Abstract
The aims of this study were to generate periodontal ligament (PDL) cells that have adenovirus- or lentivirus-mediated overexpression of human telomerase reverse transcriptase (hTERT) and to compare the osteogenic and proliferative abilities of the two cell lines to establish an efficient and stable cell model that will be more suitable for studies of PDL regeneration. After construction of the recombinant adenovirus plasmid pAd-pshuttle-cmv-hTERT, human PDL cells were infected by packaged adenovirus and lentivirus particles to establish two PDL cell lines. The expression levels of hTERT and mRNA for alkaline phosphatase, osteopontin, osteocalcin, bone sialoprotein, core-binding factor (runt-related transcription factor 2), and type I collagen were assessed for each cell line. After culture in osteoinductive culture medium for 14 days, the PDL cells were stained with alizarin red to observe formation of mineralized nodules, and proliferation activity was measured with a CCK-8 kit. A quantitative polymerase chain reaction assay indicated that the two transduced cell lines expressed hTERT levels that were significantly higher than that seen for normal PDL cells. Expression of all osteogenic genes tested, with the exception of osteopontin, was higher for both the adenovirus- and lentivirus-transduced cells relative to normal PDL cells. The expression of bone sialoprotein, osteocalcin, and runt-related transcription factor 2 in adenovirus-transduced cells was significantly higher than that for lentivirus-transduced cells. Alizarin red staining showed that the adenovirus-transduced cell line produced more mineralized nodules than the lentivirus-transduced cell line, whereas a CCK-8 test showed that the adenovirus-transduced cell line had higher proliferation activity than lentivirus-transduced cells. In conclusion, a PDL cell line established by adenovirus transduction had superior osteogenic differentiation and proliferative activity compared to the cell line produced by lentivirus transduction. The results indicate that PDL cells having adenovirus-mediated expression of hTERT would be a more suitable model for studies of PDL regeneration.
Keywords: oral periodontal ligament cells, hTERT, adenovirus vectors, lentivirus, osteogenic differentiation, periodontal regeneration
Introduction
Recent research on periodontitis treatments has focused on the regenerative periodontal ligament (PDL), which mainly comprises oral PDL cells that can differentiate into osteoblasts, adipocytes, and neurocytes under appropriate conditions.1–3 PDL cells can be used as seeding and support cells for PDL regeneration,4,5 Researchers typically produce PDL cells that are transduced by lentivirus vector that mediates overexpression of human telomerase reverse transcriptase (hTERT) to produce growth performance and regenerative activity that are similar to that of primary cells.6,7 However, the large size of the hTERT gene segment is associated with lower recombinant viral titer and hTERT expression efficiency, which both limit the applications of this approach. More importantly, the lentiviral vector is a human immunodeficiency virus–derived virus that can be potentially dangerous to humans. Recombinant adenovirus is an efficient and reliable recombinant virus expression system that can accommodate larger package volumes and has higher expression efficiency,8 and thus may be more suitable for hTERT expression. To the best of the authors' knowledge, there are no reports concerning PDL cell lines established using adenovirus, and there are no comparisons of the proliferation and osteogenic activity of cells produced using these two viral vectors.
This study aimed to establish PDL cells that have adenovirus-mediated overexpression of the hTERT gene and to compare osteogenic differentiation and proliferation activity of two PDL cell lines established by adenovirus and lentivirus infection in order to identify the most suitable PDL cell vector system for the development of PDL regeneration therapy.
Methods
Materials and reagents
Lentivirus expression vector pLVX-hTERT-puro, lentivirus packaging plasmid pMD2.G, envelope protein plasmid psPAX2, and the packaging cell line 293T were obtained from the Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences.
Dispase II (Merck Millipore), Reverse Transcription Kit (Roche), an RNeasy Mini Kit (Qiagen), a DyNAmo HS SYBR Green qPCR Kit (Thermo Fisher Scientific), α-MEM, fetal bovine serum (Gibco), and the electrocompetent Escherichia coli strain BJ5183 (Hua Yue Yang Biological Company) were used in this study. Upstream and downstream primers were synthesized and sequenced by BJI.
Isolation and culture of human normal PDL cells
First, premolars were obtained from patients who underwent orthodontic extraction treatment at the Hospital of Stomatology Lanzhou University and were collected in accordance with Lanzhou University ethical guidelines. Following extraction, the premolars were washed five times with phosphate-buffered saline (PBS). The PDL was scraped from the middle third of the root and digested in a type II collagenase and dispase II solution for 30 min at 37°C, with shaking every 10 min. The supernatant was removed after centrifugation for 5 min at 250 g. After washing three times with PBS, the cells were cultivated in α-MEM medium and passaged by trypsin after the cells had adhered and spread across the plate wall.
Construction of recombinant lentivirus particles expressing hTERT gene
293T cells were passaged into 60 mm culture dishes at a ratio of 1:3 before transfection. The cells could be transfected upon achieving 70–80% density after 24 h. The 293T cells were co-transfected with pLVX-puro-hTERT, pMD2.G and psPAX2 using FuGENE6 Transfection Reagent according to the manufacturer's instructions.9 The viral supernatant was collected 48 and 72 h after transfection, centrifuged at 250 g for 5 min at 4°C, filtered with a 0.45 μm filter, and stored at −80°C.
Construction of recombinant adenovirus particles expressing the hTERT gene
Construction of shuttle plasmid pshuttle-cmv-hTERT
Primers were designed according to the hTERT gene complementary DNA (cDNA) sequence in the NCBI database (NM_198253.2). The forward primer was 5′-CTAGGGTACCATGCCGCGCGCTCCCCGCT-3′, and the reverse primer was 5′-CTAGAAGCTTTCAGTCCAGGATGGTCTTGAAGTCT-3′. The hTERT gene sequence was amplified using Platinum Pfx DNA Polymerase according to the manufacturer's instructions. After analysis by 1% agarose gel electrophoresis, the 3.3 kb gene fragment was isolated. The hTERT and pshuttle-cmv were digested with HindIII and KpnI restriction enzyme and then ligated with T4 ligase. The ligated product was transformed into Trans-T1 competent cells. The plasmid was purified and subjected to PmeI enzyme digestion, and plasmid carrying the insert was sent to BJI for determination of the DNA sequence.
Construction of BJ5183/p super competent cells
The plasmid pAd-Easy-1 was transformed into BJ5183 competent cells using a heat-shock method and grown on agar plates containing ampicillin for 12 h before colonies were randomly selected. BJ5183 super competent cells containing pAd-Easy-1 were obtained using the method described by Inoue and were termed BJ5183/p.10
Construction of homologous recombinant adenovirus plasmid
The pshuttle-cmv-TERT plasmid was linearized by PacI enzyme digestion and then transformed into BJ5183/p cells by heat shock. The cells were grown on agar plates containing ampicillin for 12 h, and 10 small monoclonal colonies were randomly selected. Recombinant plasmids were purified and digested with PacI for sequence determination.
Collection of adenovirus particles
293T cells were passaged at a ratio of 1:3 and were transfected with recombinant plasmid using FuGENE6 Transfection Reagent according to the manufacturer's instructions. The supernatant was collected 7 days post transfection after three freeze–thaw cycles and centrifugation to reinfect the cells. After an additional 7 days, the supernatant was collected and filtered with a 0.22 μm filter before storage at −80°C.
Infection of PDL cells by adenovirus particles and lentivirus particles
PDL cells were infected by adenovirus particles and lentivirus particles after six passages. One day after infection with lentivirus particles, 2 μL puromycin was added to select stably passaged cells, termed PDL-hTERT. Cells were infected with adenovirus using the same procedure as for lentivirus, and the resulting stably transfected cells were termed PDL-PAD.
Quantitative polymerase chain reaction detection of hTERT and osteogenic gene expression
RNA was extracted from p6 PDLCS, p35 PDL-hTERT cells, and p35 PDL-PAD cells. cDNA was synthesized using reverse transcriptase and Reverse Transcription Kit (Roche) according to the manufacturer's instructions. For normal PDL cells, cDNA was amplified using quantitative polymerase chain reaction (qPCR) and the Thermo Fisher Scientific DyNAmo Flash SYBR Green qPCR Kit according to the manufacturer's instructions. Relative gene expression levels were quantitated using the 2−ΔΔCt method and normalized relative to GAPDH expression.
Primers used for qPCR to assess hTERT levels were: hTERT: F 5′-TCTGGGATGCGAACGGGC-3′, R 5′-TCCGGCTCAGGGGCAGC-3′; GAPDH: F 5′-GGAGTCCACTGGCGTCTTC-3′, R 5′-GCTGAT GATCTTG AGGCTGTTG-3′.
Primers used for qPCR of osteogenic genes are listed in Table 1.
Table 1.
Primer sequences used for quantitative polymerase chain reaction of osteogenic genes
| Gene | Forward primer (5r→3′) | Reverse primer (3p→5′) |
|---|---|---|
| ALP | CTCGTTGACACCTGGAAGAGCTTCAAACCG | GGTCCGTCACGTTGTTCCTGTTCAGC |
| Runx2 | CACTATCCAGCCACCTTTAC | ATCAGCGTCAACATC |
| BSP | CATAGCCATCGTATCCTTGTCCT | CTATGGAGAGGACGCCACGCCTGG |
| OC | GCAGAGTCCAGCAAAGGGTG | GTCAGCAACTCGTCACAG |
| OPN | CCAAGTAAGTCCAACGAAAG | GGTGATGTCCTCGTCTGTA |
| COLI | AGGGCTCCAACGAGATCGAGATCCG | TACAGGAAGCAGACAGGGCCAACGTCG |
| GAPDH | GGAGTCCACTGGCGTCTTC | GCTGATGATCTTGAGGCTGTTG |
ALP, alkaline phosphatase; BSP, bone sialoprotein; OC, osteocalin; OPN, osteopontin; COLI, collagen I.
Alizarin red staining for measurement of osteogenic differentiation activity
p7 PDL cells, p35 PDL-hTERT cells, and p35 PDL-PAD cells on six-well plates, two wells for each group, were cultured in osteoinductive culture medium (10 mM β-sodium glycerophosphate, 50 μM ascorbic acid diphosphate, and 100 μM dexamethasone) for 21 days with a media change every 3 days. At the end of the culture period, the cells were rinsed three times with PBS, fixed in absolute ethanol for 15 min, and stained with alizarin red (pH 4.1) for 10 min.
Cell proliferation assay
Samples (100 μL) of 90% confluent p7 PDL cells, p35 PDL-PAD cells, and p35 PDL-hTERT cells plated on 96-well plates at a density of 3 × 103 cells per well were taken at 24, 36, and 48 h of culture, and 10 μL of CCK-8 was added. After incubation for 2 h, the absorbance of each well was measured at 450 nm. Each experiment was carried out three times. The results are presented as the mean ± standard deviation. The results were analyzed using the software program GraphPad Prism version 5. One-way and two-way ANOVA was used to determine the significant difference among groups. The p value <0.05 was considered to be significant.
Results
Cellular morphology and phenotype after transformation
The PDL contains a variety of cell types, including fibroblasts, stem cells, osteoblasts, and cementoblasts. Isolated and cultured PDL cells have a fibroblast-like morphology. Here, isolated PDL cells were stably transduced with either adenovirus or lentivirus to generate the cell lines PDL-PAD and PDL-hTERT, which both express hTERT. PDL-PAD, PDL-hTERT, and untransduced PDL cells all had a similar morphology that had typical fibroblast characteristics such as a long fusiform shape (Fig. 1). PDLs that were not transfected with hTERT grew slowly and gradually died after 15–16 passages (Fig. 1B), whereas PDL-PAD cells (Fig. 1C) and PDL-hTERT cells (Fig. 1D) continued to grow rapidly and were stable.
Figure 1.
Cellular morphology and phenotype of periodontal ligament (PDL) cells with human telomerase reverse transcriptase (hTERT) expression. (A) Human PDL cells (100 × ). (B) Human PDL cells after 16 passages (100 × ). (C) Human PDL cells with adenovirus-mediated hTERT expression (PDL-PAD) after 25 passages (100 × ). (D) Human PDL cells with lentivirus-mediated hTERT expression (PDL-hTERT) after 25 passages (100 × ). Scale bars indicate 400 μm.
qPCR analysis of relative expression of hTERT in transduced PDLs
qPCR analysis showed that hTERT mRNA was highly expressed in both PDL-PAD cells and PDL-hTERT cells, which had 15- to 20-fold higher amounts of hTERT relative to normal PDLs. PDL-PAD cells expressed significantly higher amounts of hTERT relative to PDL-hTERT cells (p < 0.001). The hTERT expression levels of both PDL-PAD and PDL-hTERT cells remained stable through the 15th and 35th generations, indicating that cells can stably express hTERT (Fig. 2).
Figure 2.
Relative hTERT expression levels in PDL cells after transduction. Human PDL cells were transduced with the indicated vector, and the relative expression levels were determined by quantitative polymerase chain reaction (qPCR). The values represent the mean of 4 trials, and the error bars indicate the standard deviation. ***p < 0.001.
Relative expression of osteogenic genes in hTERT-transduced PDLs
qPCR analysis showed that alkaline phosphatase (ALP), type I collagen (COLI), runt-related transcription factor 2 (Runx2), bone sialoprotein (BSP), and osteocalcin (OC) mRNA were highly expressed in both PDL-PAD and PDL-hTERT cells to a level that significantly differed from that of normal PDLs (p < 0.001). Interestingly, Runx2, BSP, and OC mRNA was present at significantly higher levels in PDL-PAD cells compared to PDL-hTERT cells (p < 0.001). Meanwhile, osteopontin (OPN) expression levels were similar among PDL-PAD cells, PDL-hTERT cells, and normal PDLs (Fig. 3).
Figure 3.
Relative expression of osteogenic genes detected by reverse transcriptase qPCR. ALP, alkaline phosphatase; BSP, bone sialoprotein; OC, osteocalin; OPN, osteopontin; COLI, collagen I. The mean value for 4 trials is shown, and the error bars represent standard deviation. ***p < 0.001 compared to PDL cells; Δp < 0.001 compared to PDL-hTERT.
Alizarin red staining
Alizarin red staining showed that PDL-PAD cells formed more and bigger mineralized nodules compared to those for normal PDL cells and PDL-hTERT cells (Fig. 4).
Figure 4.
Alizarin red staining of human PDL cells. Images show alizarin red staining of (A) human PDL cells after the 7th passage, (B) PDL-PAD after the 35th passage, and (C) PDL-hTERT cells after the 35th passage. Scale bars indicate 200 μm.
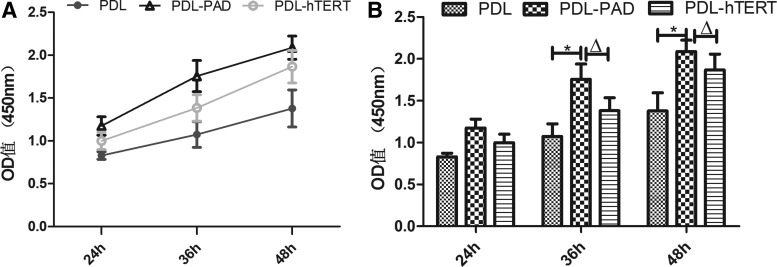
Cell proliferation assessed by CCK-8 assay
The CCK-8 assay showed faster growth for PDL-PAD and PAD-hTERT cells than PDL cells in the first 48 h. In the first 36 h, PDL-PAD cell growth was higher than for PDL-hTERT cells, and thereafter PDL-hTERT cells proliferated more rapidly (Fig. 5A). The faster growth rate for PAD-hTERT cells between 36 and 48 h could be attributed to the filling of the culture dish by PDL-PAD cells that imposed growth limitations. At the 36 and 48 h time points, the number of PDL-PAD cells was significantly higher than that of PDL cells and PDL-hTERT cells (p < 0.05; Fig. 5B). Together, these results indicate that PDL-PAD cells in particular have significant proliferation activity.
Figure 5.
Proliferation of PDL cells. A CCK-8 kit was used to assess cell proliferation. Absorbance at 450 nm was measured at the indicated time points after initiating culture. (A) The rate of PDL-PAD cell proliferation was higher between 0 and 36 h of culture. (B) Comparison of cell proliferation as a function of time. The mean value for 3 trials is shown, and the error bars represent standard deviation. *p < 0.05 compared to PDL cells; Δp < 0.05 compared to PDL-hTERT.
Discussion
Periodontitis is one of the most harmful diseases to oral health and has the highest morbidity, as reflected by positive correlations between periodontitis and the development of diabetes mellitus, cardiovascular disease, and other systemic diseases. The goal of periodontal therapy is to prevent disease development and promote regeneration of injured tissues. Although conventional therapies such as root planing and flap surgery can control the development of periodontitis, these treatments often cannot promote effective periodontal tissue regeneration. Periodontal regeneration treatments currently in use include guided tissue regeneration, bone grafts, and application of growth factors and host regulatory factors, but the biosecurity, stability, and long-term efficacy of these approaches cannot be assured.11 Thus, alternative therapies that facilitate predicable periodontal regeneration are urgently needed. The regeneration ability of PDL cells makes these cells attractive for use in periodontal regeneration methods. However, there are limitations to the use of PDL cells in that the regeneration mechanisms of these cells are unclear, and the number of passages that primary PDL cells can sustain is limited. As a result, further experimental exploration of PDL cells for use in periodontal regeneration therapies is needed to establish PDL cell lines that have proliferation ability and regenerative potential that are similar to primary cells but that can also sustain multiple passages.
Telomeres are complexes comprising DNA and proteins that are located at the end of the chromosome of eukaryotic cells and play a decisive role in maintaining chromosome stability and cell life. Due to normal apoptosis mechanisms of somatic cells, telomeres shorten as the cells undergo mitosis. When the telomere length has shortened to a certain extent, the rate of cell division and proliferation gradually slows, and the chromosome destabilizes as the cell enters senescence. Thus, progressive shortening of telomeres is an important factor that hinders cell immortalization.12 Telomerase is a ribonucleoprotein that catalyzes the synthesis of short telomere sequences. Its reverse transcriptase activity mediated through TERT allows telomerase to replicate the telomere DNA sequence independently to maintain telomere length. Telomerase expression is thus important for extended cellular life-span, and cell life could be extended through maintenance of telomere length.13,14 TERT has been shown to activate telomerase to synthesize the telomere repeat DNA sequence TTAGGG using the RNA present in its own structure as a template for synthesis of telomere sequences that maintain telomere length, and ultimately can be used to foster necessary conditions for immortalization.15 Previous studies confirmed that exogenous expression of the hTERT gene can be used to immortalize normal human cells.16
This study successfully established two PDL cell lines that have adenovirus- and lentivirus-mediated overexpression of hTERT. By passage 16, normal PDL cells appeared to be senescent, while PDL-PAD cells and PDL-hTERT cells maintained rapid proliferation activity and could be continuously passaged. Cells of both lines retained the features of primary PDL cells in terms of morphology, gene expression, and calcified sediment production and growth rate. Analysis by qPCR indicated that PDL-PAD cells had higher hTERT expression levels than did PDL-hTERT cells, but both groups showed significant differences in hTERT expression compared to the control group. The differences between the two transduced lines could be due to differences in the vector copy number, although this possibility awaits further experimentation. Overall, however, PDL-PAD cells had more efficient expression of hTERT than did PDL-hTERT cells.
hTERT can slow mesenchymal stem cell (MSC) aging and can prolong the MSC life-span during in vivo proliferation. hTERT is also reported to enhance the proliferation and osteogenic activity of MSCs in vitro.17 In this study, the expression level of the osteogenic genes ALP, RUNX2, BSP, OC, and COLI in PDL-PAD cells was apparently higher than in control PDL cells, suggesting that hTERT expression can promote osteogenic differentiation through the upregulation of expression of relevant osteogenic genes.
During osteogenic differentiation of PDL cells, expression levels of osteogenic genes, including RUNX2, BSP, ALP, OPN, OC, and COLI, increase. Among these genes, ALP and BSP are considered to be early markers of osteogenesis.18,19 ALP is involved in initiation of osteoblast differentiation, which is important for mineralization in osteogenesis, bone metabolism, and bone regeneration.20,21,22 The osteogenic activity of osteoblasts is regulated by BSP that promotes osteoblast adhesion and can be used as an index to evaluate the success of osteoblast induction and the level of mineralization activity. Within a collagen scaffold, BSP coordinates the osteogenesis mineralization process together with non-collagen proteins. RUNX2 has roles throughout osteogenesis,23,24 and increases in RUNX2 expression can be used as an indicator of differentiation from stem cells to osteoblasts.25 Recent studies indicate that during stem-cell differentiation into osteoblasts, RUNX2 can also regulate transcription of ALP and COLI to modulate their osteogenic activity.23 OC expression can indicate when osteoblast differentiation is complete and is positively correlated with bone formation based on its expression during mineralization.26 COLI can be used to assess the differentiation capacity of osteoblasts, and expression levels of this protein are positively correlated with cellular osteogenic activity. OPN is another important marker of early osteoblastic differentiation that shows significant increases in expression when bone matrix is secreted from cells that are induced to mineralization.
In this study, the mRNA expression levels of osteogenic genes such as ALP, RUNX2, BSP, OC, and COLI in PDL-PAD cells were apparently higher than that of normal PDL cells and PDL-hTERT cells. The mRNA expression of the osteogenic genes RUNX2, BSP, and OC in PDL-PAD cells was significantly higher than that in PDL-hTERT, whereas the amount of ALP and COLI mRNA was similar between PDL-PAD and PDL-hTERT cells. Alizarin red staining showed that there were more mineralized nodules in PDL-PAD cells than in PDL cells and PDL-hTERT cells, suggesting that PDL-PAD cells have enhanced osteogenic differentiation activity and thus would be a valuable cell line for research on the mechanisms of PDL regeneration.
In contrast to the four- to sixfold increase seen for the other osteogenic genes, OPN mRNA levels in both PDL-PAD and PDL-hTERT were similar to that of PDL cells. This result suggests that OPN may participate in pathways that are not affected by hTERT, although this possibility requires confirmation.
Cell proliferation activity is another critical element that reflects cellular activity. The CCK-8 method can be used to detect cell proliferation and represent the metabolic level of living cells. In this study, the CCK-8 assay showed that PDL-PAD cells had more pronounced proliferation activity than PDL and PDL-hTERT cells, particularly during the first 36 h of culture when PDL-PAD cells grew at a significantly faster rate than PAD-hTERT and PDL cells. At 36-48 h, the PDL-PAD cell growth rate is lower than PDL-hTERT cells, which could be attributed to saturation arising from overgrowth on the 96-well plate. Overall, the results showed that the proliferation ability of PDL-PAD was more robust than that of PDL and PDL-hTERT.
In conclusion, this study compared the proliferation and differentiation activity of two PDL cell lines with adenovirus- or lentivirus-mediated expression of hTERT. The PDL cells with adenovirus-mediated hTERT expression had better differentiation capacity compared to lentivirus-transduced cells through 35 passages, yet retained the morphology of the original PDL cells. These findings showed that adenovirus-mediated expression of hTERT in PDL cells produced a cell line that can serve as a valuable model for studies on periodontal regeneration.
Acknowledgments
This study was funded by the Natural Science Foundation of Gansu Province (17JR5RA217).
Author Disclosure
No competing financial interests exist.
References
- 1. Liu J, Wang L, Liu W, et al. Dental follicle cells rescue the regenerative capacity of periodontal ligament stem cells in an inflammatory microenvironment. PLoS One 2014;9:e108752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doğan A, Ozdemir A, Kubar A, et al. Healing of artificial fenestration defects by seeding of fibroblast-like cells derived from regenerated periodontal ligament in a dog: a preliminary study. Tissue Eng 2003;9:1189–1196 [DOI] [PubMed] [Google Scholar]
- 3. Hillmann G, Steinkamp-Zucht A, Geurtsen W, et al. Culture of primary human gingival fibroblasts on biodegradable membranes. Biomaterials 2002;23:1461–1469 [DOI] [PubMed] [Google Scholar]
- 4. Kunze M, Huber A, Krajewski A, et al. Efficient gene transfer to periodontal ligament cells and human gingival fibroblasts by adeno-associated virus vectors. J Dent 2009;37:502–508 [DOI] [PubMed] [Google Scholar]
- 5. Murakami Y, Kojima T, Nagasawa T, et al. Novel isolation of alkaline phosphatase-positive subpopulation from periodontal ligament fibroblasts. J Periodontol 2003;74:780–786 [DOI] [PubMed] [Google Scholar]
- 6. Kamata N, Fujimoto R, Tomonari M, et al. Immortalization of human dental papilla, dental pulp, periodontal ligament cells and gingival fibroblasts by telomerase reverse transcriptase. J Oral Pathol Med 2004;33:417–423 [DOI] [PubMed] [Google Scholar]
- 7. Saito M, Handa K, Kiyono T, et al. Immortalization of cementoblast progenitor cells with Bmi-1 and TERT. J Bone Miner Res 2005;20:50–57 [DOI] [PubMed] [Google Scholar]
- 8. Merentie M, Lottonen-Raikaslehto L, Parviainen V, et al. Efficacy and safety of myocardial gene transfer of adenovirus, adeno-associated virus and lentivirus vectors in mouse heart. Gene Ther 2016;23:296–305 [DOI] [PubMed] [Google Scholar]
- 9. Sa Z, Xiaodong Q, Xiangyi H, et al. [Construction of human mucosa oral epithelial cell lines overexpressing telomerase reverse transcriptase gene mediated by lentivirus]. Hua Xi Kou Qiang Yi Xue Za Zhi 2016;34:443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sambroo J. Molecular Cloning: A Laboratory Manual (3th Edition). Beijing, China: Science Press, 2008:93–96 [Google Scholar]
- 11. Shay JW, Zou Y, Hiyama E, et al. Telomerase and cancer. Hum Mol Genet 2001;10:677–685 [DOI] [PubMed] [Google Scholar]
- 12. Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis 2005;26:867–874 [DOI] [PubMed] [Google Scholar]
- 13. Matsushita H, Chang E, Glassford A J, et al. eNOS activity is reduced in senescent human endothelial cells: preservation by hTERT immortalization. Circ Res 2001;89:793–798 [DOI] [PubMed] [Google Scholar]
- 14. Farahzadi R, Fathi E, Mesbah-Namin SA, et al. Zinc sulfate contributes to promote telomere length extension via increasing telomerase gene expression, telomerase activity and change in the TERT gene promoter CpG island methylation status of human adipose-derived mesenchymal stem cells. PloS One 2017;12:e0188052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Farahzadi R, Fathi E, Mesbah-Namin SA, et al. Anti-aging protective effect of L-carnitine as clinical agent in regenerative medicine through increasing telomerase activity and change in the hTERT promoter CpG island methylation status of adipose tissue-derived mesenchymal stem cells. Tissue Cell 2018;54:105–113 [DOI] [PubMed] [Google Scholar]
- 16. Archibald KM, Kulbe H. Sequential genetic change at the TP53 and chemokine receptor CXCR4 locus during transformation of human ovarian surface epithelium. Oncogene 2012;31:4987–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomokiyo A, Maeda H, Fujii S, et al. Development of a multipotent clonal human periodontal ligament cell line. Differentiation 2008;76:337–347 [DOI] [PubMed] [Google Scholar]
- 18. Kilhovd BK, Berg TJ, Birkeland KI, et al. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care 1999;22:1543–1548 [DOI] [PubMed] [Google Scholar]
- 19. Kanauchi M, Tsujimoto N, Hashimoto T. Advanced glycation end innondiabetic patients with coronary disease. Diabetes Care 2001;24:1620–1623 [DOI] [PubMed] [Google Scholar]
- 20. Wescott DC, Pinkerton MN, Gaffey BJ, et al. Osteogenic gene expression by human periodontal ligament cells under tension. J Dent Res 2007;86:1212–1216 [DOI] [PubMed] [Google Scholar]
- 21. Shimoike T, Inoguchi T, Umeda F, et al. The meaning of serum levels of advanced glycosylation end products in diabetic nephropathy. Metabolism 2000;49:1030–1035 [DOI] [PubMed] [Google Scholar]
- 22. Zheng W, Wang S, Wang J, et al. Periodontitis promotes the proliferation and suppresses the differentiation potential of human periodontal ligament stem cells. Int J Mol Med 2015;36:915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Selvaraj N, Bobby Z, Das AK, et al. An evaluation of level of oxidative stress and protein glycation in nondiabetic undialyzed chronic renal failure patients. Clin Chim Acta 2002;324:45–50 [DOI] [PubMed] [Google Scholar]
- 24. Bruderer M, Richards RG, Alini M, et al. Role and regulation of RUNX2 in osteogenesis. Eur Cell Mater 2014;28:269–286 [DOI] [PubMed] [Google Scholar]
- 25. Neve A, Corrado A, Cantatore FR. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res 2011;343:289–302 [DOI] [PubMed] [Google Scholar]
- 26. Fujisawa R, Tamura M. Acidic bone matrix proteins and their roles in calcification. Front Biosci 2012;17:1891–1903 [DOI] [PubMed] [Google Scholar]