Abstract
Spirulina platensis has been advocated as safe food for human use by several investigators. In this study its beneficial dietary effect against liver injuries caused by d-galactosamine (d-GalN) was studied ensuring safety to human health using animal model. Acute hepatotoxicity was induced in Wister rats with d-GalN followed by treatment with butylated hydroxytoluene (BHT) and with Spirulina aqueous extract at various concentrations. The effect of Spirulina at different concentrations were tried and compared with BHT treatment. The animals treated with d-GalN on subsequent treatment by supplementation with Spirulina (6, 9%) in the diets, led to significant reversal in the levels of the antioxidant enzymes through hepatocytes by suppression of negative effect. Spirulina aqueous extract at 9% resulted in a significant decrease in the levels of alkaline phosphatase and infalmatory markers TNFα, IL6 and IL1β and also decreased TBARS, while it showed an increase in oxidative stress marker such as GR, GSH, GST, SOD, GPX and CAT and total protein when compared to the levels recorded with that group treated with d-GalN. Results also indicated that Spirulina aqueous extract at 9% concentration was equally effective in protecting liver damage as it was observed with BHT. Histological studies on liver treated with d-GalN, BHT and Spirulina aqueous extract showed that S. platensis is effective as diet in providing beneficial protective effect. The results obtained in the present study very clearly indicated the positive beneficial protective effect of Spirulina, when used as diet, on the safety and protection of liver from injuries caused by toxicants.
Keywords: Anti-inflammatory, Antioxidant, Antihepatotoxic, Spirulina platensis, Hepatotoxicity, Rats
1. Introduction
For many years, the cyanobacterium, Spirulina plantensis (SP), which is utilized as a nutritious source of dietary proteins and vitamins, has been consumed by human beings in the form of health drink and tablets (Hirahashi et al., 2002). Administration of SP has been shown to reduce the hepatotoxicity in rats (Iwata et al., 1990). Moreover, hot water extract of SP has been used as an anticancer as well as anti-viral agent although the pathways by which SP exerts these effects are not well identified (Hirahashi et al., 2002).
Cheng-Wu et al. (1994) studied the ability of SP in provoking the immune modulating system in mice. The study results showed that SP provoked the activation of peritoneal macrophages, which led to better growth for spleen cells in the presence of Concanavalin A (Cheng-Wu et al., 1994). Similar findings were observed by Hayashi et al (Hayashi, 1994) demonstrating enhancement of IL-1 and activation of antibodies after administration of hot water extract of SP in mice. Qureshi and Ali (1996) explained the ability of SP for increasing the phagocytic activity of bronchoalveolar lavage macrophages isolated from cats.
Hirahashi et al. (2002) discussed the ability of hot water extract of SP in activating the human innate immune system in 12 healthy volunteers. Their findings showed that SP extract altered the signaling pathway of Toll-like receptor 2 and 4 which resulted in high level of maturation in monocytes/macrophages, as well as the enhancement of IL-12 and p40 production (Hirahashi et al., 2002). Addition of hot water extract of SP to in vitro culture of spleen cells significantly increased proliferation of these cells with no effect on thymus cells (Kulshreshtha, 2008). The same study also described the ability of SP in potentiating the allergy reaction induced by shrimp extract that increased the levels of IgG1 and IgA with regard to IgE (Kulshreshtha, 2008).
Gad et al. (2011) demonstrated the antioxidant potential of SP in eradicating the free radicals elements alone as well as in combination with whey protein concentrate (Gad et al., 2011). A double-blind study on human subjects showed antioxidant and lipid lowering ability of SP treatment in chronic obstructive pulmonary disease (Ismail et al., 2015). Pak et al. (2012) investigated the protective effects of SP against hepatic damage of a rat model of non-alcoholic steohepatitis (NASH). They concluded that anti-inflammatory and antioxidant characteristics of phycocyanin (PC), which is the most abundant protein found in SP, are responsible for counteracting the hepatic damage (Pak et al., 2012). In this investigation, we studied the anti-inflammatory, antioxidant and hepatoprotective effects of SP in d-galactosamine (d-GaIN) induced hepatotoxicity in rats.
2. Materials and methods
2.1. Cultivation of Spirulina Platensis (SP)
Food grade SP powder was prepared using SP cultured at the Department of Botany and Microbiology, College of Science, King Saud University, Kingdom of Saudi Arabia, under the supervision of Prof Ali Al-Homaidan.
The microalga SP was obtained from the culture collection of algae at the University of Texas at Austin, USA, (UTEX NO. LB 2340). It was propagated in the laboratory according to the methods described by Praneetha, 2014, Spirulina platensis, xxxx. The strain was cultivated using Zarrouk medium (Al-Homaidan, 2002)
Outdoor cultures were carried out according to the methodology described for central Saudi Arabia (Spirulina platensis). Harvesting was done by filtration through nylon filters (150–200 mesh). After harvesting, SP was rinsed with deionized water and dried overnight in an oven at 80 °C. The dried biomass was ground well and sieved using a standard metal sieve (100 mesh) and stored in a desiccator to avoid moisture absorption. (Zarrouk, 1966) Preparation of Spirulina powder:
The aqueous extract of SP was prepared according to the method described by Al-Homaidan et al. (2015). In brief, 10 g of SP powder was weighed accurately in a beaker, added with 100 mL of distilled water, and the mixture was stirred using a magnetic stirrer. The mixture was heated cautiously in a steam bath for 1 min, sonicated to shrinkage on the surface of cells to facilitate the release of the protein phycocyanin, cooled at 4 °C, and kept in a refrigerator overnight.
2.2. Animals
Thirty-six Wistar albino rats, weighing 150 ± 20 g aged 5–6 weeks, were obtained from Animal Care Center, College of Pharmacy, King Saud University, Saudi Arabia. Animals were housed individually in single rat cages (Technoplast co.), bedded with Sawdust and fed with standard diet based on Institute of Nutrition (AIN)-93G formula (Ciferri, 1999). All rats were provided free access to water and food for the first week for acclimatization. All experimental procedures and protocols in this study including euthanasia were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (NIH Publications No. 80-23; 1996) as well as the Ethical Guidelines of the Experimental Animal Care Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
2.3. Experiment design
Rats were randomly divided into 6 groups consisting of 6 animals each. All animals were made to fast 24 h before the drug administration. The groups were as follows:
-
i.
Group A: ‘Control’ Group- This group included normal rats which received no treatment.
-
ii.
Group B: This group included rats with acute hepatotoxicity induced with a single dose of d-GalN (300 mg/kg body weight, i.p.).
-
iii.
Group C: This group included rats with acute hepatotoxicity and received treatment with 0.5% butylated hydroxytoluene (BHT) mixed with diet.
-
iv.
Group D: This group included rats with acute hepatotoxicity and received treatment with 3% SP mixed with diet.
-
v.
Group E: This group included rats with acute hepatotoxicity and received treatment with 6% SP mixed with diet.
-
vi.
Group F: This group included rats with acute hepatotoxicity and received treatment with 9% SP mixed with diet.
2.4. Drugs and chemicals
2.4.1. d-galactosamine (d-GaIN)
d-galactosamine (d-GaIN) induces acute hepatotoxicity, which is a commonly used experimental model based on its capacity to reduce the intracellular pool of uridine monophosphate in hepatocytes (Ciferri, 1999, Reeves et al., 1993). d-GaIN was obtained (Target Vision Est, Riyadh, KSA), and was Injected (IP) to rats from all the groups to induce hepatotoxicity except group A (control).
2.4.2. Butylated hydroxytoluene
Butylated hydroxytoluene (BHT) is a potent antioxidant and has been shown to protect experimental animals against various hepatotoxins such as allyl alcohol (Muntane et al., 2000). Since BHT is known to prevent chemically induced tumors as well as acute toxic effects of many chemicals, we used BHT as an standard antioxidant compound for comparative evaluation of SP.
2.5. Acute hepatotoxicity induction
Acute hepatotoxicity in rats was induced by a single intraperitoneal injection of d-GalN at a dose of 300 mg/kg body weight, while the rats in ‘Control group A’ were injected with the same volume of 0.9% saline alone (Lu et al., 2010). Diets were withheld for 4 h before and after d-GalN administration (8 h in total).
2.6. Samples collection
After one week of dietary SP treatment, animals were sacrificed under IP injected Ketamine (100 mg/Kg) and Xylazin (16 mg/Kg) anesthesia. Livers were dissected out, divided into two parts; one part (for histopathology) was transferred into a tube containing 10% buffered formalin (pH 7.4) and the other part (for biochemistry) was stored in a deep freezer (−85 °C).
2.7. Tissue homogenate
The liver samples were removed from the freezer and thawed at room temperature. Small portions of liver samples were homogenized with 10-fold volume of phosphate buffer saline (PBS) (pH = 7.4) (Fouad, 2004). The homogenate was centrifuged and the clear supernatant was transferred to a new labeled tube for biochemical analysis.
2.8. Biochemical tests
2.8.1. Superoxide dismutase (SOD)
Superoxide dismutase (SOD) was analyzed using commercial diagnostic kit (OxiSelec.TM, San Diego, USA) according to the manufacturer’s instructions.
2.8.2. Glutathione peroxidase (GPx)
Glutathione peroxidase (GPx) was analyzed using diagnostic kit (ALPCO US, Salem, USA). Protocol provided by the vendor was strictly followed for the assay.
2.8.3. Catalase (CAT) and Glutathione S-Transferase (GST)
Catalase (CAT) and Glutathione S-Transferase (GST) were analyzed using diagnostic kits (Sigma-Aldrich, Missouri St – Louis, USA). Protocol provided by the vendor was strictly followed for the assays.
2.8.4. Antioxidant-Glutathione (GSH)
Antioxidant-Glutathione (GSH) was analyzed using diagnostic kit (Sigma-Aldrich, Missouri St – Louis, USA). Protocol provided by the vendor was strictly followed for the assay.
2.8.5. Total protein (TP)
Total protein concentration was determined by BioAssay Systems' QuantiChrom TM protein assay kit. This Kit is based on an improved pyrogallol red-molybdate protein dye-binding assay. The color intensity at 600 nm is directly proportional to the total protein concentration in the sample.
2.8.6. Inflammatory cytokines (TNF-α, IL-6, IL-1β)
The levels of proinflammatory cytokines including, IL-6, IL-1 β and TNF-α were assayed by enzyme linked immunosorbent assay (ELISA) kits (ABCAM, San Francisco, USA).
2.9. Histologic examination
The portion of liver samples fixed in formalin where used for histopathology. The specimens were processed for dehydration and clearing steps and then embedded in paraffin. Sections of 4 μm thickness were made, stained with hematoxylin and eosin (H & E), and examined under light microscopy for morphological evidence of liver injury.
2.10. Statistical analysis
All statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS) version 19.0. The data were evaluated by one way analysis of variance (ANOVA) followed by Student–Newman–Keuls multiple comparison test. Differences were considered statistically significant at P value < 0.05.
3. Result and discussion
3.1. Histopathology examination
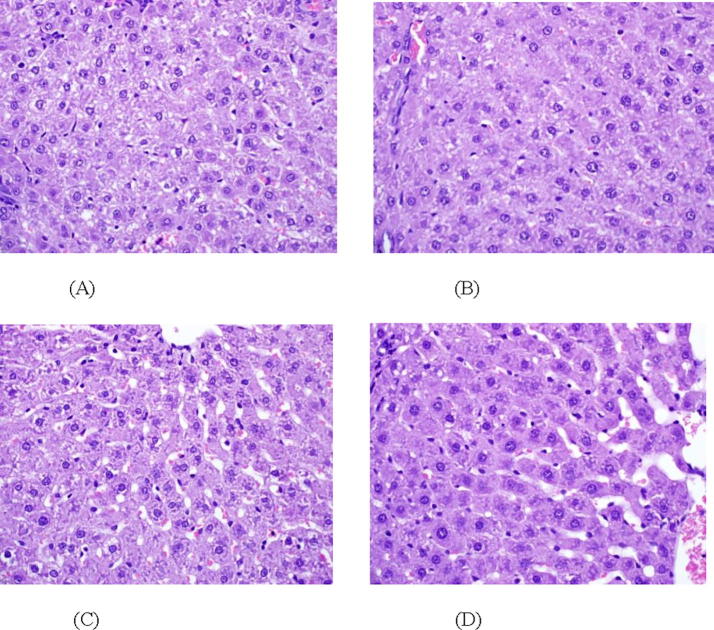
Histopathology of livers of animals treated with d-GalN showed fatty degeneration, necrosis, and apoptosis and the inflammatory cells were scattered around the congested blood vessels Fig. 1A. Fig. 1B shows a cross sectional image of rat liver that was treated with d-GalN and BHT, this image showed a marked improvement in hepatocyte architecture in different areas around the central veins and portal tracts. Concomitant treatment with SP dose-dependently protected rats against d-GalN induced structural damage in liver. The animals treated with 3% SP diet showed partial protection Fig. 1C, whereas the rats treated with 6% and 9% SP diet exhibited normal hepatocytes with neat structure Fig. 1D. These results strongly indicated the protective effect of SP on the liver tissue against d-GalN induced hepatotoxicity. There are many nutrients found in SP that may have the ability to protect the hepatocytes from any deformations whereas the phenolic compounds found in SP inhibit the liver enzymes and thus prevent any further damage to the cell structure (Humason, 1972).
Fig. 1.
(A–D) Photomicrographs of liver sections from different treatment groups; (A) Rat treated with d-GalN, (B) rat treated with d-GalN and BHT, (C) rat treated with d-GalN and SP 3% (D) Rat treated with d-GalN and SP 9%. Magnification ×400.
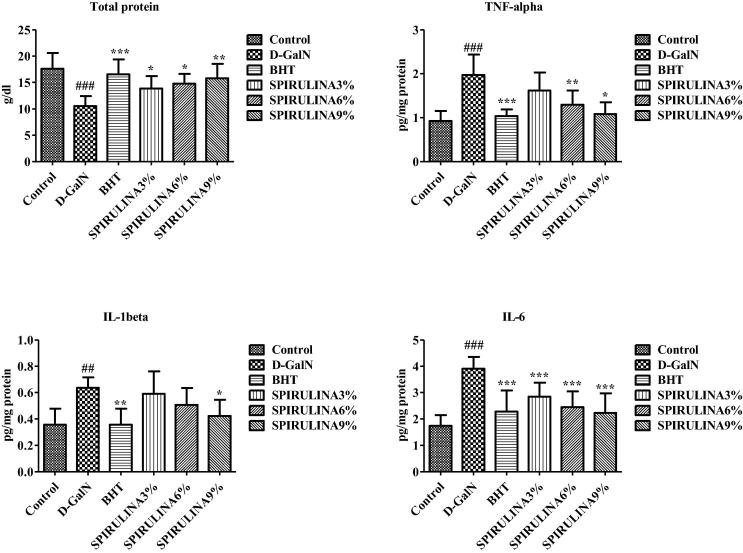
3.2. Inflammatory markers
Effects of different treatments on the levels of proinflammatory cytokines and total protein in rat liver are given in Fig. 2. Interestingly, the total protein levels were significantly reduced by d-GalN, while the co-treatment with BHT or different doses of SP significantly reversed protein-depleting effect of d-GalN. Administration of d-GalN significantly increased the levels of TNF-α, IL-1β and IL-6, which were significantly and dose-dependently reduced by SP. The protective effects of high dose of SP were comparable to BHT Fig. 2. The ability of SP in different doses to maintain the liver functions and prevent any damage due to toxic substances has been reported earlier (Pak et al., 2012). An increase in the level of inflammatory markers TNF-α, IL-1β, IL-6 and TNF-alpha indicates the activation of macrophages (Meeks et al., 2000). Significant reduction of proinflammatory cytokines by SP treatment confirms the anti-inflammatory characteristic of SP (Hirahashi et al., 2002). Only high dose SP (9%) was able to significantly reverse the d-GalN induced increase in IL-1β, which is known to cause different autoinflammatory syndromes triggered by the macrophages (Yehye et al., 2015). All doses of SP were able to significantly reduce the secretion of IL-6 that is proved to have a high impact on provoking a variety of autoimmune diseases at its high levels. This protein is having much of attention in treating many diseases such as cancer, rheumatoid arthritis, and multiple myeloma (AysunKepekci et al., 2013). Our findings suggest that beneficial effect of SP against d-GalN induced hepatotoxicity may partly be attributed to anti-inflammatory effects of SP that protected the hepatocytes during toxicant-mediated proinflammatory cascade.
Fig. 2.
Effect of different treatments on proinflammatory cytokines and total protein levels in rat liver. Data are expressed as mean ± STD. #. * Indicate significant from control vs d-GaIN and d-GaIN vs Treated groups, respectively, at P < 0.05. (n = 6).
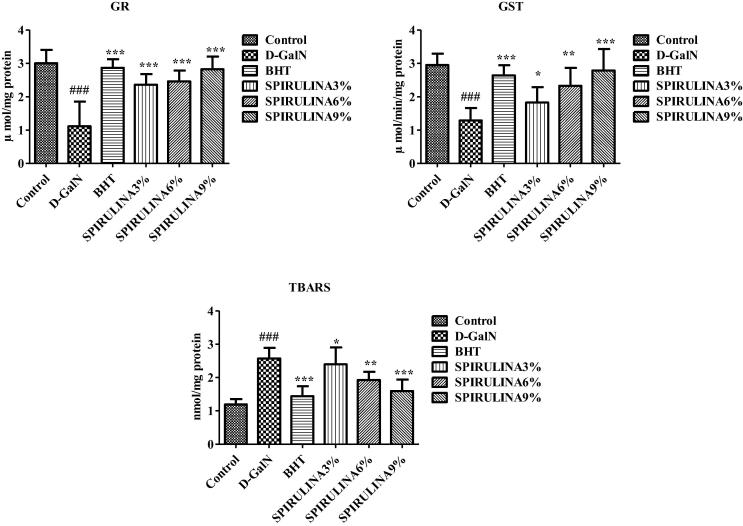
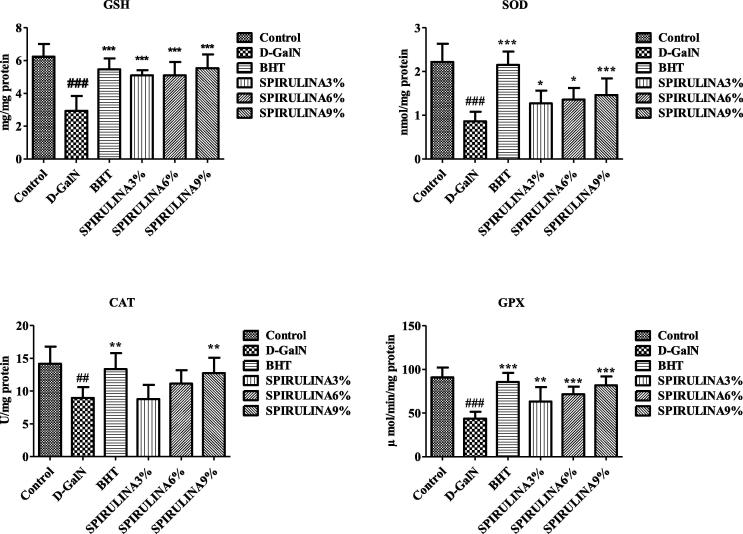
3.3. Oxidative stress
Administration of d-GalN significantly increased TBARS and reduced GR and GST in rat liver indicating the state of oxidative stress Fig. 3. The level of GSH and activities of antioxidant enzymes including SOD, CAT and GPx were also significantly reduced by d-GalN Fig. 4. In a previous study, d-GalN was found to induce significant hepatic lipid peroxidation due to the damage caused by free radicals in cirrhotic livers of rats (Praneetha, 2014). Animals treated with SP showed significant and dose-dependent protection against d-GalN induced oxidative stress in rat liver Figs. 3 and 4. Kuriakose et al. (Akao et al., 2009) have explained the major role of oxidative stress in the progression of many chronic diseases including liver damage. One of the active component of SP, C-phycocyanin, is believed to be responsible for the decrement of d-GalN induced lipid peroxidation (Praneetha, 2014, Abdel-Daim et al., 2015, Pham et al., 2017). The sulfhydryl GSH is a natural antioxidant that plays a major role in protecting cells against toxicant induced oxidative stress (Kuriakose and Kurup, 2011, Weber et al., 2003). Our results indicate that SP was able to prevent d-GalN induced hepatic damage by improving the antioxidant status and reducing the cellular oxidative stress.
Fig. 3.
Effect of different treatments on markers of oxidative stress in rat liver. Data are expressed as mean ± STD. #. * Indicate significant from control vs d-GaIN and d-GaIN vs Treated groups, respectively, at P < 0.05 (n = 6).
Fig. 4.
Effect of different treatments on antioxidant enzyme levels in rat liver. Data are expressed as mean ± STD. #. * Indicate significant from control vs d-GaIN and d-GaIN vs Treated groups, respectively, at P < 0.05 (n = 6).
4. Conclusion
SP is a green alga that is known to have many nutrients and supplements. Our results clearly showed anti-inflammatory, antioxidant and hepatoprotective properties of SP in rats. However, the clinical implication of these findings with liver disease of humans is not clear. Future investigations may involve testing the pharmacological potential of SP in patients with hepatic and other chronic inflammatory diseases.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Daim M.M., Farouk S.M., Madkour F.F., Azab S.S. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol. Immunotoxicol. 2015;37(2):126–139. doi: 10.3109/08923973.2014.998368. [DOI] [PubMed] [Google Scholar]
- Akao Y., Ebihara T., Masuda H., Saeki Y., Akazawa T., Hazeki K., Hazeki O., Matsumoto M., Seya T. Enhancement of antitumor natural killer cell activation by orally administered Spirulina extract in mice. Cancer Sci. 2009;100(8):1494–1501. doi: 10.1111/j.1349-7006.2009.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Homaidan Ali A. Large-scale cultivation of Spirulina in Saudi Arabia. Saudi J. Biol. Sci. 2002;8(2):13–23. [Google Scholar]
- Al-Homaidan A.A., Alabdullatif J.A., Al-Hazzani A.A., Al-Ghanayem A.A., Alabbad A.F. Adsorptive removal of cadmium ions by Spirulina platensis dry biomass. Saudi J. Biol. Sci. 2015;22(6):795–800. doi: 10.1016/j.sjbs.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Wu, Z., Chao-Tsi, T., Yuan-Zhen, Z., 1994. The effects of polysaccharide and phycocyanin from Spirulina platensis on peripheral blood and hematopoietic system of bone marrow in mice. In: Book of Abstracts. Second Asia Pacific Conference on Algal Biotechol. p. 58.
- Ciferri O. Microbial degradation of paintings. Appl. Environ. Microbiol. 1999;65:879–885. doi: 10.1128/aem.65.3.879-885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad D., Siendones E., Costan G., Muntane J. Role of NF-kappaB activation and nitric oxide expression during PGE1 protection against d-galactosamine-induced cell death in cultured rat hepatocytes. Liver Int. 2004;24:227–236. doi: 10.1111/j.1478-3231.2004.00913.x. [DOI] [PubMed] [Google Scholar]
- Gad Ahmed S., Khadrawy Yasser A., El-Nekeety Aziza A., Mohamed Sherif R., Hassan Nabila S., Abdel-Wahhab Mosaad A. Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition. 2011;27(5):582–589. doi: 10.1016/j.nut.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Hayashi O., Katoh T., Okuwaki Y. Enhancement of antibody production in mice by dietary Spirulina platensis. J. Nutr. Sci. Vitaminol. 1994;40(5):431–441. doi: 10.3177/jnsv.40.431. [DOI] [PubMed] [Google Scholar]
- Hirahashi Tomohiro, Matsumoto Misako, Hazeki Kaoru, Saeki Yoshiko, Ui Michio, Seya Tsukasa. Activation of the human innate immune system by Spirulina: augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int. Immunopharmacol. 2002;2(4):423–434. doi: 10.1016/s1567-5769(01)00166-7. [DOI] [PubMed] [Google Scholar]
- Humason G.L. third ed. W.H. Freeman and Company; San Francisco: 1972. Animal Tissue Techniques. [Google Scholar]
- Md. Ismail, Md. Faruk Hossain, Arifur Rahman Tanu, Hossain Uddin Shekhar, 2015. Effect of spirulina intervention on oxidative stress, antioxidant status, and lipid profile in chronic obstructive pulmonary disease patients. BioMed Res. Int., 2015, Article ID 486120, p. 7. [DOI] [PMC free article] [PubMed]
- Iwata K., Inayama T., Kato T. Effects of Spirulina platensis on plasma lipoprotein lipase activity in fructose-induced hyperlipidemic rats. J. Nutr. Sci. Vitaminol. 1990;36(2):165–171. doi: 10.3177/jnsv.36.165. [DOI] [PubMed] [Google Scholar]
- Kepekçi Remziye Aysun, Polat Sait, Çelik Ahmet, Bayat Nuray, Demirörs Saadet, Saygideger Protective effect of Spirulina platensis enriched in phenolic compounds against hepatotoxicity induced by CCl4. Food Chem. 2013;141:1972–1979. doi: 10.1016/j.foodchem.2013.04.107. [DOI] [PubMed] [Google Scholar]
- Kulshreshtha A. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008;9(5):400–405. doi: 10.2174/138920108785915111. [DOI] [PubMed] [Google Scholar]
- Kuriakose G.C., Kurup M.G. Antioxidant and antihepatotoxic effect of Spirulina laxissima against carbon tetrachloride induced hepatotoxicity in rats. Food Funct. 2011;2(3–4):190–196. doi: 10.1039/c0fo00163e. [DOI] [PubMed] [Google Scholar]
- Meeks, Robert G., Harrison, Steadman D., Bull, Richard J., 2000. Hepatotoxicity, biochemical methods of studying hepatotoxicity chapter. In: Raos, Prasada, Kodacanti, Hehendale, Harhara M., (Eds.), CRC Press Inc., Florida, USA, p. 267.
- Muntane J., Rodriguez F.J., Segado O., Quintero A., Lozano J.M., Siendones E., Pedraza C.A., Delgado M., O'Valle F., Garcia R., Montero J.L., de La M.M., Mino G. TNF-alpha dependent production of inducible nitric oxide is involved in PGE(1) protection against acute liver injury. Gut. 2000;47:553–562. doi: 10.1136/gut.47.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak Wing, Takayama Fusako, Mine Manaka, Nakamoto Kazuo, Kodo Yasumasa, Mankura Mitsumasa, Egashira Toru, Kawasaki Hiromu, Mori Akitane. Anti-oxidative and anti-inflammatory effects of spirulina on rat model of non-alcoholic steatohepatitis. J. Clin. Biochem. Nutr. 2012;51(3):227–234. doi: 10.3164/jcbn.12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T.X., Park Y.K., Bae M., Lee J.Y. The potential role of an endotoxin tolerance-like mechanism for the anti-inflammatory effect of Spirulina platensis organic extract in macrophages. J. Med. Food. 2017;20(3):201–210. doi: 10.1089/jmf.2016.0119. [DOI] [PubMed] [Google Scholar]
- Praneetha Pallerla, Rani Vanapatla Swaroopa, Reddy Yellu Narasimha, Kumar Bobbala Ravi. Hepatoprotective studies on methanolic extract of whole plant of Lindernia ciliata. Bangladesh J. Pharmacol. 2014;9(4):567–574. [Google Scholar]
- Qureshi M., Ali R. Spirulina platensis exposure enhances macrophage phagocytic function in cats. Immunopharmacol. Immunotoxicol. 1996;18(3):457–463. doi: 10.3109/08923979609052747. [DOI] [PubMed] [Google Scholar]
- Reeves P.G., Nielsen F.H., Fahey G.C., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Spirulina platensis (Arthrospira) Physiology cell-biology and biotechnology, p. 131.
- Weber L.W., Boll M., Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- Yehye W.A., Rahman N.A., Ariffin A., Abd Hamid S.B., Alhadi A.A., Kadir F.A., Yaeghoobi M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): a review. Eur. J. Med. Chem. 2015;101:295–312. doi: 10.1016/j.ejmech.2015.06.026. [DOI] [PubMed] [Google Scholar]
- Zarrouk C. University of Paris; France: 1966. Contribution a l’etude d’une cyanobacterie: influence de divers facteurs physiques et chimiques sur la croissance et la photosynthese de Spirulina maxima (Setchell et Gardner) Geitler. PhD thesis. [Google Scholar]