Abstract
Background
Pressure ulcers, also known as bedsores, decubitus ulcers and pressure injuries, are localised areas of injury to the skin or the underlying tissue, or both. A range of treatments with antimicrobial properties, including impregnated dressings, are widely used in the treatment of pressure ulcers. A clear and current overview is required to facilitate decision making regarding use of antiseptic or antibiotic therapies in the treatment of pressure ulcers. This review is one of a suite of Cochrane reviews investigating the use of antiseptics and antibiotics in different types of wounds. It also forms part of a suite of reviews investigating the use of different types of dressings and topical treatments in the treatment of pressure ulcers.
Objectives
To assess the effects of systemic and topical antibiotics, and topical antiseptics on the healing of infected and uninfected pressure ulcers being treated in any clinical setting.
Search methods
In October 2015 we searched: the Cochrane Wounds Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), Ovid MEDLINE, Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations), Ovid EMBASE, and EBSCO CINAHL Plus. We also searched three clinical trials registries and the references of included studies and relevant systematic reviews. There were no restrictions based on language or date of publication or study setting.
Selection criteria
Randomised controlled trials which enrolled adults with pressure ulcers of stage II or above were included in the review.
Data collection and analysis
Two review authors independently performed study selection, risk of bias assessment and data extraction.
Main results
We included 12 trials (576 participants); 11 had two arms and one had three arms. All assessed topical agents, none looked at systemic antibiotics. The included trials assessed the following antimicrobial agents: povidone iodine, cadexomer iodine, gentian violet, lysozyme, silver dressings, honey, pine resin, polyhexanide, silver sulfadiazine, and nitrofurazone with ethoxy‐diaminoacridine. Comparators included a range of other dressings and ointments without antimicrobial properties and alternative antimicrobials. Each comparison had only one trial, participant numbers were low and follow‐up times short. The evidence varied from moderate to very low quality.
Six trials reported the primary outcome of wound healing. All except one compared an antiseptic with a non‐antimicrobial comparator. There was some moderate and low quality evidence that fewer ulcers may heal in the short term when treated with povidone iodine compared with non‐antimicrobial alternatives (protease‐modulating dressings (risk ratio (RR) 0.78, 95% confidence interval (CI) 0.62 to 0.98) and hydrogel (RR 0.64, 95% CI 0.43 to 0.97)); and no clear difference between povidone iodine and a third non‐antimicrobial treatment (hydrocolloid) (low quality evidence). Pine resin salve may heal more pressure ulcers than hydrocolloid (RR 2.83, 95% CI 1.14 to 7.05) (low quality evidence). There is no clear difference between cadexomer iodine and standard care, and between honey and a combined antiseptic and antibiotic treatment (very low quality evidence).
Six trials reported adverse events (primary safety outcome). Four reported no adverse events; there was very low quality evidence from one showing no clear evidence of a difference between cadexomer iodine and standard care; in one trial it was not clear whether data were appropriately reported.
There was limited reporting of secondary outcomes. The five trials that reported change in wound size as a continuous outcome did not report any clear evidence favouring any particular antiseptic/anti‐microbial treatments. For bacterial resistance, one trial found some evidence of more MRSA eradication in participants with ulcer treated with a polyhexanide dressing compared with a polyhexanide swab (RR 1.48, 95% CI 1.02 to 2.13); patients in the dressing group also reported less pain (MD −2.03, 95% CI −2.66 to −1.40). There was no clear evidence of a difference between interventions in infection resolution in three other comparisons. Evidence for secondary outcomes varied from moderate to very low quality; where no GRADE assessment was possible we identified substantial limitations which an assessment would have taken into account.
Authors' conclusions
The relative effects of systemic and topical antimicrobial treatments on pressure ulcers are not clear. Where differences in wound healing were found, these sometimes favoured the comparator treatment without antimicrobial properties. The trials are small, clinically heterogenous, generally of short duration, and at high or unclear risk of bias. The quality of the evidence ranges from moderate to very low; evidence on all comparisons was subject to some limitations.
Plain language summary
Antibiotics and antiseptics for pressure ulcers
What are pressure ulcers and who is at risk?
Pressure ulcers, also known as bedsores, decubitus ulcers and pressure injuries, are wounds involving the skin and often the tissue that lies underneath. Pressure ulcers can be painful, may become infected, and affect people’s quality of life. People at risk of developing pressure ulcers include those with spinal cord injuries, and those who are immobile or have limited mobility, such as elderly people and people who are ill.
Why use antiseptics and antibiotics to treat pressure ulcers?
Where pressure ulcers are infected, antibiotics or antiseptics are used to kill or slow the growth of the micro‐organisms causing the infection and may prevent an infection from getting worse or spreading. This may also help the ulcer to heal. Where ulcers are not infected they usually still have populations of micro‐organisms present. It is thought that they may heal better if these are reduced by antimicrobial agents. However, the relationship between infection and micro‐organism populations in wounds and wound healing is not very clear.
What we found
In October 2015 we searched for as many studies as we could find that were randomised controlled trials and compared the use of an antibiotic or antiseptic with other treatments for pressure ulcers. We found 12 trials involving a total of 576 participants. Most study participants were older people in hospital. Most ulcers were not infected at the start of the trials. The different treatments assessed included povidone iodine, cadexomer iodine, gentian violet, lysozyme, silver dressings, honey, pine resin, silver sulfadiazine, polyhexanide and a combination of nitrofurazone and ethoxy‐diaminoacridine. Silver sulfadiazine and nitrofurazone are topical (locally acting) antibiotics while the other treatments are antiseptics. No trials looked at systemic (acting across the whole body) antibiotics. The treatments were compared with each other or to treatments without antimicrobial qualities. Most evidence on wound healing came from trials comparing antiseptics to treatments without antimicrobial qualities.
There was no consistent evidence of a benefit to using any particular antimicrobial treatment for pressure ulcers. However, there was some limited evidence that more ulcers healed when treated with some types of alternative dressings without antimicrobial properties than when treated with povidone iodine. All the studies had low numbers of participants, and in some cases these numbers were very small. Many studies did not report important information about how they were carried out so it was difficult to tell whether the results presented were likely to be true. More, better quality, research is needed to determine the effects of antimicrobial treatments on pressure ulcers.
Background
Description of the condition
Pressure ulcers, also known as bedsores, decubitus ulcers, pressure injuries or pressure sores are defined as "a localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in combination with shear" (EPUAP‐NPUAP‐PPPIA 2014). Pressure ulcers are a type of complex wound that heals by secondary intention (through the growth of new tissue).
Prolonged exposure of an area of the body to pressure or compression can damage cells, interrupt the local blood circulation (i.e. reduce perfusion), and trigger a cascade of biochemical changes that may lead to tissue damage and ulceration (Gebhardt 2002; Loerakker 2010). Immobility can also lead to increased damage from shear and friction, for example, when people are pulled into position in chairs and beds.
People at particular risk of pressure ulcers are those who cannot reposition themselves when they are seated in a chair or lying in bed. This includes those with limited activity and mobility or reduced bodily sensation, such as elderly people, people with spinal cord injuries (Gefen 2014), and those with acute or chronic health conditions (Allman 1997; Bergstrom 1998; Berlowitz 1990; Brandeis 1994). A recent systematic review, Coleman 2013, identified the key risk factors for pressure ulcers as: limitations of mobility or activity; reduced perfusion (including a diagnosis of diabetes); and the presence of a stage 1 pressure ulcer (see classification below). A recent cohort study found that predictors of poor healing included the severity of the ulcer and the presence of peripheral arterial disease (poor circulation/perfusion of the limb; McGinnis 2014).
Children with pressure ulcers are recognised as a discrete population that includes both neonates and older children with a range of conditions and risk factors (EPUAP‐NPUAP‐PPPIA 2014; NICE 2014); they are cared for in specialist paediatric facilities, and, accordingly, are outside the scope of this review.
Classification of pressure ulcers
One of the most widely recognised ways of classifying pressure ulcers according to severity is that of the National Pressure Ulcer Advisory Panel (NPUAP). Their international classification recognises four categories, or stages, of pressure ulcers and two categories of unclassifiable pressure injuries in which wound depth or extent, or both, cannot be accurately determined: such ulcers are generally severe and would be grouped clinically with category 3 or 4 ulcers (EPUAP‐NPUAP‐PPPIA 2014). The definitions for the categories of severity for ulcers are as follows:
Category/Stage 1: non‐blanchable erythema: "Intact skin with non‐blanchable redness of a localised area usually over a bony prominence. Darkly pigmented skin may not have visible blanching; its colour may differ from the surrounding area. The area may be painful, firm, soft, warmer or cooler as compared to adjacent tissue. Category/Stage I may be difficult to detect in individuals with dark skin tones. May indicate ’at risk’ individuals (a heralding sign of risk)."
Category 2: partial thickness tissue loss: “Partial thickness loss of dermis presenting as a shallow open ulcer with a red pink wound bed, without slough. May also present as an intact or open/ruptured serum‐filled or sero‐sanguinous filled blister. Presents as a shiny or dry shallow ulcer without slough or bruising (bruising indicates suspected deep tissue injury). This category/stage should not be used to describe skin tears, tape burns, perineal dermatitis, maceration or excoriation."
Category 3: full thickness tissue loss: "Full thickness tissue loss. Subcutaneous fat may be visible but bone, tendon or muscle are not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunnelling. The depth of a Category/Stage III pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have subcutaneous tissue and Category/Stage III ulcers can be shallow. In contrast, areas of significant adiposity can develop extremely deep Category/Stage III pressure ulcers. Bone/tendon is not visible or directly palpable."
Category 4: full thickness tissue loss with exposed muscle, tendon or bone: "Full thickness tissue loss with exposed bone, tendon or muscle. Slough or eschar may be present in some parts of the wound bed. Often includes undermining and tunnelling. The depth of a Category/Stage IV pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have subcutaneous tissue and these ulcers can be shallow. Category/Stage IV ulcers can extend into muscle and/or supporting structures (e.g., fascia, tendon or joint capsule) making osteomyelitis possible. Exposed bone/muscle is visible or directly palpable."
The two additional categories of unclassifiable wounds that are also recognised are:
Unstageable/unclassified: full thickness skin or tissue loss‐depth unknown: "Full thickness tissue loss in which actual depth of the ulcer is completely obscured by slough (yellow, tan, gray, green or brown) and/or eschar (tan, brown or black) in the wound bed. Further description: Until enough slough and/or eschar are removed to expose the base of the wound, the true depth cannot be determined; but it will be either a Category/Stage III or IV. Stable (dry, adherent, intact without erythema or fluctuance) eschar on the heels serves as “the body’s natural (biological) cover” and should not be removed."
Suspected deep tissue injury ‐ depth unknown: "Purple or maroon localized area of discoloured intact skin or blood‐filled blister due to damage of underlying soft tissue from pressure and/or shear. Further description: The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer or cooler as compared to adjacent tissue. Deep tissue injury may be difficult to detect in individuals with dark skin tones. Evolution may include a thin blister over a dark wound bed. The wound may further evolve and become covered by thin eschar. Evolution may be rapid exposing additional layers of tissue even with treatment."
Prevalence
Pressure ulcers are one of the most common types of complex wound. Prevalence refers to the number of people with a pressure ulcer at a point in time, or during a specific time period (Bonita 2006). Prevalence estimates differ according to the population assessed, the assessment methods used and the category or categories of ulcers that are included in the estimates.
In the UK, national pressure ulcer data are collected across community and acute settings — although data collection is not yet universal — as part of the National Health Service (NHS) Safety Thermometer initiative (Power 2012). In April 2014, prevalence in NHS patients was 4.6% (NHS 2014). These data represent patients cared for across a range of settings including acute hospital wards, community and residential care and at home. Most patients had category 2 ulcers (3.0%), with 1.1% having category 3 and 0.6% having category 4 ulcers (category 1 ulcers were not included in the reporting). The point prevalence of pressure ulceration in the total adult population (rather than those currently receiving medical treatment) was recently estimated using a cross‐sectional survey undertaken in Leeds, in the UK. The total adult population was 751,485, and the point prevalence (including stage I ulcers) was 0.31 per 1000 (Hall 2014). Pressure ulcer prevalence estimates specifically for community settings have reported rates of 0.77 per 1000 adults in a UK urban area (Stevenson 2013).
Worldwide figures show a range of prevalence for pressure ulcers. Data from the USA showed that incidence of facility‐acquired (i.e. hospital‐acquired) ulcers ranged from 9.2% (general cardiac care) to 10.3% (surgical intensive care unit) of which 3.3% were severe (category 3/4/unclassifiable; VanGilder 2009). Australian estimates of pressure ulcer prevalence in acute care range from 4.5% to 27% (Prentice 2001), while in Japan prevalence across 5000 hospitals was reported as being 4.26% (Sanada 2008). Lower figures (1.8%) were noted in a cross‐sectional descriptive study of pressure ulcer prevalence in a teaching hospital in China (Zhao 2010), though data from a survey of hospital patients across several European countries found an overall prevalence of 10.5% (Vanderwee 2007). A review of pressure ulcer prevalence across Scandinavia, Iceland and Ireland, found that the mean prevalence in Norway was 17% (range 4.8% to 29%), 16% in Ireland (range 4% to 37%), 15% in Denmark (range 2.2% to 35.5%), 25% in Sweden (range 0.04% to 42.7%), and 8.9% in Iceland (single study, no range available) (Moore 2013a).
The prevalence in high‐risk population groups may be very much higher: a survey of people with a spinal cord injury found a point prevalence of 23%; furthermore, the lifetime risk in this group is estimated to be 70% (Raghaven 2003).
Cost of pressure ulcers
The cost of treating pressure ulcers in the UK has been estimated to range from GBP 1214 for a category 1 ulcer to GBP 14,108 for a category 4 ulcer (Dealey 2012). These cost estimates may be conservative due to the omission of negative pressure wound therapy from costings, which were updated from a point prior to the widespread use of this therapy; they also do not include precautions required for dealing with antibiotic‐resistant infection. The main driver of these increased costs is not ulcer category per se but the increased rate of complications in higher category ulcers and the subsequent increase in time to healing. In the UK, for the year 2000, the total cost for treating pressure ulcers lay between GBP 1.4 billion and GBP 2.1 billion (Bennett 2004).
Pressure ulcers increase length of hospital stay and associated hospital costs (Allman 1999). Figures from the USA suggest that 'pressure ulcer' was noted as a diagnosis for half a million hospital stays in 2006; for adults, the total hospital cost for these stays was USD 11 billion (Russo 2008). Current data on costs from other healthcare systems are hard to identify, but costs to the Australian healthcare system for treating pressure ulceration have been estimated at AUD 285 million annually (Graves 2005). There is also a substantial societal non‐health service cost in prolonged sick leave (absence due to being unwell) for people who are in employment when they develop a pressure ulcer (Gorecki 2009).
Impact of pressure ulcers on people
The impact of pressure ulcers on affected individuals is large. A systematic review found that pressure ulcers had an impact across physical, social and psychological domains as a result of one or more of the following distressing symptoms: pain, exudate and odour, increased care burden, prolonged rehabilitation, requirement for bed‐rest, and hospitalisation (Gorecki 2009). The adjusted health‐related quality of life of people with pressure ulcers has been shown to be lower than that for comparable individuals without pressure ulcers (Essex 2009). Pressure ulcers may also become infected, and this can give rise to serious systemic (whole body) infections.
Wound infection
Complex wounds such as pressure ulcers offer an ideal environment for microbial colonisation: this is especially true for those pressure ulcers that may be particularly exposed to bacterial contamination from faecal material (Bowler 2001). However, most wounds will contain some micro‐organisms and this will not necessarily lead to adverse events (WUWHS 2008).
There are several recognised definitions for wound infection (e.g. CDC 2008; WUWHS 2008). Recently there has been a move away from the view that density of bacteria is the key factor (i.e. that a bacterial load greater than 1 x 105 g‐1 is a predictor of infection) towards the view that infection with enough — or specific types of — pathogenic micro‐organisms, or both (Bowler 2003; Davies 2007; Madsen 1996; Trengove 1996), and the possible production of biofilms (Percival 2004; Wolcott 2008), may lead to negative outcomes and potentially delay healing. However, the impact of microbial colonisation on wound healing is not independent of the host response; the ability of the host to provide an adequate immune response is likely to be as critical in determining whether a wound heals as the specifics of the flora in the wound. Regarding wound flora, investigation into the microbiology of pressure ulcers has been limited — one study of the bacteria present in 25 pressure ulcers of different categories found a mean number of 5.8 species when necrotic tissue was present, but only 1.7 species when it was not (Sapico 1986). A more recent prospective cohort study followed 145 patients with category 2 or higher pressure ulcers: 77% of these people had pressure ulcers containing Staphylococcus aureus, Gram‐negative bacilli or both (Braga 2013). The document 'Wound Infection in Clinical Practice ‐ An International Consensus' defines a scenario leading to wound infection where "bacteria multiply, healing is disrupted and wound tissues are damaged (local infection)" (WUWHS 2008). The document also notes that"Bacteria may produce problems nearby (spreading infection) or cause systemic illness (systemic infection)". Indeed, wound infection has been conceptualised as being at one end of a continuum of infection (Kingsley 2004).
Kingsley defined a continuum of infection that begins with sterility (a brief period, possibly following surgery) and progresses through contamination (presence of microbes but little active growth and no clinical problems), to colonisation (the normal status quo with wound flora being managed by the host immune system and no damage to wound tissues), culminating in critical colonisation and then infection (Kingsley 2004).
In addition, Kingsley defined critical colonisation as a point between colonisation and infection where the 'healthy' balance of wound flora is no longer maintained by the host, and the bacterial load or species present in the wound, or both, shift away from a so‐called safe level (Kingsley 2004). Others have conceptualised critical colonisation as invasion of the wound surface by micro‐organisms (AWMA 2011; Edwards 2004).
The classic clinical signs of infection include localised pain, heat, redness, swelling and purulence (pus). The concept of critical colonisation lacks clear diagnostic criteria; it is generally noted as being associated with delayed healing in the absence of overt signs of wound infection (Carville 2008; Cutting 2004), possibly with other symptoms such as increased exudate (though less than in infection) and hypergranulation/friable tissue (Cutting 2004; Gardner 2001), although associated evidence is limited.
We have been unable to identify recent or large‐scale data on the rates of clinical infection of pressure ulcers; early studies of small numbers of patients produced an estimate of 1.4 cases of infection per 1000 patient days with an ulcer (Nicolle 1994), while a point prevalence study found that 6% of all nursing home residents participating received treatment for an infected pressure ulcer (Garibaldi 1981).
Although there is a widespread view amongst those with clinical expertise in the field that healing of pressure ulcers is likely to be retarded by critical colonisation or topical/local infection, the empirical evidence to support this is extremely limited (Howell‐Jones 2005). Indeed, the Australian Wound Management Association states that "The true extent of bacterial impairment of wound healing is unknown" (AWMA 2011). In particular there is a dearth of clinical studies to demonstrate a link between infection resolution or reduction of the microbiological load and wound healing; to date, randomised evidence has not supported a link between reduction in bacterial load and faster healing in pressure ulcers (Jull 2013; O'Meara 2001; Storm‐Versloot 2010). This may stem in part from the difficulty of culturing micro‐organisms from the biofilms present in pressure ulcers (Smith 2010), meaning that microbiological load is not accurately represented in samples.
There is a limited and conflicting evidence base for the relationship between bacterial load, or diversity or structure, and wound healing in other types of chronic wounds such as venous leg and diabetic foot ulcers (Davies 2007; Halbert 1992; Hansson 1995; Madsen 1996; Moore 2010; Sotto 2012). The applicability of this evidence to pressure ulcers is uncertain, as there are known microbiological differences between the wound types. In particular the proportion of anaerobic bacteria (thought to be correlated with non‐healing) and mycobacteria appears to be higher in pressure ulcers than in venous leg ulcers (Dowd 2008). There are known differences in the microbiology of pressure ulcers at different stages of healing, but no demonstration that these differences are implicated in the healing process (Sapico 1986).
Description of the intervention
Standard care for adults with pressure ulcers includes the use of pressure redistribution devices such as high‐specification foam mattresses or cushions, or both (McInnes 2011); debridement where appropriate and non‐gauze dressings (BNF 2013), with foam, hydrocolloid or alginate bases (NICE 2014). Other general strategies include the provision of patient education, management of pain, optimising circulation/perfusion, optimising nutrition and, where appropriate, performing surgical wound closure (AWMA 2011; EPUAP‐NPUAP‐PPPIA 2014). Treatment of clinical infection is also a key strategy as it is thought that a locally infected wound might show retarded healing and may give rise to a systemic infection.
Routine use of antibiotics and antiseptics is not currently recommended for the treatment of uninfected pressure ulcers in adults, and systemic antibiotics are recommended only when there is clinical evidence of systemic sepsis (serious infection), spreading cellulitis (deep skin infection) or underlying osteomyelitis (bone infection; NICE 2014). Antibiotic use should be restricted to cases of clear clinical need in the treatment of pressure ulcers, as with all conditions. Internationally, antibiotic‐resistant bacteria and multidrug‐resistant bacteria are increasing as a clinical problem; these bacteria have been found in isolates from a substantial proportion of patients with pressure ulcers, even in community settings (Cataldo 2011; Ellis 2003; Heym 2004). Inappropriate use of antibiotics is not restricted to those given systemically; topical antibiotics are also not recommended for use on non‐infected wounds (NICE 2014).
There are two main approaches when an antimicrobial intervention is considered clinically appropriate: an antibiotic may be administered systemically (orally, intravenously or intramuscularly), or a topical antibiotic or antiseptic may be applied (NICE 2014).
Antibiotics are substances that destroy or inhibit the growth of micro‐organisms (Macpherson 2004). Systemic antibiotic treatments include groups of drugs that share similar modes of action such as penicillins, cephalosporins, aminoglycosides, macrolides and quinolones. Other antibiotics that do not belong to one of these main groups include clindamycin, metronidazole, trimethoprim and co‐trimazole (BNF 2013).
Topical antimicrobial agents that are applied directly to the ulcer include both antibiotics and antiseptics. Antiseptics are thought to prevent the growth of pathogenic micro‐organisms without damaging living tissue (Macpherson 2004). Topical applications broadly fall into lotions used for wound irrigation or cleaning with a brief contact time (unless used as a pack/soak placed into or onto the wound), or both, and products that are in prolonged contact with the wound such as creams, ointments and impregnated dressings (BNF 2013). Agents used primarily for wound irrigation/cleaning are commonly based on povidone‐iodine, chlorhexidine and peroxide agents. Less commonly used agents include traditional products such as gentian violet and hypochlorites. Creams and ointments for longer contact include fusidic acid, mupirocin, neomycin sulphate and iodine (often as cadexomer iodine; BNF 2013).
The British National Formulary (BNF) categorises antimicrobial dressings under honey‐based, iodine‐based, silver‐based and 'other', which includes dressings impregnated with agents such as chlorhexidine or peroxides. Recommendations about dressing types for wounds thought to be infected are based primarily on the level of wound exudate, as this determines the dressing substrate, as well as the antimicrobial agent (BNF 2013).
Despite guidance from NICE there is a high use of silver dressings (11%) compared with other antimicrobial dressings (2% for next most commonly prescribed antimicrobial dressing) (MeReC 2010). High prescription costs mean that silver dressings account for a disproportionate amount (22%) of the annual NHS expenditure on dressings (MeReC 2010). It seems possible that some of these dressings are being used prophylactically (i.e. to prevent infection in wounds that are not clinically infected). There is also a high level of use of both systemic antibiotics and topical agents in patients with chronic wounds. General practice morbidity data for Wales from 2000 showed that twice as many patients with chronic wounds were prescribed systemic antibiotics in the previous year compared with matched controls, with a mean number of prescriptions per year of 2.3 (range 0 to 22) compared with 0.6 (range 0 to 14) for control patients. The same data showed high levels of prescription for topical agents such as silver sulfadiazine (185 times per 1000 patients per year) and metronidazole (223 times per 1000 patients per year) in this group (Howell‐Jones 2006). Again it appears possible that some prescriptions may be for the treatment of wounds that are not clinically infected.
How the intervention might work
The rationale for treating clinically infected wounds with antimicrobial and antiseptic agents is to kill or slow the growth of the pathogenic micro‐organisms, thus preventing an infection from worsening and spreading (Kingsley 2004). Improved healing may be a secondary benefit, although evidence of an association between wound healing and infection is limited (see Description of the intervention; Jull 2013; O'Meara 2001; Storm‐Versloot 2010).
There is a widely held view that wounds that do not show clear signs of clinical infection, but have characteristics such as retarded healing, may also benefit from a reduction in bacterial load. Again, evidence for this is limited (see Description of the condition; AWMA 2011, Howell‐Jones 2005).
Normally antibiotics work by inhibiting DNA or protein synthesis, or by disrupting bacterial cell walls. Antiseptics can be bacteriocidal (in that they kill micro‐organisms) or they can work by slowing the growth of organisms (bacteriostatic). Antiseptics can have a wide spectrum of action that is not restricted to bacteria, and often work by damaging the surface of microbes (Macpherson 2004).
Why it is important to do this review
Whether systemic or topical antimicrobials or topical antiseptics can promote healing in pressure ulcers remains uncertain. An earlier systematic review of antimicrobial agents used for the treatment of all types of chronic wounds was not able to generate definitive conclusions about the use of systemic or topical agents in pressure ulcers because of methodological problems in the primary literature (O'Meara 2001). Since the first review was published, a substantial number of additional relevant trials have been published that relate to pressure ulcers; these include trials of silver‐ or honey‐based topical preparations. This review is one of a number of Cochrane reviews investigating the use of antibiotics and antiseptics in the treatment of different types of complex wounds, each of which updates elements of the original O'Meara review (O'Meara 2001). While there will be some overlap with Cochrane reviews of individual antimicrobial agents in wounds (Jull 2013; Storm‐Versloot 2010), and with reviews of different types of dressings (Dumville 2015a; Dumville 2015b; Moore 2013b), this review will provide a single synthesis of the randomised evidence relating to all systemic and topical antimicrobials for pressure ulcers. Two notable systematic reviews of a range of treatments for pressure ulcers have included some types of antimicrobial treatments in wider assessments of dressings or topical treatments (Reddy 2008; Smith 2013). A comprehensive review of all antiseptic and antibiotic treatment of pressure ulcers is, however, lacking.
There is a wide range of options available to health professionals who are considering using antimicrobial therapy for pressure ulcers, either as a treatment for or prophylaxis against clinical infection. Evidence‐based decision‐making on the impact of antimicrobial agents on healing of pressure ulcers can be challenging. Key problems include decisions about whether or when to use an antimicrobial agent instead of standard care, and whether different anti‐microbial preparations have different impacts on healing.
Objectives
To assess the effects of systemic and topical antibiotics, and topical antiseptics on the healing of infected and uninfected pressure ulcers being treated in any clinical setting.
Methods
Criteria for considering studies for this review
Types of studies
Elements of this Methods section are based on the standard Cochrane Wounds Protocol Template.
We included published and unpublished randomised controlled trials (RCTs), including cluster RCTs, irrespective of language of report. We included cross‐over trials only if they reported outcome data at the end of the first treatment period, prior to cross‐over. Quasi‐randomised studies were excluded. We included RCTs reported only as abstracts only when available data were sufficient for reasonable data extraction either from the abstract itself or from the study authors.
Types of participants
We included studies that recruited adults diagnosed with a pressure ulcer of category 2 or above (i.e. worse) managed in any care setting. We excluded participants with category 1 ulcers. We accepted study authors' definitions of what they classed as a category 2 or above pressure ulcer unless it was clear that wounds with unbroken skin were included. This included accepting authors’ decisions that a wound was a pressure ulcer rather than, for example, an incontinence related sore/wound. Studies that recruited participants with category 2 or above pressure ulcers alongside people with other types of wounds were included if the proportion of participants with pressure ulcers of category 2 or above was at least 75%. We did not restrict the review to trials that recruited only participants with colonised, critically colonised or infected wounds at baseline, but where information about wound status is reported it was recorded. Unstageable ulcers were included and recorded as such.
Types of interventions
The primary interventions of interest were topical antiseptic agents or antibacterial (antibiotic) agents delivered either systemically or topically. We included any RCT in which the use of a topical or systemic antibiotic or a topical antiseptic was the key systematic difference between treatment groups. Systemic antibiotics may be administered orally or by other routes (e.g. intravenously, intramuscularly). Both intervention and control regimens could consist of antibiotics or antiseptics administered singly or in combination; control regimens might also include placebo, another therapy, standard care or no treatment. Studies that evaluated co‐interventions (e.g. pressure‐relieving devices) were included, provided that these treatments were delivered in a standardised way across the trial arms. We decided to include studies where dressings as well as antiseptic or antibiotic treatment differed between groups for this review.
We excluded evaluations of antibiotics/antiseptics used to prepare for the surgical treatment of ulcers (i.e. the surgical closure of ulcers or skin grafting), and physical and biological therapies sometimes purported to have incidental antimicrobial properties such as heat therapy and larval therapy.
We anticipated that interventions would consist of antiseptic and antibiotic agents, which might include (but not be limited to) the following topical agents that may be available in the form of creams, sprays, ointment, or impregnated into different types of dressings: chlorhexidine; povidone‐iodine; hydrogen peroxide and potassium permanganate; benzoyl peroxide; hypochlorites (e.g. Eusol); gentian violet; mupirocin and fusidic acid; neomycin sulphate; peroxides; iodine, silver and honey.
Systemic antibiotics might include penicillins, cephalosporins, aminoglycosides, macrolides and quinolones, clindamycin, metronidazole, trimethoprim and co‐trimazole.
Types of outcome measures
We list primary and secondary outcome measures below. If a trial was otherwise eligible (correct study design, population and intervention/comparator) but did not report a relevant listed outcome, then we contacted the study authors where possible in order to establish whether the outcome was measured but not reported.
We report outcome measures at the latest time point available for a study (assumed to be length of follow‐up if not specified) and the time point specified in the methods as being of primary interest (if this is different from latest time point available). For all outcomes we classed (and categorised) outcomes from:
one to eight weeks as short‐term;
between eight and 26 weeks as medium‐term; and
over 26 weeks as long‐term.
Review authors used their judgement based on consideration of heterogeneity to determine whether statistical pooling within these time categories was appropriate.
Primary outcomes
The primary effectiveness outcome for this review was wound healing. Trialists used a range of different methods of measuring and reporting this outcome. RCTs that reported one or more of the following were considered to provide the most relevant and rigorous measures of wound healing.
Time to complete wound healing (correctly analysed using survival, time‐to‐event approaches). Ideally the outcome will be adjusted (by study authors) for appropriate covariates e.g. baseline ulcer area/duration.
Proportion of wounds completely healed during follow‐up (frequency of complete healing).
We used, and reported, authors’ definitions of complete wound healing. We reported outcome measures at the latest time point available (assumed to be length of follow‐up, if not specified) and the time point specified in the methods as being of primary interest (if this was different from latest time point available).
Where both the outcomes above were reported we planned to present all data for reference, but to focus on reporting time to healing. When time was analysed as a continuous measure, but it was unclear whether all wounds healed, we documented the use of the outcome in the study, but did not extract, summarise or use the data in a meta‐analysis.
The primary safety outcome for the review was all reported adverse events. Reported data were extracted on all serious and non‐serious adverse events when a clear methodology for the collection of adverse event data was provided. This methodology had to make it clear whether events were reported at the participant level or whether multiple events/person were reported, in which case appropriate adjustments needed to be made for data clustering. Individual types of adverse events other than pain or infection were not extracted (see Secondary outcomes).
Secondary outcomes
The following secondary outcomes were included.
Change (and rate of change) in wound size, with adjustment for baseline size (we attempted to contact study authors to request adjusted means when not presented). When change or rate of change in wound size was reported without adjustment for baseline size, use of the outcome in the study was documented, but data were not extracted, summarised or used in any meta‐analysis.
Changes in infection status; signs or symptoms of clinical infection (we used study authors' definitions of clinical infection). We did not include data on bacterial load, diversity or the presence of individual species, where it was not clear how the outcome related to infection.
Changes in bacterial (antibiotic) resistance.
Health‐related quality of life: quality of life was included when it was reported using a validated scale such as the SF‐36 (Ware 1992) or EQ‐5D (EuroQoL Group 1990) or a validated disease‐specific questionnaire such as the Cardiff Wound Impact Schedule (Price 2004). Ideally the reported data were adjusted by the study authors for the baseline score. We did not include ad hoc measures of quality of life that were unlikely to be validated and would not be common to multiple trials.
Mean pain scores (including pain at dressing change) were included only when reported as either a presence or absence of pain, or as a continuous outcome using a validated scale such as a visual analogue scale (VAS).
Resource use (when presented as mean values with standard deviation) including measures such as number of dressing changes, number of nurse visits, length of hospital stay, need for other interventions.
Costs associated with resource use (including estimates of cost‐effectiveness).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
The Cochrane Wounds Specialised Register (searched 20 October 2015);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library) (2015, Issue 9);
Ovid MEDLINE (1946 to 20 October 2015);Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 20 October 2015);
Ovid EMBASE (1974 to 20 October 2015);
EBSCO CINAHL Plus (1937 to 20 October 2015).
The search strategies for CENTRAL, Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/Default.aspx);
EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/ctr‐search/search).
Searching other resources
We attempted to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses and health technology assessment reports.
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts of the citations retrieved by the searches for relevance. After this initial assessment, we obtained full text copies of all studies considered to be potentially relevant. Two review authors independently checked the full papers for eligibility; disagreements were resolved by discussion and, where required, through the input of a third review author. When the eligibility of a study was unclear we attempted to contact the study authors. We recorded all reasons for exclusion of studies for which we obtained full copies of the text. We completed a PRISMA flowchart to summarise this process (Liberati 2009) (Figure 1).
1.
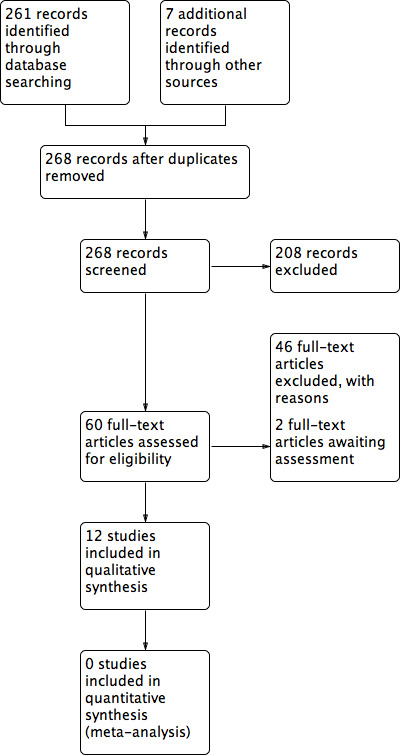
PRISMA study flow diagram.
When studies were reported in multiple publications/reports, we obtained all publications. Whilst the study was included only once in the review, we extracted data from all reports to ensure all available relevant data were obtained.
Data extraction and management
We extracted and summarised details of the eligible studies. Where possible we extracted data by treatment group for the pre‐specified interventions and outcomes in this review. Data were extracted by one review author and checked by a second review author. Discrepancies were resolved through discussion or by consultation with a third review author. When data were missing from reports, we attempted to contact the study authors to request this information.
When a study with more than two intervention arms was included, only data from the intervention and control groups that met the eligibility criteria were extracted. When the reported baseline data related to all participants rather than to those in relevant treatment arms, the data for the whole trial were extracted and this was noted. Outcome data were collected for relevant time points as described in Types of outcome measures.
Where possible we extracted the following data:
bibliographic data including date of completion/publication;
country of origin;
unit of randomisation (participant/ulcer);
unit of analysis;
trial design, e.g. parallel; cluster;
care setting;
number of participants randomised to each trial arm and number included in final analysis;
eligibility criteria and key baseline participant data including category or categories and location(s) of pressure ulcers;
details of treatment regimen received by each group;
duration of treatment;
details of any co‐interventions;
primary and secondary outcome(s) (with definitions and, where applicable, time‐points);
outcome data for primary and secondary outcomes (by group);
duration of follow‐up;
number of withdrawals (by group) and number of withdrawals (by group) due to adverse events;
publication status of study;
source of funding for trial.
Assessment of risk of bias in included studies
Two review authors independently assessed included studies using the Cochrane tool for assessing risk of bias (Higgins 2011a). This tool addresses six specific domains: sequence generation; allocation concealment; blinding; incomplete data; selective outcome reporting; and other issues — in this review we recorded unit of analysis issues, for example where a cluster trial has been undertaken but analysed at the individual level in the study report. We assessed blinding of outcome assessment and completeness of outcome data for each of the review outcomes separately. We present our assessment of risk of bias using two 'Risk of bias' summary figures; one is a summary of bias for each item across all studies, and a second shows a cross‐tabulation of each trial by all of the 'Risk of bias' items. We summarised a study’s risk of selection bias, detection bias, attrition bias, reporting bias and other bias. In many of the comparisons included in this review we anticipated that blinding of participants and personnel would not be possible. For this reason, the assessment of the risk of detection bias focused on whether blinded outcome assessment was reported. For trials using cluster randomisation, we also planned to consider the risk of bias in relation to: recruitment bias; baseline imbalance; loss of clusters; incorrect analysis; and comparability with individually randomised trials (Higgins 2011b) (Appendix 2).
Measures of treatment effect
Time‐to‐event data (e.g. time to complete wound healing) were reported as hazard ratios (HRs) when possible, in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). If studies reporting time‐to‐event data (e.g. time to healing) did not report a hazard ratio, then, where feasible, we planned to estimate this using other reported outcomes, such as numbers of events, through the application of available statistical methods (Parmar 1998; Tierney 2007). For dichotomous outcomes we calculated the risk ratio (RR) with 95% confidence intervals (CIs). For continuous outcome data, we used the mean difference (MD) with 95% CIs for trials that used the same assessment scale and, when trials used different assessment scales, we planned to use the standardised mean difference (SMD) with 95% CIs.
Unit of analysis issues
Where studies randomised at the participant level and measured outcomes at the wound level, for example for wound healing, and the number of wounds appeared to be equal to the number of participants, we treated the participant as the unit of analysis.
We had anticipated a possible unit of analysis issue if individual participants with multiple wounds were randomised. The allocated treatment used on the multiple wounds per participant (or perhaps only on some participants) and then data were presented and analysed by wound not person. This is a type of clustered data and presents a unit of analysis error which inflates precision. In cases where included studies contained some or all clustered data we reported this alongside whether data had been (incorrectly) treated as independent. We recorded this as part of the risk of bias assessment. We did not undertake further calculation to adjust for clustering.
Dealing with missing data
It is common to have data missing from trial reports. The exclusion of participants from the analysis post randomisation or ignoring those lost to follow‐up compromises the randomisation and potentially introduces bias into the trial. If we thought that study authors might be able to provide some missing data, we contacted them; however, data were often likely to be missing because of loss to follow‐up. In individual studies, when data were presented for the proportion of ulcers healed, we assumed that randomly assigned participants who were not included in the analysis had an unhealed wound at the end of the follow‐up period (i.e. they were considered in the denominator but not in the numerator). When a trial did not specify participant group numbers before dropout, we present only complete case data. For time‐to‐healing analysis using survival analysis methods, we planned to account for dropouts as censored data. Hence, all participants would contribute to the analysis. We acknowledge that such analysis assumes that dropouts are missing at random and that there is no pattern of missingness. We presented data for area change of ulcer and for all secondary outcomes as complete case analyses.
We presented available data from the study reports/study authors for continuous variables — for example length of hospital stay — and for all secondary outcomes, and did not plan to impute missing data. Where measures of variance were missing we planned to calculate these wherever possible (Higgins 2011a); where this was not possible we attempted to contact study authors. When these measures of variation remained unavailable and could not be calculated, we planned to exclude the study from any relevant meta‐analyses.
Assessment of heterogeneity
Assessment of heterogeneity is a complex, multi‐faceted process. Firstly, we considered clinical and methodological heterogeneity, that is the degree to which the included studies varied in terms of participant, intervention, outcome and characteristics such as length of follow‐up. We planned to supplement this assessment of clinical and methodological heterogeneity with information regarding statistical heterogeneity — we intended to assess this using the Chi² test (P values less than 0.10 would have been considered to indicate statistically significant heterogeneity) in conjunction with the I² statistic (Higgins 2003). I² examines the percentage of total variation across RCTs that is due to heterogeneity rather than chance (Higgins 2003). Very broadly we intended to consider that I² values of 25%, or less, may mean a low level of heterogeneity (Higgins 2003), and values of 75% or more indicate very high heterogeneity (Deeks 2011). Where there was evidence of high heterogeneity we planned to attempt to explore this further: see Data synthesis.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Publication bias is one of a number of possible causes of 'small study effects', that is, a tendency for estimates of the intervention effect to be more beneficial in smaller RCTs. Funnel plots allow a visual assessment of whether small study effects may be present in a meta‐analysis. A funnel plot is a simple scatter plot of the intervention effect estimates from individual RCTs against some measure of each trial’s size or precision (Sterne 2011). Funnel plots are only informative when there are a substantial number of studies included in an analysis; we planned to present funnel plots for meta‐analyses that included at least 10 RCTs using RevMan 2014 5.
Data synthesis
We combined details of included studies in a narrative review according to the comparison between intervention and comparator, the population and the time point of the outcome measurement. We considered clinical and methodological heterogeneity and planned to undertake pooling if studies appeared appropriately similar in terms of ulcer category, intervention type and antimicrobial agent, duration of treatment and outcome assessment.
In terms of our meta‐analytical approach, in the presence of clinical heterogeneity (review author judgement) or evidence of statistical heterogeneity, or both, we planned to use the random‐effects model. We planned only to use a fixed‐effect approach when clinical heterogeneity was thought to be minimal and statistical heterogeneity was estimated as non‐statistically significant for the Chi² test and 0% for the I² statistic (Kontopantelis 2013). We planned to adopt this approach as it is recognised that statistical assessments can miss potentially important between‐study heterogeneity in small samples, hence the preference for the more conservative random‐effects model (Kontopantelis 2012). Where clinical heterogeneity was thought to be acceptable or of interest we planned to make a decision as to whether to meta‐analyse even when statistical heterogeneity was high but to attempt to interpret the causes behind this heterogeneity and to consider using meta‐regression for that purpose, if possible (Thompson 1999; Thompson 2002).
We presented data using forest plots where possible. For dichotomous outcomes we presented the summary estimate as a risk ratio (RR) with 95% CI. Where continuous outcomes were measured in the same way across studies we planned to present a pooled mean difference (MD) with 95% CI; we planned to pool standardised mean difference (SMD) estimates where studies measured the same outcome using different methods. For time‐to‐event data, we planned to plot (and, if appropriate, pool) estimates of hazard ratios and 95% CIs as presented in the study reports using the generic inverse variance method in RevMan 2014 5. Where time to healing was analysed as a continuous measure but it was not clear if all wounds healed, use of the outcome in the study was documented, but those data were not summarised and we did not plan to use the data in any meta‐analysis.
'Summary of findings' tables
We planned to present the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We planned to present the following outcomes in the 'Summary of findings' tables:
Time to complete ulcer healing when analysed using appropriate survival analysis methods;
Proportion of ulcers completely healing during the trial period;
Changes in clinical infection status;
Adverse events.
Because in each case only a single study evaluated a comparison we did not present a full 'Summary of findings' table but instead provide a narrative summary of the results of the GRADE assessment. Where it was not possible to calculate an estimate of effect for an outcome (including where this was due to zero events reported) we did not provide a GRADE assessment; where this was the case for all the outcomes for a comparison we did not provide GRADE assessments but gave a single summary of the issues which would have been taken into account in assessments.
Subgroup analysis and investigation of heterogeneity
When possible, we planned to perform subgroup analyses to explore the influence of ulcer category on effect size. If there were sufficient data these analyses would have assessed whether there were differences in effect sizes for category 2 pressure ulcers and the more severe category 3 and 4 (and unclassifiable) pressure ulcers.
When possible, we planned to perform subgroup analyses to explore the influence of risk of bias on effect size. These analyses would have assessed the influence of removing studies classed as having high and unclear risk of bias from the meta‐analyses. These analyses would have included only studies that were assessed as having low risk of bias in all key domains, namely, adequate generation of the randomisation sequence, adequate allocation concealment and blinding of outcome assessor for the estimates of treatment effect.
Results
Description of studies
See Included studies; Excluded studies
Results of the search
The search generated 261 records. Reference checking of reviews and included studies identified a further seven records. Twelve studies reported in 18 publications were included in the review (Figure 1). Ten studies were published in English, two were published in Japanese (Imamura 1989; Toba 1997). Two studies are pending classification once translation has occurred (Bigolari 1991; Goldmeier 1997); these were reported in Italian and Portuguese respectively. We are not aware of any relevant ongoing studies.
Included studies
This review includes 12 studies which together contained 576 randomised participants (Barrois 1993; Chuangsuwanich 2011; Chuangsuwanich 2013; Imamura 1989; Kaya 2005; Kucan 1981; Moberg 1983; Nisi 2005; Sipponen 2008; Toba 1997; Wild 2012; Yapucu Güneş 2007). Eleven studies had two arms, one (Kucan 1981) had three arms. Four studies involved multiple ulcers being treated on some or all participants (Chuangsuwanich 2013; Kaya 2005; Moberg 1983; Sipponen 2008); all of these carried out randomisation at the participant level and did not make it clear whether the analysis was adjusted to reflect the clustered data from some participants.
Interventions assessed
All the included studies assess the use of topical agents; there were no eligible studies of systemic antibiotics. Most of the interventions assessed were antiseptics.
There were four types of comparisons assessed:
1. Antiseptic versus non‐antimicrobial intervention
2. Antiseptic versus alternative antiseptic
3. Antiseptic versus antibiotic
4. Antibiotic versus non‐antimicrobial intervention.
No trials compared different antibiotics with each other.
The largest amount of data available related to comparison of antiseptics compared with non anti‐microbial interventions. The most commonly evaluated agent was povidone iodine which was evaluated by four trials (Barrois 1993; Kaya 2005; Kucan 1981; Nisi 2005). All these trials employed a different non‐antimicrobial comparator and in one trial the iodine was combined with sugar. Comparator treatments (without antimicrobial properties) to which the interventions were compared included hydrogel (Kaya 2005), hydrocolloid (Barrois 1993), protease‐modulating matrix (Nisi 2005), saline gauze (Kucan 1981), and standard care (Moberg 1983). Single trials compared cadexomer iodine to standard care (Moberg 1983); and pine resin to hydrocolloid dressing (Sipponen 2008).
For comparison 2 one trial compared povidone iodine sugar to gentian violet (Toba 1997); one compared povidone iodine sugar to lysozyme ointment (Imamura 1989); and one compared two different formulations of polyhexanide (Wild 2012).
For comparison 3 two trials compared silver to silver sulfadiazine (silver mesh, Chuangsuwanich 2011; and silver alginate, Chuangsuwanich 2013). One trial compared povidone iodine to silver sulfadiazine (Kucan 1981). A fourth trial compared honey to ethoxy‐diaminoacridine administered with nitrofurazone (Yapucu Güneş 2007).
For comparison 4 a single trial compared silver sulfadiazine to saline (Kucan 1981).
No individual comparison was evaluated by more than one trial so all were considered separately; they are grouped by comparison type.
Outcomes reported
Seven studies reported the primary effectiveness outcome of this review: wound healing (Barrois 1993, Imamura 1989; Kaya 2005; Moberg 1983; Nisi 2005; Sipponen 2008;Yapucu Güneş 2007). In all cases this was reported as proportion of wounds healed. No trials appropriately reported time‐to‐healing data.
The primary safety outcome of the review was adverse effects. This was reported for all participants by six trials (Barrois 1993; Chuangsuwanich 2011; Imamura 1989; Moberg 1983;Toba 1997; Yapucu Güneş 2007), four of which reported that there were no adverse events. Individual events which would normally be considered as adverse events were reported in some of the trials reporting that no adverse events occurred, as well as by other trials which did not report data for all participants.
The review evaluated a number of secondary outcomes. Six studies reported change in wound size data (Chuangsuwanich 2011; Chuangsuwanich 2013; Imamura 1989; Moberg 1983; Toba 1997; Yapucu Güneş 2007). Infection eradication data were reported in five studies (Imamura 1989; Kucan 1981; Sipponen 2008; Toba 1997; Wild 2012); three enrolled only participants with infected pressure ulcers at baseline. In Toba 1997 and Wild 2012 this related specifically to the presence of MRSA, which all ulcers were positive for at baseline, and therefore to changes in microbiological status. All other studies either did not report this outcome or reported only qualitative data relating to species of microorganisms present. No studies reported incidence of new infections. Only two studies reported on pain (Moberg 1983; Wild 2012); and two studies reported some data on resource use (Barrois 1993; Nisi 2005). Costs related to resource use were reported by Chuangsuwanich 2011 and Chuangsuwanich 2013. No trials reported data on health‐related quality of life.
Outcome data are summarised in Table 1.
1. Summary of outcome data.
| Study | Interventions | wound healing | adverse events | Wound size (change) | Infection status or resistance (change) | Pain | Resource use | Costs |
| Barrois 1993 | Povidone iodine Hydrocolloid |
9/38 vs 10/38 | 0/38 vs 0/38 | Dressings/week 5.07 vs 2.43 (SD not reported) |
||||
| Chuangsuwanich 2011 | Silver mesh Silver sulfadiazine |
0/20 vs 0/20 | 20.1% vs 34.6% reduction (SD not reported; N = 20 in both groups) | USD 263 vs USD 1812 (SD not reported) | ||||
| Chuangsuwanich 2013 | Silver alginate Silver sulfadiazine |
51.7% (N = 13) vs 44.27% (N = 15) reduction (SD not reported) | USD 377.17 vs USD 467.74 (SD not reported) | |||||
| Imamura 1989 | Povidone iodine sugar Lysozyme ointment |
15/72 vs 12/69 | 1/72 vs 3/69 | 28/61 vs 17/61 improved | ||||
| Kaya 2005 | Povidone iodine Hydrogel |
13/24 vs 21/25 ulcers | ||||||
| Kucan 1981 | Povidone iodine Silver sulfadiazine Saline |
7/11 vs 15/15 vs 11/14 resolved | ||||||
| Moberg 1983 | Cadexomer iodine Standard care |
6/19 vs 1/19 | 5/14 vs 0/13 | |||||
| Nisi 2005 | Povidone iodine Protease‐modulating matrix |
28/40 vs 36/40 | 76.2% (SE 8.2; n = 14) vs 57.4% (SE 9.4; n = 13) reduction | 3.1 (SE 1.7) vs 7.5 (SE 2.8) | hospital stay: 1164 vs 360 days dressing use; 14 to 52 vs 6 to 15 |
|||
| Sipponen 2008 | Spruce resin salve Hydrocolloid |
17/27 vs 4/18 | 1/18 vs 1/18 infected/resolved | |||||
| Toba 1997 | Povidone iodine sugar Gentian violet |
44.3% vs 55.4% reduction (decrease to 55.7 (24.0)% vs decrease to 44.6 (12.9)% | 8/11 vs 7/8 no longer MRSA affected | |||||
| Wild 2012 | Polyhexanide dressing Polyhexanide swabs |
15/15 vs 10/15 no longer MRSA affected | 1.3 (0.36) vs 3.33 (1.2) | Dressing change time: 6 vs 25 min (SD not reported) | ||||
| Yapucu Güneş 2007 | Honey Ethoxy‐diaminoacridine plus nitrofurazone |
5/25 vs 0/≥26 ulcers | 0/15 vs 0/11 | 56% vs 13% reduction (SD not reported) |
Characteristics of participants
Most trials enrolled participants who were elderly and hospitalised. All the trials appeared to be conducted in secondary care settings. Two did not report whether participants were hospitalised (Barrois 1993; Yapucu Güneş 2007); one reported enrolling only outpatients (Chuangsuwanich 2011); and two both inpatients and outpatients (Chuangsuwanich 2013, Wild 2012). All other trials enrolled only hospital inpatients.
One trial enrolled participants with spinal cord injuries (Kaya 2005). Participants in this trial were much younger than those in other studies, with a mean age of 32.8 years. Apart from Nisi 2005 (mean age 45 years) all other studies where it was reported, had mean ages over 60 years and in some cases over 80 years. One trial did not report age (Barrois 1993) while another reported an age range of 16 to 102 years (Kucan 1981). Five trials did not report the age of participants. There was variation in the stage of ulcers present in included participants with two trials reporting a minority of participants with stage I ulcers (Imamura 1989; Kaya 2005).
There was heterogeneity between the trials in terms of infection at baseline. One trial specifically excluded participants with infected or necrotic ulcers (Chuangsuwanich 2013); and one stated that both infected and uninfected ulcers were eligible (Sipponen 2008). Barrois 1993 included only participants with necrotic ulcers but did not report whether these were infected while Kaya 2005 reported that none of the ulcers were infected. Three trials only enrolled participants with infected ulcers (Kucan 1981; Toba 1997; Wild 2012) and two of these specified that MRSA must be present (Toba 1997; Wild 2012); in one trial this was required to be intractable (Wild 2012). The primary outcomes of these three studies related to infection resolution. The other studies did not specify whether ulcers were infected at baseline.
Sample sizes
The included trials had small sample sizes. The total number of participants was 578 and the median sample size was 34 (range 19 to 141); all except three studies (Barrois 1993; Imamura 1989; Nisi 2005) had fewer than 50 participants; all of these assessed povidone iodine.
Trial duration
The duration of the trials was generally short. All except two trials which reported a clearly specified length had treatment durations/outcome assessments which would be considered to be short term according to the prespecified criteria used in this review, ranging from 14 days to 8 weeks; reported follow‐up ranged from 17 days to six months. Two trials did not explicitly report durations which were then inferred from the time‐to‐healing data reported (Kaya 2005; Nisi 2005); in Nisi 2005 the reported treatment durations in the randomised phase ranged from 2 to 8 weeks with follow‐up at 8 weeks; while in Kaya 2005 treatment durations ranged from 15 up to 106 days with most data from the lower end of this range. These trials could therefore both be reasonably considered to be reporting short‐term outcome data. Toba 1997 reported treatment duration of 14 weeks; follow‐up was reported to be 2 years but data were reported for 14 weeks; outcomes in this trial are therefore considered to be medium‐term. The single trial with a specified longer‐term treatment duration and follow‐up lasted for six months (Sipponen 2008).
Excluded studies
Forty‐six studies were excluded because they were not RCTs, did not have at least 75% of participants with pressure ulcers, did not report relevant outcomes or did not assess at least one antiseptic/antibiotic intervention (Characteristics of excluded studies).
Risk of bias in included studies
All studies were assessed for risk of bias. Barrois 1993 could not be fully assessed because it was reported in abstract form only; on the basis of the abstract it was considered to be at unclear risk of bias across all domains except for attrition bias where it was assessed as being at low risk of bias. Results of the assessment are shown in Figure 2 and Figure 3.
2.
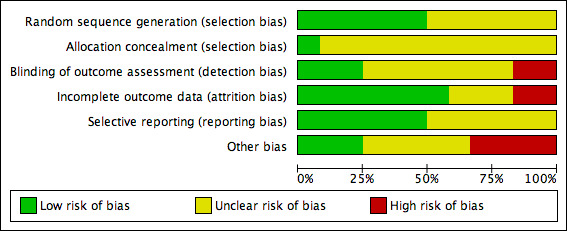
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
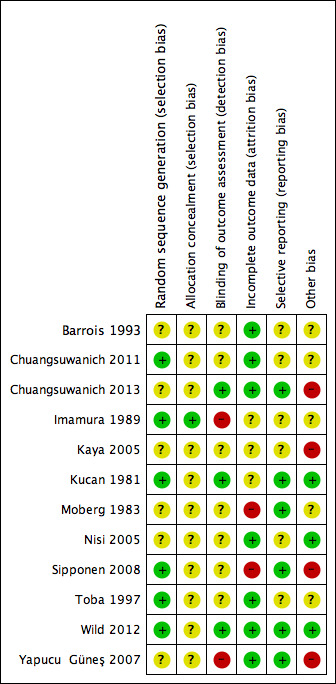
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation sequence
Six studies were classed as being at low risk of bias for randomisation (Chuangsuwanich 2011; Imamura 1989; Kucan 1981; Sipponen 2008;Toba 1997; Wild 2012). Four trials reported using a computer‐generated randomisation sequence and two a table of random numbers (Imamura 1989; Toba 1997). The remainder of the studies were at unclear risk of bias.
Allocation concealment
Only one of the included trials clearly reported adequate allocation concealment (Imamura 1989); in all other cases it was unclear whether appropriate allocation concealment had been undertaken (where sealed envelopes were used it was unclear if they were opaque).
Blinding
Blinded outcome assessment: three studies were judged to be at low risk of detection bias (Chuangsuwanich 2013; Kucan 1981; Wild 2012). Two trials were considered to be at high risk of detection bias because of nonblinded outcome assessment (Imamura 1989; Yapucu Güneş 2007). All other studies had an unclear risk of bias.
Incomplete outcome data
Two studies were judged to be at high risk of attrition bias (Moberg 1983; Sipponen 2008). In both studies a high proportion of randomised participants were not included in the analysis. Seven studies were at low risk of attrition bias (Barrois 1993; Chuangsuwanich 2011; Chuangsuwanich 2013; Nisi 2005; Toba 1997; Wild 2012; Yapucu Güneş 2007) and the remainder had an unclear risk.
Selective reporting
Six studies were judged to be at low risk of reporting bias (Chuangsuwanich 2013; Kucan 1981; Moberg 1983; Sipponen 2008; Wild 2012; Yapucu Güneş 2007) and all other studies had an unclear risk of selective outcome reporting.
Other potential sources of bias
Four studies were identified as having potential unit of analysis issues as some of the randomised participants had more than one wound and it seemed that data were presented at the wound level rather than the participant level (Chuangsuwanich 2013; Kaya 2005; Moberg 1983; Sipponen 2008). They were therefore considered to be at high risk of bias. Four studies were classed as being at unclear risk of other sources of bias due to poor reporting of methods (Barrois 1993; Chuangsuwanich 2011; Imamura 1989; Toba 1997); the remainder had a low risk.
Effects of interventions
1. Antiseptics compared with non anti‐microbial interventions (6 trials, 284 participants)
Four trials compared povidone iodine with another treatment which did not contain an antiseptic or antibiotic component (Barrois 1993; Kaya 2005; Kucan 1981; Nisi 2005). As each trial used a different comparator, and as there was also heterogeneity in the application of povidone iodine, they are presented separately. One trial compared cadexomer iodine with standard care (Moberg 1983); and one trial compared pine resin with antiseptic properties to a hydrocolloid dressing (Sipponen 2008).
Comparison 1. Povidone iodine versus hydrocolloid (1 trial; 76 participants)
One trial compared a gauze containing povidone iodine with a hydrocolloid dressing (Barrois 1993). This trial was published as an abstract only. It randomised 76 participants with open necrotic pressure ulcers (stage not specified) to treatment with paraffin gauze dressing with povidone iodine or a hydrocolloid dressing for 56 days or until healing occurred.
Primary outcome: wound healing (proportion of wounds completely healed)
Barrois 1993 reported that after 56 days, 9/38 (23.7%) ulcers treated with povidone iodine healed versus 10/38 (26.3%) treated with hydrocolloid. There was no clear evidence of a difference in wound healing between groups: RR 0.90 (95% CI 0.41 to 1.96) (Analysis 1.1).GRADE assessment: low quality evidence, downgraded twice due to imprecision for the outcome of wound healing. A GRADE assessment of low quality evidence means that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
1.1. Analysis.
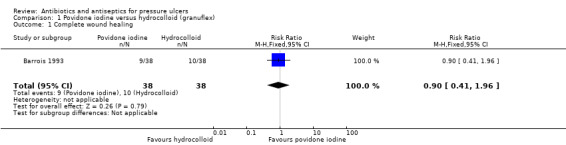
Comparison 1 Povidone iodine versus hydrocolloid (granuflex), Outcome 1 Complete wound healing.
Primary outcome: adverse events
Barrois 1993 reported that no adverse events were observed but also reported data for participants who dropped out of the trial due to deterioration in the pressure sore; these data were not further analysed.
Secondary outcome: resource use (dressings per week)
Barrois 1993 reported that participants treated with povidone iodine required a mean of 5.07 dressings per participant per week, compared with 2.43 for those treated with hydrocolloid. No measure of variance was reported and the data were not further analysed.
Comparison 2. Povidone iodine versus hydrogel (1 trial, 27 participants)
One trial compared povidone iodine with a hydrogel‐type dressing (Kaya 2005). Twenty‐seven hospitalised participants with spinal cord injury and pressure ulcers were randomised to povidone iodine gauze or hydrogel treatment for the duration of the hospital stay. Treatment duration was not further specified and neither was the length of follow‐up (reported treatment times for the two groups ranged from 16 to 106 days for the povidone iodine group and from 15 to 91 days for the hydrogel group). Most participants had more than one ulcer treated. Twelve participants with 24 ulcers were randomised to povidone iodine gauze and 15 participants with 25 ulcers to hydrogel. Most (N = 34) of the 49 ulcers were stage II with a minority (N = 12) of stage I ulcers and a smaller number of stage III ulcers (N = 3). Stage II and III ulcers made up 75.5% of all the ulcers evaluated. We therefore report results for all ulcers.
Primary outcome: wound healing (proportion of wounds completely healed)
Kaya 2005 reported that 13/24 (54.2%) ulcers treated with povidone iodine healed versus 21/25 (84%) treated with hydrogel. The RR for wound healing after was 0.64 (95% CI 0.43 to 0.97) in favour of hydrogel (Analysis 2.1). Numbers of stage II/III ulcers healed were also reported. The trial also reported mean time to healing data but this was not extracted or analysed as not all ulcers healed. GRADE assessment: low quality evidence due to imprecision for the outcome of wound healing; (downgraded once for imprecision due to the wide confidence intervals and once because participants had multiple ulcers and it was not clear whether the analysis was adjusted for the clustered data; precision estimates are likely to change upon correct analysis of data for the outcome).
2.1. Analysis.
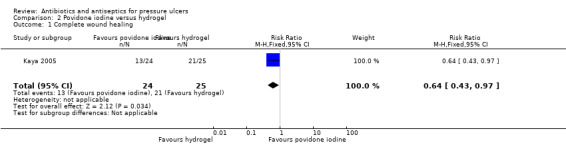
Comparison 2 Povidone iodine versus hydrogel, Outcome 1 Complete wound healing.
Primary outcome: adverse events
Kaya 2005 did not report adverse events.
No review‐relevant secondary outcomes were reported
Comparison 3. Povidone iodine versus saline (1 trial, 45 participants)
One three‐arm trial compared povidone iodine with saline (Kucan 1981). Forty‐five hospitalised participants with infected pressure ulcers were randomised to povidone iodine gauze, saline gauze or silver sulfadiazine (see comparison 5 and 11). Participants were treated for three weeks or until the wound was considered clean and ready for closure or the treatment was considered a failure. All wounds were assessed at three weeks (duration of follow‐up). Debridement of necrotic tissue was carried out as required and systemic antibiotics were prescribed for intercurrent infections; their use was reported for 15 participants who were reported to be equally distributed across the three groups.
Primary outcome: wound healing
Kucan 1981 did not report wound healing.
Primary outcome: adverse events
Kucan 1981 did not report adverse events.
Secondary outcome: infection eradication
Kucan 1981 defined infection eradication as a bacterial count of less than 105/g after three weeks. The trial reported that after three weeks 7/11 (63.6%) ulcers treated with povidone iodine were judged to be free of infection compared with 11/14 (78.6%) ulcers treated with saline. There was no clear evidence of a difference between groups in eradication of infection: RR 0.81 (95% CI 0.48 to 1.37) (Analysis 3.1). GRADE assessment: low quality evidence due to imprecision (downgraded twice for imprecision).
3.1. Analysis.
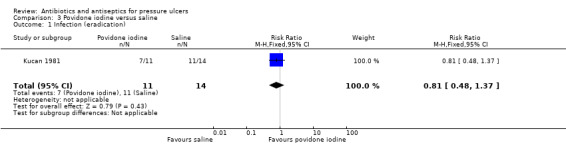
Comparison 3 Povidone iodine versus saline, Outcome 1 Infection (eradication).
Comparison 4. Povidone iodine versus protease‐modulating matrix dressing (1 trial, 80 participants)
One trial compared povidone iodine with a protease‐modulating matrix dressing (Nisi 2005). Eighty hospital inpatients with pressure ulcers of stages II to IV were randomised to daily disinfection with 50% povidone iodine solution, saline washes and Vaseline gauze, covered with a hydropolymer patch versus a protease‐modulating matrix treatment (PROMOGRAN) changed two or three times weekly, covered with a hydropolymer patch. This followed a debridement phase for all participants which used surgical debridement, disinfection with povidone iodine, saline washes and use of hydrogels. Planned treatment duration was not reported but actual duration of randomised treatment ranged from two to eight weeks in the povidone iodine group and two to six weeks in the protease‐modulating group. Follow‐up was for eight weeks.
Primary outcome: wound healing (Proportion of wounds completely healed)
In Nisi 2005 by eight weeks in the randomised groups 28/40 (70%) ulcers treated with povidone iodine had healed compared with 36/40 (90%) treated with the protease‐modulating dressing. The RR for wound healing was 0.78 (95% CI 0.62 to 0.98) in favour of protease‐modulating dressings (Analysis 4.1). GRADE assessment: moderate quality evidence, downgraded once due to imprecision for the outcome of wound healing. A GRADE assessment of moderate quality evidence means further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
4.1. Analysis.
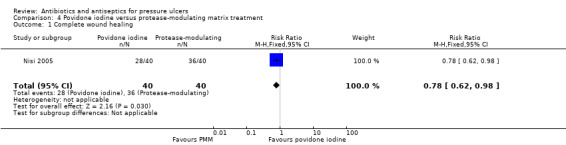
Comparison 4 Povidone iodine versus protease‐modulating matrix treatment, Outcome 1 Complete wound healing.
Primary outcome: adverse events
Nisi 2005 did not report adverse events
Secondary outcome: resource use (length of hospital stay)
Total length of hospital stay was reported as 1164 days in the povidone iodine group; 360 days in the protease‐modulating group (Nisi 2005). No measures of variance were reported and the data are not further analysed.
Secondary outcome: resource use (dressing use)
The total number of dressing changes for the povidone iodine group was reported as ranging from 14 to 52 and for the protease‐modulating group as ranging from six to 15 (Nisi 2005). Mean number of dressings was not reported for either group and the data were not further analysed.
Comparison 5. Cadexomer iodine versus standard care (1 trial, 38 participants)
One trial compared cadexomer iodine with standard care in 38 hospitalised participants (Moberg 1983). Participants were treated and followed up for eight weeks. Stages of pressure ulcers were not reported, rather they were classed as "deep" (N = 18) or "superficial" (N = 16). Classification data were not reported for participants who withdrew early in the trial. Data for ulcer area change were reported at both three and eight weeks.
Primary outcome: wound healing (proportion of wounds completely healed):
In Moberg 1983 6/19 (31.6%) participants randomised to cadexomer iodine had ulcers completely healed at eight weeks versus 1/19 (5.3%) in the group treated with standard care. The RR for complete wound healing was 6.00 (95% CI 0.80 to 45.20) (Analysis 5.1). GRADE assessment: very low quality evidence downgraded twice due to imprecision and once due to attrition bias. A GRADE assessment of very low quality means that we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
5.1. Analysis.
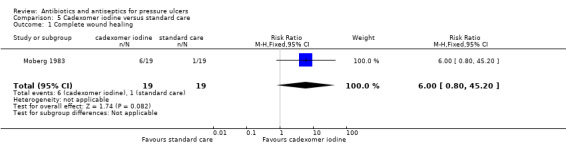
Comparison 5 Cadexomer iodine versus standard care, Outcome 1 Complete wound healing.
Primary outcome: adverse events
Moberg 1983 reported that there were no "side effects" in participants in the standard care group with data available for 13 participants. 5/14 participants in the cadexomer iodine group reported events that were considered as side effects. The RR for adverse events was 10.27 (95% CI 0.62 to 169.16) (Analysis 5.2). GRADE assessment: very low quality evidence downgraded twice for imprecision and once for attrition bias.
5.2. Analysis.
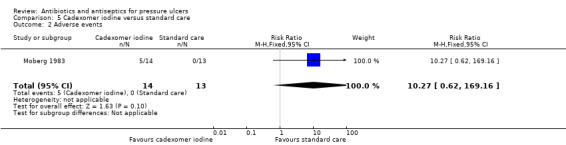
Comparison 5 Cadexomer iodine versus standard care, Outcome 2 Adverse events.
Secondary outcome: change in wound size
Moberg 1983 reported change in wound area. The mean reduction in wound area at 8 weeks was 76.2% (SE 8.2%) in the cadexomer iodine group (data for 14 participants) compared with 57.4% (SE 9.4%) in the standard care group (data for 13 participants). The mean difference in wound area reduction was 18.80% (95% CI −5.65 to 43.25) greater reduction in size in the cadexomer iodine treated group (Analysis 5.3). GRADE assessment: very low quality evidence downgraded twice due to imprecision and once due to attrition bias.
5.3. Analysis.
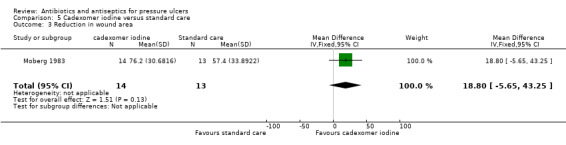
Comparison 5 Cadexomer iodine versus standard care, Outcome 3 Reduction in wound area.
Secondary outcome: pain
In Moberg 1983 pain was reported using a visual analogue scale for 13 participants in each group. Pain score at baseline was 14.6 (SE 4.6) in the cadexomer iodine group and 13.3 (SE 5.6) in the standard care group. Pain at 8 weeks was 3.1 (SE 1.7) in the cadexomer iodine group and 7.5 (SE 2.8) in the standard care group. The mean difference in pain score at 8 weeks was −4.40 (95% CI −10.82 to 2.02), (Analysis 5.4). GRADE assessment: very low quality evidence downgraded twice due to imprecision and once due to attrition bias.
5.4. Analysis.
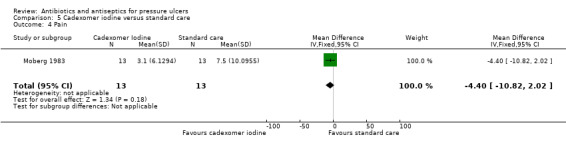
Comparison 5 Cadexomer iodine versus standard care, Outcome 4 Pain.
Comparison 6. Pine resin salve versus hydrocolloid (1 trial, 37 participants)
One trial (Sipponen 2008) compared spruce resin salve 1 mm thick between loose sterile cotton gauze changed every day with either a hydrocolloid dressing without antiseptic agent or a silver hydrocolloid dressing (where there was evidence of wound infection). The trial enrolled primary care hospital patients with stages II‐IV pressure ulcers. Several participants had more than one ulcer; 37 participants with 45 ulcers were randomised and it was not clear whether the analysis correctly adjusted for this. Participants were treated for up to six months and followed up for six months. Fifteen participants with 16 ulcers were not included in the analysis; seven of these participants died during the course of the trial.
Primary outcome: wound healing (proportion of wounds completely healed)
Sipponen 2008 reported 17/27 (63.0%) ulcers in the spruce resin group were healed by six months compared with 4/18 (22.2%) in the hydrocolloid group. The RR for wound healing was 2.83 (95% CI 1.14 to 7.05) in favour of the spruce resin (Analysis 6.1). GRADE assessment: low quality evidence downgraded once due to attrition bias and once due to imprecision as the precision estimates are likely to change upon correct analysis of data for the outcome.
6.1. Analysis.
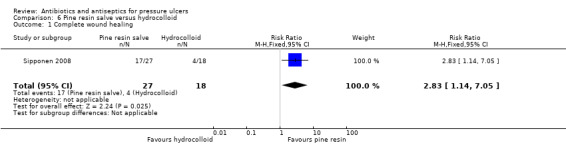
Comparison 6 Pine resin salve versus hydrocolloid, Outcome 1 Complete wound healing.
Primary outcome: adverse events
Sipponen 2008 did not report adverse events although the dropout analysis was fully reported and this included data on events such as deaths and skin reactions. These data were not further analysed.
Secondary outcome: change in bacterial resistance
Sipponen 2008 reported that of 18 ulcers assessed in each trial arm one was found to be positive for MRSA at baseline. In both cases this ulcer tested negative after one month (Analysis 6.2). GRADE assessment: very low quality evidence downgraded due to multiple sources of imprecision and due to attrition bias.
6.2. Analysis.
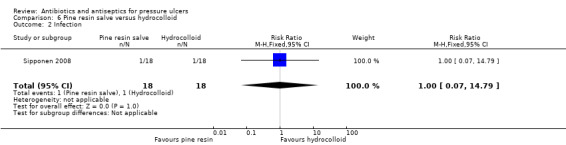
Comparison 6 Pine resin salve versus hydrocolloid, Outcome 2 Infection.
Summary of comparisons of antiseptics with non‐antimicrobial treatments (6 trials, 284 participants)
Four trials reported comparisons of povidone iodine with a dressing without antimicrobial properties (hydrocolloid (Barrois 1993; N = 78), hydrogel (Kaya 2005; N = 25), saline gauze (Kucan 1981; N = 45), protease‐modulating matrix (Nisi 2005; N = 80). Three of these reported the proportion of wounds completely healed (Barrois 1993; Kaya 2005; Nisi 2005). We had planned to pool these studies in a random‐effects meta‐analysis. However, one of the studies had substantive unit of analysis issues which would have affected the pooled analysis (in Kaya 2005 49 ulcers were analysed from 25 randomised participants). Barrois 1993 and Nisi 2005 had such significant clinical heterogeneity in the application of povidone iodine, as well as the comparators used, that it was determined that pooling would not produce a meaningful result. Whilst Barrois 1993 used a dressing impregnated with iodine, in Nisi 2005 the iodine was applied as a daily disinfection in addition to a non‐antimicrobial dressing. One trial reported the primary outcome of adverse events; Barrois 1993 reported no events.
There was no clear evidence that povidone iodine applied as a dressing or as a disinfection solution improved the numbers of pressure ulcers which healed in studies with short term follow‐up periods; data for two trials suggested comparator treatments (hydrogel and protease‐modulating matrix) were more effective than antiseptics in healing ulcers. GRADE assessment: moderate (comparison with protease‐modulating dressings) or low (comparisons with hydrocolloid and hydrogel) quality evidence. Low quality evidence showed no clear evidence of a difference in infection eradication between povidone iodine and saline gauze.
One trial compared cadexomer iodine to standard care in 38 participants (Moberg 1983). The trial did not find evidence of a difference between the groups in wound healing or any secondary outcomes. There were fewer adverse effects in the standard care group. GRADE assessment: very low quality evidence downgraded twice due to imprecision and once due to attrition bias.
One trial (37 participants with 45 ulcers) compared pine resin to hydrocolloid dressings. Sipponen 2008 found a benefit of pine resin in long‐term wound healing. There was no evidence of a difference in change in bacterial resistance. GRADE assessment: low and very low quality evidence downgraded due to attrition bias and imprecision.
2. Antiseptics compared with alternative antiseptics (3 trials, 190 participants)
Comparison 7. Iodine sugar versus lysozyme ointment
One trial compared iodine sugar to lysozyme ointment. Imamura 1989 randomised 141 participants with stage I to IV pressure ulcers to 3% povidone iodine sugar paste or lysozyme ointment. Ulcers were treated and followed up for 8 weeks.
Primary outcome: wound healing (proportion of wounds completely healed)
Imamura 1989 reported that 15/72 (21%) of participants' ulcers treated with iodine sugar healed compared with 12/69 (17%) ulcers in the lysozyme ointment group. There was no clear difference between the groups; the RR for wound healing was 1.20 (95% CI 0.60 to 2.37) (Analysis 7.1). GRADE assessment: very low quality evidence downgraded twice for imprecision and once for performance bias.
7.1. Analysis.
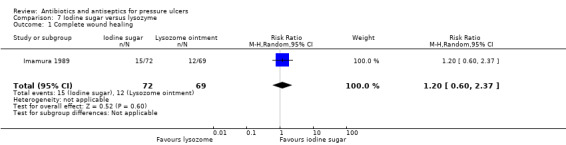
Comparison 7 Iodine sugar versus lysozyme, Outcome 1 Complete wound healing.
Primary outcome: adverse events
Imamura 1989 reported one adverse event in the iodine sugar group and three in the lysozyme ointment group; one of these was classed as serious. The RR for all adverse effects was 0.32 (95% CI 0.03 to 3.00) (Analysis 7.2). The denominators for this outcome have been inferred from the ITT population.
7.2. Analysis.
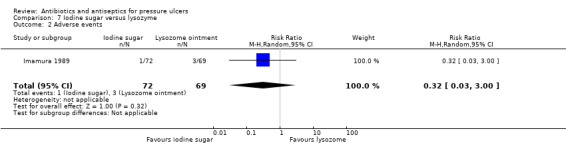
Comparison 7 Iodine sugar versus lysozyme, Outcome 2 Adverse events.
Secondary outcome: change in wound size
Imamura 1989 reported the proportion of patients achieving a reduction in wound area of at least 25% at one, two, four, six, eight and the last week. At the last follow‐up point 46/61 (75%) patients in the iodine sugar group and 34/60 (57%) in the lysozyme group had this reduction in area. The RR for this degree of reduction was 1.33 (95% CI 1.02 to 1.73) in the direction of iodine sugar (Analysis 7.4). GRADE assessment: very low quality evidence downgraded twice for imprecision and once for performance bias.
7.4. Analysis.
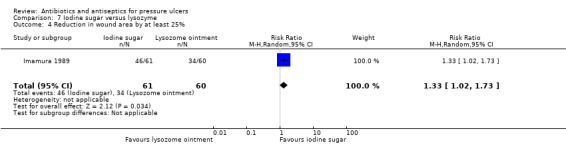
Comparison 7 Iodine sugar versus lysozyme, Outcome 4 Reduction in wound area by at least 25%.
Secondary outcome: change in infection status
Imamura 1989 reported change in infection status using a five point scale from "exacerbated" to "extremely improved". At the last follow‐up point 28/61 (46%) participants in the iodine sugar group were judged to be improved compared with 17/61 (28%) in the lysozyme group (extremely improved 10 vs 3, moderately improved 10 vs 6, slightly improved 8 vs 8). The RR of improvement was 1.65 (95% CI 1.01 to 2.68) (Analysis 7.5). It is unclear how these judgements were made. GRADE assessment: very low quality evidence downgraded twice for imprecision and once for performance bias.
7.5. Analysis.
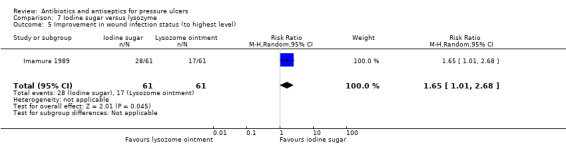
Comparison 7 Iodine sugar versus lysozyme, Outcome 5 Improvement in wound infection status (to highest level).
Comparison 8. Iodine sugar versus gentian violet (1 trial, 19 participants)
One trial compared an iodine sugar treatment with a gentian violet treatment in 19 participants with pressure ulcers in which MRSA had been detected (Toba 1997). Treatment duration was reported as 14 weeks.
Primary outcome: wound healing
Toba 1997 did not report this outcome
Primary outcome: adverse events
Toba 1997 reported that there were no adverse events in either treatment group (Analysis 8.1).
8.1. Analysis.
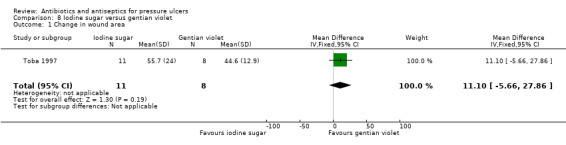
Comparison 8 Iodine sugar versus gentian violet, Outcome 1 Change in wound area.
Secondary outcome: change in wound size
Toba 1997 reported change in wound area. There was a decrease to 55.7% (SD 24.0) of the original wound area (a decrease of 44.3%) in the iodine sugar group compared with a decrease to 44.6% (SD 12.9) of original size (a decrease of 55.4%) in the gentian violet treatment group (MD −11.0; 95% CI −5.66 to 27.86) after 14 weeks (Analysis 8.1). GRADE assessment: low quality evidence downgraded twice due to imprecision.
Secondary outcome: change in bacterial resistance
Toba 1997 reported that after 14 weeks the proportion of ulcers which no longer had MRSA in wound cultures was 8/11 (72.7%) in the iodine sugar group compared with 7/8 (87.5%) in the gentian violet group (RR 0.83, 95% CI 0.53 to 1.30) (Analysis 8.2). GRADE assessment: low quality evidence downgraded twice due to imprecision.
8.2. Analysis.
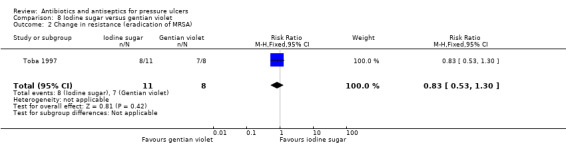
Comparison 8 Iodine sugar versus gentian violet, Outcome 2 Change in resistance (eradication of MRSA).
Comparison 9. Polyhexanide dressing versus polyhexanide swabs (1 trial, 30 participants)
One trial compared a polyhexanide‐impregnated biocellulose wound dressing plus foam dressing with a 20 minute cleansing with polyhexanide swabs at dressing change followed by a foam dressing (Wild 2012). The trial enrolled 30 hospital inpatients and outpatients with stage II ‐ IV pressure ulcers with long‐term intractable MRSA colonisation in spite of multiple previous disinfection attempts. Dressing changes were every two days, on average. Ulcers were treated for 14 days and then followed up for a further three days (total follow‐up of 17 days).
Primary outcome: wound healing
Wild 2012 did not report wound healing.
Primary outcome: adverse events
Wild 2012 did not report adverse events.
Secondary outcome: change in bacterial resistance
MRSA eradication was assessed at one and two weeks and at 17 days. After 14 and 17 days 15/15 (100%) ulcers in the dressing group were MRSA free compared with 10/15 (66.7%) in the swab group. The RR for MRSA eradication was 1.48 (95% CI 1.02 to 2.13) in the direction of polyhexanide dressings (Analysis 9.1). GRADE assessment: moderate quality evidence downgraded once for imprecision.
9.1. Analysis.
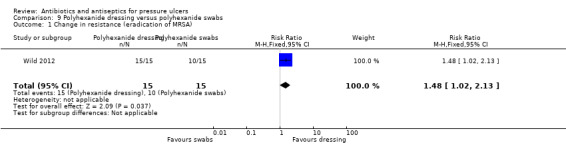
Comparison 9 Polyhexanide dressing versus polyhexanide swabs, Outcome 1 Change in resistance (eradication of MRSA).
Secondary outcome: pain
Wild 2012 reported pain using a visual analogue scale. Mean baseline scores were 7.4 (SD 0.47) in the dressing group compared with 6.8 (SD 0.53) in the swabs group. After 14 days the mean score in the dressing group was lower at 1.3 (SD 0.36) than in the swabs group, at 3.33 (SD 1.2). The mean difference between the groups was −2.03 (95% CI −2.66 to −1.40), favouring the dressing (Analysis 9.2). GRADE assessment: moderate quality evidence downgraded once for imprecision.
9.2. Analysis.
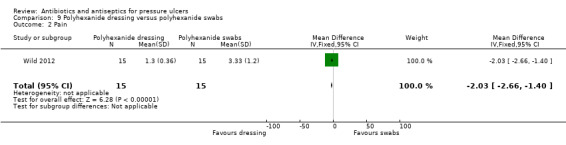
Comparison 9 Polyhexanide dressing versus polyhexanide swabs, Outcome 2 Pain.
Secondary outcome: resource use
Wild 2012 reported mean dressing change times as six minutes in the dressing group and 25 minutes in the swab group. No measures of variance were reported and no further analysis was possible.
Summary of comparison of different antiseptics (3 trials, 190 participants)
One trial with 141 participants compared povidone iodine sugar with lysozyme ointment. Imamura 1989 found no clear evidence of a difference in wound healing but evidence of more patients with a specified reduction in wound area and evidence of more wounds showing "improved" infection status in the iodine sugar group. GRADE assessment: low and very low quality evidence downgraded for imprecision and performance bias.
One trial with 19 participants compared povidone iodine sugar with gentian violet. Toba 1997 did not report the primary outcomes of wound healing or adverse events and did not find clear evidence of a difference in change in wound area or in eradication of MRSA. GRADE assessment: low quality evidence downgraded twice due to imprecision.
One trial with 30 participants compared polyhexanide swabs with polyhexanide dressings. Wild 2012 did not report the primary outcomes of wound healing or adverse events. There was evidence which favoured dressings for the eradication of MRSA from ulcers and for pain score at follow‐up. GRADE assessment: moderate quality evidence downgraded for imprecision.
3. Antiseptics compared with antibiotics (4 trials, 134 participants)
One trial compared povidone iodine with silver sulfadiazine (Kucan 1981). Two trials compared a silver dressing with silver sulfadiazine (Chuangsuwanich 2011; Chuangsuwanich 2013); as different silver dressings were used in the trials they are presented separately. One trial compared honey with ethoxy‐diaminoacridine plus nitrofurazone (a combination of an antiseptic and antibiotic) (Yapucu Güneş 2007).
Comparison 10. Povidone iodine versus silver sulfadiazine (1 trial, 45 participants)
One three‐arm trial compared povidone iodine with silver sulfadiazine (Kucan 1981) in 45 hospitalised participants with infected pressure ulcers; participants in the third arm were treated with saline gauze (see comparison 3 and comparison 14).
Primary outcome: wound healing
Kucan 1981 did not report wound healing.
Primary outcome: adverse events
Kucan 1981 did not report adverse events.
Secondary outcome: infection eradication
At three weeks' follow‐up 7/11 (63.6%) ulcers treated with povidone iodine were judged to be free of infection compared with 15/15 (100%) ulcers treated with silver sulfadiazine. The RR for infection eradication was 0.65 (95% CI 0.41 to 1.01). (Analysis 10.1). GRADE assessment: low quality evidence due to imprecision (downgraded twice for imprecision).
10.1. Analysis.
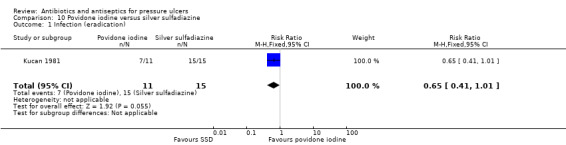
Comparison 10 Povidone iodine versus silver sulfadiazine, Outcome 1 Infection (eradication).
Comparison 11. Silver mesh versus silver sulfadiazine (1 trial, 40 participants)
One trial compared a silver mesh dressing with silver sulfadiazine (Chuangsuwanich 2011). Forty inpatients and outpatients with stage III or IV pressure ulcers were randomised to either silver mesh dressing (changed every three days) or silver sulfadiazine (dressings changed twice a day). Participants were followed up for eight weeks. Cotton dressings were used as outer dressings in both groups. Wound debridement was carried out as necessary. Without effect estimates for the reported outcomes full GRADE assessments are difficult to provide but the quality of the evidence may be downgraded as the small numbers of participants is likely to result in imprecision.
Primary outcome: wound healing
Chuangsuwanich 2011 did not report wound healing.
Primary Outcome: adverse events
Chuangsuwanich 2011 reported no complications as a result of treatment in either group. (RR not estimable).
Secondary outcome: change in wound size
Chuangsuwanich 2011 reported reduction in mean ulcer area. At baseline the mean area was 12.17 cm² in the silver mesh group and 22.82 cm² in the silver sulfadiazine group. At eight weeks the mean area in the silver mesh group was 7.96 cm² (reduction of 4.21 cm² (34.6%)) compared with 18.22 cm² (reduction of 4.58 cm² (20.1%)). No measures of variance were reported and the data were not further analysed.
Secondary outcome: infection
Chuangsuwanich 2011 reported only qualitative microbiological data; these were not extracted or analysed.
Secondary outcome: costs
Chuangsuwanich 2011 reported that the estimated mean cost of treatment in the silver mesh group was USD 263 compared with USD 1812 in the silver sulfadiazine group. Costs were estimated as drug cost + outer dressing cost x time of dressing change/20.
Comparison 12. Silver alginate versus silver sulfadiazine (1 trial, 22 participants)
One trial compared a silver alginate dressing with silver sulfadiazine (Chuangsuwanich 2013). This trial recruited participants with stage III or IV pressure ulcers and randomised 22 participants of whom 20 with a total of 28 ulcers were analysed (two participants died). Treatment duration and follow‐up were for eight weeks. Randomisation was conducted at the participant level but the analysis of outcome data was conducted at the ulcer level. It was not clear whether the analysis was correctly adjusted to account for this. None of the outcomes reported for this comparison had sufficient data to enable us to calculate an effect size. Without effect estimates for the reported outcomes full GRADE assessments are difficult to provide, but the quality of the evidence would likely be downgraded to take account of the fact that a correct analysis of the data accounting for multiple ulcers for some participants is likely to change any calculated effect sizes, as well as the small numbers of participants which is likely to contribute to imprecision.
Primary outcome: wound healing
Chuangsuwanich 2013 did not report wound healing.
Primary outcome: adverse events
Chuangsuwanich 2013 did not report adverse events although one death in each group was recorded.
Secondary outcome: change in wound size
Chuangsuwanich 2013 reported reduction in mean ulcer area. The mean wound area (in 15 ulcers) in the silver alginate group was reduced by 44.27% (from a baseline of 36.11cm²) compared with a reduction of 51.07% (from a baseline of 35.50cm²) in 13 ulcers in the silver sulfadiazine group. No measures of variance were reported and the data were not further analysed.
Secondary outcome: infection
Chuangsuwanich 2013 reported only qualitative microbiological data; these were not extracted or analysed.
Secondary outcome: costs associated with resource use
Chuangsuwanich 2013 reported mean overall cost of treatment over eight weeks as USD 377.17 in the silver alginate group and USD 467.74 in the silver sulfadiazine group. These figures were calculated based on dressing unit costs, costs of dressing changes and debridement and numbers of dressing changes. No measures of variance were reported and the data were not further analysed.
Comparison 13. Honey versus ethoxy‐diaminoacridine plus nitrofurazone (1 trial, 27 participants)
Yapucu Güneş 2007 enrolled 27 hospital patients with at least 51 stage II or III ulcers. Ninety‐six percent of participants in both groups had stage III ulcers. Participants were randomised to honey or to ethoxy‐diaminoacridine plus nitrofurazone dressings and treated for up to five weeks. Follow‐up duration was also five weeks. Randomisation was conducted at the participant level but outcome data were reported at the ulcer level. It was not clear whether the analysis was appropriately adjusted to take this into account.
Primary outcome: wound healing
Yapucu Güneş 2007 reported that 5/25 (20%) ulcers in the honey group healed compared with 0/ ≥26 (0%) in the comparison group (25 ulcers assessed but one randomised participant with ≥1 ulcer not included in analysis). There was no clear evidence of a difference between groups: RR 11.42 (0.66 to 196.4) (Analysis 13.1). GRADE assessment: very low quality evidence (downgraded twice for imprecision resulting from small numbers and also for the fact that the precision of the estimate is likely to change with correct analysis; and once for performance bias).
13.1. Analysis.
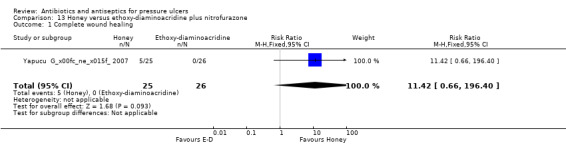
Comparison 13 Honey versus ethoxy‐diaminoacridine plus nitrofurazone, Outcome 1 Complete wound healing.
Primary outcome: adverse events
Yapucu Güneş 2007 reported that no participant in either group experienced adverse systemic or local side effects directly attributed to treatment. The death of one participant was also reported.
Secondary outcome: change in wound size
Yapucu Güneş 2007 reported reduction in wound area. Twenty‐five ulcers in the honey arm showed a mean 56% reduction in area after five weeks compared with a mean 13% reduction in the ethoxy‐diaminoacridine plus nitrofurazone group. Measures of variance were not reported and the data were not further analysed.
Summary of comparisons of antiseptics with antibiotics (4 trials, 134 participants)
One trial (N = 45) assessed povidone iodine (Kucan 1981). Kucan 1981 did not report wound healing or adverse events and did not find clear evidence of a difference between the treatment groups for infection eradication in the short‐term. GRADE assessment: low quality evidence downgraded twice due to imprecision.
Two trials (N = 62) compared silver dressings (silver mesh and silver alginate) with silver sulfadiazine. Neither reported wound healing. One reported no adverse events and the other did not report this outcome. Both trials reported data on wound area and costs but no measures of variance were reported and the data could not be fully analysed. No individual or pooled estimates of effect could be calculated and a GRADE assessment is difficult to provide. However, the issues identified for the individual comparisons of silver dressings with silver sulfadiazine (imprecision resulting from small numbers and unadjusted analyses) would apply to any assessment were an effect size to be calculated.
One trial (27 participants with at least 51 ulcers) compared honey to ethoxy‐diaminoacridine plus nitrofurazone dressings. Yapucu Güneş 2007 found a short‐term benefit of honey for wound healing and reported no adverse events. The trial reported reduction in wound area but without measures of variance that would allow calculation of an effect estimate. GRADE assessment: very low quality evidence (downgraded for imprecision resulting from small numbers and unadjusted analyses and for performance bias).
4. Antibiotics versus non‐antimicrobial agents (1 trial, 45 participants)
Comparison 14. Silver sulfadiazine versus saline (1 trial, 45 participants)
One three‐arm trial compared silver sulfadiazine with saline in hospitalised participants with infected pressure ulcers, participants in the third arm were treated with saline gauze (Kucan 1981) (see comparisons 3 and 10).
Primary outcome: wound healing
Kucan 1981 did not report wound healing.
Primary outcome: adverse events
Kucan 1981 did not report adverse events.
Secondary outcome: infection eradication):
After three weeks 15/15 (100%) ulcers treated with silver sulfadiazine were judged to be free of infection compared with 11/14 (78.6%) ulcers treated with saline. There was no clear evidence of a difference between groups: RR 1.26 (95% CI 0.94 to 1.69) (Analysis 14.1). GRADE assessment: low quality evidence due to imprecision (downgraded twice for imprecision).
14.1. Analysis.
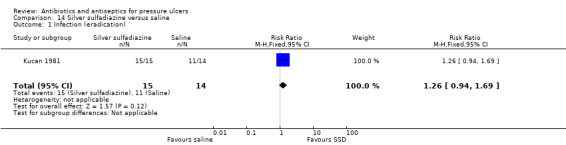
Comparison 14 Silver sulfadiazine versus saline, Outcome 1 Infection (eradication).
Summary of comparisons of antibiotics with non‐antimicrobial interventions
One trial compared silver sulfadiazine to saline in 45 participants. Kucan 1981 did not report the primary outcomes of wound healing or adverse events and did not find evidence of a difference between the treatment groups for infection eradication. GRADE assessment: low quality evidence due to imprecision (downgraded twice for imprecision).
Discussion
Summary of main results
This review includes all available RCT evidence for antiseptic/antibiotic (anti‐microbial) agents in the treatment of populations with stage II and above pressure ulcers. Eleven trials with a total of 437 participants were included.
Primary effectiveness outcome
All trials reporting wound healing did so as the proportion of wounds completely healed; no trials reported eligible time‐to‐healing data.
Most wound‐healing data were from comparisons of antiseptics with non‐antimicrobial agents. Trials compared povidone iodine (Barrois 1993, Imamura 1989, Kaya 2005, Nisi 2005), cadexomer iodine (Moberg 1983), and pine resin (Sipponen 2008) to a range of non‐antimicrobial comparators. Each individual comparison was reported by a single trial. One trial comparing an antiseptic (honey) with an antibiotic (nitrofurazone and ethoxy‐diaminoacridine) reported wound‐healing data (Yapucu Güneş 2007). None of the trials comparing an antibiotic with no treatment or two antiseptics assessed wound healing.
Trials comparing povidone iodine to hydrogel (Kaya 2005) or to a protease‐modulating dressing (Nisi 2005) reported a reduction in the 'risk' of healing in the povidone iodine group (that is they favoured the comparator treatment) however the GRADE assessments were low quality and moderate quality respectively. This indicates that further research is likely or very likely to have an important impact on our confidence in the estimate of effect and may, or is likely to, change the estimate of effect. Low quality evidence from a trial comparing povidone iodine and a hydrocolloid dressing showed no clear evidence of a difference between the treatments (Barrois 1993). There was also low quality evidence of a benefit of pine resin salve compared with a hydrocolloid dressing (Sipponen 2008). Again, further research is likely to change the estimates of effect for these comparisons.
There was very low quality evidence of no clear evidence of a difference between honey and the combination of nitrofurazone and ethoxy‐diaminoacridine treatment (Yapucu Güneş 2007). Two trials which found no clear evidence of a difference between cadexomer iodine compared with standard care (Moberg 1983) or between iodine sugar and lysozyme ointment (Imamura 1989) were also assessed as representing very low quality evidence. In these cases it is likely that the true effect is substantially different from the estimates of effect in the trials.
In most instances the quality of the evidence was primarily impacted by high levels of imprecision as a consequence of comparisons being assessed by single, small and underpowered trials.
Primary safety outcome
The primary safety outcome was adverse effects. Six trials reported extractable data (Barrois 1993; Chuangsuwanich 2011; Imamura 1989; Moberg 1983; Toba 1997; Yapucu Güneş 2007), four of which stated that there were no adverse effects. No GRADE assessment was possible but substantial limitations were identified which an assessment would have taken into account. There was very low quality evidence showing no clear evidence of a difference in patients treated with standard care compared with cadexomer iodine (Moberg 1983). Several trials reported some partial data on adverse events but did not make it clear whether this related to all participants.
Secondary outcomes
Limited secondary review outcomes were reported in the included studies. All studies were small and these comparisons were invariably underpowered and subject to imprecision.
The trials that reported change in wound size as a continuous outcome did not report any clear evidence favouring any particular antiseptic/anti‐microbial treatments. Where a GRADE assessment was possible this was low quality evidence (comparison of povidone iodine with gentian violet, Toba 1997) or very low quality evidence (cadexomer iodine compared with standard care, Moberg 1983). Further research is likely to change or very likely to substantially change these estimates of effect. Where no GRADE assessment was possible (comparison of silver dressings with silver sulfadiazine (Chuangsuwanich 2011; Chuangsuwanich 2013); and of honey versus nitrofurazone and ethoxy‐diaminoacridine (Yapucu Güneş 2007)) we identified substantial limitations which a GRADE assessment would have taken into account.
Four trials measured the resolution of infection (in three trials all ulcers were infected at baseline); in one trial there was some evidence of more MRSA eradication (at two weeks) in participants with an ulcer treated with a polyhexanide dressing compared with a polyhexanide swab (Wild 2012). This was classed as moderate quality evidence due to some imprecision; further research may change the estimate of effect. There was low quality evidence showing no clear evidence of a difference between interventions in the following comparisons: pine resin salve versus hydrocolloid dressing (Sipponen 2008), povidone iodine sugar versus gentian violet (Toba 1997), and povidone iodine versus silver sulfadiazine versus saline (Kucan 1981). Further research is likely to change these estimates of effect.
There was moderate quality evidence of less pain for patients treated with polyhexanide dressing compared with a polyhexanide swab (Wild 2012); and very low quality evidence of no conclusive findings in the comparison of cadexomer iodine with standard care (Moberg 1983).
Overall completeness and applicability of evidence
Most of the participants in the included trials were hospitalised older people. Where reported they had high levels of comorbidity. They are likely to be representative of patients seen in clinical practice.
Quality of the evidence
RCTs need to be adequately powered so that they are able to detect treatment effects of a specified size if they exist. This means that sample size calculations should be used to help estimate the number of people recruited to a trial. Trials should also have an adequate follow‐up period so that there is sufficient time for important outcome events — such as wound healing — to occur. All the trials included in this review were small and all except one had a short follow‐up period of eight weeks or less. As a result of this, only around half the trials (6/11) reported any wound‐healing data, and none were able to report appropriate time‐to‐wound‐healing data.
All studies included in this review were at high or unclear risk of bias across multiple domains. In general studies did not follow good practice for conduct and reporting guidelines (e.g. CONSORT (Schulz 2010)). Key areas of good practice are the robust generation of a randomisation sequence, robust allocation concealment, and blinded outcome assessment where possible. All this information should be clearly included in the trial report, as trial authors should anticipate the inclusion of their trials in systematic reviews. Studies should also clearly report how they planned to collect adverse event data and how this process was implemented in a standardised way across treatment arms. Where possible analysis of all data should be conducted on an intention‐to‐treat basis and measures of variance such as the standard deviation or standard error of the means should be reported. Trials should be appropriately designed and correctly analysed to take account of any clustered data; where this is not the case the reliability of results is uncertain. As far as possible trialists should take steps to reduce missing data.
Potential biases in the review process
This review considered as much evidence as possible. It was not limited by language or publication status. One of the included studies was published in Japanese (Toba 1997), as were several studies subsequently excluded from the review; other excluded studies were in German or Spanish. Many of the included studies were conducted in countries in which English is not the first language. Both studies awaiting classification are likely to be eligible for inclusion but require translation assistance from Italian (Bigolari 1991), or Portuguese (Goldmeier 1997) (we have not yet obtained the full text for this trial). It is possible that there may be unpublished data that we have been unable to identify. In many of the included studies one or more of the antimicrobial agents was being evaluated as a control/standard care arm. This was particularly the case for the assessment of povidone iodine and silver sulfadiazine. Because of this the presence of publication bias would perhaps be seen in an undue representation of small trials with negative rather than positive findings for these treatments. Whilst we acknowledge the possibility of publication bias despite our attempts to locate unpublished studies, there were too few trials to test for its presence.
Only two studies stated that they were funded by commercial companies (Nisi 2005; Wild 2012). However another two trials acknowledged some form of assistance from a company; in one case this was supply of one of the assessed treatments (Chuangsuwanich 2013); in the other the form of assistance was unclear (Moberg 1983). One other trial reported that its authors had subsequently formed a company in order to commercially develop the tested product (Sipponen 2008).
Agreements and disagreements with other studies or reviews
No other review has specifically focused on antimicrobial agents for pressure ulcers. Our review overlaps in content with general reviews of treatments for pressure ulcers and broader categories of wounds and with reviews of specific antiseptic agents for pressure ulcers or wounds generally. The category of antiseptics also covers both dressings and wound cleansing and therefore reviews in both these areas. We discuss the agreements and disagreements with the major reviews which we are aware of across these categories but acknowledge that there may be other reviews which we do not discuss.
General pressure ulcers/wounds
Smith 2013 included trials included in our review (Kaya 2005; Nisi 2005; Sipponen 2008; Yapucu Güneş 2007). The review authors also included Rhodes 2001 which was excluded by this review because of a quasi‐randomised design. It was difficult to determine whether other trials were identified and subsequently excluded or were not identified. Smith 2013 had a much broader scope than the present review, covering multiple types of treatments and including non‐randomised designs as well as RCTs; therefore many of the studies identified and excluded by our review were included in theirs.
Reddy 2008 included a trial of oxyquinolone ointment which was excluded from this review because of the high proportion of included participants with stage I pressure ulcers (Gerding 1992) and the trial by Rhodes 2001. Two additional trials were included which were excluded from our review (Kim 1996; Yastrub 2005); Kim 1996 included a high proportion of stage I ulcers whilst Yastrub 2005 did not report any outcomes relevant to our review. Reddy 2008 included five trials also included in our review (Kaya 2005; Moberg 1983; Nisi 2005; Sipponen 2008; Yapucu Güneş 2007). Several of the other trials in our review were published after Reddy 2008 was completed; it is not clear whether the remaining trials were identified and excluded or were not identified. As with Smith 2013 the scope was much broader than the present review and therefore many of the studies we excluded as not assessing a relevant intervention were included in theirs. There was no overlap with Moore 2013b's review of wound cleansing.
Honey
Jull 2013 reviewed honey for multiple wound types. The review authors included one trial in pressure ulcers which was excluded here because the great majority of the pressure ulcers were stage I (Weheida 1991). Because of unit of analysis issues this review also excluded the one RCT assessing honey which is included in this review (Yapucu Güneş 2007). We identified these issues and downgraded the quality of the evidence in our GRADE assessment.
Silver
Vermuelen 2007 and Storm‐Versloot 2010's reviews of silver were carried out before the publication date of trials assessing silver included in this review. Neither included any trials in pressure ulcers.
Iodine
Vermuelen 2010's review of iodine in wound care included three of the trials included in this review (Kaya 2005; Kucan 1981; Moberg 1983). The two other trials of iodine in the current review were not included (Barrois 1993; Nisi 2005); it was not clear whether they were identified and then excluded.
Authors' conclusions
Implications for practice.
A comprehensive review of current evidence did not find convincing evidence in favour of the use of any particular antimicrobial treatment compared with other antimicrobial treatment or to other comparators for pressure ulcers. Where differences in outcomes which matter (including wound healing) were found, these sometimes favoured the comparator treatment which did not have antimicrobial properties. However, the quality of the evidence varied from moderate to very low; all of the evidence was subject to some limitations. There is no randomised evidence on the use of systemic antibiotics for people with pressure ulcers.
Implications for research.
Currently there is no consistent evidence of a difference in pressure ulcer healing between ulcers treated with interventions with antimicrobial properties and those treated with alternative interventions, or between different antimicrobial treatment options. In terms of treatment choice, any investment in future primary research must maximise its value to patients, health‐care professionals, service commissioners and other decision‐makers. Given the large number of treatment options, the design of future trials should be driven by high‐priority questions from patients and other decision‐makers. It is also important for research to ensure that the outcomes that are collected in research studies are those that matter to patients, carers and health professionals. Where trials are conducted, good practice guidelines must be followed in their design, implementation and reporting. Further evidence synthesis (overviews of reviews, network meta‐analysis or both) may aid decision‐making about the choice of topical treatments for pressure ulcers, including the decision whether to use interventions with antimicrobial properties.
Acknowledgements
The authors are grateful for the following contributions:
Peer referees: Joan Webster, Reetu Child, Giovanni Cassazza, Lukas Schmülling, Janet Gunderson and the peer referees for the protocol: Una Adderley; Janet Cuddigan; Marina Israel; Linda Faye Lehman and Liz McInnes.
The copy editors: Elizabeth Royle and Jason Elliot‐Smith.
Assistance with data extraction: Jayne Jeffries
Translation assistance: initial assistance with Japanese language studies Erika Ota and Kimi Sawada; assistance with Spanish language papers Rocio Rodriguez Lopez; assistance with German language papers Jennifer Brown.
Appendices
Appendix 1. Search Strategies
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library)
#1 MeSH descriptor: [Anti‐Infective Agents] explode all trees #2 MeSH descriptor: [Penicillins] explode all trees #3 MeSH descriptor: [Cephalosporins] explode all trees #4 MeSH descriptor: [Aminoglycosides] explode all trees #5 MeSH descriptor: [Quinolones] explode all trees #6 MeSH descriptor: [Clindamycin] explode all trees #7 MeSH descriptor: [Metronidazole] explode all trees #8 MeSH descriptor: [Trimethoprim] explode all trees #9 MeSH descriptor: [Mupirocin] explode all trees #10 MeSH descriptor: [Neomycin] explode all trees #11 MeSH descriptor: [Fusidic Acid] explode all trees #12 MeSH descriptor: [Framycetin] explode all trees #13 MeSH descriptor: [Polymyxins] explode all trees #14 MeSH descriptor: [Chlortetracycline] explode all trees #15 (antibiotic* or antimicrobial* or antibacterial* or penicillin* or cephalosporin* or aminoglycoside* or quinolone* or clindamycin or metronidazole or trimethoprim or mupirocin or "pseudomonic acid" or neomycin or "fusidic acid" or framycetin or polymyxin* or chlortetracycline):ti,ab,kw #16 MeSH descriptor: [Antisepsis] explode all trees #17 antiseptic*:ti,ab,kw #18 MeSH descriptor: [Soaps] explode all trees #19 MeSH descriptor: [Iodophors] explode all trees #20 MeSH descriptor: [Chlorhexidine] explode all trees #21 MeSH descriptor: [Alcohols] explode all trees #22 MeSH descriptor: [Hydrogen Peroxide] explode all trees #23 MeSH descriptor: [Benzoyl Peroxide] explode all trees #24 MeSH descriptor: [Gentian Violet] explode all trees #25 MeSH descriptor: [Hypochlorous Acid] explode all trees #26 MeSH descriptor: [Hexachlorophene] explode all trees #27 MeSH descriptor: [Potassium Permanganate] explode all trees #28 MeSH descriptor: [Silver] explode all trees #29 MeSH descriptor: [Silver Sulfadiazine] explode all trees #30 MeSH descriptor: [Honey] explode all trees #31 (soap or soaps or iodophor* or povidone or iodine or chlorhexidine or betadine or "alcohol" or disinfectant* or "hydrogen peroxide" or "benzoyl peroxide" or "gentian violet" or hypochlorit* or eusol or dakin* or hexachlorophene or benzalkonium or "potassium permanganate" or "silver sulfadiazine" or "silver sulphadiazine" or honey*):ti,ab,kw #32 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 #33 MeSH descriptor: [Pressure Ulcer] explode all trees #34 (pressure next (ulcer* or sore* or injur*)):ti,ab,kw #35 (decubitus next (ulcer* or sore*)):ti,ab,kw #36 ((bed next sore*) or bedsore):ti,ab,kw #37 #33 or #34 or #35 or #36 #38 #32 and #37
Ovid MEDLINE
1 exp Anti‐Infective Agents/ 2 exp Penicillins/ 3 exp Cephalosporins/ 4 exp Aminoglycosides/ 5 exp Quinolones/ 6 exp Clindamycin/ 7 exp Metronidazole/ 8 exp Trimethoprim/ 9 exp Mupirocin/ 10 exp Neomycin/ 11 exp Fusidic Acid/ 12 exp Framycetin/ 13 exp Polymyxins/ 14 exp Chlortetracycline/ 15 (antibiotic* or antimicrobial* or antibacterial* or penicillin* or cephalosporin* or aminoglycoside* or quinolone* or clindamycin or metronidazole or trimethoprim or mupirocin or pseudomonic acid or neomycin or fusidic acid or framycetin or polymyxin* or chlortetracycline).ti,ab. 16 exp Antisepsis/ 17 antiseptic*.ti,ab. 18 exp Soaps/ 19 exp Iodophors/ 20 exp Chlorhexidine/ 21 exp Alcohols/ 22 exp Hydrogen Peroxide/ 23 exp Benzoyl Peroxide/ 24 exp Gentian Violet/ 25 exp Hypochlorous Acid/ 26 exp Hexachlorophene/ 27 exp Potassium Permanganate/ 28 exp Silver/ 29 exp Silver Sulfadiazine/ 30 exp Honey/ 31 (soap*1 or iodophor* or povidone or iodine or chlorhexidine or betadine or alcohol*1 or disinfectant* or hydrogen peroxide or benzoyl peroxide or gentian violet or hypochlorit* or eusol or dakin* or hexachlorophene or benzalkonium or potassium permanganate or silver sulfadiazine or silver sulphadiazine or honey*).ti,ab. 32 or/1‐31 33 exp Pressure Ulcer/ 34 (pressure adj (ulcer* or sore* or injur*)).tw. 35 (decubitus adj (ulcer* or sore*)).tw. 36 (bedsore* or bed sore*).tw. 37 or/33‐36 38 32 and 37 39 randomized controlled trial.pt. 40 controlled clinical trial.pt. 41 randomi?ed.ab. 42 placebo.ab. 43 clinical trials as topic.sh. 44 randomly.ab. 45 trial.ti. 46 or/39‐45 47 exp animals/ not humans.sh. 48 46 not 47 49 38 and 48
Ovid EMBASE
1 exp Antiinfective Agent/ 2 exp Penicillin G/ 3 exp Cephalosporin/ 4 exp Aminoglycoside/ 5 exp Quinolone/ 6 exp Clindamycin/ 7 exp Metronidazole/ 8 exp Trimethoprim/ 9 exp Pseudomonic Acid/ 10 exp Neomycin/ 11 exp Fusidic Acid/ 12 exp Framycetin/ 13 exp Polymyxin/ 14 exp Chlortetracycline/ 15 (antibiotic* or antimicrobial* or antibacterial* or penicillin* or cephalosporin* or aminoglycoside* or quinolone* or clindamycin or metronidazole or trimethoprim or mupirocin or neomycin or fusidic acid or framycetin or polymyxin* or chlortetracycline).ti,ab. 16 exp antisepsis/ 17 antiseptic*.ti,ab. 18 exp Soap/ 19 exp Iodophor/ 20 exp Chlorhexidine/ 21 exp Alcohol/ 22 exp Hydrogen Peroxide/ 23 exp Benzoyl Peroxide/ 24 exp Gentian Violet/ 25 exp Hypochlorous Acid/ 26 exp Hexachlorophene/ 27 exp Potassium Permanganate/ 28 exp Silver/ 29 exp Silver Sulfadiazine/ 30 exp Honey/ 31 (soap*1 or iodophor* or povidone or iodine or chlorhexidine or betadine or alcohol*1 or disinfectant* or hydrogen peroxide or benzoyl peroxide or gentian violet or hypochlorit* or eusol or dakin* or hexachlorophene or benzalkonium or potassium permanganate or silver sulfadiazine or silver sulphadiazine or honey*).ti,ab. 32 or/1‐31 33 exp decubitus/ 34 (pressure adj (ulcer* or sore* or injur*)).tw. 35 (decubitus adj (ulcer* or sore*)).tw. 36 (bedsore* or bed sore*).tw. 37 or/33‐36 38 32 and 37 39 Randomized controlled trials/ 40 Single‐Blind Method/ 41 Double‐Blind Method/ 42 Crossover Procedure/ 43 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab. 44 (doubl* adj blind*).ti,ab. 45 (singl* adj blind*).ti,ab. 46 or/39‐45 (1487429) 47 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 48 human/ or human cell/ 49 and/47‐48 50 47 not 49 51 46 not 50 52 38 and 51
EBSCO CINAHL Plus
S45 S32 AND S44 S44 S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 S43 MH "Quantitative Studies" S42 TI placebo* or AB placebo* S41 MH "Placebos" S40 TI random* allocat* or AB random* allocat* S39 MH "Random Assignment" S38 TI randomi?ed control* trial* or AB randomi?ed control* trial* S37 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S36 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S35 TI clinic* N1 trial* or AB clinic* N1 trial* S34 PT Clinical trial S33 MH "Clinical Trials+" S32 S26 AND S31 S31 S27 OR S28 OR S29 OR S30 S30 TI ( bedsore* or (bed n1 sore*) ) OR AB ( bedsore* or (bed n1 sore*) ) S29 TI ( decubitus n1 (ulcer* or sore*) ) OR AB ( decubitus n1 (ulcer* or sore*) ) S28 TI ( pressure n1 (ulcer* or sore* or injur*) ) OR AB ( pressure n1 (ulcer* or sore* or injur*) ) S27 (MH "Pressure Ulcer+") S26 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 S25 TI ( soap* or iodophor* or povidone or iodine or chlorhexidine or betadine or alcohol* or disinfectant* or hydrogen peroxide or benzoyl peroxide or gentian violet or hypochlorit* or eusol or dakin* or hexachlorophene or benzalkonium or potassium permanganate or silver sulfadiazine or silver sulphadiazine or honey*) or AB ( soap* or iodophor* or povidone or iodine or chlorhexidine or betadine or alcohol* or disinfectant* or hydrogen peroxide or benzoyl peroxide or gentian violet or hypochlorit* or eusol or dakin* or hexachlorophene or benzalkonium or potassium permanganate or silver sulfadiazine or silver sulphadiazine or honey*) S24 (MH "Honey") S23 (MH "Silver Sulfadiazine") S22 (MH "Silver") S21 (MH "Hexachlorophene") S20 (MH "Gentian Violet") S19 (MH "Hydrogen Peroxide") S18 (MH "Alcohols+") S17 (MH "Chlorhexidine") S16 (MH "Povidone‐Iodine") S15 (MH "Iodine") S14 (MH "Soaps") S13 TI antiseptic* S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 S11 TI ( antibiotic* or antimicrobial* or antibacterial* or penicillin* or cephalosporin* or aminoglycoside* or quinolone* or clindamycin or metronidazole or trimethoprim or mupirocin or pseudomonic acid or neomycin or fusidic acid or framycetin or polymyxin* or chlortetracycline ) or AB ( antibiotic* or antimicrobial* or antibacterial* or penicillin* or cephalosporin* or aminoglycoside* or quinolone* or clindamycin or metronidazole or trimethoprim or mupirocin or pseudomonic acid or neomycin or fusidic acid or framycetin or polymyxin* or chlortetracycline) S10 (MH "Polymyxins+") S9 (MH "Neomycin") S8 (MH "Mupirocin") S7 (MH "Trimethoprim+") S6 (MH "Metronidazole") S5 (MH "Clindamycin") S4 (MH "Aminoglycosides+") S3 (MH "Cephalosporins+") S2 (MH "Penicillins+") S1 (MH "Antiinfective Agents+")
Appendix 2. Risk of bias assessment (individually randomised controlled trials)
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process, such as referring to a random number table; using a computer random number generator; tossing a coin; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear risk of bias
Insufficient information about the sequence generation process provided to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or were not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear risk of bias
Insufficient information provided to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or is not described in sufficient detail to allow a definitive judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding (participants, personnel and outcome assessors) — was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others is unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others is likely to introduce bias.
Unclear risk of bias
Either of the following.
Insufficient information provided to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear risk of bias
Either of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of the suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear risk of bias
Insufficient information provided to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear risk of bias
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 3. Risk of bias assessment for cluster‐randomised controlled trials
In cluster‐randomised trials, particular biases to consider include:
recruitment bias;
baseline imbalance;
loss of clusters;
incorrect analysis; and
comparability with individually randomised trials.
Recruitment bias: can occur when individuals are recruited to the trial after the clusters have been randomised, as the knowledge of whether each cluster is an 'intervention' or 'control' cluster could affect the types of participants recruited.
Baseline imbalance: cluster‐randomised trials often randomise all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue. However, because small numbers of clusters are randomised, there is a possibility of chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals. Although this is not a form of bias as such, the risk of baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters. Reporting of the baseline comparability of clusters, or statistical adjustment for baseline characteristics, can help reduce concern about the effects of baseline imbalance.
Loss of clusters: occasionally complete clusters are lost from a trial, and have to be omitted from the analysis. Just as for missing outcome data in individually randomised trials, this may lead to bias. In addition, missing outcomes for individuals within clusters may also lead to a risk of bias in cluster randomised trials.
Incorrect analysis: many cluster‐randomised trials are analysed by incorrect statistical methods that do not take the clustering into account. Such analyses create a 'unit of analysis error' and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis.
Comparability with individually randomised trials: in a meta‐analysis that includes both cluster‐randomised and individually randomised trials, or including cluster‐randomised trials with different types of clusters, possible differences between the intervention effects being estimated need to be considered. For example, in a vaccine trial of infectious diseases, a vaccine applied to all individuals in a community would be expected to be more effective than a vaccine applied to only half the people. Another example is provided by a Cochrane review of hip protectors (Hahn 2005), where cluster trials showed a large positive effect, whereas individually randomised trials did not show any clear benefit. One possibility is that there was a 'herd effect' in the cluster‐randomised trials (which were often performed in nursing homes, where compliance with using the protectors may have been enhanced). In general, such 'contamination' would lead to underestimates of effect. Thus, if an intervention effect is still demonstrated despite contamination in those trials that were not cluster‐randomised, a confident conclusion about the presence of an effect can be drawn. However, the size of the effect is likely to be underestimated. Contamination and 'herd effects' may be different for different types of cluster.
Appendix 4. Glossary of intervention and comparator terms
Cadexomer iodine is a compound of iodine which is used as an antiseptic. Dressings impregnated with cadexomer iodine release free iodine during use.
Ethoxy‐diaminoacridine is derived from Ethacridine lactate and is used as an antiseptic.
Gentian violet is an antiseptic and also a dye.
Povidone iodine is a compound of iodine which is used as an antiseptic. Povidone iodine‐impregnated dressings release free iodine when exposed to wound exudate.
Protease‐modulating matrix: Protease‐modulating dressings alter the activity of enzymes which act to break down proteins in the wound.
Hydrocolloid: Hydrocolloid dressings contain gel‐forming agents in an adhesive compound laminated onto a flexible, water‐resistant outer layer (film or foam backing). Some formulations contain an alginate to increase absorption capabilities. When in contact with the wound surface they form a gel to provide a moist environment.
Hydrogel: Hydrogel dressings contain a large amount of water that keeps ulcers moist rather than letting them become dry.
Lysozyme ointment is an ointment containing lysozyme, an enzyme which has antibacterial properties.
Nitrofurazone: a form of Nitrofural, a compound used as an antibiotic, most commonly in the form of ointments.
Data and analyses
Comparison 1. Povidone iodine versus hydrocolloid (granuflex).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound healing | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.41, 1.96] |
Comparison 2. Povidone iodine versus hydrogel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound healing | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.43, 0.97] |
Comparison 3. Povidone iodine versus saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection (eradication) | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.37] |
Comparison 4. Povidone iodine versus protease‐modulating matrix treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound healing | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.98] |
Comparison 5. Cadexomer iodine versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound healing | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.80, 45.20] |
| 2 Adverse events | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.27 [0.62, 169.16] |
| 3 Reduction in wound area | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 18.80 [‐5.65, 43.25] |
| 4 Pain | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐4.4 [‐10.82, 2.02] |
Comparison 6. Pine resin salve versus hydrocolloid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound healing | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [1.14, 7.05] |
| 2 Infection | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.79] |
Comparison 7. Iodine sugar versus lysozyme.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound healing | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.60, 2.37] |
| 2 Adverse events | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.03, 3.00] |
| 3 Serious adverse events | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.72] |
| 4 Reduction in wound area by at least 25% | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.02, 1.73] |
| 5 Improvement in wound infection status (to highest level) | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [1.01, 2.68] |
7.3. Analysis.
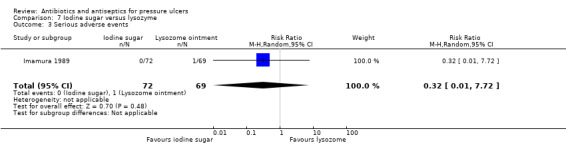
Comparison 7 Iodine sugar versus lysozyme, Outcome 3 Serious adverse events.
Comparison 8. Iodine sugar versus gentian violet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in wound area | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 11.10 [‐5.66, 27.86] |
| 2 Change in resistance (eradication of MRSA) | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.53, 1.30] |
Comparison 9. Polyhexanide dressing versus polyhexanide swabs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in resistance (eradication of MRSA) | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.02, 2.13] |
| 2 Pain | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐2.03 [‐2.66, ‐1.40] |
Comparison 10. Povidone iodine versus silver sulfadiazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection (eradication) | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.41, 1.01] |
Comparison 13. Honey versus ethoxy‐diaminoacridine plus nitrofurazone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound healing | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.42 [0.66, 196.40] |
Comparison 14. Silver sulfadiazine versus saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection (eradication) | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.94, 1.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrois 1993.
| Methods | Multicentre parallel RCT (countries not reported) Duration: 56 days Unit of randomisation: participant Unit of analysis: participant |
|
| Participants | Inclusion criteria: people presenting with open necrotic pressure ulcers Participants: 76 people with ulcers; Ulcer size (cm²): 15 (reported comparable between groups) Age, gender, ulcer location, ulcer stage not reported ("open necrotic pressure sore" interpreted by review authors as stage II or higher). |
|
| Interventions | Intervention arm 1: gauze (Tulle) with povidone iodine Intervention arm 2: hydrocolloid dressing (Granuflex) Co‐interventions: cleansing with saline and debridement with forceps if necessary in both groups. |
|
| Outcomes | Primary outcome: proportion of wounds completely healed Primary outcome: adverse events Secondary outcome: resource use (dressings per week) |
|
| Notes | Reported in abstract only; authors not contacted due to date of publication Funding: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported in abstract only |
| Allocation concealment (selection bias) | Unclear risk | Reported in abstract only |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported in abstract only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Thirty‐eight patients received Granuflex and 38 received a Tulle dressing impregnated with povidone‐iodine antiseptic ointment......Two patients receiving Granuflex and five patients receiving the control dressing dropped out of the trial due to a deterioration in the pressure sore" Comment: 7 of 72 patients were not included in the analyses; reasons specified. |
| Selective reporting (reporting bias) | Unclear risk | Reported in abstract only |
| Other bias | Unclear risk | Reported in abstract only |
Chuangsuwanich 2011.
| Methods | Country where data collected: Thailand Parallel group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 8 weeks |
|
| Participants | Inclusion criteria: people with stage III/IV pressure ulcers. Exclusion criteria: not reported Participants: 40 hospital inpatients and outpatients Mean age (years): 62.6 vs 69.1 Number of Males: 8/20 vs 9/20 Ulcer size (cm²): 12.17 vs 22.82 Ulcer location: sacrum 16/20 vs 14/20, trochanter 1/20 vs 5/20, ischium 3/20 vs 1/20 Ulcer stages: distribution not reported |
|
| Interventions | Intervention arm 1: silver mesh dressing changed every 3 days. Intervention arm 2: silver sulfadiazine cream changed twice daily. Co‐interventions: wound cleansing at every dressing change and cotton gauze outer dressing in both groups. Debridement as required. |
|
| Outcomes | Primary outcome: adverse events Secondary outcome: wound area reduction Secondary outcome: infection (qualitative bacteriological data only) |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The ulcers graded III‐IV were divided randomly by computer into two 20 patient‐groups.” Comment: Computer generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The ulcers graded III‐IV were divided randomly by computer into two 20 patient‐groups.” Comment: Although appropriate generation of sequence no information on concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “The ulcer healing was assessed by using the Pressure Ulcer Scale for Healing (PUSH 3.0) every two weeks also. PUSH tool was used for evaluation of the condition of the wounds.” Comment: No indication if assessment was blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Forty patients enrolled to the present study. Twenty patients in each group finished the eight‐week study.” Comment: Outcome data reported for all randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Comment: No direct quote but primary/secondary outcomes not stated a priori |
| Other bias | Unclear risk | Quote: “Wounds were debrided as necessary.” Comment: not clear how often this happened or how it differed between groups |
Chuangsuwanich 2013.
| Methods | Country where data collected: Thailand Parallel group RCT Unit of randomisation: participant Unit of analysis: ulcer Duration: 8 weeks |
|
| Participants | Inclusion criteria: hospital outpatients aged over 20 years and able to visit hospital regularly with stage III or IV pressure ulcers which were sacral or trochanter, not necrotic or with clinical signs of infection Exclusion criteria: no known sensitivity to treatment/control, no glucose‐6‐phosphate dehydrogenase deficiency. Participants: N = 22 (randomised), 20 (analysed) (28 ulcers) Mean age (years): 76 vs 73 years Number of males: 4/11 in each arm Ulcer stage: stage III 8/13 vs 8/15; IV: 5/13 vs 7/15 Ulcer location: sacral 9/13 vs 10/15, trochanter 4/13 vs 5/15 Ulcer size (cm²): Not reported |
|
| Interventions | Intervention arm 1: silver alginate dressing changed every 3 days, wound cleansing at every dressing change Intervention arm 2: silver sulfadiazine cream changed once per day Cointervention: wound cleansing at every dressing change; debridement as required |
|
| Outcomes | Secondary outcome: wound area reduction Secondary outcome: infection (qualitative bacteriological data only) |
|
| Notes | Funding: NR but silver alginate product donated by B Braun Co. Ltd, Thailand | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The enrolled patients were randomly divided into two groups by drawing from a sealed envelope for each group….. All of the 20 patients were randomly divided into the two groups according to the study protocol.” Comment: Unclear how randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The enrolled patients were randomly divided into two groups by drawing from a sealed envelope for each group….. All of the 20 patients were randomly divided into the two groups according to the study protocol.” Comment: Unclear whether envelopes were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "After the wound was cleansed by nurse, it was examined and scored by an independent operator, plastic surgeon, who was blinded to the dressing protocol.” Comment: blinding appears to have occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: flow diagram shows 22 patients randomised, 2 died during study, data from 20 were analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: Outcomes reported in methods present in results |
| Other bias | High risk | No specific quote: Randomisation was conducted at a patient level but the analysis was carried out at the level of the ulcer; it did not appear that paired data (multiple ulcers from individual participants) were accounted for in the analysis. |
Imamura 1989.
| Methods | Country where data collected: Japan Parallel group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 8 weeks (8 weeks follow‐up duration) |
|
| Participants | Inclusion criteria: participants with pressure ulcers stages 1‐4 treated in hospital Exclusion criteria: not reported Participants: N = 141 (randomised); 139 analysed of whom 109 had stage 2 or above pressure ulcers Mean age: Not reported Number of males: 37/71 vs 30/68 Ulcer stage: stage 1 16 vs 14; stage 2 or 3 31 vs 26; stage 4 24 vs 28 Ulcer location: sacrum 51 vs 45; ischium 6 vs 6; back 6 vs 4; greater trochanter 4 vs 4; ilium 1 vs 6; other 3 vs 4. Ulcer size (cm²): 25.48 (SE 4.34) vs 29.29 (SE 4.65) |
|
| Interventions | Intervention arm 1: KT‐136 (sugar (70g/100g) and povidone iodine (3g/100g)) ointment applied directly on to the wound or to the gauze sheet applied to the wound. Application once or twice a day. Intervention arm 2: lysozyme ointment (5g/100g) applied directly on to the wound or to the gauze sheet applied to the wound. Application once or twice a day. Cointerventions: none reported |
|
| Outcomes | Primary outcome: proportion of wounds completely healed Primary outcome: adverse events and serious adverse events Secondary outcome: wound area reduction Secondary outcome: infection |
|
| Notes | Trial published entirely in Japanese; assessment for inclusion, data extraction and risk of bias assessment all conducted by one review author Funding: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used |
| Allocation concealment (selection bias) | Low risk | Central telephone allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons were provided for missing data |
| Selective reporting (reporting bias) | Unclear risk | This was unclear due to poor reporting |
| Other bias | Unclear risk | Multiple sources of uncertainty |
Kaya 2005.
| Methods | Country where data collected: Turkey Parallel group trial Unit of randomisation: participant Unit of analysis: ulcer Duration: trial duration not reported; reported treatment duration ranged up to 106 days; primary outcome reported at durations from 21 to 85 (arm 1) and 15 to 83 (arm 2) days. |
|
| Participants | Inclusion criteria: spinal‐cord injury patients with pressure ulcers Exclusion criteria: not reported N = 27 (49 ulcers) N males: 24/27 (group distribution NR) Mean age (years): 35.3 vs 29.7 years Ulcer size (cm²) 4.13 vs 6.45 Ulcer stage: I: 6/25 vs 6/24, II: 17/25 vs 17/24; III: 2/25 vs 1/24 Ulcer location: sacral 6/25 vs 7/24, ischia 6/25 vs 3/24, heel 6/25 vs 2/24, greater trochanter 3/25 vs 6/24, iliac crest 0/25 vs 4/24, knee 1/25 vs 2/24, head of fibula 0/25 vs 2/24, lateral malleolus 2/25 vs 0/24, dorsum of foot 0/25 vs 1/24. All ulcers were non‐infected; patients were hospitalised |
|
| Interventions | Intervention arm 1: hydrogel‐type dressing (Elasto‐Gel) changed every 4 days or more frequently if membrane contaminated or non‐occlusive Intervention arm 2: povidone‐iodine‐soaked gauze changed daily Cointerventions: necrotic areas debrided |
|
| Outcomes | Primary outcome: proportion of wounds completely healed | |
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “We prospectively studied 27 hospitalised patients with spinal‐cord injury (24 males and three females) who had a total of 49 pressure ulcers. Each patient was randomly assigned to one of two groups.” Comment: how randomised unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: “We prospectively studied 27 hospitalised patients with spinal‐cord injury (24 males and three females) who had a total of 49 pressure ulcers. Each patient was randomly assigned to one of two groups.” Comment: no information on concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Ulcers were graded using the NPUAP system to ensure consistency in both groups. Surface area was used as an indicator of healing, and measured in cm².” Comment: no indication if assessment was blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “For ulcers that healed during the hospital stay, the rate of healing (cm²/days) was calculated as the initial surface area (cm²) divided by healing time (days). Where patients were discharged before healing was complete, it was calculated by subtracting the ulcer surface area at the most recent examination from the baseline surface area, then dividing this by the treatment time (days) while in hospital.” Comment: outcome data reported for all randomised patients, but it is unclear how many patients’ data were estimated |
| Selective reporting (reporting bias) | Unclear risk | Quote: "For each lesion, we recorded location, rate of healing, healing time and treatment time. Healing time (days) was defined as the time from the start of treatment to when 100% epithelialisation was observed." Comment: outcomes appear consistently listed or reported throughout text. Not clear what is primary and secondary outcome. |
| Other bias | High risk | Quote: “We prospectively studied 27 hospitalised patients with spinal‐cord injury (24 males and three females) who had a total of 49 pressure ulcers .” Comment: randomisation was conducted at a patient level but the analysis was carried out at the level of the ulcer; it did not appear that paired data (multiple ulcers from individual participants) were accounted for in the analysis. |
Kucan 1981.
| Methods | Country where data collected: USA Parallel group trial (three arms) Unit of randomisation: participant Unit of analysis: participant Duration: 3 weeks |
|
| Participants | Inclusion criteria: hospitalised patients with an infected PU on the sacral, ischial or femoral trochanteric areas. Infection was defined as bacterial count > 10⁵ bacteria/g tissue. Exclusion criteria: patients with concomitant infections outside the wound, acute cellulitis surrounding the ulcer or radiographic bone involvement beneath the ulcer were excluded. N = 45* Mean age (years): NR; range 16 to 102. Further details not reported but no statistically significant difference between groups on age, sex, paraplegia/tetraplegia or ulcer location |
|
| Interventions | Intervention arm 1: silver sulfadiazine (Silvadene cream) applied every 8 hours and covered with 2 layers fine mesh gauze Intervention arm 2: povidone iodine (Betadine solution) saturated coarse mesh gauze dressing changed every 6 hours Intervention arm 3: 0.9% sodium chloride solution: cleansing with sterile saline then coarse mesh gauze dressing saturated with solution, changed every 4 hours Cointerventions: debridement of necrotic tissue as indicated. Systemic antibiotics only for intercurrent infections (15 patients received them, distributed equally). |
|
| Outcomes | Secondary outcome: change in infection status (eradication of infection) | |
| Notes | *Numbers randomised to each arm were not reported, numbers analysed were reported per arm together with total number of dropouts. Funding: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the patients were assigned to one of three treatment groups according to a computer‐generated randomized table” Comment: computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: “the patients were assigned to one of three treatment groups according to a computer‐generated randomized table” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the biopsy specimen was delivered to the microbiologist who had no knowledge of which treatment the patient was receiving” Comment: the assessing microbiologist was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “although 45 patients were included in the study initially, only 40 were finally included in the efficacy analysis. The 40 patients were divided among the treatment groups as follows: silver sulfadiazine cream, 15; povidone‐iodine solution, 11; and physiologic saline, 14” Comment: 5/45 withdrawals but reasons for withdrawals and group allocations not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: no direct quotes but all stated outcomes of interest were reported. |
| Other bias | Low risk | Comment: no direct quotes but no evidence of additional sources of bias. Systemic antibiotic therapy was assessed and did not differ between groups |
Moberg 1983.
| Methods | Country where data collected: Sweden Parallel group RCT but included provision for cross‐over between treatments* Unit of randomisation: participant Unit of analysis: participant Duration: 8 weeks |
|
| Participants | Inclusion criteria: hospitalised patients with pressure ulcer Exclusion criteria: patients who were “moribund”, who had suspected malignancy or a psychiatric illness or other condition which could prevent informed consent were excluded. N = 34 N males 8/34 (group distribution not reported) Mean age (years) 80.1 vs 72.6; Ulcer size/stage: not reported, 8/18 versus 10/16 classed as "deep", 10/18 versus 6/16 classed as "superficial"; mean duration of ulcers: 6.2 months; review author judgement that ulcers were stage II or above. Ulcer location: not reported |
|
| Interventions | Arm 1: “standard treatment” as used in each hospital. This was individualised for each patient and depended on the appearance of the ulcer and surrounding skin. It included saline dressings, enzyme‐based debriding agents and nonadhesive dressings. Arm 2: cadexomer iodine applied daily in a 3 mm layer and removed after 24 hours with water/saline/wet swab. Cointerventions: Attention to nutrition, hygiene improvement, removal of localised pressure with specialised mattresses, turning and optimal mobilisation. |
|
| Outcomes | Primary outcome: proportion of wounds completely healed Primary outcome: adverse events Secondary outcome: wound area reduction Secondary outcome: pain |
|
| Notes | *Cross‐overs between treatments did not appear to occur at prespecified time‐points. Data for primary outcome were reported on an ITT basis Funding: NR but assistance from personnel at TIL Medical Ltd acknowledged |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were allocated blindly and at random for treatment” Comment: no information on how the randomisation sequence was produced or conducted. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “patients were allocated blindly and at random for treatment” Comment: no information on how the randomisation sequence was produced or conducted. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no specific quote but no information on whether assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “After three weeks.... three of the patients receiving standard treatment were switched to cadexomer iodine because of lack of response.... Two patients whose ulcers did not respond to cadexomer iodine were switched to standard treatment..... Two patients whose ulcers did not respond to the standard treatment dropped out of the trial after three weeks” Comment: 7/34 patients were reported as dropping out or switching treatment (reasons were given); pain data were unavailable for a further 1 patient. |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but outcomes specified in the methods were all reported in the results |
| Other bias | Unclear risk | Quote: “patients were allocated blindly and at random for treatment with cadexomer iodine or for the standard treatment used in each hospital. Standard treatment was individualized for each patient” Comment: because standard treatment was not fully reported and varied between patients it is unclear whether it included concomitant therapies which may have affected the results. |
Nisi 2005.
| Methods | Country where data collected: Italy Parallel group RCT Unit of randomisation: participant Unit of analysis: participant Duration: trial duration not reported; reported durations of randomised treatments 2 to 8 weeks (pre‐randomisation phase 1 to 6 weeks) |
|
| Participants | N = 80 Inclusion criteria: patients with pressure wounds Exclusion criteria: decompensating diabetes, hypertension, severe hypoalbuminosis, clinical evidence of arterial/venous insufficiency, haematocrit values below 4%/36% for males/females, treatment with steroids or immunosuppressant drugs. N males: 53/80 Mean age (years): 45 (group distribution not reported) Ulcer stage: II‐IV (numbers and group distribution not reported) Ulcer location: sacrum 28/80; back 2/80; upper limbs 8/80; lower limbs 42/80 (trochanteric area 24; heel 18); (group distribution not reported) Ulcer size (cm²): Not reported |
|
| Interventions | Intervention arm 1: protease‐modulating matrix treatment changed 2 or 3 times weekly according to exudation. Intervention arm 2: daily disinfection with 50% povidone iodine solution, saline wash and dressings with Vaseline gauze. Cointervention: treatment initiated after wounds were cleansed (no necrosis and no infection) using surgical debridement and disinfection with PVP‐I solution (treatment period of 1 to 6 weeks). All dressings covered with hydropolymer patch during randomised treatment phase. |
|
| Outcomes | Primary outcome: proportion of wounds completely healed Secondary outcome: resource use (hospital stay) Secondary outcome: resource use (dressing changes) |
|
| Notes | Funding: Systagenix Ltd (manufacturer of PMM treatment Promogran) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "a cohort of 80 selected patients was randomly divided into two groups" Comment: no information on how the randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Quote "a cohort of 80 selected patients was randomly divided into two groups" Comment: no information on whether allocation concealment was achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no direct quote but no information on whether assessors were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no direct quote but data were reported for all 80 randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Comment: no direct quote but outcomes were not prespecified |
| Other bias | Low risk | No evidence of other sources of bias |
Sipponen 2008.
| Methods | Country where data collected: Finland Parallel group RCT Unit of randomisation: participant Unit of analysis: ulcer Duration: 6 months |
|
| Participants | Inclusion criteria: patients with one or several severe pressure ulcers (stage II‐IV) with or without infection. Excusion criteria: patients with life expectancy of less than 6 months or with a malignant disease. N: 37 participants with 45 ulcers (randomised); 22 participants with 29 ulcers (analysed) N males: 6/13 vs 3/9 Mean age (years): 80 vs 74 No statistically significant difference between groups on ulcer location, stage or size but these were not reported; ulcer area was also not reported.* |
|
| Interventions | Intervention arm 1: spruce resin salve mixed (1 mm thick) between loose sterile cotton gauze changed every third day. Changed daily if infected or discharge present. Intervention arm 2: sodium carboxymethylcellulose hydrocolloid polymer with or without ionic silver (Aquacel or Aquacel AG) Aquacel Ag used where clinical and laboratory‐confirmed evidence of infection. Changed every third day or daily if discharge present. Cointerventions: oral antibiotics only if wound infected |
|
| Outcomes | Primary outcome: proportion of wounds completely healed Secondary outcome: infection (eradication) |
|
| Notes | *Demographic data refer to participants included in analysis only Funding: NR but authors have now founded a company to commercially develop the resin salve |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was in permuted block sizes of four. The randomization protocol was designed by a specialist in biometrics (S.S.). The responsible physicians in the primary care hospitals allocated patients to receive either resin treatment or control treatment according to the randomization list (closed envelopes).” Comment: appropriate block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The responsible physicians in the primary care hospitals allocated patients to receive either resin treatment or control treatment according to the randomization list (closed envelopes)." Comment: unclear if sealed envelopes were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “As there are, by necessity, discernible properties of the resin salve (e.g. fragrance and consistency), the treatment could not be blinded.” “Eleven independent physicians, one in each primary care centre, collected the data during the study period (6 months)” Comment: Clear that patients/physicians not blinded but unclear if outcome assessors were blinded as they are described as ‘independent’ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “A prospective, randomized, controlled multicentre trial involving 37 patients ... Thirteen patients of the resin group and nine patients of the control group completed the 6‐month trial” Comment: 15/37 patients did not complete the trial and were not included in the analysis. Reasons for these were comprehensively provided but the level of attrition remains high. |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but all outcomes specified in methods section are reported. |
| Other bias | High risk | Comment: no specific quote: randomisation was conducted at a patient level but the analysis was carried out at the level of the ulcer; it did not appear that paired data (multiple ulcers from individual participants) were accounted for in the analysis. |
Toba 1997.
| Methods | Country where data collected: Japan Parallel group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 14 weeks (duration), 2 years (follow‐up) |
|
| Participants | Inclusion criteria: detected MRSA from ulcers in previous month Excusion criteria: not reported N: 19 participants N males: 0/19 Mean age (years): 83.5 ± 3.0 years old Participants were hospital inpatients with cerebrovascular disorder, without diabetes, malignant tumours and liver dysfunctions |
|
| Interventions | Intervention arm 1: GVcAMP (base: polyethylene glycol (SOLBASE), 0.1% Gentian Violet (Piokutanin): Dibutyryl cAMP (Actsin) = 1:1) Intervention arm 2: IS (Iodine Sugar(U‐PASTA)) |
|
| Outcomes | Primary outcome: adverse events Secondary outcome: wound area reduction Secondary outcome: infection (microbiological data only) |
|
| Notes | Trial published in Japanese; data extraction and risk of bias assessment conducted by single translator. Assessed as meeting inclusion criteria for pressure ulcers (and stages) by translator. Funding: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by using a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Using a table of random numbers which was unclear whether open or not |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgement |
| Other bias | Unclear risk | Some sources of uncertainty |
Wild 2012.
| Methods | Country of data collection: Austria Parallel group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 14 days (treatment); 17 days (follow‐up) |
|
| Participants | Inclusion criteria: patients with pressure ulcers with long‐term intractable MRSA colonisation despite multiple previous disinfection attempts Exclusion criteria: NR N: 30 Mean age (years):70.9 vs 66.5 N male: 7/15 vs 8/15 Ulcer stage: stage 2: 3/15 vs 2/15, stage 3: 6/15 vs 6/15 sacral/ischial; stage 4: 7/15 vs 7/15 (all sacral) Ulcer location: heel 3/15 vs 2/15; sacral 11/15 vs 10/15; ischial 1/15 vs 3/15 Mean ulcer area (cm²) 47.67 vs 35.80 |
|
| Interventions | Intervention arm 1: biocellulose wound dressing + polyhexanide covered with foam dressing; dressing changes every 2 days (on average) Intervention arm 2: cleansing for 20 min with polyhexanide swabs and then a foam dressing; dressing changes every 2 days (on average) Cointerventions: two‐week washout period following previous disinfection attempts. Periwound skin protected with zinc cream where applicable. |
|
| Outcomes | Secondary outcome: infection (eradication) Secondary outcome: pain Secondary outcome: resource use (dressing change times) |
|
| Notes | Funding: Lohmann & Rauser GmbH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization, using a computer‐generated code, occurred following patient consent and eligibility confirmation” Comment: computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Upon inclusion to the study by opening sealed envelopes, which contained information on the proposed treatment, patients were assigned to 1 of the 2 groups” Comment: although sealed envelopes were used it was unclear if they were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “For surveillance of the antimicrobial effectiveness of treatment, swabs were taken on 3 consecutive days after the end of the 14‐day observation period...... The assessor was blinded to the treatment given.” Comment: assessor was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no specific quote but all randomised patients were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but appears all outcomes discussed in Methods section appear in results. |
| Other bias | Low risk | Comment: no evidence of other sources of bias. |
Yapucu Güneş 2007.
| Methods | Country where data collected: Turkey Parallel group RCT Unit of randomisation: participant Unit of analysis: ulcer Duration: 5 weeks |
|
| Participants | Inclusion criteria: patients aged ≥ 18 years with stage II/III pressure ulcers. Exclusion criteria: patients with diabetes or terminal illness were excluded N: 27 participants with ≥ 51 ulcers (randomised); 26 participants with 50 ulcers (analysed) N males: 9/15 vs 8/11* Mean age (years): 65.8 vs 66.6 Ulcer stage: 96% of ulcers in both groups were stage III Ulcer location: sacral 12 vs 12; shoulder 3 vs 4; trochanter 5 vs 2; heel 5 vs 7. |
|
| Interventions | Intervention arm 1: honey Intervention arm 2: ethoxy‐diaminoacridine plus nitrofurazone dressings Cointerventions: preventative regimen of turning/repositioning and pressure‐relieving mattress |
|
| Outcomes | Primary outcome: wound healing Primary outcome: adverse events Secondary outcome: changes in ulcer size |
|
| Notes | *demographic data refers to patients included in analysis (1 patient died and was excluded) Funding: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The authors completed a randomized parallel group evaluation comparing honey dressing with an ethoxydiaminoacridine plus nitrofurazone dressing for the treatment of pressure ulcers.” Comment: no further information on how randomisation was carried out |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on methods used to conceal allocation; quote above is all the information provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The authors could not be blinded to the treatment. Blinding would have required irrigation of the PU with sterile 0.9% sodium chloride(NaCl) immediately before an outcome assessor examined the wound. They needed to identify the presence of exudate and slough, which could not be done in a blinded study” Comment: unblinded assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote “75% of the patients (n = 27) we approached met the inclusion criteria and were enrolled in the study. Subsequently, 1 patient in the control group died. As a result, the final analysis sample is drawn from 26 patients” Comment: 1 patient died, data from all other patients was included in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but outcomes discussed in methods were all reported |
| Other bias | High risk | Comment: no specific quote: randomisation was conducted at a patient level but the analysis was carried out at the level of the ulcer; it did not appear that paired data (multiple ulcers from individual participants) were accounted for in the analysis. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anzai 1989 | Wrong population: fewer than 75% patients with PU |
| Baker 1981 | Not a randomised controlled trial |
| Bale 2004 | Wrong population: minority of patients with PU |
| Bazzigaluppi 1991 | Not a randomised controlled trial |
| Becker 1984 | Not a randomised controlled trial |
| Beele 2010 | Wrong population: minority of patients with PU |
| Boykin 1989 | Not a randomised controlled trial |
| Chirwa 2010 | Wrong population: minority of patients with PU |
| Colombo 1993 | Wrong population: minority of patients with PU |
| de Laat 2011 | Wrong intervention |
| Della Marchina 1997 | Not a randomised controlled trial |
| Gerding 1992 | Wrong population: mixed stage 1 and higher PU stage 2+ under 75%. |
| Gorse 1987 | Use of antiseptic/antibiotic not the only difference between intervention arms |
| Hartman 2002 | Not a randomised controlled trial |
| Helaly 1988 | Wrong population: minority of patients with PU |
| Ishibashi 1996 | Wrong population: fewer than 75% patients with PU |
| Itani 2010 | Wrong population: minority of patients with PU |
| Kim 1996 | Wrong population: mixed stage 1 and higher PU stage 2+ under 75%. |
| Konychev 2013 | Wrong population: minority of patients with PU |
| Kuroyanagi 1995 | Not a randomised controlled trial |
| Lazareth 2012 | Wrong population, not PU |
| Lee 2014 | Wrong population: minority of patients with PU |
| LeVasseur 1991 | Wrong intervention |
| Meaume 2005 | Wrong population: minority of patients with PU |
| Motta 2004 | Wrong population: minority of patients with PU |
| Munter 2006 | Wrong population: minority of patients with PU |
| Parish 1984a | Wrong population: minority of patients with PU |
| Parish 1984b | Wrong population: minority of patients with PU |
| Rhodes 2001 | Quasi‐randomised |
| Robson 1999 | Wrong intervention |
| Romanelli 2008 | Wrong population: minority of patients with PU |
| Saha 2012 | Quasi‐randomised |
| Saydak 1990 | Not a randomised controlled trial |
| Serra 2005 | Not a randomised controlled trial |
| Shrivastava 2011 | Wrong comparator |
| Sibbald 2011 | Wrong population: not PU |
| Stevens 2002 | Wrong population: minority of patients with PU |
| Thomas 1998 | Wrong intervention |
| Trial 2010 | Wrong population: minority of patients with PU |
| van der Cammen 1987 | Wrong population: patients at risk of pressure sores |
| Wang 2014 | Wrong intervention |
| Weheida 1991 | Wrong population: Stage 1 pressure ulcers |
| Worsley 1991 | Wrong population: minority of patients with PU |
| Yastrub 2005 | No relevant outcomes |
| Yura 1984 | Wrong population: fewer than 75% patients with PU |
| Zeron 2007 | Wrong intervention |
Characteristics of studies awaiting assessment [ordered by study ID]
Bigolari 1991.
| Methods | RCT |
| Participants | N = 28 patients |
| Interventions | Intervention arm 1: cadexomer iodine Intervention arm 2: standard care |
| Outcomes | Primary outcome: adverse events Secondary outcome: wound area reduction |
| Notes | trial published in Italian, English abstract |
Goldmeier 1997.
| Methods | RCT |
| Participants | N = 27 patients Patients with heart disease and pressure ulcers |
| Interventions | Intervention arm 1: medium chain triglycerides with essential fatty acids Intervention arm 2: povidone iodine |
| Outcomes | Secondary outcome: wound area reduction |
| Notes | Trial published in Portuguese (English translation of abstract obtained); full paper not yet obtained |
Differences between protocol and review
Where participants with stage II and above pressure ulcers were a subgroup of randomised individuals we had planned to analyse the subgroup of participants where data could be obtained, or to analyse the whole trial data where the relevant participants made up at least 75% of the trial population and separate data were not available. Instead of this we have included only trials where relevant participants constituted at least 75% of participants and have analysed the data from the whole trial population. We also carried out a GRADE assessment on all eligible outcomes where possible, rather than limiting this to wound healing, adverse effects and changes in infection status. This allows a more complete evaluation of the quality of the evidence base.
Contributions of authors
Gill Norman: designed and coordinated the review; extracted data; checked the quality of data extraction; undertook and checked quality assessment; analysed or interpreted data; completed the first draft of the review; performed part of writing and editing the review; made an intellectual contribution to the review; approved the final version prior to submission; wrote to study authors/experts/companies; and is a guarantor of the review.
Jo Dumville: conceived and designed the review; analysed or interpreted data; performed part of data analysis or interpretation; performed part of writing and editing the review; made an intellectual contribution to the review; approved the final version prior to submission; advised on the review; secured funding; and is a guarantor of the review.
Zena Moore: performed part of writing and editing the review; made an intellectual contribution to the review; approved the final version prior to submission; and advised on the review.
Judith Tanner: performed part of writing and editing the review; made an intellectual contribution to the review; approved the final version prior to submission; and advised on the review.
Janice Christie: extracted data; checked the quality of data extraction; undertook and checked quality assessment; analysed or interpreted data; performed part of writing and editing the review; made an intellectual contribution to the review; and approved the final version prior to submission.
Saori Goto: extracted data; undertook quality assessment; made an intellectual contribution to the review; performed translations and approved the final version prior to submission.
Contributions of editorial base:
Nicky Cullum: edited the protocol and the review; advised on methodology, interpretation and protocol content. Approved the final protocol prior to submission. Sally Bell‐Syer and Gill Rizzello: coordinated the editorial process. Advised on interpretation and content. Edited the protocol and the review. Rocio Rodriguez‐Lopez: designed the search strategy. Reetu Child edited the search strategy and the search methods section and ran the searches.
Sources of support
Internal sources
School of Nursing, Midwifery and Social Work, University of Manchester, UK.
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Cochrane Programme Grant funding (NIHR Cochrane Programme Grant 13/89/08 ‐ High Priority Cochrane Reviews in Wound Prevention and Treatment) to Cochrane Wounds. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Declarations of interest
Janice Christie: nothing to declare. Jo Dumville: nothing to declare. Saori Goto: nothing to declare. Zena Moore: has received an honorarium for speaking at professional meetings for Vancive. Gill Norman: My employment at the University of Manchester is funded by NIHR and focuses on high‐priority Cochrane reviews in the prevention and treatment of wounds. Judith Tanner: has received payment for speaking about surgical site infections at study days organised by Smith and Nephew and also Mölnlycke Heathcare.
Edited (no change to conclusions)
References
References to studies included in this review
Barrois 1993 {published data only}
- Barrois B. Comparison of Granuflex and medicated paraffin gauze in pressure sores. Proceedings of the 2nd European Conference on Advances in Wound Management, Oct 20‐23 1992, Harrogate, UK. London: Macmillan Magazines, 1993:209‐10.
Chuangsuwanich 2011 {published data only}
- Chuangsuwanich A, Charnsanti O, Lohsiriwat V, Kangwanpoom C, Thong‐In N. The efficacy of silver mesh dressing compared with silver sulfadiazine cream for the treatment of pressure ulcers. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 2011;94(5):559‐65. [PubMed] [Google Scholar]
Chuangsuwanich 2013 {published data only}
- Chuangsuwanich A, Chortrakarnkij P, Kangwanpoom J. Cost‐effectiveness analysis in comparing alginate silver dressing with silver zinc sulfadiazine cream in the treatment of pressure ulcers. Archives of Plastic Surgery 2013;40(5):589‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imamura 1989 {published data only}
- Imamura S, Uchino H, Imura Y. The clinical effect of KT‐136 (sugar and povidone‐iodine ointment) on decubitus ulcers: A comparative study with lysozyme ointment. Japanese Pharmacology and Therapeutics 1989;17(Supp 1):255‐80. [Google Scholar]
Kaya 2005 {published data only}
- Kaya AZ, Turani N, Akyüz M. The effectiveness of a hydrogel dressing compared with standard management of pressure ulcers. Journal of Wound Care 2005;14(1):42‐4. [DOI] [PubMed] [Google Scholar]
Kucan 1981 {published data only}
- Kucan J O, Robson M C, Heggers J P, Ko F. Comparison of silver sulfadiazine, povidone‐iodine and physiologic saline in the treatment of chronic pressure ulcers. Journal of the American Geriatrics Society 1981;29(5):232‐5. [DOI] [PubMed] [Google Scholar]
Moberg 1983 {published data only}
- Moberg S, Hoffman L, Grennert M‐L, Holst A. A randomized trial of cadexomer iodine in decubitus ulcers. Journal of the American Geriatrics Society 1983;31(8):462‐5. [DOI] [PubMed] [Google Scholar]
Nisi 2005 {published data only}
- Nisi G, Brandi C, Grimaldi L, Calabro M, D'Anielo C. Use of a protease‐modulating matrix in the treatment of pressure sores. Chirugia Italiana 2005;57(4):465‐8. [PubMed] [Google Scholar]
Sipponen 2008 {published data only}
- Sipponen A, Jokinen JJ, Sipponen P, Papp A, Sarna S, Lohi J. Beneficial effect of resin salve in treatment of severe pressure ulcers: A prospective, randomized and controlled multicentre trial. British Journal of Dermatology 2008;158(5):1055‐62. [DOI] [PubMed] [Google Scholar]
- Sipponen A, Jokinene JJ, Lohi J, Sipponen P. Efficiency of resin salve from Norway spruce in treatment of pressure ulcers and chronic surgical and traumatic wounds. EWMA Journal 2009;9(2):Abstract 41. [Google Scholar]
Toba 1997 {published data only}
- Toba K, Sudoh N, Nagano K, Eto M, Mizuno Y, Nakagawa H, et al. [Randomized prospective trial of gentian violet with dibutyryl cAMP and povidone‐iodine with sugar as treatment for pressure sores infected with methicillin‐resistant Staphylococcus aureus in elderly patients]. Nippon Ronen Igakkai Zasshi [Japanese Journal of Geriatrics] 1997;34(7):577‐82. [DOI] [PubMed] [Google Scholar]
Wild 2012 {published data only}
- Wild T, Bruckner M, Payrich M, Schwarz C, Eberlein T. Prospective randomized study for eradication of MRSA with polyhexanide‐containing cellulose dressing compare with polyhexanide wound solution. http://ewma.org/fileadmin/user_upload/EWMA/pdf/conference_abstracts/2009/Poster/P_145.pdf (accessed 22 March 2016). [DOI] [PubMed]
- Wild T, Bruckner M, Payrich M, Schwarz C, Eberlein T, Andriessen A. Eradication of methicillin‐resistant Staphylococcus aureus in pressure ulcers comparing a polyhexanide‐containing cellulose dressing with polyhexanide swabs in a prospective randomized study. Advances in Skin and Wound Care 2012;25(1):17‐22 (19 ref). [DOI] [PubMed] [Google Scholar]
Yapucu Güneş 2007 {published data only}
- Yapucu Güneş U, Eşer I. Effectiveness of a honey dressing for healing pressure ulcers. Journal of Wound, Ostomy and Continence Nursing 2007;34(2):184‐90. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anzai 1989 {published data only}
- Anzai T, Shiratori A, Otomo E, Honda S, Yamamoto T, Nishikawa T, et al. [Evaluation of Clinical Utility of NI‐009 on Various Cutaneous Ulcers: Comparative Study with Base]. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicine) 1989;5(12):2585‐612. [Google Scholar]
Baker 1981 {published data only}
- Baker PG. Does metronidazole help leg ulcers and pressure sores?. Drug and Therapeutics Bulletin 1982;20(3):9‐10. [PubMed] [Google Scholar]
- Baker PG, Haig G. Metronidazole in the treatment of chronic pressure sores and ulcers. A comparison with standard treatment in general practice. Practitioner 1981;225(1354):569‐73. [PubMed] [Google Scholar]
Bale 2004 {published data only}
- Bale S, Tebbie N, Price P. A topical metronidazole gel used to treat malodorous wounds. British Journal of Nursing 2004;13(11):s4‐11. [DOI] [PubMed] [Google Scholar]
Bazzigaluppi 1991 {published data only}
- Bazzigaluppi F, Biraghi MG, Fano M, Moschin AM, Toscani P, Cervone C, et al. Cadexomer iodine in the treatment of cutaneous ulcers: open, multicentric trial. Gazetta Medica Italiana: Archivo per le Scienze Mediche 1991;150(11):471‐80. [Google Scholar]
Becker 1984 {published data only}
- Becker L, Goodemote C. Treating pressure sores with or without antacid. American Journal of Nursing 1984;84(3):351‐2. [PubMed] [Google Scholar]
Beele 2010 {published data only}
- Beele H, Meuleneire F, Nahuys M, Percival SL. A prospective randomised open label study to evaluate the potential of a new silver alginate/carboxymethylcellulose antimicrobial wound dressing to promote wound healing. International Wound Journal 2010;7(4):262‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boykin 1989 {published data only}
- Boykin A, Winland‐Brown J. Pressure sores: nursing management using nursing research. Washington, DC: National League of Nursing, 1989. Publication no. 15‐2232:252‐6.
Chirwa 2010 {published data only}
- Chirwa Z, Bhengu T, Litiane K. The cost effectiveness of using calcium alginate silver matrix in the treatment of wounds. EWMA Journal 2010;10(2):257, Abstract P299. [Google Scholar]
Colombo 1993 {published data only}
- Colombo P. Topical chloroxidating solution in wounds treatment: a controlled trial. Acta Toxicologica et Therapeutica 1993;14(2):65‐72. [Google Scholar]
de Laat 2011 {published data only}
- Laat EH, Boogaard MH, Spauwen PH, Kuppevelt DH, Goor H, Schoonhoven L. Faster wound healing with topical negative pressure therapy in difficult‐to‐heal wounds: a prospective randomized controlled trial. Annals of Plastic Surgery 2011;67(6):626‐31. [DOI] [PubMed] [Google Scholar]
Della Marchina 1997 {published data only}
- Della Marchina M, Renzi G. A new antiseptic preparation used for the disinfection of cutaneous distrophic ulcers. Chronica dermatologica 1997;7(6):873‐85. [Google Scholar]
Gerding 1992 {published data only}
- Gerding GA, Browning JS. Oxyquinolone‐containing ointment versus standard therapy for stage I and stage II skin lesions. Dermatology Nursing 1992;4(5):389‐98. [PubMed] [Google Scholar]
Gorse 1987 {published data only}
- Gorse GJ, Messner RL. Improved pressure sore healing with hydrocolloid dressings. Archives of Dermatology 1987;123(6):766‐71. [PubMed] [Google Scholar]
Hartman 2002 {published data only}
- Hartman D, Coetzee JC. Two US practitioners' experience of using essential oils for wound care. Journal of Wound Care 2002;11(8):317‐20. [DOI] [PubMed] [Google Scholar]
Helaly 1988 {published data only}
- Helaly P, Vogt E, Schneider G, Ballanzin D, Balmer A, Benninger R, et al. Wound healing disorders and their enzymatic therapy: a multicenter double‐blind study. Schweizerische Rundschau für Medizin Praxis 1988;77(52):1428‐34. [PubMed] [Google Scholar]
Ishibashi 1996 {published data only}
- Ishibashi Y, Soeda S, Oura T, Nimura M, Nakajima H, Mizoguchi M, et al. Clinical effects of KCB‐1, a solution of recombinant human basic fibroblast growth factor, on skin ulcers. A phase III study comparing with sugar and povidone iodine ointment. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicine) 1996;12(10):2159‐87. [Google Scholar]
Itani 2010 {published data only}
- Itani KM, Dryden MS, Bhattacharyya H, Kunkel MJ, Baruch AM, Weigelt JA. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft‐tissue infections proven to be caused by methicillin‐resistant Staphylococcus aureus. American Journal of Surgery 2010;199(6):804‐16. [DOI] [PubMed] [Google Scholar]
Kim 1996 {published data only}
- Kim YC, Shin JC, Park CI, Oh SH, Choi SM, Kim YS. Efficacy of hydrocolloid occlusive dressing technique in decubitus ulcer treatment: a comparative study. Yonsei Medical Journal 1996;37(3):181‐5. [DOI] [PubMed] [Google Scholar]
Konychev 2013 {published data only}
- Konychev A, Heep M, Moritz R, Kreuter A, Shulutko A, Fierlbeck G, et al. Safety and efficacy of daptomycin as first‐line treatment for complicated skin and soft tissue infections in elderly patients: an open‐label, multicentre, randomized phase IIIb trial. Drugs and Aging 2013;30(10):829‐36. [DOI] [PubMed] [Google Scholar]
Kuroyanagi 1995 {published data only}
- Kuroyanagi Y, Shioya N, Nakakita N, Nakamura M, Takahashi H, Yasutomi Y, et al. Development of a new wound dressing composed of silver sulfadiazine‐impregnated polyurethane membrane laminated with a non‐woven fabric: multi‐centre's clinical reports. Japanese Pharmacology and Therapeutics 1995;23(10):399‐408. [Google Scholar]
Lazareth 2012 {published data only}
- Lazareth I, Meaume S, Sigal‐Grinberg ML, Combemale P, Guyadec T, Zagnoli A. Efficacy of a silver lipidocolloid dressing on heavily colonised wounds: a republished RCT. Journal of Wound Care 2012;21(2):96‐102. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only (unpublished sought but not used)}
- Lee RLP, Leung PHM, Wong TKS. A randomized controlled trial of topical tea tree preparation for MRSA colonized wounds. International Journal of Nursing Sciences 2014;1(1):7‐14. [Google Scholar]
LeVasseur 1991 {published data only}
- LeVasseur SA, Helme RD. A double‐blind clinical trial to compare the efficacy of an active based cream F14001 against a placebo non‐active based cream for the treatment of pressure ulcers in a population of elderly subjects. Journal of Advanced Nursing 1991;16(8):952‐6. [DOI] [PubMed] [Google Scholar]
Meaume 2005 {published data only}
- Meaume S, Vallet D, Morere MN, Teot L. Evaluation of a silver‐releasing hydroalginate dressing in chronic wounds with signs of local infection. Journal of Wound Care 2005;14(9):411‐9 (33 ref). [DOI] [PubMed] [Google Scholar]
- Meaume S, Vallet D, Morere MN, Teot L. Evaluation of a silver‐releasing hydroalginate dressing in chronic wounds with signs of local infection. Zeitschrift fur wundheilung 2006;11(5):236‐45. [DOI] [PubMed] [Google Scholar]
Motta 2004 {published data only}
- Motta GJ, Milne CT, Corbett LQ. Impact of antimicrobial gauze on bacterial colonies in wounds that require packing. Ostomy Wound Management 2004;50(8):48‐62. [PubMed] [Google Scholar]
Munter 2006 {published data only}
- Munter K‐C, Beele H, Russell L, Crespi A, Grochenig E, Basse P, et al. Effect of a sustained silver releasing dressing on ulcers with delayed healing: the CONTOP study. Journal of Wound Care 2006;15(5):199‐206. [DOI] [PubMed] [Google Scholar]
- Scalise A, Forma O, Happe M, Hahn TW. The CONTOP study: real life experiences from an international study comparing a silver containing hydro‐activated foam dressing with standard wound care. Teamwork in wound treatment: the art of healing. Proceedings of the 13th Conference of the European Wound Management Association 2003;212:Poster 81. [Google Scholar]
Parish 1984a {published data only}
- Parish LC, Parks DB, Layne PP, Uri JV. Ceftizoxime treatment of cutaneous and subcutaneous tissue infections. Clinical Therapeutics 1984;6(5):613‐9. [PubMed] [Google Scholar]
Parish 1984b {published data only}
- Parish LC, Witkowski JA. Ceforanide compared with cefazolin in skin and soft tissue infections. Cutis: Cutaneous Medicine for the Practitioner 1984;33(3):313‐5. [PubMed] [Google Scholar]
Rhodes 2001 {published data only}
- Rhodes RS, Heyneman CA, Culbertson VL, Wilson SE, Phatak HM. Topical phenytoin treatment of stage II decubitus ulcers in the elderly. Annals of pharmacotherapy 2001;35(6):675‐81. [DOI] [PubMed] [Google Scholar]
Robson 1999 {published data only}
- Robson MC, Mannari RJ, Smith PD, Payne WG. Maintenance of wound bacterial balance. American Journal of Surgery 1999;178(5):399‐402. [DOI] [PubMed] [Google Scholar]
Romanelli 2008 {published data only}
- Romanelli M, Dimi V, Bertone MS, Mazzatenta C, Martini P. Efficacy and tolerability of "Fitostimoline antibiotico" soaked gauzes in the topical treatment of cutaneous sores, ulcers and burns, complicated with bacterial contamination: an open‐label, controlled, randomised, multicentre, parallel group. Gazzetta Medica Italiana Archivio per le Scienze Mediche 2008;167(6):251‐60. [Google Scholar]
Saha 2012 {published data only}
- Saha A, Chattopadhyay S, Azam Md, Sur P. The role of honey in healing of bedsores in cancer patients. South Asian Journal of Cancer 2012;1(2):66‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saydak 1990 {published data only}
- Saydak SJ. A pilot test of two methods for the treatment of pressure ulcers. Journal of Enterostomal Therapy 1990;17(3):140‐2. [DOI] [PubMed] [Google Scholar]
Serra 2005 {published data only}
- Serra N, Torres OG, Romo MI, Llovera JM, Vigil Escalera LJ, Soto MA, et al. Hydro‐colloidal dressings which release hydro‐active silver. Revista de Enfermeria 2005;28(2):13‐8. [PubMed] [Google Scholar]
Shrivastava 2011 {published data only}
- Shrivastava R. Clinical evidence to demonstrate that simultaneous growth of epithelial and fibroblast cells is essential for deep wound healing. Diabetes Research and Clinical Practice 2011;92(1):92‐9. [DOI] [PubMed] [Google Scholar]
Sibbald 2011 {published data only}
- Sibbald RG, Coutts P, Woo KY. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing‐clinical trial results. Advances in Skin and Wound Care 2011;24(2):78‐84. [DOI] [PubMed] [Google Scholar]
Stevens 2002 {published data only}
- Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin‐resistant Staphylococcus aureus infections. Clinical Infectious Diseases 2002;34(11):1481‐90. [DOI] [PubMed] [Google Scholar]
Thomas 1998 {published data only}
- Thomas DR, Goode PS, LaMaster K, Tennyson T. Acemannan hydrogel dressing versus saline dressing for pressure ulcers. A randomized, controlled trial. Advances in Wound Care 1998;11(6):273‐6. [PubMed] [Google Scholar]
Trial 2010 {published data only}
- Teot L, Trial C, Lavigne JP. Results of RCT on the antimicrobial effectiveness of a new silver alginate wound dressing. EWMA Journal 2008;8(Supp 2):54: Abstract 69. [DOI] [PubMed] [Google Scholar]
- Trial C, Darbas H, Lavigne JP, Sotto A, Simoneau G, Tillet Y, et al. Assessment of the antimicrobial effectiveness of a new silver alginate wound dressing: a RCT. Journal of Wound Care 2010;19(1):20‐6. [DOI] [PubMed] [Google Scholar]
van der Cammen 1987 {published data only}
- Cammen TJ, O'Callaghan U, Whitefield M. Prevention of pressure sores. A comparison of new and old pressure sore treatments. British Journal of Clinical Practice 1987;41(11):1009‐11. [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang C, Huang S, Zhu T, Sun X, Zou Y, Wang Y. Efficacy of photodynamic antimicrobial therapy for wound flora and wound healing of pressure sore with pathogen infection. National Medical Journal of China 2014;94(31):2455‐9. [PubMed] [Google Scholar]
Weheida 1991 {published data only}
- Weheida SM, Nagubib HH, El‐Banna HM, Marzouk S. Comparing the effects of two dressing techniques on healing of low grade pressure ulcers. Journal of the Medical Research Institute 1991;12(2):259‐78. [Google Scholar]
Worsley 1991 {published data only}
- Worsley M. Comparing efficacies: Hydrocolloid dressing (Comfeel ulcer dressing) vs non‐adherent dressing (Betadine + Melonin dressing). Nursing Standard 1991;5(Supp 25 Tissue Viability):5‐6. [Google Scholar]
Yastrub 2005 {published data only}
- Yastrub DJ. Relationship between type of treatment and degree of wound healing among institutionalized geriatric patients with stage II pressure ulcers. Clinical Excellence for Nurse Practitioners 2005;9(2):89‐94. [DOI] [PubMed] [Google Scholar]
Yura 1984 {published data only}
- Yura J, Ando M, Ishikawa S, Ōhashi M, Ishigaki M, Ueda H, et al. Clinical evaluation of silver sulfadiazine in the treatment of decubitus ulcer or chronic dermal ulcers. Double‐blind study comparing to placebo. Chemotherapy (Tokyo) 1984;32(4):208‐22. [Google Scholar]
Zeron 2007 {published data only}
- Zeron HM, Krotzsch Gomez FE, Hernandez Munoz RE. Pressure ulcers: a pilot study for treatment with collagen polyvinylpyrrolidone. International Journal of Dermatology 2007;46(3):314‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bigolari 1991 {published data only}
- Bigolari M, Mosca P, Astengo F, Biraghi M, Balestreri R. Randomized clinical trial with Cadexomer iodine in decubitus ulcers [Studio clinico randomizzato con cadexomero iodico nelle ulcere da decubito]. Gazzetta Medica Italiana Archivio per le Scienze Mediche 1991;150(5):177‐85. [Google Scholar]
Goldmeier 1997 {published data only}
- Goldmeier S. The use of CTM with essential fatty acid in decubitus ulcers of cardiac patients. Revista Paulista de Enfermagem 1997;16(1):30‐34. [Google Scholar]
Additional references
Allman 1997
- Allman RM. Pressure ulcer prevalence, incidence, risk factors, and impact. Clinical Geriatric Medicine 1997;13(3):421‐36. [PubMed] [Google Scholar]
Allman 1999
- Allman RM, Goode PS, Burst N, Bartolucci AA, Thomas DR. Pressure ulcers, hospital complications, and disease severity: impact on hospital costs and length of stay. Advances in Wound Care 1999;12:22‐30. [PubMed] [Google Scholar]
AWMA 2011
- Australian Wound Management Association (AWMA). Bacterial impact on wound healing: from contamination to infection. Version 1.5. http://www.awma.com.au/publications/2011_bacterial_impact_position_1.5.pdf (accessed 22 March 2016).
Bennett 2004
- Bennett G, Dealey C, Posnett J. The cost of pressure ulcers in the UK. Age and Ageing 2004;33(3):230‐5. [DOI] [PubMed] [Google Scholar]
Bergstrom 1998
- Bergstrom N, Braden B, Champagne M, Kemp M, Ruby E. Predicting pressure ulcer risk: a multisite study of the predictive validity of the Braden Scale. Nursing Research 1998;47(5):261‐9. [DOI] [PubMed] [Google Scholar]
Berlowitz 1990
- Berlowitz DR, Wilikin SVB. Risk factors for pressure sores: a comparison of cross‐sectional and cohort derived data. Journal of the American Geriatrics Society 1990;37(11):1043‐50. [DOI] [PubMed] [Google Scholar]
BNF 2013
- Royal Pharmaceutical Society of Great Britain, British Medical Association. British National Formulary 66. London: British Medical Association, 2013. [Google Scholar]
Bonita 2006
- Bonita R, Beaglehole R, Kjellstrom T. Basic Epidemiology. Second Edition. Geneva: World Health Organisation, 2006. [Google Scholar]
Bowler 2001
- Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clinical Microbiology Reviews 2001;14(2):244‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowler 2003
- Bowler PG. The 105 bacterial growth guideline: reassessing its clinical relevance in wound healing. Ostomy Wound Management 2003;49(1):44‐53. [PubMed] [Google Scholar]
Braga 2013
- Braga IA, Pirett CC, Ribas RM, Gontijo Filho PP, Dioggio Filho A. Bacterial colonization of pressure ulcers: assessment of risk for bloodstream infection and impact on patient outcomes. Journal of Hospital Infection 2013;83(4):314‐20. [DOI] [PubMed] [Google Scholar]
Brandeis 1994
- Brandeis GH, Ooi WL, Hossain M, Morris JN, Lipsitz LA. A longitudinal study of risk factors associated with the formation of pressure ulcers in nursing homes. Journal of the American Geriatrics Society 1994;42:388‐93. [DOI] [PubMed] [Google Scholar]
Carville 2008
- Carville K, Cuddigan J, Fletcher J, Fuchs P, Harding K, Ishikawa O, et al. Wound infection in clinical practice: shaping the future. An International Consensus Document. International Wound Journal 2008;5(S3):1‐11. [Google Scholar]
Cataldo 2011
- Cataldo MC, Bonura C, Caputo G, Aleo A, Rizzo G, Geraci D, et al. Colonization of pressure ulcers by multidrug‐resistant microorganisms in patients receiving home care. Scandinavian Journal of Infectious Diseases 2011;43:947‐52. [DOI] [PubMed] [Google Scholar]
CDC 2008
- Centers for Disease Control and Prevention (CDC), Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infection Control 2008;36(5):309‐32. [DOI] [PubMed] [Google Scholar]
Coleman 2013
- Coleman S, Gorecki C, Nelson EA, Defloor T, Halfens R, Farrin A, et al. Patient risk factors for pressure ulcer development: systematic review. International Journal of Nursing Studies 2013;50(7):974‐1003. [DOI] [PubMed] [Google Scholar]
Cutting 2004
- Cutting KF, White R. Defined and refined: criteria for identifying wound infection revisited. British Journal of Community Nursing 2004;9(3):S6‐S15. [DOI] [PubMed] [Google Scholar]
Davies 2007
- Davies CE, Hill KE, Newcombe RG, Stephens P, Wilson MJ, Harding KG, et al. A prospective study of the microbiology of chronic venous leg ulcers to reevaluate the clinical predictive value of tissue biopsies and swabs. Wound Repair and Regeneration 2007;15(1):17‐22. [DOI] [PubMed] [Google Scholar]
Dealey 2012
- Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. Journal of Wound Care 2012;21:261‐2, 264, 266. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dowd 2008
- Dowd SE, Sun Y, Secor PR, Rhoads DD, Wolcott BM, James GA, et al. Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiology 2008;8:43. [DOI: 10.1186/1471-2180-8-43] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumville 2015a
- Dumville JC, Keogh SJ, Liu Z, Stubbs N, Walker RM, Fortnam M. Alginate dressings for treating pressure ulcers. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011277] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumville 2015b
- Dumville JC, Stubbs N, Keogh SJ, Walker RM, Liu Z. Hydrogel dressings for treating pressure ulcers. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011226] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2004
- Edwards R, Harding KG. Bacteria and wound healing. Current Opinion in Infectious Disease 2004;17:91‐6. [DOI] [PubMed] [Google Scholar]
Ellis 2003
- Ellis SL, Finn P, Noone M, Leaper DJ. Eradication of methicillin‐resistant Staphylococcus aureus from pressure sores using warming therapy. Surgical Infections 2003;4(1):53‐5. [DOI] [PubMed] [Google Scholar]
EPUAP‐NPUAP‐PPPIA 2014
- European Pressure Ulcer Advisory Pane (EPUAP), lNational Pressure Ulcer Advisory Panel (NPUAP), and Pan Pacific Pressure Injury Alliance (PPPIA), Haesler E. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Second Edition. Perth, Australia: Cambridge Media, 2014. [Google Scholar]
Essex 2009
- Essex HN, Clark M, Sims J, Warriner A, Cullum N. Health‐related quality of life in hospital inpatients with pressure ulceration: assessment using generic health‐related quality of life measures. Wound Repair and Regeneration 2009;17:797‐805. [DOI] [PubMed] [Google Scholar]
EuroQoL Group 1990
- EuroQuoL Group. EuroQoL: a new facility for the measurement of health‐related quality of life. Health Policy 1990;16:199‐208. [DOI] [PubMed] [Google Scholar]
Gardner 2001
- Gardner SE, Franz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair and Regeneration 2001;9(3):178‐86. [DOI] [PubMed] [Google Scholar]
Garibaldi 1981
- Garibaldi RA, Brodine S, Matsumiya S. Infections among patients in nursing homes: policies, prevalence, problems. New England Journal of Medicine 1981;305:731‐5. [DOI] [PubMed] [Google Scholar]
Gebhardt 2002
- Gebhardt K. Pressure ulcer prevention. Part 1. Causes of pressure ulcers. Nursing Times 2002;98(11):41‐4. [PubMed] [Google Scholar]
Gefen 2014
- Gefen A. Tissue changes in patients following spinal cord injury and implications for wheelchair cushions and tissue loading: a literature review. Ostomy Wound Management 2014;60(2):34‐45. [PubMed] [Google Scholar]
Gorecki 2009
- Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. Journal of the American Geriatrics Society 2009;57(7):1175‐83. [DOI] [PubMed] [Google Scholar]
Graves 2005
- Graves N, Birrell F, Whitby M. Modelling the economic losses from pressure ulcers among hospitalized patients in Australia. Wound Repair and Regeneration 2005;13(5):462‐7. [DOI] [PubMed] [Google Scholar]
Hahn 2005
- Hahn S, Puffer S, Torgerson DJ, Watson J. Methodological bias in cluster randomised trials. BMC Medical Research Methodology 2005;5:10. [DOI: 10.1186/1471-2288-5-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halbert 1992
- Halbert AR, Stacey MC, Rohr JB, Jopp‐McKay A. The effect of bacterial colonization on venous leg ulcer healing. American Journal of Dermatology 1992;33(2):75‐80. [DOI] [PubMed] [Google Scholar]
Hall 2014
- Hall J, Buckley HL, Lamb KA, Stubbs N, Saramago P, Dumville JC, et al. Point prevalence of complex wounds in a defined United Kingdom population. Wound Repair and Regeneration 2014;22(6):694‐700. [DOI] [PubMed] [Google Scholar]
Hansson 1995
- Hansson C, Hoborn J, Moller A, Swanbeck G. The microbial flora in venous leg ulcers without clinical signs of infection. Acta Dermato‐venerologica 1995;75(1):24‐30. [DOI] [PubMed] [Google Scholar]
Heym 2004
- Heym B, Rimareix F, Lortat‐Jacob A, Nicolas‐Chanoine M‐H. Bacteriological investigation of infected pressure ulcers in spinal cord‐injured patients and impact on antibiotic therapy. Spinal Cord 2004;42(4):230‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne, JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howell‐Jones 2005
- Howell‐Hones RS, Wilson MJ, Hill KE, Price PE, Thomas DW. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. Journal of Antimicrobial Chemotherapy 2005;55(2):143‐9. [DOI] [PubMed] [Google Scholar]
Howell‐Jones 2006
- Howell‐Jones RS, Price PE, Howard AJ, Thomas DW. Antibiotic prescribing for chronic skin wounds in primary care. Wound Repair and Regeneration 2006;14(4):387‐93. [DOI] [PubMed] [Google Scholar]
Jull 2013
- Jull AB, Walker N, Deshpande S. Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD005083.pub3] [DOI] [PubMed] [Google Scholar]
Kingsley 2004
- Kingsley A, White R, Gray D. The wound infection continuum: a revised perspective. APW Supplement. Wounds UK 2004;1(1):13‐8. [Google Scholar]
Kontopantelis 2012
- Kontopantelis E, Reeves D. Performance of statistical methods for meta‐analysis when true study effects are non‐normally distributed: a simulation study. Statistical Methods in Medical Research 2012;21(4):409‐26. [DOI] [PubMed] [Google Scholar]
Kontopantelis 2013
- Kontopantelis E, Springate DA, Reeves D. A re‐analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta‐analyses. PLoS One 2013;26:e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loerakker 2010
- Loerakker S, Stekelenburg A, Strijkers GJ, Rijpkema JJM, Baaijens FPT, Bader DL, et al. Temporal effects of mechanical loading on deformation‐induced damage in skeletal muscle tissue. Annals of Biomedical Engineering 2010;38(8):2577‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macpherson 2004
- Macpherson G (editor). Black's Student Medical Dictionary. London: A&C Black Publishers Limited, 2004. [Google Scholar]
Madsen 1996
- Madsen SM, Westh H, Danielsen L, Rosdahl VT. Bacterial colonisation and healing of venous leg ulcers. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica 1996;104(2):895‐9. [DOI] [PubMed] [Google Scholar]
McGinnis 2014
- McGinnis E, Greenwood DC, Nelson EA, Nixon J. A prospective cohort study of prognostic factors for the healing of heel pressure ulcers. Age and Aging 2014;43(2):267‐71. [DOI] [PubMed] [Google Scholar]
McInnes 2011
- McInnes E, Dumville JC, Jammali‐Blasi A, Bell‐Syer SE. Support surfaces for treating pressure ulcers. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009490] [DOI] [PubMed] [Google Scholar]
MeReC 2010
- MeReC, National Prescribing Centre. Evidence‐based prescribing of advanced wound dressings for chronic wounds in primary care. MeReC Bulletin 2010; Vol. 21, issue 01.
Moore 2010
- Moore K, Hall V, Paull A, Morris T, Brown S, McCullock D, et al. Surface bacteriology of venous leg ulcers and healing outcome. Journal of Clinical Pathology 2010;63(9):830‐4. [DOI] [PubMed] [Google Scholar]
Moore 2013a
- Moore Z, Johansen E, Etten M. A review of PU prevalence and incidence across Scandinavia, Iceland and Ireland (Part I). Journal of Wound Care 2013;22(7):1‐7. [DOI] [PubMed] [Google Scholar]
Moore 2013b
- Moore ZEH, Cowman S. Wound cleansing for pressure ulcers. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD004983.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHS 2014
- National Health Service (NHS). National Safety Thermometer Data. http://www.safetythermometer.nhs.uk/index.php?option=com_dashboards&view=classic&Itemid=126 (accessed 4 March 2014).
NICE 2014
- National Institute of Health and Clinical Excellence (NICE). Pressure ulcers: prevention and management of pressure ulcers: clinical guideline 179. http://www.nice.org.uk/guidance/cg179/resources/guidance‐pressure‐ulcers‐prevention‐and‐management‐of‐pressure‐ulcers‐pdf (accessed 22 March 2016).
Nicolle 1994
- Nicolle LE, Orr P, Duckworth H, Brunka J, Kennedy J, Urias B, et al. Prospective study of decubitus ulcers in two long term care facilities. Canadian Journal of Infection Control 1994;9(2):35‐8. [PubMed] [Google Scholar]
O'Meara 2001
- O'Meara S, Cullum NA, Majid M, Sheldon TA. Systematic review of antimicrobial agents used for chronic wounds. British Journal of Surgery 2011;88(1):4‐21. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Percival 2004
- Percival SL, Bowler PG. Biofilms and their potential role in wound healing. Wounds 2004;16(7):234‐40. [Google Scholar]
Power 2012
- Power M, Stewart K, Brotherton A. What is the NHS Safety Thermometer?. Clinical Risk 2012;18(5):163‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prentice 2001
- Prentice JL, Stacey MC. Pressure ulcers: the case for improving prevention and management in Australian health care settings. Primary Intention 2001;9(3):111‐20. [Google Scholar]
Price 2004
- Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition‐specific questionnaire to assess health‐related quality of life in patients with chronic wounds of the lower limb. International Wound Journal 2004;1(1):10‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raghaven 2003
- Raghaven P, Raza WA, Ahmed YS, Chamberlain MA. Prevalence of pressure sores in a community sample of spinal injury patients. Rehabilitation 2003;17(8):879‐84. [DOI] [PubMed] [Google Scholar]
Reddy 2008
- Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA 2008;300:2647‐62. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan) 5.3. Version 5.3. Copenhagen: The Nordic Cochrane Centre, 2014.
Russo 2008
- Russo A, Steiner C, Spector W, Healthcare Cost and Utilization Project (HCUP). Hospitalizations related to pressure ulcers among adults 18 years and older, 2006. http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb64.jsp. Acessed 6th June 2014 (accessed 6 June 2014). [PubMed]
Sanada 2008
- Sanada H, Miyachi Y, Ohura T, Moriguchi T, Tokunaga K, Shido K, et al. The Japanese pressure ulcer surveillance study: a retrospective cohort study to determine prevalence of pressure ulcers in Japan. Wounds 2008;20(7):20‐6. [PubMed] [Google Scholar]
Sapico 1986
- Sapico FL, Ginunas VJ, Thornhill‐Jones M, Canawati HN, Klein NE, Khawam S, et al. Quantitative microbiology of pressure sores in different stages of healing. Diagnostic Microbiology and Infectious Disease 1986;5(1):31‐8. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Medicine 2010;7(3):e10000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
SIGN 2015
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 22 March 2016).
Smith 2010
- Smith DM, Snow DE, Rees E, Zischkau Am, Hanson JD, Wolcott RD, et al. Evaluation of the bacterial diversity of pressure ulcers using bTEFAP pyrosequencing. BMC Medical Genomics 2010;3:41. [DOI: 10.1186/1755-8794-3-41] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013
- Smith ME, Totten A, Hickam DH, Fu R, Wasson N, Rahman B, et al. Pressure ulcer treatment strategies: a systematic comparative effectiveness review. Annals of Internal Medicine 2013;159(1):39‐50. [DOI] [PubMed] [Google Scholar]
Sotto 2012
- Sotto A, Richard J‐L, Messad N, Molinari N, Jourdan N, Schuldiner S, et al. Distinguishing colonization from infection with Staphylococcus aureus in diabetic foot ulcers with miniaturized oligonucleotide arrays: a French multicenter study. Diabetes Care 2012;35(3):617‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stevenson 2013
- Stevenson R, Collinson M, Henderson V, Wilson L, Dealey C, McGinnis E, et al. The prevalence of pressure ulcers in community settings: an observational study. International Journal of Nursing Studies 2013;50(11):1550‐7. [DOI] [PubMed] [Google Scholar]
Storm‐Versloot 2010
- Storm‐Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD006478.pub2] [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. [DOI] [PubMed] [Google Scholar]
Thompson 2002
- Thompson SG, Higgins JPT. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐74. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;7(8):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trengove 1996
- Trengove NJ, Stacey MC, McGechie DF, Stingemore NF, Mata S. Qualitative bacteriology and leg ulcer healing. Journal of Wound Care 1996;5(6):277‐80. [DOI] [PubMed] [Google Scholar]
Vanderwee 2007
- Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. Journal of Evaluation in Clinical Practice 2007;13(2):227‐35. [DOI] [PubMed] [Google Scholar]
VanGilder 2009
- VanGilder C, Amlung S, Harrison P, Meyer S. Results of the 2008‐2009 International Pressure Ulcer Prevalence Survey and a 3‐year, acute care, unit‐specific analysis. Ostomy Wound Management 2009;55(11):39‐45. [PubMed] [Google Scholar]
Vermuelen 2007
- Vermeulen H, Hattem JM, Storm‐Versloot MN, Ubbink DT, Westerbos SJ. Topical silver for treating infected wounds. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005486.pub2] [DOI] [PubMed] [Google Scholar]
Vermuelen 2010
- Vermeulen H, Westerbos SJ, Ubbink DT. Benefit and harm of iodine in wound care: a systematic review. Journal of Hospital Infection 2010;76(3):191‐9. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Wolcott 2008
- Wolcott RD, Rhoads DD, Dowd SE. Biofilms and chronic wound inflammation. Journal of Wound Care 2008;17(8):333‐41. [DOI] [PubMed] [Google Scholar]
WUWHS 2008
- World Union of Wound Healing Societies (WUWHS). Principles of Best Practice. Wound infection in clinical practice: An international consensus. Wound infection in clinical practice. An international consensus. London: MEP Ltd, 2008. [Google Scholar]
Zhao 2010
- Zhao G, Hiltabidel E, Liu Y, Chen L, Liao Y. A cross‐sectional descriptive study of pressure ulcer prevalence in a teaching hospital in China. Ostomy Wound Management 2010;56(3):38‐42. [PubMed] [Google Scholar]


