Abstract
Once believed to be limited to articular cartilage, osteoarthritis is now considered to be an organ disease of the “whole joint.” Damage to the articular surface can lead to, be caused by, or occur in parallel with, damage to other tissues in the joint. The relationship between cartilage and the underlying subchondral bone has particular importance when assessing joint health and determining treatment strategies. The articular cartilage is anchored to the subchondral bone through an interface of calcified cartilage, which as a whole makes up the osteochondral unit. This unit functions primarily by transferring load-bearing weight over the joint to allow for normal joint articulation and movement. Unfortunately, irreversible damage and degeneration of the osteochondral unit can severely limit joint function. Our understanding of joint pain, the primary complaint of patients, is poorly understood and past efforts toward structural cartilage restoration have often not been associated with a reduction in pain. Continued research focusing on the contribution of subchondral bone and restoration of the entire osteochondral unit are therefore needed, with the hope that this will lead to curative, and not merely palliative, treatment options. The purpose of this narrative review is to investigate the role of the osteochondral unit in joint health and disease. Topics of discussion include the crosstalk between cartilage and bone, the efficacy of diagnostic procedures, the origins of joint pain, current and emerging treatment paradigms, and suitable preclinical animal models for safety and efficacy assessment of novel osteochondral therapies. The goal of the review is to facilitate an appreciation of the important role played by the subchondral bone in joint pain and why the osteochondral unit as a whole should be considered in many cases of joint restoration strategies.
Impact Statement
In this comprehensive review, we are providing a holistic overview of osteochondral tissue development, disease, pain localization, as well as structural evaluation and current repair strategies. This review is intended to serve as a broad introduction to this multidisciplinary research area. It is a thorough examination of the biological aspects of the osteochondral unit from a tissue engineering perspective, highlighting the importance of the subchondral bone in chondral and osteochondral lesion repair and pain relief.
Keywords: cartilage, bone, osteochondral unit, crosstalk, tissue engineering
Introduction
The homeostasis between joint tissues, particularly between cartilage and the underlying subchondral bone, is fundamentally important to understand when determining treatment strategies for chondral, osteochondral, and subchondral bone lesions. If left untreated, these lesions can progress to osteoarthritis (OA), as the damage advances to widespread tissue degeneration along with severe joint pain and stiffness.1
Previous treatment strategies have had a strong focus on structural repair of the articular cartilage only, but in many cases, bone lesions cause the associated pain, and not articular cartilage damage.2 As nociceptors are present within the subchondral bone and not the cartilage,3 it would therefore seem prudent to consider the cartilage and subchondral bone as one entity to be successful in ameliorating joint pain while restoring the load-bearing and frictionless movement capacity of the healthy joint.
Recently improved imaging techniques have contributed to our understanding of the importance of bone pathologies and their contribution to OA progression and pain. For instance, posttraumatic bone bruising or bone marrow lesions (which can be visualized with magnetic resonance imaging [MRI]) can cause sustained bone remodeling that can lead to a loss of subchondral bone support for the cartilage. Moderate-to-severe bone marrow lesions that do not resolve over time are linked to ongoing symptoms and future cartilage degradation.4–6
In this review, we will focus on literature describing the crosstalk between cartilage, calcified cartilage and bone tissues, their response to injury, repertoire of repair responses, and their relationship to pain. This enables consideration of current and emerging treatment modalities for osteochondral defects as well as new tissue engineering tactics and animal models. We will link these broad topics in an effort to shed light on the limitations of today's treatment techniques and propose directions of future research to address the complexities of the development, regeneration, and repair of the osteochondral unit.
Methods
A variety of databases were used to collect and review all referenced material, including University of Guelph Primo, PubMed through NCBI, Medline, ProQuest Biological Sciences, the Cochrane Database, and Google Scholar. The keywords used in the search of the databases were cartilage, bone, osteochondral unit, endochondral ossification, osteochondral crosstalk, interzone, joint biomechanics, joint pain, joint nociceptors, evaluation, MRI, arthroscopy, radiography, clinical trials, microfracture, autologous chondrocyte implantation (ACI), BioCartilage, particulate cartilage, cartilage autologous implantation system (CAIS), dual-tissue transplantation, osteochondral autograft transfer (OAT), mosaicplasty, tissue engineering, animal models, synovium, T2 mapping, and chondrocytes. References listed were identified through primary searches, articles known to the authors, or from reference lists from other review articles.
Synovial joints and the osteochondral unit
Synovial joints are complex structures that permit near frictionless motion between bones to facilitate human ambulation. Hyaline cartilage, lining the bone ends in these joints, has the unique ability to withstand high loads and while maintaining a near-frictionless and compliant articulating interface between the bones. Load dispersal is shared with the subchondral bone plate and it is believed that the morphology of the subchondral bone is a direct expression and adaptation of past loading history in adults.7
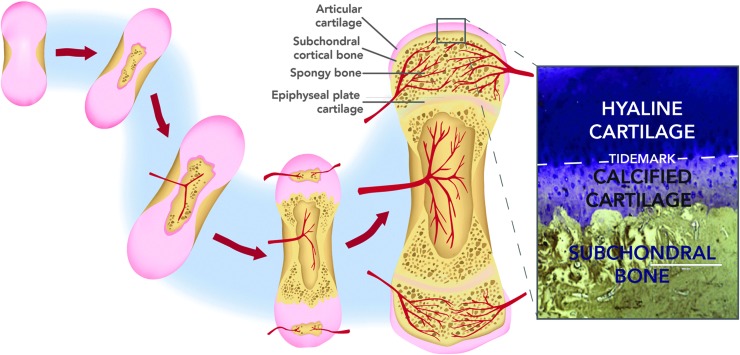
This dynamic relationship between cartilage and bone (the osteochondral unit) is crucial to maintaining overall joint health and integrity. The osteochondral unit is composed of hyaline cartilage connected through a zone of calcified cartilage to the subchondral cortical bone known as the subchondral plate, which gives way to metaphyseal trabecular bone. The distinct histological boundary between hyaline and calcified cartilage is known as the tidemark (Fig. 1, inset).
FIG. 1.
Diagram of endochondral ossification and the formation of the osteochondral unit. Inset: Histological view of the osteochondral unit (stained with Toluidine Blue and von Kossa).
The osteochondral unit arises as the final product of endochondral ossification, where the fetal cartilage “anlagen” is diminished by endochondral ossification after birth, leaving permanent articular cartilage at the ends of fully developed long bones. Upon initiation of ossification in the cartilage anlagen, bone tissue development occurs first in the diaphysis (primary ossification center) and then in the epiphyses (secondary ossification centers). Differentiating chondrocytes undergo hypertrophy and express collagen type X, which aids in promoting calcification of the surrounding matrix.8,9 Blood vessels invade the developing tissue, infiltrating it with osteoblasts and driving the expression of osteogenic factors such as Runx2, eventually replacing the transient cartilage with bone.10,11 A thin layer of calcified cartilage matrix remains at the distal ends of the long bones after ossification, anchoring the newly developed bone to the stable articular cartilage (Fig. 1).
Stable articular cartilage arises from the interzone in the developing limb, which separates epiphyseal ossification centers and serves as the eventual location of the joint. As chondrification proceeds toward the ends of the developing long bone, the interzone arises from the remaining condensed mesenchymal cells, forming a tightly packed cellular region between the ossification centers.12 Cells in this region form three distinct layers: two outer chondrogenic layers that become the lining of the epiphyses of long bones, and a middle layer that undergoes cavitation.13 It is speculated that the two outer layers mark the transition zone between calcified cartilage and the newly formed subchondral bone, while the middle layer forms the mature articular cartilage.14 The resulting articular cartilage and underlying subchondral bone form the components of the mature osteochondral unit.
Damage to or dysregulation of the osteochondral unit is often traumatic in nature, resulting in cartilage and/or subchondral bone lesions that, if unable to heal, most frequently results in OA, possibly the most chronic, debilitating disease among adults worldwide.15 Osteochondral defects typically arise in adults as a result of acute trauma to the cartilage and underlying bone or in association with meniscal/ligament tears. In young, active children and adults, osteochondral defects may form as a result of osteochondritis dissecans (OCD), a painful condition characterized by bone sclerosis that can lead to cartilage fragmentation. OCD likely develops through improper development, repetitive trauma, inflammation, and/or a decrease in blood supply, rather than an acute osteochondral fracture.16
Whatever the cause, focal damage to the osteochondral unit initiates a cascade of repair and remodeling attempts that often have detrimental effects on the long-term health and function of the joint that can lead to OA.17
Crosstalk within the osteochondral unit
The onset of disease or degeneration of one component of the osteochondral unit can impact the functions of other components. Biomechanically, subchondral bone normally supports the articular cartilage in distributing joint forces over the joint. However, damage to the subchondral bone can alter its elastic modulus and thus its force distribution properties, which can lead to cartilage degeneration through abnormal loading on the tissue.18,19 Conversely, damage to the cartilage articular surface may occur before or concurrent with bone changes and remodeling.20 This biomechanical relationship is a well-established paradigm for OA progression; however, several studies have also evaluated the concept of a biochemical crosstalk between cartilage and bone tissues.
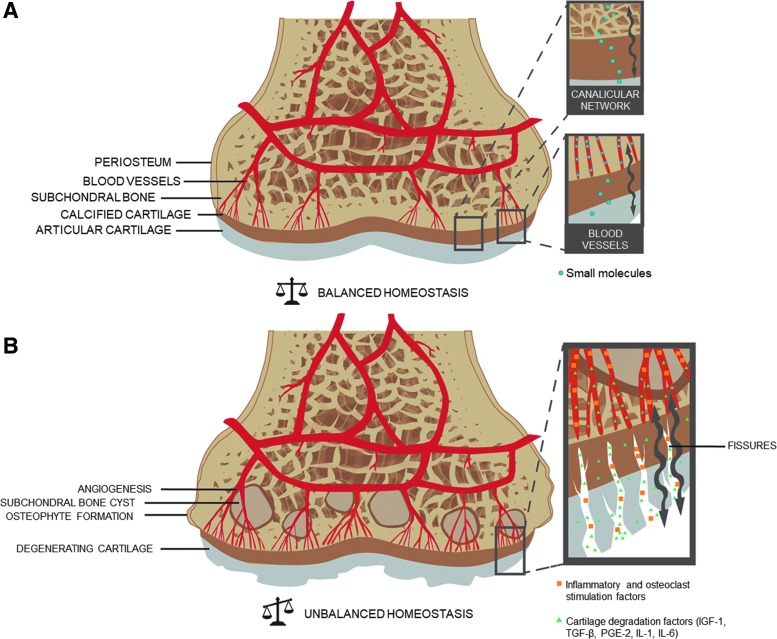
The proximity of the subchondral bone vasculature suggests that small molecules can perfuse into the cartilage in healthy osteochondral tissue. Key studies performed by Arkill and Winlove and Pan et al. demonstrated the transportation of fluorescent dyes from the subchondral circulation to deep zone cartilage.21,22 This diffusion has been shown to be elevated in OA.23 In addition, transport of larger molecules may occur through the osteocyte canalicular/lacunar network,24 which may be affected by the progression of OA with increasing subchondral bone porosity.25
OA progression can also introduce fissures, microcracks, and new blood vessels that penetrate the calcified cartilage and may increase exchange of signaling molecules between the cartilage and bone.26 It has been suggested that cytokines and prostaglandins involved in bone tissue remodeling can reach the overlying cartilage through these new channels, further resulting in its catabolism. Conversely, inflammatory and osteoclast stimulation factors released by the synovial membrane and/or the articular cartilage could affect the subchondral bone27 (Fig. 2). This crosstalk between bone and cartilage may be partially responsible for the etiology and progression of OA, and is an important consideration when exploring new treatment modalities to heal osteochondral defects.
FIG. 2.
Proposed mechanism of crosstalk within the osteochondral unit. Small molecules can diffuse through blood vessels and the canalicular network in healthy synovial joints (A). In osteoarthritic joints, in addition to the formation of osteophytes, subchondral bone cysts, and cartilage degeneration, angiogenesis and the formation of fissures may cause an increase in transport of inflammatory, osteoclast, and cartilage degradation factors (B).
Pain and the osteochondral unit
The relationship between cartilage and bone in the context of osteochondral unit degeneration is very important when assessing the cause of joint pain, the primary symptom of osteochondral lesions, and OA. As healthy hyaline cartilage does not contain nociceptors (pain receptors),3 joint pain originates from the underlying subchondral bone or other soft tissue components of the joint capsule, such as the synovium. Synovitis and its associated pain can occur concurrently with OA and subchondral bone lesions,28–30 but this is thought to be distinct from processes that regulate subchondral bone pain.31
In addition, subchondral bone lesions have been shown to be more highly correlated with pain than synovitis or joint effusion.32,33 Subchondral bone angiogenesis during early OA progression may facilitate not only the increased crosstalk leading to cartilage degradation, but also the innervation of the overlying cartilage.34 Walsh et al. examined the link between new microvasculature and nerve growth in osteochondral tissue and found increased vascular endothelial growth factor (VEGF) and nerve growth factor (NGF) in the subchondral bone space, breaching into the noncalcified cartilage.31 They postulated that the increase in VEGF (a proangiogenesis factor) contributed to the growth of new blood vessels in articular cartilage, which was accompanied by nociceptor growth through NGF (a neurotrophic factor).
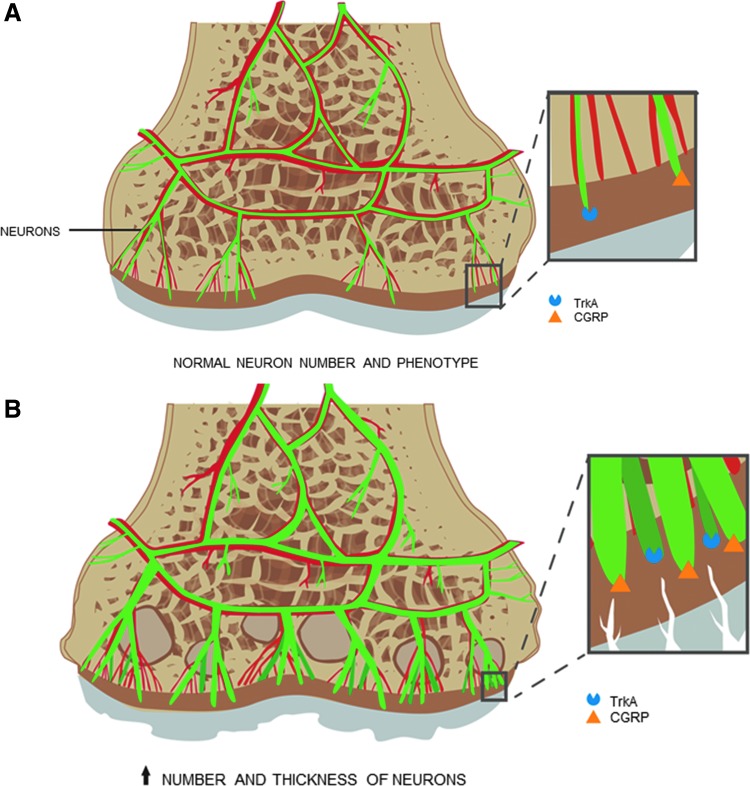
Nerve growth was further confirmed in a rat model, where upregulation of calcitonin gene-related peptide and tyrosine receptor kinase A (both nociceptive markers) concurrent with increased size of neurons was found in the subchondral bone of OA knee joints (Fig. 3).35 NGF and its effects are important mediators of pain,36 thus increases in NGF and nerve growth associated with angiogenesis in osteochondral tissue are likely causes of joint pain.
FIG. 3.
Innervation of the osteochondral unit. In synovial joints, sensory neurons innervate the healthy subchondral bone and show a normal expression of TrkA and CGRP (A), which are important effectors in the transmission of nociception. In osteoarthritic joints, neurons increase in number and size, and TrkA and CGRP are upregulated (B). TrkA, tropomyosin receptor kinase A; CGRP, calcitonin gene-related peptide.
It remains unclear at what point subchondral bone damage and/or focal lesions present as pain, making it difficult to diagnose and treat before further tissue degeneration and OA progression. However, the presence of asymptomatic bone marrow lesions can predict cartilage loss and subsequent OA symptoms.37 Cartilage loss leads to subchondral bone exposure within the osteochondral unit, which may also be associated with pain. Moisio et al. conducted cross-sectional analyses of patients with OA to determine the association of subchondral bone exposure (as evidenced by MRI) with knee pain, and found that moderate-to-severe knee pain was associated with the percentage of denuded bone, suggesting a relationship between the extent of subchondral bone exposure and increased pain.38
These results suggest that exposure of the bone can result in contact with the synovial fluid or rubbing with other structural components of the joint, leading to biochemical and/or mechanical stimulation of subchondral bone nociceptors, which may partly explain the pain associated with damage to the osteochondral unit. Lastly, subchondral bone sclerosis may contribute to increased vascular pressure in the marrow cavity and subchondral bone plate. This is exacerbated by inactivity, and may be a contributing factor for night pain in OA.39,40 All of this evidence points to the important role of the subchondral bone in the progression of joint pain and cartilage damage, making it a crucial therapeutic target for both pain relief and structural restoration of the osteochondral unit.
Evaluation of the osteochondral unit
Once patients are experiencing symptoms of joint pain and limited mobility, evaluation of the affected joint is necessary to determine the extent of damage (if any) to the osteochondral unit and its associated tissues. Generally, radiography with the patient weightbearing is the most commonly used initial imaging technique for the diagnosis of OA.41 OA severity is often graded using the Kellgren and Lawrence system, which scores the presence and severity of osteophytes, joint space narrowing, sclerosis, and other bone changes.42
However, radiography cannot detect early pathological bony changes and does not depict soft tissues such as cartilage. Radiographs therefore do not provide a complete evaluation of osteochondral lesions preceding or accompanying OA, and are not a sufficient imaging modality to determine the appropriate treatment of the damaged osteochondral unit.
The gold standard for noninvasive imaging of the joint is MRI. MRI can supply a whole organ assessment of both hard and soft tissues in the joint, and it eliminates patient exposure to radiation. Many studies have determined that high-field MRI (with a greater signal-to-noise ratio) provides a more sensitive and reliable image that can more easily detect small, early stage lesions in the chondral phase.43–46
To further quantify cartilage composition, advanced MRI techniques, such as T2 mapping can be used. T2 mapping is a relaxometry measurement that reflects the water content, collagen content, and fiber orientation in articular cartilage. More specifically, it can be used to detect early tissue degeneration, as increases in T2 relaxation times have been correlated with improper collagen stratification.47 While still mostly only utilized in a research setting, T2 mapping has shown promising results in predicting the development of macroscopic changes visible with conventional MRI. One prospective study found that T2 mapping significantly improved detection of cartilage lesions over routine MRI, indicating its potential for use in diagnosing and monitoring early cartilage degeneration.48
Arthroscopy has an advantage over other imaging techniques in that it allows for direct observation of the pathology of the affected joint. However, it does not allow assessment beyond the cartilage surface layer, except for determining tissue integrity. Therefore, this minimally invasive procedure is usually performed if the MRI results on soft tissue are inconclusive,49 as it can be difficult to evaluate early cartilage damage on MRI because of the tissue's thinness, even with advances such as T2 mapping. For the diagnosis of osteochondral lesions of the talus, MRI and arthroscopic evaluation results were well correlated,50,51 however, the diagnosis for large chondral defects should be confirmed with arthroscopy, as MRI alone can provide false-positive or false-negative findings.52,53
Arthroscopy can also serve as a one-time surgery for both diagnosis and treatment, and thus is indicated when noninvasive imaging techniques suggest that surgical intervention may be necessary to debride or repair cartilage. It is important to consider that there may be incomplete or no correlation between clinical symptoms, such as pain and diagnostic imaging,2 which complicates the therapeutic approach taken when aiming to alleviate symptoms and/or repair an asymptomatic lesion. An invasive treatment may actually worsen pain.54
Surgical treatment of osteochondral unit damage/disease
At present, there are no pharmaceutical drugs to treat chondral, subchondral, or osteochondral lesions. In the case of focal osteochondral injuries or severe joint disease, surgical intervention is the only treatment option for structural restoration and possible reduction of pain. Surgical restoration is an extremely complex process, largely due to the unique structure, mechanical strength, and crosstalk within the osteochondral unit and also with adjacent tissues.
Currently, microfracture, ACI, or variations of ACI, such as matrix-associated or gel-associated chondrocyte implantation are used to treat focal chondral lesions. In humans, microfracture has shown success in improving pain and function outcomes in the majority of patients treated, although the majority of the repair tissue was fibrocartilaginous.55 However, upon short- (1 year) and long-term (4–6 years) follow-up, patients treated with microfracture for talar osteochondral lesions showed deteriorating subchondral bone health, with the development of cysts and subchondral bone plate thickening long term.56,57
Even though the technique of ACI does not involve direct intervention into the bone, it has also been associated with complications in the subchondral bone. Subchondral bone plate advancement, intralesional osteophytes, and subchondral bone cysts were found in 30–60% of human patients treated with ACI after mid- to long-term follow-up.58–60 The upward migration of the subchondral bone due to the renewal of endochondral ossification at the tidemark has been shown to be associated with the degradation of the repaired articular surface.61
Particulate, chip, or minced tissue has been explored for cartilage resurfacing and repair of the osteochondral unit in a number of studies. For example, BioCartilage™ is a relatively new procedure, whereby minced cadaveric allogeneic cartilage (sourced from adult donors) is implanted into a microfracture site along with platelet-rich plasma for its perceived anti-inflammatory and anticatabolic effects.62 Early results in horses suggest that BioCartilage-treated full-thickness defects have greater collagen type II content and better repair/host integration than traditional microfracture, although both groups had subchondral bone voids at the defect site.63
A similar technique, DeNovo® NT, involves implanting particulate cartilage from juvenile donors (typically younger than 2 years) delivered in fibrin glue. This method relies on the observation that juvenile chondrocytes induce matrix formation in adult cartilage.64 Although subjectively assessed, this allogeneic technique has shown good clinical outcomes in the treatment of osteochondral lesions in the knee65 and ankle66 in small retrospective studies. However, MRI and histological results demonstrated an uneven cartilage surface, subchondral bone edema, and heterogeneous repair tissue despite improved patient scores. A recent study comparing DeNovo NT and microfracture in talar osteochondral defects found no differences in patient-reported outcomes or repair tissue quality.67
A new autologous strategy has shown some success as well. CAIS involves isolating cartilage tissue from a healthy nonweight-bearing area, mincing the cartilage, then implanting it into the defect site in a one-step procedure. CAIS was compared with ACI in horses to treat large defects (15 mm in diameter). Both techniques resulted in superior tissue regeneration compared with empty defects, with CAIS having the higher score.68 In humans, CAIS outperformed microfracture in a randomized controlled trial in knee function, reduced pain and stiffness, sports and recreational activities, and knee-related quality of life.69
In addition to morselizing autologous or allogeneic cartilage, a recent report has described the efficacy of implanting both fragmented bone and cartilage into osteochondral defects. Christensen et al.70 utilized autologous bone fragments press-fitted into defect beds with cartilage chips embedded in fibrin glue seeded on top. After 12 months, all eight patients had improved Magnetic Resonance Observation of Cartilage Repair scores in defect fill and cartilage tissue surface, as well as good patient-reported outcomes, although subchondral bone-specific scores (edema, bone interface) were not significantly improved.70
The authors further elucidated the role of the cartilage chips in the quality of the repair tissue. In a minipig model, defects treated with autologous bone fragments and cartilage chips had significantly more hyaline and fibrocartilage than defects treated with bone fragments alone, although no difference was noted in the bone defect volume between the groups at 6 or 12 months.71
OAT and mosaic arthroplasty have a distinct advantage over the aforementioned techniques in that they aim to repair both the cartilage and the bone phase with healthy, intact osteochondral grafts. Both techniques involve harvesting one (OAT) or more (mosaic arthroplasty/mosaicplasty) osteochondral plugs from a healthy, nonweight-bearing area of the joint and implanting it into the lesion. OAT is often indicated when a medium (∼2–3 cm2) full-thickness defect or multiple lesions are diagnosed through MRI or arthroscopy.72,73
This technique was pioneered in horses by Bodo et al. to treat subchondral bone cysts, and has shown promise in the treatment of focal cartilage defects in dogs, pigs, and sheep.73–77 In a recent systematic review, 72% of human patients who underwent OAT in the knee joint (10 studies, 610 patients) had successful clinical outcomes after an average of 10 years (based on Lysholm/International Knee Documentation Committee [IKDC] scoring), although activity scores did not improve significantly from preoperative to final follow-up.78 While the average return-to-sport rate was reported to be 85% in the studies that assessed this, highly active patients reported lowering their activity level, while sedentary patients did not change their activity level.78,79 Mean failure rate among the studies reviewed was 28%. Failure rates are influenced by age, lesion size, and/or localization, and concomitant surgeries,80 which also act as compounding variables when evaluating OAT as an effective treatment protocol for OCLs.
To date, seven randomized studies (evidence level: I or II) have compared OAT with microfracture in pediatric and adult patients.81–87 Out of these studies, five demonstrated that OAT produced better clinical outcomes and a higher return-to-sport rate than microfracture at mid- (3–5 years) to long-term (≥10 years) follow-up.81–84,87 Two studies did not find significant differences in clinical outcomes between OAT and microfracture at mid- (5 years) and long-term (10 years) follow-up, however, they were both level II randomized studies with a small number of patients in each group.85,86
There have been even fewer studies published on OAT versus ACI. To the best of our knowledge, only four randomized trials have been conducted to date, with mixed outcomes.85,88–90 After 1 year, Bentley et al. found that ACI-treated patients had better clinical results than mosaicplasty-treated patients,88 while Horas et al. determined that OAT was superior to ACI, delivering higher Lysholm scores after a 2-year follow-up.89 Dozin et al. could not conclude any significant differences between mosaicplasty and ACI, although they reported a higher Lysholm score for mosaicplasty patients after 2 years (88% vs. 68% for ACI patients).90 Lim et al. compared OAT versus ACI versus microfracture in 70 knees, and did not observe any significant differences in clinical outcomes between the three treatments after 5 years.85 To date, there have been no published Level I clinical trials comparing the long-term outcomes of OAT versus ACI.
Major concerns with OAT with respect to donor-site morbidity and critical lesion size can be addressed by using allografts harvested by cadaver limbs. Osteochondral allografts have increased in popularity as long-term storage protocols have been optimized and immune rejection rates remain low.91,92 A single-center, randomized, prospective trial comparing the efficiency of autografts versus allografts to heal large and/or recurrent osteochondral lesions of the talus found similar healing rates.93 However, as for all human donor-sourced material, adequate supply of allografts is a concern for widespread adaptation and rollout.
Osteochondral unit tissue engineering
Sourcing appropriate healthy allograft tissue remains a key limitation to osteochondral allograft transfer.94 Fortunately, much progress has been made in the realm of tissue engineering of osteochondral constructs. The majority of these constructs contain cells and one or more types of scaffolds for the cartilage and/or bone phase. Various studies have investigated methods for generating biphasic, multiphasic, and/or gradient tissues (reviewed in refs.95–99), different biomaterials/scaffolds (reviewed in refs.100,101), as well as the controlled release of growth factors for in vivo tissue regeneration (reviewed in refs.102,103). While these in vitro studies show promise, relatively few in vivo studies have been conducted to show how engineered osteochondral constructs function long-term in the joint.104–109
Questions remain concerning methods to reproducibly generate stable, mechanically competent, metabolically equivalent, and functionally relevant osteochondral units. Generally speaking, most publications report that the compressive and/or shear modulus of tissue-engineered cartilage is inferior to that of native cartilage. While biomechanical competency of cartilage is important in the creation of implant-grade tissue, the integration to and vascularization of the new subchondral bone is imperative in recapitulating the health and function of the native osteochondral unit. By focusing on building the unit as a whole, we can eventually overcome some of the limitations observed by attempting to regenerate only the chondral phase.
Animal models, human trials, and the case for health technology value in osteochondral unit repair
Research into pathologies and treatment of human cartilage disease conditions often utilizes animal models as a transition phase between in vitro studies and human clinical practice. Small animal models such as mice and rats are most commonly used as they are inexpensive and can be generated as transgenic or gene knockout animals, which allow for the exploration of direct or indirect genetic causes of cartilage-associated pathologies.110 However, while they are suitable models for hypothesis-generating studies of OA pathology and development, their small joint size and relatively thin articular cartilage means it is difficult to study clinically relevant-sized defects. Larger animals, such as rabbits, goats, pigs, sheep, dogs, and horses have also been studied extensively as models for cartilage repair (reviewed in Chu et al.111).
When choosing a large animal model for the treatment of osteochondral defects, several factors must be taken into consideration. Joint anatomy, cartilage thickness, subchondral bone properties, and biomechanical loading environment should be as close as possible to humans. Goat and sheep stifles are very similar in anatomy to the human knee, however some differences (mainly the femoral intercondylar notch width as well as trochlear groove length) limit very precise modeling of the human joint.112 In terms of cartilage thickness, horses are most similar to humans with a thickness of 1.75–2 mm113; human cartilage thickness ranges from 2.4 to 2.6 mm.114 Unlike normal human subchondral bone, horses and goats have higher bone mineral density, bone volume fraction, and thicker trabeculae.115
However, as these are features of OA,116 they may in fact pose an advantage to developing osteochondral defect treatments, where the subchondral bone environment is altered. Goats have similar joint biomechanics to humans, whereas horse joints are subjected to increased loads as a result of the animal's size and activity level,117 making defect repair particularly challenging yet perhaps excellent for modeling repair.
In addition to their similar joint characteristics to humans, horses have been deemed the superior preclinical animal model due to analog developmental disorders of the osteochondral unit and their propensity to acquire joint injuries through athletic activities.118,119 In addition, arthroscopic techniques to treat and evaluate lesions in the horse are similar to human surgical instrumentation, aiding in technology transfer between horses and humans.120 Horses are also amenable to controlled rehabilitation protocols, an integral postoperative part of many surgical treatment approaches.
Veterinary patients also suffer from natural spontaneous disease, which has significant value in preclinical modeling for assessing the true clinical importance of new therapies. Assessing new cartilage and osteochondral defect treatments in these patients is beneficial because they can be compared with current best clinical practices. In addition, disease conditions are associated with all of the complexities of human patients, such as chronicity, comorbidities, polypharmaceutical use, epigenetic factors, genetic heterogeneity, and postsurgical compliance issues, as reviewed elsewhere.121
Recently, it has been highlighted that human research studies and clinical trials are often conducted in younger people, whereas the majority of patients for these procedures are older.122 Only 4% of patients in a clinical practice fulfill inclusion criteria of clinical trials on focal cartilage repair.123 This likely reflects bias toward early commercial or research successes, but is ultimately failing development of effective treatments.122
Of note, some now argue that cartilage repair should be exempt from the rigor of randomized clinical trials (RCTs).124 Complete replacement of all animal studies with microdosing strategies in humans has also been advocated recently.125 However, this argument seems more relevant to traditional drug testing than cell and tissue-engineering strategies.
Instead of abandoning the rigor of RCTs and arguing for regulatory lenience in approving new cartilage repair methods, better strategies to determine safety and efficacy of new treatments in a cost-effective manner should be explored. In addition to enhancing the welfare of our veterinary patients, such an approach may also allow for separate veterinary revenue streams that could be used to support clinical trials in humans.
Conclusion
When pathologies arise in one component of the osteochondral unit, reducing its function, it is often only a matter of time before the other components are affected and OA occurs. Early detection and diagnosis of osteochondral lesions is important for the appropriate treatment of the disease. Unfortunately, current nonsurgical treatments are only capable of temporarily relieving symptoms and perhaps slowing down joint disease progression, whereas surgical treatments come with a plethora of complex difficulties, limitations, risks, and expenses. By using treatment techniques that focus solely on repairing the cartilage surface and do not address existing or future changes to the subchondral bone, pain can persist and cartilage damage may reoccur. The possible result is a joint that looks normal when observed arthroscopically, yet the patient still complains of joint pain.32
Further development of diagnostic tools, disease-modifying drugs, and surgical alternatives is necessary for a more effective, targeted therapeutic approach that can reverse disease progression and restore joint function long term, without the need for life-long treatment or revision surgeries. Basic research into the complexities of cartilage/bone crosstalk and the in vivo development of the osteochondral unit is fundamental to the improvement of joint therapies and tissue restoration. From a clinical and translational perspective, naturally occurring disease in companion animals, such as horses and dogs, provides unique opportunities for developing and assessing the long-term safety and efficacy of new osteochondral treatments to the benefit of animals themselves as well as humans. Rarely is osteochondral pathology a fatal condition and new therapies should therefore be thoroughly evaluated for safety and efficacy before implementation in human medicine.
Acknowledgments
Grant support for T.G.K.: Discovery Grant from Natural Sciences and Engineering Research Council of Canada, grant number: RGPIN-2014-04587. Operating grant from Equine Guelph Research Foundation, grant number: EG-2014-13. Infrastructure Operating Fund from the Canadian Foundation for Innovation (Project number 29995). Partial salary support for S.I.M.L. Early Researcher Award from the Ontario Ministry of Research and Innovation (File number ER15-11-032). Partial salary support for S.I.M.L.
Disclosure Statement
No competing financial interests exist.
References
- 1. Prakash D., and Learmonth D. Natural progression of osteo-chondral defect in the femoral condyle. Knee 9, 7, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Guermazi A., Niu J., Hayashi D., et al. . Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ 345, e5339, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grässel S.G. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res Ther 16, 485, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonadio M.B., Filho A.G.O., Helito C.P., Stump X.M., and Demange M.K. Bone marrow lesion: image, clinical presentation, and treatment. Magn Reson Insights 10, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kazakia G.J., Kuo D., Schooler J., et al. . Bone and cartilage demonstrate changes localized to bone marrow edema-like lesions within osteoarthritic knees. Osteoarthritis Cartilage 21, 94, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y., Nevitt M., Niu J., et al. . Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum 63, 691, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Román-Blas J.A., Castañeda S., Largo R., and Herrero-Beaumont G. Subchondral bone remodelling and osteoarthritis. Arthritis Res Ther 14(Suppl 2), A6, 2012 [Google Scholar]
- 8. Long F., and Linsenmayer T.F. Tissue-specific regulation of the type X collagen gene. Analyses by in vivo footprinting and transfection with a proximal promoter region. J Biol Chem 270, 31310, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Shen G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod Craniofac Res 8, 11, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Chen H., Ghori-Javed F.Y., Rashid H., et al. . Runx2 regulates endochondral ossification through control of chondrocyte proliferation and differentiation. J Bone Miner Res 29, 2653, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clarkin C., and Olsen B.R. On bone-forming cells and blood vessels in bone development. Cell Metab 12, 314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pacifici M., Koyama E., Shibukawa Y., et al. . Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann N Y Acad Sci 1068, 74, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamb K.J., Lewthwaite J.C., Bastow E.R., and Pitsillides A.A. Defining boundaries during joint cavity formation: going out on a limb. Int J Exp Pathol 84, 55, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ito M.M., and Kida M.Y. Morphological and biochemical re-evaluation of the process of cavitation in the rat knee joint: cellular and cell strata alterations in the interzone. J Anat 197(Pt 4), 659, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson V.L., and Hunter D.J. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol 28, 5, 2014 [DOI] [PubMed] [Google Scholar]
- 16. Edmonds E.W., and Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clin Orthop Relat Res 471, 1118, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stiebel M., Miller L.E., and Block J.E. Post-traumatic knee osteoarthritis in the young patient: therapeutic dilemmas and emerging technologies. Open Access J Sports Med 5, 73, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma J.X., He W.W., Zhao J., et al. . Bone microarchitecture and biomechanics of the necrotic femoral head. Sci Rep 7, 13345, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lajeunesse D. The role of bone in the treatment of osteoarthritis. Osteoarthritis Cartilage 12, S34, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Radin E.L., Martin R.B., Burr D.B., Caterson B., Boyd R.D., and Goodwin C. Effects of mechanical loading on the tissues of the rabbit knee. J Orthop Res 2, 221, 1984 [DOI] [PubMed] [Google Scholar]
- 21. Pan J., Zhou X., Li W., Novotny J.E., Doty S.B., and Wang L. In situ measurement of transport between subchondral bone and articular cartilage. J Orthop Res 27, 1347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arkill K.P., and Winlove C.P. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthritis Cartilage 16, 708, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Pan J., Wang B., Li W., et al. . Elevated cross-talk between subchondral bone and cartilage in osteoarthritic joints. Bone 51, 212, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang B., Zhou X., Price C., Li W., Pan J., and Wang L. Quantifying load-induced solute transport and solute-matrix interaction within the osteocyte lacunar-canalicular system. J Bone Miner Res 28, 1075, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calvo E., Castañeda S., Largo R., Fernández-Valle M.E., Rodríguez-Salvanés F., and Herrero-Beaumont G. Osteoporosis increases the severity of cartilage damage in an experimental model of osteoarthritis in rabbits. Osteoarthritis Cartilage 15, 69, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Yuan X.L., Meng H.Y., Wang Y.C., et al. . Bone–cartilage interface cross talk in osteoarthritis: potential pathways and future therapeutic strategies. Osteoarthritis Cartilage 22, 1077, 2014 [DOI] [PubMed] [Google Scholar]
- 27. Lajeunesse D., and Reboul P. Subchondral bone in osteoarthritis: a biologic link with articular cartilage leading to abnormal remodeling. Curr Opin Rheumatol 15, 628, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Wenham C.Y.J., and Conaghan P.G. The role of synovitis in osteoarthritis. Ther Adv Musculoskelet Dis 2, 349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yusup A., Kaneko H., Liu L., et al. . Bone marrow lesions, subchondral bone cysts and subchondral bone attrition are associated with histological synovitis in patients with end-stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 23, 1858, 2015 [DOI] [PubMed] [Google Scholar]
- 30. Wang X., Blizzard L., Jin X., et al. . Quantitative assessment of knee effusion-synovitis in older adults: association with knee structural abnormalities. Arthritis Rheumatol 68, 837, 2016 [DOI] [PubMed] [Google Scholar]
- 31. Walsh D.A., Mcwilliams D.F., Turley M.J., et al. . Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology 49, 1852, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hunter D.J., Guermazi A., Roemer F., Zhang Y., and Neogi T. Structural correlates of pain in joints with osteoarthritis. Osteoarthritis Cartilage 21, 1170, 2013 [DOI] [PubMed] [Google Scholar]
- 33. Sayre E.C., Guermazi A., Esdaile J.M., et al. . Associations between MRI features versus knee pain severity and progression: data from the Vancouver longitudinal study of early knee osteoarthritis. PLoS One 12, e0176833, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suri S., Gill S.E., Massena de Camin S., Wilson D., McWilliams D.F., and Walsh D.A. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis 66, 1423, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aso K., Izumi M., Sugimura N., Okanoue Y., Ushida T., and Ikeuchi M. Nociceptive phenotype alterations of dorsal root ganglia neurons innervating the subchondral bone in osteoarthritic rat knee joints. Osteoarthritis Cartilage 24, 1596, 2016 [DOI] [PubMed] [Google Scholar]
- 36. Mizumura K., and Murase S. Role of nerve growth factor in pain. Handb Exp Pharmacol 227, 57, 2015 [DOI] [PubMed] [Google Scholar]
- 37. Wluka A.E., Hanna F., Davies-Tuck M., et al. . Bone marrow lesions predict increase in knee cartilage defects and loss of cartilage volume in middle-aged women without knee pain over 2 years. Ann Rheum Dis 68, 850, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Moisio K., Eckstein F., Chmiel J.S., et al. . Denuded subchondral bone and knee pain in persons with knee osteoarthritis. Arthritis Rheum 60, 3703, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Findlay D.M. Vascular pathology and osteoarthritis. Rheumatology 46, 1763, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Nosova E.V., Yen P., Chong K.C., et al. . Short-term physical inactivity impairs vascular function. J Surg Res 190, 672, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hayashi D., Roemer F.W., and Guermazi A. Imaging for osteoarthritis. Ann Phys Rehabil Med 59, 161, 2016 [DOI] [PubMed] [Google Scholar]
- 42. Kellgren J.H., and Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16, 494, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Disler D.G., McCauley T.R., Wirth C.R., and Fuchs M.D. Detection of knee hyaline cartilage defects using fat-suppressed three-dimensional spoiled gradient-echo MR imaging: comparison with standard MR imaging and correlation with arthroscopy. AJR Am J Roentgenol 165, 377, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Cashman G., and Attariwala R. Influence of MRI field strength on clinical decision making in knee cartilage injury—a case study. J Can Chiropr Assoc 58, 395, 2014 [PMC free article] [PubMed] [Google Scholar]
- 45. Galea A., Giuffre B., Dimmick S., Coolican M.R.J., and Parker D.A. The accuracy of magnetic resonance imaging scanning and its influence on management decisions in knee surgery. Arthroscopy 25, 473, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Link T.M., Sell C.A., Masi J.N., et al. . 3.0 vs 1.5T MRI in the detection of focal cartilage pathology–ROC analysis in an experimental model. Osteoarthritis Cartilage 14, 63, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Mosher T.J., and Dardzinski B.J. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 8, 355, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Kijowski R., Blankenbaker D.G., Munoz Del Rio A., Baer G.S., and Graf B.K. Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology 267, 503, 2013 [DOI] [PubMed] [Google Scholar]
- 49. Voigt J.D., Mosier M., and Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy 12, 523, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee K.-B., Bai L.-B., Park J.-G., and Yoon T.-R. A comparison of arthroscopic and MRI findings in staging of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc 16, 1047, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Verhagen R.A.W., Maas M., Dijkgraaf M.G.W., Tol J.L., Krips R., and van Dijk C.N. Prospective study on diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br 87, 41, 2005 [PubMed] [Google Scholar]
- 52. Nikolaou V.S., Chronopoulos E., Savvidou C., et al. . MRI efficacy in diagnosing internal lesions of the knee: a retrospective analysis. J Trauma Manag Outcomes 2, 4, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Quatman C.E., Hettrich C.M., Schmitt L.C., and Spindler K.P. The clinical utility and diagnostic performance of magnetic resonance imaging for identification of early and advanced knee osteoarthritis: a systematic review. Am J Sports Med 39, 1557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mar C.D. Guideline: an expert panel strongly recommends against arthroscopic knee surgery for degenerative knee disease. Ann Intern Med 167, JC38, 2017 [DOI] [PubMed] [Google Scholar]
- 55. Steadman J.R., Rodkey W.G., Singleton S.B., and Briggs K.K. Microfracture technique forfull-thickness chondral defects: technique and clinical results. Oper Tech Orthop 7, 300, 1997 [Google Scholar]
- 56. Reilingh M.L., van Bergen C.J.A., Blankevoort L., et al. . Computed tomography analysis of osteochondral defects of the talus after arthroscopic debridement and microfracture. Knee Surg Sport Traumatol Arthrosc 24, 1286, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shimozono Y., Coale M., Yasui Y., O'Halloran A., Deyer T.W., and Kennedy J.G. Subchondral bone degradation after microfracture for osteochondral lesions of the talus an MRI analysis. Am J Sports Med 46, 642, 2018 [DOI] [PubMed] [Google Scholar]
- 58. Minas T., Gomoll A.H., Rosenberger R., Royce R.O., and Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med 37, 902, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Henderson I.J.P., and La Valette D.P. Subchondral bone overgrowth in the presence of full-thickness cartilage defects in the knee. Knee 12, 435, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Vasiliadis H.S., Danielson B., Ljungberg M., McKeon B., Lindahl A., and Peterson L. Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med 38, 943, 2010 [DOI] [PubMed] [Google Scholar]
- 61. Qiu Y.S., Shahgaldi B.F., Revell W.J., and Heatley F.W. Observations of subchondral plate advancement during osteochondral repair: a histomorphometric and mechanical study in the rabbit femoral condyle. Osteoarthritis Cartilage 11, 810, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Abrams G.D., Mall N.A., Fortier L.A., Roller B.L., and Cole B.J. BioCartilage: background and operative technique. Oper Tech Sports Med 21, 116, 2013 [Google Scholar]
- 63. Fortier L.A., Chapman H.S., Pownder S.L., et al. . Biocartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am J Sports Med 44, 2366, 2016 [DOI] [PubMed] [Google Scholar]
- 64. Bonasia D.E., Martin J.A., Marmotti A., et al. . Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments: in vitro matrix production. Am J Sports Med 39, 2355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Farr J., Tabet S.K., Margerrison E., and Cole B.J. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med 42, 1417, 2014 [DOI] [PubMed] [Google Scholar]
- 66. Saltzman B.M., Lin J., and Lee S. Particulated juvenile articular cartilage allograft transplantation for osteochondral talar lesions. Cartilage 8, 61, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Karnovsky S.C., DeSandis B., Haleem A.M., Sofka C.M., O'Malley M., and Drakos M.C. Comparison of juvenile allogenous articular cartilage and bone marrow aspirate concentrate versus microfracture with and without bone marrow aspirate concentrate in arthroscopic treatment of talar osteochondral lesions. Foot Ankle Int 39, 393, 2018 [DOI] [PubMed] [Google Scholar]
- 68. Frisbie D.D., Lu Y., Kawcak C.E., DiCarlo E.F., Binette F., and McIlwraith C.W. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med 37(1_Suppl), 71S, 2009 [DOI] [PubMed] [Google Scholar]
- 69. Cole B.J., Farr J., Winalski C.S., et al. . Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med 39, 1170, 2011 [DOI] [PubMed] [Google Scholar]
- 70. Christensen B.B., Foldager C.B., Jensen J., and Lind M. Autologous dual-tissue transplantation for osteochondral repair: early clinical and radiological results. Cartilage 6, 166, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Christensen B.B., Foldager C.B., Olesen M.L., Hede K.C., and Lind M. Implantation of autologous cartilage chips improves cartilage repair tissue quality in osteochondral defects: a study in Göttingen minipigs. Am J Sports Med 44, 1597, 2016 [DOI] [PubMed] [Google Scholar]
- 72. Hangody L., Vásárhelyi G., Hangody L.R., et al. . Autologous osteochondral grafting-technique and long-term results. Injury 39(Suppl. 1), S32, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Bodo G., Hangody L., Modis L., and Hurtig M. Autologous osteochondral grafting (mosaic arthroplasty) for treatment of subchondral cystic lesions in the equine stifle and fetlock joints. Vet Surg 33, 588, 2004 [DOI] [PubMed] [Google Scholar]
- 74. McCarty E.C., Fader R.R., Mitchell J.J., Glenn R.E., Potter H.G., and Spindler K.P. Fresh osteochondral allograft versus autograft: twelve-month results in isolated canine knee defects. Am J Sports Med 44, 2354, 2016 [DOI] [PubMed] [Google Scholar]
- 75. Fitzpatrick N., Van Terheijden C., Yeadon R., and Smith T.J. Osteochondral autograft transfer for treatment of osteochondritis dissecans of the caudocentral humeral head in dogs. Vet Surg 39, 925, 2010 [DOI] [PubMed] [Google Scholar]
- 76. Burks R.T., Greis P.E., Arnoczky S.P., and Scher C. The use of a single osteochondral autograft plug in the treatment of a large osteochondral lesion in the femoral condyle: an experimental study in sheep. Am J Sports Med 34, 247, 2006 [DOI] [PubMed] [Google Scholar]
- 77. Harman B.D., Weeden S.H., Lichota D.K., and Brindley G.W. Osteochondral autograft transplantation in the porcine knee. Am J Sports Med 34, 913, 2006 [DOI] [PubMed] [Google Scholar]
- 78. Pareek A., Reardon P.J., Maak T.G., Levy B.A., Stuart M.J., and Krych A.J. Long-term outcomes after osteochondral autograft transfer: a systematic review at mean follow-up of 10.2 years. Arthroscopy 32, 1174, 2016 [DOI] [PubMed] [Google Scholar]
- 79. Cognault J., Seurat O., Chaussard C., Ionescu S., and Saragaglia D. Return to sports after autogenous osteochondral mosaicplasty of the femoral condyles: 25 cases at a mean follow-up of 9 years. Orthop Traumatol Surg Res 101, 313, 2015 [DOI] [PubMed] [Google Scholar]
- 80. Emre T.Y., Ege T., Kose O., Tekdos Demırcıoglu D., Seyhan B., and Uzun M. Factors affecting the outcome of osteochondral autografting (mosaicplasty) in articular cartilage defects of the knee joint: retrospective analysis of 152 cases. Arch Orthop Trauma Surg 133, 531, 2013 [DOI] [PubMed] [Google Scholar]
- 81. Gudas R., Gudaitė A., Pocius A., et al. . Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med 40, 2499, 2012 [DOI] [PubMed] [Google Scholar]
- 82. Gudas R., Gudaitė A., Mickevičius T., et al. . Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: a prospective study with a 3-year follow-up. Arthroscopy 29, 89, 2013 [DOI] [PubMed] [Google Scholar]
- 83. Gudas R., Kalesinskas R.J., Kimtys V., et al. . A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy 21, 1066, 2005 [DOI] [PubMed] [Google Scholar]
- 84. Gudas R., Simonaityte R., Cekanauskas E., and Tamosiūnas R. A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children. J Pediatr Orthop 29, 741, 2009 [DOI] [PubMed] [Google Scholar]
- 85. Lim H.C., Bae J.H., Song S.H., Park Y.E., and Kim S.J. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res 470, 2261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ulstein S., Årøen A., Røtterud J.H., Løken S., Engebretsen L., and Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc 22, 1207, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Solheim E., Hegna J., Strand T., Harlem T., and Inderhaug E. Randomized study of long-term (15–17 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am J Sports Med 46, 826, 2018 [DOI] [PubMed] [Google Scholar]
- 88. Bentley G., Biant L.C., Carrington R.W.J., et al A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee patients and methods. J Bone Jt Surg Br 85, 223, 2003 [DOI] [PubMed] [Google Scholar]
- 89. Horas U., Pelinkovic D., Herr G., Aigner T., and Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am 85–A, 185, 2003 [DOI] [PubMed] [Google Scholar]
- 90. Dozin B., Malpeli M., Cancedda R., et al. . Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med 15, 220, 2005 [DOI] [PubMed] [Google Scholar]
- 91. Torrie A.M., Kesler W.W., Elkin J., and Gallo R.A. Osteochondral allograft. Curr Rev Musculoskelet Med 8, 413, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Demange M., and Gomoll A.H. The use of osteochondral allografts in the management of cartilage defects. Curr Rev Musculoskelet Med 5, 229, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ahmad J., and Jones K. Comparison of osteochondral autografts and allografts for treatment of recurrent or large talar osteochondral lesions. Foot Ankle Int 37, 40, 2016 [DOI] [PubMed] [Google Scholar]
- 94. De Caro F., Bisicchia S., Amendola A., and Ding L. Large fresh osteochondral allografts of the knee: a systematic clinical and basic science review of the literature. Arthroscopy 31, 757, 2015 [DOI] [PubMed] [Google Scholar]
- 95. Khorshidi S., and Karkhaneh A. A review on gradient hydrogel/fiber scaffolds for osteochondral regeneration. J Tissue Eng Regen Med 12, e1974, 2018 [DOI] [PubMed] [Google Scholar]
- 96. Daly A.C., Freeman F.E., Gonzalez-Fernandez T., Critchley S.E., Nulty J., and Kelly D.J. 3D bioprinting for cartilage and osteochondral tissue engineering. Adv Healthcare Mater 6, 2017. [Epub ahead of print]; DOI: 10.1002/adhm.201700298: 1700298 [DOI] [PubMed] [Google Scholar]
- 97. Gadjanski I., and Vunjak-Novakovic G. Challenges in engineering osteochondral tissue grafts with hierarchical structures. Expert Opin Biol Ther 15, 1583, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yousefi A.M., Hoque M.E., Prasad R.G.S.V., and Uth N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: a review. J Biomed Mater Res A 103, 2460, 2015 [DOI] [PubMed] [Google Scholar]
- 99. Shimomura K., Moriguchi Y., Murawski C.D., Yoshikawa H., and Nakamura N. Osteochondral tissue engineering with biphasic scaffold: current strategies and techniques. Tissue Eng Part B Rev 20, 468, 2014 [DOI] [PubMed] [Google Scholar]
- 100. Camarero-Espinosa S., and Cooper-White J. Tailoring biomaterial scaffolds for osteochondral repair. Int J Pharm 523, 476, 2017 [DOI] [PubMed] [Google Scholar]
- 101. Yang J., Zhang Y.S., Yue K., and Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater 57, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Santo V.E., Gomes M.E., Mano J.F., and Reis R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering-part I: recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng Part B Rev 19, 308, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Santo V.E., Gomes M.E., Mano J.F., and Reis R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering–Part II: challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng Part B Rev 19, 327, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Guo X., Wang C., Duan C., et al. . Repair of osteochondral defects with autologous chondrocytes seeded onto bioceramic scaffold in sheep. Tissue Eng 10, 1830, 2004 [DOI] [PubMed] [Google Scholar]
- 105. Kandel R.A., Grynpas M., Pilliar R., et al. . Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a sheep model. Biomaterials 27, 4120, 2006 [DOI] [PubMed] [Google Scholar]
- 106. Marquass B., Somerson J.S., Hepp P., et al. . A novel MSC-seeded triphasic construct for the repair of osteochondral defects. J Orthop Res 28, 1586, 2010 [DOI] [PubMed] [Google Scholar]
- 107. Shimomura K., Moriguchi Y., Nansai R., et al. . Comparison of 2 different formulations of artificial bone for a hybrid implant with a tissue-engineered construct derived from synovial mesenchymal stem cells: a study using a rabbit osteochondral defect model. Am J Sports Med 45, 666, 2017 [DOI] [PubMed] [Google Scholar]
- 108. Shimomura K., Moriguchi Y., Ando W., et al. . Osteochondral repair using a scaffold-free tissue-engineered construct derived from synovial mesenchymal stem cells and a hydroxyapatite-based artificial bone. Tissue Eng Part A 20, 2291, 2014 [DOI] [PubMed] [Google Scholar]
- 109. Shao X., Goh J.C.H., Hutmacher D.W., Lee E.H., and Zigang G. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng 12, 1539, 2006 [DOI] [PubMed] [Google Scholar]
- 110. Helminen H.J., Säämänen A.M., Salminen H., and Hyttinen M.M. Transgenic mouse models for studying the role of cartilage macromolecules in osteoarthritis. Rheumatology 41, 848, 2002 [DOI] [PubMed] [Google Scholar]
- 111. Chu C.R., Szczodry M., and Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev 16, 105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Proffen B.L., McElfresh M., Fleming B.C., and Murray M.M. A comparative anatomical study of the human knee and six animal species. Knee 19, 493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Frisbie D.D., Cross M.W., and McIlwraith C.W. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol 19, 142, 2006 [PubMed] [Google Scholar]
- 114. Shepherd D.E., and Seedhom B.B. Thickness of human articular cartilage in joints of the lower limb. Ann Rheum Dis 58, 27, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Changoor A., Nelea M., Méthot S., et al. . Structural characteristics of the collagen network in human normal, degraded and repair articular cartilages observed in polarized light and scanning electron microscopies. Osteoarthritis Cartilage 19, 1458, 2011 [DOI] [PubMed] [Google Scholar]
- 116. Neogi T. Clinical significance of bone changes in osteoarthritis. Ther Adv Musculoskelet Dis 4, 259, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ahern B.J., Parvizi J., Boston R., and Schaer T.P. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage 17, 705, 2009 [DOI] [PubMed] [Google Scholar]
- 118. Lacourt M., Gao C., Li A., et al. . Relationship between cartilage and subchondral bone lesions in repetitive impact trauma-induced equine osteoarthritis. Osteoarthritis Cartilage 20, 572, 2012 [DOI] [PubMed] [Google Scholar]
- 119. McIlwraith C.W., Frisbie D.D., and Kawcak C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt Res 1, 297, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. McIlwraith C., Wright I., and Nixon A. Diagnostic and surgical arthroscopy in the horse. https://books.google.ca/books?hl=en&lr=&id=qODTBQAAQBAJ&oi=fnd&pg=PP1&dq=joint+arhtroscopy+horses&ots=HQtMx4QDaR&sig=jwHkOCMtxjIenHIgPxtjo6BxRAs (Last accessed July4, 2016)
- 121. Koch T.G., and Betts D.H. Stem cell therapy for joint problems using the horse as a clinically relevant animal model. Expert Opin Biol Ther 7, 1621, 2007 [DOI] [PubMed] [Google Scholar]
- 122. Foldager C.B., Farr J., and Gomoll A.H. Patients scheduled for chondrocyte implantation treatment with MACI have larger defects than those enrolled in clinical trials. Cartilage 7, 140, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Engen C.N., Engebretsen L., and Årøen A. Knee cartilage defect patients enrolled in randomized controlled trials are not representative of patients in orthopedic practice. Cartilage 1, 312, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lyman S., Nakamura N., Cole B.J., Erggelet C., Gomoll A.H., and Farr J. Cartilage-repair innovation at a standstill: methodologic and regulatory pathways to breaking free. J Bone Joint Surg Am 98, e63, 2016 [DOI] [PubMed] [Google Scholar]
- 125. Kramer L.A., and Greek R. Human stakeholders and the use of animals in drug development. Bus Soc Rev 123, 3, 2018 [Google Scholar]