Abstract
Background:
Adjuvant sunitinib has shown no overall survival (OS) benefit, uncertain disease-free survival (DFS) benefit, and increased toxicity versus placebo in phase III trials of resected high-risk renal cell cancer. To identify patients that may derive benefit or harm from adjuvant therapy, we assessed the effects of age and sex on treatment outcomes in the phase III ASSURE trial (Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Cancer).
Methods:
We conducted a post-hoc subgroup analysis of age and sex among patients in ASSURE. Adjusted hazard ratios (HR) for OS and DFS were evaluated with sunitinib or sorafenib versus placebo in four subgroups defined by sex and median age of the study.
Results:
Sunitinib treatment was associated with decreased OS (HR 2.21; 95% CI, 1.29–3.80) among women >56 years, but not in women ≤56 years or men of any age. Similar associations with age and sex were seen for DFS, but these were not statistically significant (women >56 years: HR 1.41; 95% CI, 0.94–2.10). No such association was found for sorafenib. The interaction by age and sex on mortality was statistically significant for sunitinib (p=0.01), but not sorafenib (p=0.10).
Conclusion:
Adjuvant sunitinib may increase mortality among older women with renal cell carcinoma. Given the recent approval of adjuvant sunitinib for high-risk resected renal cell carcinoma, additional studies are needed to confirm these findings.
Keywords: Renal Cell Carcinoma, Adjuvant Therapy, Sunitinib, VEGF Inhibitors, ASSURE Trial
Condensed Abstract
In this post-hoc analysis of ASSURE, the first and largest adjuvant VEGF inhibitor trial for resected renal cancer, we found increased mortality in older women treated with adjuvant sunitinib with more than 20% lower 5-year survival rates versus placebo. These findings caution that there may be subgroups of patients at risk of harm when an adjuvant therapy fails to improve overall survival.
INTRODUCTION
Although adjuvant sunitinib for patients with resected renal cell carcinoma (RCC) failed to improve overall survival (OS) in both the phase III ASSURE and S-TRAC trials, it has been approved by the U.S. FDA for this indication based on benefit in disease-free survival (DFS) in S-TRAC but not in ASSURE 1,2. A meta-analysis of these trials showed that adjuvant sunitinib failed to improve DFS while substantially increasing the risk of severe (grade 3 or higher) toxicities3. Therefore, it is important to identify subgroups that derive benefit or harm from adjuvant sunitinib.
The National Institutes of Health requires consideration of sex as a biological variable in biomedical research4. Differences in treatment outcomes and adverse events by sex have been observed for decades, with higher rates of toxicities from anticancer therapy among women compared to men5.
Sex-differences in the pharmacokinetics of vascular endothelial growth factor (VEGF) inhibitors have been previously described6. Both older age and female sex have been associated with severe toxicity in renal cancer patients treated with sunitinib in the metastatic setting7. Other studies have reported age- and sex- differences in survival in advanced stage patients with other solid malignancies following VEGF inhibitor treatment8–10. We hypothesized that treatment outcomes with adjuvant VEGF inhibitors in RCC might differ based on age and sex of the patient population. To test this hypothesis, we used individual patient data from the ASSURE trial to investigate the joint effect of age and sex on DFS and OS in patients receiving sunitinib, sorafenib, or placebo in order to identify such predictive subgroups.
PATIENTS AND METHODS
ASSURE included 1,943 patients with ≥ pT1b G3–4 N0 and/or N+ resected RCC. Patients were randomly assigned to receive oral sunitinib (50mg), sorafenib (800mg) daily, or equivalent placebo, for one year. In the overall analysis, there was no DFS or OS benefit with adjuvant sunitinib or sorafenib versus placebo as has been previously reported1. In this study, we conducted a post-hoc subgroup analysis of age and sex among patients in ASSURE.
Statistical analysis
OS and DFS were the two endpoints for this study. DFS was defined as the time from randomization to recurrence, development of second primary cancer, or death from any cause, consistent with the original ASSURE trial1. Patients alive without disease recurrence at the time of analysis were censored on the date of last disease evaluation.
For each endpoint, a multivariable stratified Cox regression model was used to assess the joint effect of gender, age, treatment and their interactions. Models were adjusted for race (white, black, or other), time from surgery to treatment start, and history of cardiovascular disease and thromboembolic events, and stratified on the four stratification variables used for randomization: histology (clear cell vs not clear-cell), modified UCLA Integrated Staging System (UISS) risk group (intermediate high risk vs very high risk)11,12, ECOG performance status (0 vs 1), and surgical approach (laparoscopic vs open). Treatment effect hazard ratios (HRs) for DFS and OS for sunitinib vs placebo and sorafenib vs placebo were estimated from this model for four subgroups defined by sex and the median age of the study population: females ≤56 years [n=317], females >56 years [n=317], males ≤56 years [n=689], and males >56 years [n=620]. The cut-point for age was determined using the median age of the entire trial population (56 years). Age as a continuous variable was modeled using a subpopulation treatment effect pattern plot (STEPP)13, which graphically explores the treatment effect pattern as a function of age. OS distributions for each subgroup were estimated using the Kaplan-Meier method. No adjustments for multiplicity were made.
In a toxicity analysis, the proportion of patients experiencing treatment-related grade 3 or above adverse events, and treatment discontinuation rates, were summarized by subgroup. All statistical tests were two-sided and analyses were conducted using the R statistical software (Version 3.4.0). Analyses were conducted using R statistical software (v3.4.0).
RESULTS
Baseline characteristics were similar within age and sex subgroups, shown in Table 1, with few exceptions. Among women >56 years, there was a higher proportion of cardiovascular disease and T3 cancers in the sunitinib arm; among women ≤56 years, there was a higher proportion of patients with ECOG performance status 1 in the sorafenib arm; and among men ≤56 years, there was a higher proportion of node positive cancers in the placebo arm.
Table 1a:
Baseline patient characteristics – Females
| > 56 years | ≤ 56 years | |||||
|---|---|---|---|---|---|---|
| Characteristic | Sunitinib | Sorafenib | Placebo | Sunitinib | Sorafenib | Placebo |
| Total No. |
112 |
99 |
106 |
106 |
113 |
98 |
| Race | ||||||
| White | 103 (94.5) | 93 (94.9) | 95 (90.5) | 96 (92.3) | 96 (88.1) | 85 (90.4) |
| Black | 5 (4.6) | 4 (4.1) | 6 (5.7) | 7 (6.7) | 10 (9.2) | 8 (8.5) |
| Other |
1 (0.9) |
1 (1.0) |
4 (3.9) |
1 (1.0) |
3 (2.7) |
1 (1.1) |
| ECOG Performance Status | ||||||
| 0 | 84 (75.0) | 71 (71.7) | 74 (71.8) | 92(86.8) | 84 (76.4) | 78 (82.1) |
| 1 |
28 (25.0) |
27 (27.3) |
29 (28.2) |
14 (13.2) |
26 (23.6) |
16 (16.8) |
| Cardiovascular disease |
35 (31.2) |
25 (25.3) |
26 (24.5) |
14 (13.2) |
15 (13.3) |
8 (8.2) |
| Thromboembolic disease |
6 (5.4) |
3 (3.0) |
4 (3.8) |
2 (1.9) |
5 (4.4) |
1 (1.0) |
| Surgical approach | ||||||
| Open | 72 (64.3) | 52 (52.5) | 61 (57.5) | 58 (54.7) | 65 (57.5) | 53 (54.1) |
| Laparoscopic |
40 (35.7) |
47 (47.5) |
45 (42.5) |
48 (45.3) |
48 (42.5) |
45 (45.9) |
| Surgery to therapy (median weeks) |
10.4 |
10.3 |
10.6 |
10.0 |
10.6 |
10.4 |
| Histology | ||||||
| Clear Cell | 96 (85.7) | 83 (83.8) | 86 (81.1) | 73 (68.9) | 84 (74.3) | 72 (73.5) |
| Non-Clear Cell |
16 (14.3) |
16 (16.2) |
20 (18.9) |
33 (31.1) |
29 (25.7) |
26 (26.5) |
| Pathologic T stage | ||||||
| 1 | 8 (7.1) | 7 (7.1) | 14 (13.2) | 13 (12.3) | 15 (13.3) | 14 (14.3) |
| 2 | 22 (19.6) | 29 (29.3) | 23 (21.7) | 38 (35.8) | 43 (38.1) | 35 (35.7) |
| 3 | 81 (72.3) | 61 (61.6) | 69 (65.1) | 54 (50.9) | 52 (46.0) | 48 (49.0) |
| 4 |
1 (0.9) |
2 (2.0) |
0 (0.0) |
1 (0.9) |
3 (2.7) |
1 (1.0) |
| Pathologic N + |
6 (5.4) |
4 (4.0) |
7 (6.6) |
7 (6.6) |
10 (8.8) |
7 (7.1) |
| UISS Risk | ||||||
|
Intermediate High a |
55 (49.1) |
54 (54.5) |
56 (52.8) |
66 (62.3) |
66 (58.4) |
65 (66.3) |
| Very High b | 57 (50.9) | 45 (45.5) | 50 (47.2) | 40 (37.7) | 47 (41.6) | 33 (33.7) |
T1b, Grade 3–4, any ECOG PS; T2, Grade 1–4, any ECOG PS; T3, Grade 1–4, ECOG PS 0 or Grade 1, ECOG PS ≥1
T3, Grade 2–4, ECOG PS ≥1; T4, any Grade, any ECOG PS; N+, any T, any Grade, any ECOG PS.
Adjusted HRs for DFS and OS in each age and sex subgroup are displayed in Table 2. Sunitinib treatment was associated with decreased OS (HR 2.21; 95% CI, 1.29–3.80) among women >56 years, but not in women ≤56 years or men of any age. Similar associations with age and sex were seen for DFS, but these were not statistically significant (women >56 years: HR 1.41; 95% CI, 0.94–2.10). For sorafenib, although the HR remained over 1 in women >56 years for both OS and DFS, neither were statistically significant (HR OS 1.62, 95% CI, 0.89–2.95; HR DFS 1.34, 95% CI, 0.87–2.05). The interaction by age and sex on mortality was statistically significant for sunitinib (p=0.01), but not for sorafenib (p=0.10).
Table 2.
Hazard ratios for (A) disease-freea and (B) overall survival by treatment arm in age and sex subgroups
| No. | Events | Sorafenib vs Placebo (HRb, 95% CI) |
Sunitinib vs Placebo (HRb, 95% CI) |
|
|---|---|---|---|---|
| (A) DFS | ||||
|
Females | ||||
| ≤56 | 317 | 106 | 1.08 (0.70–1.66) |
0.98 (0.62–1.56) |
| >56 | 317 | 143 | 1.34 (0.87–2.05) |
1.41 (0.94–2.10) |
|
Males | ||||
| ≤56 | 689 | 297 | 1.26 (0.88–1.82) |
1.36 (0.94–1.97) |
| >56 | 620 | 338 | 0.97 (0.60–1.58) |
0.98 (0.55–1.77) |
|
(B) OSc | ||||
| Females | ||||
| ≤56 | 317 | 58 | 0.96 (0.51–1.81) |
1.21 (0.64–2.29) |
| >56 | 317 | 85 | 1.62 (0.89–2.95) |
2.21 (1.29–3.80) |
|
Males | ||||
| ≤56 | 689 | 147 | 1.10 (0.65–1.87) |
1.44 (0.86–2.42) |
| >56 | 620 | 183 | 0.78 (0.41–1.49) |
0.69 (0.32–1.47) |
DFS event is the first of recurrence, development of second primary cancer, or death from any cause.
Stratified Cox regression models assessed the joint effect on DFS and OS by sex, age, treatment and their interactions, while adjusting for race (white, black, or other), time from surgery to treatment start (number of weeks), and history of cardiovascular disease and thromboembolic events, and stratifying on the four stratification factors used for randomization in ASSURE (ECOG 2805): histology (clear cell vs not clear-cell), modified UCLA Integrated Staging System (UISS) risk group (intermediate high risk vs very high risk), ECOG performance status (0 vs 1), and surgical approach (laparoscopic vs open). Intermediate high risk is defined as T1b, Grade 3–4, any ECOG PS; T2, Grade 1–4, any ECOG PS; T3, Grade 1–4, ECOG PS 0 or Grade 1, ECOG PS ≥1. Very high risk is defined as T3, Grade 2–4, ECOG PS ≥1; T4, any Grade, any ECOG PS; N+, any T, any Grade, any ECOG PS.
P value of interaction by age and sex on mortality with sorafenib=0.10; with sunitinib=0.01.
Kaplan-Meier estimates for OS are shown for each subgroup in Figure 1. Among men, there were no differences in OS between treatment arms by age (≤56 years, P=0.55; >56 years, P=0.59). Among women ≤56 years, OS was nearly identical (5-year rates ~ 84%, P=0.96). In contrast, among women >56 years, OS was highest in the placebo group and lowest in the sunitinib group (5-year rates for placebo: 89.8%, sorafenib: 77.9%, and sunitinib: 68.5%; P =0.006).
Figure 1:
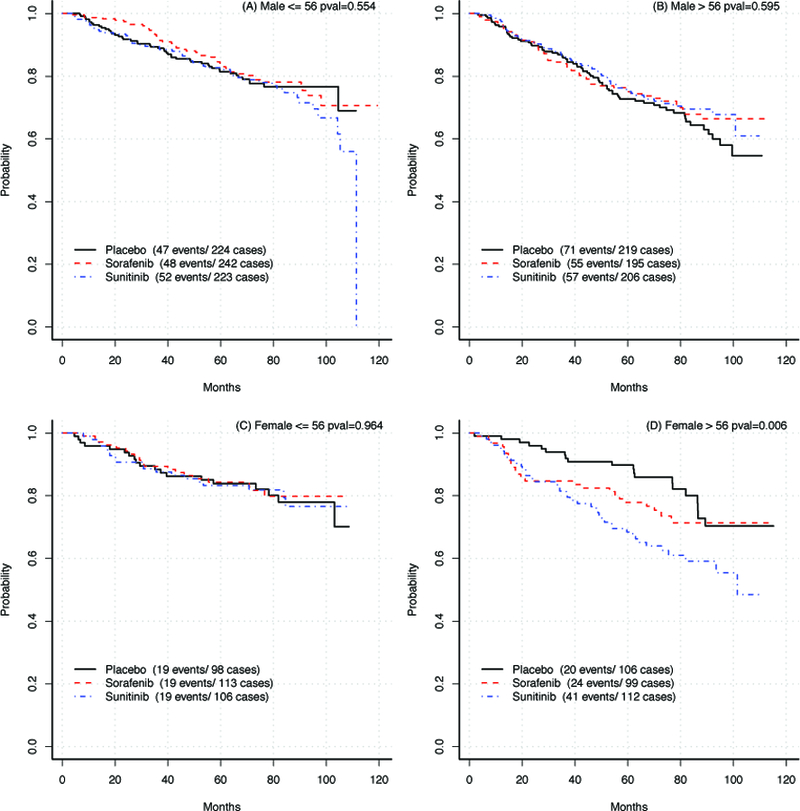
Kaplan-Meier estimates for OS by treatment arm in each subgroup
Kaplan-Meier curves for overall-survival by treatment arm in: A, Males ≤56; B, Males >56; C, Females ≤56; D, Females >56.
Treatment effects for sunitinib and sorafenib on OS are shown as a function of age by sex in Figure 2. Differences in OS varied according to age such that among patients >56 years treated with sunitinib, mortality was more than twice as high in women as compared to men. A similar but less dramatic difference was observed for sorafenib. When included as a continuous variable in multivariable Cox models, age was not associated with DFS or OS in women or men treated with sunitinib or sorafenib.
Figure 2:
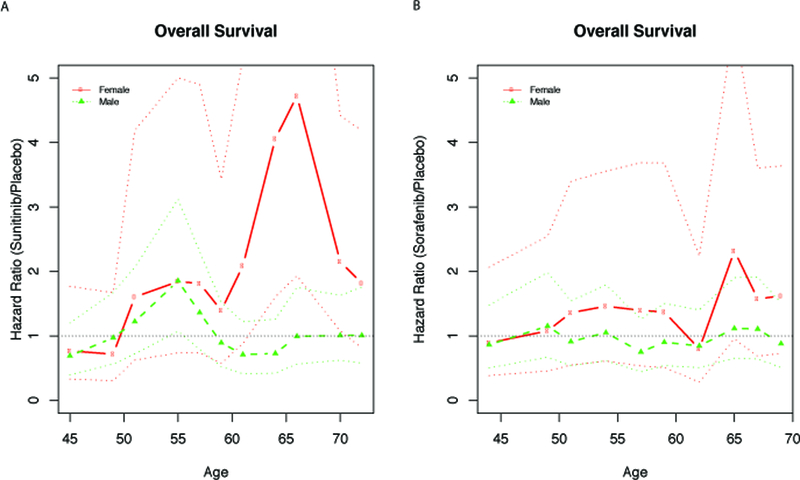
Subpopulation treatment effect pattern plot for overall survival with age as the covariate of interest. (A) sunitinib vs placebo. (B) sorafenib vs placebo.
Note: Broken solid lines are estimated hazard ratios. Dashed lines are 95% point-wise confidence intervals.
Sex-based differences in hazard ratios for OS varied according to age such that in the group of patients >56 years (trial median overall age) treated with sunitinib, the hazard ratio was more than twice as high in women as compared to men. While mortality risk was numerically higher with sorafenib relative to placebo among females older than 56, the magnitude of this association was not as large as with sunitinib.
In the toxicity analysis (Table 3), a slightly smaller proportion of men experienced adverse events than women, across subgroups. Among patients on sunitinib, older patients were modestly more likely to experience adverse events (women: 69.6%; men: 60.2%) compared to younger patients (women: 59.6%; men 47.5%). Among patients on sorafenib, older men had lower rates of toxicity relative to younger men (≤56 years, 59.0%; >56 years, 66.1%), while women had similar frequencies across the two age groups.
Table 3.
Proportion of patients (95% confidence intervals) experiencing at least one grade 3 or above adverse event [and discontinuing therapy due to adverse event]
| Sorafenib | Sunitinib | Placebo | ||||
|---|---|---|---|---|---|---|
| F ≤ 56 | 71.7% | [25%] | 59.4% | 11.2% | ||
| (63.4–80.0) | (50.1–68.8) | [17%] | (5.0–17.5) | [5%] | ||
| F > 56 | 68.7% | 69.6% | 16.0% | |||
| (59.6–77.8) | [25%] | (61.1–78.2) | [31%] | (9.1–23.0) | [9%] | |
| M ≤ 56 | 66.1% | 47.5% | 12.5% | |||
| (60.2–72.1) | [15%] | (41.0–54.1) | [12%] | (8.2–16.8) | [4%] | |
| M > 56 | 59.0% | 60.2% | 11.9% | |||
| (52.1–65.9) | [22%] | (53.5–66.9) | [23%] | (7.6–16.2) | [5%] |
DISCUSSION
This subgroup analysis observed increased mortality in older women treated with adjuvant VEGF inhibitors, particularly sunitinib. The increased risk of mortality with adjuvant sunitinib among older women was substantial with more than 20% lower 5-year survival rates versus placebo and therefore warrants serious consideration. No such association was found with sorafenib.
Although the mechanism of the observed increased mortality among older women exposed to sunitinib in our study is unknown, possible explanations include sex-based differences in drug metabolism6, drug toxicity7, or RCC tumor biology14. Sunitinib clearance is slightly lower in females relative to males, resulting in potentially higher systemic exposure15. In patients with metastatic disease, higher sunitinib exposure was associated with improved clinical outcomes and increased risk of adverse events16. Among women, it is less clear how age and reproductive status (pre-, peri- or post-menopause) influences drug clearance. Preclinical studies suggest that estrogen reduces expression of the sunitinib efflux transporter genes ABCB1 and ABCG217,18, resulting in increased drug exposure in younger women (i.e., higher estrogen) and decreased drug exposure in older women (i.e., lower estrogen). However, if sunitinib were ineffective in older women due to decreased drug exposure, similar rather than detrimental effects on survival would be expected relative to placebo. Sorafenib exposure and outcome in hepatocellular cancer are also affected by variations in the ABCB1 and ABCG2 efflux transporter genes19. Although the mortality risk appeared numerically higher with sorafenib relative to placebo among older females (HR 1.62, 95% CI, 0.89–2.95), the magnitude of this association was not as large as with sunitinib (HR 2.21, 95% CI 1.29–3.80).
In a recent meta-analysis of the ASSURE and S-TRAC trials3, the pooled HR for OS was > 1 with adjuvant sunitinib relative to placebo, suggesting possible harm. Further, the pooled relative risk of developing a grade 3 or higher adverse event was >2.5 fold higher with sunitinib. Severe sunitinib toxicity has been linked to the combination of female sex and older age, irrespective of body weight7. In our study, we observed only modestly higher toxicity in older compared to younger sunitinib treated women. Although most VEGF inhibitor related adverse events resolve after discontinuation of therapy, data on the long-term sequelae of short-term sunitinib exposure (i.e., 1 year) are limited. In a long-term safety analysis including mostly metastatic RCC patients who received < 2 years of sunitinib, incidence rates of hypertension, the most common cardiovascular event associated with sunitinib, persisted from 24% in year 1 to 30% in year 620. Indeed, comorbidity such as hypertension is an independent risk factor for mortality among patients with cancer, including kidney cancer, regardless of cancer stage21.
Surgical reports prior to the advent of adjuvant therapy in RCC have demonstrated improved survival in younger relative to older females22–24. A commonly cited reason is the age-associated changes in sex hormones. The estrogen-estrogen receptor (ER) β axis is known to influence RCC tumor biology. In preclinical RCC models, estrogen inhibits RCC progression through ER β activation14 suggesting a protective role of estrogen on RCC outcome. Older women are likely to have low levels of estrogen and ER β expression, which could contribute to the higher mortality observed among older women in the sunitinib or sorafenib arm but does not explain the lower mortality observed among older women in the placebo arm. Thus, further research is needed to examine the potential interaction between sex-hormones, VEGF inhibitor treatment, and RCC tumor biology.
Our study has important clinical and regulatory implications. Adjuvant sunitinib after surgery for high risk RCC was approved by the U.S FDA in a controversial decision after the Oncologic Drug Advisory Committee votes were split 6–625. This decision was based on improved DFS alone in the S-TRAC trial, despite negative DFS in ASSURE and negative OS in both the trials. In contrast, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) and the European Association of Urology (EAU) Guidelines Panel recommended against adjuvant sunitinib based on the poor benefit-to-harm ratio26,27. Further, a recent quality of life report from the S-TRAC trial showed reduced quality of life outcomes among patients treated with sunitinib versus placebo28. Our study shows that certain groups of patients, such as older women, may be at a risk of harm with adjuvant sunitinib. Thus, when approval decisions are made based on a surrogate endpoint neglecting the absence of benefit in overall survival or quality of life, there may be subgroups of patients at increased risk of harm.
All limitations of post-hoc data analysis apply to this study, including risks of confounding and chance. Confounding can occur if measured or unmeasured baseline characteristics are imbalanced within subgroups, such as the observed higher rate of cardiovascular disease among women >56 years on sunitinib compared to sorafenib or placebo. However, age- and sex-based differences in survival persisted despite adjusting for a large range of covariates, including but not limited to race, tumor stage, UISS risk, and cardiovascular disease, which are among the strongest predictors of death in patients with resected kidney cancer. Additionally, we were unable to determine the cause of death and RCC treatment patterns at disease recurrence, each of which could provide mechanistic insight into the observed associations. For example, women treated with adjuvant sunitinib may be at higher risk of death from adverse events not captured on trial or from recurrent metastatic RCC that is less responsive to subsequent VEGF inhibitor therapy29.
In summary, this subgroup analysis observed an increased mortality in older women treated with adjuvant sunitinib. Given the recent approval of sunitinib for the adjuvant treatment of RCC, additional studies are needed to confirm these potential risks in older women.
Table 1b:
Baseline patient characteristics – Males
| > 56 years | ≤ 56 years | |||||
|---|---|---|---|---|---|---|
| Characteristic | Sunitinib | Sorafenib | Placebo | Sunitinib | Sorafenib | Placebo |
| Total No. | 206 | 195 | 219 | 223 | 242 | 224 |
| Race | ||||||
| White | 193 (93.7) | 183 (93.8) | 202 (93.5) | 206 (93.6) | 217 (91.9) | 203 (91.9) |
| Black | 7 (3.4) | 8 (4.1) | 8 (3.7) | 8 (3.6) | 5 (2.1) | 9 (4.1) |
| Other |
6 (2.9) |
4 (2.1) |
6 (2.8) |
6 (2.8) |
14 (5.9) |
9 (4.1) |
| ECOG Performance Status | ||||||
| 0 | 169 (83.3) | 164 (86.3) | 172 (79.3) | 175 (81.4) | 209 (88.9) | 183 (83.2) |
| 1 | 34 (16.7) | 26 (13.7) | 45 (20.7) | 37 (17.2) | 26 (11.1) | 37 (16.8) |
|
Cardiovascular disease |
66 (32.0) |
69 (35.4) |
69 (31.5) |
42 (18.9) |
39 (16.2) |
41 (18.3) |
| Thromboembolic disease |
7 (3.4) |
8 (4.1) |
12 (5.5) |
9(4.1) |
10 (4.1) |
12 (5.4) |
| Surgical approach | ||||||
| Open | 120 (58.3) | 107 (54.9) | 131 (59.8) | 126 (56.5) | 137 (56.6) | 129 (57.6) |
| Laparoscopic |
86 (41.7) |
88 (45.1) |
88 (40.2) |
97 (43.5) |
105 (43.4) |
95 (42.4) |
| Surgery to therapy (median weeks) |
10.4 |
10.1 |
10.1 |
10.1 |
10.3 |
10.1 |
| Histology | ||||||
| Clear Cell | 166 (80.6) | 154 (79.0) | 176 (80.4) | 177 (79.4) | 198 (81.8) | 175 (78.1) |
| Non-Clear Cell |
40 (10.4) |
41 (21.0) |
43 (19.6) |
46 (20.6) |
44 (18.2) |
49 (21.9) |
| Pathologic T stage | ||||||
| 1 | 21 (10.2) | 18 (9.2) | 16 (7.3) | 18 (8.1) | 27 (11.2) | 27 (12.1) |
| 2 | 34 (16.5) | 39 (20.0) | 50 (22.8) | 72 (32.3) | 70 (28.9) | 65 (29.0) |
| 3 | 149 (72.3) | 134 (68.7) | 151 (68.9) | 129 (57.8) | 142 (58.7) | 132 (58.9) |
| 4 | 2 (1.0) | 4 (2.1) | 2 (0.9) | 4 (1.8) | 3 (1.2) | 0 (0.0) |
|
Pathologic N + |
17 (8.2) |
15 (7.7) |
22 (10.0) |
17 (7.6) |
22 (9.1) |
31 (13.9) |
| UISS Risk | ||||||
| Intermediate Higha | 76 (36.9) | 88 (45.1) | 100 (45.7) | 126 (56.5) | 116 (47.9) | 105 (46.9) |
| Very Highb | 130 (63.1) | 107 (54.9) | 119 (54.3) | 97 (43.5) | 126 (52.1) | 119 (53.1) |
T1b, Grade 3–4, any ECOG PS; T2, Grade 1–4, any ECOG PS; T3, Grade 1–4, ECOG PS 0 or Grade 1, ECOG PS ≥1
T3, Grade 2–4, ECOG PS ≥1; T4, any Grade, any ECOG PS; N+, any T, any Grade, any ECOG PS.
Acknowledgments
Funding: This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PHD, Group Co-Chairs) and supported by the National Institutes of Health under the following award numbers CA180820, CA180794, and K23-CA187185 (to RM). The consent is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, nor does mention of trade names, commercial products, or organizations imply endorsement of the US government.
Footnotes
Disclosures: Dr. Mamtani has served as a consultant for Roche/Genentech. Dr. Haas has served as a consultant for Novartis and Exilexis. VW, BG, RSD, CNE, and JPD report no conflicts of interest.
Meeting presentation: This research was presented at the annual Genitourinary Cancers Symposium as an Oral Abstract on February 10th 2018.
References
- 1.Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet May 14 2016;387(10032):2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Haas NB, Donskov F, Gross-Goupil M, Varlamov S, Kopyltsov E, et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology September 13 2017:JCO2017735324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gyawali B, Ando Y. Adjuvant sunitinib for high-risk-resected renal cell carcinoma: a meta-analysis of ASSURE and S-TRAC trials. Annals of oncology : official journal of the European Society for Medical Oncology. Apr 1 2017;28(4):898–899. [DOI] [PubMed] [Google Scholar]
- 4.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature May 15 2014;509(7500):282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozdemir BC, Csajka C, Dotto GP, Wagner AD. Sex Differences in Efficacy and Toxicity of Systemic Treatments: An Undervalued Issue in the Era of Precision Oncology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology September 10 2018;36(26):2680–2683. [DOI] [PubMed] [Google Scholar]
- 6.Segarra I, Modamio P, Fernandez C, Marino EL. Sunitinib Possible Sex-Divergent Therapeutic Outcomes. Clinical drug investigation October 2016;36(10):791–799. [DOI] [PubMed] [Google Scholar]
- 7.van der Veldt AA, Boven E, Helgason HH, van Wouwe M, Berkhof J, de Gast G, et al. Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. British journal of cancer July 22 2008;99(2):259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England journal of medicine December 14 2006;355(24):2542–2550. [DOI] [PubMed] [Google Scholar]
- 9.Wakelee HA, Dahlberg SE, Brahmer JR, Schiller JH, Perry MC, Langer CJ, et al. Differential effect of age on survival in advanced NSCLC in women versus men: analysis of recent Eastern Cooperative Oncology Group (ECOG) studies, with and without bevacizumab. Lung cancer June 2012;76(3):410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, Dahlberg SE, Gray RJ, Schiller JH, Perry MC, Sandler A, et al. Sex differences in outcome with bevacizumab therapy: analysis of patients with advanced-stage non-small cell lung cancer treated with or without bevacizumab in combination with paclitaxel and carboplatin in the Eastern Cooperative Oncology Group Trial 4599. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer January 2011;6(1):103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zisman A, Pantuck AJ, Wieder J, Chao DH, Dorey F, Said JW, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology December 1 2002;20(23):4559–4566. [DOI] [PubMed] [Google Scholar]
- 12.Lam JS, Shvarts O, Leppert JT, Pantuck AJ, Figlin RA, Belldegrun AS. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. The Journal of urology August 2005;174(2):466–472; discussion 472; quiz 801. [DOI] [PubMed] [Google Scholar]
- 13.Bonetti M, Gelber RD. A graphical method to assess treatment-covariate interactions using the Cox model on subsets of the data. Statistics in medicine October 15 2000;19(19):2595–2609. [DOI] [PubMed] [Google Scholar]
- 14.Yu CP, Ho JY, Huang YT, Cha TL, Sun GH, Yu DS, et al. Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-beta activation. PloS one 2013;8(2):e56667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houk BE, Bello CL, Kang D, Amantea M. A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clinical cancer research : an official journal of the American Association for Cancer Research April 01 2009;15(7):2497–2506. [DOI] [PubMed] [Google Scholar]
- 16.Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer chemotherapy and pharmacology July 2010;66(2):357–371. [DOI] [PubMed] [Google Scholar]
- 17.Imai Y, Ishikawa E, Asada S, Sugimoto Y. Estrogen-mediated post transcriptional down-regulation of breast cancer resistance protein/ABCG2. Cancer research January 15 2005;65(2):596–604. [PubMed] [Google Scholar]
- 18.Mutoh K, Tsukahara S, Mitsuhashi J, Katayama K, Sugimoto Y. Estrogen-mediated post transcriptional down-regulation of P-glycoprotein in MDR1-transduced human breast cancer cells. Cancer science November 2006;97(11):1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tandia M, Mhiri A, Paule B, Saffroy R, Cailliez V, Noe G, et al. Correlation between clinical response to sorafenib in hepatocellular carcinoma treatment and polymorphisms of P-glycoprotein (ABCB1) and of breast cancer resistance protein (ABCG2): monocentric study. Cancer chemotherapy and pharmacology April 2017;79(4):759–766. [DOI] [PubMed] [Google Scholar]
- 20.Porta C, Gore ME, Rini BI, Escudier B, Hariharan S, Charles LP, et al. Long-term Safety of Sunitinib in Metastatic Renal Cell Carcinoma. European urology February 2016;69(2):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. Jama May 26 2004;291(20):2441–2447. [DOI] [PubMed] [Google Scholar]
- 22.Qu Y, Chen H, Gu W, Gu C, Zhang H, Xu J, et al. Age-dependent association between sex and renal cell carcinoma mortality: a population-based analysis. Scientific reports March 17 2015;5:9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rampersaud EN, Klatte T, Bass G, Patard JJ, Bensaleh K, Bohm M, et al. The effect of gender and age on kidney cancer survival: younger age is an independent prognostic factor in women with renal cell carcinoma. Urologic oncology January 2014;32(1):30 e39–13. [DOI] [PubMed] [Google Scholar]
- 24.May M, Aziz A, Zigeuner R, Chromecki T, Cindolo L, Schips L, et al. Gender differences in clinicopathological features and survival in surgically treated patients with renal cell carcinoma: an analysis of the multicenter CORONA database. World journal of urology October 2013;31(5):1073–1080. [DOI] [PubMed] [Google Scholar]
- 25.Gyawali B, Goldstein DA. The US Food and Drug Administration’s Approval of Adjuvant Sunitinib for Renal Cell Cancer: A Case of Regulatory Capture? JAMA oncology May 1 2018;4(5):623–624. [DOI] [PubMed] [Google Scholar]
- 26.Withdrawal of application for a change to the marketing authorisation for Sutent (sunitinib) European Medicines Association. 27 July 2018; https://http://www.ema.europa.eu/documents/medicine-qa/questions-answers-withdrawal-application-change-marketing-authorisation-sutent-sunitinib_en.pdf. [Google Scholar]
- 27.Bex A, Albiges L, Ljungberg B, Bensalah K, Dabestani S, Giles RH, et al. Updated European Association of Urology Guidelines Regarding Adjuvant Therapy for Renal Cell Carcinoma. European urology May 2017;71(5):719–722. [DOI] [PubMed] [Google Scholar]
- 28.Staehler M, Motzer RJ, George DJ, Pandha HS, Donskov F, Escudier B, et al. Adjuvant sunitinib in patients with high-risk renal cell carcinoma: safety, therapy management, and patient-reported outcomes in the S-TRAC trial. Annals of Oncology [DOI] [PMC free article] [PubMed]
- 29.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer cell March 03 2009;15(3):232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]


