Health, Università degli Studi di Milano and Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Via Commenda 12, 20122 Milan, Italy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were allocated randomly to two groups." Comment: computer‐generated randomisation list (query clarified by Paolo Vercellini). |
| Allocation concealment (selection bias) | Low risk | Quote: "method of allocation concealment not reported." Comment: sequentially numbered opaque sealed envelops (query clarified by Paolo Vercellini). |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "An IUD (ML CU 205, Multilan S.A., Pfäffikon, Switzerland) was inserted postoperatively in the ten women in group I; they also received conjugated estrogens, 1.25 mg twice daily for 30 days, with medroxyprogesterone acetate, 10 mg/d on days 26‐30. The ten women in group II were followed without other therapeutic measures." Quote: "Follow‐up HSG was scheduled for after withdrawal bleeding and IUD removal in group I and after the first spontaneous menstrual period in group II, with repeat hysteroscopy in the next cycle in the case of abnormal HSG findings." Comment: neither physicians nor participants were blinded. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Quote: "An IUD (ML CU 205, Multilan S.A., Pfäffikon, Switzerland) was inserted postoperatively in the ten women in group I; they also received conjugated estrogens, 1.25 mg twice daily for 30 days, with medroxyprogesterone acetate, 10 mg/d on days 26‐30. The ten women in group II were followed without other therapeutic measures." Quote: "Follow‐up HSG was scheduled for after withdrawal bleeding and IUD removal in group I and after the first spontaneous menstrual period in group II, with repeat hysteroscopy in the next cycle in the case of abnormal HSG findings." Comment: neither physicians nor participants were blinded. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "Follow‐up HSG was scheduled for after withdrawal bleeding and IUD removal in group I and after the first spontaneous menstrual period in group II, with repeat hysteroscopy in the next cycle in the case of abnormal HSG findings." Comment: outcome assessors who evaluated HSG or did second‐look hysteroscopy in case of abnormal HSG were same surgeons who performed septum resection (query clarified by Paolo Vercellini). |
| Blinding of outcome assessment (detection bias) Adhesions | High risk | Quote: "Follow‐up HSG was scheduled for after withdrawal bleeding and IUD removal in group I and after the first spontaneous menstrual period in group II, with repeat hysteroscopy in the next cycle in the case of abnormal HSG findings." Comment: outcome assessors who evaluated HSG or did second‐look hysteroscopy in case of abnormal HSG were same surgeons who performed septum resection (query clarified by Paolo Vercellini). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "From January 1986 to December 1987 we studied 20 women aged 25‐36 years (mean, 29) with two or more unexplained spontaneous abortions, a double uterine cavity at hysterosalpingography (HSG) and ultrasonographic evidence of a normal uterine fundus with a half‐full bladder (Ansaldo 920 real‐time scanner with 3.5‐MHz convex transducer)." Quote: "At follow‐up HSG, five group I women had a regular uterine cavity and five a residual fundal notch ≥ 1 cm. In group II four had a normal uterine cavity and six a residual fundal notch ≥ 1 cm. No IUAs were detected in any of the patients." Comment: no exclusion; no loss‐to‐follow‐up. |
| Selective reporting (reporting bias) | Low risk | Quote: "No intrauterine adhesions were detected in any of the patients. IUD insertion and hormonal therapy after hysteroscopic metroplasty do not seem to be needed to prevent septal fusion." Comment: no difference between outcomes reported in abstract vs results section. |
| Other bias | Unclear risk | Quote: "In three group I and two group II patients, undue bleeding occurred, mainly from small, traumatized sites in the surrounding endometrium. Postoperative bleeding was observed in two women in group I [intervention] and one in group II [control]; the IUD was removed from the two group I patients 8 and 11 hours postoperatively, and methylergonovine, 0.2 mg intramuscularly, was administered. That was sufficient to arrest the bleeding in those cases whereas in the group II patient we had to insert in the uterine cavity a no. 16 Foley catheter distended with 5 mL of fluid; it remained there for 24 hours. The subsequent course was uneventful." Comment: baseline characteristics not reported. Sensitivity analyses of an ITT analysis vs a per‐protocol analysis not possible since outcomes of these 3 women were not reported. These 3 women should have been excluded since early removal of IUD in intervention group and leaving a balloon catheter in situ for 24 hours could have affected outcomes. Since study was completed in the late 1980s, it is no longer possible to retrieve these data (query clarified by Paolo Vercellini). |
Wang 2016.
| Methods | Parallel‐group randomised controlled trial. Single centre, university referral centre: Gynecological Minimally Invasive Center, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, China. Ethical board/IRB approval: yes. Study protocol registered in a clinical trial register: not reported; query not answered. Statistical power calculation before start of the trial: not reported; query not answered. Conflicts of interest: not reported; query not answered. External funding: Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (zylx201406); The Capital Health Research and Development of Special (2014‐1‐2112); National Science and Technology Infrastructure Program (2004BAI05B02). |
|
| Participants | Number recruited: 57 women. Number randomly assigned: 57 women. Number excluded after randomisation: 0 women. Number lost to follow‐up: 0 women. Number analysed: 57 women (intervention: amniotic scaffold balloon, n = 29; control: Foley's balloon without amniotic membrane: n = 28). 57 women with IUA score ≥ 10 on hysteroscopy selected at the Beijing Maternity Hospital (affiliated to the Beijing Medical University). Inclusion criterion:
Exclusion criterion:
Mean age (± SD) in intervention group: 29 (± 3) years. Mean age (± SD) in control group: 31 (± 3) years. Proportion of women with infertility: all women had infertility related to severe IUAs. Primary vs secondary infertility: not reported. Study duration: recruitment of 12 months; June 2013 to June 2014. Follow‐up: 12‐18 months. Mean (± SD) follow‐up 14.6 (± 2.7) months. |
|
| Interventions |
Foley balloon catheter wrapped in amniotic membrane (intervention: n = 29) vs Foley balloon without amniotic membrane (control: n = 28) Tracheal intubation combined with intravenous general anaesthesia. Routine cervical priming performed preoperatively. Hysteroscopic adhesiolysis combined with laparoscopy and B‐mode ultrasound. In hysteroscopy, uterine cavity inspected looking for sites and severity of adhesions or anatomical abnormality. Adhesiolysis performed with needle electrodes and loop electrodes until normal morphology of uterine cavity restored, both uterine horns were visible and fallopian tube opening visible or invisible, or both. Emphasis placed on preserving residual endometrium. Amniotic membranes used were obtained from the Jiangxi Ruizeng Biological Engineering Technology Co., Ltd., specifications for the 30 mm × 20 mm dry sterilised biological amniotic membrane. Intervention group: before operation, 2 sheets of amniotic membrane were soaked in 25‐30 °C normal saline for 15‐20 minute to allow for rehydration and wrapped onto surface of a Foley catheter. Cervix dilated using Hegar 12 dilators, following which any fluid/gas was aspirated by the Foley catheter. Subsequently, balloon catheter was inflated with 8‐10 mL saline resulting in amniotic membrane products adhering completely to the uterine wound. After waiting 1‐2 minutes, residual volume of 3‐4 mL was retained in balloon catheter to maintain a separation between uterine walls. Catheter was left in place attached to an external drainage bag for 7 days, and routine antibiotic prophylaxis given. Balloon removed 7 days postoperatively. Control group: treatment as in intervention group except Foley balloon catheter alone had no external wrapping of amniotic membrane. |
|
| Outcomes | No prioritisation of the outcomes reported. Menstrual flow changes; 1 month and 3 months postoperatively; IUA score by hysteroscopy performed after 3 cycles. If IUA score ≥ 5, participants were considered to have recurrence of adhesions, thereafter, participants were followed up every 3 months by telephone call or outpatients visits where pregnancy rates were recorded; IUA diagnosis and grading according to 1988 AFS grading method, which is a summation of adhesion score by hysteroscopy and menstrual pattern score by WHO menstrual blood loss chart (PBAC); reformation of IUAs scored by hysteroscopy; pregnancy; not further specified; ongoing pregnancy; spontaneous miscarriage; preterm birth; reformation of IUAs scored by hysteroscopy. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "57 IUA patients with IUA score ≥10 on hysteroscopy were selected from June 2013 to June 2014 at the Beijing Maternity Hospital (affiliated to the Capital Medical University). Using the SPSS random number generator, patients were divided into 2 groups." Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported; query not answered. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Surgeons were not blinded. Unclear if women were blinded to allocated treatment; query not answered. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Surgeons were not blinded. Unclear if women were blinded to allocated treatment; query not answered. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Unclear if surgeons performing second‐look hysteroscopy were different from surgeons who performed hysteroscopic adhesiolysis; query not answered. |
| Blinding of outcome assessment (detection bias) Adhesions | Unclear risk | Unclear if surgeons performing second‐look hysteroscopy were different from surgeons who performed hysteroscopic adhesiolysis; query not answered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Registration of study protocol not reported. Data collected as mentioned in methods section were all reported in results section. |
| Other bias | Unclear risk | Quote: "All patients were taking artificial cycle treatment." Comment: all women were cotreated with fertility treatment but unclear if this means that all women were offered same treatment regimen (e.g. all women received clomiphene with or without IUI or gonadotropin treatment with or without IUI or IVF) and that the proportions of different treatment regimens with different fertility prognoses were equally distributed among both groups; query not answered. |
Xiao 2015.
| Methods | Parallel‐group randomised controlled trial. Multicentre, Third Xiangya Hospital of Central South University, Changsha 410013, China, Beijing, China and 2 affiliated hospitals: Hunan Provincial People's Hospital and Hunan First People's Hospital of Chenzhou City, China. Ethical board/IRB approval: yes. Study protocol registered in clinical trial register: not reported; query not answered. Statistical power calculation before start of trial: not reported; query not answered. Conflicts of interest: not reported; query not answered. External funding: not reported; query not answered. |
|
| Participants | Number recruited: 120 women. Number randomly assigned: 120 women. Number excluded after randomisation: 0 women. Number lost to follow‐up: 9 women. Number analysed: 111 women (intervention group: n = 55; control group: n = 56). From November 2011 to November 2012, women with IUA from 3 hospitals affiliated with Xiangya Medical College and other hospitals who fulfilled inclusion criteria were included. Inclusion criteria:
Exclusion criteria:
Mean age (± SD) in intervention group: 33 (± 5) years. Mean age (± SD) in control group: 33 (± 5) years. Proportion of women with infertility: not reported; unclear if infertile women were included; query not answered. Primary vs secondary infertility: not reported. Study duration: recruitment of 12 months. |
|
| Interventions |
Foley balloon catheter + AC HA gel (intervention: n = 55) vs Foley balloon catheter only (control: n = 56) Participants with moderate‐to‐severe IUAs underwent routine hysteroscopic adhesiolysis. After surgery, participants randomly assigned into intervention and control groups according to treatment allocation table. Intervention group: Foley balloon catheter placed in uterine cavity, 3 mL saline injected to inflate balloon to seal mouth of cervix. Then, 2 mL medical self‐cross‐linking sodium hyaluronate gel (product of Changzhou Biarui Biomedical Co., Ltd.) injected from another lumen of Foley balloon catheter to fill uterine cavity and cover surgical wound. Control group: Foley balloon catheters placed in uterine cavity in same manner as intervention group with no self‐cross‐linking sodium hyaluronate gel. According to literature, after 72 hours, Foley balloon catheters were removed from and same routine postoperative treatment given to all participants. At 1 and 3 months, participants attended for clinical follow‐up 3‐7 days after menstrual bleeding had stopped for that cycle. Participant's general condition, symptoms, signs and possible complications checked and recorded. At third month of follow‐up, second‐look hysteroscopy performed and IUAs were graded (light, moderate and severe) according to AFS criteria. |
|
| Outcomes | Primary outcome: effectiveness of treatment as seen by recurrence of adhesions on hysteroscopy 3 months after surgery. Treatment success defined as decrease in total AFS score of ≥ 4 points. Formula to calculate rate of treatment success: cases with AFS total score < 4 divided by total number of cases × 100%. Secondary outcomes: comparison of AFS score, including extent of IUAs, adhesion type; menstrual pattern score before and after surgery, and between groups. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After the surgery, the patients were randomly assigned into treatment and control groups according to a treatment allocation table." Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported; query not answered. |
| Blinding of participants and personnel (performance bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "The surgeons performing the second hysteroscopy at 3 months were blinded to the choice of treatment of the patient. In addition, patients were also blinded to allocation to treatment or control. Therefore, this study can still be defined as double‐blinded (those assessing treatment response and those receiving treatment did not know of the treatments). Due to the properties of the self‐crosslinking sodium hyaluronate gel material, no gel material with the same properties can be used as a control. In addition, blank control material was not used in order to ensure efficacy of treatment and adherence to medical ethics. As a result, the surgeon performing adhesiolysis was not blinded." Comment: surgeons performing surgery not blinded but participants were blinded. |
| Blinding of participants and personnel (performance bias) Adhesions | High risk | Quote: "The surgeons performing the second hysteroscopy at 3 months were blinded to the choice of treatment of the patient. In addition, patients were also blinded to allocation to treatment or control. Therefore, this study can still be defined as double‐blinded (those assessing treatment response and those receiving treatment did not know of the treatments). Due to the properties of the self‐crosslinking sodium hyaluronate gel material, no gel material with the same properties can be used as a control. In addition, blank control material was not used in order to ensure efficacy of treatment and adherence to medical ethics. As a result, the surgeon performing adhesiolysis was not blinded." Comment: surgeons performing surgery not blinded but participants were blinded. |
| Blinding of outcome assessment (detection bias) Live birth, pregnancy or miscarriage | Low risk | Quote: "The surgeons performing the second hysteroscopy at 3 months were blinded to the choice of treatment of the patient. In addition, patients were also blinded to allocation to treatment or control. Therefore, this study can still be defined as double‐blinded (those assessing treatment response and those receiving treatment did not know of the treatments). Due to the properties of the self‐crosslinking sodium hyaluronate gel material, no gel material with the same properties can be used as a control. In addition, blank control material was not used in order to ensure efficacy of treatment and adherence to medical ethics. As a result, the surgeon performing adhesiolysis was not blinded." Comment: surgeons performing second‐look hysteroscopy were blinded. |
| Blinding of outcome assessment (detection bias) Adhesions | Low risk | Quote: "The surgeons performing the second hysteroscopy at 3 months were blinded to the choice of treatment of the patient. In addition, patients were also blinded to allocation to treatment or control. Therefore, this study can still be defined as double‐blinded (those assessing treatment response and those receiving treatment did not know of the treatments). Due to the properties of the self‐crosslinking sodium hyaluronate gel material, no gel material with the same properties can be used as a control. In addition, blank control material was not used in order to ensure efficacy of treatment and adherence to medical ethics. As a result, the surgeon performing adhesiolysis was not blinded." Comment: surgeons performing second‐look hysteroscopy were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The study period was from November 2011 to November 2012. 120 subjects were randomized into the experimental group and the control group of 60 cases each." Quote: "There were 111 patients who completed follow‐up and met the requirements of statistical analysis, including 55 patients in the trial group and 56 patients in the control group." Comment: 9 participants lost to follow‐up or excluded (5 in intervention and 4 in control group; reasons not reported). |
| Selective reporting (reporting bias) | Low risk | Results of all predefined endpoints were all reported. |
| Other bias | Low risk | No statistically significant differences in age, weight, height, number of previous pregnancies and preoperative AFS score. No cotreatments. |
ACP: auto‐cross‐linked polysaccharide; AFS: American Fertility Society; ART: assisted reproductive technology; ASRM: American Society for Reproductive Medicine; CMC: carboxymethyl cellulose; β‐hCG: beta‐human chorionic gonadotropin; FSH: follicle‐stimulating hormone; HA: hyaluronic acid; HSG: hysterosalpingography; IPD: individual participant data; IRB: institutional review board; ITT: intention to treat; IUA: intrauterine adhesion; IUD: intrauterine device; IVF: in vitro fertilisation; n: number of women; PBAC: pictorial blood‐loss assessment chart; PID: pelvic inflammatory disease; RPOC: retained products of conception; SD: standard deviation; VAS: visual analogue scale; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bednarek 2011 | Quote: "We performed a randomised non‐inferiority trial involving women undergoing uterine aspiration for induced or spontaneous abortion at 5 to 12 weeks of gestation who desired an IUD. Subjects were randomly assigned (in a 5:6 ratio) to IUD insertion immediately after the procedure or 2 to 6 weeks afterward (delayed insertion). The primary outcome was the rate of IUD expulsion 6 months after IUD insertion." Comment: not answering PICO research question. |
| Chen 2017 | Quote: "Effects of aspirin and intrauterine balloon on endometrial repair and reproductive prognosis in patients with severe intrauterine adhesion: a prospective cohort study." Comment: observational study. |
| Cheong 2016 | Quote: "The use of Hyalobarrier post salpingo‐ovariolysis did not influence follicular development as inferred from the results of the day 21 progesterone and folliculogram on day 10‐12 3‐month postsurgery." Comment: not answering PICO research question. |
| Hu 2014a | Intervention: hysteroscopic adhesiolysis followed by collagen scaffold loaded with autologous bone marrow stem cell treatment. Study design: observational; case series. Comment: observational study. |
| Hu 2014b | Intervention: hysteroscopic adhesiolysis followed by collagen scaffold loaded with umbilical cord blood‐derived mesenchymal stem cell treatment. Study design: observational; case series. Comment: observational study. |
| Johns 2001 | Quote: "OBJECTIVE: To assess the safety and efficacy of the Intergel adhesion prevention solution, a 0.5% ferric hyaluronate gel, in reducing adhesions in patients undergoing peritoneal cavity surgery by laparotomy with a planned second‐look laparoscopy. DESIGN: Randomized, third‐party blinded, placebo‐controlled, parallel group. SETTING: Eleven centres in the United States, and five centres in Europe. PATIENT(S): Women aged 18‐46 years who wanted to retain their fertility. INTERVENTION(S): Patients received 300 mL of Intergel solution (N = 143) or lactated Ringer's solution (N = 138) as an intraperitoneal instillate at the completion of surgery. MAIN OUTCOME MEASURE(S): At second‐look laparoscopy 6‐12 weeks later, the presence of adhesions was evaluated at 24 abdominal sites." Comment: not answering PICO research question. |
| Kim 2012 | Quote: "The exclusion criteria were women who planned to use an intrauterine device for contraception during the study period; (...); women who were pregnant or who planned pregnancy during the study period (...)." Comment: excluded women with subfertility. |
| Kurtz 2002 | Quote: "This randomised controlled blind prospective study is undertaken to evaluate the safety and efficacy of Seprafilm™ ‐ a novel bioresorbable membrane of chemically modified hyaluronic acid and carboxymethylcellulose ‐ in prevention and reduction of postoperative endometrial and endocervical synechiae formation after general suction evacuation or curettage for incomplete, missed, and recurrent abortion." Comment: not answering PICO research question. |
| Liu 2016 | Quote: "A retrospective analysis was carried out to explore the clinical data of 120 cases of severe IUA patients who were treated in Woman and Infant Hospital of Zhengzhou and The Third Affiliated Hospital of Zhengzhou University during the period between January 2010 and December 2013." Comment: observational study. |
| NCT02328742 | Quote: "The main objective of this study is to describe the level of expression of the biological factors involved in the formation of adhesions (Transforming growth factor beta, Activin A, inhibin) at the time of a first diagnostic hysteroscopy among women with synechia, another intracavitary disease or no intracavitary disease"; "Study design: observational model: cohort; time perspective: prospective." Comment: observational study. |
| NTR3120 | Quote: "Consented patients, who had at least one previous suction or abrasive (blunt or sharp) curettage for a miscarriage in the history, visiting the outpatient clinic with a miscarriage and planned for curettage, will be included in the study. The ultrasound is a key in the diagnosis of miscarriage; at least one recent ultrasound examination (made within 7 days before randomisation) is required for inclusion. The maximum gestational age at inclusion is 14 weeks." Comment: not answering PICO research question. |
| Pabuccu 2008 | Quote: "We randomized patients sequentially, according to their entry into the study, after the study started." Comment: quasi‐randomised study. |
| Tonguc 2010 | Quote: "A statistician allotted the participants to their postsurgical treatment groups according to their application numbers." Comment: quasi‐randomised study. |
| Tsapanos 2002 | Quote: "This randomised controlled blind prospective study is undertaken to evaluate the safety and efficacy of Seprafilm™ ‐ a novel bioresorbable membrane of chemically modified hyaluronic acid and carboxymethylcellulose ‐ in prevention and reduction of postoperative endometrial and endocervical synechiae formation after general suction evacuation or curettage for incomplete, missed, and recurrent abortion." Quote: "Endometrial synechiae formation was evaluated with the use of hysterosalpingography (HSG) in patients of all groups without pregnancy success 8 months after the intervention." Comment: not answering PICO research question. |
| Yaşar 2004 | Quote: "OBJECTIVE: To evaluate the role of prophylactic estrogen administration on preventing intrauterine adhesion formation following D&C [dilatation and curettage]." Comment: not answering PICO research question. |
IUD: intrauterine device; PICO: population, intervention, comparator, outcome.
Characteristics of studies awaiting assessment [ordered by study ID]
Hanstede 2016.
| Methods | Parallel‐group randomised controlled trial. Single centre, referral centre, the Netherlands. |
| Participants | 110 women undergoing hysteroscopic adhesiolysis for intrauterine adhesions. |
| Interventions | IUD, Cu‐IUD flexi‐T with copper removed, inserted in uterine cavity in both groups. Intervention: hormone treatment with schedule of oestrogen + norethisterone. Control: no hormone treatment. |
| Outcomes | Primary outcome: recurrence of IUAs. Secondary outcomes: pregnancy, restoration of menstrual flow and endometrial thickness. |
| Notes | Study results and conclusions presented as an abstract at an ESGE meeting. Authors are preparing a publication in a peer‐reviewed journal. |
ESGE: European Society for Gynaecological Endoscopy; IUA: intrauterine adhesion; IUD: intrauterine device.
Characteristics of ongoing studies [ordered by study ID]
NCT01464528.
| Trial name or title | Safety Study of Use of Hyaluronic Acid Gel to Prevent Intrauterine Adhesions in Hysteroscopic Surgery. |
| Methods | Allocation: randomised. Endpoint classification: safety study. Intervention model: parallel assignment. Masking: single‐blind (participant). Primary purpose: prevention. |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: hyaluronic acid gel. Control: no hyaluronic acid gel. |
| Outcomes | Primary outcome: participant satisfaction following gel application. |
| Starting date | November 2011. Estimated recruitment: 10 women. |
| Contact information | Ariel Revel, MD. Hadassah Medical Organization, Israel. Telephone: 97226777111 ext 76389. e‐mail: arielr2@hadassah.org.il. |
| Notes | 7 May 2017: overall status: not yet recruiting. The completion date has passed and the status has been last updated on 2 November 2011. |
NCT01637974.
| Trial name or title | Efficiency of INTERCOAT (Oxiplex/AP Gel) in Preventing Intrauterine Adhesion Formation in Hysteroscopic Surgery. |
| Methods | Allocation: randomised. Endpoint classification: efficacy study. Intervention model: parallel assignment. Masking: double‐blind (participant, carer). Primary purpose: prevention. |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: injection of Intercoat into the uterine cavity at the end of hysteroscopy. Control: no injection of Intercoat. |
| Outcomes | Not provided. |
| Starting date | December 2012. Status on 7 May 2017: still recruiting the estimated sample size of 130 women. |
| Contact information | Moran Paz, MD. Carmel Medical Center, Israel. Telephone: 972‐4‐8250637. e‐mail: MORANPA@CLALIT.GOV.IL. |
| Notes | Estimated primary completion date according to ClinicalTrials.gov was March 2016. Last update received 30 July 2015. |
PID: pelvic inflammatory disease.
Differences between protocol and review
In the protocol, we defined two primary outcomes: live birth (positive outcome) and presence of intrauterine adhesions (IUAs) at second‐look hysteroscopy (adverse outcome). We defined as secondary outcomes the following: clinical pregnancy, miscarriage, mean adhesion scores and severity of adhesions at second‐look hysteroscopy. In the full review, we decided to include only one primary outcome, namely, live birth or ongoing pregnancy ‐ the primary outcome of interest for women with subfertility. Clinical pregnancy, miscarriage, presence of IUAs at second‐look hysteroscopy, mean adhesion scores and severity of adhesions present at second‐look hysteroscopy were defined as secondary outcomes. We made this change on the basis of advice provided by the peer review editorial team in the interest of simplification and readability. We similarly avoided use of the outcome 'incidence of de novo adhesions'; several included studies enrolled participants with existing IUAs, and at second‐look hysteroscopy the distinction between de novo and recurrent adhesions may not be possible and may not be clinically relevant.
Term delivery and ongoing pregnancy were used in the review as a surrogate outcome for live birth because the number of studies reporting live birth was very limited. We used sensitivity analyses to study the impact of including only studies reporting live birth versus all studies reporting live birth or a surrogate outcome.
The protocol prespecified that data would be extracted simultaneously and independently by two review authors. For practical reasons, data were extracted by at least one pair of review authors: for the previous review. JB extracted data from all studies, and TD/FB/JK/SW divided all studies between them, and each extracted data from only a portion of the included studies. In cases of disagreement, BWM acted as a 'third' review author for arbitration. For the updated version, we used a similar approach for practical reasons. See Potential biases in the review process.
We clarified the inclusion criteria to specify that studies in which at least a proportion of women were undergoing operative hysteroscopy for subfertility were eligible.
In the review we reported numbers needed to treat for a beneficial effect (NNTB) when there were statistically significant differences between both comparison groups. This was not prespecified in the protocol.
In the 2017 update authors updated the Methods sections to current Cochrane standards, and changed the format of Effects of the interventions to improve readability of the review.
Contributions of authors
JB: co‐ordinating author.
SW, FB and TD: co‐authored protocol, provided comments and criticisms on the methods and content of the review, and were involved in data extraction and risk of bias assessment.
BWM: responsible for overall supervision of the methods and consulted 'ad hoc' for assistance in resolving disagreements.
HT: involved in data extraction and risk of bias assessment for the updated version.
SJC: assisted in the search for and selection of studies, translated two Chinese articles, sent queries in Chinese to the corresponding authors of three Chinese articles, and was involved in data extraction and risk of bias assessment.
Sources of support
Internal sources
-
CEBAM, Cochrane Belgium, Belgium.
Logistical support by the Managing Secretary
External sources
No sources of support supplied
Declarations of interest
JB: no conflicts of interest.
SW: no conflicts of interest.
TD is a Professor in Reproductive Medicine, Department of Development and Regeneration, University of Leuven (KU Leuven), Belgium, and Professor Adjunct, Department of Obstetrics and Gynecology, Yale University, New Haven, USA. Since October 2015, he has been appointed as Vice‐President and Head of Global Medical Affairs Fertility, Merck KGaA, Darmstadt, Germany. His participation in this publication is part of his academic work. Merck KGaA is not involved in the development or marketing of products related to hysteroscopy. Professor D'Hooghe's employment by Merck is not in breach of Cochrane's Commercial Sponsorship Policy (clause 2) as he does not have a real or potential financial interest in the outcome of this review. This matter was referred to Cochrane's Funding Arbiter for advice.
HT has received conference travel assistance from Merck.
FJB has received monetary compensation for the following: member of the external advisory board for Merck Serono and Ferring, the Netherlands; educational activities for Ferring BV, the Netherlands; consultancy work for Gedeon Richter, Belgium; strategic co‐operation with Roche on automated anti‐Müllerian hormone (AMH) assay development; and research co‐operation with Ansh Labs.
SJC: no conflicts of interest.
BWM has received consultancy from ObsEva Geneva, Guerbet, and Merck; payment for review preparation from European Journal of Obstetrics and Gynecology and Reproductive Biology; and travel/accommodation/meeting expenses for various non‐commercial scientific meetings.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abu Rafea 2013 {published data only}
- Abu Rafea BF, Vilos GA, Oraif AM, Power SG, Hollet Cains J, Vilos AG. Fertility and pregnancy outcomes following resectoscopic septum division with and without intrauterine balloon stenting: a randomized pilot study. Annals Saudi Medicine 2013;33(1):34‐9. [DOI: 10.5144/0256-4947.2013.34] [DOI] [PMC free article] [PubMed] [Google Scholar]
Acunzo 2003 {published data only}
- Acunzo G, Guida M, Pellicano M, Tommaselli GA, Spiezio Sardo A, Bifulco G, et al. Effectiveness of auto‐cross‐linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a prospective, randomized, controlled study. Human Reproduction 2003;18(9):1918‐21. [DOI: 10.1093/humrep/deg368; EMBASE: 2003371784 ; PUBMED: 12923149] [DOI] [PubMed] [Google Scholar]
Amer 2010 {published data only}
- Amer MI, Abd‐El‐Maeboud KHI, Abdelfatah I, Salama FA, Abdallah AS. Human amnion as a temporary biologic barrier after hysteroscopic lysis of severe intrauterine adhesions: pilot study. Journal of Minimally Invasive Gynecology 2010;17(5):605‐11. [DOI: 10.1016/j.jmig.2010.03.019; PUBMED: 20576472] [DOI] [PubMed] [Google Scholar]
Dabir‐Ashrafi 1996 {published data only}
- Dabir‐Ashrafi H, Mohammad K, Moghadami‐Tabrizi N, Zandinejad K, Moghadami‐Tabrizi M. Is estrogen necessary after hysteroscopic incision of the uterine septum?. Journal of the American Association of Gynecologic Laparoscopists 1996;3(4):623‐5. [PUBMED: 9050699] [DOI] [PubMed] [Google Scholar]
De Iaco 2003 {published data only}
- Iaco PA, Muzzupapa G, Bovicelli A, Marconi S, Bitti SR, Sansovini M, et al. Hyaluronan derivative gel (Hyalobarrier gel) in intrauterine adhesion (IUA) prevention after operative hysteroscopy. Ellipse 2003;19(1):15‐8. [Google Scholar]
Di Spiezio Sardo 2011 {published data only}
- Spiezio Sardo A, Spinelli M, Bramante S, Scognamiglio M, Greco E, Guida M, et al. Efficacy of a polyethylene oxide‐sodium carboxymethylcellulose gel in prevention of intrauterine adhesions after hysteroscopic surgery. Journal of Minimally Invasive Gynecology 2011;18(4):462‐9. [DOI: 10.1016/j.jmig.2011.04.007; PUBMED: 21777835] [DOI] [PubMed] [Google Scholar]
Do 2005 {published data only}
- Do JW, Lee YW, Park HJ. The effectiveness of hyaluronic acid + sodium carboxymethyl cellulose in the prevention of intrauterine adhesion after intrauterine surgery. Korean Journal of Gynecologic Endoscopy and Minimally Invasive Surgery 2005;17:112‐7. [Google Scholar]
Fuchs 2014 {published data only}
- Fuchs N, Smorgick N, Ben Ami I, Vaknin Z, Tovbin Y, Halperin R, et al. Intercoat (Oxiplex/AP gel) for preventing intrauterine adhesions after operative hysteroscopy for suspected retained products of conception: double‐blind, prospective, randomized pilot study. Journal of Minimally Invasive Gynecology 2014;21(1):126‐30. [PUBMED: 23954387] [DOI] [PubMed] [Google Scholar]
- Pansky M. Efficiency of Intercoat (Oxiplex/AP gel) in decreasing intrauterine adhesions. clinicaltrials.gov/ct2/show/NCT01377779 Date first received: 15 June 2011. [CTG: NCT01377779]
- Pansky M, Fuchs N, Ben Ami I, Tovbin Y, Halperin R, Vaknin Z, et al. Intercoat (Oxiplex/AP Gel) for preventing intrauterine adhesions following operative hysteroscopy for suspected retained products of conception ‐ a pilot study. Journal of Minimally Invasive Gynecology 2011;18(S21):68. [DOI] [PubMed] [Google Scholar]
Gan 2017 {published data only}
- Gan L, Duan H, Sun FQ, Xu Q, Tang YQ, Wang S. Efficacy of freeze‐dried amnion graft following hysteroscopic adhesiolysis of severe intrauterine adhesions. International Journal of Gynaecology and Obstetrics 2017;137:116‐22. [DOI: 10.1002/ijgo.12112; PUBMED: 28170094] [DOI] [PubMed] [Google Scholar]
Guida 2004 {published data only}
- Guida M, Acunzo G, Spiezio Sardo A, Bifulco G, Piccoli R, Pellicano M, et al. Effectiveness of auto‐crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: a prospective, randomized, controlled study. Human Reproduction 2004;19(6):1461‐4. [DOI: 10.1093/humrep/deh238; PUBMED: 15105384] [DOI] [PubMed] [Google Scholar]
Lin 2015a {published data only}
- Lin YH, Jang TN, Hwang JL, Huang LW, Seow KM, Hsieh BC, et al. Bacterial colonization with balloon uterine stent placement in the uterus for 30 days: a randomized controlled clinical trial. Fertility and Sterility 2015;103(2):513‐8. [PUBMED: 25467040 ] [DOI] [PubMed] [Google Scholar]
Lin 2015b {published data only}
- Lin X. A comparison of Cook balloon and coil in the prevention of adhesion reformation following hysteroscopic surgery for Asherman syndrome [A comparison of intrauterine balloon stent and intrauterine contraceptive device in the prevention of adhesion reformation following hysteroscopic surgery for Asherman syndrome: a randomized case‐control study]. Controlled Trials. 2013. [ISRCTN: ISRCTN69690272 ] [DOI] [PubMed]
- Lin X, Wei ML, Li TC, Zhou F, Zhang SY. A prospective randomized control trial to compare the efficacy of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation following hysteroscopic adhesiolysis. Gynecological Surgery 2014;11(Suppl 1: S1‐S358):89‐90. [DOI: 10.1007/s10397-014-0857-1] [DOI] [Google Scholar]
- Lin XN, Zhou F, Wei ML, Yang Y, Li Y, Li TC, et al. Randomized, controlled trial comparing the efficacy of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation after hysteroscopic adhesiolysis. Fertility and Sterility 2015;104(1):235‐40. [DOI: 10.1016/j.fertnstert.2015.04.008; PUBMED: 25936237] [DOI] [PubMed] [Google Scholar]
Roy 2014 {published data only}
- Roy KK, Negi N, Subbaiah M, Kumar S, Sharma JB, Singh N. Effectiveness of estrogen in the prevention of intrauterine adhesions after hysteroscopic septal resection: a prospective, randomized study. Journal of Obstetrics and Gynaecology Research 2014;40(4):1085‐8. [PUBMED: 13418076] [DOI] [PubMed] [Google Scholar]
Vercellini 1989 {published data only}
- Vercellini P, Fedele L, Arcaini L, Rognoni MT, Candiani GB. Value of intrauterine device insertion and estrogen administration after hysteroscopic metroplasty. Journal of Reproductive Medicine 1989;34(7):447‐50. [PUBMED: 2549238 ] [PubMed] [Google Scholar]
Wang 2016 {published data only}
- Wang X, Duan H. Clinical evaluation of amniotic products after transcervical resection of intensive degree of intrauterine adhesions. Zhonghua Fu Chan Ke za Zhi 2016;51(1):27‐30. [PUBMED: 26899003 ] [DOI] [PubMed] [Google Scholar]
Xiao 2015 {published data only}
- Xiao S, Wan Y, Zou F, Ye M, Deng H, Ma J, et al. A randomized multi‐center controlled study on the efficacy and safety of a new crosslinked hyaluronan gel to prevent intrauterine adhesion following hysteroscopic adhesiolysis. Giornale Italiano di Ostetricia e Ginecologia 2015;37(4):216‐9. [Google Scholar]
- Xiao S, Wan Y, Zou F, Ye M, Deng H, Ma J, et al. Prevention of intrauterine adhesion with auto‐crosslinked hyaluronic acid gel: a prospective, randomized, controlled clinical study. Zhonghua Fu Chan Ke za Zhi 2015;50(1):32‐6. [DOI: 10.3760/cma.j.issn.0529-567x.2015.01.008; PUBMED: 25877422 ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bednarek 2011 {published data only}
- Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT. Immediate versus delayed IUD insertion after uterine aspiration. New England Journal of Medicine 2011;364(23):2208‐17. [DOI: 10.1056/NEJMoa1011600] [DOI] [PubMed] [Google Scholar]
Chen 2017 {unpublished data only}
- Chen Y, Liu L, Luo Y, Chen M, Yang Huan Y, Fang R. Effects of aspirin and intrauterine balloon on the post‐operative uterine endometrial repair and reproductive prognosis in patients with severe intrauterine adhesion: a prospective cohort study. BioMed Research International 2017;ID 8526104:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02744716. Effects of aspirin on uterine endometrial repair severe intrauterine adhesion. clinicaltrials.gov/ct2/show/NCT02744716 Date first received: 9 April 2016.
Cheong 2016 {published data only}
- Cheong Y, Bailey S, Forbes J. Randomized controlled trial of Hyalobarrier® versus no Hyalobarrier® on the ovulatory status of women with periovarian adhesions: a pilot study. Advances in Therapy 2016;Online First Articles:1‐8. [DOI: 10.1007/s12325-016-0453-z; ISSN: 1865‐8652] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2014a {unpublished data only}
- ChiCTR‐OOC‐14005571. Clinical study of the treatment of infertility caused by severe intrauterine adhesions by collagen scaffold loaded with autologous bone marrow stem cells. www.chictr.org.cn/showprojen.aspx?proj=9959 Date first registered: 28 November 2014. [ChiCTR‐OOC‐14005571]
Hu 2014b {unpublished data only}
- ChiCTR‐OPC‐14005553. Clinical study of the treatment of infertility caused by severe intrauterine adhesions by collagen scaffold loaded with umbilical cord blood‐derived mesenchymal stem cells. www.chictr.org.cn/showprojen.aspx?proj=9934 Date first registered: 28 November 2014. [ChiCTR‐OPC‐14005553]
Johns 2001 {published data only}
- Johns DB, Keyport GM, Hoehler F, diZerega GS. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertility and Sterility 2001;76(3):595‐604. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim T, Ahn KH, Choi DS, Hwang KJ, Lee BI, Jung MH, et al. A randomized, multi‐center, clinical trial to assess the efficacy and safety of alginate carboxymethylcellulose hyaluronic acid compared to carboxymethylcellulose hyaluronic acid to prevent postoperative intrauterine adhesion. Journal of Minimally Invasive Gynecology 2012;19(6):731‐6. [DOI: 10.1016/j.jmig.2012.08.003; PUBMED: 23084677] [DOI] [PubMed] [Google Scholar]
Kurtz 2002 {published data only}
- Kurtz SM, Muratoglu OK, Gsell R, Greer K, Shen FW, Cooper C, et al. The role of Seprafilm™ bioresorbable membrane in the prevention and therapy of endometrial synechiae. Journal of Biomedical Materials Research 2002;63(1):10‐4. [DOI] [PubMed] [Google Scholar]
Liu 2016 {published data only}
- Liu AZ, Zhao HG, Gao Y, Liu M, Guo BZ. Effectiveness of estrogen treatment before transcervical resection of adhesions on moderate and severe uterine adhesion patients. Gynecological Endocrinology 2016;32(9):737‐40. [DOI: 10.3109/09513590.2016.1160375; PUBMED: 26982384] [DOI] [PubMed] [Google Scholar]
NCT02328742 {unpublished data only}
- NCT02328742. Development of a bioabsorbable medical device for the prevention of postoperative intra‐uterine adhesions. PréSynUT‐1. clinicaltrials.gov/ct2/show/NCT02328742 Date first received: 26 December 2014. [ISRCTN: NCT02328742]
NTR3120 {unpublished data only}
- NTR3120. Post abortion prevention of adhesions: evaluation of hyaluronic acid, hyalobarier gel endo ‐ PAPA ‐ study [Het voorkomen van littekenvorming in de baarmoeder bij vrouwen die een curettage ondergaan vanwege een miskraam. (Evaluatie van anti‐verklevingsmiddel)]. www.trialregister.nl/trialreg/admin/rctview.asp?TC=3120 Date first registered: 27 October 2011. [NTR3120]
Pabuccu 2008 {published data only}
- Pabuccu R, Onalan G, Kaya C, Selam B, Ceyhan T, Ornek T, et al. Efficiency and pregnancy outcome of serial intrauterine device‐guided hysteroscopic adhesiolysis of intrauterine synechiae. Fertility and Sterility 2008;90(5):1973‐7. [DOI: 10.1016/j.fertnstert.2007.06.074] [DOI] [PubMed] [Google Scholar]
Tonguc 2010 {published data only}
- Tonguc EA, Var T, Yilmaz N, Batioglu S. Intrauterine device or oestrogen treatment after hysteroscopic uterine septum resection. International Journal of Gynecology and Obstetrics 2010;109:226‐9. [DOI: 10.1016/j.ijgo.2009.12.015] [DOI] [PubMed] [Google Scholar]
Tsapanos 2002 {published data only}
- Tsapanos VS, Stathopoulou LP, Papathanassopoulou VS, Tzingounis VA. The role of Seprafilm™ bioresorbable membrane in the prevention and therapy of endometrial synechiae. Journal of Biomedical Materials Research 2002;63(1):10‐4. [PUBMED: 11787023] [DOI] [PubMed] [Google Scholar]
Yaşar 2004 {published data only}
- Yaşar L, Sönmez S, Koç S, Çebi Z, Küpelioğlu L, Toklar A, et al. Prophylactic estrogen administration for preventing intrauterine adhesion formation following missed abortions. Jinekoloji ve Obstetrik Dergisi 2004;18:154‐7. [Google Scholar]
References to studies awaiting assessment
Hanstede 2016 {published and unpublished data}
- Hanstede MMF, Emanuel MH. Tertiary prevention of morbus Asherman. Evaluation of hormonal support with estrogen and gestagen post adhesiolyis. Gynecological Surgery 2016;13(1):S130. [Google Scholar]
References to ongoing studies
NCT01464528 {unpublished data only}
- NCT01464528. Safety study of use of hyaluronic acid gel to prevent intrauterine adhesions in hysteroscopic surgery. clinicaltrials.gov/ct2/show/NCT01464528 Date first received: 15 August 2011. [NCT: 01464528]
NCT01637974 {unpublished data only}
- NCT01637974. Efficiency of INTERCOAT (Oxiplex/AP Gel) in preventing intrauterine adhesion formation in hysteroscopic surgery ‐ a prospective double blind randomized study. clinicaltrials.gov/ct2/show/NCT01637974 Date first received: 9 July 2012. [NCT: 01637974; CMC‐11‐0050‐CTIL]
Additional references
Ahmad 2015
- Ahmad G, O'Flynn H, Hindocha A, Watson A. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD000475.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amer 2006
- Amer MI, Abd‐El‐Maeboud KH. Amnion graft following hysteroscopic lysis of intrauterine adhesions. Journal of Obstetrics and Gynaecology Research 2006;32(6):559‐66. [DOI: 10.1111/j.1447-0756.2006.00454; PUBMED: 17100817] [DOI] [PubMed] [Google Scholar]
Arora 1994
- Arora M, Jaroudi KA, Hamilton CJ, Dayel F. Controlled comparison of Interceed and amniotic membrane graft in the prevention of postoperative adhesions in the rabbit uterine horn model. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1994;55(3):179‐82. [PUBMED: 7958162; SSDI: 0028‐2243(94)01849‐3] [DOI] [PubMed] [Google Scholar]
Baakdah 2005
- Baakdah H, Tulandi T. Adhesion in gynaecology complication, cost, and prevention: a review. Surgical Technology International 2005;14:185‐90. [PUBMED: 16525972] [PubMed] [Google Scholar]
Badawy 1989
- Badawy SZ, Baggish MS, ElBakry MM, Baltoyannis P. Evaluation of tissue healing and adhesion formation after an intra‐abdominal amniotic membrane graft in the rat. Journal of Reproductive Medicine 1989;34(3):189‐202. [PUBMED: 2724232] [PubMed] [Google Scholar]
Belluco 2001
- Belluco C, Meggiolaro F, Pressato D, Pavesio A, Bigon E, Donà M, et al. Prevention of postsurgical adhesions with an autocrosslinked hyaluronan derivative gel. Journal of Surgical Research 2001;100:217‐21. [DOI: 10.1006/jsre.2001.6248; PUBMED: 11592796] [DOI] [PubMed] [Google Scholar]
Binda 2007
- Binda MM, Molinas CR, Bastidas A, Jansen M, Koninckx PR. Efficacy of barriers and hypoxia‐inducible factor inhibitors to prevent CO(2)pneumoperitoneum‐enhanced adhesions in a laparoscopic mouse model. Journal of Minimally Invasive Gynecology 2007;14(5):591‐9. [DOI: 10.1016/j.jmig.2007.04.002; PUBMED: 17848320] [DOI] [PubMed] [Google Scholar]
Binda 2009
- Binda MM, Koninckx PR. Prevention of adhesion formation in a laparoscopic mouse model should combine local treatment with peritoneal cavity conditioning. Human Reproduction 2009;24(6):1473‐9. [DOI: 10.1093/humrep/dep053; PUBMED: 19258346] [DOI] [PubMed] [Google Scholar]
Binda 2010
- Binda MM, Koninckx PR. Hyperoxia and prevention of adhesion formation: a laparoscopic mouse model for open surgery. British Journal of Obstetrics and Gynaecology 2010;117(3):331‐9. [DOI: 10.1111/j.1471-0528.2009.02370.x; PUBMED: 19832833] [DOI] [PubMed] [Google Scholar]
Bosteels 2015a
- Bosteels J, Kasius J, Weyers S, Broekmans FJ, Mol BWJ, D'Hooghe TM. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD009461.pub3] [DOI] [PubMed] [Google Scholar]
Bristow 2007
- Bristow RE, Santillan A, Diaz‐Montes TP, Gardner GJ, Giuntoli RL, Peeler ST. Prevention of adhesion formation after radical hysterectomy using a sodium hyaluronate‐carboxymethylcellulose (HA‐CMC) barrier: a cost‐effectiveness analysis. Gynecologic Oncology 2007;104(3):739‐46. [DOI: 10.1016/j.ygyno.2006.09.029; PUBMED: 7097723] [DOI] [PubMed] [Google Scholar]
D'Hooghe 2009
- D'Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolazkaia A, Bokor A, et al. Nonhuman primate models for translational research in endometriosis. Reproduction Science 2009;16(2):152‐61. [PUBMED: 19208783] [DOI] [PubMed] [Google Scholar]
De Iaco 1998
- Iaco PA, Stefanetti M, Pressato D, Piana S, Donà M, Pavesio A, et al. A novel hyaluronan‐based gel in laparoscopic adhesion prevention: preclinical evaluation in an animal model. Fertility and Sterility 1998;69:318‐23. [DOI: 10.1016/S0015-0282(98)00496-8; PUBMED: 9496348] [DOI] [PubMed] [Google Scholar]
Deane 2013
- Deane JA, Gualano RC, Gargett CE. Regenerating endometrium from stem/progenitor cells: is it abnormal in endometriosis, Asherman's syndrome and infertility?. Current Opinion in Obstetrics & Gynecology 2013;25:193‐200. [DOI: 10.1097/GCO.Ob13e32836024e7] [DOI] [PubMed] [Google Scholar]
Deans 2010
- Deans R, Abbott J. Review of intrauterine adhesions. Journal of Minimally Invasive Gynecology 2010;17:555‐69. [DOI: 10.1016/j.jmig.2010.04.016; PUBMED: 20656564] [DOI] [PubMed] [Google Scholar]
DeCherney 1997
- DeCherney AH, diZerega GS. Clinical problem of intraperitoneal postsurgical adhesion formation following general surgery and the use of adhesion prevention barriers. Surgical Clinics of North America 1997;77:671‐88. [PUBMED: 9194886] [DOI] [PubMed] [Google Scholar]
Di Spiezio Sardo 2016
- Spiezio Sardo A, Calagna G, Scognamiglio M, O'Donovan P, Campo C, Wilde RL. Prevention of intrauterine post‐surgical adhesions in hysteroscopy. A systematic review. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2016;203:182‐92. [DOI: 10.1016/j.ejogrb.2016.05.050; PUBMED: 27337414] [DOI] [PubMed] [Google Scholar]
Diamond 1988
- Diamond MP, DeCherney AH, Linsky CB, Cunningham T, Constantine B. Adhesion re‐formation in the rabbit uterine horn model: I. Reduction with carboxymethylcellulose. International Journal of Fertility 1988;33:372‐5. [PUBMED: 2904426] [PubMed] [Google Scholar]
diZerega 1994
- diZerega GS. Contemporary adhesion prevention. Fertility and Sterility 1994;61:219‐35. [PUBMED: 8299773] [DOI] [PubMed] [Google Scholar]
Ducarme 2006
- Ducarme G, Davitian C, Zarrouk S, Uzan M, Poncelet C. Interest of auto‐crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: a case‐control study. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2006;35(7):691‐5. [DOI: ; PUBMED: 17088770] [DOI] [PubMed] [Google Scholar]
Farhi 1993
- Farhi J, Bar‐Hava I, Homburg R, Dicker D, Ben‐Rafael Z. Induced regeneration of endometrium following curettage for abortion: a comparative study. Human Reproduction 1993;8:1143. [PUBMED: 8408501 ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 02 May 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Healy 2015
- Healy MW, Schexnayder B, Connell MT, DeCherney A, Yauger B, Hill MJ. Preventative measures for intrauterine adhesions following hysteroscopy: a systematic review and meta‐analysis. Fertility and Sterility 2015;104(3 Suppl 1):e172. [Google Scholar]
Healy 2016
- Healy MW, Schexnayder B, Connell MT, Terry N, DeCherney AH, Csokmay JM, et al. Intrauterine adhesion prevention after hysteroscopy: a systematic review and meta‐analysis. American Journal of Obstetrics and Gynecology 2016;21(3):267‐75. [DOI: 10.1016/j.ajog.2016.05.001; PUBMED: 27173082] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hori 2006
- Hori J, Wang M, Kamiya K, Takahashi H, Sakuragawa N. Immunological characteristics of amniotic epithelium. Cornea 2006;25(10 Suppl 1):S53‐8. [PUBMED: 17001194] [DOI] [PubMed] [Google Scholar]
Johary 2014
- Johary J, Xue M, Zhu X, Xu D, Palani Velu P. The efficacy of estrogen therapy in patients with intrauterine adhesions: a systematic review. Journal of Minimally Invasive Gynecology 2014;21(1):44‐54. [DOI: 10.1016/j.jmig.2013.07.018; PUBMED: 23933351] [DOI] [PubMed] [Google Scholar]
Kelekci 2004
- Kelekci S, Yilmaz B, Oguz S, Zergeroğlu S, Inan I, Tokucoğlu S. The efficacy of a hyaluronate/carboxymethylcellulose membrane in prevention of postoperative adhesion in a rat uterine horn model. Tohoku Journal of Experimental Medicine 2004;204:189‐94. [DOI: 10.1620/tjem.204.189; PUBMED: 15502417] [DOI] [PubMed] [Google Scholar]
Kodaman 2007
- Kodaman PH, Arici A. Intra‐uterine adhesions and fertility outcome: how to optimize success?. Current Opinion in Obstetrics & Gynecology 2007;19(3):207‐14. [PUBMED: 17495635 ] [DOI] [PubMed] [Google Scholar]
Koçak 1999
- Koçak I, Unlü C, Akçan Y, Yakin K. Reduction of adhesion formation with cross‐linked hyaluronic acid after peritoneal surgery in rats. Fertility and Sterility 1999;72:873‐8. [DOI: 10.1016/S0015-0282(99)00368-4; PUBMED: 10560992] [DOI] [PubMed] [Google Scholar]
Laurent 1992
- Laurent TC, Fraser JRE. Hyaluronan. Journal of the Federation of American Societies for Experimental Biology 1992;6:2397‐404. [PubMed] [Google Scholar]
Leach 1998
- Leach RE, Burns JW, Dawe EJ, SmithBarbour MD, Diamond MP. Reduction of postsurgical adhesion formation in the rabbit uterine horn model with use of hyaluronate/carboxymethylcellulose gel. Fertility and Sterility 1998;69(3):415‐7. [DOI: 10.1016/S0015-0282(97)00573-6; PUBMED: 9531869] [DOI] [PubMed] [Google Scholar]
Mais 2012
- Mais V, Cirronis MG, Peiretti M, Ferrucci G, Cossu E, Melis GB. Efficacy of auto‐cross linked hyaluronan gel for adhesion prevention in laparoscopy and hysteroscopy; a systematic review and meta‐analysis of randomized controlled trials. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2012;160:1‐5. [DOI: 10.1016/j.ejogrb.2011.08.002; PUBMED: 21945572] [DOI] [PubMed] [Google Scholar]
Mascarenhas 2012
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Medicine 2012;9(12):e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meller 1999
- Meller D, Tseng SC. Conjunctival epithelial cell differentiation on amniotic membrane. Investigative Ophthalmology & Visual Science 1999;40:878‐86. [PUBMED: 10102284] [PubMed] [Google Scholar]
Nappi 2007
- Nappi C, Spiezio Sardo A, Greco E, Guida M, Bettocchi S, Bifulco G. Prevention of adhesions in gynaecological endoscopy. Human Reproduction Update 2007;13(4):1‐16. [DOI: 10.1093/humupd/dml061; PUBMED: 17452399] [DOI] [PubMed] [Google Scholar]
Nimrod 1992
- Nimrod A, Ezra E, Ezov N, Nachum G, Parisada B. Absorption, distribution, metabolism and excretion of bacteria‐derived hyaluronic acid in rats and rabbits. Journal of Ocular Pharmacology 1992;8:161‐72. [PUBMED: 1506757] [DOI] [PubMed] [Google Scholar]
Okulicz 2002
- Okulicz WC. Regeneration. In: Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S editor(s). The Endometrium. London: Taylor and Francis, 2002:110‐20. [Google Scholar]
Orhue 2003
- Orhue AAE, Aziken ME, Igbefoh JO. A comparison of two adjunctive treatments for intrauterine adhesions following lysis. International Journal of Gynaecology and Obstetrics 2003;82:49‐56. [DOI: 10.1016/S0020-7292(03)00030-4; PUBMED: 12834941] [DOI] [PubMed] [Google Scholar]
Padykula 1991
- Padykula HA. Regeneration in the primate uterus: the role of stem cells. Annals of the New York Academy of Sciences 1991;622:47‐56. [PUBMED: 2064204] [DOI] [PubMed] [Google Scholar]
Rajab 2010
- Rajab TK, Wallwiener M, Planck C, Brochhausen C, Kraemer B, Wallwiener CW. A direct comparison of Seprafilm, Adept, Intercoat, and Spraygel for adhesion prophylaxis. Journal of Surgical Research 2010;161:246‐9. [DOI: 10.1016/j.jss.2008.11.839; PUBMED: 9375716] [DOI] [PubMed] [Google Scholar]
Renier 2005
- Renier D, Bellato PA, Bellini D, Pavesio A, Pressato D, Borrione A. Pharmacokinetic behaviour of ACP gel, an autocrosslinked hyaluronan derivative, after intraperitoneal administration. Biomaterials 2005;26:5368‐74. [DOI: 10.1016/j.biomaterials.2005.01.053; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Revaux 2008
- Revaux A, Ducarme G, Luton D. Prevention of intrauterine adhesions after hysteroscopic surgery. Gynecologie, Obstetrique et Fertilité 2008;36(3):311‐7. [DOI: 10.1016/j.gyobfe.2007.11.014; PUBMED: 18308609; WoS: 000254969900012 ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salma 2014
- Salma U, Xue M, Sayed AS, Xu D. Efficacy of intrauterine device in the treatment of intrauterine adhesions. Biomedical Research International 2014;2014:589296. [DOI: 10.1155/2014/589296] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schenker 1982
- Schenker JG, Margalioth EJ. Intrauterine adhesions: an updated appraisal. Fertility and Sterility 1982;37:593‐610. [PUBMED: 6281085] [DOI] [PubMed] [Google Scholar]
Schonman 2008
- Schonman R, Corona R, Bastidas A, Cicco C, Mailova K, Koninckx PR. Intercoat gel (Oxiplex): efficacy, safety, and tissue response in a laparoscopic mouse model. Journal of Minimally Invasive Gynecology 2008;16(2):188‐94. [DOI: 10.1016/j.jmig.2008.12.014; PUBMED: 19249707] [DOI] [PubMed] [Google Scholar]
Shamiyeh 2007
- Shamiyeh A, Danis J, Benkö L, Vattay P, Röth E, Tulipan L, et al. Effect of hyaluron derivate gel in prevention of postsurgical peritoneal adhesions ‐ an experimental study in pigs. Hepatogastroenterology 2007;54(76):1121‐4. [PUBMED: 17629052] [PubMed] [Google Scholar]
Szabo 2002
- Szabo A, Haj M, Waxsman I, Eitan A. Evaluation of Seprafilm and amniotic membrane as adhesion prophylaxis in mesh repair of abdominal wall hernia in rats. European Surgical Research 2002;32(2):125‐8. [DOI: 10.1159/000008751; PUBMED: 10810219] [DOI] [PubMed] [Google Scholar]
Taskin 2000
- Taskin O, Sadik S, Onoglu A, Gokdeniz R, Erturan E, Burak F, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. Journal of the American Association of Gynecologic Laparoscopists 2000;7(3):351‐4. [DOI: 10.1016/S1074-3804(05)60478-1; PUBMED: 10924629] [DOI] [PubMed] [Google Scholar]
Trelford Sauder 1977
- Trelford Sauder M, Trelford JD, Matolo NM. Replacement of the peritoneum with amnion following pelvic exenteration. Surgery, Gynecology & Obstetrics 1977;145:699‐701. [PUBMED: 910213] [PubMed] [Google Scholar]
Wallwiener 2006
- Wallwiener M, Brucker S, Hierlemann H, Brochhausen C, Solomayer E, Wallwiener C. Innovative barriers for peritoneal adhesion prevention: liquid or solid? A rat uterine horn model. Fertility and Sterility 2006;86(4 Suppl):1266‐76. [DOI: 10.1016/j.jgyn.2014.10.014; PUBMED: 17008150] [DOI] [PubMed] [Google Scholar]
Wood 1964
- Wood J, Pena G. Treatment of traumatic uterine synechias. International Journal of Fertility 1964;9:405‐10. [PUBMED: 14145804] [PubMed] [Google Scholar]
Yang 2013
- Yang JH, Chen MJ, Chen CD, Chen SU, Ho HN, Yang YS. Optimal waiting period for subsequent fertility treatment after various hysteroscopic surgeries. Fertility and Sterility 2013;99(7):2092‐6.e3. [DOI: 10.1016/j.fertnstert.2013.01.137; PUBMED: 2343383] [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2009
- Zegers‐Hochschild F, Adamson GD, Mouzon J, Mansour R, Nygren K, Sullivan E, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology 2009. Fertility and Sterility 2009;92(5):1520‐4. [DOI: 10.1016/j.fertnstert.2009.09.009; PUBMED: 19828144] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bosteels 2013a
- Bosteels J, Weyers S, Kasius J, Broekmans FJ, Mol BWJ, D'Hooghe TM. Anti‐adhesion therapy following operative hysteroscopy for treating female subfertility. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD011110] [DOI] [PubMed] [Google Scholar]
Bosteels 2013b
- Bosteels J, Pelckmans S, Weyers S, Mol BWJ, D'Hooghe T. The effectiveness of anti‐adhesion therapy following operative hysteroscopy for treating female infertility: a systematic review and meta‐analysis. Human Reproduction (Oxford, England) 2013;28(Suppl 1):i364. [Google Scholar]
Bosteels 2014
- Bosteels J, Weyers S, Mol BWJ, D'Hooghe T. Anti‐adhesion barrier gels following operative hysteroscopy for treating female infertility: a systematic review and meta‐analysis. Gynecological Surgery 2014;11(2):113‐27. [DOI: 10.1007/s10397-014-0832-x; PUBMED: 24795547] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosteels 2015b
- Bosteels J, Weyers S, Kasius J, Broekmans FJ, Mol BWJ, D'Hooghe TM. Anti‐adhesion therapy following operative hysteroscopy for treatment of female subfertility. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011110.pub2] [DOI] [PubMed] [Google Scholar]


