Abstract
The physiological and metabolic processes of host plants are manipulated and remodeled by phytopathogenic fungi during infection, revealed obvious signs of biotrophy of the hemibiotrophic pathogen. As we known that effector proteins play key roles in interaction of hemibiotrophic fungi and their host plants. BAS4 (biotrophy-associated secreted protein 4) is an EIHM (extrainvasive hyphal membrane) matrix protein that was highly expressed in infectious hyphae. In order to study whether BAS4 is involved in the transition of rice blast fungus from biotrophic to necrotrophic phase, The susceptible rice cultivar Lijiangxintuanheigu (LTH) that were pre-treated with prokaryotic expression product of BAS4 and then followed with inoculation of the blast strain, more serious blast disease symptom, more biomass such as sporulation and fungal relative growth, and lower expression level of pathogenicity-related genes appeared in lesion of the rice leaves than those of the PBS-pretreated-leaves followed with inoculation of the same blast strain, which demonstrating that BAS4 invitro changed rice defense system to facilitate infection of rice blast strain. And the susceptible rice cultivar (LTH) were inoculated withBAS4-overexpressed blast strain, we also found more serious blast disease symptom and more biomass also appeared in lesion of leaves inoculated with BAS4-overexpressed strain than those of leaves inoculated with the wild-type strain, and expression level of pathogenicity-related genes appeared lower in biotrophic phase and higher in necrotrophic phase of infection, indicating BAS4 maybe in vivo regulate defense system of rice to facilitate transition of biotrophic to necrotrophic phase. Our data demonstrates that BAS4 in vitro and in vivo participates in transition from the biotrophic to the necrotrophic phase of Magnaporthe oryzae.
Keywords: Magnaporthe oryzae, Rice, Effector, Hemibiotrophic fungi
Abbreviations: LTH, Lijiangxintuanheigu; Bgh, Blumeria graminis; IH, invasive hyphae; BAS, biotrophy-associated secreted; EIHM, extra-invasive hyphal membrane; BIC, biotrophic interfacial complex; ORF, open reading frame; ROS, reactive oxygen species; YLG, Yue Liang Gu; GFP, green fluorescence protein; GST, glutathione-S-transferase; PBS, phosphate buffer saline; PDA, potato dextrose agar; hpi, hours post inoculation; PCD, programmed cell death; ATMT, agrobacterium tumefaciens-mediated transformation; DAB, diaminobenzidine; PR gene, pathogenicity related gene; M.oryzae, Magnaporthe oryzae; OsMPK6, rice mitogen-activated protein kinase 6; OsMPK12, rice mitogen-activated protein kinase 12
1. Introduction
Plant pathogens are classified as biotrophic, hemibiotrophic, and necrotrophic parasites based on their lifestyles. Biotrophic parasites utilizing their host cells for survival and reproduction, necrotrophic parasites obtain nutrients from dead tissues for survival, and hemibiotrophic parasites live through the biotrophic and then necrotrophic phases. Pathogenic effector proteins determine the life history of pathogens and are secreted to directly interact with the host to facilitate infection (Collmer et al., 2009, Hogenhout et al., 2009). Manipulating and remodeling of the physiological and metabolic processes of the host by pathogen are major hallmarks of biotrophy (Akin et al., 2017, Demir et al., 2017, Gao et al., 2017, Mukattash et al., 2018, Raza et al., 2018, Yang et al., 2017). Pathogens secret effector proteins to avoid host recognition or to inhibit an immune response of host during the biotrophic phase, the transition of pathogenic fungi from the biotrophic to necrotrophic phase is related to the induction of the host defense response, which is considered to have the following characteristics: the host cell programmed death and the differential regulation of the signaling pathway (Keon et al., 2007, Rudd et al., 2008). The process of necrotrophic phase involves the degradation of the cell wall and the accumulation of H2O2, which leads to the collapse of large region of the mesophyll and a rapid increase in biomass from the host dead cells, as well as a rapid increase in pathogenic fungi biomass and sporulation (Shetty et al., 2003, Kema et al., 2008, Shetty et al., 2010, Marshall et al., 2011). The levels of H2O2 sharply increase during pathogen-host interactions until H2O2 levels peak at the later biotrophic stage, indicating that the rapid increase in H2O2 is a stress response rather than a defense reaction (Shetty et al., 2003, Kema et al., 2008, Shetty et al., 2010), and other responses include the expression of wheat disulfide isomerase and pathogenesis-related genes (PR genes) and structural defense response (Ray et al., 2003, Shetty et al., 2009).
Pathogens secret effector proteins into the extracellular spaces and host cells, and those that enter the extracellular space are called apoplastic effectors. Apoplastic effectors include cell wall-degrading enzymes, toxins, and various cysteine-rich proteins. Cell wall-degrading enzymes and toxins are the most important toxic factors of necrotrophic bacteria, but these are not important for biotrophic parasites and hemibiotrophic parasites (Barras et al., 1994, Cantu et al., 2008). Biotrophic parasites and hemibiotrophic parasites secrete a variety of cysteine-rich effector proteins into the host apoplastic space (Kamoun, 2005). The apoplastic effector proteins are recognized by the pattern-recognition receptors (PRRs) of pathogen-associated molecular patterns (PAMPs) of host plants (Kamoun, 2005, Qutob et al., 2006). Biotrophic parasites and hemibiotrophic parasites mainly secrete intracellular effector proteins, which are then transported into the apoplastic space through the N-signal peptide and subsequently enter the host cells. A large number of studies have shown that PAMP-triggered immunity (PTI) is the major mechanism by which host plants resist biotrophic, hemibiotrophic, and necrotrophic parasites. PTI responses include reactive oxygen species (ROS) production (Daudi et al., 2012), MAP kinase cascade (Nühse et al., 2000), transcriptional activation of some defense-related genes, synthesis and secretion of antimicrobial compounds, and reinforcement of plant cell walls. When a plant interacts with hemibiotrophic parasites, host cell death is inhibited during the biotrophic phase and in turn is promoted during the necrotrophic phase (Coll et al., 2011). The number of death of host cells is the key to the transition of hemibiotrophic parasites from the biotrophic to necrotrophic phase, as some effector proteins can be recognized by NL-like proteins and PRRs in the host cells, triggering an HR response to limit further colonization of pathogens. For hemibiotrophic parasites, the regulation of the biotrophic and necrotrophic phases is a critical step (Wang et al., 2011). Intracellular effector proteins that inhibit host cell death are often expressed during the biotrophic phase, whereas the NLPs and the induction of the RXLR effector protein occur in the necrotrophic phase. The NLPs of P. sojae and C. higginsiαnum are upregulated during the transition (Kleemann et al., 2012). The effector protein of Septoriα tritici induces the transition from the biotrophic to necrotrophic phase, the appearance of the lesions, and host cell death (Deller et al., 2011).
Rice blast is caused by the rice blast fungus Magnaporthe oryzae, resulting in major losses in global rice production (Dean et al., 2005, Ebbole, 2007, Talbot, 2002). In the genome of rice blast strain 70–15, most of the genes (about 1306) encode putative secretory proteins (Dean et al., 2005, Ebbole, 2007, Ebbole, 2007, Yoshida et al., 2009). PWL1, PWL2 (Kang et al., 1995, Sweigard et al., 2002), Avr-Pita, Avr-Pii, Avr-Pik/km/kp (Ahn et al., 2004) and AvrPiz-t have been proven as avirulence proteins. Some of the secreted proteins are essential for the pathogenicity of rice blast fungi such as MPG 1 (Talbot, 2002), EMPl (Ahn et al., 2004), MHPl (Kim et al., 2010), MSPl (Jeong et al., 2010), MC69 (Saitoh et al., 2012), Slp1 (Mentlak et al., 2012) and four biotroph-related secreted proteins BAS1-BAS4 (Mosquera et al., 2009) were identified. Numerous studies have shown that the effector proteins secreted by infectious hyphae play an important role in the early stages of infection; however, the underlying mechanism remains unclear. Meinhardt et al. showed that the expression of secreted protein genes is associated with infection, cell growth in mycelial growth, and plant cell death. The secretory proteins expressed in the biotrophic phase are mainly related to the destruction of the intercellular matrix and fungal mycelial deformation, allowing the fungi to avoid plant recognition and defense. These secretory proteins are expressed during the necrotrophic phase are considered to be a class of proteins that can degrade plant cell walls and cell components, causing host necrosis (Meinhardt et al., 2014). In-depth analysis of secreted proteins during infection is thus key to the elucidation of the mechanism underlying the transition from the biotrophic to necrotrophic phase. BAS1–BAS4 are highly expressed during the biotrophic phase, and BAS4 expression is 61-fold higher in the infectious hyphae (Mosquera et al., 2009), The BAS4 gene encodes 102 amino acids, of which 8 are cysteine residue. BAS4 is a putative extra-invasive hyphal membrane (EIHM) matrix protein that is secreted and distributed on the external surface of the hyphae. To date, no report on BAS4 regulating physiological and metabolic processes in rice blast fungus during biotrophic and necrotrophic phase has been published to date. In this paper, the leaves and calli of rice were treated with the purified prokaryotic expression product GST-BAS4-mCherry, and callose deposition, ROS production, and defense-related gene expression were then monitored in leaves that were treated for 24 h. The purified BAS4 prokaryotic expression product was sprayed on the rice leaves and then inoculated with the blast strain, then disease incidence of rice leaves was assessed after 7 days and expression of defense-related genes were analyzed in infected leavespretreated with BAS4 fusion protein. At the same time, rice leaves were inoculated with the BAS4 overexpression strain, and disease incidence of rice leaves inoculated after 7 days and expression of defense-related genes were analyzed in BAS4 overexpression strain-infected leaves, and the biomass in lesion was also analyzed to elucidate how BAS4 regulates the metabolic and defense systems during the biotrophic and necrotrophic stages. The present study has determined that effector proteins that regulate the rice defense system are strongly associated with specific physiological processes.
2. Material and methods
2.1. Rice blast strain and rice cultivar
The present study used the rice cultivar LTH, which is highly susceptible to M. oryzae strains. The rice blast strain used in this investigation was the highly pathogenic strain 66b. The BAS4 overexpression strain (35S: BAS5/Mo-2, the overexpression strain was conserved in our laboratory) is under the control of a 35S promoter, wild-type strain A1343R-7 (PCR analysis showed that the strain did not harbor the BAS4 gene). All these strains have been maintained in our laboratory. GST-BAS4-mCherry was purified prokaryotic expression product.
2.2. Culture and sporulation of rice blast strain
Mycelia of M. oryzae were inoculated on Petri plates containing PDA solid medium (200 g potato, 20 g glucose, 15 g agar, and 1000 mL water), which was cultured in a 28 °C incubator until the mycelia covered the entire agar surface. Mycelium blocks were transferred into a flask, which was cultured on a 28 °C shaker for 5–7 d, and then stored in at 4 °C prior to use. The mycelium liquid of M. oryzae was evenly spread on Petri plates containing tomato-oat medium (tomato-oat medium: 300–400 mL tomato juice, 40 g oats, 0.6 g CaCO3, 20 g agar, and 1000 mL water). The plates were incubated at 25 °C for 7–10 days to allow sporulation. Approximately 20 mL of sterile water was added into the dish, and then the plates were gently scraped, washed, and filtered to obtain the spore suspension. The suspension was adjusted to a density of 1 × 105 cells/mL.
2.3. Cultivation of rice seedlings and blast strain in oculating rice leaves
The rice seeds were sterilized with 1.5% sodium hypochlorite and incubated at 28 °C for germination. The germinated seedlings were sown in a seedling tray. When the rice grew to the 3–4 leaf stage, these were transferred to an inoculation box. The rice seedlings were sprayed with the M. oryzae spore suspension and sufficient moisture was provided for the next 24 h and then these were transferred to a greenhouse. Disease incidence in rice leaves inoculated with the blast strain at 6 dpi was investigated, and leaf samples were collected at different times after inoculation. Each treatment was performed in triplicate, and 15 seedlings were assessed in each repeat. Four seedlings were sampled for each repeat at each time point.
2.4. Real-time RT-PCR analysis of defense-related genes in infected rice leaves
Total RNA of infected rice leaves was extracted using the TRIZOL (Invitrogen, Shanghai, China) extraction kit. Real-time RT-PCR primer sequences for the defense-related genes in rice a shown in Table 1. The 25.0 µL reaction system of real-time RT-PCR (Bio-Rad) followed: 2.0 µL template cDNA, 0.5 µL forward primer, 0.5 µL reverse primer, 12.50 µL 2 × EasyTaq PCR SuperMix, and 9.5 µL sterilized ddH2O. Amplification cycle parameters: pre-denaturation at 95 °C for 3 min, denaturation at 95 °C for 20 s, annealing extension at 59 °C for 20 s, and a collection of fluorescence signals at 65 °C; a total of 44 cycles were performed. Dissolution curve parameters: the temperature was increased starting from 59 °C; fluorescent signals were collected at each cycle with the temperature increased by 0.5 °C, and a total of 80 cycles were performed. Three repeats were performed for each sample. Ct values were recorded to calculate the relative expression levels. Real-time PCR data was analyzed with the 2–△△Ct method. Expression levels of the resistance genes in rice were calculated. The relative gene expression level = treated sample (target gene Ct-actin Ct) – blank sample (target gene Ct-actin Ct).
Table 1.
Primers used for real-time RT-PCR.
| Gene | Accession number | Description | Primer-F/Primer-R |
|---|---|---|---|
| PR1a | Os07g03710 | Pathogenesis related | F:5′-GCTACGTGTTTATGCATGTATGG-3′ R:5′-TCGGATTTATTCTCACCAGCA-3′ |
| PR10a | Os12g36880 | Pathogenesis related | ′F:5′-AATGAGAGCCGCAGAAATGT-3′ R:5′-GGCACATAAACACAACCACAA-3′ |
| RPR10b | Os12g36830 | Pathogenesis related | F:5′-TCTCCGTATTGCTGCTTCCT-3′ R:5′-CACTCTCACAAAATCAAACACCA-3′ |
| CEBiP | Os03g04110 | Chitin receptor | F:5′-GATGACTGGTTTATCCAGCTTTG-3′ R:5′-TTCAAGCAGCCGTACAAGTG-3′ |
| MPK6 | Os10g38950 | – | F:5′-AAAAAGCAGGCTCCATGGATTCCTCCTCCG-3′ R:5′-AGAAAGCTGGGTGCAATCAACCGGTATAAT-3 |
| MPK12 | Os06g49430 | – | 'F:5′-CCAAGCGCAAGATGCCTCT-3′R:5′-AGCACGGAGAAGTTGGTAC-3′ |
| Chit1 | Os02g39330 | – | F:5′-CTGGTACTGGACCAACAACG-3′ R:5′-GTTCTTGCCGTCGCACTC-3′ |
| actin | Os11g06390 | / | F:5′-GAGTATGATGAGTCGGGTCCAG-3′ R:5′-ACACCAACAATCCCAAACAGAG-3′ |
2.5. Callose and ROS observation
Rice sheaths were selected at the two-leaf stage and shortened to a length of 4 cm. The rice sheathes were then immersed in sterile water for 2 h and then placed in a Petri dish lined with wet filter paper. The purified BAS1 prokaryotic expression products were sprayed onto the leaves, which were then placed in an incubator at 26 °C and humidity for 24 h. The leaves were stained with aniline blue and DAB, respectively.
Callose observation: The leaves were soaked in ethanol lactophenol solution (12.5 g phenol, 12.5 mL glycerol, 12.5 mL lactic acid, and 12.5 mL water, mixed well) and kept in a 65 °C water bath until the chloroplasts were detached. The treated sheaths were rinsed with 50% ethanol, followed by rinsing with sterile water. The sheaths were then stained with 0.1% aniline blue (dissolved in 150 mmol/L K2HPO4, pH 9.5) for 0.5 h. The stained samples were then immersed in 50% glycerol. Callose deposition was observed with UV light under a fluorescent microscope (DFC450C, Leica).
ROS observation: After treatment with the protein solution, the leaves were then rinsed with sterile water, gently wiped with filter paper, and then stained with 1 mg/mL DAB solution for 24 h. The stained leaves were then soaked in ethanol lactophenol solution and kept in a 65 °C water bath until the chloroplasts were removed. ROS was observed under a fluorescent microscope (DFC450C, Leica).
2.6. The analysis of fungal relative growth in lesion using qPCR
The spores cultured in tomato-oat medium were washed and adjusted to a density of 1 × 105/mL as observed under a 10 × objective lens (Leica, DM750). The rice leaves were cut into blades of 4 cm in length and placed in a Petri plate lined with wet filter paper. Two wounded punch spots (spaced 1 cm apart) were made in the 4-cm long leaf (not to penetrate the leaves). The wounded punch spots were then inoculated with the spore suspension using a pipette. The inoculated leaves were kept in an incubator set at 28 °C, a relative humidity of 90%, and in the dark for 24 h, followed by 28 °C with illumination and uninterrupted moisturizing spray for the next seven days. The length and width of the lesions were measured at seven days after onset of disease. Genomic DNA and total RNA were extracted from the lesions (2 × 1 cm). Each 25 µL qPCR reaction system consisted of the following: 1.5 µL of template cDNA, 0.5 µL of the forward primer, 0.5 µL of the reverse primer; 12.50 µL of a 2 × Easy Taq PCR SuperMix, and 10 µL of ddH2O. The amplification cycle parameters were as follows: pre-denaturation at 95 °C for 3 min, denaturation at 95 °C for 20 s, annealing extension at 58 °C for 20 s, and collection of fluorescence signals at 65 °C; for a total of 44 cycles were performed. The dissolution curve parameters were as follows: the temperature was increased starting from 59 °C; and fluorescent signals were collected at each cycle with a temperature increase of 0.5 °C, for a total of 80 cycles. Three repeats were performed for each sample. Ct values were recorded to calculate absolute quantitative expression levels. The relative growth of the fungus was calculated as 2[Ct(MoPot2) Ct(OsUBQ)] × 100, where MoPot2 is the Pot2 gene of M. oryzae, OsUBQ is the rice ubiquitin gene. The primer pair of 5′ACGACCCGTCTTTACTTATTTGG3′ and 5′AAGTAGCGTTGGTTTTGTTGGAT3 was designed as nucleotide acid sequence of Pot2 gene, and primer pair of 5′TTCTGGTCCTTCCACTTTCAG3′ and 5′ACGATTGATTTAACCAGTCCATGA3′ was designed as nucleotide acid sequence of OsUBQ gene.
2.7. BAS4 distribution in infected rice root using confocal
The rice seeds surface was sterilized with 1.5% sodium hypochlorite and then soaked in sterile water. The seeds were germinated at 28 °C in an incubator. After 72 h, the seedlings were transferred to a water agar medium for cultivation. The BAS4 overexpressed strains were cultured on tomato-oat culture medium, and rice roots were inoculated with the mycelia pellets. The BAS4 fusion protein was observed under a confocal fluorescence microscope (Leica, SP5II).
3. Results
3.1. BAS4 immediat elyelicits rice basic defense responses during the early stage of interaction
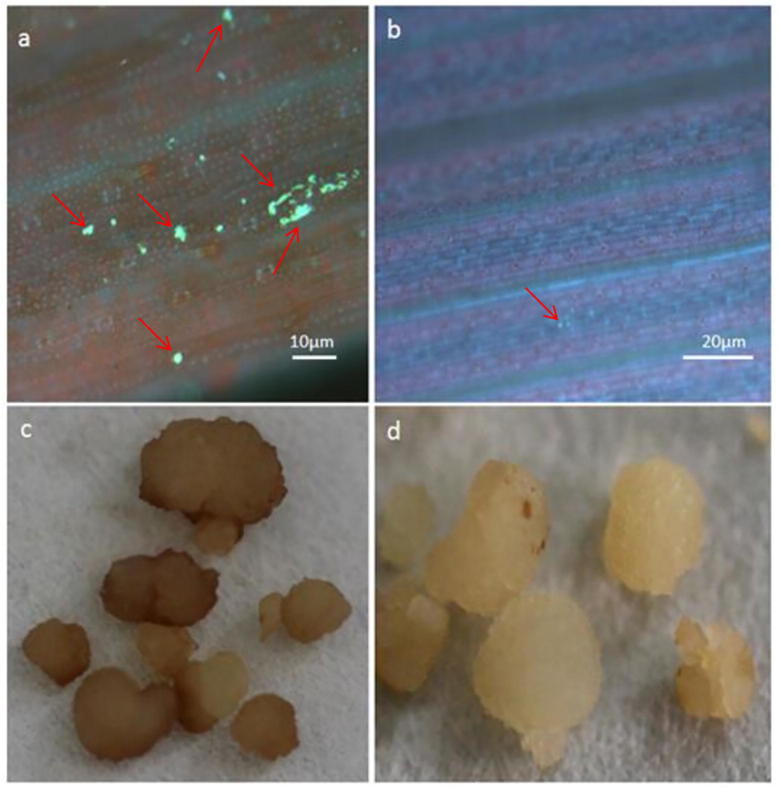
To determine whether BAS4 can induce LTH to elicit basic defense responses, the callose deposition and ROS production in the rice seedlings were assessed. Purified BAS4 prokaryotic expression product at a concentration of 1 mg/mL was sprayed onto the rice leaves and then later stained with aniline blue. Extensive callose deposition was observed on the BAS4 sprayed leaves (Fig. 1a), whereas only a little amount of callose (Fig. 1b) was detected in the leaves treated with PBS solution (CK). The rice calli that were treated with DAB exhibited high levels of ROS production after 6 h (Fig. 1c), whereas no ROS production was observed on rice calli treated with PBS solution (CK) (Figureld). These results show that BAS4 in vitro induces early and instantaneous immune responses in Rice.
Fig. 1.
Callose deposition and ROS production in rice leaves and calli treated with purified eukaryotic products of BAS4 at 24 h and 6 h. Note: (a): Callose deposition in rice leaves treated with purified eukaryotic products of BAS4 at 24 h, (b): Callose deposition in rice leaves treated with PBS solution at 24 h, (c): ROS production in rice calli treated with purified eukaryotic products of BAS4 at 6 h, (d): ROS production in rice calli treated with PBS at 6 h. Red arrows mean callose deposition.
3.2 BAS4 promotes cell death in the late stage of compatible interaction
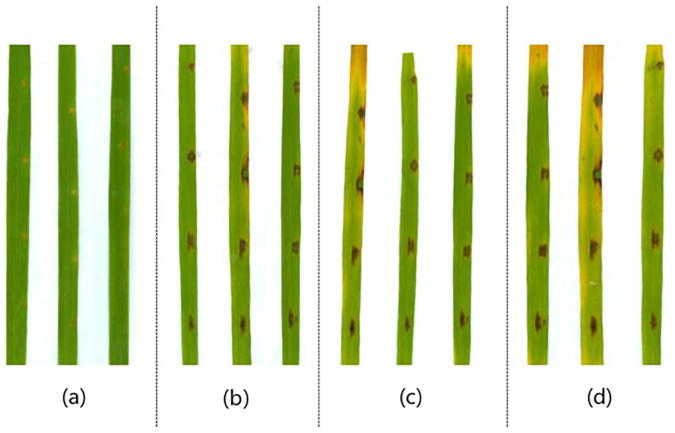
Based on the observation that the purified BAS4 prokaryotic expression product instantaneously induced early callose deposition and ROS production in rice leaves, we further analyzed whether BAS4 in vitro enhances rice resistance or increases rice leaves necrosis formation when the leaves are sprayed with the BAS4 protein solution. The leaves of the wounded rice leaves were inoculated with the purified prokaryotic expression product (1 mg/mL BAS4) and then monitored for phenotypic changes. The results showed that necrotic symptoms appeared on the fourth day after leaf treatment with the BAS4 prokaryotic expression product, and the necrotic spots become more distinct at 7 d, 8 d, and 9 d. Some of the necrotic spots on the leaves were roughly arranged as a single line, whereas no necrotic spots were observed on leaves treated with PBS for 9 d (Fig. 2). These results show that the BAS4 prokaryotic expression product could instantaneously induce early immune responses in rice, although necrotic symptoms appear during the later stage after inoculation of rice leaves with the BAS4 protein solution.
Fig. 2.
Necrotic symptoms in Lijiang xintuanheigu leaves treated with 1 mg/ml BAS4 fusion protein at 7d, 8d and 9d. (a): The wounded leaves were treated with PBS and symptom on leaves was observed at 9 day, (b): The wounded leaves were treated with BAS4 prokaryotic expression product and symptom on leaves was observed at 7 day, (c): The wounded leaves were treated with BAS4 prokaryotic expression product and symptom was observed at 8 day, (d) The leaves were treated with BAS4 prokaryotic expression product and symptom was observed at 9 day.
3.3. Effects of BAS4 invitro on infected rice in biotrophic and necrotrophic phase
Our observation found that the BAS4 protein solution could trigger basic immune responses during the early stage of infection and subsequently result in necrotic lesion formation during the later stage of infection indicates that BAS4 plays a dual role in rice leaves. We further analyzed the effect of BAS4 on the defense system and metabolism of rice during the biotrophic and necrotrophic stages.
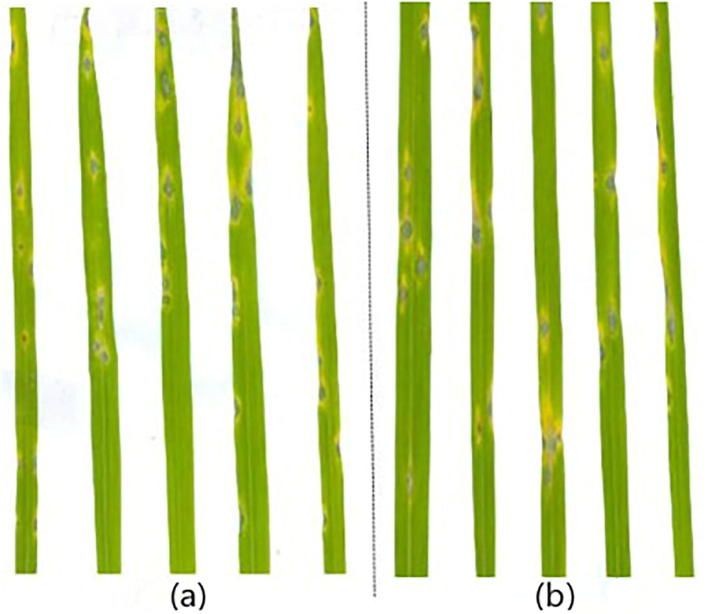
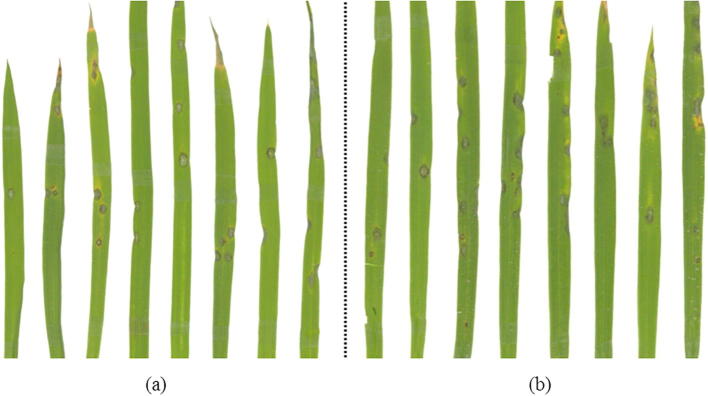
The rice leaves were sprayed with 1 mg/mL BAS4 prokaryotic expression product for 24 h, which was then followed by inoculation with blast strain 66b. Rice leaves directly inoculated with blast strain 66b were used as control. The disease symptom of leaves that were pretreated with BAS4 were more severe (i.e., size and number of lesions) compared to the controls (Fig. 3).
Fig. 3.
Rice seedlings were pretreated using prokaryotic expression product of BAS4 for 24 h before being inoculated by blast strain of 66b. The leaves of symptoms were photographed at seventh day post-inoculation. (a): rice leaves pre-treated with BAS4 prokaryotic expression product are inoculated with blast strain 66b, (b): rice leaves pre-treated with PBS were inoculated with blast strain 66b.
The BAS4-pretreated leaves also showed a 38.07% of disease incidence rate compared to the control (25.67%) (Table 2). These findings indicate that BAS4 induces more severe symptoms upon infection than leaves directly inoculated with the blast strain, thereby indicating that BAS4 in vitro contributes to infection during the biotrophic phase.
Table 2.
Disease incidence on leaves inoculated with blast strain.
| Treatment | Disease incidence (%) |
|---|---|
| BSA4 + 66b | 38.07 ± 1.32a |
| PBS + 66b | 25.67 ± 1.68b |
Note: BAS4 + 66b mean rice leaves pretreated with BAS4 prokaryotic expression products are inoculated with blast strain 66b, PBS + 66b mean rice leaves pretreated with PBS were inoculated with blast strain 66b.
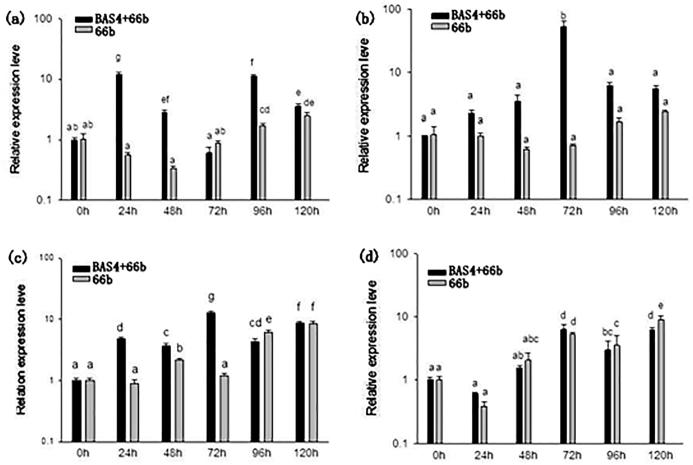
To further analyze the effect of BAS4 on expression of the rice defense-related genes during biotrophic and necrotrophic phase, the expression of PR1a, PR10a, RPR10b and Chit1 were detected using real-time RT-PCR. The results showed that the expression level of PR1a, PR10a and RPR10b (P < 0.05) appeared higher up-regulation in leavespretreated than in those (P < 0.05) PBS-pretreated inoculated with blast strain 66bat 24hpi, 48hpi, 72hpi, 96hpi and 120hpi (Fig. 4). And expression level of PR1a in leaves pretreated with BAS4 showed up-regulation from 24hpi to 48hpi and 96hpi to 120hpi, but the expression level of PR1a appeared lower expression at 72hpi. Expression level of PR10a and RPR10b appeared highest at 72hpi. There was higher expression level from 72hpi to 120hpi than from 24hpi to 48hpi. The Chit1 gene appeared higher expression level in BAS4-pretreated leaves than in PBS-pretreated ones at 24hpi and 72hpi, but lower expression appeared in The results showed that BAS4-pretreated leaves than in PBS-pretreated ones at 48hpi, 96hpi and 120hpi. And the expression tendency of Chit1 appeared the highest at 72hpi and the lowest at 24hpi. Our results indicated that expression level of pathogenicity-related genes and Chit1 contribute to blast strain infect rice and transition of biotrophic to necrotrophic phase.
Fig. 4.
The expression level ofPR1α, PR10α, RPR10b induced by BAS4 solution in rice leaves treated with GST-BAS4-mCherry were challenged with 66b were detected. (a): relative expression of PR1a gene in infected rice, (b): relative expression of PR10a gene in infected rice, (c): relative expression of PR10b gene in infected rice, (d): relative expression ofchit1 gene in infected rice.
We then further verified whether BAS4 promotes the colonization of rice blast fungus in vitro. The leaves that were pretreated with BAS4 and inoculated with 66b at 72 dpi showed lesion areas of 16.9 ± 0.11 mm, whereas that of the controls was 9.9 ± 0.07 mm (Table 3).
Table 3.
Lesion area on leaves inoculated with blast strain.
| Treatment | Lesion area (mm2) |
|---|---|
| BAS4 + 66b | 16.9 ± 0.11a |
| PBS + 66b | 9.9 ± 0.07b |
Note: BAS4 + 66b mean leaves pretreated with GST-BAS4-mCherrγ were punch inoculated with 66b, PBS + 66b mean leaves pretreated with PBS and then punch inoculated with 66b. h: hours postinoculation. Error bars represent ± SD of the mean. The data was carried out three independent experiments.
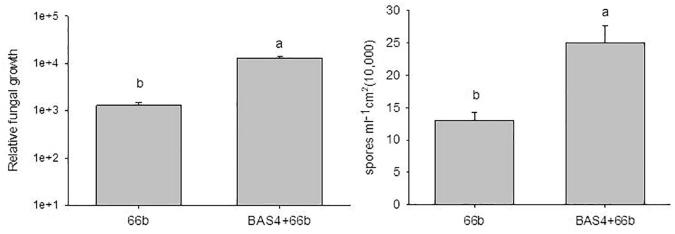
The number of spores on leaf lesions after pretreatment with BAS4 was 2.5 × 105/mL, which was significantly higher than that of the control (1.3 × 105/mL). The relative growth of infected leaves that were pretreated with BAS4 was 13085.63, which was significantly higher than that of the control (1294.68) (Fig. 5).
Fig. 5.
Spores and relative fungal growth on leaves treated with BAS4 were challenged with 66b at 6d after punch inoculation. Note: BAS4 + 66b mean leaves treated with GST-BAS4-mCheny were punch inoculation with 66b, 66b mean PBS-pretreated leaves were punch inoculation with 66b. h: hours post-inoculation. Error bars represent ± SD of the mean. The data was carried out three independent experiments.
The above results indicate that the BAS4 protein solution-pretreated rice leaves, which showed larger lesion areas, higher spore numbers, and enhanced fungal growth on leaves possess a greater biomass of infected leaves. These results confirm that BAS4 in vitro promotes the colonization of rice blast fungus during the necrotrophic phase.
3.4. Effects of BAS4 overexpression strain on infection and colonization during biotrophic and necrotrophic phase
Based on the results that BAS4 promotes the colonization of rice blast fungus during the necrotrophic phase in vitro, we further investigated how BAS4 in vivo influences rice blast fungus infection and colonization during the biotrophic and necrotrophic phases. Spraying rice leaves with spore suspensions of the BAS4 overexpression strain resulted in a high disease incidence of 49.34%, whereas disease spraying with wild-type strain (Al343R-7) was 45.07% (Table4, Fig. 6).
Table 4.
The disease incidence of rice blast on infected leaves.
| Strain | Disease incidence (%) |
|---|---|
| A1343R-7 | 45.07 ± 5.77b |
| 35S: BAS4/Mo-1 | 49.34 ± 7.36a |
Note: three independent experiments. Wild type strain: A1343R-7, BAS4 overexpression strain: 35S: BAS4/Mo-1.
Fig. 6.
Symptoms on leaves challenged with 35S: BAS4/Mo-1 overexpression strain. The data was carried out three independent experiments. (a): Rice leaves were inoculated with wild - type strainofA1343R-7, (b): Rice leaves were inoculated with BAS4 overexpression strain of 35S: BAS4/Mo-1. Scale bar represents 0.5 cm.
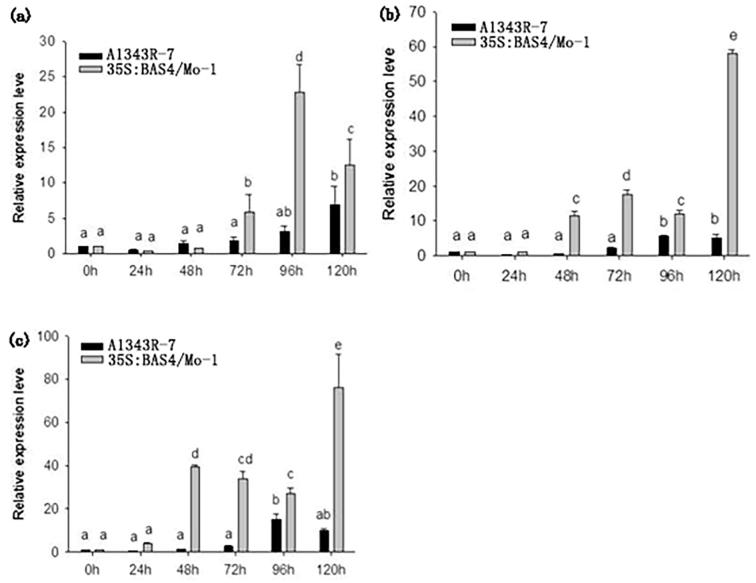
The expression of defense-related genes in leaves infected by 35S: BAS4/Mo-1 and the wild-type strain A1343R-7 were detected at 0 h, 24 h, 48 h, 72 h, 96 h, and 120 h using real-time RT-PCR. The results showed that expression level of PR1a, PR10a, RPR10b in leaves infected by 35S: BAS4/Mo-1 increased from 48 hpi to 120 hpi, whereas that in leaves infected with the wild-type strain of A1343R-7 was significantly lower than those of the BAS4-pretreated leaves (Fig. 7). The expression of PR1a, PR10a and RPR10b increased from 72 hpi to 120 hpi, indicating that rice elicited a stress response to pathogen infection, as well as possibly activated downstream signaling pathways that lead extensive cell death, thereby promoting mycelia growth. Therefore, BAS4 overexpression in the blast strain facilitates the transition of M. oryzae from the biotrophic phase to the necrotrophic phase.
Fig. 7.
Expression level of rice genes PR in leaves infected with overexpression strain. h: hours postinoculation. (a): PR1a gene relative expression in infected leaves, (b): PR10a gene relative expression in infected leaves, (c) RPR10b gene relative expression in infected leaves. Error bars represent ± SD of the mean. The data was carried out three independent experiments.
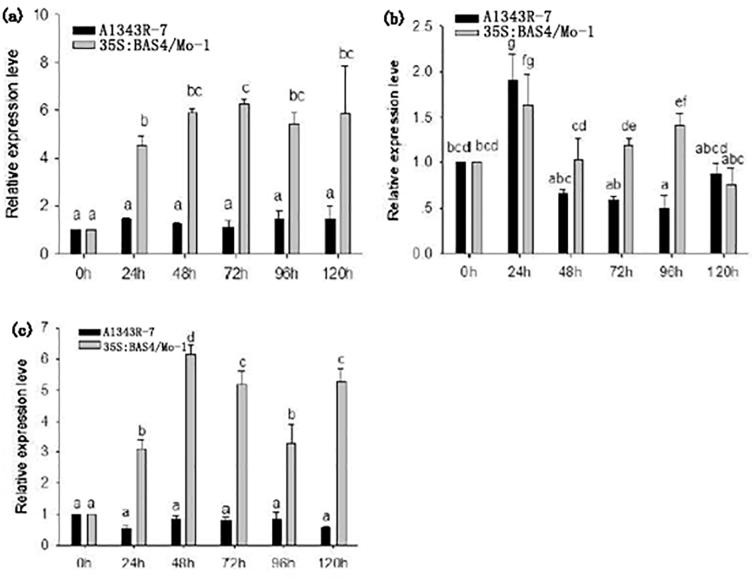
The expression level of OsCEBiP, OsMPK6, and OsMPK12 in leaves infected by 35S: BAS4/Mo-1 and wild-type strain A1343R-7 were also assessed using real-time RT-PCR. The results showed that the expression levels of the three genes in leaves infected by 35S: BAS4/Mo-1 were significantly higher than those infected with the wild-type strain (Fig. 8).
Fig. 8.
Expression level of rice genes MPK6, CEBiP and MPK12 in leaves infected by overexpression strain. h: hours post-inoculation. (a): MPK6 gene relative expression in infected leaves, (b): MPK12 gene relative expression in infected leaves, (c)CEBiP gene relative expression in infected leaves. Error bars represent ± SD of the mean. The data was carried out three independent experiments.
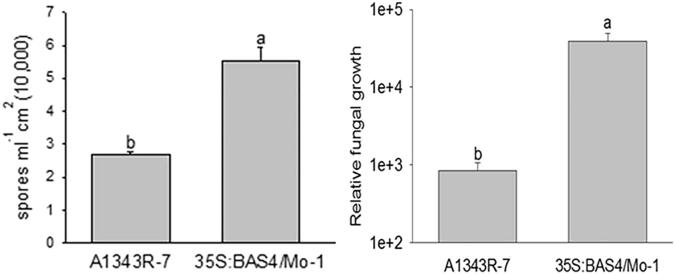
To further clarify the effect of the BAS4 overexpression strain on the colonization of M. oryzae, the number of spores and fungal relative growth in the lesions on leaves infected by strain 35S: BAS4/Mo-1 were evluated. The results showed that the spore number (2.7667 × 105) in lesion of leaves infected by 35S: BAS4/Mo-1 was higher than in that of lesions from leaves infected by the wild-type strain A1343R-7 (1.3333 × 105). qPCR analysis indicated that the relative growth rate of the fungi (39261.5) in lesion infected with 35S: BAS4/Mo-1 was significantly higher than that in lesions infected with the wild-type strain (839.1) (Fig. 9). These results further confirm that BAS4 overexpression in the blast strain promotes the colonization of rice blast fungus infection during the necrotrophic phase.
Fig. 9.
The sporulation and fungal relative growth of overexpression strain of 35S: BAS4/Mo-1 after punch inoculation. The data was carried out three independent experiments. Note: Values are the means of three replications, and error bars represent ± SD of the mean.
These results further confirmed that BAS4 overexpression in blast strain can promote the transition of biotrophic to necrotrophic phase and colonization of rice blast fungus.
To investigate cell death rates during infection with the overexpression strain 35S: BAS4/Mo-1, rice leaves infected by the blast strain at different time points were stained with DAB, which showed that there was a small amount of cell death at 10 hpi and 20 hpi and almost no cell death was observed around the infection site. The number of dead cells slightly increased at 48 hpi, significantly increased at 72 hpi, and then peaked at 96 hpi and 120 hpi. Most of the dead cells were situated distal to the infection hyphae, at the same time of increase of cell death, large number of mycelia were also simultaneously growing and colonizing (Fig. 10). The apoptotic rates in the leaves infected with 35S: BAS4/Mo-1 were higher than that of leaves infected with the wild-type strain A1343R-7.
Fig. 10.
Colonization of hyphae and rice cell death in rice leaves at six different time points during 35S: BAS4/Mo-1 infecting rice leaves. (a): Rice leaves inoculated with 35S: BAS4/Mo-1 were stained with DAB and observed at 10 hpi (hour post inoculation), (b): Rice leaves inoculated with 35S: BAS4/Mo-1 were stained with DAB and observed at 20 hpi (hour post inoculation),(c): Rice leaves inoculated with 35S: BAS4/Mo-1 were stained with DAB and observed at 48 hpi (hour post inoculation), white arrow mean hyphae in infected rice (d): Rice leaves inoculated with 35S: BAS4/Mo-1 were stained with DAB and observed at 72 hpi (hour post inoculation) (e): Rice leaves inoculated with 35S: BAS4/Mo-1 were stained with DAB and observed at 96 hpi (hour post inoculation), white arrow mean hyphae in infected rice (f): Rice leaves inoculated with 35S: BAS4/Mo-1 were stained with DAB and observed at 120 hpi (hour post inoculation), (g): Cell death of rice leaves inoculated with 35S: BAS4/Mo-1 were stained with DAB and observed at 72 hpi (hour post inoculation), (h): Cell death of rice leaves inoculated with 35S: BAS4/Mo-1 were stained with DAB and observed at 72 hpi (hour post inoculation),(i): Cell death of rice leaves inoculated with 35S: BAS4/Mo-1 were stained with DAB and observed at 120 hpi (hour post inoculation). White arrow mean infectious hyphae.
3.5 Spatial distribution of BAS4 in Magnaporthe oryzae biotrophic and necrotrophic phase
BAS4 is highly expressed in the mycelia of M. oryzae. We thus further investigated the spatial distribution and phenotype of the fusion protein of BAS4-mCherry in the roots of seedlings infected with overexpression strain 35S: BAS4/Mo-1 at 1 dpi, 2 dpi, 3 dpi 4 dpi, 5 dpi, 6 dpi, and 7 dpi. The infected roots began to turn brown at 3 dpi, which further darkened in color at 7 dpi. The fusion protein BAS4-mCherry was observed in the roots of seedlings infected with 35S: BAS4/Mo-1 at 2 dpi (Fig. 11a). More intense BAS4-mCherry staining was observed in the infected roots at 3 dpi and 4 dpi, and at the later time points (Fig. 11b). Numerous mycelia exhibiting BAS4-mCherry staining were observed in the infected roots at 7 dpi (Fig. 11c). These results show that BAS4 that is overexpressed in the blast strain facilitates infection during the biotrophic phase, and simultaneously, increases mycelia production during the necrotrophic phase, indicating that BAS4 overexpression in M. oryzae strain contributes to infection and colonization during the biotrophic and necrotrophic phases.
Fig. 11.
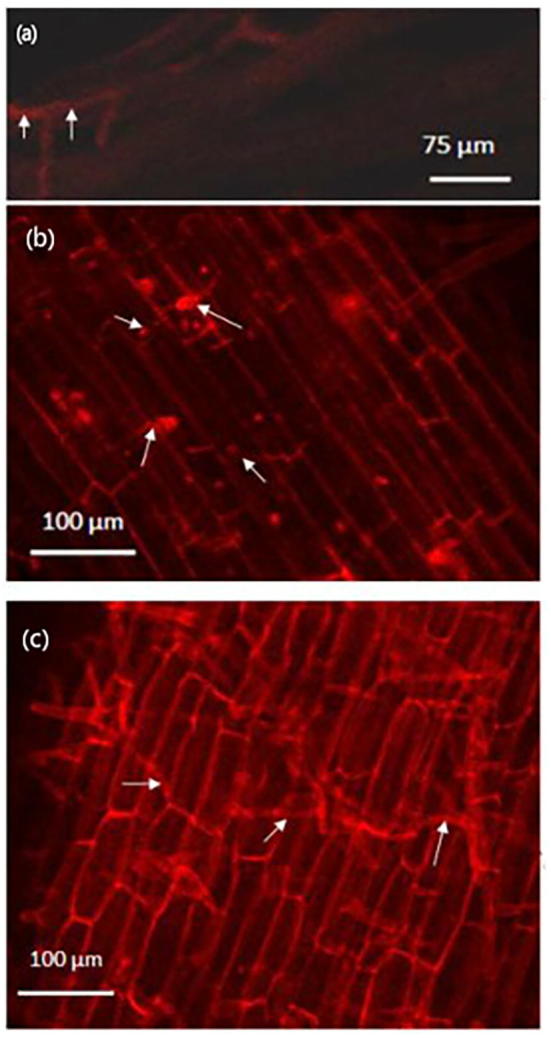
Distribution of BAS4-mCherry fusion protein in 35S: BAS4/Mo-1 overexpression strain infecting root at 2dpi, 4dpi and 7dpi. (a): Spatial distribution and phenotype of the fusion protein of BAS4-mCherry in roots infected by overexpression strain of 35S: BAS4/Mo-1 at 2 dpi. (b): Spatial distribution and phenotype of the fusion protein of BAS4-mCherry in roots infected by overexpression strain of 35S: BAS4/Mo-1 at 4 dpi. (c): Spatial distribution and phenotype of the fusion protein of BAS4-mCherry in roots infected by overexpression strain of 35S: BAS4/Mo-1 at 7 dpi.
4. Discussion
Small secretory proteins play a key role in the interaction of biotrophic parasites or hemibiotrophic parasites with their host plants. Hemibiotrophic parasites absorb nutrients from the host living cells during the biotrophic phase, and then kill the host cells in the next necrotophic phase to obtain nutrition from these dead cells (Laluk and Mengiste, 2010). Biotrophic and hemibiotrophic parasites secret effector proteins to manipulate host cell structures and functions, obtain nutrients, and inhibit host defense responses to facilitate their colonization (Khang et al., 2010, Spanu et al., 2010, Schirawski et al., 2010, Ravensdale et al., 2011). HR-induced host cell death is a major obstacle for such fungus to further infect and colonize hosts. Rice blast is caused by the infection of hemibiotrophic parasites, and M. oryzae is one of the most important model fungi. Therefore, it is important to study the infection mechanism of rice blast fungus during the biotrophic and necrotrophic phases, which may facilitate in the elucidation of the molecular role of effector proteins in the interaction between M. oryzae and rice.
To verify how BAS4 regulates rice metabolic pathways to facilitate rice blast strains to infect and colonize, we analyzed the effect of the biotrophic-related secreted protein BAS4 on the rice defense system during the compatible interaction of M. oryzae and rice. Previous studies have shown that expression levels of rice defense-related genes increase during the early stage of infection, indicating that rice defense responses are not completely inhibited during the biotrophic phase (Mosquera et al., 2009, Marcel et al., 2010). The present study observed that BAS4 triggers instantaneous rice defense responses during the early stages of infection in vitro, but caused more severe necrotic lesion, indicating this was a transient response. We thus hypothesized that BAS4 plays a major role in inducing severe necrotic lesions on rice leaves during the lates stage of interaction between rice and BAS4 in vitro. Some studies have shown that the up-regulation of PR1a activates its downstream PCD signal, which in turn causes extensive cell death in infected rice plants. In the present study, PR1a exhibited lower expression levels in BAS4-pretreated leaves at 72 hpi, indicating a lower rate of cell death at this time, whereas PR1a showed a higher expression level at 24 hpi to 48 hpi and 96 hpi to 120 hpi, suggesting that BAS4 induces early defense responses at early stage of biotrophic phase and made the infected rice a status of stress at the late stage of necrotrophic phase. The Chit1 gene showed the lowest expression levels at 24 hpi but higher expression levels from 72 hpi to 120 hpi in BAS4-pretreated leaves, indicating that there was minimal cell wall degradation of cell wall at the early stage of biotrophic phase but then increased at the later stage of necrotrophic phase. These results indicate the rice seedlings were triggered defense responses at the early stages of the biotrophic phase, but stress responses at the necrotrophic phase. The up-regulation of defense-related genes such as PR1a, PR10a, RPR10b, and Chit1 induced by BAS4 invitro led to an increase in the number of necrotic spots on rice leaves, indicating that BAS4 invitro regulates the expression level of PR1α, PR10α, RPR10b, and Chit1 of rice that facilitates the transition of rice blast strain from the biotrophic to the necrotrophic phase. Yang et al. (2013) found that biomass of lesion, H2O2 accumulation and expression of defense-related genes (PR genes, β − 1,3-glucanase gene and Chitinase gene)from wheat leaves infected by Pseudomonαs syringαe may regard as bioassay for detecting transition from biotrophic to necrotrophic phase during interaction of wheat leaf blotch and wheat. And their results also found that there was increase of biomass of lesion, H2O2 accumulation and expression level of PR genes in transition from biotrophic to necrotrophic phase (Yang et al., 2013). In our study, we also found that there are also increase of biomass of lesion from leave-pretreated with BAS4 protein solution infected by blast strain, and 72 h was switch for transition of biotrophic to necrotrophic phase of M. oryzae. Our results were in agreement with those of Yang et al. (2013), which showed that BAS4 in vitro induced early basal defense responses in rice but did not increase rice resistance to pathogen infections, and more severe necrotic spots were observed on the wounded rice leaves that were pretreated with the BAS4 prokaryotic expression product, indicating that BAS4 in vitro participated in the transition from the biotrophic to the necrotrophic phase.
To verify effect of BAS4 overexpression in the blast strain on the biotrophic and necrotrophic phase of M. oryzae, the expression of rice defense-related genes and the lesion biomass of leaves inoculated with BAS4 overexpression strain were analyzed, which showed more severe symptoms, increased lesion biomass, and higher PR1a, PR10a, and RPR10b expression levels during the biotrophic phase than that during the necrotrophic phase, thereby indicating that BAS4 overexpression in rice blast strain also facilitates the transition of M. oryzae from the biotrophic to the necrotrophic phase. The expression level of OsCEBiP, OsMPK6, and OsMPK12 increased during necrotrophic phase (72–120 hpi). OsCEBiP up-regulation could activate downstream signal, thereby inhibiting the accumulation of ROS, phytocassanes, and momilactones. OsMPK6 inhibits rice defense, wherein knocking down this gene in rice results in an enhancement of resistance to Xanthomonαs oryzae infection (Yuan et al., 2007). The present study observed a significant up-regulation of OsMPK6 during the necrotrophic phase, which indicating that it could decrease rice resistance. The up-regulation of OsMPK12 induces OsEREBP1 phosphorylation, which in turn enhances DNA to combine with the GCC box element of the PR gene, resulting in the up-regulation of the PR gene that enhances rice resistance at the early stage of infection but to make rice stress status at late stage of pathogen infection (Cheong et al., 2003). Therefore, PR1α, PR10α, RPR10b, OsMPK6, OsMPK12 and OsCEBiP contribute to the transition of M. oryzae from the biotrophic to the necrotrophic phase. Dothistromα septosorum is a hemibiotrophic parasite, and its biomass increases during the early stage of infection and sporulation and extensive amounts of tissue undergo disassembly and cell death (Kabir et al., 2015). The biomass of the other hemibiotrophic parasites such as Zymoseptoriα tritici (Keon et al., 2007) and Mycosphaerella fijiensis (Kantã°n-Moreno et al., 2013) increases during the necrotrophic phase and is accompanied by a loss of host cell membrane integrity and electrolyte leakage. Mycosphαerella fijiensis produces toxin and secrete proteins that destroy host tissues (Chuc-Uc et al., 2011, Cruz-Cruz et al., 2011). There is no any report on the role of BAS4 in transition of Mαgnαporthe oryzαe from biotrophic to necrotrophic phase. The findings of the present study have verified that both the BAS4 prokaryotic expression product and BAS4 overexpression in M. oryzae regulate the infection process. Furthermore, large amounts of hyphae harboring the BAS4-mCherry fusion protein were distributed in the root tissues of seedlings infected with 35S: BAS4/Mo-1, thereby further confirming that BAS4 is involved in the transition of rice blast strain from the biotrophic to the necrotrophic phase.
In conclusion, BAS4 invitro (BAS4 prokaryotic expression product) and invivo (BAS4 overexpression strain) facilitate in the transition of rice blast strain from the biotrophic to necrotrophic phase, thereby providing information on the molecular mechanism underlying the interaction between rice and M. oyzαe.
5. Conclusions
The study describes role of a biotrophy-related secreted 4 (BAS4) in transition from biotrophic to necrotrophic phase of Magnaporthe oryzae. Our results reveal that BAS4 in vitro facilitates more serious blast disease symptomin rice, more biomass such as sporulation and fungal relative growth, and lower expression level of pathogenicity-related genes appeared in lesion of the rice leaves than those of the PBS-pretreated-leaves followed with inoculation of the same blast strain. And we also find more serious blast disease symptom and more biomass also appeared in lesion of leaves inoculated with BAS4-overexpressed strain than those of leaves inoculated with the wild-type strain, and expression level of pathogenicity-related genes appeared lower in biotrophic phase and higher in necrotrophic phase of infection, indicating BAS4 maybe in vivo regulate defense system of rice to facilitate transition of biotrophic to necrotrophic phase. Next, we will focus on the knock-out of the BAS4 using CRISPR, analyze morphological change and pathogenicity of the mutant strain, effect of mutant strain on rice defense system. The present study may facilitate in the elucidation of the molecular mechanism of the rice blast fungal effector protein in the life history of hemibiotrophic parasites.
Author contributions
JY carried out most experiments and analyzed the data. JY, LL and CL wrote the manuscript. YL, CW and YW analyzed the data. CW, YL and YW are responsible for charting and layout. All authors read and approved the final manuscript.
Acknowledgements
This work was provided by National Key R&D Program of China (2017YFD0200400), supported by Natural Science Foundation of China (Grant No. 31400073) and also supported by the Yunnan Natural Science foundation (Grant no. 2013FB039) from the Yunnan Science and Technology Department of China and Program for Innovative Research Team (in Science and Technology, IRTSTYN) in University of Yunnan Province.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahn S.J., Shin R., Schachtman D.P. Expression of KT/KUP genes in arabidopsis and the role of root hairs in K+ Uptake. Plant Physiol. 2004;134(3):1135–1145. doi: 10.1104/pp.103.034660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin S., Aksoy D.Y., Akin S., Kilic M., Yetisir F., Bayraktar M. Prediction of central lymph node metastasis in patients with thyroid papillary microcarcinoma. Turk. J. Med. Sci. 2017;47(6):1723–1727. doi: 10.3906/sag-1702-99. [DOI] [PubMed] [Google Scholar]
- Barras F., Gijsegem F.V., Chatterjee A.K. Extracellular enzymes and pathogenesis of soft-rot erwinia. Annu. Rev. Phytopathol. 1994;32(1):201–234. [Google Scholar]
- Cantu D., Vicente A.R., Labavitch J.M., Bennett A.B., Powell A.L.T. Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci. 2008;13(11):610–617. doi: 10.1016/j.tplants.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Cheong Y.H., Moon B.C., Kim J.K., Kim C.Y., Kim M.C., Kim I.H. BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol. 2003;132(4):1961–1972. doi: 10.1104/pp.103.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuc-Uc J., Brito-Argáez L., Canto-Canché B., Tzec-Simá M., Rodríguez-García C., Peraza-Echeverría L. The in vitro secretome of mycosphaerellafijiensis induces cell death in banana leaves. Plant Physiol. Biochem. 2011;49(6):572–578. doi: 10.1016/j.plaphy.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Collmer A., Schneider D.J., Lindeberg M. Lifestyles of the effector rich: genome-enabled characterization of bacterial plant pathogens. Plant Physiol. 2009;150(4):1623–1630. doi: 10.1104/pp.109.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll N.S., Epple P., Dangl J.L. Programmed cell death in the plant immune system. Cell Death Differ. 2011;18(8):1247–1256. doi: 10.1038/cdd.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Cruz C.A., García-Sosa K., Escalante-Erosa F., Peña-Rodríguez L.M. Physiological effects of the hydrophilic phytotoxins produced by mycosphaerella fijiensis, the causal agent of black sigatoka in banana plants. J. Gen. Plant Pathol. 2011;77(2):93–100. [Google Scholar]
- Daudi A., Cheng Z., O'Brien J.A., Mammarella N., Khan S., Ausubel F.M. The apoplastic oxidative burst peroxidase in arabidopsis is a major component of pattern-triggered immunity. Plant Cell. 2012;24(1):275–287. doi: 10.1105/tpc.111.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R.A., Talbot N.J., Ebbole D.J., Farman M.L., Mitchell T.K., Orbach M.J. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434(7036):980. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- Deller S., Hammond-Kosack K.E., Rudd J.J. The complex interactions between host immunity and non-biotrophic fungal pathogens of wheat leaves. J. Plant Physiol. 2011;168(1):63–71. doi: 10.1016/j.jplph.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Demir D., Tosun A., Kahraman N., Ozsin K.K., Sanri U.S., Kocaslan A., Korkmaz U.T., Goncu M.T. Technique of circular narrowing with a polytetrafluoroethylene graft in patients with high-flow arteriovenous fistula: mid-term results. Acta Med. Mediterranea. 2017;33(4):587–591. [Google Scholar]
- Ebbole D.J. Magnaporthe as a model for understanding host-pathogen interactions. Annu. Rev. Phytopathol. 2007;45(1):437–456. doi: 10.1146/annurev.phyto.45.062806.094346. [DOI] [PubMed] [Google Scholar]
- Gao W., Wang Y., Wang W., Shi L. The first multiplication atom-bond connectivity index of molecular structures in drugs. Saudi Pharm. J. 2017;25(4):548–555. doi: 10.1016/j.jsps.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenhout S.A., Ra V.D.H., Terauchi R., Kamoun S. Emerging concepts in effector biology of plant-associated organisms. Mol. Plant-Microbe Interact.: MPMI. 2009;22(2):115. doi: 10.1094/MPMI-22-2-0115. [DOI] [PubMed] [Google Scholar]
- Jeong J.S., Mitchell T.K., Dean R.A. The Magnaporthe grisea snodprot1 homolog, MSP1, is required for virulence. FEMS Microbiol. Lett. 2010;273(2):157–165. doi: 10.1111/j.1574-6968.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- Kabir M.S., Ganley R.J., Bradshaw R.E. The hemibiotrophic lifestyle of the fungal pine pathogen Dothistroma septosporum. Forest Pathol. 2015;45(3):190–202. [Google Scholar]
- Kamoun S. A catalogue of the effector secretome of plant pathogenic oomycetes. Annu. Rev. Phytopathol. 2005;44(1):41–60. doi: 10.1146/annurev.phyto.44.070505.143436. [DOI] [PubMed] [Google Scholar]
- Kang S., Sweigard J.A., Valent B. The pwl host specificity gene family in the blast fungus Magnaporthe grisea. Mol. Plant Microbe Interact. 1995;8(6):939–948. doi: 10.1094/mpmi-8-0939. [DOI] [PubMed] [Google Scholar]
- Kantún-Moreno N., Vázquez-Euán R., Tzec-Simá M., Peraza-Echeverría L., Grijalva-Arango R., Rodríguez-García C. Genome-wide in silico identification of GPI proteins in mycosphaerell afijiensis and transcriptional analysis of two GPI-anchored β-1,3-glucanosyltransferases. Mycologia. 2013;105(2):285–296. doi: 10.3852/12-103. [DOI] [PubMed] [Google Scholar]
- Kema G.H., Ta V.D.L., Mendes O., Verstappen E.C., Lankhorst R.K., Sandbrink H. Large-scale gene discovery in the septoriatritici blotch fungus Mycosphaerell agraminicola with a focus on in planta expression. Mol. Plant-Microbe Interact.: MPMI. 2008;21(9):1249. doi: 10.1094/MPMI-21-9-1249. [DOI] [PubMed] [Google Scholar]
- Keon J., Antoniw J., Carzaniga R., Deller S., Ward J.L., Baker J.M. Transcriptional adaptation of Mycosphaerell agraminicola to programmed cell death (PCD) of its susceptible wheat host. Mol. Plant. Microbe Interact. 2007;20(2):178–193. doi: 10.1094/MPMI-20-2-0178. [DOI] [PubMed] [Google Scholar]
- Khang C.H., Berruyer R., Giraldo M.C., Kankanala P., Park S.Y., Czymmek K. Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell. 2010;22(4):1388–1403. doi: 10.1105/tpc.109.069666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Ahn I.P., Rho H.S., Lee Y.H. MHP1, a Magnaporthe grisea hydrophobin gene, is required for fungal development and plant colonization. Mol. Microbiol. 2010;57(5):1224–1237. doi: 10.1111/j.1365-2958.2005.04750.x. [DOI] [PubMed] [Google Scholar]
- Kleemann J., Rincon-Rivera L.J., Takahara H., Neumann U., Loren E.V., Charlotte H. Sequential delivery of host -induced virulence effectors by appressoria and intracellular hyphae of the phytopathogen Colletotrichum higginsianum. PLOS Pathog. 2012;8:el002643. doi: 10.1371/journal.ppat.1002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laluk K., Mengiste T. Necrotroph attacks on plants: wanton destruction or covert extortion? Arabidopsis Book. 2010;8(8):e0136. doi: 10.1199/tab.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel S., Sawers R., Oakeley E., Angliker H., Paszkowski U. Tissue-adapted invasion strategies of the rice blast fungus Magnaporthe oryzae. Plant Cell. 2010;22(9):3177–3187. doi: 10.1105/tpc.110.078048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R., Kombrink A., Motteram J., Loza-Reyes E., Lucas J., Hammond-Kosack K.E. Analysis of two in planta expressed lysm effector homologs from the fungus Mycosphaerell agraminicola reveals novel functional properties and varying contributions to virulence on wheat. Plant Physiol. 2011;156(2):756–769. doi: 10.1104/pp.111.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt L.W., Costa G.G.L., Thomazella D.P., Teixeira P.J.P., Carazzolle M.F., Schuster S.C. Genome and secretome analysis of the hemibiotrophic fungal pathogen, moniliophthora roreri, which causes frosty pod rot disease of cacao: mechanisms of the biotrophic and necrotrophic phases. BMC Genom. 2014;15(1):164. doi: 10.1186/1471-2164-15-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentlak T.A., Kombrink A., Shinya T. Effector-mediated suppression of chitin -triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell. 2012;24:322–335. doi: 10.1105/tpc.111.092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquera G., Giraldo M.C., Khang C.H., Coughlan S., Valent B. Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1-4 as biotrophy-associated secreted proteins in rice blast disease. Plant Cell. 2009;21(4):1273–1290. doi: 10.1105/tpc.107.055228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukattash T.L., AlGhzawi N.Y., Abu Farha R.K., Jarab A.S., Hameen-Anttila K., Vainio K., Gammoh O.S. An audit on parental attitudes towards medicines used in children. Saudi Pharm. J. 2018;26(1):133–137. doi: 10.1016/j.jsps.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse T.S., Peck S.C., Hirt H., Boller T. Microbial elicitors induce activation and dual phosphorylation of the arabidopsis thaliana MAPK 6. J. Biol. Chem. 2000;275(11):7521–7526. doi: 10.1074/jbc.275.11.7521. [DOI] [PubMed] [Google Scholar]
- Qutob D., Kemmerling B., Brunner F., Küfner I., Engelhardt S., Gust A.A. Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell. 2006;18(12):3721–3744. doi: 10.1105/tpc.106.044180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravensdale M., Nemri A., Thrall P.H., Ellis J.G., Dodds P.N. Co-evolutionary interactions between host resistance and pathogen effector genes in flax rust disease. Mol. Plant Pathol. 2011;12(1):93–102. doi: 10.1111/j.1364-3703.2010.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S., Anderson J.M., Urmeev F.I., Goodwin S.B. Rapid induction of a protein disulfide isomerase and defense-related genes in wheat in response to the hemibiotrophic fungal pathogen Mycosphaerell agraminicola. Plant Mol. Biol. 2003;53(5):741–754. doi: 10.1023/B:PLAN.0000019120.74610.52. [DOI] [PubMed] [Google Scholar]
- Raza S., Iqbal Y., Ullah I., Mubarak M.S., Hameed M.U., Raza M. Effects of gamma irradiation on the physico-chemical and biological properties of levofloxacin. Pakistan J. Pharm. Sci. 2018;31(1):181–186. [PubMed] [Google Scholar]
- Rudd J.J., Keon J., Hammond-Kosack K.E. The wheat mitogen-activated protein kinases tampk3 and tampk6 are differentially regulated at multiple levels during compatible disease interactions with Mycosphaerell agraminicola. Plant Physiol. 2008;147(2):802–815. doi: 10.1104/pp.108.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh H., Fujisawa S., Mitsuoka C., Ito A., Hirabuchi A., Ikeda K. Large-scale gene disruption in Magnaporthe oryzae identifies MC69, a secreted protein required for infection by monocot and dicot fungal pathogens. Plos Pathogens. 2012;8(5):e1002711. doi: 10.1371/journal.ppat.1002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirawski J., Mannhaupt G., Münch K., Brefort T., Schipper K., Doehlemann G. Pathogenicity determinants in smut fungi revealed by genome comparison. Science. 2010;330(6010):1546–1548. doi: 10.1126/science.1195330. [DOI] [PubMed] [Google Scholar]
- Shetty N.P., Mehrabi R., Lütken H., Haldrup A., Kema G.H.J., Collinge D.B. Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen, Septoria tritici and wheat. New Phytol. 2010;174(3):637–647. doi: 10.1111/j.1469-8137.2007.02026.x. [DOI] [PubMed] [Google Scholar]
- Shetty N.P., Jensen J.D., Knudsen A., Finnie C., Geshi N., Blennow A. Effects of β-1,3-glucan from Septoria tritici on structural defence responses in wheat. J. Exp. Bot. 2009;60(15):4287. doi: 10.1093/jxb/erp269. [DOI] [PubMed] [Google Scholar]
- Shetty N.P., Kristensen B.K., Newman M.A., Møller K., Gregersen P.L., Jørgensen H.J.L. Association of hydrogen peroxide with restriction of Septoria tritici, in resistant wheat. Physiol. Mol. Plant Pathol. 2003;62(6):333–346. [Google Scholar]
- Spanu P.D., Abbott J.C., Amselem J., Burgis T.A., Soanes D.M., Stüber K. Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science. 2010;330(6010):1543–1546. doi: 10.1126/science.1194573. [DOI] [PubMed] [Google Scholar]
- Sweigard J.A., Carroll A.M., Kang S., Farrall L., Chumley F.G., Valent B. Identification, cloning, and characterization of pwl2, a gene for host species specificity in the rice blast fungus. Plant Cell. 1995;7(8):1221–1233. doi: 10.1105/tpc.7.8.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot N.J. On the trail of a cereal killer: exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2002;57(1):177–202. doi: 10.1146/annurev.micro.57.030502.090957. [DOI] [PubMed] [Google Scholar]
- Wang Q., Han C., Ferreira A.O., Yu X., Ye W., Tripathy S. Transcriptional programming and functional interactions within the phytophthora sojae RXLR effector repertoire. Plant Cell. 2011;23(6):2064–2086. doi: 10.1105/tpc.111.086082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A., Han Y., Li S., Xing H., Pan Y., Liu W. Synthesis and comparison of photocatalytic properties for bi2wO6 nanofibers and hierarchical microspheres. J. Alloy. Compd. 2017;695:915–921. [Google Scholar]
- Yang F., Li W. Transcriptional reprogramming of wheat and the hemibiotrophic pathogen Septoria tritici during two phases of the compatible interaction. Plos One. 2013;8(11):e81606. doi: 10.1371/journal.pone.0081606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Saitoh H., Fujisawa S., Kanzaki H., Matsumura H., Yoshida K. Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell. 2009;21(5):1573–1591. doi: 10.1105/tpc.109.066324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B., Shen X., Li X., Xu C., Wang S. Mitogen-activated protein kinase OsMPK6 negatively regulates rice disease resistance to bacterial pathogens. Planta. 2007;226(4):953–960. doi: 10.1007/s00425-007-0541-z. [DOI] [PubMed] [Google Scholar]