Abstract
Five distinct cDNA clones for leghemoglobin (Lb) were isolated from pea (Pisum sativum) nodules. They were classified into two groups designated PsLbA and PsLbB according to sequence homology, O2-binding affinities of the recombinant proteins, and in situ localization of the mRNAs. The PsLbB group was comprised of four cDNA clones: PsLb120-1, -8, -29, and -34. They showed a high similarity of deduced amino acid sequences and O2-binding affinities of their recombinant proteins. Among them, the spatial expression pattern of PsLb120-1 was investigated in great detail, indicating that its transcripts were localized in the region from infection zone II to the distal part of nitrogen fixation zone III in effective nodules. PsLb5-10, which is the only cDNA clone of the PsLbA type, differed considerably from the PsLbB type in amino acid sequence, and the recombinant protein had a higher O2-binding affinity than those of the PsLbB type. The transcripts of PsLb5-10 were detected throughout the central tissue of effective nodules. However, in ineffective nodules on the pea mutant E135 (sym13), transcripts of PsLb5-10 were restricted to the distal portion of the central tissue as well as those of PsLb120-1. These findings indicate that the pea genome contains two types of Lb genes and suggest that they have different roles in the development of nitrogen-fixing symbiosis in pea nodules.
Nitrogen-fixing legume nodules contain a large amount of an oxygen-binding hemoprotein, leghemoglobin (Lb). Owing to its extremely fast O2 association rate and rather slow O2 dissociation rate, Lb appears to supply oxygen for oxidative respiration of bacteroids while maintaining a free oxygen concentration within the nodules low enough to protect nitrogenase against oxygen damage (Appleby, 1984). Similar “symbiotic” plant hemoglobins (Hbs) exist in nitrogen-fixing nodules formed on a nonlegume, Parasponia andersonii, that has a symbiotic association with rhizobia (Appleby et al., 1983; Landsmann et al., 1986), and actinorhizal plants, Casuarina glauca (Fleming et al., 1987; Christensen et al., 1991), and Myrica gale (Pathirana and Tjepkema, 1995), which are symbiotically associated with Frankia. In addition to symbiotic Hbs, “non-symbiotic” Hbs have been found in both legumes (Andersson et al., 1996) and nonlegumes, such as Trema tomentosa (Bogusz et al., 1988), barley (Hordeum sp.; Taylor et al., 1994), rice (Oryza sativa; Arredondo-Peter et al., 1997a), and Arabidopsis (Trevaskis et al., 1997). Although the function of non-symbiotic Hbs is unclear, it is likely that Hbs are widely distributed in plants and that more specialized symbiotic Hbs are derived from the preexisting non-symbiotic Hbs (Appleby, 1992; Arredondo-Peter et al., 1998; Hill, 1998).
In legume nodules, Lbs are encoded by a family of genes and multiple isomers of Lbs are obtained. The number of detectable isoleghemoglobins (isoLbs) varies among legume species: eight in soybean (Glycine max; Fuchsman and Appleby, 1979), five in pea (Pisum sativum; Uheda and Syono, 1982a), nine in alfalfa (Medicago sativa; Egli et al., 1991), three in cowpea (Vigna unguiculata; Dakora et al., 1991), and seven in Sesbania rostrata (Bogusz et al., 1987). It is presently unknown whether isoLbs have different roles.
Lb a and Lb IV, major isoLbs in the nodules of soybean and pea, respectively, have higher O2-binding affinities than other major isoLbs, Lb c and Lb I (Appleby, 1962; Uheda and Syono, 1982a). In both plants, Lb a and Lb IV are more effective for supporting nitrogen fixation and oxygen consumption of isolated bacteroids than Lb c and Lb I (Uheda and Syono, 1982b). Furthermore, the ratios of the two isoLbs, namely, Lb a/Lb c in soybean and Lb IV/Lb I in pea, change during nodule development. Lb a and Lb IV are synthesized mainly in older nodules that actively fix nitrogen (Fuchsman et al., 1976; Fuchsman and Appleby, 1979; Verma et al., 1979; Uheda and Syono, 1982a). From these results, it was proposed that the heterogeneity of Lbs contributes to more effective nitrogen fixation by changes in the capacity for oxygen transport. However, further evidence proving physiological significance of Lb heterogeneity has not been provided, although multiple cDNA clones have been isolated from nodules of several species.
We previously isolated two distinct cDNA clones, PsN5 and PsN120, for Lb from pea nodules (Suganuma et al., 1995). PsN5 is expressed at a lower level in ineffective nodules on the mutant E135 (sym13) compared with the level in effective nodules. In contrast, the expression of PsN120 is similar in effective and ineffective nodules. These results imply that isoLbs have different roles in nitrogen fixation in pea nodules, and prompted us to characterize further these two Lb genes. In the present study, we isolated five distinct Lb cDNA clones from pea nodules, and compared the sequences, O2-binding affinities of the recombinant proteins, and spatial expression pattern of the genes represented in the two groups. In addition, patterns of spatial expression between effective and ineffective E135 nodules were compared to discuss physiological significance of two types of Lb genes with respect to nitrogen fixation.
RESULTS
Cloning and Characterization of Lb Genes
As a consequence of screening a cDNA library of pea nodules with PsN5 and PsN120 probes, we isolated 16 and 14 cDNA clones for PsN5 and PsN120, respectively. All the nucleotide sequences of the 16 cDNAs for PsN5 were identical. The longest one, PsLb5-10, was 563 bp in length and contained an open reading frame of 441 bp. The clones for PsN120 contained four independent sequences, and they were designated PsLb120-1, -8, -29, and -34. PsLb120-1, -8, -29, and -34 were 542, 540, 540, and 568 nucleotides long, respectively, and each contained an open reading frame of 438 bp. The nucleotide sequences of these five cDNA clones showed marked similarity with those of Lb genes found in a database (data not shown). However, the nucleotide sequence of PsLb5-10 was considerably different from those of PsLb120s. Each nucleotide sequence was deposited in the database with the accession numbers AB010831 (PsLb5-10), AB015719 (PsLb120-1), AB015720 (PsLb120-8), AB009844 (PsLb120-29), and AB015721 (PsLb120-34).
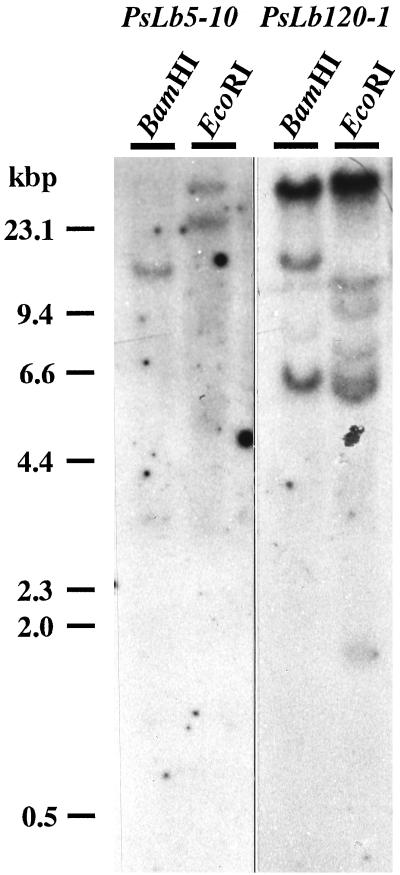
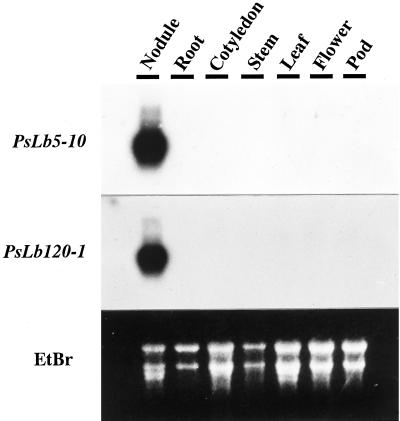
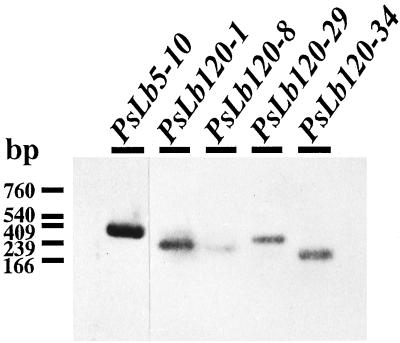
PsLb5-10 cDNA did not hybridize with any PsLb120 cDNAs, but PsLb120 cDNAs cross-hybridized with each other (data not shown). Thus, genomic Southern-blot analyses were performed using PsLb5-10 and PsLb120-1 as probes. One or two genomic fragments were detected when PsLb5-10 was used as a probe, whereas PsLb120-1 hybridized multiple genomic fragments (Fig. 1). Northern-blot analysis revealed the transcripts of approximately 900 and 700 bases hybridized with cDNAs of PsLb5-10 and PsLb120-1, respectively (Fig. 2). These transcripts were detected exclusively in nodules. The relative abundance of transcripts of each gene in nodules was examined by reverse transcriptase (RT)-PCR. Expression of the PsLb5-10 gene was the most abundant, and that of PsLb120-8 was the lowest (Fig. 3).
Figure 1.
Genomic Southern-blot analysis of PsLb5-10 and PsN120-1 genes. Genomic DNA was isolated from roots of 7-d-old pea cv Sparkle seedlings. Ten micrograms of genomic DNA was digested with BamHI and EcoRI, fractionated in a 0.8% (w/v) agarose gel, transferred to a nylon membrane, and hybridized with radiolabeled probes.
Figure 2.
Northern-blot analysis of PsLb5-10 and PsLb120-1 mRNA in the total RNA isolated from each tissue of pea cv Sparkle plants. Nodules, roots, stems, and leaves were harvested from 5-week-old plants, and flowers and pods were from 6-week-old plants. Cotyledons were obtained from 7-d-old seedlings. Ten micrograms of each sample of total RNA was subjected to electrophoresis on a 1.25% (w/v) agarose gel that contained formaldehyde, transferred to a nylon membrane, and hybridized with radiolabeled probes. The rRNA stained with ethidium bromide (EtBr) are shown as controls.
Figure 3.
RT-PCR analysis of mRNA abundance of PsN5-10, PsN120-1, -8, -29, and -34 in total RNA isolated from nodules of pea cv Sparkle plants. Five nanograms of total RNA was reverse transcribed and then the cDNA was amplified by 20-cycle PCR with each pair of forward and reverse primers described in “Materials and Methods.” The amplified DNAs were electrophoresed on a 1% (w/v) agarose gel, transferred to a nylon membrane, and hybridized with each digoxigenin-labeled probe.
The Predicted Lb Proteins
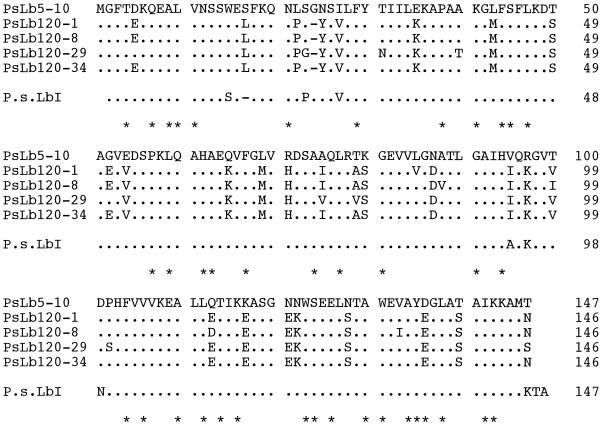
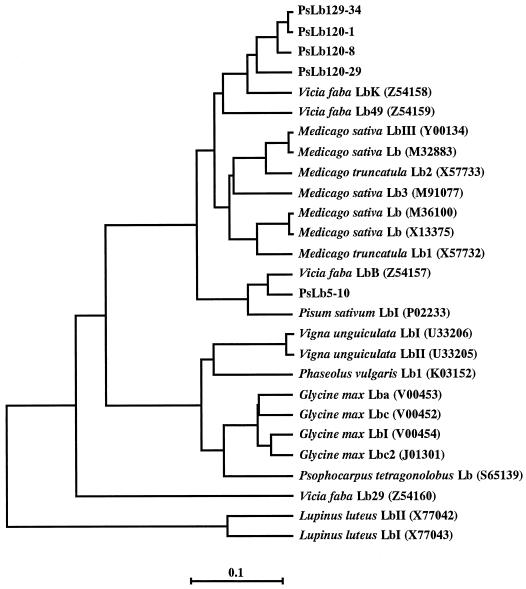
The deduced amino acid sequences of five cDNA clones isolated were found to contain residues conserved in plant Hbs except for one residue (Arredondo-Peter and Escamilla, 1991; Fig. 4). Asp (position 136) in PsLb5-10 was conserved but was replaced with Glu (position 135) in four PsLb120s. Although an amino acid sequence of pea Lb was determined (Lehtovaara et al., 1980), none of these five sequences was identical with it. The predicted sequences of PsLb120-1, -8, -29, and -34 were more than 91% homologous to each other, but were only about 80% homologous to those of PsLb5-10 (Table I). In addition, the numbers of amino acids of PsLb120s were 146, whereas the number of amino acids of PsLb5-10 was 147. The calculated pI of PsLb5-10 was more basic than those of PsLb120s. An alignment of the deduced amino acid sequences of these five cDNA clones and those of Lb genes reported in nodules of other legumes further showed that PsLb5 and PsLb120 clustered to different positions (Fig. 5).
Figure 4.
Alignment of the amino acid sequences deduced from PsN5-10, PsN120-1, -8, -29, and -34, and that of pea Lb I (Lehtovaara et al., 1980). Identical residues are indicated by dots. Dashed lines indicate gaps introduced into the sequences to maximize homology. Residues conserved in plant Hbs (Arredondo-Peter and Escamilla, 1991) are indicated by asterisks.
Table I.
Major features of isolated cDNAs for Lb and their predicted amino acid sequence homology
| Lb Clone | Length of Polypeptide | Molecular Mass | Calculated pI | Amino Acid Sequence Homology
|
||||
|---|---|---|---|---|---|---|---|---|
| 5–10 | 120–1 | 120–8 | 120–29 | 120–34 | ||||
| no. of amino acids | kDa | % | ||||||
| PsLb5-10 | 147 | 15.9 | 6.36 | – | 80.0 | 78.9 | 80.0 | 80.3 |
| PsLb120-1 | 146 | 15.9 | 5.46 | – | – | 96.6 | 93.2 | 99.3 |
| PsLb120-8 | 146 | 16.0 | 5.45 | – | – | – | 91.1 | 97.3 |
| PsLb120-29 | 146 | 15.8 | 5.74 | – | – | – | – | 93.8 |
| PsLb120-34 | 146 | 15.9 | 5.46 | – | – | – | – | – |
Figure 5.
Phylogenetic tree of the alignment of the amino acid sequences deduced from PsN5-10, PsN120-1, -8, -29, and -34, with other Lbs from main legumes. The phylogram was created by the unweighted pair group maximum average method in the GeneWorks program. Branch lengths reflect sequence diversity counted as the number of substitutions per site. The database accession numbers are indicated in parentheses after the Lb names.
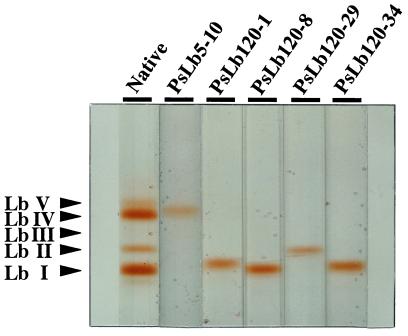
Pea nodules contained five Lb components separated by non-denaturing PAGE and these were labeled Lb I, Lb II, Lb III, Lb IV, and Lb V starting from the anode side (Uheda and Syono, 1982a). To estimate coincidence between these isoLbs and the five isolated cDNA clones, recombinant Lb proteins were produced from each cDNA and the mobility on non-denaturing PAGE was compared with native isoLbs. All recombinant Lb proteins colored red, presuming that heme molecules were derived by bacteria, and reacted with antiserum against soybean Lb (data not shown). From their mobilities, PsLb5-10 appeared to coincide with Lb IV and PsLb120-29 with Lb II (Fig. 6). However, the recombinant Lb proteins of PsLb120-1, -8, and -34 were moved to a position similar to Lb I.
Figure 6.
Mobility of the recombinant Lbs of PsN5-10, PsN120-1, -8, -29, and -34 on non-denaturing PAGE. Five Lb components in native Lb isolated from nodules of 5-week-old pea cv Sparkle plants are labeled Lb I, Lb II, Lb III, Lb IV, and Lb V, starting from the anode side as described by Uheda and Syono (1982a). Forty micrograms of each protein was pretreated with nicotinate and ferricyanide to produce ferric Lb nicotinate complexes and electrophoresed on a 7.5% (w/v) polyacrylamide gel.
O2-Binding Affinities of Recombinant Lb Proteins
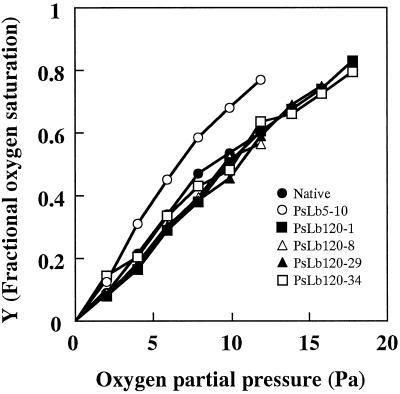
The red color of the recombinant Lb proteins suggested that these proteins were functional. The O2-binding affinity of each recombinant protein was compared. After deoxygenation, recombinant Lb proteins of the five cDNA clones bound O2 with increasing O2 partial pressure in the gas phase, like native Lb proteins (Fig. 7). Four recombinant Lb proteins of PsLb120s responded similarly to O2 partial pressure, whereas those of PsLb5-10 bound O2 more rapidly. On the basis of the O2 association curve, oxygen partial pressures for the half oxygenation of each recombinant Lb protein were estimated, indicating that PsLb5-10 showed the lowest value among them (Table II).
Figure 7.
Oxygenation curve of the recombinant Lbs of PsN5-10, PsN120-1, -8, -29, and -34. Native Lb was isolated from nodules of 5-week-old pea cv Sparkle plants. Forty nanomoles of each protein was reduced by an enzymatic reduction system and deoxygenated by flushing with pure argon. After the deoxygenation, aliquots of air were injected and oxygenation of Lbs was monitored using a spectrophotometer. The Y value representing fractional oxygen saturation was calculated using absorbance at 575 and 556 nm. Data shown are representatives of two independent experiments with similar results.
Table II.
Oxygen partial pressure for half oxygenation of native and recombinant Lbs
| Lb Clone | P50
|
|
|---|---|---|
| Experiment 1 | Experiment 2 | |
| Pa | ||
| Native | 9.21 | 9.82 |
| PsLb5-10 | 6.47 | 6.47 |
| PsLb120-1 | 10.71 | 10.59 |
| PsLb120-8 | 9.92 | 10.29 |
| PsLb120-29 | 10.00 | 10.00 |
| PsLb120-34 | 10.43 | 10.29 |
P50 values were calculated from Hill plots of the oxygenation reaction described in Figure 7. Experiments were performed independently two times and each experiment's results is shown.
Spatial Expression Pattern of Lb Genes
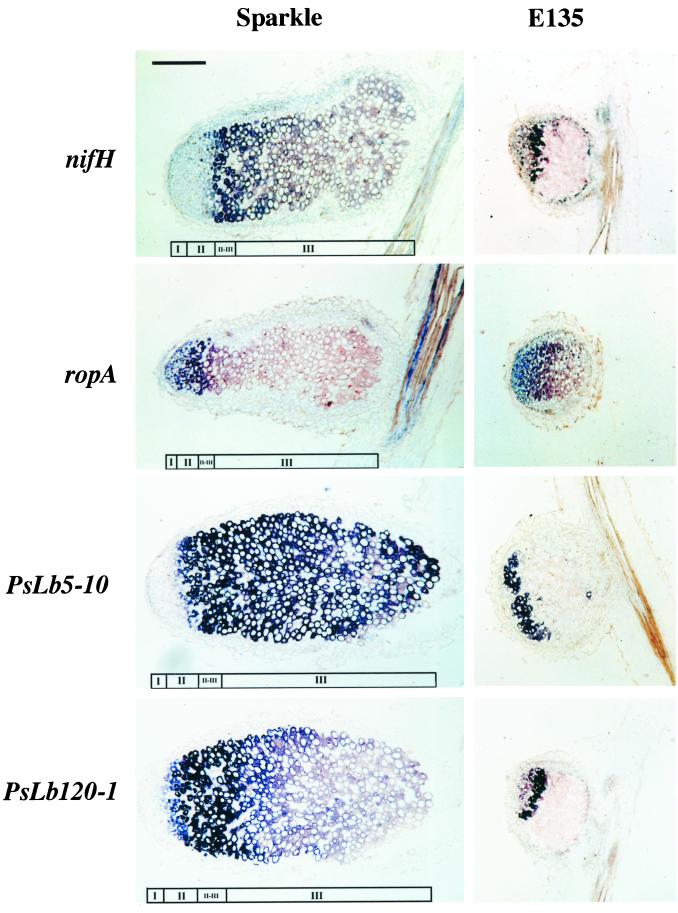
The spatial distribution in pea nodules of the transcripts of PsLb5-10 and PsLb120s in pea nodules was examined by in situ hybridization. Because the four PsLb120 cDNAs were cross-hybridized with each other, PsLb120-1 was used as the hybridization probe. To clarify the developmental zonation of the nodule tissues, the distributions in nodules of bacterial nifH and ropA genes were also examined. In pea nodules, the central tissue consists of infection zone II, interzone II–III, nitrogen fixation zone III, and senescent zone IV (Franssen et al., 1992), and expression of nifH begins at the beginning of interzone II– III, spreading to the distal part of zone III. The expression of ropA, encoding bacterial outer-membrane protein, is detected at infection zone II and decreases at the transition of the infection zone II to the interzone II–III (de Maagd et al., 1994). In the present study, infection zone II, interzone II–III, and nitrogen fixation zone III were defined on the basis of the distribution pattern of bacterial nifH and ropA transcripts (Fig. 8). Significant signals were not detected by any sense probe (data not shown).
Figure 8.
In situ localization of PsN5-10 and PsN120-1 mRNA in effective pea cv Sparkle nodules and ineffective nodules on the mutant E135. Nodules were harvested from 4-week-old plants. Bacterial nifH and ropA genes were used for definition of meristem (I), infection zone II (II), interzone II to III (II–III), and nitrogen fixation zone III (III) in the nodules. Longitudinal sections (10 μm) through the nodules were hybridized with digoxigenin-labeled antisense probes. Hybridization signals are visible as purple or blue. The bar represents 400 μm and all micrographs were taken at the same magnification.
In young circular nodules of 3-week-old plants, both transcripts of PsLb5-10 and PsLb120-1, as well as nifH, were detected in the whole central tissue except the meristem. The transcripts of ropA were in the infection zone II (data not shown). With development of the nodules, expression of PsLb120-1 was distributed in infection zone II, interzone II–III, and the distal part of nitrogen fixation zone III in effective nodules of 4-week-old Sparkle plants. In contrast, expression of PsLb5-10 was widely distributed and was detected throughout the central tissue of the effective nodules. In ineffective nodules on the mutant E135, the spatial expression patterns of nifH, ropA, and PsLb120-1 were similar to those in effective nodules. However, the distribution of PsLb5-10 transcripts was only detected at the distal portion of the central tissue.
DISCUSSION
Five distinct, nearly full-length Lb cDNA clones were isolated by two partial Lb cDNAs, with PsN5 and PsN120 as probes. A single clone, PsLb5-10, was isolated using PsN5 as a probe, whereas multiple clones, PsLb120-1, -8, -29, and -34, were identified by PsN120. This agrees with the results of genomic Southern-blot analyses showing that a higher number of genomic fragments were detected by PsLb120-1 (Fig. 1). They all encode symbiotic Lbs because their expression was exclusively specific in nodules (Fig. 2). According to the alignment of their nucleotide sequences and predicted amino acid sequences (Figs. 4 and 5; Table I), PsLb5-10 and four PsLb120s appeared to be distinct types of Lb genes, and were classified into two groups designated PsLbA and PsLbB, respectively.
An amino acid sequence of pea Lb I determined by Lehtovaara et al. (1980) was not identical with any of their deduced amino acid sequences (Fig. 4), but it was more homologous to PsLb5-10 (Fig. 4). Because one or two hybridizing fragments with PsLb5-10 were detected by genomic Southern-blot analysis (Fig. 1), one more gene for the PsLbA type might be present. We also found one more band close to Lb I at the anode side, although Uheda and Syono (1982a) did not detect this. In addition, none of the recombinant Lb proteins of five cDNA clones coincided with Lb III or Lb V (Fig. 6). These results suggest that other types of Lb genes may exist in the pea genome. However, it remains to be confirmed whether Lb I consists of three products of genes, PsLb120-1, -8, and -34. The native Lb I protein recovered from the gel was run on an isoelectric gel electrophoresis and further SDS-gel electrophoresis, but a single spot was detected (data not shown). The predicted proteins of PsLb120-1, -8, and -34 have similar molecular masses and pI (Table I), indicating that separation of these gene products would be difficult. In an alternate manner, it might be possible that protein modification occurs after translation of these genes.
O2-binding affinities of the recombinant Lb proteins were compared to investigate the difference between the products of PsLbA- and PsLbB-type genes. Recombinant Lb proteins bound oxygen in a similar manner to native Lb (Fig. 7), indicating that properties of recombinant proteins are identical to native proteins. The structural and spectral identity between expressed Lb proteins and native Lb have been demonstrated using cDNAs from lupine (Lupinus luteus; Sikorski et al., 1995), cowpea (Arredondo-Peter et al., 1997b), and soybean (Hargrove et al., 1997; Jones et al., 1998). Assay of O2-binding affinities showed that recombinant Lb proteins of PsLb5-10 had a higher O2-binding affinity than those of the PsLbB type (Fig. 7; Table II). Uheda and Syono (1982a, 1982b) compared O2-binding affinities of two major pea Lb components, Lb I and Lb IV, and showed that Lb IV had a higher O2-binding affinity than Lb I. PsLb5-10 appears to encode the Lb IV component (Fig. 6). Thus, the present results are consistent with their previous study, and indicate that proteins of these two types of Lb genes are functionally different.
Furthermore, expressions of these two types of Lb genes are differentially regulated. Expression of PsLb5-10 was almost evenly distributed in the central tissue of effective pea nodules whereas that of PsLb120-1 was restricted to the region from infection zone II to the distal part of nitrogen fixation zone III (Fig. 8). Northern-blot and RT-PCR analyses showed that transcripts of PsLb5-10 were more abundant than those of PsLb120s (Figs. 2 and 3). These results correlate well to the broader distribution of PsN5-10 transcripts. Uheda and Syono (1982a) reported that the ratio of the two major pea Lb components, Lb IV to Lb I, increased during nodule development and was higher in the proximal region than in the distal region of pea nodules. Their results are in good agreement with the spatial expression pattern of the two types of Lb genes.
In young circular nodules, both transcripts of PsLb5-10 and PsLb120-1 were distributed in the whole central tissue of the nodules (data not shown), whereas in older elongated nodules those of PsLb120-1, but not of PsLb5-10, were localized at the distal portion in the central tissue (Fig. 8). Thus, these results indicate that the expression of PsLb120-1 is restricted in early stages of nitrogen-fixing symbiosis, but PsLb5-10 expression is continued even in the later stages of nodule development. In the nitrogen fixation zone III, only the presence of the PsLbA type of Lb may be enough to support nitrogenase activity as well as to protect nitrogenase from oxygen damage because oxygen consumption of actively nitrogen-fixing bacteroids may contribute to lowering the pO2 inside the nodules. However, in the early stages of nodule development or in the infection zone II, a higher abundance of Lb, such as the PsLbB type of Lb, may be required to lower the pO2 while bacteroids begin to fix nitrogen. In determinate nodules of soybean, the content of Lb a, which shows a higher O2-binding affinity than Lb c, was increased relative to that of Lb c during nodule development (Fuchsman et al., 1976; Fuchsman and Appleby, 1979; Verma et al., 1979). Therefore, distinct Lb species with different O2-binding affinity also appear to be regulated differentially during development of determinate nodules.
We have previously shown that expression of PsN5, but not of PsN120, is reduced in ineffective nodules on the pea mutant E135 compared with the expression in effective nodules (Suganuma et al., 1995). In the present study, the spatial expression pattern of PsLb5-10 in ineffective nodules was similar to that of PsLb120-1 and was restricted to the distal portion of the central tissue (Fig. 8). This indicates that the reduced level of expression of PsN5 in ineffective nodules is due to the lack of expression in the nitrogen fixation zone III observed in effective nodules. In the ineffective E135 nodules, nitrogenase activity is absent, although nitrogenase proteins were detected in the bacteroids (Suganuma et al., 1998). This study confirmed that the bacterial nifH gene was actually expressed (Fig. 8). Furthermore, expression of the bacterial ropA gene, which is down-regulated during bacteroid development and is uncoupled from nif gene activation (de Maagd et al., 1994), was detected in the distal portion of ineffective E135 nodules like effective nodules (Fig. 8). These results indicate that development of bacteroids may proceed normally in E135 nodules, with the exception of nitrogenase activity. Therefore, it is suggested that both PsLbA- and PsLbB-type genes are expressed independent of nitrogenase activity in early stages of nodule development, but continuous expression of the PsLbA-type gene in later stages of nodule development requires nitrogenase activity.
The present study demonstrated that the pea genome has two types of Lb genes for proteins of different O2-binding affinities that are differentially regulated. Our results reinforce the idea that isoLbs have different roles (Fuchsman et al., 1976; Uheda and Syono, 1982b). It is likely that the PsLbA type of Lb, showing a higher O2-binding affinity, plays a greater role in nitrogen fixation and oxygen consumption of bacteroids by rapid transfer of oxygen to the bacteroids than the PsLbB type of Lb. However, it is unclear whether this is applicable to every legume. Davidowitz et al. (1994) showed that accumulation of transcripts of four divergent alfalfa Lb genes was spatially identical, and was detected primarily in interzone II–III of the nodules, resembling the spatial expression pattern of PsLb120-1. In broad bean (Vicia faba), an Lb gene VfLb29 was found to be induced not only in nodules, but also in roots colonized by mycorrhizal fungus (Frühling et al., 1997). It will be worth studying the physiological significance of Lb heterogeneity in other legume nodules for a better understanding of nitrogen-fixing symbiosis.
MATERIALS AND METHODS
Plant Materials
Seeds of pea (Pisum sativum L. cv Sparkle) and the Fix− mutant E135 (sym13) derived from it (Kneen et al., 1990) were surface sterilized and inoculated with Rhizobium leguminosarum bv viciae strain 128C53. The plants were grown in vermiculite with a nitrogen-free nutrient solution in a greenhouse under natural daylight conditions, as described previously (Suganuma et al., 1993).
Cloning of Lb cDNA Clones and Sequence Analysis
Two distinct cDNA clones for Lb, PsN5, and PsN120, which had been isolated by subtractive hybridization (Suganuma et al., 1995), did not have complete protein-coding regions. Therefore, approximately 2 × 104 independent recombinant phages derived from the λgt10 Sparkle nodule cDNA library, constructed as described previously (Suganuma et al., 1997), were rescreened with each cDNA insert as a probe. Relatively long cDNA inserts among positive recombinants were sub-cloned into pBluescript II SK(+) (Stratagene, La Jolla, CA) and their nucleotide sequences were determined by the method of Sanger et al. (1977) with a BcaBEST Dideoxy sequencing kit (TaKaRa, Kyoto). Nucleotide and deduced amino acid sequences were analyzed with the GeneWorks program (Intelligenetics, Mountain View, CA).
Southern- and Northern-Blot Analyses
Genomic DNA was isolated from the roots of 7-d-old Sparkle seedlings according to the method of Walbot and Warren (1987). Genomic DNA was further treated with ribonuclease A and proteinase K and restricted with the endonucleases BamHI and EcoRI. Total RNAs were isolated from various tissues of Sparkle plants by phenol extraction and LiCl precipitation (Suganuma et al., 1995). DNA fragments were separated in a 0.8% (w/v) agarose gel and total RNAs were subjected to electrophoresis on a 1.25% (w/v) agarose gel that contained formaldehyde, and transferred to a membrane (Hybond-N, Amersham Pharmacia Biotech, Uppsala) according to standard procedures (Sambrook et al., 1989). DNA probes were prepared from the isolated PsLb5-10 or PsLb120-1 cDNA with 32P-dCTP and a BcaBEST labeling kit (TaKaRa). Hybridization and washing were done as described previously (Suganuma et al., 1997).
RT-PCR Analysis
Reverse transcription, PCR, and detection of amplified DNAs were performed as described by Takane et al. (1997). Total RNA isolated from nodules of 4-week-old Sparkle plants were reverse transcribed with avian meloblastosis virus reverse transcriptase and the synthesized cDNA was amplified using oligonucleotide primers designed to specifically amplify each mRNA for Lb. The sequences of forward primers and reverse primers were: 5′-ATAGTTCATGGGAGTCATTC-3′ and 5′-CATCGTAAGCTACTTCCCAT-3′ for PsLb5-10, 5′-AGCATCAGGAGAAGTAGCTG- 3′ and 5′-AAGATTTAGTTATACAAGTC-3′ for PsLb120-1, 5′-TCACATTC-AGAAGGGAGCTA-3′ and 5′-AAGATTTAGTTATACAAGTC-3′ for PsLb120-8, 5′-TTTCTTAAGGATTCGGCAGG-3′ and 5′-CACAAGTTCAACTCATTGCC-3′ for PsLb120-29, and 5′-AAGTTTTTGGAATGGTGCAC-3′ and 5′-CTCCTGATGCTTCTTTTA-3′ for PsLb120-34. Specific amplification of each Lb was confirmed by digesting with HindIII for PsLb5-10, PsLb120-29, and PsLb120-34, or sequencing for PsLb120-1 and PsLb120-8. The amplified DNAs were electrophoresed, transferred to nylon membrane, and probed with digoxigenin-labeled PsLb5-10 or PsLb120-1 cDNA. The hybridization signals were detected by antidigoxigenin-alkaline phosphatase conjugate (Boehringer Mannheim, Mannheim, Germany) with CDP-Star (Tropix, Bedford, MA).
Preparation of Recombinant Lbs
The coding region for PsLb5-10 was amplified by PCR using forward primer (5′-CATATGGGTTTTACTGATAAACAAGAGG-3′) and reverse primer (5′-GGATCCTTAAGTCATTGCCTTCTTA-3′) and degenerated with restriction sequences for NdeI or BamHI. The amplified PCR fragment was ligated into the NdeI and BamHI cloning sites of the pRSETC expression vector (Invitrogen, San Diego). The construct was introduced into Escherichia coli BL21(DE3) pLysS (Novagen, Madison, WI). The isolated cDNA inserts, PsLb120-1, -8, -29, and -34, which have EcoRI adaptors at both ends, were ligated into the EcoRI cloning site of the pET-21(+) transcription vector (Novagen). Each construct was introduced into E. coli strain BL21(DE3) (Novagen).
The host cells carrying the construct were grown in Luria-Bertani broth medium with ampicillin at 37°C and expression of the recombinant Lb proteins was induced by addition of isopropylthio-β-galactoside to a final concentration of 1 mm. The cells collected by centrifugation were sonicated and additionally centrifuged. The red proteins in the supernatant were purified by fractionation with ammonium sulfate and chromatography using Sephacryl S-200 (Amersham Pharmacia Biotech) as described by Suganuma et al. (1987).
Separation of IsoLbs
IsoLbs were separated by non-denaturing PAGE on a 7.5% (w/v) polyacrylamide gel as described by Uheda and Syono (1982a). To achieve sharper separation, Lbs were pretreated with nicotinate and ferricyanide to produce ferric Lb nicotinate complexes (Fuchsman and Appleby, 1979). Unfractionated native Lb was prepared from nodules of 5-week-old Sparkle plants as described previously (Suganuma et al., 1987). Concentrations of Lb were determined from the spectra of pyridine hemochromes (Appleby and Bergersen, 1980). Protein was measured using a modified version of Lowry's procedure (Bensadoun and Weinstein, 1976), with bovine serum albumin as the standard.
Assay of O2-Binding Affinity of Lb
O2-binding affinities of native Lb and recombinant Lbs were determined essentially as described by Uheda and Syono (1982a). Solutions of Lb were reduced aerobically by an enzymatic reduction system composed of an NADPH2-generating system, an electron-mediating system, and catalase. After completion of the reduction, a 4-mL sample of Lb solution was placed in a glass cell (Pyrex 7740, Corning Inc., Corning, NY) and the cell was joined with a 1-L filtering bottle upside down using a silicone cap. Purified argon gas (99.9999% [w/v]) was fed continuously through another mouth of the filtering bottle and the deoxygenation of Lb was monitored by a spectrophotometer. Then, aliquots of air were injected with a microsyringe into the filtering bottle that was shaken gently. After equilibration, the absorbance at 575 and 556 nm were recorded. The fractional oxygen saturation was calculated according to the method of Appleby (1962).
In Situ Hybridization
In situ hybridization was carried out as described by Kouchi and Hata (1993). RNA probes were prepared from linearized plasmids with digoxigenin-UTP and the hybridization signals were detected by antidigoxigenin-alkaline phosphatase conjugate with nitroblue tetrazolium salt and 5-bromo-4-chloro-3-indolyl phosphate toluidinium salt (Boehringer Mannheim). Genes of nifH and ropA from R. leguminosarum bv viciae were provided by Drs. T. Bisseling and W.C. Yang (Department of Molecular Biology, Agricultural University of Wageningen, The Netherlands). Photographic images were captured and figures were arranged with Photoshop software (Adobe Systems, Mountain View, CA) on a Macintosh computer.
ACKNOWLEDGMENTS
The authors thank Drs. T. Bisseling and W.C. Yang (Agricultural University of Wageningen, The Netherlands) for providing nifH and ropA genes from R. leguminosarum bv viciae. They also thank Drs. S. Yoshida (Nagoya University, Japan) and J. Katoh (Aichi University of Education) for technical assistance and Dr. T.A. LaRue for critical reading of the manuscript.
Footnotes
This work was supported in part by Special Coordination Funds of the Science and Technology Agency of the Japanese government.
LITERATURE CITED
- Andersson CR, Jensen EO, Llewellyn DJ, Dennis ES, Peacock WJ. A new hemoglobin gene from soybean: a role for hemoglobin in all plants. Proc Natl Acad Sci USA. 1996;93:5682–5687. doi: 10.1073/pnas.93.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby CA. The oxygen equilibrium of leghemoglobin. Biochim Biophys Acta. 1962;60:226–235. doi: 10.1016/0006-3002(62)90398-0. [DOI] [PubMed] [Google Scholar]
- Appleby CA. Leghemoglobin and Rhizobium respiration. Ann Rev Plant Physiol. 1984;35:443–478. [Google Scholar]
- Appleby CA. The origin and functions of haemoglobin in plants. Sci Prog. 1992;76:365–398. [Google Scholar]
- Appleby CA, Bergersen FJ. Preparation and experimental use of leghaemoglobin. In: Bergersen FJ, editor. Methods for Evaluating Biological Nitrogen Fixation. Chichester, UK: John Wiley & Sons; 1980. pp. 315–335. [Google Scholar]
- Appleby CA, Tjepkema JD, Trinick MJ. Hemoglobin in a nonleguminous plant, Parasponia: possible genetic origin and function in nitrogen fixation. Science. 1983;220:951–953. doi: 10.1126/science.220.4600.951. [DOI] [PubMed] [Google Scholar]
- Arredondo-Peter R, Escamilla E. A consensus sequence of plant hemoglobins. Plant Mol Biol Rep. 1991;9:195–207. [Google Scholar]
- Arredondo-Peter R, Hargrove MS, Moran JF, Sarath G, Klucas RV. Plant hemoglobins. Plant Physiol. 1998;118:1121–1125. doi: 10.1104/pp.118.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo-Peter R, Hargrove MS, Sarath G, Moran JF, Lohrman J, Olson J, Klucas RV. Rice hemoglobins. Gene cloning, analysis, and O2-binding kinetics of a recombinant protein synthesized in Escherichia coli. Plant Physiol. 1997a;115:1259–1266. doi: 10.1104/pp.115.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo-Peter R, Moran JF, Sarath G, Luan P, Klucas RV. Molecular cloning of the cowpea leghemoglobin II gene and expression of its cDNA in Escherichia coli: purification and characterization of the recombinant protein. Plant Physiol. 1997b;114:493–500. doi: 10.1104/pp.114.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bogusz D, Appleby CA, Landsmann J, Dennis ES, Trinick MJ, Peacock WJ. Functioning hemoglobin genes in non-nodulating plants. Nature. 1988;331:178–180. doi: 10.1038/331178a0. [DOI] [PubMed] [Google Scholar]
- Bogusz D, Kortt AA, Appleby CA. Sesbania rostrata root and stem nodule leghemoglobins: purification and relationship among the seven major components. Arch Biochem Biophys. 1987;254:263–271. doi: 10.1016/0003-9861(87)90102-0. [DOI] [PubMed] [Google Scholar]
- Christensen T, Dennis ES, Peacock WJ, Landsmann J, Marcker KA. Hemoglobin genes in non-legumes: cloning and characterization of a Casuarina glauca hemoglobin gene. Plant Mol Biol. 1991;16:339–344. doi: 10.1007/BF00020566. [DOI] [PubMed] [Google Scholar]
- Dakora FD, Appleby CA, Atkins CA. Effects of pO2 on the formation and status of leghemoglobin in nodules of cowpea and soybean. Plant Physiol. 1991;95:723–730. doi: 10.1104/pp.95.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidowitz EJ, Cullis CA, Lang-Unnasch N. Messenger RNA from diverse classes of alfalfa leghemoglobin genes show a similar pattern of spatial expression in symbiotic root nodules. Plant Soil. 1994;162:303–307. [Google Scholar]
- de Maagd RA, Yang WC, Goosen-de Roo L, Mulders IHM, Roest HP, Spaink HP, Bisseling T, Lugtenberg BJJ. Down-regulation of expression of the Rhizobium leguminosarum outer membrane protein gene ropA occurs abruptly in interzone II-III of pea nodules and can be uncoupled from nif gene activation. Mol Plant-Microbe Interact. 1994;7:276–281. [Google Scholar]
- Egli MA, Larson RJ, Hruschka WR, Vance CP. Synthesis of nodulins and nodule-enhanced polypeptides by plant gene-controlled ineffective alfalfa nodules. J Exp Bot. 1991;42:969–977. [Google Scholar]
- Fleming AI, Wittenberg JB, Wittenberg BA, Dudman WF, Appleby CA. The purification, characterization and ligand-binding kinetics of hemoglobin from root nodules of the non-leguminous Casuarina glauca-Frankia symbiosis. Biochim Biophys Acta. 1987;911:209–220. [Google Scholar]
- Franssen HJ, Vijn I, Yang WC, Bisseling T. Developmental aspects of the Rhizobium-legume symbiosis. Plant Mol Biol. 1992;19:89–107. doi: 10.1007/BF00015608. [DOI] [PubMed] [Google Scholar]
- Frühling M, Roussel H, Gianinazzi-Pearson V, Pühler A, Perlick AM. The Vicia faba leghemoglobin gene VfLb29 is induced in root nodules and in root colonized by the arbuscular mycorrhizal fungus Glomus fasciculatum. Mol Plant-Microbe Interact. 1997;10:124–131. doi: 10.1094/MPMI.1997.10.1.124. [DOI] [PubMed] [Google Scholar]
- Fuchsman WH, Appleby CA. Separation and determination of the relative concentrations of the homogeneous components of soybean leghemoglobin by isoelectric focusing. Biochim Biophys Acta. 1979;579:314–324. doi: 10.1016/0005-2795(79)90059-x. [DOI] [PubMed] [Google Scholar]
- Fuchsman WH, Barton CR, Stein MM, Thompson JT, Willett Leghemoglobin: different roles for different components? Biochem Biophys Res Commun. 1976;68:387–392. doi: 10.1016/0006-291x(76)91157-8. [DOI] [PubMed] [Google Scholar]
- Hargrove MS, Barry JK, Brucker EA, Berry MB, Phillips GN, Jr, Olson JS, Arredondo-Peter R, Dean JM, Klucas RV, Sarath G. Characterization of recombinant soybean leghemoglobin a and apolar distal histidine mutants. J Mol Biol. 1997;266:1032–1042. doi: 10.1006/jmbi.1996.0833. [DOI] [PubMed] [Google Scholar]
- Hill RD. What are hemoglobins doing in plants? Can J Bot. 1998;76:707–712. [Google Scholar]
- Jones DK, Badii R, Rosell FI, Lloyd E. Bacterial expression and spectroscopic characterization of soybean leghaemoglobin a. Biochem J. 1998;330:983–988. doi: 10.1042/bj3300983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneen BE, LaRue TA, Hirsch AM, Smith CA, Weeden NF. sym 13: a gene conditioning ineffective nodulation in Pisum sativum. Plant Physiol. 1990;94:899–905. doi: 10.1104/pp.94.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi H, Hata S. Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol Gen Genet. 1993;238:106–119. doi: 10.1007/BF00279537. [DOI] [PubMed] [Google Scholar]
- Landsmann J, Dennis ES, Higgins TJV, Appleby CA, Kortt AA, Peacock WJ. Common evolutionary origin of legume and non-legume plant hemoglobins. Nature. 1986;324:166–168. [Google Scholar]
- Lehtovaara P, Lappalainen A, Ellfolk N. The amino acid sequence of pea (Pisum sativum) leghemoglobin. Biochim Biophys Acta. 1980;623:98–106. doi: 10.1016/0005-2795(80)90012-4. [DOI] [PubMed] [Google Scholar]
- Pathirana SM, Tjepkema JD. Purification of hemoglobin from the actinorhizal root nodules of Myrica gale L. Plant Physiol. 1995;107:827–831. doi: 10.1104/pp.107.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski MM, Topunov AF, Strózycki PM, Vorgias CE, Wilson KS, Legocki AB. Cloning and expression of plant leghemoglobin cDNA of Lupinus luteus in Escherichia coli and purification of the recombinant protein. Plant Sci. 1995;108:109–117. [Google Scholar]
- Suganuma N, Kitou M, Yamamoto Y. Carbon metabolism in relation to cellular organization of soybean root nodules and respiration of mitochondria aided by leghemoglobin. Plant Cell Physiol. 1987;28:113–122. [Google Scholar]
- Suganuma N, Okada Y, Kanayama Y. Isolation of a cDNA for nodule-enhanced phosphoenolpyruvate carboxylase from pea and its expression in effective and plant-determined ineffective pea nodules. J Exp Bot. 1997;48:1165–1173. [Google Scholar]
- Suganuma N, Sonoda N, Nakane C, Hayashi K, Hayashi T, Tamaoki M, Kouchi H. Bacteroids isolated from ineffective nodules of Pisum sativum mutant E135 (sym13) lack nitrogenase activity but contain the two protein components of nitrogenase. Plant Cell Physiol. 1998;39:1093–1098. [Google Scholar]
- Suganuma N, Tamaoki M, Kouchi H. Expression of nodulin genes in plant-determined ineffective nodules of pea. Plant Mol Biol. 1995;28:1027–1038. doi: 10.1007/BF00032664. [DOI] [PubMed] [Google Scholar]
- Suganuma N, Tamaoki M, Takaki M. Comparison of the protein composition and enzymatic activities during development between effective and plant-determined ineffective nodules in pea. Plant Cell Physiol. 1993;34:781–788. [Google Scholar]
- Takane K, Tajima S, Kouchi H. Two distinct uricase II (nodulin 35) genes are differentially expressed in soybean plants. Mol Plant-Microbe Interact. 1997;10:735–741. doi: 10.1094/MPMI.1997.10.6.735. [DOI] [PubMed] [Google Scholar]
- Taylor ER, Nie XZ, MacGregor AW, Hill RD. A cereal hemoglobin gene is expressed in seed and root tissues under anaerobic conditions. Plant Mol Biol. 1994;24:853–862. doi: 10.1007/BF00014440. [DOI] [PubMed] [Google Scholar]
- Trevaskis B, Watts RA, Andersson CR, Llewellyn DJ, Hargrove MS, Olson JS, Dennis ES, Peacock WJ. Two hemoglobin genes in Arabidopsis thaliana: the evolutionary origins of leghemoglobins. Proc Natl Acad Sci USA. 1997;94:12230–12234. doi: 10.1073/pnas.94.22.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uheda E, Syono K. Physiological role of leghaemoglobin heterogeneity in pea root nodule development. Plant Cell Physiol. 1982a;23:75–84. [Google Scholar]
- Uheda E, Syono K. Effects of leghaemoglobin components on nitrogen fixation and oxygen consumption. Plant Cell Physiol. 1982b;23:85–90. [Google Scholar]
- Verma DPS, Ball S, Guérin C, Wanamaker L. Leghemoglobin biosynthesis in soybean root nodules: characterization of the nascent and released peptides and the relative rate of synthesis of the major leghemoglobins. Biochemistry. 1979;18:476–483. doi: 10.1021/bi00570a016. [DOI] [PubMed] [Google Scholar]
- Walbot V, Warren C. Regulation of Mu element copy number in maize line with an active or inactive mutator transposable element system. Mol Gen Genet. 1987;211:27–34. doi: 10.1007/BF00338389. [DOI] [PubMed] [Google Scholar]