Abstract
Cisplatin, an effective antineoplastic drug used in the treatment of many cancers, has ototoxic potential, thus placing cancer patients, receiving this treatment, at risk of hearing loss. It is therefore important for health care professionals managing these patients to be aware of cisplatin's ototoxic properties and its clinical signs to identify patients at risk of developing a hearing impairment. Eighty-five English peer-reviewed articles and two books, from January 1975 to July 2015, were identified from PubMed, ScienceDirect, and EBSCOhost. An overview of cisplatin-associated ototoxicity, namely its clinical features, incidence rates, molecular and cellular mechanisms, and risk factors, is presented in this article. This review further highlights the importance of a team-based approach to complement an audiological monitoring program in reducing any further loss in the quality of life of affected patients, as there is currently no otoprotective agent routinely recommended for the prevention of cisplatin-associated ototoxicity.
Keywords: cisplatin, ototoxicity, hearing loss, ototoxicity monitoring program, cancer, otoprotectant
Cancer has been identified as the leading cause of death in both more and less economically developed countries. 1 Projections based on the GLOBOCAN 2012 estimates predict a substantive increase to 19.3 million new cancer cases per year by 2025, due to growth and aging of the global population. 2 This is likely to result in an increase in the use of cancer chemotherapy agents, such as cisplatin, which assist in preventing the proliferation, invasion, and metastases of the cancer cells. 3 While considered to be one of the most potent cancer chemotherapeutics in children and adults, due to its effectiveness against many cancers, 4 namely, osteogenic sarcoma, medulloblastoma, and testicular, cervical, and ovarian cancers, 5 cisplatin has an expansive toxicity profile, involving the gastrointestinal, hematologic, renal, and auditory systems. 5
Ototoxicity refers to the hearing disorder that results from the temporary or permanent inner ear dysfunction after treatment with an ototoxic drug 6 such as aminoglycosides, loop diuretics, quinine, nonsteroidal anti-inflammatory drugs, 7 and antiretroviral therapy (ART). 8 Therefore, it is possible that some individuals may be receiving treatments which consist of the simultaneous use of more than one ototoxic drug, increasing the likelihood of ototoxicity.
All healthcare professionals managing patients with cancer should be knowledgeable about the ototoxic properties of cisplatin. Hence, this review aims to serve as a resource for health professionals to enhance their understanding of ototoxicity as well as their roles within an ototoxicity-monitoring program by providing an overview and description of this condition in patients diagnosed with cancer and receiving cisplatin chemotherapy.
Method
The review identified peer-reviewed articles published between January 1975 and July 2015 in the area of cisplatin-associated ototoxicity and ototoxicity monitoring, and included English articles only. Studies were identified using keyword, and MeSH term searches of electronic databases depicted in Table 1 , followed by a manual search of relevant authors and journals. Additional potential publications were identified by reviewing the references cited by each publication, review or book chapter. A criterion for selection was that the article had to present data on either cisplatin ototoxicity and/or ototoxicity monitoring in human participants, and no research designs were excluded.
Table 1. Search and MeSH Terms Used in the Literature Search.
| Electronic database | Search term | MeSH term |
|---|---|---|
| PubMed (Medline) | Ototoxicity [All Fields] AND monitoring [All Fields] | ((“cisplatin”[MeSH Terms] OR “cisplatin”[All Fields]) AND ototoxicity [All Fields]) OR ((“cisplatin”[MeSH Terms] OR “cisplatin”[All Fields]) AND (“hearing loss”[MeSH Terms] OR (“hearing”[All Fields] AND “loss”[All Fields]) OR “hearing loss”[All Fields])) |
| ScienceDirect | “cisplatin ototoxicity” or “cisplatin hearing loss” “ototoxicity monitoring” | |
| EBSCOhost | Cisplatin ototoxicity or cisplatin hearing loss Ototoxicity monitoring |
A total of 2,106 records were initially identified, of which 1,581 were excluded based on the title and/or abstract as well as duplication. Eighty-five relevant articles, comprising of six national (South Africa) and 79 international articles, as well as four internationally published books were selected to provide an overview in the following eight areas: the mechanisms of cisplatin ototoxicity, clinical presentation, risk factors, incidence rates in adults and children, the effect on quality of life, ototoxicity monitoring, otoprotective strategies, and the management of an ototoxic hearing loss.
The Mechanisms of Cisplatin Ototoxicity
Cisplatin ototoxicity is produced by several distinct mechanisms 9 as depicted in Fig. 1 . One such mechanism, the antioxidant model, involves the formation of reactive oxygen species (ROS) within the cochlea and consequent reduction in antioxidant enzymes following exposure to cisplatin chemotherapy. 9 10 11 12 13 Another mechanism of cisplatin ototoxicity involves the significant contribution of nicotinamide adenine dinucleotide phosphate oxidase 3 isoform (NOX3) to the generation of ROS within the cochlea, when activated by cisplatin, 10 14 while a third mechanism relates to the activation of transient receptor potential vanilloid 1 (TRPV1) channel. 15 16 17
Figure 1.
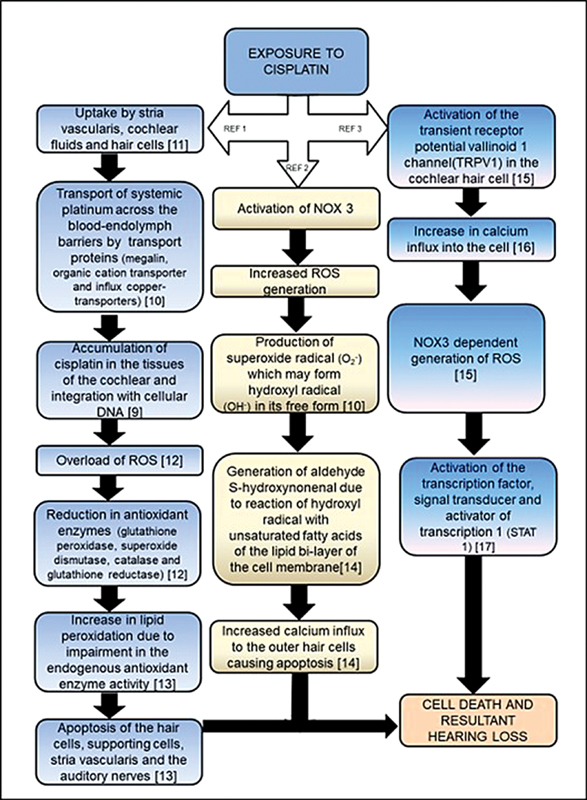
Mechanisms of cisplatin ototoxicity. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
The molecular mechanisms of cisplatin ototoxicity therefore include “creation of ROS, depletion of antioxidant glutathione and its regenerating enzymes, increased rate of lipid peroxidation, oxidative modifications of proteins, nucleic acids damage by caspase system activation, and S-Nitrosylation of cochlear proteins.” 18 With the cellular mechanisms of cisplatin-associated ototoxicity including damage to the outer hair cells, supporting cells, marginal cells of the stria vascularis, spiral ligament, and the spiral ganglion cells, 18 it is evident that the structures of the inner ear are most susceptible to damage by cisplatin chemotherapy; with apoptotic degeneration of the hair cell in the Organ of Corti being most prominent. 19 The outer hair cells in the basal turn of the cochlea are most affected. This leads to an initial elevation of high-frequency audiometric thresholds, followed by a progressive loss into the lower frequencies with continued therapy. 20 21 Knowledge of the different mechanisms of cisplatin ototoxicity is important for health care professionals as it will create an awareness of its complexity and the resulting clinical presentation.
Clinical Presentation and Risk Factors
Cisplatin-associated ototoxicity usually manifests as irreversible, progressive, 5 bilateral, high-frequency sensorineural hearing loss 22 with tinnitus. 23 Tinnitus may occur with or without a hearing loss, 22 and may be permanent or transient. While most of the hearing loss is permanent, there is sometimes sporadic and partial recovery. 24 Furthermore, rare cases of unilateral hearing loss have been reported, and these are usually explained by tumor location and surgical or therapeutic intervention on the affected side. 25
The degree of hearing loss varies and is often dose dependent, that is, the higher the cumulative dose, the greater the ototoxic effect. 26 27 The duration, number of cycles administered, 28 and method of administration 29 also influence cisplatin-associated ototoxicity. Additional factors that may increase the risk for ototoxicity include exposure to concomitant noise, 26 30 chemicals and other ototoxic medications, 26 as well as having a higher melanin content, 31 and/or presenting with renal insufficiency, that is, high levels of serum creatinine, 26 and preexposure hearing loss. 29 32 Genetic risk factors, such as megalin and glutathione S-transferases gene polymorphism, also influence cisplatin ototoxicity, 33 as do physiological factors such as age, with younger children 34 and older adults (older than 46 years) 35 presenting with a greater severity of hearing damage. Awareness of these risk factors may assist health care professionals with informational counselling of the patient receiving cisplatin chemotherapy.
Cisplatin-Associated Hearing Loss in Adults and Children
The incidence of cisplatin ototoxicity is variable in adults ( Table 2 ) 5 26 27 36 37 38 39 40 41 42 43 44 and children ( Table 3 ). 34 38 45 46 47 The variations may be due to several factors, such as differences in the dose, both within a cycle and the total amount administered over multiple cycles; time interval between courses; method of administration; treatment duration; as well as differences in patient population. Further exploration in this regard is therefore necessary.
Table 2. Studies Reflecting Cisplatin-Associated Hearing Loss in Adults.
| Study | Country | Type of study | Audiological tests conducted | Patient population | No. of patients who developed ototoxicity |
|---|---|---|---|---|---|
| Malgonde et al 36 | India | Prospective | Pure tone audiometry (frequencies not specified) and short increment sensitivity index test | 34 patients with head and neck cancers receiving cisplatin-containing chemotherapy and concomitant radiation therapy | 34 (100%) |
| Whitehorn et al 37 | South Africa | Retrospective cross sectional | Air (0.25–8 kHz) and bone conduction pure tone audiometry | 107 patients receiving cisplatin-containing chemotherapy, irrespective of the type of the cancer | 59 (55.1%) |
| Nitz et al 38 | Germany | Prospective longitudinal trinational population based | Air (0.125–8 kHz) and bone conduction pure tone audiometry | 1 patient with soft-tissue sarcoma and 16 with osteosarcoma, receiving cisplatin- and/or carboplatin-containing chemotherapy | 6 (35.3%) |
| Arora et al 5 | India | Prospective, randomized, and observational | Pure tone air (0.25–16 kHz) and bone conduction audiometry Results are reflective of frequencies 4–16 kHz |
57 patients receiving cisplatin-containing chemotherapy: 10 patients (low-dose group—carcinoma of the larynx) 35 patients (middle-dose group—head and neck cancers, carcinoma of the cervix) 12 patients (high-dose group—carcinoma of the lung and carcinoma of the testis |
- 6 (60%) 35 (100%) 12 (100%) |
| Dell'Aringa et al 39 | Brazil | Case series | Tympanometry, acoustic reflex threshold testing, DPOAEs, air (0.25–8 kHz) and bone conduction pure tone audiometry, speech audiometry | 17 patients with extracranial head and neck cancers receiving cisplatin-containing chemotherapy and concomitant radiation therapy | 12 left ears (70.5%), 11 right ears (64.7%) |
| Schultz et al 40 | Brazil | Prospective | Full audiometric evaluations, with only air (0.25–8 kHz) and bone conduction pure tone audiometry thresholds computed | 31 patients receiving cisplatin-containing chemotherapy, irrespective of the type of cancer | NCI 12 criteria—12 (38%), Brock et al's criterion—19 (65%), ASHA criteria—17 (54%), David and Silverman's criteria—9 (29%) |
| Zuur et al 41 | The Netherlands | Prospective | Air (0.125–16 kHz) and bone conduction pure tone audiometry | 60 patients with locally advanced head and neck cancer, receiving cisplatin-containing chemotherapy and concomitant radiation therapy | Up to 8 kHz—19 (31%) Up to 16 kHz—28 (47%) |
| Dutta et al 27 | India | Prospective | Pure tone audiometry (frequencies not specified) | 60 patients receiving cisplatin-containing chemotherapy—type of cancer not indicated 51—low-dose group 9—high-dose group |
9 (15%) 6 (12%) 3 (33%) |
| Strumberg et al 42 | Germany | Retrospective | Pure tone air (0.125–12 kHz) and bone conduction audiometry, TEOAE test | 32 patients with testicular cancer receiving cisplatin-containing chemotherapy | 21 (70%) |
| Nagy et al 43 | USA | Retrospective | Tympanometry, air (0.25–8 kHz) conduction pure tone audiometry | 53 patients with oesophageal, lung, or head and neck cancer receiving cisplatin-containing chemotherapy and concomitant radiation therapy (only for head and neck cancer) | 19 (36%) |
| Bokemeyer et al 26 | Germany | Retrospective | Pure tone air (0.5–8 kHz) and bone audiometry | 86 patients with testicular cancer receiving cisplatin-containing chemotherapy | 57 (66%) |
| Waters et al 44 | Canada | Retrospective | Pure tone air (0.25–8 kHz) and bone conduction audiometry, immittance audiometry, and speech audiometry | 60 patients with advanced ovarian carcinomas receiving cisplatin-containing chemotherapy 39—low-dose, short treatment (25 from LDE group and 14 new cases after treatment modification) 8—low-dose, blocks 25—low-dose, extended treatment 13—high-dose, short treatment |
6 (15%) 0 (0%) 9 (36%) 12 (92%) |
Abbreviations: ASHA, American Speech Language Hearing Association; DPOAEs, distortion product otoacoustic emissions; LDE, low dose extended treatment; TEOAE, transient evoked otoacoustic emission.
Table 3. Studies Reflecting Cisplatin-Associated Hearing Loss in Children.
| Study | Country | Type of study | Audiological tests conducted | Patient population | No. of patients who developed ototoxicity |
|---|---|---|---|---|---|
| Nitz et al 38 | Germany | Prospective longitudinal trinational population based | Air (0.125–8 kHz) conduction pure tone audiometry | 93 patients with osteosarcoma and 19 with soft-tissue sarcoma receiving cisplatin and/or carboplatin-containing chemotherapy | 55 (49.1%) |
| Knight et al 45 | USA | Prospective | Otoscopy, tympanometry, pure tone audiometry (0.5–8 kHz), DPOAEs, and ABR Otoscopy, tympanometry, extended pure tone audiometry (0.5–16 kHz), and DPOAEs |
32 children with different types of cancers treated with cisplatin- and/or carboplatin-containing chemotherapy 17 children with different types of cancers treated with cisplatin- and/or carboplatin-containing chemotherapy |
20 (62.5%) 16 (94.1%) |
| Coradini et al 34 | Brazil | Retrospective | Tympanometry, pure tone audiometry (0.25–8 kHz), TEOAEs, and DPOAEs | 23 children with malignant hepatic tumor, osteosarcoma, and germ cell tumors receiving cisplatin-containing chemotherapy | Pure tone—12 (52%), TEOAEs—5 (22%), DPOAEs—16 (71%) |
| Bertolini et al 46 | France | Prospective | Otoscopy, immittance audiometry, speech audiometry, play audiometry or free-field audiometry, conventional pure tone audiometry or ABR (depending on the age of the participant) | 102 children with either neuroblastoma, hepatoblastoma, germ cell tumor, or osteosarcoma, 96 received cisplatin- and/or carboplatin-containing chemotherapy, 52 received cisplatin only |
- 39 (41%) 19 (37%) |
| Stavroulaki et al 47 | Greece | Prospective | Otoscopy, immittance audiometry, pure tone audiometry (0.25–8 kHz), TEOAEs, and DPOAEs | 12 children with neuroblastoma, osteosarcoma, medulloblastoma, rhabdomyosarcoma, or primitive neuroectodermal tumor receiving cisplatin-containing chemotherapy | 6 (50%) |
Abbreviations: ABR, auditory brainstem response; DPOAEs, distortion product otoacoustic emissions; TEOAE, transient evoked otoacoustic emission.
Quality of Life
Ototoxicity poses a major problem to the cancer patient, as the quality of life after receiving cisplatin chemotherapy may be negatively affected. Tasks that normal hearing persons take for granted may become challenging and frustrating, 48 with the hearing loss possibly resulting in psychosocial and physical health problems, as well as depression and social isolation. 49 Hence, hearing loss, often referred to as the invisible condition, has serious visible ramifications on the quality of life of an individual with hearing loss. 48
The impact of an ototoxic hearing loss may be more profound for infants and young children who are at a critical stage of their speech and language development. 50 Furthermore, the high-frequency nature of an ototoxic hearing loss may result in speech recognition and comprehension being compromised, 51 resulting in possible neurocognitive and psychosocial delays. 52 There is also an elevated risk for academic learning problems and psychosocial difficulties in school-aged children and adolescents. 53 Hence, cisplatin-associated ototoxicity further complicates the morbidity of patients with cancer, 5 as it may isolate them from family members and significant others at a time when they require the greatest support.
Ototoxicity Monitoring
An audiological monitoring program can avert, to a large extent, the reduced quality of life as a result of hearing loss, as patients on cisplatin chemotherapy can be identified early, counselled, monitored, and managed appropriately through medical and hearing interventions in a logical, systematic, and coherent manner. 54 Prospective audiological evaluations remain the only reliable method for detecting ototoxicity before it becomes symptomatic 55 and a communication problem evident. An ototoxicity monitoring program should involve a health care team comprising of an oncology nurse, oncologists, audiologist, and pharmacist to ensure effective sustainability of such a program, if implemented, with the patient being the central focus, as depicted in Fig. 2 . 56 57 The principles of early identification and early intervention are a part of ototoxicity monitoring, and the audiologist can manage such a program. 46
Figure 2.
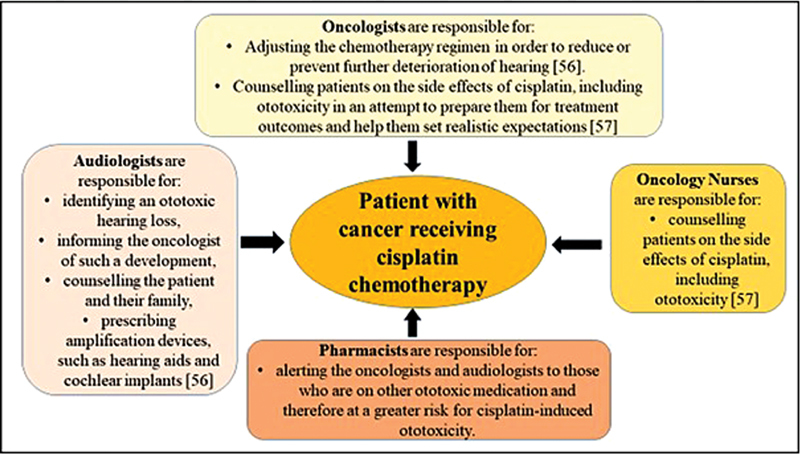
Team approach for ototoxicity monitoring, with the patient being the central focus. 56 57
In countries without ototoxicity management guidelines, the “Guidelines for the audiological management of individuals receiving cochleotoxic drug therapy” developed by ASHA 55 may, consequently, guide the audiologist in the implementation of an ototoxicity monitoring program. For widespread acceptance and use, ototoxicity monitoring programs need to incorporate efficient and cost-effective ototoxicity identification techniques, 58 while considering the health care system and demographics of the patient population being managed. For any population receiving ototoxic medication, the following should be considered: (1) the patient's level of alertness or ability to respond reliably; (2) the most appropriate times during the treatment protocol for test administration; and (3) the test should comprise the baseline, monitoring, and posttreatment evaluations. 59 Appropriate time intervals for audiological assessments may differ depending on the type of cancer as well as the frequency and dose of cisplatin ( Fig. 3 ). 55
Figure 3.
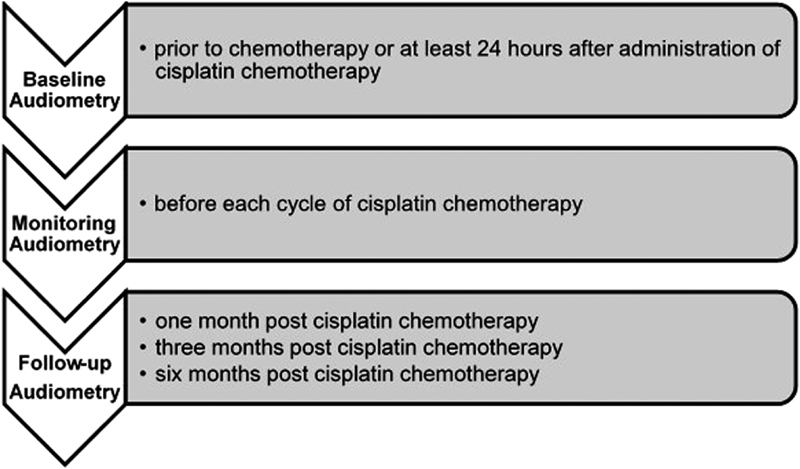
Timelines for audiological assessments. 55
The audiological assessments should incorporate a detailed case history, otoscopic examination, immittance audiometry, speech audiometry, distortion product otoacoustic emissions (DPOAEs) testing, and conventional and extended high-frequency audiometry (HFA; i.e., up to 20,000 Hz). 55 59 These procedures are all conducted for the baseline assessment and the 6-month follow-up evaluation. 55 59 While auditory brainstem response test may be used, it is not considered a standard procedure for monitoring ototoxicity. 59
Monitoring audiological evaluations during treatment and the 1 and 3-month follow-up evaluations include case interview, otoscopy and immittance audiometry, as well as air conduction pure tone and objective testing. 59 However, full-frequency threshold testing is impractical for many patients on cisplatin chemotherapy, as these individuals are often extremely ill and easily fatigued. The use of abbreviated threshold monitoring procedures that are clinically practical for these patients, such as the sensitive range for ototoxicity (SRO), are therefore recommended. This is the highest frequency with a threshold at or below 100 dB SPL followed by the next six lower adjacent frequencies in 1/6-octave steps or the one octave range near the highest audible frequency. 59 SRO is usually determined during the baseline evaluation and is dependent on each patient's hearing threshold configuration. During monitoring evaluations, air conduction thresholds should be determined within the patient's defined SRO, with full frequency testing conducted within the same session if an ASHA significant hearing change is noted within the SRO. 55
The protocol presented earlier would be suitable for a patient who is alert; however, a patient who has limited responsiveness may be required to undergo the same audiological evaluations, excluding speech audiometry. Only objective testing, such as otoscopy, tympanometry, acoustic reflexes, and DPOAEs or ABRs, 59 is considered suitable for the assessment of those patients who are too ill or too young to respond.
While pure tone audiometry in the conventional frequency range is suitable for evaluating hearing in the range responsible for speech understanding, as well as for differential diagnosis, it is less sensitive to detecting early ototoxic change. 7 56 The two tests identified as being the most important for the early detection of cisplatin ototoxicity are HFAs and otoacoustic emissions, each also having limitations (see Table 4 ). 5 7 28 34 45 56 58 60 61 62 63 Therefore, using each test in isolation may not be as effective as utilizing a test battery approach, as it increases the chances of obtaining reliable audiologic monitoring data over time. Furthermore, utilizing these two tests to complement one another in every cycle of chemotherapy would possibly ensure the earliest detection of ototoxicity. 64
Table 4. Clinical Significance and Limitations of HFA and OAEs.
| HFA (>8 kHz) | OAEs |
|---|---|
| Clinical significance for ototoxicity | |
|
• HFA is considered to be the most sensitive test to identify ototoxic hearing loss
5
45
60
• HFA is not as affected by middle ear pathologies as OAEs 7 • The criteria of change for ototoxicity is established 7 |
• OAES is considered a noninvasive objective measure of cochlear outer hair cell function
63
• DPOAEs can be regarded as a more sensitive measure for the early detection of hearing loss than conventional pure tone audiometry 34 • OAEs is time efficient 7 • DPOAEs provide frequency-specific information 58 |
| Limitations | |
|
• HFA is not standardized.
7
• HFA is not commonly used, due to the need for additional equipment such as circum-aural headphones 61 • HFA may not always be applicable, as patients with hearing loss in the conventional frequency range may not have measurable hearing in the extended high-frequency range 62 • Test efficiency may be affected due to HFA being time consuming 56 |
• OAEs is significantly affected by middle ear pathology
28
• There is no universal value for the criteria of change indicating ototoxicity 63 • OAEs is absent in patients with moderate degrees of hearing loss 58 • OAEs has a limited frequency range (generally up to 8,000 Hz) 58 |
Abbreviations: DPOAEs, distortion product otoacoustic emissions; HFA, high - frequency audiometry; OAEs, otoacoustic emissions .
In developing countries such as South Africa and India, no programs have been formally implemented to identify and monitor ototoxicity in patients on cancer chemotherapy. 65 As a result, there is no contextually relevant research to steer the implementation of an accountable and effective ototoxicity monitoring program in the country. This is probably one of the main reasons for ototoxicity monitoring programs not being commonplace in local hospitals and clinics. However, the creation of an audiological monitoring program allows for better control of cancer-related comorbidities, while research focuses on identifying the most suitable otoprotective strategy against cisplatin ototoxicity.
Otoprotective Strategies
Over the years, several studies have investigated the use of otoprotectants with cisplatin, their purpose being to protect the inner ear from any injury while not interfering with the antitumor effects of cisplatin. 51 Otoprotective strategies include reducing the formation of free radicals by maintaining glutathione levels and antioxidant activity. 20 Three mechanisms may provide protection against cisplatin, namely, endogenous molecules, exogenous agents, or a combination of exogenous agents that trigger endogenous protective mechanisms. However, endogenous agents are not effective against cisplatin when the dose exceeds a certain threshold. 10 66
Nearly all of the otoprotective agents are sulfur- or sulfhydryl-containing compounds (thio compounds), known as antioxidants, and potent heavy metal chelators. 67 The numerous otoprotective agents utilized in clinical and animal studies include Amifostine, D -or L-methionine, methylthiobenzoic acid, lipoic acid, tiopronin, glutathione ester, sodium thiosulfate, 68 melatonin, 69 vitamin E, 70 N-acetylcysteine, 71 dexamethasone, 72 and resveratrol. 73 However, none of these agents have been found to be unequivocally beneficial in preventing cisplatin ototoxicity and no agent is currently recommended for routine use. 74 Further research is needed to find new methods and optimize old ones to prevent and/or treat hearing loss during cisplatin therapy. In addition, administering medication intratympanically together with gene therapy needs to be further explored. 18 Intratympanic administration involves the diffusion of the otoprotective agent across the round window into the inner ear, where its therapeutic effect is exerted. Alternatively, gene therapy may prove to be beneficial in protecting an individual against cisplatin-induced hearing loss as several genes, namely megalin, glutathione-S-transferases, Thiopurine S -methyltransferase, and catechol- O -methyl transferase, may be responsible for susceptibility to hearing loss. 75
Management of an Ototoxic Hearing Loss
If a cisplatin-associated hearing loss results in communication difficulties, it is the audiologist's ethical responsibility to begin or recommend aural rehabilitation. 55 However, this intervention should not only occur once hearing loss has been detected but before the patient begins the cisplatin chemotherapy. Aural rehabilitation techniques such as speech reading and counselling on compensatory communication strategies should be conducted. The counselling should include spouses and significant others, as hearing loss may not only impact the person with cancer but also frequent communication partners. 76 Patients with sensorineural hearing loss due to the use of cisplatin may benefit from the use of assistive listening devices such as hearing aids or cochlear implants. 6 Children with ototoxic hearing loss also may require the use of remote microphone technology to improve the signal-to-noise ratio in the classroom.
Furthermore, with the recent developments in hearing aid technology, a patient with an ototoxic hearing loss is more likely to receive the desired amplification benefit. These developments in technology include extended bandwidth hearing aids 77 and hearing aids with frequency lowering technology achieved by linear frequency transposition, nonlinear frequency compression, or spectral envelope warping. 78
Conclusion
This review has highlighted that cisplatin ototoxicity is a common side effect of cisplatin chemotherapy that may negatively affect the quality of life of patients with cancer. The different molecular and cellular mechanisms involved in cisplatin-associated ototoxicity highlight the complexity of this condition and the consequent difficulty in identifying an effective otoprotective agent. The varying incidence rates reported in both adults and pediatrics may be due to the different audiological tests employed in the monitoring of the patient's hearing status and therefore highlight the importance of the use of extended HFA and DPOAEs in ototoxicity monitoring. An audiological monitoring program comprising of a team of health care professionals, knowledgeable about cisplatin ototoxicity, may serve to improve evidence-based service delivery to these patients.
Conflict of Interest None.
Disclosures
The study is supported by the Medical Research Council of South Africa in terms of the National Health Scholarship Program provided for this purpose by the National Department of Health. The study also received financial support from Oticon Foundation and the University of Kwazulu-Natal. This paper has been presented at the ENT/SAAA/SASLHA Congress 2015 in South Africa, Audiology Australia National Conference 2016, and the World Congress of Audiology 2016.
This paper is a summarized version of Jessica Paken, Cyril D. Govender, Mershen Pillay, and Vikash Sewram, “Cisplatin-Associated Ototoxicity: A Review for the Health Professional,” Journal of Toxicology, Vol. 2016, Article ID 1809394, 13 pages, 2016. https://doi.org/10.1155/2016/1809394 .
References
- 1.Torre L A, Bray F, Siegel R L, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(02):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R . Lyon, France International Agency for Research on Cancer; 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. [Google Scholar]
- 3.Rybak L P. United States: Thomson Delmar Learning; 2007. Cancer and Ototoxicity of chemotherapeutics. In: Campbell KCM, ed. Pharmacology and Ototoxicity for Audiologists; pp. 138–162. [Google Scholar]
- 4.Reavis K M, McMillan G, Austin D et al. Distortion-product otoacoustic emission test performance for ototoxicity monitoring. Ear Hear. 2011;32(01):61–74. doi: 10.1097/AUD.0b013e3181e8b6a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora R, Thakur J S, Azad R K, Mohindroo N K, Sharma D R, Seam R K. Cisplatin-based chemotherapy: add high-frequency audiometry in the regimen. Indian J Cancer. 2009;46(04):311–317. doi: 10.4103/0019-509X.55551. [DOI] [PubMed] [Google Scholar]
- 6.Yorgason J G, Fayad J N, Kalinec F. Understanding drug ototoxicity: molecular insights for prevention and clinical management. Expert Opin Drug Saf. 2006;5(03):383–399. doi: 10.1517/14740338.5.3.383. [DOI] [PubMed] [Google Scholar]
- 7.Schellack N, Naude A. An overview of pharmacotherapy-induced ototoxicity: review article. S Afr Fam Pract. 2013;55(04):357–365. [Google Scholar]
- 8.Stearn N, Swanepoel D W. San Diego: Plural Publishing; 2010. Sensory and neural auditory disorders associated with HIV/AIDS; pp. 243–288. [Google Scholar]
- 9.Gonçalves M S, Silveira A F, Teixeira A R, Hyppolito M A. Mechanisms of cisplatin ototoxicity: theoretical review. J Laryngol Otol. 2013;127(06):536–541. doi: 10.1017/S0022215113000947. [DOI] [PubMed] [Google Scholar]
- 10.Rybak L P. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg. 2007;15(05):364–369. doi: 10.1097/MOO.0b013e3282eee452. [DOI] [PubMed] [Google Scholar]
- 11.Olgun Y. Cisplatin ototoxicity: where we are? J Int Adv Otol. 2013;9(03):403–416. [Google Scholar]
- 12.Rybak L P, Husain K, Morris C, Whitworth C, Somani S. Effect of protective agents against cisplatin ototoxicity. Am J Otol. 2000;21(04):513–520. [PubMed] [Google Scholar]
- 13.Campbell K C, Kalkanis J, Glatz F R. Ototoxicity: mechanisms, protective agents, and monitoring. Curr Opin Otolaryngol Head Neck Surg. 2000;8(05):436–440. [Google Scholar]
- 14.Ikeda K, Sunose H, Takasaka T. Effects of free radicals on the intracellular calcium concentration in the isolated outer hair cell of the guinea pig cochlea. Acta Otolaryngol. 1993;113(02):137–141. doi: 10.3109/00016489309135781. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjea D, Jajoo S, Whitworth C et al. Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J Neurosci. 2008;28(49):13056–13065. doi: 10.1523/JNEUROSCI.1307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karasawa T, Steyger P S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett. 2015;237(03):219–227. doi: 10.1016/j.toxlet.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt N C, Rubel E W, Nathanson N M. Cisplatin-induced hair cell death requires STAT1 and is attenuated by epigallocatechin gallate. J Neurosci. 2009;29(12):3843–3851. doi: 10.1523/JNEUROSCI.5842-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chirtes F, Albu S. Prevention and restoration of hearing loss associated with the use of cisplatin. BioMed Res Int. 2014;2014:925485. doi: 10.1155/2014/925485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callejo A, Sedó-Cabezón L, Juan I D, Llorens J. Cisplatin-induced ototoxicity: effects, mechanisms and protection strategies. Toxics. 2015;3(03):268–293. doi: 10.3390/toxics3030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rybak L P, Ramkumar V. Ototoxicity. Kidney Int. 2007;72(08):931–935. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- 21.Schacht J, Talaska A E, Rybak L P. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken) 2012;295(11):1837–1850. doi: 10.1002/ar.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakamoto M, Kaga K, Kamio T. Extended high-frequency ototoxicity induced by the first administration of cisplatin. Otolaryngol Head Neck Surg. 2000;122(06):828–833. doi: 10.1016/S0194-59980070009-X. [DOI] [PubMed] [Google Scholar]
- 23.Reddel R R, Kefford R F, Grant J M, Coates A S, Fox R M, Tattersall M HN. Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treat Rep. 1982;66(01):19–23. [PubMed] [Google Scholar]
- 24.Moroso M J, Blair R L. A review of cis-platinum ototoxicity. J Otolaryngol. 1983;12(06):365–369. [PubMed] [Google Scholar]
- 25.Schmidt C-M, Knief A, Lagosch A K, Deuster D, am Zehnhoff-Dinnesen A. Left-right asymmetry in hearing loss following cisplatin therapy in children--the left ear is slightly but significantly more affected. Ear Hear. 2008;29(06):830–837. doi: 10.1097/AUD.0b013e31818005a4. [DOI] [PubMed] [Google Scholar]
- 26.Bokemeyer C, Berger C C, Hartmann J T et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77(08):1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutta A, Venkatesh M D, Kashyap R C. Study of the effects of chemotherapy on auditory function. Indian J Otolaryngol Head Neck Surg. 2005;57(03):226–228. doi: 10.1007/BF03008019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen G C, Tiu C, Koike K, Ritchey A K, Kurs-Lasky M, Wax M K. Transient-evoked otoacoustic emissions in children after cisplatin chemotherapy. Otolaryngol Head Neck Surg. 1998;118(05):584–588. doi: 10.1177/019459989811800504. [DOI] [PubMed] [Google Scholar]
- 29.Kopelman J, Budnick A S, Sessions R B, Kramer M B, Wong G Y.Ototoxicity of high-dose cisplatin by bolus administration in patients with advanced cancers and normal hearing Laryngoscope 198898(8, Pt 1):858–864. [DOI] [PubMed] [Google Scholar]
- 30.Gratton M A, Salvi R J, Kamen B A, Saunders S S.Interaction of cisplatin and noise on the peripheral auditory system Hear Res 199050(1-2):211–223. [DOI] [PubMed] [Google Scholar]
- 31.Barr-Hamilton R M, Matheson L M, Keay D G. Ototoxicity of cis-platinum and its relationship to eye colour. J Laryngol Otol. 1991;105(01):7–11. doi: 10.1017/s0022215100114689. [DOI] [PubMed] [Google Scholar]
- 32.Melamed L B, Selim M A, Schuchman D. Cisplatin ototoxicity in gynecologic cancer patients. A preliminary report. Cancer. 1985;55(01):41–43. doi: 10.1002/1097-0142(19850101)55:1<41::aid-cncr2820550106>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 33.Kirkim G, Olgun Y, Aktas S et al. Is there a gender-related susceptibility for cisplatin ototoxicity? Eur Arch Otorhinolaryngol. 2015;272(10):2755–2763. doi: 10.1007/s00405-014-3283-0. [DOI] [PubMed] [Google Scholar]
- 34.Coradini P P, Cigana L, Selistre S G, Rosito L S, Brunetto A L. Ototoxicity from cisplatin therapy in childhood cancer. J Pediatr Hematol Oncol. 2007;29(06):355–360. doi: 10.1097/MPH.0b013e318059c220. [DOI] [PubMed] [Google Scholar]
- 35.Helson L, Okonkwo E, Anton L, Cvitkovic E. cis-Platinum ototoxicity. Clin Toxicol. 1978;13(04):469–478. doi: 10.3109/15563657808988252. [DOI] [PubMed] [Google Scholar]
- 36.Malgonde M S, Nagpure P S, Kumar M. Audiometric patterns in ototoxicity after radiotherapy and chemotherapy in patients of head and neck cancers. Indian J Palliat Care. 2015;21(02):164–167. doi: 10.4103/0973-1075.156479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitehorn H, Sibanda M, Lacerda M et al. High prevalence of cisplatin-induced ototoxicity in Cape Town, South Africa. S Afr Med J. 2014;104(04):288–291. doi: 10.7196/samj.7389. [DOI] [PubMed] [Google Scholar]
- 38.Nitz A, Kontopantelis E, Bielack S et al. Prospective evaluation of cisplatin- and carboplatin-mediated ototoxicity in paediatric and adult soft tissue and osteosarcoma patients. Oncol Lett. 2013;5(01):311–315. doi: 10.3892/ol.2012.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dell'Aringa A HB, Isaac M L, Arruda G V et al. Audiological findings in patients treated with radio- and concomitant chemotherapy for head and neck tumors. Radiat Oncol. 2009;4(01):53. doi: 10.1186/1748-717X-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz C, Goffi-Gomez M VS, Liberman P HP, Carvalho A L. Report on hearing loss in oncology. Rev Bras Otorrinolaringol (Engl Ed) 2009;75(05):634–641. doi: 10.1016/S1808-8694(15)30510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuur C L, Simis Y J, Verkaik R S et al. Hearing loss due to concurrent daily low-dose cisplatin chemoradiation for locally advanced head and neck cancer. Radiother Oncol. 2008;89(01):38–43. doi: 10.1016/j.radonc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Strumberg D, Brügge S, Korn M W et al. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann Oncol. 2002;13(02):229–236. doi: 10.1093/annonc/mdf058. [DOI] [PubMed] [Google Scholar]
- 43.Nagy J L, Adelstein D J, Newman C W, Rybicki L A, Rice T W, Lavertu P. Cisplatin ototoxicity: the importance of baseline audiometry. Am J Clin Oncol. 1999;22(03):305–308. doi: 10.1097/00000421-199906000-00020. [DOI] [PubMed] [Google Scholar]
- 44.Waters G S, Ahmad M, Katsarkas A, Stanimir G, McKay J. Ototoxicity due to cis-diamminedichloroplatinum in the treatment of ovarian cancer: influence of dosage and schedule of administration. Ear Hear. 1991;12(02):91–102. doi: 10.1097/00003446-199104000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Knight K R, Kraemer D F, Winter C, Neuwelt E A. Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25(10):1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- 46.Bertolini P, Lassalle M, Mercier G et al. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26(10):649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 47.Stavroulaki P, Apostolopoulos N, Segas J, Tsakanikos M, Adamopoulos G. Evoked otoacoustic emissions--an approach for monitoring cisplatin induced ototoxicity in children. Int J Pediatr Otorhinolaryngol. 2001;59(01):47–57. doi: 10.1016/s0165-5876(01)00455-4. [DOI] [PubMed] [Google Scholar]
- 48.Tye-Murray N.Foundations of Aural Rehabilitation: Children, Adults, and Their Family Members: Cengage learning:2014
- 49.Herbst K G, Humphrey C.Hearing impairment and mental state in the elderly living at home BMJ 1980281(6245):903–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neuwelt E A, Brock P. Critical need for international consensus on ototoxicity assessment criteria. J Clin Oncol. 2010;28(10):1630–1632. doi: 10.1200/JCO.2009.26.7872. [DOI] [PubMed] [Google Scholar]
- 51.Langer T, am Zehnhoff-Dinnesen A, Radtke S, Meitert J, Zolk O. Understanding platinum-induced ototoxicity. Trends Pharmacol Sci. 2013;34(08):458–469. doi: 10.1016/j.tips.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Yancey A, Harris M S, Egbelakin A, Gilbert J, Pisoni D B, Renbarger J. Risk factors for cisplatin-associated ototoxicity in pediatric oncology patients. Pediatr Blood Cancer. 2012;59(01):144–148. doi: 10.1002/pbc.24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurney J G, Tersak J M, Ness K K, Landier W, Matthay K K, Schmidt M L; Children's Oncology Group.Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children's Oncology Group Pediatrics 200712005e1229–e1236. [DOI] [PubMed] [Google Scholar]
- 54.Jacob L CB, Aguiar F P, Tomiasi A A, Tschoeke S N, Bitencourt R F. Auditory monitoring in ototoxicity. Rev Bras Otorrinolaringol (Engl Ed) 2006;72(06):836–844. doi: 10.1016/s1808-8694(15)31053-3. [DOI] [PubMed] [Google Scholar]
- 55.American Speech Language Hearing Association.Audiologic management of individuals receiving cochleotoxic drug therapy ASHA 1994361211–19. [Google Scholar]
- 56.American Academy of Audiology.Position Statement and guidelines: Ototoxicity monitoring 2009. Available at:https://audiology-web.s3.amazonaws.com/migrated/OtoMonGuidelines.pdf_539974c40999c1.58842217.pdf. Accessed March 28, 2019
- 57.Dabrowski T, Hussain-Said F. The audiologist's role in ototoxicity monitoring. Advance for Audiologists. 2010;10(03):54. [Google Scholar]
- 58.Fausti S A, Wilmington D J, Helt P V, Helt W J, Konrad-Martin D. Hearing health and care: the need for improved hearing loss prevention and hearing conservation practices. J Rehabil Res Dev. 2005;42(04) 02:45–62. doi: 10.1682/jrrd.2005.02.0039. [DOI] [PubMed] [Google Scholar]
- 59.Fausti S A, Helt W J, Gordon J S. United States: Thomson Delmar Learning; 2007. Audiologic monitoring for ototoxicity and patients management; pp. 230–251. [Google Scholar]
- 60.Fausti S A, Larson V D, Noffsinger D, Wilson R H, Phillips D S, Fowler C G. High-frequency audiometric monitoring strategies for early detection of ototoxicity. Ear Hear. 1994;15(03):232–239. doi: 10.1097/00003446-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Frank T. High-frequency (8 to 16 kHz) reference thresholds and intrasubject threshold variability relative to ototoxicity criteria using a Sennheiser HDA 200 earphone. Ear Hear. 2001;22(02):161–168. doi: 10.1097/00003446-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Osterhammel D. High frequency audiometry. Clinical aspects. Scand Audiol. 1980;9(04):249–256. doi: 10.3109/01050398009076360. [DOI] [PubMed] [Google Scholar]
- 63.Campbell K CM. Detection of ototoxicity. Semin Hear. 2011;32(02):196–202. [Google Scholar]
- 64.Yu K K, Choi C H, An Y-H et al. Comparison of the effectiveness of monitoring Cisplatin-induced ototoxicity with extended high-frequency pure-tone audiometry or distortion-product otoacoustic emission. Korean J Audiol. 2014;18(02):58–68. doi: 10.7874/kja.2014.18.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh Chauhan R, Saxena R K, Varshey S. The role of ultrahigh-frequency audiometry in the early detection of systemic drug-induced hearing loss. Ear Nose Throat J. 2011;90(05):218–222. doi: 10.1177/014556131109000506. [DOI] [PubMed] [Google Scholar]
- 66.Rybak L P, Whitworth C A, Mukherjea D, Ramkumar V.Mechanisms of cisplatin-induced ototoxicity and prevention Hear Res 2007226(1-2):157–167. [DOI] [PubMed] [Google Scholar]
- 67.Huang M Y, Schacht J. Drug-induced ototoxicity. Pathogenesis and prevention. Med Toxicol. 1989;4(06):452–467. doi: 10.1007/BF03259926. [DOI] [PubMed] [Google Scholar]
- 68.Rybak L P, Whitworth C A. Ototoxicity: therapeutic opportunities. Drug Discov Today. 2005;10(19):1313–1321. doi: 10.1016/S1359-6446(05)03552-X. [DOI] [PubMed] [Google Scholar]
- 69.Reiter R J, Tan D X, Korkmaz A, Fuentes-Broto L. Drug-mediated ototoxicity and tinnitus: alleviation with melatonin. J Physiol Pharmacol. 2011;62(02):151–157. [PubMed] [Google Scholar]
- 70.Kalkanis J G, Whitworth C, Rybak L P. Vitamin E reduces cisplatin ototoxicity. Laryngoscope. 2004;114(03):538–542. doi: 10.1097/00005537-200403000-00028. [DOI] [PubMed] [Google Scholar]
- 71.Choe W T, Chinosornvatana N, Chang K W. Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol Neurotol. 2004;25(06):910–915. doi: 10.1097/00129492-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 72.Hughes A L, Hussain N, Pafford R, Parham K. Dexamethasone otoprotection in a multidose cisplatin ototoxicity mouse model. Otolaryngol Head Neck Surg. 2014;150(01):115–120. doi: 10.1177/0194599813511948. [DOI] [PubMed] [Google Scholar]
- 73.Olgun Y, Kırkım G, Kolatan E et al. Friend or foe? Effect of oral resveratrol on cisplatin ototoxicity. Laryngoscope. 2014;124(03):760–766. doi: 10.1002/lary.24323. [DOI] [PubMed] [Google Scholar]
- 74.Malhotra H. Cisplatin ototoxicity. Indian J Cancer. 2009;46(04):262–263. doi: 10.4103/0019-509X.55545. [DOI] [PubMed] [Google Scholar]
- 75.Mukherjea D, Rybak L P. Pharmacogenomics of cisplatin-induced ototoxicity. Pharmacogenomics. 2011;12(07):1039–1050. doi: 10.2217/pgs.11.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Govender N, Maistry N, Soomar N, Paken J. Hearing loss within a marriage: perceptions of the spouse with normal hearing. S Afr Fam Pract. 2014;56(01):50–56. [Google Scholar]
- 77.Neumann S, Wolfe J. What's new and notable in hearing aids: a friendly guide for parents and hearing aid wearers. Volta Voices. 2013;20(03):24–29. [Google Scholar]
- 78.Parsa V, Scollie S, Glista D, Seelisch A. Nonlinear frequency compression: effects on sound quality ratings of speech and music. Trends Amplif. 2013;17(01):54–68. doi: 10.1177/1084713813480856. [DOI] [PMC free article] [PubMed] [Google Scholar]


