Abstract
Background
By reducing the amount of nicotine that reaches the brain when a person smokes a cigarette, nicotine vaccines may help people to stop smoking or to prevent recent quitters from relapsing.
Objectives
The aims of this review are to assess the efficacy of nicotine vaccines for smoking cessation and for relapse prevention, and to assess the frequency and type of adverse events associated with the use of nicotine vaccines.
Search methods
We searched the Cochrane Tobacco Addiction Review Group specialised register for trials, using the term 'vaccine' in the title or abstract, or in a keyword (date of most recent search April 2012). To identify any other material including reviews and papers potentially relevant to the background or discussion sections, we also searched MEDLINE, EMBASE, and PsycINFO, combining terms for nicotine vaccines with terms for smoking and tobacco use, without design limits or limits for human subjects. We searched the Annual Meeting abstracts of the Society for Research on Nicotine and Tobacco up to 2012, using the search string 'vaccin'. We searched Google Scholar for 'nicotine vaccine'. We also searched company websites and Google for information related to specific vaccines. We searched clinicaltrials.gov in March 2012 for 'nicotine vaccine' and for the trade names of known vaccine candidates.
Selection criteria
We included randomized controlled trials of nicotine vaccines, at Phase II and Phase III trial stage and beyond, in adult smokers or recent ex‐smokers. We included studies of nicotine vaccines used as part of smoking cessation or relapse prevention interventions.
Data collection and analysis
We extracted data on the type of participants, the dose and duration of treatment, the outcome measures, the randomization procedure, concealment of allocation, blinding of participants and personnel, reporting of outcomes, and completeness of follow‐up.
Our primary outcome measure was a minimum of six months abstinence from smoking. We used the most rigorous definition of abstinence, and preferred cessation rates at 12 months and biochemically validated rates where available. We have used the risk ratio (RR) to summarize individual trial outcomes. We have not pooled the current group of included studies as they cover different vaccines and variable regimens.
Main results
There are no nicotine vaccines currently licensed for public use, but there are a number in development. We found four trials which met our inclusion criteria, three comparing NicVAX to placebo and one comparing NIC002 (formerly NicQbeta) to placebo. All were smoking cessation trials conducted by pharmaceutical companies as part of the drug development process, and all trials were judged to be at high or unclear risk of bias in at least one domain. Overall, 2642 smokers participated in the included studies in this review. None of the four included studies detected a statistically significant difference in long‐term cessation between participants receiving vaccine and those receiving placebo. The RR for 12 month cessation in active and placebo groups was 1.35 (95% Confidence Interval (CI) 0.82 to 2.22) in the trial of NIC002 and 1.74 (95% CI 0.73 to 4.18) in one NicVAX trial. Two Phase III NicVAX trials, for which full results were not available, reported similar quit rates of approximately 11% in both groups. In the two studies with full results available, post hoc analyses detected higher cessation rates in participants with higher levels of nicotine antibodies, but these findings are not readily generalisable. The two studies with full results showed nicotine vaccines to be well tolerated, with the majority of adverse events classified as mild or moderate. In the study of NIC002, participants receiving the vaccine were more likely to report mild to moderate adverse events, most commonly flu‐like symptoms, whereas in the study of NicVAX there was no significant difference between the two arms. Information on adverse events was not available for the large Phase III trials of NicVAX.
Vaccine candidates are likely to undergo significant changes before becoming available to the general public, and those included in this review may not be the first to reach market; this limits the external validity of the results reported in this review in terms of both effectiveness and tolerability.
Authors' conclusions
There is currently no evidence that nicotine vaccines enhance long‐term smoking cessation. Rates of serious adverse events recorded in the two trials with full data available were low, and the majority of adverse events reported were at mild to moderate levels. The evidence available suggests nicotine vaccines do not induce compensatory smoking or affect withdrawal symptoms. No nicotine vaccines are currently licensed for use in any country but a number are under development.
Further trials of nicotine vaccines are needed, comparing vaccines with placebo for smoking cessation. Further trials are also needed to explore the potential of nicotine vaccines to prevent relapse. Results from past, current and future research should be reported in full. Adverse events and serious adverse events should continue to be carefully monitored and thoroughly reported.
Keywords: Humans, Randomized Controlled Trials as Topic, Secondary Prevention, Smoking, Smoking/immunology, Smoking Cessation, Smoking Cessation/methods, Smoking Prevention, Vaccines, Vaccines/adverse effects, Vaccines/therapeutic use
Plain language summary
Can nicotine vaccines help people stop smoking or help stop recent quitters from relapsing?
Nicotine is the main addictive component in tobacco. When a person smokes a cigarette, nicotine causes chemicals in the brain to be released, which gives a feeling of reward to the smoker. This reward is part of the reason why people keep smoking. Nicotine vaccines are designed to work by reducing the effects of nicotine on the brain, meaning the smoker will feel less of a reward when they smoke a cigarette. By reducing the pleasure felt when smoking, vaccines may help smokers to stop smoking or help stop recent quitters from starting to smoke again.
There are no nicotine vaccines currently licensed for public use, but there are a number in development. We found four trials (2642 participants) comparing nicotine vaccines to a placebo. These did not show that vaccines help people to stop smoking in the long term. All four trials were conducted by pharmaceutical companies as part of the drug development process and involved vaccines administered by injection. There were no trials testing whether nicotine vaccines helped keep people who had stopped smoking from starting to smoke again. Only two of the four trials had full results available. The two trials showed nicotine vaccines to be generally safe, with most side effects being mild or moderate. In one trial, flu‐like symptoms were found to be a side effect of the nicotine vaccine. If nicotine vaccines become available to the general public they may have changed from the ones tested in these studies, meaning the results reported in this review, including those on side effects, may not apply to all nicotine vaccines.
Background
Tobacco is the leading preventable cause of death in the world, estimated to cause nearly six million deaths a year. A continuation of the current trend would result in more than eight million deaths a year by 2030, with 80% of these premature tobacco‐related deaths occurring in low‐ and middle‐income nations (WHO 2011). It has been projected that with a progressive 50% reduction in uptake and consumption rates of tobacco, as many as 200 million lives could be saved by the year 2050 (WHO 2006). A number of behavioural and pharmacological therapies are widely available to assist with smoking cessation, and have been shown to improve the chances of becoming smoke‐free. Among licensed pharmacotherapies, all forms of nicotine replacement therapy (Stead 2008a) and bupropion (Hughes 2007) improve the chances of quitting by between 1½‐ to two‐fold, while evidence indicates that varenicline more than doubles the chances of quitting (Cahill 2012). Behavioural interventions, such as individual or group counselling (Lancaster 2005; Stead 2005), physician advice (Stead 2008b) and telephone counselling (Stead 2006), have also been shown to improve the chances of quitting by between 1⅓‐ and two‐fold. However, the absolute quit rates achieved by these methods remain relatively low, and there is still scope for novel methods to tackle the smoking epidemic. Immunological approaches to treating dependence on drugs such as heroin and cocaine have opened up new possibilities for the treatment of nicotine dependence (Haney 2004).
Nicotine is the primary addictive agent in tobacco (Stolerman 1995; Harvey 2004), and delivers its effects by stimulating the rapid release of dopamine in the part of the brain called the nucleus acumbens (Balfour 2004; Benowitz 2010; Fagerström 2005). This gives reward to the smoker, and positively reinforces the habitual use of tobacco (Laviolette 2004; Pentel 2004; Scherer 1999). Nicotine vaccines are designed to work by altering the pharmacokinetics of nicotine in the brain; the vaccine stimulates the immune system to generate nicotine‐specific antibodies. These elicited antibodies circulate in the blood stream, and bind to serum nicotine entering the body via the lungs through smoking. The resulting molecules are too large to cross the blood‐brain barrier (Cerny 2009). The binding of the antibody to nicotine is reversible, and nicotine does eventually reach the brain, but less quickly and at lower levels than occurs during normal smoking (Cerny 2009). In addition, studies on rats demonstrate that vaccination significantly lengthens nicotine terminal half‐life and slows elimination, which could reduce the rate of smoking by prolonging nicotine's effects from each cigarette (Keyler 1999; LeSage 2006; Siu 2007).
The linked combination of nicotine with a larger carrier protein or a virus‐like particle is known as a conjugate vaccine (Cerny 2009). The conjugate vaccine is administered as a series of injections, e.g. one shot every month for three or four months, aiming to gradually produce a serum level of antibodies for several months. Booster shots are required periodically to maintain the antibody level. Selecta Biosciences in the USA is investigating the linked combination of nicotine with a synthetic carrier (as opposed to a biological carrier), with a Phase I trial underway (Selecta 2011). An alternative regimen, so far tested only on animals, is passive immunization, which involves the injection of antibodies produced in vitro or in other animal species (Pentel 2000). This may confer the benefits of immediately effective levels of antibodies, and the ability to fine‐tune the dosage, but has the disadvantages of requiring frequent injections and of being much more expensive than active immunization (LeSage 2006). Nicotine vaccines delivered via mechanisms other than injection are also being investigated (Brozek 2004; NIDA 2011), including adeno‐associated virus gene transfer vectors, which are currently showing promise in animal testing (Hicks 2012).
At the time of writing, no nicotine vaccines have been approved for use by the general public. So far, four conjugate vaccines have been tested in Phase I and Phase II clinical trials (Caponnetto 2012):
NIC002 (formerly known as Nicotine‐Qβ or Nic‐Qβ), developed by Cytos Biotechnology in Switzerland, and now in collaboration with Novartis, using a virus‐like particle. Niccine, developed by Independent Pharmaceutica AB in Sweden, using tetanus taxoid. NicVAX, developed by Nabi Biopharmaceuticals in the USA, using pseudomonas exoprotein A. TA‐NIC, developed by Xenova and now in the portfolio of Celtic Pharma in the UK, using a recombinant cholera toxin B subunit.
There are theoretical concerns that prolonged blocking of nicotine by vaccination might induce compensatory smoking or precipitate withdrawal symptoms, leading smokers to smoke more. Compensatory smoking is a change in smoking behaviour in order to maintain nicotine levels, by smoking more cigarettes or taking more or deeper puffs (Scherer 1999); it could occur following vaccination if smokers try to compensate for the reduction in nicotine reaching the brain. Withdrawal symptoms might occur for the same reason, that nicotine is not reaching the brain at the normal levels. A potential benefit of the immunological approach is that targeting the nicotine molecule rather than central nervous system function should mean that there are minimal central nervous system side effects (Haney 2004).
The most obvious application of this therapy is likely to be relapse prevention, as reducing the reward from smoking a cigarette may discourage lapses, but currently it is mainly being tested for promotion as an aid to quitting. Its potential as a preventive measure for smoking uptake may be limited by practical and ethical considerations (Hall 2011; Hasman 2004).
Objectives
To review the efficacy of nicotine vaccines for smoking cessation and for relapse prevention. To assess the frequency and type of adverse events associated with the use of nicotine vaccines.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
We include studies with adult smokers or recent ex‐smokers.
Types of interventions
Nicotine vaccines, at Phase II and III trial stage and beyond. They may be used as part of smoking cessation or relapse prevention interventions.
Types of outcome measures
Our primary outcome measure is a minimum of six months' continuous abstinence. We have used the most rigorous sustained cessation rates in preference to point prevalence, with a preference for biochemical verification over self report of quitting and with a preference for cessation rates at 12 months as opposed to at six months, where possible. We also performed sensitivity analysis by vaccine responder status (level of antibodies). We consider participants lost to follow‐up to be continuing smokers. We have evaluated any adverse effects of treatment noted in the studies.
Search methods for identification of studies
We searched the Cochrane Tobacco Addiction Review Group specialised register for trials, using the term 'vaccine' in the title or abstract, or in a keyword. The date of the most recent search was April 2012. This register has been developed from electronic searching of CENTRAL, MEDLINE, EMBASE, PsycINFO and Web of Science, together with handsearching of specialist journals, conference proceedings and reference lists of previous trials and overviews. Details of search strategies and dates for each database are given in the Tobacco Addiction Group Module in the Cochrane Library. Records in the specialised register are reports of controlled trials. To identify any other papers including reviews and papers potentially relevant to the background or discussion sections, we also searched MEDLINE (via OVID, 1996 to March Week 3 2012), and In‐Process & Other Non‐Indexed Citations April 02, 2012, EMBASE (via OVID, 1996 to 2012 Week 13), and PsycINFO (via OVID, 1987 to March Week 4 2012), combining terms for nicotine vaccines with terms for smoking and tobacco use, without design limits or limits for human subjects. Searches were limited by date because research on nicotine vaccines is a recent development (see Appendix 1 for full strategy). We searched the Annual Meeting abstracts of the Society for Research on Nicotine and Tobacco (SRNT) up to 2012, using the search string 'vaccin'. We searched Google Scholar for 'nicotine vaccine'. We also searched company websites and Google for information related to specific vaccines, in particular press releases and company reports, and e‐mailed pharmaceutical companies and other contacts for further information where possible. We searched the website clinicaltrials.gov in March 2012 for 'nicotine vaccine' and for the trade names of known vaccine candidates.
Data collection and analysis
One author (KC) checked the abstracts for relevance, and attempted to acquire full trial reports. Two authors (KC and JHB) independently extracted study data and compared the findings. Any discrepancies were resolved by mutual consent. Reasons for the non‐inclusion of studies are given in the Characteristics of excluded studies table. Where available, the following information is recorded in the Characteristics of included studies table:
Country and setting
Aims
Study ID
Recruitment method
Definition of smoker used
Participant demographics (i.e. average age, sex, average cigarettes per day)
Intervention and control description (including dose, schedule, and vaccine type)
Outcomes, including length of follow‐up, definition of abstinence, and chemical validation of smoking cessation
Sources of funding
Proportion of participants with follow‐up data
Unless noted otherwise, quit rates are calculated based on numbers randomized to an intervention or control, and exclude any deaths or untraceable moves. We regard participants who dropped out or were lost to follow‐up as continuing to smoke. We have where possible conducted intention‐to‐treat analyses, i.e. all participants initially assigned to intervention or control are included in their original groups. Adverse events and deaths are noted in the Results section and details are reported in Appendix 2.
We have used the risk ratio (RR) to summarize individual trial outcomes ((number of events in intervention condition/ intervention denominator) / (number of events in control condition/control denominator)) with 95% confidence intervals. Where the event is defined as smoking cessation, an RR greater than one indicates that more people successfully quit in the treatment group than in the control group. We have not pooled the current group of included studies, as they cover different vaccines and variable regimens, using different molecules to which to bind the nicotine, and hence have been judged to be clinically heterogeneous. We have therefore confined the analyses to descriptive forest plots. Had it been appropriate to estimate a pooled effect size we would have used a Mantel‐Haenszel fixed‐effect model.
We have included the Tobacco Addiction Group glossary of tobacco‐specific terms in Appendix 3.
Assessment of risk of bias in included studies
Included studies were evaluated for risk of bias in accordance with Cochrane guidelines (Higgins 2011, Section 8.6) using risk of bias tables (see Characteristics of included studies). Studies were rated as being at high, low or unclear risk of bias in the following areas:
Selection bias (random sequence generation and allocation concealment)
Performance bias (blinding of participants and personnel)
Detection bias (blinding of outcome assessment)
Attrition bias (incomplete outcome data)
Reporting bias (selective reporting of outcomes)
Results
Description of studies
Results of the search
Our search of the specialised register retrieved 19 records, of which 13 reported eight studies which were sufficiently relevant to be assessed for inclusion or exclusion. We found one additional abstract reporting one of these studies from our search of SRNT meeting abstracts. The searches of bibliographic databases retrieved 285 records; of those not already identified from the register search, none was relevant for inclusion. We identified eight relevant trials not linked to a full trial publication from the clinicaltrials.gov website. We found additional information about unpublished and ongoing trials from Google, company reports and contact with trialists. We display a flow diagram of search results in Figure 1.
1.

PRISMA search flow diagram (excluding searches via Google, company websites, and contact with trialists)
Included studies
We found four trials which met our inclusion criteria, two of which had full results available (Cornuz 2008; Hatsukami 2011) and two of which have not currently made results publicly available (NCT00836199; NCT01102114). The studies covered a total of 2642 participants. All are studies of smoking cessation as opposed to relapse prevention. The two studies for which data were available (and hence included in analyses) covered 642 participants, 430 of whom received nicotine vaccines. Both studies with published results were Phase II, placebo‐controlled trials assessing the clinical efficacy, safety, tolerability and immunogenicity of nicotine vaccines as an aid to smoking cessation. Both studies with results not yet published were Phase III trials, also assessing clinical efficacy, safety, tolerability and immunogenicity. Despite efforts to contact Nabi Pharmaceuticals (NicVAX developers), we were unable to obtain data for NCT00836199 and NCT01102114 beyond what had been released on clinicaltrials.gov and what was included in Nabi press releases. We look forward to further information being released in due course. Each of the four studies is fully described in the Characteristics of included studies table.
Hatsukami 2011 evaluated NicVAX across nine sites in the USA. Three hundred and one smokers of at least 15 cigarettes per day were randomized either to placebo (alum injections, 100 participants) or to 200 or 400ɥg vaccine (201 participants). Within each group, participants were further randomized on a one‐to‐one ratio to two different treatment schedules of either four or five injections over 26 weeks, with a target quit date one week after the second injection. Participants received five face‐to‐face counselling sessions of approximately ten minutes each over the course of treatment. Relapsers were offered a second quit date up to week 18, with additional counselling sessions and two supportive phone calls. Prolonged abstinence, defined as not a single puff during the period from two weeks after the target quit date, was the strictest definition of abstinence provided and was reported at six and 12 months. Abstinence was also recorded from weeks 19 to 26 and from weeks 19 to 52 (not a single puff between the designated time points) and is used in subgroup analyses where data on prolonged abstinence are not available. Self report of smoking cessation was validated by exhaled carbon monoxide (CO) of fewer than 8 parts per million (ppm).
NCT00836199 and NCT01102114 were two trials of identical design also evaluating NicVAX, conducted at multiple sites across the USA. Each had 1000 participants and recruited generally healthy adults smoking at least 10 cigarettes per day and motivated to quit. It is unclear what proportions of participants were allocated to intervention and placebo groups. Intervention groups received six injections of NicVAX over the course of six months, with participants in the placebo group receiving injections on the same schedule. To better align with peak antibody levels as determined in previous studies, the target quit date was set at 14 weeks after the first injection. Nabi reports that dosage was based on a previous Phase II schedule optimisation immunogenicity study (Nabi 2008), and hence we assume that the dosage in these two studies was 400 µg. Behavioural counselling was provided to all participants. The primary outcome measure for both trials was abstinence at 12 months, confirmed by exhaled CO. Serum antibody levels were measured and data were collected on adverse events, withdrawal symptoms, cigarette consumption, smoking satisfaction, and nicotine dependence.
Whereas the other three included studies evaluated NicVAX, Cornuz 2008 evaluated NIC002 (previously known as Nicotine‐Qβ and developed by Cytos Biotechnology) in 341 generally healthy adults smoking 10 to 40 cigarettes per day for three years or more. The trial was conducted in three clinical study centres in Switzerland. Two hundred and twenty‐nine participants randomized to active treatment received five injections of 100ɥg NIC002 at months 0, 1, 2, 3 and 4, and 112 participants randomized to placebo received alum injections on the same schedule. The target quit date was set at one month and individual behavioural counselling was provided to all participants from week three to month four. The strictest definition of abstinence was continuous abstinence, data for which were recorded at monthly visits from months three to six, at month nine and at month 12. Abstinence was validated by exhaled CO of fewer than 10 ppm.
Excluded studies
We excluded seven studies identified by our search. A list of these studies, with brief details and rationale for exclusion, can be found in the Characteristics of excluded studies table. They include four NicVAX trials (Hatsukami 2005a; Lindmayer 2003; Nabi 2008; Wagena 2008), two TA‐NIC trials (Bunce 2005; St Clair Roberts 2003), and one NIC002 trial (Maurer 2005), all of which studied safety and immunogenicity rather than rates of smoking cessation.
Ongoing studies
We found six studies in clinical trial registers that appeared relevant to our review but were ongoing or for which results had yet to be released (earliest study start date was May 2007). Details of these studies can be found in the Characteristics of ongoing studies table. Three are studies of NicVAX: one studies the efficacy of NicVAX alone for smoking cessation (NCT01304810); one studies the efficacy of NicVAX for relapse prevention when co‐administered with varenicline (NCT00995033); and one (NCT01178346) assesses health‐related quality of life and healthcare resource utilization in a subset of participants following two Phase III trials (NCT00836199 and NCT01102114). One study is a Phase II study of TA‐NIC (NCT00633321), and a further two ongoing studies evaluate safety and efficacy of NIC002 (NCT00736047; NCT01280968).
Risk of bias in included studies
Both Cornuz 2008 and Hatsukami 2011 provided details of the blinding of participants, personnel, and outcome assessment, and were judged to be at low risk for performance or detection bias. Cornuz 2008 described the method of sequence generation and allocation concealment and was judged to be at low risk for selection bias, whereas Hatsukami 2011 did not provide sufficient details to determine the risk of selection bias, and hence was rated as at 'unclear risk'. Though Hatsukami 2011 did not indicate the number of participants with imputed serology values (only that data was imputed from last measurement), both trials were judged to be at low risk of attrition bias since the proportion of participants lost to follow‐up was similar in active and placebo groups and participants with missing data were counted as smokers in intent‐to‐treat analyses, apart from 44 participants excluded from analysis in Cornuz 2008 due to departure from the study protocol by using NRT; the authors report that this did not affect outcome measurements.
The Cochrane Handbook (Higgins 2011) states that studies which report primary outcomes using analysis methods or subsets of data that are not pre‐specified should be rated as being at 'high risk' of reporting bias. Both Cornuz 2008 and Hatsukami 2011 stratified active treatment groups by the level of antibodies (Ab) present, and detected statistically significantly higher cessation rates in 'high Ab' groups when compared to placebo. A report produced by Cytos Biotechnology on Cornuz 2008 states that since intent‐to‐treat analysis did not achieve statistically significant results, further subgroup analysis was performed based on antibody levels to establish a clinical proof of concept (Cytos 2007). There was insufficient detail in the protocol to determine if this method of stratification was planned in the original study design or introduced post‐hoc, and hence Cornuz 2008 was judged to be at 'unclear risk' for reporting bias. Hatsukami 2011 also stratified participants according to Ab level, defining high as the top 30% Ab level in the area under the curve (AUC). The authors indicate that this approach was chosen based on "the largest group of high‐Ab responders between the 25 and 50% levels that demonstrated statistical significance compared with the subjects using placebo." We therefore rated Hatsukami 2011 as being at high risk of reporting bias.
We did not have enough information to judge risk of bias for NCT00836199 and NCT01102114, and hence rated them at 'unclear risk' for all bias domains.
Effects of interventions
Long‐term abstinence
None of the four included studies detected a statistically significant difference in abstinence at six or 12 months between active and placebo arms. Two Phase III trials of NicVAX versus placebo in 1000 participants each (NCT00836199; NCT01102114) measured continuous abstinence from weeks 37 to 52. In 2011, press releases from Nabi Biopharmaceuticals announced that neither study detected a statistically significant difference in abstinence rates between intervention and placebo groups (Nabi 2011a; Nabi 2011b). The first of the trials (NCT00836199) found a similar rate of abstinence in both groups (approximately 11%, Nabi 2011a). A percentage was not provided for the second trial (NCT01102114), but results were reported to be similar to the first trial, and the press release stated that there was no statistically significant difference in abstinence between NicVAX and placebo groups (Nabi 2011b). At the time of writing, we are unable to find information on any other outcomes from these two trials.
The two Phase II studies for which data were available (Cornuz 2008 and Hatsukami 2011) reported long‐term abstinence at six and 12 months in active versus placebo groups. In both studies, participants in the treatment groups had a higher rate of long‐term abstinence at 12 months than participants in placebo groups, but confidence intervals were wide and not statistically significant (Analysis 1.1). The relative risk (RR) for prolonged abstinence at 12 months follow‐up in active versus placebo groups in Hatsukami 2011 was 1.74 (95% CI 0.73 to 4.18). The RR for continuous abstinence at 12 months in Cornuz 2008 was 1.35 (95% CI 0.82 to 2.22). The Cornuz estimate excludes participants known to have used nicotine replacement therapy during the treatment period (30 intervention, 14 placebo) due to concerns that nicotine replacement therapy may reduce or eliminate the effect of nicotine vaccines by saturating nicotine‐specific antibodies produced during vaccination. The authors state that removing this group did not affect outcome measurements (Cornuz 2008).
1.1. Analysis.

Comparison 1 Active vs. placebo, Outcome 1 Abstinence at 12 months.
In both Cornuz 2008 and Hatsukami 2011, the difference in quit rates between intervention and control groups followed the same pattern at six months as at twelve, with participants receiving active treatment more likely to have quit than those receiving placebo, but with wide confidence intervals including the line of no effect (Cornuz 2008: RR 1.15, 95% CI 0.80 to 1.67; Hatsukami 2011: RR 1.91, 95% CI 0.80 to 4.53, Analysis 1.2). Cornuz 2008 performed logistic regression analysis to investigate the influence of different variables (age, gender, treatment, weight, cigarettes per day, duration of smoking, Fagerström score) on continuous abstinence. Only antibody levels (discussed below) had a significant influence.
1.2. Analysis.

Comparison 1 Active vs. placebo, Outcome 2 Abstinence at 6 months.
Effect of the antibody titer
Both Cornuz 2008 and Hatsukami 2011 conducted subgroup analyses of long‐term abstinence based on antibody (Ab) titers, comparing high Ab groups with placebo (and for some results comparing high Ab groups with groups with lower Ab levels). Data used for Ab calculations in both studies are from per‐protocol analyses (as opposed to intent‐to‐treat), as Ab levels determined at follow‐up visits up to month six were an essential element of determining Ab groupings. Cornuz 2008 split subjects receiving active treatment who complied with the scheduled visits and blood samplings into three equal groups (high, medium, and low) based on area under the curve (AUC) tertiles; Ab levels were calculated from blood samples collected at monthly visits up to month six and the high Ab group was defined as the top 33% by AUC. In Hatsukami 2011, the high Ab group was defined as the top 30% by AUC from 0 to 26 weeks, and the low Ab group consisted of the remaining 70% of participants receiving active treatment. Unlike Cornuz 2008 , the Hatsukami 2011 analysis by Ab level included participants with missing data. In this trial, injection windows were defined for each schedule and missing values were imputed by using the next available serology result in that window or, if the next value was not available, the value of the nearest previous time point in that window. The proportion of participants with data imputed in this manner was not reported.
At six months, high Ab groups in both studies demonstrated statistically significantly higher continuous abstinence rates than groups with lower Ab levels, with an RR of 1.76 in Cornuz 2008 (95% CI 1.23 to 2.54, high Ab vs low + medium Ab groups) and an RR of 2.65 in Hatsukami 2011 (95% CI 1.34 to 5.22, Analysis 2.1). However, at 12 months the difference in abstinence between low and high Ab groups in Hatsukami 2011 was no longer statistically significant (RR 1.80, 95% CI 0.81 to 3.99, analysis not shown). Twelve‐month data were not available for this comparison in Cornuz 2008 .
2.1. Analysis.

Comparison 2 High Ab vs low Ab, Outcome 1 Abstinence at 6 months.
When comparing high Ab groups with placebo at 12 months, participants in high Ab groups also demonstrated significantly higher rates of continuous abstinence, with an RR of 1.95 in Cornuz 2008 (95% CI 1.15 to 3.32) and an RR of 3.01 in Hatsukami 2011 (95% CI 1.17 to 7.71, Analysis 1.3). As seen in Analysis 1.4, at six months the high Ab group in Cornuz 2008 also demonstrated a statistically significantly higher continuous abstinence rate than participants receiving placebo (RR 1.81, 95% CI 1.21 to 2.71), whereas the difference between the two groups in Hatsukami 2011 was not statistically significant (RR 1.89, 95% CI 0.97 to 3.70). In the overall treatment population in Cornuz 2008 , Ab levels were highest during months three to six, whereas the difference in abstinence rates between placebo and vaccine groups narrowed during this period.
1.3. Analysis.
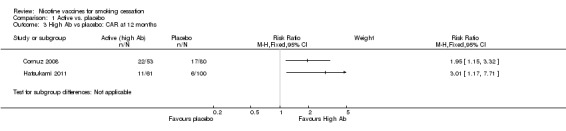
Comparison 1 Active vs. placebo, Outcome 3 High Ab vs placebo: CAR at 12 months.
1.4. Analysis.
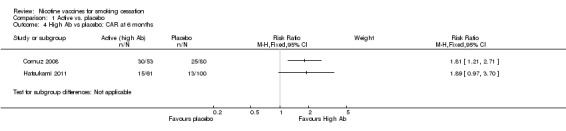
Comparison 1 Active vs. placebo, Outcome 4 High Ab vs placebo: CAR at 6 months.
Comparison of doses and schedules
Cornuz 2008 and the two Phase III trials of NicVAX (NCT00836199; NCT01102114) used only one dose and injection schedule. As well as comparing active treatment with placebo, Hatsukami 2011 split the treatment group equally into two schedules and two doses (200µg and 400µg), allowing a comparison between schedules and doses of NicVAX. Participants on schedule one received four doses over the course of six months (at weeks 0, 6, 12 and 26). Participants on schedule two received five doses, also over the course of six months (at weeks 0, 4, 8, 16 and 26). Regardless of schedule, 12 month continuous abstinence rates were not significantly different between 200µg and 400µg groups; pooling schedules 1 and 2, comparison of 400µg with 200µg resulted in an RR of 1.08, with a 95% CI of 0.49 to 2.42, (Analysis 3.1). The difference in continuous abstinence at 12 months between participants receiving five vaccine injections (schedule 2) and participants receiving four vaccine injections (schedule 1) was more pronounced (RR 2.47, 95% CI 1.00 to 6.12, Analysis 4.1). Participants receiving vaccine injections on schedule 2 also had higher overall Ab concentrations on average.
3.1. Analysis.

Comparison 3 High vs. low dose, Outcome 1 400ųg vs 200ųg: CAR at 12 months.
4.1. Analysis.
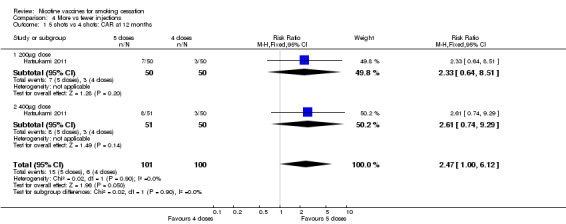
Comparison 4 More vs fewer injections, Outcome 1 5 shots vs 4 shots: CAR at 12 months.
Cigarettes smoked per day in non‐abstainers
Due to the biodynamics of nicotine vaccines, the investigators explored their possible effect on compensatory smoking. Cornuz 2008 and Hatsukami 2011 recorded the number of cigarettes smoked per day in non‐abstainers. Neither study indicated the occurrence of compensatory smoking (increased levels of smoking to compensate for the reduction of nicotine reaching the brain due to vaccination) amongst participants receiving treatment. Both provided data on smoking amongst non‐abstainers in the high Ab participants as compared with participants receiving placebo. Hatsukami 2011 reported a significantly greater reduction in daily cigarette consumption amongst non‐abstainers in the high Ab group compared to placebo (average of 4.6 cigarettes per day difference in reduction between high Ab and placebo groups), and stated that median cigarettes per day were very similar in placebo and low Ab groups. In Cornuz 2008, non‐abstainers in the high Ab group smoked fewer cigarettes per day than non‐abstainers in placebo, low Ab and medium Ab groups from months two to six.
Adverse events
All four studies collected data on adverse events (AEs), but data are not currently available for NCT00836199 or NCT01102114. Cornuz 2008 assessed safety and tolerability through systematic collection of vital signs and reported symptoms, a specific safety check‐up one week after each injection, and self assessment diaries of local reactions. In Hatsukami 2011, subjects were instructed to record reactogenicity events for seven days after each injection which were followed up until resolution or study completion; non‐serious treatment‐emergent AEs were recorded for four weeks after the last injection and data on serious AEs were recorded up to 52 weeks. In Cornuz 2008, mild AEs were reported in 316 out of 342 participants (96.5% of vaccine users and 84.8% of placebo users) and moderate AEs were reported in 233 participants (72.9% of vaccine recipients and 58.9% of placebo recipients). Mild to moderate AEs were experienced by 266 out of 301 subjects in Hatsukami 2011 (87.1% of vaccine users and 91.0% of placebo users). Data on all serious AEs and other common AEs possibly, probably, or definitely attributable to study medication from Cornuz 2008 and Hatsukami 2011 are recorded in Appendix 2 . Results have not been pooled due to between‐study differences in vaccine type, dosage, and AE reporting method.
Local events
Among reactogenicity events aggregated across all injections reported in Hatsukami 2011, aches and tenderness were the most commonly reported local event (in over 90% of both placebo and vaccine groups). Swelling, erythema, heat and burning at the injection site occurred in approximately half of the participants in Hatsukami 2011. In Cornuz 2008, pain at the injection site was the most commonly reported local event (19.7% of vaccine recipients and 1.8% of placebo participants), with no other local reactions reported in 10% or more of participants from either group.
Systemic events
The most common systemic events reported in Hatsukami 2011 were myalgia, general discomfort/malaise, and headache, with similar numbers in both groups (67% to 86%). Nausea was reported in 44% of participants in both groups, fever in 10% of both groups, and vomiting in 6% of the placebo group and 7% in the vaccine group. Flu‐like symptoms, usually occurring within 12 hours of injection and disappearing 24 hours post‐dose, were the most common AE, systemic or otherwise, reported in Cornuz 2008 (69.4% of vaccine subjects and 12.5% of placebo subjects). Other systemic events in Cornuz 2008 which appeared to be related to the flu‐like symptoms but were reported separately included fever, headache, chills and myalgia.
Between group differences in specific AEs
There were significantly higher levels of reported AEs among the active group compared with the placebo group in Cornuz 2008 for flu‐like symptoms (RR 5.55, 95% CI 3.38 to 9.13), pyrexia (RR 5.22, 95% CI 2.74 to 9.94), injection site pain (RR 11.00, 95% CI 2.72 to 44.55) and myalgia (RR 2.53, 95% CI 1.09, 5.88; analyses not shown). There were no statistically significant differences between active and placebo groups for any other AEs as reported in either Cornuz 2008 or Hatsukami 2011.
Withdrawal symptoms
Cornuz 2008 found no detectable difference in withdrawal symptoms between vaccine and placebo groups, according to the Wisconsin Withdrawal Scale. Similarly, Hatsukami 2011 reported no significant inter‐group differences in overall withdrawal severity between placebo, high Ab and low Ab groups as measured with the Minnesota Nicotine Withdrawal Scale. Data on withdrawal symptoms were not available for NCT00836199 or NCT01102114.
Serious adverse events
A serious adverse event (SAE) is defined as any untoward medical occurrence that results in death, persisting or significant disability or incapacity, is life‐threatening, or requires in‐patient hospitalisation (Cornuz 2008).
Eighteen AEs reported in Hatsukami 2011 were classified as serious, i.e. eight in the treatment group among seven subjects, and 10 in the placebo group among five subjects. Only SAE, anaphylactic shock, was considered by study investigators to be related to treatment: this occurred in a participant with a history of urticaria reaction to penicillin and seasonal allergies, and was resolved by an epinephrine and diphenhydramine injection.
Nine SAEs were reported in Cornuz 2008 (six in the treatment group, three in the placebo group). Of the nine events, the authors report that only one might be attributable to treatment: a 60‐year old woman reported flu‐like symptoms and chest pains, but there was no evidence of heart disease and no cardiovascular or pulmonary disease found at six month follow‐up.
No treatment‐related deaths were reported in either study.
Discussion
Nicotine vaccines have not yet been licensed for use in any country. Multiple vaccines are in development, and hence the only studies currently relevant to our review are those completed, or being conducted, as part of the drug development process. Despite attempts to contact companies and investigators, our review has been hampered by a lack of published results for Phase II and Phase III clinical trials of nicotine vaccines, and results reported in our review must be viewed in this context.
Smoking cessation
Despite encouraging preclinical data, none of the four included studies detected a statistically significant difference in long‐term cessation between participants receiving vaccine and participants receiving placebo. The risk ratio (RR) for 12‐month cessation in active and placebo groups was 1.35 (95% CI 0.82 to 2.22) in Cornuz 2008 and 1.74 (95% CI 0.73 to 4.18) in Hatsukami 2011. The two Phase III NicVAX trials, for which full results were not available, reported similar quit rates in both groups at approximately 11% (NCT00836199 and NCT01102114). Although overall 2642 smokers took part in the included studies in this review, we only had sufficient data on two of the four studies to conduct statistical analyses, covering 642 participants in total. Candidate vaccines are likely to undergo significant changes before becoming available to the general public, and those included in this review may not be the first to reach market, which limits the external validity of the results reported here.
Analyses in Cornuz 2008 and Hatsukami 2011 which detected significantly higher cessation rates in participants in active arms with the highest antibody (Ab) levels compared with placebo or compared with participants in active arms with lower Ab levels are also not readily generalisable. The method of stratification for subgroup analysis in Hatsukami 2011 was determined post‐hoc and calculated so as to achieve statistical significance, rendering it at high risk of selection bias. In Cornuz 2008, subgroup analysis by Ab level was used to determine clinical proof of concept, and we were unable to determine if the methods of stratification and analysis were predetermined. These subgroup analyses are hypothesis generating as opposed to hypothesis confirming; the decisions on how to split data into high and low Ab groups and how to stratify participants to achieve a significant result place the findings at risk of bias and are unlikely to translate to different participant groups. However, it should be noted that both these Phase II studies were primarily aimed towards demonstrating proof of the concept that anti‐nicotine antibodies may enhance cessation rates. Optimal vaccines, dosing regimens and schedule of vaccinations may not have been demonstrated in these studies.
Various explanations have been offered for the lack of significant effects on smoking cessation in clinical studies of nicotine vaccines to date. The contribution of nicotine to the rewards of smoking may be smaller than initially thought: nicotine is only one component of tobacco addiction, and it has been suggested that blocking the effects of nicotine alone may be too simplistic an approach (Raupach 2012). Based on their findings of statistically significant higher abstinence rates in participants with higher concentrations of nicotine antibodies, Cornuz 2008 and Hatsukami 2011 suggest that the lack of a statistically significant effect in the overall treatment groups when compared with placebo may be attributable to insufficient antibodies produced, and that vaccines capable of inducing higher levels of antibodies may be more effective. However, in Cornuz 2008, though Ab levels peaked between months three and six, the difference in abstinence between treatment and control groups declined through that period. In Cornuz 2008, the non‐significant difference in abstinence rates between vaccine and placebo groups may partially be explained by the high percentage of smoking abstinence in the placebo group (18% abstinent in placebo group versus 23% in the treatment group at 12 months). This suggests that smokers who volunteered for the trial may have placed very high expectations in the study and/or may have been positively influenced by the smoking cessation counselling provided. Such effects may statistically bias the difference between groups towards the null hypothesis. Finally, all four included studies measured smoking cessation as opposed to relapse prevention as their primary outcome. Due to their mechanism of action, nicotine vaccines may be more effective for relapse prevention than as an aid to smoking cessation in itself (Raupach 2012). Given this, Raupach 2012 suggests that future trials should consider testing vaccines in smokers who have already quit or, to maximise the level and effect of the antibodies, in smokers willing to reduce cigarette consumption with the ultimate goal of quitting (with the quit date set at the period of peak antibody levels, rather than at or towards the beginning of treatment). However, it should be noted that the two Phase III trials of NicVAX (NCT00836199 and NCT01102114) deferred the target quit date in order to align with peak antibody levels and did not report a difference in cessation rates between active and placebo groups. This finding suggests that timing of quit attempts with the nicotine vaccine may be challenging.
Adverse events and effects
Hatsukami 2011 and Cornuz 2008 showed nicotine vaccines to be well tolerated, with the majority of adverse events classified as mild or moderate. Information on adverse events was not available for the large Phase III trials of NicVAX (NCT00836199 and NCT01102114). Hatsukami 2011 reported similar rates of adverse events in those receiving NicVAX and those receiving placebo. In Cornuz 2008, participants receiving NIC002 were significantly more likely to report flu‐like symptoms than those in the placebo arm (RR 5.55, 95% CI 3.38 to 9.13). A newer formulation of NIC002 has been reported to reduce the incidence of flu‐like symptoms to 10% from the 70% reported in Cornuz 2008 (Cytos 2007). In Hatsukami 2011, one serious adverse event (SAE) was attributed to study treatment: a participant in the active treatment group with a history of allergy to penicillin presented with anaphylaxis following an injection. In Cornuz 2008, one SAE was considered by investigators to be possibly related to study treatment: a 60‐year old woman reported flu‐like symptoms and chest pain. Both events were resolved and there were no deaths reported in either study.
Neither Hatsukami 2011 nor Cornuz 2008 found evidence of compensatory smoking in participants receiving the vaccine, which is consistent with findings from other studies (Hatsukami 2005b). This may be attributable to the slow rate at which the antibodies reach optimum levels (Fagerström 2005) or to the prolonged clearance of nicotine (Hatsukami 2005b). Safety findings are not generalisable across nicotine vaccines as a whole, however, as there are a variety of vaccines in development with a range of delivery mechanisms and biological components.
Publication bias
Risk of bias assessments for included studies in this review rate most elements at unclear risk of bias. Overall, however, results reported in this review are at high risk of bias due to a lack of published trial results, especially for larger and more robust trials. We are unable to quantify the level of missing results, but are aware of relevant studies which have been completed and for which results have not been released. The publication bias affecting this review can be broken down into three main categories: studies which we do not know about at all, studies which we know have been conducted but for which we have no results, and studies which we know have been conducted for which we have limited results. We are unable to quantify the extent of the first problem, and there are currently too few adequately reported studies to support a meaningful funnel plot analysis. in an attempt to address this problem we have searched clinical trials registers and company websites and reports. These have contributed to our list of ongoing studies, but the list is still limited to trials which have been publicly announced through formal routes of communication. We are aware from other sources of a Phase II study of Niccine announced in 2008 (Caponnetto 2012) and of a 2011 Phase II study of a vaccine candidate developed at the Scripps Institute in California (Quenqua 2011; Scripps 2011), but are unable to gather sufficient information on them to list them under ongoing or excluded studies.
Two of our included studies, NCT00836199 and NCT01102114, demonstrate the limitations posed by the third category, where we do not have full results for relevant completed studies. Together, NCT00836199 and NCT01102114 account for 2000 of the 2654 participants in the included studies for this review. To demonstrate the effect of their omission, if we were to extend the approximate 11% quit rate attributed to both arms of both studies for inclusion in a meta‐analysis for NicVAX, the RR of continuous abstinence at 12 months, when pooled with the results of Hatsukami 2011, would be 1.03 (95% CI 0.86 to 1.22, analysis not shown), a risk ratio much closer to the line of null effect with a much narrower confidence interval than that we have with Hatsukami 2011 alone (RR 1.74, 95% CI 0.73 to 4.18). Results for NIC002 may also be affected by this limitation: a press release from Cytos Biotechnologies (Cytos 2009) indicates that a Phase II NIC002 trial listed in our ongoing studies and sponsored by Novartis (NCT00736047) did not meet its primary endpoint of a statistically significant difference in continuous abstinence from weeks eight to 12 in the active group as compared with placebo. However, as limited results were reported for week 12 only we did not include this in our analysis.
Future directions
Information on the future directions of nicotine vaccines is for the most part speculative in nature and, in the absence of information published in academic journals, relies on information from company reports and the popular press.
At the time of writing we are unable to determine future development plans for NicVAX. Nabi Biopharmaceuticals, owners of NicVAX, announced they were exploring strategic alternatives after the lack of significant results from Phase III trials (Nabi 2011b). A further trial of NicVAX is ongoing in the Netherlands (NCT00995033), studying co‐administration with varenicline, but Nabi has announced they will reveal their strategic alternatives in the second quarter of 2012 and that these will be announced regardless of results from the ongoing trial, for which results are expected in the second half of 2012 (Nabi 2012). Prior to the Phase III trials, GlaxoSmithKline (GSK) took out an option on NicVAX and stated that, independent of their choice to option NicVAX, they would develop the next generation nicotine vaccine based on Nabi's intellectual property (GlaxoSmithKline 2009). As of March 2012, Nabi had not been informed that GSK was abandoning these plans (Nabi 2012), but we were unable to find information on GSK's development of a nicotine vaccine more recent than the 2009 press release.
After results from Cornuz 2008 demonstrated higher cessation rates in participants with higher Ab levels, Cytos Biotechnologies conducted studies to optimise dose, regimen, and formulation for increased Ab titres (Cytos 2007). A study of a higher dose of NIC002 (300ɥg as opposed to the 100ɥg used in Cornuz 2008) induced a mean antibody level four times higher than that in Cornuz 2008, and another study of varied treatment regimens found participants receiving injections of NIC002 once a week over five weeks (as opposed to the monthly regimen in Cornuz 2008) obtained mean antibody levels 10 times higher than those in Cornuz 2008 (Cytos 2007). A further Phase II study of NIC002, designed to improve its efficacy, is underway at two sites in the USA (NCT01280968).
Beyond NicVAX and NIC002, ongoing studies are recorded in this review for TA‐NIC and Niccine, other nicotine vaccines delivered by injection. Independent Pharmaceutica, owners of Niccine, were reported to be in liquidation as of February 2010 (Bloomberg 2012). An article published in the Wall Street Journal records a partner of Celtic Pharma, developers of TA‐NIC, stating that a Phase II study of TA‐NIC "failed totally" because of a manufacturing error and that TA‐NIC would be revisited if a study of the firm's cocaine vaccine yields promising results (Long 2011).
We are currently aware of at least four other nicotine vaccine candidates in development: Selecta Biosciences in the USA is investigating the linked combination of nicotine with a synthetic carrier (as opposed to a biological carrier, Selecta 2011) and The Scripps Insitute in California has developed a nicotine vaccine (discussed above, Scripps 2011). Vaccine candidates in development also include those delivered by mechanisms other than injection. Investigators at the University of Nebraska were awarded a grant from the National Institute of Drug Abuse (NIDA) for further research into a peptide‐based nicotine vaccine delivered via patch in 2004 (Brozek 2004). An investigation led by the University of Connecticut (in conjunction with Thomas Cerny, who has reported working closely with Chilka Ltd.) of a synthetic, peptide‐based vaccine delivered intranasally was funded by NIDA in 2011 (Buckley 2011; Cerny 2009; NIDA 2011).
Agreements and disagreements with other studies or reviews
As nicotine vaccines are still in clinical development, conclusions regarding their efficacy change frequently. Recently published reviews covering nicotine vaccines (Caponnetto 2012; Fahim 2011; Ottney 2011; Raupach 2012) are broadly in alignment with our review: inclusion criteria differ, but overall the same studies are addressed, and the same overall lack of statistically significant results in Phase II and III trials in active versus placebo groups is reported. The reviews express some optimism regarding the future of nicotine vaccines, and Raupach 2012 speculates they may one day become part of a multi‐faceted approach to treating tobacco addiction.
In light of the limited evidence available, we look forward to carefully monitoring the development of nicotine vaccines over the coming years, and to the release of an increasing number of trial results.
Authors' conclusions
Implications for practice.
From the two trials with fully reported findings, there is no clear evidence that people injected with nicotine vaccine are more likely to stop smoking in the long term than those injected with placebo. There are similar findings from two studies for which only summary quit rates are available.
Rates of serious adverse events recorded in the two trials with full data available were low, with no significant difference between active and treatment arms. The majority of adverse events reported were mild to moderate. The most commonly reported adverse events were injection site discomfort and flu‐like symptoms. In the study of NIC002, participants receiving the vaccine were more likely to report mild to moderate adverse events, whereas in the study of NicVAX there was no significant difference between the active and placebo groups.
In two included studies, cessation rates increased with participants' levels of nicotine antibodies. Because of the methods used to stratify and analyse antibody levels, this finding may not be generalisable to other studies or populations.
Higher doses or more frequent injections of vaccine may be associated with higher levels of antibodies, and subsequently with higher cessation rates.
The evidence available suggests nicotine vaccines do not induce compensatory smoking or affect withdrawal symptoms.
No nicotine vaccines are currently licensed for use in any country but a number are under development.
Implications for research.
Results from past, current and future research should be reported in full.
Further trials of nicotine vaccines are needed, comparing vaccines with placebo for smoking cessation.
Further trials are also needed to explore the potential of nicotine vaccines as an aid to relapse prevention.
Adverse events and serious adverse events should continue to be carefully monitored and thoroughly reported.
There should be a common standard for reporting and categorising nicotine‐specific antibody levels. The method used for antibody calculations should be specified and published in advance of study start.
What's new
| Date | Event | Description |
|---|---|---|
| 20 September 2012 | Amended | PLS typo amended (total participants corrected from 2664 to 2642) |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 8, 2012
| Date | Event | Description |
|---|---|---|
| 21 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Dr Erich Cerny and Dr Tobias Raupach for detailed comments on the review and for providing information during the writing process. We thank Professor Paul Aveyard for editorial and content comments. We thank Shirley Manknell for her thorough review comments from a consumer perspective, and we thank Beth Shinkins for providing statistical advice. We thank Dr Jacques Le Houezec for reading and commenting on the previously published protocol of this review, Dr Paul Pentel for providing additional information for the protocol version, and Dr Anne Ottney for information on an earlier review. We also thank Professor Peter Burkhard, Professor Kim Janda, Dr Thomas Cerny and Dr Nancy Rigotti for responding to queries during the writing process.
Appendices
Appendix 1. Search strategy for OVID databases
Database: Embase <1996 to 2012 Week 13>, Ovid MEDLINE(R) without Revisions <1996 to March Week 3 2012>, Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations <April 02, 2012>, PsycINFO <1987 to March Week 4 2012> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (nicotine adj3 vaccin*).ti,ab. (234) 2 (nicotine adj3 immun*).ti,ab. (320) 3 (nicotine qb or nicotine qbeta or nicotine qbeta vaccine or nicotine vaccine or nicotine vaccine qb).sh. (186) 4 (cigar* or smok* or tobacco).mp. (404747) 5 1 or 2 or 3 (608) 6 4 and 5 (451) 7 remove duplicates from 6 (285)
Appendix 2. Adverse events*
| Study ID | Data collection | Serious Adverse Events (SAEs)** | Other adverse events | ||
| Placebo | Intervention | Placebo | Intervention | ||
| Cornuz 2008 | Specific safety check‐up one week after each injection (0‐4 months), self‐assessment diaries of local reactions | Overall: 3 SAEs. None attributable to treatment. |
Overall: 6 SAEs. Flu‐like symptoms associated with chest pain (1) |
Flu‐like symptoms (14/112); Pyrexia (9); Headache (30); Nasopharyngitis (29); Myalgia (6); Injection site discomfort (2) |
Flu‐like symptoms (159/229); Pyrexia (96); Headache (92); Nasopharyngitis (73); Myalgia (31); Injection site discomfort (45) |
| Hatsukami 2011 | Subjects instructed to record reactogenicity events for 7 days after each injection (followed up until resolution or study completion); non‐serious treatment‐emergent AEs were recorded for 4 weeks after last injection; SAEs recorded up to 52 weeks |
Overall: 10 SAEs in 5 subjects. Herpes zoster (1) |
Overall: 8 SAEs in 7 subjects. Anaphylaxis (1) Herpes zoster (4) |
Pyrexia (10/100); Headache (61); Nasopharyngitis (14); Myalgia (86); Injection site discomfort (49) |
Pyrexia (21/201); Headache (138); Nasopharyngitis (18); Myalgia (171); Injection site discomfort (122) |
* AEs listed individually are possibly, probably, or definitely attributable to study medication.
** No deaths reported in either study
Appendix 3. Glossary of tobacco‐related terms
| Term | Definition |
| Abstinence | A period of being quit, i.e. stopping the use of cigarettes or other tobacco products, May be defined in various ways; see also: point prevalence abstinence; prolonged abstinence; continuous/sustained abstinence |
| Biochemical verification | Also called 'biochemical validation' or 'biochemical confirmation': A procedure for checking a tobacco user's report that he or she has not smoked or used tobacco. It can be measured by testing levels of nicotine or cotinine or other chemicals in blood, urine, or saliva, or by measuring levels of carbon monoxide in exhaled breath or in blood. |
| Bupropion | A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant) |
| Carbon monoxide (CO) | A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence. |
| Cessation | Also called 'quitting' The goal of treatment to help people achieve abstinence from smoking or other tobacco use, also used to describe the process of changing the behaviour |
| Continuous abstinence | Also called 'sustained abstinence' A measure of cessation often used in clinical trials involving avoidance of all tobacco use since the quit day until the time the assessment is made. The definition occasionally allows for lapses. This is the most rigorous measure of abstinence |
| 'Cold Turkey' | Quitting abruptly, and/or quitting without behavioural or pharmaceutical support. |
| Craving | A very intense urge or desire [to smoke]. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
| Dopamine | A neurotransmitter in the brain which regulates mood, attention, pleasure, reward, motivation and movement |
| Efficacy | Also called 'treatment effect' or 'effect size': The difference in outcome between the experimental and control groups |
| Harm reduction | Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco. |
| Lapse/slip | Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence, but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse. |
| nAChR | [neural nicotinic acetylcholine receptors]: Areas in the brain which are thought to respond to nicotine, forming the basis of nicotine addiction by stimulating the overflow of dopamine |
| Nicotine | An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking. |
| Nicotine Replacement Therapy (NRT) | A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco‐free The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges. |
| Outcome | Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial. |
| Pharmacotherapy | A treatment using pharmaceutical drugs, e.g. NRT, bupropion |
| Point prevalence abstinence (PPA) | A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long‐term quitters. cf. prolonged abstinence, continuous abstinence |
| Prolonged abstinence | A measure of cessation which typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. See: Hughes et al 'Measures of abstinence in clinical trials: issues and recommendations'; Nicotine & Tobacco Research, 2003: 5 (1); 13‐25 |
| Relapse | A return to regular smoking after a period of abstinence |
| Secondhand smoke | Also called passive smoking or environmental tobacco smoke [ETS] A mixture of smoke exhaled by smokers and smoke released from smouldering cigarettes, cigars, pipes, bidis, etc. The smoke mixture contains gases and particulates, including nicotine, carcinogens and toxins. |
| Self‐efficacy | The belief that one will be able to change one's behaviour, e.g. to quit smoking |
| SPC [Summary of Product Characteristics] | Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively. |
| Tapering | A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment |
| Tar | The toxic chemicals found in cigarettes. In solid form, it is the brown, tacky residue visible in a cigarette filter and deposited in the lungs of smokers. |
| Titration | A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects. |
| Withdrawal | A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
For terms related to clinical trials and methodology, see The Cochrane Collaboration's Glossary.
Data and analyses
Comparison 1. Active vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence at 12 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Abstinence at 6 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 High Ab vs placebo: CAR at 12 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 High Ab vs placebo: CAR at 6 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 2. High Ab vs low Ab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence at 6 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. High vs. low dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 400ųg vs 200ųg: CAR at 12 months | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.49, 2.42] |
| 1.1 Schedule 1 (4 injections) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.72] |
| 1.2 Schedule 2 (5 injections) | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.44, 2.86] |
Comparison 4. More vs fewer injections.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 5 shots vs 4 shots: CAR at 12 months | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.00, 6.12] |
| 1.1 200µg dose | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.64, 8.51] |
| 1.2 400µg dose | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.61 [0.74, 9.29] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cornuz 2008.
| Methods | Medication: NIC002 (formerly Nicotine‐Qβ) Setting: 3 clinical study centres in Switzerland Aims: To establish the clinical efficacy, safety and tolerability of NIC002 for smoking cessation in smokers willing to quit, and to determine its immunogenicity. Study: Phase II randomized, double‐blind, placebo‐controlled study. Study ID: NCT00369616. |
|
| Participants | Pts generally healthy, aged 18‐70, smoking 10‐40 cpd for >3 yrs, Fagerström score ≧5. Randomized 2:1 to vaccine (229) or placebo (112); 1 pt dropped from placebo group as he had already quit. Mean age 42, 58% M, mean cpd 25, mean previous quit attempts 3. | |
| Interventions | Treatment: Five injections of 100ɥg NIC002 in alum, at months 0, 1, 2, 3 and 4. Placebo: Five injections of alum alone at months 0, 1, 2, 3 and 4. TQD was month 1, with individual counselling from wk 3 to month 4. |
|
| Outcomes | Primary: CAR months 3‐6, validated by CO<10ppm. Immunogenicity, safety and tolerability of NIC002. Secondary: PPA on day of testing, CAR wks 8‐52. AEs and SAEs monitored throughout. Craving and withdrawal symptoms assessed by QSU and WWS. |
|
| Notes | Study was funded by Cytos Biotechnology AG. Participants using NRT removed from final data and ITT analysis (n=44). Data on number of events for 12m CAR derived from Cytos Biotechnology publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization list, prepared using standard software, with a block size of 15" by a local pharmacist |
| Allocation concealment (selection bias) | Low risk | see above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All study personnel, participants, study statisticians and data monitoring committee were blinded to treatment assignment for the duration of the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | see above, plus biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Subjects with missing visits or who were lost to follow‐up at any time during the study were considered to be smoking." Similar number of dropouts in both groups (40/229 vs 17/97). |
| Selective reporting (reporting bias) | Unclear risk | "An intent‐to‐treat analysis of the entire study population....did not achieve statistical significance. Therefore, a sub‐group analysis was performed based on antibody levels and this analysis established a clinical proof‐of‐concept for this novel vaccine candidate" (Cytos Biotechnology). Though protocol available, unclear if method of subgroup analysis planned at protocol stage or determined post hoc. |
Hatsukami 2011.
| Methods | Medication: NicVAX [3'AmNic‐rEPA] Setting: "nine geographically diverse US sites" Aims: To establish proof of concept, to test the efficacy and safety of NicVAX for smoking cessation, and to assess effects of different levels of serum concentrations of antinicotine Abs and different delivery schedules. Study: Phase IIb study; multicentre, randomized, double‐blind, placebo‐controlled, parallel‐arm trial design. Study IDs: NCT00318383; NCT00598325; Nabi‐4512 |
|
| Participants | 301 heavy smokers randomized to placebo (100) or to intervention (201). Pts generally healthy, motivated to quit, smoked at least 15 cpd (mean 24 cpd), exhaled CO>10ppm, aged 18+ (mean age 48), 47.5% M, mean Fagerström score 6. |
|
| Interventions | Pts randomized 1:1:1 into 3 groups (placebo, 200ɥg, 400ɥg). Within each group, randomized 1:1 to schedule 1 or schedule 2 (Schedule 1 = 4 shots at wks 0, 6, 12, 26; Schedule 2 = 5 shots at wks 0, 4, 8, 16, 26) resulting in 6 groups: 1. 200ɥg Schedule 1 (50 participants) 2. 400ɥg Schedule 1 (50) 3. Placebo Schedule 1 (50) 4. 200ɥg Schedule 2 (50) 5. 400ɥg Schedule 2 (51) 6. Placebo Schedule 2 (50) TQD set at 1 wk after 2nd injection (end of wk 7 for Schedule 1, or end of wk 5 for Schedule 2). 2nd quit date allowed for relapsers up to wk 18. All pts got standard behavioural counselling (5 sessions <10mins) and for relapsers additional 1 session + 2 phone calls. Pts followed up for 21 visits over 52 wks. |
|
| Outcomes | Primary: CAR wks 19‐26 Secondary: CAR wks 19‐52; 7‐day PPA; any 8‐wk period of sustained abstinence up to wk 52; impact on compensatory smoking among non‐abstainers; immunogenicity; prolonged abstinence at 6 and 12m (with grace period of 2 wks following TQD, used in analysis). Validation by exhaled CO <8ppm. Regular measures of Fagerström score and withdrawal/craving. 16‐17 serum samples taken from baseline to wk 52. Treatment‐emergent AEs recorded for 4 wks after last dose, and SAEs over 52 wks. |
|
| Notes | Study funded by NIDA and by Nabi Biopharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized" but no further information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)." Biochemical validation used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and drop‐outs fully reported; drop‐outs counted as smokers. Missing diary data imputed on basis of last observation carried forward. Missing serology data imputed from next available value (though scale of imputation not specified). Similar number lost to follow‐up in both groups (33/100 in placebo group, 72/200 in active treatment group). |
| Selective reporting (reporting bias) | High risk | Stratification into high (30% AUC) and low (70% AUC) Ab responders, based on "largest group of high‐Ab responders between the 25% and 50% levels that demonstrated statistical significance." |
NCT00836199.
| Methods | Medication: NicVAX [3'AmNic‐rEPA] Setting: 22 sites in the USA Aims: Assess efficacy, safety and immunogenicity of NicVAX as an aid to smoking cessation Study: Phase III, multi‐centre, randomized, double‐blind, placebo‐controlled study Study IDs: NCT00836199; Nabi‐4514 |
|
| Participants | 1000 participants randomized to placebo or intervention. 18‐65 years, male and female, current smoker for ≥1yr and of ≥10 cpd, motivated to quit, in good general health. |
|
| Interventions | NicVAX (probably 400ɥg) given 6 times over 6m; placebo treatment as per NicVAX administration. TQD set at wk 14. Behavioural counselling provided for both groups. |
|
| Outcomes | Primary: CAR wks 37‐52 Validation: CO (information on cut off level not available at time of writing) Secondary: abstinence at other time intervals; safety (adverse events); immunogenicity (serum Ab levels); withdrawal symptoms; smoking satisfaction; cigarette consumption; and nicotine dependence |
|
| Notes | First of two Phase III NicVAX trials. Full data not available at time of writing. Information included obtained from clinicaltrials.gov and from Nabi Biopharmaceuticals press release. Funded by Nabi Biopharmaceuticals and National Institute on Drug Abuse. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not published. |
| Allocation concealment (selection bias) | Unclear risk | Details not published. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind." Details not published. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind." Details not published. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not published. |
| Selective reporting (reporting bias) | Unclear risk | Full results not yet published. |
NCT01102114.
| Methods | Medication: NicVAX [3'AmNic‐rEPA] Setting: 25 sites in the US Aim: Evaluate NicVAX as an aid to long‐term smoking cessation Study: Phase III, multi‐centre, randomised, double‐blind, placebo‐controlled study Study IDs: NCT01102114, Nabi‐4515 |
|
| Participants | 1000 participants randomized to placebo or intervention. 18‐65 years, male and female, current smoker for ≥1yr and of ≥10 cpd, motivated to quit, in good general health. |
|
| Interventions | NicVAX (probably 400ɥg) given 6 times over 6m; placebo treatment as per NicVAX administration. TQD set at week 14. Behavioural counselling provided for both groups. |
|
| Outcomes | Primary: CAR wks 37‐52 Validation: CO (information on cut off level not available at time of writing) Secondary: abstinence at other time intervals; safety (adverse events); immunogenicity (serum Ab levels); withdrawal symptoms; smoking satisfaction; cigarette consumption; and nicotine dependence |
|
| Notes | Second Phase III NicVAX trial. Full data not available at time of writing. Information included obtained from clinicaltrials.gov and from Nabi Biopharmaceuticals press release. Funded by Nabi Biopharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not published. |
| Allocation concealment (selection bias) | Unclear risk | Details not published. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind." Details not published. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind." Details not published. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not published. |
| Selective reporting (reporting bias) | Unclear risk | Full results not yet published. |
Abs: antibodies; AE: adverse events; CAR: continuous abstinence rate; CO: carbon monoxide; cpd: cigarettes per day; ITT: intent‐to‐treat; M: male; m: month(s); NRT: nicotine replacement therapy; PPA: point prevalence abstinence; pt(s): participant(s); QSU: Questionnaire on Smoking Urges; SAE: serious adverse event; TQD: target quit date; wks: weeks; WWS: Wisconsin Withdrawal Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bunce 2005 | TA‐NIC: Does not address cessation. Phase I safety and immunogenicity trial to identify optimal dose for future efficacy trials. 60 smokers allocated to 1 of 3 doses over 7 time points. |
| Hatsukami 2005a | NicVAX: Study of safety and immunogenicity, not of smoking cessation. 68 smokers assigned to 1 of 3 doses over 4 time points, tested for Ab levels, withdrawal, compensatory smoking and 30‐day abstinence. |
| Lindmayer 2003 | NicVAX: Does not address cessation. Phase I safety and immunogenicity double‐blind trial in 20 healthy adults. Side‐effects and Ab levels measured over 63 days. |
| Maurer 2005 | NIC002: Does not address cessation. Phase I safety and immunogenicity trial in 32 healthy non‐smokers, assessed over 14 days. |
| Nabi 2008 | NicVAX: Phase II study to assess Ab response and safety of 400ɥg, 6‐dose schedule. Limited information available but based on the information provided seems likely that abstinence was not an outcome. |
| St Clair Roberts 2003 | TA‐NIC: Does not address cessation. Phase I safety and immunogenicity trial in smokers and non‐smokers, measured over 9m. |
| Wagena 2008 | NicVAX: Does not address cessation. Phase I/II of 30 healthy adults (smoking and non‐smoking), given 4 doses of vaccine, and followed for 266 days for safety, Ab levels, reactivity. |
Ab: antibodies; m: month(s).
Characteristics of ongoing studies [ordered by study ID]
NCT00633321.
| Trial name or title | Vaccine: TA‐NIC Study of TA‐NIC to Assess the Efficacy and Safety of the Vaccine as an Aid to Smoking Cessation |
| Methods | Multicentre, double‐blind, randomized, placebo‐controlled, 3 armed, Phase II study, assessing safety and efficacy of TA‐NIC vaccine to aid smoking cessation. |
| Participants | ≥18 yrs, male and female, regular smoker for ≥1yr and ≥10 cpd, motivated to quit smoking within 12 wks, avoid smoking cessation therapies other than those in the study. Enrolment: 522 |
| Interventions | TA‐NIC vaccine administered to each subject as 7 single doses, at weeks 0, 2, 4, 6, 8, 12 and 16; placebo treatment as for TA‐NIC administration. |
| Outcomes | 4‐wk assessment period. Quit rate at wk 26 measured by self‐reported abstinence in the 4 wks immediately prior to the 26 wk visit, supported by CO breath test; measures of antibody levels, quit status at 52 wks, smoking urge, continuous abstinence wks 26 ‐ 52 |
| Starting date | May 2007 |
| Contact information | "only displayed when the study is recruiting subjects" |
| Notes | Celtic Pharmaceuticals |
NCT00736047.
| Trial name or title | Vaccine: NIC002 Study to Evaluate the Efficacy, Safety, Tolerability and Immunogenicity of 100ɥg NIC002 Vaccine in Cigarette Smokers Who Are Motivated to Quit Smoking |
| Methods | Randomized, double‐blind, safety and efficacy study |
| Participants | 18 ‐ 65 yrs, male and female, ≥10 cpd for past 12m, exhaled CO ≥10 ppm + positive urine cotinine at screening Enrolment: 200 |
| Interventions | NIC002 intervention vs placebo |
| Outcomes | Smoking status at time intervals from TQD, safety and tolerability, immunogenicity |
| Starting date | August 2008 |
| Contact information | "only displayed when the study is recruiting subjects" |
| Notes | Novartis. Did not meet detect statistically significant difference in abstinence from weeks 8 to 12 in intervention compared with placebo groups (Cytos 2009, long‐term results not available). |
NCT00995033.
| Trial name or title | Vaccine: NicVAX Efficacy and Safety of NicVAX® Co‐administered With Varenicline (Champix®) |
| Methods | Randomized, double‐blind, safety and efficacy study |
| Participants | 18 ‐ 65 yrs, male and female, ≥10 CPD Enrolment: 600 |
| Interventions | Biological: NicVax vaccine, 6 times over 6m vs placebo identical to vaccine, 6 times over 6m; Drug: Varenicline (Champix) given to both arms |
| Outcomes | Long‐term abstinence; safety, immunogenicity, lapse and relapse rate, withdrawal symptoms |
| Starting date | October 2009 |
| Contact information | Dr Phillipe Hoogsteder: Phillipe.Hoogsteder@HAG.unimaas.nl; Dr Daniel Kotz: D.Kotz@HAG.unimaas.nl |
| Notes | Sponsors and collaborators: Maastricht University Medical Center; ZonMw: The Netherlands Organisation for Health Research and Development; Nabi Biopharmaceuticals. Results expected in the second half of 2012. |
NCT01178346.
| Trial name or title | Vaccine: NicVAX Health‐Related Quality‐of‐Life (HRQoL) and Health‐Care Resource Utilization Assessment in Nabi‐4514 and Nabi‐4515 Phase 3 Studies |
| Methods | Interventional, Phase III, non‐randomized, double‐blind, parallel assignment trial looking at pharmacoeconomic data |
| Participants | Subjects receiving NicVAX or placebo who had not yet reached week 12 in Nabi‐4514 or Nabi‐4515 studies (NCT00836199a; NCT01102114a) Enrolment: 500 (estimated) |
| Interventions | As per NCT00836199a and NCT01102114a |
| Outcomes | Effect of NicVAX vs placebo on HRQoL over the study period of 1 year; estimate utility scores; evaluate healthcare resource utilisation |
| Starting date | July 2010 |
| Contact information | "only displayed when the study is recruiting subjects" |
| Notes | Nabi Biopharmaceuticals and GlaxoSmithKline. Nabi ID: Nabi‐4519 |
NCT01280968.
| Trial name or title | Vaccine: NIC002 Improving the Efficacy of Anti‐Nicotine Immunotherapy |
| Methods | Phase II, randomized, parallel assignment, single‐blind (subject) |
| Participants | 18 ‐ 55 yrs, male and female, ≥10 cpd during past 12m, desire to quit smoking, in good general health. Enrolment: 65 |
| Interventions | NIC002 (55 participants), 4 injections over 16 weeks, vs placebo (10 participants) on same schedule |
| Outcomes | Primary outcome: kinetics of nicotine distribution during smoking Secondary outcomes: efficacy for smoking cessation (at 1, 2 and 6m post quit date); binding properties of vaccination‐induced anti‐nicotine Abs; side effects of vaccination |
| Starting date | December 2010 |
| Contact information | Alexey G Mukhin, Duke University |
| Notes | Duke University, Wake Forest University, National Institute on Drug Abuse, Novartis |
NCT01304810.
| Trial name or title | Vaccine: NicVAX A Follow‐up Study to Assess Long‐term Immunogenicity and Safety of Nic‐Vax/Placebo as an Aid to Smoking Cessation |
| Methods | Observational model: cohort, time perspective: prospective |
| Participants | Invited participants from previous studies, who received vaccinations of NicVax in prior Phase III Enrolment: 300 |
| Interventions | Prior treatment of NicVAX vs prior treatment of matching placebo |
| Outcomes | Blood nicotine antibody levels for 24m post initial injection |
| Starting date | January 2011 |
| Contact information | "only displayed when the study is recruiting subjects" |
| Notes | Nabi Biopharmaceuticals. Nabi ID: Nabi‐4522 |
Ab: antibodies; cpd: cigarettes per day; m: month(s); TQD: target quit date; wks: weeks.
Differences between protocol and review
In the absence of published results of relevant trials that we were aware had been conducted and completed, we expanded our search methods to clinical trial registers and company websites. We have not conducted meta‐analyses as initially planned as we currently included only two studies with full data sets, which investigate different vaccines and dosing schedules. In addition to reporting on adverse events, we have also included information on compensatory smoking in the results section. Although not mentioned in the original protocol, risk of bias was formally assessed for each included study, as recommended by the Cochrane Handbook (Higgins 2011).
Contributions of authors
KC and JHB performed searches of Google and clinicaltrials.gov, extracted data and wrote the review. All authors contributed to text and findings.
Sources of support
Internal sources
Department of Primary Health Care, Oxford University, Oxford, UK.
Department of Psychiatry, University of Minnesota Tobacco Use Programs, Minneapolis, MN, USA.
Department of Ambulatory Care and Community Medicine, Lausanne University Hospital, Lausanne, Switzerland.
External sources
NHS Research and Development Programme, UK.
Declarations of interest
Jacques Cornuz and Dorothy Hatsukami are principal investigators of trials included in this review.
Dorothy Hatsukami has received funding from Nabi Biopharmaceuticals to conduct clinical Phase II and III trials testing the nicotine vaccine, NicVAX. She also attended a meeting convened by Selecta Biosciences to discuss a nicotine vaccine that is being developed by this company. No honoraria have been received from either of these companies although costs for travel have been covered.
Jacques Cornuz has received funding from Cytos to conduct a clinical Phase II trial testing the nicotine vaccine, NicQb [NIC002]. He attended two international scientific meetings to present the results of such a trial developed by this company. Costs of travel have been covered by Cytos.
Jamie Hartmann‐Boyce and Kate Cahill have no conflicts of interest to declare for this review.
Edited (no change to conclusions)
References
References to studies included in this review
Cornuz 2008 {published data only}
- Cornuz J, Klingler K, Mueller P, Jungi F, Cerny T. A therapeutic vaccine for nicotine dependence: results of a phase I and a randomized phase II study [abstract]. Annual Meeting Proceedings of the American Society of Clinical Oncology 2005;23:108. [Google Scholar]
- Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, Melle G, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS ONE 2008;3(6):e2547. [DOI: 10.1371/journal.pone.0002547] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cytos Biotechnology. NIC002: a novel vaccine for nicotine addiction. http://www.cytos.ch/doc/NIC002_Nicaddiction_Facts_August07.pdf (accessed 25 Feb /2012) 2007.
- Willers J. Clinical trial protocol synopsis: Evaluation of efficacy and safety of Nicotine‐Qbeta (NicQb) vaccine versus placebo in healthy smokers. http://www.plosone.org/article/info:doi%2F10.1371%2Fjournal.pone.0002547#pone.0002547.s001 (accessed 15 March 2012) 2004.
Hatsukami 2011 {published data only}
- Fahim R. Promising new treatments including vaccines. http://www.seiservices.com/blendingalbuquerque/doc/R.Fahim%20Session%2019.pdf 2010.
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, et al. Immunogenicity and smoking‐cessation outcomes for a novel nicotine immunotherapeutic. Clinical Pharmacology and Therapeutics 2011;89(3):392‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard SI, Jorenby JE, Gonzales D, Rigotti NA, Vos A, Bortey EB. Abstract 3712: A randomized placebo‐controlled trial of a conjugate nicotine vaccine (Nic‐VAX®) in smokers who want to quit: 12 month results. Circulation 2007;116:ii_844. [Google Scholar]
- Rigotti N, Reus V, Glover E, Tashkin D, Oncken C, Rennard S, et al. Nicotine vaccine vs placebo for smoking cessation: long‐term abstinence and quitting [POS5‐1]. Society for Research on Nicotine and Tobacco 14th Annual Meeting February 27‐ March 1, Portland, Oregon. Rapid Communication Abstracts. 2008.
NCT00836199 {published and unpublished data}
- Nabi Biopharmaceuticals. Clinical Trials: NicVAX® (Nicotine Conjugate Vaccine). http://www.nabi.com/pipeline/clinicaltrials.php (accessed 15 March 2012).
- Nabi Biopharmaceuticals. News Release: Nabi Biopharmaceuticals Announces Results of First NicVAX(R) Phase III Clinical Trial. http://phx.corporate‐ir.net/phoenix.zhtml?c=100445&p=irol‐newsArticle&ID=1586001&highlight= (accessed 15 March 2012) 2011.
NCT01102114 {published and unpublished data}
- Nabi Biopharmaceuticals. Clinical Trials: NicVAX® (Nicotine Conjugate Vaccine). http://www.nabi.com/pipeline/clinicaltrials.php (accessed 15 March 2012).
- Nabi Biopharmaceuticals. News Release: Nabi Biopharmaceuticals Announces Results of Second NicVAX(R) Phase III Clinical Trial. http://phx.corporate‐ir.net/phoenix.zhtml?c=100445&p=irol‐newsArticle&ID=1626882&highlight= (accessed 15 March 2012) 2011.
References to studies excluded from this review
Bunce 2005 {published data only}
- Bunce C, Ackrel S, Fryer S, Rameal S, Treasure P, Long S. Interim data from a phase i study to identify the optimal dose of the nicotine vaccine, TA‐NIC to use for efficacy trials to establish impact of vaccine on quit rates [RP‐073]. Society for Research on Nicotine and Tobacco 11th annual meeting, March 2005; Prague, Czech Republic. Rapid Communications posters. 2005:19.
- Xenova Group PLC. News release: Anti‐smoking vaccine TA‐NIC preliminary 12 month clinical trial results. http://hugin.info/133161/R/982993/146255.pdf (accessed 15 March 2012) 2005.
Hatsukami 2005a {published data only}
- Hatsukami D, Rennard S, Jorenby D, Pentel P, Vos A, Horwith G. Results of a phase 2, multi‐center, randomized, double‐blinded, placebo‐controlled, parallel‐arm, dose comparison study to assess the immunogenicity and safety of 3'‐aminomethylnicotine‐P.aeruginosa R‐exoprotein A conjugate vaccine (Nicvax) administered to smokers. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005.
- Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, Vos A, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clinical Pharmacology and Therapeutics 2005;78(5):456‐67. [DOI] [PubMed] [Google Scholar]
Lindmayer 2003 {unpublished data only}
- Lindamyer K, Horwith G, Fattom A, Naso R, Fuller S, Muenz L, et al. Results of a phase I, double‐blinded, controlled safety and immunogenicity trial of Nicvax, a conjugated nicotine vaccine [POS1‐30]. Society for Research on Nicotine and Tobacco 9th Annual Meeting, New Orleans LA. Feb 19‐22 2003.
Maurer 2005 {published data only}
- Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, et al. A therapeutic vaccine for nicotine dependence: pre‐clinical efficacy, and phase I safety and immunogenicity. European Journal of Immunology 2005;35:2031‐40. [DOI] [PubMed] [Google Scholar]
Nabi 2008 {published data only}
- Nabi Biopharmaceuticals. Clinical Trials: NicVAX® (Nicotine Conjugate Vaccine). http://www.nabi.com/pipeline/clinicaltrials.php (accessed 15 March 2012).
St Clair Roberts 2003 {unpublished data only}
- Clair Roberts J, Akers CVR, Vanhinsbergh L, McKenna KA, Wood DM, Jack LM. Longitudinal safety and immunogenicity data of Ta‐Nic, a novel nicotine vaccine [POS1‐30]. Society for Research on Nicotine and Tobacco 9th Annual Meeting, New Orleans LA. Feb 19‐22 2003.
Wagena 2008 {published data only}
- Wagena EJ, Vos A, Akhavain R, Winkens R, Schayck CP. The safety and immunogenicity of a conjugated nicotine vaccine (Nicvax[TM]): Preliminary results of a randomised placebo‐controlled trial in healthy smokers and non‐smokers. Society for Research on Nicotine and Tobacco 5th European Meeting November 20‐22 2003 Padua: Abstract book. 2003.
- Wagena EJ, Vos A, Horwith G, Schayck CP. The immunogenicity and safety of a nicotine vaccine in smokers and nonsmokers: results of a randomized, placebo‐controlled 1/2 trial. Nicotine & Tobacco Research 2008;10(1):213‐8. [DOI] [PubMed] [Google Scholar]
- Wagena EJ, Schayck CP, Akhavasin R, Vos A. The safety of immunogenicity of a conjugated nicotine vaccine results from a double blind placebo controlled trial in healthy smokers and non smokers [Abstract]. European Respiratory Journal 2004;24(Suppl 48):28s. [Google Scholar]
- Wagena EJ, Schayck O, Akhavain R, Vos A. Preliminary results of a phase 2 safety trial with nicvax, a conjugated nicotine vaccine. A double‐blind, placebo‐controlled study in healthy smokers and non‐smokers (POS2‐068). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004:79.
References to ongoing studies
NCT00633321 {unpublished data only}
- Vaccine: TA‐NICStudy of TA‐NIC to Assess the Efficacy and Safety of the Vaccine as an Aid to Smoking Cessation. Ongoing study May 2007.
NCT00736047 {published and unpublished data}
- Cytos Biotechnology. Media Release: Interim analysis of an ongoing phase II study with nicotine vaccine shows primary endpoint not achieved. http://www.cytos.com/userfiles/file/Cytos_Press_E_091015.pdf (accessed 15 March 2012) 2009.
NCT00995033 {unpublished data only}
- Vaccine: NicVAXEfficacy and Safety of NicVAX® Co‐administered With Varenicline (Champix®). Ongoing study October 2009.
NCT01178346 {unpublished data only}
- Vaccine: NicVAXHealth‐Related Quality‐of‐Life (HRQoL) and Health‐Care Resource Utilization Assessment in Nabi‐4514 and Nabi‐4515 Phase 3 Studies. Ongoing study July 2010.
NCT01280968 {unpublished data only}
- Vaccine: NIC002Improving the Efficacy of Anti‐Nicotine Immunotherapy. Ongoing study December 2010.
NCT01304810 {unpublished data only}
- Vaccine: NicVAXA Follow‐up Study to Assess Long‐term Immunogenicity and Safety of Nic‐Vax/Placebo as an Aid to Smoking Cessation. Ongoing study January 2011.
Additional references
Balfour 2004
- Balfour DJK. The neurobiology of tobacco dependence: a preclinical perspective on the role of the dopamine projections to the nucleus. Nicotine & Tobacco Research 2004;6(6):899‐912. [DOI] [PubMed] [Google Scholar]
Benowitz 2010
- Benowitz NL. Nicotine Addiction. The New England Journal of Medicine 2010;362:2295‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloomberg 2012
- Bloomberg Businessweek. Company Overview of Independent Pharmaceutica AB. http://investing.businessweek.com/research/stocks/private/snapshot.asp?privcapId=6026103 (accessed 29 March 2012) 2012.
Brozek 2004
- Brozek A. Grant given for research on possible nicotine vaccine. Daily Nebraskan News 2004:http://www.dailynebraskan.com/news/grant‐given‐for‐research‐on‐possible‐nicotine‐vaccine‐1.1000520#.T3SBjWHZAfV (accessed 29 March 2012).
Buckley 2011
- Buckley C. A Vaccine for Nicotine?. UConn Today 3 October 2011:http://today.uconn.edu/blog/2011/10/a‐vaccine‐for‐nicotine/ (accessed 29 March 2012).
Cahill 2012
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006103.pub6] [DOI] [PubMed] [Google Scholar]
Caponnetto 2012
- Caponnetto P, Russo C, Polosa R. Smoking cessation: present status and future perspectives. Current Opinion in Pharmacology 2012;12:1‐9. [DOI: 10.1016/j.coph.2012.02.005] [DOI] [PubMed] [Google Scholar]
Cerny 2009
- Cerny EH, Cerny T. Vaccines against nicotine. Human Vaccines 2009;5(4):200‐5. [DOI] [PubMed] [Google Scholar]
Cytos 2007
- Cytos Biotechnology. NIC002: a novel vaccine for nicotine addiction. http://www.cytos.ch/doc/NIC002_Nicaddiction_Facts_August07.pdf (accessed 25 Feb 2012) 2007.
Cytos 2009
- Cytos Biotechnology. Media Release: Interim analysis of an ongoing phase II study with nicotine vaccine shows primary endpoint not achieved. http://www.cytos.com/userfiles/file/Cytos_Press_E_091015.pdf (accessed 15 March 2012) 2009.
Fagerström 2005
- Fagerström K, Balfour DJK. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert Opinion on Investigational Drugs 2005;15(2):107‐16. [DOI] [PubMed] [Google Scholar]
Fahim 2011
- Fahim RE, Kessler PD, Fuller SA, Kalnik MW. Nicotine vaccines. CNS & Neurological Disorders Drugs Targets 2011;10(8):905‐15. [DOI] [PubMed] [Google Scholar]
GlaxoSmithKline 2009
- GSK and Nabi announce agreement for NicVAX®, a vaccine for nicotine addiction. Press Release Archive 16 November 2009:http://www.gsk.com/media/pressreleases/2009/2009_pressrelease_10130.htm (accessed 15 March 2012).
Hall 2011
- Hall W, Gartner C. Ethical and policy issues in using vaccines to treat and prevent cocaine and nicotine dependence. Current Opinion in Psychiatry 2011;24(3):191‐6. [DOI] [PubMed] [Google Scholar]
Haney 2004
- Haney M, Kosten TR. Therapeutic vaccines for substance abuse. Expert Review of Vaccines 2004;3:11‐18. [DOI] [PubMed] [Google Scholar]
Harvey 2004
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug‐taking behavior in human cigarette smokers. Psychopharmacology (Berl) 2004;175:134‐42. [DOI] [PubMed] [Google Scholar]
Hasman 2004
- Hasman A, Holm S. Nicotine conjugate vaccine: is there a right to a smoking future?. Journal of Medical Ethics 2004;30(4):344‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatsukami 2005b
- Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, Vos A, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clinical Pharmacology and Therapeutics 2005;78(5):456‐67. [DOI] [PubMed] [Google Scholar]
Hicks 2012
- Hicks MJ, Rosenberg JB, De BP, Pagovich OE, Young CN, Qiu J‐P, et al. AAV‐directed persistent expression of a gene encoding anti‐nicotine antibody for smoking cessation. Science Translational Medicine 2012;4(140):140ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. http://www.cochrane.org/resources/handbook/hbook.htm (accessed 12 March 2012).
Hughes 2007
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD000031.pub3] [DOI] [PubMed] [Google Scholar]
Keyler 1999
- Keyler DE, Hieda Y, Peter J, Pentel PR. Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine & Tobacco Research 1999;1(3):241‐9. [DOI] [PubMed] [Google Scholar]
Lancaster 2005
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001292.pub2] [DOI] [PubMed] [Google Scholar]
Laviolette 2004
- Laviolette SR, Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nature Reviews: Neuroscience 2004;5:55‐65. [DOI] [PubMed] [Google Scholar]
LeSage 2006
- LeSage MG, Keyler DE, Pentel PR. Current status of immunologic approaches to treating tobacco dependence: vaccines and nicotine‐specific antibodies. American Association of Pharmaceutical Scientists Journal 2006;8(1):E65‐E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2011
- Long M. Quest for Vaccines to Treat Addiction. Wall Street Journal 3 May 2011:http://online.wsj.com/article/SB10001424052748704436004576298980739463392.html (accessed 15 March 2012).
Nabi 2011a
- Nabi Biopharmaceuticals. News Release: Nabi Biopharmaceuticals Announces Results of First NicVAX(R) Phase III Clinical Trial. http://phx.corporate‐ir.net/phoenix.zhtml?c=100445&p=irol‐newsArticle&ID=1586001&highlight= (accessed 15 March 2012) 2011.
Nabi 2011b
- Nabi Biopharmaceuticals. News Release: Nabi Biopharmaceuticals Announces Results of Second NicVAX(R) Phase III Clinical Trial. http://phx.corporate‐ir.net/phoenix.zhtml?c=100445&p=irol‐newsArticle&ID=1626882&highlight= (accessed 15 March 2012) 2011.
Nabi 2012
- Q4 2011 Nabi Biopharmaceuticals Earnings Conference Call. http://edge.media‐server.com/m/p/sw2pg4dk/lan/en (accessed 15 March 2012) 14 March 2012.
NIDA 2011
- NIDA Avant‐Garde‐Medications Development Award winners announced: Grants support novel approaches for treating methamphetamine and nicotine addiction. http://m.drugabuse.gov/news‐events/news‐releases/2011/09/nida‐avant‐garde‐medications‐development‐award‐winners‐announced (accessed 29 March 2012) 20 September 2011.
Ottney 2011
- Ottney AR. Nicotine conjugate vaccine as a novel approach to smoking cessation. Pharmacotherapy 2011;31(7):703‐13. [DOI] [PubMed] [Google Scholar]
Pentel 2000
- Pentel PR, Malin DH, Ennifar S, Hieda Y, Keyler DE, Lake JR, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacology Biochemistry and Behavior 2000;65(1):191‐8. [DOI] [PubMed] [Google Scholar]
Pentel 2004
- Pentel PR. Vaccines and depot medication for drug addiction: rationale, mechanisms of action, and treatment implications. In: Harwood H, Myers T editor(s). New treatments for addiction: behavioural, ethical, legal and social questions. Washingto DC: National Academies Press, 2004. [PubMed] [Google Scholar]
Quenqua 2011
- Quenqua D. An Addiction Vaccine, Tantalizingly Close. New York Times 2011:http://www.nytimes.com/2011/10/04/health/04vaccine.html?pagewanted=all (accessed 29 March 2012).
Raupach 2012
- Rachpach T, Hoogsteder PHJ, Schayck CP. Nicotine vaccines to assist with smoking cessation: Current status of research. Drugs 2012;72(4):e1‐e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scherer 1999
- Scherer G. Smoking behavior and compensation: a review of the literature. Psychopharmacology 1999;145:1‐20. [DOI] [PubMed] [Google Scholar]
Scripps 2011
- The Scripps Research Institute. Scientists Create Vaccine Against Heroin High. News & Views: Online weekly of the Scripps Research Institute 2011; Vol. 11, issue 23:http://www.scripps.edu/newsandviews/e_20110801/heroin.html (accessed 29 March 2012).
Selecta 2011
- Selecta Biosciences. Selecta Biosciences Initiates Phase 1 Clinical Study of SEL‐068, a First‐in‐Class Synthetic Nicotine Vaccine for Smoking Cessation and Relapse Prevention. http://www.selectabio.com/news/recent‐news/Selecta‐Biosciences‐Initiates‐Phase‐1‐Clinical‐Study‐of‐SEL‐068.cfm (accessed 15 March 2012) 2011.
Siu 2007
- Siu ECK, Tyndale RF. Non‐nicotinic therapies for smoking cessation. Annual Review of Pharmacology and Toxicology 2007;47:541‐64. [DOI] [PubMed] [Google Scholar]
Stead 2005
- Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001007.pub2] [DOI] [PubMed] [Google Scholar]
Stead 2006
- Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002850.pub2] [DOI] [PubMed] [Google Scholar]
Stead 2008a
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000146.pub3] [DOI] [PubMed] [Google Scholar]
Stead 2008b
- Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD000165.pub3] [DOI] [PubMed] [Google Scholar]
Stolerman 1995
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2‐10; 14‐20. [DOI] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. WHO Framework Convention on Tobacco Control: why is it important?. http://www.who.int/features/qa/34/en/print.html (accessed 24th November 2007) 2006.
WHO 2011
- World Health Organization Tobacco Free Initiative. Tobacco Facts. http://www.who.int/tobacco/mpower/tobacco_facts/en/index.html (accessed 12 March 2012) 2011.


