Abstract
Affecting 5% of all preschool-aged children, developmental stuttering -- also called childhood onset fluency disorder-- is a complex, multifactorial neurodevelopmental disorder characterized by frequent disruption of the fluent flow of speech. Over the past two decades, neuroimaging studies of both children and adults who stutter have begun to provide significant insights into the neurobiological bases of stuttering. This review highlights convergent findings from this body of literature with a focus on functional and structural neuroimaging results that are supported by theoretically-driven neurocomputational models of speech production. Updated views on possible mechanisms of stuttering onset and persistence, and perspectives on promising areas for future research into the mechanisms of stuttering are discussed.
Verbal communication, facilitated by effortless and fluent speech production, is one of the defining characteristics of being human. Speech production requires the coordination of hundreds of muscles of the head, face, neck, and abdomen on a millisecond time scale, and in an overlapping manner. Humans are nearly flawless in our ability to constantly adapt to situational changes in speaking rate, articulation, and emotional load. Not only must we coordinate speech sounds like consonants and vowels, but also regulate pitch, rhythm, loudness, and prosody in order to produce natural sounding fluent speech. This requires input from multiple cortical and subcortical brain regions and finely tuned interactions among large scale neural networks. While most speakers take this complex feat for granted, people who stutter are acutely aware of how the process can go awry and lead to speech disruptions.
Stuttering is a disorder that specifically affects a speaker’s ability to produce fluent speech. More than 5% of the general population, cutting across differing languages, ethnicity, and socioeconomic status, report ever having stuttered. Most cases of stuttering are not associated with brain injury, nor is stuttering linked to any obvious deficits affecting cognitive, linguistic, or psychiatric functions. Despite the relatively high lifetime prevalence (see Box 1) and its detrimental effects on communication for those afflicted, stuttering remains an under-investigated and poorly understood disorder. The etiology and mechanisms behind stuttering are largely unexplained, and limited options for effective treatments are available for those affected.
Box 1. Stuttering Epidemiology.
Life-time incidence
Stuttering is a childhood onset disorder that affects up to 8% of preschool age children (Dworzynski and others 2007; Yairi and Ambrose 2013).
Prevalence
Stuttering is present in approximately 1% of the general population (due to natural recovery during childhood; see below) (Craig and others 2002).
Time of onset
Onset of stuttering occurs for most children between 2-4 years of age, a period coinciding with a dynamic time of language development (Yairi and Ambrose 1992).
Natural recovery
Most children (~80%) grow out of stuttering naturally, typically between 2-4 years post stuttering onset (G. Andrews and Harris 1964; Yairi and Ambrose 1999).
Sex ratio
Like in the case of many neurodevelopmental disorders, stuttering affects many more males than females. Near onset, the sex ratio is closer to 2:1 (male: female) (Yairi and Ambrose 1999), while by adulthood this ratio is increased to 4:1 (Bloodstein 1995).
Co-occurrence with other neurodevelopmental conditions
Stuttering commonly co-occurs with conditions such as ADHD (Felsenfeld and others 2010; Donaher and Richels 2012) and phonological delay in children (Byrd and others 2007). Anxiety, social anxiety in particular, is a major concomitant and prevalent in adults who stutter (Iverach and Rapee 2013; Craig and Tran 2014), however it is not commonly reported in children and is at present not considered a causal factor in the onset of stuttering (Kefalianos and others 2014).
In the past two decades, an increasing number of studies and several recent meta-analyses and review papers highlight convergent findings of functional neuroanatomical differences in speakers who stutter relative to fluent speakers. Despite these important advances, a neural mechanistic understanding of stuttering remains elusive. It is unclear why up to 5% of preschool age children begin to stutter but only about 20% of those children develop lifelong chronic stuttering. We do not know why stuttering severity fluctuates in an individual, why stuttering can be alleviated through manipulation of auditory feedback or with an external pacing signal, or why stuttering can be exacerbated during stressful speaking situations. In this review, we summarize critical functional neuroanatomical differences linked to stuttering that provide clues to answering these questions. Based on results reported to date, updated views on possible mechanisms of stuttering onset and persistence are discussed.
Clues to the neural bases of stuttering
Aberrant sensory-motor integration for speech production
The most commonly reported finding with regard to neural bases of stuttering involves decreased white matter ‘integrity’ along parts of the left arcuate/superior longitudinal fasciculus in both children and adults who stutter (Sommer and others 2002; Chang and others 2008; Watkins and others 2008; Cykowski and others 2010). Sometimes referred to as the dorsal auditory tract (Hickok and Poeppel 2007; Saur and others 2008), this pathway interconnects speech motor and auditory areas to enable mapping between speech sounds and motor plans for articulation. The relevant areas include major perisylvian cortical structures critical to speech and language development, encompassing the inferior frontal gyrus (IFG), ventral premotor cortex (vPMC), ventral motor cortex (vMC), and posterior superior temporal gyrus (pSTG) (Figure 1). All of these areas have been found through multiple measures of structure and function to differ in stuttering speakers. There is empirical evidence and theoretical perspectives pointing to probable deficits in integrating auditory feedback into the speech motor program in stuttering (Max and others 2004). The accumulating findings from neuroimaging research focused on left perisylvian motor and auditory cortical structures seem to corroborate these earlier perspectives. Below, we review some of these findings by each cortical area to interpret how deficits found in critical structures supporting auditory-motor integration may contribute to stuttering.
Figure 1.
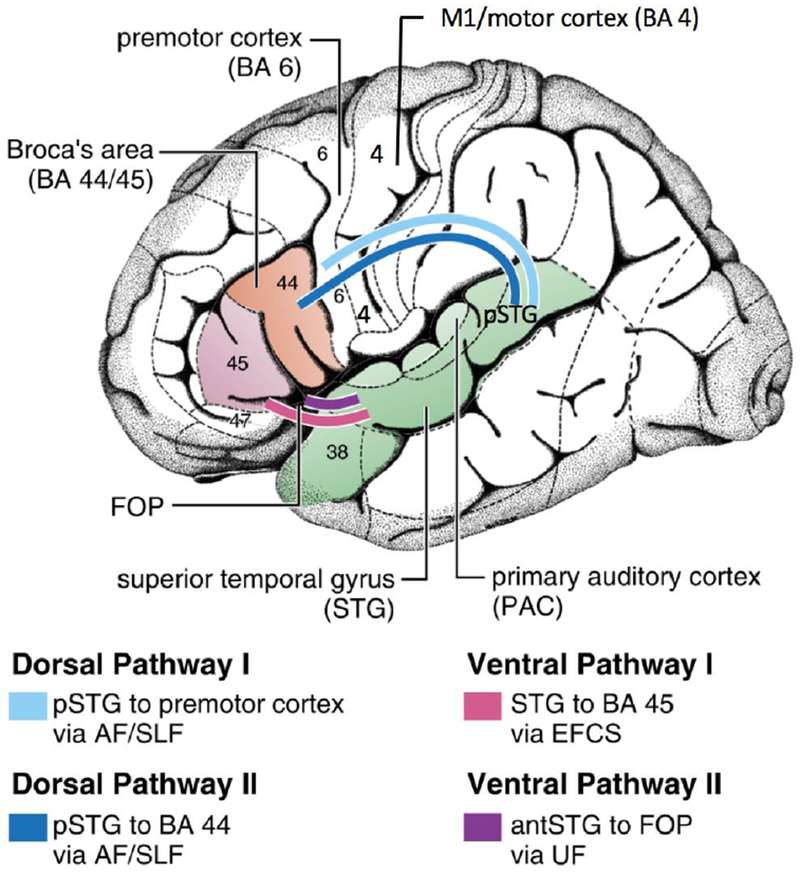
Speech-language cortical areas and the dorsal and ventral pathways that interconnect them. Multiple studies examining stuttering speakers have reported decreased white matter integrity in the left dorsal pathways, and morphological changes affecting the motor and auditory cortical areas that are interconnected by these tracts. AF/SLF: arcuate fasciculus/superior longitudinal fasciculus; BA: Brodmann area; EFCS: extreme fiber capsule system; FOP: frontal operculum; UF: uncinate fasciculus. Figure reprinted and modified with permission from The American Physiological Society and Copyright Clearance Center. The Brain Basis of Language Processing: From Structure to Function. Friederici AD. Physiological Reviews. 2011.
Left IFG/ventral premotor cortex (vPMC)
The left IFG updates speech articulatory plans as a function of the sensory context (Guenther 2016), and is a critical structure supporting speech planning (Flinker and others 2015). The left IFG (BA 44,45) and vPMC regions showed significantly decreased white matter integrity (Sommer and others 2002; Chang and others 2008; Watkins and others 2008; Cykowski and others 2010; Connally and others 2013; Chang and others 2015) and decreased gray matter volume (Chang and others 2008; Kell and others 2009; Beal and others 2013) in both adults and children who stutter. The pars opercularis (BA44) showed aberrant developmental trajectories of cortical thickness (Beal and others 2013) and white matter integrity (Chang and others 2015) in people who stutter (PWS) compared to controls. This area also shows aberrant brain activity during speech production tasks in speakers who stutter (Watkins and others 2008; Neef and others 2016). Broca’s area and the right homologue showed abnormally decreased cerebral blood flow in PWS, which was especially the case for severe stutterers (Desai and others 2016). Hemodynamic responses measured with functional near infrared spectroscopy (fNIRS) during a picture description task showed significant decreases in the left dorsal IFG and premotor cortex in school-age children who stutter compared to the control group (Walsh and others 2017). These results collectively suggest deficiencies in left IFG function in stuttering, which in turn may implicate deficits in the speech planning phase for stuttering speakers.
Functional coupling between vPMC/IFG and other left hemisphere speech areas is also reported to be deficient: for instance, IFG connectivity with primary motor cortex (M1) (Lu and others 2009), and with the inferior parietal lobe (Neef and others 2016), significantly differed in stuttering speakers relative to fluent speakers. Further, people who stutter exhibit aberrant functional connectivity between the basal ganglia and IFG during both speech (Lu and others 2010) and non-speech tasks (Metzger and others 2017). These functional connectivity differences may affect efficient integration of speech planning and motor execution, as well as speech planning and sensory input areas.
Primary motor cortex/M1
Stuttering has long been considered a disorder of motor control (Zimmermann 1980; Kent 1984; Ludlow and Loucks 2003). Aberrant M1 activity has been linked to disfluent speech production in stuttering speakers (Belyk and others 2017). The anomalous function of M1 observed in stuttering speakers is likely influenced by deficient functional links to speech planning areas (Vanhoutte and others 2015). For example, activity in the cortical speech motor execution area in M1 preceded that in IFG/speech planning region in people who stutter, a reversal of what was seen in controls (Salmelin and others 2000).
Further demonstrating possible motor deficiencies during speech preparation, stuttering speakers did not show the typical left-lateralized excitability in the left M1 tongue representation during speech preparation. In addition, the extent to which the left M1 tongue representation was facilitated was negatively correlated with stuttering severity, e.g., the more left lateralized, the less severe (Neef, Hoang, and others 2015).
Neural oscillations during motor preparation preceding speech production were also reported to be aberrant in stuttering speakers (Mersov and others 2016; Mersov and others 2018). Neural oscillations are rhythmic fluctuations of neural excitability that reflect synchronized activity across distributed groups of neurons (Buzsáki and Draguhn 2004; Lakatos and others 2008). When oscillatory phases align, this indicates neurons are firing in a synchronous manner, in turn reflecting increased communication and neuronal processing (Singer 1999; Varela and others 2001; Fries 2005). Neural oscillations are categorized based on the characteristic frequencies at which the rhythms occur; among these, beta oscillations that occur in the 12-30 Hz range are prevalent in the motor system (Pogosyan and others 2009; Joundi and others 2012; Kilavik and others 2013). Aberrant beta oscillations were reported in both children (Ozge and others 2004; Etchell and others 2016) and adults who stutter (Joos and others 2014; Kikuchi and others 2016; Mersov and others 2016; Mock and others 2016; Saltuklaroglu and others 2017). Specifically, in the case of adults who stutter, beta desynchronization and synchronization that occur characteristically during movement preparation and execution respectively, were both exaggerated relative to controls in stuttering speakers (Mersov et al., 2016). The authors suggested that adults who stutter may have an overly inhibited motor system that requires heightened beta suppression (preparation) to disengage prior to speech initiation. In sum, these results point to anomalous neural activity in the M1, and abnormal neural coordination during speech preparation and execution in stuttering.
Posterior superior temporal gyrus (pSTG)
The pSTG interconnects with the premotor and inferior frontal areas via a number of major white matter tracts, such as the superior longitudinal fasciculus dorsally, and the extreme fiber capsule system (EFCS)/uncinate fasciculus (UF) ventrally (See figure 1). As alluded to in previous sections, preparation and execution of speech in stuttering speakers cannot be considered in isolation from sensory regions, as speech movements are planned and executed in the context of the expected sensory (auditory, somatosensory) targets (i.e., speech sounds generated as a result of the speech movements). Much evidence points to aberrant interaction between auditory-motor integration for speech in stuttering speakers. It is well-established that auditory feedback manipulations (e.g., masking noise, frequency-shifted feedback, or delayed auditory feedback) can induce fluency in people who stutter, at least temporarily (Kalinowski and others 1993; Stuart and others 2008; Saltuklaroglu and others 2009; R.J. Ingham and others 2012; Foundas and others 2013; van de Vorst and Gracco 2017) (Box 2). Studies investigating sensorimotor adaptation to auditory perturbations indicate that adults who stutter show reduced adaptation compared to controls (Nudelman and others 1992; Cai and others 2012; Loucks and others 2012; Cai and others 2014) whereas children who stutter show the same amount of adaptation as fluent children (Daliri and others 2017). Notably, speech-induced suppression of the auditory cortex was delayed in latency in children who stutter, indicating aberrant interaction of the auditory and motor areas were present even in children (Beal and others 2011).
Box 2. Fluency inducing conditions and clues to possible deficits in internal timing of movement.
Auditory feedback manipulation
Delayed auditory feedback (DAF) devices present real-time feedback of one’s own speech that is slightly (~60ms) delayed. In speakers who stutter, this effect elicits near-fluent speech (Unger and others 2012; Foundas and others 2013), but disrupts speech in fluent speakers. Under auditory masking conditions, such as white noise, stuttering is also reduced (Bloodstein 1995).
Speech paced with external timing cues
When speakers who stutter speak to the beat of an external rhythm such as a metronome, during singing, or in unison with another person (choral speech), stuttering is markedly reduced and almost completely fluent even in the most severe cases (Park & Logan, 2015). Such techniques are also associated with more ‘normalized’ brain activation patterns (i.e., similar to activation patterns found in nonstuttering speakers), such as increased left frontotemporal activation, and reduced motor activation, including right frontal opercular areas (De Nil and others 2003; Neumann and others 2005; Giraud and others 2008; Kell and others 2009; Toyomura and others 2011; Toyomura and others 2015).
Deactivation of left auditory cortices (STG, MTG) during speech tasks in people who stutter is one of the “neural signatures” highlighted in meta-analyses of fMRI and PET studies on stuttering (Brown and others 2005; Budde and others 2014). Interestingly, activity in the bilateral pSTG and right STG in particular heightens during fluent speech in people who stutter. When compared to solo reading, induced fluency conditions such as metronome paced speech and choral speech showed heightened activity in the auditory cortex that overshot the controls’ activity in the same areas (Toyomura and others 2011). Although speculative, this may suggest that deactivation of the auditory cortex occurs when stuttering is anticipated in order to reduce sensory mismatch between actual and predicted speech sounds (Eliades and Wang 2008).
The studies reviewed in this section relevant to sensorimotor integration in speech production point to deficient functional neuroanatomy of the left premotor, motor, and auditory cortical areas, and the white matter tracts interconnecting these structures, in speakers who stutter. These structures collectively form critical scaffolding for fluent speech and language development during childhood. Evidence of anomalous development of this cortical network in speakers who stutter corroborate previous reports of weaker performance in tasks requiring sensorimotor integration. These results also offer clues to why auditory feedback manipulation (e.g., delayed auditory feedback) could have a powerful influence on fluent speech production in speakers who stutter.
Aberrant timing and sequencing of speech sounds
Speech motor control requires coordination of movement sequences that occur with precise timing, often expertly adjusting to changes in speech rate, emotional state, and modulations in rhythm, intonation and prosody of the continuous speech stream. Stuttering has thus also long been considered a disorder of timing (Van Riper 1982; Donald G Mackay 1984; Kent 1984; Caruso and others 1994). More recent theoretical perspectives corroborate the critical role of timing-related neural circuits in stuttering (Alm 2004; Etchell and others 2014). These perspectives are supported by accumulating evidence pointing to deficient interaction among cortical and subcortical regions in stuttering speakers (Wu and others 1997; Lu and others 2010; Chang and Zhu 2013; Wieland and others 2015) that are linked to both rhythm and speech processing.
Basal Ganglia
The classic left hemisphere speech motor control regions discussed in the previous sections interface with subcortical structures at multiple levels, and are all key components of the basal ganglia-thalamo-cortical motor circuit (hereafter, BGTC loop) (Maguire and others 2000; Maguire and others 2002; Alm 2004; Maguire and others 2004; Chang and Zhu 2013; Civier and others 2013). The function of the BGTC loop is implicated in the selection and initiation of movement sequences (e.g., (Brotchie and others 1991; Marsden and Obeso 1994; Mink 1996) including the sequence of gestures for a word or syllable (Bohland and Guenther 2006; Bohland and others 2010). According to a biologically plausible neurocomputational model of speech sound sequencing and motor initiation (GODIVA model) (Bohland and others 2010; Civier and others 2013), the BGTC loop is responsible for initiating the articulatory gestures within a syllabic motor program at the appropriate time by activating neurons in an initiation map in SMA. Projections from sensory, motor, and premotor cortical areas to the putamen provide a detailed “sensorimotor context” that the basal ganglia monitor to determine exactly when to initiate the next gesture in the sequence.
For example, left vPMC provides information about the syllable currently being produced, SMA and vMC provide information about the ongoing articulatory gesture, ventral somatosensory cortex (vSC) provides information about the current somatosensory state, and posterior auditory cortex (pAC) provides information about the current acoustic signal being produced. When the BG recognize that the current gesture is nearly complete, a “completion signal” is sent to the supplementary motor area (SMA) that extinguishes activity in the initiation map neurons coding the current gesture and activates the neurons coding the next gesture. In the context of GODIVA, the structural and functional differences reviewed in previous sections concerning vMC and vPMC in stuttering speakers may make it relatively difficult for the BGTC loop to identify the proper sensorimotor context for initiating the next gesture in a speech sequence, leading to moments of stuttering. Prediction and planning of proper timing of upcoming sounds relate to an internalized timing mechanism localized in core structures such as the basal ganglia and SMA, which intersects with other areas to support timing in a context dependent manner (Figure 3) (Merchant and others 2013).
Figure 3.
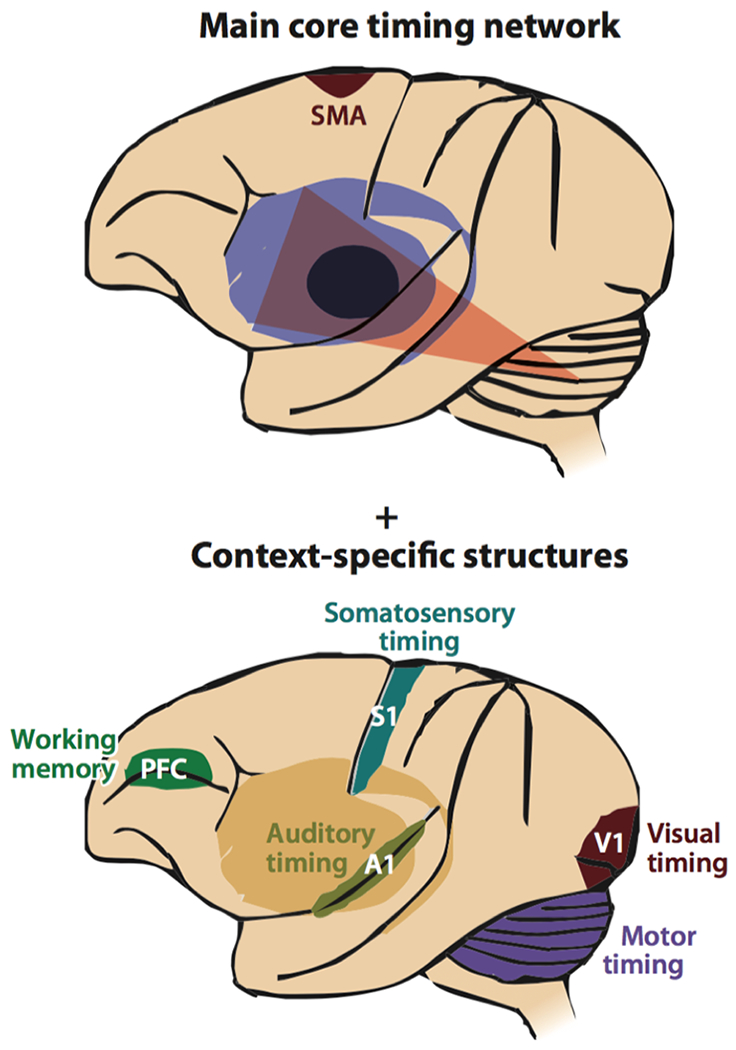
Major neural structures supporting estimation of time. The core structures include the basal ganglia and supplementary motor area (SMA). These structures interface with a distributed network of areas in a context specific manner. Reprinted and modified with permission from Annual Reviews, Inc: Annual Review of Neuroscience. Neural Basis of the Perception and Estimation of Time. Merchant H, Harrington DL, Meck WH. 2013.
Relevant to this discussion is beta oscillations that were mentioned under the previous section on M1. Given that beta power reliably decreases before movement and is increased after movement, examining the time course of beta modulation provides a mechanism for maintaining predictive timing. Beta activity seems to cue the initiation and end of a movement sequence, enabling internally driven timing of movement sequences (Bartolo and Merchant 2015). Furthermore, striatal beta activity allows utilization of sensory cues to guide behavior (Leventhal and others 2012). According to Fujioka et al. (2012), “beta oscillations reflect functional coordination between auditory and motor systems, and … coherence in beta oscillations dynamically configure the sensorimotor networks for auditory-motor coupling” (Fujioka and others 2012) (p.1791). Thus, it could be speculated that beta oscillations provide a mechanism for coordinating auditory and motor systems in producing speech sequences that are internally timed. If beta oscillations are affected in stuttering (see previous section for relevant studies), stuttering speakers could have deficits in tasks that require internal timing of events that require auditory-motor integration. Using rhythm discrimination tasks, this was indeed found to be the case in both children (Wieland and others 2015) and adults who stutter (Chang and others 2016).
Impaired structural connectivity affecting core areas that support internal timing, such as the SMA and putamen, has been reported in stuttering speakers. The left SMA/preSMA area is also interconnected with the left posterior IFG via the frontal aslant tract ([FAT]; (Dick and others 2014)), which was shown in recent studies to support language production (Catani and others 2013). The FAT was found to be aberrant in white matter integrity in stuttering speakers (Kronfeld-Duenias and others 2014), and axonal stimulation that transiently lesioned the FAT led to transitory stuttering (Kemerdere and others 2015). These findings add to the literature that accounts for why conditions that provide external timing cues (metronome-paced speech, choral reading, singing) may play a compensatory role during speech production in adults who stutter. If neural pathways that support internally timed movement are affected in stuttering, external pacing conditions may provide PWS with cues upon which to time their speech movements, so that they do not need to rely on deficient internal timing (Adams and Ramig 1980; C.C. Andrews and others 2012).
Cerebellum
Dysfunctional BG circuits may lead to an increased influence of the cerebellum and a compensatory network encompassing the cerebellum-thalamus-SMA (Kotz and others 2009). In Parkinson’s Disease for example, cerebellar circuits are proposed to compensate for timing deficits present in the disorder (Kotz and Schwartze 2010; Merchant and others 2013). The cerebellum may play a compensatory role due to its function in predicting and tuning movements on the basis of an efferent copy of sensory and motor information (Miall and Wolpert 1996; Wolpert and others 1998). The cerebellum selectively contributes during self-initiated sounds; it makes rapid predictions about the sensory consequences of self-generated movement (Blakemore and Sirigu 2003), which makes it critical for action-perception coupling (Christensen and others 2014).
In this context, it is interesting to note that most studies examining brain function in stuttering have reported anomalous cerebellar activity. To point out a few, Lu et al. (2009) in an fMRI study reported that two parallel neural circuits-- the cerebellum-left premotor circuit and the BG-IFA/premotor circuit—were involved in atypical planning and production processing in stuttering speakers (Lu and others 2009). Fox and colleagues (2000) on the other hand, showed that cerebellar activity was correlated with fluent utterances in adults who stutter (Fox, R.J. Ingham, J.C. Ingham, Zamarripa, Xiong, and Lancaster 2000a). Heightened cerebellar activity was observed in stuttering speakers, which was even further heightened and then lowered following therapy (De Nil and others 2003). The cerebellar peduncles that carry efferent and afferent fibers interconnecting cerebellum to cerebrum were found significantly reduced in white matter integrity in speakers who stutter (Connally and others 2013). An interesting case study reported a patient in whom stuttering emerged after right cerebellar infarction (Tani and Sakai 2011). The current body of evidence suggests that the cerebellum could play a compensatory role to deficient BGTC function in stuttering. The compensation is not completely successful however, because it does not altogether alleviate stuttering. Furthermore, there is ample evidence that performance on tasks that require a fully functioning efference copy mechanism, which relies on cerebellar function, is affected in stuttering speakers (Daliri and Max 2017). Future research that further investigates the role of cerebellar circuits in stuttering would clarify some of these outstanding questions.
The compensatory and/or maladaptive role of right hemisphere homologues
Historically, stuttering has been attributed to abnormal laterality of the brain (ORTON 1927; Travis 1978). This idea has persisted, and has been in part corroborated by results reported in some neuroimaging studies. Morphometry studies of adults who stutter consistently find right hemisphere anomalies in the speech network, mostly in the form of larger regional volume/thickness (Jancke and others 2004) and higher white matter integrity (Jancke and others 2004; Neef and others 2017), which contrasts sharply with the smaller volume/thickness and decreased integrity of white matter tracts found in the left hemisphere of children who stutter (Chang and others 2008; Beal and others 2013; Chang and others 2015; Chow and Chang 2017) (for meta-analyses see (Brown and others 2005; Belyk and others 2014). With regard to measures of brain function, adults who stutter showed increased beta oscillations across the cortex, particularly in the right temporo-parietal lobe during a reading task (Rastatter and others 1998). Another study found that adults who stutter tended to have more right-lateralized suppression of beta power in the mouth motor cortex during single-word reading, while fluent speakers showed a primarily left-lateralized pattern (Salmelin and others 2000). Similar right laterality was observed in decreased alpha and beta oscillations during rest in children who stutter, which was proposed to reflect reduced cortical maturation (Ozge and others 2004).
There has been much debate about whether such changes are adaptive or maladaptive. Some authors assert the right IFG over-activation is adaptive and has negative correlations with stuttering severity (e.g. (Preibisch and others 2003; Kell and others 2009)). In a seminal study, Fox et al., (1996) reported right hemisphere activation was stronger in fluent than stuttered speech ((Fox and others 1996), see also (Braun and others 1997)). Vanhoutte and colleagues found enhanced right contingent negative variation (CNV; an event related potential linked to motor preparation) to be associated with fluent relative to stuttered speech (Vanhoutte and others 2015; Vanhoutte and others 2016). Other authors suggest right hemisphere involvement is maladaptive. For example, adults who stutter with greater rightward planum temporale asymmetry tended to exhibit significantly greater disfluency than those who had more leftward asymmetry (Foundas and others 2004). Overactivation of the right hemisphere is positively correlated with stuttering rate (Fox, R.J. Ingham, J.C. Ingham, Zamarripa, Xiong, and Lancaster 2000a), and stuttering therapy reduces right sided activation (De Nil and others 2003).
In a recent meta-analysis, Neef et al., (2015) reported that activity in some right hemisphere regions like the pars orbitalis and SMA was related to disfluency, but that activation in left hemisphere regions like Heschl’s gyrus, planum temporale, posterior STG, and pars opercularis as well as the middle, superior medial gyrus and the inferior parietal lobule was related to fluency (Neef, Anwander, and others 2015). To further elucidate the basis for right inferior frontal hyperactivity reported in many previous studies —maladaptive or compensatory-- Neef et al. (2017) conducted a combined fMRI-DTI study to investigate structural connectivity of subsections of the right IFG. They found white matter tracts that interconnect right posterior IFG, SMA, and preSMA (right frontal aslant tract) and between the right frontal pole and anterior thalamic nuclei (right anterior thalamic radiation), were associated with greater stuttering severity, suggesting a possible maladaptive role of these tracts in stuttering speakers. On the other hand, connection strength of the right uncinate fasciculus, which interconnects the right frontal pole to auditory cortices, was found to be negatively correlated with stuttering severity, suggesting a possible compensatory role of this tract in stuttering speakers (Neef and others 2017).
The overall interpretation of this pattern of results within the DIVA/GODIVA framework is that the core deficit in stuttering is an impairment of the left hemisphere feedforward control system (and thus left hemisphere anomalies are found in both adults and children who stutter), and this deficit forces over-reliance on right hemisphere feedback control mechanisms, eventually leading to right hemisphere morphological changes seen in adults who stutter. These interpretations await confirmation through studies involving children who stutter, to possibly help clarify the role of right hemisphere homologues in fluent and stuttered speech.
Brain developmental trajectories linked to stuttering persistence and recovery
There is a relatively high rate of natural recovery from stuttering during childhood (Box 1). Knowing the basis for persistence versus recovery has significant implications for clinical management of stuttering. These may include helping find an early prognostic marker for persistent stuttering and later, neural target for developing novel intervention.
Recent studies have begun to shed light on indices predicting persistence or recovery in children. In one study, Mohan and Weber (2015) examined neural activity mediating phonological processing in 7-8 year old children who had recovered from stuttering, those with persistent stuttering, and controls. An ERP response indexing phonological segmentation and rehearsal was observed to be attenuated for persistent children who stutter. This response was present in recovered children who stutter similar to controls, but it was localized with an atypical lateralization relative to controls. There is also kinematic evidence of a maturational lag in speech motor coordination in 5- to 7- year old children with persistent stuttering compared to controls and children recovered from stuttering (Usler and others 2017). Specifically, children with persistent stuttering exhibited higher variability in articulatory movement regardless of length or syntactic complexity of the speech stimuli, which the authors attributed to a higher variability in speech planning and execution and due to increased “neuromotor noise”. This disrupts proper integration of sensory information to optimal execution of the speech motor plan ((Wolpert 2007), cited in (Usler and others 2017)).
In an earlier study, children with persistent stuttering were found to exhibit immature patterns of neural response for syntactic processing relative to fluent controls and children who recovered from stuttering (Usler and Weber-Fox 2015). A lag in syntactic structure processing could be linked to anomalous development of the left IFG. Increased syntactic ability has been linked to increased specialization of the left IFG (Nuñez and others 2011), and the white matter tracts that interconnect auditory and IFG, compared to the connection between auditory to PMC, tends to increase with age (and language acquisition) and are uniquely present in humans (Friederici 2012). Thus, through very different measures encompassing speech kinematic variability and language/syntactic processing, results from Weber and colleagues largely corroborate neuroimaging results pointing to deficits affecting sensory-motor integration for speech planning and production in children with persistent stuttering.
Developmental trajectories relevant to auditory-motor integration were examined by Chow and Chang (2017), who showed in a longitudinal investigation that children with persistent stuttering had flat or depressed maturational curves associated with white matter integrity in the left arcuate fasciculus. The recovered children, while showing common deficits along this tract and other areas that were shared with that found in persistent children, demonstrated age-related increases in white matter integrity in the same regions, e.g., exhibited normalized developmental trajectory (Chow and Chang 2017) (Figure 4).
Figure 4.
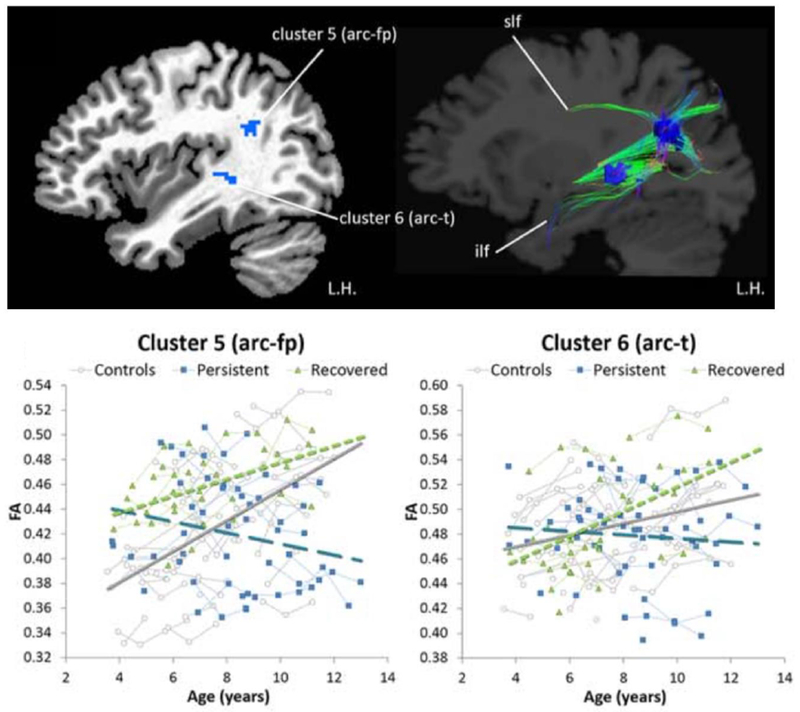
While age-related increases in white matter integrity was observed in areas (left temporoparietal junction, posterior STG) along the left arcuate fasciculus in normally fluent children as well as children who recover from stuttering, the developmental trajectories in the same regions lacked age-related increases in children with persistent stuttering. Arc-fp: arcuate fasciculus-frontoparietal; arc-t: arcuate fasciculus-temporal; ilf: inferior longitudinal fasciculus; Slf: superior longitudinal fasciculus. Figure reprinted and modified with permission from John Wiley and Sons and Copyright Clearance Center. White matter developmental trajectories associated with persistence and recovery of childhood stuttering. Chow HM, Chang S-E. Human Brain Mapping, 2017.
Another study examining differences between persistent and recovered groups used surface-based measures of cortical size and shape (Garnett and others, 2018). Here, persistent children were found to have decreased cortical thickness in left ventral motor cortex (vMC) and ventral premotor cortex (vPMC) areas relative to controls (Figure 5). This was not found to be the case in recovered children. Recovered children had decreased gyrification in the SMA and Pre-SMA areas with age, which may indicate better long-range connectivity with regions such as the left IFG (Garnett et al., 2018). These results support previous convergent findings in the literature that point to possible deficits in left speech motor areas in speakers who stutter. The motor cortical areas found to differentiate persistent children from controls play critical roles in interfacing with sensory, motor execution, and basal ganglia-cortical timing areas.
Figure 5.
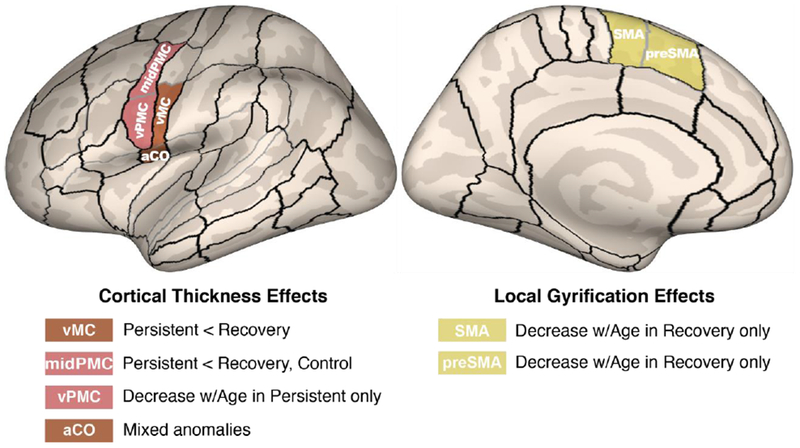
Morphometric differences in speech motor control regions differentiated children with persistent stuttering from those who recover. A compensatory mechanism involving left medial premotor cortex may contribute to recovery. From “Anomalous morphology in left hemisphere motor and premotor cortex of children who stutter,” by Garnett et al., 2018, Brain, in press, Copyright 2018 by the Oxford University Press. Reprinted with permission.
In sum, compared to their fluent counterparts, children with persistent stuttering exhibit more immature speech motor coordination, lack of age-appropriate growth in white matter tracts relevant to auditory-motor integration, and aberrant cortical thickness in left ventral motor and premotor areas. Recovered children on the other hand seem to achieve more typical patterns of white matter development with age, despite initially having similar neuroanatomical risk factors associated with stuttering. Recovered children may also achieve greater long-range connectivity involving PreSMA and SMA, regions that are critical to timing. Studies investigating persistent and recovery during childhood are only just emerging. More research in this area is warranted to reveal the basis for the risk for stuttering, and mechanisms behind recovery during childhood. The results from these studies are expected to contribute to important clinical implications for early detection and intervention development in stuttering.
Bringing it all together: a plausible neural framework for stuttering
Neuroimaging studies to date that employed widely varying methods, including the imaging modality itself, participant demographics, sample sizes, and age ranges have reported at times conflicting and divergent findings on the functional neuroanatomy of stuttering. Nevertheless, some convergent findings have emerged that strongly suggest neural circuit level deficits that affect planning and execution of self-initiated, intrinsically timed sound sequences. As reviewed in previous sections, these neural circuits encompass: 1) auditory-motor cortical areas primarily in the left hemisphere that enable speech motor planning and execution guided by the sensory context; 2) basal ganglia-thalamocortical loop and cerebellum that provides the temporal structure of speech including initiation and timing of speech sequences. Coordination between these neural circuits crucially relies upon interaction between component areas within each circuit.
A useful subcortical-cortical framework for speech perception and production that might be considered in this context is one proposed by Kotz and colleagues (Kotz and Schwartze 2010; Kotz and others 2016). Here, it is argued that the auditory cortical areas interact with the temporal processing system (basal ganglia and cerebellum) to establish basic timed routines during speech acquisition that form the basis for acquiring more advanced sophisticated behavior (e.g., longer sequences of speech sounds). In this framework, the BG’s contribution is thought to be reduced to a supplementary function once the routines are acquired, whereas the cerebellum continues to be actively engaged in computing sensory information. According to Kotz and colleagues, auditory information can be transmitted to the cerebellar temporal processing system via neural pathways between the cerebellum and cochlear nuclei. The cerebellum (dentate; the nonmotor part of the cerebellum) in turn projects to the frontal cortex (PreSMA/SMA) via the thalamus; the frontal cortex then connects to BG, forming the circuit that encompass the auditory-cerebellar- thalamus- frontal-striatal (BG) regions (Figure 6).
Figure 6.
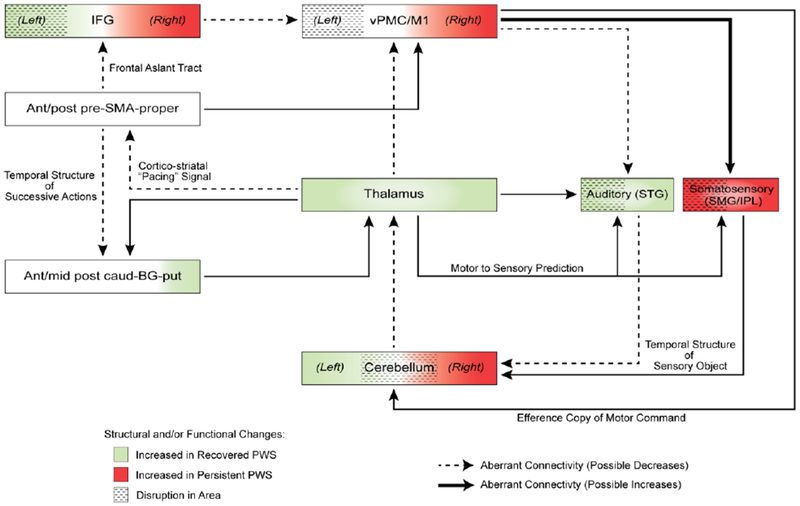
A plausible neural framework relevant to stuttering risk, persistence, and recovery. Neural structures critical for sensorimotor integration for speech planning and production interface with subcortical structures that provide temporal structure and enable internal timing of speech sound production. Colors and thickness of lines are hypothetical, based on previous reported findings in the literature. Modified based on a subcortical-cortical model proposed by Kotz and colleagues, this model provides a useful framework that incorporates most convergent empirical findings to date on neural deficits linked to stuttering. Elucidating the functional and structural connectivity among component areas and causal relationships represented here could lead to novel insights into possible neural mechanisms linked to stuttering. BG: basal ganglia; caud: caudate; CE: cerebellum; IFG: inferior frontal gyrus; M1: primary motor cortex; PMC: premotor cortex; put: putamen; PWS: people who stutter; SMA: supplementary motor area; SMG/IPL: supramarginal gyrus/inferior parietal lobe; STG: superior temporal gyrus; vPMC: ventral premotor cortex.
According to the reviewed studies in this paper, most of the connections as well as component areas in Figure 6 were found to be abnormal in structure and/or function in stuttering speakers. Given previous reports of cerebellar compensation for BG dysfunction, specifically involving cerebellar-thalamic-SMA proper projection for speech production (Kotz and others 2009), it is not surprising that many studies to date have reported hyperactivity in each of these regions during speech tasks in stuttering speakers (Fox, R.J. Ingham, J.C. Ingham, Zamarripa, Xiong, and Lancaster 2000b; Budde and others 2014). This is also consistent with what was proposed in an influential theory paper where cerebellar compensation to a core BG deficit was proposed (Alm 2004). The subcortical-cortical model shown in figure 6 provides an updated framework in which future studies might consider investigating component areas and networks that are relevant to stuttering. Characterizing the functional anatomical differences and connectivity strengths of component areas might help elucidate possible mechanisms of speech disfluencies manifested in stuttering.
Outstanding questions for future research
The mechanisms behind the core symptoms of stuttering may very well involve abnormal function of the neural circuits reviewed in previous sections. The individual manifestation of stuttering behavior can vary however, depending on factors such as emotional reactions and motor compensatory behaviors and therapy, which may interact with core neural deficits. For instance, stuttering severity can be exacerbated by greater demands on attention, linguistic complexity, and emotional significance of the speech context (Smith and Weber 2017). Complex interactions among these factors as they relate to stuttering are likely to be better explored with methods that enable examination of large scale neural network interactions. Complex brain function likely emerges from whole-network topology rather than isolated regions and connections, but to date there has been little empirical study combining these powerful approaches to determine mechanisms of neurodevelopmental and psychiatric disorders, including stuttering. In a recent study, we applied a whole brain connectomics approach to examine functional interactions within and between large scale neural networks across the whole brain (Chang and others 2017). This approach revealed a common deficit in stuttering children regardless of later recovery or persistence involving the somatomotor network (which encompasses the speech motor networks). The persistent children were differentiated from recovered children in how the attention networks interacted with the default mode network; the latter anti-correlate with task positive networks such as attention networks in typical development. The anomalous functional interactions involving neural networks other than those directly involved in speech motor control seem to suggest their critical role in developing persistent stuttering. Future studies that involve such large scale connectomics approaches, potentially through data sharing across different research labs, are expected to lead to greater insights into network markers for persistent stuttering. Furthermore, these studies may be better able to give us insights into why there are comorbidities in stuttering such as attention, phonological issues, emotional issues in adults, etc. and basis for recovery vs persistence.
Another area of investigation involves examining the links between brain anomalies and genetics. Genes causative of persistent stuttering have begun to be identified (Kang and others 2010; Raza and others 2015). By studying the genetics of large families with high incidence of persistent stuttering in Pakistan and Cameroon, mutations in four genes related to intracellular trafficking functions have been linked to persistent stuttering. Mutations in these four genes have been reported to cumulatively account up to 20% of unrelated individuals with persistent stuttering (Frigerio-Domingues and Drayna 2017). How these mutations in these genes affect the ability to produce fluent speech remains unknown. As discussed in the previous sections, persistent stuttering is associated with subtle functional and anatomical anomalies. Because stuttering is a highly heritable disorder, genetics is likely to contribute to the brain anomalies associated with stuttering. However, this proposition has not been tested.
This question could be answered through imaging genetics approaches (Meyer-Lindenberg and Weinberger 2006). One such approach might be applying neuroinformatic methods where expression of genes linked to stuttering can be examined in terms of their expression patterns across the brain, using data provided by the Allen Institute for Brain Science (AIBS; http://www.brain-map.org/). Regional expression of the genes can be examined in the context of brain areas that differed in stuttering speakers. In a recent such analysis, Chow and colleagues found that two of the four genes related to persistent stuttering (GNPTG and NAGPA) exhibited a strong positive correlation with between-group absolute differences in gray matter volume (GMV). Further gene set enrichment analysis revealed that genes that highly correlate with the GMV differences between children who stutter and controls were enriched for energy metabolism in mitochondria. The results suggested that the effect of stuttering related gene mutations could be exacerbated in brain areas with high energy consumption such as the motor cortices during a sharp increase in metabolic rate during early childhood that co-occurs with the “explosion” of language development from 2 to 5 years of age. Since stuttering onset occurs during a period of rapid increase in brain energy utilization, this stage of development may be a period of vulnerability for children with a genetic predisposition to stuttering due to steep changes energy demands related to synaptogenesis and myelination. This study is significant, as it shows for the first time how genes that have been linked to stuttering may affect speech via aberrant brain structure as found in persistent stuttering children (Chow and Chang 2018).
Future research on the biological bases of stuttering is expected to lead to meaningful clinical applications that may include designing novel interventions based on a mechanistic understanding of the basis of stuttering. Better insights into the neural basis of stuttering is also expected to lead to better objective markers that could help identify children at risk for stuttering near symptom onset. Such advances could help children who are most at risk for persistent stuttering by providing intensive therapy that can be delivered to them before disfluent speech patterns become established. If successful, these approaches in the future may help children avoid developing a chronic life-time disorder that affects a most fundamental human faculty-- efficient communication via fluent speech production.
Figure 2.
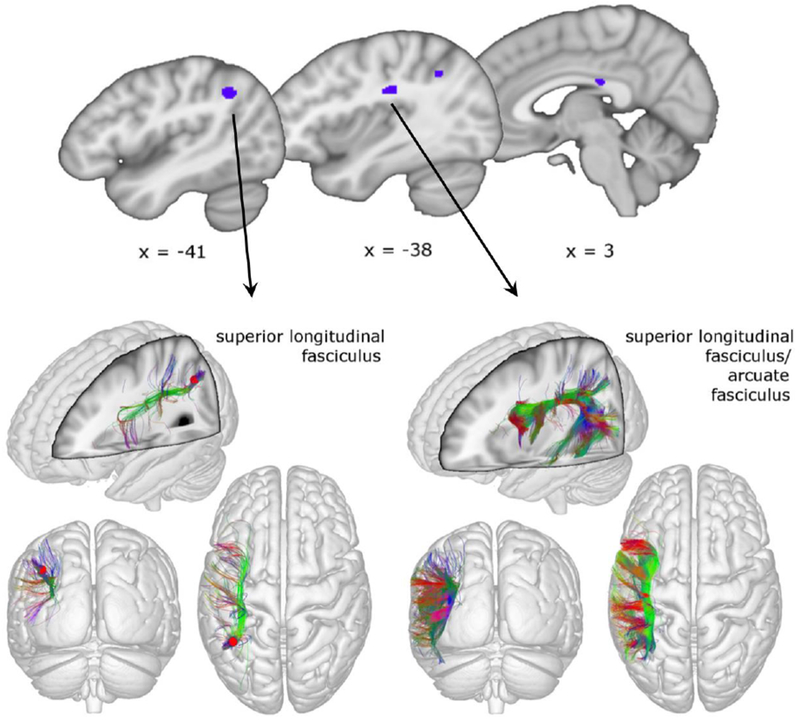
Convergent findings from past diffusion tensor imaging (DTI) studies of stuttering focus on deficits in sections along the left superior longitudinal fasciculus. Reprinted and adapted by permission from Springer Customer Service Centre GmbH: Springer Nature. The Neurobiological Grounding of Persistent Stuttering: from Structure to Function. Neef NE, Anwander A, Friederici AD. Current Neurology and Neuroscience Reports. 2015.
Figure 7.
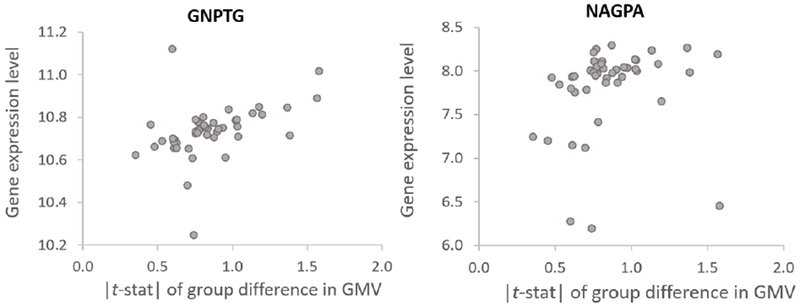
Relationship between gene expression of two stuttering-linked genes (GNPTG and NAGPA) and absolute regional gray matter volume differences observed in persistent children who stutter. Dots represent brain regions in the left hemisphere defined by the AAL atlas. The brain regions with relatively high expression of GNPTG and NAGPA and between-group differences in GMV were primarily in the sensorimotor, parietal, the cingulate cortex, and the middle frontal gyrus. The Spearman’s rank correlation coefficients for gene expression-brain volume group difference were ρ = 0.57 for GNPTG, and ρ = 0.42 for NAGPA. Chow et al., 2018.
Acknowledgments
This study was supported by Award Numbers R01DC011277 (SC), R21DC015312 (SC), R21DC015853 (HMC) from the National Institute on Deafness and Other Communication Disorders (NIDCD), and the Matthew Smith Stuttering Research Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health. We thank Cecilie Simonsen for graphic support.
References
- Adams MR, Ramig P. 1980. Vocal characteristics of normal speakers and stutterers during choral reading. J Speech Hear Res 23:457–469. [DOI] [PubMed] [Google Scholar]
- Alm PA. 2004. Stuttering and the basal ganglia circuits: a critical review of possible relations. Journal of Communication Disorders 37:325–370. [DOI] [PubMed] [Google Scholar]
- Andrews CC, O’Brian SS, Harrison EE, Onslow MM, Packman AA, Menzies RR. 2012. Syllable-timed speech treatment for school-age children who stutter: a phase I trial. Language, Speech, and Hearing Services in Schools 43:359–369. [DOI] [PubMed] [Google Scholar]
- Andrews G, Harris M. 1964. The syndrome of stuttering. Spastics Society Medical Education:191. [Google Scholar]
- Bartolo R, Merchant H. 2015. β oscillations are linked to the initiation of sensory-cued movement sequences and the internal guidance of regular tapping in the monkey. J Neurosci [Internet] 35:4635–4640. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=β+oscillations+are+linked+to+the+initiation+of+sensory-cued+movement+sequences+and+the+internal+guidance+of+regular+tapping+in+the+monkey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal DS, Gracco VL, Brettschneider J, Kroll RM, De Nil LF. 2013. A voxel-based morphometry (VBM) analysis of regional grey and white matter volume abnormalities within the speech production network of children who stutter. CORTEX 49:2151–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal DS, Quraan MA, Cheyne DO, Taylor MJ, Gracco VL, De Nil LF. 2011. Speech-induced suppression of evoked auditory fields in children who stutter. NeuroImage [Internet] 54:2994–3003. Available from: http://www.sciencedirect.com/science/article/pii/S1053811910014564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyk M, Kraft SJ, Brown S. 2014. Stuttering as a trait or state - an ALE meta-analysis of neuroimaging studies. European Journal of Neuroscience:n/a–n/a. [DOI] [PubMed] [Google Scholar]
- Belyk M, Kraft SJ, Brown S. 2017. Stuttering as a trait or a state revisited: motor system involvement in persistent developmental stuttering. European Journal of Neuroscience 45:622–624. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, Sirigu A. 2003. Action prediction in the cerebellum and in the parietal lobe. Exp Brain Res 153:239–245. [DOI] [PubMed] [Google Scholar]
- Bloodstein O 1995. A handbook of stuttering. San Diego: Singular Publishing Group, Inc. [Google Scholar]
- Bohland JW, Bullock D, Guenther FH. 2010. Neural Representations and Mechanisms for the Performance of Simple Speech Sequences. Journal of Cognitive Neuroscience 22:1504–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland JWJ, Guenther FHF. 2006. An fMRI investigation of syllable sequence production. NeuroImage 32:821–841. [DOI] [PubMed] [Google Scholar]
- Braun AR, Varga M, Stager S, Schulz G, Selbie S, Maisog JM, and others 1997. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H2(15)O positron emission tomography study. 120 ( Pt 5):761–784. [DOI] [PubMed] [Google Scholar]
- Brotchie JM, Mitchell IJ, Sambrook MA. 1991. Alleviation of parkinsonism by antagonism of excitatory amino acid transmission in the medial segment of the globus pallidus in rat and primate. Movement …. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham R, Ingham J, Laird A, Fox P. 2005. Stuttered and fluent speech production: An ALE meta-analysis of functional neuroimaging studies. Hum Brain Mapp 25:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde KS, Barron DS, Fox PT. 2014. Stuttering, induced fluency, and natural fluency: A hierarchical series of activation likelihood estimation meta-analyses . Brain and Language 139:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. 2004. Neuronal Oscillations in Cortical Networks. Science [Internet] 304:1926–1929. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15218136 [DOI] [PubMed] [Google Scholar]
- Byrd CT, Conture EG, Ohde RN. 2007. Phonological priming in young children who stutter: holistic versus incremental processing. Am J Speech Lang Pathol 16:43–53. [DOI] [PubMed] [Google Scholar]
- Cai S, Beal DS, Ghosh SS, Guenther FH, Perkell JS. 2014. Impaired timing adjustments in response to time-varying auditory perturbation during connected speech production in persons who stutter. Brain and Language 129:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Beal DS, Ghosh SS, Tiede MK, Guenther FH, Perkell JS. 2012. Weak Responses to Auditory Feedback Perturbation during Articulation in Persons Who Stutter: Evidence for Abnormal Auditory-Motor Transformation. PLoS ONE 7:e41830–e41830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso AJA, Chodzko-Zajko WJW, Bidinger DAD, Sommers RKR. 1994. Adults who stutter: responses to cognitive stress. J Speech Hear Res 37:746–754. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C, and others 2013. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain [Internet] 136:2619–2628. Available from: http://brain.oxfordjournals.org/content/136/8/2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Angstadt M, Chow HM, Etchell AC, Garnett EO, Choo AL, and others 2017. Anomalous network architecture of the resting brain in children who stutter. Journal of Fluency Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Chow HM, Wieland EA, McAuley JD. 2016. Relation between functional connectivity and rhythm discrimination in children who do and do not stutter. NeuroImage: Clinical 12:442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. 2008. Brain anatomy differences in childhood stuttering. NeuroImage 39:1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Zhu DC, Choo AL, Angstadt M. 2015. White matter neuroanatomical differences in young children who stutter. Brain [Internet]. Available from: http://brain.oxfordjournals.org/cgi/reprint/awu400?ijkey=4ytazRbZPJ7F6Ey&keytype=ref [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Zhu DC. 2013. Neural network connectivity differences in children who stutter. Brain 136:3709–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow HM, Chang S-E. 2017. White matter developmental trajectories associated with persistence and recovery of childhood stuttering. Hum Brain Mapp 13:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow HM, Chang S-E. 2018. Genes Linked to Stuttering are Associated with Anomalous Brain morphometry in children with persistent stuttering.
- Christensen A, Giese MA, Sultan F, Mueller OM, Goericke SL, Ilg W, and others 2014. An intact action-perception coupling depends on the integrity of the cerebellum. Journal of Neuroscience 34:6707–6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civier O, Bullock D, Max L, Guenther FH. 2013. Computational modeling of stuttering caused by impairments in a basal ganglia thalamo-cortical circuit involved in syllable selection and initiation. Brain and Language [Internet] 126:263–278. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0093934X13001144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connally EL, Ward D, Howell P, Watkins KE. 2013. Disrupted white matter in language and motor tracts in developmental stuttering. Brain and Language:1–11. [DOI] [PubMed] [Google Scholar]
- Craig A, Hancock K, Tran Y, Craig M, Peters K. 2002. Epidemiology of Stuttering in the Community Across the Entire Life Span. J Speech Lang Hear Res 45:1097. [DOI] [PubMed] [Google Scholar]
- Craig A, Tran Y. 2014. Trait and social anxiety in adults with chronic stuttering: conclusions following meta-analysis. Journal of Fluency Disorders 40:35–43. [DOI] [PubMed] [Google Scholar]
- Cykowski MD, Fox PT, Ingham RJ, Ingham JC, Robin DA. 2010. A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: A potential role for impaired myelination. NeuroImage 52:1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daliri A, Max L. 2017. Stuttering adults’ lack of pre-speech auditory modulation normalizes when speaking with delayed auditory feedback. CORTEX 99:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daliri A, Wieland EA, Cai S, Guenther FH, Chang S-E. 2017. Auditory-motor adaptation is reduced in adults who stutter but not in children who stutter. Developmental Science 121:e12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nil L, Kroll R, Lafaille S, Houle S. 2003. A positron emission tomography study of short- and long-term treatment effects on functional brain activation in adults who stutter. Journal of Fluency Disorders [Internet] 28:357–380. Available from: http://www.sciencedirect.com/science/article/pii/S0094730X03000597 [DOI] [PubMed] [Google Scholar]
- Desai J, Huo Y, Wang Z, Bansal R, Williams SCR, Lythgoe D, and others 2016. Reduced perfusion in Broca’s area in developmental stuttering. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AS, Bernal B, Tremblay P. 2014. The language connectome: new pathways, new concepts. The Neuroscientist [Internet] 20:453–467. Available from: http://nro.sagepub.com/content/20/5/453.abstract [DOI] [PubMed] [Google Scholar]
- Donaher J, Richels C. 2012. Traits of attention deficit/hyperactivity disorder in school-age children who stutter. Journal of Fluency Disorders 37:242–252. [DOI] [PubMed] [Google Scholar]
- Donald G Mackay MCM. 1984. Stuttering as a sequencing and timing disorder.
- Dworzynski K, Remington A, Rijsdijk FH, Howell P, Plomin R. 2007. Genetic Etiology in Cases of Recovered and Persistent Stuttering in an Unselected, Longitudinal Sample of Young Twins. Am J Speech Lang Pathol [Internet] 16:169–178. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=Genetic+Etiology+in+Cases+of+Recovered+and+Persistent+Stuttering+in+an+Unselected%2C+Longitudinal+Sample+of+Young+Twins [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. 2008. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature 453:1102–1106. [DOI] [PubMed] [Google Scholar]
- Etchell AC, Johnson BW, Sowman PF. 2014. Beta oscillations, timing, and stuttering. Front Hum Neurosci 8:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchell AC, Ryan M, Martin E, Johnson BW, Sowman PF. 2016. Abnormal time course of low beta modulation in non-fluent preschool children: A magnetoencephalographic study of rhythm tracking. NeuroImage 125:953–963. [DOI] [PubMed] [Google Scholar]
- Felsenfeld SS, van Beijsterveldt CEMC, Boomsma DID. 2010. Attentional regulation in young twins with probable stuttering, high nonfluency, and typical fluency. CORD Conference Proceedings 53:1147–1166. [DOI] [PubMed] [Google Scholar]
- Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, and others 2015. Redefining the role of Broca’s area in speech. Proc Natl Acad Sci U S A 112:2871–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Bollich AM, Feldman J, Corey DM, Hurley M, Lemen LC, and others 2004. Aberrant auditory processing and atypical planum temporale in developmental stuttering. Neurology 63:1640–1646. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Mock JR, Corey DM, Golob EJ, Conture EG. 2013. Brain & Language. Brain and Language [Internet] 126:141–150. Available from: http://www.sciencedirect.com/science/article/pii/S0093934X13000886 [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, Martin C, and others 1996. A PET study of the neural systems of stuttering. Nature 382:158–162. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Zamarripa F, Xiong J-H, Lancaster JL. 2000a. Brain correlates of stuttering and syllable production A PET performance-correlation analysis. [DOI] [PubMed]
- Fox PT, Ingham RJ, Ingham JC, Zamarripa F, Xiong JH, Lancaster JL. 2000b. Brain correlates of stuttering and syllable production. A PET performance-correlation analysis. Brain 123 (Pt 10):1985–2004. [DOI] [PubMed] [Google Scholar]
- Friederici AD. 2012. Language development and the ontogeny of the dorsal pathway. Front Evol Neurosci 4:3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P 2005. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci (Regul Ed) 9:474–480. [DOI] [PubMed] [Google Scholar]
- Frigerio-Domingues C, Drayna D. 2017. Genetic contributions to stuttering: the current evidence. Mol Genet Genomic Med 5:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T, Trainor LJ, Large EW, Ross B. 2012. Internalized Timing of Isochronous Sounds Is Represented in Neuromagnetic Beta Oscillations. Journal of Neuroscience 32:1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A, Neumann K, Bachoud-Levi A, Gudenberg von A, Euler H, Lanfermann H, and others 2008. Severity of dysfluency correlates with basal ganglia activity in persistent developmental stuttering. Brain and Language [Internet] 104:190–199. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17531310 [DOI] [PubMed] [Google Scholar]
- Guenther FH. 2016. Neural control of speech. Cambridge, MA: MIT Press [Google Scholar]
- Hickok G, Poeppel D. 2007. The cortical organization of speech processing. Nat Rev Neurosci 8:393–402. [DOI] [PubMed] [Google Scholar]
- Ingham RJ, Bothe AK, Wang Y, Purkhiser K, New A. 2012. Phonation interval modification and speech performance quality during fluency-inducing conditions by adults who stutter. Journal of Communication Disorders 45:198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverach L, Rapee RM. 2013. Social anxiety disorder and stuttering: Current status and future directions. Journal of Fluency Disorders. [DOI] [PubMed] [Google Scholar]
- Jancke L, Hänggi J, Steinmetz H. 2004. Morphological brain differences between adult stutterers and non-stutterers. BMC Neurology 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos K, De Ridder D, Boey RA, Vanneste S. 2014. Functional connectivity changes in adults with developmental stuttering: a preliminary study using quantitative electro-encephalography. Front Hum Neurosci 8:783–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joundi RA, Jenkinson N, Brittain J-S, Aziz TZ, Brown P. 2012. Driving Oscillatory Activity in the Human Cortex Enhances Motor Performance. Current Biology 22:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowski J, Armson J, Roland-Mieszkowski M, Stuart A, Gracco VL. 1993. Effects of alterations in auditory feedback and speech rate on stuttering frequency. Lang Speech 36 ( Pt 1):1–16. [DOI] [PubMed] [Google Scholar]
- Kang C, Riazuddin S, Mundorff J, Krasnewich D, Friedman P, Mullikin JC, and others 2010. Mutations in the lysosomal enzyme-targeting pathway and persistent stuttering. N Engl J Med 362:677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalianos E, Onslow M, Ukoumunne O, Block S, Reilly S. 2014. Stuttering, temperament and anxiety: Data from a community cohort aged 2-4 years. Journal of Speech Language and Hearing Research. [DOI] [PubMed] [Google Scholar]
- Kell CA, Neumann K, Kriegstein Von K, Posenenske C, Gudenberg Von AW, Euler H, and others 2009. How the brain repairs stuttering. Brain 132:2747–2760. [DOI] [PubMed] [Google Scholar]
- Kemerdere R, de Champfleur NM, Deverdun J, Cochereau J, Moritz-Gasser S, Herbet G, and others 2015. Role of the left frontal aslant tract in stuttering: a brain stimulation and tractographic study. J Neurol [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=Role+of+the+left+frontal+aslant+tract+in+stuttering%3A+a+brain+stimulation+and+tractographic+study. [DOI] [PubMed] [Google Scholar]
- Kent R 1984. Stuttering as a temporal programming disorder. (Curlee RF, Perkins WH, editors.). San Diego: College-Hill Press [Google Scholar]
- Kikuchi Y, Okamoto T, Ogata K, Hagiwara K, Umezaki T, Kenjo M, and others 2016. Abnormal auditory synchronization in stuttering: A magnetoencephalographic study. Hearing Research. [DOI] [PubMed] [Google Scholar]
- Kilavik BE, Zaepffel M, Brovelli A, MacKay WA, Riehle A. 2013. The ups and downs of beta oscillations in sensorimotor cortex. Experimental Neurology [Internet] 245:15–26. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=The+ups+and+downs+of+beta+oscillations+in+sensorimotor+cortex [DOI] [PubMed] [Google Scholar]
- Kotz SA, Brown RM, Schwartze M. 2016. Cortico-striatal circuits and the timing of action and perception. Current Opinion in Behavioral Sciences 8:42–45. [Google Scholar]
- Kotz SA, Schwartze M, Schmidt-Kassow M. 2009. Non-motor basal ganglia functions: A review and proposal for a model of sensory predictability in auditory language perception. CORTEX 45:982–990. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Schwartze M. 2010. Cortical speech processing unplugged:a timely subcortico-cortical framework. Trends Cogn Sci (Regul Ed) 14:392–399. [DOI] [PubMed] [Google Scholar]
- Kronfeld-Duenias V, Amir O, Ezrati-Vinacour R, Civier O, Ben-Shachar M. 2014. The frontal aslant tract underlies speech fluency in persistent developmental stuttering. Brain Struct Funct [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=The+frontal+aslant+tract+underlies+speech+flu-+ency+in+persistent+developmental+stuttering [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. 2008. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320:110–113. [DOI] [PubMed] [Google Scholar]
- Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. 2012. Basal Ganglia Beta Oscillations Accompany Cue Utilization. Neuron [Internet] 73:523–536. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=Basal+Ganglia+Beta+Oscillations+Accompany+Cue+Utilization [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks T, Chon H, Han W. 2012. Audiovocal integration in adults who stutter. Int J Lang Commun Disord 47:451–456. [DOI] [PubMed] [Google Scholar]
- Lu C, Chen C, Ning N, Ding G, Guo T, Peng D, and others 2009. The neural substrates for atypical planning and execution of word production in stuttering. Experimental Neurology 221:11–11. [DOI] [PubMed] [Google Scholar]
- Lu C, Peng D, Chen C, Ning N, Ding G, Li K, and others 2010. Altered effective connectivity and anomalous anatomy in the basal ganglia-thalamocortical circuit of stuttering speakers. CORTEX 46:49–67. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Loucks T. 2003. Stuttering: a dynamic motor control disorder. Journal of Fluency Disorders [Internet] 28:273–95–quiz 295 Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14643066 [DOI] [PubMed] [Google Scholar]
- Maguire G, Riley G, Yu B. 2002. A neurological basis of stuttering? Lancet Neurology [Internet] 1:407–407. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12849360 [DOI] [PubMed] [Google Scholar]
- Maguire GA, Riley GD, Franklin DL, Gottschalk LA. 2000. Risperidone for the Treatment of Stuttering. Journal of Clinical Psychopharmacology [Internet] 20:479 Available from: http://www.ncbi.nlm.nih.gov/pubmed/10917410 [DOI] [PubMed] [Google Scholar]
- Maguire GA, Yu BP, Franklin DL. 2004. Alleviating stuttering with pharmacological interventions. Expert opinion on …. [DOI] [PubMed]
- Marsden CD, Obeso JA. 1994. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson’s disease. Brain 117:877–897. [DOI] [PubMed] [Google Scholar]
- Max L, Guenther F, Gracco V, Ghosh S, Wallace M. 2004. Unstable or insufficiently activated internal models and feedback-biased motor control as sources of dysfluency: A theoretical model of stuttering. Contemporary Issues in Communication Science and Disorders 31:105–122. [Google Scholar]
- Merchant H, Harrington DL, Meck WH. 2013. Neural Basis of the Perception and Estimation of Time. Annual Review of Neuroscience 36:313–336. [DOI] [PubMed] [Google Scholar]
- Mersov A, Cheyne D, Jobst C, De Nil L. 2018. A preliminary study on the neural oscillatory characteristics of motor preparation prior to dysfluent and fluent utterances in adults who stutter. Journal of Fluency Disorders 55:145–155. [DOI] [PubMed] [Google Scholar]
- Mersov A-M, Jobst C, Cheyne DO, De Nil L. 2016. Sensorimotor Oscillations Prior to Speech Onset Reflect Altered Motor Networks in Adults Who Stutter. Front Hum Neurosci 10:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger FL, Auer T, Helms G, Paulus W, Frahm J, Sommer M, and others 2017. Shifted dynamic interactions between subcortical nuclei and inferior frontal gyri during response preparation in persistent developmental stuttering. Brain Struct Funct:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. 2006. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 7:818–827. [DOI] [PubMed] [Google Scholar]
- Miall R, Wolpert D. 1996. Forward models for physiological motor control. Neural Networks 9:1265–1279. [DOI] [PubMed] [Google Scholar]
- Mink JW. 1996. The basal ganglia: focused selection and inhibition of competing motor programs. Progress in Neurobiology 50:381–425. [DOI] [PubMed] [Google Scholar]
- Mock JR, Foundas AL, Golob EJ. 2016. Cortical activity during cued picture naming predicts individual differences in stuttering frequency. CLINICAL NEUROPHYSIOLOGY 127:3093–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef NE, Anwander A, Bütfering C, Schmidt-Samoa C, Friederici AD, Paulus W, and others 2017. Structural connectivity of right frontal hyperactive areas scales with stuttering severity. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef NE, Anwander A, Friederici AD. 2015. The Neurobiological Grounding of Persistent Stuttering: from Structure to Function. Curr Neurol Neurosci Rep 15:579. [DOI] [PubMed] [Google Scholar]
- Neef NE, Bütfering C, Anwander A, Friederici AD, Paulus W, Sommer M. 2016. Left posterior-dorsal area 44 couples with parietal areas to promote speech fluency, while right area 44 activity promotes the stopping of motor responses. NeuroImage [Internet]:1–63. Available from: https://www.ncbi.nlm.nih.gov/pubmed/?term=Left+posterior-dorsal+area+44+couples+with+parietal+areas+to+promote+speech+fluency%2C+while+right+area+44+activity+promotes+the+stopping+of+motor+responses [DOI] [PubMed] [Google Scholar]
- Neef NE, Hoang TNL, Neef A, Paulus W, Sommer M. 2015. Speech dynamics are coded in the left motor cortex in fluent speakers but not in adults who stutter. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann K, Preibisch C, Euler HA, Gudenberg AWV, Lanfermann H, Gall V, and others 2005. Cortical plasticity associated with stuttering therapy. Journal of Fluency Disorders [Internet] 30:23–39. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15769497 [DOI] [PubMed] [Google Scholar]
- Nudelman HB, Herbrich KE, Hess KR, Hoyt BD, Rosenfield DB. 1992. A model of the phonatory response time of stutterers and fluent speakers to frequency-modulated tones. J Acoust Soc Am 92:1882–1888. [DOI] [PubMed] [Google Scholar]
- Nuñez SC, Dapretto M, Katzir T, Starr A, Bramen J, Kan E, and others 2011. fMRI of syntactic processing in typically developing children: structural correlates in the inferior frontal gyrus. Developmental cognitive neuroscience 1:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORTON ST. 1927. STUDIES IN STUTTERING. Arch NeurPsych 18:671. [Google Scholar]
- Ozge A, Toros F, Cömelekoğlu U. 2004. The role of hemispheral asymmetry and regional activity of quantitative EEG in children with stuttering. Child Psychiatry Hum Dev 34:269–280. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Gaynor LD, Eusebio A, Brown P. 2009. Boosting Cortical Activity at Beta-Band Frequencies Slows Movement in Humans. Current Biology 19:1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisch C, Neumann K, Raab P, Euler H, Gudenberg von A, Lanfermann H, and others 2003. Evidence for compensation for stuttering by the right frontal operculum. NeuroImage 20:1356–1364. [DOI] [PubMed] [Google Scholar]
- Rastatter MP, Stuart A, Kalinowski J. 1998. Quantitative electroencephalogram of posterior cortical areas of fluent and stuttering participants during reading with normal and altered auditory feedback. Percept Mot Skills 87:623–633. [DOI] [PubMed] [Google Scholar]
- Raza MH, Domingues CEF, Webster R, Sainz E, Paris E, Rahn R, and others 2015. Mucolipidosis types II and III and non-syndromic stuttering are associated with different variants in the same genes. Eur. J. Hum. Genet. [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=Mucolipidosis+types+II+and+III+and+non-syndromic+stuttering+are+associated+with+different+variants+in+the+same+genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Freund H. 2000. Single word reading in developmental stutterers and fluent speakers. Brain 123:1184–1202. [DOI] [PubMed] [Google Scholar]
- Saltuklaroglu T, Harkrider AW, Thornton D, Jenson D, Kittilstved T. 2017. EEG Mu (µ) Rhythm Spectra and Oscillatory Activity Differentiate Stuttering from Non-Stuttering Adults. NeuroImage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltuklaroglu T, Kalinowski J, Robbins M, Crawcour S, Bowers A. 2009. Comparisons of stuttering frequency during and after speech initiation in unaltered feedback, altered auditory feedback and choral speech conditions. Int J Lang Commun Disord 44:1000–1017. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, and others 2008. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA 105:18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W 1999. Neuronal synchrony: A versatile code for the definition of relations? Neuron 24:49–65. [DOI] [PubMed] [Google Scholar]
- Smith A, Weber C. 2017. How Stuttering Develops: The Multifactorial Dynamic Pathways Theory. Journal of Speech Language and Hearing Research:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Büchel C. 2002. Disconnection of speech-relevant brain areas in persistent developmental stuttering. The Lancet 360:380–383. [DOI] [PubMed] [Google Scholar]
- Stuart A, Frazier CL, Kalinowski J, Vos PW. 2008. The effect of frequency altered feedback on stuttering duration and type. J Speech Lang Hear Res 51:889–897. [DOI] [PubMed] [Google Scholar]
- Tani T, Sakai Y. 2011. Analysis of five cases with neurogenic stuttering following brain injury in the basal ganglia. Journal of Fluency Disorders. [DOI] [PubMed] [Google Scholar]
- Toyomura A, Fujii T, Kuriki S. 2011. Effect of external auditory pacing on the neural activity of stuttering speakers. NeuroImage 57:1507–1516. [DOI] [PubMed] [Google Scholar]
- Toyomura A, Fujii T, Kuriki S. 2015. Effect of an 8-week practice of externally triggered speech on basal ganglia activity of stuttering and fluent speakers. NeuroImage 109:458–468. [DOI] [PubMed] [Google Scholar]
- Travis LE. 1978. The cerebral dominance theory of stuttering: 1931−-1978. J Speech Hear Disord 43:278–281. [DOI] [PubMed] [Google Scholar]
- Unger JP, Glück CW, Cholewa J. 2012. Immediate effects of AAF devices on the characteristics of stuttering: a clinical analysis. Journal of Fluency Disorders 37:122–134. [DOI] [PubMed] [Google Scholar]
- Usler E, Smith A, Weber C. 2017. A Lag in Speech Motor Coordination During Sentence Production Is Associated With Stuttering Persistence in Young Children. Journal of Speech Language and Hearing Research 60:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usler E, Weber-Fox C. 2015. Neurodevelopment for syntactic processing distinguishes childhood stuttering recovery versus persistence. J Neurodev Disord 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Vorst R, Gracco VL. 2017. Journal of Fluency Disorders. Journal of Fluency Disorders 53:14–25. [DOI] [PubMed] [Google Scholar]
- Van Riper C 1982. The nature of stuttering.
- Vanhoutte S, Cosyns M, van Mierlo P, Batens K, Corthals P, De Letter M, and others 2016. When will a stuttering moment occur? The determining role of speech motor preparation. Neuropsychologia [Internet] 86:93–102. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=When+will+a+stuttering+moment+occur%3F+The+determining+role+of+speech+motor+preparation. [DOI] [PubMed] [Google Scholar]
- Vanhoutte S, Santens P, Cosyns M, van Mierlo P. 2015. Increased motor preparation activity during fluent single word production in DS: a correlate for stuttering frequency and severity. Neuropsychologia. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux J, Rodriguez E, Martinerie J. 2001. The brainweb: Phase synchronization and large-scale integration. Nat Rev Neurosci 2:229–239. [DOI] [PubMed] [Google Scholar]
- Walsh B, Tian F, Tourville JA, Yücel MA, Kuczek T, Bostian AJ. 2017. Hemodynamics of speech production: An fNIRS investigation of children who stutter. Sci. Rep 7:4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. 2008. Structural and functional abnormalities of the motor system in developmental stuttering. Brain 131:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland EA, McAuley JD, Dilley LC, Chang S-E. 2015. Brain & Language. Brain and Language 144:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert D, Miall R, Kawato M. 1998. Internal models in the cerebellum. Trends Cogn Sci (Regul Ed) 2:338–347. [DOI] [PubMed] [Google Scholar]
- Wolpert DM. 2007. Probabilistic models in human sensorimotor control. Human Movement Science 26:511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Maguire G, Riley G, Lee A, Keator D, Tang C, and others 1997. Increased dopamine activity associated with stuttering. NeuroReport [Internet] 8:767–770. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9106763 [DOI] [PubMed] [Google Scholar]
- Yairi E, Ambrose N. 1992. A longitudinal study of stuttering in children: a preliminary report. J Speech Hear Res 35:755–760. [DOI] [PubMed] [Google Scholar]
- Yairi E, Ambrose N. 2013. Epidemiology of stuttering: 21st century advances. Journal of Fluency Disorders 38:66–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yairi E, Ambrose NG. 1999. Early childhood stuttering I: persistency and recovery rates. J Speech Lang Hear Res 42:1097–1112. [DOI] [PubMed] [Google Scholar]
- Zimmermann G 1980. Stuttering: a disorder of movement. J Speech Hear Res 23:122–136. [PubMed] [Google Scholar]


