Abstract
Medical interventions to combat serious infection or malignancies carry significant morbidities, including ototoxicity. While these lifesaving drugs are often necessary to preserve life, the impact on quality of life for survivors is increasingly concerning for families and healthcare providers. Of primary importance for medical prescribers are appropriately sensitive ototoxicity grading scales and audiological monitoring protocols for surveillance for hearing loss. The intent of grading scales is to help communicate complicated audiological information to non-audiologist healthcare providers (such as oncologists) to help them make good decisions with regards to chemotherapy dosing. Appropriate audiological monitoring helps reduce the time delay between the adventitious onset of hearing loss and the diagnosis and intervention. Finally, pediatric ototoxicity grading and monitoring protocols help ensure timely access to adequate hearing habilitation, verification and validation of the management of permanent medication-induced hearing loss and tinnitus in children.
Keywords: ototoxicity, cisplatin chemotherapy, aminoglycosides
The ototoxicity literature, including manuscripts in this special edition of Seminars in Hearing , has provided numerous examples of medications that carry side effects that include damage to the cochlea and/or vestibular system. With regard to ototoxicity in children, there are specific examples that are most likely to be observed clinically:
Newborns who are extremely premature and/or carry life-threatening diagnoses and require extensive medical interventions prior to initial discharge home.
Children, of any age, who have been diagnosed with cancer and are being treated with chemotherapy and/or radiation.
Children, of any age, who have a condition that makes them highly vulnerable to opportunistic infections, and require medications to combat the infection while balancing the risk for sensorineural hearing loss and associated detriment in quality of life.
Each of these populations have unique needs, but there are similarities that offer the clinical audiologist opportunities to establish protocols that aid in providing consistent results in diagnostic tests and in providing timely, effective audiological management. This manuscript reviews the pertinent pediatric ototoxicity literature and describes recent decisions made by consensus bodies to establish universal ototoxicity grading systems and minimum audiological test batteries.
Hearing loss due to medications is almost always sensorineural and bilateral and symmetric, due to the nature of the delivery of the drug (systemic, via intravenous or oral administration). Hearing is initially affected in the high frequencies and progresses to lower frequencies with increasing duration and dose of the medication. 1 2 3 Most ototoxic medications affect the outer hair cells within the cochlea first, 4 resulting in a loss of audibility of soft sounds and possibly reducing frequency discrimination ability. Compared with adults and adolescents, prelingual and primary-school-aged children require greater audibility for speech recognition and comprehension. Children with prelingual onset of hearing loss have reduced ability to eavesdrop on spoken-language models, and consequently are at risk for speech and language delays, given that incidental learning from eavesdropping on spoken-language models (e.g., parents) is a primary mode of language development. Young children do not have the language base for auditory closure when there are gaps in comprehending the spoken language. 5 Even minimal or high-frequency hearing loss can interfere with speech and language acquisition in younger children, 6 and is associated with poor academic performance in school-aged children. 7 It is in light of the developing knowledge of the impact of minimal and high-frequency hearing loss on language development, academic achievement, and quality of life in children that ototoxicity monitoring protocols and ototoxicity grading criteria have steadily become stricter 8 since the first ototoxicity grading scale was introduced by Brock and colleagues in 1991. 9
Review
Clinical protocols are adopted by academic consortia and by professional societies to help clarify standard of care. Oncologists, and those involved in care for people with cancer, have robust cancer therapy evaluation programs, administered by the National Cancer Institute (NCI). With respect to adverse events stemming from cancer therapies (such as diarrhea, hair loss, or hearing loss), the degree of severity is described in Grade 1, 2, 3, 4, or 5; Grade 1 is the least severe, and 5 is death (NCI Common Terminology Criteria for Adverse Events [NCI CTCAE] v5.0). 10 There are 837 categories of adverse events in CTCAE v5.0, with hearing impaired and tinnitus representing 2 of these 837 categories. Not all adverse events have representation at all grades; for instance, hair loss is categorized as Grade 1 (<50% loss) or Grade 2 (≥50% loss). According to the most recent version of the NCI CTCAE (version 5.0), hearing loss is graded differently for children versus adults, on a scale of Grade 1 to Grade 4. There is no Grade 5 hearing loss, as death is not considered a possible outcome from hearing loss. In children, Grades 1 to 4 are defined in Table 1 .
Table 1. NCI CTCAE v5.0 Ototoxicity Grading Scale.
| Grade 1 | Threshold shift >20 dB HL (i.e., 25 dB HL or greater); SNHL above 4 kHz (i.e., 6 or 8 kHz) in at least one ear |
| Grade 2 | Threshold shift >20 dB at 4 kHz in at least one ear |
| Grade 3 | HL sufficient to indicate therapeutic intervention, including hearing aids; threshold shift >20 dB at 2 to < 4 kHz in at least one ear |
| Grade 4 | Audiologic indication for cochlear implant; > 40 dB HL (i.e., 45 dB HL or more); SNHL at 2 kHz and above |
Abbreviations: HL, hearing loss; SNHL, sensorineural hearing loss.
Notwithstanding the mismatch in Grade 4's indication for cochlear implant when the degree of hearing loss is nowhere near cochlear implant audiological candidacy, the reader can appreciate that the increasing grade of adverse event correlates with increasing severity and need for clinical intervention.
Thus, physicians (particularly oncologists) are familiar with and rely on grading scales for severity of a condition stemming from medical therapy, and understandably need clear definitions of severity. This reliance on grading scales carries the stark reality that decisions related to whether or not to continue with chemotherapy may hinge on whether or not an adverse event reaches Grade 2 versus Grade 3 in severity. Cancer therapy protocols advise the oncologist to consider reducing the dose of (or eliminating) of an ototoxic chemotherapy if a patient is documented to have increasingly severe adverse events (such as having a Grade 3 hearing loss). 11 Reduction in chemotherapy may jeopardize treatment efficacy; that is, the oncologist risks the child's life to lessen the risk for hearing loss from chemotherapy.
Risk of hearing loss from increasing cumulative dose of ototoxic medications extends beyond chemotherapy. Many bacterial infections (including tuberculosis, sepsis in newborns, pulmonary infections stemming from cystic fibrosis, and infections secondary to heart conditions) are treated with aminoglycoside antibiotics. 12 13 Aminoglycosides may be used to treat acute disease, or may be used as prophylaxis against opportunistic infections; they are widely used around the world, are inexpensive, and highly effective at treating a wide range of gram-negative bacteria. 13 With increasing cumulative dose of aminoglycoside antibiotics, risk of hearing loss increases. Aminoglycoside ototoxicity may be amplified by coadministration of other potentially ototoxic agents, such as platinum-based chemotherapy, loop-inhibiting diuretics, vancomycin, and noise exposure. 12 14 15 The prescribing physician faces a dilemma analogous to the oncologist: increase cumulative dose of the medication and increase likelihood of survival, at the risk of inflicting life-long, life-altering disability.
The audiologist may find himself or herself in a self-contradictory position with regard to advising the prescribing physician. The audiologist's principal role in the care of a patient receiving ototoxic medications is to provide data to the managing physician in the form of audiological data (audiogram, auditory brainstem response [ABR] threshold measures, or tinnitus survey results). Concurrent but secondary roles for the audiologist include anticipating audiological interventions (hearing aids, assistive listening devices, tinnitus therapy) and counseling the patient and/or family of the likelihood of a hearing loss, and what can be done to ameliorate the negative consequences of hearing loss and tinnitus.
The audiologist's contradictory position may be one of advocating for minimizing the risk for hearing loss (such as alerting the physician of a significant decrease in hearing relative to the last audiogram), while at the same time promoting the success of well-fitted, objectively verified hearing aids. To the audiologist, a CTCAE v5.0 Grade 3 hearing loss is typically not a clinical challenge. Mitigating the negative consequences of sensorineural hearing loss is fundamental to the audiologist's role, with carefully documented clinical practice guidelines 16 and seminal studies in the scientific literature supporting the efficacy of audiological treatment. 17 Receiver-in-the-ear/receiver-in-the-canal behind-the-ear hearing aids can very effectively provide access to fricatives, morphemes, and other subtle acoustic cues of speech, while managing the occlusion effect, in children with normal hearing in the lower speech frequencies and a high-frequency hearing loss. Custom earmolds can be adequately vented to allow access to low-frequency ambient sound and mitigate occlusion, while still providing stability in the ear and consistent performance.
Provision of remote microphone technology for use in classroom or other challenging listening environments, with or without hearing aids, can be very effective at providing a child with hearing loss with equal access to classroom instruction. The prescribing physician almost certainly does not have the benefit of the perspective of the audiologist, that a hearing loss can be managed, with good results. When outcomes are successful, the hearing loss does not singularly define the individual; it is one characteristic of an otherwise complex person.
Numerous ototoxicity monitoring protocols have been published, but in the clinical setting, uniformity is key. In children requiring audiological monitoring, they may be ill and have minimal ability to participate in behavioral audiometry. Several ototoxicity monitoring protocols rely on a change in hearing from a baseline audiogram. 1 While establishing baseline hearing sensitivity is ideal, this is often not possible. Other scales have subjectivity associated with them (e.g., scales which classify a grade according to need for hearing aids). Others (e.g., Brock's scale) are not sufficiently sensitive to early ototoxicity. The most recently proposed international grading scale is the SIOP Boston grading scale. 1 It does not require baseline audiogram, is sensitive to small changes in high-frequency hearing, is relevant to reduced audibility of important speech sounds, and requires very few frequencies to successfully assign an adverse event grade. The SIOP Boston grading scale is shown in Table 2 .
Table 2. SIOP Boston Ototoxicity Grading Scale.
| Grade 0 | ≤ 20 dB HL at all frequencies |
| Grade 1 | > 20 dB HL (i.e., 25 dB HL or greater) SNHL above 4,000 Hz (i.e., 6 or 8 kHz) |
| Grade 2 | > 20 dB HL SNHL at 4,000 Hz and above |
| Grade 3 | > 20 dB HL SNHL at 2,000 Hz or 3,000 Hz and above |
| Grade 4 | > 40 dB HL (i.e., 45 dB HL or more) SNHL at 2,000 Hz and above |
Abbreviations: HL, hearing loss; SNHL, sensorineural hearing loss.
While an easily applied grading system is important in the clinical setting, it is also imperative for research into improved outcomes. Extensive work in otoprotectant agents to reduce occurrence and severity of chemotherapy-induced hearing loss (and noise-induced hearing loss) has been underway for decades. 18 To compare across multiple clinical trial sites, and across multiple studies, outcomes must be uniform. If a safe and effective otoprotective agent could be identified, oncologists may be able to treat the cancer more aggressively without hearing loss consequences.
Determining factors that influence an individual's risk for hearing loss from an ototoxic agent can require large numbers of research subjects, as there is wide variability in susceptibility across the population. Sixty percent of children treated with platinum-based chemotherapy have sensorineural hearing loss, 1 but what are the factors that cause a person to be in the 40% who does not have hearing loss following treatment? Individual clinics may not have a large enough number of patients to provide an appropriate sample size, so multisite studies are sometimes necessary to tease out subtle results. Outcome measures must be standardized across study sites. When specific risk factors for ototoxicity are identified, patients (and families) can be appropriately counseled in advance of the onset of hearing loss, which can improve the timeliness of hearing loss diagnosis, and lessen the emotional impact of the hearing loss diagnosis. 19
Application
To successfully apply a grading scale, an adequate number of frequencies must be tested and threshold of hearing sensitivity documented. The SIOP Boston panel of experts recommended a minimum test battery to assist the clinical audiologist in prioritizing order of frequencies to test to successfully apply the SIOP Boston grading scale. 1 This minimum test battery is not intended to replace full diagnostic audiological assessment. Rather, this minimum test battery recognizes the challenge of engaging a sick child in behavioral audiometry. The minimum test battery is shown in Fig. 1 .
Figure 1.
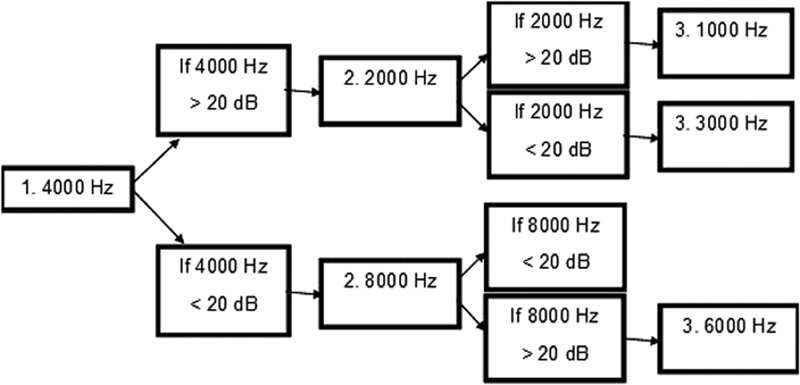
SIOP Boston minimum test battery. This test battery requires only two to three frequencies to apply the SIOP Boston grading scale to the patient's audiogram.
Fig. 2(A–E) reflects possible hearing outcomes of children treated with platinum-based chemotherapy. SIOP Boston Grades 0 to 4 are reflected in the greater degrees of hearing loss, and none require more than three frequencies to be tested.
Figure 2.
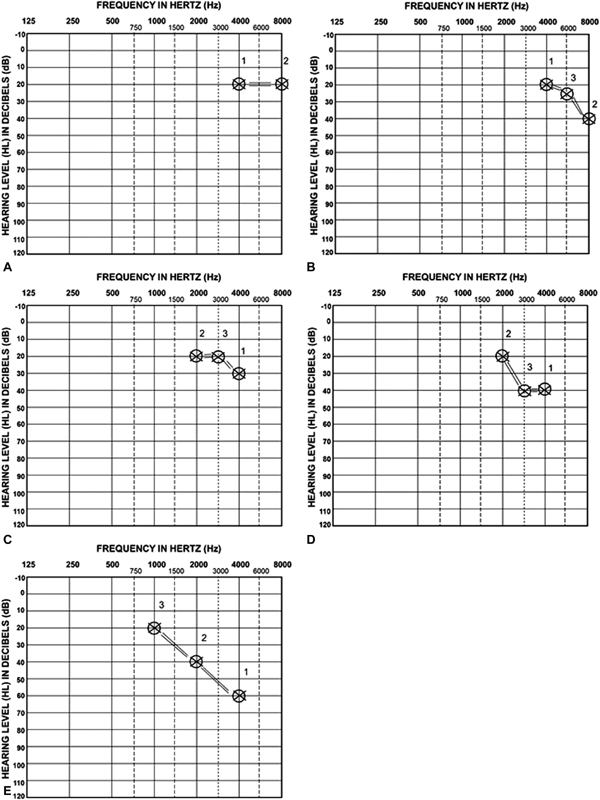
( A ) SIOP Boston Grade 0. According to the minimum test battery, 4,000 Hz is tested first (denoted “1”) and with hearing threshold ≤ 20 dB HL, 8,000 Hz is tested second (denoted “2). With 8,000 Hz ≤ 20 dB HL, testing is done. ( B ) SIOP Boston Grade 1. According to the minimum test battery, 4,000 Hz is tested first (denoted “1”) and with hearing threshold ≤ 20 dB HL, 8,000 Hz is tested second (denoted “2). With 8,000 Hz > 20 dB HL, 6,000 Hz is tested third (denoted “3”). ( C ) SIOP Boston Grade 2. According to the minimum test battery, 4,000 Hz is tested first (denoted “1”) and with hearing threshold > 20 dB HL, 2,000 Hz is tested second (denoted “2). With 2,000 Hz ≤ 20 dB HL, 3,000 Hz is tested third (denoted “3”). ( D ) SIOP Boston Grade 3. According to the minimum test battery, 4,000 Hz is tested first (denoted “1”) and with hearing threshold > 20 dB HL, 2,000 Hz is tested second (denoted “2). With 2,000 Hz ≤ 20 dB HL, 3,000 Hz is tested third (denoted “3”). ( E ) SIOP Boston Grade 4. According to the minimum test battery, 4,000 Hz is tested first (denoted “1”) and with hearing threshold > 20 dB HL, 2,000 Hz is tested second (denoted “2). With 2,000 Hz > 20 dB HL, 1,000 Hz is tested third (denoted “3”).
Additional Tests of Auditory Function
Currently, efforts are underway to validate the SIOP Boston Scale, and to compare the effectiveness of this scale against previous ototoxicity grading scales and other measures of auditory function. 20 While ABR evoked potential threshold measures, distortion product otoacoustic emissions (DPOAEs), and extended high-frequency (EHF) audiometry show potential for greater sensitivity to detecting early ototoxicity, their inclusion in ototoxicity test batteries is of limited benefit for the sake of grading an ototoxic hearing loss and providing the medical team with necessary data to make treatment recommendations. Currently, these measures are adjunct to the conventional audiogram, and cannot be used instead of behavioral audiometry 20 (with the potential exception of ABR, when necessary).
ABR evoked potential threshold measures have been used in lieu of behavioral audiometry in children too ill or too young to provide an audiogram. While objective test measures are standard in pediatric audiology, use of ABR thresholds has not yet been validated for use in chemotherapy protocols. 21 Click-evoked ABR is not frequency specific enough to detect differences in SIOP Boston Grade 0 versus Grade 1, and cannot detect differences between Grade 2 and Grade 3. Click-ABR should be able to consistently detect the difference between Grade 0 and Grade 4. Tone-burst ABR (e.g., 1,000, 2,000, and 4,000 Hz tone-burst) should be able to consistently detect the difference between Grades 0, Grade 2, and Grade 4. Audiologists are advised to use best clinical decision making, and communicate to the referring physician the limitations of the testing method. For instance, it is unlikely that commercially available tone-burst ABR using higher frequency tone-bursts (e.g., 8,000 Hz signal applied to the insert earphone) actually tests frequencies above 4,000 Hz. 22 23
DPOAEs have the potential to detect changes in cochlear function before a threshold shift is observed on the pure-tone audiogram. 24 DPOAEs have been shown to be efficacious at showing chemotherapy-induced cochlear changes. 24 25 However, as with ABR evoked potential threshold measures, chemotherapy protocols have not yet validated the use of DPOAEs for the sake of applying a grading scale. For instance, if DPOAEs show a significant shift in OAE amplitude, what should the oncologist do with this information? What is the functional effect on the patient's hearing ability? Lacking answers to these questions, the use of DPOAEs in ototoxicity grading is moot. The clinical audiologist is advised to use best practice and include all appropriate audiometric test results, but currently, DPOAEs cannot be used in place of pure-tone audiometry.
EHF audiometry is more sensitive to early ototoxicity than conventional audiometry. 24 26 Given that ototoxic medications lesion the cochlea from the base (high-frequency tuned region) to the apex (low-frequency tuned region), it would logically follow that EHF audiometry will detect ototoxic changes earlier than conventional audiometry. However, EHF is best applied by comparing the individual's baseline to serial audiograms. As a matter of practicality, EHF audiometric equipment is not consistently available in audiology clinics.
Absent from most reviews of ototoxicity, and most ototoxicity grading scales, is the diagnosis and management of tinnitus. It has been estimated that 40% of patients receiving chemotherapy develop tinnitus. 21 This auditory condition is not so easily documented for severity as a pure-tone threshold shift on an audiogram, but patients with tinnitus and hearing loss often describe the tinnitus as having a more significant impact on quality of life. 27
The NCI CTCAE v5.0 is one grading scale that does include tinnitus as an adverse event associated with chemotherapy ototoxicity, 10 and defines it according to Grade 1: “mild symptoms, interventions not indicated”; Grade 2: “moderate symptoms, limiting instrumental activities of daily life [ADL]”; and Grade 3: “severe symptoms, limiting self-care ADL.” While it is encouraging that tinnitus appears on a grading scale for chemotherapy adverse events, the grading is highly subjective, and does not consider that more severe tinnitus could exist (e.g., Grade 4 or 5). Included in several validated tinnitus severity inventories are questions related to suicidal thought or ideation, 28 and patients with comorbid depression and tinnitus have taken their own life.
The most recent and widely used tinnitus inventory is the tinnitus functional index (TFI). 29 This self-administered survey was written with adults as the target audience, but wording can be adjusted to reflect a child's experience (for instance, exchanging words “work or other tasks” for “school work or homework”). 30 A child or adolescent's reaction to his or her tinnitus is often similar to an adult, in that the tinnitus interferes with sleep and concentration, and those with more severe reactions find it interferes with their ability to relax or cope with daily life. The TFI was designed to categorize severity of tinnitus (from no problem to very severe, requiring specialized intervention), and to document response to therapy. Future adverse events in ototoxicity grading scales specific to drug-induced tinnitus should include validation of the TFI as a starting point for assessing severity of tinnitus.
Conclusions
Audiologists provide a vital service to the referring physician, but one that is quite limited in scope, relative to the breadth of the physician's focus. With 837 categories of adverse events in the NCI CTCAE v5.0, it is understandable that simple, clearly defined grades of severity are necessary for any one category. While sensorineural hearing loss is a complex condition requiring careful diagnosis and management, it is necessary that the reporting of results be brief and specific.
Pure-tone audiometry is challenged in young children and in those who are ill, so a minimum test battery has been proposed to increase likelihood of successfully providing a brief and specific result of the hearing test. A complete audiological evaluation is indicated when the child is well enough or mature enough to engage in a longer test battery. Pure-tone audiometry cannot yet be replaced with ABR thresholds, DPOAEs, or EHF audiometry. These diagnostic tools should be incorporated into the test battery to supplement the conventional pure-tone audiogram.
Quick identification of hearing loss, should it occur, is facilitated by routine audiological monitoring. When hearing loss is likely, based on the child's diagnosis and specific treatment course, the audiologist should review with the family (and child, as appropriate) the potential impact on hearing and communication, and review audiological treatment options. The referring physician should be kept informed of the audiological interventions, including hearing aids, educational interventions, and tinnitus therapy.
The children who require administration of ototoxic medications are among the most ill, and families are often in a fragile state. The audiologist can provide guidance for this extremely important area of the child's development, and when an adverse event occurs, happily effective treatments are available.
Footnotes
Conflict of Interest None.
References
- 1.Brock P R, Knight K R, Freyer D R et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30(19):2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garinis A C, Kemph A, Tharpe A M, Weitkamp J H, McEvoy C, Steyger P S. Monitoring neonates for ototoxicity. Int J Audiol. 2018;57 04:S41–S48. doi: 10.1080/14992027.2017.1339130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handelsman J A, Nasr S Z, Pitts C, King W M. Prevalence of hearing and vestibular loss in cystic fibrosis patients exposed to aminoglycosides. Pediatr Pulmonol. 2017;52(09):1157–1162. doi: 10.1002/ppul.23763. [DOI] [PubMed] [Google Scholar]
- 4.Laurell G, Bagger-Sjöbäck D. Dose-dependent inner ear changes after i.v. administration of cisplatin. J Otolaryngol. 1991;20(03):158–167. [PubMed] [Google Scholar]
- 5.Boothroyd A. Developmental factors in speech recognition. Int J Audiol. 1970;9:30–38. [Google Scholar]
- 6.Stelmachowicz P G, Pittman A L, Hoover B M, Lewis D E, Moeller M P. The importance of high-frequency audibility in the speech and language development of children with hearing loss. Arch Otolaryngol Head Neck Surg. 2004;130(05):556–562. doi: 10.1001/archotol.130.5.556. [DOI] [PubMed] [Google Scholar]
- 7.Bess F H, Dodd-Murphy J, Parker R A. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19(05):339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Gurney J G, Tersak J M, Ness K K, Landier W, Matthay K K, Schmidt M L; Children's Oncology Group.Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children's Oncology Group Pediatrics 200712005e1229–e1236. [DOI] [PubMed] [Google Scholar]
- 9.Brock P R, Bellman S C, Yeomans E C, Pinkerton C R, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol. 1991;19(04):295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute Common Terminology Criteria for Adverse Events v5.0. Cancer Therapy Evaluation Program. Available at:https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5.0.xlsx. Published November 27,2017, accessed November 14, 2018
- 11.Minasian L M, Frazier A L, Sung L et al. Prevention of cisplatin-induced hearing loss in children: informing the design of future clinical trials. Cancer Med. 2018;7:2951–2959. doi: 10.1002/cam4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang M, Karasawa T, Steyger P S. Aminoglycoside-induced cochleotoxicity: a review. Front Cell Neurosci. 2017;11:308. doi: 10.3389/fncel.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause K M, Serio A W, Kane T R, Connolly L E. Aminoglycosides: an overview. Cold Spring Harb Perspect Med. 2016;6(06):a027029. doi: 10.1101/cshperspect.a027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah S U, Anjum S, Littler W A. Use of diuretics in cardiovascular diseases: (1) heart failure. Postgrad Med J. 2004;80(942):201–205. doi: 10.1136/pgmj.2003.010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Steyger P S. Synergistic ototoxicity due to noise exposure and aminoglycoside antibiotics. Noise Health. 2009;11(42):26–32. doi: 10.4103/1463-1741.45310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Academy of Audiology.Pediatric amplification: clinical practice guidelinesPublished June 2013. Available at:https://www.audiology.org/sites/default/files/publications/PediatricAmplificationGuidelines.pdf. Accessed November 21, 2018
- 17.Sininger Y S, Grimes A, Christensen E. Auditory development in early amplified children: factors influencing auditory-based communication outcomes in children with hearing loss. Ear Hear. 2010;31(02):166–185. doi: 10.1097/AUD.0b013e3181c8e7b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell K C, Martin S M, Meech R P, Hargrove T L, Verhulst S J, Fox D J. D-methionine (D-met) significantly reduces kanamycin-induced ototoxicity in pigmented guinea pigs. Int J Audiol. 2016;55(05):273–278. doi: 10.3109/14992027.2016.1143980. [DOI] [PubMed] [Google Scholar]
- 19.Fligor B J, Neault M W, Mullen C H, Feldman H A, Jones D T. Factors associated with sensorineural hearing loss among survivors of extracorporeal membrane oxygenation therapy. Pediatrics. 2005;115(06):1519–1528. doi: 10.1542/peds.2004-0247. [DOI] [PubMed] [Google Scholar]
- 20.Knight K R, Chen L, Freyer D et al. Group-wide, prospective study of ototoxicity assessment in children receiving cisplatin chemotherapy (ACCL05C1): a report from the Children's Oncology Group. J Clin Oncol. 2017;35(04):440–445. doi: 10.1200/JCO.2016.69.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dille M F, Ellingson R M, McMillan G P, Konrad-Martin D. ABR obtained from time-efficient train stimuli for cisplatin ototoxicity monitoring. J Am Acad Audiol. 2013;24(09):769–781. doi: 10.3766/jaaa.24.9.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rance G, Roper R, Symons L et al. Hearing threshold estimation in infants using auditory steady-state responses. J Am Acad Audiol. 2005;16(05):291–300. doi: 10.3766/jaaa.16.5.4. [DOI] [PubMed] [Google Scholar]
- 23.Vander Werff K R, Prieve B A, Georgantas L M. Infant air and bone conduction tone burst auditory brain stem responses for classification of hearing loss and the relationship to behavioral thresholds. Ear Hear. 2009;30(03):350–368. doi: 10.1097/AUD.0b013e31819f3145. [DOI] [PubMed] [Google Scholar]
- 24.Knight K R, Kraemer D F, Winter C, Neuwelt E A. Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25(10):1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- 25.Dille M F, McMillan G P, Reavis K M, Jacobs P, Fausti S A, Konrad-Martin D. Ototoxicity risk assessment combining distortion product otoacoustic emissions with a cisplatin dose model. J Acoust Soc Am. 2010;128(03):1163–1174. doi: 10.1121/1.3473693. [DOI] [PubMed] [Google Scholar]
- 26.Dille M F, Wilmington D, McMillan G P, Helt W, Fausti S A, Konrad-Martin D. Development and validation of a cisplatin dose-ototoxicity model. J Am Acad Audiol. 2012;23(07):510–521. doi: 10.3766/jaaa.23.7.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatt J M, Lin H W, Bhattacharyya N. Tinnitus epidemiology: prevalence, severity, exposures and treatment patterns in The United States. JAMA Otolaryngol Head Neck Surg. 2016;142:959–965. doi: 10.1001/jamaoto.2016.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall D A, Fackrell K, Li A B et al. A narrative synthesis of research evidence for tinnitus-related complaints as reported by patients and their significant others. Health Qual Life Outcomes. 2018;16(01):61. doi: 10.1186/s12955-018-0888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meikle M B, Henry J A, Griest S E et al. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33(02):153–176. doi: 10.1097/AUD.0b013e31822f67c0. [DOI] [PubMed] [Google Scholar]
- 30.Fligor B J. Audiological evaluation and management of teenagers with tinnitus. ENT Audiol News. 2017;25:93–94. [Google Scholar]


