Abstract
The need for monitoring hearing and auditory function during drug therapy and other treatments that have the potential to cause hearing loss is well documented. Besides the main purpose of ototoxic monitoring, which is to provide feedback to the attending physician about the effects the treatment is having on the auditory system, it is also helpful in setting expectations for the patient and his/her family about the communication issues that may result from the drug therapy. This article will review tests available to an audiologist, both subjective and objective, that can be used to effectively monitor hearing levels and auditory function during treatment. Published guidelines and various ototoxic monitoring protocols are reviewed regarding tests administered, what constitutes a significant change in test results and how these findings are reported, and the impact significant changes may have on the course of treatment. Test protocols from different institutions are compared for both similarities and contrasts. Effective scheduling and test location are key to a successful monitoring program. Finally, the need to streamline ototoxic monitoring of hearing and auditory function to reduce test time and make it less stressful and tiresome on the patient will be considered.
Keywords: ototoxicity, distorting product otoacoustic emissions, extended high-frequency audiometry, ototoxic monitoring program
Ototoxicity is the cellular degeneration of cochlear and/or vestibular tissues, leading to functional deterioration, due to an adverse reaction to certain therapeutic agents. 1 While most of the attention is focused on medication-induced damage, any nonmechanical damage to the inner ear also is considered to be an ototoxic agent leading to possible auditory and vestibular dysfunction. 2 Other examples of nonmedication ototoxicity include noise exposure, chemical toxins, many solvents, some heavy metals, and certain asphyxiants. 2 Auditory symptoms include hearing loss, tinnitus, hyperacusis, and aural fullness. 3 For the purpose of this article, only medication-induced hearing loss will be addressed.
The number of drugs identified as being potentially ototoxic has increased over the past several decades. Some estimate the number to be over 600, 3 while other estimates are in the 200+ range. 4 The most commonly used ototoxic medications include platinum-based chemotherapeutic agents, aminoglycoside antibiotics, loop diuretics, macrolide antibiotics, antimalarials, and nonsteroidal anti-inflammatory drugs (NSAIDs). 3 5 6 7 Of these known ototoxic medications, chemotherapeutic agents (platinum-based drugs) and aminoglycosides will cause permanent hearing loss, while loop diuretics, quinine, and salicylate pain relievers are known to cause temporary hearing loss. 4
The percentage of patients who experience hearing loss as a result of receiving treatment with ototoxic medications varies widely and is impacted by several factors, including the particular medication utilized, the dosage level, frequency of treatments, method of delivery, the patient's age, hearing status at the time treatment begins, past history of ototoxic drug treatment, and other risk factors including past and concurrent noise exposure. 8 Genetic factors also may contribute to individual vulnerability to ototoxicity. 9 10
The potential for increased ototoxicity occurs if drugs and/or treatment methods are used in combination. Cranial radiation combined with cisplatin exacerbates the progression of cochlear damage and associated hearing loss. 11 Concurrent noise exposure and even ambient noise levels in the NICU acting synergistically with the ototoxic medication can potentiate progressive hearing loss. 12 Higher cumulative doses of cisplatin and carboplatin increase the risk of hearing loss, especially in younger patients. 13
Permanent sensorineural hearing loss secondary to medication used to treat a variety of infections and cancers (cochleotoxicity) is a risk for well over 4 million people in the United States each year. 7 The American Cancer Society reported that 1.7 million people would be diagnosed with some form of cancer in 2017. While the 5-year survival rate varies based on the type of cancer as well as other factors, that rate has steadily improved over the past 40 years. Based on data reported for the period 2005–2011, approximately 70% of cancer patients are now likely to survive. 14 As an increasing number of cancer patients survive, there will be a greater need for audiologic intervention services to improve the patient's communication and overall quality of life. This assumes that the cancer survivors are part of a comprehensive audiologic ototoxic monitoring program (AOMP). Unfortunately, surveys conducted in the United States and United Kingdom have shown that less than 50% of respondents have an AOMP in place. This is despite the evidence that supports the importance and value of an effective AOMP to the patient. 3
The incidence of permanent sensorineural hearing loss is difficult to accurately estimate and is quite variable due to the acquired disease/illness, treatment prescribed, individual patient differences, and the many different metrics used to determine and report changes in hearing. 8 Medication-induced hearing loss cannot be predicted based on dosage, serum levels, or the development of other toxic outcomes. 7 15
According to Konrad-Martin et al, 8 reporting on ototoxicity monitoring in the VA Healthcare System, approximately 4,000 veterans will receive cisplatin this year with an estimated 50% developing permanent sensorineural hearing loss as a result. Other estimates of the percentage of patients who develop hearing loss secondary to antineoplastic medications range from 23 to 50% in adults and 60% in children. 3 Other reports have shown an incidence rate of 26 to 90% in pediatric patients receiving cisplatin and/or carboplatin. 16
Theunissen et al found hearing loss incidence ranging from 17 to 88% in adult head and neck cancer patients receiving cisplatin chemoradiation. 17 Knight et al 16 performed audiologic evaluations on 67 patients receiving platinum-based chemotherapy. Sixty-one percent of the patients showed bilateral hearing loss.
The aminoglycoside family of antibiotics is used to treat various infections contracted by both children and adults. In the United States, there are over 4 million births annually of which 12% are admitted to the neonatal intensive care unit. Most of those admitted to the NICU are treated with antibiotics to prevent a bacterial infection 12 and neonatal sepsis. 17 18 Other at-risk pediatric populations treated with aminoglycosides include those with cystic fibrosis, tuberculosis, endocarditis, severe pulmonary infections, and sepsis. 8 17 The incidence of hearing loss in the standard frequency range in both children and adults who are treated with aminoglycosides ranges from 2 to 20%, the highest incidence occurring in patients with cystic fibrosis. 18
The onset of symptoms can be quite variable. Symptoms may occur alone or in combination (auditory and/or vestibular), and with rapid or gradual onset. In most cases, these symptoms are of a permanent nature, although some are reversible. 3 In cases when patients' complaints of hearing difficulties are reported and used as the only monitoring method, it is generally too late to take any preventative action since the cochlear damage that has occurred has already impinged upon the speech frequency region. Nonetheless, additional steps may then be taken to limit damage and retain the residual hearing that remains.
While many patients are faced with life-threatening conditions that require aggressive drug therapeutic intervention, preventive efforts should be made to mitigate the effects these drugs have on a patient's hearing status, when possible. This can only be accomplished through the implementation of an effective AOMP. Therefore, ototoxicity should be monitored for each individual patient. 8 16
Audiologic Monitoring Programs
It has been almost 25 years since the American Speech-Language-Hearing Association published guidelines for audiologic management of individuals receiving cochleotoxic drug therapy and 9 years since the American Academy of Audiology's position statement on ototoxicity monitoring was published. Two points emerge when reviewing the literature on audiologic ototoxic monitoring over the past two decades. One is the fact that the test protocol utilized has been essentially unchanged. The other is that audiologic ototoxic monitoring is still not standard of care. Konrad-Martin et al 8 reviewed national audiology guidelines for ototoxic monitoring and found that audiologic ototoxicity monitoring is still not well established and continues to be an inconsistent practice for many adult oncology and infectious disease patients.
The evidence documenting hearing loss that affects the critical speech frequency range secondary to treatment with ototoxic medications, particularly those in the aminoglycoside and platinum-based chemotherapy family, is indisputable. 3 And while the primary goal is always to treat the disease/illness as aggressively as possible, the onset and progression of a hearing loss will have an adverse effect on overall quality of life and interfere with the ability to communicate with family, friends, coworkers, as well as medical personnel, thereby limiting their ability to convey and understand basic health information. 9
This may seem somewhat trivial when put in context with the potential life-threatening nature of the disease/illness for which the patient is receiving treatment; however, with more patients surviving due to earlier diagnosis and improved treatment methods, quality of life after completion of treatment should not be minimized or overlooked. If possible, efforts should be made to maintain quality of life after treatment by designing medical treatment protocols that successfully treat the disease/illness while minimizing hearing loss. 5 In patients where progressive hearing loss is documented, the physician may decide to modify the treatment protocol (drug type, dosage level, extend the therapy sessions) or maintain the status quo and, in conjunction with the audiologist, prepare the patient and his/her family about the inevitable negative impact the hearing loss will have on communication and the use of nonmedical treatment strategies (e.g., amplification, aural rehabilitation) to deal with the hearing loss after-effects.
It is in the best interest of patients being treated with ototoxic medications to receive audiologic monitoring prior to, during, and after treatment, with the goals of early identification, prevention, and, if necessary, intervention. 6 In fact, an effective audiologic monitoring program should detect changes in auditory function before the damage affects the speech frequency range and before the patient is aware of changes in hearing and/or experience other auditory symptoms. 5
According to the American Academy of Audiology's position statement published in 2009, “audiologic monitoring for ototoxicity is primarily performed for two purposes: (1) early detection of changes to hearing status presumably attributed to a drug/treatment regimen so that changes in the drug regimen may be considered and (2) audiologic intervention when handicapping hearing impairment has occurred.” 6 Intervention may include counseling, communication strategies, amplification, and assistive listening devices. 19
Planning and Implementation
The planning, implementation, and success of an ongoing AOMP is a team effort that includes the attending physician, nursing staff, audiologist, and other medical personnel who may be involved in the care of the patient. Of this team, “only the audiologist is endowed by their professional training with the ability to achieve both objectives of ototoxicity monitoring.” 6 Therefore, the audiologist should facilitate the development and implementation of the audiologic ototoxicity monitoring program. Part of that will be to develop an excellent working relationship with the physicians to receive referrals for patients they are treating with ototoxic medications. 6 The audiologist may need to educate oncologists, infectious disease specialists, and other medical personnel about the communication difficulties hearing loss has in a patient's everyday life, including the ability to communicate effectively in family, social, vocational, educational, and health-care situations. This may include offering to present at grand rounds stressing the importance of early identification and intervention for all patients, but especially neonates and young children. 18 Untreated hearing loss also can lead to increased hospital readmissions. 20
For pediatric patients, particularly in the first 2 years of life, it is imperative the attending physician and supportive medical personnel understand the many developmental, educational, financial, and, eventually, vocational consequences secondary to developing hearing loss. These include delays in speech and language development, limited educational achievement, and social–emotional development. 15 The consequences of ototoxic hearing loss for the pediatric patient can be devastating and lead to a lifetime of medical expenses. 15 Societal costs are estimated at over $1.3 million (in 2015 U.S. dollars after inflation) for each child with severe prelingual hearing loss. 20
Without an audiologist's efforts to educate physicians about the value of a structured, consistent AOMP, it is likely that many patients being treated with ototoxic medications will not receive audiologic care at any point prior to, during, or after treatment. 3 12 19
Scheduling
The audiologist should be the team member responsible for defining the timing of audiologic testing and coordinating the scheduling of patients throughout the AOMP. The logistics of the AOMP can be very challenging for the staff and, most importantly, the patient. The audiologist must coordinate, through effective communication, with members of the medical team involved in the patient's care, as well as the patient and family, scheduling of audiometric testing so that it is performed at the appropriate time.
There are many challenges faced by the AOMP team to accomplish the goal of timely and appropriate scheduling, not the least of which is the condition of the patient and how they are feeling during their treatment. The importance of baseline testing to the overall success of the AOMP cannot be understated. Without baseline measures, it is impossible to know if any hearing loss detected during treatment was preexisting or secondary to the ototoxic medication/s. 3 5 6 20 Therefore, the first scheduling goal is to arrange for a baseline test prior to the first treatment or within an acceptable timeframe after the first treatment. The acceptable timeframe is dependent on the prescribed medication. For platinum-based drugs, the baseline test should be completed within 24 hours of the first treatment, while a window of 72 hours is acceptable following the first aminoglycoside treatment. 5 20
If possible, the baseline test should be performed in an audiometric test booth to ensure accurate measurements. The results of the baseline test should be as accurate and reliable as possible. Although the ability to test higher frequencies is not compromised by ambient noise levels, by testing in an audiometric test booth that meets the ANSI standard for maximum permissible ambient noise levels, the audiologist can be certain that the test environment does not have any impact on the accuracy of threshold testing. 5
Baseline testing of any kind is almost impossible to perform on neonates since approximately 80% admitted to the NICU are immediately placed on aminoglycoside antibiotics to ward off infection and neonatal sepsis. 20
It is wise to consult with the attending physician to identify patients who are at risk for ototoxicity. 21 22 These include all patients who will be treated with known ototoxic agents paying particular attention to those who have other risk factors that can potentiate ototoxicity. These include poor general health with low levels of red blood cells and serum proteins, poor renal function, coadministration of multiple ototoxic agents, hereditary factors, and previous noise exposure. 21 Having this information available ahead of treatment may allow the creation of a treatment plan that strikes a “balance between a curative approach and quality-of-life outcomes following treatment.” 22
Konrad-Martin et al proposed the use of “prediction audiograms” that are formulated based on the planned treatment dosage. 22 They reported a hearing loss prediction error of 4.0 to 8.0 dB when compared with actual pure tone threshold shifts. 22 Knowing ahead of time if a patient has a greater risk for ototoxicity is important information that can be used to develop an individualized treatment plan and provide critical information about the potential for ototoxicity that the audiologist can share with the patient and family ( Fig. 1 ).
Figure 1.
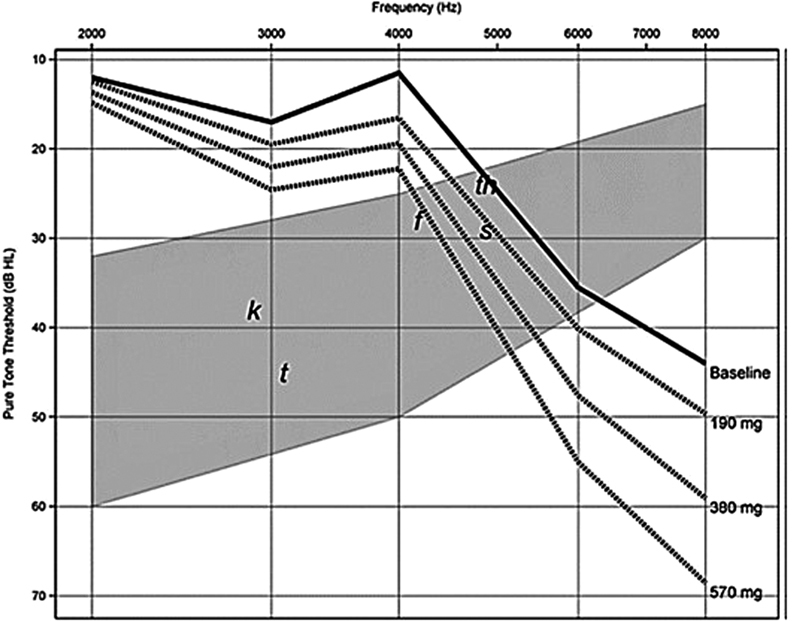
Pretreatment risk assessment audiograms using threshold information from Case Study 1. Series of prediction audiograms were generated using planned cisplatin dosing regimen ( dashed lines ) and patient's actual baseline audiogram ( solid line ) in decibels hearing level (dB HL) shown as function of test frequency. Gray shading indicates “speech banana” with phonemes. This model of conventional frequency thresholds yielded overall accuracy of 4.9 to 8.0 dB prediction error. 22
Scheduling monitoring testing during treatment presents a greater challenge to both the audiologist and patient. Scheduling must be done within the context of other appointments requiring coordination with multiple specialties. 23
Timing of the monitoring testing is critical. Ideally, monitoring tests should be administered at specific intervals that correspond to the treatment schedule but, to some extent, will be dependent on the prescribed medication. If possible, the patient should be scheduled before each treatment session. By doing so, the patient will probably feel well enough for testing. Nonetheless, at times, patients may be too ill to complete the entire audiologic test battery. Some patients may be unable to respond reliably or may be entirely unresponsive. 5 Similarly, the patient may not be able to come to the audiology clinic thereby requiring testing in an area that will have higher ambient noise levels. In either case, the test battery must be modified to accommodate the general health of the patient and the test environment in which the testing is administered. For these reasons, the monitoring test protocol needs to be as quick and efficient as possible. 3
During treatment, the following are common audiologic monitoring test schedules:
Prior to every scheduled cisplatin treatment.
Prior to every third scheduled carboplatin treatment (some recommend prior to every treatment).
Weekly or biweekly for patients on an aminoglycoside drug. 21
A similar schedule is followed with pediatric patients, regardless of age.
Finally, the audiologist is responsible for coordinating posttreatment monitoring testing to identify progressive hearing loss. The frequency of posttreatment audiologic testing is related to the treatment agent received (cisplatin, carboplatin, cranial radiation, and aminoglycoside). As it is possible for ototoxic hearing loss to occur after completion of treatment, posttreatment testing may reveal a hearing loss that was not apparent during the treatment phase. 21 In addition, posttreatment testing will identify progressive hearing loss and, in some cases, may even show improvement in hearing. 3 20
Posttreatment scheduling varies somewhat among AOMPs, but follows a schedule like the following:
Within 1 month of last treatment and then every 3 months for 1 year for patients treated with cisplatin, carboplatin, and aminoglycoside antibiotics.
Within 1 month of last treatment and then every 6 to 12 months for 10 years for patients who were treated with cranial radiation (high risk every 6 months through year 5, then annually through year 10).
Changes in hearing must be confirmed by repeat testing within 24 hours at any point during the AOMP. If hearing changes are confirmed at any point during treatment or posttreatment, the test schedule may need to be modified and intervention strategies implemented.
The audiologist is responsible for developing and implementing an evidence-based AOMP that may have to be modified based on the dynamic nature of individual treatment plans, the patient's age, other otologic diseases (both chronic and acute), and the responsiveness of the patient. 7 15 21 Test methods and protocols, particularly those used when monitoring during treatment, should be easy to administer, quick, sensitive, reliable, and as objective as possible 1
The testing portion of an AOMP can be divided into three distinct phases: (1) baseline (pretreatment), (2) serial (during treatment), and (3) maintenance (posttreatment). Each of these phases is critical to the success of the AOMP and the inability to fully implement each phase may miss changes in hearing due to ototoxicity, significantly impact the ability to prevent further hearing loss, and create unnecessary stress for the patient and his/her family.
A baseline audiologic assessment should be scheduled in advance of the first drug treatment and be comprehensive in nature. 3 5 15 17 18 Any test that may be used to monitor hearing during the treatment phase must be included in the baseline test protocol. 6
By most accounts, the baseline assessment should include the following 3 8 :
A thorough case history emphasizing risk factors contributing to hearing loss (family history, noise exposure, previous drug treatments, history of ear disease, etc.) These factors may hasten the onset of ototoxic-related hearing loss. It is important to establish if the patient is currently exposed to noise in the workplace, recreationally, and/or by listening to loud music.
A review of the proposed treatment plan including diagnosis, type of treatment, dosage, number of treatment cycles, and date of completion. This information should be available from the patient's medical record or by communicating with the patient's attending physician.
Otoscopic examination.
Pure tone air conduction testing—standard frequencies 250 Hz to 8 kHz.
Include mid-octave frequencies 3 and 6 kHz. 21
-
Pure tone air conduction testing—extended high frequencies (EHF) 9 to 16 kHz (or 20 kHz).
○ The American National Standards Institute publishes reference equivalent threshold sound pressure levels (RETSPLs) for circumaural headphones for the frequency range 125 Hz to 16 kHz. The frequencies in the range between 16 and 20 kHz are calibrated to the equipment manufacturer's values 24 ( Table 1 , Fig. 2 ).
-
Pure tone bone conduction testing 500 Hz to 4 kHz.
○ May not be necessary if air conduction thresholds are 10 dB HL (hearing level) or better.
Speech audiometry including speech reception threshold (SRT) and word recognition score (WRS; if possible).
-
Full immittance test battery.
○ Tympanometry, acoustic reflexes with both ipsilateral and contralateral stimulation.
-
Distortion product otoacoustic emissions (DPOAE) from 1,500 Hz—as high a frequency as the instrumentation allows (typically 8 or 10 kHz).
○ Testing with an f2 less than 1,500 Hz may increase test time due to the higher noise levels encountered in that frequency range.
-
Transient evoked otoacoustic emissions (TEOAE) using a broad band stimulus.
○ TEOAEs are reported to be more sensitive to outer hair cell (OHC) damage that can contribute to marginal and mild hearing losses.
○ Upper frequency limit is only 5 kHz.
-
Auditory brainstem response (ABR) using tone burst or narrow band CE Chirp stimuli (500 Hz–4 kHz) to establish estimated hearing level (eHL) for patents who, due to their age and/or level of responsiveness, cannot be tested by conventional means.
○ Auditory steady state response as an alternative tone burst or narrow band CE Chirp ABR.
Pretreatment counseling to review the outcome of the baseline audiologic testing, ototoxic side effects of the upcoming treatment, the potential communication difficulties secondary to the ototoxicity, the impact these will have on quality of life, nonmedical treatment strategies, and the need to reduce exposure to other risk factors that can accelerate and/or exacerbate hearing loss.
Table 1. Reference Equivalent Threshold Sound Pressure Levels for Circumaural Headphones.
| ANSI S3.6 2010 | |
|---|---|
| Frequency | Sennheiser HDA200 and RadioEar DD450 |
| 125 | 30.5 |
| 250 | 18 |
| 500 | 11 |
| 750 | 6 |
| 1,000 | 5.5 |
| 1,500 | 5.5 |
| 2,000 | 4.5 |
| 3,000 | 2.5 |
| 4,000 | 9.5 |
| 5,000 | 14 |
| 6,000 | 17 |
| 8,000 | 17.5 |
| 9,000 | 19 |
| 10,000 | 22 |
| 11,200 | 23 |
| 12,500 | 27.5 |
| 14,000 | 35 |
| 16,000 | 56 |
| Speech | 19 |
Notes: These values are added to the audiometer dial setting to convert HL to SPL. ANSI S3.6 2010 Specifications for audiometers.
Figure 2.

Circumaural headphones can be used for standard and extended high frequency air conduction audiometric testing.
For some patients, especially infants and very young children, many of the aforementioned tests, specifically the behavioral tests, cannot be performed due to their age and/or inability to provide reliable responses. In these cases, modifications to the conventional test battery may be necessary. In other cases, objective testing protocols are the only option. These should include a full immittance test battery using the probe tone stimulus appropriate for the patient's age, DPOAE testing, and a diagnostic ABR threshold estimation assessment. 9 15 16 17 18
Ideally, the baseline assessment should be administered prior to the commencement of the first treatment; however, that may not be possible in all cases. When this occurs, the following is a common and acceptable alternative:
Patients receiving an aminoglycoside drug, baseline levels should be established within 72 hours after the initial treatment.
For treatment plans using either cisplatin or carboplatin, 1 week prior to or within 24 hours after the first treatment.
In all cases of cranial radiation treatments, the patient should have the baseline test prior to the first treatment. 5 21
Test Environment
Ideally, both baseline and all serial testing should be conducted in a sound-treated test booth (to obtain valid thresholds unaffected by ambient noise). 5 6 8 Nonetheless, there may be cases when a test booth is not available, or the patient is unable to make it to the audiology clinic. Testing outside of a sound-treated test booth may not allow for certain tests to be administered, although use of the latest audiometric testing equipment and by implementing certain modifications to the most common and conventional test methodologies allow for accurate and reliable testing outside of a test booth. For example, if the bone oscillator is placed on the forehead instead of the mastoid, circumaural headphones, which in the past were exclusively used for EHF testing, can now be used to test all frequencies.
Calibration values for frequencies above 8 kHz through 16 kHz were published in ANSI S3.6 1996 and are displayed in Table 1 . 24 For frequencies above 16 kHz, the equipment manufacturer provides calibration values.
If forehead placement of the bone oscillator is employed, the circumaural headphones can be kept in place, covering both ears during bone conduction testing, thereby increasing attenuation of ambient noise in the treatment room and reducing test time.
There are other advantages of forehead placement of the bone conduction oscillator including higher test–retest reliability, lower intersubject variability, more stable placement, and less middle ear contribution to the bone conduction test. 25 Therefore, if bone conduction threshold testing must be conducted outside of a sound-treated test booth during baseline or follow-up testing, forehead placement should be strongly considered.
One final note about forehead bone conduction testing, the maximum output of the bone conduction oscillator, is reduced by approximately 10 to 20 dB HL at certain frequencies since more acoustic output is required to reach threshold compared with mastoid placement. It is imperative that your audiometer be calibrated for forehead placement. Many of the newer generation diagnostic and clinical audiometers allow calibration of the bone oscillator for both mastoid and forehead placement. Please consult your audiometer's user manual and local calibration vendor.
Furthermore, when testing is performed outside of a sound-treated test booth, measuring ambient noise levels using an octave or one-third octave band analyzer at the standard audiometric frequencies is recommended. The measured values can then be compared with ANSI S3.1–1999 (R2003), which specifies maximum permissible ambient noise levels (MPANLs) for both ears covered (supra-aural headphones or insert phones) and uncovered (bone conduction with mastoid placement or sound field testing) which determines if audiometric threshold can be tested to 0 dB HL (see Table 2 ). For example, if the measured ambient noise level is 5 dB above the ANSI MPANL standard at 1 kHz, then a patient with an actual threshold of 0 dB HL at that frequency would yield a threshold of 5 dB HL. Unfortunately, most audiology clinics will not have access to a sound level meter with an octave or one-third octave band analyzer. The goal is then to ensure that the test area is as consistently quiet as possible. 25
Table 2. Maximum Permissible Ambient Noise Levels (MPANL; dB SPL) in ANSI S3.1–1999 for Ears not Covered.
| Ears not covered | |
|---|---|
| Center frequency | MPANL |
| 125 | 24.0 |
| 250 | 16.0 |
| 500 | 11.0 |
| 800 | 10.0 |
| 1,000 | 8.0 |
| 1,600 | 9.0 |
| 2,000 | 9.0 |
| 3,150 | 8.0 |
| 4,000 | 6.0 |
| 6,300 | 8.0 |
| 8,000 | 9.0 |
Although MPANLs are not provided for circumaural headphones in the 1999 ANSI Standard, Konrad-Martin et al estimated MPANLs for circumaural headphones from 125 Hz to 16 kHz using published attenuation data for circumaural headphones and the ANSI computational formula. Fig. 3 plots the MPANLs for both insert phones and circumaural headphones along with hospital ward noise measured in half-octave bands. In this case, insert earphones could be used to test all frequencies while circumaural headphones could be used for testing at frequencies ≥2 kHz 22 ( Table 2 , Fig. 3 ).
Figure 3.
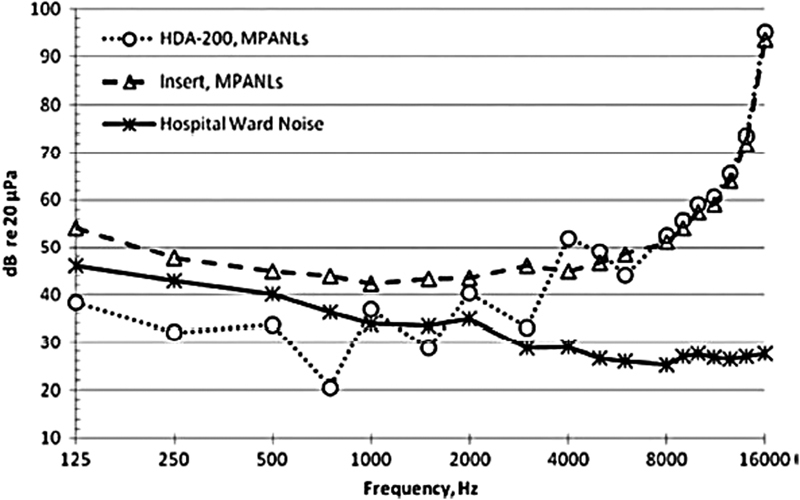
Maximum permissible ambient noise levels (MPANLs) (in decibels re: 20 μPa, American National Standards Institute S3.1-1999) for audiometric test room is shown as function of frequency when HDA 200 (circles) circumaural earphones and Etymotic ER2 (triangles) earphones are used. Hospital noise (asterisk) levels as function of frequency measured in 1/3-octave frequency bands are also shown.45 Data show that reliable behavioral hearing thresholds can be obtained for frequencies above 2,000 Hz in most circumstances. In addition, room noise using OtoID is measured must before presentation of each tone as extra measure that noise levels in room are well controlled. Using insert earphones for collecting distortion-product otoacoustic emissions, all f2 frequencies can be used.
Speech audiometry also can be administered away from the audiology clinic using recorded speech tests. Many of the current generation of portable audiometers, either standalone or PC based, come equipped with installed wave files of the basic adult and child speech audiometry tests (e.g., spondee word lists, CID W-22 and NU-6). This eliminates the need for an external CD player and is essentially as fast as using monitored live voice (MLV) presentation since the next word in sequence is presented as soon as the most recent word is scored as correct or incorrect. Speech audiometry, particularly word recognition tests, can be a powerful counseling tool that may motivate a patient to seek a treatment change while demonstrating the need for intervention. 8
Pure Tone Air Conduction Audiometry
It is essential and ideal to obtain a baseline pure tone air conduction audiogram and to do so prior to the start of treatment. This will determine if a preexisting hearing loss is present and documents HLs prior to treatment 5 26 to which future serial testing can be compared. Frequencies tested should be from 250 Hz to 16 kHz including both 3 and 6 kHz. 8 It is reasonable to eliminate 250 Hz due to ambient noise concerns if testing is conducted outside of the test booth. Some clinicians may choose to test out to 20 kHz; however, ANSI has not published calibration values for the frequencies above 16 kHz. The audiometer manufacturer provides calibration values beyond 16 kHz.
Pure tone testing within the standard frequency range only will not allow for early identification of ototoxicity. 6 By the time hearing loss is detected within the standard frequency range, the patient already may be experiencing communication difficulties. 27
Animal research and clinical trials on humans administered ototoxic drugs have shown that damage to the cochlea initially occurs in the basal end of the cochlea which causes hearing loss to progress from high to low frequencies. 7 Knight et al completed baseline and follow-up standard frequency and EHF testing on 17 children as part of a study comparing standard frequency audiometry, EHF audiometry, and DPOAEs. 16 Ninety-four percent of the children showed bilateral ototoxicity in the EHF range.
Comparatively, only 47% of the children showed bilateral ototoxicity in the standard frequency range. 16 This supports research and clinical data that EHF audiometry is more sensitive to early identification of ototoxic hearing loss. Nonetheless, it is important to document audiometric thresholds within the standard frequency range for comparison with future tests and to determine how well the patient hears speech for normal conversation. 6
Unlike the standard frequency range, which is based on octave and mid-octave frequencies only, the resolution of the EHF range is broken down into approximately 1/6 octave points between 8 and 16 kHz. The frequencies above 8 kHz typically include the following: 9, 10, 11.2, 12.5, 14, 16, 18, and 20 kHz (please check your audiometer's technical specifications for the frequencies included above 8 kHz). Testing within this extended frequency range is critical to the overall success of the monitoring program as it is the most sensitive test frequency range for early identification of ototoxicity. 5 6 18 It is recommended that all frequencies between 8 and 16 kHz be tested during the baseline audiogram and subsequent serial audiograms.
In a 1994 study by Fausti et al, they reported on a shortened, yet sensitive, protocol to identify a higher percentage of patients with ototoxic hearing loss as early as possible during treatment. 5 They tested a total of 222 ears of patients who were treated with either aminoglycoside antibiotics and cisplatin and compared standard frequency audiometry to EHF audiometry. These data were analyzed to determine which of the two protocols (standard frequency or EHF) alone would initially identify the highest percentage of patients with a change in HLs. The results showed a clear advantage to testing in the EHF range as 86.5% of the ears tested showed a change in HLs in the EHF range compared with only 34.6% for the standard frequency range. 27
As part of the same study, a third protocol to identify a hearing loss was utilized and referred to as the high-frequency slope. This metric was composed of the highest five adjacent frequencies that had thresholds ≤100 dB sound pressure level (SPL). These five frequencies were patient specific. If only these frequencies were used to monitor changes in threshold, 89.2% of the ears tested were identified as having a significant change in hearing thresholds, the most sensitive of the three protocols. 28 The authors concluded that the “use of the five-frequency monitoring protocol should result in a high detection rate in a significantly shortened testing procedure that could be tolerated by a greater number of patients.” 28
This shortened procedure was eventually expanded to include the seven highest frequencies with a threshold of ≤100 dB SPL and became known as the sensitive region for ototoxicity (SRO). In 2014, Konrad-Martin et al proposed a comprehensive ototoxic monitoring program utilizing the SRO technique as the proposed behavioral hearing testing method of choice. 22 This recommendation is based on research showing a 94% sensitivity in detecting hearing loss when utilizing the SRO technique. 22 This is true whether the SRO is above or below 8 kHz. They felt that hearing tests lose their sensitivity for subjects with good pretreatment hearing when testing does not include frequencies near the high-frequency hearing limit. 22
The baseline test should be expanded to incorporate the SRO since, by doing so, it may be possible to limit pure tone air conduction testing during treatment to the highest seven frequencies comprising the SRO.
Instrumentation will play a role in an audiologist's ability to incorporate the SRO into their test protocol. Most audiometers that can test EHF have at least a 1/6 octave resolution between 8 and 16 kHz. Most, if not all, of the clinical audiometers are capable of increased resolution per octave across the entire test frequency spectrum; however, not all audiometers have that level of resolution below 8 kHz (please check the technical specifications of your audiometer). 25
Pure Tone Bone Conduction Testing
Pure tone threshold measurement via bone conduction should be administered as part of the baseline assessment at octave frequencies from 500 Hz to 4 kHz. This is especially true for any frequency where the air conduction threshold is greater than 10 dB HL.
While it may not be necessary to measure bone conduction thresholds during the monitoring phase of the AOMP, it is important to establish bone conduction baseline levels prior to treatment. Any change in air conduction HLs detected during a monitoring session will require retesting of bone conduction thresholds to determine if the change in hearing is conductive or sensorineural in nature. In the pediatric population, for example, which is already prone to otitis media with effusion, the use of immunosuppressive chemotherapeutic drugs and/or cranial radiation places them at greater risk for this condition. 27 28 Older children and adults also may develop otitis media with effusion if treated similarly.
A stable, conductive hearing loss should not complicate the monitoring process; however, a fluctuating conductive hearing loss will make it difficult to track changes in HLs compared with baseline that are directly related to the ototoxic treatment. 29
Speech Audiometry
Speech reception threshold and WRS tests are included in the baseline assessment. Measuring a WRS to a monosyllabic word list is very important when counseling the patient and the family about the impact and consequences of a hearing loss affecting the speech frequency region of the audiogram. The use of supporting materials that may include the use of the speech intelligibility index (SII) score and plotting the audiogram with the speech region superimposed are methods that can simplify and bring meaning to the practical reality of hearing loss in the patient's daily life.
The ANSI S3.6 Specifications for Audiometers standard recommends the use of recorded speech material when performing speech audiometry. Yet only 42% of American Academy of Audiology members who responded to a 2012 survey stated they consistently use recorded materials when performing WRS tests. 30 Since it is possible that serial testing will be completed outside of the test booth under what may be less than ideal conditions, and to ensure the most accurate and reliable test, recorded speech material is the method of choice for the AOMP.
Today's audiometers, both standalone and PC based, have wave files stored internally of the basic adult and child monosyllabic word lists. Presentation of the words is either via a mouse click or automatically after scoring the previous word correct or incorrect based on the patient's response. By using this method instead of an external CD player, test time is very similar to MLV testing, the WRS is more accurate, and with excellent test–retest reliability. 30
Speech audiometry is administered only during the treatment phase if a change in pure tone air conduction thresholds is measured. If this change affects the speech frequencies, it is important to determine if the WRS has also gotten worse. If it has, the patient and family should be made aware of not only the change in pure tone air conduction hearing but also the effect it is having on the speech frequencies and the patient's ability to understand speech, especially when in adverse listening situations. This is a very valuable piece of information to share as patient counseling as part of the monitoring program increases the probability the patient will pursue treatment for the hearing loss. 8
Immittance Testing
Tympanometry and acoustic reflex testing should be part of the baseline assessment as there may be a need to administer tympanometry and reflexes during the treatment phase. Brooks and Knight recommended tympanometry be routinely performed as part of the monitoring process for pediatric patients due to children having a higher prevalence of otitis media with effusion. 32 This provides the audiologist the ability to rule out middle ear dysfunction when audiometric and otoacoustic emission (OAE) tests show new or additional hearing loss and/or reduced or absent OAEs, respectively, when compared with baseline.
As mentioned in the previous section, certain treatment methods can cause otitis media with effusion in children and adults. Therefore, if hearing loss develops or worsens when monitored during treatment, a conductive component must be ruled out. Otherwise, any change in hearing may be misrepresented as being related to ototoxicity caused by treatment when it may be conductive in nature. 27 28
When testing children less than 7 months of age, it is necessary to do so using a 1-kHz probe tone. Literature has shown that this probe tone frequency is more sensitive to middle ear dysfunction in neonates and infants (up to 6 months of age) compared with a 226-Hz probe tone. 32 Some advocate for the use of both probe tones between 3 and 9 months. Suffice to say, this approach is well documented and beyond the scope of this article.
Acoustic reflex thresholds (ART) should be recorded with both ipsilateral and contralateral stimulation and used in conjunction with tympanometry, when possible. The ART test provides corroborating evidence for possible middle ear dysfunction. In patients who cannot be tested behaviorally, the ART test can be a valuable tool by tracking changes in the ARTs during the treatment phase. The ART will be elevated beyond the normal range (70–100 dB HL) or absent when severe to profound hearing loss develops. 33 However, for this purpose, the ART test must be interpreted with caution as acoustic reflexes can be recorded in ears with moderate to severe sensorineural hearing losses with recruitment, such as occurs when the cochlea is compromised.
Otoacoustic Emissions
In the 1994 ASHA guidelines, OAE testing was one of three objective procedures recommended for testing unresponsive patients. 5 Indeed, OAEs, specifically DPOAE, are ideally suited to assess cochlear function at the level of the OHCs in patients who will not or cannot respond to behavioral testing. This includes patients receiving treatment with the ototoxic medications mentioned earlier in this article.
OHCs located at the base of the cochlea are damaged before any other cochlear structure, 3 31 thereby affecting hearing thresholds in the higher frequencies first. Eventually, as the damage progresses toward the apical end of the cochlea, hearing loss in the lower frequencies develops. 3 31 34
While TEOAEs as well as DPOAEs meet all the criteria for an excellent ototoxic monitoring test, TEOAEs are limited to assessing cochlear function through 5 kHz only. DPOAEs are not as sensitive to marginal and mild hearing losses, thereby allowing for monitoring patients who have up to a 45-dB HL loss. 28 In other words, TEOAEs are more likely to be reduced or absent for marginal and milder sensory hearing loss. Therefore, DPOAE is the test of choice for use in the AOMP.
DPOAE testing is objective, fast (<1 minute/ear), reveals subclinical ototoxic cochlear damage in advance of changes to pure tone air conduction thresholds from 500 Hz to 8 kHz, is frequency specific, does not require a response from the patient, can assess OHC function through 10 kHz, and can be easily conducted at bedside or in the treatment room. 28 34 35
Many patients may not feel well at the time of the monitoring testing, perhaps making it difficult, if not impossible for them to respond to any behavioral tests. Therefore, the use of an objective test, like DPOAE, that takes less than a minute per ear, is easy to administer, has good test–retest reliability over several test sessions, and is very sensitive to damage within the cochlea, is extremely valuable. 35 This is also an important monitoring tool when testing neonates, infants, and young children who cannot be tested behaviorally due to their age.
DPOAE levels should be established at the time of the baseline assessment to which measurements taken during treatment can be compared. The recommended frequency range for testing is 1,500 Hz to 10 kHz (if the equipment allows) with primary tone intensity levels set to f1 = 65 dB SPL and f2 = 55 dB SPL at a f2/f1 frequency ratio of 1.22. The recommended frequency resolution is 1/6 octave or six points per octave. Testing with an f2 setting lower than 1,500 Hz is problematic, as background ambient noise either from the environment or the patient may interfere. And since ototoxic hearing loss affects high frequencies first, the need to test below 1,500 Hz is not critical or necessary.
An important goal of the baseline assessment is to obtain as much information as possible for as many tests as possible, including DPOAE. Testing performed at the time of the monitoring visit should include a limited number of tests compared with the baseline assessment. This is more efficient and less of a burden on the patient who may not be able to tolerate lengthy audiologic testing. 36 The DPOAE frequency range can be limited to the octave just below the highest frequency that had an acceptable OAE. This is similar to the technique used in pure tone air conduction testing and is referred to as the SRO DP (SRO—distortion product). 3 If any significant changes occur within this octave region, then the entire frequency range should be tested.
What is considered a significant change in the DPOAE response? ASHA (1994) defines a significant change in pure tone air conduction levels within the standard frequency range; however, currently, there is no standard defining a significant change in DPOAE levels compared with baseline. 32 37 Dhar and Hall suggested a 4- or 5-dB decrease in DPOAE amplitude for a limited number of the highest frequencies. 29 Others suggested a decrease of at least 6 dB from baseline at SRO DP . 3 9
Still others suggested the need to establish change criteria that exceeds the normal variability that exists in healthy subjects. 37 Changes in DPOAE response levels must be interpreted within the context of sources of test–retest reliability and minimize these sources as much as possible. 38 This is particularly important when testing neonates, infants, and young children in which the normal variability of the DPOAE changes as a function of age at baseline, the f2 tested, and the follow-up interval. 37 A decrease in the DPOAE response amplitude of 12 dB from baseline, for example, may be significant for some pediatric patients but not for others. The normal variation in DPOAE response amplitudes is greater for children compared with adults, thereby suggesting the need for different change criteria for the two populations. 37
Research and clinical data have shown that DPOAEs reveal changes in cochlear function at frequencies before hearing loss appears at the corresponding frequencies on the audiogram ( Fig. 4 ). 29 35 This factor makes DPOAEs an excellent ototoxic monitoring tool, as it reveals physiological change before a perceptual difference. 38 But in some cases, a hearing loss never develops even though OHC function has been compromised. 38
Figure 4.
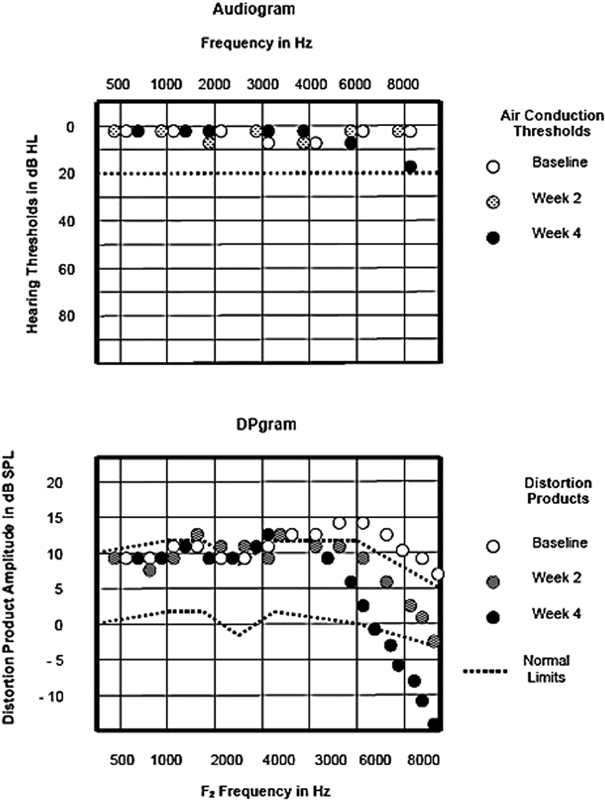
Pure-tone hearing thresholds and distortion product otoacoustic emissions (DPOAEs) in a 7-year-old boy with a brainstem tumor undergoing medical management with chemotherapy that included cisplatin. Changes in DP amplitude occur in advance of pure tone hearing loss. 29
Therefore, reporting changes in DPOAE response levels to the physician as in early indicator of ototoxicity may not engender any change in treatment, as this finding alone may not be a good predictor for the development of measurable hearing loss. Regardless, changes in DPOAE response amplitude that are considered significant must be reported to the attending physician.
Reavis et al measured DPOAEs out to 10 kHz to identify a patient's DPOAE high-frequency limit and reported that “when measured near the high-frequency limit, DPOAEs are a reliable indicator as to whether an ASHA-defined significant hearing change has occurred.” 38 They also developed a multivariate discriminate function that included the patient's baseline HLs, the cumulative medication dose, and DPOAE changes to the high-frequency limit at follow-up visits to predict the probability of hearing change. This model predicted hearing change occurring at the time of a treatment visit with more accuracy than DPOAEs alone. 38
Other notable findings from the study of Reavis et al are as follows:
The frequency that yielded the most predictive accuracy was the highest DPOAE able to be recorded. This is similar to the SRO described earlier for pure tone audiometry. Their study demonstrated that “higher DPOAE frequencies are statistically more sensitive to incipient ototoxicity than lower frequencies.” 38 This finding supports what Gorga et al reported in 1997 that being DPOAE test performance improves with increasing test frequency. 39
Although DPOAE measurements were made out to 10 kHz, the median upper frequency where a response occurred was 4 kHz compared with the behavioral SRO (by pure tone audiometry) in their test population of 12.5 kHz. Nevertheless, this discrepancy in the respective high-frequency limits did not affect the predictive accuracy of the DPOAE. The interesting point is the DPOAEs were predicting hearing loss at frequencies higher than the DPOAE test frequencies that were being monitored. 38
When combined with cumulative platinum dose and pretreatment hearing, the ability for DPOAE results to correctly identify hearing as being stable or changing was better than DPOAE results alone. 38
Clearly, it would be advantageous to develop criterion that defines what represents a significant change to DPOAE baseline levels while, at the same time, also having a high degree of accuracy as a predictor of hearing change. Considering that OAEs were recommended as an objective monitoring tool in the ASHA guidelines 24 years ago, as well as the AAA Guidelines in 2009, it is surprising that change criteria have not yet been developed. I believe there are three possible reasons as to why: (1) unlike audiometers, no calibration standards have been published for OAE equipment. As a result, probe design has not been standardized. The difference in probe design can contribute to variations in OAE test results (personal observation); (2) technique with respect to proper ear tip selection and probe insertion depth can also lead to poor test–retest reliability by up to 20 dB in some reported cases 40 ; and (3) the method for in situ calibration of the primary tone levels can cause errors above 3 kHz in adult ears and 6 kHz in children younger than 2 years. 37 Other methods of in situ calibration for DPOAE testing have been reported in the literature but have not been integrated into commercially available OAE instruments at this time.
Auditory Brain Response
It is estimated that approximately 30 to 40% of patients taking ototoxic medications become too ill to provide reliable responses during behavioral audiologic testing. 41 In addition, neonates, infants, and most young children less than 3 years old cannot be tested behaviorally. DPOAE testing is an excellent objective method to assess cochlear function, but it is not a test of hearing and cannot estimate behavioral threshold.
The ABR is an objective test (although it requires subjective interpretation) of auditory nerve and brainstem function that can estimate frequency-specific thresholds using tone burst stimuli in patients who cannot be tested behaviorally. Clinical ABR equipment utilizes broadband and frequency-specific tone burst stimuli at octave and midoctave frequencies from 250 to 8 kHz; however, this frequency range is not ideal for early identification of hearing loss due to ototoxic medications. It can be a time-consuming test if the goal is to estimate threshold at multiple frequencies. It is also a test that is not included in the baseline assessment when the patient is able to be tested by conventional means.
For very young patients, the ABR can establish a baseline response to multiple stimuli but only within the speech frequency range. Research has shown that higher frequency stimuli, including frequency-specific tone bursts from 8 to 14 kHz, and a high-frequency filtered click can be used to measure the ABR and possibly be incorporated into an AOMP. 41 Fausti et al compared ABR recordings with conventional clicks, HF clicks, and HF tone bursts. A fixed intensity level of 50 dB SL was used to determine if a response was present or not, as past research by the same author demonstrated that confirming the presence or absence of a response was the most reliable ABR measurement to confirm ototoxic change. 41 In their opinion, it is not feasible to perform threshold searches in this population because of the time involved and questionable reliability of the responses near threshold. 41
The need for a quiet, cooperative patient may prevent measurement of the ABR in infants and young children. Sedation may be required for some patients to collect valid ABR recordings. Since many children require sedation during medical treatment, it is certainly possible to perform ABR threshold measurement at the same time. 30 The focus should be on testing the higher frequencies first at 4 and 8 kHz. Similar to pure tone audiometry and DPOAEs, the higher frequencies will show evidence of ototoxic hearing loss before lower frequencies. 30
Another electrophysiologic test option for estimating behavioral threshold is the auditory steady-state response (ASSR). This procedure is completely objective as subjective interpretation of a waveform is not required. Modulated pure tone or narrow band octave band chirp stimuli can be delivered at multiple frequencies (up to four/ear) binaurally. Second-generation ASSR technology is faster and more accurate compared with first-generation ASSR equipment that became commercially available approximately 17 years ago. In addition, the use of the chirp stimuli enhances amplitude of the response also contributing to reduced test time.
Sininger et al compared the accuracy and test time of octave band CE chirp stimuli presented binaurally at four different frequencies (octave frequencies from 500 to 4 kHz) with single tone burst ABR at the same frequencies. They found that ASSR was more accurate than tone burst ABR in that the ASSR thresholds measured were lower in intensity than tone burst ABR. ASSR was also faster than ABR with an average test time of 19.93 minutes. ABR average test time was 32.15 minutes. 42 If behavioral estimation is the goal, ASSR testing offers an advantage in time, accuracy in threshold estimation, and removes the subjective interpretation component by the audiologist.
What Constitutes a Significant Change and the Use of Grading Scales?
The primary goal of the AOMP is to identify patients at risk for developing hearing loss that affects the speech frequency range that creates communication issues thereby affecting overall quality of life. 3 5 6 16 18 20 In so doing, evidence of developing or worsening hearing loss and/or changes in cochlear function using objective tests and reporting these changes in a meaningful way may cause the physician to modify the treatment regimen to either slow down or prevent further hearing loss. But it is also important to convey to the physician the impact the hearing loss is having on the patient's communication ability. 27
The ASHA guidelines define a significant change in hearing compared with baseline as follows:
≥20 dB HL decrease at any one frequency.
≥10 dB HL decrease at any two adjacent test frequencies.
Loss of response at three consecutive test frequencies where responses were previously obtained. 5
Significant changes in hearing need to be confirmed within 24 hours. 5 Once confirmed, the audiologist is responsible for reporting these findings to the physician caring for the patient. But what is the preferred method of reporting these results? King and Brewer stated that audiologists must shape “clinical data into a language that can best be consumed by the recipient.” 27 Jargon should be avoided, and the results should be presented in the context of each individual patient. 27
According to the ASHA guidelines, these criteria were to be applied to changes in hearing only in the standard frequency range up to 8 kHz; however, the same criteria can be used to determine if a significant change in hearing has occurred in the EHF range, also. 16 The guidelines do not address the degree of hearing loss secondary to the significant threshold shift or the functional impact it may have on a patient's communication.
The National Cancer Institute at the National Institutes of Health published the most recent version of Common Terminology Criteria for Adverse Events v5.0 (CTCAE) in 2017. 43 The CTCAE is a set of criteria for the standardized classification of adverse effects of drugs used in cancer therapy. Adverse events are grouped based on the severity of the event and an expected level of intervention required. Criteria are provided for adults in a monitoring program, adults not in a monitoring program (sans baseline test), and pediatric patients. Unlike the ASHA guidelines, CTCAE criteria for adults in a monitoring program and pediatric patients categorize changes in HLs, functional impact to some extent, along with descriptions of severity and the need for intervention. The pediatric categories also consider the frequencies involved in the hearing loss. The hearing impairment category includes four grades (1–4) ranging from adverse effects considered to be mild (Group 1) through severe, “urgent intervention indicated” (Grade 4).
The four grade classifications for hearing impairment are as follows (for adults in a monitoring program/baseline established):
Grade 1—Threshold shift of 15 to 25 dB averaged at two contiguous test frequencies in at least one ear. Intervention not indicated.
Grade 2—Threshold shift of >25 dB averaged at two contiguous test frequencies in at least one ear. Moderate: minimal intervention indicated.
Grade 3—Threshold shift of >25 dB averaged at three contiguous test frequencies in at least one ear or therapeutic intervention indicated. Disabling: intervention indicated.
Grade 4—Decrease in hearing to profound bilateral loss (absolute threshold >80 dB HL at 2 kHz and above); nonserviceable hearing. Urgent intervention indicated.
For adults not enrolled in a monitoring program/baseline not established, the grade classifications are as follows:
Grade 1—Subjective change in hearing in the absence of documented hearing loss.
Grade 2—Hearing loss with hearing aid or intervention not indicated.
Grade 3—Hearing loss with hearing aid or intervention indicated.
Grade 4—Decrease in hearing to profound bilateral loss (absolute threshold >80 dB HL at 2 kHz and above); nonserviceable hearing. Urgent intervention indicated.
For pediatric patients, the criteria for grade classifications are as follows:
Grade 1—Threshold shift >20 dB HL loss (i.e., 25 dB HL or greater); sensorineural hearing loss (SNHL) above 4 kHz (i.e., 6 or 8 kHz) in at least one ear. Mild: intervention not indicated.
Grade 2—Threshold shift >20 dB at 4 kHz in at least one ear. Moderate: minimal intervention indicated.
Grade 3—Hearing loss sufficient to indicate therapeutic intervention, including hearing aids; threshold shift >20 dB at 2 to < 4 kHz in at least one ear. Disabling: intervention indicated.
Grade 4—Audiologic indication for cochlear implant; > 40 dB HL (i.e., 45 dB HL or more); SNHL at 2 kHz and above. Urgent intervention indicated.
This grading system accounts for increasing shifts in HLs and describes the severity of the shift. As such, there is an implied relationship between the amount of change in HLs and the severity of the adverse event (AE) assuming that baseline hearing is normal. If a preexisting hearing loss exists, a Grade 1 AE, for example, may not be associated with just a “mild” functional impairment. Therefore, communication with the physician may require slightly more than just a simple grade level classification, such as a statement addressing the current functional impact of the absolute HLs.
Frequencies included in the CTCAE criteria are 1, 2, 3, 4, 6, and 8 kHz. The EHF range is not included in the calculation of threshold shift; however, the purpose of the adverse effect grading system is to identify circumstances that have an impact on the patient that may lead to or require intervention. 44
Other grading scales have been developed for use with either adult or pediatric patients. Many expand the scope of the parameters to include a portion of the EHF range and absolute HLs with more emphasis on higher frequencies including those in the EHF range. Others do not grade findings based on shifts due to ototoxicity but only focus on functional impact.
Grading scales should be simple to use by the audiologist and easy to understand by the physician: must identify hearing changes and absolute HLs and the resultant handicapping effect. Interested readers are referred to the article by Brewer and King for an excellent, comprehensive analysis of ototoxic grading scales. 27
Despite the value of including DPOAE testing as part of the AOMP, currently there are no published guidelines that define a significant change in DPOAE levels compared with baseline results. Many have suggested a change in the overall DPOAE amplitude ranging from 5 to 9 dB at two or more frequencies in the SRO DP range. 3 9 18 29
Reavis et al tested four inpatients not receiving ototoxic medications over a period of 7 months to determine criteria for defining a “significant” change in DPOAE levels. They found that a decrease in DPOAE level of 4 dB or greater, or a loss of response at two or more adjacent frequencies, would result in a false-positive rate of 5% or less over a 4-week period. 38
A fixed intensity frequency sweep measuring DP amplitude is most frequently used in an AOMP; however, other metrics have been investigated that may be more sensitive to detect changes in cochlear function. The other DPOAE measures investigated include DP threshold measurement by using a fixed frequency input/output protocol or the calculation of group delay as a function of f2/f1 ratio. (Group delay is calculated based on the time it takes between the presentation of the stimuli and the time the microphone measures the DPOAE in the ear canal. 36 38 ) Research has shown that a DPOAE measurement other than just DP amplitude may be more sensitive and less problematic. 38
There is a need for a standardized set of audiologic guidelines 30 defining a significant shift for OAE measures, to categorize the functional impact of hearing loss regardless of the degree of ototoxicity and to expand the ASHA guidelines defining significant changes for EHF and OAE.
Instrumentation
Audiologic testing protocols for patients on ototoxic medication treatment plans must be quick, efficient, and accurate. In addition, the tests administered must be sensitive for detecting significant changes in hearing and/or cochlear function related to ototoxicity, while at the same time limiting the number of false-positive outcomes. Furthermore, portable equipment should be available for testing at bedside or in the treatment area. 32
Audiometers must be able to test both the standard (125 Hz–8 kHz) and extended high-frequency (9–20 kHz) regions; immittance instruments must be fully diagnostic in scope and include ipsilateral and contralateral acoustic reflex testing; and OAE devices must be able to administer a distortion product OAE test at least up to 8 kHz and, preferably, to 10 kHz. Audiologists should have access to auditory evoked potential equipment in the event a patient requires a frequency-specific ABR (essential in pediatric AOMPs).
Baseline and monitoring tests should be conducted in a sound-treated audiometric test booth, if possible. When testing is administered outside of a booth, knowledge of octave band ambient noise levels determines to what HL audiometric threshold can be tested. Since serial threshold testing during treatment is mostly conducted at EHF or the highest frequencies in the standard frequency range, ambient noise levels in a quiet room will not adversely impact air conduction threshold measurement.
There are several compact, portable audiometers that have a frequency range of 125 Hz to 20 kHz. Circumaural headphones are required for testing beyond 8 kHz (some can be calibrated to test the full frequency range). Most audiometers are also able to store test results either internally or on a PC allowing for convenient comparison to the baseline test.
Fully capable, diagnostic, portable DPOAE devices have been in common use for almost two decades. Many can be hand carried and are able to store test results for easy transfer to a PC. When an audiologist is not available to provide the serial test, a well-trained nonaudiology staff member can perform the DPOAE test which the audiologist can review to determine if any significant changes occurred. The device must be capable of a DP gram resolution of at least 6 points/octave and be capable of generating a f2 frequency at least through 8 kHz, although 10 kHz if preferable. There are some devices able to extend the highest f2 frequency to 16 kHz; however, this requires a special OAE probe which is not commonly available on commercial DPOAE devices.
Research has shown that standing waves can occur within a patient's ear canal caused by interactions between the incident and reflected sound waves because of the calibration method. When this occurs, errors in estimates of both stimulus and DPOAE level of up to 20 dB are possible. 7
When partial cancellation or enhancement of the sound wave occurs, it results in a pressure magnitude that is either lower or higher at the plane of the OAE probe than it is at the eardrum. Due to these inaccuracies at the plane of the probe, the system software adjusts the input voltage level delivered to the transducer which will either increase or decrease the primary tone test levels to compensate for this inaccurate calibration. Errors in DPOAE calibration like this affect higher frequency regions, primarily in adults, from 3 to 7 kHz and at 10 kHz. 7 It is very important to confirm that the primary tone levels (L1 and L2) are within ± 3 dB or ± 5 dB of the preferred target levels. Otherwise, the DPOAE results may be invalid resulting in an incorrect interpretation of test findings. Other calibration methods exist that overcome this problem but are not currently available on commercial products.
Finally, new FDA-approved automated audiometers are available that meet the ANSI standard for audiometers and offer great promise for use in AOMPs. These new devices address the challenges of staffing, location, and logistics. One is a patient-driven device that is equipped for testing pure tone air, bone, and speech audiometry using circumaural headphones with forehead bone oscillator placement. Masking is applied when needed. A standalone audiometer is required and operates via proprietary software via PC control.
Another version also uses circumaural headphones but is connected to a tablet computer and is limited to pure tone air conduction screening or threshold testing. It also meets the ANSI standard for audiometers, is patient driven, and can be easily used in a quiet room outside of the audiology clinic and a test booth. Currently, neither of these models can test above 8 kHz. Test results are stored electronically for review by the supervising audiologist. Quality indicators provide critical feedback and ensure accuracy. The automated, self-directed feature makes it ideal for use in AOMPs.
Testing instruments are not an obstacle to implementation of a successful AOMP. Fully capable, portable, and, in some cases, automated audiometers and DPOAE devices are FDA approved and commercially available. Automated, self-directed audiometric testing is a reality and offers convenience, accuracy, and reliability.
Summary
On the surface, audiologic monitoring for patients taking ototoxic medications for cancer or infection treatment may seem straightforward. That being to establish a baseline test followed by serial testing to document any change in hearing status throughout treatment and thereafter. When a change occurs that is considered significant and permanent during treatment, it must be reported to the physician who will then decide if modifying the treatment plan is safe and feasible. But there is much more to a successful AOMP.
Identifying the occurrence of ototoxicity does not necessarily provide any insight into the absolute HLs of the patient. Can a significant change in hearing occur that is not considered handicapping? For example, if a patient has baseline HLs between 0 and 5 dB HL at all test frequencies and a significant change (e.g., ASHA 1994) occurs in the EHF range, this will not influence the patient's communication ability. But it is important for these findings to be reported to the physician in the context of the change itself as an early identifier of ototoxicity.
Hopefully, the physician can change the treatment regimen before the ototoxicity extends into the speech frequency range. Conversely, if the hearing loss does extend into the speech frequency range and continues to deteriorate, how important is it to not only inform the physician of the additional significant changes taking place in hearing but to convey the functional impact it is having? And if that additional information is conveyed, will it have more influence on the physician to make changes in the treatment regimen now that the patient is having difficulty communicating? Of course, there are cases when altering medical treatment is not an option.
Regardless, the audiologist will be aware of the handicapping nature of the hearing loss and must provide counseling to the patient and family about the ototoxicity that has occurred, the potential for additional ototoxicity and hearing loss while at the same time discussing available audiologic treatment options that will positively impact quality of life.
Even with the advent of otoprotectents to mitigate the effects of ototoxic medications, the need for comprehensive AOMPs will remain.
Conclusion
Ototoxic monitoring programs need to become standard of care for all patients receiving treatment with ototoxic medications. Audiologists must take the lead to implement programs that are thorough, efficient, and accurate. Significant changes in hearing must be reported to the physician immediately for a decision about changing the treatment plan. Ototoxicity that affects the speech frequencies may require intervention; therefore, patients and their families should be counseled throughout the AOMP to increase the likelihood they will seek audiologic care for the hearing loss.
Conflict of Interest None.
Highlights
• Audiologic ototoxicity monitoring is not well established and continues to be an inconsistent practice for many adult oncology and infectious disease patients. 8
• Audiologists should take the lead in efforts to implement audiologic ototoxic monitoring programs through education of medical personnel involved in the care of patients on ototoxic medication and on the impact ototoxic hearing loss may have on a patient's quality of life.
• As the survival rate for cancer patients continues to rise, the need for early identification, serial monitoring, counseling, and possible intervention with appropriate audiologic care and treatment is critical to maintain and improve the patient's quality of life.
• Baseline testing should be conducted prior to treatment. Modification to the conventional audiologic test battery is necessary to accommodate patient needs throughout treatment.
• EHF shows evidence of change due to ototoxicity before DPOAE testing and both before standard frequency audiometry. 5
• EHF audiometry is the most important test in the AOMP and should be administered at the baseline assessment and all subsequent monitoring tests.
• Changes in DPOAE levels precede changes in hearing levels.
• Threshold shifts in adjacent frequencies indicate more systematic change and increase the likelihood of a true decrease in hearing sensitivity.
• Any significant change in hearing thresholds must be confirmed with a repeat test within 24 hours 5 and reported to the physician and medical team immediately.
• Physicians in charge of the medication regimen may state ahead of time that any changes in hearing level during treatment will not bring about a change in the treatment plan. In that case, Campbell recommended that only the standard frequency range be tested. 23
• Noise exposure can exacerbate the ototoxic effects of both aminoglycosides and cisplatin. 23
• Preexisting hearing loss affecting the high frequencies negatively affects the ability to utilize EHF audiometry and DPOAE as an effective monitoring tool. 23
• Most changes in hearing occur in the octave preceding the highest audible frequency. 6
• The presence of preexisting hearing loss is important in identifying risks, presenting them to the patient, and setting the stage for follow-up counseling and/or rehabilitation. 45
• Effective grading scales that report the functional impact ototoxicity has on a patient need to be included in the AOMP.
• New audiometric tools should make testing more efficient and convenient for the patient and staff.
References
- 1.Arslan E, Orzan E, Santarelli R. Global problem of drug-induced hearing loss. Ann N Y Acad Sci. 1999;884(884):1–14. doi: 10.1111/j.1749-6632.1999.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 2.Hammill T L, Campbell K C. Protection for medication-induced hearing loss: the state of the science. Int J Audiol. 2018;57:S67–S75. doi: 10.1080/14992027.2018.1455114. [DOI] [PubMed] [Google Scholar]
- 3.Ganesan P, Schmiedge J, Manchaiah V, Swapna S, Dhandayutham S, Kothandaraman P P. Ototoxicity: a challenge in diagnosis and treatment. J Audiol Otol. 2018;22(02):59–68. doi: 10.7874/jao.2017.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cone B, Dorn P, Konrad-Martin Det al. Ototoxic medications 2015. ASHA Audiol Info Series. Available at:www.asha.org. Accessed February 28, 2019
- 5.Fausti S, Bouchard K, Farrer Set al. Audiologic Management of Individuals Receiving Cochleotoxic Drug TherapyAmerican Speech-Language-Hearing Association;1994
- 6.Durrant J, Campbell K, Fausti Set al. Ototoxicity monitoringAmerican Academy of Audiology Position Statement and Practical Guidelines. American Academy of Audiology;2009
- 7.Fausti S A, Wilmington D J, Helt P V, Helt W J, Konrad-Martin D. Hearing health and care: the need for improved hearing loss prevention and hearing conservation practices. J Rehabil Res Dev. 2005;42(04) 02:45–62. doi: 10.1682/jrrd.2005.02.0039. [DOI] [PubMed] [Google Scholar]
- 8.Reavis K M, McMillan G, Austin D et al. Distortion-product otoacoustic emission test performance for ototoxicity monitoring. Ear Hear. 2011;32(01):61–74. doi: 10.1097/AUD.0b013e3181e8b6a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischel-Ghodsian N. Genetic factors in aminoglycoside toxicity. Pharmacogenomics. 2005;6(01):27–36. doi: 10.1517/14622416.6.1.27. [DOI] [PubMed] [Google Scholar]
- 10.Talach T, Rottenberg J, Gal B et al. Genetic risk factors of cisplatin induced ototoxicity in adult patients. Neoplasma. 2016;63(02):263–268. doi: 10.4149/212_140820N391. [DOI] [PubMed] [Google Scholar]
- 11.Konrad-Martin D, Poling G L, Garinis A C et al. Applying U.S. national guidelines for ototoxicity monitoring in adult patients: perspectives on patient populations, service gaps, barriers and solutions. Int J Audiol. 2018;57 04:S3–S18. doi: 10.1080/14992027.2017.1398421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garinis A C, Cornell A, Allada G, Fennelly K P, Maggiore R J, Konrad-Martin D. Ototoxicity monitoring through the eyes of the treating physician: Perspectives from pulmonology and medical oncology. Int J Audiol. 2018;57 04:S19–S24. doi: 10.1080/14992027.2017.1381769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bass J, White S, Jones S.Monitoring ototoxicity in the pediatric oncology population. American Speech-Language-Hearing Association. June2013. Available at:https://www.asha.org/articles/monitoring-ototoxicity-in-the-oediatric-oncology-population. Accessed February 28, 2019
- 14.American Cancer Society.Cancer Facts and Figures 2017Available at:www.cancer.org/research/cancer-facts-statistics. Accessed February 28, 2019
- 15.Poling G.The five W's of ototoxicity monitoring: who, what, where, when, & whyPaper presented at the 26th Mayo Clinic Audiology Conference February2016
- 16.Knight K R, Kraemer D F, Winter C, Neuwelt E A. Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25(10):1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- 17.Theunissen E A, Bosma S C, Zuur C L et al. Sensorineural hearing loss in patients with head and neck cancer after chemoradiotherapy and radiotherapy: a systematic review of the literature. Head Neck. 2015;37(02):281–292. doi: 10.1002/hed.23551. [DOI] [PubMed] [Google Scholar]
- 18.Knight K.Ototoxic monitoring in children.2015. Available at:https://www.audiologyonline.com/audiology-ceus/course/ototoxicity-monitoring-in-children-26996. Accessed February 28, 2019
- 19.Campbell K. Hamilton, Canada: BC Decker, Inc.; 2004. Audiologic monitoring for ototoxicity; pp. 153–160. [Google Scholar]
- 20.Garinis A C, Kemph A, Tharpe A M, Weitkamp J H, McEvoy C, Steyger P S. Monitoring neonates for ototoxicity. Int J Audiol. 2018;57 04:S41–S48. doi: 10.1080/14992027.2017.1339130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konrad-Martin D, Gordon J, Reavis K et al. Audiological monitoring of patients receiving ototoxic drugs. Hearing and hearing disorders: research and diagnostics. Amer Speech Lang Hear Assn Spec Int Grp. 2005;6:17–21. [Google Scholar]
- 22.Konrad-Martin D, Reavis K M, McMillan G, Helt W J, Dille M. Proposed comprehensive ototoxicity monitoring program for VA healthcare (COMP-VA) J Rehabil Res Dev. 2014;51(01):81–100. doi: 10.1682/JRRD.2013.04.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell K. New York, NY: Thieme Medical Publishers, Inc.; 2007. Pharmacology in audiology; pp. 167–168. [Google Scholar]
- 24.ANSI/ASA S3.6 2010 Specifications for audiometersAccredited Standards Committee S3, Bioacoustics, Approved November 2, 2010, Amer Nat Stand Inst
- 25.Wilmington D J, Konrad-Martin D, Helt W et al. Ototoxicity monitoring: program approaches and considerations. Semin Hear. 2011;32(03):248–261. [Google Scholar]
- 26.Studebaker G A. Placement of vibrator in bone-conduction testing. J Speech Hear Res. 1962;5(04):321–331. doi: 10.1044/jshr.0504.321. [DOI] [PubMed] [Google Scholar]
- 27.King K A, Brewer C B. Clinical trials, grading scales, and the audiologist's role in therapeutic decision making. Int J Audiol. 2018;57:19–28. doi: 10.1080/14992027.2017.1417644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fausti S A, Larson V D, Noffsinger D, Wilson R H, Phillips D S, Fowler C G. High-frequency audiometric monitoring strategies for early detection of ototoxicity. Ear Hear. 1994;15(03):232–239. doi: 10.1097/00003446-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Dhar S, Hall J. San Diego: Plural Publishing; 2012. OAEs and cochlear pathophysiology: children; pp. 103–133. [Google Scholar]
- 30.Bass J K, Bhagat S P.Challenges in ototoxicity monitoring in the pediatric oncology population J Am Acad Audiol 20142508760–774., quiz 782–783 [DOI] [PubMed] [Google Scholar]
- 31.Hornsby B, Mueller H G.Monosyllabic word testing: five simple steps to improve accuracy and efficiency. 2013Available at:https://www.audiologyonline.com/articles/word-recognition-testing-puzzling-disconnect-11978. Accessed February 28, 2019
- 32.Brooks B, Knight K. Ototoxicity monitoring in children treated with platinum chemotherapy. Int J Audiol. 2018;57 04:S34–S40. doi: 10.1080/14992027.2017.1355570. [DOI] [PubMed] [Google Scholar]
- 33.Margolis R, Hunter L. London: Pearson; 1999. Tympanometry: basic principles and clinical applications; pp. 85–126. [Google Scholar]
- 34.Clark J, Roeser R, Mendrygal M. New York, NY: Thieme Medical Publishers, Inc.; 2007. Middle ear measures; pp. 380–399. [Google Scholar]
- 35.Leigh-Paffenroth E, Reavis K, Gordon J et al. Objective measures of ototoxicity. hearing and hearing disorders: research and diagnostics. Amer Speech Lang Hear Assn Spec Int Grp. 2005;6:10–16. [Google Scholar]
- 36.Dreisbach L, Zettner E, Chang Liu M, Meuel Fernhoff C, MacPhee I, Boothroyd A. High-frequency distortion-product otoacoustic emission repeatability in a patient population. Ear Hear. 2018;39(01):85–100. doi: 10.1097/AUD.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 37.Konrad-Martin D, Knight K, McMillan G P, Dreisbach L E, Nelson E, Dille M.Long-term variability of distortion-product otoacoustic emissions in infants and children and its relation to pediatric ototoxicity monitoringEar Hear 2017 [DOI] [PMC free article] [PubMed]
- 38.Reavis K M, Phillips D S, Fausti S A et al. Factors affecting sensitivity of distortion-product otoacoustic emissions to ototoxic hearing loss. Ear Hear. 2008;29(06):875–893. doi: 10.1097/AUD.0b013e318181ad99. [DOI] [PubMed] [Google Scholar]
- 39.Gorga M P, Neely S T, Ohlrich B, Hoover B, Redner J, Peters J. From laboratory to clinic: a large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear. 1997;18(06):440–455. doi: 10.1097/00003446-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Neely S T, Gorga M P. Comparison between intensity and pressure as measures of sound level in the ear canal. J Acoust Soc Am. 1998;104(05):2925–2934. doi: 10.1121/1.423876. [DOI] [PubMed] [Google Scholar]
- 41.Fausti S A, Flick C L, Bobal A M, Ellingson R M, Henry J A, Mitchell C R.Comparison of ABR stimuli for the early detection of ototoxicity: conventional clicks compared with high frequency clicks and single frequency tone bursts J Am Acad Audiol 20031405239–250., quiz 281–282 [PubMed] [Google Scholar]
- 42.Sininger Y S, Hunter L L, Hayes D, Roush P A, Uhler K M. Evaluation of speed and accuracy of next-generation auditory steady state response and auditory brainstem response audiometry in children with normal hearing and hearing loss. Ear Hear. 2018;39(06):1207–1223. doi: 10.1097/AUD.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Cancer Institute.Common terminology criteria for adverse events (CTCAE) v5.0November2017
- 44.Brock P R, Knight K R, Freyer D R et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30(19):2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durrant J D, Rodgers G, Myers E N, Johnson J T. Hearing loss--risk factor for cisplatin ototoxicity? Observations. Am J Otol. 1990;11(05):375–377. [PubMed] [Google Scholar]


