Significance
We developed L-2-tellurienylalanine (TePhe), an analog of phenylalanine that contains a tellurium atom, as a small-molecule probe for directly measuring protein synthesis quickly and in a wide range of contexts. Using TePhe to monitor protein synthesis overcomes challenges with existing techniques, as TePhe is an excellent Phe isostere, allowing it to be incorporated into proteins with the native translation machinery without amino acid starvation in vitro and in vivo. TePhe incorporation can be measured by mass cytometry techniques, allowing it to be multiplexed in deep profiling procedures. Furthermore, the unique properties of tellurium suggest promising applications of TePhe in X-ray crystallography, NMR spectroscopy, and imaging of translation in vivo with temporal resolution.
Keywords: mass cytometry, protein synthesis, tellurium
Abstract
Protein synthesis is central to maintaining cellular homeostasis and its study is critical to understanding the function and dysfunction of eukaryotic systems. Here we report L-2-tellurienylalanine (TePhe) as a noncanonical amino acid for direct measurement of protein synthesis. TePhe is synthetically accessible, nontoxic, stable under biological conditions, and the tellurium atom allows its direct detection with mass cytometry, without postexperiment labeling. TePhe labeling is competitive with phenylalanine but not other large and aromatic amino acids, demonstrating its molecular specificity as a phenylalanine mimic; labeling is also abrogated in vitro and in vivo by the protein synthesis inhibitor cycloheximide, validating TePhe as a translation reporter. In vivo, imaging mass cytometry with TePhe visualizes translation dynamics in the mouse gut, brain, and tumor. The strong performance of TePhe as a probe for protein synthesis, coupled with the operational simplicity of its use, suggests TePhe could become a broadly applied molecule for measuring translation in vitro and in vivo.
Measuring spatial and temporal changes in protein synthesis is necessary to understand the function and dysfunction of multicellular organisms. Protein synthesis can be monitored using radiolabeled amino acids, functional noncognate amino acids, sequencing data from ribosome-associated mRNA, or puromycin labeling approaches (1). However, application of these methods can be complicated by the limitations of the experimental protocols. For example, classical incorporation of 35S methionine commonly calls for amino acid starvation which complicates the timing and conditions of experiments in vitro (2). Incorporation of noncognate amino acids with bioorthogonal reactivity also requires cell starvation in vitro, and in vivo long incubation times and high doses are required to observe incorporation (3, 4). Improved incorporation can be achieved in vivo through the expression of engineered aminoacyl tRNA synthetases (5) and exceptional temporally resolved data can be obtained from cells expressing combinations of fluorescent RNA binding proteins and epitope tags (6–9). However, the barrier to implementing these techniques is significant compared with metabolic approaches. Puromycin-based tagging approaches to follow protein synthesis are operationally simple and can be readily applied in vivo; however, the detection of puromycin conjugates requires either antibody staining or bioorthogonal chemistry, adding to the complexity of the protocol (10, 11). Puromycin conjugates are also protein truncations that can be degraded or retained in the endoplasmic reticulum (ER), which may influence the measurement (12). To simplify the direct measurement of protein synthesis both in vitro and in vivo, we have developed a noncognate phenylalanine surrogate, L-2-tellurienylalanine (TePhe), which is efficiently incorporated by the native protein synthesis machinery (Fig. 1A). TePhe uptake can be monitored directly by atomic mass spectrometry and, most importantly, by the deep profiling methods of mass cytometry (MC) and imaging mass cytometry (IMC).
Fig. 1.
TePhe labels cells in vitro. (A) Chemical and density functional theory (DFT)-calculated structures of l-Phe and l-2-tellurienylalanine (TePhe). (B) Overlay of DFT-calculated structures for Phe and TePhe. (C and D) MC analysis of Jurkat cells after 24-h treatment of TePhe in media.
Using antibodies labeled with the heavy isotopes, MC and IMC allow >40 parameters to be measured simultaneously at the cellular level, putting these techniques at the forefront of deep profiling methods for analysis of heterogeneous cell populations (13, 14). Beyond labeled bioaffinity reagents applied ex vivo, MC probes for use in vivo are promising tools, as they facilitate investigations of the heterogeneous biochemistry within an organism. Probes for DNA synthesis (iododeoxyuridine) and hypoxia (Telox-2) have been used in vivo with subsequent detection by MC (15, 16). Furthermore, when multiple isotopes of a probe are available (isotopologs), unbiased pulsed experiments to monitor dynamic biochemistry in vivo are possible (15).
Telluromethionine (TeMet) has the potential to be used to monitor protein synthesis by MC as it is taken up into synthesized proteins and Te can be readily detected by MC, but TeMet is unstable and toxic, making its use challenging (17). Due to favorable stability and toxicity characteristics of tellurophenes, a Te-containing analog of 2-thienylalanine was investigated (18). In vivo, 2-thienylalanine is observed to be incorporated into protein as a phenylalanine surrogate (19, 20). We hypothesized that TePhe would be similarly incorporated and, through direct detection of Te, could be used to measure protein synthesis in eukaryotic systems.
Results
TePhe Labels Proteins in Vitro.
TePhe was synthesized as l-enantiomer (>95%) in four steps from N-Boc-l-propargylglycine (SI Appendix, Figs. S1 and S2). Structurally, the tellurophene ring is similar in shape to a phenyl ring, due to the distortion provided by the long C–Te bonds (Fig. 1 A and B). Similar to other tellurophenes, TePhe is stable under ambient aqueous buffered conditions for weeks (SI Appendix, Fig. S3) (18).
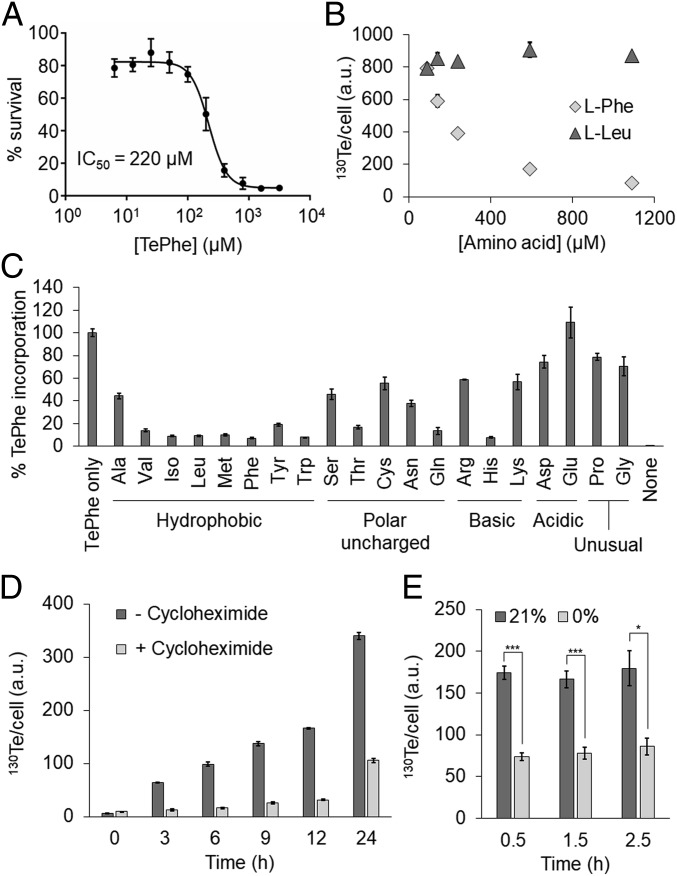
Robust uniform TePhe labeling of Jurkat cells was observed (24 h) by MC with as low as 6.3 μM TePhe and negligible Te background was present in untreated cells (Fig. 1 C and D). These experiments were carried out with TePhe-containing natural isotopic abundance in tellurium and the 130Te (34% of total Te) was monitored. Unlike labeling with other noncognate amino acids, TePhe labeling was observed without cell starvation or the use of Phe-depleted media; TePhe was simply supplemented to the incubating culture. The observed Te signal was likely due to covalent incorporation of TePhe to cellular components (i.e., recently synthesized proteins) as the fixation and washing steps before MC analysis remove small molecules, including free TePhe. As observed with previous tellurophenes, the toxicity of TePhe was substantially lower than the required working concentrations, with IC50 values ranging from 100 to 400 μM in several human cancer cell lines (Fig. 2A and SI Appendix, Fig. S4) (18).
Fig. 2.
TePhe functions as a protein synthesis probe in vitro. (A) Toxicity of TePhe in Jurkat cells, measured using the WST-1 assay after 24-h incubation. (B) Competition of TePhe (12.5 µM) incorporation in Jurkat cells with l-Phe or l-Leu performed in media (contains 90 µM l-Phe), for 24 h and analyzed by MC. (C) ICP-MS analysis of TePhe transport into Jurkat cells treated with TePhe (12.5 μM) and one of the 20 amino acids (1 mM). The 125Te channel was used for TePhe and the 24Mg channel was used to standardize cell number. TePhe/Mg ratios were expressed as percent TePhe uptake compared with TePhe alone. (D) MC analysis of Jurkat cells treated with TePhe (12.5 µM) ± CHX (3.5 µM). (E) MC analysis of PANC-1 cells treated with TePhe for 30 min after 0, 1, and 2 h in a hypoxic (<0.02%) or normoxic (21%) chamber. a.u. represents arbitrary units. Significance is reported as *P < 0.05 and ***P < 0.001.
Competition experiments (24 h, 12.5 µM TePhe) with l-Phe showed that the natural amino acid inhibited TePhe labeling in a dose-dependent manner. l-Leu, another large hydrophobic amino acid, had no effect (Fig. 2B), nor did the other aromatic amino acids l-Tyr, l-Trp, and l-His (SI Appendix, Fig. S5), demonstrating that the incorporation is amino-acid–specific. The transport of TePhe into cells was evaluated in a competition experiment with the canonical amino acids, where cells were exposed to TePhe and a competitive amino acid for 1 min, then analyzed intact to measure total intracellular Te. Large neutral amino acids efficiently inhibited TePhe transport (Fig. 2C), consistent with TePhe transport through the large amino acid transporters LAT1 and LAT2 (21, 22). Further support for TePhe incorporation into recently synthesized protein was obtained by inhibiting protein synthesis with cycloheximide, which caused a marked decrease in TePhe labeling (Fig. 2D) to an extent similar to that previously observed with cycloheximide inhibition of puromycin labeling (11).
The level of incorporation of TePhe across the proteome was evaluated by fractionating cell lysate from cells treated with TePhe (24 h, 12.5 µM) by anion exchange. Measuring Te and protein concentrations in eluted fractions gave an average incorporation of 1 TePhe residue for every 130 ± 13 Phe residues (mean ± SE) across the fractionated lysate under these conditions, assuming a 4% Phe content in the protein (SI Appendix, Fig. S6) (23). Given the background Phe (90 µM) in the media, TePhe incorporation is calculated to occur at ∼5–10% the rate of Phe. We attempted to unequivocally demonstrate the specific replacement of Phe with TePhe via mass spectrometry analysis of protein isolated from cell lysate. However, the low level of TePhe incorporation (<1%) and the fact that Te is polyisotopic (eight stable isotopes, the most abundant of which is 34%), leads to the signal from Te-containing proteins and peptides being less than 1% of the Phe-containing equivalent. To overcome the challenges of characterizing a TePhe-substituted protein from human cells, we explored overexpressed readily isolable proteins in Escherichia coli. The E. coli maltose-binding protein MalE and the Bacillus circulans xylanase Bcx were expressed from IPTG-inducible plasmids in E. coli grown in M9 minimal media in the absence of Phe and with 1 or 5 mM TePhe. After purification of the resulting proteins, liquid chromatography-MS analysis supported the partial substitution of Phe for TePhe in both MalE and Bcx (SI Appendix, Fig. S7). MalE contains 15 Phe residues and when expressed from cells grown in 1 mM TePhe, protein masses observed were consistent with 0–14 Phe to TePhe substitutions per protein. Bcx is a smaller protein containing four Phe residues. When cells expressing Bcx were grown in 5 mM TePhe, proteins with masses consistent with 0–4 Phe to TePhe substitutions were observed. At the higher TePhe concentration, more than 70% of the Bcx proteins contained three or four TePhe residues. Importantly, no masses corresponding to greater than four substitutions, or masses corresponding to TePhe to other amino acid substitutions, were observed, supporting the specific TePhe for Phe substitution.
A common use of protein synthesis probes is to monitor changes in the rate of protein synthesis in response to external stimuli in vitro. One such stimulus is hypoxia, which decreases protein synthesis at the translational level (24). To assess the effect of hypoxia in vitro, PANC-1 cells were pulsed with TePhe (10 µM) for 30 min after varying durations of exposure to hypoxia. Te labeling was attenuated at the earliest timepoint after hypoxic incubation, consistent with the described rapid kinetics of PERK activation and phosphorylation and inhibition of eIF2α to prevent mRNA translation initiation and attenuate protein synthesis (Fig. 2E) (24).
Monitoring Protein Synthesis in Mice with TePhe.
Next, we used IMC to investigate spatial variations in protein synthesis in vivo. IMC images are produced through an analogous sample preparation workflow to immunofluorescence images, but IMC uses mass tags instead of fluorescent dyes, and employs pulsed laser ablation to introduce the tissue into the mass cytometer (13). All raw IMC files are available (25). Mice bearing PANC-1 xenografts were treated with TePhe (60 mg/kg, i.v.) and then killed after 3 h. This dose was separately assessed as having no adverse toxicity (SI Appendix, Fig. S8). Sections of the jejunum, cerebellum, and tumor were analyzed by IMC after staining with an Ir-containing DNA intercalator and additional isotopically tagged antibodies. All free small molecules including TePhe are washed away during the multiple washing steps required for slide preparation; therefore, any tellurium detected by IMC represents incorporation into macromolecular structures. In the jejunum, the proliferative crypt compartment displayed a strong Te signal that was attenuated toward the tips of the villi (Fig. 3 A and B), consistent with current understanding of protein synthesis in the gut (11). In addition to visualizing this gradient, TePhe revealed higher levels of protein synthesis in the apical cytoplasm of enterocytes than their basal cytoplasm, consistent with these cells’ roles in production and secretion of digestive enzymes into the lumen of the gut. Goblet cells are unlabeled, possibly due to the mucus they produce being largely carbohydrate-based. To confirm labeling was protein synthesis dependent, mice were injected with cycloheximide (60 mg/kg, i.p.) 2 h before a TePhe dose (60 mg/kg, i.v.). Cycloheximide attenuated TePhe labeling in the jejunum (Fig. 4A). Pixel analysis showed comparable labeling of the nuclei with the Ir-containing DNA intercalator between cycloheximide (CHX)-treated and untreated mice (Fig. 4C) but a twofold attenuation of Te signal was observed in the CHX-treated sample (Fig. 4B), concurrent with a loss of strongly labeled regions.
Fig. 3.
TePhe visualizes native spatial variation of protein synthesis in mice. (A) IMC image of the jejunum showing DNA, alpha smooth muscle actin, and TePhe. Boxed region expanded in B, arrows indicate a goblet cell (Right arrow) and an enterocyte (left arrow). (C) H&E image of an adjacent section to B. (D) IMC image of the cerebellum and pons showing DNA and TePhe. Boxed region expanded in E molecular layer (M), Purkinje layer (P), granular layer (G), and white matter (W). (F) H&E image of an adjacent section to E. (Scale bars, 200 μm.)
Fig. 4.
TePhe reports on induced changes in protein synthesis in mice. (A) IMC image of mouse jejunum dosed with or without CHX before TePhe labeling. (B) Pixel histogram of tellurium channel from A showing a 2.1-fold decrease in median TePhe signal with CHX dosing. (C) Pixel histogram of DNA channel from A showing no change in DNA with CHX dosing. (D) IMC image of a human tumor mouse xenograft section dosed simultaneously with 124Telox 2 (hypoxia probe) and TePhe. V marks viable tissue, N marks necrosis, PN marks perinecrotic region. Otsu thresholding was used to determine the threshold for fast protein synthesis (E) and for hypoxia (F). (G) Bivariate histograms of pixels in image D scored by translation (TePhe) and hypoxia (Telox 2), gated according to Otsu thresholds shown in E and F [protein synthesis (PS)]. (Scale bars, 200 μm.)
Te labeling of the Purkinje cell layer was observed in the cerebellum and in neuronal bodies in the pons (Fig. 3 D and E), a pattern of labeling consistent with 14C-Leu administration (26). The TePhe labeling correlated with SOX2 positive Bergmann glia, which were stained using a 150Nd tagged antibody (SI Appendix, Fig. S9) (27).
Simultaneous Measuring of Hypoxia and Protein Synthesis in Mice.
We took advantage of the polyisotopic nature of tellurium to perform a multiplexed experiment using TePhe and our previously established MC-compatible hypoxia probe, Telox 2, to visualize the correlation between hypoxia and protein synthesis in the tumor microenvironment of PANC-1 xenografts (15). As we observed in vitro, hypoxia inhibits protein synthesis, but no direct measurements of changes in protein synthesis influenced by tumor hypoxia have been made in vivo. Administration of isotopically enriched 124Te–Telox-2 alongside natural abundance TePhe (5% 124Te corrected for in image processing) allowed the use of the 124Te signal to visualize hypoxia, and the 126Te channel to visualize protein synthesis in the same tissue. Near-orthogonal labeling was observed between the hypoxia and protein synthesis probes (Fig. 4 D–G), demonstrating suppression of translation in the hypoxic microenvironment and confirming that tellurophene-containing probes are specific for their target biochemistry (28).
Discussion
The central dogma dictates that protein synthesis underpins biological processes necessitating methods to measure spatial and temporal differences in translation in living systems. TePhe is incorporated into recently synthesized proteins in vitro and in vivo and can be readily measured with MC. We expect the simplicity of the technique—which requires no starvation, lengthy dosing regimens, secondary detection, or genetic manipulation—to lead to its broad application in biological science.
In comparison with other common noncanonical amino acids, TePhe is significantly more like its cognate analog. The methionine analogs azidohomoalanine and homopropargylglycine are incorporated at ∼500× lower efficiency than methionine (3, 11, 29). Our protein fractionation studies suggest that TePhe is incorporated ∼10–20-fold less efficiently than Phe. This high incorporation efficiency allows labeling in the presence of endogenous Phe and dramatically simplifies labeling protocols. The low background of Te in biology and the sensitivity of MC instruments allows labeling to be observed with simple supplementation to normal cell culture (as low as 10 μM, 30 min) or i.v. injection into mice (at 60 mg/kg for as low as 1 h). In the future, synthesis of isotopically enriched TePhe samples should allow further increases in sensitivity. Unlike bioorthogonal amino acids and puromycin, labeling methods do not yet exist for the isolation of TePhe labeled proteins, but given the unique reactivity of the tellurophene ring, new biorthogonal reactions may be possible with this amino acid (30).
Our observations in vitro and in vivo demonstrate that TePhe is dynamically sensitive to a biologically relevant range of translation rates. Similar analysis of protein synthesis in the murine intestine with alkyne-functionalized puromycin gave gradients of protein synthesis toward the intestinal crypt. However, localization was observed in the ER, suggesting the puromycin conjugates may not be trafficked efficiently (11). ER localization was not observed with TePhe and an expected gradient of synthesis toward the apical cytoplasm of the enterocytes was observed. In addition TePhe enters the central nervous system (CNS) from the blood with no special procedure or animal manipulation. This is likely due to the high concentration of LAT transporters at the blood brain barrier (BBB) and the similarity of TePhe to Phe (31, 32). Access to the CNS offers a substantial improvement over puromycin, which does not cross the BBB and needs to be directly injected into the CNS to monitor protein synthesis in the brain.
At working concentrations TePhe is not toxic in vitro as measured by cellular metabolic activity and proliferation. At high doses we expect the toxicity mechanism is similar to that proposed for thienyl alanine, where replacement of key phenylalanine residues perturbs the function of some proteins (20). Ongoing studies evaluating proteins with high TePhe incorporation levels will reveal the degree and mechanisms of perturbation. Preliminary protein expression experiments in E. coli confirm that TePhe can be incorporated at high levels in expressed proteins. These constructs will enable explorations of the other promising properties of the tellurium nucleus. For example, the 125Te isotope is spin-1/2 and may prove to be a highly sensitive probe of local protein environments due to the large chemical shift range of the nuclide. Furthermore, the electron density of tellurium is sufficient to generate signals in the isomorphous and anomalous difference Patterson maps at the commonly used CuKα wavelengths, suggesting TePhe can be leveraged for structural studies (17).
MC and IMC are quantitative techniques and in the case of TePhe allow measurement of translation. MC and IMC are becoming pervasive in high-dimensionality workflows, and we anticipate that TePhe will add a new dimension to their use since TePhe is compatible with standard MC reagents, including other tellurium-based probes. Incorporating panels of heavy isotope-tagged antibodies into TePhe-based experiments will allow detailed reports of cell phenotype and metabolic state to be realized. Furthermore, tellurium exists as a mixture of eight isotopes, six of which are commercially available in an enriched form. We expect that synthesis of isotopologs of TePhe, which are chemically identical but distinguishable with MC, will allow the visualization of protein synthesis with temporal and spatial resolution. We hope that administration of a mixture of tellurium-based probes for a range of cellular biochemistries will become common practice to deepen the profile provided by MC and IMC.
Materials and Methods
Cell Culture and Maintenance.
Human cell lines HCT 116 (CC-247), Jurkat (CRL-2899), MDA-MB-231 (HTB-26), PANC-1 (CRL-1469), and SiHa (HTB-35) were purchased from ATCC. Media Roswell Park Memorial Institute (RPMI), DMEM, and DMEM/F12 supplemented with 10% FBS ± 5% sodium pyruvate and penicillin/streptomycin were used in cell maintenance and experiments in a humidified 37 °C incubator with 5% CO2.
Labeling Cells with TePhe in Vitro.
The detailed synthesis of TePhe is shown in SI Appendix text. Toxicity of TePhe in adherent cells was performed using IncuCyte ZOOM Analysis system (Sartorius) while toxicity of TePhe in Jurkat cells was performed using WST-1 (Roche Inc.). See SI Appendix for full details.
For labeling experiments, Jurkat cells (5.0 × 106/mL) were incubated with TePhe in RPMI supplemented with 10% FBS at 37 °C. CHX (C7698; Millipore Sigma) experiments were performed with a pretreatment of 3.5 µM CHX for 10 min, followed by addition of TePhe to a final concentration of 12.5 μM. At various time points, 3.0 × 106 cells were harvested by centrifugation at 2,000 × g for 3 min, washed twice with PBS, and fixed in 10% formalin for 20 min at room temperature. Cells were washed twice with ice-cold PBS then incubated with 0.2-μM Cell-ID Ir intercalator for 10 min at room temperature, and washed once with PBS and once with dH2O. All cells were stored as pellets at −20 °C and analyzed on the Fluidigm Helios system. Each sample was made up in deionized water (1 mL) with 1× EQ Four Element Calibration Beads (Fluidigm). Data were analyzed using FlowJo (Tree Star Inc.) software.
Competition experiments with l-Phe and l-Leu were performed with TePhe (12.5 µM) and 0–1 mM l-Phe or l-Leu in RPMI supplemented with 10% FBS at 37 °C for 24 h. Cells were then harvested and analyzed by mass cytometry (CyTOF) as described above. The concentration of l-Phe in RPMI is 90 µM and this number is added to the concentrations of exogenous l-Phe.
Competition experiments with 20 amino acids to probe transporter selectivity for TePhe were performed by treating Jurkat cells (5.0 × 106/mL) with both TePhe (12.5 µM) and one of the 20 amino acids (1 mM) for 1 min at 37 °C in PBS. Cells were harvested by centrifugation at 2,000 × g for 3 min, washed three times with PBS, resuspended in 500 µL 37% HNO3, and analyzed by inductively coupled plasma-MS. The 125Te channel was used for TePhe and the 24Mg channel was used to standardize the number of cells. TePhe/Mg ratios were expressed as percent TePhe incorporation compared with cells treated with TePhe alone.
In preparation for hypoxia experiments, PANC-1 cells (1 × 106) were cultured for 18 h at 21% O2 in 60-mm plastic or glass dishes (Corning Inc.). Cells on glass plates were transferred to hypoxia chamber (H85 hypoxia workstation; Don Whitley Scientific) maintained at <0.02% O2. Medium was removed, replaced with preequilibrated hypoxic medium, and incubated for 0.5–2.5h. Cells were treated with TePhe (10 µM) for 30 min before the end of hypoxia exposure. A simultaneous treatment was done for cells on plastic plates under normoxic conditions. Cells were washed twice with ice-cold PBS and incubated with Ir intercalator for 10 min and processed for CyTOF as described.
Cell-based experiments were performed at least three times in technical duplicate. FlowJo was used to generate population histograms and determine the population mean. Averages and SEs of replicate means were determined using Microsoft Excel and shown as error bars. Where shown, P values were calculated with Welch’s t test.
In Vivo Labeling Experiments.
University Health Network institutional guidelines and Animal Research Committee-approved protocols were followed for mouse studies. Mice bearing PANC-1 xenografts were injected with TePhe (60 mg/kg, 40% Captisol as a vehicle) ± Telox-2 (60 mg/kg; for multiplex experiments only) i.v. through the tail vein. Three hours after injection, mice were killed, and relevant organs (tumor, intestine, brain) were harvested and fixed in 10% formalin. The fixed organs were dehydrated in 70% ethanol, then embedded in paraffin and sectioned (5 μm). Some sections were subjected to H&E staining to assess the morphology of the tissue, and some were stained for IMC analysis according to Fluidigm protocols (see SI Appendix for full details).
CHX was dissolved in saline and administered IP at a concentration of 60 mg/kg. CHX injections were given 2 h before TePhe (60 mg/kg) i.v. 1-h dose. Control mice were given saline injections before TePhe dose. The mice were killed and organs were extracted followed by formalin-fixed paraffin-embedded tissue processing and section staining. The dose of 30 mg/kg has ∼80% inhibition of brain protein synthesis (33).
Supplementary Material
Acknowledgments
We thank Taunia Closson and Jessica Watson for their assistance with IMC and CyTOF. We are also grateful for the technical assistance with animal experiments from Laura Caporiccio and Napoleon Law at the University Health Network. This work was supported by the Canadian Cancer Society, Natural Sciences and Engineering Research Council, and Fluidigm Canada. Computational resources were provided by Compute Canada and Gaussian, Inc.
Footnotes
Conflict of interest statement: L.M.W., R.N.V., L.J.E., B.G.W., and M.N. have pending intellectual property on the use of tellurium reagents for mass cytometry applications which has been licensed to the Fluidigm Canada.
This article is a PNAS Direct Submission.
Data deposition: Unprocessed IMC image files have been deposited in the Open Science Framework, https://osf.io/2ary3/.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821151116/-/DCSupplemental.
References
- 1.Dermit M, Dodel M, Mardakheh FK. Methods for monitoring and measurement of protein translation in time and space. Mol Biosyst. 2017;13:2477–2488. doi: 10.1039/c7mb00476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bizios N. Eukaryotic cell labeling and preparation for 2-D. Methods Mol Biol. 1999;112:49–52. doi: 10.1385/1-59259-584-7:49. [DOI] [PubMed] [Google Scholar]
- 3.Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT) Proc Natl Acad Sci USA. 2006;103:9482–9487. doi: 10.1073/pnas.0601637103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calve S, Witten AJ, Ocken AR, Kinzer-Ursem TL. Incorporation of non-canonical amino acids into the developing murine proteome. Sci Rep. 2016;6:32377. doi: 10.1038/srep32377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott TS, et al. Proteome labeling and protein identification in specific tissues and at specific developmental stages in an animal. Nat Biotechnol. 2014;32:465–472. doi: 10.1038/nbt.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halstead JM, et al. Translation. An RNA biosensor for imaging the first round of translation from single cells to living animals. Science. 2015;347:1367–1671. doi: 10.1126/science.aaa3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hocine S, Raymond P, Zenklusen D, Chao JA, Singer RH. Single-molecule analysis of gene expression using two-color RNA labeling in live yeast. Nat Methods. 2013;10:119–121. doi: 10.1038/nmeth.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu B, Eliscovich C, Yoon YJ, Singer RH. Translation dynamics of single mRNAs in live cells and neurons. Science. 2016;352:1430–1435. doi: 10.1126/science.aaf1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morisaki T, et al. Real-time quantification of single RNA translation dynamics in living cells. Science. 2016;352:1425–1429. doi: 10.1126/science.aaf0899. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods. 2009;6:275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Xu Y, Stoleru D, Salic A. Imaging protein synthesis in cells and tissues with an alkyne analog of puromycin. Proc Natl Acad Sci USA. 2012;109:413–418. doi: 10.1073/pnas.1111561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews TM, Tata JR. Protein synthesis by membrane-bound and free ribosomes of secretory and non-secretory tissues. Biochem J. 1971;121:683–694. doi: 10.1042/bj1210683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giesen C, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. 2014;11:417–422. doi: 10.1038/nmeth.2869. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer MH, Nolan GP. Mass cytometry: Single cells, many features. Cell. 2016;165:780–791. doi: 10.1016/j.cell.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgar LJ, et al. Isotopologous organotellurium probes reveal dynamic hypoxia in vivo with cellular resolution. Angew Chem Int Ed Engl. 2016;55:13159–13163. doi: 10.1002/anie.201607483. [DOI] [PubMed] [Google Scholar]
- 16.Chang Q, et al. Single-cell measurement of the uptake, intratumoral distribution and cell cycle effects of cisplatin using mass cytometry. Int J Cancer. 2015;136:1202–1209. doi: 10.1002/ijc.29074. [DOI] [PubMed] [Google Scholar]
- 17.Moroder L. Isosteric replacement of sulfur with other chalcogens in peptides and proteins. J Pept Sci. 2005;11:187–214. doi: 10.1002/psc.654. [DOI] [PubMed] [Google Scholar]
- 18.Park H, Edgar LJ, Lumba MA, Willis LM, Nitz M. Organotellurium scaffolds for mass cytometry reagent development. Org Biomol Chem. 2015;13:7027–7033. doi: 10.1039/c5ob00593k. [DOI] [PubMed] [Google Scholar]
- 19.Wheatley DN, Inglis MS. Amino acid analogues and the progression of HeLa cells into mitosis. I. Pre-mitotic arrest and thermosensitivity of HeLa cells in relation to the uptake and incorporation of various amino acid analogues. Exp Cell Res. 1977;107:191–199. doi: 10.1016/0014-4827(77)90400-1. [DOI] [PubMed] [Google Scholar]
- 20.Godin C, Dolan G. The effects of phenylalanine analogues on the metabolism of phenylalanine in rats. Can J Biochem. 1967;45:71–79. doi: 10.1139/o67-085. [DOI] [PubMed] [Google Scholar]
- 21.Segawa H, et al. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- 22.Kanai Y, et al. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 23.Smith MH. The amino acid composition of proteins. J Theor Biol. 1966;3:261–282. [Google Scholar]
- 24.Koritzinsky M, et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006;25:1114–1125. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassan J, et al. 2019 TePhe_IMC. Open Science Framework. Available at https://osf.io/2ary3/. Deposited March 11, 2019.
- 26.Smith CB, Kang J. Cerebral protein synthesis in a genetic mouse model of phenylketonuria. Proc Natl Acad Sci USA. 2000;97:11014–11019. doi: 10.1073/pnas.97.20.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahlfeld J, et al. Neurogenesis from Sox2 expressing cells in the adult cerebellar cortex. Sci Rep. 2017;7:6137. doi: 10.1038/s41598-017-06150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wouters BG, et al. Control of the hypoxic response through regulation of mRNA translation. Semin Cell Dev Biol. 2005;16:487–501. doi: 10.1016/j.semcdb.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Beatty KE, et al. Fluorescence visualization of newly synthesized proteins in mammalian cells. Angew Chem Int Ed Engl. 2006;45:7364–7367. doi: 10.1002/anie.200602114. [DOI] [PubMed] [Google Scholar]
- 30.Carrera EI, Lanterna AE, Lough AJ, Scaiano JC, Seferos DS. A mechanistic study of halogen addition and photoelimination from π-Conjugated tellurophenes. J Am Chem Soc. 2016;138:2678–2689. doi: 10.1021/jacs.5b11649. [DOI] [PubMed] [Google Scholar]
- 31.Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci USA. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts LM, et al. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155:423–438. doi: 10.1016/j.neuroscience.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Sershen H, et al. On the interaction between nicotine and cycloheximide. Brain Res. 1982;251:183–185. doi: 10.1016/0006-8993(82)91290-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.