Abstract
Liraglutide, a relatively long-lasting analogue of glucagon-like peptide-1 (GLP-1), has received recent attention as a treatment for obesity. It has been proposed that activation of GLP-1 receptors in mesolimbic reward pathways contributes to this outcome by reducing hedonic value of food. However, other findings suggest that activation of GLP-1 signaling pathways may suppress appetitive behavior by enhancing a hippocampal-dependent form of learned inhibition. The present experiment compares these two alternatives. Rats first solved a hippocampal-dependent discrimination problem in which a target stimulus signaled the delivery of sucrose, except when it was preceded by an inhibitory cue that signaled nonreward. The effects of 12 daily intraperitoneal (i.p.) injections of liraglutide on responding to the target cue was then compared with and without the inhibitory stimulus. Relative to saline, liraglutide suppressed responding to the target cue only on trials when the inhibitory stimulus was also present (p<.05). This outcome was independent of sex and maintenance diet (Western diet or standard chow). The failure of liraglutide to suppress responding in the absence of the inhibitory cue argues against the notion that this GLP-1 agonist reduced the value of food reward and favors the idea that it enhanced a hippocampal-dependent form of behavioral inhibition.
Glucagon-like peptide-1 (GLP-1) has been identified as an important hormonal satiety signal based on findings that GLP-1 agonists decrease food intake and body fat1. A prevailing interpretation is that GLP-1 decreases intake by attenuating the value of food rewards2. However, recent evidence suggests that GLP-1 agonists may also enhance hippocampal-dependent behavioral inhibition3. Furthermore, several findings have linked this form of behavioral inhibition to energy regulation4,5. The present study attempted to distinguish the effects of the GLP-1 agonist, liraglutide, on reward value from its effects on learned inhibition. We did this by training rats to solve a serial feature negative (sFN) discrimination problem4,6, which required rats to learn when a single target cue would be followed food reward or nonreward. To solve this discrimination, rats were given another stimulus cue that preceded the target cue only on nonrewarded trials. Utilization of this inhibitory stimulus, termed a negative feature cue, is known to be hippocampal-dependent, while the ability of simple rewarded cues to excite conditioned responding is hippocampal-independent6.
During subsequent testing, we substituted a separately trained and extinguished transfer stimulus for the originally-trained target. Similar to the target in the sFN problem, acquisition followed by extinction should embed the transfer cue in concurrent excitatory and inhibitory associations with reward. Because negative feature cues are thought to suppress responding by activating the inhibitory target-reward association, this training makes the transfer cue susceptible to inhibition7. Substituting the transfer target for the originally-trained target enabled us to separate inhibitory control by the negative feature cue from response suppression by a potential target+feature compound cue that could have formed during training. This separation is important because while sFN performance is hippocampal-dependent, discrimination based on training with simple compound cues is not.
If liraglutide suppresses appetitive responding by reducing reward value, responding to the target cue should be reduced, even in the absence of the inhibitory stimulus. Alternatively, if liraglutide reduces appetitive behavior by enhancing hippocampal-dependent inhibition, suppressed responding to the target should occur only on trials when the inhibitory cue is present. We also examined if the effects of liraglutide on reward value or inhibition depended on sex and/or maintenance diet. Western diets (WD) can impair hippocampal-dependent learning mechanisms that underlie intake4. Liraglutide could prevent such WD-induced impairments by affecting those mechanisms. The present experiment also aimed to increase what is known about sex differences in the excitatory and inhibitory control of learned appetitive behavior8
Methods
Subjects and apparatus.
Male and female Sprague-Dawley rats (n=32 each; Harlan) were housed individually and maintained on a 12:12 light:dark cycle. Daily training and testing trials began at start of the light phase. The rats received standard low-fat chow (LabDiets 5001; 3.0kcal/g, 13%kcal/gm fat, 56%kcal/gm carbohydrates) and water ad libitum for two weeks. Food rationing was then used to reduce rats to 95% of their ad libitum weight. All procedures for the care and treatment of the rats were approved by the American University Institutional Animal Care and Use Committee.
The apparatus consisted of 8 standard, computer-controlled, conditioning chambers, each housed in a sound-attenuating cubicle. A recessed food magazine attached to a dispenser of 45mg sucrose pellets (Research Diets) was located in the center of one end wall. Photobeam transceivers inside the magazine recorded entries. An EchoMRI-900 nuclear magnetic resonance machine was used to measure body composition.
Transfer cue training.
The rats were given 12 daily, 4min trials with a 10s clicker (3hz) that co-terminated with the delivery of five sucrose pellets (C+). Number of magazine entries during the clicker (target) minus number of entries in the 10s immediately prior to the onset of the clicker (pretarget) was the index of learning. Next, responding to the clicker was reduced to approximately half of the level of responding observed on the last reward trial by presenting it on four trials without reward (C-).
sFN training:
During sFN training, a 10s tone (3000Hz) co-terminated with sucrose reward at the end of each 4min trial (T+), except on trials where the tone was preceded by presentation of a 4min of a light (L→T-). Thus, the rats had to learn that the light was an inhibitory cue that signaled nonreinforcement of the tone. The rats received at total of 64 training trials, 32 rewarded (T+) and 32 nonrewarded (L→T-).
Testing.
After significant, stable, discrimination was achieved, half of the rats of each sex were switched to a high-fat (42% kcal/gm) high-carbohydrate (39% kcal/gm) WD (Harlan, Teklad, TD10768) and half remained on chow. Half of each sex in each diet group also received daily i.p. injections of saline while the other half received liraglutide (Bachem:10μg/kg in saline vehicle). This dose of liraglutide was reported to reduce 24h food intake without producing conditioned taste avoidance9. This combination of treatments produced a 2×2×2 between-subjects factorial design with Diet (WD, Chow), Drug (liraglutide, saline), and Sex as factors. Group assignments were matched on body weight, body fat, and all behavioral performance.
All rats were then probe tested 4d and 12d after diet and drug treatment initiation. Parameters for probe test trials were the same as for sFN training, except the transfer target stimulus (clicker) was substituted for the presentation of the tone. The order of trials for each test was C+, L→C-, C+, L→C-. Body weight and composition were measured at terminal training (before diet and drug manipulations) and after the conclusion of each probe test.
Results
Transfer cue training.
Mean number of responses (+/− SEM) to the transfer cue was 4.11±2.67 at terminal acquisition, decreasing to 2.11±1.93 at the end of partial extinction. ANOVA analysis yielded no significant sex effect.
sFN training.
Figure 1A shows that, by the end of training, rats learned to respond significantly more on T+ trials compared to L→T- trials (+/−×Block interaction (F(15,930)=9.08,p<.0001). A significant +/−×Sex interaction (F(1,62)=4.03,p<.05) was also obtained. Although the magnitude of the difference in responding on T+ relative to L→T- trials was greater for females, Bonferroni tests confirmed both sexes responded more on T+ than on L→T- trials.(ps<.0001).
Figure 1.
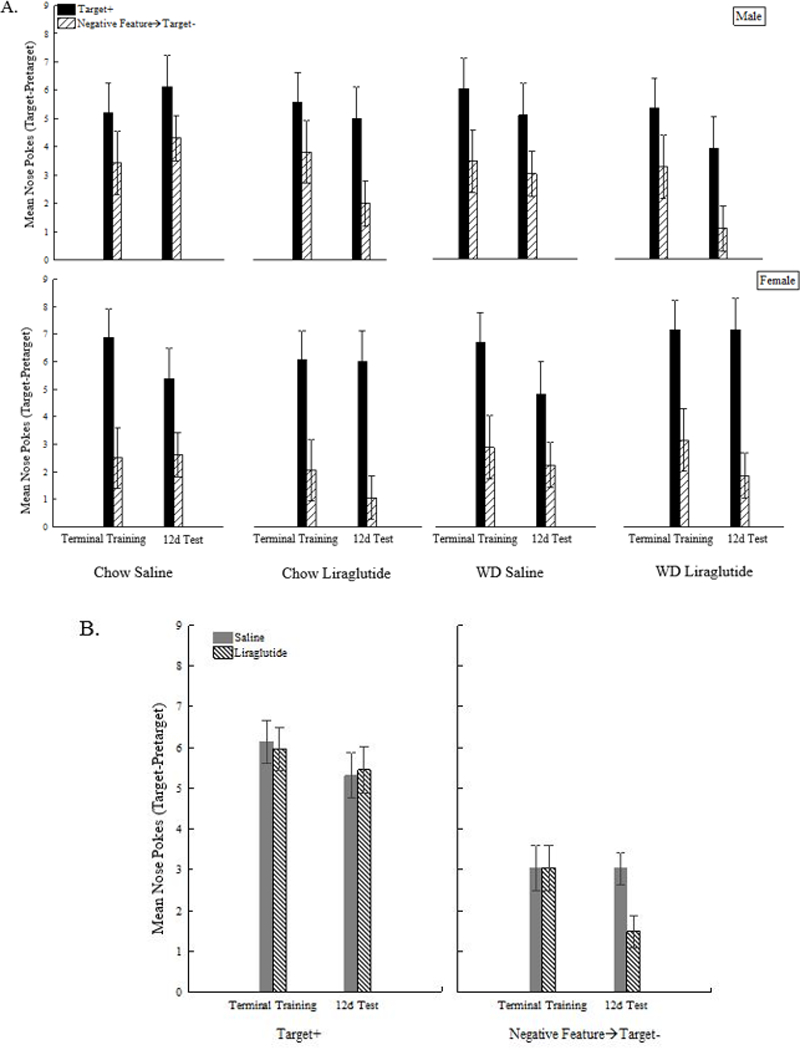
(A) Effects of liraglutide on the last two trials of sFN training prior to administration of diet or drug and test performance following 12d of diet and drug administration as a function of sex and diet. (B) Effects of liraglutide on responding to the target cue in the presence and absence of the inhibitor collapsed across sex and diet.
Transfer cue test.
Because no significant main effects or interactions involving Diet or Drug treatment were observed on the 4d test, only findings from the 12d test are presented. On that test, all rats responded significantly more on C+ trials compared to L→C- trials (F(1,56)=71.1,p<.0001). However, the magnitude of the discrimination was larger for rats injected with liraglutide compared to saline (+/−×Drug (F(1,56)=5.18,p<.05)). This effect of liraglutide did not vary significantly as a function of Diet or Sex (Figure 1A). Newman-Keuls tests confirmed that rats injected with liraglutide responded less on L→C- trials compared to saline injected rats (p<.05), whereas liraglutide had no effect relative to saline on C+ trials (p>.83) (Figure 1B).
BW and adiposity.
After 12d, WD increased body weight gain relative to chow in males, but not females (Diet×Sex (F(1,56)=10.45, p<.01) (Figure 2A). No significant effects of liraglutide on body weight were observed for either sex or diet. In contrast, body fat gain was significantly lower for rats injected with liraglutide compared to saline (F(1,56)=7.75,p<.01)) (Figure 2B). No interaction with Sex or Diet was observed.
Figure 2.
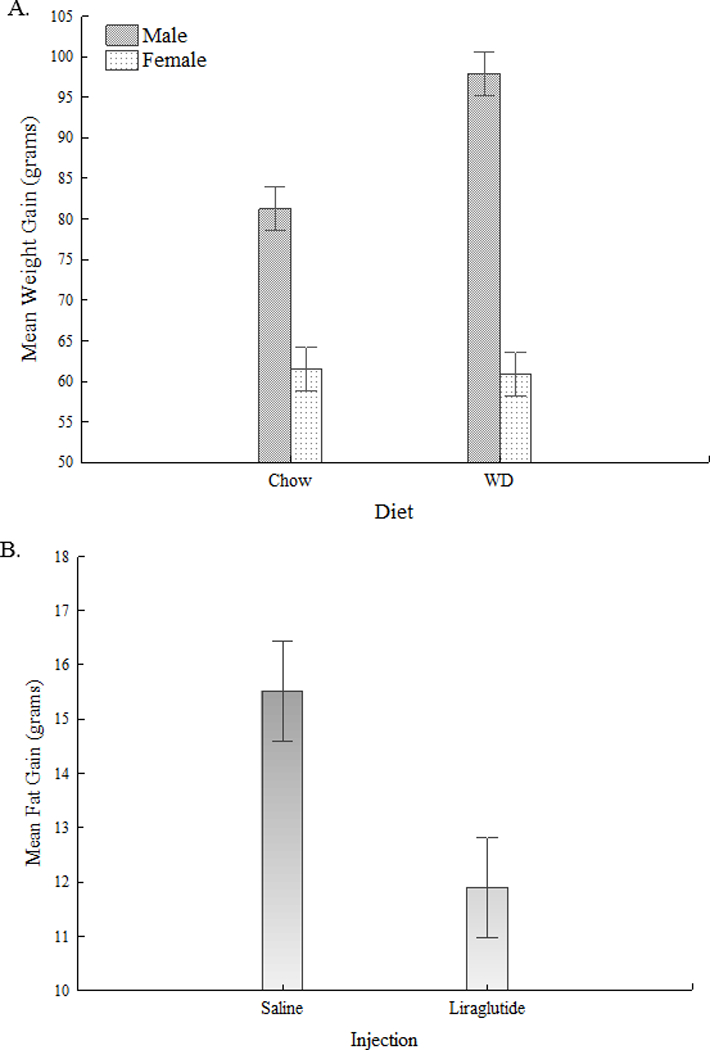
(A) Effects of WD and chow on weight gain. (B) Effects of liraglutide on percent body fat.
Discussion
The current study provides evidence that peripheral injections of liraglutide enhanced the ability of an inhibitory stimulus to suppress responding to a rewarded cue. In contrast, liraglutide had little impact on responding to that rewarded cue when the inhibitory cue was absent. In other words, the effects of liraglutide were confined to a stimulus that functioned as a learned inhibitory cue. The finding that responding to the rewarded cue was not reduced when the inhibitor was absent challenges the view that liraglutide suppresses appetitive behavior by decreasing reward value. While the present results provide no evidence that liraglutide weakens appetitive behavior by reducing the value of food reward, it is possible that measures of strictly consummatory behavior could yield a different outcome.
Previous research reported that the hippocampus is a substrate for response inhibition under conditions that require the withholding of responding to cues that have been associated with both hedonically positive and negative outcomes4,10. In the present study, the transfer target cue represents this type of stimulus as it was associated with both reward and nonreward. Animals solve this problem by using the light to predict that the target cue would be nonrewarded. This utilization of the light to inhibit responding to the target depends on the hippocampus6. The results of our study indicated that liraglutide enhanced this hippocampal-dependent inhibition. However, because significant effects were observed after 12d, but not after 4d of administration, it appears that more extended dosing may be needed to reveal liraglutide’s effects on this inhibitory mechanism.
GLP-1 may also suppress feeding behavior via its effects on hippocampal-dependent inhibitory processes. Our results are consistent with reports that GLP-1 signaling suppresses feeding by activating an inhibitory, monosynaptic, glutamatergic pathway between the ventral hippocampus and the medial prefrontal cortex3. It may be that hippocampal-dependent forms of learned inhibition involving activation of this pathway contribute to food satiety. Accordingly, investigating the capacity of GLP-1 agonists to promote hippocampal-dependent learning and memory, or to protect against pathophysiologies that can disrupt those functions, may lead to new ways to combat both obesity and certain forms of cognitive decline.
Acknowledgments
Support for this research was provided by NIH grant R01DK110412 to Terry L. Davidson.
Footnotes
Competing interests: The authors declare no conflict of interest.
References
- 1.Ladenheim EE (2015). Liraglutide and obesity: a review of the data so far. Drug Des Devel Ther. 30, 1867–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickson SL, Shirazi RH,…Skibicka KP (2012).The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J.Neurosci, 32 4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu TM, Noble EE,…Kanoski SE (2017). A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Molecular Psychiatry. 00, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson TL, Sample CH, Swithers SE (2014). An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobio. Learn. Mem, 108, 172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgs S (2016). Cognitive processing of food rewards. Appetite, 104, 10–17. [DOI] [PubMed] [Google Scholar]
- 6.Holland PC, Lamoureux JA, Han JS, & Gallagher M (1999). Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus, 9(2), 143–157. [DOI] [PubMed] [Google Scholar]
- 7.Swartzentruber D, Rescorla RA (1994). Modulation of trained and extinguished stimuli by facilitators and inhibitors Anim. Learn. Behav. 22, 309–316. [Google Scholar]
- 8.Sample CH, & Davidson TL (2018). Considering sex differences in the cognitive controls of feeding. Physiology & behavior, 187, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanoski SE, Rupprecht LE….Hayes MR (2012). The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide.Neuropharmacology, 62, 1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito R, Lee AC (2016). The role of the hippocampus in approach-avoidance conflict decision-making: evidence from rodent and human studies. Behav. Brain Research, 313, 345–357. [DOI] [PubMed] [Google Scholar]


