Significance
Strong electrophilicity usually goes hand in hand with positive charge. In contrast, most negative ions behave like nucleophiles. Here we challenge this conventional wisdom by introducing an apparently counterintuitive idea that anions can, under well-defined circumstances, behave as superelectrophiles and even show superior binding strength and kinetic product stabilization in comparison with typical superelectrophilic cations. Emanating from the most stable gas-phase dianion [B12(CN)12]2−, synthesized here, we generate its superelectrophilic fragment anion [B12(CN)11]−, which binds Ar covalently at room temperature. This opens up additional directions for activation of inert compounds and elements.
Keywords: superelectrophilic anions, multiple-charged anions, Ar compounds, photoelectron spectroscopy, dodecaborates
Abstract
Chemically binding to argon (Ar) at room temperature has remained the privilege of the most reactive electrophiles, all of which are cationic (or even dicationic) in nature. Herein, we report a concept for the rational design of anionic superelectrophiles that are composed of a strong electrophilic center firmly embedded in a negatively charged framework of exceptional stability. To validate our concept, we synthesized the percyano-dodecoborate [B12(CN)12]2−, the electronically most stable dianion ever investigated experimentally. It serves as a precursor for the generation of the monoanion [B12(CN)11]−, which indeed spontaneously binds Ar at 298 K. Our mass spectrometric and spectroscopic studies are accompanied by high-level computational investigations including a bonding analysis of the exceptional B-Ar bond. The detection and characterization of this highly reactive, structurally stable anionic superelectrophile starts another chapter in the metal-free activation of particularly inert compounds and elements.
Converting small saturated hydrocarbons or inert chemical waste into valuable functional molecules is of overarching economic and environmental significance and represents one of the outstanding challenges of contemporary molecular science. This requires the design and realization of stable reactants that can activate the most inert compounds (1). Canonically, free radicals are employed to cleave or form chemical bonds homolytically, but such reactions tend to be difficult to control (2). An alternative approach involves electronically closed-shell molecules, which are usually less reactive than radicals, but provide access to a completely different and potentially more controllable kind of chemistry by either donating (nucleophiles) or accepting (electrophiles) an electron pair. Nucleophiles are naturally limited in their reactivity, since electrons that are very loosely bound tend to autodetach. In contrast, the reactive strength of electrophilic centers is not limited in such a way in principle.
The binding of the argon atom (Ar) at room temperature (RT) serves as a critical benchmark for novel superstrong electrophiles and is highly challenging. Argon, the most abundant noble gas (NG) on Earth, received its name from the Greek word “ἀργόν” which means “lazy” or “inactive” (3). Accordingly, Ar binding to molecules is extremely rare with exceptions being simple bi- and triatomic species in outer space or in cold matrices near the absolute zero degree, where very weak interactions with argon can hold the compound together. It is, therefore, not surprising that only a single neutral Ar-containing compound HArF, isolated in a low-temperature matrix, is presently known (4–7) and that the strongest interactions with Ar have been reported in transient cationic species (8–13). The stabilization of a sufficiently strong electrophilic binding site carrying a positive charge within a larger molecular framework is extremely challenging even in an inert environment, since such cationic species tend to rearrange to more stable isomers. Therefore, the main pathways leading to Ar compounds investigated at RT are SN2-type reactions involving either small radical cations or dications, in which the formation of long-lived unstable free binding sites can be circumvented. However, these product ions survive only sufficiently long to be detected in the absence of competing nucleophiles such as water. Therefore, room temperature Ar chemistry is currently limited to a small number of isolated cations, whose observation and characterization are rather challenging. Due to the negative electron affinity of Ar (14–16), the possibility of binding Ar directly to an anion has never been seriously considered because anions usually behave like nucleophiles. In this contribution, we report a groundbreaking concept for the rational design of highly reactive but stable molecules, so-called superelectrophilic anions, a class of compounds that are able to bind rare gas atoms, like argon, covalently and at room temperature in the presence of competing strong nucleophiles like water. Our approach is based on a counterintuitive idea that anions can, under well-defined circumstances, behave as superelectrophiles with advantages to form and stabilize products with extremely weak nucleophiles like Ar.
Recently, we reported on the exotic properties of anions (17) generated by the abstraction of a halogenide substituent X− from the exceptionally stable, weakly coordinating closo-dodecaborate dianions [B12X12]2− (Fig. 1A). The free boron coordination site in [B12X11]− carries a substantial positive partial charge and is, therefore, electrophilic, albeit the overall charge of the ion is negative. In the following, we propose a concept based on the extraordinary combination of five characteristic properties that rationalizes why anions of the type [B12X11]− are advantageous in the formation and stabilization of chemical bonds with inert noncharged species, such as NGs. These properties include the following:
-
i)
Strong electrophilicity. The free boron-binding site carries a partial positive charge (Fig. 1A) and the lowest unoccupied molecular orbital (LUMO) with negative energy is localized on this boron center (details in SI Appendix, section S1).
-
ii)
Structural stability. The rigid and highly stable dodecaborate scaffold prevents potential intramolecular rearrangement reactions. The highly reactive electrophilic center is preserved and stays chemically available.
-
iii)
Advantages in formation and stabilization of the collision complex. The strongly electrophilic (positive) binding site within the overall anionic framework generates an unusual electric field in the vicinity of the reactive boron atom. Polar nucleophiles approaching the anion in the preferred orientation (Fig. 1B) have to overcome a barrier and need to change orientation so that the nucleophilic site can react with the anion (17). This lowers the cross-section for reactive collisions. Nonpolar molecules like noble gases are not prone to this effect. The large molecular framework of these polyatomic cage anions allows the efficient redistribution of the collision energy supplied by the reaction partner(s) over the many, in particular, lower-energy vibrational degrees of freedom so that the collision complex lives long enough to be stabilized by collisional cooling.
-
iv)
Protection against substitution. After the NG bond to boron has been formed, its substitution with a nucleophile through a typical SN2 mechanism is inhibited by the cage structure of the borate, since the NG-B bond is sterically protected (Fig. 1C).
-
v)
Favorable electrostatics and dispersion forces. In addition to the dative NG-B bond, dispersion interactions and electrostatics contribute to the stabilization of NG complexes. The electrophilic binding site is located within a “crater” defined by five partially negatively charged substituents X (Fig. 2A), providing a large interaction area for attractive London dispersion forces (18). The bound NG atom, on the other hand, provides electron density to the adjacent boron atom and becomes slightly positive, leading to a stabilizing attractive electrostatic interaction with the surrounding substituents (Fig. 1C).
Fig. 1.
(A) Fragmentation reaction that yields the electrophilic anion [B12X11]− depicting the differently charged areas within the reactant and product. Note that the color coding is just for illustration purposes and is neither quantitative nor linear. (B) Visualization of the electric field resulting from the molecular charge distribution (see SI Appendix, section S1 for more detailed information). Colored arrows depict the force on a negative point charge, which is large and attractive near the positively charged binding site (green arrows) and becomes repulsive (red arrows) at larger distances due to the overall negative charge. The change in direction of the electric field close to the binding site leads to reversal of the preferred orientation of a dipole. (C) The NG atom binds to the positive binding site, provides electron density (dative bond, dark gray), and is charged slightly positive, allowing for an attractive electrostatic interaction with the surrounding negatively charged substituent shell. SN2-type substitution of the bound NG atom is prevented by the dodecaborate scaffold (reaction path crossed out in red). Additionally, a large contact surface is available for dispersion interactions (light blue; see Fig. 2A for a 3D image of the binding cavity).
Fig. 2.
(A) Electrostatic potential (ESP) plotted on the molecular surface (electron density isosurface at ρ = 0.001 a.u.) of [B12(CN)11]−. (B) Isolines of equal ESP outside the molecular surface (gray). Black lines correspond to negative whereas red lines correspond to positive ESP values (details in SI Appendix, section S1). Blue background marks the region of anionic field (force on a negative charge points away from the ion), while yellow background marks regions of attraction. These regions were obtained from the derivative of the ESP.
These aspects rationalize our recent observation: [B12Cl11]− binds Xe and even Kr at RT in the presence of the competing strong nucleophile water. Highly electrophilic cations with comparable thermodynamic binding strength toward NG (e.g., C6H5+) show no NG products under the same experimental conditions (details in SI Appendix, section S2), but exclusively form water adducts (17). This may be explained by properties iii and iv (see above), which account for kinetic advantages in forming and stabilizing the NG product in the anion case, highlighting the potential of these anions to provide experimental access to exotic compounds. One prominent example is Ar compounds for which no molecular anions are currently known at RT. Therefore, we decided to explore the potential of this chemical tool by increasing the electrophilicity of [B12X11]− type anions, first identifying alternative substituents X that should yield improved properties.
The thermodynamic stability of the precursor dianion [B12X12]2− plays a key role in obtaining highly electrophilic anions [B12X11]− through gas-phase fragmentation. As a rule of thumb, an increase in electronic stability of the dianion correlates with an increase in electrophilicity of the corresponding monoanion. Therefore, the preparation of the most electrophilic anion requires synthesizing the most stable precursor dianion. The search for small, stable multiply charged anions (MCAs) represents a long-standing challenge in chemistry and physics (19–22). Closo-dodecaborates have been shown to exhibit particularly robust electronic structures and several anionic fragments have been shown to be highly reactive (17, 23, 24). Electronic stability critically depends on the choice of the substituent X (25–29). Recently, the stability of the percyano (CN) and perboronyl (BO) dianions, [B12(CN)12]2− and [B12(BO)12]2− was predicted to exceed all known MCA records with binding energies of the second electron exceeding 5 eV (27, 28). The synthesis of such exceptionally stable dianions will open up additional perspectives in battery development and energy storage (27). To test whether these dianions represent promising precursors for the generation of exceptionally electrophilic anions we computed the atomic charges of the free boron binding sites in [B12(CN)11]− and [B12(BO)11]−. Natural population analysis (NPA) assigns charges (QNPA) of +0.82 e (X = CN) and +0.75 e (X = BO) to the boron binding sites, suggesting an enhanced electrophilicity of these anions in comparison with [B12Cl11]− (QNPA + 0.65 e), which binds Kr but not Ar. Fig. 2 visualizes the electrostatic potential (ESP) of and the electric field around [B12(CN)11]−. The comparison with [B12Cl11]− is shown in SI Appendix, section S1.
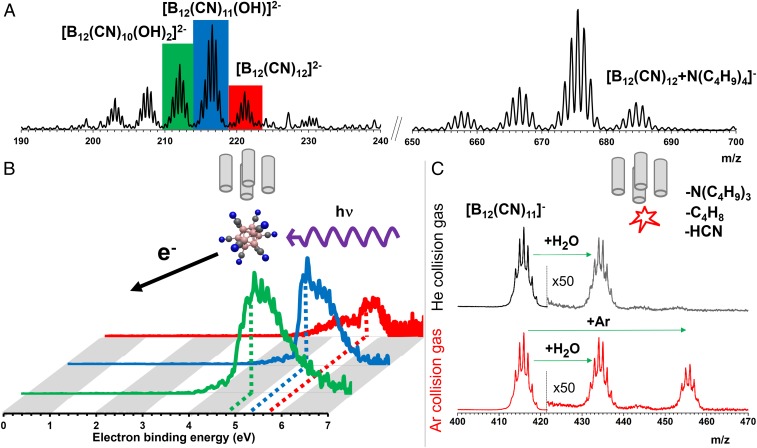
There are no established synthetic procedures for introducing boronyl substituents into closo-borates (30). The cyanation of dodecaborates was explored solely by Trofimenko and Cripps (31) and Trofimenko (32) in the 1960s, who reported the substitution of halogens in [B12Cl12]2− and [B12Br12]2− with cyanide anions in an aqueous solution upon irradiation with a low-pressure mercury lamp. Up to seven and nine halogens were substituted by CN groups in [B12Cl12]2− and [B12Br12]2−, respectively. The increase in the substituent substitution efficiency with halogen size was attributed to weakening of the boron–halogen bond. We decided to further explore this synthetic strategy to obtain [B12(CN)12]2−. Following the original idea, we irradiated an aqueous solution of K2[B12I12] and KCN for 3 h with a 150-W medium-pressure mercury lamp (see experimental details in Methods and SI Appendix, section S3) and obtained a mixture containing [B12(CN)12-n(OH)n]2− (n = 0–5) anions. After resalting with tetrabutylammonium bromide, electrospray ionization mass spectrometry (ESI-MS) in the negative ion mode (Fig. 3A) produced these dianions and the anionic [B12(CN)12-n(OH)n+N(C4H9)4]− ion pairs in the gas phase. This constitutes conclusive experimental evidence for the formation and observation of [B12(CN)12]2−.
Fig. 3.
(A) (−) ESI mass spectrum of products after photochemically induced cyanation of [B12I12]2− in water. In the lower mass-to-charge ratio region, the free dianions were detected while in the higher mass region anionic ion pairs with the counterion tetrabutylammonium were found. (B) Negative ion photoelectron spectra of [B12(CN)10(OH)2]2− (green), [B12(CN)11OH]2− (blue), and [B12(CN)12]2− (red) measured at a fixed photodetchment wavelength of 157 nm. Note that the photodetachment cross-section for [B12(CN)12]2− is smaller as the photoelectron signal is suppressed by the repulsive Coulomb barrier, resulting in low signal intensity and relatively more pronounced spectral features originating from photofragmentation visible at slightly lower electron-binding energies than those of the parent dianion spectral band (details in SI Appendix, section S4). Vertical detachment energies are indicated by dotted lines. (C) Time-of-flight mass spectra after isolation of [B12(CN)11]− and subsequent trapping at RT for up to 100 ms using helium (black) or argon (red) as a buffer gas. The water adducts are due to water vapor, which is present as an impurity (details in SI Appendix, section S7).
The electronic properties of the [B12(CN)12-n(OH)n]2− dianions isolated in the gas phase were characterized using negative ion photoelectron (PE) spectroscopy. The 157-nm PE spectra are shown in Fig. 3B (see SI Appendix, section S4 for details), which yield vertical (adiabatic) detachment energies for n = 0–2 of 5.75 eV (5.55 eV), 5.23 eV (4.97 eV), and 4.95 eV (4.50 eV), respectively. These values, and in particular those of [B12(CN)12]2−, substantially exceed the previous experimental record of 3.4 eV (2.9 eV) of [ZrF6]2− for measured detachment energies of MCAs (33). Moreover, they are in satisfactory agreement with the predicted values for these dianions (SI Appendix, section S5 and ref. 27). These results provide experimental evidence of the remarkable electronic stability of dodecaborate cyanides synthesized in this study and establish the percyano-dodecaborate dianion as the electronically most stable MCA investigated experimentally.
Tandem mass spectrometry was used to isolate the detected species and examine their fragmentation behavior with the goal to generate [B12(CN)11]−. These experiments revealed that, in contrast to the halogenated dodecaborates, the target anion could not be generated by direct substituent abstraction from [B12(CN)12]2−. Rather, collisional activation produced several doubly charged fragments in low abundance, confirming the exceptional electronic and structural stability of [B12(CN)12]2− (SI Appendix, section S6). However, collisional activation of the ion pair [B12(CN)12+N(C4H9)4]− led to fragmentation of the counter cation. Loss of tributylamine and subsequent loss of butene resulted in formation of [B12(CN)12H]−. This protonated dodecaborate subsequently fragmented by HCN loss yielding the target anion [B12(CN)11]−, which was then isolated in the ion trap for spectroscopic as well as reactivity measurements.
To quantify the electrophilicity of the free boron binding site spectroscopically, we measured the site-induced vibrational frequency shift ΔνCO upon binding carbon monoxide to [B12(CN)11]− (see SI Appendix, section S7 for details). The CO stretching frequency is sensitive to the strength of the binding site and shows a red shift (ΔνCO < 0) for nucleophilic and a blue shift (ΔνCO > 0) for electrophilic sites (34). For [B12(CN)11CO]− we detect a blue shift of +115 cm−1, a value that is roughly twice (!) as large as that for the corresponding Kr-binding perchloro compound [B12Cl11]− (+66 cm−1) and still more than 35% larger than that of the strongly electrophilic phenyl cation (+84 cm−1). These measurements experimentally verify the exceptional strength of the electrophilic binding site in the [B12(CN)11]− monoanion, in accordance with our predictions from electronic structure calculations (Fig. 2 and SI Appendix, section S1).
To probe the reactivity of [B12(CN)11]− toward smaller NG atoms we trapped the mass-selected anions at RT for up to 100 ms using either helium (3.0·10−3 mbar) or argon (1.7·10−3 mbar) as a collision gas. Note that water vapor as well as N2 and O2 are typically present at RT as trace impurities in such experiments. The corresponding time-of-flight mass spectra are shown in Fig. 3C. For pure helium, predominantly H2O adduct formation is observed, corresponding to the broad B12 isotopomer distributions, which result from the natural isotope distribution of boron (80% 11B, 20% 10B), centered at +m/z 18 (+H2O) and, to lesser extent, at +m/z 36 (+2 H2O). No evidence for He binding is found. For argon, on the other hand, an additional distribution of peaks is observed at +m/z 40, which we assign to the Ar adduct [B12(CN)11Ar]−. This first observation of an anion which is able to bind Ar at RT manifests the exceptional property of [B12(CN)11]− ions to create previously unobservable bonding motifs.
Experimental observations of B-Ar bond formation at RT are scarce. Koskinen and Cooks (8) observed the formation of B-Ar+ (Fig. 4A), while Levee et al. (35) reported the formation of F2B-Ar+ (Fig. 4B). In both cases, these ions were formed in an endothermic reaction following the loss of a halogen radical from a precursor cation. A more recent study showed the exothermic binding of Ar to cyclic boroxol cations, [B5O7]+ (Fig. 4C), at cryogenic temperatures; theoretical investigations suggested the presence of a strong boron–argon bond in [Ar-B5O7]+ that may exist even at RT (36). We calculated the 0-K enthalpy of Ar binding for these three cations at different levels of theory and compared them to that for [B12(CN)11]− (Fig. 4D). The results are listed in Table 1. Ar attachment to [B5O7]+ and [B12(CN)11]− exhibits similar enthalpies of around 60 kJ∙mol−1, making the comparison of the boron–argon binding motif in these very different ions especially interesting.
Fig. 4.
(A–D) Molecular structures of the experimentally observed ions that contain a B-Ar bond.
Table 1.
Computational values of bond parameters
| Formula | B-Ar | F2B-Ar | B5O7Ar | B12(CN)11Ar |
| Charge state | + | + | + | − |
| Ar attachment energy, 0-K enthalpy, kJ/mol | −22 | −40 | −62 | −61 |
| Bond length, Å | 2.52 | 2.11 | 2.03 | 1.98 |
| ρbcp, e⋅Å−3 | 0.30 | 0.40 | 0.42 | 0.40 |
| Hbcp/ρbcp, Ha⋅e−1 | −0.18 | −0.52 | −0.64 | −0.60 |
| QNPA free B, e | 1.00 | 1.67 | 1.50 | 0.82 |
| QNPA bound Ar, e | 0.13 | 0.26 | 0.33 | 0.41 |
| Attractive energy components, EDA | ||||
| Orbital interaction, % | 77 | 82 | 80 | 65 |
| Electrostatics, % | 20 | 15 | 17 | 25 |
| Dispersion, % | 3 | 3 | 3 | 10 |
The observed trends are predominantly independent of the theoretical method. The given values for Ar attachment enthalpies are CCSD(T)/cc-pVTZ results. The given values should be understood with an uncertainty of ±5 kJ/mol based on a comprehensive method and basis set dependency analysis including basis set superposition error (BSSE)-corrected SCS-MP2 and B3LYP with QZ and 5Z basis sets (details in SI Appendix, section S8). The CCSD(T) binding energies are obtained at the MP2 optimized geometries, for which the B-Ar bond lengths are listed. All other parameters are electron-density related and are derived from DFT calculations [B3LYP/6-311++G(2d,2p) including GD3BJ dispersion correction]. ρbcp, electron density at the bcp; Hbcp, total energy density at the bcp; QNPA, atomic charge derived from NPA calculations.
A quantum theory of atoms in molecules (QTAIM) analysis provides evidence for a bond path for all boron–argon interactions in the four molecules with a bond critical point (bcp) and a significant accumulation of electron density inside the bond (see electron density ρ at the bcp in Table 1 and SI Appendix, section S8). The negative values of the total energy density (37) H at the bcp reveal a considerable amount of covalent interaction in all four cases. While the Ar attachment energies correlate with the B-Ar bond lengths (Table 1), the electrophilicity of the binding site is related to the positive partial charge on the reactive boron. Remarkably, a comparison of the NPA charges QNPA in the free ions points to a considerably stronger electrophilic boron atom in [B5O7]+ (1.50 e) than in [B12(CN)11]− (0.82 e), indicating that other stabilizing parameters must be present in the anion to reach the very similar attachment energy and to account for a comparatively high positive partial charge of the Ar atom (0.41 e) in the anion. We attribute these additional stabilizing forces to electrostatic and dispersion interactions between the Ar atom and the surrounding substituent shell. This is confirmed by an energy decomposition analysis (EDA) (38, 39) showing that the relative contributions of the orbital overlap vs. dispersion and electrostatic forces to the binding energy (Table 1) are substantially different for [B12(CN)11]− compared with [B5O7]+. Electrostatic and dispersion forces are significantly larger in the anion compared with all cations in relation to the orbital overlap. The method and basis set dependence of binding energies and a complementary bonding analysis are provided in SI Appendix, section S8 and underline the different B-Ar bond character in [B5O7Ar]+ and [B12(CN)11Ar]−.
In conclusion, we have developed an approach for the rational design of superelectrophiles that is based on the incorporation of an electrophilic center into an anionic framework and then demonstrated its feasibility using dodecaborate-based anions. In the course of this study, we developed the synthesis and performed a comprehensive spectroscopic characterization of [B12(CN)12]2−, the electronically most stable MCA available today. Furthermore, we used tandem mass spectrometry to abstract a substituent, generating the RT argon-binding anion [B12(CN)11]−. Its strong electrophilicity, which appears counterintuitive at first sight due to its anionic nature, can be traced back to the pronounced electronic stability of the parent dianion. Formation of a localized, structurally protected but chemically accessible, positively charged binding site embedded in a spherical anionic framework results in outstanding binding properties. The chemically highly potent electrophilic anion [B12(CN)11]− may open another chapter in the metal-free activation of particularly inert compounds and elements. In addition, the formation of strong noble gas bonds with anions opens fundamentally more opportunities to stabilize them in the condensed phase: Before our findings, the strongest interactions of noble gases were observed for transient cations. The existence of such cations in the condensed phase is unlikely, because counteranions need to be present to form a condensed-phase compound. Even the weakest coordinating anions may successfully compete with noble gases for the positively charged, electrophilic binding site in such reactive cations, leading to substitution and release of noble gas atoms. In contrast, a noble gas bond in an electrophilic anion avoids this problem because the required counterion would be a cation. A cation should not compete with the noble gas for the positive binding site within the electrophilic anion (cations are usually electrophiles themselves). Gaseous ion deposition on surfaces (ion soft landing) has recently been shown to generate condensed-phase material layers from mass selected anions (40). [B12(CN)11]− binds Xe with enthalpies substantially above 100 kJ/mol (SI Appendix, section S2), suggesting that [B12(CN)11Xe]− may survive an ion soft-landing process. Joint deposition with inert counter cations may enable the generation of ionic material. These exceptional anions may complement other anionic clusters used for the construction of functional condensed-phase materials (41).
Methods
Synthesis of [NBu4][B12(CN)(12-n)(OH)n] (n = 0–4).
Potassium cyanide (AnalR NORMAPUR) and tetrabutylammonium bromide (Alfa Aesar) were purchased and used without further purification. K2[B12I12] (42) was prepared by a known procedure. The reaction was performed using a TQ 150 (Heraeus; power supply from UV-Technik) 150-W mercury medium pressure immersion lamp.
In a quartz tube equipped with a stirring bar, K2[B12I12] (1.00 g, 0.58 mmol) and KCN (7.52 g, 115 mmol) were dissolved in water (70 mL). The reaction mixture was stirred under irradiation for 3 h at room temperature. The solution was cooled down to 4 °C and 14.4 mL (173 mmol) concentrated hydrochloric acid was added. Subsequently, the reaction mixture was heated to 120 °C overnight to evaporate the solvent. The residue was dissolved in 40 mL water and tetrabutylammonium bromide (0.93 g, 2.89 mmol) was added to the solution and stirred for 30 min. The grayish precipitate (170 mg) was filtered off and dried at 120 °C overnight.
Detailed NMR data and ESI mass spectra of [NBu4][B12(CN)(12-n)(OH)n] (n = 0–4) can be found in SI Appendix, section S3.
Mass Spectrometry.
MS/MS experiments to explore fragmentation pathways yielding [B12(CN)11]− were conducted using a Bruker HCT Ion Trap and a Thermo Hybrid Linear Ion Trap (LTQ)/Orbitrap. Samples were dissolved in acetonitrile at concentrations of ∼10−5–10−6 mol⋅L−1 and injected into the mass spectrometer via a syringe pump at a flow rate of 3 μL⋅min−1.
The mass spectrometry of [B12(CN)11Ar]− and infrared photodissociation (IRPD) spectroscopy, to determine the stretching vibration of CO bound to the electrophilic binding site, were studied using the Leipzig cryogenic ion trap triple mass spectrometer described in detail elsewhere (43, 44). In brief (for more details, see SI Appendix, section S7.1) [B12(CN)11]− anions were produced via skimmer collision-induced dissociation (sCID) from precursor ions formed using a nanospray ion source with a 0.5 mmol/L solution of [B12(CN)12-n(OH)n][N(C4H9)4]2 (n = 0–5) in CH3OH/H2O (2:1, vol/vol). After the ions passed two helium buffer gas-filled radio-frequency (RF) ion guides, they were selected according to their m/z ratios in a quadrupole mass filter and subsequently trapped in a temperature-controllable RF ring-electrode ion trap filled with either helium or argon at room temperature or a mixture of carbon monoxide in helium at 55 K to form [B12(CN)11Ar]− or [B12(CN)11(CO)2]− complexes. All ions were extracted from the ion trap after 100 ms and focused into the center of an orthogonally mounted reflectron TOF tandem photofragmentation spectrometer, where IRPD spectra were obtained by irradiating the anions with tunable IR radiation from an Nd:YAG laser pumped OPO/OPA/AgGaSe2 laser system.
Detailed data on mass spectrometry and IRPD spectroscopy can be found in SI Appendix, sections S6 and S7.
Negative Ion Photoelectron Spectroscopy.
The negative ion photoelectron spectroscopy (NIPES) experiments were performed using the Pacific Northwest National Laboratory cryogenic NIPES instrument for mass-selected anions, coupled with an electrospray ionization source (45). Electrospraying 0.1-mM acetonitrile solutions of tetrabutylammonium (TBA) salts into vacuum afforded the corresponding [B12(CN)12-n(OH)n]2− (n = 0, 1, 2) anions. Photoelectron spectra of the three resulting ions were obtained at 20 K, using an F2 excimer laser at 157 nm (7.866 eV). Photoelectrons were collected with almost 100% efficiency with a magnetic bottle and analyzed in a 5.2-m-long flight tube. The resulting time-of-flight photoelectron spectra data were calibrated by recording the known spectra of I− (46) and Au(CN)2− (47). In all instances, the laser was operated at 20 Hz to enable shot-to-shot background correction of the observed intensities, and the resulting spectra have a resolution of ∼50 meV for electrons with kinetic energies of ∼2.5 eV.
Detailed descriptions of the conducted NIPES experiments can be found in SI Appendix, section S4.
Theoretical Investigations.
Analysis of the B-Ar bond was accomplished by computing the 0-K attachment enthalpies of the molecules [BAr]+, [F2BAr]+, [B5O7Ar]+, and [B12(CN)11Ar]− using density-functional theory (DFT) (B3LYP with D3 dispersion correction), spin-component-scaled Møller–Plesset perturbation theory (SCS-MP2), and coupled-cluster with single, double, and perturbative triple excitations [CCSD(T)] quantum chemistry methods. Hybrid DFT methods (B3LYP and PBE0) with dispersion correction were again used to compute adiabatic and vertical detachment energies. B3LYP was used to compute harmonic frequencies, too.
Since chemical bonds are not quantum-mechanical observables, there is no single method to satisfactorily capture the nature of an interatomic interaction, which is especially problematic for medium-to-weak polar covalent bonds as found here (B-Ar). Therefore, we resort to the full potential of a complementary bonding analysis that includes different bond analysis techniques from real, orbital, and energy space (48). Among others, frontier molecular and natural bond orbital theories, electron density and electron localizability topologies, and energy decomposition analysis are utilized. For references to all methods and the software employed, see SI Appendix, section S8. The analyses are based on fully relaxed geometries with a D3BJ-dispersion–corrected B3LYP functional and triple-zeta quality basis sets.
Supplementary Material
Acknowledgments
J.W. thanks Prof. Julia Laskin for many helpful discussions. K.R.A. acknowledges instrumental support from the Fritz-Haber-Institute of the Max-Planck-Society. S.G. acknowledges the German Research Foundation for an Emmy Noether Fellowship funded within GR 4451/1-1. E.A. and S.S.X. acknowledge support from the Center for Scalable Predictive Methods for Excitations and Correlated Phenomena (SPEC), which is funded by the US Department of Energy (DOE), Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division, as part of the Computational Chemical Sciences Program at Pacific Northwest National Laboratory (PNNL). X.-B.W. acknowledges support from the US DOE, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences at PNNL. Battelle operates the PNNL for the US DOE. This research used computer resources provided by PNNL Institutional Computing and by the National Energy Research Scientific Computing Center, which is supported by the Office of Science of the US DOE under Contract DE-AC02-05CH11231. A portion of this research (E.A. and X.-B.W.) was performed in Environmental Molecular Sciences Laboratory (EMSL), a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research and located at PNNL. J.W. acknowledges a Feodor Lynen Fellowship from the Alexander von Humboldt Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820812116/-/DCSupplemental.
References
- 1.Schwarz H, Shaik S, Li J. Electronic effects on room-temperature, gas-phase C-H bond activations by cluster oxides and metal carbides: The methane challenge. J Am Chem Soc. 2017;139:17201–17212. doi: 10.1021/jacs.7b10139. [DOI] [PubMed] [Google Scholar]
- 2.Renaud P, Sibi MP. Radicals in Organic Synthesis. Wiley-VCH; Weinheim, Germany: 2001. [Google Scholar]
- 3.Räsänen M. Argon out of thin air. Nat Chem. 2014;6:82. doi: 10.1038/nchem.1825. [DOI] [PubMed] [Google Scholar]
- 4.Khriachtchev L, Pettersson M, Runeberg N, Lundell J, Räsänen M. A stable argon compound. Nature. 2000;406:874–876. doi: 10.1038/35022551. [DOI] [PubMed] [Google Scholar]
- 5.Runeberg N, Pettersson M, Khriachtchev L, Lundell J, Räsänen M. Theoretical study of HArF, a newly observed neutral argon compound. J Chem Phys. 2001;114:836–841. [Google Scholar]
- 6.Bochenkova AV, Bochenkov VE, Khriachtchev L. HArF in solid argon revisited: Transition from unstable to stable configuration. J Phys Chem A. 2009;113:7654–7659. doi: 10.1021/jp810457h. [DOI] [PubMed] [Google Scholar]
- 7.Bihary Z, Chaban GM, Gerber RB. Vibrational spectroscopy and matrix-site geometries of HArF, HKrF, HXeCl, and HXel in rare-gas solids. J Chem Phys. 2002;116:5521–5529. [Google Scholar]
- 8.Koskinen JT, Cooks RG. Novel rare gas ions BXe+, BKr+, and BAr+ formed in a halogen/rare gas exchange reaction. J Phys Chem A. 1999;103:9565–9568. [Google Scholar]
- 9.Ascenzi D, Tosi P, Roithová J, Schröder D. Gas-phase synthesis of the rare-gas carbene cation ArCH2+ using doubly ionised bromomethane as a superelectrophilic reagent. Chem Commun (Camb) 2008:4055–4057. doi: 10.1039/b811115d. [DOI] [PubMed] [Google Scholar]
- 10.Ascenzi D, et al. Generation of the organo-rare gas dications HCCRg2+ (Rg = Ar and Kr) in the reaction of acetylene dications with rare gases. Phys Chem Chem Phys. 2008;10:7121–7128. doi: 10.1039/b810398d. [DOI] [PubMed] [Google Scholar]
- 11.Roithová J, Schröder D. Silicon compounds of neon and argon. Angew Chem Int Ed Engl. 2009;48:8788–8790. doi: 10.1002/anie.200903706. [DOI] [PubMed] [Google Scholar]
- 12.Lockyear JF, et al. Generation of the ArCF22+ dication. J Phys Chem Lett. 2010;1:358–362. [Google Scholar]
- 13.Wagner JP, McDonald DC, 2nd, Duncan MA. An argon-oxygen covalent bond in the ArOH+ molecular ion. Angew Chem Int Ed Engl. 2018;57:5081–5085. doi: 10.1002/anie.201802093. [DOI] [PubMed] [Google Scholar]
- 14.Dickerson RE, Gray HB, Haight GP., Jr . Chemical Principles. 3rd Ed Benjamin/Cummings Publishing Company, Inc.; Menlo Park, CA: 1979. [Google Scholar]
- 15.Bratsch SG, Lagowski JJ. Predicted stabilities of monatomic anions in water and liquid ammonia at 298.15 K. Polyhedron. 1986;5:1763–1770. [Google Scholar]
- 16.Buendía E, Gálvez FJ, Maldonado P, Sarsa A. Explicitly correlated wave functions for atoms and singly charged ions from Li through Sr: Variational and diffusion Monte Carlo results. Chem Phys Lett. 2014;615:21–25. [Google Scholar]
- 17.Rohdenburg M, et al. Superelectrophilic behavior of an anion demonstrated by the spontaneous binding of noble gases to [B12 Cl11 ]−. Angew Chem Int Ed Engl. 2017;56:7980–7985. doi: 10.1002/anie.201702237. [DOI] [PubMed] [Google Scholar]
- 18.Wagner JP, Schreiner PR. London dispersion in molecular chemistry–Reconsidering steric effects. Angew Chem Int Ed Engl. 2015;54:12274–12296. doi: 10.1002/anie.201503476. [DOI] [PubMed] [Google Scholar]
- 19.Scheller MK, Compton RN, Cederbaum LS. Gas-phase multiply charged anions. Science. 1995;270:1160–1166. [Google Scholar]
- 20.Boldyrev AI, Gutowski M, Simons J. Small multiply charged anions as building blocks in chemistry. Acc Chem Res. 1996;29:497–502. [Google Scholar]
- 21.Wang X-B, Wang L-S. Experimental search for the smallest stable multiply charged anions in the gas phase. Phys Rev Lett. 1999;83:3402–3405. [Google Scholar]
- 22.Dreuw A, Cederbaum LS. Multiply charged anions in the gas phase. Chem Rev. 2002;102:181–200. doi: 10.1021/cr0104227. [DOI] [PubMed] [Google Scholar]
- 23.Warneke J, Dülcks T, Knapp C, Gabel D. Collision-induced gas-phase reactions of perhalogenated closo-dodecaborate clusters–A comparative study. Phys Chem Chem Phys. 2011;13:5712–5721. doi: 10.1039/c0cp02386h. [DOI] [PubMed] [Google Scholar]
- 24.Zhao T, Zhou J, Wang Q, Jena P. Like charges attract? J Phys Chem Lett. 2016;7:2689–2695. doi: 10.1021/acs.jpclett.6b00981. [DOI] [PubMed] [Google Scholar]
- 25.Warneke J, et al. Electronic structure and stability of [B12X12]2- (X = F-At): A combined photoelectron spectroscopic and theoretical study. J Am Chem Soc. 2017;139:14749–14756. doi: 10.1021/jacs.7b08598. [DOI] [PubMed] [Google Scholar]
- 26.Fang H, Jena P. [B12(SCN)12]-: An ultrastable weakly coordinating dianion. J Phys Chem C. 2017;121:7697–7702. [Google Scholar]
- 27.Zhao H, Zhou J, Jena P. Stability of B12 (CN)122− : Implications for lithium and magnesium ion batteries. Angew Chem Int Ed Engl. 2016;55:3704–3708. doi: 10.1002/anie.201600275. [DOI] [PubMed] [Google Scholar]
- 28.Moon J, Baek H, Kim J. Unusually high stability of [B12(BO)12]2- achieved by boronyl ligand manipulation: Theoretical investigation. Chem Phys Lett. 2018;698:72–76. [Google Scholar]
- 29.Boeré RT, et al. On the oxidation of the three-dimensional aromatics [B(12)X(12)](2-) (X=F, Cl, Br, I) Chem Eur J. 2014;20:4447–4459. doi: 10.1002/chem.201304405. [DOI] [PubMed] [Google Scholar]
- 30.Zhai HJ, Chen Q, Bai H, Li SD, Wang L-S. Boronyl chemistry: The BO group as a new ligand in gas-phase clusters and synthetic compounds. Acc Chem Res. 2014;47:2435–2445. doi: 10.1021/ar500136j. [DOI] [PubMed] [Google Scholar]
- 31.Trofimenko S, Cripps HN. Photoinduced nucleophilic substitution in polyhedral boranes. J Am Chem Soc. 1965;87:653–654. [Google Scholar]
- 32.Trofimenko S. Photoinduced nucleophilic substitution in halogenated clovoboranes. J Am Chem Soc. 1966;88:1899–1904. [Google Scholar]
- 33.Wang X-B, Wang L-S. Experimental observation of a very high second electron affinity for ZrF6 from photodetachment of gaseous ZrF62- doubly charged anions. J Chem Phys A. 2000;104:4429–4432. [Google Scholar]
- 34.Gruene P, Fielicke A, Meijer G, Rayner DM. The adsorption of CO on group 10 (Ni, Pd, Pt) transition-metal clusters. Phys Chem Chem Phys. 2008;10:6144–6149. doi: 10.1039/b808341j. [DOI] [PubMed] [Google Scholar]
- 35.Levee L, et al. Formation of argon-boron bonds in the reactions of BFn+/2+ cations with neutral argon. Int J Mass Spectrom. 2012;323–324:2–7. [Google Scholar]
- 36.Jin J, Li W, Liu Y, Wang G, Zhou M. Preparation and characterization of chemically bonded argon-boroxol ring cation complexes. Chem Sci (Camb) 2017;8:6594–6600. doi: 10.1039/c7sc02472j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cremer D, Kraka E. Chemical bonds without bonding electron density–Does the difference electron-density analysis suffice for a description of the chemical bond? Angew Chem Int Ed Engl. 1984;23:627–628. [Google Scholar]
- 38.Te Velde G, et al. Chemistry with ADF. J Comput Chem. 2001;22:931–967. [Google Scholar]
- 39.Bickelhaupt FM, Baerends EJ. Kohn-Sham density functional theory: Predicting and understanding chemistry. Rev Comput Chem. 2000;15:1–86. [Google Scholar]
- 40.Warneke J, et al. Self-organizing layers from complex molecular anions. Nat Commun. 2018;9:1889. doi: 10.1038/s41467-018-04228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jena P, Sun Q. Super atomic clusters: Design rules and potential for building blocks of materials. Chem Rev. 2018;118:5755–5870. doi: 10.1021/acs.chemrev.7b00524. [DOI] [PubMed] [Google Scholar]
- 42.Juhasz MA, Matheson GR, Chang PS, Rosenbaum A, Juers DH. Microwave-assisted iodination: Synthesis of heavily iodinated 10-vertex and 12-vertex boron clusters. Synth React Inorg Met Org Nano Metal Chem. 2016;46:583–588. [Google Scholar]
- 43.Heine N, Asmis KR. Cryogenic ion trap vibrational spectroscopy of hydrogen-bonded clusters relevant to atmospheric chemistry. Int Rev Phys Chem. 2015;34:1–34. [Google Scholar]
- 44.Heine N, Asmis KR. Corrigendum to: Cryogenic ion trap vibrational spectroscopy of hydrogen-bonded clusters relevant to atmospheric chemistry. Int Rev Phys Chem. 2016;35:507. [Google Scholar]
- 45.Wang X-B, Wang L-S. Development of a low-temperature photoelectron spectroscopy instrument using an electrospray ion source and a cryogenically controlled ion trap. Rev Sci Instrum. 2008;79:073108. doi: 10.1063/1.2957610. [DOI] [PubMed] [Google Scholar]
- 46.Hanstorp D, Gustafsson M. Determination of the electron affinity. J Phys B At Mol Opt Phys. 1992;25:1773–1783. [Google Scholar]
- 47.Wang X-B, et al. Evidence of significant covalent bonding in Au(CN)2−. J Am Chem Soc. 2009;131:16368–16370. doi: 10.1021/ja908106e. [DOI] [PubMed] [Google Scholar]
- 48.Fugel M, Beckmann J, Jayatilaka D, Gibbs GV, Grabowsky S. A variety of bond analysis methods, one answer? An investigation of the element-oxygen bond of hydroxides Hn XOH. Chem Eur J. 2018;24:6248–6261. doi: 10.1002/chem.201800453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.