Abstract
Background
Exercise programmes are a relatively inexpensive, low‐risk option compared with other, more invasive therapies for treatment of leg pain on walking (intermittent claudication (IC)). This is the fourth update of a review first published in 1998.
Objectives
Our goal was to determine whether an exercise programme was effective in alleviating symptoms and increasing walking treadmill distances and walking times in people with intermittent claudication. Secondary objectives were to determine whether exercise was effective in preventing deterioration of underlying disease, reducing cardiovascular events, and improving quality of life.
Search methods
For this update, the Cochrane Vascular Information Specialist searched the Specialised Register (last searched 15 November 2016) and the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 10) via the Cochrane Register of Studies Online, along with trials registries.
Selection criteria
Randomised controlled trials of an exercise regimen versus control or versus medical therapy for people with IC due to peripheral arterial disease (PAD). We included any exercise programme or regimen used for treatment of IC, such as walking, skipping, and running. Inclusion of trials was not affected by duration, frequency, or intensity of the exercise programme. Outcome measures collected included treadmill walking distance (time to onset of pain or pain‐free walking distance and maximum walking time or maximum walking distance), ankle brachial index (ABI), quality of life, morbidity, or amputation; if none of these was reported, we did not include the trial in this review.
Data collection and analysis
For this update (2017), RAL and AH selected trials and extracted data independently. We assessed study quality by using the Cochrane 'Risk of bias' tool. We analysed continuous data by determining mean differences (MDs) and 95% confidence intervals (CIs), and dichotomous data by determining risk ratios (RRs) and 95% CIs. We pooled data using a fixed‐effect model unless we identified significant heterogeneity, in which case we used a random‐effects model. We used the GRADE approach to assess the overall quality of evidence supporting the outcomes assessed in this review.
Main results
We included two new studies in this update and identified additional publications for previously included studies, bringing the total number of studies meeting the inclusion criteria to 32, and involving a total of 1835 participants with stable leg pain. The follow‐up period ranged from two weeks to two years. Types of exercise varied from strength training to polestriding and upper or lower limb exercises; supervised sessions were generally held at least twice a week. Most trials used a treadmill walking test for one of the primary outcome measures. The methodological quality of included trials was moderate, mainly owing to absence of relevant information. Most trials were small and included 20 to 49 participants. Twenty‐seven trials compared exercise versus usual care or placebo, and the five remaining trials compared exercise versus medication (pentoxifylline, iloprost, antiplatelet agents, and vitamin E) or pneumatic calf compression; we generally excluded people with various medical conditions or other pre‐existing limitations to their exercise capacity.
Meta‐analysis from nine studies with 391 participants showed overall improvement in pain‐free walking distance in the exercise group compared with the no exercise group (MD 82.11 m, 95% CI 71.73 to 92.48, P < 0.00001, high‐quality evidence). Data also showed benefit from exercise in improved maximum walking distance (MD 120.36 m, 95% CI 50.79 to 189.92, P < 0.0007, high‐quality evidence), as revealed by pooling data from 10 studies with 500 participants. Improvements were seen for up to two years.
Exercise did not improve the ABI (MD 0.04, 95% CI 0.00 to 0.08, 13 trials, 570 participants, moderate‐quality evidence). Limited data were available for the outcomes of mortality and amputation; trials provided no evidence of an effect of exercise, when compared with placebo or usual care, on mortality (RR 0.92, 95% CI 0.39 to 2.17, 5 trials, 540 participants, moderate‐quality evidence) or amputation (RR 0.20, 95% CI 0.01 to 4.15, 1 trial, 177 participants, low‐quality evidence).
Researchers measured quality of life using Short Form (SF)‐36 at three and six months. At three months, the domains 'physical function', 'vitality', and 'role physical' improved with exercise; however this was a limited finding, as it was reported by only two trials. At six months, meta‐analysis showed improvement in 'physical summary score' (MD 2.15, 95% CI 1.26 to 3.04, P = 0.02, 5 trials, 429 participants, moderate‐quality evidence) and in 'mental summary score' (MD 3.76, 95% CI 2.70 to 4.82, P < 0.01, 4 trials, 343 participants, moderate‐quality evidence) secondary to exercise. Two trials reported the remaining domains of the SF‐36. Data showed improvements secondary to exercise in 'physical function' and 'general health'. The other domains ‐ 'role physical', 'bodily pain', 'vitality', 'social', 'role emotional', and 'mental health' ‐ did not show improvement at six months.
Evidence was generally limited in trials comparing exercise versus antiplatelet therapy, pentoxifylline, iloprost, vitamin E, and pneumatic foot and calf compression owing to small numbers of trials and participants.
Review authors used GRADE to assess the evidence presented in this review and determined that quality was moderate to high. Although results showed significant heterogeneity between trials, populations and outcomes were comparable overall, with findings relevant to the claudicant population. Results were pooled for large sample sizes ‐ over 300 participants for most outcomes ‐ using reproducible methods.
Authors' conclusions
High‐quality evidence shows that exercise programmes provided important benefit compared with placebo or usual care in improving both pain‐free and maximum walking distance in people with leg pain from IC who were considered to be fit for exercise intervention. Exercise did not improve ABI, and we found no evidence of an effect of exercise on amputation or mortality. Exercise may improve quality of life when compared with placebo or usual care. As time has progressed, the trials undertaken have begun to include exercise versus exercise or other modalities; therefore we can include fewer of the new trials in this update.
Plain language summary
Exercise for reducing intermittent claudication symptoms
Background
Intermittent claudication is a cramping leg pain that develops when walking and is relieved with rest. It is caused by inadequate blood flow to the leg muscles caused by atherosclerosis (fatty deposits restricting blood flow through the arteries). People with mild to moderate claudication are advised to keep walking, stop smoking, and reduce cardiovascular risk factors. Other treatments include antiplatelet therapy, pentoxifylline or cilostazol, angioplasty (inserting a balloon into the artery to open it up), and bypass surgery.
Studies and key results
Review authors identified 32 controlled trials that randomised 1835 adults with stable leg pain to exercise, usual care or placebo, or other interventions (current until November 2016). Researchers measured outcomes at times ranging from two weeks to two years. Types of exercise varied from strength training to polestriding and upper or lower limb exercises; in general, supervised sessions were held at least twice a week. The quality of included trials was moderate, mainly because of absence of relevant information. Ten trials reported that in the exercise groups, pain‐free walking distance and the maximum distance that participants could walk was increased. Improvements were seen for up to two years. Exercise did not improve ankle to brachial blood pressure index. No evidence of an effect of exercise was seen on death or need for amputation because data were limited. Researchers assessed quality of life using the SF‐36 Questionnaire at three and six months. At three months, indicators of quality of life ‐ 'physical function', 'vitality', and 'role physical' ‐ had all improved with exercise, but these data are limited, as only two trials reported this. Five studies reported improved 'physical summary score' and four studies reported improved 'mental health score' following exercise at six months, with two trials also reporting improvements in 'physical function' and 'general health'. All other domains showed no improvement at six months following exercise.
Comparisons of exercise with antiplatelet therapy, pentoxifylline, iloprost, vitamin E, and pneumatic foot and calf compression were limited because numbers of identified trials and participants were small.
Quality of the evidence
The present review shows that exercise programmes appear to improve walking distance for people considered fit for exercise regimens. This benefit appears to be sustained over two years. Evidence presented in this review was of moderate to high quality. Although differences between trials were evident, populations and outcomes were comparable overall, and findings were relevant to people with intermittent claudication. Combined results were derived from large sample sizes ‐ over 300 participants for most outcomes ‐ using reproducible methods.
Summary of findings
Summary of findings for the main comparison. Is exercise an effective intervention in intermittent claudication?
| Exercise compared with no exercise for intermittent claudication | ||||||
|
Patient or population: adults with intermittent claudication Settings: hospital or community‐based physical therapy exercise programmes Intervention: exercisea Comparison: no exercise (previously known as usual care) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI)b | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No exercise | Exercisea | |||||
|
Pain‐free walking distance (m) follow‐up: 6 weeks to 24 months |
Mean pain‐free walking distance ranged across control groups from 63.3 m to 253 m. | Mean pain‐free walking distance (m) in exercise groups ranged from 116 m to 413 m. MD was 82.11 m further in the exercise group. (95% CI 71.73 to 92.48) |
391 (9 RCTs) | ⊕⊕⊕⊕ highc | 4/9 studies (Cucato 2013; Gardner 2002; Jansen 1991 and Mika 2005) reported a clear improvement. | |
|
Maximum walking distance (m) follow‐up: 6 weeks to 24 months |
Mean maximum walking distance ranged across control groups from 122 m to 771 m. | Mean maximum walking distance (m) in exercise groups ranged from 136 m to 1100 m. MD was 120.36 m further in the exercise group. (95% CI 50.79 to 189.92) |
500 (10 RCTs) | ⊕⊕⊕⊕ highd | 5/10 studies (Cucato 2013; Gardner 2002; Jansen 1991; Tew 2015; Zwierska 2005) reported a clear improvement. | |
|
Ankle brachial index follow‐up: 3 to 12 months |
Mean ABI ranged across control groups from 0.32 to 0.89. | Mean ABI in exercise groups ranged from 0.34 to 0.96. MD was 0.04 higher in the exercise group. (95% CI 0.00 to 0.08) |
570 (13 RCTs) | ⊕⊕⊕⊝ moderatee | A change in ABI of 0.04 is of limited clinical significance. | |
|
Mortality: all‐cause deaths follow‐up: 3 to 12 months |
A total of 9/273 deaths occurred in the no exercise group. | A total of 8/267 deaths occurred in the exercise group. | RR 0.92 (0.39 to 2.17) | 540 (5 RCTs) |
⊕⊕⊕⊝ moderatef | |
|
Amputation follow‐up: 12 months |
A total of 2/88 amputations occurred in the no exercise group. | No amputations occurred in the exercise group (0/89). | RR 0.20 (0.01 to 4.15) | 177 (1 RCT) | ⊕⊕⊝⊝ lowg | |
|
Quality of Life SF‐36 Physical Summary score (scale 0 to 100, higher score indicates better quality of life) follow‐up: 6 months |
Mean physical summary score ranged across control groups from 34.2 to 47.1. |
Mean Physical Summary score in exercise groups ranged from 41 to 54.1. MD score was 2.15
higher in the exercise group. (95% CI 1.26 to 3.04) |
429 (5 RCTs) |
⊕⊕⊕⊝ moderateh | ||
|
Quality of Life SF‐36 Mental Summary score (scale 0 to 100, higher score indicates better quality of life) follow‐up: 6 months |
Mean Mental Summary score ranged across control groups from 38.8 to 62.0. |
Mean Mental Summary score in exercise groups ranged from 39.6 to 67. MD score was 3.76
higher in the exercise group. (95% CI 2.7 to 4.82) |
343 (4 RCTs) |
⊕⊕⊕⊝ moderatei | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ABI: ankle brachial index; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SF: Short Form. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aVariability in type, duration, and frequency of exercise programmes as prescribed. Exercise programmes included a duration of six weeks up to one year, generally twice or three times a week, varying in length between 30 minutes and one hour. bWhen possible, the no exercise (control) groups results were measured at the same time point and were used in the meta‐analysis to calculate assumed risk. cThe level of evidence was high, as most trials were of high quality. dThe level of evidence was high, as most trials were of high quality. Heterogeneity was significant (P = 0.01, I2 = 89%), and CIs were broad (50.79 to 189.92). However further research is unlikely to change this. eWe downgraded by one step owing to significant heterogeneity (P ≤ 0.01, I2 = 64%); however CIs were narrow (0.00 to 0.09). Given the size of the data set, it is unlikely that further data will demonstrate a difference between the two groups. fWe downgraded the evidence by one step, as analysis included only five small studies (of which one was underpowered) and showed wide confidence intervals (imprecision). gWe downgraded the evidence by two steps, as data are from a single study with wide CIs (imprecision) and events were few overall. hWe downgraded owing to heterogeneity (P = 0.02, I2 = 66%), narrow CI (1.26 to 3.04), and a symmetrical distribution on funnel plot. Other quality of life measures were used and have been described more fully in the additional Table 2. Improvement was also seen in the following domains: vitality and role physical at three months, physical function, and general health at six months. iWe downgraded owing to heterogeneity (P < 0.01, I2 = 87%), narrow CI (2.70 to 4.82), and a symmetrical distribution on funnel plot. Additional improvements in other domains may be seen in the future as more studies report their outcomes for quality of life scores.
Background
Peripheral arterial disease is an important cause of morbidity and mortality for people in many Western countries. It is estimated that most adults have some degree of atherosclerosis by the time they reach middle age, and approximately 4% will develop intermittent claudication (Leng 1993). As the population ages, the prevalence of claudication will increase. Risk factors for development of lower limb arterial disease are similar to those for coronary heart disease, and include smoking, raised cholesterol levels, hypertension, and diabetes.
Several epidemiological studies have demonstrated an association between sedentary habits, functional decline, and worsening claudication (McDermott 2006; McDermott 2011). The impact of exercise therapy on physical functional ability, muscle strength, and walking times has drawn increased focus since a review was prepared in 2013 (Parmenter 2013). Over the past few years, further research into calf muscle strength has supported known changes in muscle architecture while enhancing focus on the impact of these changes in relation to walking distance and physical function. Researchers have described reduction in myofibre cross‐sectional area, enzymes, and power (Clyne 1985; Dahllof 1974; King 2015; Koutakis 2015), along with alterations in gait (Gommans 2016; King 2012), among patients with intermittent claudication.
Description of the condition
Peripheral arterial disease covers a spectrum ranging from asymptomatic disease through to claudication, critical limb ischaemia, and finally limb loss. Within this spectrum, most people have relatively stable disease, termed 'claudication'. Intermittent claudication occurs secondary to atherosclerosis of the lower limb arteries, resulting from impaired blood flow. Whether at rest or when walking slowly, this reduction may go unnoticed; however during periods of exercise or walking with additional loads, for example, carrying while shopping, a cycle of pain requiring short rests occurs. This muscular, cramp‐like tightening of the calf, the buttocks, or the foot on walking is known as 'claudication'.
Description of the intervention
Treatment options for intermittent claudication include bypass surgery, angioplasty, and drug therapy, but the mainstay of treatment for many patients with mild to moderate claudication remains advice to 'stop smoking and keep walking' (Housley 1988; NCGC 2012) while modifying cardiovascular risk factors.
Exercise therapy or programmes usually require a regular weekly commitment, lasting from six weeks to a year. In general, programmes are run twice or three times per week. Duration can vary; usually a minimum of 30 minutes is required per session.
How the intervention might work
Researchers have conducted numerous studies of exercise therapy using various regimens that differ in duration and intensity; many of these studies suggest that exercise can prove beneficial for individuals with intermittent claudication (Ernst 1992). Underlying mechanisms through which exercise may effect improvement include increased and more effective distribution of blood flow to the legs (Ernst 1987a), improved rheological characteristics of the blood (Ernst 1987), less reliance on anaerobic metabolism (Ruell 1984), and greater use of oxygen (Dahllof 1974).
A systematic review of the effect of exercise on lower limb haemodynamics in individuals with mild to moderate claudication identified 33 trials. In these trials, investigators reported no change when comparing effects of control versus exercise therapy on resting ankle brachial index (ABI), postexercise ABI, resting calf arterial blood flow, reactive hyperaemic blood flow post ischaemia, and resting toe systolic pressure (Parmenter 2011). An extensive non‐systematic review of the literature focussed on exercise training in people with claudication and physiological changes associated with exercise (Haas 2012). This review discussed how regular exercise improves endothelial flow‐mediated dilatation (FMD). Exercise training also improves FMD in those with claudication.
Exercise is proposed to improve walking among claudicants through angiogenesis. Underlying biomechanisms by which this may occur include ischaemia, sheer stress secondary to exercise, and remodelling of skeletal muscle (Hiatt 1994; Regensteiner 1993). Researchers have done extensive work on the changes that occur in skeletal muscle secondary to claudication. These changes can be summarised as a change in capillary density (Clyne 1985), as a ratio of type I to type II fibres (Clyne 1985; Hiatt 1994; Sjöström 1982), or as increases in arteriogenesis and mitochondrial activity. Trialists have described subsequent changes in skeletal muscle that can occur with training in humans (Lundgren 1989a; Terjung 1988; Wang 2009), as well as in animal models with artificial hindlimb stenoses (Yang 1991).
More recently, researchers have explored changes secondary to differing exercise programmes, while focussing on calpain activity, which has been associated with muscle atrophy in animal models. When trial authors explored effects of walking regimens versus strength training combined with walking, they noted that neither was seen to alter calpain activity (Delaney 2014).
The impact of peripheral arterial disease (PAD) on lower limb skeletal muscle becomes apparent when focussing on daily tasks or measuring strength. Individuals with PAD have reduced lower limb strength and ability or endurance for performing lower limb tasks, that is, knee flexion, dorsiflexion, or plantar flexion, when compared with healthy controls (Câmara 2012). In addition, people with PAD and reduced muscle density are more likely to have higher all‐cause and cardiovascular disease mortality (McDermott 2012).
Clinicians have identified reduced muscle strength (Câmara 2012; Gohil 2013a; Parmenter 2013a), decreased walking distances (Parmenter 2013a), greater imbalance (Gohil 2013; Mockford 2014), and alterations in gait (King 2012; Koutakis 2010), secondary to PAD.
Why it is important to do this review
A meta‐analysis of exercise rehabilitation programmes for claudication pain showed that exercise training to near‐maximal pain for at least six months was beneficial in improving claudication (Gardner 1995). That review provided good evidence for the best type of exercise therapy but did not compare findings with non‐exercised control groups. The present Cochrane review focusses on randomised controlled trials only and encompasses additional endpoints. The National Institute for Health and Care Excellence (NICE) (NCGC 2012) currently advocates that all patients with PAD should undergo an exercise programme as first‐line treatment. Numerous other studies have echoed this advice (Haas 2012; Lauret 2012; Willigendael 2005). However, uptake remains low across the UK. This review aims to add weight to the current body of available evidence recommending exercise, while demonstrating effects of different durations of exercise on walking distance and quality of life.
Objectives
Our goal was to determine whether an exercise programme was effective in alleviating symptoms and increasing walking treadmill distances and walking times in people with intermittent claudication. Secondary objectives were to determine whether exercise was effective in preventing deterioration of underlying disease, reducing cardiovascular events and improving quality of life.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) of an exercise regimen versus control, or versus medical therapy. We excluded trials that used alternation (e.g. allocation by date of birth or days of the week). We included trials that did not perform intention‐to‐treat analysis, provided all randomised participants were accounted for. We excluded trials for which numerical data were not available in a usable format, despite contact with study authors, but that were otherwise suitable.
We included studies in this review if they focussed on exercise versus usual care or other medical interventions. We excluded studies focussing on exercise compared with other forms of exercise, unsupervised exercise, angioplasty, or surgery, to prevent overlap with other Cochrane reviews (Antoniou 2017; Fakhry 2013; Fokkenrood 2013; Fowkes 1998). We excluded studies in which usual care included walking advice or suggestions to increase daily exercise, as this advice constitutes unsupervised exercise. Although the exclusion of walking advice as part of usual care may be deemed controversial, this has been undertaken according to what has been reported by the trialists in the study papers.
Types of participants
We included trials involving participants with symptomatic intermittent claudication due to atherosclerotic disease. Intermittent claudication may be diagnosed objectively by an ABI < 0.9 or evidence of PAD on Doppler ultrasound or angiography, or both, or by questionnaire or clinically if objective measures such as ABI or imaging were not used or reported. We excluded studies of participants with asymptomatic lower limb atherosclerosis that was identified by testing.
Types of interventions
We included any exercise programme used for treatment of patients with intermittent claudication, such as walking, skipping, and running, and home‐based therapies provided researchers compared treatment against placebo or no therapy. Inclusion of trials was not affected by duration, frequency, or intensity of the exercise programme, but these issues were taken into account in the meta‐analysis. This review did not consider supervised versus unsupervised exercise because this is the topic of another Cochrane review (Fokkenrood 2013). Walking advice provided by consultants in clinic can be seen as the best medical treatment or control. However, this may also be deemed unsupervised exercise. Therefore, we excluded from this review all studies that provided best medical treatment, which includes walking advice.
To avoid overlap with other Cochrane reviews (Antoniou 2017; Fakhry 2013; Fowkes 1998), this review excluded all modalities by which exercise can be compared with percutaneous transluminal angioplasty (PTA) or surgery. We therefore reviewed studies originally included in this review comparing exercise versus PTA or surgery, and excluded them when appropriate.
Types of outcome measures
We included only studies that reported one or more of the following outcome measures: treadmill walking distance (time to onset of pain or pain‐free walking distance and maximum walking time or maximum walking distance), ABI, quality of life, morbidity, or amputation.
Primary outcomes
Treadmill walking distance (time to onset of pain or pain‐free walking distance and maximum walking time or maximum walking distance)
Secondary outcomes
ABI
Mortality
Amputation
Quality of life (includes QoL measured by the Short Form (SF)‐36 Questionnaire and other validated measurements such as the EuroQoL Group Quality of Life Questionnaire based on five dimensions (EQ‐5D), the Vascular Quality of Life Questionnaire (VascuQol), and the Intermittent Claudication Questionnaire)
Peak exercise blood flow
Cardiovascular events
Search methods for identification of studies
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
Cochrane Vascular Specialised Register (15 November 2016).
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 10) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The CIS maintains the Cochrane Vascular Specialised Register, which is constructed from weekly electronic searches of MEDLINE Ovid, Embase, Ovid, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Allied and Complementary Medicine Database (AMED), and through handsearching of relevant journals. The full list of databases, journals, and conference proceedings searched, as well as the search strategies used, is presented in the Specialised Register section of the Cochrane Vascular Module in the Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial registries for details of ongoing and unpublished studies.
ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
ISRCTN Register (www.isrctn.com/).
See Appendix 2.
Searching other resources
We checked the reference lists of relevant studies retrieved via electronic searches.
Data collection and analysis
Selection of studies
For this update, one of the review authors (RAL) independently identified relevant trials and determined their eligibility for inclusion in the review; another review author (AH) checked this work. We resolved disagreements by discussion or by consultation with a third review author (GL); however this was not required. As necessary, we sought additional information from authors of all trials that appeared to meet the inclusion criteria.
Data extraction and management
Two review authors (RAL and AH) independently extracted data. Review authors resolved disagreements by discussion and included the final results in the review.
Assessment of risk of bias in included studies
For this update (2017), two review authors (RAL and AH) independently performed risk of bias assessments. We discussed discrepancies, and we planned that if we could not reach agreement, we would ask a third review author (GL) to assess the trial. Risk of bias assessment comprises seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and the additional option of assessing any other aspects deemed to produce bias.
Measures of treatment effect
When appropriate, we pooled trial results in a statistical meta‐analysis using guidelines published by Cochrane Vascular. We analysed continuous data by determining mean differences (MDs) and 95% confidence intervals (CIs) using a fixed‐effect model. When significant heterogeneity was present, we used a random‐effects model. We analysed dichotomous data by determining risk ratios (RRs) and 95% CIs using a fixed‐effect model, unless we suspected heterogeneity. In addition, for the primary outcome measure treadmill walking distance (time to onset of pain or pain‐free walking distance and maximum walking time or maximum walking distance), we also analysed percentage change by using the 95% CI.
Unit of analysis issues
We analysed all data by using means and standard deviations. When original study papers did not provide these, we calculated them using the method recommended by the statistician for Cochrane Vascular. The unit of analysis was the individual participant.
Dealing with missing data
When data were not available from the original study paper, we contacted study authors to request the relevant data. If these were not available, we excluded the paper from the review. If data for at least one predefined outcome of the review were available, we included the study and examined available data in the meta‐analysis.
Assessment of heterogeneity
We subjectively tested heterogeneity between trial results by using clinical judgement of differences in patient populations, interventions (including type, duration, and intensity of exercise programmes), and outcome assessments. We assessed heterogeneity statistically by using the Chi2 test and the I2 statistic. We deemed heterogeneity as significant if the P value of the Chi2 test was less than 0.01, or if I2 was greater than 70%. An I2 of 50% to 70% equated to moderate heterogeneity.
Assessment of reporting biases
When we identified sufficient studies (> 10), we assessed publication bias using a funnel plot. For meta‐analyses, when the number of studies was less than 10, we did not use funnel plots, as this would have led to reduced power and inability to differentiate artefactual from true asymmetry.
Data synthesis
For this update, two review authors (RAL and AH) independently collected and pooled data, provided agreement was met. We then uploaded data into Review Manager 5 (RevMan 2014) software for analysis. We performed meta‐analysis by using a fixed‐effect model unless we detected heterogeneity (Chi2 test P < 0.01); we used a random‐effects model in the analysis if heterogeneity was present.
Subgroup analysis and investigation of heterogeneity
Owing to the numerous domains associated with assessment of quality of life as per the Medical Outcomes Study (MOS) Short Form (SF)‐36, we analysed all domains by performing subgroup analysis. When possible, we also analysed and presented data by subgroups for usual care and placebo.
Sensitivity analysis
Exercise programmes consisted of 12‐week interventions or 24‐week interventions. In one case, the duration of the programme was two years. In view of the variable length of programmes, we separated and analysed data as two separate analyses ‐ one for 12 weeks and one for 24 weeks.
'Summary of findings' table
For this update we included a table summarising the findings presented by this review for our main comparison of exercise versus no exercise in adults with symptomatic intermittent claudication. The study population continues to be at low risk with regards to their claudication; however, this is only one aspect of a progressive debilitating disease that can lead to limb loss and is accompanied by the coexistent risk of cardiovascular disease. We selected for inclusion in Table 1 the most important and clinically relevant outcomes thought to be essential for decision‐making. We described these under Types of outcome measures; they include pain‐free walking distance, maximum walking distance, ABI, mortality, amputation, and quality of life. We calculated assumed control intervention risks by using the mean number of events in control groups of selected studies for each outcome. We used the system developed by the GRADE Working Group in grading the quality of evidence as high, moderate, low, or very low, on the basis of within‐study risk of bias, directness of evidence, heterogeneity, precision of effects estimates, and risk of publication bias (GRADE 2004). We used GRADEpro GDT (GRADEpro GDT 2015) software to create Table 1.
Results
Description of studies
Results of the search
See Figure 1.
1.
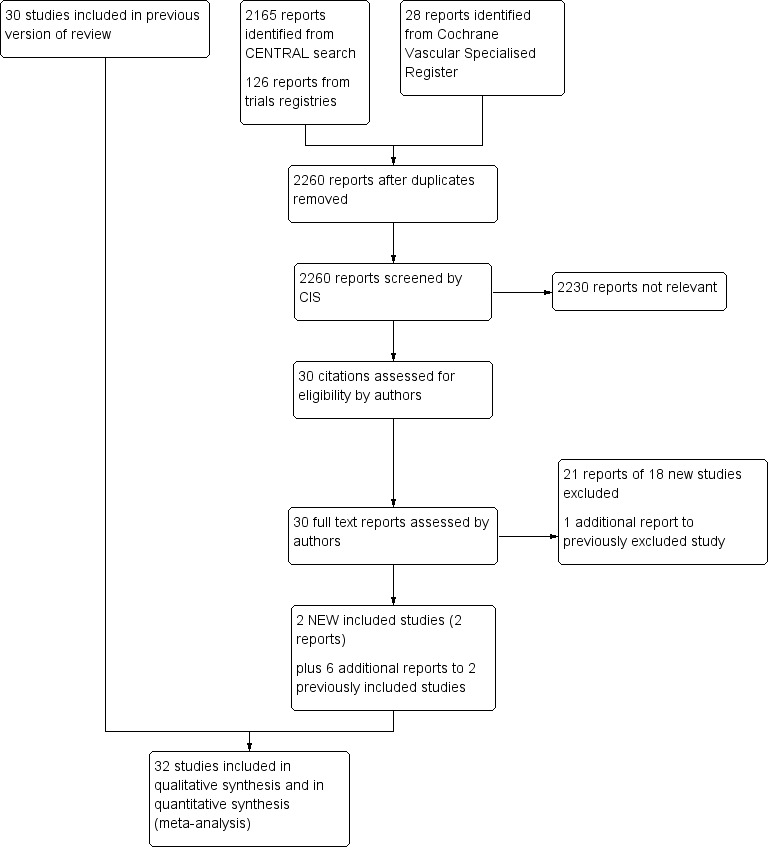
Study flow diagram.
Included studies
See Characteristics of included studies.
We included two additional trials for this update (McGuigan 2001; Tew 2015). We also included five publications related to the previously included GOALS 2013 trial (McDermott 2013; McDermott 2013a; McDermott 2014; McDermott 2014a; Rejeski 2014). However only one of these publications provided data (on mortality at six months) that could be included within the results section (McDermott 2014a). This brings the total number of included studies to 32 (Arosio 2001; Castro‐Sanchez 2013; Ciuffetti 1994; Collins 2005; Crowther 2012; Cucato 2013; Dahllof 1974; Gardner 2002; Gelin 2001; GOALS 2013; Guidon 2010; Hiatt 1990; Hiatt 1994; Hobbs 2005; Jansen 1991; Kakkos 2005; Larsen 1966; Leicht 2011; Mannarino 1991; McDermott 2008; McGuigan 2001; Mika 2005; Mika 2006; Mika 2011; Sanderson 2006; Schlager 2011; Tew 2009; Tew 2015; Tisi 1997; Tsai 2002; Wood 2006; Zwierska 2005) and the total number of participants to 1835.
Eighteen trials included fewer than 50 participants (Arosio 2001; Ciuffetti 1994; Crowther 2012; Cucato 2013; Dahllof 1974; Guidon 2010; Hiatt 1990; Hiatt 1994; Hobbs 2005; Jansen 1991; Kakkos 2005; Larsen 1966; Leicht 2011; Mannarino 1991; McGuigan 2001; Sanderson 2006; Schlager 2011; Wood 2006), ten between 50 and 100 participants (Castro‐Sanchez 2013; Collins 2005; Gardner 2002; Mika 2005; Mika 2006; Mika 2011; Tew 2009; Tew 2015; Tisi 1997; Tsai 2002), three over 100 participants (GOALS 2013; McDermott 2008; Zwierska 2005), and one over 200 participants (Gelin 2001).
Researchers compared exercise versus six different modes of treatment, the most common being usual care or placebo. Two early trials compared exercise versus placebo tablets (Dahllof 1974; Larsen 1966), but in more recent studies, trial authors used usual care as the control comparator (Collins 2005; Crowther 2012; Gardner 2002; Gelin 2001; GOALS 2013; Guidon 2010; Hiatt 1990; Hiatt 1994; Jansen 1991; Leicht 2011; McDermott 2008; McGuigan 2001; Mika 2005; Mika 2006; Mika 2011; Sanderson 2006; Schlager 2011; Tew 2009; Tew 2015; Tisi 1997; Tsai 2002; Wood 2006; Zwierska 2005). Investigators compared exercise with the following drug therapies: antiplatelet agents (Mannarino 1991), pentoxifylline (Ciuffetti 1994), iloprost (Arosio 2001), and vitamin E (Collins 2005). One study compared exercise versus pneumatic foot and calf compression (Kakkos 2005); one used a placebo treatment protocol of disconnected ultrasound electrotherapy (Castro‐Sanchez 2013); and one used a 'stretching class' as usual care (Cucato 2013).
Inclusion and exclusion criteria of the included studies varied widely, but usually excluded people with serious comorbidities that would compromise an exercise programme, or would make it impractical.
We noted some variation in the exercise regimens used, although all recommended at least two sessions weekly. All specified some element of supervision, except the earliest trial (Larsen 1966), in which participants were simply advised to exercise at home and were given some training or walking regimen. Types of exercise varied from strength training to polestriding, cycling, and upper or lower limb exercises. We did not identify studies that included skipping or running. Duration of treatment generally fell within 3 to 12 months. Trialists measured outcomes at times ranging from 14 days to 2 years and reported variable compliance with exercise.
Nearly all trials used a treadmill walking test to assess one of the outcome measures, but results show considerable variation in outcomes. Some reported walking distance, and others reported walking times. Trial authors reported calf blood flow and ABI and often provided haematological and biochemical measures. Trialists provided little information about mortality, amputations, and fatal or non‐fatal cardiovascular events. Eleven studies reported quality of life measures (Collins 2005; Gardner 2002; Gelin 2001; GOALS 2013; Guidon 2010; Kakkos 2005; McDermott 2008; Tew 2015; Tisi 1997; Tsai 2002; Zwierska 2005). As trials increasingly used the Short Form (SF)‐36 Questionnaire, we have combined these results for meta‐analysis in this review. We did not include in the meta‐analysis trials that used an alternative quality of life assessment; we provided individual study findings in Table 2.
1. Functional status and quality of life data (QoL) for all studies.
| Study | Measure reported | Effect reported |
| Collins 2005 | Walking Impairment Questionnaire (WIQ) ‐ perceived distance and walking speed | On the basis of analysis of change scores, the polestriding group reported significantly greater perceived ability to walk distance than the control group at 4 (P = 0.05), 12 (P = 0.001), and 24 (P = 0.002) weeks. Moreover, the polestriding group rated their perceived ability to walk faster to be significantly greater than the control group at 4 (P = 0.03), 12 (P = 0.19), and 24 (P = 0.02) weeks. The groups’ ratings were equivalent at baseline (P > 0.05). In the polestriding group, polestriding aggregate scores for both distance and speed improved significantly between baseline and 12 weeks (P < 0.0001) and baseline and 24 weeks (P < 0.0001), but not between 12 and 24 weeks (P > 0.015). The perception of walking speed and distance did not improve in the control group. |
| Rand Short Form‐36 (SF‐36) ‐ perceived physical function | Exercise significantly improved the Physical Component Summary score (PSS) of SF‐36 when compared with usual care. Difference using the change score between polestriding and control groups for the PSS was significant; P = 0.03. There was no difference in the change on the Mental Component Summary score between groups. | |
| Gardner 2002 | Health‐related quality of life (QoL) assessed with the Medical Outcomes Study Short Form‐36 (MOS SF‐36) | Baseline measures of health‐related QoL were comparable in both groups. The physical and mental health composite scores of the MOS SF‐36 were similar between the 2 groups and did not change during the study. Consequently, no analyses were performed on the individual subscales. |
| WIQ | No change in the WIQ was identified. Baseline scores on the 3 WIQ subscales ranged between 32% and 52%. Although the exercise group increased by 22% and 34% on the distance and speed subscales, respectively, these changes were not significant and did not differ from the changes in controls. | |
| Gelin 2001 | Intermittent Claudication‐specific Sickness Impact Profile (SIP) scale, SIPIC | Supervised physical training produced significant improvements from baseline in only 2 health‐related quality of life (HRQoL) domains (SIP Recreation and pastimes (P < 0.05) and the single‐item rating scale Physical Condition) and an ambiguous pattern of positive and negative trends in others. Unexpectedly, deterioration was most striking in functional health, where reductions appeared in 10 of 15 SIP categories. Compared with no treatment, physical training produced significantly greater improvement in only 1 HRQoL category: SIP recreation and pastimes. Improvement in this category, however, may possibly be accounted for by the opportunities for increased leisure activity afforded by participation in the ongoing training programme. |
| Health‐related QoL, QoL overall | No significant improvement was observed between training and control groups. Training produced small SRMs (mean change between assessments divided by the standard deviation of change) (0.2 to < 0.5) on 4 HRQoL dimensions, of which 2 represented improvement. | |
| Guidon 2010 | Medical Outcomes Study Short Form‐36 | No significant differences between groups were demonstrated for any of the MOS SF‐36 scores over the 3 time points (baseline, 12 weeks, and 1 year). This study was not included in the meta‐analysis, as the MOS SF‐36 differs from the standard SF‐36 Questionnaire. |
| Disease‐specific QoL (ICQ) | Results show a statistically significant decrease (P = 0.003) in ICQ scores from baseline to 12‐week follow‐up (mean difference ‐9.74, 95% CI ‐3.76 to ‐15.71) in the exercise group, indicating improved quality of life. No significant difference was demonstrated in the control group. | |
| WIQ | In the exercise group, increases were observed in all WIQ scores, with a statistically significant increase (P = 0.015) in the WIQ Distance score (mean difference 14.28, 95% CI 2.96 to 25.61). In the control group, scores for the WIQ Stair‐climbing and Distance categories decreased, with a marginal increase in the WIQ Speed score. None of these changes were significant. | |
| Kakkos 2005 | Short Form‐36 (SF‐36) | Score for the general health domain of the SF‐36 was significantly improved at 1 year in individuals who used intermittent pneumatic compression (IPC). This study was not included in the meta‐analysis, as the full dataset was not available in the study and could not be obtained from the study author. |
| WIQ | IPC improved speed score of WIQ significantly. WIQ scores for walking distance, walking speed, and stair climbing were reduced in the unsupervised exercise group, remained stable in the supervised exercise group, and were increased in the IPC group. | |
| Intermittent Claudication Questionnaire (ICQ) | Supervised exercise and IPC reduced (improved) the ICQ score, but this was significant only in the IPC group. | |
| McDermott 2008 | WIQ | Distance score improved (P = 0.02) in the treadmill group when compared to the control group. This was not apparent in the other 2 domains (speed and stair climbing). |
| SF‐36 physical functioning score | Improved (P = 0.04) in the treadmill group when compared to the control group. | |
| GOALS 2013 | WIQ scores | Participants in the intervention group, when compared with those in the control group, improved their WIQ distance score (35.3 to 47.4 vs 33.3 to 34.4; mean difference 11.1, 95% CI 3.9 to 18.1; P = 0.003) and their WIQ speed score (36.1 to 47.7 vs 35.3 to 36.6; mean difference 10.4, 95% CI 3.4 to 17.4; P = 0.004) but not their WIQ stair‐climbing score (48.9 to 57.3 vs 47.9 to 48.5; mean difference 7.9, 95% CI 0.00 to 15.8; P = 0.05). |
| Physical Health Composite Score (PCS) and Mental Health Composite Score (MCS) scales from the 12‐item Medical Outcomes Study Short Form Health Survey (SF‐12) | Results show no between‐group differences in change in the SF‐12 PCS or MCS subscales. | |
| Tew 2015 | Intermittent Claudication Questionnaire (ICD) | The intervention group demonstrated improvement in the ICD score of ‐10.6 (95% CI ‐18.9 to ‐ 2.3). |
| Tisi 1997 | Nottingham Health Profile (NHP) | A daily home exercise programme, supervised weekly by a physiotherapist for the first month, achieves good compliance, increased walking distances, and improved quality of life as assessed by the Nottingham Health Profile Questionnaire. |
| Tsai 2002 | WIQ | Improved speed (P < 0.001) and stairs (P < 0.001) in the exercise group when compared to the control group at 12 weeks. No significant difference in the distance domain |
| SF‐36 ‐ version 1 | Perception of QoL increased significantly in the exercise group compared to usual care for the domains of physical function, role limitation, bodily pain, general health, and vitality. This study was included in the 3‐month meta‐analysis; however the SF‐36 version 1 differs from the standard SF‐36 Questionnaire. | |
| Zwierska 2005 | WIQ | Improvement in all 3 domains was seen in the upper limb group at 24 and 48 weeks when compared to the control group. The lower limb group improved in stair and speed domains at 24 and 48 weeks only when compared to the control group. |
| SF‐36 ‐ version 2 | At 6 weeks, improvement in general health was seen in the lower‐limb group when compared to the control group. At 24 weeks, a significant improvement was seen in general health and vitality in the lower limb group when compared to the control group. The upper limb group significantly improved in physical function and mental health when compared to the control group. |
We sought additional information from trialists in most of the included studies for the updated version of this review.
Excluded studies
See Characteristics of excluded studies.
For this update (2017), we excluded 18 additional studies (Aruna 2015; Cucato 2015; Dantas 2016; Gardner 2014; Gardner 2014a; Gibbs 2013; Guidon 2013; Guirro 2015; Kono 2013; LIFE Study; Mays 2015; NCT02075502; NCT02879019; PROPEL study; Rodrigues 2014; Schlager 2011a; Sonaglia 2013; Ventura 1984). We added one publication to a previously excluded study (EXITPAD 2010).
We excluded a total of 111 studies from the current review.
We excluded studies from this review because they compared percutaneous transluminal angioplasty versus exercise (CLEVER 2009; Creasy 1990; Greenhalgh 2008; Hobbs 2006; Kruidenier 2011; Mazari 2010; Spronk 2009; SUPER study), surgery versus exercise (Lundgren 1989), different exercise regimens (Allen 2010; Andreozzi 2008; Beutel 1985; Buchwalsky 1974; Cachovan 1999; Cheetham 2004; Choi 2012; Collins 2012; Cucato 2011; Cucato 2011a; Cucato 2015; Dedes 2010; Degischer 2002; Fakhry 2011; Gardner 2005; Gardner 2012; Gardner 2014; Gardner 2014a; Gottstein 1987; Jones 1996; Kiesewetter 1987; Labs 1999; Martinez 2009; Nawaz 1999; Nawaz 2001; NCT01241747; NCT02879019; Nicolai 2010; Nielsen 1977; Parr 2009; Patterson 1997; Pinto 1997; Riebe 2001; Ritti‐Dias 2010; Rodrigues 2014; Saleem 2011; Savage 2001; Scheffler 1991; Slordahl 2005; Sonaglia 2013; Thomson 1999; Zwierska 2004), and exercise versus walking advice as the form of conservative best medical treatment (Bronas 2011; Crowther 2008; EXERT 2009; EXITPAD 2010; Gardner 2011; Gardner 2012; Hodges 2008; Mays 2015; Nawaz 1999; NCT02075502; Nordanstig 2011; Parr 2009; Stewart 2008; Wang 2008). We excluded nine studies because relevant suitable numerical data were not available despite attempts to contact study authors (Collins 2010; Fowler 2002; Gibbellini 2000; Holm 1973; Maejima 2005; Schlager 2011a; Streminski 1992; Tebbutt 2011; Ventura 1984). We excluded the remainder of excluded studies because they did not fit the inclusion criteria (e.g. participants did not have intermittent claudication, not an RCT, insufficient evidence of randomly allocated population, no non‐exercise control group) (Aruna 2015; Brotons 2011; Bulling 1991; Carmeli 2004; Cina 1996; Cunningham 2012; Dahllof 1976; Dantas 2016; Dittmar 1977; Ericsson 1970; Ernst 1987; Ernst 1990; Fitzgerald 1971; Gibbs 2013; Guidon 2013; Guirro 2015; Kono 2013; Krause 1976; Lee 2007; Leon 2005; Lepantalo 1984; LIFE Study; Mannarino 1988; Mannarino 1989; McDermott 2004; NCT01065740; Necker 2003; Presern‐Strukelj 200; PROPEL study; Riccioni 2010; Richardson 1991; Schoneberger 1994; Silvestro 2002; Snabl 1958; Taft 2004; Treat‐Jacobson 2012; Walker 2000; Waller 1988; Wang 2008; Winterfeld 1983).
Ongoing studies
We included within the ongoing studies section two studies that are still awaiting data: NCT01231360 (completed 2014) and NCT01822457 (completed August 2016). See Characteristics of ongoing studies.
Risk of bias in included studies
Figure 2 and Figure 3 provide an overall summary of bias present within each of the included studies (see also Characteristics of included studies). The high level of unclear bias was due to unclear reporting about sequence generation and allocation. We have expanded upon these aspects below.
2.
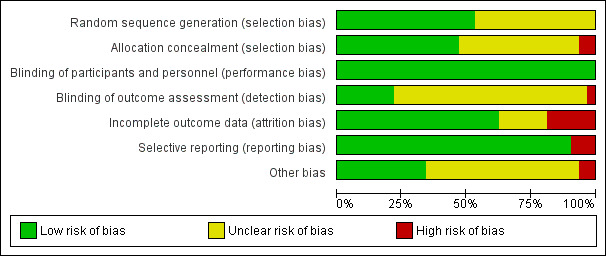
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
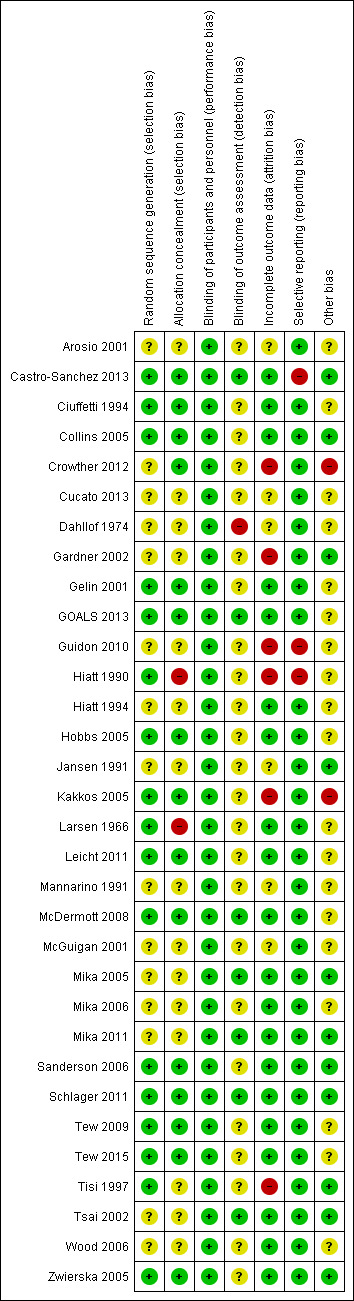
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were RCTs, but many reports provided no details on random sequence generation other than a statement of 'randomised' (Arosio 2001; Crowther 2012; Cucato 2013; Dahllof 1974; Gardner 2002; Guidon 2010; Hiatt 1994; Jansen 1991; Mannarino 1991; McGuigan 2001; Mika 2005; Mika 2006; Mika 2011; Tsai 2002; Wood 2006). See Figure 3. To ensure that all trials were dealt with fairly, we deemed that any described as 'randomised' with no explanation as to how this was done had unclear risk of bias. We deemed that the remaining studies were at low risk of bias for random sequence generation.
A total of 15 studies did not report details of allocation concealment (Arosio 2001; Cucato 2013; Dahllof 1974; Gardner 2002; Guidon 2010; Hiatt 1994; Jansen 1991; Mannarino 1991; McGuigan 2001; Mika 2005; Mika 2006; Mika 2011; Tisi 1997; Tsai 2002; Wood 2006); we deemed these studies to be at unclear risk of selection bias. We deemed two studies to be at high risk of bias because the randomisation procedure was not blinded (Hiatt 1990; Larsen 1966). The remaining studies provided details of allocation concealment, and we judged them to be at low risk of bias.
Blinding
As the nature of exercise‐based studies involved an activity versus standard care, medication, or an intervention, review authors deemed that blinding of participants was not possible. To ensure that all trials used a standardised approach, we scored all as having low risk of bias secondary to participant blinding. Inevitably in trials of exercise, blinding was not possible; therefore significant placebo responses may have occurred in trials comparing exercise versus usual care.
Blinding of participants was not possible, and included trials have additional risk of bias as outcome assessors may not be blinded to the group to which a participant was randomised. Seven studies did specify that outcome assessors were blinded; we judged these to be at low risk of bias (Castro‐Sanchez 2013; GOALS 2013; McDermott 2008; Mika 2005; Mika 2011; Schlager 2011; Tsai 2002). For some, however, blinding involved a separate aspect of the trial that was not focused on exercise, that is, vitamin E in Collins 2005, or carnitine analysis in Hiatt 1990; we judged these studies as having unclear risk. We deemed Dahllof 1974 to be at high risk of bias because the outcome assessor was not blinded to treatment groups. We judged the remainder of the included studies to be at unclear risk of bias owing to lack of reporting on blinding of outcome assessors.
Incomplete outcome data
Most trials reported no or minimal losses to follow‐up (Arosio 2001; Castro‐Sanchez 2013; Ciuffetti 1994; Collins 2005; Cucato 2013; Dahllof 1974; Gelin 2001; GOALS 2013; Hiatt 1994; Hobbs 2005; Jansen 1991; Larsen 1966; Leicht 2011; Mannarino 1991; McDermott 2008; Mika 2005; Mika 2006; Mika 2011; Sanderson 2006; Schlager 2011; Tew 2009; Tew 2015; Tsai 2002; Wood 2006; Zwierska 2005). We judged studies that had an attrition rate of 20% or more to be associated with higher risk of bias (Crowther 2012; Gardner 2002; Guidon 2010; Hiatt 1990; Kakkos 2005; McGuigan 2001; Tisi 1997).
We judged five studies to be at unclear risk of bias (Arosio 2001; Cucato 2013; Dahllof 1974; Jansen 1991; Mannarino 1991); Arosio 2001, Dahllof 1974, Jansen 1991, and Mannarino 1991 because investigators did not mention whether all enrolled participants completed the studies, and Cucato 2013 because only participants from the exercise group were lost to follow‐up.
Selective reporting
All studies reported their prespecified outcome measures and were at low risk of reporting bias. Guidon 2010 discussed results of the Walking Impairment Questionnaire (WIQ) and the Intermittent Claudication Questionnaire (ICQ) for 30 of 44 randomised participants; therefore, we judged this study to be at high risk of selective reporting bias. Castro‐Sanchez 2013 did not report on intermittent claudication distance; we therefore classified it as having high risk. Hiatt 1990 did not specifically report on maximum walking distance or intermittent claudication in the treatment group but correlated it with treadmill performance; therefore, we classified this study as having high risk of reporting bias.
Other potential sources of bias
We initially labelled studies as having high risk of bias when they failed to meet sample size calculations (Kakkos 2005; McDermott 2008; McGuigan 2001). Kakkos 2005 reported an attrition rate of 26% (8 of 34 discontinued); we therefore kept it at high risk. However, we reclassified McDermott 2008 to unclear risk of bias, as the attrition rate was low; according to power calculations, 50 were needed in each group, and 50 completed the supervised exercise therapy (SET) whilst 48 in the control group completed the study. We also reclassified McGuigan 2001 to unclear risk of bias as the reported attrition rate was low.
Reporting of treatment group numbers varied in the results section of Crowther 2012; effects on outcomes were unclear. We therefore judged the study to be at high risk of bias.
Eleven studies were at low risk of bias (Castro‐Sanchez 2013; Collins 2005; Gardner 2002; Jansen 1991; Mika 2005; Mika 2011; Sanderson 2006; Schlager 2011; Tisi 1997; Tsai 2002; Zwierska 2005).
Eleven studies included small sample sizes; we therefore deemed these studies to be at unclear risk of bias (Arosio 2001; Ciuffetti 1994; Cucato 2013; Dahllof 1974; Hiatt 1990; Hiatt 1994; Hobbs 2005; Larsen 1966; Leicht 2011; Mannarino 1991; Wood 2006). We deemed the remaining six studies to be at unclear risk of bias for a variety of other reasons (Gelin 2001; GOALS 2013; Guidon 2010; Mika 2006; Tew 2009; Tew 2015). Please see the risk of bias tables for additional details.
Effects of interventions
See: Table 1
Exercise regimen compared with placebo or usual care
Overall outcomes
The wide range of reported time points meant that the overall analysis includes the last data time point at which data were presented in the study publications. This section shows a high degree of heterogeneity, with low heterogeneity noted in Analysis 1.2 and Analysis 1.8.
1.2. Analysis.
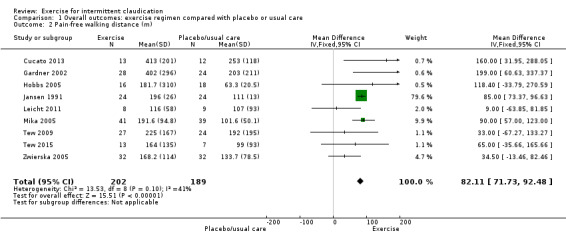
Comparison 1 Overall outcomes: exercise regimen compared with placebo or usual care, Outcome 2 Pain‐free walking distance (m).
1.8. Analysis.
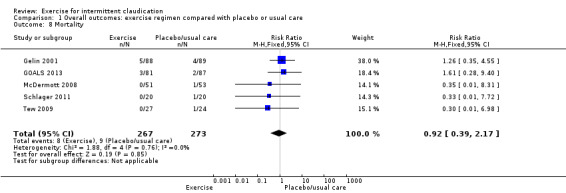
Comparison 1 Overall outcomes: exercise regimen compared with placebo or usual care, Outcome 8 Mortality.
Maximum walking distance
(Analysis 1.1) Ten trials with 500 participants reported this outcome and showed significant statistical heterogeneity (I2 = 89%, P < 0.00001) therefore we used a random‐effects model (Cucato 2013; Gardner 2002; Gelin 2001; Hobbs 2005; Jansen 1991; Leicht 2011; Schlager 2011; Tew 2009; Tew 2015; Zwierska 2005). The exercise group showed overall improvement in maximum walking distance (MD 120.36 metres, 95% CI 50.79 to 189.92, P < 0.00007, high quality of evidence as assessed via GRADE).
1.1. Analysis.
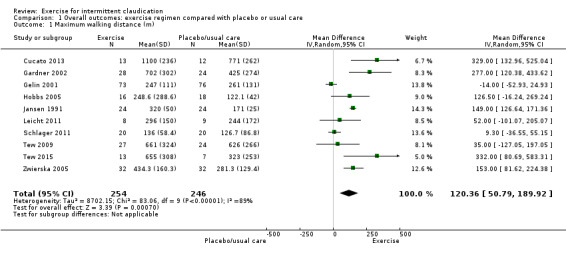
Comparison 1 Overall outcomes: exercise regimen compared with placebo or usual care, Outcome 1 Maximum walking distance (m).
Pain‐free walking distance
Nine trials with a total of 391 participants reported on this outcome (Cucato 2013; Gardner 2002; Hobbs 2005; Jansen 1991; Leicht 2011; Mika 2005; Tew 2009; Tew 2015; Zwierska 2005) and noted improvement in pain‐free walking distance in the exercise group (MD 82.11 metres, 95% CI 71.73 to 92.48, P < 0.00001, low quality of evidence as assessed via GRADE). Trials showed no significant heterogeneity (I2 = 41%, P = 0.1) therefore a fixed‐effect model was used.
Maximum walking time
1.3. Analysis.

Comparison 1 Overall outcomes: exercise regimen compared with placebo or usual care, Outcome 3 Maximum walking time (min).
Twelve studies with a total of 577 participants reported on maximum walking time (Collins 2005; Crowther 2012; GOALS 2013; Hiatt 1990; Hiatt 1994; Larsen 1966; McDermott 2008; Mika 2006; Mika 2011; Sanderson 2006; Tsai 2002; Wood 2006). Data show overall improvement in walking time for those who underwent exercise (MD 4.51 minutes, 95% CI 3.11 to 5.92, P < 0.00001, high‐quality evidence). Heterogeneity between studies was found to be significant (I2 = 82%, P < 0.00001) therefore a random‐effects model was used. Walking time improved in eight trials (Crowther 2012; Hiatt 1990; Hiatt 1994; Larsen 1966; McDermott 2008; Mika 2006; Mika 2011; Tsai 2002), which demonstrated exercise to be effective.
Pain‐free walking time
1.4. Analysis.
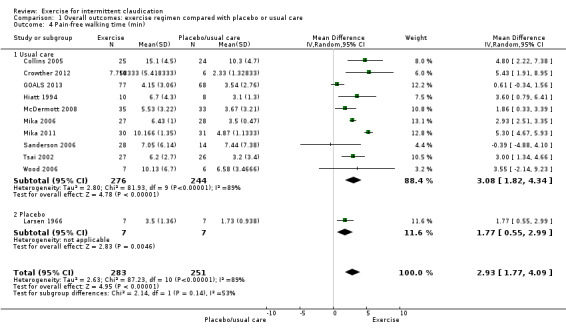
Comparison 1 Overall outcomes: exercise regimen compared with placebo or usual care, Outcome 4 Pain‐free walking time (min).
Eleven studies with a total of 534 participants reported on pain‐free walking time (Collins 2005; Crowther 2012; GOALS 2013; Hiatt 1994; Larsen 1966; McDermott 2008; Mika 2006; Mika 2011; Sanderson 2006; Tsai 2002; Wood 2006). Meta‐analysis demonstrated improvement in pain‐free walking time for the exercise groups (MD 2.93 minutes, 95% CI 1.77 to 4.09, P < 0.0001, high‐quality evidence). Heterogeneity between studies was found to be significant (I2 = 89%, P < 0.00001) therefore a random‐effects model was used. Heterogeneity was most likely secondary to differences in reporting times and variable length of exercise programmes. Data show 100% improvement in pain‐free walking time, which was likely to be of clinical significance. Only two of the studies found no real improvement with exercise (Sanderson 2006; Wood 2006).
Percentage change in maximum walking distance or time
1.5. Analysis.
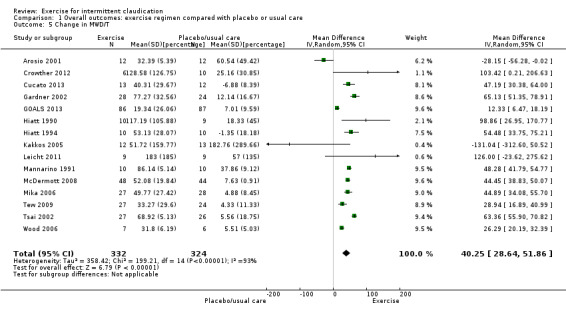
Comparison 1 Overall outcomes: exercise regimen compared with placebo or usual care, Outcome 5 Change in MWD/T.
We calculated the percentage change in maximum walking distance or time for 15 studies with a total of 656 participants (Arosio 2001; Crowther 2012; Cucato 2013; Gardner 2002; GOALS 2013; Hiatt 1990; Hiatt 1994; Kakkos 2005; Leicht 2011; Mannarino 1991; McDermott 2008; Mika 2006; Tisi 1997; Tsai 2002; Wood 2006). Meta‐analysis reported overall improvement in maximum walking distance or time for those who underwent exercise (MD 40.25%, 95% CI 28.64 to 51.86, P < 0.00001). Heterogeneity between these studies was found to be significant (P < 0.00001, I2 = 93%) therefore a random‐effects model was used.
Percentage change in pain‐free walking distance (intermittent claudication distance (ICD)) or time (ICT)
1.6. Analysis.
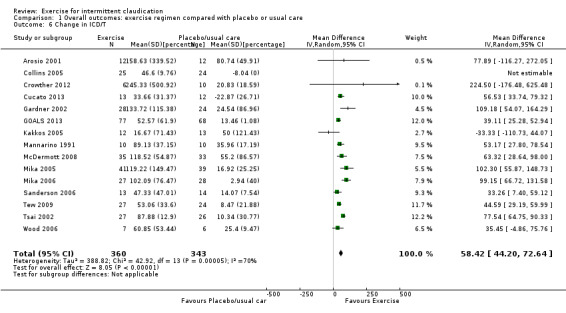
Comparison 1 Overall outcomes: exercise regimen compared with placebo or usual care, Outcome 6 Change in ICD/T.
We calculated the percentage change in ICD or ICT for 15 studies with 703 participants (Arosio 2001; Collins 2005; Crowther 2012; Cucato 2013; Gardner 2002; GOALS 2013; Kakkos 2005; Mannarino 1991; McDermott 2008; Mika 2005; Mika 2006; Sanderson 2006; Tew 2009; Tsai 2002; Wood 2006). Results showed overall improvement in percentage change for ICD or ICT in favour of exercise (MD 58.42%, 95% CI 44.20 to 72.64, P < 0.00001). Heterogeneity between studies was found to be significant (P < 0.001, I2 = 70%) when a random‐effects model was used.
Ankle brachial index (ABI)
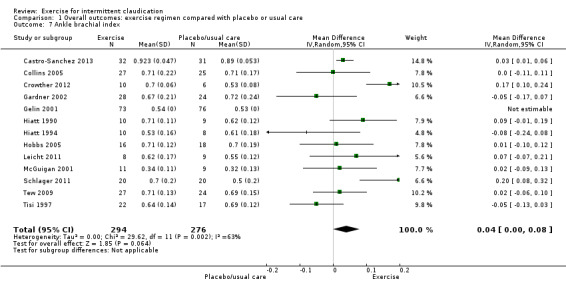
1.7. Analysis.
Comparison 1 Overall outcomes: exercise regimen compared with placebo or usual care, Outcome 7 Ankle brachial index.
Thirteen trials with a total of 570 participants reported this outcome (Castro‐Sanchez 2013; Collins 2005; Crowther 2012; Gardner 2002; Gelin 2001; Hiatt 1990; Hiatt 1994; Hobbs 2005; Leicht 2011; McGuigan 2001; Schlager 2011; Tew 2009; Tisi 1997). Meta‐analysis showed a small change in ABI (MD 0.04, 95% CI 0.00 to 0.08, P = 0.06, moderate‐quality evidence), which was supported by three studies (Castro‐Sanchez 2013; Crowther 2012; Schlager 2011). Studies showed significant statistical heterogeneity (P = 0.002, I2 = 63%) therefore a random‐effects model was used. Heterogeneity may be attributed to variations in exercise programme type or duration. Of note, as the number of trials included in updates has increased, the significance of change in ABI with exercise has diminished to show no improvement.
Mortality
Five studies with 540 participants reported on mortality (Gelin 2001; GOALS 2013; McDermott 2008; Schlager 2011; Tew 2009). Results show no differences in effect between groups (risk ratio (RR) 0.92, 95% CI 0.39 to 2.17, P = 0.85, moderate‐quality evidence). We updated these figures for this update, as recent published data included mortality figures for GOALS 2013. We noted no significant heterogeneity between trials (I 2 = 0%, P = 0.76).
Amputation
1.9. Analysis.
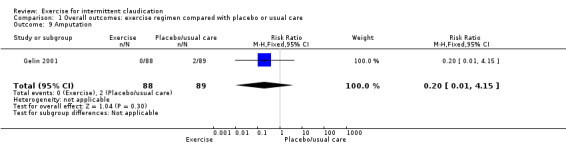
Comparison 1 Overall outcomes: exercise regimen compared with placebo or usual care, Outcome 9 Amputation.
Only Gelin 2001 reported on amputation. Two amputations occurred in the usual care group, and none in the exercise group (RR 0.20, 95% CI 0.01 to 4.15, P = 0.3, low‐quality evidence).
Quality of life
Overall, researchers reported quality of life (QoL) in numerous different ways (see Table 2). SF‐36 provided the only consistently reported outcome for QoL, but this was reported at three and six months, rather than at one year. We have reported below results at these time points.
Other QoL questionnaires included the ICD Questionnaire (Tew 2015; Guidon 2010; Kakkos 2005), the Nottingham Health Profile (NHP) (Tisi 1997), and the Intermittent Claudication‐Specific Sickness Impact Profile (SIP) scale (SIPIC) (Gelin 2001).
Tew 2015 used the ICD Questionnaire to assess changes between zero and six weeks. Data show no differences between intervention and control groups in EQ‐5D score at six weeks, but improvement in ICD score in the intervention group (ICD score ‐10.6, 95% CI ‐18.9 to ‐ 2.3, P < 0.05) (see Table 2).
Cardiovascular events
None of the included studies reported on non‐fatal cardiovascular events. We have examined all‐cause mortality in Analysis 1.8.
Peak exercise calf blood flow
1.10. Analysis.
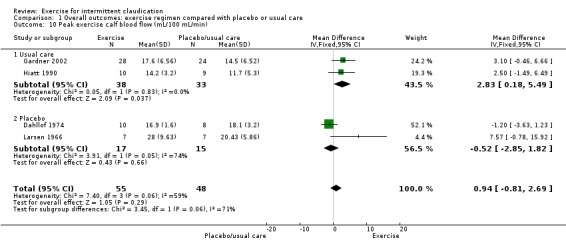
Comparison 1 Overall outcomes: exercise regimen compared with placebo or usual care, Outcome 10 Peak exercise calf blood flow (mL/100 mL/min).
We included in the meta‐analysis for this outcome four studies with a total of 103 participants (Dahllof 1974; Gardner 2002; Hiatt 1990; Larsen 1966). Results show no significant overall improvement in blood flow between groups (MD 0.94 mL/100 mL/min, 95% CI ‐0.81 to 2.69, P = 0.29, low‐quality evidence) (I2 = 59%, P = 0.06, moderate heterogeneity between trials). We downgraded the evidence owing to small sample size and wide confidence intervals.
Three‐monthly outcomes
Seven trials provided one or more outcomes at the three‐month time point for exercise compared with placebo or usual care (Collins 2005; Hiatt 1990; Hiatt 1994; Mika 2011; Schlager 2011; Tew 2009; Tsai 2002).
Maximum walking distance
2.1. Analysis.
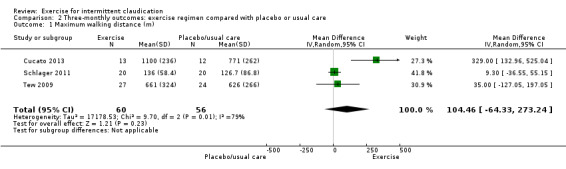
Comparison 2 Three‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 1 Maximum walking distance (m).
Three studies with 116 participants reported outcomes for maximum walking distance at the three‐month time point (Cucato 2013; Schlager 2011; Tew 2009). Results showed no clear differences in distance between groups (MD 104.46 metres, 95% CI ‐64.33 to 273.24, P = 0.23) along with significant heterogeneity (P = 0.008, I2 = 79%) between studies when a random‐effects model was used.
Pain‐free walking distance
2.2. Analysis.
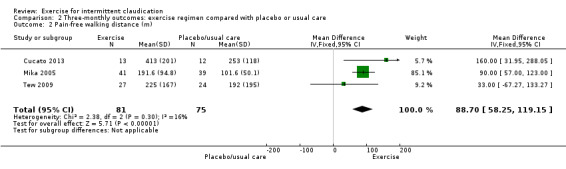
Comparison 2 Three‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 2 Pain‐free walking distance (m).
Three trials with 156 participants provided pain‐free walking distance outcomes at three months (Cucato 2013; Mika 2005; Tew 2009). Data showed significant improvement in the exercise group (MD 88.70 metres, 95% CI 58.25 to 119.15, P < 0.00001) (I2 = 16, P = 0.3, no significant heterogeneity).
Maximum walking time
2.3. Analysis.
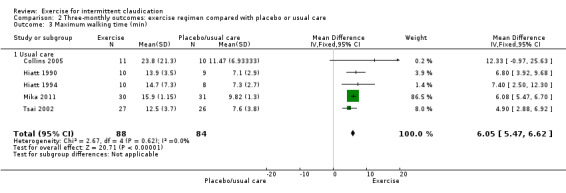
Comparison 2 Three‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 3 Maximum walking time (min).
Meta‐analysis of five trials with 172 participants reporting maximum walking time at three months (Collins 2005; Hiatt 1990; Hiatt 1994; Mika 2011; Tsai 2002) showed improvement with exercise (MD 6.05 minutes, 95% CI 5.47 to 6.62, P < 0.00001) and no significant heterogeneity (P = 0.62, I2 = 0%).
Pain‐free walking time
2.4. Analysis.
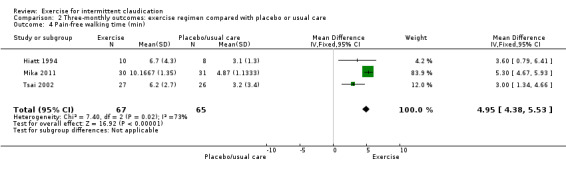
Comparison 2 Three‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 4 Pain‐free walking time (min).
Three trials with 132 participants provided data on pain‐free walking time at three months (Hiatt 1994; Mika 2011; Tsai 2002). Meta‐analysis demonstrated improvement in the exercise group (MD 4.95 minutes, 95% CI 4.38 to 5.53, P < 0.00001) and significant heterogeneity between trials (P = 0.02, I2 = 73%).
Ankle brachial index (ABI)
2.5. Analysis.
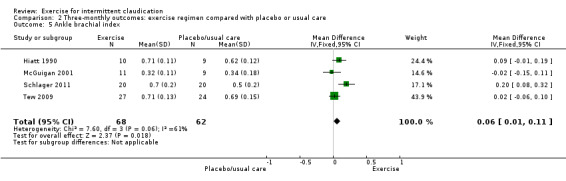
Comparison 2 Three‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 5 Ankle brachial index.
Four studies with a total of 130 participants reported on ABI outcomes at the three‐month time point (Hiatt 1990; McGuigan 2001; Schlager 2011; Tew 2009). In contrast to the overall analysis, data show small differences in ABI between groups (MD 0.06, 95% CI 0.01 to 0.11, P = 0.02) (P = 0.06, I2 = 61%, moderate heterogeneity).
Mortality
2.6. Analysis.
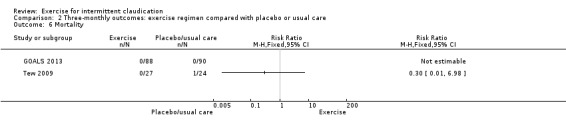
Comparison 2 Three‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 6 Mortality.
Tew 2009 and GOALS 2013 reported on this outcome for a total of 229 participants. No deaths occurred in either group in GOALS 2013, and Tew 2009 reported one death in the control group compared with none in the exercise group (RR 0.30, 95% CI 0.01 to 6.98).
Quality of life
2.7. Analysis.

Comparison 2 Three‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 7 Quality of Life SF‐36.
Tsai 2002 and Guidon 2010 reported a quality of life analysis for the SF‐36 at three months. Domains found to improve secondary to exercise included 'physical function' (MD 6.60, 95% CI 2.37 to 10.83), 'vitality' (MD 5.55, 95% CI 1.54 to 9.56), and 'role physical' (MD 10.31, 95% CI 3.64 to 16.98). A random‐effects model applied to assess for any variation revealed that the domain 'vitality' continued to show improvement secondary to exercise, but significance was lost for 'role physical' (MD 16.06, 95% CI ‐8.41 to 40.53) and 'physical function' (MD 5.95, 95% CI‐2.45 to 14.34).
Peak exercise calf blood flow
2.8. Analysis.
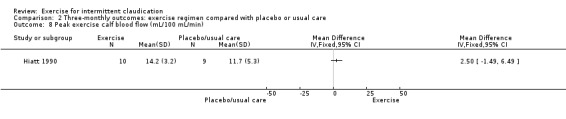
Comparison 2 Three‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 8 Peak exercise calf blood flow (mL/100 mL/min).
Only Hiatt 1990 reported on this outcome at three months and included 19 participants. Results show no clear differences between exercise and control groups at the end of the study (MD 2.50 mL/100 mL/min, 95% CI ‐1.49 to 6.49).
Six‐monthly outcomes
Maximum walking distance
3.1. Analysis.
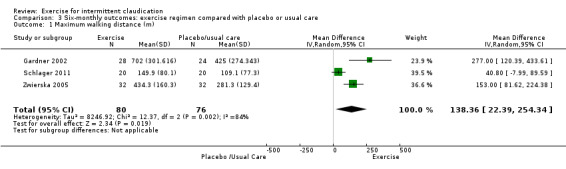
Comparison 3 Six‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 1 Maximum walking distance (m).
Three studies with a total of 156 participants reported maximum walking distance at six months (Gardner 2002; Schlager 2011; Zwierska 2005). Results showed an increase in the exercise group (MD 138.36 metres, 95% CI 22.39 to 254.34, P = 0.02) and significant heterogeneity between studies (P = 0.002, I2 = 84%) when a random‐effects model was used.
Pain‐free walking distance
3.2. Analysis.
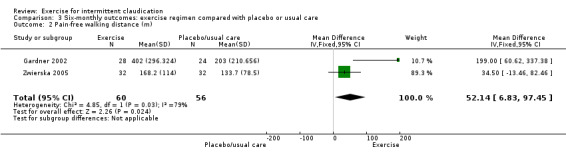
Comparison 3 Six‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 2 Pain‐free walking distance (m).
Two studies with 116 participants reported on pain‐free walking distance at six months (Gardner 2002; Zwierska 2005), noting improvement in the exercise group (MD 52.14 metres, 95% CI 6.83 to 97.45, P = 0.02) (I2= 79%, P = 0.03, high heterogeneity).
Maximum walking time
3.3. Analysis.
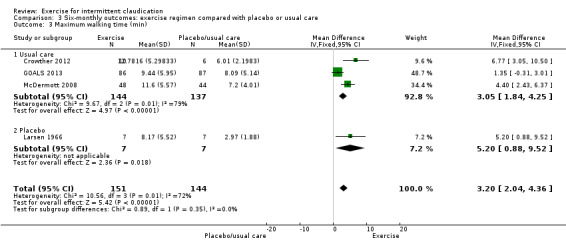
Comparison 3 Six‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 3 Maximum walking time (min).
Four studies with a total of 295 participants reported outcomes for maximum walking time at six months (Crowther 2012; GOALS 2013; Larsen 1966; McDermott 2008). Meta‐analysis showed improvement in favour of exercise (MD 3.20 minutes, 95% CI 2.04 to 4.36, P < 0.00001) and high heterogeneity (P = 0.01, I2 = 72%).
Pain‐free walking time
3.4. Analysis.
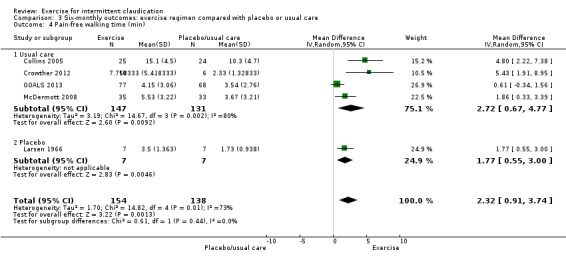
Comparison 3 Six‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 4 Pain‐free walking time (min).
Five trials with 292 participants provided pain‐free walking time outcomes at six months (Collins 2005; Crowther 2012; GOALS 2013; Larsen 1966; McDermott 2008), showing improvement with exercise (MD 2.32 minutes, 95% CI 0.91 to 3.74, P < 0.001) and significant heterogeneity between studies (P = 0.005, I2 = 73%) when a random‐effects model was used.
Ankle brachial index (ABI)
3.5. Analysis.
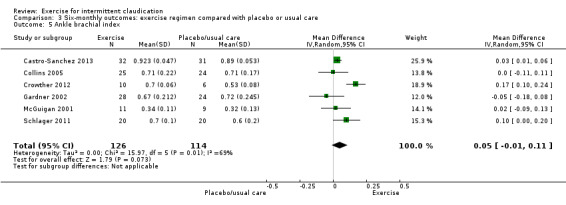
Comparison 3 Six‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 5 Ankle brachial index.
Six studies with 240 participants reported on ABI at six months (Castro‐Sanchez 2013; Collins 2005; Crowther 2012; Gardner 2002; McGuigan 2001; Schlager 2011). Data showed no clear differences in ABI between the two groups (MD 0.05, 95% CI ‐0.01 to 0.11, P = 0.07) and significant heterogeneity (P = 0.007, I2 = 69%) between studies when a random‐effects model was used.
Mortality
3.6. Analysis.
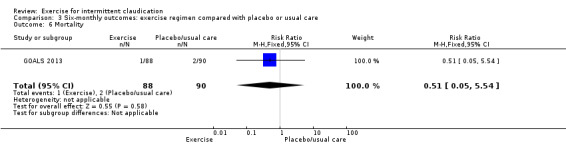
Comparison 3 Six‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 6 Mortality.
Only GOALS 2013 provided mortality data for the six‐month time point. Overall data show no clear difference in mortality between exercise and non‐exercise groups at the six‐month time period (RR 0.51, 95% CI 0.05 to 5.54, P = 0.58).
Quality of life
3.7. Analysis.
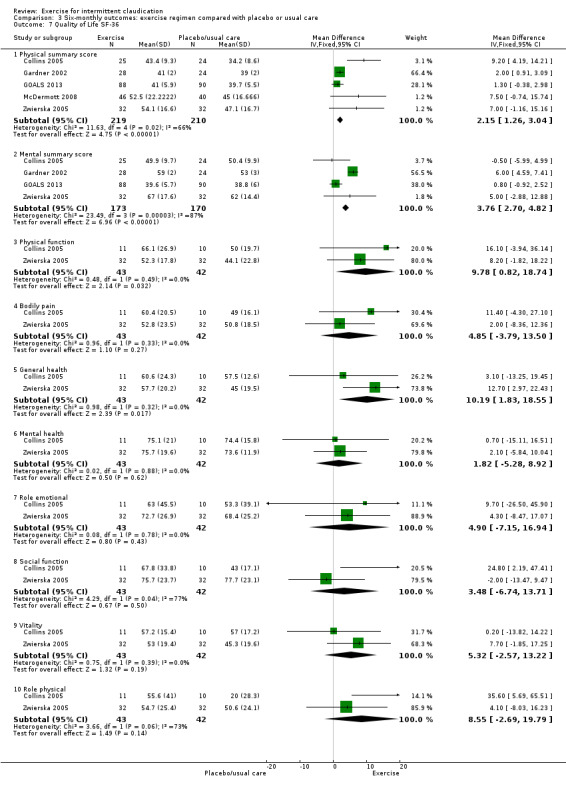
Comparison 3 Six‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 7 Quality of Life SF‐36.
Five studies with a total of 429 participants provided outcomes for the SF‐36 generic quality of life measure (Collins 2005; Gardner 2002; GOALS 2013; McDermott 2008; Zwierska 2005), which showed improvement in quality of life secondary to exercise in certain domains, namely, 'physical summary score' (MD 2.15, 95% CI 1.26 to 3.04, P < 0.00001, moderate‐quality evidence; supported with a random‐effects model) and 'mental summary score' (MD 3.76, 95% CI 2.70 to 4.82, P < 0.00001, 4 studies, 343 participants, moderate‐quality evidence). The 'mental summary score' no longer showed improvement when a random‐effects model was applied (MD 2.85, 95% CI ‐1.01 to ‐6.71). Heterogeneity was significant (87%) for 'mental summary score'. Only two studies (43 participants) gave details on all domains; 'physical function' (MD 9.78, 95% CI 0.82 to 18.74) and 'general health' (MD 10.19, 95% CI 1.83 to 18.55) improved with exercise (Collins 2005; Zwierska 2005); this was supported again when a random‐effects model was used. The other domains ‐ 'role physical', 'bodily pain', 'vitality', 'social function', 'role emotional', and 'mental health' ‐ did not show improvement.
Peak exercise calf blood flow
3.8. Analysis.
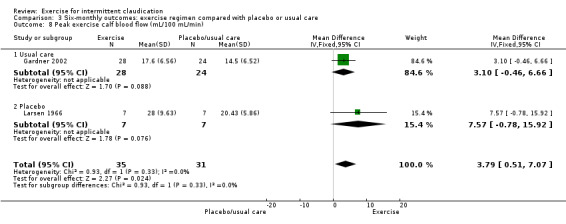
Comparison 3 Six‐monthly outcomes: exercise regimen compared with placebo or usual care, Outcome 8 Peak exercise calf blood flow (mL/100 mL/min).
Two studies with 66 participants reported on blood flow measurements at six months (Gardner 2002; Larsen 1966). As with overall and three‐month analyses, six‐month blood flow measurements showed no differences between groups (MD 3.79 mL/100 mL/min, 95% CI 0.51 to 7.07, P = 0.02). Results showed no significant heterogeneity (P = 0.33, I2 = 0%) between studies when a fixed‐effect model was used.
Publication bias
We assessed overall publication bias using a funnel plot in three meta‐analyses (Analysis 1.3; Analysis 1.4; Analysis 1.7) (see Figure 4; Figure 5; and Figure 6).
4.
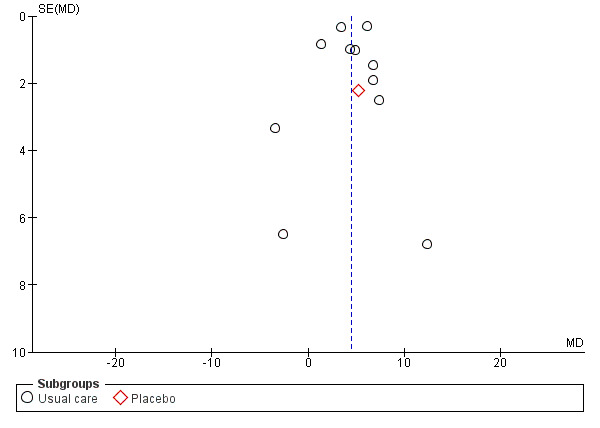
Funnel plot of comparison: 1 Overall outcomes: exercise regimen compared with placebo or usual care, outcome: 1.1 Maximum walking time (min).
5.
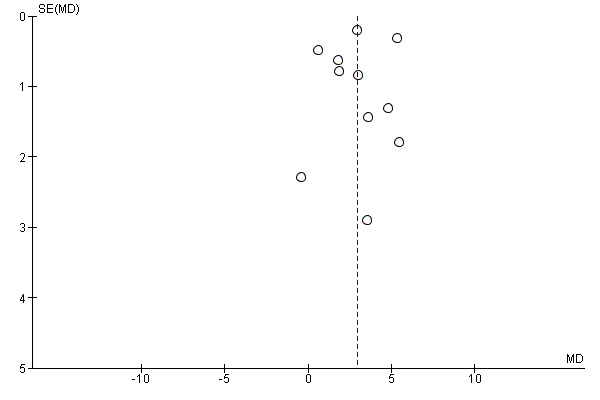
Funnel plot of comparison: 1 Overall outcomes: exercise regimen compared with placebo or usual care, outcome: 1.4 Pain‐free walking time (min).
6.
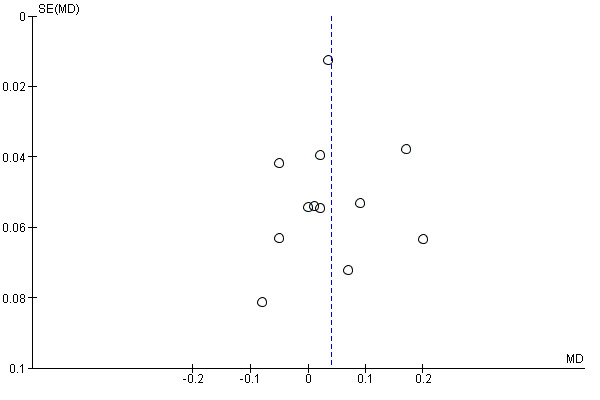
Funnel plot of comparison: 1 Overall outcomes: Exercise regimen compared with placebo or usual care, outcome: 1.5 Ankle brachial index.
The funnel plot in Figure 4 shows that 10 studies were within the 95% CI, and two were outside this range. Overall plot data were symmetrical, suggesting no evidence of publication bias or asymmetry secondary to the presence of smaller studies.
The funnel plot in Figure 5 indicates that seven studies were within the 95% CI, and three were outside this range. Overall plot data were asymmetrical, suggesting bias, possibly attributed to the presence of smaller studies.
The funnel plot in Figure 6 revealed that nine studies were within the 95% CI, and three were outside this range. Overall plot data were asymmetrical, suggesting bias, possibly attributed to the presence of smaller studies.
Exercise regimen compared with antiplatelet therapy
One trial involving 10 participants compared exercise with antiplatelet therapy (Mannarino 1991) and reported the following results.
Maximum walking time
4.1. Analysis.
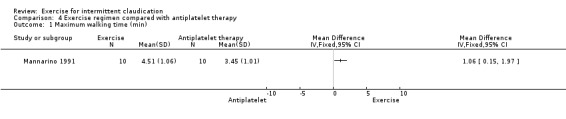
Comparison 4 Exercise regimen compared with antiplatelet therapy, Outcome 1 Maximum walking time (min).
After six months of treatment, maximum walking time was improved in the exercise group compared with the group treated with antiplatelet therapy (MD 1.06 minutes, 95% CI 0.15 to 1.97). Maximum walking time was increased by 86% in the exercise group and by 38% in antiplatelet therapy group.
Ankle brachial index (ABI)
4.2. Analysis.
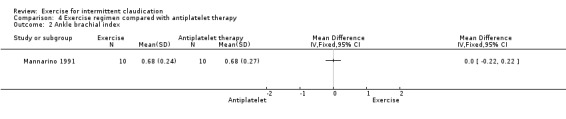
Comparison 4 Exercise regimen compared with antiplatelet therapy, Outcome 2 Ankle brachial index.
Results show no differences between exercise and antiplatelet therapy groups at the end of the trial (MD 0.00, 95% CI ‐0.22 to 0.22).
Peak exercise calf blood flow
4.3. Analysis.
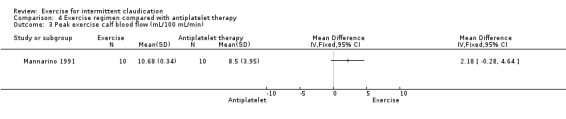
Comparison 4 Exercise regimen compared with antiplatelet therapy, Outcome 3 Peak exercise calf blood flow (mL/100 mL/min).
Results show no clear differences in calf blood flow between antiplatelet therapy and exercise groups after six months, although flow tended to be higher in the exercise group (MD 2.18 mL/100 mL/min, 95% CI ‐0.28 to 4.64).
Mannarino 1991 did not report the remaining outcomes of this review.
Exercise regimen compared with pentoxifylline
One trial involving 30 participants compared exercise versus pentoxifylline (Ciuffetti 1994) and reported the following results.
Maximum walking time
5.1. Analysis.
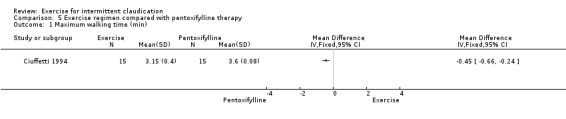
Comparison 5 Exercise regimen compared with pentoxifylline therapy, Outcome 1 Maximum walking time (min).
After 13 weeks of therapy, maximum walking time was greater in the pentoxifylline group than in the exercise group (MD ‐0.45 minutes, 95% CI ‐0.66 to ‐0.24). Walking distance increased by 62% in the exercise group and by 88% in pentoxifylline group.
Adverse events
Two participants experienced gastroenteritis during treatment with pentoxifylline, but investigators did not consider this to be a side effect of study treatments.
Ciuffetti 1994 did not report the remaining outcomes of this review.
Exercise regimen compared with iloprost therapy
One study with 24 participants compared iloprost versus exercise (Arosio 2001) and reported the following results.
Pain‐free walking distance
6.2. Analysis.
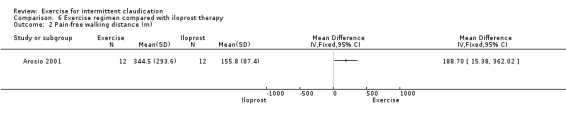
Comparison 6 Exercise regimen compared with iloprost therapy, Outcome 2 Pain‐free walking distance (m).
Data showed improvement in pain‐free walking distance in the exercise group at two weeks (MD 188.7 metres, 95% CI 15.38 to 362.02).
Maximum walking distance
6.1. Analysis.
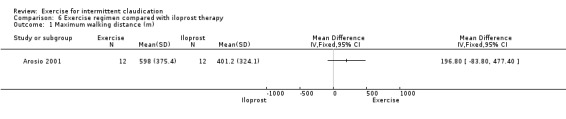
Comparison 6 Exercise regimen compared with iloprost therapy, Outcome 1 Maximum walking distance (m).
Data showed no clear effect on maximum walking distance in the exercise group at two weeks (MD 196.80 metres, 95% CI ‐83.8 to 477.40).
Arosio 2001 did not report the remaining outcomes of this review.
Exercise regimen compared with pneumatic foot and calf compression
One study with 25 participants compared exercise versus pneumatic foot and calf compression (Kakkos 2005).
Pain‐free walking distance
7.2. Analysis.
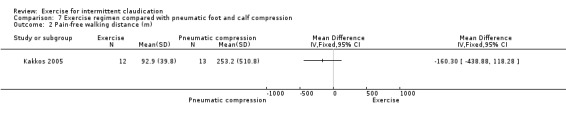
Comparison 7 Exercise regimen compared with pneumatic foot and calf compression, Outcome 2 Pain‐free walking distance (m).
Data showed no clear effect on pain‐free walking distance in the pneumatic compression group compared with the exercise group (MD ‐160.30 metres, 95% CI ‐438.88 to 118.28).
Maximum walking distance
7.1. Analysis.
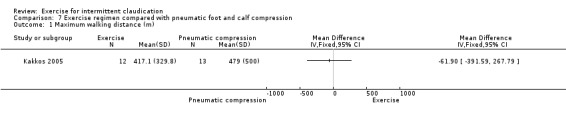
Comparison 7 Exercise regimen compared with pneumatic foot and calf compression, Outcome 1 Maximum walking distance (m).
Data showed no clear effect on maximum walking distance in the pneumatic compression group compared with the exercise group (MD ‐61.90 metres, 95% CI ‐391.59 to 267.79).
Mortality
7.3. Analysis.
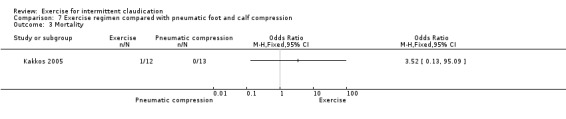
Comparison 7 Exercise regimen compared with pneumatic foot and calf compression, Outcome 3 Mortality.
One death occurred within the pneumatic calf compression group, and no deaths occurred in the exercise group (odds ratio (OR) 3.52, 95% CI 0.13 to 95.09).
Kakkos 2005 did not report the remaining outcomes of this review.
Exercise regimen compared with vitamin E
One study involving 24 participants compared effects of vitamin E and exercise (Collins 2005).
Maximum walking time
8.1. Analysis.
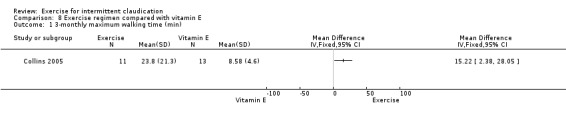
Comparison 8 Exercise regimen compared with vitamin E, Outcome 1 3‐monthly maximum walking time (min).
8.2. Analysis.
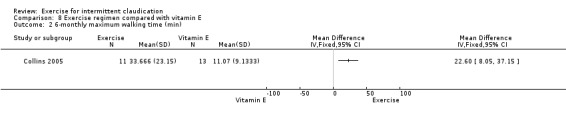
Comparison 8 Exercise regimen compared with vitamin E, Outcome 2 6‐monthly maximum walking time (min).
Collins 2005 demonstrated improvement secondary to exercise, which was greater than with vitamin E alone, at three and six months, respectively (MD 15.22 minutes, 95% CI 2.38 to 28.05; MD 22.60 minutes, 95% CI 8.05 to 37.15).
Ankle brachial index (ABI)
8.3. Analysis.
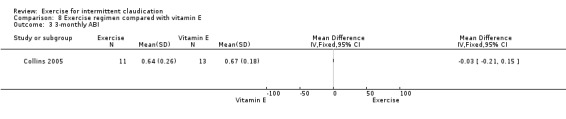
Comparison 8 Exercise regimen compared with vitamin E, Outcome 3 3‐monthly ABI.
8.4. Analysis.
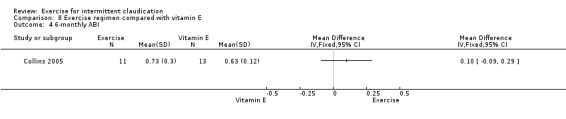
Comparison 8 Exercise regimen compared with vitamin E, Outcome 4 6‐monthly ABI.
ABI was unchanged at three and six months, respectively (MD ‐0.03, 95% CI ‐0.21 to 0.15; MD 0.10, 95% CI ‐0.09 to 0.29).
Collins 2005 did not report the remaining outcomes of this review.
Discussion
Summary of main results
The data presented in this update generally confirm the findings of previous versions of this review ‐ that exercise has a significant positive effect on walking times and walking distances in people considered to be fit for exercise intervention, compared with placebo or usual care. Although most studies examined outcomes at three or six months, it is important to note that this benefit would appear to be sustained for up to two years (Jansen 1991). Of note, however, data show improvement in maximum walking distance at six months but not at three months.
Mean improvements in walking distance and walking time with exercise were clinically and statistically significant; however in most cases, the data were not normally distributed. Some individuals responded with improvement of considerably larger magnitude than the mean, whereas others responded less well, which may reflect varying compliance with exercise programmes. Successful programmes generally comprised physiotherapy with supervised exercise two or three times per week for 30 to 60 minutes, often with walking, leg exercises, or treadmill training. Some programmes encouraged additional home exercise.
We used percentage change from baseline to allow a more holistic understanding of change in walking ability. These additional parameters have demonstrated an increase in both initial claudication and maximum walking percentage changes, in keeping with changes documented for initial claudication walking distance and time, as well as for maximum walking distance and time. Of note, use of combined data allowed inclusion of 15 studies and a larger cohort of participants in the analysis.
Data related to ankle brachial index (ABI) and to other important outcomes are sparse. Investigators provided no data on non‐fatal cardiovascular events and inconclusive data regarding mortality and amputation.
Antiplatelet therapy was less effective than exercise in improving walking distance and other measures of lower limb function, but researchers presented no data pertaining to fatal and non‐fatal cardiovascular events. A previous meta‐analysis has shown that antiplatelet agents reduce the incidence of cardiovascular events in people with claudication (Trialists 1994). Therefore, aspirin should be of benefit despite lack of effect on the lower limb.
The single small trial comparing pentoxifylline with exercise showed that participants on drug therapy had significantly longer walking distances after three months than those on exercise therapy (Ciuffetti 1994). Investigators reported no cardiovascular or adverse events among those on pentoxifylline, but disadvantages of drug treatment might include cost and lack of general cardiovascular improvement. Arosio 2001 showed that exercise improved pain‐free walking time significantly more than iloprost, but this was a small study (24 participants).
Pneumatic foot and calf compression showed no clear effect on walking distances compared with exercise, again in a small trial (Kakkos 2005). However, alternatives for improving blood flow among those with poor mobility represent an important area for future research in those who are unsuitable for exercise programmes. Therefore further research into such avenues may yield more certain results and may therefore be of interest.
Researchers reported quality of life data on a range of scales, which made it difficult to incorporate this information into a meta‐analysis. Results were variable depending on the comparisons made; this is a topic for future research.
Overall completeness and applicability of evidence
Overall, it is not contested that exercise may provide benefit for individuals with intermittent claudication. However, the overall benefit and duration of exercise programmes differ. A standardised approach with a minimum duration of six months appears to confer benefit and would improve both pain‐free and maximum walking distance. Review authors found scant data on the benefits of a six‐month programme compared with a three‐month programme when measured outcomes focused on quality of life, mortality, or amputation risk. The general assumption is that exercise training is safe in peripheral arterial disease, but the data presented are often focussed on mortality and limb loss. Other cardiovascular morbidity appears to be under‐reported, and additional details on safety data within exercise studies would be of value.
Criteria for participant selection resulted in exclusion of many individuals with stable claudication for whom exercise was not practical or safe owing to pre‐existing medical conditions. As most people with intermittent claudication are elderly, comorbidities are common. For up to a third of people, exercise may not be a suitable option. Other research trials and reviews have examined optimum modes of exercise for people with intermittent claudication, for example, a Cochrane review explored supervised versus unsupervised exercise for intermittent claudication (Fokkenrood 2013). The present review cannot resolve uncertainties about different exercise regimens.
This review did not investigate the issue of non‐compliance, which has a large impact on the use of supervised exercise classes and how funding should be allocated for this treatment as advocated by the National Institute for Health and Care Excellence (NICE) (NCGC 2012). Further information on the cost‐effectiveness of exercise programmes is needed.
Quality of the evidence
We the review authors continue to believe that the quality of evidence is high in supporting exercise as first‐line treatment for symptomatic claudicants. See Table 1. In this review update, we have added only two studies that support the body of literature already presented.
Compared with the previous version of this review, the evidence presented has improved in quality, as reporting criteria have become more stringent. In general, the bias present within papers was often difficult to assess owing to absence of relevant information. In these cases, we marked risk of bias as unclear, as evidence on which to base a judgement was insufficient. Overall, included studies were of moderate methodological quality.
Pooled outcomes for walking distance and maximum walking distance at all time points demonstrated significant heterogeneity. This most likely was secondary to pooling of walking times from some studies at two years and reporting of outcomes after three months in other studies. When possible we addressed this heterogeneity by analysing data at specific time points, such as three and six months post intervention. Regardless of this, data showed improvement in initial walking distance and time at three months, and in maximum walking distance and time by six months.
Publication bias remains an additional confounding factor for the quality of evidence pooled within this review. Older studies have been reported more poorly, as journal reporting criteria were previously less rigorous, resulting in greater inherent bias.
Potential biases in the review process
We searched the Specialised Register and the Cochrane Central Register of Controlled Trials (CENTRAL) for new publications about exercise in patients with intermittent claudication. Two review authors independently assessed all new studies in keeping with recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The main cause of bias within this review is that walking advice provided by consultants in clinics is now seen as the best medical advice but may also be deemed as unsupervised exercise. Therefore this review excluded all studies that reported best medical treatment, which includes walking advice, as we believe this would overlap with the Cochrane review on supervised versus unsupervised exercise (Fokkenrood 2013). It remains possible that usual care included walking advice, but this was not explicitly stated by trialists in their study papers, suggesting one possible aspect of reporting bias within studies.
It is the review authors' feeling that as claudication research develops, a usual care non‐exercise control group will cease to exist, as its inclusion will be deemed unethical.
Finally, earlier studies used treadmill walking distance as the primary outcome measure, and this has continued to serve as a method for assessment of improvement in claudication. Whilst treadmill distances remain a reliable means of assessment, this method is being superseded by other functional and subjective assessments. Newer assessment tools to assess changes in walking distance include GPS tracking, six‐minute walks, subjective reporting, and assessments based on the Walking Impairment Questionnaire (WIQ). It is important that future reviews consider inclusion of these as outcome measures (Cucato 2013b; Tew 2013).
Agreements and disagreements with other studies or reviews
This review is consistent with previous versions (Lane 2014; Leng 2000; Watson 2008) and adds to the body of available evidence supporting exercise for people with intermittent claudication. This evidence is also in line with NICE guidelines, which recommend that exercise should be provided as first‐line treatment for people with intermittent claudication (NCGC 2012). Other meta‐analyses such as Parmenter 2011 and other reviews such as Lauret 2012 and Lauret 2012a also support this finding.
Authors' conclusions
Implications for practice.
This review provides high‐quality evidence showing that exercise therapy should play an important part in the care of selected patients with intermittent claudication, to improve walking times and distances. Effects were demonstrated following three months of supervised exercise, although some programmes lasted longer than one year. Limited data suggest that an effect is sustained for up to two years. Exercise did not improve ankle brachial index (ABI), and investigators detected no differences in the effect of exercise between groups in terms of amputation or mortality. Exercise may improve quality of life when compared with placebo or usual care.
Antiplatelet agents were less effective than exercise in improving walking distance but should continue to be used because of benefits in reducing cardiovascular events and death. In contrast, pentoxifylline was more effective than exercise but may have fewer beneficial effects on the cardiovascular system in general. Iloprost led to less improvement in walking time than exercise. Data show no clear effect on walking distances when pneumatic foot and calf compression was compared with exercise. However the number of participants in these studies is small and data are limited.
Implications for research.
Important questions involve the degree of supervision required in any exercise regimen and how long any change in exercise habits can be expected to last. Therefore, a trial with long follow‐up ‐ of five years ‐ is needed to compare the effectiveness of different supervised and unsupervised regimens in terms of changing long‐term exercise patterns. Behavioural changes and attitudes towards exercise have been items of key interest in areas such as cardiovascular rehabilitation (Jolly 2009). More recent studies have demonstrated that brief psychological interventions may play a key role in improving walking distances among patients with intermittent claudication up to one year (Cunningham 2012). A telling aspect of research into claudicants' beliefs is their poor overall understanding of the disease and of why walking is recommended (Cunningham 2014). Future holistic exercise programmes, which may include cognitive‐behavioural therapy and lifestyle and risk factor modification, could provide great benefit in encouraging people to start and maintain a better overall lifestyle.
Future research should also focus on compliance with exercise and how this could be improved. Outcome measures should include fatal and non‐fatal cardiovascular events. In addition, expansion of this review to assess the benefit of exercise for asymptomatic patients and its impact on cardiovascular morbidity is an important goal.
Further cost‐effectiveness analysis is required to determine whether the cost of supervised sessions might offset the cost of deterioration in terms of surgery or occupation of in‐patient beds for complications such as myocardial infarction.
A trial is needed to compare exercise treatment with pentoxifylline to determine whether the benefit of drug treatment is sustained over a longer period, and whether data show any differences in cardiovascular events. Further investigation of pneumatic foot and calf compression for treatment of individuals with intermittent claudication is also needed. Consistency in use of quality of life measures among different trials would be helpful in linking outcomes to patient‐assessed improvements.
What's new
| Date | Event | Description |
|---|---|---|
| 15 November 2016 | New citation required but conclusions have not changed | Searches rerun, 2 new studies included, 18 additional studies excluded. New author (AH) joined the review team. Text updated to reflect current Cochrane standards. 'Summary of findings' table added. Conclusions not changed |
| 15 November 2016 | New search has been performed | Searches rerun, 2 new studies included, 18 additional studies excluded |
History
Protocol first published: Issue 2, 1997 Review first published: Issue 1, 1998
| Date | Event | Description |
|---|---|---|
| 9 November 2013 | New search has been performed | Searches rerun, 11 new studies included, 45 additional studies excluded |
| 9 November 2013 | New citation required but conclusions have not changed | New author (RAL) joined the review team. Searches rerun, 11 new studies included, 45 additional studies excluded. Risk of bias tables completed for all included studies In comparison with previous versions of this review, this version no longer focuses on supervised versus non‐supervised exercise, or invasive interventions compared with exercise, as these are covered in other Cochrane reviews. |
| 18 June 2008 | New search has been performed | 12 new trials considered. Conclusions confirm findings of the previous review. |
| 29 April 2008 | New citation required but conclusions have not changed | Two new review authors |
| 8 April 2008 | Amended | Converted to new review format |
| 25 October 1999 | New citation required but conclusions have not changed | Substantive updates made |
| 17 June 1998 | New citation required and minor changes | After consultation with GCL, original November 1997 version substituted for June 1998 'update', as latter was an incorrect version. Some phrasing changed in June 1998, no changes to figures, so earlier version more appropriate. Edited abstract added too (24.2.99) |
Acknowledgements
We are very grateful to Cochrane Vascular for assistance provided in the search for new trials and to Dr Brian Ellis for his input in previous versions of this review.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MESH DESCRIPTOR Arteriosclerosis | 868 |
| #2 | MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES | 0 |
| #3 | MESH DESCRIPTOR Arteriosclerosis Obliterans | 71 |
| #4 | MESH DESCRIPTOR Atherosclerosis | 614 |
| #5 | MESH DESCRIPTOR Arterial Occlusive Diseases | 722 |
| #6 | MESH DESCRIPTOR Intermittent Claudication | 710 |
| #7 | MESH DESCRIPTOR Ischemia | 786 |
| #8 | MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES | 2195 |
| #9 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY | 9000 |
| #10 | ((arter* or vascular or vein* or veno* or peripher*) near (occlus* or reocclus* or re‐occlus* or steno* or obstruct* or lesio* or block* or harden* or stiffen*) ):TI,AB,KY | 8590 |
| #11 | (peripheral near3 dis*):TI,AB,KY | 3324 |
| #12 | (claudic* or IC):TI,AB,KY | 3004 |
| #13 | (isch* or CLI):TI,AB,KY | 23368 |
| #14 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 | 42290 |
| #15 | MESH DESCRIPTOR exercise EXPLODE ALL TREES | 16154 |
| #16 | MESH DESCRIPTOR Exercise Therapy EXPLODE ALL TREES | 8362 |
| #17 | MESH DESCRIPTOR dancing EXPLODE ALL TREES | 110 |
| #18 | MESH DESCRIPTOR Sports EXPLODE ALL TREES | 11532 |
| #19 | MESH DESCRIPTOR Physical Exertion | 3523 |
| #20 | MESH DESCRIPTOR Leisure Activities | 214 |
| #21 | ((fitness near3 (train* or intervention* or protocol* or program* or therap* or activit* or regim* or centre* or center*))):TI,AB,KY | 1051 |
| #22 | (((training or conditioning) near3 (circuit or intervention* or protocol* or program* or activit* or regim*))):TI,AB,KY | 8527 |
| #23 | ((walk* or run* or treadmill or aerobic or swim* or danc* or weight or squat* or lunge or bend* or raise or cycling or step)):TI,AB,KY | 94487 |
| #24 | kinesiotherap*:TI,AB,KY | 1533 |
| #25 | ((physical near3 (exertion or endurance or therap* or conditioning or activit* or fitness or train*))):TI,AB,KY | 25488 |
| #26 | exercise:TI,AB,KY | 47402 |
| #27 | #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 | 132771 |
| #28 | #14 AND #27 | 7702 |
| #29 | NOT SR‐PVD:CC AND 30/09/2013 TO 31/10/2016:DL | 268200 |
| #30 | #28 AND #29 | 2165 |
Appendix 2. Trials registries searches
Clinicaltrials.gov
42 studies found for: intermittent claudication | exercise
WHO
62 records for 61 trials found for: exercise AND intermittent claudication
ISRCTN
22 results exercise AND intermittent claudication
Data and analyses
Comparison 1. Overall outcomes: exercise regimen compared with placebo or usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum walking distance (m) | 10 | 500 | Mean Difference (IV, Random, 95% CI) | 120.36 [50.79, 189.92] |
| 2 Pain‐free walking distance (m) | 9 | 391 | Mean Difference (IV, Fixed, 95% CI) | 82.11 [71.73, 92.48] |
| 3 Maximum walking time (min) | 12 | 577 | Mean Difference (IV, Random, 95% CI) | 4.51 [3.11, 5.92] |
| 3.1 Usual care | 11 | 563 | Mean Difference (IV, Random, 95% CI) | 4.47 [3.00, 5.94] |
| 3.2 Placebo | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 5.20 [0.88, 9.52] |
| 4 Pain‐free walking time (min) | 11 | 534 | Mean Difference (IV, Random, 95% CI) | 2.93 [1.77, 4.09] |
| 4.1 Usual care | 10 | 520 | Mean Difference (IV, Random, 95% CI) | 3.08 [1.82, 4.34] |
| 4.2 Placebo | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 1.77 [0.55, 2.99] |
| 5 Change in MWD/T | 15 | 656 | Mean Difference (IV, Random, 95% CI) | 40.25 [28.64, 51.86] |
| 6 Change in ICD/T | 15 | 703 | Mean Difference (IV, Random, 95% CI) | 58.42 [44.20, 72.64] |
| 7 Ankle brachial index | 13 | 570 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.00, 0.08] |
| 8 Mortality | 5 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.39, 2.17] |
| 9 Amputation | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.15] |
| 10 Peak exercise calf blood flow (mL/100 mL/min) | 4 | 103 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [‐0.81, 2.69] |
| 10.1 Usual care | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | 2.83 [0.18, 5.49] |
| 10.2 Placebo | 2 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐2.85, 1.82] |
Comparison 2. Three‐monthly outcomes: exercise regimen compared with placebo or usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum walking distance (m) | 3 | 116 | Mean Difference (IV, Random, 95% CI) | 104.46 [‐64.33, 273.24] |
| 2 Pain‐free walking distance (m) | 3 | 156 | Mean Difference (IV, Fixed, 95% CI) | 88.70 [58.25, 119.15] |
| 3 Maximum walking time (min) | 5 | 172 | Mean Difference (IV, Fixed, 95% CI) | 6.05 [5.47, 6.62] |
| 3.1 Usual care | 5 | 172 | Mean Difference (IV, Fixed, 95% CI) | 6.05 [5.47, 6.62] |
| 4 Pain‐free walking time (min) | 3 | 132 | Mean Difference (IV, Fixed, 95% CI) | 4.95 [4.38, 5.53] |
| 5 Ankle brachial index | 4 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.01, 0.11] |
| 6 Mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Quality of Life SF‐36 | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Physical function | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 6.60 [2.37, 10.83] |
| 7.2 Bodily pain | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 3.89 [‐1.91, 9.68] |
| 7.3 General health | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 4.52 [‐0.01, 9.04] |
| 7.4 Mental health | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 1.44 [‐0.93, 3.81] |
| 7.5 Role emotional | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 1.26 [‐4.84, 7.36] |
| 7.6 Social function | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 1.49 [‐4.16, 7.14] |
| 7.7 Vitality | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 5.55 [1.54, 9.56] |
| 7.8 Role physical | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 10.31 [3.64, 16.98] |
| 7.9 Physical Summary Score | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.58 [‐4.29, 9.45] |
| 7.10 Mental Summary Score | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.05 [‐4.73, 8.83] |
| 8 Peak exercise calf blood flow (mL/100 mL/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Six‐monthly outcomes: exercise regimen compared with placebo or usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum walking distance (m) | 3 | 156 | Mean Difference (IV, Random, 95% CI) | 138.36 [22.39, 254.34] |
| 2 Pain‐free walking distance (m) | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | 52.14 [6.83, 97.45] |
| 3 Maximum walking time (min) | 4 | 295 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [2.04, 4.36] |
| 3.1 Usual care | 3 | 281 | Mean Difference (IV, Fixed, 95% CI) | 3.05 [1.84, 4.25] |
| 3.2 Placebo | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 5.20 [0.88, 9.52] |
| 4 Pain‐free walking time (min) | 5 | 292 | Mean Difference (IV, Random, 95% CI) | 2.32 [0.91, 3.74] |
| 4.1 Usual care | 4 | 278 | Mean Difference (IV, Random, 95% CI) | 2.72 [0.67, 4.77] |
| 4.2 Placebo | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 1.77 [0.55, 3.00] |
| 5 Ankle brachial index | 6 | 240 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.01, 0.11] |
| 6 Mortality | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.54] |
| 7 Quality of Life SF‐36 | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Physical summary score | 5 | 429 | Mean Difference (IV, Fixed, 95% CI) | 2.15 [1.26, 3.04] |
| 7.2 Mental summary score | 4 | 343 | Mean Difference (IV, Fixed, 95% CI) | 3.76 [2.70, 4.82] |
| 7.3 Physical function | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 9.78 [0.82, 18.74] |
| 7.4 Bodily pain | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 4.85 [‐3.79, 13.50] |
| 7.5 General health | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 10.19 [1.83, 18.55] |
| 7.6 Mental health | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 1.82 [‐5.28, 8.92] |
| 7.7 Role emotional | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 4.90 [‐7.15, 16.94] |
| 7.8 Social function | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 3.48 [‐6.74, 13.71] |
| 7.9 Vitality | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 5.32 [‐2.57, 13.22] |
| 7.10 Role physical | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 8.55 [‐2.69, 19.79] |
| 8 Peak exercise calf blood flow (mL/100 mL/min) | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | 3.79 [0.51, 7.07] |
| 8.1 Usual care | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [‐0.46, 6.66] |
| 8.2 Placebo | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 7.57 [‐0.78, 15.92] |
Comparison 4. Exercise regimen compared with antiplatelet therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum walking time (min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Ankle brachial index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Peak exercise calf blood flow (mL/100 mL/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Exercise regimen compared with pentoxifylline therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum walking time (min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Exercise regimen compared with iloprost therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum walking distance (m) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Pain‐free walking distance (m) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. Exercise regimen compared with pneumatic foot and calf compression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum walking distance (m) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Pain‐free walking distance (m) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Exercise regimen compared with vitamin E.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 3‐monthly maximum walking time (min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 6‐monthly maximum walking time (min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 3‐monthly ABI | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 6‐monthly ABI | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arosio 2001.
| Methods | Study design: RCT Method of randomisation: 'randomly divided' Exclusions post randomisation: not reported Losses to follow‐up: not reported |
|
| Participants | Country: Italy Setting: hospital No. of participants: 24 Age: mean 65 years; 64.5 (55 to 69) years in exercise group; 66.4 (57 to 72) years in iloprost group Sex: all male Inclusion criteria: Fontaine stage II PAD with IC during past 4 to 6 years, confirmed by Doppler ultrasound, angiography, and ABI Exclusion criteria: not clearly stated but inferred to be smoking, severe hypertension, stroke, ischaemic attack, cerebrovascular disease, any drugs for PAD or other disease except transdermal clonidine for mild to moderate hypertension |
|
| Interventions | Intervention 1: physical exercise (n = 10) ‐ walking, running, squat thrusts, 2 × day for 30 min, cycle ergometer for 30 min, plus 30 min constant load treadmill (2 mph, slope 0%) Intervention 2: iloprost (0.5 to 2 ng/kg/min) (n = 10) Duration: 14 days |
|
| Outcomes | Endogenous NO products Neutrophil adhesion ICD (m) and ACD (m) from constant load treadmill test (2 mph, slope 0%) |
|
| Notes | Exercise was interrupted at onset of pain, participant rested for 3 min, or until pain had gone, then resumed activity until each 30‐min block. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation method provided. Simply stated, 'the groups were randomly divided in two treatment regimens' |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of whether all enrolled completed the study |
| Selective reporting (reporting bias) | Low risk | Prespecified outcome measures reported |
| Other bias | Unclear risk | Small numbers (n = 10 per treatment arm) and duration for only 14 days |
Castro‐Sanchez 2013.
| Methods | Study design: RCT Method of randomisation: randomised balanced (stratified) selection process Groups were balanced for type of medication received and Leriche‐Fontaine stage (I or IIa), via a stratification system that generates a sequence of letters (from a table of correlatively ordered permutations) for each category and combination of categories. Exclusions post randomisation: 5; 3 in placebo (reasons: working hours, care for grandparents, and sprained ankle) and 2 in the physical therapy modality (reasons: terminally ill husband, and bed rest) Losses to follow‐up: not reported |
|
| Participants | Country: Spain Setting: healthcare district No. of participants: 68 Age: mean (SD) age, 53 (12) years; range, 41 to 65 years Sex: 30 women and 38 men Inclusion criteria: diagnosis of type 2 diabetes, postexercise ABI of 0.6 to 0.9, HbA1c of 7% to 13%, BMI of 25 to 40, and sedentary lifestyle Exclusion criteria: other stages of PAD; peripheral venous insufficiency; cardiac, renal, or hepatic insufficiency; a cardiovascular event in the previous year; arterial pressure > 160/90 mmHg; LDL cholesterol > 160 mg/dL; an active smoking habit (in 3 previous years); and the presence of walking disorder, impaired skin integrity, or psychological or neurological disorder |
|
| Interventions | Intervention 1: Exercise group (n = 34) performed a session of 3 physical therapy modalities 2 times/d at home. Intervention 2: Placebo group (n = 34) received a treatment protocol 1 day/week with disconnected ultrasound electrotherapy equipment in dorsal and lumbar regions (15 minutes per region); these patients were instructed on the use of ultrasound equipment and were unaware that it was switched off. Duration: 20 weeks |
|
| Outcomes | Initial claudication walking distance (not reported in results); constant load treadmill test (3 km/h, 10% grade) Blood parameters: fibrinogen (mg/dL), haemoglobin (g/dL), glucose (mg/dL), cholesterol (mg/dL), high‐density lipoprotein (HDL) cholesterol (mg/dL), LDL cholesterol (mg/dL), triglycerides (mg/dL), and HbA1c (%) Doppler flow velocity ABPI Cardiovascular risk score Heart rate during exercise test Assessments at 3 time points: baseline (before treatments), immediately after the final treatment session, and at 6 months after the conclusion of treatment |
|
| Notes | Home exercise protocol described by Boutroux 1980 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised balanced (stratified) selection process |
| Allocation concealment (selection bias) | Low risk | Groups were balanced for type of medication received and Leriche‐Fontaine stage (I or IIa) via a stratification system that generates a sequence of letters (from a table of correlatively ordered permutations) for each category and combination of categories. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to the treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five dropouts were reported and excluded. |
| Selective reporting (reporting bias) | High risk | All outcomes stated ‐ with the exception of initial claudication walking distance |
| Other bias | Low risk | No evidence of other bias |
Ciuffetti 1994.
| Methods | Study design: RCT Method of randomisation: allocated by predetermined computer code. Not blinded Exclusions post randomisation: not reported Losses to follow‐up: no dropouts reported |
|
| Participants | Country: Italy Setting: hospital No. of participants: 30 Age: 48 to 64 years Sex: male and female Inclusion criteria: stable maximal walking time (90 to 130 s) at 2 previous 6‐monthly checks, plus stage II PAD confirmed by velocimetry and angiography Exclusion criteria: no h/o vascular surgery, coronary or cerebrovascular disease, DM; no factors affecting oxygen demand (e.g. anaemias); recent infection; treatment with vasodilators, antiplatelets, anticoagulants, or drugs affecting haemorrheological parameters for previous 1 month |
|
| Interventions | Treatment: 1 h exercise at home daily plus twice‐weekly supervision as out‐patients. Home regimen: week 1, 500 m in 20 min; week 2, 1000 m in 40 min; week 3, 2000 m in 60 min on the flat Control: pentoxifylline 800 mg tds Duration: 3 months |
|
| Outcomes | Primary: treadmill test maximal walking distance (2 km/h on 12° slope) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by means of a predetermined computer code |
| Allocation concealment (selection bias) | Low risk | Predetermined computer code |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated in paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated |
| Other bias | Unclear risk | n = 15 per group; small numbers; groups matched for baseline gender and age |
Collins 2005.
| Methods | Study design: RCT Method of randomisation: participants randomised after baseline testing completed; 4 groups, randomised 2 × 2 factorial analysis, permuted blocks: computer generated for vit E, sealed envelopes for exercise Exclusions post randomisation: none Losses to follow‐up: 6 (4 polestriding group, 2 control group) |
|
| Participants | Country: USA Setting: community No. of participants: 52 (49 analysed) Age: 65.8 ± 7.1 years polestriding group; 68.0 ± 8.6 control group Sex: 51 M, 1 F Inclusion criteria: history of IC, ABI < 0.95 at rest or < 0.85 after exercise, IC pain a factor for arrested walking Exclusion criteria: vascular surgery, angioplasty in previous 6 months; other comorbid conditions that would interfere with participation in an exercise programme; currently taking vit E, warfarin, or pentoxifylline; unable to give informed consent |
|
| Interventions | Treatment: polestriding (n = 27), supervised training 3 times per week for 4 weeks, twice weekly for 8 weeks, once weekly for 4 weeks, biweekly for 4 weeks, unsupervised for 4 weeks Control: no exercise (n = 25), usual care. Seen biweekly for 3 months and monthly thereafter Duration: 24 weeks |
|
| Outcomes | Primary: ABI, maximum walking distance, oxygen uptake Secondary: health‐related quality of life |
|
| Notes | All participants given $6 travel for each visit and $5 for each test battery completed, starting at $25
Secondary analysis of the 2 × 2 factorial design of Collins 2003; because results showed no influence of vit E on exercise, researchers combined exercise groups and compared findings with those of the non‐exercise groups The treadmill protocol began at a speed of 1.8 meter per hour (mph) and 0% grade. After the first 6 minutes, speed increased by 0.2 mph every 3 minutes. Per cent grade increased by 0.5% every 30 seconds |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised after baseline testing completed; 4 groups, randomised 2 × 2 factorial analysis, permuted blocks, computer generated |
| Allocation concealment (selection bias) | Low risk | Randomised 2 × 2 factorial analysis, permuted blocks, computer generated for vit E. For exercise: sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded to tablets, but not to exercise |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessors blinded to serum vit E levels |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data clearly reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated |
| Other bias | Low risk | Controlled for additional visits by making it fair across all 4 groups |
Crowther 2012.
| Methods | Study design: RCT Method of randomisation: "randomly allocated using a blinded protocol" Exclusions post randomisation: nil stated Losses to follow‐up: For personal reasons, 6 participants (n = 5 CPAD‐IC, and n = 1 TPAD‐IC) withdrew over the course of the research study. |
|
| Participants | Country: Australia Setting: hospital No. of participants: N = 22; control (CPAD‐IC, n = 11) or treatment (TPAD‐IC, n = 11) group Age: CPAD‐IC group: 67.1 (± 6.8), TPAD‐IC group: 71.3 (± 8.5) Sex: 50% male Inclusion criteria: symptoms of IC, appropriate history of IC, imaging confirmation of PAD on lower limb duplex or CTA, and ability and willingness to attend for regular supervised exercise Exclusion criteria: selection for surgical or endovascular intervention (n = 30), patient preference (n = 20) and requirement for mobility aids, obvious gait abnormalities (e.g. steppage, vaulting, circumduction, hip hiking), and medical conditions that influenced gait (e.g. orthopaedic conditions, neurological impairment) |
|
| Interventions | Treatment: 6‐month supervised exercise programme Control: standard medical treatment as outlined in the Trans‐Atlantic Inter‐Society Consensus (TASC II) guidelines Duration: 6 months |
|
| Outcomes | Primary: submaximal walking economy and walking performance during a graded treadmill exercise test Secondary: body composition; resting ABI |
|
| Notes | Exercise programme initially consisted of intermittent supervised treadmill walking 3 days per week for a total time of 25 minutes at 3.2 km/h (0.88 m/s). Participants were required to walk until the pain level was perceived as 3 or 4 on the CPS. Exercise intensity (via treadmill grade and walking speed) and duration (25 minutes up to a maximum of 40 minutes) were progressively increased once the participant could walk continuously for 25 minutes at a level below 3 on the CPS. This exercise progression strategy was continued over the 6‐month period of the study. The graded treadmill walking protocol consisted of a constant speed of 3.2 km/h (0.88 m/s) at an incline of 0% for the first 2 min, which was then increased by 2% every 2 minutes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Description of randomisation limited to: "Participants were randomly allocated using a blinded protocol" |
| Allocation concealment (selection bias) | Low risk | Blinded protocol; no further details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 participants lost to follow‐up. Groups left uneven. However baseline data for the 2 groups were similar after participants who dropped out were excluded. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | In Results section, numbers in CPAD and TPAD groups alternate. In Table 1, CPAD (n = 6), TPAD (n = 10); Table 2, CPAD (n = 10), TPAD (n = 6) |
Cucato 2013.
| Methods | Study design: RCT Method of randomisation: randomised; nil else stated Exclusions post randomisation: nil stated Losses to follow‐up: 4 from the walking group (reason: personal reasons) |
|
| Participants | Country: Brazil Setting: hospital No. of participants: 29 Age: control 61 ± 8 years; walking training 64 ± 6 years Sex: male Inclusion criteria: Stable symptoms of IC were recruited from a tertiary centre specialising in vascular disease. Male patients with Fontaine stage II symptoms of IC for longer than 6 months, an ABI < 0.90 at rest in 1 or 2 legs, and who were able to walk for at least 2 min at 3.2 km/h on a treadmill were invited to participate. Exclusion criteria: obesity (BMI < 30 kg/m2); use of beta‐blockers, non‐dihydropyridine calcium channel blockers, or peripheral vasodilators; inability to obtain the ABI; exercise tolerance limited by factors other than claudication (i.e. arrhythmias, cardiac symptoms, or exaggerated blood pressure rise); electrocardiogram response suggestive of myocardial ischaemia, and h/o revascularisation in the previous year |
|
| Interventions | Treatment: 15 × 2‐min walking bouts, with 2‐min intermittent rest periods. Exercise intensity was adjusted to maintain heart rate within 4 bpm above or below the heart rate of claudication pain onset (e.g. if the patient reported claudication pain onset in the maximal treadmill test at 100 bpm, the heart rate exercise zone was set at 96 to 104 bpm). Treadmill speed was set at 3.2 km/h, while the grade was adjusted to achieve the target heart rate. Twice‐weekly 12‐week walking exercise training programme Control: stretching exercise classes Duration: 12 weeks |
|
| Outcomes | Primary: to analyse the pain, cardiovascular and metabolic responses experienced by patients during walking exercise performed at the heart rate corresponding to claudication pain onset, and to investigate the effects of a 12‐week walking training programme at this intensity on walking capacity in patients with IC
Secondary: to determine whether low‐volume walking exercise training programme at this intensity would induce walking performance improvements in this patient group Graded treadmill test: PFWD, MWD |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised into 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants given the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants from exercise group lost to follow‐up only. Baseline demographics between 2 groups matched in spite of attrition |
| Selective reporting (reporting bias) | Low risk | All stated |
| Other bias | Unclear risk | Small numbers |
Dahllof 1974.
| Methods | Study design: RCT Method of randomisation: states "randomised". Not blinded Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: Sweden Setting: not stated No. of participants: 18 Age: 54 to 71 years Sex: male and female Inclusion criteria: IC for > 1 year Exclusion criteria: DM or IHD |
|
| Interventions | Treatment: 30‐min training sessions 3 times weekly, supervised by a physiotherapist, including dynamic leg exercises beyond the appearance of pain Control: placebo tablets Duration: 6 months |
|
| Outcomes | Primary: treadmill test pain‐free and maximal walking distance (max 1000 m), at 4 km/h Secondary: calf blood flow ‐ venous occlusion plethysmography after ischaemic foot exercises |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, nil else reported |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded to tablets, but not to exercise |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Physiotherapist ran exercise aspect. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of whether all enrolled completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes stated |
| Other bias | Unclear risk | Small numbers (n = 18) |
Gardner 2002.
| Methods | Study design: RCT Method of randomisation: not described Exclusions post randomisation: nil Losses to follow‐up: 3 in treatment group, 6 in control group dropped out. More at 18 months. 31 remained. |
|
| Participants | Country: USA Setting: vascular clinics, newspaper and radio adverts No. of participants: 61 originally, 31 remained at 18 months Age: over 60 years Sex: 90% male Inclusion criteria: Fontaine II PAD Rose Questionnaire. ABI < 0.97 at rest. IC limiting factor on treadmill Exclusion criteria: other significant medical conditions limiting exercise tolerance, poorly controlled DM |
|
| Interventions | Treatment: 30‐min training sessions 3 times weekly for 6 months, then twice weekly for 12 months Control: usual care Duration: 6 months and 18 months |
|
| Outcomes | Treadmill distance to claudication Maximum claudication distance ABI Peak oxygen uptake Walking economy Six‐minute walking test distance Accelerometer‐derived physical activity Walking Impairment Questionnaire Calf blood flow Health‐related quality of life on SF‐36 Self‐perceived ambulatory measures | |
| Notes | Participants performed a progressive, graded treadmill protocol (2 mph, 0% grade with 2% increase every 2 minutes) until maximal claudication pain. Distance walked to onset of claudication pain, distance walked to maximal claudication pain, time to relief of claudication pain after the test, and peak oxygen uptake were measured. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants given the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated in paper |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numbers for attrition provided for all outcomes except the HRQoL, but reasons not stated 61 originally, 31 remained at 18 months: high loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported on |
| Other bias | Low risk | No evidence of other bias |
Gelin 2001.
| Methods | Study design: RCT Method of randomisation: block, computer Exclusions post randomisation: nil stated Losses to follow‐up: 39 (13 control, 15 training, 11 invasive therapy) did not complete evaluation at 1 year. A total of 90 patients did not complete treatment as allocated, although analysis was based on intention to treat. 15 were unsuitable for surgery and 17 received angioplasty in surgery arm. 25 withdrew from quality of life section. | |
| Participants | Country: Sweden Setting: vascular out‐patients No. of participants: 253 Age: mean 67 (range 45 to 81) years Sex: 67% male Inclusion criteria: stable IC for > 6 months, ABI < 0.6 Maximum postischaemic blood flow < 25 mL/min/100 g, willing to undergo operations Exclusion criteria: contraindication to surgery; other disorder limiting treadmill walking | |
| Interventions | Treatment: • Supervised exercise training 30 min 3 times per week for 6 months, then 6 to 12 months 2 sessions per week • Invasive surgery/endovascular procedure, based on angiographic findings Control: observation only Duration: 1 year | |
| Outcomes | Primary: mortality, ABI, amputation, treadmill distance; max postischaemic calf blood flow, big toe systolic pressure Secondary: blood pressure, haemoglobin, cholesterol, triglycerides, creatine, quality of life | |
| Notes | Quality of life (data for 171 only, 18 changed group but were analysed in original)
Randomised before pretreatment investigations. Low compliance in exercise group but classes offered for longer than most studies
Results for ABI omitted standard deviations. Walking test was performed on a treadmill with a progressively inclinating slope from 0° to 12°, simulating a gradually increasing workload. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised into 3 groups 'utilising a computer based algorithm, taking 21 assumed long‐term prognostic variables into account' |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by an independent nurse, who communicated the allocation group to the responsible physician |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated in paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data clearly reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | No competing interests declared. Funding transparent. High number of cross‐overs described and discussed. However, greater degree of treatment dropouts in the exercise group; total numbers for this section (58%); has the potential to bias reported outcomes (PTA/surgery 80%) |
GOALS 2013.
| Methods | Study design: RCT Method of randomisation: parallel‐design randomised controlled clinical trial Exclusions post randomisation: 5 lost interest (control), 8 lost interest in exercise group Losses to follow‐up: in CONSORT diagram; 3 deaths overall |
|
| Participants | Country: USA Setting: community No. of participants: 194 Age: 69.3 (9.5) exercise group; 71.0 (9.6) control group Sex: 49 (50.5%) men in exercise group; 48 (49.5%) in control group Inclusion criteria: ABI ≤ 0.90 in either leg. Individuals with a resting ABI ≥ 0.91 to ≤ 1.00 at baseline were eligible if their ABI dropped by at least 20% after a heel‐rise test. Individuals with a resting ABI > 0.90 were eligible if they provided medical record documentation of lower extremity revascularisation or evidence of PAD from an accredited vascular laboratory. Exclusion criteria: potential participants with a below‐ or above‐knee amputation; wheelchair confinement; inability to walk at least 50 feet without stopping; use of a walking aid other than a cane; inability to attend weekly sessions; walking impairment for a reason other than PAD, foot ulcer, or critical limb ischaemia; significant visual or hearing impairment; non‐completion of the study run‐in (attendance at 2 weekly health education sessions over a 3‐week period); major surgery or lower extremity revascularisation during the previous 3 months or planned during the next 12 months; major medical illness including cancer treatment during the prior 12 months; current participation in another clinical trial or in another exercise trial within the past 3 months; completion of cardiac rehabilitation during the past 3 months; Parkinson disease; requirement of oxygen with activity or exercise; determination that exercise may be unsafe including having more than a class II New York Heart Association level of heart failure or angina; an increase in angina pectoris during the prior 6 months; an abnormal baseline exercise stress test; an exercise level similar to that targeted in the intervention at the time of recruitment; and a Mini‐Mental State Examination score of < 23 at baseline |
|
| Interventions | Treatment: Our intervention applied principles from social cognitive theory, the group dynamics literature, and research on self‐regulation to motivate participants to adhere to home‐based walking exercise. Participants met once weekly for 90 minutes in a group with other PAD participants for the entire 6 months of the intervention. Forty‐five minutes was devoted to facilitator‐led discussions and 45 minutes to walking around an indoor track. Control: The health education control group attended weekly 60‐minute group sessions with other PAD participants. Physicians and other healthcare professionals provided lectures on topics including managing hypertension, cancer screening, and vaccinations. Control group attended weekly lectures about topics not related to exercise. Duration: 6 months |
|
| Outcomes | Primary: 6‐minute walk test Secondary: maximal treadmill walking time, pain‐free treadmill walking time, physical activity, Walking Impairment Questionnaire (WIQ) scores, and Physical Health Composite Score (PCS) and Mental Health Composite Score (MCS) from the 12‐item Medical Outcomes Study Short‐Form Health Survey (SF‐12) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: parallel‐design randomised controlled clinical trial randomised by computer via a randomly permuted block method |
| Allocation concealment (selection bias) | Low risk | Eligible participants were randomised by computer via a randomly permuted block method, stratifying by baseline 6‐minute walk performance. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes were measured before randomisation and at 6‐month follow‐up by assessors unaware of participants’ group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up rates were 90.7% and 92.8% in the intervention and control groups. After exclusion of 3 participants who died before follow‐up testing, follow‐up rates were 91.7% and 94.7%. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Multiple imputation was used by study authors to account for missing data at 6‐month follow‐up, including additional analyses in which a value of zero was assigned for missing 6‐month follow‐up data for decedents. |
Guidon 2010.
| Methods | Study design: RCT Method of randomisation: "randomly allocated" Exclusions post randomisation: nil stated Losses to follow‐up: 13 withdrew before follow‐up |
|
| Participants | Country: Ireland Setting: not stated No. of participants: 44 initially randomised, 30 completed study Age: 67 ± 8.12 years Sex: 70.5% male Inclusion criteria: IC with ABI < 0.9 Exclusion criteria: nil stated |
|
| Interventions | Treatment: twice‐weekly supervised exercise programme for 12 weeks Control: usual care Duration: 12 weeks |
|
| Outcomes | Walking Impairment Questionnaire Intermittent Claudication Questionnaire SF‐36 |
|
| Notes | RCT performed by School of Physiotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated"; no further information available |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25% lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Only WIQ and ICQ discussed for 30/44 participants |
| Other bias | Unclear risk | RCT performed by School of Physiotherapy: who will require funding and may therefore be undertaking the study to provide weight for sustaining or undertaking additional classes |
Hiatt 1990.
| Methods | Study design: RCT Method of randomisation: randomised in pairs by coin toss Exclusions post randomisation: 6 were discontinued from the study. 4 treated participants were discontinued: 3 for non‐compliance with exercise training sessions and 1 for development of medical problems unrelated to PAD. Two control participants were discontinued: 1 for medical problems not due to PAD and the second for progression of arterial disease, as previously defined. Losses to follow‐up: nil |
|
| Participants | Country: USA Setting: not stated No. of participants: 19 (25 enrolled, 6 discontinued); 10 treatment and 9 control Age: mean 61 years (treatment), 59 years (control) Sex: all male Inclusion criteria: IC due to PAD (ABI < 0.95 at rest or < 0.85 after exercise) Exclusion criteria: critical limb ischaemia; resting ankle blood pressure < 50 mmHg; unable to walk on the treadmill at a speed of 2 mph or an exercise capacity limited by angina, congestive heart failure, COPD, or arthritis; DM; vascular intervention in previous year; treatment with beta‐blockers or pentoxifylline |
|
| Interventions | Treatment: programme of exercise 3 times each week (5 min warm up, 50 min intermittent isotonic resistive exercise, 5 min cool down) Control: maintain usual level of exercise Duration: 12 weeks |
|
| Outcomes | Primary: treadmill test maximal walking time (2 mph at 0% slope, with a subsequent 3.5% increase in slope every 3 min until forced to stop) Secondary: calf blood flow venous occlusion plethysmography, concentration of plasma carnitine at rest Subjective indicators: perceived pain during exercise; walking‐limited distance |
|
| Notes |
Hiatt 1990 included distance walked and claudication pain. Unfortunately these results were not reported by treatment group but simply correlated with treadmill performance. The correlation was good; therefore it may be assumed that significant improvement was experienced by those receiving exercise therapy. Treadmill test: graded treadmill test (2 mph 0% grade, with a subsequent 3.5% increase in grade every 3 minutes) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | High risk | Coin toss |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only for the carnitine analysis |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All outcome data clearly reported. 25 enrolled, 6 discontinued (24% lost) |
| Selective reporting (reporting bias) | High risk | Maximum walking distance and claudication pain not reported for individual treatment group but correlated with treadmill performance. |
| Other bias | Unclear risk | Small numbers (n = 19) |
Hiatt 1994.
| Methods | Study design: RCT Method of randomisation: not stated Exclusions post randomisation: 3 did not consent for muscle biopsy Losses to follow‐up: 2 in control group |
|
| Participants | Country: USA Setting: not stated No. of participants: 29 Age: mean 67 years ± 6 years Sex: male only Inclusion criteria: 3‐month h/o stable IC (Rose Questionnaire) limiting exercise sufficiently to affect ability to perform routine activity; ABI < 0.94 at rest, < 0.73 after exercise Exclusion criteria: rest pain, ulcer, gangrene; inability to walk on treadmill at > 2 mph; exercise limited by angina, CHF, COAD, or arthritis; no DM, vascular surgery, or PTA in previous year |
|
| Interventions | Treatment: supervised treadmill walking exercise (n = 10) or strength training (n = 9) and encouraged to walk alone for 2 days each week (treadmill 1 h 3 times a week, walking until moderate pain, then rest) Control: maintain usual level of activity (n = 10) Duration: 12 weeks |
|
| Outcomes | Primary: treadmill test pain‐free and maximal walking distance (2 mph, 0% slope, with a subsequent 3.5% increase in slope every 3 min until forced to stop) ABI (resting and post exercise) | |
| Notes | Trial continued after 12 weeks without a control group; therefore later results not included Strength training was less effective than treadmill exercise; only the latter was included in the meta‐analysis for statistical reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Small numbers |
Hobbs 2005.
| Methods | Study design: RCT Method of randomisation: 2 × 2 factorial design, random number table Exclusions post randomisation: not reported Losses to follow‐up: 4 of original 38 withdrew |
|
| Participants | Country: UK Setting: university department of vascular surgery, patients referred to IC clinic from primary or secondary care No. of participants: 34 Age: median 67 (63 to 72) years Sex: 27 men, 7 women Inclusion criteria: IC diagnosed by Edinburgh Claudication Questionnaire and reduced ABI < 0.9, reviewed after 3 to 6 months; max walking distance 20 to 500 m Exclusion criteria: significant aorto‐iliac disease, inability to complete treadmill distance to absolute claudication distance, MI, transient ischaemic attack, stroke or PTA in past 3 months, CHF, bleeding diathesis, glomerular filtration rate < 20 mL/min, CYP3A4 or CYP2C19 inhibitor use |
|
| Interventions | Treatment:
• Supervised exercise: 3‐month, twice‐weekly 1‐hour physiotherapist‐led exercise programme. Given videotape of programme and encouraged to take log of exercise at home
• Cilostazol 100 mg twice daily, if side effects dosing halved for 1 week
• Exercise as above plus cilostazol Control: best medical therapy Duration: 6 months |
|
| Outcomes | MWD Pain‐free MWD ABI Thrombin antithrombin complex Prothrombin fragments 1 and 2 Plasminogen activator inhibitor Tissue plasminogen activator | |
| Notes | Study authors contacted for means and SDs ‐ received for BMT and supervised exercise groups Treadmill test: 3 km/h, 10% incline to MWD or 1000 m |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 2 × 2 factorial design randomisation, random number table |
| Allocation concealment (selection bias) | Low risk | Eligible participants were randomised in a 2 × 2 factorial design to continue BMT only or to receive BMT + supervised exercise, BMT+ cilostazol, or BMT + supervised exercise + cilostazol. The 2 × 2 factorial design is well recognised as one of the most robust study designs and allows for greater interrogation of the data, as well as allowing interaction of different treatments to be assessed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated in paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data clearly reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Small numbers |
Jansen 1991.
| Methods | Study design: RCT Method of randomisation: not described Exclusions post randomisation: not described Losses to follow‐up: not described |
|
| Participants | Country: Germany Setting: community No. of participants: 48 Age: not described Sex: not described Inclusion criteria: PAD stage II Exclusion criteria: not stated |
|
| Interventions | Treatment: training on treadmill 3.5 km/h 10% slope for 2 hours twice per week under medical/physiotherapy supervision Control: no training Duration: 2 years |
|
| Outcomes | Primary: treadmill walking distance, pain‐free and maximum Secondary: ultrasound Doppler of arm and leg arteries |
|
| Notes | Treadmill test: 3.5 km/h, 10% incline Translated from German |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further information available |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of whether all enrolled completed the study |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes stated |
| Other bias | Low risk | No evidence of other bias |
Kakkos 2005.
| Methods | Study design: RCT Method of randomisation: "blind block telephone procedure" by means of computer Exclusions post randomisation: 2 participants in the IPC group withdrew their consent before their 6 weekly appointments Losses to follow‐up: 4 in supervised exercise group; 4 in IPC group |
|
| Participants | Country: England Setting: vascular out‐patient clinic No. of participants: 34 Age: median and IQR: 66 (10.5) unsupervised exercise (USE) group; 69 (11.8) supervised exercise (SE) group; 66 (7) intermittent pneumatic compression (IPC) group Sex: 27 M in total; 8 M USE group, 11 M SE group, 8 M IPC group Inclusion criteria: stable IC for > 6 months due to superficial femoral artery occlusion of ≥ 6 cm in length on ultrasound and/or angiogram Exclusion criteria: duration of symptoms < 6 months, previous angioplasty or arterial surgery to symptomatic leg, MI within previous 6 months, inability to manage treadmill or training, any psychiatric illness or other reason making follow‐up difficult, ischaemic rest pain, gangrene, ischaemic ulceration, inability to attend supervised programme, severe peripheral neuropathy, ABI > 0.9 at enrolment, non‐compressible calf arteries, iliac occlusions or stenoses amenable to surgery or angioplasty, femoral artery occlusion < 6 cm; exercise capacity limited by angina, congestive heart failure, COPD, disease of spinal column, venous disease, neurological disease, mental illness, or arthritis |
|
| Interventions | Treatment: • Supervised (n = 12): 5‐minute warm‐up, 50‐minute intermittent exercise, 5‐minute cool‐down. Attendance was 3 times a week for 6 months. • Unsupervised exercise (n = 9); advised to walk for approximately 45 minutes each day • Pneumatic foot compression (n = 13); to be used daily for 3‐hour periods Duration: 6‐month treatment period plus further follow‐up at 12 months after treatment began (exercise advice given for second 6‐month period) |
|
| Outcomes | Primary: ICD, ACD, ABI Secondary: SF‐36, WIQ, IC Questionnaire |
|
| Notes | Study authors were successfully contacted for means and SDs. Treadmill test: 3.5 km/h, 10% incline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised: blind, block telephone computer generated randomisation, undertaken by Clinical Trials and Evaluation Unit |
| Allocation concealment (selection bias) | Low risk | Independent allocation A blind, block 'telephone' randomisation procedure was performed by means of a computer. Randomisation was performed by the Clinical Trials and Evaluation Unit at the Royal Brompton Hospital in London. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 of 34 discontinued at 6 months (attrition rate of 26%) |
| Selective reporting (reporting bias) | Low risk | All data reported |
| Other bias | High risk | n = 8 per group. Power calculation required 15 participants per group. |
Larsen 1966.
| Methods | Study design: RCT Method of randomisation: randomised in pairs matched by age and disease, not blinded Exclusions post randomisation: 0 Losses to follow‐up: 0 |
|
| Participants | Country: Denmark Setting: not stated No. of participants: 14 Age: 44 to 65 years Sex: male and female Inclusion criteria: typical IC, stable for > 6 months Exclusion criteria: none stated |
|
| Interventions | Treatment: instructed to walk daily, in addition to normal activities Control: 1 placebo (lactose) tablet bd Duration: 6 months |
|
| Outcomes | Primary: treadmill test MWD (4.6 km/h, elevation of 0, 8, or 16 cm/m) Secondary: calf blood flow xenon clearance method |
|
| Notes | Treadmill test differed for each participant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in pairs matched by age and disease. Not blinded |
| Allocation concealment (selection bias) | High risk | Not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants to exercise |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes stated |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Treadmill test different for each participant, small numbers (n = 14) |
Leicht 2011.
| Methods | Study design: RCT Method of randomisation: (info from study author) Consecutive IC patients were randomly allocated to each group via sealed envelopes that were developed by a researcher not associated with the study. Exclusions post randomisation: nil stated Losses to follow‐up: described in detail in CONSORT chart (Leicht 2011) |
|
| Participants | Country: Australia Setting: local hospital and university centre No. of participants: 25 PAD, 24 healthy age‐matched controls Age: 66.9 years (± 8 years) Sex: 14 male (56%) Inclusion criteria: PAD was confirmed based on absence of lower limb peripheral pulses, lower limb artery stenosis, or occlusion on duplex or computed tomographic angiography, and ankle brachial index (ABI) < 0.94. Exclusion criteria: inability to attend potential regular supervised exercise (n = 48), selected for surgical or endovascular intervention (n = 30), declined (n = 20), other medical condition influencing gait (n = 20), exhibited significant ectopy at rest (n = 3) |
|
| Interventions | • Conservative medical treatment (n = 13) • Supervised exercise (n = 12) consisted of treadmill walking 3 days per week for 25 to 40 minutes per day at a workload that induced intense to maximal claudication pain. Control: healthy age‐matched controls (n = 24) Duration: 12 months |
|
| Outcomes | Primary: to compare heart rate variability (HRV) in patients with IC and in age‐matched healthy adults Secondary: to examine the influence of an intense long‐term (12‐month) exercise programme on HRV in patients with IC PFWD, MWD, ABI |
|
| Notes | Treadmill test: 3.2 km/h and incline of 0%. The incline increased by 2% every 2 minutes until voluntary exhaustion or a maximum time of 25 minutes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, consecutive IC patients were randomly allocated to each group via sealed envelopes that were developed by a researcher not associated with the study. |
| Allocation concealment (selection bias) | Low risk | Consecutive IC patients were randomly allocated to each group via sealed envelopes that were developed by a researcher not associated with the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data reported |
| Selective reporting (reporting bias) | Low risk | All outcome data reported |
| Other bias | Unclear risk | Small numbers |
Mannarino 1991.
| Methods | Study design: RCT Method of randomisation: states random. Not blinded Exclusions post randomisation: not stated Losses to follow‐up: none |
|
| Participants | Country: Italy Setting: not stated No. of participants: 20 Age: 48 to 75 years Sex: male and female Inclusion criteria: IC for > 2 years, stable for past 3 months, pain‐free walking distance < 300 m Exclusion criteria: h/o angina, recent MI, or stroke; vascular surgery or PTA in previous 6 months; impaired cardiac or lung function; major liver, kidney, or metabolic disorders; infection; cancer; peptic ulcer |
|
| Interventions | Treatment: 1 h home exercises daily supervised via out‐patients. Week 1: 500 m in 20 min; week 2: 1000 m in 40 min; week 3: 2000 m in 60 min Control: dipyridamole 75 mg tds plus aspirin 330 mg od Duration: 6 months |
|
| Outcomes | Primary: treadmill test pain‐free and maximal walking time (2 km/h on 12° slope), ABI Secondary: calf blood flow strain‐gauge plethysmography |
|
| Notes | A third group of 10 participants received antiplatelet treatment and exercise. These results are discussed but are not formally included in a meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further information available |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of whether all enrolled completed the study |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Small numbers |
McDermott 2008.
| Methods | Study design: RCT Method of randomisation: parallel assignment by computer via a randomly permuted block method; outcome assessor blinded Exclusions post randomisation: not described Losses to follow‐up: described in flow chart (McDermott 2008) |
|
| Participants | Country: Chicago Setting: urban academic medical centre No. of participants: 156 Age: 70.6 ± 10.3 years Sex: male and female Inclusion criteria: 150 peripheral arterial disease patients with and without IC Exclusion criteria: above or below knee amputation, wheelchair confinement, inability to walk on a treadmill or perform progressive resistance training, inability to attend 3 times a week for 6 months, Class II NYHA heart failure or angina at rest or on minimal exertion, silent coronary ischaemia (ST depression of 1 mm) during baseline exercise test, ST‐T wave changes or LBBB on baseline ECG, walking impairment not attributed to PAD, planned lower extremity revascularisation or major surgery within 12 months, MI or CABG during previous 3 months, current foot ulcer, ABI > 0.95, life expectancy < 12 months, does not speak English, dementia, poorly controlled BP, treated for cancer in the past 12 months, current significant exercise |
|
| Interventions | Treatment group 1: supervised treadmill exercise (6 months, 3 times a week; followed by a 6‐month home‐based programme). Initially 15 minutes of exercise, increased to 40 minutes by week 8. Initial treadmill walking speed 2.0 mph. Between weeks 8 and 24, intensity was increased weekly; either by grade or by speed, n = 51 Treatment group 2: lower extremity resistance training (6 months, 3 times a week; followed by a 6‐month home‐based programme). Participants performed 3 sets of 8 repetitions of knee extensions, leg press, leg curl exercises. Weights adjusted monthly until lifting 80% of 1 maximum repetition. 3 sets of 8 repetitions of squat and toe rises were also performed, n = 52 (1 dropped out) Control: usual care with diet and nutrition advice (11 sessions lasting 1 hour each over 6 months), n = 53 (1 dropped out) Duration: 6 months |
|
| Outcomes | 6‐Minute walking test distance Short physical performance battery Brachial artery flow‐mediated dilatation Treadmill walking performance distance WIQ SF‐36 physical functioning score |
|
| Notes | Treadmill test: Gardner Skinner Protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised parallel assignment by computer via a randomly permuted block method |
| Allocation concealment (selection bias) | Low risk | Parallel assignment by computer via a randomly permuted block method |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examiners were blinded to participant group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data clearly reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Underpowered. Power calculations assumed that 50 people in each group would complete 6‐month follow‐up, and that 2 separate 2‐sample t‐tests using a 2‐sided alpha of 0.05 would be conducted. The study was designed to have 80% power to detect a difference of 30 meters change in 6‐minute walk distance and a difference of 0.97 change in the SPPB between baseline and 6‐month follow‐up between each exercise and control group. Only 50 completed the SET; 48 were in the control group, and 46 were in the lower extremity resistance group. |
McGuigan 2001.
| Methods | Study design: RCT Method of randomisation: randomly assigned to either of the 2 experimental groups Exclusions post randomisation: 2 participants from the training group and 2 from the control group withdrew from the study because of circumstances unrelated to the investigation. Losses to follow‐up: not stated |
|
| Participants | Country: Australia Setting: not stated No. of participants: 20 (sample size of 18 needed) Age: not stated Sex: male and female Inclusion criteria: ABI < 0.94 at rest that decreased to < 0.73 after exercise Exclusion criteria: • Leg pain at rest • Ischaemic ulceration or gangrene • Inability to walk at least 2 km/h on a treadmill • Limited exercise capacity by factors other than IC (e.g. symptoms of angina, CCF, COPD, arthritis) • Vascular surgery or angioplasty undergone within the previous year • Smoking |
|
| Interventions | Treatment: n = 11. Resistance training program 3 days per week throughout the 24‐week period. Minimum of 48 hours between sessions Control: n = 9 Duration: 24 weeks |
|
| Outcomes | Aims: to investigate effects of resistance training on walking performance, strength, and skeletal muscle adaptation Primary: fibre area and shifts in MHC isoforms Secondary: • Graded treadmill protocol ‐ initially 3 km/h at 0% for 2 min, then increased 2% every 2 min with speed constant • Rate of perceived pain (scale 0 to 10) • Time in seconds to onset of claudication pain or maximal claudication pain • 6‐minute walk • ABI • Fibre type and distribution • Muscle capillarisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. Randomly assigned to either of the 2 experimental groups |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two participants from the training group and 2 from the control group withdrew from the study because of circumstances unrelated to the investigation. Unclear what total numbers were |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Sample size was 18, which was not met, as 4 withdrew. |
Mika 2005.
| Methods | Study design: RCT Method of randomisation: not described Exclusions post randomisation: not described Losses to follow‐up: 18 withdrew (10 control, 8 exercise) |
|
| Participants | Country: Poland Setting: university department out‐patient clinics No. of participants: 98 Age: 50 to 70 years Sex: male and female Inclusion criteria: PAD and IC (Fontaine stage II) stable for 3 months, PFWD 50 to 200 m at 3.2 km/h Exclusion criteria: angina, recent MI, vascular surgery in past 3 months, impaired cardiac or lung function, DM, cancer, kidney and liver disease, arthritis limiting walking, other contraindication to walking, those taking beta‐blockers, pentoxifylline or other haemorheologically active drugs |
|
| Interventions | Treatment: 12‐week programme of supervised pain‐free treadmill exercise, 1 hour per day 3 times a week of repetitive walking exercise Control: usual care Duration: 3 months |
|
| Outcomes | PFWD Total leukocyte count Neutrophil count Microalbuminuria | |
| Notes | Treadmill test: 3.2 km/h, 12% incline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further details available |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor. Testing and analyses were conducted by qualified medical staff blinded to participants' group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data clearly reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Mika 2006.
| Methods | Study design: RCT Method of randomisation: not described Exclusions post randomisation: not described Losses to follow‐up: 5 |
|
| Participants | Country: Poland Setting: university department clinics No. of participants: 60 Age: 50 to 70 years Sex: male and female Inclusion criteria: Fontaine stage II PAD, IC limiting walking and stable for 3 months, ABI < 0.9 Exclusion criteria: angina; recent MI; vascular surgery in past 3 months; impaired cardiac or lung function; DM; cancer, kidney, and liver disease; arthritis limiting walking; inability to walk at 3.2 km/h; taking beta‐blockers, pentoxifylline, or other haemorheologically active drugs |
|
| Interventions | Treatment: 12‐week programme of supervised pain‐free MD treadmill exercise, 1 hour per day 3 times a week of repetitive walking exercise Control: usual care, to maintain normal activity level Duration: 3 months |
|
| Outcomes | Pain‐free walking time Maximum walking time Red cell deformability ‐ erythrocyte elongation index | |
| Notes | Treadmill test: 3.2 km/h 0% for 3 minutes followed by an increase in grade of 3.5% every 3 minutes (speed remained constant) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further details available |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 lost to follow‐up (3 in exercise group, 2 in control group). All data on remaining 55 reported |
| Selective reporting (reporting bias) | Low risk | All data on remaining 55 reported |
| Other bias | Unclear risk | Patients who were unable to walk on the treadmill at a speed of at least 3.2 km/h were also excluded. |
Mika 2011.
| Methods | Study design: RCT Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: 7 dropped out |
|
| Participants | Country: Poland Setting: hospital; vascular out‐patient clinic No. of participants: 68 Age: 63.5 years (training group), 62.1 years (control group) 60 male, 8 female Inclusion criteria: PAD and IC – Fontaine stage II, aged 50 to 70 years, ABI < 0.9 at rest and 0.75 after exercise. All included patients had stable claudication distance and were able to walk no less than 150 m without pain. Exclusion criteria: rest pain, gangrene, ulceration, history of angina pectoris, recent MI or vascular surgery within the previous year, impaired cardiac or lung function, DM, cancer, or kidney and liver disease. Also, patients with arthritis who were unable to walk on the treadmill at a speed of at least 3.2 km/h. Additionally, women in menopausal status and those taking oestrogen were excluded because of the possible effects of these factors on HDL cholesterol level. None of the participants in the study was taking beta‐blockers. |
|
| Interventions | Treatment: 12‐week SEP, sessions conducted in the morning, 3 times per week Repetitive walking exercise with 3‐min resting intervals. During each session, after 5 min of warm‐up activities (free cycling on a stationary cycle ergometer), participants walked on the treadmill at a speed of 3.2 km/h at a grade that induced claudication pain within approximately 3 to 5 min Control: no change in physical activity All study participants were encouraged to stop smoking. Their diet was neither controlled nor modified throughout the study period. Duration: 3 months |
|
| Outcomes | Pain‐free walking time (PFWT) Maximal walking time Haematocrit Plasma lipoproteins |
|
| Notes | Graded treadmill protocol (Gardner protocol): 3.2 km/h throughout the test, but the inclination from 0% (during initial stage) was raised by 2% every 2 min until maximal claudication pain occurred; without handrail | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to either an intervention group (n = 34) or a control group (n = 34) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treadmill testing and biochemical analyses were conducted by medical staff blinded to participants' group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 dropouts discussed; rest of data complete |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | During an initial visit, participants were familiarised with treadmill walking (speed 3.2 km/h, 0% inclination) for 10 min. The treadmill test was repeated on the next day, and the mean of 2 measurements was used in data analysis. The treadmill was calibrated before each testing. Participants were instructed not to use handrail support during the test. |
Sanderson 2006.
| Methods | Study design: RCT Method of randomisation: closed envelope system Exclusions post randomisation: nil Losses to follow‐up: 1 in treadmill group |
|
| Participants | Country: Australia
Setting: not stated No. of participants: 42 Age: mean 63 years Inclusion criteria: claudication lasting > 1 year, ABI < 0.9 Exclusion criteria: reduced cardiac function, rest pain, recent surgery or cardiac event, other medical conditions rendering exercise unsuitable |
|
| Interventions | Treatment:
• Treadmill exercise • Cycling Both groups exercised 3 times a week for 6 weeks Control: no exercise Duration: 6 weeks |
|
| Outcomes | MWD and PFWD and cycling tests Submaximal and peak physiological response ABI | |
| Notes | Stratified by gender, presence of diabetes, then randomised
Results given as mean differences The maximal graded walking test was performed on a motorised treadmill (TrackMaster TMX425CP, Newton, Kan) at a constant speed of 2.7 km/h. The treadmill gradient was set at 0% for the first 5 minutes of the test, then it was increased by 2% every 3 min until the participant failed to sustain the task. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Closed envelope system |
| Allocation concealment (selection bias) | Low risk | Closed envelope system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated in paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data clearly reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Schlager 2011.
| Methods | Study design: RCT single centre Method of randomisation: computer‐generated random digits in sealed envelopes (block‐wise randomisation by 2) Exclusions post randomisation: nil Losses to follow‐up: 1 (MI 3 months after study inclusion) |
|
| Participants | Country: Austria Setting: single‐centre hospital No. of participants: 40 Age: mean age of 69.1 years (standard deviation of ± 9.5 years) Inclusion criteria: symptomatic PAD (Rutherford category I to III), diagnosed by clinical evaluation, oscillometric pulse wave measurements, ABI, duplex sonography, and or CT or MR angiography Exclusion criteria: asymptomatic PAD, critical limb ischaemia and reduced exercise tolerance caused by other limitations than claudication (coronary artery disease, congestive heart failure, dyspnoea, uncontrolled blood pressure, any kind of restriction of the musculoskeletal system, which might have an influence on the efficiency of exercise training) |
|
| Interventions | Treatment: SEP with BMT (twice‐weekly programme for 6 months). Warm‐up period of 5 to 10 min; initial duration included 35 min of intermittent walking, which was increased by 5 min each session until 50 min of intermittent walking was accomplished. The workload of exercise training was set to a walking speed that elicited claudication symptoms within 3 to 5 min. Control: BMT Duration: 12 months |
|
| Outcomes | Endothelial progenitor cells (CD34+, CD133+, KDR+) Plasma levels of vascular endothelial growth factor (VEGF), asymmetrical dimethylarginine (ADMA), stromal cell derived factor‐1 (SDF‐1) Maximum walking distance |
|
| Notes | Treadmill test: 3.2 km/h, 12% incline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random digits in sealed envelopes (block‐wise randomisation by 2) |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random digits in sealed envelopes (block‐wise randomisation by 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treadmill exercise tests were routinely performed by 2 experienced medical technical assistants, who were blinded to the assigned treatment arm and otherwise were not involved in the study. Participants were instructed to report all symptoms and discomforts (claudication and other than claudication) occurring during the test, but assistants refrained from encouraging participants during treadmill exercise tests. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One out of 40 participants did not complete the study. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Tew 2009.
| Methods | Study design: RCT Method of randomisation: computer programme (nQuery Advisor 6.0 Statistical solutions) Not blinded Exclusions post randomisation: none Losses to follow‐up: 2 withdrew from the exercise group, and 4 withdrew from the control group; 1 participant died of a heart attack, 1 developed a lower limb ulcer that required revascularisation surgery, 1 was identified as having a popliteal artery aneurysm, and 1 returned to full‐time employment. The remaining 2 participants cited lack of time as their reason for withdrawal. |
|
| Participants | Country: England Setting: single‐centre hospital No. of participants: 57 Age: 69 ± 9 years Sex: male and female Inclusion criteria: Fontaine stage II PAD, 12‐month history of stable IC, ambulation limited by IC, resting ABI < 0.90 or ABPI drop > 15 mmHg post maximal walking exercise Exclusion criteria: absence of PAD, ABI unobtainable owing to incompressible vessels, Fontaine I or III, exercise limited by cause other than IC, history of IC < 12 months, revascularisation/other major surgery within past 12 months, pharmacological therapy specifically for IC (e.g. cilostazol) |
|
| Interventions | Treatment:
• Arm crank exercise (twice‐weekly exercise programme for 12 weeks at an intensity of 60% to 70% of the peak work rate achieved of the initial arm crank assessment. Patients trained in cycles of 2‐minute exercise at 50 rev/min followed by 2 min of rest for a total of 40 min/session) Control: • Non‐exercise group Duration: 3 months |
|
| Outcomes | Primary: lower limb oxygen delivery Secondary: NIRS, peak VO2 kinetics |
|
| Notes | Treadmill test: 3.2 km/h, 0% grade with 1% increase every 1 min | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised via a computer programme (nQuery Advisor 6.0; Statistical Solutions) |
| Allocation concealment (selection bias) | Low risk | Computer programme (nQuery Advisor 6.0; Statistical Solutions) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 from the exercise group and 4 from the control group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Methodological limitations need to be considered: NIRS data;exact contribution of intracellular myoglobin to the StO2 signal is unclear, and subcutaneous fat thickness changes might have influenced findings. No relationship exists between calf skinfold and calf muscle StO2 during walking in patients with IC. |
Tew 2015.
| Methods | Study design: RCT Method of randomisation: randomised ratio 2:3. Achieved via a block randomisation sequence (block size 10) generated before recruitment Exclusions post randomisation: none Losses to follow‐up: 1 lost to follow‐up, all accounted for within CONSORT diagram |
|
| Participants | Country: UK Setting: not stated No. of participants: 23 Age: > 18 Sex: men and women Inclusion criteria: age > 18 years and stable IC for > 3 months Exclusion criteria: CLI, planned or previous lower‐limb revascularisation, presence of contraindications to exercise (e.g. unstable angina) or co‐morbidities that limited walking to a greater extent than the IC (e.g. severe arthritis); major surgery, MI, or CVA in the previous 6 months |
|
| Interventions | Treatment: usual care + SEDRIC (structured education for rehabilitation in intermittent claudication programme that promotes self‐managed walking in people with IC) Control: usual care (included a brief info leaflet on PAD) Duration: 6 weeks |
|
| Outcomes | Mean daily step count Pain‐free and maximum walking distances 6‐min walking distance WELCH questionnaire, WIQ, EQ‐5D, Intermittent Claudication Questionnaire |
|
| Notes | Gardner incremental treadmill test: 3.2 km/h, 0% grade, with 2% increase every 2 min | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised ratio 2:3. Achieved via a block randomisation sequence (block size 10) generated before recruitment |
| Allocation concealment (selection bias) | Low risk | Implemented by an individual who was not involved in recruitment or data gathering processes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Group allocation was concealed from participants while baseline information was gathered. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All stated |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Baseline data showed that the intervention group had slightly higher levels of physical activity, walking capacity, and quality of life than the control group. |
Tisi 1997.
| Methods | Study design: RCT Method of randomisation: randomised by sealed envelope, weighted 70:40:40 (angioplasty:exercise:observation) 19/25 randomised to PTA had unsuitable lesions, and the technique failed in one other. Not blinded Intention‐to‐treat analysis Exclusions post randomisation: none stated Losses to follow‐up: none stated |
|
| Participants | Country: England Setting: general hospital No. of participants: 67 Age: mean 69.3 years Sex: male and female Inclusion criteria: stable IC > 6 months, positive Edinburgh Claudication Questionnaire, ABI < 0.8 and > 3 0 mmHg drop in ankle systolic pressure on exercise, walking distance 50 to 250 m on treadmill (3 km/h, 10% gradient) Exclusion criteria: intervention for IC in past 6 months, exercise limited by other factors, concurrent medical disease, treatment with steroids, inability to complete assessment visits |
|
| Interventions | Treatment:
• Exercise ‐ series of active and passive leg exercises performed to the limit of exercise pain, supervised by a physiotherapist once weekly for 4 weeks. Also encouraged to exercise for 45 min daily at home, plus to walk 1 mile a day; n = 22 • PTA, n = 28 • Control: observation, plus advice given to all 3 groups (leaflet advising on weight loss, smoking, and exercise, plus 75 mg aspirin daily), n = 17 • Healthy controls, n = 15 Duration: 12 months |
|
| Outcomes | Primary: treadmill test ‐ PFWD and MWD (3 km/h on 10% slope); ABI Secondary: Nottingham Health Profile |
|
| Notes | Results for the exercise versus angioplasty comparison have not been included in this review. Claudication and walking distance results not in usable format | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by sealed envelope, weighted 70:40:40 (angioplasty:exercise:observation) |
| Allocation concealment (selection bias) | Unclear risk | Randomised by sealed envelope, weighted 70:40:40 (angioplasty:exercise:observation); opacity of envelopes not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated in paper |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 22 enrolled at baseline, 10 were present at 12 months in the exercise group. In the observation group, n = 17 at baseline, and n = 9 at 12 months. No reasons given for loss to F/U |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Tsai 2002.
| Methods | Study design: RCT Method of randomisation: not described Exclusions post randomisation: not described Losses to follow‐up: 11 ‐ 5 in treatment group, 6 in control group |
|
| Participants | Country: Taiwan Setting: community No. of participants: 64 Age: 76 years ± 4 Sex: 81% male Inclusion criteria: Fontaine II PAD on Rose Questionnaire. ABI < 0.95 Exclusion criteria: intervention for IC in past 3 months, exercise limited by other factors, rest pain, MI or unstable claudication in the past 3 months, history of angina on exertion |
|
| Interventions | Treatment: 12‐week progressive rehabilitation programme, 3 times a week. Up to 30 minutes on the treadmill Control: usual care Duration: 12 weeks |
|
| Outcomes | Time to onset of pain Time to maximum pain 6‐Minute walking test distance WIQ Physical function Bodily pain Role limitation physical and emotional General health Mental health Social function Vitality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further details available |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data clearly reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Wood 2006.
| Methods | Study design: RCT Method of randomisation: not stated by paper Not blinded Exclusions post randomisation: none stated Losses to follow‐up: nil stated |
|
| Participants | Country: Australia Setting: vascular out‐patient clinic at the Royal Brisbane and Women's Hospital No. of participants: 18 Age: 56 ± 9 control, 64 ± 6 treatment Sex: male and female Inclusion criteria: history (> 6 months) of stable PAD and IC Exclusion criteria: rest pain, unstable angina and/or uncontrolled hypertension, experienced loss of consciousness as a result of dizziness, had a bone or joint problem that could be exacerbated by exercise, had undergone vascular surgery within the past 6 months, had suffered a cerebrovascular or coronary event in the past 12 months, or resided more than 1 h by car from the laboratory. Patients not limited by claudication and those who experienced angina or demonstrated ischaemic ECG abnormalities during exercise were excluded from the study. |
|
| Interventions | Treatment: • 6 weeks of treadmill walking training, three 40‐min supervised walking training sessions/week (n = 7) Control: sedentary control (n = 6) Participants in both groups were asked to continue their normal daily activities. Duration: 6 weeks |
|
| Outcomes | • To determine whether plasma vascular endothelial growth factor (VEGF) increases in response to acute exercise in patients with IC, given the potentially large hypoxic stimulus for VEGF release during exercise in this population • To determine whether this response is attenuated following 6 weeks of high‐intensity exercise training |
|
| Notes | Treadmill test: 2.7 km/h, 0%, with grade increased 2% every 3 min | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In part B participants were randomised to one of two study arms". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to bind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | n = 6 for control and n = 7 for training. Nil lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Small numbers in each arm |
Zwierska 2005.
| Methods | Study design: RCT Method of randomisation: Randomisation was undertaken via the fishbowl technique (Baumgartner 1998). This involved drawing patient names out of a 'hat' and allocating them to respective groups at random. Randomisation was undertaken by an independent academic based at another site in Sheffield. Intention‐to‐treat analysis: yes Exclusions post randomisation: nil Losses to follow‐up: 10 |
|
| Participants | Country: UK Setting: not stated No. of participants: 104 Age: median 69 (50 to 89) Sex: 81% male in leg group, 78% in arm group, 73% in control group Inclusion criteria: stable symptomatic peripheral arterial disease Exclusion criteria: not stated |
|
| Interventions | Treatment:
• Supervised upper limb aerobic exercise
• Supervised lower limb aerobic exercise Control: no exercise Duration: 24 weeks |
|
| Outcomes | ICD MWD Peak heart rate Peak oxygen consumption Perceived exertion and pain Physical activity status | |
| Notes | Results given as medians ‐ study authors contacted successfully for more information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised Randomisation was undertaken via the fishbowl technique (Baumgartner and Strong 1998). This involved drawing patient names out of a 'hat' and allocating them to respective groups at random. |
| Allocation concealment (selection bias) | Low risk | Randomisation was undertaken by an independent academic based at another site in Sheffield. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment of outcome measures was conducted by the same 2 staff members. Procedures were checked for consistency at random by the lead investigator at Sheffield Hallam University (J.S.), who was blinded to group assignment. Blinding of the 2 staff members who assessed outcome measures was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data clearly reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No evidence of other bias |
ABI: ankle brachial index. ABPI: ankle brachial pressure index. ACD: absolute claudication distance (or maximum walking distance). bd: twice a day. BMI: body mass index. BMT: best medical therapy. bpm: beats per minute. CABG: coronary artery bypass graft. CCF: congestive cardiac failure. CHF: congestive heart failure. CLI: critical limb ischaemia. COAD: chronic obstructive airways disease. COPD: chronic obstructive pulmonary disease. CPAD‐IC: peripheral arterial disease with intermittent claudication control group. CPS: composite pain scale. CT: computed tomography. CTA: computed tomographic angiography. CVA: cerebrovascular accident. DM: diabetes mellitus. ECG: electrocardiography. EQ‐5D: EurQoL Group Quality of Life Questionnaire based on 5 dimensions. HbA1c: glycated haemoglobin. HDL: high‐density lipoprotein. h/o: history of. HRQoL: health‐related quality of life. HRV: heart rate variability. ICQ: Intermittent Claudication Questionnaire. IQR: interquartile ratio. LBBB: left bundle branch block. LDL: low‐density lipoprotein. IC: intermittent claudication. ICD: intermittent claudication distance. IHD: ischaemic heart disease. IPC: intermittent pneumatic compression. MI: myocardial infarction. min: minutes. MR: magnetic resonance. MWD: maximum or maximal walking distance. NIRS: near‐infrared spectroscopy. NO: nitric oxide. NYHA: New York Heart Association. od: once a day. PAD: peripheral arterial disease. PFWD; pain‐free walking distance. PTA: percutaneous transluminal angioplasty. RCT: randomised controlled trial. SD: standard deviation. SDF‐1: stromal cell derived factor‐1. SE: supervised exercise. SEP: supervised exercise programme. SET: supervised exercise therapy. SF‐36: Short Form‐36. SPPB: Short Physical Performance Battery. TASC: Trans‐Atlantic Inter‐Society Consensus. tds: three times a day TPAD‐IC: peripheral arterial disease with intermittent claudication treatment group. USE: unsupervised exercise. VEGF: vascular endothelial growth factor. vit E: vitamin E. VO2: maximum volume of oxygen. WELCH: Walking Estimated‐Limitation Calculated by History. WIQ: Walking Impairment Questionnaire.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 2010 | Compares home and supervised based programmes with a focus on nitrite flux |
| Andreozzi 2008 | All 4 study arms utilised exercise. |
| Aruna 2015 | Unclear whether symptomatic PAD. Methods state only that patients had type 2 diabetes mellitus for > 4 years and ABI between 0.90 and 0.40. |
| Beutel 1985 | Both groups included exercise; the RCT compared CO2 mineral baths vs CO2 gas baths. |
| Bronas 2011 | The usual care group was provided with an exercise regimen that participants could decide whether to follow or not. Therefore, the study compares supervised vs non‐supervised exercise. "Control participants also received written instructions on how to exercise independently if they so chose to do". |
| Brotons 2011 | This RCT focussed on all patients with "a diagnosis of ischaemic heart disease, stroke or peripheral arterial disease", and the "primary endpoint was the combination of all‐cause mortality and hospital cardiovascular readmission". Separate outcomes for each group were not provided. |
| Buchwalsky 1974 | 2 training groups |
| Bulling 1991 | Comparison of exercise plus gingko biloba and exercise vs placebo. No non‐exercise control group |
| Cachovan 1999 | Comparison of 2 different sequences of exercise. No non‐exercise control group |
| Carmeli 2004 | Not randomised |
| Cheetham 2004 | 2 exercise groups and no non‐exercise control group |
| Choi 2012 | 2 exercise groups and no non‐exercise control group. "Controlled clinical trial with two intervention arms: calf ergometry and treadmill training". |
| Cina 1996 | Comparison of physical training vs low‐dose heparin and physical training and placebo. No non‐exercise control group |
| CLEVER 2009 | This study compares relative clinical and cost‐effectiveness of invasive revascularisation with stents vs supervised exercise rehabilitation. |
| Collins 2010 | Satisfied inclusion criteria, but data not available in usable format despite attempt to contact trial author |
| Collins 2012 | Comparison of 2 different sequences of exercise. No non‐exercise control group. "Patients were randomized to a traditional walking program or walking with poles program of exercise training". |
| Creasy 1990 | This study compared PTA vs supervised exercise therapy. |
| Crowther 2008 | Supervised exercise vs usual care, which included exercise advice for both groups |
| Cucato 2011 | Methodology not of an RCT: 8 participants randomly underwent 2 experimental sessions: a session of resistance exercise (6 exercises, 3 sets of 12, 10 and 8 repetitions with perceived exertion of 11 to 13 on the 15‐grade Borg scale) and a control session (resting on exercise machines) |
| Cucato 2011a | The aim of this study was to compare effects of walking and strength training on cardiovascular responses assessed at rest and during exercise in patients with intermittent claudication. |
| Cucato 2015 | Participants with IC participated in 2 experimental sessions in a random order. Walking exercise (WE) (15 × 2‐min bouts of WE interpolated with 2‐min rest intervals) and control (standing rest on a treadmill for 60 min). BP, cardiac output (CO: CO2 rebreathing), and cardiovascular autonomic modulation (spectral analysis of heart ratevariability) were assessed before and after both experimental sessions during supine rest, and stroke volume and systemic vascular resistance were calculated. |
| Cunningham 2012 | This trial assessed whether a brief psychological intervention could increase daily walking at 4 months. The control group received usual care plus researcher contact, and the treatment group received usual care and a brief psychological intervention to modify illness and walking beliefs and to develop a personalised walking action plan. |
| Dahllof 1976 | This trial was not fully randomised, as the first 10 of the 34 participants were allocated to exercise before randomisation was introduced. There were several differences between treatment and control groups, including significantly higher cholesterol levels and maximal calf blood flow in the control group. Treatment effects may also have been masked because many control participants spontaneously undertook increased exercise during the trial period. |
| Dantas 2016 | Randomised cross‐over intervention study with outcome measures of systolic BP, diastolic BP, and heart rate |
| Dedes 2010 | Compares calf ergometry vs standard treadmill training (both groups involve exercise) |
| Degischer 2002 | Not a randomised trial, and both groups had exercise |
| Dittmar 1977 | Exercise in addition to drug treatment |
| Ericsson 1970 | This trial compared exercise with no treatment but was excluded from the review because of insufficient evidence that participants were randomised. The paper stated that participants were "divided into two groups", and trialists were approached to confirm the method of allocation. No reply was received; therefore it was decided to exclude results from the review, unless evidence to the contrary becomes available. |
| Ernst 1987 | This trial compared participants on an exercise programme with those not exercised, but allocation was not strictly randomised, as participants were "assigned according to space on the exercise programme". |
| Ernst 1990 | Comparison of treadmill exercise plus pentoxifylline and treadmill exercise plus placebo. No non‐exercise control group |
| EXERT 2009 | Participants in the control group will continue to receive usual care from their regular doctor for treatment of PAD and will be provided with written exercise instructions. |
| EXITPAD 2010 | Both groups have compare supervised exercise vs walking advice. |
| Fakhry 2011 | Home‐based exercise compared to supervised exercise |
| Fitzgerald 1971 | This trial compared an exercise regimen with no treatment, but no evidence in the report indicates that the groups were randomly allocated. The quality of the trial appears generally poor, with variable periods of follow‐up and inclusion of some patients who did not have intermittent claudication. |
| Fowler 2002 | Some patients were asymptomatic, and it was not possible to exclude them for data extraction purposes. |
| Gardner 2005 | 2 exercise groups compared |
| Gardner 2011 | Home‐based exercise compared to supervised exercise, and third control group given walking advice |
| Gardner 2012 | No exercise, usual care control group encouraged to walk more on their own but did not receive specific recommendations regarding an exercise programme during the study |
| Gardner 2014 | 80 participants were randomised to home‐based and supervised exercise programmes. Both groups received training. |
| Gardner 2014a | 180 participants were randomised. The NEXT Step programme and the supervised exercise programme consisted of intermittent walking to mild to moderate claudication pain for 12 weeks, whereas controls performed light resistance training. |
| Gibbellini 2000 | Satisfied inclusion criteria but no usable data, despite attempts to contact trial authors. Results divided into asymptomatic and symptomatic groups, not aggregated |
| Gibbs 2013 | This study is not focussed on patients with PAD. Participants (n = 140) with uncomplicated Type 2 Diabetes Mellitus, and without known cardiovascular disease or PAD, aged 40 to 65 years, were randomised to supervised aerobic and resistance training 3 times per week for 6 months, or to a usual care control group. ABI was measured before and after the intervention. |
| Gottstein 1987 | Both groups received training. |
| Greenhalgh 2008 | Both groups included exercise with the addition of PTA as the modality being investigated. |
| Guidon 2013 | Focused on dropout rate of trials. Recruitment to clinical trials of exercise presents significant challenges in the PAD population owing to the presence of coexisting cardiovascular and cerebrovascular disease, reluctance to exercise due to leg pain, and acceptance of reduced mobility as part of ageing. Early identification in primary care before the onset of significant comorbidity may ameliorate some of these issues. |
| Guirro 2015 | A cross‐over study was carried out with 1 session of each therapeutic resource (high‐voltage electrical stimulation (HVES), continuous short‐wave diathermy, or physical exercise), with a 7‐day washout period between protocols. |
| Hobbs 2006 | This study compared exercise with angioplasty. |
| Hodges 2008 | Groups were given supervised exercise or normal care, which included walking advice. |
| Holm 1973 | Suitable for review but no usable numerical data, despite attempt to contact trial author. Stated significant improvement in walking time in exercise group compared to placebo group |
| Jones 1996 | Comparison of treadmill vs StairMaster. No non‐exercise control group |
| Kiesewetter 1987 | Both groups received intensive physical therapy. No non‐exercise control group |
| Kono 2013 | This study is not focussed on patients with PAD. An observer‐blind randomised controlled trial that enrolled 70 patients (48 men, mean age 63.5 years) with acute non‐cardioembolic mild ischaemic stroke. Participants were allocated in equal numbers to a lifestyle intervention group or a control group. |
| Krause 1976 | Combines exercise with drug |
| Kruidenier 2011 | Treatment groups received angioplasty or exercise and angioplasty. A third group of exercise is required to make the trial eligible for inclusion in the review. |
| Labs 1999 | Comparison of constant‐load and graded‐load treadmill testing with and without beraprost sodium. No non‐exercise control group |
| Lee 2007 | Non‐randomised (clinical and cost‐effectiveness) |
| Leon 2005 | Focussed on cardiovascular disease, not intermittent claudication |
| Lepantalo 1984 | Comparison of exercise plus flunarizine and exercise plus placebo. No non‐exercise control group |
| LIFE Study | Original study is a multi‐centred RCT that compared the ability of a structured physical activity intervention vs a successful ageing intervention to prevent mobility disability in older sedentary people, not specifically those with PAD. |
| Lundgren 1989 | This study compared exercise vs surgical reconstruction. |
| Maejima 2005 | Satisfied inclusion criteria, but no data available after attempt to contact trial authors. Reported improvement in walking time in exercise group at 12 weeks |
| Mannarino 1988 | Controlled, not randomised |
| Mannarino 1989 | This trial compared an exercise regimen with nothing (placebo tablets), but the report suggested that groups were not allocated by an acceptable randomisation method. Trialists have been approached to confirm the method of allocation; therefore it may become possible to include the trial at a later date. |
| Martinez 2009 | 3 groups with different durations of a walking programme |
| Mays 2015 | Participants with PAD randomised to a community‐based walking exercise programme compared to usual care advice. Control group received verbal advice to exercise but no formal training. |
| Mazari 2010 | This trial compares PTA, a supervised exercise programme (SEP), and combined treatment (PTA plus SEP) for intermittent claudication. |
| McDermott 2004 | Participants did not have intermittent claudication. |
| Nawaz 1999 | Both groups received exercise advice. No non‐exercise control group |
| Nawaz 2001 | Comparison of upper limb and lower limb exercise. Separate non‐randomised control group |
| NCT01065740 | Outcome measure of 6‐minute walking distance, not treadmill walking distance |
| NCT01241747 | Treadmill training vs light resistance training without any walking |
| NCT02075502 | Participants with PAD randomised to an exercise programme in the community setting incorporating training, monitoring, and coaching compared with participants who received the standard of care (exercise advice) |
| NCT02879019 | Both groups use exercise. |
| Necker 2003 | Training after angioplasty |
| Nicolai 2010 | This paper compared 4 different graded treadmill protocols in random order. |
| Nielsen 1977 | Both groups had exercise. No non‐exercise control group |
| Nordanstig 2011 | "All patients received verbal training advice and a written programme for IC. The patients were instructed to walk 1/hr day and up to MWD as often as possible". No arm received no exercise advice. |
| Parr 2009 | All groups had exercise, no non‐exercise control group. 3‐armed trial of upper body strength training programme, conventional exercise programme, and walking advice for the control group |
| Patterson 1997 | Comparison of supervised exercise programme plus lectures and home‐based exercise plus lectures. No non‐exercise control group |
| Pinto 1997 | 2 exercise regimens |
| Presern‐Strukelj 200 | Comparison of standard exercise alone and standard exercise plus electrostimulation in amputees with PAD |
| PROPEL study | Comparison between those with and without PAD. Looks at baseline data without exercise |
| Riccioni 2010 | No mention of randomisation in methods |
| Richardson 1991 | This compared rocker‐bottomed shoes vs normal shoes with respect to walking distances. |
| Riebe 2001 | Comparison of 2 progressive treadmill tests. No non‐exercise control group |
| Ritti‐Dias 2010 | No control arm; strength training vs walking |
| Rodrigues 2014 | Randomised cross‐over of participants with PAD who performed 2 experimental sessions: control (C) and resistance exercise (R). Both sessions were identical (8 exercises, 3 × 10 reps), except that R session was performed with intensity between 5 and 7 on the OMNI‐RES scale, and the C session was performed without any load. |
| Saleem 2011 | Each group received exercise training. No non‐exercise control group |
| Savage 2001 | Compared supervised exercise vs home‐based exercise. No non‐exercise control group |
| Scheffler 1991 | Each group received exercise training. No non‐exercise control group |
| Schlager 2011a | Satisfied inclusion criteria but data not available in usable format despite attempt to contact trial author |
| Schoneberger 1994 | Controlled study, not randomised |
| Silvestro 2002 | Age‐matched control group |
| Slordahl 2005 | 2 exercise groups |
| Snabl 1958 | Not a randomised controlled trial |
| Sonaglia 2013 | 2 exercise groups. Eligible PAD La Fontaine IIa‐IIb participants were randomised into 2 groups. Group A was treated with physical therapy plus oral pRLX, 20 ug b.i.d. for 12 weeks, and group B received physical therapy alone. |
| Spronk 2009 | This study compared endovascular revascularisation vs supervised exercise. |
| Stewart 2008 | Supervised exercise vs exercise advice. No control group |
| Streminski 1992 | Satisfied inclusion criteria but data not available in usable format despite attempt to contact trial author. Actovegin vs exercise. Results given as mean change. Reported improvement in pain‐free walking distance in exercise group |
| SUPER study | This study compared initial PTA vs initial supervised exercise therapy. |
| Taft 2004 | This study was not truly randomised and did not contain any outcomes of relevance to this review. |
| Tebbutt 2011 | Satisfied inclusion criteria but data not available in usable format despite attempt to contact trial author |
| Thomson 1999 | 2 exercise groups |
| Treat‐Jacobson 2012 | Participants with IC were assessed before and after a 12‐week progressive, 3 times a week, supervised aerobic arm exercise training programme. No control arm |
| Ventura 1984 | Satisfied inclusion criteria but data not available in usable format despite attempt to contact trial author |
| Walker 2000 | The non‐exercise group was not randomised. |
| Waller 1988 | Comparison of exercise when participants had smoked immediately before treadmill test vs exercise when participants had not smoked before treadmill test |
| Wang 2008 | Treatment: Plantar Flexion Ergometer, this occurred 3 times a week for 8 weeks. Each session lasted 40 minutes with equal time on each leg. Control: received advice in accordance with existing exercise guidelines for PAD patients provided by the AHA |
| Wang 2010 | The study design was that of a cross‐over from control to exercise, not a randomised controlled trial. |
| Winterfeld 1983 | Not a randomised controlled trial |
| Zwierska 2004 | Both groups had exercise. No non‐exercise control group |
ABI: ankle brachial index. AHA: American Heart Association. BP: blood pressure. b.i.d.: twice daily. CO2: carbon dioxide. IC: intermittent claudication. MWD: maximum walking distance. PAD: peripheral arterial disease. PTA: percutaneous transluminal angioplasty. RCT: randomised controlled trial. SEP: supervised exercise programme. WE: walking exercise.
Characteristics of ongoing studies [ordered by study ID]
NCT01231360.
| Trial name or title | The Effect of Exercise Training on Skeletal Muscle Metabolism in PAD |
| Methods | Allocation: randomised Intervention model: parallel assignment |
| Participants | Ages eligible for study: 40 years and older Genders eligible for study: both Accepts healthy volunteers: yes Inclusion criteria:
Exclusion criteria:
|
| Interventions | Active comparator: exercise training
Participants randomised to exercise training will participate in a 3‐month treadmill exercise programme in 1‐h training sessions 3 times per week as previously described. After a 5‐minute warm‐up period, exercise is initiated at a low workload of 2 mph at 0% grade. Participants walk until moderate claudication severity develops, then rest until the discomfort resolves, repeating until the total exercise period is completed. The intensity of treadmill exercise is increased as tolerated by increasing walking speed by 0.5 to 1 mph and/or grade by 1% to 2%. Participants are encouraged to continue the walking programme at home for at least 30 minutes on 2 separate occasions each week. Active comparator: normal routine Participants randomised to the routine activity control group will be asked to keep a log of their daily activities and return to the Vascular Research Center at weeks 4, 8, and 12, at which time they will be asked to return their log and undergo repeat treadmill testing and complete the 6‐minute walking test. |
| Outcomes | Specific aim 1: to test the hypothesis that subjects with PAD and IC have altered expression of genes that regulate skeletal muscle metabolism Specific aim 2: to test the hypothesis that exercise training improves calf skeletal muscle insulin resistance and genes that regulate skeletal muscle metabolic function in PAD patients with intermittent claudication |
| Starting date | Estimated enrolment: 75 Study start date: October 2010 Estimated study completion date: June 2013 Estimated primary completion date: December 2012 (final data collection date for primary outcome measure) |
| Contact information | Reena Pande, MD, Brigham and Women's Hospital skadivar@partners.org Contact: 617‐732‐6320 |
| Notes | NCT01231360 |
NCT01822457.
| Trial name or title | Effect of Nike Fuel Band on Exercise and Function in Claudicants: A Randomised Controlled Trial |
| Methods | Allocation: randomised
Intervention model: parallel assignment
Masking: single‐blind (outcomes assessor)
Primary purpose: supportive care
|
| Participants | Inclusion criteria:
Exclusion criteria:
*Participants should be able to use the NFB technology with minimal assistance. Gender: both Ages: 40 to 80 years |
| Interventions | Device: Nike Fuel Band (NFB). The NFB is a wrist‐worn sensor with a built‐in accelerometer for motion quantification. It is programmed to estimate the number of steps taken per day, and also to predict energy expenditure in units known as Nike Fuel. Accompanying software allows the user to set daily targets and monitor his or her activity through a graphical user interface. |
| Outcomes | All outcomes assessed at 3 months Primary: MWD ‐ standardised treadmill test Secondary:
|
| Starting date | August 2013 |
| Contact information | Pasha Normahani: Pn106@imperial.ac.uk, Richard M Kwasnicki: rmk107@imperial.ac.uk |
| Notes | Estimated completion: February 2015 (final data collection date for primary outcome measure) |
ABI: ankle brachial index. CLI: critical limb ischaemia. HMG‐CoA: rate‐controlling enzyme of the mevalonate pathway. IC: intermittent claudication. mph: miles per hour. MI: myocardial infarction. MWD: maximum walking distance. NFB: Nike Fuel Band. NYHA: New York Heart Association. PAD: peripheral arterial disease. PFWD: pain‐free treadmill walking distance.
Differences between protocol and review
Since the time of the original protocol, various changes have had to be instigated with regards to bias assessments. These have moved from the Schutlz assessment to Jadad and finally to the current Cochrane risk of bias assessment method (Higgins 2011). We have reassessed all trials to comply with the current bias scoring system.
The protocol itself has not been altered; however we have applied strict adherence to the protocol with regards to usual care. As walking advice has often been advocated as part of usual care, we have considered inclusion of only non‐exercise control groups within this review. This has been clarified and searched for in each paper, as walking advice would constitute unsupervised exercise, which was not the focus of this review.
To avoid overlap with other existing Cochrane reviews (Antoniou 2017; Fowkes 1998), we removed from this review the comparisons exercise versus angioplasty and exercise versus surgery.
In addition to planned treadmill walking distance and walking time, we have presented the results as a percentage change.
In keeping with current Cochrane policy, this version includes a 'Summary of findings' table and GRADE assessment of the quality of evidence. We have amended secondary outcomes to reflect clinical relevance.
Contributions of authors
Risha Lane (RL): selected trials, assessed quality, extracted data, and revised text for the third and fourth updates. Amy Harwood (AH): selected trials, assessed quality, and extracted data for the fourth update. Lorna Watson (LW): commented on content of the fourth update. Gillian Leng (GCL): commented on content of the fourth update.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The Chief Scientist Office, Scottish Executive Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research, UK.
The Cochrane Vascular editorial base is supported by a programme grant from the NIHR.
Declarations of interest
RL: none known. AH: none known. LW: none known. GCL: I am responsible for the National Institute for Health and Care Excellence (NICE) Implementation programme and I am the Executive Director of NICE. I am a trustee of the Royal Society of Medicine and the Guidelines International Network.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Arosio 2001 {published data only}
- Arosio E, Cuzzolin L, Marchi S, Minuz P, Degan M, Crivellente F, et al. Increased endogenous nitric oxide production induced by physical exercise in peripheral arterial occlusive disease patients. Life Sciences 1999;65(26):2815‐22. [DOI] [PubMed] [Google Scholar]
- Arosio E, Minuz P, Prior M, Zuliani V, Gaino S, Marchi S, et al. Vascular adhesion molecule‐1 and markers of platelet function before and after a treatment with iloprost or a supervised physical exercise program in patients with peripheral arterial disease. Life Sciences 2001;69(4):421‐33. [DOI] [PubMed] [Google Scholar]
Castro‐Sanchez 2013 {published data only}
- Castro‐Sanchez AM, Mataran‐Penarrocha GA, Feriche‐Fernandez‐Castanys B, Fernandez‐Sola C, Sanchez‐Labraca N, Moreno‐Lorenzo C. A program of 3 physical therapy modalities improves peripheral arterial disease in diabetes type 2 patients: a randomized controlled trial. Journal of Cardiovascular Nursing 2013;28(1):74‐82. [DOI] [PubMed] [Google Scholar]
- Castro‐Sanchez AM, Moreno‐Lorenzo C, Mataran‐Penarrocha GA, Feriche‐Fernandez‐Castanys B, Sanchez Labraca N, Sanchez Joya Mdel M. Efficacy of a massage and exercise programme on the ankle‐brachial index and blood pressure in patients with diabetes mellitus type 2 and peripheral arterial disease: a randomized clinical trial. [Spanish]. Medicina Clinica 2010;134(3):107‐10. [DOI] [PubMed] [Google Scholar]
Ciuffetti 1994 {published data only}
- Ciuffetti G, Paltriccia R, Lombardini R, Lupattelli G, Pasqualini L, Mannarino E. Treating peripheral arterial occlusive disease: pentoxifylline vs exercise. International Angiology 1994;13(1):33‐9. [PubMed] [Google Scholar]
Collins 2005 {published data only}
- Collins EG, Langbein WE, Orebaugh C, Bammert C, Hanson K, Reda D, et al. Cardiovascular training effect associated with polestriding exercise in patients with peripheral arterial disease. Journal of Cardiovascular Nursing 2005;20(3):177‐85. [DOI] [PubMed] [Google Scholar]
- Collins EG, Langbein WE, Orebaugh C, Bammert C, Hanson K, Reda D, et al. Polestriding exercise and vitamin E for management of peripheral vascular disease. Medicine and Science in Sports and Exercise 2003;35(3):384‐93. [DOI] [PubMed] [Google Scholar]
- Langbein WE, Collins EG, Orebaugh C, Littooy FN, Edwards L, Reda D. Polestriding exercise and vitamin E for management of claudication pain. Journal of Rehabilitation Research and Development 1998;35(Suppl 1):181‐2. [Google Scholar]
- Langbein WE, Collins EG, Orebaugh C, Maloney C, Williams KJ, Littooy FN, et al. Increasing exercise tolerance of persons limited by claudication pain using polestriding. Journal of Vascular Surgery 2002;35(5):887‐93. [DOI] [PubMed] [Google Scholar]
Crowther 2012 {published data only}
- Crowther RG, Leicht AS, Spinks WL, Sangla K, Quigley F, Golledge J. Effects of a 6‐month exercise program pilot study on walking economy, peak physiological characteristics, and walking performance in patients with peripheral arterial disease. Vascular Health and Risk Management 2012;8:225‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cucato 2013 {published data only}
- Cucato GG, Chehuen Mda R, Costa LA, Ritti‐Dias RM, Wolosker N, Saxton JM, et al. Exercise prescription using the heart of claudication pain onset in patients with intermittent claudication. Clinics (Sao Paulo, Brazil) 2013;68(7):974‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahllof 1974 {published data only}
- Dahllof AG, Bjorntorp P, Holm J, Schersten T. Metabolic activity of skeletal muscle in patients with peripheral arterial insufficiency. European Journal of Clinical Investigation 1974;4(1):9‐15. [DOI] [PubMed] [Google Scholar]
Gardner 2002 {published data only}
- Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, et al. Exercise rehabilitation Improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomised controlled trial. Journal of the American Geriatric Society 2001;49(6):755‐62. [DOI] [PubMed] [Google Scholar]
- Gardner AW, Katzel LI, Sorkin JD, Goldberg AP. Effects of long‐term exercise rehabilitation on claudication distances in patients with peripheral arterial disease: a randomized controlled trial. Journal of Cardiopulmonary Rehabilitation 2002;22(3):192‐8. [DOI] [PubMed] [Google Scholar]
Gelin 2001 {published data only}
- Gelin J, Jivegard L, Taft C, Karlsson J, Sullivan M, Dahllöf AG, et al. Treatment efficacy of intermittent claudication by surgical intervention, supervised physical exercise training compared to no treatment in unselected randomised patients I: one year results of functional and physiological improvements. European Journal of Vascular and Endovascular Surgery 2001;22(2):107‐13. [DOI] [PubMed] [Google Scholar]
- Taft C, Karlsson J, Gelin J, Jivegard L, Sandström R, Arfvidsson B, et al. Treatment efficacy of intermittent claudication by invasive therapy, supervised physical exercise training compared to no treatment in unselected randomised patients II: one year results of health‐related quality of life. European Journal of Vascular and Endovascular Surgery 2001;22(2):114‐23. [DOI] [PubMed] [Google Scholar]
GOALS 2013 {published data only}
- McDermott M. A home‐based exercise intervention significantly improves walking performance in peripheral arterial disease: one‐year follow‐up from a randomized controlled trial. Circulation 2013;128:A14789. [Google Scholar]
- McDermott M, Criqui M, Domanchuk K, Guralnik J, Kibbe M, Liu K, et al. Home‐based exercise improves walking speed and prevents mobility loss in peripheral artery disease: a randomized controlled trial. Circulation 2014; Vol. 130:A11744.
- McDermott MM, Guralnik JM, Criqui MH, Ferrucci L, Liu K, Spring B, et al. Unsupervised exercise and mobility loss in peripheral artery disease: a randomized controlled trial. Journal of the American Heart Association 2015;4(5):e001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Guralnik JM, Criqui MH, Ferrucci L, Zhao L, Liu K, et al. Home‐based walking exercise in peripheral artery disease: 12‐month follow‐up of the GOALS randomized trial. Journal of the American Heart Association 2014;3(3):e000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, et al. Home‐based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013;310(1):57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJS, Spring B, Domanchuk K, Tao H, Tian L, Zhao L, et al. A group‐mediated, home‐based physical activity intervention for patients with peripheral artery disease: effects on social and psychological function. Journal of Translational Medicine 2014;12(29):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guidon 2010 {published data only}
- Guidon M, McGee H. One‐year effect of a supervised exercise programme on functional capacity and quality of life in peripheral arterial disease. Disability and Rehabilitation 2013;35(5):397‐404. [DOI] [PubMed] [Google Scholar]
- Guidon MM, McGee H, Kelly C. Quality of life and functional capacity following peripheral arterial disease exercise programme. European Journal of Cardiovascular Prevention and Rehabilitation 2010;17:S102. [Google Scholar]
Hiatt 1990 {published data only}
- Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation 1990;81(2):602‐9. [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. Journal of Vascular Medicine and Biology 1990;2:142‐52. [Google Scholar]
Hiatt 1994 {published data only}
- Hiatt WR, Regensteiner JG, Wolfel EE, Carry MR, Brass EP. Effect of exercise training on skeletal muscle histology and metabolism in peripheral arterial disease. Journal of Applied Physiology 1996;81(2):780‐8. [DOI] [PubMed] [Google Scholar]
- Hiatt WR, Wolfel EE, Meier RH, Regensteiner JG. Superiority of treadmill walking exercise versus strength training for patients with peripheral arterial disease. Implications for the mechanism of the training response. Circulation 1994;90(4):1866‐74. [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. Journal of Vascular Surgery 1996;23(1):104‐15. [DOI] [PubMed] [Google Scholar]
Hobbs 2005 {published data only}
- Hobbs SD, Marshall T, Fegan C, Adam DJ, Bradbury AW. The effect of supervised exercise and cilostazol on coagulation and fibrinolysis in intermittent claudication: a randomised controlled trial. Journal of Vascular Surgery 2007;45(1):65‐70, discussion 70. [DOI] [PubMed] [Google Scholar]
- Hobbs SD, Marshall T, Fegan C, Adam DJ, Bradbury AW. The effect of supervised exercise and cilostazol on coagulation and fibrinolysis in patients with intermittent claudication. Yearbook 2005, The Vascular Society of Great Britain & Ireland. 2005. Abstract 68.
Jansen 1991 {published data only}
- Jansen T, Weiss T, Amendt K, Hsu E, Hubsch‐Muller C, Diehm C. Effect of a 2‐year ambulatory vascular sports program on walking distance in claudication patients ‐ a controlled study [German]. Vasa Supplementum 1991;33:175. [PubMed] [Google Scholar]
Kakkos 2005 {published data only}
- Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. European Journal of Vascular and Endovascular Surgery 2005;30(2):164‐75. [DOI] [PubMed] [Google Scholar]
Larsen 1966 {published data only}
- Larsen OA, Lassen NA. Effect of daily muscular exercise in patients with intermittent claudication. Lancet 1966;2(7473):1093‐6. [DOI] [PubMed] [Google Scholar]
- Larsen OA, Lassen NA. Effect of daily muscular exercise in patients with intermittent claudication. Scandinavian Journal of Clinical and Laboratory Investigation 1967;Suppl 99:168‐71. [PubMed] [Google Scholar]
Leicht 2011 {published data only}
- Leicht A, Crowther R, Golledge J. Influence of regular exercise on body fat and eating patterns of patients with intermittent claudication. International Journal of Molecular Sciences 2015;16(5):11339‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht AS, Crowther RG, Golledge J. Influence of peripheral arterial disease and supervised walking on heart rate variability. Journal of Vascular Surgery 2011;54(5):1352‐9. [DOI] [PubMed] [Google Scholar]
Mannarino 1991 {published data only}
- Mannarino E, Pasqualini L, Innocente S, Scricciolo V, Rignanese A, Ciuffetti G. Physical training and antiplatelet treatment in stage II peripheral arterial occlusive disease: alone or combined?. Angiology 1991;42(7):513‐21. [DOI] [PubMed] [Google Scholar]
McDermott 2008 {published data only}
- McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301(2):165‐74, Erratum in JAMA 2012;307(16):1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Ades PA, Dyer A, Guralnik JM, Kibbe M, Criqui MH. Corridor‐based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. Journal of Vascular Surgery 2008;48(5):1231‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00106327. Improving functioning in peripheral arterial disease. clinicaltrials.gov/ct2/show/NCT00106327. Date first received: 22 March 2005.
- Roitman JL. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without in intermittent claudication: a randomized controlled trial. Journal of Cardiopulmonary Rehabilitation and Prevention 2010;30(1):62. [Google Scholar]
McGuigan 2001 {published data only}
- McGuigan MR, Bronks R, Newton RU, Sharman MJ, Graham JC, Cody DV, et al. Resistance training in patients with peripheral arterial disease: effects on myosin isoforms, fiber type distribution, and capillary supply to skeletal muscle. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2001;56:B302‐10. [DOI] [PubMed] [Google Scholar]
Mika 2005 {published data only}
- Mika P, Spodaryk K, Cencora A. Effects of treadmill training on walking distance and lower limb blood flow in patients with intermittent claudication [Zmiany dystansu marszu i przepływu tętniczego w kończynach dolnychpodczas treningu marszowego u pacjentów z chromaniem przestankowym]. Medical Rehabilitation 2005;9(1):3‐9. [Google Scholar]
- Mika P, Spodaryk K, Cencora A, Unnithan VB, Mika A. Experimental model of pain‐free treadmill training in patients with claudication. American Journal of Physical Medicine and Rehabilitation 2005;84(10):756‐62. [DOI] [PubMed] [Google Scholar]
Mika 2006 {published data only}
- Mika P, Spodaryk K, Cencora A, Mika A. Red blood cell deformability in patients with claudication after pain‐free treadmill training. Clinical Journal of Sport Medicine 2006;16(4):335‐40. [DOI] [PubMed] [Google Scholar]
Mika 2011 {published data only}
- Mika P, Wilk B, Marchewka A, Nizankowski R. The effect of pain‐free treadmill training on fibrinogen, haematocrit, and lipid profile in patients with claudication. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(5):754‐60. [DOI] [PubMed] [Google Scholar]
Sanderson 2006 {published data only}
- Sanderson B, Askew C, Stewart I, Walker P, Gibbs H, Green S. Short‐term effects of cycle and treadmill training on exercise tolerance in peripheral arterial disease. Journal of Vascular Surgery 2006;44(1):119‐27. [DOI] [PubMed] [Google Scholar]
Schlager 2011 {published data only}
- Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Gröger M, et al. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis 2011;217(1):240‐8. [DOI] [PubMed] [Google Scholar]
- Schlager O, Hammer A, Giurgea A, Schuhfried O, Fialka‐Moser V, Gschwandtner M, et al. Impact of exercise training on inflammation and platelet activation in patients with intermittent claudication. Swiss Medical Weekly 2012;142:w13623. [DOI] [PubMed] [Google Scholar]
- Steiner‐Boeker S, Schlager O, Giurgea A, Schuhfried O, Seidinger D, Koppensteiner R. Exercise training on top of standard medication increases endothelial progenitor cells and decreases ADMA levels in patients with PAD. European Heart Journal 2009;30(984):985. [Google Scholar]
Tew 2009 {published data only}
- Tew G, Nawaz S, Zwierska I, Saxton JM. Limb‐specific and cross‐transfer effects of arm‐crank exercise training in patients with symptomatic peripheral arterial disease. Clinical Science 2009;117(12):405‐13. [DOI] [PubMed] [Google Scholar]
Tew 2015 {published data only}
- Tew GA, Humphreys L, Crank H, Hewitt C, Nawaz S, Al‐Jundi W, et al. The development and pilot randomised controlled trial of a group education programme for promoting walking in people with intermittent claudication. Vascular Medicine (United Kingdom). SAGE Publications Ltd, 2015; Vol. 20, issue 4:348‐57. [DOI] [PubMed]
Tisi 1997 {published data only}
- Tisi P, Shearman C. The impact of treatment of intermittent claudication on subjective health of the patient. Health Trends 1998/99;30:109‐14. [Google Scholar]
- Tisi PV, Hulse M, Chulakadabba A, Gosling P, Shearman CP. Exercise training for intermittent claudication: does it adversely affect biochemical markers of the exercise‐induced inflammatory response?. European Journal of Vascular and Endovascular Surgery 1997;14(5):344‐50. [DOI] [PubMed] [Google Scholar]
Tsai 2002 {published data only}
- Tsai JC, Chan P, Wang CH, Jeng C, Hsieh MH, Kao PF, et al. The effects of exercise training on walking function and perception of health status in elderly patients with peripheral arterial occlusive disease. Journal of Internal Medicine 2002;252(5):448‐55. [DOI] [PubMed] [Google Scholar]
Wood 2006 {published data only}
- Wood RE, Sanderson BE, Askew CD, Walker PJ, Green S, Stewart IB. Effect of training on the response of plasma vascular endothelial growth factor to exercise in patients with peripheral arterial disease. Clinical Science 2006;111(6):401‐9. [DOI] [PubMed] [Google Scholar]
Zwierska 2005 {published data only}
- Saxton JM, Zwierska I, Blagojevic M, Choksy SA, Nawaz S, Pockley AG. Upper‐ versus lower‐limb aerobic exercise training on health‐related quality of life in patients with symptomatic peripheral arterial disease. Journal of Vascular Surgery 2011;53(5):1265‐73. [DOI] [PubMed] [Google Scholar]
- Saxton JM, Zwierska I, Hopkinson K, Espigares E, Choksy S, Nawaz S, et al. Effect of upper‐ and lower‐limb exercise training on circulating soluble adhesion molecules, hs‐CRP and stress proteins in patients with intermittent claudication. European Journal of Vascular and Endovascular Surgery 2008;35(5):607‐13. [DOI] [PubMed] [Google Scholar]
- Zwierska I, Walker RD, Choksy SA, Male JS, Pockley AG, Saxton JM. Upper‐ vs lower‐limb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: a randomized controlled trial. Journal of Vascular Surgery 2005;42(6):1122‐30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 2010 {published data only}
- Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, et al. Plasma nitrite flux predicts exercise performance in peripheral arterial disease after 3 months of exercise training. Free Radical Biology and Medicine 2010;49(6):1138‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreozzi 2008 {published data only}
- Andreozzi GM, Leone A, Laudani R, Martin R, Deinit G, Cataldi V. Levo‐propionyl‐carnitine improves the effectiveness of supervised physical training on the absolute claudication distance in patients with intermittent claudication. Angiology 2008;59(1):84‐9. [DOI] [PubMed] [Google Scholar]
Aruna 2015 {published data only}
- Aruna S, Thenmozhi P. Effectiveness of Allen Buerger exercise in preventing peripheral arterial disease among people with type II diabetes mellitus. International Journal of Pharma and Bio Sciences 2015;6(2):(B) 966‐70. [Google Scholar]
Beutel 1985 {published data only}
- Beutel G, Sobanski R. Results of the complex spa‐therapy in peripheral obstructive disease Stage II. Zeitschrift fur Physiotherapie 1985;37:309‐11. [Google Scholar]
Bronas 2011 {published data only}
- Bronas UG, Treat‐Jacobson D, Leon AS. Comparison of the effect of upper body‐ergometry aerobic training vs treadmill training on central cardiorespiratory improvement and walking distance in patients with claudication. Journal of Vascular Surgery 2011;53(6):1557‐64. [DOI] [PubMed] [Google Scholar]
- Treat‐Jacobson D, Bronas UG, Leon AS. Efficacy of arm‐ergometry versus treadmill exercise training to improve walking distance in patients with claudication. Vascular Medicine 2009;14(3):203‐13. [DOI] [PubMed] [Google Scholar]
Brotons 2011 {published data only}
- Brotons C, Soriano N, Moral I, Rodrigo MP, Kloppe P, Rodríguez AI, et al. PREseAP Study Research Team. Randomized clinical trial to assess the efficacy of a comprehensive programme of secondary prevention of cardiovascular disease in general practice: the PREseAP study. Revista Española de Cardiología 2011;64(1):13‐20. [DOI] [PubMed] [Google Scholar]
Buchwalsky 1974 {published data only}
- Buchwalsky R, Blümchen, G, Schlosser V, Pohle W, Isbary J, Barmeyer J, et al. The influence of long‐term training on the degree of compensation of peripheral occlusive arterial disease. Klinische Wochenschrift 1971; Vol. 49:1045.
- Buchwalsky R, Schnellbacher K, Roskamm H, Barmeyer J. Long‐term training effects in chronic ischemia. [German]. Medizinische Welt 1974;25(3):83‐6. [PubMed] [Google Scholar]
Bulling 1991 {published data only}
- Bulling B, Bary S. Treatment of chronic peripheral occlusive disease with physical training and ginkgo biloba extract 761. Medizinische Welt 1991;42(8):702‐8. [Google Scholar]
Cachovan 1999 {published data only}
- Cachovan M, Rogatti W, Creutzig A, Diehm C, Heidrich H, Scheffler P, et al. Treadmill testing for evaluation of claudication: comparison of constant‐load and graded‐exercise tests. European Journal of Vascular and Endovascular Surgery 1997;14(4):238‐43. [DOI] [PubMed] [Google Scholar]
- Cachovan M, Rogatti W, Woltering F, Creutzig A, Diehm C, Heidrich H, et al. Randomized reliability study evaluating constant‐load and graded‐exercise treadmill test for intermittent claudication. Angiology 1999;50(3):193‐200. [DOI] [PubMed] [Google Scholar]
Carmeli 2004 {published data only}
- Carmeli E, Barchad S, Masharawi Y, Coleman R. Impact of a walking program in people with down syndrome. Journal of Strength and Conditioning Research 2004;18(1):180‐4. [DOI] [PubMed] [Google Scholar]
Cheetham 2004 {published data only}
- Cheetham DR, Burgess L, Ellis M, Williams A, Greenhalgh RM, Davies AH. Does supervised exercise offer adjuvant benefit over exercise advice alone for the treatment of intermittent claudication? A randomised trial. European Journal of Vascular and Endovascular Surgery 2004;27(1):17‐23. [DOI] [PubMed] [Google Scholar]
Choi 2012 {published data only}
- Choi KKS, Etnyre G, Figoni SF, Kunkel CF, Ornelas CC, Scremin E. Comparison of calf exercise and treadmill training in peripheral arterial disease. American Academy of Physical Medicine and Rehabilitation Conference 2012; Vol. 4, issue 10 Suppl:S346 (Poster 457).
Cina 1996 {published data only}
- Cina G, Vernich M, Campisi C, Cascone C, Ofria F, Leopardi N, et al. Physical training and low‐dose heparin‐calcium in patients suffering from chronic obliterating arteriopathy of the lower limbs with intermittent claudication [Training fisico ed eparina calcica a basse dosi in pazienti affetti da arteriopatia obliterante cronica degli arti inferiori con claudicatio intermittens]. Minerva Cardioangiologica 1996;44(4):179‐85. [PubMed] [Google Scholar]
CLEVER 2009 {published data only}
- Bronas UG, Hirsch AT, Murphy T, Badenhop D, Collins TC, Ehrman JK, et al. CLEVER Research Group. Design of the multicenter standardized supervised exercise training intervention for the claudication: exercise vs endoluminal revascularization (CLEVER) study. Vascular Medicine 2009;14(4):313‐21. [DOI] [PubMed] [Google Scholar]
- Melton C. Supervised exercise program helps patients with peripheral artery disease walk longer. Annals of Long‐Term Care 2011;19(12):15‐6. [Google Scholar]
- Melton C. Supervised exercise program helps patients with peripheral artery disease walk longer. Clinical Geriatrics 2011;19(12):17. [Google Scholar]
- Murphy TP. Supervised exercise vs. primary stenting for claudication due to aorto‐iliac peripheral artery disease: 6‐month outcomes from the CLEVER Study. European Heart Journal 2012;33(1):144‐5. [Google Scholar]
- Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, et al. CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six‐month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012;125(1):130‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TP, Hirsch AT, Cutlip DE, Regensteiner JG, Comerota AJ, Mohler E, et al. CLEVER Investigators. Claudication: exercise vs endoluminal revascularization (CLEVER) study update. Journal of Vascular Surgery 2009;50(4):942‐5, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TP, Hirsch AT, Ricotta JJ, Cutlip DE, Mohler E, Regensteiner JG, et al. CLEVER Steering Committee. The Claudication: Exercise Vs. Endoluminal Revascularization (CLEVER) study: rationale and methods. Journal of Vascular Surgery 2008;47(6):1356‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2010 {published data only}
- Collins T. Walking therapy for patients with diabetes mellitus and peripheral arterial disease who are limited by leg pain and/or fatigue. Atherosclerosis Supplements 2011;12(1):118‐9. [Google Scholar]
- Collins T, Ghidei W, Ahluwalia J. Benefits of walking therapy in patients with diabetes mellitus and peripheral arterial disease who are limited by leg pain or fatigue. Journal of General Internal Medicine 2011;26:S176‐7. [Google Scholar]
- Collins T, Lunos S. Home‐based walking therapy improves walking ability and quality of life in patients with diabetes mellitus and peripheral arterial disease. Journal of General Internal Medicine 2010;25(3 Suppl):S296‐7. [Google Scholar]
- Collins T, Lunos S. Home‐based walking therapy improves walking ability and quality of life in persons with diabetes mellitus and peripheral arterial disease. Vascular Medicine 2010;15:155. [DOI] [PubMed] [Google Scholar]
- Collins TC, Lunos S, Carlson T, Henderson K, Lightbourne M, Nelson B, et al. Effects of a home‐based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care 2011;34(10):2174‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins TC, Lunos S, Hodges J. Pilot study of whether a walking intervention reduces inflammation in patients with diabetes and peripheral arterial disease (PAD). Vascular Medicine 2011;16(3):230. [Google Scholar]
- NCT00611988. Self‐managed walking improves function. clinicaltrials.gov/ct2/show/NCT00611988. Date first received: 25 January 2008.
Collins 2012 {published data only}
- Collins EG, McBurney C, Butler J, Jelinek C, O'Connell S, Fritschi C, et al. The effects of walking or walking‐with‐poles training on tissue oxygenation in patients with peripheral arterial disease. International Journal of Vascular Medicine 2012;2012:Article 985025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins EG, O'Connell S, McBurney C, Jelinek C, Butler J, Reda D, et al. Comparison of walking with poles and traditional walking for peripheral arterial disease rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(4):210‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Creasy 1990 {published data only}
- Creasy TS, Fletcher EW, Walton J, Collin J, Morris P. Prospective randomized trial of PTA versus supervised exercise therapy for intermittent claudication. British Journal of Radiology 1992;65(Suppl):108. [Google Scholar]
- Creasy TS, McMillan PJ, Fletcher EWL, Collin J, Morris PJ. Is percutaneous transluminal angioplasty better than exercise for claudication? Preliminary results from a prospective randomised trial. European Journal of Vascular Surgery 1990;4(2):135‐40. [DOI] [PubMed] [Google Scholar]
- Perkins JM, Collin J, Creasy TS, Fletcher EW, Morris PJ. Exercise training versus angioplasty for stable claudication. Long and medium term results of a prospective, randomised trial. European Journal of Endovascular Surgery 1996;11(4):409‐13. [DOI] [PubMed] [Google Scholar]
Crowther 2008 {published data only}
- Crowther RG, Spinks WL, Leicht AS, Sangla K, Quigley F, Golledge J. Effects of a long‐term exercise program on lower limb mobility, physiological responses, walking performance, and physical activity levels in patients with peripheral arterial disease. Journal of Vascular Surgery 2008;47(2):303‐9. [DOI] [PubMed] [Google Scholar]
- Crowther RG, Spinks WL, Leicht AS, Sangla K, Quigley F, Golledge J. The influence of a long term exercise program on lower limb movement variability and walking performance in patients with peripheral arterial disease. Human Movement Science 2009;28(4):494‐503. [DOI] [PubMed] [Google Scholar]
Cucato 2011 {published data only}
- Cucato GG, Ritti‐Dias RM, Wolosker N, Santarem JM, Jacob Filho W, Forjaz CL, et al. Post‐resistance exercise hypotension in patients with intermittent claudication. Clinics (Sao Paulo, Brazil) 2011;66(2):221‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cucato 2011a {published data only}
- Cucato GG, Forjaz CL, Kanegusuku H, Rocha Chehuen M, Riani Costa LA, Wolosker N, et al. Effects of walking and strength training on resting and exercise cardiovascular responses in patients with intermittent claudication. Vasa 2011;40(5):390‐7. [DOI] [PubMed] [Google Scholar]
Cucato 2015 {published data only}
- Cucato GG, Saxton JM. Post‐walking exercise hypotension in patients with intermittent claudication. Medicine and Science in Sports and Exercise 2015;47(3):460‐7. [DOI] [PubMed] [Google Scholar]
Cunningham 2012 {published data only}
- Cunningham MA, Swanson V, Holdsworth RJ, O'Carroll RE. Late effects of a brief psychological intervention in patients with intermittent claudication in a randomized clinical trial. British Journal of Surgery 2013;100(6):756‐60. [DOI] [PubMed] [Google Scholar]
- Cunningham MA, Swanson V, O'Carroll RE, Holdsworth RJ. Randomized clinical trial of a brief psychological intervention to increase walking in patients with intermittent claudication. British Journal of Surgery 2012;99(1):49‐56. [DOI] [PubMed] [Google Scholar]
Dahllof 1976 {published data only}
- Dahllof AG, Holm J, Schersten T, Sivertsson R. Peripheral arterial insufficiency, effect of physical training on walking tolerance, calf blood flow and blood flow resistance. Scandinavian Journal of Rehabilitation Medicine 1976;8(1):19‐26. [PubMed] [Google Scholar]
Dantas 2016 {published data only}
- Dantas FF, Silva Santana F, Silva TS, Cucato GG, Farah BQ, Ritti‐Dias RM. Acute effects of T'ai Chi Chuan exercise on blood pressure and heart rate in peripheral artery disease patients. Journal of Alternative and Complementary Medicine (New York, N.Y.) 2016;22:375‐9. [DOI] [PubMed] [Google Scholar]
Dedes 2010 {published data only}
- Dedes H, Figoni SF, Kalioundji G, Kunkel C, Peter A, Phillips A, et al. Prospective trial of calf ergometry training on walking ability in peripheral arterial disease. Physical Medicine and Rehabilitation 2010;2(9 Suppl 1):S26. [Google Scholar]
Degischer 2002 {published data only}
- Degischer S, Labs KH, Hochstrasser J, Aschwanden M, Tschoepl M, Jaeger KA. Physical training for intermittent claudication: a comparison of structured rehabilitation versus home‐based training. Vascular Medicine 2002;7(2):109‐15. [DOI] [PubMed] [Google Scholar]
Dittmar 1977 {published data only}
- Dittmar K, Krause D. Success of physiotherapeutic interval training combined with a standardized hemoderivative in intermittent claudication. Munchener Medizinische Wochenschrift 1977;119(11):369‐72. [PubMed] [Google Scholar]
Ericsson 1970 {published data only}
- Ericsson B, Haeger K, Lindell SE. Effect of physical training of intermittent claudication. Angiology 1970;21(3):188‐92. [DOI] [PubMed] [Google Scholar]
Ernst 1987 {published data only}
- Ernst EE, Matrai A. Intermittent claudication, exercise, and blood rheology. Circulation 1987;76(5):1110‐4. [DOI] [PubMed] [Google Scholar]
Ernst 1990 {published data only}
- Ernst E, Kollar L, Resch KL, Bergmann H. Arterial occlusive disease. Comparison between pentoxifylline and exercise vs. exercise alone in patients with stage II of disease. Munchener Medizinische Wochenschrift 1990;132(28‐29):456‐8. [Google Scholar]
EXERT 2009 {published data only}
- NCT00895635. Evaluating two exercise training programs to reduce leg pain in people with peripheral arterial disease (The EXERT study). clinicaltrials.gov/ct2/show/NCT00895635 Date first received: 6 May 2009.
EXITPAD 2010 {published data only}
- Gommans LNM, Scheltinga MRM, Sambeek MRHM, Maas AHEM, Bendermacher BLW, Teijink JAW. Gender differences following supervised exercise therapy in patients with intermittent claudication. Journal of Vascular Surgery 2015;62:681‐8. [DOI] [PubMed] [Google Scholar]
- Nicolai SP, Hendriks EJ, Prins MH, Teijink JA, EXITPAD Study Group. Optimizing supervised exercise therapy for patients with intermittent claudication. Journal of Vascular Surgery 2010;52(5):1226‐33. [DOI] [PubMed] [Google Scholar]
- Nicolai SP, Teijink JA, Prins MH, Exercise Therapy in Peripheral Arterial Disease Study Group. Multicenter randomized clinical trial of supervised exercise therapy with or without feedback versus walking advice for intermittent claudication. Journal of Vascular Surgery 2010;52(2):348‐55. [DOI] [PubMed] [Google Scholar]
- Nicolai SPA, Prins MH, Teijink JAW. Supervised exercise therapy is more effective than walking advice in patients with intermittent claudication; a randomized multicenter study. [Dutch]. Nederlands Tijdschrift voor Geneeskunde 2011;155(2):55‐62. [Google Scholar]
- Asselt AD, Nicolai SP, Joore MA, Prins MH, Teijink JA, Exercise Therapy in Peripheral Arterial Disease Study Group. Cost‐effectiveness of exercise therapy in patients with intermittent claudication: supervised exercise therapy versus a 'go home and walk' advice. European Journal of Vascular and Endovascular Surgery 2011;41(1):97‐103. [DOI] [PubMed] [Google Scholar]
Fakhry 2011 {published data only}
- Fakhry F, Spronk S, Ridder M, den‐Hoed PT, Hunink MG. Long‐term effects of structured home‐based exercise program on functional capacity and quality of life in patients with intermittent claudication. Archives of Physical Medicine and Rehabilitation 2011;92(7):1066‐73. [DOI] [PubMed] [Google Scholar]
Fitzgerald 1971 {published data only}
- Fitzgerald DE, Keates JS, MacMillan D. Angiographic and plethysmographic assessment of graduated physical exercise in the treatment of chronic occlusive arterial disease of the leg. Angiology 1971;22(2):99‐106. [DOI] [PubMed] [Google Scholar]
Fowler 2002 {published data only}
- Fowler B, Jamrozik K, Norman P, Allen Y, Wilkinson E. Improving maximum walking distance in early peripheral arterial disease: randomised controlled trial. Australian Journal of Physiotherapy 2002;48(4):269‐75. [DOI] [PubMed] [Google Scholar]
Gardner 2005 {published data only}
- Gardner AW, Montgomery PS, Flinn WR, Katzel LI. The effect of exercise intensity on the response to exercise rehabilitation in patients with intermittent claudication. Journal of Vascular Surgery 2005;42(4):702‐9. [DOI] [PubMed] [Google Scholar]
Gardner 2011 {published data only}
- Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home‐based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation 2011;123(5):491‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardner 2012 {published data only}
- Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication. Journal of Vascular Surgery 2012;55(5):1346‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication: a randomized controlled trial. Journal of Cardiopulmonary Rehabilitation and Prevention 2011; Vol. 31:E4‐5.
Gardner 2014 {published data only}
- Gardner AW, Parker DE, Montgomery PS, Blevins SM. Diabetic women are poor responders to exercise rehabilitation in the treatment of claudication. Journal of Vascular Surgery 2014;59(4):1036‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardner 2014a {published data only}
- Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step‐monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. Journal of the American Heart Association 2014;3(5):e001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbellini 2000 {published data only}
- Gibellini R, Fanello M, Bardile AF, Salerno M, Aloi T. Exercise training in intermittent claudication. International Angiology 2000;19(1):8‐13. [PubMed] [Google Scholar]
Gibbs 2013 {published data only}
- Gibbs BB, Dobrosielski DA, Althouse AD, Stewart KJ. The effect of exercise training on ankle‐brachial index in type 2 diabetes. Atherosclerosis 2013;230(1):125‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottstein 1987 {published data only}
- Gottstein P, Schollmayer E. Additive effect of Buflomedil under simultaneous training. Buflomedil‐therapy in combination with intensive training in patients with intermittent claudication. Zeitschrift fur Allgemeinmedizin 1987;63(28):836‐9. [Google Scholar]
Greenhalgh 2008 {published data only}
- Greenhalgh RM. MIMIC Trials: Angioplasty effective in randomised controlled trials for peripheral arterial disease. http://www.cxvascular.com/interventionalnews/latestnews.cfm?ccs=485&cs=4222. accessed 2 February 2009.
- Greenhalgh RM, Belch JJ, Brown LC, Gaines PA, Gao L, Reise JA, et al. The adjuvant benefit of angioplasty in patients with mild to moderate intermittent claudication (MIMIC) managed by supervised exercise, smoking cessation advice and best medical therapy: results from two randomised trials for stenotic femoropopliteal and aortoiliac arterial disease. European Journal of Vascular and Endovascular Surgery 2008;36(6):680‐8. [DOI] [PubMed] [Google Scholar]
Guidon 2013 {published data only}
- Guidon M, McGee H. Recruitment to clinical trials of exercise: challenges in the peripheral arterial disease population. Physiotherapy 2013;99(4):305‐10. [DOI] [PubMed] [Google Scholar]
Guirro 2015 {published data only}
- Guirro EC, Guirro RR, Dibai‐Filho AV, Pascote SCS, Rodrigues‐Bigaton D. Immediate effects of electrical stimulation, diathermy, and physical exercise on lower limb arterial blood flow in diabetic women with peripheral arterial disease: a randomized crossover trial. Journal of Manipulative and Physiological Therapeutics 2015;38(3):195‐202. [DOI] [PubMed] [Google Scholar]
Hobbs 2006 {published data only}
- Hobbs SD, Bradbury AW. The EXercise versus Angioplasty in Claudication Trial (EXACT): reasons for recruitment failure and the implications for research into and treatment of intermittent claudication. Journal of Vascular Surgery 2006;44(2):432‐3. [DOI] [PubMed] [Google Scholar]
- Hobbs SD, Marshall T, Fegan C, Adam DJ, Bradbury AW. The constitutive procoagulant and hypofibrinolytic state in patients with intermittent claudication due to infrainguinal disease significantly improves with percutaneous transluminal balloon angioplasty. Journal of Vascular Surgery 2006;43(1):40‐6. [DOI] [PubMed] [Google Scholar]
Hodges 2008 {published data only}
- Hodges LD, Sandercock GR, Das SK, Brodie DA. Randomized controlled trial of supervised exercise to evaluate changes in cardiac function in patients with peripheral atherosclerotic disease. Clinical Physiology and Functional Imaging 2008;28(1):32‐7. [DOI] [PubMed] [Google Scholar]
Holm 1973 {published data only}
- Holm J, Dahllof AG, Bjorntorp P, Schersten T. Enzyme studies in muscles of patients with intermittent claudication. Effect of training. Scandinavian Journal of Clinical Laboratory Investigation 1973;31(Suppl 128):201‐5. [PubMed] [Google Scholar]
Jones 1996 {published data only}
- Jones PP, Skinner JS, Smith LK, John FM, Bryant CX. Functional improvements following StairMaster vs. treadmill exercise training for patients with intermittent claudication. Journal of Cardiopulmonary Rehabilitation 1996;16(1):47‐55. [DOI] [PubMed] [Google Scholar]
Kiesewetter 1987 {published data only}
- Kiesewetter H, Blume J, Jung F, Gerhards M, Leipnitz G. Training by walking and drug therapy of peripheral arterial occlusive disease. [German]. Vasa Supplementum 1987;20:384‐7. [PubMed] [Google Scholar]
Kono 2013 {published data only}
- Kono Y, Yamada S, Yamaguchi J, Hagiwara Y, Iritani N, Ishida S, et al. Secondary prevention of new vascular events with lifestyle intervention in patients with noncardioembolic mild ischemic stroke: a single‐center randomized controlled trial. Cerebrovascular Diseases 2013;36(2):88‐97. [DOI] [PubMed] [Google Scholar]
Krause 1976 {published data only}
- Krause D, Dittmar K. Combination of physiotherapeutic exercise therapy with bencyclane in intermittent claudication. Munchener Medizinische Wochenschrift 1976;118(40):1281‐4. [PubMed] [Google Scholar]
Kruidenier 2011 {published data only}
- Kruidenier LM, Nicola SP, Rouwet EV, Peters RJ, Prins MH, Teijink JAW. Additional supervised exercise therapy after a percutaneous vascular intervention for peripheral arterial disease: a randomized clinical trial. Journal of Vascular and Interventional Radiology 2011;22(7):961‐8. [DOI] [PubMed] [Google Scholar]
Labs 1999 {published data only}
- Labs KH, Nehler MR, Roessner M, Jaeger KA, Hiatt WR. Reliability of treadmill testing in peripheral arterial disease: a comparison of a constant load with a graded load treadmill protocol. Vascular Medicine 1999;4(4):239‐46. [DOI] [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee HL, Mehta T, Ray B, Heng MS, McCollum PT, Chetter IC. A non‐randomised controlled trial of the clinical and cost effectiveness of a Supervised Exercise Programme for claudication. European Journal of Endovascular and Vascular Surgery 2007;33(2):202‐7. [DOI] [PubMed] [Google Scholar]
Leon 2005 {published data only}
- Leon AS. Does long‐term aerobic exercise slow progression of atherosclerosis?. Clinical Journal of Sport Medicine 2005;15(4):285‐6. [DOI] [PubMed] [Google Scholar]
Lepantalo 1984 {published data only}
- Lepantalo M, Sundberg S, Gordin A. The effects of physical training and flunarizine on walking capacity in intermittent claudication. Scandinavian Journal of Rehabilitation Medicine 1984;16(4):159‐62. [PubMed] [Google Scholar]
LIFE Study {published data only}
- McDermott M, Bonds D, Buford T, Church T, Dodson J, Fielding J, et al. A physical activity intervention improves 400‐meter walking velocity as compared to a successful aging intervention in patients with peripheral artery disease: the LIFE study randomized trial. Circulation 2014; Vol. 130:A19294.
- Newman ABD, Kritchevsky S, Beavers MM. Cardiovascular events in a physical activity intervention as compared to a successful aging intervention: the LIFE study randomized trial. Circulation 2014; Vol. 130:A19294. [DOI] [PMC free article] [PubMed]
Lundgren 1989 {published data only}
- Lundgren F, Dahllof AG, Lundholm K, Schersten T, Volkmann R. Intermittent claudication ‐ surgical reconstruction or physical training? A prospective randomised trial of treatment efficiency. Annals of Surgery 1989;209(3):346‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren F, Dahllof AG, Schersten T, Bylund‐Fellenius AC. Muscle enzyme adaptation in patients with peripheral arterial insufficiency: spontaneous adaptation, effect of different treatments and consequences on walking performance. Clinical Science 1989;77(5):485‐93. [DOI] [PubMed] [Google Scholar]
Maejima 2005 {published data only}
- Maejima Y, Yasu T, Ueba H, Kobayashi N, Hashimoto S, Kubo N, et al. Exercise after heparin administration: new therapeutic program for patients with‐option arteriosclerosis oblitrans. Circulation Journal 2005;69(9):1099‐104. [DOI] [PubMed] [Google Scholar]
Mannarino 1988 {published data only}
- Mannarino E, Menna M, Pasqualini L, Innocente S, Regni O. Effect of physical exercise in patients with peripheral occlusive arteriopathy. Controlled study. Riabilitazione 1988;21(2):123‐31. [Google Scholar]
Mannarino 1989 {published data only}
- Mannarino E, Pasqualini L, Menna M, Maragoni G, Orlandi U. Effects of physical training on peripheral vascular disease: a controlled study. Angiology 1989;40(1):5‐10. [DOI] [PubMed] [Google Scholar]
Martinez 2009 {published data only}
- Martinez CA, Carmeli E, Barak S, Stopka CB. Changes in pain‐free walking based on time in accommodating pain‐free exercise therapy for peripheral arterial disease. Journal of Vascular Nursing 2009;27(1):2‐7. [DOI] [PubMed] [Google Scholar]
Mays 2015 {published data only}
- Mays RJ. Community‐based walking exercise for patients with peripheral artery disease: a pilot study moderated. Journal of the American College of Cardiology 2014; Vol. 63, issue 12(Suppl 1):A2035.
- Mays RJ, Hiatt WR, Casserly IP, Rogers RK, Main DS, Kohrt WM, et al. Community‐based walking exercise for peripheral artery disease: an exploratory pilot study. Vascular Medicine 2015;20(4):339‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazari 2010 {published data only}
- Lee HLD, Gulati S, Mehta T, Mekako AI, Rahman MNA, McCollum P, et al. Early result of a randomised controlled trial of treatment for intermittent claudication. The Vascular Society of Great Britain & Ireland Yearbook. 2007:78.
- Mazari F, Khan J, Abdul Rahman MNA, Mehta T, Gulati S, McCollum P, et al. Cost utility analysis of a randomised control trial of percutaneous transluminal angioplasty (PTA), supervised exercise programme (SEP) and combined treatment (PTA+SEP) for patients with intermittent claudication (IC) due to femoropopliteal disease. The Vascular Society of Great Britain and Ireland Yearbook. 2009:44.
- Mazari FA, Gulati S, Rahman MN, Lee HL, Mehta TA, McCollum PT, et al. Early outcomes from a randomized, controlled trial of supervised exercise, angioplasty, and combined therapy in intermittent claudication. Annals of Vascular Surgery 2010;24(1):69‐79. [DOI] [PubMed] [Google Scholar]
- Mazari FA, Khan JA, Carradice D, Samuel N, Abdul Rahman MN, Gulati S, et al. Randomized clinical trial of percutaneous transluminal angioplasty, supervised exercise and combined treatment for intermittent claudication due to femoropopliteal arterial disease. British Journal of Surgery 2012;99(1):39‐48. [DOI] [PubMed] [Google Scholar]
- Mazari FAK, Mehta T, Rahman MNA, McCollum P, Chetter IC. A RCT of non‐surgical treatment for intermittent claudication in femoro‐popliteal disease: 12‐month results. The Vascular Society of Great Britain & Ireland Yearbook. 2008:75.
McDermott 2004 {published data only}
- McDermott MM, Tiukinhoy S, Greenland P, Liu K, Pearce WH, Guralnik JM, et al. A pilot exercise intervention to improve lower extremity functioning in peripheral arterial disease unaccompanied by intermittent claudication. Journal of Cardiopulmonary Rehabilitation 2004;24(3):187‐96. [DOI] [PubMed] [Google Scholar]
Nawaz 1999 {published data only}
- Nawaz S, Walker RD, Pockley AG, Wood RF. A safer way to exercise claudicants?. British Journal of Surgery 1999;86(Suppl 1):10‐1. [Google Scholar]
Nawaz 2001 {published data only}
- Nawaz S, Walker RD, Wilkinson CH, Saxton JM, Pockley AG, Wood RF. The inflammatory response to upper and lower limb exercise and the effects of exercise training in patients with claudication. Journal of Vascular Surgery 2001;33(2):392‐9. [DOI] [PubMed] [Google Scholar]
NCT01065740 {published data only}
- NCT01065740. Optimized supervised education program for peripheral arterial disease (POPART). clinicaltrials.gov/ct2/show/NCT01065740. Date first received: 8 February 2010.
NCT01241747 {published data only}
- NCT01241747. Exercise for women with peripheral arterial disease. clinicaltrials.gov/show/NCT01241747. Date first received: 15 November 2010.
NCT02075502 {published data only}
- NCT02075502. Community walking exercise for patients with peripheral artery disease (GAIT). clinicaltrials.gov/ct2/show/NCT02075502. Date first received: 13 February 2014.
NCT02879019 {published data only}
- NCT02879019. Walking training in peripheral artery disease (GrEnADa Sub‐study) (GrEnADa). clinicaltrials.gov/ct2/show/NCT02879019. Date first received: 11 August 2016.
Necker 2003 {published data only}
- Necker A, Schumm T, Liu Y, Baur C, Steinacker JM, Lehmann M. Effects of training after angioplasty on performance and circulation of the lower extremities in peripheral occlusive arterial disease. Deutsche Zeitschrift fur Sportmedizin 2001;52:s67. [Google Scholar]
- Necker A, Stilgenbauer F, Schumm T, Liu Y, Steinacker JM. Effects of training after angioplasty on walking distance, blood flow and muscular HSP70 expression in peripheral occlusive arterial disease. Deutsche Zeitschrift fur Sportmedizin 2003;54:S46. [Google Scholar]
Nicolai 2010 {published data only}
- Nicolai SP, Leffers P, Kruidenier LM, De‐Bie RA, Prins MH, Teijink JA. Extending the range of treadmill testing for patients with intermittent claudication. Medicine and Science in Sports and Exercise 2010;42(4):640‐5. [DOI] [PubMed] [Google Scholar]
Nielsen 1977 {published data only}
- Nielsen SL, Gyntelberg F, Larsen B, Lassen NA. Hospital versus home training, a clinical trial. Aktuelle Probleme in der Angiologie 1975;30:121‐6. [Google Scholar]
- Nielsen SL, Larsen B, Prahl M, Jensen CT, Jensen BE, Wenkens V. Hospital training compared with home training in patients with intermittent claudication. [Danish]. Ugeskrift for Laeger 1977;139(46):2733‐6. [PubMed] [Google Scholar]
Nordanstig 2011 {published data only}
- NCT01219842. Invasive revascularization or not in intermittent claudication ‐ a randomised controlled trial IRONIC. clinicaltrials.gov/ct2/show/NCT01219842. Date first received: 12 October 2010.
- Nordanstig J, Gelin J, Hensater M, Taft C, Osterberg K, Jivegard L. Walking performance and health‐related quality of life after surgical or endovascular invasive versus non‐invasive treatment for intermittent claudication ‐ a prospective randomised trial. European Journal of Vascular and Endovascular Surgery 2011;42(2):220‐7. [DOI] [PubMed] [Google Scholar]
Parr 2009 {published data only}
- Parr BM, Noakes TD, Derman EW. Peripheral arterial disease and intermittent claudication: efficacy of short‐term upper body strength training, dynamic exercise training, and advice to exercise at home. South African Medical Journal 2009;99(11):800‐4. [PubMed] [Google Scholar]
Patterson 1997 {published data only}
- Patterson RB, Pinto B, Marcus B, Colucci A, Braun T, Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. Journal of Vascular Surgery 1997;25(2):312‐9. [DOI] [PubMed] [Google Scholar]
Pinto 1997 {published data only}
- Pinto BM, Marcus BH, Patterson RB, Roberts M, Colucci A, Braun C. On‐site versus home exercise programs: psychological benefits for individuals with arterial claudication. Journal of Aging and Physical Activity 1997;5(4):311. [Google Scholar]
Presern‐Strukelj 200 {published data only}
- Presern‐Strukelj M, Poredos P. The influence of electrostimulation on the circulation of the remaining leg in patients with one‐sided amputation. Angiology 2002;53(3):329‐35. [DOI] [PubMed] [Google Scholar]
PROPEL study {published data only}
- Domanchuk K, Ferrucci L, Guralnik JM, Criqui MH, Tian L, Liu K, et al. Progenitor cell release plus exercise to improve functional performance in peripheral artery disease: the PROPEL study. Contemporary Clinical Trials 2013;36(2):502‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber R, Liu K, Ferrucci L, Criqui MH, Zhao L, Tian L, et al. Ischemia‐related changes in circulating stem and progenitor cells and associated clinical characteristics in peripheral artery disease. Vascular Medicine 2015;20(6):534‐43. [DOI: 10.1177/1358863X15600255] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riccioni 2010 {published data only}
- Riccioni C, Sarcinella R, Izzo A, Palermo G, Liguori M, Caliumi C, et al. Rehabilitative treatment in peripheral artery disease: protocol application and follow‐up. Minerva Cardioangiologica 2010;58(5):551‐65. [PubMed] [Google Scholar]
Richardson 1991 {published data only}
- Richardson JK. Rocker‐soled shoes and walking distance in patients with calf claudication. Archives of Physical Medicine and Rehabilitation 1991;72(8):554‐8. [PubMed] [Google Scholar]
Riebe 2001 {published data only}
- Riebe D, Patterson RB, Braun CM. Comparison of two progressive treadmill tests in patients with peripheral arterial disease. Vascular Medicine 2001;6(4):215‐21. [DOI] [PubMed] [Google Scholar]
Ritti‐Dias 2010 {published data only}
- Meneses AL, Lima GH, Forjaz CL, Lima AH, Silva GQ, Cucato GG, et al. Impact of a supervised strength training or walking training over a subsequent unsupervised therapy period on walking capacity in patients with claudication. Journal of Vascular Nursing 2011;29(2):81‐6. [DOI] [PubMed] [Google Scholar]
- Ritti‐Dias RM, Wolosker N, Moraes Forjaz CL, Carvalho CR, Cucato GG, Leão PP, et al. Strength training increases walking tolerance in intermittent claudication patients: randomized trial. Journal of Vascular Surgery 2010;51(1):89‐95. [DOI] [PubMed] [Google Scholar]
Rodrigues 2014 {published data only}
- Rodrigues L, Forjaz C. A single bout of resistance exercise does not modify cardiovascular responses during daily activities in patients with peripheral artery disease. Blood Pressure Monitoring 2014;19(2):64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saleem 2011 {published data only}
- Saleem H, Delaney C, Puckridge P, Foreman RT, Spark JI. What is the most appropriate exercise programme for patients with intermittent claudication? A study based on clinical response and inflammatory markers [Abstract]. ANZ Journal of Surgery 2011;81:A95. [Google Scholar]
Savage 2001 {published data only}
- Savage P, Ricci MA, Lynn M, Gardner A, Knight S, Brochu M, et al. Effects of home versus supervised exercise for patients with intermittent claudication. Journal of Cardiopulmonary Rehabilitation 2001;21(3):152‐7. [DOI] [PubMed] [Google Scholar]
Scheffler 1991 {published data only}
- Scheffler P, Hamette D, Muller H. Controlled vascular training in IIb peripheral arterial occlusive disease: additive effect of intravenous PGE1 versus intravenous pentoxifylline during training [German]. Vasa Supplementum 1991;33:350‐2. [PubMed] [Google Scholar]
Schlager 2011a {published data only}
- Schlager O, Giurgea A, Schuhfried O, Seidinger D, Gschwandtner ME, Fialka‐Moser V, et al. Supervised exercise training has no effect on inflammation and platelet activation in peripheral arterial disease. Circulation 2011;124:A11379. [Google Scholar]
Schoneberger 1994 {published data only}
- Schoneberger A, Horeis M, Raschka C. The effects of kinetotherapy and electrotherapy on the maximum walking distance of patients with peripheral arterial occlusion at stage II according to Fontaine. International Journal of Sports Medicine. 15 1994; Vol. 15:356.
Silvestro 2002 {published data only}
- Silvestro A, Scopacasa F, Oliva G, Cristofaro T, Iuliano L, Brevetti G. Vitamin C prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication. Atherosclerosis 2002;165(2):277‐83. [DOI] [PubMed] [Google Scholar]
Slordahl 2005 {published data only}
- Slordahl SA, Wang E, Hoff J, Kemi OJ, Amundsen BH, Helgerud J. Effective training for patients with intermittent claudication. Scandinavian Cardiovascular Journal 2005;39(4):244‐9. [DOI] [PubMed] [Google Scholar]
Snabl 1958 {published data only}
- Snabl P, Fiala Z, Polak F. The treatment of lower extremity arterial disease. Vnitrni Lekarstvi 1958;4(3):253‐8. [PubMed] [Google Scholar]
Sonaglia 2013 {published data only}
- Sonaglia F, Milia P, Caserio M, Bigazzi B, Bigazzi B, Ricotta S, et al. Efficacy and safety of oral porcine relaxin (pRLX) in adjunct to physical exercise in the treatment of peripheral arterial disease (PAD). Italian Journal of Anatomy and Embryology 2013;118(1 Suppl):84‐91. [PubMed] [Google Scholar]
Spronk 2009 {published data only}
- ISRCTN64443682. Comparing exercise training and angioplasty for claudication: a randomised controlled trial. http://www.controlled‐trials.com/ISRCTN64443682 (accessed December 2013).
- Spronk S, Bosch JL, Hoed PT, Veen HF, Pattynama PM, Hunink MG. Cost‐effectiveness of endovascular revascularization compared to supervised hospital‐based exercise training in patients with intermittent claudication: a randomized controlled trial. Journal of Vascular Surgery 2008;48(6):1472‐80. [DOI] [PubMed] [Google Scholar]
- Spronk S, Bosch JL, Hoed PT, Veen HF, Pattynama PM, Hunink MG. Intermittent claudication: clinical effectiveness of endovascular revascularization versus supervised hospital‐based exercise training ‐ randomized controlled trial. Radiology 2009;250(2):586‐95. [DOI] [PubMed] [Google Scholar]
Stewart 2008 {published data only}
- Stewart AH, Smith FC, Baird RN, Lamont PM. Local versus systemic mechanisms underlying supervised exercise training for intermittent claudication. Vascular and Endovascular Surgery 2008;42(4):314‐20. [DOI] [PubMed] [Google Scholar]
Streminski 1992 {published data only}
- Streminski JA, Haye R, Rettig K, Kuntz G. Comparison of the effectiveness of physical training with parental drug therapy in Fontaine stage IIb peripheral arterial occlusive disease. VASA 1992;21(4):392‐402. [PubMed] [Google Scholar]
SUPER study {published data only}
- Frans FA, Bipat S, Reekers JA, Legemate DA, Koelemay MJ, SUPER Study Collaborators. SUPERvised exercise therapy or immediate PTA for intermittent claudication in patients with an iliac artery obstruction ‐ a multicentre randomised controlled trial; SUPER study design and rationale. European Journal of Vascular and Endovascular Surgery 2012;43(4):466‐71. [DOI] [PubMed] [Google Scholar]
- NTR2776. SUPERvised exercise therapy or immediate PTA for intermittent claudication in patients with an iliac artery obstruction: a randomized controlled trial. SUPER study. http://apps.who.int/trialsearch/Trial.aspx?TrialID=NTR2776 (accessed December 2013). [DOI] [PubMed]
Taft 2004 {published data only}
- Taft C, Sullivan M, Lundholm K, Karlsson J, Gelin J, Jivegard L. Predictors of treatment outcome in intermittent claudication. European Journal of Vascular and Endovascular Surgery 2004;27(1):24‐32. [DOI] [PubMed] [Google Scholar]
Tebbutt 2011 {published data only}
Thomson 1999 {published data only}
- Thomson IA, Rij AM, Morrison ND, Packer SGK, Christie R. A ten year randomised controlled trial of percutaneous femoropopliteal angioplasty for claudication. Australia and New Zealand Journal of Surgery 1999;69(Suppl):98. [Google Scholar]
Treat‐Jacobson 2012 {published data only}
- Treat‐Jacobson DJ, Bronas UG, Krause BJ, Robinson CA, Santili SM, Leon AS. Aerobic arm exercise training to improve outcomes for patients with severe claudication and ischemic rest pain. Vascular Medicine 2012;17(3):204. [Google Scholar]
Ventura 1984 {published data only}
- Ventura MR, Young DE, Feldman MJ, Pastore P, Pikula S, Yates MA. Effectiveness of health promotion interventions. Nursing Research 1984;33(3):162‐7. [PubMed] [Google Scholar]
Walker 2000 {published data only}
- Walker RD, Nawaz S, Wilkinson CH, Saxton JM, Pockley AG, Wood RF. Influence of upper‐ and lower‐limb exercise training on cardiovascular function and walking distances in patients with intermittent claudication. Journal of Vascular Surgery 2000;31(4):662‐9. [DOI] [PubMed] [Google Scholar]
Waller 1988 {published data only}
- Waller PC, Solomon SA, Ramsay LE. Cigarette smoking and treadmill exercise distances in patients with intermittent claudication: the effects of suggestion. Proceedings of The British Pharmacological Society. 1988:632P‐3P.
Wang 2008 {published data only}
- Wang E, Hoff J, Loe H, Kaehler N, Helgerud J. Plantar flexion: an effective training for peripheral arterial disease. European Journal of Applied Physiology 2008;104(4):749‐56. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang E, Helgerud J, Loe H, Indseth K, Kaehler N, Hoff J. Maximal strength training improves walking performance in peripheral arterial disease patients. Scandinavian Journal of Medicine and Science in Sports 2010;20(5):764‐70. [DOI] [PubMed] [Google Scholar]
Winterfeld 1983 {published data only}
- Winterfeld HJ, Siewert H, Strangfeld D, Schmidt HH. Experiences with the application of physiotherapy in peripheral arterial circulatory disorders of the lower extremities (stage I and IIa) under ambulatory conditions. Zeitschrift für die gesamte innere Medizin und ihre Grenzgebiete 1983;38(7):221‐5. [PubMed] [Google Scholar]
Zwierska 2004 {published data only}
- Zwierska I, Nawaz S, Walker RD, Wood RF, Pockley AG, Saxton JM. Treadmill versus shuttle walk tests of walking ability in intermittent claudication. Medicine and Science in Sports and Exercise 2004;36(11):1835‐40. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01231360 {published data only}
- NCT01231360. The effect of exercise training on skeletal muscle metabolism in peripheral artery disease (PAD). clinicaltrials.gov/ct2/show/NCT01231360. Date first received: 28 October 2010.
NCT01822457 {published data only}
- NCT01822457. Effect of Nike Fuel Band on exercise and function in claudicants: a randomised controlled trial. clinicaltrials.gov/ct2/show/NCT01822457. Date first received: 19 February 2013.
Additional references
Antoniou 2017
- Antoniou GA, Georgiadis GS, Antoniou SA, Makar RR, Smout JD, Torella F. Bypass surgery for chronic lower limb ischaemia. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD002000.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumgartner 1998
- Baumgartner TA, Strong CH. Conducting and Reading Research. 2nd Edition. New York: WCB/Mcgraw‐Hill, 1998. [Google Scholar]
Boutroux 1980
- Boutroux YC, Baccard D, Bouchet JY. Programmed physical training in physiotherapy for obliterative arteriopathies of the lower limbs at the stage of claudication (authors' transl). Journal des Maladies Vasculaires 1980;5(3):173‐6. [PubMed] [Google Scholar]
Clyne 1985
- Clyne CAC, Mears H, Weller RO, O'Donnell TF. Calf muscle adaptation to peripheral vascular disease. Cardiovascular Research 1985;19(8):507‐12. [DOI] [PubMed] [Google Scholar]
Collins 2003
- Collins EG, Langbein WE, Orebaugh C, Bammert C, Hanson K, Reda D, et al. Polestriding exercise and vitamin E for management of peripheral vascular disease. Medicine and Science in Sports and Exercise 2003;35(3):384‐93. [DOI] [PubMed] [Google Scholar]
Cucato 2013b
- Cucato GG, Zerati AE, Rocha Chehuen M, Ritti‐Dias RM, Saez G, Ragazzo L, et al. Comparison between subjective and objective methods to assess functional capacity during clinical treatment in patients with intermittent claudication [Comparação entre os métodos subjetivo e objetivo para avaliação da capacidade funcional durante tratamento clínico em pacientes com claudicação intermitente]. Einstein (Sao Paulo) 2013;11(4):495‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunningham 2014
- Cunningham MA, Swanson V, Pappas E, O'Carroll RE, Holdsworth RJ. Illness beliefs and walking behavior after revascularization for intermittent claudication: a qualitative study. Journal of Cardiopulmonary Rehabilitation and Prevention 2014;34(3):195‐201. [DOI] [PubMed] [Google Scholar]
Câmara 2012
- Câmara LC, Ritti‐Dias RM, Menêses AL, D'Andréa Greve JM, Filho WJ, Santarém JM, et al. Isokinetic strength and endurance in proximal and distal muscles in patients with peripheral artery disease. Annals of Vascular Surgery 2012;26(8):1114‐9. [DOI] [PubMed] [Google Scholar]
Delaney 2014
- Delaney CL, Miller MD, Chataway TK, Spark JI. A randomised controlled trial of supervised exercise regimens and their impact on walking performance, skeletal muscle mass and calpain activity in patients with intermittent claudication. European Journal of Vascular and Endovascular Surgery 2014;47(3):304‐10. [DOI] [PubMed] [Google Scholar]
Ernst 1987a
- Ernst E. Physical exercise for peripheral vascular disease: a review. VASA 1987;16(3):227‐31. [PubMed] [Google Scholar]
Ernst 1992
- Ernst E. Exercise: the best therapy for intermittent claudication?. British Journal of Hospital Medicine 1992;48(6):303‐4, 307. [PubMed] [Google Scholar]
Fakhry 2013
- Fakhry F, Fokkenrood HJP, Rouwet EV, Teijink JAW, Spronk S, Hunink MGM. Endovascular revascularisation versus conservative management for intermittent claudication. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD010512] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fokkenrood 2013
- Fokkenrood HJP, Bendermacher BLW, Lauret GJ, Willigendael EM, Prins MH, Teijink JAW. Supervised exercise therapy versus non‐supervised exercise therapy for intermittent claudication. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD005263.pub3] [DOI] [PubMed] [Google Scholar]
Fowkes 1998
- Fowkes G, Gillespie IN. Angioplasty (versus non surgical management) for intermittent claudication. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000017] [DOI] [PubMed] [Google Scholar]
Gardner 1995
- Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta analysis. JAMA 1995;274(12):975‐80. [PubMed] [Google Scholar]
Gohil 2013
- Gohil RA, Mockford KA, Mazari FA, Khan JA, Vanicek N, Chetter IC, et al. Percutaneous transluminal angioplasty results in improved physical function but not balance in patients with intermittent claudication. Journal of Vascular Surgery 2013;58(6):1533‐9. [DOI] [PubMed] [Google Scholar]
Gohil 2013a
- Gohil RA, Lane TRA, Coughlin PA. Review of the adaptation of skeletal muscle in intermittent claudication. World Journal of Cardiovascular Diseases 2013;3:347‐60. [Google Scholar]
Gommans 2016
- Gommans LN, Smid AT, Scheltinga MR, Brooijmans FA, Disseldorp EM, Linden FT, et al. Altered joint kinematics and increased electromyographic muscle activity during walking in patients with intermittent claudication. Journal of Vascular Surgery 2016;63(3):664‐72. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 4 July 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Haas 2012
- Haas TL, Lloyd PG, Yang HT, Terjung RL. Exercise training and peripheral arterial disease. Comprehensive Physiology 2012;2(4):2933‐3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Housley 1988
- Housley E. Treating claudication in five words. BMJ (Clinical Research Edition) 1988;296(6635):1483‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jolly 2009
- Jolly K, Lip GYH, Taylor RS, Raftery J, Mant J, Lane D, et al. The Birmingham rehabilitation uptake maximisation study (BRUM): a randomised controlled trial comparing home‐based with centre‐based cardiac rehabilitation. Heart 2009;95(1):36‐42. [DOI] [PubMed] [Google Scholar]
King 2012
- King S, Vanicek N, Mockford KA, Coughlin PA. The effect of a 3‐month supervised exercise programme on gait parameters of patients with peripheral arterial disease and intermittent claudication. Clinical Biomechanics (Bristol, Avon) 2012;27(8):845‐51. [DOI] [PubMed] [Google Scholar]
King 2015
- King S, Vanicek N, O'Brien TD. Dynamic muscle quality of the plantar flexors is impaired in claudicant patients with peripheral arterial disease and associated with poorer walking endurance. Journal of Vascular Surgery 2015;62(3):689‐97. [DOI: 10.1016/j.jvs.2015.03.039] [DOI] [PubMed] [Google Scholar]
Koutakis 2010
- Koutakis P, Pipinos II, Myers SA, Stergiou N, Lynch TG, Johanning JM. Joint torques and powers are reduced during ambulation for both limbs in patients with unilateral claudication. Journal of Vascular Surgery 2010;51(1):80‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koutakis 2015
- Koutakis P, Myers SA, Cluff K, Ha DM, Haynatzki G, McComb RD, et al. Abnormal myofiber morphology and limb dysfunction in claudication. Journal of Surgical Research 2015;196(1):172‐9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauret 2012
- Lauret GJ, Dalen DC, Willigendael EM, Hendriks EJ, Bie RA, Spronk S, et al. Supervised exercise therapy for intermittent claudication: current status and future perspectives. Vascular 2012;20(1):12‐9. [DOI] [PubMed] [Google Scholar]
Lauret 2012a
- Lauret GJ, Dalen HC, Hendriks HJ, Sterkenburg SM, Koelemay MJ, Zeebregts CJ, et al. When is supervised exercise therapy considered useful in peripheral arterial occlusive disease? A nationwide survey among vascular surgeons. European Journal of Vascular and Endovascular Surgery 2012;43(3):308‐12. [DOI] [PubMed] [Google Scholar]
Leng 1993
- Leng GC, Fowkes FGR. The epidemiology of peripheral arterial disease. Vascular Medicine Review 1993;4(1):5‐18. [Google Scholar]
Lundgren 1989a
- Lundgren F, Dahllöf AG, Scherstén T, Bylund‐Fellenius AC. Muscle enzyme adaptation in patients with peripheral arterial insufficiency: spontaneous adaptation, effect of different treatments and consequences on walking performance. Clinical Science 1989;77(5):485‐93. [DOI] [PubMed] [Google Scholar]
McDermott 2006
- McDermott MM. The magnitude of the problem of peripheral arterial disease: epidemiology and clinical significance. Cleveland Clinic Journal of Medicine 2006;73(Suppl 4):S2‐7. [DOI] [PubMed] [Google Scholar]
McDermott 2011
- McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Liao Y, et al. Greater sedentary hours and slower walking speed outside the home predict faster declines in functioning and adverse calf muscle changes in peripheral arterial disease. Journal of the American College of Cardiology 2011;57(23):2356‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDermott 2012
- McDermott MM, Liu K, Tian L, Guralnik JM, Criqui MH, Liao Y, et al. Calf muscle characteristics, strength measures, and mortality in peripheral arterial disease: a longitudinal study. Journal of the American College of Cardiology 2012;59(13):1159‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDermott 2013
- McDermott M. A home‐based exercise intervention significantly improves walking performance in peripheral arterial disease: one‐year follow‐up from a randomized controlled trial. Circulation 2013;128:A14789. [Google Scholar]
McDermott 2013a
- McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, et al. Home‐based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013;310(1):57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDermott 2014
- McDermott M, Criqui M, Domanchuk K, Guralnik J, Kibbe M, Liu K, et al. Home‐based exercise improves walking speed and prevents mobility loss in peripheral artery disease: a randomized controlled trial. Circulation 2014; Vol. 130:A11744.
McDermott 2014a
- McDermott MM, Guralnik JM, Criqui MH, Ferrucci L, Zhao L, Liu K, et al. Home‐based walking exercise in peripheral artery disease: 12‐month follow‐up of the GOALS randomized trial. Journal of the American Heart Association 2014;3(3):e000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mockford 2014
- Mockford KA, Gohil RA, Mazari F, Khan JA, Vanicek N, Coughlin PA, et al. Effect of supervised exercise on physical function and balance in patients with intermittent claudication. British Journal of Surgery 2014;101(4):356‐62. [DOI] [PubMed] [Google Scholar]
NCGC 2012
- National Clinical Guideline Centre. Lower limb peripheral arterial disease. Diagnosis and management. NICE Clinical Guideline 147. http://www.nice.org.uk/nicemedia/live/13856/60426/60426.pdf (accessed September 2013).
Parmenter 2011
- Parmenter BJ, Raymond J, Dinnen P, Singh MA. A systematic review of randomized controlled trials: walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis 2011;218(1):1‐12. [DOI] [PubMed] [Google Scholar]
Parmenter 2013
- Parmenter BJ, Raymond J, Fiatarone Singh MA. The effect of exercise on fitness and performance‐based tests of function in intermittent claudication: a systematic review. Sports Medicine (Auckland, N.Z.) 2013;43(6):513‐24. [DOI] [PubMed] [Google Scholar]
Parmenter 2013a
- Parmenter BJ, Raymond J, Dinnen PJ, Lusby RJ, Fiatarone Singh MA. Preliminary evidence that low ankle‐brachial index is associated with reduced bilateral hip extensor strength and functional mobility in peripheral arterial disease. Journal of Vascular Surgery 2013;57(4):963‐73. [DOI] [PubMed] [Google Scholar]
Regensteiner 1993
- Regensteiner JG, Wolfel EE, Brass EP, Carry MR, Ringel SP, Hargarten ME, et al. Chronic changes in skeletal muscle histology and function in peripheral arterial disease. Circulation 1993;87(2):413‐21. [DOI] [PubMed] [Google Scholar]
Rejeski 2014
- Rejeski WJS, Spring B, Domanchuk K, Tao H, Tian L, Zhao L, et al. A group‐mediated, home‐based physical activity intervention for patients with peripheral artery disease: effects on social and psychological function. Journal of Translational Medicine 2014;12(29):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruell 1984
- Ruell PA, Imperial ES, Bonar FJ, Thursby PF, Gass GC. Intermittent claudication. The effect of physical training on walking tolerance and venous lactate concentration. European Journal of Applied Physiology and Occupational Physiology 1984;52(4):420‐5. [DOI] [PubMed] [Google Scholar]
Sjöström 1982
- Sjöström M, Neglén P, Fridén J, Eklöf B. Human skeletal muscle metabolism and morphology after temporary incomplete ischaemia. European Journal of Clinical Investigation 1982;12(1):69‐79. [DOI] [PubMed] [Google Scholar]
Terjung 1988
- Terjung RL, Mathien GM, Erney TP, Ogilvie RW. Peripheral adaptations to low blood flow in muscle during exercise. American Journal of Cardiology 1988;62(8):15E‐9E. [DOI] [PubMed] [Google Scholar]
Tew 2013
- Tew G, Copeland R, Faucheur A, Gernigon M, Nawaz S, Abraham P. Feasibility and validity of self‐reported walking capacity in patients with intermittent claudication. Journal of Vascular Surgery 2013;57(5):1227‐34. [DOI] [PubMed] [Google Scholar]
Trialists 1994
- Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy ‐ I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308(6921):81‐106. [PMC free article] [PubMed] [Google Scholar]
Wang 2009
- Wang J, Zhou S, Bronks R, Graham J, Myers S. Effects of supervised treadmill walking training on calf muscle capillarization in patients with intermittent claudication. Angiology 2009;60(1):36‐41. [DOI] [PubMed] [Google Scholar]
Willigendael 2005
- Willigendael EM, Bendermacher BL, Berg C, Welten RJ, Prins MH, Bie RA, et al. The development and implementation of a regional network of physiotherapists for exercise therapy in patients with peripheral arterial disease, a preliminary report. BMC Health Services Research 2005;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 1991
- Yang HT, Ogilvie RW, Terjung RL. Low‐intensity training produces muscle adaptations in rats with femoral artery stenosis. Journal of Applied Physiology 1991;71(5):1822‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lane 2014
- Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD000990.pub3] [DOI] [PubMed] [Google Scholar]
Leng 2000
- Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000990] [DOI] [PubMed] [Google Scholar]
Watson 2008
- Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD000990.pub2] [DOI] [PubMed] [Google Scholar]


