Abstract
In Arabidopsis, the rhizobacterial strain Pseudomonas fluorescens WCS417r triggers an induced systemic resistance (ISR) response that is effective against different types of pathogens. The ISR signaling pathway functions independent of salicylic acid, but requires responsiveness to jasmonate (JA) and ethylene. Using the genetic variability of ISR inducibility between Arabidopsis accessions, we recently identified a locus (ISR1) on chromosome III that is involved in ISR signaling. Accessions RLD and Wassilewskija (Ws) are recessive at the ISR1 locus and are, therefore, unable to develop ISR. Here we investigated whether the ISR1 locus is involved in JA or ethylene signaling. Compared with the ISR-inducible accession Columbia (Col), accessions RLD and Ws were not affected in JA-induced inhibition of root growth and expression of the JA-responsive gene Atvsp, suggesting that the ISR1 locus is not involved in JA signaling. However, RLD and Ws showed an affected expression of the triple response and a reduced expression of the ethylene responsive genes Hel and Pdf1.2 after exogenous application of the ethylene precursor 1-aminocyclopropane-1-carboxylate. Moreover, in contrast to Col, RLD and Ws did not develop resistance against P. syringae pv. tomato DC3000 after treatment of the leaves with 1-aminocyclopropane-1-carboxylate. Analysis of the F2 and F3 progeny of a cross between Col (ISR1/ISR1) and RLD (isr1/isr1) revealed that reduced sensitivity to ethylene cosegregates with the recessive alleles of the ISR1 locus. These results suggest that the ISR1 locus encodes a component of the ethylene response, which is required for the expression of rhizobacteria-mediated ISR.
Localized treatment of plants with specific biotic or abiotic agents can result in the development of enhanced resistance against pathogens in distal plant parts. Resistance induced by such treatments is generally characterized by a restriction of pathogen growth and a reduction of disease severity (Hammerschmidt, 1999). Induced resistance against pathogens can be subdivided into two categories. The classical way to induce disease resistance is by predisposal infection with a necrotizing pathogen, resulting in a systemic resistance in distal plant parts. This form of induced resistance is generally referred to as systemic acquired resistance (SAR; Ryals et al., 1996; Sticher et al., 1997). SAR is characterized by the endogenous accumulation of salicylic acid (SA; Malamy et al., 1990; Métraux et al., 1990) and a concomitant expression of genes encoding pathogenesis-related proteins (for review, see Van Loon, 1997). The second type of induced resistance develops in response to colonization of plant roots by selected strains of non-pathogenic rhizobacteria, and is often referred to as induced systemic resistance (ISR; Van Loon et al., 1998). Rhizobacteria-mediated ISR has been demonstrated to be effective in a variety of plant species under conditions in which the rhizobacteria remained spatially separated from the challenging pathogen. Pathogen-induced SAR and rhizobacteria-mediated ISR confer an enhanced defensive capacity that is effective against a broad spectrum of plant pathogens (Ryals et al., 1996; Van Loon et al., 1998). In Arabidopsis the level of induced resistance can be enhanced further when both types of induced resistance are activated simultaneously (Van Wees et al., 2000), indicating that SAR and ISR are additive and constitute two different mechanisms of induced resistance.
Rhizobacteria-mediated ISR has been studied extensively in Arabidopsis using the non-pathogenic rhizobacterial strain Pseudomonas fluorescens WCS417r as the inducing agent and Pseudomonas syringae pv. tomato DC3000 (Pst) as the challenging pathogen (Pieterse et al., 2000). In this combination the ISR signaling pathway clearly differs from the one that controls pathogen-induced SAR. SA-nonaccumulating NahG plants expressing the bacterial salicylate hydroxylase gene (NahG) fail to express SAR (Gaffney et al., 1993; Lawton et al., 1995), but show normal levels of ISR after treatment of the roots with WCS417r bacteria (Pieterse et al., 1996). This indicates that SA is a necessary signal for the SAR response, but is not required for ISR signaling. Arabidopsis mutants that are impaired in their response to the plant hormones jasmonate (JA) or ethylene, develop normal levels of SAR (Lawton et al., 1995; Pieterse et al., 1998), but are unable to express WCS417r-mediated ISR (Pieterse et al., 1998; Knoester et al., 1999). This demonstrates that, in contrast to SAR, ISR signaling requires components of the JA and the ethylene response. Despite these differences, the SAR and the ISR pathway are both controlled by the regulatory protein NPR1/NIM1 (Cao et al., 1994; Delaney et al., 1995; Pieterse et al., 1998). Downstream of NPR1/NIM1 both pathways diverge, indicating that NPR1/NIM1 differentially regulates defense responses depending on the pathway that is activated upstream of it (Pieterse et al., 1998).
We recently identified a novel factor in the ISR signaling pathway by screening 10 Arabidopsis accessions for their ability to express ISR. Two accessions, RLD and Wassilewskija (Ws), failed to develop ISR after treatment of the roots with WCS417r bacteria, whereas they expressed normal levels of pathogen-induced SAR (Ton et al., 1999). This WCS417r-non-responsive phenotype could not be attributed to poor root colonization by the ISR-inducing rhizobacteria, since colonization of the roots of both accessions was unaffected. Furthermore, the WCS417r-non-responsive phenotype of RLD and Ws was associated with a relatively high level of susceptibility to Pst. Genetic analysis of progeny of crosses between inducible and noninducible accessions revealed that the potential to express ISR, as well as the relatively high level of basal resistance against Pst, are controlled by a single dominant locus (ISR1) that maps on chromosome III between cleaved amplified polymorphism sequence (CAPS) markers B4 and GL1. This suggested that the ISR1 locus encodes a factor that is involved in both ISR and basal resistance against Pst.
Not only are the Arabidopsis mutants etr1-1 and jar1-1 affected in their ability to express WCS417r-mediated ISR against Pst, they also develop more severe disease symptoms upon primary infection with Pst, and allow significantly more bacterial growth compared with wild-type Columbia (Col) plants (Pieterse et al., 1998). This strikingly resembles the isr1 phenotype of accessions RLD and Ws, which are similarly affected in the expression of ISR and basal resistance against Pst. The involvement of JA and ethylene in basal defense responses has repeatedly been demonstrated. In many cases, blocking the response to either of these signals can render plants more susceptible to certain pathogens and even insects. For instance, mutants that are affected in JA biosynthesis or signaling are more susceptible to pathogens such as Pythium mastophorum (Vijayan et al., 1998) and P. irregulare (Staswick et al., 1998), as well as to insect herbivory (McConn et al., 1997; Stout et al., 1999). In a similar manner, ethylene-insensitive tobacco plants transformed with the mutant etr1-1 gene from Arabidopsis lost their ability to resist the soil-borne pathogen P. sylvaticum (Knoester et al., 1998). Furthermore, the ethylene-insensitive Arabidopsis mutant ein2-1 gained enhanced susceptibility to the necrotrophic fungal pathogen Botrytis cinerea (Thomma et al., 1999) and the bacterial leaf pathogen Erwinia carotovora pv. carotovora (Norman-Setterblad et al., 2000). Although these examples demonstrate the importance of JA and ethylene in specific basal resistance responses, other pathogens seem to be resisted predominantly through a SA-dependent pathway (Thomma et al., 1998).
The isr1 phenotype, i.e. inability to express ISR and enhanced susceptibility to Pst infection, of accessions RLD and Ws on the one hand and that of mutants etr1-1 and jar1-1 on the other hand, prompted us to investigate whether the ISR1 locus is involved in JA or ethylene signaling. Arabidopsis accessions Col (ISR1/ISR1), RLD (isr1/isr1), and Ws (isr1/isr1) were tested for their ability to respond to JA and ethylene by examining JA- and ethylene-induced inhibition of root growth and by studying JA- and ethylene-responsive gene expression. Here we show that the isr1 phenotype of accessions RLD and Ws is associated with a reduced sensitivity to ethylene, suggesting that the ISR1 locus is involved in ethylene signaling.
RESULTS
The isr1 Phenotype Is Not Associated with Reduced Sensitivity to Methyl JA (MeJA)
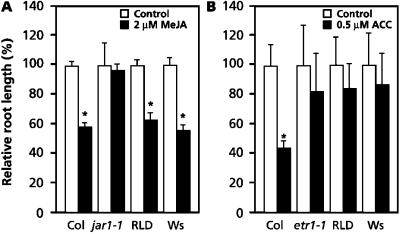
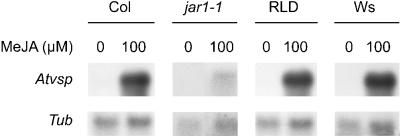
Accessions RLD and Ws resemble the JA-insensitive mutant jar1-1 in that they are blocked in the ISR signaling pathway and exhibit enhanced susceptibility to Pst (Pieterse et al., 1998; Ton et al., 1999). Therefore, we investigated whether the isr1 phenotype of accessions RLD and Ws is based on reduced sensitivity to JA. It was previously demonstrated that MeJA-induced inhibition of primary root growth and MeJA-induced expression of the Atvsp gene is substantially decreased in the jar1-1 mutant (Staswick et al., 1992, 1998). Both characteristics were examined in Col, jar1-1, RLD, and Ws plants. Five days after germination, accession Col showed a 42% inhibition of primary root growth on Murashige and Skoog (MS) agar plates with 2 μm MeJA (Fig. 1A). Growth of primary roots of mutant jar1-1 was not inhibited, whereas RLD and Ws showed a similar response to MeJA as accession Col, resulting in 38% and 45% inhibition of primary root growth, respectively. Moreover, exogenous application of 100 μm MeJA to leaves of Col resulted in a strong activation of the JA-responsive gene Atvsp, whereas in jar1-1 plants Atvsp transcripts accumulated to a much lower level (Fig. 2). RLD and Ws showed similar responses to MeJA as Col. These results demonstrate that the isr1 phenotype of RLD and Ws cannot be attributed to reduced responsiveness to JA.
Figure 1.
MeJA- and ACC-induced inhibition of primary root growth. A, MeJA-induced inhibition of primary root growth in Col, jar1-1, RLD, and Ws. Seeds were surface-sterilized, distributed on MS-agar plates containing 0 or 2 μm MeJA, and germinated for 2 d at 4°C in the dark. After an additional growth period of 5 d at 20°C with an 8-h photoperiod, the length of the primary roots was measured. Data are means (± sd; n = 15–25) of the relative root length compared with control plants (0 μm MeJA), which was set at 100%. Asterisks indicate statistically significant differences compared with the control plants (Student’s t test; α = 0.05). B, ACC-induced inhibition of primary root length in Col, etr1-1, RLD, and Ws. Seeds were germinated on MS-agar plates containing 0 or 0.5 μm ACC for 2 d at 4°C in the dark. After an additional growth period of 3 to 7 d in the dark at 20°C, the length of the primary root was measured. Data are means (± sd; n = 15–25) of the relative root length compared with control plants (0 μm ACC), which was set at 100%. Asterisks indicate statistically significant differences compared with the control plants (Student's t test; α = 0.05).
Figure 2.
RNA gel-blot analysis of MeJA-induced Atvsp gene expression in leaves of Col, jar1-1, RLD, and Ws. Five-week-old plants were treated by dipping the leaves in a solution containing 0 or 100 μm MeJA. Two days after MeJA-treatment the leaves were harvested. An Arabidopsis Atvsp gene-specific probe was used for the RNA gel-blot hybridization. To check for equal loading, the blot was stripped and hybridized with an Arabidopsis gene-specific probe for β-tubulin (Tub). The experiment was repeated with similar results.
RLD and Ws Show Reduced Sensitivity to Ethylene
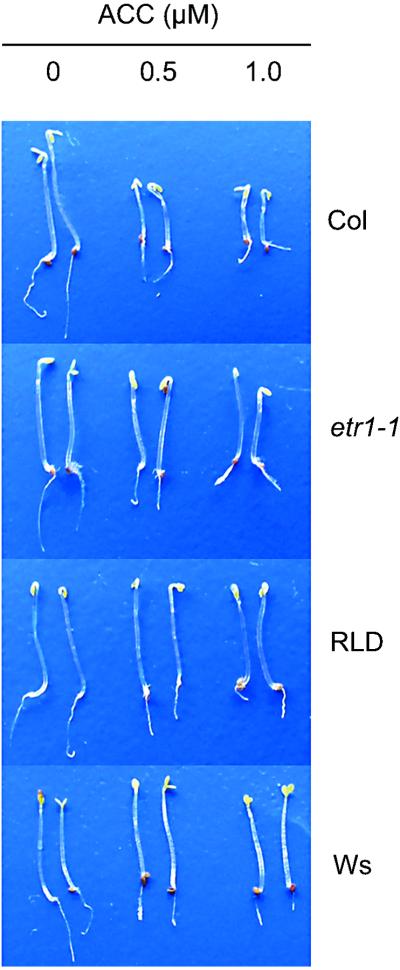
Like jar1-1, the ethylene insensitive mutant etr1-1 is blocked in the expression of ISR and exhibits enhanced disease susceptibility to Pst (Pieterse et al., 1998; Knoester et al., 1999). Therefore, we investigated whether the isr1 phenotype of accessions RLD and Ws is based on reduced sensitivity to ethylene. The “triple response” is a reaction of etiolated seedlings to ethylene and has been shown to be a reliable marker for ethylene sensitivity (Guzmán and Ecker, 1990). To assess ethylene sensitivity, we first examined ethylene-induced root length inhibition of etiolating seedlings, which is one of the characteristics of the triple response. Col, etr1-1, RLD, and Ws seedlings were grown on MS-agar plates containing 0.5 μm of the ethylene precursor 1-aminocyclopropane-1-carboxylate (ACC), as this concentration was found to differentiate best for root length inhibition between Col and etr1-1 (data not shown). At 0.5 μm ACC, Col plants showed a statistically significant inhibition of root growth, whereas mutant etr1-1, RLD, and Ws responded only weakly (Fig. 1B). In etr1-1, RLD, and Ws the weak inhibition of root growth was never statistically significant, indicating that RLD and Ws exhibit a certain degree of insensitivity to ACC. To determine the extent of this insensitivity, the effect of increasing concentrations of ACC on root length inhibition was examined. Figure 3 shows that primary root length of etiolated Col seedlings was reduced by 55% to 80% when grown on 0.5 to 5 μm ACC, respectively. In this concentration range, the inhibition of root elongation of etr1-1 and RLD was significantly less dramatic (ranging between 25%–45%). Up to 2.5 μm ACC, the effect of ACC on root elongation in accession Ws was even less evident than in RLD and etr1-1 (ranging between 10%–35%). However, at 5 μm ACC Ws showed the same level of inhibition of root length as Col. Other characteristics of the triple response, i.e. inhibition of hypocotyl elongation and exaggeration of the apical hook, were clearly apparent at 0.5 μm of ACC in Col. In etr1-1, RLD, and Ws these characteristics were absent at 0.5 μm and 1 μm (Fig. 4) and only occurred consistently at concentrations above 2.5 μm (data not shown).
Figure 3.
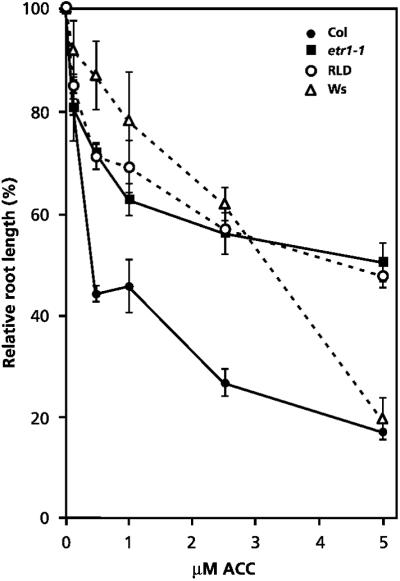
Dose-response curves of ACC-induced inhibition of primary root growth in Col, etr1-1, RLD, and Ws. Seeds were germinated on MS-agar plates containing 0, 0.1, 0.5, 1.0, 2.5, or 5.0 μm ACC for 2 d at 4°C in the dark. After an additional growth period of 3 to 7 d in the dark at 20°C, the length of the primary roots was measured. Data are means (±sd; n = 15–25) of the relative root length compared with that of control-treated plants (0 μm ACC), which was set at 100%.
Figure 4.
Triple response expression of Col, etr1-1, RLD, and Ws. Seeds were germinated on MS-agar plates containing 0, 0.5, or 1.0 μm ACC for 2 d at 4°C in the dark. Photographs were taken after an additional growth period of 4 d in the dark at 20°C.
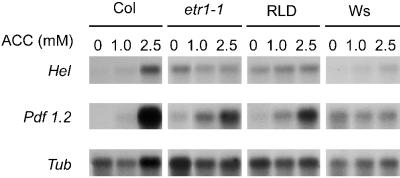
To further investigate the apparent differences in ACC sensitivity, the expression patterns of the ethylene-inducible, defense-related genes Hel (Potter et al., 1993) and Pdf1.2 (Penninckx et al., 1996) were analyzed after application of 0, 1.0, or 2.5 mm ACC to the leaves of Col, etr1-1, RLD, and Ws (Fig. 5). In Col, Hel and Pdf1.2 transcripts were evident at 1.0 mm ACC and accumulated to a relatively high level at 2.5 mm ACC. In contrast, at increasing ACC concentrations etr1-1 showed no increase in the steady-state Hel mRNA level. Moreover, only a relatively weak induction of Pdf1.2 gene expression was apparent in etr1-1, which was significantly less pronounced than that observed in Col plants. RLD showed Hel and Pdf1.2 expressions patterns similar to that observed in etr1-1. In Ws, steady-state levels of Pdf1.2 transcripts did not increase at all after ACC treatment, whereas the Hel gene was weakly induced. These results confirm that accessions RLD and Ws show reduced sensitivity to ACC, with RLD resembling etr1-1 more than Ws.
Figure 5.
RNA gel-blot analysis of ACC-induced Hel and Pdf1.2 gene expression in the leaves of Col, etr1-1, RLD, and Ws. Five-week-old plants were treated by dipping the leaves in a solution containing 0, 1.0, or 2.5 mm ACC. Two days after ACC-treatment the leaves were harvested. Arabidopsis Hel and Pdf1.2 gene-specific probes were used for RNA gel-blot hybridizations. To check for equal loading, the blots were stripped and hybridized with an Arabidopsis gene-specific probe for β-tubulin (Tub). The experiment was repeated with similar results.
After application of 1 mm ACC, all four genotypes showed similar time kinetics in the emission of ethylene (data not shown), indicating that ACC uptake and ACC converting capacity was similar for all four genotypes. It can thus be concluded that the observed differences in responsiveness to ACC are the result of differences in ethylene sensitivity.
RLD and Ws Are Affected in Ethylene-Induced Protection against Pst
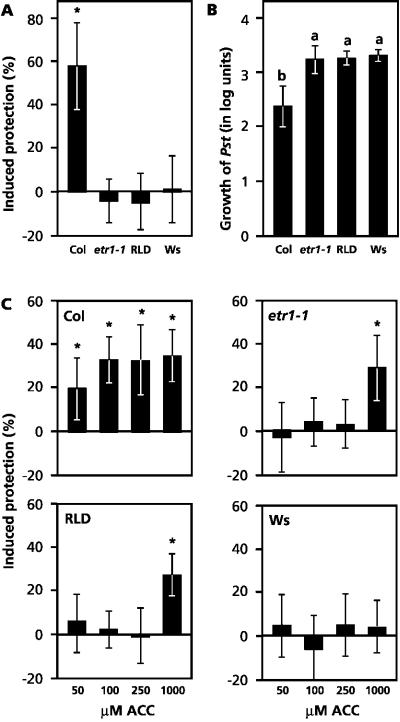
Comparison of mutant etr1-1 and accessions RLD and Ws revealed that they are phenotypically similar in that they are non-responsive to WCS417r-mediated induction of ISR (Fig. 6A) and allow statistically significant higher levels of growth of Pst compared with Col (Fig. 6B). Both characteristics are likely to be caused by the common reduced sensitivity to ethylene. We previously demonstrated that exogenous application of ACC to the leaves induces protection against Pst in Col, but not in etr1-1 (Pieterse et al., 1998). To investigate whether RLD and Ws are similarly affected in ACC-induced protection, we determined the level of resistance against Pst after exogenous application of increasing concentrations of ACC (Fig. 6C). In Col, all ACC concentrations tested induced a statistically significant level of protection against Pst compared with water-treated plants. In contrast, etr1-1 and RLD failed to develop resistance after treatment with the three lowest ACC concentrations (50, 100, and 250 μm), whereas application of 1 mm ACC induced protection in etr1-1 and RLD only. In Ws, all ACC concentrations tested failed to induce protection against Pst. It can thus be concluded that, like etr1-1, accessions RLD and Ws are impaired in ethylene-induced resistance against Pst.
Figure 6.
Level of WCS417r-induced protection, basal resistance, and ACC-induced protection against Pst in Col, etr1-1, RLD, and Ws. A, Quantification of WCS417r-mediated ISR. ISR was triggered by growing plants in soil containing ISR-inducing P. fluorescens WCS417r bacteria at 5 × 107 cfu g−1. Five-week-old plants were challenge-inoculated with a bacterial suspension of virulent Pst at 2.5 × 107 cfu mL−1. Three days after challenge inoculation the percentage of diseased leaves was assessed and the level of induced protection was calculated. Induced protection is presented as a reduction of disease symptoms relative to challenged control plants. Asterisks indicate statistically significant differences compared with noninduced control plants (Student's t test; α = 0.05; n = 20–25). Data presented are means (±sd) from representative experiments that were performed at least twice with similar results. B, Proliferation of Pst over a 3-d time interval. Five-week-old plants were infected by pressure infiltrating a suspension of virulent Pst at 5 × 105 cfu mL−1 into the leaves. Immediately after pressure infiltration and 3 d later, the number of Pst bacteria per gram of leaf fresh weight was determined and the proliferation over a 3-d time interval was calculated. Data presented are the means (±sd) of the proliferation values (log cfu g−1) of a representative experiment that was repeated twice with similar results. Different letters indicate statistically significant differences between genotypes (Fisher's lsd test; α = 0.05; n = 6). C, Quantification of ACC-induced protection against Pst. Plants were dipped in a solution containing different concentrations of ACC 3 d before challenge inoculation with Pst. Three days after challenge, the level of induced protection was assessed as described above.
Reduced Sensitivity to Ethylene Cosegregates with the isr1 Phenotype
To investigate whether the reduced sensitivity to ethylene observed in RLD and Ws is associated with the recessive alleles of the ISR1 locus we tested the F2 progeny of the RLD × Col cross for ethylene sensitivity. The triple response was tested in the F2 seedlings and in the RLD and Col parents at 0 and 0.5 μm ACC. In the F2 population, responsiveness to 0.5 μm ACC segregated in a statistically significant 3:1 ratio (X2 = 0.16, P = 0.69; Table I), indicating that the reduced sensitivity to ethylene is caused by a single recessive locus. This experiment was repeated twice, yielding comparable segregation ratios (X2 = 0.04 and 0.19; P = 0.83 and 0.66, respectively). Five F3 families homozygous at the ISR1 locus (ISR1/ISR1), five F3 families homozygous at the isr1 locus (isr1/isr1), and the corresponding Col and RLD parent were subsequently tested for triple response expression at 0, 0.5, 1, and 5 μm ACC. On MS-agar plates without ACC, none of the genotypes showed triple response expression (Table II). On 0.5 μm ACC, only the Col parent and the five ISR1/ISR1 F3 families exhibited consistent triple response expression, whereas the RLD parent and the isr1/isr1 F3 families did not (Table II). At higher concentrations of ACC, RLD and the isr1/isr1 F3 families showed triple response expression, although in many cases this was inconsistent (Table II). It can thus be concluded that the reduced ethylene sensitivity observed in RLD is a recessive trait that cosegregates with the isr1 phenotype.
Table I.
Genetic segregation of triple response expression at 0.5 μm ACC in Col, RLD, and F2 plants of the RLD × Col crossa
| Genotype | Treatment (μM ACC) | Total No. of Seedlings | Triple Responseb | No Triple Responsec | Expected Ratio | χ2 value | P |
|---|---|---|---|---|---|---|---|
| Col | 0 | 48 | 0 | 48 | 0:1 | – | – |
| 0.5 | 56 | 53 | 3 | 1:0 | – | – | |
| RLD | 0 | 52 | 3 | 49 | 0:1 | – | – |
| 0.5 | 53 | 4 | 49 | 1:0 | – | – | |
| F2 | 0 | 52 | 4 | 48 | 0:1 | – | – |
| 0.5 | 74 | 54 | 20 | 3:1 | 0.16 | 0.69 |
Surface-sterilized seeds were plated onto MS-agar plates with or without 0.5 μm ACC. After germination for 2 d at 4°C, seedlings were grown for 5 d at 20°C in the dark and examined for triple-response expression. The experiment was repeated twice, yielding similar results.
No. of seedlings exhibiting triple response characteristics.
No. of seedlings showing no triple response characteristics.
Table II.
Triple response expression in accessions Col and RLD, and in F3 families of the RLD × Col cross that are homozygous at the ISR1 locus
| Accessions/F3 Familiesa | Triple Response
Assayb
|
|||
|---|---|---|---|---|
| 0 μm | 0.5 μm | 1 μm | 5 μm | |
| Col (ISR1/ISR1) | − | + | + | + |
| ISR1/ISR1 fam 1 | − | + | + | + |
| ISR1/ISR1 fam 2 | − | + | + | + |
| ISR1/ISR1 fam 3 | − | + | + | + |
| ISR1/ISR1 fam 4 | − | + | + | + |
| ISR1/ISR1 fam 5 | − | + | + | + |
| RLD (isr/isr1) | − | − | + | + |
| isr/isr1 fam 1 | − | − | +/− | +/− |
| isr/isr1 fam 2 | − | − | + | + |
| isr/isr1 fam 3 | − | − | + | + |
| isr/isr1 fam 4 | − | − | +/− | +/− |
| isr/isr1 fam 5 | − | − | − | +/− |
The F3 families homozygous for the dominant ISR1 alleles of Col are capable of expressing ISR after treatment of the roots with P. fluorescens WCS417r and exhibit relatively high basal resistance against Pst. The F3 families homozygous for the recessive isr1 alleles of RLD fail to express ISR after treatment of the roots with WCS417r and exhibit relatively low levels of basal resistance against Pst (Ton et al., 1999).
Genotypes were assayed for sensitivity to ACC using the triple response assay. Surface-sterilized seeds (50–100) were plated onto MS-agar plates containing different concentrations of ACC. After germination for 2 d at 4°C, seedlings were grown for 3 to 7 d at 20°C in darkness before being examined for triple response expression. +, Consistent triple response in all plants; +/−, inconsistent triple response; −, none of the plants expressed the triple response.
The ISR1 Locus Is Not Allelic with Ein3
The ISR1 locus was previously mapped at chromosome III between CAPS markers GL1 and B4 (Ton et al., 1999). The Ein3 gene, encoding an activator of the ethylene response pathway (Chao et al., 1997), also maps in this region. Moreover, the ein3 mutant is unable to express WCS417r-mediated ISR (Knoester et al., 1999). Therefore, we investigated whether ISR1 and Ein3 are allelic. For this purpose we designed a CAPS marker based on the nucleotide sequence of the Ein3 gene (Chao et al., 1997) and performed a CAPS marker analysis on 32 homozygous F3 families of the RLD × Col cross (16 ISR1/ISR1 F3 families and 16 isr1/isr1 F3 families). Of the 64 chromosomes tested, 13 were recombinant with the Ein3 CAPS marker, yielding a recombination frequency at 20.3%. Moreover, the F1 progeny of a complementation cross between RLD and the ein3 mutant was fully capable of expressing ISR and exhibited a similar level of basal resistance against Pst as the Col parent (data not shown). It can thus be concluded that the ISR1 locus is not allelic with the Ein3 gene.
DISCUSSION
We previously demonstrated that the ISR1 locus on chromosome III of Arabidopsis controls the expression of WCS417r-mediated ISR and basal resistance against Pst (Ton et al., 1999). This study aimed at elucidating the physiological role of the ISR1 locus in the ISR signaling pathway. In Arabidopsis the ISR pathway requires an intact response to the plant hormones JA and ethylene (Pieterse et al., 1998). Analysis of MeJA-induced inhibition of primary root growth and Atvsp gene expression in the WCS417r-non-responsive accessions RLD (isr1/isr1) and Ws (isr1/isr1), and the ISR-inducible accession Col (ISR1/ISR1), revealed that RLD and Ws are not affected in their response to MeJA (Figs. 1 and 2). It can thus be concluded that the ISR1 locus is not involved in JA signaling. In contrast, analysis of the triple response and ethylene-inducible gene expression demonstrated that sensitivity to ethylene is significantly reduced in RLD and Ws in comparison with Col (Fig. 3–5). Moreover, RLD and Ws were impaired in their ability to express ethylene-induced resistance against Pst (Fig. 6). Genetic analysis of the F2 and F3 progeny of a RLD × Col cross revealed that the reduced sensitivity to ethylene is monogenically inherited as a recessive trait and genetically linked to the recessive alleles of the ISR1 locus. These results indicate that the ISR1 locus contains a gene encoding a component involved in ethylene signaling. Using a large set of ethylene response mutants, we previously demonstrated that insensitivity to ethylene causes non-responsiveness to WCS417r (Pieterse et al., 1998; Knoester et al., 1999). Therefore, it is likely that ethylene responsiveness and ISR inducibility are determined by a single gene on the ISR1 locus. However, the possibility of close linkage between two different genes cannot be eliminated completely.
The ISR1 locus maps on chromosome III between CAPS markers B4 and GL1 (Ton et al., 1999). Two genes from the ethylene signaling pathway, Ein3 and Ein4, map in the vicinity of the ISR1 locus. The possibility that the ISR1 locus is allelic with Ein4 can be ruled out by the observation that accessions RLD and Ws exhibit recessive phenotypes (Ton et al., 1999; this study), whereas the ein4 mutation is dominant (Roman et al., 1995). The considerable recombination between the ISR1 locus and the Ein3 gene in F3 families of the RLD × Col cross also rules out the possibility that ISR1 is allelic with Ein3. Moreover, accession RLD and mutant ein3 showed full complementation of ISR-inducibility and basal resistance against Pst in their F1 progeny (data not shown). Therefore, we hypothesize that the Arabidopsis ISR1 locus encodes a novel component of the ethylene response pathway, which plays an important role in disease resistance signaling.
Although RLD and Ws showed reduced sensitivity to ethylene in comparison to Col, the magnitude of the reduced ethylene response differed between both accessions. For instance, like etr1-1, RLD was affected in the inhibition of ethylene-induced primary root growth at all ACC concentrations tested (0.5–5.0 μm), whereas for Ws this was only apparent at ACC concentrations up to 2.5 μm. At 5 μm, Ws displayed a normal triple response. Analysis of ethylene-responsive gene expression revealed a similar pattern, with RLD resembling etr1-1 more than Ws. The apparent differences in ethylene responsiveness between RLD and Ws likely result from the considerable genetic diversity between both accessions (Erschadi et al., 2000). Alternatively, RLD and Ws might be affected at different sites in the ISR1 locus.
In Arabidopsis considerable genetic variation between accessions has been reported for several developmental, physiological, and biochemical traits (Alonso-Blanco and Koornneef, 2000). This naturally occurring variation of Arabidopsis has contributed to the identification of a large number of loci conferring resistance to viral, bacterial, or fungal pathogens (Kunkel, 1996). In this study we provide evidence that reduced sensitivity to ethylene in RLD and Ws is causing the isr1 phenotype in these accessions. Naturally occurring variation in ethylene sensitivity is known to occur in various plant species. For instance, Voesenek et al. (1996) reported significant differences in ethylene sensitivity between three species of Rumex, one of which showed exceptionally high responsiveness to ethylene. This phenotype appeared to be a necessary adaptation for escaping water submergence by mediation of ethylene-induced shoot elongation. Moreover, Emery et al. (1996) reported considerable differences in ethylene responsiveness between ecotypes of Stellaria longipes. The enhanced ethylene responsiveness of one ecotype was explained as a critical adaptation to wind stress.
Arabidopsis plants carrying the recessive alleles of the ISR1 locus show an enhanced susceptibility to Pst infection (Ton et al., 1999), suggesting that ethylene signaling is involved in basal resistance against Pst. This is supported by the observation that the ethylene response mutant etr1-1 allows 10-fold higher levels of growth of Pst than wild-type plants (Pieterse et al., 1998; this study). Moreover, treatment of Arabidopsis with the ethylene precursor ACC induces resistance against Pst (Pieterse et al., 1998; Van Wees et al., 1999). These findings seem to contradict those reported by Bent et al. (1992), who showed that the ethylene response mutant ein2 allows similar levels of growth of Pst, and develops fewer symptoms compared with wild-type plants. However, Bent et al. (1992) used a 5-fold lower density of Pst inoculum. This suggests that the ethylene-dependent basal resistance that we observed is only apparent when the initial inoculum density is above a certain threshold level, although other so far unidentified differences in the experimental setup could also be responsible for the different results. The plant hormones SA and JA are also involved in basal resistance against Pst, because genotypes that are impaired in their response to these signaling molecules are more susceptible to Pst infection (Delaney et al., 1994; Pieterse et al., 1998). It is apparent that the mechanisms contributing to basal resistance against Pst are controlled by a coordinated action of SA-, JA-, and ethylene-dependent signaling pathways.
Knoester et al. (1999) previously tested eight ethylene response mutants on their ability to express WCS417r-mediated ISR. None of these mutants were able to express ISR after application of WCS417r to the roots, demonstrating that an intact ethylene-signaling pathway is required for the expression of ISR. Mutant eir1-1 that is insensitive to ethylene in the roots only was capable of mounting ISR after application of WCS417r to the leaves, but not after application of WCS417r to the roots, indicating that the ISR signaling pathway requires ethylene sensitivity at the site of WCS417r application. From these results it was hypothesized that ethylene signaling is involved in the generation or translocation of the systemically transported ISR signal. Future research will be focused on cloning of the ISR1 gene. This will open the way to study the role of the ISR1 protein in both ISR and ethylene signaling.
MATERIALS AND METHODS
Cultivation of Rhizobacteria, Pathogens, and Plants
Non-pathogenic, ISR-inducing Pseudomonas fluorescens WCS417r bacteria (Pieterse et al., 1996) were grown on King's medium B (KB) agar plates (King et al., 1954) for 24 h at 28°C. Bacterial cells were collected and resuspended in 10 mm MgSO4 to a final density of 109 colony-forming units (cfu) per mL. The virulent pathogen P. syringae pv. tomato strain DC3000 (Pst; Whalen et al., 1991) used for challenge inoculations was grown overnight at 28°C in liquid KB. After centrifugation, the bacterial cells were resuspended in 10 mm MgSO4 with 0.015% (v/v) of the surfactant Silwet L-77 (Van Meeuwen Chemicals, Weesp, The Netherlands) to a final density of 2.5 × 107 cfu mL−1.
Seedlings of wild-type Arabidopsis accessions Col (Col-0), Ws (Ws-0), and RLD (RLD1), and the Col mutants etr1-1 (Bleecker et al., 1988) and jar1-1 (Staswick et al., 1992) were grown in quartz sand for 2 weeks. For transfer of the seedlings, a sand/potting soil mixture (5:12, v/v) that had been autoclaved twice for 1 h with a 24-h interval was supplemented with a suspension of ISR-inducing WCS417r bacteria or an equal volume of 10 mm MgSO4. Seedlings were then transferred into 60-mL pots containing the sand/potting soil mixture with or without WCS417r. Plants were cultivated in a growth chamber with an 9-h day (200 μE m−2 s−1 at 24°C) and 15-h night (20°C) cycle at 65% relative humidity. For the duration of the experiments, all genotypes remained vegetative and developed at least 10 to 15 mature leaves. Plants were watered on alternate days, and once a week supplied with modified one-half strength Hoagland solution: 2 mm KNO3, 5 mm Ca[NO]3, 1 mm KH2PO4, 1 mm MgSO4, and trace elements, pH 7 (Hoagland and Arnon, 1938), containing 10 μm sequestreen [Fe-ethylenediamide-di(0-hydroxyphenylacetic acid); Novartis, Basel].
Induction Treatments
For treatment with ISR-inducing rhizobacteria, 2-week-old seedlings were transplanted into soil containing WCS417r bacteria at 5 × 107 cfu g−1. Induction of ethylene-mediated resistance was performed 2 d before challenge by dipping the leaves of 5-week-old plants in a solution containing the ethylene precursor ACC with 0.015% (v/v) Silwet L-77. For RNA-blot analysis, chemical treatments were performed by dipping the leaves of 5-week-old plants in a solution containing 0.015% (v/v) Silwet L77 and different concentrations of MeJA or ACC.
Challenge Inoculations and Disease Assessment
For assaying induced resistance, WCS417r- and control-treated plants were challenged when 5 weeks old by dipping the leaves in a suspension of virulent Pst bacteria at 2.5 × 107 cfu mL−1 in 10 mm MgSO4, 0.015% (v/v) Silwet L-77. One day before challenge inoculation, the plants had been placed at 100% relative humidity. Three or 4 d after challenge inoculation, the percentage of leaves with symptoms was determined per plant (n = 20–25). Leaves showing necrotic or water-soaked lesions surrounded by chlorosis were scored as diseased (Pieterse et al., 1996). For assaying basal resistance against Pst, leaves of 5-week-old, control-treated plants were inoculated by pressure-infiltration with a suspension of virulent Pst at 5 × 105 cfu mL−1 in 10 mm MgSO4. Immediately after pressure infiltration and 3 d later, replicate leaf samples from five plants per genotype were collected, weighed, and homogenized in 10 mm MgSO4. Serial dilutions were plated on selective KB-agar plates supplemented with 100 mg L−1 cycloheximide and 50 mg L−1 rifampicin. After incubation at 28°C for 2 d, the number of rifampicin-resistant colony-forming units per gram of infected leaf tissue was determined and bacterial proliferation over the 3-d time interval was calculated.
Inhibition of Primary Root Length by MeJA and ACC
Seeds of Arabidopsis were surface sterilized for 5 min in 5% (v/v) sodium hypochlorite, washed in 70% (v/v) ethanol, and air dried. Seeds were subsequently distributed evenly on 1.0% (w/v) agar medium containing 0.5% (w/v) MS salts (Duchefa bv, Haarlem, The Netherlands), 0.5% (w/v) Suc, and different concentrations of MeJA or ACC (pH 5.7). MeJA (Serva, Brunschwig Chemie bv, Amsterdam) was added to the autoclaved medium from a filter-sterilized 1-mm stock (containing 0.96% [v/v] ethanol). ACC (Sigma-Aldrich Chemie bv, Zwijndrecht, The Netherlands) was added from a 10 mm stock in a similar manner. Seeds were germinated in the dark for 2 d at 4°C. The effect of MeJA on primary root growth was determined essentially as described by Staswick et al. (1992). Plates were incubated in a climate chamber at 22°C with an 8-h day (approximately 200 μE m−2 s−1) and a 16-h night cycle. After 5 d the primary root length was measured under a dissection microscope. In each case, 15 to 25 randomly selected seedlings were measured. The effect of ethylene on primary root length of etiolated seedlings was tested essentially according to Guzmán and Ecker (1990). After germination in the dark for 2 d at 4°C the seedlings were grown for an additional 3 to 7 d at 20°C without light. The primary root length was measured as described above.
Ethylene Measurements
Leaves of plants pretreated with 1 mm ACC were detached, weighed, and placed in 25-mL gas-tight serum flasks, and subsequently incubated at climate chamber conditions. At different time points over a 28-h interval, cumulative ethylene production was measured by gas chromatography as described by De Laat and Van Loon (1982).
RNA Gel-Blot Analysis
Total RNA was extracted by homogenizing frozen leaf tissue in extraction buffer (0.35 m Gly, 0.048 n NaOH, 0.34 m NaCl, 0.04 m EDTA, 4% [w/v] SDS, and 1 mL g−1 of leaf tissue). The homogenates were extracted with phenol and chloroform and the RNA was precipitated using LiCl, as described by Sambrook et al. (1989). For RNA gel-blot analysis, 15 μg of RNA was denatured using glyoxal and dimethyl sulfoxide (Sambrook et al., 1989). Samples were subsequently electrophoretically separated on 1.5% (w/v) agarose gels and blotted onto Hybond-N+ membranes (Amersham, ‘s-Hertogenbosch, The Netherlands) by capillary transfer. The electrophoresis buffer and blotting buffer consisted of 10 and 25 mm sodium phosphate (pH 7.0), respectively. RNA gel blots were hybridized and washed as described previously (Pieterse et al., 1994). DNA probes were labeled with α-32P-dCTP by random primer labeling (Feinberg and Vogelstein, 1983). Probes for the detection of Atvsp, Hel, and Tub transcripts were prepared by PCR with primers based on sequences obtained from GenBank accession numbers Z18377, U01880, and M21415, respectively. Probes to detect Pdf1.2 transcripts were derived from an Arabidopsis Pdf1.2 cDNA clone (Penninckx et al., 1996).
Genetic Analysis
The F1, F2, and F3 progenies of a cross between Col (ISR1/ISR1) and RLD (isr1/isr1) were previously tested for ISR inducibility and basal resistance against Pst (Ton et al., 1999). From this cross the F2 progeny, five randomly selected F3 families homozygous at the ISR1 locus, and five randomly selected F3 families homozygous at the isr1 locus were tested for ethylene sensitivity and compared with that of the corresponding parents. Ethylene sensitivity was quantified by assaying triple response expression as described by Guzmán and Ecker (1990), using MS-agar plates containing different concentrations of ACC. Recombination between the ISR1 and the EIN3 locus was determined on 16 F3 families homozygous at the ISR1 locus and 16 F3 families homozygous at the isr1 locus, using a CAPS marker (Konieczny and Ausubel, 1993) for the EIN3 gene (accession no. AF004217; Chao et al., 1997). Amplification of the EIN3 sequence was performed using the primers 5′-CTCCTTCTTTTTCCCATCACCATA-3′ (nucleotides 349–372) and 5′-TTCCCATCTCATTAAACATCATTG-3′ (nucleotides 975–952). Subsequent digestion with BglII resulted in a polymorphism between accessions Col (627 bp) and RLD (359 and 268 bp).
ACKNOWLEDGMENTS
We acknowledge the Nottingham Arabidopsis Stock Centre for providing Arabidopsis seeds, Andrew Bent for kindly providing Pst DC3000, and Willem Broekaert for the Arabidopsis Pdf1.2 cDNA clone. We also thank Hans van Pelt and Patricia Tersteeg for technical assistance.
LITERATURE CITED
- Alonso-Blanco C, Koornneef M. Naturally occurring variation in Arabidopsis: an unexploited resource for plant genetics. Trends Plant Sci. 2000;5:22–29. doi: 10.1016/s1360-1385(99)01510-1. [DOI] [PubMed] [Google Scholar]
- Bent AF, Innes RW, Ecker JR, Staskawicz BJ. Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Mol Plant-Microbe Interact. 1992;5:372–378. doi: 10.1094/mpmi-5-372. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Sommerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an Arabidopsis mutant that is non-responsive to inducers of systemic acquired resistance. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ethylene-insensitive3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- De Laat AMM, Van Loon LC. Regulation of ethylene biosynthesis in virus-infected tobacco leaves: II. Time course of levels of intermediates and in vivo conversion rates. Plant Physiol. 1982;69:240–245. doi: 10.1104/pp.69.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney T, Friedrich L, Ryals J. Arabidopsis signal transduction mutant defective in plant disease resistance. Proc Natl Acad Sci USA. 1995;92:6602–6606. doi: 10.1073/pnas.92.14.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney T, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Ward M, Ryals J. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Emery RJN, Reid DM, Chinnappa CC. Phenotypic plasticity of stem elongation in two ecotypes of Stellaria longipes: the role of ethylene and response to wind. Plant Cell Environ. 1996;19:1423–1430. [Google Scholar]
- Erschadi S, Haberer G, Schöniger M, Torres-Ruiz RA. Estimating genetic diversity of Arabidopsis thaliana ecotypes with amplified fragment length polymorphisms (AFLP) Theor Appl Genet. 2000;100:633–640. [Google Scholar]
- Feinberg AP, Vogelstein G. A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gaffney TP, Friedrich L, Vernooij B, Negrotto D, Neye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt R. Induced disease resistance: how do induced plants stop pathogens? Physiol Mol Plant Pathol. 1999;55:77–84. [Google Scholar]
- Hoagland DR, Arnon DI. The water culture method for growing plants without soil. Calif Agric Exp Stn Bull. 1938;347:36–39. [Google Scholar]
- King EO, Ward MK, Raney DE. Two simple media for the demonstration of phycocyanin and fluorescin. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- Knoester M, Pieterse CMJ, Bol JF, Van Loon LC. Systemic resistance in Arabidopsis induced by rhizobacteria requires ethylene-dependent signaling at the site of application. Mol Plant-Microbe Interact. 1999;12:720–727. doi: 10.1094/MPMI.1999.12.8.720. [DOI] [PubMed] [Google Scholar]
- Knoester M, Van Loon LC, Van den Heuvel J, Hennig J, Bol JF, Linthorst HJM. Ethylene-insensitive tobacco lacks non-host resistance against soil-borne fungi. Proc Natl Acad Sci USA. 1998;95:1933–1937. doi: 10.1073/pnas.95.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- Kunkel BN. A useful weed put to work: genetic analysis of disease resistance in Arabidopsis thaliana. Trends Genet. 1996;12:63–69. doi: 10.1016/0168-9525(96)81402-8. [DOI] [PubMed] [Google Scholar]
- Lawton K, Weimann K, Friedrich L, Vernooij B, Uknes S, Ryals J. Systemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Mol Plant-Microbe Interact. 1995;8:863–870. doi: 10.1094/mpmi-8-0863. [DOI] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J. Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J-P, Signer H, Ryals J, Ward E, Wyss-Benz MM, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B. Increase of salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Norman-Setterblad C, Vidal S, Palva TE. Interacting signal pathways control defense gene expression in Arabidopsis in response to cell wall-degrading enzymes from Erwinia carotovora. Mol Plant-Microbe Interact. 2000;13:430–438. doi: 10.1094/MPMI.2000.13.4.430. [DOI] [PubMed] [Google Scholar]
- Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, De Samblanx GW, Buchala A, Métraux J-P, Manners JM, Broekaert WF. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Derksen A-MCE, Folders J, Govers F. Expression of the Phytophthora infestans ipiB and ipiO genes in planta and in vitro. Mol Gen Genet. 1994;244:269–277. doi: 10.1007/BF00285454. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Wees SCM, Hoffland E, Van Pelt JA, Van Loon LC. Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell. 1996;8:1225–1237. doi: 10.1105/tpc.8.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Wees SCM, Ton J, Léon-Kloosterziel KM, Van Pelt JA, Keurentjes JJB, Knoester M, Van Loon LC. Rhizobacteria-mediated induced systemic resistance (ISR) in Arabidopsis: involvement of jasmonate and ethylene. In: De Wit PJGM, Bisseling T, Stiekema WJ, editors. Biology of Plant-Microbe Interactions. Vol. 2. St. Paul: International Society for Molecular Plant-Microbe Interactions; 2000. pp. 291–296. [Google Scholar]
- Pieterse CMJ, Van Wees SCM, Van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, Van Loon LC. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell. 1998;10:1571–1580. doi: 10.1105/tpc.10.9.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S, Uknes S, Lawton K, Winter AM, Chandler D, DiMaio J, Novitzky R, Ward E, Ryals J. Regulation of a hevein-like gene in Arabidopsis. Mol Plant-Microbe Interact. 1993;6:680–685. doi: 10.1094/mpmi-6-680. [DOI] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner H, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Staswick PE, Su W, Howell SH. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA. 1992;95:1933–1937. doi: 10.1073/pnas.89.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Yuen GY, Lehman CC. Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. 1998;15:747–754. doi: 10.1046/j.1365-313x.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- Sticher L, Mauch-Mani B, Métraux J-P. Systemic acquired resistance. Annu Rev Phytopathol. 1997;35:235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- Stout MJ, Fidantsef AL, Duffey SS, Bostock RM. Signal interactions in pathogen and insect attack: systemic plant-mediated interactions between pathogens and herbivores of the tomato, Lycopersicon esculentum. Physiol Mol Plant Pathol. 1999;54:115–130. [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Tierens FM-J, Broekaert WF. Requirement of a functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999;121:1093–1101. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, Pieterse CMJ, Van Loon LC. Identification of a locus in Arabidopsis controlling both the expression of rhizobacteria-mediated induced systemic resistance (ISR) and basal resistance against Pseudomonas syringae pv. tomato. Mol Plant-Microbe Interact. 1999;12:911–918. doi: 10.1094/MPMI.1999.12.10.911. [DOI] [PubMed] [Google Scholar]
- Van Loon LC. Induced resistance in plants and the role of pathogenesis-related proteins. Eur J Plant Pathol. 1997;103:753–765. [Google Scholar]
- Van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol. 1998;36:453–485. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- Van Wees SCM, De Swart EAM, Van Pelt JA, Van Loon LC, Pieterse CMJ. Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA, 2000;97:8711–8716. doi: 10.1073/pnas.130425197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wees SCM, Luijendijk M, Smoorenburg I, Van Loon LC, Pieterse CMJ. Rhizobacteria-mediated induced systemic resistance (ISR) in Arabidopsis is not associated with a direct effect on expression of known defense-related genes but stimulates the expression of the jasmonate-inducible Atvsp upon challenge. Plant Mol Biol. 1999;41:537–549. doi: 10.1023/a:1006319216982. [DOI] [PubMed] [Google Scholar]
- Vijayan P, Shockey J, Lévesque CA, Cook RJ, Browse J. A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA. 1998;95:7209–7214. doi: 10.1073/pnas.95.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Banga M, Rijnders JGHM, Visser EJW, Blom CWPM. Hormone sensitivity and plant adaptations to flooding. Folia Geobot Phytotax. 1996;31:47–56. [Google Scholar]
- Whalen MC, Innes RW, Bent AF, Staskawicz BJ. Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell. 1991;3:49–59. doi: 10.1105/tpc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]