Abstract
Background
The prevalence and incidence of pain and skeletal complications of metastatic bone disease such as pathologic fractures, spinal cord compression and hypercalcemia is high and an important contributor to morbidity, poor performance status and decreased quality of life. Moreover, pathologic fractures are associated with increased risk of death in people with disseminated malignancies. Therefore, prevention of pain and fractures are important goals in men with prostate cancer at risk for skeletal complications.
Objectives
To assess the effects of bisphosphonates in men with bone metastases from prostate cancer.
Search methods
We identified studies by electronic search of bibliographic databases including the Cochrane Controlled Trials Register and MEDLINE on 13 July 2017 and trial registries. We handsearched the Proceedings of American Society of Clinical Oncology (to July 2017) and reference lists of all eligible trials identified. This is an update of a review last published in 2006.
Selection criteria
We included randomized controlled studies comparing the effectiveness of bisphosphonates in men with bone metastases from prostate cancer.
Data collection and analysis
Two review authors independently extracted data and assessed the quality of trials. We defined the proportion of participants with pain response as the primary end point; secondary outcomes were skeletal‐related events, mortality, quality of life, adverse events, analgesic consumption and disease progression. We assessed the quality of the evidence for the main outcomes using the GRADE approach.
Main results
We included 18 trials reporting on 4843 participants comparing the effect of bisphosphonate administration to control regimens.
Primary outcome: there was no clear difference in the proportion of participants with pain response (RR 1.15, 95% CI 0.93 to 1.43; P = 0.20; I2 = 0%; 3 trials; 876 participants; low quality evidence). In absolute terms, bisphosphonates resulted in a pain response in 40 more participants per 1000 (19 fewer to 114 more).
Secondary outcomes: bisphosphonates probably reduced the incidence of skeletal‐related events in participants with prostate cancer metastatic to bone (RR 0.87, 95% CI 0.81 to 0.94; P = 0.27; I2 = 19%; 9 trials; 3153 participants; moderate quality evidence). In absolute terms, bisphosphonates resulted in 58 fewer SREs per 1000 (85 fewer to 27 fewer).
We found no clinically relevant differences in mortality (RR 0.97, 95% CI 0.91 to 1.04; P = 0.43; I2 = 1%; 9 trials; 2450 participants; moderate quality evidence). In absolute terms, bisphosphonates resulted in 16 fewer deaths per 1000 (47 fewer to 21 more).
Outcome definition of quality of life and the measurement tools varied greatly across trials and we were unable to extract any quantitative data for meta‐analysis.
Bisphosphonates probably increased the number of participants affected by nausea (RR 1.19, 95% CI 1.00 to 1.41; P = 0.05; I2 = 0%; 9 trials; 3008 participants; moderate quality evidence). In absolute terms, bisphosphonates resulted in seven more cases of nausea per 1000 (0 fewer to 14 more). Bisphosphonates probably increased the number of renal adverse events (RR 1.65, 95% CI 1.11 to 2.46; P = 0.01; I2 = 0%; 7 trials; 1794 participants; moderate quality evidence). In absolute terms, bisphosphonates resulted in 22 more renal adverse events per 1000 (4 more to 50 more). We found no clear difference in the number of participants with osteonecrosis of the jaw between groups (RR 1.92, 95% CI 0.75 to 4.90; P = 0.17; I2 = 0%; 5 trials; 1626 participants; very low quality evidence). In absolute terms, bisphosphonates resulted in seven more cases with osteonecrosis of the jaw per 1000 (2 fewer to 29 more). We observed no clinically relevant difference in the proportion of participants with decreased analgesic consumption (RR 1.19, 95% CI 0.87 to 1.63; P = 0.28; I2 = 37%; 4 trials; 416 participants). Statistical analysis revealed that bisphosphonates probably reduced the number of participants with disease progression (RR 0.94, 95% CI 0.90 to 0.98; P = 0.006; I2 = 0%; 7 trials; 2115 participants; moderate quality evidence). In absolute terms, bisphosphonates resulted in 36 fewer cases of disease progression per 1000 (71 fewer to 7 fewer).
Findings of our predefined subgroup and sensitivity analyses were no different from those of the primary analyses.
Authors' conclusions
Based on low quality evidence, there may be no clinically relevant difference in the proportion of men with pain response between bisphosphonates and control regimens in men with bone metastases from prostate cancer. Bisphosphonates probably decrease the number of skeletal‐related events and disease progression. These benefits need to be weighed against the increased risk of renal impairment and nausea in men receiving bisphosphonates. Future studies should explicitly evaluate patient important outcomes such as quality of life and pain by using standardized and comparable assessment tools.
Plain language summary
Bisphosphonates for advanced prostate cancer
Review question
This review and analysis compared the chance of pain reduction, number of bone complications (skeletal‐related events), number of deaths, quality of life, side effects, use of analgesics (pain killers) and progression of cancer in men with bone metastases (bone cancer) from prostate cancer.
Background
The prostate is a gland in the male reproductive system. Prostate cancer can spread to other parts of the body (called metastases) including the bones. Bone fractures and compression of the spinal cord are feared complications in addition to death due to prostate cancer. Bisphosphonates are medicines that interact with the formation of new bone and might be useful to prevent the men from experiencing bone pain, fractures or other skeletal complications. We focused this review on pain because pain frequently occurs and can restrict the daily life activities and might require further treatment.
Study characteristics
We searched medical databases to 13 July 2017. Two review authors independently screened, summarized and analyzed the findings. This led to the inclusion of 18 clinical trials.
Key results
We found low quality evidence that bisphosphonates provided no clinically relevant difference in pain response (three studies involving 876 men) compared to placebo (pretend treatment) or no additional treatment. Bisphosphonates reduced pain in 40 more men per 1000 men (19 fewer to 114 more).
We found moderate quality evidence that bisphosphonates probably resulted in 58 fewer skeletal‐related events per 1000 (85 fewer to 27 fewer). Bisphosphonates showed no clear difference in the number of men who died or the number of men with decreased use of pain killers. We observed moderate quality evidence that bisphosphonates probably increased the number of men with nausea. Bisphosphonates resulted in seven more men with nausea per 1000 men (0 fewer to 14 more). We found moderate quality evidence that bisphosphonates probably increased the number of men with kidney problems. In this case, bisphosphonates resulted in 22 more men with renal complications per 1000 men (4 more to 50 more). For osteonecrosis of the jaw (where the jaw bone weakens and dies), we found very low quality evidence that bisphosphonates showed no clear difference. We observed moderate quality evidence that bisphosphonates probably decreased the number of men affected by disease progression (where the disease got worse). This means that bisphosphonates resulted in 36 fewer men with disease progression per 1000 men (71 fewer to 7 fewer). We found no useable data on quality of life.
Quality of the evidence
We judged the quality of evidence as moderate to very low.
Summary of findings
Summary of findings for the main comparison. Bisphosphonates compared to placebo/no treatment for advanced prostate cancer.
| Bisphosphonates compared to control for advanced prostate cancer | |||||
|
Patient or population: men with advanced prostate cancer Settings: ‐ Intervention: bisphosphonate Comparison: control | |||||
| Outcomes | No of participants (studies) Follow‐up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with control | Risk difference with bisphosphonates | ||||
|
Proportion of participants with pain response Follow‐up: 5‐12 months |
876 (3 RCTs) | ⊕⊕⊝⊝ Low1,2 | RR 1.15 (0.93 to 1.43) | Study population | |
| 265 per 1000 | 40 more per 1000 (19 fewer to 114 more) | ||||
| Skeletal‐related events: any, composite outcome Follow‐up: 5‐60 months | 3153 (9 RCTs) | ⊕⊕⊕⊝ Moderate3 | RR 0.87 (0.81 to 0.94) | Study population | |
| 448 per 1000 | 58 fewer per 1000 (85 fewer to 27 fewer) | ||||
| Mortality Follow‐up: 12‐60 months | 2450 (9 RCTs) | ⊕⊕⊕⊝ Moderate3 | RR 0.97 (0.91 to 1.04) | Study population | |
| 517 per 1000 | 16 fewer per 1000 (47 fewer to 21 more) | ||||
| Quality of life | ‐ | ‐ | Not estimable | ‐ | |
| Adverse events: nausea Follow‐up: 5‐36 months | 3008 (9 RCTs) | ⊕⊕⊕⊝ Moderate3 | RR 1.19 (1.00 to 1.41) | Study population | |
| 35 per 1000 | 7 more per 1000 (0 fewer to 14 more) | ||||
|
Adverse events: renal Follow‐up: 5‐36 months |
1794 (7 RCTs) | ⊕⊕⊕⊝ Moderate3 | RR 1.65 (1.11 to 2.46) | Study population | |
| 34 per 1000 | 22 more per 1000 (4 more to 50 more) | ||||
| Adverse events: osteonecrosis of the jaw Follow‐up: 5‐24 months | 1626 (5 RCTs) | ⊕⊝⊝⊝ Very low3,4 | RR 1.92 (0.75 to 4.90) | Study population | |
| 7 per 1000 | 7 more per 1000 (2 fewer to 29 more) | ||||
| Proportion of participants with disease progression Follow‐up: 12‐60 months | 2115 (7 RCTs) | ⊕⊕⊕⊝ Moderate3 | RR 0.95 (0.90 to 0.99) | Study population | |
| 710 per 1000 | 36 fewer per 1000 (71 fewer to 7 fewer) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1Potential risk of performance, detection and attrition bias leading to downgrading (one point).
2Small number of events leading to downgrading (one point).
3Potential risk of performance and attrition bias leading to downgrading (one point).
4Very small number of events leading to downgrading (two points).
Background
Description of the condition
Prostate cancer is the second most common cancer in men. Approximately 1.1 million men worldwide were diagnosed with prostate cancer in 2012 (Ferlay 2013). The reported age‐adjusted incidence rate of prostate cancer in the US was 137.9 per 100,000 from 2008 to 2012 (Howlader 2015). Mortality was 21.4 per 100,000 persons per year from 2008 to 2012 (Howlader 2015). About 80% of men with advanced prostate cancer develop bone metastases (Bubendorf 2000).
The prevalence and incidence of skeletal complications of metastatic bone disease such as bone pain, pathologic fractures, spinal cord compression and hypercalcemia is high and an important contributor to morbidity, poor performance status and decreased quality of life (QoL) (Coleman 1997). Despite the bone metastases themselves, androgen deprivation therapy, which is often given to men with bone metastases, is known to reduce bone mineral density and increase the risk of fractures (Alibhai 2017). As pathologic fractures are associated with increased risk of death in men with malignant bone disease (Fizazi 2015; Saad 2010), preventing fractures is an important goal in men with prostate cancer at risk for skeletal complications.
Description of the intervention
Therapeutic options for men with bone metastases are bone‐modifying agents such as bisphosphonates or inhibitors of RANK‐ligands (receptor activator of NF‐κB ligand) (Coleman 2012).
The clinical use of bisphosphonates started in the 1970s for the treatment of Paget disease (Reid 2003). Since then, their effectiveness has been shown in other diseases, including osteoporosis, hypercalcemia of malignancy, multiple myeloma and bone metastases (Devogelaer 2000). More than 70% of people with hypercalcemia of malignancy responded to bisphosphonate treatment (Saunders 2004). Meta‐analyses have also shown their effectiveness in reducing pain, bone loss and vertebral fractures in people with multiple myeloma (Mhaskar 2012) and breast cancer (Wong 2012). The mechanism through which bisphosphonates prevent fractures and therefore bone pain is related to the inhibition of disease‐induced, osteoclast‐mediated bone loss (Clohisy 2002). Nowadays, there are hints that despite the bone resorption properties, bisphosphonates even have a preventive potential against breast and colon cancer (Newcomb 2010; Thosani 2013).
Adverse events of the intervention
Bisphosphonates might have beneficial effects, but they are associated with adverse events. These can be structured according to affected organs. Bisphosphonates might increase the risk of atypical femur fracture or osteonecrosis of the jaw (ONJ) as skeletal complications (Bartl 2007; Bartl 2008; Hellstein 2011; Lee 2014; Reyes 2016). Bisphosphonates are associated with a prevalence of approximately 0.10% agent‐induced ONJ (Hellstein 2011). Non‐skeletal adverse events might affect the gastrointestinal tract (Bartl 2007; Bartl 2008; Reyes 2016). Two percent to 10% of people receiving bisphosphonates experience nausea, emesis, diarrhea or gastric pain (Bartl 2008). Additional reported gastrointestinal complications are esophagitis, gastrointestinal bleeding or ulcers (Bartl 2008; Reyes 2016). Other non‐skeletal adverse events probably caused by bisphosphonates are hypocalcemia or reduction of renal function (Bartl 2008; Gartrell 2014). In particular, intravenous (IV) administration of bisphosphonates seems to be associated with an increased risk of renal impairment and requires hemostasis of the person's fluid balance (Bartl 2008).
How the intervention might work
Bisphosphonates are analogues of pyrophosphate and they target osteoclastic cells. They can be subgrouped to amino‐bisphosphonates or non‐amino‐bisphosphonates (Reyes 2016).
Examples for amino‐bisphosphonates are zoledronate, risedronate, pamidronate and alendronate. They affect osteoclast metabolism by targeting farnesyl diphosphate synthase, which is responsible for post‐translational modification of guanosine‐5'‐triphosphate‐binding proteins (Reyes 2016). The group of non‐amino‐bisphosphonates includes etidronate, clodronate and tiludronate. These substances function by forming an analog of adenosine triphosphate. The resulting metabolite has toxic properties and induces apoptosis of osteoclasts (Reyes 2016). Both groups of bisphosphonates inhibit the effect of prostacyclins and cytokines in bone tissue and reduce the number of osteoclasts by downregulation of the reticuloendothelial system (Bartl 2007). They also bind hydroxyapatite in bone matrix (Gartrell 2015).
Addressing pharmacokinetics, orally administered bisphosphonates have a low bioavailability, which can even be decreased by concomitant consumption of calcium‐containing food (Bartl 2008). Consequently, oral bisphosphonates should not be taken with food or milk, but with water with a low content of calcium. Bisphosphonates are eliminated by the kidneys (Bartl 2007; Bartl 2008). The kidneys eliminate 50% to 80% of serum bisphosphonates, depending on the type of bisphosphonate (Bartl 2008).
Why it is important to do this review
Skeletal complications from bone metastases lead to a significant clinical burden such as pain, decreased QoL and increased mortality (Fizazi 2015; Saad 2010). The decision‐making process for prevention of pain and skeletal‐related events (SREs) in men with prostate cancer and bone metastases is usually challenging men and their physicians, as there are deviating recommendations on different approaches of bone‐modifying agents in national and international guidelines (Conford 2017; Cookson 2013; Wirth 2016). One systematic review on the use of bisphosphonates in men with prostate cancer described an increased risk for ONJ (Lee 2014). Liu 2015 and Gartrell 2015 found that bisphosphonates reduced the incidence of SREs. Furthermore, current evidence suggests that bisphosphonates delay the onset of SREs (Alibhai 2017; Gartrell 2015). One systematic review from Vignani 2016 and colleagues. emphasized that zoledronate has no impact on overall survival. All of these systematic reviews frequently focused on clinically important outcomes, but did not assess information on patient‐important outcomes (e.g. pain or QoL). Most of these systematic reviews performed descriptive analysis and only two provided data from pooled data analysis (Lee 2014; Liu 2015). None of the systematic reviews used the GRADE approach. In awareness of these weaknesses, we carried out an update of this review considering patient‐important outcomes and conducted this Cochrane Review using the GRADE approach.
The aim of our systematic review and meta‐analysis was to provide a comprehensive overview on the effects of bisphosphonates compared to placebo or no treatment or compared to chemotherapy. By systematically identifying all randomized trials and critically reviewing their reliability and validity considering similar trials in the meta‐analysis, we overcame statistical limitations of individual studies. This comprehensive overview is necessary for clinical decision making, and it will have a great impact on international guidelines and clinical pathways. Moreover, it will contribute to a high‐grade decision support for effective therapeutic strategies for the individual person.
Current guidelines and recommendations
Although bone‐targeted therapy is common in men with prostate cancer at risk for skeletal complications, recommendations in current guidelines are inconsistent. The guidelines by the European Association of Urology and by the German Oncologic Guidelines Program recommend the usage of zoledronic acid or the RANK‐ligand‐inhibitor, denosumab, in men with advanced, relapsed or castration‐resistant prostate cancer, with no evidence to demonstrate greater efficacy of one drug over another (Conford 2017; Wirth 2016). The guidelines by the American Urology Association (AUA) and the guidelines by the European Society of Medical Oncology (ESMO) suggest denosumab or zoledronic acid for men with bone metastases from castration‐resistant prostate cancer at high risk for clinically relevant SREs (Cookson 2013; Horwich 2013). Neither the National Comprehensive Cancer Network (NCCN) (Mohler 2016) nor the European Organisation for Research and Treatment of Cancer (EORTC) (Fitzpatrick 2014) give strong recommendations to use denosumab or bisphosphonates for SREs in men with prostate cancer.
Even though bisphosphonates are recognized as a reliable treatment option in bone metastases from prostate cancer, there is disagreement whether potentially beneficial effects would be outweighed by adverse events. In this context, the choice of the bisphosphonate is still a subject of discussion. In consideration of the presented guidelines, zoledronic acid might be the drug of choice. This review analyzed data of different bisphosphonates to determine the advantages and disadvantages.
Objectives
To assess the effects of bisphosphonates in men with bone metastases from castration‐resistant or castration‐sensitive prostate cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) without any language restrictions. We excluded cross‐over trials and quasi‐randomized trials. We included full‐text, abstracts and unpublished data if sufficient information on study design, participant characteristics, interventions and outcomes was available.
Types of participants
We included men with a confirmed diagnosis of bone metastases from castration‐resistant, hormone‐sensitive or hormone‐naive prostate cancer. Diagnosis of bone metastasis was based either on imaging or tissue specimens. There were no restrictions on age, performance status, life expectancy or previous treatment of the participants. We excluded studies evaluating non‐metastatic prostate cancer or other primary site(s) of cancer and animal studies.
Types of interventions
We included trials comparing bisphosphonates to control regimens for the treatment of bone metastases from prostate cancer. We considered any type of bisphosphonate, except radioactive bisphosphonates, eligible. There were no restrictions on dose, route, frequency or duration of bisphosphonate treatment. We had no restrictions on duration of follow‐up.
The control arm could have been placebo, no bisphosphonate treatment (open control) or a chemotherapeutical regimen. In contrast to prior versions of this review, we excluded studies with bisphosphonates as control treatment (active control) from qualitative and quantitative synthesis. These are listed in the Characteristics of excluded studies table.
Comparison:
Bisphosphonate versus control (placebo or no treatment)
We included studies in which the intended chemotherapy regimen and supportive care did not differ between study arms. Trials with more than two arms were included, provided at least two arms with the relevant comparison had the same chemotherapy protocol.
As agreed with the Editorial base, we removed the comparison of different dosages and of one bisphosphonate versus another due to potentially imbalanced results with restricted applicability.
Types of outcome measures
We included all trials fitting the inclusion criteria, irrespective of the outcomes reported (see Differences between protocol and review).
Primary outcomes
-
Proportion of participants with pain response:
we considered all trials reporting on the proportion of participants with pain response; there were no restrictions on pain assessment tools or definition of pain response in the trials.
Secondary outcomes
-
Skeletal‐related events (SRE):
any SRE;
pathologic fractures (total and subgrouped by vertebral or non‐vertebral fractures);
spinal cord compression;
bone radiation therapy;
bone surgery.
Mortality.
-
Quality of life (QoL):
we considered all trials reporting on QoL; there were no restrictions on QoL assessment tools or definition of response in the trials.
-
Adverse events:
nausea;
-
renal:
we considered all trials reporting renal adverse events; as bisphosphonates were described with nephrotoxicity with variable expression, we considered creatinine elevation and renal failure as renal adverse events;
bone pain;
osteonecrosis of the jaw (ONJ).
-
Proportion of participants with decreased analgesic consumption:
we considered all trials reporting on the proportion of participants with decreased analgesic consumption; there were no restrictions on assessment tools or definition of analgesic consumption in the trials.
-
Proportion of participants with disease progression:
we considered all trials reporting on the proportion of participants with disease progression; we included trials reporting on clinical progression (pain, analgesic consumption, treatment for progression such as radiation or surgery), biochemical progression (prostate‐specific antigen (PSA) elevation; no threshold value defined) or radiographic progression (new bone metastasis or growth of known bone metastasis).
Search methods for identification of studies
We performed an electronic search of bibliographic databases and handsearching. We repeated the previously used search strategy from the initial version of this review including the Cochrane Central Register of Controlled Trials (CENTRAL, see Appendix 1), MEDLINE (1966 to May 2005), Embase (1980 to April 2005), LILACS (to June 2005), DARE (to June 2005) and AMED (to June 2005). For this updated review, we revised the search strategy using those described in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We applied no language constraints. We extended the electronic search including published references in CENTRAL and MEDLINE to 13 July 2017 (see Appendix 2; Appendix 3).
Electronic searches
We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library, 2017, Issue 7; see Appendix 2);
MEDLINE (1980 to 13 July 2017; see Appendix 3).
Since we revised our searches, we re‐ran them for CENTRAL and MEDLINE for the entire period (i.e. 1980 to 13 July 2017).
Searching other resources
We searched the conference proceedings of the American Society of Clinical Oncology (ASCO) from 2000 to July 2017, which were not included in CENTRAL.
We electronically searched in the following databases of ongoing trials:
Metaregister of controlled trials: www.controlled‐trials.com/mrct/;
EU clinical trials register: www.clinicaltrialsregister.eu/ctr‐search/search;
ClinicalTrials.gov: clinicaltrials.gov/.
We handsearched the references of all identified trials, relevant review articles and current treatment guidelines.
Data collection and analysis
Selection of studies
Two review authors (SM, NS) independently screened the results of the search strategies for eligibility by reading the abstracts. In the case of disagreement, we obtained the full‐text publication. If no consensus could be reached, we consulted a third review author, in accordance with Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We documented the study selection process in a flow chart as recommended in the PRISMA statement (Moher 2009), showing the total numbers of retrieved references and the numbers of included and excluded studies.
We mapped all references reporting on the same study cohort together.
Data extraction and management
Two review authors (SM, NS) independently extracted the data according to the guidelines proposed by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If required, we contacted authors of individual studies for additional information. We used a standardized data extraction form containing the following items.
General information: author; title; source; publication date; country; language; duplicate publications.
Quality assessment ('Risk of bias' assessment): sequence generation; allocation concealment; blinding (participants, personnel, outcome assessors); incomplete outcome data; selective outcome reporting; other potential sources of bias.
Study characteristics: trial design; aims; setting and dates; source of participants; inclusion and exclusion criteria; comparability of groups; subgroup analysis; statistical methods; power calculations; treatment cross‐overs; compliance with assigned treatment; length of follow‐up; time point of randomization.
Participant characteristics: age; diagnosis; stage of disease; prior treatments; number of participants recruited, allocated, and evaluated; participants lost to follow‐up.
Interventions: duration; type; dose and timing of bisphosphonates; concomitant treatment (setting, duration, type of chemotherapy); and supportive care.
Outcomes: pain response, SREs (including pathologic fractures, spinal cord compression, bone radiation therapy, bone surgery), mortality, QoL, analgesic consumption, disease progression, radiologic response, adverse events, performance status.
Assessment of risk of bias in included studies
Two review authors (SM and NS) independently assessed the risk of bias for each study using the following criteria outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Random sequence generation.
Allocation concealment.
-
Blinding (participants, personnel, outcome assessors):
we differentiated between subjective (proportion of participants with pain response, SREs, QoL, adverse events, proportion of participants with decreased analgesic consumption, proportion of participants with disease progression) and objective (mortality) outcomes in measurement of detection bias (blinding of outcome assessment).
Incomplete outcome data for each evaluated outcome.
Selective outcome reporting.
Other potential sources of bias.
We made a judgment for every criterion, using one of three categories.
'Low risk': if the criterion was adequately fulfilled in the study (i.e. the study was at a low risk of bias for the given criterion).
'High risk': if the criterion was not fulfilled in the study (i.e. the study was at high risk of bias for the given criterion).
'Unclear risk': if the study report did not provide sufficient information to allow for a judgment of 'Yes' or 'No,' or if the risk of bias was unknown for one of the criteria listed above.
Measures of treatment effect
For binary outcomes, we calculated risk ratios (RR) with 95% confidence intervals (CI) for each trial. For continuous outcomes we would have calculated mean differences, or in case different scales would have been used, standardized mean difference (SMD). For time‐to‐event outcomes, we would have extracted the hazard ratio (HR) from published data according to Parmar 1998 and Tierney 2007.
Unit of analysis issues
Unit of analysis was the participant being randomized to one of the intervention arms. In multi‐arm trials, participants from the intervention arm receiving different dosages of the drug were merged to one intervention arm. One trial consisted of four interventions which we analyzed as two comparisons consisting of the control group and the intervention arm receiving the same drugs as the control arm plus bisphosphonates (Smith 1989).
Dealing with missing data
As suggested in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), there are many potential sources of missing data which are to be taken into account at: the study level; outcome level; summary data level; individual level and study‐level characteristics (e.g. for subgroup analysis). It is important to distinguish the difference between 'missing at random' and 'not missing at random.'
If data were assumed to be missing at random, we analyzed only the available data (i.e. ignored the missing data).
In the case that data were assumed not to be missing at random, we imputed the missing data with replacement values and treated these as if they were observed (e.g. last observation carried forward, imputing an assumed outcome such as assuming all were poor outcomes, imputing the mean, imputing based on predicted values from a regression analysis).
Assessment of heterogeneity
We assessed heterogeneity of treatment effects between trials using the Chi2 test with a significance level at P < 0.1. We used the I2 statistic to quantify possible heterogeneity (30% < I2 < 75%: moderate heterogeneity, I2 > 75%: considerable heterogeneity) (Deeks 2011).
Assessment of reporting biases
In meta‐analyses with at least 10 trials, we would have explored potential publication bias by generating a funnel plot and applying a linear regression test. A P value less than 0.1 would have been considered significant for this test (Sterne 2011). However, none of the analyses included 10 trials or more.
Data synthesis
We performed analyses according to the recommendations of Chapter nine of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We used aggregated data for analysis. For statistical analysis, we entered data into Review Manager 5 (RevMan 2014). One review author entered data and a second review author checked it for accuracy. We performed meta‐analyses using a fixed‐effect model (e.g. the generic inverse variance method for survival data outcomes and Mantel‐Haenszel method for dichotomous data outcomes).
Subgroup analysis and investigation of heterogeneity
We assessed heterogeneity of treatment effects between trials using a Chi2 test with a significance level at P < 0.1. We used the I2 statistic to quantify possible heterogeneity. We considered performing subgroup analyses according to the type of bisphosphonate and the route of administration.
As previously described (see How the intervention might work), amino‐bisphosphonates and non‐amino‐bisphosphonates work through similar but also different mechanism of action. Subgroup analysis was intended to reveal whether these differences in mechanism of actions might affect participant outcomes.
Amino‐bisphosphonates: alendronate, ibandronate, pamidronate, risedronate, zoledronate.
Non‐amino‐bisphosphonate: clodronate, etidronate.
Bisphosphonates are potentially nephrotoxic substances. There are reports in the literature that IV bisphosphonates increased the risk of nephrotoxicity in comparison with oral application (Bartl 2008). Moreover, Lee 2014 found people receiving IV bisphosphonates were at higher risk for ONJ.
IV administration.
Oral administration.
Sensitivity analysis
We performed sensitivity analyses using the following quality criteria:
quality components with regard to low and high risk of bias;
full‐text publication versus abstract publication only.
'Summary of findings' table
In the original protocol, the authors did not pre‐specify patient‐relevant outcomes for the 'Summary of findings' table and decided to present pain response, SREs, overall survival, QoL, adverse events, analgesic consumption and disease progression as most important outcomes. For this update of the review, we included most clinically relevant outcomes and those with the highest patient importance in the Table 1. These were:
proportion of participants with pain response;
SREs: any;
mortality;
QoL;
adverse events: nausea;
adverse events: renal;
adverse events: ONJ;
proportion of participants with disease progression.
The 'Summary of findings' table reports the grade of evidence of the outcomes reported according to the principles of the GRADE system (Schünemann 2011).
Results
Description of studies
Results of the search
Our updated literature research strategy identified 1973 articles regarding the use of bisphosphonates in men with advanced prostate cancer. Filtering these references, we excluded 1917 obviously irrelevant references. We checked the abstracts or full‐text publications of the remaining 56 articles for further information. After detailed revision of each reference, we excluded 26 articles (23 studies) and included 18 trials (30 references) in this review (see Figure 1).
1.
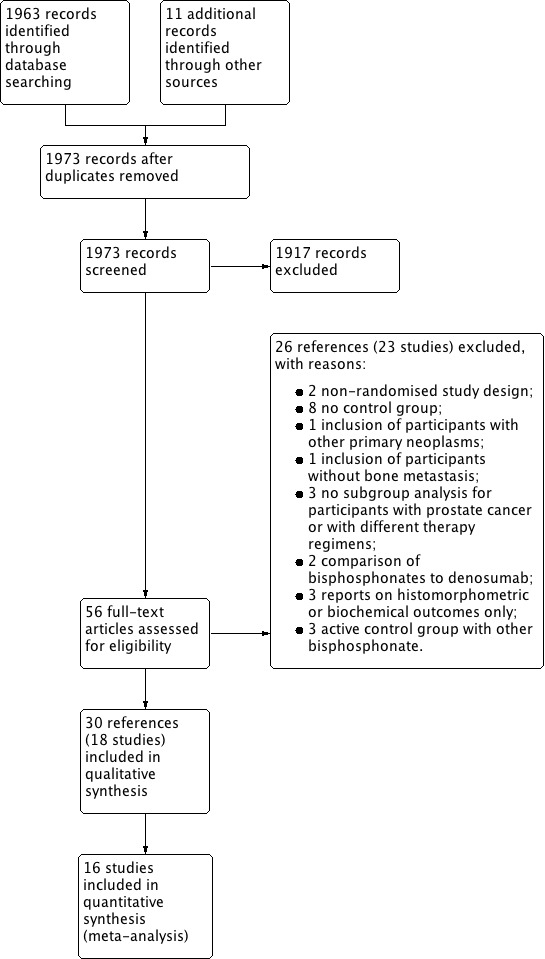
Study flow diagram.
Included studies
We included 18 trials in this review. Of these, nine were part of prior versions of this review (Elomaa 1992; Ernst 2003; Kylmala 1993; Kylmala 1997; PR05; Saad 2010; Small 2003; Smith 1989; Strang 1997). The update search revealed nine additional studies fulfilling the inclusion criteria (Abetz 2006; CALGB 90202; Figg 2005; GU02‐4; Meulenbeld 2012; Pan 2014; TRAPEZE 2016; ZABTON‐PC; ZAPCA).
The earliest trial was published in 1992 (Elomaa 1992) and the latest trial recruited from 2004 to 2012 (CALGB 90202). The Saad 2010 trial was a three‐armed trial, and we merged the data of both active arms for meta‐analysis. The Smith 1989 trial was a four‐armed trial, and we merged the data of all three active arms for meta‐analysis.
Design
Fifteen studies were two‐armed controlled trials (Abetz 2006; CALGB 90202; Elomaa 1992; Ernst 2003; Figg 2005; GU02‐4; Kylmala 1993; Kylmala 1997; Meulenbeld 2012; Pan 2014; PR05; Small 2003; Strang 1997; ZABTON‐PC; ZAPCA). Of these, 10 trials investigated the difference of bisphosphonates versus placebo and five trials (Figg 2005; Kylmala 1993; Meulenbeld 2012; ZABTON‐PC; ZAPCA) tested bisphosphonates against a control regimen without placebo.
The remaining three studies were three‐ or four‐armed trials.
Saad 2010 investigated the effect of zoledronic acid 4 mg IV versus zoledronic acid 8 mg IV versus placebo in a three‐armed trial. Noteworthy, the second group experienced a dose reduction from 8 mg to 4 mg due to renal toxicity of zoledronic acid. We merged the data of the active arms for meta‐analysis.
Smith 1989 evaluated the effect of etidronate and randomized 57 participants to a four‐armed trial: arm I (etidronate 7.5 mg/kg IV followed by sodium etidronate 400 mg PO) versus arm II (etidronate 7.5 mg/kg IV followed by placebo PO) versus arm III (placebo IV followed by sodium etidronate 400 mg PO) versus arm IV (placebo IV followed by placebo PO). We considered arms I, II and III as one intervention arm in the statistical analysis of this review.
TRAPEZE 2016 compared the effect of zoledronic acid and strontium chloride Sr89 in a four‐armed trial. Therefore, participants in the four arms were treated as follows: arm I (control regimen: docetaxel and prednisone) versus arm II (zoledronic acid IV, docetaxel and prednisone) versus arm III (strontium chloride Sr89 IV, docetaxel and prednisone) versus arm IV (zoledronic acid IV, strontium chloride Sr89 IV, docetaxel and prednisone). However, as the authors summarized all participants receiving zoledronate and compared these to all participants not receiving zoledronate, we extracted data for participants from arm I and arm III as the 'control group' and events in arm II and arm IV as the 'bisphosphonate group.'
Sample sizes
The 18 studies reported on 4843 participants. The smallest trial included 55 participants (Strang 1997) and the largest trial randomized 757 participants (TRAPEZE 2016). The median sample size per trial was 102 participants.
Setting
The included trials were performed by a range of research groups and in different countries. Five studies took place in a single country: Canada (Ernst 2003), China (Pan 2014), US (Small 2003), and Japan (ZABTON‐PC; ZAPCA). Two trials took place in a continental setting: Europe (Meulenbeld 2012, Netherlands and Norway) and North America (CALGB 90202, US and Canada). Two trials were conducted in an intercontinental setting: PR05 (UK and New Zealand), Saad 2010 (Argentina, Australia, Austria, Belgium, Brazil, Canada, Chile, France, Germany, Italy, New Zealand, Peru, Sweden, Switzerland, UK, Uruguay, US). There was no precise information regarding the country for nine trials (Abetz 2006; Elomaa 1992; Figg 2005; GU02‐4; Kylmala 1993; Kylmala 1997; Smith 1989; Strang 1997; TRAPEZE 2016).
Participants
All participants had a confirmed diagnosis of primary prostate cancer. All participants had at least one bone metastasis confirmed by imaging or histologic exam. Participants in 12 trials had hormone‐refractory prostate cancer or the trial investigators documented at least one failure of hormonal therapy prior to study treatment (Elomaa 1992; Ernst 2003; Figg 2005; Kylmala 1993; Kylmala 1997; Meulenbeld 2012; Pan 2014; Saad 2010; Small 2003; Smith 1989; Strang 1997; TRAPEZE 2016). In four trials, participants either responded to previous androgen blockade or received hormonal therapy concomitantly to study treatment (CALGB 90202; PR05; ZABTON‐PC; ZAPCA). Abetz 2006 provided no information on prior surgical or pharmaceutical castration in their study population. The CALGB 90202, Pan 2014, and TRAPEZE 2016 trials each included participants with other sites of metastases additional to bone metastases.
Interventions
Bisphosphonates
Seven trials used zoledronic acid (Abetz 2006; CALGB 90202; Pan 2014; Saad 2010; TRAPEZE 2016; ZABTON‐PC; ZAPCA). Five studies used a 4 mg dose of zoledronic acid IV (Abetz 2006; CALGB 90202; Pan 2014; ZABTON‐PC; ZAPCA), but the studies had different treatment intervals, mostly every three or four weeks. Saad 2010 compared the effect of zoledronic acid 4 mg IV (every three weeks) with zoledronic acid 8 mg IV and placebo, but observed renal toxicity led to a dose reduction of zoledronic acid from 8 mg to 4 mg IV during the study. TRAPEZE 2016 investigated the interaction of zoledronic acid IV with strontium chloride IV in a four‐armed setting.
Six trials used clodronate (Elomaa 1992; Ernst 2003; Kylmala 1993; Kylmala 1997; PR05; Strang 1997). Elomaa 1992 and Kylmala 1993 tested clodronate 3,200 mg orally (for one month) followed by clodronate 1,600 mg orally (two to six months). Kylmala 1997 investigated clodronate 300 mg IV (one to five days) followed by clodronate 1,600 mg PO (for five months). Ernst 2003 tested clodronate 1,500 mg IV versus placebo. PR05 used clodronate 2,080 mg orally as active drug. Strang 1997 investigated the effect of clodronate 300 mg IV (one to three days) followed by clodronate 3,200 mg orally in comparison with placebo.
Two trials used risedronate (GU02‐4; Meulenbeld 2012). Both trials investigated the effects of risedronate 30 mg orally.
One trial compared the effects of alendronate 40 mg with placebo (Figg 2005).
One trial tested pamidronate 90 mg (every three weeks for 27 weeks) against placebo (Small 2003).
One trial, a four‐armed trial, explored the effect of etidronate 7.5 mg/kg IV (one to three days) followed by etidronate 400 mg orally in comparison with etidronate 7.5 mg/kg IV (one to three days) followed by placebo, placebo IV followed by etidronate 400 mg IV or placebo IV followed by oral placebo (Smith 1989).
Androgen deprivation therapy
Eight studies reported on the use of androgen deprivation therapy (CALGB 90202; Elomaa 1992; GU02‐4; Kylmala 1993; Kylmala 1997; PR05; ZABTON‐PC; ZAPCA). Three trials used a therapy regimen consisting of estramustine 560 mg orally, daily for six months (Elomaa 1992; Kylmala 1993; Kylmala 1997). Two trials used a double androgen blockade with bicalutamide and a luteinizing hormone releasing hormone (LHRH) agonist (ZABTON‐PC; ZAPCA). Three trials provided no precise information regarding androgen deprivation therapy (CALGB 90202; GU02‐4; PR05).
Chemotherapy
Four studies reported on the use of chemotherapy (Ernst 2003; Meulenbeld 2012; Pan 2014; TRAPEZE 2016). Participants in Ernst 2003 received mitoxantrone 12 mg/m2 IV (21‐day cycles) and prednisone 10 mg daily. Three trials used docetaxel (21‐day cycles) in combination with daily prednisone (doses from 5 mg to 10 mg) (Meulenbeld 2012; Pan 2014; TRAPEZE 2016).
Supplemental therapy
Three trials used daily supplemental therapy with calcium 500 mg orally and vitamin D 400 IU to 500 IU (CALGB 90202; Pan 2014; Saad 2010).
Other interventional therapies
One trial tested the effect of antimycotic therapy with ketoconazole 1,200 mg daily in combination with hydrocortisone 30 mg daily (Figg 2005).
Outcomes
Primary outcome
Proportion of participants with pain response
Eleven of the 18 included trials initially planned to analyze pain response (Abetz 2006; Elomaa 1992; Ernst 2003; Kylmala 1993; Kylmala 1997; Meulenbeld 2012; Pan 2014; Small 2003; Smith 1989; Strang 1997; ZAPCA). Only three studies provided the proportion of participants with pain response, which was the primary outcome of this review. Hence, these trials could be included in the statistical analysis (Ernst 2003; Meulenbeld 2012; Smith 1989).
Ernst 2003 and Meulenbeld 2012 used Present Pain Intensity (PPI) scales from the "McGill Melzack Questionnaire" to measure pain. Smith 1989 described a numeric and a linear scale as assessment tools.
In these three trials, definitions of pain response were as follows:
Ernst 2003: PPI score = 0 or decrease of 2 points without an increase in analgesic score or evidence for disease progression;
Meulenbeld 2012: at least 2‐point reduction from baseline PPI score without increase in analgesic class or decrease in analgesic class without increased PPI score;
Smith 1989: no definition provided.
Secondary outcomes
Skeletal‐related events
Nine trials analyzed the rate of and time to SREs (mostly defined as spinal cord compression, pathologic fracture, surgery to bone and radiation to bone), as targetable outcome measure (CALGB 90202; GU02‐4; Pan 2014; PR05; Saad 2010; Small 2003; TRAPEZE 2016; ZABTON‐PC; ZAPCA). We included all nine trials in the quantitative synthesis.
Mortality
Thirteen of the 18 included trials analyzed mortality (CALGB 90202; Elomaa 1992; Ernst 2003; Figg 2005; GU02‐4; Kylmala 1993; Meulenbeld 2012; Pan 2014; PR05; Saad 2010; Small 2003; TRAPEZE 2016; ZAPCA). We included nine studies in a quantitative synthesis (CALGB 90202; Elomaa 1992; Ernst 2003; GU02‐4; Kylmala 1993; Meulenbeld 2012; PR05; Small 2003; ZABTON‐PC).
Quality of life
Four trials provided QoL data (Abetz 2006; Ernst 2003; Saad 2010; Small 2003). The study investigators used different assessment tools to assess QoL.
Further information on measurement tools and outcome definition were available for four trials:
Abetz 2006: investigated pain and evaluated the influence on daily life activities. They provided no definition of QoL data;
Ernst 2003: used a health‐related quality of life (HRQoL) questionnaire. HRQoL response was defined as a 1‐cm improvement from baseline on the 10‐cm visual analog scale (VAS) for overall well‐being maintained on two successive visits no less than three weeks apart;
Saad 2010: used the Functional Assessment of Cancer Therapy‐General (FACT‐G), version 4 (27) and the EURO Quality of Life EQ‐5D (EURO QOL), but did not define the outcome QoL;
Small 2003: evaluated mobility, measuring the number of seconds required to walk 10 feet (3 m) and the number of steps required to make a 360 degree turn to the left.
Adverse events
Sixteen studies investigated the incidence of adverse events (CALGB 90202; Elomaa 1992; Ernst 2003; Figg 2005; GU02‐4; Kylmala 1993; Kylmala 1997; Meulenbeld 2012; Pan 2014; PR05; Saad 2010; Small 2003; Smith 1989; TRAPEZE 2016; ZABTON‐PC; ZAPCA), but we included only the previously described adverse events in qualitative and quantitative synthesis.
Renal adverse events could represent different expressions of renal impairment. The outcome definition of the seven trials included in quantitative analysis were:
CALGB 90202: Grade 3, 4 or 5 events in creatinine elevation or renal failure;
Elomaa 1992: National Prostatic Cancer Project (NPCP) criteria for adverse events and renal failure;
Figg 2005: Grade 3, 4 or 5 events in creatinine elevation or renal failure;
Kylmala 1997: no definition provided;
Pan 2014: renal failure;
Saad 2010: change from baseline serum creatinine of 0.5 mg/dL or greater (if the baseline value was less than 1.4 mg/dL) or of 1.0 mg/dL or greater (if the baseline value was 1.4 mg/dL or less);
ZAPCA: Grade 3, 4 or 5 events in acute renal failure.
Proportion of participants with decreased analgesic consumption
Seven studies investigated analgesic consumption (Elomaa 1992; Ernst 2003; Kylmala 1993; Kylmala 1997; PR05; Small 2003; Smith 1989). Of these, we included four studies in the quantitative synthesis, as only these described the rate of patients with decreased or increased analgesic consumption (Elomaa 1992; Ernst 2003; Kylmala 1997; Smith 1989). The other trials used different scales/scores and were not comparable to these four studies.
Measurement tools and outcome definition were:
Elomaa 1992: no definition provided, but use of analgesic drugs was documented;
Ernst 2003: at least 50% decrease in analgesic score from the baseline with no increase in pain;
Kylmala 1997: no definition provided, but scoring based on a 0‐ to 4‐point grading scale (0 = no analgesic to 4 = narcotic analgesics);
Smith 1989: no definition provided, but analgesic requirement was documented.
Proportion of participants with disease progression
Twelve trials evaluated disease progression or time to progression (CALGB 90202; Ernst 2003; Figg 2005; GU02‐4; Kylmala 1997Meulenbeld 2012; Pan 2014; PR05; Saad 2010; TRAPEZE 2016; ZABTON‐PC; ZAPCA). Of these, seven studies reported the proportion of participants with disease progression and were included in quantitative analysis (CALGB 90202; Ernst 2003; Kylmala 1997; Meulenbeld 2012; Pan 2014; PR05; ZAPCA).
Disease progression could represent different events ranging from biochemical disease progression (increase in serum PSA level) to death due to prostate cancer. The outcome definition of the seven trials included in the quantitative analysis were:
CALGB 90202: new bone metastasis or PSA progression (defined as three consecutive rises in PSA with each PSA measurement at least two weeks apart and at least one PSA value greater than 4 ng/mL);
Ernst 2003: 1‐point or greater increase in PPI, 25% increase in analgesic consumption, need for palliative radiation therapy or unequivocal evidence of radiologic progression;
Kylmala 1997: new bone metastasis or greater than 25% increase of known lesions;
Meulenbeld 2012: objective progression by Response Evaluation Criteria in Solid Tumours (RECIST) criteria, PSA progression (defined as an increase of 25% or greater over nadir PSA concentration provided that the increase in the absolute PSA value was 5 ng/mL or greater for men without PSA response, or 50% or greater over nadir for PSA responders) or pain progression;
Pan 2014: more than 2‐point increase in VAS, restart or 40% increase in analgesic consumption, need for palliative radical therapy or new occurrence of bone metastasis;
PR05: osseous disease requiring an increase in regular analgesic use, treatment with radiation therapy, or change in hormone therapy, or that was associated with a pathologic fracture or spinal cord compression or to death from prostate cancer;
ZAPCA: PSA or clinical progression, appearance of adverse events or withdrawal of informed consent by the participant. PSA progression was defined as three consecutive increases (of 0.1 ng/mL or greater) in PSA from the lowest level, and was measured at four‐week intervals. Clinical progression was defined as an increase of at least 20% in the sum of the longest diameters of the target lesions, appearance of one or more new lesions, clear progression of non‐target lesions, or appearance of two or more new bone metastases by bone scan. Clinical progression was also determined if the person's condition was worsening due to prostate cancer.
Excluded studies
We contacted the study authors from the BO18039 and CALGB 70604 trials to request further information (on 22 January 2016), and received no reply. We excluded 23 studies (26 references), which are presented in the Characteristics of excluded studies table, for the following reasons:
no control group (Adami 1985; Carey 1988; Clarke 1991; Cresswell 1995; Kylmala 1994; Pelger 1998; Vorreuther 1992; Vorreuther 1993);
inclusion of participants without bone metastasis (STAMPEDE);
inclusion of participants with other primary neoplasms (Jagdev 2001);
no subgroup analysis for participants with prostate cancer or participants with different therapy regimens (BO18039; CALGB 70604; NCT00242567);
non‐randomized study design (Heidenreich 2001; Heidenreich 2002);
comparison of bisphosphonates to denosumab (Fizazi 2009; Fizazi 2011);
report on histomorphometric or biochemical outcomes only (Fernandez‐Conde 1997; Magnusson 1998; Taube 1994);
active control group with other bisphosphonate (Adami 1989; MER‐101‐03; Wang 2013).
Risk of bias in included studies
See the 'Risk of bias' tables in the Characteristics of included studies table. The 'Risk of bias' is summarized in Figure 2. This figure presents our judgments for each study in a cross‐tabulation. In summary, we considered the quality of included trials to be moderate.
2.
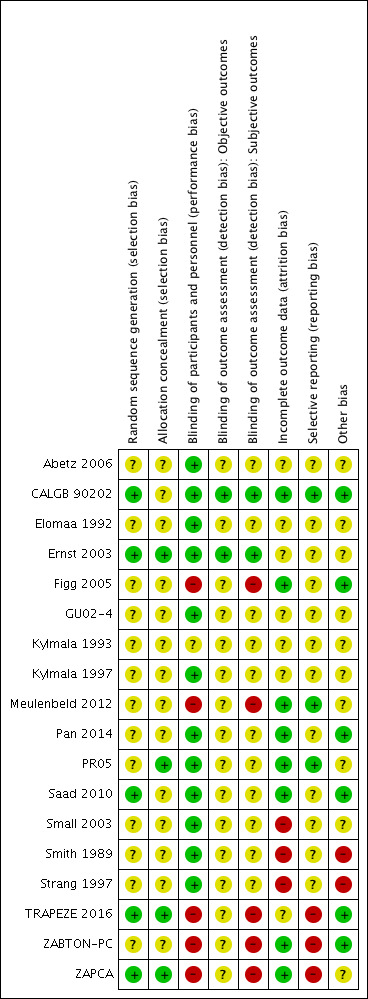
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Random sequence generation
Five trials described a random component in the sequence generation process and were at low risk of selection bias (CALGB 90202; Ernst 2003; Saad 2010; TRAPEZE 2016; ZAPCA). The other 13 trials were randomized studies, but without any further report on the sequence generation process (Abetz 2006; Elomaa 1992; Figg 2005; GU02‐4; Kylmala 1993; Kylmala 1997; Meulenbeld 2012; Pan 2014; PR05; Small 2003; Smith 1989; Strang 1997; ZABTON‐PC). Hence, we judged the risk of selection bias for these studies as unclear.
Allocation concealment
Four studies reported on the method to conceal allocation and were at low risk of selection bias (Ernst 2003; PR05; TRAPEZE 2016; ZAPCA). Fourteen trials provided no further information addressing allocation concealment and were at unclear risk of selection bias (Abetz 2006; CALGB 90202; Elomaa 1992; Figg 2005; GU02‐4; Kylmala 1993; Kylmala 1997; Meulenbeld 2012; Pan 2014; Saad 2010; Small 2003; Smith 1989; Strang 1997; ZABTON‐PC).
Blinding
Blinding of participants and personnel (performance bias)
Twelve trials described some type of blinding or placebo usage and were at low risk of performance bias (Abetz 2006; CALGB 90202; Elomaa 1992; Ernst 2003; GU02‐4; Kylmala 1997; Pan 2014; PR05; Saad 2010; Small 2003; Smith 1989; Strang 1997). One trial provided no information and was at unclear risk of performance bias (Kylmala 1993). Five trials were designed as open‐label studies and were at high risk of bias (Figg 2005; Meulenbeld 2012; TRAPEZE 2016; ZABTON‐PC; ZAPCA).
Blinding of outcome assessment (detection bias)
Objective outcomes
Two studies provided detailed information on blinding of outcome assessment in case of objective outcomes and were at low risk of detection bias (CALGB 90202; Ernst 2003). Sixteen trials provided no further information and were at unclear risk of detection bias because objective outcomes are by nature unaffected by blinding (Abetz 2006; Elomaa 1992; Figg 2005; GU02‐4; Kylmala 1993; Kylmala 1997; Meulenbeld 2012; Pan 2014; PR05; Saad 2010; Small 2003; Smith 1989; Strang 1997; TRAPEZE 2016; ZABTON‐PC; ZAPCA).
Subjective outcomes
Two trials reported on blinding of outcome assessment in case of subjective outcomes and were at low risk of detection bias (CALGB 90202; Ernst 2003). Seventeen trials had missing information (Abetz 2006; Elomaa 1992; Ernst 2003; Figg 2005; GU02‐4; Kylmala 1993; Kylmala 1997; Meulenbeld 2012; Pan 2014; PR05; Saad 2010; Small 2003; Smith 1989; Strang 1997; TRAPEZE 2016; ZABTON‐PC; ZAPCA). Of these, five trials were open‐label studies, which we judged at high risk of bias (Figg 2005; Meulenbeld 2012; TRAPEZE 2016; ZABTON‐PC; ZAPCA). We judged the remaining 11 trials at unclear risk of bias.
Incomplete outcome data
We assessed attrition bias for each outcome separately. However, as studies reported the same number of participants for all evaluated outcomes, we summed the judgment for attrition bias and reported the judgments here on a study level. Eight trials addressed incomplete outcome data adequately, describing reasons for missing data or including all randomized participant in the statistical analysis, and were at low risk of attrition bias (CALGB 90202; Figg 2005; Meulenbeld 2012; Pan 2014; PR05; Saad 2010; ZABTON‐PC; ZAPCA). Seven studies provided insufficient information and were at unclear risk of attrition bias (Abetz 2006; Elomaa 1992; Ernst 2003; GU02‐4; Kylmala 1993; Kylmala 1997; TRAPEZE 2016). Small 2003 excluded 7.4% of randomized participants from statistical efficacy analysis because of protocol violations. Therefore, we judged the risk of bias as high. Smith 1989 excluded 10.5% of randomized participants from statistical analysis because they did not complete one month of treatment. Consequently, we judged the risk of bias as high. Strang 1997 mentioned two different numbers of randomized participants (55 and 52 participants). We judged the risk of bias as high because of a potential loss of data of three participants without any information what happened to these participants.
Selective reporting
Three trials published a study protocol or included all expected outcomes and were at low risk of reporting bias (CALGB 90202; Meulenbeld 2012; PR05). Thirteen trials provided little information on primary or secondary outcomes and their definition and were at unclear risk for reporting bias (Abetz 2006; Elomaa 1992; Ernst 2003; Figg 2005; GU02‐4; Kylmala 1993; Kylmala 1997; Pan 2014; Saad 2010; Small 2003; Smith 1989; Strang 1997; TRAPEZE 2016). ZABTON‐PC initially planned per protocol to analyze survival data, but excluded survival data in the final publication. TRAPEZE 2016 and ZAPCA did not analyze all prespecified outcomes (e.g. QoL). Hence, we judged the risk of bias for these three studies as high.
Other potential sources of bias
Ten trials were neither at important or obvious risk of other sources of bias, nor completely free of other sources of bias (Abetz 2006; Elomaa 1992; Ernst 2003; GU02‐4; Kylmala 1993; Kylmala 1997; Meulenbeld 2012; PR05; Small 2003; ZAPCA). Authors of these studies had conflicts of interest or the studies were funded trials. Influence of the funding source and the conflicts of interest on the study design, conduction and outcome evaluation remained unclear. Consequently, we judged these studies at unclear risk of other bias. Six trials seemed to be free of other sources of bias and were at low risk of other bias (CALGB 90202; Figg 2005; Pan 2014; Saad 2010; TRAPEZE 2016; ZABTON‐PC).
Abetz 2006 did not sufficiently report on methods. Smith 1989 provided no information on statistical analysis of observed results. Strang 1997 was prematurely terminated because of low accrual. We judged the risk of bias for these three studies as high.
Effects of interventions
See: Table 1
Bisphosphonates versus control (placebo, chemotherapy or no treatment)
Primary outcome: proportion of participants with pain response
Meta‐analysis
Three RCTs provided data on the proportion of participants with pain response (Ernst 2003; Meulenbeld 2012; Smith 1989). Finally, 133/449 participants in the bisphosphonates group and 113/427 participants in the control groups demonstrated with pain response. Bisphosphonates showed no clear clinically relevant benefit in the proportion of participants with pain response (RR 1.15, 95% CI 0.93 to 1.43, P = 0.20, I2 = 0%, low quality evidence; Analysis 1.1; Figure 3). In absolute terms, bisphosphonates resulted in pain response in 39 more participants per 1000 (19 fewer to 114 more). We downgraded the quality of evidence by one point due to the risk of performance, detection and attrition bias in these trials and downgraded one more point due to the small number of events (see Table 1).
1.1. Analysis.

Comparison 1 Bisphosphonates versus control, Outcome 1 Proportion of participants with pain response.
3.
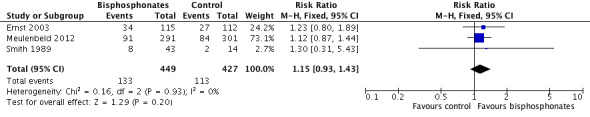
Forest plot of comparison: 1 Bisphosphonates versus control, outcome: 1.1 Proportion of participants with pain response.
Single study results
Data of eight studies could be included in the analysis of the proportion of participants with pain response (Abetz 2006; Elomaa 1992; Kylmala 1993; Kylmala 1997; Meulenbeld 2012; Saad 2010; Small 2003; Strang 1997). These studies provided information on the participants' pain responses, but no precise information on the proportion of participants with pain response. Hence, inclusion in pooled data analysis of this outcome was not possible. Due to the heterogeneous nature of these studies and presentation of only qualitative data, we reported these studies narratively.
Abetz 2006 demonstrated a significant reduction in "pain at its worst," "pain at its least" and "pain on average" in weeks 6, 8 and 12 for participants receiving zoledronic acid in comparison with placebo (P < 0.05), favoring the zoledronic arm. Saad 2010 detected a statistically significant reduction in brief pain inventory scores in weeks 3, 9, 21 and 24 in participants receiving zoledronic acid versus participants receiving placebo (week 3: P = 0.003, week 9: P = 0.03, week 21: P = 0.014, week 24: P = 0.024). Moreover, participants receiving zoledronic acid had significantly smaller increases in pain scores than participants receiving placebo (P = 0.05, Saad 2010). Elomaa 1992, Kylmala 1993, Kylmala 1997, and Strang 1997 detected no statistical significant difference in pain reduction between participants on clodronate or control group. The Meulenbeld 2012 trial showed no significant difference in pain reduction for participants receiving risedronate versus placebo. Small 2003 showed no significant difference in pain reduction for participants receiving pamidronate in comparison with placebo.
Secondary outcome: skeletal‐related events
Meta‐analysis
Nine RCTs provided sufficient data on the number of SREs (CALGB 90202; GU02‐4; Pan 2014; PR05; Saad 2010; Small 2003; TRAPEZE 2016; ZABTON‐PC; ZAPCA). A total of 687/1681 participants receiving bisphosphonates and 689/1472 participants receiving control experienced any SRE. Bisphosphonates probably reduced the incidence of any SREs in men with prostate cancer (RR 0.87, 95% CI 0.81 to 0.94, P = 0.27, I2 = 19%, moderate quality evidence; Analysis 1.2; Figure 4). In absolute terms, bisphosphonates resulted in 58 fewer SREs per 1000 (85 fewer to 27 fewer). We downgraded the quality of evidence due to the risk of performance and attrition bias in these trials (see Table 1).
1.2. Analysis.
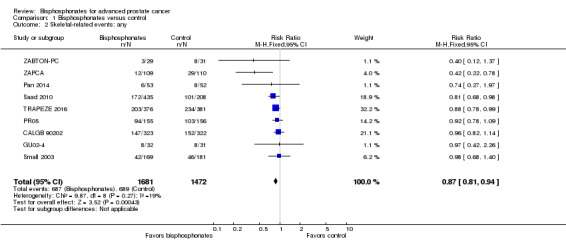
Comparison 1 Bisphosphonates versus control, Outcome 2 Skeletal‐related events: any.
4.
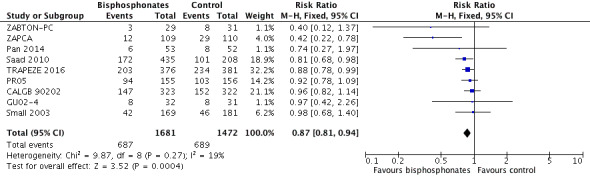
Forest plot of comparison: 1 Bisphosphonates versus control, outcome: 1.2 Skeletal‐related events: any.
Pathologic fractures: total
Six RCTs with 2226 participants investigated the incidence of pathologic fractures in detail (Pan 2014; PR05; Saad 2010; Small 2003; TRAPEZE 2016; ZABTON‐PC). A total of 107/1217 participants receiving bisphosphonates and 113/1009 participants receiving control experienced any pathologic fracture. Bisphosphonates probably reduced the number pathologic fractures in comparison with control regimens (RR 0.68, 95% CI 0.53 to 0.87, P = 0.002, I2 = 35%, moderate heterogeneity; Analysis 1.3).
1.3. Analysis.
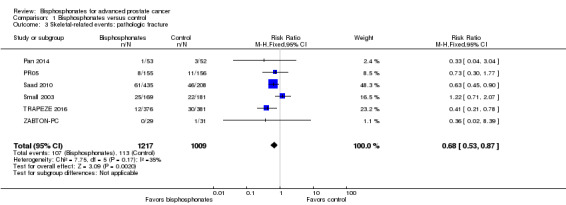
Comparison 1 Bisphosphonates versus control, Outcome 3 Skeletal‐related events: pathologic fracture.
Pathologic fractures: vertebral fracture
Two RCTs with 993 participants reported the number of vertebral fractures (Saad 2010; Small 2003). A total of 36/604 participants receiving bisphosphonates and 27/389 participants receiving control had vertebral fractures. There was no clear difference in the number of vertebral fractures between groups (RR 0.84, 95% CI 0.52 to 1.36, P = 0.49, I2 = 0%; Analysis 1.4).
1.4. Analysis.
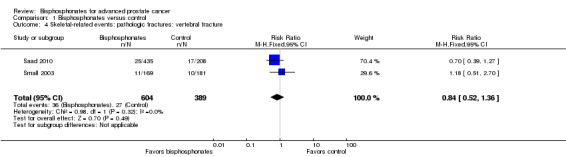
Comparison 1 Bisphosphonates versus control, Outcome 4 Skeletal‐related events: pathologic fractures: vertebral fracture.
Pathologic fractures: non‐vertebral fracture
Two RCTs with 993 participants reported the number of non‐vertebral fractures (Saad 2010; Small 2003). A total of 58/604 participants receiving bisphosphonates and 45/389 participants receiving control had non‐vertebral fractures. Bisphosphonates showed no clinically relevant difference in the number of non‐vertebral fractures (RR 0.76, 95% CI 0.53 to 1.10, P = 0.14, I2 = 58%, moderate heterogeneity; Analysis 1.5).
1.5. Analysis.
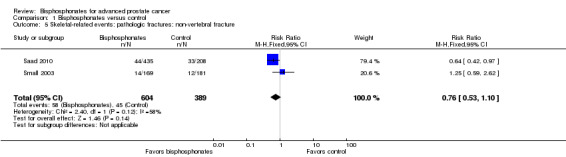
Comparison 1 Bisphosphonates versus control, Outcome 5 Skeletal‐related events: pathologic fractures: non‐vertebral fracture.
Spinal cord compression
Six RCTs with 2226 participants provided data regarding the proportion of participants with spinal cord compression (Pan 2014; PR05; Saad 2010; Small 2003; TRAPEZE 2016; ZABTON‐PC). A total of 75/1217 participants receiving bisphosphonates and 99/1009 participants receiving control experienced spinal cord compression. Bisphosphonates probably reduced the number of participants affected by spinal cord compression (RR 0.67, 95% CI 0.50 to 0.89, P = 0.005, I2 = 0%; Analysis 1.6).
1.6. Analysis.
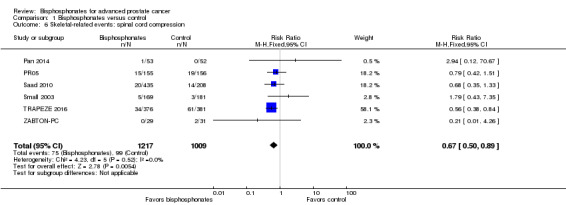
Comparison 1 Bisphosphonates versus control, Outcome 6 Skeletal‐related events: spinal cord compression.
Bone radiation therapy
Six RCTs with 1696 participants provided data regarding the number of participants treated with radiation to bone (Ernst 2003; Pan 2014; PR05; Saad 2010; Small 2003; ZABTON‐PC). A total of 230/956 participants receiving bisphosphonates and 196/740 participants receiving control received radiation to bone. Bisphosphonates show no clear difference in the number of participants treated with radiation therapy to bone (RR 0.90, 95% CI 0.77 to 1.06, P = 0.21, I2 = 0%; Analysis 1.7).
1.7. Analysis.
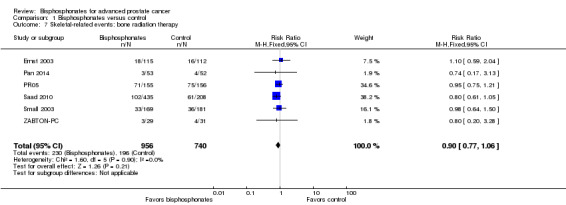
Comparison 1 Bisphosphonates versus control, Outcome 7 Skeletal‐related events: bone radiation therapy.
Bone surgery
Five RCTs with 1915 participants provided data regarding the number of participants undergoing surgery to bone (Pan 2014; Saad 2010; Small 2003; TRAPEZE 2016; ZABTON‐PC). A total of 22/1062 participants receiving bisphosphonates and 35/853 participants receiving control received surgery to bone. Bisphosphonates probably reduced the proportion of participants with bone surgery (RR 0.50, 95% CI 0.29 to 0.86, P = 0.01, I2 = 5%; Analysis 1.8).
1.8. Analysis.
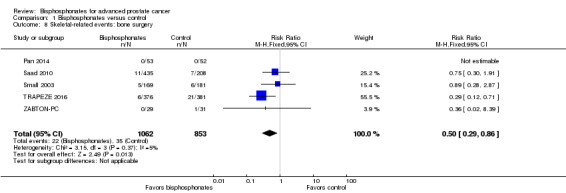
Comparison 1 Bisphosphonates versus control, Outcome 8 Skeletal‐related events: bone surgery.
Single study results
Three trials investigated the time to SREs (CALGB 90202; Saad 2010; TRAPEZE 2016). CALGB 90202 showed a median time to SRE of 31.9 months for participants receiving zoledronic acid in comparison with 28.8 months for participants receiving placebo (HR 0.97, 95% CI 0 to 1.174, P = 0.385). Saad 2010 detected a median time to SRE of 448 days for participants receiving zoledronic acid 4 mg versus 321 days for participants receiving placebo (HR 0.667, 95% CI 0.505 to 0.908, P = 0.009). TRAPEZE 2016 reported a median time to SRE of 13.6 months for the zoledronic acid arm versus 11.2 months for the control arm. In summary, the qualitative analysis probably indicated a prolonged time to SREs in participants receiving bisphosphonates.
Pathologic fracture: total
None of the RCTs reported pathologic fractures.
Pathologic fractures: vertebral fracture
None of the RCTs reported vertebral fractures.
Pathologic fractures: non‐vertebral fracture
None of the RCTs reported non‐vertebral fractures.
Spinal cord compression
None of the RCTs reported spinal cord compression.
Bone radiation therapy
None of the RCTs reported radiation to bone.
Bone surgery
None of the RCTs reported surgery to bone.
Secondary outcome: mortality
Meta‐analysis
Nine RCTs with 2450 participants provided quantitative data on the mortality (CALGB 90202; Elomaa 1992; Ernst 2003; GU02‐4; Kylmala 1993; Meulenbeld 2012; PR05; Small 2003; ZABTON‐PC). A total of 615/1213 participants receiving bisphosphonates and 640/1237 participants receiving control died during study treatment or follow‐up. Bisphosphonates demonstrated no clinically relevant difference in mortality (RR 0.97, 95% CI 0.91 to 1.04, P = 0.43, I2 = 1%, moderate quality evidence; Analysis 1.9; Figure 5). In absolute terms, bisphosphonates resulted in 16 fewer deaths per 1000 (47 fewer to 21 more). We downgraded the quality of evidence due to a potential risk of performance and attrition bias in these trials (see Table 1).
1.9. Analysis.
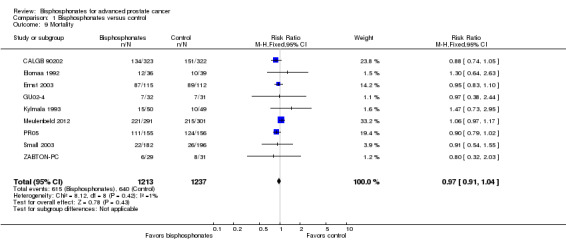
Comparison 1 Bisphosphonates versus control, Outcome 9 Mortality.
5.
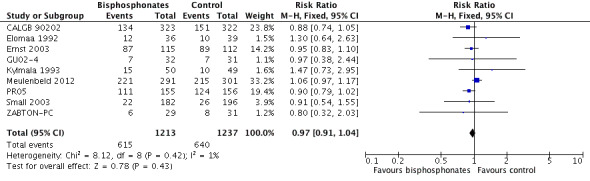
Forest plot of comparison: 1 Bisphosphonates versus control, outcome: 1.9 Mortality.
Single study results
Data of 11 studies could be included in the analysis of mortality (CALGB 90202; Elomaa 1992; Ernst 2003; Figg 2005; Kylmala 1993; Meulenbeld 2012; Pan 2014; PR05; Saad 2010; TRAPEZE 2016; ZAPCA). On the one hand, Pan 2014 (median survival of 19 months for participants receiving bisphosphonates versus 15 months for participants receiving placebo, P = 0.02) and PR05 (HR 0.77, 95% CI 0.60 to 0.98, P = 0.032) detected a statistically significant survival advantage in favor of bisphosphonates. On the other hand, four trials showed no significant difference in survival advantage for participants receiving bisphosphonates (CALGB 90202; Meulenbeld 2012; Saad 2010; ZAPCA). CALGB 90202 reported a median survival of 37.9 months for participants receiving zoledronic acid versus 36.0 months for participants receiving placebo (HR 0.88, 95% CI 0.7 to 1.12, P = 0.29). Meulenbeld 2012 reported a median survival of 19.2 months for participants receiving risedronate in comparison with 18.4 months for participants receiving control (HR 1.09, P = 0.33). Saad 2010 found a median survival of 546 days for participants receiving zoledronic acid 4 mg versus 464 days for participants receiving placebo (P = 0.091). ZAPCA documented no clinically relevant difference in overall survival between participants receiving zoledronic acid in comparison with the control group (HR 0.78, 95% CI 0.49 to 1.23, P = 0.28). In Figg 2005, participants receiving alendronate reached a median survival of 19 months, whereas participants receiving control did not reach median survival. Elomaa 1992 showed no statistical significant difference between the clodronate group and the placebo group. Furthermore, three studies showed a survival advantage for participants in control arms (Ernst 2003; Kylmala 1993; TRAPEZE 2016). Ernst 2003 demonstrated a median survival of 10.8 months for participants receiving clodronate in comparison with 11.5 months for participants receiving placebo (HR 0.95, 95% CI 0.71 to 1.28). Kylmala 1993 reported a median survival of 10 months for clodronate and 12 months for control. TRAPEZE 2016 showed a median survival of 16.99 months for participants receiving zoledronic acid compared to 17.06 months for participants receiving placebo (HR 0.99, 95% CI 0.84 to 1.16, P = 0.91).
Secondary outcome: quality of life
Meta‐analysis
None of the RCTs provided quantitative data on QoL.
Single study results
Four RCTs provided data on QoL (Abetz 2006; Ernst 2003; Saad 2010; Small 2003). Abetz 2006 showed no significant difference in any of the QoL items other than "Interference with general activities" at weeks 30 to 32 and 42 to 44 (favoring zoledronic acid arm, P < 0.05). On the one hand, Ernst 2003 demonstrated a significant reduction in "pain" for participants on clodronate in comparison with placebo (P = 0.022). On the other hand, none of the other items of the QoL analysis showed a significant difference between clodronate and placebo. Saad 2010 showed no significant difference between participants receiving zoledronic acid 4 mg versus participants receiving placebo regarding analgesic scores, pain scores, FACT‐G quality‐of‐life and EURO‐QOL scores. Small 2003 reported no significant difference in "mobility measurements" between participants receiving pamidronate versus placebo.
Secondary outcome: adverse events
Meta‐analysis
Nausea
Nine RCTs with 3008 participants provided data addressing the number of participants with nausea (CALGB 90202; Ernst 2003; Kylmala 1993; Kylmala 1997; Meulenbeld 2012; Pan 2014; PR05; Saad 2010; Small 2003). A total of 268/1606 participants receiving bisphosphonates and 142/1402 participants receiving control developed nausea. Bisphosphonates probably increased the number of participants affected by nausea (RR 1.19, 95% CI 1.00 to 1.41, P = 0.05, I2 = 0%, moderate quality evidence; Analysis 1.10). In absolute terms, bisphosphonates resulted in seven more cases of nausea per 1000 (0 fewer to 14 more). We downgraded the quality of evidence due to a potential risk of performance and attrition bias in these trials (see Table 1).
1.10. Analysis.

Comparison 1 Bisphosphonates versus control, Outcome 10 Adverse events: nausea.
Renal
Seven RCTs with 1794 participants provided data regarding the number of participants with renal adverse events (CALGB 90202; Elomaa 1992; Figg 2005; Kylmala 1997; Pan 2014; Saad 2010; ZAPCA). A total of 87/1010 participants receiving bisphosphonates and 27/784 participants receiving control had renal adverse events. Statistical analysis revealed that bisphosphonates probably increased the number of renal adverse events (RR 1.65, 95% CI 1.11 to 2.46, P = 0.01, I2 = 0%, moderate quality evidence; Analysis 1.11; Figure 6). In absolute terms, bisphosphonates resulted in 22 more renal adverse events per 1000 (4 more to 50 more). We downgraded the quality of evidence due a potential risk of performance and attrition bias in these trials (see Table 1).
1.11. Analysis.

Comparison 1 Bisphosphonates versus control, Outcome 11 Adverse events: renal.
6.

Forest plot of comparison: 1 Bisphosphonates versus control, outcome: 1.11 Adverse events: renal.
Bone pain
Five RCTs with 1445 participants provided data on the number of participants with bone pain (Kylmala 1997; PR05; Saad 2010; Small 2003; ZABTON‐PC). A total of 261/827 participants receiving bisphosphonates and 146/618 participants receiving control experienced bone pain. Bisphosphonates showed no clinically relevant difference in the frequency of bone pain (RR 0.93, 95% CI 0.81 to 1.06, P = 0.29, I2 = 24%; Analysis 1.12).
1.12. Analysis.
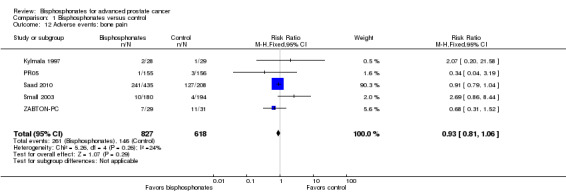
Comparison 1 Bisphosphonates versus control, Outcome 12 Adverse events: bone pain.
Osteonecrosis of the jaw
Five RCTs with 1626 participants provided data on the proportion of participants with ONJ (CALGB 90202; Meulenbeld 2012; Pan 2014; ZABTON‐PC; ZAPCA). A total of 12/808 participants receiving bisphosphonates and 6/818 participants receiving control had ONJ. Bisphosphonates did not clearly increase the number of participants with ONJ (RR 1.92, 95% CI 0.75 to 4.90, P = 0.17, I2 = 0%, very low quality evidence; Analysis 1.13). In absolute terms, bisphosphonates resulted in seven more cases with ONJ per 1000 (2 fewer to 29 more). We downgraded the quality of evidence to very low due a potential risk of performance and attrition bias in these trials and the very small number of events (see Table 1).
1.13. Analysis.
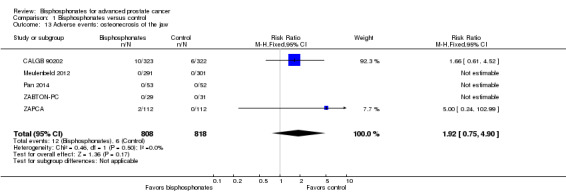
Comparison 1 Bisphosphonates versus control, Outcome 13 Adverse events: osteonecrosis of the jaw.
Single study results
Nausea
None of the RCTs provided qualitative data on the proportion of participants with nausea.
Renal
Two studies reported qualitative data on the proportion of participants with renal adverse events (Pan 2014; Saad 2010). Both trials used zoledronic acid. Pan 2014 showed no significant difference in the number of participants with renal impairment (P = 0.12). Saad 2010 presented the relative risk of participants receiving zoledronic acid 4 mg versus placebo. Participants receiving zoledronic acid had an RR of 1.07 (95% CI 0.46 to 2.47, P = 0.882) to experience renal adverse events in comparison with the placebo group. Participants with an initial dose of zoledronic acid 8 mg, which was decreased due to renal toxicity, had an RR of 1.76 (95% CI 0.79 to 3.93, P = 0.165) compared to the placebo group. Analyzing the different doses of zoledronic acid, the RR was 1.63 (95% CI 0.80 to 3.30, P = 0.176) for participants with zoledronic acid 8/4 mg with participants receiving zoledronic 4 mg as reference population.
Bone pain
None of the RCTs provided qualitative data on the proportion of participants with bone pain.
Osteonecrosis of the jaw
None of the RCTs provided qualitative data on the proportion of participants with ONJ.
Secondary outcome: proportion of participants with decreased analgesic consumption
Meta‐analysis
Four RCTs with 416 participants reported on the proportion of participants with decreased analgesic consumption (Elomaa 1992; Ernst 2003; Kylmala 1997; Smith 1989). A total of 62/222 participants receiving bisphosphonates and 49/194 participants receiving control were able to reduce their analgesic consumption. Bisphosphonates did not lead to a clinically relevant difference in analgesic consumption (RR 1.19, 95% CI 0.87 to 1.63, P = 0.28, I2 = 37%, moderate heterogeneity; Analysis 1.14).
1.14. Analysis.

Comparison 1 Bisphosphonates versus control, Outcome 14 Proportion of participants with decreased analgesic consumption.
Single study results
Five studies provided qualitative data regarding analgesic consumption (Elomaa 1992; Kylmala 1993; Kylmala 1997; PR05; Small 2003). Elomaa 1992, Kylmala 1993 and Kylmala 1997 showed no significant difference in analgesic consumption between participants on clodronate and placebo. PR05 demonstrated a 12% reduction of analgesic consumption in participants receiving clodronate in comparison with placebo. Small 2003 showed no significant difference in analgesic consumption between participants receiving pamidronate and placebo.
Secondary outcome: proportion of participants with disease progression
Meta‐analysis
Seven studies with 2115 participants provided data on disease progression (CALGB 90202; Ernst 2003; Kylmala 1997; Meulenbeld 2012; Pan 2014; PR05; ZAPCA). A total of 778/1055 participants receiving bisphosphonates and 832/1060 participants receiving control experienced disease progression. Bisphosphonates probably reduced the number of participants with disease progression (RR 0.94, 95% CI 0.90 to 0.98, P = 0.006, I2 = 0%, moderate quality evidence; Analysis 1.15). In absolute terms, bisphosphonates resulted in 36 fewer cases of disease progression per 1000 (71 fewer to 7 fewer). We downgraded the quality of evidence due to the potential risk of performance and attrition bias in these trials (see Table 1).
1.15. Analysis.
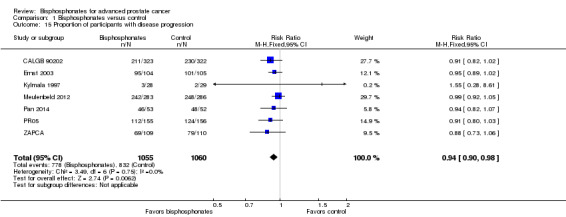
Comparison 1 Bisphosphonates versus control, Outcome 15 Proportion of participants with disease progression.
Single study results
Seven studies reported qualitative data regarding disease progression (CALGB 90202; Ernst 2003; Figg 2005; Meulenbeld 2012; Pan 2014; PR05; TRAPEZE 2016). Six trials documented a beneficial effect of bisphosphonates on disease progression, but none of them showed a significant difference between the study cohorts. CALGB 90202 showed a median time to disease progression of 10.6 months for the zoledronic acid group versus 9.2 months for placebo group (HR 0.89, 95% CI 0.74 to 1.07, P = 0.22). Ernst 2003 reported a median progression‐free survival of five months for participants receiving clodronate versus four months for participants receiving placebo (HR 1.23, 95% CI 0.934 to 1.64, P = 0.136). Figg 2005 showed a median progression‐free survival of 4.6 months in the alendronate group in comparison with 3.8 months in the placebo group (P = 0.27). Pan 2014 reported a median time to disease progression of nine months for the zoledronic acid group versus six months for the placebo group (P < 0.05). PR05 showed a median progression‐free survival of 23.6 months for participants receiving clodronate compared to 19.3 months for participants receiving control (HR 0.79, 95% CI 0.61 to 1.02, P = 0.066). TRAPEZE 2016 demonstrated an HR of 0.98 (95% CI 0.85 to 1.14) for time to disease progression comparing participants receiving zoledronic acid with control group (P = 0.81). One study demonstrated a prolonged time to disease progression for the placebo group (7.4 months) in comparison with the risedronate group (6.5 months), however, without any evidence for a difference in the time‐to‐event analysis (HR 1.04, 95% CI 0.87 to 1.24) (Meulenbeld 2012).
Subgroup and sensitivity analyses
We carried out subgroup analyses considering the different classes of bisphosphonates (amino‐bisphosphonate versus non‐amino‐bisphosphonate) and the route of administration (oral versus IV). Additionally, we performed sensitivity analyses comparing studies at high risk of bias with studies at low risk of bias and comparing full‐text publications with abstract publications. We planned to focus on the most participants relevant outcomes and the most clinically relevant outcomes. We intended to perform subgroup and sensitivity analyses for the proportion of participants with pain response, SREs, mortality, renal adverse events and ONJ.
Proportion of participants with pain response
We performed sensitivity analysis according to the risk of bias but not for publication status analyzing pain response because all trials reporting the proportion of participants with pain response were full‐text publications. This analysis showed that bisphosphonates did not lead to a clinically relevant difference in the proportion of participants with pain response (test for subgroup differences: Chi2 = 0.11, P = 0.74, I2 = 0%; Analysis 1.16). There was no clear difference in any of the performed subgroup analyses between groups in the proportion of participants with pain response, neither considering different classes of bisphosphonates (Chi2 = 0.15, P = 0.70, I2 = 0%; Analysis 1.17) nor according to the route of administration (Chi2 = 0.15, P = 0.70, I2 = 0%; Analysis 1.18).
1.16. Analysis.
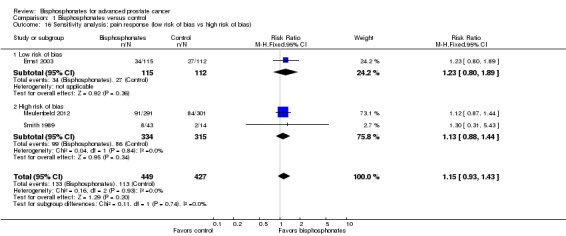
Comparison 1 Bisphosphonates versus control, Outcome 16 Sensitivity analysis: pain response (low risk of bias vs high risk of bias).
1.17. Analysis.

Comparison 1 Bisphosphonates versus control, Outcome 17 Subgroup analysis: pain response (amino‐bisphosphonate vs non‐amino‐bisphosphonate).
1.18. Analysis.
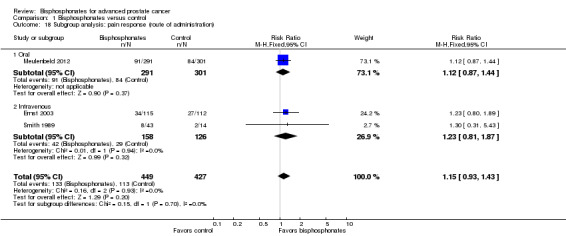
Comparison 1 Bisphosphonates versus control, Outcome 18 Subgroup analysis: pain response (route of administration).
Skeletal‐related events
For SREs, sensitivity and subgroup analyses were based on the total number of SREs. Statistical analyses revealed that bisphosphonates probably decreased the number of SREs. This finding was evident comparing studies at high risk of bias to studies at low risk of bias (Chi2 = 0.06, P = 0.81, I2 = 0%; Analysis 1.19) as well as comparing full‐text to abstract publications (Chi2 = 1.56, P = 0.21, I2 = 35.9%; Analysis 1.20). In both subgroup analyses, bisphosphonates probably decreased number of SREs, in the subset of different classes of bisphosphonates (Chi2 = 0.39, P = 0.53, I2 = 0%; Analysis 1.21) or for different routes of administration (Chi2 = 0.47, P = 0.49, I2 = 0%; Analysis 1.22).
1.19. Analysis.
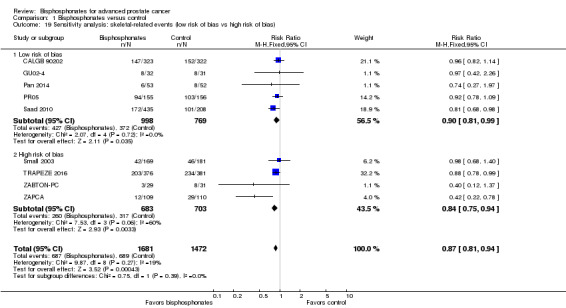
Comparison 1 Bisphosphonates versus control, Outcome 19 Sensitivity analysis: skeletal‐related events (low risk of bias vs high risk of bias).
1.20. Analysis.
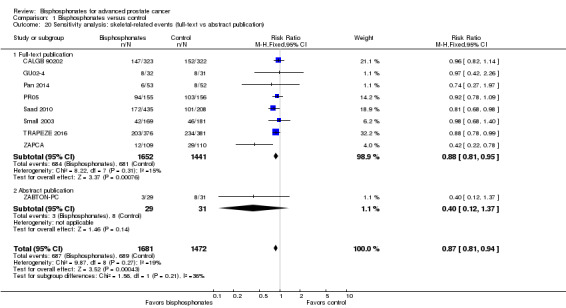
Comparison 1 Bisphosphonates versus control, Outcome 20 Sensitivity analysis: skeletal‐related events (full‐text vs abstract publication).
1.21. Analysis.
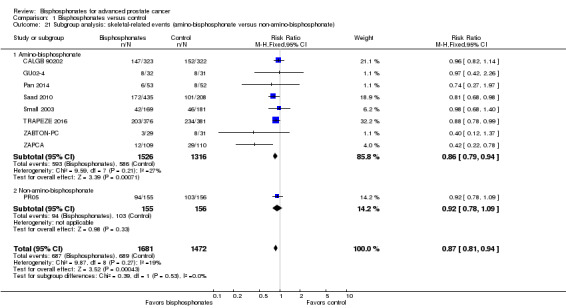
Comparison 1 Bisphosphonates versus control, Outcome 21 Subgroup analysis: skeletal‐related events (amino‐bisphosphonate versus non‐amino‐bisphosphonate).
1.22. Analysis.

Comparison 1 Bisphosphonates versus control, Outcome 22 Subgroup analysis: skeletal‐related events (route of administration).
Mortality
For mortality, sensitivity analyses revealed that there was no clear difference between bisphosphonates and control (placebo or no treatment). This finding was evident comparing studies at high risk of bias to studies at low risk of bias (Chi2 = 2.46, P = 0.12, I2 = 59.4%; Analysis 1.23) and full‐text to abstract publications (Chi2 = 0.17, P = 0.68, I2 = 0%; Analysis 1.24). In both subgroup analyses, there was no clinically relevant difference in mortality, neither in the subset of different classes of bisphosphonates (Chi2 = 0.08, P = 0.78, I2 = 0%; Analysis 1.25) nor in different routes of administration (Chi2 = 2.75, P = 0.10, I2 = 63.7%; Analysis 1.26).
1.23. Analysis.

Comparison 1 Bisphosphonates versus control, Outcome 23 Sensitivity analysis: mortality (low risk of bias vs high risk of bias)).
1.24. Analysis.
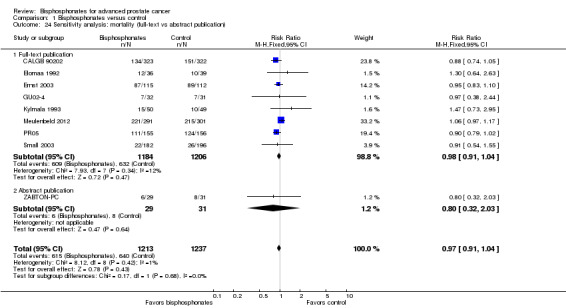
Comparison 1 Bisphosphonates versus control, Outcome 24 Sensitivity analysis: mortality (full‐text vs abstract publication).
1.25. Analysis.

Comparison 1 Bisphosphonates versus control, Outcome 25 Subgroup analysis: mortality (amino‐bisphosphonate vs non‐amino‐bisphosphonate).
1.26. Analysis.

Comparison 1 Bisphosphonates versus control, Outcome 26 Subgroup analysis: mortality (route of administration).
Adverse events: nausea
For nausea, sensitivity and subgroup analyses were based on the total number of participants affected by nausea. Statistical analyses revealed that bisphosphonates probably increased the number of participants affected by nausea. This finding was evident comparing studies at high risk of bias to studies at low risk of bias (Chi2 = 0.11, P = 0.74, I2 = 0%; Analysis 1.27). In both subgroup analyses, bisphosphonates probably decreased number of SREs in the subset of different classes of bisphosphonates (Chi2 = 0.00, P = 0.97, I2 = 0%; Analysis 1.28) or for different routes of administration (Chi2 = 3.65, P = 0.89, I2 = 0%; Analysis 1.29).
1.27. Analysis.
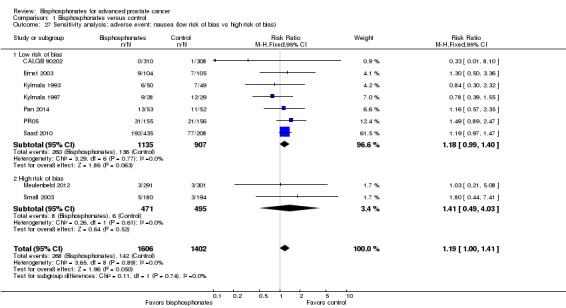
Comparison 1 Bisphosphonates versus control, Outcome 27 Sensitivity analysis: adverse event: nausea (low risk of bias vs high risk of bias).
1.28. Analysis.
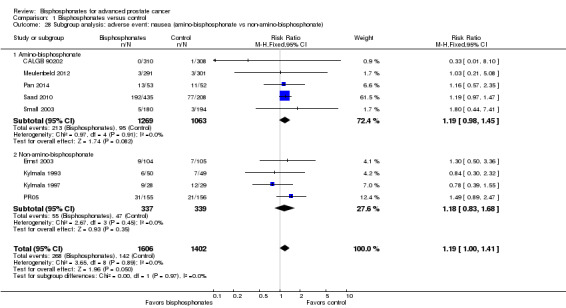
Comparison 1 Bisphosphonates versus control, Outcome 28 Subgroup analysis: adverse event: nausea (amino‐bisphosphonate vs non‐amino‐bisphosphonate).
1.29. Analysis.
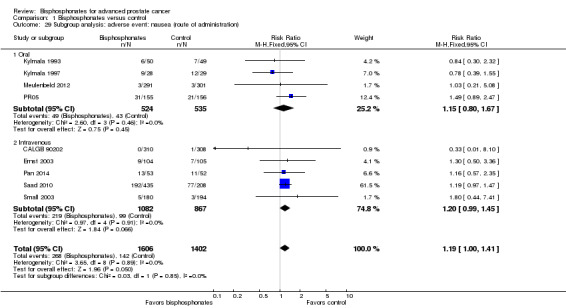
Comparison 1 Bisphosphonates versus control, Outcome 29 Subgroup analysis: adverse event: nausea (route of administration).
Adverse events: renal
Sensitivity analysis was performed according to the risk of bias but not for publication status analyzing renal adverse events because all trials reporting on these events were full‐text publications. This analysis showed that bisphosphonates may have increased the number of renal adverse events (Chi2 = 0.04, P = 0.84, I2 = 0%; Analysis 1.30). In both subgroup analyses, bisphosphonates may have increased renal adverse events, in the subset of different classes of bisphosphonates (Chi2 = 0.18, P = 0.67, I2 = 0%; Analysis 1.31) or for different routes of administration (Chi2 = 0.17, P = 0.68, I2 = 0%; Analysis 1.32).
1.30. Analysis.
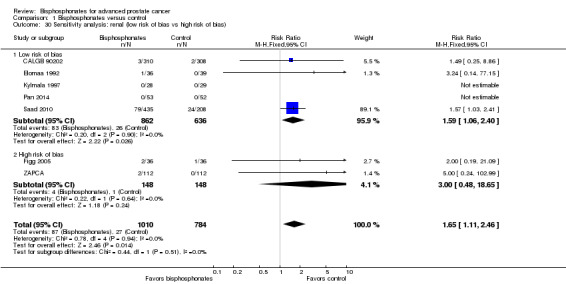
Comparison 1 Bisphosphonates versus control, Outcome 30 Sensitivity analysis: renal (low risk of bias vs high risk of bias).
1.31. Analysis.

Comparison 1 Bisphosphonates versus control, Outcome 31 Subgroup analysis: renal (amino‐bisphosphonate vs non‐amino‐bisphosphonate).
1.32. Analysis.
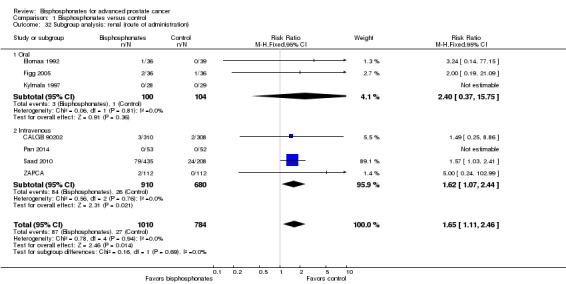
Comparison 1 Bisphosphonates versus control, Outcome 32 Subgroup analysis: renal (route of administration).
Adverse events: osteonecrosis of the jaw
As only a small number of events occurred in two trials only with one trial reporting only on two events in one arm, sensitivity and subgroup analyses seemed to be inappropriate.
Discussion
Summary of main results
The following main results emerged from this Cochrane Review and meta‐analysis investigating the effect of bisphosphonates compared to placebo, chemotherapy or standard of care in men with bone metastases from primary hormone‐naive, hormone‐sensitive, but mostly hormone‐refractory prostate cancer.
The use of bisphosphonates showed no clear difference in the proportion of participants with pain response.
Bisphosphonates probably decreased the number of participants with SREs in general. Analysis in detail revealed that bisphosphonates lead to a clinically relevant reduction of pathologic fractures, spinal cord compressions or bone surgery, but there was no clear difference in radiation to bone or in vertebral and non‐vertebral fractures.
There was no clinically relevant difference in mortality between participants receiving bisphosphonates in comparison with participants receiving control regimens.
We were unable to perform a quantitative analysis of the studies' QoL assessments. Analysis revealed that the studies used several assessment tools. Generally accepted and used QoL outcomes could not be identified.
Bisphosphonates probably increased the number of participants with renal adverse events, but also the number of participants with nausea. There was no clear difference in the proportion of participants with ONJ or bone pain.
We observed no clinically relevant difference in the proportion of participants with decreased analgesic consumption.
Pooled‐data analysis revealed that bisphosphonates probably decreased the number of participants with disease progression in participants with prostate cancer metastatic to bone.
We identified no subgroup differences in terms of amino‐bisphosphonates versus non‐amino‐bisphosphonates or route of administration (oral versus IV). We also found no difference in sensitivity analyses (low risk of bias versus high risk of bias; full‐text publication versus abstract publication).
Overall completeness and applicability of evidence
This systematic review addressed the review question by analyzing data on participants with bone metastases from treatment‐naive, castration‐sensitive or castration‐resistant advanced prostate cancer. Castration‐resistant prostate cancer accounted for most cancers. The group of bisphosphonates included zoledronate (seven trials), clodronate (six trials), risedronate (two trials), alendronate (one trial), pamidronate (one trial) and etidronate (one trial). Current German, American and European guidelines recommend zoledronic acid for the prevention of skeletal complications and for bone pain relief in participants with castration‐resistant prostate cancer (Conford 2017; Cookson 2013; Wirth 2016). Hence, zoledronic acid is the most frequently used bisphosphonate in clinical practice. On the contrary, the US Food and Drug Administration (FDA) has still not approved clodronate (El‐Amm 2016). Clodronate was included in one third of all trials, but plays a minor role in clinical routine. Overall, this review answered the review question considering different subgroups of participants with advanced prostate cancer and different types of bisphosphonates.
Pain frequently occurs in men with bone metastasis from prostate cancer and its control is one target in palliative therapy. Consequently, the proportion of participants with pain response has been defined as primary outcome of this review and the meta‐analysis. Pooled data analysis consisted of three trials with 876 participants. We found a low quality of evidence and observed no clear difference in pain response between bisphosphonates and control. This finding might have been influenced by differing end point definitions in the trials. Smith 1989 provided no definition and Ernst 2003 considered disease progression as 'pain event.' Meulenbeld 2012 defined an at least 2‐ point reduction from baseline PPI score without increase in analgesic class or decrease in analgesic class without increased PPI score as pain response. Noteworthy, the three trials used different bisphosphonates: clodronate (Ernst 2003), risedronate (Meulenbeld 2012), and etidronate (Smith 1989) and all participants had castration‐resistant prostate cancer. Zoledronate is the most frequently used bisphosphonate in men with prostate cancer, but none of the studies with participants receiving zoledronic acid could be included in the statistical synthesis. Descriptive analysis revealed that in two trials participants receiving zoledronic acid presented with clinically relevant lower pain scores in comparison with placebo groups (Abetz 2006; Saad 2010). As zoledronate is the clinically most important bisphosphonate, applicability of our quantitative results on pain response is restricted to participants receiving clodronate, risedronate and etidronate. To clarify the role of zoledronic acid in pain management in men with bone metastases from prostate cancer, future studies should evaluate pain response by clearly mentioning the proportion of participants with pain response, defining pain response and providing clear definitions of pain response. Further analyses in participants with bone metastases from treatment‐naive or castration‐sensitive prostate cancer are needed, as we could not include any of the fours trials reporting on this subset of participants (CALGB 90202; PR05; ZABTON‐PC; ZAPCA).
Bone metastases may result in SREs. Pooled data analysis of SREs showed that bisphosphonates probably decreased the total number of SREs. Qualitative analysis demonstrated that zoledronic acid probably delayed the onset of SREs and four out of five studies included in the meta‐analysis compared zoledronic acid to control groups. These findings derived from trials reporting on participants with either castration‐resistant or castration‐sensitive prostate cancer. Finally, zoledronic acid probably increased the time to any SRE and decreased the number of SREs in men with bone metastases from prostate cancer.
SREs were defined as a composite outcome consisting of pathologic fractures, spinal cord compression, bone surgery or bone radiation. From the man's perspective, these are less or more important. The differing relevance predominantly arises from the restrictions in daily life activities. Hence, acute interventions (surgery or radiation) might be rated as more important than an asymptomatic fracture or spinal cord compression. Considering this, zoledronic acid might contribute to the man's daily life activities by reducing SREs in general and especially surgical interventions to bone. Future analyses of SREs would be more beneficial if they stratify the relevance of SREs and take the man's perspective as a correlated outcome into account.
The trials heterogeneously assessed QoL by using different measurement tools and explored different dimensions of the man's daily life considering, for example, mobility, disability or pain. In doing so, each study focused on different facets of QoL. QoL assessment might be important for the man and the physician, because it allows detection of deficiencies of anticancer therapy, evaluation of treatment response and some type of patient satisfaction. Our analysis highlighted the limited applicability of QoL data from each trial and emphasized the need for further research on this outcome. Future studies should evaluate QoL by clearly mentioning the proportion of participants with changed QoL and providing clear definitions of QoL. We recommend the use of one standardized questionnaire to raise external validity of results.
Bisphosphonates are associated with a potential nephrotoxic effect (Bartl 2008; Gartrell 2014). Meta‐analysis of seven trials revealed that bisphosphonates probably increased the number of renal adverse events in comparison with control regimens. Analyzing each study separately, only Saad 2010 showed a clear difference in the occurrence of renal adverse events, the other trials demonstrated no clinically relevant difference between groups (CALGB 90202; Elomaa 1992; Figg 2005; Kylmala 1997; Pan 2014; ZAPCA). In overall analysis, most of the observed events derived from Saad 2010 and the weight of this trial was 89.1%. Four trials used zoledronic acid 4 mg IV (CALGB 90202; Pan 2014; Saad 2010; ZAPCA). Saad 2010 added a zoledronic acid 8 mg IV arm, but, after an increase in renal adverse events in this arm, Saad and colleagues amended the initial dose from zoledronic acid 8 mg to 4 mg (8/4 mg group). For meta‐analysis, we initially planned to combine results of bisphosphonate or control participants from three‐ or four‐armed trials. This approach allowed us to compare two‐armed trials to three‐ or four‐armed trials, but might have also led to misleading results in this outcome.
The rates of renal adverse events were 15.2% with zoledronic acid 4 mg versus 20.7% with zoledronic acid 8/4 mg versus 11.5% with placebo in Saad 2010. The authors reported that participants receiving zoledronate 4 mg IV had an RR of 1.07 (95% CI 0.46 to 2.47; P = 0.882) in comparison with placebo, but the corresponding RR was 1.76 (95% CI 0.79 to 3.93, P = 0.165) for the zoledronic acid 8/4 mg group. Noteworthy, zoledronic acid 4 mg, which represents the clinical standard dose, was not associated with a clinically relevant increase in renal adverse events in CALGB 90202, Pan 2014, Saad 2010, and ZAPCA. Given the facts that the temporary high dose of zoledronic acid 8 mg might have influenced the overall effect, Saad 2010 contributed more than 90% of renal adverse events and had the most sensitive definition of renal adverse events, future studies should address the nephrotoxic effects of zoledronic acid 4 mg in the context of a homogeneous outcome definition. The inclusion of the zoledronic acid 8/4 mg group in the analysis of renal adverse events restricted the applicability of our results, therefore, these should be interpreted with caution. Moreover, participants with castration‐sensitive prostate cancer and bisphosphonates other than zoledronate were under‐represented in our analysis. Future studies should evaluate the renal toxicity of different bisphosphonates in these participants in the near future.
We included seven trials in statistical analysis of disease progression. Definition of disease progression included biochemical progression (PSA progression), radiographic progression (new or expanding bone lesions) and clinical progression in these trials. Clinical progression mainly included pain progression and increased analgesic consumption, but some studies also considered SREs and death due to prostate cancer. Given this heterogeneity in outcome definition, the observed probable reduction of the proportion of participants with disease progression on bisphosphonate treatment must be interpreted carefully. This finding does not reflect the clinical importance of the use of bisphosphonates, as physicians do not treat biochemical results only clinical findings. From the man's perspective, biochemical progression or radiographic progression might not be experienced as discomfort or might not immediately affect survival. On the contrary, the man's daily life activities might be impaired by increased bone pain or need for treatment of disease progression (e.g. radiation or surgery to bone). As the relevance of our finding remains uncertain in the context of the man's perspective, we emphasize the need for new studies taking the man's view into account for the measurement of disease progression.
In addition to the published trials, we checked registries to identify ongoing clinical trials. We found no ongoing clinical trials. Therefore, the current evidence will not change in the near future.
Quality of the evidence
We used the GRADE approach to evaluate the quality of evidence. Overall, we judged the quality of the 18 studies included as moderate. In detail, the quality of evidence for pain response was low because the studies were at a potential risk of performance and attrition bias and included a small number of events (see Table 1). We considered the quality of evidence for SREs, mortality, nausea, renal adverse events and disease progression as moderate due to the potential bias by influence of funding sources. Consequently, we downgraded each of these outcomes by one point. We downgraded ONJ by three points, resulting in very low quality evidence. This is justified by the risk of performance and attrition bias in the trials and the very small number of events included in this outcome analysis. We were unable to judge the quality of evidence for QoL, because the studies did not report this outcome in a way allowing a meta‐analysis.
Potential biases in the review process
We used different strategies to minimize the risk of bias in the review process. We performed a highly sensitive literature search considering full‐text and abstract publications from medical databases and cancer congresses. We checked study registries to identify completed but unpublished trials. In doing so, we potentially missed unpublished, ongoing or not completed trials. If results of these trials are going to be published in the future, we will consider them in an update of this systematic review.
Two review authors independently assessed study eligibility. They separately performed data extraction and analysis. During the review process, we produced funnel plots. None of the defined end points had at least 10 studies in the analysis and therefore, this statistical analysis could not be performed. Addressing analysis of defined end points, the trials used different methods in QoL assessment. Because of the deviation in reported data, we were unable to obtain data to meta‐analyze, but performed a qualitative outcome analysis.
The current analysis is an update of the initial review on the use of bisphosphonates in men with bone metastases from prostate cancer. By nature, updates of systematic reviews are often prepared without providing a specific protocol, which defines the methodologic background of the review. This could lead to a potential sources of bias, for example, in selection criteria of studies included or end point definitions. This updated review differs from the prior version by including all trials using bisphosphonates in prostate cancer participants irrespective of outcomes. The first version only included studies reporting on pain response in these participants. Furthermore, we did not provide data on the comparison of different routes of administration or dosages of bisphosphonates. According to the protocol of the prior version of the review, we initially identified three trials comparing different doses and types of bisphosphonates, but these studies were designed heterogeneously. In the final analysis, we excluded these trials due to potentially imbalanced results with restricted applicability.
Agreements and disagreements with other studies or reviews
Lee and colleagues evaluated the risk of ONJ in people with cancer (Lee 2014). The study included 1389 participants receiving bisphosphonates and 569,620 receiving a control regimen from eight observational studies. In their systematic review, they found an increased risk for participants receiving bisphosphonates (odds ratio (OR) 4.25, 95% CI 3.67 to 5.36). The risk was increased further in participants receiving IV bisphosphonates (OR 4.27, 95 % CI 3.38 to 5.40) compared to participants receiving bisphosphonates orally (OR 1.18, 95% CI 0.89 to 1.56). We included five studies in the analysis of ONJ, but only two trials documented events. The number of participants in experimental groups was almost similar in both systematic reviews, but our quantitative analysis revealed no clear difference in the rate of ONJ (RR: 1.92; 95% CI 0.75 to 4.90, very low quality of evidence). This clinically relevant deviation is surprising, even though Lee and colleagues included different types of primary cancer and should be addressed in future investigations on this topic.
Liu 2015 and colleagues evaluated the role of bisphosphonates to reduce the risk of any SREs in participants with lung cancer, prostate cancer, breast cancer and bone metastases. Although they included only seven trials for the analysis of men with prostate cancer, their results are in line with ours, saying that bisphosphonates reduced the risk of SREs in these participants (OR for men with prostate cancer 0.62, 95% CI 0.45 to 0.86). In their analysis, Liu and colleagues calculated ORs and used a random‐effects model. On the contrary, we calculated RRs and used a fixed‐effect model. Moreover, Liu and coworkers defined SREs "as pathologic bone fracture, the bone surgery, the bone radiation therapy, or change in anticancer therapy to relieve bone pain." These discrepancies might have caused the more distinct effect of bisphosphonates on SREs in their analysis in comparison with our analysis.
Gartrell 2015 conducted a systematic review on bone‐targeted therapies in men with prostate cancer with bone metastases. The authors concluded that zoledronic acid reduced the number of SREs and the time to SREs in men with castration‐resistant prostate cancer. Additionally, Alibhai 2017 carried out a systematic review on the use of bone‐targeted therapies in men with prostate cancer. In summary, the authors reported that zoledronic acid delayed the time to SRE and prevented participants with castration‐resistant prostate cancer from SRE development. On the one hand, results of the Alibhai 2017 and Gartrell 2015 reviews were in line with our findings. On the other hand, both reviews provided no information on the effect of bisphosphonates in castration‐sensitive prostate cancer on SREs. Our data suggested a beneficial effect of zoledronic acid in men with castration‐sensitive prostate cancer.
Vignani 2016 recommended the use of zoledronic acid for men with castration‐resistant prostate cancer in their systematic review, but found no survival benefit with zoledronic acid. Concordantly, our quantitative analysis revealed no clinically relevant difference in mortality for participants receiving bisphosphonates. Only Pan 2014 (zoledronic acid) and PR05 (clodronate) detected an improved survival in participants receiving bisphosphonates, all other trials showed similar survival data for both groups.
None of the identified and discussed reviews used the GRADE approach. As presented, the systematic reviews focused on SRE, survival and ONJ, but provided no data on patient‐important outcomes such as pain, analgesic consumption or QoL. A further advantage of this Cochrane Review was the consideration of men with castration‐sensitive prostate cancer.
Authors' conclusions
Implications for practice.
This systematic review found low quality evidence that there is no clinically relevant difference in the proportion of participants with pain response between bisphosphonates and control regimens in men with bone metastases from prostate cancer. Our analysis revealed moderate quality evidence that bisphosphonates decreased the total number of skeletal‐related events and the proportion of participants with disease progression in comparison with control regimens. The advantageous effect of bisphosphonates on the composite outcome of skeletal‐related events was more distinct in pathologic fractures, spinal cord compressions and bone surgery. The benefits should be weighed against the risk of renal impairment and nausea in men receiving bisphosphonates. We found very low to moderate quality evidence that there is no clear difference in mortality, quality of life and osteonecrosis of the jaw using bisphosphonates in contrast to control regimens.
Implications for research.
Our review enlightened the need for more patient‐important data, especially for pain and quality of life. To support further research on pain and quality of life in these men, we recommend the use of standardized assessment tools. As the primary outcome pain was assessed by only including bisphosphonates other than zoledronic acid, further research is needed on the analgesic potential of zoledronic acid as it is the predominantly used bisphosphonate in clinical practice. Future studies might investigate the incidence of osteonecrosis of the jaw as typical adverse event in people receiving bisphosphonates and preventional strategies. A higher number of events may raise the quality of evidence of this outcome in a following update of this review. More research is needed to evaluate cost effectiveness. In addition, information is needed to guide the choice of bisphosphonates and the optimal treatment schedule.
What's new
| Date | Event | Description |
|---|---|---|
| 13 July 2017 | New citation required and conclusions have changed | Conclusions not changed |
| 13 July 2017 | New search has been performed | Search updated and new trials included |
History
Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 23 November 2009 | Amended | The first author was incorrectly cited as Kwok Yung Yuen. It has been corrected to Kwok Keung Yuen. |
| 13 May 2008 | Amended | Converted to new review format. |
| 15 August 2006 | New citation required and conclusions have changed | Substantive amendment |
Notes
Some passages in this review, especially in the methods part, are from the standard template of the Cochrane Haematological Malignancies Review Group.
Acknowledgements
The authors of the first version of this review would like to thank Mrs Bernadette Coles MSc for developing and running the search strategies for this review.
We thank the authors of the first published version of this review, Mike Shelley, Wai Man Sze, Timothy J Wilt and Malcolm D Mason.
We thank the Cochrane Urology Group for their support, including the editors for their feedback on this review. We also thank the referees for their appreciated feedback: Stefan Krause, Stefanie Schmidt and Gunhild von Amsberg.
Appendices
Appendix 1. Previous search strategies
The electronic search included MEDLINE (1966 to May 2005), EMBASE (1980 to April 2005) LILACS (up to June 2005), DARE (up to June 2005), AMED (up to June 2005) and the Cochrane Central Register of Controlled Trials (CENTRAL).
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized controlled trials/
random allocation/
double blind method/
single‐blind method/
clinical trial.pt.
exp clinical trials/
(clin$ adj25 trial$).tw.
((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).tw.
placebos/
placebos.tw.
random.tw.
research design/
comparative study/
exp evaluation studies/
follow up studies/
prospective studies/
(control$ or prospectiv$ or volunteer$).tw.
or/1‐19
limit 20 to animal
limit 20 to human
21 and 22
21 not 23
20 not 24
exp prostate neoplasms/
(prostat$ adj3 (cancer$ or carcinoma$ or malignan$ or tumo?r$ or neoplas$ or adeno$)).mp.
26 or 27
exp Bone Neoplasms/
Osseous metastasis or Osseous metastases
((bone$ or skelet$ or osseous or osteo$) adj3 (second$ or metast$)).af.
29 or 30 or 31
28 and 32
exp diphosphonates/
exp bisphosphonates/
(bisphosphonat$ or diphosphonat$).af.
alendron$.af.
fosamax.af.
clodron$.af.
bonefos.af.
CL2MDP
loron.af.
ostac.af.
etidron$.af.
didrocal.af.
didronel.af.
EHDP.af.
ibandron$.af.
bondranat.af.
incadron$.af.
YM175.af.
YM 175.af.
minodron$.af.
YM529.af.
YM 529.af.
neridron$.af.
AHDP.af.
olpadron$.af.
OPD.af.
pamidron$.af.
APD.af.
aredia.af.
pamisol.af.
risedron$.af.
actonel.af.
tiludron$.af.
skelid.af.
zoldron$.af.
zometa.af.
or/34‐69
(pain or "anlages*").mp. [mp=title, abstract, registry number word, mesh subject heading]
25 and 33 and 70
71 and 72
Appendix 2. Current CENTRAL search strategy
#1 MeSH descriptor: [Prostatic Neoplasms] explode all trees
#2 (prostat* near/3 (cancer* or carcinoma* or malignan* or tumor* or tumour* or neoplas* or intraepithelial* or adenocarcinoma*))
#3 MeSH descriptor: [Prostatitis] explode all trees
#4 (prostatitis or prostatitides or prostatosis)
#5 #1 or #2 or #3 or #4
#6 MeSH descriptor: [Diphosphonates] explode all trees
#7 (diphosphonate* or diphosph*nate*)
#8 (bisphosph*nate* or biphosph*nate*)
#9 #6 or #7 or #8
#10 MeSH descriptor: [Alendronate] explode all trees
#11 (alendronat* or aledronic*)
#12 (fosamax* or binosto* or adronat* or alendros* or onclast*)
#13 #10 or #11 or #12
#14 MeSH descriptor: [Clodronic Acid] explode all trees
#15 (clodronic* or clodronat*)
#16 (bonefos* or clasteon* or difosfonal* or ossiten* or mebonat* or loron* or ostac*)
#17 Cl2MDP
#18 #14 or #15 or #16 or #17
#19 MeSH descriptor: [Etidronic Acid] explode all trees
#20 (etidronic* or etidronat*)
#21 (didronel* or xidifon* or dicalcium or xidiphon*)
#22 (HEDP or EHDP)
#23 #19 or #20 or #21 or #22
#24 MeSH descriptor: [Technetium Tc 99m Medronate] explode all trees
#25 (medronat* or medronic*)
#26 (Technetium near/2 Tc 99m near/2 Medronat*)
#27 #24 or #25 or #26
#28 (pamidronat* or pamidronic* or amidronat*)
#29 (aredia* or ADP sodium* or aminomux*)
#30 (GCP23339A or GCP‐23339A or YM529 or YM‐529)
#31 #28 or #29 or #30
#32 (zoledronic* or zoledronat*)
#33 (zometa* or zomera* or aclasta* or reclast* or aredia* or zoldron*)
#34 (m05BA08 or CGP‐42446* or CGP42446* or zol‐446 or zol446)
#35 #32 or #33 or #34
#36 (ibandronic* or ibandrovic* or ibandronat*)
#37 (bon*iva* or bondronat* or bondranat* or adronil*)
#38 (RPR102289A or RPR‐102289A)
#39 (BM210955 or BM‐210955)
#40 #36 or #37 or #38 or #39
#41 (risedronic* or risedronat*)
#42 (actonel* or atelvia* or benet*)
#43 (NE58095 or NE‐58095)
#44 #41 or #42 or #43
#45 (neridronat* or neridronic*)
#46 (AHHexBP or 6AHHDP or 6‐AHHDP)
#47 #45 or #46
#48 MeSH descriptor: [RANK Ligand] explode all trees
#49 (rank near/3 ligand*)
#50 RANK ligand inhibitor*
#51 (protein* near/2 (RANKL or TRANCE))
#52 Tumor Necrosis Factor‐Related Activation‐Induced Cytokin*
#53 #48 or #49 or #50 or #51 or #52
#54 denosumab*
#55 (xgeva* or prolia*)
#56 (AMG162 or AMG‐162)
#57 #54 or #55 or #56
#58 tiludronat* or tiludronic* or skelid*
#59 Incadronat* or YM175 or YM‐175
#60 olpadronat* or olpadronic*
#61 #9 or #13 or #18 or #23 or #27 or #31 or #35 or #40 or #44 or #47 or #53 or #57 or #58 or #59 or #60
#62 #5 and #61 in Trials
Appendix 3. Current MEDLINE search strategy
| 1 | exp PROSTATIC NEOPLASMS/ |
| 2 | (prostat$ adj3 (cancer$ or carcinoma$ or malignan$ or tumo?r$ or neoplas$ or intraepithelial or adenocarcinoma$)).tw. |
| 3 | PROSTATITIS/ |
| 4 | (prostatitis or prostatitides or prostatosis).tw. |
| 5 | or/1‐4 |
| 6 | exp DIPHOSPHONATES/ |
| 7 | (diphosphonate$ or diphosph#nate$).tw,kf,ot,nm. |
| 8 | (bisphosph#nate$ or biphosph#nate$).tw,kf,ot,nm. |
| 9 | or/6‐8 |
| 10 | ALENDRONATE/ |
| 11 | (alendronat$ or aledronic$).tw,kf,ot,nm. |
| 12 | (fosamax$ or binosto$ or adronat$ or alendros$ or onclast$).tw,kf,ot,nm. |
| 13 | or/10‐12 |
| 14 | CLODRONIC ACID/ |
| 15 | (clodronic$ or clodronat$).tw,kf,ot,nm. |
| 16 | (bonefos$ or clasteon$ or difosfonal$ or ossiten$ or mebonat$ or loron$ or ostac$).tw,kf,ot,nm. |
| 17 | Cl2MDP.tw,kf,ot,nm. |
| 18 | or/14‐17 |
| 19 | ETIDRONIC ACID/ |
| 20 | (etidronic$ or etidronat$).tw,kf,ot,nm. |
| 21 | (didronel$ or xidifon$ or dicalcium$ or didrocal$ or xidiphon$).tw,kf,ot. |
| 22 | (HEDP or EHDP).tw,kf,ot. |
| 23 | or/19‐22 |
| 24 | TECHNETIUM TC 99M MEDRONATE/ |
| 25 | (medronat$ or medronic$).tw,kf,ot,nm. |
| 26 | (Technetium adj2 Tc 99m adj2 Medronat$).tw,kf,ot,nm. |
| 27 | or/24‐26 |
| 28 | (pamidronat$ or pamidronic$ or amidronat$).tw,kf,ot,nm. |
| 29 | (aredia$ or ADP sodium$ or incadron$ or aminomux$).tw,kf,ot,nm. |
| 30 | (GCP23339A or GCP‐23339A or YM529 or YM‐529).tw,kf,ot,nm. |
| 31 | or/28‐30 |
| 32 | (zoledronic$ or zoledronat$).tw,kf,ot,nm. |
| 33 | (zometa$ or zomera$ or aclasta$ or zoldron$ or reclast$ or aredia$).tw,kf,ot,nm. |
| 34 | (m05BA08 or CGP‐42446$ or CGP42446$ or zol‐446 or zol446).tw,kf,ot,nm. |
| 35 | or/32‐34 |
| 36 | (ibandronic$ or ibandrovic$ or ibandronat$).tw,kf,ot,nm. |
| 37 | (bon?iva$ or bondronat$ or bondranat$ or adronil$).tw,kf,ot,nm. |
| 38 | (RPR102289A or RPR‐102289A).tw,kf,ot,nm. |
| 39 | (BM210955 or BM‐210955).tw,kf,ot,nm. |
| 40 | or/36‐39 |
| 41 | (risedronic$ or risedronat$).tw,kf,ot,nm. |
| 42 | (actonel$ or atelvia$ or benet$).tw,kf,ot,nm. |
| 43 | (NE58095 or NE‐58095).tw,kf,ot,nm. |
| 44 | or/41‐43 |
| 45 | (neridronat$ or neridronic$).tw,kf,ot,nm. |
| 46 | (AHHexBP or 6AHHDP or 6‐AHHDP).tw,kf,ot,nm. |
| 47 | or/45‐46 |
| 48 | RANK LIGAND/ |
| 49 | (rank$ adj3 ligand$).tw,kf,ot,nm. |
| 50 | RANK ligand inhibitor$.tw,kf,ot,nm. |
| 51 | (protein$ adj2 (RANKL or TRANCE)).tw,kf,ot,nm. |
| 52 | Tumor Necrosis Factor‐Related Activation‐Induced Cytokin$.tw,kf,ot,nm. |
| 53 | or/48‐51 |
| 54 | denosumab$.tw,kf,ot,nm. |
| 55 | (xgeva$ or prolia$).tw,kf,ot,nm. |
| 56 | (AMG162 or AMG‐162).tw,kf,ot,nm. |
| 57 | or/54‐56 |
| 58 | (tiludronat$ or tiludronic$ or skelid$).tw,kf,ot,nm. |
| 59 | (Incadronat$ or YM175 or YM‐175).tw,kf,ot,nm. |
| 60 | (olpadronat$ or olpadronic$).tw,kf,ot,nm. |
| 61 | 9 or 13 or 18 or 23 or 27 or 31 or 35 or 40 or 44 or 47 or 53 or 57 or 58 or 59 or 60 |
| 62 | 5 and 61 |
| 63 | randomized controlled trial.pt. |
| 64 | controlled clinical trial.pt. |
| 65 | randomi?ed.ab. |
| 66 | placebo.ab. |
| 67 | drug therapy.fs. |
| 68 | randomly.ab. |
| 69 | trial.ab. |
| 70 | groups.ab. |
| 71 | or/63‐70 |
| 72 | humans.sh. |
| 73 | 71 and 72 |
| 74 | 5 and 61 and 73 |
Data and analyses
Comparison 1. Bisphosphonates versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with pain response | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| 2 Skeletal‐related events: any | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 3 Skeletal‐related events: pathologic fracture | 6 | 2226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.87] |
| 4 Skeletal‐related events: pathologic fractures: vertebral fracture | 2 | 993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.52, 1.36] |
| 5 Skeletal‐related events: pathologic fractures: non‐vertebral fracture | 2 | 993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.53, 1.10] |
| 6 Skeletal‐related events: spinal cord compression | 6 | 2226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.89] |
| 7 Skeletal‐related events: bone radiation therapy | 6 | 1696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.06] |
| 8 Skeletal‐related events: bone surgery | 5 | 1915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.86] |
| 9 Mortality | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 10 Adverse events: nausea | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| 11 Adverse events: renal | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| 12 Adverse events: bone pain | 5 | 1445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.06] |
| 13 Adverse events: osteonecrosis of the jaw | 5 | 1626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.75, 4.90] |
| 14 Proportion of participants with decreased analgesic consumption | 4 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.87, 1.63] |
| 15 Proportion of participants with disease progression | 7 | 2115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.98] |
| 16 Sensitivity analysis: pain response (low risk of bias vs high risk of bias) | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| 16.1 Low risk of bias | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.80, 1.89] |
| 16.2 High risk of bias | 2 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.88, 1.44] |
| 17 Subgroup analysis: pain response (amino‐bisphosphonate vs non‐amino‐bisphosphonate) | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| 17.1 Amino‐bisphosphonate | 1 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.44] |
| 17.2 Non‐amino‐bisphosphonate | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.81, 1.87] |
| 18 Subgroup analysis: pain response (route of administration) | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| 18.1 Oral | 1 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.44] |
| 18.2 Intravenous | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.81, 1.87] |
| 19 Sensitivity analysis: skeletal‐related events (low risk of bias vs high risk of bias) | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 19.1 Low risk of bias | 5 | 1767 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 0.99] |
| 19.2 High risk of bias | 4 | 1386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.75, 0.94] |
| 20 Sensitivity analysis: skeletal‐related events (full‐text vs abstract publication) | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 20.1 Full‐text publication | 8 | 3093 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.81, 0.95] |
| 20.2 Abstract publication | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.12, 1.37] |
| 21 Subgroup analysis: skeletal‐related events (amino‐bisphosphonate versus non‐amino‐bisphosphonate) | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 21.1 Amino‐bisphosphonate | 8 | 2842 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 21.2 Non‐amino‐bisphosphonate | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.09] |
| 22 Subgroup analysis: skeletal‐related events (route of administration) | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 22.1 Oral | 2 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.09] |
| 22.2 Intravenous | 7 | 2779 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 23 Sensitivity analysis: mortality (low risk of bias vs high risk of bias)) | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 23.1 Low risk of bias | 6 | 1420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.85, 1.02] |
| 23.2 High risk of bias | 3 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.15] |
| 24 Sensitivity analysis: mortality (full‐text vs abstract publication) | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 24.1 Full‐text publication | 8 | 2390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.04] |
| 24.2 Abstract publication | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.03] |
| 25 Subgroup analysis: mortality (amino‐bisphosphonate vs non‐amino‐bisphosphonate) | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 25.1 Amino‐bisphosphonate | 5 | 1738 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.07] |
| 25.2 Non‐amino‐bisphosphonate | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.06] |
| 26 Subgroup analysis: mortality (route of administration) | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 26.1 Oral | 5 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.11] |
| 26.2 Intravenous | 4 | 1310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.02] |
| 27 Sensitivity analysis: adverse event: nausea (low risk of bias vs high risk of bias) | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| 27.1 Low risk of bias | 7 | 2042 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.40] |
| 27.2 High risk of bias | 2 | 966 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.49, 4.03] |
| 28 Subgroup analysis: adverse event: nausea (amino‐bisphosphonate vs non‐amino‐bisphosphonate) | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| 28.1 Amino‐bisphosphonate | 5 | 2332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.98, 1.45] |
| 28.2 Non‐amino‐bisphosphonate | 4 | 676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.83, 1.68] |
| 29 Subgroup analysis: adverse event: nausea (route of administration) | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| 29.1 Oral | 4 | 1059 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.67] |
| 29.2 Intravenous | 5 | 1949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.99, 1.45] |
| 30 Sensitivity analysis: renal (low risk of bias vs high risk of bias) | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| 30.1 Low risk of bias | 5 | 1498 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.06, 2.40] |
| 30.2 High risk of bias | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.48, 18.65] |
| 31 Subgroup analysis: renal (amino‐bisphosphonate vs non‐amino‐bisphosphonate) | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| 31.1 Amino‐bisphosphonate | 5 | 1662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.09, 2.44] |
| 31.2 Non‐amino‐bisphosphonate | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.14, 77.15] |
| 32 Subgroup analysis: renal (route of administration) | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| 32.1 Oral | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.37, 15.75] |
| 32.2 Intravenous | 4 | 1590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.07, 2.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abetz 2006.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest: see meeting.ascopubs.org/cgi/content/abstract/24/18_suppl/4638?sid=2d509f53‐6021‐4c00‐8de9‐6bbec9c7cf92.
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Insufficient information on outcome assessment. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient information on outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information regarding discontinuations and ITT. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Insufficient report on methods. |
CALGB 90202.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | Prematurely completed after corporate supporter withdrew study drug supply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized block design was used." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from protocol: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote from protocol: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote from protocol: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)." |
| Incomplete outcome data (attrition bias) | Low risk | All participants with bone metastasis from prostate cancer were included in the analysis of efficacy and all participants on treatment were used for analysis of safety. |
| Selective reporting (reporting bias) | Low risk | Report on every end point (primary and secondary) mentioned in the original protocol. |
| Other bias | Low risk | No further information provided. |
Elomaa 1992.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Insufficient information on outcome assessment. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient information on outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information regarding discontinuations and ITT. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Quote from the article: "We are grateful to the Finnish Cancer Foundation and to Leiras Pharmaceutical Company for their support of this work." |
Ernst 2003.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Median age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned using a block‐randomization procedure with equal probability of assignment to either arm." |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned using a block‐randomization procedure with equal probability of assignment to either arm." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The treating staff and patients were blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | "The treating staff and patients were blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | "The treating staff and patients were blinded to treatment allocation." |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information regarding discontinuations and ITT. |
| Selective reporting (reporting bias) | Unclear risk | Protocol available (NCT00003232), but outcomes not prespecified in the protocol. |
| Other bias | Unclear risk | Quote: "Supported by a grant from Immunex Corporation, Seattle, WA, and Aventis Pharma, Laval, Quebec, Canada." |
Figg 2005.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was an open label, randomized, phase II study [...]" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information on blinding of outcome assessor. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "This was an open label, randomized, phase II study [...]" |
| Incomplete outcome data (attrition bias) | Low risk | Complete analysis of all randomized participants. |
| Selective reporting (reporting bias) | Unclear risk | Protocol available (NCT00019695), more outcomes reported than prespecified in the protocol (e.g. overall survival). |
| Other bias | Low risk | No further information provided. |
GU02‐4.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information on blinding of outcome assessor. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No information on blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) | Unclear risk | Complete analysis of all randomized participants. |
| Selective reporting (reporting bias) | Unclear risk | Protocol available (NCT00019695), more outcomes reported than prespecified in the protocol (e.g. overall survival). |
| Other bias | Unclear risk | No information provided. |
Kylmala 1993.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information on blinding of investigated outcome. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No information on blinding of investigated outcome. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information regarding discontinuations and ITT. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Quote from the article: "We are grateful to [...] the Finnish Cancer Foundation and to Leiras Pharmaceutical Company for their support of this study." |
Kylmala 1997.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information on blinding of investigated outcome. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No information on blinding of investigated outcome. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information regarding discontinuations and ITT. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Quote: "This study was supported by the Finnish Academy of Sciences, Finnish Cancer Foundation, Finnish Medical Society Duodecim, Reino Lathikari Foundation and by Leiras Clinical Research." |
Meulenbeld 2012.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This randomised, open label, phase II/III trial [...]." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Quote: "This randomised, open label, phase II/III trial [...]." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "This randomised, open label, phase II/III trial [...]." |
| Incomplete outcome data (attrition bias) | Low risk | All participants with bone metastasis from prostate cancer were included in the analysis of efficacy and safety. |
| Selective reporting (reporting bias) | Low risk | Protocol available (ISRCTN22844568), prespecified outcomes reported. |
| Other bias | Unclear risk | Quote: "Funding was provided by Grants from Sanofi‐Aventis." |
Pan 2014.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient report on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Insufficient report on blinding of outcome. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient report on blinding of outcome. |
| Incomplete outcome data (attrition bias) | Low risk | No participants lost to follow‐up. All participants were included in the ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Low risk | No further information provided. |
PR05.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Median age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Quote: "This trial was sponsored by the U.K. Medical Research Council (MRC)." "The trial was initiated with the support of Boehringer Mannheim. The company provided trial tablets (Loron 520 and matching placebo) free of charge, plus financial support (£250) on a per patient basis, which was sufficient to contribute toward the administrative costs of the trial. The financial support was distributed proportionately between the participating clinicians and the coordinating center [...] During the trial, Boehringer Mannheim was taken over by Roche Products Ltd., which honored all commitments regarding this trial." |
|
| Declarations of interest | Insufficient report on potential conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally at the MRC CTU [...] No patient information, other than their drug number and hospital, was revealed to the pharmaceutical companies." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Insufficient information on blinding of outcome assessment. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient information on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Study protocol available. All prespecified outcomes were reported. |
| Other bias | Unclear risk | Quote: "The trial was initiated with the support of Boehringer Mannheim. The company provided trial tablets (Loron 520 and matching placebo) free of charge, plus financial support (£250) on a per patient basis, which was sufficient to contribute toward the administrative costs of the trial. The financial support was distributed proportionately between the participating clinicians and the coordinating center.[...] During the trial, Boehringer Mannheim was taken over by Roche Products Ltd., which honored all commitments regarding this trial." |
Saad 2010.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Quote: "Supported by a grant from Novartis Pharmaceuticals Corporation, East Hanover, NJ." | |
| Declarations of interest | Quote: "The following have conducted or are currently conducting research sponsored by Novartis Pharmaceuticals Corp.: F. Saad, D. M. Gleason, R. Murray, L. Lacombe, J. L. Chin, and J. J. Vinholes. F. Saad is a consultant on an advisory board to Novartis Pharmaceuticals Corp." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 643 patients who met the inclusion criteria after the screening visit were randomly assigned to treatment according to a computer‐generated list of randomization numbers provided to each center." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient report on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Insufficient report on blinding of outcome assessment. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient report on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Low risk | No further information provided. |
Small 2003.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Only CGP 032:
Participants randomized:
Median age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | 2 multicenter, randomized, double‐blind, placebo‐controlled trials (INT‐05 as international trial and CGP 032 as national trial in the US) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient report on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Insufficient report on blinding of outcome assessor. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient report on blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Because of protocol violations, 350 patients were included in the intent‐to‐treat efficacy analysis (169 patients in the pamidronate group and 181 patients in the placebo group)." |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Quote: "The following authors or their immediate family members have indicated a financial interest. No conflict exists for drugs or devices used in a study if they are not being evaluated as part of the investigation. Owns stock (not including shares held through a public mutual fund): John Seaman, Novartis Pharmaceuticals; Mildred Kowalski, Novartis Pharmaceuticals; Stephanie Petrone, Novartis Pharmaceuticals. Acted as a consultant within the last 2 years: Matthew Smith, Novartis Pharmaceuticals; Eric Small, Novartis Pharmaceuticals. Received more than $2,000 a year from a company for either of the last 2 years: John Seaman, Novartis Pharmaceuticals; Mildred Kowalski, Novartis Pharmaceuticals; Matthew Smith, Novartis Pharmaceuticals." |
Smith 1989.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information on blinding of investigated outcome. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No information on blinding of investigated outcome. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Six patients [...] were considered unevaluable because they failed to complete 1 month of treatment." |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | High risk | No statistical analysis of observed results. |
Strang 1997.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient report on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Insufficient report on blinding of outcome assessor. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient report on blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) | High risk | Different report on number of randomized participants. In the text, 55 participants were randomized and, according to Table 1, 52 participants were randomized. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | High risk | Quote: "The study had to be prematurely terminated before the planned number of patients were included in secondary to difficulties finding enough patients according to inclusion and exclusion criteria." "The work was supported by Leiras OY Finland and ASTRA Lakemedel Sweden." |
TRAPEZE 2016.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Median age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were stratified by investigation center and ECOG performance status at trial entry in a 1:1:1:1 allocation ratio using a computerized minimization algorithm accessed by telephone to the trials unit." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were stratified by investigation center and ECOG performance status at trial entry in a 1:1:1:1 allocation ratio using a computerized minimization algorithm accessed by telephone to the trials unit." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "TRAPEZE was a randomized, open‐label, phase 3 trial using a 2 × 2 factorial design." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Insufficient report on blinding of outcome assessment. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "TRAPEZE was a randomized, open‐label, phase 3 trial using a 2 × 2 factorial design." |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient report on outcome data. |
| Selective reporting (reporting bias) | High risk | No report on all prespecified outcomes (e.g. QoL). |
| Other bias | Low risk | No further information provided. |
ZABTON‐PC.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | Inclusion of "bone pain" in SREs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This study was under a still ongoing randomized multicenter collaborative open‐labeled project [...]." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Insufficient information on blinding of the outcome assessor. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "This study was under a still ongoing randomized multicenter collaborative open‐labeled project [...]." |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in statistical analysis. |
| Selective reporting (reporting bias) | High risk | No report on survival data (planned per protocol). |
| Other bias | Low risk | No further information provided. |
ZAPCA.
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
|
|
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Median age:
Country of participants:
|
|
| Interventions | Previous interventions:
Interventions during study period:
|
|
| Outcomes | Reported and analyzed in this review:
|
|
| Funding sources | Funding sources:
|
|
| Declarations of interest | Conflicts of interest:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐based randomization was conducted at the Translational Research Informatics Center (TRI; Kobe, Japan) with stratification according to the treatment institution, baseline PSA concentration (<200 or ≥200 ng/mL), baseline extent of disease (EOD) grade [13] (≤2 or ≥3), and biopsy Gleason score (≤7 or ≥8). [...] The system automatically evaluated the eligibility of each patient and randomly assigned participants to each group." |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐based randomization was conducted at the Translational Research Informatics Center (TRI; Kobe, Japan) with stratification according to the treatment institution, baseline PSA concentration (<200 or ≥200 ng/mL), baseline extent of disease (EOD) grade [13] (≤2 or ≥3), and biopsy Gleason score (≤7 or ≥8). [...] The system automatically evaluated the eligibility of each patient and randomly assigned participants to each group." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Insufficient information on blinding of outcome assessor. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All 224 patients who received at least one dose of LH–RH agonist were included in the Safety Assessment Set (SAS)." |
| Selective reporting (reporting bias) | High risk | Study investigators initially planned to analyze QoL and pain as outcomes, but the authors did not provide any data on these end points in their publications. |
| Other bias | Unclear risk | Quote: "The ZAPCA trial was supported by Grant for Urologic Research No. 200040700148 from Kyoto University Hospital. [...] Tomomi Kamba accepted an honorarium from Astellas Pharma. Toshiyuki Kamoto accepted research funding and honoraria from Astellas Pharma. Fuminori Sato accepted research funding from Janssen Pharmaceutical and Astellas Pharma. Naoya Masumori accepted honoraria from Novartis Pharma and Daiichi Sankyo, and research funding from Daiichi Sankyo. Shin Egawa accepted research funding from Astellas Pharma and Takeda Pharmaceutical. Hideki Sakai accepted research funding from Astellas Pharma and Takeda Pharmaceutical, and honoraria from Astellas Pharma and AstraZeneca. Osamu Ogawa accepted an honorarium from Astellas Pharma." |
ALT: alanine aminotransferase; AST: aspartate transaminase; BPI: Brief Pain Inventory; BUN: blood urea nitrogen; CNS: central nervous system; CT: computed tomography; ECG: electrocardiogram; ECOG: Eastern Cooperative Oncology Group; Hb: hemoglobin; Inst: institution; ITT: intention to treat; IV: intravenous; LHRH: luteinizing hormone releasing hormone; MRI: magnetic resonance imaging; PFS: progression‐free survival; PO: orally; PPI: Present Pain Intensity; PSA: prostate‐specific antigen; QoL: quality of life; SRE: skeletal‐related event; ULN: upper limit of normal; VAS: visual analog scale; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adami 1985 | No control arm. |
| Adami 1989 | Active control group with different dosages of clodronate. |
| BO18039 | No subgroup analysis for men with prostate cancer. |
| CALGB 70604 | No subgroup analysis for men with prostate cancer. |
| Carey 1988 | No control arm. |
| Clarke 1991 | No control arm. |
| Cresswell 1995 | No control arm. |
| Fernandez‐Conde 1997 | Randomized controlled study with histomorphometric outcomes. Pain not an outcome. |
| Fizazi 2009 | Bisphosphonates compared to denosumab. |
| Fizazi 2011 | Bisphosphonates compared to denosumab. |
| Heidenreich 2001 | Non‐randomized study. |
| Heidenreich 2002 | Non‐randomized study. |
| Jagdev 2001 | Randomized study comparing intravenous pamidronate with oral clodronate in a mixed tumor population. Not specific for prostate cancer. |
| Kylmala 1994 | No control arm. |
| Magnusson 1998 | Randomized controlled study with biochemical outcomes, clinical outcomes including pain were reported in another article by Strang 1997, 1 of the included studies. |
| MER‐101‐03 | Active control groups on different administration routes of zoledronic acid. |
| NCT00242567 | Participants in both arms received zoledronic acid, early or delayed, no results for the comparison before receiving delayed treatment. |
| Pelger 1998 | No control arm. |
| STAMPEDE | Participants with and without bone metastases included, no subgroup results for people with metastases. |
| Taube 1994 | Randomized controlled study with histomorphometric outcomes. Pain not an outcome. |
| Vorreuther 1992 | No control arm. |
| Vorreuther 1993 | No control arm. |
| Wang 2013 | Active control group with other bisphosphonate (zoledronic acid). |
Differences between protocol and review
We included all trials fitting the inclusion criteria, irrespective of outcomes reported. The protocol and first version of the review included only trials that evaluated pain. However, as this is not in line with the methodologic expectations of Cochrane intervention reviews (MECIR) guidelines, we included all trials irrespective of the outcomes reported.
For continuous outcomes we would have calculated mean differences, or in case different scales would have been used, standardized mean difference (SMD). For time‐to‐event outcomes, we would have extracted the hazard ratio (HR) from published data according to Parmar 1998 and Tierney 2007, but neither continuous outcomes nor time‐to‐event outcomes have been reported.
In contrast to the protocol and the prior version of this review, we did not include trials with active control groups (other bisphosphonates). We initially identified three trials comparing different doses and types of bisphosphonates, but these studies have been designed heterogeneously. As agreed with the Editorial Base, in the final analysis, we subsequently decided to omit analysis of these trials due to potentially imbalanced results with restricted applicability.
Contributions of authors
SM: data extraction and analysis, drafting of final review.
IM: developed and ran search strategies and provided databases.
FJ: clinical expertise.
KJ: clinical expertise.
KKY: clinical expertise for the first version of this review.
AH: clinical expertise.
NS: data extraction and analysis, content and methodologic input.
Sources of support
Internal sources
University Hospital of Cologne, Department I of Internal Medicine, Germany.
External sources
No sources of support supplied
Declarations of interest
SM: none known.
IM: none known.
FJ: received payment for lectures from MSD, Riemser and Tesaro; received travel, accommodation or meeting expenses from Pfizer, Roche, Tesaro.
KJ: received payment for lectures from Amgen.
KKY: none known.
AH: none known.
NS: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abetz 2006 {published data only}
- Abetz L, Barghout V, Arbuckle R, Bosch V, Shirina N, Saad F. Impact of zoledronic acid (Z) on pain in prostate cancer patients with bone metastases in a randomised placebo‐control trial. Journal of Clinical Oncology 2006:4638. [Google Scholar]
- Barghout V, Abetz L, Arbuckle R, Bosch V, Hei Y, Saad F. Effect of zoledronic acid (Z) on pain in prostate cancer patients with bone metastases based on performance status. Journal of Clinical Oncology ‐ Supplement 2006;24(18):14544. [Google Scholar]
CALGB 90202 {published data only}
- Smith MR, Halabi S, Ryan CJ, Hussain A, Vogelzang N, Stadler W, et al. Randomized controlled trial of early zoledronic acid in men with castration‐sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). Journal of Clinical Oncology 2014;32:1143‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elomaa 1992 {published data only}
- Elomaa I, Kylmata T, Tammela T, Vitanen J, Ottelin M, Ruutu K, et al. Effect of oral clodronate on bone pain: a controlled study in patients with metastatic prostate cancer. International Journal of Urology and Nephrology 1992;24(2):159‐66. [DOI] [PubMed] [Google Scholar]
Ernst 2003 {published data only}
- Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, Moore MJ, et al. Randomized, double‐blind, controlled trial of mitoxantron/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone‐refractory prostate cancer and pain. Journal of Clinical Oncology 2003;21(17):3335‐42. [DOI] [PubMed] [Google Scholar]
Figg 2005 {published data only}
- Figg WD, Liu Y, Arlen P, Gulley J, Steinberg SM, Liewehr DJ, et al. A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. Journal of Urology 2005;173:790‐6. [DOI] [PubMed] [Google Scholar]
GU02‐4 {published data only}
- Hahn NM, Yiannoutsos CT, Kirkpatrick K, Sharma J, Sweeney CJ. Failure to suppress markers of bone turnover on first‐line hormone therapy for metastatic prostate cancer is associated with shorter time to skeletal‐related event. Clinical Genitourinary Cancer 2014;12(1):33‐40.e4. [PUBMED: 24126237] [DOI] [PubMed] [Google Scholar]
- Sharma J, Yiannoutsos CT, Hahn NM, Sweeney C. Prognostic value of suppressed markers of bone turnover (BTO) after 6 months of androgen deprivation therapy (ADT) in prostate cancer. Journal of Clinical Oncology 2011;29:4594. [Google Scholar]
- Sweeney C, Dugan WM, Dreicer R, Chu F, Parks G, Baker K, et al. A randomized placebo‐controlled trial of daily high‐dose oral risedronate in men with metastatic prostate cancer commencing androgen deprivation therapy (ADT). Journal of Clinical Oncology 2010;28:e15000. [Google Scholar]
Kylmala 1993 {published data only}
- Kylmala T, Tammela T, Risteli L, Risteli J, Taube T, Elomma I. Evaluation of the effect of oral clodronate on skeletal metastases with type I collagen metabolites. A controlled trial of the Finnish Prostate Cancer Group. European Journal of Cancer 1993;29A(6):821‐5. [DOI] [PubMed] [Google Scholar]
Kylmala 1997 {published data only}
- Kylmala T, Taube T, Tammela TL. Concomitant i.v. and oral clodronate in the relief of bone pain: a double‐blind placebo‐controlled study in patients with metastatic prostate cancer. British Journal of Cancer 1997;76:939‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meulenbeld 2012 {published data only}
- Meulenbeld HJ, Werkhoven ED, Coenen JLLM, Creemers GJ, Loosveld OJL, Jong PC, et al. Randomised phase II/III study of docetaxel with or without risedronate in patients with metastatic Castration Resistant Prostate Cancer (CRPC), the Netherlands Prostate Study (NePro). European Journal of Cancer 2012;48:2993‐3000. [DOI] [PubMed] [Google Scholar]
Pan 2014 {published data only}
- Pan Y, Jin H, Chen W, Yu Z, Ye T, Zheng Y, et al. Docetaxel with or without zoledronic acid for castration‐resistant prostate cancer. International Urology and Nephrology 2014;46:2319‐26. [DOI] [PubMed] [Google Scholar]
PR05 {published data only}
- Dearnaley DP, Mason MD, Parmar MKB, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long‐term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncology 2009;10:872‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley DP, Sydes MR, Mason MD, Stott M, Powell CS, Robinson ACR, et al. A double‐blind, placebo‐controlled, randomised trial of oral sodium clodronate for metastatic prostate cancer (MRC PRO5 Trial). Journal of the National Cancer Institute 2003;95(17):1300‐11. [DOI] [PubMed] [Google Scholar]
Saad 2010 {published data only}
- Saad F, Eastham J. Zoledronic acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urology 2010;76:1175‐81. [DOI] [PubMed] [Google Scholar]
- Saad F, Gleason DM, Murray R. A randomized, placebo‐controlled trial of zoledronic acid in patients with hormone‐refractory metastatic prostate carcinoma. Journal of the National Cancer Institute 2002;94(19):1458‐68. [DOI] [PubMed] [Google Scholar]
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long‐term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone‐refractory prostate cancer. Journal of the National Cancer Institute 2004;96:879‐82. [DOI] [PubMed] [Google Scholar]
- Weinfurt KP, Anstrom KJ, Castel LD, Schulman KA, Saad F. Effect of zoledronic acid on pain associated with bone metastasis in patients with prostate cancer. Annals of Oncology 2006;17:986‐9. [DOI] [PubMed] [Google Scholar]
Small 2003 {published data only}
- Small EJ, Matthew RS, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo‐controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostatic cancer. Journal of Clinical Oncology 2003;21(23):4277‐84. [DOI] [PubMed] [Google Scholar]
Smith 1989 {published data only}
- Smith JA Jr. Palliation of painful bone metastases from prostate cancer using sodium etidronate: results of a randomized, prospective, double‐blind, placebo‐controlled study. Journal of Urology 1989;141:85‐7. [DOI] [PubMed] [Google Scholar]
Strang 1997 {published data only}
- Strang P, Nilsson S, Brandstedt S. The analgesic efficacy of clodronate compared with placebo inpatients with painful bone metastases from prostatic cancer. Anticancer Research 1997;17:4717‐21. [PubMed] [Google Scholar]
TRAPEZE 2016 {published data only}
- James N, Pirrie S, Pope A, Barton D, Andronis L, Goranitis I, et al. TRAPEZE: A randomised controlled trial of the clinical effectiveness and cost‐effectiveness of chemotherapy with zoledronic acid, strontium‐89, or both, in men with bony metastatic castration‐refractory prostate cancer. Health Technology Assessment 2016;20(53):1‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ND, Andronis L, Goranitis I, Pirrie S, Pope A, Barton D, et al. Cost‐effectiveness of zoledronic acid and strontium‐89 as bone protecting treatments in addition to chemotherapy in patients with metastatic castrate‐refractory prostate cancer. (ISRCTN 12808747) TRAPEZE. Journal of Clinical Oncology 2015;33:e16108. [DOI] [PubMed] [Google Scholar]
- James ND, Pirrie S, Barton D, Brown JE, Billingham L, Collins SI, et al. Clinical outcomes in patients with castrate‐refractory prostate cancer (CRPC) metastatic to bone randomized in the factorial TRAPEZE trial to docetaxel (D) with strontium‐89 (Sr89), zoledronic acid (ZA), neither, or both (ISRCTN 12808747). Journal of Clinical Oncology 2013;31:LBA5000. [Google Scholar]
- James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitis I, et al. Clinical outcomes and survival following treatment of metastatic castrate‐refractory prostate cancer with docetaxel alone or with strontium‐89, zoledronic acid, or both: the TRAPEZE randomized clinical trial. JAMA Oncology 2016;2(4):493‐9. [PUBMED: 26794729] [DOI] [PubMed] [Google Scholar]
- Porfiri E, Collins SI, Barton D, Billingham L, McLaren D, Nixon GG, et al. Initial feasibility and safety results from a phase II/III clinical trial to evaluate docetaxel (D) therapy in combination with zoledronic acid (ZA) {+/‐} strontium‐89 (Sr89) in hormone‐refractory prostate cancer patients: ISRCTN12808747. Journal of Clinical Oncology 2010;28:4677. [Google Scholar]
ZABTON‐PC {published data only}
- Ueno S, Mizokami A, Fukagai T, Fujimoto N, Oh‐Oka H, Kondo Y, et al. Efficacy of combined androgen blockade with zoledronic acid treatment in prostate cancer with bone metastasis: the ZABTON‐PC (zoledronic acid/androgen blockade trial on prostate cancer) study. Anticancer Research 2013;33:3837‐44. [PubMed] [Google Scholar]
ZAPCA {published data only}
- Kamba T, Kamoto T, Maruo S, Kikuchi T, Shimizu Y, Namiki S, et al. A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: results of the ZAPCA trial. International Journal of Clinical Oncology 2017;22:166‐73. [DOI] [PubMed] [Google Scholar]
- Kamba T, Kamoto T, Shimizu Y, Namiki S, Fujimoto K, Kawanishi H, et al. A phase III, multicenter, randomized, controlled study of maximum androgen blockade with versus without zoledronic acid in treatment‐naive prostate cancer patients with bone metastases: Results of ZAPCA study. Journal of Clinical Oncology 2015;33:150. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adami 1985 {published data only}
- Adami S, Salvagno G, Guarrera G. Dichloromethylene‐diphosphonate in patients with prostatic carcinoma metastatic to the skeleton. Journal of Urology 1985;134:1152‐4. [DOI] [PubMed] [Google Scholar]
Adami 1989 {published data only}
- Adami S, Mian M. Clodronate therapy of metastatic bone disease in patients with prostatic carcinoma. Recent Results in Cancer Research 1989;116:67‐72. [DOI] [PubMed] [Google Scholar]
BO18039 {published data only (unpublished sought but not used)}
- BO18039. Randomized, two arm, placebo controlled double dummy study to compare the efficacy of intravenous loading doses followed by maintenance treatment with oral ibandronic acid versus zoledronic acid in patients with malignant and painful bone disease. www.clinicaltrialsregister.eu/ctr‐search/search?query=2004‐001854‐10 Date first received: 16 February 2005.
CALGB 70604 {published data only}
- Himelstein AL, Qin R, Novotny PJ, Seisler DK, Khatcheressian JL, Roberts JD, et al. CALGB 70604 (Alliance): a randomized phase III study of standard dosing vs. longer interval dosing of zoledronic acid in metastatic cancer. Journal of Clinical Oncology 2015;33:9501. [Google Scholar]
Carey 1988 {published data only}
- Carey PO, Lippert MC. Treatment of painful prostatic bone metastases with oral etidronate disodium. Urology 1988;32:403‐7. [DOI] [PubMed] [Google Scholar]
Clarke 1991 {published data only}
- Clarke NW, Holbrook IB, McClure J, George NJ. Osteoclast inhibition by pamidronate in metastatic prostate cancer: a preliminary study. British Journal of Cancer 1991;63(3):420‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cresswell 1995 {published data only}
- Cresswell SM, English PJ, Hall RR. Pain relief and quality‐of‐life assessment following intravenous and oral clodronate in hormone‐escaped metastatic prostate cancer. British Journal of Urology 1995;76:360‐5. [DOI] [PubMed] [Google Scholar]
Fernandez‐Conde 1997 {published data only}
- Fernandez‐Conde M, Alcover J, Aaron JE, Ordi J, Carretero P. Skeletal response to clodronate in prostate cancer with bone metastases. American Journal of Clinical Oncology 1997;20(5):471‐6. [DOI] [PubMed] [Google Scholar]
Fizazi 2009 {published data only}
- Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab treatment of prostate cancer with bone metastases and increased urine N‐telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. Journal of Urology 2009;182:509‐15; discussion 515‐6. [DOI] [PubMed] [Google Scholar]
Fizazi 2011 {published data only}
- Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration‐resistant prostate cancer: a randomised, double‐blind study. Lancet 2011;377:813‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizazi K, Carducci MA, Smith MR, Damiao R, Brown JE, Karsh L, et al. A randomized phase III trial of denosumab versus zoledronic acid in patients with bone metastases from castration‐resistant prostate cancer [abstract no. LBA4507]. Journal of Clinical Oncology 2010;28(18):951. [Google Scholar]
- Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. Journal of Clinical Oncology 2009;27:1564‐71. [DOI] [PubMed] [Google Scholar]
Heidenreich 2001 {published data only}
- Heidenreich A, Hoffmann R, Engelmann UH. The use of bisphosphonate for the palliative treatment of painful bone metastasis due to hormone refractory prostate cancer. Journal of Urology 2001;165(1):136‐40. [DOI] [PubMed] [Google Scholar]
Heidenreich 2002 {published data only}
- Heidenreich A, Elert A, Hofmann R. Ibandronate in the treatment of prostate cancer associated painful osseous metastases. Prostate Cancer and Prostatic Diseases 2002;5(3):231‐5. [DOI] [PubMed] [Google Scholar]
Jagdev 2001 {published data only}
- Jagdev SP, Purohit OP, Heatley S, Herling C, Coleman RE. Comparison of the effects of intravenous pamidronate and oral clodronate on symptoms and bone resorption in patients with metastatic bone disease. Annals of Oncology 2001;12:1433‐8. [DOI] [PubMed] [Google Scholar]
Kylmala 1994 {published data only}
- Kylmala T, Tammela TL, Lindholm TS. The effect of combined intravenous and oral clodronate treatment on bone pain in patients with metastatic prostate cancer. Annales Chirurgiae et Gynaecologiae 1994;83(4):316‐9. [PubMed] [Google Scholar]
Magnusson 1998 {published data only}
- Magnusson P, Larssson L, Englund G, Larsson B, Strang P, Selin‐Sjogren L. Differences of bone alkaline phosphatase isoforms in metastatic bone disease and discrepant effects of clodronate on different skeletal sites indicated by location of pain. Clinical Chemistry 1998;44(8 Pt 1):1621‐8. [PubMed] [Google Scholar]
MER‐101‐03 {published data only}
- McHugh C, Madigan K, Walsh A, Fox J, Leonard TW, Quint JB. MER‐101‐03, a multicenter, phase II study to compare MER‐101 20mg tablets to intravenous zoledronic acid 4mg in prostate cancer patients. Journal of Clinical Oncology 2009;27:5161. [Google Scholar]
NCT00242567 {published data only}
Pelger 1998 {published data only}
- Pelger RC, Hamdy NA, Zwinderman AH. Effects of the bisphosphonate olpadronate in patients with carcinoma of prostate metastatic to the skeleton. Bone 1998;22:403‐8. [DOI] [PubMed] [Google Scholar]
STAMPEDE {published data only}
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first‐line long‐term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387(10024):1163‐77. [PUBMED: 26719232] [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ND, Sydes MR, Mason MD, Clarke NW, Dearnaley DP, Spears MR, et al. Docetaxel and/or zoledronic acid for hormone‐naive prostate cancer: first overall survival results from STAMPEDE (NCT00268476). Journal of Clinical Oncology 2015;33:5001. [Google Scholar]
Taube 1994 {published data only}
- Taube T, Kylmala T, Lamberg‐Allardt C. The effect of clodronate on bone in metastatic prostate cancer: histomorphonmetric report of a double‐blind randomised placebo‐controlled study. European Journal of Cancer 1994;30:751‐8. [DOI] [PubMed] [Google Scholar]
Vorreuther 1992 {published data only}
- Vorreuther R, Klotz T, Engelking R. Clodronate in the palliative therapy of bone‐metastasized prostate carcinoma. Urologe A 1992;31(2):63‐6. [PubMed] [Google Scholar]
Vorreuther 1993 {published data only}
- Vorreuther R. Bisphosphonates as an adjunct to palliative therapy of bone metastases from prostate carcinoma. A pilot study on clodronate. British Journal of Urology 1993;72((5 Pt 2)):792‐5. [DOI] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang F, Chen W, Chen H, Mo L, Jin H, Yu Z, et al. Comparison between zoledronic acid and clodronate in the treatment of prostate cancer patients with bone metastases. Medical Oncology 2013;30:657. [DOI] [PubMed] [Google Scholar]
Additional references
Alibhai 2017
- Alibhai SMH, Zukotynski K, Walker‐Dilks C, Emmenegger U, Finelli A, Morgan SC, et al. Bone Health and Bone‐targeted Therapies for Prostate Cancer: a Programme in Evidence‐based Care ‐ Cancer Care Ontario Clinical Practice Guideline. Clinical oncology (Royal College of Radiologists (Great Britain)) 2017;29(6):348‐55. [PUBMED: 28169118] [DOI] [PubMed] [Google Scholar]
Bartl 2007
- Bartl R, Frisch B, Tresckow E, Bartl C. Bisphosphonates in Medical Practice ‐ Actions, Side effects, Indications, Strategies. Berlin (Germany): Springer, 2007. [Google Scholar]
Bartl 2008
- Bartl R, Bartl C, Gradinger R. Use of bisphosphonates in orthopedic surgery [Einsatz der Bisphosphonate in der Orthopädie und Unfallchirurgie]. Der Orthopäde 2008;37(6):595‐614. [DOI] [PubMed] [Google Scholar]
Bubendorf 2000
- Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Human Pathology 2000;31(5):578‐83. [DOI] [PubMed] [Google Scholar]
Clohisy 2002
- Clohisy DR, Mantyh PW. Bone cancer pain. Cancer 2002;97(3 Suppl):866‐73. [DOI] [PubMed] [Google Scholar]
Coleman 1997
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80(8 Suppl):1588‐94. [DOI] [PubMed] [Google Scholar]
Coleman 2012
- Coleman R, Gnant M, Morgan G, Clezardin P. Effects of bone‐targeted agents on cancer progression and mortality. Journal of the National Cancer Institute 2012;104(14):1059‐67. [DOI] [PubMed] [Google Scholar]
Conford 2017
- Cornford P, Bellmunt J, Bolla M, Briers E, Santis M, Gross T. EAU‐ESTRO‐SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration‐resistant prostate cancer. European Urology 2017;71(4):631‐42. [DOI] [PubMed] [Google Scholar]
Cookson 2013
- Cookson MS, Roth BJ, Dahm P, Engstrom C, Freedland SJ, Hussain M. Castration‐resistant prostate cancer: AUA guideline. Journal of Urology 2013;190(2):429‐38. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Devogelaer 2000
- Devogelaer D. Treatment of bone diseases with bisphosphonates, excluding osteoporosis. Current Opinion in Rheumatology 2000;12:331‐5. [DOI] [PubMed] [Google Scholar]
El‐Amm 2016
- El‐Amm J, Aragon‐Ching JB. Targeting bone metastases in metastatic castration‐resistant prostate cancer. Clinical Medicine Insight: Oncology 2016;10(Suppl 1):11‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferlay 2013
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Cancer incidence and mortality worldwide: IARC CancerBase No. 11, 2013. globocan.iarc.fr (accessed 12 August 2017).
Fitzpatrick 2014
- Fitzpatrick JM, Bellmunt J, Fizazi K, Heidenreich A, Sternberg CN, Tombal B, et al. Optimal management of metastatic castration‐resistant prostate cancer: highlights from a European Expert Consensus Panel. European Journal of Cancer 2014;50(9):1617‐27. [DOI] [PubMed] [Google Scholar]
Fizazi 2015
- Fizazi K, Massard C, Smith M, Rader M, Brown J, Milecki P, et al. Bone‐related parameters are the main prognostic factors for overall survival in men with bone metastases from castration‐resistant prostate cancer. European Urology 2015;68(1):42‐50. [DOI] [PubMed] [Google Scholar]
Gartrell 2014
- Gartrell BA, Coleman RE, Fizazi K, Miller K, Saad F, Sternberg CN, et al. Toxicities following treatment with bisphosphonates and receptor activator of nuclear factor‐kappaB ligand inhibitors in patients with advanced prostate cancer. European Urology 2014;65(2):278‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gartrell 2015
- Gartrell BA, Coleman R, Efstathiou E, Fizazi K, Logothetis CJ, Smith MR, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. European Urology 2015;68(5):850‐8. [DOI] [PubMed] [Google Scholar]
Hellstein 2011
- Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. Journal of the American Dental Association 2011;142(11):1243‐51. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horwich 2013
- Horwich A, Parker C, Reijke T, Kataja V. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2013;24 Suppl 6:vi106‐14. [DOI] [PubMed] [Google Scholar]
Howlader 2015
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER cancer statistics review, 1975‐2012, based on November 2014 SEER data submission, posted to the SEER website, April 2015. seer.cancer.gov/csr/1975_2012/ (accessed prior to 26 November 2017).
Lee 2014
- Lee SH, Chan RC, Chang SS, Tan YL, Chang KH, Lee MC, et al. Use of bisphosphonates and the risk of osteonecrosis among cancer patients: a systemic review and meta‐analysis of the observational studies. Supportive Care in Cancer 2014;22(2):553‐60. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liu 2015
- Liu J, Huang W, Zhou R, Jia S, Tang W, Luo Y, et al. Bisphosphonates in the treatment of patients with metastatic breast, lung, and prostate cancer: a meta‐analysis. Medicine (Baltimore) 2015;94(46):e2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mhaskar 2012
- Mhaskar R, Redzepovic J, Wheatley K, Clark OAC, Miladinovic B, Glasmacher A, et al. Bisphosphonates in multiple myeloma: a network meta‐analysis. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD003188.pub3; CD003188] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [DOI] [PubMed] [Google Scholar]
Mohler 2016
- Mohler JL, Armstrong AJ, Bahnson RR, D'Amico AV, Davis BJ, Eastham JA, et al. Prostate cancer, version 1.2016. Journal of the National Comprehensive Cancer Network 2016;14(1):19‐30. [DOI] [PubMed] [Google Scholar]
Newcomb 2010
- Newcomb P A, Trentham‐Dietz A, Hampton J M. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. British Journal of Cancer 2010;102(5):799‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Reid 2003
- Reid IR. Bisphosphonates: new indications and methods of administration. Current Opinion in Rheumatology 2003;15:458‐63. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes 2016
- Reyes C, Hitz M, Prieto‐Alhambra D, Abrahamsen B. Risks and benefits of bisphosphonate therapies. Journal of Cellular Biochemistry 2016;117(1):20‐8. [DOI] [PubMed] [Google Scholar]
Saunders 2004
- Saunders Y, Ross JR, Broadley KE, Edmonds PM, Patel S. Systematic review of bisphosphonates for hypercalcaemia of malignancy. Palliative Medicine 2004;18(5):418‐31. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Thosani 2013
- Thosani N, Thosani SN, Kumar S, Nugent Z, Jimenez C, Singh H, et al. Reduced risk of colorectal cancer with use of oral bisphosphonates: a systematic review and meta‐analysis. Journal of Clinical Oncology 2013;31(5):623‐30. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vignani 2016
- Vignani F, Bertaglia V, Buttigliero C, Tucci M, Scagliotti GV, Maio M. Skeletal metastases and impact of anticancer and bone‐targeted agents in patients with castration‐resistant prostate cancer . Cancer Treatment Reviews 2016;44:61‐73. [DOI] [PubMed] [Google Scholar]
Wirth 2016
- Wirth M, Berges R, Fröhner M, Miller K, Rübben H, Stöckle M, et al. Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms, Langversion 4.0. Berlin (Germany): AWMF, 2016. [Google Scholar]
Wong 2012
- Wong MHF, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD003474.pub3; CD003474] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Yuen 2006
- Yuen KK, Shelley M, Sze WM, Wilt TJ, Mason M. Bisphosphonates for advanced prostate cancer. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006250] [DOI] [PubMed] [Google Scholar]


