Abstract
The equilibrium of signaling through activating and inhibitory receptors dictates whether a given NK cell will execute cellular cytotoxicity. In this issue of the JCI, Kamiya et al. describe a novel approach to efficiently inhibiting surface expression of the inhibitory receptor CD94/NK group 2 member A (NKG2A) through retention of the protein in the endoplasmic reticulum. In adoptive transfer experiments into tumor-bearing immunodeficient mice, NKG2Anull NK cells were significantly more effective at eliminating HLA-E–expressing tumor cells than NKG2A+ NK cells. This study provides proof of concept for a new immunotherapeutic approach using NKG2Anull NK cells.
A central role for NKG2A in NK cell inhibition
CD94/NK group 2 member A (NKG2A) and NKG2C were identified in the mid-1990s as cell surface glycoproteins that form disulfide-bonded heterodimers with CD94 and bind the nonclassical MHC class Ib molecule HLA-E. NKG2C engagement in CMV-seropositive individuals imparts NK cell activation (with activated cells termed adaptive NK cells), while engagement of CD94/NKG2A transduces an inhibitory signal, consistent with the presence of two I/VxYxxL immunoreceptor tyrosine-based inhibitory motifs (ITIMs) within the cytoplasmic domain of NKG2A (1–4). While NKG2A expression and NKG2C expression are usually mutually exclusive, the frequency of NKG2A+ NK cells is considerably higher than that of NKG2C+ NK cells, especially in CMV-seronegative donors (5). Therefore, alongside killer immunoglobulin-like receptors (KIR), NKG2A represents a dominant inhibitory receptor on NK cells. The NKG2A phospho-ITIMs interact directly with the SH2 domains of the tyrosine phosphatases SHP-1 and SHP-2 (6). One of the major targets of SHP-1–mediated dephosphorylation in NK cells is the guanine exchange factor and adaptor protein Vav1. Dephosphorylation of Vav1 prevents Rac1-dependent rearrangement of the actin cytoskeleton and amplification of activating signals (7). Engagement of CD94/NKG2A by HLA-E within inhibitory signaling clusters can also lead to the phosphorylation of the signaling adaptor protein Crk and disruption of actin-dependent signaling upstream of Vav1 (8). NKG2A is uniformly high on immunoregulatory CD56bright NK cells, whereas cytotoxic CD56dim NK cells exhibit more heterogeneous expression, with a general decrease associated with terminal differentiation (9).
HLA-E molecules are expressed at low levels in most tissues and primarily present peptides derived from the leader sequences of classical class Ia HLA molecules (10). However, HLA-E is commonly expressed at high levels on the surface of a variety of different cancers (11–13). The mechanistic basis of HLA-E overexpression is not entirely clear. However, it has been shown that IFN-γ treatment can induce high levels of HLA-E in ovarian carcinoma cell lines (14). Intriguingly, the human cytomegalovirus protein gpUL40 can also upregulate HLA-E (15). Thus, it is possible that HLA-E overexpression in some types of cancer could be driven by viral infection. While CMV-induced NKG2C+NKG2Alo/– NK cells may be highly effective in fighting cancer (16), high levels of HLA-E on tumor cells from cancer patients are negatively correlated with survival and are assumed to restrain the cytotoxic effector function of both NK cells and subsets of CD8+ T cells that upregulate NKG2A in response to inflammatory cytokines (13, 17). Given these correlative clinical findings, there is a strong rationale for targeting the NKG2A/HLA-E axis as a means of enhancing immunotherapy.
Trapping NKG2A to unleash NK cell antitumor function
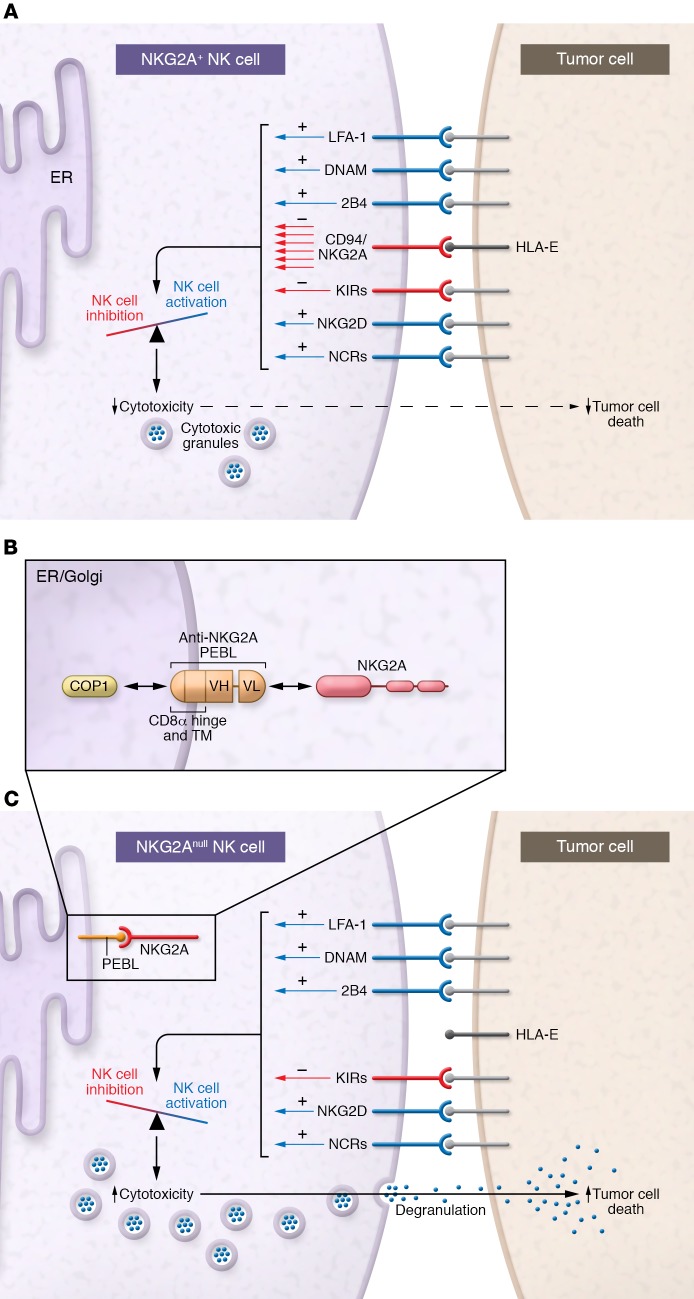
The current study by Kamiya et al. employs a clever approach to eliminating NKG2A surface expression on primary peripheral blood NK cells (18). The authors designed a novel construct termed NKG2A protein expression blocker (PEBL). The construct consists of a single-chain variable fragment (scFv) derived from the sequence of the Z199 anti-NKG2A antibody clone linked to different endoplasmic reticulum (ER) retention domains. The idea behind the use of a NKG2A PEBL is that, once endogenous NKG2A proteins are translated by ribosomes in the ER, they will be immediately “captured” by the anti-NKG2A antibody fragment and retained within the ER instead of trafficking to the cell surface. Indeed, NK cells retrovirally transduced with NKG2A PEBLs exhibited marked reduction in NKG2A surface expression. In particular, constructs containing KKMP (lysine, lysine, methionine, proline) domains together with a CD8α hinge and transmembrane domain, allowing for direct binding to the ER protein COP1, almost completely blocked NKG2A surface expression (Figure 1B).
Figure 1. Trapping NKG2A in the ER enhances NK cell cytotoxicity against HLA-E–expressing tumors.
(A) High HLA-E+ tumors deliver a potent inhibitory signal that leads to inhibition of NK cell function (five relative activating signal units vs. seven inhibitory). (B) Kamiya et al. developed a series of NKG2A PEBLs consisting of an scFv derived from an anti-NKG2A antibody linked to ER-retention domains. Transduction of human peripheral blood NK cells with retrovirus containing PEBL cassettes leads to efficient intracellular retention of NKG2A. (C) After NKG2A knockdown by PEBLs, the resulting NKG2Anull NK cells exhibit greater cytotoxicity against HLA-E–expressing tumor cells due to a net lack of inhibitory signaling (five relative activating signal units vs. one inhibitory).
After confirming that no other NK cell receptors were altered in response to NKG2A PEBL transduction, the authors generated multiple tumor cell lines with strong NKG2A-binding potential by transducing them with HLA-E plus HLA-G signal peptide. Relative to control NKG2A+ NK cells (expressing only GFP), NKG2Anull NK cells (NKG2A PEBL-transduced) exhibited markedly higher cytotoxicity against these HLA-E–overexpressing lines. Therefore, the balance in signaling inputs was tipped; NKG2A+ NK cells that received a negative signal in response to HLA-E ligation were restrained in their killing (Figure 1A), while activating signals prevailed in NKG2Anull cells to trigger cytotoxicity (Figure 1C). The authors also tested the function of control and NKG2Anull NK cells against tumor cell lines with endogenous HLA-E expression that could be further elevated after exposure to IFN-γ. Again, NKG2Anull NK cells exerted significantly higher cytotoxicity with or without the addition of IFN-γ in three out of the four lines tested. Importantly, more robust cytotoxicity was also observed by NKG2Anull NK cells in response to acute myeloid leukemia (AML) specimens that were collected from patient bone marrow and treated with IFN-γ.
Kamiya et al. wrap up this study with xenogeneic adoptive transfer experiments designed to assess the antitumor capabilities of NKG2Anull NK cells in vivo. They engrafted the Ewing sarcoma cell line ES8 or the osteosarcoma line U2OS (both transduced with HLA-E plus HLA-G signal peptide) into immunodeficient mice. Mice were then given two infusions of either control NKG2A+ NK cells or NKG2Anull NK cells at days 1 and 5 after tumor injection along with IL-2 (3 times per week). In both tumor models, control NKG2A+ NK cells were effective only at delaying tumor development, while the administration of NKG2Anull NK cells resulted in long-term survival for most mice. These impressive in vivo results provide proof of concept for the use of NKG2Anull NK cells as a novel immunotherapy for treating patients with cancer.
Future directions
There are three ways to block or diminish NKG2A inhibition: Trapping NKG2A as described here, CMV exposure to bias the repertoire towards NKG2C+/NKG2Alow/- adaptive NK cells, and with therapeutic antibody blockade. Two recent publications in Cell (19, 20) demonstrate the beneficial impact of targeting NKG2A with blocking antibodies for enhanced cytotoxic immune response against cancer. André et al. recently reported results from a phase 2 trial using monalizumab, a humanized anti-NKG2A antibody, in combination with the anti-EGFR antibody cetuximab in previously treated squamous cell carcinoma patients. They observed a 31% objective response rate in this patient population (20). van Montfoort et al. showed that antibody blockade of NKG2A potentiated CD8+ T cell responses induced by a peptide-based cancer vaccine in a mouse model for HPV16-induced carcinoma (19). As a treatment option, administration of a monoclonal anti-NKG2A antibody to cancer patients would be more cost effective and logistically straightforward relative to cell therapy in which NK cells would need to be expanded and transduced under GMP conditions. However, NK numbers and function are frequently suppressed in patients with advanced cancer (21). Thus, the administration of blocking antibodies may not be effective for patients with very low cytotoxic lymphocyte numbers and/or defective cytotoxicity. Adoptive transfer of NKG2Anull NK cells, as suggested by Kamiya and colleagues, may be a promising therapy for cancers that are refractory to checkpoint inhibitory receptor blockade. It should be noted that, in order to efficiently transduce NK cells and generate large numbers of cells, the authors of the current study cocultured NK cells with the genetically modified K562-mbIL-15-41BBL cell line. What effects this potent stimulation might have on NK cell persistence and homing after adoptive transfer into humans are still largely unknown. Going forward, it is clear that NKG2A is an important inhibitory receptor on cytotoxic lymphocytes and that cancers can highly overexpress its HLA-E ligand. Future studies to compare the clinical benefit of utilizing NKG2Anull cells generated by intracellular protein retention with strategies aimed at increasing CMV-induced NKG2C+NKG2Alo/– adaptive NK cells or therapeutic antibody-mediated NKG2A blockade are needed.
Acknowledgments
We would like to acknowledge the following sources of NIH funding: K99/R00 HL123638 (to FC) and P01 CA111412, P01 CA65493, and R35 CA197292 (to JSM).
Version 1. 04/15/2019
Electronic publication
Version 2. 05/01/2019
Print issue publication
Footnotes
Conflict of interest: JSM serves on the scientific advisory board of, and consults for, GT BioPharma Inc. and Fate Therapeutics. He has received research funds from these relationships. He also serves on the scientific advisory boards for CytoSen Therapeutics and Onkimmune. FC consults for Fate Therapeutics and has received research funds from this relationship.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2019;129(5):1839–1841. https://doi.org/10.1172/JCI128480.
See the related article at Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells.
Contributor Information
Frank Cichocki, Email: cich0040@umn.edu.
Jeffrey S. Miller, Email: mille011@tc.umn.edu.
References
- 1.Lazetic S, Chang C, Houchins JP, Lanier LL, Phillips JH. Human natural killer cell receptors involved in MHC class I recognition are disulfide-linked heterodimers of CD94 and NKG2 subunits. J Immunol. 1996;157(11):4741–4745. [PubMed] [Google Scholar]
- 2.Braud VM, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391(6669):795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 3.Lee N, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A. 1998;95(9):5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paust S, Blish CA, Reeves RK. Redefining memory: building the case for adaptive NK cells. J Virol. 2017;91(20):e00169-17. doi: 10.1128/JVI.00169-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104(12):3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 6.Le Dréan E, et al. Inhibition of antigen-induced T cell response and antibody-induced NK cell cytotoxicity by NKG2A: association of NKG2A with SHP-1 and SHP-2 protein-tyrosine phosphatases. Eur J Immunol. 1998;28(1):264–276. doi: 10.1002/(SICI)1521-4141(199801)28:01<264::AID-IMMU264>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 2003;23(17):6291–6299. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu D, Peterson ME, Long EO. The adaptor protein Crk control activation and inhibition of natural killer cells. Immunity. 2012;36(4):600–611. doi: 10.1016/j.immuni.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Béziat V, Descours B, Parizot C, Debré P, Vieillard V. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS One. 2010;5(8):e11966. doi: 10.1371/journal.pone.0011966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braud V, Jones EY, McMichael A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol. 1997;27(5):1164–1169. doi: 10.1002/eji.1830270517. [DOI] [PubMed] [Google Scholar]
- 11.Seliger B, et al. HLA-E expression and its clinical relevance in human renal cell carcinoma. Oncotarget. 2016;7(41):67360–67372. doi: 10.18632/oncotarget.11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson E, et al. Non-classical HLA-class I expression in serious ovarian carcinoma: Correlation with the HLA-genotype, tumor infiltrating immune cells and prognosis. Oncoimmunology. 2015;5(1):e1052213. doi: 10.1080/2162402X.2015.1052213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talebian Yazdi M, et al. The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma. Oncotarget. 2016;7(3):3477–3488. doi: 10.18632/oncotarget.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malmberg KJ, et al. IFN-gamma protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J Clin Invest. 2002;110(10):1515–1523. doi: 10.1172/JCI15564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomasec P, et al. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000;287(5455):1031. doi: 10.1126/science.287.5455.1031. [DOI] [PubMed] [Google Scholar]
- 16.Cichocki F, et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia. 2016;30(2):456–463. doi: 10.1038/leu.2015.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooden M, et al. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8+ T lymphocytes. Proc Natl Acad Sci U S A. 2011;108(26):10656–10661. doi: 10.1073/pnas.1100354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamiya T, Seow SV, Wong D, Robinson M, Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J Clin Invest. 2019;129(5):2094–2106. doi: 10.1172/JCI123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Montfoort N, et al. NKG2A blockade potentiates CD8 T cell immunity induced by cancer vaccines. Cell. 2018;175(7):1744–1755.e15. doi: 10.1016/j.cell.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.André P, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175(7):1731–1743.e13. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Introna M, Allavena P, Biondi A, Colombo N, Villa A, Mantovani A. Defective natural killer activity within human ovarian tumors: low numbers of morphologically defined effectors present in situ. J Natl Cancer Inst. 1983;70(1):21–26. [PubMed] [Google Scholar]