Abstract
The main goal of head and neck reconstruction is the restoration of form and function. Oncologic surgery makes this process more complex, as the preplanned defect can be very different from its intraoperative counterpart. This emphasizes the role of preoperative planning and a diverse reconstructive “tool box” that can accommodate a variety of complicated defects. The other reconstructive goals are determined by the patient with the aid of an interdisciplinary team. While multiple local and regional reconstructive options are available, free tissue transfer provides a versatile and reliable option for reconstruction—especially for complex orbital defects. Here the authors discuss free soft tissue transfer options for orbital exenteration. This review will catalog the advantages and disadvantages of the radial forearm, rectus abdominis, latissimus, and anterolateral thigh.
Keywords: orbital reconstruction, orbital exenteration, free tissue transfer, head and neck reconstruction, soft tissue reconstruction
Orbital exenteration (OE) involves resection of the globe and orbital soft tissues. 1 2 Ablative surgery of the orbit can create a challenging anatomic defect, involving bony anatomy, the skull base, the periorbital soft tissues, and the skin. 3 4 5 6 Reconstructive options range from split thickness skin grafts and healing by secondary intention to local and regional pedicle muscle or fascia flaps and free tissue transfer. 6 7 8 9 10 While multiple options have proven effective, large exenteration defects often expose the paranasal sinuses, the skull base, and/or the cavernous sinus. Without appropriate coverage and separation of the intracranial anatomy from the oral and paranasal cavities, there is a risk of infection and pneumocephalus. 3 6 11 Patients with a history of prior radiation or surgery represent a distinct risk because skin grafts, adjacent tissue transfer, or regional flaps may be less reliable due to poor local tissue quality and unfavorable angles of rotation ( Fig. 1 ). 6 Free tissue transfer provides a versatile tool that can be designed to accommodate the defect and provide adequate coverage with vascularized nonirradiated tissue. 8 12 13
Fig. 1.
Skin graft reconstruction of the orbit: provides contour to accommodate prosthetic restoration, however may not completely heal in areas where the underlying tissue bed is compromised.
Typically, OE is performed for locally advanced malignancy, commonly squamous cell carcinoma, basal cell carcinoma, or melanoma. 5 14 15 As a result, patients are often left with a significant functional and cosmetic defect that may have a significant psychological impact on a patient and their family. 9 16 Free tissue transfer affords a variety of tissue flaps to manage complex defects as well as provide protection of the skull base, replace the orbital volume, and create a skin envelope adequate for future prosthetic restoration. 3 9 12 13 17 18
To this end, multiple soft tissue free flaps have been employed, most commonly the radial forearm (RF), rectus abdominis (RA), latissimus (LAT), and anterolateral thigh (ALT). 2 8 10 19 20 21 22 23 Herein we will discuss closed cavity reconstruction and highlight the advantages and limitations of free tissue transfer for replacing the globe and periorbital volume. 16 18
Goals of Reconstruction
The goals of orbital reconstruction after OE include restoration of safe anatomic barriers, cosmetic restoration, and tolerating adjuvant radiation if needed. 5 18 With these goals in mind, surgical reconstructive planning should be centered around the patient's goals. 11 12 16 This starts with the understanding the patient's expectations and postoperative goals. Next, the patient's medical comorbidities take paramount importance in deciding the appropriate reconstructive option. While free tissue transfer may be ideal from an anatomic perspective, there are select patients who cannot safely tolerate the cardiopulmonary burden of such a surgery. Though this does not preclude them from free tissue transfer, it does make non-free flap reconstructions more appealing.
The reconstructive goal of separating key anatomic boundaries is critical in preventing infection, wound breakdown, and the long-term morbidity of communication between the external environment, nasal passage, and skull base. OE defects can be accompanied by large midface or palatal defects, depending on the location and size of the primary lesion. As a result, OE defects often leave a large “dead space” cavity to be filled. Ablation of the dead space is performed to ensure a watertight and airtight closure to limit opportunistic infections within the cavity. 18 Soft tissue reconstruction can be coupled with a palatal obturator to additionally improve functional outcomes. 24 Ultimately, vascularized soft tissue can achieve both of these goals as a durable volume filling barrier and a platform for reconstruction.
The need for postoperative adjuvant therapy such as radiation can help guide options as well. Soft tissue flaps with well vascularized adipose tissue can withstand high doses of radiation without critical wound breakdown. 3 The discussion of postoperative and oncologic management should be started early with the patient's interdisciplinary team.
Finally, the reconstructive surgeon must consider the patient's plans for postoperative cosmesis. If a patient prefers wearing a patch rather than using a prosthesis, this allows a soft tissue flap to be more astutely tailored. 16
While no specific reconstruction can do it all, patient-focused outcomes can help the reconstructive surgeon choose between the multitude of free tissue transfer options. For OE defects, free tissue transfer can have several advantages—volume, versatility, and durable tissue in comparison to local grafting, advancements, and regional flaps.
Preoperative Planning
Orbital exenteration is an extensive surgery. In turn, thorough preoperative planning with the patient and an interdisciplinary team is essential. The work-up begins with a diagnostic work-up that may include a computed tomography (CT) scan for hard tissue evaluation, a magnetic resonance imaging (MRI) scan for soft tissue evaluation, and a positron emission tomography (PET) scan for evaluation of distant and subclinical disease. Once a formal histopathologic diagnosis is achieved, patients may benefit from evaluation by an institutional tumor board where the likelihood of disease control and the role of adjuvant therapy are determined. Once OE has been agreed upon, patient comorbidities should be optimized, including an appropriate cardiac, pulmonary, and/or general medical work-up. In addition, patients should be introduced to a postoperative management plan and consult with occupational therapy, physical therapy, radiation oncology, and ophthalmology teams.
After an appropriate diagnostic work-up, surgical planning is the next. The literature is replete with approaches and systems for staging OE defects and algorithms for reconstruction. 1 16 25 26 Hanasono et al created an approach to reconstruction, based on patient-centered goals, use of a prosthesis, needs for adjuvant therapy, and soft tissue revision. 16 More recently, Kesting et al described a defect-driven system that classifies reconstructive options. 1 Both discuss the role of free tissue transfer as essential to managing OE patients, and long-term patient goals and management-driven surgical therapy.
Free Tissue Transfer
While OE ablations can result in complicated soft tissue and bony defects, the focus for this review is on the application of soft tissue free tissue transfer to meet the established goals. Donor site harvest has been well described, including those with anatomic variability, thus this review will focus on qualities of the more common soft tissue flaps in OE reconstruction rather than dissection technique. 27
Radial Forearm
The RF free flap has been used and described for nearly half a century. 28 It has been well established as a versatile and reliable reconstructive option with predictable anatomy. Known as a pliable soft tissue flap with a long vascular pedicle, the RF is commonly used for smaller midface defects. 29 30 In the head and neck, regional flaps can be bulky and limited by rotation, while the RF can surpass these limitations with a long pedicle tunneled through midface subcutaneous tissues and in turn effectively eliminate dead space. 31 In addition, a distant harvest site allows for a two-team simultaneous harvest.
The RF is unique in that with a lengthy pedicle and reliable perforators, multiple iterative folding patterns, skin islands, and subcutaneous dissection beyond the skin paddle can be carried out. 25 30 32 Cordeiro and Santamaria described the comprehensive roles the RF can play, with successful contouring, symmetry, and texture in the head and neck ( Figs. 2 3 4 5 6 ). 25
Fig. 2.
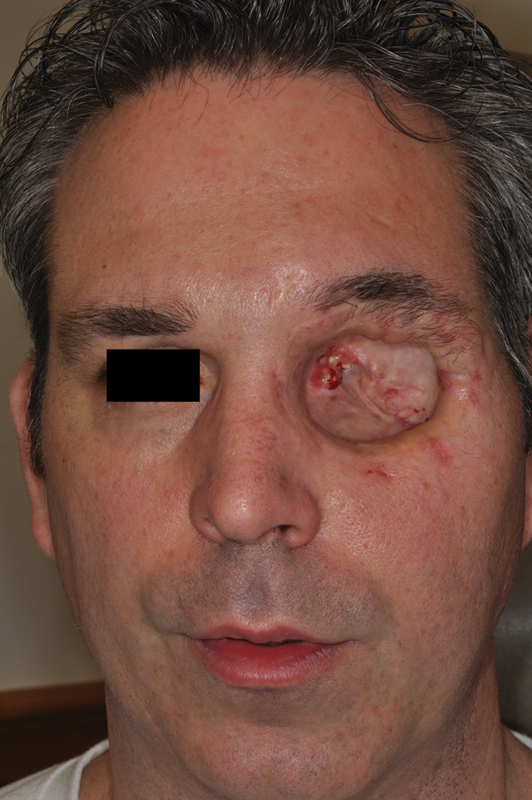
Preoperative photo of invasive malignancy requiring extended orbital exenteration.
Fig. 3.
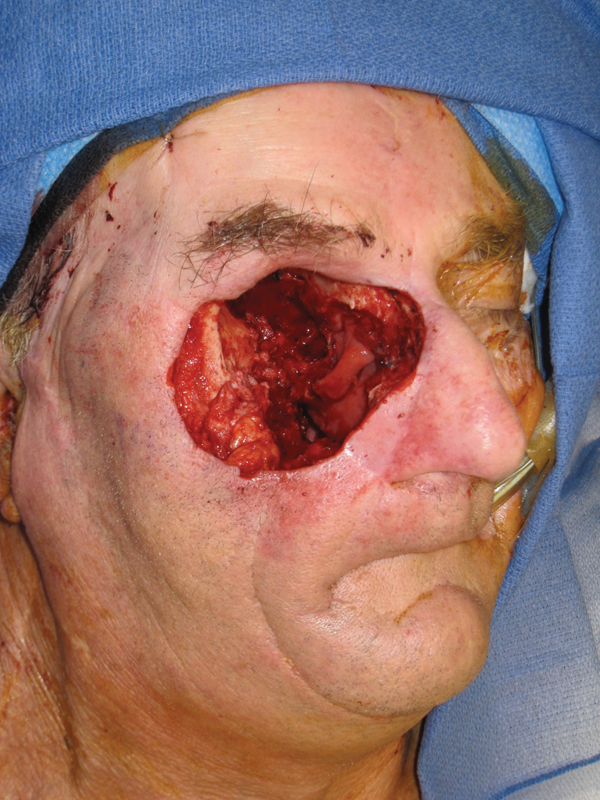
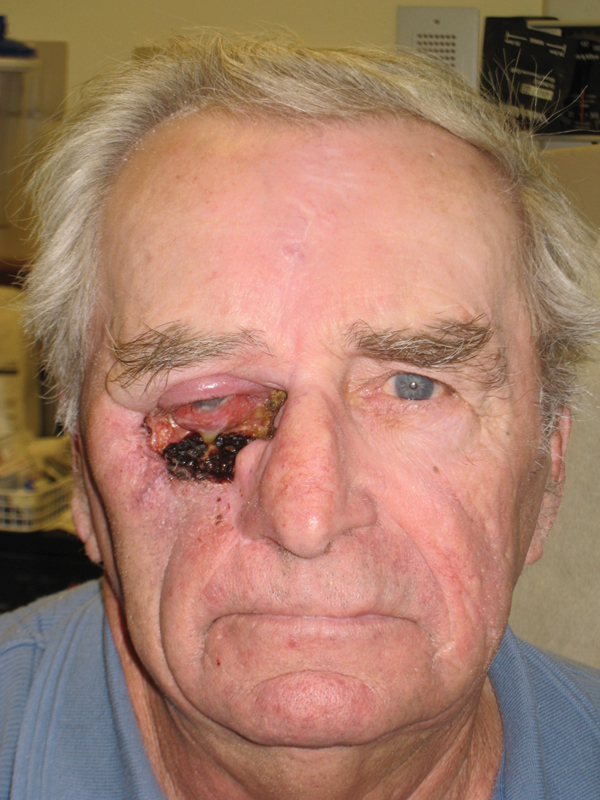
Intraoperative photo demonstrating composite resection and complex defect.
Fig. 4.
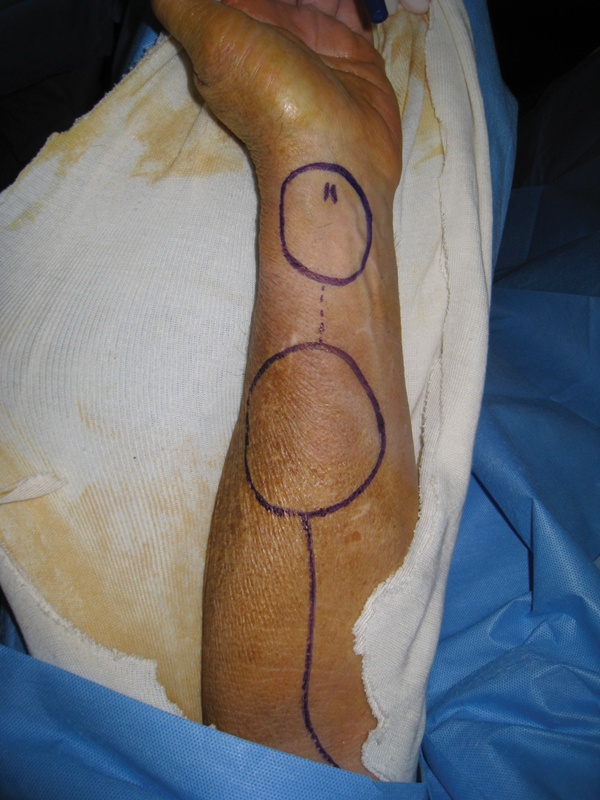
Intraoperative photo showing radial forearm free flap markings and planning of double-paddle reconstruction for watertight closure.
Fig. 5.
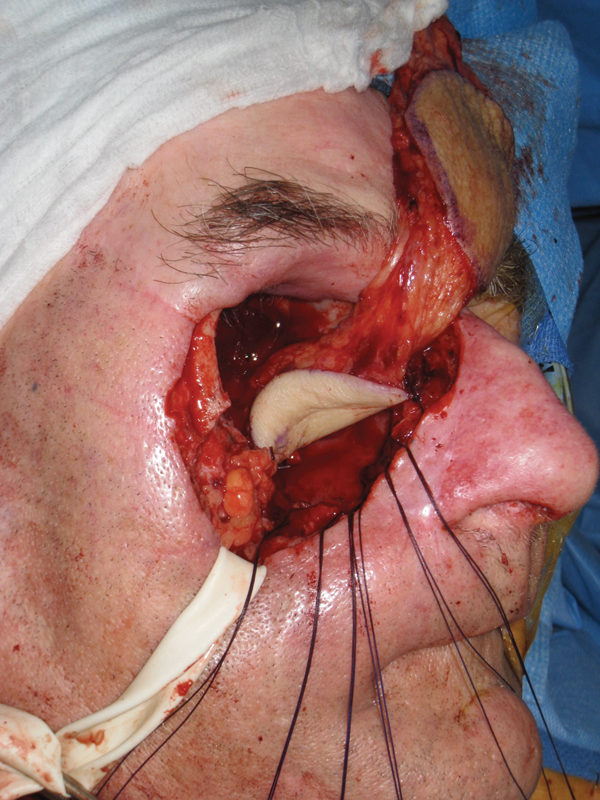
Intraoperative photo of dual-paddle parachuting inset.
Fig. 6.
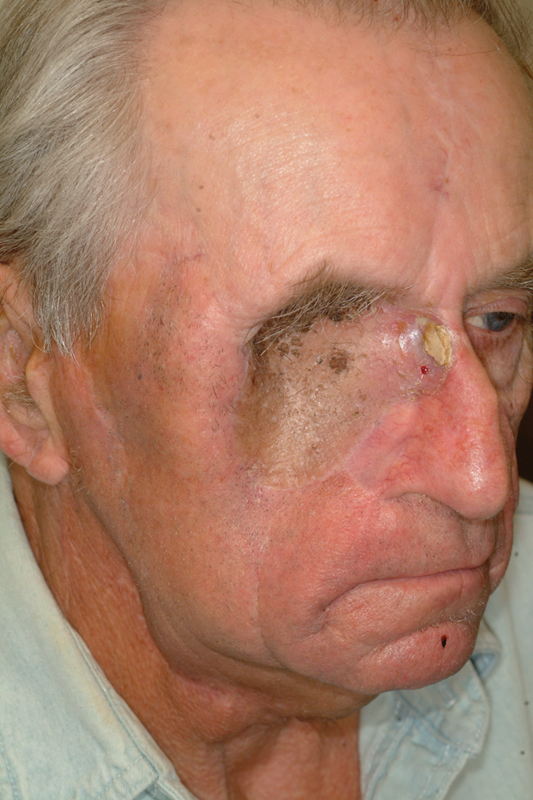
Postoperative photo.
Although the most commonly raised soft tissue flap in head and neck reconstruction, the RF is not without its limitations. In large OE defects, it cannot adequately fill dead space due to a lack of a large adipose or muscular component. In addition, it has a higher than usual donor-site morbidity when compared to others—sacrifice of a major artery, need for skin grafting, tendon exposure, cosmesis, and poor aesthetics. The advent of negative pressure dressings has greatly improved donor-site healing; however, wound management can still comprise a significant part of the postoperative period. 3 30
Finally, in many cases, patients treated with OE for malignancy have either been previously treated with external beam radiotherapy or will require postoperative adjuvant radiotherapy. In the former situation, healing of the recipient orbital bed will be compromised. Vascularized tissue is critical; however, we believe that vascularized muscle may heal more reliably in a compromised bed than a fasciocutaneous flap. This is particularly true for larger defects with more complex defects. To our knowledge, there is no randomized data to support this contention.
Although beyond the scope of this review, multiple studies have recently described the pros and cons of the osteocutaneous RF, with additional variations including sandwiching of soft tissue and a double barrel technique to reestablish the midface boney framework. 25 26 33
Rectus Abdominis
The RA flap is a musculocutaneous flap that has a long history of use in the repair of OE defects. 13 34 35 36 Though its popularity has waned due to well-documented morbidities and alternatives, in certain patients, it meets critical reconstructive needs. 3 As a hardy muscular flap, the RA can be used similarly to a regional pedicled flap like pectoralis but without the limitations of unappealing bulk or rotational arc. 10 In addition, the abdominal wall adipose tissue and fascia compose a large voluminous free tissue transfer. 35 The RA has a lengthy pedicle that can easily be tunneled through the midface. Similar to RF, it can be easily harvested as a two-team approach without having to change patient position.
The major benefit of the RA flap is its vascularized muscle and the ability to harvest a large cutaneous skin paddle. This donor site is ideal for larger defects with a complex skin defect. Another advantage is that the donor site can be closed primarily. 26 Multiple studies have cited the utility of the RA as a volume replacement flap, preventing the associated wound breakdown of thinner reconstructive options. 36 Using the tensile strength of the rectus sheath, flap volume can be effectively shaped and contoured to the midface, including extended OE defects where the palate needs reconstruction as well. 21 The ability to harvest a narrow proximal segment allows it to be easily tunneled to the neck ( Figs. 7 8 9 10 ). 37
Fig. 7.
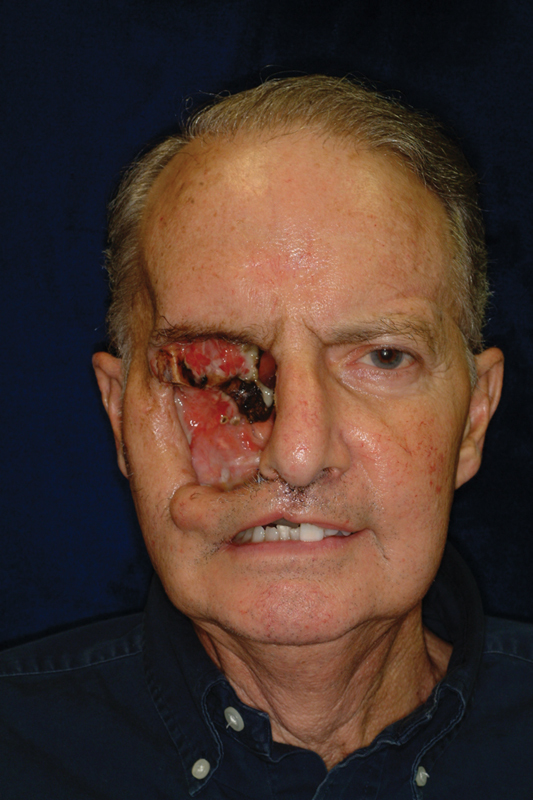
Preoperative photo of invasive eroding mass requiring complex ablation and reconstruction.
Fig. 8.
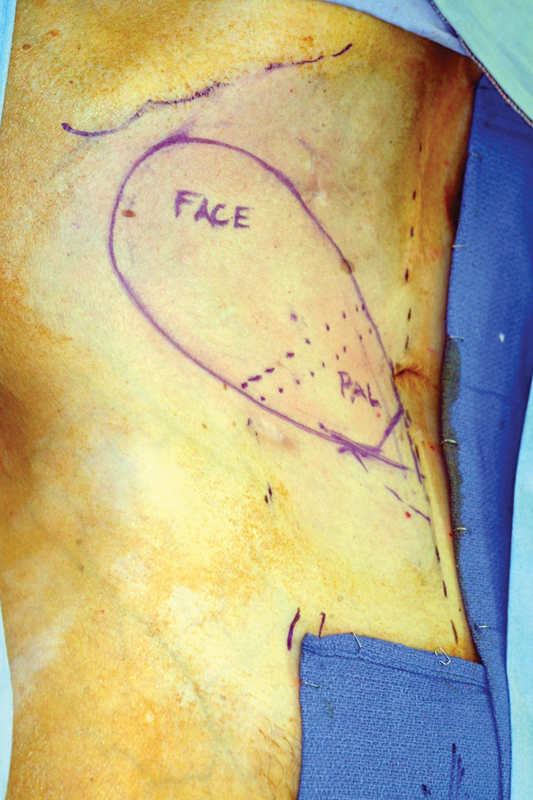
Intraoperative photo showing rectus free flap markings and reconstruction of palate and external skin.
Fig. 9.
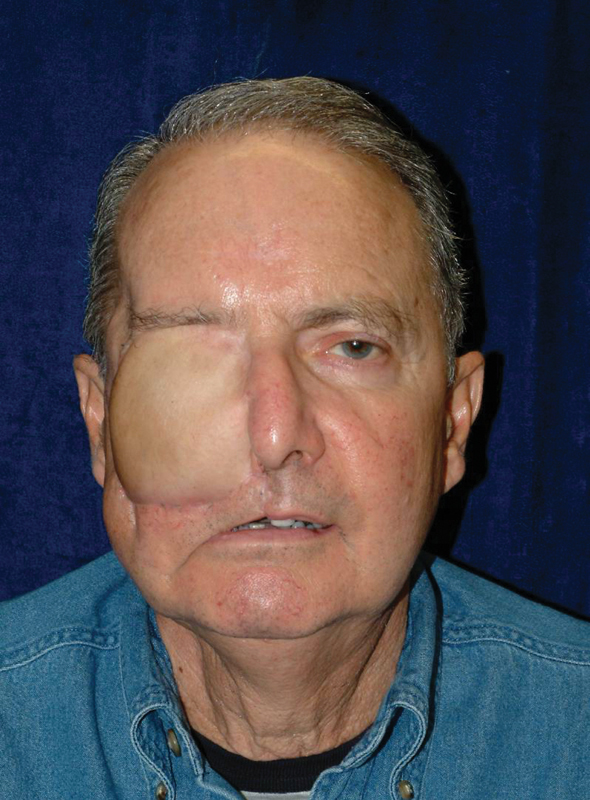
Postoperative photo after discharge.
Fig. 10.

Postoperative photo after adjuvant therapy and fitting with a prosthetic.
The donor site morbidity associated with a RA flap has led many to consider other donor-site options. From the poor aesthetics of an abdominal scar and hair bearing skin over the orbit to complications of bowel herniation, the RA has well-documented donor-site morbidity. 3 10 38 Unlike the RF, the RA cannot be coupled with bone to provide for midface buttress reconstruction. In addition, cachectic patients with thin abdominal walls may not have adequate fatty and muscular bulk to fill a large OE cavity. Finally, a history of abdominal surgery makes the RA a suboptimal option, as patients are at higher risk for wall dehiscence and unpredictable changes in vascular supply. 10 39 In spite of these limitations, Pryor et al recently described the meaningful and relevant role of the RA flap in reconstruction of the orbit. 6 There are few donor-site options that provide a long, vascularized pedicle, vascularized muscle and skin, and the volume required for extended cranio-orbital defect reconstruction.
Latissimus
The LAT free flap is a well-established large soft tissue donor that can be harvested in its entirety with skin, subcutaneous tissue, and muscle, regularly used in head and neck reconstruction. 40 41 While the LAT dorsi flap was initially described as a regional flap, it is most commonly used as a free flap and may be an ideal option for OE reconstruction. 42 The flap thickness is determined by the patient's body habitus; however, this donor site commonly provides skin and muscle that are ideally situated between those of the RF and RA for large complex OE defects, particularly when a concomitant maxilla defect must be addressed. 22 39
The vascular supply of the LAT flap is the thoracodorsal artery, the terminal branch off the subscapular arterial system. Ligation of the branch to the serratus can further extend the pedicle up to 10 cm, allowing a wide range of designs. 43 44 When used as a free flap, the LAT flap easily overcomes the usual limitations of a pedicled flap. 39 45 The LAT can act as a surrogate to patients with previous abdominal surgeries in whom a RA flap carries higher risk. The donor site can easily be closed primarily even when a large skin paddle is harvested. 22
The biggest disadvantage of the LAT is the need to move patients into the lateral decubitus position, preventing a two-team approach. 39 Similar to the RA, multiple revision surgeries may be needed to achieve proper contour and shape the excessive tissue bulk.
The advantages of the LAT include its reliable pedicle anatomy and the versatility to treat multiple defects. It can be designed with multiple skin paddles to address a skull-base defect and a cutaneous defect by creating a watertight seal for both the palate and skull base. 24 42 Uniquely, the LAT muscle can be reinnervated to provide a dynamic facial reanimation. Finally, for combined soft tissue and bony defects, the subscapular system is unique in that it can provide the LAT muscle, the serratus muscle, 39 the scapular and/or parascapular skin paddles, and two distinct vascularized bone flaps. 46 Referred to as the “mega flap,” only the subscapular system of flaps can provide such a diverse flap to address the most complex defects.
Anterolateral Thigh
The ALT is a fasciocutaneous flap based on either musculocutaneous or septocutaneous perforators from the descending branch of the lateral circumflex femoral artery. Introduced in 1984, the ALT has more recently become a workhorse flap in head and neck reconstruction. 47 The ALT is a large volume donor of vascularized adipose tissue and can be sculpted to include skin, fat, tensor fascia lata, and, if needed, vastus lateralis muscle. It can be raised to cover a defect of > 30 cm, closed primarily, carries minimal site morbidity, and requires no wound vacuum-assisted closure, cast, or skin graft. The ALT vascular pedicle can be dissected up to 16 cm, making it ideal for a vessel-depleted neck. In addition, a distant harvest site allows for an expedited seamless two-team approach, without a tourniquet.
In meeting the goals of orbital reconstruction, the ALT is an extremely versatile option with more than adequate soft tissue to both fill dead space and create firm anatomic boundaries. The large soft tissue volume can easily protect critical structures from associated complications and can be thinned to accommodate a prothesis. For multifaceted orbital defects as in OE, the ALT is especially useful. It can be dissected into individual perforators creating multiple soft tissue paddles or a chimeric flap. These chimeras can be a mix of fascia, skin, de-epithelized soft tissue, and adjacent tensor fascia lata. 48 The tensor fascia lata can be used to perform a static facial sling for those cases requiring facial reanimation.
The ALT has well-described limitations; the vascular variation of its perforators is among those most studied. While a well-trained surgeon can carefully dissect out unusual perforator anatomy, it can make a simple harvest challenging. The ALT also lacks a boney component, which necessitates another flap or hardware for larger midface defects. Use of the iliac crest and femur has been described, but regular use is uncommon. ALT donor-site morbidity is minimal; however, postoperative seroma multiple weeks postop is a common occurrence. Finally, the pallor and skin texture of the lateral thigh skin create a stark color mismatch at the recipient site ( Fig. 11 ).
Fig. 11.
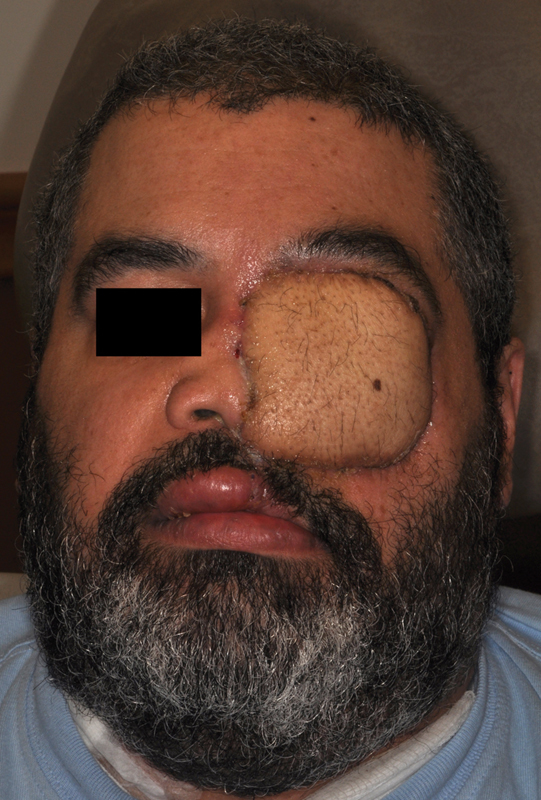
Anterolateral thigh free flap reconstruction of the orbit: provides well-vascularized tissue ideal for large defects and those previously radiated; however, tissue is often bulky and requires surgical revision.
Multiple studies have demonstrated the ALT's newly predominant role in orbital reconstruction. 49 Parkes et al described 33 ALT OE reconstructions, highlighting cases involving anterior skull base defects and layered ALT reconstructions involving de-epithelized segments with a 91% success rate. 2 In 2017, Simsek et al described a case series utilizing the chimeric ALT flap, with a skin and muscular component to obliterate an OE cavity. 8 Studies have shown the role of a chimeric ALT flap in patient-tailored three-dimensional orbito-cranial reconstructions, with multiple paddles off of a single vascular pedicle. 20 Finally, Joo et al showed in 22 patients the role of the chimeric ALT–fascia lata flap for complicated orbital defects to recreate the nasal lining and orbital floor. 48
In conclusion, the ALT's versatility makes it an ideal workhorse flap for orbital reconstruction, regardless of the dissection challenges and minor limitations.
Conclusion
Soft tissue reconstruction of OE presents a complex problem for the head and neck surgeon. While there exists a wide range of reconstructive options, free tissue transfer provides a unique benefit to achieve the goals of a safe and successful reconstruction. Regardless of the method used, the goals remain the same; reliable separation of anatomic boundaries, restoration of function, cosmetic restoration, and reliable wound healing. A thoughtful approach with a multidisciplinary team and a deep understanding of the patient's goals improve the likelihood of a god outcome.
Acknowledgment
The authors would like to thank Jill Gregory (illustrator).
Footnotes
Conflict of Interest None declared.
References
- 1.Kesting M R, Koerdt S, Rommel N et al. Classification of orbital exenteration and reconstruction. J Craniomaxillofac Surg. 2017;45(04):467–473. doi: 10.1016/j.jcms.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Parkes W J, Krein H, Heffelfinger R, Curry J. Use of the anterolateral thigh in cranio-orbitofacial reconstruction. Plast Surg Int. 2011;2011:941742. doi: 10.1155/2011/941742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López F, Suárez C, Carnero S, Martín C, Camporro D, Llorente J L. Free flaps in orbital exenteration: a safe and effective method for reconstruction. Eur Arch Otorhinolaryngol. 2013;270(06):1947–1952. doi: 10.1007/s00405-012-2308-9. [DOI] [PubMed] [Google Scholar]
- 4.Rapidis A D, Liarikos S. Malignant orbital and orbitomaxillary tumors: surgical considerations. Orbit. 1998;17(02):77–88. doi: 10.1076/orbi.17.2.77.2764. [DOI] [PubMed] [Google Scholar]
- 5.Moyer J S, Chepeha D B, Prince M E, Teknos T N. Microvascular reconstruction of the orbital complex. Facial Plast Surg Clin North Am. 2009;17(02):225–237. doi: 10.1016/j.fsc.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Pryor S G, Moore E J, Kasperbauer J L. Orbital exenteration reconstruction with rectus abdominis microvascular free flap. Laryngoscope. 2005;115(11):1912–1916. doi: 10.1097/01.MLG.0000181512.55041.7D. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki C T, Ariyan S, Spencer D, Buckwalter J. Pectoralis major myocutaneous reconstruction of the anterior skull base. Laryngoscope. 1985;95(02):162–166. doi: 10.1288/00005537-198502000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Simsek T, Engin M S, Yildirim K, Kodalak E A, Demir A. Reconstruction of extensive orbital exenteration defects using an anterolateral thigh/vastus lateralis chimeric flap. J Craniofac Surg. 2017;28(03):638–642. doi: 10.1097/SCS.0000000000003430. [DOI] [PubMed] [Google Scholar]
- 9.Rabey N, Abood A, Gillespie P, Athanassoglou V, Rene C, Malata C M. Reconstruction of complex orbital exenteration defects: a single center's experience with a five-year follow-up. Ann Plast Surg. 2014;73(02):158–163. doi: 10.1097/SAP.0b013e31826a1a56. [DOI] [PubMed] [Google Scholar]
- 10.Taylan G, Yildirim S, Aköz T. Reconstruction of large orbital exenteration defects after resection of periorbital tumors of advanced stage. J Reconstr Microsurg. 2006;22(08):583–589. doi: 10.1055/s-2006-956232. [DOI] [PubMed] [Google Scholar]
- 11.Kwon D, Iloreta A, Miles B, Inman J. Open anterior skull base reconstruction: a contemporary review. Semin Plast Surg. 2017;31(04):189–196. doi: 10.1055/s-0037-1607273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browne J D, Burke A J. Benefits of routine maxillectomy and orbital reconstruction with the rectus abdominis free flap. Otolaryngol Head Neck Surg. 1999;121(03):203–209. doi: 10.1016/S0194-5998(99)70172-5. [DOI] [PubMed] [Google Scholar]
- 13.Jones N F, Johnson J T, Shestak K C, Myers E N, Swartz W M. Microsurgical reconstruction of the head and neck: interdisciplinary collaboration between head and neck surgeons and plastic surgeons in 305 cases. Ann Plast Surg. 1996;36(01):37–43. doi: 10.1097/00000637-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Savage R C. Orbital exenteration and reconstruction for massive basal cell and squamous cell carcinoma of cutaneous origin. Ann Plast Surg. 1983;10(06):458–466. doi: 10.1097/00000637-198306000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Bartley G B, Garrity J A, Waller R R, Henderson J W, Ilstrup D M. Orbital exenteration at the Mayo Clinic. 1967–1986. Ophthalmology. 1989;96(04):468–473. doi: 10.1016/s0161-6420(89)32872-7. [DOI] [PubMed] [Google Scholar]
- 16.Hanasono M M, Lee J C, Yang J S, Skoracki R J, Reece G P, Esmaeli B. An algorithmic approach to reconstructive surgery and prosthetic rehabilitation after orbital exenteration. Plast Reconstr Surg. 2009;123(01):98–105. doi: 10.1097/PRS.0b013e3181904b95. [DOI] [PubMed] [Google Scholar]
- 17.Gliklich R E, Rounds M F, Cheney M L, Varvares M A.Combining free flap reconstruction and craniofacial prosthetic technique for orbit, scalp, and temporal defects Laryngoscope 1998108(4, Pt 1):482–487. [DOI] [PubMed] [Google Scholar]
- 18.Chepeha D B, Wang S J, Marentette L J et al. Restoration of the orbital aesthetic subunit in complex midface defects. Laryngoscope. 2004;114(10):1706–1713. doi: 10.1097/00005537-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Chana J S, Wei F C. A review of the advantages of the anterolateral thigh flap in head and neck reconstruction. Br J Plast Surg. 2004;57(07):603–609. doi: 10.1016/j.bjps.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Cherubino M, Turri-Zanoni M, Battaglia P et al. Chimeric anterolateral thigh free flap for reconstruction of complex cranio-orbito-facial defects after skull base cancers resection. J Craniomaxillofac Surg. 2017;45(01):87–92. doi: 10.1016/j.jcms.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Olsen K D, Meland N B, Ebersold M J, Bartley G B, Garrity J A.Extensive defects of the sino-orbital region. Results with microvascular reconstruction Arch Otolaryngol Head Neck Surg 199211808828–833., discussion 859–860 [DOI] [PubMed] [Google Scholar]
- 22.Baker S R. Closure of large orbital-maxillary defects with free latissimus dorsi myocutaneous flaps. Head Neck Surg. 1984;6(04):828–835. doi: 10.1002/hed.2890060405. [DOI] [PubMed] [Google Scholar]
- 23.Piantanida R, Roselli R, Pellini R, Ferrario F, Boschini P, Spriano G. Reconstruction of major orbital-maxillary defects with free latissimus dorsi myocutaneous flap. Facial Plast Surg. 1999;15(04):297–302. doi: 10.1055/s-2008-1064330. [DOI] [PubMed] [Google Scholar]
- 24.Triana R J, Jr, Uglesic V, Virag M et al. Microvascular free flap reconstructive options in patients with partial and total maxillectomy defects. Arch Facial Plast Surg. 2000;2(02):91–101. doi: 10.1001/archfaci.2.2.91. [DOI] [PubMed] [Google Scholar]
- 25.Cordeiro P G, Santamaria E.A classification system and algorithm for reconstruction of maxillectomy and midfacial defects Plast Reconstr Surg 2000105072331–2346., discussion 2347–2348 [DOI] [PubMed] [Google Scholar]
- 26.Santamaria E, Cordeiro P G. Reconstruction of maxillectomy and midfacial defects with free tissue transfer. J Surg Oncol. 2006;94(06):522–531. doi: 10.1002/jso.20490. [DOI] [PubMed] [Google Scholar]
- 27.Urken M L, Sullivan M J, Biller H F, MLC,Atlas of Regional and Free Flaps for Head and Neck Reconstruction New York, NY: Raven Press; 1994 [Google Scholar]
- 28.Yang G, Chen B, Gao Y. Free transfer of forearm flap. Report of 56 cases. National Med J China. 1981;61(03):139–142. [Google Scholar]
- 29.Evans H B, Lampe H B. The radial forearm flap in head and neck reconstruction. J Otolaryngol. 1987;16(06):382–386. [PubMed] [Google Scholar]
- 30.Santamaria E, Granados M, Barrera-Franco J L. Radial forearm free tissue transfer for head and neck reconstruction: versatility and reliability of a single donor site. Microsurgery. 2000;20(04):195–201. doi: 10.1002/1098-2752(2000)20:4<195::aid-micr10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 31.Yokoo S, Komori T, Furudoi S et al. Three-dimensional reconstruction after oral oncologic surgery using single free radial forearm flaps or free rectus abdominis musculocutaneous flaps. J Oral Sci. 2004;46(01):65–70. doi: 10.2334/josnusd.46.65. [DOI] [PubMed] [Google Scholar]
- 32.Seikaly H, Rieger J, O'Connell D, Ansari K, Alqahtani K, Harris J. Beavertail modification of the radial forearm free flap in base of tongue reconstruction: technique and functional outcomes. Head Neck. 2009;31(02):213–219. doi: 10.1002/hed.20953. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Castro J, Petrisor D, Ballard D, Wax M K. The double-barreled radial forearm osteocutaneous free flap. Laryngoscope. 2016;126(02):340–344. doi: 10.1002/lary.25388. [DOI] [PubMed] [Google Scholar]
- 34.Meland N B, Fisher J, Irons G B, Wood M B, Cooney W P. Experience with 80 rectus abdominis free-tissue transfers. Plast Reconstr Surg. 1989;83(03):481–487. doi: 10.1097/00006534-198903000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Urken M L, Turk J B, Weinberg H, Vickery C, Biller H F. The rectus abdominis free flap in head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 1991;117(09):1031. doi: 10.1001/archotol.1991.01870210103021. [DOI] [PubMed] [Google Scholar]
- 36.Nakatsuka T, Harii K, Yamada A, Asato H, Ebihara S. Versatility of a free inferior rectus abdominis flap for head and neck reconstruction: analysis of 200 cases. Plast Reconstr Surg. 1994;93(04):762–769. doi: 10.1097/00006534-199404000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Uusitalo M, Ibarra M, Fulton L et al. Reconstruction with rectus abdominis myocutaneous free flap after orbital exenteration in children. Arch Ophthalmol. 2001;119(11):1705–1709. doi: 10.1001/archopht.119.11.1705. [DOI] [PubMed] [Google Scholar]
- 38.Slavin S A, Goldwyn R M. The midabdominal rectus abdominis myocutaneous flap: review of 236 flaps. Plast Reconstr Surg. 1988;81(02):189–199. doi: 10.1097/00006534-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Civantos F J. Latissimus dorsi microvascular flap. Facial Plast Surg. 1996;12(01):65–68. doi: 10.1055/s-2008-1064495. [DOI] [PubMed] [Google Scholar]
- 40.Maxwell G P, Stueber K, Hoopes J E. A free latissimus dorsi myocutaneous flap: case report. Plast Reconstr Surg. 1978;62(03):462–466. doi: 10.1097/00006534-197809000-00033. [DOI] [PubMed] [Google Scholar]
- 41.Barrow D L, Nahai F, Fleischer A S. Use of free latissimus dorsi musculocutaneous flaps in various neurosurgical disorders. J Neurosurg. 1983;58(02):252–258. doi: 10.3171/jns.1983.58.2.0252. [DOI] [PubMed] [Google Scholar]
- 42.Shestak K C, Schusterman M A, Jones N F, Johnson J T. Immediate microvascular reconstruction of combined palatal and midfacial defects using soft tissue only. Microsurgery. 1988;9(02):128–131. doi: 10.1002/micr.1920090214. [DOI] [PubMed] [Google Scholar]
- 43.Bartlett S P, May J W, Jr, Yaremchuk M J. The latissimus dorsi muscle: a fresh cadaver study of the primary neurovascular pedicle. Plast Reconstr Surg. 1981;67(05):631–636. [PubMed] [Google Scholar]
- 44.Tobin G R, Schusterman M, Peterson G H, Nichols G, Bland K I. The intramuscular neurovascular anatomy of the latissimus dorsi muscle: the basis for splitting the flap. Plast Reconstr Surg. 1981;67(05):637–641. doi: 10.1097/00006534-198105000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Baudet J, Guimberteau J C, Nascimento E. Successful clinical transfer of two free thoraco-dorsal axillary flaps. Plast Reconstr Surg. 1976;58(06):680–688. doi: 10.1097/00006534-197612000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Kosutic D, Uglesic V, Knezevic P, Milenovic A, Virag M. Latissimus dorsi-scapula free flap for reconstruction of defects following radical maxillectomy with orbital exenteration. J Plast Reconstr Aesthet Surg. 2008;61(06):620–627. doi: 10.1016/j.bjps.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Song Y G, Chen G Z, Song Y L. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br J Plast Surg. 1984;37(02):149–159. doi: 10.1016/0007-1226(84)90002-x. [DOI] [PubMed] [Google Scholar]
- 48.Joo Y H, Cho K J, Park J O, Kim M S. Usefulness of the anterolateral thigh flap with vascularized fascia lata for reconstruction of orbital floor and nasal surface after total maxillectomy. Laryngoscope. 2013;123(09):2125–2130. doi: 10.1002/lary.23797. [DOI] [PubMed] [Google Scholar]
- 49.Hynes S L, Forrest C R, Borschel G H. Use of the anterolateral thigh flap for reconstruction of the pediatric anophthalmic orbit. J Plast Reconstr Aesthet Surg. 2016;69(01):84–90. doi: 10.1016/j.bjps.2015.09.011. [DOI] [PubMed] [Google Scholar]


