Abstract
Background and Aims
Children with very early onset inflammatory bowel disease [VEO-IBD] represent a unique cohort, often with a severe phenotype that is refractory to conventional medications, and some cases have underlying primary immunodeficiencies. Previous work has identified distinct histopathological patterns in the gastrointestinal tract in patients with primary immunodeficiencies. The aim of this study is to characterise the diagnostic histological findings in patients with VEO-IBD as compared with older onset paediatric IBD, and determine if there are unique pathological changes that can shed light on the driving forces of the disease, particularly immunodeficiencies.
Methods
Clinical retrospective chart review, including disease characteristics and endoscopic findings, was performed on all included subjects. Two paediatric pathologists reviewed biopsies from diagnostic upper endoscopies and colonoscopies of subjects with very early onset inflammatory bowel disease and older onset inflammatory bowel disease, to evaluate for the presence of 11 histological features previously associated with inflammatory bowel disease and primary immunodeficiencies.
Results
The diagnostic gastrointestinal biopsies of subjects with very early onset inflammatory bowel disease differed from those in older onset paediatric IBD, demonstrated by increased frequency of apoptosis, severe chronic architectural changes, small intestine villous blunting, and eosinophils in the crypts, lamina propria, and surface epithelium.
Conclusions
The diagnostic biopsies of children with very early onset inflammatory bowel disease can identify characteristic features that may be important in guiding the diagnostic work-up in this population.
Keywords: Paediatric inflammatory bowel disease, diagnostic pathology, immunodeficiency
1. Background
Inflammatory bowel disease [IBD] is a chronic intestinal inflammatory disorder that is the multifactorial result of a dysregulated immune response to environmental exposures in a genetically susceptible host. The genetic contribution to the disease in older paediatric patients and adults is most often polygenic, involving over 200 risk loci that include over 300 genes identified through genome-wide association studies.1,2 In contrast, genetic studies performed in patients with very early onset IBD [VEO-IBD], diagnosed at <6 years of age, have identified causative monogenic or digenic defects, often involving genes associated with primary immunodeficiencies [PID].3–5 Possibly as a consequence of the unique aetiology of the disease, a subset of patients with VEO-IBD have a more severe and extensive phenotype than older onset IBD. Choosing the optimal therapy for these children can be challenging, especially given the almost inevitable delay in the determination of the underlying causative defect or, more often, in cases in which a defect is not identified.6–8 Therefore, all elements of the diagnostic evaluation must be utilised when choosing therapy, particularly those patients with marked immune dysregulation and aggressive disease.
Our earlier work, using whole-exome sequencing [WES], has demonstrated an enrichment of genes associated with PID in patients with VEO-IBD.9 Although the literature on the histological features of VEO-IBD is rather limited, pathologists have previously identified specific patterns of intestinal involvement on histopathology in patients with PID and immune dysregulation. The characteristic histological features of PID include epithelial apoptosis, lack of plasma cells, various degrees of small intestinal villous blunting, granulomas, and eosinophils.10–12 These distinct findings within the gastrointestinal tract may provide clues to an underlying immunodeficiency or epithelial intrinsic defect that is unique to patients with VEO-IBD. The purpose of this study is to describe the diagnostic histopathological findings in patients with VEO-IBD as compared with older children with IBD. We hypothesise that there will be findings more frequently present at diagnosis in children with VEO-IBD which are reflective of an underlying immune deficiency or dysregulation. These findings may shed light on the driving forces of VEO-IBD.
This study is the first to include a comprehensive description of the microscopic features of the diagnostic biopsies obtained from a subset of VEO-IBD subjects as compared with older onset paediatric IBD. Ultimately, these data could lead to a more focused diagnostic evaluation and could assist in guiding the clinician in identifying the underlying disease process and, subsequently, directed therapy for the patient.
2. Methods
A retrospective clinical and histological review of patients with newly diagnosed IBD and naïve to IBD treatment at our institution between 1998 and 2016 was performed. This study was approved by the Children’s Hospital of Philadelphia Institutional Review Board. There were two cohorts included: VEO-IBD defined for the purposes of this study by diagnosis of chronic intestinal inflammatory disease of any known or unknown aetiology before the age of 6 years, and older onset paediatric IBD group diagnosed at ≥6 years of age. Inclusion criteria for this study required a confirmed diagnosis of IBD by a paediatric gastroenterologist determined by clinical history, esophagogastroduodenoscopy and colonoscopy, and radiological examinations, with biopsy slides from the diagnostic procedure available for histopathological review by the pathologists in this study. Crohn’s disease was diagnosed when any of the following features were present in the diagnostic evaluation: granulomas, fistulising disease, ileal inflammation in the presence of a normal caecum, thickened small bowel loops, skip lesions, or perianal disease. Ulcerative colitis was diagnosed in cases of diffuse continuous mucosal ulceration of varying severity extending through the colon proximally from the rectum. Inflammatory bowel disease unspecified [IBD-U] was diagnosed in cases consistent with ulcerative colitis but with least one of the following features: rectal sparing, macroscopic duodenal or oesophageal ulcers without comorbidities, or numerous gastric aphthous lesions without additional aetiology. In addition, IBD-U was diagnosed in children with aphthous ulcers in the colon without additional findings.13,14
Additional inclusion criteria to the VEO-IBD group included completed genetic evaluation using whole-exome sequencing. Exclusion criteria were: subjects who did not have their diagnostic pathology reviewed at the Children’s Hospital of Philadelphia; or subjects with concurrent intestinal comorbidity including positive celiac serology and histology, Hirschsprung disease, previous diagnosis of eosinophilic oesophagitis, preceding diagnosis of immunodeficiency, short bowel syndrome, or infection.
Demographic data were obtained from the electronic medical records including sex, age at diagnosis, initial IBD diagnosis, Paris classification based on diagnostic endoscopic evaluation, and anatomical location of disease. Macroscopic findings were recorded by retrospective review of endoscopic reports, in order to ascertain presence of haemorrhagic mucosa, linear ulceration and cobblestoning, aphthous ulcers, ileitis or ileal ulcers, pseudopolyps, narrowing or stenosis of the colon or terminal ileum, colitis with rectal sparing, oesophagitis, gastritis or gastric ulcers, and duodenitis or duodenal ulcers.
2.1. Histopathology
Of the VEO-IBD and older onset paediatric IBD subjects meeting criteria for this study, the original haematoxylin and eosin [H&E]-stained slides and accompanying pathology reports were retrieved and reviewed by two pathologists separately, followed by a joint review for the final diagnosis and assignment of grading. Depending on the extent of evaluation performed at the time of endoscopy, the biopsy set consisted of specimens that were collected from the following sites: oesophagus, gastric antrum, gastric body, duodenum, terminal ileum, right colon, transverse colon, left colon, sigmoid colon, and rectum. A full set of biopsies had representative tissue from the oesophagus, stomach, and small and large intestines, but cases with specimens limited to the large intestine only were included as well. These cases were only included in colonic analyses and excluded from all small intestinal [i.e. assess villous blunting] and pan-enteric analyses.
We identified 11 histological features, previously described as classic features of IBD, as well as findings associated with immunodeficiency, which were incorporated into the evaluation of each case.12,15,16 The histological features included: active inflammation [neutrophil-predominant cryptitis and crypt micro-abscesses], chronic architectural changes, apoptosis, granulomas, lymphoid aggregates, intraepithelial lymphocytes, plasma cells infiltrates in the lamina propria, eosinophils in the lamina propria or crypts or surface epithelium, mucus and goblet cell depletion, small bowel villous blunting, and colonic villous surface transformation [Table 1]. Any distinctive features aside from the previously mentioned list were also recorded.
Table 1.
Definition and classification of histological description of intestinal biopsies.
| Active inflammation: presence of neutrophils | |
| 0 | No active inflammation/absent neutrophils |
| 1 | Mild; neutrophils limited to lamina propria or isolated cryptitis |
| 2 | Moderate; cryptitis with crypt abscesses |
| 3 | Severe; diffuse active inflammation with ulceration or granulation tissue |
| Crypt architectural distortion: branching, elongated, shortened, or atrophic crypts, defined as lack of space between bottom of the crypts and upper edge of muscularis mucosae | |
| 0 | None |
| 1 | Mild; focal and mild distorted crypts |
| 2 | Moderate; areas of crypt distortion alternating with more normal areas |
| 3 | Severe; diffuse crypt architectural distortion |
| Apoptosis in crypt epithelium [or dyskeratotic cells if in oesophagus] | |
| 0 | Absent |
| 1 | Present but not increased [<1 apoptotic figure/10 crypts] |
| 2 | Increased [>1 apoptotic figure/10 crypts] |
| Eosinophilic inflammation | |
| 0 | Absent |
| 1 | Present within normal limits in the lamina propria |
| 2 | Increased in lamina propria [>1/hpf in the oesophagus; >5/hpf in the stomach; >15/hpf in the duodenum; >18/hpf in the ileum; >29/hpf in the caecum; >22/hpf in the transverse colon; and >14/hpf in the sigmoid colon] |
| 3 | Increased in lamina propria as above with eosinophil crypt abscesses |
| 4 | Increased in lamina propria as above and involving surface epithelium |
| Plasma cell infiltrates | |
| 0 | Absent |
| 1 | Present with normal distribution in the lamina propria |
| 2 | Plasma cells in dense clusters or sheet-like pattern of distribution with associated displacement of glandular crypts |
| Intraepithelial lymphocytes | |
| 0 | Absent |
| 1 | Normal; [10–20 lymphocytes per 100 epithelial cells in the stomach; 20–40 lymphocytes per 100 epithelial cells in the small intestine; and 5–10 lymphocytes per 100 epithelial cells in the large intestine] |
| 2 | Increased; [>20 lymphocytes per 100 intraepithelial cells in the stomach; >40 lymphocytes per 100 intraepithelial cells in the small intestine; > 10 lymphocytes per 100 intraepithelial cells in the large intestine] |
| Lymphoid aggregates | |
| 0 | None |
| 1 | Aggregates of lymphocytes distorting crypts |
| Granulomas | |
| 0 | None |
| 1 | Sarcoid-like granulomas in uninflamed areas, separate from damaged or ruptured crypts |
| Small intestinal villous blunting | |
| 0 | Normal-appearing villi |
| 1 | Flattening of villi |
| Surface mucus/goblet cell depletion | |
| 0 | Normal distribution of goblet cells in surface epithelium |
| 1 | Loss of goblet cells in surface epithelium |
| Colonic villous surface transformation | |
| 0 | No transformation |
| 1 | Presence of villous surface transformation |
HPF, high power field.
Validated histological grading systems for paediatric inflammatory bowel disease do not currently exist. The grading of the inflammatory and architectural changes in this study was adapted from published studies, some involving mainly adult patients, others more specifically directed towards paediatric patients.16–19 For the remainder of the biopsy characteristics, we designed a grading system for the purpose of standardisation for this study as summarised in Table 1.
An interim preliminary statistical analysis was conducted after completion of review of the first 39 VEO-IBD subjects and 22 subjects with older onset IBD. This revealed that of the features we studied, the following histological features were consistently present and/or among these features there was a statistically significant difference between the two study groups: active inflammation, chronic architectural changes, plasma cells, crypt epithelium apoptosis, eosinophils, small intestinal villous blunting, and granulomas. The subsequent review of the remainder of biopsies from an additional 18 VEO-IBD and 35 older onset IBD subjects thus focused on this subset of seven features.
The findings were tabulated and the differences between the two groups were analysed statistically using Fisher’s exact test [p <0.05].
2.2. Whole-exome sequencing in VEO-IBD subjects
Whole-exome sequencing was performed on the 57 subjects with VEO-IBD as outlined in our earlier publication.9 The older onset IBD subjects did not undergo WES.
2.3. Immunohistochemistry
Biopsies of five VEO-IBD subjects with known B cell defects, based on their immunophenotyping and candidate genetic variant detected by WES, were further analysed by the pathologists. A more focused evaluation of plasma cells and B cell maturation by morphology [H&E] and immunohistochemistry was performed using the following immunostains: CD79a, Kappa light chain, Lambda light chain, IgG, IgA, and IgM.
3. Results
A total of 230 subjects with VEO-IBD were identified, and 57 subjects met criteria to be included in this study. The median age of diagnosis was 2.2 years [1.3, 3.4]. Of these 57 subjects with VEO-IBD, 39 [68%] had been diagnosed with Crohn’s disease, 16 [28%] with inflammatory bowel disease unspecified [IBD-U], and 2 [4%] with ulcerative colitis based on the primary gastroenterologist’s interpretation of the clinical, endoscopic, histological, and radiological data at the time of diagnosis during the study window [Table 2]. Of the 742 older onset IBD subjects, 58 met inclusion criteria for this study. The median age of diagnosis was 13.0 years [9.5, 13.6]. Of these 58 subjects, 44 [76%] had Crohn’s disease, five [9%] had IBD-U, and nine [15%] had ulcerative colitis.
Table 2.
Patient characteristics and Paris classification for paediatric inflammatory bowel disease.
| VEO-IBD [n = 57] | Older onset IBD [n = 58] | |
|---|---|---|
| Male, n [%] | 40 [70] | 40 [69] |
| Age at diagnosis, median, yr [IQR] | 2.2 [1.3, 3.4] | 13.0 [9.5, 13.6] |
| A1a 0–<10 yr, n | 57 | 5 |
| A1b 10–<17 yr, n | 0 | 53 |
| IBD subtype, n [%] | ||
| Crohn’s disease | 39 [68] | 44 [76] |
| IBD-unclassified | 16 [28] | 5 [9] |
| Ulcerative colitis | 2 [4] | 9 [15] |
| Ulcerative colitis/IBD-U extent of disease | ||
| E1: Ulcerative proctitis | 0 [0] | 1 [7] |
| E2: Left-sided colitis | 6 [33] | 0 |
| E3: Extensive | 3 [17] | 1 [7] |
| E4: Pancolitis | 9 [50] | 12 [86] |
| Crohn’s disease characteristics | ||
| Lower GI involvement, n [%] | ||
| L1: Distal 1/3 Ileum/limited caecal disease | 0 [0] | 4 [9] |
| L2: Colonic only | 24 [49] | 8 [18] |
| L3: Ileocolonic | 7 [44] | 19 [43] |
| None | 3 [8] | 0 [0] |
| Upper GI involvement, n [%] | ||
| L4a: Upper disease proximal to ligament of Treitz [LOT] | 19 [49] | 11 [25] |
| L4b: Upper disease distal to LOT and proximal to distal 1/3 ileum | 5 [13] | 19 [43] |
| Behaviour phenotype, n [%] | ||
| B1: Non-stricturing, non-penetrating | 31 [80] | 31[70] |
| B2: Stricturing | 4 [10] | 7 [16] |
| B3: Penetrating | 4 [10] | 4 [9] |
| B4: Both Stricturing and penetrating | 0 [0] | 2 [5] |
| Perianal involvement, n [%] | 14 [36] | 5 [11] |
VEO-IBD, very early onset inflammatory bowel disease; yr, years; IQR, interquartile range.
3.1. Endoscopic findings
Disease location and behaviour for both study groups are described in Table 2. The most common macroscopic findings among VEO-IBD subjects were haemorrhagic mucosa, cobblestoning with linear ulcers, and duodenitis or duodenal ulcers. [Table 3] There were significantly more VEO-IBD subjects [27/57] with haemorrhagic mucosa present than older onset IBD subjects [14/58, p = 0.011]. Visual ileitis was described in the endoscopic reports more frequently in the older onset IBD subjects [14/58] than in the VEO-IBD subjects [5/57, p = 0.042] [Table 3]. In addition, the majority of the older onset Crohn’s disease subjects had ileocolonic disease, which differed from the predominantly colonic VEO-IBD [Table 2].
Table 3.
Macroscopic findings during diagnostic endoscopy.
| VEO-IBD | Older onset IBD | |||||
|---|---|---|---|---|---|---|
| Macroscopic findings | Crohn’s disease [n = 39] | IBD-U[n = 16] | Ulcerative colitis [n = 2] | Crohn’s disease[n = 41] | IBD-U [n = 5] | Ulcerative colitis [n = 9] |
| Haemorrhagic mucosa | 18 | 8 | 1 | 4 | 2 | 8 |
| Cobblestoning and linear ulcers | 14 | 5 | 1 | 15 | 2 | 3 |
| Pseudopolyps | 2 | 1 | 0 | 1 | 0 | 1 |
| Ileitis | 5 | 0 | 0 | 14 | 0 | 0 |
| Terminal Ileum narrowing/stricture | 2 | 0 | 0 | 8 | 0 | 0 |
| Other narrowing stricture | 0 | 0 | 0 | 1 | 0 | 0 |
| Colitis with rectal sparing | 2 | 2 | 0 | 8 | 0 | 0 |
| Oesophagitis | 3 | 0 | 0 | 1 | 0 | 1 |
| Gastritis/gastric ulcer | 8 | 2 | 0 | 5 | 2 | 2 |
| Duodenitis/duodenal ulcer | 7 | 4 | 0 | 3 | 0 | 2 |
VEO-IBD, very early onset inflammatory bowel disease; IBD-U, IBD unspecified.
In the VEO-IBD cohort, there were 10/57 subjects with monogenic defects, identified through WES, included in this analysis. The endoscopic findings in 5/10 of these subjects included cobblestoning with linear ulcers in the colon, and 4/10 had duodenitis. Despite these trends, there were no statistically significant differences in frequency of any of the described macroscopic findings between VEO-IBD subjects with identified monogenic defect and the other VEO-IBD subjects.
3.2. Histopathological features that are common to both VEO-IBD and older onset IBD
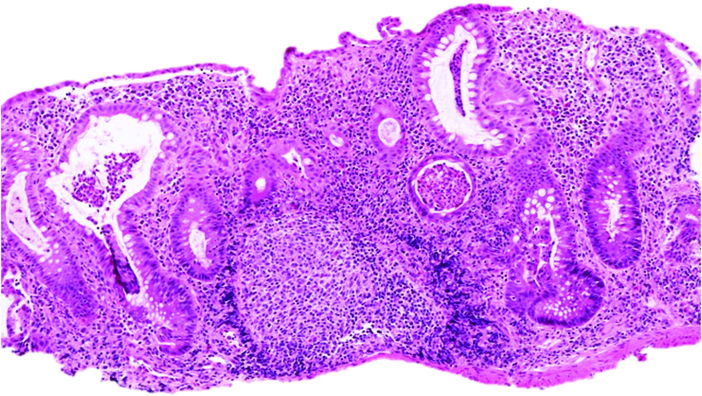
Review of pathology slides revealed that there were similar rates of severe active inflammation among the two groups. Moderate to severe findings of chronic architectural changes were seen more often in VEO-IBD as compared with the older onset group [70% vs 32%, p <0.001] [Figure 1]. Additionally, granulomas were present in VEO-IBD and older onset IBD at similar rates [30% vs 35%, p = 0.07].
Figure 1.
Colonic biopsy of a 2-year-old female demonstrating multiple crypt micro-abscesses and marked architectural distortion [haematoxylin-eosin, original magnification x 100].
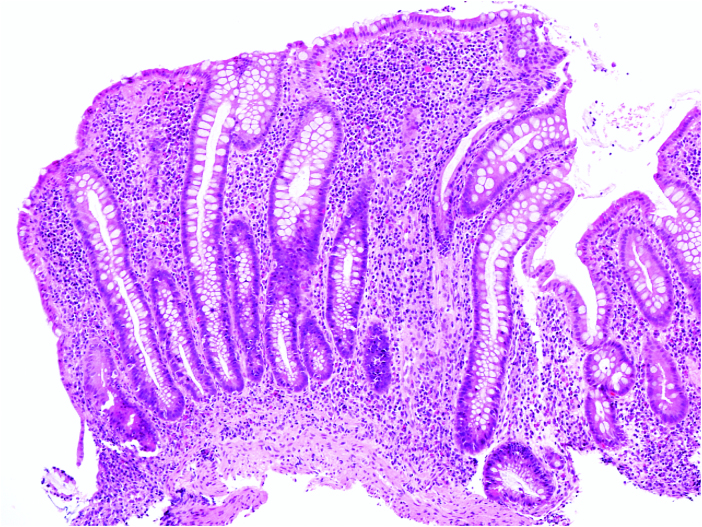
Among the VEO-IBD subjects with active inflammation in the colon, there was a mixed inflammatory cell infiltrate including plasma cells, lymphocytes, monocytes, and eosinophils, of varying proportions, in addition to neutrophils that define active inflammation. In some cases of VEO-IBD, the influx of inflammatory cells significantly expanded the lamina propria, imparting a ‘vertically stretched’ or taller appearance of the mucosa [Figure 2]. The severe active inflammation was often accompanied by significant architectural distortion even at a very young age at diagnosis. In rare cases, obvious architectural distortion was also observed even with a less severe active inflammation.
Figure 2.
Colonic biopsy of a 5-year-old male demonstrating a relatively taller appearance of the mucosa with elongation of the crypts and an expanded highly cellular lamina propria [haematoxylin-eosin, original magnification x 100].
Lymphoid aggregates were found universally in all subjects. Additionally, there were no significant differences in mucus depletion or colonic villous transformation between the two groups.
3.3. Distinct histopathological findings in VEO-IBD
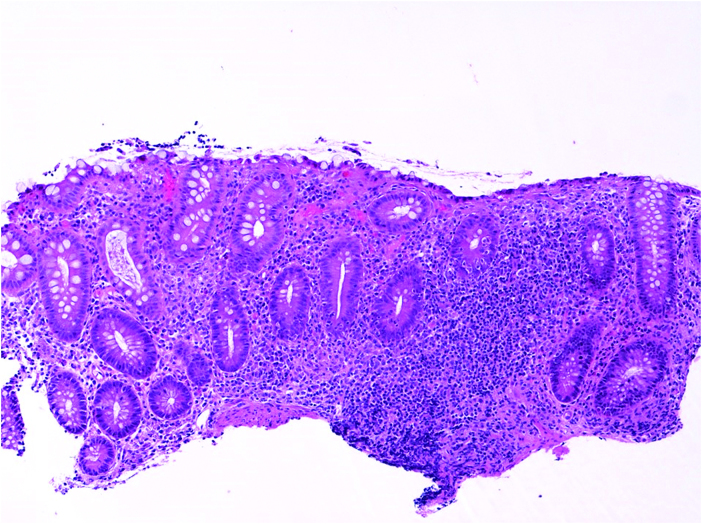
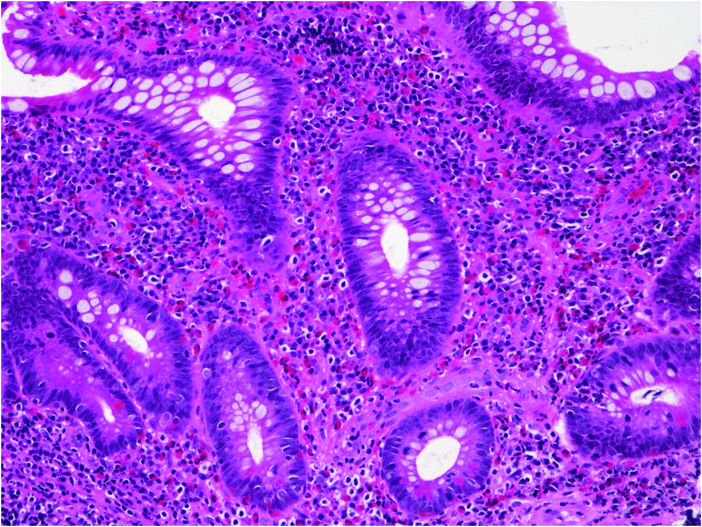
Features that were detected more frequently in VEO-IBD, as compared with older onset IBD, included apoptosis [53% vs 28%, p <0.05] [Figure 3] and villous blunting [Figure 4] [40% vs 16%, p <0.01]. Further analyses of the subjects with villous blunting demonstrated that although there was associated active inflammation at the site in all the older onset IBD cases, 30% of the VEO-IBD subjects with blunting had no inflammation associated at that same site. Another distinguishing feature was eosinophilia. The VEO-IBD cohort showed more prominent and frequently diffuse eosinophilic infiltration of the mucosa with involvement of the crypt, lamina propria, and surface epithelium [p <0.001] [Figure 5] compared with older onset IBD. In some cases, eosinophils were noted as a component of crypt micro-abscesses, and scattered eosinophilic granules were observed in areas where dense aggregates of eosinophils were present [Figure 3].
Figure 3.
A rectal biopsy from a 5-month-old male with confirmed monogenic mutation causative for VEO-IBD demonstrating abundant apoptosis and scattered eosinophils in the lamina propria. Eosinophilic granules are also present within the lumens of the crypts [haematoxylin-eosin, original magnification x 600]. VEO-IBD, very early onset inflammatory bowel disease.
Figure 4.
Small bowel biopsy of a 2-year-old male demonstrating severe villous blunting [haematoxylin-eosin, original magnification x 100].
Figure 5.
Colonic biopsy of a 10-month-old male infant demonstrating marked eosinophilia in the lamina propria [haematoxylin-eosin, original magnification x 200].
Among these 11 histological features evaluated, there were no significant differences in the presence of any of these findings between VEO-IBD with monogenic defects and those without monogenic defects. The presence of these findings among monogenic VEO-IBD as well as by IBD diagnosis in non-monogenic VEO-IBD and older onset paediatric IBD are described in Table 4.
Table 4.
Histological findings during diagnostic endoscopy. Data are presented as n [%].
| VEO-IBD | Older onset IBD | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Histological findings | Monogenic[n = 11] | Non-monogenic [n = 46] | Crohn’sdisease[n = 44] | IBD-U [n = 4] | Ulcerative colitis [n = 9] | Monogenic vs Non-Monogenic VEO-IBD | Monogenic VEO-IBD vs Older Onset | Non-Monogenic VEO-IBD vs Older Onset | ||
| Crohn’s disease [n = 31] | IBD-U[n = 13] | Ulcerative colitis [n = 2] | ||||||||
| Granulomas | 1 [9.1] | 15 [48.4] | 1 [7.7] | 0 | 19 [45.2] | 0 | 0 | NS | NS | NS |
| Moderate-severe chronic architectural changes | 8 [72.7] | 21 [67.7] | 10 [76.9] | 1 [50] | 9 [20.5] | 2 [50] | 7 [77.8] | NS | <0.05 | <0.001 |
| Increased apoptosis | 4 [36.4] | 19 [61.3] | 5 [38.5] | 2 [100] | 10 [22.7] | 3 [75] | 3 [33.3] | NS | NS | <0.01 |
| Eosinophils in epithelium, lamina propria, and crypts | 3 [27.3] | 9 [29] | 7 [53.8] | 2 [100] | 0 | 0 | 1 [11.1] | NS | <0.05 | <0.0001 |
| Small intestinal villous blunting | 3 [42.9] | 15 [53.6] | 2 [15.4] | 0 | 7 [17.9] | 1 [33.3] | 0 | NS | NS | <0.05 |
| Moderate-severe active inflammation | 9 [81.8] | 26 [83.9] | 13 [100] | 2 [100] | 32 [72.7] | 4 [100] | 7 [77.8] | NS | NS | NS |
| Increased intraepithelial lymphocytes with villous blunting | 2 [18.2] | 13 [41.9] | 0 | 0 | 17 [38.6] | 1 [25] | 1 [11.1] | NS | NS | NS |
VEO-IBD, very early onset inflammatory bowel disease; IBD-U, IBD unspecified; NS, non-significant.
3.4. Plasma cell and B cell analysis
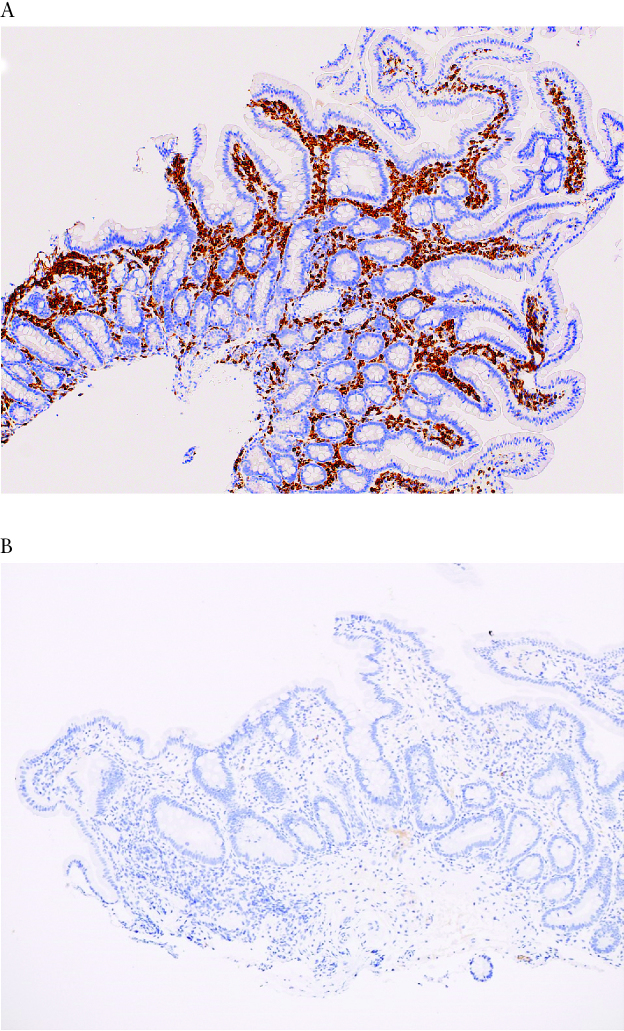
Plasma cells were generally increased in the gastric and intestinal biopsies of both groups [p >0.05]. Interestingly, a subset of five VEO-IBD patients with suspected B cell defects, based on abnormal immunological evaluation, did not show absence or paucity of plasma cells on initial review. However in one case of VEO-IBD with hypogammaglobulinaemia, who was found to have a monogenic defect in ZBTB24, subsequent immunostaining using CD79a as a marker for B cells showed a marked decrease in plasma cell numbers, with almost complete absence of IgA-staining plasma cells. In addition, there was markedly decreased staining for IgG, IgM, and kappa and lambda light chains in this subject as compared with other VEO-IBD subjects without a B cell defect or known monogenic aetiology.20 [Figure 6A and B].
Figure 6.
A: Duodenal biopsy from a VEO-IBD patient, with normal B cell function and no known genetic aetiology, shows abundant plasma cells in the lamina propria as highlighted by a CD79a immunostain [immunohistochemistry, original magnification x 100]. B: Case of VEO-IBD with monogenic defect in ZBTB24: Immunohistochemical stain for IgA shows absence of IgA-producing plasma cells in the same duodenal biopsy [immunohistochemistry, original magnification × 100]. In a normal duodenal biopsy, IgA is the predominant immunoglobulin produced by plasma cells in the intestinal mucosa. Similar patterns were seen with immunohistochemical stains for IgG and IgM. VEO-IBD, very early onset inflammatory bowel disease.
4. Discussion
Very early onset inflammatory bowel disease is a heterogeneous disease, both phenotypically and genetically. The young age of onset suggests a strong genetic component, and indeed there is a unique enrichment of rare genetic variants in immune-related pathways in a subset of these children. Advances in molecular genetics have expanded our ability to identify those causative variants and to understand their pathophysiological role in IBD. Nevertheless, despite these advances, a causative genetic defect has been identified in only a fraction of these cases, and only very rarely identified at diagnosis. Thus an integrated comprehensive diagnostic evaluation is critical in order to direct therapy for the majority of children with VEO-IBD. Critical among these studies is the initial histopathological assessment of the gastrointestinal biopsies. This analysis can help identify features that suggest an immune dysregulation or deficiency in the very young children in whom allergy or infection were initially suspected, indicating that further testing, including genetics, is warranted.
Thus far, in contrast to the histopathology, endoscopic findings have not been used to distinguish VEO-IBD from other age groups. In this study, the majority of subjects of all ages had Crohn’s disease, but a considerable number of cases [28%] of IBD-U were diagnosed in the VEO-IBD population. Early in the course of disease, it can be difficult to predict progression of isolated colonic disease or colonic disease with non-specific upper tract findings. At our large paediatric institution, this observation has led to frequent diagnoses of IBD-U instead of typical or atypical ulcerative colitis, especially when the subjects are diagnosed very young.
Whereas there was not a specific signature identified on the endoscopic review, we did identify a histological signature of VEO-IBD in the diagnostic gastrointestinal biopsies as compared with older onset paediatric IBD. Microscopic features of severe disease were detected more frequently in the colonic biopsies of VEO-IBD group as compared with the older onset group. We also saw findings that are characteristic of PID in the VEO-IBD cohort, which should prompt an expansive investigation in these patients. These features include moderate to severe chronic architectural changes, increased eosinophils within the lamina propria, crypts, and surface epithelium, and increased apoptosis in the crypt epithelium. Small bowel villous blunting involving the duodenum, terminal ileum, or both, is also seen more frequently in the VEO-IBD group, and interestingly, we observed villous blunting in the absence of inflammation in young patients. These findings have been previously associated with immunodeficiencies and disorders of immune regulation, such as severe combined immunodeficiency or common variable immunodeficiency; therefore their presence in the histology of VEO-IBD patients may be indicators of different disease drivers compared with older onset IBD.12 Granulomas are a phenomenon observed in chronic auto-inflammatory disorders and are likely a result of individual host responses. Within IBD, the presence of granulomas is one of the hallmarks of distinguishing colonic Crohn’s disease from ulcerative colitis. However when identified among children with VEO-IBD, chronic granulomatous disease or Hermansky Pudlak syndrome, among other immune deficiency syndromes, should also be considered. Interestingly, this study found similar rates of granulomas in VEO-IBD patients as compared with older onset IBD, and in our limited sample set was not a marker for underlying immunodeficiency.
Previous descriptions of the histological findings seen in VEO-IBD have had varied results. Loss of function defects in interleukin 10 [IL-10] and its receptor were one of the first genetic variants identified as causative for very early onset inflammatory bowel disease. Histological analyses in patients with functional defects in IL-10 signalling pathways demonstrated epithelioid granulomas with giant cell formation, on a background of extensive inflammation consisting of mononuclear and polymorphonuclear [neutrophil and eosinophil] cells.21 Deep ulceration extending to the muscularis propria, and crypt micro-abscesses in the colon, were also frequently seen with relative sparing of the small bowel mucosa. However, there have not been any distinct histological features described that could be more specific to an abnormality in the IL-10 signalling pathway.
A larger cohort study characterised the phenotypic and genotypic features of patients with infantile onset IBD [IO-IBD] diagnosed before 2 years of age. Of these subjects, 77% exhibited extensive disease with significant inflammation of both the upper and lower gastrointestinal [GI] tract. Similar to our work, the epithelial abnormalities observed in these patients included abundant epithelial cell apoptosis as well as epithelial shedding and tufting. Villous blunting was identified in at least 40% of the unclassifiable IO-IBD cases, similar to what we detected in our VEO-IBD group.11
In this current study, more than half of the VEO-IBD cases featured prominent eosinophilia in the colon with variable involvement of the lamina propria, glandular crypts, and surface epithelium. Eosinophils are a source of numerous cytokines, chemokines, matrix metalloproteinases, and reactive oxygen species. They can produce proteins which are toxic to parasites and cause damage to host tissue. The presence of eosinophils in the gastrointestinal tract is not uncommon and has previously been seen in IBD, but their role is incompletely understood. In fact, eosinophils can be protective and indicative of remission in some cases,22 and although eosinophils are involved in a pro-inflammatory process, as suggested above, they are likely important in the anti-inflammatory cascade as well.23 However, the extent of eosinophilic inflammation identified here in some cases of VEO-IBD, potentially indicates a more robust pro-inflammatory response consistent with an underlying hyperinflammatory or auto-inflammatory pathway. Interestingly, three patients with identified monogenic defects, all similarly in the hyperinflammatory pathway, had marked eosinophilia throughout their colon.
We also observed apoptosis of the crypt epithelium more frequently in the VEO-IBD cohort as well, which appears distinctly prominent in some cases. Increased crypt epithelium apoptosis has been recognised as an important histological feature in other immune-mediated disorders such as autoimmune enteropathy and acute graft versus host disease.24 Recent publications on VEO-IBD also have described increased apoptosis in the crypt epithelium as a key histological feature as well.10,11 None of the specimens in this study that demonstrated apoptosis of crypt epithelial cells had associated crypt dropout. Apoptosis with crypt dropout has been characteristically seen in VEO-IBD subjects with a mutation in tetratricopeptide repeat domain-7A [TTC7A], in whom a severe exfoliative apoptotic enterocolitis and multiple intestinal atresias are associated with severe combined immunodeficiency.25–27
Similar to epithelial apoptosis, villous blunting is not a frequent feature of IBD, and evokes a differential diagnosis that includes coeliac disease and other entities such as collagenous sprue, PID, autoimmune enteropathy, and tropical sprue, among others. Viral and bacterial gastroenteritis can also lead to villous atrophy, particularly in patients with immunodeficiency such as severe combined immunodeficiency. Coeliac disease, collagenous sprue, and tropical sprue present with villous blunting but without the other chronic signs of inflammation or eosinophilia described here. In addition to apoptosis noted above, villous atrophy has also been described in VEO-IBD patients with homozygous TTC7A mutations.25 At present, the exact mechanism of villous atrophy in the setting of VEO-IBD is unclear, although it is believed to reflect a severely compromised small intestinal function overall, which could also partly explain the more severe clinical presentation of the affected individuals, including failure to thrive in the first few months of life. The mechanism of villous atrophy likely varies among VEO-IBD patients. Other disease models have demonstrated villous atrophy due to the release of lymphocyte perforin cytolytic granules, causing enterocyte apoptosis or enterocyte auto-antibody dependent cellular cytotoxicity.
The power of using histological findings to guide the aetiology, evaluation, and ultimately targeted therapy is perhaps seen best through one of our VEO-IBD patients, who ultimately had a variant identified through WES, in ZBTB24. This monogenic defect has been shown to be causative for immunodeficiency, centromere instability, and facial anomalies, type 2. The mutation is believed to be disruptive to both T and B cell development. While initial review of the patient’s biopsies demonstrated presence of plasma cells, further evaluation showed marked abnormalities that were critical to the diagnosis, including decreased staining for cd79a, IgG, and IgM, almost absent IgA, and decreased kappa and lambda light chains when compared with the other representative cases with known B cell pathway defects and representative cases from the older onset group [Figure 6A and B]. This was the first reported case associated with inflammatory bowel disease, and this subject was treated with intravenous immunoglobulin for his hypogammaglobulinaemia with simultaneous improvement in his IBD.20 The other nine subjects with monogenic defects included in this analysis had GI biopsies that demonstrated apoptosis, severe active inflammation with chronic architectural distortion, and/or small intestinal villous blunting, which were clues for further investigation and prompted genetic and immune evaluations revealing monogenic PID.£
The presentation of VEO-IBD can be severe, rapidly progressive, and heterogeneous, and their response to conventional IBD therapies is variable. Therefore, identifying features that are suggestive of immune deficiency or dysregulation is integral to forming a systematic approach to the comprehensive evaluation of patients with VEO-IBD, and distinguishes this group further from older onset paediatric IBD. The presence of the histological findings at initial evaluation described here should prompt further testing in this subpopulation, including immunological assessment and appropriate referral for whole-exome sequencing to evaluate for rare causative variants for the disease. Here, the subjects with monogenic defects all had at least one of the histopathological features suggestive of immune dysregulation, along with those features more commonly associated with older onset IBD, pointing to the need for further testing and ultimately targeted therapies. As more genetic defects are described with VEO-IBD, future studies of the histological findings of these patients will be necessary to fully characterise the immune-mediated pathways associated with this disease.
Funding
This work was supported by funding from the National Institutes of Health for JK [grant number K23DK1000461-01A1].
Conflict of Interest
There is no actual or potential conflict of interest for any of this manuscript’s authors
Acknowledgements
The authors would like to thank Dr Nancy Spinner, Chief of the Division of Genomic Diagnostics at the Children’s Hospital of Philadelphia, and Dr Jinbo Fan, whose contributions were instrumental to this manuscript as well as the VEO-IBD Program at the Children’s Hospital of Philadelphia.
Author Contributions
MC, JK—study design, interpreting data, drafting manuscript; CKC, PR, NS—collecting/interpreting data, drafting manuscript; ND—data analysis. All authors [MC, CKC, ND, NS, PR, and JK] approved the final draft submitted.
References
- 1. Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium [IIBDGC] Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu JZ, van Sommeren S, Huang H, et al. ; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015;47:979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Ridder L, Weersma RK, Dijkstra G, et al. Genetic susceptibility has a more important role in pediatric-onset Crohn’s disease than in adult-onset Crohn’s disease. Inflamm Bowel Dis 2007;13:1083–92. [DOI] [PubMed] [Google Scholar]
- 4. Biank V, Broeckel U, Kugathasan S. Pediatric inflammatory bowel disease: clinical and molecular genetics. Inflamm Bowel Dis 2007;13: 1430–8. [DOI] [PubMed] [Google Scholar]
- 5. Begue B, Verdier J, Rieux-Laucat F, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am J Gastroenterol 2011;106:1544–55. [DOI] [PubMed] [Google Scholar]
- 6. Glocker E, Grimbacher B. Inflammatory bowel disease: is it a primary immunodeficiency? Cell Mol Life Sci 2012;69:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cannioto Z, Berti I, Martelossi S, et al. IBD and IBD mimicking enterocolitis in children younger than 2 years of age. Eur J Pediatr 2009;168: 149–55. [DOI] [PubMed] [Google Scholar]
- 8. Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 2009;361:2033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelsen JR, Dawany N, Moran CJ, et al. Exome sequencing analysis reveals variants in primary immunodeficiency genes in patients with very early onset inflammatory bowel disease. Gastroenterology 2015;149: 1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uhlig HH, Schwerd T, Koletzko S, et al. ; COLORS in IBD Study Group and NEOPICS The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014;147:990–1007.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kammermeier J, Dziubak R, Pescarin M, et al. Phenotypic and genotypic characterisation of inflammatory bowel disease presenting before the age of 2 years. J Crohns Colitis 2017;11:60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal S, Mayer L. Diagnosis and treatment of gastrointestinal disorders in patients with primary immunodeficiency. Clin Gastroenterol Hepatol 2013;11:1050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine A, Koletzko S, Turner D, et al. ; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 2014;58:795–806. [DOI] [PubMed] [Google Scholar]
- 14. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- 15. Daniels JA, Lederman HM, Maitra A, Montgomery EA. Gastrointestinal tract pathology in patients with common variable immunodeficiency [CVID]: a clinicopathologic study and review. Am J Surg Pathol 2007;31:1800–12. [DOI] [PubMed] [Google Scholar]
- 16. Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyle B, Collins MH, Wang Z, et al. ; PROTECT Study Group Histologic correlates of clinical and endoscopic severity in children newly diagnosed with ulcerative colitis. Am J Surg Pathol 2017;41:1491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masia R, Peyton S, Lauwers GY, Brown I. Gastrointestinal biopsy findings of autoimmune enteropathy: a review of 25 cases. Am J Surg Pathol 2014;38:1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeBrosse CW, Case JW, Putnam PE, Collins MH, Rothenberg ME. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol 2006;9:210–8. [DOI] [PubMed] [Google Scholar]
- 20. Conrad MA, Dawany N, Sullivan KE, Devoto M, Kelsen JR. Novel ZBTB24 mutation associated with immunodeficiency, centromere instability, and facial anomalies type-2 syndrome identified in a patient with very early onset inflammatory bowel disease. Inflamm Bowel Dis 2017;23:2252–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pigneur B, Escher J, Elawad M, et al. Phenotypic characterization of very early-onset IBD due to mutations in the IL10, IL10 receptor alpha or beta gene: a survey of the Genius Working Group. Inflamm Bowel Dis 2013;19:2820–8. [DOI] [PubMed] [Google Scholar]
- 22. Woodruff SA, Masterson JC, Fillon S, Robinson ZD, Furuta GT. Role of eosinophils in inflammatory bowel and gastrointestinal diseases. J Pediatr Gastroenterol Nutr 2011;52:650–61. [DOI] [PubMed] [Google Scholar]
- 23. Masterson JC, McNamee EN, Fillon SA, et al. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut 2015;64:1236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gomez AJ, Arai S, Higgins JP, Kambham N. Clinicopathologic threshold of acute colorectal graft-versus-host disease. Arch Pathol Lab Med 2016;140:570–7. [DOI] [PubMed] [Google Scholar]
- 25. Avitzur Y, Guo C, Mastropaolo LA, et al. Mutations in tetratricopeptide repeat domain 7A result in a severe form of very early onset inflammatory bowel disease. Gastroenterology 2014;146:1028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang W, Lee PP, Thong MK, et al. Compound heterozygous mutations in TTC7A cause familial multiple intestinal atresias and severe combined immunodeficiency. Clin Genet 2015;88:542–9. [DOI] [PubMed] [Google Scholar]
- 27. Fernandez I, Patey N, Marchand V, et al. Multiple intestinal atresia with combined immune deficiency related to TTC7A defect is a multiorgan pathology: study of a French-Canadian-based cohort. Medicine [Baltimore] 2014;93:e327. [DOI] [PMC free article] [PubMed] [Google Scholar]