Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a common disease that occurs all over the world. Models of care, initially accessed from the clinical point of view, must also be evaluated in terms of their economic effectiveness, as health care systems are limited. The Integrated Care Model (ICM) is a procedure dedicated to patients suffering from advanced COPD that offers home-oriented support from a multidisciplinary team. The main aim of the present study was to evaluate the cost-effectiveness of the ICM.
Material/Methods
We included 44 patients in the study (31 males, 13 females) with an average age 72 years (Me=71). Costs of care were estimated based on data received from public payer records and included general costs, COPD-related costs, and exacerbation-related costs. To evaluate cost-effectiveness, cost-effectiveness analysis (CEA) was used. The incremental cost-effectiveness ratio (ICER) was calculated based on changes in health care resources utilization and the value of costs observed in 2 consecutive 6-month periods before and after introducing ICM.
Results
Costs of care of all types decreased after introducing ICM. Demand for ambulatory visits changed significantly (p=0.037) together with a substantial decrease in the number of emergency department appointments and hospitalizations (p=0.033). ICER was more profitable for integrated care than for standard care when assessing costs of avoiding negative parameters such as hospitalizations (−227 EUR), exacerbations-related hospitalizations (−312 EUR), or emergency procedures (−119 EUR).
Conclusions
ICM is a procedure that meets the criteria of cost-effectiveness. It allows for avoiding negative parameters such as unplanned hospitalizations with higher economic effectiveness than the standard type of care used in managing COPD.
MeSH Keywords: Delivery of Health Care, Integrated; Economics, Pharmaceutical; Pulmonary Disease, Chronic Obstructive
Background
Integrated Care as an optimal way of managing advanced chronic obstructive pulmonary disease (COPD)
The concept of care integration at a patient’s home was first described in 1967 [1], but CEE (Central-Eastern Europe) countries are still working on improving it and shaping its role [2]. As such medical technologies allow avoiding hospitalizations [3], numerous institutions recommend home-oriented care in COPD management. It is also commonly accepted to introduce IC in COPD due to the high prevalence of the disease and high risk of comorbidities. According to the World Health Organization (WHO), 65 million people worldwide are have moderate or severe COPD [4]. Nevertheless, few studies have focused on COPD and integrated care, even fewer studies have assessed its economic effectiveness.
One of these interventions is a program carried out in Gdansk (Poland) called the Integrated Care Model (ICM). The most important features of the ICM are clinical effectiveness and high level of acceptance by patients and their relatives [5].
ICM is a home-oriented procedure for patients with advanced COPD (stage III and IV of obturation according to recent GOLD classification). For this reason, medical and non-medical personnel not only educate but also support patients and their families. The main goal is to maintain a stable health condition of the patients, and to avoid exacerbations and hospitalizations. Moreover, all of them are under regular control of family doctors (GPs) and pneumonologists. Appointments are planned alternatively every 4 months. Between medical appointments, patients are visited every 2 weeks by social workers who assist them in everyday activities. Additionally, a study coordinator phones patients once a month and controls their compliance. The exact principle of operation is presented in Figure 1. Currently, support of patients is the responsibility of the study coordinator and social workers. The study coordinator, among others, prepares a schedule of medical visits for patients and plans visits of social workers. The study coordinator is also responsible for planning activities of all members of the ICM (for example: dietitians, psychologists, GPs, pneumonologists, priests). In previous studies, the ICM demonstrated a high level of acceptance by patients and their relatives [5,6].
Figure 1.
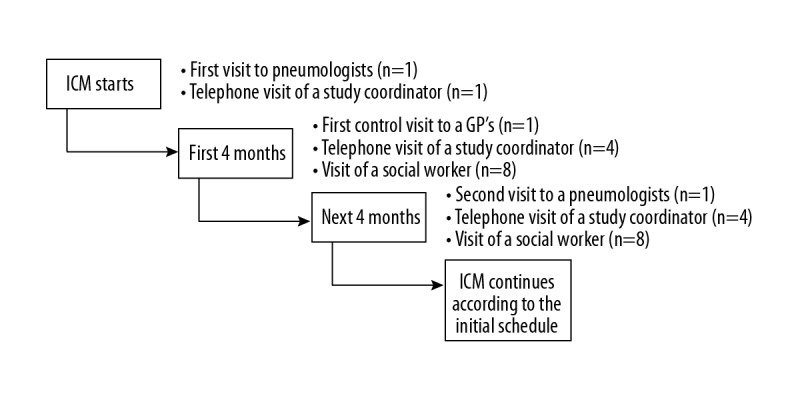
Organization of Integrated Care Model.
Steps of pharmacoeconomic assessment in medicine and ICM
A pharmacoeconomic approach to assessing medical interventions includes analysis and comparison of costs and results (effects) of introducing medical technology such as integrated care in managing COPD. The cost-effectiveness method (CEA) is one of the most commonly used to indicate procedures of optimal efficacy and acceptable costs, as it structurally indicates the hierarchy of medical technologies depending on their costs and effectiveness. As a result, it allows allocating a fixed healthcare system budget between interventions in a way that builds better public health [7].
The first step is to perform cost analysis, which is analysis of expenditures related to specific interventions; this step is related to analysis of direct medical costs, administrative costs, and indirect costs (if necessary) [8].
The second step is to assess effects of the intervention demonstrated by outcomes that may vary depending on the type of intervention. These may be changes in state of health of the patients or quality of life, or changes in demand for medical services.
Analysis of ICM was done from the public payer perspective. As a result, cost analysis included assessment of expenditures related to standard care (SC) and intergraded care (IC), which replaced SC after 6 months of observation. Cost analysis included assessment of both medical and administrative costs (costs of running the ICM). Assessment of effects involved analysis of changes in demand for medical services (e.g., number of hospitalizations, visits to emergency departments, and visits to GPs).
The main aim of the present study was to assess cost-effectiveness of ICM with use of Cost-Effectiveness Analysis. The secondary goals were to:
– determine costs of standard and integrated care;
– evaluate effects of the intervention by assessing changes in demand for medical services;
– estimate the value of incremental cost-effectiveness ratio (ICER).
Material and Methods
Study group
During the observation period, ICM was available for 44 patients fulfilling all the inclusion criteria. All the patients (31 males and 13 females) had to: give their informed consent to enter the study, have stage III–IV of obturation according to GOLD, experience at least 2 exacerbations in 12 months before entering ICM, and at least 1 hospitalization related to COPD. The average age was 72 years (Me=71). All of the patients had multiple comorbidities (from 2 to 11 different health conditions), most often from hypertension (n=22) and ischemic heart disease (n=9). The most frequently diagnosed health problems are presented in Table 1.
Table 1.
Number of cases of most common comorbidities.
| Group of health conditions | Number of cases |
|---|---|
| Chronic conditions | 53 |
| Chronic renal failure | 6 |
| Hyperthyroidism | 2 |
| Osteoarthritis | 5 |
| Non-malignant prostatic hypertrophy | 6 |
| Cholelithiasis | 8 |
| Hyperlipidaemia | 8 |
| Atherosclerosis | 5 |
| Diabetes mellitus | 13 |
| Cardiovascular diseases | 41 |
| Ischemic heart disease | 9 |
| Myocardial infarction in history | 7 |
| Congestive heart failure | 3 |
| Hypertension | 22 |
| Cancers | 8 |
| Lung | 1 |
| Prostate | 3 |
| Stomach | 1 |
| Breast | 1 |
| Penile | 1 |
| Kidney | 1 |
| Respiratory diseases other than COPD | 4 |
| Bronchiectasis | 2 |
| Asthma | 2 |
Cost analysis
Cost analysis for ICM purposes was performed based on cost of treatment and costs of running the ICM.
Data on costs related to running the ICM were provided by the study coordinator based on official bills and invoices documenting expenses.
Data on expenditures were provided by the Pomeranian Branch of the National Health Fund (NHF) and covered 1 year (2 consecutive 6-month periods) for each patient in a before-after study. All analyzes were performed from the public payer perspective. In order to estimate all types of direct medical expenditures that might occur in COPD patients, a 3-stage cost analysis was introduced, which included 3 types of costs:
– I – general ones, meaning charges on all medical procedures provided in 1 year.
– II – expenses from medical procedures provided due to COPD and other diseases of the respiratory system. On the basis of clinical expertise, 2 of the authors included ICD-10 codes of diseases and related health problems requiring procedures, qualified to the group costs of COPD and other diseases of respiratory system, independently.
– III – expenditures on medical procedures provided due to COPD exacerbations. Inclusion of ICD-10 codes was conducted similarly to the COPD-related costs described above.
Health care resources utilization
Demand for medical procedures (expressed as the number of medical procedures) was analyzed in subgroups separately for outpatient procedures and hospitalizations/emergency appointments within 6-month periods prior and following ICM introduction. To assess the significance of observed changes, statistical calculations were used.
Statistical calculations
All calculations were carried out by means of STATISTICA, StatSoft, Inc. ver. 8.0. statistical package (data analysis software system). The chi-squared test was used to determine the significance of changes in demand described by change in the number of medical services realized before and after introducing ICM. In all the calculations, the statistical significance level was set to p<0.05.
CEA analysis
CEA analysis involved a comparison of costs and effects/benefits occurring during the period before and after entering the ICM. The indicator used to determine cost-effectiveness of a given intervention is called the incremental cost-effectiveness ratio (ICER), which is described by the following formula [9]:
Where:
C1 – cost of more effective intervention
C2 – cost of less effective intervention
E1 – effect of more effective intervention
E2 – effect of less effective intervention
Effects (E) can be expressed in different ways depending on the type of technology being analyzed and research needs. They can be expressed as the number of avoided hospitalizations, shortening the time of hospitalization, or the number of deaths avoided.
Results
Cost analysis
We analyzed the expenditures of NHF in 2 consecutive 6-month periods of time before and after the patients entered the ICM. The costs of all types decreased after changing type of care from standard to integrated (Table 2).
Table 2.
Value of direct medical costs in PLN and (EUR) before and after introducing ICM (n=44).
| Observation period | General costs | COPD costs | ERP costs |
|---|---|---|---|
| Before ICM | 5 627 (1 295) | 3 191 (734) | 2 444 (526) |
| After ICM | 3 577 (741) | 1 741 (401) | 735 (169) |
The next stage of cost analysis was to assess expenditures related to running the ICM. Based on the data collected by the entities involved in project management, they amounted to 915.18 PLN (EUR 2018) per patient per 6 months. This amount included the costs of patient care (visits of ICM staff members, among others: social workers, dietitians, psychologists) and administrative costs.
Changes in demand for services
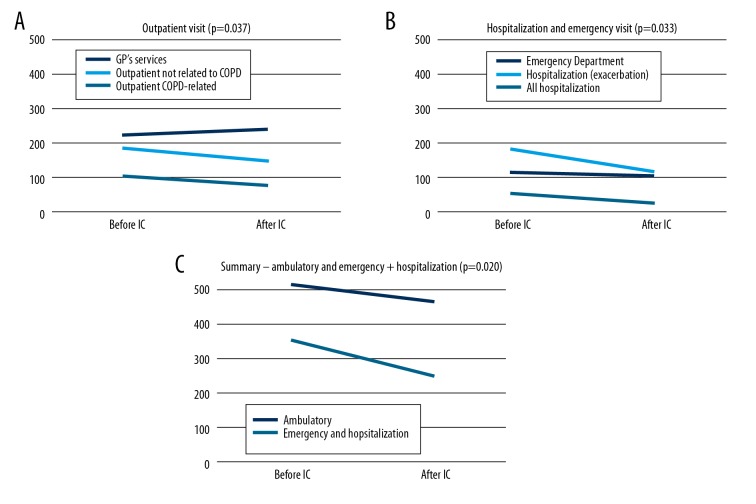
The next stage of the study was to assess changes in the demand for medical services resulting from changing the care type from SC to IC. We found that after including patients in the ICM, their demand for ambulatory visits (including GP appointments) changed significantly (p=0.037), (Figure 2A). Patients more often visited their GPs, but at the same time, the number of visits to outpatient clinics of other types decreased. Also, the demand for emergency department appointments and hospitalizations declined substantially (p=0.033), which is particularly visible in case of hospitalizations (Figure 2B). Furthermore, the number of hospitalizations due to COPD exacerbations dropped significantly (27 vs. 54). The summary analysis of changes in demand, in both groups (emergency/hospitalizations and ambulatory services) proved that in both cases their numbers were reduced significantly (p=0.020), as presented in Figure 2C.
Figure 2.
(A) Changes in demand for medical services after introducing ICM – ambulatory (outpatient and GPs) appointments. (B) Changes in demand for medical services after introducing ICM – emergency appointments and hospitalizations. (C) Changes in demand for medical services after introducing ICM – summary comparison of ambulatory and emergency appointments (together with hospitalizations).
CEA analysis
CEA analysis using incremental indicators was determined for an introductory 6-month period, referred to as initial. Under the ICER, a value of negative parameters avoided (e.g., hospitalizations) in general and due to COPD exacerbations or emergency department appointments was more profitable for IC than SC (Table 3).
Table 3.
The summary data on CEA analysis results in PLN and (EUR).
| CEA analysis (profitable when ICER<0) | |||||
|---|---|---|---|---|---|
| Type of procedures | Cost | Effect (avoided procedure) | ICER | ||
| Before ICM | After ICM | Before ICM | After ICM | ||
| Hospitalizations | 130 849 (31 107) | 108 909 (25 891) | 0 | 66 | −332 (−79) |
| Exacerbation related hospitalizations | 63 534 (15 104) | 28 050 (6 668) | 0 | 27 | −1314 (−312) |
| Emergency procedures (Emergency Department appointment or hospitalization) | 230 719 (54 849) | 184 163 (43 781) | 0 | 93 | −501 (−119) |
ICER has been calculated, taking into account the relevant types of costs and the effects. For the effect in the form of avoiding hospitalization, COPD costs were used, as most of them resulted from this condition or were related to typical comorbidities. For hospitalization related to the treatment of acute exacerbations, we used ERP costs, and general expenses were applied for analysis of visits to emergency departments (all mentioned in Table 1). Costs after ICM used for calculation of ICER consisted of medical direct costs and running ICM model costs. In the case of ERP cost analysis, the number of patients used for ICER assessment changed, because after the introduction of the ICM, only 17 people from the initial 26 had exacerbation of the disease. In case of other indicators, a constant number of patients (n=41) was used, as that was the number of patients who experienced either hospitalization or unplanned visits to an emergency department.
Effects, regardless of type, were expressed as avoiding negative events by replacing standard care with IC. Thus, in the case of standard care, this effect is always zero.
Discussion
Introducing home-oriented IC causes different effects that constitute a subject of international studies. In the setting of increasing incidence of COPD in Europe and the limited budgets of health systems, our primary goal was to assess the effectiveness of home-oriented IC, which seems to meet the above-mentioned expectations, but not in all cases. In our study we used CEA analysis, which allows assessment of the economic effectiveness of integrated care in relation to demand-related results. CEA is recommended, among others, by the WHO [7] to estimate health-related consequences (so-called natural effects, including demand-related) of introducing treatment. This tool is highly accepted and often used. Also, the baseline assumptions of ICM are similar to the other IC programs described earlier, although not in all details. Similarly to the presented study, Steinel et al. demonstrated the influence of multidisciplinary team involvement in the model of care on patient health and used pharmacoeconomic tools to assess its effectiveness, although with different methods [10]. Although IC is used in numerous countries, the study of its economic effectiveness is still incomplete. In the study of McDowell, ICER was above zero, which indicate high costs, but the authors observed improved quality of life [11]. Hoogendoorn et al. found growth of the average care costs after introducing IC; therefore, ICER was higher than zero. The reason for this might be providing many additional medical services for patients receiving IC [12]. Additionally, Goossens et al. reported there were only minimal savings (EUR 65) in indirect expenses [13]. More recent studies have not fully confirmed the economic effectiveness, and there was a cost increase of GBP 494 and no clear change in demand for medical services [14]. Finally, in the rest of the studies, the economic efficacy of IC was fully or partially proven. For example, Boland et al. [15] showed positive effects of multidisciplinary teams on education and lifestyle change based on results of the Clinical COPD Questionnaire and dyspnea scale MRC. Similar results were obtained by Kruis et al. [16]. It is difficult to indicate one specific reason for such significant differences in the cost-effectiveness of integrated care models. Most likely, this is due to their different designs (including the type of support offered) but also due to the financial condition of the healthcare system itself. Nevertheless, in our study, CEA analysis confirmed ICM to be beneficial (ICER<0), which might indicate that ICM is well-adjusted to the medical needs of COPD patients; therefore, it results in fewer adverse medical events, as demonstrated by emergency appointments and hospitalizations avoided (changes in demand). All of the analyzed factors of CEA analysis seem to support that medical needs of patients were satisfied by ICM, as they less often looked for help in the emergency department and were hospitalized less frequently.
Changes in demand for medical services are commonly assessed as a surrogate indicator of effectiveness [17]. After introducing ICM, we observed desirable changes in utilization of resources, as they resulted in partial replacing of emergency and unplanned appointments to GPs with scheduled appointments. Unscheduled appointments dropped substantially (p=0.033), which corresponds with the results of Bird et al., who found a 10% reduction in use of emergency services [18]. In the present study, the demand for emergency department services fell by 7.83%; however, for all unplanned services (emergency department appointments and hospitalizations), the reduction was 28.98%. Another study, presenting early assisted discharge from hospital after COPD exacerbation, indicated an increase in the average number of GPs services; in the case of standard appointments by 0.11, home appointments by 0.36, and telephone consultations 0.38 per patient [13]. The demand for GPs services rose by 7.14%, which along with reduction in prevalence of hospitalization, can be regarded as a positive effect of ICM.
The Integrated Care Model for patients affected by severe COPD provided in Gdansk and conducted at the patient’s home with support of specialists such as physiotherapist, psychologist, nutritionist, patient’s assistant supervised by a coordinator seems to be a beneficial model, as results CEA indicated that after including patients in the ICM, the care costs were lowered and the benefits exceeded the charges. ICM was also more cost-effective in avoiding hospitalizations and unplanned appointments. Introducing ICM resulted in clear and positive changes in demand for medical services, in which patients more often visited GP offices and were less frequently hospitalized.
Conclusions
All the indicators of cost-effectiveness assessment showed that ICM is a beneficial model of care, both for the patients and the system. It helps avoid unplanned visits, which can be considered as a surrogate indicator of improved health. ICM also saves money by replacing expensive and unplanned visits (e.g., to emergency departments) with less expensive visits to GPs and pneumonologists, which is also more convenient for the patients.
Implications and limitations
The undeniable strength of the presented study is that there are no other studies in Poland or other CEE countries that used pharmacoeconomic tools to assess IC effectiveness in COPD. Such studies, mostly mentioned in this article, were conducted in western EU countries such as the USA and Canada. Moreover, in the CEE region there is an urgent need to deepen research in this area that would include regional specificities and financial considerations. Nevertheless, there is only one study carried out in Poland dealing with the assessment of economic effectiveness of care for patients affected by COPD using pharmacoeconomic tools. It was carried out in Bydgoszcz, but it only considered one aspect – pulmonary rehabilitation. However, outcomes of the CEA analysis did not prove pulmonary rehabilitation to be beneficial from the public payer point of view [19]. The program provided in Bydgoszcz did not include most of the services available in Gdansk: education of patients and their families, treatment coordination by the study coordinator, volunteers, and social workers. ICM is the first such broad program providing IC for patients suffering from advanced COPD in Poland. Our study is thus the first to use pharmacoeconomic assessment of IC in the region.
The weakness of the study is the small size of the study group, which is because the public payer does not finance ICM, so it is available only locally, as it uses funds from grants, non-governmental organizations, and local government. Currently, the ongoing arrangements for initiation of the negotiation procedure with the public payer are being settled. This would allow increasing the number of patients, conducting the longitudinal research, and exploring further implications. Nevertheless, the effects of the present study show clear evidence of the potential ICM cost-effectiveness.
Footnotes
Source of support: ST 02-0025/07 grant, Novartis Grant, ST-553 grant, Town Council of Gdańsk
Conflicts of interest
None.
References
- 1.Sia T, Tonniges T, Osterhus E, Taba S. History of the advanced medical home concept. Pediatrics. 2004;113:1473–78. [PubMed] [Google Scholar]
- 2.Kr Kringos D, Boerma W, Hutchinson A, Saltman R. Building primary care in a changing Europe: European Observatory on Health Systems and Policies. WHO; 2015. [PubMed] [Google Scholar]
- 3.Cox K, McLeaod S, Sim C, et al. Avoiding hospital admission in COPD: Impact of a specialist nursing team. Br J Nurs. 2017;26(3):152–58. doi: 10.12968/bjon.2017.26.3.152. [DOI] [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015.pdf.
- 5.Damps-Konstańska I, Cynowska B, Kuziemski K, et al. [Integrated care for patients with chronic obstructive pulmonary disease (COPD) in family doctor’s practice]. Forum Medycyny Rodzinnej. 2012;6(1):14–23. [in Polish] [Google Scholar]
- 6.Bandurska E, Damps-Konstańska I, Popowski P, et al. Impact of integrated care model (ICM) on direct medical costs in management of advanced chronic obstructive pulmonary disease (COPD) Med Sci Monit. 2017;23:2850–62. doi: 10.12659/MSM.901982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edejer T, Baltussen R, Adam T, et al. WHO – Guide to Cost-Effectiveness Analysis. WHO; 2003. [Google Scholar]
- 8.Simoens S. How to assess the value of medicines? Front Pharmacol. 2010;1:115. doi: 10.3389/fphar.2010.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein M, Stason W. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296(13):716–21. doi: 10.1056/NEJM197703312961304. [DOI] [PubMed] [Google Scholar]
- 10.Steinel JA, Madigan EA. Resource utilization in home health chronic obstructive pulmonary disease management. Outcomes Manag. 2003;7:23–27. [PubMed] [Google Scholar]
- 11.McDowell J, McClean S, FitzGibbon F. A randomised clinical trial of the effectiveness of home-based health care with telemonitoring in patients with COPD. J Telemed Telecare. 2015;21(2):80–87. doi: 10.1177/1357633X14566575. [DOI] [PubMed] [Google Scholar]
- 12.Hoogendoorn M, van Wetering CR, Schols AM, Rutten-van Molken MPMH. Is INTERdisciplinary COMmunity-based COPD management (INTERCOM) cost-effective? Eur Respir J. 2010;35:79–87. doi: 10.1183/09031936.00043309. [DOI] [PubMed] [Google Scholar]
- 13.Goossens L, Utens C, Smeenk F, et al. Cost-effectiveness of early assisted discharge for COPD exacerbations in The Netherlands. Value Health. 2013;16(4):517–28. doi: 10.1016/j.jval.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Sørensen S, Pedersen K, Weinreich U, Ehlers L. Economic evaluation of community-based case management of patients suffering from chronic obstructive pulmonary disease. Appl Health Econ Health Policy. 2017;15(3):413–24. doi: 10.1007/s40258-016-0298-2. [DOI] [PubMed] [Google Scholar]
- 15.Boland M, Kruis A, Tsiachristas A, et al. Cost-effectiveness of integrated COPD care: The RECODE cluster randomised trial. BMJ Open. 2015;5:e007284. doi: 10.1136/bmjopen-2014-007284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruis LA, Boland MR, Schoonvelde1 CH, et al. RECODE: Design and baseline results of a cluster randomized trial on cost-effectiveness of integrated COPD management in primary care. BMC Pulm Med. 2013;13:17. doi: 10.1186/1471-2466-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deb P, Li C, Trivedi P, Zimmer D. The effect of managed care on use of health care services: Results from two contemporaneous household surveys. Health Econ. 2006;15:743–60. doi: 10.1002/hec.1096. [DOI] [PubMed] [Google Scholar]
- 18.Bird S, Noronha M, Sinnott H. An integrated care facilitation model improves quality of life and reduces use of hospital resources by patients with chronic obstructive pulmonary disease and chronic heart failure. Aust J Prim Health. 2010;16(4):326–33. doi: 10.1071/PY10007. [DOI] [PubMed] [Google Scholar]
- 19.Wesołowski A, Wesołowska I, Kamzol-Kończak D, Maćkowiak M. Cost-effectiveness of pulmonary rehabilitation in COPD treatment from the perspective of the payer and the provider in a healthcare system. Nowiny Lekarskie. 2013;82(4):303–9. [Google Scholar]