Abstract
Purpose:
We assessed the decision-making of individuals pursuing genomic sequencing without a requirement for pre-test genetic counseling. We sought to describe the extent to which individuals who decline genetic counseling reported decisional conflict or struggled to make a decision to pursue genomic testing.
Methods:
We administered a 100-item survey to 3,037 individuals who consented to the Return of Actionable Variants Empirical (RAVE) study, a genomic medicine implementation study supported by the National Institutes of Health (U.S.A.) eMERGE consortium. The primary outcomes of interest were self-reported decisional conflict about the decision to participate in the study and time required to reach a decision.
Results:
We received 2,895 completed surveys (response rate = 95.3%), and of these respondents 97.8% completed the decisional conflict scale in its entirety. A majority of individuals (63%) had minimal or no decisional conflict about the pursuit of genomic sequencing and were able to reach a decision quickly (78%). Multivariable logistic regression analyses identified several characteristics associated with decisional conflict, including lower education, lower health literacy, lower self-efficacy in coping, lack of prior experience with genetic testing, not discussing study participation with a family member or friend, and being male.
Conclusion:
As genomic sequencing is used more widely, genetic counseling resources may not be sufficient to meet demand. Our results challenge the notion that all individuals need genetic counseling in order to make an informed decision about genomic sequencing.
Keywords: Genetic counseling, Informed consent, Genomic implementation, ELSI
INTRODUCTION
Pre-test genetic counseling is widely used to ensure that individuals are informed about the goals of genetic testing, its potential benefits and risks, the range of findings that might be generated, and the actions that may be recommended if genetic test results are positive[1]. The value added by genetic counseling has been demonstrated through years of clinical experience, and is codified in the recommendations of prominent professional organizations such as the American Heart Association and the American Society for Cancer and Oncology, which have issued statements endorsing the importance of pre-test genetic counseling[2, 3].
As new forms of genetic testing are employed in a larger number of clinical settings, it may be difficult to provide traditional pre-test genetic counseling to every person considering genetic testing. Although efforts have been made to improve the efficiency of genetic counseling services,[4, 5] the expectation that all patients are referred to a genetic counselor or other medical specialist for in-person counseling prior to genomic evaluation may not be sustainable as genomic evaluation becomes a more common element of clinical care. This pending inconsistency between historical expectations for patient support and a scarcity of available medical resources is spurring efforts to identify alternatives to conventional pre-test counseling, including advanced online support and use of clinicians who are not formally trained in medical genetics or genetic counseling[6].
In addition to looking for ways to enhance the delivery of pre-test genetic counseling services, it is important to consider more pragmatic alternatives for supporting the large number of patients who will be offered some type of genomic evaluation in the future. One such alternative is to forgo the requirement of pre-test genetic counseling altogether in cases where the risks of genomic evaluation are limited and make genetic counseling an option to interested patients. This approach could be ethically justifiable, for example, if there were good reasons to believe that alternative means of patient education could position patients to make a quality decision about genetic testing. Consistent with the aims of pre-test genetic counseling, and ethical commitments to promoting patient autonomy, that decision would need to be well informed and reflective of the values and preferences of the patient.
To assess the feasibility of such an approach, we evaluated the decision-making process of individuals pursuing genomic sequencing in the context of a large clinical implementation study in which genetic counseling was optional and consent to genomic testing was obtained via mail. Our goals were to describe sources of decisional conflict, the length of time required to make a decision to pursue genomic testing, and the network of support (family, friends, and others) involved in the decision-making process of patients who elected to pursue genetic testing in the absence of pre-test genetic counseling. The data we report can inform decisions about the use of genetic counseling resources in future genomic sequencing programs.
METHODS
Setting and Participants
We embedded a survey within a genomic medicine implementation study conducted as part of the eMERGE consortium. The parent study provided genomic sequencing of 109 medically actionable genes to 3,037 individuals in an effort to identify previously undetected inherited disease risks and assess the medical and psychosocial implications of genomic risk screening[7]. Although genomic sequencing was conducted under a research protocol, an associated clinical report was placed in participants’ electronic medical record. Clinically actionable results were communicated in-person to participants by a genetic counselor but variants of uncertain significance were not returned. Participants were informed about these and other potential clinical implications of study results at the time of consent.
The study sample consisted of individuals with one of two phenotypes—documented colon polyps and hyperlipidemia. To create the sample, the Mayo Clinic biobank, a genetic biobank with approximately 55,000 samples,[8] along with a smaller Vascular Disease Biorepository,[9] were screened to identify 5,106 eligible participants exhibiting one or both of the phenotypes of interest. Eligible participants were mailed a study packet containing a letter of invitation,a study brochure, a “Frequently Asked Questions” document, and an informed consent document. These items described the risks and benefits of participating in the study, including information about the potential results that participants might receive, and discussed potential consequences for family members. The aim of these documents was to present common elements of an in-person genetic counseling session. Those electing to participate in the study returned a signed consent form in a postage-paid envelop. Optional pre-test genetic counseling was available at no cost and was described several times in the study invitation materials, along with a phone number to call to schedule an appointment with a genetic counselor.
Survey
We designed a 100-item survey comprised largely of validated psychosocial measures from the behavioral health literature, including items from the Behavioral Risk Factor Surveillance System (2014),[10] the multi-dimensional health locus of control scale,[11] and a scale measuring knowledge about the capabilities and limitations of genomic sequencing[12]. We collected standard demographic variables, and assessed previous experience with genetic testing, health literacy,[13] self-reported health status,[14] and subjective assessment of discretionary income[15]. We also developed new items to assess domains not identified in the literature but that were viewed as important psychosocial measures for the parent study. Not all survey data are presented in this paper.
The primary outcomes of interest for our analysis were decisional conflict and time required to reach a decision about the pursuit of genomic sequencing. We measured decisional conflict using O’Connor’s Decisional Conflict Scale (DCS)[16]. The DCS is a validated, 16-item tool utilizing a 5-point agreement scale (0 = Strongly Agree, 1 = Agree, 2 = Neither Agree Nor Disagree, 3 = Disagree, 4 = Strongly Disagree). In this survey, we used the full 16-item standard DCS, but modified the scale slightly to clarify that we were interested in decision-making related to participating in a research study, a modification that has precedence in the literature[17, 18]. The modified items are presented in Supplemental Figure 1, to facilitate comparison against the original item wordings and question order.
Since we were interested in assessing the decision-making process of patients pursuing genomic sequencing, we examined the amount of time it took participants to decide to participate (decisional latency). To measure decisional latency, we used the following response options: “I was able to decide right away,” “I had to think about it for several hours before deciding,” or “I had to think about it for several days before deciding.” We also asked participants to indicate if they had talked with: “No one,” a “Family Member,” a “Friend,” a “Healthcare provider,” “Research staff from the RAVE study,” or “Other (please specify).” The survey was an element of the parent genomic sequencing study and was approved by the Mayo Clinic Institutional Review Board(#15–005013).
Data Collection
The survey was administered at or near the time of consent to the parent study. Recruitment for the parent study began in March 2016 and was completed in October 2016. An invitation packet containing a study consent form was sent to eligible participants via standard U.S. postal mail. Interested participants were asked to review the materials and return a signed consent form to the research team via a postage-paid return envelope. For a random subset of study invitations (n=1,557), we included the psychosocial survey and study invitation materials in the same mailed packet as part of a methodological experiment designed to assess its impact on enrollment rates (results forthcoming). For these participants, the survey was completed at the time participants signed the consent form for the study; and both items were mailed back to the study team in the same return envelope. For the remaining participants, the study invitation materials and consent form were sent to study invitees together, and the surveys were sent to study participants at a later time, after the research team had received their signed consent form in the mail. Because of transit time of postal mail, these participants completed surveys no sooner than 5–8 days following their consent to the parent study. Study participants who returned their consent form but did not complete the psychosocial survey were sent reminders at 30 and 60 days following their enrollment.
Trained biobank staff processed consent materials for the parent study and tracked survey completion. Each returned item was date-stamped and documented in a tracking database. Data from completed surveys were doubled-entered by data-entry staff, and research staff conducted periodic quality checks. Individual responses to survey items that were unclear to data-entry personnel were flagged, and the paper surveys were reviewed by a research team member (JP).
Data Analysis
Data were analyzed using SAS 9.4 (SAS Institute Inc., Cary NC). Means, medians, standard deviations, and ranges were calculated for continuous variables, and frequencies and percentages were calculated for categorical variables. Our sample was derived from research registries, which allowed us to conduct bias analyses to identify demographic factors that might be associated with enrollment in the parent study. These demographic factors were compared between consenters and responders who were deemed ineligible (e.g. members of the research registry who reported that they no longer receive care at Mayo Clinic), non-responders, and active refusers, using chi-square tests for nominal characteristics, and Kruskal-Wallis or ANOVA F-tests for ordinal or continuous characteristics, as appropriate.
After calculating decisional conflict scores, the total score was dichotomized as <25 versus ≥25. This approach is consistent with prior studies in which a score of less than 25 has been associated with following through with one’s choice[19]. Univariate logistic regression models were used to assess unadjusted associations with a decisional conflict score ≥25. Conceptually relevant variables were included in a multivariable logistic regression model to identify factors associated with increased odds of having decisional conflict score ≥25. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. P-values of 0.05 or lower were considered statistically significant.
RESULTS
Of the 5,106 participants who met eligibility criteria for the parent study, 3,037 (59.5%) responded to the study invitation and consented to participate in the study. Seventy six individuals (1.5%) were determined to be ineligible after the study invitation was extended; 1567 (30.7%) did not respond; and 426 (8.3%) actively declined to participate in the study. Of the 5,106 individuals who were invited to participate, only 8 individuals elected to have pre-test genetic counseling[6].
Table 1 presents demographic information on the individuals who consented to the parent study and compares these inviduals to those who were determined to be ineligible after the study invitation, those who actively declined to participate in the study, and those who did not respond to the study invitation. Fewer males consented to the parent study than females (57.6% vs. 60.9%), and more males were non-responders than females (33.0% vs. 28.9%; p = 0.02). Fewer single individuals consented to the study compared to married or partnered individuals (51.6% vs. 60.6%), and more single individuals were non-responders compared to married or partnered individuals (31.4% vs. 29.1%, p < 0.01). Consenters and active decliners differed significantly with respect to education, with increased consent rates corresponding with higher education (see Table 1, p < 0.01). Consent rates were significantly higher for members of the Mayo Clinic Biobank (a genetic biorepository) compared to members of the Vascular Disease Biorepository (61.1% vs. 35.0%, p < 0.01). Length of time in the recruitment registry and participant age were also significantly different between consenters and non-consenters.
Table 1.
Demographic characteristics and analysis of sample bias, n = 5,106
| Total n=5106 |
Consenters n=3037 |
Ineligible n=76 |
Non Responders n=1567 |
Active Refusers n=426 |
p value | |
|---|---|---|---|---|---|---|
| Sex | 0.02a | |||||
| Female | 2,884 (100) | 1,757 (60.9) | 45 (1.6) | 834 (28.9) | 248 (8.6) | |
| Male | 2,222 (100) | 1,280 (57.6) | 31 (1.4) | 733 (33.0) | 178 (8.0) | |
| Age (y) at study invite | <0.01c | |||||
| Mean (SD) | 60.0 (8.5) | 60.4 (8.1) | 61.5 (7.0) | 58.7 (9.1) | 61.6 (8.3) | |
| Range | (24.7–71.1) | (26.0–71.1) | (36.5–71.0) | (24.7–71.1) | (25.9–71.0) | |
| Race | 0.53a | |||||
| White | 4,944 (100) | 2,949 (59.6) | 75 (1.5) | 1,506 (30.5) | 414 (8.4) | |
| Black or African American | 24 (100) | 12 (50.0) | 0 (0.0) | 11 (45.8) | 1 (4.2) | |
| Asian | 44 (100) | 23 (52.3) | 1 (2.3) | 18 (40.9) | 2 (4.5) | |
| Other or unknown | 94 (100) | 53 (56.4) | 0 (0.0) | 32 (34.0) | 9 (9.6) | |
| Ethnicity | 0.72a | |||||
| Not Hispanic or Latino | 4,967 (100) | 2,956 (59.5) | 72 (1.4) | 1,522 (30.6) | 417 (8.4) | |
| Hispanic or Latino | 21 (100) | 11 (52.4) | 1 (4.8) | 8 (38.1) | 1 (4.8) | |
| Unknown | 118 (100) | 70 (59.3) | 3 (2.5) | 37 (31.4) | 8 (6.8) | |
| Marital status | 0.01a | |||||
| Married / partnered | 4,015 (100) | 2,435 (60.6) | 58 (1.4) | 1,170 (29.1) | 352 (8.8) | |
| No longer partneredd | 677 (100) | 389 (57.5) | 12 (1.8) | 235 (34.7) | 41 (6.1) | |
| Single, never married | 413 (100) | 213 (51.6) | 6 (1.5) | 161 (39.0) | 33 (8.0) | |
| Education | <0.01b | |||||
| Missing | 300 | 114 | 3 | 150 | 33 | |
| Grades 1–8 | 8 (100) | 2 (25.0) | 0 (0.0) | 5 (62.5) | 1 (12.5) | |
| Grades 9–11 | 38 (100) | 11 (28.9) | 1 (2.6) | 24 (63.2) | 2 (5.3) | |
| Grade 12/GED | 646 (100) | 351 (54.3) | 8 (1.2) | 206 (31.9) | 81 (12.5) | |
| College 1–3 years | 1,962 (100) | 1,134 (57.8) | 27 (1.4) | 632 (32.2) | 169 (8.6) | |
| College 4+ years | 1,223 (100) | 778 (63.6) | 19 (1.6) | 328 (26.8) | 98 (8.0) | |
| Grad/professional school | 929 (100) | 647 (69.6) | 18 (1.9) | 222 (23.9) | 42 (4.5) | |
| Registry membership | <0.01a | |||||
| Mayo Clinic Biobank | 4,786 (100) | 2,925 (61.1) | 70 (1.5) | 1,427 (29.8) | 364 (7.6) | |
| Mayo VDB | 320 (100) | 112 (35.0) | 6 (1.9) | 140 (43.8) | 62 (19.4) | |
| Tenure (months) in biobank | 0.01b | |||||
| Mean (SD) | 58.1 (19.7) | 57.1 (19.8) | 62.2 (21.6) | 59.1 (19.4) | 61.2 (19.1) | |
| Range | (5.0–116.0) | (5.0–107.6) | (10.4–107.3) | (7.2–116.0) | (9.4–110.9) | |
Chi-Square;
Kruskal Wallis;
ANOVA F-Test;
Divorced, separated, or widowed
Of the 3,037 individuals who consented to the parent study, 2,895 (95.3%) completed the survey. Of the 2,895 individuals who completed survey, 2,830 (97.8%) individuals completed all 16 items of the decisional conflict scale (DCS). Those without complete decisional conflict data (n = 65) were excluded from analyses involving the total DCS score. The mean total DCS score for the sample was 16.2 (SD 13.1), including 525 (18.6%) respondents with a DCS score of 0.
Over 95% of respondents felt they had made an informed choice about the study (as reflected by a response of either “strongly agree” or “agree”) and 98.0% of respondents indicated that they were satisfied with their decision. In addition, a high proportion of respondents agreed that they knew both the risks (85.4%) and benefits (90.8%) of participating in the parent study, and felt knowledgeable about the specific risks (81.2%) and benefits (83.9%) that were most important to them. Over 56% of respondents agreed that they had sufficient decisional support and over 71% of respondents agreed that they had sufficient advice in making their decision to participate in the parent study.
A decisional conflict score of less than 25 has been identified in the literature as associated with following through with one’s decision[19]. In our sample of 2,830 individuals with complete DCS data, 1,782 (63.0%) had a DCS score below 25. We examined demographic characteristics, knowledge, experience with genetics, health literacy, self-efficacy in coping, and discussion with others as predictors of DCS ≥25 using logistic regression. Table 2 presents statistically significant predictors in the final model, along with adjusted odds ratios of having a DCS score ≥ 25. Significant predictors included higher levels of genetic knowledge (OR= 0.90,CI: 0.87—0.95), education (OR= 0.86, CI: 0.79—0.95), and sex (OR= 1.43, CI: 1.20—1.71, with males having higher odds of DCS ≥25). Additionally, respondents with less than full confidence in coping (OR= 2.58, CI: 2.15—3.11) or no previous experience with genetic testing (OR= 1.42, CI: 1.05—1.92) were more likely to have a DCS score ≥25. Finally, respondents who reported that they had not discussed their decision with a family member (OR: 1.58, CI: 1.30—1.91) or a friend (OR: 1.88, CI: 1.01—3.73) before enrolling in the parent study were more likely to have a DCS score ≥25.
Table 2.
Odds of having decisional conflict about the choice to pursue genomic testing in a multivariable logistic regression analysis (n=1048)
| OR (95% CI)c |
|
|---|---|
| Education (ordinal)a | 0.86 (0.79, 0.95) |
| Grades 1–8 | --- |
| Grades 9–11 | --- |
| Grade 12/GED | --- |
| College 1–3 years | --- |
| College 4+ years | --- |
| Grad/professional school | --- |
| Gx knowledgeb | 0.90 (0.87, 0.94) |
| Sexb | |
| Female | REF |
| Male | 1.43 (1.20, 1.71) |
| Confidence w/ medical formb | |
| Extremely | REF |
| Quite a bit | 1.82 (1.46, 2.25) |
| Not at all to somewhat | 2.29 (1.59, 3.30) |
| Full confidence in copingb | |
| Yes | REF |
| No | 2.58 (2.15, 3.11) |
| Previous Gx testa | |
| Yes | REF |
| No | 1.42 (1.05, 1.92) |
| Before enrolling, talked w/ | |
| Family Memberb | |
| Yes | REF |
| No | 1.58 (1.30, 1.91) |
| Frienda | |
| Yes | REF |
| No | 1.88 (1.01, 3.73) |
= p<0.05;
= p<0.01;
= Adjusted for all variables in the final model. Final model variables that were not statistically significant are: age, months in biobank, race, marital status, insurance coverage, and discussion of choice with health care provider, study staff, or others. For additional details, see supplementary digital content.
In the final logistic regression model, three variables measuring self-efficacy in coping (i.e. confidence in ability to cope with positive findings related to elevated lipids or presence of colon polyps, positive findings related to other disease risks, and positive findings related to unpreventable disease risk) were collapsed into a single dichotomous variable (full confidence in coping with results vs. less-than-full confidence), since the three variables, when separated, created multi-collinearity in the model. Confidence in coping with negative results was excluded from the final model because its conceptual relevance became increasingly suspect as we consolidated the self-efficacy questions focused on positive results. Employment status was also excluded from the final model because it was correlated with insurance coverage. Additional details can be found in Supplemental Tables 1 and 2, which presents the full set of variables used, unadjusted and adjusted confidence intervals, and p-values for the two logistic regression models that were run.
A second outcome of interest was decisional latency, understood as the time participants needed to reach a decision about the pursuit of genomic sequencing. The relationship between decisional latency and decisional conflict is shown in Figure 1. Respondents who reported that they were able to make a decision right away (n=2,158, 76.5%) had a mean DCS score of 14.4 (SD 12.7), while those who took several hours to decide (n=372, 13.2%) had a mean DCS score of 20.1 (SD 12.5), and those who took several days to decide (n=291, 10.3%) had a mean DCS score of 24.4 (SD 12.2).
Figure 1.
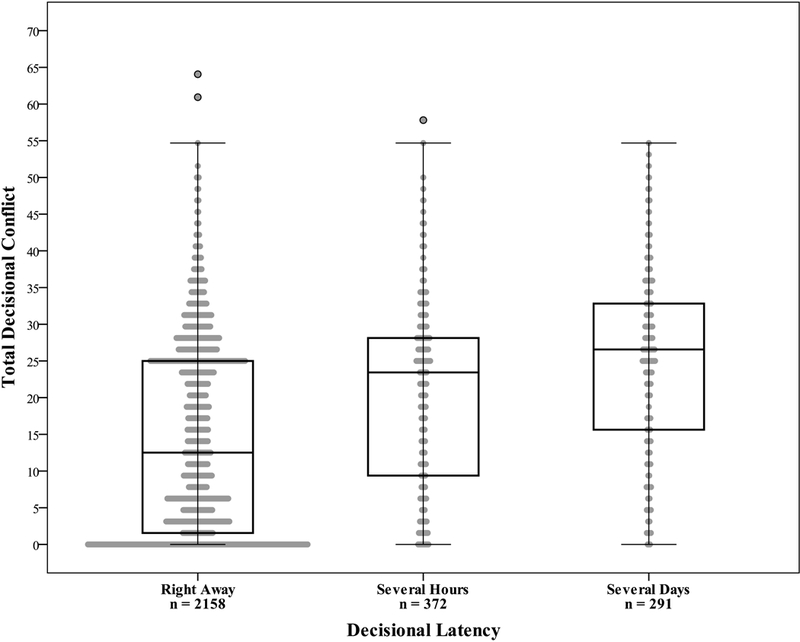
Box plots of mean decisional conflict scores by categories of decisional latency, p<0.0001. Frequencies provided reflect cases with complete data for both the DCS and decisional latency variables (n=2,821).
We examined factors that might be associated with decisional latency using the variables that were included in the final logistic regression model for decisional conflict (Table 3). Differences between decisional latency categories and multiple variables were found to be statistically significant, including decisional conflict score, participant age, education, gender, health literacy, self-efficacy in coping, previous experience with genetic testing, and discussion of study participation with family and friends. Although most respondents decided to pursue genomic sequencing right away (more than 76%), these findings suggest a strong relationship between decisional conflict and decisional latency, since several significant predictors of decisional conflict in our final logistic regression model were also statistically significant in differentiating between decisional latency categories.
Table 3.
Distribution of decisional conflict predictors across categories of decisional latency
| Decisional Latency “I was able to make my decision...” |
||||
|---|---|---|---|---|
| Characteristica | Right away (n=2,206) |
After several hours (n=382) |
After several days (n=296) |
p value |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Decisional Conflict | 14.4 (12.7) | 20.1 (12.5) | 24.4 (12.2) | <0.01b |
| Age | 59.9 (8.1) | 60.7 (8.1) | 61.1 (7.7) | 0.02d |
| Months in registry | 57.7 (19.9) | 58.1 (19.5) | 57.0 (19.8) | 0.60b |
| Gx knowledge | 8.1 (2.5) | 8.2 (2.2) | 7.8 (2.6) | 0.08b |
| N (%) | N (%) | N (%) | ||
| Education (ordinal) | 0.01b | |||
| Grades 1–8 | 1 (0.0) | 0 (0.0) | 0 (0.0) | |
| Grades 9–11 | 6 (0.2) | 1 (0.3) | 1 (0.3) | |
| Grade 12/GED | 242 (11.1) | 44 (11.8) | 44 (15.2) | |
| College 1–3 years | 823 (37.8) | 123 (32.9) | 121 (41.7) | |
| College 4+ years | 600 (27.5) | 117 (31.3) | 67 (23.1) | |
| Grad/professional school | 508 (23.3) | 89 (23.8) | 54 (19.7) | |
| Gender Male | 915 (42.1) | 179 (47.2) | 110 (37.8) | 0.04c |
| Race White | 2,102 (96.7) | 369 (97.4) | 281 (96.9) | 0.81c |
| Marital status | 0.38c | |||
| Married/Partnered | 1,807 (83.2) | 326 (86.0) | 239 (82.1) | |
| No longer partnered | 270 (12.4) | 34 (9.0) | 37 (12.7) | |
| Single, never married | 96 (4.4) | 19 (5.0) | 15 (5.2) | |
| Insurancee | 0.47c | |||
| Employer only | 1,368 (62.8) | 217 (57.7) | 174 (60.2) | |
| Government | 582 (26.7) | 119 (31.6) | 83 (28.7) | |
| Private | 211 (9.7) | 37 (9.8) | 31 (10.7) | |
| No insurance | 19 (0.9) | 3 (0.8) | 1 (0.3) | |
| Confidence w/ med forms | <0.01c | |||
| Extremely | 1,711 (78.6) | 279 (74.2) | 177 (60.8) | |
| Quite a bit | 356 (16.4) | 68 (18.1) | 77 (26.5) | |
| Not at all to somewhat | 109 (5.0) | 29 (7.7) | 37 (12.7) | |
| Full confidence in coping | 891 (40.7) | 89 (23.4) | 58 (19.9) | <0.01c |
| Previous Gx test | 227 (10.3) | 28 (7.3) | 19 (6.4) | 0.03c |
| Before enrolling, talked w/ | ||||
| Family Member | 546 (24.8) | 203 (53.1) | 157 (53.0) | <0.01c |
| Friend | 26 (1.2) | 15 (3.9) | 22 (7.4) | <0.01c |
| Healthcare provider | 30 (1.4) | 2 (0.5) | 8 (2.7) | 0.05c |
| RAVE Staff | 57 (2.6) | 5 (1.3) | 8 (2.7) | 0.31c |
| Other | 31 (1.4) | 5 (1.3) | 8 (2.7) | 0.22c |
Characteristics are included for analysis here based on their inclusion in the final logistic regression model;
Kruskall Wallis;
Chi-Square;
ANOVA F-Test.
At least 80% of expected cell counts are ≥ 5.
DISCUSSION
Results from our study challenge the notion that all individuals need pre-test genetic counseling to make an informed decision about whether to pursue genomic sequencing as a way to identify actionable genetic variants. We found low levels of decisional conflict in a majority of our participants, most of whom were able to make a decision about genomic testing quickly. It is noteworthy, however, that our participants had previously donated biological materials to a DNA biobank, and may not be typical of other patients. Although the genomic sequencing performed was done under a research protocol, participants were informed that the study would generate a clinical report and might require medical follow-up. As a result, findings from the study have important implications for the allocation of pre-test genetic counseling resources in both clinical and research settings, particularly for high-volume genomic screening programs.
At least three conclusions may be drawn from our data. First, in a population that resembles our sample, decisional conflict about participating in a disease risk screen may be low, if not altogether absent, in a significant proportion of individuals who are considering genomic sequencing. Decisional conflict is widely used as a method of gauging the quality of patient decision-making, especially choices about medical treatment and screening for diseases like prostate cancer[20–26] and breast cancer.[27–32] Decisional conflict also has been an outcome of much interest in genetic testing,[33–47] and at least two studies have shown that decisional conflict is modifiable by genetic counseling[48, 49]. Our data suggest that many patients believe they can make an informed decision about genomic sequencing in the absence of genetic counseling, a belief that appears to be confirmed by very low levels of decisional conflict.
Second, in a population that resembles our sample, it may be possible to identify a subset of individuals who would benefit from pre-test genetic counseling prior to making a decision about genetic risk evaluation. Individuals in our study with decisional conflict ≥25 tended to have lower education, lower knowledge about the capabilities and limitations of genomic sequencing, and lower self-efficacy in coping with positive findings. Men and individuals who did not have prior experience with genetic testing were also more likely to be ambivalent about their decision to participate. Consistent with findings by Puski and colleagues, participants who discussed their interest in genomic screening with a family member or friend were less likely to report decisional ambivalence[50]. These data suggest that pre-test genetic counseling resources and other decisional support tools might be prioritized for a subgroup of patients with specific risk factors related to the quality of their decision-making.
Toward identifying patients who may be at risk of making a decision that they subsequently regret, some promising work has been done in the creation of a four-item screener for decisional conflict in clinical settings[51]. Although such a tool has not been tested in the context of genomic sequencing, adopting this approach might help to maximize the value added by pre-test genetic counseling. Future research should aim to assess whether participants who might benefit the most from pre-test genetic counseling would opt to pursue such counseling if they understood its value and these services were convenient and accessible.
Third, our data suggest that many individuals make decisions about genomic sequencing quickly, with nearly 78% of our study participants deciding to pursue genomic risk evaluation “right away.” A majority (67.8%) of those who decided to participate “right away” had a decisional conflict score below 25. Although these data highlight a statistically significant association between decisional latency and decisional conflict, it is unclear how this association should be interpreted. For example, we do not believe these data support the idea that quick decision-making is indicative of a more informed or “better” decision; nor do we conclude that participants’ lack of decisional conflict was the cause of their quick decision. What does seem clear, however, is that requiring pre-consent genetic counseling may force some individuals—including individuals with little or no decisional conflict—to spend more time making medical decisions than they typically would, potentially disrupting their normal decision-making process and alienating some patients who might otherwise benefit from genetic testing. In addition, mandating a more deliberate decision-making process may reinforce the idea that genetic testing poses unique risks and is somehow very different from other types of diagnostic evaluations, thereby reinforcing a form of genetic exceptionalism.
In the context of our genomic sequencing study, requiring pre-test genetic counseling in addition to the mailed study invitation would have likely prolonged the decision-making process of those who elected to participate. Our data challenge the notion that patients need to meet with a genetic counselor prior to genomic evaluation and suggest instead that offering interested patients the opportunity speak with a genetic counselor should they choose to do so may be sufficient, particularly for adults with adequate levels of genetic knowledge, coping abilities, and health literacy. Future research should seek to replicate our findings in more diverse populations and assess the longitudinal impact of eliminating a requirement for pre-test genetic counseling.
LIMITATIONS
Results from our study should be interpreted with an awareness of several limitations. For example, participants responding to our survey were individuals who chose to participate in the parent genomic sequencing study, and we were not able to obtain data on decisional conflict from individuals who declined to participate in the parent study. In addition, although the differences between responders and non-responders that we observed were limited (see Table 1), self-selection into the screening study may have created bias in the data we report. Another limitation is the inability of a mailed survey to explore participants’ decision making processes in greater depth. While we saw little evidence of decisional conflict in our sample, it is unclear whether these findings reflect an authentic lack of conflict. In addition, while the Decisional Conflict Scale has demonstrated validity in many clinical settings, its use in the context of genomic sequencing research is more novel and invites further exploration and evaluation.
It is also important to note that the parent study in which this survey was embedded recruited individuals who were likely to be supportive of biomedical research, as evidenced by their enrollment in a DNA biobank or disease repository. In addition, the prior decision-making process that these individuals used to decide whether to participate in these research repositories may have influenced their decision-making about the pursuit of genomic sequencing. Although our study was designed to simulate a clinical screening program, participants may not have fully appreciated the potential medical implications of the results generated in the study. Our population was also predominantly white, over 50 years of age, and generally well educated. Nearly 99% of participants had some form of health insurance and nearly 90% had adequate health literacy. These features may limit the generalizability of our findings
Ultimately, additional data are needed to confirm or refute the notion that quality decisions to receive genetic disease risk assessment always require pre-test genetic counseling. For example, our data do not address whether participants with low levels of baseline decisional conflict would have nonetheless appreciated genetic counseling despite their lack of ambivalence about their decision. Our data also do not indicate whether study participants would have found pre-test genetic testing to be burdensome or would have passed on the opportunity to receive genetic testing altogether because of the inconvenience of pre-test counseling. Finally, our data are limited to patient beliefs and attitudes collected at a single point in time, prior to their receipt of genomic sequencing results, and do not reflect the psychosocial impact that might have followed from the receipt of specific test results. While our data on psychosocial outcomes associated with a multi-gene disease risk panel do not include post-test outcomes, our data are consistent with prior studies focused on psychosocial outcomes of genetic testing for single genes. For example, Hirschberg and colleagues found that the single best predictor of post-results anxiety was patient-reported anxiety at baseline[52]. We did relate self-efficacy in coping with decisional conflict, which together may serve as a good proxy for psychosocial status at baseline.
CONCLUSION
The precision of precision medicine should extend not just to the technical capabilities of genomic sequencing and risk stratification, but to the manner in which genetics professionals support patients. Some patients will require more pre-test decisional support than others; and it will be critical to identify those individuals who are most at risk of making an uninformed choice that fails to align with their core values and preferences. Other patients will be reluctant to use traditional forms of genetic counseling, and a requirement of pre-test genetic counseling may disenfranchise some patients who would otherwise be inclined to pursue, and would potentially benefit from, genomic sequencing. As greater numbers of institutions implement new forms of genomic sequencing, they should simultaneously consider how best to meet the decisional support needs of their patients. This may not involve providing traditional forms of pre-test genetic counseling, but alternative—and, in some cases, optional—decisional support services tailored to the unique needs of each individual patient.
Supplementary Material
Acknowledgments
This study was supported by a grant from the National Institutes of Health, U.S.A. (U01 HG006379) and by the Mayo Clinic Center for Individualized Medicine.
Footnotes
Conflict of Interest Statement
The authors report no conflicts of interest in the conduct and reporting of this study.
References
- 1.Genetic Counseling: An Indispensable Step in the Genetic Testing Process. Journal of Oncology Practice. 2008;4(2):96–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley EA, Hershberger RE, Caleshu C, Ellinor PT, Garcia JG, Herrington DM, Ho CY, Johnson JA, Kittner SJ, Macrae CA, Mudd-Martin G, Rader DJ, Roden DM, Scholes D, Sellke FW, Towbin JA, Van Eyk J, Worrall BB. Genetics and cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2012;126(1):142–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robson ME, Bradbury AR, Arun B, Domchek SM, Ford JM, Hampel HL, Lipkin SM, Syngal S, Wollins DS, Lindor NM. American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(31):3660–7. [DOI] [PubMed] [Google Scholar]
- 4.Schmidlen T, Sturm AC, Hovick S, Scheinfeldt L, Scott Roberts J, Morr L, McElroy J, Toland AE, Christman M, O’Daniel JM, Gordon ES, Bernhardt BA, Ormond KE, Sweet K. Operationalizing the Reciprocal Engagement Model of Genetic Counseling Practice: a Framework for the Scalable Delivery of Genomic Counseling and Testing. Journal of Genetic Counseling. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan AH, Datta SK, Skinner CS, Hollowell GP, Beresford HF, Freeland T, Rogers B, Boling J, Marcom PK, Adams MB. Randomized Trial of Telegenetics vs. In-Person Cancer Genetic Counseling: Cost, Patient Satisfaction and Attendance. Journal of Genetic Counseling. 2015;24(6):961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton EJ, Kullo IJ, Sharp RR. Making pretest genomic counseling optional: lessons from the RAVE study. Genetics In Medicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kullo I, Olson J, Fan X, Jose M, Safarova M, Radecki Breitkopf C, Winkler E, Snipes S, Kochan D, Carney M, Pacyna J, Chute C, Gupta J, Jose S, Venner E, Murugan M, Jiang Y, Zordok M, Farwati M, Philogene M, Smith E, Shaibi G, Caraballo P, Freimuth R, Lindor N, Sharp R, Thibodeau S. The Return of Actionable Variants Empiric (RAVE) Study, a Mayo Clinic Genomic Medicine Implementation Study: Design and Initial Results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson JE, Ryu E, Johnson KJ, Koenig BA, Maschke KJ, Morrisette JA, Liebow M, Takahashi PY, Fredericksen ZS, Sharma RG, Anderson KS, Hathcock MA, Carnahan JA, Pathak J, Lindor NM, Beebe TJ, Thibodeau SN, Cerhan JR. The Mayo Clinic Biobank: a building block for individualized medicine. Mayo Clinic proceedings. 2013;88(9):952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye Z, Kalloo FS, Dalenberg AK, Kullo IJ. An electronic medical record-linked biorepository to identify novel biomarkers for atherosclerotic cardiovascular disease. Global cardiology science & practice. 2013;2013(1):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Questionnaire (BRFSS) 2014. [cited CDC. Available from: https://www.cdc.gov/brfss/questionnaires/pdf-ques/2014_BRFSS.pdf.
- 11.Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Educ Monogr. 1978;6(2):160–70. [DOI] [PubMed] [Google Scholar]
- 12.Kaphingst KA, Facio FM, Cheng MR, Brooks S, Eidem H, Linn A, Biesecker BB, Biesecker LG. Effects of informed consent for individual genome sequencing on relevant knowledge. Clin Genet. 2012;82(5):408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, Bradley KA, Nugent SM, Baines AD, Vanryn M. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23(5):561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeSalvo KB, Fan VS, McDonell MB, Fihn SD. Predicting mortality and healthcare utilization with a single question. Health Serv Res. 2005;40(4):1234–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szanton SL, Allen JK, Thorpe RJ, Seeman T, Bandeen-Roche K, Fried LP. Effect of financial strain on mortality in community-dwelling older women. J Gerontol B Psychol Sci Soc Sci. 2008;63(6):S369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor AM. Validation of a decisional conflict scale. Medical decision making : an international journal of the Society for Medical Decision Making. 1995;15(1):25–30. [DOI] [PubMed] [Google Scholar]
- 17.Cappelli M, Hunter AGW, Stern H, Humphreys L, Van Houten L, O’Rourke K, Viertelhausen S, Perras H, Lagarde AE. Participation rates of Ashkenazi Jews in a colon cancer community-based screening/prevention study. Clinical Genetics. 2002;61(2):104–14. [DOI] [PubMed] [Google Scholar]
- 18.Flynn KE, Weinfurt KP, Seils DM, Lin L, Burnett CB, Schulman KA, Meropol NJ. Decisional Conflict Among Patients Who Accept or Decline Participation in Phase I Oncology Studies. Journal of empirical research on human research ethics : JERHRE. 2008;3(3):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor AM. User Manual - Decisional Conflict Scale 1993. [Available from: https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_decisional_conflict.pdf.
- 20.Volk RJ, Hawley ST, Kneuper S, Holden EW, Stroud LA, Cooper CP, Berkowitz JM, Scholl LE, Saraykar SS, Pavlik VN. Trials of Decision Aids for Prostate Cancer Screening. A Systematic Review. American journal of preventive medicine. 2007;33(5):428–34.e11. [DOI] [PubMed] [Google Scholar]
- 21.Stephens RL, Xu Y, Volk RJ, Scholl LE, Kamin SL, Holden EW, Stroud LA. Influence of a Patient Decision Aid on Decisional Conflict Related to PSA Testing: A Structural Equation Model. Health Psychology. 2008;27(6):711–21. [DOI] [PubMed] [Google Scholar]
- 22.Allen JD, Mohllajee AP, Shelton RC, Drake BF, Mars DR. A computer-tailored intervention to promote informed decision making for prostate cancer screening among African American men. American Journal of Men’s Health. 2009;3(4):340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams RM, Davis KM, Luta G, Edmond SN, Dorfman CS, Schwartz MD, Lynch J, Ahaghotu C, Taylor KL. Fostering informed decisions: A randomized controlled trial assessing the impact of a decision aid among men registered to undergo mass screening for prostate cancer. Patient education and counseling. 2013;91(3):329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sultan DH, Rivers BM, Osongo BO, Wilson DS, Schenck A, Carvajal R, Rivers D, Roetzheim R, Lee Green B. Affecting African American men’s prostate cancer screening decision-making through a mobile tablet-mediated intervention. Journal of Health Care for the Poor and Underserved. 2014;25(3):1262–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watts KJ, Meiser B, Wakefield CE, Barratt AL, Howard K, Cheah BC, Mann GJ, Lobb EA, Gaff CL, Patel MI. Online prostate cancer screening decision aid for at-risk men: A randomized trial. Health Psychology. 2014;33(9):986–97. [DOI] [PubMed] [Google Scholar]
- 26.Gökce MI, Wang X, Frost J, Roberson P, Volk RJ, Brooks D, Canfield SE, Pettaway CA. Informed decision making before prostate-specific antigen screening: Initial results using the American Cancer Society (ACS) Decision Aid (DA) among medically underserved men. Cancer. 2017;123(4):583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathieu E, Barratt A, Davey HM, McGeechan K, Howard K, Houssami N. Informed choice in mammography screening: A randomized trial of a decision aid for 70-year-old women. Archives of Internal Medicine. 2007;167(19):2039–46. [DOI] [PubMed] [Google Scholar]
- 28.Nekhlyudov L, Li R, Fletcher SW. Informed decision making before initiating screening mammography: Does it occur and does it make a difference? Health Expectations. 2008;11(4):366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schonberg MA, Hamel MB, Davis RB, Griggs C, Wee CC, Fagerlin A, Marcantonio ER. Development and evaluation of a decision aid on mammography screening for women 75 years and older. JAMA Internal Medicine. 2014;174(3):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eden KB, Scariati P, Klein K, Watson L, Remiker M, Hribar M, Forro V, Michaels L, Nelson HD. Mammography decision aid reduces decisional conflict for women in their forties considering screening. Journal of Women’s Health. 2015;24(12):1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scariati P, Nelson L, Watson L, Bedrick S, Eden KB. Impact of a decision aid on reducing uncertainty: Pilot study of women in their 40s and screening mammography Clinical decision-making, knowledge support systems, and theory. BMC medical informatics and decision making. 2015;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meisel SF, Freeman M, Waller J, Fraser L, Gessler S, Jacobs I, Kalsi J, Manchanda R, Rahman B, Side L, Wardle J, Lanceley A, Sanderson SC. Impact of a decision aid about stratified ovarian cancer risk-management on women’s knowledge and intentions: A randomised online experimental survey study. BMC Public Health. 2017;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humphreys L, Cappelli M, Hunter AGW, Allanson J, Zimak A. What is the significance of attendance by the partner at genetic counselling for advanced maternal age? Psychology, Health and Medicine. 2003;8(3):265–78. [Google Scholar]
- 34.Kaiser AS E Ferris L, Katz R, Pastuszak A, Llewellyn-Thomas H, Johnson JA, Shaw BF. Psychological responses to prenatal NTS counseling and the uptake of invasive testing in women of advanced maternal age. Patient education and counseling. 2004;54(1):45–53. [DOI] [PubMed] [Google Scholar]
- 35.Van Den Berg M, Timmermans DRM, Ten Kate LP, Van Vugt JMG, Van Der Wal G. Are pregnant women making informed choices about prenatal screening? Genetics in Medicine. 2005;7(5):332–8. [DOI] [PubMed] [Google Scholar]
- 36.Peterson SK, Pentz RD, Marani SK, Ward PA, Blanco AM, LaRue D, Vogel K, Solomon T, Strong LC. Psychological functioning in persons considering genetic counseling and testing for Li-Fraumeni syndrome. Psycho-oncology. 2008;17(8):783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Portnoy DB, Roter D, Erby LH. The role of numeracy on client knowledge in BRCA genetic counseling. Patient education and counseling. 2010;81(1):131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katapodi MC, Northouse L, Pierce P, Milliron KJ, Liu G, Merajver SD. Differences between women who pursued genetic testing for hereditary breast and ovarian cancer and their at-risk relatives who did not. Oncology Nursing Forum. 2011;38(5):572–81. [DOI] [PubMed] [Google Scholar]
- 39.Watts KJ, Meiser B, Mitchell G, Kirk J, Saunders C, Peate M, Duffy J, Kelly PJ, Gleeson M, Barlow-Stewart K, Rahman B, Friedlander M, Tucker K, Antill Y, Gregory P, Lipton L, McKay L, Senior J, Lobb EA, Crowe P, Matthews A, Neil G, Parasyn A, Thomson D, Zilliacus E, Andrews L, Gale J, Fox J, Harris M, Hart S, Smythe C, White M, Creighton L, Crowe K, D’Arcy J, Grieve S, Secomb E, Cicciarelli L, Henderson M, O’Brien J, Poliness C, Hattam A, Susman R, Ung O, Dickson R, Field M, Moore K, Bastick P, Inder S, Lynch J, Schwartz P, Zia R, Mak C, Snook K, Spillane A, Hopper J, Geelhoed L, Bowman M, Cheung D, Edirimanne S, Edwards E, Elder E, French J, Moon D. How should we discuss genetic testing with women newly diagnosed with breast cancer? Design and implementation of a randomized controlled trial of two models of delivering education about treatment-focused genetic testing to younger women newly diagnosed with breast cancer. BMC Cancer. 2012;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanderson SC, Linderman MD, Kasarskis A, Bashir A, Diaz GA, Mahajan MC, Shah H, Wasserstein M, Zinberg RE, Zweig M, Schadt EE. Informed decision-making among students analyzing their personal genomes on a whole genome sequencing course: A longitudinal cohort study. Genome Medicine. 2013;5(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sie AS, Prins JB, Spruijt L, Kets CM, Hoogerbrugge N. Can we test for hereditary cancer at 18 years when we start surveillance at 25? Patient reported outcomes. Familial Cancer. 2013;12(4):675–82. [DOI] [PubMed] [Google Scholar]
- 42.Connors LM, Voian N, Shi Y, Lally RM, Edge S. Decision making after BRCA genetic testing. Clinical journal of oncology nursing. 2014;18(3):E58–E63. [DOI] [PubMed] [Google Scholar]
- 43.Kuppermann M, Pena S, Bishop JT, Nakagawa S, Gregorich SE, Sit A, Vargas J, Caughey AB, Sykes S, Pierce L, Norton ME. Effect of enhanced information, values clarification, and removal of financial barriers on use of prenatal genetic testing: A randomized clinical trial. JAMA - Journal of the American Medical Association. 2014;312(12):1210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinney AY, Steffen LE, Brumbach BH, Kohlmann W, Du R, Lee JH, Gammon A, Butler K, Buys SS, Stroup AM, Campo RA, Flores KG, Mandelblatt JS, Schwartz MD. Randomized noninferiority trial of telephone delivery of BRCA1/2 genetic counseling compared with in-person counseling: 1-Year follow-up. Journal of Clinical Oncology. 2016;34(24):2914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muller C, Cameron LD. It’s complicated - Factors predicting decisional conflict in prenatal diagnostic testing. Health Expectations. 2016;19(2):388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cloutier M, Gallagher L, Goldsmith C, Akiki S, Barrowman N, Morrison S. Group genetic counseling: An alternate service delivery model in a high risk prenatal screening population. Prenatal Diagnosis. 2017;37(11):1112–9. [DOI] [PubMed] [Google Scholar]
- 47.Eure G, Germany R, Given R, Lu R, Shindel AW, Rothney M, Glowacki R, Henderson J, Richardson T, Goldfischer E, Febbo PG, Denes BS. Use of a 17-Gene Prognostic Assay in Contemporary Urologic Practice: Results of an Interim Analysis in an Observational Cohort. Urology. 2017;107:67–75. [DOI] [PubMed] [Google Scholar]
- 48.Suckiel SA, Linderman MD, Sanderson SC, Diaz GA, Wasserstein M, Kasarskis A, Schadt EE, Zinberg RE. Impact of Genomic Counseling on Informed Decision-Making among ostensibly Healthy Individuals Seeking Personal Genome Sequencing: the HealthSeq Project. Journal of Genetic Counseling. 2016;25(5):1044–53. [DOI] [PubMed] [Google Scholar]
- 49.Kaiser AS, Ferris LE, Pastuszak AL, Llewellyn-Thomas H, Johnson JA, Conacher S, Shaw BF. The effects of prenatal group genetic counselling on knowledge, anxiety and decisional conflict: Issues for nuchal translucency screening. Journal of Obstetrics and Gynaecology. 2002;22(3):246–55. [DOI] [PubMed] [Google Scholar]
- 50.Puski A, Hovick S, Senter L, Toland AE. Involvement and Influence of Healthcare Providers, Family Members, and Other Mutation Carriers in the Cancer Risk Management Decision-Making Process of BRCA1 and BRCA2 Mutation Carriers. J Genet Couns. 2018;27(5):1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferron Parayre A, Labrecque M, Rousseau M, Turcotte S, Légaré F. Validation of SURE, a four-item clinical checklist for detecting decisional conflict in patients. Medical Decision Making. 2014;34(1):54–62. [DOI] [PubMed] [Google Scholar]
- 52.Hirschberg AM, Chan-Smutko G, Pirl WF. Psychiatric implications of cancer genetic testing. Cancer. 2015;121(3):341–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


