Abstract
Many of the components, which render honey its specific aroma, flavor, and biological activity, are unstable over time and thermolabile. This study was aimed to compare the chemical composition, effect of heating as well as the time of heat exposure, and storage period on the quality of honey samples from Apis mellifera (A.m.) and Apis florea (A.f.). Methods of the Association of the Official Analytical Chemists (AOAC) were used in this study. The mean values for both A.m. and A.f. honeys were, respectively: moisture (18.5, 13.7%); glucose (35.2, 36.3%); fructose (33.7, 33.8%); sucrose (7.3, 2.9%); invert sugar (68.9, 70.4%); ash (0.26, 1.1%); acidity (51.8, 98.4 meq/kg); pH (3.6, 4.4) and Hydroxy methyl furfural (HMF) (3.78, 3.17 mg/100 g). Honey from A. florea contained less moisture, have higher acidity and ash contents than A. mellifera honey. Significant alterations (P < 0.05) in glucose, fructose, sucrose, and acidity were noticed after six months. Honeys exposed to heating for 15 and 30 min at 50 and 80 °C have shown increased thermo-generated HMF after 15, 30, and 45 days. HMF reached 16.30 ± 1.1 in A. mellifera and 7.41 ± 1.4 mg/100 g in A. florea honeys that exposed for 30 min at 80 °C. Honey from A. florea showed more heat tolerance to thermo-generation of HMF than honey from A. mellifera.
Keywords: Honeybee species, HMF, Thermo-generation tolerance
1. Introduction
There are approximately 20,000 species of bees exhibiting terrestrial life, only 6–11 species of them are known to produce honey (Ball, 2007). In Sudan, Apis mellifera and Apis florea are coexist for at least the last three decades. However, most of the commercialized honey and wax in the country comes from A. mellifera, and the exotic A. florea (which was identified as alien species by Moga et al., 1989) contributes largely in pollination of orchards and vegetables. The distinctive characteristics of honey are not, primarily, due to its major sugar components which can be found in many sweet products, but it is rather due to presence of multitude of minor components originated from the nectar and bees themselves. Many of the minor components such as phenolic compounds, which give its specific aroma, flavor and some of its biological activities, are unstable over time and thermolabile (Tosi et al., 2004). Heating has a negative effect on honey due to the loss of the minor substances (Tosi et al., 2004). Honey quality can be affected not only by fraudulent practices but also by the use of inadequate storage conditions or the application of severe heat treatments. Freshly extracted honey is liquid, but during storage honey becomes crystallized sooner or later. Crystallized honeys are not popular with consumers and can only be marketed liquefied. Gentle heating (32–40 °C) is mostly used to liquefy crystallized honey and to destroy yeast. To avoid heat damage of sensitive substances, it is recommended not to heat honey to more than 40–50 °C. However, high temperatures (80–100 °C) are also applied for liquefaction and/or pasteurization purposes. Besides long period of storage high temperatures can adversely affect the chemical composition of honeys (Castro-Vazquez et al., 2008, Morales et al., 2009). Long periods of storage or high temperatures are known to produce furan derivatives, HMF which is acyclic aldehyde formed from hexoses by the action of normal acidity on honey sugars. Fructose is more sensitive than glucose to the reaction that forms HMF (Jeering and Kuppers, 1980, Crane, 1982). HMF is used as an indicator of honey freshness or heating.
In the literature, there is much information regarding factors influencing the quality of honey such as heating and prolong storage on the chemical composition of the honey, particularly on HMF formation and diastase enzyme deactivation. Little information available on the effect of these two factors on sugars and other honey constituents. Nevertheless, none of the reviews have dealt with the response of honeys of different honeybee species on the ratio of HMF formation. Perhaps, this is the first study to concern with this issue. Thus the aim of the present study was (i) to compare the chemical composition of honey of the two species of honeybees (ii) to assess the magnitude of effect of heating and storage on selected chemical parameters (iii) to detect whether there is heat tolerant honey owing to the honeybee species produced it. Considering this point, if, certain honey sort has low changes during adverse storage condition over a certain period of storage, such honey will have long shelf life which is important from consumer's point of view.
2. Materials and methods
2.1. Honey samples
Freshly extracted comb honey samples (5 samples) produced by the honeybee A. mellifera were taken from the apiary of Bee Research, Environment and Natural Resources Research Institute-Sudan in January 2015. At the same season, another five comb honey samples were collected from feral colonies of A. florea nesting in the apiary vicinity. The combs were cleaned from broods, pollen grains, and debris; then squeezed and the honey was strained through a mesh (5 mm pore size).
2.2. Honey heating and storage treatments
Heating of honey was carried in a thermo-static water bath provided with shaker (DlabTEch®, Model:LSB-030S made in Korea) at two temperatures (50 °C and 80 °C) and two times (15 and 30 min) in glass vials containing aliquots of 5 g honey of both honeybee species and stored at ambient temperature (25 °C) for 45 days for HMF determination. HMF was determined every 15 days, in the heated samples as well as the control ones (unheated batch) samples. Changes in the chemical parameters of the unheated stored honey samples were followed monthly for a total period of six months.
2.3. Chemical analysis
Refractive index was determined with an Abbe refractometer (Hilger, M 64.315/56304, made in England) at 20 °C, the corresponding moisture content (%) was calculated using the Wedmore Table (AOAC, 1990). Invert sugar, glucose, fructose, and sucrose were determined following FAO (1995) methods. Ash, pH, and acidity were determined according to the standard methods of the Association of the Official Analytical Chemists (AOAC, 1990). HMF was determined using White (1979) spectroscopic method. All chemicals used were an analytical grade or general purpose reagents?
2.4. Statistical analysis
Data are analyzed as means of triplicate readings with standard deviation (SD). And a comparison of data was performed by Student t-test; pair-wise mean comparison of Bradfoni test by applying SPSS version 9 statistical packages (SPSS, Chicago, IL, USA, copyright 2010).
3. Results and discussion
3.1. Chemical composition
Table1 shows means and standard deviations of the various chemical parameters analyzed: moisture, glucose, fructose, sucrose, invert sugar, ash, acidity, pH, and HMF. Comparison between honeys produced by two different species of honey bees A. mellifera and A. florea has resulted in significant differences (P < 0.05) in the studied parameters. A. florea honey contained less moisture 13.7% than A. mellifera honey which contained 18.5%. This variation between the two types of honey could indicate that both honeybee species process honey differently during the mechanical stage of honey ripening. In the hive, nectar is delivered to the house bees who store it in in the honeycomb cells. In the cells water has to be evaporated off the nectar in a process called mechanical stage of honey ripening (Australian Honey Bee Industry Council, 2017). Furthermore, it could be due to the different nesting habitats (the open nest may facilitate for easy evaporation of nectar) or different nectarines foraged by the bees. The moisture content of honey depends on nectar, harvesting season and beekeeping practices. Moreover, moisture content is a critical factor for honey fermentation and crystallization as reported by Nombre et al. (2010).
Table 1.
Chemical composition of Apis mellifera and Apis florea honeys.
| Parameter | Apis mellifera | Apis florea | t-test |
|---|---|---|---|
| Moisture (%) | 18.50 ± 1.53 | 13.70 ± 0.79 | ** |
| Glucose (%) | 35.24 ± 1.06 | 36.38 ± 2.91 | NS |
| Fructose (%) | 33.70 ± 1.09 | 33.82 ± 3.16 | NS |
| Sucrose (%) | 7.32 ± 4.13 | 2.90 ± 1.85 | * |
| Invert sugar (%) | 68.94 ± 1.99 | 70.42 ± 5.55 | NS |
| Ash (%) | 0.26 ± 0.15 | 1.16 ± 0.13 | ** |
| Acidity (meq/kg) | 51.80 ± 1.947 | 98.40 ± 1.817 | * |
| pH | 3.62 ± 0.466 | 4.40 ± 0.10 | * |
| HMF (mg/100 g) | 3.78 ± 1. 4 | 3.17 ± 1.2 | NS |
Data presented are mean ± SD of triplicate readings.
NS = no significant differences.
Significant difference (P < 0.05).
Significant difference (P < 0.01).
Ash contents, consecutively, for A. mellifera and A. florea honey, were 0.26, 1.16%. The highly significant variation observed in honey's ash contents implicit the different honey extraction methods applied for both honey types. In this study, A. mellifera honey samples were extracted by centrifugation while A. florea samples were obtained by squeezing and straining (which introduces more pollen to the honey) as A. florea can’t be reared in the modern hives with movable frames. Several reports mentioned that ash content depends on the material foraged by the bees (Madejczyk and Baralkiewicz, 2008, Rashed and Soltan, 2004). Moreover, it depends on bee’s foraging preference (Cook et al., 2003). However, ash values reported in this study corresponds to those reported by Khalil et al., 2001, Feas et al., 2010.
Acidity was found 51.80, 98.40 meq/kg and pH estimated 3.62, 4.40 for both honey sorts. Honey acidity is important for its characteristic flavor and stability against microbial attack (White, 1975). It is obvious that honey from A. florea contains noticeably higher acidity and ash contents than honey from Apis mellifera. This is contradicting the fact that higher mineral contents correspond to lower acidity (Finola et al., 2007). However, Stivenho et al. (2009) found a linear correlation between the ash content and electrical conductivity of heather honey.
The mean glucose 35.24 and 36.38%, fructose 33.70 and 33.82, respectively for the two honey types A. mellifera and A. florea showed no significant variation. Both sugars collectively represent invert sugar. The percentage of invert sugar is a prerequisite for the quality control of honey. The mean values 68.94 and 70.42% of invert sugar for both honey types satisfy the standard level for honey quality and agree with other results (Nombre et al., 2010, Stivenho et al., 2012).
Sucrose content (7.32, 2.90) varied significantly (P < 0.05) between the two honey types. A similar result was reported by Iftikhar et al. (2011) They reported that honey from A. mellifera contains significantly higher sucrose than honey from Apis florea. This variation could be due to the variation in invertase activity of the two honeybee species. It was reported by Wakhle (1997) that the invertase activity of some honeybee species is higher than the others.
The results of chemical composition obtained comply with earlier findings in Sudanese honey (Mohamed and Ali, 2005, Mohammed and Babiker, 2009) and are comparable to those of other authors as (Adebiyi et al., 2004, Terrab et al., 2004, Finola et al., 2007).
3.2. Effect of storage period on the chemical composition
Data in Table 2 elucidate influences of storage period on honey chemical composition. As the two types of honey compared, glucose and fructose significantly (P < 0.05) decreased during first, and 4th–6th months of storage. It was reported that the sugar spectrum of ripened honey is not static; rather, it does change with time. A slight (<15%) decrease in the quantity of fructose and glucose occurs over time owing to the acid catalyzed the formation of maltose and other reducing disaccharides (Ball, 2007). Sucrose dropped significantly from 9.2 to 1.1% in A. mellifera honey and from 2.9 to 1.0% in A. florea honey after six months storage, this result is comparable to the results of Rybak-Chmielewska and Szczesna (1995). Total acidity increased significantly during the six-month storage period for both A. mellifera and A. florea. Free and lactonic acids in honey increase significantly during storage, mainly at 40 °C (Castro-Vazquez et al., 2008). However, values of pH did not show a clear variation in stored honey (Gulati and Kumari, 2007).
Table 2.
Analysis of glucose, fructose, sucrose and total acidity in Apis mellifera and Apis florea honeys stored at room temperature (25 °C) for six months.
| Glucose (%) |
Fructose (%) |
Sucrose (%) |
Acidity (meq/kg) |
|||||
|---|---|---|---|---|---|---|---|---|
| A.m. | A.f. | A.m. | A.f. | A.m. | A.f. | A.m. | A.f. | |
| 1st month | 35.1 ± 3.2* | 36.4 ± 2.9* | 31.3 ± 2.4* | 33.8 ± 3.1* | 9.2 ± 3.7* | 2.9 ± 1.8* | 63.8 ± 7.8* | 98.4 ± 18.1* |
| 2nd month | 34.1 ± 3.5 | 34.0 ± 3.3 | 26.7 ± 3.0 | 33.3 ± 3.3 | 5.9 ± 2.1* | 2.4 ± 1.1* | 71.8 ± 6.5* | 98.4 ± 17.9* |
| 3rd month | 33.7 ± 3.3 | 33.5 ± 2.9 | 25.0 ± 2.9 | 31.5 ± 2.6 | 5.6 ± 2.1* | 2.3 ± 1.1* | 79.4 ± 9.6* | 100.4 ± 18.9* |
| 4th month | 25.7 ± 5.8* | 26.4 ± 1.3* | 20.2 ± 4.4* | 28.5 ± 3.1* | 3.3 ± 1.5 | 1.8 ± 0.8 | 84.6 ± 12.5 | 101.2 ± 18.5 |
| 5th month | 25.7 ± 5.8* | 26.92 ± 1.9* | 17.7 ± 3.7* | 27.7 ± 3.5* | 1.5 ± 0.9 | 1.1 ± 0.6 | 88.6 ± 12.1 | 103.2 ± 18.2 |
| 6th month | 25.5 ± 5.8 | 26.1 ± 1.3 | 14.9 ± 25.2 | 25.2 ± 3.7 | 1.1 ± 0.5 | 1.0 ± 0.6 | 91.8 ± 12.1 | 97.9 ± 5.6 |
| % decrease (+)/increase (−) | −29.90 | −28.25 | −55.78 | −25.48 | −84.97 | −65.51 | +43.57 | −5.08 |
Data presented are means of five samples ± SD of triplicate readings.
A.m. = Apis mellifera, A.f. = Apis florea.
(Astric) are significantly different (P < 0.05) as compared by Bradfoni Pair-wise mean test.
3.3. Effect of heating regimes and storage period on HMF formation
A. mellifera and A. florea honeys revealed initial 3.78 and 3.17 mg/100 g HMF contents, respectively; which satisfy the international limit of 40 mg/kg (Table 1). The level of HMF in unheated honey has increased by two folds after 45 days storage under room temperature (Fig. 3). This is due to the hot tropical weather which can increase the HMF level of honey in the hive. Consequently, Codex Alimentarius (2001) has increased the HMF limit in honey from tropical zones to 80 mg/kg. Heating honey for 15 and 30 min at 50 °C has raised, respectively HMF level of A. mellifera honey to 8.1 and 9.37 mg/100 g after 15 days; 9.61 and 11.51 after 30 days; and 4–5 folds after 45 days (Fig. 1, Fig. 2, Fig. 3). When samples heated for 15 and 30 min at 80 °C, 4–5 folds increment in the level of HMF was reached after 15–30 days storage (Fig. 1, Fig. 2). This indicated that the level of HMF formation during storage depends on the heat applied during honey processing and duration of heating. In contrary, it is interesting, to note that honey produced by Apis florea, has tolerated the heating regimes applied. Consequently, a lesser increment in HMF has occurred during storage. Some reports attribute variation in HMF contents of honey due to variation in the floral sources (Ajlouni and Sujrapinyokul, 2010). However, in the surveyed literature, we did not find any report matching with the present results and supporting variation in HMF due to the honey produced by different honeybee species. The explanation for this tolerance may be due to the difference in the chemical composition of honey produced by the different bees' species and possibly their forage preference.
Fig. 3.
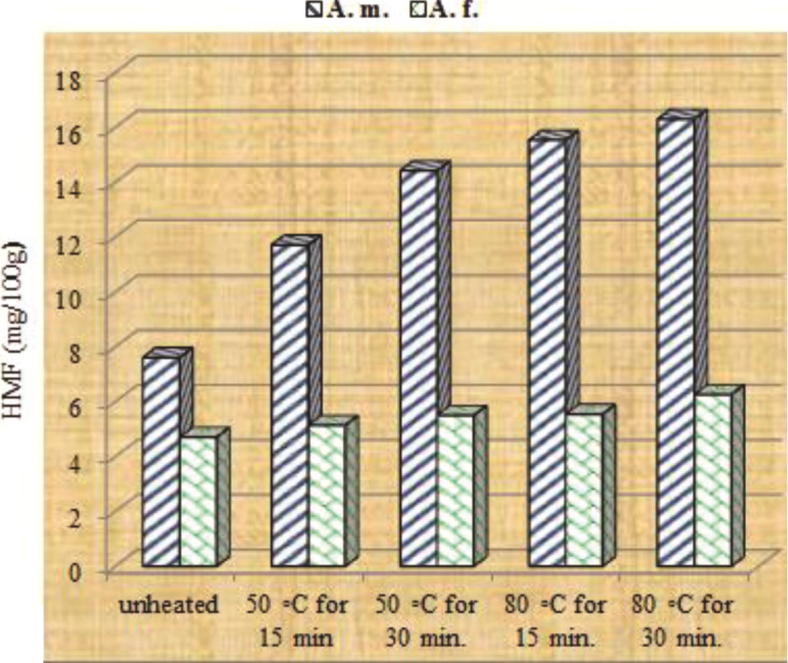
Thermo-generation of HMF in Apis mellifera and Apis florea honeys stored for 45 days after exposure to different thermal treatments.
Fig. 1.
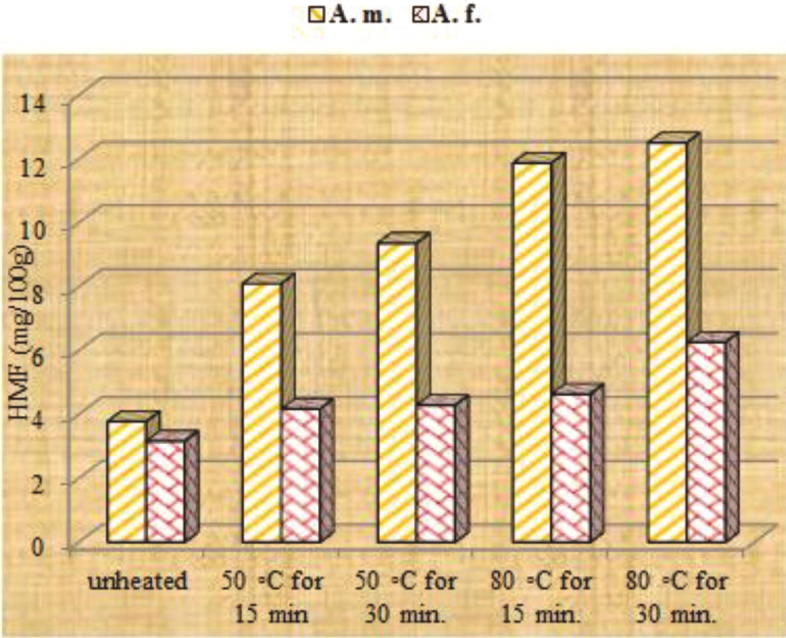
Thermo-generation of HMF in Apis mellifera and Apis florea honeys stored for 15 days after exposure to different thermal treatments.
Fig. 2.
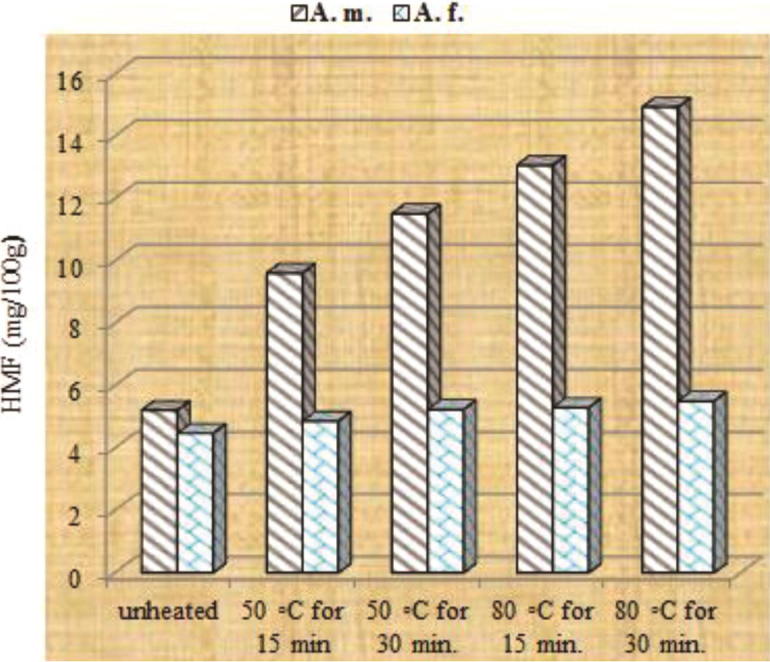
Thermo-generation of HMF in Apis mellifera and Apis florea honeys stored for 30 days after exposure to different thermal treatments.
4. Conclusion
It can be concluded that A. florea honey contains less moisture content than A. mellifera honey due to the different nesting habitats of both species and different nectarines foraged. Possibly they process honey differently during the mechanical stage of honey ripening. Also, A. florea honey contains higher acidity and ash due to the different foraging preference of the two species. Many of the components of honey are unstable over time and are thermolabile. After six month storage, glucose, fructose and sucrose of both honey; dropped significantly. Moreover, A. florea honey tolerated temperature regimes applied regarding HMF production.
Conflict of interest
The authors confirm that there is no conflict of interests and are also liable for the content and writing of this article.
Acknowledgement
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adebiyi I., Akpan O.E.I., Olaniyi H.B. Chemical/physical characterization of Nigerian honey. Pakistan J. Nutr. 2004;3:278–281. [Google Scholar]
- Ajlouni S., Sujrapinyokul P. Hydroxymethylfurfuraldehyde and amylase contents in Australian honey. Food Chem. 2010;119:1000–1005. [Google Scholar]
- AOAC, 1990. Official Methods of analysis. 15th (Ed.). Association of the Official Analytical Chemists, Arlington, VA.
- Australian Honey Bee Industry Council, 2017. How bees make honey? <https://honeybee.org.au/education/wonderful-world-of-honey/how-bees-make-honey/>.
- Ball D.W. The chemical composition of honey. J. Chem. Educ. 2007;84:1643–1646. [Google Scholar]
- Castro-Vazquez L., Diaz-Maroto M.C., Gonzalez-inas E.D., Perez-Coello M.S. Influence of storage conditions on chemical composition and sensory properties of citrus honey. J. Agric. Food Chem. 2008;56:1999–2006. doi: 10.1021/jf072227k. [DOI] [PubMed] [Google Scholar]
- Codex Alimentarius Commission, 2001. Revised Codex Standard for Honey. Codex Standard. Rome FAO and WHO, 12-1981.
- Cook S.M., Awmack C.S., Murray D.A., Williams I.H. Are honey bees’ foraging preferences affected by pollen amino acid composition? Ecol. Entomol. 2003;28:622. [Google Scholar]
- Crane E. Learning about honey through fructose. Bee World. 1982;63:147–167. [Google Scholar]
- FAO Value-added products from beekeeping. Agric. Serv. Bull. 1995;124:343. [Google Scholar]
- Feas Z., Pires J., Iglesias A., Estevinho M.L. Characterization of artisanal honey produced on the north of Portugal by mellisopalynological and physico-chemical data. Food Chem. Toxicol. 2010;48:3462–3470. doi: 10.1016/j.fct.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Finola M.S., Lasagno M.C., Marioli J.M. Microbiological and chemical characterization of honeys from central Argentina. Food Chem. 2007;100:1649–1653. [Google Scholar]
- Gulati R., Kumari B. Chemical composition of unifloral, stored and commercial Apis mellifera L. honeys. J. Food Sci. Technol. 2007;42:492–495. [Google Scholar]
- Iftikhar F., Masood M.A., Waghchoure E.S. Comparison of Apis cerana, Apis dorsata, Apis florea, and Apis mellifera honey from different areas of Pakistan. Asian J. Exp. Biol. Sci. 2011;2:399–403. [Google Scholar]
- Jeering N.J., Kuppers F.J.E. High performance liquid chromatography of furfural in honey and spirits. J. Assoc. Off. Anal. Chem. 1980;63:126. [PubMed] [Google Scholar]
- Khalil M.I., Motallib M.A., Anisuzzaman A.S.M., Sathi Z.S., Hye M.A., Shahjahan M. Biochemical analysis of different brands of unifloral honey available at the northern region of Bangladesh. Sciences. 2001;1:383–388. [Google Scholar]
- Madejczyk M., Baralkiewicz D. Characterisation of honey from different areas of Poland by their physico-chemical parametres and trace elements. Proc. Ecopole. 2008;22:59–63. [Google Scholar]
- Moga, J.B., Abdin, A.M.Z., Nagi, S.K.A., Ali, A.M., 1989. Apis florea in Sudan: some biological observations. In: Proc. 4 Int. Api. Conf. In Trop. Climates, Cairo, Egypt, pp. 422–424.
- Mohamed S.A., Ali E.E. Study on the quality characteristics of some Sudanese honeys. Sudan J. Agric. Res. 2005;5:83–88. [Google Scholar]
- Mohammed S.A., Babiker E.E. Protein structure, physicochemical properties and mineral composition of Apis mellifera honey samples of different floral origin. Austr. J. Basic Appl. Sci. 2009;3:2477–2483. [Google Scholar]
- Morales V., Sanz M.L., Martin-Alvarez P.J., Corzo N. Combination use of HMF and furosine to assess fresh honey quality. J. Sci. Food Agric. 2009;89:1332–1338. [Google Scholar]
- Nombre I., Schweitzer P., Boussim J.I., Rasolodimby J.M. Characteristics of honey samples from Burkina Faso. Afr. J. Food Sci. 2010;4:458–463. [Google Scholar]
- Rashed M.N., Soltan M.E. Major and trace elements in different types of Egyptian mono- floral and non-floral bee honeys. J. Food Compos. Anal. 2004;17:725–735. [Google Scholar]
- Rybak-Chmielewska, H., Szczesna, T., 1995. Composition and properties of Polish Buckwheat honey. Current advances in Buckwheat Research, pp. 793–799.
- Stivenho M.L., Feas Z., Seijas J.A., Vazquez-Tato M.P. Organic honey from Tras-Os-Montes region (Portugal): chemical, palynological, microbiological and bioactive compounds characterization. Food Chem. Toxicol. 2012;50:258–264. doi: 10.1016/j.fct.2011.10.034. [DOI] [PubMed] [Google Scholar]
- Stivenho M.L., Leticia M., Feas Z., Jesus C., Iglesias A. Pollen spectra and physico-chemical attributes of heather (Erica sp.) honeys of north Portugal. J. Sci. Food Agric. 2009;89:1862–1870. [Google Scholar]
- Terrab A., Recamales A.F., Hernanz D., Heredia F.J. Characterization of Spanish thyme honeys by their physicochemical and mineral contents. Food Chem. 2004;88:537–542. [Google Scholar]
- Tosi E.A., Ré E., Lucero H., Bulacio L. Effect of honey high temperature and short time heating on parameters related to quality, crystallization phenomena and fungal inhibition. Lebensm-wiss. U-Technol. 2004;37:669–678. [Google Scholar]
- Wakhle D.M. Beekeeping technology – production, characteristics and uses of honey and other products. In: Mishra R.C., editor. Perspectives in Indian Apiculture. Agro-Botanica; Bikaner: 1997. pp. 134–139. [Google Scholar]
- White, J.W., 1975. The hive and the honey bee. In: Grout, R.A. (Ed.), Standard Printing Company Hannibal, Missori, pp. 556.
- White J.W. Spectrometric method for determination of HMF in honey. J. Assoc. Off. Anal. Chem. 1979;62:509–526. [PubMed] [Google Scholar]


