Abstract
Objectives:
We sought to determine the 1-year outcomes of patients receiving successful chronic total occlusion (CTO) percutaneous coronary intervention (PCI) procedures comparing subintimal versus intraplaque wire tracking patterns.
Background:
CTO PCI utilizes both intraluminal and subintimal wire tracking to achieve successful percutaneous revascularization. Intravascular ultrasound (IVUS) can be used to precisely determine the path of wire tracking.
Methods:
From 2014 to 2016, data from patients undergoing CTO PCI were collected in a single-center database. The primary composite endpoint was target vessel failure (TVF) defined as cardiovascular death, target vessel myocardial infarction (MI), or target vessel revascularization (TVR).
Results:
157 patients with successful CTO PCI and concomitant IVUS imaging completed 1-year follow-up. Subintimal tracking was detected in 53.5% of cases and those patients had a higher incidence of prior PCI, prior CABG, and higher J-CTO score. At 1-year, the unadjusted rate of TVF in the subintimal tracking group was higher than the intraplaque group (17.9% vs. 6.9%, HR 2.74, 95% CI 1.00–7.54, P = 0.04), driven by numerically higher rates of TVR and peri-procedural MI. After multivariable adjustment, no significant differences in the rates of the TVF between subintimal vs. intraplaque groups were present at 1-year (TVF: HR 1.51, 95% CI 0.38–6.00, P =0.55). Landmark analysis excluding in-hospital events showed no significant differences in TVF to 1-year.
Conclusions:
IVUS-detected subintimal tracking was observed in over half of successful CTO PCI cases and correlated with baseline and angiographic factors that contributed to the overall rate of TVF at 1-year.
INTRODUCTION:
The “hybrid algorithm” for chronic total occlusion (CTO) percutaneous coronary intervention (PCI) utilizes dual coronary catheters to facilitate the transition between antegrade and retrograde techniques (1). Achieving technical success in advanced CTO lesions requires familiarity with the full range of approaches within the hybrid algorithm. The wire escalation technique typically uses an intraplaque course for CTO crossing. The dissection re-entry technique (antegrade or retrograde) exploits the subintimal space for coronary wire passage with subsequent re-entry into the true lumen (2).
Importantly, the intended crossing technique frequently does not reflect the actual guidewire positioning as determined by intravascular ultrasound (IVUS) imaging with up to 23% of antegrade and 56% of retrograde wire escalation approaches utilizing the subintimal space (3,4). As such, subintimal guidewire tracking is likely more common than expected in CTO-PCI. In a previous analysis, subintimal wire tracking was seen to have a higher rate of early adverse clinical events than intraplaque tracking during CTO PCI (3).
In this study, we sought to determine the 1-year outcomes of patients who underwent successful CTO PCI with concomitant IVUS imaging comparing intraplaque versus subintimal wire tracking and PCI.
METHODS
Patients.
Clinical and procedural data were collected from 479 consecutively treated CTO patients at Columbia-Presbyterian Hospital (New York, New York) from March 2014 to March 2016. Patients who underwent successful CTO recanalization, defined as restoration of antegrade TIMI (Thrombolysis In Myocardial Infarction) grade 3 flow with < 30% remaining stenosis within the treated segment were included in the study (5). Exclusion criteria were inadequate or unavailable IVUS imaging, inability for the IVUS catheter to cross the lesion, unsuccessful CTO crossing, and in-stent restenosis within the CTO lesion.
Baseline patient demographics, angiographic/IVUS characteristics, procedural data, and in-hospital outcomes were prospectively collected into a local database. Clinical adverse events, medication adherence information, and quality of life surveys were then retrospectively obtained via a combination of patient phone calls, mailed patient surveys, medical record review, and obituary review. The study complied with the Declaration of Helsinki. The institutional review board of Columbia University Medical Center approved the research protocol.
Procedures
Treatment approach, techniques, and devices utilized in CTO crossing were determined according to the operator’s preference based on the “hybrid” treatment algorithm. Single or dual guiding catheters were placed as required to facilitate transition among four techniques: antegrade wire escalation, retrograde wire escalation, antegrade dissection re-entry, and retrograde dissection re-entry. The initial directional strategy and subsequent strategies of antegrade or retrograde were recorded during the procedure. The subintimal tracking and re-entry (STAR) technique was not utilized for CTO in this cohort. IVUS was performed after successful wire crossing into the true lumen with post-balloon dilatation and was repeated at the conclusion of the procedure after successful stenting. All patients had normal cardiac enzymes (creatine kinase [CK]) before the beginning of the procedure and had routine follow-up CK drawn within 24 hours per institutional protocol. Additional CK-MB or troponin I/T were collected post-procedure in patients with clinical evidence suggestive of myocardial infarction (MI).
Endpoints and Definitions
The study primary endpoint was the rate of target vessel failure (TVF) defined as the composite cardiovascular death, target vessel myocardial infarction, or target vessel revascularization. Secondary 1-year endpoints include the individual components of the primary endpoint and major adverse cardiovascular events (MACE) defined as the composite of all-cause death, MI, and unplanned revascularization (6). Additional secondary endpoints include cardiovascular rehospitalization and Academic Research Consortium defined definite/probable stent thrombosis (7). Periprocedural clinical events included periprocedural MI, clinically significant perforation, cardiac tamponade, wire perforation, emergent surgery/ coronary artery bypass grafting, and acute renal failure. Individual events were confirmed by investigator consensus based on the predefined criteria detailed in the preceding text with additional endpoints/definitions located in the accompanying supplemental appendix.
Angiographic and IVUS Examination
Dissection re-entry (intentional or “bail-out”) was defined as angiographic presence of a wire tip in the subintima, a knuckle in the subintimal space followed by CrossBoss/Stingray (Boston Scientific, Natick, Massachusetts) device, or reverse controlled antegrade and retrograde subintimal tracking assisted re-entry (reverse CART). Successful crossing of the CTO was reported intraprocedurally by the operator when true lumen wire position was obtained.
IVUS was performed after guidewire crossing and pre-dilation with a 1.5–2.5 mm compliant balloon using commercially available IVUS systems. The IVUS catheter was advanced at least 5 millimeters beyond the CTO segment. IVUS imaging runs were performed before and after stenting. Qualitative and quantitative IVUS analyses were performed by experienced cardiologists (L.S. and A.M.) who were blinded to patient baseline clinical characteristics according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement, and Reporting of Intravascular Ultrasound Studies (8).
Translesional guidewire tracking was categorized as intraplaque or subintimal by IVUS (Supplemental Figure). Intra-plaque tracking was defined as the IVUS catheter in the center of plaque (Figures 2A and 2B). Subintimal tracking was defined as the IVUS catheter located in the subintimal space, which was identified by the absence of all 3-layers of the arterial wall (Figures 2C and 2D) (9). Additional IVUS definitions, methods, procedures performed in this study have been described previously (3).
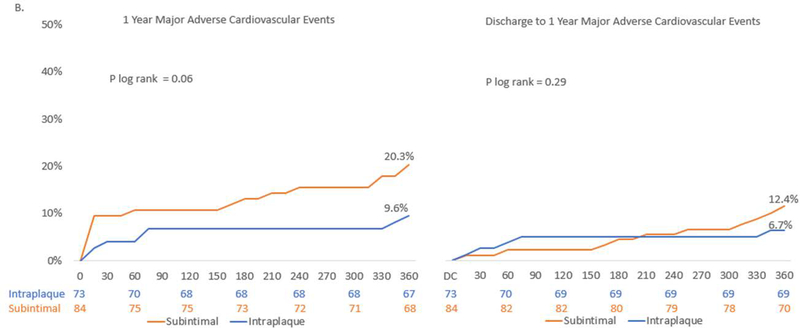
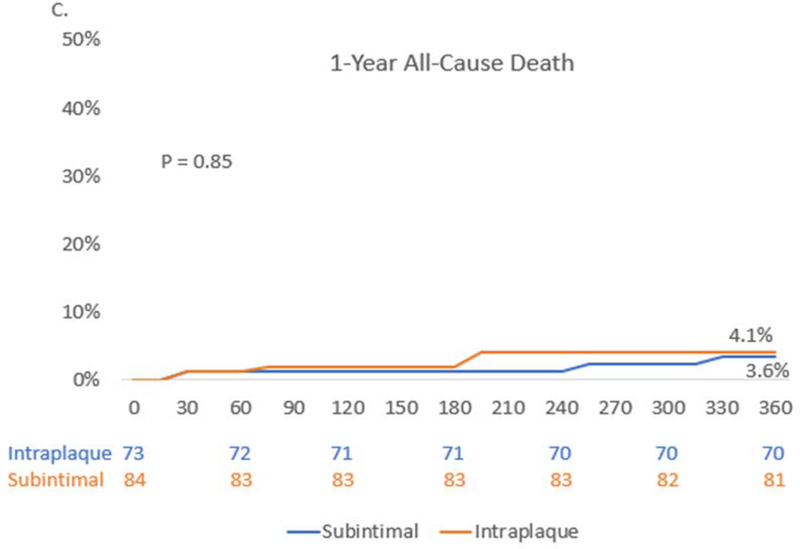
Figure 2. Outcomes to 1-year.
Kaplan-Meier estimates from index procedure through1-year follow-up and landmark analysis from discharge to 1-year for (A.) Composite Target Vessel Failure – comprised of cardiovascular death, target vessel MI, or cardiovascular death, target vessel myocardial infarction, or target vessel revascularization. (B.) Major Adverse Cardiovascular Events – All Cause Death, Myocardial infarction or unplanned Revascularization. (C.) All-cause death Kaplan Meier estimate from index procedure to 1-year. DC – discharge
Quality of life surveys were also retrospectively performed evaluating subjective change in angina and exercise tolerance. Surveyed individuals were asked if their symptoms were: worse/unchanged, mildly improved, moderately improved, or significantly improved compared to prior to their PCI.
Statistical Analysis
Categorical characteristics were compared using the χ2 test or the Fisher exact test as appropriate. Continuous variables were presented as mean ± standard deviation and evaluated with the Student t test. Non-normal continuous variables were presented as medians [interquartile range] and evaluated with the Mann-Whitney U test or Kruskal-Wallis test. Time-to-event data were assessed with log-rank testing and are shown as Kaplan-Meier estimates with the risk for outcomes assessed to 1-year. To adjust for differences between the subintimal and intraplaque tracking groups, cox proportional hazard models were constructed with candidate variables selected based on clinically important baseline differences, as determined by prior literature (3). To further account for baseline differences in lesion complexity, a sensitivity analysis was performed for the composite outcomes including only those patients with Japanese CTO (JCTO) scores ≥ 2. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Data Collection and Patient Characteristics:
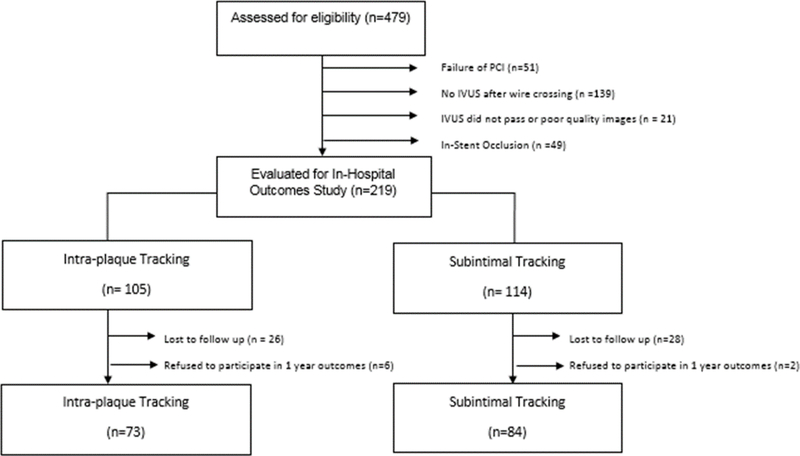
Of the 219 patients who underwent CTO PCI with concomitant IVUS imaging, 62 patients were excluded due lack of available follow-up data or refusal to participate with survey questionnaire (Figure 1). 157 (71.7%) patients were included in the 1-year outcomes analysis. Within the study cohort, 84 (53.5%) patients had IVUS determined subintimal wire tracking compared to 73 (46.5%) with intraplaque tracking.
Figure 1. Consort Diagram.
Abbreviations: IVUS – intravascular ultrasound
Compared to patients in the intraplaque tracking group patients in the subintimal tracking group were more likely to have had prior PCI (78.6% vs. 60.3% p = 0.01) or prior coronary artery bypass grafting (46.4% vs. 23.6%, p = 0.003), and had a lower median ejection fraction (50.0 vs. 55.0, p = 0.001) (Table 1). There were no significant differences in other important baseline covariates, including clinical indication for CTO-PCI.
Table 1.
Clinical Characteristics in Successfully Recanalized Chronic Total Occlusion Lesions
| Intraplaque Tracking (n = 73) | Subintimal Tracking (n = 84) | P Value | |
|---|---|---|---|
| Age | 63.9±10.2 | 65.5±8.9 | 0.29 |
| Male | 61 (83.6) | 75 (89.3) | 0.29 |
| Diabetes Mellitus | 25 (34.3) | 25 (29.8) | 0.55 |
| Hypertension | 65 (89.0) | 79 (94.1) | 0.26 |
| Hyperlipidemia | 69 (94.5) | 81 (96.4) | 0.56 |
| Smoking History | 32 (43.8) | 43 (51.2) | 0.36 |
| Active Smoking | 7 (9.6%) | 6 (7.1) | 0.78 |
| Glomerular Filtration Rate <60 ml/h | 6 (8.2) | 8 (9.5) | 0.77 |
| Current Dialysis | 4 (5.5) | 5 (6.0) | 1.0 |
| Peripheral Artery Diseases | 7 (9.6) | 11 (13.1) | 0.49 |
| Prior Myocardial Infarction | 22 (30.1) | 30 (35.7) | 0.46 |
| Prior Percutaneous Coronary Intervention | 44 (60.3) | 66 (78.6) | 0.01 |
| Prior Coronary Artery Bypass Grafting | 17 (23.6) | 39 (46.4) | 0.003 |
| Prior Heart Failure | 14 (19.2) | 25 (29.8) | 0.13 |
| Ejection Fraction Median, [IQR] | 55.0 [49, 60] | 50.0 [40, 55] | 0.001 |
| Ejection Fraction < 45%* | 7/70 (10.0) | 19/81 (23.5) | 0.03 |
| Clinical Presentation | |||
| Unstable Angina | 32 (43.8) | 35 (41.7) | 0.78 |
| Stable Angina | 31 (42.5) | 41 (48.8) | 0.43 |
| Silent Ischemia | 10 (13.7) | 8 (9.5) | 0.41 |
Continuous variable represented as mean± standard deviation or median [interquartile range];
Visual or Simpsons Biplane Ejection Fraction
Abbreviations: IQR - interquartile Range
Angiographic, Procedural, and IVUS Characteristics
Dissection re-entry (antegrade or retrograde) was the successful technique in 68 (43.3%) and wire escalation in 89 (56.7%) of patients in the overall cohort. IVUS imaging determined intraplaque wire tracking occurred in 10 (14.7%) dissection re-entry techniques and subintimal tracking occurred in 26 (29.2%) of wire escalation techniques (Table 2).
Table 2.
Angiographic/Procedural Characteristics of Successfully Recanalized Chronic Total Occlusion Lesions
| Intraplaque Tracking (n = 73) | Subintimal Tracking (n = 84) | p Value | |
|---|---|---|---|
| Multi-vessel disease* | 57 (78.1) | 72 (85.7) | 0.21 |
| Target vessel | |||
| Right Coronary | 29 (39.7) | 44 (52.4) | 0.11 |
| Left Anterior Descending/ Diagonal | 25 (34.3) | 24 (28.6) | 0.44 |
| Left Circumflex / Obtuse Marginal | 19 (26.0) | 13 (15.5) | 0.10 |
| Left Main | 0 (0.0) | 1 (1.9) | 1.0 |
| Ramus | 0 (0.0) | 1 (1.9) | 1.0 |
| CTO Location in Vessel | 0.12† | ||
| Ostial | 5 (6.9) | 12 (14.3) | |
| Proximal | 14 (19.2) | 8 (9.5) | |
| Middle | 45 (61.6) | 51 (60.7) | |
| Distal | 9 (12.3) | 13 (15.5) | |
| Side Branch at Proximal Stump | 31 (42.5) | 36 (42.9) | 0.96 |
| Blunt Proximal Stump | 19 (26.0) | 41 (48.8) | 0.005 |
| Moderate or Severe Lesion Calcification | 40 (54.8) | 71 (84.5) | <0.0001 |
| Tortuosity bend > 45 Degrees | 23 (31.51) | 40 (47.6) | 0.04 |
| Occluded Length, mm | 13.4±7.7 | 23±25.1 | <0.0001 |
| Length ≥ 20 mm | 15 (20.6) | 48 (57.1) | <0.0001 |
| Prior Attempt Failure | 15 (20.6) | 17 (20.2) | 0.96 |
| Japan CTO Score (mean) | 1.5±1.1 | 2.6±1.1 | <0.0001 |
| ≥2 | 26 (49.3) | 72 (85.7) | <0.0001 |
| Rentrop Class 3 | 37 (50.7) | 30 (35.7) | 0.06 |
| Successful Technique | |||
| Antegrade Wire Escalation | 56 (76.7) | 18 (21.4) | <0.0001 |
| Antegrade Dissection Re-Entry | 8 (11.0) | 26 (31.0) | 0.002 |
| Retrograde Wire Escalation | 7 (9.6) | 8 (9.5) | 0.99 |
| Retrograde Dissection Re-Entry | 2 (2.7) | 32 (38.1) | <0.0001 |
| Success Retrograde | 9 (12.3) | 40 (47.6) | <0.0001 |
| Reverse CART | 1 (1.4) | 30 (35.7) | <0.0001 |
| Non-CTO Vessel Treated at Same Setting | 13 (17.8) | 16 (19.1) | 0.84 |
| Drug-Eluting Stent Implantation | 73 (100.0) | 83 (98.8) | 0.35 |
| Number of Stents in CTO vessel | 2.1±0.9 | 3.0±1.0 | <0.001 |
| Total Stent Length, mm | 55.3±25.6 | 87.8±29.6 | <0.0001 |
| Fluoroscopy Time, min | 33.0 [25.7, 46.6] | 68.5 [53.6, 93.4] | <0.0001 |
| Contrast Volume, mL | 250.0 [180.0, 360.0] | 382.5 [290, 500] | <0.0001 |
| Radiation Exposure Dose, Gy | 1.1 [0.7,2.1] | 2.0 [1.4, 3.1] | 0.0001 |
Continuous variable represented as mean± standard deviation or median [interquartile range]
≥50% luminal diameter stenosis in ≥2 major epicardial arteries.
Overall P value
Abbreviations: CTO = chronic total occlusion. CART - controlled antegrade and retrograde subintimal tracking
Patients with subintimal tracking had overall higher complexity CTO lesions than patients with intraplaque tracking as determined by JCTO score (2.6± 1.1 vs. 1.5 ± 1.1, P < 0.0001) and numerically less Rentrop grade 3 collaterals (35.7% vs. 50.7% P = 0.06). CTO PCI with subintimal tracking required a greater number of stents (mean stent number per patients 3.0 ± 1.0 vs. 2.1 ± 0.9, P = <0.001) and a greater mean stent length (87.8 ± 29.6 vs. 55.3 ± 25.6, P <0.0001) than intraplaque tracking. Subintimal tracking procedures also had greater utilization of the retrograde approach, fluoroscopy time, iodinated contrast use, and radiation exposure.
IVUS imaging demonstrated that the subintimal tracking group had a significantly longer total stent length (74.1 mm vs. 46.1 mm, P <0.0001) and a smaller minimal stent area than the intraplaque tracking group (4.8 mm2 vs 5.5 mm2 P= 0.03) (Table 3). However, the minimal stent area within only the CTO segment was not different between groups (6.1 mm2 vs. 6.2 mm2, P= 0.31). Subintimal tracking was associated with a significantly increased frequency of IVUS vascular injury (91.7% vs. 48.9%, P <0.0001) and its individual components of intramural hematoma, perivascular hematoma, or perivascular blood speckle compared to intra-plaque tracking. The angiographic, procedural, and IVUS findings in 1-year follow up cohort are consistent with the prior publication of early events from the full cohort (3).
Table 3.
IVUS Findings in Successfully Recanalized CTO Lesions
| Intraplaque Tracking (n = 73) | Subintimal Tracking (n = 84) | p Value | |
|---|---|---|---|
| Total Stent Length, mm | 46.1 [31.9, 58.7] | 74.1 [55.7, 86.8] | <0.0001 |
| Subintimal Length, mm | — | 17.4 [8.2, 34.6] | — |
| >10 mm | — | 39 (46.4) | — |
| MSA in entire stent length, mm2 | 5.5 [4.1, 7.3] | 4.8 [3.8, 6.01] | 0.03 |
| MSA < 5 mm2 entire stent length | 31/70 (44.3) | 33/62 (53.2) | 0.30 |
| MSA in CTO segment, mm2 | 6.1 [4.9–8.3] | 6.2 [5.1–6.9] | 0.31 |
| MSA <5 mm2 in CTO segment | 22 (30.1) | 37 (44.1) | 0.07 |
| IVUS-VI * | 35 (48.9) | 77 (91.7) | <0.0001 |
| Intramural Hematoma | 13 (17.8) | 48 (57.1) | <0.0001 |
| Perivascular Hematoma | 5 (6.9) | 30 (35.7) | <0.0001 |
| Total Length Of Hematoma | 9.0 [5.2,12.3] | 14.0 [6.8, 22.3] | 0.07 |
| Perivascular Blood Speckle | 29 (39.7) | 66 (78.7) | <0.0001 |
| Malapposition | 12 (17.1) | 10 (16.1) | 0.88 |
| Significant† | 8 (11.4) | 4 (6.5) | 0.38 |
| Tissue Protrusion | 5 (7.1) | 10 (16.1) | 0.17 |
| Significant‡ | 3 (4.3) | 5 (8.1) | 0.47 |
| Stent Edge Dissection | 6 (8.2) | 11 (13.1) | 0.33 |
| Significant§ | 3 (4.3) | 5 (8.1) | 0.47 |
Continuous values are reported as mean ± (standard deviation) or median [interquartile range]
IVUS-VI includes any of the following: intramedial hematoma, perivascular hematoma, perivascular blood speckle.
Malapposition area >10% lumen area.
Protruded tissue area >10% stent area.
Dissection >3 mm in length and >60° in angle.
MSA - minimum stent area, CTO- chronic total occlusion, IVUS-VI – intravascular ultrasound vascular injury
One Year Outcomes
At 1-year in CTO PCI patients with IVUS determined subintimal tracking pattern, there was a significantly higher rate of TVF (17.9% vs. 6.9%, HR 2.74, 95% CI: 1.00–7.54, P = 0.04) and a numerically important trend of MACE (20.3% vs. 9.6% HR 2.25 95% CI 0.93–5.41, P =0.06) when compared to intraplaque tracking pattern (Table 4, Figure 2). This difference was driven by numerically greater periprocedural MI (7.1% vs. 2.7%, P = 0.29) and target vessel revascularization (8.5% vs. 2.8%, P = 0.13) in the subintimal tracking group. However, there was no difference in the rate of all cause or cardiovascular death at 1-year between groups (3.6 vs. 4.1%, P =0.85 and 2.4% vs. 2.8%, P= 0.88, respectively). Additionally, both groups showed small improvements in ejection fraction at 1-year (subintimal vs. intraplaque: +3.7% ± 12.2 vs. +2.0% ± 11 P= 0.59).
Table 4.
Unadjusted 1-Year Outcomes in Successfully Recanalized CTO Lesions
| Intraplaque Tracking (n = 73) | Subintimal Tracking (n = 84) | HR/OR;* 95% CI | P Value log rank | |
|---|---|---|---|---|
| 1-Year outcomes | ||||
| Target Vessel Failure† | 5 (6.9) | 15 (17.9) | 2.74 (1.00–7.54) | 0.04 |
| Major Adverse Cardiovascular Events | 7 (9.6) | 17 (20.3) | 2.24 (0.93–5.41) | 0.06 |
| All-Cause Death | 3 (4.1) | 3 (3.6) | 0.86 (0.17– 4.24) | 0.85 |
| Cardiovascular Death | 2 (2.8) | 2 (2.4) | 0.86 (0.12–6.10) | 0.88 |
| Target Vessel MI | 2 (1.9) | 8 (9.6) | 3.52 (0.75–16.60) | 0.08 |
| Peri-Procedural MI | 2 (2.7) | 6 (7.14) | 2.73 (0.53–13.97)* | 0.29 |
| Definite/ Probable Stent Thrombosis | 1 (1.4%) | 0 (0.0) | 0.29 (0.012–7.13)* | 0.47 |
| Target Vessel Revascularization | 2 (2.8) | 7 (8.5) | 3.12 (0.64–15.03) | 0.13 |
| Coronary Artery Bypass Grafting | 0 (0.0) | 1 (1.19) | 2.64 (0.11–65.8)* | 1.0 |
| Cardiovascular Rehospitalization | 9 (12.7) | 8 (9.7) | 0.71 (0.28–1.85) | 0.51 |
| Change In Ejection Fraction | +2.0% ± 11 | +3.7% ± 12.2 | 0.59 | |
| Periprocedural Clinical Events | ||||
| Clinically Significant Perforation‡ | 1 (1.4) | 7 (8.3) | 6.54 (0.79–54.52)* | 0.07 |
| Tamponade | 1 (1.4) | 2 (2.4) | 1.76 (0.16–19.77)* | 1.0 |
| Wire Perforation | 3 (4.1) | 13 (15.5) | 4.27 (1.17 −15.65)* | 0.03 |
| Emergent Surgery/CABG | 1 (1.37) | 1 (1.19) | 0.88 (0.05 −14.12)* | 1.0 |
| Branch Occlusion (diameter >1.5 mm) | 12 (16.4) | 41 (48.8) | 4.85 (2.28 −10.29)* | <0.0001 |
| Acute Renal Failure | 1 (1.0) | 1 (0.9) | 0.87 (0.05–14.10)* | 1.0 |
| Length of Stay§ | 1.9 ± 3.8 | 2.4 ± 3.8 | 0.04 | |
| Active Medication Use At 1-year | ||||
| Aspirin | 68/71 (95.8) | 74/76 (97.3) | 1.63 (0.26–10.07)* | 0.50 |
| Dual Antiplatelet Therapy | 64/70 (91.4) | 71/76 (93.4) | 1.33 (0.38–4.57)* | 0.65 |
| Clopidogrel | 47/70 (67.14) | 52 /76 (68.4) | ||
| Ticagrelor | 7/70 (10.0) | 8/76 (10.5) | ||
| Prasugrel | 10/70 (14.3) | 11/76 (14.5) | ||
| Coumadin | 5/68 (7.4) | 5/71 (7.0) | 0.95 (0.26–3.46)* | 1.0 |
PMI = peri-procedural myocardial infarction, MI – myocardial infarction, CABG = Coronary Artery Bypass Grafting
represent odds ratio
composite of cardiovascular death, target vessel MI, and Target vessel revascularization‡composite of all-cause death, myocardial infarction and unplanned revascularization
Perforation requiring treatment with prolonged balloon inflation, pericardiocentesis, coils, beads thrombin, or surgery.
From date of index procedure to discharge
A landmark analysis was performed to examine events after discharge to 1-year, and there was no significant difference in the unadjusted rates of TVF (9.6% vs. 5.5%, P = 0.36) or MACE (12.4% vs. 6.7%, P =0.29) over this period.
To assess further whether the increased crude hazard associated with subintimal tracking was primarily attributed to higher risk angiographic disease, patients with J-CTO scores ≥2 were independently analyzed in a sensitivity analysis. In this limited subset, there was no longer a significant crude association between subintimal and intraplaque wire tracking pattern and 1-year unadjusted TVF (19.4% vs. 11.3%, P=0.29) or MACE (20.9% vs.13.9%, P = 0.39). When events were analyzed in the low JCTO score <2 subset, once again there was no significant difference in the rates of TVF (P = 0.38) or MACE (P = 0.20) at 1-year with very low event rates for the composite endpoints (2 TVF events and 4 MACE events at 1-year in this subgroup).
Multivariable Analysis
In a multivariable analysis, there was no significant difference in the adjusted hazard of the composite endpoints TVF (HR 1.51 95% CI 0.38–6.00, P =0.55) or MACE (HR 1.83 95% CI 0.52–6.46, P =0.34) (Table 5) after we controlled for age, gender, prior history of diabetes, ejection fraction, history of coronary artery bypass grafting, history of percutaneous coronary intervention, JCTO, IVUS vascular injury (IVUS-VI), retrograde approach and minimal stent area (mm).
Table 5.
Cox Proportional Hazard Models Clinical and Procedural Predictors of Target Vessel Failure and MACE at 1-year.
| Predictors of Target Vessel Failure* | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P value | |
| Subintimal Tracking | 2.74 | 1.00–7.54 | 0.04 | 1.51 | 0.38–6.00 | 0.55 |
| Japanese CTO Score‡ | 1.75 | 1.20 −2.56 | 0.004 | 1.40 | 0.74–2.62 | 0.30 |
| History of Coronary Artery Bypass Grafting | 1.51 | 0.63–3.66 | 0.85 | 0.75 | 0.20–2.77 | 0.66 |
| History of Percutaneous Coronary Intervention | 2.51 | 0.74–8.56 | 0.14 | 4.87 | 0.81–29.21 | 0.08 |
| Retrograde approach | 2.38 | 0.99–5.7 | 0.053 | 1.55 | 0.39–6.1 | 0.53 |
| IVUS Vascular injury | 3.83 | 0.88–16.50 | 0.07 | 2.50 | 0.25–24.86 | 0.43 |
| Minimal Stent Area | 0.87 | 0.63–1.19 | 0.37 | 0.98 | 0.68–1.41 | 0.92 |
| Angiographic and IVUS predictors of MACE* | HR | 95% CI | P Value | HR | 95% CI | Adjusted P value |
| Subintimal Tracking | 2.24 | 0.93–5.41 | 0.06 | 1.83 | 0.52–6.46 | 0.34 |
| Japanese CTO Score‡ | 1.60 | 1.13–2.26 | 0.008 | 1.37 | 0.80–2.36 | 0.25 |
| History of Coronary Artery Bypass Grafting | 1.60 | 0.70–3.50 | 0.27 | 0.83 | 0.26–2.62 | 0.74 |
| History of Percutaneous Coronary Intervention | 1.69 | 0.63–4.51 | 0.30 | 2.14 | 0.57–8.04 | 0.26 |
| Retrograde approach | 2.04 | 0.91–4.55 | 0.08 | 1.30 | 0.38–4.27 | 0.78 |
| IVUS Vascular injury | 2.14 | 0.73–6.27 | 0.16 | 1.02 | 0.22–4.76 | 0.97 |
| Minimal Stent Area | 0.99 | 0.76–1.28 | 0.01 | 1.12 | 0.84–1.49 | 0.46 |
Multivariable Models also adjusted for Age, Gender, DM, and Ejection Fraction
Log rank test used
treated as an ordinal variable
Abbreviations: CTO – chronic total occlusion, IVUS – intravascular ultrasound.
Quality of Life Analysis
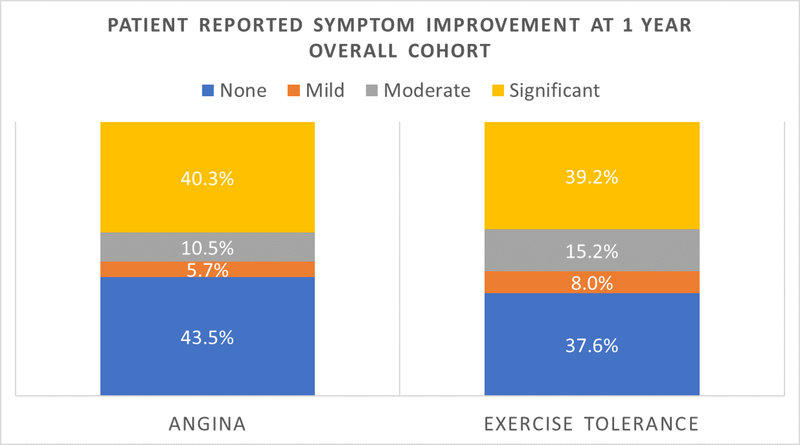
At 1-year, there was no significant difference in reported angina or exercise tolerance based on wire tracking patterns (Figure 3A). However, there was important improvement in reported symptoms after CTO PCI in the overall cohort (Figure 3B) with 70.7% of patients experiencing a moderate or greater subjective improvement in one or both of the two surveyed categories. Furthermore, 50.8% and 54.4% of patients reported moderate or greater symptom improvements in reported subjective angina and exercise tolerance, respectively.
Figure 3.
Patient reported symptom improvement at 1 year (A.) comparing intra plaque vs. subintimal wire tracking. (B.) Symptom status improvement at 1 year in the overall cohort.
Discussion
The current study represents one of the largest analyses of subintimal versus intraplaque tracking in CTO PCI and the effect of tracking path on long-term clinical outcomes. The principal finding of this study was that in adjusted analyses, subintimal tracking was not associated with significantly worsened TVF at 1-year, despite numerically higher upfront crude rates of MI and TLR. However, going subintimal was a marker of higher CTO complexity with greater utilization of the retrograde approach.
To our knowledge, only two other reports have utilized IVUS determined wire position to assess outcomes beyond index hospitalization (2,9), but neither study utilized the contemporary “hybrid approach” to CTO PCI. The 2014 J-Proctor analysis of 163 patients revealed no difference in the frequency of 1-year target vessel revascularization between subintimal and intraplaque groups (12.9% vs. 10.4% P= 0.75). However, in patients that underwent routine repeat angiography at an average of 9 months post-CTO PCI, there was a significantly greater degree of ISR in the subintimal tracking group (subintimal vs. intraplaque minimal luminal diameter 2.41 vs. 2.03 mm, P = 0.02). The J-PROCTOR 2 registry evaluated 318 CTO PCI patients with IVUS imaging and found that there was a numerically increased risk of TVR (9.9% vs. 3.7% P= 0.08) and MACE (11.3% vs. 4.8% P = 0.07) at 1-year in patients with subintimal stenting. While these results are similar to the primary results of our study, there was no adjusted analysis performed to correct for confounders from imbalanced patient or lesion characteristics. Furthermore, time-to-event analysis was not performed and therefore, limited information was obtained as to the rate of events over the follow up period.
Our study sought to further explore the hypothesis that subintimal stenting may lead to higher rates of adverse 1-year outcomes. The crude Kaplan Meier rates of TVF were higher in the subintimal group compared to the intraplaque group with a clear, early separation of the Kaplan Meier curves. This separation can be attributed to a higher upfront procedural risk with subintimal tracking, i.e. greater branch vessel loss, clinically significant perforation, and periprocedural MI. This upfront risk was also due to higher risk coronary anatomy (as determined by significantly higher J-CTO scores), as well as more extensive coronary disease in the subintimal stenting group (as determined by a significantly higher rate of prior PCI and CABG). To specifically examine if there were differences in late (post-discharge) event rates, based on wire tracking pattern, event rates were evaluated with a landmark analysis from discharge to 1-year with no significant difference in the rates of unadjusted or adjusted TVF or MACE. This result demonstrates that after accepting a higher upfront procedural risk with subintimal procedures, no important differences the composite outcomes occurred. This analysis was limited by relatively low late event rates with only 12 TVF events occurring after discharge.
In addition to tracking pattern, retrograde approach and IVUS –VI were assessed as univariable and multivariable predictors of the TVF. Retrograde approach and IVUS-VI both had numerically higher trends toward the primary outcome. However, after adjusting for important clinical confounders no effect on the composite was observed. As with subintimal stenting, the use of retrograde approach and the development of IVUS-VI occurred in patients with a greater comorbidity burden who carrying a greater overall risk of adverse outcomes, but were not independently predictive of the composite.
Lastly, patients in the study cohort experienced significant improvements in overall subjective angina and exercise tolerance without important differences based on procedural tracking pattern. These results, although consistent with recent publications on quality of life after CTO (10,11), should be viewed as hypothesis generating only, as a validated quality of life score such as the Seattle Angina Questionnaire or Kansas City Cardiomyopathy Questionnaire were not used. Furthermore, these symptomatic improvements cannot account for a potential post-procedural placebo effect.
Limitations
There are several other limitations to this study that should be mentioned. First, this is a single center retrospective cohort study, which is subject to the biases inherent to such analyses (i.e. recall bias and ascertainment bias). Second, this study was performed at a quaternary referral center with many patients referred from out-of-state. This likely contributed to some of the stated loss to follow up and resulted in diminished overall study power for the adjusted outcomes. Third, the CTO operators performing procedures in this study were all high-volume operators with > 350 CTO PCI case experience. Therefore, these results may not be readily applicable to all CTO programs. Fourth, routine follow-up angiography was not performed in this study, which may have limited the ability to discern clinically silent in-stent restenosis. Lastly, the subintimal and intraplaque tracking curves of the Kaplan Meier landmark analysis cross diminishing the statistical power of the log rank test. To address this, standard logistic regression analyses were performed for the events occurring from discharge to 1-year TVF and MACE without a change in the outcomes of the statistical tests.
Conclusions
IVUS-detected subintimal tracking was observed in over half of successful CTO PCI cases and correlated with baseline and angiographic factors that contributed to the overall rate of TVF at 1-year. Larger, prospective studies are required to establish the safety of subintimal wire tracking and stenting in CTO PCI.
Supplementary Material
Acknowledgments
Sources of Funding: None.
Disclosures:
Scientific, St Jude Medical for fellows; consultant - Boston Scientific, OCT Medical Imaging Inc.; speaker fee - St. Jude Medical. Matthew T. Finn: NIH grant support −2T32HL007854–21. Manish A. Parikh: Speakers bureau – Medtronic, Boston Scientific, Abbott Vascular, CSI; advisory board – Philips, Abbott Vascular, Medtronic. A. Kirtane: Institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, ReCor Medical; Personal: Conference honoraria and Travel/Meal reimbursements only. Philip Green: Research supported by a career development award from the National Heart Lung and Blood Institute K23 HL121142. Ziad A. Ali: Institutional research grants to Columbia University from St. Jude Medical, and Cardiovascular Systems Inc. Consultant to St Jude Medical, ACIST. Gary S. Mintz: Consultant - Boston Scientific, ACIST; fellowship/grant support - Volcano, Boston Scientific, InfraReDx; honoraria - Boston Scientific, ACIST. Dimitri Karmpaliotis: Speaker’s bureau - Abbott Vascular, Boston Scientific, Medtronic; consultant - Vascular Solutions. All other authors have no relationships to disclose.
Bibliography
- 1.Brilakis ES, Grantham JA, Rinfret S et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovascular interventions 2012;5:367–79. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa K, Tsuchikane E, Okamura A et al. Incidence and impact on midterm outcome of intimal versus subintimal tracking with both antegrade and retrograde approaches in patients with successful recanalisation of chronic total occlusions: J-PROCTOR 2 study. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2017;12:e1868–e1873. [DOI] [PubMed] [Google Scholar]
- 3.Song L, Maehara A, Finn MT et al. Intravascular Ultrasound Analysis of Intraplaque Versus Subintimal Tracking in Percutaneous Intervention for Coronary Chronic Total Occlusions and Association With Procedural Outcomes. JACC Cardiovascular interventions 2017;10:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muhammad KI, Lombardi WL, Christofferson R, Whitlow PL. Subintimal guidewire tracking during successful percutaneous therapy for chronic coronary total occlusions: insights from an intravascular ultrasound analysis. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2012;79:43–8. [DOI] [PubMed] [Google Scholar]
- 5.Sianos G, Werner GS, Galassi AR et al. Recanalisation of chronic total coronary occlusions: 2012 consensus document from the EuroCTO club. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2012;8:139–45. [DOI] [PubMed] [Google Scholar]
- 6.Moussa ID, Klein LW, Shah B et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2014;83:27–36. [DOI] [PubMed] [Google Scholar]
- 7.Laskey WK, Yancy CW, Maisel WH. Thrombosis in coronary drug-eluting stents: report from the meeting of the Circulatory System Medical Devices Advisory Panel of the Food and Drug Administration Center for Devices and Radiologic Health, December 7–8, 2006. Circulation 2007;115:2352–7. [DOI] [PubMed] [Google Scholar]
- 8.Mintz GS, Nissen SE, Anderson WD et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. Journal of the American College of Cardiology 2001;37:1478–92. [DOI] [PubMed] [Google Scholar]
- 9.Muramatsu T, Tsuchikane E, Oikawa Y et al. Incidence and impact on midterm outcome of controlled subintimal tracking in patients with successful recanalisation of chronic total occlusions: J-PROCTOR registry. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2014;10:681–8. [DOI] [PubMed] [Google Scholar]
- 10.Rossello X, Pujadas S, Serra A et al. Assessment of Inducible Myocardial Ischemia, Quality of Life, and Functional Status After Successful Percutaneous Revascularization in Patients With Chronic Total Coronary Occlusion. The American journal of cardiology 2016;117:720–6. [DOI] [PubMed] [Google Scholar]
- 11.Sapontis J, Salisbury AC, Yeh RW et al. Early Procedural and Health Status Outcomes After Chronic Total Occlusion Angioplasty: A Report From the OPEN-CTO Registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovascular interventions 2017;10:1523–1534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.