Abstract
Increased consumption of fossil fuels is an emerging problem. Scientists look for the existence of other alternatives to fossil fuels, including so-called renewable energy. Accordingly, we report the production of bio-ethanol from the remnants of castor oil bean seed cake (CBC) by the carboxymethylcellulase enzyme (CMCase). A bacterial strain isolated from rice straw showing higher CMCase activity was identified. The 16S rRNA result showed a 93% homology with the 16SrRNA gene sequences of Pseudomonas poae RE∗1-1-14, the strain was identified as Pseudomonas poae AB3. In addition, our results showed that the highest enzyme activity was achieved after 48 h and inoculum size of 3.7 × 105 CFU. The optimum temperature, pH and Carboxymethylcellulose (CMC) concentration for the highest enzyme activity was 25 °C, pH 7 and 10 g/l respectively. Furthermore, The CMCase was purified by ammonium sulphate at a concentration of 60%. The SDS-PAGE of the purified enzyme showed a molecular weight of 88 kDa. Additionally, the (CBC) was hydrolyzed by the purified CMCase at the enzyme optimum conditions. The results showed the liberation of 5.2 g/L of reducing sugar by using dinitrosalicylic acid (DNS) assay. Finally, the total sugar produces 35 g/L after 48 h when Saccharomyces cerevisiae was used as a fermentation agent. Hence for the first time, we have been successfully able to produce bioethanol from CBC with CMCase of Pseudomonas poae.
Keywords: Carboxymethylcellulase, Pseudomonas poae, Castor bean cacke, Bioethanol
1. Introduction
Shortage of fossil fuel resources and adverse effects of their use on the environment are the two most important challenges. These challenges encourage researchers to concentrate on ways to discover alternative new sources of energy instead of fossil oil. Food waste and oilseed are considered as preferable sources of renewable energy by many scientists. The fuels produced from them reduce the greenhouse phenomena, air pollution, and fuel import that reduce the energy cost.
For this purpose, many vegetable species are grown on climatic regions including canola, castor, coconut, corn, cottonseed, soybean, and sunflower (Singh and Sharma, 2009). The previous vegetable sp. have special amount of oil with a specific characters. The waste edible oil is possibly another source of renewable energy (Abada, 2014a, Abada, 2014b); but the production seems to be more economic and efficient (Demirbas, 2007, Singh and Sharma, 2009, Wang and Yang, 2007). Ricinus communis L. belongs to family Euphorbiaceae. It is known as wonder tree and castor oil bean. The castor oil seeds include 40% oil and about 5% of ricin protein. This oil of castor bean has unpleasant taste, its colour ranging from pale yellow to colorless, highly viscous. While, ricin considered as one of the deadly toxin compound (Aslani et al., 2007). Castor bean cake (CBC) is a by-product after pressing of castor bean seed during oil production. It has been reported that pressing one ton of castor beans for oil production produces approximately 550 kg of cake which may vary according to oil extraction process and seed oil content (Pina et al., 2005). CBC can be used as fungicide, fertilizer, animal feed after detoxification, in nematode control and as raw material for ethanol production since it contain 11% cellulose, 10% hemicellulose and 23% lignin (Beltrão, 2008, Severino, 2005). Additionally, CBC contains high concentrations of starch, which is considered as a potential feedstock for the production of bioethanol (Silva et al., 1996).
Some environmental problems such as global warming and its consequences; i.e. gas emissions, show the importance of biofuels on greenhouse attenuation (Blottnitz and Curran, 2007, Wesseler, 2007). In this context, the availability of biotechnological resources and biomass put the biodiesel and bioethanol as the most promising biofuels. Interfacing the need of treating the deposit produced from Castor Bean Oil extraction to the capability of its Cake as a fermentable wellspring of sugars. Recently, the utilization of cellulases in production of bioethanol from many lignocellulosic materials has been reported (Rodhe et al., 2011). Basically, cellulases change over cellulosic part of lignocellulosic biomass to fermentable sugars such as carboxymethylcellulase (E.C. 3.2.1.4). Predominantly, CMCase has been used in the production of ethanol (Singh and Bishnoi, 2012). This study aims to produce bioethanol from CBC by using the enzyme CMCase. Since, it will decompose cellulose and hemicellulose content of CBC to monosaccharide, which in turn will be fermented by yeast to produce bioethanol.
2. Materials and methods
2.1. CMCase production medium
With some modifications, bacterial standard medium (BSM) (8.0 g/L peptone, 1.0 g/L glucose, 1.0 g/L beef extract, 1.0 g/L yeast extract and 10.0 g/L CMC) was used to prepare the CMCase production medium, pH was adjusted to 7.0 using 0.2 M phosphate buffer (pH 7.0) then sterilized at 121 °C for 15 min (Piddington et al., 1995).
2.2. Isolation and purification of bacterial strains
Isolation of CMCase producing bacteria was done according to Abada (2014a) with some modifications. Ten grams of cellulose rich waste materials including rice straw (RS), wheat straw (WS) and bagass was transferred to 500 mL conical flask containing 100 mL of sterile saline solution (8.5 g NaCl). The flask was incubated at 30 °C on a rotary shaker at 200 rpm. Serial dilution 10−1 of this sample was inoculated on the BSM agar medium containing CMC, and then the plates were incubated at 30 °C for 24 h. Also, the purification steps were carried out for the grown bacterial isolates according to Abada (2014a).
2.3. Identification of the potential CMCase isolate by 16S rRNA
Genomic DNA was isolated from the candidate isolate that showed highest CMCase activity by using protocol of Gene Jet genomoic DNA purification kit (Thermo). Amplification of the 16S rRNA gene was carried out by means of 16SrRNA pair of primer named as Forward (F) (5′AGAGTTT GATCCTGGCTCAG3′) and Reverse (R) (3′GGTTACCTTGTTACGACTT5′). PCR reaction was performed in a 50 µL reaction mixture containing 5 µL Template DNA, 25 µL 2X Maxima hot Start PCR master Mix, 1 µL of primer F and R (20 µM) and 18 µL water nuclease-free. Amplification program was done according to following program: Initial denaturation (10 min at 94 °C), followed by 35 cycles of denaturation at 94 °C for 30 s., Annealing at 65 °C for 1 min, extension at 72 °C for 1.5 min and a final extension at 72 °C for 10 min (Abada, 2014b). The PCR product was electrophorized at 1% low melting agarose, and purified by using GeneJET™ PCR purification Kit (Thermo). GATC Company (GATC Company, South Korea) sequenced the purified PCR product by the use of ABI 3730 xl DNA sequencer. The gene homology and related sequences were carried out by public databases BLAST at the NCBI server (http://www.ncbi.nlm.nih.gov/blast/).
2.4. CMCase activity assay
The CMCase activity of the bacterial isolate was carried out using colorimetric assay as described before (Singh et al., 2014). The liberated reducing sugar was measured by DNS method described by Miller (1959).
2.5. Effect of different physical factors on the CMCase production
The following factors were examined to optimize the conditions for CMCase activity by the most potent bacterial strain (with highest CMCase activity). The deduced optimal conditions resulted from each experiments were taken in consideration as reported previously by Abada (2014a).
2.5.1. Incubation period
The most potent CMCase producing bacterial strain was grown on BSM supplemented with CMC for different incubation periods (12, 24, 48, 72 and 96 h). At the end of each incubation period CMCase activity was estimated.
2.5.2. Incubation temperature
The most potent CMCase producing bacterial strain was grown at different incubation temperatures including 25, 30, 35, 40 and 45 °C then CMCase activity was estimated.
2.5.3. pH
The effect of pH on CMCase activity was studied at different pH values (5, 6, 7, 8 and 9).
2.5.4. Inoculum concentration
This experiment was carried out to investigate the effect of different inoculums size on CMCase activity by the most potent strain. Different inoculum concentrations were applied (0.1, 0.2, 0.4, 0.6, 0.8 and 1 mL) at 600 nm absorbance (OD600).
2.5.5. Substrate (CMC) concentrations
The effect of different substrate concentrations (CMC) on CMCase activity produced by the most potent strain was investigated. Different CMC concentrations (2.5, 5, 10, 15 and 20 g/L) were applied.
2.6. Protein content estimation
Protein content estimation was performed according to Lowry et al. (1951). The reagents were prepared freshly at each experiment. The specific activity of the enzyme protein in terms of (U/mg protein enzyme/ml) was determined from the following equation:
2.7. Purification of CMCase produced by the most potent strain
2.7.1. Ammonium sulphate precipitation of the crude enzyme
Ranges of 20% to 100% saturation of ammonium sulphate were added to 100 mL of the cell-free filtrate and the precipitated protein was obtained by centrifugation for 15 min at 16,000 rpm. The pellet was dissolved in 5 mL of 0.2 M phosphate buffer (pH 7.0). Centrifugation was repeated and the pellet was re-suspended in phosphate buffer.
2.7.2. Dialysis against sucrose
This purification step was carried out to remove the traces of ammonium sulphate from the crude CMCase enzyme. Crude enzyme was filled in a dialysis bag previously soaked in phosphate buffer overnight and concentrated by dialysis against sucrose crystals till it achieved constant volume. The concentrated crude enzyme became ready to be applied for further purification step using column chromatography technique.
2.7.3. Purification of the dialyzed crude enzyme by using Sephadex G-200 gel chromatography
The dialyzed crude enzyme was applied onto pharmacia column (2.5 X 80 cm) packed with Sephadex G-200 (Sigma, Germany). The column was equilibrated with (0.2 M) phosphate buffer (pH 7.0). Five grams of Sephadex G-200 were suspended into 500 mL (0.2 M) phosphate buffer at (pH 7.0) and allowed to swell over-night in the refrigerator. Sodium azide (0.02%) was added to prevent any microbial growth. The column was connected to the buffer reservoir and the flow of the buffer was maintained at a rate of approximately 20 mL/h for two hours to allow the settlement of the bed. One mL of the enzyme preparation sample was applied carefully to the top of the gel. Buffer was then added without disturbing the gel surface and then the column was connected to the reservoir. Thirty fractions were collected (each containing 5 mL). Both enzyme activity and protein content were estimated for each separate fraction. The active enzyme fractions were concentrated by dialysis against sucrose crystals to achieve constant volume (Abada, 2014a).
2.8. Hydrolysis of the cellulose and hemicellulose content of CBC by the purified CMCase using solid state fermentation
Hydrolytic assays were performed in a thermostatized bath, in 250 mL conical flasks containing 10 g of sterilized CBC. Experiments were run with a solid–liquid ratio of 1:6. Due to the specificity of the CMCase enzyme and the structure of cellulose and hemicellulose from the seeds, the purified CMCase was utilized in the hydrolysis at 30 °C and pH 7. The hydrolysate of flask was collected for reducing sugar estimation test (Melo et al., 2008).
2.9. Batch fermentation for bioethanol production by using Saccharomyces cerevisiae
Fermentation media were obtained by centrifugation and filter sterilization of the remaining solid residue of CBC, after hydrolytic stage, followed by pH adjustment to 5.0 with calcium oxide of the liquid fraction. Fermentation step was carried out using fermentor (10.0 L Biotron Liflus SL) with 7.0 L working volume. The system was maintained at 30 °C under stirring of 100 rpm for 72 h. Saccharomyces cerevisiae was used as a 10% (v/v). Samples were taken every 6 h for bioethanol determination (Melo et al., 2008).
2.9.1. Detection of bioethanol by using gas chromatography (GC)
First different concentrations of ethanol absolute (GC grade) were prepared and then detected in GC (Agilent 7890 A) FID detector. Standardized water MilliQ was used as mobile phase in a flow rate of 0.6 mL/min. Sample volume was 5 µL, oven and detector temperatures were 80 °C and 40 °C, respectively.
3. Results and discussion
3.1. Isolation and identification of CMCase producing bacteria from different agricultural wastes
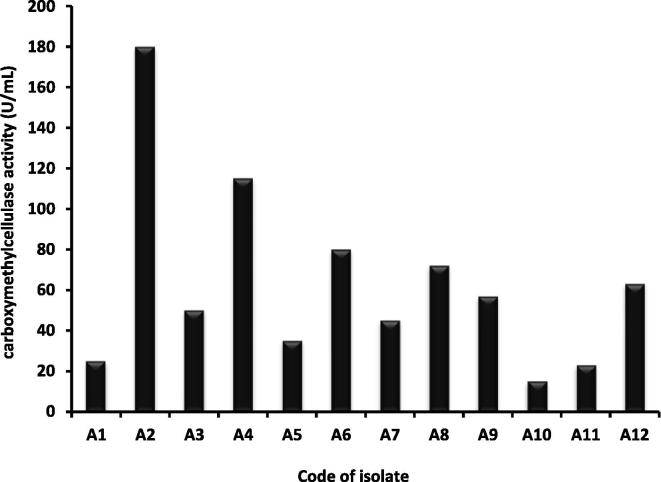
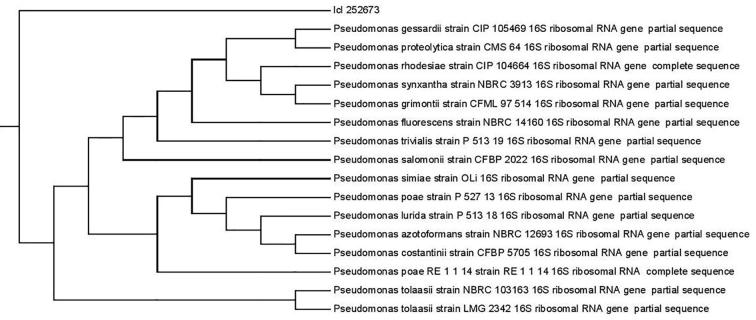
Five, 3 and 4 bacterial isolates were isolated from different sources rich in cellulose content including RS, WS and bagass respectively. A total of 12 bacterial isolates were subjected to a round for the CMCase production by using BSM supplemented by CMC. The enzyme activity was estimated in terms of (U/mL). After 1st round, one bacterial isolate shows the highest CMCase activity than the other isolates; isolate no 2 named as A2 was selected as a potential isolate for CMCase enzyme production with activity of 180 U/ml (Fig. 1). After genomic DNA separation and purification of A2 isolate, the sequence of the 16S rRNA gene was determined in order to achieve the phylogeny. Search for similar sequences in the databases using BLAST at the NCBI server (http://www.ncbi.nlm.nih.gov/blast/) and subsequent alignment of the retrieved sequences indicated that the DNA sequence of A2 isolate shows 93% homology with the 16S rRNA gene sequences of Pseudomonas poae RE∗1-1-14, the strain was identified as Pseudomonas poae as indicated in (Fig. 2).
Fig. 1.
Screening test for carboxymethylcellulase producing isolates.
Fig. 2.
Phylogenetic tree of the 16S rRNA sequence of Pseudomonas poae.
3.2. Effect of physical conditions on CMCase activity of Pseudomonas poae
3.2.1. Incubation time and inoculums volume
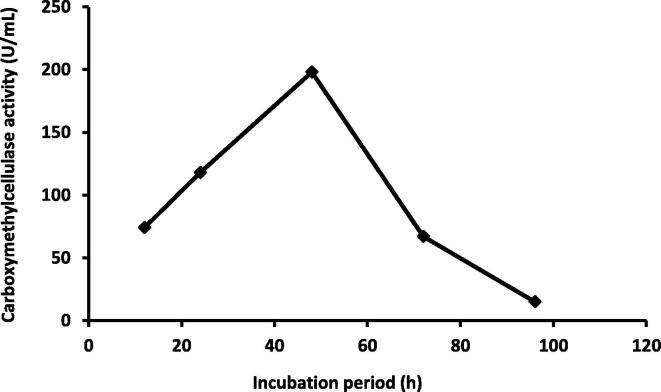
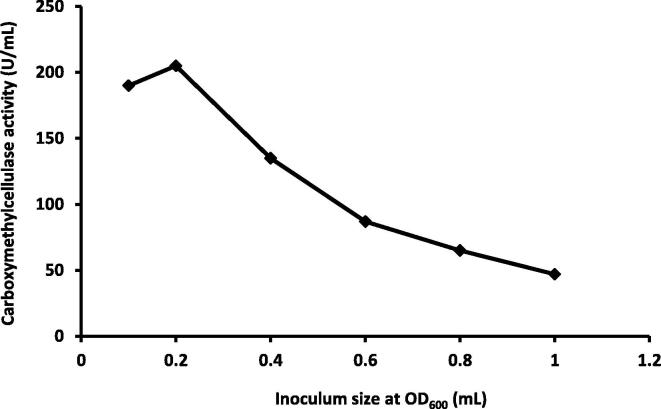
The CMCase production was increased with the increase in incubation time starting from the first 24 h. The maximum production of CMCase was observed after 48 h (198 U/mL), but after 72 h, it was decreased (Fig. 3). While, the maximum enzyme activity was achieved (205 U/mL) when 0.2 mL OD600 (3.7 × 105 CFU) was used as an inoculums (Fig. 4). Our results were in agreement with that of Goyal et al. (2014); CMCase enzyme production was increased with the increase in incubation time with maximum CMCase activity of 2.40 U/ml was reached after 60 h under stationary conditions and 2.97 U/ml at 48 h under shaking conditions. Shabeb et al. (2010) found maximum CMCase activity in Bacillus subtilis KO strain after 24 h of incubation period. Heck et al., 2002, Amritkar et al., 2004 found maximum CMCase activity in Bacillus sp. B21, Bacillus pumilus, and Bacillus subtilis after 72 h of incubation. Poorna and Prema (2007) were reported that the maximum Bacillus pumilus CMCase activity was reached after 120 h of incubation.
Fig. 3.
Relation between different incubation periods and carboxymethylcellulase activity by Pseudomonas poae.
Fig. 4.
Relation between different inoculum size (absorbance) and carboxymethylcellulase activity by Pseudomonas poae.
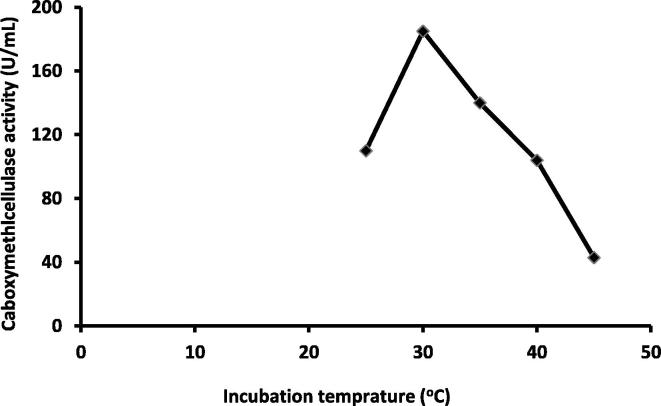
3.2.2. Incubation temperature and pH
CMCase activity of Pseudomonas poae was decreased as the temperature increased from 25 to 45 °C. The maximum CMCase activity was observed at 30 °C (185 U/ml) (Fig. 5). Lee et al. (2010) found that the highest activity of CMCase was 131.7 U/ml at 30 °C under aerobic conditions. Also, our results were in agreement with that of Goyal et al. (2014) who report that the highest CMCase activity was achieved at 30 °C under aerobic condition. Moreover, results are close to those of Kanmani et al. (2011) who found that the CMCase produced by Bacillus pumilis showed highest activity at optimum temperature of 35 °C.
Fig. 5.
Relation between incubation temperature (°C) and carboxymethylcellulase activity by Pseudomonas poae.
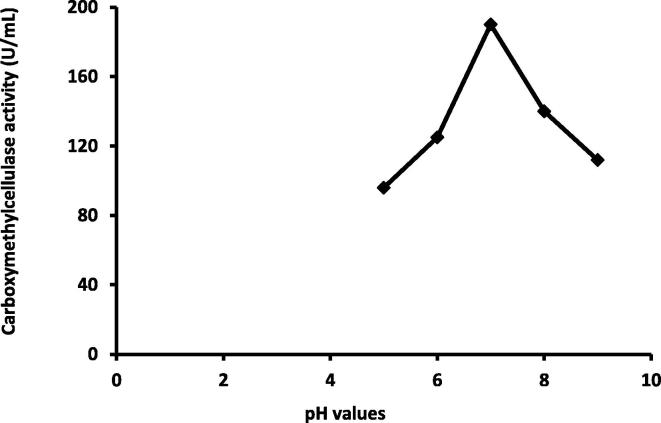
The initial pH of production medium plays an important role in the activity of CMCase. The Pseudomonas poae strain AB3 was found to produce CMCase over wide range of pH from 5 to 9 with optimum at pH 7.0 (190 U/ml) (Fig. 6). These results are in agreement with those of Immanuel et al. (2006) who found the cellulolytic enzyme, endoglucanase, obtained from Cellulomonas, Bacillus, and Micrococcus sp. hydrolyzed substrate in the pH range of 4.0–9.0, with maximum activity transpiring at pH 7. Ray et al. (2007) reported that pH 7–7.5 was more suitable for optimization of cellulase production by Bacillus subtilis and Bacillus circulans.
Fig. 6.
Relation between pH and carboxymethylcellulase activity by Pseudomonas poae.
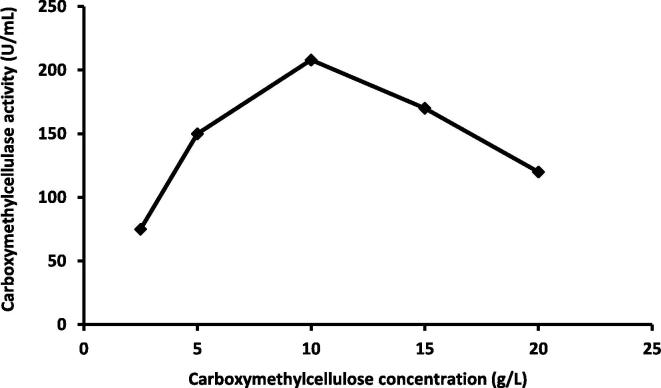
3.2.3. Effect of CMC concentrations on CMCase activity
CMC was added to the culture medium with the concentration ranging from 2.5 to 20 g/L. The maximum activity of the CMCase reached at 10 g/L (208 U/ml); while as the substrate concentration increase there was a decrease in the activity at concentration of 20 g/L (Fig. 7). Effect of different carbon sources (0.1% w/v) on the activity of CMCase was studied. CMC was most effective as a sole carbon source for CMCase production by Bacillus alcalophilus S39 (Abou-Taleb et al., 2009). CMCase was the best carbon source followed by cellulose for CMCase production (Narasimha et al., 2006, Niranjane et al., 2007). 1% (w/v) CMC was found to be optimal for carboxymethyl cellulase enzyme production in Bacillus sp. (Shikata et al., 1990) which is in agreement with our results. Goyal et al. (2014) reported that CMC was the best carbon sources at concentration of (0.1% w/v).
Fig. 7.
Relation between different carboxymethylcellulose concentrations and carboxymethylcellulase activity by Pseudomonas poae.
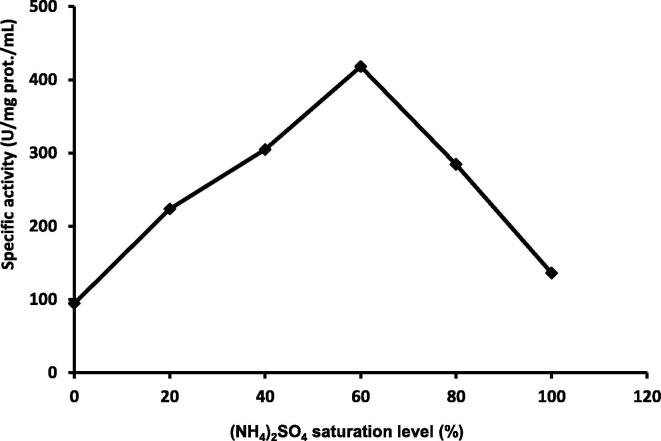
3.3. Purification of the CMCase enzyme by ammonium sulphate
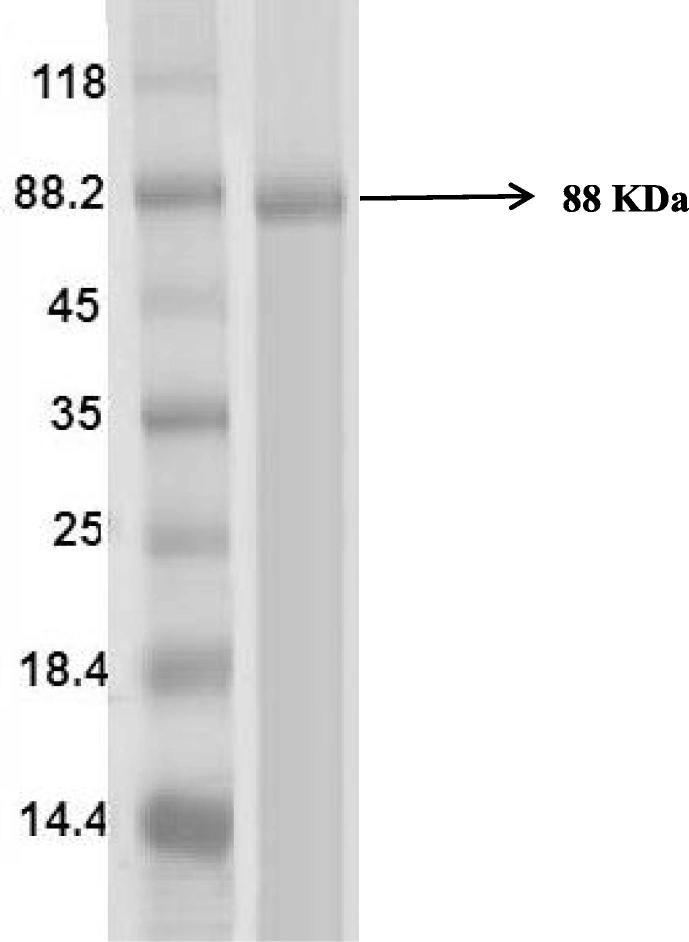
The CMCase was purified at a concentration of 60% ammonium sulphate. The CMCase showed 4-fold activities at 60% ammonium sulphate saturation (140 U/ml) and has specific activity of 418 U mg/mL protein (Fig. 8). It has been reported that Ammonium sulphate precipitation at a concentration between 40 and 80% showed high CMCase activity with 7-fold purification (Singh et al., 2001). The purified CMCase showed a single protein band on SDS-PAGE with an estimated molecular mass of 88 kDa (Fig. 9). This finding is almost similar to monomeric CMCases (86 kDa) reported in Bacillus sp. KSM-S237 (Hakamada et al., 1997). CMCases with molecular weights ranging between 40 and 92 kDa have been reported in different Bacillus sps. by various workers (Fukumori et al., 1985 Van Solingen, 1999).
Fig. 8.
Relation between ammonium sulphate saturation levels and the corresponding specific carboxymethylcellulase activities.
Fig. 9.
SDS-PAGE analysis of the purified CMCase. Molecular mass markers (lane 1), purified CMCase (lane 2).
3.4. Hydrolysis of CBC by CBCase and bioethanol production
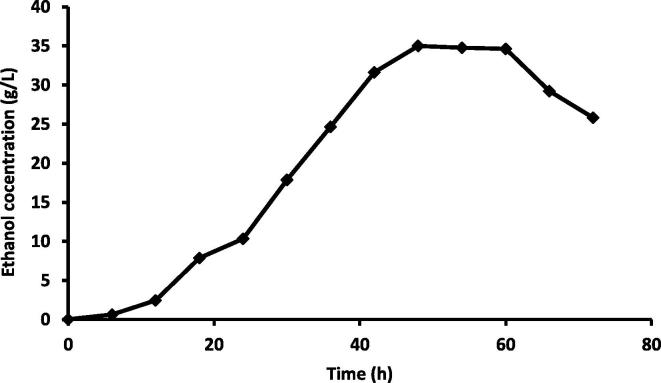
The enzymatic hydrolysis of cellulose and hemicelluolse of CBC was done with the use of purified CMCase of this study at 30 °C. This experiment were carried out utilizing 1:6 solid–liquid ratio in order to improve the enzyme action. Our results showed that, the total reducing sugar liberated from the reaction was 5220 mg/L after 48 h incubation (Table 1). While, after 48 h of fermentation, the ethanol concentration reached its maximum value (35 g/L) (Fig. 10). It has been reported that after 5 h of fermentation, the ethanol concentration reached its maximum value (34.5 g/L), corresponding to a yield of product on substrate consumed of 0.465 g and a volumetric productivity of 6.98 g/L/h.
Table 1.
Hydrolysis of caster residue by Pseudomonas poae using solid state fermentation.
| Time (h) | Total reducing sugar (mg/L) |
|---|---|
| 6 | 105.12 |
| 12 | 976.40 |
| 24 | 1852.65 |
| 36 | 3745.29 |
| 48 | 5220.64 |
Fig. 10.
Ethanol production from the fermentation step using Saccharomyces cerevisiae.
4. Conclusion
Our results indicate the successful production of bioethanol from CBC with CMCase of Pseudomonas poae. Accordingly, we can industrially benefit from the CBC waste after crushing the seed through the processes of castor oil production. Also, further studies should be done for the production of bioethanol in large scale.
Acknowledgments
Acknowledgement
The authors thank Dr. Samy El-henawy at Egyptian Petroleum Research Institute (EPRI), Biotechnology Lab, Cairo, Egypt; for their helping and supporting in many ways during this work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abada E. Production and Purification of lipase from Pseudomonas sp. AB2 with potential application in biodiesel production. J. Pure Appl. Microbio. 2014;8(spl. Edn 1):133–142. [Google Scholar]
- Abada E. Production optimization of extracellular amidase enzyme by newly isolated Pseudomonas putida Ap-2 from agricultural soil. Rendiconti Lincei Scienze Fisiche E Naturali. 2014;25(4):523–530. [Google Scholar]
- Abou-Taleb A.A.K., Mashhoor W.A., Nasr A.S., Sharaf M.S., Abdel-Azeem H.M.H. Nutritional and environmental factors affecting cellulase production by two strains of cellulolytic Bacilli. Aust. J. Basic Appl. Sci. 2009;3(3):2429–2436. [Google Scholar]
- Amritkar N., Kamat N.M., Lali A. Expanded bed affinity purification of bacterial α-amylase and cellulase on composite substrate analogue–cellulose matrices. Process Biochem. 2004;39(5):565–570. [Google Scholar]
- Aslani M.R., Malekib M., Mohria M., Sharifia K., Najjar-Nezhad V., Afshari E. Castor bean (Ricinuscommunis) toxicosis in a sheep flock. Toxicon. 2007;49(3):400–406. doi: 10.1016/j.toxicon.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Beltrão, N.E.M., 2008. Torta de mamona (Ricinuscommunis L.): fertilizante e alimento. Comunicado Técnicon. 177 da Embrapa de 2003. <http://www.cnpa.embrapa.br/plataforma_mamona/publicacoes/comunicacoes/04.PDF> (Acessoem 02 de setembro de 2008).
- Blottnitz H., Curran M.A. A review of assessments conducted on bio-ethanol as a transportation fuel from a net energy, greenhouse gas, and environmental life cycle perspective. J. Cleaner Prod. 2007;15(7):607–619. [Google Scholar]
- Demirbas A. Progress and recent trends in biofuels. Prog. Energy Combust. Sci. 2007;33:1–18. [Google Scholar]
- Fukumori F., Kudo T., Horikoshi K. Purification and properties of a cellulase from alkalophilic Bacillus sp. No. 1139. J. Gen. Microbiol. 1985;131:3339–3345. [Google Scholar]
- Goyal V., Mittal A., Bhuwal A.K., Singh G., Yadav A., Aggarwal N.K. Parametric optimization of cultural conditions for carboxymethylcellulase production using pretreated rice straw by Bacillus sp. 313 SI under stationary and shaking conditions. Biotechnol. Res. Int. 2014 doi: 10.1155/2014/651839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamada Y., Koike K., Yoshimatsu T., Mori H., Kobayashi T., Ito S. Thermostable alkaline cellulase from an alkaliphilic isolate, Bacillus sp. KSM-S237. Extremophiles. 1997;1:151–156. doi: 10.1007/s007920050028. [DOI] [PubMed] [Google Scholar]
- Heck J.X., Hertz P.F., Ayub M.A.Z. Cellulase and xylanase production by isolated amazon Bacillus strains using soybean industrial residue based solid-state cultivation. Br. J. Microbiol. 2002;33(3):213–218. [Google Scholar]
- Immanuel G., Dhanusha R., Prema P., Palavesam A. Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. Int. J. Environ. Sci. Technol. 2006;3(1):25–34. [Google Scholar]
- Kanmani R., Vijayabaskar P., Jayalakshmi S. Saccharification of banana-agro waste and clarification of apple juice by cellulase enzyme produced from Bacillus pumilis. World Appl. Sci. J. 2011;12(11):2120–2128. [Google Scholar]
- Lee B., Kim B., Lee Y., Chung C., Lee J. Industrial scale of optimization for the production of carboxymethylcellulase from rice bran by a marine bacterium, Bacillus subtilis sub sp. subtilis A-53. Enz. Microb. Technol. 2010;46(1):38–42. [Google Scholar]
- Lowry O., Rosebrough N., Farr A., Randal R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Melo W.C., dos Santos A.S., Anna S.L.M.M., Nei Pereira J.R. Acid and enzymatic hydrolysis of the residue from castor bean (Ricinuscommunis L.) oil extraction for ethanol production: detoxification and biodiesel process integration. J. Br. Chem. Soc. 2008;19(3):418–425. [Google Scholar]
- Miller G. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31(3):226–428. [Google Scholar]
- Narasimha G., Sridevi A., Viswanath B., Chandra S.M., Reddy R.B. Nutrient effects on production of cellulolytic enzymes by Aspergillus niger. Afr. J. Biotechnol. 2006;5(5):472–476. [Google Scholar]
- Niranjane A.P., Madhou P., Stevenson T.W. The effect of carbohydrate carbon sources on the production of cellulase by Phlebiagigantean. Enzyme Microb. Technol. 2007;40(6):1464–1468. [Google Scholar]
- Piddington C.S., Kovacevich B.R., Rambosek J. Sequencing and molecular characterization of a DNA region encoding the dibenzothiophene-desulfurization operon of Rhodococcus sp. IGTS8. Appl. Environ. Microbiol. 1995;61:468–475. doi: 10.1128/aem.61.2.468-475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina M., Severino L.S., Beltrão N.E.M., Villeneuve P., Lago R. Novasalternativas de valorização para dinamizar a cultura da mamona no Brasil. Cadernos de Ciência e Tecnologia. 2005;22:453–462. [Google Scholar]
- Poorna C.A., Prema P. Production of cellulase-free endoxylanase from novel alkalophilic thermotolerent Bacillus pumilus by solid-state fermentation and its application in waste paper recycling. Bioresour. Technol. 2007;98(3):485–490. doi: 10.1016/j.biortech.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Ray A.K., Bairagi A., Ghosh K.S., Sen S.K. Optimization of fermentation conditions for cellulase production by Bacillus subtilis CY5 and Bacillus circulans TP3 isolated from fish gut. Acta Ichthyologica Et Piscatoria. 2007;37(1):47–53. [Google Scholar]
- Rodhe A.V., Sateesh L., Sridevi J., Venkateswarlu B., Venkateswar Rao L. Enzymatic hydrolysis of sorghum straw using native cellulase produced by T. reesei NCIM 992 under solid state fermentation using rice straw. 3 Biotech. 2011;1:207–215. doi: 10.1007/s13205-011-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severino, L.S., 2005. O que sabemossobre a torta de mamona. Campina Grande: EmbrapaAlgodão, (Documentos, 134).
- Shabeb M.S.A., Younis M.A.M., Hezayen F.F., Eldein M.A.N. Production of cellulase in low-cost medium by Bacillus subtilis KO strain. World Appl. Sci. J. 2010;8(1):35–42. [Google Scholar]
- Shikata S., Saeki K., Okoshi H. Alkaline cellulase for laundry detergents: production by alkalophilic strains of Bacillus and some properties of crude enzymes. Agric. Biol. Chem. 1990;52:91–96. [Google Scholar]
- Silva J.G., Machado O.L., Izumi C., Padovan J.C., Chait B.T., Mirza U.A., Greene L.J. Amino acid sequence of a new 2S albumin from Ricinuscommunis which is part of a 29-kDa precursor protein. Arch. Biochem. Biophys. 1996;336(1):8–10. doi: 10.1006/abbi.1996.0526. [DOI] [PubMed] [Google Scholar]
- Singh A., Bishnoi N.R. Enzymatic hydrolysis optimization of microwave alkali pretreated wheat straw and ethanol production by yeast. Bioresour. Technol. 2012;108:94–101. doi: 10.1016/j.biortech.2011.12.084. [DOI] [PubMed] [Google Scholar]
- Singh B., Sharma Y.C. Development of biodiesel: current scenario. Renew. Sustain Energy Rev. 2009;13:1646–1651. [Google Scholar]
- Singh J., Batra N., Sobti R.C. A highly thermostable, alkaline CMCase produced by a newly isolated Bacillus sp. VG1. World J. Microbiol. Biotechnol. 2001;17:761–765. [Google Scholar]
- Singh S., Moholkar V.S., Goyal A. Optimization of carboxymethylcellulase production from Bacillus amylolique faciens SS35. 3 Biotech. 2014;4:411–424. doi: 10.1007/s13205-013-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Solingen, P., 1999. Alkalinecellulase and method of producing the same. US patent 5,858,165.
- Wang L., Yang J. Transesterification of soybean oil with nano-MgO or not in supercritical and subcritical methanol. Fuel. 2007;86:328–333. [Google Scholar]
- Wesseler J. Opportunities (‘costs) matter: a comment on Pimentel and Patzek ‘‘Ethanol production using corn, switchgrass, and wood; biodiesel production using soybean and sunflower’’. Energy Policy. 2007;35(2):1414–1416. [Google Scholar]