Abstract
Aside from its catalytic function in protein synthesis, leucyl-tRNA synthetase (LRS) has a nontranslational function in regulating cell growth via the mammalian target of rapamycin (mTOR) complex 1 (mTORC1) pathway by sensing amino acid availability. mTOR also regulates skeletal myogenesis, but the signaling mechanism is distinct from that in cell growth regulation. A role of LRS in myogenesis has not been reported. Here we report that LRS negatively regulated myoblast differentiation in vitro. This function of LRS was independent of its regulation of protein synthesis, and it required leucine-binding but not tRNA charging activity of LRS. Local knock down of LRS accelerated muscle regeneration in a mouse injury model, and so did the knock down of Rag or Raptor. Further in vitro studies established a Rag-mTORC1 pathway, which inhibits the IRS1-PI3K-Akt pathway, to be the mediator of the nontranslational function of LRS in myogenesis. BC-LI-0186, an inhibitor reported to disrupt LRS-Rag interaction, promoted robust muscle regeneration with enhanced functional recovery, and this effect was abolished by cotreatment with an Akt inhibitor. Taken together, our findings revealed what we believe is a novel function for LRS in controlling the homeostasis of myogenesis, and suggested a potential therapeutic strategy to target a noncanonical function of a housekeeping protein.
Keywords: Muscle Biology, Therapeutics
Keywords: Skeletal muscle
Introduction
Skeletal myogenesis is a highly coordinated process that includes satellite cell activation, cell-cycle exit, and fusion of mononucleated myoblasts resulting in multinucleated myofibers (1). Many signaling pathways regulate the expression of myogenic genes and eventually the myogenesis process (2, 3). Dysregulation of this process may exacerbate pathological conditions such as muscular dystrophy, cachexia, and sarcopenia (4).
Aminoacyl-tRNA synthetases (AARSs) are essential for the initiation of protein synthesis by catalyzing ligation of each amino acid to its cognate tRNAs, a process called aminoacylation. This catalytic activity of AARSs entails a 2-step process: amino acid activation by condensing with ATP to form aminoacyl adenylates, and transferring activated amino acids to the cognate tRNAs. In recent years, noncanonical functions of AARSs independent of protein synthesis are increasingly recognized to play diverse roles in cellular regulation (5, 6). One example is leucyl-tRNA synthetase (LRS), which has been reported to be a leucine sensor upstream of mTOR complex 1 (mTORC1) that regulates cell growth in yeast and mammals (7, 8). Multiple mechanisms have been proposed for this LRS function upstream of mTORC1, including GTPase-activating protein (GAP) activity toward RagD (7), activation of PI3-kinase Vps34 (9), interaction with folliculin (a GAP for RagC/D) (10), and leucylation of RagA/B (11). Whereas a role of LRS in activating mTORC1 has been validated independently by several groups in diverse systems (7–11), LRS as a GAP for RagD remains controversial (7, 12).
While it can be assumed that the translational function of LRS is necessary for myogenesis, a process involving robust protein synthesis, whether LRS has any noncanonical function in myogenesis is unknown. Here we reveal that LRS is a negative regulator of myogenic differentiation and injury-induced skeletal muscle regeneration. We show that this function of LRS is independent of protein synthesis and is mediated by Rag activation of mTORC1, and that the Rag-mTORC1 pathway negatively regulates myogenesis through inhibition of Akt signaling. Disruption of the LRS-Rag interaction using a recently reported small molecule, BC-LI-0186, results in larger regenerating myofibers and functional enhancement of the regenerating muscles.
Results and Discussion
LRS negatively regulates myogenesis in a translation-independent manner.
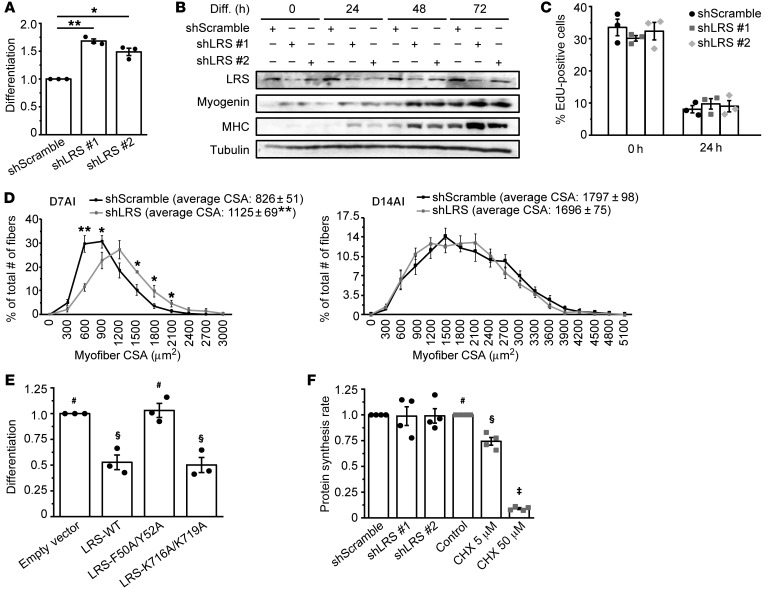
To investigate a potential role of LRS in myoblast differentiation, we knocked down LRS using lentivirus-delivered shRNA in C2C12 myoblasts, which were then induced to differentiate by serum withdrawal. We found that knock down of LRS by 2 independent shRNAs markedly enhanced C2C12 differentiation, as indicated by an increased number of myosin heavy chain–positive (MHC-positive) cells and elevated differentiation index (Figure 1A, Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/JCI122560DS1), as well as higher levels of MHC expression (Figure 1B). The expression of myogenin, an early myogenic marker, was accelerated by LRS knock down (Figure 1B), suggesting LRS may act at an early step during myogenesis. LRS expression level did not alter during differentiation (Supplemental Figure 1B), which is not surprising for an abundant housekeeping protein. We then asked whether LRS affected cell-cycle withdrawal, one of the earliest events of myogenesis (13). As shown in Figure 1C, LRS knock down had no significant effect on C2C12 cells exiting the cell cycle, reflected by decreased EdU incorporation during the first 24 hours of serum withdrawal.
Figure 1. LRS negatively regulates myogenesis in a translation-independent manner.
(A) C2C12 cells were transduced with lentiviruses expressing shLRS or shScramble (control), puromycin-selected for 2 days, and differentiated for 72 hours, followed by measurement of differentiation index as described in the Supplemental Methods. Data were normalized to shScramble (n = 3). (B) Cells treated as in A were lysed every 24 hours and subjected to Western blotting analysis (n = 3). (C) Cells were treated as in A, and at 0 and 24 hours of differentiation, EdU incorporation was conducted for 2 hours as described in the Supplemental Methods (n = 3). (D) TA muscles were coinjected with BaCl2 and lentiviruses expressing shLRS or shScramble, and isolated on day 7 after injury (D7AI) (n = 5) and day 14 after injury (D14AI) (n = 6), followed by measurement of cross-sectional area (CSA) of regenerating myofibers as described in the Supplemental Methods. Data were presented as the size distribution of all myofibers with average CSA. (E) Cells were transfected with Myc-LRS-WT, F50A/Y52A, K716A/K719A, or empty vector (EV; control), selected with G418, and differentiated for 72 hours, followed by measurement of differentiation index. Data were normalized to EV (n = 3). (F) Cells were either transduced with lentiviruses expressing shLRS or shScramble, followed by puromycin-selection for 2 days (black bars), or treated with different concentrations of cycloheximide (CHX) for 24 hours (gray bars). Confluent cells were subjected to measurement of protein synthesis rate by [35S]Met/Cys metabolic labeling (n = 4). *P < 0.05, **P < 0.01 by 1-way ANOVA (A) or 2-tailed paired t test (D). The data in E and F denoted by #, §, ‡ are significantly different from each other by 1-way ANOVA (P < 0.05). All error bars represent SEM.
To probe a potential LRS function in myogenesis in vivo, we used a well-established mouse model of barium chloride (BaCl2) injury-induced skeletal muscle regeneration (14, 15). When lentiviruses expressing shRNA for LRS were coinjected with BaCl2, a significant increase in the number of large regenerating myofibers as well as the average size was observed on day 7 after injury (AI) (Figure 1D and Supplemental Figure 1C). This difference was no longer observed on day 14 AI. Thus, reduction of LRS expression during muscle injury leads to an accelerated regenerative process although it does not change the final outcome of regeneration. These results are consistent with our in vitro observations, and they strongly suggest that LRS is a negative regulator of skeletal myogenesis.
A negative role of LRS in myogenesis is inconsistent with its housekeeping function in protein synthesis, the latter required for the differentiation process. To further examine this noncanonical function of LRS, we made use of 2 previously reported LRS mutants: the leucine binding–deficient mutant (F50A/Y52A) and the tRNA charging–deficient mutant (K716A/K719A) (7, 9). Corroborating the effect of LRS knock down, overexpression of the wild-type LRS suppressed myoblast differentiation in vitro (Figure 1E, Supplemental Figure 1, D and E). Interestingly, K716A/K719A-LRS had an effect similar to that of wild-type LRS when overexpressed. Hence, tRNA charging activity is not involved in LRS inhibition of differentiation, indicating that this function of LRS is independent of protein translation. On the other hand, F50A/Y52A-LRS lost its effect on differentiation, implying that leucine binding/sensing may be necessary for the myogenic function of LRS. When leucine concentration was varied in the differentiation medium, a positive correlation was observed between leucine concentration and degree of differentiation (Supplemental Figure 1F). This is not surprising because, aside from regulating the LRS-Rag pathway, leucine most likely has other, indispensable, roles supporting differentiation.
LRS knock down did not affect protein synthesis rates in C2C12 cells (Figure 1F and Supplemental Figure 1I). Knock down of 2 other AARSs (IleRS and EPRS) was also examined, and it did not significantly affect protein synthesis rates (Supplemental Figure 1, G and I). Importantly, knock down of EPRS or IleRS did not result in the same phenotype of enhanced differentiation as LRS knock down (Supplemental Figure 1H). Finally, cycloheximide (CHX) inhibited protein synthesis in a dose-dependent manner (Figure 1F), and even at a very low concentration CHX displayed cytotoxicity and did not enhance differentiation (data not shown). Taken together, our observations reveal a translation-independent and unique function of LRS that negatively regulates myogenesis.
The Rag-mTORC1 pathway negatively regulates myogenesis in vivo.
In searching for a molecular mechanism underlying the newly unraveled LRS function in myogenesis, we considered the role of LRS as a leucine sensor that activates the Rag GTPases upstream of mTORC1 in cell growth regulation (7, 8). Previously, we reported that Rag and Raptor (a key component of mTORC1) are both negative regulators of myogenic differentiation in vitro (16, 17), which would make an inhibitory LRS-Rag-mTORC1 pathway a compelling model. However, in vivo evidence for a negative function of the Rag-mTORC1 pathway in myogenesis is lacking. On the contrary, skeletal muscle–specific knock out of Raptor leads to muscle dystrophy (18), suggesting a positive role of mTORC1 in muscle maintenance. It is important to note that in the aforementioned study the human skeletal actin (HSA) promoter was used to drive the expression of Cre recombinase for Raptor deletion, which is only active after differentiation (19) and thus could not be used to address a role of Raptor in myogenic differentiation.
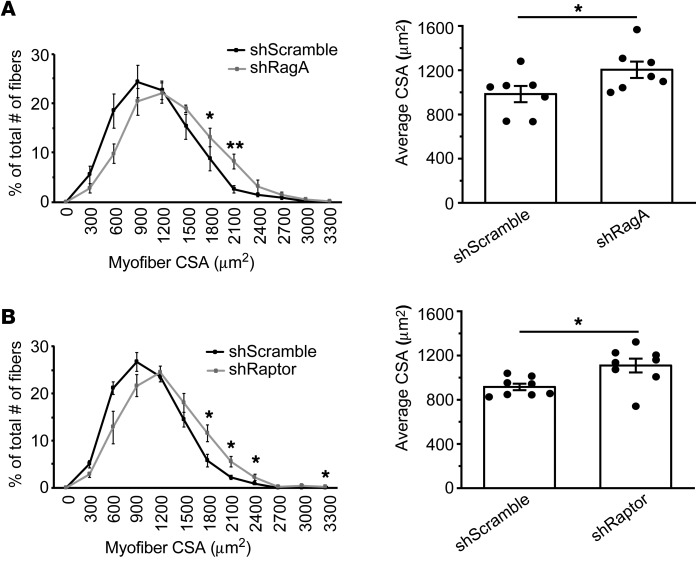
To clarify the roles of Rag and mTORC1 in myogenesis in vivo, we again employed our injury model using coinjection of BaCl2 and shRNA-expressing lentiviruses. Unlike the HSA-mediated knock out, this approach would lead to knock down of a target protein in muscle stem cells before they undergo myogenic differentiation. Rag GTPases activate mTORC1 as a heterodimer of RagA or RagB in complex with RagC or RagD (20). Although RagA and RagB are functionally redundant, skeletal muscles predominantly express RagA (21). Hence, we delivered shRagA and, separately, shRaptor. As shown in Figure 2, A and B, and Supplemental Figure 2, A and B, knock down of RagA or Raptor resulted in significantly larger regenerating myofibers on day 7 AI. These results provide direct evidence for the negative role of the Rag-mTORC1 pathway in myogenesis in vivo. Hence, mTORC1 appears to have dual functions in muscles — a negative role in myogenic differentiation (ref. 16 and the present study) and a positive role in muscle maintenance (18).
Figure 2. The Rag-mTORC1 pathway negatively regulates myogenesis in vivo.
TA muscles were coinjected with BaCl2 and lentiviruses expressing shRagA (A), shRaptor (B), or shScramble (control). Injected muscles were isolated on day 7 after injury and subjected to measurement of CSA of regenerating myofibers (n = 7–8). Data were presented as the size distribution of all myofibers (left panels) and average CSA (right graphs). *P < 0.05, **P < 0.01 by 2-tailed paired t test. All error bars represent SEM.
LRS inhibits myogenic differentiation through the Rag-mTORC1 pathway.
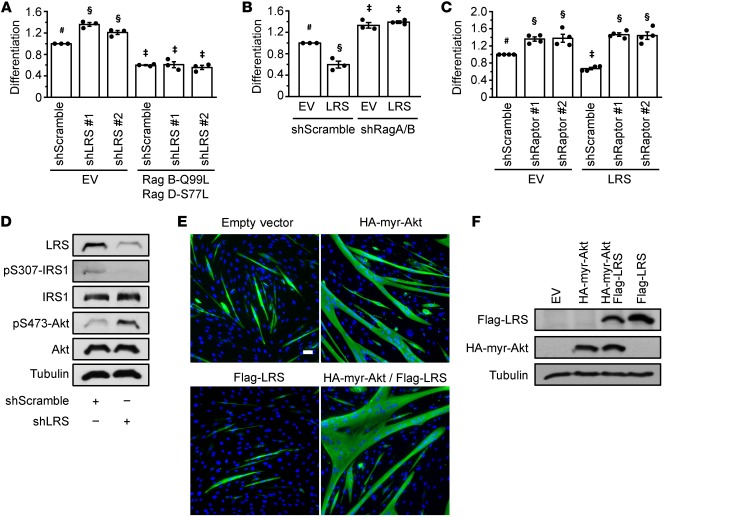
The results of Rag and Raptor knock down noted above (Figure 2) mirror the phenotype of LRS knock down in vivo (Figure 1D), which is consistent with LRS acting upstream of mTORC1 in the negative regulation of myogenic differentiation. To further probe a role of mTORC1 in mediating LRS function in myogenesis, we overexpressed a constitutively active Rag dimer — RagB-Q99L (GTP-bound) and RagD-S77L (GDP-bound) (20) — in C2C12 cells where LRS was knocked down. As shown in Figure 3A and Supplemental Figure 3A, active Rags reversed the effect of LRS knock down on myogenic differentiation. Conversely, when shRNAs for RagA and RagB were simultaneously delivered into the cells, overexpression of LRS no longer inhibited myoblast differentiation (Figure 3B, Supplemental Figure 3B). RagB was not detected in myoblasts by Western analysis, consistent with the reported low expression of RagB in muscles (21). At the same time, LRS overexpression did not inhibit myoblast differentiation when Raptor was knocked down (Figure 3C, Supplemental Figure 3C). Taken together, these observations strongly suggest that Rag GTPases and Raptor mediate the myogenic effect of LRS.
Figure 3. LRS inhibits myogenic differentiation through the Rag-mTORC1 pathway.
(A) C2C12 cells were transduced with lentiviruses expressing shLRS or shScramble, and puromycin-selected for 2 days. Cells were then transfected with either empty vector (EV) or HA-GST-RagB-Q99L and HA-GST-RagD-S77L for 24 hours, differentiated for 72 hours, and subjected to measurement of differentiation index. Data were normalized to control (shScramble and empty vector) (n = 3). (B) LRS was transfected in cells where RagA and RagB were knocked down and subjected to measurement of differentiation index as in A (n = 3). (C) LRS was transfected in cells where Raptor was knocked down and subjected to measurement of differentiation index as in A (n = 4). (D) Cells were transduced with lentiviruses expressing shLRS or shScramble, and puromycin-selected for 2 days. Upon confluence, cells were lysed and subjected to Western blotting analysis (n = 2). (E, F) Cells were transfected with myr-Akt and/or LRS. Confluent cells were either induced to differentiate for 72 hours, followed by immunofluorescence staining with anti-MHC (green) and DAPI (blue) (E, n = 3), or lysed for Western blotting analysis (F, n = 3). Scale bar: 50 μm. The data denoted by #, §, ‡ are significantly different from each other by 2-way ANOVA (P < 0.005). All error bars represent SEM.
Previously, we reported that the Rag-mTORC1 pathway inhibits myogenic differentiation via the feedback inhibition of IRS1-Akt signaling (16). Therefore, we set out to test whether LRS could be placed upstream of IRS1 and Akt. Indeed, IRS1 phosphorylation on Ser307 was dampened by LRS knock down in C2C12 cells, accompanied by increased Akt phosphorylation (Figure 3D). Furthermore, overexpression of a constitutively active Akt (myristoylated Akt [myr-Akt]) rescued differentiation in cells overexpressing LRS (Figure 3, E and F). Conversely, treatment with an Akt inhibitor, Akti-1/2, eliminated the stimulatory effect of LRS knock down on myoblast differentiation (Supplemental Figure 3D). Collectively, our observations suggest that LRS negatively regulates myogenic differentiation via the Rag-mTORC1 pathway and subsequent inhibition of the IRS1-PI3K-Akt pathway.
An inhibitor of LRS-Rag interaction enhances muscle regeneration.
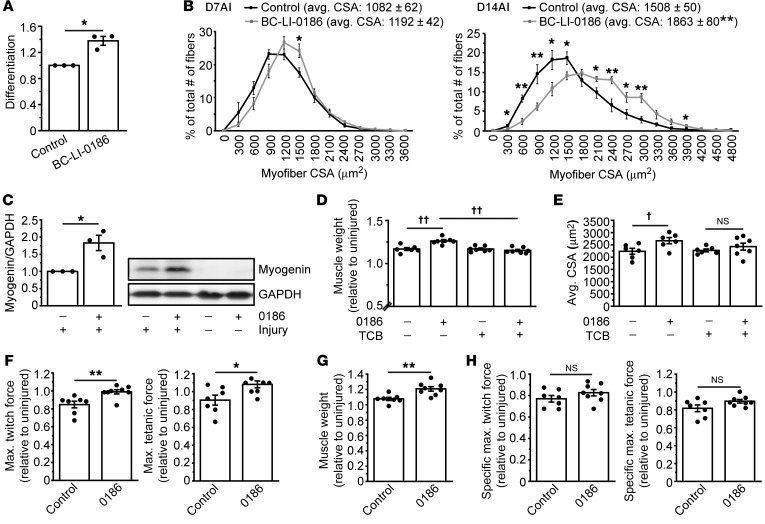
A small-molecule inhibitor, BC-LI-0186, has been developed to directly interact with LRS, disrupt binding of LRS to Rag, and inhibit leucine-dependent activation of mTORC1 in cells with high specificity, without affecting the aminoacylation activity of LRS (22). We reasoned that this inhibitor could specifically target the nontranslational function of LRS and recapitulate the effect of LRS knock down in myogenesis. Indeed, the addition of BC-LI-0186 in differentiation medium significantly enhanced myoblast differentiation (Figure 4A, Supplemental Figure 4A).
Figure 4. An inhibitor of LRS-Rag interaction enhances muscle regeneration.
(A) C2C12 cells were induced to differentiate for 72 hours in the presence or absence of 4 μM BC-LI-0186 (0186) and subjected to measurement of differentiation index. Data were normalized to control (n = 3). (B) Mice were intraperitoneally injected with 0186 at 5 mg/kg body weight every 3 days. The first 0186 injection coincided with BaCl2 injection into TA muscles. Muscles were isolated on day 7 after injury (D7AI) and day 14 after injury (D14AI), and subjected to measurement of CSA of regenerating myofibers. Data were presented as the size distribution of all myofibers with average (avg.) CSA (n = 5–6). (C) TA muscles from mice treated as in B were isolated on day 4 after injury and subjected to Western blotting analysis (n = 3). (D, E) Mice were intraperitoneally injected with 0186 at 5 mg/kg body weight every 3 days and with triciribine (TCB) at 1 mg/kg body weight every day. The first injection of 0186 and TCB coincided with BaCl2 injection into TA muscles. Muscles were isolated on D14AI, weighed (D, n = 6–7), and subjected to measurement of CSA of regenerating myofibers (E, n = 6–7). (F–H) Mice treated as in B were subjected to in situ force measurements of regenerating TA muscles at D14AI (F, n = 7–8), followed by muscle weight measurement (G, n = 7–8). Maximum twitch force and tetanic force were converted to specific maximum twitch force and tetanic force as described in the Supplemental Methods (H, n = 7–8). All values were presented as relative to uninjured contralateral muscles. *P < 0.05, **P < 0.01 by 2-tailed paired t test (A) or 2-tailed unpaired t test (B, C, F–H). ††P < 0.001 (D), †P < 0.005 (E) by 2-way ANOVA. All error bars represent SEM.
Next, we investigated the effect of BC-LI-0186 on muscle regeneration in vivo by administering the inhibitor to mice from the initiation of muscle injury by BaCl2 injection. Strikingly, BC-LI-0186 enhanced muscle regeneration, as indicated by a significant increase in the average regenerating myofiber size (day 14 AI) and in the number of large regenerating myofibers (days 7 and 14 AI) (Figure 4B, Supplemental Figure 4B). These effects of BC-LI-0186 in vitro and in vivo further validate the mechanism by which LRS negatively regulates myogenesis. It is noteworthy that while the effect of LRS knock down was transient (Figure 1D), the effect of BC-LI- persisted through day 14 AI. This difference may be due to the fact that knock down might not have persisted to a later time point, whereas the inhibitor was continuously delivered throughout the regeneration. Alternatively, the better enhancement of regeneration could be owing to the more effective blockade of the nontranslational function of LRS by the inhibitor than by partial reduction of LRS protein levels. Consistent with the enhanced muscle regeneration by BC-LI-0186, we also found that the inhibitor induced a higher expression level of myogenin on day 4 AI (Figure 4C).
We identified the IRS1-PI3K-Akt pathway as a mediator of LRS inhibition of myoblast differentiation in vitro (Figure 3, D and E, and Supplemental Figure 3D). To investigate whether Akt mediates LRS function in vivo, we administered BC-LI-0186 together with an Akt inhibitor, triciribine, for 14 days after BaCl2 injection. As shown in Figure 4, D and E, and Supplemental Figure 4, C and D, triciribine prevented BC-LI-0186–induced increases in both muscle weight and regenerating myofiber size. Combined with our in vitro results, these findings establish Akt as a critical mediator of LRS function in myogenesis.
An important question remaining was whether the increase in regenerating muscle mass and myofiber size by BC-LI-0186 can be translated into functional enhancements of the regenerating muscles. To address this question, we performed in situ force measurement of the regenerating TA muscles. Remarkably, both maximum twitch force and tetanic force significantly increased with BC-LI-0186 treatment (Figure 4F). The specific muscle forces remained unchanged by the inhibitor treatment (Figure 4H) as a result of the increased muscle mass (Figure 4G).
In conclusion, we demonstrated for what we believe is the first time that LRS has a nontranslational function in negatively regulating myoblast differentiation and skeletal muscle regeneration. This function of LRS in myogenesis is mediated by the Rag-mTORC1 pathway, which inhibits the IRS1-PI3K-Akt pathway. We also provided in vivo evidence for a negative role of the Rag-mTORC1 pathway in myogenesis, which is in contrast to a well-established role of mTORC1 signaling in muscle growth and maintenance. Importantly, we showed that pharmacological intervention to specifically impair this negative function of LRS promotes robust muscle regeneration accompanied by enhanced functional recovery of the regenerating muscles. Impaired muscle regeneration occurs in aging, muscular dystrophies, and other pathological conditions. The LRS-Rag pathway identified in our study can be a potential target for regenerative medicine. Our findings also provide a proof of principle for selective targeting of the noncanonical function of a housekeeping protein as a potentially effective therapeutic strategy.
Methods
Study approval.
All animal experiments in this study followed protocols approved by the Animal Care and Use Committee at the University of Illinois at Urbana-Champaign.
See the Supplemental Methods for a detailed description of all experimental procedures.
Author contributions
KS, JSY, and JC conceptualized the study; KS, JSY, MSY, CD, NK, and AB collected data; JHK, GH, JMH, SK, and SAM contributed resources; KS, JSY, SK, and JC wrote the manuscript.
Supplementary Material
Acknowledgments
We thank Adriana Reyes-Ordoñez for technical assistance. This work was supported by grants from the National Institutes of Health to JC (R01AR048914, R01GM089771) and from the Keck Foundation to SAM and JC.
Version 1. 04/15/2019
Electronic publication
Version 2. 05/01/2019
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2019;129(5):2088–2093.https://doi.org/10.1172/JCI122560.
Contributor Information
Kook Son, Email: kookson2@illinois.edu.
Jae-Sung You, Email: youj@illinois.edu.
Mee-Sup Yoon, Email: msyoon@gachon.ac.kr.
Chong Dai, Email: chongd2@illinois.edu.
Jong Hyun Kim, Email: kimjohn@biocon.snu.ac.kr.
Nidhi Khanna, Email: nidhi65@gmail.com.
Aditi Banerjee, Email: abanerj7@illinois.edu.
Gyoonhee Han, Email: gyoonhee@gmail.com.
Jung Min Han, Email: jhan74@yonsei.ac.kr.
Sunghoon Kim, Email: sungkim@biocon.snu.ac.kr.
Jie Chen, Email: jiechen@illinois.edu.
References
- 1.Horsley V, Pavlath GK. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs (Print) 2004;176(1-3):67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- 2.Naya FJ, Olson E. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol. 1999;11(6):683–688. doi: 10.1016/S0955-0674(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993;75(7):1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- 4.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Park SG, Ewalt KL, Kim S. Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers. Trends Biochem Sci. 2005;30(10):569–574. doi: 10.1016/j.tibs.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Guo M, Schimmel P. Essential nontranslational functions of tRNA synthetases. Nat Chem Biol. 2013;9(3):145–153. doi: 10.1038/nchembio.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han JM, et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149(2):410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 8.Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell. 2012;46(1):105–110. doi: 10.1016/j.molcel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Yoon MS, Son K, Arauz E, Han JM, Kim S, Chen J. Leucyl-tRNA synthetase activates Vps34 in amino acid-sensing mTORC1 signaling. Cell Rep. 2016;16(6):1510–1517. doi: 10.1016/j.celrep.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khayati K, et al. The amino acid metabolite homocysteine activates mTORC1 to inhibit autophagy and form abnormal proteins in human neurons and mice. FASEB J. 2017;31(2):598–609. doi: 10.1096/fj.201600915R. [DOI] [PubMed] [Google Scholar]
- 11.He XD, et al. Sensing and transmitting intracellular amino acid signals through reversible lysine aminoacylations. Cell Metab. 2018;27(1):151–166.e6. doi: 10.1016/j.cmet.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Tsun ZY, et al. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell. 2013;52(4):495–505. doi: 10.1016/j.molcel.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrés V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132(4):657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldwell CJ, Mattey DL, Weller RO. Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol Appl Neurobiol. 1990;16(3):225–238. doi: 10.1111/j.1365-2990.1990.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 15.Ge Y, et al. mTOR regulates skeletal muscle regeneration in vivo through kinase-dependent and kinase-independent mechanisms. Am J Physiol, Cell Physiol. 2009;297(6):C1434–C1444. doi: 10.1152/ajpcell.00248.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge Y, Yoon MS, Chen J. Raptor and Rheb negatively regulate skeletal myogenesis through suppression of insulin receptor substrate 1 (IRS1) J Biol Chem. 2011;286(41):35675–35682. doi: 10.1074/jbc.M111.262881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon MS, Chen J. Distinct amino acid-sensing mTOR pathways regulate skeletal myogenesis. Mol Biol Cell. 2013;24(23):3754–3763. doi: 10.1091/mbc.e13-06-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bentzinger CF, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8(5):411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Miniou P, Tiziano D, Frugier T, Roblot N, Le Meur M, Melki J. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res. 1999;27(19):e27. doi: 10.1093/nar/27.19.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sancak Y, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efeyan A, et al. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493(7434):679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JH, et al. Control of leucine-dependent mTORC1 pathway through chemical intervention of leucyl-tRNA synthetase and RagD interaction. Nat Commun. 2017;8(1):732. doi: 10.1038/s41467-017-00785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.