Abstract
Dwarf mutants of pea (Pisum sativum), with impaired gibberellin (GA) biosynthesis in the shoot, were studied to determine whether the roots of these genotypes had altered elongation and GA levels. Mutations na, lh-2, and ls-1 reduced GA levels in root tips and taproot elongation, although in lh-2 and ls-1 roots the reduction in elongation was small (less than 15%). The na mutation reduced taproot length by about 50%. The roots of na plants elongated in response to applied GA1 and recombining na with mutation sln (which blocks GA catabolism) increased GA1 levels in root tips and completely restored normal root development. In shoots, Mendel's le-1 mutation impairs the 3β-hydroxylation of GA20 to the bioactive GA1, resulting in dwarfism. However, GA1 and GA20 levels were normal in le-1 roots, as was root development. The null mutation le-2 also did not reduce root GA levels or elongation. The results support the theory that GAs are important for normal root elongation in pea, and indicate that a 3β-hydroxylase gene other than LE operates in pea roots.
Bioactive gibberellins (GAs) such as GA1 are important regulatory factors in the control of shoot growth (Hedden and Proebsting, 1999). Evidence from several species indicates that GAs are also required for the normal development of roots. For example, root development is altered in the maize d5 mutant (Baluska et al., 1993), and excised roots of the gib-1 (tomato) and d1 (maize) mutants grew more slowly than the corresponding wild-type lines (Mertz, 1966; Butcher et al., 1990; Barlow et al., 1991). Shoots of these mutants are known to be GA1 deficient (Fujioka et al., 1988; Hedden and Lenton, 1988), and it has been assumed that this deficiency extends to the roots. GA biosynthesis inhibitors are also known to inhibit root elongation in wild-type lines of tomato (Butcher et al., 1990), lettuce (Tanimoto, 1990), and maize (Baluska et al., 1993), and this inhibition is overcome by application of bioactive GA, providing further evidence that GAs are important for root growth.
However, in the garden pea (Pisum sativum), the roots of the best known GA1-deficient dwarf mutant, Mendel's le-1, are usually considered to be normal in appearance (Tanimoto, 1990). Although this has not been confirmed previously using isogenic LE/le-1 lines, the general vigor of le-1 lines, which are favored for agricultural purposes, is certainly consistent with normal root development. Furthermore, le-1 roots do not respond to applied GA (Tanimoto, 1990). Nevertheless, as in wild-type peas, le-1 roots can be shortened by treatment with an inhibitor of GA biosynthesis, an effect reversed by GA application (Tanimoto, 1988, 1990). These observations led to suggestions that roots are more sensitive to GAs than are shoots, and that the small amount of bioactive GA produced by the leaky le-1 mutation is sufficient for normal root growth (Tanimoto, 1990). In shoots, le-1 reduces the content of GA1 by at least 10-fold (Ross et al., 1992), and if there are similar effects in roots, it would appear that even quite large reductions in bioactive GA do not alter root development. This would suggest that even though GAs are required for normal root development in pea, they may not limit or regulate that process. It might follow that only a very large decrease in GA levels can reduce root growth in pea.
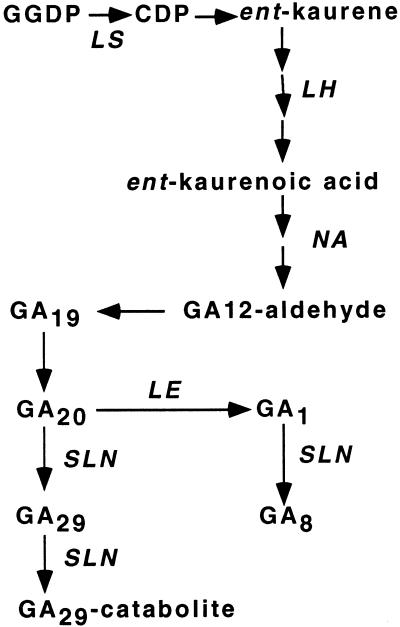
We should not assume, however, that the le-1 mutation actually reduces root GA levels. Smith et al. (1992) found no difference in root GA1 content between wild-type and le-1 plants, although these plants were too old for root development to be readily compared. At present we are unaware of any other reports regarding root GA levels in GA mutants in any species. The aim of the present study was, therefore, to investigate the effects of the GA mutations le-1, le-2, lh-2, ls-1, and na on endogenous GA levels in, and the development of, pea roots. In shoots, these mutations block GA biosynthesis at the steps shown in Figure 1. The ls and lh mutations block the production and oxidation, respectively, of ent-kaurene (Ait-Ali et al., 1997; Swain et al., 1997). na is thought to block between ent-7α-hydroxykaurenoic acid and GA12-aldehyde (Ingram and Reid, 1987). The le alleles block the step GA20 to GA1 (Ingram et al., 1984; Ross et al., 1989). The le-1 mutation arose from a G to A substitution at position 685, and reduces enzyme activity by about 95%, whereas le-2 involves a base deletion of the le-1 allele at position 376, and results in a severely truncated protein with undetectable enzyme activity (Lester et al., 1997, 1999a; Martin et al., 1997). We also use the sln mutation to examine the effects of increased endogenous GA levels on root growth because this mutation blocks the catabolism of GA20 (Fig. 1; Ross et al., 1995; Lester et al., 1999b), resulting in elevated GA levels at the seedling stage (Reid et al., 1992).
Figure 1.
GA biosynthesis pathway in pea, showing the steps affected by the genes studied. GGDP, geranylgeranyldiphosphate; CDP, copalyl diphosphate.
RESULTS AND DISCUSSION
Distribution of GAs in Roots
The level of GA1 was approximately 15-fold greater in the apical 25 mm (“tip”) of the taproot than in the remainder of the taproot, and was also relatively high in the lateral roots (Table I). This is consistent with the proposition that GA1 is the important bioactive GA in pea because the root tips analyzed would have included the zone of maximal taproot growth (Heyes, 1977). Levels of the GA1 catabolite, GA8, were also higher in tips and laterals than in the remaining portions. GA19 was relatively abundant in all portions, and its level consistently exceeded that of its product, GA20. This contrasts with the shoots, where GA20 levels are often greater than those of GA19, especially in leafy apical portions (for example, see Ross, 1998). Except for GA19, the level of GAs in the root, even in the tips, was typically at least an order of magnitude lower than in typical shoot apical portions (Ross, 1998). In roots, GA3 and GA4 were not detected, although the corresponding internal standards, added at a rate of 500 pg g−1 fresh weight, were recovered.
Table I.
Distribution of GAs in wild-type roots
| GA | Root Portion
|
|||
|---|---|---|---|---|
| Top | Intermediate | Tips | Laterals | |
| GA19 | 2,735 ± 15 | 1,425 ± 195 | 1,935 ± 125 | 3,510 ± 720 |
| GA20 | 805 ± 85 | 220 ± 40 | 350 ± 50 | 235 ± 5 |
| GA1 | 110 ± 0 | 95 ± 5 | 1,500 ± 110 | 640 ± 10 |
| GA29 | 555 ± 15 | 105 ± 5 | 480 ± 60 | 415 ± 25 |
| GA8 | 320 ± 30 | 245 ± 5 | 2,190 ± 10 | 990 ± 280 |
Line 107 roots were partitioned into taproot tips (25 mm), lateral roots, and the top and intermediate portions of the remainder. Data are in pg g−1 fresh wt, and are the means and ses of two replicate harvests. Plants were grown in a growth chamber at 18°C and were 12 d old at harvest.
The le Mutations Are Not Expressed in Roots
Comparison of isogenic lines (205+/205−) confirmed that Mendel's le-1 mutation did not affect root development. The overall phenotype of le-1 roots was indistinguishable from that of the wild type (Fig. 2A), and le-1 and LE taproots were of similar length (Table II). Furthermore, in le-1 (205−) root tips GA1 and GA20 levels were similar to those in the isogenic wild type (205+), in contrast with the shoots, where le-1 dramatically reduces GA1 content but elevates that of GA20 (Ingram et al., 1984; Ross et al., 1992). The normal root GA content explains why le-1 roots elongated to the same extent as wild-type roots.
Figure 2.
Photograph showing root phenotypes. A, Left, wild type (205+); right, le-1 (205−). B, Left, wild type; right, na. C, From left to right, wild type, na SLN, na sln, and NA sln. Plants were 8 (C) or 9 (A and B) d old and were grown at 21°C in a growth cabinet.
Table II.
Morphology of, and root GA levels in, LE, le-1, and le-2 seedlings
| Characteristic | LE (205+) | le-1 (205−) | le-1 (Dippes gelbe Viktoria) | le-2 (M66A) |
|---|---|---|---|---|
| Shoot height (mm) | 184 ± 4 | 63 ± 2 | 114 ± 3 | 57 ± 1 |
| Taproot length (mm) | 258 ± 4 | 252 ± 5 | 283 ± 4 | 283 ± 5 |
| Length of the six longest lateral roots (mm) | 128 ± 5 | 124 ± 7 | 94 ± 4 | 91 ± 3 |
| GA level (pg g−1 fresh wt) | ||||
| GA20 | 48 ± 25 | 34 ± 34 | 344 ± 17 | 360 ± 3 |
| GA1 | 483 ± 2 | 448 ± 84 | 179 ± 18 | 176 ± 1 |
There were two pairs of isolines: LE and le-1 (205+/205−), and le-1 and le-2 (Dippes gelbe Viktoria/M66A). Morphological data are means ± se of 12 replicates; plants were grown in a growth chamber at 21°C. GAs were quantified from 40-mm taproot tips and plants were grown in a heated greenhouse. GA levels are shown as means ± se of two replicate harvests. All plants were 12 d old.
It was formally possible that the “leakiness” of the le-1 allele in some way enabled le-1 roots to maintain normal levels of GA1. This possibility was investigated by observing root development in mutant le-2. The le-2 mutation appears to be biochemically null, because when the le-2 cDNA was expressed in Escherichia coli, the expression product failed to convert GA20 to GA1 (Martin et al., 1997; Lester et al., 1999a). A direct comparison of LE and le-2 roots was not possible in the present study, because LE/le-2 isolines were not available. Nevertheless, a valid comparison could be made using the two pairs of isolines LE/le-1 (205+/205−) and le-1/le-2 (Dippes gelbe Viktoria/M66A), because lines 205− and Dippes gelbe Viktoria have an identical le-1 cDNA sequence (Lester et al., 1997, 1999a). In the le-2 mutant, root development and root GA levels were similar to those in its le-1 isoline (Dippes gelbe Viktoria; Table II). Because le-1 itself did not affect root development or root GA levels (see above), we can conclude that root development and root GA levels were normal (i.e. similar to wild type) in the le-2 mutant.
The level of GA1 did vary between the two le-1 lines, 205− and Dippes Gelbe Viktoria (Table II), which are derived from quite distinct pea varieties. This demonstrates the effect that genetic background may exert on hormone levels and reinforces the need for using isogenic lines when examining the effects of major genes; such lines are still not always used.
The lack of effect of le-1 and le-2 on root GA1 levels strongly supports the idea that GA1 can be synthesized in root tissues because it is difficult to envisage how le-1 and le-2 roots could contain normal GA1 levels if they were dependent on the shoot for this GA (because le-1 and le-2 shoots contain less than 10% and 2%, respectively, of the GA1 content of wild-type shoots; Ross et al., 1989, 1992).
The level of LE mRNA is reported to be quite high in roots (at least in dark-grown plants; Martin et al., 1997), which suggests a role for LE in these organs. However, the normal GA1 level in le-2 roots indicates that at least one other 3β-hydroxylase gene also operates in roots, and that this second gene can completely compensate for the loss of LE activity in le-2 roots. Therefore, the mere presence of mRNA for a gene should not be interpreted to mean that the tissue depends on that gene for a certain step to proceed.
Mutations na, lh-2, and ls-1 Are Expressed in Roots
In contrast with the le mutations, the mutation na markedly affected root growth (Fig. 2B). The length of the taproot, measured 8 (Fig. 2C), 9 (Fig. 2B), and 12 d (Table III) after sowing, was consistently reduced in na plants, compared with wild-type plants. Lateral roots were also substantially shorter in na plants (Table III). It is important that the na mutation markedly reduced GA1 content in root tips (Tables III and IV). This appears to be the first time that reduced root growth has been directly correlated with reductions in endogenous root GA levels. Furthermore, the diameter of na roots (20 mm from the root tip) was significantly greater than that of wild-type roots (Table III), consistent with previous reports linking GA deficiency with root thickening (Butcher et al., 1990; Tanimoto, 1994). The GA levels in Tables III and IV are from 12.5-mm root tips (na) and 25-mm tips (NA), which were harvested this way to avoid including too much mature root tissue in the na harvest. Both sections would have included the entire growth zone (Heyes, 1977). Very similar results were obtained when root tips of the same length (25 mm) from both genotypes were harvested (data not shown). These results support the theory that GAs are important for root development. Consistent with that theory, roots of the na mutant responded to exogenous GA1 application (Table V).
Table III.
Morphology of, and root GA levels in, NA and na seedlings
| Characteristic | NA | na |
|---|---|---|
| Shoot height (mm) | 124 ± 3 | 25 ± 1 |
| Taproot length (mm) | 201 ± 7 | 104 ± 4 |
| Taproot diameter, 20 mm from tip (mm) | 1.1 ± 0.04 | 1.8 ± 0.05 |
| Number of lateral roots longer than 10 mm | 41.3 ± 0.7 | 11.7 ± 1.5 |
| Length of the four longest lateral roots (mm) | 29 ± 2 | 13 ± 1 |
| GA level (pg g−1 fresh wt) | ||
| GA20 | 55 ± 5 | 2 ± 2 |
| GA1 | 570 ± 30 | 75 ± 25 |
| GA29 | 120 ± 10 | 40 ± 40 |
| GA8 | 1,325 ± 365 | 170 ± 40 |
Shown are means ± se of 12 replicates for morphological data, and means ± se of two replicate harvests for GA levels. GAs were quantified from taproot tips (25-mm tips for NA and 12.5-mm tips for na). Plants were grown in a growth chamber at 21°C. All plants were 12 d old.
Table IV.
Morphology of, and root GA levels in, NA SLN, na SLN, na sln, and NA sln seedlings
| Characteristic | NA SLN (WT) | na SLN | na sln | NA sln |
|---|---|---|---|---|
| Shoot height (mm) | 41 ± 1 | 23 ± 2 | 92 ± 3 | 95 ± 4 |
| Taproot length (mm) | 181 ± 6 | 99 ± 6 | 159 ± 7 | 172 ± 4 |
| Taproot diameter, 20 mm from tip (mm) | 1.1 ± 0.08 | 1.7 ± 0.07 | 1.1 ± 0.04 | 1.0 ± 0.06 |
| Number of lateral roots longer than 10 mm | 20.1 ± 2.6 | 6.5 ± 1.9 | 17.4 ± 3.7 | 18.4 ± 3.0 |
| GA level (pg g−1 fresh wt) | ||||
| GA19 | 1,445 ± 285 | n.d. | 35 ± 35 | 7,885 ± 1,055 |
| GA20 | 119 ± 31 | 30 ± 30 | 505 ± 15 | 2,315 ± 5 |
| GA1 | 1,790 ± 80 | 51 ± 11 | 3,820 ± 270 | 8,500 ± 1,030 |
| GA29 | 2,540 ± 40 | 1,065 ± 135 | 53,350 ± 10,950 | 49,450 ± 350 |
| GA8 | 3,865 ± 205 | 5,550 ± 180 | 15,265 ± 65 | 16,900 ± 800 |
Morphological data are means ± se of 12 replicates; plants were grown in a growth chamber at 21°C for 12 d. GAs were quantified from 12.5-mm (na SLN) or 25-mm (all other genotypes) taproot tips; plants were grown in a heated greenhouse for 9 d. GA levels are shown as means ± se of two replicate harvests. n.d., no dilution of internal standard.
Table V.
Effects of GA1 application on shoot and root elongation in genotype na
| Characteristic | Wild Type | na | na+ GA1 |
|---|---|---|---|
| Shoot height (mm) | 54 ± 3 | 24 ± 1 | 40 ± 3 |
| Taproot length (mm) | 189 ± 7 | 97 ± 2 | 125 ± 7 |
GA1 (1 μg in 5 μL of ethanol) was applied to the dry seed before sowing. Plants were grown in a heated greenhouse for 11 d before measurements were made. n = 12.
However, the most striking rescue of the na root phenotype was observed after recombining na with the sln mutation (Table IV and Fig. 2C). It was reported previously that sln results in elevated levels of GA1 in the shoots of seedlings (Reid et al., 1992), and here we show that the same is true for roots (Table IV). The observation that na sln roots were essentially wild type in appearance (Fig. 2C) confirms that GA deficiency is the primary reason for the shorter roots of na SLN plants. The shoots of na sln plants were elongated at 8 (Fig. 2C) and 9 d (Table IV), but their internodes shortened dramatically as the plants matured (Ross et al., 1995). Roots of sln plants contained high levels of other GAs derived (directly or indirectly) from GA20, namely GA29 and GA8 (Table IV). The GA20 that gave rise to these elevated levels originated in the seed (Reid et al., 1992; Ross et al., 1995). Seeds of genotype na sln would have contained high GA20 levels because the na mutation does not block GA biosynthesis in seeds (Potts and Reid, 1983). It is also probable that even in na SLN plants some GA20 moved from the seed into the root. This would explain why in Table IV there were relatively high levels of GA8 and GA29 in na SLN roots.
Mutations lh-2 and ls-1 also affected both root development and endogenous GA levels (Table VI). The phenotypic effects of these mutations were subtle, but both significantly (P < 0.05) reduced taproot length at 12 d, the number of lateral roots longer than 10 mm, and the length of the longest lateral roots (Table VI). Mutation lh-2 reduced GA1 content substantially but reduced root elongation less than na did (Tables III and VI). The reason for this is not clear, but possibly on the LH LS/lh-2/ls-1 genetic background the roots are more sensitive to GA than on the NA/na background.
Table VI.
Morphology of, and root GA levels in, WT (line 107), lh-2 and ls-1 seedlings
| Characteristic | WT | lh-2 | ls-1 |
|---|---|---|---|
| Shoot height (mm) | 138 ± 3 | 61 ± 2 | 39 ± 1 |
| Taproot length (mm) | 237 ± 4 | 208 ± 5 | 221 ± 5 |
| Number of lateral roots longer than 10 mm | 73 ± 2 | 50 ± 2 | 54 ± 3 |
| Mean length of the six longest lateral roots (mm) | 114 ± 4 | 81 ± 2 | 93 ± 3 |
| GA level (pg g−1 fresh wt) | |||
| GA19 | 3,745 ± 955 | 20 ± 13 | 13 ± 1 |
| GA20 | 153 ± 54 | 37 ± 37 | 17 ± 12 |
| GA1 | 768 ± 106 | 79 ± 2 | 156 ± 13 |
| GA29 | 250 ± 38 | 46 ± 46 | 10 ± 10 |
| GA8 | 2,630 ± 190 | 373 ± 24 | 339 ± 38 |
Shown are means ± se of 10 to 12 replicates for morphological data, and means ± se of two replicate harvests for GA levels. GAs were quantified from root tips (40 mm). Plants were grown in a heated greenhouse. Morphological data and GA levels were obtained in separate experiments. All plants were 12 d old.
In both lh-2 and ls-1 roots the reduction in GA19 content was greater than the drop in GA1. This indicates that in roots, as in shoots (Hedden and Croker, 1992), GA1 negatively regulates its own biosynthesis, with the metabolism of GA19 occurring at a faster rate in GA1-deficient roots. This suggestion also explains why, on an NA background, the level of GA19 was higher in sln roots (where the GA1 level was elevated) than in SLN roots (Table IV).
The shorter roots of na plants are most likely not a consequence of reduced photosynthesis by na shoots (which are very small; Fig. 2), because in dark-grown plants na caused a similar reduction to that observed in the light (data not shown). In general, there was no evidence from the present study that dwarfism in the shoot is necessarily associated with shorter roots. For example, the roots of the le-1 and le-2 mutants were normal even though shoot height was markedly reduced by le-1 and particularly by le-2 (Table II).
CONCLUSION
These results show, for the first time, that some mutants with GA-deficient shoots also possess GA-deficient roots. This GA deficiency was associated with impaired root elongation (Tables III, IV, and VI). Mutant na roots responded to exogenous GA (Table V), and were rescued by mutation sln (Fig. 2C), which increases root GA content (Table IV). Therefore, our results support the theory that GAs are important for normal root growth. It is interesting that on an NA (wild-type) background, sln did not increase taproot length (in contrast with stem length; Table IV; Fig. 2C). This result is consistent with the inability of applied GA to promote elongation in wild-type pea roots, unless those roots are pretreated with a GA biosynthesis inhibitor (Tanimoto, 1988). These findings suggest that the GA1 level in wild-type roots is sufficient for maximal elongation. Because this is not the case in shoots, even though they contain more GA1 than roots, the present results support the previous theory (Tanimoto, 1990) that roots are more GA sensitive than shoots. However, there is no longer any evidence from the le-1 mutation for this theory (Tanimoto, 1990). It now appears that mutant le-1 roots are normal not because moderate reductions in root GA1 level have no effect, but because le-1 does not reduce root GA1 levels (Table II).
MATERIALS AND METHODS
Plant Material and Growing Conditions
Three pairs of isogenic lines, and a fourth group of three isogenic lines, were used in this study. These were: NGB1769 (NA) and NGB1766 (na); 205+ (LE) and 205− (le-1); Dippes gelbe Viktoria (DGV, le-1) and M66A (le-2, previously led; Ross and Reid 1987); and 107 (LH LS, selected from cv Torsdag), NGB5843 (lh-2), and 181 (ls-1) (Reid and Potts, 1986; Swain and Reid, 1992). Each pair or triplet possesses a different genetic background. Genotypes na sln, na SLN, NA sln, and NA SLN originated from a cross between line NGB6074 (sln) and an na segregate from cross NGB1766 × NGB1769.
The growth medium was a 1:1 (v/v) mixture of dolerite chips:vermiculite, topped with 20 to 30 mm of sterilized potting mix. Plants were grown either in a heated greenhouse (Beveridge and Murfet, 1996) with an 18-h photoperiod, provided by extending the natural photoperiod at its beginning and end with a mixture of fluorescent (40-W cool-white, Osram, Munich) and incandescent (100-W, Thorn, Melbourne, Australia) light (25 μmol m−2 s−1 at pot top); or in a growth cabinet in an 18-h photoperiod provided by a mixture of fluorescent and incandescent light (36 and 60 W, respectively; Thorn, Australia; 200 μmol m−2 s−1 at pot top).
Extraction and Quantification of GAs
Root tips were harvested by excising 12.5, 25, or 40 mm from the taproot apex; typically, approximately 1 g of material from 30 to 60 plants was harvested. The distribution of GAs in wild-type roots was investigated using line 107. In this case 25-mm taproot tips were excised; these constituted about 10% of the total root fresh weight. Lateral roots were also excised (about 10% of total root fresh weight). The remainder of the root was partitioned into “top” and “intermediate” sections, consisting of approximately 55% and 25%, respectively, of the total root fresh weight.
Harvested material was immediately immersed in cold (−20°C) methanol and placed in a freezer. The tissue was macerated using a blender and GAs were extracted at 2°C to 4°C for 24 h, prior to filtering. The internal standards were [2H2]GA19, [2H2]GA20, [2H2]GA29, [2H2]GA1, [2H2]GA8, [2H2]GA3, and [2H2]GA4. Extracts were purified as before (Ross, 1998), except that (in some cases) after the Sep-Pak step GAs were extracted into ethyl acetate at pH 2.9, prior to HPLC, performed as before (Ross, 1998). In other cases, GAs were further purified at the (post-HPLC) methyl ester stage by taking up the extract in 1 mL of distilled water and partitioning three times against 400 μL of diethyl ether. Gas chromatography-mass spectrometry was performed as described previously (Ross, 1998), except that in some cases extracts were derivatized twice, first in 10 μL of dry pyridine and 40 μL of N,O-bistrimethylsilyltrifluoroacetamide + 1% (v/v) trimethylchlorosilane, and then, (after drying), in 15 μL of bistrimethylsilyltrifluoroacetamide + 1% (v/v) trimethylchlorosilane. Peak areas were corrected for contributions from naturally occurring isotopes and for small amounts of unlabeled material in the internal standards. The limit of detection (for GA1) was approximately 30 pg gFW−1 for a harvest of 1 g of tissue.
ACKNOWLEDGMENTS
We thank Dr. Noel Davies (Central Science Laboratory, University of Tasmania), Jennifer Smith, Tracey Jackson, Ian Cummings, Carla Wolbang, Shona Batge, and Jennifer Yaxley (School of Plant Science, University of Tasmania) for technical assistance, Professor L.N. Mander (Australian National University, Canberra) for deuterated GAs, Professor Ian Murfet (School of Plant Science, University of Tasmania) for helpful discussions, and the Australian Research Council for financial support.
LITERATURE CITED
- Ait-Ali T, Swain SM, Reid JB, Sun T-P, Kamiya Y. The LS locus of pea encodes the gibberellin biosynthesis enzyme ent-kaurene synthase A. Plant J. 1997;11:443–454. doi: 10.1046/j.1365-313x.1997.11030443.x. [DOI] [PubMed] [Google Scholar]
- Baluska F, Parker JS, Barlow PW. A role for gibberellic acid in orienting microtubules and regulating cell growth polarity in the maize root cortex. Planta. 1993;191:149–157. [Google Scholar]
- Barlow PW, Brain P, Parker JS. Cellular growth in roots of a gibberellin-deficient mutant of tomato (Lycopersicon esculentum Mill.) and its wild type. J Exp Bot. 1991;42:339–351. [Google Scholar]
- Beveridge CA, Murfet IC. The gigas mutant in pea is deficient in the floral stimulus. Physiol Plant. 1996;96:637–645. [Google Scholar]
- Butcher DN, Clark JA, Lenton JR. Gibberellins and the growth of excised tomato roots: comparison of gib-1 mutant and wild type and responses to applied GA3 and 2S, 3S paclobutrazol. J Exp Bot. 1990;41:715–722. [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Gaskin P, MacMillan J, Phinney BO, Takahashi N. Qualitative and quantitative analyses of gibberellins in vegetative shoots of normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 seedlings of Zea mays L. Plant Physiol. 1988;88:1367–1372. doi: 10.1104/pp.88.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P, Croker SJ. Regulation of gibberellin biosynthesis in maize seedlings. In: Karssen C, Van Loon L, Vreugdenhil D, editors. Progress in Plant Growth Regulation. Dordrecht, The Netherlands: Kluwer; 1992. pp. 534–554. [Google Scholar]
- Hedden P, Lenton JR. Genetic and chemical approaches to the metabolic regulation and mode of action of gibberellins in plants. In: Steffens G, Rumsey T, editors. Biomechanisms Regulating Growth and Development. Dordrecht, The Netherlands: Kluwer; 1988. pp. 175–204. [Google Scholar]
- Hedden P, Proebsting WM. Genetic analysis of gibberellin biosynthesis. Plant Physiol. 1999;119:365–370. doi: 10.1104/pp.119.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes JK. Metabolic aspects of cell growth and development in the root. In: Sutcliffe J, Pate J, editors. The Physiology of The Garden Pea. London: Academic Press; 1977. pp. 153–181. [Google Scholar]
- Ingram TJ, Reid JB. Internode length in Pisum: gene na may block gibberellin synthesis between ent-7α-hydroxykaurenoic acid and GA12-aldehyde. Plant Physiol. 1987;83:1048–1053. doi: 10.1104/pp.83.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram TJ, Reid JB, Murfet IC, Gaskin P, Willis CL, MacMillan J. Internode length in Pisum: the Le gene controls the 3β-hydroxylation of gibberellin A20 to gibberellin A1. Planta. 1984;160:455–463. doi: 10.1007/BF00429763. [DOI] [PubMed] [Google Scholar]
- Lester DR, MacKenzie-Hose AK, Davies PJ, Ross JJ, Reid JB. The influence of the null le-2 mutation on gibberellin levels in developing pea seeds. Plant Growth Regul. 1999a;27:83–89. [Google Scholar]
- Lester DR, Ross JJ, Davies PJ, Reid JB. Mendel's stem length gene (Le) encodes a gibberellin 3β-hydroxylase. Plant Cell. 1997;9:1435–1443. doi: 10.1105/tpc.9.8.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester DR, Ross JJ, Smith JJ, Elliott RC, Reid JB. Gibberellin 2-oxidation and the SLN gene of Pisum sativum. Plant J. 1999b;19:65–73. doi: 10.1046/j.1365-313x.1999.00501.x. [DOI] [PubMed] [Google Scholar]
- Martin DN, Proebsting WM, Hedden P. Mendel's dwarfing gene: cDNAs from the Le alleles and function of the expressed proteins. Proc Natl Acad Sci. 1997;94:8907–8911. doi: 10.1073/pnas.94.16.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz D. Hormonal control of root growth. Plant Cell Physiol. 1966;7:125–135. [Google Scholar]
- Potts WC, Reid JB. Internode length in Pisum: III. The effect and interaction of the Na/na and Le/le gene differences on endogenous gibberellin-like substances. Physiol Plant. 1983;57:448–454. [Google Scholar]
- Reid JB, Potts WC. Internode length in Pisum: two further mutants, lh and ls, with reduced gibberellin synthesis, and a gibberellin-insensitive mutant, lk. Physiol Plant. 1986;66:417–426. [Google Scholar]
- Reid JB, Ross JJ, Swain SM. Internode length in Pisum: a new, slender mutant with elevated levels of C19 gibberellins. Planta. 1992;188:462–467. doi: 10.1007/BF00197036. [DOI] [PubMed] [Google Scholar]
- Ross JJ. Effects of auxin transport inhibitors on gibberellins in pea. J Plant Growth Regul. 1998;17:141–146. [Google Scholar]
- Ross JJ, Reid JB. Internode length in Pisum: a new allele at the le locus. Ann Bot. 1987;59:107–109. [Google Scholar]
- Ross JJ, Reid JB, Dungey HS. Ontogenetic variation in levels of gibberellin A1 in Pisum: implications for the control of stem elongation. Planta. 1992;186:166–171. doi: 10.1007/BF00196245. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Reid JB, Gaskin P, MacMillan J. Internode length in Pisum: estimation of GA1 levels in genotypes Le, le and led. Physiol Plant. 1989;76:173–176. [Google Scholar]
- Ross JJ, Reid JB, Swain SM, Hasan O, Poole AT, Hedden P, Willis CL. Genetic regulation of gibberellin deactivation in Pisum. Plant J. 1995;7:513–523. [Google Scholar]
- Smith VA, Knatt CJ, Gaskin P, Reid JB. The distribution of gibberellins in vegetative tissues of Pisum sativum L: I. Biological and biochemical consequences of the le mutation. Plant Physiol. 1992;99:368–371. doi: 10.1104/pp.99.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Reid JB. Internode length in Pisum: a new allele at the Lh locus. Physiol Plant. 1992;86:124–130. [Google Scholar]
- Swain SM, Reid JB, Kamiya Y. Gibberellins are required for embryo growth and seed development in pea. Plant J. 1997;12:1329–1338. [Google Scholar]
- Tanimoto E. Gibberellin regulation of root growth with change in galactose content of cell walls in Pisum sativum. Plant Cell Physiol. 1988;29:269–280. [Google Scholar]
- Tanimoto E. Gibberellin requirement for the normal growth of roots. In: Takahashi N, Phinney B, Mac- Millan J, editors. Gibberellins. New York: Springer-Verlag; 1990. pp. 229–240. [Google Scholar]
- Tanimoto E. Interaction of gibberellin A3 and ancymidol in the growth and cell-wall extensibility of dwarf pea roots. Plant Cell Physiol. 1994;35:1019–1028. [Google Scholar]