Abstract
This study was conducted at the apiary of the Agricultural and Veterinary Training and Research Station of King Faisal University in the Al-Ahsa oasis of eastern Saudi Arabia. We performed a comparison between Carniolan (Apis mellifera carnica Pollmann) and Yemeni (Apis mellifera jemenitica Ruttner) honeybee races to determine the monthly fluctuations in foraging activity, pollen collection, colony growth and honey yield production under the environmental conditions of the Al-Ahsa oasis of eastern Saudi Arabia. We found three peaks in the flight activity of the two races, and the largest peaks occurred during September and October. Compared to Carniolan bee colonies, the performance of Yemeni bee colonies was superior in terms of stored pollen, worker and drone brood rearing, and the adult population size. The Carniolan bee colonies produced 27.77% and 27.50% more honey than the Yemeni bee colonies during the flow seasons of alfalfa and sidir, respectively, with an average increase of 27.64%. It could be concluded that the race of bees is an important factor affecting the activity and productivity of honeybee colonies. The Yemeni bee race produced more pollen, a larger brood and more bees, which exhibited a longer survival. The imported Carniolan bees can be reared in eastern Saudi Arabia, but the Yemeni bee race is still better.
Keywords: Apis mellifera carnica, A. m. jemenitica, Foraging, Brood, Pollen, Honey, Al-Ahsa
1. Introduction
The activity of a honeybee (Apis mellifera L.) colony depends on several factors that arise simultaneously and in reaction to some environmental conditions. These factors include nectar and pollen floral resources (Taha, 2000, Taha, 2005, Taha, 2007, Taha et al., 2006, Taha et al., 2016, Taha and Bayoumi, 2009), colony population size (Georgijev et al., 2003, Taha, 2005, Jevtić et al., 2009, Taha and AL-Kahtani, 2013, de Mattos et al., 2015), season (AL-Kahtani, 2003, Shawer et al., 2003, Indu et al., 2007, Taha and AL-Kahtani, 2013), and species/subspecies of honeybees (Khanbash, 1989, Rahman and Rahman, 1993, Tan et al., 2012, Alqarni et al., 2014, Taha et al., 2016, Al-Ghamdi et al., 2017, Awad et al., 2017). In tropical and sub-tropical areas, foraging for nectar and pollen is a continuous process throughout the year where bee floral resources are available (Neupane and Thapa, 2005). The foraging activities of honeybees for pollen are greatly affected by the availability of pollen and weather conditions (Taha, 2007, Taha, 2014). However, the Al-Ahsa oasis is not in tropical or sub-tropical areas. It is one of the world’s largest oases with a cultivated area exceeding 10,000 hectares (Anon, 2014), so the activities of honeybee colonies are influenced by the type of cultivated crops grown in this area as well as weather factors (Taha, 2014).
The highest rate of foraging activity under the environmental conditions of Al-Ahsa was recorded during September and October, followed by August and then May (Taha, 2014). The foraging behavior of Carniolan honeybees on alfalfa flowers in Al-Ahsa, eastern Saudi Arabia showed two peaks: the first one at 0800–0900 hrs and the second and highest peak at 1700–1800 hrs. Additionally, the peak of gathering pollen was at 0800–0900 hrs (Taha et al., 2016).
In the semi-arid conditions that cover large parts of Saudi Arabia, the temperatures during the summer season often surpass 40 °C in different regions of the country. The honeybee colonies not only decrease foraging activity during the high temperature hours but also spend some of that time consuming honey for thermoregulation. Consequently, the productivity of the colony is affected (Taha, 2014, Taha, 2015c).
This study aimed to compare the activity and productivity of Carniolan and Yemeni (native) subspecies under the environmental conditions of Al-Ahsa, eastern Saudi Arabia.
2. Materials and methods
Experiments were conducted at the apiary of the Agricultural and Veterinary Training and Research Station, King Faisal University, Al-Ahsa oasis, eastern Saudi Arabia from the beginning of April 2015 to the end of March 2016. Al-Ahsa lies at latitude 25° 25′ 46″N and longitude 49° 37′ 19″E at an altitude of 121 m above sea level. Ten Carniolan (Apis mellifera carnica Pollmann) bee colonies headed by newly mated sister queens obtained from a honeybee breeder (Germany) and ten Yemeni (Apis mellifera jemenitica Ruttner) bee colonies headed by newly mated sister queens were used to perform the following experiments.
The number of forager bees for each colony was estimated by counting the number of incoming workers entering the colony within one minute. The number of pollen foragers was estimated by counting the number of incoming workers with pollen loads during the same period. The counts were carried out periodically once a week at 0700–0800 hrs during April to September, and 0900–1000 hrs during October to March. The counts were taken during periods when peak flight activity was noted (Taha, 2014).
The areas (square inches) of stored pollen and worker and drone sealed broods were measured in 12-day intervals using an empty standard frame divided into square inches, and the overall monthly areas were determined. The monthly population size of the colony was determined by counting the numbers of combs covered with bees/colony every month. Honey yield was harvested at the end of alfalfa (Medicago sativa L.) season flow at the end of June. Sidir (Ziziphus spp.) honey was harvested at the end of October. The average honey yield per colony was estimated by counting the difference between the weight of honeycombs in each colony before and after extraction.
The meteorological factors of air temperature, relative humidity, wind velocity, rainfall, soil temperature at a 5-cm depth, solar radiation and net radiation were obtained from the meteorological station of the Agricultural and Veterinary Training and Research Station of King Faisal University in Al-Ahsa, Saudi Arabia. The system used for data recording was the Cambell Scientific CR 3000.
Data were statistically analyzed with analysis of variance (ANOVA). Treatment means were compared using Duncan's Multiple Range Test (Duncan, 1955). Pearson correlation between the number of foragers, number of pollen foragers, areas of stored pollen and worker and drone sealed broods, colony population size, honey yield and some weather factors was determined using SAS® software (SAS Institute, 2003).
3. Results
The data listed in Table 1 indicated that the highest monthly mean values of relative humidity (51.21%), rainfall (0.0028 mm) and wind velocity (2.89 m/s.) were recorded during January, March and April, respectively. The maximum values of solar radiation (0.25 kW/m2) and net radiation (102.40 W/m2) were recorded during June. July and August were characterized by the maximum values of air temperature (37.68 °C and 37.15 °C, respectively) and soil temperature (37.49 °C and 38.23 °C, respectively). In contrast, January was characterized by the minimum values of air temperature (15.31 °C), soil temperature (20.66 °C) and solar radiation (0.13 kW/m2).
Table 1.
The monthly mean values of some meteorological factors in the Al-Ahsa oasis, Saudi Arabia during 2015/2016.
| Months | Air temperature, oC | Relative humidity% | Wind velocity, m/s | Rainfall, mm | Soil temperature, oC | Solar radiation, kW/m2 | Net radiation, W/m2 |
|---|---|---|---|---|---|---|---|
| April 2015 | 21.23 | 35.08 | 2.89 | 0.001 | 25.03 | 0.17 | 70.33 |
| May | 34.28 | 18.59 | 2.79 | 0.0003 | 33.34 | 0.23 | 110.1 |
| June | 35.61 | 14.93 | 1.72 | 0.00 | 35.23 | 0.25 | 102.4 |
| July | 37.68 | 22.04 | 1.6 | 0.00 | 37.49 | 0.24 | 100.2 |
| August | 37.15 | 27.93 | 0.99 | 0.00 | 38.23 | 0.23 | 83.24 |
| September | 33.56 | 27.98 | 1.42 | 0.00 | 36.8 | 0.21 | 64.55 |
| October | 27.43 | 27.54 | 0.79 | 0.00 | 32.49 | 0.18 | 38.79 |
| November | 21.35 | 41.20 | 1.03 | 0.0023 | 28.2 | 0.14 | 28.96 |
| December | 17.71 | 48.20 | 1.65 | 0.00 | 24.18 | 0.13 | 42.17 |
| January 2016 | 15.31 | 51.21 | 2.36 | 0.0001 | 20.66 | 0.13 | 52.91 |
| February | 17.44 | 41.75 | 2.41 | 0.0012 | 21.03 | 0.16 | 71.39 |
| March | 22.38 | 42.78 | 1.77 | 0.0028 | 25.02 | 0.17 | 90.74 |
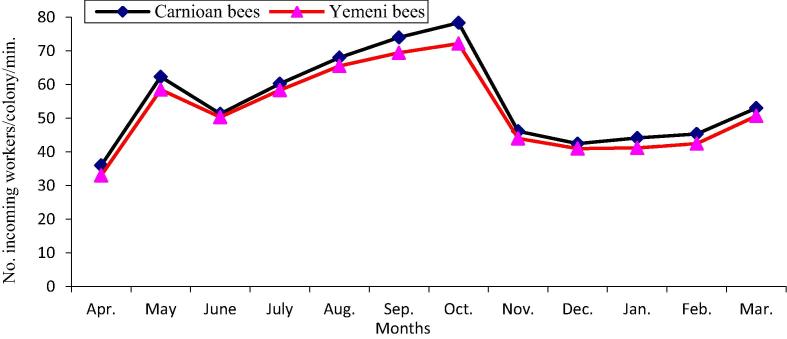
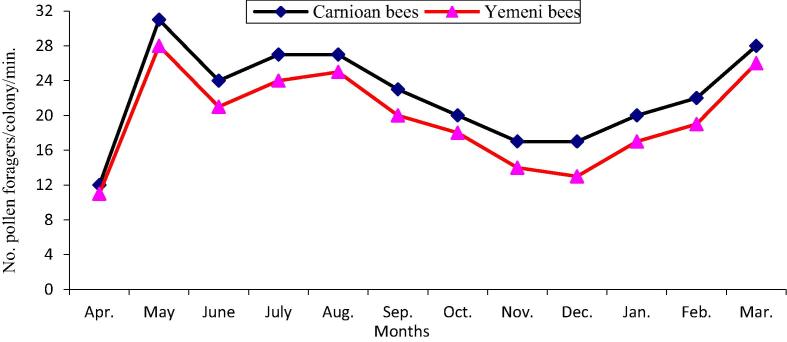
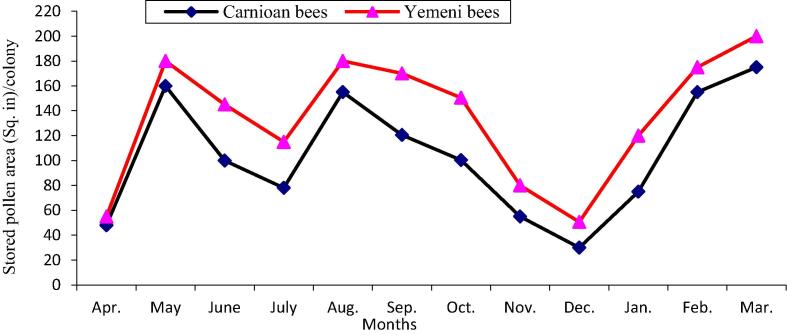
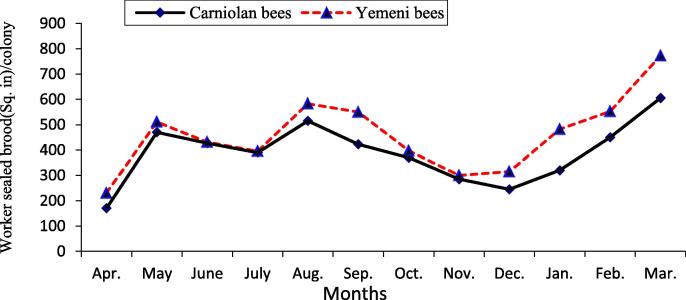
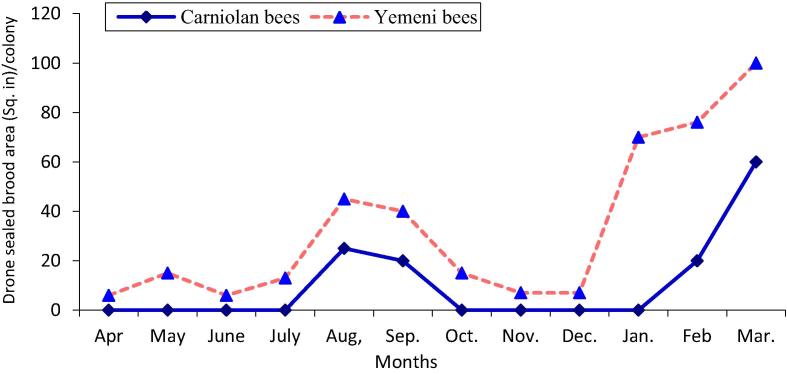
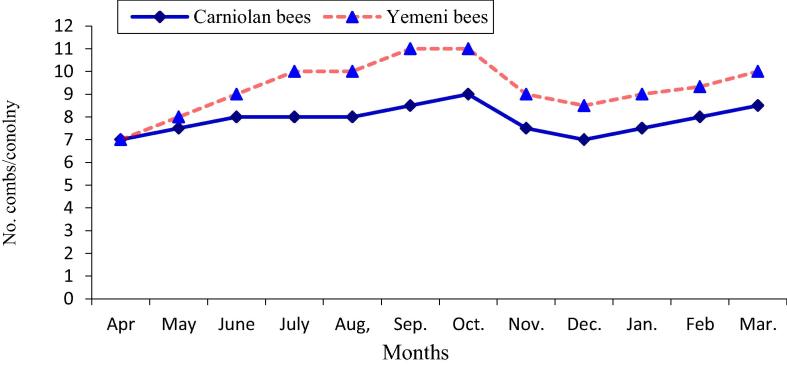
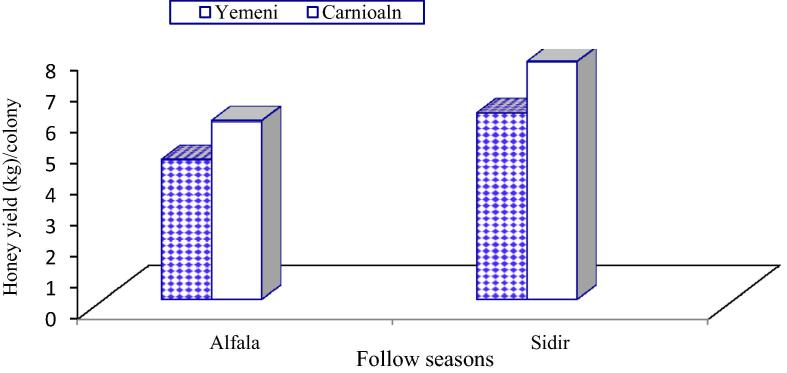
The data illustrated graphically in Fig. 1, Fig. 2 show that the monthly average number of forager workers and the number of pollen foragers in Carniolan bee colonies were significantly (P < 0.01) higher compared to Yemeni bee colonies. In contrast, the monthly averages of stored pollen and worker and drone sealed brood areas and of colony population size in Yemeni bee colonies were significantly (P < 0.01) higher compared to the Carniolan bee colonies (Fig. 3, Fig. 4, Fig. 5, Fig. 6). The Carniolan bee colonies produced more honey than the Yemeni colonies (5.75 and 7.65 kg/colony vs. 4.50 and 6.00 kg/colony for clover and sidir flow seasons, respectively) (Fig. 7).
Fig. 1.
Monthly fluctuation of foraging activity of Carniolan and Yemeni bees in Al-Ahsa region during 2015 year.
Fig. 2.
Monthly fluctuation of pollen gathering rate of Carniolan and Yemeni bees in Al-Ahsa region during 2015 year.
Fig. 3.
Monthly fluctuation stored pollen area of Carniolan and Yemeni bees in Al-Ahsa region during 2015 year.
Fig. 4.
Monthly fluctuation of worker sealed brood area of Carniolan and Yemeni bees in Al-Ahsa region during 2015 year.
Fig. 5.
Monthly fluctuation of drone sealed brood area of Carniolan and Yemeni bees in Al-Ahsa region during 2015 year.
Fig. 6.
Monthly fluctuation of population size of Carniolan and Yemeni bees in Al-Ahsa region during 2015 year.
Fig. 7.
Honey yield (kg)/colony of Carniolan and Yemeni bees in Al-Ahsa region during 2015 year.
The seasonal variations in the activities of the honeybee colonies showed that the highest number of retuned workers/colony/min and the largest population size (number of combs/colony/month) were recorded during October in both the Carniolan and Yemeni bee colonies. Meanwhile, May was found to be the best month for pollen gathering activity (pollen foragers) in both bee races, while the largest areas of stored pollen and worker and drone sealed broods were recorded during March for both bee races.
The data presented in Table 2 show significantly (P < 0.01) positive correlations between the number of forager bees and the number of pollen foragers, stored pollen area, worker and drone sealed brood areas, colony population size, honey yield, solar radiation, and net radiation. The correlations between the number of forager bees and both the air temperature and soil temperature were significantly (P < 0.05) positive as well. Honey yield was significantly (P < 0.01) and positively correlated with the number of forager bees, number of pollen foragers, stored pollen area, worker and drone sealed brood areas, and colony population size. On the other hand, the correlations between relative humidity and the number of pollen foragers, stored pollen area, and worker sealed brood area were significantly negative.
Table 2.
Pearson correlation coefficients for number of foragers, number of pollen foragers, areas of stored pollen, worker and drone sealed brood, colony population size, honey yield and some weather factors.
| Items | No. foragers | No. Pollen foragers | Stored pollen area | Worker sealed brood area | Drone sealed brood area | Colony population size | Honey yield |
|---|---|---|---|---|---|---|---|
| No. foragers | |||||||
| No. pollen foragers | 0.94** | ||||||
| Stored pollen area | 0.89** | 0.96** | |||||
| Worker sealed brood area | 0.88** | 0.95** | 0.95** | ||||
| Drone sealed brood area | 0.70** | 0.80** | 0.74** | 0.68** | |||
| Colony population size | 0.94** | 0.81** | 0.78** | 0.74** | 0.55** | ||
| Honey yield | 0.88** | 0.76** | 0.74** | 0.86** | 0.48** | 0.92** | |
| Air temperature | 0.38* | 0.35* | 0.34* | 0.46** | 0.14 | −0.08 | |
| Relative humidity | −0.28 | −0.30* | −0.31* | −0.40** | −0.20 | 0.12 | 0.18 |
| Wind velocity | 0.16 | 0.07 | 0.05 | −0.04 | 0.18 | −0.17 | −0.09 |
| Rainfall | −0.22 | −0.22 | −0.21 | −0.25 | −0.21 | −0.04 | −0.12 |
| Soil temperature | 0.33* | 0.30* | 0.32* | 0.48** | 0.10 | 0.05 | 0.28 |
| Solar radiation | 0.40** | 0.35* | 0.33* | 0.45** | 0.14 | −0.16 | 0.25 |
| Net radiation | 0.40** | 0.36* | 0.32* | 0.34* | 0.10 | −0.18 | 0.23 |
Indicate correlation is significant at the 0.05 level (2-tailed).
Indicate correlation is significant at the 0.01 level (2-tailed).
4. Discussion
Foraging for nectar and/or pollen continued throughout the year. In addition to bee race, the foraging activity of honeybee colonies is influenced by the availability of bee floral resources, colony population size and weather factors. In this study, nutritional factors, population size and weather factors were similar across all colonies, so the effects should have been the same regardless of bee race. Therefore, the differences should have been related to bee race. The rate of foraging activity in Carniolan bee colonies was higher compared to the Yemeni bee colonies. These results correlated with the results obtained by Alqarni et al. (2014) in the Assir region of southwestern Saudi Arabia. Additionally, Tan et al. (2012) found that A. cerana started foraging earlier and at lower temperatures than A. mellifera. The peak foraging also occurred earlier and at a lower temperature than A. mellifera foraging. Under the environmental conditions of the Al-Ahsa oasis, the highest numbers of forager workers were recorded during September and October, which coincided with the maximum population size during sidir (Ziziphus spp.) flow season, followed by August when sunflower (Helianthus annuus L.) and some Cucurbitaceae plants were blooming and then May, which coincided with alfalfa (Medicago sativa L.) flow season (Taha, 2015a, b). These results are similar to the results obtained by Helal et al. (2003), who found that the highest numbers of forager bees were recorded during May, followed by July and August. A significantly positive correlation (r = 0.94) between the number of foragers and colony population size was found. Our results confirmed the results obtained by Murray and Eaton, 1995, Taha, 2014. The lowest numbers of forager workers were recorded during April. The decrease in flight activity during April might be due to the shortage of pollen and nectar floral resources (Taha and AL-Kahtani, 2013, Taha, 2014) as well as the presence of migratory bee-eater (Merops spp.) birds in the apiary area, which cause a decline in worker flights (Ali and Taha, 2012, Taha, 2014). The correlation between the number of foragers and air temperature was significantly positive (r = 0.38), while the correlation with relative humidity was negative and not significant (r = -0.28). Honeybees were found to prefer a relatively higher air temperature for nectar collection than for pollen collection, whereas they preferred a higher relative humidity for pollen collection than for nectar collection (Gebremedhn et al., 2014).
The area of stored pollen in colonies depends on the amount of collected pollen and the rate of pollen consumption in the colony. The correlation (r = 0.96) between pollen foragers and stored pollen area was significantly (P < 0.01) positive. The average monthly area of stored pollen in Yemeni bee colonies exceeded the pollen stored by Carniolan bee colonies by 29.50%. Similar results were obtained by Al-Ghamdi et al. (2017) in the Al-Baha region of southwestern Saudi Arabia and Awad et al. (2017) in central Arabia. The highest numbers of incoming workers with pollen loads were recorded in May when alfalfa was blooming, followed by March, which coincided with the blooming period of date palms (Phoenix dactylifera L.), rape (Brassica napus L.), Cucurbitaceae and sunflower (Taha, 2015a, Taha, 2015b). The largest area of stored pollen in the colonies was recorded during March. These results are similar to results obtained by Taha (2015a) in Saudi Arabia and by Rahman and Rahman, 1993, Sattigi and Lingappa, 1993 in India. Meanwhile, the largest area of stored pollen was recorded during May in Egypt (Shawer et al., 2003, Taha, 2005) and in Yemen (Khanbash and Bin Ghodel, 1994, Al-Humyarie et al., 1999). On the other hand, the maximum areas of stored pollen were found during September in Hawaii (Arita and Fujii 1992). The large areas of stored pollen during previous periods were correlated (r = 0.96) with the high numbers of pollen foragers and the availability of major pollen sources during these periods. The amount of stored pollen in the colony increased in parallel to the rate of brood rearing (Shawer, 1987, Georgijev et al., 2003, Taha and AL-Kahtani, 2013, Taha, 2014, Taha, 2015c).
The lowest rates of pollen gathering activity, including pollen foragers and the area of stored pollen, were recorded during April and then December. The decrease in the pollen gathering rate during April may be the result of decreases in the amount of gathered pollen because of the decrease of the flight activity rate due to the scarcity of pollen sources (Taha, 2015a) and the occurrence of migratory bee-eater birds in the apiary area, which leads to a decline in worker flights (Ali and Taha, 2012, Taha, 2014). The decreasing rate of gathering and storing pollen during December was due to the shortage of pollen sources in this period. Additionally, pollen gathering activity is affected by environmental conditions, including weather factors and bee floral resources (Taha, 2014).
The area of sealed broods and the population of adult bees were used as indicators of the strength of the honeybee colony (Taha and Al-Kahtani, 2013). In addition to the race of bees, brood rearing and population growth in the colonies are influenced by several factors, including the availability of bee floral resources, weather factors and colony population size. Except for the race of bees, all factors affected the colonies in a similar manner, so the effects were likely due to the bee race. The Yemeni bee colonies were found to be significantly (P < 0.01) superior to Carniolan bee colonies in terms of worker and drone brood rearing as well as adult population size. The monthly average area of worker sealed broods in Yemeni bee colonies exceeded the average in Carniolan bee colonies by 20.33%. Similar results were recorded by Alqarni, 1995, Alqarni et al., 2014 in the Assir region, Al-Ghamdi et al. (2017) in the Al-Baha region of southwestern Saudi Arabia, and Awad et al. (2017) in central Arabia. The monthly average brood rearing of drones in Yemeni bee colonies exceeded the average in Carniolan bee colonies by 215.93%.
The largest average monthly worker and drone sealed brood areas were obtained during March, which formed the major peak, followed by August, which formed the second peak for both races. These results are in agreement with the results obtained by AL-Kahtani, 2003, Taha, 2014, Taha, 2015c in Saudi Arabia and Shawer et al. (2003) in Egypt. In India, the largest worker sealed brood area was found in May (Rana and Goyal, 1994). The peaks of the sealed brood area coincided with the peaks of stored pollen in the colonies (Taha, 2014). The worker sealed brood area was significantly (P < 0.01) and positively correlated with the number of forager bees (r = 0.88), number of pollen foragers (r = 0.95), stored pollen area (r = 0.95), and air temperatures (r = 0.46). Similar results were reported by Jevtić et al., 2009, Taha and AL-Kahtani, 2013, and Taha (2014) because they found significant positive correlations between the area of stored pollen and worker sealed brood areas. Meanwhile, a significant (P < 0.01) negative correlation (r = 0.40) between the worker sealed brood area and relative humidity was found. These results are in accord with the results of Indu et al. (2007), and Taha (2014), who found negative correlations between brood area and relative humidity.
The monthly increase of the population size (No. of combs) in Yemeni bee colonies compared to Carniolan bee colonies was estimated as 18.97%. The large population size in Yemeni bee colonies was correlated with the large amount of reared brood. Our results are in agreement with the findings of Al-Ghamdi et al. (2017) in the Al-Baha region, southwestern Saudi Arabia and Awad et al. (2017) in central Arabia. Some genotypes had higher adult bee populations in their origin area than outside it (Hatjina et al., 2014). Yemeni bees have a local genotype in Saudi Arabia, so this could explain why the Yemeni bees survive longer and build a larger colony population. A significantly (P < 0.01) positive correlation (r = 0.74) between the worker sealed brood area and colony population size was obtained. The larger population size in Yemeni bee colonies reflects the longer survival of bees due to the adaptation of the Yemeni bees to harsh weather in Saudi Arabia. The largest adult population size occurred during September and October (sidir season flow) due to the large worker sealed broods in August and September. According to Fathy (1996), the highest rates of brood rearing and stored pollen were found in May, and consequently, the maximum number of adult bees was observed in June and July. Due to the decrease in pollen collection as a result of the dearth of most pollen floral resources and the season of migratory bee-eater birds in April, a low rate of brood rearing was observed, which resulted in low bee population sizes (Taha, 2014).
The harvested honey yield per colony was significantly (P < 0.01) influenced by the race of bees. The Carniolan bee colonies surpassed Yemeni bee colonies in honey yield by 27.78 and 27.50% during the clover and sidir seasons, respectively. Our results were confirmed by the data recorded by Alqarni, 1995, Alqarni et al., 2014 in the Assir region and Al-Ghamdi et al. (2017) in the highland of the Al-Baha region, southwestern Saudi Arabia. Meanwhile, the opposite trend was observed in the lowland of the Al-Baha region (Al-Ghamdi et al., 2017) and in central Arabia (Awad et al., 2017). The high honey yield produced by Carniolan bee colonies compared to that produced by Yemeni bee colonies might be correlated to the body size of workers and subsequently to the size of the nectar sac, which is larger for Carniolan bees. The honey yield was found to be correlated with the weight of the nectar sac of returned workers (Taha, 2000). However, the lower amount of honey harvested from the Yemeni bee race may be explained by the larger population size. A larger colony population (and with more drones) consumes more honey to survive; moreover, the population consumes a large part of its own honey during the rearing of more broods. The harvested honey yield during sidir season was larger than the honey yield during clover season. Similar results were found by Taha and AL-Kahtani, 2013, Taha, 2015c. This might be the result of variations in colony population size, which was larger in sidir season, as well as in the cultivated area and nectar secretion of each crop (Taha, 2005, Taha, 2007).
5. Conclusion
Based on the obtained data in this study, it can be concluded that the Carniolan bee race produces slightly more honey than the Yemeni bee race; however the Yemeni bee race produces more pollen, larger broods and more bees, which exhibited a longer survival, and these bees can thus pollinate more. Finally, our results indicate that the imported Carniolan bees can be reared in eastern Saudi Arabia, but the Yemeni bee race is still better.
Acknowledgement
The authors express their sincere appreciation to International Cooperation and Knowledge Exchange Administration, King Faisal University for moral support and financial funding for the Project No. 2/2-008.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Humyarie A.A., El-Sherif M.E., Naser Kh.S.A. Brood rearing, food storage and worker longevity of Yemeni bee colonies and their Carniolan hybrid. J. Agric. Sci. Mansoura Univ. 1999;24:1345–1358. [Google Scholar]
- Al-Ghamdi A., Adgaba N., Tadesse Y., Getachew A., Al-Maktary A. Comparative study on the dynamics and performances of Apis mellifera jemenitica and imported hybrid honeybee colonies in southwestern Saudi Arabia. Saudi J. Biol. Sci. 2017;24:1086–1093. doi: 10.1016/j.sjbs.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.A., Taha E.A. Bee-eating birds (Coraciiformes: Meropidae) reduce virgin honeybee queen survival during mating flights and foraging activity of honeybees (Apis mellifera L.) Inter. J. Sci. Eng. Res. 2012;3:1–8. [Google Scholar]
- AL-Kahtani, S.N., 2003. Ecological studies on some activities of honeybee colonies under Al-Hasa district conditions- Kingdom of Saudi Arabia. Unpublished M.Sc. Thesis, Fac. Agric. & Foods Sci., King Faisal Univ. KSA, 195 pp.
- Alqarni, A.S., 1995. Morphometrical and biological studies of the native honeybee race, Apis mellifera L., the Carnioan, A. m. carnica Pollmann, and their F1 hybrid. M.Sc. Thesis. Fac. Agric. Sci. King Saud Univ., Riyadh, KSA.
- Alqarni A.S., Balhareth H.M., Owayss A.A. Performance evaluation of indigenous and exotic honeybee (Apis mellifera L.) races in Assir region, southwestern Saudi Arabia. Saudi J. Biol. Sci. 2014;21:256–264. doi: 10.1016/j.sjbs.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon. Saudi Arabia; PP: 2014. 27th Agricultural statistics yearbook; p. 68. [Google Scholar]
- Arita L.H., Fujii J.K. Quantity and seasonal variation of pollen Types collected by honeybees at two localities on island of Hawaii. Proc. Hawaiian Entomol. Soc. 1992;31:119–123. [Google Scholar]
- Awad A.M., Owayss A.A., Alqarni A.S. Performance of two honey bee subspecies during harsh weather and Acacia gerrardii nectar-rich flow. Sci. Agric. 2017;74:474–480. [Google Scholar]
- Duncan B.D. Multiple Range and Multiple F. test, Biometrics. 1955;11:1–42. [Google Scholar]
- Fathy H.M. Honeybee colony population in relation to brood rearing and stored pollen. Apiacta. 1996;31:36–44. [Google Scholar]
- Gebremedhn H., Tadesse A., Belay T. Relating climatic factors to foraging behavior of honeybees (Apis mellifera) during blooming period of Guizotia abyssinica (L.F.) Livestock Res. Rural Develop. 2014;26 Article No. 20. [Google Scholar]
- Georgijev, A., Mladenović, M., Nedić, N., 2003. Experimental calculation of the correlation between the cell surface and the intake of nectar and pollen in bee colonies. In: 38th Apimondia Int. Apic. Cong., Aug. 24–29, Ljubljana, Slovenia, 760.
- Hatjina F., Costa C., Büchler R., Uzunov A., Drazic M., Filipi J., Charistos L., Ruottinen L., Andonov S., Meixner M.D., Bienkowska M., Dariusz G., Panasiuk B., Le Conte Y., Wilde J., Berg S., Bouga M., Dyrba W., Kiprijanovska H., Korpela S., Kryger P., Lodesani M., Pechhacker H., Petrov P., Kezic N. Population dynamics of European honey bee genotypes under different environmental conditions. J. Apic. Res. 2014;53:233–247. [Google Scholar]
- Helal R.M., El-Dakhakhni T.N., Shawer M.B., Taha E.A. Effect of moving the apiaries on activity of honeybee colonies. 2- Flight activity, gathering of nectar and sugar concentration contents and honey. J. Agric. Res. Tanta Univ. 2003;29:268–282. [Google Scholar]
- Indu V., Kumar P.A., (Late) Rathore R.R. Effect of weather parameters on brood development of European honeybee, Apis mellifera L. in different seasons. J. Entomol. Res. 2007;31:347–354. [Google Scholar]
- Jevtić G., Mladenović M., Anđelković B., Nedić N., Sokolović D., Štrbanović R. The correlation between colony strength, food supply and honey yield in honeybee colonies. Biotechnol. Animal Husb. 2009;25:1141–1147. [Google Scholar]
- Khanbash, M.S., 1989. The relationship between population size and amount of pollen collected by Carniolan and Italian honeybee colonies. Agrartudomanyi Egyetem tudomanyos Kozlemenyei 28, 179−195 (English Summary). (Apic. Abst. 514/93).
- Khanbash M.S., Bin Ghodel A.Y. Seasonal collection and storage of pollen in honeybee colonies with different sizes. J. Yemeni Agric. Res. Aden Univ. 1994;5:1–14. [Google Scholar]
- de Mattos I.M., de Souza J., Soares A.E. Analysis of the effects of colony population size and feeding supplementation on bee pollen production. J. Apic. Res. 2015;54:411–419. [Google Scholar]
- Murray J.E., Eaton L.J. Honeybee hive strength assessments and relationship to lowbush blueberry pollination. Canadian Honey Council Symp. Proc. 1995:105–115. [Google Scholar]
- Neupane K.R., Thapa R.B. Pollen collection and brood production by honeybees (Apis mellifera L.) under Chitwan condition of Nepal. J. Inst. Agric. Anim. Sci. 2005;26:143–148. [Google Scholar]
- Rahman S., Rahman A. Comparative pollen gathering activity of Apis cerana indica Fabr. and Apis mellifera L., Under Johrot conditions of Assam (India) Indian Bee J. 1993;55:42–46. [Google Scholar]
- Rana B.S., Goyal N.P. Comparative brood rearing activity of Apis mellifera and Apis cerana indica at Nauni (Solan), mid-hills of Himachal Pradesh. Indian Bee J. 1994;55:42–46. [Google Scholar]
- SAS Institute, 2003. SAS/STAT User’s Guide release 9.1. SAS Institute Inc, Cary, NC 27513.
- Sattigi H.N., Lingappa S. Foraging activities of Indian honeybee Apis Cerana Fabr. Under Dhrwad Conditions Karnataka. J. Agric. Sci. 1993;6:352–354. [Google Scholar]
- Shawer M.B. Major pollen sources in Kafrelsheikh, Egypt and the effect of pollen supply on brood area and honey yield. J. Apic. Res. 1987;26:43–46. [Google Scholar]
- Shawer M.B., El-Dakhakhni N.M., Helal R.M., Taha E.A. Effect of moving the apiaries on activity of honeybee colonies. 1- Gathering and storing pollen, brood rearing and wax secretion. J. Agric. Res. Tanta Univ. 2003;29:250–267. [Google Scholar]
- Taha, E.A., 2000. Effect of transferring the apiaries on activity of honeybee colonies. Unpublished M.Sc. Thesis, Fac. Agric. Tanta Univ. Egypt, 117 pp.
- Taha, E.A., 2005. Studies on honeybee (Apis mellifera L.). Unpublished Ph.D. Thesis, Fac. Agric. Tanta Univ. Egypt, 151 pp.
- Taha E.A. Importance of banana Musa sp. (Musaceae) for honeybee Apis mellifera L. (Hymenoptera: Apidae) in Egypt. Bull. Ent. Soc. Egypt. 2007;II:125–133. [Google Scholar]
- Taha E.A. Seasonal variation of foraging activity, pollen collection and growth of honeybee colonies in Al-Ahsa. Saudi Arabia. Bull. Ent. Soc. Egypt. 2014;91:163–175. [Google Scholar]
- Taha E.A. A study on nectar and pollen sources for honeybee Apis mellifera L. in Al-Ahsa. Saudi Arabia. J. Entomol. Zool. Stud. 2015;3:272–277. [Google Scholar]
- Taha E.A. Chemical composition and amounts of mineral elements in honeybee-collected pollen in relation to botanic origin. J. Apic. Sci. 2015;59:75–81. [Google Scholar]
- Taha E.A. The impact of feeding certain pollen substitutes on maintaining the strength and productivity of honeybee colonies (Apis mellifera L.). Bull. Ent. Soc. Egypt. Econ. Ser. 2015;41:63–74. [Google Scholar]
- Taha E.A., Bayoumi Y.A. The value of honeybee (Apis mellifera L.) as pollinator of summer seed watermelon (Citrullus lanatus colothynthoides L.: Cucurbitaceae) in Egypt. Acta Biol. Szeg. 2009;53:33–37. [Google Scholar]
- Taha E.A., AL-Kahtani S.N. Relationship between population size and productivity of honeybee colonies. J. Entomol. 2013;10:163–169. [Google Scholar]
- Taha E.A., Nour M.E., Shawer M.B. Loofah, Luffa aegyptiaca Mill. (Cucurbitaceae), a source of nectar and pollen for honeybee Apis mellifera L. (Hymenoptera: Apidae) in Egypt. Bull. Ent. Soc. Egypt. 2006;83:337–345. [Google Scholar]
- Taha E.A., Al-Abdulsalam M., Al-Kahtani S.N. Insect pollinators and foraging behavior of honeybees on alfalfa (Medicago sativa L.) in in Saudi Arabia. J. Kansas Entomol. Soc. 2016;89:92–99. [Google Scholar]
- Tan K., Yang S., Wang Z., Radloff S.E., Oldroyd B.P. Differences in foraging and broodnest temperature in the honey bees Apis cerana and A. mellifera. Apidologie. 2012;43:618–623. [Google Scholar]