Abstract
BACKGROUND.
Women with breast cancer (BCa) experience heightened distress which is related to greater inflammation and poorer outcomes. The s100 protein family facilitates the inflammatory response by regulating myeloid cell function by binding Toll-like receptor 4 and the receptor for advanced glycation end products (RAGE). The heterodimer s100A8/A9 RAGE ligand is associated with hastened tumor development and metastasis. Previously, a 10-week stress management intervention using cognitive behavioral therapy (CBT) and relaxation training (RT) was associated with less leukocyte inflammatory gene expression in BCa patients; however, its impact on s100A8/A9 was not examined. Because a 10-week intervention may be impractical during primary BCa treatment we developed briefer forms of CBT and RT and showed their efficacy in reducing distress over 12 months of primary treatment. Here we tested their effects on s100A8/A9 levels over the initial 12 months of BCa treatment.
METHODS.
Post-surgical BCa patients (Stage 0–IIIb) were randomized to a 5-week group-based condition: CBT, RT, or health education control (HE). At baseline and 12-months, women provided sera from which s100A8/A9 levels were determined by ELISA.
RESULTS.
Participants (M age=54.81, SD 9.63) assigned to either CBT (N=41) or RT (N=38) showed significant s100A8/A9 decreases over 12 months compared to HE (N=44) (F(1, 114)=4.500, p=0.036) controlling for age, stage, time since surgery, and receipt of chemotherapy or radiation. Greater stress management skills increase pre-to-post intervention predicted greater reductions in s100A8/A9 levels over 12 months (β=−0.379, t[101]=−4.056, p<0.001).
CONCLUSIONS.
Brief post-surgical group-based stress management reduces RAGE-associated s100A8/A9 ligand during primary BCa treatment.
Keywords: Breast cancer, stress management, cognitive behavioral therapy, RAGE, s100
Precis:
Post-surgical stage 0-III breast cancer patients (M age=54.81, SD 9.63) randomized to either 5-week cognitive behavioral therapy (N=41) or 5-week relaxation training (N=38) showed significant s100A8/A9 decreases over 12 months compared to 5-week health education control (N=44) and greater stress management skills increase pre-to-post intervention predicted greater reductions in s100A8/A9 levels over 12 months, controlling for covariates. Brief post-surgical group-based stress management during primary BCa treatment reduces RAGE-associated s100A8/A9, a ligand that is associated with inflammatory signaling and hastened tumor development and metastasis.
INTRODUCTION
Cancer diagnosis and treatment induce distress, which may decrease health behaviors, and dysregulate neuroendocrine and immune functioning, which could worsen health outcomes1–3. Learning of a cancer diagnosis and undergoing treatment generates multiple uncontrollable and unpredictable stressors, which place intense demands on a patient’s psychological adaptation2. Physiological adaptations (e.g., sympathetic nervous system [SNS] and hypothalamic pituitary adrenal [HPA] axis-mediated neuroendocrine and neural signaling) may change in parallel with variations in psychological adaptation. These changes may alter the activity of myeloid immune cells and tumor cells in ways that can enhance tumor growth and increase the odds of metastasis (e.g., increased angiogenesis, migration and invasion, inflammation, and immune evasion), thereby affecting long-term quality of life and health outcomes in cancer patients2, 4.
Since modifiable cognitive, behavioral, and interpersonal factors can facilitate adaptation during active treatment and throughout cancer survivorship, several studies have tested the effects of various behavioral and psychosocial stress management interventions in cancer patients at various points in the cancer continuum2, 3. Stress management approaches that combine relaxation training (RT), cognitive behavioral therapy (CBT), and Health Education (HE)5, or RT and CBT (Cognitive Behavioral Stress Management [CBSM]6) have been shown to improve psychological adaptation5, 7–9, neuroendocrine10, 11, and immune5, 12–14 indicators in non-metastatic breast cancer (BCa) patients undergoing primary treatment. Specifically, these forms of stress management delivered in the period after surgery for BCa into active adjuvant therapy down-regulate inflammatory processes (e.g., leukocyte pro-inflammatory cytokine and chemokine gene expression13; leukocyte cell populations15). These interventions have ranged in duration from 10 weeks6 to 12 months7. Importantly each of these interventions was associated with improved overall survival and disease-free interval at an 11-yr median follow-up16, 17 and declines in inflammatory signaling over the first 12 months predicted longer disease-free survival in one trial14, suggesting that intervening early in primary treatment for non-metastatic BCa can produce long-term clinical benefit.
Despite these encouraging results, psychological intervention programs of 10 weeks – 12 months in duration may not be feasible in the clinical oncology setting. However, after demonstrating that a 10-week intervention improved psychological adaptation indicators over the 12-month primary breast cancer treatment period8, one team noted that women attending 5 of 10 sessions showed similar effects to those attending 8 – 10 sessions. They further observed that reported increases in self-efficacy for using RT and CBT skills were associated with greater improvements in psychological and physiological adaptation8, 9, 11. A subsequent “dismantling” trial comparing the effects of 5-week group-based RT or CBT vs a 5-week attention-matched HE control revealed that BCa patients assigned to either RT or CBT showed significant reductions in distress and psychological adversity vs those in HE18. Therefore, two different brief forms of group-based stress management, RT and CBT, were effective, and significantly better than HE in improving psychological adaptation during primary treatment. Prior work showed that BCa patients assigned to 10 weeks of CBSM revealed reductions in leukocyte pro-inflammatory (cytokine [IL1, IL6, TNFA], chemokine, COX2) and pro-metastatic (MMP9) gene expression during primary treatment13, thus the present study examined whether these briefer forms of stress management could modulate inflammatory signaling.
One process that is suggested to contribute to stress-associated elevated leukocyte inflammatory signaling is the effusion of myeloid-derived suppressor cells (MDSCs)19 from the bone marrow, following SNS activation20. These myeloid cells are hypothesized to stimulate immune and cancer cells, among others, through the Receptor for Advanced Glycation End Products (RAGE), a cell surface receptor part of the immunoglobulin sub-family of proteins21. Increased RAGE activation, via ligands such as the heterodimer s100A8/A922, is associated with greater breast tumor differentiation, lymph node metastasis and distant metastasis21, 23.
Given emerging models suggesting that mood states and other stress factors may relate to disease via RAGE-mediated processes24, and prior knowledge that stress-related SNS neuroendocrines (norepinephrine) can stimulate bone marrow to facilitate effusion of myeloid cells capable of producing RAGE ligands20, we examined the effects of the two brief stress management interventions (5-week RT and CBT) vs 5-week HE on circulating levels of s100A8/A9 in a sample of post-surgical breast cancer patients. We hypothesized that those assigned to either of the active stress management conditions, RT or CBT, would show significant reductions in s100A8/A9 over 12 months vs those in the HE control condition. We also hypothesized that the magnitude of stress management skill improvements would relate to the relative reduction in s100A8/A9 levels over time.
METHODS
Procedures and Participants
This single-center, single-blind, randomized controlled trial was conducted at the University of Miami and approved by the Institutional Review Board and is registered as National Institutes of Health Clinical Trial NCT02103387. Detailed information on methodology including pre-specified primary and secondary outcome measures and allocation to study conditions can be found in a previously published report on the trial18. The present study is based upon secondary analyses of preserved blood samples that were conceptualized after the study commenced. From 2006 to 2013, women with stage 0-III BCa were recruited from the Sylvester Comprehensive Cancer Center and private clinics in South Florida. Recruitment ended when targeted sample size was reached. Women were age 21 or older and up to 10 weeks post-surgery. Women were excluded during screening for severe psychiatric illness, non-fluency in English, prior history of cancer (except non-melanoma skin cancer), stage IV BCa, other serious chronic medical conditions, and initiation of neoadjuvant or adjuvant therapy.
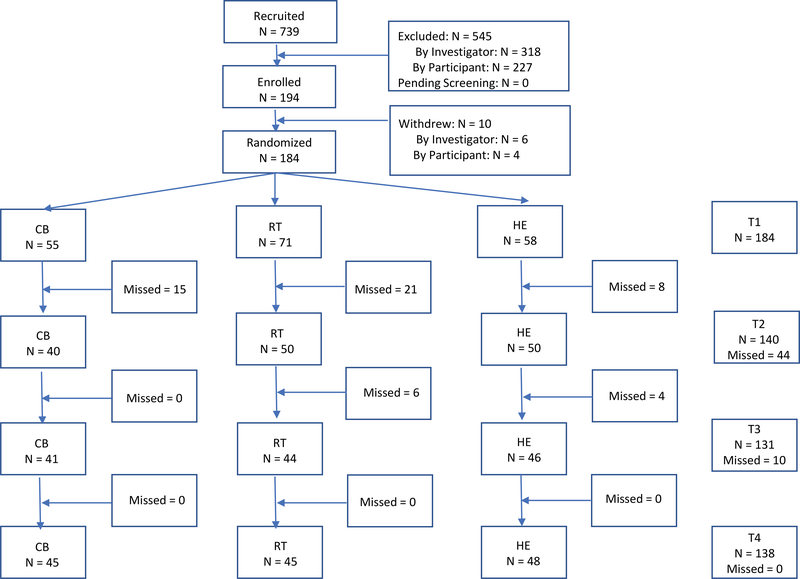
A priori power analysis using the NQuery program indicated that a sample size of 60 participants per condition (180 total) would provide 75% power to detect a medium effect size at a significance level of p< 0.05. Of the 739 women approached, 545 were excluded (318 for not meeting study criteria and 227 for participant refusal or non-availability) and 194 gave written informed consent and were enrolled in the study and 11 withdrew prior to randomization (See Figure 1 for CONSORT). Following baseline assessment, these 183 participants were randomized into one of three conditions, Cognitive Behavioral Therapy (CBT), Relaxation Training (RT), or a time-attention matched Health Education control (HE). A project coordinator not involved in intervention administration or assessment generated the random allocation sequence, enrolled participants, and assigned participants to groups. The sequence of the groups was pre-determined by a drawing. Of this cohort, 123 had an available baseline and 12-month serum sample for analysis (CBT: N=41; RT: N=38; HE: N=44). This was the subsample that was used for the present analyses. Chi-squared and independent t-tests were run to assess for differential attrition. Attrition did not differ significantly based on intervention condition, stage, number of positive lymph nodes, marital status, income, education, chemotherapy receipt, nor radiation receipt (p>0.05).
Figure 1:
CONSORT
Study Conditions
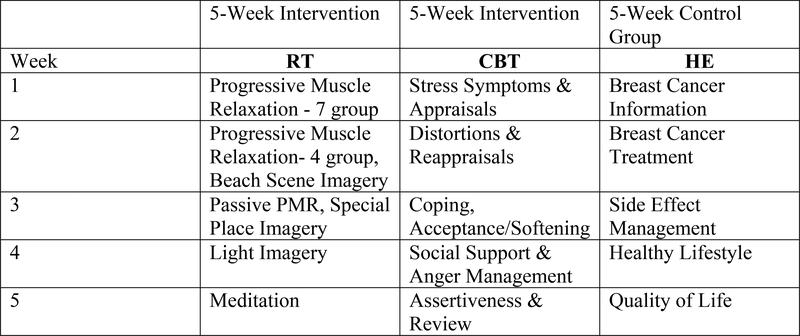
All conditions consisted of 1.5 hour group sessions that met once weekly for five consecutive weeks. (See Figure 2 for intervention content). Groups consisting of 3–7 participants were led by facilitators from a rotating pool of pre-doctoral students in an APA-approved Clinical Psychology Ph.D. program over the years of the trial (total facilitators=7) who were trained (~20 hours) in and implemented one or more of the three intervention protocols (CBT, RT, HE). Intervention sessions were videotaped and reviewed for protocol fidelity by study investigators with licenses in clinical psychology during weekly supervision with the interventionists. All three of the intervention conditions included an in-person weekly session and a written participant manual containing all of the key information from session modules.
Figure 2:
Intervention content
CBT:
The CBT condition drew on core concepts from Beck’s Cognitive Behavioral Therapy, Stress and Coping Theory, and Social Learning Theory and was derived from the CBT components of the 10-week CBSM manualized intervention6. Intervention content included thought monitoring, cognitive restructuring, adaptive coping skills, communication skills, and social network building methods. Participants were also given exercises to complete at home to reinforce concepts learned in sessions, which were then discussed at subsequent sessions.
RT:
The RT condition was derived from the relaxation components of the 10-week CBSM manualized intervention6. The intervention included in-session instruction and discussion of and at-home daily practice of relaxation techniques, including progressive muscle relaxation, diaphragmatic breathing, guided imagery, and meditation. Progress on practice of RT techniques was discussed at each session
HE:
The HE condition was the time-attention matched control and consisted of educational content related to BCa diagnosis and treatment, available resources, side effect management, and healthy lifestyle behaviors. The intervention included in-session instruction and discussion of the material and at-home review of session modules.
Assessments
Participants provided a blood sample at baseline prior to randomization (T1) and again 12 months post-baseline (T2). Participants completed a self-report assessment of perceived stress management skills at baseline and again post-intervention (approximately 6 weeks post-baseline). Participants also provided self-report demographic information and self-report medical information, which was verified via medical chart review. A blinded study coordinator generated the randomization sequence and randomized participants to study condition.
Measurements
S100A8/A9 Levels
Blood samples of approximately 35ml were obtained by a licensed phlebotomist between 4pm and 6:30pm at baseline and 12-month follow-up. Same-day centrifugation was used to separate the serum from blood samples. An ultra-sensitive enzyme-linked immunosorbent assay (HycultBiotech Calprotectin Human ELISA) was conducted by trained lab personnel on the serum samples to quantify the concentration of S100A8/A9 ligands.
Stress Management Skill Confidence
A subset of the Measure of Current Status-Part A25 assessed women’s perceived confidence in using stress management skills targeted by the interventions. Changes in selected items from this scale have been shown to relate to improvements in psychological8 and physiological adaptation11 in breast cancer patients in a prior CBSM intervention trial8. Scores on relevant CBT-based items including, “I can easily recognize situations that make me feel stressed or upset,” “I am aware of the stream of thoughts that pass through my mind as events occur,” and RT-based items including “I am able to use mental imagery to reduce any tension I experience,” and “I am able to use muscle relaxation techniques to reduce any tension I experience” were combined for a composite perceived stress management skills score. Participants were administered all items regardless of group assignment and rated each item from 1 (“I cannot do this at all”) to 5 (“I can do this extremely well”) for a total possible score of 20.
Statistical Analyses
Data was analyzed using the Statistical Package for the Social Sciences (SPSS)-version 24. Analyses included all participants from the parent trial for whom baseline and 12-month follow-up serum samples were available (N=123), including participants who did not attend all sessions. Group differences on demographic characteristics and medical factors, were assessed with chi-square tests and one-way ANOVAs. Data were examined for skewness, and kurtosis. Intervention effects were tested using 2 (Condition: CBT/RT: active treatment, HE: control) by 2 (Time: baseline, 12 months) repeated-measures analysis of co-variance (RANCOVA) controlling for age (years), stage (0 vs I-III), time from surgery to baseline (days), and receipt of chemotherapy and radiation (yes/no). ANOVA was used to assess differences in stress management skill uptake between intervention and control conditions and associations between 12-month changes in s100A8/A9 and pre-post intervention stress management skills in the full sample were determined using linear regression, including the same covariates. Covariates were selected based on previous literature that suggests that the variables of age, disease stage, and the amount of time since surgery may be related to stress adaptation26 and inflammatory markers,27 and these covariates have been controlled in our prior studies relating distress states28, 29 to immune markers in breast cancer patients.
RESULTS
Sample Characteristics
Sample demographic and medical characteristics can be found in Table 1. There were no differences in intervention condition assignment on demographic characteristics or disease or treatment factors. Across all study conditions, attendance at the 5 weekly sessions was high (Mean=4.30, SD=1.16) and the number of sessions attended did not significantly differ across the study conditions (F(2, 98)=1.868, p=0.253). Session attendance was not significantly associated with the magnitude of T1–T2 changes in s100A8/A9. There was a significant baseline group difference for natural log (ln) s100A8/A9, F(2, 120)=9.16, p<0.001 such that those assigned to the active stress management conditions showed higher levels compared to HE at baseline.
Table 1:
Baseline s100A8/A9, Demographic and Medical Variables by Treatment Group
| Variable | CBT (n=41) | RT (n=38) | HE (n=44) | Total (n=123) | Statistic | p |
|---|---|---|---|---|---|---|
| Age (years), 28–77 | 54.54(9.48) | 54.39(10.68) | 55.43(8.99) | 54.81(9.63) | F(2, 120)=0.142 | 0.868 |
| Surgery to baseline (days) | 34.51 (22.21) | 34.55(16.49) | 35.18(17.15) | 34.76(18.65) | F(2, 120)=0.017 | 0.983 |
| Race/Ethnicity | X2(6)=5.494 | 0.482 | ||||
| Non-Hispanic White | 20(48.78%) | 17(44.74%) | 23(52.27%) | 60(48.78%) | ||
| Hispanic | 13(31.71%) | 18(47.37%) | 18(40.91%) | 49(39.84%) | ||
| African American | 4(9.76%) | 1(2.63%) | 2(4.55%) | 7(5.69%) | ||
| Other | 4(9.76%) | 2(5.26%) | 1(2.27%) | 7(5.69%) | ||
| BMI | 26.51(5.56) | 27.04(4.98) | 26.91(5.96) | 26.81(5.51) | F(2, 107)=0.088 | 0.916 |
| Income (thousands) | 102.58(56.16) | 92.59(59.61) | 125.23(84.10) | 107.60(69.25) | F(2, 120)=2.486 | 0.088 |
| Education (years) | 15.98(2.53) | 15.11(3.04) | 15.18(2.99) | 15.42(2.87) | F(2, 120)=1.154 | 0.319 |
| Married/partnered | 29(70.73%) | 24(63.16%) | 28(63.64%) | 81(65.85%) | X2(2)=0.653 | 0.721 |
| Employed | 31(75.61%) | 26(68.42%) | 29(65.91%) | 86(69.92%) | X2(2)=1.008 | 0.604 |
| Stage | X2(6)=7.269 | 0.297 | ||||
| 0 | 9(21.95%) | 7(18.42%) | 6(13.64%) | 22(17.89%) | ||
| I | 24(58.54%) | 15(39.47%) | 24(54.55%) | 63(51.22%) | ||
| II | 7(17.07%) | 14(36.84%) | 10(22.73%) | 31(25.20%) | ||
| III | 1(2.44%) | 2(5.26%) | 4(9.09%) | 7(5.69%) | ||
| Positive Nodes | 4(9.76%) | 8(21.05%) | 13(29.55%) | 25(20.33%) | X2(2)=4.923 | 0.085 |
| Hormonal Status | ||||||
| Her2 neu | 6(14.63%) | 2(5.26%) | 4(9.09%) | 12(9.76%) | X2(2)=1.832 | 0.400 |
| ER Positive | 34(82.93%) | 28(73.68%) | 33(75.00%) | 95(77.24%) | X2(2)=0.689 | 0.708 |
| PR Positive | 28(68.29%) | 26(68.42%) | 31(70.45%) | 85(69.11%) | X2(2)=0.179 | 0.915 |
| ER or PR Positive | 32(78.05%) | 27(71.05%) | 32(72.73%) | 91(73.98%) | X2(2)=0.282 | 0.868 |
| Surgical Procedure | X2(2)=0.458 | 0.785 | ||||
| Lumpectomy | 20(48.78%) | 18(47.37%) | 24(54.55%) | 62(50.41%) | ||
| Mastectomy | 21(51.22%) | 20(52.63%) | 20(45.45%) | 61(49.59%) | ||
| Adjuvant treatment | 33(80.49%) | 31(81.58%) | 39(88.64%) | 103(83.74%) | X2(2)=2.635 | 0.268 |
| Chemotherapy | 11(26.83%) | 17(44.74%) | 15(34.09%) | 43(34.96%) | X2(2)=2.804 | 0.246 |
| Radiation | 17(41.46%) | 13(34.21%) | 27(61.36%) | 57(46.34%) | X2(2)=5.860 | 0.053 |
| Antihormonal Therapy | 32(78.05%) | 23(60.53%) | 34(77.27%) | 89(72.36%) | X2(2)=2.917 | 0.233 |
| Session Attendance | 4.25(1.25) | 4.09(1.36) | 4.56(0.76) | 4.30(1.16) | F(2, 98)=1.868 | 0.253 |
| Ln s100A8/A9 | 8.17(0.71) | 8.37(0.88) | 7.65(0.77) | F(2, 120)=9.16 | <0.001 |
Effects of Brief Stress Management Interventions
Group assignment was associated with changes in s100A8/A9 over 12 months as indicated by the significant group (CBT/RT vs HE control) x time (0 vs 12 months) interaction effect. Women assigned to either 5-week RT or CBT showed decreases in s100A8/A9 over this period while those assigned to HE showed increases, F(1, 114)=4.500, p=0.036. The contrast between 5-week CBT and HE was marginally significant with the CBT group showing declines and the HE condition showing increases (F(1, 78)=3.789, p=0.055). The contrasts between CBT vs RT and RT vs HE were not significant (see Table 2). Although the single condition contrasts between CBT vs HE and RT vs HE showed a pattern of decreases in s100 A8/A9 in the active treatments vs control, these did not reach the level of statistical significance, likely due to the small sample size. Combining participants in CBT/RT showed that those in the active conditions had greater increases in perceived stress management skills pre-to-post intervention compared to HE (F(1, 135)=14.992, p<0.001, see Table 3). Finally, across all cases, greater increases in perceived stress management skills pre-to-post intervention was associated with greater decreases in s100A8/A9 over the 12 month follow-up, (F(6, 101)=4.045, β=−0.379, t(101)=−4.056, p<0.001, see Table 4).
Table 2:
Effects of Study Conditions on ln_s100A8/A9 over 12 months of Primary Treatment for Breast Cancer
| T1 ln_s100A8A9 mean(SD) | T2 ln_s100A8A9 mean(SD) | |
|---|---|---|
| CBT/RT | 8.2645(0.79549) | 8.0568(0.68626) |
| CBT | 8.1693(0.70810) | 7.9885(0.62950) |
| RT | 8.3671(0.87812) | 8.1304(0.74410) |
| HE | 7.6521(0.77544) | 7.7622(0.70167) |
| Statistic | Sig. | |
| CBT/RT v HE | F(1, 114)=4.500 | 0.036 |
| CBT v HE | F(1, 78)=3.789 | 0.055 |
| CBT v RT | F(1, 70)=0.000 | 0.997 |
| RT v HE | F(1, 73)=2.512 | 0.117 |
Note: In a post-hoc analysis, a univariate ANOVA was conducted comparing CBT/RT vs HE controls on raw s100A8/A9 values at 12 month follow-up controlling for baseline s100A8/A9 values, and found that while baseline s100A8/A9 values contributed marginally to 12 month values (F=3.05, p=0.083), treatment condition (CBT/RT vs HE) retained a nearly significant effect on s100A8/A9 (F=3.72, p=0.056). This suggests that baseline s100 A8/A9 differences in conditions may have contributed, in part, to the overall effects.
Table 3:
Those in the active conditions (CBT/RT) had greater increases in perceived stress management skills pre-to-post intervention compared to HE
| MOCS change pre-to-post intervention | CBT/RT change mean (SD) | HE change mean (SD) |
|---|---|---|
| 1.45(2.87) | −0.51(2.79) | |
| CBT/RT v HE ANOVA | Statistic | Sig. |
| F(1, 135)=14.992 | <0.001 |
Table 4:
Greater increases in perceived stress management skills on Measure of Current Status (MOCS) pre-to-post intervention was associated with greater decreases in ln_s100A8/A9 over the 12 month follow-up
| Regression Model (full sample) | Statistic | Sig. | ||
|---|---|---|---|---|
| F(6,101)=4.045 | 0.001 | |||
| Standardized β | Standard Error | T | Sig. | |
| MOCS change | −0.379 | 0.027 | −4.056 | <0.001 |
| Stage of Disease (0 vs I-III) | 0.252 | 0.212 | 2.579 | 0.011 |
| Chemotherapy (yes/no) | 0.072 | 0.184 | 0.706 | 0.482 |
| Radiation (yes/no) | −0.047 | 0.160 | −0.496 | 0.621 |
| Time since surgery (days) | −0.018 | 0.004 | −0.180 | 0.857 |
| Age (years) | 0.093 | 0.008 | 0.986 | 0.327 |
DISCUSSION
To extend prior research demonstrating that stress management may reduce distress and inflammatory processes in BCa patients during treatment13, and that stress factors may influence disease outcomes through RAGE-mediated processes24, we examined the effects of the two brief stress management interventions (5-week RT and CBT) vs 5-week HE on circulating levels of s100A8/A9, a RAGE ligand, in a sample of post-surgical breast cancer patients. Women assigned to either RT or CBT showed decreases in s100A8/A9 over this period compared to women assigned to HE. In the full sample, greater increases in perceived stress management skills pre-to-post intervention were associated with greater decreases in s100A8/A9 over the first 12 months of primary treatment and participants in CBT/RT had greater increases in perceived stress management skills compared to HE.
A few studies in the past 5 years have reported the effects of brief (6–12 weeks) psychosocial interventions on inflammatory markers in BCa patients. One RCT in younger BCa patients showed that a 6-week mindfulness intervention was associated with significant reductions in leukocyte pro-inflammatory gene expression as well as lower levels of IL-6 post-treatment vs wait-list control, though these effects were documented to only the post-intervention assessment point30. In one RCT with BCa survivors, those assigned to 12 weeks of yoga showed lower serum IL-6, TNF-α, and IL-1β vs wait-list control at 3 months31. In another 12-week yoga RCT among BCa survivors, the yoga condition showed reduced leukocyte gene expression for the transcription factor nuclear factor kappa B (NF-κB), in tandem with increased activity of the anti-inflammatory glucocorticoid receptor and reduced activity of cAMP response element-binding protein (CREB) family transcription factors compared to health education controls over 3 months32.
Of these studies, the longest follow up was 3 months. Our study significantly adds to this emerging area of research by showing reductions in inflammatory markers by brief 5-week stress management interventions out to 12 months in women undergoing primary treatment for BCa. Additionally, the use of an attention-matched control to keep nonspecific effects of interventionist attention, time, and group support equivalent across conditions, is a strength in study design over many previous psychosocial intervention trials in BCa. The current intervention had excellent adherence and the brevity of it makes it attractive for uptake in clinical oncology settings.
Our study sought to investigate s100A8/A9 as it has been identified as a potential upstream player in inflammatory processes. Increased RAGE activation, via ligands such as the heterodimer s100A8/A9 (calprotectin)22, is putatively associated with greater risk of metastasis because it alters properties associated with the malignant process, including increased cell migration and invasion, proliferation, and resistance to apoptosis21, 23. RAGE expression in breast cancer cells increases endothelial-to-mesenchymal transition (EMT)-related transcription factors in a MEK-dependent manner, and increases transwell invasion, soft agar colony formation and lung metastasis in mice independent of tumor growth33. S100A8/A9 ligands act on RAGE to up-regulate mitogen-activated protein kinases (MAPK) and NF-κB pathways, which in turn are associated with pro-inflammatory cytokine production and increased EMT34, 35. Moreover, blocking RAGE signaling in cancer cells reduces tumor growth and impairs metastasis both in vitro and in murine models33, 36, 37, and therefore represents an attractive therapeutic target in cancer, especially because inhibiting RAGE affects not only the tumor cells but also many other cell types of the tumor microenvironment that are crucial for tumor progression and metastasis38. Building on the in vitro and murine models, our study was the first to demonstrate that brief forms of stress management intervention delivered post-surgically may be able to impact s100 levels in women undergoing treatment for BCa.
Our results are limited by the fact that despite random assignment to condition there was a baseline group difference for s100A8/A9 levels. Thus, regression to the mean or natural improvement in well-being over time in the CBT and RT groups cannot be ruled out as possible explanations for the observed group differences. However, secondary analyses showed that S100A8/A9 reductions were proportional to perceived stress management skill improvements, suggesting that changes in s100A8/A9 may have been attributable to improvements in stress management skills or self-efficacy over time. The women in the study were highly motivated and predominately middle-aged and middle-class which may not be fully representative of a clinic sample. While a strength of the study is the large percentage of participants who were of an ethnic minority (51% overall with approximately 40% Hispanic), English fluency was a requirement of enrollment, again limiting generalizability. Translating and culturally-adapting the intervention are future directions for this work. In the current study, no minimal level of distress was required for study eligibility and it is possible that effects would be larger in a more distressed sample. Future work should examine stress management effects on s100 ligands in a particularly distressed cancer sample. Finally, this study excluded women with metastatic breast cancer and future work should test whether such brief interventions can improve psychological adaptation and decrease s100A8/A9 levels in addition to examining effects on disease outcomes in women with more advanced disease. Due to some methodological limitations and because of the novel nature of this investigation, replication is needed.
Conclusion
Despite limitations, the current findings add to the literature by suggesting that brief group-based stress-management interventions offered in the post-surgical period for women with non-metastatic BCa may be efficacious in reducing levels of s100A8/A9, an inflammatory marker reportedly associated with heightened risk for cancer progression and metastasis. The current cohort is being followed to investigate whether these changes in inflammatory signaling during primary treatment are predictive of long-term clinical outcomes.
Acknowledgments
Sources of Support: NCI R01 CA64710, Sylvester Cancer Center
Footnotes
Previously presented at: Paper Session at Society of Behavioral Medicine Annual Meeting, April 2018 in New Orleans, Louisiana
Disclaimers/Conflict of interest declaration: Dr. Antoni reports that he receives royalties from a Book on stress management in breast cancer patients
References
- 1.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nature Reviews Cancer. 2006;6: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoni MH. Psychosocial intervention effects on adaptation, disease course and biobehavioral processes in cancer. Brain, behavior, and immunity. 2013;30: S88–S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutgendorf SK, Andersen BL. Biobehavioral approaches to cancer progression and survival: Mechanisms and interventions. American psychologist. 2015;70: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. Journal of Clinical Oncology. 2010;28: 4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Psychological, Behavioral, and Immune Changes After a Psychological Intervention: A Clinical Trial. Journal of Clinical Oncology. 2004;22: 3570–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoni MH. Stress Management Intervention for Women with Breast Cancer. American psychological association, 2003. [Google Scholar]
- 7.Andersen BL, Shelby RA, Golden-Kreutz DM. RCT of a psychological intervention for patients with cancer: I. Mechanisms of change. Journal of Consulting and Clinical Psychology. 2007;75: 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoni MH, Lechner Suzanne C., Kazi Aisha, Wimberly Sarah R., Tammy Sifre, Urcuyo Kenya R., Phillips Kristin, Glück Stefan, and Charles S. Carver. How stress management improves quality of life after treatment for breast cancer. Journal of Consulting and Clinical Psychology. 2006;74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoni MH, Wimberly SR, Lechner SC, Kazi A, Sifre T, Urcuyo KR, Phillips K, Smith RG, Petronis VM, Guellati S and Wells KA Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. American Journal of Psychiatry. 2006;163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruess DG, Antoni MH, McGregor BA, et al. Cognitive-Behavioral Stress Management Reduces Serum Cortisol By Enhancing Benefit Finding Among Women Being Treated for Early Stage Breast Cancer. Psychosomatic Medicine. 2000;62: 304–308. [DOI] [PubMed] [Google Scholar]
- 11.Phillips KM, Antoni MH, Carver CS, et al. Stress management skills and reductions in serum cortisol across the year after surgery for non-metastatic breast cancer. Cognitive Therapy and Research. 2011;35: 595–600. [Google Scholar]
- 12.Antoni MH, Lechner S, Diaz A, et al. Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain, behavior, and immunity. 2009;23: 580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoni MH, Lutgendorf SK, Blomberg B, et al. Cognitive-Behavioral Stress Management Reverses Anxiety-Related Leukocyte Transcriptional Dynamics. Biological Psychiatry. 2012;71: 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antoni MH, Bouchard LC, Jacobs JM, et al. Stress management, leukocyte transcriptional changes and breast cancer recurrence in a randomized trial: an exploratory analysis. Psychoneuroendocrinology. 2016;74: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton LM, Andersen BL, Carson WE. Immune, endocrine, and behavioral precursors to breast cancer recurrence: a case-control analysis. Cancer Immunology, Immunotherapy. 2008;57: 1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen BL, Yang H-C, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients. Cancer. 2008;113: 3450–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stagl JM, Lechner SC, Carver CS, et al. A randomized controlled trial of cognitive-behavioral stress management in breast cancer: survival and recurrence at 11-year follow-up. Breast cancer research and treatment. 2015;154: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudenkauf LM, Antoni MH, Stagl JM, et al. Brief cognitive–behavioral and relaxation training interventions for breast cancer: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2015;83: 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nature Reviews Cancer. 2013;13: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nature Reviews Cancer. 2015;15: 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin C, Li H, Zhang B, et al. RAGE-binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial–mesenchymal transition. Breast cancer research and treatment. 2013;142: 297–309. [DOI] [PubMed] [Google Scholar]
- 22.Leukert N, Vogl T, Strupat K, Reichelt R, Sorg C, Roth J. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. Journal of molecular biology. 2006;359: 961–972. [DOI] [PubMed] [Google Scholar]
- 23.Drews-Elger K, Iorns E, Dias A, et al. Infiltrating S100A8+ myeloid cells promote metastatic spread of human breast cancer and predict poor clinical outcome. Breast cancer research and treatment. 2014;148: 41–59. [DOI] [PubMed] [Google Scholar]
- 24.Hudson BI, Lippman ME. Targeting RAGE signaling in inflammatory disease. Annual review of medicine. 2018;69: 349–364. [DOI] [PubMed] [Google Scholar]
- 25.Carver CS. Measure of Current Status. http://www.psy.miami.edu/faculty/ccarver/sclMOCS.html. 2006.
- 26.Montazeri A Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. Journal of experimental & clinical cancer research. 2008;27: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Connor M-F, Bower JE, Cho HJ, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain, behavior, and immunity. 2009;23: 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouchard LC, Antoni MH, Blomberg BB, et al. Post-Surgical Depressive Symptoms and Pro-Inflammatory Cytokine Elevations in Women Undergoing Primary Treatment for Breast Cancer. Psychosomatic Medicine. 2016;78: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blomberg BB, Alvarez JP, Diaz A, et al. Psychosocial adaptation and cellular immunity in breast cancer patients in the weeks after surgery: An exploratory study. Journal of psychosomatic research. 2009;67: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiecolt-Glaser JK, Bennett JM, Andridge R, et al. Yoga’s Impact on Inflammation, Mood, and Fatigue in Breast Cancer Survivors: A Randomized Controlled Trial. Journal of Clinical Oncology. 2014;32: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bower JE, Greendale G, Crosswell AD, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology. 2014;43: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwak T, Drews-Elger K, Ergonul A, et al. Targeting of RAGE-ligand signaling impairs breast cancer cell invasion and metastasis. Oncogene. 2017;36: 1559. [DOI] [PubMed] [Google Scholar]
- 34.Ghavami S, Rashedi I, Dattilo BM, et al. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase‐dependent pathway. Journal of leukocyte biology. 2008;83: 1484–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaminska J, Nowacki MP, Kowalska M, et al. Clinical Significance of Serum Cytokine Measurements in Untreated Colorectal Cancer Patients: Soluble Tumor Necrosis Factor Receptor Type I – An Independent Prognostic Factor. Tumor Biology. 2005;26: 186–194. [DOI] [PubMed] [Google Scholar]
- 36.Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE–amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405: 354–360. [DOI] [PubMed] [Google Scholar]
- 37.Kalea AZ, See F, Harja E, Arriero M, Schmidt AM, Hudson BI. Alternatively spliced RAGEv1 inhibits tumorigenesis through suppression of JNK signaling. Cancer research. 2010: 0008–5472. CAN-0010–0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebhardt C, Riehl A, Durchdewald M, et al. RAGE signaling sustains inflammation and promotes tumor development. Journal of Experimental Medicine. 2008;205: 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]