Abstract
Background
Asthma is one of the most common long‐term conditions worldwide, which places considerable pressure on patients, communities and health systems. The major international clinical guidelines now recommend the inclusion of self management programmes in the routine management of patients with asthma. These programmes have been associated with improved outcomes in patients with asthma. However, the implementation of self management programmes in clinical practice, and their uptake by patients, is still poor. Recent developments in mobile technology, such as smartphone and tablet computer apps, could help develop a platform for the delivery of self management interventions that are highly customisable, low‐cost and easily accessible.
Objectives
To assess the effectiveness, cost‐effectiveness and feasibility of using smartphone and tablet apps to facilitate the self management of individuals with asthma.
Search methods
We searched the Cochrane Airways Group Register (CAGR), the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO, CINAHL, Global Health Library, Compendex/Inspec/Referex, IEEEXplore, ACM Digital Library, CiteSeerx and CAB abstracts via Web of Knowledge. We also searched registers of current and ongoing trials and the grey literature. We checked the reference lists of all primary studies and review articles for additional references. We searched for studies published from 2000 onwards. The latest search was run in June 2013.
Selection criteria
We included parallel randomised controlled trials (RCTs) that compared self management interventions for patients with clinician‐diagnosed asthma delivered via smartphone apps to self management interventions delivered via traditional methods (e.g. paper‐based asthma diaries).
Data collection and analysis
We used standard methods expected by the Cochrane Collaboration. Our primary outcomes were symptom scores; frequency of healthcare visits due to asthma exacerbations or complications and health‐related quality of life.
Main results
We included two RCTs with a total of 408 participants. We found no cluster RCTs, controlled before and after studies or interrupted time series studies that met the inclusion criteria for this systematic review. Both RCTs evaluated the effect of a mobile phone‐based asthma self management intervention on asthma control by comparing it to traditional, paper‐based asthma self management. One study allowed participants to keep daily entries of their asthma symptoms, asthma medication usage, peak flow readings and peak flow variability on their mobile phone, from which their level of asthma control was calculated remotely and displayed together with the corresponding asthma self management recommendations. In the other study, participants recorded the same readings twice daily, and they received immediate self management feedback in the form of a three‐colour traffic light display on their phones. Participants falling into the amber zone of their action plan twice, or into the red zone once, received a phone call from an asthma nurse who enquired about the reasons for their uncontrolled asthma.
We did not conduct a meta‐analysis of the data extracted due to the considerable degree of heterogeneity between these studies. Instead we adopted a narrative synthesis approach. Overall, the results were inconclusive and we judged the evidence to have a GRADE rating of low quality because further evidence is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. In addition, there was not enough information in one of the included studies to assess the risk of bias for the majority of the domains. Although the other included study was methodologically rigorous, it was not possible to blind participants or personnel in the study. Moreover, there are concerns in both studies in relation to attrition bias and other sources of bias.
One study showed that the use of a smartphone app for the delivery of an asthma self management programme had no statistically significant effect on asthma symptom scores (mean difference (MD) 0.01, 95% confidence interval (CI) ‐0.23 to 0.25), asthma‐related quality of life (MD of mean scores 0.02, 95% CI ‐0.35 to 0.39), unscheduled visits to the emergency department (OR 7.20, 95% CI 0.37 to 140.76) or frequency of hospital admissions (odds ratio (OR) 3.07, 95% CI 0.32 to 29.83). The other included study found that the use of a smartphone app resulted in higher asthma‐related quality of life scores at six‐month follow‐up (MD 5.50, 95% CI 1.48 to 9.52 for the physical component score of the SF‐12 questionnaire; MD 6.00, 95% CI 2.51 to 9.49 for the mental component score of the SF‐12 questionnaire), improved lung function (PEFR) at four (MD 27.80, 95% CI 4.51 to 51.09), five (MD 31.40, 95% CI 8.51 to 54.29) and six months (MD 39.20, 95% CI 16.58 to 61.82), and reduced visits to the emergency department due to asthma‐related complications (OR 0.20, 95% CI 0.04 to 0.99). Both studies failed to find any statistical differences in terms of adherence to the intervention and occurrence of other asthma‐related complications.
Authors' conclusions
The current evidence base is not sufficient to advise clinical practitioners, policy‐makers and the general public with regards to the use of smartphone and tablet computer apps for the delivery of asthma self management programmes. In order to understand the efficacy of apps as standalone interventions, future research should attempt to minimise the differential clinical management of patients between control and intervention groups. Those studies evaluating apps as part of complex, multicomponent interventions, should attempt to tease out the relative contribution of each intervention component. Consideration of the theoretical constructs used to inform the development of the intervention would help to achieve this goal. Finally, researchers should also take into account: the role of ancillary components in moderating the observed effects, the seasonal nature of asthma and long‐term adherence to self management practices.
Keywords: Humans; Cell Phone; Computers, Handheld; Mobile Applications; Asthma; Asthma/therapy; Randomized Controlled Trials as Topic; Self Care; Self Care/instrumentation; Self Care/methods
Plain language summary
Can smartphone apps improve access to asthma self management?
Background
Self management programmes have been advocated as a means to help people with asthma achieve better levels of asthma control and better asthma‐related outcomes. However, there are a number of barriers affecting the successful implementation and uptake of these programmes. These barriers call for innovative approaches for the delivery of self management programmes. Of particular interest is the use of consumer devices such as smartphones and tablet computers as a means of delivering these programmes within the existing healthcare configuration.
Review question
This review assessed whether smartphone and tablet computer apps are effective tools for supporting patients with asthma to self manage their own condition.
Description of the studies
We included two studies with a total of 408 participants. Both studies evaluated the effect of a mobile phone‐based asthma self management intervention on asthma control by comparing it to traditional, paper‐based asthma self management. One study allowed participants to keep daily entries of their asthma symptoms, asthma medication usage, peak flow readings and peak flow variability on their mobile phone, from which their level of asthma control was calculated remotely and displayed together with the corresponding asthma self management recommendations. In the other study, participants recorded the same readings twice daily, and they received immediate self management feedback in the form of a three‐colour traffic light display on their phones. Participants falling into the amber zone of their action plan twice, or into the red zone once, received a phone call from an asthma nurse who enquired about the reasons for their uncontrolled asthma.
Key results
Due to the lack of enough included studies and the considerable differences between them, we were unable to obtain conclusive answers to our research question. One study showed that the use of a smartphone app can result in better asthma‐related quality of life and lung function, and reduced visits to the emergency department. The other study failed to show any significant improvements in asthma‐related outcomes after using a smartphone app as a delivery mechanism.
Quality of the evidence
The current evidence base is not sufficient to advise clinicians, policy‐makers and the general public with regards to the effectiveness of smartphone and tablet computer apps for the delivery of asthma self management programmes.
This plain language summary is current as of June 2013.
Summary of findings
for the main comparison.
| Smartphone apps compared with paper‐based diaries for asthma self management | |||
|
Patient or population: patients with clinician‐diagnosed asthma Settings: primary and tertiary care Intervention: smartphone app for asthma self management Comparison: paper‐based diaries for asthma self management | |||
| Outcomes | Effects of smartphone apps for asthma self management | No of participants (studies) | Quality of the evidence (GRADE) |
|
Symptom scores Asthma Control Questionnaire (ACQ) ‐ 6‐item version Mean differences in scores at 6 months |
One study found no statistically significant difference in the mean difference in ACQ scores between the intervention and control group at 6 months (MD 0.01, 95% CI ‐0.23 to 0.25) | 278 participants (1 study) | ⊕⊕⊝⊝ low1 |
|
Patients with unscheduled visits to the emergency department 6‐month follow‐up |
One study found that participants in the intervention group were less likely to attend the emergency department than those in the control group (OR 0.20, 95% CI 0.04 to 0.99). Another study found no statistically significant difference between the intervention and control groups (Fisher's exact test P = 0.12) | 370 participants (2 studies) |
⊕⊕⊝⊝ low1 |
|
Hospital admissions 6‐month follow‐up |
None of the included studies found a statistically significant difference between the intervention and control groups (Fisher's exact test yielding a one‐sided P = 0.52; OR 3.07 (95% CI 0.32 to 29.83)) | 370 participants (2 studies) | ⊕⊕⊝⊝ low1 |
|
GP consultations for asthma 6‐month follow‐up |
One study did not find a statistically significant difference between the intervention and control groups (OR 1.40, 95% CI 0.85 to 2.31) | 281 participants (1 study) |
⊕⊕⊝⊝ low1 |
|
Unscheduled general practice nurse consultation 6‐month follow‐up |
One study found that participants in the intervention group were less likely to attend unscheduled general practice nurse consultations than those in the control group (OR 0.60, 95% CI 0.37 to 0.98) | 281 participants (1 study) | ⊕⊕⊝⊝ low1 |
|
Out of hours attendances 6‐month follow‐up |
One study did not find a statistically significant difference between the intervention and control groups (OR 0.60, 95% CI 0.14 to 2.54) | 281 participants (1 study) | ⊕⊕⊝⊝ low1 |
|
HRQoL measured on the SF‐12 questionnaire ‐ SF ‐ 12 6‐month follow‐up |
One study found that participants in the intervention group had significantly higher scores in both the mental and physical components of the SF‐12 questionnaire than those in the control group (MD 6.00, 95% CI 2.51 to 9.49 and MD 5.50, 95% CI 1.48 to 9.52, respectively) | 89 participants (1 study) |
⊕⊕⊝⊝ low1 |
|
HRQoL measured on the mini‐AQLQ 6‐month follow‐up |
One study did not find a statistically significant difference between the intervention and control groups in their mean scores on the mini‐AQLQ (MD 0.02, 95% CI ‐0.35 to 0.39) | 201 participants (1 study) |
⊕⊕⊝⊝ low1 |
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; HRQoL: health‐related quality of life; MD: mean difference; OR: odds ratio | |||
1All outcomes were graded as low quality because further evidence is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. The two included studies were clinically heterogeneous and their results were therefore not combined. We also took into consideration the high/unclear risk of bias in our assessment of the quality of the evidence.
Background
Asthma is a common, chronic disorder of the airways characterised by paroxysmal and reversible obstruction of the airways in response to an inflammatory trigger (Van der Meer 2009). It is one of the most common chronic diseases worldwide, estimated to affect approximately 235 million individuals (WHO 2011). The increase in the global prevalence of asthma has been estimated at 50% per decade, most of which is accounted for by changing prevalence in low‐income settings (Braman 2006; Pearce 2000). This high burden of disease places significant pressure not only on health systems but also on patients, their families and communities (Masoli 2004; Ring 2011).
The cornerstone of asthma treatment is a stepwise approach, which aims to control symptoms, prevent acute asthma exacerbations and improve lung function (BTS‐SIGN 2011). Inhaled bronchodilators are the first component of this approach and can be complemented with inhaled or oral corticosteroids, leukotriene receptor antagonists and other drug classes in more severe cases. Traditionally, asthma treatment has been delivered under a paternalistic model of care in which patient involvement is usually limited to following physician recommendations (Foster 2007; Wilson 2010).
The recent shift towards partnership models of care has seen an increase in the importance given to self management as a means to encourage patients’ proactive participation in their own health care (Foster 2007). Self management programmes use a range of techniques in order to help patients develop condition‐specific management skills, make changes in health behaviour and accomplish desired health‐related goals (Creer 2008; Foster 2007; Wilson 2010). Asthma clinical guidelines advocate the inclusion of self management education as part of the routine management of patients with asthma (BTS‐SIGN 2011; GINA 2011; NHLBI 2007; Powell 2009). Although variability exists across different programmes, it is recommended that self management training incorporates: (1) structured information about the nature of the condition; (2) self monitoring of asthma symptoms or peak flow readings (or both); (3) regular medication review and (4) written asthma action plans (BTS‐SIGN 2011; Partridge 2008; Powell 2009). Self management programmes can help reduce the demand for, and increase the capacity of, healthcare resources whilst improving clinical outcomes for patients: self management programmes have been associated with improved asthma control, improved asthma‐related quality of life, and a reduction in the number of unscheduled healthcare visits and hospital admissions (Partridge 2008; Powell 2009; Ring 2007).
Despite the potential benefits of asthma self management programmes, their implementation in clinical practice is still poor, particularly in primary care (Partridge 2008; Ring 2011). Haughney 2004 found that in a sample of 517 asthma patients in the UK, 71% had received no asthma advice from their healthcare providers, and 80% had never been given a written, personalised asthma action plan. Similarly, a European survey conducted by Partridge 2011 revealed that approximately 64% of the patients interviewed had regular follow‐ups with their GPs; however, less than 33% had received a personalised, written asthma action plan, and only 28% had been given written information about asthma.
Even when implemented, action plans are underutilised by individuals with asthma (Kaya 2009; Lahdensuo 1999; Ring 2007; Verschelden 1996; Weinstein 2005). Action plans are self management strategies created jointly between the patient and physician that enable patients to identify changes in their asthma status and adjust their therapy accordingly (Gibson 2008). However, Haughney 2004 found that approximately 60% of patients with an asthma action plan do not adjust their medication in response to changes in their asthma. Moreover, Partridge 2011 found that of those asthma patients who reported adjusting their therapy, 55% did so according to how they felt and not on the basis of a plan or professional guidance.
The poor implementation of asthma self management programmes, and their underutilisation by patients, calls for innovative approaches. The rapid evolution of technology over the past few decades provides new opportunities for the design and delivery of self management initiatives within existing healthcare systems (Charles 2007; Pinnock 2007). Of particular interest is the use of consumer mobile communication devices for these purposes, operating within a field that has come to be known as mHealth (Estrin 2010; Istepanian 2005).
Smartphones (i.e. mobile phones with advanced computing and internet access) and tablet computers (i.e. general purpose computers contained in a single panel and usually operated through a touch screen) have become the most popular and widespread types of mobile device (mHealth Alliance 2010). Around 51% of British adults claim to own a smartphone (Ofcom 2013) and 10% are thought to own a tablet (MacLeod 2013). In the United States, a report by the Pew Research Center revealed that approximately 56% of all adults now own a smartphone (Smith 2013). Similarly, approximately 34% of American adults own a tablet computer (Zickuhr 2013). Worldwide, approximately 16.7% of the six billion mobile subscriptions correspond to smartphone subscriptions (mobiThinking 2013). As retail prices decline, ownership of these devices is likely to continue to increase (Ofcom 2010) in high‐income and low‐ and middle‐income countries.
Sophisticated computing features mean that both smartphones and tablet computers are capable of supporting self management functions and deliver them at a population level. Self management interventions could be offered within software extensions that users add to their devices, popularised under the term 'apps' (short for applications).
Description of the intervention
Asthma apps are software programs designed for smartphones and tablets, which aim to promote or support one or more asthma self management skills. Apps act as an optional add‐on to the device that interacts with users via a set of interfaces (e.g. a visual user interface).
Health apps can be characterised as a medium with broad capabilities to communicate information, provide interactive experiences and collect information from patients. They provide a platform for the delivery of self management interventions that are highly customisable, low‐cost and easily accessible.
How the intervention might work
Understanding how apps can support asthma self management can be structured by considering the theories that underpin self management itself. These theories (e.g. theory of planned behaviour, transtheoretical model, social cognitive theory) attempt to identify the skills and techniques needed for inducing changes in behaviours that, in turn, lead to desired outcomes. For asthma, these include self monitoring of symptoms and peak flow, appropriate medication taking and avoidance of triggers (Newman 2010). Apps can support the acquisition and maintenance of these practices through the provision of written and multimedia educational information, reminders for medication taking, and/or the creation of a space for patients to log their symptoms and peak flow. In this respect, they are similar to other media used for supporting asthma self management. Therefore, we need to consider app‐specific aspects that separate them from other media with regards to their potential impact on asthma‐related outcomes. Two aspects are salient: accessibility and perceived convenience.
Due to the wide uptake of mobile phone technology, health apps may be able to reach a large proportion of the population, particularly in settings where infrastructure and access to printed materials or face‐to‐face consultations is restricted (Masoli 2004). Most people tend to carry their mobile phones with them at all times, leaving them on even at night (Ofcom 2013). Ready access to apps running on a phone may promote engagement and reduce barriers to specific activities such as self monitoring. Unlike websites, apps can store login details and, by default, store the last used location within the app so that they can be resumed instantly. Apps can also alert an individual (or the clinical team) about the deterioration of asthma symptoms, prompting them to take timely action or seek timely care. Reminders linked to an electronic diary could help address non‐adherence or non‐attendance caused by forgetfulness.
Like websites and other software‐based interventions, apps offer a range of interactive possibilities that may enhance their effectiveness compared to static media. Electronic diary functions may support monitoring of symptoms, lung function, or both, and display the collected data back to patients in a meaningful fashion. Patients could then reflect upon these data and gain a better understanding of their own asthma. For example, they can identify trends in their asthma severity, or identify associations between a particular trigger and the development of asthma symptoms. Educational materials can be offered in a variety of multimedia formats and customised in order to match the patient’s informational needs. For example, users can choose to have these materials presented through a variety of multimedia modalities in order to satisfy their preferences. Additionally, they can choose to download and view only those modules or topics that are relevant to them. By taking advantage of modalities such as videos or animations, health apps can make structured education more accessible for illiterate patients or those with certain learning disabilities such as dyslexia.
Smartphone and tablet apps support the collection and immediate transfer of real‐time data. As part of more complex interventions, the transfer to a healthcare provider of personal health data might improve the quality of regular medical reviews. These data could potentially provide an objective measure of a patient’s asthma status. On reviewing these data, patients and their clinicians could have the opportunity of engaging in a more rewarding dialogue and set asthma‐related goals that are more appropriate to the patient’s condition.
Adverse effects of the intervention
Poor usability and technical difficulties with a mobile health app, or the hardware on which it operates, may compromise self management interventions and negatively affect health outcomes.
Health apps that provide incorrect or misleading information may harm patients if they subsequently follow that guidance. An appraisal of asthma apps conducted by Huckvale 2012 found that 32 out of 72 asthma apps made recommendations that were not supported by current evidence.
Smartphone and/or tablet adoption is usually associated with particular socio‐demographic groups. In the United States, for example, higher than average levels of smartphone adoption are seen amongst the wealthy and well‐educated; individuals younger than 45 years; and urban and suburban residents (Smith 2013). If a service chose to utilise apps to the exclusion of other treatment options, they could inadvertently exclude important sectors of the population for whom they care.
Why it is important to do this review
There is a large body of literature demonstrating the beneficial effect of self management education on defined outcomes in individuals with asthma (Boyd 2009; Gibson 2008; Gibson 2009; Tapp 2007; Welsh 2011; Wolf 2008). Previous reviews have also examined the role of information technologies in supporting self management education. Bussey‐Smith 2007 and Sanders 2006 assessed the effectiveness of computer‐based asthma self management programmes and found no consistent evidence of their beneficial effect on clinical outcomes. More recently, McLean 2010 conducted a systematic review to evaluate the effectiveness of a number of different technologies supporting the delivery of asthma care, including the Internet, telephone, videoconferencing, text messages and other networked systems. They concluded that these interventions may not result in significant improvements of clinical outcomes in individuals with relatively well‐controlled asthma.
However, the studies included in these reviews predate the advent of modern smartphones and apps. Apps have been widely adopted by smartphone and tablet users, and have been proposed as a new delivery mode for self management interventions by policy‐makers (Huckvale 2012). Moreover, the portability, and the advanced computing and connectivity capabilities of smartphone and/or tablet devices, have the potential to overcome some of the limitations of older technology‐based asthma self management interventions, making them more accessible and convenient. Therefore, a review of this technology is warranted in order to address the current gap in knowledge and to tease out the potential benefits (or harmful effects) of health apps in asthma self management.
Objectives
To assess the effectiveness, cost‐effectiveness and feasibility of using smartphone and tablet apps to facilitate the self management of individuals with asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel randomised controlled trials (RCTs), cluster RCTs (cRCTs), controlled before and after (CBA) studies (with at least two intervention and two comparison sites) and interrupted time series (ITS) studies (with at least three before‐ and three post‐intervention time points) (EPOC 2009). However, we did not identify any cRCTs, CBA or ITS studies that met the inclusion criteria for this systematic review. Our rationale for including CBA and ITS studies was twofold. First, apps are a contemporary technology that is unlikely to have been extensively evaluated in RCTs. The inclusion of CBA and ITS study designs could have provided further evidence of the effectiveness of asthma self management apps. Second, the findings of these CBA and ITS studies could have been used to inform the design of future RCTs evaluating these technologies.
We also planned to include qualitative studies and economic analyses that were reported separately but were linked to a primary study adopting one of the above designs; or those that were embedded within a primary trial. We planned the inclusion of reports of ongoing or unpublished work and pilot studies if they were associated with data important to the outcomes of interest. In these instances, we would have contacted the authors.
We excluded any other type of study design.
Types of participants
We included participants with clinician‐diagnosed asthma in any care setting. We included individuals without a diagnosis of asthma only if they were a parent to, or caregiver for, a patient with asthma. We excluded trials of apps aimed at clinicians. We did not make exclusions on the basis of age, gender or any other socio‐demographic characteristic of the participants.
Types of interventions
We included studies that utilised a health app accessible via a smartphone and/or tablet computer, to facilitate asthma self management. We included interventions if they equipped individuals with, or helped them to sustain and develop, one or more asthma self management skills (Lahdensuo 1999 ‐ see Table 2).
1. Self management skills adopted from Lahdensuo 1999 (box 2).
| Patients should do the following | |
| I | Accept that asthma is a long‐term and treatable disease |
| II | Be able to accurately describe asthma and its treatment |
| III | Actively participate in the control and management of their asthma |
| IV | Identify factors that make their asthma worse |
| V | Be able to describe strategies for avoidance or reduction of exacerbating factors |
| VI | Recognise the signs and symptoms of worsening asthma |
| VII | Follow a prescribed written treatment plan |
| VIII | Use correct technique for taking drugs, including inhalants by metered dose inhalers, dry powder inhalers, diskhalers, spacers or nebulisers |
| IX | Take appropriate action to prevent and treat symptoms in different situations |
| X | Use medical resources appropriately for routine and acute care |
| XI | Monitor symptoms and objective measures of asthma control |
| XII | Identify barriers to compliance (adherence) to the treatment plan |
| XIII | Address specific problems that have an impact on their individual condition |
To try to maximise external validity, we included only interventions using commercially available devices. Devices that required bespoke connecting or ancillary devices (e.g. peak flow meter devices offering Bluetooth connectivity with a mobile handset) were acceptable provided that the consumer device itself was left unaltered. We permitted devices with global system for mobile communication (GSM) and wireless connectivity (e.g. smartphones) as well as those without (e.g. some personal digital assistants (PDAs)). We also included tablet devices meeting the above criteria.
We included interventions where a health app was the sole means by which an intervention was delivered, or where apps formed a smaller part of a composite intervention. We included studies if their interventions were compared to other self management interventions delivered using traditional or alternative methods.
We excluded interventions that:
lay outside the self management domain;
were not aimed at individuals with asthma or their caregivers;
targeted health or allied professionals;
fell within the National Institutes of Health (NIH) definition of complementary or alternative medicine (NIH 2010) and are not generally considered part of conventional medicine;
relied on devices using bespoke hardware;
required physical modification of consumer hardware for intervention delivery;
used existing software available on a smartphone or tablet in a new way (e.g. using a calendar as an asthma diary);
relied solely on messaging (e.g. short message service (SMS) and multimedia messaging service (MMS));
did not offer a mode of interaction but acted simply as a transmitter of data (e.g. from patient to clinician) ‐ this is more consistent with telemonitoring (Paré 2007); and
consisted of desktop computers, laptops, notebooks and netbooks as these currently offer interaction methods not comparable with smartphones or tablets (e.g. portability).
Types of outcome measures
Primary outcomes
Symptom scores measured using any validated standard instrument.
Frequency of healthcare visits, both planned and unplanned (emergency department, general practitioners (GPs), hospitalisations), due to asthma exacerbations or complications. For the purpose of this review, we defined unplanned healthcare visits as those instances which were not normally part of a patient's regular review as recommended by clinical guidelines. Therefore, if patients called their GP to book an appointment, with asthma being the purpose of the appointment, before their annual review was due, we considered it an unplanned healthcare visit. To protect the treatment effect from being distorted by people experiencing many exacerbations, we dichotomised this outcome variable to: number of people experiencing no event and number of people experiencing one or more events.
Health‐related quality of life (QoL) scores measured using any validated standard instrument.
Secondary outcomes
Time‐off school, work or other commitments due to asthma exacerbations or complications.
Adherence to the intervention (e.g. proportion of diary data completeness; proportion of study days on which the tool was used).
Satisfaction with the intervention (theoretically assessed by a valid instrument or scale).
Health economic properties of the intervention (e.g. length of stay; rates of readmission).
Acceptability of the intervention using any validated standard instrument.
Lung function measurements (peak expiratory flow (PEF), forced expiratory volume in one second (FEV1) and forced vital capacity (FVC)).
Adverse events other than frequency of unplanned healthcare visits (e.g. number of days in which rescue asthma medication was needed).
In this review we included outcomes that were observed at the time of completion of an intervention, as well as those measured at subsequent time points as follow‐up. We regarded outcomes recorded within 30 days of cessation of the intervention as short‐term follow‐up; those continuing for at least six months as long‐term follow‐up; and outcomes recorded between 30 days and six months as medium‐term follow‐up.
Search methods for identification of studies
Electronic searches
We searched: the Cochrane Airways Group Register (CAGR), the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO, CINAHL, Global Health Library, Compendex, Inspec, Referex, IEEEXplore, ACM Digital Library, CiteSeerX and ERIC. Initially, we intended to search CAB Direct Global Health. However, due to the limitations of its interface we decided to run the search on CAB abstracts via Web of Knowledge. This represents the main component database of CAB Direct Global Health. We searched the grey literature in OpenGrey, Mobile Active and ProQuest Dissertations using search terms from the strategies in Appendix 1.
The database search strategies are described in Appendix 1. As pointed out by McLean 2010, terminology in this area evolves very rapidly and there is considerable overlap between terms such as 'telemedicine' and 'telenursing'. At the expense of obtaining a large number of potentially irrelevant studies, we included these terms in our search strategy so as not to risk excluding our interventions of interest.
Health apps are an emerging technology that is unlikely to have been extensively evaluated in the scientific literature. In order not to limit the number of citations retrieved by our search, we did not apply any methodological filters to our search strategy.
We also searched registers of current and ongoing trials such as the WHO (World Health Organization) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov, and planned to contact authors if necessary.
We did not exclude any studies based on their language of publication.
We documented the search results for each database and included them as Appendix 2 and Appendix 3.
We searched for studies published from 2000, since technologies existing prior to that time are unlikely to be representative of contemporary technologies that support health apps (Terry 2010; Zeldes 2005); furthermore, the concept of self management had not been widely adopted prior to 2000. The latest search was conducted in June 2013.
Searching other resources
We applied the same data restrictions as before. We checked reference lists of all primary studies and review articles for additional references. We contacted authors of identified trials and asked them to identify other published and unpublished studies. We planned to contact authors who might have ongoing trials or relevant publications in press for additional information on potentially relevant studies. We also planned to contact manufacturers and experts in the field. We did not exclude any studies based on language of publication.
Data collection and analysis
Selection of studies
Liz Stovold (LS), Trials Search Co‐ordinator for the Cochrane Airways Group (CAG), implemented the search strategy described above in the CAG Register (CAGR), CENTRAL, MEDLINE, EMBASE, PsycINFO, Global Health Library, ACM Digital Library and ERIC. Tim Reeves (TR), Research Support Librarian at Imperial College London, implemented the search strategy described above in the remaining bibliographic databases listed in Electronic searches and Searching other resources. All the references were imported into EndNote and duplicate records of the same report were removed. JMB and GG independently examined titles and abstracts to remove studies which did not meet the inclusion criteria. Afterwards JMB and GG independently screened the full text of those studies that were considered relevant during the first screening, to assess for compliance with the inclusion and exclusion criteria. JMB and GG resolved disagreements through discussion. If no agreement could be reached, KH and LG acted as arbiters.
Data extraction and management
Two authors, JMB and GG, independently extracted data from included studies using a structured form. We compared the data extraction forms completed by each review author and followed up discrepancies with reference to the original article. We contacted authors of studies containing missing or incomplete data in an effort to obtain the incomplete information.
Where possible, we extracted the following data from each trial:
General information about the study.
Study methods including aims of the study, study design, methods of and setting for recruitment, inclusion and exclusion criteria, details of the control and comparison groups, and incentives for participation.
Description and number of participants (at each stage of the trial) and geographical setting and place where the intervention was delivered.
Details of the intervention providers.
Name of the intervention, asthma self management skill(s) it promotes, mode of interaction it offers, main receiver of the intervention, hardware and software technologies it uses, mode of data entry, process and timing for data download.
Time points at which quantitative outcome measurements were taken, including the outcome values.
Qualitative outcomes and the assessment methodology used.
We summarised this information in a Characteristics of included studies table.
Assessment of risk of bias in included studies
We assessed risk of bias for all included studies using The Cochrane Collaboration's tool for assessing the risk of bias in RCTs (Higgins 2011). Therefore, we assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
Other sources of bias included the imbalance of outcome measures at baseline, the comparability of intervention and control group characteristics at baseline, and protection against contamination as recommended by the Cochrane Effective Practice and Organization of Care (EPOC) Group. The latter refers to those circumstances in which the control group is likely to have received the intervention.
For CBA or ITS studies, we planned to use the quality criteria suggested by the Cochrane EPOC Group (EPOC 2009). For ITS study designs, we would have assessed the risk of bias in relation to the following domains: 1) the intervention being independent of other changes; 2) sufficient data points to enable statistical inference; 3) the intervention being unlikely to affect data collection; 4) blinding of outcome assessors to intervention allocation; 5) incomplete outcome data being adequately addressed and 6) selective reporting of outcomes. We also would have presented a narrative description of the results from ITS and CBA studies and would have referred to the guidance provided by the Cochrane Consumer and Communication Review Group (Ryan 2011) to describe the results of our 'Risk of bias' assessment. However, we did not include any CBA or ITS studies.
For all studies we attempted to locate the original study protocol to compare reported methods and outcomes against those originally planned.
Two review authors, JMB and GG, independently assigned each domain of The Cochrane Collaboration's tool of each individual study to one of three categories: low, high or unclear risk of bias. For each study, we created a 'Risk of bias' table summarising our judgements.
Measures of treatment effect
We compared the characteristics of included studies in order to determine the feasibility of performing a meta‐analysis. For dichotomous outcomes, we calculated the odds ratio (OR) and 95% confidence interval (CI). For continuous outcomes, we calculated the mean difference (MD) and 95% CI. If studies using different measurement scales had been included, we would have calculated the standardised mean difference (SMD). We performed all statistical analyses using RevMan 2011.
Unit of analysis issues
We stated that for cRCTs, we would calculate a summary measurement for each cluster, taking the sample size as the unit of analysis. However, there were no cRCTs included in this review.
Dealing with missing data
We contacted the original investigators to request missing data. However, due to insufficient information on missing data, we used an available case analysis.
Assessment of heterogeneity
We assessed heterogeneity in the results for the primary outcomes by qualitatively comparing study participant and intervention characteristics between the two included studies. Because of the small number of studies, we did not use a formal statistical test to quantify statistical heterogeneity. Since the included studies were deemed to contain substantial clinical and methodological diversity, we did not conduct a meta‐analysis.
Assessment of reporting biases
Because of the small number of studies we did not use visual or statistical techniques to explore possible reporting biases. The small number of studies in this review is consistent with the early stage of development of this research area.
As part of the search strategy, we examined trial registrations. No unpublished trials were found.
We assessed outcome reporting bias as part of the per‐study 'Risk of bias' assessment.
Data synthesis
Because meta‐analysis was not appropriate due to heterogeneity, we performed a narrative synthesis of the evidence. We adapted the narrative synthesis framework to guide this process (Rodgers 2009), omitting the first step of grouping studies by intervention type because only two studies were retained in the review.
We used the following steps to describe the included studies:
Described the inclusion criteria (PICOS elements) along with the reported findings for each of the included studies.
Explored the relationships between characteristics of individual studies and their reported findings, as well as those between the findings of different studies.
Described the moderators as well as the mediators that would have an impact on the intervention effects.
We had originally planned to use the GRADE system to assess the quality of the evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes, to produce a 'Summary of findings' table (Higgins 2011).
We initially stated that we would perform a qualitative thematic synthesis (Thomas 2008) of all studies that explored individuals' attitudes towards an intervention using a recognised qualitative methodology. We would have grouped studies assessing the same outcome and coded their findings accordingly. We would have extracted the free text from included studies and iteratively coded them using NViVo. Finally, we would have segregated information by the type of intervention being reported. However, we did not include any qualitative studies.
Subgroup analysis and investigation of heterogeneity
We originally planned to perform a subgroup analysis by baseline asthma severity amongst recruited participants. There may be greater scope for improvement and motivation to engage with interventions like health apps in those with more severe asthma.
The two studies retained in the review had different levels of baseline severity (on the basis of mean FEV1). However, since there were only two studies and a meta‐analysis was not appropriate, no subgroup analysis was performed.
Sensitivity analysis
We planned to conduct a sensitivity analysis if one or more studies were dominant due to their size; if one or more studies had results that differed from those observed in other studies; or if one or more studies had quality issues that may have affected their interpretation judged using the Cochrane 'Risk of bias' approach (Higgins 2011). To account for the inclusion of non‐RCTs (i.e. CBA and ITS studies) we would have conducted sensitivity analyses that only included RCTs in order to determine whether the results differed. Additionally, we would have conducted analyses, excluding those studies labelled as having high risk of bias for a particular outcome, irrespective of their study design. However, we did not conduct a sensitivity analysis since none of the conditions mentioned above were met.
Results
Description of studies
Results of the search
The initial electronic searches were run between June and July 2012. After removing duplicate records, we screened 713 papers that resulted from implementing the search strategy. All the records were identified through the searches listed in Electronic searches. After the initial screening of titles and abstracts, we excluded 691 records, retrieved the full‐text reports for 22 potential includable studies and assessed them for eligibility. Of these, we excluded 19 papers for not meeting the eligibility criteria for this review.
The electronic searches were updated in June 2013. As a result of this, we screened 260 records. After the initial screening of titles and abstracts, we excluded 254 records, retrieved the full‐text reports for six potential includable studies and assessed them for eligibility. All of these reports were excluded (see Characteristics of excluded studies table).
Therefore, only three records were included in the final review: Liu 2011; Ryan 2010 and Ryan 2012 (see Figure 1 and Figure 2).
1.
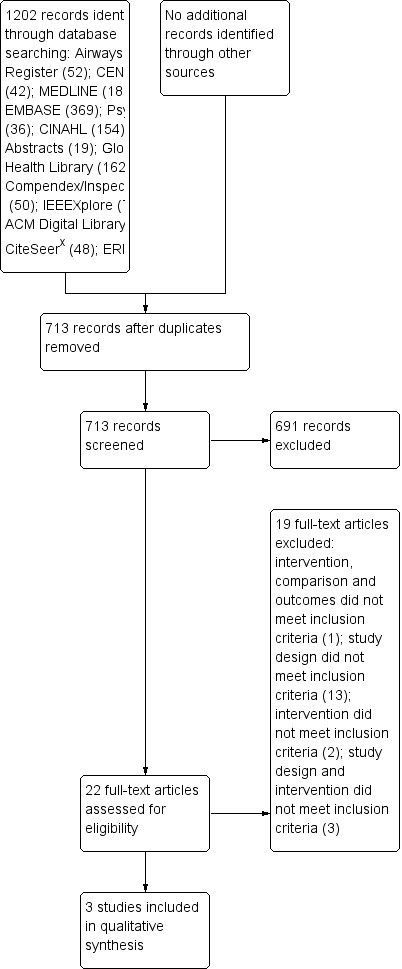
PRISMA flow diagram.
2.

PRISMA flow diagram ‐ June/July 2013 update
Included studies
Study design
Liu 2011 conducted a prospective randomised controlled study and Ryan 2012 conducted a multicentre randomised controlled trial with cost‐effectiveness analysis. Participants in both trials were followed up for six months, with regular follow‐up assessments taking place every month.
Population
For Liu 2011, 120 patients (age range not available from the study report) with moderate to severe persistent asthma were recruited from the outpatient clinics of the Chang Gung Memorial Hospital, Linkou, in northern Taiwan. For Ryan 2012, 288 participants with poorly controlled asthma, aged 12 and over, were recruited from 32 GP practices across the UK (located in Norfolk, Suffolk, Yorkshire and Tyneside). Both trials differed in terms of the diagnostic criteria for asthma that they used. Liu 2011 defined asthma and its severity according to the criteria set out by the American Thoracic Society on the basis of clinical symptoms and physical examination. Moreover, patients in this trial were treated according to their level of asthma severity, following the guidelines of the Global Initiative for Asthma (GINA). Ryan 2012, on the other hand, used the results of the Asthma Control Questionnaire (ACQ) to define patients with poorly controlled asthma: someone with an ACQ score of 1.5 or above at the time of recruitment. Participants in this trial were treated according to asthma severity by following the stepwise recommendations of the British Thoracic Society (BTS)/SIGN asthma guideline.
When comparing across the included studies, participants differed in terms of their baseline lung function. The main forced expiratory volume in one second (FEV1) percentage predicted in Liu 2011 was 58.2% (standard error (SE) 3.1%) for those participants assigned to the control condition, and 57.9% (SE 3.0%) for those assigned to the study intervention. In Ryan 2012 the median FEV1 predicted was 80.8% (interquartile range (IQR) 65.3% to 94.9%) for participants in the control condition, and 83.1% (IQR 71.0% to 96.6%) for those in the intervention group. However, there were no baseline differences between participants in the control and intervention groups within each of the included studies.
Intervention
Both Liu 2011 and Ryan 2012 evaluated the effect of a mobile phone‐based asthma self management intervention on participants' level of asthma control by comparing it to traditional, paper‐based asthma self management. However, the two studies differed in specific aspects of their intervention design. Liu 2011 evaluated the effect of an interactive mobile phone‐based asthma self management system, which allowed participants to keep daily entries of their asthma symptoms, asthma medication usage, peak flow readings and peak flow variability. Data entered into this system were immediately transmitted to patients' personal files, which were located in a secure central server, and used to calculate a person's level of asthma control (i.e. controlled, partly controlled or uncontrolled) using a scoring system developed by the research team. We were unable to find validation information for this scoring method. Once a participant's asthma status had been scored, it was transmitted back and displayed on their mobile phone together with the corresponding asthma self management recommendations.
In Ryan 2012, intervention group participants were asked to record twice daily their asthma symptoms, asthma medication use and peak flow readings using an app running on a supplied smartphone. Upon entering data, participants received immediate self management feedback in the form of a three‐colour traffic light display on their phones. Each of these zones represented a level of asthma control, the limits of which were determined using valid measures such as validated questionnaires and peak flow readings. Participants falling into the amber zone of their action plan twice, or into the red zone once, received a phone call from an asthma nurse who enquired about the reasons for their uncontrolled asthma. In both studies (Liu 2011; Ryan 2012) participants in the comparison groups were asked to keep a paper diary of the same data collected by participants in the intervention group. Participants in this group were also given a personalised written asthma action plan that included detailed instructions on the daily self management of asthma, as well as on the management of acute asthma exacerbations and other asthma‐related emergencies.
Outcomes
Primary outcomes
Only Ryan 2012 reported symptom scores. They compared changes in the asthma control questionnaire (ACQ, six‐question version) scores between baseline and six months between participants in the intervention group and those in the control group.
Regarding frequency of healthcare visits, both Liu 2011 and Ryan 2012 reported the total number of patients in each intervention group who presented at least one unscheduled visit to the emergency department due to asthma‐related complications after six months, as well as the those who presented at least one asthma‐related hospital admission throughout the duration of the trial. In addition, Ryan 2012 also reported on the number of participants in each intervention group who had: at least one consultation with their GP for asthma; at least one unscheduled GP nurse consultation for asthma; or one or more out of hours attendances due to asthma‐related complications.
Both Liu 2011 and Ryan 2012 reported on health‐related quality of life. Liu 2011 compared the changes in the scores for the physical and mental components of the short‐form (SF)‐12 questionnaires between the control and the intervention groups. This outcome was measured at baseline and then monthly for six months. Ryan 2012 compared the changes in scores on the mini‐Asthma Quality of Life Questionnaire (mini‐AQLQ) between baseline assessment and after six months between the control and intervention groups.
Secondary outcomes
None of the included studies reported on the following outcomes: time off school, work or other commitments due to asthma‐related complications; satisfaction with the intervention and acceptability of the intervention.
Both Liu 2011 and Ryan 2012 reported the proportion of participants who were still adherent to the intervention three and six months after the start of the intervention. Liu 2011 defined adherence as those participants in the intervention group who were still submitting data to the website through their smartphone app or those participants in the control group who were still completing the paper diary. Ryan 2012 defined adherence for both groups as the proportion of participants who returned the postal questionnaires sent to them.
Concerning the health economic properties of the intervention, Ryan 2012 compared the mean costs of service provision (in pounds sterling) between the intervention and control groups. Only Liu 2011 compared the differences in lung function between the control and intervention groups in terms of their peak expiratory flow rate (PEFR) and forced expiratory volume in one second (FEV1) percentage predicted. These measures were taken at baseline and then monthly for six months. Finally, both included studies reported adverse events other than frequency of unplanned healthcare visits. Liu 2011 collected data on those experiencing respiratory failure and mortality. Ryan 2012 reported on the number of participants who experienced at least one acute asthma exacerbation and those who required at least one course of steroids.
See the Characteristics of included studies table for further information on each of the included studies.
Excluded studies
The most common reason for exclusion of articles (19 out of 25) was that the study design did not meet the eligibility criteria for this systematic review. Indeed, the majority of these papers came from conference proceedings and provided theoretical descriptions of asthma self management systems and their development. However, these systems were not evaluated in a formal trial. The remaining studies were excluded because of ineligible interventions (two studies), comparisons and outcomes (one study); and ineligible study design and intervention (three studies).
Further information on excluded studies and reasons for exclusion can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
Overall, there was not enough information available in Liu 2011 to make an assessment of the risk of bias for the majority of the domains, even after follow‐up with the study authors. See Figure 3 and Figure 4 for the 'Risk of bias' assessments for each domain of each included study.
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation in Ryan 2012 took place using a central telephone randomisation service that was provided by the Health Services Research Unit at the University of Aberdeen. This service used blocks of varying size and stratified by practice to ensure equal randomisation within each GP practice. Therefore, we deemed the risk of selection bias to be low for the random sequence generation domain. There was not enough information available in Liu 2011 to make an assessment of the risk of selection bias for this domain, so it was deemed unclear.
The telephone randomisation service used by Ryan 2012 allowed the researchers to conceal allocation until the treatment was assigned. For this reason we judged the risk of selection bias for allocation concealment to be low. There was not enough information available from the study report to make an assessment of the risk of selection bias for Liu 2011 on this domain, so we deemed it to be unclear.
Blinding
Given the nature of the interventions, blinding of participants in both Liu 2011 and Ryan 2012 was not possible. In both cases, healthcare providers conducting the follow‐up assessment visits were informed about the condition to which each participant had been allocated. For this reason we assessed the risk of performance bias for blinding of participants and personnel to be high for both studies.
Research staff collecting data on primary outcomes in Ryan 2012 were blinded to the allocation of participants. Therefore, we assessed the risk of detection bias for blinding of outcome assessment as low for this study. There was not enough information available in order to assess the risk of detection bias appropriately for Liu 2011, so we judged this risk to be unclear.
Incomplete outcome data
Ryan 2012 appears to have used a number of methods to analyse their primary and secondary outcomes. The analysis of ACQ scores was based on 278 participants (as opposed to the 288 participants that were originally randomised between the two groups), as 10 people had to be excluded due to incomplete baseline ACQ scores. Ryan 2012 claimed to have followed an intention‐to‐treat analysis; however, missing data seem to have been addressed by last observation carried forward (LOCF). Analysis of other primary and secondary outcomes appears to have been based on a complete case analysis. Although reasons for excluding participants were provided at three months, there was still a substantial amount of missing data without a reason for loss to follow‐up. Furthermore, no reasons for missing data at six months were provided. For these reasons, we deemed the risk of attrition bias for this study as unclear.
One hundred and twenty participants were recruited in Liu 2011; however, only 89 of them were included in the statistical analyses. They used a complete case analysis in handling missing data, and they clearly described the numbers of missing participants and their associated reasons for dropout at both three‐ and six‐month assessments. However, due to the proportion of participants lost to follow‐up (36% and 56% of missing data at three months in the intervention and control groups, respectively; and 50% and 60% of missing data in the intervention and control groups, respectively, at six months), we assessed the risk of attrition bias for this study as unclear.
Selective reporting
We located the original study protocol for Ryan 2012. Since we found no discrepancies between the outcomes that the authors intended to measure originally and those reported in the included study, we judged the risk of reporting bias to be low on this domain. We were unable to find the study protocol for Liu 2011. Therefore, we assessed the risk of reporting bias for this study as unclear.
Other potential sources of bias
Seasonal variation of asthma severity is a recognised effect (Fleming 2000) that should be taken into consideration when conducting studies in patients with asthma. Both Liu 2011 and Ryan 2012 were conducted over a six‐month period; both were multicentre trials in which recruitment and initiation of the trial across the different health centres are likely to have occurred at different times of year. In Ryan 2012, recruitment of participants rolled over a 13‐month period (this information was obtained in direct communication with the contact author). If randomisation were balanced over the period (which approximated a year), asthma seasonality might be discounted as a source of potential confounding. However, we were unable to verify this assumption. Not enough information was available in Liu 2011 to make an appropriate assessment.
There were additional intervention components in Ryan 2012 that could have influenced the observed results. Participants in the mobile phone monitoring group who fell into the amber zone of their personalised asthma action plan on two consecutive occasions, or once into the red zone, received a phone call from an asthma nurse. While apps may support remote care (telehealth) in the future, this kind of additional support was not present in Liu 2011 and was not the intended focus of this review. The potential impact of this additional support should be taken into account when interpreting outcomes data.
Conversation with the contact author of Ryan 2012 revealed that 9% of individuals assessed during the baseline appointment were excluded because their asthma control had significantly improved by the time their appointment was due. Feedback from patients in this study suggested that the initial invitation phone call (in which they completed a telephone version of the ACQ) highlighted their poor asthma control and acted as a prompt to seek better control independently of the study. If an invitation phone call can itself act as an intervention, observed effect sizes may therefore be minimised due to improvements in both the control and the intervention groups. In addition, the sample of participants who took part in this study could represent a subgroup of asthma patients prone to particularly poorly controlled asthma or less able to take action to self regulate their condition. Consequently, they may not be representative of the wider population of poorly controlled asthma patients that the study targeted.
For all the reasons outlined in this section, we assessed the risk of bias for other potential sources of bias as high for both Liu 2011 and Ryan 2012.
Effects of interventions
See: Table 1
We originally intended to collect data on three primary outcomes (symptom scores, frequency of healthcare visits and health‐related quality of life) and on seven secondary outcomes (time‐off school or work, adherence to the intervention, satisfaction with the intervention, health economic properties of the intervention, acceptability of the intervention, lung function measurements and other adverse events). However, the included studies did not contain any data on time‐off work or school, satisfaction with the intervention or acceptability of the intervention. These outcomes are therefore not reported in this section.
There was a considerable degree of clinical diversity between the included studies across three domains. First, participants recruited into Liu 2011 had a baseline mean FEV1 percentage predicted of 58.2% (SE 3.1) in the control group and 57.9% (SE 3.0) in the intervention group, whereas participants in Ryan 2012 had a median baseline FEV1 percentage predicted of 80.8% (IQR 65.3% to 94.9%) and 83.1% (IQR 71.0% to 96.6%) in the control and intervention groups, respectively. This suggests that each study might have sampled participants from different populations of patients with asthma, with those in Liu 2011 being at a substantially worse level of asthma control at commencement of the study. The difference may have arisen by chance, or reflect subtle differences in the targeting for study recruitment. Participants in Liu 2011 were recruited from tertiary care centres, whilst those taking part in Ryan 2012 were recruited from primary care practices. Alternatively, differences in population levels of asthma control between the Taiwan and UK may be relevant.
Second, there was a considerable difference in the levels of missing data between the two included studies. Ryan 2012 analysed their outcome data using an ITT analysis, with the exception of ACQ scores for which six participants (4.2%) in the intervention group and four participants (2.8%) in the control group were excluded after randomisation due to incomplete baseline questionnaires. In Liu 2011, on the other hand, 120 participants were initially randomised into the trial (60 participants in each intervention group); however, only 43 participants (71.7%) in the intervention group and 46 participants (76.7%) in the control group were included in the statistical analyses.
Third, the intensity of the intervention in Ryan 2012 was higher than in Liu 2011. Participants in the former were reviewed on a regular basis by an asthma nurse until their asthma was under control. At this point, patients were discharged and advised to continue the self management practices they learned during this intervention.
Recruitment of participants is likely to have occurred in different seasons in the different healthcare centres (see Other potential sources of bias). However, it is unclear whether authors of the included studies made allowances for a potential seasonal asthma effect.
The combination of between‐study heterogeneity, concerns about potential biases and the small number of studies prompted the decision not to undertake a meta‐analysis. This should be reviewed in the future as more studies become available.
Primary outcomes
Symptom scores
Ryan 2012 compared the effect of a smartphone self management app with a traditional paper diary plus a written asthma action plan on the mean difference in ACQ scores between the two groups at six months (MD 0.01, 95% CI ‐0.23 to 0.25; see Analysis 1.1) and found no statistically significant difference. They also compared the mean change in ACQ score between baseline and six months for each group. They found that although both groups improved by more than the minimum important difference (MID) of 0.5 (Juniper 1999) (mean change 0.75 (95% CI 0.61 to 0.89) for the intervention group and 0.73 (95% CI 0.57 to 0.89) for the control group), the mean difference in change was statistically non‐significant (MD of mean change ‐0.02, 95% CI ‐0.23 to 0.19). There was no significant difference (P = 0.78) between the intervention and the control group in the proportion of people whose ACQ score improved by more than the MID of 0.5. Liu 2011 did not report on asthma symptom scores.
1.1. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 1 Symptom scores using the ACQ.
Frequency of healthcare visits
Both Liu 2011 and Ryan 2012 compared the difference between participants in the mobile phone‐based self monitoring group (intervention) and the paper‐based asthma self management group (control) using unscheduled visits to the emergency department and hospital admissions due to asthma‐related complications. With regards to the unscheduled visits to the emergency department, Liu 2011 found that participants in the intervention group were less likely to attend the emergency department than those in the control group (OR 0.20, 95% CI 0.04 to 0.99; Analysis 1.2). The results for this outcome in Ryan 2012 were statistically non‐significant (Fisher's exact test yielding a P value of 0.12 ‐ although with no observed events for the control group and only three events observed in the intervention group, there is insufficient information to find conclusive results regarding emergency department visits).
1.2. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 2 Patients with unscheduled visits to the emergency department.
Concerning hospital admissions, the results for both Liu 2011 and Ryan 2012 were statistically non‐significant: Fisher's exact test yielding a one‐sided P value of 0.52 or a two‐sided P value of 1.0 (Liu 2011) ‐ since there were no observed events in the intervention group and only one observed event in the control group, there is not enough information to draw firm conclusions regarding the likelihood of reduced/increased hospital admissions in one group compared to the other (hospital admissions OR 3.07, 95% 0.32 to 29.83) (Analysis 1.3) (Ryan 2012).
1.3. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 3 Hospital admissions.
Ryan and colleagues also compared the difference between the two groups in terms of GP consultations for asthma (OR 1.40, 95% CI 0.85 to 2.31) (Analysis 1.4), unscheduled general practice nurse consultations (OR 0.60, 95% CI 0.37 to 0.98) (Analysis 1.5) and out of hours attendances (OR 0.60, 95% CI 0.14 to 2.54) (Analysis 1.6). Only the difference in unscheduled general practice nurse consultations was statistically significant; however, a trend toward non‐significance exists as the 95% CI approaches 1.
1.4. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 4 GP consultations for asthma.
1.5. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 5 Unscheduled general practice nurse consultation.
1.6. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 6 Out of hours attendances.
Health‐related quality of life
Liu 2011 explored the difference in health‐related quality of life between the two groups using the mental and physical components of the SF‐12 questionnaire. Measurements using this questionnaire were taken at baseline and then monthly for six months. They found a statistically significant difference in the mean scores of the physical component of the SF‐12 questionnaire at months three, four, five and six that favoured those in the intervention group (see Analysis 1.7). Similarly, they found a statistically significant difference in the scores of the mental component of this questionnaire at months four, five and six that favoured participants in the intervention group (Analysis 1.7).
1.7. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 7 HRQoL measured on the SF‐12 questionnaire.
Ryan 2012 explored the difference in health‐related quality of life using the mini‐Asthma Quality of Life Questionnaire (mini‐AQLQ). They found that after six months participants in both groups improved their scores on the mini‐AQLQ by more than the MID of 0.5 (mean change ‐0.75 for participants in the intervention group (95% CI ‐0.94 to ‐0.57); mean change ‐0.65 for participants in the control group (95% CI ‐0.84 to ‐0.46)). They also found a significant difference (P = 0.03) in the proportion of people whose mini‐AQLQ score improved by more than the MID at six months between the intervention and control group, with the greater improvement seen in the intervention group. However, the mean difference in mean change between the intervention group and the control group was statistically non‐significant (MD of mean change 0.10, 95% CI ‐0.16 to 0.34). Similarly, there was no statistically significant difference in the mean difference of mean scores between the two groups (0.02, 95% CI ‐0.35 to 0.39)(see Analysis 1.8).
1.8. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 8 HRQoL measured on the mini‐AQLQ.
Secondary outcomes
Adherence to the intervention
Both Liu 2011 and Ryan 2012 measured the proportion of participants who still were adherent to the intervention after three and six months. The former did not find any statistically significant differences between the two groups (three months OR 0.79, 95% 0.30 to 2.06 and six months OR 0.77, 95% 0.34 to 1.75) (Analysis 1.9). Unlike for other outcomes in this study, Liu 2011 included all 120 participants in their calculations of adherence to the intervention. Similarly, Ryan 2012 did not find any statistically significant difference between the intervention and the control groups (three months OR 0.91, 95% CI 0.55 to 1.50 and six months OR 1.19, 95% CI 0.67 to 2.13).
1.9. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 9 Proportion of participants adherent to the intervention.
Health economic properties of the intervention
Ryan 2012 evaluated the mean costs of service provision (in pounds sterling (GBP)) between the intervention and the comparison groups using the following indicators: (1) total healthcare costs; (2) total cost of delivering trial interventions according to allocation; (3) trial nursing costs; (4) telemonitoring service costs; (5) total cost of healthcare provision; (6) GP respiratory consultations; (7) practice nurse respiratory consultations; (8) secondary care costs; (9) emergency services and (10) total cost of prescription for respiratory drugs. They only found statistically significant results for three of these: total healthcare costs, cost of trial intervention and nursing costs, costs of trial intervention and nursing costs (Analysis 1.10). Total healthcare costs of an app‐based intervention were higher than the costs associated with the control condition (MD (GBP) 70.00, 95% CI 19.98 to 120.02). Likewise, the total cost of delivering trial interventions according to allocation was higher in the intervention group than in the control group (MD (GBP) 66.00, 95% CI 63.19 to 68.81). The trial nursing costs, however, were marginally lower in the intervention group than in the control group (MD (GBP) ‐3.00, 95% CI ‐5.81 to ‐0.19).
1.10. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 10 Healthcare costs.
Lung function
Liu 2011 compared the differences in peak expiratory flow rate (PEFR) and forced expiratory volume in one second (FEV1) percentage predicted between the participants using the self management app and those completing paper diaries. In Liu 2011, PEFR measurements were taken monthly for six months; they found that there was an incremental improvement of PEFR (L/min) throughout the duration of the study that favoured the group using the smartphone app. However, only the mean differences in PEFR at months four to six were statistically significant (see Analysis 1.11). With regards to FEV1 percentage predicted, measurements were taken at months three and six (see Analysis 1.12). Whilst the results at month three were statistically non‐significant (MD 3.70, 95% CI ‐4.91 to 12.31), participants in the intervention group showed significantly higher mean FEV1 percentage predicted values than those in the control group at six months (MD 8.70, 95% CI 0.37 to 17.03). Ryan 2012 did not report data on lung function.
1.11. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 11 Peak expiratory flow rate (PEFR).
1.12. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 12 Forced expiratory volume in 1 second (FEV1) percentage predicted.
Other adverse events
Liu 2011 collected data on two additional adverse events: respiratory failure and mortality. No participant experienced any of these events. Ryan 2012 collected data on the number of participants who experienced at least one acute asthma exacerbation and those who required at least one course of steroids. However, they found no statistically significant difference between the control and intervention groups for these outcomes (exacerbation OR 0.95, 95% CI 0.57 to 1.57 and steroids OR 0.93, 95% CI 0.52to 1.65) (Analysis 1.13; Analysis 1.14).
1.13. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 13 Proportion of participants experiencing at least one acute asthma exacerbation.
1.14. Analysis.

Comparison 1 Smartphone asthma apps versus control, Outcome 14 Proportion of participants who required at least one course of steroids.
Discussion
Summary of main results
The objective of this systematic review was to assess the effectiveness, cost‐effectiveness and feasibility of using smartphone or tablet computer apps to support patients with asthma in the self management of their condition. Our aim was to determine whether this technology has a positive impact on the following primary outcomes: patient‐reported measures of asthma control (e.g. Asthma Control Questionnaire (ACQ)), frequency of healthcare visits, and health‐related quality of life (e.g. mini‐Asthma Quality of Life Questionnaire (AQLQ)). Secondly, we wanted to explore if these apps had any effect on secondary outcomes, such as lung function (e.g. peak expiratory flow rate (PEFR)), health economic properties, and patient adherence and patient satisfaction with asthma self management interventions, as it has been shown that poor adherence to self management interventions is one of the principal causes for the poor levels of asthma control amongst individuals with asthma.
We were, however, unable to answer any of these questions due to the small number of included studies (n = 2), which only partially addressed the intended outcomes. The two studies showed a considerable degree of heterogeneity, particularly in relation to participants' baseline level of asthma control and intensity of the intervention. These reasons meant that we could not conduct a meta‐analysis of the data extracted; instead, we performed a narrative synthesis.
Overall, the results of this review are inconclusive (please see Table 1). On the one hand, Ryan 2012 concluded that the use of a smartphone app has no statistically significant effect on asthma symptom scores, asthma‐related quality of life (using the mini‐AQLQ questionnaire) or frequency of healthcare visits. Liu 2011, meanwhile, concluded that patients using an asthma self management app had significantly higher asthma‐related quality of life scores (both in the mental and physical components of the SF‐12 questionnaire) and were less likely to visit the emergency department due to asthma‐related complications. Neither Liu 2011 nor Ryan 2012 found any statistically significant differences between those using an asthma self management app and those using paper‐based self management tools in terms of adherence to the intervention and occurrence of other asthma‐related complications. However, Liu and colleagues found that those using an asthma app consistently showed significantly higher mean PEFR and FEV1 percentage predicted values than participants in the control group.
A number of factors could explain the differences between the findings of Ryan 2012 and those of Liu 2011, including differences in the design and intensity of the interventions, and the baseline severity of participants. The lower baseline mean PEFR of the participants in Liu 2011 may have been by chance, reflecting differences in participant selection or differences in overall levels of population asthma control. As with McLean 2010, these findings could be an indication of asthma apps being more effective for those with more severe asthma, higher risk of hospital admission or both.
Differences in attitudes towards self management and smartphone‐based technologies between the UK and Taiwan may also be relevant. GPs in the UK are the gatekeepers to secondary care services, and there is a growing emphasis on self management (Department of Health 2006). In the context of this review, it is possible that participants in Ryan 2012 were more familiar with, and better equipped for, asthma self management, whether through a smartphone app or through more traditional tools. In Taiwan, on the other hand, some of the most pressing issues of the healthcare system relate to the quality of outpatient care and gatekeeping (Wu 2010). It is common practice for people in Taiwan to seek frequent medical help. Consequently, the average outpatient visit rate is significantly higher than in the UK (Wu 2010). Improvements in lung function observed in Liu 2011 might also reflect attitudinal differences in the acceptability of technology in healthcare between the two countries. The intensity of the intervention in Ryan 2012 also deserves consideration. Participants were reviewed regularly by the asthma nurse until their asthma was considered to be under complete control, at which point they were discharged and encouraged to follow self management practices. It is plausible that participants in both the intervention and control groups had reached a similar level of self management proficiency by the time they were discharged, therefore reducing the effect size attributable to the smartphone app.
Ryan 2012 found that the total costs of delivering the mobile phone‐based intervention were higher than the associated costs of delivering the paper‐based intervention. A possible explanation for this finding is that the smartphone asthma app intervention was delivered in the context of a randomised controlled trial. It is assumed that both the intervention and the control groups had the same trial‐associated costs. However, similar to other technology‐based interventions, the cost of developing the app might have been relatively high when compared to the cost of paper diaries. The problem is that this was a relatively short intervention (not long enough to offset the cost of development), which did not have a positive impact on asthma‐related health outcomes or on costs associated with healthcare provision.
Overall, this is a new form of technology that has only recently been introduced in the healthcare sector, and as a result it has hardly been evaluated in formal intervention trials. Moreover, physicians might not be at the point of recommending that patients use apps outside of trial settings. In a recent review of 103 asthma apps, Huckvale 2012 found that only 72 addressed items contained in a pre‐defined set of evidence‐based recommendations; of these, only 40 were in line with current guidance.
Overall completeness and applicability of evidence
Both studies included in this review recruited a small sample (120 in Liu 2011 and 288 in Ryan 2012). Although Ryan 2012 exceeded their intended sample size based on power calculations, they only managed to recruit 2.4% of those individuals who were invited. Ryan and colleagues attributed this to the low response rate and to their inclusion criteria (i.e. ACQ score of 1.5 or higher and owning a compatible mobile phone device). Although Liu 2011 acknowledged the small sample size as one of the limitations of their study, they did not provide possible explanations for this finding. However, both studies had acceptable response rates during the follow‐up assessments: over 70% for both Liu 2011 and Ryan 2012 after six months. Nonetheless, any interpretation of the acceptability of mobile health interventions should take into account the fact that people seemed to have enjoyed them once they were engaged; however, sparking that initial interest proved harder than expected.
The available results concern participants in primary care or outpatient settings with moderately uncontrolled asthma. While Ryan 2012 used a threshold from a validated questionnaire to define poor asthma control, Liu 2011 used their own schema. However, the baseline PEFR of the participants in Liu 2011 is consistent with moderately poorly controlled asthma. Approximately 9% of those individuals who were invited to attend the baseline assessment in Ryan 2012 had to be excluded as they significantly improved their ACQ scores between the telephone invitation and the baseline appointment. The results of Ryan 2012 should be interpreted in light of the possible exclusion of individuals who were able to take action to improve their asthma without external support. Participants in both groups improved their ACQ scores by more than the minimum important difference. Therefore, they may represent a subset of individuals who require additional support to control their asthma, irrespective of the delivery mode.
The explanations provided for the intervention effect observed in each of the included studies did not take into account the theoretical constructs underlying asthma self management. Consequently, it becomes harder to identify the determinants of behavioural change that these interventions were targeting, and to establish the relative contribution of each intervention component to the observed effects.
As mentioned in the Summary of main results, smartphone and tablet computer apps are not only a new form of technology, but also have only recently been introduced in the field of self management of chronic conditions. This means that the effectiveness of these apps has yet to be evaluated in formal trials of interventions. Indeed, in our searches we identified numerous theoretical papers that described the development of mobile phone‐based asthma self management systems (all of which included asthma self management apps). It is expected that future updates of this review will include trials evaluating the systems described in these papers.
Quality of the evidence
There was not enough information available in Liu 2011 to make an assessment of the quality of the evidence across all the domains specified in the Cochrane 'Risk of bias' assessment tool. For Ryan 2012, we were able to obtain the information needed (either from the publication or in direct communication with the first author) to assess all these quality domains. However, there were still concerns about both studies in relation to blinding of participants and personnel, attrition bias and other potential sources of bias.
The nature of the interventions meant that participants and those conducting the follow‐up assessments could not be blinded to allocation. This could have affected participants' performance and the observed effect. Concerning other potential sources of bias, both studies lasted for six months and recruited participants from different primary care centres or outpatient clinics. This poses a risk to the observed effect since no allowances were made for a potential seasonal effect of asthma. Furthermore, it is possible that certain aspects of research administration may have contaminated the intervention effects. For example, participants in Ryan 2012 who incurred in the amber or red zone received a phone call from an asthma nurse. Since the number of people who received this call is unknown, we cannot determine with certainty the extent to which these administrative processes moderated the observed effects.
Potential biases in the review process
None to report.
Agreements and disagreements with other studies or reviews
Huckvale 2012 adapted systematic review methodology to evaluate the content and tools of asthma apps available in the official app stores for Android, Apple, Blackberry and Windows Phone devices. They concluded that none of the apps evaluated combined reliable and comprehensive asthma information with self management tools. They also concluded that since current apps do not meet the need of every patient and some of them could be unsafe, practitioners should exert caution when recommending these apps.
Mosa 2012 reviewed articles published in MEDLINE that discussed the design, development, evaluation or use of smartphone‐based software for healthcare professionals. The authors concluded that various medical apps have been developed and widely used by health professionals and patients. We agree with their conclusions that smartphone apps can potentially have a key role in patient education, self management and remote monitoring of patients.
The use of smartphone and tablet computers apps for the self management of long‐term conditions has been explored in conditions other than asthma. Baron 2012, for example, conducted a systematic review on the clinical effectiveness of apps for the collection of blood glucose readings and their immediate transfer to a central server. The authors concluded that clinical diversity, poor outcome reporting and methodological weaknesses made comparison difficult. As a result of this, the evidence still remains weak. However, to the authors' knowledge this is the first systematic review to evaluate the effectiveness of smartphone apps in the context of asthma self management.
McLean 2010 assessed the effectiveness of telehealthcare interventions for people with asthma. They found that these interventions did not demonstrate improved clinical outcomes for people with mild asthma; however, they may have a role to play in those with more severe asthma and high risk of hospital admission. The difference between the findings of Liu 2011 and Ryan 2012 seems to support the conclusions of McLean 2010, as participants in Liu 2011 were more severe.
Authors' conclusions
Implications for practice.
Asthma self management apps have the potential to reach a considerable proportion of patients with asthma, making self management interventions more accessible, convenient and less costly than traditional or standard techniques for asthma management throughout the healthcare system. The use of this technology to support asthma self management is in its early days. The studies included in this review provided contradictory information regarding their effectiveness and cost‐effectiveness. Although once engaged with the app‐based intervention, patients were largely compliant. However, these two studies are not enough evidence to draw firm conclusions about the potential effect of asthma apps, particularly if we consider their methodological limitations. Clinical practitioners wishing to recommend these apps in their daily practice must do so with caution and ensure that they provide appropriate support.
Implications for research.
Currently, the evidence base is insufficient to advise clinical practitioners, policy‐makers and the general public with regards to the use of smartphone and tablet computer apps for asthma self management. This review highlights the need to fill this knowledge gap, especially if we consider the potential benefits that this form of technology can offer.
There is a need to establish whether or not asthma apps are effective for the delivery of self management interventions (compared with usual care) when delivered as part of complex telehealth scenarios. However, researchers should also focus on understanding the efficacy of apps as standalone interventions. In order to achieve this, differential clinical management of patients between control and intervention groups should be minimised in future studies. Where asthma self management apps are evaluated as part of complex, multicomponent interventions, researchers should focus on ways of teasing out the relative contribution of each intervention component. To be able to do this, there should be explicit description of the underlying theoretical constructs used to develop the intervention. Care must also be taken when designing such interventions to acknowledge the role of ancillary components (notably those that are part of research administration, rather than the designed intervention per se) in moderating any observed effects.
In the design of smartphone app‐based interventions, researchers should consider specific strategies to encourage and promote adherence to self management practices and evaluate these in studies of suitable duration to examine whether behaviour is sustained over time. It remains unclear whether patients with asthma are prepared to use app‐based interventions in the longer term.
Finally, researchers should also consider the seasonal nature of asthma when designing their studies and account for this throughout recruitment and the duration of the study.
Acknowledgements
We would like to acknowledge Mohana Ratnapalan and Thomas Cowling for their contribution in the development of the initial versions of the protocol. We would also like to thank LS and TR for their help running the electronic searches.
Anne Holland was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. Database search strategies
Cochrane Airways Group Register (CAGR)
((cell* or mobile*) and phone*) or handheld* or hand‐held* or PDA or "personal digital*" or "palm OS" or "Palm Pre Classic" or (palm* and computer*) or Blackberry or Nokia or Symbian or (Windows and (mobile* or phone*)) or INQ or HTC or Sidekick or Android or iphone* or ipod* or ipad* or ipod* or MP3* or (tablet and (device* or computer*)) or mhealth or m‐health or "m health" or "mobile health" or telemed* or tele‐med* or telehealth* or tele‐health* or telecare* or tele‐care* or telenursing or tele‐nursing or ehealth or "e health" or e‐health or (app* and (smartphone* or smart‐phone or mobile* or phone*))
[Limited to asthma records]
CENTRAL on The Cochrane Library
#1 MeSH descriptor Asthma explode all trees
#2 asthma* or wheez*
#3 (#1 OR #2)
#4 MeSH descriptor Cellular Phone explode all trees
#5 MeSH descriptor MP3‐Player explode all trees
#6 MeSH descriptor Computers, Handheld explode all trees
#7 ((cell* or mobile*) near/3 phone*)
#8 (handheld* or hand‐held*)
#9 (smartphone* or smart‐phone*)
#10 PDA
#11 (personal* near/3 digital*)
#12 "Palm OS" or "Palm Pre classic"
#13 blackberry
#14 nokia
#15 symbian
#16 (windows near/3 (mobile* or phone*))
#17 INQ
#18 HTC
#19 sidekick
#20 android
#21 iphone*
#22 ipad*
#23 ipod*
#24 (tablet near/3 (device* or comput*))
#25 mhealth or m‐health or "m health"
#26 "mobile health"
#27 MeSH descriptor Telemedicine, this term only
#28 MeSH descriptor Telenursing, this term only
#29 telehealth* or tele‐health*
#30 telecare* or tele‐care*
#31 e‐health or ehealth or "e health"
#32 (app* near/3 (smartphone* or smart‐phone or mobile* or phone*))
#33 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32)
#34 (#3 AND #33)
#35 (#34), from 2000 to 2012
MEDLINE (Ovid) search strategy
1. exp asthma/
2. (asthma$ or wheez$).tw.
3. 1 or 2
4. Cellular Phone/
5. MP3‐Player/
6. Computers, Handheld/
7. ((cell$ or mobile$) adj3 phone$).tw.
8. (handheld$ or hand‐held$).tw.
9. (smartphone$ or smart‐phone$).tw.
10. PDA.tw.
11. (personal$ adj3 digital$).tw.
12. ("Palm OS" or "Palm Pre classic").tw.
13. (palm$ adj3 computer$).tw.
14. blackberry.tw.
15. nokia.tw.
16. symbian.tw.
17. (windows adj3 (mobile$ or phone$)).tw.
18. INQ.tw.
19. HTC.tw.
20. sidekick.tw.
21. android.tw.
22. iphone$.tw.
23. ipad.tw.
24. ipod.tw.
25. (tablet adj3 (device$ or comput$)).tw.
26. (mhealth or m‐health or "m health").tw.
27. "mobile health".tw.
28. Telemedicine/
29. Telenursing/
30. (telehealth$ or tele‐health$).tw.
31. (telecare$ or tele‐care$).tw.
32. (e‐health or ehealth or "e health").tw.
33. (app$ adj3 (smartphone$ or smart‐phone or mobile$ or phone$)).tw.
34. or/4‐33
35. 3 and 34
36. limit 35 to yr="2000 ‐Current"
EMBASE (Ovid) search strategy
1. exp asthma/
2. (asthma$ or wheez$).tw.
3. 1 or 2
4. mobile phone/
5. mp3 player/
6. personal digital assistant/
7. microcomputer/
8. ((cell$ or mobile$) adj3 phone$).tw.
9. (handheld$ or hand‐held$).tw.
10. (smartphone$ or smart‐phone$).tw.
11. PDA.tw.
12. (personal$ adj3 digital$).tw.
13. ("Palm OS" or "Palm Pre classic").tw.
14. (palm$ adj3 computer$).tw.
15. blackberry.tw.
16. nokia.tw.
17. symbian.tw.
18. (windows adj3 (mobile$ or phone$)).tw.
19. INQ.tw.
20. HTC.tw.
21. sidekick.tw.
22. android.tw.
23. iphone$.tw.
24. ipad.tw.
25. ipod.tw.
26. (tablet adj3 (device$ or comput$)).tw.
27. (mhealth or m‐health or "m health").tw.
28. "mobile health".tw.
29. exp telehealth/
30. (telehealth$ or tele‐health$).tw.
31. (telecare$ or tele‐care$).tw.
32. (e‐health or ehealth or "e health").tw.
33. (app$ adj3 (smartphone$ or smart‐phone or mobile$ or phone$)).tw.
34. or/4‐33
35. 3 and 34
36. limit 35 to yr="2000 ‐Current"
PsycINFO (Ovid) search strategy
1. exp Asthma/
2. (asthma$ or wheez$).tw.
3. 1 or 2
4. exp mobile devices/
5. ((cell$ or mobile$) adj3 phone$).tw.
6. (handheld$ or hand‐held$).tw.
7. (smartphone$ or smart‐phone$).tw.
8. PDA.tw.
9. (personal$ adj3 digital$).tw.
10. ("Palm OS" or "Palm Pre classic").tw.
11. (palm$ adj3 computer$).tw.
12. blackberry.tw.
13. nokia.tw.
14. symbian.tw.
15. (windows adj3 (mobile$ or phone$)).tw.
16. INQ.tw.
17. HTC.tw.
18. sidekick.tw.
19. android.tw.
20. iphone$.tw.
21. ipad.tw.
22. ipod.tw.
23. (tablet adj3 (device$ or comput$)).tw.
24. (mhealth or m‐health or "m health").tw.
25. "mobile health".tw.
26. telemedicine/
27. (telehealth$ or tele‐health$).tw.
28. (telecare$ or tele‐care$).tw.
29. (e‐health or ehealth or "e health").tw.
30. (app$ adj3 (smartphone$ or smart‐phone or mobile$ or phone$)).tw.
31. or/4‐30
32. 3 and 31
33. limit 32 to yr="2000 ‐Current"
CINAHL (EBSCO)
S1 (MH "Asthma+")
S2 asthma* OR wheez*
S3 S1 or S2
S4 (MH "Wireless Communications")
S5 (MH "Computers, Hand‐Held")
S6 (cell* N3 phone*) OR (mobile* N3 phone*)
S7 handheld* or hand‐held*
S8 smartphone* or smart‐phone*
S9 PDA
S10 personal* N3 digital*
S11 "Palm OS" or "Palm Pre Classic"
S12 palm* N3 computer*
S13 blackberry
S14 Nokia
S15 Symbian
S16 Windows N3 (mobile* OR phone*)
S17 INQ
S18 HTC
S19 Sidekick
S20 Android
S21 iphone or ipod or ipad
S22 tablet* N3 (device* or computer*)
S23 mhealth or m‐health or "m health"
S24 "mobile health"
S25 (MH "Telehealth+")
S26 telehealth* or tele‐health*
S27 telecare* or tele‐care*
S28 e‐health or ehealth or "e health"
S29 app* N3 (smartphone* or smart‐phone* or mobile* or phone*)
S30 S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29
S31 S3 and S30
S32 S3 and S30
ERIC
Advance search interface
((Keywords:asthma) and (Keywords:smartphone or Keywords:smart‐phone or Keywords:handheld or Keywords:hand‐held or Keywords:PDA or Keywords:palm or Keywords:blackberry or Keywords:nokia or Keywords:android or Keywords:Symbian or Keywords:INQ or Keywords:HTC or Keywords:sidekick or Keywords:android or Keywords:iphone or Keywords:ipod or Keywords:ipad or Keywords:mp3 or Keywords:mhealth or Keywords:m‐health or Keywords:telemedicine or Keywords:tele‐medicine or Keywords:telecare or Keywords:tele‐care or Keywords:e‐health or Keywords:ehealth or Keywords:app))Publication Date:2000‐2012
Global Health Library
Asthma AND ("cell phone" or "mobile phone" or smartphone or smart‐phone or handheld or hand‐held or "tablet computer" or "tablet device" or ipad or iphone or ipad or mp3 or PDA or blackberry or nokia or android or symbian or INQ or HTC or sidekick or mhealth or m‐health or "mobile health" or ehealth or e‐health or telemedicine or tele‐medicine or telecare or tele‐care or app)
ACM Digital Library
Advance search interface
(Abstract:asthma) and (Abstract:smartphone or Abstract:smart‐phone or Abstract:handheld or Abstract:hand‐held or Abstract:PDA or Abstract:palm or Abstract:blackberry or Abstract:nokia or Abstract:android or Abstract:Symbian or Abstract:INQ or Abstract:HTC or Abstract:sidekick or Abstract:android or Abstract:iphone or Abstract:ipod or Abstract:ipad or Abstract:mp3 or Abstract:phone or Abstract:mobile or Abstract:cell or Abstract:mhealth or Abstract:m‐health or Abstract:telemedicine or Abstract:tele‐medicine or Abstract:telecare or Abstract:tele‐care or Abstract:e‐health or Abstract:ehealth or Abstract:app)
Basic keyword search string
(asthma) AND (smartphone or smart‐phone or “tablet computer” or tablet device” or handheld or hand‐held or PDA or palm or blackberry or nokia or android or Symbian or INQ or HTC or sidekick or android or iphone or ipod or ipad or mp3 or “cell phone” or “mobile phone” or mhealth or m‐health or “mobile health” or telemedicine or tele‐medicine or telecare or tele‐care or e‐health or ehealth or app)
Appendix 2. Results of database searching
| Database | Years searched | Date of search | Results (before de‐duplication) | Results (after de‐duplication) |
| Airways Register | 2000 to present | 15 June 2012 | 52 | 52 |
| CENTRAL (Issue 6 of 12) | 2000 to present | 15 June 2012 | 42 | 7 |
| MEDLINE (Ovid) | 2000 to present | 15 June 2012 | 181 | 149 |
| EMBASE (Ovid) | 2000 to present | 15 June 2012 | 369 | 183 |
| PsycINFO (Ovid) | 2000 to present | 15 June 2012 | 36 | 17 |
| CINAHL (EBSCO) | 2000 to present | 15 June 2012 | 154 | 94 |
| CAB Abstracts | 2000 to present | 27 July 2012 | 19 | 15 |
| Global Health Library | 2000 to present | 20 July 2012 | 162 | 21 |
| Compendex/Inspec/Referex | 2000 to present | 27 July 2012 | 50 | 45 |
| IEEEXplore | 2000 to present | 27 July 2012 | 70 | 66 |
| ACM Digital Library | 2000 to present | 20 July 2012 | 17 | 16 |
| CiteSeerx | 2000 to present | 27 July 2012 | 48 | 47 |
| ERIC | 2000 to present | 20 July 2012 | 2 | 1 |
| Total | 1202 | 713 |
Appendix 3. Results of updated search ‐ June/July 2013
| Database | Years searched | Date of search | Results (before de‐duplication) | Results (after de‐duplication) |
| Airways Register | June 2012 to June 2013 | 19 June 2013 | 11 | 3 |
| CENTRAL (Issue 5, 2013) | June 2012 to June 2013 | 19 June 2013 | 9 | 3 |
| MEDLINE (Ovid) | June 2012 to June 2013 | 19 June 2013 | 30 | 12 |
| EMBASE (Ovid) | June 2012 to June 2013 | 19 June 2013 | 166 | 104 |
| PsycINFO (Ovid) | June 2012 to June 2013 | 19 June 2013 | 10 | 9 |
| CINAHL (EBSCO) | June 2012 to June 2013 | 19 June 2013 | 32 | 28 |
| CAB Abstracts | June 2012 to July 2013 | 29 July 2013 | 4 | 4 |
| Global Health Library | 2000 to present | 19 June 2013 | 231 | 45 |
| Compendex/Inspec/Referex | January 2012 to July 2013 | 29 July 2013 | 44 | 41 |
| IEEEXplore | January 2012 to July 2013 | 29 July 2013 | 18 | 18 |
| ACM Digital Library | January 2012 to July 2013 | 29 July 2013 | 9 | 4 |
| CiteSeerx | January 2012 to July 2013 | 29 July 2013 | 1 | 1 |
| ERIC | January 2012 to July 2013 | 19 June 2013 | 0 | 0 |
| Total | 565 | 272 (260 after de‐duplication of combined EndNote library) |
Data and analyses
Comparison 1. Smartphone asthma apps versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom scores using the ACQ | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Patients with unscheduled visits to the emergency department | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Hospital admissions | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 GP consultations for asthma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Unscheduled general practice nurse consultation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Out of hours attendances | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 HRQoL measured on the SF‐12 questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 SF‐12 (1 month) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 SF‐12 (2 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 SF‐12 (3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 SF‐12 (4 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 SF‐12 (5 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 SF‐12 (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 HRQoL measured on the mini‐AQLQ | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Proportion of participants adherent to the intervention | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 3 months | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 6 months | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Healthcare costs | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Total healthcare costs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Total cost of delivering trial intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Trial nursing costs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Telemonitoring service costs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.5 Total cost of healthcare provision | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.6 GP respiratory consultations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.7 Practice nurse respiratory consultations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.8 Secondary care costs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.9 Emergency services | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.10 Total cost of prescriptions for respiratory drugs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Peak expiratory flow rate (PEFR) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 PEFR at month 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 PEFR at month 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 PEFR at month 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 PEFR at month 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 PEFR at month 5 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.6 PEFR at month 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Forced expiratory volume in 1 second (FEV1) percentage predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 FEV1 percentage predicted at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 FEV1 percentage predicted at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Proportion of participants experiencing at least one acute asthma exacerbation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Proportion of participants who required at least one course of steroids | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Liu 2011.
| Methods | Study design: randomised controlled trial | |
| Participants | 120 participants with moderate‐to‐severe persistent asthma as defined by the American Thoracic Society (ATS) on the basis of clinical symptoms and physical examination. Patients were receiving treatment appropriate to their current level of asthma severity as specified by the Global Initiative for Asthma (GINA) guidelines After randomisation, 60 participants were assigned to the control condition and 60 to the intervention group. 51 participants in the control group and 49 participants in the intervention group attended the 3‐month review. At the time of the final review (6 months), there were 46 participants in the control group and 43 in the intervention group |
|
| Interventions |
Intervention group ‐ mobile telephone‐based interactive asthma self care: participants in this group were asked to complete and diary with daily peak expiratory flow rate (PEFR) measurements and asthma symptoms. For this purpose, the research team developed a mobile telephone‐based interactive self care software. This software provided an electronic diary to record patients' daily asthma symptom score, use of reliever medication, PEFR and PEFR variability. The data uploaded by patients were stored in their own personal files hosted in a secured server at the National Center for High‐Performance Computing. The research team developed their own scoring system in order to determine a patient's level of asthma control. This score system took into consideration a patient's PEFR, need for reliever medication and the number of daily asthma symptoms in the past 7 days. This system would categorise patients as being controlled, partially controlled or uncontrolled. Patients received immediate feedback on their asthma status as well as management advice. Feedback was displayed in their phones via General Packer Radio Service (GPRS) Control group ‐ (booklet for written asthma diary and action plan) group: participants in this group were given an asthma symptom diary booklet and were requested to record their PEFR daily. Patients also received their own individualised asthma action plan with detailed instructions for daily self management and guidelines for handling exacerbations and emergencies. All participants were taught how to adjust their medication. They also received asthma education, self management plan and standard medication. Moreover, all participants were assessed monthly for 6 months. Assessments involved completion of the short‐form (SF)‐12 quality of life questionnaire and review of: the number of episodes of acute exacerbation; medications used for asthma control, asthma symptom score; number of unscheduled clinic visits; visits to the emergency department and hospital admissions. Forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were performed every 3 months. Setting: outpatients clinics Location: Chang Gung Memorial Hospital, Linkou, Taiwan |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information available in the study report |
| Allocation concealment (selection bias) | Unclear risk | Not enough information available in the study report |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor personnel could have been blinded considering the nature of the intervention. This is likely to have affected participants' performance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not enough information available in the study report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not enough information available in the study report |
| Selective reporting (reporting bias) | Unclear risk | Not enough information available in the study report |
| Other bias | High risk | The study duration was 6 months. Since participants were recruited from different outpatient clinics it is likely that recruitment of participants spanned over a longer period. Therefore, there could have been a seasonal effect of asthma acting as a confounding variable |
Ryan 2012.
| Methods | Study design: multicentre randomised controlled trial | |
| Participants | Participants were recruited from 32 GP practices located in Norfolk, Suffolk, Yorkshire and Tyne‐and‐Wear with a population of 311,926 registered patients. The initial computer screening identified 13,101 potential eligible patients. After examination of these patients' records, 12,081 postal invitations were sent out. A total of 1016 patients expressed interest in the study and 393 baseline visits were booked. Finally, 288 participants were recruited and randomised (143 participants to the intervention group and 145 participants to the control group). Approximately 9% of patients who were invited for a baseline appointment had to be excluded since they showed improvement in their asthma control between phone assessment and baseline appointment Patients were eligible if they were 12 years old or older, had poorly controlled asthma (defined as a score of 1.5 or higher on the asthma control questionnaire), and had (or were willing to borrow) a compatible mobile phone handset. Exclusion criteria included: having a respiratory disease other than asthma; inability to communicate in English; receiving specialist care for sever asthma and being advised against inclusion by their GP |
|
| Interventions |
Intervention group ‐ mobile phone monitoring: participants allocated to this condition were contacted by an asthma nurse working for the manufacturer of the app being evaluated (OBS Medical). The asthma nurse helped participants to download the asthma app onto their mobile phone, tested its functionality, trained patients in its use, provide details of web access and followed up with a technical support call after 1 week of use. The asthma app evaluated in this study allowed for twice daily recording and transmission of symptoms, drug use and peak flow. The 80% and 60% thresholds for the asthma action plan were defined by taking the mean of the 5 best peak flow values in the most recent 50 readings. Patients' peak flow readings were displayed within a traffic light display and patients were prompted to follow the corresponding action point. Those patients who incurred twice into the amber zone or once into the red zone received a phone call from an asthma nurse working for OBS Medical. Unfortunately, there was no information available regarding the proportion of participants in the intervention group who received a phone call from an asthma nurse. Both the patient and their clinician had web access to the patient data record Control group ‐ paper based monitoring: patients assigned to this group were required to keep a paper diary of the same data as those in the intervention group Before randomisation, the asthma nurse delivered a 30‐minute standardised education session that covered topics on asthma, asthma treatment, inhaler technique, monitoring, and a personalised asthma action plan based on both symptoms and peak flows. Information about mobile‐ or paper‐based self monitoring was provided after randomisation according to allocation. Throughout the duration of the trial, the practices' asthma nurse provided clinical care following the stepwise approach recommended by the BTS‐SIGN asthma guideline. All patients were reviewed monthly until the asthma nurse judged that control was achieved on the basis of clinical monitoring. Once good asthma control was achieved, the patient was discharged from monthly follow‐up but was encouraged to continue monitoring on a maintenance basis, seeking professional advice if needed Setting: primary care Location: the United Kingdom |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone randomisation provided by the University of Aberdeen's Health Services Research Unit |
| Allocation concealment (selection bias) | Low risk | Telephone‐controlled randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants or personnel was not possible due to the nature of the intervention. This could have affected participants' performance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers blinded to allocation collected data on primary outcomes at the final trial visit |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data appear to have been analysed using an available case analysis |
| Selective reporting (reporting bias) | Low risk | Authors reported the outcomes and performed the statistical analyses they had listed in the study protocol |
| Other bias | High risk | Authors of this paper set out to evaluate the effectiveness of a technological component of asthma self management. However, participants' incursion into the amber or red zone triggered a telephone call by an asthma nurse. This could be considered an intervention in itself that could have confounded the effect of the smartphone app. Additionally, although the study duration was 6 months, recruitment across all the GP practices was rolled over 13 months. This could have introduced another confounder in the form of a seasonal effect of asthma. Authors did not have the means to make allowances for a potential seasonal effect. Moreover, in direct conversation with the contact author, it became apparent that 9% of the participants who were invited to attend a baseline assessment had to be excluded because they improved their asthma control by the time their appointment was due. Therefore, the sample tested in this study could represent a population of individuals who are more susceptible to poor levels of asthma self management rather than the general population of individuals with asthma |
FEV1: forced expiratory volume in one second GP: General Practitioner PEFR: peak expiratory flow rate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Dowaihi 2013 | The study design did not meet the eligibility criteria for this systematic review. This paper describes a prototype that would allow patients to self monitor their symptoms and manage their condition. This paper was identified in the updated search conducted in June/July 2013 |
| Awoyinka 2011 | The intervention, comparison and outcomes did not meet the eligibility criteria for this systematic review. This study assessed the difference in perceived asthma control between overweight and normal weight inner‐city teens diagnosed with asthma. Both the control and the intervention groups were receiving mobile technology (M‐CHESS), with the latter receiving asthma education and case management |
| Bumatay 2012 | The study design did not meet the eligibility criteria for this systematic review. This paper describes the development of an external mobile device accessory that records and stores the user's PEF, and graphs these data over time |
| Burbank 2013 | The study design did not meet the eligibility criteria for this systematic review. This was a pilot study. This paper was identified during the updated search conducted in June/July 2013 |
| Burnay 2013 | The study design did not meet the eligibility criteria for this systematic review. This paper described the development of a smartphone app and was identified during the updated search conducted in June/July 2013 |
| Chiu 2011 | The study design did not meet the eligibility criteria for this systematic review. This paper described the development of a smartphone‐based asthma monitoring system |
| Chu 2006 | The study design did not meet the eligibility criteria for this systematic review. This paper described the creation of a medical application of the global positioning system (GPS) to prevent possible morbidity of asthma during outdoor activities |
| Cleland 2007 | The study design did not meet the eligibility criteria for this systematic review. This study was a qualitative study that was not linked to a primary trial |
| Finkelstein 2002 | The study design and the intervention did not meet the eligibility criteria for this systematic review. This paper describes the development of a platform for the rapid development of interactive computer‐based health education programmes |
| Finkelstein 2009 | The study design did not meet the eligibility criteria for this systematic review. This paper described the development of an interactive patient learning system for use on mobile phones to deliver asthma‐related information to patients with this condition |
| Glykas 2004 | The study design did not meet the eligibility criteria for this systematic review. This paper described the creation of a web‐based asthma tool to enhance public information and awareness to support illness prevention and patients' independent living |
| Gupta 2011A | The study design did not meet the eligibility criteria for this systematic review. This paper described the development of a multi‐configuration Android‐based portable spirometer which support patient self monitoring of respiratory conditions |
| Gupta 2011B | The study design did not meet the eligibility criteria for this systematic review. This paper described the development of a multi‐configuration Android‐based portable spirometer which support patient self monitoring of respiratory conditions |
| Holtz 2009 | The intervention did not meet the eligibility criteria for this systematic review (i.e. it relied on short messaging service) |
| Larson 2013 | The study design did not meet the eligibility criteria for this systematic review. This paper, identified during the updated search (June/July 2013), described the development of a smartphone app that measures common lung function using the phone's built‐in microphone |
| Lee 2005 | The study design and the intervention did not meet the eligibility criteria for this systematic review. This paper describes the development of a web‐based mobile asthma management system |
| Licskai 2011 | The study design did not meet the eligibility criteria for this systematic review. This paper was a feasibility study identified during the updated search conducted in June/July 2013 |
| Lim 2009 | The study design and the intervention did not meet the eligibility criteria for this systematic review. This pilot project was aimed at reinforcing asthma counselling for patients attending asthma clinics in a tertiary care hospital. The project utilised personalised videos of asthma inhaler technique, which could be viewed online or downloaded to a mobile device for review anytime. Registration details were sent to participants via SMS |
| Lin 2009 | The study design did not meet the eligibility criteria for this systematic review. This paper describes the development of a platform for asthma patients using mobile phones to monitor their condition in real time |
| Oletic 2012 | The study design did not meet the eligibility criteria for this systematic review. This chapter of a book presents the development of a wheeze detection method for wearable body sensor nodes used in asthma management. This paper was identified during the updated search conducted in June/July 2013 |
| Pinnock 2006 | The study design did not meet the eligibility criteria for this systematic review. This was a qualitative study exploring professional and patient attitudes to using mobile technology to monitor asthma |
| Pinnock 2007 | The study design did not meet the eligibility criteria for this systematic review. This was a qualitative study exploring professional and patient attitudes to using mobile phones for the self management of asthma |
| Postolache 2009 | The study design did not meet the eligibility criteria for this systematic review. This paper describes the development of a wireless sensing network Bluetooth enabled for the continuous monitoring of indoor humidity and temperature, pollutant gases and vapours |
| Ryan 2005 | The study design did not meet the eligibility criteria for this systematic review. This was an observational study using electronic peak flow monitoring and mobile phone technology in a UK population attending primary care |
| Sundberg 2005 | The intervention did not meet the inclusion criteria for this systematic review: interactive computer programme providing information about asthma |
PEF: peak expiratory flow
Differences between protocol and review
We used ORs for dichotomous outcomes because this measure of effect size is less sensitive to variations in underlying event rates.
We dealt with missing data using an available case analysis rather than an ITT analysis because 1) we could not obtain missing data from the original authors and 2) we cannot be confident that the data were missing at random in order to follow a multiple imputation approach.
Contributions of authors
JMB and GG performed the selection of included studies and data extraction from them. JMB wrote the final draft of the review. KH and LG critically appraised the review. KH developed the search strategy. LG provided support regarding statistical methods. JC and KH conceived the idea for the review.
Declarations of interest
None known.
New
References
References to studies included in this review
Liu 2011 {published data only (unpublished sought but not used)}
- Liu WT, Huang CD, Wang CH, Lee KY, Lin SM, Kuo HP. A mobile telephone‐based interactive self‐care system improves asthma control. European Respiratory Journal 2011;37(2):310‐7. [DOI: 10.1183/09031936.00000810] [DOI] [PubMed] [Google Scholar]
Ryan 2012 {published data only}
- Ryan D, Pinnock H, Lee AJ, Tarassenko L, Ayansina D, Musgrave S, et al. Can your mobile phone help your asthma: preliminary results. Primary Care Respiratory Journal 2010;19(2):A15. [Google Scholar]
- Ryan D, Pinnock H, Lee AJ, Tarassenko L, Pagliari C, Sheikh A, et al. The CYMPLA trial. Mobile phone‐based structured intervention to achieve asthma control in patients with uncontrolled persistent asthma: a pragmatic randomised controlled trial. Primary Care Respiratory Journal 2009;18(4):343‐45. [DOI: 10.4104/pcrj.2009.00064] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D, Price D, Musgrave SD, Malhotra S, Lee AJ, Ayansina D, et al. Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: multicentre randomised controlled trial. BMJ 2012;344(e1756):1‐15. [DOI: 10.1136/bmj.e1756] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Dowaihi 2013 {published data only}
- Al‐Dowaihi D, Al‐Ajlan M, Al‐Zahrani N, Al‐Quwayfili N, al‐Jwiser N, Kanjo E. mBreath: Asthma Monitoring System on the Go. International Conference on Computer Medical Applications. January 2013. [DOI: 10.1109/ICCMA.2013.6506169] [DOI]
Awoyinka 2011 {published data only}
- Awoyinka IA, Shanovich KK, Chih M, McTavish F, Sorkness C, Gustafson D. Perception of asthma control in overweight inner‐city teens. Journal of Allergy and Clinical Immunology 2011;127(2):AB267. [Google Scholar]
Bumatay 2012 {published data only}
- Bumatay A, Chan R, Lauher K, Kwan AM, Stoltz T, Delplanque JP, et al. Coupled mobile phone platform with peak flowmeter enables real‐time lung function assessment. IEEE Sensors Journal 2012;12(3):685‐91. [Google Scholar]
Burbank 2013 {published data only}
- Burbank AJ, Hall‐Barrow J, Denman RR, Lewis SD, Hewes M, Schellhase DE, et al. There's an app for that: a pilot and feasibility study of mobile‐based asthma action plans. Journal of Allergy and Clinical Immunology 2013;131(2):AB161. [Google Scholar]
Burnay 2013 {published data only}
- Burnay E, Cruz‐Correia R, Jacinto T, Sá Sousa A, Fonseca J. Challenges of a mobile application for asthma and allergic rhinitis patient enablement ‐ interface and synchronization. Telemedicine and e‐Health 2013;19(1):13‐8. [DOI] [PubMed] [Google Scholar]
Chiu 2011 {published data only}
- Chiu HW, Chen IJ, Hsieh YJ, Lee TC. G‐Asthma: A smart phone‐based asthma management system for Taiwanese. 5th European Conference of the Internation Federation for Medical and Biological Engineering. 2011:898‐901.
Chu 2006 {published data only}
- Chu HT, Huang CC, Lian ZH, Tsai JJP. A ubiquitous warning system for asthma‐inducement. Proceedings of the IEEE International Conference on Sensor Networks, Ubiquitous, and Trustworthy Computing (SUTC '06). 2006:186‐91.
Cleland 2007 {published data only}
- Cleland J, Caldow J, Ryan D. A qualitative study of the attitudes of patients and staff to the use of mobile phone technology for recording and gathering asthma data. Journal of Telemedicine and Telecare 2007;13:85‐9. [DOI: 10.1258/135763307780096230] [DOI] [PubMed] [Google Scholar]
Finkelstein 2002 {published data only}
- Finkelstein J, Nambu S, Khare R, Gupta D. CO‐ED: A development platform for interactive patient education. Proceedings of the International Conference on Computers in Education. 2002.
Finkelstein 2009 {published data only}
- Finkelstein J, Wood J. Mobile eLearning platform for interactive patient education. 2009 International Conference on Mobile, Hybrid, and On‐line Learning. 2009:23‐7. [DOI: 10.1109/eLmL.2009.24] [DOI]
Glykas 2004 {published data only}
- Glykas M, Chytas P. Technological innovations in asthma patient monitoring and care. Expert Systems with Applications 2004;27:121‐31. [DOI: 10.1016/j.eswa.2003.12.007] [DOI] [Google Scholar]
Gupta 2011A {published data only}
- Gupta S, Chang P, Anyigbo N, Sabharwal A. mobileSpiro: Portable open‐interface spirometry for Android. Proceedings of the 2nd Conference on Wireless Health. 2011:Article No. 24.
Gupta 2011B {published data only}
- Gupta S, Chang P, Anyigbo N, Sabharwal A. mobileSpiro: Accurate mobile spirometry for self‐management of asthma. Proceedings of the First ACM Workshop on Mobile Systems, Applications, and Services for Healthcare. 2011:Article No. 1.
Holtz 2009 {published data only}
- Holtz B, Whitten P. Managing asthma with mobile phones: a feasibility study. Telemedicine and e‐Health 2009;15(9):907‐9. [DOI: 10.1089/tmj.2009.0048] [DOI] [PubMed] [Google Scholar]
Larson 2013 {published data only}
- Larson EC, Goel M, Redfield M, Boriello G, Rosenfeld M, Patel SN. Tracking lung function on any phone. Proceedings of the 3rd ACM Symposium on Computing for Development. 2013.
Lee 2005 {published data only}
- Lee HR, Yoo SK, Jung SM, Kwon NY, Hong CS. A web‐based mobile asthma management system. Journal of Telemedicine and Telecare 2005;11(Suppl 1):56‐9. [DOI] [PubMed] [Google Scholar]
Licskai 2011 {published data only}
- Licskai C, Sands T, Ferrone M. Integrating the air quality health index into asthma action plans. American Journal of Respiratory Critical Care Medicine 2011;183:A4767. [Google Scholar]
Lim 2009 {published data only}
- Lim T, Kong M, Lim F, Chee M, Tan L, Tan K. A pilot project of inhaler technique in the multimedia format for asthma education. Journal of Allergy and Clinical Immunology 2009;123(2):S65. [Google Scholar]
Lin 2009 {published data only}
- Lin SP, Yang HY. Exploring key factors in the choice of e‐health using an asthma care mobile service model. Telemedicine and e‐Health 2009;2009:884‐90. [DOI] [PubMed] [Google Scholar]
Oletic 2012 {published data only}
- Oletic D, Arsenali B, Bilas V. Towards continuous wheeze detection body sensor node as a core of asthma monitoring system. Wireless Mobile Communication and Healthcare. Springer, 2012. [Google Scholar]
Pinnock 2006 {published data only}
- Pinnock H, Slack R, Pagliari C, Price D, Sheikh A. Professional and patient attitudes to using mobile phone technology to monitor asthma: questionnaire survey. Primary Care Respiratory Journal 2006;15:237‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinnock 2007 {published data only}
- Pinnock H, Slack R, Pagliari C, Price D, Sheikh A. Understanding the potential role of mobile phone‐based monitoring on asthma self‐management: qualitative study. Clinical and Experimental Allergy 2007;37:794‐802. [DOI] [PubMed] [Google Scholar]
Postolache 2009 {published data only}
- Postolache O, Girão P, Dias Pereira M, Ferraria G, Barroso N. Indoor monitoring of respiratory distress triggering factors using a wireless sensing network and smart phone. International Instrumentation and Measurement Technology Conference. 2009.
Ryan 2005 {published data only}
- Ryan D, Cobern W, Wheeler J, Price D, Tarassenko L. Mobile phone technology in the management of asthma. Journal of Telemedicine and Telecare 2005;11(Suppl 1):43‐6. [DOI] [PubMed] [Google Scholar]
Sundberg 2005 {published data only}
- Sundberg R, Tunsäter A, Palmqvist M, Ellbjär S, Löwhagen O, Torén K. A randomized controlled study of a computerized limited education program among young adults with asthma. Respiratory Medicine 2005;99:321‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Baron 2012
- Baron J, McBain H, Newman S. The impact of mobile monitoring technologies on glycosylated hemoglobin in diabetes: a systematic review. Journal of Diabetes Science & Technology 2012;6(5):1185‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyd 2009
- Boyd M, Lasserson TJ, McKean MC, Gibson PG, Ducharme FM, Haby M. Interventions for educating children who are at risk of asthma‐related emergency department attendance. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001290.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Braman 2006
- Braman SS. The global burden of asthma. Chest 2006;130(1):4S‐12S. [DOI] [PubMed] [Google Scholar]
BTS‐SIGN 2011
- British Thoracic Society ‐ Scottish Intercollegiate Guideline Network. British guideline on the management of asthma: a national clinical guideline. www.sign.ac.uk/guidelines/fulltext/101/index.html (accessed November 2011).
Bussey‐Smith 2007
- Bussey‐Smith KL, Rossen RD. A systematic review of randomized control trials evaluating the effectiveness of interactive computerized asthma patient education programs. Annals of Allergy, Asthma and Immunology 2007;98(6):507‐16. [DOI] [PubMed] [Google Scholar]
Charles 2007
- Charles T, Quinn D, Weatherall M, Aldington S, Beasley R, Holt S. An audiovisual reminder function improves adherence with inhaled corticosteroid therapy in asthma. Journal of Allergy and Clinical Immunology 2007;119(4):811‐6. [DOI] [PubMed] [Google Scholar]
Creer 2008
- Creer TL. Behavioral and cognitive processes in the self‐management of asthma. Journal of Asthma 2008;45:81‐94. [DOI: 10.1080/02770900701247236] [DOI] [PubMed] [Google Scholar]
Department of Health 2006
- Department of Health. Supporting people with long term conditions to self care ‐ a guide to developing local strategies and good practice. Department of Health 2006.
EndNote [Computer program]
- Thomson Reuters Corporation. EndNote. New York, USA: Thomson Reuters Corporation, 2010.
EPOC 2009
- Cochrane Effective Practice and Organization of Care (EPOC) Review Group. Data collection checklist. http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/datacollectionchecklist.pdf (accessed March 2012).
Estrin 2010
- Estrin D, Sim I. Health care delivery. Open mHealth architecture: an engine for health care innovation. Science 2010;330(6005):759‐60. [DOI] [PubMed] [Google Scholar]
Fleming 2000
- Flaming DM, Cross KW, Sunderland R, Ross AM. Comparison of the seasonal patterns of asthma identified in general practitioner episodes, hospital admissions, and deaths. Thorax 2000;55:662‐5. [DOI: 10.1136/thorax.55.8.662] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2007
- Foster G, Taylor SJC, Eldridge S, Ramsay J, Griffiths CJ. Self‐management education programmes by lay leaders for people with chronic conditions. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005108.pub2] [DOI] [PubMed] [Google Scholar]
Gibson 2008
- Gibson PG, Powell H, Wilson A, Hensley MJ, Abramson MJ, Bauman A, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD001005] [DOI] [PubMed] [Google Scholar]
Gibson 2009
- Gibson PG, Powell H, Wilson A, Abramson MJ, Haywood P, Bauman A, et al. Self‐management education and regular practitioner review for adults with asthma. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001117] [DOI] [PubMed] [Google Scholar]
GINA 2011
- Global Initiative for Asthma. Global strategy for asthma management and prevention. http://www.ginasthma.org/uploads/users/files/GINA_Report2011_May4.pdf (accessed 19 December 2012).
Haughney 2004
- Haughney J, Barnes G, Partridge M, Cleland J. The Living & Breathing Study: a study of patients' views of asthma and its treatment. Primary Care Respiratory Journal 2004;13:28‐35. [DOI: 10.1016/j.pcrj.2003.11.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huckvale 2012
- Huckvale K, Car M, Morrison C, Car J. Apps for asthma self‐management: a systematic assessment of content and tools. BMC Medicine 2012;10:144. [DOI: 10.1186/1741-7015-10-144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Istepanian 2005
- Istepanian R, Laxminarayan S, Pattichis CS (editors). M‐Health: Emerging Mobile Health Systems. Springer, 2005. [Google Scholar]
Juniper 1999
- Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. European Respiratory Journal 1999;14(4):902‐7. [DOI] [PubMed] [Google Scholar]
Kaya 2009
- Kaya Z, Erkan F, Ozkan M, Ozkan S, Kocaman M, Ertekin BA, et al. Self‐management plans for asthma control and predictors of patient compliance. Journal of Asthma 2009;46:270‐5. [DOI] [PubMed] [Google Scholar]
Lahdensuo 1999
- Lahdensuo A. Guided self‐management of asthma ‐ how to do it?. BMJ 1999;319(7212):759‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacLeod 2013
- MacLeod H, Feldman R. Tablets to nearly double in 2013. http://yougov.co.uk/news/2012/09/11/tablets‐nearly‐double‐2013/ 11 September 2013 (accessed 10 October 2013).
Masoli 2004
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy 2004;59(5):469‐78. [DOI] [PubMed] [Google Scholar]
McLean 2010
- McLean S, Chandler D, Nurmatov U, Liu J, Pagliari C, Car J, et al. Telehealthcare for asthma. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007717.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
mHealth Alliance 2010
- Glossary of terms. www.mhealthalliance.org/media_center/glossary‐terms (accessed January 2012).
mobiThinking 2013
- Global mobile statistics 2013 Part A: Mobile subscribers; handset market share; mobile operators. http://mobithinking.com/mobile‐marketing‐tools/latest‐mobile‐stats/a#mobilephonepenetration (accessed 10 October 2013).
Mosa 2012
- Mosa ASM, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Medical Informatics and Decision Making 2012;12:67. [DOI: 10.1186/1472-6947-12-67] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newman 2010
- Serlachius A, Sutton S. Self‐management and behaviour change: theoretical models. In: Newman S, Steed L, Mulligan K editor(s). Chronic Physical Illness: Self‐Management and Behavioural Interventions. McGraw Hill, 2010. [Google Scholar]
NHLBI 2007
- National Heart, Lung and Blood Institute & National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf (accessed 19 December 2012).
NIH 2010
- National Institute of Health. What is complementary and alternative medicine?. www.nccam.nih.gov/health/whatiscam/ (accessed 2 December 2011).
Ofcom 2010
- Ofcom. Communications Market Report. www.stakeholders.ofcom.org.uk/market‐data‐research/market‐data/communications‐market‐reports/cmr10/ (accessed January 2012).
Ofcom 2013
- Ofcom. Communications Market Report 2013. http://stakeholders.ofcom.org.uk/binaries/research/cmr/cmr13/2013_UK_CMR.pdf 2013 (accessed 10 October 2013).
Partridge 2008
- Partridge MR, Caress AL, Brown C, Hennings J, Luker K, Woodcock A, et al. Can lay people deliver asthma self‐management education as effectively as primary care based practice nurses?. Thorax 2008;63(9):778‐83. [DOI] [PubMed] [Google Scholar]
Partridge 2011
- Partridge MR, Dal Negro RW, Olivieri D. Understanding patients with asthma and COPD: insights from a European study. Primary Care Respiratory Journal 2011;20(3):315‐23. [DOI: 10.4104/pcrj.2011.00056] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paré 2007
- Paré G, Jaana M, Sicotte C. Systematic review of home telemonitoring for chronic diseases: the evidence base. Journal of the American Medical Informatics Association 2007;14(3):269‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearce 2000
- Pearce N, Sunyer J, Cheng S, Chinn S, Bjorksten B, Burr M, et al. Comparison of asthma prevalence in the ISAAC and the ECRHS. European Respiratory Journal 2000;16(3):420‐6. [DOI] [PubMed] [Google Scholar]
Powell 2009
- Powell H, Gibson PG. Options for self‐management education for adults with asthma. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD004107] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ring 2007
- Ring N, Malcolm C, Wyke S, MacGillivray S, Dixon D, Hoskins G, et al. Promoting the use of Personal Asthma Action Plans: a systematic review. Primary Care Respiratory Journal 2007;16(5):271‐83. [DOI: 10.3132/pcrj.2007.00049] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ring 2011
- Ring N, Jepson R, Hoskins G, Wilson C, Pinnock H, Sheikh A, et al. Understanding what helps or hinders asthma action plan use: a systematic review and synthesis of the qualitative literature. Patient Education and Counseling 2011;85(2):131‐43. [DOI: 10.1016/j.pec.2011.01.025] [DOI] [PubMed] [Google Scholar]
Rodgers 2009
- Rodgers M, Sowden A, Petticrew M, Arai L, Roberts H, Britten N, et al. Testing methodological guidance on the conduct of narrative synthesis in systematic reviews: effectiveness of interventions to promote smoke alarm ownership and function. Evaluation 2009;15(1):49‐73. [Google Scholar]
Ryan 2011
- Ryan R. Guide for review authors on assessing study quality. Cochrane Consumers and Communication Review Group Study Quality Guide. http://www.latrobe.edu.au/chcp/assets/downloads/StudyQualityGuide_May2011.pdf (accessed March 2012).
Sanders 2006
- Sanders DL, Aronsky D. Biomedical informatics applications for asthma care: a systematic review. Journal of the American Medical Informatics Association 2006;13(4):418‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013
- Smith A. Smartphone ownership ‐ 2013 update. http://pewinternet.org/˜/media//Files/Reports/2013/PIP_Smartphone_adoption_2013_PDF.pdf 5 June2013 (accessed 10 October 2013).
Tapp 2007
- Tapp S, Lasserson TJ, Rowe B. Education interventions for adults who attend the emergency room for acute asthma. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003000.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Terry 2010
- Terry M. Medical apps for smartphones. Telemedicine Journal and e‐Health 2010;16(1):17‐22. [DOI] [PubMed] [Google Scholar]
Thomas 2008
- Thomas J, Harden A. Methods for thematic synthesis of qualitative research in systematic reviews. BMC Medical Research Methodology 2008;8:45. [DOI: 10.1186/1471-2288-8-45] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Meer 2009
- Meer V, Bakker MJ, Hout WB, Rabe KF, Sterk PJ, Kievit J, et al. Internet‐based self‐management plus education compared with usual care in asthma: a randomized trial. Annals of Internal Medicine 2009;151(2):110‐20. [DOI] [PubMed] [Google Scholar]
Verschelden 1996
- Verschelden P, Cartier A, L'Archeveque J, Trudeau C, Malo JL. Compliance with and accuracy of daily self‐assessment of peak expiratory flows (PEF) in asthmatic subjects over a three month period. European Respiratory Journal 1996;9:880‐5. [DOI] [PubMed] [Google Scholar]
Weinstein 2005
- Weinstein AG. Should patients with persistent severe asthma be monitored for medication adherence. Annals of Allergy, Asthma and Immunology 2005;94(2):251‐7. [DOI] [PubMed] [Google Scholar]
Welsh 2011
- Welsh EJ, Hasan M, Li P. Home‐based educational interventions for children with asthma. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD008469.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. Asthma, Fact sheet N°307. www.who.int/mediacentre/factsheets/fs307/en/index.html (accessed 14 February 2011).
Wilson 2010
- Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. American Journal of Respiratory and Critical Care Medicine 2010;181(6):566‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 2008
- Wolf F, Guevara JP, Grum CM, Clark NM, Cates CJ. Educational interventions for asthma in children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD000326] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2010
- Wu TY, Majeed A, Kuo KN. An overview of the healthcare system in Taiwan. London Journal of Primary Care 2010;3:115‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeldes 2005
- Zeldes N. The first PDA. www.nzeldes.com/HOC/Newton.htm (accessed 6 September 2011).
Zickuhr 2013
- Zickuhr K. Tablet ownership 2013. http://www.pewinternet.org/˜/media//Files/Reports/2013/PIP_Tablet%20ownership%202013.pdf 10 June 2013 (accessed 10 October 2013).


