Significance
Application of antigen-specific immune tolerance in autoimmune disease is a long-sought goal. We studied diseases with abundant information on the autoimmune target: in multiple sclerosis (MS), various myelin antigens are known targets of T cells and antibodies, whereas in neuromyelitis optica (NMO), the aquaporin-4 channel is attacked by T cells and antibodies. We tested whether engineered dendritic cells might induce a tolerogenic immune response in these two conditions. In this in-human clinical study, individual regulatory T cells, secreting IL-10, a key tolerogenic cytokine, were detected after treatment. These results might lead to more extensive trials with this approach in autoimmune conditions where the antigenic target has been identified, including MS, NMO, myasthenia gravis, and Graves disease.
Keywords: immune tolerance, dendritic cells, neuromyelitis optica, multiple sclerosis, Tr1 cells
Abstract
There are adaptive T-cell and antibody autoimmune responses to myelin-derived peptides in multiple sclerosis (MS) and to aquaporin-4 (AQP4) in neuromyelitis optica spectrum disorders (NMOSDs). Strategies aimed at antigen-specific tolerance to these autoantigens are thus indicated for these diseases. One approach involves induction of tolerance with engineered dendritic cells (tolDCs) loaded with specific antigens. We conducted an in-human phase 1b clinical trial testing increasing concentrations of autologous tolDCs loaded with peptides from various myelin proteins and from AQP4. We tested this approach in 12 patients, 8 with MS and 4 with NMOSD. The primary end point was the safety and tolerability, while secondary end points were clinical outcomes (relapses and disability), imaging (MRI and optical coherence tomography), and immunological responses. Therapy with tolDCs was well tolerated, without serious adverse events and with no therapy-related reactions. Patients remained stable clinically in terms of relapse, disability, and in various measurements using imaging. We observed a significant increase in the production of IL-10 levels in PBMCs stimulated with the peptides as well as an increase in the frequency of a regulatory T cell, known as Tr1, by week 12 of follow-up. In this phase 1b trial, we concluded that the i.v. administration of peptide-loaded dendritic cells is safe and feasible. Elicitation of specific IL-10 production by peptide-specific T cells in MS and NMOSD patients indicates that a key element in antigen specific tolerance is activated with this approach. The results warrant further clinical testing in larger trials.
Antigen-specific immunotherapies for autoimmune diseases aim to restore peripheral immune tolerance for pathogenic adaptive T- and B-cell responses without dampening the overall immune surveillance against microbes and cancer (1). Therefore, by using a highly selective approach, the safety and efficacy of immunotherapies should be enhanced. Several approaches have been tested aimed to restore immune tolerance, including peptide-based tolerance vaccines (either oral, i.v., intradermal, or i.m.), altered peptide ligands, DNA vaccination, or cell-based tolerance [T-cell vaccines, leukocytes coupled with peptides, mesenchymal stem cells, regulatory macrophages, T or B cells, or tolerogenic dendritic cells (tolDCs) (1–3)]. A probabilistic model of the immune system states that immune responses (including autoimmune responses) require a minimum number of MHC–peptide–T cell receptor complexes to be formed (4), providing support for testing antigen-specific tolerance based on increasing the exposure of self-antigens within an antiinflammatory environment, which can be provided by tolDCs (5). The induction of tolDCs is an approach tested for antigen-specific tolerance in autoimmune diseases such as rheumatoid arthritis, type 1 diabetes, and Crohn’s disease. These trials with tolDCs have shown satisfactory safety and some evidence of immune tolerance (6–10).
Genetic and immunological studies support the involvement of myelin-specific immune responses in the pathogenesis of multiple sclerosis (MS) (1). Even if the target antigen remains unknown, by promoting immune tolerogenic responses in adaptive immune cells that migrate to the inflamed CNS, an amelioration of the inflammatory process can be achieved (2). Previous trials of tolerance with myelin peptides in MS have been shown to be safe and that the peptides are capable of induction of immunomodulatory responses (11).
Neuromyelitis optica spectrum disorders (NMOSDs) represent an excellent opportunity for testing immune tolerance protocols. In NMOSD, the target autoantigen, is well identified, the aquaporin-4 (AQP4) (12, 13), and up to 80% of the patients have anti-AQP4 antibodies in the blood or the cerebrospinal fluid. All NMOSD patients in this trial were required to have anti-AQP4 antibodies.
In this study, we tested the safety of CNS peptide-loaded tolDC therapy and searched for signs of efficacy and markers of immune tolerance in patients with MS and NMOSD. We focused on tolDCs based on our previous experience in Crohn’s disease for which tolDCs showed excellent safety and signs of clinical efficacy (5, 9). Moreover, we combined in the same protocol patients with either MS or NMOSD to obtain evidence for the safety of tolerance induction with a diversity of CNS-related peptides. The use of a battery of tolDCs for different peptides would allow a potential approach for a broader swathe of patients.
Materials and Methods
Ethical Statement.
The protocol was reviewed and approved by the Ethics Committee of the Hospital Clinic of Barcelona and the Spanish Agency of Drugs and Sanitary Products (SI Appendix). All procedures were done following the rules of the Declaration of Helsinki Guidelines. The trial is registered under European Clinical Trials Database no. 2013-005165-39 and National Clinical Trial no. NCT02283671. All patients signed a written informed consent before inclusion in the study.
Study Design.
The study was an open-label, single-center, multiple ascending-dose phase 1b clinical trial of 3 mo of follow-up (SI Appendix, Fig. S1). We included a total of 12 patients: 8 with MS and 4 with NMOSD. The trial was performed at the Hospital Clinic of the University of Barcelona, and patient recruitment was performed between September 2015 and July 2017.
Participants were assigned to four blocks of three patients each (two MS and one NMOSD per block), and they were consecutively included once the safety of the previous cohort of patients was confirmed based on the absence of serious adverse events in that previous dose after 3 mo of follow-up.
Intravenous administration of the cells was done slowly over 1 h, after resuspension of the product in physiological saline solution. The cell dose escalation was 50 × 106, 100 × 106, 150 × 106, and 300 × 106 tolDCs in total, separated in three independent doses administered every 2 wk. The last group did not receive the programmed doses [300 million tolDCs (100 million for each dose)] due to a technical limitation to obtain the required number of cells on the leukapheresis, and for this reason, they received the maximum yield of the cultures, always above 150 million cells in total. We conducted 1-h monitoring after the administration for safety purposes. Cell viability, sterility, purity, and functionality of the final product were evaluated and registered for each patient and procedure, as previously described (9).
A baseline visit was conducted after patients fulfilled the inclusion criteria and signed the informed consent. Brain MRI and an optical coherence tomography (OCT) were performed at baseline and by week 12, as previously described (14). Blood tests for analyses and immunological assessment were done every 2 wk. The neurological examination included the Expanded Disability Status Scale (EDSS) (15) and the Multiple Sclerosis Functional Composite (MSFC) (16), which were done at baseline and by week 12. Quality of life was assessed with the Short Form-36 questionnaire at baseline and end of follow-up (17).
Primary and Secondary End Points.
The primary end point of the study was to evaluate the safety and tolerability of the treatment. Safety and tolerability were determined by the number and severity of adverse effects (AEs) related to tolDC administration using the MedDRA dictionary (https://www.meddra.org). Secondary end points were (i) assessment of clinical efficacy in terms of relapse rate and changes in the disability scales (EDSS and MSFC): (a) remaining relapse-free by week 12; (b) change on EDSS from baseline to week 12; and (c) change of MSFC from baseline to week 12; (ii) assessment of imaging efficacy: (a) number of new gadolinium-enhancing lesions at week 12; (b) number of new/enlarging T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) lesions at week 12; and (c) changes in peripapillary retinal nerve fiber layer and macular volume (MV) from baseline to week 12; and (iii) changes in the immune responses (cytokine production and cell proliferation) to peptides and differential immune cell counts at week 12.
Inclusion and Exclusion Criteria.
Inclusion criteria were (i) patients diagnosed with MS based on the 2010 McDonald criteria (18) or NMOSD with anti-AQP4 antibodies based on Wingerchuk criteria (19); (ii) disease duration ≥1 y; (iii) age between 18 and 65 y; and (iv) EDSS between 3 and 8.5. All NMOSD patients were required to have anti-AQP4 antibodies. Excluding criteria were (i) presence of a relapse or use of steroids the month before the screening; (ii) use of immunomodulatory/immunosuppressive therapy in the case of MS in the last 12 mo, but NMOSD patients were allowed to maintain their immunomodulatory treatment stable for 6 mo for ethical requirements (20); (iii) impossibility to perform brain MRI with gadolinium contrast, severe systemic diseases, or history of personal cancer or hereditary familial cancer; (iv) impossibility to proceed to the leukapheresis (e.g., absence of peripheral vein access); (v) participation in other experimental studies within the previous 3 mo; and (vi) pregnant or breastfeeding women.
Generation of Tolerogenic Dendritic Cells.
Autologous monocyte-derived dendritic cells (DCs) were obtained by leukapheresis (Amicus; Fresenius Kabi), and tolDCs were generated under good manufacturing practice (GMP) conditions by conditioning monocyte-derived DCs, as previously described (9, 21), and reported following minimum information about tolerogenic antigen-presenting cells criteria (22). Briefly, monocytes were cultured in the presence of 500 IU (international units)/mL of IL-4 and 800 IU/mL of GM-CSF (Miltenyi Biotec) during 7 d in X-VIVO-15 (BioWhittaker; Lonza) supplemented with 2% autologous serum. At day 3, fresh medium and cytokines were added; furthermore, 10−6 M dexamethasone (Merck) was added to the cells to induce the tolerogenic phenotype. At day 6, to boost peptide-specific DC tolerogenic properties, a mixture of cytokines including IL-1β, IL-6, TNF-α, and prostaglandin E2 (Miltenyi Biotec), as well as the immunogenic peptides (SI Appendix, Table S1), were added for 24 h and washed before administration. DCs from MS patients were stimulated with the seven myelin peptides from myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG) (described in ref. 11), and DCs from NMOSD patients were stimulated with the seven myelin peptides plus the T-cell immunogenic peptide from AQP4, as described in ref. 23. The peptides MBP13–32 (MBP1), MBP83–99 (MBP2), MBP11–129 (MBP3), MBP146–170 (MBP4), MOG1–20 (MOG1), MOG35–55 (MOG2), PLP139–154 (PLP1), and AQP463–76 were produced in GMP conditions (Hemmo Pharmaceuticals) and added at 5 µM to the cultures. After sterility testing and phenotype and purity characterization, tolDCs were administered by i.v. injection. Progressively increasing total DCs doses were injected in all of the cohorts (50 × 106, 100 × 106, 150 × 106, and 300 × 106 DCs) divided into three consecutive doses at weeks 0, 2, and 4 (SI Appendix, Fig. S1).
Characterization of tolDC Phenotype and Cytokine Production.
tolDCs from each patient were characterized assessing for their phenotype, cytokine production, and immunogenicity. As depicted in SI Appendix, Fig. S2A, clinical grade-produced tolDCs presented semimature phenotype (lower expression of maturation marker CD83 and MHCII compared with mature DCs) and increased expression of the glucocorticoid-induced receptor MERTK, which was described as a marker for tolDCs (24). Moreover, clinical grade-generated tolDCs produced higher amounts of the immunosuppressive cytokine IL-10 at baseline and after LPS challenge compared with mature immunogenic DCs (SI Appendix, Fig. S2B). Neither IL-12 nor IL-23 was produced by tolDCs (data not shown).
MRI.
MRI examinations were performed on a 3T Siemens Trio MRI scanner at the Hospital Clinic of the University of Barcelona, as previously described (14). Scans were performed at baseline and at week 12 of follow-up. A 1.5T MRI scanner was used for patient MS01 because she has an implanted abdominal stimulator, which prevented the use of a high-field scanner. The sequences obtained were T1-magnetization prepared rapid gradient echo before and spin echo after gadolinium administration and T2-FLAIR sequences as previously described (14).
OCT.
OCT was performed using a spectral domain device (Spectralis; Heidelberg Engineering), as previously described (14), and performed before therapy initiation and by week 12 follow-up. We calculated the thickness of the peripapillary retinal nerve fiber layer thickness and MV from each eye at baseline and week 12 following criteria as previously described (25).
Flow Cytometry Analysis.
Different immune cell subsets frequencies were analyzed from the patient’s peripheral blood mononuclear cells (PBMCs). The antibody panels for flow cytometry were (i) PBMC subtypes CD3, CD4, CD9, CD14, CD19, and CD56; (ii) T-lymphocyte subpopulations; (iii) Treg subsets: natural Tregs (CD4+CD25+Foxp3+) and Tr1 (CD4+IL−10+); (iv) T-helper subpopulations: Th1 (CD4+IFN-γ+), Th2 (CD4+IL-4+), and Th17 (CD4+IL-17+); (v) CD4 and CD8 GM-CSF “encephalitogenic” T cells; and (vi) T-cell subtypes by activation memory phenotype: CD4+CD161+ and CD8+CD161+. The list of antibodies and panels is shown in SI Appendix, Table S2. Cells were analyzed in a Canto cytometer and analyzed using the FACSDiva software.
Antigen-Specific Proliferation.
PBMCs 2 × 105 were cultured in a 96-well round plate in 200 µL of X-VIVO 15 medium (BioWhittaker; Lonza) supplemented with 2% AB human serum (Sigma-Aldrich). Cells were stimulated with each individual peptide at 5 µM or a pool of all peptides in triplicates for each condition for 6 d. Cells were cultured for 6 d at 37 °C and 5% CO2. On day 6, 50 µL of supernatant from each condition was collected and frozen at −20 °C. Then, 1 µCi of [3H]thymidine was added to the culture for 12–16 h at 37 °C and 5% CO2. Afterward, the plates were frozen until being read. Thymidine incorporation was measured using a Wallac Beta Liquid Scintillation counter (Perkin-Elmer). Measurements were obtained as cpm and calculated the stimulation index (SI) as cpm peptides/cpm unstimulated (26).
ELISA.
Supernatant from PBMCs cultures was collected after 6 d of stimulation and analyzed by ELISA the production of IFN‐γ (BD OptEIA; BD Biosciences) and IL‐10 (eBioscience), following the manufacturer’s guidelines. Results are reported as pg/mL.
Enzyme-Linked Immunospot Assay.
Enzyme-linked immunospot (ELISPOT) assays for IL-10, IFN‐γ, IL-17, and IL-4 (U-CyTech) were performed after 2-d stimulation of PBMCs with each individual peptide, according to the manufacturer’s guidelines. Peptides were added as 1:10 dilution of the 5 mM peptide stimulus, and 2 μL of each peptide was added to each well. Results are reported as the number of cells with spots for a given cytokine, as described in refs. 27 and 28. Stimuli well spot number = (mean of three wells for each stimulus) − (mean of three wells of negative control) for 200,000 PBMCs per well.
HLA-DRB1* Typing.
DNA HLA-DRB1* typing was conducted at the Department of Immunology of our center using high-resolution PCR.
Genomic DNA was collected from the cell pellets after in vitro assays using a QIAmp DNABlood Mini Kit (QIAGEN), following the manufacturer’s instructions, and stored at −20 °C. Allelic variants were genotyped using the TaqMan SNP Genotyping Assay (Applied Biosystems). The specific primers and FAM and VIC dye-labeled probes used were designed by the Applied Biosystems assay-on-demand service.
Statistical Analysis.
All data analysis was conducted based on the intent‐to‐treat principle, except where stated otherwise. Variables were tested for normality using the Kolmogorov–Smirnoff test. Differences in immunological assays from baseline to week 12 were assessed with the Wilcoxon test due to biological variability and the small sample size, preventing the assumption of normality. Changes in cytokines from baseline to week 12 were tested using Wilcoxon test by pooling all doses together (analysis for each dose or a dose–effect analysis was not possible due to the small sample size). All analyses were done in R software. The sample sizes for this dose escalation trial (three patients per dose) were chosen to detect major intolerability signals. Consistent with the design of phase 1 trials, this study was underpowered to elucidate clinical efficacy.
Results
We included 12 patients in the trial, 8 with MS and 4 with NMOSD (all of them testing positive for anti-AQP4 antibodies, as required by the inclusion criteria). The summary of the demographic and clinical data appears in Table 1. All patients received at least one dose, and all patients except one received the three doses and completed the 12-wk assessment. All patients included completed the 24-wk safety assessment. Patient NMO03 withdrew from the study after the first dose of tolDCs for personal reasons (no safety concern) but completed the safety follow-up.
Table 1.
Demographic and clinical variables of the cohort
| Patient ID | Sex | Age, y | MS subtype | Duration, y | EDSS | Previous DMD | Concomitant DMD | HLA DRB1 |
| MS01 | F | 48 | SPMS | 14.79 | 5.0 | MP | NA | *0101*0301 |
| MS02 | F | 50 | SPMS | 9.67 | 6.0 | IFN-β1a s.c. | NA | NA |
| MS03 | M | 27 | PPMS | 6.6 | 6.0 | GA | NA | NA |
| MS04 | F | 57 | PPMS | 4.29 | 5.5 | NA | NA | *1501*1301 |
| MS05 | M | 40 | SPMS | 8.18 | 6.5 | GA, IFN-β1a s.c. | NA | *0701*1001 |
| MS06 | M | 57 | SPMS | 29.29 | 6.0 | IFN-β1a i.m. | NA | *1104*1301 |
| IFN-β1a s.c. | ||||||||
| MS07 | F | 56 | PPMS | 7.46 | 6.5 | NA | NA | *0102*0404 |
| MS08 | M | 59 | RRMS | 1.81 | 6.0 | GA | NA | *0101*1101 |
| NMO01 | M | 38 | NA | 3.37 | 3.5 | AZA, RTX, IFN-β1a s.c. | RTX | *03*13 |
| NMO02 | F | 39 | NA | 21.91 | 6.0 | AZA, RTX, PDN, IVIG, MTX | MMF | NA |
| NMO03 | F | 40 | NA | 3.22 | 4.5 | RTX, MP | RTX | *0404*1001 |
| NMO04 | F | 43 | NA | 8.47 | 5.0 | RTX, CP, CyA, IVIG, MP | RTX | *0301*1101 |
AZA, azathioprine; CP, cyclophosphamide; CyA, cyclosporine A; F, female; GA, glatiramer acetate; IVIG, i.v. immunoglobulins; M, male; MMF, mycophenolate mofetil; MP, methylprednisolone; MTX, mitoxantrone; NA, not available; PDN, prednisone; PPMS, primary-progressive multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; RTX, rituximab; SPMS, secondary-progressive multiple sclerosis.
Safety.
During the 24-wk follow-up, 16 nonsevere AEs were reported. All AEs were considered unrelated to therapy (Table 2). Half of the study population reached the 2-y long-term safety milestone, and there were two severe AEs not related to the therapy (MS01 and NMO01 had elected surgeries). Additionally, we did not observe any clinically meaningful changes in vital signs (temperature, blood pressure, heart rate), blood chemistry, or blood cell counts at any time after tolDC administration.
Table 2.
Adverse events during the study
| Patient ID | AE | Severity | Relation with the study drug | Time to AEs, wk | Recovery of AEs by week 12 |
| MS01 | Fatigue | Mild | Not related | 1 | Complete |
| MS01 | Headache | Mild | Possibly related | 1 | Complete |
| MS01 | Herpes labialis | Mild | Not related | 4 | Complete |
| MS01 | Sensitive fluctuant symptoms | Mild | Not related | 8 | Persist |
| MS01 | Instability | Mild | Not related | 12 | Complete |
| MS02 | None | ||||
| MS03 | None | ||||
| MS04 | Casual fall and headache | Severe | Not related | 8 | Complete |
| MS04 | Melena with treatment for Helicobacter pylori (OCA7) | Severe | Not related | 12 | Complete |
| MS05 | None | ||||
| MS06 | Fatigue | Mild | Not related | 1 | Complete |
| MS07 | Pain in left leg | Mild | Possibly related | 2 | Complete |
| MS07 | Back pain | Mild | Not related | 4 | Complete |
| MS08 | Cold | Mild | Possibly related | 4 | Complete |
| MS08 | Fatigue | Mild | Not related | 6 | Persist |
| NMO01 | Left leg pain | Mild | Possibly related | 4 | Complete |
| NMO01 | Back pain | Severe | Not related | 6 | Persist |
| NMO02 | None | ||||
| NMO03 | Palpitations | Mild | Possibly related | 2 | Complete |
| NMO03 | Influenza | Mild | Possibly related | 2 | Complete |
| NMO04 | None |
Efficacy and Clinical Follow-Up.
All patients remained clinically stable in terms of relapses and disability worsening after 24 wk of follow-up. Similarly, no changes were observed in quality of life (Table 3). All patients included in the study (exception of NMO03) completed the MRI and OCT assessments. Of the 12 patients treated with tolDCs, 2 MS patients (MS06 and MS08) showed one new T2 lesion in the MRI by week 12, but none of them showed gadolinium-enhancing lesions at any time. We did not observe significant changes in retinal thicknesses measured by OCT (SI Appendix, Table S3).
Table 3.
Disability assessed with the EDSS and MSFC from baseline to week 12
| Patient ID | Baseline | Week 12 | Week 24 | ||||||||||||
| EDSS | T25FW | 9HPT dominant | 9HPT nondominant | Average 9HPT | PASAT3 | MSFC | EDSS | T25FW | 9HPT dominant | 9HPT nondominant | Average 9HPT | PASAT3 | MSFC | EDSS | |
| MS01 | 5.0 | 8.670 | 21.850 | 20.930 | 21.390 | 40 | −0.422 | 4.0 | 9.650 | 19.500 | 20.600 | 20.050 | 57 | −0.160 | 5.5 |
| MS02 | 6.0 | 31.065 | NA | 25.365 | 25.365 | 5 | 0.943 | 6.0 | 41.450 | 120.540 | 22.700 | 71.620 | 60 | 1.662 | 6.0 |
| MS03 | 6.0 | 12.035 | 50.560 | NA | 50.560 | 14 | 0.263 | 6.0 | 11.845 | 42.710 | NA | 42.710 | 30 | −0.399 | 6.0 |
| MS04 | 5.5 | 7.400 | 25.030 | 24.855 | 24.943 | 48 | −0.148 | 5.5 | 7.425 | 31.125 | 30.040 | 30.583 | 57 | −0.003 | 5.5 |
| MS05 | 6.5 | 11.940 | 27.230 | 21.760 | 24.495 | 39 | −0.205 | 6.5 | 13.000 | 30.815 | 23.620 | 27.218 | 46 | −0.243 | 6.5 |
| MS06 | 6.0 | 16.865 | 25.265 | 26.170 | 25.718 | 18 | −0.453 | 6.0 | 10.500 | 25.300 | 26.500 | 25.900 | 28 | −0.816 | 6.0 |
| MS07 | 6.5 | 32.100 | 22.400 | 22.750 | 22.575 | 37 | 0.398 | 6.5 | 43.350 | 20.415 | 23.370 | 21.893 | 48 | 0.395 | 6.5 |
| MS08 | 6.0 | 13.270 | 24.090 | 22.785 | 23.438 | 56 | 0.184 | 6.0 | 9.540 | 19.985 | 20.610 | 20.298 | 55 | −0.213 | 6.0 |
| NMO01 | 3.5 | 4.540 | 18.790 | 19.825 | 19.308 | 58 | −0.247 | 3.5 | 4.795 | 18.705 | 20.535 | 19.620 | 59 | −0.225 | 3.5 |
| NMO02 | 6.0 | 8.725 | 22.565 | 23.180 | 22.873 | 39 | −0.384 | 6.0 | NA | NA | NA | NA | NA | NA | 6.0 |
| NMO03 | 4.5 | 7.050 | 35.205 | 34.685 | 34.945 | 58 | 0.459 | 4.5 | NA | NA | NA | NA | NA | NA | 4.5 |
| NMO04 | 5.0 | 4.835 | 22.925 | 22.935 | 22.930 | 45 | −0.387 | 5.0 | NA | NA | NA | NA | NA | NA | 5.0 |
9HPT, nine hole-peg test; NA, not available; PASAT3, paced auditory serial addition test 3 seconds; T25FW, timed 25 feet walking.
Immunological Responses After tolDC Therapy.
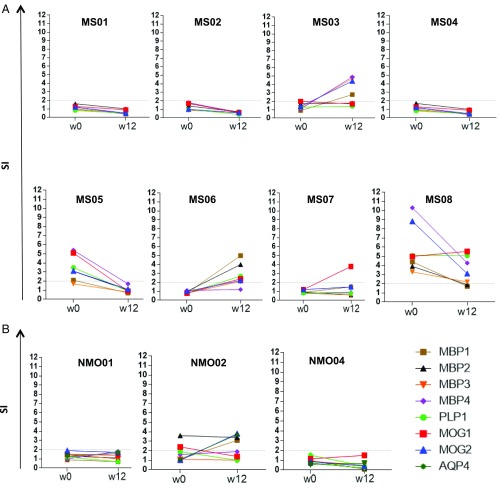
First, we evaluated changes in the antigen-specific cell proliferation induced by tolDCs by assessing [3H]thymidine incorporation in response to each peptide or the pool of peptides. We did not find significant differences on T-cell proliferation by week 12 after treatment for any of the peptides compared with baseline, although we found a trend for decreased proliferation in response to the AQP4 peptide by week 12 in NMOSD-treated patients (P = 0.067) (Fig. 1).
Fig. 1.
Peptide-specific T-cell proliferation assays at baseline and week 12. MBP13–32, MBP83–99, MBP11–129, MBP146–170, MOG1–20, MOG35–55, PLP139–154, and AQP463–76–specific T-cell responses, at baseline (before treatment) and 12 wk after tolDC treatment in patients with MS (A) or NMO (B), are shown. Proliferative responses were measured by [3H]thymidine incorporation assay. Graphs (y axis) represent the SI.
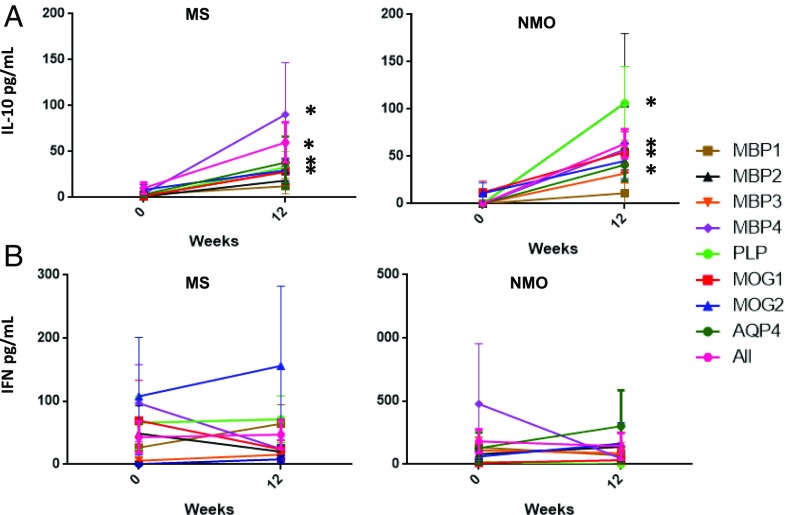
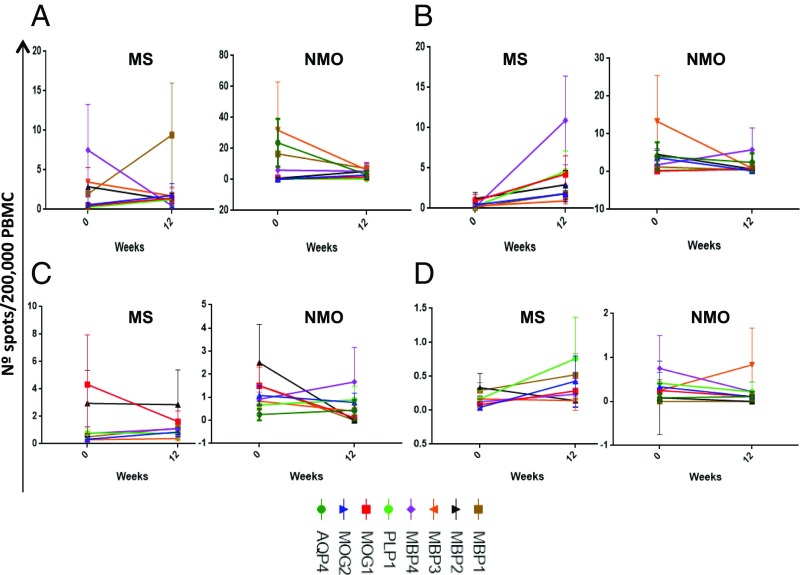
Second, we evaluated changes in cytokine secretion in response to specific peptide stimulation in PBMC culture supernatants by ELISA (INF-γ and IL-10) and in cultures by ELISPOT (IFN-γ, IL-17, IL-4, and IL-10). We found a significant increase of IL-10 production by week 12 compared with baseline for all myelin peptides (except MBP1) in patients with MS as well as for AQP4 in patients with NMOSD (Fig. 2). IL-10 production has been previously described as associated with the induction of Tr1 cells by tolDCs (5, 29). Regarding changes in cytokine production by ELISPOT, we did not find significant differences for any of the cytokines analyzed at the group level (Fig. 3).
Fig. 2.
Peptide-specific cytokine detection at baseline and week 12. Cytokine levels were measured by ELISA in the supernatant of PBMC cultures at each time point and stimulated in vitro with the given peptide for 6 d. (A) IL-10 was significantly increased by week 12 in response to MBP2 (P = 0.028), MBP3 (P = 0.18), MBP4 (P = 0.43), PLP (P = 0.18), MOG1 (P = 0.025) and showed a trend for MOG2 (P = 0.69) and AQP4 (P = 0.063) but not for MBP1 (P = 0.465). (B) IFN-γ levels were not significantly different between time points for any of the peptides.
Fig. 3.
Peptide-specific ELISPOT assays at baseline and week 12. The frequency of T cells producing IL-10 (A), IFNγ (B), IL-17 (C), or IL-4 (D) in response to peptides was analyzed in PBMC cultures at each time point and stimulated in vitro with the given peptide for 2 d.
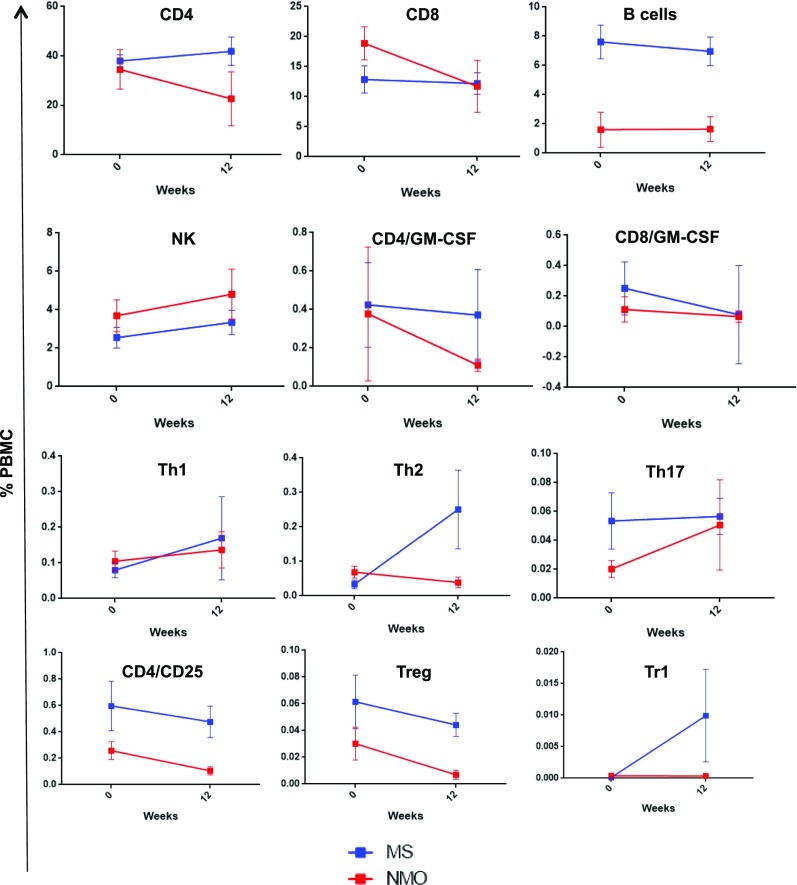
Finally, we assessed whether tolDC therapy induced changes in peripheral immune cell subset frequencies from baseline to week 12 after, including CD4 (Th1, Th2, Th17, naïve vs. memory cells, GM-CSF–expressing cells), Treg (natural Treg and Tr1), CD8, monocytes (CD14), B cells (CD19), and NK (CD56) cells, with or without memory phenotype (CD161). We observed an upward trend in the frequency of Tr1 cells (CD4+IL-10+; P = 0.14) and a significant decrease in memory CD8 (CD8+CD161+; P = 0.021), NK cells [CD56+CD161+ (P = 0.012) and CD14+CD56+ (P = 0.041)] in MS patients by week 12 compared with baseline, while the remaining subpopulations did not show significant changes (Fig. 4).
Fig. 4.
Immune-cell subset analysis by flow cytometry at baseline and week 12. Percentage of cells (means ± SEM) at baseline and 12 wk after treatment: CD4+, CD8+, B cells (CD19+), NK cells (CD56+), encephalitogenic cells (CD4+GM−CSF+ and CD8+GM−CSF+), Th1 (CD4+IFN-γ+), Th2 (CD4+IL-4+), Th17 (CD4+IL-17+), Treg (CD4+CD25+Foxp3+), and Tr1 (CD4+IL-10+).
Discussion
In this trial, we have shown feasibility and safety of treating patients with MS and NMOSD with tolDCs loaded with either myelin antigens or AQP4 antigens at the highest dose of feasible cells. Considering previous findings reporting reactivation of MS activity after altered ligand peptide therapy (30), it is important to highlight that therapy using tolDCs loaded with myelin peptide was safe. The design of the study combined patients with MS and NMOSD, treating both groups for tolerance induction with seven myelin peptides, while adding for patients with NMOSD, the combination of the AQP4 peptide plus the seven myelin peptides in the tolerizing regimen. This design allowed leveraging treatments for both conditions, with a constellation of different myelin antigens, and supplementing the regimen for patients with NMOSD by adding a peptide from AQP4. This allowed us to obtain safety data as a proof of concept for future antigen-tolerance induction clinical trials for both diseases. The trial was not powered for probing efficacy at the clinical level or at the imaging level, and for this reason, no conclusions in terms of efficacy should be raised. Finally, the immunological assessment revealed the induction of Tr1 cells producing high levels of IL-10, as it has been described in previous tolDCs trials (5, 29).
Therefore, we obtained evidence that tolDC therapy induces peptide-specific tolerance by promoting the adaptive regulatory T-cell function. Considering that Tr1 function is impaired in MS (31), augmenting Tr1 function by tolDCs may contribute to restoring peripheral immune tolerance in patients with autoimmune diseases (32–34). Future trials powered to show clinical efficacy would clarify whether the augmentation of the Tr1 response could modify the disease course in MS or NMOSD.
Indeed, NMOSD is becoming a promising candidate as a disease that is optimal for testing immune tolerization, considering that the target antigen is well known (AQP4) for this condition. The target antigen AQP4 is actually part of the criteria for making a diagnosis of this disease, making it both a “biomarker” and a target for therapy. In addition, the clinical severity of NMOSD makes a case for development of new therapies that are effective and safe. Targeting the major pathologic immune response seems a compelling strategy.
The improved knowledge and fine mapping of AQP4 epitopes and associated HLA types should allow the design of specific tolDC protocols as a path for personalized medicine. Indeed, the fact that in our trial, the combination of peptides from different antigens was well tolerated and induced Tr1 cells, suggests that a pool of AQP4 peptides in different HLA class II contexts might achieve even better results in NMOSD, than those so far seen with one AQP4 epitope.
Regulatory DCs or tolDCs have shown the ability to dampen the immune response and restore cell tolerance in animal models of autoimmune diseases as well as in humans (3, 5, 35–37). TolDCs can be generated in vitro by promoting its differentiation with GM-CSF and IL-4 and inducing the regulatory phenotype with several stimuli such as IL-10, TGF-β, vitamin D3, prostaglandin E2 or dexamethasone among others (35). tolDCs generated with dexamethasone and other stimuli have a stable phenotype, high expression of HLA class II molecules, intermediate expression of the costimulatory molecules CD80 and CD86, low expression of CD40, express high levels of IL-10 and TGF-β, and low levels of IL-12, IL-23, and TNF-α, overall conferring a low capacity to stimulate T cells, with the capacity to tolerize them (3, 35). Specifically, tolDCs induced with dexamethasone have a low expression of CD1a and CD14, and they express CD209 (34), which has been associated with the increase of Treg cells in patients with Crohn’s disease (9).
Regarding the migratory capacity of DCs, in vivo imaging of pulsed tolDCs in the experimental autoimmune encephalomyelitis model showed that these cells reached the liver and the spleen at 24 h after i.v. injection, and in smaller amounts the lymph nodes, thymus, and bone marrow, and remained stable for 7 d (38). Although it is unknown whether tolDCs are able to migrate to the inflamed CNS in MS and NMOSD, the migration to primary and secondary lymphoid organs suggest they can modulate the peripheral immune response in such lymphoid organs and alter the autoimmune attack to the brain. Regarding the mechanism by which tolDCs exert their tolerogenic effects, they may include contact-dependent mechanisms [e.g., PD-L1, Fas-ligand, ICOS-ligand, Ig-like transcript-2 (ILT-2), ILT-3, ILT4, HLA-G, and others] or contact-independent mechanisms such as immunomodulatory cytokines (e.g., IL-10 and TGF-β) or enzymes that generate immunomodulatory molecules or related to nutrient deprivation (e.g., IDO, heme-oxygenase-1, iNOS, and arginase 1), all mainly mediated by regulatory cells (3). In this study, we have obtained evidence for the induction of IL-10 and Tr1 responses in patients with MS or NMO treated with tolDCs.
Considering the critical role of DCs in the induction and maintenance of central and peripheral tolerance, clinical-grade human tolDCs have been tested in phase 1b clinical trials in type 1 diabetes, rheumatoid arthritis, and Crohn’s disease with evidence for feasibility and safety (6–9). Most of the trials have shown as the main immunological effect an increase in regulatory cells and their related cytokines (10), in agreement with our finding of increased IL-10 production and frequency of Tr1 cells.
The search for immune-tolerance therapies or antigen-specific vaccination to treat autoimmune diseases such as MS or NMOSD is based on the need of more selective immunotherapies designed to restore self-tolerance without causing general immune suppression (1). The probabilistic model of the immune system states that the outcome of the immune response is based on the probability of presenting a given antigen in the appropriate context (pro- or antiinflammatory), showing a remarkable robustness in the preference for pathogen targeted responses compared with autoimmune responses (4). Considering that the immune system exhibits the remarkable ability to detect sudden increases in the abundance of self-antigens, this model provides a framework for testing antigen-specific tolerance induction based in the exposition of a higher than normal dose of self-antigens [“the 100-fold increase in effective abundance” proposed in the model (4)] within an antiinflammatory environment provided by the tolDCs (5).
In the case of MS, the target antigen is not known yet, although proteins within the myelin sheath, such as MBP, MOG, and PLP, are important targets of the autoreactive immune response (30, 39–41). To develop antigen-specific tolerance for MS, several myelin immunodominant peptides have been identified ex vivo from patients with MS (41, 42) and tested in clinical trials using autologous leukocytes chemically coupled with such peptides (11). Indeed, tolerance induction with alpha B-crystallin (HspB5) has been tested in patients with MS showing good safety as well (43).
In the case of NMOSD, almost 80% of patients have specific antibodies against AQP4, providing a good target for antigen-specific tolerance-induction strategies (12, 13). As such, the T-cell immunogenic peptide AQP463–76 described by Zamvil and coworkers (23) was used in this trial, because it was the only known human disease-related epitope when we started the trial. New AQP4 epitopes have been described (44, 45), which would be tested in future trials.
In summary, this study shows that therapy with CNS peptide-specific loaded tolDCs is safe in patients with MS and NMOSD and is able to induce immune tolerance as indicated by the induction of regulatory T cell, Tr1, activity.
Supplementary Material
Acknowledgments
We thank Mark Sefton for English revisions, Prof. Jerry Nepom (Benarroya Institute and the Immune Tolerance Network) for his advice in immunological biomarkers, and the Group Affectats Esclerosi Multiple (GAEM) Foundation for logistic support for the study. This study was supported by the GAEM Foundation, La Caixa Foundation Grant LCF/PR/GN12/10250001, the Guthy Jackson Charity Foundation, the Instituto de Salud Carlos III [Fondo Europeo de Desarrollo Regional (FEDER) funds “Otra manera de hacer Europa,” Grant PI15/0061 (to P.V. and A.S.); and Red Española de Esclerosis múltiple Grants RD16/0015/0002, /0003, and /0015], and the Centres Excellencia Recerca Catalunya Programme/Generalitat de Catalunya. I.Z. is a recipient of the Rio Hortega research contracts from the Instituto de Salud Carlos III, Spain (Grant CM16/00113). N.S.-V. received funding from the Instituto de Salud Carlos III, Spain; and FEDER Grant FI16/00251, Predoctoral Grant for Health Research.
Footnotes
Conflict of interest statement: E.F. and L.S. published an obituary on two leaders in MS [Kildebeck EJ, et al. (2017) The emergence of neuroepidemiology, neurovirology and neuroimmunology: The legacies of John F. Kurtzke and Richard “Dick” T. Johnson. J Neurol 264:817–828]. I.Z. has received travel reimbursement from Genzyme, Biogen, and Merck for national and international meetings over the last 3 y. E.H.M.-L. has received speaker honoraria from Biogen, Roche, Novartis, and Sanofi and a travel reimbursement from Biogen, Roche, Novartis, and Sanofi. E.H.M.-L. has participated in advisory boards for Roche and Sanofi. A.S. has received compensation for consulting services and speaker honoraria from Bayer-Schering, Merck-Serono, Biogen-Idec, Sanofi-Aventis, Teva Pharmaceutical Industries Ltd., Roche, and Novartis. S.L. has received speaker honoraria and travel reimbursement from Biogen, Merck, Novartis, and Teva. I.P.-V. has received travel reimbursement from Roche Spain and Genzyme-Sanofi, European Academy of Neurology, and the European Committee for Treatment and Research in Multiple Sclerosis for international and national meetings over the last 3 y; I.P.-V. holds a patent for an affordable eye-tracking system to measure eye movement in neurologic diseases and holds stock in Aura Innovative Robotics. N.S.-V. received compensation for consulting services and speaker honoraria from Genzyme-Sanofi, Biogen idec, Merck-Serono, and Bayer-Schering. P.V. holds stocks and has received compensation from Bionure Farma SL; Health Engineering SL; Spiral Therapeutics, Inc.; and QMenta SL. L.S. received compensation from Novartis, Celgene, Bionure, Tolerion, Katexco, Atreca, and TG Therapeutics. All other authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820039116/-/DCSupplemental.
References
- 1.Steinman L. The re-emergence of antigen-specific tolerance as a potential therapy for MS. Mult Scler. 2015;21:1223–1238. doi: 10.1177/1352458515581441. [DOI] [PubMed] [Google Scholar]
- 2.Willekens B, Cools N. Beyond the magic bullet: Current progress of therapeutic vaccination in multiple sclerosis. CNS Drugs. 2018;32:401–410. doi: 10.1007/s40263-018-0518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marín E, Cuturi MC, Moreau A. Tolerogenic dendritic cells in solid organ transplantation: Where do we stand? Front Immunol. 2018;9:274. doi: 10.3389/fimmu.2018.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iranzo J, Villoslada P. Autoimmunity and tumor immunology: Two facets of a probabilistic immune system. BMC Syst Biol. 2014;8:120. doi: 10.1186/s12918-014-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flórez-Grau G, Zubizarreta I, Cabezón R, Villoslada P, Benitez-Ribas D. Tolerogenic dendritic cells as a promising antigen-specific therapy in the treatment of multiple sclerosis and neuromyelitis optica from preclinical to clinical trials. Front Immunol. 2018;9:1169. doi: 10.3389/fimmu.2018.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–2032. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benham H, et al. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med. 2015;7:290ra87. doi: 10.1126/scitranslmed.aaa9301. [DOI] [PubMed] [Google Scholar]
- 8.Bell GM, et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann Rheum Dis. 2017;76:227–234. doi: 10.1136/annrheumdis-2015-208456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jauregui-Amezaga A, et al. Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn’s disease: A phase I study. J Crohn’s Colitis. 2015;9:1071–1078. doi: 10.1093/ecco-jcc/jjv144. [DOI] [PubMed] [Google Scholar]
- 10.Phillips BE, Garciafigueroa Y, Trucco M, Giannoukakis N. Clinical tolerogenic dendritic cells: Exploring therapeutic impact on human autoimmune disease. Front Immunol. 2017;8:1279. doi: 10.3389/fimmu.2017.01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutterotti A, et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: A phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5:188ra75. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinman L, et al. Restoring immune tolerance in neuromyelitis optica: Part I. Neurol Neuroimmunol Neuroinflamm. 2016;3:e276. doi: 10.1212/NXI.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar-Or A, et al. Restoring immune tolerance in neuromyelitis optica: Part II. Neurol Neuroimmunol Neuroinflamm. 2016;3:e277. doi: 10.1212/NXI.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez-Lapiscina EH, et al. The multiple sclerosis visual pathway cohort: Understanding neurodegeneration in MS. BMC Res Notes. 2014;7:910. doi: 10.1186/1756-0500-7-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 16.Fischer JS, Rudick RA, Cutter GR, Reingold SC. National MS Society Clinical Outcomes Assessment Task Force The multiple sclerosis functional composite measure (MSFC): An integrated approach to MS clinical outcome assessment. Mult Scler. 1999;5:244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 18.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wingerchuk DM, et al. International Panel for NMO Diagnosis International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinshenker BG, et al. Guthy-Jackson Charitable Foundation International Clinical Consortium Challenges and opportunities in designing clinical trials for neuromyelitis optica. Neurology. 2015;84:1805–1815. doi: 10.1212/WNL.0000000000001520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabezón R, Ricart E, España C, Panés J, Benitez-Ribas D. Gram-negative enterobacteria induce tolerogenic maturation in dexamethasone conditioned dendritic cells. PLoS One. 2012;7:e52456. doi: 10.1371/journal.pone.0052456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lord P, et al. Minimum information about tolerogenic antigen-presenting cells (MITAP): A first step towards reproducibility and standardisation of cellular therapies. PeerJ. 2016;4:e2300. doi: 10.7717/peerj.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varrin-Doyer M, et al. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann Neurol. 2012;72:53–64. doi: 10.1002/ana.23651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabezón R, et al. MERTK as negative regulator of human T cell activation. J Leukoc Biol. 2015;97:751–760. doi: 10.1189/jlb.3A0714-334R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Dalmau B, et al. Predictors of vision impairment in multiple sclerosis. PLoS One. 2018;13:e0195856. doi: 10.1371/journal.pone.0195856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno B, et al. Methylthioadenosine reverses brain autoimmune disease. Ann Neurol. 2006;60:323–334. doi: 10.1002/ana.20895. [DOI] [PubMed] [Google Scholar]
- 27.Gabanti E, et al. Predictive value of human cytomegalovirus (HCMV) T-cell response in the control of HCMV infection by seropositive solid-organ transplant recipients according to different assays and stimuli. New Microbiol. 2016;39:247–258. [PubMed] [Google Scholar]
- 28.Malm M, Tamminen K, Vesikari T, Blazevic V. Norovirus-specific memory T cell responses in adult human donors. Front Microbiol. 2016;7:1570. doi: 10.3389/fmicb.2016.01570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, et al. Selective depletion of CD11c+ CD11b+ dendritic cells partially abrogates tolerogenic effects of intravenous MOG in murine EAE. Eur J Immunol. 2016;46:2454–2466. doi: 10.1002/eji.201546274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bielekova B, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Forero I, et al. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur J Immunol. 2008;38:576–586. doi: 10.1002/eji.200737271. [DOI] [PubMed] [Google Scholar]
- 32.Gregori S, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935–944. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 33.García-González P, Ubilla-Olguín G, Catalán D, Schinnerling K, Aguillón JC. Tolerogenic dendritic cells for reprogramming of lymphocyte responses in autoimmune diseases. Autoimmun Rev. 2016;15:1071–1080. doi: 10.1016/j.autrev.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 34.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD-L1. Eur J Immunol. 2009;39:3147–3159. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 35.Raïch-Regué D, Glancy M, Thomson AW. Regulatory dendritic cell therapy: From rodents to clinical application. Immunol Lett. 2014;161:216–221. doi: 10.1016/j.imlet.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 37.Van Brussel I, et al. Tolerogenic dendritic cell vaccines to treat autoimmune diseases: Can the unattainable dream turn into reality? Autoimmun Rev. 2014;13:138–150. doi: 10.1016/j.autrev.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Mansilla MJ, et al. Beneficial effect of tolerogenic dendritic cells pulsed with MOG autoantigen in experimental autoimmune encephalomyelitis. CNS Neurosci Ther. 2015;21:222–230. doi: 10.1111/cns.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villoslada P, et al. Frequency, heterogeneity and encephalitogenicity of T cells specific for myelin oligodendrocyte glycoprotein in naive outbred primates. Eur J Immunol. 2001;31:2942–2950. doi: 10.1002/1521-4141(2001010)31:10<2942::aid-immu2942>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 40.Wallström E, et al. Increased reactivity to myelin oligodendrocyte glycoprotein peptides and epitope mapping in HLA DR2(15)+ multiple sclerosis. Eur J Immunol. 1998;28:3329–3335. doi: 10.1002/(SICI)1521-4141(199810)28:10<3329::AID-IMMU3329>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 41.Grau-López L, et al. Specific T-cell proliferation to myelin peptides in relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18:1101–1104. doi: 10.1111/j.1468-1331.2010.03307.x. [DOI] [PubMed] [Google Scholar]
- 42.Bielekova B, et al. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J Immunol. 2004;172:3893–3904. doi: 10.4049/jimmunol.172.6.3893. [DOI] [PubMed] [Google Scholar]
- 43.van Noort JM, Bsibsi M, Nacken PJ, Verbeek R, Venneker EH. Therapeutic intervention in multiple sclerosis with alpha B-crystallin: A randomized controlled phase IIa trial. PLoS One. 2015;10:e0143366. doi: 10.1371/journal.pone.0143366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arellano B, et al. Human aquaporin 4281-300 is the immunodominant linear determinant in the context of HLA-DRB1*03:01: Relevance for diagnosing and monitoring patients with neuromyelitis optica. Arch Neurol. 2012;69:1125–1131. doi: 10.1001/archneurol.2012.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuya N, et al. Increased T-cell immunity against aquaporin-4 and proteolipid protein in neuromyelitis optica. Int Immunol. 2011;23:565–573. doi: 10.1093/intimm/dxr056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.