Abstract
Purpose:
Cervical cancer is the leading cause of cancer death in sub-Saharan Africa. Risk of cancer development is increased for women living with HIV (WLWH). It is unknown what factors predict initiation of curative chemoradiotherapy (CRT) in resource-limited settings and if HIV is associated with initiating curative CRT in settings with a high HIV burden.
Methods:
All women living with and without HIV infection who were initiating curative and non-curative CRT for locally advanced cervical cancer in Botswana were prospectively enrolled in an observational study. Factors associated with receiving CRT were evaluated in all patients and the subgroup of WLWH.
Results:
Of 519 women enrolled, 284 (55%) initiated CRT with curative intent. The curative cohort included 200 (70.4%) WLWH with median CD4 count of 484.0 cells/μL (IQR 342.0–611.0). In the non-curative cohort, 157 of 235 patients (66.8%) were WLWH with median CD4 count of 476.5 cells/μL (IQR 308.0–649.5). HIV status was not associated with initiating curative CRT (OR=0.95, 95% CI=0.58–1.56). Factors associated with receiving curative CRT treatment on multivariable analysis in all patients included baseline hemoglobin levels ≥10 g/dL (OR=1.80, 95% CI=1.18–2.74) and stage 1 or 2 vs. stage 3 or 4 disease (OR=3.16, 95% CI=2.10–4.75). Women >61 years old were less likely to receive curative treatment (OR=0.43, 95% CI=0.24–0.75). Among WLWH, higher CD4 cell counts are associated with higher rates of CRT initiation.
Conclusions:
Initiation of curative CRT is not dependent on HIV status. Significant predictors of CRT initiation include baseline hemoglobin level, stage, and age.
Keywords: HIV, Cervical Cancer, Chemoradiotherapy, CD4, Botswana
Condensed Abstract:
HIV status is not associated with initiating curative chemoradiotherapy for invasive cervical cancer in a cohort of women in Botswana. Our study suggests that women with well-managed HIV infection are not preferentially prescribed non-curative treatment over curative treatment and may have implications for cervical cancer treatment in populations with HIV world-wide.
Introduction
Cervical cancer, the fourth most common malignancy presenting in women around the world, is the leading cause of cancer-related death for women in sub-Saharan Africa (SSA)1. The development of cervical carcinoma is highly correlated with longstanding human papilloma virus (HPV) infection2. Women with HIV (WLWH) are more likely to maintain a chronic HPV infection that results in cellular transformation and subsequent cancer3. In regions that have a high burden of HIV infection4 as well as limited HPV screening capabilities and vaccination access5, addressing the long-term effects of co-infection is a public health imperative.
In Botswana, an estimated 22% of people aged 15–49 years are living with HIV infection6, yet HIV prevalence exceeds 34% in women aged 25–45 years4,6, putting this population at particularly high risk of developing cervical cancer. While an effective national anti-retroviral treatment (ART) program7 covers 83% of patients with HIV infection and has dramatically increased the lifespan of patients living with HIV, many of these patients subsequently suffer from immune- and infection-mediated cancers, including cervical cancer6. Despite increased access to ART, the incidence of cervical cancer has not declined in Botswana8,9. Given the high rates of HIV and cervical cancer in Botswana, there is a pressing need to identify and implement the optimal treatment for cervical cancer in WLWH.
Currently, the global standard of care for the treatment of locally invasive cervical cancer with the goal of cure requires radiotherapy (RT) and concurrent cisplatin-based chemotherapy10–12. However, it remains controversial if cervical cancer patients with HIV infection have similar outcomes to cervical cancer patients without HIV infection. While some studies suggest that HIV infection may be associated with decreased survival13,14, our previous findings in Botswana have suggested that patients with HIV infection who initiated chemoradiation treatment (CRT) with curative intent have similar outcomes to patients without HIV infection15. Factors associated with improved survival in this group were hemoglobin levels ≥10 g/dL and total radiation dose, but not HIV infection15. However, it is unknown if HIV infection plays a role in initiation of curative CRT in the modern ART era with well managed HIV infection either because of physician bias or due to sequela of HIV infection that make it challenging for patients with HIV infection to receive chemotherapy or curative dose radiation16–18. This study aims to identify predictors of initiation of curative CRT in an effort to inform future care decisions of patients in limited resource settings, particularly in regions with a high burden of HIV infection.
Methods
Women with histologically confirmed cervical carcinoma who presented for radiation treatment (RT) or CRT at Gaborone Private Hospital (GPH) or Princess Marina Hospital (PMH) gynecological multi-disciplinary clinic were approached for enrollment in this prospective observational cohort study between July 2013 and July 2017. Because GPH houses the sole radiation oncology facility in Botswana, the government maintains a public-private partnership that covers the cost of primary therapy for cervical cancer patients at GPH, including radiation and chemotherapy8,19. All patients from PMH are referred to GPH for treatment. Patients under 18 years of age and those who did not wish to participate were excluded. After participants provided written informed consent they were interviewed by designated research associates in their preferred language about demographics, marital status, and distance from home to treatment facility. Stage, baseline laboratory values, CD4 count history, and ART history were collected through review of medical records. Disease stage was determined according to International Federation of Gynaecology and Obstetrics (FIGO) guidelines. Karnofsky Performance Status (KPS) scale was used to evaluate functional impairment due to disease20.
Main Outcome
The primary goal of this study was to determine predictors of initiation of curative treatment with concurrent CRT. After appropriate disease staging, patients were prescribed treatment with curative intent or non-curative treatment. Patients initiating curative treatment were defined as receiving at least one cycle of chemotherapy. Patients initiating non-curative CRT were defined as not receiving any chemotherapy, which includes patients receiving high dose RT alone and palliative RT with or without brachytherapy.
Cervical Cancer Treatment
Government-funded cervical cancer care is provided by GPH, the only clinical center in the country with the capability to provide radiotherapy treatment. Patients’ cervical cancer disease was staged according to FIGO guidelines21. Before treatment began, patients underwent a chest x-ray and abdominal ultrasound to assess for hydronephrosis. Basic laboratory studies such as complete blood count, liver function tests and renal function tests were also obtained. Patients were given transfusion to hemoglobin of 10g/dL when possible. Curative CRT included 45–50 Gy whole-pelvis radiation, weekly concurrent cisplatin treatment (35–40 mg/m2) for five cycles, and high dose rate (HDR) brachytherapy (7 Gy x 3–4 fractions or 6 Gy x 4–5 fractions) using either a tandem and ring applicator or a tandem and ovoid applicator. Details of radiation treatment planning at GPH for cervical cancer have been described previously15.
Antiretroviral Treatment
HIV status was determined by patients’ health records. Patients without infection or unknown status who had not received an HIV test within the past six months were offered an HIV test on site. All WLWH with CD4 count ≤350 cells/μl, cervical cancer diagnosis, or any other HIV-associated condition22 were eligible for free ART from the Botswana National ART Program from 2013 to 20154,6,23. As of 2016, a test and treat policy was implemented such that all positive patients were eligible for free ART at the time of HIV testing and subsequent diagnosis. First-line ART included tenofovir, emtricitabine, and efavirenz from 2013 to 20165. Regimens including dolutegravir, tenofovir and emtricitabine were also first-line options from 2016 onwards24. Any WLWH who were not already on treatment at the time of their cervical cancer diagnosis were referred for ART initiation.
Statistical Analysis
The primary outcome of our study was initiation of curative CRT. Independent predictors evaluated included HIV status, baseline CD4 cell count (>200 and ≤200 cells/μl), age (>61 and ≤61 years), marital status, disease stage, baseline hemoglobin (<10 vs. ≥10 g/dL), baseline creatinine, performance status using KPS, and distance to treatment center. Baseline values represent laboratory results obtained before treatment initiation. Based on knowledge of clinical practice, we explored older age thresholds for reduced CRT. Age >61 was found to be predictive of reduced CRT and used to dichotomize the age variable for our primary analysis. Additional analysis with continuous CD4 count and age has been included in Supplementary Table 1.
Bivariate associations between independent variables and initiation of curative CRT were assessed using logistic regression analysis. Multivariable logistic regression model was fitted with CRT initiation as the dependent variable. Independent covariates for inclusion in the final logistic model were selected based on a priori knowledge, which included HIV status, baseline CD4 cell count, age, marital status, disease stage, baseline hemoglobin, baseline creatinine, and performance status.
To understand the role of HIV infection and CD4 count on initiation of curative CRT, models with all patients and only patients with HIV were explored. Model 1a compares patients with and without HIV regardless of CD4 count in the population with HIV. Model 1b incorporates information about CD4 count in patients with HIV infection and compares CD4 counts >200 and ≤200 cells/μl to HIV-uninfected patients. Model 2 includes only patients with HIV and compares those with CD4 count ≤200 cells/μl to those with CD4 count >200 cells/μl. Independent variables showing statistical association with the outcome in bivariate analyses were also included in the multivariate models.
Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). An alpha cutoff for statistical significance was not defined as recommended by current statistical guidelines25.
Ethics
This study was approved by the Health Research Development Committee of the Botswana Ministry of Health and the Institutional Review Board of the University of Pennsylvania.
Results
Demographic and clinical characteristics of the cohort:
We enrolled 532 patients between July 2013 and July 2017. Subjects with unknown HIV status, marital status, and performance status were excluded from analysis (n=13). Of the remaining 519 patients, 54.7% (n=284) initiated curative CRT (Table 1). Of those who initiated curative treatment, 70.4% (n=200) were WLWH with 94.9% (n=187) on ART at the start of cervical cancer treatment. Of the 235 patients who did not initiate curative CRT, 66.8% (n=157) were WLWH with 92.9% (n=145) on ART. Median CD4 count in the curative group was 484.0 cells/μL (IQR 342.0–611.0) compared to 476.5 cells/μL (IQR 308.0–649.5) in the non-curative group. Patients presenting with stage 1 or 2 disease, stage 3 or 4 disease, or missing staging information accounted for 64.4% (n=183), 34.5% (n=98), and 1.1% (n=3) of the curative CRT cohort, respectively. In the non-curative cohort, 27.7% (n=65) presented at stage 1 or 2, 54.9% (n=129) presented at stage 3 or 4, and 17.4% (n=41) were missing staging information. Tumor histology of the cohort was comprised of 88.8% (n=461) squamous cell carcinoma, 6.7% (n=36) adenocarcinoma, and 3.7% (n=19) other; 0.6% (n=3) were missing histological information. A strong majority of participants in the curative CRT arm (70.1%, n=199) had a hemoglobin count ≥10 g/dL before starting treatment while 55.3% (n=130) of those receiving non-curative CRT had comparable hemoglobin levels. The median distance from patients’ home town to Gaborone was 271 kilometers (IQR= 68–410) in both groups. The median time from biopsy to treatment initiation for those who did and did not receive curative treatment was 92 days (IQR=55–141) and 77 days (IQR=43–132), respectively (Table 1).
Table 1.
Characteristics of study participants (n=519)
| Characteristic | Curative treatment initiated n = 284 | Curative treatment not initiated n = 235 | |
|---|---|---|---|
| HIV status | Uninfected | 84 (29.6%) | 78 (33.2%) |
| Infected | 200 (70.4%) | 157 (66.8%) | |
| Age in years, median (IQR) | 48 (42–57) | 49 (42–62) | |
| Age | ≤ 40 years | 58 (20.4%) | 53 (22.5%) |
| 41–50 years | 113 (39.8%) | 78 (33.2%) | |
| 51–60 years | 64 (22.5%) | 39 (16.6%) | |
| ≥ 61 years | 49 (17.3%) | 65 (27.7%) | |
| Marital status | Single | 169 (59.5%) | 152 (64.7%) |
| Married/partnered | 78 (27.5%) | 52 (22.1%) | |
| Divorced/widowed | 37 (13.0%) | 31 (13.2%) | |
| Stage | Stage 1, 2 | 183 (64.4%) | 65 (27.7%) |
| Stage 3, 4 | 98 (34.5%) | 129 (54.9%) | |
| Missing | 3 (1.1%) | 41 (17.4%) | |
| Baseline hemoglobin, g/dL | < 10 | 85 (29.9%) | 105 (44.7%) |
| ≥ 10 | 199 (70.1%) | 130 (55.3%) | |
| Baseline creatinine, μmol/L, median (IQR) | 54 (46.5–63) | 66.2 (50–87) | |
| Performance status | ≤ 80 | 50 (17.6%) | 61 (26.0%) |
| > 80 | 234 (82.4%) | 174 (74.0%) | |
| On ART* | Yes | 187 (94.9%) | 145 (92.9%) |
| No | 10 (5.1%) | 11 (7.1%) | |
| Baseline CD4 cells/μL, median (IQR)* | 484.0 (342.0–611.0) | 476.5 (308.0–649.5) | |
| Baseline CD4 cells/μL* | < 200 | 10 (%) | 15 (%) |
| 200–349 | 34 (%) | 20 (%) | |
| 350–499 | 45 (%) | 23 (%) | |
| ≥ 500 | 82 (%) | 50 (%) | |
| Distance in km, median (IQR) | 271 (68–410) | 271 (68–410) |
Abbreviations: ART=anti-retroviral treatment; IQR=Interquartile range
Only applicable to participants with HIV.
Predictors of curative chemo-radiation:
Bivariate analysis:
Bivariate analysis of factors associated with initiation of curative CRT in all patients and HIV-infected patients only is shown in Table 2. In Model 1, the odds of initiating curative CRT in all patients were lower for patients > 61 years of age (OR=0.52, 95% CI=0.33–0.80). Factors associated with significantly higher odds of starting curative CRT included baseline hemoglobin level ≥10 g/dL (OR=1.89, 95% CI=1.32–2.71), presence of stage 1 or 2 disease vs. stage 3 or 4 disease (OR=3.71, 95% CI=2.52–5.45), KPS >80 (OR=1.64, 95% CI=1.08–2.50), and CD4 count >200 cells/μl (OR=1.6, 95% CI=1.08–2.40).
Table 2.
Factors associated with initiating curative treatment among study participants
| All patients (N=519) | Patients with HIV only (n=357) | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| Model 1 | Model 1a | Model 1b | ||||
| Variables | Crude OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Age | ≤ 61 years | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| > 61 years | 0.52 (0.33–0.80) | 0.43 (0.24–0.75) | 0.43 (0.24–0.77) | 1.02 (0.44–2.40) | 0.91 (0.34–2.42) | |
| Marital status | Single | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Married/partnered | 1.35 (0.89–2.04) | 1.52 (0.94–2.45) | 1.54 (0.95–2.49) | 1.32 (0.78–2.25) | 1.28 (0.71–2.31) | |
| Divorced/widowed | 1.07 (0.64–1.82) | 1.27 (0.68–2.38) | 1.34 (0.71–2.54) | 1.35 (0.63–2.90) | 1.58 (0.65–3.85) | |
| HIV status | Uninfected | 1.00 | 1.00 | N/A | N/A | N/A |
| Infected | 0.91 (0.55–1.49) | N/A | N/A | N/A | ||
| Baseline CD4, cells/μL | HIV uninfected | 1.00 | N/A | 1.00 | N/A | N/A |
| ≤ 200 | 0.62 (0.26–1.46) | N/A | 0.48 (0.18–1.29) | 1.00 | 1.00 | |
| > 200 | 1.61 (1.08–2.40) | N/A | 1.25 (0.74–2.12) | 2.59 (1.12–6.01) | 2.84 (1.14–7.09) | |
| Stage |
Stage 3, 4 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Stage 1, 2 | 3.71 (2.52–5.45) | 3.24 (2.15–4.87) | 3.36 (2.21–5.11) | 3.35 (2.11–5.33) | 3.16 (1.92–5.19) | |
| Missing | 0.10 (0.03–0.32) | 0.08 (0.02–0.26) | 0.09 (0.03–0.29) | 0.13 (0.04–0.44) | 0.13 (0.04–0.47) | |
| Baseline hemoglobin, g/dL | < 10 g/dL | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥ 10 g/dL | 1.89 (1.32–2.71) | 1.75 (1.15–2.65) | 1.85 (1.21–2.83) | 1.74 (1.14–2.67) | 1.63 (0.99–2.69) | |
| Baseline creatinine, μmol/L | 0.998 (0.996–1.000) | 0.999 (0.997–1.001) | 0.999 (0.998–1.001) | 0.999 (0.998–1.001) | 1.00 (0.999–1.001) | |
| Performance status | ≤ 80 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| > 80 | 1.64 (1.08–2.50) | 1.04 (0.64–1.69) | 1.02 (0.62–1.68) | 1.75 (1.02–2.98) | 1.32 (0.71–2.45) | |
| Distance in km | 1.000 (0.999–1.001) | Not included in model | Not included in model | 1.000 (0.999–1.001) | Not included in model | |
Abbreviations: OR=Odds ratio; CI=Confidence interval.
Model 1a: HIV+ vs. HIV−
Model 1b: HIV− vs HIV+ by CD4 count ≤ 200 vs. >200 cells/μl
Model 2: HIV+ only with CD4 count ≤ 200 vs. >200 cells/μl
As shown in Model 2, subset bivariate analysis of patients with HIV comparing those with CD4 count ≤200 cells/μl to those with CD4 count >200 cells/μl showed that CRT initiation is associated with CD4 count >200 cells/μl in patients with HIV infection (OR=2.59, 95% CI=1.12–6.01), baseline hemoglobin level ≥10 g/dL (OR=1.74, 95% CI=1.14–2.67), presence of stage 1 or 2 disease vs. stage 3 or 4 disease (OR=3.35, 95% CI=2.11–5.33), and a performance status >80 (OR=1.75, 95% CI=1.02–2.98).
Multivariable analysis:
Multivariable analysis (Table 2) in Model 1a revealed that after adjusting for HIV status, age, marital status, stage, baseline hemoglobin, baseline creatinine, and performance status, the odds of initiating curative CRT was higher among patients with baseline hemoglobin levels ≥10 g/dL (OR=1.80, 95% CI=1.18–2.74) and those with stage 1 or 2 disease vs. stage 3 or 4 disease (OR=3.16, 95% CI=2.10–4.75). Similar to bivariate analysis findings, age > 61 years remained a factor associated with lower odds of initiating curative CRT (OR=0.43, 95% CI=0.24–0.75). Notably, our findings did not show that HIV status was associated with initiating curative CRT among participants (OR=0.95, 95% CI=0.58–1.56). Figure 1 also demonstrates no variation in receipt of CRT by HIV status.
Figure 1. Percent of patients that initiate chemoradiation (CRT) by HIV status.
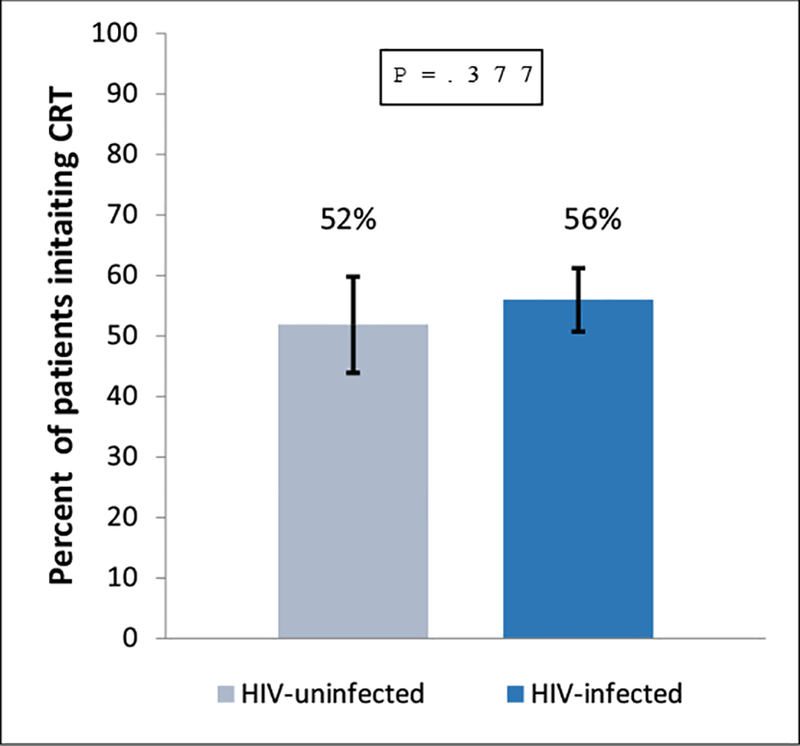
No significant difference in the percent of patients who initiated CRT was found when patients with and without HIV were compared (p=0.377). Error bars indicate 95% CI.
In Model 1b, patients with HIV were stratified by CD4 count >200 cells/μl or ≤ 200 cells/μl and compared to patients without HIV. Factors associated with CRT initiation in this model included CD4 count >200 cells/μl in patients with HIV (OR=1.61, 95% CI=1.08–2.40) compared to patients without HIV, age >61 (CI=0.43, 95% CI=0.24–0.77), and presence of stage 1 or 2 disease vs. stage 3 or 4 disease (OR=3.36 95% CI=2.21–5.11).
The multivariable analysis in Model 2 included only patients with HIV infection stratified by CD4 count. In this subgroup, factors associated with initiating CRT included CD4 count >200 cells/μl (OR=2.84, 95% CI=1.14–7.09) and presence of stage 1 or 2 disease compared to stage 3 or 4 disease (OR=3.16, 95% CI=1.92–5.19). Figure 2 shows the difference in the percent of patients with HIV who initiated CRT by CD4 count.
Figure 2. Percent of patients with HIV that initiate chemoradiation (CRT) by CD4 count.

Of the subset patients with HIV, the percent of patients who initiated CRT was significantly higher in those with CD4 counts > 200 cells/μl as compared to patients with CD4 counts ≤ 200 (p=0.022). Error bars indicate 95% CI.
Age and CD4 count were included as continuous variables in a multivariate analysis shown in Supplementary Table 1. Similar to the above results, initiation of CRT for all patients was significantly associated with early stage 1 or 2 disease (OR=3.16, 95% CI=2.10–4.75) and baseline hemoglobin (OR=1.80, 95% CI=1.18–2.74). In analysis of only patients with HIV, stage (OR=3.55, 95% CI=2.03–6.22) and baseline hemoglobin (OR=1.82, 95% CI=1.04–3.17) alone were found to be significant predictors of CRT initiation.
Discussion
In this large prospective observational cohort study of women with locally advanced cervical cancer in Botswana, 55% of patients initiated curative CRT. HIV infection was not associated with initiation of curative treatment. We found that the odds of initiation were significantly higher for patients with baseline hemoglobin levels ≥10 g/dL and those with stage 1 or 2 disease as compared to stage 3 or 4 disease. Furthermore, the odds of treatment initiation were significantly decreased in patients >61 years of age. Among WLWH, CD4 count >200 cells/μl and stage 1 or 2 disease vs. stage 3 or 4 disease were associated with higher odds of initiating CRT.
While several studies have reported that HIV is a significant negative predictor of survival after cervical cancer treatment14,26, our recent findings in Botswana suggest that HIV has no impact on two-year overall survival in patients initiating curative CRT15. The results from that analysis identified baseline hemoglobin level, receipt of total prescribed radiation dose, and age as the significant predictors of two-year overall survival15. The study was limited in its ability to determine whether HIV infection was associated with the initiation of curative CRT among patients who were intended to receive it. Results of the present study indicate that, overall, WLWH in Botswana are not preferentially prescribed non-curative treatment over curative treatment as compared to those without HIV. This result drives a striking contrast with studies in the United States that revealed that cancer patients with HIV were significantly less likely to be prescribed cancer treatment27,28. Taken together, these studies suggest that disparities that may exist in treatment of these patients in the United States are not biological in origin, but rather based on inaccurate clinical perceptions of cancer patients living with HIV or lack of guidelines for management of patients living with HIV. Lack of bias in Botswana maybe be due to the higher volume of patients living with HIV relative to those without HIV being treated for cancer compared to the United States, and high ART coverage leading to well-managed HIV infection in Botswana6. Nevertheless, certain degree of bias towards initiation of curative CRT for WLWH with low CD4 cells may remain, even in settings where WLWH account for the majority of patients with cervical cancer.
Baseline hemoglobin level was a significant predictor of CRT initiation. Previous studies of locally advanced cervical cancer in WLWH, including results from our curative cohort of patients with and without HIV, have demonstrated that a low hemoglobin level at baseline is a significant negative predictor of overall survival14,15,29. While survival outcomes may be linked to tumor biology such as hypoxic microenvironments or aggressive phenotypes30,31, the choice to initiate curative therapy is made clinically. Patients with low baseline hemoglobin in our study were routinely given transfusions before beginning treatment whenever blood was accessible. Studies have shown conflicting results regarding the clinical utility of this practice, with some highlighting benefit32,33 and others cautioning its usage34,35. Persistently low hemoglobin levels despite transfusion may be cause for delaying treatment or omitting concurrent chemotherapy.
Patients with early stage disease were significantly more likely to initiate curative CRT than those with late stage disease. This is likely due to better performance status in patients with early stage disease. Also, urgent palliative radiation therapy is often needed in cases of advanced cervical cancer. Patients presenting with advanced or metastatic cancers frequently report living with pain, anorexia, and fatigue36,37. Short-course radiation has been shown to be effective at controlling vaginal bleeding and pelvic pain38,39.
The odds of initiating CRT decreased with age > 61 years in our study. Despite evidence that elderly women tolerate pelvic radiotherapy and brachytherapy well40,41, studies have shown that older women are less likely to receive surgery and adjuvant radiotherapy for cervical cancer than their younger counterparts42,43. This trend may be due to treatment bias of physicians who worry about perceived risk associated with curative treatment of an older patient, but also may take into account older patients’ desire to forego cancer treatment in the face of multiple comorbidities43,44. More studies are needed to determine the underlying cause of reduced treatment initiation of older women in this population.
Within the subgroup of patients with HIV, CD4 count >200 cells/μl and stage 1 or 2 disease as compared to stage 3 or 4 disease were associated with higher odds of initiating CRT after adjusting for hemoglobin level, creatinine and performance status. These findings are consistent with a previous study’s finding that a CD4 count below 144 cells/μl and advanced stage disease were associated with reduced odds of receiving cancer treatment27. It is possible that patients with HIV with CD4 count ≤200 cells/μl are unable to initiate CRT due to physician discomfort with giving chemotherapy to patients with CD4 counts below 200 cells/μl. Although there are limited studies in this area, previous data from South Africa suggested that patients with HIV tolerated chemotherapy poorly and majority of patients with HIV had CD4 counts ≤200 cells/μl45. Further studies are needed to assess tolerability of chemotherapy and CRT in patients with HIV with CD4 counts ≤200 cells/μl.
This study was notable for its large cohort size specifically of WLWH that is well-controlled with ART. Despite the multiplicity of survival data, few studies have explored biases in clinical judgment regarding HIV status. However, this study was limited by lack of availability of data from patients who were not able to present for treatment. We were not able to capture information about those who were unable or unwilling to travel, possibly due to HIV- or cancer-related disability. Additionally, although stage was a predictor of curative treatment initiation, performance status was not. This may be attributable to different cultural interpretations of KPS questions, resulting in downward bias, and potential lack of power to detect such association. Given Botswana’s public funding of ART and high rate of ART adherence, our results may not be generalizable to regions with high rates of untreated HIV. Additional studies in HIV endemic areas are needed to confirm our conclusions.
In summary, initiation of curative CRT for women with cervical cancer who present for treatment was predicted by hemoglobin level, stage, and age but not HIV status. These results demonstrate that women living with well-managed HIV infection are not preferentially prescribed non-curative treatment over curative treatment. In concert with our previous findings demonstrating that treatment outcomes in cervical cancer are not dependent on HIV status, this analysis has potential implications for cancer treatment of patients living with well-managed HIV around the globe.
Supplementary Material
Acknowledgments
We acknowledge with thanks the contributions of Erle Robertson, Keba Ngoni, Lame Bakwenabatsile, Kesego Phologo and Tapologo Leselwa.
Funding Support: Center for AIDS Research (5-P30-AI-045008–17), Conquer Cancer Foundation Young Investigator Award, Sub-Saharan African Collaborative HIV and Cancer Consortia-U54 (1 U54 CA190158–01), National Institute of Allergy and Infectious Diseases (K01AI118559).
Footnotes
Conflicts of interest: All authors report no conflicts of interest.
References:
- 1.World Health Organization GLOBOCAN. Cervical Cancer, Mortality and Prevalence Worldwide in 2012. 2012. http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp.
- 2.Bosch FX, Manos MM, Muñoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. JNCI: Journal of the National Cancer Institute 1995; 87(11): 796–802. [DOI] [PubMed] [Google Scholar]
- 3.Singh DK, Anastos K, Hoover DR, et al. Human papillomavirus infection and cervical cytology in HIV‐infected and HIV‐uninfected Rwandan women. The Journal of infectious diseases 2009; 199(12): 1851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botswana Ministry of Health. Botswana HIV/AIDS Impact Survey III Results. 2008. http://www.gov.bw/Global/NACAMinistry/wana/BAISIIIStatsPress.pdf.
- 5.Denny L, de Sanjose S, Mutebi M, et al. Interventions to close the divide for women with breast and cervical cancer between low-income and middle-income countries and high-income countries. The Lancet 2017; 389(10071): 861–70. [DOI] [PubMed] [Google Scholar]
- 6.UNAIDS. HIV and AIDS Estimates. 2014.
- 7.Gaolathe T, Wirth KE, Holme MP, et al. Botswana’s progress toward achieving the 2020 UNAIDS 90–90-90 antiretroviral therapy and virological suppression goals: a population-based survey. The lancet HIV 2016; 3(5): e221–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grover S, Raesima M, Bvochora-Nsingo M, et al. Cervical cancer in Botswana: current state and future steps for screening and treatment programs. Frontiers in oncology 2015; 5: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS One 2015; 10(8): e0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang LT, Temin S, Camacho R, et al. Management and care of women with invasive cervical cancer: American Society of Clinical Oncology resource-stratified clinical practice guideline. Journal of global oncology 2016; 2(5): 311–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyongesa C, Ruff P, Donde B, Kotzen J. A phase I study of concurrent cisplatin chemotherapy in patients with carcinoma of the cervix receiving pelvic radiotherapy. International Journal of Gynecological Cancer 2006; 16(4): 1614–9. [DOI] [PubMed] [Google Scholar]
- 12.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90–01. Journal of Clinical Oncology 2004; 22(5): 872–80. [DOI] [PubMed] [Google Scholar]
- 13.Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, et al. HIV infection and survival among women with cervical cancer. Journal of Clinical Oncology 2016; 34(31): 3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim A, Sia S. Outcomes of chemoradiotherapy in cervical cancer—the Western Australian experience. International Journal of Radiation Oncology* Biology* Physics 2012; 82(4): 1431–8. [DOI] [PubMed] [Google Scholar]
- 15.Grover S, Bvochora-Nsingo M, Yeager A, et al. Impact of Human Immunodeficiency Virus Infection on Survival and Acute Toxicities From Chemoradiation Therapy for Cervical Cancer Patients in a Limited-Resource Setting. International Journal of Radiation Oncology* Biology* Physics 2018; 101(1): 201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogart LM, Catz SL, Kelly JA, Benotsch EG. Factors influencing physicians’ judgments of adherence and treatment decisions for patients with HIV disease. Medical Decision Making 2001; 21(1): 28–36. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman R, Welton ML, Klencke B, Weinberg V, Krieg R. The significance of pretreatment CD4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer. International Journal of Radiation Oncology* Biology* Physics 1999; 44(1): 127–31. [DOI] [PubMed] [Google Scholar]
- 18.Shrivastava SK, Engineer R, Rajadhyaksha S, Dinshaw KA. HIV infection and invasive cervical cancers, treatment with radiation therapy: toxicity and outcome. Radiotherapy and oncology 2005; 74(1): 31–5. [DOI] [PubMed] [Google Scholar]
- 19.Efstathiou JA, Bvochora-Nsingo M, Gierga DP, et al. Addressing the growing cancer burden in the wake of the AIDS epidemic in Botswana: The BOTSOGO collaborative partnership. International Journal of Radiation Oncology* Biology* Physics 2014; 89(3): 468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer 1948; 1(4): 634–56. [Google Scholar]
- 21.Benedet J, Bender H, Jones III H, Ngan H, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. Int J Gynecol Obstet 2000; 70(2): 209–62. [PubMed] [Google Scholar]
- 22.World Health Organization. Interim WHO clinical staging of HVI/AIDS and HIV/AIDS case definitions for surveillance: African Region: Geneva: World Health Organization, 2005. [Google Scholar]
- 23.Statistics Botswana. Botswana AIDS Impact Survey IV (BAIS IV), 2013 Summary Results. 2013. http://www.cso.gov.bw/images/aids_summary.pdf.
- 24.Botswana Ministry of Health. Handbook of the Botswana 2016 Integrated HIV Clinical Care Guidelines. 2016. https://aidsfree.usaid.gov/resources/handbook-botswana-2016-integrated-hiv-clinical-care-guidelines.
- 25.Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. European journal of epidemiology 2016; 31(4): 337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States. Journal of Clinical Oncology 2015; 33(21): 2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suneja G, Shiels MS, Angulo R, et al. Cancer treatment disparities in HIV-infected individuals in the United States. Journal of Clinical Oncology 2014; 32(22): 2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suneja G, Boyer M, Yehia BR, et al. Cancer treatment in patients with HIV infection and non–AIDS-defining cancers: a survey of US oncologists. Journal of oncology practice 2015; 11(3): e380–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalil J, El Kacemi H, Afif M, Kebdani T, Benjaafar N. Five years’ experience treating locally advanced cervical cancer with concurrent chemoradiotherapy: results from a single institution. Archives of gynecology and obstetrics 2015; 292(5): 1091–9. [DOI] [PubMed] [Google Scholar]
- 30.Höckel M, Schlenger K, Aral B, Mitze M, Schäffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer research 1996; 56(19): 4509–15. [PubMed] [Google Scholar]
- 31.Barkati M, Fortin I, Mileshkin L, Bernshaw D, Carrier J-F, Narayan K. Hemoglobin level in cervical cancer: a surrogate for an infiltrative phenotype. International Journal of Gynecological Cancer 2013; 23(4): 724–9. [DOI] [PubMed] [Google Scholar]
- 32.Grogan M, Thomas GM, Melamed I, et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer 1999; 86(8): 1528–36. [DOI] [PubMed] [Google Scholar]
- 33.Kapp KS, Poschauko J, Geyer E, et al. Evaluation of the effect of routine packed red blood cell transfusion in anemic cervix cancer patients treated with radical radiotherapy. International Journal of Radiation Oncology* Biology* Physics 2002; 54(1): 58–66. [DOI] [PubMed] [Google Scholar]
- 34.Bishop AJ, Allen PK, Klopp AH, Meyer LA, Eifel PJ. Relationship between low hemoglobin levels and outcomes after treatment with radiation or chemoradiation in patients with cervical cancer: has the impact of anemia been overstated? International Journal of Radiation Oncology* Biology* Physics 2015; 91(1): 196–205. [DOI] [PubMed] [Google Scholar]
- 35.Santin AD, Bellone S, Parrish R, et al. Influence of allogeneic blood transfusion on clinical outcome during radiotherapy for cancer of the uterine cervix. Gynecologic and obstetric investigation 2003; 56(1): 28–34. [DOI] [PubMed] [Google Scholar]
- 36.Kim YJ, Munsell MF, Park JC, et al. Retrospective review of symptoms and palliative care interventions in women with advanced cervical cancer. Gynecologic oncology 2015; 139(3): 553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DH, Lee JH, Ki YK, et al. Short-course palliative radiotherapy for uterine cervical cancer. Radiation oncology journal 2013; 31(4): 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eleje GU, Eke AC, Igberase GO, Igwegbe AO, Eleje LI. Palliative interventions for controlling vaginal bleeding in advanced cervical cancer. Cochrane Database Syst Rev 2015; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Lonkhuijzen L, Thomas G. Palliative radiotherapy for cervical carcinoma, a systematic review. Radiotherapy and Oncology 2011; 98(3): 287–91. [DOI] [PubMed] [Google Scholar]
- 40.Goodheart M, Jacobson G, Smith B, Zhou L. Chemoradiation for invasive cervical cancer in elderly patients: outcomes and morbidity. International Journal of Gynecological Cancer 2008; 18(1): 95–103. [DOI] [PubMed] [Google Scholar]
- 41.Ikushima H, Takegawa Y, Osaki K, et al. Radiation therapy for cervical cancer in the elderly. Gynecologic oncology 2007; 107(2): 339–43. [DOI] [PubMed] [Google Scholar]
- 42.Sharma C, Deutsch I, Horowitz DP, et al. Patterns of care and treatment outcomes for elderly women with cervical cancer. Cancer 2012; 118(14): 3618–26. [DOI] [PubMed] [Google Scholar]
- 43.Wright JD, Gibb RK, Geevarghese S, et al. Cervical carcinoma in the elderly: an analysis of patterns of care and outcome. Cancer 2005; 103(1): 85–91. [DOI] [PubMed] [Google Scholar]
- 44.Samet J, Hunt WC, Key C, Humble CG, Goodwin JS. Choice of cancer therapy varies with age of patient. Jama 1986; 255(24): 3385–90. [PubMed] [Google Scholar]
- 45.Simonds HM, Neugut AI, Jacobson JS. HIV status and acute haematological toxicity among cervix cancer patients undergoing radical chemoradiation. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society 2015; 25(5): 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


