Abstract
Background:
Dual-task paradigms are used to investigate gait and cognitive declines in older adults (OA). Optic-flow is a virtual reality environment where the scene flows past the subject while walking on a treadmill, mimicking real-life locomotion.
Aims:
To investigate cost of environment (no optic-flow v. optic-flow) while completing single- and dual-task walking and dual-task costs (DTC; single- v. dual-task) in optic-flow and no optic-flow environments.
Methods:
Twenty OA and seven younger adults (YA) walked on a self-paced treadmill in 3minute segments per task and both environments. Five task conditions included: no task, semantic fluency (category), phonemic fluency (letters), word reading, and serial-subtraction.
Results:
OAs had a benefit of optic-flow compared to no optic-flow for step width (p=0.015) and step length (p=0.045) during letters compared to the YA. During letters, OA experienced improvement in step width DTC; whereas, YA had a decrement in step width DTC from no optic-flow to optic-flow (p=0.038). During serial-subtraction, OA had less step width DTC when compared to YA in both environments (p=0.02).
Discussion:
During letters, step width and step length improved in OA while walking in optic-flow. Also, step width DTC differed between the two groups. Sensory information from optic-flow appears to benefit OA. Letters relies more on verbal ability and word knowledge, which are preserved in aging. However, YA use a complex speech style during dual tasking, searching for complex words and an increased speed of speech.
Conclusions:
OA can benefit from optic-flow by improving spatial gait parameters, specifically, step width, during dual-task walking.
Keywords: dual task cost, gait, virtual reality, environment, spatiotemporal
Introduction
In 2014, 28.7% of older adults aged 65 years and older reported a fall, totaling 29 million reported falls resulting in 7 million fall injuries [1]. Declines in cognitive functioning associated with aging have been linked to falls and fall risk [2]. Along with cognitive deficits, some older adults have sensory and visual deficits compared to young adults [3], further contributing to fall risk. Using dual task protocols, where subjects are asked to walk and perform a concurrent cognitive task, performance of both domains can be measured. Compared to young adults, older adults’ gait is more affected by dual task paradigms [4, 5], potentially due to a deficit in attention allocation, gait requiring additional attentional resources, and/or a decline in cognitive functioning. Additionally, older adults demonstrate greater dual task costs (DTC) than young adults [6]. DTC, sometimes referred to as dual task interference, is the relative change in performance (usually a cost) associated with the addition of a secondary task. As adults progress through life, DTC increases for both cognitive and gait domains [7]. DTC can distinguish between healthy older adults and those with impaired cognitive function [8].
Because many dual task studies have been conducted in a laboratory on a treadmill, the lack of ecologically valid sensory inputs may impact DTC. Few dual task protocols have used optic flow (OF), which is a type of virtual reality. OF is the appearance of objects moving past the subject as they walk, providing important information about the speed and direction of motion [9]. This happens naturally during overground walking or is artificially created in an OF environment while stationary, i.e., on a treadmill. Enhancing ecological validity to apply results outside the laboratory setting is one reason OF environments have been used. Participants have subjectively rated walking with OF to be more like walking overground compared to walking on a treadmill without OF [10]. Other studies have used OF as a rehabilitation tool, which has been shown decrease falls and fear of falling post training [11,12]. To our knowledge, it is not known if the presence of OF will have an effect on gait performance while dual tasking.
The cost of environment (COE), a measure created for this study, quantifies the change in performance of dual tasking between different environments (i.e., OF or no optic flow (NOF)). Similar to DTC, COE quantifies the benefit or cost of the OF environment. Walking and/or cognitive domain performance can be measured depending on the research question. COE is important to measure in dual task studies incorporating OF to investigate whether the use of OF is helpful to dual task performance. Older adults are thought to have sensory deficits not present in young adults, due to age-related physical and cognitive changes [3]. These deficits could be ameliorated with the addition of an OF environment, thus enhancing performance of walking with or without dual task.
The first aim of this study was to compare COE differences in spatial gait performance between healthy young and healthy older adults during single and dual task conditions. It was hypothesized that older adults would have a greater benefit from the OF environment while young adults’ performance would not change due to OF. The second aim of the study was to compare the DTC for four dual tasks presented (category and letter fluency, word reading, and serial subtraction) between the healthy young and healthy older adults in each of the environments, OF and NOF. It was hypothesized that the older adults would have greater DTC while walking in both environments when compared to the young adults for all cognitive tasks.
Methods
Demographics
Twenty healthy, older adults aged 71.05 ± 4.90 years (range = 65–80 years; 14 females) and seven healthy, young adults aged 22.85 ± 1.46 years (range = 21–25 years; 4 females) took part in this study (Table 1). Older adult subjects were recruited through senior wellness centers in the community and were included if they were older than 65 years, living independently in the community, and able to walk for at least 60 meters without an assistive device. Young adults were included if they were between the ages of 19 and 35 years and were recruited through word-of-mouth. Exclusion criteria for all subjects included: native speaker of any language other than English, diagnosis of any cardiac, pulmonary, neurological, musculoskeletal, or neuromuscular diseases. Additional exclusion criteria included taking any medications for depression that made them dizzy, interfered with cognition, or made them otherwise unable to participate in this study. All subjects had normal or corrected vision and hearing. All procedures were approved by the University Institutional Review Board and subjects were consented prior to study participation.
Table 1.
Mean (standard deviation) of subject demographic variables. Significance of p <.05 indicated with an asterisk (*).
| Young | Older | p-value | |
|---|---|---|---|
| N | 7 | 20 | |
| Age (years) | 22.85 (1.5) | 71.05 (4.9) | p=: 0.001* |
| Mass (kg) | 76.46 (16.2) | 73.40 (17.0) | p = 0.69 |
| Height (m) | 1.73(0.11) | 1.67(0.11) | p = 0.26 |
| WAIS-R1 Total (points) | 125.8 (18.8) | ||
| WAIS-R Symbol (points) | 61.7 (11.5) | ||
| WAIS-R Vocabulary (points) | 4S.1 (10.2) | ||
| WAIS-R Digit (points) | 5.6 (2.3) | ||
| PMA2-TotaKpoints) | 54.6 (26.7) | ||
| PMA-Object (points) | 16.0 (9.5) | ||
| PMA-Figure (points) | 13.1 (11.4) | ||
| PMA-Letter (points) | 11.7 (6.2) | ||
| PMA-Word (points) | 12.0 (5.4) | ||
| Montreal Cognitive Assessment (points) | 25.2 (2.6) | ||
| Hand Grip Strength Right (kg) | 29.0 (10.3) | ||
| Hand Grip Strength Left (kg) | 28.1 (10.6) | ||
| Trail Making Test-A (sec) | 44.5 (13.0) | ||
| Trail Making Test-B (sec) | 98.5 (33.4) | ||
| Timed lip and go (sec) | 9.9 (1.2) | ||
| Fullerton Advanced Balance Scale (points) | 32.3 (4.7) |
WAIS-R = Wechsler Adult Intelligence Scale-Revised;
PMA = Primary Mental Abilities
Protocol
The protocol consisted of three visits. During the first visit, all subjects completed a medical background questionnaire, a hearing test using an audiometer, and the Snellen eye exam. Older adults completed the following additional screening tests during the first visit: a hand grip strength test using a dynamometer with each hand three times; cognitive assessments; and balance assessments (Table 1). The cognitive assessments for the older adults included the following: the vocabulary, digit symbol, and forward and backward digit span of the Wechsler Adult Intelligence Scale-Revised [13]; the Schaie-Thurstone Adult Mental Abilities Test Form Older Adults which included object and figure rotation and letter and word series [14]; the Montreal Cognitive Assessment version 7.1 original version [15]; and Trail Making Test: Parts A and B [16]. Balance was assessed by the Timed Up and Go test [17] and the Fullerton Advanced Balance scale [18].
After screening, subjects completed four single cognitive tasks during visit 1 (single task condition): category and letter fluencies; word reading; and serial subtraction by 3’s while seated. Details of these tasks are provided below. During the first visit, all the single tasks except for serial subtractions were presented using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA) on a 12-inch laptop. The serial subtraction task was given verbally by the test administrator. Tasks were presented randomly.
During visits 2 and 3 (dual task conditions), subjects were provided a form-fitting suit (i.e., wrestling singlet) that allowed the researchers to place retroreflective markers in a modified Helen Hayes configuration. Subjects were asked to walk on a self-paced treadmill (Bertec Corp., Columbus, OH). The self-paced treadmill automatically adjusted to the subject’s speed via real-time feedback [19]. A five-minute adaptation period to the treadmill was provided before starting any experimental trials. Subjects were considered adapted if they showed no problems (e.g., stutterstepping, unintentional stopping or slowing) while walking on the treadmill. Using a self-paced treadmill allowed subjects to make adjustments in gait speed while dual tasking instead of being constrained to one speed as with traditional treadmills. Once adapted, subjects were asked to perform the same cognitive tasks as in visit 1. In addition, a walking-only, baseline trial was randomized within the dual task conditions. The environment was manipulated by using NOF or an OF environment. OF was present as an endless hallway scene that was flowing past the subject on the screen; during NOF subjects encountered a static hallway image (Figure 1). In OF, the speed of the flow was the same as the speed of the treadmill. Environments were randomized between visit 2 and 3.
Fig 1.
Experimental setup: a equipment and 180° virtual reality screen; b subject performing the serial subtraction task with optic flow.
For all gait trials, gait data were collected using an eight-camera motion analysis system (100 Hz; Vicon, Oxford, United Kingdom). Only older adults were required to wear a safety harness to prevent falls (Solo-Step, Inc., North Sioux City, SD). Baseline and all four dual task conditions were presented in a randomized order and no instructions regarding prioritization of either walking or cognitive performance were given. Cognitive tasks were administered on a 180° immersive virtual reality screen via D-flow software (Motek Medical, Amsterdam) (Figure 1). For all trials, subjects wore headphones equipped with a microphone to record their responses. Each walking trial was performed for three minutes. A minimum two-minute rest was provided between trials to prevent fatigue.
Cognitive task descriptions
Category Fluency Task
During the category fluency task, subjects were given a category and instructed to name as many words as possible that belong to said category. They had one minute for each category and performed this for three different categories, for a total of three minutes, in each visit. Categories were selected [20, 21] and grouped so that each grouping contained an easy, medium, and hard category as determined by the researchers. They were instructed not to repeat words, use synonyms, and/or proper nouns. Category groupings were randomized between visits. See Appendix A for a list of categories used.
Letter Fluency Task
During the letter fluency task, subjects were given a letter and instructed to name as many words as possible that start with that letter. They had one minute for each letter and performed this for three different letters, for a total of three minutes, in each visit. Letters were grouped by difficulty so that each grouping contained two letters that were considered easy and one letter that was considered moderate [22, 23]. They were instructed not to repeat words, use synonyms, and/or proper nouns. Letter groupings were randomized between visits. See Appendix A for list of letters used.
Word Reading
For the reading task, a word was presented on the screen and subjects were instructed to read the word as fast and accurately as they could. There were 30 different words in each session for the total three minutes. The words were chosen so that each of the word groupings used contained the same number of words that had the same frequency, length, and difficulty. Words were gathered from the North American Adult Reading Test [24] and The English Lexicon Project [25]. Word groupings were randomized between visits. A list of all words in each grouping can be found in Appendix A.
Serial Subtraction
Subjects were given a three-digit number and told to subtract by three from this number continuously for one minute. At the end of the minute, subjects were immediately given a new three-digit number. They did this a total of three times, one minute each, for a total of three minutes. Starting numbers were chosen so that each number had a different starting subtraction pattern. In the single-task session, the test administrator verbally gave the subject the 3-digit number to subtract from, three times, for a total of three minutes during their first visit. During their second and third visits, the numbers were displayed on the virtual reality screen. See Appendix A for all numbers used.
Data Processing and Analysis
Walking trials during dual tasks were trimmed to ensure only time spent performing the dual task was analyzed. Baseline walking trials were trimmed to ensure data lengths were similar to dual task waking trials. Right and left step length and step width were quantified through custom MATLAB script (MathWorks, Inc., Natick, MA). Step length was calculated as the anterior-posterior distance from the heel strike of the right foot to the subsequent heel strike of the left foot and vice versa. Step width was calculated as the medial-lateral distance between the heel strike of the right foot to the subsequent heel strike of the left foot and vice versa. All consecutive right and left steps for step length and step width were used (N=200). Data were checked for spikes or outliers greater than three standard deviations and replaced with a cubic spline algorithm. The mean and standard deviation of all step lengths and step widths were calculated for each trial (Table 2).
Table 2.
Mean (standard deviation) of step length and step width in the baseline trial and each cognitive task trial for healthy, young and older adult subjects for both the no optic flow and optic flow environments.
|
STEP LENGTH MEAN(SD) (cm) | |||||
| NO OPTIC FLOW | |||||
| Baseline | Categories | Letters | Reading | Serial Sub. | |
| Young | 61.51 (4.34) | 60.79 (2.57) | 61.63 (3.45) | 61.05 (3.13) | 60.62 (1.96) |
| Older | 57.14(8.28) | 56.39 (7.75) | 55.72 (7.19) | 57.71 (6.50) | 55.27 (7.45) |
| OPTIC FLOW | |||||
| Young | 62.32 (3.38) | 62.70 (3.00) | 61.34 (6.35) | 60.50 (3.82) | 59.84 (4.03) |
| Older | 61.47 (6.39) | 59.25 (5.73) | 59.99 (7.33) | 57.04 (6.98) | 57.59 (6.73) |
|
STEP WIDTH MEAN(SD) (cm) | |||||
| NO OPTIC FLOW | |||||
| Baseline | Categories | Letters | Reading | Serial Sub. | |
| Young | 14.11 (4.85) | 14.79 (4.57) | 14.58 (4.92) | 14.69 (4.64) | 15.59 (5.71) |
| Older | 15.99 (3.98) | 15.97 (3.93) | 17.30 (3.83) | 15.84 (3.61) | 16.25 (4.36) |
| OPTIC FLOW | |||||
| Young | 15.03 (4.35) | 15.53 (4.20) | 16.43 (4.30) | 16.33 (4.93) | 16.19(4.58) |
| Older | 15.58 (4.47) | 16.04 (4.56) | 15.71 (4.96) | 16.52 (4.81) | 16.21 (4.56) |
Cost of Environment and Dual Task Cost Equations
The COE was calculated using Equation 1. This was done for both step length and step width for each of the conditions between the two environments (baseline, categories, letters, reading, and serial subtraction).
| (1) |
The DTC was calculated using Equation 2 for both step length and step width for each of the four cognitive tasks.
| (2) |
Equations were chosen so that a positive COE or DTC value would indicate a benefit and a negative COE or DTC value would indicate a cost. For variables in which a higher score was deemed as a poor outcome (i.e. step width), a negative was inserted at the beginning of the equation. This is done so that the benefits/costs for each variable could be compared.
Statistical Analysis
Data were checked for normality. Independent t-tests were used to compare the COE between healthy young and older adults for each cognitive task and the baseline condition. Cohen’s d effect sizes were calculated for the t-tests. An effect size was considered small for values between 0.2 ≤ d < 0.5, medium for 0.5 ≤ d < 0.8, and large for d >0.8. A 2X2 repeated measures ANOVA (group x environment) was used to compare the differences between the healthy young and older adults’ DTC for each cognitive task between the OF and NOF environments. Effect size (partial η2) were calculated. A partial η2 effect size was considered small for values between 0.01 ≤ η2 < 0.06, medium for 0.06 ≤ η2 < 0.14, and large for η2 > 0.14. Data were analyzed with IBM SPSS Statistics for Windows (Version 23.0). Statistical significance was set at alpha = 0.05.
Results
The COE was significantly beneficial (a positive COE value) in older adults for step width (p=0.015; d=1.28) and step length (p=0.045; d=0.83) when performing the letter fluency task as compared to healthy, young adults, not assuming equal variance between groups (Figure 2). No other significant findings were found for COE. See supplemental figures for plots of each subject’s data.
Fig 2.
Cost of environment (COE): The cost (−) and benefit (+) of the optic flow (OF) environment for step width (SW) and step length (SL) for both healthy young (left) and older adults (right). Cost/benefit of environment is calculated between the conditions of OF and no OF (NOF). Horizontal lines indicate p<0.05 difference for the letters task between healthy young and older adults
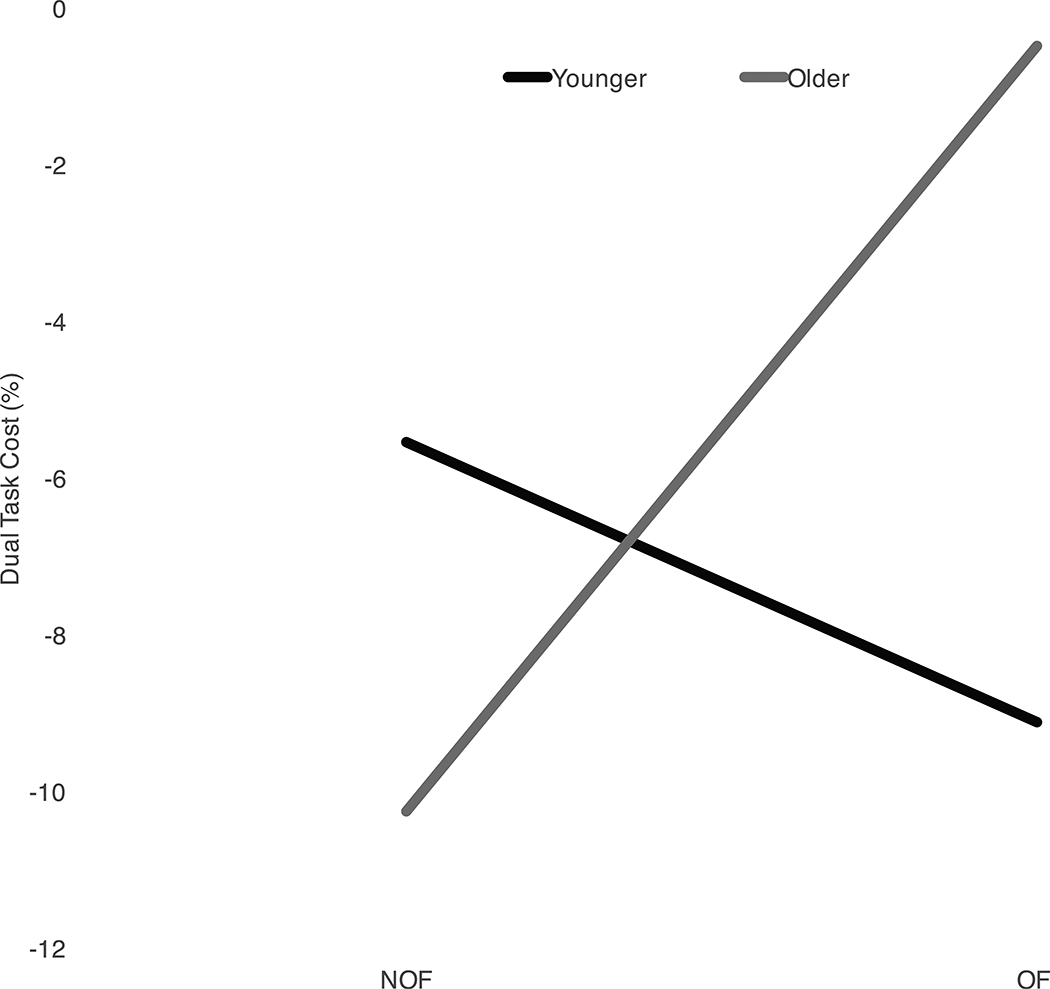
A significant interaction between environment and group was found for DTC of step width while performing the letter fluency task (p=0.038; η2=0.17) (Figure 3). The older adults demonstrated improvement (less cost), whereas, the young adults demonstrated increased cost, from NOF to OF while performing the letter fluency task. Although the interaction was significant, the main effect of optic flow (p=0.53) and group (p=0.40) were not. Young adults had a greater DTC for step width as compared to older adults when performing serial subtractions in both environments (p=0.02; η2=0.22). No main effect of environment was found for the cost of step width during serial subtraction. Additionally, no significant effect of environment nor group was found for step width DTC during categories or reading. There was no significant effect of environment nor group for step length DTC for any of the cognitive tasks.
Fig 3.
The dual task cost (DTC) (−) and benefit (+) in no optic flow (NOF) (A) and optic flow (OF) (B) environments for step width (SW) and step length (SL) for all cognitive tasks compared to baseline walking for healthy young and older subjects. Younger adults had a greater DTC for step width as compared to older adults when performing serial subtractions in both NOF and OF (p=0.02). (C) During the letter fluency task, older adults demonstrated less cost whereas, the young adults demonstrated increased cost due to OF
Discussion
Cost of Environment
The primary aim of this study was to investigate the COE for step length and step width between healthy older and young adults during single and dual tasking. Older adults have multisensory deficits and therefore, it was hypothesized that older adults would have a greater benefit from the OF environment, while young adults’ performance would not change due to OF. The results partially support this hypothesis, step width and step length improved (became narrower and longer, respectively) in older adults while walking in OF, but only for the letter fluency task. A wider step width has been associated with cerebellar disorders [26], falls [27], and fear of falling [28]. Furthermore, longer strides, which are related to longer step lengths, are protective against a meaningful cognitive decline associated with walking speed, an indicator of gait health [29].
Information received from the environment via the visual-sensory system provides information and feedback regarding movements in space and from this, necessary corrections to gait patterns can be made. Older adults may benefit from the additional sensory information OF provides due to their age-related visual and cognitive deficits. When walking overground, OF is generated naturally as we move through the environment and contributes to self-motion [30], obstacle avoidance [31], and navigation [32]. Thus, in the absence of optic flow, one would be missing this information, causing more cognitive resource allocation to determine where they were in space. The older adults in our study appear to have used this information while performing the letter fluency task to improve their step width and length while walking.
The fact that these parameters were improved for the older adults in the OF environment only for the letter fluency task may allude to the nature of the cognitive task performed while walking. The letter fluency task has been associated with the frontal lobes of the brain [33], which in turn is associated with executive function. Many cognitive processes, including allocating attentional resources, inhibition of relative information (providing the words that start with the correct letter and inhibiting those that do not), encoding and retrieval, problem solving, decision making and other goal directed activities are vital activities included under the umbrella of executive function [3]. Additionally, executive function is important in novel tasks for which habitual processes are not already available [3]. Success in the letter fluency task is thought to be well maintained in older adults as it relies more heavily on verbal ability and word knowledge, both of which are preserved in aging [34]. Although accessing information may be slower in older adults, their knowledge is believed to remain unchanged and seems to exceed that of young adults [35].
Dual Task Cost
The second aim of this study was to investigate the DTC of step length and step width between the healthy older and young adults during each of the dual tasks while walking in NOF and OF. It was hypothesized that the older adults would have a greater cost (larger negative values) in both environments when compared to the young adults. Findings from this study do not support this hypothesis. Younger adults, as compared to older adults, experienced a greater cost of step width (i.e., a wider step width) while performing the letter fluency task in OF and when performing the serial subtraction task in both NOF and OF. Opposite of what was found in young adults, older adults experienced a benefit in step width while performing the letter fluency task in both environments.
Verbal ability and word knowledge are crucial to the successful completion of the letter fluency task. According to Vaportzis et al., complexity and difficulty of dual tasks causes different mental operations in young and older adults [36]. Cost of speech production indicates young adults try to use a more complex speech style during dual tasking, increasing word frequency and sentence length [37]. Searching for more complex words and the increased speed of speech could be a potential reason for the increasing step width cost that occurred during the letter fluency task in young adults.
Furthermore, young adults had a greater DTC for step width when performing serial subtractions in both environments. Fedorenko et al. reported that an arithmetic-processing task was more difficult than spatial integration processes because of shared working memory resources with language processing or because of attention-switching costs [38]. Although more impairment was reported with the tasks requiring working memory or attention switching in older adults [35], extended mental arithmetic practice in older adults in comparison with their young counterparts can reduce the deficits of divided attention and attention switching. With today’s technologies, young adults do not have to perform simple mental math frequently, as most older adults have done their whole lives. More practice is reported to be effective in reducing attention demands during dual task [39].
Why did the reading and category tasks show no significant differences between the groups? Task difficulty can affect dual task performance, yet task difficulty is challenging to define. In general, a difficult task is one that is new, unpracticed, and involves multiple processes, rapidly changing sensory input, and an inconsistent or illogical mapping between stimuli and the appropriate responses between them [40]. Regarding the reading task, this is not new to either older adults or young adults as they have been reading for most of their lives. In fact, practice makes tasks easier by themselves and easier to combine with other tasks [40], as is the case in our study with walking and word reading. Category fluency is also not particularly novel. People often group items into categories, whether it be what items to get at the grocery store (i.e., isle by isle, diary, fruits & vegetables) or where things belong (i.e., locations of things in the kitchen, bathroom, etc.). Thus, the commonality of categorizing everyday things is a plausible reason as to why this condition did not show any differences. Further, each of the cognitive tasks measures a different type of cognitive ability. With verbal fluency, this type of task is vulnerable to age-related declines in processing speed due to making it a timed task with a limit. If given unlimited time, this would not be the case, because verbal fluency also represents vocabulary, which is crystallized intelligence, and may increase with age. Less challenging tasks, due to the nature of the task itself or due to common frequency in daily life, could be plausible reasons why age-related differences did not emerge.
Limitations of this study include the small young adult sample. Due to the small sample size, this work could be considered a pilot study. However, all significant findings had a large effect size demonstrating that these findings were fully powered. For variables such as COE while walking only, a total of 28 subjects per group for differences in step width,or 32 subjects per group for differences in step length, would be needed for power at 0.80 with α < 0.05. On the other hand, to have that power for step width COE during categories, 584 subjects per group would be needed. Further, cognitive data results were not reported. These data were available for the older adult subjects however, due to technical difficulties, young adult cognitive data was not recorded. Perhaps the comparison of cognitive data between the two groups could have added to the explanation of differences between groups. Additionally, although a self-paced treadmill removes the speed constraint of a fixed-speed treadmill, overground walking would be the gold standard. Due to the difficulties in presenting cognitive tasks (i.e., reading) while walking overground, a treadmill was needed for this study. Future studies could consider alternative approaches to presenting cognitive tasks while walking overground. Lastly, most of the cognitive tasks used were not representative of everyday tasks (e.g. fluency tasks). In addition, the experiment took place in a laboratory setting; although optic flow was present, the scenery was not like that of everyday life. The generalizability of the findings is likely limited by these factors, but this study is an improvement upon most dual-task study environments. Future studies should aim to test more real-world tasks such as conversations and in a more naturalistic environment, perhaps even using mobile means of virtual reality like headsets and goggles.
Supplementary Material
Acknowledgments
The authors would like to thank Angie Helseth for her assistance in data collection and processing.
Funding: This work was supported by the National Institutes of Health (P20 GM109090 to SAM, JBB, JMY, and R01 HD090333 to SAM) and the University of Nebraska at Omaha Graduate Research and Creative Activity Fund (TL).
Appendix A
Version A
Phonemic (Letter) Fluency: F, L, S
Semantic (Category) Fluency: Animals, Parts of the Body, Kitchen Utensils
Serial Subtractions: 233, 650, 502
Word Reading: indices, hose, lamb, acquisitiveness, equivocal, fountain, corps, pout, subtle, détente, casserole, debt, browbeat, asked, statistics, epitome, shield, chord, flower, subpoena, banal, chrysanthemum, rarefy, diverse, irrelevant, generate, bell, eccentricities, bouquet, thyme
Version B
Phonemic (Letter) Fluency: W, G, T
Semantic (Category) Fluency: Sports, Furniture, Supermarket Items
Serial Subtractions: 805, 297, 483
Word Reading: asphyxiation, doll, paradigm, simile, reify, gauge, recipe, procreate, fifth, magnanimity, gouge, naïve, idyll, machine, hospital, sieve, youth, colony, cloud, façade, indict, confide, amicable, synthesis, deny, epic, fly, labile, crayon, clasped
Version C
Phonemic (Letter) Fluency: M, B, R
Semantic (Category) Fluency: Professions, Fruits & Vegetables, Things you Wear
Serial Subtractions: 738, 369, 922
Word Reading: ache, aisle, ballad, bibliography, caveat, cellist, colonel, courteous, curtain, debris, depot, efficacy, evolve, gigantic, gist, heir, hiatus, joker, ladder, lily, lingerie, nausea, placebo, pony, psalm, pugnacious, stranger, superfluous, yesterday, zealot
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: All authors declare they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged >/=65 years - united states, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(37):993–998. doi: 10.15585/mmwr.mm6537a2 [doi]. [DOI] [PubMed] [Google Scholar]
- 2.Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: The role of aging, falls, and executive function. Mov Disord. 2006;21(7):950957. doi: 10.1002/mds.20848 [doi]. [DOI] [PubMed] [Google Scholar]
- 3.Glisky EL. Changes in cognitive function in human aging In: Riddle DR, ed. Brain aging: Models, methods, and mechanisms. Boca Raton, FL: CRC Press/Taylor & Francis; 2007. [PubMed] [Google Scholar]
- 4.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture. 2002;16(1):1–14. doi: S0966636201001564 [pii]. [DOI] [PubMed] [Google Scholar]
- 5.Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997;349(9052):2. doi: S0140–6736(97)24009–2 [pii]. [DOI] [PubMed] [Google Scholar]
- 6.Walshe EA, Patterson MR, Commins S, Roche RA. Dual-task and electrophysiological markers of executive cognitive processing in older adult gait and fall-risk. Front Hum Neurosci. 2015;9:200. doi: 10.3389/fnhum.2015.00200 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: Increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15(3):417–436. [DOI] [PubMed] [Google Scholar]
- 8.Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. The journals of gerontology. Series A, Biological sciences and medical sciences. 2011;66(8):879–887. http://www.ncbi.nlm.nih.gov/pubmed/21593013. doi: 10.1093/gerona/glr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GIBSON JJ. Visually controlled locomotion and visual orientation in animals. Br J Psychol. 1958;49(3):182–194. [DOI] [PubMed] [Google Scholar]
- 10.Sloot LH, van der Krogt MM, Harlaar J Effects of adding a virtual reality environment to different modes of treadmill walking. Gait Posture. 2014;39(3):939–945. doi: 10.1016/j.gaitpost.2013.12.005 [doi]. [DOI] [PubMed] [Google Scholar]
- 11.Levy F, Leboucher P, Rautureau G, Komano O, Millet B, Jouvent R. Fear of falling: Efficacy of virtual reality associated with serious games in elderly people. Neuropsychiatric disease and treatment. 2016;12:877–881. http://www.ncbi.nlm.nih.gov/pubmed/27143889. doi: 10.2147/NDT.S97809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parijat P, Lockhart TE, Liu J. Effects of perturbation-based slip training using a virtual reality environment on slip-induced falls. Annals of biomedical engineering. 2015;43(4):958 http://www.ncbi.nlm.nih.gov/pubmed/25245221. doi: 10.1007/s10439-014-1128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wechsler D Manual for the wechsler adult intelligence scale-revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 14.Schaie KW. Schaie-thurstone adult mental abilities test (STAMAT). Palo Alto, California: Consulting Psychologists Press; 1985. [Google Scholar]
- 15.Nasreddine ZS, Phillips NA, Bedirian V, et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: JGS53221 [pii]. [DOI] [PubMed] [Google Scholar]
- 16.REITAN RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19(5):393–394. [DOI] [PubMed] [Google Scholar]
- 17.Podsiadlo D, Richardson S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez D, Rose DJ. Predicting which older adults will or will not fall using the fullerton advanced balance scale. Arch Phys Med Rehabil. 2008;89(12):2309–2315. doi: 10.1016/j.apmr.2008.05.020 [doi]. [DOI] [PubMed] [Google Scholar]
- 19.Wiens C, Denton W, Schieber M et al. Reliability of a feedback-controlled treadmill algorithm dependent on the user’s behavior. Proc IEEE Int Conf Electro Inf Technol. (2017) 10.1109/EIT.2017.8053423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: Evidence from younger and older healthy adults. Neuropsychology. 1997;11(1):138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- 21.Troyer AK. Normative data for clustering and switching on verbal fluency tasks. Journal of Clinical and Experimental Neuropsychology. 2000;22(3):370–378. doi: 3;1-V;FT370. [DOI] [PubMed] [Google Scholar]
- 22.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14(2):167–177. doi: S0887617797000954 [pii]. [PubMed] [Google Scholar]
- 23.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5(2):+. doi: 10.1016/0028-3932(67)90015-2. [DOI] [Google Scholar]
- 24.Uttl B North american adult reading test: Age norms, reliability, and validity. J Clin Exp Neuropsychol. 2002;24(8):1123–1137. doi: 10.1076/jcen.24.8.1123.8375 [doi]. [DOI] [PubMed] [Google Scholar]
- 25.Balota DA, Yap MJ, Cortese MJ, et al. The english lexicon project. Behav Res Methods. 2007;39(3):445–459. [DOI] [PubMed] [Google Scholar]
- 26.Diener HC, Nutt JG. Vestibular and cerebellar disorders of equilibrium and gait In: Masdeu JC, Sudarsky L, Wolfson L, eds. Gait disorders of aging: Falls and therapeutic strategies. Philadelphia: Lippincott-Raven; 1997:261–272. [Google Scholar]
- 27.Gehlsen GM, Whaley MH. Falls in the elderly: Part I, gait. Arch Phys Med Rehabil. 1990;71(10):735–738. [PubMed] [Google Scholar]
- 28.Chamberlin ME, Fulwider BD, Sanders SL, Medeiros JM. Does fear of falling influence spatial and temporal gait parameters in elderly persons beyond changes associated with normal aging? J Gerontol A Biol Sci Med Sci. 2005;60(9):1163–1167. doi: 60/9/1163 [pii]. [DOI] [PubMed] [Google Scholar]
- 29.Jerome GJ, Ko SU, Kauffman D, Studenski SA, Ferrucci L, Simonsick EM. Gait characteristics associated with walking speed decline in older adults: Results from the baltimore longitudinal study of aging. Arch Gerontol Geriatr. 2015;60(2):239–243. doi: 10.1016/j.archger.2015.01.007 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrone JA, Stone LS A model of self-motion estimation within primate extrastriate visual cortex. Vis Res (1994) 34(21):2917–2938. 10.1016/0042-6989(94)90060-4 [DOI] [PubMed] [Google Scholar]
- 31.Sun HJ, Carey DP, Goodale MA. A mammalian model of optic-flow utilization in the control of locomotion. Exp Brain Res. 1992;91(1):171–175. [DOI] [PubMed] [Google Scholar]
- 32.Warren WH, Kay BA, Zosh WD, Duchon AP, Sahuc S. Optic flow is used to control human walking. Nat Neurosci. 2001;4(2):213–216. doi: 10.1038/84054 [doi]. [DOI] [PubMed] [Google Scholar]
- 33.Coslett HB, Bowers D, Verfaellie M, Heilman KM. Frontal verbal amnesia. phonological amnesia. Arch Neurol. 1991;48(9):949–955. [DOI] [PubMed] [Google Scholar]
- 34.Hughes DL, Bryan J. Adult age differences in strategy use during verbal fluency performance. J Clin Exp Neuropsychol. 2002;24(5):642–654. doi: 10.1076/jcen.24.5.642.1002 [doi]. [DOI] [PubMed] [Google Scholar]
- 35.Riddle DR. Brain aging. Boca Raton: CRC Press; 2007. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=frbrainage. [Google Scholar]
- 36.Vaportzis E, Georgiou-Karistianis N, Stout JC. Dual task performance in normal aging: A comparison of choice reaction time tasks. PLoS One. 2013;8(3):e60265. doi: 10.1371/journal.pone.0060265 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemper S, Schmalzried R, Hoffman L, Herman R. Aging and the vulnerability of speech to dual task demands. Psychol Aging. 2010;25(4):949–962. doi: 10.1037/a0020000 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fedorenko E, Gibson E, Rohde D. The nature of working memory in linguistic, arithmetic and spatial integration processes. Journal of Memory and Language. 2007;56(2):246–269. doi: 10.1016/j.jml.2006.06.007. [DOI] [Google Scholar]
- 39.Kramer AF, Larish JL, Weber TA et al. Training for executive control: task coordination strategies and aging In: Gopher D, Koriat A (eds) Attention and performance XVII. Cambridge, MIT Press, (1999) pp 616–652 [Google Scholar]
- 40.Andrade J, May J BIOS instant notes in cognitive psychology, 1st edn. London: CRC Press, (2003) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.