Abstract
Wounds are common clinical entities of life which may be subacute or acute. Wound healing is a complex biochemical process where the cell structures are restored to normalcy, which depend on cell proliferation and migration, basically fibroblast cell. The present investigation was undertaken to evaluate the healing efficacy of red pigment isolated from marine isolate Vibrio sps on experimental wounds in albino rats. The red pigment was applied topically, twice daily for 14 days. Treatment with framycetin ointment was used as reference control. The red pigment treated group showed faster reduction in wound area in comparison with control and framycetin ointment treated groups. In conclusion, red pigment possesses significant healing potential in wounds and has a positive influence on the different phases of wound repair.
Keywords: Pigments, Marine microbes, Vibrio sps, Wound healing, Anti bacterial
1. Introduction
Wounds are major case of physical disabilities which are caused by environmental insults such as mechanical and chemical injuries (Sheeba et al., 2009). Wound healing is an interactive process of complex cascade system among cellular and bio chemical actions which involves continuous cell - cell and cell matrix interactions that allow the process to proceed in different overlapping phases such as inflammation, wound contraction, Re-epithelialization tissue, re modeling, & formation of granulation tissue with angiogenesis (Kumarasamyraja et al., 2012). Several factors including bacterial infection, necrotic tissue & interference with blood supply, lymphatic blockage & diabetes mellitus delay/reduce the wound healing process. Among them bacterial infection is the external factor which can effectively delay the wound healing process. Hence protecting the wound from bacterial infection by any agent can promote an increase in healing rate. But in recent years, human pathogenic microorganisms have developed resistance mechanisms for commercially available antibiotics. Moreover, the production costs of these antibiotics are too high and also they cause adverse side effects when compared to natural obtained bioactive drugs (Grasian Immanuel et al., 2012). Natural products have always played a significant role in medicine and in meticulous, marine metabolites have increasingly turned out to be major players in recent drug discovery. Marine biodiversity is the richest and complex ecosystem; its harsh environmental conditions such as salinity, varying physical and chemical properties are the driving forces for the production of various natural products with unique features (Grasian Immanuel et al., 2012). In fact, marine microbial flora were reported to produce a wide variety of bioactive secondary metabolites as antimicrobial, and cytotoxic agents and the bioactive substances included alkaloids, polyketides, cyclic peptide, polysaccharide, phlorotannins, diterpenoids, pigments, sterols, quinones, lipids and glycerols (Ines Mancini et al., 2007). Among them pigments produced by microbial flora play a significant role in human health care therefore, the utilization of natural pigments in pharmaceutical manufacturing processes has been increasing in recent years. Hence, microbial pigment production is now one of the emerging fields of research to demonstrate its potential for various industrial applications (Chidambaram and Perumalsamy, 2009). On a continuous effort in this direction, a red pigment isolated from marine microbial strain was investigated for its wound healing activity along with antimicrobial property to understand its role as antimicrobial activity assisted wound healing process.
2. Materials and methods
2.1. Chemicals
All the chemicals and solvents used in the study were of analytical grade and purchased from Merck Millipore (Billerica, MA, USA).
2.2. Isolation of marine bacteria
Seawater samples were collected in the intertidal zone at coastal locations of Nellore Krishnapatnam, India. The samples were spread over the entire surface of marine agar plates consisting of g l−1: peptone 5.0, yeast extract1.0, ferric citrate 0.1, sodium chloride 19.45, magnesium chloride 8.8, sodium sulphate 3.24, calcium chloride 1.8, potassium chloride 0.55, sodium bicarbonate 0.16, potassium bromide 0.08, strontium chloride 0.034, boric acid 0.022, sodium silicate 0.004, sodium fluoride 0.0024, ammonium nitrate 0.0016, disodium phosphate 0.008, agar 15.0. After incubation at 25 °C for 48 h, all colonies were screened and those with different pigmentation and morphology were isolated.
2.3. Biochemical characterization
Different tests were performed to characterize the biochemical properties of the isolated strain, including those for establishing temperature tolerance, pH tolerance and response to NaCl concentrations (minimum, optimum and maximum). Specific tests for Gram staining, spore staining, motility, indole production, MR-VP test, gelatin hydrolysis, citrate utilization, triple sugar iron agar, oxidase activity, catalase production, nitrate reduction, urease production and starch hydrolysis were performed according to standard methodology. The ability to ferment the sugars such as arabinose, sucrose, glucose, fructose, rhamnose, xylose, raffinose and mannitol as sole carbon sources was also evaluated for the isolated strains.
2.4. Molecular-based characterization
The molecular identification of 16S rRNA was performed at the Microbial Type Culture Collection Centre, IMTECH, Chandigarh, India. DNA was extracted from the culture and its quality was evaluated by agarose (1.2%) gel electrophoresis. A fragment of the 16S rDNA gene was amplified by PCR and purified. Forward and reverse DNA sequencing reactions were carried out with primers reverse and forward primers Giovannoni, 1996 using BDT v3.1 Cycle sequencing kit on ABI 3730xl Genetic Analyzer. Standard nucleotide BLAST searches using the identified 16S rRNA sequence as a query were performed against NCBI’s 16S ribosomal RNA sequences (Bacteria and Archaea) database. Additional searches were conducted against NCBI genomes, whole genome shotgun contigs, and the non-redundant nucleotide collection databases also available on NCBI Gene bank (www.ncbi.nlm.nih.gov). Sequences were aligned using the online version of MAFFT. The multiple sequence alignment was trimmed and manually adjusted where necessary. A phylogenetic tree displaying the evolutionary relationships between sequences was obtained with the maximum likelihood method implemented in RAxML. Node support was estimated with 1000 rapid bootstrap replicates.
2.5. Fermentation process
A loopful of culture was transferred to pre-sterilized 50 ml Zobell marine broth in 250 ml flask. The flasks were incubated in stationary condition at 28 °C for 16–18 h or until the absorbance of the culture reached optical density of 1.0 at 600 nm. One ml of the inoculum was transferred to the production medium (100 ml Zobell marine broth) in a 250 ml Erlenmeyer flask. The flask was incubated at 28 °C for 72 h.
2.6. Extraction and measurement of produced pigment
Pigment was extracted according to Slater et al., 2003. Briefly, 10 ml of the culture was removed from the broth and centrifuged at 100 × 100 g for 10 min. Pigment extraction was performed by adding methanol to the pellet and incubating at 60 °C for 20 min followed by centrifugation (100 × 100 g, 10 min). The colored supernatant was analyzed in UV –Visible spectrophotometer for detecting the absorption maximum (λ max) by scanning in the range of 400–600 nm and absorbance peak (λ max) was used for further investigation.
2.7. Purification and characterization
Pigment produced by bacterium was purified by few steps, the microbial pigment from the crude methanol extracts were filtered (whatman filter paper) to remove any residual biomass and then concentrated by using rotary evaporator (Sanco Rotavapor). A chloroform/water liquid –liquid extraction was carried out to remove hydrophilic impurities. The organic phase, containing the pigment, was concentrated by rotary evaporator. In the next step the dried extract was dissolved in chloroform and then applied to silica gel column 18 cm height and 20 mm width. The column was then packed with silica gel (column chromatography grade) and washed twice with n-hexane. The concentrated sample obtained was placed on the top of the column and separation initiated with the addition of solvent. Different fractions consisting of different colors ranging from light yellow to bright red color and the red colored fraction was collected from the column.
2.8. IH Nuclear Magnetic Resonance Spectroscopy (NMR Spectroscopy)
Nuclear magnetic resonance spectroscopy (NMR spectroscopy) is the technique which exploits the magnetic properties of certain nuclei. When placed in a magnetic field, NMR active nuclei absorb at a frequency characteristic of the isotope. The resonant frequency, energy of the absorption and the intensity of the signal are proportional to the strength of the magnetic field. Since this frequency shift is proportional to the strength of the magnetic field, it is converted into a field-independent dimensionless value known as the chemical shift. By understanding different chemical environments, the chemical shift can be used to obtain some structural information about the molecule in a sample. The conversion of the raw data to this information is called assigning the spectrum. The 'H-NMR spectra of the purified Red pigment was analyzed at Indian institute of chemical technology (IICT), HYDERABAD Andhra Pradesh, INDIA (Krishna et al., 2015).
2.9. Wound healing activity
2.9.1. Experiment animals
Wistar albino rats weighing about 8–10 weeks (150–250 g), were used for the study (Mahaveer Agencies, Hyderabad). The rats were fed with standard rodent pellet diet and were housed in polypropylene cages maintained under standard conditions (12 h light - dark cycle; 25 ± 3 °C; 35–60% humidity). The animals were left for ten days at room conditions for acclimatization. A minimum of six animals were used in each group. The experimental rats used and the protocols followed in this study were reviewed and approved by the Institutional Animal Ethics Committee (44/SPIPS/IAEC/13) prior to the initiation of the experiment.
2.9.2. Preparation of ointment
For assessment of wound healing activity by excision wound model the extract were formulated in the form of ointment. The ointment is prepared by fusion method. For preparation of simple ointment the ingredients used includes wool fat, hard paraffin, cetosteryl alcohol, white soft paraffin which were heated according to increasing order of their melting point and mixed gently with stirring followed by cooling and packing in wide mouth container. In same manner 10% ointment of red pigment was prepare and pack in wide mouth container.
2.9.3. In vivo wound healing activity
The cutaneous excision model was used to assess the wound healing activity of the red pigment. The wound was inflicted at dorsal side on the rats as described in the literature (Mori et al., 2002). Before inflicting the excision wounds, the experimental rats were anesthetized by intraperitoneal administration of ketamine (70 mg/kg body weight), and the fur on the dorsal side of the animals was shaved using an aseptic surgical blade and disinfected with 40% of ethanol. A circular excision wound, of 300 mm2 and 0.2 cm depth, was created with a surgical blade on the dorsal surface at the thoracolumbar region of each of the experimental rats, under sterile conditions (Morton and Melon, 1972, Hamill et al., 2003, Hassan et al., 2011) (Fig. 1). To each experimental animal, 10% red pigment ointments were applied topically twice a day on the wound till they completely healed. The progressive changes in wound were monitored planimetrically by tracing the wound margin on graph paper every alternate day. Epithelialisation time was noted as a number of days after wound required for the scar to fall off leaving no raw wound behind. From the healed wound, a specimen sample of tissue is isolated from each group of rats for histopathological examination.
Fig. 1.
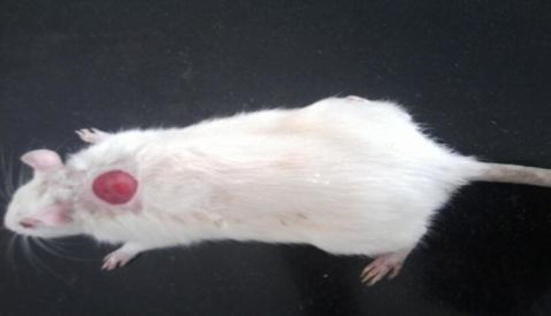
Photographic representation of excision wound in rat.
2.9.4. Dosage used in wound healing studies
The effect of red pigment on wound healing activity was studied in male Wistar rats. A total of 24 rats weighing 150–200 g were randomly selected and divided into four groups consisting of six rats in each group. Rats in each of the different experimental groups topically received an application of 10% of cream containing the following test compounds:
The animals are divided into four groups each group consisting of 6 rats
-
•
Group I: Control group Without treatment
-
•
Group II: Ointment base Apply ointment base
-
•
Group III: Standard Treated with 2% framycetin ointment
-
•
Group IV: Test group Treated with 10% red pigment extract ointment
2.9.5. Measurement of wound index
The wound indices were measured after every 2 days of wound formation following a random scoring system. The healing property was evaluated as percentage of wound contraction, measuring the length and size of the wound with digital callipers following the Walker and Mason formula (Walker et al., 1968). Significance in wound healing of the test groups was derived by comparing the healed wound area on the respective days, with the healed wound area of the control group. The rate of wound contraction was calculated using the given formula:
2.9.6. Skin irritation studies
This study was performed to observe any skin irritation for the animal model. Three sites were selected on the dorsal side of the rat. One side serve as control and other two sides was applied with standard ointment and extracted 10% red pigment ointment and observed the results for skin irritation.
2.9.7. Histopathology studies
The histological changes, i.e. epithelialization, granulation tissue formation and cell migration, were observed during the process of wound healing in individual experimental rats that treated with red pigment. On the 16th day postwounding, all the animals were sacrificed, and the granulation tissue formed on and around the excision wounds of the untreated and treated rats was carefully dissected with a sterile surgical knife and carefully collected without any folding, and weighed. Later, the sample tissues were fixed in 10% buffered formalin solution (pH 7.4) and stored. After the usual processing of the tissue in dehydrated alcohol, these tissues were cleared in xylene and were embedded in paraffin wax (melting point 55 °C). Skin samples from wound healing sites were taken for histopathological studies. The skin tissue biopsies were cut into 5 µm thick sections and stained with hematoxylin and eosin. The sections were then observed under a light microscope (Olympus BX51) for qualitative assessment of the degree of necrosis, epithelialization, collagen formation and fibroblast proliferation in the wound tissues. Congestion, edema, PNL, mononuclear cells, fibroblasts and vascularization were also qualitatively evaluated for treated and untreated rats.
2.9.8. Statistical analysis
All the results represented in the study were the mean ± Standard error mean (S.E.M) of six rats in each group. The significance of the difference of the mean value with respect to control group was analyzed by one way ANOVA followed by Dunnet’s t-test using Statistica 8.0. Statistically significant at a level of P < .05 or above was considered to be significant.
3. Results
3.1. Isolation and screening
Marine samples collected from Nellore marine area were used to isolate pigment producing microbial strains using Zobell marine agar plates. A total of 70 different pigmented bacterial colonies were isolated upon incubation at 30 °C, purified and preserved. Among the isolated strains, the strain which produced red pigment was selected for further studies and designated as SKMASRSP9. The extracted crude red pigment was initially evaluated for antimicrobial nature and noticed that this crude extract has significant antibacterial activity against both gram positive and gram negative bacteria. Antibacterial activity in marine bacteria is a well-known phenomenon and has been demonstrated in a number of studies (Isnansetyo and Kamei, 2003, Uzair et al., 2006). The isolated strain was further evaluated for wound healing activity and antimicrobial studies. The resulting hydrophobic crude extracts (120 mg) were collected and by thin layer chromatography (20% ethyl acetate and n-hexane) it was observed the presence of three compounds. The three compounds were separated by column chromatography using gradient elution method using n-hexane and ethyl acetate solvents. The column was packed by choosing the column width 8 cm and height 25 cm. The column was loaded up to 12 cm with silica gel (100–200 mesh) and 120 mg crude material was also loaded on the column. First pure compound, 8 mg was obtained with n-hexane as eluent solvent. The second pure compound 15 mg was obtained with 6% ethyl acetate and n-hexane solvent mixture as elution solvent. The third compound 85 mg was obtained with 50% ethyl acetate and n-hexane solvent mixture as eluent. Of the three compounds the second pure compound was obtained as a red colour liquid and was found to exhibit significant biological actions. These spectroscopic analyses led to the assignment of structure for the new compound as pyranone benzoate derivative i.e., Ethyl 4-(6-oxo tetra hydro-2H-pyron-2carbonyl) benzoate where reported in our earlier (Krishna et al., 2015).
3.2. Skin irritation studies
All the three (ointment base, 10% red pigment ointment & 2% framycetin) was shown negative results for skin irritation studies. Neither redness nor any type of inflammation was observed for the three (Sudha Bhargavi et al., 2013) (Table 1).
Table 1.
Skin irritation test.
| Group | Sign | Score |
|---|---|---|
| Control | ----- | 0 |
| Red pigment ointment | No noticeable redness and inflammation | 0 |
3.3. Wound healing activity
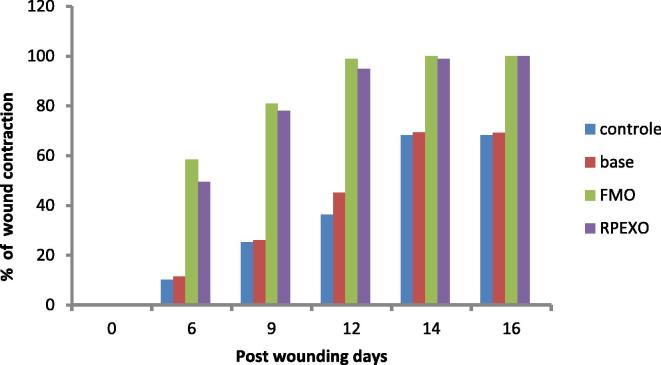
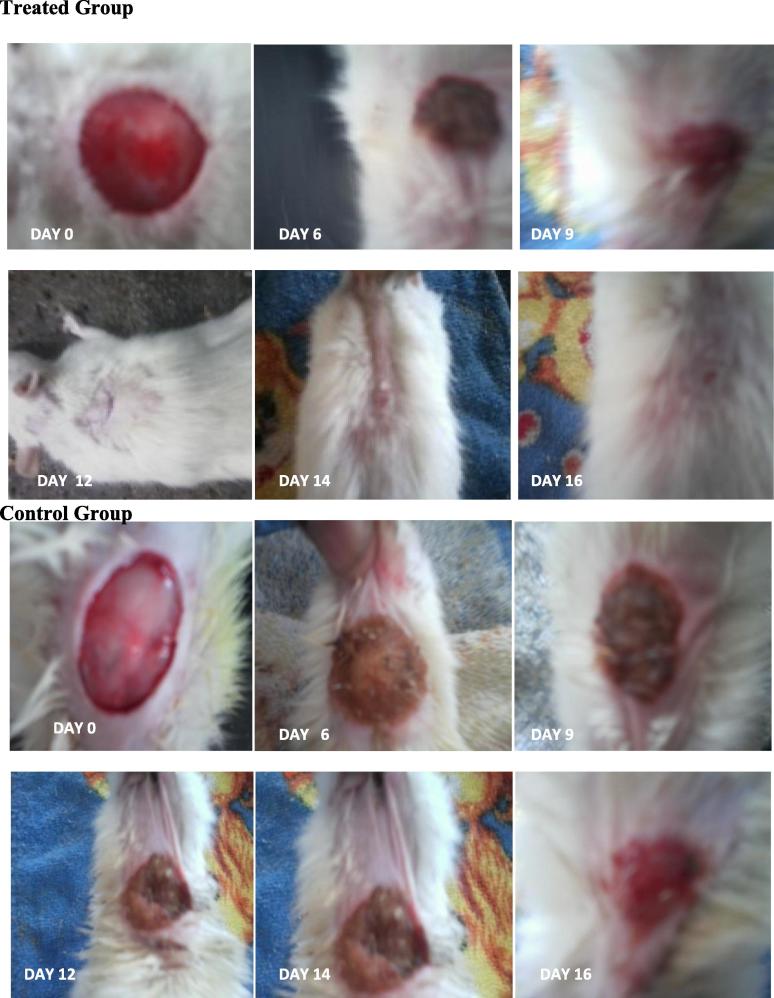
Wound healing activity of the pigment was evaluated using excision wound model (Sudha Bhargavi et al., 2013). It was observed that red pigment ointment and positive control (Framycetin) showed significant wound healing activity when compare to control and ointment base Fig. 2. Percentage of wound contraction was tabulated in Table 2. It was observed from the table that both red pigment ointment and framycetin ointment showed 50% of wound healing within six days of application. Further observation revealed that complete wound healing was observed after 14 days for framycetin and 16 days for red pigment ointment (Table 2). A partial wound healing was observed for control group and ointment base group only after incubation for 25 days (Fig. 3).
Fig. 2.
Percentage of wound contraction. Control – Control group, Base - Ointment base, FMO - Framycetin ointment, RPEXO – Red pigment extract ointment
Table 2.
Effect of red pigment extract on wound healing by excision wound method in albino rats.
| Percentage of wound contraction | ||||
|---|---|---|---|---|
| days | Control | Base | FMO | RPEXO |
| 0 | 0 | 0 | 0 | 0 |
| 6 | 10.2 | 11.42 | 58.42 | 49.4 |
| 9 | 25.2 | 26.05 | 80.95 | 78.02 |
| 12 | 36.3 | 45.22 | 98.8 | 94.82 |
| 14 | 68.24 | 69.45 | 100 | 98.92 |
| 16 | 68.22 | 69.24 | 100 | 100 |
Control – Control group, Base – Ointment base FMO – Framycetin ointment RPEXO – Red pigment extract ointment.
Fig. 3.
photographic representations of contration rate and different days in treatment group
3.4. Antibiotic property evaluation
The extracted red pigment produced by isolated marine Vibrio sp. was evaluated for its antibacterial activity using the cup plate method (Prakasham, et al., 2012) using streptomycin as reference standard. The antibacterial studies revealed that the pigment showed significant antibacterial activity against both gram positive (Bacillus cereus, Micrococcus luteus, Bacillus subtilis, Bacillus stearothermophilus, Bacillus megatherium and Staphylococcus aureus) and gram negative (Klebsiella pneumoniae, Proteus vulgaris, Salmonella paratyphi, Salmonella typhi, Pseudomonas aeruginosa and Escherichia coli) bacteria Table 3. However, the raw pigment did not surpass the standard streptomycin in any case, but showed the same level of inhibition against Micrococcus luteus. Further, it was observed that the red pigment showed effective antimicrobial properties against gram negative than the gram positive bacteria mainly due to less compact cell wall nature of gram negative bacteria than the gram positive ones.
Table 3.
Antibacterial activity of red pigment produced by isolated marine Vibrio sp.
| S.No | Test Organisms | Pigment | Standard |
|---|---|---|---|
| 1 | Klebsiella pneumoniae | 12 mm | 26 mm |
| 2 | Proteus vulgaris | 06 mm | 14 mm |
| 3 | Salmonella paratyphi | 20 mm | 24 mm |
| 4 | Escherichia coli | 22 mm | 14 mm |
| 5 | Salmonella typhi | 16 mm | 22 mm |
| 6 | Pseudomonas aeruginosa | 10 mm | 12 mm |
| 7 | Micrococcus luteus | 09 mm | 08 mm |
| 8 | Bacillus subtilis | 12 mm | 19 mm |
| 9 | Bacillus cereus | 14 mm | 24 mm |
| 10 | Bacillus stearothermophilus | 14 mm | 10 mm |
| 11 | Staphylococcus aureus | 06 mm | 12 mm |
| 12 | Bacillus megatherium | 08 mm | 13 mm |
4. Discussion
Any agent that accelerates the wound healing process can be termed as promoter of wound healing. In spite of tremendous advances in chemical industries, the availability of substances capable of stimulating the process of would repair is still needed. Many traditional plant based remedies are known in folk medicine and used for treatment. In fact some of them have been validated by scientific studies to actually exert biological action against wound healing or its complications. As a search for novel agents, many promising lead compounds have been reported from marine sources having good biological activities like anti bacterial, antifungal and anti-inflammatory activity. Similarly, in the present study, evaluation of the isolated pigment revealed good antibacterial activity against various pathogenic bacteria (Table 3) similar to that noticed in a number of studies (Isnansetyo and Kamei, 2003, Uzair et al., 2006, Jeong et al., 2009). Although several microbial strains such as Serratia marcescens and Vibrio gazogenes had been evaluated for the production of pigments, the isolation of red pigment producing marine bacterial species has been rarely reported in literature (Harwood, 1978, Li et al., 2011).
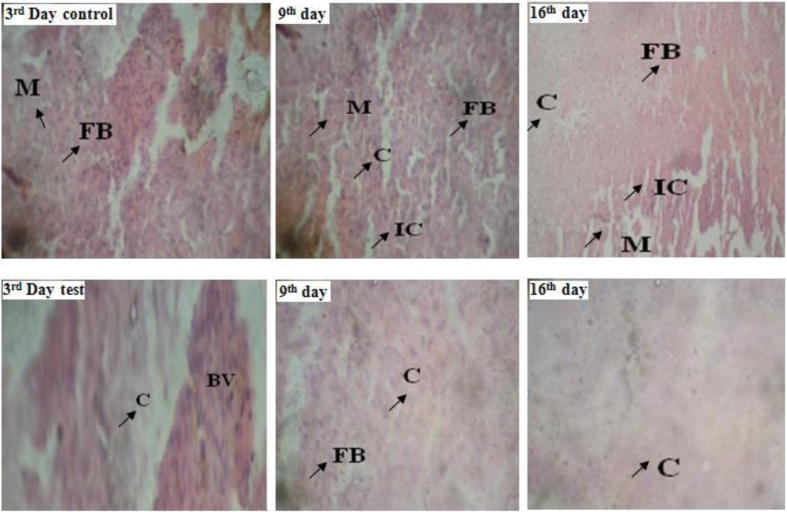
In general, prolongation of wound healing process may be associated with several factors including bacterial inhabitation. Several pathogenic bacteria such as Staphylococcus, Streptococcus, and Pseudomonas in wounds normally may lead to infection of wounds which may result in the formation of chronic wounds ((Frantz, 2005). The topical application of antimicrobial agents or extracts is an efficient therapy method of destroying microbial populations because of the availability of the active agents at the wound site which leads to enhanced wound healing activity (Nayak et al., 2009, Okuda, 2005). Our data on wound healing by isolated red pigment may be attributed to associated antibacterial properties. This is further confirmed based on the fact that this pigment showed antimicrobial property against several bacterial strains (Table 3), suggesting this pigment could be effective agent for reduction of the inflammatory cells on the wound site. In addition, early dermal and epidermal regeneration in the treated group confirmed that the ointment containing the pigment extract had a positive effect toward cellular proliferation, granulation tissue formation, and marked epithelialization, a moderate amount of extracellular matrix synthesis, and new blood vessel formation. Incomplete epithelialization with less extracellular matrix synthesis was observed in control rats and clumps of degenerating neutrophils, necrotic changes, and the persistence of inflammatory exudates in the upper dermis with loss of epidermis were also observed up to Day 16 in control group (Fig. 3). These observations noticed in the present study further confirm high efficiency of wound healing activity of red pigment prepared from the marine bacterial against albino rats. In fact, histo-pathological studies (conducted by taking a tissue) concluded reduction of congestion, edema, mononuclear leukocyte infiltration and necrosis with the groups treated with red pigment and standard framycetin ointment suggesting imperative role of the isolated pigment in biochemical processes of the wound healing and its commercial importance (Fig. 4). In addition, the pigment treated groups of animal’s revealed mild vascular proliferation and reduction of accessory skin structures along with increase in the dermal collagen content denoting the positive response of animals towards wound healing.
Fig. 4.
Histopathogical study
5. Conclusion
Herein, we reported the wound healing and antibacterial activity of the natural product pyranone, purified from the marine bacteria. The wound healing activity of the red pigment e associated with the antibacterial property of the compound. Topical application of 10% pyranone containing ointment resulted in effective reduction of congestion, edema, mononuclear leukocyte infiltration and necrosis along with mild vascular proliferation and reduction of accessory skin structures along with increase in the dermal collagen content. The compound increased the epithelialization and granulation tissue formation without any side effects like non toxicity, non-irritant, non-staining and non-skin-sensitive. Collectively, our results demonstrated that the bioactive pyranone could be useful in the management of excision wounds or as an alternative wound healing agent for future therapeutics.
Conflicts of interests
The authors declare no conflicts of interests.
Footnotes
Peer review under responsibility of King Saud University.
References
- Chidambaram V., Perumalsamy L. An insightful overview on microbial pigment. Prodigiosin Electron. J. Biol. 2009;5:49–61. [Google Scholar]
- Frantz, R.A., 2005. Identifying infection in chronic wounds. Nursing 2016, 35(7), p. 73. [DOI] [PubMed]
- Giovannoni Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 1996;62:625–630. doi: 10.1128/aem.62.2.625-630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill F.A., Apio S., Mubiru N.K., Bukenya-Ziraba R., Mosango M., Maganyi O.W., Soejarto D.D. Traditional herbal drugs of Southern Uganda, II: literature analysis and antimicrobial assays. J. Ethnopharmacol. 2003;84(1):57–78. doi: 10.1016/s0378-8741(02)00289-1. [DOI] [PubMed] [Google Scholar]
- Harwood C.S. Beneckea gazogenes sp. nov., a red, facultatively anaerobic, marine bacterium. Current Microbiol. 1978;1:233–238. [Google Scholar]
- Hassan K.A., Deogratius O., Nyafuono J.F., Francis O., Engeu O.P. Wound healing potential of the ethanolic extracts of Bidens pilosa and Ocimum suave. African J. Pharm. Pharmacol. 2011;5:132–136. [Google Scholar]
- Immanuel Grasian, Thaddaeus Berkmans Jude, Usha Muthusamy, Ramasubburayan Ramasamy, Prakash Santhiyagu, Palavesam Arunachalam. Antipyretic, wound healing and antimicrobial activity of processed shell of the marine mollusc Cypraea moneta. Asian Pacific J. Tropical Biomed. 2012:1643–1646. [Google Scholar]
- Isnansetyo A., Kamei Y. Pseudoalteromonas phenolica sp. nov., a novel marine bacterium that produces phenolic anti-methicillin-resistant Staphylococcus aureus substances. Int. J. Syst. Evol. Microbiol. 2003;53:583–588. doi: 10.1099/ijs.0.02431-0. [DOI] [PubMed] [Google Scholar]
- Jeong M.R., Kim H.Y., Cha J.D. Antimicrobial activity of methanol extract from Ficus carica leaves against oral bacteria. J. Bacteriol. Virol. 2009;39:97–102. [Google Scholar]
- Krishna P.S., Kumar B.S., Raju P., Murty M.S.R., Rao T.P., Charya M.S., Prakasham R.S. Fermentative Production of Pyranone Derivate I from Marine Vibrio sp. SKMARSP9: Isolation, Characterization and Bioactivity Evaluation. Indian J. Microbiol. 2015;55:292–301. doi: 10.1007/s12088-015-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasamyraja D., Jeganathan N.S., Manavalan R. A review on medicinal plants with potential wound healing activity. Int. J. Pharm. Pharm. Sci. 2012;2:105–111. [Google Scholar]
- Li N., Kojima S., Homma M. Sodium-driven motor of the polar flagellum in marine bacteria Vibrio. Genes Cells. 2011;16:985–999. doi: 10.1111/j.1365-2443.2011.01545.x. [DOI] [PubMed] [Google Scholar]
- Mancini I., Defant A., Guella G. Recent synthesis of marine natural products with antibacterial activities. Anti-Infective Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Infective Agents) 2007;6:17–48. [Google Scholar]
- Mori R., Kondo T., Ohshima T., Ishida Y., Mukaida N. Accelerated wound healing in tumor necrosis factor receptor p55-deficient mice with reduced leukocyte infiltration. FASEB J. 2002;16(9):963–974. doi: 10.1096/fj.01-0776com. [DOI] [PubMed] [Google Scholar]
- Morton J.J.P., Melon H.M. Archives Internationales de Pharmcodynamic et de Therapie. 1972;176:117. [Google Scholar]
- Nayak B.S., Sandiford S., Maxwell A. Evaluation of the wound-healing activity of ethanolic extract of Morinda citrifolia L. leaf. Evidence-Based Complement. Altern. Med. 2009;6:351–356. doi: 10.1093/ecam/nem127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry. 2005;66:2012–2031. doi: 10.1016/j.phytochem.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Prakasham R.S., Buddana S.K., Yannam S.K., Guntuku G.S. Characterization of silver nanoparticles synthesized by using marine isolate Streptomyces albidoflavus. J. Microbiol. Biotechnol. 2012;22:614–621. doi: 10.4014/jmb.1107.07013. [DOI] [PubMed] [Google Scholar]
- Sheeba M., Emmanuel S., Revathi K., Ignacimuthu S. Wound healing activity of Cassia occidentalis L. in albino Wistar rats. Int. J. Integrative Biol. 2009;8:1–6. [Google Scholar]
- Slater H., Crow M., Everson L., Salmond G.P. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and-independent pathways. Mol. Microbiol. 2003;47:303–320. doi: 10.1046/j.1365-2958.2003.03295.x. [DOI] [PubMed] [Google Scholar]
- Sudha Bhargavi C.H., Swamy V., Syed B., Ushasri S. Ranjith K Wound healing activity of alcoholic extract of Solanum erianthum in excision and incision wound method. IJRAP. 2013;4:130–135. [Google Scholar]
- Uzair B., Ahmed N., Ahmad V.U., Kousar F. A new antibacterial compound produced by an indigenous marine bacteria—fermentation, isolation, and biological activity. Nat. Prod. Res. 2006;20:1326–1331. doi: 10.1080/14786410601102017. [DOI] [PubMed] [Google Scholar]
- Walker, H.L., Mason, Jr., A.D., 1968. A standard animal burn. J. Trauma. vol. 8, n. 6, p. 1049–1051. [DOI] [PubMed]